- 1School of Pharmacy, Guangdong Pharmaceutical University, Guangzhou, Guangdong, China
- 2NMPA Key Laboratory for Technology Research and Evaluation of Pharmacovigilance, Guangzhou, Guangdong, China
- 3Guangzhou Pinyi Information Technology Co., Guangzhou, Guangdong, China
- 4Department of Pharmacy, Maoming Hospital of Guangzhou University of Chinese Medicine (Maoming Hospital of Traditional Chinese Medicine), Maoming, Guangdong, China
- 5Department of Pharmacy, The First Afffliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China
Objective: To explore the method of active monitoring of adverse drug events (ADEs) in patients with a rare disease: pulmonary arterial hypertension (PAH).
Methods: First, initial trigger items were extracted and organized through literature-based evidence and drug inserts. Then, the trigger items were refined using expert consultation based on a Delphi method. Second, patients with PAH admitted to a hospital between 1 January 2022 and 1 June 2023 were extracted as study samples. A retrospective case review was conducted to verify the clinical applicability of triggers. We calculated indicators such as sensitivity, specificity, and positive predictive value (PPV), and also observed the ADEs. Risk factors for the development of ADEs in hospitalized patients with PAH were also analyzed.
Results: We extracted and organized 31 initial trigger items. After two rounds of modification through expert consultation, 24 triggers were identified. We converted the trigger items into risk signals to automatically identify cases with possible ADEs with the help of the Chinese Hospital Pharmacovigilance System (CHPS). Manual review of suspected cases revealed that the overall PPV of the triggers was 30.30%, the sensitivity was 98.21%, and the specificity was 72.57%. The prevalence of ADEs detection was 17.57%, which was equivalent to 25.75 ADEs occurrences in 1,000 patient days and 11.84 ADEs occurrences in 1,000 medications. ADEs-involved organs included the blood system, and there was also damage to the gastrointestinal system, hepatic system, and renal system. ADEs severity was categorized into 24 grade D cases, 102 grade E cases, 15 grade F cases, and two grade H cases. The longer the duration of hospitalization, the higher the number of comorbidities, the higher the number of positive triggers, and the higher the likelihood of ADEs.
Conclusion: This study provides a new method for actively identifying ADEs in hospitalized patients with PAH, as well as a reference for research on pharmacovigilance for rare diseases.
1 Introduction
The use of any medication carries potential risks. Adverse drug event (ADE) monitoring began in the 1960s with the thalidomide incident. Pregnant women took thalidomide to treat vomiting during pregnancy, but this resulted in tens of thousands of “seal limbed babies” (Routledge, 1998; Fornasier et al., 2018). Since then, drug safety has become a major global concern. The World Health Organization (WHO) defines an ADE as a “harmful, unplanned adverse event that occurs at a therapeutic dose of a drug” (Nebeker et al., 2004).
The most common and simplest method of ADE monitoring is passive monitoring. This involves spontaneous reporting of ADEs by marketing authorization holders and healthcare providers, among others (National Center for Adverse Drug Reaction Monitoring, 2023). Spontaneous reporting is the main source of ADE reports and an important basis for post-marketing safety evaluation of drugs (Montané and Santesmases, 2020). However, passive surveillance has numerous shortcomings, such as incomplete and outdated reporting and many ADEs going undetected (Garcia-Abeijon et al., 2023).
To counteract the inadequacy of passive monitoring, scholars in various countries have explored active monitoring methods. Active monitoring methods for clinical ADEs include the Global Trigger Tool (GTT), Harvard Medical Practice Research, Comparative Safety Indicator Database, direct observation, and case review. The GTT method is considered to have a particularly high efficacy for monitoring (Ad et al., 2020).
The GTT method is an active monitoring tool introduced by the Institute for Healthcare Improvement (IHI) in 2003. It is a method for purposefully identifying potential ADEs in cases using “triggers” as clues (Classen et al., 2011; Hu et al., 2019). In 2009, the IHI released the second edition of the Global Trigger Tool for Measuring Adverse Events (GTT White Paper). The latter categorizes triggers into six modules, namely, nursing (C), medications (M), surgery (S), intensive care (I), perinatal (P), and emergency (E), and recommends 51 triggers (Griffin and Resar, 2009). The GTT has been applied widely for more than 2 decades after it was released by the IHI. To conduct active monitoring of ADEs in a more targeted manner, researchers have specified monitoring to specific departments, such as in emergency medicine (Rosen and Mull, 2016; Howard et al., 2017; Spangler et al., 2020), surgery (Pérez Zapata et al., 2015), pediatrics (Liu et al., 2018; Sakuma et al., 2024), and intensive care unit (ICU) (Aikawa et al., 2021; Liston et al., 2023), among other departments. For example, in Sweden, Rutberg et al. (2014) used the GTT method for active monitoring of ADEs in patients in one hospital. The prevalence of detection of ADEs was 20.5%, while only 6.3% of ADEs were reported in the spontaneous reporting system during the same period. In the United States, Takata et al. (2008) developed a specific trigger tool for detecting ADEs in children. In Sweden, Nilsson et al. (2012) conducted a structured review of ICU cases in a medium-sized hospital based on GTT. They reviewed 128 cases and identified 41 ADEs in 25 patients.
GTT studies have focused on certain populations, such as children (Ji et al., 2018; Liu et al., 2020), older people (Hibbert et al., 2016), or populations of patients with certain common diseases. Very few studies have been conducted on the active monitoring of ADEs in patients with rare diseases. Pre-marketing studies of drugs for rare diseases are very limited in size; sometimes, only a few dozen patient volunteers can be recruited for clinical trials, and the duration of the studies is short. Furthermore, information on rare adverse reactions, long-term toxicity, and effects on special populations (e.g., children, pregnant women, older people) is often difficult to obtain in pre-marketing studies (Anzelewicz et al., 2017). In addition, in the treatment of rare diseases, patients are often forced to accept off-label medications whose safety is difficult to confirm because rare medications without corresponding indications are approved for marketing (Kleiber et al., 2021). Therefore, it is important to monitor ADEs in patients with rare diseases.
Taking pulmonary arterial hypertension (PAH) as an example, we aimed to establish a triggering mechanism for ADEs and conduct a clinical-applicability validation test for it. In this way, we explored a method for the active monitoring of ADEs in patients with a potentially severe disease.
2 Methods
2.1 Establishment of triggers
2.1.1 Selection of target drugs
Based on the in-hospital drug supply catalog and a review of historical cases, we decided to use PAH-targeted drugs as the target drugs for the study. There are eight targeted drugs in use in their premises currently: (1) phosphodiesterase type-5 inhibitors (e.g., Sildenafil, Tadalafil); (2) Endothelin Receptor Antagonists (ERA) (Ambrisentan, Macitentan); (3)soluble guanylate cyclase (sGC) stimulant (Riociguat); (4) prostacyclin IP receptor agonists (Selexipag); and (5) Artificially synthesized prostacyclin analogs (Beraprost, Treprostinil).
2.1.2 Preliminary establishment of trigger items
First, the Web of science, PubMed, and China National Knowledge Infrastructure (CNKI) were searched. The English search terms were as follows: “trigger tool,” “adverse drug event,” “adverse event,” “adverse drug reaction,” “adverse reaction,” “pulmonary hypertension,” and “pulmonary arterial hypertension”. The Chinese search terms were as follows: “global trigger tool,” “adverse event,” “adverse reaction,” and “pulmonary arterial hypertension”. The information and triggers related to adverse events of PAH-targeted drugs were extracted and summarized through evidence-based literature.
Second, based on the 51 triggers recommended in the GTT white paper (Griffin and Resar, 2009), the triggers relevant to the present study were extracted.
Third, we extracted ADE information from drug inserts. The risk signals for ADE occurrence were organized and extracted based on the adverse events, contraindications, and precautions mentioned in the drug inserts of the target drugs Ambrisentan, Macitentan, Sildenafil, Tadalafil, Riociguat, Selexipag, Beraprost, Treprostinil.
