Abstract
Introduction:
Bacteriocin P7 was extracted from the cell-free supernatant (CFS) of Bacillus velezensis G7, which is a strain isolated from mangrove plants.
Methods:
In this study, the culture conditions of B. velezensis G7 were optimised using an orthogonal test. The (CFS) was subsequently purified by using TA-GF75 gel chromatography, Tiderose Q HP anion chromatography and reversed-phase high-performance liquid chromatography (RP-HPLC). Finally, the bacteriocin was identified by using LC-MS/MS.
Results and discussion:
The optimal culture conditions for B. velezensis G7 are 4.5 g/100 mL glucose, 1.5 g/100 mL yeast, and 1.2 g/100 mL MgSO4·7H2O. The stability of the CFS is affected by several factors, including heat, UV treatment and different storage conditions. High temperatures and long UV irradiation treatments significantly reduce the stability of CFS, which is more sensitive to strong acids, bases and enzymatic degradation. The minimum inhibitory concentration (MIC) of purified bacteriocin P7 against S. aureus was determined to be 30.352 μg/mL. On the basis of the results of the haemolytic activity assay, it was concluded that the use of bacteriocin P7 at concentrations equal to or below the 2 × MIC is safe. The addition of organic solvents and inorganic salts did not affect the bacteriocin P7, while the incorporation of SDS could enhance its antimicrobial efficacy. The bacteriocin was subjected to analysis by LC-MS/MS, which revealed that it was similar to the class I bacteriocin amyloliquecidin GF610. The findings of the present study indicate that the endophytic B. velezensis G7 from mangrove plant can produce bacteriocins, thereby providing a reference point for the expansion of novel bacteriocin sources.
1 Introduction
Mangrove forests are wetland forests composed of saline tree and shrub species distributed between tropical and subtropical land and sea around the world (Friess, 2016). Due to the specialised nature of their habitats, their metabolic pathways different from those of terrestrial microorganisms and are rich in novel metabolites. A fungus, Aspergillus fumigatus JRJ111048, whose metabolites include one new lipid amide 11-methyl-11-hydroxydodecanoic acid amide, was isolated from the leaves of Acrostichum specioum, a mangrove plant endemic to Hainan, and this new compound showed strong insecticidal activity against newly hatched larvae of Bacillus Species (Islam et al., 2022). Different endophytes are present in mangrove plants and soils, for example, in the mangrove forests of the Andaman Nicobar Islands, India, a total of three Bacillus species were found, two of which were identified as Bacillus subtilis and B. velezensis (Guo et al., 2017). Extracts of mangrove fruits, roots, stems and leaves have been observed to possess antimicrobial, antioxidant, anti-inflammatory and anticancer activities (Acharya et al., 2023). The majority of plants in mangrove ecosystems are angiosperms that produce specific secondary metabolites, which are employed to control and even communicate with phytopathogenic bacteria and fungi. Plants are subjected to attack by phytopathogenic bacteria, fungi and viruses and, in response, produce a substantial number of antimicrobial secondary metabolites (Sulaiman et al., 2022). In one study, a total of 386 strains of Lactobacillus spp. were isolated from mangrove forests in southern Thailand. Four strains were selected for screening on the basis of their potential to produce antimicrobial secondary metabolites, specifically bacteriocins, which demonstrated inhibitory activity against Lactobacillus sakei, Listeria monocytogenes and Brochothrix thermokilleri (Hwanhlem et al., 2013). Bacillus species may produce bacteriocins that resemble those produced by lactic acid bacteria, such as lantibiotics and pediocin-like bacteriocins. Bacillus is widespread in soil, plants, food and the intestines of animals, and its trophozoites divide and multiply approximately every half hour. Changes in environmental factors such as nutrient status, temperature, pH, oxygen content and salt concentration can trigger the transformation between the spores and the nutrients. Bacillus is characterised by high temperature resistance, rapid resurrection and strong secretion of enzymes, and can survive under both aerobic and anaerobic conditions. In the lack of nutrients, drought and other conditions to form spores, in the right conditions and can re-emerge into nutrients. Bacillus has a large number of strains with special functions, and these strains show a wide range of potential applications in various fields such as livestock and poultry breeding, agriculture and medicine.
Bacteriocins are peptides synthesized by ribosomes with antimicrobial activity (Li et al., 2024). The genus Bacillus is the primary source of bacteriocins. Many Bacillus species produce a variety of bacteriocins, including Bacillus thuringiensis, Bacillus thermophilus, Bacillus licheniformis, Bacillus cereus, Bacillus amyloliquefaciens, B. subtilis, and Bacillus coagulans (Elazzazy et al., 2024). Bacillus bacterin has broad-spectrum antimicrobial activity, showing effectiveness against both gram-positive and gram-negative bacterial strains (Bastos et al., 2008; Wang Z. et al., 2024). Bacteriocins from B. velezensis significantly inhibited Streptomyces scabies, with a minimum inhibitory concentration of 10.58 μg/mL, and were stable to UV radiation and high temperature (Zhao et al., 2022).
The nutrient composition of a medium influences the microbial fermentation process (Gomes et al., 2022). More specifically, small changes in the composition of the fermentation medium can significantly affect the production and metabolic composition of microorganisms (Vlajkov et al., 2022a). To increase the capacity of Bacillus to produce bacteriocins, the development of an efficacious medium to augment the production of bacteriocins by Bacillus through the screening of carbon, nitrogen, and inorganic salt components is regarded as a viable strategy (Lajis, 2020). Taswar Ahsan (Ahsan et al., 2023) screened B. velezensis BP-1 fermentation medium by determining 15 g/L crude flour as a carbon source, 13.68 g/L peanut root extract as the nitrogen source, and 0.50 g/L magnesium sulfate as the inorganic salt component of the medium, which led to 90% inhibition of Peyronellaea arachidicola. Adequate addition of magnesium sulphate can change the medium from neutral to weakly alkaline, creating a more favourable environment for the growth of certain microorganisms. Mg2+ in magnesium sulphate plays an important role in the manufacture of proteins by activating a variety of enzymes and participating in the synthesis of amino acids, the transcription and translation of genes, the production of proteins that are structural components of the ribosome, and other processes (Chen et al., 2025).
A total of 227 culturable endophytes were isolated from mangrove roots tissues of the species Avicennia marina from Zhanjiang Mangrove Nature Reserve, Guangdong Province, China. In this experiment, an endophytic strain G7 was used, which exhibited good antimicrobial activity against both Gram-positive and Gram-negative bacteria, demonstrating broad-spectrum antimicrobial activity, and its culture medium was optimised. The stability and haemolysis of the cell-free supernatant were determined, and the bacteriocin was purified by gel chromatography, ion chromatography and high-performance liquid chromatography. The bacteriocin was also identified by LC-MS/MS. The experimental scheme of this study is shown in Figure 1. These results provide a theoretical basis for the research and application of bacteriocins in food, agriculture, and animal husbandry.
FIGURE 1

The experimental scheme of this study.
2 Materials and methods
2.1 Reagents and strains
B. velezensis G7 was isolated from Zhanjiang Mangrove Forest Nature Reserve, Guangdong, China. The bacterial strains used in this study, including Escherichia coli (CMCC(B)44,103), Pseudomonas aeruginosa (ATCC9027), Staphylococcus aureus (ATCC25923), and L. monocytogenes (ATCC19115), were obtained from Huankai Microbiology Technology Co (Guangzhou, Guangdong, China). All strains were cultured in LB liquid medium and stored at −80 °C with a solution of glycerol at a concentration of 30%. Chicken blood obtained from hongquan bio-tel(Guangzhou, Guangdong, China).
2.2 Optimisation of single components of culture media
B. velezensis G7 was inoculated in LB liquid medium overnight at 30 °C, then inoculated with 2% of the inoculum into different media and incubated at 180 rpm and 30 °C for 24 h. Following this, the mixture was centrifuged at 8000 rpm and 4 °C for 30 min, after which the supernatant was filtered through a 0.22 μm membrane to obtain the CFS. The extent of bacterial inhibition was determined by measuring the diameter of the inhibition zone formed around the inoculation site on solid agar plates. Specifically, the overnight-cultured indicator bacteria were inoculated into an unconsolidated LB solid medium at an inoculum of 1%, and wells were punched with a sterilised punch. Subsequently, 200 μL of CFS was added to the wells, and the culture was incubated at 37°C for 24 h. Thereafter, the inoculum was filtered through a 0.22 μm filter membrane at 4°C to obtain the CFS.
The carbon source medium consisted of 2 g/100 mL yeast and 1.0 g/100 mL NaCl. Glucose, sucrose, fructose, cyclodextrin, and maltose were added to the basal medium at a concentration of 2 g/100 mL. The nitrogen source medium was supplemented with glucose, the optimal carbon source. The nitrogen source basal medium consisted of 2 g/100 mL glucose and 1.0 g/100 mL NaCl. Yeast, casein, peptone, beef meal, and fish protein at a concentration of 2 g/100 mL were added to the basal medium. The inorganic salt medium consisted of 2 g/100 mL glucose and yeast. Magnesium sulfate heptahydrate, dipotassium hydrogen phosphate, sodium chloride, calcium chloride, and ammonium persulfate were added to the basal medium at a concentration of 1 g/100 mL.
2.3 Optimisation of the carbon, nitrogen and inorganic salt contents in culture media
The optimum carbon source, nitrogen source and inorganic salts were selected as the basic medium. To optimize the amount of carbon source additive, concentrations of 1, 2, 3, 4, 5, and 6 g/100 mL glucose were added. To optimize the amount of nitrogen source additive, concentrations of 1, 2, 3, 4, 5, and 6 g/100 mL yeast were added. For the optimization of inorganic salt, MgSO4·7H2O was added at concentrations of 0.6, 0.8, 1.0, 1.2, 1.4, and 1.6 g/100 mL. The antimicrobial activity of the above culture supernatants was determined under the same experimental conditions, and all three sets of replicates were performed.
2.4 Orthogonal test
According to the optimised medium screened by the one-way test, Orthogonal tests were designed by Latin was carried out as shown in Table 1. The three factors were the optimal carbon, nitrogen and inorganic salts screened, the three levels were the optimal additive amounts of the factors, and three-factor and three-level analyses were carried out. The experiment was repeated three times.
TABLE 1
| Level | Ingredient | ||
|---|---|---|---|
| Glucose (g/100 mL) | Yeast (g/100 mL) | MgSO4·7H2O (g/100 mL) | |
| Group 1 | 1.5 | 0.5 | 0.6 |
| Group 2 | 1.5 | 1.0 | 1.2 |
| Group 3 | 1.5 | 1.5 | 1.8 |
| Group 4 | 3.0 | 0.5 | 1.2 |
| Group 5 | 3.0 | 1.0 | 1.8 |
| Group 6 | 3.0 | 1.5 | 0.6 |
| Group 7 | 4.5 | 0.5 | 1.8 |
| Group 8 | 4.5 | 1.0 | 0.6 |
| Group 9 | 4.5 | 1.5 | 1.2 |
| K1 | 45.31 | 28.76 | 45.00 |
| K2 | 47.19 | 54.54 | 49.34 |
| K3 | 50.33 | 59.53 | 48.49 |
| R | 5.02 | 30.77 | 3.49 |
Orthogonal experimental design factor level table.
