Abstract
Introduction:
Programmed death-ligand 1 (PD-L1) blockade is a growing treatment for extensive-stage small cell lung cancer (ES-SCLC). This study evaluates the cost-effectiveness of benmelstobart and anlotinib plus etoposide/carboplatin (EC) compared versus anlotinib plus EC and EC alone for patients with ES-SCLC in China.
Methods:
Using a Markov model over 5-year boundary and data from the ETER701 trials, we analyzed quality-adjusted life-years (QALYs), incremental cost-effectiveness ratio (ICER), total cost, incremental net health benefit (INHB) and incremental monetary benefit (INMB). To address uncertainties, we conducted one-way analysis and probabilistic sensitivity analysis (PSA). Scenario analyses were used to evaluate the resilience of our model's findings.
Results:
The administration of triple therapy for ES-SCLC demonstrated a significant improvement in QALY, with respective gains of 0.26, 0.39, compared with the other two schemes. However, enhanced therapeutic benefit was accompanied by increased costs. And triple therapy showed less cost-effectiveness with ICER of $189797.99 and $149249.24 per QALY respectively when compared with other schemes. Moreover, the analysis revealed an INHB of −1.04, −1.12 QALYs, and the INMB of −39755.48 $, −42819.93 $ respectively. Sensitivity analysis demonstrated that benmelstobart's cost was the main driver of cost-effectiveness. The cost-effectiveness acceptability curve displayed that the likelihood of triple therapy being cost-effective increased from 34.20% to 97.60% when the threshold value for cost per QALY gained varied from $180000 to $240000. The scenario analysis supported these findings.
Discussion:
Triple therapy was a less cost-effective option for patients with ES-SCLC compared with anlotinib plus EC and EC alone in China.
1 Introduction
Drawing from the most recent data provided by the International Agency for Research on Cancer (IARC), lung cancer has reaffirmed its status as the preeminent malignancy, with a staggering 2.481 million new cases diagnosed globally in 2022 (Bray et al., 2024). Among the various subtypes of lung cancer, small cell lung cancer (SCLC) stands out as the most invasive, constituting approximately 15% of all lung cancer cases. Its reputation is largely due to its aggressive nature and tendency for early metastasis (Rudin et al., 2021). A significant proportion, nearly two-thirds, of patients are diagnosed with extensive-stage small cell lung cancer (ES-SCLC) at the outset, facing a disheartening 5-year survival rate that hovers below 5% (Zhou et al., 2020). This grim prognosis is predominantly attributed to the high frequency of disease recurrence and metastasis (Ko et al., 2021).
Historically, platinum-based doublet chemotherapy, notably the regimen combining platinum agents with etoposide, has served as the cornerstone of first-line therapy for ES-SCLC. Yet, this strategy has only modestly extended survival, typically to about 8–10 months (Pavan et al., 2019). Recently, the therapeutic landscape has been revolutionized by the convergence of chemotherapy with immune checkpoint inhibitors, specifically those targeting the programmed death protein 1 (PD-1) and its ligand (PD-L1). A wealth of clinical research has attested to the significant survival benefits afforded by this synergistic approach. The IMpower 133 trial demonstrated that atezolizumab combined with chemotherapy achieved a median overall survival (OS) of 12.3 months in ES-SCLC patients (Horn et al., 2018; Liu et al., 2021). Similarly, durvalumab and serplulimab in combination with chemotherapy showed median OS of 12.9 months and 15.4 months, respectively, for ES-SCLC treatment (Paz-Ares et al., 2019; Goldman et al., 2021; Cheng et al., 2022). However, the specter of resistance that can emerge during treatment poses a constraint on the enduring efficacy of these interventions. T-cell exhaustion and elevated fibronectin type III domain-containing protein 4 (FNDC4) expression are independently associated with poor prognosis in lung cancer (Liu et al., 2024; Xu and Lu, 2024). Anti-angiogenic therapy, by targeting the formation of tumor vasculature, not only refines the tumor microenvironment but also potentiates the impact of immunotherapy, offering a promising strategy to counteract resistance to both chemotherapy and immunotherapy (Augustin and Koh, 2022).
Benmelstobart, a fully humanized monoclonal antibody targeting PD-L1, represents an innovative therapeutic approach developed by Zhengda Tianqing. The ETER701 trial assessed the efficacy and safety of benmelstobart in conjunction with anlotinib and standard chemotherapy for the treatment of previously untreated ES-SCLC (Cheng et al., 2024). The findings indicate that the integration of benmelstobart with anlotinib and the EC regimen significantly extended the median OS of patients to 19.3 months, compared to 11.9 months with the EC regimen. While the combination of anlotinib and EC demonstrated a trend towards survival improvement, it did not achieve statistical significance (13.3 months vs 11.9 months) (Cheng et al., 2024). Notably, the triple therapy did not exhibit a significant increase in treatment-related adverse events compared to the double therapy, suggesting that the triple therapy’s safety profile is tolerable and manageable. These insights suggest that the incorporation of anti-angiogenic therapy into immunochemotherapy may offer a potent and secure treatment strategy for ES-SCLC patients. Despite the marked therapeutic benefits of PD-L1 inhibitors and anti-angiogenic agents, their substantial costs place a considerable financial strain on patients. To date, a systematic evaluation of the economic viability of these medications is lacking. This study, therefore, seeks to conduct a comprehensive economic assessment of the triple therapy, double therapy, and chemotherapy alone from the perspective of China’s healthcare system. The goal is to inform the rational adjustment of new drug pricing and the enhancement of the medical insurance catalog, thereby alleviating the financial burden on patients, enhancing drug accessibility, and optimizing cost-effectiveness.
2 Methods
2.1 Analytical overview
The study focused its analytical lens on an envisaged cohort comprising individuals afflicted by the intricate nexus of ES-SCLC, and who had hitherto not undergone systemic therapeutic interventions. This patient profile harmonized seamlessly with the distinct demographic fabric of the ETER701 clinical trial. In the realm of this economic assessment, an intricate Markov model, demarcated into three distinct health epochs, was meticulously erected to initiate the pivotal deliberation between the therapeutic avenues paved by benmelstobart, anlotinib and chemotherapy.
