- 1Pingyuan Laboratory, Xinxiang, China
- 2Department of Nephrology, China-Japan Friendship Hospital, Beijing, China
- 3Department of Nephrology, First Medical Center of Chinese PLA General Hospital, Nephrology Institute of the Chinese People’s Liberation Army, National Key Laboratory of Kidney Diseases, National Clinical Research Center for Kidney Diseases, Beijing Key Laboratory of Kidney Disease Research, Beijing, China
- 4Department of Microbiology, Immunology and Infectious Diseases, University of Calgary, Calgary, AB, Canada
The global impact of COVID-19 has highlighted the urgent need for effective therapeutic interventions against SARS-CoV-2. Azvudine, a dual-target nucleoside drug initially developed for human immunodeficiency virus (HIV), has gained attention for its potential in treating COVID-19. On 25 July 2022, Azvudine received conditional approval from the National Medical Products Administration (NMPA) of China, making it the first oral SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) inhibitor for COVID-19 treatment. This review explores the pharmacological activity, antiviral mechanisms, and clinical effectiveness of azvudine in the context of COVID-19. Clinical trials have demonstrated its ability to reduce the viral load, shorten the time to nucleic acid negativity, and improve clinical outcomes in patients. Additionally, azvudine has shown excellent pharmacokinetic properties and a favorable safety profile with mild side effects. The review also addresses the importance of drug interactions and safety considerations, particularly in high-risk populations. Research should focus on optimizing second-generation inhibitors with enhanced effectiveness against SARS-CoV-2 variants, improving oral bioavailability, and minimizing adverse effects, ensuring more robust treatment options for COVID-19.
1 Introduction
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has driven the rapid development and approval of several antiviral drugs to mitigate the impact of COVID-19. Among these, RNA-dependent RNA polymerase (RdRp) inhibitors and protease inhibitors have played a crucial role in reducing viral replication, disease severity, and mortality. Notable oral antivirals such as Remdesivir, Molnupiravir, and Nirmatrelvir/Ritonavir (Paxlovid), Azvudine have been widely used in clinical practice.
Azvudine is the first dual-target nucleoside drug (inhibiting nucleoside reverse transcriptase and restoring cytidine deaminase expression) with broad-spectrum antiviral activity against human immunodeficiency virus (HIV), hepatitis C virus (HCV), enterovirus 71 (EV71), and hepatitis B virus (HBV) infections (Zhang J. L. et al., 2021). It is also known to modulate P-glycoprotein (P-gp) expression and is effective against SARS-CoV-2 (Liu et al., 2017; Liu et al., 2018). Its chemical name is 1-(4-azido-2-deoxy-2-fluoro-β-D-arabinofuranosyl) cytosine, with a molecular formula of C9H11FN6O4 and a relative molecular weight of 286.22 (Yu and Chang, 2022). RdRP is a promising therapeutic target for SARS-CoV-2 infection. Azulfidine (FNC, RO-0622), a first-in-class nucleoside-based prodrug developed by Henan Sincere Biotech Co., Ltd., received conditional marketing authorization from the National Drug Administration (NMPA) on 25 July 2022, becoming the first oral SARS-CoV-2 RdRp inhibitor for treating adult COVID-19 patients in China (Yu and Chang, 2022; Yu and Chang, 2020). Figure 1 shows the life cycle of SARS-CoV-2 and the targets of azvudine. Recent clinical trials have shown that 40% of people treated with azvudine have improved clinical symptoms (NCT04668235) (Zhang J. L. et al., 2021). Following these results, Chinese authorities approved azvudine for COVID-19 treatment. The drug has demonstrated desirable pharmacokinetic properties, excellent effectiveness, and safety in initial clinical trials (NCT04303598, CXHS2000016, CXHS2000017). During the clinical studies, COVID-19 patients received 5 mg per day of azvudine combined with standard treatment for up to 14 days (NCT05033145, NCT04668235). Real-world evidence has provided critical insights into their performance outside controlled clinical settings. A real-world study analyzed Remdesivir’s effectiveness, confirming significant clinical improvement and viral load reduction (Mazzitelli et al., 2023a). Another observational study reported Molnupiravir and nirmatrelvir/ritonavir’s real-life data on tolerability, safety, and adverse eventsin high-risk populations (Mazzitelli et al., 2023b). Additionally, a multicenter real-world study showed the use of oral antivirals to treat COVID-19 was associated with a reduced risk of hospitalization and inpatient disease progression among older patients living in nursing homes (Ma et al., 2023). A summary of the dose, frequency, clinical indications and effectiveness of antivirals and mAbs in COVID-19 patients is shown in Table 1.

Figure 1. Overview of the life cycle of SARS-CoV-2 and targets for the antiviral drug azvudine. In the host cell, the spike protein (S) of SARS-CoV-2 interacts with the cellular receptor ACE2, followed by viral entry into the host cell. The virus enters the host cell in two ways, either through serine proteases that activate the virus to fuse with the plasma membrane, or through the host’s endocytic machinery that activates the virus to fuse with the cell membrane. The viral genome is released in the cytoplasm and translated into the viral replicase polyproteins (PP1a and PP1ab), which are subsequently cleaved by the viral proteases to form non-structural proteins (nsps). Some of the nsps then form the replicase-transcriptase complex, the RNA-dependent RNA polymerase (RdRp). Through intermittent transcription, the polymerase produces a family of subgenomic mRNAs that are ultimately translated into functional viral proteins. In the cytoplasm, the viral nucleocapsid is formed along with genomic RNA and N proteins, and buds in the lumen of the ERGIC. The virus is then released from the host’s infected cells into the extracellular space by exocytosis. Created with BioRender software (https://app.biorender.com/)
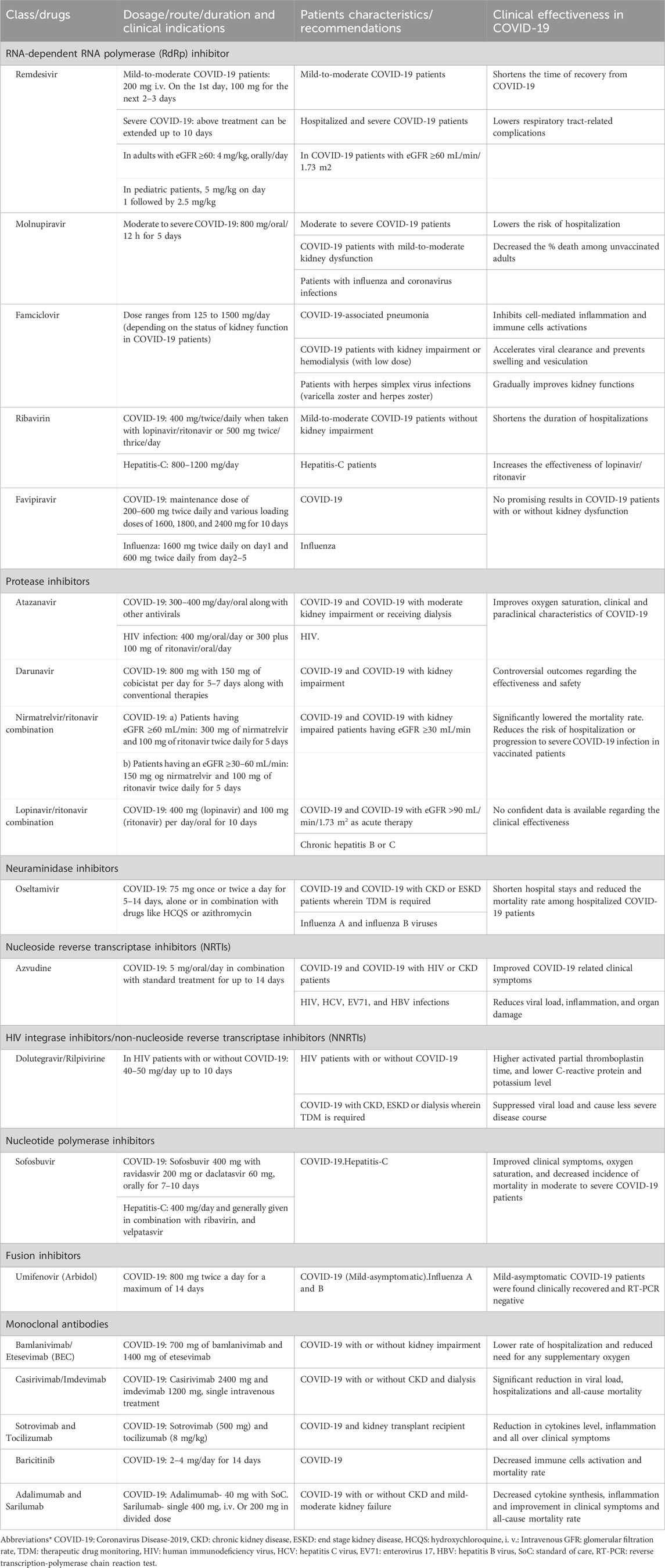
Table 1. Dosage, clinical indications and effectiveness of antivirals and monoclonal antibodies in COVID-19 patients.
