Abstract
Background:
Cymbopogon is used in traditional medicine in tropical and subtropical regions to treat various ailments. However, no research has yet provided a detailed pharmacodynamic explanation for its antidiarrheal and antispasmodic effects. This study aimed to evaluate the antidiarrheal and antispasmodic properties of the essential oil of Cymbopogon proximus (EOCP), with the goal of scientifically validating its traditional use in folk medicine.
Methods:
An animal study of antidiarrheal activity was carried out using a castor oil-induced diarrhea model in rats, and the isolated small intestine of rats was used to investigate the specific mechanisms of the antispasmodic effects.
Results:
The EOCP, exhibited a protective effect against castor oil-induced diarrhea in rats at 100 and 200 mg/kg, similar to the standard drug, dicyclomine. In rat ileum preparations, EOCP decreased the basal tonus with a maximal response of (Rmax; % of ACh.-contraction) of 18.5% ± 1.5% compared with 17.5% ± 2.5% achieved with dicyclomine. In contractions elicited by different agents, EOCP showed higher potency in relaxing carbachol (CCh)-induced contractions compared to those induced by high K+ concentrations. It showed a similar relaxation profile to dicyclomine but differed from the inhibitory effects of verapamil and/or atropine. Pre-incubation of isolated ileum with increasing doses of EOCP showed that at a lower concentration (0.03 mg/mL), EOCP induced a rightward parallel shift in carbachol (CCh) concentration-response curves. At a higher concentration (0.1 mg/mL), it caused a non-parallel shift with a reduction in the maximum response, similar to the effects of dicyclomine, a dual inhibitor of muscarinic receptors and Ca++ channels. The Ca++ channel inhibitory effect of EOCP was confirmed by its rightward shift of Ca++ concentration-response curves and suppression of the maximal response, resembling the effects of verapamil, a well-established Ca++ antagonist.
Conclusion:
These results suggest that EOCP produces antidiarrheal and antispasmodic effects, possibly mediated via direct effect on intestinal smooth musclesf followed by dual inhibition of muscarinic receptors and Ca++ channels, thus providing a pharmacological basis for its traditional use in hyperactive gut disorders such as diarrhea and spasms.
1 Introduction
Cymbopogon, known for its rich essential oil content, has long been used in traditional medicine across the tropical and subtropical regions of Asia, Africa, and the Americas (Avoseh et al., 2015). Its medicinal uses include treatment of ailments such as cough, fever, infections, cancer, and digestive disorders (Dutta et al., 2016). Cymbopogon lowers blood pressure in normotensive rats and provides protection against L-NAME-induced hypertension (El-Nezhawy et al., 2014; Althurwi et al., 2020). Laboratory and animal studies demonstrate its pharmacological benefits, including anticancer, heart-protective, cholesterol-lowering, antioxidant, anti-inflammatory, anti-diabetic, and antimicrobial effects (Mansour et al., 2002; Adeneye and Agbaje, 2007; Miguel, 2010; Selim, 2011; Ekpenyong et al., 2014).
Cymbopogon proximus (commonly known as Halfabar or Maharaib) is a highly aromatic grass commonly found in Southern Egypt and Northern Sudan. C. proximus is traditionally used as a diuretic and antispasmodic, which is attributed to its potent smooth muscle-relaxing effects (El-Askary et al., 2003). Additionally, it possesses various biological activities, including hypoglycemic, antipyretic, bronchodilatory, antibacterial, anticonvulsant, and antiemetic properties (El-Askary et al., 2003; Selim, 2011; Ibrahim and El-Khateeb, 2013; El-Nezhawy et al., 2014; Warrag et al., 2014). In our earlier conducted study on the essential oil of this plant (Althurwi et al., 2020), the detailed phytochemical GC-MS analysis revealed the presence of elemol, piperitone, α-eudesmol and β-eudesmol in as the plant major constituents. Interestingly, some of the identified components in C. proximus are known to have spasmolytic properties such as, piperitone, with previously reported findings of having concentration-dependent spasmolytic activity (Ponce-Monter et al., 2008). Moreover, β-eudesmol, found in C. Proximus essential oil, has been explored for its relaxing effect in previous studies (Morita et al., 1996). Although its antispasmodic and smooth muscle relaxant effects have been established in previous studies (Abdel-Moneim et al., 1969), no detailed pharmacodynamic explanation for its antidiarrheal and antispasmodic effects has been provided. Therefore, the current study aimed to scientifically investigate the antidiarrheal and antispasmodic properties of the essential oil of Cymbopogon proximus (EOCP), to provide a pharmacological basis for its traditional use in the treatment of hyperactive gut disorders like diarrhea and spasms. While Cymbopogon proximus has long been used in traditional medicine, no previous research has provided a clear mechanistic explanation for its effectiveness in treating hyperactive gut disorders. This study identifies EOCP’s dual inhibitory effect on muscarinic receptors and calcium channels and positions it as a natural alternative to established pharmaceuticals such as dicyclomine. The broader significance of these findings extends to the potential development of EOCP as a plant-based therapeutic for gastrointestinal conditions, offering a safer, natural remedy at a time of increasing interest in alternative and complementary medicine. Additionally, this research could open avenues for further exploration of other Cymbopogon species and their medicinal properties, thereby contributing to the advancement of ethnopharmacology and drug development from natural products.
2 Materials and methods
2.1 Plant material and extraction
Cymbopogon proximus belonging to the Poaceae family, was sourced from a local market in Alexandria, Egypt. The plant’s identity was confirmed by Prof. Saniya Kamal of the Department of Botany, College of Science, Alexandria University, Alexandria, Egypt.
2.2 Extraction of the essential oil Cymbopogon proximus
Essential oil was extracted from 250 g of dried, powdered C. proximus by hydrodistillation for 5 h of (Meyer-Warnod, 1984). The oil was then separated and dried with anhydrous sodium sulfate, giving a final yield of 5.4% w/w (Althurwi et al., 2020).
2.3 Chemicals
Acetylcholine chloride (ACh), atropine, carbachol (CCh), dicyclomine, Tween 80 and verapamil were sourced from Sigma Chemicals Co. (St. Louis, MO, United States). The physiological salt solutions were prepared using potassium chloride (Sigma Chemicals Co.), calcium chloride, magnesium chloride, magnesium sulfate, glucose, sodium bicarbonate, potassium dihydrogen phosphate, and sodium dihydrogen phosphate (Merck, Darmstadt, Germany), ethylenediaminetetraacetic acid (EDTA), and sodium chloride (BDH Laboratory Supplies, Poole, England). All chemicals used were of analytical grade. Castor oil was obtained from a local pharmacy in Al-Kharj. EOCP was administered in a volume of 10 mL/kg after dissolving in a vehicle of 1% (w/v) Tween 80 in saline in the in-vivo assay whereas for the ex-vivo studies, EOCP stock solution and dilutions were prepared in Tyrode’s solution. The prepared stock solutions of EOCP were sonicated just before use.
2.4 Animals
Male Wistar albino rats, weighing 200–250 g, were obtained from the Lab Animal Care Unit of the College of Pharmacy, Prince Sattam Bin Abdulaziz University (Al-Kharj, KSA). The animals were housed under a 12-h light/dark cycle and received food and water ad libitum. After a 24-h fasting period, the rats were humanely euthanized by a blow to the head, followed by cervical dislocation. All experiments were conducted in accordance with the guidelines set by the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council (1996). The assay protocol was approved by the Standing Committee of Bioethics Research (SCBR) at Prince Sattam Bin Abdulaziz University with reference number SCBR-394/2024.