Fourth, we extracted adverse-event risk signals from databases focusing on ADE reporting systems. The Chinese Adverse Drug Reaction Feedback Database, the European Database of Suspected Adverse Drug Reaction Reports, and the FDA Adverse Event Reporting System were searched. Then, we extracted the risk signals for ADEs of PAH-targeted drugs reported in these databases.
The trigger entries extracted from the four areas stated above and the list of PAH-targeted ADEs were combined and summarized to form the initial trigger items. Trigger items were categorized into the categories of “Laboratory Indicators (L),” “Antidotes (A),” “Clinical Symptoms (S),” and “Treat (T).”
2.1.3 Delphi method for optimization of trigger items
We designed a Delphi expert consultation questionnaire based on the initial trigger items. The selection criteria for experts are as follows: (1) Possess a bachelor’s degree or above; (2) Clinical doctors or pharmacists who have rich experience in clinical medicine or clinical pharmacy and are engaged in front-line clinical work of pulmonary arterial hypertension. The opinions of experts are quantified through the positive coefficient, authority coefficient (Cr), coefficient of variation (Vj) and coordination coefficient (W) of experts.
Ultimately, we selected 19 experts in clinical medicine and pharmacy from six tertiary grade A hospitals in Guangzhou, Shanghai and Jiangmen for consultation. After two rounds of consultation, the positive coefficient of the experts was 100%, Cr = 0.82 ± 0.03, Vj = 0.16 ± 0.04, and W = 0.365. It indicates that the enthusiasm and authority of the experts are both relatively high, and the differences in their opinions are relatively small, with a relatively high overall coordination.
We determined the final trigger based on the cut-off values of three indicators, namely, the mean importance score (Mj), the frequency of full marks (Kj), and the coefficient of variation (Vj), as well as expert opinions. The calculation method for the cut-off values of Mj and Kj for each trigger is “cut-off value = mean - standard deviation”, and those with scores higher than the cut-off values are selected. The calculation method for the cut-off value of Vj is “cut-off value = mean +standard deviation”, and those with scores lower than the cut-off values are selected. Triggers whose three indicators do not fall within the threshold range can be deleted; otherwise, a decision on whether to discard them should be made after discussion in combination with expert opinions.
2.2 Validation of the clinical suitability of triggers
We extracted data of patients with PAH admitted to the First Affiliated Hospital of Guangzhou Medical University (Guangzhou, China) from January 1, 2022, to June 1, 2023, with the help of the Chinese Hospital Pharmacovigilance System (CHPS).
The inclusion criteria for cases were as follows: (1) confirmed diagnosis of PAH; (2) treatment with ambrisentan, macitentan, sildenafil, tadalafil, riociguat, selexipag, beraprost, treprostinil for more than 24 h; (3) age >28 days; (4) hospitalization duration ≥24 h; and (5) hospital admission after 1 January 2022 and hospital discharge before 1 June 2023.
The study protocol was approved by the Ethics Committee of the First Hospital of Guangzhou Medical University (ES-2023-076-02).
2.2.1 Review of suspicious cases
Based on the risk signals set by the triggers, CHPS can automatically identify cases where ADEs are likely to occur. Nevertheless, false-positives can occur, such as abnormalities in the indicators caused by disease progression rather than due to the medication. Such false-positives require a professional reviewer to audit the suspicious case to determine if the patient has experienced an ADE.
The Case Review Team comprised two junior reviewers (one clinical pharmacist and one graduate pharmacy student) and two senior reviewers (two chief pharmacists). All members were trained in ADEs prior to case review. The two junior reviewers reviewed suspicious cases independently to determine whether the trigger result was a true-positive or a false-positive. In the event of a disagreement between the two junior reviewers, the decision of whether an ADE had occurred was made by the two senior reviewers with strict adherence to the “20-min rule” to ensure thorough review of the case and to avoid omission of ADE information. The sex, age, hospitalization duration, and number of comorbidities were recorded.
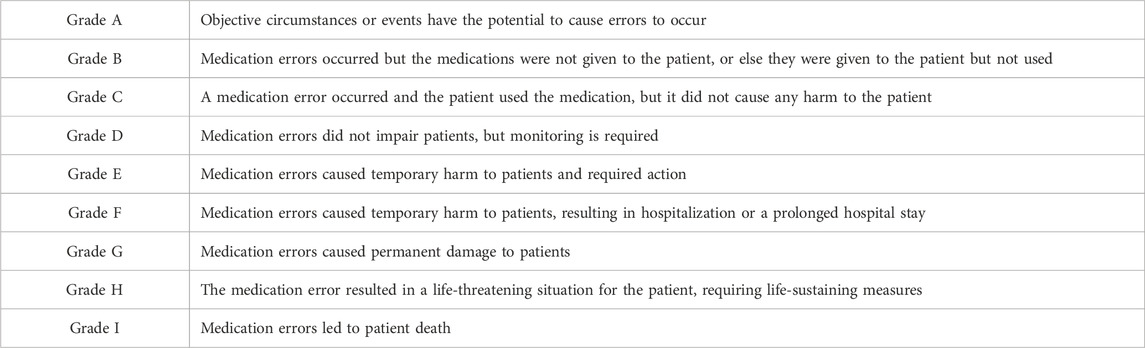
Evaluation of drug-ADE causality was carried out using the criteria set by the World Health Organization-Uppsala Monitoring Centre (WHO-UMC). The latter categorizes causality into six levels: “definitely related,” “very probably related,” “probably related,” “probably not related,” “to be evaluated,” and “not evaluable.” Injury levels caused by ADEs were classified using the error-grading system developed by the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) of the United States, which categorizes ADEs into grades A–I, as detailed in Table 1. Only E–I injuries were included in this study.
2.2.2 Statistical analyses
Excel 2021 (Microsoft, Redmond, WA, United States) and SPSS 26.0 (IBM, Armonk, NY, United States) were used for data analysis. Descriptive statistics were employed for the basic information of patients. Measurement data are presented as the mean ± standard deviation. Count data are expressed as frequencies and percentages.
The GTT method was evaluated using the positive predictive value (PPV, where PPV = number of true positives/number of positives × 100%), sensitivity, specificity, and prevalence of ADE detection. We calculated the following: number of ADE occurrences in 1,000 patient days (1000-patient-day ADE occurrences = total number of ADE occurrences/total time of each case × 1,000); ADE occurrences in 100 patients (100-patient ADE occurrences = total number of ADE occurrences/total number of cases × 100); ADE occurrences over 1,000 drugs (1000-drug ADE occurrences = total number of ADE occurrences/number of medication types in each case × 100); and ADE prevalence = number of patients who had at least one ADE/total number of cases × 100%. Risk factors for PAH development in patients were analyzed using univariate analysis and multifactorial binary logistic regression. Detection using the GTT method was analyzed in comparison with spontaneous reporting during the same period to verify the validity of the GTT method.
3 Results
3.1 Creation of trigger items
ADE-related triggers for hospitalized patients with PAH were extracted in a multifaceted manner through evidence-based literature, recommended triggers in the GTT white paper, drug inserts, and the database for the spontaneous reporting of ADEs. Thirty-one initial trigger items were extracted and collated.
After expert consultation, the calculation results of the boundary values are: Mj = 3.693, Kj = 11.848%, Vj = 0.2. Based on the threshold values and expert opinions, we revised the initial trigger items and identified 24 triggers: seven laboratory indicators, six antidotes, eight clinical symptoms, and three interventions.
