Abstract
Copper (Cu), an essential trace element for normal bodily functions, plays a pivotal role in cardiac muscle biology and is critical for cardiac function and metabolism. Recent research increasingly links Cu-related cell death (cuproptosis) to diseases like myocardial infarction (MI). Cu overload drives cuproptosis via mitochondrial dysfunction, lipoylated protein aggregation, and Fe-S cluster reduction, inducing proteotoxic stress and linking inflammatory/ROS pathways to MI progression. Therefore, it can be hypothesized that cuproptosis is a novel therapeutic target for MI. In this review, we explore the primary molecular mechanisms, treatment strategies and potential therapeutic targets involved in cuproptosis. Moreover, the insights obtained from this review provide a novel perspective on the pathogenesis of MI and new targets for its intervention.
1 Introduction
Myocardial infarction (MI), characterized by the high morbidity and mortality rates, remains a significant challenge in global healthcare, and continues to claim lives (Roth et al., 2020). The development of atherosclerotic plaques in the coronary vessel wall, which can lead to vessel stenosis or potential rupture, constitutes the primary mechanism behind MI through thrombotic vessel occlusion (Doenst et al., 2019). Importantly, myocardial cell death plays a crucial role in MI pathogenesis. The deceased cardiomyocytes can not be replaced by viable ones due to their limited regenerative capacity (Addis and Epstein, 2013). Intervention of myocardial cell death is vital for improving MI prognosis. Immediate coronary blood flow restoration, namely, the early success in reperfusion treatment, is the cornerstone for minimizing myocardial injury and enhancing MI patient outcomes (Xiang et al., 2024). Currently, electrocardiogram results and high-sensitivity troponin measurements are the preferred tests for MI evaluation and diagnosis. Nevertheless, they have inherent limitations in the detection of mild myocardial injury, early MI, and stable coronary artery disease (Bhatt et al., 2022). Therefore, identifying effective cardiac biomarkers and therapeutic targets for MI will significantly contribute to its early diagnosis and improved prognosis.
The cell death mode in MI has become an important point of research. While apoptosis and necrosis are the primary forms of cell death, cuproptosis, the novel programmed cell death form resulting from copper (Cu), was identified in 2022 (Kahlson and Dixon, 2022). Cu plays a vital role as a micronutrient in various physiological processes across nearly all cell types. However, the excessive intracellular Cu level may cause oxidative stress while disrupting cell functions, emphasizing the significance of maintaining Cu homeostasis (Zhong et al., 2022). Cuproptosis mainly occurs when intracellular Cu binds to lipoylated components in the tricarboxylic acid (TCA) cycle. The process results in Cu-bound lipoylated mitochondrial protein aggregation, therefore reducing iron–sulfur (Fe–S) clusters, inducing proteotoxic stress, and finally resulting in cell death (Tsvetkov et al., 2022). Recently, cuproptosis has attracted considerable attention for its involvement in the pathophysiology of tumors, Menkes disease, Wilson disease, neurodegenerative diseases, and other conditions (Chen et al., 2022). Furthermore, research on its role in cardiovascular disease is advancing steadily. Therefore, this review focuses on recent research progress concerning cuproptosis in MI and discusses its vital role in MI modulation.
2 The physiological functions of copper
Cu, an essential trace element essential for maintaining normal bodily functions, exists in organisms in two forms: Cu ions (Cu1+, reduced form) or Cu ions (Cu2+, oxidized form). Both forms are essential for numerous physiological responses in the human body (Chen et al., 2020). The dietary Cu dose recommended for adults is 0.9 mg/day, and the overall body Cu content is estimated to be approximately 100 mg (Bost et al., 2016).
The Cu level in cells is modulated through specific absorption systems, which can be balanced by ion-activated P-type ATPases associated with Cu elimination. The absorption and efflux transporters are also important for maintaining Cu homeostasis (Abeyrathna et al., 2020). The Cu content remains relatively stable in the body; the insufficient free Cu level can impair metal-binding enzyme function, while excess free Cu level can cause cellular damage and even death (Wang et al., 2023). To shield cells against free Cu-induced detrimental impacts, the sophisticated intracellular metallochaperone system has evolved. These proteins acquire Cu from donor proteins, facilitate its uptake, and transport it to specific cellular sites through direct interactions with target proteins. This mechanism ensures the acquisition of necessary Cu cofactors and mitigates adverse effects in association with elevated free Cu levels (Fukai et al., 2018).
In addition to its general physiological functions, Cu plays a pivotal role in cardiac muscle biology, significantly impacting cardiac function and metabolism (Mohammadifard et al., 2019). It catalyzes reactions in various physiological events influencing mitochondrial energy generation (Tian et al., 2023), neurotransmitter and tyrosine metabolism (Campolo et al., 2014), redox homeostasis (Méndez et al., 2024), and extracellular matrix remodeling (Liu et al., 2024). Studies have shown that Cu is not only a risk factor for MI but also serves as a specific biomarker for numerous biological processes. It acts as a cofactor for antioxidant enzymes, participates in glycosylation, influences cytochrome and mitochondrial activity, and modulates vascular responses to inflammatory stimuli. Disruption of Cu homeostasis, either through deficient or excess Cu level, can perturb these critical processes, potentially exacerbating disease progression (Lim et al., 2023). Cu is closely associated with MI, both as a causative factor and as a consequence.
3 Molecular mechanisms
3.1 Angiogenesis enhancement
Myocardial regeneration and angiogenesis are important for the MI pathogenic mechanism, serving as the fundamental mechanisms for cardiac function restoration post-infarction. In particular, angiogenesis occupies a central role in creating a rejuvenated microenvironment under ischemic conditions, which can therefore promote myocardial rejuvenation (Wu et al., 2021). Hypoxia-inducible factor 1 (HIF-1) is a primary transcription factor governing angiogenesis. It oversees oxygen delivery by controlling angiogenesis and vascular remodeling, and regulates oxygen utilization through glucose metabolism and redox homeostasis (Semenza, 2014). In prolonged MI, the activity of HIF-1 is chiefly modulated by HIF-1α, which serves as a crucial subunit of HIF-1. Cu has a regulatory role in numerous aspects of HIF-1, such as the stabilization of HIF-1α, the assembly of transcription complexes, and the binding to the hypoxic response element sequences present in the target genes (Chen et al., 2011). In addition, it also contributes to the selective regulation of HIF-1 binding to the target angiogenic genes, thus influencing Cu-dependent angiogenic factor expression (Martin et al., 2005; Xiao et al., 2020). Studies have shown that during myocardial ischemic injury, Cu depletion results in the deactivation of HIF-1-regulated angiogenesis, causing the upregulation of genes like vascular endothelial growth factor (VEGF) related to angiogenesis (Zhang et al., 2018; Sato and Takeda, 2023). Cu supplementation can enhance the HIF-1 transcriptional activity, restore angiogenic capacity, and increase capillary density in the heart. Dietary Cu supplementation has been found to replenish cardiac Cu, stimulate HIF-1 activity, upregulate VEGF expression, and accelerate angiogenesis, while reversing hypertrophic cardiomyopathy in mice (Jiang et al., 2007). Li et al. found a gradual decrease in heart Cu level over time in a MI mouse model, while serum Cu levels increased (Li et al., 2018). Furthermore, prolonged ischemia is correlated with the decreased Cu content in ischemic heart, significantly inhibiting angiogenesis (Kobayashi et al., 2017). Considering the crucial role of Cu in promoting angiogenesis, increasing Cu concentration in the heart can effectively reactivate angiogenesis in ischemic myocardium, which can provide a potential alternative therapeutic approach for MI.
3.2 Oxidative stress activation
In physiological situations, cells are under the oxidation-antioxidant defense balance. Oxidative stress is triggered upon the disruption of the balance, causing cell damage and the onset of different disorders. During both ischemia-reperfusion in acute MI and the subsequent chronic remodeling phase, oxidative stress significantly induces cardiac injury. Reactive oxygen species (ROS) produced in mitochondria exert a vital role in mechanisms contributing to ischemia-reperfusion injury, including induction of mitochondrial permeability transition and oxidative injury to intramitochondrial molecules and structures. Apart from acute settings, mechanisms such as extracellular remodeling, inflammatory signal transduction, and pro-apoptotic signaling facilitating post-infarction remodeling, can be modulated through mitochondrial ROS (Bugger and Pfeil, 2020). Normally, the lower ROS levels can be balanced by detoxification mechanisms, which are critical for cellular signaling, excitation-contraction coupling, gene expression regulation, cell proliferation, migration, apoptosis, and differentiation. This process, which is known as redox signaling, involves the special yet reversible oxidation/reduction modifications of signal transduction components in cells. Under pathological conditions, ROS may result in oxidative modifications of key cell macromolecules such as lipids, DNA and proteins, affecting subcellular organelles including the mitochondria, sarcolemma, nucleus, and sarcoplasmic reticulum (Dubois-Deruy et al., 2020). It has been reported in some studies that, excessive Cu may trigger an oxidative stress response (Yang et al., 2019). As a transition metal element, Cu participates in Fenton-like reactions, which are essential in a variety of diseases (Pi et al., 2023). Cu ions which are redox-active catalyze Fenton reactions, generating ROS (Zhou et al., 2022). These ions have convertible valences, serving as the active centers in Fenton-like reactions. Cu2+ catalyzes the generation of oxygen from the overexpressed H2O2 and combating hypoxia, while Cu1+ catalyzes H2O2 to produce hydroxyl radicals and other highly toxic ROS. Cu2+ reacts with glutathione (GSH) to form GSSH, which can produce against the generated ROS and induce cell apoptosis (Fu et al., 2021). Zhou et al. (2022) demonstrated that Cu exposure induces ROS-mediated NF-κB activation in microglia, which initially serves as a pro-survival signal by upregulating anti-oxidant genes. However, sustained Cu accumulation disrupts mitochondrial homeostasis via PINK1/Parkin inactivation, leading to mitophagy failure and NLRP3 inflammasome-dependent pyroptosis. Chen et al. (2021a) demonstrated that depletion of Cysteine-rich protein 2 (CRIP2) and Cu-induced degradation of CRIP2 increased ROS levels and induced autophagy in cancer cells. Mechanistically, Cu1+ promotes CRIP2 ubiquitination and proteasomal degradation, thereby relieving its suppressive effect on autophagy. This process is associated with increased ROS production, which triggers autophagic flux independent of the AMPKα-ULK1 pathway. Based on Zhu et al. (2023a), elevated Cu2+ induces neuronal and histopathological alterations in the mouse hypothalamus by generating excessive ROS, which triggers mitophagy and disrupts mitochondrial dynamics. The process is characterized by inhibited mitochondrial fusion (via downregulation of Mfn1/Mfn2) and enhanced fission (via upregulation of Drp1/FIS1), leading to mitochondrial swelling and cristae breakage. Furthermore, Cu is a vital element in Cytochrome c oxidase (CcO) and superoxide dismutase (SOD) 1, which are of great importance for mitochondrial energy metabolism and antioxidation capacity. Dietary Cu restriction leads to cardiac hypertrophy, eventually contributing to MI (Wen et al., 2022). These studies suggest that Cu ions damage cells and mitochondria by promoting ROS generation while promoting activation of oxidative stress-related pathways, exerting potential influence on the pathogenesis of MI.