2.5 Castor oil-induced diarrhea
Rats were subjected to 24 h fasting and kept individually in separate cages with saw dust replaced with a clean blotting sheet. They were divided into five groups, each consisting of five rats. Each rat in the first group received saline (1% Tween 80 at 10 mL/kg, orally) and was recorded as a negative control. Effective doses of plant oil were previously determined on a trial basis, with the second and third groups receiving two increasing doses of EOCP as 100 and 200 mg/kg previously dissolved in vehicle (1% Tween 80 at 10 mL/kg, orally). The fourth and fifth groups received the positive control drug, dicyclomine, at oral doses of 50 and 100 mg/kg, respectively. Each rat was carefully subjected to 10 mL/kg dose of castor oil after approximately 1 h of sample treatment. After 4 h of the castor oil dosing, the cages were subsequently monitored for loose spots of diarrhea, and their absence was recorded as a positive result, indicating protection from diarrhea (Rehman et al., 2012).
2.6 Rat ileum
The rat’s abdomen was opened, and with due care, the ileum was isolated by tracing it from the ileo-cecal junction, placed in a normal buffer (Tyrode’s solution), and mesenteries were removed (Rehman et al., 2024). A 2-cm segment of the ileum was suspended in a 10 mL tissue bath filled with Tyrode’s solution (pH 7.4), maintained at 37°C, and aerated with a mixture of 95% O2 and 5% CO2 (carbogen). The composition of the Tyrode’s solution (in mM) was as follows: NaCl 136.9, KCl 2.7, MgCl2·6H2O 0.5, NaHCO3 11.9, NaH2PO4·2H2O 0.32, CaCl2 1.8, and glucose 5.05. One end of the ileum was secured to a metal tissue hook, while the other was attached via a cotton thread to an isotonic transducer connected to an emkaBath (France) system. Tissue responses were recorded using IOX software (version 2.9.10.6, emka technologies, SAS, Paris, France). Each tissue segment was subjected to an initial load of 1 g and allowed to equilibrate for 30 min before drugs were administered. After the equilibration phase, each tissue preparation was stabilized using a submaximal concentration of ACh, (0.3 μM) at 3-min intervals, until stable responses were observed. The inhibitory effect of the test substances was evaluated against contractions induced by CCh (1 μM) and high potassium (80 mM). Carbachol, a muscarinic receptor agonist, induces strong contractions in isolated ileum tissue, and any substance that reverses these contractions is considered anti-muscarinic (Arunlakshana and Schild, 1997).
To confirm whether the inhibitory substance acts competitively or non-competitively on muscarinic receptors, control concentration-response-curves (CRCs) for CCh-were generated by incrementally increasing the CCh concentration. CRCs for CCh were repeated with increasing concentrations of the test substance (EOCP) and control drugs (dicyclomine, verapamil, and atropine), as described by Choo and Mitchelson (1978).
For determining the calcium channel-blocking signaling, high potassium (80-mM) was used to contract the tissue sample, as outlined by Farre et al. (1991). Potassium concentrations (>30 mM) have been reported to cause smooth muscle depolarization by activating membrane voltage-dependent calcium signaling channels, thus facilitating the entry of extracellular calcium and triggering contractions. A substance that inhibits these contractions is considered a blocker of calcium influx through L-type calcium channels (Godfraind et al., 1986). Once the contraction reached a plateau (usually within 7–10 min), the test material was added cumulatively to achieve concentration-dependent inhibitory effects.
To further verify the calcium antagonist activity of the test substance, the tissue was first stabilized in normal Tyrode’s solution. This was then replaced with a calcium-free Tyrode’s solution containing 0.1 mM EDTA for 30 min to remove calcium from the tissue. The solution was then exchanged for a potassium-rich, calcium-free Tyrode’s solution with the following composition (in mM): NaCl 91.03, KCl 50, MgCl2·6H2O 0.50, NaHCO3 11.9, NaH2PO4·2H2O 0.32, glucose 5.05, and EDTA-Na2·2H2O 0.1. After incubating for 30 min, control calcium concentration-response curves (CRCs) were generated. Once the calcium CRCs had stabilized (typically after two cycles), the tissue was exposed to the test compound for 1 h. Calcium CRCs were then reconstructed at varying concentrations of the test substance to evaluate their calcium antagonist effect.
2.7 Statistical analyses
Data are expressed as mean ± standard error of the mean (SEM, n = number of experiments), along with median effective concentrations (EC50) and corresponding 95% confidence intervals (CI). Statistical analysis for the antidiarrheal assay was performed using the Chi-square test, while potency of the agonist control CRCs) was compared with its potency in the presence of the different antagonist(s) using Oneway ANOVA and Dunnett’s multiple comparison test. Repeated Measures ANOVA followed by Bonferroni’s post-test for comparisons of CRCs of Ca++ with their respective controls. Differences were considered statistically significant at P < 0.05.
Concentration-response curves were evaluated using non-linear regression analysis with GraphPad software (GraphPAD, San Diego, CA, United States).
3 Results
3.1 Effect on castor oil-induced diarrhea
The tested oil (EOCP) exhibited dose-dependent protective effects (40% and 80%) against castor oil-induced diarrhea in rats at 100 and 200 mg/kg, respectively. All rats of the negative control group (saline treated) were recorded with loose diarrheal spots and therefore did not exhibit protection against castor oil-induced diarrhea. However, the positive control drug, dicyclomine, showed 40% protection at a lower dose of 50 mg/kg, while its higher dose (100 mg/kg) showed complete (100%) protection (Table 1).
TABLE 1
| Treatment (p.o.), dose (mg/kg) | No. of rats out of five with diarrhea | % Protection |
|---|---|---|
| Saline (10 mL/kg) + castor oil | 5/5 | 0 |
| EOCP + castor oil | ||
| 100 + 10 200 + 10 |
3a/5 1a/5 |
40 80 |
| Dicyclomine + castor oil | ||
| 50 + 10 100 + 10 |
3a/5 0b/5 |
40 100 |
Antidiarrheal activity of the essential oil of Cymbopogon proximus (EOCP) in rats with diarrhea induced castor oil (10 mL/kg).
p <0.05 and.
p <0.01 vs. saline + castor oil treated group (χ2-test).
3.2 Relaxant effect on the baseline of rat ileum
Figures 1A–D shows the original representative tracings of the effect of EOCP, dicyclomine, verapamil and atropine on the baseline contraction of the rat isolated ileum. The essential oil of C. proxismus was shown to have a direct relaxant effect on the baseline intestinal tone (Figure 1A) with a maximum relaxant response (Rmax) corresponded to 18.5% ± 1.5% of the ACh (0.3 µM)-induced contraction (Figure 1E). This effect of EOCP was equivalent to the maximal relaxant response induced by dicyclomine (Figure 1B) which was recorded with Rmax of 17.5% ± 2.5% (Figure 1E) whereas verapamil (Figure 1C) and atropine (Figure 1D) relaxed only slightly the baseline contraction of ileum tissue with resultant Rmax of 3.5% ± 1.5% and 4.5% ± 2.5%, respectively (Figure 1E).
