Abstract
Objectives:
To provide the latest systematic review and meta-analysis comparing the effectiveness and safety of tofacitinib and adalimumab in rheumatoid arthritis (RA) patients.
Methods:
A systematic search of PubMed, Embase, Web of Science, and Cochrane databases was conducted until April 2025. Randomized controlled trials and cohorts comparing tofacitinib and adalimumab in RA patients were included. Outcomes assessed were significant improvements in American College of Rheumatology (ACR) 20 improvement criteria, changes in visual analog scale (VAS) (global activity), disease activity score (DAS) 28-C-reactive protein (CRP), Health Assessment Questionnaire-Disability Index (HAQ-DI), and adverse events. Sensitivity analyses and subgroup analysis evaluated the robustness of results and heterogeneity. Data analysis was performed using Review Manager 5.4.1 and STATA 15.0.
Results:
Nine studies with 24,643 patients were analyzed. Tofacitinib showed superior effectiveness over adalimumab in ACR20 (risk ratio (RR): 1.28; 95% CI: 1.06, 1.55; P = 0.01), HAQ-DI (standardized mean difference (SMD): 0.20; 95% CI: 0.35, −0.05; P = 0.008), and VAS (SMD: 0.30; 95% CI: 0.56, −0.03; P = 0.03). No significant differences were found in adverse events (RR: 0.96; 95% CI: 0.89, 1.03; P = 0.22) or DSA28-CRP improvement (SMD: 0.02; 95% CI: 0.45, 0.02; P = 0.07). Sensitivity analyses confirmed stable outcomes for adverse events, HAQ-DI, and ACR20, but instability for VAS and DSA28-CRP. Subgroup analysis found that tofacitinib >5 mg twice daily was superior to ≤5 mg in terms of ACR20.
Conclusion:
Tofacitinib was more effective than adalimumab in improving ACR20, VAS, and HAQ-DI, with no significant differences in adverse events or DSA28-CRP improvement.
Systematic Review Registration::
1 Introduction
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease marked by persistent synovitis, pannus formation, and destruction of cartilage and bone (Zhu and Zhou, 2024; Vittecoq et al., 2024). The exact cause and mechanism in RA patients remain unknown. Clinically, it presents as symmetrical joint swelling, pain, and stiffness, potentially leading to joint deformities in advanced stages (Soriano et al., 2024). RA is prevalent worldwide, with varying rates across regions. Current early treatment primarily involves five classes of drugs: NSAIDs, csDMARDs, tsDMARDs, bDMARDs, and glucocorticoids (Qi et al., 2024; Han et al., 2024). Among them, adalimumab and tofacitinib are representative drugs of bDMARDs and tsDMARDs, respectively.
Adalimumab, the first fully humanized monoclonal antibody, binds specifically to TNF-α and effectively inhibits this cytokine (Zhang et al., 2024). The main risks of adalimumab include opportunistic infections and injection site reactions, and studies have shown that the incidence of serious infections is comparable to the underlying risk of rheumatoid arthritis (Smolen et al., 2024; Conde-Aranda et al., 2024; Lakhmiri et al., 2024). Tofacitinib, a first-generation oral drug, selectively inhibits JAK1 and JAK3, exerting less influence on JAK2 and TYK2 (Shih et al., 2024). Tofacitinib was first approved by the FDA for the treatment of RA in November 2012. Research indicates that tofacitinib is effective as a monotherapy or combined with DMARDs for RA treatment. The US FDA has approved tofacitinib for RA patients unresponsive to MTX (Fournier et al., 2024; Chang and Wang, 2024). In January 2017, after reviewing long-term safety and efficacy data, the European Medicines Agency proposed approving tofacitinib for RA treatment. In 2016, the FDA approved an extended-release tofacitinib formulation administered via an osmotic system for RA treatment (Boussaa et al., 2024; Adami et al., 2024). However, in February 2019, the FDA issued a safety alert regarding dose-dependent thrombosis and mortality risks with JAK inhibitors, which originated from the ORAL Surveillance study of tofacitinib in rheumatoid arthritis (RA) patients. Subsequent regulatory actions extended this boxed warning to the entire JAK inhibitor class (including baricitinib and upadacitinib), mandating restricted use in patients with cardiovascular risk factors regardless of specific indications (Kim et al., 2023; Fleischmann et al., 2017; van Vollenhoven et al., 2012; Fleischmann et al., 2012). A 24-week double-blind phase IIb study by Fleischmann et al. (2012) found that tofacitinib monotherapy (≥5 mg, twice daily) effectively treated active RA for over 24 weeks with manageable safety, compared to adalimumab (Fleischmann et al., 2012). Another study reported similar ACR20 outcomes for tofacitinib and adalimumab in RA treatment (Fleischmann et al., 2017).
Despite numerous clinical studies comparing tofacitinib and adalimumab for RA, evidence-based data confirming their relative advantages and disadvantages remains insufficient. This study aims to compare adalimumab and tofacitinib regarding clinical efficacy, disease activity, quality of life, and safety through systematic review and meta-analysis, providing clinicians with a reference for selecting treatments. The goal is to identify a regimen with rapid onset, strong efficacy, good safety, and low cost for RA patients.
2 Methods
2.1 Literature search
This meta-analysis followed the PRISMA 2020 guidelines (Page et al., 2021) and is registered in PROSPERO (CRD42024605000). A systematic literature search was conducted in PubMed, Embase, Web of Science, and Cochrane up to April 2025 for studies comparing adalimumab and tofacitinib in rheumatoid arthritis patients. The search terms used were “adalimumab,” “tofacitinib,” and “rheumatoid arthritis.” Detailed search strategies are provided in Supplementary Table S1. Additionally, we manually screened the reference lists of included studies. Two authors independently retrieved and assessed eligible articles, resolving any discrepancies through discussion.
2.2 Inclusion and exclusion criteria
Articles were included if they met the following criteria: P (Population): patients with rheumatoid arthritis; I (Intervention): tofacitinib; C (Comparison): adalimumab; O (Outcome): American College of Rheumatology (ACR) 20, change in patient-reported visual analog scale (VAS) (global activity), disease activity score (DAS) 28-C-reactive protein (CRP), change in Health Assessment Questionnaire-Disability Index (HAQ-DI), and adverse events, etc.; S (Study design): randomized controlled trials and cohort studies. Exclusion criteria included study protocols, unpublished or non-original studies (e.g., meeting abstracts, corrections, replies), single-arm studies, studies with insufficient data, and reviews.
2.3 Data abstraction
Data abstraction was independently performed by two authors, with discrepancies resolved by a third author. Abstracted data included: first author, publication year, research period, region, study design, registration number, population, intervention/exposure, control, sample size, age, gender, disease duration, follow-up, ACR20, VAS changes, DAS28-CRP changes, HAQ-DI changes, and adverse events. If data were incomplete, corresponding authors were contacted for additional information.
