- 1Department of Hepatobiliary and Pancreatic Surgery and Retroperitoneal Tumor Surgery, The Affiliated Hospital of Qingdao University, Qingdao, China
- 2Department of Oncology, Women and Children’s Hospital Affiliated to Qingdao University, Qingdao, China
- 3Department of Anesthesiology Department, The Affiliated Hospital of Qingdao University, Qingdao, China
Liposarcoma, as a complex disease, is characterized by intricate interactions between distinct histopathological subtypes and corresponding clinical outcomes, emphasizing the necessity of personalized approaches in diagnosis and treatment strategies. This malignant tumor originating from adipose tissue is classified into different subtypes with specific molecular markers, which not only distinguish them but also guide treatment directions. The main approach for treating liposarcoma is surgical resection, with the aim of complete excision and achieving clean margins (R0 resection) to minimize the risk of recurrence. This surgical principle emphasizes the critical need for precise preoperative planning, and in certain cases, the integration of neoadjuvant therapy may be needed to reduce the tumor to a surgically manageable size. In addition to surgery, systemic therapy plays a key role in the advanced stages of the disease, especially when resistance to traditional treatment arises. The emergence of novel systemic therapies, including chemotherapy, targeted therapy, and immunotherapy, has opened new avenues for treating this challenging malignancy. These systemic therapies are selected on the basis of the specific molecular features of the tumor, highlighting the importance of detailed molecular diagnostics. As our understanding of the molecular basis of liposarcoma deepens, integrating clinical and molecular features is crucial for optimizing treatment outcomes. This comprehensive approach, which combines surgical precision with systemic therapy innovations, will change the treatment landscape for patients with liposarcoma, advancing toward more personalized and effective treatment strategies.
1 Introduction
Soft tissue sarcomas (STSs) account for 1% of adult malignancies and are a group of mesenchymal tumors comprising 179 histological subtypes (https://seer.cancer.gov/statfacts/html/soft.html) (Kallen and Hornick, 2021). Liposarcoma (LPS) accounts for 15%–20% of STSs and is a rare malignant tumor characterized by adipocyte differentiation (Gronchi et al., 2021). Approximately 41% of LPSs occur in the lower limbs, 36% in the retroperitoneum, 8% in the upper limbs, and 5% each in visceral organs and the trunk (De vita et al., 2016). According to the 5th edition of the WHO Classification of Soft Tissue Tumors released in 2020 (Schaefer and Gronchi, 2022), subtypes of LPS include atypical lipomatous tumor (ALT)/well-differentiated liposarcoma (WDLPS), dedifferentiated liposarcoma (DDLPS), myxoid liposarcoma (MLPS), pleomorphic liposarcoma (PLPS), and myxoid pleomorphic liposarcoma (MPLPS), with MPLPS being a new addition characterized by a nonspecific nuclear pattern, lacking the classic gene fusion of DDIT3 with FUS or EWSR1. Given that these subtypes have unique clinical, histological, biological, immunohistochemical, and molecular genetic features relevant to diagnosis, prognosis, and treatment sensitivity (Kallen and Hornick, 2021), individualized treatment methods should be formulated on the basis of the histological type (Haddox and Riedel, 2020). A series of novel antitumor drugs that target the specific molecular biology of LPS are actively being researched, offering hope for increasing treatment options for recurrent or unresectable LPS (Lee et al., 2018).
2 Clinical and molecular characteristics of each subtype of LPS
Each subtype of liposarcoma (LPS) has unique clinical and molecular characteristics, reflecting the diversity and complexity of this malignant tumor (Yao et al., 2020).
2.1 Cytogenetic characteristics of ALT/WDLPS and DDLPS
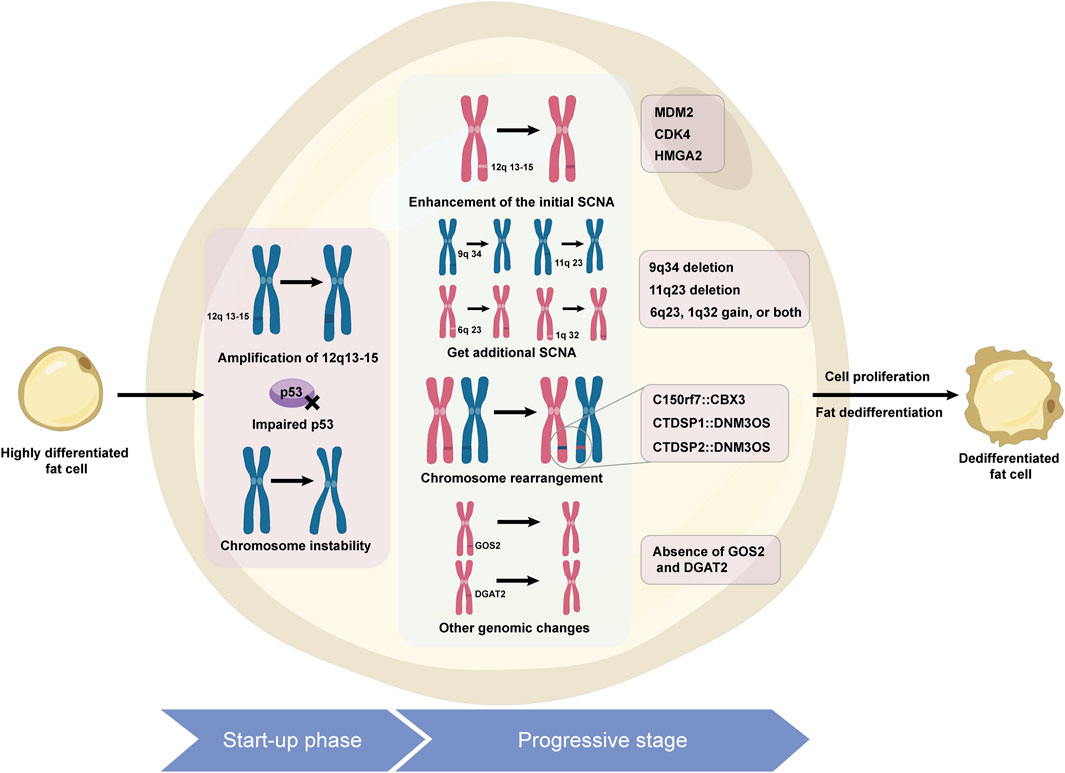
ALT/WDLPS is the most common subtype of liposarcoma, accounting for 40%–45%, and is commonly found in the limbs, buttocks, and deep soft tissues of the trunk, with 25% originating from the retroperitoneum. Its subtypes include lipomatous, sclerosing, inflammatory, and spindle cell variants (Sciot et al., 2020). They usually grow slowly, are prone to recurrence, and are resistant to radiotherapy and chemotherapy. Compared with WDLPS of the limbs, retroperitoneal WDLPS has a greater risk of dedifferentiation (Keung et al., 2018). DDLPS accounts for 15%–20% of cases, mostly in middle-aged and elderly people, with 75% occurring in the abdominal cavity and retroperitoneum (Gahvari and Parkes, 2020; Kilpatrick, 2021). DDLPS is characterized by increased aggressiveness and metastatic potential and is chemoresistant (Ghadimi et al., 2011). On a pathological level, it may exhibit homologous and heterologous dedifferentiation, with the majority being of high grade (Schaefer and Gronchi, 2022). DDLPS has a local recurrence rate of up to 40%, with a distant metastasis rate of 15%–30%, and the site of the lesion is an important prognostic factor. The molecular hallmark of WDLPS and DDLPS is amplification of chromosomal region 12q13-15 (Matthews et al., 2010), particularly amplification of the MDM2 and CDK4 genes (Aleixo et al., 2009), which drive tumor growth and dedifferentiation. Other key genes in this region, such as HMGA2, TSPAN31, FRS2, and GLI1, and new genes outside this area, such as DDR2, SDHC, and FGFR, also play significant roles in its pathogenesis (Pentimalli et al., 2003; Saâda-bouzid et al., 2015; Gao et al., 2013; Kanojia et al., 2015; Barretina et al., 2010). The signaling pathways of the FGFR/FRS2 and the PIK3R3/ERK/Nanog axis are closely linked to the development of DDLPS (Wang et al., 2011; Zhang et al., 2013; Jing et al., 2018). While DDLPS shares common cytogenetic features, it presents more genomic abnormalities and complexity, exerting a greater impact on treatment response and prognosis. Mechanisms of liposarcoma dedifferentiation can be seen in Figure 1.
2.2 The genetic characteristics of MLPS
MLPS is the predominant form of LPS among children and adolescents, accounting for 20%–30% of cases, and is found mainly in the deep soft tissue of the limbs, particularly near the proximal thigh, with a rare occurrence in the retroperitoneum (approximately 2.3%) (Henze and Bauer, 2013). Approximately 12%–25% of patients are likely to experience local recurrence, and between 30% and 60% can metastasize (Schaefer and Gronchi, 2022). Pathology findings have shown that the presence of round cell components is associated with a poor prognosis (Schaefer and Gronchi, 2022; Setsu et al., 2016). In patients with MLS, dedifferentiation is rare (Ciongariu et al., 2023). More than 95% of MLS patients present with a t (12; 16) (q13; p11) translocation, leading to the production of the FUS-DDIT3 fusion protein, impeding adipocyte terminal differentiation and facilitating tumor formation (Pérez-mancera et al., 2008; Powers et al., 2010; Xiao et al., 2018). Next-generation sequencing has identified new fusion genes and signaling pathway abnormalities, such as RET, FGFR2 (Künstlinger et al., 2015; Napolitano et al., 2021), PI3K/AKT/mTOR (Trautmann et al., 2019a; Berthold et al., 2022), Hippo/YAP1 dysregulation (Regina and Hettmer, 2019; Trautmann et al., 2019b), and TERT promoter mutations (Koelsche et al., 2014), revealing that FUS-CHOP activates the SRC/FAK/RHO/ROCK signaling axis, enhancing the invasive capacity of MLS cells (Tornin et al., 2018). Staaberg’s team reported that a subgroup of MLS cells with CSC characteristics activate the JAK-STAT signaling pathway (Dolatabadi et al., 2019), which could be a significant target for MLS treatment.
2.3 Genetic alterations in PLPS and MPLPS
PLPS, a subtype characterized by high invasiveness and poor prognosis, accounts for just 5%–10% of all LPSs, featuring elevated rates of local recurrence and metastasis (approximately 30%–50% each), with a 5-year survival rate of 60% (Schaefer and Gronchi, 2022). It affects mainly the deep soft tissues of the limbs, particularly the lower extremities. There is significant chemoresistance, which may be associated with P53 mutations. PLPS is characterized by pronounced chromosomal abnormalities, encompassing deletions and duplications (Conyers et al., 2011). Some studies indicate a correlation between RB1 mutations and PLPS (Libbrecht et al., 2021). MPLPS is a highly aggressive, rare tumor that predominantly occurs in the mediastinum of children and young adults and affects mainly females. It has complex chromosomal alterations and lacks FUS-DDIT3 gene fusion and MDM2/CDK4 gene amplification (Schaefer and Gronchi, 2022).
The diversity of the LPS subtypes in terms of clinical manifestations and molecular pathology underscores the need for a meticulous approach in diagnosis, treatment, and research to accommodate the unique characteristics of each subtype. Advances in molecular genetics offer promising avenues for targeted treatments, emphasizing the importance of continued research to fully leverage these outcomes to improve patient care.
3 LPS treatment
3.1 Surgical intervention
The primary treatment for LPS is to perform R0 surgical resection as much as possible, avoiding unplanned resection (Qureshi et al., 2012). The survival duration of primary localized RPL is relatively short, with an overall 8-year survival rate ranging from 30% to 80% for different subtypes (Siew et al., 2022). All patients with resectable RPL should undergo initial extensive surgical resection for complete R0 excision (Delisle et al., 2022; Harati et al., 2017). Research has shown a significant association between OS, DFS, the LR rate, and LRFS in RPLPS patients and R0 resection (Paik et al., 2022). Compartment resection is the current standard procedure. Precision surgical principles require surgical stratification on the basis of the biological behavior of RPL. For radiotherapy and chemotherapy-resistant LPS, such as WDLPS/DDLPS, surgical intervention remains the cornerstone of treatment. In case of surgical difficulties, neoadjuvant chemotherapy or radiotherapy may be considered to reduce the risk of recurrence. Given the high recurrence rate of RPL, measures such as radiotherapy and drug therapy may be considered, but controversy remains (Rust et al., 2022; D’ambrosio et al., 2022). Surgical intervention may have a certain effect on locally recurrent RPL, but the likelihood of long-term control decreases after each recurrence (Tseng et al., 2022). Research by Maria Anna Smolle et al. revealed that the survival rate of patients with primary localized limb MLPS who undergo metastatic liver resection after recurrence is higher than that of those who receive other treatments (Smolle et al., 2020). Studies suggest that simultaneous resection of the primary tumor and metastases in patients with LPS presenting with distant metastasis at diagnosis may prolong survival (Illuminati et al., 2010). Multiple studies have demonstrated that metastasectomy can increase survival rates (Chudgar et al., 2017; Marudanayagam et al., 2011). The Japanese JCO guidelines recommend resection of the primary lesion and metastases, but further research is needed on patient selection, considering factors such as patient condition, number of metastases, and status of the primary tumor. Studies (Tirotta et al., 2020) by Tirotta F et al. suggested that LRSM can result in prolonged patient survival, although factors such as extrahepatic metastases, large metastatic lesions, chemotherapy resistance, and short DFI contribute to reduced survival rates.
