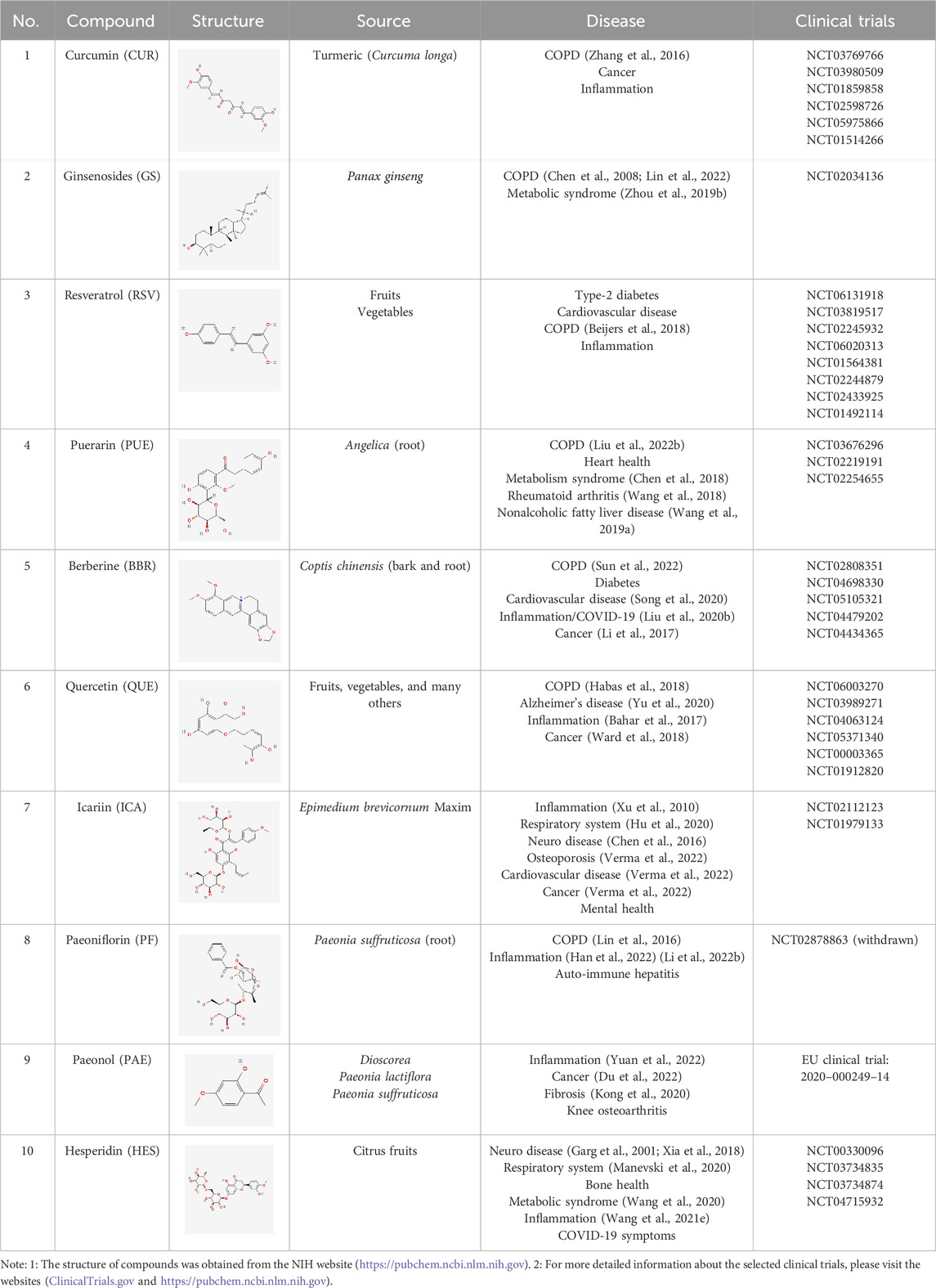
- 1Inflammation & Allergic Diseases Research Unit, The Affiliated Hospital of Southwest Medical University, Luzhou, China
- 2Department of Microbiology and Immunology, Indiana University School of Medicine, Indianapolis, IN, United States
- 3Indiana University School of Medicine, Melvin and Bren Simon Comprehensive Cancer Center, Indianapolis, IN, United States
- 4Department of Respiratory and Critical Care Medicine, The Affiliated Hospital of Southwest Medical University, Luzhou, China
- 5Department of Respiratory and Critical Care Medicine, Bishan Hospital of Chongqing, Bishan Hospital of Chongqing Medical University, Chongqing, China
- 6Department of Otolaryngology Head and Neck Surgery, The Affiliated Hospital of Southwest Medical University, Luzhou, China
Chronic obstructive pulmonary disease (COPD) is a chronic respiratory disease that leads to death and disability worldwide, and it is caused by hereditary and environmental factors. It is characterized by chronic inflammation, emphysema, and irreversible limitation of airflow. Dual or triple therapy with a traditional approach can provide relief from COPD symptoms by reducing the frequency and severity of the outbreaks, but there are no current therapies to reverse the long-term decline in lung function. Although ICS rescue inhalers demonstrate efficacy in acute attacks, these cannot be utilized for chronic management of COPD due to adverse effects. Therefore, novel agents and therapeutic strategies are urgently needed to address this disease. It is believed that malfunctioning mitochondria are associated with COPD pathogenesis, contributing to inflammation, apoptosis, and cellular senescence. A better understanding of these mechanisms could provide novel therapeutic approaches for maintaining lung and skeletal muscle function. Many natural extract compounds show therapeutic potential for COPD and are associated with few adverse reactions. Notably, these natural compounds can improve mitochondrial function and exhibit a variety of anti-inflammatory, antioxidant, and immunomodulatory properties. In this review, we systemically summarize the pathogenic role of impaired mitochondria in COPD and the potential mechanisms by which natural extract compounds may ameliorate these impairments.
1 Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic respiratory disease associated with heredity, the environment, individual development, and self-behavior. It is usually characterized as chronic inflammation, emphysema, and irreversible airflow limitation and causes high morbidity and mortality especially in the adult population (Doucet et al., 2016; Global Burden of Disease, 2017; Global Burden of Disease, 2018). In 2019, 212.3 million cases of COPD were reported globally, with an age-standardized point prevalence of 2,638.2 per 100,000 people, which was a decrease of 8.7% since 1990, but the absolute counts are on the rise (Safiri et al., 2022). Due to the large percentage of aging people, the number of COPD patients might increase in the coming years. Long-term exposure to cigarette smoke, air pollution, occupational dust, chemicals, biomass fumes, and other risk factors are considered risk factors that cause COPD (Agusti et al., 2023). According to the global initiative for chronic obstructive lung disease guidelines, drugs that are β2-agonists, anti-muscarinics, corticosteroids, antibiotics, antioxidants, and mucolytic are widely used to relieve COPD symptoms; additionally, the drugs can only control or delay COPD progression but cannot prevent long-term functional decrease (Agusti et al., 2023). Therefore, novel and safe medications are eagerly needed to effectively inhibit COPD progression, promote pulmonary rehabilitation, and reduce severity and mortality.
Mitochondria are responsible for a variety of cellular functions, including metabolism, intracellular signal transduction, energy production, and cell death. Previous studies have demonstrated that mitochondria play an important role in the development and progression of chronic respiratory diseases, including COPD (Yue and Yao, 2016; Zhou et al., 2021), and it might become a promising therapeutic target (Hu et al., 2022).
In this review, we will discuss COPD pathogenesis that is associated with impaired mitochondria and the potential therapeutic mechanisms by modulating mitochondrial activities with natural extracted compounds.
2 Mitochondrial dysregulation in COPD
COPD is a heterogeneous disease with an underlying disease process, and smoking is a high-risk factor of pathogenesis (Salvi, 2014). Cigarette smoke (CS) can impact mitochondrial structures and function of COPD epithelia after long-time exposure (Hoffmann et al., 2013). In the following sections, we will focus on the relationship between the pathogenesis of COPD and impaired mitochondrial function, such as excessive mitochondrial reactive oxygen species (mtROS), impaired mitochondrial DNA (mtDNA) and mitophagy, and impaired mitochondrial membrane potential.
2.1 mtROS is associated with COPD
Dysfunctional mitochondria are highly related with pathogenesis of COPD that might via mitochondrial damage associate molecular patterns (DAMPS) from dying or stressed cells including enhanced ROS production, inflammation response as well as cellular senescence (Lerner et al., 2016; Brusselle et al., 2011). COPD shows increased oxidative stress, especially during acute outbreaks caused by the overproduction of ROS resulting from CS, air pollution, biomass smoke, inflammatory responses of lung infections, and mitochondrial stress (Noguera et al., 2001). Compared with a nonsmoker or a population that quit smoking for a long time, the bronchial epithelia from COPD patients showed enhanced mtROS and lower levels of manganese superoxide dismutase 2 (SOD2) (Haji et al., 2020). Decreased mitochondrial complexes and increased mtROS were noticed in the airway smooth muscular cells of COPD patients (Wiegman et al., 2015) (Figure 1A).

Figure 1. Malfunctioned mitochondrial activities are associated with cellular senescence, apoptosis, and inflammation of COPD patients. (A) mtROS production and its networks that are associated with inflammation, apoptosis, and cellular senescence; (B) mtDNA contributes to inflammatory responses and also leads to cellular apoptosis and cellular senescence; (C) MMP signaling is related to apoptosis, cellular senescence, and inflammation.
Mitochondrial deacetylase sirtuin 3 (SIRT3) is an upstream signaling factor that regulates mtROS homeostasis (Bause and Haigis, 2013). It can directly bind with and deacetylate mitochondrial antioxidant enzymes such as SOD2 (Tao et al., 2010), isocitrate dehydrogenase 2 (Yu et al., 2012), and glutathione peroxidase (Liu et al., 2015), resulting in the increased activity to regulate the mtROS level (Bause and Haigis, 2013). In contrast, the overexpression of SIRT3 leads to significant abolishing of trimethylamine N-oxide (TMAO)-induced SOD2 suppression and mtROS production (Zhang et al., 2017). The study suggested that curcumin might be involved in the upregulation of PGC-1α/SIRT3 signaling to attenuate impairing skeletal muscle mitochondria in COPD rats (Zhang et al., 2017). In addition, high-level SIRT3 can prevent SOD2 from decreasing and can also improve the mitochondrial oxidative stress of cigarette smoke extract (CSE)-treated airway epithelia, which suggests that SIRT3 might contribute to the suppression of COPD pathogenesis by inhibiting mitochondrial oxidative stress via the modification of SOD2 (Zhang et al., 2020). Another family member, namely, SIRT1, is involved in the regulation of mitochondrial oxidative stress gene expression, including NOX4 (Dasgupta et al., 2020) and SOD2 (Liu et al., 2019). SIRT1 overexpression can protect airway epithelia from CS-induced senescence by interacting with FOXO3; it suggests that modulating mitochondrial function might be a potential therapeutic strategy for COPD treatment (Yao et al., 2012; Aghapour et al., 2020).
2.1.1 mtROS and inflammation
CS poses the risk of COPD that is highly associated with ROS production (Feng et al., 2023). Macrophages play a critical role in the iron homeostasis, and impaired macrophage leads to ROS overproduction that is associated with COPD development (Belchamber et al., 2019). mtROS might affect macrophage M1 polarization via MAPK, JNK/c-Jun, JNK-SOD2 and JNK-m6A-p38 signaling pathways in COPD (Feng et al., 2023; Hu et al., 2023; Liu Z. et al., 2020;). It is indicated that the airway epithelium produces more mtROS with aging, which leads to accelerated lung aging in COPD patients (Chen Q. et al., 2023). S1P can induce oxidative stress and NLRP3 inflammasome activation, leading to lung injury (Gong et al., 2023). These results indicate that S1P might affect macrophage polarization by inducing mtROS generation in COPD. It shows that the mtROS inhibitor, mitoTEMPO, can reduce NLRP3 expression in lung tissue (Zhao et al., 2018). In turn, activated NLRP3 inflammasomes can amplify mitochondrial damage via caspase-1, which is manifested by increasing mtROS production, dissipation of mitochondrial membrane potential (MMP/ΔΨm), loss of outer and inner membrane integrity, and fragmentation of the mitochondrial network (Yu et al., 2014). Other than promoting inflammatory response, the NLRP3 inflammasome can activate caspase-1 to induce emphysema as well; this process is independent of mtROS (Zhao et al., 2018). Correspondingly, COPD, especially in airway epithelia and macrophages, was found to increase activated NF-κB and NLRP3 inflammasome (Le et al., 2020; Rumora et al., 2021). The activated NLRP3 inflammasome-associated pyroptosis that promotes COPD pathogenesis was identified in CS-induced COPD mouse as well as COPD patients (Wang L. et al., 2021). The mtROS production and hypoxia-inducible factor-1 alpha (HIF-1α) stabilization is a marker of pro-inflammatory macrophage activation (Willenborg et al., 2021). The mtROS production has been shown to cause DNA damage, unfolded protein response, and inflammatory responses through the HIF-1α and MAPK/NF-κB pathways in LPS-stimulated macrophages (Wang Y. et al., 2021). Rapamycin negatively regulates macrophage activation by inhibiting mtROS production and limiting the activation of NLRP3 inflammasomes (Ko et al., 2017). mtROS is critical in the COPD pathogenesis and development, by targeting mtROS might provide novel treatment strategies against COPD (Figure 1A).
Neutrophils from patients with COPD have enhanced chemotaxis, extracellular proteolysis, and overproduction of ROS compared with those from nonsmokers and healthy smoker controls (Vernooy et al., 2002). It indicated that mtROS is involved in the oxidative burst and in degranulation of human neutrophils induced by the chemoattractant fMLP in vitro (Vorobjeva et al., 2017). Mitochondrial permeability transition pore (mPTP) in human neutrophils is a critical step for increasing mtROS production (Vorobjeva et al., 2020). Therefore, the drugs that target mtROS may reduce neutrophil inflammation in COPD.
During COPD progression, dendritic cells (DCs) participate in the activation of other immune cells, immune tolerance, and facilitating lung remodeling (Bu et al., 2020). Following Aspergillus fumigatus infection, it was observed that lung leukocytes over generated mtROS, including monocyte-derived DC (Shlezinger and Hohl, 2021). Bone marrow-derived DCs stimulated by chitin-derived polymer deacetylation activated cGAS-STING-mediated type-I interferon (IFN-I) response and NLRP3 inflammasome activation by enhancing mtROS production (Turley et al., 2021). When plasmacytoid dendritic cells (pDCs) were simultaneously exposed to hypoxia and toll-like receptor 9 (TLR9) agonist, pDCs released CXCL4 that was dependent on the overproduction of mtROS (Ottria et al., 2022). However, the molecular mechanism of mtROS in DCs from COPD is not clear, and more studies are needed for clarity.
2.1.2 mtROS affects lung cell apoptosis in COPD
Many studies indicate that the apoptosis of lung structural cells and pulmonary vascular endothelial cells is a critical event to initiate and participate during emphysema and COPD (Sauler et al., 2019; Gogebakan et al., 2014). It is also suggested that the mice alveolar cell destruction increased after intrathoracic injection with CSE, and a large amount of apoptotic epithelia were detected in the bronchoalveolar lavage fluid (Hattori et al., 2022). It has been reported that pulmonary epithelia and endothelia apoptosis, and apoptotic factor expression are increased in COPD patients, which is closely related to the destruction of lung tissue and the development of emphysema (Hodge et al., 2005; Kasahara et al., 2001; Demedts et al., 2006). Smoke treatment resulted in increased levels of proapoptotic proteins in the terminal bronchiolar region of rat lung tissue. Mitochondrial dysfunction has also been reported in skeletal muscle cells from COPD patients, including decreased mitochondrial density and biogenesis, and increased mtROS production, which is closely related to muscle dysfunction (Meyer et al., 2013). It has been shown that the accumulation of mtROS regulates the mitochondrial intrinsic apoptosis pathway (Yee et al., 2014) (Figure 1A).
Excessive mtROS induces the opening of mPTPs, nonspecific protein complex channels between the inner and outer mitochondrial membranes (Halestrap, 1999), resulting in the transportation of ions and metabolites from the mitochondria into the cytoplasm, leading to increasing colloidal osmotic pressure within the mitochondrial matrix, loss of membrane potential, uncoupling of oxidative phosphorylation, termination or depletion of ATP synthesis, eventual cell necrosis, etc. (Bernardi et al., 1999). Cyto c binds to Apaf-1 and caspase-9 in the cytoplasm to form an apoptosome complex, resulting in triggering a caspase-dependent apoptotic cascade (Li et al., 1997). Mitochondrial damage is exacerbated in results from the depletion of Cyto c, further resulting in reduced mtROS accumulation and ATP production (Green and Reed, 1998). Conversely, sesamin can increase MMP and reduce lung epithelia apoptosis by inhibiting TNF-α/IL-4-induced mtROS production (Bai et al., 2022). In addition, mtROS-activated NLRP3 inflammasome is involved in the process of apoptosis, which can increase both the BAX/Bcl-2 ratio and the cleaved caspase-3 expression level (Lin Z. et al., 2018). This suggests that mtROS is highly associated with apoptosis that is involved in the regulation of COPD development.
Overproduction of mtROS results in sustained activation of JNK signaling (Kamata et al., 2005). Mitochondrial JNK is one of the key elements in cytochrome c (Cyto c) release and activating caspase (caspase-8, caspase-9, effector caspase-3, and caspase-7) (Kamata et al., 2005; Salehi et al., 2002); moreover, the processing of Cyto c release is independent of the permeability transition of the inner mitochondrial membrane (Schroeter et al., 2003). Furthermore, JNK can catalyze the phosphorylation of Bcl-2 and Bcl-x(L) to promote apoptosis as well (Schroeter et al., 2003).
2.1.3 mtROS affects lung cell senescence in COPD
Senescence-associated secretory phenotype (SASP) is a basic characteristic of senescent cells, including secreting a large number of cytokines, chemokines, matrix metalloproteinases, and growth factors into the tissue microenvironment (Kuilman et al., 2008; Acosta et al., 2008), which are associated with the development of COPD. COPD-derived fibroblasts secrete higher levels of parts of SASPs (Kuilman et al., 2008; Acosta et al., 2008). Senescent lung cells secrete COPD-associated SASPs, such as IL-6, IL-8, monocyte chemoattractant protein-1 (MCP-1), and plasminogen activation inhibitor-1 (PAI-1), which further promote chronic inflammation in COPD (Freund et al., 2010). COPD is considered a disease that accelerates lung aging, and it is associated with features of cellular senescence, DNA damage, oxidative stress, and extracellular matrix remodeling (Ito and Barnes, 2009). Increased expression of p21CIP1/WAF1, p16INK4a, and senescence-associated β-galactosidase were found in bronchial epithelia and macrophages from healthy smokers (Tomita et al., 2002; Tsuji et al., 2006; Sundar et al., 2018). Emphysematous lungs also show an increased expression of p16, p19, and p21 (Tuder et al., 2012). Senescent cells accumulate in the lung and lose their regenerative capacity, thus limiting cellular repair and renewal in COPD lungs, which leads to the progression of emphysema and worsening lung function (Tsuji et al., 2006).
mtROS is involved in cellular senescence and is a major determinant of aging (Chen et al., 1995; Dai et al., 2012) (Figure 1A). ROS accumulation has been reported to trigger the p53/p21CIP1/WAF1 and p16INK4A/Rb pathways, leading to irreversible cell-cycle arrest in senescent cells (Ziegler et al., 2015). The accumulation of mtROS leads to mitochondrial dysfunction and promotes cellular senescence (Hekimi et al., 2011), which is mainly dependent on mitochondrial oxidative damage, such as mtDNA mutation and mitochondrial membrane permeability. Cytoplasmic chromatin fragments (CCFs) are chromatin fragments released into the cytoplasm by senescent cells via budding formation from the nucleus (Ivanov et al., 2013). CCF is a trigger of SASP, and the elevated mtROS induce CCF formation in senescent cells by promoting JNK activation (Vizioli et al., 2020). DNA fragments in CCFs of senescent cells activate the cytoplasmic DNA-sensor cGAS to generate the second messenger cGAMP; cGAMP binds and activates the adapter protein STING (Wu et al., 2013; Glück et al., 2017). The cGAS-STING signaling promotes TBK1 recruitment and phosphorylation, resulting in IRF3 nucleus translocation and enhanced IFN-I expression. On the other hand, NF-κB is activated by TBK1, leading to upregulation of type-I interferons and inflammatory cytokine expression (Yang et al., 2017). Similarly, inhibiting cGAS-STING signaling can effectively inhibit both SASP expression and astrocyte senescence (Aguado et al., 2021). Furthermore, the AMPK–mTOR pathway modulates NF-κB transcriptional activity to regulate the translational levels of pro-inflammatory factors in SASPs (van Vliet et al., 2021). TLR2, which acts as a downstream factor of STING to regulate NF-κB, was shown to be required for increasing SASP secretion in vivo (Hari et al., 2019).
Therefore, drugs targeting mtROS might promote lung recovery and delay emphysema changes by reducing pulmonary oxidative stress, delaying cellular senescence, and inhibiting inflammatory responses.