3.2 Validation tests for the clinical suitability of triggers
3.2.1 Basic information of patients
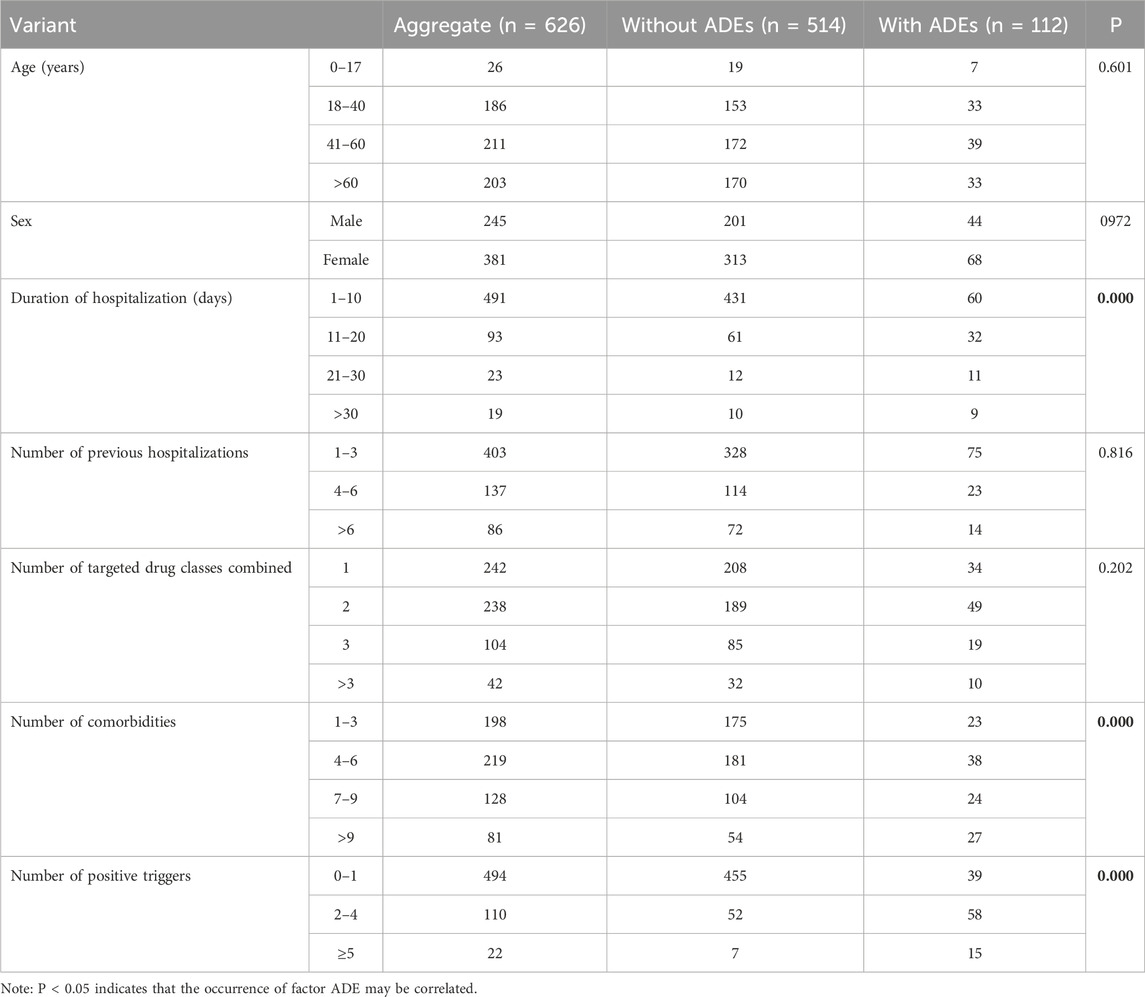
Based on the inclusion criteria, we included 626 inpatient with PAH (245 (39.14%) males and 381 (60.86%) females). The age range was 50.04 ± 18.18 years (range, 4 months to 93 years). The duration of hospitalization was 8.87 ± 8.08 (range, 1–68) days. The number of combined targeted drug classes was 1.93 ± 0.95 (1–6 types). The number of comorbidities was 5.74 ± 3.63 (range, 1–25). The number of previous hospitalizations was 3.65 ± 3.55 (range, 1–24). Respiratory medicine, general pediatrics, rheumatology, and orthopedic surgery were the main specialties involved (Table 2).
Assessment of patients with and without an ADE using a one-way chi-square test revealed no significant differences in the number of PAH-targeted drug classes combined, number of prior hospitalizations, age, or sex (P > 0.05). However, for hospitalization duration, number of comorbidities, and number of positive triggers, P < 0.05 was noted, suggesting that these may be influential factors in ADE occurrence.
3.2.2 Trigger detection
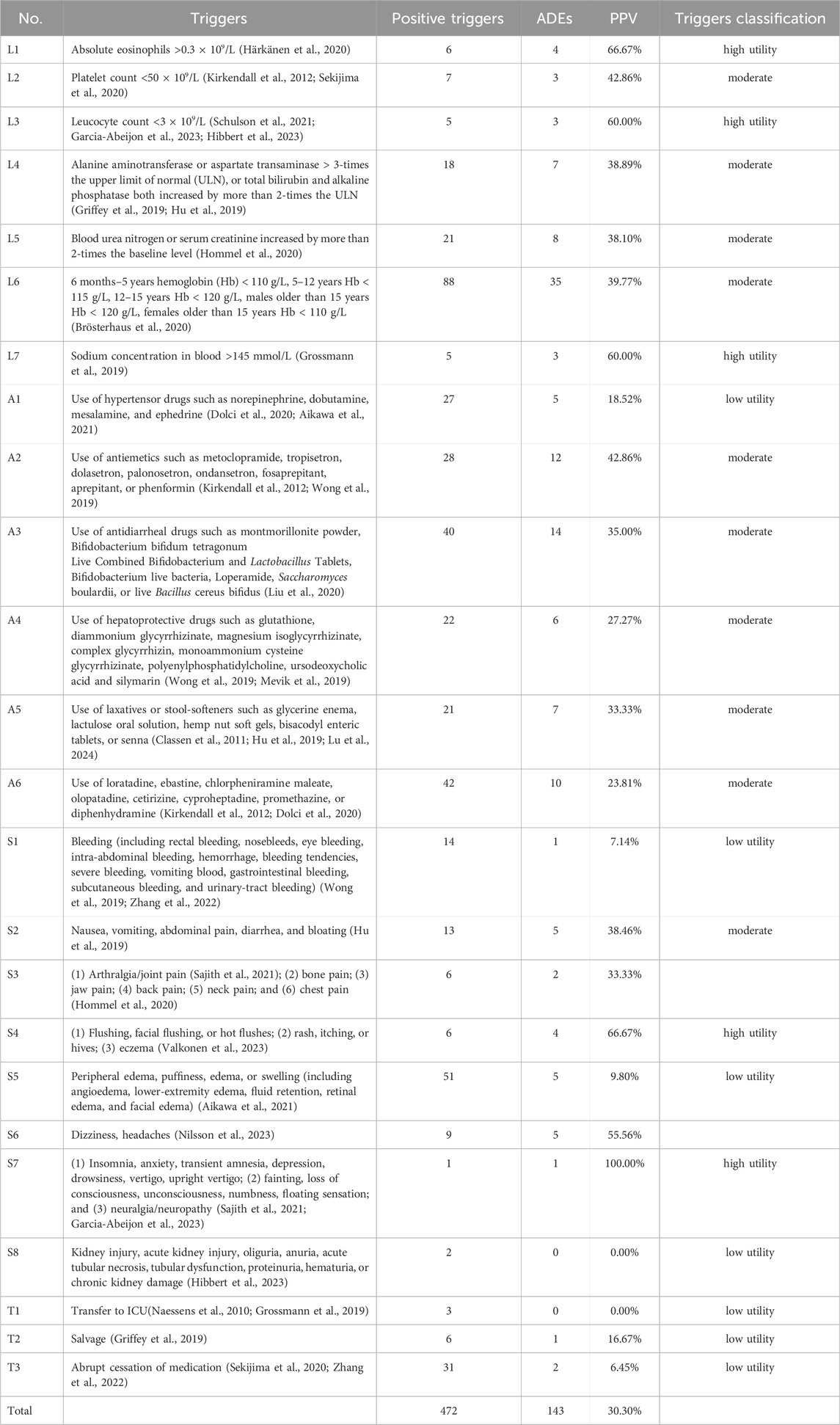
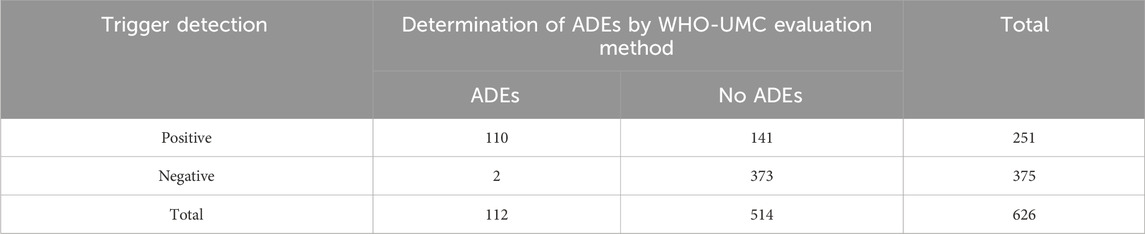
We undertook the ADE-detection test with the help of CHPS in 626 patients with PAH. All 24 trigger factors yielded positive results in actual application. Among the 626 patients, 375 had positive triggers, resulting in a total of 472 trigger instances, averaging approximately 0.75 instances per patient. A review of positive cases revealed 143 ADEs involving 110 cases, suggesting that some patients had more than one ADE. The overall PPV of the triggers was 30.30% (143/472) (Table 3).