3.2 Chemotherapeutic treatment
3.2.1 Neoadjuvant chemotherapy for high-risk patients with resectable LPS
For patients at very high risk or with early resection difficulties, neoadjuvant chemotherapy regimens resemble those of advanced treatment, frequently employing anthracycline-based agents. Meta-analyses demonstrated a 6% reduction in mortality risk with perioperative chemotherapy and an 11% reduction with the standard A + I regimen (Woll et al., 2012). Phase III trials have shown that the A + I regimen is superior to trabectedin (Gronchi et al., 2017; Gronchi et al., 2020). The results of phase II trials are promising (Tanaka et al., 2015; Tanaka et al., 2019). There is no evidence supporting the use of neoadjuvant chemotherapy for resectable RPS (Gronchi et al., 2021; Swallow et al., 2021). The TARPSWG study recommended the adoption of the A + I regimen for Grade 3 DDLPS patients (Tseng et al., 2021). The STRASS2 trial evaluated histology-tailored neoadjuvant chemotherapy, with the DDLPS regimen being doxorubicin + ifosfamide (Istl and Gronchi, 2022).
3.2.2 Systemic therapy for unresectable, advanced, or metastatic LPS
The efficacy of chemotherapy and overall survival rates vary depending on the LPS subtype (Schöffski, 2022). MLP exhibits high sensitivity to chemotherapy (Hindi and Haas, 2022); PLPS shows relative sensitivity to chemotherapy (Italiano et al., 2012); DDLPS demonstrates some response, whereas ALT/WDLPS are generally insensitive to chemotherapy. The median overall survival (mOS) for chemotherapy-sensitive subtypes in advanced stages is approximately 2 years (Abbas Manji et al., 2015). Presently, the first-line systemic therapy regimen is D + IFO (Crago and Dickson, 2016); however, there are inadequate specific research data on the various subtypes of LPS. Stacchiotti et al. reported that WD/DDLPS patients had response rates of 6.3% and 13% to first-line anthracycline and ifosfamide chemotherapy, respectively, whereas in the D + IFO group, the response rate was 22%. The response rate to first-line chemotherapy is significantly greater in MLPS patients than in WDLPS/DDLPS patients (48% vs. 11%) (Jones et al., 2005), and the overall survival with first-line chemotherapy is significantly longer in MPLS patients than in DDLPS/PLPS patients (Langmans et al., 2019).
Second-line and subsequent regimens include high-dose continuous infusion of ifosfamide, gemcitabine-based combination therapy (such as docetaxel and dacarbazine), and novel chemotherapy agents, including trabectedin, eribulin, and dacarbazine (Chamberlain et al., 2021). Second-line treatment with trabectedin significantly prolongs progression-free survival (PFS) in advanced LPS patients (Patel et al., 2019; Le cesne et al., 2021; De sande gonzález et al., 2020; Vincenzi et al., 2023). MLS is more sensitive to trabectedin (Assi et al., 2019). For unresectable/recurrent STS patients, the overall median PFS is 3.7 months, with a median PFS of 17.4 months for MLS patients and 3.7 months for DDLPS patients (Kobayashi et al., 2020). Trabectedin can be safely administered to elderly STS patients who are unsuitable for first-line anthracycline therapy (Grosso et al., 2020). Eribulin affects tumor cells and the microenvironment by inhibiting microtubule growth and through various molecular mechanisms (De vita et al., 2021), with chemotherapy-induced peripheral neuropathy (CIPN) being the principal adverse effect. Phase III trials indicate that eribulin monotherapy is superior to dacarbazine monotherapy in patients with locally advanced, recurrent, or metastatic LPS (Frapolli et al., 2019). Novel treatment strategies, such as the combination of eribulin with lenvatinib and eribulin combined with gemcitabine, show promising efficacy (Chen et al., 2022; Kim et al., 2022). Future directions involve enhancing efficacy, mitigating toxicity, and identifying biomarkers to predict treatment response. Other novel agents, such as cabazitaxel, exhibit favorable activity in advanced DDLPS (Sanfilippo et al., 2022). Ascorbic acid and carfilzomib also demonstrate potential therapeutic effects (Schoenfeld et al., 2018; Jeitany et al., 2021).
3.2.3 Others
Research by Miao Chengli et al. revealed that performing HIPEC after surgery for retroperitoneal LPS can significantly reduce mortality and recurrence rates (Miao et al., 2022). Angeles et al. discovered that SN-38 induces apoptosis in DDLPS cells by increasing C/EBPα protein expression (Angeles et al., 2022).
3.3 Targeted therapy
3.3.1 Targeting MDM2
Currently, the MDM2 inhibitor DS-3032b shows potential efficacy in patients with WDLPS/DDLPS (Bauer et al., 2018), with comparative trials underway (Gounder et al., 2022a). The efficacy of AMG 232 is also under investigation (Gluck et al., 2020), and the MANTRA trial revealed that milademetan has failed as a second-line treatment for unresectable or metastatic DDLPS patients (Jones et al., 2023). Brigimadlin has demonstrated potential antitumor activity in DDLPS/WDLPS (Lorusso et al., 2023), with global phase II/III studies currently underway. Studies by Cissé MY et al. reported that MDM2-mediated serine metabolism control is a driving force in the growth of LPS (Cissé et al., 2020), whereas Seligson ND et al. suggested that targeting HDAC2 may be a potential strategy for modulating MDM2 expression in DDLPS (Seligson et al., 2019).
3.3.2 Targeting CDK4
In WD/DDLPS, the amplification rate of CDK4 is as high as 90%, making CDK4 another viable target (Assi et al., 2020). The CSCO guidelines recommend palbociclib as a second-line treatment, but practical application studies show poor outcomes (Nassif et al., 2022). The combination of palbociclib with recombinant methionase enhanced the efficacy of palbociclib (Higuchi et al., 2022). A phase III study of abemaciclib versus placebo is underway. MDM2 inhibitors combined with CDK4/6 inhibitors show manageable toxicity and good antitumor activity in advanced-stage patients (Abdul Razak et al., 2022).
3.3.3 Targeting vascular endothelial growth factor
Studies have shown that LPS contains more microvessels (Baneth et al., 2005) and is more sensitive to antiangiogenic therapy. Anlotinib, as a second-line treatment for STS, is included in the CSCO guidelines. The ALTER0202 study showed significant efficacy, with a 12-week PFR of 63% and mPFS and mOS of 5.6 and 13 months, respectively (Chi et al., 2018). The ALTER-S006 study indicated that patients who were maintained on anlotinib after first-line chemotherapy had an mPFS of 12.5 months (Xu et al., 2023). Retrospective studies have shown that treatment with anlotinib in patients with metastatic or recurrent WDLPS/DDLPS resulted in an mPFS of 27.9 weeks, a 24-week PFR of 58.8%, and an OS of 56.6 weeks (Li et al., 2021).
3.3.4 Multitargeted tyrosine kinase inhibitors
Pazopanib is a second-line treatment option for STS recommended by the National Comprehensive Cancer Network (NCCN) guidelines (Cassinelli et al., 2022), but its efficacy as a monotherapy for LPS is limited. Phase II research revealed that pazopanib treatment resulted in a 12-week PFR of 68.3%, and the mPFS for DDLPS patients was 6.24 months (Samuels et al., 2017). A German phase II trial compared the efficacy of pazopanib combined with gemcitabine versus pazopanib alone in treating refractory LPS/LMS patients, noting an increase in toxicity with the combination treatment, which was manageable; however, phase III trials are needed to confirm its efficacy (Schmoll et al., 2021). Another phase II study evaluating preoperative pazopanib in high-risk STS patients reported no benefit (Ronellenfitsch et al., 2019). The SARC024 study indicated that regorafenib has poor efficacy in patients with advanced LPS (Riedel et al., 2020).
3.3.5 Additional potential targets
PARP-1 has emerged as a new therapeutic target for treating LPS (Bertucci et al., 2019). The TOMAS2 study from Italy revealed that the combination of trabectedin and the PARP inhibitor olaparib is effective in the treatment of LPS/LMS (D’Ambrosio et al., 2023). XPO1 represents another potential therapeutic avenue (Gounder et al., 2016), with selinexor demonstrating enhanced tumor responses in retroperitoneal DDLPS-PDXs (Thirasastr and Somaiah, 2022). The SEAL study revealed that the median PFS for advanced DDLPS patients treated with selinexor as second-line therapy was 2.8 months and that CALB1 could serve as a predictive biomarker (Gounder et al., 2022b). Selinexor treatment can reduce the pain rate in late-stage DDLPS patients, with a slower deterioration in quality of life (Gounder et al., 2021). Future endeavors should continue multidisciplinary research to explore novel drug targets and individualized treatment approaches.
3.4 Immunotherapy
Multiple clinical trials have explored immunotherapies for STSs, including ICIs, tumor vaccines, immune modulators, and TCR-T-cell therapy. Although STSs are considered “immunologically inert or cold” tumors, recent biomarker studies have shown significant immunoheterogeneity among different subtypes (Moreno tellez et al., 2022; Zhu et al., 2020). Biomarker-driven and tissue subtype-customized immunotherapy holds promise for improving the efficacy of immunotherapy (Roulleaux dugage et al., 2021). Immunotherapy combined with other treatment modalities, such as chemotherapy and radiotherapy, can transform “cold” tumors into “hot” tumors (Rytlewski et al., 2021). Efficacy biomarkers such as TLSs, PD-L1 expression, and the TMB stratify patients to optimize efficacy, design improved clinical trials, and potentially enhance the effectiveness of immunotherapy (Nakata et al., 2021).
3.4.1 Monotherapy immunotherapy
Monotherapy with ICIs has not yet demonstrated definitive clinical benefits, but pembrolizumab has been shown to have antitumor effects in DDLPS-PDX models (Choi et al., 2020). In SARC028, the DCR for advanced STS patients was 18%, with an ORR of 40% for UPS and 20% for LPS (Tawbi et al., 2017; Burgess et al., 2019). In the Alliance A091401 trial, the overall response rate (ORR) of nivolumab monotherapy in metastatic STS patients was merely 5%.
3.4.2 Combination immunotherapy
Immunotherapy is continuously evolving in the field of LPS, with efforts focused on genomic analysis and research into the tumor immune microenvironment to identify additional combination treatment strategies, aiming to improve the effectiveness of immunotherapy in LPS patients.
3.4.2.1 Immunotherapy combined with chemotherapy
In patients with STS, the ORR of combination therapy with pembrolizumab and doxorubicin was 36.7%, with an mPFS and OS of 5.7 months and 17 months, respectively (Livingston et al., 2021). Among DDLPS patients, 1 patient achieved a complete response (CR), 1 patient achieved a partial response (PR), and 2 patients had stable disease (SD). In patients with L-type sarcoma treated with avapritinib combined with trabectedin, among 11 LPS patients, 7 patients achieved the best response of stable disease (SD), and 1 patient achieved disease stability for over 2 years (Wagner et al., 2022).
3.4.2.2 ICIs combined with antiangiogenic targeted therapy
Previous studies have shown that low-grade sarcomas typically exhibit a weak immune response and that antiangiogenic drugs can convert the immune microenvironment from “cold” to “hot,” increasing the sensitivity of the immune microenvironment to immunotherapy (De vita et al., 2016). J. Wu et al.’s retrospective study (Wu et al., 2023) investigated the treatment of L-type sarcomas with carfilzomib in combination with anlotinib and aidiublin. The ORR was 19.4%, and the DCR was 72.2%. Among nonsurgical patients, the mPFS values for LPS and LMS were 5.5 months and 6.2 months, respectively. Research by Zhou et al. revealed the satisfactory efficacy of pembrolizumab combined with anlotinib and paclitaxel in treating STS, with hematologic toxicity associated with paclitaxel being the primary adverse effect (Zhou et al., 2023).
3.4.2.3 Immunotherapy combined with small molecule inhibitors targeting epigenetics
Various subtypes of STSs exhibit defects in DNA damage repair and abnormalities in epigenetic regulation. Although epigenetic drugs can stimulate the immune system, increasing the immunogenicity of tumors, they may still suppress immune responses in the absence of immune checkpoint inhibitors (Keenan et al., 2019; Nacev et al., 2020). Recent research (Starzer et al., 2021) suggests that the DNA methylation characteristics of tumors may serve as markers for the response to PD-1 ICI therapy in sarcomas. Que et al. reported HDAC gene amplification in patients with LPS, and the HDAC inhibitor chidamide increased PD-L1 expression, facilitating tumor regression (Que et al., 2021). Phase II trials have demonstrated that the combination of chidamide and trastuzumab is highly effective in treating STSs and has good tolerability, indicating promising therapeutic potential (Zhang et al., 2023). Ongoing clinical trials of tazemetostat combined with durvalumab for the treatment of STSs (NCT04705818) may offer new hope for patients.
3.4.2.4 Dual immunotherapy
In a phase II trial conducted by MD Anderson (NCT02815995), the efficacy of the PD-L1 monoclonal antibody durvalumab and the CTLA-4 monoclonal antibody tremelimumab in refractory advanced STSs was evaluated (Somaiah et al., 2022). The ORR was 12%, with a 12-week PFS rate of 49%, a median PFS of 2.8 months, and an mOS of 21.6 months. No effects were observed for LPS or the other subtypes.