2.5 Stability of CFS
2.5.1 Acid‒base stability
The treated CFS was divided into five equal parts, adjusted to pH 2, 4, 6, 8 and 10 with 1 mol/L HCl or NaOH, stored in a refrigerator at 4°C for 24 h, and then adjusted back to the natural pH for the detection of antimicrobial activity (Ming et al., 2022).
2.5.2 UV stability
The appropriate amount of treated CFS was divided into five equal portions, the CFS broth was placed under ultraviolet light (30 W) for 10, 20, 40, 60 and 90 min, and then the antimicrobial activity was tested (Ming et al., 2022).
2.5.3 Temperature stability
An appropriate amount of treated CFS was divided into five equal portions, the CFS broth was placed in a water bath at 40, 60, 80, or 100°C for 60 min and 121°C for 20 min, and then the mixture was cooled to room temperature to detect the antimicrobial activity (Ming et al., 2022).
2.5.4 Storage stability
The treated CFS was divided into three equal portions and stored at 4°C, −25°C and −80°C for 0, 3, 6, 9, 12, 18 and 24 days for the detection of antimicrobial activity (Ming et al., 2022).
2.5.5 Protease stability
The appropriate amount of treated CFS was divided into five equal portions, pepsin, trypsin, papain, protease K, and protease E were added to the CFS (1 g/mL), and the antimicrobial activity was detected by heating in a water bath at 37°C for 1 h (Ming et al., 2022).
2.6 Isolation and purification of bacteriocin
The optimised culture conditions for B. velezensis G7 consisted of a liquid volume of 30 mL, an inoculum volume of 2%, an incubation temperature of 30°C and an incubation time of 24 h. The culture was subsequently centrifuged at 4°C for 30 min at 8000 rpm, and the supernatant was concentrated 10-fold using a rotary evaporator, followed by filtration through a 0.22 μm filter membrane. The concentrated crude extract was loaded onto a deionised water-equilibrated TA-GF75 gel column and subjected to purification at a flow rate of 0.55 mL/min. The obtained fraction with bacteriostatic effects was concentrated and loaded onto a Tiderose Q HP anionic column equilibrated with 1 mol/L NaCl and subjected to anionic column purification at a flow rate of 3 mL/min (Ma et al., 2025). The fraction with bacteriostatic activity was then concentrated and subjected to RP‒HPLC separation and purification. This process consisted of mobile phase A (ddH2O+0.1% TFA) and mobile phase A (acetonitrile+0.1% TFA). The elution procedure was as follows: 5% B; 11–20 min, 5%–50% B; 21–30 min, 50%–70% B; and 31–38 min, 70%–95% B. The bacteriostatic activity of the isolated bacteriocin was detected via the solid agar perforation method and the micro broth twofold dilution method. The bacteriocins were concentrated and stored at −80°C for later use. The molecular weights of the bacteriocins were determined via Tricine-SDS‒PAGE. The gels were electrophoresed at 30 V for 1 h and then electrophoresed at 100 V, after which the gels were stained with Coomassie blue (Peng et al., 2024).
2.7 LC‒MS/MS
Qingdao Stantec Standard Testing Company, Shandong, China, conducted the LC‒MS/MS analysis of bacteriocin P7 (Selvam et al., 2021). The sample was processed before mass spectrometry analysis: total bacteriocin was extracted, enzymatically digested, and desalted, and the supernatant was left. Separation was carried out on a chromatographic column with an injection volume of 1 μL. Equilibration was performed with 92% liquid A (0.1% formic acid aqueous solution). The relevant liquid‒phase gradient was set as follows: 0–98 min, the linear gradient of liquid B (0.1% formic acid acetonitrile aqueous solution (80% acetonitrile)) was from 8% to 28%; 98–113 min, the linear gradient of liquid B was from 28% to 37%; 113–117 min, the linear gradient of liquid B was from 37% to 100%; and 117–---- 120 min, liquid B was maintained at 100%. Mass spectrometry analysis was performed on a Thermo QE HF mass spectrometer (Thermo Fisher) with an analysis duration of 120 min. Detection mode: positive ions. The mass‒charge ratios of the peptides and fragments of the peptides were determined according to the following method: 20 fragment profiles were collected after each full scan (MS2 scan). The scanning range was 400–1800, the primary resolution was 60,000, the secondary resolution was 15,000, and the collision energy was CE28eV. The bacteriocin identification results were obtained by searching the corresponding databases with Proteome Discoverer 2.5 (Banerjee et al., 2017).
2.8 Analysis of the bacteriocin propertie
2.8.1 Minimum inhibitory concentration (MIC)
The MIC was determined via a twofold micro broth dilution method. One hundred microlitres of S. aureus were diluted 1 × 10−3 times with LB, 100 μL of diluted S. aureus was added to 96-well plates, and then 100 μL of bacteriocin was serially diluted in 96-well plates containing indicator bacteria. At the same time, the wells containing bacteriocin and indicator bacteria were used as the control, and bacterial growth was observed after 16 h at 37°C. As a control, bacterial growth was observed at 37°C for 16 h. The lowest concentration at which the medium was clear to the naked eye and no bacterial growth was observed was judged to be the lowest inhibitory concentration of the drug (Banerjee et al., 2017).
2.8.2 Haemolytic effects of bacteriocin P7
The appropriate amount of domestic chicken blood was taken and centrifuged at 4 °C and 1000 × g for 10 min to obtain erythrocytes, which were washed three times with PBS, and the erythrocytes were fully suspended during the washing process. At the same time, the fragmentation of erythrocytes was avoided as much as possible, and the erythrocytes were subsequently resuspended in PBS. Different concentrations of bacteriocin solution (1 × MIC, 2 × MIC, and 4 × MIC) were mixed with an equal volume of erythrocyte suspension, added to a 2 mL centrifuge tube and incubated at 37°C for 1 h. The mixture was centrifuged at 4°C and 1000 × g for 10 min to obtain the supernatant, 100 μL was added to a 96-well plate, and an enzyme marker was used to measure the absorbance at 540 nm. Under the same treatment conditions, 0.1% Triton X-100 and PBS were used as positive controls and blank controls, respectively. The OD540 value of the PBS-treated erythrocyte solution was considered 0% haemolysis, and the OD540 value of the 0.1% Triton X-100-treated erythrocyte solution was considered 100% haemolysis (Selvam et al., 2021; Banerjee et al., 2017). The formula for calculating the haemolysis rate of bacteriocins is as follows:where A is the OD540 value of blood cells after treatment with bacteriocin, A1 is the OD540 value of blood cells after treatment with PBS, and At is the OD540 value of blood cells after treatment with Triton X-100.
2.8.3 Effects of different chemicals on the bacteriostatic activity of bacteriocin P7
The bacteriocin P7 was divided into five equal portions (1 mL) and mixed with EDTA, SDS, CO(NH2)2 and 25% CH3OH and 25% C2H5OH at a mass ratio of 1%. After 2 h at room temperature (25–27°C), the bacteriostatic activity of the mixture was determined via the solid agar perforation method with S. aureus used as an indicator organism, and the bacteriocin mixture not treated with the chemical agents was used as a blank control (Banerjee et al., 2017).
2.8.4 Effects of different inorganic salts on the bacteriostatic activity of bacteriocin P7
The bacteriocin was divided into five equal portions (1 mL), and 10 μL of NaCl, CaCl2, KCl, ZnSO4, or MgCl2 at a concentration of 2 mol/L was added and mixed. Bacteriocin without inorganic salts was used as a control, and the inhibitory activity of the mixtures was measured by solid agar perforation to test the effect of the inhibitory activity of the bacteriocin via different metal ions.
3 Results
3.1 1Identification of strain G7
A strain with remarkable bacterial inhibition was screened, and a total of 857 bacterial strains were isolated from the mangrove roots. Among these strains, G7 exhibited exceptional bacteriostatic activity. The 16S rRNA gene sequence of G7 was amplified through PCR and compared with several B. velezensis strains, revealing a high degree of similarity (Figure 2). Consequently, the isolate was designated B. velezensis G7.
FIGURE 2
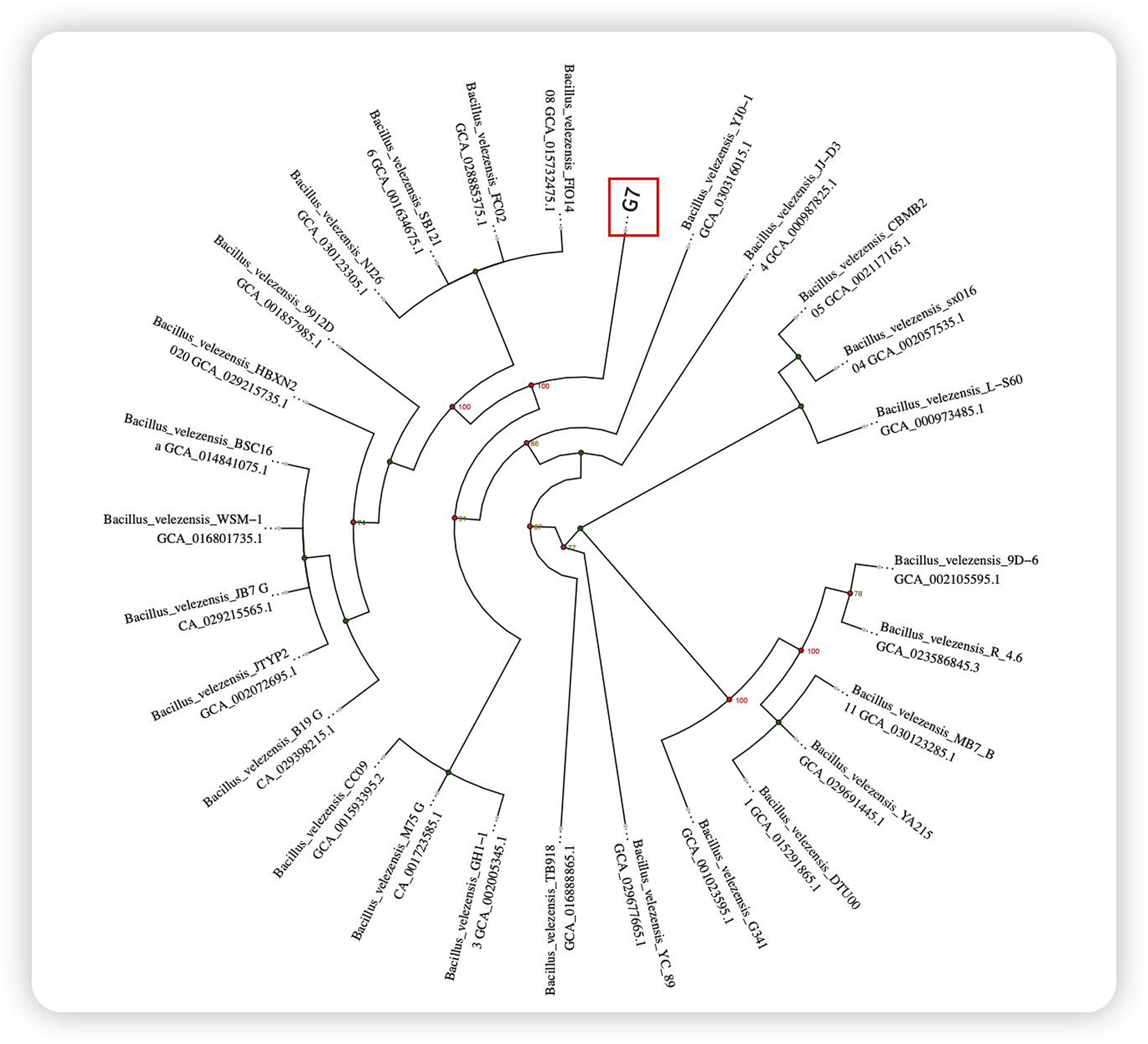
Circular evolutionary tree based on 16S rRNA.