2.2 Damaged mtDNA is associated with COPD, inflammation, lung aging, oxidative stress, and cell apoptosis
mtDNA is susceptible to oxidative damage due to its proximity to ROS production sites, special packaging structures, and asymmetric replication (Reyes et al., 1998; Parisi and Clayton, 1991). Excessive mtROS results in mtDNA site mutations, insertions, deletions, and copy number reduction (Quan et al., 2020). Different types of damage to mtDNA can also occur because of endogenous and exogenous (tobacco smoke, chemicals, etc.) noxious substances (Alexeyev et al., 2013). Damaged mtDNA fragments can escape into the cytoplasm or extracellular space, which may be related to changes in mitochondrial permeability, mitochondrial dynamics, mitophagy, BAX/BAK pores, and VDAC1 oligomers (Pérez-Treviño et al., 2020). It has been reported that the mtDNA content does not change in end-stage COPD lung tissue, but the mtDNA strand breaks and/or untrustworthy sites are significantly increased compared with normal lung tissue (Pastukh et al., 2011). In a SPIROMICS cohort study (NCT01969344), higher levels of plasma-mtDNA were found in subjects with mild or moderate COPD than in nonsmokers and smokers without airflow obstruction (Zhang W. Z. et al., 2021). Similarly, plasma levels of both cell-free mtDNA (cf-mtDNA) and nuclear DNA (cf-nDNA) were elevated in former COPD smokers compared with that in former smokers without COPD (Giordano et al., 2022). Increased cf-mtDNA copy number was significantly associated with the development of COPD. They also found that bronchial epithelia exposed to CSE led to the release of mtDNA into the extracellular space through vehicles and cellular debris (Giordano et al., 2022).
The mtDNA released from mitochondria is considered a damage-associated molecular pattern (DAMP) by activating downstream pro-inflammatory signaling cascades (Fang et al., 2016). Activation of human polymorphonuclear neutrophils (PMNs) by mtDNA in the plasma through TLR9 induces secondary IL-8 release and activates PMN p38 and p44/42 MAPKs, leading to systemic inflammation (Zhang et al., 2010). Cytosolic mtDNA also mediates inflammatory responses in the intracellular space by contributing to IL-1β and IL-18 secretion through the activation of the NLRP3/AIM2-caspase-1 pathway (Nakahira et al., 2011). The accumulation of mtDNA activates cGAS-STING signaling and IFN-I responses in DCs, B cells, and natural killer cells (West and Shadel, 2017), resulting in enhanced inflammatory responses. In addition, damaged mtDNA can increase the expression of pro-protein convertase subtilisin/kexin type 9 (PCSK9) and elevated PCSK9 can induce apoptosis by stimulating caspase-3 (Ding et al., 2016).
Mitochondrial dysfunction plays a central role in the aging process, and mtDNA mutations are critical hallmarks of cellular aging and other related diseases (Szczepanowska and Trifunovic, 2017; van der Rijt et al., 2020) (Figure 1B). As previously described, oxidatively damaged mtDNA results in reduced gene expression of mtDNA, defects in the oxidative phosphorylation system, and increased oxidative flux (Wallace, 1992); this is a common feature in many human aging tissues, including lung tissue (Bender et al., 2006). Studies have demonstrated that mtDNA drives cGAS-dependent responses and enhances senescence in lung epithelia and fibroblasts (Schuliga et al., 2021; Schuliga et al., 2020). mtDNA mutations also accelerate oocyte aging by reducing the NADH/NAD+ redox state (Yang et al., 2020). The accumulation of mtDNA damage has also been shown to promote apoptosis by increasing c-caspase-3 and Cyto c release and leading to mitochondrial outer membrane permeabilization (MOMP) (Kujoth et al., 2005). These studies suggest that drugs that protect mtDNA may have the potential effect of slowing COPD progression by affecting cellular senescence and inflammatory pathogenesis (Figure 1B).
2.3 Disturbed mitochondrial membrane potential is associated with COPD, inflammation, and apoptosis
Mitochondrial membrane potential (MMP/ΔΨm) plays an important role in maintaining mitochondrial function, mitochondrial permeability, mitochondrial viability, and other cellular functions (Bagkos et al., 2014; Gottlieb et al., 2003). It has been observed that CSE leads to the loss of cellular ATP and rapid depolarization of MMP (Wu et al., 2020). Reduced MMP/ΔΨm was noticed in mitochondria isolated from bronchial biopsies of COPD patients (Haji et al., 2020). Human airway smooth muscle cells from COPD patients have reduced MMP, ATP content, and basal and maximal respiration compared to those from healthy controls (Wiegman et al., 2015). Likewise, both MMP and ATP levels were significantly reduced in quadriceps muscle cells of CS-exposed rats compared to controls, which are associated with the decreased physical ability of COPD rats (Mao et al., 2019). Decreased or depolarized MMP is considered an important indicator of mitochondrial dysfunction, with profound effects on inflammatory responses and apoptosis (Zhou et al., 2021) (Figure 1C).
Disrupting MMP directly affects the electron transport chain (ETC) that in turn leads to loss of oxidative phosphorylation and increases mtROS production. Depolarized MMP induces a transition in mitochondrial membrane permeability that leads to the release of mtROS, mtDNA, and intermembrane proteins into the cytosol, resulting in triggering inflammatory and proapoptotic responses (Bronner and O'Riordan, 2016). Improving mitochondrial function and decreasing mtROS generation can attenuate inflammatory response (Chen Y. et al., 2023). Cleaved oxidized-mtDNA promotes NLRP3 inflammasome and cGAS-STING signaling activation, leading to the pathogenesis of chronic inflammatory diseases (Xian et al., 2022).
MMP reduction is a key event in the induction of apoptosis (Zaib et al., 2022). The pro-apoptotic Bcl-2 family protein BAK/BAX can regulate the MMP change; the activation and oligomerization of BAK/BAX can induce homomultimeric pores formation (Korsmeyer et al., 2000) or induce the MOMP (Kuwana et al., 2002). BAK/BAX can bind to and promote the opening of VDAC1, leading to decreasing MMP (Shimizu et al., 1999). The decline in MMP causes structural changes in mitochondria, including matrix condensation and cristae disintegration (Gottlieb et al., 2003). These structural changes result in the release of Cyto c into the intermembrane space and cytoplasm (Gottlieb et al., 2003). The released Cyto c results in apoptosis by activating Apaf-1-caspase-9 apoptosome and subsequently activating executioner caspase-3 (Yuan and Akey, 2013). The impact of abnormal MMP on the pathogenesis of COPD may be more complicated, and further research is needed.
2.4 Impaired mitophagy is associated with inflammation and senescence in COPD
Mitophagy-associated signals are highly related to the pathogenesis of COPD, such as AMPK signaling. Studies have shown that AMPK can directly phosphorylate the serine/threonine kinase ULK1 (Kim et al., 2011; Egan et al., 2011); then, ULK1 promotes mitophagy by promoting phosphorylation of the Parkin ACT domain (Hung et al., 2021). ULK1 can also directly phosphorylate mitophagy receptors, including FUNDC1 (Wu et al., 2014), BNIP3 (Poole et al., 2021), NIX, and Bcl-2-L-13 (Kim et al., 2011; Egan et al., 2011; Murakawa et al., 2019). AMPK can induce mitophagy by regulating MFN2 to respond to energy stress (Hu et al., 2021). Increased mTOR activity leads to decreased molecular activity of PINK1/Parkin and LC3 (Wen et al., 2020). In addition, AMPK inhibits mTOR signaling by phosphorylating the mTOR factors TSC2 and Raptor (Ha et al., 2015).
SIRT1 was shown to induce mitophagy and alleviate mitochondrial damage (Biel et al., 2016). Adiponectin reduces mtROS production and inhibits the generation of inflammatory cytokines TNF-α and IL-6 via mitophagy through SIRT1-PINK1 signaling (Jiang et al., 2021). The transcription factor Nrf2 is crucial in maintaining mitochondrial structure and function (Dinkova-Kostova and Abramov, 2015). The activation of Nrf2 leads to mitophagy (Dinkova-Kostova and Abramov, 2015; Gumeni et al., 2021).
BNIP3, one of the pro-apoptotic factors belonging to the Bcl-2 family, can mediate mitophagy by binding to LC3 through the LIR motif in its homodimer, which is closely related to mitochondrial function disorder and cell death (Quinsay et al., 2010; Hanna et al., 2012). Airway epithelia exposed to 7.5% CSE shows BNIP3L overproduction to promote mitophagy, resulting in enhanced mitochondrial dysfunction and cellular damage (Zhang et al., 2019). Patients with systemic inflammatory COPD show higher BNIP3- and BNIP3L-mediated mitophagy marker levels than those with normal COPD, which suggests that BNIP3-mediated mitophagy might promote systemic COPD inflammation (Leermakers et al., 2018).
The activated AMPK, ULK1, and SIRT1 signaling pathways contribute to enhance mitophagy that is highly associated with emphysema, aging, and inflammatory responses. This suggests that targeting mitophagy networks may improve the physical ability and life quality of COPD patients.
2.4.1 Mitophagy affects inflammation
Mitophagy has a complex relationship with COPD progression, and PINK1/Parkin signaling is considered to be a key pathway for mitophagy (Yao et al., 2021). A study found that Parkin protein expression levels were significantly lower in COPD lungs than in the lungs of nonsmokers and non-COPD smokers, and they were positively correlated with the percentage of FEV1/FVC (Ito et al., 2015).
Additional studies indicate that Parkin/PINK1-deficient mice show elevated circulating mtDNA that induces inflammatory phenotype cytokine and chemokine (IL-6, IL-12, IL-13, IFN-β, CXCL1, CCL2, and CCL4) expression by activating the cGAS–STING pathway; Parkin and PINK1 mediate mitophagy mitigating STING-induced inflammation (Sliter et al., 2018). COPD patients had higher accumulation of ubiquitinated proteins and p62 in lung homogenates than heavy smokers and light/nonsmokers, which was possibly due to insufficient CSE-induced autophagy in damaged cells (Fujii et al., 2012). Furthermore, a significant increase in the number of total cells and macrophages was observed in the bronchoalveolar lavage fluid of Parkin KO mice compared with wild-type mice exposed to CS, and Parkin plays a critical role in regulating mitophagy during COPD pathogenesis (Araya et al., 2019).
These results suggest that PINK1/Parkin, cGAS–STING pathways, and mitophagy regulations are essential during the pathogenesis of COPD. Sufficient and effective mitophagy is necessary and critical to maintain mitochondrial homeostasis (Figure 2).
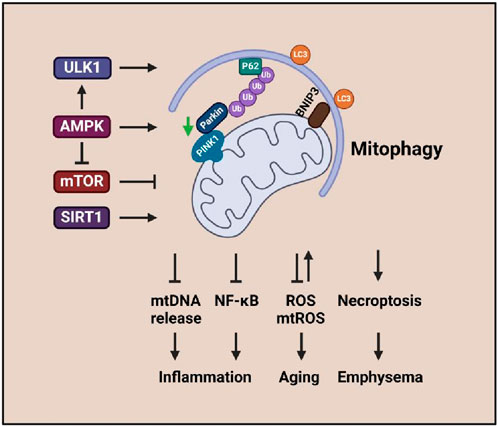
Figure 2. Mitophagy signaling associated with inflammation, aging, and emphysema. The enhanced ULK1, AMPK, and SIRT1 activities promote mitophagy that leads to emphysema, aging, and inflammatory response in COPD.
2.4.2 Mitophagy affects senescence
Emphysema and chronic bronchitis are the two major forms of COPD. Malfunctioning mitochondria and cellular senescence are associated with CS-induced pathogenesis of COPD/emphysema linked with mitophagy and PINK1/Parkin signaling (Wang et al., 2022; Ahmad et al., 2015) (Figure 2). CSE-induced PINK1-dependent mitophagy aggravates mitochondrial damage and induces necroptosis, leading to enhanced emphysema (Mizumura et al., 2014).
Imbalanced low-level mitophagy is one of the major factors that cause senescence (Figure 2). PINK1 and Parkin are involved in the pathogenesis of COPD via regulating mitophagy, but the effects are controversial. Studies indicate that insufficient mitophagy can result in accelerated aging of CSE-exposed human small airway epithelia (Ito et al., 2015; Ahmad et al., 2015), which is associated with cytoplasmic p53 inhibiting Parkin mitochondrial translocation (Ahmad et al., 2015). Reduced Parkin level-induced insufficient mitophagy can result in ROS overproduction and promotion of branchial epithelial aging (Ito et al., 2015). Mice deficient in Parkin showed changes in emphysema and aggravated airway wall thickening exposure to CS; impaired mitochondrial accumulation, enhanced mtROS, and increased senescence were found in bronchial epithelia (Saito et al., 2019). In contrast, pirfenidone can promote mitophagy and attenuate CSE-induced cellular senescence by inducing Parkin expression (Saito et al., 2019). Additionally, Parkin-mediated mitophagy deficiency-induced mtROS overproduction promotes the development of COPD-associated muscle atrophy (Ito et al., 2022). Elevated PINK1 represents the accumulation of impaired mitochondria, which is conferred by insufficient mitophagic degradation (Hoffmann et al., 2013).
Homeostasis of mitophagy is highly associated with senescence, which plays a critical role in the pathogenesis of COPD. Targeting mitophagy-associated senescence signaling might provide novel therapeutic strategies against COPD.
2.5 Mitochondrial biogenesis is associated with COPD cell aging and inflammation
Mitochondrial biogenesis plays an important role in maintaining cellular homeostasis when stimulated by environmental factors (Popov, 2020). The mTORC1/PGC-1β signaling pathway is a master regulator of mitochondrial biogenesis in mammalian cells (Correia-Melo et al., 2016). It has been found that aging lung epithelia exhibit increased mTOR/PGC-1α/β activation, which is associated with upregulation of mitochondrial biogenesis, oxidative phosphorylation, mtROS overproduction, and induction of cellular senescence (Summer et al., 2019). The activation of mTOR induces senescence of lung cells and mimics COPD lung changes with rapid development of emphysema, pulmonary hypertension, and inflammation via phosphorylated GSK3 and Aktser473 signaling (Houssaini et al., 2018). In addition, the reduced lamin B1 in airway epithelia results in the decreasing expression of DEPTOR (a natural negative regulator of mTOR kinase activity) that is involved in aberrant activation of mTOR in response to CSE (Saito et al., 2019). Correspondingly, rapamycin reversed cellular senescence by reducing the activity of mTORC1/PGC-1α/β signaling, and rapamycin suppressed the expression of inflammatory cytokine SASPs (Summer et al., 2019; Walters et al., 2016). COPD patients show low mitochondrial biogenesis due to attenuated PGC-1α and TFAM (Taivassalo and Hussain, 2016). The increased PGC-1α suggests host cells enhancing mitochondrial biogenesis through up taking foreign mitochondria. This mitochondrial transfer can reverse mitochondrial malfunction in COPD (Frankenberg Garcia et al., 2022). Improving mitochondrial biogenesis to enhance mitochondrial function and muscle performance might lead to promising therapeutic strategies for COPD patients.
PGC-1α can interact with nuclear respiratory factors (Nrf1 and Nrf2) (Wu et al., 1999), members of the nuclear receptor family (such as PPARα, PPARγ, and ERR-1α), non-nuclear receptor transcription factors (such as myocyte enhancer factor-2 and MEF-2), and the FOXO family to regulate the expression of mitochondrial genes and mitochondrial transcription genes (TFAM, TFB1M, and TFB2M), leading to controlling the transcriptional activity of energy metabolism by key metabolic factors, namely, AMPK, SIRT1, and PGC-1α (Cantó and Auwerx, 2009; Gleyzer et al., 2005; Rizk et al., 2023). In addition, Parkin regulates PGC-1α at the transcriptional level, and they also interact with each other to regulate mitochondrial mass and function (Zheng et al., 2017). SIRT1 can directly interact with PGC-1α and deacetylate PGC-1α (Rodgers et al., 2005) to activate its downstream targets such as Nrf1 and TFAM to enhance mitochondrial biogenesis (Tian et al., 2019; Ye et al., 2022). In vitro SIRT1 protein levels have been reported to decrease in lung epithelia (Yao et al., 2012), endothelial cells (Arunachalam et al., 2010), and macrophages after exposure to CSE; this finding was also noticed in the lungs of smokers and patients with COPD (Rajendrasozhan et al., 2008). CS mediates pro-inflammatory responses and senescence that is associated with impaired SIRT1/FOXO3 signaling (Yao et al., 2012; Di Vincenzo et al., 2018; Yao et al., 2014). Hesperidin inhibits CSE-induced inflammatory response in a COPD rat model via modulating SIRT1/PGC-1α/NF-κB signaling (Manevski et al., 2020). In addition, SIRT1 activators inhibit TGF-β1-mediated phosphorylation of Smad3, resulting in attenuating the CSE-mediated airway remodeling of bronchial epithelia (Guan R. et al., 2020). Therefore, SIRT1 regulates PGC-1α activity at least partly via TGF-β signaling. However, the specific mechanism through which the SIRT1/PGC-1α axis regulates mitochondrial activities in COPD is yet to be known, and we need more evidence to verify the mechanism.
AMPK expression is reduced in bronchial epithelia of emphysema and CSE-exposed mice (Cui et al., 2018). Prophylactically used AMPK activators can reduce inflammation and cellular senescence in emphysema mice, which has been associated with the upregulation of mitochondrial proteins including SOD2 and SIRT3; AMPK-α1/α2 knocked down in human bronchial epithelia increases cellular senescence-related gene expression (Cheng et al., 2017). Studies have shown that mitochondrial biogenes is related with SIRT3 (Zhang et al., 2017), p-AMPK-, and TFAM (Remels et al., 2007) levels. The results indicated that they are decreasing in the skeletal muscle of COPD rats model. These findings suggest the influence of AMPK on lung inflammation and aging. However, the insight mechanism of whether AMPK signaling regulates PGC-1α in COPD needs to be verified.
Taken together, the elevated mitochondrial biogenesis is beneficial for reversing the pathogenesis of COPD. Mitochondrial homeostasis is critical for maintaining cellular survival and function; impaired mitochondrial structure and function in various cell types may induce COPD pathogenesis. However, the mechanisms by which mitochondria regulate COPD development at different stages and cell types require deep insight investments. Therefore, it might be a great challenge for the clinical application of mitochondria-targeted therapy (Figure 3).
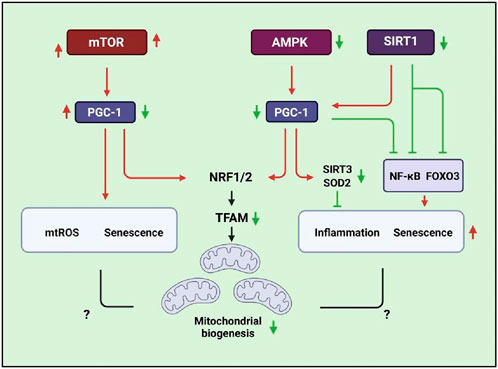
Figure 3. Schematic signaling indicating that mitochondrial biogenesis is associated with SIRT1, AMPK, mTOR, and PGC-1/TFAM pathways that are related to inflammatory responses, ROS production, and senescence.
2.6 The potential role for UPRmt in COPD development
Mitochondrial unfolded protein response, UPRmt, is a pivotal component in maintaining immune homeostasis in response to internal or external stress (Bernales et al., 2012; Inigo and Chandra, 2022). Accumulated mtROS breaks the folding proteins and unfolded protein balance, resulting in UPR (Bernales et al., 2012). Meanwhile, mtROS overproduction can trigger UPRmt signaling that is shown as ATF5, SIRT3, and ERα upregulation to process antioxidative activities in response to mtROS overload (Fiorese et al., 2016; Jenkins et al., 2021; Livezey et al., 2018). It is indicated that UPRmt is associated with enhancing ELF2α and JNK phosphorylation in response to an inflammatory environment (Bernales et al., 2012; Nguyen et al., 2024; Gaspar et al., 2023). It has been suggested that UPRmt is associated with antioxidant defense, metabolism, inflammation, etc. However, the evidence showing the mechanism of how UPRmt contributes to COPD is limited (Kelsen, 2016). Based on our knowledge, we hypothesize that UPRmt accumulation might prevent COPD pathogenesis in the early stage, but severe UPRmt might play a role in COPD progression. The potential therapeutic strategies of COPD that aim at modulation of UPRmt and mtROS need in-depth investigations.
2.7 MAMs response to stress effects on COPD pathogenesis
Mitochondria-associated endoplasmic reticulum (ER) membranes (MAMs) serve as the bridge connecting mitochondria and ER (Zhang Y. et al., 2023; Zhang et al., 2024). MAMs play a critical role in multiple functions based on different components (Elwakiel et al., 2024), such as: 1) VDAC, and IP3R are associated with stress responses and apoptosis; 2) NLRP3 is responsible for inflammation; and 3) VDAC, IP3R, GRP75, and Mitofusin 2 (MFN2) are related with Ca2+ transfer to maintain homeostasis, and many others. Studies show that malfunctioning MAMs can cause extensive Ca2+ overloading, resulting in ER Ca2+ efflux into mitochondria through the VDAC1-Grp75-IP3R1 complex interacting with Cyclophilin D, which leads to disease pathogenesis (Zhang et al., 2024; Elrod et al., 2010; Baines et al., 2005). Mitochondrial dynamics-related proteins are rich in MAMs, such as MFN2, OPA1, and FIS1 (Zhang Y. et al., 2023; Elwakiel et al., 2024). Silencing or mutation of MFN2 can cause inefficient Ca2+ uptake, leading to health problems (Gӧbel et al., 2020; de Brito and Scorrano, 2008). Overloaded mitochondrial Ca2+ can further lead to mtROS, mPTP, and mtDNA release, resulting in cell death signaling (Bronner and O'Riordan, 2016; Zhong et al., 2016). Moreover, MFN2, OPA1, FIS1, etc. are involved in the mtROS homeostasis regulation (Lin H. Y. et al., 2018). It has been shown that MFN2 and OPA1 are downregulated in COPD, whereas increasing MFN2 and OPA1 expression can somehow attenuate oxidative stress and cellular senescence (Li et al., 2023; Maremanda et al., 2021). CSE can induce bronchial epithelial mtROS and senescence by enhancing mitochondrial FIS1 expression (Hara et al., 2013). Now, we can see that the overloaded Ca2+ will worsen the mtROS balance that might lead to UPRmt extensive activities, which might result in unpredictable COPD development. Further understanding the relation between dysfunctional mitochondria–ER tethering proteins and mtROS overproduction, Ca2+ overload, and mitochondrial dynamics might provide novel therapeutic strategies of COPD.