3.2.3 Characteristics of ADEs
Among 626 patients with PAH, the triggers detected 143 ADEs in 110 patients, with a prevalence of ADE detection of 17.57% (110/626). According to the WHO-UMC assessment method, 119 cases were rated as “possibly related” (83.22%, 119/143), 19 cases were rated as “very likely related” (13.29%, 19/143), and five cases were rated as “definitely related” (3.50%, 5/143). According to the NCC MERP error-grading system, of the 143 ADEs, 24 (16.78%, 24/143) were “D injuries,” 102 (71.33%, 102/143) were “E injuries,” 15 (10.49%, 15/143) were “F injuries,” 2 (1.40%, 2/143) were “H injuries,” and no “G injuries” or “I injuries” were found. The number of ADEs in 1,000 patient days was calculated to be 25.75 (143/5,554 × 1,000), the number of ADEs in 100 patients was calculated to be 22.84 (143/626 × 100), and the number of ADEs over 1,000 drugs was calculated to be 11.84 (143/1,208 × 100).
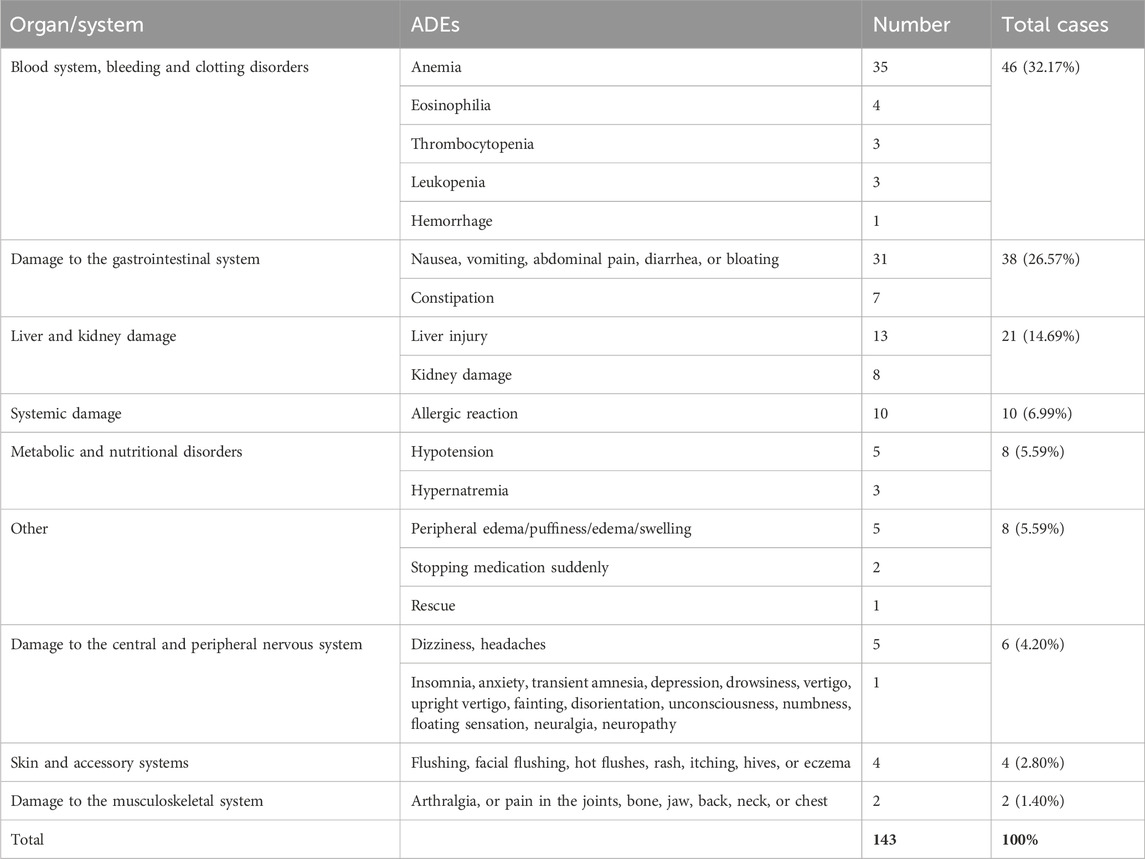
ADEs can be categorized into nine major groups in terms of the organs and systems involved. The “hematologic system, bleeding, and coagulation disorders” category was the most prevalent, amounting to 46 cases (32.17%, 46/143). These were followed by “damage to the gastrointestinal system” in 38 cases (26.57%, 38/143) and “damage to the liver and kidneys” in 21 cases (14.69%, 21/143). The remainder were “systemic damage,” “metabolic and nutritional disorders,” “skin and adnexal system,” “damage to the central and peripheral nervous system,” and “damage to the musculoskeletal system” (Table 4).
3.2.4 Evaluation of trigger utility
Our audit of the 375 patients who did not have a positive trigger revealed two patients who did not have a positive trigger but had an ADE. One patient had muscle soreness from suspected use of tadalafil and the other had headache from suspected use of selexipag tablets. The prevalence of false-negative triggering was 0.53% (2/375). The data for positive and negative cases and occurrence and non-occurrence of ADE are collated in Table 5. The sensitivity of the triggers was calculated to be 98.21% (110/112), the specificity was 72.57% (373/514), and the Correctness Index was 0.71.