3.2 Screening of single components of culture media
Figure 3 shows the single-component screening of the medium, which comprises glucose as a single carbon source, yeast as a single nitrogen source, and MgSO4·7H2O as a single inorganic salt. The antimicrobial agents were effective against all four indicator bacteria, with the greatest inhibition observed against S. aureus and L. monocytogenes, both of which were greater than 20 mm in diameter.
FIGURE 3
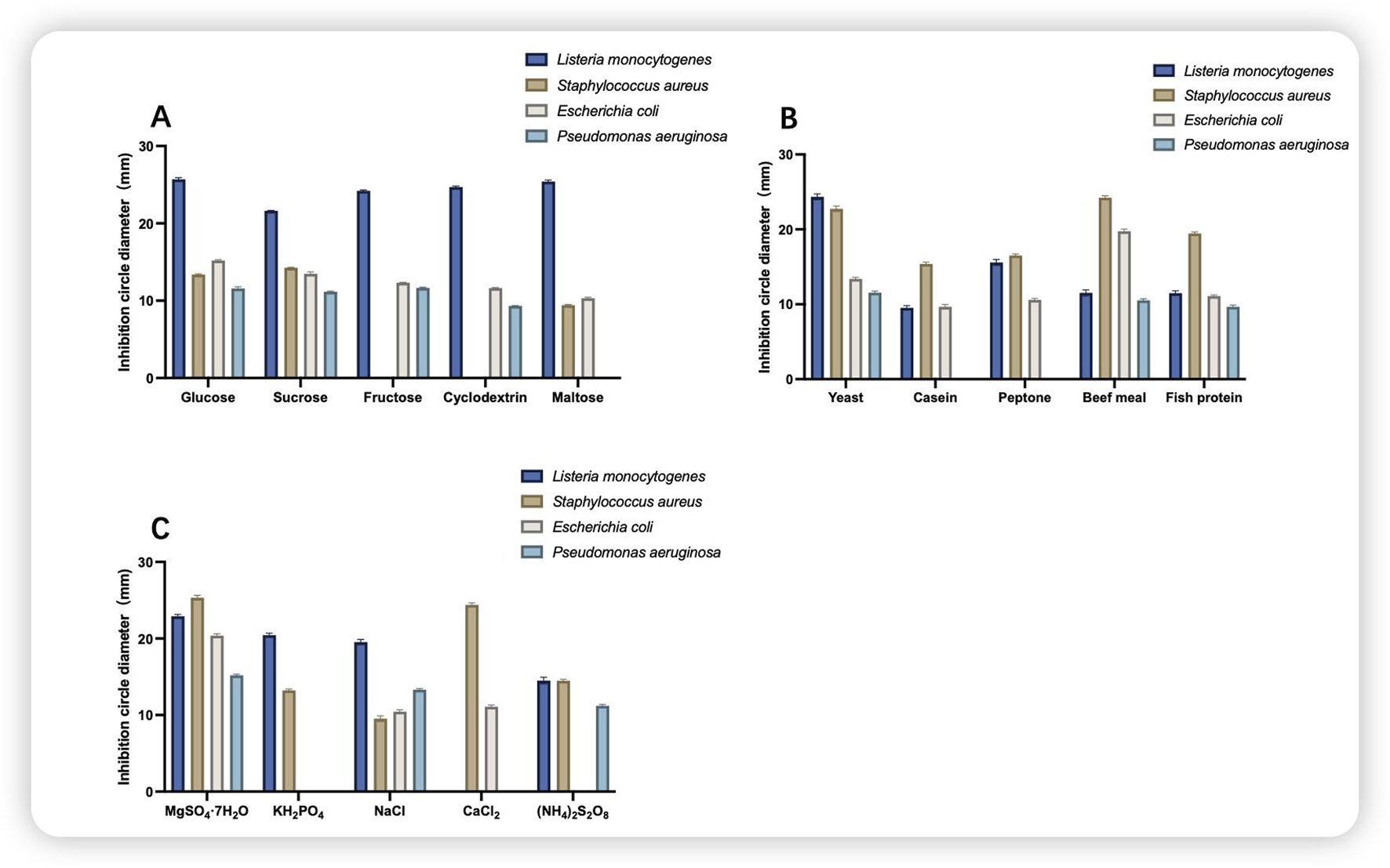
Inhibitory effects of different components of the medium. (A) Comparison of the size of the circle of inhibition on different carbon sources; (B) comparison of the size of the circle of inhibition on different nitrogen sources; (C) comparison of the size of the circle of inhibition on different inorganic salts.
3.3 Optimisation of the amount of each ingredient added
As shown in Figure 4A, glucose at the right concentration will have a certain inhibitory effect on the indicator bacteria, and the inhibitory effect may be deteriorated by using too much, and the inhibitory effect on the indicator bacteria will be most obvious when the concentration of glucose is 3.0 g/100 mL. As shown in Figure 4B, the inhibitory effect on the growth of indicator bacteria was more pronounced at a yeast concentration of 2.0 g/100 mL, and disappeared as the concentration was increased to 6.0 g/100 mL for the growth of P. aeruginosa and E. coli. As shown in Figure 4C, when the MgSO4·7H2O concentration was 1.2 g/100 mL, the most pronounced inhibitory effect was observed. Consequently, this concentration was selected as the inorganic salt concentration for the medium.
FIGURE 4
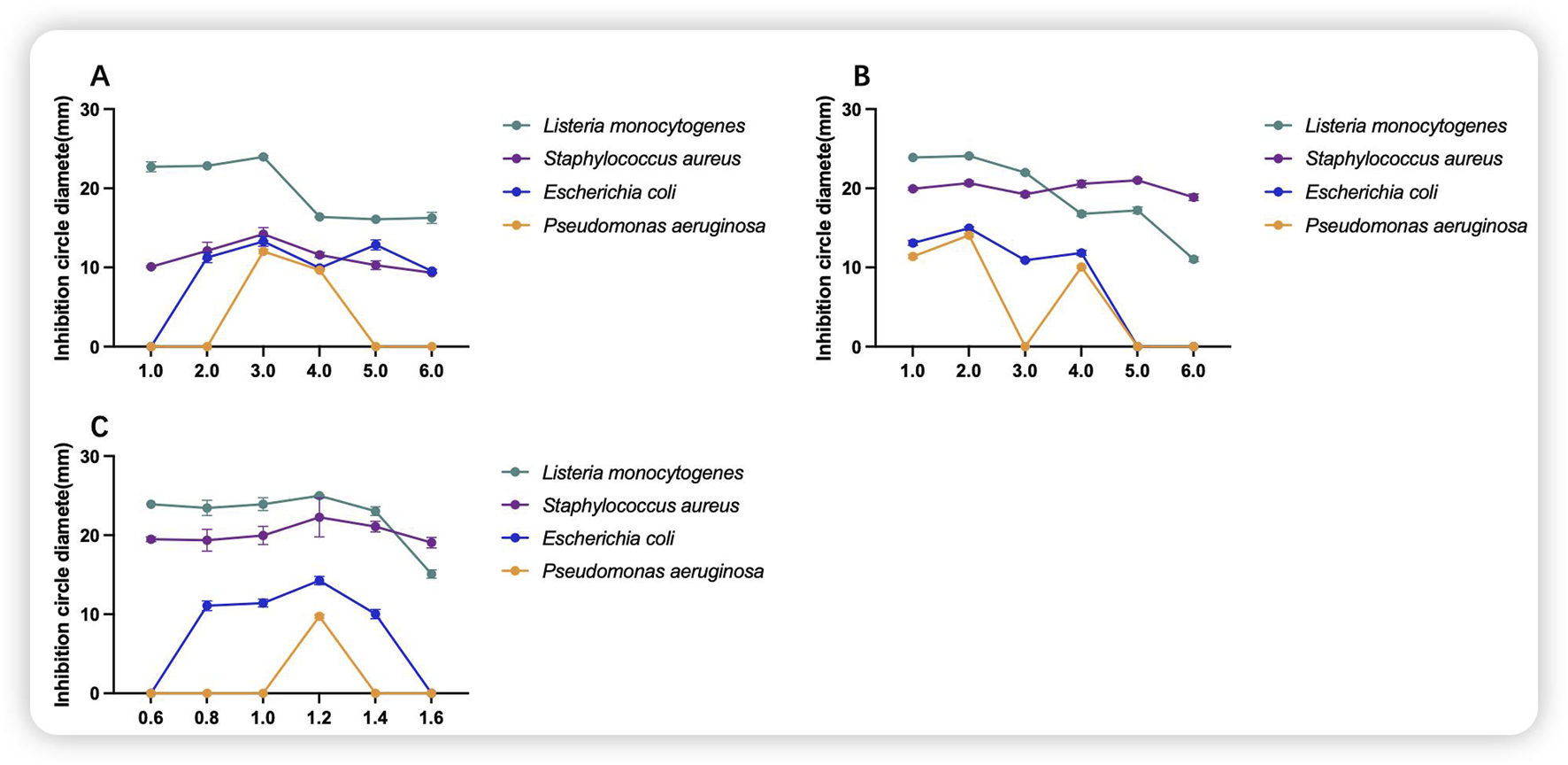
Inhibitory effects of component additions to the culture medium. (A) Glucose; (B) yeast; (C) MgSO4·7H2O; (g/100 mL).
3.4 Orthogonal test results
The results of the one-factor level test indicated that glucose, yeast and MgSO4·7H2O were the components of the fermentation medium. Latin software was employed to select three factors and three levels for orthogonal test optimisation, and the resulting level scheme is presented in Table 1. As shown in Tables 1–3, as a component of the medium, yeast was the main influencing factor followed by glucose and magnesium sulphate heptahydrate. According to the comparison between K values, the most pronounced inhibitory effect was observed in group 9. Therefore, the optimal culture conditions for strain G7 were identified as 4.5 g/100 mL glucose, 1.5 g/100 mL yeast, and 1.2 g/100 mL MgSO4·7H2O.