3 Effects of natural compounds on mitochondria
Treatments that modulate mitochondrial function are beneficial in restoring airway inflammation, promoting pulmonary recovery, and encouraging the investigations on new drugs development for COPD therapy (Manevski et al., 2020). As mitochondrial dysfunction highlights a wide range of pathological conditions in COPD, the outcome of treatment with mitochondrial-targeting drugs requires in-depth exploration. The findings revealed potential new applications from natural compounds and suggest that the regulation of mitochondria may be an important mechanism for herb extracts to treat COPD, as we discuss below (Figure 4).
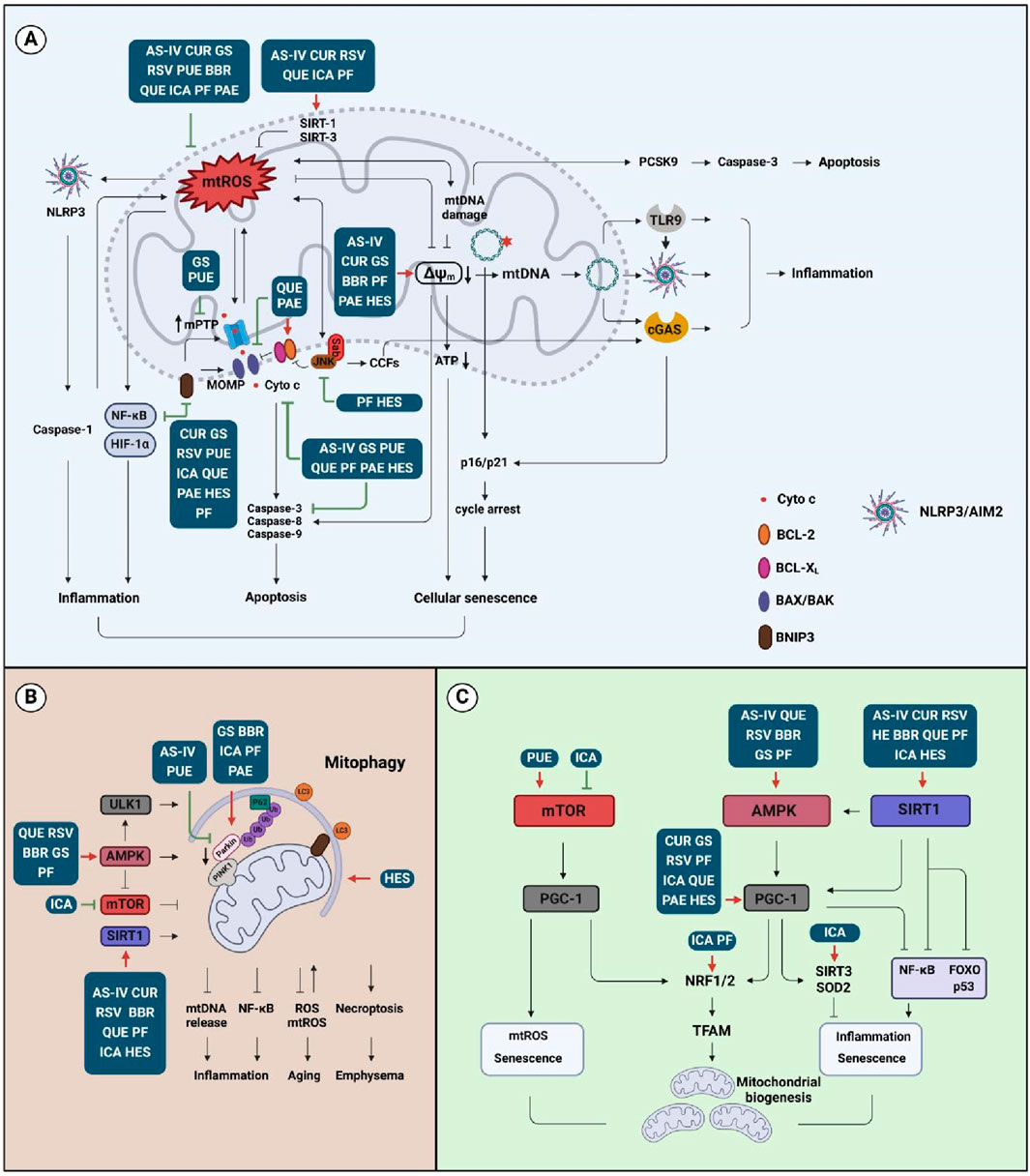
Figure 4. Effects of natural compounds on the mitochondrial activities. (A) Compound affects mtROS, mtDNA, and MMP signaling that is associated with inflammation, apoptosis, and cellular senescence; (B) Compound targets mitophagy-associated signaling that is related to inflammation, aging, and emphysema; (C) Compound regulates mitochondrial biogenesis signaling that is involved in inflammation and senescence. Abbreviation: AS-IV, astragaloside IV; BBR, berberine; CUR, curcumin; GS, ginsenosides; HES, hesperidin; ICA, icariin; PAE, paeonol; PF, paeoniflorin; PUE, puerarin; QUE, quercetin; RSV, resveratrol.
3.1 Curcumin
Curcumin (CUR), a polyphenol and nontoxic compound obtained from turmeric (Curcuma longa), has been shown to exert therapeutic effects on respiratory diseases, which might be due to mitochondrial protective properties (Lelli et al., 2017). In a randomized controlled clinical study, the combination curcuminoids with piperine were shown to alleviate systemic oxidative stress and clinical symptoms in COPD patients (Panahi et al., 2016). A preclinical study indicated that CUR can improve emphysema and airway inflammation in COPD rats and reduce mitochondrial damage in alveolar epithelia (Zhang et al., 2016). CUR can also alleviate mitochondrial damage in skeletal muscle cells of COPD rats by activating PGC-1α/SIRT3 signaling (Zhang et al., 2017) and attenuate airway inflammation and airway remodeling in a COPD mouse model, which was closely related to inhibiting BEAS-2B cell proliferation and inhibiting the activation of NF-κB and cyclooxygenase-2 (COX-2) expression (Yuan et al., 2018). CUR alleviates COPD by activating SIRT1 signaling and enhancing the expression of autophagy-related proteins LC3-I, LC3-II, and Beclin-1 (Tang and Ling, 2019). These findings suggest that the mitochondrial pathway may be a potential mechanism for the treatment of COPD with CUR.
3.2 Ginsenosides
Ginsenosides (GS) from Panax ginseng (such as Rb1, Rb2, Rc, Rd, Re, Rg1, Rg3, Rg5, Rh1, and Rh2) are the main bioactive components against COPD (Chen et al., 2008; Lin et al., 2022), and ginsenosides have been found to have potential effects on mitochondrial diseases by regulating mitochondrial activities (Liu Z. et al., 2022; Zhou et al., 2019a). The anti-inflammatory effect of ginseng and ginsenosides in the treatment of COPD includes regulating the NF-κB pathway, and inflammatory cytokine (TNF-α, IL-6, IL-8, and IL-1β) expressions (Shergis et al., 2014).
Ginsenoside Rg3 treatment in the acute exacerbation COPD (AECOPD) mouse model resulted in decreased neutrophil migration and improvement of lung function and lung morphology (Guan X. et al., 2020). Ginsenoside Rg3 promoted mitochondrial biogenesis and increased PGC-1α, Nrf1, and TFAM levels (Lee et al., 2019). It can also promote mitophagy-related protein (LC3-II/LC3-I and Beclin-1) expressions by activating AMPK (Xing et al., 2017). In addition, ginsenoside Rg3 inhibits mPTP opening via scavenging free radicals to protect the mitochondrial function of nerve cells (Tian et al., 2009). Ginsenoside Rb3 might inhibit mitochondrial-associated apoptosis (Bcl-2 and BAX) and enhance energy metabolism via activating PPARα (Chen et al., 2019). It has been shown that ginsenoside Rd attenuates focal cerebral ischemia-mediated mitochondrial dysfunction that manifests by reducing mitochondrial swelling, maintaining MMP and respiratory chain complex activity, and reducing ROS production, Cyto c release, and apoptosis-inducing factor (AIF) expression (Ye et al., 2011). Additionally, ginsenoside Rd combined with Re can attenuate rotenone-induced mitochondrial damage that is associated with inhibiting MMP depolarization and increasing cytosolic and mitochondrial Ca2+ levels (González-Burgos et al., 2017). It is suggested that ginsenosides Rb1 and Rg1 can alleviate astrocyte impairment via reducing ROS production, increasing catalase (CAT) activity, inhibiting MMP depolarization and mtDNA content, and maintaining mitochondrial respiratory chain activity (Xu et al., 2019). Ginsenoside Rh2 promotes mitophagy via increasing PINK1/Parkin production (Hou et al., 2020). Notoginsenoside R1 (NGR1), a triterpenoid saponin compound extracted from ginseng, effectively alleviates diabetic retinopathy of db/db mice by inhibiting inflammation, reducing mtROS production, and activating PINK1-mediated mitophagy (Zhou et al., 2019b).
Increased transforming growth factor-β1 (TGF-β1) in the airway epithelium and lung cells of COPD patients promotes the recruitment of macrophages (de Boer et al., 1998; Chung et al., 2018). Ginsenoside Rg1 can not only downregulate the inflammatory response but also delay the progression of CS-induced airway remodeling by inactivating TGF-β1/Smad3 signaling (Guan et al., 2017). All these findings indicate that the properties of ginsenosides on the mitochondria are potential therapeutic strategies for COPD, and more in-depth investigations are needed to promote this natural compound to potential clinical practice.
3.3 Resveratrol
Resveratrol (RSV) is a natural polyphenolic phytoalexin found in many fruits and vegetables with complex pharmacological effects including anti-cancer (Zhang L. X. et al., 2021). RSV shows potential benefits for COPD patients (Beijers et al., 2018). Studies have shown that RSV is associated with reducing inflammatory cell infiltration, improving the production of pro-inflammatory cytokines (TNF-α, IL-6, GM-CSF, IL-1β, and IL-8), and up-regulating antioxidant genes (SIRT1, PGC-1α, CAT, SOD1, and SOD2) in the treatment of COPD (Ma and Li, 2020; Wang et al., 2017). SIRT1 is a potential target of RSV in the treatment of COPD (Ma and Li, 2020). A mouse COPD model indicated that RSV maintains alveolar type-2 epithelial cell integrity by stimulating SIRT1 expression and promoting p53 destabilization (Navarro et al., 2017). RSV also restores steroid sensitivity of COPD lymphocytes and NKT-like cells and reduces systemic inflammatory responses by promoting SIRT1 expression (Hodge et al., 2020). RSV significantly increases PGC-1α and Nrf1 mRNA levels, mitochondrial mass, and coupled respiration through pharmacological activation of AMPK signaling to protect visual cortical neurons (Yu and Yang, 2010). RSV can upregulate antioxidant enzymes’ (SOD1, SOD2, and CAT) activities and restore reduced glutathione (GSH) biogenesis to protect astrocytes from oxidative damage (Bellaver et al., 2016).
AMPK/FOXO3 signaling is one of the vital pathways that regulate muscle atrophy; activated FOXO3 can induce the expression of atrophy-related genes, proteolysis, and muscle loss (Powers et al., 2012), and it is associated with intracellular ROS production (Akasaki et al., 2014) via NF-κB activation (Romanello et al., 2010). The ability of RSV to activate AMPK signaling to improve mitochondrial function and to increase mitochondrial biogenesis is dependent on SIRT1 (Price et al., 2012). These findings suggest that the development of new usages of RSV in COPD therapy might be via modulating mitochondrial function. However, the mechanisms of RSV anti-COPD effect via modulating mitochondrial activities need in-depth investigations.
3.4 Puerarin
Puerarin (PUE), an isoflavone derived from Angelica root, has various beneficial pharmacological functions (Jiang et al., 2022). The PI3K/Akt/mTOR pathway regulates autophagy, cell survival and differentiation, proliferation, and apoptosis (Hou et al., 2018), and it is involved in PM2.5-induced COPD (Mao et al., 2020). PUE can alleviate COPD by activating PI3K/Akt/mTOR signaling, inhibiting FUNDC1-mediated mitophagy and bronchial epithelial cell apoptosis (Liu J. et al., 2022).
PUE may serve as a novel and effective therapeutic agent for early-stage COPD. PUE can attenuate the acute smoking-induced infiltration of neutrophils and macrophages in mouse lung, leading to decreasing NF-κB signaling, and it also sensitively enhanced inflammatory mediator (TNF-α, COX-2, IL-6, MCP-1, and IL-8) expression; PUE also can suppress ROS production and upregulate NOX isoforms of small airway epithelia (Fang L. et al., 2022). It has been reported that PUE does not only increase mitochondrial antioxidant capacity, reduce overproduction of ROS, inhibit the expression of inflammatory factors and oxidative stress damage, and improve mitochondrial respiratory function and energy metabolism (Chang et al., 2021a) but also show mitochondrial protective properties in the treatment of nonalcoholic fatty liver disease (Wang S. et al., 2019), osteoarthritis (Wang et al., 2018), and diabetes (Chen et al., 2018).
These studies suggest that the mitochondrial pathway may be one of the mechanisms of using PUE in the treatment of COPD, but more research is needed.
3.5 Berberine
Berberine (BBR) is extracted from the bark and root of Coptis chinensis (Sun et al., 2022). A preliminary study indicates that BBR exhibits anti-inflammatory effects in treating chronic airway inflammatory diseases, including COPD (Tew et al., 2020). High-dose BBR attenuates CSE-induced airway inflammation by inactivating TGF-β1/Smads signaling (Wang W. et al., 2019).
In an in vitro study, BBR-loaded liquid crystal nanoparticles can reduce the expression of oxidative stress gene Nqo1 and inflammatory mediator TNF-α in bronchial epithelia and macrophages; it also inhibits p21 expression in bronchial epithelia (Paudel et al., 2022). BBR-loaded solid lipid nanoparticles (SLNs)-chitosan nanoparticles could dramatically ameliorate inflammation scores in lung tissues and reduce inflammatory cells (neutrophils and macrophages) and inflammatory cytokines (IL-1β, IL-6, IL-17, and TNF-α), thus improving the therapeutic anti-inflammatory impact of BBR against CS-induced airway inflammation in COPD rats (Sun et al., 2022).
BBR affects mitochondrial quality control and function by regulating the mitochondrial respiratory chain, oxidative stress, mitophagy, mitochondrial biogenesis and intracellular calcium concentration, and mitochondrial apoptosis (Fang X. et al., 2022). BBR can promote mitochondrial biogenesis (Yao et al., 2020) and restore autophagic flux (Hang et al., 2018) and mitochondrial energy homeostasis by regulating AMPK/PGC-1α signaling (Qin et al., 2020). BBR has also been shown to attenuate high-fat diet-induced muscle mitochondrial dysfunction by activating SIRT1/PGC-1α signaling, in which SIRT1 is involved in BBR-induced AMPK phosphorylation (Gomes et al., 2012). It is also reported that BBR protects the cardiac function of heart failure patients by upregulating PINK1/Parkin-mediated mitophagy (Abudureyimu et al., 2020) and attenuates myocardial ischemia/reperfusion impairment by regulating HIF-1α/BNIP3 signaling and enhancing mitophagy (Song et al., 2020). Furthermore, BBR inhibited NLRP3 inflammasome activation and ROS production by upregulating mitophagy, resulting in a reduction in lung inflammation in mice with influenza virus pneumonia (Liu H. et al., 2020). In addition, BBR can induce mitochondrial apoptosis, G0/G1 cell-cycle arrest, and inhibitory migration through modulating the PI3K/Akt and MAPK pathways of thyroid cancer cells (Li et al., 2017).
This suggests that BBR’s regulation of mitochondria may have an important place in new therapeutic strategies for COPD. Therefore, further studies are warranted to elucidate the detailed mechanism of the mitochondria-protective properties shown by BBR in the treatment of COPD.
3.6 Quercetin
Quercetin (QUE) is a plant flavanol (Zhu et al., 2018). It has been indicated that QUE protects COPD patients’ lymphocytes from 2-amino-3-methylimidazo [4, 5-f]quinoline (IQ)-induced DNA damage (Habas et al., 2018). QUE also reduces pulmonary inflammatory cell infiltration, oxidative stress, and lung function modification caused by CS exposure (da Silva Araújo et al., 2020), and it avoids emphysema changes (Araújo et al., 2022).
QUE has rhinovirus inhibitory properties in vitro and in vivo, including endocytosis, viral genome transcription, and viral protein synthesis (Ganesan et al., 2012). QUE effectively delayed lung disease progression in rhinovirus-infected COPD mice by reducing rhinovirus-induced pulmonary inflammatory cell accumulation, inhibiting mucus metaplasia and airway hyperresponsiveness (Farazuddin et al., 2018). QUE inhibits bronchial smooth muscle contraction induced by acetylcholine chloride and high K+ (Luo et al., 2018).
In addition, QUE modulates mitochondrial including mitochondrial quality control, mitochondria-mediated apoptosis pathways, MMP, oxidative respiratory chain and energy metabolism, and mitochondrial redox status etc. (de Oliveira et al., 2016). QUE has been shown to modulate mitochondrial properties in neuroprotective (Wang W. W. et al., 2021), cardioprotective (Chang et al., 2021b), enteroprotective (Vissenaekens et al., 2021), and hepatoprotective (Cai et al., 2021) effects. QUE reduces mtROS accumulation and subsequent NLRP3 assembly via enhancing mitophagy (Han et al., 2021). It is also known to inhibit NLRP3/caspase-1/GSDMD-N-mediated pyroptosis, maintain MMP, reduce mtDNA damage, and promote PGC-1α-mediated mitochondrial homeostasis, possibly through scavenging mtROS to protect liver cells from alcohol damage (Zhao et al., 2022). QUE was proven to upregulate SIRT1 expression, resulting in inhibiting NLRP3 activation and reducing neuroinflammation in aging mice (Li et al., 2021). It enhances the production of mitochondrial-related proteins (SIRT1, PGC-1α, and TFAM) expression (Ho et al., 2022) to inhibit apoptosis by modulating SIRT1/PGC-1α signaling (Tang et al., 2019); renal tubular epithelial cell senescence is associated with SIRT1/PINK1/Parkin activation (Liu T. et al., 2020). QUE can prevent sepsis-induced acute lung impairment and mitochondrial dysfunction by promoting the SIRT1/AMPK pathway (Sang et al., 2022). It can also restore amyloid-β-induced mitochondrial dysfunction by activating AMPK (Wang et al., 2014) and modulating the SIRT1/Nrf2/HO-1 pathway, leading to protecting the neurons in Alzheimer’s disease (Yu et al., 2020). It has been shown that QUE attenuates NF-κB signaling by inhibiting the HMGB1/TLR pathway (Li et al., 2016), and it inhibits NF-κB, TNF-α, IL-1β, IL-6, COX-2, and iNOS expression, reduces ROS levels, and increases SOD activity to attenuate Mn-induced oxidative stress and neuroinflammation (Bahar et al., 2017). Moreover, QUE was reported to downregulate mitochondrial apoptosis-related markers (BAX, Cyto c, cleaved caspase-3, and polymerase-1) and upregulate anti-apoptotic Bcl-2 expression, resulting in reduced Mn-induced apoptosis in SD rats (Bahar et al., 2017). However, on the other hand, QUE has also been reported to disrupt mitochondrial respiration between the ubiquinone pool and Cyto c that is associated with its cytotoxicity (Carrillo-Garmendia et al., 2022). It induces apoptosis and cell death of prostate cancer cells but not normal prostate epithelia via affecting mitochondrial integrity and interfering with ROS homeostasis (Ward et al., 2018).
However, few studies indicate the role of QUE-mediated mitochondrial function in the treatment of COPD. These results suggest that mitochondria may be the underlying mechanism of QUE in the treatment of COPD. However, further investigation needs to be carried out to test whether QUE has cytotoxic effects on normal cells.
3.7 Icariin
Icariin (ICA) is the main biologically active monomer of natural flavonoids extracted from Epimedium brevicornum Maxim (Li et al., 2015). It was found that ICA can reduce CSE-induced pro-inflammatory cytokine secretion and oxidative damage in bronchial epithelia, and it also has a positive effect on glucocorticoid resistance (Hu et al., 2020).
