- Department of Oncology, Beijing Ditan Hospital, Capital Medical University, Beijing, China
Objective: This study attempted to comprehensively assess the clinical outcomes of cases with progressive HCC (pHCC) undergoing treatment with TKI and ICI in conjunction with TACE, as compared to the combination of TKI with TACE alone.
Methods: From March 2019 to January 2022, this cohort comprised 82 cases who received TACE in conjunction with TKI and 52 cases who were treated with TACE plus TKI alone. The propensity scores was used to mitigate selection bias.
Results: The multivariate analysis further reinforced that liver cirrhosis (HR = 1.233, 95% CI: 1.024–1.484, P = 0.027), tumor diameter (HR = 1.283, 95% CI: 1.086–1.515, P = 0.003), and the treatment strategy (HR = 0.495, 95% CI: 0.264–0.793, P = 0.000) were independently linked to OS, underscoring their prognostic relevance.
Conclusion: Incorporating TACE, TKI, and ICI remarkably enhanced both PFS and OS relative to TACE with TKI alone, positioning it as a more efficacious first-line therapeutic strategy for unresectable HCC, while maintaining an acceptable safety profile in clinical settings.
Introduction
Hepatocellular carcinoma (HCC) represents a highly aggressive neoplasm, with profoundly elevated rates of both morbidity and mortality (Freddie et al., 2024; Llovet et al., 2022). The predominant etiological factors underlying the development of HCC are chronic viral hepatitis and the inexorable progression of liver cirrhosis (Daniel et al., 2023). Alarmingly, more than 80% of HCC cases are diagnosed at intermediate or advanced stages, where curative options are limited (Henrik et al., 2020; Ju Dong et al., 2019). Transarterial chemoembolization (TACE) is regarded as the standard therapeutic modality for intermediate HCC and remains the most extensively utilized intervention for cases with intermediate-to-advanced disease (Jean-Luc et al., 2019; European Association for the Study of the Liver, 2018).
Notably, the advent of targeted pharmacotherapies, involving sorafenib, donafenib, and Lenvatinib (LEN)—alongside immunotherapeutic strategies, has regarded as first-line approaches for advanced HCC (Zhou et al., 2023; Benson et al., 2021). The combinatorial regimen of atezolizumab and bevacizumab has been substantiated through clinical evidence as both efficacious and well-tolerated in advanced cases (Finn et al., 2020). Moreover, the synergistic integration of immune-based therapies with TACE holds promise for further clinical benefit, as TACE potentiates the release of tumor antigens and enhances immune recognition (Masatoshi et al., 2023).
Given the remarkable tumor burden, the presence of portal vein tumor thrombus (PVTT), and the frequent occurrence of impaired hepatic function in unresectable HCC cases, monotherapeutic approaches mainly demonstrate suboptimal efficacy. In this context, it has been demonstrated that the combination of LEN and TACE significantly augmented survival outcomes in HCC cases (Jun-Ning et al., 2023), Consequently, the therapeutic regimen of TACE, a tyrosine kinase inhibitor (TKI), and an immune checkpoint inhibitor (ICI) has proven to be as a potentially advantageous strategy. Nevertheless, the clinical efficacy of this combined modality has not yet been documented.
Therefore, predicated upon the hypothesized synergistic mechanisms of action, in the present investigation, it was attempted to figure out the clinical outcomes of cases with progressive HCC (pHCC) undergoing treatment with TKI and ICI in conjunction with TACE, as compared to the combination of TKI with TACE alone.
Materials and methods
Patients
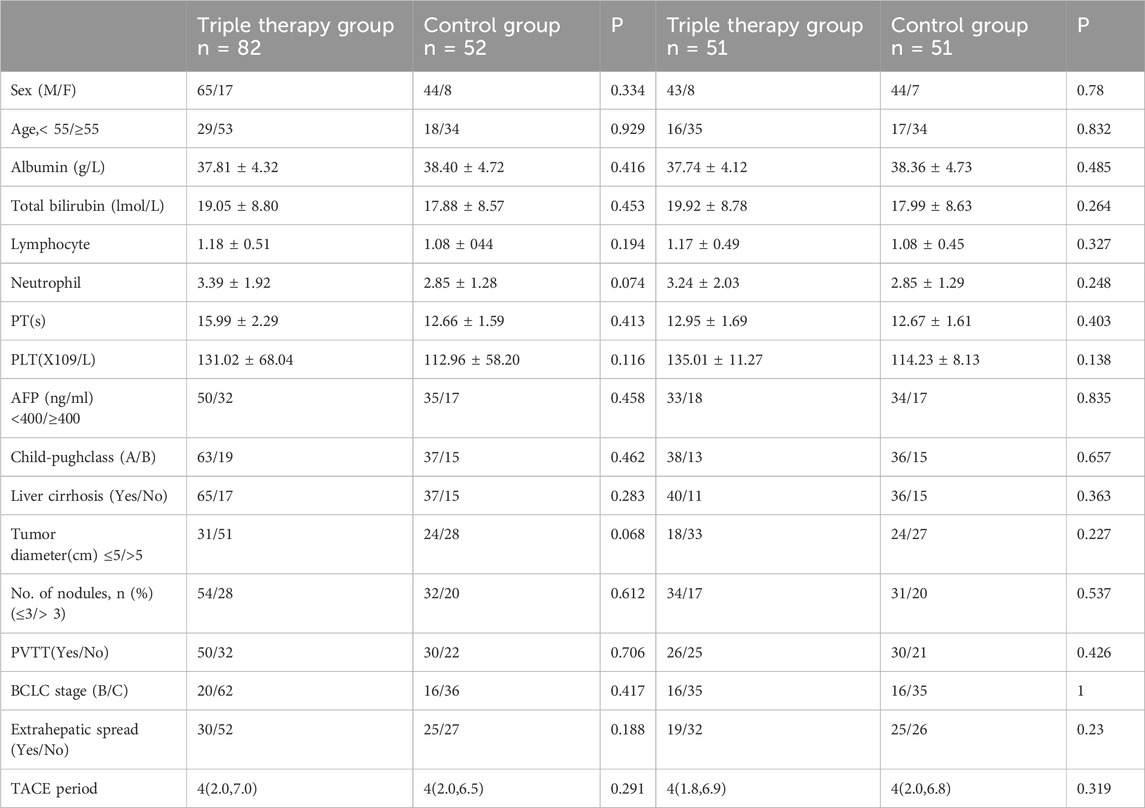
Clinical data were precisely gathered from a cohort of 134 HCC cases who underwent treatment at Beijing Ditan Hospital, Capital Medical University, between March 2019 and January 2022. This cohort comprised 82 cases who received TACE in conjunction with TKI and 52 cases who were treated with TACE plus TKI alone. The study protocol received ethical approval from the relevant Institutional Ethics Committee and was implemented in accordance with the principles articulated in the Declaration of Helsinki. Informed consent was duly attained from all participants for the utilization of their clinical data in this investigation.