3.4.3 Others
In addition to ICIs, immunotherapy involving immune cell therapy is also utilized in patients with STSs. CAR-T-cell therapy and TCR-T-cell therapy are still in their early stages and face various challenges. In the SPEARHEAD-1 study, afami-cel was used to treat patients with MRLPS or SS, resulting in 2 cases of CR, 8 cases of PR, and 11 cases of SD out of 25 patients. NY-ESO-1 is one of the most immunogenic TAAs, with a positivity rate of 89%–100% in MRLPS. Phase I/Ib studies of NY-ESO-1 TCR/IL-15 NK cells are currently underway. In recent years, the CMB305 vaccine has also been utilized in STS research, enhancing immune responses to the NY-ESO-1 antigen. A phase II trial (Chawla et al., 2022) evaluating CMB305 in combination with atezolizumab for MLS/SS patients revealed no significant extension of PFS or OS, but some patients exhibited anti-NY-ESO-1 immune responses, with seemingly favorable radiographic responses. IFN-γ alters the TME, increases antigen presentation, reduces T-cell exhaustion, and can convert tumors into “hot” tumors, potentially synergizing with PD-1 antibodies (Zhang et al., 2019). In an IB/II trial conducted by the University of Iowa (Monga et al., 2021), TVEC combined with neoadjuvant radiotherapy for STSs resulted in SD in 66.7% of patients, PR in 1 MLS patient, death due to PD in 2 patients, and pCR in 7 patients (24%), with 2-year PFS and OS rates reaching 57% and 88%, respectively, without postoperative local recurrence.
Emerging therapeutic modalities such as antibody-drug conjugates (ADCs) and oncolytic viruses demonstrate promising therapeutic potential. Initially deployed in hematologic malignancies, ADC-based therapies achieved their first breakthrough in solid tumors with HER2-positive breast cancer. As of 2024, no ADC clinical trials targeting liposarcoma have received regulatory approval (Xi et al., 2024). The leucine-rich repeat-containing protein 15 (LRRC15), overexpressed in sarcoma-associated cancer-associated fibroblasts, has emerged as a compelling anticancer target. LRRC15-directed ADCs may substantially improve clinical outcomes for sarcoma patients (Ray et al., 2022). Preclinical evidence indicates that BB-1701—a novel eribulin-based ADC engineered for HER2 targeting—represents a potential therapeutic advancement for liposarcoma management (Wang et al., 2024). Talimogene laherparepvec (T-VEC), an oncolytic herpes simplex virus type 1, holds the distinction of being the first oncolytic virus approved by the US FDA and European Medicines Agency (Greig, 2016). In a phase IB/II trial involving 30 patients with locally advanced soft tissue sarcoma (STS), preoperative intratumoral T-VEC combined with concurrent external beam radiotherapy (EBRT) demonstrated no treatment-related herpes infections. The 2-year progression-free survival (PFS) and overall survival (OS) rates were 57% and 88%, respectively (Monga et al., 2021). A phase II trial enrolling 20 patients with locally advanced or metastatic sarcoma evaluated T-VEC plus pembrolizumab, yielding an overall objective response rate (ORR) of 35%, with 20% grade 3 treatment-related adverse events (TRAEs) and no grade 4 TRAEs (Kelly et al., 2020). Another phase II study of 39 pretreated advanced sarcoma patients investigated the TNT regimen (T-VEC + trabectedin + nivolumab), reporting an ORR of 7.7%, disease control rate (DCR) of 84.6%, median PFS of 7.8 months, and median OS of 19.3 months (Chawla et al., 2023). Novel combinatorial therapeutic strategies incorporating oncolytic viruses remain under active investigation.
3.5 Radiotherapy
Postoperative LPS is prone to recurrence, and radiotherapy can improve local control rates. Therefore, radiotherapy is strongly recommended for patients with high-risk localized recurrence profiles, while therapeutic de-escalation through radiation omission represents a viable strategy for those with low recurrence probability (Salerno, 2022). For most patients, preoperative delivery of radiation therapy is preferred. In patients initially thought to be at low risk for local recurrence and found to have unexpected adverse pathologic features at resection, postoperative radiation therapy is indicated. In select patients who received preoperative ra-diation and have close or positive margins, postoperative boost may be considered (Salerno, 2022).
MLS is sensitive to radiotherapy and is an important target for radiotherapy. Multiple studies have shown that neoadjuvant radiotherapy combined with surgical resection can achieve a 5-year local control rate of 96%–98% (Guadagnolo et al., 2008; Chung et al., 2009; Moreau et al., 2012). Research (Chen et al., 2021) has shown that the interaction between FUS-CHOP and chromatin remodeling complexes regulates sarcoma cell proliferation, explaining the sensitivity of MLS to radiotherapy. Phase II/III trials (Bonvalot et al., 2019) evaluating neoadjuvant radiotherapy combined with NBTXR3 versus radiotherapy alone in advanced STSs have shown a significant increase in the R0 resection rate (81% vs. 66%; P = 0.042). The standard neoadjuvant radiation therapy dose for MLS is 50 Gy/25 fractions. Low-dose radiation therapy may reduce the complications associated with preoperative radiation therapy while maintaining disease control. A phase II trial (Lansu et al., 2021a) revealed that low-dose preoperative radiation therapy (36 Gy) had comparable efficacy in nonmetastatic MLS and could reduce complications. Another phase II trial (Lansu et al., 2021b) showed that moderate-dose preoperative radiation therapy (36 Gy) could improve the resectability of MLS while preserving clear margins and function.
Radiation therapy is also under investigation for RLPS. The STRASS phase III study (Lam et al., 2021) demonstrated that neoadjuvant radiation therapy reduced the risk of local recurrence in patients with resectable RLPS, with a 3-year ARFS rate of 71.6%. The TARPSWG study (Haas et al., 2019) enrolled 607 RLPS patients, and univariate analysis revealed that perioperative radiation therapy had local control advantages in all three cohorts, but no survival benefit was confirmed after adjustment. An analysis of 2082 RLPS patients from the American Cancer Database revealed that neoadjuvant radiation therapy conferred survival benefits, with a mOS of 129.2 months vs. 84.3 months, with more pronounced effects in those with involvement of adjacent organs. Multidisciplinary discussions are recommended to formulate initial treatment plans, and the selective use of RT may be considered for those at high risk of local recurrence (Istl and Gronchi, 2022; Callegaro et al., 2023).
In the radiotherapeutic management of liposarcoma, emerging modalities continue to undergo rigorous investigation. A clinical study validated the safety profile of proton and carbon ion particle therapy for dedifferentiated liposarcoma (DDLPS), demonstrating favorable overall survival (OS) and local control (LC) outcomes (Kubota et al., 2024). A retrospective analysis of stereotactic body radiotherapy (SBRT) in sarcoma pulmonary metastases revealed prolonged disease-free intervals among oligometastatic patients, with a median survival duration of 40.7 months (Lee et al., 2023). Evidence indicates stereotactic ablative radiotherapy (SABR) serves as a viable local control strategy for limited pulmonary oligometastatic disease, exhibiting minimal toxicity (Baumann et al., 2020; Baumann et al., 2016). A multicenter trial evaluating SABR in oligometastatic soft tissue sarcoma (STS) established its therapeutic efficacy and safety profile, with 20% of patients maintaining progression-free status at 2-year follow-up (Franceschini et al., 2024).
3.6 Alternative local therapeutic approaches
Emerging locoregional therapeutic modalities including percutaneous radiofrequency ablation (RFA), cryoablation, and high-intensity focused ultrasound (HIFU) are being increasingly utilized in liposarcoma management. A clinical case demonstrated sustained tumor-free survival exceeding 24 months following RFA treatment in a patient with third recurrence of retroperitoneal liposarcoma involving the left psoas muscle (Keil et al., 2008). Koichiro et al. conducted a retrospective multicenter analysis of percutaneous RFA in 52 recurrent bone and soft tissue sarcoma patients, reporting a 1-year overall survival (OS) rate of 73.4% with minimal major complication rate (0.9%), confirming RFA as a safe and effective option for advanced sarcomas (Yamakado et al., 2014).
A retrospective study of percutaneous cryoablation in 141 adults with recurrent/metastatic soft tissue sarcomas documented 217 ablation procedures achieving adequate ice-ball coverage in 82% (204/250) of lesions. The cohort exhibited a 2% complication rate (4/217) with favorable survival outcomes: 89% 1-year OS and 80% 2-year OS (Pal et al., 2024). Another real-world analysis of 67 recurrent/metastatic STS patients undergoing 189 cryoablation procedures for 104 lesions demonstrated an objective response rate (ORR) of 65.38% and disease control rate (DCR) of 86.54%, with survival analysis indicating prognostic improvement (Wu et al., 2021).
In HIFU applications, a study treating 29 lesions in 22 solid tumor patients achieved near-complete MRI-confirmed ablation in liposarcoma cases with symptomatic relief (Orgera et al., 2011). Yu et al. reported 51.8% ORR and 85.2% local control rate in 27 patients with locally unresectable sarcomas undergoing HIFU, with no severe treatment-related complications observed (Yu et al., 2015).
3.7 Multidisciplinary discussions
STSs are diverse and rare, and nonspecialist doctors should refrain from diagnosing and treating them. The MDT diagnostic and treatment model is essential in the management of STSs (Kawai et al., 2022).
4 Prognosis
4.1 Prognostic factor analysis of primary nonmetastatic extremity or trunk liposarcoma
For patients with nonmetastatic extremity or trunk liposarcoma, studies have shown that tumor size and subtype are independently associated with distant metastasis-free survival (DSD) and disease-specific survival (DR), whereas size, subtype, and R1 resection are independently associated with local recurrence (LR) (Bartlett et al., 2021). These findings suggest that the patterns, risks, and timing of postoperative recurrence vary by subtype, which can guide the development of targeted treatment measures for patients.
4.2 Prognostic factor analysis of patients with retroperitoneal liposarcoma
Large-scale population-based international cohort studies have consistently identified advanced age as an independent prognostic factor for overall survival (OS) and cancer-specific survival (CSS) in patients with retroperitoneal liposarcoma (Li et al., 2022; Singer et al., 2003). Gender-specific analysis reveals males exhibit inferior survival outcomes compared to females following primary resection of RLPS, particularly in subsets with low-grade histology or undergoing non-radical resection (R1/R2 resections) (Ren et al., 2024). An Asian multicenter cohort study of 211 patients demonstrated independent associations between American Society of Anesthesiologists (ASA) physical status classification, Clavien-Dindo complication grading system, and long-term OS (Zhuang et al., 2021).
Tumor-related characteristics significantly impact prognostic outcomes in retroperitoneal liposarcoma (RLS) patients. Retrospective cohort analysis demonstrates inferior disease-free survival (DFS) in dedifferentiated histology compared to well-differentiated subtypes (Osuna-soto et al., 2021). Histologic subtype emerged as an independent predictor of progression-free survival (PFS) (Singer et al., 2003; Zhuang et al., 2021). The Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) grading system and myogenic differentiation status constitute critical prognostic determinants (Gronchi et al., 2015). Additionally, tumor anatomical location and presence of necrosis may serve as independent pathologic prognostic indicators (Sun et al., 2021). Tumor rupture and major postoperative complications (Dindo-Clavien grade ≥ III) adversely affect overall survival (OS) (Brehat et al., 2023). Patients developing multifocal recurrence exhibit particularly dismal clinical outcomes (Deng et al., 2023a).
Surgical margin characteristics significantly influence oncologic outcomes in retroperitoneal liposarcoma (RLS). The presence of dedifferentiated (DD) components at resection margins correlates with diminished local recurrence-free survival (LRFS) (Dehner et al., 2021). A comparative effectiveness study demonstrated that total (ipsilateral) retroperitoneal lipectomy (TRL), when contrasted with conventional complete resection (CR), confers significant improvements in both recurrence-free survival (RFS) and overall survival (OS) for primary RPLS patients (Gao et al., 2024).
Recurrence patterns critically determine clinical prognosis in RLS management. Patients exhibiting DR patterns demonstrate more favorable survival trajectories compared to those with early multifocal recurrence (Deng et al., 2023b). Homsy. P et al. analyzed 107 RPL patients and reported that 72% experienced LR, whereas 15% experienced DR, indicating more local recurrence and fewer metastases (Homsy et al., 2020). After R0/R1 resection, histological type and grade were important predictors of DSS, with multifocal LR having a poorer prognosis and a higher DR rate with high-grade histology. Improta. L et al. studied 109 RPL cases, with a 5-year OS rate of 67%, a DFS rate of 53.2%, an LR rate of 25.7%, and a DM rate of 12.1%, with lung metastasis being the most common. Patients with complications had better DFS and OS, and HOI-3 was an independent risk factor for DM, OS, and DFS (Improta et al., 2023). One study reported a 6-year DFS rate of 19.2% and an OS rate of 54.1% for RPS-LR1 patients, with recurrence patterns associated with histological subtypes, and the CCI for the second LR of LPS was the highest (60.2%–70.9%). Column charts predicting DFS and OS were established, incorporating multiple factors (Raut et al., 2019). The TARPSWG study analyzed RPS-R2 patients and reported a 70.5% incidence of second recurrence, with an LR accounting for 80.75%, predominantly LPS (Van houdt et al., 2020). Singaporean scholars proposed a five-gene prognostic model for retroperitoneal DDLPS, which better predicted overall survival than did clinical factors (Shannon et al., 2021).