ICA attenuates LPS-induced acute lung inflammation via modulating the PI3K/Akt and NF-κB pathways (Xu et al., 2010). PI3K/Akt signaling plays an important role in the regulation of the Nrf2 pathway (Martin et al., 2004). ICA is considered a potential therapeutic agent of various diseases, such as cancers, neurodegenerative diseases, osteoporosis, and cardiovascular disease due to its ability to regulate the PI3K/Akt and Nrf2 signaling pathways (Verma et al., 2022). ICA promotes mitochondrial biogenesis in human nucleus pulposus cells by activating the PI3K/Akt and Nrf2 signaling pathways and inhibits hydrogen peroxide-induced mitochondria-mediated apoptosis (Hua et al., 2020). Icariside, a derivative of ICA, protects bone marrow mesenchymal cells from damage resulting from iron overload by regulating mitochondrial morphology and fission through the MAPK and PI3K/Akt/mTOR pathways (Yao et al., 2019).
Furthermore, ICA attenuates mitochondrial oxidative damage by enhancing SIRT1 activity and maintains mitochondrial homeostasis to protect cardiomyocytes from ischemia-reperfusion caused injury (Wu et al., 2018). ICA protects neurons from rotenone damage by upregulating SIRT3 and PGC-1α expressions (Zeng et al., 2019a). This protective effect may also be related to the restoration of autophagic flux by ICA via enhancing mitophagy-related proteins’ (LC3-II and Beclin-1) expression and inhibiting mTOR activation (Zeng et al., 2019b). ICA ameliorates ROS accumulation and mitochondrial dynamics disturbance of alcohol-impaired atrium by activating SIRT3/AMPK signaling (Yu et al., 2022). ICA can rescue high glucose-induced osteoblast differentiation via inhibiting mtROS and maintaining mitochondrial homeostasis (Liu J. et al., 2022). Additionally, ICA can enhance neuronal cell activity in a triple transgenic Alzheimer’s disease mouse model via maintaining mitochondrial key enzyme COX IV and promoting ATP generation (Chen et al., 2016). The ICA and β-azalone combination increases mitophagy-related proteins’ (Beclin-1, PINK1, and p-Parkin) expression to promote autophagosomes formation, leading to attenuating amyloid-β-induced nerve cell impairment (Wang N. et al., 2021).
These studies suggest that the mitochondrial pathway may be the mechanism for icariin to treat COPD and other mitochondria-associated diseases.
3.8 Paeoniflorin
Paeoniflorin (PF), a water-soluble monoterpene glucoside extracted from the root of Paeonia suffruticosa, has shown that the therapeutic effect of PF in a COPD rat model includes not only reducing airway inflammation and improving lung function but also improving oxidative stress condition by quenching ROS and upregulating antioxidant enzymes via Nrf2-dependent signaling (Lin et al., 2016).
PF can ameliorate ovalbumin-induced lung injury in a mouse model by restoring MMP, modulating mitochondrial function, and inhibiting pro-inflammatory cytokine release (Han et al., 2022). PF alleviates bortezomib-induced peripheral neuropathy (BiPN) by promoting Parkin-mediated mitophagy and decreasing IL-6 (Sun et al., 2022). In addition, PF can reduce the activities of caspase-9 and caspase-3, and it also inhibits the release of Cyto c in mitochondria, resulting in apoptosis inhibition (Liu Y. F. et al., 2022). PF can inhibit the expression of mitochondrial apoptotic proteins BAX, caspase-9, and caspase-3 and upregulate Bcl-2 by inhibiting JNK-related signaling pathways, resulting in apoptosis inhibition (Deng et al., 2022; Cong et al., 2019). PF can protect spiral ganglion neurons from cisplatin damage by reducing ROS and modulating the PINK1/BAD pathway (Yu et al., 2019). However, PF can promote apoptosis of synovial tissue by downregulating Bcl-2 and enhancing AMPK phosphorylation (Huang et al., 2021); it can promote mitochondrial biogenesis and improve TNFα-induced muscle atrophy via regulating TFAM, ERα, and Nrf1 expression (Park et al., 2022). It also improves mitochondrial dysfunction and oxidative stress induced by TNF-α by promoting the AMPK/SIRT1/PGC-1α pathway, (Li Q. et al., 2022), PF inhibits lipopolysaccharide-induced mitochondrial damage, and activation of the NLRP3 inflammasome of hepatocytes via the regulation of SIRT3/FOXO1a/SOD1 signaling (Li L. et al., 2022). Furthermore, it downregulates the ROS-NF-κB axis via suppressing NOX2/NOX4 and RAGE expression, which results in a decrease of the downstream HIF-1α/VEGF level, leading to protecting human umbilical vein endothelial cells from oxidative damage (Song et al., 2017) and inhibiting intracellular Ca2+ and calcium/calmodulin kinase II (CaMKII), which might show protective effects on mitochondria (Wang D. et al., 2013).
Even though few studies indicate that PF modulates mitochondrial function in COPD, the above pieces of evidence suggest that mitochondria may be the underlying molecular mechanism for PF in COPD treatment.
3.9 Paeonol
Paeonol (PAE) is one of the phenolic phytochemicals isolated from herbs such as Dioscorea, Paeonia lactiflora, and P. suffruticosa. PAE can reduce glutamate-induced apoptosis and neurotoxicity by suppressing Cyto c release and caspase-3 activation, as well as downregulating the ERK pathway (Wang et al., 2011). However, the mechanism of PAE in the treatment of COPD is yet to be understood. It has been shown that PAE inhibits hypoxia-mediated mitochondrial damage in pulmonary artery smooth muscle cells by decreasing ATP production, increasing ROS production, and enhancing mitochondrial morphological changes and polarization (Wang D. et al., 2019). PAE alleviates LPS-induced liver injury by improving mitochondrial function, maintaining MMP, reducing the expression of BAX and cleaved caspase-3, inhibiting superoxide production from mitochondria, and nuclear translocation of NF-κB (Xu et al., 2021).
PAE can reduce M1 macrophage polarization by inhibiting NLRP3 inflammasome, which results in decreasing inflammation levels in acute (Yuan et al., 2022). PAE can ameliorate streptozotocin-induced mtROS, TNF-α and IL-6, and MMP disturbance (Tayanloo-Beik et al., 2022). PAE derivatives have been shown to exert anti-inflammatory effects via suppressing TLR4/MyD88 signaling and inflammatory factor expression; they can also inhibit LPS-induced ROS production and restore the MMP of macrophages (Gong et al., 2022). In addition, PAE can upregulate PINK1/Parkin and BNIP3L/NIX autophagy signaling to protect retinal photoreceptor cells (Zhang D. et al., 2021). PAE can also promote apoptosis of hepatic stellate cells via inhibiting NF-κB signaling (Kong et al., 2013). PAE can induce apoptosis of cervical cancer cells that is associated with regulating the PI3K/Akt pathway to trigger mitochondrial apoptotic signaling (Du et al., 2022).
These studies suggest that PAE has potential mitochondrial protective activity, which supports the potential COPD therapy with PAE via modulating mitochondrial activities.
3.10 Hesperidin
Hesperidin (HES), a flavonoid glycoside present in citrus fruits, has been shown to possess antioxidant, anti-inflammatory, antiviral, anticancer, and neuroprotective properties (Garg et al., 2001; Xia et al., 2018). A recent study has indicated that HES ameliorates CSE-induced inflammation and oxidative stress by promoting SIRT1/PGC-1α/NF-κB signaling (Manevski et al., 2020). Derivatives of HES, such as hesperidin-3-O-methyl ether and hesperetin-5, 7, 3-O-trimethyl ether, inhibited airway hyperresponsiveness and inflammation in mouse models (Yang et al., 2012; Shih et al., 2020).
HES can improve mitochondrial malfunction during benzopyridine-induced lung carcinogenesis in a mouse model; for example, it upregulates antioxidant and TCA cycle enzymes, protects electron transport chains, and restores ATP levels (Kamaraj et al., 2011). The mechanism by which HES treats COPD is yet to be known.
HES alleviates 6-hydroxydopamine-induced degeneration of dopamine neurons by restoring mitochondrial respiratory chain complexes-I, -IV, and V as well as Na+-K+-ATPase activity and regulating caspase-3 and caspase-9 activities (Antunes et al., 2021). HES can protect against amyloid-β-induced neurotoxicity by suppressing VDAC1-mediated mitochondrial apoptosis signaling (Wang D. M. et al., 2013). HES reduces high glucose-induced apoptosis and oxidative damage of retinal pigment epithelia via scavenging ROS, decreasing Cyto c release, and inhibiting caspase-9/3 expression (Liu et al., 2018).
Furthermore, neohesperidin attenuated hepatic steatosis and insulin resistance that was associated with increasing PGC-1α-mediated mitochondrial biogenesis in a high-fat diet mouse model (Wang et al., 2020). HES can attenuate PM2.5-induced DNA damage, cell cycle arrest, and cellular senescence of human HaCaT keratinocytes via deactivating ROS/JNK signaling (Herath et al., 2022). HES can suppress high glucose-induced DNA damage and high mitochondrial calcium level to protect neuronal cells (Lim et al., 2022). HES also inhibits apoptosis and promotes cell viability by inactivation of ERK, JNK, and MAPK (Lim et al., 2022). Additionally, HES can reverse bupivacaine anesthesia-induced decreased MMP, mitochondrial apoptotic signaling, and HES can modulate the homeostasis between redox and inflammatory system (Wang T. et al., 2021). Furthermore, HES promotes DRP1-mediated mitophagy and improves impaired mitochondria in a functional dyspepsia rat model (Jia et al., 2022).
These studies suggest that the mitochondrial pathway might provide potential insights of HES for COPD therapy.
4 Conclusion
Although we have found some mechanisms and functions of the active monomers and combinations from herbal extractions that show mitochondrial protection ability in COPD therapy, the evidence from clinical studies that support the treatment of COPD with the natural compounds is still insufficient and remains inconclusive (according to ClinicalTrials.gov database, as accessed on 03/31/2024) (Table 1). Many natural compounds show effects on mitochondrial modification, but no COPD-related research has been performed that is worth considering in the future investigation. One such example is astragaloside IV (AS-IV), a small-molecule saponin extracted from Astragalus membranaceus, which improves mitochondrial activity in cortical neurons subjected to oxygen and glucose deprivation (OGD) by modulating PKA/CREB signaling, resulting in neuronal apoptosis inhibition by regulating mtROS and ATP production (Xue et al., 2019; Ryu et al., 2005). This might have potential effects on regulating COPD mitochondrial homeostasis.
Impaired mitochondria have different appearances and effects on different types of cells, stages of growth, and diseases. All these different conditions should be considered to maximize the efficiency of administration. Additionally, the effects of natural compounds on various mitochondrial targets should be well investigated and considered. Based on the concerns mentioned above, a comprehensive and systematic investigation strategy on herb extractions should be carried out to promote their use for mitochondrial related other diseases.
Author contributions
QW: writing – original draft and writing – review and editing. ZZ: writing – original draft and writing – review and editing. LG: supervision and writing – review and editing. KW: writing – review and editing. YZ: funding acquisition and writing – review and editing. HT: writing – review and editing. HH: writing – review and editing. GQ: writing – review and editing. KW: writing – review and editing. XW: funding acquisition, software, supervision, writing – original draft, and writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Health Commission of Sichuan Province Medical Science and Technology Program (No. 24QNMP071 to Y. Z), the Foundation of Luzhou Science and Technology Program and Southwest Medical University (No. 2024LZXNYDJ032 to X. W and No. 2021LZXNYD-J19 to Y. Z), and the Sichuan Science and Technology Program (No. 2022YFS0629 to G. Q). Funders had no role in study design, literature collection, review, analysis, interpretation, writing of the report, and so on.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abudureyimu, M., Yu, W., Cao, R. Y., Zhang, Y., Liu, H., and Zheng, H. (2020). Berberine promotes cardiac function by upregulating PINK1/parkin-mediated mitophagy in heart failure. Front. Physiol. 11, 565751. doi:10.3389/fphys.2020.565751
Acosta, J. C., O'Loghlen, A., Banito, A., Guijarro, M. V., Augert, A., Raguz, S., et al. (2008). Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133 (6), 1006–1018. doi:10.1016/j.cell.2008.03.038
Aghapour, M., Remels, A. H. V., Pouwels, S. D., Bruder, D., Hiemstra, P. S., Cloonan, S. M., et al. (2020). Mitochondria: at the crossroads of regulating lung epithelial cell function in chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell Mol. Physiol. 318 (1), L149-L164–l164. doi:10.1152/ajplung.00329.2019
Aguado, J., Chaggar, H. K., Gómez-Inclán, C., Shaker, M. R., Leeson, H. C., Mackay-Sim, A., et al. (2021). Inhibition of the cGAS-STING pathway ameliorates the premature senescence hallmarks of Ataxia-Telangiectasia brain organoids. Aging Cell 20 (9), e13468. doi:10.1111/acel.13468
Agusti, A., Celli, B. R., Criner, G. J., Halpin, D., Anzueto, A., Barnes, P., et al. (2023). GlobalInitiative for Chronic Obstructive Lung Disease 2023Report: GOLD Executive Summary. Eur Respir J 1 (4), 2300239. doi:10.1183/13993003.00239-2023
Ahmad, T., Sundar, I. K., Lerner, C. A., Gerloff, J., Tormos, A. M., Yao, H., et al. (2015). Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: implications for chronic obstructive pulmonary disease. Faseb J. 29 (7), 2912–2929. doi:10.1096/fj.14-268276
Akasaki, Y., Alvarez-Garcia, O., Saito, M., Caramés, B., Iwamoto, Y., and Lotz, M. K. (2014). FoxO transcription factors support oxidative stress resistance in human chondrocytes. Arthritis Rheumatol. 66 (12), 3349–3358. doi:10.1002/art.38868
Alexeyev, M., Shokolenko, I., Wilson, G., and LeDoux, S. (2013). The maintenance of mitochondrial DNA integrity--critical analysis and update. Cold Spring Harb. Perspect. Biol. 5 (5), a012641. doi:10.1101/cshperspect.a012641
Antunes, M. S., Ladd, F. V. L., Ladd, A. A. B. L., Moreira, A. L., Boeira, S. P., and Cattelan Souza, L. (2021). Hesperidin protects against behavioral alterations and loss of dopaminergic neurons in 6-OHDA-lesioned mice: the role of mitochondrial dysfunction and apoptosis. Metab. Brain Dis. 36 (1), 153–167. doi:10.1007/s11011-020-00618-y
Araya, J, Tsubouchi, K., Sato, N., Ito, S., Minagawa, S., Hara, H., et al. (2019). PRKN-regulated mitophagy and cellular senescence during COPD pathogenesis. Autophagy. 15 (3), 510–526. doi:10.1080/15548627.2018.1532259
Araújo, N., de Matos, N. A., Oliveira, M., de Souza, A. B. F., Castro, T. d. F., Machado-Júnior, P. A., et al. (2022). Quercetin improves pulmonary function and prevents emphysema caused by exposure to cigarette smoke in male mice. Antioxidants (Basel) 11 (2), 181. doi:10.3390/antiox11020181
Arunachalam, G., Yao, H., Sundar, I. K., Caito, S., and Rahman, I. (2010). SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: role of resveratrol. Biochem. Biophys. Res. Commun. 393 (1), 66–72. doi:10.1016/j.bbrc.2010.01.080
Bagkos, G., Koufopoulos, K., and Piperi, C. (2014). A new model for mitochondrial membrane potential production and storage. Med. Hypotheses 83 (2), 175–181. doi:10.1016/j.mehy.2014.05.001
Bahar, E., Kim, J. Y., and Yoon, H. (2017). Quercetin attenuates manganese-induced neuroinflammation by alleviating oxidative stress through regulation of apoptosis, iNOS/NF-κB and HO-1/Nrf2 pathways. Int. J. Mol. Sci. 18 (9), 1989. doi:10.3390/ijms18091989
Bai, Q., Wang, Z., Piao, Y., Zhou, X., Piao, Q., Jiang, J., et al. (2022). Sesamin alleviates asthma airway inflammation by regulating mitophagy and mitochondrial apoptosis. J. Agric. Food Chem. 70 (16), 4921–4933. doi:10.1021/acs.jafc.1c07877
Baines, C. P., Kaiser, R. A., Purcell, N. H., Blair, N. S., Osinska, H., Hambleton, M. A., et al. (2005). Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434 (7033), 658–662. doi:10.1038/nature03434
Bause, A. S., and Haigis, M. C. (2013). SIRT3 regulation of mitochondrial oxidative stress. Exp. Gerontol. 48 (7), 634–639. doi:10.1016/j.exger.2012.08.007
Beijers, R., Gosker, H. R., and Schols, A. (2018). Resveratrol for patients with chronic obstructive pulmonary disease: hype or hope? Curr. Opin. Clin. Nutr. Metab. Care 21 (2), 138–144. doi:10.1097/MCO.0000000000000444
Belchamber, K. B. R., Singh, R., Batista, C. M., Whyte, M. K., Dockrell, D. H., Kilty, I., et al. (2019). Defective bacterial phagocytosis is associated with dysfunctional mitochondria in COPD macrophages. Eur. Respir. J. 54 (4), 1802244. doi:10.1183/13993003.02244-2018
Bellaver, B., Bobermin, L. D., Souza, D. G., Rodrigues, M. D. N., de Assis, A. M., Wajner, M., et al. (2016). Signaling mechanisms underlying the glioprotective effects of resveratrol against mitochondrial dysfunction. Biochim. Biophys. Acta 1862 (9), 1827–1838. doi:10.1016/j.bbadis.2016.06.018
Bender, A., Krishnan, K. J., Morris, C. M., Taylor, G. A., Reeve, A. K., Perry, R. H., et al. (2006). High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat. Genet. 38 (5), 515–517. doi:10.1038/ng1769
Bernales, S., Soto, M. M., and McCullagh, E. (2012). Unfolded protein stress in the endoplasmic reticulum and mitochondria: a role in neurodegeneration. Front. Aging Neurosci. 4, 5. doi:10.3389/fnagi.2012.00005
Bernardi, P., Scorrano, L., Colonna, R., Petronilli, V., and Di Lisa, F. (1999). Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur. J. Biochem. 264 (3), 687–701. doi:10.1046/j.1432-1327.1999.00725.x
Biel, T. G., Lee, S., Flores-Toro, J. A., Dean, J. W., Go, K. L., Lee, M. H., et al. (2016). Sirtuin 1 suppresses mitochondrial dysfunction of ischemic mouse livers in a mitofusin 2-dependent manner. Cell Death Differ. 23 (2), 279–290. doi:10.1038/cdd.2015.96
Bronner, D. N., and O'Riordan, M. X. (2016). Measurement of mitochondrial DNA release in response to ER stress. Bio Protoc. 6 (12), e1839. doi:10.21769/BioProtoc.1839