The inclusion criteria for this investigation were summarized as follows: 1) cases must receive either TACE plus TKI or TACE plus TKI with ICI as their first-line therapeutic intervention; 2) liver function prior to treatment was required to be classified as Child-Pugh Class A or Class B; 3) cases must be at Barcelona Clinic Liver Cancer (BCLC) stage B or C; 4) a complete absence of other malignancies was mandated; 5) participants were required to be between the ages of 18 and 80 years; 6) an Eastern Cooperative Oncology Group Performance Status (ECOG PS) score ranging from 0 to 1 was necessary; and 7) the necessity of at least one target lesion with a measurable diameter of ≥1 cm.
Conversely, the exclusion criteria were delineated as follows: 1) cases with prior treatment histories for HCC, including whereas not limited to surgical intervention, ablation, or radiotherapy; 2) cases exhibiting coagulation disorders; 3) cases with a concurrent diagnosis of other malignancies; and 4) those who experienced gastric or esophageal variceal hemorrhage within the preceding month.
Clinical parameters and laboratory results
Peripheral blood samples were attained from cases between 7:30 and 9:30 AM, precisely 1 week prior to the commencement of combination therapy. A comprehensive array of clinical, laboratory, and radiological data was systematically extracted from medical record systems. This dataset involved variables, comprising age, gender, ECOG PS score, BCLC stage, Child-Pugh classification, alpha-fetoprotein levels, tumor distribution, size, multiplicity, presence of liver cirrhosis, vascular invasion, extrahepatic metastasis, as well as various hematological and biochemical indices.
Treatment
TACE procedure
All cases underwent synchronous TACE, the methodology of which has been elucidated in prior investigations (Yanjun et al., 2019). In brief, the TACE procedure was implemented by adept interventional radiologists, who delivered iodine oil (5–10 mL) in conjunction with one or more chemotherapeutic agents, involving pirarubicin, hydroxycamptothecin, or lobaplatin. Subsequently, embolization was executed utilizing embolic materials, involving gelatin sponge particles or polyvinyl alcohol particles, until complete stasis in the tumor-feeding vasculature was attained.
Administration of TKI and ICI
TKIs and ICIs were strategically withheld for a period of 5 days preceding and following the TACE intervention. The PD-1 inhibitors—specifically camrelizumab (200 mg), tislelizumab (200 mg), toripalimab (200 mg), or sintilimab (200 mg)—were administered via intravenous infusion once every 3 weeks.
In accordance with the physician’s assessment regarding drug tolerance, particularly in the context of advanced age, non-Child-Pugh A classification, diminished body weight, the presence of ascitic fluid, and gastrointestinal varices with a potential for hemorrhage, the oral TKI regimens included sorafenib (400 mg daily or 400 mg twice daily), LEN (8 mg or 4 mg daily), or apatinib (0.25 g daily), all delivered in 21-day cycles.
It was attempted to precisely evaluate adverse events (AEs), employing the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03(UMLS metathesaurus - NCI_CTCAE, 2020). The modification or cessation of TKI or ICI therapies was executed under physicians’ vigilant guidance. Furthermore, all cases with active hepatitis B virus (HBV) infection were provided with oral antiviral therapy as a standard precaution.
Follow-up and assessments
The initial diagnosis of HCC was on the basis of the criteria released by the American Association for the Study of Liver Diseases (Bruix and Sherman, 2011). Tumor evaluation was systematically undertaken every 4–6 weeks, with therapeutic responses assessed through triphasic scanning techniques, MRI, or CT, in accordance with the modified Response Evaluation Criteria in Solid Tumors (mRECIST) (Yu et al., 2022). The primary endpoints encompassed overall survival (OS) and progression-free survival (PFS). Definition of OS included the duration from the first administration of combination treatment to the date of mortality from any cause or to the last follow-up (July 31, 2022). PFS was characterized as the interval from the initial use of combination therapy to the occurrence of tumor progression or mortality. The tumor progression or unacceptable adverse reactions, the patient received second-line treatment. The second-line therapy included other targeted drug, regorafenib, or radiotherapy, or HAIC (Hepatic Artery Infusion Chemotherapy).