FIGURE 1
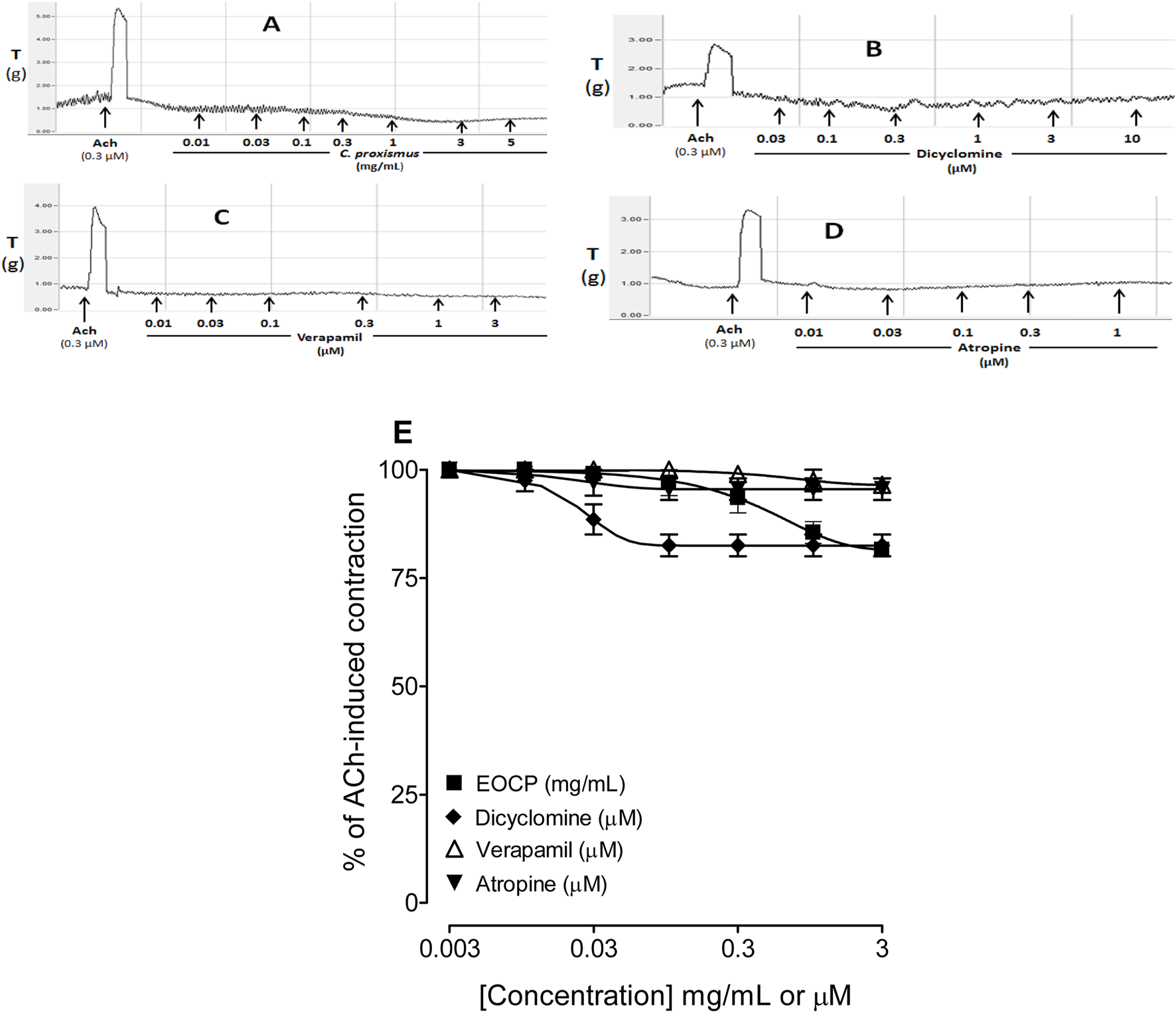
Original recordings to observe any stimulant and/or inhibitory effect on the baseline contractions of isolated rat ileum preparations by the increasing concentration of (A) essential oil of Cymbopogon proximus (EOCP), (B) dicyclomine, (C) verapamil, and (D) atropine at approximately 2–4 min intervals. The % inhibitory baseline effect was calculated by considering ACh (0.3 µM) contraction as 100%. compared with acetylcholine (Ach; 0.3 µM)-induced contraction (E). Data are presented as mean ± SEM, n = 6.
3.3 Antispasmodic effect on the induced contractoins of rat ileum
Figure 2 shows the representative concentration-dependent antispasmodic effect of the EOCP on high K+-induced contractions. In both, CCh (1 µM) and K+ (80 mM)-induced contractions, complete relaxation was observed by EOCP. However, EOCP reversed CCh-induced contractions at lower concentrations, with a recorded EC50 of 0.12 mg/mL (0.10–0.15), n = 5, demonstrating higher potency compared to its inhibitory effect against K+-induced contractions where lower potency was observed with resultant EC50 of 1.15 mg/mL (1.07–1.28), n = 5, as shown in Table 2 and Figure 3A. Dicyclomine, also showed a similar pattern of inhibitory activity against CCh- and K+-induced contractions with corresponding EC50 values of 0.31 μM (0.24–0.41), n = 5 and 3.41 μM (3.01–3.86), n = 5 (Figure 3B). However, verapamil exhibited greater potency against K+-induced contractions, with EC50 of 0.11 μM (0.08–0.13), n = 5, and its effect on CCh-induced contractions, had an EC50 0.92 μM (0.83–1.01), n = 5, Figure 3C. Control drug, atropine effectively counteracted contractions induced by CCh (1 µM) with an EC50 of 0.29 μM (0.26–0.32), n = 5, without any effect on K+-induced contractions (Figure 3D). The detailed EC50 values for EOCP and control drugs are shown in Table 2.
FIGURE 2
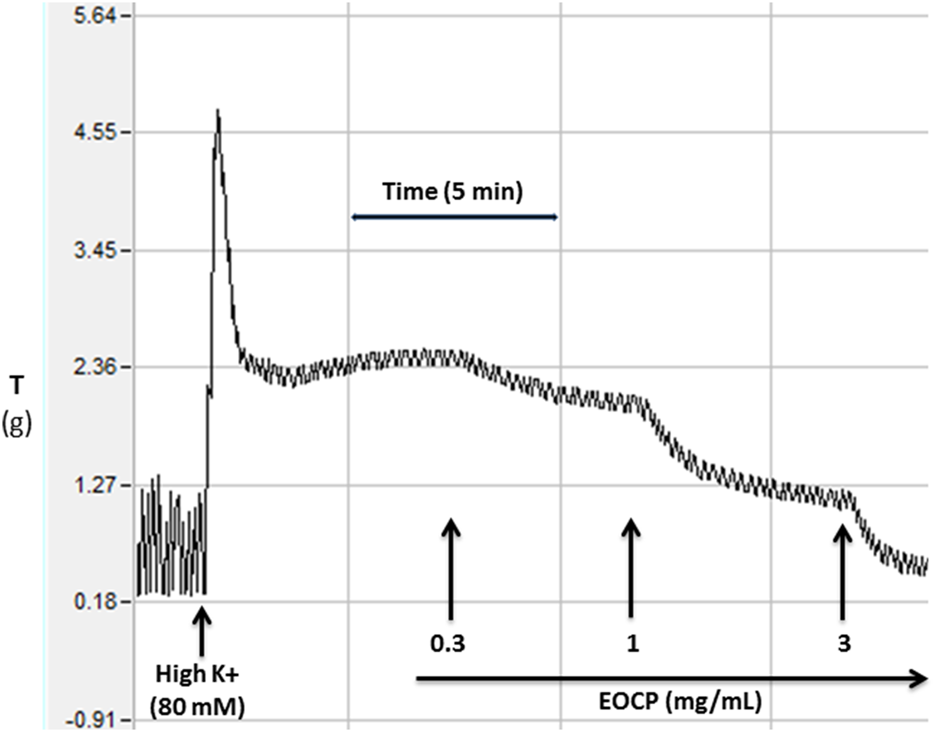
Original recordings showing induced contractions in isolated rat ileum by high K+ (80 mM) and the inhibitory effect of the essential oil of Cymbopogon proximus (EOCP). The time interval between two concentrations was approximately 5–10 min whereas Y-axis showing the tissue tension (T) in grams (g).
TABLE 2
| Parameters | EOCP | Dicyclomine | Atropine | Verapamil |
|---|---|---|---|---|
| CCh | 0.12 mg/mL (0.10–0.15), n = 5 | 0.31 μM (0.24–0.41), n = 5 | 0.29 μM (0.26–0.32), n = 5 | 0.92 μM (0.83–1.01), n = 5 |
| High K+ | 1.15 mg/mL (1.07–1.28), n = 5 | 3.41 μM (3.01–3.86), n = 5 | 0% inhibition, n = 5 | 0.11 μM (0.08–0.13), n = 5 |
Comparison of EC50 values for the inhibitory effect of the essential oil of Cymbopogon proximus (EOCP) and positive control drugs (dicyclomine, atropine, and verapamil) on carbachol (CCh, 1 µM) and high K+ (80 mM)-induced contractions in isolated rat ileum.