Brusselle, G. G., Joos, G. F., and Bracke, K. R. (2011). New insights into the immunology of chronic obstructive pulmonary disease. Lancet 378 (9795), 1015–1026. doi:10.1016/S0140-6736(11)60988-4
Bu, T., Wang, L. F., and Yin, Y. Q. (2020). How do innate immune cells contribute to airway remodeling in COPD progression? Int. J. Chron. Obstruct Pulmon Dis. 15, 107–116. doi:10.2147/COPD.S235054
Cai, P., Zhu, Q., Cao, Q., Bai, Y., Zou, H., Gu, J., et al. (2021). Quercetin and allicin can alleviate the hepatotoxicity of lead (Pb) through the PI3K signaling pathway. J. Agric. Food Chem. 69 (32), 9451–9460. doi:10.1021/acs.jafc.1c03794
Cantó, C., and Auwerx, J. (2009). PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 20 (2), 98–105. doi:10.1097/MOL.0b013e328328d0a4
Carrillo-Garmendia, A., Martinez-Ortiz, C., Canizal-Garcia, M., González-Hernández, J. C., Arvizu-Medrano, S. M., Gracida, J., et al. (2022). Cytotoxicity of quercetin is related to mitochondrial respiration impairment in Saccharomyces cerevisiae. Yeast 39 (11-12), 617–628. doi:10.1002/yea.3818
Chang, X., Zhang, T., Liu, D., Meng, Q., Yan, P., Luo, D., et al. (2021a). Puerarin attenuates LPS-induced inflammatory responses and oxidative stress injury in human umbilical vein endothelial cells through mitochondrial quality control. Oxid. Med. Cell Longev. 2021, 6659240. doi:10.1155/2021/6659240
Chang, X., Zhang, T., Meng, Q., ShiyuanWang, P., Yan, P., Wang, X., et al. (2021b). Quercetin improves cardiomyocyte vulnerability to hypoxia by regulating SIRT1/TMBIM6-related mitophagy and endoplasmic reticulum stress. Oxid. Med. Cell Longev. 2021, 5529913. doi:10.1155/2021/5529913
Chen, C. F., Chiou, W. F., and Zhang, J. T. (2008). Comparison of the pharmacological effects of Panax ginseng and Panax quinquefolium. Acta Pharmacol. Sin. 29 (9), 1103–1108. doi:10.1111/j.1745-7254.2008.00868.x
Chen, Q., Fischer, A., Reagan, J. D., Yan, L. J., and Ames, B. N. (1995). Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc. Natl. Acad. Sci. U. S. A. 92 (10), 4337–4341. doi:10.1073/pnas.92.10.4337
Chen, Q., Vasse, G. F., Nwozor, K. O., Bekker, N. J., van den Berge, M., Brandsma, C. A., et al. (2023a). FAM13A regulates cellular senescence marker p21 and mitochondrial reactive oxygen species production in airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 325 (4), L460–l466. doi:10.1152/ajplung.00141.2023
Chen, X., Wang, Q., Shao, M., Guo, D., Wu, Y., et al. (2019). Ginsenoside Rb3 regulates energy metabolism and apoptosis in cardiomyocytes via activating PPARα pathway. Biomed. Pharmacother. 120, 109487. doi:10.1016/j.biopha.2019.109487
Chen, X. F., Wang, L., Wu, Y. Z., Song, S. Y., Min, H. Y., Yang, Y., et al. (2018). Effect of puerarin in promoting fatty acid oxidation by increasing mitochondrial oxidative capacity and biogenesis in skeletal muscle in diabetic rats. Nutr. Diabetes 8 (1), 1. doi:10.1038/s41387-017-0009-6
Chen, Y., Zhang, Y., Jiang, Z., and Li, X. (2023b). Role of mitochondrial stress and the NLRP3 inflammasome in lung diseases. Inflamm. Res. 72 (4), 829–846. doi:10.1007/s00011-023-01712-4
Chen, Y. J., Zheng, H. Y., Huang, X. X., Han, S. X., Zhang, D. S., Ni, J. Z., et al. (2016). Neuroprotective effects of icariin on brain metabolism, mitochondrial functions, and cognition in triple-transgenic Alzheimer's disease mice. CNS Neurosci. Ther. 22 (1), 63–73. doi:10.1111/cns.12473
Cheng, X. Y., Li, Y. Y., Huang, C., Li, J., and Yao, H. W. (2017). AMP-activated protein kinase reduces inflammatory responses and cellular senescence in pulmonary emphysema. Oncotarget 8 (14), 22513–22523. doi:10.18632/oncotarget.15116
Chung, S., Overstreet, J. M., Li, Y., Wang, Y., Niu, A., Wang, S., et al. (2018). TGF-β promotes fibrosis after severe acute kidney injury by enhancing renal macrophage infiltration. JCI Insight 3 (21), e123563. doi:10.1172/jci.insight.123563
Cong, C., Kluwe, L., Li, S., Liu, X., Liu, Y., Liu, H., et al. (2019). Paeoniflorin inhibits tributyltin chloride-induced apoptosis in hypothalamic neurons via inhibition of MKK4-JNK signaling pathway. J. Ethnopharmacol. 237, 1–8. doi:10.1016/j.jep.2019.03.030
Correia-Melo, C., Marques, F. D. M., Anderson, R., Hewitt, G., Hewitt, R., Cole, J., et al. (2016). Mitochondria are required for pro-ageing features of the senescent phenotype. Embo J. 35 (7), 724–742. doi:10.15252/embj.201592862
Cui, W., Zhang, Z., Zhang, P., Qu, J., Zheng, C., Mo, X., et al. (2018). Nrf2 attenuates inflammatory response in COPD/emphysema: crosstalk with Wnt3a/β-catenin and AMPK pathways. J. Cell Mol. Med. 22 (7), 3514–3525. doi:10.1111/jcmm.13628
Dai, D. F., Rabinovitch, P. S., and Ungvari, Z. (2012). Mitochondria and cardiovascular aging. Circ. Res. 110 (8), 1109–1124. doi:10.1161/CIRCRESAHA.111.246140
Dasgupta, A., Shukla, S. K., Vernucci, E., King, R. J., Abrego, J., Mulder, S. E., et al. (2020). SIRT1-NOX4 signaling axis regulates cancer cachexia. J. Exp. Med. 217 (7), e20190745. doi:10.1084/jem.20190745
da Silva Araújo, N. P., de Matos, N. A., Leticia Antunes Mota, S., Farias de Souza, A. B., Dantas Cangussú, S., Cunha Alvim de Menezes, R., et al. (2020). Quercetin attenuates acute lung injury caused by cigarette smoke both in vitro and in vivo. Copd 17 (2), 205–214. doi:10.1080/15412555.2020.1749253
de Boer, W. I., van Schadewijk, A., Sont, J. K., Sharma, H. S., Stolk, J., Hiemstra, P. S., et al. (1998). Transforming growth factor beta1 and recruitment of macrophages and mast cells in airways in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 158 (6), 1951–1957. doi:10.1164/ajrccm.158.6.9803053
de Brito, O. M., and Scorrano, L. (2008). Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456 (7222), 605–610. doi:10.1038/nature07534
Demedts, I. K., Demoor, T., Bracke, K. R., Joos, G. F., and Brusselle, G. G. (2006). Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir. Res. 7 (1), 53. doi:10.1186/1465-9921-7-53
Deng, X., Li, Y., Li, X., Zhang, Z., Dai, S., Wu, H., et al. (2022). Paeoniflorin protects against acetaminophen-induced liver injury in mice via JNK signaling pathway. Molecules 27 (23), 8534. doi:10.3390/molecules27238534
de Oliveira, M. R., Nabavi, S. M., Braidy, N., Setzer, W. N., Ahmed, T., and Nabavi, S. F. (2016). Quercetin and the mitochondria: a mechanistic view. Biotechnol. Adv. 34 (5), 532–549. doi:10.1016/j.biotechadv.2015.12.014
Ding, Z., Liu, S., Wang, X., Mathur, P., Dai, Y., Theus, S., et al. (2016). Cross-talk between PCSK9 and damaged mtDNA in vascular smooth muscle cells: role in apoptosis. Antioxid. Redox Signal 25 (18), 997–1008. doi:10.1089/ars.2016.6631
Dinkova-Kostova, A. T., and Abramov, A. Y. (2015). The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 88 (Pt B), 179–188. doi:10.1016/j.freeradbiomed.2015.04.036
Di Vincenzo, S., Heijink, I. H., Noordhoek, J. A., Cipollina, C., Siena, L., Bruno, A., et al. (2018). SIRT1/FoxO3 axis alteration leads to aberrant immune responses in bronchial epithelial cells. J. Cell Mol. Med. 22 (4), 2272–2282. doi:10.1111/jcmm.13509
Doucet, M., Rochette, L., and Hamel, D. (2016). Incidence, prevalence, and mortality trends in chronic obstructive pulmonary disease over 2001 to 2011: a public health point of view of the burden. Can. Respir. J. 2016, 7518287. doi:10.1155/2016/7518287
Du, J., Song, D., Li, J., Li, Y., Li, B., and Li, L. (2022). Paeonol triggers apoptosis in HeLa cervical cancer cells: the role of mitochondria-related caspase pathway. Psychopharmacol. Berl. 239 (7), 2083–2092. doi:10.1007/s00213-021-05811-0
Egan, D. F., Shackelford, D. B., Mihaylova, M. M., Gelino, S., Kohnz, R. A., Mair, W., et al. (2011). Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331 (6016), 456–461. doi:10.1126/science.1196371
Elrod, J. W., Wong, R., Mishra, S., Vagnozzi, R. J., Sakthievel, B., Goonasekera, S. A., et al. (2010). Cyclophilin D controls mitochondrial pore-dependent Ca(2+) exchange, metabolic flexibility, and propensity for heart failure in mice. J. Clin. Invest 120 (10), 3680–3687. doi:10.1172/JCI43171
Elwakiel, A., Mathew, A., and Isermann, B. (2024). The role of endoplasmic reticulum-mitochondria-associated membranes in diabetic kidney disease. Cardiovasc Res. 119 (18), 2875–2883. doi:10.1093/cvr/cvad190
Fang, C., Wei, X., and Wei, Y. (2016). Mitochondrial DNA in the regulation of innate immune responses. Protein Cell 7 (1), 11–16. doi:10.1007/s13238-015-0222-9
Fang, L., Zhang, M., Li, J., Zhou, L., Tamm, M., and Roth, M. (2022a). Airway smooth muscle cell mitochondria damage and mitophagy in COPD via ERK1/2 MAPK. Int. J. Mol. Sci. 23 (22), 13987. doi:10.3390/ijms232213987
Fang, X., Wu, H., Wei, J., Miao, R., Zhang, Y., and Tian, J. (2022b). Research progress on the pharmacological effects of berberine targeting mitochondria. Front. Endocrinol. (Lausanne) 13, 982145. doi:10.3389/fendo.2022.982145
Farazuddin, M., Mishra, R., Jing, Y., Srivastava, V., Comstock, A. T., and Sajjan, U. S. (2018). Quercetin prevents rhinovirus-induced progression of lung disease in mice with COPD phenotype. PLoS One 13 (7), e0199612. doi:10.1371/journal.pone.0199612
Feng, H., Zhang, D., Yin, Y., Kang, J., and Zheng, R. (2023). Salidroside ameliorated the pulmonary inflammation induced by cigarette smoke via mitigating M1 macrophage polarization by JNK/c-Jun. Phytother. Res. 37 (9), 4251–4264. doi:10.1002/ptr.7905
Fiorese, C. J., Schulz, A. M., Lin, Y. F., Rosin, N., Pellegrino, M. W., and Haynes, C. M. (2016). The transcription factor ATF5 mediates a mammalian mitochondrial UPR. Curr. Biol. 26 (15), 2037–2043. doi:10.1016/j.cub.2016.06.002
Frankenberg Garcia, J., Rogers, A. V., Mak, J. C. W., Halayko, A. J., Hui, C. K. M., Xu, B., et al. (2022). Mitochondrial transfer regulates bioenergetics in healthy and chronic obstructive pulmonary disease airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 67 (4), 471–481. doi:10.1165/rcmb.2022-0041OC
Freund, A., Orjalo, A. V., Desprez, P. Y., and Campisi, J. (2010). Inflammatory networks during cellular senescence: causes and consequences. Trends Mol. Med. 16 (5), 238–246. doi:10.1016/j.molmed.2010.03.003
Fujii, S., Hara, H., Araya, J., Takasaka, N., Kojima, J., Ito, S., et al. (2012). Insufficient autophagy promotes bronchial epithelial cell senescence in chronic obstructive pulmonary disease. Oncoimmunology 1 (5), 630–641. doi:10.4161/onci.20297
Ganesan, S., Faris, A. N., Comstock, A. T., Wang, Q., Nanua, S., Hershenson, M. B., et al. (2012). Quercetin inhibits rhinovirus replication in vitro and in vivo. Antivir. Res. 94 (3), 258–271. doi:10.1016/j.antiviral.2012.03.005
Garg, A., Garg, S., Zaneveld, L. J., and Singla, A. K. (2001). Chemistry and pharmacology of the Citrus bioflavonoid hesperidin. Phytother. Res. 15 (8), 655–669. doi:10.1002/ptr.1074
Gaspar, R. S., Katashima, C. K., Crisol, B. M., Carneiro, F. S., Sampaio, I., Silveira, L. D. R., et al. (2023). Physical exercise elicits UPR(mt) in the skeletal muscle: the role of c-Jun N-terminal kinase. Mol. Metab. 78, 101816. doi:10.1016/j.molmet.2023.101816
Giordano, L., Gregory, A. D., Pérez Verdaguer, M., Ware, S. A., Harvey, H., DeVallance, E., et al. (2022). Extracellular release of mitochondrial DNA: triggered by cigarette smoke and detected in COPD. Cells 11 (3), 369. doi:10.3390/cells11030369
Gleyzer, N., Vercauteren, K., and Scarpulla, R. C. (2005). Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol. Cell Biol. 25 (4), 1354–1366. doi:10.1128/MCB.25.4.1354-1366.2005
Global Burden of Disease (2017). Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 5 (9), 691–706. doi:10.1016/S2213-2600(17)30293-X
Global Burden of Disease (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392 (10159), 1789–1858. doi:10.1016/S0140-6736(18)32279-7
Glück, S., Guey, B., Gulen, M. F., Wolter, K., Kang, T. W., Schmacke, N. A., et al. (2017). Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat. Cell Biol. 19 (9), 1061–1070. doi:10.1038/ncb3586
Gӧbel, J., Engelhardt, E., Pelzer, P., Sakthivelu, V., Jahn, H. M., Jevtic, M., et al. (2020). Mitochondria-endoplasmic reticulum contacts in reactive astrocytes promote vascular remodeling. Cell Metab. 31 (4), 791–808. doi:10.1016/j.cmet.2020.03.005
Gogebakan, B., Bayraktar, R., Ulaslı, M., Oztuzcu, S., Tasdemir, D., and Bayram, H. (2014). The role of bronchial epithelial cell apoptosis in the pathogenesis of COPD. Mol. Biol. Rep. 41 (8), 5321–5327. doi:10.1007/s11033-014-3403-3
Gomes, A. P., Duarte, F. V., Nunes, P., Hubbard, B. P., Teodoro, J. S., Varela, A. T., et al. (2012). Berberine protects against high fat diet-induced dysfunction in muscle mitochondria by inducing SIRT1-dependent mitochondrial biogenesis. Biochim. Biophys. Acta 1822 (2), 185–195. doi:10.1016/j.bbadis.2011.10.008
Gong, L., Shen, Y., Wang, S., Wang, X., Ji, H., Wu, X., et al. (2023). Nuclear SPHK2/S1P induces oxidative stress and NLRP3 inflammasome activation via promoting p53 acetylation in lipopolysaccharide-induced acute lung injury. Cell Death Discov. 9 (1), 12. doi:10.1038/s41420-023-01320-5
Gong, X., Yang, D., Yang, S., Li, J., Zhao, H., et al. (2022). Synthesis and anti-inflammatory activity of paeonol derivatives with etherized aryl urea by regulating TLR4/MyD88 signaling pathway in RAW264.7 cell. Bioorg Chem. 127, 105939. doi:10.1016/j.bioorg.2022.105939
González-Burgos, E., Fernández-Moriano, C., Lozano, R., Iglesias, I., and Gómez-Serranillos, M. P. (2017). Ginsenosides Rd and Re co-treatments improve rotenone-induced oxidative stress and mitochondrial impairment in SH-SY5Y neuroblastoma cells. Food Chem. Toxicol. 109 (Pt 1), 38–47. doi:10.1016/j.fct.2017.08.013
Gottlieb, E., Armour, S. M., Harris, M. H., and Thompson, C. B. (2003). Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 10 (6), 709–717. doi:10.1038/sj.cdd.4401231
Green, D. R., and Reed, J. C. (1998). Mitochondria and apoptosis. Science 281 (5381), 1309–1312. doi:10.1126/science.281.5381.1309
Guan, R., Wang, J., Cai, Z., Li, Z., Wang, L., Li, Y., et al. (2020a). Hydrogen sulfide attenuates cigarette smoke-induced airway remodeling by upregulating SIRT1 signaling pathway. Redox Biol. 28, 101356. doi:10.1016/j.redox.2019.101356
Guan, S., Xu, W., Han, F., Gu, W., Song, L., Ye, W., et al. (2017). Ginsenoside Rg1 Attenuates Cigarette Smoke-Induced Pulmonary Epithelial-Mesenchymal Transition via Inhibition of the TGF-β1/Smad Pathway. Biomed Res Int. 7171404. doi:10.1155/2017/7171404
Guan, X., Yuan, Y., Wang, G., Zheng, R., Zhang, J., Dong, B., et al. (2020b). Ginsenoside Rg3 ameliorates acute exacerbation of COPD by suppressing neutrophil migration. Int. Immunopharmacol. 83, 106449. doi:10.1016/j.intimp.2020.106449
Gumeni, S., Papanagnou, E. D., Manola, M. S., and Trougakos, I. P. (2021). Nrf2 activation induces mitophagy and reverses Parkin/Pink1 knock down-mediated neuronal and muscle degeneration phenotypes. Cell Death Dis. 12 (7), 671. doi:10.1038/s41419-021-03952-w
Ha, J., Guan, K. L., and Kim, J. (2015). AMPK and autophagy in glucose/glycogen metabolism. Mol. Asp. Med. 46, 46–62. doi:10.1016/j.mam.2015.08.002
Habas, K., Abdulmwli, M., Demir, E., Jacob, B. K., Najafzadeh, M., and Anderson, D. (2018). DNA damage protection by bulk and nano forms of quercetin in lymphocytes of patients with chronic obstructive pulmonary disease exposed to the food mutagen 2-amino-3-methylimidazo [4,5-f]quinolone (IQ). Environ. Res. 166, 10–15. doi:10.1016/j.envres.2018.05.012
Haji, G., Wiegman, C. H., Michaeloudes, C., Patel, M. S., Curtis, K., Bhavsar, P., et al. (2020). Mitochondrial dysfunction in airways and quadriceps muscle of patients with chronic obstructive pulmonary disease. Respir. Res. 21 (1), 262. doi:10.1186/s12931-020-01527-5
Halestrap, A. P. (1999). The mitochondrial permeability transition: its molecular mechanism and role in reperfusion injury. Biochem. Soc. Symp. 66, 181–203. doi:10.1042/bss0660181
Han, X., Hu, S., Yang, Q., Sang, X., Tang, D., and Cao, G. (2022). Paeoniflorin ameliorates airway inflammation and immune response in ovalbumin induced asthmatic mice: from oxidative stress to autophagy. Phytomedicine 96, 153835. doi:10.1016/j.phymed.2021.153835
Han, X., Xu, T., Fang, Q., Zhang, H., Yue, L., Hu, G., et al. (2021). Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox Biol. 44, 102010. doi:10.1016/j.redox.2021.102010
Hang, W., He, B., Chen, J., Xia, L., Wen, B., Liang, T., et al. (2018). Berberine ameliorates high glucose-induced cardiomyocyte injury via AMPK signaling activation to stimulate mitochondrial biogenesis and restore autophagic flux. Front. Pharmacol. 9, 1121. doi:10.3389/fphar.2018.01121
Hanna, R. A., Quinsay, M. N., Orogo, A. M., Giang, K., Rikka, S., and Gustafsson, Å. B. (2012). Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J. Biol. Chem. 287 (23), 19094–19104. doi:10.1074/jbc.M111.322933
Hara, H., Araya, J., Ito, S., Kobayashi, K., Takasaka, N., Yoshii, Y., et al. (2013). Mitochondrial fragmentation in cigarette smoke-induced bronchial epithelial cell senescence. Am. J. Physiol. Lung Cell Mol. Physiol. 305 (10), L737–L746. doi:10.1152/ajplung.00146.2013
Hari, P., Millar, F. R., Tarrats, N., Birch, J., Quintanilla, A., Rink, C. J., et al. (2019). The innate immune sensor Toll-like receptor 2 controls the senescence-associated secretory phenotype. Sci. Adv. 5 (6), eaaw0254. doi:10.1126/sciadv.aaw0254
Hattori, N., Nakagawa, T., Yoneda, M., Hayashida, H., Nakagawa, K., Yamamoto, K., et al. (2022). Compounds in cigarette smoke induce EGR1 expression via the AHR, resulting in apoptosis and COPD. J. Biochem. 172, 365–376. doi:10.1093/jb/mvac077
Hekimi, S., Lapointe, J., and Wen, Y. (2011). Taking a “good” look at free radicals in the aging process. Trends Cell Biol. 21 (10), 569–576. doi:10.1016/j.tcb.2011.06.008
Herath, H., Piao, M. J., Kang, K. A., Zhen, A. X., Fernando, P. D. S. M., Kang, H. K., et al. (2022). Hesperidin exhibits protective effects against PM(2.5)-mediated mitochondrial damage, cell cycle arrest, and cellular senescence in human HaCaT keratinocytes. Molecules 27 (15), 4800. doi:10.3390/molecules27154800