Statistical analysis
Propensity scores were computed to facilitate patient matching, employing a caliper of 0.02 in a 1:1 ratio utilizing a logistic regression model. The propensity scores used the following variables: age, gender, BCLC stage, Child-Pugh classification, alpha-fetoprotein levels, tumor distribution, size, presence of liver cirrhosis, vascular invasion, extrahepatic metastasis. Data analysis was implemented through SPSS 22.0 software that was developed by IBM Corp. It was attempted to plot Kaplan–Meier survival curves via GraphPad Prism 7.0 software that was developed by GraphPad Software Inc. Comparisons particularly between the two principal groups were executed using Student’s t-test for continuous variables, while the analysis of categorical variables was undertaken through Pearson’s χ2 test with continuity correction. Survival rates were estimated at each time point through Kaplan–Meier curve analysis. For multivariate regression analysis, a Cox proportional hazards model was implemented. Definition of statistical significance was through P-value falling behind 0.05.
Results
Patients’ characteristics
In the study, a cohort of 134 HCC cases was recruited, involving 52 cases allocated to the T+T group and 82 to the T+T+I group, as detailed in Table 1. In the T+T+I cohort, all cases received TKI, comprising LEN (n = 70), sorafenib (n = 8), and apatinib (n = 4), in conjunction with a programmed cell death-1 (PD-1) inhibitor: sintilimab (n = 45), camrelizumab (n = 31), toripalimab (n = 4), and tislelizumab (n = 2). Conversely, in the T+T cohort, 28 cases were administered LEN, while the remaining 24 received sorafenib. Post-propensity score matching (PSM), 102 cases were incorporated into the balanced cohort, involving 51 cases per group, ensuring comparable baseline characteristics between the two groups (Table 1). Regarding metastatic dissemination, the T+T+I group exhibited pulmonary metastases in 9 cases, lymph node metastases in 15, bone metastases in 8, and other forms of metastasis in 17 cases. Meanwhile, the T+T group demonstrated pulmonary metastases in 11 cases, lymph node involvement in 9, bone metastases in 3, and other metastatic manifestations in 9 cases (Table 2).
Efficacy
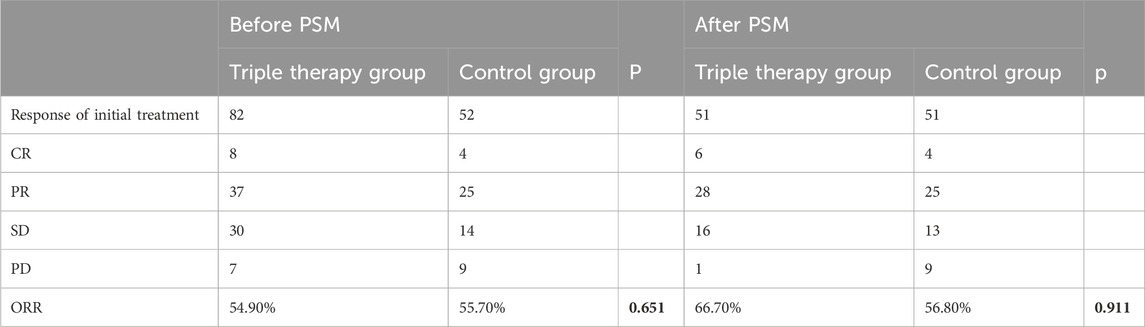
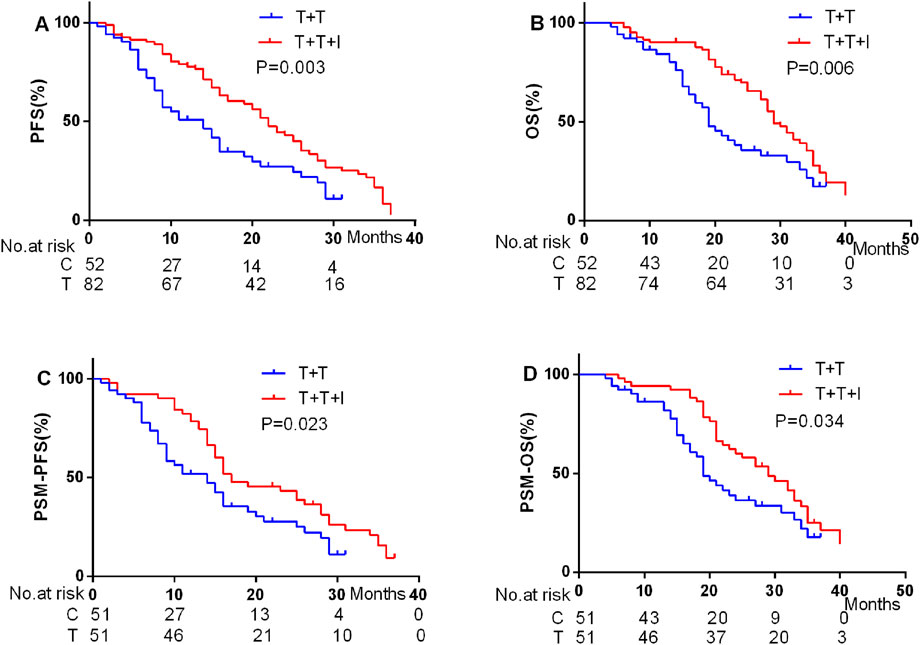
It was attempted to implement a comparative assessment of the best overall response between the two cohorts. According to the modified RECIST (mRECIST) criteria, the overall response rate (ORR) was notably higher particularly in the T+T+I group, recorded at 54.90% prior to PSM and elevated to 66.70% post-PSM. In contrast, the ORR in the T+T group was 55.70% prior to PSM and 57.80% following PSM, demonstrating a relatively smaller variation in response (Table 3). In terms of survival outcomes, prior to PSM, the median PFS (mPFS) and median OS (mOS) were 20 and 29 months, respectively, in the T+T+I group, relative to 12 and 19 months in the T+T group (P = 0.003 for mPFS; P = 0.006 for mOS) (Figures 1A, B). Following PSM, the T+T+I group continued to demonstrate superior survival outcomes, with mPFS and mOS of 17 and 28 months, respectively, relative to 12 and 19 months in the T+T group (P = 0.023; P = 0.034) (Figures 1C, D).