3.3 Effect on myocardial fibrosis
In MI, ischemic cell death initiates the multiphase reparative response, in which the fibrotic scar predominantly formed by myofibroblasts and fibroblasts substitutes the injured tissue. This process can cause biochemical, geometrical, and biomechanical alterations in unaffected ventricular walls, triggering the reactive remodeling characterized by perivascular and interstitial fibrosis (Talman and Ruskoaho, 2016). After MI, cardiac fibroblasts experience dynamic phenotype transition regulating the inflammatory, angiogenic and reparative responses. This transition is initiated by danger-associated molecular patterns released from necrotic cells, such as HMGB1 and ATP, which bind to TLR4/6 on fibroblasts, activating the NF-κB pathway and upregulating pro-inflammatory cytokines (Prabhu and Frangogiannis, 2016). Necrotic cells can produce danger signals in an inflammatory stage during infarct healing, activating innate immune pathways and triggering the potent inflammatory response. Inflammatory signals promote leukocyte-endothelial cell adhesion, leading to the monocyte and neutrophil extravasation. Suppressing inflammatory response may be associated with the activation of reparative cells (Prabhu and Frangogiannis, 2016). Furthermore, after matrix debris and dead cells are cleared out of the infarct site, the transforming growth factor β (TGF-β) cascades and anti-inflammatory pathways are activated, causing fibroblast transformation into myofibroblasts expressing α-smooth muscle actin (α-SMA). After activation, myofibroblasts can produce abundant matrix proteins, contributing to forming the collagen-based scar for protecting infarcted ventricle against potential fatal events like cardiac rupture (Venugopal et al., 2022).
Actually, a study indicates that dietary Cu restriction induces mouse cardiac hypertrophy and failure, whereas Cu supplementation abolishes hypertrophy while preventing progression into heart failure. The restoration of normal cardiac function with Cu repletion may be caused by favorable downregulation of gene expression in myocardial tissue (Elsherif et al., 2004). By contrast, dietary Cu deficiency results in cardiac hypertrophy, fibrosis, and myofibril disarray. Wold et al. (2001) suggested that the impaired cardiac contractile function observed in Cu-deficient whole hearts might not stem from the depressed contractile function at the single-cell level but rather from cardiac fibrosis. Cu deficiency impairs lysyl oxidase activity, a Cu-dependent enzyme essential for collagen cross-linking, leading to disorganized collagen deposition and fibrotic heterogeneity. Another study shows that in the rodent models of cardiac hypertrophy, the Cu chelator Trientine can supplement Cu in the heart, thereby reducing cardiac fibrosis (Liu et al., 2018). Xiao et al. (Y et al., 2023) developed a MI model in rhesus monkeys through coronary artery ligation and employed the ultrasound-guided Cu albumin microbubble technology for targeted Cu delivery to ischemic myocardial tissue. This approach could significantly increase Cu concentrations in infarcted areas, facilitate the relaxation of the collagen cross-linking network, restore vascular density, and enhance cardiac contractility. These findings suggest that Cu inhibits fibroblast differentiation into myofibroblasts, promoting a profibrinolytic environment and therefore improving cardiac function. In conclusion, based on the aforementioned evidence, variations in Cu levels can influence the extent of cardiac fibrosis, potentially serving as a mechanism for ameliorating fibrotic heart disease.
3.4 Intervention in mitochondrial energy metabolism
Micronutrients like Cu make vital impacts on mitochondrial function, particularly in mitochondrial-rich tissues like cardiac muscles (Chen et al., 2011). COX11 and SCO1, key chaperones for CcO assembly, deliver Cu to the Cu(B) site of COX1 and Cu(A) site of COX2 subunits in the mitochondrial inner membrane, respectively, to facilitate CcO maturation (Horng et al., 2004). Mitochondria are the primary sites associated with energy generation, which are important for modulating different cell death types resulted from metal metabolism, immunotherapy, radiotherapy, and targeted antitumor therapies (Tian et al., 2023). Cu accumulates in the mitochondrial matrix to support the cuproenzyme maturation, like SOD and CcO. The transfer of Cu in the matrix can be facilitated through proteins belonging to the mitochondrial carrier family. Regulatory functions of Cu and resident cuproproteins in mitochondria are increasingly recognized to extend beyond the organelle itself. Mitochondrial Cu chaperones are implicated in modulating cellular Cu uptake and export, as well as promoting inter-organ communication (Cobine et al., 2021; Garza et al., 2023). Isei et al. (2021) found that hypoxic conditions diminished mitochondrial sensitivity to Cu, while increased Cu levels further stimulated the release of H2O2 from mitochondria during the oxidative metabolism of palmitoylcarnitine. In rats, a Cu deficient diet caused a 74% reduction in complex IV (Zuo et al., 2013). Zhang et al. (2020) demonstrated that Cu deficiency in diabetic hearts impairs myocardial function by down-regulating PGC-1α, a key regulator of mitochondrial biogenesis, and this defect is reversed by Cu chelation therapy. Specifically, Cu deficiency reduces the expression of mitochondrial Cu chaperones Cox17 and Cox11, leading to decreased CcO activity and mitochondrial ROS accumulation, which in turn suppresses PGC-1α expression and impairs mitochondrial biogenesis. Concurrently, changes of mitochondrial cristae and membranes disrupt the energy metabolism and contribute to myocardial damage (Chen et al., 2011; Halling and Pilegaard, 2020).
3.5 Regulation of lipid metabolism
Lipids and Cu are related to the pathogenic mechanisms of dyslipidemia-related disorders including obesity, neurological disorders, non-alcoholic fatty liver disease, and Wilson disease (the inherited disease characterized by Cu overload) (Muchenditsi et al., 2017; Lu et al., 2022; Qiu et al., 2023; Övermöhle et al., 2023). It is essential to comprehend the impact of Cu with lipid metabolism, aiming to identify potential therapeutic targets in conditions where Cu/lipid metabolism are disrupted. The same links may also exist in cardiovascular diseases with impaired Cu and lipid transport mechanisms. Genomic variances, especially in pathways involving coagulation and lipid metabolism, have been found in MI patients. Further knowledge of these risk factors, anatomical considerations, and pathophysiological processes can improve strategies for the prevention and treatment of MI in this patient population (Sagris et al., 2022).
Ceruloplasmin (Cp) is a predominant plasma protein containing 7 Cu atoms in each molecule, constituting 95% of circulating Cu among normal adult subjects (Fox et al., 2000). Its physiological roles include Cu transport, coagulation regulation, angiogenesis, defense against oxidative stress, and iron homeostasis (White et al., 2012; Linder, 2016). The increased serum Cp levels have been consistently observed in cardiovascular disorders including arteriosclerosis (Grammer et al., 2014), MI (Tang et al., 2012), and heart failure (Savic-Radojevic et al., 2017). Biochemical studies have revealed that Cp catalyzes low-density lipoprotein (LDL) oxidation in vitro, and the optimal activity is achieved in the presence of superoxide, which reduces the surface Cu atom of Cp (Fox et al., 2000). In addition, Wells et al. (2014) reported the positive correlations between Cu levels and triglycerides, whereas the negative correlations between Cu levels and high-density lipoprotein cholesterol levels. Blades et al. (2021) further clarified the tissue-specific roles of the Cu-regulating protein ATP7B, providing insights into the complex relationship between Cu and lipid metabolism. In summary, changes in Cu levels can indirectly influence lipid metabolism in the human body, potentially impacting the cardiovascular disease risk (Figure 1).
FIGURE 1
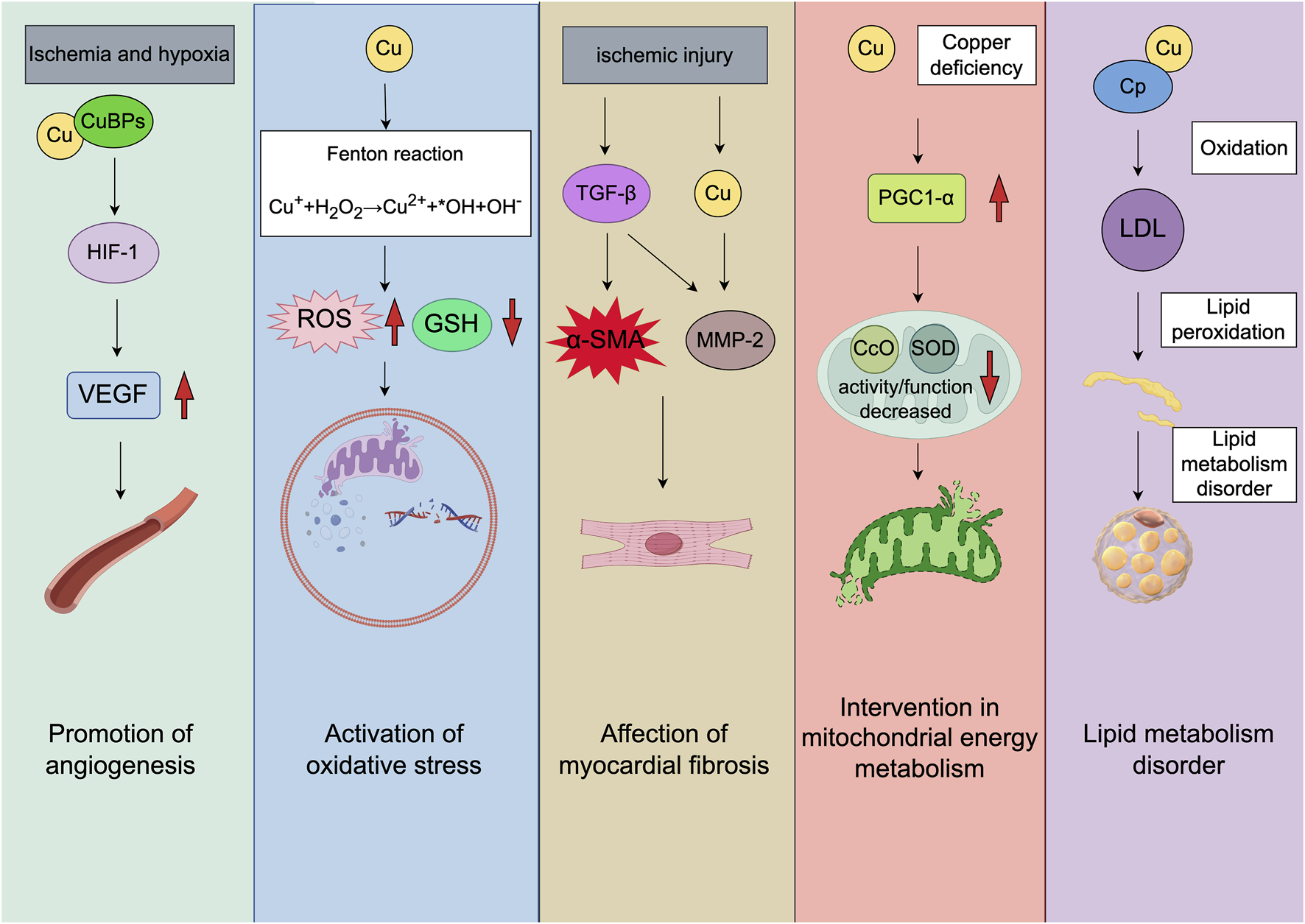
The molecular mechanisms of MI associated cuproptosis. The direction of the red arrow corresponds with increased or decreased molecules, respectively. The figure was created with Figdraw (https://www.figdraw.com/). CuBPs: copper-binding proteins; TGF-β: transforming growth factor-β; α-SMA: α-smooth muscle actin; MMP-2: matrix metalloproteinase 2; PGC1-α: peroxisome proliferator-activated receptor gamma coactivator 1-alpha; CcO: cytochrome c oxidase; SOD: superoxide dismutase.