2.4 Quality evaluation
Risk of bias (RoB) tool for RCT quality evaluation followed the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0, considering seven domains: randomization sequence generation, allocation concealment, participant and personnel blinding, outcome assessment blinding, incomplete outcome data, selective reporting, and other potential bias sources (Cumpston et al., 2019). Each aspect was rated as low, high, or unclear risk. Studies with more “low risk” evaluations were considered superior. Cohort study quality was assessed using the Newcastle-Ottawa Scale (NOS) (Wells et al., 2011), with scores of seven to nine indicating high quality (Kim et al., 2019). Two authors independently evaluated the quality of all included studies, resolving disagreements through discussion.
2.5 Statistical analysis
Data synthesis was conducted using Review Manager 5.4.1. Standardized mean difference (SMD) was applied for continuous data, and risk ratio (RR) for dichotomous data. We measured improvement in all continuous variables by calculating the change from baseline to the last follow-up visit. Each metric was reported with 95% CIs using a random-effects model. Heterogeneity across outcomes was assessed using the chi-squared (χ2) test (Cochran’s Q) and the inconsistency index (Higgins and Thompson, 2002). Substantial heterogeneity was defined as a χ2 P value below 0.1 or an I2 over 50%. For outcomes with more than two studies, sensitivity analysis was performed to assess the impact of individual studies on the overall results. In addition, subgroup analyses were performed to explore the stability of the results and potential sources of heterogeneity.
3 Results
3.1 Literature retrieval, study characteristics, and baseline
Figure 1 illustrates the flowchart of literature retrieval and selection. A total of 2,360 studies from PubMed (n = 157), Embase (n = 1,530), Web of Science (n = 567), and Cochrane (n = 106) were identified through a systematic search. After removing duplicates, 1,773 titles and abstracts were screened. Ultimately, nine studies (including 14 comparison groups) (Kim et al., 2023; Fleischmann et al., 2017; van Vollenhoven et al., 2012; Fleischmann et al., 2012; Deakin et al., 2023; Baker et al., 2023; Bergman et al., 2022; Takeuchi et al., 2021; Strand et al., 2019) involving 24,643 patients were included in the meta-analysis. Among them, six studies were RCTs and three studies were cohort studies. Table 1 summarizes the characteristics of each eligible study. Figure 2 shows the quality evaluation of all included RCTs. Quality assessments for included cohorts are provided in Supplementary Table S2.
FIGURE 1

Flowchart of the systematic search and selection process.
TABLE 1
| Study | Study period | Country | Study design | Registration number | Population | Intervention/exposure | Control | Patients | Mean follow-up | Mean/median age | Male | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tofacitinib | adalimumab | tofacitinib | adalimumab | tofacitinib | adalimumab | |||||||||
| Kim 2023 | 2018–2020 | Korea | Cohort | NCT03703817 | Patients aged ≥19 years with patients who were taking tofacitinib or adalimumab for ≥6 months | tofacitinib | adalimumab | 226 | 99 | 2 years | 53.56 | 53.27 | 28 | 15 |
| Deakin 2023 | 2015–2021 | Australian | RCT | HC17799 | Australian adults aged 18 years or older with RA in the Optimising Patient Outcomes in Australian Rheumatology (OPAL) data set | Tofacitinib (10 mg daily) | Adalimumab (40 mg every 14 days) | 273 | 569 | 3 years | 59 | 56 | 72 | 175 |
| Baker 2023 | 2003–2019 | United States | Cohort | NA | Patients aged 18 years or older with RA | tofacitinib | adalimumab | 1565 | 13,326 | 1.8 years | 57.6 | 52.7 | 271 | 3591 |
| Bergman 2023 | 2018–2022 | United States | Cohort | NA | Adults (aged C 18 years) with C 1 RA diagnosis | tofacitinib | adalimumab | 1770 | 3732 | 12 months | 52.1 | 49.7 | 301 | 897 |
| Takeuchi 2021 | NA | Multi-center | RCT | NCT02187055 | Patients were ≥18 years of age with active RA per ACR/EULAR criteria [9], despite receiving MTX for ≥4 months before screening and at stable doses of 15–25 mg/week (<15 mg/week permitted only for safety reasons) for ≥6 weeks before baseline | Tofacitinib 5 mg BID + MTX | ADA 40 mg every other week + MTX | 311 | 314 | 12 months | NA | NA | NA | NA |
| Strand 2019 | NA | Multi-center | RCT | NCT02187055 | ≥18 years of age and met ACR/European League Against Rheumatism classification criteria for active RA. | Oral tofacitinib was dosed at 5 mg two times per day + MTX | Subcutaneous ADA was dosed at 40 mg Q2W + MTX | 376 | 386 | NA | NA | NA | NA | NA |
| Fleischmann 2017 | 2014–2015 | Multi-center | RCT | NCT02187055 | aged 18 years or older who met the 2010 ACR and EULAR classification criteria for rheumatoid arthritis20 | tofacitinib 5 mg BID + MTX | ADA 40 mg every other week + MTX | 376 | 386 | 12 months | 50 | 50 | 65 | 66 |
| van Vollenhoven 2012a | 2009–2011 | Multi-center | RCT | NCT00853385 | 18 years of age or older and had received a diagnosis of active rheumatoid arthritis | 5 mg of tofacitinib twice daily | 40 mg of adalimumab administered by subcutaneous injection once every 2 weeks | 204 | 204 | 6 months | 53 | 52.5 | 30 | 42 |
| van Vollenhoven 2012b | 2009–2011 | Multi-center | RCT | NCT00853385 | 18 years of age or older and had received a diagnosis of active rheumatoid arthritis | 10 mg of tofacitinib twice daily | 40 mg of adalimumab administered by subcutaneous injection once every 2 weeks | 201 | 204 | 6 months | 52.9 | 52.5 | 33 | 42 |
| Fleischmann 2012a | NA | Multi-center | RCT | NCT00550446 | ≥18 years of age, had a diagnosis of RA for ≥6 months | 1 mg twice a day | Injected subcutaneously at 40 mg once every other week | 54 | 53 | 12 weeks | 55 | 54 | 8 | 8 |
| Fleischmann 2012b | NA | Multi-center | RCT | NCT00550446 | ≥18 years of age, had a diagnosis of RA for ≥6 months | 3 mg twice a day | Injected subcutaneously at 40 mg once every other week | 51 | 53 | 12 weeks | 53 | 54 | 7 | 8 |
| Fleischmann 2012c | NA | Multi-center | RCT | NCT00550446 | ≥18 years of age, had a diagnosis of RA for ≥6 months | 5 mg twice a day | Injected subcutaneously at 40 mg once every other week | 49 | 53 | 12 weeks | 54 | 54 | 6 | 8 |
| Fleischmann 2012d | NA | Multi-center | RCT | NCT00550446 | ≥18 years of age, had a diagnosis of RA for ≥6 months | 10 mg twice a day | Injected subcutaneously at 40 mg once every other week | 61 | 53 | 12 weeks | 52 | 54 | 8 | 8 |
| Fleischmann 2012e | NA | Multi-center | RCT | NCT00550446 | ≥18 years of age, had a diagnosis of RA for ≥6 months | 15 mg twice a day | Injected subcutaneously at 40 mg once every other week | 57 | 53 | 12 weeks | 53 | 54 | 7 | 8 |
Characteristics of included studies.
