Abstract
Objective:
The efficacy of compound Xuanju capsule (CXC) in the treatment of erectile dysfunction (ED) remains unclear. This study aimed to quantitatively assess the benefits and risks of CXC in the treatment of ED.
Methods:
Eight major databases were systematically searched for relevant literature published till 10 May 2025. Studies were screened based on established criteria; meta-analysis and trial sequential analysis of included literature were conducted.
Results:
The meta-analysis demonstrated that, compared with phosphodiesterase type 5 inhibitors alone, the combination of CXC and phosphodiesterase type 5 inhibitors significantly improved the International Index of Erectile Function (IIEF)-5 score by 3.19 points (mean difference [MD] = 3.19; 95% confidence interval [CI]: 2.42–3.96; P < 0.00001), clinical effectiveness rate by 23% (risk ratio [RR] = 1.23; 95% CI: 1.15–1.32; P < 0.00001), penile cavernous blood flow by 5.21 cm/s (MD = 5.21; 95% CI: 4.43–6.00; P < 0.00001), and serum testosterone levels by 4.09 nmol/L (MD = 4.09; 95% CI: 3.14–5.04; P < 0.00001). There was no significant difference in total adverse events between the groups (RR = 0.94; 95% CI: 0.62–1.42; P = 0.77). Trial sequential analysis confirmed that the meta-analysis results for IIEF-5, clinical effectiveness rate, penile cavernous blood flow, and serum testosterone levels were conclusive. However, the results of adverse events require further validation through additional similar studies. Funnel plot analysis and Egger’s test indicated no potential publication bias for outcomes other than the clinical effectiveness rate.
Conclusion:
CXC improves erectile function and testosterone levels in patients with ED without increasing the incidence of adverse events. These findings support the potential role of CXC as an adjunctive treatment for ED. However, due to the limitations in the quality of the current evidence, further validation through multicenter, randomized, double-blind controlled trials is necessary.
1 Introduction
Erectile dysfunction (ED) is a common disorder in men, characterized by the inability to achieve or maintain a sufficient erection for satisfactory sexual performance (Hatzimouratidis et al., 2010). The etiology of ED is multifactorial, including vasogenic, neurogenic, endocrine, pharmacologic depression, systemic diseases, and local penile injury (Lizza and Rosen, 1999). ED not only adversely affects male psychological wellbeing and mutual sexual satisfaction but also poses a potential threat to family harmony (Amoo et al., 2017). An epidemiological survey in 2,000 estimated that approximately 150 million people suffer from ED worldwide, a figure projected to rise to 322 million by 2025 (McKinlay, 2000; Shamloul and Ghanem, 2013). The combined prevalence of ED in adult males has been reported to exceed 20%, trending toward younger age (Nguyen et al., 2017), with ED patients aged 20–40 years accounting for 30% of the total number of patients (Huang et al., 2023). Phosphodiesterase-5 inhibitors (PDE5Is) are the first-line pharmacological treatment for ED, primarily by enhancing the bioavailability of nitric oxide (NO) (Mehrotra et al., 2007). However, PDE5Is do not cure ED and merely provide symptomatic relief. Additionally, while improving blood supply to the penis, PDE5Is also affect the function of tissues, such as blood vessels, smooth muscle in the airway, and skeletal muscle, which increases the risk of potential adverse events (Carson and Lue, 2005). PDE5Is may also increase the psychological burden of patients, and psychological resistance to PDE5Is has been reported in several patients with ED (Cai et al., 2020). Therefore, there is a need to develop a safe, long-term, and effective adjunctive treatment strategy for ED.
Compound Xuanju capsule (CXC) a traditional Chinese medicine preparation for treating ED, consisting of Black Ant [Formicidae; Camponotus spp], Epimedii folium [Berberidaceae; Epimedium brevicornu Maxim], Lycii Fructus [Solanaceae; Lycium barbarum L.], and Cnidii Fructus [Apiaceae; Cnidium monnieri (L.) Cuss] (Wang et al., 2020). Previous animal studies have shown that CXC improves erectile function by increasing serum testosterone and luteinizing hormone levels, as well as enhancing the index of accessory reproductive organs (Zhou et al., 2011). Additionally, pharmacological research indicates that CXC suppresses inflammatory responses by downregulating levels of TNF-α, IL-1β, and IL-17, thereby alleviating damage caused by prostatitis and autoimmune arthritis (Li et al., 2014; Wang et al., 2015; Wang CY. et al., 2016). Quantitative analysis using the multi-metabolites by single marker (QAMS) method has identified the main active metabolites of CXC as icariin, baohuoside I, osthole, catechin, epicatechin, bergapten, and imperatorin (Wang et al., 2020). Among these, icariin has been identified as the main active metabolite of CXC, with a content of over 4.0 mg/g (Ling, 2014). As research has progressed, the role of CXC in male diseases, especially ED, has received increasing attention. Previous studies have shown that CXC increases erectile hardness and duration in patients with ED, thereby improving the quality of their sexual lives (Wang et al., 2022). Additionally, CXC has been used to treat ED in conjunction with other male conditions, such as prostatitis and premature ejaculation (Liu et al., 2019; Hu et al., 2023). However, owing to the lack of high-quality evidence-based data, the specific benefits and risks of combining CXC with PDE5Is for the treatment of ED are unclear. In the present study, we performed a meta-analysis and trial sequential analysis (TSA) to evaluate the efficacy and safety of CXC, and to provide an evidence-based rationale for its use in the treatment of ED.
2 Phytochemical information of CXC
The CXC investigated in this study is a traditional Chinese medicine preparation produced by Cnstrong Company, with the national medicine approval number Z20060462. Currently, all CXC available on the market are original research drugs, and no generic versions have been approved for sale. CXC is composed of Black Ant [Formicidae; Camponotus spp], Epimedii folium [Berberidaceae; Epimedium brevicornu Maxim], Lycii Fructus [Solanaceae; Lycium barbarum L.], and Cnidii Fructus [Apiaceae; Cnidium monnieri (L.) Cuss]. The QAMS method has identified icariin, baohuoside I, osthole, catechin, epicatechin, bergapten, and imperatorin as the main active metabolites of CXC (Wang et al., 2020). Icariin is identified as the main active metabolite of CXC, with a content of over 4.0 mg/g (Ling, 2014). Detailed information on the CXC formulations used in each study is provided in Table 1.
TABLE 1
| Composition | Main active metabolites | Standard | ||
|---|---|---|---|---|
| Drug | Scientific name | Family | ||
| Black ant | Camponotus spp. | Formicidae | Icariin, baohuoside I, osthole, catechin, epicatechin, bergapten, and imperatorin | Icariin >4.0 mg/g |
| Epimedii folium | Epimedium brevicornu Maxim. | Berberidaceae | ||
| Lycii fructus | Lycium barbarum L. | Solanaceae | ||
| Cnidii fructus | Cnidium monnieri (L.)Cuss. | Apiaceae | ||
Metabolites and basic information of CXC.
CXC, compound Xuanju capsules.
Icariin can reduce the damage to endothelial and smooth muscle cells in the corpus cavernosum by inhibiting pyroptosis and apoptosis in rat corpus cavernosum tissue, thereby improving erectile function in diabetic rats (Yang et al., 2025). Additionally, icariin and baohuoside can relax corpus cavernosum smooth muscle by activating the NO/phosphodiesterase type 5/cyclic guanosine monophosphate (NO/PDE5/cGMP) signaling pathway, thus exerting a therapeutic effect on ED (Li et al., 2022). Osthole has the ability to upregulate the expression of hydrogen sulfide (H2S) generating enzymes and promote the endogenous synthesis of H2S, which is considered a key gaseous signaling molecule that facilitates smooth muscle relaxation (Alan-Albayrak et al., 2025). This suggests that osthole can regulate smooth muscle relaxation by promoting the synthesis and release of H2S, thereby improving erectile function (Alan-Albayrak et al., 2025). Catechin and epicatechin are both flavonoids that exhibit binding affinities for PDE5, comparable to that of sildenafil (Ejeje et al., 2024). Catechin and epicatechin can treat ED by inhibiting oxidative stress damage in endothelial and smooth muscle cells of the corpus cavernosum (Wang et al., 2024; Smith et al., 2023). Although there are no direct reports on the use of bergapten and imperatorin in the treatment of ED, both metabolites have demonstrated anti-inflammatory and antioxidant properties (Xu et al., 2025; Feng et al., 2025). In summary, as main active metabolites of CXC, icariin, baohuoside, osthole, catechin, and epicatechin can improve erectile function, while bergapten and imperatorin may have a positive impact on ED through their anti-inflammatory or anti-oxidative properties.
3 Materials and methods
This study strictly followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021) and has been registered in PROSPERO (CRD420251072306, www.crd.york.ac.uk/PROSPERO/view/CRD420251072306).
3.1 Literature search
The search strategy for this study was developed using a combination of subject terms and free words, with the search field limited to the title or abstract. The search formula used was: ([Xuanju] AND [Erectile Dysfunction OR Male Impotence OR Male Sexual Impotence OR Impotence]). Literature related to the treatment of ED with CXC was retrieved from four public Chinese databases (China National Knowledge Infrastructure, VIP, Wanfang, and Sinomed), and four public English databases (Embase, PubMed, Cochrane Library, and Web of Science), with no restrictions on language, region, or other factors. The initial search was conducted on 31 February 2024, followed by an updated search on 10 May 2025.
3.2 Inclusion and exclusion criteria
The inclusion criteria were as follows: (i) the study design was a randomized controlled trial (RCT); (ii) patients had a diagnosis of ED (Zhang et al., 2016); (iii) the control group received PDE5Is, including sildenafil, vardenafil, tadalafil, lodenafil, avanafil, udenafil, mirodenafil, and other similar drugs; (iv) the experimental group received treatment with CXC in combination with PDE5Is, and the PDE5Is used were the same as those in the control group; (v) the efficacy endpoints included international index of erectile function-5 (IIEF-5) score, the clinical effectiveness rate, penile cavernous blood flow, and serum testosterone level, while the safety endpoint was the incidence of adverse events. The IIEF-5 is a widely utilized tool for assessing ED. It comprises five key questions that evaluate various aspects of erectile function, including confidence, hardness, and the ability to maintain an erection. The IIEF-5 is appreciated for its simplicity, cost-effectiveness, and robust scientific foundation, making it an essential instrument in clinical practice for both screening and managing ED. The clinical effectiveness rate is defined as the proportion of patients who report satisfaction with their erectile function following treatment. Additionally, penile cavernous blood flow and serum testosterone levels are important objective measures in the evaluation of erectile dysfunction. Penile cavernous blood flow, assessed through penile color Doppler ultrasound, is one of the key parameters for evaluating ED. Serum testosterone levels are positively correlated with libido and erectile function, making them a common indicator for investigating the causes of ED. Adverse events refer to any discomfort experienced by patients during the treatment period, including headaches, dizziness, nasal congestion, facial flushing, nausea, abdominal bloating, dyspepsia, gastrointestinal bleeding, liver function abnormalities, rashes, and other related symptoms.