Analysis of prognostic factors
To further elucidate the independent determinants influencing OS and PFS, it was attempted to execute univariate and multivariate Cox proportional hazards regression analyses following PSM (Table 4). The multivariate analysis unveiled that BCLC stage (HR = 1.940, 95% CI: 1.560–2.413, P = 0.000) and the therapeutic regimen (HR = 0.67, 95% CI: 0.291–0.962, P = 0.000) emerged as significant independent prognostic factors for PFS.
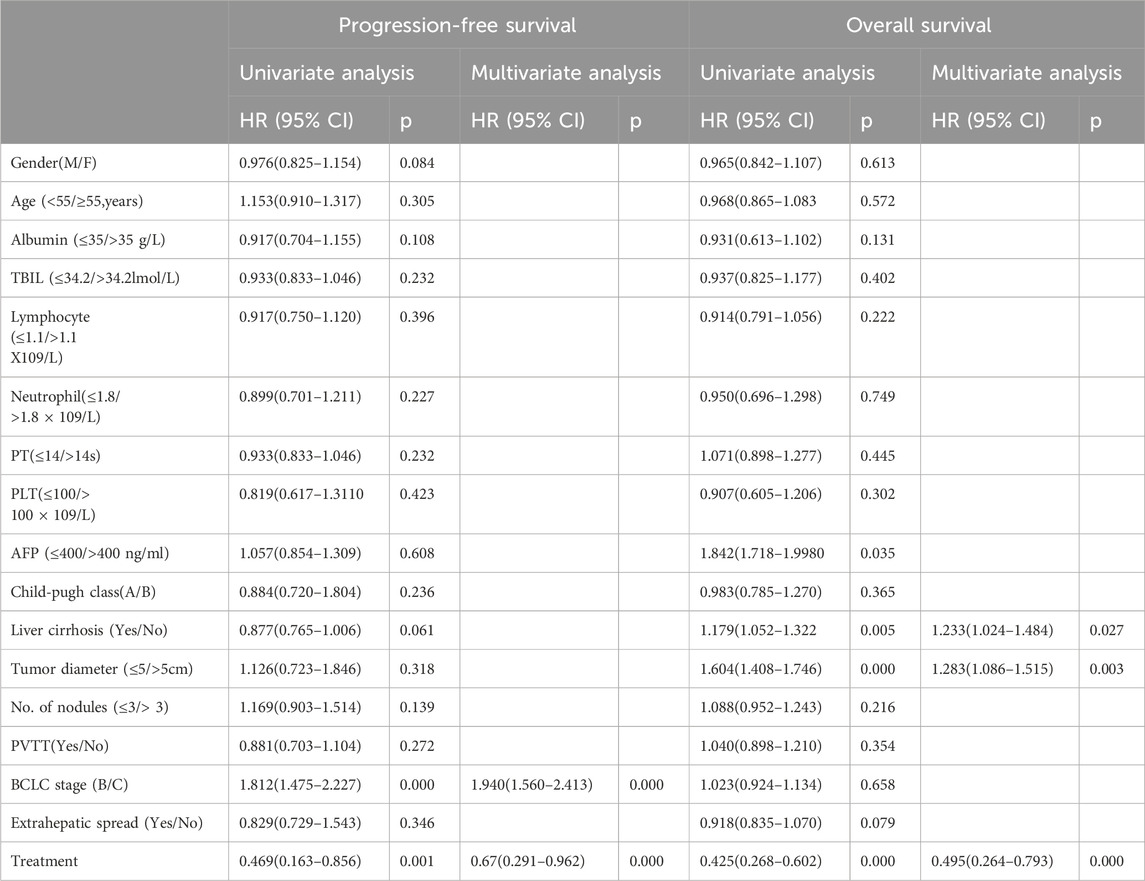
Table 4. Cox proportional hazards univariate and multivariate analysis for PFS and OS in the matched cohort.