Ho, C. L., Kao, N. J., Lin, C. I., Cross, T. W. L., and Lin, S. H. (2022). Quercetin increases mitochondrial biogenesis and reduces free radicals in neuronal SH-SY5Y cells. Nutrients 14 (16), 3310. doi:10.3390/nu14163310
Hodge, G., Tran, H. B., Reynolds, P. N., Jersmann, H., and Hodge, S. (2020). Lymphocyte senescence in COPD is associated with decreased sirtuin 1 expression in steroid resistant pro-inflammatory lymphocytes. Ther. Adv. Respir. Dis. 14, 1753466620905280. doi:10.1177/1753466620905280
Hodge, S., Hodge, G., Holmes, M., and Reynolds, P. N. (2005). Increased airway epithelial and T-cell apoptosis in COPD remains despite smoking cessation. Eur. Respir. J. 25 (3), 447–454. doi:10.1183/09031936.05.00077604
Hoffmann, R. F., Zarrintan, S., Brandenburg, S. M., Kol, A., de Bruin, H. G., Jafari, S., et al. (2013). Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respir. Res. 14 (1), 97. doi:10.1186/1465-9921-14-97
Hou, J., Yun, Y., Xue, J., Jeon, B., and Kim, S. (2020). Doxorubicin-induced normal breast epithelial cellular aging and its related breast cancer growth through mitochondrial autophagy and oxidative stress mitigated by ginsenoside Rh2. Phytother. Res. 34 (7), 1659–1669. doi:10.1002/ptr.6636
Hou, Y., Wang, K., Wan, W., Cheng, Y., Pu, X., and Ye, X. (2018). Resveratrol provides neuroprotection by regulating the JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Dis. 5 (3), 245–255. doi:10.1016/j.gendis.2018.06.001
Houssaini, A., Breau, M., Kebe, K., Abid, S., Marcos, E., Lipskaia, L., et al. (2018). mTOR pathway activation drives lung cell senescence and emphysema. JCI Insight 3 (3), e93203. doi:10.1172/jci.insight.93203
Hu, H., Guo, L., Overholser, J., and Wang, X. (2022). Mitochondrial VDAC1: a potential therapeutic target of inflammation-related diseases and clinical opportunities. Cells 11 (19), 3174. doi:10.3390/cells11193174
Hu, L., Liu, F., Zhang, L., Yan, C., Li, Q., et al. (2020). Effects of icariin on cell injury and glucocorticoid resistance in BEAS-2B cells exposed to cigarette smoke extract. Exp. Ther. Med. 20 (1), 283–292. doi:10.3892/etm.2020.8702
Hu, T., Pang, N., Li, Z., Xu, D., Jing, J., Li, F., et al. (2023). The activation of M1 macrophages is associated with the JNK-m6A-p38 Axis in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct Pulmon Dis. 18, 2195–2206. doi:10.2147/COPD.S420471
Hu, Y., Chen, H., Zhang, L., Lin, X., Li, X., Zhuang, H., et al. (2021). The AMPK-MFN2 axis regulates MAM dynamics and autophagy induced by energy stresses. Autophagy 17 (5), 1–15. doi:10.1080/15548627.2020.1749490
Hua, W., Li, S., Luo, R., Wu, X., Zhang, Y., Liao, Z., et al. (2020). Icariin protects human nucleus pulposus cells from hydrogen peroxide-induced mitochondria-mediated apoptosis by activating nuclear factor erythroid 2-related factor 2. Biochim. Biophys. Acta Mol. Basis Dis. 1866 (1), 165575. doi:10.1016/j.bbadis.2019.165575
Huang, L., Hu, S., Shao, M., Wu, X., Zhang, J., and Cao, G. (2021). Combined cornus officinalis and Paeonia lactiflora pall therapy alleviates rheumatoid arthritis by regulating synovial apoptosis via AMPK-mediated mitochondrial fission. Front. Pharmacol. 12, 639009. doi:10.3389/fphar.2021.639009
Hung, C. M., Lombardo, P. S., Malik, N., Brun, S. N., Hellberg, K., Van Nostrand, J. L., et al. (2021). AMPK/ULK1-mediated phosphorylation of Parkin ACT domain mediates an early step in mitophagy. Sci. Adv. 7 (15), eabg4544. doi:10.1126/sciadv.abg4544
Inigo, J. R., and Chandra, D. (2022). The mitochondrial unfolded protein response (UPR(mt)): shielding against toxicity to mitochondria in cancer. J. Hematol. Oncol. 15 (1), 98. doi:10.1186/s13045-022-01317-0
Ito, A., Hashimoto, M., Tanihata, J., Matsubayashi, S., Sasaki, R., Fujimoto, S., et al. (2022). Involvement of Parkin-mediated mitophagy in the pathogenesis of chronic obstructive pulmonary disease-related sarcopenia. J. Cachexia Sarcopenia Muscle 13 (3), 1864–1882. doi:10.1002/jcsm.12988
Ito, K., and Barnes, P. J. (2009). COPD as a disease of accelerated lung aging. Chest 135 (1), 173–180. doi:10.1378/chest.08-1419
Ito, S., Araya, J., Kurita, Y., Kobayashi, K., Takasaka, N., Yoshida, M., et al. (2015). PARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesis. Autophagy 11 (3), 547–559. doi:10.1080/15548627.2015.1017190
Ivanov, A., Pawlikowski, J., Manoharan, I., van Tuyn, J., Nelson, D. M., Rai, T. S., et al. (2013). Lysosome-mediated processing of chromatin in senescence. J. Cell Biol. 202 (1), 129–143. doi:10.1083/jcb.201212110
Jenkins, E. C., Chattopadhyay, M., and Germain, D. (2021). Are the estrogen receptor and SIRT3 axes of the mitochondrial UPR key regulators of breast cancer sub-type determination according to age? Aging Cancer 2 (3), 75–81. doi:10.1002/aac2.12035
Jia, Q., Li, L., Wang, X., Wang, Y., Jiang, K., Yang, K., et al. (2022). Hesperidin promotes gastric motility in rats with functional dyspepsia by regulating Drp1-mediated ICC mitophagy. Front. Pharmacol. 13, 945624. doi:10.3389/fphar.2022.945624
Jiang, T., Liu, T., Deng, X., Ding, W., Yue, Z., Yang, W., et al. (2021). Adiponectin ameliorates lung ischemia-reperfusion injury through SIRT1-PINK1 signaling-mediated mitophagy in type 2 diabetic rats. Respir. Res. 22 (1), 258. doi:10.1186/s12931-021-01855-0
Jiang, Z., Cui, X., Qu, P., Shang, C., Xiang, M., and Wang, J. (2022). Roles and mechanisms of puerarin on cardiovascular disease:A review. Biomed. Pharmacother. 147, 112655. doi:10.1016/j.biopha.2022.112655
Kamaraj, S., Anandakumar, P., Jagan, S., Ramakrishnan, G., and Devaki, T. (2011). Hesperidin attenuates mitochondrial dysfunction during benzo(a)pyrene-induced lung carcinogenesis in mice. Fundam. Clin. Pharmacol. 25 (1), 91–98. doi:10.1111/j.1472-8206.2010.00812.x
Kamata, H., Honda, S. I., Maeda, S., Chang, L., Hirata, H., and Karin, M. (2005). Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120 (5), 649–661. doi:10.1016/j.cell.2004.12.041
Kasahara, Y., Tuder, R. M., Cool, C. D., Lynch, D. A., Flores, S. C., and Voelkel, N. F. (2001). Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am. J. Respir. Crit. Care Med. 163 (3 Pt 1), 737–744. doi:10.1164/ajrccm.163.3.2002117
Kelsen, S. G. (2016). The unfolded protein response in chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 13 (Suppl. 2), S138–S145. doi:10.1513/AnnalsATS.201506-320KV
Kim, J., Kundu, M., Viollet, B., and Guan, K. L. (2011). AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13 (2), 132–141. doi:10.1038/ncb2152
Ko, J. H., Yoon, S. O., Lee, H. J., and Oh, J. Y. (2017). Rapamycin regulates macrophage activation by inhibiting NLRP3 inflammasome-p38 MAPK-NFκB pathways in autophagy- and p62-dependent manners. Oncotarget 8 (25), 40817–40831. doi:10.18632/oncotarget.17256
Kong, D., Zhang, F., Wei, D., Zhu, X., Zhang, X., Chen, L., et al. (2013). Paeonol inhibits hepatic fibrogenesis via disrupting nuclear factor-κB pathway in activated stellate cells: in vivo and in vitro studies. J. Gastroenterol. Hepatol. 28 (7), 1223–1233. doi:10.1111/jgh.12147
Korsmeyer, S. J., Wei, M. C., Saito, M., Weiler, S., Oh, K. J., and Schlesinger, P. H. (2000). Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 7 (12), 1166–1173. doi:10.1038/sj.cdd.4400783
Kuilman, T., Michaloglou, C., Vredeveld, L. C. W., Douma, S., van Doorn, R., Desmet, C. J., et al. (2008). Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133 (6), 1019–1031. doi:10.1016/j.cell.2008.03.039
Kujoth, G. C., Hiona, A., Pugh, T. D., Someya, S., Panzer, K., Wohlgemuth, S. E., et al. (2005). Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309 (5733), 481–484. doi:10.1126/science.1112125
Kuwana, T., Mackey, M. R., Perkins, G., Ellisman, M. H., Latterich, M., Schneiter, R., et al. (2002). Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111 (3), 331–342. doi:10.1016/s0092-8674(02)01036-x
Le, Y., Wang, Y., Zhou, L., Xiong, J., Tian, J., Yang, X., et al. (2020). Cigarette smoke-induced HMGB1 translocation and release contribute to migration and NF-κB activation through inducing autophagy in lung macrophages. J. Cell Mol. Med. 24 (2), 1319–1331. doi:10.1111/jcmm.14789
Lee, S. J., Bae, J. H., Lee, H., Lee, H., Park, J., Kang, J. S., et al. (2019). Ginsenoside Rg3 upregulates myotube formation and mitochondrial function, thereby protecting myotube atrophy induced by tumor necrosis factor-alpha. J. Ethnopharmacol. 242, 112054. doi:10.1016/j.jep.2019.112054
Leermakers, P. A., Schols, A. M. W. J., Kneppers, A. E. M., Kelders, M. C. J. M., de Theije, C. C., Lainscak, M., et al. (2018). Molecular signalling towards mitochondrial breakdown is enhanced in skeletal muscle of patients with chronic obstructive pulmonary disease (COPD). Sci. Rep. 8 (1), 15007. doi:10.1038/s41598-018-33471-2
Lelli, D., Sahebkar, A., Johnston, T. P., and Pedone, C. (2017). Curcumin use in pulmonary diseases: state of the art and future perspectives. Pharmacol. Res. 115, 133–148. doi:10.1016/j.phrs.2016.11.017
Lerner, C. A., Sundar, I. K., and Rahman, I. (2016). Mitochondrial redox system, dynamics, and dysfunction in lung inflammaging and COPD. Int. J. Biochem. Cell Biol. 81 (Pt B), 294–306. doi:10.1016/j.biocel.2016.07.026
Li, C., Li, Q., Mei, Q., and Lu, T. (2015). Pharmacological effects and pharmacokinetic properties of icariin, the major bioactive component in Herba Epimedii. Life Sci. 126, 57–68. doi:10.1016/j.lfs.2015.01.006
Li, C., Liu, Q., Chang, Q., Xie, M., Weng, J., Wang, X., et al. (2023). Role of mitochondrial fusion proteins MFN2 and OPA1 on lung cellular senescence in chronic obstructive pulmonary disease. Respir. Res. 24 (1), 319. doi:10.1186/s12931-023-02634-9
Li, H., Chen, F. J., Yang, W. L., Qiao, H. Z., and Zhang, S. J. (2021). Quercetin improves cognitive disorder in aging mice by inhibiting NLRP3 inflammasome activation. Food Funct. 12 (2), 717–725. doi:10.1039/d0fo01900c
Li, L., Wang, H., Zhao, S., Zhao, Y., Chen, Y., Zhang, J., et al. (2022b). Paeoniflorin ameliorates lipopolysaccharide-induced acute liver injury by inhibiting oxidative stress and inflammation via SIRT1/FOXO1a/SOD2 signaling in rats. Phytother. Res. 36 (6), 2558–2571. doi:10.1002/ptr.7471
Li, L., Wang, X., Sharvan, R., Gao, J., and Qu, S. (2017). Berberine could inhibit thyroid carcinoma cells by inducing mitochondrial apoptosis, G0/G1 cell cycle arrest and suppressing migration via PI3K-AKT and MAPK signaling pathways. Biomed. Pharmacother. 95, 1225–1231. doi:10.1016/j.biopha.2017.09.010
Li, P., Nijhawan, D., Budihardjo, I., Srinivasula, S. M., Ahmad, M., Alnemri, E. S., et al. (1997). Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91 (4), 479–489. doi:10.1016/s0092-8674(00)80434-1
Li, Q., Wu, J., Huang, J., Hu, R., You, H., Liu, L., et al. (2022a). Paeoniflorin ameliorates skeletal muscle atrophy in chronic kidney disease via AMPK/SIRT1/PGC-1α-Mediated oxidative stress and mitochondrial dysfunction. Front. Pharmacol. 13, 859723. doi:10.3389/fphar.2022.859723
Li, X., Jin, Q., Yao, Q., Xu, B., Li, Z., Tu, C., et al. (2016). Quercetin attenuates the activation of hepatic stellate cells and liver fibrosis in mice through modulation of HMGB1-TLR2/4-NF-κB signaling pathways. Toxicol Lett. 261, 1–12. doi:10.1016/j.toxlet.2016.09.002
Lim, C., Zhen, A. X., Ok, S., Fernando, P. D. S. M., Herath, H. M. U. L., Piao, M. J., et al. (2022). Hesperidin protects SH-SY5Y neuronal cells against high glucose-induced apoptosis via regulation of MAPK signaling. Antioxidants (Basel) 11 (9), 1707. doi:10.3390/antiox11091707
Lin, H., Wang, C., Yu, H., Liu, Y., Tan, L., He, S., et al. (2022). Protective effect of total Saponins from American ginseng against cigarette smoke-induced COPD in mice based on integrated metabolomics and network pharmacology. Biomed. Pharmacother. 149, 112823. doi:10.1016/j.biopha.2022.112823
Lin, H. Y., Weng, S. W., Chang, Y. H., Su, Y. J., Chang, C. M., Tsai, C. J., et al. (2018b). The causal role of mitochondrial dynamics in regulating insulin resistance in diabetes: link through mitochondrial reactive oxygen species. Oxid. Med. Cell Longev. 2018, 7514383. doi:10.1155/2018/7514383
Lin, J., Xu, F., Wang, G., Kong, L., Luo, Q., Lv, Y., et al. (2016). Paeoniflorin attenuated oxidative stress in rat COPD model induced by cigarette smoke. Evid. Based Complement. Altern. Med. 2016, 1698379. doi:10.1155/2016/1698379
Lin, Z., Altaf, N., Li, C., Chen, M., Pan, L., Wang, D., et al. (2018a). Hydrogen sulfide attenuates oxidative stress-induced NLRP3 inflammasome activation via S-sulfhydrating c-Jun at Cys269 in macrophages. Biochim. Biophys. Acta Mol. Basis Dis. 1864 (9 Pt B), 2890–2900. doi:10.1016/j.bbadis.2018.05.023
Liu, H., You, L., Wu, J., Zhao, M., Guo, R., Zhang, H., et al. (2020b). Berberine suppresses influenza virus-triggered NLRP3 inflammasome activation in macrophages by inducing mitophagy and decreasing mitochondrial ROS. J. Leukoc. Biol. 108 (1), 253–266. doi:10.1002/JLB.3MA0320-358RR
Liu, J., Cheng, Q., Wu, X., Zhu, H., Deng, X., Wang, M., et al. (2022b). Icariin treatment rescues diabetes induced bone loss via scavenging ROS and activating primary cilia/gli2/osteocalcin signaling pathway. Cells 11 (24), 4091. doi:10.3390/cells11244091
Liu, L., Peritore, C., Ginsberg, J., Kayhan, M., and Donmez, G. (2015). SIRT3 attenuates MPTP-induced nigrostriatal degeneration via enhancing mitochondrial antioxidant capacity. Neurochem. Res. 40 (3), 600–608. doi:10.1007/s11064-014-1507-8
Liu, T., Ma, X., Ouyang, T., Chen, H., Xiao, Y., Huang, Y., et al. (2019). Efficacy of 5-aminolevulinic acid-based photodynamic therapy against keloid compromised by downregulation of SIRT1-SIRT3-SOD2-mROS dependent autophagy pathway. Redox Biol. 20, 195–203. doi:10.1016/j.redox.2018.10.011
Liu, T., Yang, Q., Zhang, X., Qin, R., Shan, W., Zhang, H., et al. (2020c). Quercetin alleviates kidney fibrosis by reducing renal tubular epithelial cell senescence through the SIRT1/PINK1/mitophagy axis. Life Sci. 257, 118116. doi:10.1016/j.lfs.2020.118116
Liu, W. Y., Liou, S. S., Hong, T. Y., and Liu, I. M. (2018). Hesperidin prevents high glucose-induced damage of retinal pigment epithelial cells. Planta Med. 84 (14), 1030–1037. doi:10.1055/a-0601-7020
Liu, Y. F., Zhang, L., Wu, Q., and Feng, L. Y. (2022c). Paeoniflorin ameliorates ischemic injury in rat brain via inhibiting cytochrome c/caspase3/HDAC4 pathway. Acta Pharmacol. Sin. 43 (2), 273–284. doi:10.1038/s41401-021-00671-y
Liu, Z., Pan, H., Zhang, Y., Zheng, Z., Xiao, W., Hong, X., et al. (2022a). Ginsenoside-Rg1 attenuates sepsis-induced cardiac dysfunction by modulating mitochondrial damage via the P2X7 receptor-mediated Akt/GSK-3β signaling pathway. J. Biochem. Mol. Toxicol. 36 (1), e22885. doi:10.1002/jbt.22885
Liu, Z., Xu, S., Ji, Z., Xu, H., Zhao, W., Xia, Z., et al. (2020a). Mechanistic study of mtROS-JNK-SOD2 signaling in bupivacaine-induced neuron oxidative stress. Aging (Albany NY) 12 (13), 13463–13476. doi:10.18632/aging.103447
Livezey, M., Kim, J. E., and Shapiro, D. J. (2018). A new role for estrogen receptor α in cell proliferation and cancer: activating the anticipatory unfolded protein response. Front. Endocrinol. (Lausanne) 9, 325. doi:10.3389/fendo.2018.00325
Luo, X., Xue, L., Xu, H., Zhao, Q. Y., Wang, Q., She, Y. S., et al. (2018). Polygonum aviculare L. extract and quercetin attenuate contraction in airway smooth muscle. Sci. Rep. 8 (1), 3114. doi:10.1038/s41598-018-20409-x
Ma, B. N., and Li, X. J. (2020). Resveratrol extracted from Chinese herbal medicines: a novel therapeutic strategy for lung diseases. Chin. Herb. Med. 12 (4), 349–358. doi:10.1016/j.chmed.2020.07.003
Manevski, M., Muthumalage, T., Devadoss, D., Sundar, I. K., Wang, Q., Singh, K. P., et al. (2020). Cellular stress responses and dysfunctional Mitochondrial-cellular senescence, and therapeutics in chronic respiratory diseases. Redox Biol. 33, 101443. doi:10.1016/j.redox.2020.101443
Mao, J., Li, Y., Feng, S., Liu, X., Tian, Y., Bian, Q., et al. (2020). Bufei jianpi formula improves mitochondrial function and suppresses mitophagy in skeletal muscle via the adenosine monophosphate-activated protein kinase pathway in chronic obstructive pulmonary disease. Front. Pharmacol. 11, 587176. doi:10.3389/fphar.2020.587176
Mao, J., Li, Y., Li, S., Li, J., Tian, Y., Feng, S., et al. (2019). Bufei jianpi granules reduce quadriceps muscular cell apoptosis by improving mitochondrial function in rats with chronic obstructive pulmonary disease. Evid. Based Complement. Altern. Med. 2019, 1216305. doi:10.1155/2019/1216305
Maremanda, K. P., Sundar, I. K., and Rahman, I. (2021). Role of inner mitochondrial protein OPA1 in mitochondrial dysfunction by tobacco smoking and in the pathogenesis of COPD. Redox Biol. 45, 102055. doi:10.1016/j.redox.2021.102055
Martin, D., Rojo, A. I., Salinas, M., Diaz, R., Gallardo, G., Alam, J., et al. (2004). Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J. Biol. Chem. 279 (10), 8919–8929. doi:10.1074/jbc.M309660200
Meyer, A., Zoll, J., Charles, A. L., Charloux, A., de Blay, F., Diemunsch, P., et al. (2013). Skeletal muscle mitochondrial dysfunction during chronic obstructive pulmonary disease: central actor and therapeutic target. Exp. Physiol. 98 (6), 1063–1078. doi:10.1113/expphysiol.2012.069468
Mizumura, K., Cloonan, S. M., Nakahira, K., Bhashyam, A. R., Cervo, M., Kitada, T., et al. (2014). Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J. Clin. Invest 124 (9), 3987–4003. doi:10.1172/JCI74985
Murakawa, T., Okamoto, K., Omiya, S., Taneike, M., Yamaguchi, O., and Otsu, K. (2019). A mammalian mitophagy receptor, bcl2-L-13, recruits the ULK1 complex to induce mitophagy. Cell Rep. 26 (2), 338–345. doi:10.1016/j.celrep.2018.12.050
Nakahira, K., Haspel, J. A., Rathinam, V. A. K., Lee, S. J., Dolinay, T., Lam, H. C., et al. (2011). Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 12 (3), 222–230. doi:10.1038/ni.1980
Navarro, S., Reddy, R., Lee, J., Warburton, D., and Driscoll, B. (2017). Inhaled resveratrol treatments slow ageing-related degenerative changes in mouse lung. Thorax 72 (5), 451–459. doi:10.1136/thoraxjnl-2016-208964