Compared to other COVID-19 drugs, azvudine offers several unique advantages. Its dual-target mechanism provides a broader antiviral spectrum and potentially reduces the risk of drug resistance (Wang et al., 2025; Meng et al., 2024). Additionally, its ability to modulate P-glycoprotein expression may enhance its therapeutic effects and safety profile. The molecular mechanism of azvudine involves its incorporation into viral RNA by RdRp, leading to chain termination and inhibition of viral replication. This mechanism is distinct from some other COVID-19 drugs that target different stages of the viral life cycle, such as viral entry or assembly.
2 Search strategy
A comprehensive literature search was conducted to identify relevant studies on azvudine’s effectiveness, safety, and mechanism of action in the treatment of COVID-19. The following databases were systematically searched: PubMed, Web of Science, Embase, and Cochrane Library. The search covered articles published up to 2025/3/31, using a combination of keywords and Medical Subject Headings (MeSH) terms, including: (“Azvudine” OR “FNC” OR “RO-0622″) AND (“COVID-19″OR “SARS-CoV-2″), (“Nucleoside analog” OR “RNA-dependent RNA polymerase inhibitor”) AND (“COVID-19 treatment”). The inclusion criteria were: 1. Studies investigating the pharmacokinetics, effectiveness, and safety of azvudine. 2. Clinical trials, real-world studies, and preclinical investigations. 3. Articles published in English. The exclusion criteria were: 1. Non-peer-reviewed articles and preprints without sufficient data validation.2. Studies focusing on unrelated diseases or mechanisms. Additional references were identified through manual screening of bibliographies from relevant publications. Two independent reviewers assessed the eligibility of the studies, and discrepancies were resolved by consensus.
3 Azvudine for treating COVID-19
3.1 Pharmacokinetics
The synthesis of azvudine began with structural modification and optimization of the anti-HIV RdRp inhibitor RO-9187, which is derived from the potent nucleoside inhibitor NM-107 (Sommadossi and La Colla, 2001a; Sommadossi and La Colla, 2001b) (Figure 2). The 4′-azido-substituted R1479 exhibited significant activity against HIV in vitro (IC50 = 1.28 μM), with increased oral bioavailability and a larger therapeutic window (Klumpp et al., 2006; Smith et al., 2007). The 2′-hydroxyl inversion to form RO-9187 resulted in increased anti-HIV potency in vitro (IC50 = 0.171 μM) (Klumpp et al., 2008; Smith et al., 2009a). Additionally, RO-9187 showed increased phosphorylation efficiency, a rate-limiting step in the process. Replacing the 2′-β-hydroxyl group of RO-9187 with 2′-β-fluorine increased the in vitro anti-HCV potency (EC50 = 0.024 μM) (Smith et al., 2009b). The formation of FNC hydrochloride resulted in excellent antiviral activity against HIV (wild-type) in vitro (EC50 = 0.13 nM) (Wang et al., 2011). FNC also demonstrated excellent in vitro activity against HIV-1 (EC50 = 0.03–6.92 nM) and HIV-2 (EC50 = 0.018–0.025 nM) with low cytotoxicity (selectivity index [SI] > 1000) (Wang et al., 2014a). The safety and effectiveness of FNC have been evaluated in HIV patients since 2013, with several clinical trials (e.g., ClinicalTrials.gov: NCT04109183, NCT04303598) demonstrating its favorable long-term safety profile in a 48-week oral regimen for patients with AIDS (Zhang et al., 2021b). FNC was conditionally approved by the State Drug Administration of China on 21 July 2021 (XZXK-2021-214), for treating HIV (Yang and Wang, 2023).
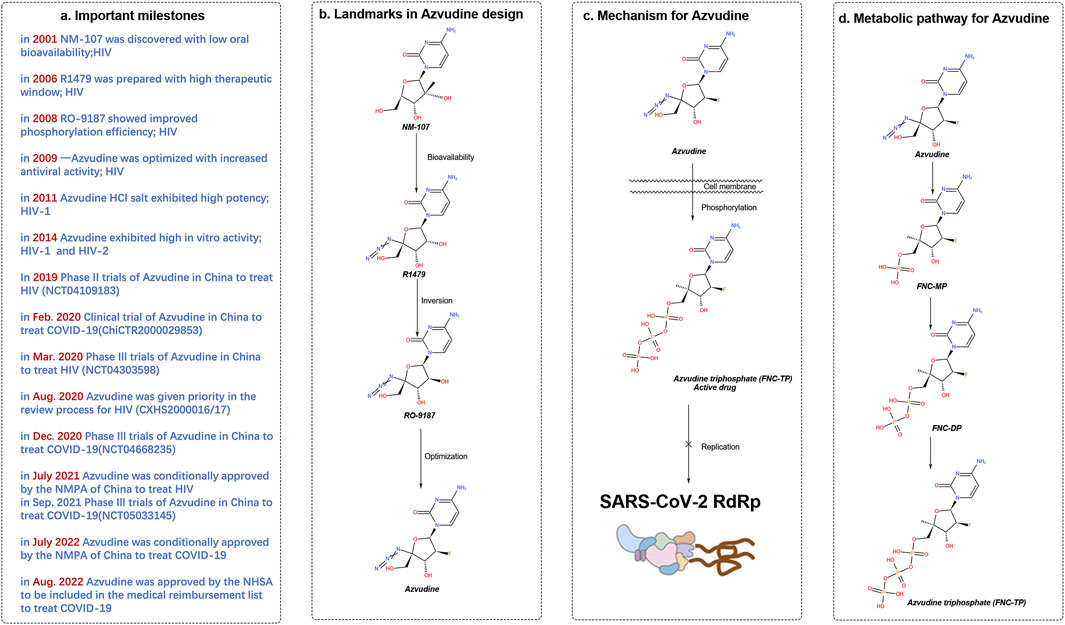
Figure 2. History of the discovery of azulfidine, an oral SARS-CoV-2 RdRp covalent inhibitor. (A) Important milestones in the discovery of azivudine from NM-107 to the first-in-class dual HIV and SARS-CoV-2 inhibitor. (B) Advances in medicinal chemistry led to the discovery of the oral RdRp inhibitor azivudine. (C) Proposed mechanism of action of the prodrug azivudine against SARS-CoV-2. (D) Metabolic pathway of azulfidine. The SARS-CoV-2 RNA replication process is blocked by the active metabolite, azulfidine triphosphate, through the formation of covalent bonds. Created with BioRender software (https://app.biorender.com/)
FNC exhibits broad-spectrum antiviral properties in vitro, including activity against HIV-1 (EC50 = 0.03–6.92 nM), HIV-2 (EC50 = 0.018–0.025 nM), HCoV-OC43 (EC50 = 4.3 μM), and SARS-CoV-2 (EC50 = 1.2 μM). It has shown good anti-SARS activity in infected rhesus monkeys with favorable anti-SARS-CoV-2 activity. Notably, FNC targeted the thymus in a rat SARS-CoV-2 model and showed excellent absolute oral bioavailability (F = 82.7%) in a dog model.
