- 1Department of Pharmacy, People’s Hospital of Zhongjiang County, Deyang, Sichuan, China
- 2Department of Rehabilitation, People’s Hospital of Zhongjiang County, Deyang, Sichuan, China
Objective: Drug-associated hearing impairment has a serious impact on children’s quality of life and poses a significant public health burden. However, there is a lack of large-scale population-based studies of medication-associated hearing impairment in children. The aim of this study was to hypothesize about medications through data mining in order to assess the potential risk of these medications increasing hearing impairment in children.
Methods: We extracted and analyzed reports on drugs linked to hearing impairment in children from the FDA Adverse Event Reporting System (FAERS). To assess the relationship between drugs and hearing impairment in children, we performed a disproportionality study utilizing the proportional reporting ratio (PRR) and reporting odds ratio (ROR). Concurrently, we conducted comparisons with medicine labels to identify medications that, although not now indicating hearing impairment in their labels, may possibly pose risks of hearing impairment in children.
Results: In the FAERS database, there are 1,884 reports of AE related to hearing impairment in children. The top three medications with the highest ROR were vinblastin [N = 6 cases, ROR = 86.72 (34.15–220.19)], risedronate [N = 3 cases, ROR = 73.59 (20.24–267.63)], and amikacin [N = 11 cases, ROR = 71.31 (36.40–139.72)]. The top 3 drugs with the highest number of reports were carboplatin [N = 125 cases, ROR = 18.41 (15.27–22.21)], cisplatin [N = 78 cases, ROR = 31.24 (24.59–39.70)], and vincristine [N = 56 cases, ROR = 6.32 (4.83–8.27)]. Based on drug labeling, 48% drugs (27/56) were classified as potentially ototoxic.
Conclusion: Our findings suggest that nearly half of the 56 drugs linked to hearing impairment signals in children are not currently labeled with ototoxicity warnings. Consequently, further research is required to evaluate the association of these medicines with this risk.
Introduction
Ototoxicity is one of the most common adverse drug reactions, with symptoms including hearing loss and vestibular dysfunction, which have a serious impact on patients’ quality of life and have become a significant public health burden (Tanoshima et al., 2019). Vestibular dysfunction increases the risk of falls (Osoba et al., 2019), and both hearing and vestibular dysfunction are strongly associated with cognitive decline (Hanes and McCollum, 2006; Loughrey et al., 2018; Rizk et al., 2020b). Recognizing medication-induced ototoxicity is critical for healthcare providers in many areas (Rizk et al., 2020a). In an era of increasing polypharmacy, the negative public health impact of drug-induced hearing impairment cannot be ignored.
The impact and severity of ototoxicity may vary widely depending on pharmacology and individual patient risk factors (Rizk et al., 2020a). In addition, the consequences of hearing loss in children compared to adults are multifaceted and particularly affect patients treated at a young age. These consequences include impairments in speech and language acquisition, psychosocial and cognitive development, and educational and occupational achievement (Gurney et al., 2007; Schreiber et al., 2014; Olivier et al., 2019). Therefore, the effective prevention of hearing impairment due to ototoxic medications is becoming an area of great interest (Heinemann et al., 2020).
A few drugs are strongly associated with ototoxicity (e.g., aminoglycosides, labeled diuretics, platinum-based chemotherapeutic agents, etc.). However, more potentially ototoxic drugs may remain undiscovered (Rizk et al., 2020a). The FDA Adverse Event Reporting System (FAERS) is a database used to collect information about spontaneously reported adverse drug events. Because of its large volume of data, diversity of data information, and free public access, it is often used by pharmacovigilance experts in studies of adverse drug event signal mining to detect potential safety signals (Sakaeda et al., 2013).
This study aimed to identify drugs suspected of causing hearing impairment in children, utilizing the FAERS database. This study may provide data on medications that need to be studied further to determine the potential risk of these medications for hearing impairment in children.
Methods
Data source
This was a retrospective study utilizing the FAERS database, a database of spontaneously reported adverse events (AEs) designed to support post-marketing safety surveillance of drugs and biologics (Shen et al., 2019). We downloaded and extracted all data from the FDA website (fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html) from the first quarter of 2014 to the second quarter of 2024. We then transferred the data to MySQL 8.0 for further analysis. In addition, FAERS data were updated quarterly, so there were duplicate reports. According to FDA recommendations, data de-duplication is performed prior to data analysis (Sakaeda et al., 2013). This procedure entailed choosing the most current FDA_DT when CASEID matched, and selecting the higher PRIMARYID when both CASEID and FDA_DT matched. Furthermore, our analysis was limited to AE reports from individuals aged 0–17 years.