FIGURE 3
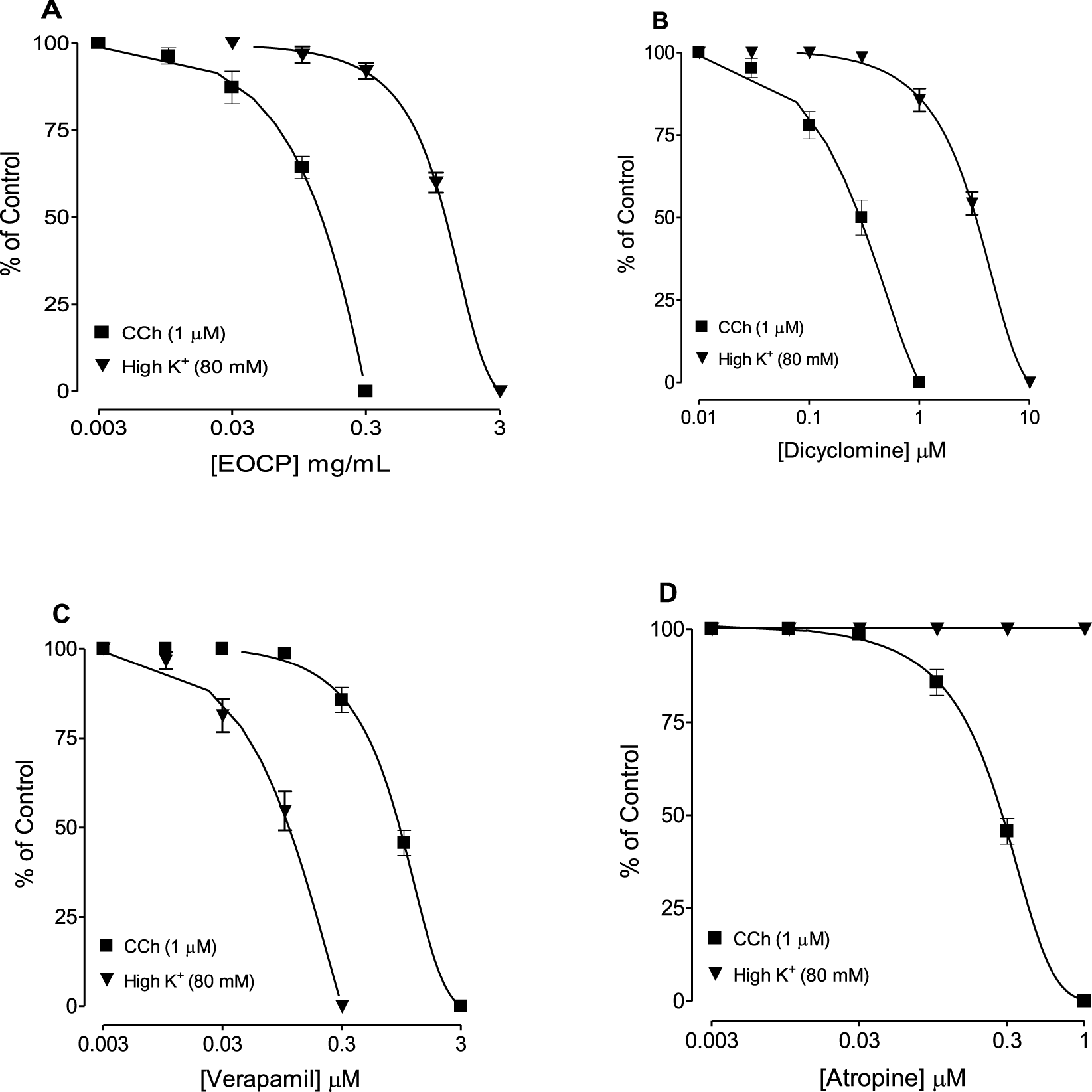
Concentration-response curves comparing the inhibitory effects of (A) essential oil of Cymbopogon proximus (EOCP), (B) dicyclomine, (C) verapamil, and (D) atropine on carbachol (CCh) and high K+-induced contractions in isolated rat ileum preparations. Data are presented as mean ± SEM, n = 5.
3.4 Anticholinergic activity confirmation
At a lower concentration of 0.03 mg/mL, EOCP caused a rightward parallel shift in the CCh-CRCs without reducing the maximum contractile response of the agonist, followed by a non-parallel shift with suppression of the maximal response at a higher concentration of 0.1 mg/mL (Figure 4A; Table 3). Similarly, dicyclomine (0.01 and 0.03 µM) showed a similar shift pattern by showing suppression of the maximum response only at higher concentration of 0.03 µM where the maximum contractile response of CCh was recorded as 56.6% whereas its lower concentration significantly effected CCh potency (p < 0.01) without decreasing the maximum response of CCh (Table 3; Figure 4B). Pre-incubation with atropine (0.01 and 0.03 µM) caused a rightward parallel shift in the CCh-CRCs without suppressing the maximal effect while the potency of CCh was significantly effected (p < 0.01) at both concentrations (Table 3; Figure 4C). In contrast, verapamil (0.01 and 0.03 µM) caused a non-parallel rightward shift in CCh-CRCs with a decrease in the maximum response of control curves to 79% and 51.3% at verapamil pre-incubated concentration of 0.01 µM and 0.03 µM, respectively (Table 3; Figure 4D).
FIGURE 4
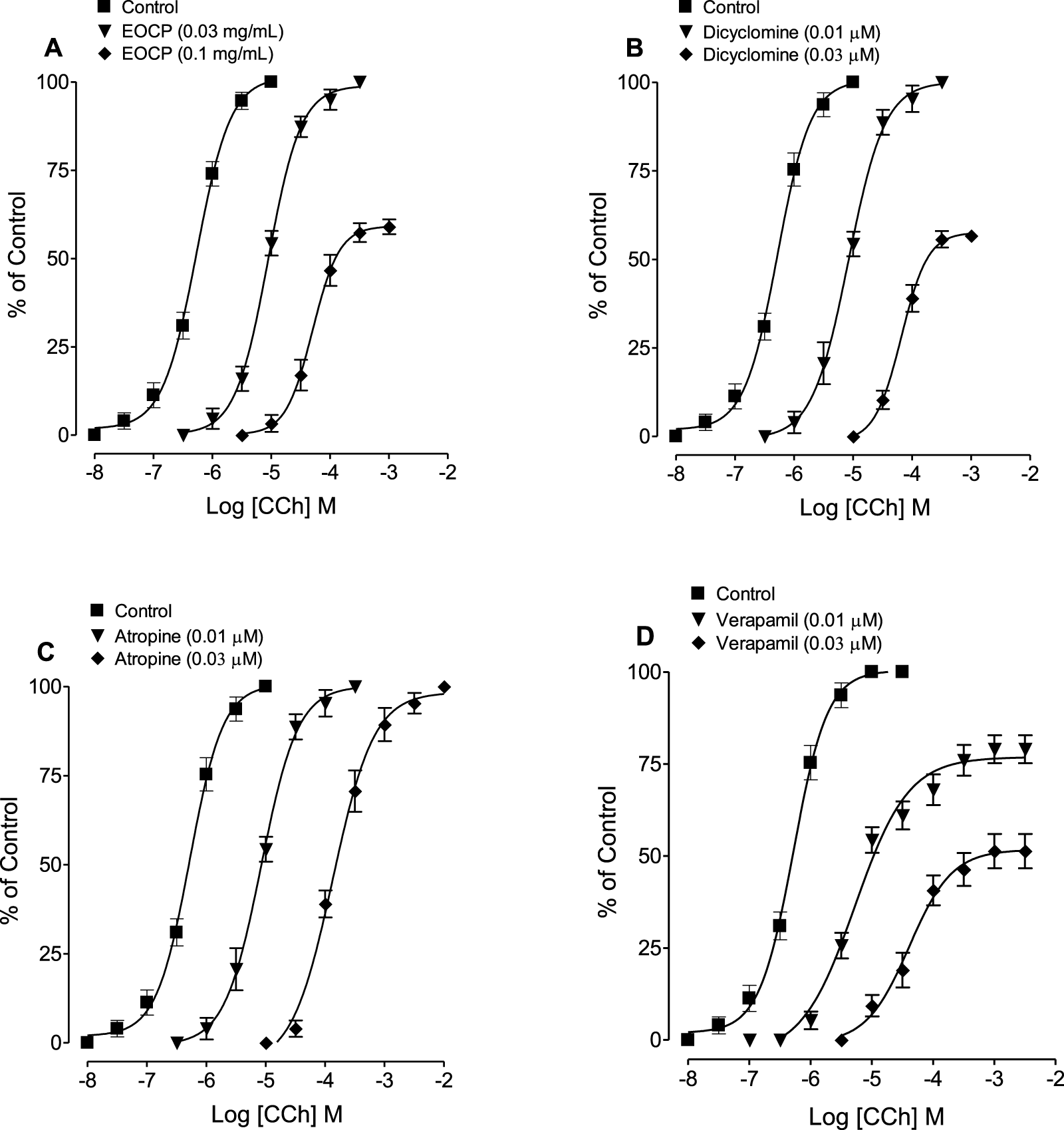
Concentration-response curves of carbachol (CCh) in the absence and presence of different concentrations of (A) the essential oil of Cymbopogon proximus (EOCP), (B) dicyclomine, (C) atropine, and (D) verapamil in isolated rat ileum preparations. Data are presented as mean ± SEM, n = 5-6.