4 Clinical treatment strategies that target Cu-triggered cell death during MI
Micronutrients and trace elements are vital for the normal functioning of the body. Despite small amounts are required, alterations in their levels may result in serious diseases including MI and its consequences. In fact, one study (Begum et al., 2023a) demonstrates that the assessment of serum Cu levels in MI patients may be beneficial for taking appropriate measures to prevent free radical-induced reperfusion injury. Serum Cu shows potential significance for the prognosis and diagnosis of MI (Wen et al., 2022), with close associations being found in the alterations of serial serum Cu contents with markers including creatine phosphokinase and lactate dehydrogenase (Singh et al., 1985). Currently, researchers in the field of cardiovascular disease are investigating the correlation of Cu ion homeostasis with cardiovascular disease treatment. A summary of clinical studies on Cu and MI is provided in Table 1. Feng et al. (Feng et al., 2023) reported that Cu ions, combined with mild heat, promoted angiogenesis and significantly improved cardiac function while inhibiting ventricular remodeling. Natural antidotes can regulate Cu homeostasis and mitigate metal toxicity. Curcuminoids and their synthetic derivatives possess different medicinal properties, such as heavy metal chelation, antioxidation and anti-inflammation activities, cytotoxicity to cancer cells, and roles in hypertension management and apoptosis regulation. These properties highlight curcumin as a potential treatment for neurodegenerative diseases, cardiovascular diseases, malignancies, and conditions associated with metal overload (Maghool et al., 2023). Fang et al. (2024a) indicated that paeoniflorin (PF) significantly reduced the expression of Ferredoxin 1 and serum Cu levels, and increased the pyruvate levels. Moreover, PF can prevent from left ventricular remodeling following MI through mitigating myocardial cell apoptosis, inflammation, and fibrosis.
TABLE 1
| Author, year | MI cases | Research methodology | Result |
|---|---|---|---|
| Afridi et al. (2006) | 193 | Flame/graphite furnace atomic absorption spectrophotometer | The Zn/Cu ratios in the scalp hair (p < 0.01) of the MI group were significantly lower than those of the healthy groups |
| Guo et al. (2002) | 34 | Plasma spectrometer detection | Cu content increased |
| Kazi et al. (2008) | 130 | Flame atomic absorption spectrophotometry | In these subjects, the concentration of Cu was increased by 3.12% in the scalp hair and 22.5% in blood samples, respectively, when compared to those who survived a third MI attack |
| Khan et al. (1984) | 27 | Atomic absorption spectrometry | In AMI, the concentration of Cu was higher after 21–30 h (as compared with the values at 0–10 h) |
| Luo et al. (2002) | 135 | Plasma spectrometer detection | Cu content increased |
| Awadallah et al. (2006) | 56 | Turbidimetry and atomic absorption spectrophotometry | Serum concentrations of Cu were significantly higher in the MI group of patients than in the controls |
| Martín-Lagos et al. (1997) | 12 | Flame atomic absorption spectrophotometry for metal analysis in biological samples | The mean serum Cu level showed no statistical difference between MI patients (p > 0.05) and the healthy group. Male patients had no statistically different serum Cu values (p > 0.05) than female patients, and patient age had no statistical influence (p > 0.05) on serum Cu |
| Singh et al. (1985) | 44 | Sodium diethyldithiocarbamate method | A highly significant increase in serum Cu levels was observed in patients with AMI compared to those with angina and controls. Mean peak serum Cu levels were significantly higher (p < 0.001) in complicated cases of AMI than in uncomplicated cases |
| Taneja et al. (2000) | 30 | An atomic absorption spectrophotometer | Urine Cu levels in patients were approximately twofold lower; Cu in the urine of MI descendants was higher than that in patients (p < 0.001), but Cu in hair was lower in MI descendants compared to control counterparts (p < 0.001) |
| Versieck et al. (1975) | 16 | Neutron activation analysis | A statistically significant increase in serum Cu was observed after MI. |
| Khaki-Khatibi et al. (2018) | 80 | Atomic absorption spectrophotometry | Cu concentrations were higher in patients than in the control group (p < 0.001), indicating a positive diagnostic value for the disease |
| Tan et al. (1992) | 138 | Flame atomic absorption spectrophotometry (Varian Spectra AA-20) | The differences in serum Cu and Zn levels between patients and controls were magnified when the Cu/Zn ratios were calculated for both groups (p < 001) |
| Zama and Towns (1986) | 71 | X-ray fluorescence spectrometry and atomic absorption spectrometry | Significant differences (p < 0.001) in elemental levels were observed between the noninfarct and recent infarct groups, with the noninfarct group having higher cardiac levels of all three elements. Cardiac levels of Zn (p < 0.001) and Cn (p < 0.01) were significantly greater in the old-infarct group than in the recent-infarct group. Magnesium levels were higher in the recent-and-old-infarct group than in the recent infarct group (p < 0.01), suggesting elemental redistribution during MI to maintain myocardial integrity and function |
| Jain and Mohan (1991) | 30 | Colorimetric method of Ventura and King | Serum Cu levels increased from the first 24 h up to the 7th day after infarction, with a gradual decline that did not return to normal by the 14th day |
| Białkowska et al. (1987) | 29 | Neutron activation analysis for Zn and Cu concentrations | The Zn/Cu ratio in survivors of MI was significantly higher than in controls |
| Bakos et al. (1988) | 30 | Atomic absorption spectrometry | The mean values of serum Cu were lower in patients with AMI than in noncardiac patients |
| Shokrzadeh et al. (2009) | 30 | Atomic absorption spectrophotometry | The mean Cu level in the ISCMP group was significantly higher than that in healthy volunteers (p = 0.048) |
| Nowicki et al. (2021) | 74 | Inductively coupled plasma mass spectrometry (ICP-MS) | Higher concentrations of Cu, Zn, Mn, Co, and Fe were significantly associated with an increased risk of MI. |
| Begum et al. (2023b) | 60 | Serum Cu ion level detection | The mean serum Cu level was higher in the case group than in the control group: 105.44 ± 24.15 μg/dL vs 146.49 ± 23.52 μg/dL (p < 0.05) |
| Yesmin et al. (2016a) | 60 | Colorimetric method for serum Cu determination | The mean serum Cu level was significantly increased in AMI patients compared to the control group (p < 0.01) |
Clinical studies on Cu homeostasis and myocardial infarction.
4.1 Copper chelators
Chelation therapy is the option of treatment for Cu overload or intoxication. Various chelating agents are currently in clinical use, under investigation, or enter clinical trials. Obviously, chelation therapy has also been proposed as a treatment for certain neurodegenerative diseases and cardiovascular disorders (Tegoni et al., 2014). Tetrathiomolybdate (TM) is an orally active agent which can be used for disorders of Cu metabolism. TM works by chelating bioavailable Cu to form a tripartite TM-Cu-protein complex (Alvarez et al., 2010). Wei et al. (Wei et al., 2012) demonstrated that TM suppressed mouse atherosclerosis by decreasing vascular inflammation and bioavailable Cu without influencing iron homeostasis or oxidative stress. In addition, due to its Cu-dependent mechanism, TM may exert influence on angiogenesis (Brewer, 2005). Li et al. reported that TM exhibited significant anti-inflammatory properties by inhibiting Cu-dependent cytokines involved in inflammation. This anti-inflammatory effect may also contribute to the anticancer properties of TM, as cancer progression usually involves inflammatory cells and excessive angiogenic agents (Brewer, 2014). Other Cu complexes, including trientine, the Cu-aspirinate complex, and Cu (II) diethyldithiocarbamate, have shown potential in preventing and treating cardiovascular disorders (Zhu et al., 2023b). Based on clinical research, Cu2+-selective chelation with trientine is safe and well-tolerated, showing promise as a future therapeutic target for hypertrophic cardiomyopathy. The treatment shows efficacy in reducing left ventricular mass and myocardial fibrosis, while its high selectivity for Cu2+ avoids affecting serum zinc or iron levels, minimizing off-target toxicity. The ongoing TEMPEST trial will further validate its long-term safety and efficacy (Reid et al., 2022).
As the broad-spectrum metal-chelating agent, ethylenediaminetetraacetic acid disodium salt (EDTA) is explored due to its efficacy in treating MI (Ga et al., 2013). For example, according to one double-blinded, placebo-controlled trial (Escolar et al., 2020), disodium EDTA chelation therapy decreases the possibility of unfavorable cardiovascular outcomes among stable MI patients. However, concerns remain regarding its non-selective metal-binding profile, which may pose risks such as electrolyte imbalance or trace element depletion (Fulgenzi et al., 2020). While these findings warrant further exploration, the current evidence is insufficient to support routine clinical use of EDTA chelation for MI. Given the significant economic burden of cardiovascular diseases, definitive validation of this treatment’s safety and efficacy through large-scale randomized trials is essential before widespread recommendation.
4.2 Small-molecule inhibitors of copper chaperone proteins
ATOX1 is the significant Cu chaperone protein for mammalian cells. It can enhance genotoxic drug resistance by activating the DNA damage repair mechanisms (Jin et al., 2022). A growing body of evidence has also indicated that ATOX1 is vital for modulating cell migration, growth, autophagy, and apoptosis, and for organism development and reproduction (Yang et al., 2023a). Sudhahar et al. (2022) identified novel downstream nuclear ATOX1 targets related to ROS generation and inflammation, indicating that nuclear ATOX1 might be the candidate target used for treating inflammatory disorders like atherosclerosis.