4.3 Potential prognostic significance of immune-related molecular markers and tumor-infiltrating immune cells in LPS
Miyake M et al. reported that PD-L1 expression was higher in retroperitoneal DDLPS and retroperitoneal LMS than in other sarcomas (Miyake et al., 2020). Serum LDH levels were moderately positively correlated with PD-L1 and PD-L2 expression. Higher PD-1 expression was associated with an increased risk of recurrence; High expression of Ki-67 and stage IIIB disease were independent predictors of RFS and DSS. The Ki67 proliferation index has been established as an independent prognostic factor for recurrence, metastasis, and overall survival (OS) in retroperitoneal liposarcoma (RLS) patients undergoing complete resection (Gao et al., 2023). Schroeder BA et al. reported that high TCR clonality combined with a low T-cell fraction predicted a lower 3-year OS rate, that CD4+ T cells were associated with better outcomes, and that CD14+ monocytes were associated with poorer prognosis (Schroeder et al., 2021). In recent years, research on tertiary lymphoid structures (TLSs) in patients with STSs has increased, with more TLS patients showing longer OS and PFS, associated with increased expression of the TNFRSF14 and DUSP9 genes, and better immunotherapy outcomes (Xiang-Xu et al., 2023). Inflammatory biomarkers such as the NLR or PLR fail to accurately predict survival (Schwartz et al., 2020), with tumor-related factors remaining the best predictors. Kim KM et al. reported that baseline inflammatory markers such as IL-6 were associated with early recurrence of STSs (Van der laan et al., 2023). The nuclear expression of 4Rα and IL13Rα1 is associated with shortened OS and RFS (Kim et al., 2021). Next-generation sequencing (NGS)-based detection of circulating cell-free DNA (cfDNA) in sarcoma patients facilitates diagnostic refinement and longitudinal disease monitoring (Mc connell et al., 2020). Circulating tumor DNA (ctDNA) demonstrates clinical utility in tracking minimal residual disease (MRD) and early recurrence patterns (Braig et al., 2019). Thrombospondin-2 (Tsp2) encoded by THBS2 serves as an independent predictor of disease-free survival (DFS) and recurrence-free survival (RFS) in RLS cohorts (Xu et al., 2022). Fibroblast growth factor receptor substrate 2 (FRS2) exhibits high positivity rates in primary RPLS tumor specimens, demonstrating significant correlation with recurrence dynamics and survival outcomes (Chen and Miao, 2023).
5 Summary and prospects
Recent research has revealed significant findings and trends in the treatment and prognosis of liposarcoma. Various treatment modalities can be seen in Figure 2. Overall, liposarcoma treatment and prognosis are influenced by various factors, including the tumor type, grade, histological characteristics, and the immune environment. Surgical resection remains the primary treatment modality for tumors such as retroperitoneal liposarcoma (RPL) and retroperitoneal sarcoma (RPS), although the risk of local recurrence after surgery is high. Advances in medical technology are expected to enhance minimally invasive surgery and precision radiotherapy, reducing treatment-related complications and side effects and thus improving patient quality of life. Adjuvant radiotherapy before and after surgery, along with novel immunotherapy, may become integral parts of treatment strategies. Preoperative radiotherapy has shown efficacy in lowering the risk of local recurrence, but the effectiveness of perioperative radiotherapy remains uncertain. Immunotherapy has exhibited potential efficacy in some studies, particularly for patients with high PD-L1 and PD-L2 expression. Future research may delve deeper into the mechanisms and efficacy of immunotherapy and identify more precise prognostic markers to personalize treatment regimens, ultimately increasing patient survival rates and quality of life.
In addition to advancements in treatment, prognosis evaluation has become more precise. By integrating various factors, such as tumor characteristics, patient factors, and treatment response, we can establish more reliable prognostic models to assist physicians and patients in making informed treatment decisions. Further research in patients with retroperitoneal liposarcoma (RPL) and retroperitoneal sarcoma (RPS) suggests that peripheral blood inflammatory markers and specific biomarkers in tumor tissue may aid in predicting early recurrence and survival rates for postoperative patients. Additionally, with a deeper understanding of tumor immunology and genomics, coupled with the ongoing development of immunotherapy and targeted therapy, personalized treatment, including targeted therapy and immunotherapy tailored to specific tumor subtypes, is poised to become a future trend. Our approach to treating liposarcoma will also become more personalized and precise. Genomic and biomarker studies will further our understanding of tumor development mechanisms and prognostic factors, providing a stronger scientific basis for personalized treatment. Furthermore, ongoing clinical trials will present opportunities for the development of novel treatment modalities and drugs, offering patients more options and improving treatment success and survival rates.
In the future, comprehensive assessment and personalized treatment plans based on multidisciplinary teams will be key to improving the prognosis of patients with liposarcoma. Additionally, conducting more large-scale clinical trials and molecular biology research is expected to provide deeper insights and breakthroughs in the treatment and prognosis of this field. Strengthening multicenter clinical research and data sharing is also a future direction to promote a comprehensive understanding of the treatment and prognostic factors of liposarcoma, accelerate the clinical translation of new treatment methods, and continuously improve treatment outcomes and survival rates for patients. With advances in science and technology and further research, we hope to find more effective treatment methods and improve the quality of life and survival of patients with liposarcoma. We anticipate a brighter future for the treatment and prognosis of liposarcoma.
Overall, significant progress has been made in the treatment and prognosis research of liposarcoma, including the exploration and application of various treatment methods, such as surgery, radiotherapy, and immunotherapy, as well as the discovery and validation of new prognostic factors. However, many challenges and unknown factors remain. The application of novel treatment methods such as personalized treatment, immunotherapy, and targeted therapy has brought new hope for patients, but further research and clinical validation are needed to determine how to select and combine these treatment options better.
In the future, we expect further in-depth research into the pathogenesis, biological characteristics, and potential of targeted therapies for liposarcoma. With the continuous development of technology, the application of high-throughput technologies such as genomics, transcriptomics, and proteomics will provide us with more comprehensive and precise tumor classification and personalized treatment strategies. Moreover, the accumulation of clinical trials and practical experience will lead to more information on the effectiveness and safety of various treatment options, helping to guide clinical practice and improve patient prognosis. In the future, with the continuous advancement of technology and research methods, we can expect more accurate diagnostic methods, more effective treatment strategies, and more accurate prognosis evaluation models to emerge. Ultimately, we hope to provide liposarcoma patients with more effective and safer treatment methods, improving their quality of life and survival rate through various efforts.
Author contributions
HL: Investigation, Writing – original draft. XiW: Formal Analysis, Writing – original draft. XiaW: Writing – review and editing. FQ: Writing – review and editing. BZ: Formal Analysis, Funding acquisition, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1529755/full#supplementary-material
References
Abbas Manji, G., Singer, S., Koff, A., and Schwartz, G. K. (2015). Application of molecular biology to individualize therapy for patients with liposarcoma. Am. Soc. Clin. Oncol. Educ. Book, 213–218. doi:10.14694/EdBook_AM.2015.35.213
Abdul Razak, A. R., Bauer, S., Suarez, C., Lin, C. C., Quek, R., Hütter-Krönke, M. L., et al. (2022). Co-targeting of MDM2 and CDK4/6 with siremadlin and ribociclib for the treatment of patients with well-differentiated or dedifferentiated liposarcoma: results from a proof-of-concept, phase ib study. Clin. Cancer Res. 28 (6), 1087–1097. doi:10.1158/1078-0432.CCR-21-1291
Aleixo, P. B., Hartmann, A. A., Menezes, I. C., Meurer, R. T., and Oliveira, A. M. (2009). Can MDM2 and CDK4 make the diagnosis of well differentiated/dedifferentiated liposarcoma? An immunohistochemical study on 129 soft tissue tumours. J. Clin. Pathol. 62 (12), 1127–1135. doi:10.1136/jcp.2009.070201
Angeles, C. V., Velez, A., Rios, J., Laxa, B., Shum, D., Ruiz, P. D., et al. (2022). A high-content screen for C/EBPα expression identifies novel therapeutic agents in dedifferentiated liposarcoma. Clin. Cancer Res. 28 (1), 175–186. doi:10.1158/1078-0432.CCR-19-2486
Assi, T., Kattan, J., El Rassy, E., Honore, C., Dumont, S., Mir, O., et al. (2019). A comprehensive review of the current evidence for trabectedin in advanced myxoid liposarcoma. Cancer Treat. Rev. 72, 37–44. doi:10.1016/j.ctrv.2018.11.003
Assi, T., Kattan, J., Rassy, E., Nassereddine, H., Farhat, F., Honore, C., et al. (2020). Targeting CDK4 (cyclin-dependent kinase) amplification in liposarcoma: a comprehensive review. Crit. Rev. Oncol. Hematol. 153, 103029. doi:10.1016/j.critrevonc.2020.103029
Baneth, V., Raica, M., and CîMPEAN, A. M. (2005). Assessment of angiogenesis in soft-tissue tumors. Rom. J. Morphol. Embryol. 46 (4), 323–327.
Barretina, J., Taylor, B. S., Banerji, S., Ramos, A. H., Lagos-Quintana, M., Decarolis, P. L., et al. (2010). Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat. Genet. 42 (8), 715–721. doi:10.1038/ng.619
Bartlett, E. K., Curtin, C. E., Seier, K., Qin, L. X., Hameed, M., Yoon, S. S., et al. (2021). Histologic subtype defines the risk and kinetics of recurrence and death for primary extremity/truncal liposarcoma. Ann. Surg. 273 (6), 1189–1196. doi:10.1097/SLA.0000000000003453
Bauer, T. M., Gounder, M. M., Weise, A. M., Schwartz, G. K., Carvajal, R. D., Kumar, P., et al. (2018). A phase 1 study of MDM2 inhibitor DS-3032b in patients with well/de-differentiated liposarcoma (WD/DD LPS), solid tumors (ST) and lymphomas (L). JCO 36, 11514. doi:10.1200/JCO.2018.36.15_suppl.11514
Baumann, B. C., Bernstein, K. A., Delaney, T. F., Simone, C. B., Kolker, J. D., Choy, E., et al. (2020). Multi-institutional analysis of stereotactic body radiotherapy for sarcoma pulmonary metastases: high rates of local control with favorable toxicity. J. Surg. Oncol. 122 (5), 877–883. doi:10.1002/jso.26078
Baumann, B. C., Nagda, S. N., Kolker, J. D., Levin, W. P., Weber, K. L., Berman, A. T., et al. (2016). Efficacy and safety of stereotactic body radiation therapy for the treatment of pulmonary metastases from sarcoma: a potential alternative to resection. J. Surg. Oncol. 114 (1), 65–69. doi:10.1002/jso.24268
Berthold, R., Isfort, I., Erkut, C., Heinst, L., Grünewald, I., Wardelmann, E., et al. (2022). Fusion protein-driven IGF-IR/PI3K/AKT signals deregulate Hippo pathway promoting oncogenic cooperation of YAP1 and FUS-DDIT3 in myxoid liposarcoma. Oncogenesis 11 (1), 20. doi:10.1038/s41389-022-00394-7