Visualized in the illustrative construct of Figure 1, these three discrete health junctures stood as mutually exclusive entities, characterized as progression-free survival (PFS), progressed disease (PD), and the finality of mortality. The survival tenure, with its manifold implications, was meticulously parsed within these health states, further bifurcated into two avenues: one of continued vitality intertwined with PFS, and the other, an existence ensnared by the clutches of PD. The intricate calculus revealed itself as an intricate tapestry where the proportion of individuals enduring at a specific point in the cyclical timeline was adroitly deduced by the cumulative extent beneath the overarching survival curve, echoing the definitive arc of OS. Concomitantly, the contingent proportion navigating the realm of vitality entwined with PFS was elegantly ascertained through analogous calculus underpinning the PFS curve. The third facet, comprising those sustaining a tenuous coexistence with PD, was ingeniously estimated as the differential quotient between the overarching OS and the PFS trajectories.
FIGURE 1
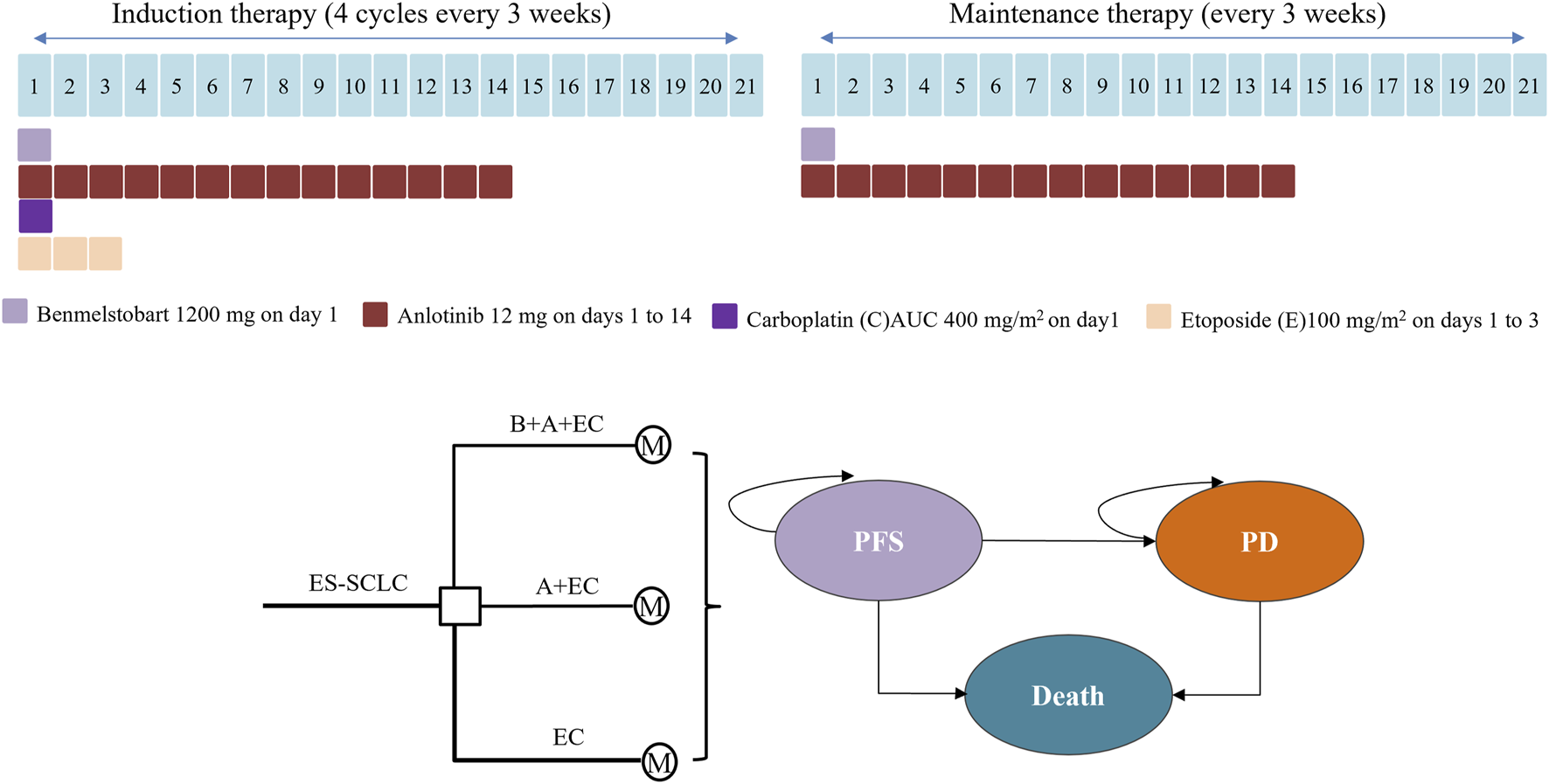
Medication regimens and model structure for ES-SCLC. ES-SCLC, extensive stage small cell lung cancer; B, benmelstobart; A, anlotinib; E, etoposide; C, carboplatin; M, Markov model; PFS, progression-free survival; PD, progressed disease.
The bedrock of this intricate proportionality found its moorings in the empirical outcomes crystallized within the annals of the ETER701 trial. This pivotal validation was realized through a harmonious juxtaposition of the model-constructed projections for PFS and OS, thoughtfully calibrated against the empirical bedrock of observed clinical data. The methodological compass of this study steadfastly adhered to the tenets prescribed by the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline (Supplementary Table 1 in the Supplement), which orchestrate the harmonious choreography of health economic evaluations.