Numerous studies are carried out on nanomedicine, which have led to the emergence of “nanocatalytic therapy,” in which catalytic responses regulated via nanomaterials can be used for the intervention with disease-related biomolecular processes (Kim et al., 2024). Exogenous nanomaterials often undergo rapid biotransformation once injected, which can impair their intended function. Notably, Cu-deposited ceria nanoparticles (CuCe NPs) have been reported to show promoted antioxidation efficacy when compared with pristine ceria nanoparticles. This is because that the released Cu buffers glutathione depletion and serves as the cofactor of SOD1. In MI models, CuCe NPs have exhibited therapeutic effects by improving perfusion and reducing tissue damage (Im et al., 2023). While CuCe NPs show promising therapeutic effects in preclinical ischemic models, clinical translation requires validation through large-scale trials. Further studies are needed to evaluate their long-term safety and define optimal delivery strategies for targeted accumulation in ischemic tissues.
Applying Cu ion chelators can reduce Cu ion contents, potentially causing severe toxicities that break essential physiological processes requiring Cu. The compound DCAC50 has been reported to specifically inhibit tumor cell growth exerting no influence on healthy cells by limiting intracellular Cu ion delivery while binding to Cu chaperone proteins CCS and ATOX1 (Karginova et al., 2019). Cu chelators show non-specific chelation with additional metal cations, causing subsequent adverse reactions. Based on studies on the mechanism underlying DCAC50, it suppresses Cu SOD1 activity, which depends on the cofactor Cu ions, through the interference with Cu ion transport, increase in ROS contents, influence on mitochondrial function, and the reduction of ATP generation (Inkol et al., 2020). Moreover, these findings can inform the development of anti-MI novel drugs.
4.3 Copper ionophore
Cu ionophores, also known as Cu transport-related drugs, enhance the bioavailability of Cu in cells. For instance, the Cu ionophore elesclomol selectively transports Cu2+ from the extracellular environment into mitochondria, where it is reduced to Cu1+, therefore generating ROS (Ge et al., 2022; Zheng et al., 2022). While Cu ionophores can address Cu deficiency by delivering Cu to small molecules within cells, they also pose a risk of increasing intracellular Cu levels, which may result in cell death.
Elesclomol, a well-known Cu ionophore with selectivity of tumor cells, is evaluated by clinical studies for cancer therapy (Zheng et al., 2022). Nevertheless, the precise mechanism underlying the selectivity of elesclomol is unclear, and thus further research is warranted to determine whether this selectivity can be leveraged to develop other Cu ionophores for the treatment of MI. Li et al. (Zulkifli et al., 2023) demonstrated that elesclomol alleviated Cu deficiency in the yeast, mouse and zebrafish models through Cu delivery to mitochondria and the restoration of CcO function. Despite the obtained findings, the exact mechanism of elesclomol in regulating Cu delivery in cells is still largely unclear.
Conventional Cu carriers face limitations concerning versatility and targeted delivery. Ineffective regulation of Cu transport can result in excessive Cu supplementation and Fenton-like reactions, leading to oxidative damage (Yuan et al., 2017). Due to accumulation and subsequent oxidative stress, non-specific Cu delivery may cause tissue damage. To address these issues, Su et al. (2019) proposed the targeted ion carrier–based metal supplements (TIMS) concept, an approach for site-specific metal delivery within organisms. Considering these considerations, Cu ionophores can provide the promising treatment for target Cu-mediated cell death during MI.
In clinical studies, serum Cu contents significantly increase among MI patients in relative to healthy individuals (Yesmin et al., 2016b; Begum et al., 2023a). Substantial clinical evidence has suggested that Cu can be a valuable target for predicting, treating, and assessing the prognosis of cardiovascular diseases (Wang et al., 2023; Yang L. et al., 2023). Propensity score-matched analyses indicate that the higher dietary Cu intake is associated with a reduced risk of MI, especially among elderly women, overweight individuals, smokers, and those with hypertension or diabetes (Wen et al., 2022). He and James Kang, (2013) found that myocardial ischemia usually resulted in the decreased Cu content in the heart, and Cu supplementation could enhance HIF-1 transcriptional activity, therefore restoring the angiogenic capacity and increasing capillary density in the heart. This finding has implications for the development of the association between elevated serum Cu levels and MI, though subgroup analysis indicated considerable effect modification by ethnicity. Despite the correlation between Cu and MI, further mechanistic research is required to demonstrate these findings.
5 Cuproptosis-related genes
Cuproptosis is the novel cell death type dependent on mitochondrial respiration. Nevertheless, research concerning the impact of cuproptosis-related genes on MI is limited. Recently, researchers have systematically evaluated the genetic alterations in MI based on bioinformatics approaches. This study reviews cuproptosis genes and targets potentially associated with MI, aiming to provide insights for the improved diagnosis and treatment of the disease (Figure 2).
FIGURE 2
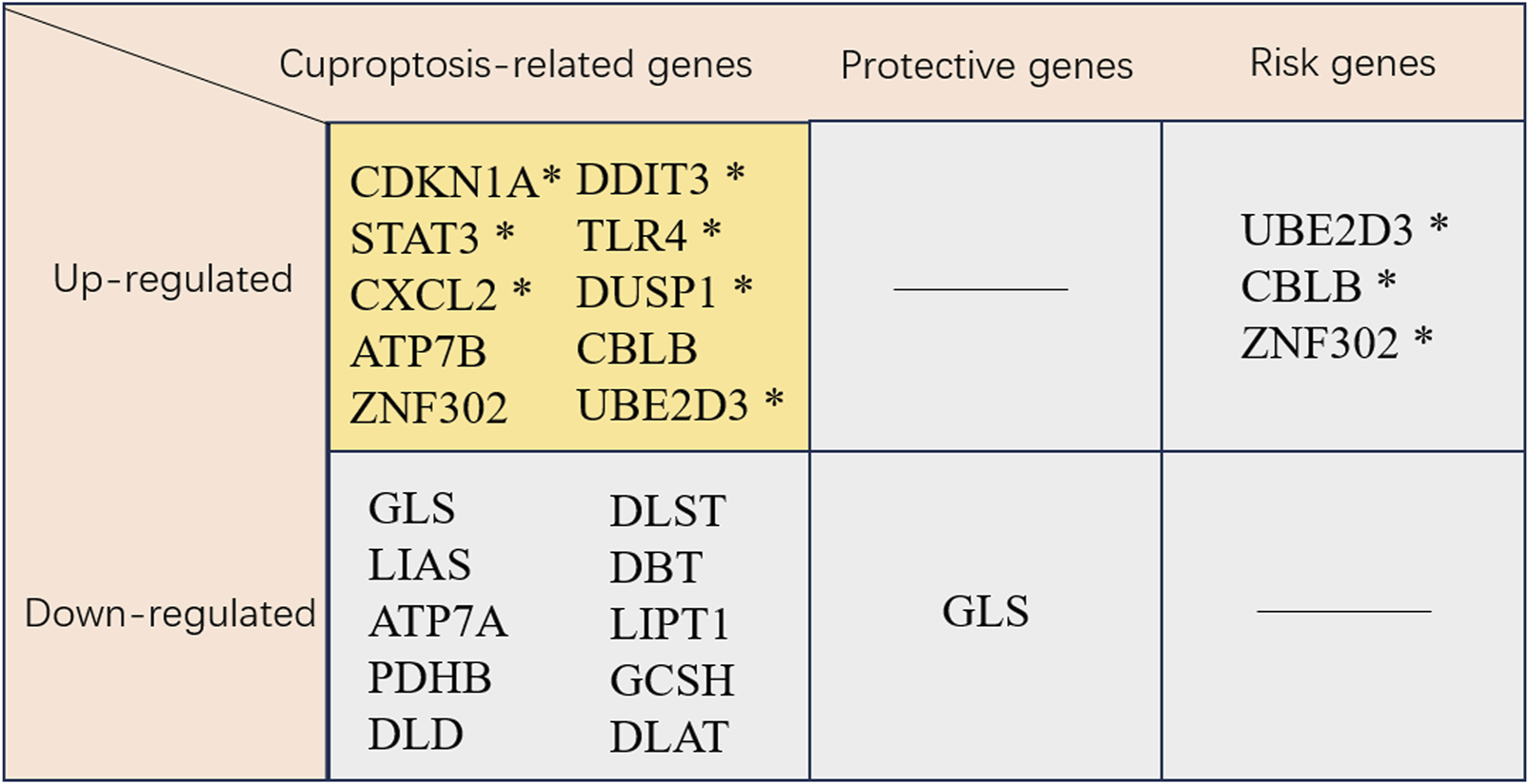
Cuproptosis-related genes. * This gene has been validated. CDKN1A, cyclin-dependent kinase inhibitor 1A; DDIT3, DNA damage inducible transcript 3; STAT3, signal transducer and activator of transcription 3; TLR4, toll like receptor 4; CXCL2, C-X-C motif chemokine ligand 2; DUSP1, dual specificity phosphatase 1; ATP7B, ATPase copper transporter 7B; CBLB, Cbl proto-oncogene B; ZNF302, zinc finger protein 302; Ube2d3, ubiquitin-conjugating enzyme E2D 3; GLS, glutaminase; DLST, dihydrolipoamide S-succinyltransferase; LIAS, lipoic acid synthetase; DBT, dihydrobiopterin; ATP7A, ATPase copper transporter 7A; LIPT1, lipoyltransferase 1; PDHB, pyruvate dehydrogenase E1 subunit beta; GCSH, glycine cleavage system protein H; DLD, dihydrolipoamide dehydrogenase; DLAT, dihydrolipoamide S-acetyltransferase.
Liu et al. (2022) indicated that the cuproptosis-related gene GLS was likely associated with MI development through the HIF-1 pathway. Notably, GLS was found to be correlated with immune-related pathways, including the chemokine and T-cell receptor pathways. In addition, GLS participated in hypoxia-related pathways, including the HIF-1 pathway, consistent with KEGG analysis. This dual involvement in immune and hypoxic signaling positions GLS as a promising biomarker for MI, with its expression potentially reflecting both ischemic stress and inflammatory burden. As a therapeutic target, modulating GLS activity may offer a dual strategy to alleviate myocardial hypoxia and regulate immune cell infiltration, particularly given its negative correlation with monocyte abundance in acute coronary syndrome (Liu et al., 2022).