In a clinical trial involving HIV-infected patients, continuous oral administration of azvudine for 7 days in two groups (2 mg twice daily and 4 mg once daily) resulted in some accumulation of azvudine in the body in the 2 mg group but not in the 4 mg group. Adverse events (AEs) were similar between fasting and postprandial administration (Ren et al., 2020a). Compared with fasting administration, postprandial administration significantly increased the exposure of the body to azvudine. Hence, it is recommended that azvudine be taken on an empty stomach. After a single oral dose in rats, azvudine showed the greatest distribution in the thymus (Zhang et al., 2021c), followed by the spleen, with lower levels in the heart, liver, and lungs. Azvudine had low plasma protein binding in humans, dogs, and rats.
The pharmacokinetic data of azvudine in COVID-19 treatment revealed excellent pharmacokinetics in different phases of clinical trials (GQ-FNC-2014-2, GQ-FNC-201, and NCT04109183), and other available information was derived from animal studies and HIV-infected patient trials.
Azvudine is primarily excreted unchanged through the kidneys. In HIV-infected patients, more than 70% of the drug is excreted within 12 h postadministration. Increased doses resulted in increased urinary excretion.
3.2 Pharmacodynamics
Azvudine inhibits SARS-CoV-2 and HCoV-OC43 coronaviruses (Chen et al., 2020) with an EC50 of 1.2–4.3 μmol/L, depending on the virus or cell type and a selection index (SI) of 15–83. The active triphosphate form of azvudine accumulates mainly in the thymus and peripheral blood mononuclear cells (PBMCs) in rats. In rhesus monkeys infected with SARS-CoV-2, azvudine reduced the viral load, restored thymic immunity, improved lymphocyte distribution, and reduced inflammation and organ damage (Zhang J. L. et al., 2021).
3.3 Clinical effectiveness evaluation
The primary pathophysiological mechanism of COVID-19 involves viral replication in the early days of infection and the subsequent host immune-inflammatory response (Ledford and Maxmen, 2022). Early administration of antiviral drugs or neutralizing antibodies and anti-inflammatory drugs in moderate to severe cases is crucial.
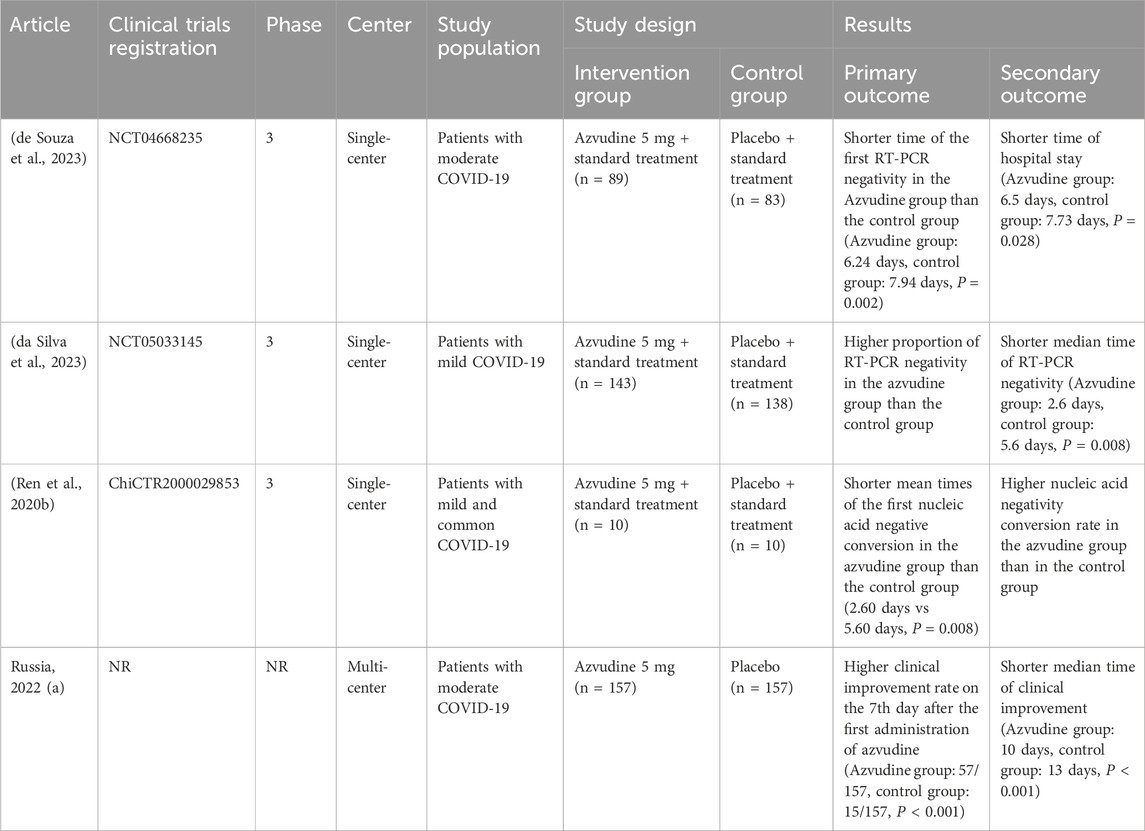
Clinical studies have shown that azvudine shortens the time to nucleic acid negativity compared with standard antiviral treatment (Zhou et al., 2023). In a randomized, open, controlled clinical trial of azvudine for mild and moderate COVID-19, the average time to consecutive negative nucleic acid tests was reduced by 4.5 days compared with that of standard treatment, with no observed drug-related adverse reactions (Ren et al., 2020a). Azvudine also showed significant clinical benefits in specific subgroups, such as males, those under 65 years of age, patients hospitalized for over 5 days, patients with severe COVID-19, and those receiving antibiotic treatment (Zhang J. L. et al., 2021).
The results of studies conducted in various countries revealed the effectiveness of azvudine in reducing the viral load and improving clinical symptoms. In Russia, the proportion of subjects with clinical improvement was significantly greater in the azvudine group than in the control group on the 7th day after administration (azvudine group: 57/157; control group: 15/157; P < 0.001). The median time to clinical improvement was also significantly shorter (azvudine group: 10 days; control group: 13 days; P < 0.001) (Hkexnews, 2024). In China, one study revealed no statistically significant difference in viral load changes, but another reported greater RT‒PCR negativity in the azvudine group, and the median RT‒PCR negativity time was significantly shorter (azvudine group: 2.6 days; control group: 5.6 days; P = 0.008). In Brazil, studies have indicated shorter times to first RT‒PCR negativity (azvudine group: 6.24 days, control group: 7.94 days, P = 0.002) and shorter hospital stays (azvudine group: 6.5 days, control group: 7.73 days, P = 0.028) for azvudine-treated patients (Ren et al., 2020a; da Silva et al., 2023) (Table 2).