TABLE 3
| CCh potency (-Log M) | Complete emax. With EC50 | Maximum % stimulant effect (mean ± SEM) |
|---|---|---|
| Control | 0.54 µM (0.45–0.64), n = 6 | |
| EOCP 0.03 mg/mL | 8.89 µM (7.62–10.38)**, n = 6 | |
| EOCP 1.00 mg/mL | 59.0% ± 2.08%, n = 5 | |
| Control | 0.52 µM (0.43–0.63), n = 6 | |
| Atropine 1 × 10−8 M | 8.42 µM (6.78–10.60)**, n = 5 | |
| Atropine 3 × 10−8 M | 135.6 µM (102.6–179.1)**, n = 6 | |
| Control | 0.52 µM (0.43–0.63), n = 6 | |
| Dicyclomine 1 × 10−8 M | 8.42 µM (6.78–10.46)**, n = 5 | |
| Dicyclomine 3 × 10−8 M | 56.6% ± 1.66%, n = 5 | |
| Control | 0.52 µM (0.44–0.61), n = 6 | |
| Verapamil 1 × 10−8 M | 79.0% ± 3.78%, n = 6 | |
| Verapamil 3 × 10−8 M | 51.3% ± 6.66%, n = 5 |
Effects of different inhibitors/antagonists (EOCP, atropine, dicyclomine, verapamil) on potency of carbachol (CCh) at producing contractile effect in rat isolated ileum preparations, expressed as EC50 values.
Values represent geometric means along with 95% confidence intervals in parenthesis. In preparation, where complete inhibition was not achieved, the data were presented as the maximum stimulant response for comparison. “n” represents number of observations. Asterisks denote significance of difference between CCh, potency in the absence (respective controls) to its potency in the presence of the different concentrations of the essential oil (EOCP), atropine, dicyclomine and verapamil (Oneway ANOVA, and Dunnett’s multiple comparison test: **p < 0.01).
3.5 Ca++ channel blocking activity confirmation
When tested for possible interactions with Ca++ channels, EOCP (0.1 and 0.3 mg/mL) induced a rightward shift of Ca++ concentration-response curves (CRCs) and suppressed the maximal response (Figure 5A), mirroring the effect of verapamil (Figure 5B) and dicyclomine (Figure 5C).
FIGURE 5
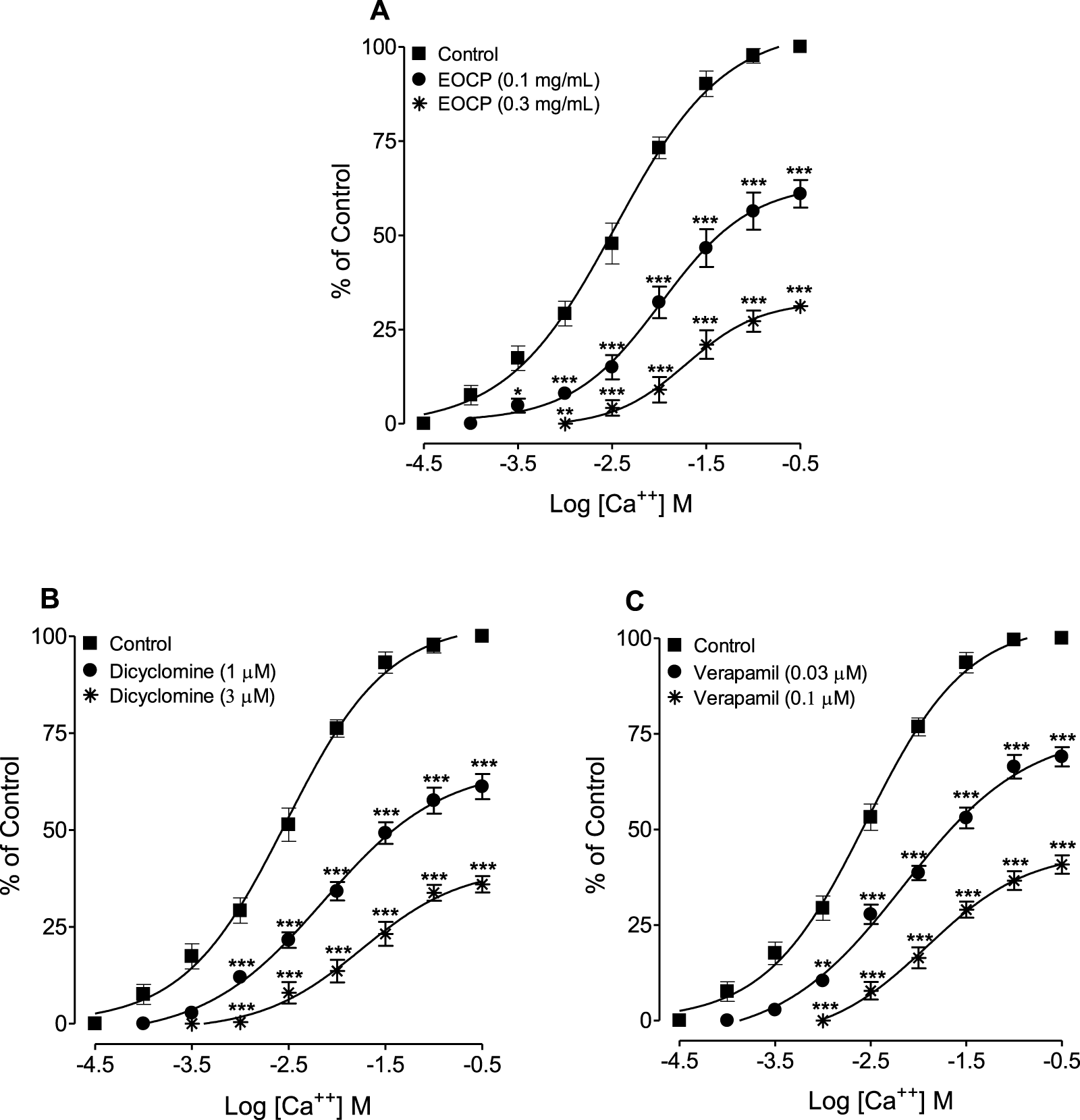
Concentration-response curves of Ca++ in the absence and presence of different concentrations of (A) the essential oil of Cymbopogon proximus (EOCP), (B) dicyclomine, and (C) verapamil in isolated rat ileum preparations. Data are presented as mean ± SEM, n = 5. **p < 0.01, and ***p < 0.001 shows comparison of the mean of Ca++-mediated contractions in the pretreated tissues with EOCP, dicyclomine and verapamil with the respective mean of Ca++-mediated contractions in control (untreated) ileum preparations (Repeated Measures ANOVA, followed by Bonferroni post-test).