Using bioinformatics analysis, Miao et al. (2023) identified six immune-related genes (CXCL2, DDIT3, DUSP1, CDKN1A, TLR4, and STAT3) associated with cuproptosis and ferroptosis as the candidate early diagnostic biomarkers for MI. Based on ROC curve analysis, they were of prominent prediction significance. Peripheral blood samples in MI patients and acute myocardial infarction (AMI) mouse model verified these six genes as candidate biomarkers, highlighting their diagnostic utility. These genes may also serve as therapeutic targets, with curcumin and N-acetyl-L-cysteine identified as potential modulators that could intervene in cuproptosis, ferroptosis, and immune infiltration pathways to improve MI outcomes (Miao et al., 2023). Yang et al. (2024) identified UBE2D3 as a key gene in MI development through promoting cuproptosis, leading to cardiomyocyte death. As an E2 family member, UBE2D3 was highly expressed in MI model animals and OGD-treated cardiomyocytes, with its expression correlated with ischemic injury. In myocardial ischemia/reperfusion injury, UBE2D3 enhanced p62 ubiquitination to exacerbate autophagic flux impairment (Wang et al., 2021). Preclinical studies showed that UBE2D3 knockdown improved cell viability and decreased LDH release in OGD models. Notably, its association with neutrophil infiltration highlighted UBE2D3 as a dual regulator of inflammation and cell death, positioning it as a promising therapeutic target for MI intervention (Wang et al., 2021). According to the MI-cuproptosis differentially expressed genes risk model and drug prediction in the Coremine Medical database, Zhang et al. (Fang C. et al., 2024) demonstrated the downregulation of nine cuproptosis-related genes (DLST, LIAS, DBT, ATP7A, LIPT1, PDHB, GCSH, DLD, and DLAT), whereas the upregulation of one gene (ATP7B) in MI patients. These ten cuproptosis-related genes demonstrated significant associations with immune cell infiltration. Recently, the application of machine learning models to predict MI prevalence based on demographic and imaging data has gained prominence. For instance, Wang et al. (2024) identified two key cuproptosis-related genes, ZNF302 and CBLB, through these models, which are vital for MI diagnosis and treatment. A GWAS analysis of congenital heart disease revealed ZNF302 as the significant differential gene (Jiang et al., 2018), suggesting its key role in cardiac development. Furthermore, a study highlighted the potential of differentially expressed genes, metabolites, and microbiota in MI regulation following CBLB intervention, demonstrating its vital role in MI regulation (You et al., 2024). Wang et al. (2024) also found that MI mice exhibited significant MI and impaired cardiac function, with an obvious increase in CBLB and ZNF302 expression in the myocardium, underscoring the importance of these genes in MI diagnosis and treatment. Zhou et al. (2024b) used nine mainstream machine learning algorithms and stacking methods to develop robust AI models for MI diagnosis. Their findings suggested that SLC31A1 might be a promising emerging diagnostic biomarker for MI. They confirmed the vital role of SLC31A1 expression in the MI immune milieu and its potential to enhance cardiac tissue recovery post-MI using animal models, therefore emphasizing its prospective utility as a diagnostic biomarker. Evidence has indicated that CTR1 (also known as SLC31A1) is the redox sensor for promoting angiogenesis in endothelial cells. Mechanistically, CTR1 is under rapid sulfenylation at Cys189 at the cytosolic C terminal following VEGF stimulation, inducing the generation of the CTR1-vascular endothelial growth factor receptor type 2 (VEGFR2) disulfide bond and the co-internalization into endosomes, thus triggering the persistent VEGFR2 signal transduction and enhancing angiogenesis (A et al., 2022).
6 Conclusion and perspectives
Despite significant advancements in modern medicine, MI is a major challenge in both medical care and public health. Obviously, there is a concerning trend towards younger age groups among patients with MI (Arora et al., 2019). Moreover, readmission rates, mortality, and hospitalization burdens for MI patients have not decreased (Chen P. et al., 2021; Tisminetzky et al., 2021; Alves et al., 2022). Recent research has increasingly performed to explore Cu-related cell death mechanisms in cancers, cardiovascular diseases, and additional conditions. It is urgently needed to identify novel targets and strategies for the treatment and prevention of MI. Based on the studies on MI, the high serum Cu level is significantly associated with MI. Cu overload influences mitochondrial function and exacerbates the development of MI (Muñoz-Bravo et al., 2023). Studies have reported that Cu ions contribute to aberrant lipoylated protein aggregation, leading to the downregulated Fe-S cluster protein level, which induces proteotoxic stress, finally causing cell death. In addition, Cu affects cell death through mechanisms involving ROS and inflammatory responses. Furthermore, the role of Cu-induced cell death in linking oxidative stress with inflammation underscores its significance in MI pathogenic mechanism. Therefore, it can be hypothesized that cuproptosis is a novel therapeutic target for MI. Dysregulation of Cu metabolism can alter cardiac gene expression, and Cu supplementation has been found to mitigate certain unfavorable cardiac outcomes caused by Cu deficiency. Given the association of Cu homeostasis with various disease states, further research plays an essential role in clarifying how Cu imbalance leads to cellular damage. In summary, targeting cuproptosis may provide the promising method to the diagnosis, treatment, prevention and assessment of MI.
Statements
Author contributions
ZS: Investigation, Visualization, Funding acquisition, Writing – original draft, Writing – review and editing. ZL: Visualization, Writing – original draft, Writing – review and editing. SC: Visualization, Writing – review and editing. HF: Formal Analysis, Writing – original draft. YG: Formal Analysis, Writing – original draft. XL: Project administration, Writing – original draft. XW: Project administration, Writing – original draft. CL: Conceptualization, Writing – original draft. WM: Conceptualization, Writing – original draft. JC: Supervision, Writing – review and editing. NL: Visualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study received funding from the National Natural Science Foundation of China (No. 82405146), the Shandong Province Medical and Health Technology Project (No. 202303011361), the Shandong Province Traditional Chinese Medicine Technology Project (No. Q-2023006) and the Weifang Science and Technology Development Plan Project (No. 2021GX058 and 2024YX035).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer FX declared a shared affiliation with the authors HF, YG, XL and NL to the handling editor at the time of review.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- AMI
acute myocardial infarction
- ATOX1
Antioxidant 1
- ATP7A
ATPase copper transporter 7A
- ATP7B
ATPase copper transporter 7B
- CBLB
Cbl proto-oncogene B
- CcO
Cytochrome c oxidase
- CDKN1A
Cyclin-dependent kinase inhibitor 1A
- Cp
Ceruloplasmin
- CRIP2
Cysteine-rich protein 2
- Cu
Copper
- CuBPs
Copper-binding proteins
- CuCe
NPs Cu-deposited ceria nanoparticles
- CXCL2
C-X-C motif chemokine ligand 2
- DBT
Dihydrobiopterin trihydrobiopterin synthase
- DDIT3
DNA damage inducible transcript 3
- DLAT
Dihydrolipoamide S-acetyltransferase
- DLD
Dihydrolipoamide dehydrogenase
- DLST
Dihydrolipoamide S-succinyltransferase
- DUSP1
Dual specificity phosphatase 1
- EDTA
Ethylenediaminetetraacetic acid disodium salt
- Fe–S
Iron–sulfur
- GCSH
Glycine cleavage system protein H
- GLS
Glutaminase
- GSH
Glutathione
- GSSH
Oxidized glutathione
- HIF-1
Hypoxia-inducible factor 1
- I/R
Ischemia/reperfusion
- LDL
Low-density lipoprotein
- LIAS
Lipoic acid synthetase
- LIPT1
Lipoyltransferase 1
- MI
Myocardial infarction
- MMP-2
Matrix metalloproteinase 2
- PDHB
Pyruvate dehydrogenase E1 subunit beta
- PF
Paeoniflorin
- PGC1-α
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- STAT3
Signal transducer and activator of transcription 3
- TCA
Tricarboxylic acid
- TGF-β
Transforming growth factor-β
- TIMS
Targeted ion carrier–based metal supplements
- TLR4
Toll like receptor 4
- TM
Tetrathiomolybdate
- UBE2D3
Ubiquitin-conjugating enzyme E2D 3
- VEGF
Vascular endothelial growth factor
- VEGFR2
Vascular endothelial growth factor receptor type 2
- ZNF302
Zinc finger protein 302
- α-SMA
α-smooth muscle actin
References
1
Abeyrathna N. Abeyrathna S. Morgan M. T. Fahrni C. J. Meloni G. (2020). Transmembrane Cu(I) P-type ATPase pumps are electrogenic uniporters. Dalton Trans.49, 16082–16094. 10.1039/d0dt01380c
2
A D. D A. Ay F. V S. Ym K. Y H. et al (2022). Cysteine oxidation of copper transporter CTR1 drives VEGFR2 signalling and angiogenesis. Nat. cell Biol.24, 35–50. 10.1038/s41556-021-00822-7
3
Addis R. C. Epstein J. A. (2013). Induced regeneration--the progress and promise of direct reprogramming for heart repair. Nat. Med.19, 829–836. 10.1038/nm.3225
4
Afridi H. I. Kazi T. G. Kazi G. H. Jamali M. K. Shar G. Q. (2006). Essential trace and toxic element distribution in the scalp hair of Pakistani myocardial infarction patients and controls. Biol. Trace Elem. Res.113, 19–34. 10.1385/BTER:113:3
5
Alvarez H. M. Xue Y. Robinson C. D. Canalizo-Hernández M. A. Marvin R. G. Kelly R. A. et al (2010). Tetrathiomolybdate inhibits copper trafficking proteins through metal cluster formation. Science327, 331–334. 10.1126/science.1179907
6
Alves L. Ziegelmann P. K. Ribeiro V. Polanczyk C. (2022). Hospital mortality from myocardial infarction in Latin America and the Caribbean: systematic review and meta-analysis. Arq. Bras. Cardiol.119, 970–978. 10.36660/abc.20220194
7
Arora S. Stouffer G. A. Kucharska-Newton A. M. Qamar A. Vaduganathan M. Pandey A. et al (2019). Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction. Circulation139, 1047–1056. 10.1161/CIRCULATIONAHA.118.037137
8
Awadallah S. M. Hamad M. Jbarah I. Salem N. M. Mubarak M. S. (2006). Autoantibodies against oxidized LDL correlate with serum concentrations of ceruloplasmin in patients with cardiovascular disease. Clin. Chim. Acta365, 330–336. 10.1016/j.cca.2005.09.021
9
Bakos S. N. Ahmed H. K. Nasser T. A. K. (1988). Serum copper, magnesium, zinc, calcium, and potassium changes following acute myocardial infarction. Angiology39, 413–416. 10.1177/000331978803900502
10
Begum S. Sultana I. Faysal M. R. Alam S. Tasnim J. Akter T. et al (2023a). Study of changes in serum copper level in patients with acute myocardial infarction. Mymensingh Med. J.32, 39–43.
11
Begum S. Sultana I. Faysal M. R. Alam S. Tasnim J. Akter T. et al (2023b). Study of changes in serum copper level in patients with acute myocardial infarction. Mymensingh Med. J.32, 39–43.