Adverse events and drug identification
The FAERS database adheres to the International Safety Reporting Guidelines outlined by the International Conference on Harmonization (ICH E2B). The classification and uniformity of ADEs in the FAERS data are based on the Medical Dictionary of Regulatory Activities (MedDRA) (Brown et al., 1999). The MedDRA nomenclature is organized into five levels: the System Organ Classifications (SOC), the High-Level Group Term (HLGT), the High-Level Term (HLT), the Preferred Term (PT), and the Lowest Level Term (LLT) (Mascolo et al., 2021). In addition, this can be achieved by adopting the Standardized MedDRA Query (SMQ) approach (Brown et al., 1999), where algorithms are used to combine several PTs to identify unique clinical syndromes. The definition of hearing impairment (code:20000171) in this study utilizes the definition of SMQ. Supplementary Table S1 displays the PTs associated with hearing impairment. To improve the accuracy of the analysis, our study only included AE reports for which the drug was the primary suspect.
Drug classification
We reviewed the USA (https://www.accessdata.fda.gov/scripts/cder/daf/) and China (https://www.yaozh.com/) databases to identify medications previously recognized as ototoxic. Drug labeling serves to furnish healthcare providers and patients with thorough and precise information regarding medication use, thereby ensuring safety and efficacy (Wu et al., 2022). In this study, a drug was classified as “known” ototoxic if the side effect was indicated on the drug label. Otherwise, it was classified as a “potential” ototoxic agent.
Data mining
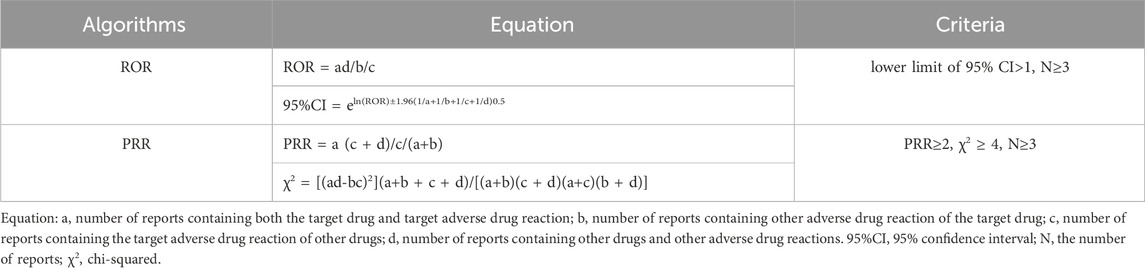
This study used disproportionality analysis, also known as case/non-case analysis, which is one of the most common methods of detecting AE signals in pharmacovigilance. pharmacovigilance (Almenoff et al., 2007). The general principle is that we consider an AE signal to be generated when the reported rate of a specific AE for a particular drug is significantly higher than the background frequency in the database and meets certain criteria or thresholds. In this study, frequentist methods [reporting odds ratio (ROR) (van Puijenbroek et al., 2002) and proportional reporting ratio (PRR) (Evans, Waller, and Davis, 2001)] were used to identify potential AE signals associated with drug-hearing impairment. To improve the accuracy of the analysis, a meaningful AE signal was considered to be generated when both of these algorithms produced an AE signal. The formulas and thresholds for the two algorithms are shown in Table 1. All data processing was performed using MYSQL 8.0, Navicat Premium 16, and Microsoft Excel 2021.
Results
Descriptive analysis
Between the first quarter of 2014 and the second quarter of 2024, the FAERS database documented 1,884 instances of childhood hearing impairment. A few cases were missing a minor amount of baseline information, leading to their classification as unknown. Table 2 presents the specific characteristics of children with drug-related hearing impairment. There were equal numbers of males (42.73%) and females (42.89%) among children with drug-related hearing impairment. The mean age was 8.34 ± 5.99 years. Hospitalization accounted for 21.71% of the reported drug-induced hearing impairment in children and deaths accounted for 2.07%. In addition, the country from which the most reports originated was the United States (35.83%), followed by Canada (14.28%) and France (8.39%). The frequency of reports of children with drug-induced hearing impairment has generally been rising over time.