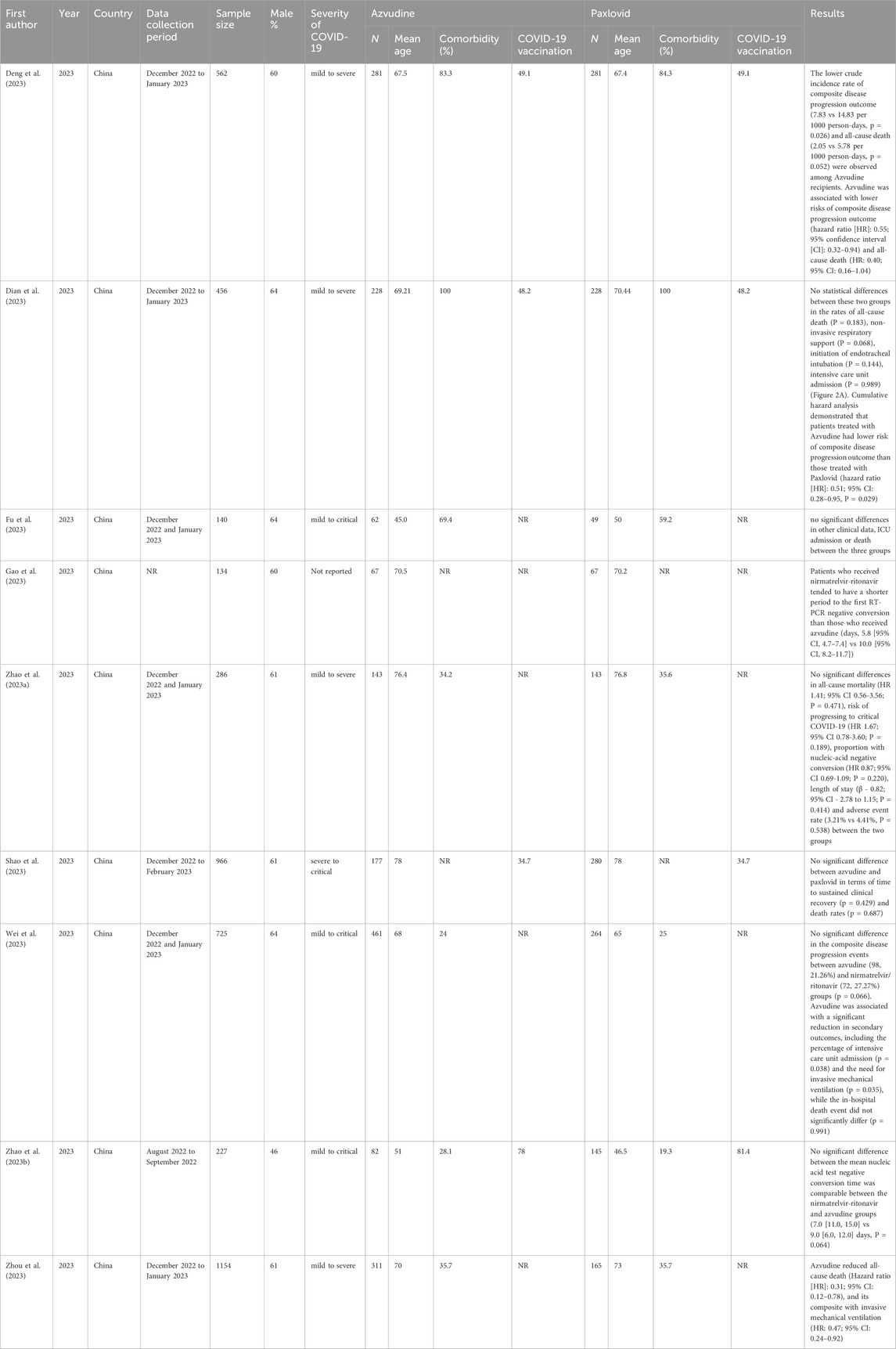
A recent study evaluating the clinical effectiveness of azvudine in hospitalized COVID-19 patients revealed benefits in males under 65 years of age, those hospitalized for more than 5 days post-symptom onset, patients with severe COVID-19 at admission, and those receiving antibiotic treatment at admission. The study included 228 azvudine recipients and 228 nirmatrelvir/ritonavir recipients, revealing that the incidence rates of composite disease progression were lower in the azvudine group (6.662 per 1,000 person-days) than in the nirmatrelvir/ritonavir group (13.493 per 1,000 person-days, P = 0.029). There were no statistically significant differences between the groups in all-cause mortality (P = 0.183), noninvasive respiratory support (P = 0.068), the intubation rate (P = 0.144), or the ICU admission rate (P = 0.144). Cumulative risk analysis revealed a lower risk of composite disease progression in the azvudine group than in the nirmatrelvir/ritonavir group (HR: 0.51, 95% CI: 0.28–0.95; P = 0.029). These findings suggest the potential of azvudine as an effective treatment in specific subgroups of hospitalized COVID-19 patients, warranting further investigation and potential clinical practice integration (Dian et al., 2023; Gao et al., 2023).
In another retrospective cohort study, azvudine demonstrated significant clinical effectiveness in reducing disease progression risk, although the difference in all-cause mortality was not statistically significant (Sun et al., 2023a; Zong et al., 2023; Sun et al., 2023b). A real-world retrospective cohort study in China revealed greater clinical benefits in hospitalized patients with COVID-19 aged <65 years, those with a history of disease, those with severe COVID-19 at admission, and those receiving antibiotics of azvudine than nirmatrelvir-ritonavir (Deng et al., 2023). Some main retrospective studies on the clinical effectiveness of azvudine versus paxlovid are shown in Table 3. A meta-analysis revealed that azvudine and paxlovid had similar effectiveness in reducing mortality rates, negative PCR conversion times and hospital stays. However, azvudine was more effective at improving other outcomes (Amani and Amani, 2024).
The inconsistent conclusions regarding the effectiveness of Azvudine and Paxlovid in Table 3 are primarily due to multiple factors. In terms of study design, differences exist in sample size, data collection periods, and types of studies. Regarding statistical methods, variations in analytical models and definitions of endpoint events are observed. Some studies conducted simple comparative analyses, while others employed complex multivariate regression models. Additionally, the definitions of endpoint events, such as “disease progression,” vary in strictness across studies. According to the studies in Table 3, Azvudine has demonstrated effectiveness in hospitalized patients with severe conditions, older age, and comorbidities, reducing mortality and the risk of disease progression. In particular, its effectiveness is comparable to that of Paxlovid in patients with severe COVID-19.
Regarding the inconsistency of the previous studies, in 2025 a new meta-analysis that included 21 studies covering 10,011 patients confirmed that compared with standard care and Paxlovid (nirmatrelvir/ritonavir) control groups, Azvudine significantly reduced the mortality risk of COVID-19 patients (Wang et al., 2025). The mortality rate in the Azvudine group was significantly lower than that in the standard care/placebo group (RR = 0.48, 95% CI: 0.40–0.57, P < 0.001). The mortality rate in the Azvudine group was also significantly lower than that in the Paxlovid group (RR = 0.73, 95% CI: 0.58–0.92, P < 0.05). In summary, Azvudine has shown significant promise as a treatment for COVID-19, particularly in high-risk patients, by reducing mortality, decreasing viral load, and improving immune response.
Azvudine was approved for import registration for treating common COVID-19 patients with high-risk factors. On 25 July 2022, it was formally launched as the second small-molecule oral medication for COVID-19 in mainland China. On 6 January 2023, the People’s Republic of China’s National Health Commission included azvudine in the Scheme for Diagnosis and Treatment of SARS-CoV-2 (The 10th Trial Edition). Compared with the 9th Edition of the previously released diagnosis and treatment plan, in addition to paxlovid (Hammond et al., 2022), monoclonal antibodies, intravenous injections of human immunoglobulin and convalescent plasma, and antiviral treatment include the addition of azvudine.
Expanding on these findings, previous studies have primarily focused on the original SARS-CoV-2 strain. A new study of characteristics of patients with non-severe infections of different SARS-CoV-2 omicron subvariants in China with 244 Omicron (BA.2.76 and BA.5.1) infected patients showed more frequent clinical symptoms and higher viral loads, but shorter viral clearance times. Azulfidine is safe and effective in the treatment of SARS-CoV-2 omicron subvariants, shortening the viral clearance time, increasing the levels of antiviral antibodies and immune cells, and decreasing the levels of inflammatory factors (Yuan et al., 2024). This latest study fills a research gap by demonstrating that Azvudine is also effective against Omicron variants.
3.4 Safety
Azvudine’s technical evaluation report for marketing approval mentioned its genotoxicity and reproductive toxicity (Center for drug evaluation, 2024). In Ames tests, CHL chromosome aberration tests, and in vivo mouse micronucleus tests, azvudine had positive results. It affects the ovarian mass and increases fetal resorption rates in rats but does not significantly harm male fertility. Clinical application has reported mild adverse reactions such as fever, headache, dizziness, nausea, vomiting, and diarrhea (Zhang J. L. et al., 2021). Severe adverse reactions are rare, and the drug has a good safety profile, with no significant impact on underlying diseases or elderly patients; however, liver and kidney functions should be closely monitored during treatment.