4 Discussion
Given the known medicinal use of C. proximus as an antispasmodic (El-Askary et al., 2003) and its smooth muscle relaxant properties (Abdel-Moneim et al., 1969), the extracted essential oil was tested on laboratory rats to evaluate its potential effects on hyperactive gut disorders, such as diarrhea and spasms. In a castor oil-induced diarrhea model, EOCP demonstrated a dose-dependent protective effect similar to dicyclomine, a standard antidiarrheal agent (Pasricha, 2006). Castor oil causes diarrhea due to the ricinoleic acid, formed during hydrolysis, which disrupts electrolyte and water transport, and leads to strong intestinal contractions (Iwao and Terada, 1962; Croci et al., 1997). Therefore, an effective antidiarrheal remedy can work by inhibiting bowel contractions.
Essential oil of C. proxismus had a direct relaxant effect on the isolated ileum preparation of rat and also relaxed tissue with induced contractions by different spasmodic agents. These effects were observed at lower concentrations and might be related to the symptomatic relief of the acute abdominal pain associated with some gastric disturbances (Câmara et al., 2003). Next, to explore the mechanism underlying EOCP’s antidiarrheal effects, its activity was tested against contractions induced by CCh and high potassium (K+). Medicinal plants extracts or thier extracted essential oils usually possess antispasmodic effect on multiple smooth muscles including intestinal smooth muscles, possibly mediated by combination of mechanism(s) such as anticholinergic (Mehmood et al., 2011), Ca++ channel blockade (Gilani et al., 2010; Rehman et al., 2021), phosphodiesterase inhibition (Arshad et al., 2016; Imam et al., 2020) and/or potassium channel activation (Ansari et al., 2024; Khan et al., 2011). Notably, EOCP reversed CCh-induced contractions more effectively than those caused by high K+. Dicyclomine, which blocks both muscarinic receptors and calcium (Ca++) influx (McGrath et al., 1964; Downie et al., 1977), displayed a similar inhibition pattern, whereas verapamil, a known Ca++ signaling inhibitor (Jenkinson, 2002), was observed to be potent for reversing high K+-provoked spasms than those induced by CCh. Atropine, a muscarinic receptor non-specific blocker (Arunlakshana and Schild, 1997), only relaxed CCh-induced contractions. These results suggested that EOCP has both muscarinic receptor blocking and Ca++ influx inhibitory effects.
Further confirmation of EOCP’s effects was obtained by constructing CCh and Ca++ CRCs in the presence of different concentrations of tested samples. At lower doses, EOCP caused a parallel shift in CCh curves without reducing the maximal effect, indicating competitive inhibition, similar to atropine (Eglen and Harris, 1993; Jenkinson, 2002). At higher concentrations, EOCP produced a non-parallel deflection with a reduced peak effect previously observed in control curves, suggesting non-competitive inhibition, as observed with Ca++ antagonists (Van den Brink, 1973; Irie et al., 2000). Dicyclomine also deflected the CCh curves in a pattern similar to EOCP, while verapamil caused a rightward, non-parallel manner shift of curves with a reduction in the maximum peaks at all pre-incubated concentrations. In contrast, atropine caused a parallel rightward shift without reducing the maximum response. EOCP shifted the Ca++ curves to the right and reduced the maximal response, akin to the effects of verapamil and dicyclomine. Our findings align with the study by Marghich et al. (2023), which demonstrated that the myorelaxant and antispasmodic effects of Artemisia campestris essential oil are likely mediated through conformational changes in cholinergic muscarinic receptors and inhibition of L-type voltage-gated calcium channels, with a predominant role attributed to the former mechanism. These findings were further supported by molecular docking analyses. Similarly, several studies on medicinal plants with antidiarrheal and antispasmodic properties (Aleem and Janbaz, 2018; Mehmood et al., 2011) have reported smooth muscle relaxant mechanisms comparable to those observed for EOCP, reinforcing the validity of our current results.
Cholinergic antagonists are well-established therapeutic agents for the treatment of diarrhea (Pietrusko, 1979); however, their use is often associated with adverse cardiac stimulation, particularly when administered orally (Nawarth, 1981). Conversely, calcium channel blockers (CCBs) are also effective in managing hypermotile gut conditions (Borsari, 1991) but are known for their cardio-suppressant effects (Billman, 1992). The presence of CCB-like constituents alongside antimuscarinic compounds in Cymbopogon proximus essential oil may represent a naturally occurring mechanism designed to counteract tachycardia, a common side effect of standalone anticholinergic agents. This phenomenon aligns with the concept that natural remedies often exhibit “effect-enhancing and/or side-effect-neutralizing” properties (Gilani and Rahman, 2005), in addition to their cost-effectiveness and potential merit in evidence-based medicine (Ernst, 2005).
This study builds on the traditional use of Cymbopogon species as natural remedies to treat gastrointestinal disorders, such as diarrhea and spasms, and supports their historical use in folk medicine (Zhao et al., 2024). For instance, Cymbopogon citratus essential oil and its major component, citral, exhibit strong antidiarrheal effects by reducing intestinal motility and mitigating gut hyperactivity (Tangpu and Yadav, 2006). Additionally, Cymbopogon martinii essential oil exhibits spasmolytic properties by inhibiting spontaneous contractions in the isolated rabbit jejunum and suppressing potassium-induced contractions, functioning similarly to calcium channel blockers such as verapamil (Janbaz et al., 2014). Research also revealed that Cymbopogon schoenanthus essential oil from Sudan has remarkable spasmolytic activity (Pavlović et al., 2017; Djemam et al., 2020).
The effects observed in C. proximus may be attributed to the rich composition of its essential oils (Al-Taweel et al., 2013; Althurwi et al., 2020). Notably, some of the identified components in C. proximus are known to have spasmolytic properties. For example, Piperitone, a major metabolite in the essential oil studied, was previously shown to have concentration-dependent spasmolytic activity (Ponce-Monter et al., 2008). Additionally, β-eudesmol, another metabolite of C. Proximus essential oil, has shown relaxing effect in previous studies (Morita et al., 1996). Limonene, another minor component of this oil, has also been proven to have spasmolytic activity (Cardoso Lima et al., 2012).
This study provides valuable insight into the dual mechanism of C. proximus essential oil (EOCP) as an antidiarrheal and antispasmodic agent, but several limitations should be acknowledged. The results based on animal models, may not be directly applicable to humans, and the study lacks examination of other relevant physiological pathways and detailed pharmacokinetic data. Additionally, more research is needed to identify the key components responsible for the observed effects. However, previous studies have documented the antispasmodic effects of C. Proximus. This study introduces novel insights by providing a more detailed mechanistic understanding, showing that EOCP acts through both muscarinic receptor inhibition and calcium influx blockade.
5 Conclusion
These results suggest that the essential oil extracted from C. proximus, independently, had a direct relaxant and spasmolytic effect on the intestinal smooth muscles that does not depend on interaction with receptors for neurotransmitters and are probably myogenic in nature. Moreover, the antidiarrheal and antispasmodic effects of EOCP is to a great extent may be mediated by the dual inhibition of muscarinic receptors and Ca++ channels. However, the possible involvement of additional inhibitory mechanism(s) cannot be ruled out. Thus, this study provides a scientific rationale for the traditional use of C. proximus in treating gut hyperactivity disorders such as diarrhea and spasms, while paving the way for future studies to explore its potential for broader therapeutic applications. Future research should also examine its long-term safety, effectiveness, and the molecular mechanisms underlying its actions.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Bio-Ethical Research Committee (BERC) of PSAU. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
HA: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review and editing. NR: Conceptualization, Formal Analysis, Methodology, Writing – original draft, Writing – review and editing. MA: Resources, Writing – review and editing. FA: Investigation, Resources, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors thank Prince Sattam Bin Abdulaziz University for funding this research work under project number (PSAU/2023/03/25854).