12
Bhatt D. L. Lopes R. D. Harrington R. A. (2022). Diagnosis and treatment of acute coronary syndromes: a review. JAMA327, 662–675. 10.1001/jama.2022.0358
13
Białkowska M. Hoser A. Szostak W. B. Dybczyński R. Sterliński S. Nowicka G. et al (1987). Hair zinc and copper concentration in survivors of myocardial infarction. Ann. Nutr. Metab.31, 327–332. 10.1159/000177287
14
Blades B. Ayton S. Hung Y. H. Bush A. I. La Fontaine S. (2021). Copper and lipid metabolism: a reciprocal relationship. Biochim. Biophys. Acta Gen. Subj.1865, 129979. 10.1016/j.bbagen.2021.129979
15
Bost M. Houdart S. Oberli M. Kalonji E. Huneau J.-F. Margaritis I. (2016). Dietary copper and human health: current evidence and unresolved issues. J. Trace Elem. Med. Biol.35, 107–115. 10.1016/j.jtemb.2016.02.006
16
Brewer G. J. (2005). Copper lowering therapy with tetrathiomolybdate as an antiangiogenic strategy in cancer. Curr. Cancer Drug Targets5, 195–202. 10.2174/1568009053765807
17
Brewer G. J. (2014). The promise of copper lowering therapy with tetrathiomolybdate in the cure of cancer and in the treatment of inflammatory disease. J. Trace Elem. Med. Biol.28, 372–378. 10.1016/j.jtemb.2014.07.015
18
Bugger H. Pfeil K. (2020). Mitochondrial ROS in myocardial ischemia reperfusion and remodeling. Biochim. Biophys. Acta Mol. Basis Dis.1866, 165768. 10.1016/j.bbadis.2020.165768
19
Campolo N. Bartesaghi S. Radi R. (2014). Metal-catalyzed protein tyrosine nitration in biological systems. Redox Rep.19, 221–231. 10.1179/1351000214Y.0000000099
20
Chen J. Jiang Y. Shi H. Peng Y. Fan X. Li C. (2020). The molecular mechanisms of copper metabolism and its roles in human diseases. Pflugers Arch.472, 1415–1429. 10.1007/s00424-020-02412-2
21
Chen L. Li N. Zhang M. Sun M. Bian J. Yang B. et al (2021a). APEX2-based proximity labeling of Atox1 identifies CRIP2 as a nuclear copper-binding protein that regulates autophagy activation. Angew. Chem. Int. Ed. Engl.60, 25346–25355. 10.1002/anie.202108961
22
Chen L. Min J. Wang F. (2022). Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target Ther.7, 378. 10.1038/s41392-022-01229-y
23
Chen P. Zhang M. Zhang Y. Su X. Chen J. Xu B. et al (2021b). Economic burden of myocardial infarction combined with dyslipidemia. Front. Public Health9, 648172. 10.3389/fpubh.2021.648172
24
Chen X. Cai Q. Liang R. Zhang D. Liu X. Zhang M. et al (2011). Copper homeostasis and copper-induced cell death in the pathogenesis of cardiovascular disease and therapeutic strategies. Cell Death Dis.14, 105. 10.1038/s41419-023-05639-w
25
Cobine P. A. Moore S. A. Leary S. C. (2021). Getting out what you put in: copper in mitochondria and its impacts on human disease. Biochim. Biophys. Acta Mol. Cell Res.1868, 118867. 10.1016/j.bbamcr.2020.118867
26
Doenst T. Haverich A. Serruys P. Bonow R. O. Kappetein P. Falk V. et al (2019). PCI and CABG for treating stable coronary artery disease: JACC review topic of the week. J. Am. Coll. Cardiol.73, 964–976. 10.1016/j.jacc.2018.11.053
27
Dubois-Deruy E. Peugnet V. Turkieh A. Pinet F. (2020). Oxidative stress in cardiovascular diseases. Antioxidants (Basel)9, 864. 10.3390/antiox9090864
28
Elsherif L. Jiang Y. Saari J. T. Kang Y. J. (2004). Dietary copper restriction-induced changes in myocardial gene expression and the effect of copper repletion. Exp. Biol. Med. (Maywood)229, 616–622. 10.1177/153537020422900705
29
Escolar E. Ujueta F. Kim H. Mark D. B. Boineau R. Nahin R. L. et al (2020). Possible differential benefits of edetate disodium in post-myocardial infarction patients with diabetes treated with different hypoglycemic strategies in the trial to assess chelation therapy (TACT). J. Diabetes Complicat.34, 107616. 10.1016/j.jdiacomp.2020.107616
30
Fang C. Sun S. Chen W. Huang D. Wang F. Wei W. et al (2024a). Bioinformatics analysis of the role of cuproptosis gene in acute myocardial infarction. Minerva Cardiol. Angiol.72, 595–606. 10.23736/S2724-5683.23.06493-1
31
Fang X. Ji Y. Li S. Wang L. He B. Li B. et al (2024b). Paeoniflorin attenuates cuproptosis and ameliorates left ventricular remodeling after AMI in hypobaric hypoxia environments. J. Nat. Med.78, 664–676. 10.1007/s11418-024-01781-7
32
Feng K. Ruan Y. Zhang X. Wu X. Liu Z. Sun X. (2023). Photothermal-ionic-pharmacotherapy of myocardial infarction with enhanced angiogenesis and antiapoptosis. ACS Appl. Mater Interfaces. 10.1021/acsami.3c14109
33
Fox P. L. Mazumder B. Ehrenwald E. Mukhopadhyay C. K. (2000). Ceruloplasmin and cardiovascular disease. Free Radic. Biol. Med.28, 1735–1744. 10.1016/S0891-5849(00)00231-8
34
Fu L.-H. Wan Y. Qi C. He J. Li C. Yang C. et al (2021). Nanocatalytic theranostics with glutathione depletion and enhanced reactive oxygen species generation for efficient cancer therapy. Adv. Mater33, e2006892. 10.1002/adma.202006892
35
Fukai T. Ushio-Fukai M. Kaplan J. H. (2018). Copper transporters and copper chaperones: roles in cardiovascular physiology and disease. Am. J. Physiol. Cell Physiol.315, C186-C201–C201. 10.1152/ajpcell.00132.2018
36
Fulgenzi A. Vietti D. Ferrero M. E. (2020). EDTA chelation therapy in the treatment of neurodegenerative diseases: an update. Biomedicines8, 269. 10.3390/biomedicines8080269
37
Ga L. C G. R B. Db M. T R. Rl N. et al (2013). Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA309, 1241–1250. 10.1001/jama.2013.2107
38
Garza N. M. Swaminathan A. B. Maremanda K. P. Zulkifli M. Gohil V. M. (2023). Mitochondrial copper in human genetic disorders. Trends Endocrinol. Metab.34, 21–33. 10.1016/j.tem.2022.11.001
39
Ge E. J. Bush A. I. Casini A. Cobine P. A. Cross J. R. DeNicola G. M. et al (2022). Connecting copper and cancer: from transition metal signalling to metalloplasia. Nat. Rev. Cancer22, 102–113. 10.1038/s41568-021-00417-2
40
Grammer T. B. Kleber M. E. Silbernagel G. Pilz S. Scharnagl H. Lerchbaum E. et al (2014). Copper, ceruloplasmin, and long-term cardiovascular and total mortality (the ludwigshafen risk and cardiovascular health study). Free Radic. Res.48, 706–715. 10.3109/10715762.2014.901510
41
Guo X. Ma G. Zhang M. (2002). Study on iron, zinc, copper, manganese and selenium contents in serum of patients suffering from acute myocardial infarction and using thrombolysis therapy. J. Chin. Physician, 38–40.
42
Halling J. F. Pilegaard H. (2020). PGC-1α-mediated regulation of mitochondrial function and physiological implications. Appl. Physiol. Nutr. Metab.45, 927–936. 10.1139/apnm-2020-0005
43
He W. James Kang Y. (2013). Ischemia-induced copper loss and suppression of angiogenesis in the pathogenesis of myocardial infarction. Cardiovasc Toxicol.13, 1–8. 10.1007/s12012-012-9174-y
44
Horng Y.-C. Cobine P. A. Maxfield A. B. Carr H. S. Winge D. R. (2004). Specific copper transfer from the Cox17 metallochaperone to both Sco1 and Cox11 in the assembly of yeast cytochrome C oxidase. J. Biol. Chem.279, 35334–35340. 10.1074/jbc.M404747200
45
Im G.-B. Kim Y. G. Yoo T. Y. Kim Y. H. Kim K. Hyun J. et al (2023). Ceria nanoparticles as copper chaperones that activate SOD1 for synergistic antioxidant therapy to treat ischemic vascular diseases. Adv. Mater35, e2208989. 10.1002/adma.202208989
46
Inkol J. M. Poon A. C. Mutsaers A. J. (2020). Inhibition of copper chaperones sensitizes human and canine osteosarcoma cells to carboplatin chemotherapy. Vet. Comp. Oncol.18, 559–569. 10.1111/vco.12579
47
Isei M. O. Chinnappareddy N. Stevens D. Kamunde C. (2021). Anoxia-reoxygenation alters H2O2 efflux and sensitivity of redox centers to copper in heart mitochondria. Comp. Biochem. Physiol. C Toxicol. Pharmacol.248, 109111. 10.1016/j.cbpc.2021.109111
48
Jain V. K. Mohan G. (1991). Serum zinc and copper in myocardial infarction with particular reference to prognosis. Biol. Trace Elem. Res.31, 317–322. 10.1007/BF02990200
49
Jiang T. Huang M. Jiang T. Gu Y. Wang Y. Wu Y. et al (2018). Genome-wide compound heterozygosity analysis highlighted 4 novel susceptibility loci for congenital heart disease in Chinese population. Clin. Genet.94, 296–302. 10.1111/cge.13384
50
Jiang Y. Reynolds C. Xiao C. Feng W. Zhou Z. Rodriguez W. et al (2007). Dietary copper supplementation reverses hypertrophic cardiomyopathy induced by chronic pressure overload in mice. J. Exp. Med.204, 657–666. 10.1084/jem.20061943
51
Jin J. Ma M. Shi S. Wang J. Xiao P. Yu H.-F. et al (2022). Copper enhances genotoxic drug resistance via ATOX1 activated DNA damage repair. Cancer Lett.536, 215651. 10.1016/j.canlet.2022.215651
52
Kahlson M. A. Dixon S. J. (2022). Copper-induced cell death. Science375, 1231–1232. 10.1126/science.abo3959
53
Karginova O. Weekley C. M. Raoul A. Alsayed A. Wu T. Lee S. S.-Y. et al (2019). Inhibition of copper transport induces apoptosis in triple-negative breast cancer cells and suppresses tumor angiogenesis. Mol. Cancer Ther.18, 873–885. 10.1158/1535-7163.MCT-18-0667
54