A meta-analysis including 5 RCTs (Ren et al., 2020a; Hkexnews, 2024; da Silva et al., 2023) reported that the incidence of adverse events in COVID-19 patients in the intervention groups who received azvudine treatment was 44.52% (256/575) and that in the control groups was 49.74% (282/567). The incidence of serious adverse events in COVID-19 patients in the intervention groups who received azvudine treatment was 1.16% (5/432), and that in the control groups was 1.86% (8/429). The safety of azvudine was better than that in the control group (adverse events: RR = 0.89, 95% CI: 0.80--0.99, P = 0.04; serious adverse events: RR = 0.63, 95% CI: 0.22--1.79, P = 0.39) (Chen and Tian, 2023; Zheng et al., 2024).
Further supporting Azvudine’s favorable safety profile, another mata-analysis comparing Azvudine with Paxlovid also showed Azvudine has better safety profile overall. The meta-analysis including 21 studies of 10,011 patients collected data on adverse events (AEs) during follow-up for both the azvudine and Paxlovid groups (Wang et al., 2025). Compared with azvudine recipients, patients in the Paxlovid group had a greater risk of Grade 1 AEs, including increased alanine aminotransferase (ALT) (p = 0.013), hypercholesterolemia (p < 0.001), and increased aspartate aminotransferase (AST) (p = 0.047). With respect to Grade 2 AEs, Paxlovid administration was related to greater risks of increased decreased platelet (p = 0.009), increased creatinine (p = 0.018), and ALT (p = 0.036) than azvudine. For Grade 3 and greater SEs, Paxlovid treatment was related to a higher incidence of decreased lymphocyte count (p < 0.001).
Recent cases have indicated that azvudine could cause sinus tachycardia (Ren et al., 2020a). It did not cause renal-related adverse events in COVID-19 patients but requires more information on its use in patients with a GFR ≤60 mL/min/1.73 m2.
3.5 Drug interactions
Azvudine is a substrate and weak inducer of P-gp (Liu et al., 2017; Liu et al., 2018). Caution is needed when P-gp substrates, inhibitors, and inducers are coadministered. Azvudine modulates the expression of P-gp, MRP2, and BCRP, affecting their absorption and potentially enhancing the antiviral activity of other antiretroviral drugs. Azvudine has demonstrated synergistic therapeutic effects on the EC50 against HIV-1IIIB-infected C8166 cells and HIV-1TC-1-infected PBMCs when used in combination with six FDA-approved antiretroviral drugs (Wang et al., 2014b).
Azvudine’s drug interaction profile is an important consideration for its clinical use. When combined with other COVID-19 drugs, such as immunosuppressants or anticoagulants, there may be potential risks. The use of azvudine with anticoagulants may require careful monitoring due to the potential for increased bleeding risk. Further research is needed to fully understand these interactions and to provide guidance on the safe use of azvudine in combination with other medications.
3.6 Special populations
3.6.1 Pregnant or lactating women, children, and elderly patients
Currently, there are no studies on the use of azvudine in pregnant or lactating women, and it is not recommended for these populations. Effective contraception is advised for women of childbearing age during treatment and for 4 days after the last dose.
Clinical studies in children and elderly individuals are lacking. It is generally not recommended for use in individuals under 18 years old. Elderly patients, especially those with underlying conditions, should be closely monitored for liver and kidney function during treatment.
3.6.2 High-risk factor patients
For the treatment of common COVID-19 patients with advanced severe high-risk factors, such as advanced age, not having received the COVID-19 vaccine, chronic kidney disease, diabetes, severe cardiovascular disease, chronic obstructive pulmonary disease, organ transplant recipients, and other individuals taking immunosuppressive medications (Ju et al., 2024), azvudine has been approved for import registration by the Scheme for Diagnosis and Treatment of SARS-CoV-2 (The 10th Trial Edition) published on 6 January 2023, by the People’s Republic of China’s National Health Commission.
For those high risk patients such as elderly patients (>60 years) and those with primary malignant tumors, the subgroup analysis from a meta-analysis suggesting a greater benefit of azvudine over Paxlovid. Subgroup analysis of a meta-analysis including 21 studies of 10,011 patients showed in elder patients (>60 years) (Wang et al., 2025), for all-cause death, potentially meaningful interactions suggesting a greater benefit of azvudine over Paxlovid in those with primary malignant tumors (p for interaction <0.001, HR: 0.33, 95% CI: 0.20–0.54), and in those without systemic steroid use (p for interaction = 0.004, HR: 0.67, 95% CI: 0.53–0.84). For composite disease progression, potentially meaningful interactions suggesting a greater benefit of azvudine over Paxlovid were observed in patients with moderate COVID-19 (p for interaction = 0.036, HR: 0.67, 95% CI: 0.45–1.00) and with primary malignant tumors (p for interaction = 0.012, HR: 0.54, 95% CI: 0.33–0.88). No differences of all-cause death between the azvudine and paxlovid group were observed in severe covid-19, diabetes, hypertension, liver diseases, cardio-cerebral diseases, kidney diseases, autoimmune diseases, and chronic respiratory diseases patients.
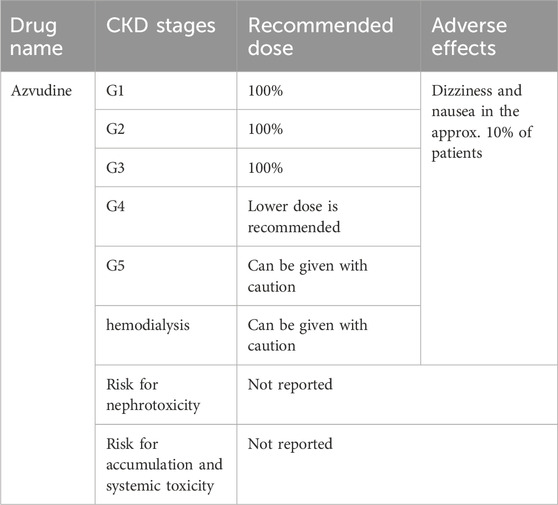
The recommended doses of azvudine for COVID-19 patients at different stages of chronic kidney disease (CKD) are listed in Table 4 (Kale et al., 2023).
3.6.3 Hemodialysis patients
For hemodialysis patients with COVID-19, a Chinese multicenter observational study revealed that the negative nucleic acid conversion rate of the azvudine group was significantly greater than that of the basic treatment group. There were no significant differences in liver function, renal function, or the number of adverse events between the two groups, suggesting that azvudine may be safe and effective compared with the basic treatment of hemodialysis patients with common COVID-19 (Shang et al., 2023).
3.6.4 Other special patients
Patients with a history of pancreatitis or viral hepatitis should use azvudine cautiously because of its structural similarities with lamivudine, a nucleoside reverse transcriptase inhibitor known to cause pancreatitis in some cases. Severe and potentially fatal liver events have been reported in patients with chronic hepatitis B or C coinfection with HIV who are receiving antiretroviral therapy (Thompson et al., 2015; Köklü et al., 2013; Park et al., 2011).
4 Antiviral and antitumor activities of azvudine
Azvudine has broad-spectrum antiviral activity against RNA viruses, such as human immunodeficiency virus (HIV), hepatitis C virus (HCV), enterovirus 71 (EV71), and hepatitis B virus (HBV) (Zhang J. L. et al., 2021). It also has underlying antitumor mechanisms through various pathways. Figure 3 shows how FNC might work against cancer and viruses.