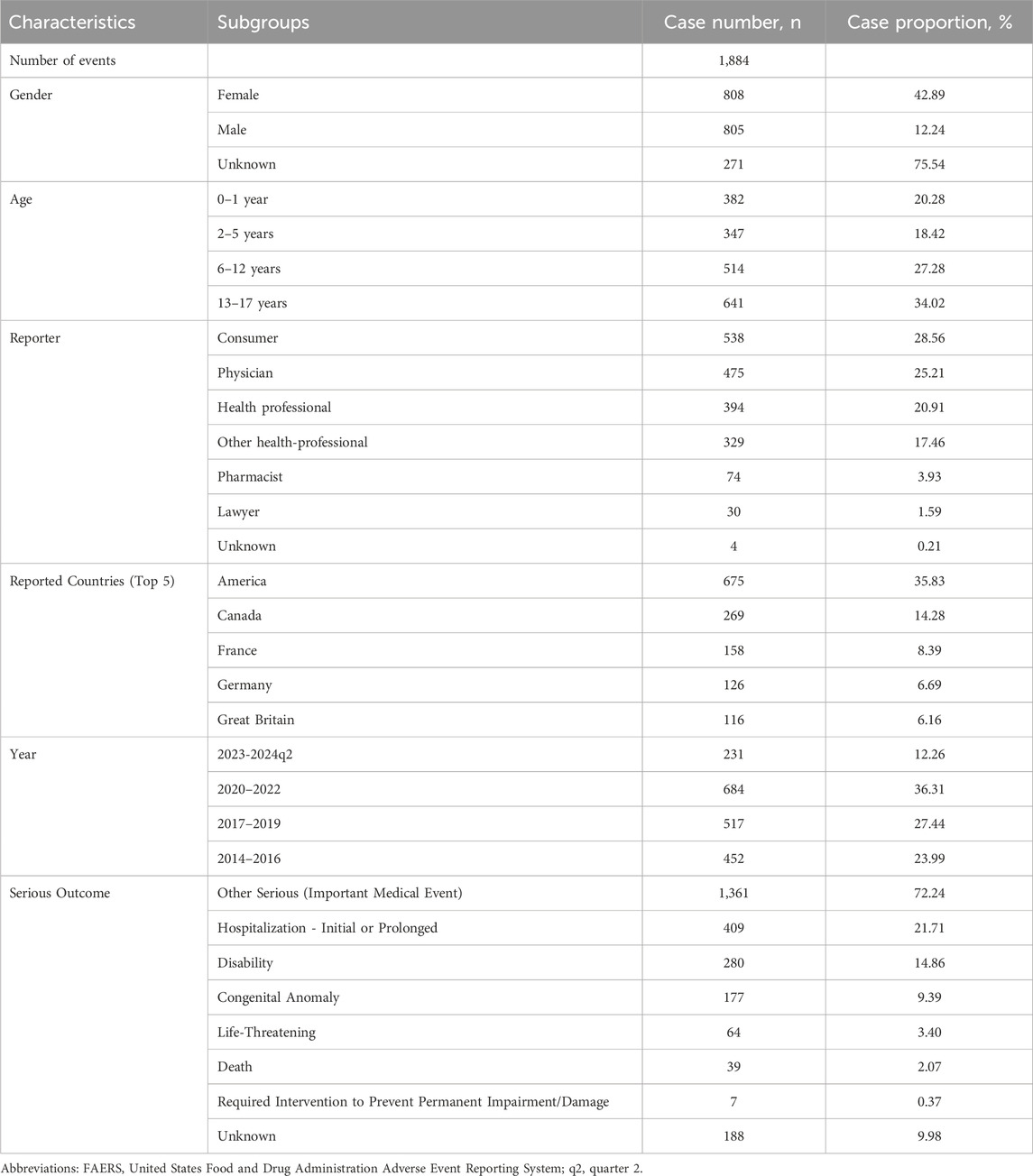
Table 2. Clinical characteristics of children with drug-associated hearing impairment from the FAERS database (January 2014 to June 2024).
Disproportionality analysis
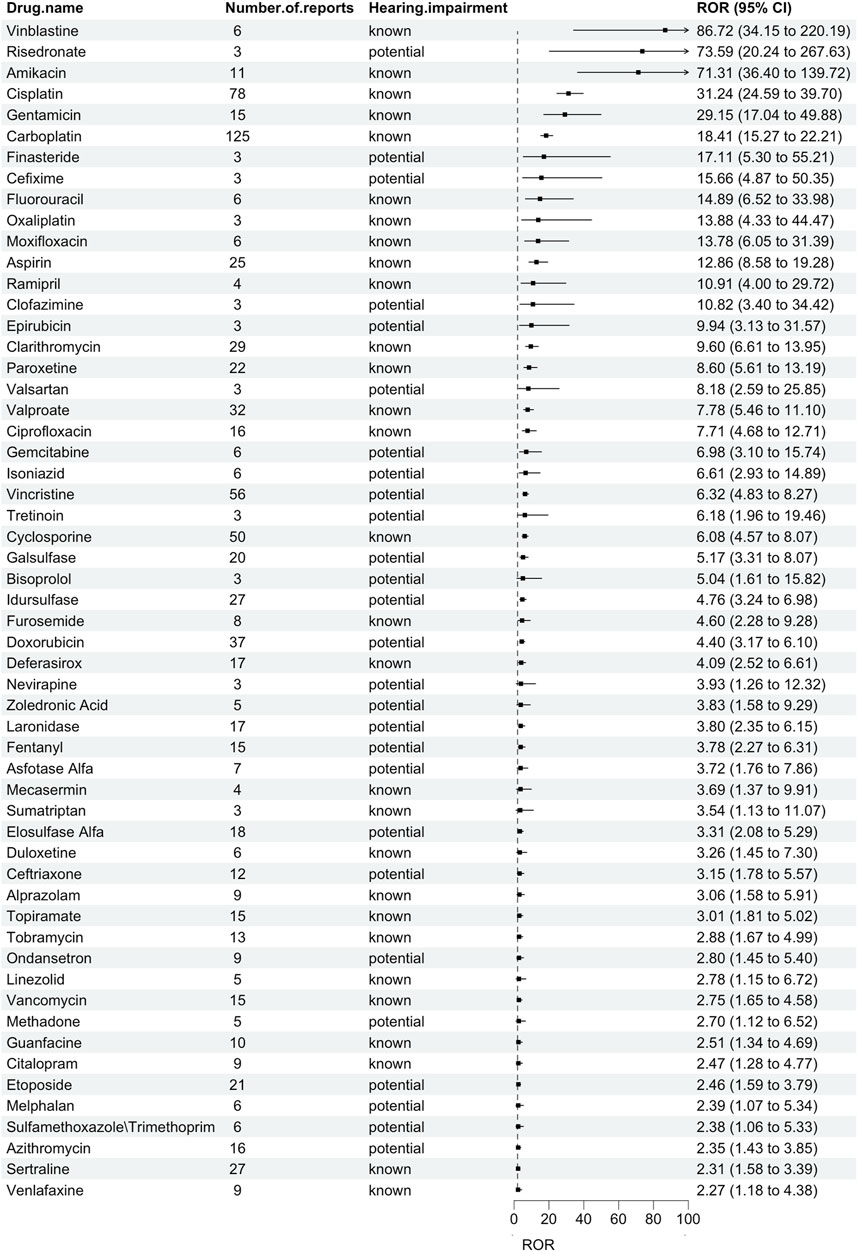
We discovered 56 pharmaceuticals in the FAERS database linked to hearing impairment in children (Figure 1). Vinblastin [N = 6, ROR = 86.72 (34.15–220.19)], risedronate [N = 3, ROR = 73.59 (20.24–267.63)], and amikacin [N = 11, ROR = 71.31 (36.40–139.72)] were the top three drugs with the highest ROR. The top 3 drugs with the highest number of reports were carboplatin [N = 125, ROR = 18.41 (15.27–22.21)], cisplatin [N = 78, ROR = 31.24 (24.59–39.70)], and vincristine [N = 56, ROR = 6.32 (4.83–8.27)]. According to drug labeling, 29 drugs were categorized as known ototoxic agents, while 27 drugs were categorized as potentially ototoxic agents.
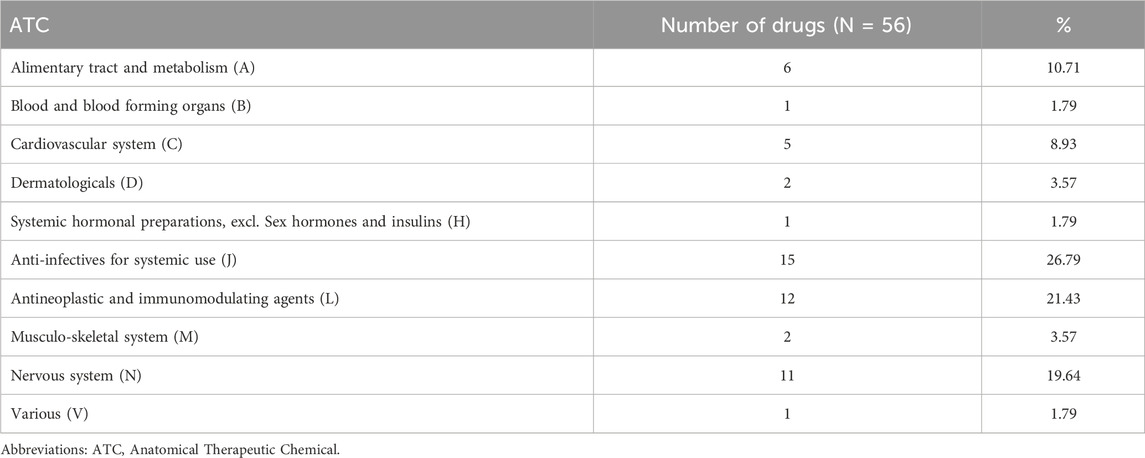
Table 3 lists the number of AEs recorded for each medication class based on the Anatomical Therapeutic Chemical (ATC) classification. Anti-infectives for systemic use, antineoplastic and immunomodulating agents, and nervous system were the three drug classes with the highest reporting rates. Reports related to hearing impairment in children were commonly reported in FAERS at 26.79%, 21.43%, and 19.64%, respectively. Among these, paroxetine [N = 22, ROR = 8.60 (5.61–13.19)] had the highest ROR among the medications for the nervous system, vinblastine had the highest ROR among the antineoplastic and immunomodulating agents, and amikacin had the highest ROR among the anti-infectives for systemic use in children with hearing impairment.
Discussion
This study utilized 10 years of data from the FAERS database to identify suspected medications that may be associated with hearing impairment in children. We identified 1,884 reports of associated AE. Furthermore, we found 56 medications linked to hearing impairment in children in the FAERS database. Among them, the categories with the largest number of reports were anti-infectives for systemic use, antineoplastic and immunomodulating agents, and nervous system. The three medications with the greatest ROR were vinblastine, risedronate, and amikacin. It is noteworthy that approximately 48% of the drugs showed a potential hearing impairment in children and were not clearly identified on the drug labeling as having associated adverse effects.