Kazi T. G. Afridi H. I. Kazi N. Jamali M. K. Arain M. B. Sarfraz R. A. et al (2008). Distribution of zinc, copper and iron in biological samples of Pakistani myocardial infarction (1st, 2nd and 3rd heart attack) patients and controls. Clin. Chim. Acta389, 114–119. 10.1016/j.cca.2007.12.004
55
Khaki-Khatibi F. Mansouri F. Hajahmadipoorrafsanjani M. Ghojazadeh M. Gholikhani-Darbroud R. (2018). Study of rs1137101 polymorphism of leptin receptor gene with serum levels of selenium and copper in the patients of non-ST-segment elevation myocardial infarction (NSTEMI) in an Iranian population. Clin. Biochem.60, 64–70. 10.1016/j.clinbiochem.2018.06.016
56
Khan S. N. Rahman M. A. Samad A. (1984). Trace elements in serum from Pakistani patients with acute and chronic ischemic heart disease and hypertension. Clin. Chem.30, 644–648. 10.1093/clinchem/30.5.644
57
Kim Y. G. Lee Y. Lee N. Soh M. Kim D. Hyeon T. (2024). Ceria-based therapeutic antioxidants for biomedical applications. Adv. Mater36, e2210819. 10.1002/adma.202210819
58
Kobayashi K. Maeda K. Takefuji M. Kikuchi R. Morishita Y. Hirashima M. et al (2017). Dynamics of angiogenesis in ischemic areas of the infarcted heart. Sci. Rep.7, 7156. 10.1038/s41598-017-07524-x
59
Li K. Li C. Xiao Y. Wang T. James Kang Y. (2018). The loss of copper is associated with the increase in copper metabolism MURR domain 1 in ischemic hearts of mice. Exp. Biol. Med. (Maywood)243, 780–785. 10.1177/1535370218773055
60
Lim S. Y. Dayal H. Seah S. J. Tan R. P. W. Low Z. E. Laserna A. K. C. et al (2023). Plasma metallomics reveals potential biomarkers and insights into the ambivalent associations of elements with acute myocardial infarction. J. Trace Elem. Med. Biol.77, 127148. 10.1016/j.jtemb.2023.127148
61
Linder M. C. (2016). Ceruloplasmin and other copper binding components of blood plasma and their functions: an update. Metallomics8, 887–905. 10.1039/C6MT00103C
62
Liu Y. Xiao Y. Liu J. Feng L. Kang Y. J. (2018). Copper-induced reduction in myocardial fibrosis is associated with increased matrix metalloproteins in a rat model of cardiac hypertrophy. Metallomics10, 201–208. 10.1039/C7MT00165G
63
Liu Z. Wang L. Xing Q. Liu X. Hu Y. Li W. et al (2022). Identification of GLS as a cuproptosis-related diagnosis gene in acute myocardial infarction. Front. Cardiovasc Med.9, 1016081. 10.3389/fcvm.2022.1016081
64
Liu Z.-Y. Liu Z.-Y. Lin L.-C. Song K. Tu B. Zhang Y. et al (2024). Redox homeostasis in cardiac fibrosis: focus on metal ion metabolism. Redox Biol.71, 103109. 10.1016/j.redox.2024.103109
65
Lu Q. Zhang Y. Zhao C. Zhang H. Pu Y. Yin L. (2022). Copper induces oxidative stress and apoptosis of hippocampal neuron via pCREB/BDNF/and Nrf2/HO-1/NQO1 pathway. J. Appl. Toxicol.42, 694–705. 10.1002/jat.4252
66
Luo J. Gu H. Ynag Y. (2002). Study on the content of calcium, magnesium, zinc, copper and selenium in serum in patients with acute myocardial infarction. J. Chin. Physician, 59–60.
67
Maghool F. Emami M. H. Alipour R. Mohammadzadeh S. Sereshki N. Dehkordi S. A. E. et al (2023). Rescue effect of curcumin against copper toxicity. J. Trace Elem. Med. Biol.78, 127153. 10.1016/j.jtemb.2023.127153
68
Martin F. Linden T. Katschinski D. M. Oehme F. Flamme I. Mukhopadhyay C. K. et al (2005). Copper-dependent activation of hypoxia-inducible factor (HIF)-1: implications for ceruloplasmin regulation. Blood105, 4613–4619. 10.1182/blood-2004-10-3980
69
Martín-Lagos F. Navarro-Alarcón M. Terrés-Martos C. López-G de la Serrana H. López-Martínez M. C. (1997). Serum copper and zinc concentrations in serum from patients with cancer and cardiovascular disease. Sci. Total Environ.204, 27–35. 10.1016/S0048-9697(97)00163-0
70
Méndez A. A. E. Argüello J. M. Soncini F. C. Checa S. K. (2024). Scs system links copper and redox homeostasis in bacterial pathogens. J. Biol. Chem.300, 105710. 10.1016/j.jbc.2024.105710
71
Miao M. Cao S. Tian Y. Liu D. Chen L. Chai Q. et al (2023). Potential diagnostic biomarkers: 6 cuproptosis- and ferroptosis-related genes linking immune infiltration in acute myocardial infarction. Genes Immun.24, 159–170. 10.1038/s41435-023-00209-8
72
Mohammadifard N. Humphries K. H. Gotay C. Mena-Sánchez G. Salas-Salvadó J. Esmaillzadeh A. et al (2019). Trace minerals intake: risks and benefits for cardiovascular health. Crit. Rev. Food Sci. Nutr.59, 1334–1346. 10.1080/10408398.2017.1406332
73
Muchenditsi A. Yang H. Hamilton J. P. Koganti L. Housseau F. Aronov L. et al (2017). Targeted inactivation of copper transporter Atp7b in hepatocytes causes liver steatosis and obesity in mice. Am. J. Physiol. Gastrointest. Liver Physiol.313, G39-G49–G49. 10.1152/ajpgi.00312.2016
74
Muñoz-Bravo C. Soler-Iborte E. Lozano-Lorca M. Kouiti M. González-Palacios Torres C. Barrios-Rodríguez R. et al (2023). Serum copper levels and risk of major adverse cardiovascular events: a systematic review and meta-analysis. Front. Cardiovasc Med.10, 1217748. 10.3389/fcvm.2023.1217748
75
Nowicki G. J. Ślusarska B. Prystupa A. Blicharska E. Adamczuk A. Czernecki T. et al (2021). Assessment of concentrations of heavy metals in postmyocardial infarction patients and patients free from cardiovascular event. Cardiol. Res. Pract.2021, 9546358–11. 10.1155/2021/9546358
76
Övermöhle C. Rimbach G. Waniek S. Strathmann E. A. Liedtke T. Stürmer P. et al (2023). Association of plasma zinc and copper with body composition, lipids and inflammation in a cross-sectional general population sample from Germany. Nutrients15, 4460. 10.3390/nu15204460
77
Pi W. Wu L. Lu J. Lin X. Huang X. Wang Z. et al (2023). A metal ions-mediated natural small molecules carrier-free injectable hydrogel achieving laser-mediated photo-fenton-like anticancer therapy by synergy apoptosis/cuproptosis/anti-inflammation. Bioact. Mater29, 98–115. 10.1016/j.bioactmat.2023.06.018
78
Prabhu S. D. Frangogiannis N. G. (2016). The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ. Res.119, 91–112. 10.1161/CIRCRESAHA.116.303577
79
Qiu Y. Su M. Xiao X. Zhou D. Xie L. (2023). Metabolomic profiling of wilson disease, an inherited disorder of copper metabolism, and diseases with similar symptoms but normal copper metabolism. Orphanet J. Rare Dis.18, 282. 10.1186/s13023-023-02900-5
80
Reid A. Miller C. Farrant J. P. Polturi R. Clark D. Ray S. et al (2022). Copper chelation in patients with hypertrophic cardiomyopathy. Open Heart9, e001803. 10.1136/openhrt-2021-001803
81
Roth G. A. Mensah G. A. Johnson C. O. Addolorato G. Ammirati E. Baddour L. M. et al (2020). Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J. Am. Coll. Cardiol.76, 2982–3021. 10.1016/j.jacc.2020.11.010
82
Sagris M. Antonopoulos A. S. Theofilis P. Oikonomou E. Siasos G. Tsalamandris S. et al (2022). Risk factors profile of young and older patients with myocardial infarction. Cardiovasc Res.118, 2281–2292. 10.1093/cvr/cvab264
83
Sato T. Takeda N. (2023). The roles of HIF-1α signaling in cardiovascular diseases. J. Cardiol.81, 202–208. 10.1016/j.jjcc.2022.09.002
84
Savic-Radojevic A. Pljesa-Ercegovac M. Matic M. Simic D. Radovanovic S. Simic T. (2017). Novel biomarkers of heart failure. Adv. Clin. Chem.79, 93–152. 10.1016/bs.acc.2016.09.002
85
Semenza G. L. (2014). Hypoxia-inducible factor 1 and cardiovascular disease. Annu. Rev. Physiol.76, 39–56. 10.1146/annurev-physiol-021113-170322
86
Shokrzadeh M. Ghaemian A. Salehifar E. Aliakbari S. Saravi S. S. S. Ebrahimi P. (2009). Serum zinc and copper levels in ischemic cardiomyopathy. Biol. Trace Elem. Res.127, 116–123. 10.1007/s12011-008-8237-1
87
Singh M. M. Singh R. Khare A. Gupta M. C. Patney N. L. Jain V. K. et al (1985). Serum copper in myocardial infarction--diagnostic and prognostic significance. Angiology36, 504–510. 10.1177/000331978503600805
88
Su T. A. Bruemmer K. J. Chang C. J. (2019). Caged luciferins for bioluminescent activity-based sensing. Curr. Opin. Biotechnol.60, 198–204. 10.1016/j.copbio.2019.05.002
89
Sudhahar V. Shi Y. Kaplan J. H. Ushio-Fukai M. Fukai T. (2022). Whole-transcriptome sequencing analyses of nuclear Antixoxidant-1 in endothelial cells: role in inflammation and atherosclerosis. Cells11, 2919. 10.3390/cells11182919
90
Talman V. Ruskoaho H. (2016). Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res.365, 563–581. 10.1007/s00441-016-2431-9
91
Tan I. K. Chua K. S. Toh A. K. (1992). Serum magnesium, copper, and zinc concentrations in acute myocardial infarction. Clin. Lab. Anal.6, 324–328. 10.1002/jcla.1860060513
92
Taneja S. K. Girhotra S. Singh K. P. (2000). Detection of potentially myocardial infarction susceptible individuals in Indian population: a mathematical model based on copper and zinc status. BTER75, 177–186. 10.1385/BTER:75:1-3:177
93
Tang W. H. W. Wu Y. Hartiala J. Fan Y. Stewart A. F. R. Roberts R. et al (2012). Clinical and genetic association of serum ceruloplasmin with cardiovascular risk. Arterioscler. Thromb. Vasc. Biol.32, 516–522. 10.1161/ATVBAHA.111.237040
94