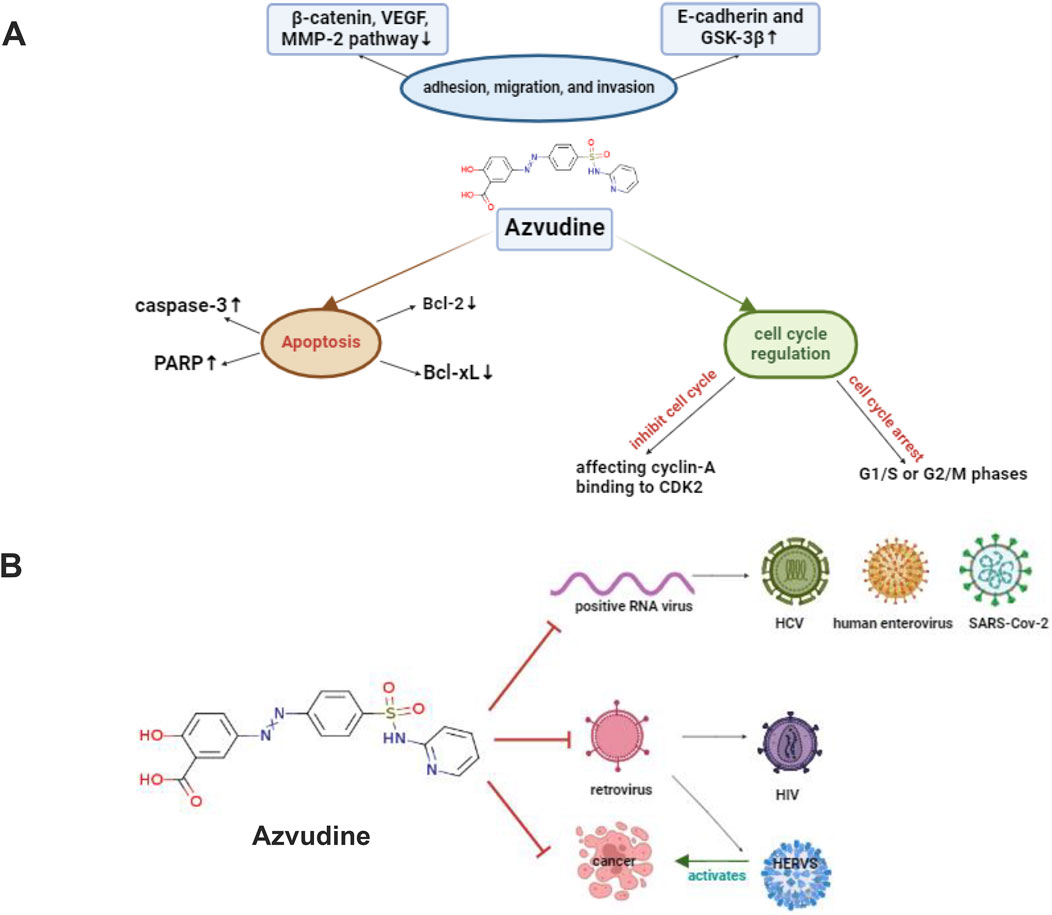
Figure 3. Overview of how FNC fights tumors through various pathways (A) FNC induces cell death by lowering Bcl-xL and Bcl-2 levels and activating caspase-3, which breaks down key proteins like PARP. FNC also prevents Raji and JeKo-1 cells from sticking, moving, and invading by increasing E-cadherin and GSK-3β and decreasing β-catenin, VEGF, MMP-2, and MMP-9. The Wnt/β-catenin pathway is important in cancer growth, and changes in this pathway are linked to tumor invasiveness. FNC causes cell cycle arrest at G1/S or G2/M phases and induces apoptosis. It stops cell cycle checkpoints, like Cyclin-A binding to CDK2, allowing cells to finish the S phase and move to the M phase (B) Graphic Abstract, shows the mechanisms of FNC anticancer and antiviral activities. Abbreviations*HIV: human immunodeficiency virus, HCV: hepatitis C virus, HERVs: human endogenous retroviruses. Created with BioRender software (https://app.biorender.com/)
It shows potent inhibitory activity against both wild-type and drug-resistant HIV strains, with EC50 values ranging from 0.063 nM to 0.735 nM. In 2021, the National Medical Products Administration (NMPA) approved its use to treat HIV-1 infected adult patients [63].
Azulfidine also has antitumor potential, which can modulate the tumor immune microenvironment and inhibit tumor growth. For example, azulfidine significantly inhibited the proliferation and invasive ability of hepatocellular carcinoma (HCC) cells in in vitro experiments (Wang et al., 2025). A subgroup analysis of a Meta-study showed that azulfidine demonstrated greater benefit in the treatment of patients with malignant tumors, significantly reducing the composite of all-cause mortality (HR = 0.33, 95% CI: 0.20–0.54) and disease progression (HR = 0.54, 95% CI: 0.33–0.88). In vitro experiments showed that azulfidine significantly inhibited the proliferation and invasive ability of HCC cell lines and lung cancer cell lines, whereas Paxlovid did not. In in vivo experiments, azulfidine significantly inhibited H22 cell-induced tumor growth in mice with a favorable safety profile. Single-cell RNA sequencing showed that the proportion of CD4+ T cells and CD8+ T cells in the tumor immune microenvironment increased after azulfidine treatment, and specific subpopulations of these cells exhibited functional alterations, suggesting that azulfidine may exert its antitumor effects by modulating immune cells. These findings suggest that azulfidine not only has a promising application in antiviral therapy, but may also provide a new strategy for tumor therapy.
5 Conclusion
Azvudine shows promise as an effective treatment for COVID-19, particularly in reducing disease progression and viral load, with a favorable safety profile characterized by mild and transient side effects. However, current research is limited by small sample sizes, which may affect the generalizability of results, and a lack of long-term effectiveness and safety data, restricting its widespread clinical application. Future studies should focus on evaluating Azvudine’s effectiveness against different SARS-CoV-2 variants, exploring its potential for combination therapies, and developing second-generation inhibitors. Additionally, conducting larger clinical trials targeting high-risk populations, such as the elderly, immunocompromised individuals, and those with severe renal impairment, will provide stronger evidence to support its clinical use and optimize treatment protocols.
Author contributions
JyL: Writing – original draft, Writing – review and editing. BZ: Conceptualization, Writing – review and editing, Data curation. JnL: Writing – review and editing. ZD: Conceptualization, Methodology, Supervision, Writing – review and editing. PL: Methodology, Project administration, Writing – review and editing. WL: Conceptualization, Supervision, Writing – original draft. CZ: Supervision, Validation, Writing – review and editing. JC: Writing – review and editing, Investigation. SS: Writing – review and editing, Conceptualization, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Open Grant from the Pingyuan Laboratory (2023PYOP-0203), Cross-sectional project of ChinaJapan Friendship Hospital (2023-HX-103), National High Level Hospital Clinical Research Funding (2024-NHLHCRF-JBGS-WZ-03 and 2023-NHLHCRFYS-01), Elite Medical Professionals Project of China-Japan Friendship Hospital (ZRJY2023-GG06), Young Elite Scientists Sponsorship Program by CAST (2023QNRC001 and 2022QNRC001), Beijing Hospitals Authority Clinical medicine Development of special funding support (ZLRK202308), Beijing Natural Science Foundation (7244407) and National Natural Science Foundation (82400846 and 82274327).
Acknowledgments
Figures were created with BioRender software (https://app.biorender.com/).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amani, B., and Amani, B. (2024). Azvudine versus Paxlovid in COVID-19: a systematic review and meta-analysis. Reviews Med. Virology 34 (4), e2551. doi:10.1002/rmv.2551
Center for drug evaluation Center for drug evaluation (2024). Available online at: https://www.cde.org.cn/main/xxgk/postmarketpage?acceptidCODE=93007324365052ca04d998ec99e4cdaf.