TABLE 2
| Indicator bacteria | |||||
|---|---|---|---|---|---|
| Staphylococcus aureus | Listeria monocytogenes | Escherichia coli | Pseudomonas aeruginosa | ||
| Inhibition circle diameter (mm) | Group 1 | 13.97 ± 0.12 | 16.11 ± 0.2 | — | — |
| Group 2 | 20.82 ± 0.57 | 18.34 ± 0.52 | 10.4 ± 0.82 | — | |
| Group 3 | 22.65 ± 0.31 | 21.35 ± 0.40 | 12.28 ± 0.36 | — | |
| Group 4 | — | 17.3 ± 0.30 | — | 10.95 ± 0.45 | |
| Group 5 | 20.41 ± 0.40 | 19.08 ± 0.45 | 11.98 ± 0.39 | 9.75 ± 0.25 | |
| Group 6 | 19.59 ± 0.45 | 20.05 ± 0.49 | 12.45 ± 0.77 | — | |
| Group 7 | — | 16.98 ± 0.27 | — | 10.98 ± 0.54 | |
| Group 8 | 22.90 ± 0.13 | 17.80 ± 0.40 | 12.13 ± 0.20 | — | |
| Group 9 | 23.90 ± 0.21 | 22.65 ± 0.29 | 13.76 ± 0.21 | 9.90 ± 0.19 | |
Inhibitory effects of different fermentation broths in the orthogonal test.
TABLE 3
| Factor | DV | TSS | df | MS | F | P-value |
|---|---|---|---|---|---|---|
| Glucose | Staphylococcus aureus | 51.511 | 2 | 25.756 | 0.913 | 0.523 |
| Listeria monocytogenes | 0.450 | 2 | 0.225 | 0.441 | 0.694 | |
| Escherichia coli | 1.722 | 2 | 0.861 | 1.234 | 0.448 | |
| Pseudomonas aeruginosa | 94.671 | 2 | 47.336 | 5.454 | 0.155 | |
| Yeast | Staphylococcus aureus | 582.419 | 2 | 291.210 | 10.322 | 0.088 |
| Listeria monocytogenes | 31.988 | 2 | 15.994 | 31.295 | 0.31 | |
| Escherichia coli | 298.696 | 2 | 149.348 | 214.126 | 0.005 | |
| Pseudomonas aeruginosa | 30.972 | 2 | 15.486 | 1.784 | 0.359 | |
| MgSO4·7H2O | Staphylococcus aureus | 35.571 | 2 | 17.786 | 0.630 | 0.613 |
| Listeria monocytogenes | 3.492 | 2 | 1.746 | 3.416 | 0.226 | |
| Escherichia coli | 0.032 | 2 | 0.016 | 0.023 | 0.978 | |
| Pseudomonas aeruginosa | 94.698 | 2 | 47.349 | 5.456 | 0.155 | |
| SEM | Staphylococcus aureus | 3037.612 | 2 | 28.212 | ||
| Listeria monocytogenes | 3235.232 | 2 | 0.511 | |||
| Escherichia coli | 893.956 | 2 | 0.697 | |||
| Pseudomonas aeruginosa | 427.037 | 2 | 8.679 |
Analysis of variance table (ANOVA).
3.5 Stability results of fermentation broth
The pH of the control protein was 6.5. As shown in Figure 5A, different pH values affected the antimicrobial activity of the CFS for all four indicator bacteria. Among pH 8 and 9, the size of the circle of inhibition of CFS against E. coli (control: 15.96 ± 0.66) was 12.24 ± 0.24 and 12.12 ± 0.38, and the size of the circle of inhibition of CFS against P. aeruginosa (control: 13.96 ± 0.06) was 11.92 ± 0.33 and 10.05 ± 0.28. Compared with that of the control, and the antimicrobial activity was highly significantly reduced (P < 0.01). As shown in Figure 5B, compared withthe control group, the antimicrobial activity of CFS against L.monocytogenes (control:23.78 ± 0.29) decreased significantly (P < 0.05) with increasing UV irradiation time with a circle of inhibition size of 22.56 ± 0.59 after 80 min and against E. coli after 90 min (P < 0.05) with a circle of inhibition size of 14.14 ± 0.39. CFS was heated in a water bath at 40–100°C for 60 min and autoclave at 121°C for 20 min before observing the change in antimicrobial activity. As shown in Figure 5C, the CFS was stable at 40–60 °C, with no significant difference. With increasing temperature, the antimicrobial activity of the CFS against E. coli and P. aeruginosa decreased significantly at 80 °C (P < 0.05), and at 100 °C, there was no antimicrobial activity against P. aeruginosa. At 120 °C, only antimicrobial activity against S. aureus was detected. As shown in Figure 5D, the control group was not subjected to enzyme treatment. compared with that of the control group, the antimicrobial activity of the culture broth treated with trypsin against E. coli decreased. The antimicrobial activity of the culture broth treated with papain showed a decline against L. monocytogenes, E. coli, and P. aeruginosa. The antimicrobial activity of the culture broth treated with pepsin and protease E against E. coli, P. aeruginosa, S. aureus and L. monocytogenes decreased. As shown in Figures 5E–G, the antimicrobial activity of the CFS stored at 4°C, −20°C and −80°C for 0–18 days remained relatively stable compared with that of the control group.
FIGURE 5
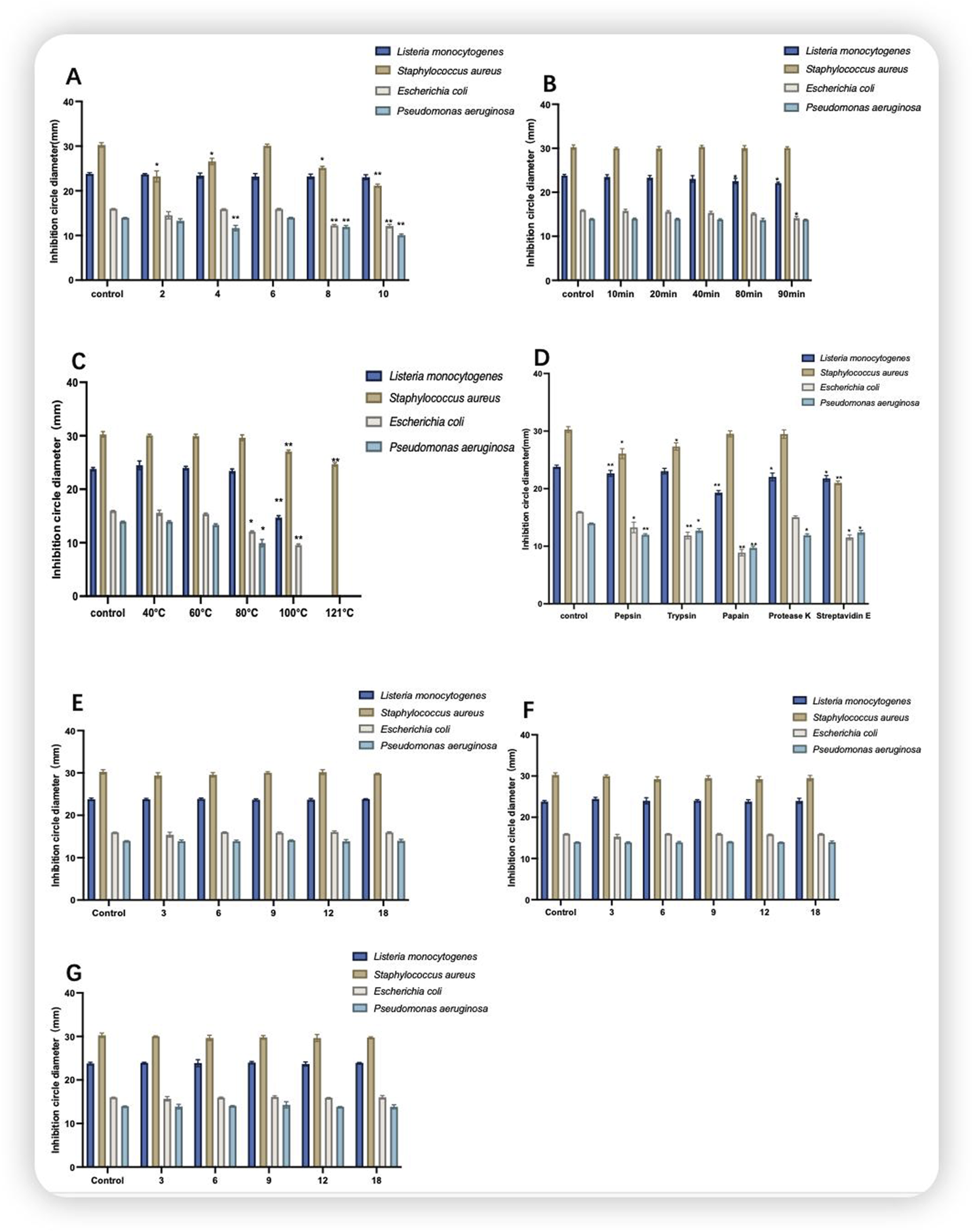
Stability of the cell-free supernatant. (A) Acid-base stability; (B) UV stability; (C) temperature stability; (D) enzyme stability; (E) 4°C storage stability; (F) −20°C storage stability; (G) −80°C storage stability; where Control is the control condition; note that *indicates a significant difference (P < 0.05) and **indicates a highly significant difference (P < 0.01).
3.6 Purification of bacteriocin P7
Gel chromatography separates molecules based on their size; within the column, molecules larger than the gel voids are excluded and left directly out of the column, small molecules seep into the gel voids, and medium-sized molecules fall somewhere in between. The main components of the gel column packing GF75 used in this experiment are highly cross-linked agarose and dextran. The separation range for linear molecules is 0.5kd∼30kd, for spherical molecules 3kd∼70kd, and for nucleic acids less than 50bp. Following gel chromatography of the CFSs via the TA-GF75 method, three distinct peaks were obtained. As shown in Figure 6, the second peak exhibited notable bacteriostatic efficacy. The fraction corresponding to the second peak was concentrated and subjected to further purification via Tiderose Q HP anion chromatography to finalise the three peaks. Ion chromatography controls the adsorption and desorption of various ionic substances on the surface of the resin to achieve the separation and detection of ions. As shown in Figure 7, the initial peak exhibited notable bacteriostatic efficacy. The fraction collected and corresponding to the initial peak was subsequently concentrated and purified via RP-HPLC, the working principle is that each component of the mixture has a different magnitude and strength of interaction between the mobile and stationary phases, resulting in different retention times in the stationary phase, which sequentially flow out of the column and into the detector for detection. As shown in Figure 8A, Three different peaks appeared, with the initial peak showing significant antimicrobial activity. We collected the initial peak and then purified it again by RP-HPLC as shown in Figure 8B as a single peak.