FIGURE 2
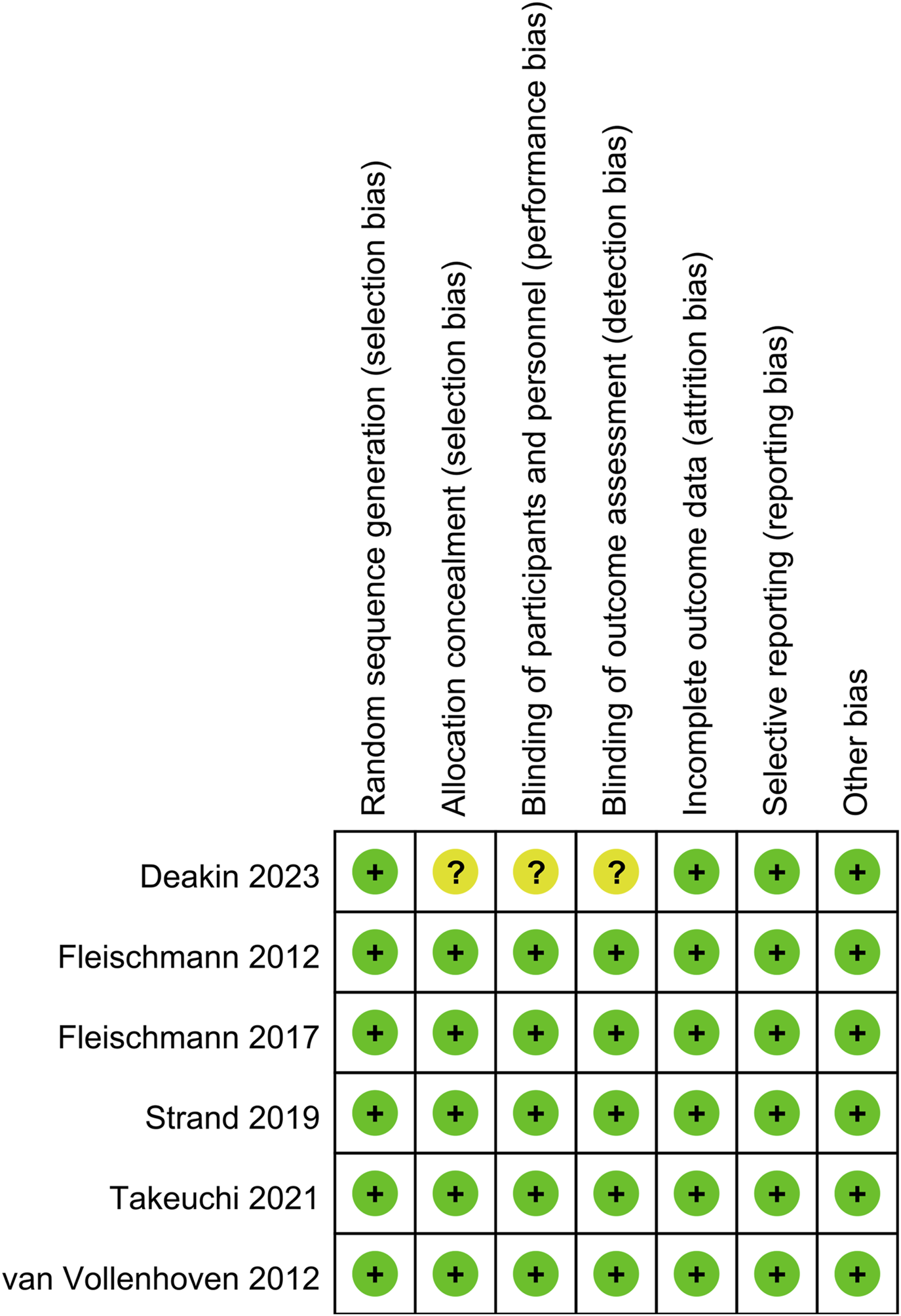
Details of the quality evaluation for included RCTs.
3.2 ACR20
ACR20 results were synthesized from three studies (7 comparison groups) involving 1,982 patients. Meta-analysis showed a significantly higher ACR20 in the tofacitinib group (RR: 1.28; 95% CI: 1.06, 1.55; P = 0.01) with substantial heterogeneity (I2 = 74%, P = 0.0008) (Figure 3a).