The exclusion criteria were as follows: (i) research data published in duplicate; (ii) research data that were unusable; and (iii) non-comparable baseline information for the test and control groups.
3.3 Literature screening
The initial step involved importing the entire set of retrieved studies into a dedicated literature management software to facilitate efficient organization and screening. Predefined inclusion and exclusion criteria were applied to identify relevant studies. Two reviewers (RL and YY) independently performed the screening process based on titles and abstracts. Discrepancies in study selection were discussed collaboratively, and unresolved disagreements were resolved through consensus with a third reviewer (GZ). This rigorous screening process ensured the quality and relevance of the studies selected for further analysis.
3.4 Data collection
Following the screening process, the full texts of the eligible studies were retrieved for detailed data extraction. Two independent reviewers (RL and YY) systematically extracted key information from each study, including study characteristics, participant details, intervention details, measured outcomes, and main results. Any ambiguities or missing data encountered during this process prompted attempts to contact the original study authors for clarification. Consistency checks were performed to ensure data accuracy, and discrepancies between the two reviewers were discussed and resolved with the input of GZ.
3.5 Risk of bias assessment
The methodological quality of included studies was appraised using the Risk of Bias Tool provided by RevMan 5.3. The evaluation covered key domains such as sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential biases. Each study was independently evaluated by RL and YY to minimize subjective bias. The assessments were rated as ‘low risk,’ ‘high risk,’ or ‘unclear risk’ for each domain. Any disagreements between the reviewers were addressed through discussion until consensus was reached, or GZ was consulted, if necessary. The results of this assessment informed the overall interpretation of study quality and the robustness of the meta-analytical findings.
3.6 Statistical analysis
The primary meta-analysis was conducted using RevMan 5.3. For continuous variables, the mean difference (MD) with 95% confidence interval (CI) was employed as the effect measure, whereas for dichotomous variables, the risk ratio (RR) with 95% CI was utilized. To assess heterogeneity across studies, the I2 statistic was calculated; an I2 value of <50% was considered indicative of low heterogeneity, while values ≥ 50% suggested substantial heterogeneity. Based on heterogeneity levels, either a fixed-effects model (for I2 < 50%) or a random-effects model (for I2 ≥ 50%) was selected to combine the data. The threshold for statistical significance was set at a two-tailed P-value of less than 0.05.
To further investigate the influence of clinical factors on the primary efficacy endpoints, predefined subgroup analyses were performed. These analyses focused on variables such as the dosage of CXC agents, the type of control medications used, and the duration of treatment. By stratifying the data accordingly, we aimed to identify potential variations in treatment effects attributable to these clinical factors, thereby enhancing the interpretability of our results. In addition, a leave-one-out sensitivity analysis was conducted to evaluate the robustness of the combined results. This method involved systematically removing one study at a time from the meta-analysis to assess how each individual study impacted the overall effect size. This process allowed us to determine whether any single study disproportionately influenced the results, thereby providing insights into the stability and reliability of our findings.
A sequential analysis of the data was performed using TSA software version 0.9 beta. This approach determines whether the accumulated evidence from the meta-analysis is conclusive. The results were considered definitive when the Z-value curve reached the boundary value, indicating that sufficient evidence had been accumulated to support the conclusions drawn from the meta-analysis.
Publication bias was assessed using funnel plots and Egger’s test. Funnel plots are graphical tools that plot the effect size of each study against a measure of its precision. Asymmetry in the funnel plot may indicate the presence of publication bias, where smaller studies with non-significant results are less likely to be published. Egger’s test, performed using Stata 15.0, is a statistical method to formally test for funnel plot asymmetry. The test generates a P-value, and a P-value > 0.1 was used as an indication that there was no significant publication bias. The non-significant P-value suggests that the observed asymmetry in the funnel plot could be due to chance rather than true publication bias.
Finally, we evaluated the certainty of the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which considers factors such as study limitations, inconsistency, indirectness, imprecision, and publication bias. This comprehensive evaluation enabled a clear assessment of the overall quality of the evidence supporting our findings.
4 Results
4.1 Literature screening
The database search initially identified 296 documents. During the screening process, 161 duplicate documents were excluded, and 122 documents were excluded based on the inclusion and exclusion criteria. Ultimately, 13 documents (Liu et al., 2019; Chen et al., 2012; Dai et al., 2018; Gao and Wang, 2016; Huang, 2020; Jiang et al., 2016; Liu, 2024; Nan et al., 2022; Sun, 2023; Wang et al., 2018; Wang, 2019; Wang Y. et al., 2016; Zhang, 2024) were included in the final analysis. The detailed process of literature screening is illustrated in Figure 1.
FIGURE 1

Literature screening process.
4.2 Basic characteristics
A total of 13 clinical studies (Liu et al., 2019; Chen et al., 2012; Dai et al., 2018; Gao and Wang, 2016; Huang, 2020; Jiang et al., 2016; Liu, 2024; Nan et al., 2022; Sun, 2023; Wang et al., 2018; Wang, 2019; Wang Y. et al., 2016; Zhang, 2024) were included in this review. All the experimental centers were located in China, the publication years were from 2012 to 2024, and the total sample size was 1,019 cases. Among them, 511 were treated with PDE5Is, while 508 were treated with CXC combined with PDE5Is. The characteristics of the included studies are shown in Table 2.
TABLE 2
| Study | Sample (E/C) | Age(years) | Disease duration (months) | Intervention | Control | Treatment duration (weeks) |
|---|---|---|---|---|---|---|
| Chen et al. (2012) | 18/18 | 40.2 | — | CXC 0.84 g tid Sildenafil 50 mg |
Sildenafil 100 mg | 8 |
| Dai et al. (2018) | 42/45 | 42.9 | — | CXC 1.26 g tid Tadalafil 10 mg |
Tadalafil 10 mg | 8 |
| Gao and Wang (2016) | 40/40 | 47.1 | 48.0 | CXC 1.26 g tid Sildenafil 50 mg Vitamin E 10 mg tid |
Sildenafil 50 mg Vitamin E 10 mg tid |
4 |
| Huang (2020) | 40/40 | 41.8 | — | CXC 1.26 g tid Tadalafil 10 mg |
Tadalafil 10 mg | 8 |
| Jiang et al. (2016) | 40/40 | — | — | CXC 1.26 g tid Tadalafil 10 mg |
Tadalafil 10 mg | 4 |
| Liu et al. (2019) | 40/40 | 33.0 | 7.5 | CXC 1.26 g tid Sildenafil 50 mg |
Sildenafil 50 mg | 6 |
| Liu (2024) | 62/62 | 40.2 | 8.7 | CXC 1.26 g tid Tadalafil 5–20 mg |
Tadalafil 5–20 mg | 12 |
| Nan et al. (2022) | 43/43 | 31.5 | 7.4 | CXC 1.26 g tid Tadalafil 10 mg |
Tadalafil 10 mg | 12 |
| Sun (2023) | 40/40 | 41.9 | 11.6 | CXC 1.26 g tid Sildenafil 50 mg |
Sildenafil 50 mg | 12 |
| Wang et al. (2018) | 40/40 | 43.8 | 13.9 | CXC 1.26 g tid Tadalafil 5 mg |
Tadalafil 5 mg | 4 |
| Wang (2019) | 31/31 | 39.8 | — | CXC 1.26 g tid Tadalafil 10 mg |
Tadalafil 10 mg | 4 |
| Wang Y. et al. (2016) | 42/42 | 37.4 | 41.4 | CXC 1.26 g tid Fatanafil 50 mg |
Fatanafil 10 mg | 8 |
| Zhang (2024) | 30/30 | 42.4 | 12.7 | CXC 1.26 g tid Sildenafil 50 mg |
Sildenafil 50 mg | 4 |
Basic characteristics of the included studies.
Baseline information, including sex, age, and disease duration, was comparable between the experimental and control groups in each of the included studies. CXC, compound Xuanju capsules.
4.3 Risk of bias
In the risk of bias assessment, seven studies were flagged with an unclear risk level in the domain of random sequence generation. This uncertainty stemmed from the insufficient details regarding the randomization process provided in these studies. Regarding allocation concealment, 12 studies were deemed to have an unclear risk. The root cause was the lack of comprehensive information about the concealment methods employed, leaving the integrity of the allocation process open to question. In the domain of blinding of participants and personnel, 13 studies were assigned an unclear risk rating. The main issue was that these studies failed to mention the use of a placebo or the specific blinding techniques, which could potentially introduce bias into the research. On a more positive note, the included studies demonstrated a low risk of bias in several other critical areas. These areas encompass blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential sources of bias, as shown in Figure 2.