Bertucci, F., Finetti, P., Monneur, A., Perrot, D., Chevreau, C., Le Cesne, A., et al. (2019). PARP1 expression in soft tissue sarcomas is a poor-prognosis factor and a new potential therapeutic target. Mol. Oncol. 13 (7), 1577–1588. doi:10.1002/1878-0261.12522
Bonvalot, S., Rutkowski, P. L., Thariat, J., Carrère, S., Ducassou, A., Sunyach, M. P., et al. (2019). NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): a multicentre, phase 2-3, randomised, controlled trial. Lancet Oncol. 20 (8), 1148–1159. doi:10.1016/S1470-2045(19)30326-2
Braig, D., Becherer, C., Bickert, C., Braig, M., Claus, R., Eisenhardt, A. E., et al. (2019). Genotyping of circulating cell-free DNA enables noninvasive tumor detection in myxoid liposarcomas. Int. J. Cancer 145 (4), 1148–1161. doi:10.1002/ijc.32216
Brehat, E., Chaltiel, L., Thoulouzan, M., Carrère, N., Philis, A., Ferron, G., et al. (2023). Survival analysis and prognostic factors of retroperitoneal liposarcoma curative surgery in a single centre. Analysis of adjacent organ invasion between imaging and definitive histopathology. Surg. Oncol. 49, 101950. doi:10.1016/j.suronc.2023.101950
Burgess, M. A., Bolejack, V., Schuetze, S., Van Tine, B. A., Attia, S., Riedel, R. F., et al. (2019). Clinical activity of pembrolizumab (P) in undifferentiated pleomorphic sarcoma (UPS) and dedifferentiated/pleomorphic liposarcoma (LPS): final results of SARC028 expansion cohorts. JCO 37, 11015. doi:10.1200/JCO.2019.37.15_suppl.11015
Callegaro, D., Raut, C. P., Ajayi, T., Strauss, D., Bonvalot, S., Ng, D., et al. (2023). Preoperative radiotherapy in patients with primary retroperitoneal sarcoma: EORTC-62092 trial (STRASS) versus off-trial (STREXIT) results. Ann. Surg. 278 (1), 127–134. doi:10.1097/SLA.0000000000005492
Cassinelli, G., Pasquali, S., and Lanzi, C. (2022). Beyond targeting amplified MDM2 and CDK4 in well differentiated and dedifferentiated liposarcomas: from promise and clinical applications towards identification of progression drivers. Front. Oncol. 12, 965261. doi:10.3389/fonc.2022.965261
Chamberlain, F., Benson, C., Thway, K., Huang, P., Jones, R. L., and Gennatas, S. (2021). Pharmacotherapy for liposarcoma: current and emerging synthetic treatments. Future Oncol. 17 (20), 2659–2670. doi:10.2217/fon-2020-1092
Chawla, S. P., Tellez, W. A., Chomoyan, H., Valencia, C., Ahari, A., Omelchenko, N., et al. (2023). Activity of TNT: a phase 2 study using talimogene laherparepvec, nivolumab and trabectedin for previously treated patients with advanced sarcomas (NCT# 03886311). Front. Oncol. 13, 1116937. doi:10.3389/fonc.2023.1116937
Chawla, S. P., Van Tine, B. A., Pollack, S. M., Ganjoo, K. N., Elias, A. D., Riedel, R. F., et al. (2022). Phase II randomized study of CMB305 and atezolizumab compared with atezolizumab alone in soft-tissue sarcomas expressing NY-ESO-1. J. Clin. Oncol. 40 (12), 1291–1300. doi:10.1200/JCO.20.03452
Chen, T. W., Hsu, C. L., Hong, R. L., Lee, J. C., Chang, K., Yu, C. W., et al. (2022). A single-arm phase ib/II study of lenvatinib plus eribulin in advanced liposarcoma and leiomyosarcoma. Clin. Cancer Res. 28 (23), 5058–5065. doi:10.1158/1078-0432.CCR-22-2092
Chen, W. D., and Miao, C. L. (2023). Expression of fibroblast growth factor receptor substrate 2 (FRS2) in primary retroperitoneal liposarcoma and its clinical implications. Eur. Rev. Med. Pharmacol. Sci. 27 (13), 6273–6281. doi:10.26355/eurrev_202307_32987
Chen, M., Foster, J. P., Lock, I. C., Leisenring, N. H., Daniel, A. R., Floyd, W., et al. (2021). Radiation-induced phosphorylation of a prion-like domain regulates transformation by FUS-CHOP. Cancer Res. 81 (19), 4939–4948. doi:10.1158/0008-5472.CAN-20-1497
Chi, Y., Fang, Z., Hong, X., Yao, Y., Sun, P., Wang, G., et al. (2018). Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft-tissue sarcoma. Clin. Cancer Res. 24 (21), 5233–5238. doi:10.1158/1078-0432.CCR-17-3766
Choi, B., Lee, J. S., Kim, S. J., Hong, D., Park, J. B., and Lee, K. Y. (2020). Anti-tumor effects of anti-PD-1 antibody, pembrolizumab, in humanized NSG PDX mice xenografted with dedifferentiated liposarcoma. Cancer Lett. 478, 56–69. doi:10.1016/j.canlet.2020.02.042
Chudgar, N. P., Brennan, M. F., Tan, K. S., Munhoz, R. R., D’Angelo, S. P., Bains, M. S., et al. (2017). Is repeat pulmonary metastasectomy indicated for soft tissue sarcoma? Ann. Thorac. Surg. 104 (6), 1837–1845. doi:10.1016/j.athoracsur.2017.07.024
Chung, P. W., Deheshi, B. M., Ferguson, P. C., Wunder, J. S., Griffin, A. M., Catton, C. N., et al. (2009). Radiosensitivity translates into excellent local control in extremity myxoid liposarcoma: a comparison with other soft tissue sarcomas. Cancer 115 (14), 3254–3261. doi:10.1002/cncr.24375
Ciongariu, A. M., Dumitru, A. V., CîRSTOIU, C., Crețu, B., Sajin, M., Țăpoi, D. A., et al. (2023). The conundrum of dedifferentiation in a liposarcoma at a peculiar location: a case report and literature review. Med. Kaunas. 59 (5), 967. doi:10.3390/medicina59050967
Cissé, M. Y., Pyrdziak, S., Firmin, N., Gayte, L., Heuillet, M., Bellvert, F., et al. (2020). Targeting MDM2-dependent serine metabolism as a therapeutic strategy for liposarcoma. Sci. Transl. Med. 12 (547), eaay2163. doi:10.1126/scitranslmed.aay2163
Conyers, R., Young, S., and Thomas, D. M. (2011). Liposarcoma: molecular genetics and therapeutics. Sarcoma 2011, 483154. doi:10.1155/2011/483154
Crago, A. M., and Dickson, M. A. (2016). Liposarcoma: multimodality management and future targeted therapies. Surg. Oncol. Clin. N. Am. 25 (4), 761–773. doi:10.1016/j.soc.2016.05.007
D’Ambrosio, L., Merlini, A., Brunello, A., Ferraresi, V., Paioli, A., Vincenzi, B., et al. (2023). LBA91 TOMAS2: a randomized phase II study from the Italian Sarcoma Group (ISG) of trabectedin plus olaparib (T+ O) or trabectedin (T) in advanced, metastatic, or unresectable soft tissue sarcomas (STS) after failure of standard treatments. Ann. Oncol. 34, S1332. doi:10.1016/j.annonc.2023.10.093
D’Ambrosio, L., Van Houdt, W., Stelmes, J. J., and Gronchi, A. (2022). First and further-line multidisciplinary treatment of retroperitoneal sarcomas. Curr. Opin. Oncol. 34 (4), 328–334. doi:10.1097/CCO.0000000000000851
Dehner, C. A., Hagemann, I. S., and Chrisinger, J. S. A. (2021). Retroperitoneal dedifferentiated liposarcoma. Am. J. Clin. Pathol. 156 (5), 920–925. doi:10.1093/ajcp/aqab051
Delisle, M., Gyorki, D., Bonvalot, S., and Nessim, C. (2022). Landmark series: a review of landmark studies in the treatment of primary localized retroperitoneal sarcoma. Ann. Surg. Oncol. 29 (12), 7297–7311. doi:10.1245/s10434-022-12517-w
Deng, H., Gao, J., Xu, X., Liu, G., Song, L., Pan, Y., et al. (2023b). Predictors and outcomes of recurrent retroperitoneal liposarcoma: new insights into its recurrence patterns. BMC Cancer 23 (1), 1076. doi:10.1186/s12885-023-11586-8
Deng, H., Xu, X., Gao, J., Huang, J., Liu, G., Song, L., et al. (2023a). Predictors and outcomes of recurrent retroperitoneal liposarcoma with multiple tumors. Front. Med. (Lausanne) 10, 1161494. doi:10.3389/fmed.2023.1161494
De Sande GonzáLEZ, L. M., Martin-Broto, J., Kasper, B., Blay, J. Y., and Le Cesne, A. (2020). Real-world evidence of the efficacy and tolerability of trabectedin in patients with advanced soft-tissue sarcoma. Expert Rev. Anticancer Ther. 20 (11), 957–963. doi:10.1080/14737140.2020.1822744
De Vita, A., Mercatali, L., Recine, F., Pieri, F., Riva, N., Bongiovanni, A., et al. (2016). Current classification, treatment options, and new perspectives in the management of adipocytic sarcomas. Onco Targets Ther. 9, 6233–6246. doi:10.2147/OTT.S112580
De Vita, A., Recine, F., Miserocchi, G., Pieri, F., Spadazzi, C., Cocchi, C., et al. (2021). The potential role of the extracellular matrix in the activity of trabectedin in UPS and L-sarcoma: evidences from a patient-derived primary culture case series in tridimensional and zebrafish models. J. Exp. Clin. Cancer Res. 40 (1), 165. doi:10.1186/s13046-021-01963-1
Dolatabadi, S., Jonasson, E., LindéN, M., Fereydouni, B., Bäcksten, K., Nilsson, M., et al. (2019). JAK-STAT signalling controls cancer stem cell properties including chemotherapy resistance in myxoid liposarcoma. Int. J. Cancer 145 (2), 435–449. doi:10.1002/ijc.32123
Franceschini, D., Greto, D., Dicuonzo, S., Navarria, F., Federico, M., La Vecchia, M., et al. (2024). Oligometastatic sarcoma treated with Curative intent Ablative Radiotherapy (OSCAR): a multicenter study on behalf of AIRO (Italian association of Radiotherapy and clinical Oncology). Radiother. Oncol. 191, 110078. doi:10.1016/j.radonc.2023.110078
Frapolli, R., Bello, E., Ponzo, M., Craparotta, I., Mannarino, L., Ballabio, S., et al. (2019). Combination of PPARγ agonist pioglitazone and trabectedin induce adipocyte differentiation to overcome trabectedin resistance in myxoid liposarcomas. Clin. Cancer Res. 25 (24), 7565–7575. doi:10.1158/1078-0432.CCR-19-0976
Gahvari, Z., and Parkes, A. (2020). Dedifferentiated liposarcoma: systemic therapy options. Curr. Treat. Options Oncol. 21 (2), 15. doi:10.1007/s11864-020-0705-7
Gao, H., Liu, S., Li, W., Zou, B., and Miao, C. (2024). Total retroperitoneal lipectomy improves prognosis in patients with primary retroperitoneal liposarcoma: a comparative study. Front. Oncol. 14, 1488143. doi:10.3389/fonc.2024.1488143
Gao, J., Aksoy, B. A., Dogrusoz, U., Dresdner, G., Gross, B., Sumer, S. O., et al. (2013). Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 6 (269), pl1. doi:10.1126/scisignal.2004088
Gao, X., Ding, P., Zhang, Z., Li, Y., Zhao, Q., Wang, D., et al. (2023). Analysis of recurrence and metastasis patterns and prognosis after complete resection of retroperitoneal liposarcoma. Front. Oncol. 13, 1273169. doi:10.3389/fonc.2023.1273169
Ghadimi, M. P., Al-Zaid, T., Madewell, J., Peng, T., Colombo, C., Hoffman, A., et al. (2011). Diagnosis, management, and outcome of patients with dedifferentiated liposarcoma systemic metastasis. Ann. Surg. Oncol. 18 (13), 3762–3770. doi:10.1245/s10434-011-1794-0
Gluck, W. L., Gounder, M. M., Frank, R., Eskens, F., Blay, J. Y., Cassier, P. A., et al. (2020). Phase 1 study of the MDM2 inhibitor AMG 232 in patients with advanced P53 wild-type solid tumors or multiple myeloma. Invest New Drugs 38 (3), 831–843. doi:10.1007/s10637-019-00840-1
Gounder, M. M., Razak, A. A., Somaiah, N., Chawla, S., Martin-Broto, J., Grignani, G., et al. (2022b). Selinexor in advanced, metastatic dedifferentiated liposarcoma: a multinational, randomized, double-blind, placebo-controlled trial. J. Clin. Oncol. 40 (22), 2479–2490. doi:10.1200/JCO.21.01829
Gounder, M. M., Schwartz, G. K., Jones, R. L., Chawla, S. P., Chua-Alcala, V. S., Stacchiotti, S., et al. (2022a). MANTRA: a randomized, multicenter, phase 3 study of the MDM2 inhibitor milademetan versus trabectedin in patients with de-differentiated liposarcomas. JCO 40, TPS11589. doi:10.1200/JCO.2022.40.16_suppl.TPS11589
Gounder, M. M., Zer, A., Tap, W. D., Salah, S., Dickson, M. A., Gupta, A. A., et al. (2016). Phase IB study of selinexor, a first-in-class inhibitor of nuclear export, in patients with advanced refractory bone or soft tissue sarcoma. J. Clin. Oncol. 34 (26), 3166–3174. doi:10.1200/JCO.2016.67.6346
Gounder, M., Abdul Razak, A. R., Gilligan, A. M., Leong, H., Ma, X., Somaiah, N., et al. (2021). Health-related quality of life and pain with selinexor in patients with advanced dedifferentiated liposarcoma. Future Oncol. 17 (22), 2923–2939. doi:10.2217/fon-2021-0284
Greig, S. L. (2016). Talimogene laherparepvec: first global approval. Drugs 76 (1), 147–154. doi:10.1007/s40265-015-0522-7