FIGURE 3
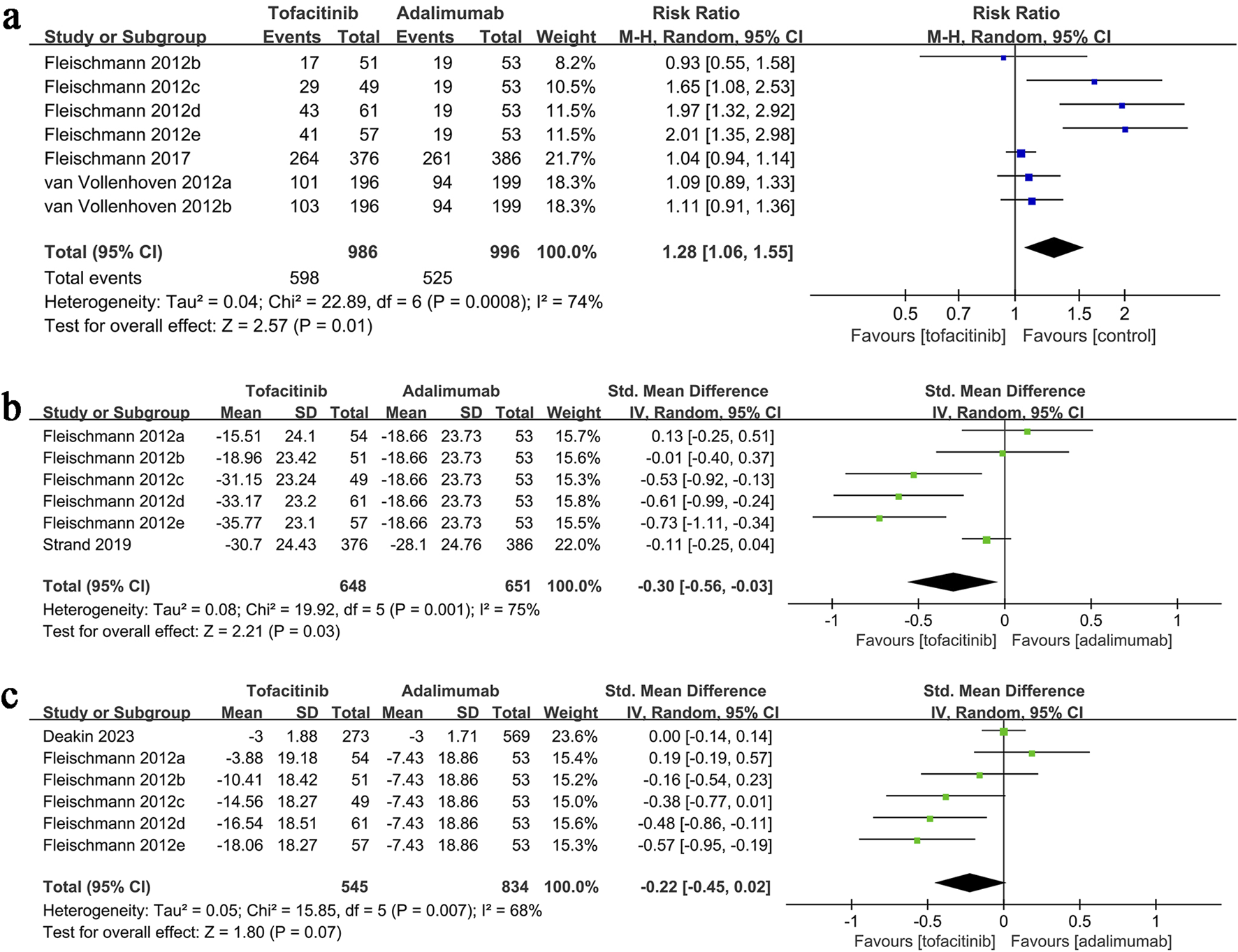
Forest plots of (a) ACR20, (b) change in VAS, (c) change in DAS28-CRP.
3.3 Change in VAS
VAS change data synthesis was performed in two studies (6 comparison groups) involving 1,299 patients. Meta-analysis showed a significantly greater reduction in VAS in the tofacitinib group (SMD: 0.30; 95% CI: 0.56, −0.03; P = 0.03) with substantial heterogeneity (I2 = 75%, P = 0.001) (Figure 3b).
3.4 Change in DAS28-CRP
Results of change in DAS28-CRP were synthesized from two studies (including six comparison groups) including 1,379 patients. Meta-analysis revealed a similar change in DAS28-CRP in the tofacitinib and adalimumab group (SMD: 0.02; 95% CI: 0.45, 0.02; P = 0.07) with a significant heterogeneity (I2 = 68%, P = 0.007) (Figure 3c).
3.5 Change in HAQ-DI
HAQ-DI change data synthesis was performed in four studies (9 comparison groups) involving 2,737 patients. Meta-analysis showed a significantly greater reduction in HAQ-DI in the tofacitinib group (SMD: 0.20; 95% CI: 0.35, −0.05; P = 0.008) with substantial heterogeneity (I2 = 69%, P = 0.001) (Figure 4a).
FIGURE 4
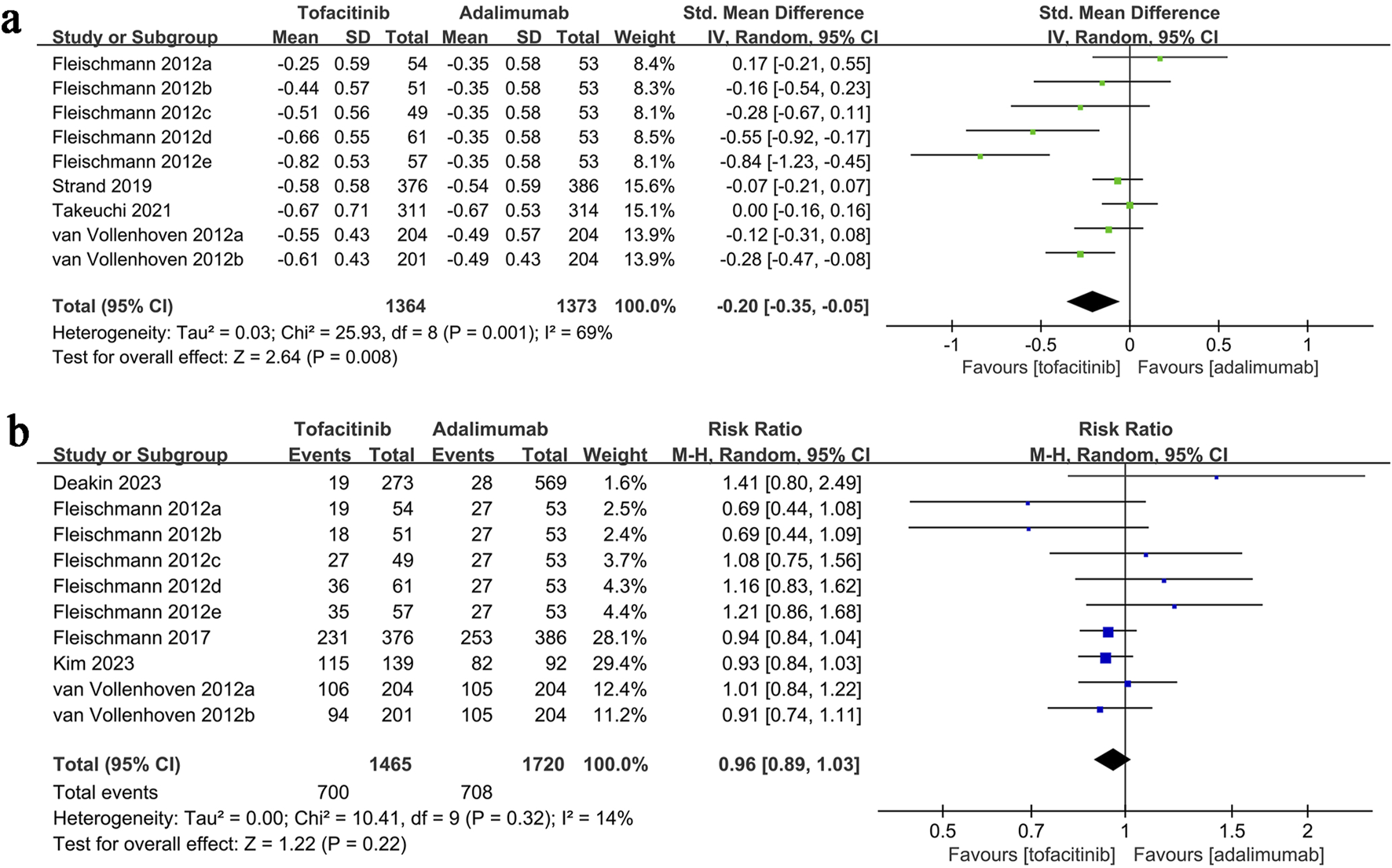
Forest plots of (a) change in HAQ-DI, (b) adverse events.