Moreover, the multivariate analysis further reinforced that liver cirrhosis (HR = 1.233, 95% CI: 1.024–1.484, P = 0.027), tumor diameter (HR = 1.283, 95% CI: 1.086–1.515, P = 0.003), and the treatment strategy (HR = 0.495, 95% CI: 0.264–0.793, P = 0.000) were independently linked to OS, underscoring their prognostic relevance.
Safety analysis
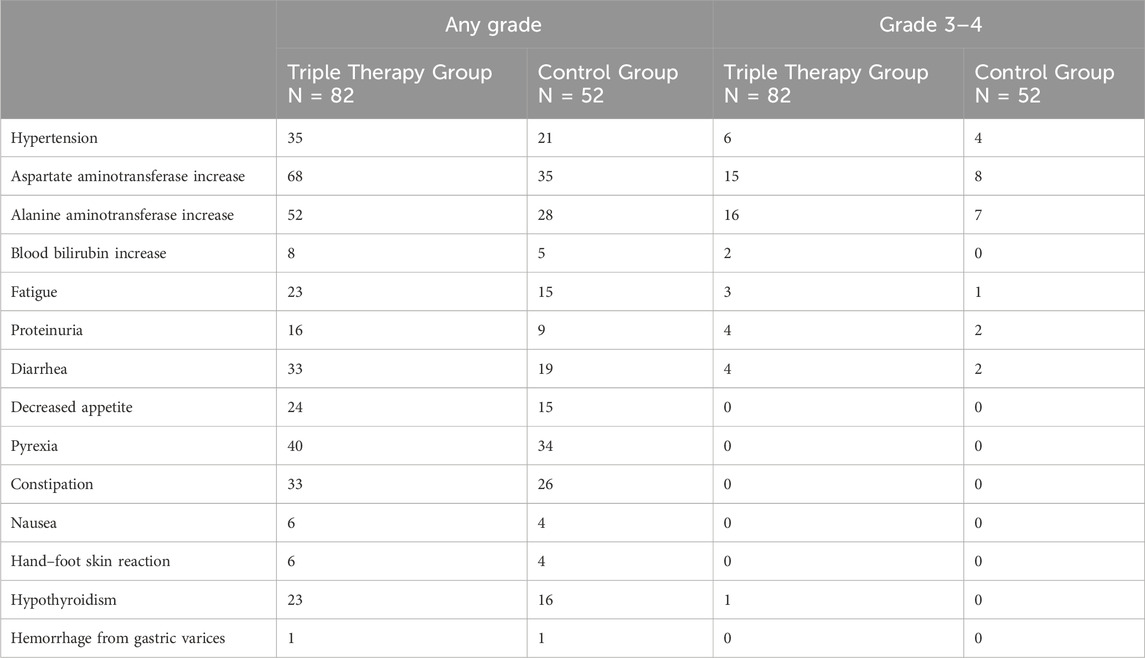
Before PSM, the incidence of treatment-related AEs between the two principal groups is summarized in Table 5. Importantly, no treatment-related mortalities were reported in either cohort. The most prevalent treatment-related AEs of any grade included an increase in aspartate aminotransferase (AST) (n = 68, 82.92% in the T+T+I group; n = 35, 67.31% in the T+T group), an increase in alanine aminotransferase (ALT) (n = 52, 63.41% in the T+T+I group; n = 28, 53.84% in the T+T group), pyrexia (n = 40, 48.78% in the T+T+I group; n = 34, 65.38% in the T+T group), and hypertension (n = 35, 42.68% in the T+T+I group; n = 21, 40.38% in the T+T group). Regarding grade 3 treatment-related AEs, elevated ALT level was observed in 19.51% (n = 16) of patients in the T+T+I group and 13.46% (n = 7) in the T+T group, while elevated AST level was noteworthy in 18.29% (n = 15) and 15.38% (n = 8) of patients in the respective groups. These AEs were transient in nature, generally resolving in a short timeframe following the cessation or adjustment of treatment.
Discussion
HCC is an aggressive malignancy characterized by a high propensity for recurrence and prompt disease progression (Freddie et al., 2024). As the current standard of care for unresectable HCC (uHCC), TACE has a noticeable function in managing this hypervascular tumor. HCC typically exhibits remarkable arterial phase enhancement and rapid contrast washout during the portal phase on imaging, reflecting its rich blood supply (Atsushi et al., 2017). TACE exerts its therapeutic effects by inducing ischemic necrosis through selective occlusion of the tumor’s arterial blood flow. This method not only disrupts the tumor’s vascular supply, but also triggers the release of tumor-associated antigens following tumor cell destruction, contributing to the cytotoxic effect (Ren et al., 2021; Jenny et al., 2023). However, distant metastases typically remain unaffected by localized interventions, necessitating the exploration of combined locoregional and systemic therapeutic strategies to improve outcomes in HCC management.
ICIs, targeting the PD-1/PD-L1 signaling pathway, represent an evolving Frontier in cancer therapy (Liu et al., 2024). ICIs facilitate an enhanced infiltration of CD4+ and CD8+ T cells in the tumor microenvironment, contributing to an amplified immune response (Kikuchi et al., 2022). CD8+ can completely wipe out malignancy cells by means of cell lysis and apoptosis (Jin et al., 2017). The antitumor function of CD8 + T cell recovered from PD-1 blockade (Se et al., 2016; Yu et al., 2024). The TACE treatment may enhance the number and function of CD8+ cells in HCC patients (Jingjing et al., 2021; Alessandro et al., 2006).