3.3 Analyses of risk factors for ADE occurrence
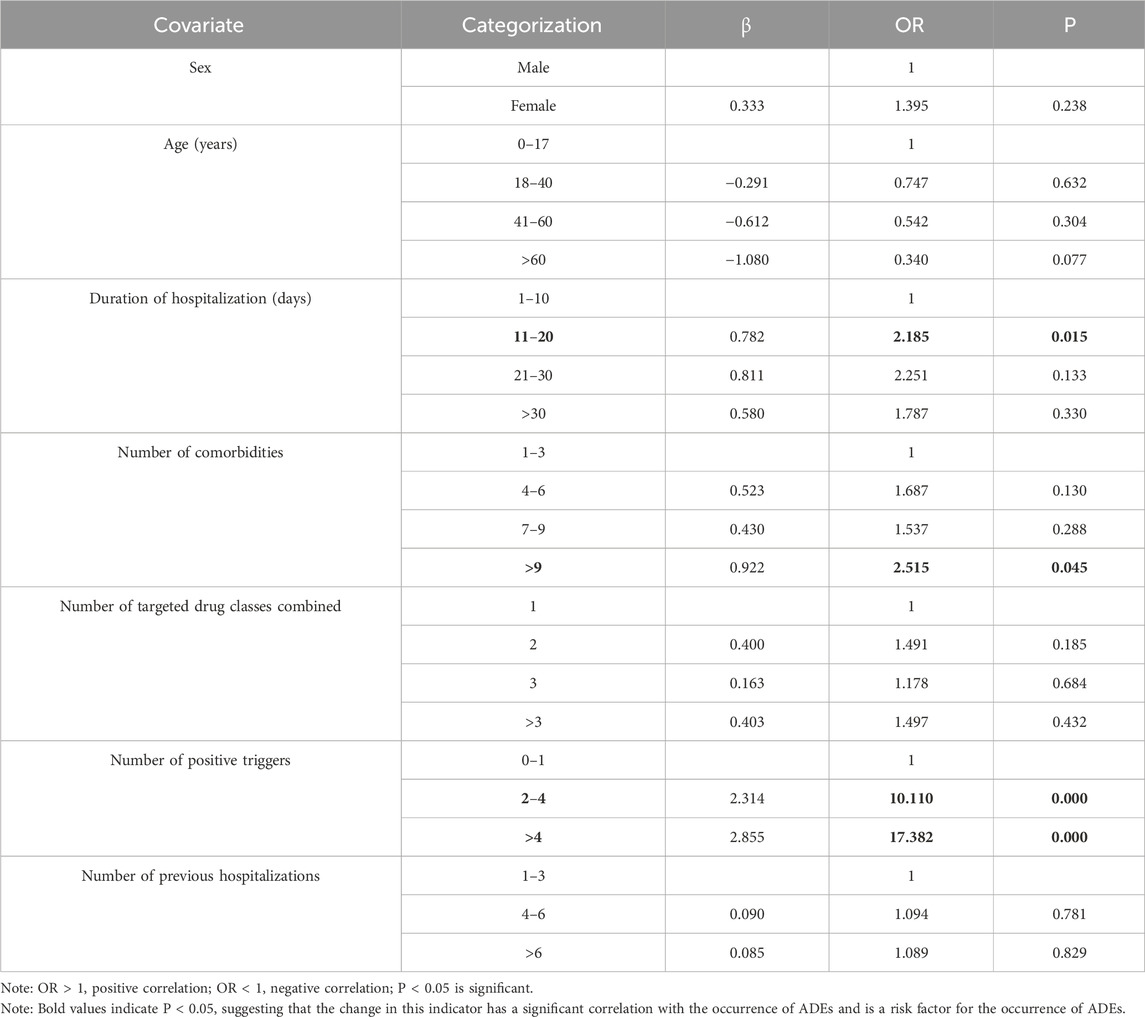
The results of the univariate statistical analysis in Table 2 showed that P < 0.05 for hospitalization duration, number of comorbidities, and number of positive triggers. Hence, these may be risk factors for ADE development in hospitalized patients with PAH. We conducted a multivariate binary logistic regression analysis with age, sex, previous hospitalizations, number of targeted drugs used concurrently, duration of hospital stay, number of comorbidities, and number of positive triggers as covariates, using ADE as the dependent variable.
The overall fit of the data to the binary logistic regression analysis model was 0.589. Hence, our data fitted well with the binary logistic regression model. A hospitalization duration of 11–20 days (odds ratio (OR) = 2.185), a number of comorbidities >9 (OR = 2.515), 2–4 positive triggers (OR = 10.110), and >4 positive triggers (OR = 17.382) all had P < 0.05 and OR > 1, suggesting that these factors were positively correlated with ADE occurrence and were risk factors for ADE occurrence (Table 6).
According to the results of binary logistic regression analysis, the probability of ADE in patients with hospitalization duration of 11–20 days was 2.185-times higher than that in patients with hospitalization duration of 1–10 days. ADE prevalence in patients with >9 comorbidities was 2.515-times higher than that in patients with 7–9 comorbidities. ADE prevalence in patients with 2–4 positive triggers was 10.110-times higher than that in patients with 0–1 positive triggers. ADE prevalence in patients with >4 positive triggers was 17.382-times that in patients with 2–4 positive triggers.
3.4 Comparative analysis of spontaneous reporting and the GTT method
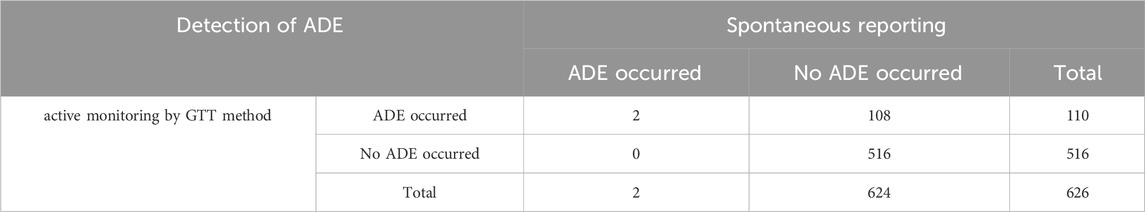
Among 626 patients with PAH treated with targeted drugs at the First Affiliated Hospital of Guangzhou Medical University between 1 January 2022 and 1 June 2023, the number of self-reported ADEs consisted of two cases involving two patients (0.32% (2/626)). The prevalence of detection of ADEs by the GTT method was 17.57%. We have organized the ADE data detected by the active monitoring and spontaneous reporting of the GTT method respectively into the following Table 7. The chi-square test revealed that the exact significance value (P value) was 0.00, which was less than 0.01, indicating that there was a significant difference in the results of the two monitoring methods. Therefore, compared with spontaneous reporting, the trigger monitoring method we have established can effectively improve the detection rate of ADEs.
The main reasons for the low spontaneous reporting rate of ADE in patients with PAH are the insufficient ability and skills of medical staff to identify ADE and concerns about responsibility. Doctors may be concerned about doctor-patient disputes and reputation damage caused by ADE reporting, as well as their unfamiliarity with the ADE reporting system and process, which leads to a relatively low enthusiasm of medical staff to report voluntarily. The GTT method established in this study can help medical staff quickly identify ADE and is conducive to improving the reporting rate of ADE.
4 Discussion
4.1 Discussion on bias factors
Whether the results of case review are correct or not is the main bias factor affecting the detection rate of ADE by the GTT method. To improve the accuracy of case review, we adopt the following measures: (1) Train the reviewers before the case review and organize them to study the training tutorial on ADE review in the GTT white paper. (2) The reviewers conduct the review independently. After the review is completed, the review results are compared. If any differences are found in the review results, they are handed over to two senior reviewers for re-examination to determine whether it is ADE. (3) When reviewing medical records, reviewers must strictly adhere to the “20-min principle” to ensure that the review time for each case is no less than 20 min. A study in the United States compared the internal review teams of hospitals with the hiring of experienced external teams and found that the sensitivity and specificity of the internal teams had both been checked by the experienced external teams (Sharek et al., 2011). In this study, a clinical pharmacist from the hospital was introduced as a junior reviewer, and a chief pharmacist and a chief physician were introduced as senior reviewers, effectively improving the sensitivity, specificity and detection rate of ADE of the trigger.
4.2 Effectiveness of trigger detection
Triggers are the basis for active monitoring of ADEs (Ji et al., 2018). We used a scientific method to extract and organize the initial triggers through several aspects, and we modified and improved the trigger items through a Delphi method. The overall PPV of the 24 triggers we established was 30.30%. According to previous studies, the overall PPV of triggers, in general, fluctuates between 10% and 40% (Magnéli et al., 2023). Hence, the overall PPV of the triggers established in our study was high.