3.6 Adverse events
Adverse events data were synthesized from five studies (10 comparison groups) involving 3,185 patients. Meta-analysis showed a similar adverse event rate between the tofacitinib and adalimumab groups (RR: 0.96; 95% CI: 0.89, 1.03; P = 0.22) with no significant heterogeneity (I2 = 14%, P = 0.32) (Figure 4b).
3.7 Subgroup analysis
To explore the effect of tofacitinib dose on efficacy, we performed a subgroup analysis of ACR20. The results showed that when the dose was >5 mg twice a day, the ACR20 of tofacitinib was significantly better than that of adalimumab (RR: 1.59; 95% CI: 1.03, 2.48; P = 0.04). However, in the subgroup with a dose of ≤5 mg twice a day, tofacitinib and adalimumab had similar ACR20 (RR: 1.09; 95% CI: 0.95, 1.26; P = 0.22) (Figure 5).
FIGURE 5
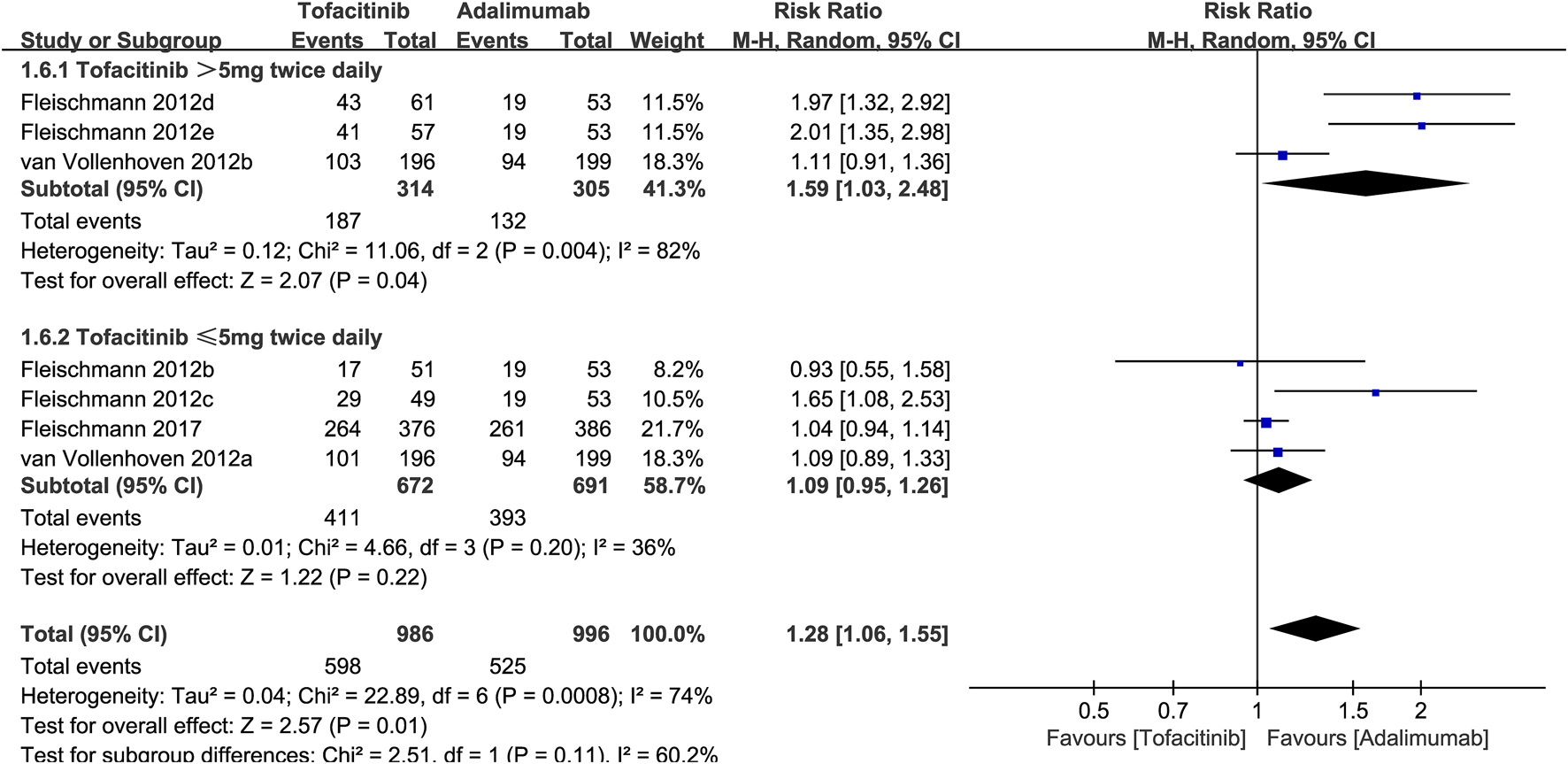
Subgroup analysis of ACR20 based on the dose of tofacitinib.
3.8 Sensitivity analysis
Sensitivity analyses were conducted for ACR20, VAS, DAS28-CRP, HAQ-DI, and adverse events to evaluate the effect of each study on overall outcomes by sequentially excluding eligible studies. The analyses showed stable outcomes after excluding each study for adverse events (Figure 6a), HAQ-DI (Figure 6b), and ACR20 (Figure 6c). However, significant instability was found for VAS (Figure 6d) and DAS28-CRP (Figure 6e). For HAQ-DI, excluding Fleischmann 2012e reduced heterogeneity from 69% to 47%, indicating that this study contributed to the significant heterogeneity. Heterogeneity in other outcomes was not linked to a specific study.