2.2 Clinical data inputs
The patient outcomes in benmelstobart, anlotinib plus etoposide/carboplatin group (B + A + EC group), anlotinib plus etoposide/carboplatin group (A + EC goup) and etoposide/carboplatin group (EC group) were influenced by findings from the ETER701 trial, at least up to the point of trial follow-up. Beyond this timeframe, standard statistical methods as outlined by Guyot et al. (2012) were employed for extrapolation. Data extraction from PFS and OS curves was conducted using GetData Graph Digitizer, version 2.20. These data points were then fitted to various parametric survival models, including Weibull, log-logistic, exponential, log-normal, Gompertz, and Generalized gamma models. Selection of the appropriate survival model was based on the Akaike information criterion (AIC) and Bayesian information criterion (BIC), with detailed goodness-of-fit outcomes presented in Supplementary Table 2 and Supplementary Table 3 within the Supplement. Our analysis concluded that the log-normal distribution provided the most accurate fit for the PFS data within the B + A + EC group. Conversely, the log-logistic distribution proved suitable for extrapolation purposes in other groups. Consequently, the combination of log-normal and log-logistic distributions was employed for the calculation of transition probabilities. To generate the parametric survival curves, the RStudio 2022.02.0 software was utilized, and the construction of the Markov model was executed using TreeAge Pro 2022. For estimation of the transition probability from PFS to mortality, we incorporated mortality rates from the general population of 2023, sourced from the mortality tables provided by the National Bureau of Statistics (Statistics, 2023).
2.3 Cost and utility inputs
Only direct medical expenses were taken into account, encompassing expenditures related to drug procurement, follow-up examinations, management of serious adverse effects (SAEs), and best supportive care (BSC). These details are outlined in Table 1. The monetary figures are presented in 2023 United States dollars (converted at a rate of 7.0467 RMB/USD) and were adjusted to 2023 values using the consumer price index (CPI). As indicated by the ETER701 trial report, the dosing regimen of patients receiving triple therapy (benmelstobart, anlotinib plus etoposide/carboplatin), dual therapy or chemotherapy alone were shown in Figure 1. Treatment cycles persisted for 21 days each, until factors such as unacceptable toxicity, consent withdrawal, disease progression, or investigator judgment necessitated a halt. Prices for benmelstobart, anlotinib, carboplatin, and etoposide were sourced from Shandong drug and medical consumables procurement management subsystem (https://ypjc.ybj.shandong.gov.cn/). Local medical institution rates were employed for follow-up expenses, while additional cost data were referenced from relevant literature. Discontinuations arising from SAEs were not factored into this assessment. The analysis encompassed expenses linked to managing grade 3 or higher adverse events (AEs), extracted from pertinent sources (see Table 1).
TABLE 1
| Parameter | Expected value | Range | Distribution | Source |
|---|---|---|---|---|
| Drug costs ($) | ||||
| Anlotinib/cycle | 563.24 | 450.59–675.89 | gamma | Local charge |
| Carboplatin injection/cycle | 74.50 | 59.60–89.40 | gamma | Local charge |
| Etoposide injection/cycle | 134.53 | 107.62–161.44 | gamma | Local charge |
| Benmelstobart | 3,485.32 | 2,788.26–4,182.38 | gamma | Local charge |
| AEs costs ($) | ||||
| Neutropenia | 16,619.16 | 13295.33-19942.99 | gamma | Ding et al. (2021) |
| Leukopenia | 16,619.16 | 13295.33-19942.99 | gamma | Ding et al. (2021) |
| Thrombocytopenia | 2,674.01 | 2139.20-3208.81 | gamma | Ding et al. (2021) |
| Anemia | 510.35 | 408.28–612.42 | gamma | Xiang et al. (2023) |
| Hypertension | 14.45 | 11.56–17.34 | gamma | Dai et al. (2024) |
| Follow up monitoring cost ($) | ||||
| Contrast CT | 447.02 | 357.61–536.42 | gamma | Local charge |
| Cranial MRI | 248.34 | 198.67–298.01 | gamma | Local charge |
| Cervical lymph node Ultrasound | 19.87 | 15.89–23.84 | gamma | Local charge |
| Tumor marker | 72.52 | 58.01–87.02 | gamma | Local charge |
| Bone scan | 120.62 | 96.50–144.75 | gamma | Local charge |
| Complete blood count | 2.70 | 2.16–3.24 | gamma | Local charge |
| Blood-biochemistry | 30.09 | 24.07–36.10 | gamma | Local charge |
| Best supportive care | 1,543.46 | 1234.77-1852.15 | gamma | Zhu et al. (2023) |
| Utility | ||||
| PFS | 0.84 | 0.67–0.88 | beta | Ding et al. (2021) |
| PD | 0.47 | 0.38–0.57 | beta | Ding et al. (2021), Xiang et al. (2023) |
| Disutility due to AEs | ||||
| Neutropenia | 0.09 | 0.07–0.11 | beta | Ding et al. (2021) |
| Leukopenia | 0.09 | 0.07–0.11 | beta | Ding et al. (2021) |
| Thrombocytopenia | 0.20 | 0.16–0.24 | beta | Zhu et al. (2022) |
| Anemia | 0.07 | 0.06–0.09 | beta | Xiang et al. (2023) |
| Hypertension | 0.04 | 0.03–0.05 | beta | Dai et al. (2024) |
| Probabilities, % | ||||
| Benmelstobart + anlotinib + EC | ||||
| Neutropenia | 0.70 | 0.63–0.76 | beta | Cheng et al. (2024) |
| Leukopenia | 0.38 | 0.34–0.42 | beta | Cheng et al. (2024) |
| Thrombocytopenia | 0.50 | 0.45–0.55 | beta | Cheng et al. (2024) |
| Anemia | 0.24 | 0.22–0.26 | beta | Cheng et al. (2024) |
| Hypertension | 0.16 | 0.14–0.17 | beta | Cheng et al. (2024) |
| Anlotinib + EC | ||||
| Neutropenia | 0.73 | 0.66–0.80 | beta | Cheng et al. (2024) |
| Leukopenia | 0.31 | 0.28–0.34 | beta | Cheng et al. (2024) |
| Thrombocytopenia | 0.54 | 0.48–0.59 | beta | Cheng et al. (2024) |
| Anemia | 0.27 | 0.24–0.29 | beta | Cheng et al. (2024) |
| Hypertension | 0.12 | 0.11–0.13 | beta | Cheng et al. (2024) |
| EC alone | ||||
| Neutropenia | 0.69 | 0.62–0.76 | beta | Cheng et al. (2024) |
| Leukopenia | 0.35 | 0.31–0.38 | beta | Cheng et al. (2024) |
| Thrombocytopenia | 0.36 | 0.32–0.39 | beta | Cheng et al. (2024) |
| Anemia | 0.24 | 0.21–0.26 | beta | Cheng et al. (2024) |
| Hypertension | 0.02 | 0.01–0.02 | beta | Cheng et al. (2024) |
| Discount | 0.05 | 0.00–0.08 | beta | China Guidelines for Pharmacoeconomic Evaluations. (2020) |
Model parameters: baseline values, ranges, and distributions for sensitivity analysis.