Nguyen, K., Tang, J., Cho, S., Ying, F., Sung, H. K., Jahng, J. W., et al. (2024). Salubrinal promotes phospho-eIF2α-dependent activation of UPR leading to autophagy-mediated attenuation of iron-induced insulin resistance. Mol. Metab. 83, 101921. doi:10.1016/j.molmet.2024.101921
Noguera, A., Batle, S., Miralles, C., Iglesias, J., Busquets, X., MacNee, W., et al. (2001). Enhanced neutrophil response in chronic obstructive pulmonary disease. Thorax 56 (6), 432–437. doi:10.1136/thorax.56.6.432
Ottria, A., Zimmermann, M., Paardekooper, L. M., Carvalheiro, T., Vazirpanah, N., Silva-Cardoso, S., et al. (2022). Hypoxia and TLR9 activation drive CXCL4 production in systemic sclerosis plasmacytoid dendritic cells via mtROS and HIF-2α. Rheumatol. Oxf. 61 (6), 2682–2693. doi:10.1093/rheumatology/keab532
Panahi, Y., Ghanei, M., Hajhashemi, A., and Sahebkar, A. (2016). Effects of curcuminoids-piperine combination on systemic oxidative stress, clinical symptoms and quality of life in subjects with chronic pulmonary complications due to sulfur mustard: a randomized controlled trial. J. Diet. Suppl. 13 (1), 93–105. doi:10.3109/19390211.2014.952865
Parisi, M. A., and Clayton, D. A. (1991). Similarity of human mitochondrial transcription factor 1 to high mobility group proteins. Science 252 (5008), 965–969. doi:10.1126/science.2035027
Park, K. S., Kim, H., Kim, H. J., Lee, K. I., Lee, S. Y., and Kim, J. (2022). Paeoniflorin alleviates skeletal muscle atrophy in ovariectomized mice through the erα/NRF1 mitochondrial biogenesis pathway. Pharm. (Basel) 15 (4), 390. doi:10.3390/ph15040390
Pastukh, V. M., Zhang, L., Ruchko, M. V., Gorodnya, O., Bardwell, G. C., Tuder, R. M., et al. (2011). Oxidative DNA damage in lung tissue from patients with COPD is clustered in functionally significant sequences. Int. J. Chron. Obstruct Pulmon Dis. 6, 209–217. doi:10.2147/COPD.S15922
Paudel, K. R., Panth, N., Manandhar, B., Singh, S. K., Gupta, G., Wich, P. R., et al. (2022). Attenuation of cigarette-smoke-induced oxidative stress, senescence, and inflammation by berberine-loaded liquid crystalline nanoparticles: in vitro study in 16HBE and RAW264.7 cells. Antioxidants (Basel) 11 (5), 873. doi:10.3390/antiox11050873
Pérez-Treviño, P., Velásquez, M., and García, N. (2020). Mechanisms of mitochondrial DNA escape and its relationship with different metabolic diseases. Biochim. Biophys. Acta Mol. Basis Dis. 1866 (6), 165761. doi:10.1016/j.bbadis.2020.165761
Poole, L. P., Bock-Hughes, A., Berardi, D. E., and Macleod, K. F. (2021). ULK1 promotes mitophagy via phosphorylation and stabilization of BNIP3. Sci. Rep. 11 (1), 20526. doi:10.1038/s41598-021-00170-4
Popov, L. D. (2020). Mitochondrial biogenesis: an update. J. Cell Mol. Med. 24 (9), 4892–4899. doi:10.1111/jcmm.15194
Powers, S. K., Wiggs, M. P., Duarte, J. A., Zergeroglu, A. M., and Demirel, H. A. (2012). Mitochondrial signaling contributes to disuse muscle atrophy. Am. J. Physiol. Endocrinol. Metab. 303 (1), E31–E39. doi:10.1152/ajpendo.00609.2011
Price, N. L., Gomes, A. P., Ling, A. J. Y., Duarte, F. V., Martin-Montalvo, A., North, B. J., et al. (2012). SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 15 (5), 675–690. doi:10.1016/j.cmet.2012.04.003
Qin, X., Jiang, M., Zhao, Y., Gong, J., Su, H., Yuan, F., et al. (2020). Berberine protects against diabetic kidney disease via promoting PGC-1α-regulated mitochondrial energy homeostasis. Br. J. Pharmacol. 177 (16), 3646–3661. doi:10.1111/bph.14935
Quan, Y., Xin, Y., Tian, G., Zhou, J., and Liu, X. (2020). Mitochondrial ROS-modulated mtDNA: a potential target for cardiac aging. Oxid. Med. Cell Longev. 2020, 9423593. doi:10.1155/2020/9423593
Quinsay, M. N., Thomas, R. L., Lee, Y., and Gustafsson, A. B. (2010). Bnip3-mediated mitochondrial autophagy is independent of the mitochondrial permeability transition pore. Autophagy 6 (7), 855–862. doi:10.4161/auto.6.7.13005
Rajendrasozhan, S., Yang, S. R., Kinnula, V. L., and Rahman, I. (2008). SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 177 (8), 861–870. doi:10.1164/rccm.200708-1269OC
Remels, A. H., Schrauwen, P., Broekhuizen, R., Willems, J., Kersten, S., Gosker, H. R., et al. (2007). Peroxisome proliferator-activated receptor expression is reduced in skeletal muscle in COPD. Eur. Respir. J. 30 (2), 245–252. doi:10.1183/09031936.00144106
Reyes, A., Gissi, C., Pesole, G., and Saccone, C. (1998). Asymmetrical directional mutation pressure in the mitochondrial genome of mammals. Mol. Biol. Evol. 15 (8), 957–966. doi:10.1093/oxfordjournals.molbev.a026011
Rizk, F. H., Soliman, N. A., Kashef, S. M., and Elsaadany, A. A. (2023). Lipoxin A4 attenuated dexamethasone-induced muscle atrophy via activation of PGC-1α/Nrf2/TFAM pathway. J. Physiol. Biochem. 79 (1), 107–115. doi:10.1007/s13105-022-00925-1
Rodgers, J. T., Lerin, C., Haas, W., Gygi, S. P., Spiegelman, B. M., and Puigserver, P. (2005). Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434 (7029), 113–118. doi:10.1038/nature03354
Romanello, V., Guadagnin, E., Gomes, L., Roder, I., Sandri, C., Petersen, Y., et al. (2010). Mitochondrial fission and remodelling contributes to muscle atrophy. Embo J. 29 (10), 1774–1785. doi:10.1038/emboj.2010.60
Rumora, L., Hlapčić, I., Hulina-Tomašković, A., Somborac-Bačura, A., Bosnar, M., and Rajković, M. G. (2021). Pathogen-associated molecular patterns and extracellular Hsp70 interplay in NLRP3 inflammasome activation in monocytic and bronchial epithelial cellular models of COPD exacerbations. Apmis 129 (2), 80–90. doi:10.1111/apm.13089
Ryu, H., Lee, J., Impey, S., Ratan, R. R., and Ferrante, R. J. (2005). Antioxidants modulate mitochondrial PKA and increase CREB binding to D-loop DNA of the mitochondrial genome in neurons. Proc. Natl. Acad. Sci. U. S. A. 102 (39), 13915–13920. doi:10.1073/pnas.0502878102
Safiri, S., Carson-Chahhoud, K., Noori, M., Nejadghaderi, S. A., Sullman, M. J. M., Ahmadian Heris, J., et al. (2022). Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990-2019: results from the Global Burden of Disease Study 2019. Bmj 378, e069679. doi:10.1136/bmj-2021-069679
Salehi, A. H., Xanthoudakis, S., and Barker, P. A. (2002). NRAGE, a p75 neurotrophin receptor-interacting protein, induces caspase activation and cell death through a JNK-dependent mitochondrial pathway. J. Biol. Chem. 277 (50), 48043–48050. doi:10.1074/jbc.M205324200
Salvi, S. (2014). Tobacco smoking and environmental risk factors for chronic obstructive pulmonary disease. Clin. Chest Med. 35 (1), 17–27. doi:10.1016/j.ccm.2013.09.011
Sang, A., Wang, Y., Wang, S., Wang, Q., Wang, X., Li, X., et al. (2022). Quercetin attenuates sepsis-induced acute lung injury via suppressing oxidative stress-mediated ER stress through activation of SIRT1/AMPK pathways. Cell Signal 96, 110363. doi:10.1016/j.cellsig.2022.110363
Sauler, M., Bazan, I. S., and Lee, P. J. (2019). Cell death in the lung: the apoptosis-necroptosis Axis. Annu. Rev. Physiol. 81, 375–402. doi:10.1146/annurev-physiol-020518-114320
Schroeter, H., Boyd, C. S., Ahmed, R., Spencer, J. P. E., Duncan, R. F., Rice-Evans, C., et al. (2003). c-Jun N-terminal kinase (JNK)-mediated modulation of brain mitochondria function: new target proteins for JNK signalling in mitochondrion-dependent apoptosis. Biochem. J. 372 (Pt 2), 359–369. doi:10.1042/BJ20030201
Schuliga, M., Kanwal, A., Read, J., Blokland, K. E. C., Burgess, J. K., Prêle, C. M., et al. (2021). A cGAS-dependent response links DNA damage and senescence in alveolar epithelial cells: a potential drug target in IPF. Am. J. Physiol. Lung Cell Mol. Physiol. 321 (5), L859–l871. doi:10.1152/ajplung.00574.2020
Schuliga, M., Read, J., Blokland, K. E. C., Waters, D. W., Burgess, J., Prêle, C., et al. (2020). Self DNA perpetuates IPF lung fibroblast senescence in a cGAS-dependent manner. Clin. Sci. (Lond) 134 (7), 889–905. doi:10.1042/CS20191160
Shergis, J. L., Zhang, A. L., Vlahos, R., Helliwell, R., Ye, J. M., et al. (2014). Therapeutic potential of Panax ginseng and ginsenosides in the treatment of chronic obstructive pulmonary disease. Complement. Ther. Med. 22 (5), 944–953. doi:10.1016/j.ctim.2014.08.006
Shih, C. H., Wang, W. H., Chen, C. M., and Ko, W. C. (2020). Hesperetin-5,7,3'-O-Trimethylether dually inhibits phosphodiesterase 3/4 and methacholine-induced airway hyperresponsiveness in sensitized and challenged mice. Drug Des. Devel Ther. 14, 519–526. doi:10.2147/DDDT.S227432
Shimizu, S., Narita, M., and Tsujimoto, Y. (1999). Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399 (6735), 483–487. doi:10.1038/20959
Shlezinger, N., and Hohl, T. M. (2021). Mitochondrial reactive oxygen species enhance alveolar macrophage activity against Aspergillus fumigatus but are dispensable for host protection. mSphere 6 (3), e0026021. doi:10.1128/mSphere.00260-21
Sliter, D. A., Martinez, J., Hao, L., Chen, X., Sun, N., Fischer, T. D., et al. (2018). Parkin and PINK1 mitigate STING-induced inflammation. Nature 561 (7722), 258–262. doi:10.1038/s41586-018-0448-9
Song, L., Chen, X., Mi, L., Liu, C., Zhu, S., Yang, T., et al. (2020). Icariin-induced inhibition of SIRT6/NF-κB triggers redox mediated apoptosis and enhances anti-tumor immunity in triple-negative breast cancer. Cancer Sci. 111 (11), 4242–4256. doi:10.1111/cas.14648
Song, S., Xiao, X., Guo, D., Mo, L., Bu, C., Ye, W., et al. (2017). Protective effects of Paeoniflorin against AOPP-induced oxidative injury in HUVECs by blocking the ROS-HIF-1α/VEGF pathway. Phytomedicine 34, 115–126. doi:10.1016/j.phymed.2017.08.010
Summer, R., Shaghaghi, H., Schriner, D., Roque, W., Sales, D., Cuevas-Mora, K., et al. (2019). Activation of the mTORC1/PGC-1 axis promotes mitochondrial biogenesis and induces cellular senescence in the lung epithelium. Am. J. Physiol. Lung Cell Mol. Physiol. 316 (6), L1049-L1060–l1060. doi:10.1152/ajplung.00244.2018
Sun, R., Liu, J., Yu, M., Xia, M., Zhang, Y., Sun, X., et al. (2022). Paeoniflorin ameliorates BiPN by reducing IL6 levels and regulating PARKIN-mediated mitochondrial autophagy. Drug Des. Devel Ther. 16, 2241–2259. doi:10.2147/DDDT.S369111
Sundar, I. K., Rashid, K., Gerloff, J., Li, D., and Rahman, I. (2018). Genetic ablation of p16(INK4a) does not protect against cellular senescence in mouse models of chronic obstructive pulmonary disease/emphysema. Am. J. Respir. Cell Mol. Biol. 59 (2), 189–199. doi:10.1165/rcmb.2017-0390OC
Szczepanowska, K., and Trifunovic, A. (2017). Origins of mtDNA mutations in ageing. Essays Biochem. 61 (3), 325–337. doi:10.1042/EBC20160090
Taivassalo, T., and Hussain, S. N. (2016). Contribution of the mitochondria to locomotor muscle dysfunction in patients with COPD. Chest 149 (5), 1302–1312. doi:10.1016/j.chest.2015.11.021
Tang, F., and Ling, C. (2019). Curcumin ameliorates chronic obstructive pulmonary disease by modulating autophagy and endoplasmic reticulum stress through regulation of SIRT1 in a rat model. J. Int. Med. Res. 47 (10), 4764–4774. doi:10.1177/0300060519869459
Tang, J., Lu, L., Liu, Y., Ma, J., Yang, L., Li, L., et al. (2019). Quercetin improve ischemia/reperfusion-induced cardiomyocyte apoptosis in vitro and in vivo study via SIRT1/PGC-1α signaling. J. Cell Biochem. 120 (6), 9747–9757. doi:10.1002/jcb.28255
Tao, R., Coleman, M. C., Pennington, J. D., Ozden, O., Park, S. H., Jiang, H., et al. (2010). Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol. Cell 40 (6), 893–904. doi:10.1016/j.molcel.2010.12.013
Tayanloo-Beik, A., Kiasalari, Z., and Roghani, M. (2022). Paeonol ameliorates cognitive deficits in streptozotocin murine model of sporadic Alzheimer's disease via attenuation of oxidative stress, inflammation, and mitochondrial dysfunction. J. Mol. Neurosci. 72 (2), 336–348. doi:10.1007/s12031-021-01936-1
Tew, X. N., Xin Lau, N. J., Chellappan, D. K., Madheswaran, T., Zeeshan, F., Tambuwala, M. M., et al. (2020). Immunological axis of berberine in managing inflammation underlying chronic respiratory inflammatory diseases. Chem. Biol. Interact. 317, 108947. doi:10.1016/j.cbi.2020.108947
Tian, J., Zhang, S., Li, G., Liu, Z., and Xu, B. (2009). 20(S)-ginsenoside Rg3, a neuroprotective agent, inhibits mitochondrial permeability transition pores in rat brain. Phytother. Res. 23 (4), 486–491. doi:10.1002/ptr.2653
Tian, L., Cao, W., Yue, R., Yuan, Y., Guo, X., Qin, D., et al. (2019). Pretreatment with Tilianin improves mitochondrial energy metabolism and oxidative stress in rats with myocardial ischemia/reperfusion injury via AMPK/SIRT1/PGC-1 alpha signaling pathway. J. Pharmacol. Sci. 139 (4), 352–360. doi:10.1016/j.jphs.2019.02.008
Tomita, K., Caramori, G., Lim, S., Ito, K., Hanazawa, T., Oates, T., et al. (2002). Increased p21(CIP1/WAF1) and B cell lymphoma leukemia-x(L) expression and reduced apoptosis in alveolar macrophages from smokers. Am. J. Respir. Crit. Care Med. 166 (5), 724–731. doi:10.1164/rccm.2104010
Tsuji, T., Aoshiba, K., and Nagai, A. (2006). Alveolar cell senescence in patients with pulmonary emphysema. Am. J. Respir. Crit. Care Med. 174 (8), 886–893. doi:10.1164/rccm.200509-1374OC
Tuder, R. M., Kern, J. A., and Miller, Y. E. (2012). Senescence in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 9 (2), 62–63. doi:10.1513/pats.201201-012MS
Turley, J. L., Moran, H. B. T., McEntee, C. P., O'Grady, K., Muñoz-Wolf, N., Jin, L., et al. (2021). Chitin-derived polymer deacetylation regulates mitochondrial reactive oxygen species dependent cGAS-STING and NLRP3 inflammasome activation. Biomaterials 275, 120961. doi:10.1016/j.biomaterials.2021.120961
van der Rijt, S., Molenaars, M., McIntyre, R. L., Janssens, G. E., and Houtkooper, R. H. (2020). Integrating the hallmarks of aging throughout the tree of life: a focus on mitochondrial dysfunction. Front. Cell Dev. Biol. 8, 594416. doi:10.3389/fcell.2020.594416
van Vliet, T., Varela-Eirin, M., Wang, B., Borghesan, M., Brandenburg, S. M., Franzin, R., et al. (2021). Physiological hypoxia restrains the senescence-associated secretory phenotype via AMPK-mediated mTOR suppression. Mol. Cell 81 (9), 2041–2052.e6. doi:10.1016/j.molcel.2021.03.018
Verma, A., Aggarwal, K., Agrawal, R., Pradhan, K., and Goyal, A. (2022). Molecular mechanisms regulating the pharmacological actions of icariin with special focus on PI3K-AKT and Nrf-2 signaling pathways. Mol. Biol. Rep. 49 (9), 9023–9032. doi:10.1007/s11033-022-07778-3
Vernooy, J. H., Küçükaycan, M., Jacobs, J. A., Chavannes, N. H., Buurman, W. A., Dentener, M. A., et al. (2002). Local and systemic inflammation in patients with chronic obstructive pulmonary disease: soluble tumor necrosis factor receptors are increased in sputum. Am. J. Respir. Crit. Care Med. 166 (9), 1218–1224. doi:10.1164/rccm.2202023
Vissenaekens, H., Smagghe, G., Criel, H., Grootaert, C., Raes, K., Rajkovic, A., et al. (2021). Intracellular quercetin accumulation and its impact on mitochondrial dysfunction in intestinal Caco-2 cells. Food Res. Int. 145, 110430. doi:10.1016/j.foodres.2021.110430
Vizioli, M. G., Liu, T., Miller, K. N., Robertson, N. A., Gilroy, K., Lagnado, A. B., et al. (2020). Mitochondria-to-nucleus retrograde signaling drives formation of cytoplasmic chromatin and inflammation in senescence. Genes Dev. 34 (5-6), 428–445. doi:10.1101/gad.331272.119
Vorobjeva, N., Galkin, I., Pletjushkina, O., Golyshev, S., Zinovkin, R., Prikhodko, A., et al. (2020). Mitochondrial permeability transition pore is involved in oxidative burst and NETosis of human neutrophils. Biochim. Biophys. Acta Mol. Basis Dis. 1866 (5), 165664. doi:10.1016/j.bbadis.2020.165664
Vorobjeva, N., Prikhodko, A., Galkin, I., Pletjushkina, O., Zinovkin, R., Sud'ina, G., et al. (2017). Mitochondrial reactive oxygen species are involved in chemoattractant-induced oxidative burst and degranulation of human neutrophils in vitro. Eur. J. Cell Biol. 96 (3), 254–265. doi:10.1016/j.ejcb.2017.03.003
Wallace, D. C. (1992). Mitochondrial genetics: a paradigm for aging and degenerative diseases? Science 256 (5057), 628–632. doi:10.1126/science.1533953
Walters, H. E., Deneka-Hannemann, S., and Cox, L. S. (2016). Reversal of phenotypes of cellular senescence by pan-mTOR inhibition. Aging (Albany NY) 8 (2), 231–244. doi:10.18632/aging.100872
Wang, D., Du, Y., Xu, H., Pan, H., and Wang, R. (2019c). Paeonol protects mitochondrial injury and prevents pulmonary vascular remodeling in hypoxia. Respir. Physiol. Neurobiol. 268, 103252. doi:10.1016/j.resp.2019.103252
Wang, D., Tan, Q. R., and Zhang, Z. J. (2013a). Neuroprotective effects of paeoniflorin, but not the isomer albiflorin, are associated with the suppression of intracellular calcium and calcium/calmodulin protein kinase II in PC12 cells. J. Mol. Neurosci. 51 (2), 581–590. doi:10.1007/s12031-013-0031-7
Wang, D. M., Li, S. Q., Wu, W. L., Zhu, X. Y., Wang, Y., and Yuan, H. Y. (2014). Effects of long-term treatment with quercetin on cognition and mitochondrial function in a mouse model of Alzheimer's disease. Neurochem. Res. 39 (8), 1533–1543. doi:10.1007/s11064-014-1343-x
Wang, D. M., Li, S. Q., Zhu, X. Y., Wang, Y., Wu, W. L., and Zhang, X. J. (2013b). Protective effects of hesperidin against amyloid-β (Aβ) induced neurotoxicity through the voltage dependent anion channel 1 (VDAC1)-mediated mitochondrial apoptotic pathway in PC12 cells. Neurochem. Res. 38 (5), 1034–1044. doi:10.1007/s11064-013-1013-4
Wang, L., Chen, Q., Yu, Q., Xiao, J., and Zhao, H. (2021a). TREM-1 aggravates chronic obstructive pulmonary disease development via activation NLRP3 inflammasome-mediated pyroptosis. Inflamm. Res. 70 (9), 971–980. doi:10.1007/s00011-021-01490-x
Wang, L., Shan, H., Wang, B., Wang, N., Zhou, Z., Pan, C., et al. (2018). Puerarin attenuates osteoarthritis via upregulating AMP-activated protein kinase/proliferator-activated receptor-γ coactivator-1 signaling pathway in osteoarthritis rats. Pharmacology 102 (3-4), 117–125. doi:10.1159/000490418
Wang, N., Wang, H., Pan, Q., Kang, J., Liang, Z., and Zhang, R. (2021d). The combination of β-asarone and icariin inhibits amyloid-β and reverses cognitive deficits by promoting mitophagy in models of Alzheimer's disease. Oxid. Med. Cell Longev. 2021, 7158444. doi:10.1155/2021/7158444