FIGURE 6
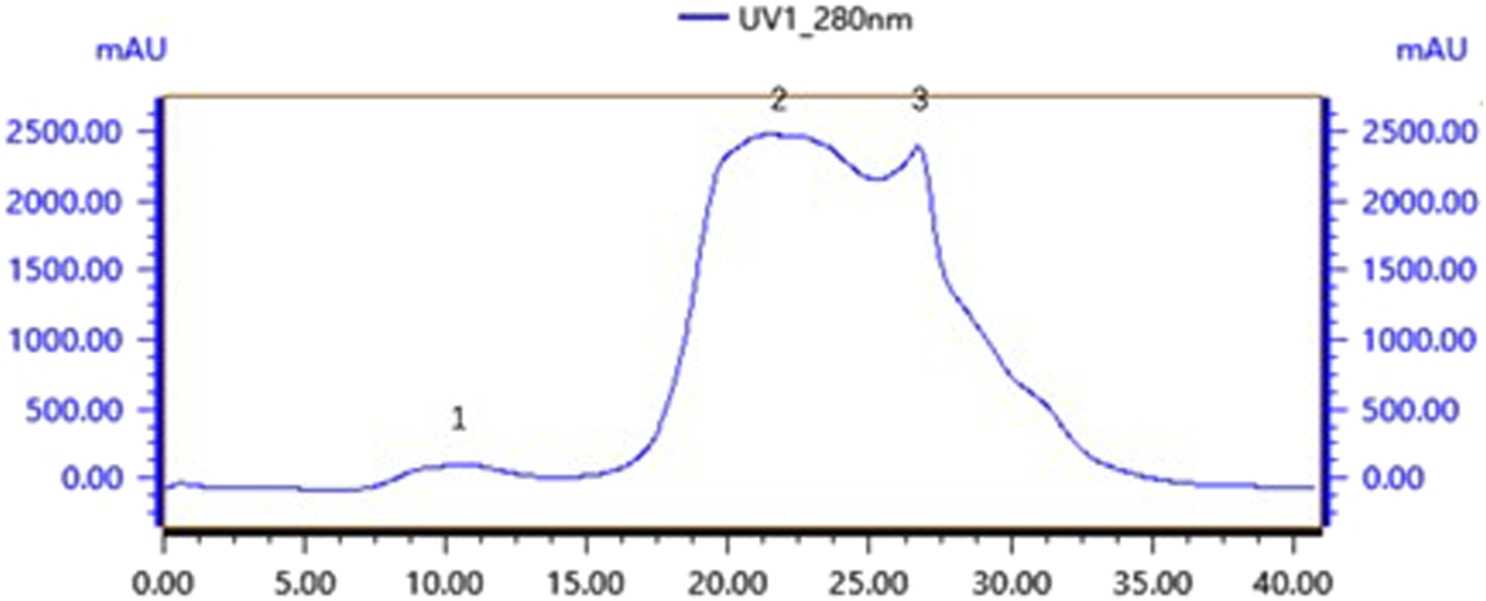
TA-GF75 gel chromatography.
FIGURE 7
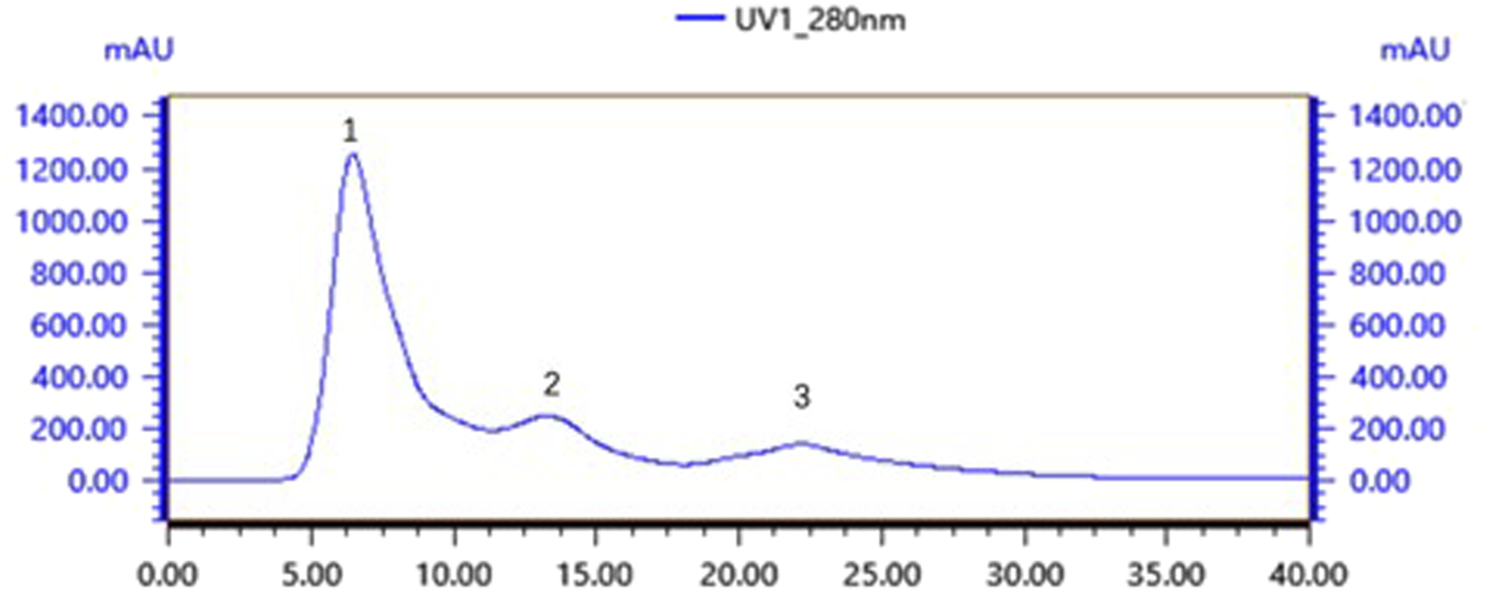
Tiderose Q HP anion chromatography.
FIGURE 8
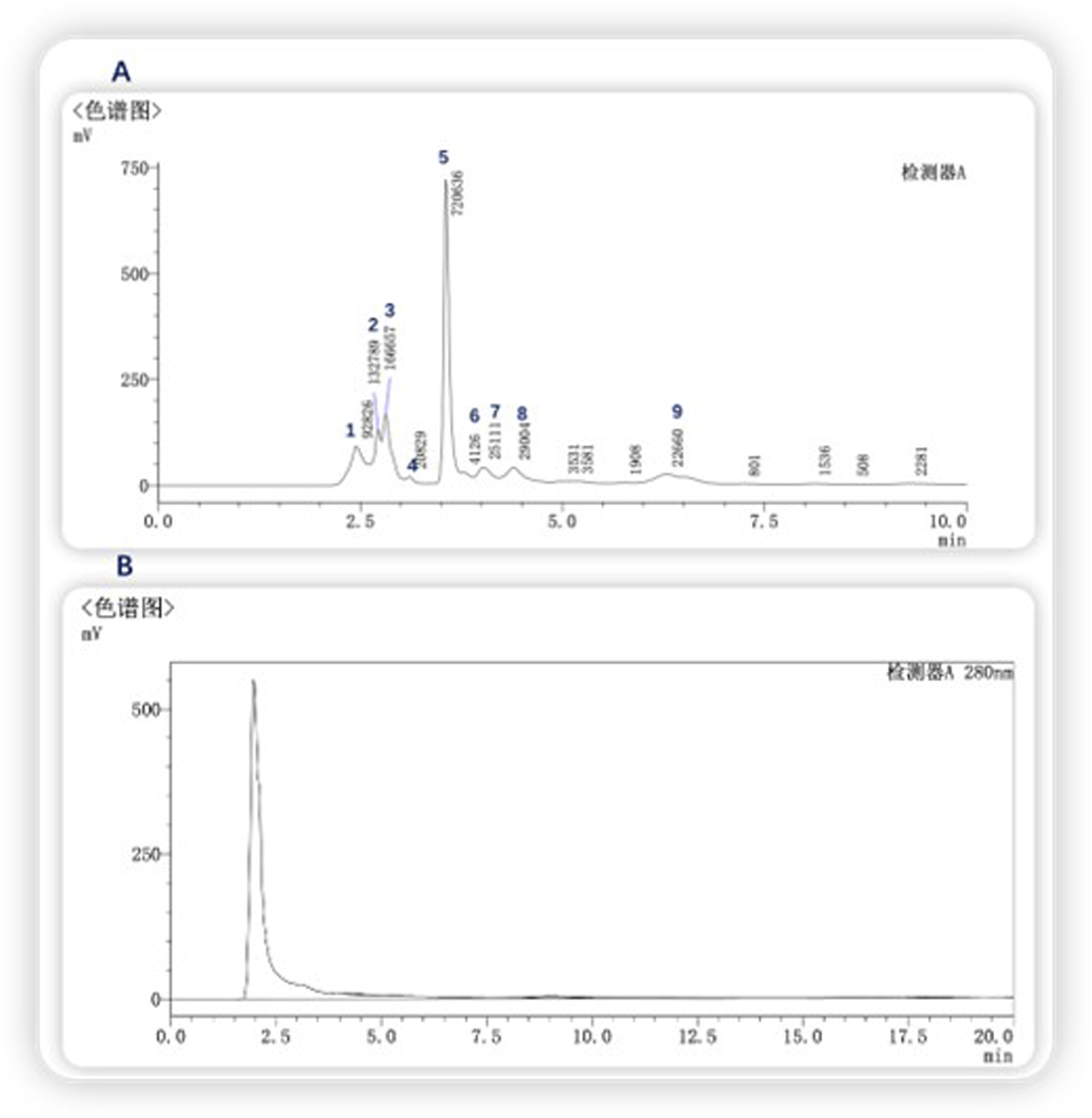
RP-HPLC purification (A) First isolation and purification (B) Second isolation and purification.
Furthermore, tricine-SDS‒PAGE analysis of bacteriocin P7 revealed that its molecular weight fell within the range of 6.5–14.4 KDa. The antimicrobial activity of the protein was measured by solid agar perforation method, The same research method was used by Haotian Ma (Ma et al., 2025) and Jinju Peng (Peng et al., 2024). The diameters of the antimicrobial circles of the protein (60.704 μg/mL) against S. aureus and L. monocytogenes were 27.49 and 19.21 mm, respectively (Figure 9).
FIGURE 9
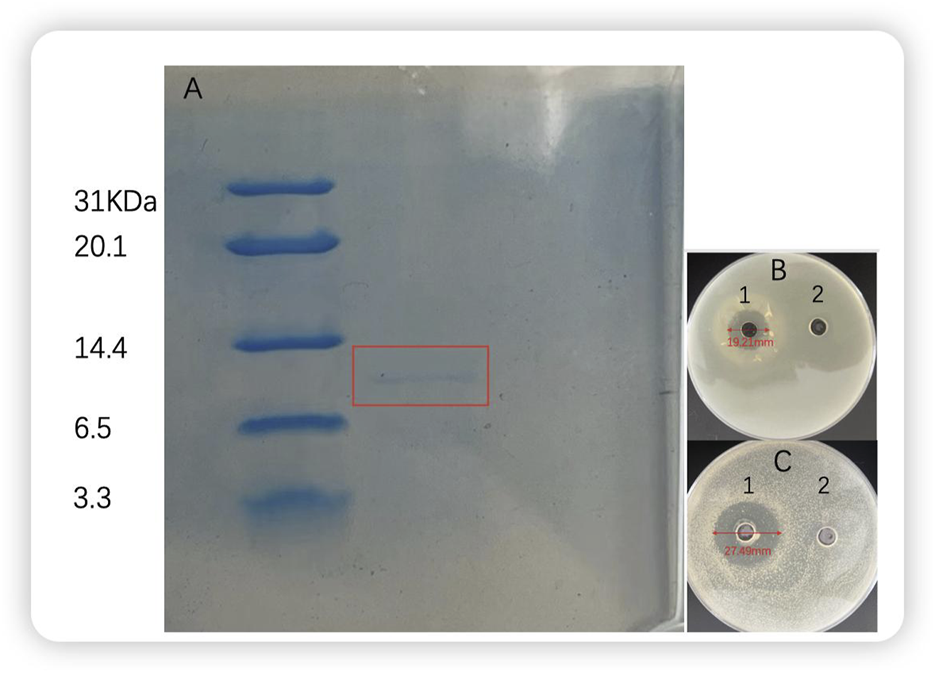
Gel electrophoresis and antibacterial activity of bacterial P7 (A) Bacterial P7 gel electrophoresis. M: marker; 1: bacteriocin P7; (B)Listeria monocytogenes(C)Staphylococcus aureus 1: bacteriocin P7; 2: blank control.