For each health state, a health utility preference was assigned on a scale ranging from 0 (indicating death) to 1 (representing perfect health). The utility values for the PFS and PD states concerning ES-SCLC were determined as 0.84 and 0.47 (Ding et al., 2021; Xiang et al., 2023), respectively. The analysis took into account the disutility values caused by severe side effects (grade 3/4 adverse effects). The assumption was made that all AEs occurred during the initial treatment cycle. Owing to the low 5-year survival rate, our analysis was conducted over a 5-year timeframe, encompassing about 90 cycles.
2.4 Base-case analysis
The incremental cost-effectiveness ratio (ICER) was computed as the added cost per extra quality adjusted life-year (QALY) gained, comparing each group pairwise. If the ICER fell below the predetermined threshold for acceptable expenditure ($38070.59 per additional QALY achieved), it was considered cost-effective in line with recommendations. Costs and QALYs were discounted at an annual rate of 5% to account for future values. Additionally, we derived the incremental net health benefit (INHB) and incremental monetary benefit (INMB) using the following expressions: INHB(λ) = (μE1 − μE0) − (μC1 − μC0)/λ = ΔE − ΔC/λ and INMB(λ) = (μE1 − μE0) × λ − (μC1 − μC0) = ΔE × λ−ΔC, where μCi and μEi represented the cost and effectiveness of each group i represents evaluation group, and 0 represents control group). The parameter λ represented the willingness-to-pay (WTP) threshold (Craig and Black, 2001).
2.5 Sensitivity analysis
To thoroughly validate the integrity of our foundational findings, we undertook comprehensive sensitivity analyses encompassing both one-way sensitivity assessments and probabilistic sensitivity analysis (PSA). One-way sensitivity analysis is an analytical method that evaluates the impact of individual model parameters on study outcomes by systematically varying each parameter within a predefined range. The one-way sensitivity analyses were executed across all parameters, with the parameter range derived from either reported or estimated 95% confidence intervals from the referenced studies, or determined by a considered 10% or 20% deviation from the base-case value (outlined in Table 1). Probabilistic sensitivity analysis was conducted through 1,000 Monte Carlo simulations, where all model parameters were randomly sampled within predefined distributions. The outcome measures from these multiple simulations were analyzed to evaluate the robustness of the model. The choice of distribution was guided by a gamma distribution for cost parameters and a beta distribution for probability, proportion, and preference value parameters. Drawing insights from the data amassed through these 1,000 iterations, a cost-effectiveness acceptability curve emerged, presenting the probability that triple therapy would qualify as cost-effective across various thresholds of WTP concerning health advancements (QALYs).
2.6 Scenario analysis
We meticulously evaluated the resilience of our model’s findings through a series of scenario analyses. Initially, we explored the potential impact of China’s pharmaceutical policies on the pricing of benmelstobart, hypothesizing a significant reduction in drug costs by either 50% or 90%. Subsequently, we delved into the ramifications of discounts, ranging from 3% to 8%, on pharmacoeconomic outcomes, aligning with the parameters stipulated in the China Guidelines for Pharmacoeconomic Evaluations. (2020). Our comprehensive data analysis was expertly executed utilizing TreeAge Pro 2022, ensuring the accuracy and reliability of our insights.
3 Results
3.1 Base-case analysis
The 21-day therapy costs were as follows: anlotinib ($563.24), carboplatin ($74.50), etoposide ($134.53) and benmelstobart ($3,485.32) (Table 1). In comparison with chemotherapy therapy, both triple therapy and dual therapy showed less cost-effectiveness, with ICER of $149225.65 and $62961.20 respectively. While compared with dual therapy, triple therapy also showed less cost-effectiveness, with ICER $189763.99. The findings for both INHB and INMB showed negative values, suggesting that neither the triple therapy nor the dual therapy offered a higher cost-effectiveness ratio (Table 2).
TABLE 2
| Strategy | Cost | Incr cost | Eff | Incr eff | ICER | INHB, QALY | INMB, $ | Remarks |
|---|---|---|---|---|---|---|---|---|
| EC | 46,771.61 | NA | 0.45 | NA | NA | NA | NA | NA |
| A + EC | 54,523.56 | 7,751.95 | 0.57 | 0.12 | 62,959.34 | −0.08 | −3,064.46 | compared with EC |
| B + A + EC | 104,254.26 | 57,482.64 | 0.83 | 0.39 | 149,249.24 | −1.12 | −42819.93 | compared with EC |
| B + A + EC | 104,254.26 | 49,730.70 | 0.83 | 0.26 | 189,797.99 | −1.04 | −39755.48 | compared with A + EC |
Base results of triple therapy, dual therapy or chemotherapy alone.
Abbreviations: E, etoposide/carboplatin; A, anlotinib; B, benmelstobart; INHB, incremental net health benefit; INMB, incremental net monetary benefit; NA, not applicable; QALY, quality-adjusted life-years.
3.2 Sensitivity analysis
The one-way sensitivity analyses demonstrated that the cost of benmelstobart was a pivotal determinant of the model’s outcomes for the triple therapy group, exerting a more pronounced influence compared to the other treatment groups (Figure 2). The utility of PFS and PD were also important determinant among 3 groups. Additionally, the substantial impact of adverse reactions (AEs) in this study can likely be attributed to the elevated likelihood of severe grade 3 and higher AEs, with certain occurrences, such as Neutropenia, exceeding a staggering 70%. Adopting a lower boundary value for the cost of benmelstobart (i.e., $2,788.26) resulted in an ICER of $129786.80 per additional QALY gained for triple therapy compared with chemotherapy. Conversely, adopting an upper boundary ($4,182.38) yielded an ICER was $168711.68 per additional QALY gained. However, none of these changes surpassed the threshold of $38070.59/QALY, which was the predetermined limit. Other parameters, including probability of AEs, discount, and the cost of best support care, AEs or laboratory tests, exhibited only moderate or weak associations with the outcome and did not lead to ICERs exceeding the threshold.