Despite the potential of immunotherapy, its efficacy is often compromised by the formation of an immunosuppressive microenvironment dominated by regulatory T (Treg) cells, which blunt anti-tumor immunity. TACE may potentiate the immune response by depleting Treg cells and modulating their immunosuppressive activities, thereby synergizing with immune checkpoint blockade (Ren et al., 2021). The targeted depletion of Tregs along with modulation of their functional activity through TACE can potentiate a more robust and efficacious anti-tumor immune response (Yuan et al., 2024). The combination of TACE with PD-1 inhibitors has exhibited to be not only safe, but also capable of significantly delaying tumor progression and reducing disease stage in carefully selected patients (Huang et al., 2024). Moreover, targeted therapies that inhibit FGF receptor 4 (FGFR4) can enhance the effectiveness of anti-PD-1 therapy by reducing PD-L1 expression and impeding Treg differentiation, further augmenting the anti-tumor immune response (Yi et al., 2021).
HCC could be treated by adjusting the proportion of the Kupffer macrophage, the treatment of TACE could decrease the expression of CYP3A4 with HepG2 HCC cells (Alican et al., 2023). There were Kupffer cells (KCs) expressed high levels of B7-H1 and CD8+ T cells with high expression of PD-1 in the HCC stroma. Inhibitory B7-H1/PD-1 interaction could improve the effector function of T cells (Ke et al., 2009).
In this investigation, the T+T+I treatment regimen demonstrated a substantial survival benefit relative to the T+T regimen, both before and after PSM. Post-PSM analysis unveiled that the T+T+I group achieved a mPFS of 17 months and a mOS of 28 months, relative to 12 months (mPFS) and 19 months (mOS) in the T+T group (P = 0.023 for mPFS; P = 0.034 for mOS). Furthermore, multivariate analysis identified the T+T+I treatment as an independent prognostic factor for prolonged PFS and OS, emphasizing its superior efficacy. Consequently, the findings revealed that the T+T+I regimen provided a significant therapeutic advantage over T+T as a first-line treatment option for unresectable HCC cases.
In the CHANCE2211 study, a cohort of 26 cases with intermediate-to-advanced HCC who underwent TACE plus camrelizumab and apatinib demonstrated markedly prolonged median PFS (13.5 vs. 7.7 months) and median OS (24.1 vs. 15.7 months) relative to those treated with monotherapy (Jin et al., 2023). Dawood et al. (2024) corroborated these findings, reporting that the tri-modal approach integrating ICIs, TKIs, and TACE conferred superior tumor response rates and enhanced survival outcomes in HCC cases, paralleling the outcomes of this investigation.
Nonetheless, this investigation is not without limitations. First, although PSM was employed to mitigate potential confounding variables, the inherently retrospective design might introduce the possibility of residual selection bias, which could not be entirely eliminated. Therefore, in the next study, all available covariates should be performed more sample heterogeneity analysis via performing PCA analysis. The lasso regression method should be used to reduce the multicollinearity between covariables. In the future, external databases should be used to support research. In addition, the subsequent treatment methods may affect the patients’ prognosis. This study only utilized PD-1 inhibitors and did not employ other immune checkpoint inhibitors. The enrolled patients lacked PD-1 expression. Thus, the prospective clinical studies will further explore the efficacy of targeted drugs combined with other immunosuppressants, such as PD-1, PD-L1 and CTLA-4. The expression of PD-1 in the patients who participated in the study was followed by PD-1 staining of the pathological sections of the patients and IHC scoring. Finally, differences in various TKIs and ICIs used may potentially confound the results. According to the findings so far, the future prospective studies may further explore a target combined with a PD-1 combined with TACE to corroborate the findings. Thus, precisely designed prospective trials are imperative to robustly validate these preliminary conclusions.
AEs reported in this investigation were largely manageable, with no instances of fatal outcomes, and the incidence rates of AEs aligned with those previously documented (Deng et al., 2024.). Notably, the data suggests that the T+T+I regimen did not exacerbate the risk of TRAEs relative to the T+T approach.
Conclusion
In conclusion, incorporating TACE, TKI, and ICI remarkably enhanced both PFS and OS relative to TACE with TKI alone, positioning it as a more efficacious first-line therapeutic strategy for unresectable HCC, while maintaining an acceptable safety profile in clinical settings.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YS: Conceptualization, Investigation, Software, Writing – original draft, Writing – review & editing. YX: Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing. YT: Formal Analysis, Project administration, Validation, Writing – original draft, Writing – review & editing. XD: Funding acquisition, Resources, Visualization, Writing – original draft, Writing – review & editing. Jinglong Chen: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The current investigation was funded by the Capital Health Development Scientific Research Project (Shou fa 2022-2-2175).