FIGURE 2
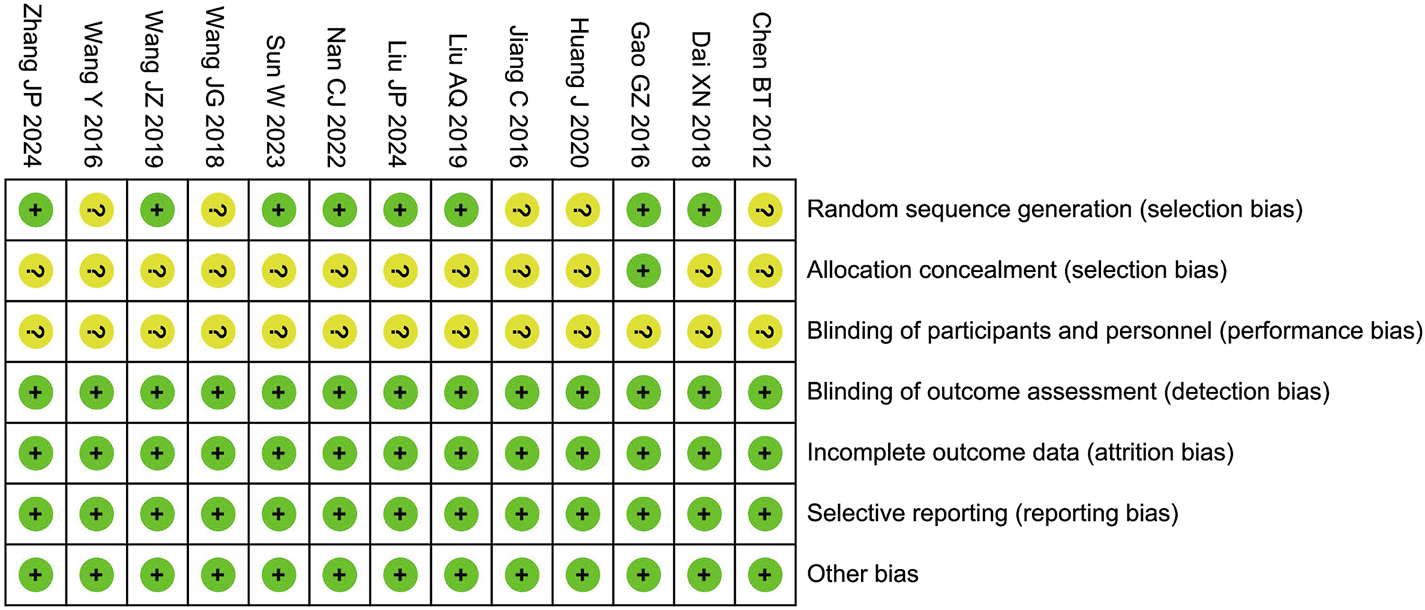
Risk assessment of bias.
4.4 Meta-analysis
4.4.1 IIEF-5
The meta-analysis of the IIEF-5 score included 13 studies with 1,019 participants. The results showed that the combination of CXC with PDE5Is significantly improved the IIEF-5 score by 3.19 points compared to PDE5Is alone (MD = 3.19; 95% CI: 2.42–3.96; P < 0.00001), as shown in Figure 3. The sensitivity analysis showed that the meta-analysis results of IIEF-5 were robust.
FIGURE 3
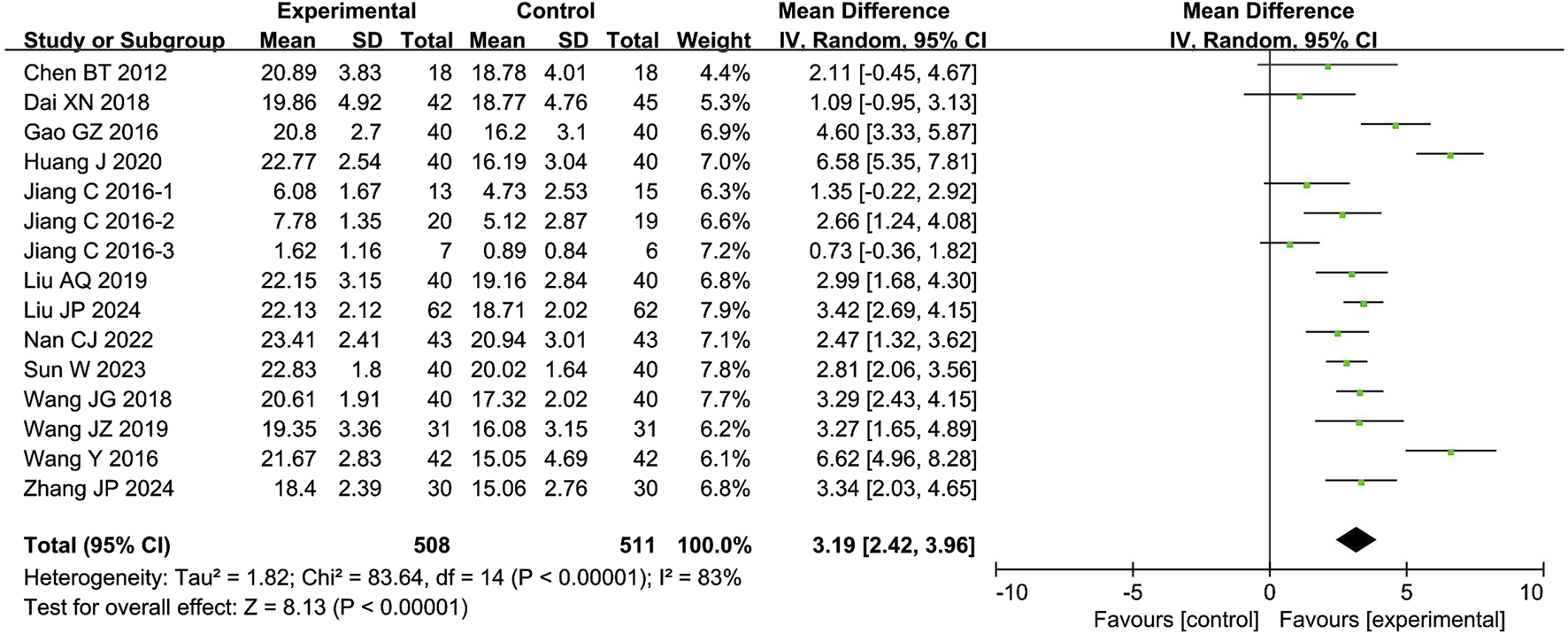
Forest plot of the meta-analysis on International Index of Erectile Function-5 (IIEF-5).
4.4.2 Clinical effectiveness rate
The meta-analysis of the clinical effectiveness rate included 10 studies with 585 participants. The results showed that the combination of CXC with PDE5Is significantly increased the clinical effectiveness rate by 23% compared with PDE5Is (RR = 1.23; 95% CI: 1.15–1.32; P < 0.00001), as shown in Figure 4. The sensitivity analysis showed that the meta-analysis results of IIEF-5 were robust.
FIGURE 4
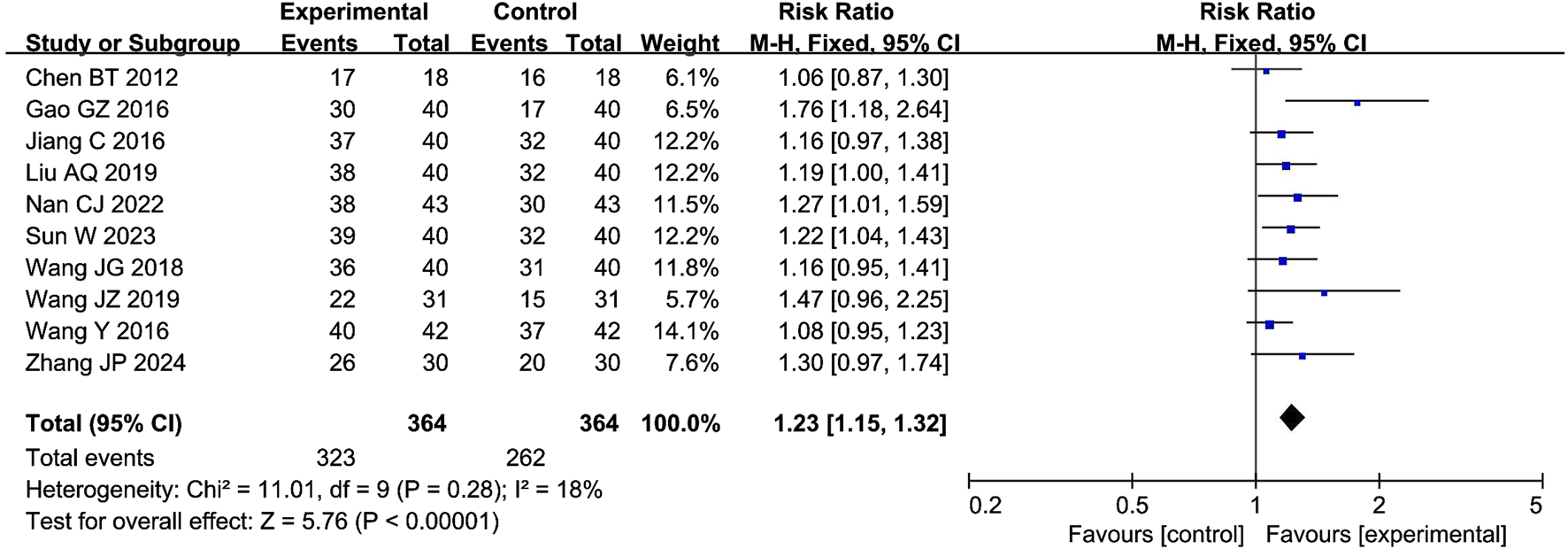
Forest plot of the meta-analysis on the clinical effectiveness rate.
4.4.3 Penile cavernous blood flow
The meta-analysis of penile cavernous blood flow included 3 studies with 284 participants. The results showed that the combination of CXC with PDE5Is significantly increased the penile cavernous blood flow by 5.21 cm/s compared with PDE5Is (MD = 5.21; 95% CI: 4.43–6.00; P < 0.00001), as shown in Figure 5. The sensitivity analysis showed that the meta-analysis results of penile cavernous blood flow were robust.
FIGURE 5
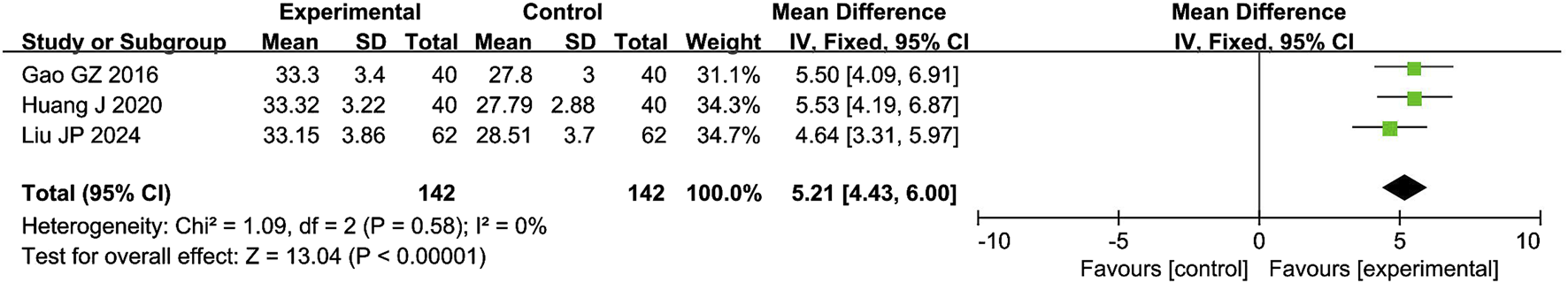
Forest plot of the meta-analysis on the penile cavernous blood flow.