Gronchi, A., Collini, P., Miceli, R., Valeri, B., Renne, S. L., Dagrada, G., et al. (2015). Myogenic differentiation and histologic grading are major prognostic determinants in retroperitoneal liposarcoma. Am. J. Surg. Pathol. 39 (3), 383–393. doi:10.1097/PAS.0000000000000366
Gronchi, A., Ferrari, S., Quagliuolo, V., Broto, J. M., Pousa, A. L., Grignani, G., et al. (2017). Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 18 (6), 812–822. doi:10.1016/S1470-2045(17)30334-0
Gronchi, A., Miah, A. B., Dei Tos, A. P., Abecassis, N., Bajpai, J., Bauer, S., et al. (2021). Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up(☆). Ann. Oncol. 32 (11), 1348–1365. doi:10.1016/j.annonc.2021.07.006
Gronchi, A., Palmerini, E., Quagliuolo, V., Martin Broto, J., Lopez Pousa, A., Grignani, G., et al. (2020). Neoadjuvant chemotherapy in high-risk soft tissue sarcomas: final results of a randomized trial from Italian (ISG), Spanish (GEIS), French (FSG), and polish (PSG) sarcoma groups. J. Clin. Oncol. 38 (19), 2178–2186. doi:10.1200/JCO.19.03289
Grosso, F., D’Ambrosio, L., Zucchetti, M., Ibrahim, T., Tamberi, S., Matteo, C., et al. (2020). Pharmacokinetics, safety, and activity of trabectedin as first-line treatment in elderly patients who are affected by advanced sarcoma and are unfit to receive standard chemotherapy: a phase 2 study (TR1US study) from the Italian Sarcoma Group. Cancer 126 (21), 4726–4734. doi:10.1002/cncr.33120
Guadagnolo, B. A., Zagars, G. K., Ballo, M. T., Patel, S. R., Lewis, V. O., Benjamin, R. S., et al. (2008). Excellent local control rates and distinctive patterns of failure in myxoid liposarcoma treated with conservation surgery and radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 70 (3), 760–765. doi:10.1016/j.ijrobp.2007.07.2337
Haas, R. L. M., Bonvalot, S., Miceli, R., Strauss, D. C., Swallow, C. J., Hohenberger, P., et al. (2019). Radiotherapy for retroperitoneal liposarcoma: a report from the transatlantic retroperitoneal sarcoma working group. Cancer 125 (8), 1290–1300. doi:10.1002/cncr.31927
Haddox, C. L., and Riedel, R. F. (2020). Individualizing systemic therapy for advanced soft tissue sarcomas based on tumor histology and biology. Expert Rev. Anticancer Ther. 20 (1), 5–8. doi:10.1080/14737140.2020.1708198
Harati, K., Goertz, O., Pieper, A., Daigeler, A., Joneidi-Jafari, H., Niggemann, H., et al. (2017). Soft tissue sarcomas of the extremities: surgical margins can Be close as long as the resected tumor has No ink on it. Oncologist 22 (11), 1400–1410. doi:10.1634/theoncologist.2016-0498
Henze, J., and Bauer, S. (2013). Liposarcomas. Hematol. Oncol. Clin. North Am. 27 (5), 939–955. doi:10.1016/j.hoc.2013.07.010
Higuchi, T., Igarashi, K., Yamamoto, N., Hayashi, K., Kimura, H., Miwa, S., et al. (2022). Review: precise sarcoma patient-derived orthotopic xenograft (PDOX) mouse models enable identification of novel effective combination therapies with the cyclin-dependent kinase inhibitor palbociclib: a strategy for clinical application. Front. Oncol. 12, 957844. doi:10.3389/fonc.2022.957844
Hindi, N., and Haas, R. L. (2022). Management of synovial sarcoma and myxoid liposarcoma. Surg. Oncol. Clin. N. Am. 31 (3), 547–558. doi:10.1016/j.soc.2022.03.012
Homsy, P., Heiskanen, I., Sampo, M., Rönty, M., Tukiainen, E., and Blomqvist, C. (2020). Single centre 30-year experience in treating retroperitoneal liposarcomas. J. Surg. Oncol. 122 (6), 1163–1172. doi:10.1002/jso.26118
Illuminati, G., Ceccanei, G., Pacilè, M. A., Calio, F. G., Migliano, F., Mercurio, V., et al. (2010). Surgical outcomes for liposarcoma of the lower limbs with synchronous pulmonary metastases. J. Surg. Oncol. 102 (7), 827–831. doi:10.1002/jso.21706
Improta, L., Pasquali, S., Iadecola, S., Barisella, M., Fiore, M., Radaelli, S., et al. (2023). Organ infiltration and patient risk after multivisceral surgery for primary retroperitoneal liposarcomas. Ann. Surg. Oncol. 30 (7), 4500–4510. doi:10.1245/s10434-023-13314-9
Istl, A. C., and Gronchi, A. (2022). Neoadjuvant therapy for primary resectable retroperitoneal sarcomas-looking forward. Cancers (Basel) 14 (7), 1831. doi:10.3390/cancers14071831
Italiano, A., Toulmonde, M., Cioffi, A., Penel, N., Isambert, N., Bompas, E., et al. (2012). Advanced well-differentiated/dedifferentiated liposarcomas: role of chemotherapy and survival. Ann. Oncol. 23 (6), 1601–1607. doi:10.1093/annonc/mdr485
Jeitany, M., Prabhu, A., Dakle, P., Pathak, E., Madan, V., Kanojia, D., et al. (2021). Novel carfilzomib-based combinations as potential therapeutic strategies for liposarcomas. Cell Mol. Life Sci. 78 (4), 1837–1851. doi:10.1007/s00018-020-03620-w
Jing, W., Lan, T., Chen, H., Zhang, Z., Chen, M., Peng, R., et al. (2018). Amplification of FRS2 in atypical lipomatous tumour/well-differentiated liposarcoma and de-differentiated liposarcoma: a clinicopathological and genetic study of 146 cases. Histopathology 72 (7), 1145–1155. doi:10.1111/his.13473
Jones, R. L., Fisher, C., Al-Muderis, O., and Judson, I. R. (2005). Differential sensitivity of liposarcoma subtypes to chemotherapy. Eur. J. Cancer 41 (18), 2853–2860. doi:10.1016/j.ejca.2005.07.023
Jones, R., Sanfilippo, R., Schuetze, S., Sebio, A., Alvarez, R., Bui, N., et al. (2023). LBA89 Efficacy and safety findings from MANTRA: a global, randomized, multicenter, phase III study of the MDM2 inhibitor milademetan vs trabectedin in patients with dedifferentiated liposarcomas. Ann. Oncol. 34, S1331. doi:10.1016/j.annonc.2023.10.091
Kallen, M. E., and Hornick, J. L. (2021). The 2020 WHO classification: what’s new in soft tissue tumor pathology? Am. J. Surg. Pathol. 45 (1), e1–e23. doi:10.1097/PAS.0000000000001552
Kanojia, D., Nagata, Y., Garg, M., Lee, D. H., Sato, A., Yoshida, K., et al. (2015). Genomic landscape of liposarcoma. Oncotarget 6 (40), 42429–42444. doi:10.18632/oncotarget.6464
Kawai, A., Araki, N., Ae, K., Akiyama, T., Ozaki, T., Kawano, H., et al. (2022). Japanese Orthopaedic Association (JOA) clinical practice guidelines on the management of soft tissue tumors 2020 - secondary publication. J. Orthop. Sci. 27 (3), 533–550. doi:10.1016/j.jos.2021.11.023
Keenan, T. E., Burke, K. P., and Van Allen, E. M. (2019). Genomic correlates of response to immune checkpoint blockade. Nat. Med. 25 (3), 389–402. doi:10.1038/s41591-019-0382-x
Keil, S., Bruners, P., Brehmer, B., and Mahnken, A. H. (2008). Percutaneous radiofrequency ablation for treatment of recurrent retroperitoneal liposarcoma. Cardiovasc Interv. Radiol. 31 (Suppl. 2), S213–S216. doi:10.1007/s00270-007-9263-7
Kelly, C. M., Antonescu, C. R., Bowler, T., Munhoz, R., Chi, P., Dickson, M. A., et al. (2020). Objective response rate among patients with locally advanced or metastatic sarcoma treated with talimogene laherparepvec in combination with pembrolizumab: a phase 2 clinical trial. JAMA Oncol. 6 (3), 402–408. doi:10.1001/jamaoncol.2019.6152
Keung, E. Z., Ikoma, N., Benjamin, R., Wang, W. L., Lazar, A. J., and Feig, B. W. (2018). The clinical behavior of well differentiated liposarcoma can be extremely variable: a retrospective cohort study at a major sarcoma center. J. Surg. Oncol. 117 (8), 1799–1805. doi:10.1002/jso.25082
Kilpatrick, S. E. (2021). Dedifferentiated liposarcoma: a comprehensive historical review with proposed evidence-based guidelines regarding a diagnosis in need of further clarification. Adv. Anat. Pathol. 28 (6), 426–438. doi:10.1097/PAP.0000000000000314
Kim, C. G., Sim, N. S., Kim, J. E., Yun, K. H., Lee, Y. H., Kim, S. H., et al. (2022). Phase II clinical trial of eribulin-gemcitabine combination therapy in previously treated patients with advanced liposarcoma or leiomyosarcoma. Clin. Cancer Res. 28 (15), 3225–3234. doi:10.1158/1078-0432.CCR-22-0518
Kim, K. M., Hussein, U. K., Park, S. H., Moon, Y. J., Zhang, Z., Ahmed, A. G., et al. (2021). Expression of IL4Rα and IL13Rα1 are associated with poor prognosis of soft-tissue sarcoma of the extremities, superficial trunk, and retroperitoneum. Diagn Pathol. 16 (1), 2. doi:10.1186/s13000-020-01066-z
Kobayashi, H., Iwata, S., Wakamatsu, T., Hayakawa, K., Yonemoto, T., Wasa, J., et al. (2020). Efficacy and safety of trabectedin for patients with unresectable and relapsed soft-tissue sarcoma in Japan: a Japanese Musculoskeletal Oncology Group study. Cancer 126 (6), 1253–1263. doi:10.1002/cncr.32661
Koelsche, C., Renner, M., Hartmann, W., Brandt, R., Lehner, B., Waldburger, N., et al. (2014). TERT promoter hotspot mutations are recurrent in myxoid liposarcomas but rare in other soft tissue sarcoma entities. J. Exp. Clin. Cancer Res. 33 (1), 33. doi:10.1186/1756-9966-33-33
Kubota, H., Demizu, Y., Iwashita, K., Fukumitsu, N., Takahashi, D., Park, S., et al. (2024). Definitive particle therapy using protons or carbon ions for dedifferentiated liposarcoma. Clin. Transl. Radiat. Oncol. 49, 100864. doi:10.1016/j.ctro.2024.100864
KüNSTLINGER, H., Fassunke, J., Schildhaus, H. U., Brors, B., Heydt, C., Ihle, M. A., et al. (2015). FGFR2 is overexpressed in myxoid liposarcoma and inhibition of FGFR signaling impairs tumor growth in vitro. Oncotarget 6 (24), 20215–20230. doi:10.18632/oncotarget.4046
Lam, M. B., Baldini, E. H., Reijers, S. J. M., Haas, R. L., and DeLaney, T. F. (2021). Role of radiation therapy for newly diagnosed retroperitoneal sarcoma. Curr. Treat. Options Oncol. 22 (9), 75. doi:10.1007/s11864-021-00877-6
Langmans, C., Cornillie, J., Van Cann, T., Wozniak, A., Hompes, D., Sciot, R., et al. (2019). Retrospective analysis of patients with advanced liposarcoma in a tertiary referral center. Oncol. Res. Treat. 42 (7-8), 396–404. doi:10.1159/000500608
Lansu, J., BovéE, J., Braam, P., van Boven, H., Flucke, U., Bonenkamp, J. J., et al. (2021a). Dose reduction of preoperative radiotherapy in myxoid liposarcoma: a nonrandomized controlled trial. JAMA Oncol. 7 (1), e205865. doi:10.1001/jamaoncol.2020.5865
Lansu, J., Braam, P. M., Van Werkhoven, E., Scholten, A. N., Schrage, Y., van Houdt, W. J., et al. (2021b). A moderate dose of preoperative radiotherapy may improve resectability in myxoid liposarcoma. Eur. J. Surg. Oncol. 47 (10), 2633–2639. doi:10.1016/j.ejso.2021.06.020
Le Cesne, A., Blay, J. Y., Cupissol, D., Italiano, A., Delcambre, C., Penel, N., et al. (2021). A randomized phase III trial comparing trabectedin to best supportive care in patients with pre-treated soft tissue sarcoma: T-SAR, a French Sarcoma Group trial. Ann. Oncol. 32 (8), 1034–1044. doi:10.1016/j.annonc.2021.04.014
Lee, A. T. J., Thway, K., Huang, P. H., and Jones, R. L. (2018). Clinical and molecular spectrum of liposarcoma. J. Clin. Oncol. 36 (2), 151–159. doi:10.1200/JCO.2017.74.9598
Lee, T. H., Kim, H. J., Kim, J. H., Kim, M. S., Jang, W. I., Kim, E., et al. (2023). Treatment outcomes of stereotactic body radiation therapy for pulmonary metastasis from sarcoma: a multicenter, retrospective study. Radiat. Oncol. 18 (1), 68. doi:10.1186/s13014-023-02255-y
Li, Z. K., Liu, J., Deng, Y. T., and Jiang, Y. (2021). Efficacy and safety of anlotinib in patients with unresectable or metastatic well-differentiated/dedifferentiated liposarcoma: a single-center retrospective study. Anticancer Drugs 32 (2), 210–214. doi:10.1097/CAD.0000000000001023
Libbrecht, S., Van Dorpe, J., and Creytens, D. (2021). The rapidly expanding group of RB1-deleted soft tissue tumors: an updated review. Diagn. (Basel) 11 (3), 430. doi:10.3390/diagnostics11030430
Livingston, M. B., Jagosky, M. H., Robinson, M. M., Ahrens, W. A., Benbow, J. H., Farhangfar, C. J., et al. (2021). Phase II study of pembrolizumab in combination with doxorubicin in metastatic and unresectable soft-tissue sarcoma. Clin. Cancer Res. 27 (23), 6424–6431. doi:10.1158/1078-0432.CCR-21-2001