3.7 LC‒MS/MS
The results were subjected to analysis via LC‒MS/MS and are presented in Table 4. On the basis of the coverage and relative molecular weight of the bacteriocin, it was postulated that the substance should be a class I bacteriocin, similar to amyloliquecidin GF610. This bacteriocin was shown to be active against L. monocytogenes, Clostridium perfringens, Clostridium difficile, S. aureus and Bacillus acidophilus, with MICs ranging from 0.5 to 7.0 μmol/L,the mic against S. aureus was 10 umol/L (Gerst et al., 2022). Bacteriocin p7 isolated in the present study had similar antimicrobial activity and was more effective against S. aureus and L. monocytogenes, where the mic against S. aureus was 30.352 μg/mL. Consequently, this bacteriocin is considered a novel bacteriocin with similarities to amyloliquecidin GF610.
TABLE 4
| Protein name | Sequence | Coverage [%] | MW [kDa] |
|---|---|---|---|
| Single-stranded | MKPASKKRGGYGLFNHVMLVGRLTKDPELRFTSAGIPVAHITLAVNRNFKSA | ||
| DNA-binding protein | SGETGTDFVNCTIWRKNAENTALYCQKGSMVGVSGRIQTRSYEKTDGVKVYVTEVMADTVRFMDQKRKEPLAE | 5 | 13.9 |
| YfzA-like protein | MDNEMDKVNKKRPLYKKGWFLTVLAFLVSQLYFNFAELTGWGPNYREMNG | 7 | 12.1 |
| FPANIAELDFFQTYLSFYDNPWFNIVTVFLGVFTVIQIITGITKDIRNESNNF | |||
| Small ribosomal subunit protein | MKHMPNIKSAIKRTKTNNERRAHNATIKSAMRTAIKQVEASVANNEADKAKT | 9 | 9.9 |
| ALSEAAKRIDKAVKTGLVHKNAAARYKSRLAKQVNGLSA | |||
| Amyloliquecidin GF610 | MKGGDIMSNREKAELYRNASKRTELGFVNPVGEVSEDELRNLAGAADVTPHT | 13 | 8.1 |
| TPSSLPCGVFVTAAFCPSTKCTSSC | |||
| Fur-regulated basic protein | MAPLLREAINRKKQHLRTKLIRSGFYQDHVQELSGYTLSELEKEYEAVKRLKKAELH | 11 | 6.8 |
| Small, acid-soluble spore protein | MSFFNKEKGKNSDKNKNVIQGALEDAGAALKDDPLQEAVQKKKNNR | 17 | 5.1 |
| DUF3941 domain-containing protein | MKDDDKKPLDNNAVNQKKNRLAEKNRQAGKNQNSKKPDHL | 15 | 4.6 |
Identification of several proteins by LC-MS/MS.
3.8 Physicochemical properties of bacteriocin P7
The MIC of purified bacteriocin P7 against S. aureus was determined to be 30.352 μg/mL. As shown in Figure 10A, the haemolytic activity of the bacteriocin at concentrations of MIC-2 × MIC was less than 10%, and at a concentration of 4 × MIC, the haemolytic activity was 10.949%, which was slightly toxic. Overall, the concentrations of the MIC-2 × MIC bacteriocin are safe. As illustrated in Figure 10B, the five organic solvents had no notable effect on the antimicrobial activity. Interestingly, organic solvents such as SDS increased the inhibitory activity of bacteriocin P7. As shown in Figure 10C, compared with the control group, the MgCl2 and CaCl2 groups presented highly significant (P < 0.05) and significant (P < 0.01) reductions in the antimicrobial activity of bacteriocin P7, respectively. In contrast, the remaining substances had no significant effect.
FIGURE 10
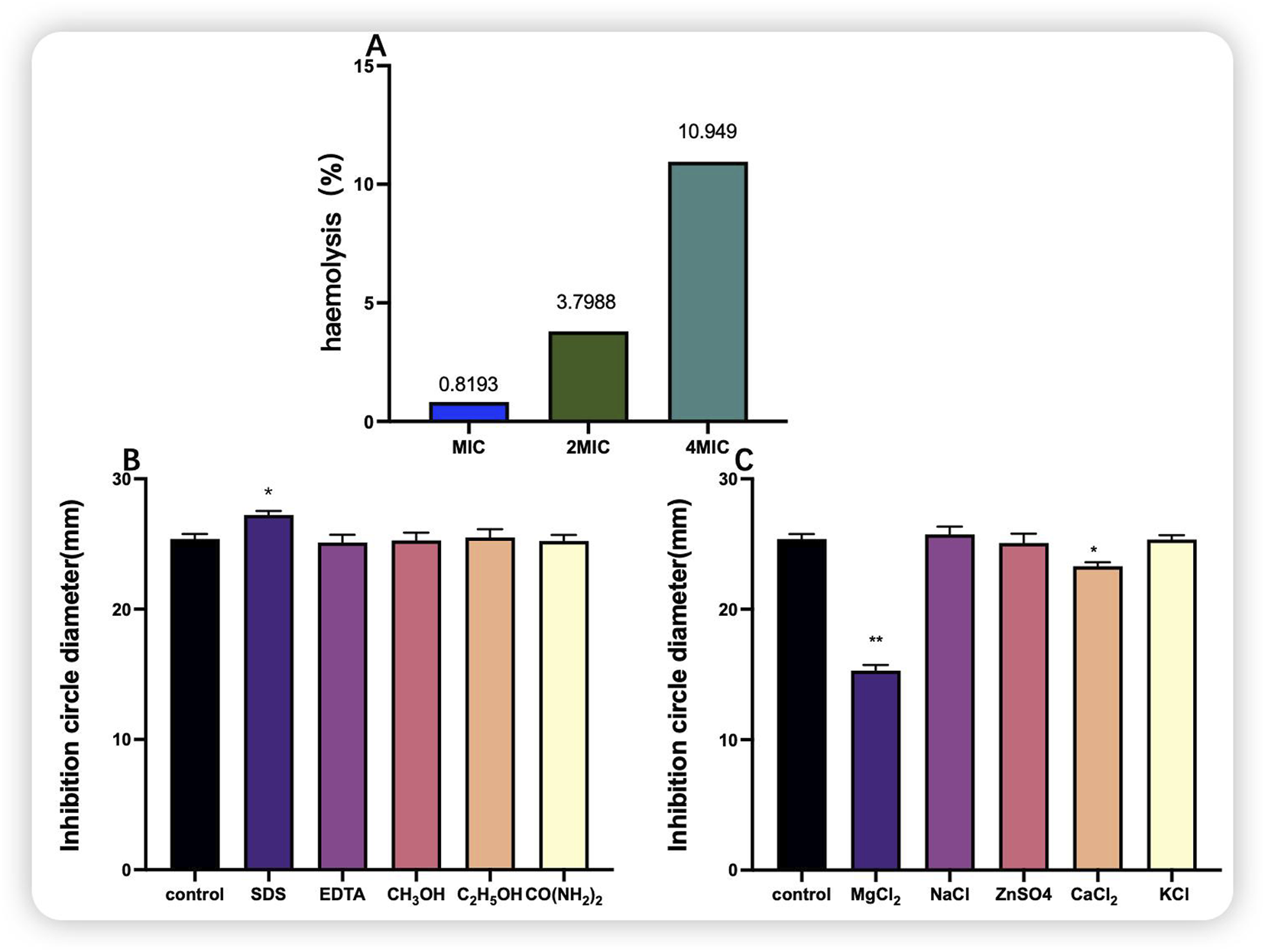
Haemolytic activity of bacteriocin P7 and the effects of organic solvents and inorganic salts on bacteriocin P7. (A) Haemolytic activity; (B) organic solvent; (C) inorganic salt. Note: *indicates a significant difference (P < 0.05), **indicates a highly significant difference (P < 0.01).
4 Discussion
Bacteriocins are small peptides synthesized by ribosomes. They are secreted to inhibit the growth of competing bacteria, fungi and some parasites. Therefore, bacteriocins have potential applications in food preservation, animal husbandry, agriculture, and medicine (Fernandes and Jobby, 2022). In the food industry, bacteriocins are always used as natural preservatives to extend the shelf life of food and to prevent spoilage of dairy products. Nisin is the first FDA-approved bacteriocin that has been used in the preservation of pasteurized processed cheese spread (Lahiri et al., 2022). Also, some bacteriocins are used in animal clinical studies and experiments, for instance, the therapeutic efficacy of pyocins had been confirmed in a murine model of P. aeruginosa sepsis (Six et al., 2021).The composition of the medium has a very large effect on bacteriocin production and bacterial growth, so the production medium needs to be optimised to maximise metabolite production. Carbon is the most important medium component, and the rate of metabolism of carbon sources usually affects the formation of biomass and the production of primary or secondary metabolites (Wang et al., 2018). In this study, glucose was chosen as the carbon source of the fermentation medium, with better antimicrobial activity, and the best effect was achieved at a concentration of 4.5 g/mL, and its antimicrobial effect was affected by either too high or too low concentration. Similar outcomes were achieved by Chang Qing Zhao (Zhao et al., 2017), who optimised the medium for the production of microbial flocculants from B. subtilis and identified 20 g/L glucose as the most effective carbon source. The selection of carbon sources as key macronutrients for the growth and reproduction of productive microorganisms and their metabolic activities is considered the first step in the optimisation of culture media. The assimilative capacity of certain carbon sources, as well as their nature and origin, affects biomass growth as well as the type and yield of metabolites (Vlajkov et al., 2022b).
In terms of nitrogen sources, yeast resulted in higher production of bacteriocin P7. This may be because microorganisms can utilise inorganic or organic nitrogen sources. In some cases, the use of specific amino acids can increase productivity; in contrast, unsuitable amino acids may inhibit the synthesis of secondary metabolites (Singh et al., 2017). In the present study, when casein was used as a nitrogen source, the antimicrobial activity of strain G7 was decreased and activity against P. aeruginosa disappeared.
Moreover, MgSO4·7H2O was chosen as the inorganic salt component of this medium and was able to increase the expression of bacteriocin P7. Mg2+ is involved in a variety of physiological roles, such as signalling, energy metabolism, fatty acid synthesis, ribosome stabilisation and protein synthesis. The effects of many of these metal ions on the physiological activity of microorganisms are concentration dependent, with low concentrations often showing stimulatory effects and high concentrations showing inhibitory effects (De Leersnyder et al., 2018). Fakher Kamoun (Kamoun et al., 2009) optimized the culture conditions with a carbon/nitrogen ratio of 9, which resulted in more than a fourfold increase in bacteriocin production compared with the use of TSB medium. Yonghong Li (Li et al., 2022) optimised the formulation of the medium by flask fermentation, and Clostridium butyricum DL-1 reached a viable count of 1.5 × 108 cfu/mL, which was 375 times greater than that of the initial medium culture. The spore production rate was 92.6%. Therefore, the maximum product concentration can be achieved by media optimisation.