Chen, Z., and Tian, F. (2023). Efficacy and safety of azvudine in patients with COVID-19: a systematic review and meta-analysis. Heliyon 9 (9), e20153. doi:10.1016/j.heliyon.2023.e20153
Chen, Y., Liu, Q., and Guo, D. (2020). Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 92 (4), 2249–2423. doi:10.1002/jmv.26234
da Silva, R. M., Gebe Abreu Cabral, P., de Souza, S. B., Arruda, R. F., Cabral, S. P. d. F., de Assis, A. L. E. M., et al. (2023). Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19. Front. Med. (Lausanne) 10, 1143485. doi:10.3389/fmed.2023.1143485
de Souza, S. B., Cabral, P. G. A., da Silva, R. M., Arruda, R. F., Cabral, S. P. d. F., de Assis, A. L. E. M., et al. (2023). Phase III, randomized, double-blind, placebo-controlled clinical study: a study on the safety and clinical efficacy of AZVUDINE in moderate COVID-19 patients. Front. Med. (Lausanne) 10, 1215916. doi:10.3389/fmed.2023.1215916
Deng, G., Li, D., Sun, Y., Jin, L., Zhou, Q., Xiao, C., et al. (2023). Real-world effectiveness of Azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: a retrospective cohort study. J. Med. Virol. 95 (4), e28756. doi:10.1002/jmv.28756
Dian, Y., Meng, Y., Sun, Y., Deng, G., and Zeng, F. (2023). Azvudine versus Paxlovid for oral treatment of COVID-19 in Chinese patients with pre-existing comorbidities. J. Infect. 87 (2), e24–e27. doi:10.1016/j.jinf.2023.05.012
Fu, Y., Pan, J., Chen, W., Li, W., Zheng, C., and Chang, J. (2023). Comparison of the different medications for COVID-19 in kidney transplant recipients. Authorea.
Gao, Y., Luo, Z., Ren, S., Duan, Z., Han, Y., Liu, H., et al. (2023). Antiviral effect of azvudine and nirmatrelvir-ritonavir among hospitalized patients with COVID-19. J. Infect. 86 (6), e158–e160. doi:10.1016/j.jinf.2023.03.023
Hammond, J., Leister-Tebbe, H., Gardner, A., Abreu, P., Bao, W., Wisemandle, W., et al. (2022). Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19. N. Engl. J. Med. 386 (15), 1397–1408. doi:10.1056/NEJMoa2118542
Hkexnews (2024). Available online at: https://www1.hkexnews.hk/app/sehk/2022/104646/documents/sehk22080402059.pdf.
Ju, C., Wang, M., Yuan, J., Xu, Y., Xue, W., Lu, H., et al. (2024). Chinese expert consensus on diagnosis and treatment strategies for novel coronavirus infection in immunocompromised populations (2023 edition). Int. J. Rheumatic Dis. 27 (2). doi:10.1111/1756-185x.14998
Kale, A., Shelke, V., Dagar, N., Anders, H. J., and Gaikwad, A. B. (2023). How to use COVID-19 antiviral drugs in patients with chronic kidney disease. Front. Pharmacol. 14, 1053814. doi:10.3389/fphar.2023.1053814
Klumpp, K., Lévêque, V., Le Pogam, S., Ma, H., Jiang, W. R., Kang, H., et al. (2006). The novel nucleoside analog R1479 (4'-azidocytidine) is a potent inhibitor of NS5B-dependent RNA synthesis and hepatitis C virus replication in cell culture. J. Biol. Chem. 281 (7), 3793–3799. doi:10.1074/jbc.M510195200
Klumpp, K., Kalayanov, G., Ma, H., Le Pogam, S., Leveque, V., Jiang, W. R., et al. (2008). 2′-deoxy-4′-azido nucleoside analogs are highly potent inhibitors of hepatitis C virus replication despite the lack of 2′-α-hydroxyl groups. J. Biol. Chem. 283 (4), 2167–2175. doi:10.1074/jbc.M708929200
Köklü, S., Tuna, Y., Gülşen, M. T., Demir, M., Köksal, A. Ş., Koçkar, M. C., et al. (2013). Long-term efficacy and safety of lamivudine, entecavir, and tenofovir for treatment of hepatitis B virus-related cirrhosis. Clin. Gastroenterol. Hepatol. 11 (1), 88–94. doi:10.1016/j.cgh.2012.10.003
Ledford, H., and Maxmen, A. (2022). African clinical trial denied access to key COVID drug Paxlovid. Nature 604 (7906), 412–413. doi:10.1038/d41586-022-00919-5
Liu, Y., Liu, B., Zhang, Y., Peng, Y., Huang, C., Wang, N., et al. (2017). Intestinal absorption mechanisms of 2'-deoxy-2'-β-fluoro-4'-azidocytidine, a cytidine analog for AIDS treatment, and its interaction with P-glycoprotein, multidrug resistance-associated protein 2 and breast cancer resistance protein. Eur. J. Pharm. Sci. 105, 150–158. doi:10.1016/j.ejps.2017.05.009
Liu, Y., Wang, Y., Peng, Y., Liu, B., Ma, F., Jiang, J., et al. (2018). Effects of the antiretroviral drug 2'-deoxy-2'-β-fluoro-4'-azidocytidine (FNC) on P-gp, MRP2 and BCRP expressions and functions. Pharmazie 73 (9), 503–507. doi:10.1691/ph.2018.8555
Ma, B. H., Yip, T. C. F., Lui, G. C. Y., Lai, M. S. M., Hui, E., Wong, V. W. S., et al. (2023). Clinical outcomes following treatment for COVID-19 with nirmatrelvir/ritonavir and Molnupiravir among patients living in nursing homes. JAMA Netw. Open 6 (4), e2310887. doi:10.1001/jamanetworkopen.2023.10887
Mazzitelli, M., Trunfio, M., Sasset, L., Scaglione, V., Ferrari, A., Mengato, D., et al. (2023a). Risk of hospitalization and sequelae in patients with COVID-19 treated with 3-day early remdesivir vs. controls in the vaccine and Omicron era: a real-life cohort study. J. Med. Virol. 95 (3), e28660. doi:10.1002/jmv.28660
Mazzitelli, M., Mengato, D., Sasset, L., Ferrari, A., Gardin, S., Scaglione, V., et al. (2023b). Molnupiravir and nirmatrelvir/ritonavir: tolerability, safety, and adherence in a retrospective cohort study. Viruses 15 (2), 384. doi:10.3390/v15020384
Meng, Y., Sun, N., Liang, L., Yu, B., and Chang, J. (2024). 2'-Fluorinated nucleoside chemistry for new drug discovery: achievements and prospects. Natl. Sci. Rev. 11 (10), nwae331. doi:10.1093/nsr/nwae331
Park, J. W., Kim, H. S., Seo, D. D., Jang, J. S., Shin, W. G., Kim, K. H., et al. (2011). Long-term efficacy of entecavir in adefovir-refractory chronic hepatitis B patients with prior lamivudine resistance. J. Viral Hepat. 18 (10), e475–e481. doi:10.1111/j.1365-2893.2011.01479.x
Ren, Z., Luo, H., Yu, Z., Song, J., Liang, L., Wang, L., et al. (2020a). A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv. Sci. (Weinh) 7 (19), e2001435. doi:10.1002/advs.202001435
Ren, Z., Luo, H., Yu, Z., Song, J., Liang, L., Wang, L., et al. (2020b). A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv. Sci. 7 (19), 2001435. doi:10.1002/advs.202001435
Shang, S., Fu, B., Geng, Y., Zhang, J., Zhang, D., Xiao, F., et al. (2023). Azvudine therapy of common COVID-19 in hemodialysis patients. J. Med. Virol. 95 (8), e29007. doi:10.1002/jmv.29007
Shao, J. S., Fan, R., Guo, C., Huang, X., Guo, R., Zhang, F., et al. (2023). Composite interventions on outcomes of severely and critically ill patients with COVID-19 in Shanghai, China. MICROORGANISMS 11 (7), 1859. doi:10.3390/microorganisms11071859
Smith, D. B., Martin, J. A., Klumpp, K., Baker, S. J., Blomgren, P. A., Devos, R., et al. (2007). Design, synthesis, and antiviral properties of 4'-substituted ribonucleosides as inhibitors of hepatitis C virus replication: the discovery of R1479. Bioorg Med. Chem. Lett. 17 (9), 2570–2576. doi:10.1016/j.bmcl.2007.02.004
Smith, D. B., Kalayanov, G., Sund, C., Winqvist, A., Pinho, P., Maltseva, T., et al. (2009a). The design, synthesis, and antiviral activity of 4′-azidocytidine analogues against hepatitis C virus replication: the discovery of 4′-azidoarabinocytidine. J. Med. Chem. 52 (1), 219–223. doi:10.1021/jm800981y
Smith, D. B., Kalayanov, G., Sund, C., Winqvist, A., Maltseva, T., Leveque, V. J. P., et al. (2009b). The design, synthesis, and antiviral activity of monofluoro and difluoro analogues of 4′-azidocytidine against hepatitis C virus replication: the discovery of 4′-Azido-2′-deoxy-2′-fluorocytidine and 4′-Azido-2′-dideoxy-2′,2′-difluorocytidine. J. Med. Chem. 52 (9), 2971–2978. doi:10.1021/jm801595c
Sommadossi, J., and La Colla, P. (2001a). Methods and compositions using modified nucleosides for treating flaviviruses and pestiviruses. Novirio Pharm. Ltd. Univ. Degli Studi Di Cagliari.