FIGURE 2
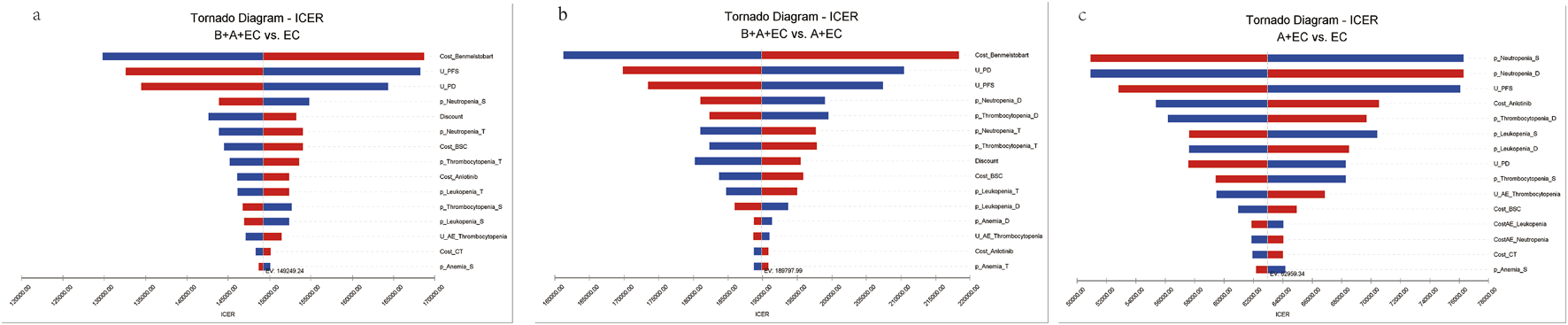
Tornado diagram of triple therapy, dual therapy or chemotherapy alone in the one-way deterministic sensitivity analysis. (a) B + A + EC vs. EC; (b) B + A + EC vs. A + EC; (c) A + EC vs. EC. B, benmelstobart; A, anlotinib; E, etoposide; C, carboplatin; U, utility; PFS, progression-free survival; PD, progressed disease; BSC, best supportive care; AE, adverse effect; p, probability; S, single therapy; D, dual therapy; T, triple therapy.
Compared to EC chemotherapy, the probabilistic sensitivity analysis revealed that the combination of benmelstobart and anlotinib contributed a mean increase of 0.39 QALYs (ranging from 0.30 to 0.49) and an additional average cost of $57482.64 (with a range of $46541.86 to $71511.23). Consequently, the calculated mean ICER was $149249.24/QALY (from $145941.29/QALY to$155139.53/QALY). As demonstrated in Figure 3, each group exhibited a negligible or minimal likelihood of achieving cost-effectiveness. Moreover, the cost-effectiveness acceptability curve displayed that the likelihood of triple therapy being cost-effective increased from 34.20% to 97.60% when the threshold value for cost per QALY gained varied from $180000 to $240000 (Figure 4).
FIGURE 3
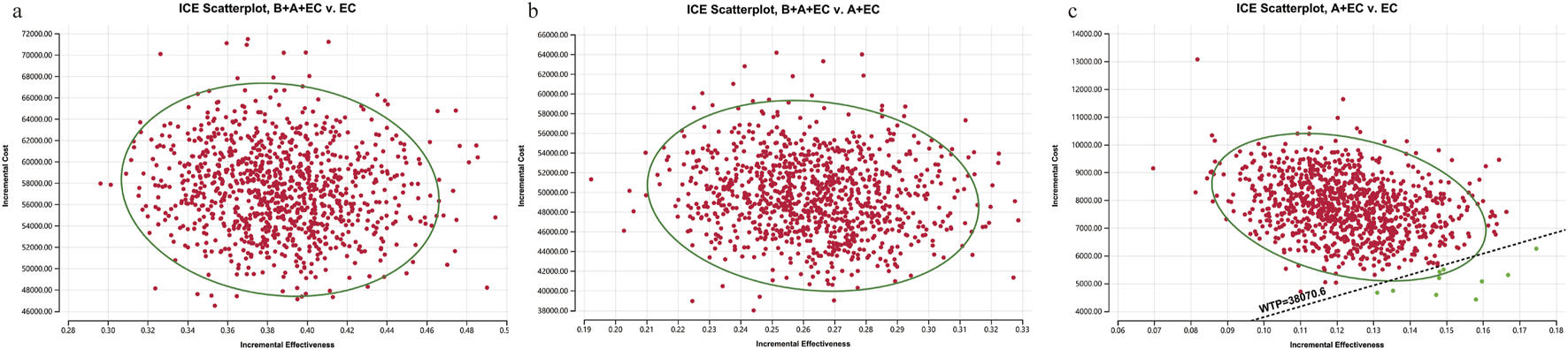
1,000 Monte Carlo simulation diagram of the probabilistic sensitivity analysis. ICE, ICE, incremental cost-effectiveness. (a) B + A + EC vs. EC; (b) B + A + EC vs. A + EC; (c) A + EC vs. EC. B, benmelstobart; A, anlotinib; E, etoposide; C, carboplatin.