Tegoni M. Valensin D. Toso L. Remelli M. (2014). Copper chelators: chemical properties and bio-medical applications. Curr. Med. Chem.21, 3785–3818. 10.2174/0929867321666140601161939
95
Tian Z. Jiang S. Zhou J. Zhang W. (2023). Copper homeostasis and cuproptosis in mitochondria. Life Sci.334, 122223. 10.1016/j.lfs.2023.122223
96
Tisminetzky M. Mehawej J. Miozzo R. Gurwitz J. H. Gore J. M. Lessard D. et al (2021). Temporal trends and patient characteristics associated with 30-Day hospital readmission rates after a first acute myocardial infarction. Am. J. Med.134, 1127–1134. 10.1016/j.amjmed.2021.03.024
97
Tsvetkov P. Coy S. Petrova B. Dreishpoon M. Verma A. Abdusamad M. et al (2022). Copper induces cell death by targeting lipoylated TCA cycle proteins. Science375, 1254–1261. 10.1126/science.abf0529
98
Venugopal H. Hanna A. Humeres C. Frangogiannis N. G. (2022). Properties and functions of fibroblasts and myofibroblasts in myocardial infarction. Cells11, 1386. 10.3390/cells11091386
99
Versieck J. Barbier F. Speecke A. Hoste J. (1975). Influence of myocardial infarction on serum manganese, copper, and zinc concentrations. Clin. Chem.21, 578–581. 10.1093/clinchem/21.4.578
100
Wang B. Zhou J. An N. (2024). Investigating molecular markers linked to acute myocardial infarction and cuproptosis: bioinformatics analysis and validation in the AMI mice model. PeerJ12, e17280. 10.7717/peerj.17280
101
Wang D. Tian Z. Zhang P. Zhen L. Meng Q. Sun B. et al (2023). The molecular mechanisms of cuproptosis and its relevance to cardiovascular disease. Biomed. and Pharmacother.163, 114830. 10.1016/j.biopha.2023.114830
102
Wang X. Yang P. Jiang Y. Xu Y. Wang N. Rao P. et al (2021). UBE2D3 contributes to myocardial ischemia-reperfusion injury by regulating autophagy in dependence of p62/SQSTM1. Cell Signal87, 110118. 10.1016/j.cellsig.2021.110118
103
Wei H. Zhang W.-J. McMillen T. S. Leboeuf R. C. Frei B. (2012). Copper chelation by tetrathiomolybdate inhibits vascular inflammation and atherosclerotic lesion development in apolipoprotein E-deficient mice. Atherosclerosis223, 306–313. 10.1016/j.atherosclerosis.2012.06.013
104
Wells E. M. Navas-Acien A. Apelberg B. J. Herbstman J. B. Jarrett J. M. Lin Y. H. et al (2014). Association of selenium and copper with lipids in umbilical cord blood. J. Dev. Orig. Health Dis.5, 281–287. 10.1017/S2040174414000233
105
Wen H. Niu X. Hu L. Sun N. Zhao R. Wang Q. et al (2022). Dietary copper intake and risk of myocardial infarction in US adults: a propensity score-matched analysis. Front. Cardiovasc. Med.9, 942000. 10.3389/fcvm.2022.942000
106
White K. N. Conesa C. Sánchez L. Amini M. Farnaud S. Lorvoralak C. et al (2012). The transfer of iron between ceruloplasmin and transferrins. Biochim. Biophys. Acta1820, 411–416. 10.1016/j.bbagen.2011.10.006
107
Wold L. E. Saari J. T. Ren J. (2001). Isolated ventricular myocytes from copper-deficient rat hearts exhibit enhanced contractile function. Am. J. Physiol. Heart Circ. Physiol.281, H476–H481. 10.1152/ajpheart.2001.281.2.H476
108
Wu X. Reboll M. R. Korf-Klingebiel M. Wollert K. C. (2021). Angiogenesis after acute myocardial infarction. Cardiovasc Res.117, 1257–1273. 10.1093/cvr/cvaa287
109
Xiang Q. Yi X. Zhu X.-H. Wei X. Jiang D.-S. (2024). Regulated cell death in myocardial ischemia-reperfusion injury. Trends Endocrinol. Metab.35, 219–234. 10.1016/j.tem.2023.10.010
110
Xiao Y. Wang T. Song X. Yang D. Chu Q. Kang Y. J. (2020). Copper promotion of myocardial regeneration. Exp. Biol. Med. (Maywood)245, 911–921. 10.1177/1535370220911604
111
Yang D. Xiao P. Qiu B. Yu H.-F. Teng C.-B. (2023a). Copper chaperone antioxidant 1: multiple roles and a potential therapeutic target. J. Mol. Med. Berl.101, 527–542. 10.1007/s00109-023-02311-w
112
Yang F. Pei R. Zhang Z. Liao J. Yu W. Qiao N. et al (2019). Copper induces oxidative stress and apoptosis through mitochondria-mediated pathway in chicken hepatocytes. Toxicol Vitro54, 310–316. 10.1016/j.tiv.2018.10.017
113
Yang L. Yang P. Lip G. Y. H. Ren J. (2023b). Copper homeostasis and cuproptosis in cardiovascular disease therapeutics. Trends Pharmacol. Sci.44, 573–585. 10.1016/j.tips.2023.07.004
114
Yang M. Wang Y. He L. Shi X. Huang S. (2024). Comprehensive bioinformatics analysis reveals the role of cuproptosis-related gene Ube2d3 in myocardial infarction. Front. Immunol.15, 1353111. 10.3389/fimmu.2024.1353111
115
Yesmin M. Mia A. R. Chakraborty P. K. Hossain M. S. Hoque M. R. Akhter S. et al (2016a). Serum copper status among acute myocardial infarction Male patients in Bangladesh. Mymensingh Med. J.25, 611–614.
116
Yesmin M. Mia A. R. Chakraborty P. K. Hossain M. S. Hoque M. R. Akhter S. et al (2016b). Serum copper status among acute myocardial infarction Male patients in Bangladesh. Mymensingh Med. J.25, 611–614.
117
You H. Chang F. Chen H. Wang Y. Han W. (2024). Exploring the role of CBLB in acute myocardial infarction: transcriptomic, microbiomic, and metabolomic analyses. J. Transl. Med.22, 654. 10.1186/s12967-024-05425-y
118
Yuan S. Chen S. Xi Z. Liu Y. (2017). Copper-finger protein of Sp1: the molecular basis of copper sensing. Metallomics9, 1169–1175. 10.1039/C7MT00184C
119
Y X. Q F. L H. X M. P H. W Z. et al (2023). Copper promotes cardiac functional recovery via suppressing the transformation of fibroblasts to myofibroblasts in ischemia-infarcted monkey hearts. J. Nutr. Biochem.111, 109180. 10.1016/j.jnutbio.2022.109180
120
Zama N. Towns R. L. R. (1986). Cardiac copper, magnesium, and zinc in recent and old myocardial infarction. Biol. Trace Elem. Res.10, 201–208. 10.1007/BF02795618
121
Zhang D. Lv F.-L. Wang G.-H. (2018). Effects of HIF-1α on diabetic retinopathy angiogenesis and VEGF expression. Eur. Rev. Med. Pharmacol. Sci.22, 5071–5076. 10.26355/eurrev_201808_15699
122
Zhang S. Liu H. Amarsingh G. V. Cheung C. C. H. Wu D. Narayanan U. et al (2020). Restoration of myocellular copper-trafficking proteins and mitochondrial copper enzymes repairs cardiac function in rats with diabetes-evoked heart failure. Metallomics12, 259–272. 10.1039/c9mt00223e
123
Zheng P. Zhou C. Lu L. Liu B. Ding Y. (2022). Elesclomol: a copper ionophore targeting mitochondrial metabolism for cancer therapy. J. Exp. Clin. Cancer Res.41, 271. 10.1186/s13046-022-02485-0
124
Zhong C.-C. Zhao T. Hogstrand C. Chen F. Song C.-C. Luo Z. (2022). Copper (cu) induced changes of lipid metabolism through oxidative stress-mediated autophagy and Nrf2/PPARγ pathways. J. Nutr. Biochem.100, 108883. 10.1016/j.jnutbio.2021.108883
125
Zhou Q. Zhang Y. Lu L. Zhang H. Zhao C. Pu Y. et al (2022). Copper induces microglia-mediated neuroinflammation through ROS/NF-κB pathway and mitophagy disorder. Food Chem. Toxicol.168, 113369. 10.1016/j.fct.2022.113369
126
Zhou S. Wang L. Huang X. Wang T. Tang Y. Liu Y. et al (2024). Comprehensive bioinformatics analytics and in vivo validation reveal SLC31A1 as an emerging diagnostic biomarker for acute myocardial infarction. Aging (Albany NY)16, 8361–8377. 10.18632/aging.205199
127
Zhu S. Wu H. Cui H. Guo H. Ouyang Y. Ren Z. et al (2023a). Induction of mitophagy via ROS-Dependent pathway protects copper-induced hypothalamic nerve cell injury. Food Chem. Toxicol.181, 114097. 10.1016/j.fct.2023.114097
128
Zhu W. Zhang Y. Luo X. Peng J. (2023b). Role of copper and its complexes in cardiovascular diseases. Zhong Nan Da Xue Xue Bao Yi Xue Ban.48, 1731–1738. 10.11817/j.issn.1672-7347.2023.230159
129
Zulkifli M. Spelbring A. N. Zhang Y. Soma S. Chen S. Li L. et al (2023). FDX1-dependent and independent mechanisms of elesclomol-mediated intracellular copper delivery. Proc. Natl. Acad. Sci. U. S. A.120, e2216722120. 10.1073/pnas.2216722120
130
Zuo X. Dong D. Sun M. Xie H. Kang Y. J. (2013). Homocysteine restricts copper availability leading to suppression of cytochrome C oxidase activity in phenylephrine-treated cardiomyocytes. PLoS One8, e67549. 10.1371/journal.pone.0067549
Summary
Keywords
copper, cuproptosis, myocardial infarction, mitochondrion, molecular mechanisms
Citation
Shen Z, Liu Z, Cai S, Fu H, Gan Y, Li X, Wang X, Liu C, Ma W, Chen J and Li N (2025) Copper homeostasis and cuproptosis in myocardial infarction: molecular mechanisms, treatment strategies and potential therapeutic targets. Front. Pharmacol. 16:1525585. doi: 10.3389/fphar.2025.1525585
Received
10 November 2024
Accepted
30 June 2025
Published
26 September 2025
Volume
16 - 2025
Edited by
Xiangxiang Wei, Fudan University, China
Reviewed by
Xilan Yang, Fourth Affiliated Hospital of Nanjing Medical University, China
Fan Xinbiao, Tianjin University of Traditional Chinese Medicine, China
Chenbin Bian, Sichuan University, China
Updates
Copyright
© 2025 Shen, Liu, Cai, Fu, Gan, Li, Wang, Liu, Ma, Chen and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinhong Chen, chenjh0818@163.com; Ningcen Li, 517654179@qq.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.