In an age of rising polypharmacy, severe medication responses such ototoxicity pose considerable public health concerns. This study using disproportionality analysis to identify safety signals linking medications and hearing impairment in children. In our study, we first identified some drugs that are clearly ototoxic, such as platinum-based chemotherapeutic agents, aminoglycoside antibiotics, and vancomycin (Freyer et al., 2020; Diepstraten et al., 2021; Lestner et al., 2016). The ototoxicity induced by these drugs is mainly characterized by cochlear and vestibular toxicity, and the contribution of aminoglycoside antibiotics to the risk of ototoxicity in children is particularly significant. According to the World Health Organization, among the preventable causes of hearing loss in children, about 4% of ototoxicity cases are attributed to the use of ototoxic drugs such as aminoglycosides in pregnant women and infants (Lieu et al., 2020). Clinical experience and previous studies have shown that clinical experience and previous studies suggest that aminoglycoside antibiotics may induce cochlear toxicity and/or vestibular toxicity in some pediatric patients (Lanvers-Kaminsky et al., 2017). Cochlear toxicity is caused by hair cell rupture and can lead to neurologic hearing impairment and even blindness in severe cases (Jiang, Karasawa, and Steyger, 2017). This is in agreement with the results of the present study, where AE signals for hearing impairment were detected for amikacin, gentamicin, and tobramycin, and amikacin and gentamicin were the two drugs with the highest ROR for hearing impairment among the antimicrobials.
Platinum-based drugs are commonly used as chemotherapeutic agents for the treatment of childhood cancers, and 60% of children treated with cisplatin develop permanent bilateral hearing impairment (Knight, Kraemer, and Neuwelt, 2005; Knight et al., 2007). In the present study, we found that carboplatin and cisplatin were the two drugs with the highest number of reports, and oxaliplatin also showed AE signals of potential hearing impairment. This result is in line with other research and indicates that there may be some variation in hearing impairment brought on by platinum compounds, with oxaliplatin being the least ototoxic and cisplatin being the most ototoxic (Grewal et al., 2010; Romano et al., 2020).
Aspirin is one of the most commonly used antipyretic and analgesic drugs for children. Because children with Kawasaki disease need to take aspirin for a long period of time and in large quantities, it is easy to have headaches, dizziness, tinnitus, visual hearing loss, and other symptoms, known as the salicylic acid reaction, whose mechanism may be the vasoconstriction of vasculature supplying the helix caused by salicylic acid, and the children may have monaural or binaural hearing loss, but if the drug is stopped in a timely manner, most of them can be restored (Salvi et al., 2021). Aspirin has been demonstrated to substantially diminish the risk of hearing impairment produced by gentamicin (Chen et al., 2007). Nonetheless, owing to the distinct processes of ototoxicity associated with cisplatin and gentamicin, research indicates that aspirin does not safeguard hearing in individuals undergoing cisplatin treatment (Crabb et al., 2017). Despite the variability in clinical study outcomes, we must be cautious regarding the potential risk of hearing impairment associated with aspirin in children.
Antidepressants are widely known for causing ototoxicity due to tinnitus and positional vertigo (Clewes, 2012), and the present study also found multiple antidepressants associated with ototoxicity in children. In addition, iron chelators have been strongly associated with neurologic hearing loss at high frequencies (Osma et al., 2015), and our study also observed a significant proportion of AE reports involving deferasirox-induced hearing impairment. The current study indicates that valsartan and bisoprolol may possess ototoxic potential, corroborating findings from a prior review that identified angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers as potentially ototoxic agents (Cianfrone et al., 2011). In a related study, decreased beta receptor function may lead to hearing deficits (Al-Ghamdi et al., 2018). Hearing impairments have been linked to the use of medications that enhance peripheral vascularization. Additionally, hypertension patients may experience tinnitus as a result of taking medications that stimulate the renin-angiotensin system or raise peripheral vascular tone (β-blockers) (Borghi et al., 2005).
Notably, the data-mining results of this study revealed that approximately 60% of drugs with potential pediatric hearing impairment signals do not mention this adverse effect in their drug labeling. These drugs include neurologic drugs such as valproate and sertraline, and cardiovascular drugs such as valsartan and bisoprolol. The association of these drugs with ototoxicity has not been well documented, and there are only a few case reports suggesting that these drugs may cause hearing impairment (Armon et al., 1990; Hori et al., 2003; Yeap et al., 2014). In addition, this study found that drugs used to treat mucopolysaccharide storage disease (e.g., galsulfase, idursulfase, laronidase, and elosulfase alfa), and the third-generation bisphosphonates risedronate and zoledronic acid, used to treat hearing loss due to pediatric sclerosis, have also been associated with ototoxicity signaling correlations (Quesnel et al., 2012). Although these signals may be more associated with primary disease (Ago et al., 2024; Karosi, Szekanecz, and Sziklai, 2009).