FIGURE 4
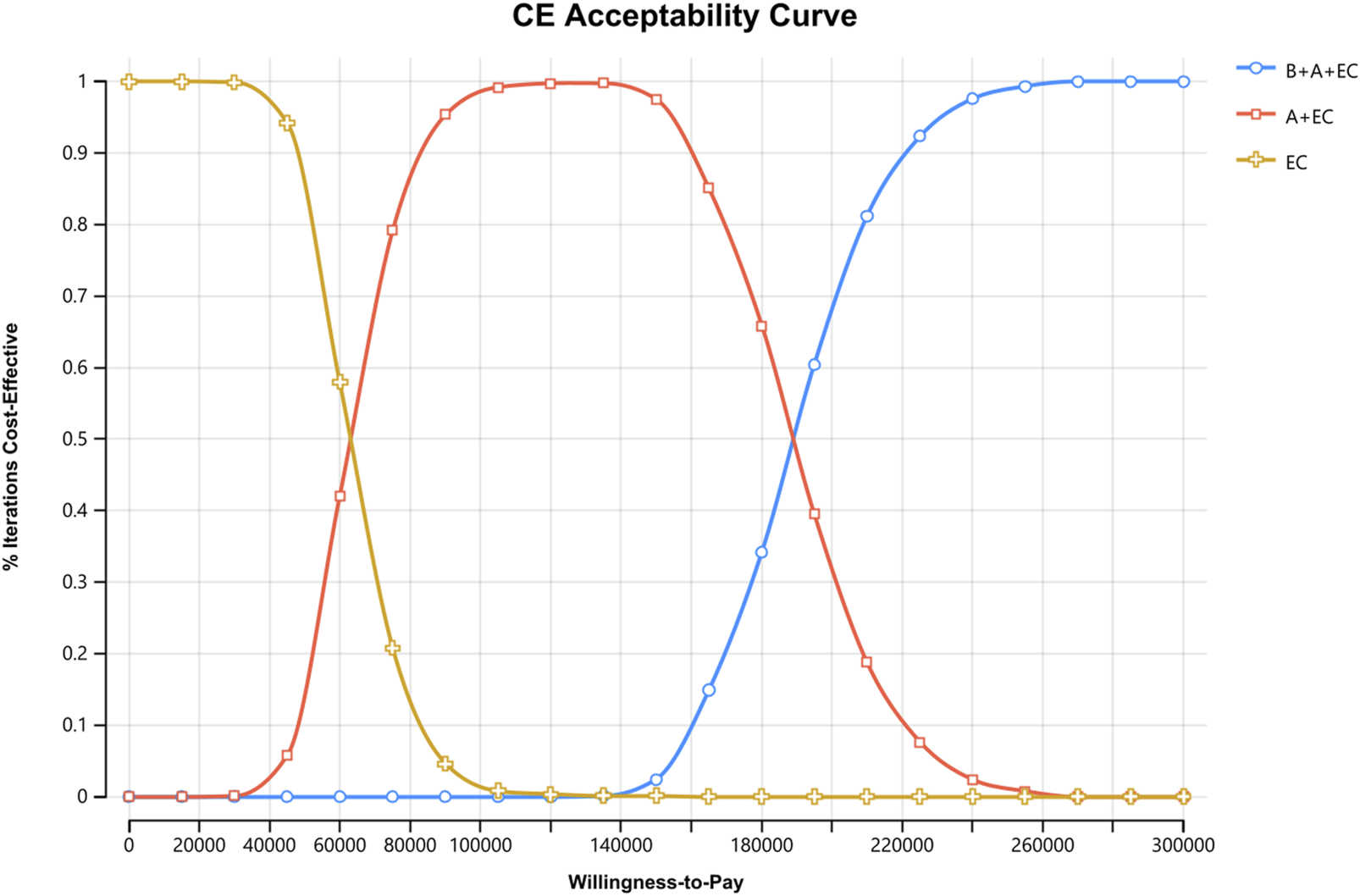
Cost-effectiveness Acceptability Curves for Patients with ES-SCLC. CE, cost-effectiveness, B, benmelstobart; A, anlotinib; E, etoposide; C, carboplatin.
3.3 Scenario analysis
The scenario analysis outcomes are presented in Table 3. Notably, a 90% reduction in the price of benmelstobart results in an ICER of $61667.75 compared with EC, which remains above the WTP threshold, set at three times the GDP per capita, suggesting potential decreased cost-effective. Additionally, when the discount rates are adjusted to 3% and 8%, the ICER for benmelstobart in comparison to chemotherapy is calculated to be $146580.26 and $153263.18, respectively. These results clearly demonstrate that benmelstobart is less cost-effective across both discount rates. It is important to highlight that the impact of discounts on the outcomes is relatively minor, and only substantial decreases in drug’s prices could potentially make benmelstobart a more economically viable option.
TABLE 3
| No. | Strategy | Cost | Incr cost | Eff | Incr eff | ICER | Remarks |
|---|---|---|---|---|---|---|---|
| 1 | EC | 46,771.61 | 0.45 | price dropped by 50% | |||
| B + A + EC | 85,514.48 | 38,742.87 | 0.83 | 0.39 | 100,592.85 | ||
| 2 | EC | 46,771.61 | 0.45 | price dropped by 90% | |||
| B + A + EC | 70,522.66 | 23,751.04 | 0.83 | 0.39 | 61,667.75 | ||
| 3 | EC | 45,991.93 | 0.44 | 8% discount | |||
| B + A + EC | 101,796.73 | 55,804.81 | 0.80 | 0.36 | 153,263.18 | ||
| 4 | EC | 47,326.70 | 0.46 | 3% discount | |||
| B + A + EC | 106,020.64 | 58,693.94 | 0.86 | 0.40 | 146,580.26 |
Scenario analysis results.
4 Discussion
With the rising incidence of ES-SCLC nationwide, the associated costs are also progressively on the rise. Multiple studies have examined the cost-effectiveness of novel anti-cancer drugs for ES-SCLC treatment. Comparatively, the economic findings for durvalumab in combination with platinum/etoposide and chemotherapy alone suggest them to be less cost-effective regimen from the United States healthcare system perspective (Ding et al., 2021). In Xiang’s study, serplulimab plus chemotherapy was determined to be lacking in cost-effectiveness, and it was suggested that cost-effectiveness may improve with price discounts on serplulimab from the perspective of Chinese healthcare system (Xiang et al., 2023). Adebrelimab plus chemotherapy was not an economical strategy compared with chemotherapy for first-line treatment of ES-SCLC in China, however, You’s study held a different view (You et al., 2022; Long et al., 2024).