Acknowledgments
The authors extend their appreciation to Prince Sattam Bin Abdulaziz University for funding this research work under project number (PSAU/2023/03/25854).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abdel-Moneim F. M. Ahmed Z. F. Fayez M. B. E. Ghaleb H. (1969). Constituents of local plants. XIV. The antispasmodic principle in Cymbopogon proximus. Planta Med.17, 209–216. 10.1055/s-0028-1099848
2
Adeneye A. A. Agbaje E. O. (2007). Hypoglycemic and hypolipidemic effects of fresh leaf aqueous extract of Cymbopogon citratus stapf. in rats. J. Ethnopharmacol.112, 440–444. 10.1016/j.jep.2007.03.034
3
Aleem A. Janbaz K. H. (2018). Dual mechanisms of anti-muscarinic and Ca++ antagonistic activities to validate the folkloric uses of Cyperus niveus retz. as antispasmodic and antidiarrheal. J. Ethnopharmacol.1, 138–148. 10.1016/j.jep.2017.11.006
4
Al-Taweel A. M. Fawzy G. A. Perveen S. El Tahir K. E. H. (2013). Gas chromatographic mass analysis and further pharmacological actions of Cymbopogon proximus essential oil. Drug Res. Stuttg.63, 484–488. 10.1055/s-0033-1347239
5
Althurwi H. N. Abdel-Kader M. S. Alharthy K. M. Salkini M. A. Albaqami F. F. (2020). Cymbopogon proximus essential oil protects rats against isoproterenol-induced cardiac hypertrophy and fibrosis. Molecules25, 1786. 10.3390/molecules25081786
6
Ansari M. N. Rehman N. U. Samad A. Ahmad W. (2024). Pharmacological basis for the antidiarrheal and antispasmodic effects of cuminaldehyde in experimental animals: in silico, in vitro and in vivo studies. Front. Biosci.29, 43. 10.31083/j.fbl2901043
7
Arshad U. Bashir S. Rehman N. U. Yaqub T. Gilani A. H. (2016). Dual inhibition of Ca+2 influx and phosphodiesterase enzyme provides scientific base for the medicinal use of Chrozophora prostrata dalz. In respiratory disorders. Phytother. Res.30, 1010–1015. 10.1002/ptr.5610
8
Arunlakshana O. Schild H. O. (1997). Some quantitative uses of drug antagonists. 1958. Br. J. Pharmacol.120 (Suppl. ment), 151–161. 10.1111/j.1476-5381.1997.tb06793.x
9
Avoseh O. Oyedeji O. Rungqu P. Nkeh-Chungag B. Oyedeji A. (2015). Cymbopogon species; ethnopharmacology, phytochemistry and the pharmacological importance. Molecules20, 7438–7453. 10.3390/molecules20057438
10
Billman G. E. (1992). “The antiarrythmic effects of the calcium antagonists,” in Calcium antagonists in clinical medicine. Editor EpsteinM. (Philadelphia, United States: Hanley and Belfus Inc.), 183–212.
11
Borsari G. (1991). L'effet thérapeutique de l'anticalcique vérapamil dans la diarrhée chronique. Communication préliminaire [The therapeutic effects of the calcium blocker verapamil in chronic diarrhea. Preliminary communication]. Schweiz. Med. wochenschr.31, 1238–1242.
12
Câmara C. C. Nascimento N. R. Macêdo-Filho C. L. Almeida F. B. Fonteles M. C. (2003). Antispasmodic effect of the essential oil of Plectranthus barbatus and some major constituents on the guinea-pig ileum. Planta Med.69, 1080–1085. 10.1055/s-2003-45186
13
Cardoso Lima T. Mota M. M. Barbosa-Filho J. M. Viana Dos Santos M. R. De Sousa D. P. (2012). Structural relationships and vasorelaxant activity of monoterpenes. Daru20, 23. 10.1186/2008-2231-20-23
14
Choo L. K. Mitchelson F. (1978). Antagonism of cholinomimetics by troxypyrrolidinium in guinea-pig atria and longitudinal ileal muscle: Comparison with hemicholinium-3. Eur. J. Pharmacol.52, 313–322. 10.1016/0014-2999(78)90284-4
15
Croci T. Landi M. Emonds-Alt X. Le Fur G. Maffrand J. P. Manara L. (1997). Role of tachykinins in Castor oil diarrhoea in rats. Br. J. Pharmacol.121, 375–380. 10.1038/sj.bjp.0701130
16
Djemam N. Lassed S. Gül F. Altun M. Monteiro M. Menezes-Pinto D. et al (2020). Characterization of ethyl acetate and n-butanol extracts of Cymbopogon Schoenanthus and Helianthemum lippii and their effect on the smooth muscle of the rat distal colon. J. Ethnopharmacol.252, 112613. 10.1016/j.jep.2020.112613
17
Downie J. W. Twiddy D. A. Awad S. A. (1977). Antimuscarinic and noncompetitive antagonist properties of dicyclomine hydrochloride in isolated human and rabbit bladder muscle. J. Pharmacol. Exp. Ther.201, 662–668. 10.1016/s0022-3565(25)30905-5
18
Dutta S. Munda S. Lal M. Bhattacharyya P. R. (2016). A short review on chemical composition therapeutic use and enzyme inhibition activities of cymbopogon species. Indian J. Sci. Technol.9, 1–9. 10.17485/ijst/2016/v9i46/87046
19
Eglen R. M. Harris G. C. (1993). Selective inactivation of muscarinic M2 and M3 receptors in guinea-pig ileum and atria in vitro. Br. J. Pharmacol.109, 946–952. 10.1111/j.1476-5381.1993.tb13712.x
20
Ekpenyong C. E. Davies K. Antai E. E. (2014). Cymbopogon citratus Stapf (DC) extract ameliorates atherogenic cardiovascular risk in diabetes-induced dyslipidemia in rats. Br. J. Med. Med. Res.4, 4695–4709. 10.9734/BJMMR/2014/11262
21
El-Askary H. I. Meselhy M. R. Galal A. M. (2003). Sesquiterpenes from Cymbopogon proximus. Molecules8, 670–677. 10.3390/80900670
22
El-Nezhawy A. O. H. Maghrabi I. A. Mohamed K. M. Omar H. A. (2014). Cymbopogon proximus extract decreases L-NAME-induced hypertension in rats. Int. J. Pharm. Sci. Rev. Res.27, 66–69.
23
Ernst E. (2005). The efficacy of herbal medicine-an overview. Fundam. Clin. Pharmacol.19, 405–409. 10.1111/j.1472-8206.2005.00335.x
24
Farre A. J. Colombo M. Fort M. Gutierrez B. (1991). Differential effects of various Ca2+ antagonists. Gen. Pharmacol.22, 177–181. 10.1016/0306-3623(91)90331-y
25
Gilani A. H. Mandukhail S. U. Iqbal J. Yasinzai M. Aziz N. Khan A. et al (2010). Antispasmodic and vasodilator activities of Morinda citrifolia root extract are mediated through blockade of voltage dependent calcium channels. Bmc. Complement. Altern. Med.10, 2. 10.1186/1472-6882-10-2
26
Gilani A. H. Rahman A. (2005). Trends in ethnopharmocology. J. Ethnopharmacol.100, 43–49. 10.1016/j.jep.2005.06.001
27
Godfraind T. Miller R. Wibo M. (1986). Calcium antagonism and calcium entry blockade. Pharmacol. Rev.38, 321–416. 10.1016/s0031-6997(25)06867-x
28
Ibrahim F. Y. El-Khateeb A. Y. (2013). Effect of herbal beverages of Foeniculum vulgare and Cymbopogon proximus on inhibition of calcium oxalate renal crystals formation in rats. Ann. Agric. Sci.58, 221–229. 10.1016/j.aoas.2013.07.006
29
Imam F. Rehman N. U. Ansari M. N. Qamar W. Afzal M. Alharbi K. S. (2020). Effect of roflumilast in airways disorders via dual inhibition of phosphodiesterase and Ca2+-channel. Saudi. Pharm. J.28, 698–702. 10.1016/j.jsps.2020.04.011
30
Irie K. Yoshioka T. Nakai A. Ochiai K. Nishikori T. Wu G. R. et al (2000). A Ca(2+) channel blocker-like effect of dehydrocurdione on rodent intestinal and vascular smooth muscle. Eur. J. Pharmacol.403, 235–242. 10.1016/s0014-2999(00)00445-3
31
Iwao I. Terada Y. (1962). On the mechanism of diarrhea due to Castor oil. Jpn. J. Pharmacol.12, 137–145. 10.1254/jjp.12.137
32
Janbaz K. H. Qayyum A. Saqib F. Imran I. Zia-Ul-Haq M. De Feo V. (2014). Bronchodilator, vasodilator and spasmolytic activities of Cymbopogon martinii. J. Physiol. Pharmacol.65, 859–866.