4.4.4 Serum testosterone levels
The meta-analysis of serum testosterone levels included 2 studies with 140 participants. The results showed that the combination of CXC with PDE5Is significantly increased the penile cavernous blood flow by 4.09 nmol/L compared with PDE5Is (MD = 4.09; 95% CI: 3.14–5.04; P < 0.00001), as shown in Figure 6. The sensitivity analysis showed that the meta-analysis results of serum testosterone levels were robust.
FIGURE 6
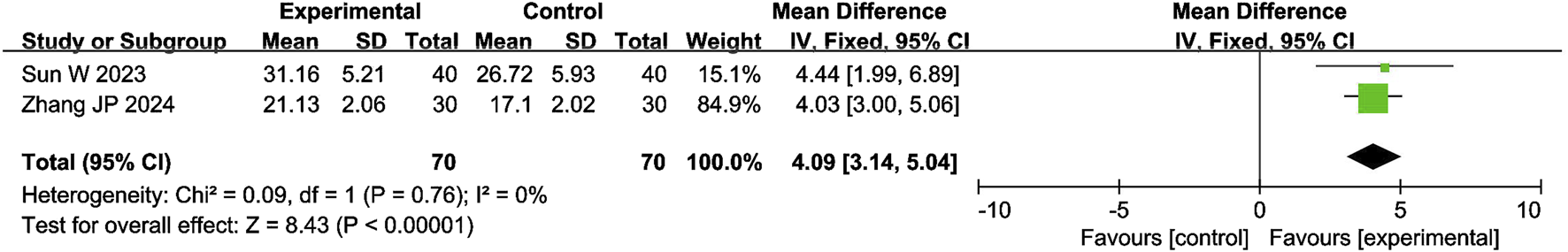
Forest plot of the meta-analysis on serum testosterone levels.
4.4.5 Adverse events
The meta-analysis of adverse events included 10 studies with 837 participants. The results showed that the total adverse events were 9.11% (38/417) for the combination of compound CXC with PDE5Is and 9.76% (41/420) for the PDE5Is, which were comparable between the two groups (RR = 0.94; 95% CI: 0.62–1.42; P = 0.77), as shown in Figure 7. The sensitivity analysis showed that the meta-analysis results of adverse events were robust. In the analysis of individual adverse events, headache (RR = 0.73; 95% CI: 0.38–1.38; P = 0.33), dizziness (RR = 0.33; 95% CI: 0.01–7.96; P = 0.50), nasal congestion (RR = 0.39; 95% CI: 0.09–1.66; P = 0.20), facial flushing (RR = 1.00; 95% CI: 0.26–3.87; P = 1.00), nausea (RR = 1.80; 95% CI: 0.39–8.26; P = 0.45), abdominal distension (RR = 1.33; 95% CI: 0.30–5.87; P = 0.70), dyspepsia (RR = 1.33; 95% CI: 0.34–5.21; P = 0.68), gastrointestinal bleeding (RR = 0.33; 95% CI: 0.04–3.14; P = 0.34), liver dysfunction (RR = 0.50; 95% CI: 0.09–2.65; P = 0.42), and rash (RR = 5.00; 95% CI: 0.24–102.07; P = 0.30) were all comparable between the two groups, as shown in Table 3.
FIGURE 7
![Forest plot showing the risk ratios of various studies comparing experimental and control groups. Each study is represented with a blue square indicating the risk ratio and a horizontal line showing the confidence interval. The plot also includes a diamond at the bottom, representing the overall effect estimate. The x-axis shows the risk ratio scale ranging from 0.005 to 200, with a vertical line at 1. The overall risk ratio is 0.94 with a 95% confidence interval of [0.62, 1.42]. Study weights and heterogeneity statistics are noted.](https://www.frontiersin.org/files/Articles/1537789/xml-images/fphar-16-1537789-g007.webp)
Forest plot of the meta-analysis on total adverse events.
TABLE 3
| Adverse event | Experimental group | Control group | I2 | RR (95% CI) | P value |
|---|---|---|---|---|---|
| Headache | 14/337 | 20/340 | 0 | 0.73 (0.38, 1.38) | 0.33 |
| Dizziness | 0/42 | 1/42 | 0 | 0.33 (0.01, 7.96) | 0.50 |
| Nasal congestion | 2/124 | 6/127 | 0 | 0.39 (0.09, 1.66) | 0.20 |
| Facial flushing | 4/82 | 4/82 | 0 | 1.00 (0.26, 3.87) | 1.00 |
| Nausea | 4/111 | 2/111 | 0 | 1.80 (0.39, 8.26) | 0.45 |
| Abdominal distension | 3/142 | 2/118 | 0 | 1.33 (0.30, 5.87) | 0.70 |
| Dyspepsia | 4/115 | 3/118 | 0 | 1.33 (0.34, 5.21) | 0.68 |
| Gastrointestinal bleeding | 0/80 | 2/80 | 0 | 0.33 (0.04, 3.14) | 0.34 |
| Liver dysfunction | 2/80 | 4/80 | 0 | 0.50 (0.09, 2.65) | 0.42 |
| Rash | 2/62 | 0/62 | 0 | 5.00 (0.24,102.07) | 0.30 |
Meta-analysis of individual adverse events associated with compound Xuanju capsules in the treatment of erectile dysfunction.
RR, risk ratio; CI, confidence interval.
4.5 Subgroup analysis
Subgroup analyses in this study were conducted to investigate the drug dosage, control drug, and treatment duration on the effects using IIEF-5 as an indicator. The results showed that in terms of dosage, 1.26 g/time of CXC significantly improved IIEF-5 score (MD = 3.24; 95% CI: 2.45–4.03; P < 0.00001), while 0.84 g/time CXC did not have a significant effect on the IIEF-5 score (MD = 2.11; 95% CI: -0.45–4.67; P = 0.11). In terms of control drugs, tadalafil (MD = 2.78; 95% CI: 1.79–3.76, P < 0.00001), sildenafil (MD = 3.26; 95% CI: 2.54–3.99; P < 0.00001), or vardenafil (MD = 6.62; 95% CI: 4.96–8.28; P < 0.00001) in combination with CXC significantly improved IIEF-5 scores. In terms of duration of treatment, both ≤6 weeks (MD = 2.78; 95% CI: 1.90–3.66; P < 0.00001) and >6 weeks (MD = 3.66; 95% CI: 2.35–4.98; P < 0.0001) of treatment with CXC significantly improved the IIEF-5 score, as shown in Table 4.
TABLE 4
| Subject | Subgroup | I2 | MD (95% CI) | P value |
|---|---|---|---|---|
| CXC dosage | 0.84 g/time | 0 | 2.11 (−0.45, 4.67) | 0.11 |
| 1.26 g/time | 84 | 3.24 (2.45, 4.03) | <0.00001 | |
| Control drug | Tadalafil | 87 | 2.78 (1.79, 3.76) | <0.00001 |
| Sildenafil | 38 | 3.26 (2.54, 3.99) | <0.00001 | |
| Vardenafil | 0 | 6.62 (4.96, 8.28) | <0.00001 | |
| Treatment duration | ≤6 weeks | 74 | 2.78 (1.90, 3.66) | <0.00001 |
| >6 weeks | 88 | 3.66 (2.35, 4.98) | <0.00001 |
Subgroup analysis of compound Xuanju capsules in the treatment of erectile dysfunction.
CXC, compound Xuanju capsules; CI, confidence interval; MD, mean difference.
4.6 TSA
In the present study, the TSA revealed that the Z-value curves for the IIEF-5 and the clinical effectiveness rate crossed the boundaries in the third and fourth studies, respectively. In contrast, both the penile cavernous blood flow and serum testosterone levels crossed the boundaries in the first study. These findings suggest that the meta-analysis results for these parameters are conclusive. Regarding adverse events, the cumulative Z-value was significantly smaller than the TSA boundary value of 83,577. This indicates that the results for adverse events require validation through more studies. Relevant details are presented in Figure 8.
FIGURE 8
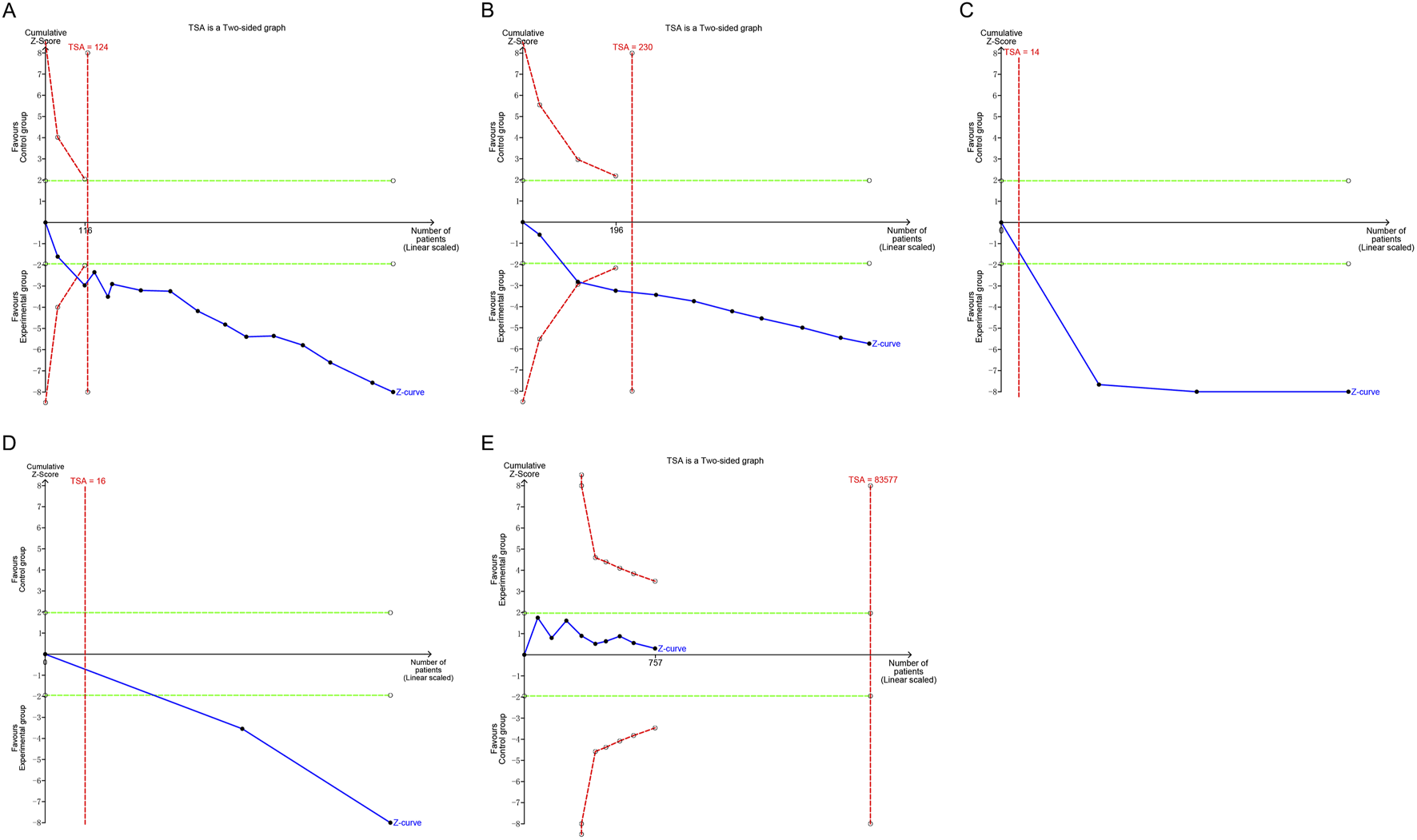
Trial sequential analysis of the efficacy and safety endpoints: (A) IIEF-5; (B) Clinical effectiveness rate; (C) Penile cavernous blood flow; (D) Serum testosterone levels; (E) Total adverse events. IIEF-5, International Index of Erectile Function-5.