Li, Y., Wu, G., Zhang, Y., Yang, W., Wang, X., Duan, L., et al. (2022). Development and validation of a prognostic model to predict the prognosis of patients with retroperitoneal liposarcoma: a large international population-based cohort study. Front. Oncol. 12, 857827. doi:10.3389/fonc.2022.857827
Lorusso, P., Yamamoto, N., Patel, M. R., Laurie, S. A., Bauer, T. M., Geng, J., et al. (2023). The MDM2-p53 antagonist brigimadlin (BI 907828) in patients with advanced or metastatic solid tumors: results of a phase ia, first-in-human, dose-escalation study. Cancer Discov. 13 (8), 1802–1813. doi:10.1158/2159-8290.CD-23-0153
Marudanayagam, R., Sandhu, B., Perera, M. T., Bramhall, S. R., Mayer, D., Buckels, J. A. C., et al. (2011). Liver resection for metastatic soft tissue sarcoma: an analysis of prognostic factors. Eur. J. Surg. Oncol. 37 (1), 87–92. doi:10.1016/j.ejso.2010.11.006
Matthews, A., Tang, M., and Cooper, K. (2010). Cytogenetic aberrations in soft tissue tumors harvested from fresh tissue submitted for surgical pathology: a single institutional experience. Int. J. Surg. Pathol. 18 (4), 260–267. doi:10.1177/1066896909346270
Mc Connell, L., Gazdova, J., Beck, K., Srivastava, S., Harewood, L., Stewart, J. P., et al. (2020). Detection of structural variants in circulating cell-free DNA from sarcoma patients using next generation sequencing. Cancers (Basel) 12 (12), 3627. doi:10.3390/cancers12123627
Miao, C. L., Chen, W. D., Liu, W. Q., H, M., Zou, B. Y., Li, W. J., et al. (2022). Clinical study of unilateral tumour and retroperitoneal fat resection and hyperthermic intraperitoneal chemotherapy for retroperitoneal tumour. Chin. Res. Hosp. 9 (04), 14–17. doi:10.19450/j.cnki.jcrh.2022.04.004
Miyake, M., Oda, Y., Nishimura, N., Morizawa, Y., Ohnishi, S., Hatakeyama, K., et al. (2020). Integrative assessment of clinicopathological parameters and the expression of PD-L1, PD-L2 and PD-1 in tumor cells of retroperitoneal sarcoma. Oncol. Lett. 20 (5), 190. doi:10.3892/ol.2020.12052
Monga, V., Miller, B. J., Tanas, M., Boukhar, S., Allen, B., Anderson, C., et al. (2021). Intratumoral talimogene laherparepvec injection with concurrent preoperative radiation in patients with locally advanced soft-tissue sarcoma of the trunk and extremities: phase IB/II trial. J. Immunother. Cancer 9 (7), e003119. doi:10.1136/jitc-2021-003119
Moreau, L. C., Turcotte, R., Ferguson, P., Wunder, J., Clarkson, P., Masri, B., et al. (2012). Myxoid\round cell liposarcoma (MRCLS) revisited: an analysis of 418 primarily managed cases. Ann. Surg. Oncol. 19 (4), 1081–1088. doi:10.1245/s10434-011-2127-z
Moreno Tellez, C., Leyfman, Y., D’Angelo, S. P., Wilky, B. A., and Dufresne, A. (2022). Immunotherapy in sarcoma: where do things stand? Surg. Oncol. Clin. N. Am. 31 (3), 381–397. doi:10.1016/j.soc.2022.03.004
Nacev, B. A., Jones, K. B., Intlekofer, A. M., Yu, J. S. E., Allis, C. D., Tap, W. D., et al. (2020). The epigenomics of sarcoma. Nat. Rev. Cancer 20 (10), 608–623. doi:10.1038/s41568-020-0288-4
Nakata, E., Fujiwara, T., Kunisada, T., Ito, T., Takihira, S., and Ozaki, T. (2021). Immunotherapy for sarcomas. Jpn. J. Clin. Oncol. 51 (4), 523–537. doi:10.1093/jjco/hyab005
Napolitano, A., Ostler, A. E., Jones, R. L., and Huang, P. H. (2021). Fibroblast growth factor receptor (FGFR) signaling in GIST and soft tissue sarcomas. Cells 10 (6), 1533. doi:10.3390/cells10061533
Nassif, E. F., Cope, B., Traweek, R., Witt, R. G., Erstad, D. J., Scally, C. P., et al. (2022). Real-world use of palbociclib monotherapy in retroperitoneal liposarcomas at a large volume sarcoma center. Int. J. Cancer 150 (12), 2012–2024. doi:10.1002/ijc.33956
Orgera, G., Monfardini, L., Della Vigna, P., Zhang, L., Bonomo, G., Arnone, P., et al. (2011). High-intensity focused ultrasound (HIFU) in patients with solid malignancies: evaluation of feasibility, local tumour response and clinical results. Radiol. Med. 116 (5), 734–748. doi:10.1007/s11547-011-0634-4
Osuna-Soto, J., Caro Cuenca, T., Sanz-Zorrilla, A., Torrecilla-Martínez, A., Ortega Salas, R., and Leiva-Cepas, F. (2021). Prognosis and survival of patients diagnosed with well-differentiated and dedifferentiated retroperitoneal liposarcoma. Cir. Esp. Engl. Ed. 100, 622–628. doi:10.1016/j.cireng.2022.06.034
Paik, B., Seo, C. J., Tan, J. W., Juan, W. K. D., Soo, K. C., Ong, C. A. J., et al. (2022). A systematic review of margin status in retroperitoneal liposarcomas: does the R0 margin matter? Front. Oncol. 12, 891710. doi:10.3389/fonc.2022.891710
Pal, K., Awad, A., Yevich, S., Kuban, J. D., Tam, A. L., Huang, S. Y., et al. (2024). Safety and efficacy of percutaneous cryoablation for recurrent or metastatic soft-tissue sarcoma in adult patients. AJR Am. J. Roentgenol. 223 (4), e2431490. doi:10.2214/AJR.24.31490
Patel, S., Von Mehren, M., Reed, D. R., Kaiser, P., Charlson, J., Ryan, C. W., et al. (2019). Overall survival and histology-specific subgroup analyses from a phase 3, randomized controlled study of trabectedin or dacarbazine in patients with advanced liposarcoma or leiomyosarcoma. Cancer 125 (15), 2610–2620. doi:10.1002/cncr.32117
Pentimalli, F., Dentice, M., Fedele, M., Pierantoni, G. M., Cito, L., Pallante, P., et al. (2003). Suppression of HMGA2 protein synthesis could be a tool for the therapy of well differentiated liposarcomas overexpressing HMGA2. Cancer Res. 63 (21), 7423–7427.
PéREZ-Mancera, P. A., Bermejo-RodríGUEZ, C., SáNCHEZ-MartíN, M., Abollo-Jiménez, F., Pintado, B., and Sánchez-García, I. (2008). FUS-DDIT3 prevents the development of adipocytic precursors in liposarcoma by repressing PPARγ and C/EBPα and activating eIF4E. PLoS One 3 (7), e2569. doi:10.1371/journal.pone.0002569
Powers, M. P., Wang, W. L., Hernandez, V. S., Patel, K. S., Lev, D. C., Lazar, A. J., et al. (2010). Detection of myxoid liposarcoma-associated FUS-DDIT3 rearrangement variants including a newly identified breakpoint using an optimized RT-PCR assay. Mod. Pathol. 23 (10), 1307–1315. doi:10.1038/modpathol.2010.118
Que, Y., Zhang, X. L., Liu, Z. X., Zhao, J. J., Pan, Q. Z., Wen, X. Z., et al. (2021). Frequent amplification of HDAC genes and efficacy of HDAC inhibitor chidamide and PD-1 blockade combination in soft tissue sarcoma. J. Immunother. Cancer 9 (2), e001696. doi:10.1136/jitc-2020-001696
Qureshi, Y. A., Huddy, J. R., Miller, J. D., Strauss, D. C., Thomas, J. M., and Hayes, A. J. (2012). Unplanned excision of soft tissue sarcoma results in increased rates of local recurrence despite full further oncological treatment. Ann. Surg. Oncol. 19 (3), 871–877. doi:10.1245/s10434-011-1876-z
Raut, C. P., Callegaro, D., Miceli, R., Barretta, F., Rutkowski, P., Blay, J. Y., et al. (2019). Predicting survival in patients undergoing resection for locally recurrent retroperitoneal sarcoma: a study and novel nomogram from TARPSWG. Clin. Cancer Res. 25 (8), 2664–2671. doi:10.1158/1078-0432.CCR-18-2700
Ray, U., Pathoulas, C. L., Thirusangu, P., Purcell, J. W., Kannan, N., and Shridhar, V. (2022). Exploiting LRRC15 as a novel therapeutic target in cancer. Cancer Res. 82 (9), 1675–1681. doi:10.1158/0008-5472.CAN-21-3734
Regina, C., and Hettmer, S. (2019). Myxoid liposarcoma: it’s a hippo’s world. EMBO Mol. Med. 11 (5), e10470. doi:10.15252/emmm.201910470
Ren, L., Qi, Y., Zhao, J., Weng, C., Wang, J., Yuan, D., et al. (2024). Gender differences in prognosis after primary resection for retroperitoneal liposarcoma. Am. Surg. 90 (4), 575–584. doi:10.1177/00031348231201883
Riedel, R. F., Ballman, K. V., Lu, Y., Attia, S., Loggers, E. T., Ganjoo, K. N., et al. (2020). A randomized, double-blind, placebo-controlled, phase II study of regorafenib versus placebo in advanced/metastatic, treatment-refractory liposarcoma: results from the SARC024 study. Oncologist 25 (11), e1655–e1662. doi:10.1634/theoncologist.2020-0679
Ronellenfitsch, U., Karampinis, I., Dimitrakopoulou-Strauss, A., Sachpekidis, C., Jakob, J., Kasper, B., et al. (2019). Preoperative pazopanib in high-risk soft tissue sarcoma: phase II window-of opportunity study of the German interdisciplinary sarcoma group (NOPASS/GISG-04). Ann. Surg. Oncol. 26 (5), 1332–1339. doi:10.1245/s10434-019-07183-4
Roulleaux Dugage, M., Nassif, E. F., Italiano, A., and Bahleda, R. (2021). Improving immunotherapy efficacy in soft-tissue sarcomas: a biomarker driven and histotype tailored review. Front. Immunol. 12, 775761. doi:10.3389/fimmu.2021.775761
Rust, D. J., Kato, T., and Yoon, S. S. (2022). Treatment for local control of retroperitoneal and pelvis sarcomas: a review of the literature. Surg. Oncol. 43, 101814. doi:10.1016/j.suronc.2022.101814
Rytlewski, J., Milhem, M. M., and Monga, V. (2021). Turning ‘Cold’ tumors ‘Hot’: immunotherapies in sarcoma. Ann. Transl. Med. 9 (12), 1039. doi:10.21037/atm-20-6041
SaâDA-Bouzid, E., Burel-Vandenbos, F., RanchèRE-Vince, D., Birtwisle-Peyrottes, I., Chetaille, B., Bouvier, C., et al. (2015). Prognostic value of HMGA2, CDK4, and JUN amplification in well-differentiated and dedifferentiated liposarcomas. Mod. Pathol. 28 (11), 1404–1414. doi:10.1038/modpathol.2015.96
Salerno, K. E. (2022). Radiation therapy for soft tissue sarcoma: indications, timing, benefits, and consequences. Surg. Clin. North Am. 102 (4), 567–582. doi:10.1016/j.suc.2022.04.001
Samuels, B. L., Chawla, S. P., Somaiah, N., Staddon, A. P., Skubitz, K. M., Milhem, M. M., et al. (2017). Results of a prospective phase 2 study of pazopanib in patients with advanced intermediate-grade or high-grade liposarcoma. Cancer 123 (23), 4640–4647. doi:10.1002/cncr.30926
Sanfilippo, R., Hayward, R. L., Musoro, J., Benson, C., Leahy, M. G., Brunello, A., et al. (2022). Activity of cabazitaxel in metastatic or inoperable locally advanced dedifferentiated liposarcoma: a phase 2 study of the eortc soft tissue and bone sarcoma group (stbsg). JAMA Oncol. 8 (10), 1420–1425. doi:10.1001/jamaoncol.2022.3218
Schaefer, I. M., and Gronchi, A. (2022). WHO pathology: highlights of the 2020 sarcoma update. Surg. Oncol. Clin. N. Am. 31 (3), 321–340. doi:10.1016/j.soc.2022.03.001
Schmoll, H. J., Lindner, L. H., Reichardt, P., Heißner, K., Kopp, H. G., Kessler, T., et al. (2021). Efficacy of pazopanib with or without gemcitabine in patients with anthracycline- and/or ifosfamide-refractory soft tissue sarcoma: final results of the PAPAGEMO phase 2 randomized clinical trial. JAMA Oncol. 7 (2), 255–262. doi:10.1001/jamaoncol.2020.6564
Schoenfeld, J. D., Sibenaller, Z. A., Mapuskar, K. A., Bradley, M. D., Wagner, B. A., Buettner, G. R., et al. (2018). Redox active metals and H(2)O(2) mediate the increased efficacy of pharmacological ascorbate in combination with gemcitabine or radiation in pre-clinical sarcoma models. Redox Biol. 14, 417–422. doi:10.1016/j.redox.2017.09.012
Schöffski, P. (2022). Established and experimental systemic treatment options for advanced liposarcoma. Oncol. Res. Treat. 45 (9), 525–543. doi:10.1159/000524939
Schroeder, B. A., Lafranzo, N. A., Lafleur, B. J., Gittelman, R. M., Vignali, M., Zhang, S., et al. (2021). CD4+ T cell and M2 macrophage infiltration predict dedifferentiated liposarcoma patient outcomes. J. Immunother. Cancer 9 (8), e002812. doi:10.1136/jitc-2021-002812
Schwartz, P. B., Poultsides, G., Roggin, K., Howard, J. H., Fields, R. C., Clarke, C. N., et al. (2020). PLR and NLR are poor predictors of survival outcomes in sarcomas: a new perspective from the USSC. J. Surg. Res. 251, 228–238. doi:10.1016/j.jss.2020.01.008
Sciot, R., Gerosa, C., and Faa, G. (2020). Adipocytic, vascular and skeletal muscle tumors: a practical diagnostic approach [M]. Alexandria, VA, Springer Nature.