Certain limitations of this study should be acknowledged while analyzing the results. Firstly, because the total number of patients treated with a particular medication was not available, we were unable to calculate the incidence of drug-induced hearing impairment for each medication. Secondly, FAERS is constrained by the initiative, precision, and promptness with which physicians, other healthcare professionals, and consumers report adverse events. This may lead to potential misreporting and underreporting. Finally, the study design only allowed for the monitoring of safety signals, and therefore future studies are needed to validate associations and confirm causality between them (Fusaroli et al., 2024). Despite these limitations, our study will provide useful guidance to healthcare providers and the public, reveal potential medications associated with hearing impairment in children, and provide valuable data to support the monitoring and clinical application of relevant medications in the future.
Conclusion
This pharmacovigilance study explored reports of AEs associated with pediatric hearing impairment in the FAERS database. Our research indicated that almost 48% of the 56 examined pharmaceuticals with possible safety signals for pediatric hearing impairment did not mention ototoxicity as an adverse effect in their labeling. Consequently, further research is required to evaluate the association of these drugs with this risk.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
JL: Writing – original draft, Conceptualization, Investigation, Validation. JT: Writing – original draft, Data curation, Formal Analysis. QX: Data curation, Formal Analysis, Writing – original draft. YB: Project administration, Resources, Writing – review and editing. EC: Conceptualization, Investigation, Validation, Writing – review and editing. CS: Writing – review and editing. YW: Conceptualization, Investigation, Writing – review and editing. HZ: Data curation, Methodology, Visualization, Writing – review and editing. WW: Data curation, Methodology, Visualization, Conceptualization, Formal Analysis, Funding acquisition, Project administration, Software, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Sichuan Provincial Hospital Association Young Pharmacist Research Special Fund Project (Phase II) (No. YP2202405).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1532461/full#supplementary-material
References
Ago, Y., Rintz, E., Musini, K. S., Ma, Z., and Tomatsu, S. (2024). Molecular mechanisms in pathophysiology of mucopolysaccharidosis and prospects for innovative therapy. Int. J. Mol. Sci. 25 (2), 1113. doi:10.3390/ijms25021113
Al-Ghamdi, B. S., Rohra, D. K., Abuharb, G. A. I., Alkofide, H. A., AlRuwaili, N. S., Shoukri, M. M., et al. (2018). Use of beta blockers is associated with hearing loss. Int. J. Audiol. 57 (3), 213–220. doi:10.1080/14992027.2017.1405162
Almenoff, J. S., Pattishall, E. N., Gibbs, T. G., DuMouchel, W., Evans, S. J., and Yuen, N. (2007). Novel statistical tools for monitoring the safety of marketed drugs. Clin. Pharmacol. Ther. 82 (2), 157–166. doi:10.1038/sj.clpt.6100258
Armon, C., Brown, E., Carwile, S., Miller, P., and Shin, C. (1990). Sensorineural hearing loss: a reversible effect of valproic acid. Neurology 40 (12), 1896–1898. doi:10.1212/wnl.40.12.1896
Borghi, C., Brandolini, C., Prandin, M. G., Dormi, A., Modugno, G. C., and Pirodda, A. (2005). Prevalence of tinnitus in patients withhypertension and the impact of different anti hypertensive drugs on the incidence of tinnitus: a prospective, single-blind, observational study. Curr. Ther. Res. Clin. Exp. 66 (5), 420–432. doi:10.1016/j.curtheres.2005.10.001
Brown, E. G., Wood, L., and Wood, S. (1999). The medical dictionary for regulatory activities (MedDRA). Drug Saf. 20 (2), 109–117. doi:10.2165/00002018-199920020-00002
Chen, Y., Huang, W. G., Zha, D. J., Qiu, J. H., Wang, J. L., Sha, S. H., et al. (2007). Aspirin attenuates gentamicin ototoxicity: from the laboratory to the clinic. Hear Res. 226 (1-2), 178–182. doi:10.1016/j.heares.2006.05.008
Cianfrone, G., Pentangelo, D., Cianfrone, F., Mazzei, F., Turchetta, R., Orlando, M. P., et al. (2011). Pharmacological drugs inducing ototoxicity, vestibular symptoms and tinnitus: a reasoned and updated guide. Eur. Rev. Med. Pharmacol. Sci. 15 (6), 601–636.