The ICER represents the additional cost per QALY gained when comparing two treatment strategies. When the ICER exceeds the cost-effectiveness threshold, the intervention is typically deemed not cost-effective due to the excessive financial burden on patients or healthcare systems relative to the clinical benefit achieved. Our study addresses the unmet need for an economic assessment of benmelstobart triple therapy. Drawing on data from the ETER701 trial, our analysis demonstrates the less cost-effectiveness of triple therapy for the treatment of advanced ES-SCLC at a WTP threshold of $38070.59 per QALY. These findings are generally robust and supported by the results of both one-way sensitivity and probabilistic sensitivity analysis. The results of scenario analysis show that adjusting the discount rate or lowering the price of benmelstobart still support the outcomes of base-case analysis. This may be due to the high cost of benmelstobart, which has a crucial influence on the model results.
The prevention of disease progression by the combination of benmelstobart triple therapy played a crucial role in determining the economic outcomes. The results from the 1-way sensitivity analysis demonstrated that the cost of benmelstobart was the most influential factor. Additionally, the utility of PD, and utility of PFS were also deemed significant. Furthermore, the PSA results indicated that the ICER values of triple therapy were predominantly situated above the threshold curve when compared with the other treatment groups, which was set at 3 times China’s per capita GDP. Benmelstobart triple therapy is also found to not be a cost-effective option.
To the best of our knowledge, this study represents the inaugural analysis to comprehensively examine the economic implications of administering benmelstobart triple therapy for the management of advanced ES-SCLC. By leveraging an economic modeling methodology, we have synthesized the most up-to-date evidence available. Despite this milestone, we must acknowledge the lack of sufficient data pertaining to the economic outcomes associated with immune checkpoint inhibitors (ICIs) for the treatment of advanced ES-SCLC. Further investigation is warranted to ascertain the specific patient cohorts that would drive optimal benefits from the administration oftriple therapy.
The analysis at hand does exhibit certain limitations that warrant consideration. Firstly, due to the absence of head-to-head data, we were unable to incorporate other ICIs like atezolizumab, durvalumab as first-line treatments, despite their demonstrated positive health outcomes for patients with advanced ES-SCLC. Thus, it is essential that our analysis be updated once first-line data become available. Secondly, in this study, we employed parameter distribution fitting to extrapolate the PFS and OS curves. Although this allows for the extrapolation of survival trends beyond the ETER701 study period, it also introduces uncertainty into the model results. Therefore, our next step should involve further exploration of the clinical efficacy of triple therapy through real-world research, as well as validation of the economic feasibility of this regimen with the aid of more mature survival curves from longer follow-up results in the ETER701 study. Thirdly, it is important to acknowledge that in real-world scenarios, individual patient characteristics may lead to variations in subsequent treatment plans. While this study was based on data from the ETER701 clinical trial, the model may not fully capture the heterogeneity of individual patient characteristics. Although sensitivity analyses were performed across predefined parameter ranges to address uncertainty, the complexity of real-word patient profiles remains incompletely represented. Future research should focus on developing more sophisticated models that integrate individualized patient characteristics, comorbidities, and relevant clinical indicators. Such enhanced modeling approaches would enable the generation of personalized, economically optimized treatment recommendations that better reflect clinical decision-making processes in routine practice. Fourthly, due to the lack of time series data, the present analysis did not account for variations in costs associated with survival time and duration, such as follow-up costs. Lastly, in clinical practice, grade 1–2 AEs typically require minimal or no intervention and incur relatively low management costs. However, their exclusion from economic evaluations may lead to underestimation of total treatment costs and potential overestimation of a regimen’s cost-effectiveness. Nevertheless, this limitation is unlikely to significantly alter the study conclusions. In real-world clinical practice, it is essential to adopt a patient-centered approach that integrates individual patient characteristics, AE management costs, and treatment cost-effectiveness to optimize therapeutic option for each patient. Moreover, since this evaluation reflects the general clinical practice for managing advanced ES-SCLC, it can serve as a valuable reference for physicians and policymakers. Furthermore, pharmacoeconomic analyses provide substantial, objective, and comprehensive evidence, aiding long-term planning in domains like public health and healthcare insurance.
Our results indicate that benmelstobart and anlotinib plus EC chemotherapy is not cost-effective compared with chemotherapy at the WTP threshold of $38070.59 per QALY. These findings may aid clinicians in making optimal decisions regarding the treatment of advanced ES-SCLC.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
JN: Conceptualization, Funding acquisition, Methodology, Writing – original draft, Visualization. CK: Data curation, Methodology, Visualization, Writing – original draft. KT: Data curation, Writing – original draft, Methodology. SD: Data curation, Writing – original draft. XZ: Data curation, Writing – original draft. JW: Conceptualization, Project administration, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the China zhongguancun Precision Medicine science and technology foundation (320.99.2024.0305.004).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1524108/full#supplementary-material
References
1
Augustin H. G. Koh G. Y. (2022). Antiangiogenesis: vessel regression, vessel normalization, or both?Cancer Res.82 (1), 15–17. 10.1158/0008-5472.Can-21-3515
2
Bray F. Laversanne M. Sung H. Ferlay J. Siegel R. L. Soerjomataram I. et al (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin.74 (3), 229–263. 10.3322/caac.21834
3
Cheng Y. Han L. Wu L. Chen J. Sun H. Wen G. et al (2022). Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: the ASTRUM-005 randomized clinical trial. Jama328 (12), 1223–1232. 10.1001/jama.2022.16464
4
Cheng Y. Chen J. Zhang W. Xie C. Hu Q. Zhou N. et al (2024). Benmelstobart, anlotinib and chemotherapy in extensive-stage small-cell lung cancer: a randomized phase 3 trial. Nat. Med.30, 2967–2976. 10.1038/s41591-024-03132-1
5
Craig B. A. Black M. A. (2001). Incremental cost-effectiveness ratio and incremental net-health benefit: two sides of the same coin. Expert Rev. pharmacoeconomics & outcomes Res.1 (1), 37–46. 10.1586/14737167.1.1.37
6
Dai Z. Xu J. Chang F. Zhou W. Ren T. Qiu J. et al (2024). The cost-effectiveness of iruplinalkib versus alectinib in anaplastic lymphoma kinase-positive crizotinib-resistant advanced non-small-cell lung cancer patients in China. Front. Public Health12, 1333487. 10.3389/fpubh.2024.1333487
7
Ding D. Hu H. Li S. Zhu Y. Shi Y. Liao M. et al (2021). Cost-effectiveness analysis of durvalumab plus chemotherapy in the first-line treatment of extensive-stage small cell lung cancer. J. Natl. Compr. Cancer Netw.19 (10), 1141–1147. 10.6004/jnccn.2020.7796
8
Goldman J. W. Dvorkin M. Chen Y. Reinmuth N. Hotta K. Trukhin D. et al (2021). Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol.22 (1), 51–65. 10.1016/s1470-2045(20)30539-8
9
Guyot P. Ades A. E. Ouwens M. J. N. M. Welton N. J. (2012). Enhanced secondary analysis of survival data: reconstructing the data from published kaplan-meier survival curves. BMC Med. Res. Methodol.12, 9. 10.1186/1471-2288-12-9
10
Horn L. Mansfield A. S. Szczęsna A. Havel L. Krzakowski M. Hochmair M. J. et al (2018). First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med.379 (23), 2220–2229. 10.1056/NEJMoa1809064
11
Ko J. Winslow M. M. Sage J. (2021). Mechanisms of small cell lung cancer metastasis. EMBO Mol. Med.13 (1), e13122. 10.15252/emmm.202013122
12
Liu G. E Hu S. L. Wu J. H. et al (2020). China Guidelines for Pharmacoeconomic Evaluations2020. Bejing, China: China Market Press.