In this real-world trial validating the clinical applicability of triggers, all 24 triggers were positive, and 22 triggers (91.67%) detected ADEs, of which 19 had an overall PPV >15%. L1 (absolute eosinophil value >0.3 × 109/L) and S4 (flushing, facial flushing, hot flushes, rash, pruritus, urticaria, eczema) had high sensitivity, high specificity, and a PPV of 66.67%. Some triggers had a high prevalence of positive detection and a low prevalence of true-positives. An example of this phenomenon was S5 (peripheral edema, puffiness, edema, or swelling (including angioedema, lower-limb edema, fluid retention, retinal edema, and facial edema)), with 51 positive detections and only five true-positives, for a PPV of only 9.8%. This finding was considered to be due to pulmonary hypertension causing right-heart failure (Chinese Medical Association, Chinese Physicians Association, Pulmonary Vascular Disease Working Committee, 2021), which then leads to edema. Therefore, edema in some patients was caused by disease development rather than ADEs. A1 (use of antihypertensive drugs such as norepinephrine, dobutamine, mesoxamine, or ephedrine) is employed commonly for surgery or resuscitation, and less often to combat ADEs. Although Beraprost in S1 can increase the tendency of bleeding, including gastrointestinal bleeding, fundus bleeding, cerebral hemorrhage, etc., there are many interfering factors for bleeding in patients with PAH, making it difficult to determine the correlation between bleeding and medication, such as bleeding caused by excessive gastrointestinal ulcers in patients themselves. Theoretically, drug withdrawal due to ADE in T3 should be relatively common, such as withdrawal due to pulmonary fibrosis caused by Ambrisentan and withdrawal due to headache caused by Macitentan, etc. However, only two cases of ADE were detected in T3. The possible reason considered is that the number of included cases is relatively small. If the data volume is expanded, T3 may detect more ADE. For triggers with a relatively high false positive rate such as A1, S1, S5 and T3, it will increase the burden on reviewers when determining the “drug-ADE” association, but it will not lead to overtreatment because the prerequisite for intervening in patients is to determine that ADE has occurred, that is, to eliminate the interference of false positives.
T1 (transfer to ICU or higher) and S8 (renal injury, acute kidney injury, oliguria, anuria, acute tubular necrosis, tubular dysfunction, proteinuria, hematuria, chronic kidney damage) did not detect ADEs despite positive detection. A possible explanation for this observation is that during our review, patients with PAH were transferred to the ICU, more often than not, after surgery, usually unrelated to ADEs. Renal injury is usually judged using indicators that must be combined with serum urea nitrogen, serum creatinine, and urine output, and excluding renal impairment due to disease is important. However, considering that the amount of included case data is limited and the above two types of ADE are not included, if the data volume T1 and S8 is expanded, ADE will be detected. Therefore, two triggers are temporarily retained.
4.3 Characterization of ADEs
Of the 143 ADEs detected by a trigger, >80% of the drug–ADE associations were rated as “possibly relevant.” Possible explanations are that pulmonary hypertension is a rare disease and that patients often have multiple comorbidities simultaneously, so the occurrence of some ADEs can be explained by the effects of other diseases or other medications. Therefore, it is more rational to conclude that drug–ADE associations were “possibly relevant.” When encountering suspected ADEs in the clinic, the principle of “report when suspected” should be followed so that no ADEs are omitted.
According to the NCC MERP error-grading system, the ADEs detected in our study were predominantly “mild” and “moderate” (grades D, E, and F in 98.6% of cases). No ADEs were detected for permanent impairment or death (grades G and I). These observations may have been due to the small number of cases in our study and short timespan, which hampered the detection of long-term, rare, and unintended or severe ADEs. Ji et al. (2018) used triggers in the GTT method for active monitoring of ADEs in pediatric hospitalized patients. They detected ADE severity grades of E (82.6%), F (11.7%), and H (5.7%), which is consistent with the results of our study. Compared with spontaneous reporting for passive surveillance during the same time period, spontaneous reporting at the First Affiliated Hospital of Guangzhou Medical University identified only two E-level injuries, and no F- or H-level injuries were identified. Hence, the GTT method that we employed allowed for more comprehensive monitoring of ADEs.
ADEs after use of PAH-targeted drugs most frequently involved the hematologic system (46 cases, 32.17%), followed by the gastrointestinal system (38 cases, 26.57%), as well as the hepatic and renal systems (21 cases, 14.69%). The main manifestations were anemia, thrombocytopenia, leukopenia, nausea, diarrhea, increased serum urea nitrogen, abnormal creatinine level, and an abnormal ALT level. Therefore, it is recommended that physicians and nurses treating patients with PAH should pay attention to gastrointestinal symptoms as well as changes in levels of blood markers, ALT, AST, total bilirubin, serum creatinine, and serum urea nitrogen to prevent and cure ADEs in a timely manner.
4.4 Risk factors for ADE occurrence
Multifactorial binary logistic regression analyses showed that the risk factors for ADE occurrence in hospitalized patients with PAH included (but were not limited to) hospitalization duration of 11–20 days, number of comorbidities >9, 2–4 positive triggers, and >4 positive triggers.
In general, the longer the duration of hospitalization, the higher the prevalence of ADEs. Patients who stay longer in hospital usually require more treatments and interventions, and so are more likely to develop ADEs (Hwang et al., 2014). Tola et al. (2023) studied the incidence and factors influencing ADEs in pediatric cancer patients. They showed that 8 days of hospitalization was a risk factor for ADE occurrence.
We showed that the number of comorbidities >9 was a risk factor for ADE occurrence. Patients with more comorbidities are usually in poorer physical condition, have lower autoimmunity, and are less likely to tolerate the side effects of a medication and, thus, are more prone to ADE occurrence, than patients with fewer comorbidities. Sendekie et al. (2023) studied ADE incidence in adult patients at Gondar University General Specialized Hospital (Ethiopia). They found that the number of comorbidities had a significant correlation with ADE incidence, which is in line with the results of the present study.
5 Research shortcomings and outlook
First, this study is a single-center research and lacks multi-center external validation. Considering that different hospitals have different electronic medical record structures, the wide application of ADE automated detection in the hospital system requires adaptive optimization. Second, only 8 targeted drugs for the treatment of PAH were included, and no other commonly used drugs were included. There are certain limitations in the analysis. Third, unintended severe ADEs were not monitored, probably due to the small number of cases and short timespan. The successful identification of unintended severe ADEs usually requires long-term continuous follow-up and the exclusion of other intervening factors.
The ADE triggers for PAH inpatients constructed in this study, which have been embedded in the sample hospital’s HIS via CHPS, enable the real-time monitoring of ADEs, allowing for the continuous monitoring of long-term, chronic, cumulative adverse events, as well as that of unintended serious ADEs. Therefore, future studies will focus on monitoring unintended severe ADEs.
6 Conclusion
The triggers for ADEs in hospitalized patients with PAH established in our study based on the GTT method had high validity and practicability. These triggers could provide assistance for ADE monitoring in patients with PAH, improve the efficiency of ADE reporting, help better reflect the situation of ADEs, avoid the occurrence of predictable ADEs, improve the safety of medication administration in patients with PAH, and provide a reference for the improvement of clinical-treatment protocols. We explored one method of active monitoring of ADEs in patients with a rare disease (PAH). Our data could provide a reference for other similar diseases.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Medical Ethics Review Committee of the First Afffliated Hospital of Guangzhou Medical University (Guangdong, China) (Ethics No. ES-2023-076-02). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MX: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review and editing. SX: Investigation, Software, Validation, Visualization, Writing – review and editing. GC: Investigation, Software, Visualization, Writing – review and editing.GL: Conceptualization, Data curation, Investigation, Software, Visualization, Writing – original draft, Writing – review and editing. SH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Project administration, Resources, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by Guangzhou Science and Technology Plan Project (No. 2023A03J0370).The Science and Technology Innovation Project of Guangdong Medical Products Administration (No. 2024ZDZ10); Philosophy and Social Science Planning Project of Guangdong Province in 2023 (No. GD23SQGL01).