4.7 Publication bias
The funnel plots for IIEF-5, clinical effectiveness rate, and adverse events displayed an asymmetric scatter distribution, suggesting the presence of potential publication bias. In contrast, the funnel plot for penile cavernous blood flow showed a symmetrical scatter distribution, indicating no publication bias. The Egger’s test revealed no potential publication bias for IIEF-5 (P = 0.989), penile cavernous blood flow (P = 0.630), and adverse events (P = 0.617), suggesting that the observed publication bias in the funnel plots may not have a significant impact on the results. However, the Egger’s test also indicated the presence of potential publication bias for the clinical effectiveness rate (P = 0.004), as shown in Figure 9. Since only two studies were included for serum testosterone levels, the assessment of publication bias was not conducted.
FIGURE 9
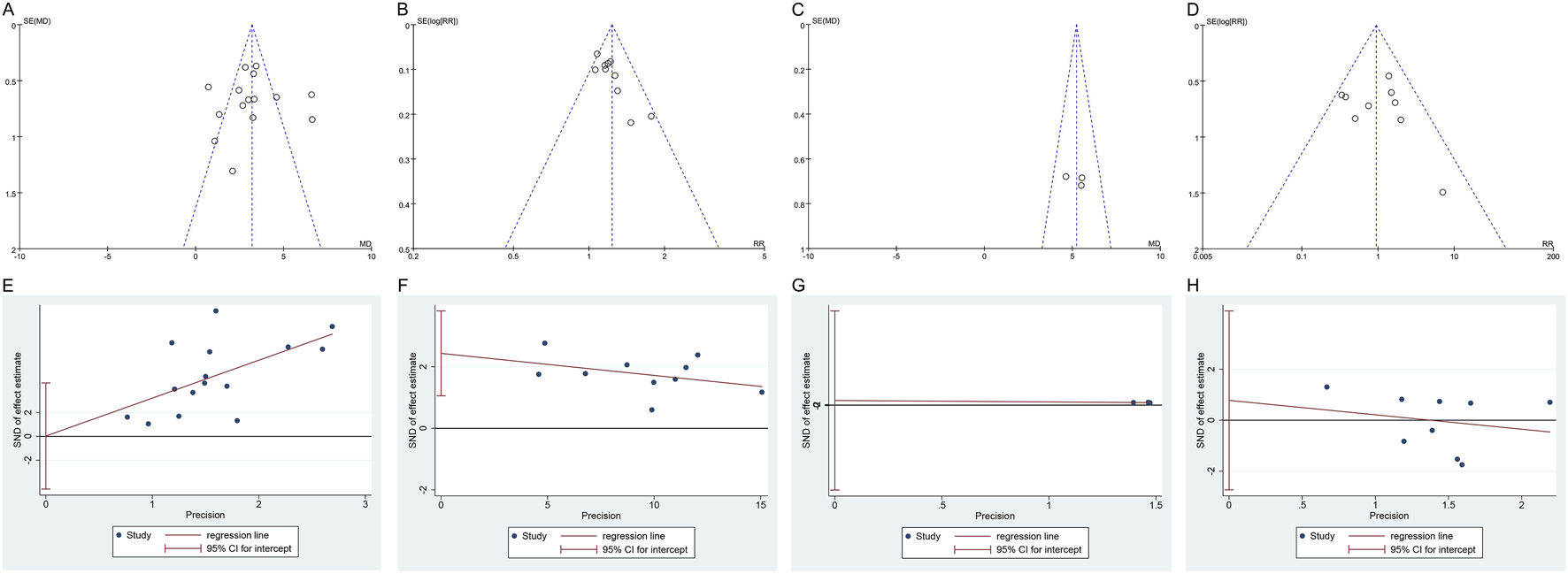
Funnel plot and Egger’s test of publication bias: (A) Funnel plot of IIEF-5; (B) Funnel plot of clinical effectiveness rate; (C) Funnel plot of penile cavernous blood flow; (D) Funnel plot of total adverse events; (E) Egger’s test of IIEF-5; (F) Egger’s test of clinical effectiveness rate; (G) Egger’s test of penile cavernous blood flow; (H) Egger’s test of total adverse events. IIEF-5, International Index of Erectile Function-5.
4.8 Certainty of evidence
According to the GRADE system, the certainty of evidence for the IIEF-5, clinical effectiveness rate, penile cavernous blood flow, serum testosterone levels, and adverse events was determined to be low. Detailed results of this assessment are presented in Table 5.
TABLE 5
| Outcome | Risk of bias | Inconsistency | Indirectness | Imprecision | Others | RR/MD (95% CI) | Certainty of evidence |
|---|---|---|---|---|---|---|---|
| IIEF-5 | Serious | Serious | None | None | None | 3.19 (2.42, 3.96) | Low |
| Clinical effectiveness rate | Serious | None | None | None | Publication bias | 1.23 (1.15, 1.32) | Low |
| Penile cavernous blood flow | Serious | None | None | Serious | None | 5.21 (4.43, 6.00) | Low |
| Serum testosterone levels | Serious | None | None | Serious | None | 4.09 (3.14, 5.04) | Low |
| Adverse events | Serious | None | None | Serious | None | 0.94 (0.62, 1.42) | Low |
Certainty of evidence.
IIEF-5, International Index of Erectile Function-5; CI, confidence interval; MD, mean difference; RR, risk ratio.
5 Discussion
5.1 Research background and significance
ED is a common condition that leads to decreased quality of life and is an important cause of depression in men (Amoo et al., 2017). A European research study found that patients with ED had significantly lower physical and mental domain quality of life scores than men without ED (Jannini et al., 2014). A related meta-analysis showed a 192% increased risk of depression in men with ED, compared to those without ED (Liu et al., 2018). Given the potential detriment to mental health in men with ED, it is necessary to intervene aggressively. PDE5Is are widely recognized as first-line treatments for ED, as they maintain the cyclic guanosine monophosphate (cGMP) concentration in penile tissue by inhibiting PDE-5 activity, which in turn promotes NO bioavailability and facilitates the onset and maintenance of an erection (Omote, 1999). Although PDE5Is offer hope to patients with ED, their potential limitations and adverse events remain a concern. While PDE5Is provide temporary relief from the symptoms of ED, they cannot cure the disease itself (Cai et al., 2020). Long-term administration of PDE5Is not only leads to decreased sensitivity to the drug, but may also cause patients to develop resistance (Cai et al., 2020). Additionally, although most patients tolerate PDE5Is well, adverse events such as headache, dyspepsia, and visual abnormalities may still occur (Dhaliwal and Gupta, 2024). Therefore, it is important to explore adjunctive therapeutic strategies that can improve erectile function.
CXC is a traditional Chinese medicine preparation produced by Cnstrong company, and currently only the original drug with the national medicine approval number Z20060462 is circulating on the market. It is composed of Black Ant [Formicidae; Camponotus spp], Epimedii folium [Berberidaceae; Epimedium brevicornu Maxim], Lycii Fructus [Solanaceae; Lycium barbarum L.], and Cnidii Fructus [Apiaceae; Cnidium monnieri (L.) Cuss]. The main active metabolites include icariin, baohuoside I, osthole, catechin, epicatechin, bergapten, and imperatorin (Wang et al., 2020). In 2006, Cai et al. (Cai et al., 2006) published the first clinical trial of CXC for the treatment of ED, reporting promising therapeutic potential. There have subsequently been numerous positive reports on the use of CXC for the treatment of male disorders. Nevertheless, due to the lack of high-quality evidence-based data, the potential risks and relative benefits of CXC in patients with ED remain unclear. This study includes the first meta-analysis and TSA evaluating the combination of CXC with a PDE5I for treating ED, aiming to strengthen the clinical evidence supporting the use of CXC.
5.2 Evaluation of effectiveness
The IIEF-5 score is a widely used tool for diagnosing ED. Owing to its high sensitivity and specificity in the diagnosis of ED, the IIEF-5 score is widely used to both diagnose ED and determine its severity (Rc et al., 1997). Clinical effectiveness rate, defined as the percentage of people satisfied with erectile function relative to the total population, is important for assessing the effectiveness of a given treatment (Liu, 2016). The penile cavernous blood flow and serum testosterone levels are critical factors in the evaluation of ED, as they play pivotal roles in achieving and maintaining an erection (Hoppe et al., 2023; Moreno et al., 2011). Adequate penile blood flow is crucial for erectile function, as it directly responds to the physiological processes involved in achieving an erection (Hoppe et al., 2023). Additionally, low testosterone levels can adversely affect libido and contribute to ED by impairing the regulation of nitric oxide synthase (NOS) (Moreno et al., 2011). This meta-analysis showed that compared with PDE5I alone, CXC combined with PDE5Is significantly improved the IIEF-5 scores by 3.19, clinical effectiveness rate by 23%, penile cavernous blood flow by 5.21 cm/s, and serum testosterone levels by 4.09 noml/L, suggesting that the addition of CXC to PDE5I can effectively enhance and maintain erectile function.