Clewes, J. (2012). A case report of onset of tinnitus following discontinuation of antidepressant and a review of the literature. Prim. Care Companion CNS Disord. 14 (1), PCC.11br01218. doi:10.4088/PCC.11br01218
Crabb, S. J., Martin, K., Abab, J., Ratcliffe, I., Thornton, R., Lineton, B., et al. (2017). COAST (cisplatin ototoxicity attenuated by aspirin trial): a phase II double-blind, randomised controlled trial to establish if aspirin reduces cisplatin induced hearing-loss. Eur. J. Cancer 87, 75–83. doi:10.1016/j.ejca.2017.09.033
Diepstraten, F. A., Hoetink, A. E., van Grotel, M., Huitema, A. D. R., Stokroos, R. J., van den Heuvel-Eibrink, M. M., et al. (2021). Aminoglycoside- and glycopeptide-induced ototoxicity in children: a systematic review. JAC Antimicrob. Resist 3 (4), dlab184. doi:10.1093/jacamr/dlab184
Evans, S. J., Waller, P. C., and Davis, S. (2001). Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 10 (6), 483–486. doi:10.1002/pds.677
Freyer, D. R., Brock, P. R., Chang, K. W., Dupuis, L. L., Epelman, S., Knight, K., et al. (2020). Prevention of cisplatin-induced ototoxicity in children and adolescents with cancer: a clinical practice guideline. Lancet Child. Adolesc. Health 4 (2), 141–150. doi:10.1016/s2352-4642(19)30336-0
Fusaroli, M., Salvo, F., Begaud, B., AlShammari, T. M., Bate, A., Battini, V., et al. (2024). The REporting of A disproportionality analysis for DrUg safety signal detection using individual case safety reports in PharmacoVigilance (READUS-PV): explanation and elaboration. Drug Saf. 47 (6), 585–599. doi:10.1007/s40264-024-01423-7
Grewal, S., Merchant, T., Reymond, R., McInerney, M., Hodge, C., and Shearer, P. (2010). Auditory late effects of childhood cancer therapy: a report from the children's oncology group. Pediatrics 125 (4), e938–e950. doi:10.1542/peds.2009-1597
Gurney, J. G., Tersak, J. M., Ness, K. K., Landier, W., Matthay, K. K., Schmidt, M. L., et al. (2007). Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: a report from the children's oncology group. Pediatrics 120 (5), e1229–e1236. doi:10.1542/peds.2007-0178
Hanes, D. A., and McCollum, G. (2006). Cognitive-vestibular interactions: a review of patient difficulties and possible mechanisms. J. Vestib. Res. 16 (3), 75–91. doi:10.3233/ves-2006-16301
Heinemann, A. W., Feuerstein, M., Frontera, W. R., Gard, S. A., Kaminsky, L. A., Negrini, S., et al. (2020). Rehabilitation is a global health priority. Can. J. Occup. Ther. 87 (2), 89–90. doi:10.1177/0008417420907804
Hori, A., Kataoka, S., Sakai, K., Hirose, G., Iwasaki, N., Horiguchi, A., et al. (2003). Valproic acid-induced hearing loss and tinnitus. Intern Med. 42 (11), 1153–1154. doi:10.2169/internalmedicine.42.1153
Jiang, M., Karasawa, T., and Steyger, P. S. (2017). Aminoglycoside-induced cochleotoxicity: a review. Front. Cell Neurosci. 11, 308. doi:10.3389/fncel.2017.00308
Karosi, T., Szekanecz, Z., and Sziklai, I. (2009). Otosclerosis: an autoimmune disease? Autoimmun. Rev. 9 (2), 95–101. doi:10.1016/j.autrev.2009.03.009
Knight, K. R., Kraemer, D. F., and Neuwelt, E. A. (2005). Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that May influence academic and social development. J. Clin. Oncol. 23 (34), 8588–8596. doi:10.1200/jco.2004.00.5355
Knight, K. R., Kraemer, D. F., Winter, C., and Neuwelt, E. A. (2007). Early changes in auditory function as a result of platinum chemotherapy: use of extended high-frequency audiometry and evoked distortion product otoacoustic emissions. J. Clin. Oncol. 25 (10), 1190–1195. doi:10.1200/jco.2006.07.9723
Lanvers-Kaminsky, C., Zehnhoff-Dinnesen, A. A., Parfitt, R., and Ciarimboli, G. (2017). Drug-induced ototoxicity: Mechanisms, pharmacogenetics, and protective strategies. Clin. Pharmacol. Ther. 101 (4), 491–500. doi:10.1002/cpt.603
Lestner, J. M., Hill, L. F., Heath, P. T., and Sharland, M. (2016). Vancomycin toxicity in neonates: a review of the evidence. Curr. Opin. Infect. Dis. 29 (3), 237–247. doi:10.1097/qco.0000000000000263
Lieu, J. E. C., Kenna, M., Anne, S., and Davidson, L. (2020). Hearing loss in children: a review. Jama 324 (21), 2195–2205. doi:10.1001/jama.2020.17647
Loughrey, D. G., Kelly, M. E., Kelley, G. A., Brennan, S., and Lawlor, B. A. (2018). Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol. Head. Neck Surg. 144 (2), 115–126. doi:10.1001/jamaoto.2017.2513