Wang, Q., Unwalla, H., and Rahman, I. (2022). Dysregulation of mitochondrial complexes and dynamics by chronic cigarette smoke exposure Utilizing MitoQC reporter mice. Mitochondrion 63, 43–50. doi:10.1016/j.mito.2022.01.003
Wang, S., Yang, F. J., Shang, L. C., Zhang, Y. H., Zhou, Y., and Shi, X. L. (2019a). Puerarin protects against high-fat high-sucrose diet-induced non-alcoholic fatty liver disease by modulating PARP-1/PI3K/AKT signaling pathway and facilitating mitochondrial homeostasis. Phytother. Res. 33 (9), 2347–2359. doi:10.1002/ptr.6417
Wang, S. W., Sheng, H., Bai, Y. F., Weng, Y. Y., Fan, X. Y., Lou, L. J., et al. (2020). Neohesperidin enhances PGC-1α-mediated mitochondrial biogenesis and alleviates hepatic steatosis in high fat diet fed mice. Nutr. Diabetes 10 (1), 27. doi:10.1038/s41387-020-00130-3
Wang, T., Zheng, L., and Zhang, W. (2021e). Hesperidin alleviates bupivacaine anesthesia-induced neurotoxicity in SH-SY5Y cells by regulating apoptosis and oxidative damage. J. Biochem. Mol. Toxicol. 35 (7), e22787. doi:10.1002/jbt.22787
Wang, W., Zha, G., Zou, J. J., Wang, X., Li, C. N., and Wu, X. J. (2019b). Berberine attenuates cigarette smoke extract-induced airway inflammation in mice: involvement of TGF-β1/smads signaling pathway. Curr. Med. Sci. 39 (5), 748–753. doi:10.1007/s11596-019-2101-8
Wang, W. W., Han, R., He, H. J., Li, J., Chen, S. Y., Gu, Y., et al. (2021c). Administration of quercetin improves mitochondria quality control and protects the neurons in 6-OHDA-lesioned Parkinson's disease models. Aging (Albany NY) 13 (8), 11738–11751. doi:10.18632/aging.202868
Wang, X., Zhu, G., Yang, S., Cheng, H., Wang, F., et al. (2011). Paeonol prevents excitotoxicity in rat pheochromocytoma PC12 cells via downregulation of ERK activation and inhibition of apoptosis. Planta Med. 77 (15), 1695–1701. doi:10.1055/s-0030-1271033
Wang, X. L., Li, T., Li, J. H., Miao, S. Y., and Xiao, X. Z. (2017). The effects of resveratrol on inflammation and oxidative stress in a rat model of chronic obstructive pulmonary disease. Molecules 22 (9), 1529. doi:10.3390/molecules22091529
Wang, Y., Zhang, X., and Horng, T. (2021b). Mitochondrial metabolism regulates macrophage biology. J. Biol. Chem. 297 (1), 100904. doi:10.1016/j.jbc.2021.100904
Ward, A. B., Mir, H., Kapur, N., Gales, D. N., Carriere, P. P., and Singh, S. (2018). Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J. Surg. Oncol. 16 (1), 108. doi:10.1186/s12957-018-1400-z
Wen, D., Tan, R. Z., Zhao, C. Y., Li, J. C., Zhong, X., Diao, H., et al. (2020). Astragalus mongholicus bunge and Panax notoginseng (burkill) F.H. Chen formula for renal injury in diabetic nephropathy-in vivo and in vitro evidence for autophagy regulation. Front. Pharmacol. 11, 732. doi:10.3389/fphar.2020.00732
West, A. P., and Shadel, G. S. (2017). Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat. Rev. Immunol. 17 (6), 363–375. doi:10.1038/nri.2017.21
Wiegman, C. H., Michaeloudes, C., Haji, G., Narang, P., Clarke, C. J., Russell, K. E., et al. (2015). Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 136 (3), 769–780. doi:10.1016/j.jaci.2015.01.046
Willenborg, S., Sanin, D. E., Jais, A., Ding, X., Ulas, T., Nüchel, J., et al. (2021). Mitochondrial metabolism coordinates stage-specific repair processes in macrophages during wound healing. Cell Metab. 33 (12), 2398–2414.e9. doi:10.1016/j.cmet.2021.10.004
Wu, B., Feng, J. Y., Yu, L. M., Wang, Y. C., Chen, Y. Q., Wei, Y., et al. (2018). Icariin protects cardiomyocytes against ischaemia/reperfusion injury by attenuating sirtuin 1-dependent mitochondrial oxidative damage. Br. J. Pharmacol. 175 (21), 4137–4153. doi:10.1111/bph.14457
Wu, J., Sun, L., Chen, X., Du, F., Shi, H., Chen, C., et al. (2013). Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339 (6121), 826–830. doi:10.1126/science.1229963
Wu, K., Luan, G., Xu, Y., Shen, S., Qian, S., Zhu, Z., et al. (2020). Cigarette smoke extract increases mitochondrial membrane permeability through activation of adenine nucleotide translocator (ANT) in lung epithelial cells. Biochem. Biophys. Res. Commun. 525 (3), 733–739. doi:10.1016/j.bbrc.2020.02.160
Wu, W., Tian, W., Hu, Z., Chen, G., Huang, L., Li, W., et al. (2014). ULK1 translocates to mitochondria and phosphorylates FUNDC1 to regulate mitophagy. EMBO Rep. 15 (5), 566–575. doi:10.1002/embr.201438501
Wu, Z., Puigserver, P., Andersson, U., Zhang, C., Adelmant, G., Mootha, V., et al. (1999). Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98 (1), 115–124. doi:10.1016/S0092-8674(00)80611-X
Xia, R., Sheng, X., Xu, X., Yu, C., and Lu, H. (2018). Hesperidin induces apoptosis and G0/G1 arrest in human non-small cell lung cancer A549 cells. Int. J. Mol. Med. 41 (1), 464–472. doi:10.3892/ijmm.2017.3250
Xian, H., Watari, K., Sanchez-Lopez, E., Offenberger, J., Onyuru, J., Sampath, H., et al. (2022). Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity 55 (8), 1370–1385.e8. doi:10.1016/j.immuni.2022.06.007
Xing, W., Yang, L., Peng, Y., Wang, Q., Gao, M., Yang, M., et al. (2017). Ginsenoside Rg3 attenuates sepsis-induced injury and mitochondrial dysfunction in liver via AMPK-mediated autophagy flux. Biosci. Rep. 37 (4). doi:10.1042/BSR20170934
Xu, C. Q., Liu, B. J., Wu, J. F., Xu, Y. C., Duan, X. H., Cao, Y. X., et al. (2010). Icariin attenuates LPS-induced acute inflammatory responses: involvement of PI3K/Akt and NF-kappaB signaling pathway. Eur. J. Pharmacol. 642 (1-3), 146–153. doi:10.1016/j.ejphar.2010.05.012
Xu, M., Ma, Q., Fan, C., Chen, X., Zhang, H., and Tang, M. (2019). Ginsenosides Rb1 and Rg1 protect primary cultured astrocytes against oxygen-glucose deprivation/reoxygenation-induced injury via improving mitochondrial function. Int. J. Mol. Sci. 20 (23), 6086. doi:10.3390/ijms20236086
Xu, S., Xu, J., Hao, T., Yan, Y., Zhang, S., Li, A., et al. (2021). Paeonol alleviates lipopolysaccharide-induced hepatocytes injury through alteration of mitochondrial function and NF-κB translocation. Mol. Med. Rep. 24 (5), 779. doi:10.3892/mmr.2021.12419
Xue, B., Huang, J., Ma, B., Yang, B., Chang, D., and Liu, J. (2019). Astragaloside IV protects primary cerebral cortical neurons from oxygen and glucose deprivation/reoxygenation by activating the PKA/CREB pathway. Neuroscience 404, 326–337. doi:10.1016/j.neuroscience.2019.01.040
Yang, H., Wang, H., Ren, J., Chen, Q., and Chen, Z. J. (2017). cGAS is essential for cellular senescence. Proc. Natl. Acad. Sci. U. S. A. 114 (23), E4612-E4620–e4620. doi:10.1073/pnas.1705499114
Yang, L., Lin, X., Tang, H., Fan, Y., Zeng, S., Jia, L., et al. (2020). Mitochondrial DNA mutation exacerbates female reproductive aging via impairment of the NADH/NAD(+) redox. Aging Cell 19 (9), e13206. doi:10.1111/acel.13206
Yang, Y. L., Hsu, H. T., Wang, K. H., Wang, C. S., Chen, C. M., and Ko, W. C. (2012). Hesperidin-3'-o-methylether is more potent than hesperidin in phosphodiesterase inhibition and suppression of ovalbumin-induced airway hyperresponsiveness. Evid. Based Complement. Altern. Med. 2012, 908562. doi:10.1155/2012/908562
Yao, H., Chung, S., Hwang, J. w., Rajendrasozhan, S., Sundar, I. K., Dean, D. A., et al. (2012). SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J. Clin. Invest 122 (6), 2032–2045. doi:10.1172/JCI60132
Yao, H., Sundar, I. K., Ahmad, T., Lerner, C., Gerloff, J., Friedman, A. E., et al. (2014). SIRT1 protects against cigarette smoke-induced lung oxidative stress via a FOXO3-dependent mechanism. Am. J. Physiol. Lung Cell Mol. Physiol. 306 (9), L816–L828. doi:10.1152/ajplung.00323.2013
Yao, R. Q., Ren, C., Xia, Z. F., and Yao, Y. M. (2021). Organelle-specific autophagy in inflammatory diseases: a potential therapeutic target underlying the quality control of multiple organelles. Autophagy 17 (2), 385–401. doi:10.1080/15548627.2020.1725377
Yao, S., Yuan, Y., Zhang, H., Meng, X., Jin, L., Yang, J., et al. (2020). Berberine attenuates the abnormal ectopic lipid deposition in skeletal muscle. Free Radic. Biol. Med. 159, 66–75. doi:10.1016/j.freeradbiomed.2020.07.028
Yao, X., Jing, X., Guo, J., Sun, K., Deng, Y., Zhang, Y., et al. (2019). Icariin protects bone marrow mesenchymal stem cells against iron overload induced dysfunction through mitochondrial fusion and fission, PI3K/AKT/mTOR and MAPK pathways. Front. Pharmacol. 10, 163. doi:10.3389/fphar.2019.00163
Ye, P., Li, W., Huang, X., Zhao, S., Chen, W., Xia, Y., et al. (2022). BMAL1 regulates mitochondrial homeostasis in renal ischaemia-reperfusion injury by mediating the SIRT1/PGC-1α axis. J. Cell Mol. Med. 26 (7), 1994–2009. doi:10.1111/jcmm.17223
Ye, R., Zhang, X., Kong, X., Han, J., Yang, Q., Zhang, Y., et al. (2011). Ginsenoside Rd attenuates mitochondrial dysfunction and sequential apoptosis after transient focal ischemia. Neuroscience 178, 169–180. doi:10.1016/j.neuroscience.2011.01.007
Yee, C., Yang, W., and Hekimi, S. (2014). The intrinsic apoptosis pathway mediates the pro-longevity response to mitochondrial ROS in C. elegans. Cell 157 (4), 897–909. doi:10.1016/j.cell.2014.02.055
Yu, J., Nagasu, H., Murakami, T., Hoang, H., Broderick, L., Hoffman, H. M., et al. (2014). Inflammasome activation leads to Caspase-1-dependent mitochondrial damage and block of mitophagy. Proc. Natl. Acad. Sci. U. S. A. 111 (43), 15514–15519. doi:10.1073/pnas.1414859111
Yu, L., and Yang, S. J. (2010). AMP-activated protein kinase mediates activity-dependent regulation of peroxisome proliferator-activated receptor gamma coactivator-1alpha and nuclear respiratory factor 1 expression in rat visual cortical neurons. Neuroscience 169 (1), 23–38. doi:10.1016/j.neuroscience.2010.04.063
Yu, L. M., Dong, X., Xu, Y. L., Zhou, Z. J., Huang, Y. T., Zhao, J. K., et al. (2022). Icariin attenuates excessive alcohol consumption-induced susceptibility to atrial fibrillation through SIRT3 signaling. Biochim. Biophys. Acta Mol. Basis Dis. 1868 (10), 166483. doi:10.1016/j.bbadis.2022.166483
Yu, W., Dittenhafer-Reed, K. E., and Denu, J. M. (2012). SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J. Biol. Chem. 287 (17), 14078–14086. doi:10.1074/jbc.M112.355206
Yu, X., Li, Y., and Mu, X. (2020). Effect of quercetin on PC12 Alzheimer's disease cell model induced by aβ (25-35) and its mechanism based on sirtuin1/nrf2/HO-1 pathway. Biomed. Res. Int. 2020, 8210578. doi:10.1155/2020/8210578
Yu, X., Man, R., Li, Y., Yang, Q., Li, H., Yang, H., et al. (2019). Paeoniflorin protects spiral ganglion neurons from cisplatin-induced ototoxicity: possible relation to PINK1/BAD pathway. J. Cell Mol. Med. 23 (8), 5098–5107. doi:10.1111/jcmm.14379
Yuan, C., Xu, X., Wang, N., Zhu, Q., Zhang, J., Gong, W., et al. (2022). Paeonol protects against acute pancreatitis by inhibiting M1 macrophage polarization via the NLRP3 inflammasomes pathway. Biochem. Biophys. Res. Commun. 600, 35–43. doi:10.1016/j.bbrc.2022.02.019
Yuan, J., Liu, R., Ma, Y., Zhang, Z., and Xie, Z. (2018). Curcumin attenuates airway inflammation and airway remolding by inhibiting NF-κB signaling and COX-2 in cigarette smoke-induced COPD mice. Inflammation 41 (5), 1804–1814. doi:10.1007/s10753-018-0823-6
Yuan, S., and Akey, C. W. (2013). Apoptosome structure, assembly, and procaspase activation. Structure 21 (4), 501–515. doi:10.1016/j.str.2013.02.024
Yue, L., and Yao, H. (2016). Mitochondrial dysfunction in inflammatory responses and cellular senescence: pathogenesis and pharmacological targets for chronic lung diseases. Br. J. Pharmacol. 173 (15), 2305–2318. doi:10.1111/bph.13518
Zaib, S., Hayyat, A., Ali, N., Gul, A., Naveed, M., and Khan, I. (2022). Role of mitochondrial membrane potential and lactate dehydrogenase A in apoptosis. Anticancer Agents Med. Chem. 22 (11), 2048–2062. doi:10.2174/1871520621666211126090906
Zeng, R., Wang, X., Zhou, Q., Fu, X., Wu, Q., Lu, Y., et al. (2019a). Icariin protects rotenone-induced neurotoxicity through induction of SIRT3. Toxicol. Appl. Pharmacol. 379, 114639. doi:10.1016/j.taap.2019.114639
Zeng, R., Zhou, Q., Zhang, W., Fu, X., Wu, Q., Lu, Y., et al. (2019b). Icariin-mediated activation of autophagy confers protective effect on rotenone induced neurotoxicity in vivo and in vitro. Toxicol. Rep. 6, 637–644. doi:10.1016/j.toxrep.2019.06.014
Zhang, D., Wu, J., Wu, J., and Zhang, S. (2021c). Paeonol induces protective autophagy in retinal photoreceptor cells. Front. Pharmacol. 12, 667959. doi:10.3389/fphar.2021.667959
Zhang, J. R., Shen, S. Y., Zhai, M. Y., Shen, Z. Q., Li, W., Liang, L. F., et al. (2024). Augmented microglial endoplasmic reticulum-mitochondria contacts mediate depression-like behavior in mice induced by chronic social defeat stress. Nat. Commun. 15 (1), 5199. doi:10.1038/s41467-024-49597-z
Zhang, L. X., Li, C. X., Kakar, M. U., Khan, M. S., Wu, P. F., Amir, R. M., et al. (2021b). Resveratrol (RV): a pharmacological review and call for further research. Biomed. Pharmacother. 143, 112164. doi:10.1016/j.biopha.2021.112164
Zhang, M., Hei, R., Zhou, Z., Xiao, W., Liu, X., and Chen, Y. (2023a). Macrophage polarization involved the inflammation of chronic obstructive pulmonary disease by S1P/HDAC1 signaling. Am. J. Cancer Res. 13 (9), 4478–4489.
Zhang, M., Shi, R., Zhang, Y., Shan, H., Zhang, Q., Yang, X., et al. (2019). Nix/BNIP3L-dependent mitophagy accounts for airway epithelial cell injury induced by cigarette smoke. J. Cell Physiol. 234 (8), 14210–14220. doi:10.1002/jcp.28117
Zhang, M., Tang, J., Li, Y., Xie, Y., Shan, H., Chen, M., et al. (2017). Curcumin attenuates skeletal muscle mitochondrial impairment in COPD rats: PGC-1α/SIRT3 pathway involved. Chem. Biol. Interact. 277, 168–175. doi:10.1016/j.cbi.2017.09.018
Zhang, M., Xie, Y., Yan, R., Shan, H., Tang, J., Cai, Y., et al. (2016). Curcumin ameliorates alveolar epithelial injury in a rat model of chronic obstructive pulmonary disease. Life Sci. 164, 1–8. doi:10.1016/j.lfs.2016.09.001
Zhang, M., Zhang, Y., Roth, M., Zhang, L., Shi, R., Yang, X., et al. (2020). Sirtuin 3 inhibits airway epithelial mitochondrial oxidative stress in cigarette smoke-induced COPD. Oxid. Med. Cell Longev. 2020, 7582980. doi:10.1155/2020/7582980
Zhang, Q., Itagaki, K., and Hauser, C. J. (2010). Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock 34 (1), 55–59. doi:10.1097/SHK.0b013e3181cd8c08
Zhang, W. Z., Hoffman, K. L., Schiffer, K. T., Oromendia, C., Rice, M. C., Barjaktarevic, I., et al. (2021a). Association of plasma mitochondrial DNA with COPD severity and progression in the SPIROMICS cohort. Respir. Res. 22 (1), 126. doi:10.1186/s12931-021-01707-x
Zhang, Y., Yao, J., Zhang, M., Wang, Y., and Shi, X. (2023b). Mitochondria-associated endoplasmic reticulum membranes (MAMs): possible therapeutic targets in heart failure. Front. Cardiovasc Med. 10, 1083935. doi:10.3389/fcvm.2023.1083935
Zhao, P., Li, J., Yang, L., Li, Y., Tian, Y., and Li, S. (2018). Integration of transcriptomics, proteomics, metabolomics and systems pharmacology data to reveal the therapeutic mechanism underlying Chinese herbal Bufei Yishen formula for the treatment of chronic obstructive pulmonary disease. Mol. Med. Rep. 17 (4), 5247–5257. doi:10.3892/mmr.2018.8480
Zhao, X., Wang, C., Dai, S., Liu, Y., Zhang, F., Peng, C., et al. (2022). Quercetin protects ethanol-induced hepatocyte pyroptosis via scavenging mitochondrial ROS and promoting PGC-1α-regulated mitochondrial homeostasis in L02 cells. Oxid. Med. Cell Longev. 2022, 4591134. doi:10.1155/2022/4591134
Zheng, L., Bernard-Marissal, N., Moullan, N., D'Amico, D., Auwerx, J., Moore, D. J., et al. (2017). Parkin functionally interacts with PGC-1α to preserve mitochondria and protect dopaminergic neurons. Hum. Mol. Genet. 26 (3), 582–598. doi:10.1093/hmg/ddw418
Zhong, Z., Umemura, A., Sanchez-Lopez, E., Liang, S., Shalapour, S., Wong, J., et al. (2016). NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell 164 (5), 896–910. doi:10.1016/j.cell.2015.12.057
Zhou, P., Xie, W., Meng, X., Zhai, Y., Dong, X., Zhang, X., et al. (2019b). Notoginsenoside R1 ameliorates diabetic retinopathy through PINK1-dependent activation of mitophagy. Cells 8 (3), 213. doi:10.3390/cells8030213
Zhou, P., Xie, W., Sun, Y., Dai, Z., Li, G., Sun, G., et al. (2019a). Ginsenoside Rb1 and mitochondria: a short review of the literature. Mol. Cell Probes 43, 1–5. doi:10.1016/j.mcp.2018.12.001
Zhou, W. C., Qu, J., Xie, S. Y., Sun, Y., and Yao, H. W. (2021). Mitochondrial dysfunction in chronic respiratory diseases: implications for the pathogenesis and potential therapeutics. Oxid. Med. Cell Longev. 2021, 5188306. doi:10.1155/2021/5188306
Zhu, J. X., Wen, L., Zhong, W. J., Xiong, L., Liang, J., and Wang, H. L. (2018). Quercetin, kaempferol and isorhamnetin in elaeagnus pungens thunb. Leaf: pharmacological activities and quantitative determination studies. Chem. Biodivers. 15 (8), e1800129. doi:10.1002/cbdv.201800129
Keywords: Mitochondria, mtROS, cigarette smoke, lung disease, natural extract compound, COPD
Citation: Wang Q, Zeng Z, Guo L, Williams KE, Zhang Y, Tang H, Hu H, Qin G, Wang K and Wang X (2025) Modulating mitochondria with natural extract compounds: from bench to clinical therapeutic opportunities for COPD. Front. Pharmacol. 16:1531302. doi: 10.3389/fphar.2025.1531302
Received: 26 November 2024; Accepted: 17 April 2025;
Published: 21 May 2025.
Edited by:
Julie Gunnells Ledford, University of Arizona, United StatesReviewed by:
Er Yue, City of Hope National Medical Center, United StatesPaul Victor Santiago Raj, University of Arizona, United States
Copyright © 2025 Wang, Zeng, Guo, Williams, Zhang, Tang, Hu, Qin, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linlin Guo, bGxpbmd1b0BpdS5lZHU=, cGFpbm5vZ2Fpbm5vY3VyaWVAaG90bWFpbC5jb20=; Kaijin Wang, d2FuZ2thaXhpbjIwMDY1MjFAMTI2LmNvbQ==; Xing Wang, d2FuZy54aW5nQHN3bXUuZWR1LmNu, d3hfZWxpb3RfODgxMDE0QDE2My5jb20=
†These authors have contributed equally to this work
 Qiao Wang1†
Qiao Wang1† Linlin Guo
Linlin Guo Yun Zhang
Yun Zhang Gang Qin
Gang Qin Xing Wang
Xing Wang