Furthermore, CXC has also demonstrated the ability to reduce PDE5I dependence and maintain long-term effects. A study by Porter et al. (Chen et al., 2012) showed that the combination of CXC with 50 mg of sildenafil improved the IIEF-5 scores and Total Sexual Satisfaction (TSS) partner scores of patients more substantially over a 2-month treatment period than 100 mg of sildenafil alone. This suggests that a combination regimen containing CXC reduces the necessary PDE5I dosage and helps reduce patient dependence and psychological resistance to PDE5Is. Additionally, Nan et al. (Nan et al., 2022)found that 6 months after the discontinuation of treatment, patients with ED who had been treated with CXC for a period of 3 months still had better erectile function than the control group. These results suggest that CXC may have a long-term effect on improving erectile function, rather than a purely symptomatic treatment.
Additionally, the included studies reported that CXC also conferred benefits in terms of Erection Hardness Score (EHS), Sexual Encounter Profile Question 3 (SEP-Q3) score, Quality of Erection Questionnaire (QEQ), and TSS score. The EHS is a common tool used to assess erectile hardness; SEP-Q3 is an important measure of the ability of a patient to perform and maintain an erection during sexual intercourse; the QEQ is a questionnaire used to assess erectile function and quality of sexual life in men; and the TSS is an indicator of the satisfaction of patients and their sexual partners with erectile function and sexual life. The improvements in these indicators suggest that CXC effectively improves penile erection hardness, male sexual life quality, and partner sexual life satisfaction.
Subgroup analyses showed that in terms of dosage, 1.26 g/time of CXC significantly improved the IIEF-5 scores, while 0.84 g/time of CXC had no significant effect on IIEF-5 scores, suggesting that 1.26 g/time may be the lowest effective dose of CXC for the treatment of ED. In terms of control medications, combining CXC with tadalafil, sildenafil, or vardenafil improved the IIEF-5 scores of patients, suggesting that the benefit of CXC was not limited by the specific type of PDE5I. In terms of treatment duration, patients with ED treated with CXC for either less than or more than 6 weeks experienced improvements in IIEF-5 scores, suggesting that CXC may have both short- and long-term effects. This suggests that CXC can be flexibly paired with different PDE5I and is adaptable to different treatment durations.
5.3 Mechanism analysis
Penile erections involve the synergistic action of tissues, such as the vascular endothelium, autonomic nerves, bundles of smooth muscle cells, and fibroblasts. Following sexual stimulation, nerve impulses release neurotransmitters in the corpus cavernosum, leading to the production of NO by endothelial cells and its diffusion into the neighboring smooth muscle cells. Subsequently, NO stimulates vascular smooth muscle cells to synthesize cGMP, resulting in vasodilation and increased blood flow to the cavernous body of the penis, and, in turn, penile erection (McMahon, 2019). As a classical ED therapeutic drug, PDE5Is mainly reduce cGMP degradation by inhibiting PDE-5 activity, which, in turn, maintains penile blood supply and improves erectile function. The mechanism of action of CXC in treating ED may be related to the promotion of NO release and an increase in serum testosterone levels. CXC has been shown to increase serum NO and NOS levels. NOS plays a key role in NO synthesis, and generates NO by catalyzing the oxidation of L-arginine (Naseem, 2005). NO increases blood flow to the cavernous body of the penis by stimulating cGMP synthesis in vascular smooth muscle, thus maintaining the erectile state of the penis (Cooke and Losordo, 2002). Additionally, CXC has been reported to increase serum testosterone and follicle-stimulating hormone levels (Wu et al., 2013). Testosterone not only maintains the anatomical structure and physiological function of sex organs but also plays a role in central and peripheral penile erection regulation (Gerald and Raj, 2022). Therefore, this suggests that CXC may assist in regulating penile erectile function by increasing testosterone levels.
The primary active components of CXC include icariin, baohuoside I, osthole, catechin, epicatechin, bergapten, and imperatorin (Wang et al., 2020). Notably, icariin stands out as the principal active metabolite, with a concentration exceeding 4.0 mg/g (Ling, 2014). First, icariin enhances the transmembrane transport of cholesterol by upregulating the expression of StAR, P450C17, and the benzodiazepine receptor (PBR), which subsequently boosts testosterone synthesis (Chen et al., 2014). Additionally, it facilitates the neogenesis and stabilization of the vascular endothelium by increasing the expression of sphingosine-1-phosphate (S1P) and its receptor, S1PR1, thereby improving erectile function (Yao et al., 2023). Second, baohuoside I promotes relaxation of the corpus cavernosum smooth muscle by activating the NO/PDE5/cGMP signaling pathway, providing a therapeutic effect on ED (Li et al., 2022). Third, osthole enhances the expression of enzymes responsible for generating H2S, promoting its endogenous synthesis, which aids in regulating smooth muscle relaxation and improving erectile function (Alan-Albayrak et al., 2025). Fourth, both catechin and epicatechin, as flavonoids, exhibit binding affinities for PDE5 that are comparable to those of sildenafil (Ejeje et al., 2024), which indicates that these metabolites can mitigate oxidative stress damage in endothelial and smooth muscle cells within the corpus cavernosum (Wang et al., 2024; Smith et al., 2023). Although there are no direct reports on the use of bergapten and imperatorin for treating ED, they have been proven to possess anti-inflammatory or antioxidant properties (Xu et al., 2025; Feng et al., 2025). This protective effect may also be beneficial for ED.
Furthermore, several additional active metabolites in CXC have been validated by modern pharmacological studies for their roles in treating ED. Black ants (Formicidae; Camponotus spp) are rich in essential nutrients, including zinc and vitamins D and E (Yang et al., 2020). Notably, black ants contain a substantial amount of zinc, ranging from 120 to 198 mg/kg (Yang et al., 2020). Research indicates that zinc can enhance testosterone synthesis and release by inhibiting oxidative stress driven by xanthine oxidase/uric acid and downregulating the inhibitory effects on the pituitary-testis axis, ultimately improving libido and erectile function in male rats (Besong et al., 2023). Vitamin D has been shown to upregulate the NO/cGMP pathway by promoting the synthesis and release of endothelium-derived NO, thereby enhancing penile erectile function (Talib et al., 2017). Additionally, vitamin E increases NO levels and superoxide dismutase activity in penile tissue by elevating intracavernous pressure/mean arterial pressure in rat models, which further improves erectile function (Helmy et al., 2012). Lycium barbarum polysaccharide, the primary active metabolite in Lycii Fructus (Solanaceae; Lycium barbarum L), has been demonstrated to enhance penile blood supply by upregulating eNOS, nNOS, and cGMP expression, while also improving corpus cavernosum function by mitigating cavernous nerve injury through increased antioxidant enzyme activity (Zhao et al., 2016; Moon et al., 2017).
5.4 Security evaluation
We conducted a statistical analysis of adverse events associated with the combination of CXC and PDE5Is in the treatment of ED. The overall incidence of adverse events was found to be 9.11%. The rates of specific adverse events were as follows: headache 4.15%, nasal congestion 1.61%, facial flushing 4.88%, nausea 3.60%, abdominal distension 2.11%, dyspepsia 3.48%, liver dysfunction 2.50%, rash 3.23%, dizziness 0%, and gastrointestinal bleeding 0%. The meta-analysis indicated that the adverse effects of CXC combined with PDE5Is were comparable to those of PDE5Is alone, suggesting that the addition of CXC did not significantly increase the risk of additional adverse events (RR = 0.94; 95% CI: 0.62–1.42; P = 0.77). Furthermore, the combination of CXC and PDE5Is did not elevate the incidence of individual adverse events, including headache (RR = 0.73; 95% CI: 0.38–1.38; P = 0.33), dizziness (RR = 0.33; 95% CI: 0.01–7.96; P = 0.50), nasal congestion (RR = 0.39; 95% CI: 0.09–1.66; P = 0.20), facial flushing (RR = 1.00; 95% CI: 0.26–3.87; P = 1.00), nausea (RR = 1.80; 95% CI: 0.39–8.26; P = 0.45), abdominal distension (RR = 1.33; 95% CI: 0.30–5.87; P = 0.70), dyspepsia (RR = 1.33; 95% CI: 0.34–5.21; P = 0.68), gastrointestinal bleeding (RR = 0.33; 95% CI: 0.04–3.14; P = 0.34), liver dysfunction (RR = 0.50; 95% CI: 0.09–2.65; P = 0.42), and rash (RR = 5.00; 95% CI: 0.24–102.07; P = 0.30). The adverse events reported during the treatment period in the included studies were generally mild and manageable. Researchers speculate that these adverse events may be related to PDE5Is, as these inhibitors can cross-react with PDE isoenzymes, leading to transient vasodilation in the heart and other organs (Schwarz et al., 2007). Currently, there is no evidence to suggest that CXC may cause additional adverse events during the treatment of ED, indicating that CXC may serve as a safe complementary therapy.
To comprehensively assess the safety of CXC, we conducted a thorough review of all RCTs reporting adverse events associated with CXC, without restricting the analysis to specific diseases. In these trials, the experimental group received either CXC alone or CXC in combination with conventional medications, while the control group received only conventional treatments. Ultimately, 41 studies were included in this analysis (Supplementary Table S1). Of these, 16 focused on the safety of CXC in treating ED, seven on prostatitis, six on polycystic ovary syndrome, five on oligoasthenospermia, four on infertility, two on premature ejaculation, and one on rheumatoid arthritis. The results of the meta-analysis indicated that CXC did not increase the incidence of adverse events such as headache, dizziness, nasal congestion, flushing, nausea, abdominal distension, indigestion, gastrointestinal bleeding, abnormal liver function, dysuria, diarrhea, palpitations, skin allergies, fatigue, dry mouth, or constipation (P > 0.05), as shown in Supplementary Table S2. These findings suggest that CXC is a safe therapeutic option.