Bacillus species are capable of producing endospores that exhibit exceptional resilience to unfavourable conditions. These endospores display high-temperature resistance, rapid resurrection, and the secretion of enzymes, enabling the species to survive in both aerobic and anaerobic environments (Owusu-Darko et al., 2020). As a consequence, the CFS of B. velezensis G7 in this study was stable at temperatures between 40°C and 60°C. However, a notable decline in antimicrobial activity was observed following an increase in temperature to 80°C. The antimicrobial activity of CFS treated at 80°C was reduced compared to the control, but it was able to maintain its activity for 60min at this temperature, and the antimicrobial activity of CFS at 80°C was reduced, which indicates that its antimicrobial activity could be preserved after a batch pasteurization process (63°C–30 min) or an HTST (High Temperature Short Time) continuous flow pasteurization (72°C–15 s) in dairy products. Moreover, liquid egg products undergo heat treatments at specific times (3.55–6.2 min) and temperatures (55.6–63.3°C). Therefore, these treatments would not affect the antibacterial activity of CFS since temperatures applied are below 80 °C (León Madrazo et al., 2022).
Furthermore, the antimicrobial activity of the CFS was diminished in the presence of UV treatment under various conditions. This may be attributed to the potential inactivation of certain antimicrobial substances resulting from elevated temperatures and UV exposure (Ming et al., 2022). UV has bactericidal and sporicidal properties. The main target of UV damage in microbial cells is DNA, and UV light can act directly on DNA molecules in cells, causing neighbouring pyrimidines on the same DNA strand to form thymine dimers, which leads to changes in the structure of double-stranded DNA, and affects the normal replication of DNA, and ultimately leads to cell death (Begyn et al., 2020). Melanin has been reported to act as a UV-protectant, possibly by absorbing radiation before penetrating the DNA in the spore core or protoplast. melanin synthesis can also be induced by mutations, metal ions and amino acids (Idris et al., 2024). Organic substances and compounds are essential to reduce the negative effects of UV radiation on Bacillus cells. TiO2 nanoparticle mixture (anatase and rutile) increase the persistence of B. thuringiensis to the UV radiation (Jalali et al., 2020).
CFS exhibited the highest activity at pH 6 and demonstrated a decrease in inhibitory activity in acidic and alkaline environments. However, it maintained more than 50% activity between pH 2 and 10, which may be attributed to the diverse antimicrobial substances produced by different Bacillus species. For example, Lactobacillus rhamnosus LS8, which was studied by Lihui Zhang et al. (Wang et al., 2022), was only active under acidic conditions. CFS was found to be sensitive to pepsin, protease E, proteinase K and trypsin, as determined by an enzyme stability assay, indicating that the antimicrobial substances produced by B. velezensis G7 are natural proteins. The antimicrobial substance produced by B. velezensis is sensitive to pepsin, papain, trypsin, proteinase k, and protease E. The antimicrobial substance has also been confirmed to be a peptide (Wayah and Philip, 2018). The CFS of Pediococcus pentosaceus LB44 is also sensitive to proteinase K, papain and trypsin (Kaur and Tiwari, 2018). Bacillus secretes one or more bacteriostatic active substances during metabolism, and bacteriocin, as one of its secondary metabolites, has the advantages of a wide range of bacteriostatic inhibition, stable physicochemical properties, and excellent safety (Wang H. et al., 2024). B. velezensis bacteriocins have a wider range of bactericidal activity than other bacteriocins and are active against both gram-positive and gram-negative bacteria (Vaca et al., 2022).
The haemolytic activity of bacteriocin P7 produced by B. velezensis G7 was determined, and it was found that the haemolysis rate was less than 5% at concentrations in the MIC-2MIC range. Therefore, it can be concluded that bacteriocin P7 can be used safely in this concentration range. The antimicrobial activity of bacteriocin P7 was diminished in the presence of CaCl₂ and MgCl₂; however, it was more stable in the presence of other inorganic salts and organic solvents. Furthermore, SDS was shown to enhance the antimicrobial activity of bacteriocin P7. This probably due to the SDS is an anionic surfactant, low concentration of SDS affects the permeability of cell membrane and promotes the signal transmission between cells and cells. Meanwhile, facilitates the secretion of extracellular proteins and polysaccharides and other substances, which are involved in the process of biofilm formation. It is worth mentioning that when studying the effect of organic solvents on bacteriocins, relevant studies also found that SDS increased the activity of bacteriocins (Xi et al., 2018).
These favourable characteristics indicate that this antimicrobial protein may have potential applications in feed processing, drug therapy and other related fields. Bacteriocins can be used in food preservation to treat infections and antibiotic-resistant pathogenic bacteria (Chikindas et al., 2018; Kaškonienė et al., 2017).
With the further development of biotechnology and in-depth research on the structure and function of various proteins, protein isolation and purification techniques have developed rapidly, and chromatography, electrophoresis, molecular blotting and other techniques can be used to isolate target proteins (Liu et al., 2020). For example, the antimicrobial protein PAG14, which was isolated from the metabolites of B. velezensis G14 via dextran gel and ion chromatography, was classified by LC‒MS/MS as a class III bacteriocin, similar to lysozyme C (Peng et al., 2024). A novel bacteriocin, LFX01, of Lactobacillus plantarum LF-8, which was isolated from the intestine of tilapia, showed excellent stability under heat and acid‒base stresses and exhibited sensitivity to a variety of enzymes, such as proteinase K, pepsin and trypsin (Jiang et al., 2022). In this study, the bacteriocin was purified via gel chromatography, anion chromatography and RP-HPLC and identified as an amyloliquecidin GF610 beta analogue via LC‒MS/MS (Gerst et al., 2022). Amyloliquecidin GF610 is a two-component wool‒sulfur antimicrobial peptide, and its α and β peptides have single equivalent volume masses of 3026 and 2451 Da, with molecular formulas of C130H191N35O39S5 and C110H158N26O30S4, respectively. Bacteriocin P7 has significant inhibitory effects on a variety of pathogenic bacteria and is a potential antimicrobial drug with broad application prospects.
5 Conclusion
The optimal medium composition for bacteriocin production in B. velezensis G7 includes 4.5 g/100 mL glucose, 1.5 g/100 mL yeast extract, and 1.2 g/100 mL MgSO4·7H2O. The CFS exhibited enhanced stability at 40–60°C, 4, −25, and −80°C storage. And remains active under prolonged UV exposure. The bacteriostatic activity remained consistent at pH 6. Among the various substances tested, Proteinase E had the greatest effect on the activity of bacteriocin P7. Furthermore, the class I bacteriocin produced by B. velezensis G7 was purified and identified as responsible for the production of bacteriocin P7, which demonstrated a pronounced inhibitory effect on S. aureus, with a molecular weight of approximately 6.5–14.4 kDa. Given its stability, broad-spectrum antimicrobial activity, and safety profile, bacteriocin P7 holds potential for applications in food preservation, as a feed additive, or even as a novel antimicrobial agent for controlling infections. Further studies should focus on genetically engineering bacteriocin P7 for large-scale production, as well as evaluating its efficacy in in vivo models to fully assess its potential for industrial and clinical applications.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies on animals in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
YL: Data curation, Writing–original draft, Writing–review and editing. HM: Data curation, Formal Analysis, Writing–original draft. RP: Data curation, Writing–original draft. YL: Formal Analysis, Writing–original draft. YZ: Data curation, Writing–original draft. MY: Data curation, Writing–original draft. JP: Data curation, Writing–original draft. YM: Data curation, Methodology, Project administration, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by the Natural Science Foundation of Guangdong Province (2023A1515012181).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Acharya S. Jali P. Pradhan M. Pradhan C. Mohapatra P. K. (2023). Antimicrobial and Antioxidant property of a True mangrove Rhizophora apiculata Bl. Chem. & Biodivers.20 (9), e202201144. 10.1002/cbdv.202201144
2
Ahsan T. Liang C. Yu S. Pei X. Xie J. Lin Y. et al (2023). Screening and optimization of fermentation medium for Bacillus velezensis BP-1 and its biocontrol effects against Peyronellaea arachidicola. Appl. Sci.13 (0), 4653. 10.3390/app13084653
3
Banerjee G. Nandi A. Ray A. K. (2017). Assessment of hemolytic activity, enzyme production and bacteriocin characterization of Bacillus subtilis LR1 isolated from the gastrointestinal tract of fish. Archives Microbiol.199 (1), 115–124. 10.1007/s00203-016-1283-8
4
Bastos E. M. Simone M. Jorge D. M. Soares A. E. E. Spivak M. (2008). In vitro study of the antimicrobial activity of Brazilian propolis against Paenibacillus larvae. J. Invertebr. pathology97 (3), 273–281. 10.1016/j.jip.2007.10.007
5
Begyn K. Kim D. T. Heyndrickx M. Michiels C. Aertsen A. Rajkovic A. et al (2020). Directed evolution by UV-C treatment of Bacillus cereus spores. Int. J. Food Microbiol.317, 108424. 10.1016/j.ijfoodmicro.2019.108424
6
Chen S. Shi F. Liu F. Yang N. Xu X. Jin Y. (2025). Kinetics study on microbial growth and surfactin production of Bacillus subtilis ATCC 21332 under the synergistic effect of magnetic field and Mg2+. Food Biosci.63, 105546. 10.1016/j.fbio.2024.105546
7
Chikindas M. L. Weeks R. Drider D. Chistyakov V. A. Dicks L. M. (2018). Functions and emerging applications of bacteriocins. Curr. Opin. Biotechnol.49, 23–28. 10.1016/j.copbio.2017.07.011
8
De Leersnyder I. De Gelder L. Van Driessche I. Vermeir P. (2018). Influence of growth media components on the antibacterial effect of silver ions on Bacillus subtilis in a liquid growth medium. Sci. Rep.8 (1), 9325. 10.1038/s41598-018-27540-9