Acknowledgments
We thank the experts who participated in the two rounds of Delphi consultancy for improving the trigger items. We thank LetPub (www.letpub.com.cn) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
Author GC was employed by Guangzhou Pinyi Information Technology Co.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ad, P., K, P., D, R., Sd, G., Sd, M., and P, P. (2020). Global trigger tool: proficient adverse drug reaction autodetection method in critical care patient units. Indian J. Crit. care Med. peer-reviewed, official Publ. Indian Soc. Crit. Care Med. 24, 172–178. doi:10.5005/jp-journals-10071-23367
Aikawa, G., Ouchi, A., Sakuramoto, H., Ono, C., Hatozaki, C., Okamoto, M., et al. (2021). Impact of adverse events on patient outcomes in a Japanese intensive care unit: a retrospective observational study. Nurs. Open 8, 3271–3280. doi:10.1002/nop2.1040
Anzelewicz, S., Garnier, H., Rangaswami, A., and Czauderna, P. (2017). Cultural, geographical and ethical questions when looking to enroll pediatric patients in rare disease clinical trials. Expert Opin. Orphan Drugs 5, 613–621. doi:10.1080/21678707.2017.1348293
Brösterhaus, M., Hammer, A., Kalina, S., Grau, S., Roeth, A. A., Ashmawy, H., et al. (2020). Applying the global trigger tool in German hospitals: a pilot in surgery and neurosurgery. J. Patient Saf. 16, e340–e351. doi:10.1097/PTS.0000000000000576
Chinese Medical Association, Chinese Physicians Association, Pulmonary Vascular Disease Working Committee (2021). Chinese guidelines for the diagnosis and treatment of pulmonary arterial Hypertension(2021). Chin. Med. J. 101, 11–51. doi:10.3760/cma.j.cn112137-20201008-02778
Classen, D. C., Resar, R., Griffin, F., Federico, F., Frankel, T., Kimmel, N., et al. (2011). Global trigger tool’ shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff. 30, 581–589. doi:10.1377/hlthaff.2011.0190
Dolci, E., Schärer, B., Grossmann, N., Musy, S. N., Zúñiga, F., Bachnick, S., et al. (2020). Automated fall detection algorithm with global trigger tool, incident reports, manual chart review, and patient-reported falls: algorithm development and validation with a retrospective diagnostic accuracy study. J. Med. Internet Res. 22, e19516. doi:10.2196/19516
Fornasier, G., Francescon, S., Leone, R., and Baldo, P. (2018). An historical overview over pharmacovigilance. Int. J. Clin. Pharm. 40, 744–747. doi:10.1007/s11096-018-0657-1
Garcia-Abeijon, P., Costa, C., Taracido, M., Herdeiro, M. T., Torre, C., and Figueiras, A. (2023). Factors associated with underreporting of adverse drug reactions by health care professionals: a systematic review update. Drug Saf. 46, 625–636. doi:10.1007/s40264-023-01302-7
Griffey, R. T., Schneider, R. M., Todorov, A. A., Yaeger, L., Sharp, B. R., Vrablik, M. C., et al. (2019). Critical review, development, and testing of a taxonomy for adverse events and near misses in the emergency department. Acad. Emerg. Med. 26, 670–679. doi:10.1111/acem.13724
Griffin, F. A., and Resar, R. K. (2009). “IHI global trigger tool for measuring adverse events.” in IHI Innovation Series white paper. 2nd Edition. (Cambridge, MA: Institute for Healthcare Improvement). Available online at: ihi.org.
Grossmann, N., Gratwohl, F., Musy, S. N., Nielen, N. M., Donzé, J., and Simon, M. (2019). Describing adverse events in medical inpatients using the global trigger tool. Swiss Med. Wkly. 149, w20149. doi:10.4414/smw.2019.20149
Härkänen, M., Turunen, H., and Vehviläinen-Julkunen, K. (2020). Differences between methods of detecting medication errors: a secondary analysis of medication administration errors using incident reports, the global trigger tool method, and observations. J. Patient Saf. 16, 168–176. doi:10.1097/PTS.0000000000000261
Hibbert, P. D., Molloy, C. J., Hooper, T. D., Wiles, L. K., Runciman, W. B., Lachman, P., et al. (2016). The application of the global trigger tool: a systematic review. Int. J. Qual. Health Care 28, 640–649. doi:10.1093/intqhc/mzw115
Hibbert, P. D., Molloy, C. J., Schultz, T. J., Carson-Stevens, A., and Braithwaite, J. (2023). Comparing rates of adverse events detected in incident reporting and the global trigger tool: a systematic review. Int. J. Qual. Health Care 35, mzad056. doi:10.1093/intqhc/mzad056
Hommel, A., Magnéli, M., Samuelsson, B., Schildmeijer, K., Sjöstrand, D., Göransson, K. E., et al. (2020). Exploring the incidence and nature of nursing-sensitive orthopaedic adverse events: a multicenter cohort study using global trigger tool. Int. J. Nurs. Stud. 102, 103473. doi:10.1016/j.ijnurstu.2019.103473
Howard, I. L., Bowen, J. M., Al Shaikh, L. A. H., Mate, K. S., Owen, R. C., and Williams, D. M. (2017). Development of a trigger tool to identify adverse events and harm in emergency medical services. Emerg. Med. J. 34, 391–397. doi:10.1136/emermed-2016-205746
Hu, Q., Wu, B., Zhan, M., Jia, W., Huang, Y., and Xu, T. (2019). Adverse events identified by the global trigger tool at a university hospital: a retrospective medical record review. J. Evid. Based Med. 12, 91–97. doi:10.1111/jebm.12329
Hwang, J.-I., Chin, H. J., and Chang, Y.-S. (2014). Characteristics associated with the occurrence of adverse events: a retrospective medical record review using the global trigger tool in a fully digitalized tertiary teaching hospital in Korea. J. Eval. Clin. Pract. 20, 27–35. doi:10.1111/jep.12075
Ji, H., Song, L., Xiao, J., Guo, Y., Wei, P., Tang, T., et al. (2018). Adverse drug events in Chinese pediatric inpatients and associated risk factors: a retrospective review using the global trigger tool. Sci. Rep. 8, 2573. doi:10.1038/s41598-018-20868-2
Kirkendall, E. S., Kloppenborg, E., Papp, J., White, D., Frese, C., Hacker, D., et al. (2012). Measuring adverse events and levels of harm in pediatric inpatients with the global trigger tool. Pediatrics 130, e1206–e1214. doi:10.1542/peds.2012-0179
Kleiber, N., Gariépy-Assal, L., Coulombe, J., Marcoux, S., Essouri, S., McCuaig, C., et al. (2021). Off-label use and safety of drug use in vascular anomalies. Dermatology 237, 649–657. doi:10.1159/000515980
Liston, E., O’Connor, E., and Ward, M. E. (2023). Exploring safety culture in the ICU of a large acute teaching hospital through triangulating different data sources. Healthc. (Basel) 11, 3095. doi:10.3390/healthcare11233095
Liu, Y., Yan, J., Xie, Y., and Bian, Y. (2020). Establishment of a pediatric trigger tool based on global trigger tool to identify adverse drug events of children: experience in a Chinese hospital. BMC Pediatr. 20, 454. doi:10.1186/s12887-020-02354-9
Liu, yi, Bian, yuan, and Yan, junfeng (2018). Establishing pediatric adverse drug event triggers based on a global trigger tool. Chin. J. New Drugs Clin. 37, 432–438. doi:10.14109/j.cnki.xyylc.2018.07.014
Lu, H., Zeng, Y., Shi, Q.-Z., Liu, L., Gong, Y.-Q., Li, S., et al. (2024). Low albumin combined with low-molecular-weight heparin as risk factors for liver injury using azvudine: evidence from an analysis of COVID-19 patients in a national prospective pharmacovigilance database. Int. J. Clin. Pharmacol. Ther. 62, 222–228. doi:10.5414/CP204544
Magnéli, M., Kelly-Pettersson, P., Rogmark, C., Gordon, M., Sköldenberg, O., and Unbeck, M. (2023). Timing of adverse events in patients undergoing acute and elective hip arthroplasty surgery: a multicentre cohort study using the global trigger tool. BMJ Open 13, e064794. doi:10.1136/bmjopen-2022-064794
Mevik, K., Hansen, T. E., Deilkås, E. C., Ringdal, A. M., and Vonen, B. (2019). Is a modified global trigger tool method using automatic trigger identification valid when measuring adverse events? a comparison of review methods using automatic and manual trigger identification. Int. J. Qual. Health Care 31, 535–540. doi:10.1093/intqhc/mzy210
Montané, E., and Santesmases, J. (2020). Adverse drug reactions. Med. Clin. Barc. 154, 178–184. doi:10.1016/j.medcli.2019.08.007
Naessens, J. M., O’Byrne, T. J., Johnson, M. G., Vansuch, M. B., McGlone, C. M., and Huddleston, J. M. (2010). Measuring hospital adverse events: assessing inter-rater reliability and trigger performance of the global trigger tool. Int. J. Qual. Health Care 22, 266–274. doi:10.1093/intqhc/mzq026
National Center for Adverse Drug Reaction Monitoring (2023). National adverse drug reaction monitoring annual report. Available online at: https://cdr-adr.org.cn/drug_1/aqjs_1/drug_aqjs_sjbg/202403/t20240326_50614.html (Accessed February 28, 2024).