13
Liu S. V. Reck M. Mansfield A. S. Mok T. Scherpereel A. Reinmuth N. et al (2021). Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J. Clin. Oncol.39 (6), 619–630. 10.1200/jco.20.01055
14
Liu X. Xi X. Xu S. Chu H. Hu P. Li D. et al (2024). Targeting T cell exhaustion: emerging strategies in non-small cell lung cancer. Front. Immunol.15, 1507501. 10.3389/fimmu.2024.1507501
15
Long Y. Wang H. Xie X. Li J. Xu Y. Zhou Y. (2024). Updated cost-effectiveness analysis of adebrelimab plus chemotherapy for extensive-stage small cell lung cancer in China. BMJ Open14 (4), e077090. 10.1136/bmjopen-2023-077090
16
Pavan A. Attili I. Pasello G. Guarneri V. Conte P. F. Bonanno L. (2019). Immunotherapy in small-cell lung cancer: from molecular promises to clinical challenges. J. Immunother. Cancer7 (1), 205. 10.1186/s40425-019-0690-1
17
Paz-Ares L. Dvorkin M. Chen Y. Reinmuth N. Hotta K. Trukhin D. et al (2019). Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet394 (10212), 1929–1939. 10.1016/s0140-6736(19)32222-6
18
Rudin C. M. Brambilla E. Faivre-Finn C. Sage J. (2021). Small-cell lung cancer. Nat. Rev. Dis. Prim.7 (1), 3. 10.1038/s41572-020-00235-0
19
Statistics N. B. O. (2023). Birth rate, mortality rate and natural growth rate. Available online at: https://data.stats.gov.cn/easyquery.htm?cn=C01&zb=A0302&sj=2023.
20
Xiang G. Jiang T. Gan L. Wu Y. Zhang N. Xing H. et al (2023). Cost-effectiveness of serplulimab as first-line therapy for extensive-stage small cell lung cancer in China. Front. Immunol.14, 1223020. 10.3389/fimmu.2023.1223020
21
Xu S. Lu Z. (2024). Exploring FNDC4 as a biomarker for prognosis and immunotherapy response in lung adenocarcinoma. Asian J. Surg.48, 1799–1801. 10.1016/j.asjsur.2024.09.054
22
You M. Chen R. Wu Q. Zhu W. He Y. Huang Y. (2022). Cost-effectiveness analysis of adebrelimab combined with chemotherapy for extensive-stage small cell lung cancer. Front. Pharmacol.13, 1019826. 10.3389/fphar.2022.1019826
23
Zhou T. Zhang Z. Luo F. Zhao Y. Hou X. Liu T. et al (2020). Comparison of first-line treatments for patients with extensive-stage small cell lung cancer: a systematic review and network meta-analysis. JAMA Netw. Open3 (10), e2015748. 10.1001/jamanetworkopen.2020.15748
24
Zhu Y. Liu K. Qin Q. Zhu H. (2022). Serplulimab plus chemotherapy as first-line treatment for extensive-stage small-cell lung cancer: a cost-effectiveness analysis. Front Immunol13, 1044678. 10.3389/fimmu.2022.1044678
25
Zhu Y. Liu K. Yang Q. Zeng M. Peng L. (2023). First-line Immuno-chemotherapy for extensive-stage small-cell lung cancer: a network meta-analysis and cost-effectiveness analysis. Front. Public Health11, 1028202. 10.3389/fpubh.2023.1028202
Summary
Keywords
extensive-stage small-cell lung cancer, benmelstobart, anlotinib, carboplatin, etoposide, cost-effectiveness
Citation
Nie J, Kou C, Tang K, Dai S, Zhao X and Wu J (2025) Cost-effectiveness evaluation of benmelstobart, anlotinib and chemotherapy in patients with extensive-stage small-cell lung cancer. Front. Pharmacol. 16:1524108. doi: 10.3389/fphar.2025.1524108
Received
07 November 2024
Accepted
30 July 2025
Published
19 August 2025
Volume
16 - 2025
Edited by
Yang Chen, Chinese Academy of Sciences (CAS), China
Reviewed by
Hesong Wang, Fourth Hospital of Hebei Medical University, China
Qian Guo, First Affiliated Hospital of Zhengzhou University, China
Updates
Copyright
© 2025 Nie, Kou, Tang, Dai, Zhao and Wu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiyong Wu, wujiyongsdcn@gmail.com
ORCID: Jiyong Wu, orcid.org/0000-0001-9360-090X
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.