It is important to note that the complication rate or adverse event rate presented in this study may not fully reflect real-world outcomes associated with the combination of CXC and PDE5Is. Firstly, the included studies in our meta-analysis were predominantly RCTs. These trials often have strict inclusion and exclusion criteria, which may lead to a selected sample that is not fully representative of the general population. For example, patients with certain comorbidities or those taking other medications may be excluded from the trials. In real-world clinical practice, patients often present with complex medical histories and may be concurrently using multiple medications, which could potentially interact with CXC and PDE5Is and increase the risk of adverse events. Secondly, the follow-up periods (4–12 weeks) in the included studies were relatively limited. Some adverse events may have a long-term latency period and may not be detected within the short follow-up durations of these trials. For instance, chronic liver or kidney function impairment may take months or even years to manifest, but the follow-up in most of our included studies was likely not long enough to capture such events. Thirdly, the reporting of adverse events in the original studies may be incomplete. There could be under-reporting of mild or self-limiting adverse events, as patients may not always disclose these symptoms to their healthcare providers, and researchers may not actively collect data on all possible adverse events. Moreover, the sample sizes of some of the included studies were relatively small. Small sample sizes may lead to insufficient statistical power to detect rare but serious adverse events. Consequently, the complication rates we calculated may underestimate the true risk of adverse events associated with the combination of CXC and PDE5Is. While our meta-analysis provides valuable information on the safety of CXC and PDE5Is combination, the complication rate presented here should be interpreted with caution. Future large-scale, long-term, and real-world studies are needed to more accurately assess the safety profile of this combination therapy.
5.5 Limitations and outlook
Although the results of this study contribute valuable clinical evidence for the treatment of ED with CXC, some potential limitations must be acknowledged. First, due to insufficient reporting of allocation concealment and blinding of participants and personnel in the included studies, there may be an increased risk of selection and performance bias. Future studies should enhance the description and implementation of intervention blinding to ensure the scientific validity and rigor of the study design. Second, the present study only included studies conducted in China, and the study samples were primarily from clinical centers in China, which may have led to a geographic bias. Future studies should aim to expand the geographic scope by including multiple countries or regions and incorporating patient populations from different ethnic and cultural backgrounds to enhance the generalizability of the findings. Third, although the present study evaluated the clinical effects of CXC in combination with PDE5Is, the benefits and risks of CXC alone could not be explained. Future studies should focus on the effects of CXC alone and explore its benefits and risks in ED maintenance therapy. Fourth, the TSA in the present study indicated that the sample size for adverse events did not meet expectations, suggesting that the findings from the analysis of adverse events need to be validated by additional similar studies. Future studies should strengthen the monitoring and reporting of adverse events to comprehensively assess the safety of CXC. Additionally, the long-term effects and safety of CXC need to be further explored to provide more reliable clinical guidance.
6 Conclusion
CXC effectively enhances erectile function and testosterone levels in patients with ED, without increasing the incidence of adverse events. These findings support the potential of CXC as a supplementary treatment for ED, with a recommended dosage of 1.26 g administered three times daily. However, due to the limited quality of the current evidence, further validation through multicenter, randomized, double-blind, controlled trials is necessary.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
RL: Conceptualization, Data curation, Methodology, Supervision, Writing – original draft. ML: Data curation, Formal analysis, Writing – review and editing. YY: Data curation, Methodology, Writing – original draft. BG: Formal Analysis, Writing – original draft. RY: Formal Analysis, Writing – review and editing. QH: Conceptualization, Supervision, Writing – review and editing. GZ: Conceptualization, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Hunan Provincial Traditional Chinese Medicine Research Program (E2022007), the Key Project of the First Class Discipline of Integrated Traditional Chinese and Western Medicine at Hunan University of Chinese Medicine (2021ZXYJH10), the Graduate Innovation Project of Hunan University of Chinese Medicine (2024CX042), and the Yifang Innovation Project of Hunan University of Chinese Medicine (2024YF10).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1537789/full#supplementary-material
References
1
Alan-Albayrak E. Pinilla E. Comerma-Steffensen S. Erac Y. Yetik-Anacak G. Simonsen U. et al (2025). Osthole improves erectile function by increasing endogenous H2S production. Eur. J. Pharmacol.997, 177619. 10.1016/j.ejphar.2025.177619
2
Amoo E. O. Omideyi A. K. Fadayomi T. O. Ajayi M. P. Oni G. A. Idowu A. E. (2017). Male reproductive health challenges: appraisal of wives coping strategies. Reprod. Health14, 90. 10.1186/s12978-017-0341-2
3
Besong E. E. Akhigbe T. M. Ashonibare P. J. Oladipo A. A. Obimma J. N. Hamed M. A. et al (2023). Zinc improves sexual performance and erectile function by preventing penile oxidative injury and upregulating circulating testosterone in lead-exposed rats. Redox Rep.28, 2225675. 10.1080/13510002.2023.2225675
4
Cai J. Deng Z. X. Jian H. B. (2006). Observation on the efficacy of Compound Xuanju Capsule in the treatment of erectile dysfunction. Zhonghua Nan Ke Xue12, 568–569. 10.3969/j.issn.1009-3591.2006.06.028
5
Cai Z. Song X. Zhang J. Yang B. Li H. (2020). Practical approaches to treat ED in PDE5i nonresponders. Aging Dis.11, 1202–1218. 10.14336/AD.2019.1028
6
Carson C. C. Lue T. F. (2005). Phosphodiesterase type 5 inhibitors for erectile dysfunction. BJU Int.96, 257–280. 10.1111/j.1464-410X.2005.05614.x
7
Chen B. T. Yang H. Wu J. S. (2012). Combination of Compound Xuanju Capsule and sildenafil for erectile dysfunction that progressively fails to respond to sildenafil. Natl. J. Androl.18, 1101–1104. 10.13263/j.cnki.nja.2012.12.012
8
Chen M. Hao J. Yang Q. Li G. (2014). Effects of icariin on reproductive functions in male rats. Molecules19, 9502–9514. 10.3390/molecules19079502
9
Cooke J. P. Losordo D. W. (2002). Nitric oxide and angiogenesis. Circulation105, 2133–2135. 10.1161/01.cir.0000014928.45119.73
10
Dai X. N. Li W. J. Zhu X. S. (2018). Efficacy analysis of tadalafil combined with fuxuanju capsule in the treatment of erectile dysfunction. Zhejiang Med. J.40, 2593–4+2596. 10.12056/i.issn.1006-2785.2018.40.23.2017-2221
11
Dhaliwal A. Gupta M. (2024). “PDE5 inhibitors,” in StatPearls. Treasure island (Florida). StatPearls Publishing. Available online at: http://www.ncbi.nlm.nih.gov/books/NBK549843/(Accessed April 10, 2024).
12
Ejeje J. N. Agbebi E. A. Mathenjwa-Goqo M. S. Oje O. A. Agboinghale P. E. Ebe I. T. et al (2024). Computational investigation of the therapeutic potential of detarium senegalense in the management of erectile dysfunction. Int. J. Mol. Sci.25, 12362. 10.3390/ijms252212362
13
Feng Y. Zhang M. Yuan W. Zhao D. Luo Z. Tang Z. et al (2025). Effects and mechanisms of imperatorin on vitrified mouse oocytes. Anim. (Basel)15, 661. 10.3390/ani15050661
14
Gao G. Z. Wang F. (2016). Efficacy observation on treating ED with the fufang Xuanju capsule. Clin. J. Chin. Med.8, 104–105. 10.3969/j.issn.1674-7860.2016.34.047
15
Gerald T. Raj G. (2022). Testosterone and the androgen receptor. Urol. Clin. North Am.49, 603–614. 10.1016/j.ucl.2022.07.004
16
Hatzimouratidis K. Amar E. Eardley I. Giuliano F. Hatzichristou D. Montorsi F. et al (2010). Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur. Urol.57, 804–814. 10.1016/j.eururo.2010.02.020
17
Helmy M. M. Senbel A. M. (2012). Evaluation of vitamin E in the treatment of erectile dysfunction in aged rats. Life Sci.90, 489–494. 10.1016/j.lfs.2011.12.019
18
Hoppe H. Hirschle D. Schumacher M. C. Schönhofen H. Glenck M. Kalka C. et al (2023). Erectile dysfunction: role of computed tomography cavernosography in the diagnosis and treatment planning of venous leak. CVIR Endovasc.6, 56. 10.1186/s42155-023-00403-9
19
Hu J. B. Zhang C. T. Lu J. Y. Xu K. X. (2023). The effect of Fufangxuanju Capsules on sperm DNA fragmentation index and sexual function aftervaricocelectomy. China Med. Pharm.13, 78–80+130. 10.20116/j.issn2095-0616.2023.16.18
20
Huang G. Q. Gong T. Wang S. S. Xia Q. H. Lin L. J. Wang G. B. (2023). Pudendal nerve lesions in young men with erectile dysfunction: imaging with 3T magnetic resonance neurography. Asian J. Androl.25, 650–652. 10.4103/aja202293
21
Huang J. (2020). Therapeutic efficacy of compound Xuanju capsule combined with tadalafil in patients with erectile dysfunction. Contemp. Med.26, 151–152. 10.3969/j.issn.1009-4393
22
Jannini E. A. Sternbach N. Limoncin E. Ciocca G. Gravina G. L. Tripodi F. et al (2014). Health-related characteristics and unmet needs of men with erectile dysfunction: a survey in five European countries. J. Sex. Med.11, 40–50. 10.1111/jsm.12344
23
Jiang C. Cao Z. G. Chen Q. C. Luo Y. Wang K. Xu L. et al (2016). The curative effect of compound Xuanju capsule combined with tadalafil in the treatment of diabetic erectile dysfunction. J. Bengbu Med. Univ.41, 1323–1325. 10.13898/j.cnki.issn.1000-2200.2016.10.020
24
Li J. Wu Y. Yu X. Zheng X. Xian J. Li S. et al (2022). Isolation, bioassay and 3D-QSAR analysis of 8-isopentenyl flavonoids from Epimedium sagittatum maxim. as PDE5A inhibitors. Chin. Med.17, 147. 10.1186/s13020-022-00705-5
25
Li T. F. Wu Q. Y. Li W. W. Zhang C. Li N. Shang X. J. et al (2014). Therapeutic efficacy of Compound Xuanju Capsule on autoimmune prostatitis in rats:An experimental study. Natl. J. Androl.20, 442–447. 10.13263/j.cnki.nja.2014.05.012
26
Ling Y. (2014). Determination of icariin in fufang Xuanju jiaonang by HPLC. Asia-Pacific Tradit. Med.10, 23–24.
27
Liu A. Q. Li J. Chen T. Zhu Q. F. Ma D. Y. Wu X. F. (2019). Curative effect of Sildenafil combined with Compound Xuanju Capsules for patients with type mchronic prostatitis and erectile dysfunction. Chin. J. Hum. Sex.28, 12–14. 10.3969/i.issn.1672-1993.2019.05.003
28
Liu B. L. (2016). Endpoints and common methods for assessing efficacyin clinical trials of new drugs. Chin. J. New Drugs25, 2074–2077.