Nebeker, J. R., Barach, P., and Samore, M. H. (2004). Clarifying adverse drug events: a clinician’s guide to terminology, documentation, and reporting. Ann. Intern Med. 140, 795–801. doi:10.7326/0003-4819-140-10-200405180-00009
Nilsson, L., Lindblad, M., Johansson, N., Säfström, L., Schildmeijer, K., Ekstedt, M., et al. (2023). Exploring nursing-sensitive events in home healthcare: a national multicenter cohort study using a trigger tool. Int. J. Nurs. Stud. 138, 104434. doi:10.1016/j.ijnurstu.2022.104434
Nilsson, L., Pihl, A., Tågsjö, M., and Ericsson, E. (2012). Adverse events are common on the intensive care unit: results from a structured record review. Acta Anaesthesiol. Scand. 56, 959–965. doi:10.1111/j.1399-6576.2012.02711.x
Pérez Zapata, A. I., Gutiérrez Samaniego, M., Rodríguez Cuéllar, E., Andrés Esteban, E. M., Gómez de la Cámara, A., and Ruiz López, P. (2015). Detection of adverse events in general surgery using the “ Trigger Tool” methodology. Cir. Esp. 93, 84–90. doi:10.1016/j.ciresp.2014.08.007
Rosen, A. K., and Mull, H. J. (2016). Identifying adverse events after outpatient surgery: improving measurement of patient safety. BMJ Qual. Saf. 25, 3–5. doi:10.1136/bmjqs-2015-004752
Routledge, P. (1998). 150 years of pharmacovigilance. Lancet 351, 1200–1201. doi:10.1016/S0140-6736(98)03148-1
Rutberg, H., Borgstedt Risberg, M., Sjödahl, R., Nordqvist, P., Valter, L., and Nilsson, L. (2014). Characterisations of adverse events detected in a university hospital: a 4-year study using the global trigger tool method. BMJ Open 4, e004879. doi:10.1136/bmjopen-2014-004879
Sajith, S. G., Fung, D. S. S., and Chua, H. C. (2021). The mental health trigger tool: development and testing of a specialized trigger tool for mental health settings. J. Patient Saf. 17, e360–e366. doi:10.1097/PTS.0000000000000606
Sakuma, M., Ohta, Y., Takeuchi, J., Yuza, Y., Ida, H., Bates, D. W., et al. (2024). Adverse events in pediatric inpatients: the Japan adverse event study. J. Patient Saf. 20, 38–44. doi:10.1097/PTS.0000000000001180
Schulson, L. B., Novack, V., Folcarelli, P. H., Stevens, J. P., and Landon, B. E. (2021). Inpatient patient safety events in vulnerable populations: a retrospective cohort study. BMJ Qual. Saf. 30, 372–379. doi:10.1136/bmjqs-2020-011920
Sekijima, A., Sunga, C., and Bann, M. (2020). Adverse events experienced by patients hospitalized without definite medical acuity: a retrospective cohort study. J. Hosp. Med. 15, 42–45. doi:10.12788/jhm.3235
Sendekie, A. K., Netere, A. K., Tesfaye, S., Dagnew, E. M., and Belachew, E. A. (2023). Incidence and patterns of adverse drug reactions among adult patients hospitalized in the university of gondar comprehensive specialized hospital: a prospective observational follow-up study. PLoS One 18, e0282096. doi:10.1371/journal.pone.0282096
Sharek, P. J., Parry, G., Goldmann, D., Bones, K., Hackbarth, A., Resar, R., et al. (2011). Performance characteristics of a methodology to quantify adverse events over time in hospitalized patients. Health Serv. Res. 46, 654–678. doi:10.1111/j.1475-6773.2010.01156.x
Spangler, D., Edmark, L., Winblad, U., Colldén-Benneck, J., Borg, H., and Blomberg, H. (2020). Using trigger tools to identify triage errors by ambulance dispatch nurses in Sweden: an observational study. BMJ Open 10, e035004. doi:10.1136/bmjopen-2019-035004
Takata, G. S., Mason, W., Taketomo, C., Logsdon, T., and Sharek, P. J. (2008). Development, testing, and findings of a pediatric-focused trigger tool to identify medication-related harm in US children’s hospitals. Pediatrics 121, e927–e935. doi:10.1542/peds.2007-1779
Tola, W. O., Melaku, T., Fufa, D., and Sheleme, T. (2023). Adverse drug events and contributing factors among pediatric cancer patients at jimma university medical center, southwest Ethiopia. BMC Pediatr. 23, 77. doi:10.1186/s12887-023-03891-9
Valkonen, V., Haatainen, K., Saano, S., and Tiihonen, M. (2023). Evaluation of global trigger tool as a medication safety tool for adverse drug event Detection—A cross-sectional study in a tertiary hospital. Eur. J. Clin. Pharmacol. 79, 617–625. doi:10.1007/s00228-023-03469-5
Wong, C. I., Zerillo, J. A., Stuver, S. O., Siegel, J. H., Jacobson, J. O., and McNiff, K. K. (2019). Role of adverse events in unscheduled hospitalizations among patients with solid tumors who receive medical oncology treatment. JOP 15, e39–e45. doi:10.1200/JOP.18.00319
Zhang, N., Pan, L.-Y., Chen, W.-Y., Ji, H.-H., Peng, G.-Q., Tang, Z.-W., et al. (2022). A risk-factor model for antineoplastic drug-induced serious adverse events in cancer inpatients: a retrospective study based on the global trigger tool and machine learning. Front. Pharmacol. 13, 896104. doi:10.3389/fphar.2022.896104
Keywords: global trigger tool, pulmonary arterial hypertension, adverse drug events, active monitoring, Chinese hospital pharmacovigilance system
Citation: Xu M, Xiong S, Chen G, Li G and He S (2025) Active surveillance of adverse drug events in hospitalized patients with pulmonary arterial hypertension based on the global trigger tool. Front. Pharmacol. 16:1533634. doi: 10.3389/fphar.2025.1533634
Received: 24 November 2024; Accepted: 04 July 2025;
Published: 14 August 2025.
Edited by:
Narasaiah Kolliputi, University of South Florida, United StatesReviewed by:
Vinod Kumar Yata, Malla Reddy University, IndiaSantenna Chenchula, All India Institute of Medical Sciences, Bhopal, India
Copyright © 2025 Xu, Xiong, Chen, Li and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suzhen He, c3V6aGVuMDE5QDE2My5jb20=; Guozhi Li, MTc5Mzg3NDY2QHFxLmNvbQ==
 Mengdan Xu
Mengdan Xu Shunyu Xiong
Shunyu Xiong Guanquan Chen3
Guanquan Chen3 Guozhi Li
Guozhi Li