9
Elazzazy A. M. Mobarki M. O. Baghdadi A. M. Bataweel N. M. Al-Hejin A. M. (2024). Optimization of culture conditions and batch process control for the augmented production of bacteriocin by Bacillus species. Microorganisms12 (4), 651. 10.3390/microorganisms12040651
10
Fernandes A. Jobby R. (2022). Bacteriocins from lactic acid bacteria and their potential clinical applications. Appl. Biochem. Biotechnol.194 (10), 4377–4399. 10.1007/s12010-022-03870-3
11
Friess D. A. (2016). Mangrove forests. Curr. Biol.26 (16), R746–R748. 10.1016/j.cub.2016.04.004
12
Gerst M. M. Somogyi Á. Yang X. Yousef A. E. (2022). Detection and characterization of a rare two-component lantibiotic, Amyloliquecidin GF610 produced by Bacillus velezensis, using a combination of culture, molecular and bioinformatic analyses. J. Appl. Microbiol.132 (2), 994–1007. 10.1111/jam.15290
13
Gomes R. J. Ida E. I. Spinosa W. A. (2022). Nutritional supplementation with amino acids on bacterial cellulose production by Komagataeibacter intermedius: effect analysis and application of response surface methodology. Appl. Biochem. Biotechnol.194 (11), 5017–5036. 10.1007/s12010-022-04013-4
14
Guo Z. Gai C. Cai C. Chen L. Liu S. Zeng Y. et al (2017). Metabolites with insecticidal activity from Aspergillus fumigatus JRJ111048 isolated from mangrove plant Acrostichum specioum endemic to Hainan Island. Mar. Drugs15 (12), 381. 10.3390/md15120381
15
Hwanhlem N. Biscola V. El-Ghaish S. Jaffrès E. Dousset X. Haertlé T. et al (2013). Bacteriocin-producing lactic acid bacteria isolated from mangrove forests in Southern Thailand as potential bio-control agents: Purification and characterization of bacteriocin produced by Lactococcus lactis subsp. lactis KT2W2L. Probiotics Antimicrob. proteins5 (4), 264–278. 10.1007/s12602-013-9150-2
16
Idris A. L. Li W. Huang F. Lin F. Guan X. Huang T. (2024). Impacts of UV radiation on Bacillus biocontrol agents and their resistance mechanisms. World J. Microbiol. & Biotechnol.40 (2), 58. Published 2024 Jan 2. 10.1007/s11274-023-03856-1
17
Islam T. Rabbee M. F. Choi J. Baek K. H. (2022). Biosynthesis, molecular regulation, and application of Bacilysin produced by Bacillus species. Metabolites12 (5), 397. 10.3390/metabo12050397
18
Jalali E. Maghsoudi S. Noroozian E. (2020). A novel method for biosynthesis of different polymorphs of TiO2 nanoparticles as a protector for Bacillus thuringiensis from Ultra Violet. Sci. Rep.10 (1), 426. 10.1038/s41598-019-57407-6
19
Jiang Y. H. Xin W. G. Zhang Q. L. Lin L. B. Deng X. Y. (2022). A novel bacteriocin against Shigella flexneri from Lactiplantibacillus plantarum isolated from Tilapia intestine: purification, antibacterial properties and antibiofilm activity. Front. Microbiol.12, 779315. 10.3389/fmicb.2021.779315
20
Kamoun F. Zouari N. Saadaoui I. Jaoua S. (2009). Improvement of Bacillus thuringiensis bacteriocin production through culture conditions optimization. Prep. Biochem. & Biotechnol.39 (4), 400–412. 10.1080/10826060903209653
21
Kaškonienė V. Stankevičius M. Bimbiraitė-Survilienė K. Naujokaitytė G. Šernienė L. Mulkytė K. et al (2017). Current state of purification, isolation and analysis of bacteriocins produced by lactic acid bacteria. Appl. Microbiol. Biotechnol.101 (4), 1323–1335. 10.1007/s00253-017-8088-9
22
Kaur R. Tiwari S. K. (2018). Membrane-acting bacteriocin purified from a soil isolate Pediococcus pentosaceus LB44 shows broad host-range. Biochem. biophysical Res. Commun.498 (4), 810–816. 10.1016/j.bbrc.2018.03.062
23
Lahiri D. Nag M. Dutta B. Sarkar T. Pati S. Basu D. et al (2022). Bacteriocin: A natural approach for food safety and food security. Front. Bioeng. Biotechnol.10, 1005918. 10.3389/fbioe.2022.1005918
24
Lajis A. F. B. (2020). Biomanufacturing process for the production of bacteriocins from Bacillaceae family. Bioresour. Bioprocess.7 (1), 8–4365. 10.1186/s40643-020-0295-z
25
León Madrazo A. Fuentes Ortíz A. B. Morales Mendoza L. F. Segura Campos M. R. (2022). Antibacterial peptide fractions from chia seeds (Salvia hispanica L.) and their stability to food processing conditions. J. food Sci. Technol.59 (11), 4332–4340. 10.1007/s13197-022-05506-0
26
Li Y. Wang Y. Liu Y. Li X. Feng L. Li K. (2022). Optimization of an economical medium composition for the coculture of Clostridium butyricum and Bacillus coagulans. Amb. Express12 (1), 19. 10.1186/s13568-022-01354-5
27
Li Y. Wu Y. Peng Z. Long L. Guo Q. Tian L. et al (2024). Isolation and identification of bacteriocin-producing lactic acid bacteria from Daqu and mining of bacteriocin gene. Biologa79, 2891–2905. 10.1007/s11756-024-01746-x
28
Liu S. Li Z. Yu B. Wang S. Shen Y. Cong H. (2020). Recent advances on protein separation and purification methods. Adv. colloid interface Sci.284, 102254. 10.1016/j.cis.2020.102254
29
Ma H. Ding Y. Peng J. Li Y. Pan R. Long Y. et al (2025). Identification and characterization of a novel bacteriocin PCM7-4 and its antimicrobial activity against Listeria monocytogenes. Microbiol. Res.290, 127980. 10.1016/j.micres.2024.127980
30
Ming S. Chen X. Zhang N. Li S. Zhu Z. Cheng S. (2022). Structure and stability analysis of antibacterial substance produced by selenium enriched Bacillus cereus BC1. Archives Microbiol.204 (3), 196. 10.1007/s00203-022-02798-w
31
Owusu-Darko R. Allam M. Ismail A. Ferreira C. A. S. Oliveira S. D. d. Buys E. M. (2020). Comparative genome analysis of Bacillus sporothermodurans with its closest phylogenetic neighbor, Bacillus oleronius, and Bacillus cereus and Bacillus subtilis groups. Microorganisms8 (8), 1185. 10.3390/microorganisms8081185
32
Peng J. Xie X. Fan T. Ma H. Li Y. Luo S. et al (2024). Optimization of culture conditions for endophytic bacteria in mangrove plants and isolation and identification of bacteriocin. Front. Pharmacol.15, 1429423. 10.3389/fphar.2024.1429423
33
Selvam D. Thangarasu A. Shyu D.J. H. Neelamegam R. Muthukalingan K. Nagarajan K. (2021). Antimicrobial substance produced by Pseudomonas aeruginosa isolated from slaughterhouse sediment: physicochemical characterization, purification, and identification. Int. J. Peptide Res. Ther.27, 887–897. 10.1007/s10989-020-10135-2
34
Singh V. Haque S. Niwas R. Srivastava A. Pasupuleti M. Tripathi C. K. M. (2017). Strategies for fermentation medium optimization: An in-depth review. Front. Microbiol.7, 2087. Published 2017 Jan 6. 10.3389/fmicb.2016.02087
35
Six A. Mosbahi K. Barge M. Kleanthous C. Evans T. Walker D. (2021). Pyocin efficacy in a murine model of Pseudomonas aeruginosa sepsis. J. Antimicrob. Chemother.76 (9), 2317–2324. 10.1093/jac/dkab199
36
Sulaiman M. Nissapatorn V. Rahmatullah M. Paul A. K. Rajagopal M. Rusdi N. A. et al (2022). Antimicrobial secondary metabolites from the mangrove plants of Asia and the Pacific. Mar. drugs20 (10), 643. 10.3390/md20100643
37
Vaca J. Ortiz A. Sansinenea E. (2022). A study of bacteriocin like substances comparison produced by different species of Bacillus related to B. cereus group with specific antibacterial activity against foodborne pathogens. Archives Microbiol.205 (1), 13. 10.1007/s00203-022-03356-0
38
Vlajkov V. Anđelić S. Pajčin I. Grahovac M. Budakov D. Jokić A. et al (2022b). Medium for the production of Bacillus-based biocontrol agent effective against aflatoxigenic Aspergillus flavus: Dual approach for modelling and optimization. Microorganisms10 (6), 1165. 10.3390/microorganisms10061165
39
Vlajkov V. Pajčin I. Loc M. Budakov D. Dodić J. Grahovac M. et al (2022a). The effect of cultivation conditions on Antifungal and maize seed germination activity of Bacillus-based biocontrol agent. Bioeng. Basel, Switz.9 (12), 797. 10.3390/bioengineering9120797
40
Wang H. Li X. Wang Y. Tao Y. Lu S. Zhu X. et al (2018). Improvement of n-caproic acid production with Ruminococcaceae bacterium CPB6: selection of electron acceptors and carbon sources and optimization of the culture medium. Microb. cell factories17 (1), 99. 10.1186/s12934-018-0946-3
41
Wang H. Wang L. Zhang F. Li X. Wang S. Gao D. et al (2024b). ParalichenysinDY4, a novel bacteriocin-like substance, is employed to control Clostridium perfringens. Int. J. Biol. Macromol.279 (Pt 4), 135412. 10.1016/j.ijbiomac.2024.135412
42
Wang T. Zheng J. Dong S. Ismael M. Shan Y. Wang X. et al (2022). Lacticaseibacillus rhamnosus LS8 Ameliorates Azoxymethane/dextran sulfate sodium-induced colitis-associated tumorigenesis in mice via regulating gut microbiota and inhibiting inflammation. Probiotics Antimicrob. proteins14 (5), 947–959. 10.1007/s12602-022-09967-9
43
Wang Z. Zhang W. Wang Z. Zhang Z. Liu Y. Liu S. et al (2024a). Analysis of antimicrobial biological activity of a marine Bacillus velezensis NDB. Archives Microbiol.206 (3), 131. 10.1007/s00203-024-03861-4
44
Wayah S. B. Philip K. (2018). Characterization, yield optimization, scale up and biopreservative potential of fermencin SA715, a novel bacteriocin from Lactobacillus fermentum GA715 of goat milk origin. Microb. cell factories17 (1), 125. 10.1186/s12934-018-0972-1
45
Xi Q. Wang J. Du R. Zhao F. Han Y. Zhou Z. (2018). Purification and characterization of bacteriocin produced by a strain of Enterococcus faecalis TG2. Appl. Biochem. Biotechnol.184 (4), 1106–1119. 10.1007/s12010-017-2614-1
46
Zhao C. Yang Q. Zhang H. (2017). Optimization of microbial flocculant-producing medium for Bacillus subtilis. Indian J. Microbiol.57 (1), 83–91. 10.1007/s12088-016-0631-3
47
Zhao J. Zhou Z. Bai X. Zhang D. Zhang L. Wang J. et al (2022). A novel of new class II bacteriocin from Bacillus velezensis HN-Q-8 and its antibacterial activity on Streptomyces scabies. Front. Microbiol.13, 943232. Published 2022 Jul 29. 10.3389/fmicb.2022.943232
Summary
Keywords
mangroves, bacteriocin, optimization, Bacillus velezensis , culture
Citation
Li Y, Ma H, Pan R, Long Y, Zhao Y, Yu M, Peng J and Ma Y (2025) Optimisation of cultivation conditions for Bacillus velezensis G7 from mangrove plants and exploration of potential bacteriocins. Front. Pharmacol. 16:1530043. doi: 10.3389/fphar.2025.1530043
Received
18 November 2024
Accepted
07 February 2025
Published
26 February 2025
Volume
16 - 2025
Edited by
Anutthaman Parthasarathy, University of Bradford, United Kingdom
Reviewed by
Ruqeya Nazir, University of Kashmir, India
Andrés Barajas-Solano, Francisco de Paula Santander University, Colombia
Updates
Copyright
© 2025 Li, Ma, Pan, Long, Zhao, Yu, Peng and Ma.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Ma, mayi761@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.