FIGURE 6
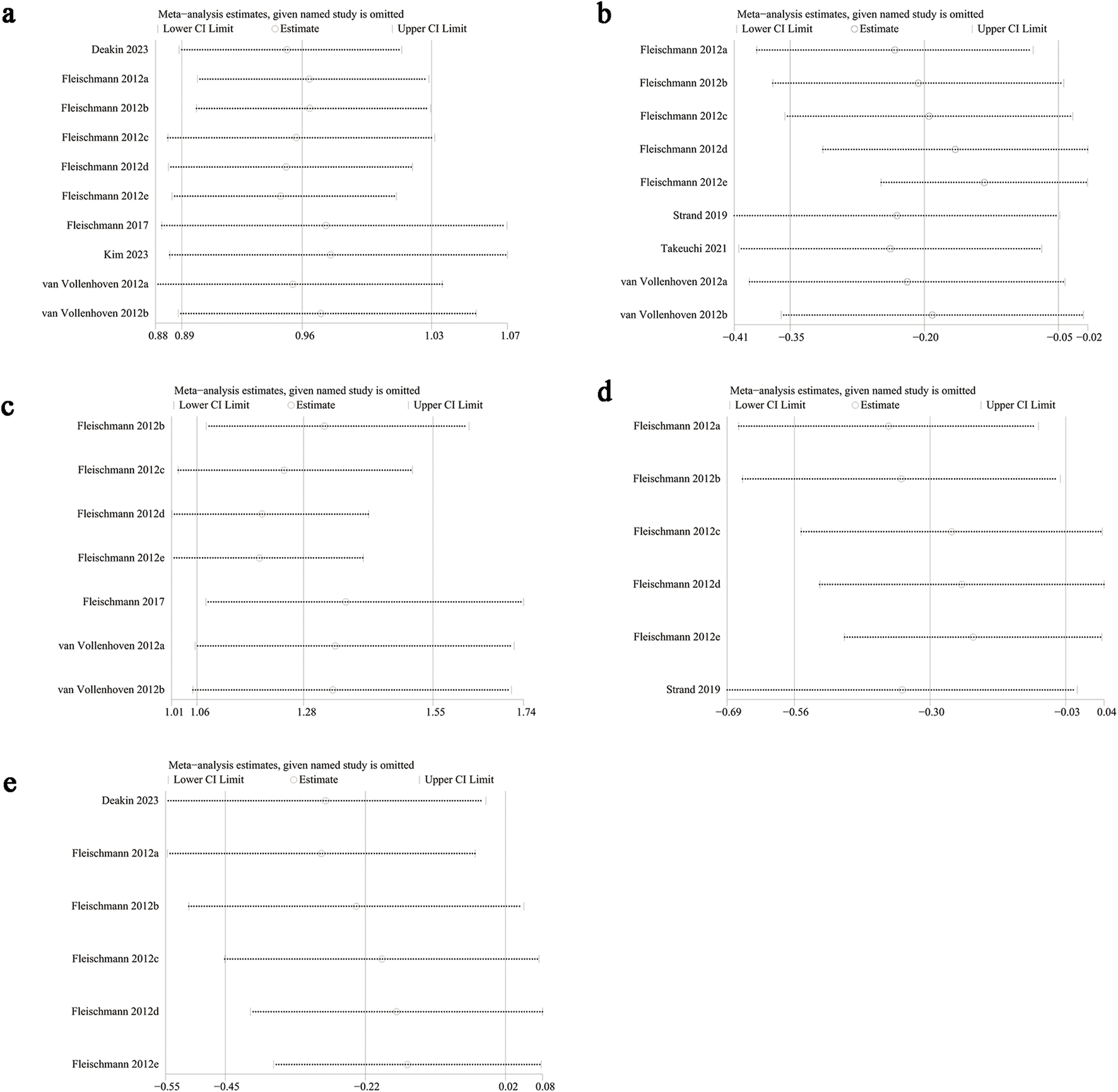
Sensitivity analysis of (a) adverse events, (b) change in HAQ-DI, (c) ACR20, (d) change in VAS, (e) change in DAS28-CRP.
4 Discussion
Current treatments for RA primarily consist of drug therapies, including NSAIDs, csDMARDs, tsDMARDs, bDMARDs, and glucocorticoids. TNF-α is a key inflammatory factor in RA pathogenesis, regulating osteoclast production and inhibiting osteoblast differentiation, which disrupts the balance between osteoclasts and osteoblasts, leading to bone and joint damage (Bertolini et al., 1986). Research indicates that patients with high TNF-α expression have a significantly higher risk of erosive arthritis (Laverdière et al., 2015). Adalimumab is the first fully humanized monoclonal antibody that specifically targets TNF-α. Tofacitinib, a first-generation oral drug, selectively inhibits JAK1 and JAK3, with minimal effect on JAK2 and TYK2 (Clark et al., 2014). Studies show that tofacitinib is effective as monotherapy or in combination with DMARDs for RA (Chang and Wang, 2024).
Nine studies were included in this meta-analysis. Results showed that tofacitinib significantly outperformed adalimumab in ACR20, HAQ-DI, and VAS improvements. No significant difference was observed in adverse events or DAS28-CRP improvements between the two groups. ACR20 was the primary efficacy indicator in this analysis. Van Vollenhoven et al. (van Vollenhoven et al., 2012) found that at month 6, similar proportions of patients on tofacitinib and adalimumab achieved ACR20, both exceeding placebo. However, this meta-analysis found significantly higher ACR20 in the tofacitinib group than in the adalimumab group. This discrepancy was largely due to the inclusion of Fleischmann et al.'s (Fleischmann et al., 2012) dose-response study. At doses of ≥5 mg (twice daily), tofacitinib’s efficacy was significantly superior to adalimumab 40 mg (once every 2 weeks). van Vollenhoven et al. (2012) also observed a trend suggesting tofacitinib’s superiority over adalimumab in ACR20, though this was not statistically significant. Thus, we believe that when the tofacitinib dose exceeds 5 mg (twice daily), its ACR20 is significantly higher than that of the conventional adalimumab dose. However, the long-term efficacy of both drugs in treating rheumatoid arthritis requires further investigation in future clinical studies with extended follow-up durations and additional time points.
Although ACR20 is a commonly used indicator in RA clinical trials, its sensitivity to functional improvement is limited. In addition, the ACR20 standard currently only applies to the US FDA regulatory system and has not been adopted by agencies such as MHRA and EMA. This study also included patient-reported outcomes such as HAQ-DI to more comprehensively evaluate the impact of treatment on quality of life. Joint pain, swelling, and functional limitation are key clinical manifestations of RA (Skyrme et al., 2024; Looijen et al., 2024; Frazzei et al., 2024). This study found tofacitinib significantly superior to adalimumab in improving pain scores. However, Strand et al. (2019) did not observe a significant advantage of tofacitinib in improving VAS, possibly due to sample size limitations. In assessing quality of life, this meta-analysis found tofacitinib significantly superior to adalimumab in improving HAQ-DI. However, Strand et al. (2016) reported similar HAQ-DI improvements in both treatment groups at all time points, differing from the results of this study. Differences in race, disease course, and body mass index may explain varying patient responses to treatment. Additionally, no significant difference was observed between tofacitinib and adalimumab in improving DAS28-CRP, suggesting similar effects in reducing RA activity and delaying bone destruction. Tofacitinib’s primary advantage lies in improving quality of life and symptom relief. The long-term prognostic differences between these two drugs in rheumatoid arthritis treatment require further study.
This study found comparable incidence rates of adverse events between the two groups in the safety evaluation. The safety of these two drugs remains controversial in current reports. While some studies align with this article (Fleischmann et al., 2017), van Vollenhoven et al. (2012) reported a higher probability of serious adverse events with tofacitinib than adalimumab within the first 3 months of treatment. Given the sample size limitations, further research is needed to confirm this finding.