Acknowledgments
We express our profound gratitude to the dedicated researchers and participants whose invaluable contributions were integral to the success of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alessandro, Z., Pilli, M., Penna, A., Pelosi, G., Schianchi, C., Molinari, A., et al. (2006). Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 66 (2), 1139–1146. doi:10.1158/0008-5472.CAN-05-2244
Alican, Ö., Stolley, D. L., Cressman, E. N. K., McMillin, M., Yankeelov, T. E., and Rylander, M. N. (2023). Vascularized hepatocellular carcinoma on a chip to control chemoresistance through cirrhosis, inflammation and metabolic activity. Small Struct. 4 (9), 2200403. doi:10.1002/sstr.202200403
Atsushi, H., Kumada, T., Kudo, M., Hirooka, M., Koizumi, Y., Hiasa, Y., et al. (2017). Hepatic function during repeated TACE procedures and prognosis after introducing sorafenib in patients with unresectable hepatocellular carcinoma: multicenter analysis. Dig. Dis. 35 (6), 602–610. doi:10.1159/000480256
Benson, A. B., D’Angelica, M. I., Abbott, D. E., Anaya, D. A., Anders, R., Are, C., et al. (2021). Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc Netw. 19 (5), 541–565. doi:10.6004/jnccn.2021.0022
Bruix, J., and Sherman, M.American Association for the Study of Liver Diseases (2011). Management of hepatocellular carcinoma: an update. Hepatology 53, 1020–1022. doi:10.1002/hep.24199
Daniel, Q. H., Singal, A. G., Kanwal, F., Lampertico, P., Buti, M., Sirlin, C. B., et al. (2023). Hepatocellular carcinoma surveillance — utilization, barriers and the impact of changing aetiology. Nat. Rev. Gastroenterol. Hepatol. 20 (12), 797–809. doi:10.1038/s41575-023-00818-8
Dawood, Z. S., Brown, Z. J., Alaimo, L., Lima, H. A., Shaikh, C., Katayama, E. S., et al. (2024). Comparison of tumor response and outcomes of patients with hepatocellular carcinoma after multimodal treatment including immune checkpoint inhibitors - a systematic review and meta-analysis. HPB Oxf. 26 (5), 618–629. doi:10.1016/j.hpb.2024.02.003
Deng, L., Sun, Y., Wang, H., Liao, C., Li, D., Xu, G., et al. (2024). Efficacy and safety of transarterial chemoembolization plus donafenib with or without immune checkpoint inhibitors as the first-line treatment for unresectable hepatocellular carcinoma: a propensity score matching analysis. J. Hepatocell. Carcinoma 11, 29–38. doi:10.2147/JHC.S443779
European Association for the Study of the Liver (2018). EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 69 (1), 182–236. doi:10.1016/j.jhep.2018.03.019
Finn, R. S., Qin, S., Ikeda, M., Galle, P. R., Ducreux, M., Kim, T. Y., et al. (2020). Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382 (20), 1894–1905. doi:10.1056/NEJMoa1915745
Freddie, B., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., et al. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74 (3), 229–263. doi:10.3322/caac.21834
Henrik, P., Fritsch, R., Guckenberger, M., De Oliveira, M. L., Dutkowski, P., and Clavien, P. A. (2020). Modern therapeutic approaches for the treatment of malignant liver tumours. Nat. Rev. Gastroenterol. Hepatol. 17 (12), 755–772. doi:10.1038/s41575-020-0314-8
Huang, H., Liao, W., Zhang, K., Wang, H., Cheng, Q., and Mei, B. (2024). Adjuvant transarterial chemoembolization plus immunotherapy for huge hepatocellular carcinoma: a propensity score matching cohort study. J. Hepatocell. Carcinoma 8 (11), 721–735. doi:10.2147/JHC.S455878
Jean-Luc, R., Forner, A., Bolondi, L., Cheung, T. T., Kloeckner, R., and de Baere, T. (2019). Updated use of TACE for hepatocellular carcinoma treatment: how and when to use it based on clinical evidence. Cancer Treat. Rev. 72, 28–36. doi:10.1016/j.ctrv.2018.11.002
Jenny, S., Laureano, R. S., Vanmeerbeek, I., Govaerts, J., Naulaerts, S., Borras, D. M., et al. (2023). Trial watch: chemotherapy-induced immunogenic cell death in oncology. Oncoimmunology 12 (1), 2219591. doi:10.1080/2162402X.2023.2219591
Jin, L., Liao, R., Wang, G., Yang, B. H., Luo, X., Varki, N. M., et al. (2017). Preventive inhibition of liver tumorigenesis by systemic activation of innate immune functions. Cell Rep. 21 (7), 1870–1882. doi:10.1016/j.celrep.2017.10.064
Jin, Z.-C., Zhong, B.-Y., Chen, J.-J., Zhu, H. D., Sun, J. H., Yin, G. W., et al. (2023). Real-world efficacy and safety of TACE plus camrelizumab and apatinib in patients with HCC (CHANCE2211): a propensity score matching study. Eur. Radiol. 33 (12), 8669–8681. doi:10.1007/s00330-023-09754-2
Jingjing, G., Wang, S., Han, Y., Jia, Z., and Wang, R. (2021). Effects of transarterial chemoembolization on the immunological function of patients with hepatocellular carcinoma. Oncol. Lett. 22 (1), 554. doi:10.3892/ol.2021.12815