Sommadossi, J., and La Colla, P. (2001b). Preparation of antiviral nucleosides and methods for treating hepatitis C virus. Novirio Pharm. Ltd. Univ. Degli Studi Di Cagliari.
Sun, Y., Jin, L., Dian, Y., Shen, M., Zeng, F., Chen, X., et al. (2023a). Oral Azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study. EClinicalMedicine 59, 101981. doi:10.1016/j.eclinm.2023.101981
Sun, Y., Jin, L., Dian, Y., Shen, M., Zeng, F., Chen, X., et al. (2023b). Corrigendum to ‘Oral Azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study.’ [EClinicalMedicine 59 (2023) 101981]. eClinicalMedicine 62, 102110. doi:10.1016/j.eclinm.2023.102110
Thompson, A. B., Wynn, B. A., O Akerele, D., A Rostad, C., Anderson, E. J., Camacho-Gonzalez, A. F., et al. (2015). Acute pancreatitis associated with dolutegravir and lamivudine/abacavir administration. AIDS 29 (3), 390–392. doi:10.1097/QAD.0000000000000542
Wang, Q., Hu, W., Wang, S., Pan, Z., Tao, L., Guo, X., et al. (2011). Synthesis of new 2′-deoxy-2′-fluoro-4′-azido nucleoside analogues as potent anti-HIV agents. Eur. J. Med. Chem. 46 (9), 4178–4183. doi:10.1016/j.ejmech.2011.06.020
Wang, R.-R., Yang, Q. H., Luo, R. H., Peng, Y. M., Dai, S. X., Zhang, X. J., et al. (2014a). Azvudine, A novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro. Plos One 9 (8), e105617. doi:10.1371/journal.pone.0105617
Wang, R.-R., Yang, Q. H., Luo, R. H., Peng, Y. M., Dai, S. X., Zhang, X. J., et al. (2014b). Azvudine, A novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro. PLoS ONE 9 (8), e105617. doi:10.1371/journal.pone.0105617
Wang, H., Cui, G., Cheng, M., Aji, T., Li, G., Hu, X., et al. (2025). Real-world effectiveness and safety of oral azvudine versus nirmatrelvir‒ritonavir (Paxlovid) in hospitalized patients with COVID-19: a multicenter, retrospective, cohort study. Signal Transduct. Target Ther. 10 (1), 30. doi:10.1038/s41392-025-02126-w
Wei, A. H., Zeng, L., Wang, L., Gui, L., Zhang, W. T., Gong, X. P., et al. (2023). Head-to-head comparison of azvudine and nirmatrelvir/ritonavir for the hospitalized patients with COVID-19: a real-world retrospective cohort study with propensity score matching. Front. Pharmacol. 14, 1274294. doi:10.3389/fphar.2023.1274294
Yang, L., and Wang, Z. (2023). Bench-to-bedside: innovation of small molecule anti-SARS-CoV-2 drugs in China. Eur. J. Med. Chem. 257, 115503. doi:10.1016/j.ejmech.2023.115503
Yu, B., and Chang, J. (2020). Azvudine (FNC): a promising clinical candidate for COVID-19 treatment. Signal Transduct. Target. Ther. 5 (1), 236. doi:10.1038/s41392-020-00351-z
Yu, B., and Chang, J. (2022). The first Chinese oral anti-COVID-19 drug Azvudine launched. Innovation 3 (6), 100321. doi:10.1016/j.xinn.2022.100321
Yuan, W., Liu, Y., Zhan, H., Wei, F., Zhang, Q., Gao, H., et al. (2024). Characteristics of patients with non-severe infections of different SARS-CoV-2 omicron subvariants in China. Front. Med. (Lausanne) 11, 1511227. doi:10.3389/fmed.2024.1511227
Zhang, J. L., Li, Y. H., Wang, L. L., Liu, H. Q., Lu, S. Y., Liu, Y., et al. (2021a). Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal Transduct. Target Ther. 6 (1), 414. doi:10.1038/s41392-021-00835-6
Zhang, J.-L., Li, Y. H., Wang, L. L., Liu, H. Q., Lu, S. Y., Liu, Y., et al. (2021b). Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal Transduct. Target. Ther. 6 (1), 414. doi:10.1038/s41392-021-00835-6
Zhang, J.-L., Li, Y. H., Wang, L. L., Liu, H. Q., Lu, S. Y., Liu, Y., et al. (2021c). Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal Transduct. Target. Ther. 6 (1), 414. doi:10.1038/s41392-021-00835-6
Zhao, Q. Q., Zheng, B., Han, B., Feng, P., Xia, Z., Jiang, H., et al. (2023a). Is azvudine comparable to nirmatrelvir-ritonavir in real-world efficacy and safety for hospitalized patients with COVID-19? A retrospective cohort study. Infect. Dis. Ther. 12 (8), 2087–2102. doi:10.1007/s40121-023-00845-7
Zhao, X., Cheng, Y., Zhang, M., Qianda, B., Zhouma, B., Yangzhen, B., et al. (2023b). Efficacy of nirmatrelvir-ritonavir versus azvudine for COVID-19 treatment in tibet: a retrospective study. Infect. DRUG Resist. 16, 6053–6060. doi:10.2147/IDR.S423725
Zheng, B., Zhao, Q., Yang, W., Feng, P., Xin, C., Ying, Y., et al. (2024). Small-molecule antiviral treatments for COVID-19: a systematic review and network meta-analysis. Int. J. Antimicrob. Agents 63 (3), 107096. doi:10.1016/j.ijantimicag.2024.107096
Zhou, Y., Liu, Y., Jiang, L., Zhang, R., Zhang, H., Shi, Q., et al. (2023). Azvudine and nirmatrelvir-ritonavir in hospitalized patients with moderate-to-severe COVID-19: emulation of a randomized target trial. J. Med. Virol. 95 (12), e29318. doi:10.1002/jmv.29318
Keywords: azvudine, COVID-19, SARS-CoV-2 RdRp, pharmacokinetics, special patients
Citation: Li J, Zhu B, Lu J, Dong Z, Li P, Li W, Zheng C, Chang J and Shang S (2025) Advances in the effectiveness and safety of azvudine treatment: a comprehensive review. Front. Pharmacol. 16:1524072. doi: 10.3389/fphar.2025.1524072
Received: 07 November 2024; Accepted: 01 April 2025;
Published: 25 April 2025.
Edited by:
Bin Su, Capital Medical University, ChinaReviewed by:
Daniele Mengato, University Hospital of Padua, ItalyGencheng Han, Institute of Basic Medical Sciences, China
Tao Yu, Harvard Medical School, United States
Copyright © 2025 Li, Zhu, Lu, Dong, Li, Li, Zheng, Chang and Shang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenge Li, d2VuZ2VfbGVlMjAwMkAxMjYuY29t; Chunfu Zheng, emhlbmcuYWxhbkBob3RtYWlsLmNvbQ==; Junbiao Chang, Y2hhbmdqdW5iaWFvQHp6dS5lZHUuY24=; Shunlai Shang, MTg4MTA1Njg2MDBAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jiayi Li1,2†
Jiayi Li1,2† Zheyi Dong
Zheyi Dong Ping Li
Ping Li Wenge Li
Wenge Li Chunfu Zheng
Chunfu Zheng Junbiao Chang
Junbiao Chang Shunlai Shang
Shunlai Shang