This meta-analysis has several limitations. First, the study included a small number of retrospective cohort studies, inherent in clinical research. A major limitation of retrospective studies is the potential for confounding factors and bias. In addition, not all included studies were international multicenter studies. Some studies only included populations from a single country or region, resulting in a certain degree of selective bias. Besides, due to insufficient data, we were unable to analyze the differences between tofacitinib and adalimumab in important clinical indicators such as ACR50, which needs to be confirmed by further studies. In addition, merging different types of studies may produce “mixed effects” that are difficult to explain. For example, the effect size of RCTs is based on strictly controlled intervention conditions, while the effect size of cohort studies is easily interfered by real-world confounding factors (such as lifestyle and comorbidities). The combined results of the two may not be pure causal effects or true exposure associations. At the same time, the non-compressibility of OR values may cause the combined effect to deviate from the true value. Furthermore, if the patient stops treatment prematurely, the results at the last follow-up may not reflect the sustained treatment effect, which may affect the overall efficacy analysis of the drug to some extent. Finally, due to insufficient data, the economic costs of tofacitinib and adalimumab could not be analyzed, requiring further investigation. Due to the limited number of studies, detailed subgroup and dose-response analyses could not be performed, leaving the effects of factors such as population, intervention duration, follow-up time, and drug dose unconfirmed. Although significant heterogeneity was present in this meta-analysis, sensitivity analysis did not fully identify its source, warranting caution when interpreting the results. Despite these limitations, this is the latest meta-analysis comparing the efficacy and safety of tofacitinib and adalimumab. The findings provide clinicians with the most up-to-date, comprehensive evidence-based reference for selecting treatments, aiming to identify options with rapid onset, strong efficacy, safety, and affordability for RA patients.
5 Conclusion
This meta-analysis found tofacitinib significantly superior to adalimumab in improving ACR20, VAS, and HAQ-DI, with no significant difference in adverse event rates or DAS28-CRP improvement between the two. Given the limitations of ACR20, clinical decision making needs to comprehensively consider patient-reported outcomes, long-term safety, and individualized treatment goals. Given the potential heterogeneity, small sample size, and lack of subgroup analysis, larger multicenter prospective studies are needed to confirm the advantages and disadvantages of tofacitinib and adalimumab in RA treatment.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
CZ: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review and editing. YZ: Software, Supervision, Validation, Writing – original draft. ZW: Investigation, Resources, Visualization, Writing – original draft. GC: Data curation, Formal Analysis, Supervision, Writing – original draft. YL: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1524214/full#supplementary-material
References
1
Adami G. Orsolini G. Rossini M. Fratucello A. Fassio A. Viapiana O. et al (2024). Effects of tofacitinib on bone turnover markers and bone modulators in patients with rheumatoid arthritis. BMC Rheumatol.8 (1), 40. 10.1186/s41927-024-00414-6
2
Baker M. C. Liu Y. Lu R. Lin J. Melehani J. Robinson W. H. (2023). Incidence of interstitial lung disease in patients with rheumatoid arthritis treated with biologic and targeted synthetic disease-modifying antirheumatic drugs. JAMA Netw. open6 (3), e233640. 10.1001/jamanetworkopen.2023.3640
3
Bergman M. Chen N. Thielen R. Zueger P. (2022). One-year medication adherence and persistence in patients with rheumatoid arthritis in clinical practice: retrospective analysis of upadacitinib, adalimumab, baricitinib, and tofacitinib. J. Manag. Care Specialty Pharm.28 (10), S102–S103.
4
Bertolini D. R. Nedwin G. E. Bringman T. S. Smith D. D. Mundy G. R. (1986). Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature319 (6053), 516–518. 10.1038/319516a0
5
Boussaa H. Hamdi O. Miladi S. Makhlouf Y. Abdelghani K. B. Fazaa A. et al (2024). Tofacitinib-induced acute pancreatitis in a patient with rheumatoid arthritis: a case report and review of the literature. Curr. Drug Saf.20, 366–370. 10.2174/0115748863300565240819114551
6
Chang J. Wang G. (2024). A retrospective study of efficacy of tofacitinib combined with bDMARDs in the treatment of rheumatoid arthritis patients with inadequate response to bDMARDs. Int. J. rheumatic Dis.27 (9), e15311. 10.1111/1756-185X.15311
7
Clark J. D. Flanagan M. E. Telliez J.-B. (2014). Discovery and development of Janus Kinase (JAK) inhibitors for inflammatory diseases: miniperspective. J. Med. Chem.57 (12), 5023–5038. 10.1021/jm401490p
8
Conde-Aranda J. Scotece M. Varela-García M. Torrijos-Pulpón C. Arosa L. Camba-Gómez M. et al (2024). Lipocalin-2 serum levels in rheumatoid arthritis patients treated with adalimumab and its correlation with proinflammatory factors. Mediat. Inflamm.2024, 7264704. 10.1155/2024/7264704
9
Cumpston M. Li T. Page M. J. Chandler J. Welch V. A. Higgins J. P. et al (2019). Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane database Syst. Rev.10, Ed000142. 10.1002/14651858.ED000142
10
Deakin C. T. De Stavola B. L. Littlejohn G. Griffiths H. Ciciriello S. Youssef P. et al (2023). Comparative effectiveness of adalimumab vs tofacitinib in patients with rheumatoid arthritis in Australia. JAMA Netw. open6 (6), e2320851. 10.1001/jamanetworkopen.2023.20851
11
Fleischmann R. Cutolo M. Genovese M. C. Lee E. B. Kanik K. S. Sadis S. et al (2012). Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis rheumatism64 (3), 617–629. 10.1002/art.33383
12
Fleischmann R. Mysler E. Hall S. Kivitz A. J. Moots R. J. Luo Z. et al (2017). Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet London, Engl.390 (10093), 457–468. 10.1016/S0140-6736(17)31618-5
13