Mascolo, A., Scavone, C., Ferrajolo, C., Rafaniello, C., Danesi, R., Del Re, M., et al. (2021). Immune checkpoint inhibitors and cardiotoxicity: an analysis of spontaneous reports in eudravigilance. Drug Saf. 44 (9), 957–971. doi:10.1007/s40264-021-01086-8
Olivier, T. W., Bass, J. K., Ashford, J. M., Beaulieu, R., Scott, S. M., Schreiber, J. E., et al. (2019). Cognitive implications of ototoxicity in pediatric patients with embryonal brain tumors. J. Clin. Oncol. 37 (18), 1566–1575. doi:10.1200/jco.18.01358
Osma, U., Kurtoglu, E., Eyigor, H., Yilmaz, M. D., and Aygener, N. (2015). Sensorineural hearing loss in β-thalassemia patients treated with iron chelation. Ear Nose Throat J. 94 (12), 481–485. doi:10.1177/014556131509401206
Osoba, M. Y., Rao, A. K., Agrawal, S. K., and Lalwani, A. K. (2019). Balance and gait in the elderly: a contemporary review. Laryngoscope Investig. Otolaryngol. 4 (1), 143–153. doi:10.1002/lio2.252
Quesnel, A. M., Seton, M., Merchant, S. N., Halpin, C., and McKenna, M. J. (2012). Third-generation bisphosphonates for treatment of sensorineural hearing loss in otosclerosis. Otol. Neurotol. 33 (8), 1308–1314. doi:10.1097/MAO.0b013e318268d1b3
Rizk, H. G., Lee, J. A., Liu, Y. F., Endriukaitis, L., Isaac, J. L., and Bullington, W. M. (2020a). Drug-induced ototoxicity: a comprehensive review and reference guide. Pharmacotherapy 40 (12), 1265–1275. doi:10.1002/phar.2478
Rizk, H. G., Sharon, J. D., Lee, J. A., Thomas, C., Nguyen, S. A., and Meyer, T. A. (2020b). Cross-sectional analysis of cognitive dysfunction in patients with vestibular disorders. Ear Hear 41 (4), 1020–1027. doi:10.1097/aud.0000000000000825
Romano, A., Capozza, M. A., Mastrangelo, S., Maurizi, P., Triarico, S., Rolesi, R., et al. (2020). Assessment and management of platinum-related ototoxicity in children treated for cancer. Cancers (Basel) 12 (5), 1266. doi:10.3390/cancers12051266
Sakaeda, T., Tamon, A., Kadoyama, K., and Okuno, Y. (2013). Data mining of the public version of the FDA adverse event reporting system. Int. J. Med. Sci. 10 (7), 796–803. doi:10.7150/ijms.6048
Salvi, R., Radziwon, K., Manohar, S., Auerbach, B., Ding, D., Liu, X., et al. (2021). Review: neural mechanisms of tinnitus and hyperacusis in acute drug-induced ototoxicity. Am. J. Audiol. 30 (3s), 901–915. doi:10.1044/2020_aja-20-00023
Schreiber, J. E., Gurney, J. G., Palmer, S. L., Bass, J. K., Wang, M., Chen, S., et al. (2014). Examination of risk factors for intellectual and academic outcomes following treatment for pediatric medulloblastoma. Neuro Oncol. 16 (8), 1129–1136. doi:10.1093/neuonc/nou006
Shen, J., Yang, J., and Zhao, B. (2019). A survey of the fDA's adverse event reporting system database concerning urogenital tract infections and sodium glucose Cotransporter-2 inhibitor use. Diabetes Ther. 10 (3), 1043–1050. doi:10.1007/s13300-019-0611-9
Tanoshima, R., Khan, A., Biala, A. K., Trueman, J. N., Drögemöller, B. I., Wright, G. E. B., et al. (2019). Analyses of adverse drug reactions-nationwide active surveillance network: canadian pharmacogenomics network for drug safety database. J. Clin. Pharmacol. 59 (3), 356–363. doi:10.1002/jcph.1336
van Puijenbroek, E. P., Bate, A., Leufkens, H. G., Lindquist, M., Orre, R., and Egberts, A. C. (2002). A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 11 (1), 3–10. doi:10.1002/pds.668
Wu, Y., Xiao, W., Tong, W., Borlak, J., and Chen, M. (2022). A systematic comparison of hepatobiliary adverse drug reactions in FDA and EMA drug labeling reveals discrepancies. Drug Discov. Today 27 (1), 337–346. doi:10.1016/j.drudis.2021.09.009
Keywords: drug-associated hearing impairment, children, disproportionality, FAERS, pharmacovigilance
Citation: Liu J, Tan J, Xiao Q, Bai Y, Chang E, Su C, Wei Y, Zhong H and Wei W (2025) Drug-associated hearing impairment in children: a disproportionality analysis of the FDA adverse event reporting system. Front. Pharmacol. 16:1532461. doi: 10.3389/fphar.2025.1532461
Received: 22 November 2024; Accepted: 19 June 2025;
Published: 26 June 2025.
Edited by:
Sarath Vijayakumar, Creighton University, United StatesReviewed by:
Juraj Tihányi, Slovak Medical University in Bratislava, SlovakiaJosue Eli Villegas-Dominguez, Universidad Veracruzana, Mexico
Copyright © 2025 Liu, Tan, Xiao, Bai, Chang, Su, Wei, Zhong and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Wei, d2Vpd2VpZXJpYzA0MDVAMTYzLmNvbQ==
 Jinfeng Liu1
Jinfeng Liu1 Yuxun Wei
Yuxun Wei Hu Zhong
Hu Zhong Wei Wei
Wei Wei