33
Jenkinson D. H. (2002). “Classical approaches to the study of drug-receptor interactions,” in Textbook of receptor pharmacology, Editors Foreman JC, Johansen T, (Boca Raton, FL: CRC Press), 3–78.
34
Khan A. Rehman N. U. AlKharfy K. M. Gilani A. H. (2011). Antidiarrheal and antispasmodic activities of Salvia officinalis are mediated through activation of K+ channels. Bang. J. Pharmacol.6, 111–116. 10.3329/bjp.v6i2.9156
35
Mansour H. A. Newairy A.-S. A. Yousef M. I. Sheweita S. A. (2002). Biochemical study on the effects of some Egyptian herbs in alloxan-induced diabetic rats. Toxicology170, 221–228. 10.1016/s0300-483x(01)00555-8
36
Marghich M. Amrani O. Karim A. Harit T. Beyi L. Mekhfi H. et al (2023). Myorelaxant and antispasmodic effects of the essential oil of Artemisia campestris L., and the molecular docking of its major constituents with the muscarinic receptor and the L-type voltage-gated Ca2+ channel. J. Ethnopharmacol.15, 116456. 10.1016/j.jep.2023.116456
37
McGrath W. R. Lewis R. E. Kuhn W. L. (1964). The dual mode of the antispasmodic effect of dicyclomine hydrochloride. J. Pharmacol. Exp. Ther.146, 354–358. 10.1016/s0022-3565(25)26933-6
38
Mehmood M. H. Siddiqi H. S. Gilani A. H. (2011). The antidiarrheal and spasmolytic activities of Phyllanthus emblica are mediated through dual blockade of muscarinic receptors and Ca2+ channels. J. Ethnopharmacol.27, 856–865. 10.1016/j.jep.2010.11.023
39
Meyer-Warnod B. (1984). Natural essential oils: extraction processes and applications to some major oils. Perfum. Flavorist, 93–103.
40
Miguel M. G. (2010). Antioxidant and anti-inflammatory activities of essential oils: a short review. Molecules15, 9252–9287. 10.3390/molecules15129252
41
Morita M. Nakanishi H. Morita H. Mihashi S. Itokawa H. (1996). Structures and spasmolytic activities of derivatives from sesquiterpenes of Alpinia speciosa and Alpinia japonica. Chem. Pharm. Bull.44, 1603–1606. 10.1248/cpb.44.1603
42
National Research Council (1996). Guide for the care and use of laboratory animals. Washington, DC: National Academy Press, 1–7.
43
Nawarth H. (1981). Action potential, membrane currents and force of contraction in cat ventricular heart muscle treated with papaverine. J. Pharmacol. Exp. Ther.218, 544–549. 10.1016/s0022-3565(25)32705-9
44
Pasricha P. J. (2006). “Treatment of disorders of bowel motility and water flux; antiemetics; agents used in biliary and pancreatic disease,” in Goodman and gilman’s the pharmacological basis of therapeutics. 11th ed. (New York: McGraw-Hill), 983–1008.
45
Pavlović I. Omar E. Drobac M. Radenković M. Branković S. Kovačević N. (2017). Chemical composition and spasmolytic activity of Cymbopogon schoenanthus (L.) spreng. (poaceae) essential oil from Sudan. Arch. Biol. Sci. (Beogr).69, 409–415. 10.2298/ABS160506113P
46
Pietrusko R. G. (1979). Drug therapy reviews: pharmacotherapy of diarrhea. Am. J. Hosp. Pharm.36, 757–767. 10.1093/ajhp/36.6.757
47
Ponce-Monter H. Campos M. G. Pérez S. Pérez C. Zavala M. Macías A. et al (2008). Chemical composition and antispasmodic effect of Casimiroa pringlei essential oil on rat uterus. Fitoterapia79, 446–450. 10.1016/j.fitote.2008.04.005
48
Rehman N. U. Ansari M. N. Ahmad W. Ali A. (2024). Calcium channel inhibitory effect of marjoram (Origanum majorana L.): its medicinal use in diarrhea and gut hyperactivity. Front. Biosci.29, 47. 10.31083/j.fbl2902047
49
Rehman N. U. Ansari M. N. Hailea T. Karim A. Abujheisha K. Y. Ahamad S. R. et al (2021). Possible tracheal relaxant and antimicrobial effects of the essential oil of Ethiopian thyme specie (Thymus serrulatus hoschst. Ex benth.): a multiple mechanistic approach. Front. Pharmacol.12, 615228. 10.3389/fphar.2021.615228
50
Rehman N. U. Mehmood M. H. Alkharfy K. M. Gilani A. H. (2012). Studies on antidiarrheal and antispasmodic activities of Lepidium sativum crude extract in rats. Phytother. Res.26, 136–141. 10.1002/ptr.3642
51
Selim S. A. (2011). Chemical composition, antioxidant and antimicrobial activity of the essential oil and methanol extract of the Egyptian lemongrass Cymbopogon proximus stapf. Grasas Aceites62, 55–61. 10.3989/gya.033810
52
Tangpu V. Yadav A. K. (2006). Antidiarrhoeal activity of Cymbopogon citratus and its main constituent, citral. Pharmacologyonline2, 290–298.
53
Van den Brink F. G. (1973). The model of functional interaction. I. Development and first check of a new model of functional synergism and antagonism. Eur. J. Pharmacol.22, 270–278. 10.1016/0014-2999(73)90026-5
54
Warrag N. M. Tag Eldin I. M. Ahmed E. M. (2014). Effect of Cymbopogon proximus (mahareb) on ethylene glycol-induced nephrolithiasis in rats. Afr. J. Pharm. Pharmacol.8, 443–450. 10.5897/AJPP2014.4053
55
Zhao J. Fan Y. Cheng Z. Kennelly E. J. Long C. (2024). Ethnobotanical uses, phytochemistry and bioactivities of cymbopogon plants: a review. J. Ethnopharmacol.330, 118181. 10.1016/j.jep.2024.118181
Summary
Keywords
Cymbopogon proximus , antidiarrheal, isolated ileum, antimuscarinic, calcium channel blocker
Citation
Althurwi HN, Rehman NU, Abdel-Kader MS and Albaqami FF (2025) Pharmacological basis for medicinal use of Cymbopogon proximus Hochst. ex A. Rich. Essential oil in hyperactive gastrointestinal disorders. Front. Pharmacol. 16:1533511. doi: 10.3389/fphar.2025.1533511
Received
24 November 2024
Accepted
13 June 2025
Published
30 June 2025
Volume
16 - 2025
Edited by
Maria De Lourdes Pereira, University of Aveiro, Portugal
Reviewed by
Miriam Durante, University of Siena, Italy
Miguel Angel Plaza, University of Zaragoza, Spain
Updates
Copyright
© 2025 Althurwi, Rehman, Abdel-Kader and Albaqami.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hassan N. Althurwi, h.althurwi@psau.edu.sa
ORCID: Hassan N. Althurwi, orcid.org/0000-0003-3548-0852; Najeeb Ur Rehman, orcid.org/0000-0001-6925-7697; Maged S. Abdel-Kader, orcid.org/0000-0002-5128-2656; Faisal F. Albaqami, orcid.org/0000-0001-9343-5713
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.