Seligson, N. D., Stets, C. W., Demoret, B. W., Awasthi, A., Grosenbacher, N., Shakya, R., et al. (2019). Inhibition of histone deacetylase 2 reduces MDM2 expression and reduces tumor growth in dedifferentiated liposarcoma. Oncotarget 10 (55), 5671–5679. doi:10.18632/oncotarget.27144
Setsu, N., Miyake, M., Wakai, S., Nakatani, F., Kobayashi, E., Chuman, H., et al. (2016). Primary retroperitoneal myxoid liposarcomas. Am. J. Surg. Pathol. 40 (9), 1286–1290. doi:10.1097/PAS.0000000000000657
Shannon, N. B., Tan, Q. X., Tan, J. W., Hendrikson, J., Ng, W. H., Ng, G., et al. (2021). Gene expression changes associated with dedifferentiation in liposarcoma predict overall survival. Cancers (Basel) 13 (12), 3049. doi:10.3390/cancers13123049
Siew, C. C. H., Apte, S. S., Baia, M., Gyorki, D. E., Ford, S., and van Houdt, W. J. (2022). Retroperitoneal and mesenteric liposarcomas. Surg. Oncol. Clin. N. Am. 31 (3), 399–417. doi:10.1016/j.soc.2022.03.005
Singer, S., Antonescu, C. R., Riedel, E., and Brennan, M. F. (2003). Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann. Surg. 238 (3), 358–371. discussion 70-1. doi:10.1097/01.sla.0000086542.11899.38
Smolle, M. A., Leithner, A., and Bernhardt, G. A. (2020). Abdominal metastases of primary extremity soft tissue sarcoma: a systematic review. World J. Clin. Oncol. 11 (2), 74–82. doi:10.5306/wjco.v11.i2.74
Somaiah, N., Conley, A. P., Parra, E. R., Lin, H., Amini, B., Solis Soto, L., et al. (2022). Durvalumab plus tremelimumab in advanced or metastatic soft tissue and bone sarcomas: a single-centre phase 2 trial. Lancet Oncol. 23 (9), 1156–1166. doi:10.1016/S1470-2045(22)00392-8
Starzer, A. M., Berghoff, A. S., Hamacher, R., Tomasich, E., Feldmann, K., Hatziioannou, T., et al. (2021). Tumor DNA methylation profiles correlate with response to anti-PD-1 immune checkpoint inhibitor monotherapy in sarcoma patients. J. Immunother. Cancer 9 (3), e001458. doi:10.1136/jitc-2020-001458
Sun, P., Ma, R., Liu, G., Wang, L., Chang, H., and Li, Y. (2021). Pathological prognostic factors of retroperitoneal liposarcoma: comprehensive clinicopathological analysis of 124 cases. Ann. Transl. Med. 9 (7), 574. doi:10.21037/atm-21-972
Swallow, C. J., Strauss, D. C., Bonvalot, S., Rutkowski, P., Desai, A., Gladdy, R. A., et al. (2021). Management of primary retroperitoneal sarcoma (RPS) in the adult: an updated consensus approach from the transatlantic australasian RPS working group. Ann. Surg. Oncol. 28 (12), 7873–7888. doi:10.1245/s10434-021-09654-z
Tanaka, K., Mizusawa, J., Fukuda, H., Araki, N., Chuman, H., Takahashi, M., et al. (2015). Perioperative chemotherapy with ifosfamide and doxorubicin for high-grade soft tissue sarcomas in the extremities (JCOG0304). Jpn. J. Clin. Oncol. 45 (6), 555–561. doi:10.1093/jjco/hyv042
Tanaka, K., Mizusawa, J., Naka, N., Kawai, A., Katagiri, H., Hiruma, T., et al. (2019). Ten-year follow-up results of perioperative chemotherapy with doxorubicin and ifosfamide for high-grade soft-tissue sarcoma of the extremities: Japan Clinical Oncology Group study JCOG0304. BMC Cancer 19 (1), 890. doi:10.1186/s12885-019-6114-2
Tawbi, H. A., Burgess, M., Bolejack, V., Van Tine, B. A., Schuetze, S. M., Hu, J., et al. (2017). Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 18 (11), 1493–1501. doi:10.1016/S1470-2045(17)30624-1
Thirasastr, P., and Somaiah, N. (2022). Overview of systemic therapy options in liposarcoma, with a focus on the activity of selinexor, a selective inhibitor of nuclear export in dedifferentiated liposarcoma. Ther. Adv. Med. Oncol. 14, 17588359221081073. doi:10.1177/17588359221081073
Tirotta, F., Hodson, J., Parente, A., Pasquali, S., Sutcliffe, R., Desai, A., et al. (2020). Liver resection for sarcoma metastases: a systematic review and experience from two European centres. Eur. J. Surg. Oncol. 46 (10 Pt A), 1807–1813. doi:10.1016/j.ejso.2020.05.024
Tornin, J., Hermida-Prado, F., Padda, R. S., Gonzalez, M. V., Alvarez-Fernandez, C., Rey, V., et al. (2018). FUS-CHOP promotes invasion in myxoid liposarcoma through a SRC/FAK/RHO/ROCK-Dependent pathway. Neoplasia 20 (1), 44–56. doi:10.1016/j.neo.2017.11.004
Trautmann, M., Cheng, Y. Y., Jensen, P., Azoitei, N., Brunner, I., Hüllein, J., et al. (2019b). Requirement for YAP1 signaling in myxoid liposarcoma. EMBO Mol. Med. 11 (5), e9889. doi:10.15252/emmm.201809889
Trautmann, M., Cyra, M., Isfort, I., Jeiler, B., Krüger, A., Grünewald, I., et al. (2019a). Phosphatidylinositol-3-kinase (PI3K)/Akt signaling is functionally essential in myxoid liposarcoma. Mol. Cancer Ther. 18 (4), 834–844. doi:10.1158/1535-7163.MCT-18-0763
Tseng, W. W., Barretta, F., Conti, L., Grignani, G., Tolomeo, F., Albertsmeier, M., et al. (2021). Defining the role of neoadjuvant systemic therapy in high-risk retroperitoneal sarcoma: a multi-institutional study from the Transatlantic Australasian Retroperitoneal Sarcoma Working Group. Cancer 127 (5), 729–738. doi:10.1002/cncr.33323
Tseng, W. W., Swallow, C. J., Strauss, D. C., Bonvalot, S., Rutkowski, P., Ford, S. J., et al. (2022). Management of locally recurrent retroperitoneal sarcoma in the adult: an updated consensus approach from the transatlantic australasian retroperitoneal sarcoma working group. Ann. Surg. Oncol. 29 (12), 7335–7348. doi:10.1245/s10434-022-11864-y
Van Der Laan, P., Van Der Graaf, W., Van Den Broek, D., van Boven, H., Steeghs, N., and Van Houdt, W. (2023). 1953P Prognostic value of pretreatment inflammatory markers and other clinicopathological factors for early recurrence in soft tissue sarcoma. Ann. Oncol. 34, S1046–S1047. doi:10.1016/j.annonc.2023.09.1182
Van Houdt, W. J., Fiore, M., Barretta, F., Rutkowski, P., Blay, J. Y., Lahat, G., et al. (2020). Patterns of recurrence and survival probability after second recurrence of retroperitoneal sarcoma: a study from TARPSWG. Cancer 126 (22), 4917–4925. doi:10.1002/cncr.33139
Vincenzi, B., Napolitano, A., Comandone, A., Sanfilippo, R., Celant, S., Olimpieri, P. P., et al. (2023). Trabectedin use in soft-tissue sarcoma patients in a real-world setting: data from an Italian national drug-access registry. Int. J. Cancer 152 (4), 761–768. doi:10.1002/ijc.34309
Wagner, M. J., Zhang, Y., Cranmer, L. D., Loggers, E. T., Black, G., McDonnell, S., et al. (2022). A phase 1/2 trial combining avelumab and trabectedin for advanced liposarcoma and leiomyosarcoma. Clin. Cancer Res. 28 (11), 2306–2312. doi:10.1158/1078-0432.CCR-22-0240
Wang, X., Asmann, Y. W., Erickson-Johnson, M. R., Oliveira, J. L., Zhang, H., Moura, R. D., et al. (2011). High-resolution genomic mapping reveals consistent amplification of the fibroblast growth factor receptor substrate 2 gene in well-differentiated and dedifferentiated liposarcoma. Genes Chromosom. Cancer 50 (11), 849–858. doi:10.1002/gcc.20906
Wang, Y., Xia, B., Cao, L., Yang, J., Feng, C., Jiang, F., et al. (2024). Preclinical studies of BB-1701, a HER2-targeting eribulin-containing ADC with potent bystander effect and ICD activity. Antib. Ther. 7 (3), 221–232. doi:10.1093/abt/tbae019
Woll, P. J., Reichardt, P., Le Cesne, A., Bonvalot, S., Azzarelli, A., Hoekstra, H. J., et al. (2012). Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol. 13 (10), 1045–1054. doi:10.1016/S1470-2045(12)70346-7
Wu, J., Hao, C., Jia, W., Lv, A., Qiu, H., Li, C., et al. (2023). 1943P Efficacy and safety of eribulin plus anlotinib and camrelizumab in advanced or metastatic retroperitoneal liposarcoma (RLPS) or leiomyosarcoma (RLMS): a single-center retrospective cohort study. Ann. Oncol. 34, S1043. doi:10.1016/j.annonc.2023.09.1172
Wu, Q., Li, J., and Yang, W. (2021). Survival analysis of patients with recurrent or metastatic soft tissue sarcoma who were treated by cryoablation: a real-world retrospective study. J. Cancer Res. Ther. 17 (7), 1736–1741. doi:10.4103/jcrt.jcrt_409_21
Xiang-Xu, W., Jing-Yi, R., Xiao-Wen, W., and Hong-Mei, Z. (2023). 1935P Exploration of tertiary lymphatic structure in soft tissue sarcoma for the prognostic and immunotherapy response. Ann. Oncol. 34, S1039–S1040. doi:10.1016/j.annonc.2023.09.1164
Xiao, X., Garbutt, C. C., Hornicek, F., Guo, Z., and Duan, Z. (2018). Advances in chromosomal translocations and fusion genes in sarcomas and potential therapeutic applications. Cancer Treat. Rev. 63, 61–70. doi:10.1016/j.ctrv.2017.12.001
XI, M., Zhu, J., Zhang, F., Shen, H., Chen, J., Xiao, Z., et al. (2024). Antibody-drug conjugates for targeted cancer therapy: recent advances in potential payloads. Eur. J. Med. Chem. 276, 116709. doi:10.1016/j.ejmech.2024.116709
Xu, B., Pan, Q., Pan, H., Li, H., Li, X., Chen, J., et al. (2023). Anlotinib as a maintenance treatment for advanced soft tissue sarcoma after first-line chemotherapy (ALTER-S006): a multicentre, open-label, single-arm, phase 2 trial. EClinicalMedicine 64, 102240. doi:10.1016/j.eclinm.2023.102240
Xu, C., Yan, L., Guan, X., Wang, Z., Wu, J., Lv, A., et al. (2022). Tsp2 facilitates tumor-associated fibroblasts formation and promotes tumor progression in retroperitoneal liposarcoma. Int. J. Biol. Sci. 18 (13), 5038–5055. doi:10.7150/ijbs.70083
Yamakado, K., Matsumine, A., Nakamura, T., Nakatsuka, A., Takaki, H., Matsubara, T., et al. (2014). Radiofrequency ablation for the treatment of recurrent bone and soft-tissue sarcomas in non-surgical candidates. Int. J. Clin. Oncol. 19 (5), 955–962. doi:10.1007/s10147-013-0640-8
Yao, X., Ghert, M., Dickson, B. C., Popovic, S., Purgina, B. M., Verma, S., et al. (2020). An evidence-based guideline on the application of molecular testing in the diagnosis, prediction of prognosis, and selection of therapy in non-GIST soft tissue sarcomas. Cancer Treat. Rev. 85, 101987. doi:10.1016/j.ctrv.2020.101987
Yu, W., Tang, L., Lin, F., Yao, Y., Shen, Z., and Zhou, X. (2015). High-intensity focused ultrasound: noninvasive treatment for local unresectable recurrence of osteosarcoma. Surg. Oncol. 24 (1), 9–15. doi:10.1016/j.suronc.2014.10.001
Zhang, K., Chu, K., Wu, X., Gao, H., Wang, J., Yuan, Y. C., et al. (2013). Amplification of FRS2 and activation of FGFR/FRS2 signaling pathway in high-grade liposarcoma. Cancer Res. 73 (4), 1298–1307. doi:10.1158/0008-5472.CAN-12-2086
Zhang, S., Kohli, K., Black, R. G., Yao, L., Spadinger, S. M., He, Q., et al. (2019). Systemic interferon-γ increases MHC class I expression and T-cell infiltration in cold tumors: results of a phase 0 clinical trial. Cancer Immunol. Res. 7 (8), 1237–1243. doi:10.1158/2326-6066.CIR-18-0940
Zhang, X., Peng, R., Pan, Q., Xu, B., Hong, D., Que, Y., et al. (2023). A phase II clinical trial of chidamide in combination with toripalimab in patients with advanced soft tissue sarcoma. JCO 41, 11566. doi:10.1200/JCO.2023.41.16_suppl.11566
Zhou, Y., Li, W., Fang, M., Zhuang, R., Feng, Y., Guo, X., et al. (2023). Phase I/II study to evaluate penpulimab combined with anlotinib and epirubicin in the first-line treatment of soft tissue sarcoma: updated. JCO 41, 11569. doi:10.1200/JCO.2023.41.16_suppl.11569
Zhu, M. M. T., Shenasa, E., and Nielsen, T. O. (2020). Sarcomas: immune biomarker expression and checkpoint inhibitor trials. Cancer Treat. Rev. 91, 102115. doi:10.1016/j.ctrv.2020.102115
Zhuang, A., Zhuang, A., Wu, Q., Lu, W., Tong, H., and Zhang, Y. (2021). Prognostic factor analysis and nomogram construction of primary retroperitoneal liposarcoma: a review of 10 Years of treatment experience in a single asian cohort of 211 cases. Front. Oncol. 11, 777647. doi:10.3389/fonc.2021.777647
Keywords: liposarcoma, clinical and molecular characteristics, surgery, systemic treatment, prognosis
Citation: Liu H, Wang X, Wang X, Qiu F and Zhou B (2025) Challenges and hope: latest research trends in the clinical treatment and prognosis of liposarcoma. Front. Pharmacol. 16:1529755. doi: 10.3389/fphar.2025.1529755
Received: 17 November 2024; Accepted: 21 April 2025;
Published: 12 May 2025.
Edited by:
Hong Duan, Sichuan University, ChinaReviewed by:
Dimitris Tatsis, Aristotle University of Thessaloniki, GreeceZhongkui Xiong, Shaoxing Second Hospital, China
Copyright © 2025 Liu, Wang, Wang, Qiu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fabo Qiu, cWl1ZmFib0BzaW5hLmNvbQ==; Bin Zhou, YmluYWxpbkAxNjMuY29t
†These authors have contributed equally to this work
 Hongliang Liu
Hongliang Liu Xi Wang
Xi Wang Xiaoyu Wang3
Xiaoyu Wang3 Bin Zhou
Bin Zhou