29
Liu J. P. (2024). Observation of the effect of compound Xuanju capsules combined with tadalafil in downgrading treatment for type Ⅲ chronic prostatitis with erectile dysfunction patients. Reflexology Rehabilitation Med.5, 106–8+112.
30
Liu Q. Zhang Y. Wang J. Li S. Cheng Y. Guo J. et al (2018). Erectile dysfunction and depression: a systematic review and meta-analysis. J. Sex. Med.15, 1073–1082. 10.1016/j.jsxm.2018.05.016
31
Lizza E. F. Rosen R. C. (1999). Definition and classification of erectile dysfunction: report of the nomenclature committee of the international society of impotence research. Int. J. Impot. Res.11, 141–143. 10.1038/sj.ijir.3900396
32
McKinlay J. B. (2000). The worldwide prevalence and epidemiology of erectile dysfunction. Int. J. Impot. Res.12 (Suppl. 4), S6–S11. 10.1038/sj.ijir.3900567
33
McMahon C. G. (2019). Current diagnosis and management of erectile dysfunction. Med. J. Aust.210, 469–476. 10.5694/mja2.50167
34
Mehrotra N. Gupta M. Kovar A. Meibohm B. (2007). The role of pharmacokinetics and pharmacodynamics in phosphodiesterase-5 inhibitor therapy. Int. J. Impot. Res.19, 253–264. 10.1038/sj.ijir.3901522
35
Moon H. W. Park J. W. Lee K. W. Jeong H. C. Choi J. B. Choi S. W. et al (2017). Administration of goji (Lycium chinense mill.) extracts improves erectile function in old aged rat model. World J. Mens. Health35, 43–50. 10.5534/wjmh.2017.35.1.43
36
Moreno S. A. Morgentaler A. (2011). “Hormonal evaluation and therapy in erectile dysfunction,” in Contemporary treatment of erectile dysfunction: a clinical guide. Editor McVaryK. T. (Totowa, NJ: Humana Press), 161–177. 10.1007/978-1-60327-536-1_12
37
Nan C. J. Chen C. H. Yang S. Chen Y. H. (2022). Observation on clinical efficacy of fufang Xuanju capsule combined with tadalafil in treatment of erectile dysfunction. Chin. Archives Traditional Chin. Med.40, 189–191. 10.13193/j.issn.1673-7717.2022.06.043
38
Naseem K. M. (2005). The role of nitric oxide in cardiovascular diseases. Mol. Asp. Med.26, 33–65. 10.1016/j.mam.2004.09.003
39
Nguyen H. M. T. Gabrielson A. T. Hellstrom W. J. G. (2017). Erectile dysfunction in young men-A review of the prevalence and risk factors. Sex. Med. Rev.5, 508–520. 10.1016/j.sxmr.2017.05.004
40
Omote M. (1999). Pharmacological profiles of sildenafil (VIAGRA) in the treatment of erectile dysfunction: efficacy and drug interaction with nitrate. Nihon Yakurigaku Zasshi114, 213–218. 10.1254/fpj.114.213
41
Page M. J. McKenzie J. E. Bossuyt P. M. Boutron I. Hoffmann T. C. Mulrow C. D. et al (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ372, n71. 10.1136/bmj.n71
42
Rc R. A R. G W. Ih O. J K. A M. (1997). The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology49, 822–830. 10.1016/s0090-4295(97)00238-0
43
Schwarz E. R. Kapur V. Rodriguez J. Rastogi S. Rosanio S. (2007). The effects of chronic phosphodiesterase-5 inhibitor use on different organ systems. Int. J. Impot. Res.19, 139–148. 10.1038/sj.ijir.3901491
44
Shamloul R. Ghanem H. (2013). Erectile dysfunction. Lancet381, 153–165. 10.1016/S0140-6736(12)60520-0
45
Smith E. Lewis A. Narine S. S. Emery R. J. N. (2023). Unlocking potentially therapeutic phytochemicals in capadulla (doliocarpus dentatus) from Guyana using untargeted mass spectrometry-based metabolomics. Metabolites13, 1050. 10.3390/metabo13101050
46
SunW Li W. Z. (2023). Curative effect of compound Xuanju capsule combined with sildenafil on male sexual dysfunction and the influence of sex hormone level, international index of erectile dysfunction-5 score and psychological state. Chin. J. Hum. Sex.32, 128–132. 10.3969/i.issn.1672-1993.2023.08.033
47
Talib R. A. Khalafalla K. Cangüven Ö. (2017). The role of vitamin D supplementation on erectile function. Turk J. Urol.43, 105–111. 10.5152/tud.2017.76032
48
Wang C. Y. Wang X. Y. Zhang Y. Wang L. P. (2016a). The effect of Th17 cell percentage/Interleukin-17 in patients with rheumatoid arthritis after compound Xuanju Capsule therapy. Pharmacol. Clin. Chin. Materia Medica32, 198–201. 10.13412/j.cnki.zyyl.2016.06.054
49
Wang D. Y. Liu L. H. Song B. (2020). Simultaneous determination of seven components in fufang Xuanju capsul by QAMS. Food Drug22, 192–197. 10.3969/j.issn.1672-979X.2020.03.006
50
Wang J. Guo Y. Huang J. Yan J. Ma J. (2024). Using network pharmacology and in vivo experiments to uncover the mechanisms of radix paeoniae rubra and radix angelicae sinensis granules in treating diabetes mellitus-induced erectile dysfunction. Drug Des. Devel Ther.18, 6243–6262. 10.2147/DDDT.S493198
51
Wang J. G. Tan Y. Wang W. R. Xie Z. P. Li T. Xie S. (2018). Clinical study of compound Xuanju capsule combined with tadalafi in the treatment of abdominal obesity with erectile dysfunction. J. Hubei Univ. Med.37, 338–40+345. 10.13819/j.issn.1006-9674.2018.04.009
52
Wang J. Z. (2019). Study on the safety and efficacy of compound Xuanju capsule combined with tadalafil in the treatment of diabetic erectile dysfunction. Chin. J. Hum. Sex.28, 114–117. 10.3969/j.issn.1672-1993.2019.03.033
53
Wang Y. Dong Z. L. Xia Y. (2016b). Clinical study of compound Xuanju capsule combining vardenafil in the treatment of erectile dysfunction. Chin. J. Hum. Sex.25, 5–7. 10.3969/j.issn.1672-1993.2016.11.001
54
Wang Y. Li Q. B. Guo G. J. Wang G. R. Xu H. Bu X. et al (2022). Efficacy and safety of black tomato concentrate in the treatment of erectile dysfunction: a prospective randomized controlled open trial. Zhonghua Nan Ke Xue28, 415–421. 10.13263/j.cnki.nja.2022.05.005
55
Wang Y. Zhang H. J. Liu R. Zhang B. (2015). Effect of fufang Xuanju capsule on serum TNF-α and IL-1β of immunologic arthritis rats. Pharm. Today25, 334–337.
56
Wu G. L. Li T. Y. She Y. G. Lu W. W. (2013). Clinical effects of fufang XuanJu capsule on quality of sperm and reproductive hormone in rats with infertility. West. J. Traditional Chin. Med.26, 12–14.
57
Xu F. Luo S. Huang Z. Wang J. Li T. Zhong L. et al (2025). The molecular mechanisms of bergapten against abdominal aortic aneurysm: evidence from network pharmacology, molecular docking/dynamics, and experimental validation. J. Cell Biochem.126, e70029. 10.1002/jcb.70029
58
Yang H. Xiong W. Jiang J. Jiang R. (2025). Icariin inhibits hyperglycemia-induced cell death in penile cavernous tissue and improves erectile function in type 1 diabetic rats. Sex. Med.13, qfaf017. 10.1093/sexmed/qfaf017
59
Yang Z. J. Xu H. Xu G. J. Ji X. X. Bian S. J. Lv S. W. (2020). Progress of research on chemical composition, pharmacological effects and clinical application of black ants. China Pharm.31, 1148–1152. 10.6039/i.issn.1001-0408.2020.09.23
60
Yao J. C. Liu Q. S. Yan T. Wang L. Li Y. T. Yang Q. et al (2023). Effect and mechanism of icariin on vascular erectile dysfunction in rats, 44. Academic Journal of Naval Medical University, 1373–1377. 10.16781/j.CN31-2187/R.20220109
61
Zhang J. P. (2024). Clinical study of compound Xuanju capsule combining vardenafil in the treatment of erectile dysfunction. Chin. J. Hum. Sex. China's Naturopathy32, 83–86. 10.19621/j.cnki.11-3555/r.2024.0126
62
Zhang M. J. Chang D. G. He Z. J. Shang X. J. Zhou S. H. Lv B. D. et al (2016). Guidelines on integrated Chinese and western medicine for erectile dysfunction (trial version). Zhonghua Nan Ke Xue22, 751–757. 10.13263/j.cnki.nja.2016.08.015
63
Zhao Z. K. Yu H. L. Liu B. Wang H. Luo Q. Ding X. G. (2016). Antioxidative mechanism of Lycium barbarum polysaccharides promotes repair and regeneration following cavernous nerve injury. Neural Regen. Res.11, 1312–1321. 10.4103/1673-5374.189197
64
Zhou S. H. Weng Z. W. Chen Y. Q. Li L. Fang Y. Q. (2011). Compound Xuanju Capsule improves sex hormones and sex organ indexes in castrated male rats. Natl. J. Androl.17, 953–956. 10.13263/j.cnki.nja.2011.10.005
Summary
Keywords
compound Xuanju capsule, phosphodiesterase type 5 inhibitor, erectile dysfunction, meta-analysis, trial sequential analysis
Citation
Li R, Liao M, Yu Y, Guo B, Yu R, He Q and Zhang G (2025) Compound Xuanju capsules combined with phosphodiesterase-5 inhibitors as a new strategy for erectile dysfunction: a meta-analysis and trial sequential analysis. Front. Pharmacol. 16:1537789. doi: 10.3389/fphar.2025.1537789
Received
01 December 2024
Accepted
04 June 2025
Published
26 June 2025
Volume
16 - 2025
Edited by
Adolfo Andrade-Cetto, National Autonomous University of Mexico, Mexico
Reviewed by
Thomas Hsueh, Taipei City Hospital, Taiwan
Ezzat Abulmajd Ismail, Suez Canal University, Egypt
Updates
Copyright
© 2025 Li, Liao, Yu, Guo, Yu, He and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guomin Zhang, 834095773@qq.com; Qinghu He, hqh19651111@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.