Fournier J. Boussat B. Dervaux B. Gaudin P. Romand X. (2024). Cost-utility of tofacitinib in the treatment of moderate-to-severe rheumatoid arthritis in France: a multi-state Markov model analysis. Clin. Exp. rheumatology43, 62–69. 10.55563/clinexprheumatol/3f60yv
14
Frazzei G. Cramer S. H. M. Landewé R. B. M. Maijer K. I. Gerlag D. M. Tak P. P. et al (2024). The effect of rituximab on patient reported outcomes in the preclinical phase of rheumatoid arthritis: 2 year data from the PRAIRI study. RMD open10 (4), e004622. 10.1136/rmdopen-2024-004622
15
Han P. Liu X. He J. Han L. Li J. (2024). Overview of mechanisms and novel therapies on rheumatoid arthritis from a cellular perspective. Front. Immunol.15, 1461756. 10.3389/fimmu.2024.1461756
16
Higgins J. P. Thompson S. G. (2002). Quantifying heterogeneity in a meta-analysis. Statistics Med.21 (11), 1539–1558. 10.1002/sim.1186
17
Kim S. K. Lee S. H. Sun J. Lee S. H. Jeon J. Y. Yoo H. J. et al (2023). Comparisons of treatment satisfaction and health-related quality of life in patients with rheumatoid arthritis treated with tofacitinib and adalimumab. Arthritis Res. Ther.25 (1), 68. 10.1186/s13075-023-03047-1
18
Kim S. R. Kim K. Lee S. A. Kwon S. O. Lee J. K. Keum N. et al (2019). Effect of red, processed, and white meat consumption on the risk of gastric cancer: an overall and dose-response meta-analysis. Nutrients11 (4), 826. 10.3390/nu11040826
19
Lakhmiri R. Cherrah Y. Serragui S. (2024). Tumor necrosis alpha (TNF-α) antagonists used in chronic inflammatory rheumatic diseases: risks and their minimization measures. Curr. Drug Saf.19 (4), 431–443. 10.2174/0115748863274863231222023853
20
Laverdière I. Guillemette C. Tamouza R. Loiseau P. de Latour R. P. Robin M. et al (2015). Cyclosporine and methotrexate-related pharmacogenomic predictors of acute graft-versus-host disease. haematologica100 (2), 275–283. 10.3324/haematol.2014.109884
21
Looijen A. E. M. Snoeck Henkemans S. V. J. van der Helm-van Mil A. H. M. Welsing P. M. J. Koc G. H. Luime J. J. et al (2024). Combining patient-reported outcome measures to screen for active disease in rheumatoid arthritis and psoriatic arthritis. RMD open10 (4), e004687. 10.1136/rmdopen-2024-004687
22
Page M. J. McKenzie J. E. Bossuyt P. M. Boutron I. Hoffmann T. C. Mulrow C. D. et al (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ Clin. Res. ed372, n71. 10.1136/bmj.n71
23
Qi P. Chen X. Tian J. Zhong K. Qi Z. Li M. et al (2024). The gut homeostasis-immune system axis: novel insights into rheumatoid arthritis pathogenesis and treatment. Front. Immunol.15, 1482214. 10.3389/fimmu.2024.1482214
24
Shih P. C. Hung P. C. Leong P. Y. Hsu J. N. Yang C. C. Wei J. C. C. et al (2024). Incidence and risk factors of discontinuation of tofacitinib and biologic disease-modifying anti-rheumatic drugs among patients with rheumatoid arthritis: a population-based cohort study. Clin. Rheumatol.43, 3625–3637. 10.1007/s10067-024-07161-6
25
Skyrme S. Dixon W. G. van der Veer S. N. Sanders C. Sharp C. A. Dowding D. (2024). The role of patient reported symptom data in co-producing meaning in rheumatoid arthritis. J. Eval. Clin. Pract.31, e14182. 10.1111/jep.14182
26
Smolen J. S. Taylor P. C. Tanaka Y. Takeuchi T. Hashimoto M. Cara C. et al (2024). Impact of high rheumatoid factor levels on treatment outcomes with certolizumab pegol and adalimumab in patients with rheumatoid arthritis. Rheumatology63, 3015–3024. 10.1093/rheumatology/keae435
27
Soriano E. R. Mysler E. Rios C. Xavier R. M. Cardiel M. H. Citera G. (2024). Rheumatoid arthritis in Latin America: pharmacotherapy and clinical challenges. Expert Opin. Pharmacother.25, 2023–2033. 10.1080/14656566.2024.2412247
28
Strand V. Mysler E. Moots R. J. Wallenstein G. V. DeMasi R. Gruben D. et al (2019). Patient-reported outcomes for tofacitinib with and without methotrexate, or adalimumab with methotrexate, in rheumatoid arthritis: a phase IIIB/IV trial. RMD open5 (2), e001040. 10.1136/rmdopen-2019-001040
29
Strand V. van Vollenhoven R. F. Lee E. B. Fleischmann R. Zwillich S. H. Gruben D. et al (2016). Tofacitinib or adalimumab versus placebo: patient-reported outcomes from a phase 3 study of active rheumatoid arthritis. Rheumatol. Oxf. Engl.55 (6), 1031–1041. 10.1093/rheumatology/kev442
30
Takeuchi T. Fleischmann R. Iikuni N. Shi H. Soma K. Paulissen J. et al (2021). Differences and similarities in clinical and functional responses among patients receiving tofacitinib monotherapy, tofacitinib plus methotrexate, and adalimumab plus methotrexate: a post hoc analysis of data from ORAL Strategy. Arthritis Res. & Ther.23 (1), 220. 10.1186/s13075-021-02591-y
31
van Vollenhoven R. F. Fleischmann R. Cohen S. Lee E. B. García Meijide J. A. Wagner S. et al (2012). Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. New Engl. J. Med.367 (6), 508–519. 10.1056/NEJMoa1112072
32
Vittecoq O. Brevet P. Gerard B. Lequerre T. (2024). On difficulties to define prognostic factors for clinical practice in rheumatoid arthritis. RMD open10 (3), e004472. 10.1136/rmdopen-2024-004472
33
Wells G. Shea B. O'Connell D. Peterson J. Welch V. Losos M. et al (2011). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
34
Zhang W. Xu Z. Shu Y. Shu S. Zhang Q. (2024). Adverse event profiles of adalimumab in children: a disproportionality analysis. Pharm. Basel, Switz.17 (8), 1028. 10.3390/ph17081028
35
Zhu Q. Zhou H. (2024). The role of cGAS-STING signaling in rheumatoid arthritis: from pathogenesis to therapeutic targets. Front. Immunol.15, 1466023. 10.3389/fimmu.2024.1466023
Summary
Keywords
tofacitinib, adalimumab, rheumatoid arthritis, meta-analysis, systematic review
Citation
Zhu C, Zheng Y, Wang Z, Chen G and Li Y (2025) Comparative effectiveness and safety of tofacitinib vs. adalimumab in patients with rheumatoid arthritis: A systematic review and meta-analysis. Front. Pharmacol. 16:1524214. doi: 10.3389/fphar.2025.1524214
Received
07 November 2024
Accepted
28 May 2025
Published
09 June 2025
Volume
16 - 2025
Edited by
Stefania Tacconelli, University of Studies G.d’Annunzio Chieti and Pescara, Italy
Reviewed by
Marc Henri De Longueville, UCB Pharma, Belgium
Moetaza M. Soliman, Mansoura University, Egypt
Caroline Tianeze de Castro, Federal University of Bahia, Brazil
Updates
Copyright
© 2025 Zhu, Zheng, Wang, Chen and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yushi Li, liyushi2304@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.