Ju Dong, Y., Hainaut, P., Gores, G. J., Amadou, A., Plymoth, A., and Roberts, L. R. (2019). A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 16 (10), 589–604. doi:10.1038/s41575-019-0186-y
Jun-Ning, L., Ji-Jiang, Li, Shu, Y., Zhang, G. N., and Yi, P. S. (2023). Transarterial chemoembolization combined with lenvatinib versus transarterial chemoembolization combined with sorafenib for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Front. Oncol. 13 (0), 1074793. doi:10.3389/fonc.2023.1074793
Ke, Wu, Kryczek, I., Chen, L., Zou, W., and Welling, T. H. (2009). Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 69 (20), 8067–8075. doi:10.1158/0008-5472.CAN-09-0901
Kikuchi, H., Matsui, A., Morita, S., Amoozgar, Z., Inoue, K., Ruan, Z., et al. (2022). Increased CD8+ T-cell infiltration and efficacy for multikinase inhibitors after PD-1 blockade in hepatocellular carcinoma. J. Natl. Cancer Inst. 114 (9), 1301–1305. doi:10.1093/jnci/djac051
Liu, Y., Yang, H., Li, T., and Zhang, N. (2024). Immunotherapy in liver cancer: overcoming the tolerogenic liver microenvironment. Front. Immunol. 15, 1460282. doi:10.3389/fimmu.2024.1460282
Llovet, J. M., Castet, F., Heikenwalder, M., Maini, M. K., Mazzaferro, V., Pinato, D. J., et al. (2022). Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 19 (3), 151–172. doi:10.1038/s41571-021-00573-2
Masatoshi, K., Tomoko, A., Kazuomi, U., Tsuchiya, K., Morita, M., Chishina, H., et al. (2023). Achievement of complete response and drug-free Status by atezolizumab plus bevacizumab combined with or without curative conversion in patients with transarterial chemoembolization-unsuitable, intermediate-stage hepatocellular carcinoma: a multicenter proof-of-concept study. Liver Cancer 12 (4), 321–338. doi:10.1159/000529574
Ren, Z., Yue, Y., Zhang, Y., Dong, J., Liu, Y., Yang, X., et al. (2021). Changes in the peripheral blood treg cell proportion in hepatocellular carcinoma patients after transarterial chemoembolization with microparticles. Front. Immunol. 12, 624789. doi:10.3389/fimmu.2021.624789
Se, J.Im, Hashimoto, M., Gerner, M. Y., Lee, J., Kissick, H. T., Burger, M. C., et al. (2016). Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537 (7620), 417–421. doi:10.1038/nature19330
UMLS metathesaurus - NCI_CTCAE (2020). (Common Terminology criteria for adverse events 4.3 subset) - synopsis. Available online at: https://www.nlm.nih.gov/research/umls/sourcereleasedocs/current/NCI_CTCAE/index.html (Accessed February 14, 2020).
Yanjun, S., Wang, H., Chen, X., Li, W., and Chen, J. (2019). Prognostic significance of lymphocyte-to-monocyte ratio and platelet-to-lymphocyte ratio in patients with hepatocellular carcinoma undergoing transcatheter arterial chemoembolization and radiofrequency ablation. Onco Targets Ther. 12, 7129–7137. doi:10.2147/OTT.S217935
Yi, C., Chen, L., Lin, Z., Liu, L., Shao, W., Zhang, R., et al. (2021). Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology 74 (5), 2544–2560. doi:10.1002/hep.31921
Yu, H., Bai, Y., Xie, X., Feng, Y., Yang, Y., and Zhu, Q. (2022). RECIST 1.1 versus mRECIST for assessment of tumour response to molecular targeted therapies and disease outcomes in patients with hepatocellular carcinoma: a systematic review and meta-analysis. BMJ Open 12 (6), e052294. doi:10.1136/bmjopen-2021-052294
Yu, P., Shan, J., Qin, H., Li, F., Qu, J., Guo, R., et al. (2024). PD-1 signaling limits expression of phospholipid phosphatase 1 and promotes intratumoral CD8+ T cell ferroptosis. Immunity 57 (9), 2122–2139.e9. doi:10.1016/j.immuni.2024.08.003
Yuan, G., Li, R.-C., Xia, W.-Li, Yang, X., Zhu, W. B., Li, F. T., et al. (2024). Immune effect and prognosis of transcatheter arterial chemoembolization and tyrosine kinase inhibitors therapy in patients with hepatocellular carcinoma. World J. Gastrointest. Oncol. 16 (7), 3256–3269. doi:10.4251/wjgo.v16.i7.3256
Keywords: hepatocellular carcinoma, transarterial chemoembolization, tyrosine kinase inhibitor, immune checkpoint inhibitor, propensity scores
Citation: Shen Y, Xu Y, Teng Y, Ding X and Chen J (2025) First-line treatment of hepatocellular carcinoma: a propensity-matched analysis of tyrosine kinase inhibitors combined with TACE, with or without PD-1 inhibitors. Front. Pharmacol. 16:1533471. doi: 10.3389/fphar.2025.1533471
Received: 24 November 2024; Accepted: 24 April 2025;
Published: 13 May 2025.
Edited by:
Ian James Martins, University of Western Australia, AustraliaReviewed by:
Tongyi Huang, The First Affiliated Hospital of Sun Yat-sen University, ChinaXiujuan Chang, Fifth Medical Center of the PLA General Hospital, China
Copyright © 2025 Shen, Xu, Teng, Ding and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinglong Chen, ZHR6bGtAc2luYS5jb20=
 Yanjun Shen
Yanjun Shen Yawen Xu
Yawen Xu Ying Teng
Ying Teng Xiaoyan Ding
Xiaoyan Ding Jinglong Chen
Jinglong Chen