- 1Institute of Translational Medicine, Shanghai University, Shanghai, China
- 2Department of Neurosurgery, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
Post-stroke depression (PSD) is a debilitating condition affecting more than one-third of stroke survivors, leading to significant impairments in mood, cognitive function, and overall quality of life. While conventional treatments like selective serotonin reuptake inhibitors (SSRIs) are commonly used, their efficacy is often limited, and they are associated with adverse side effects. Emerging research underscores the critical roles of neuroinflammation, neurotransmitter imbalances, and disruptions in the gut-brain axis in the development and progression of PSD, suggesting that targeting these pathways could lead to more effective therapeutic outcomes. Traditional Chinese Medicine (TCM) presents a promising multi-faceted approach, addressing these complex biological mechanisms by regulating neurotransmitter systems, modulating immune responses, and restoring gut microbiota balance. Key herbs such as Salvia miltiorrhiza Bunge (Lamiaceae; Dan Shen) and Bupleurum chinense DC. (Apiaceae; Chai Hu) have shown significant potential in modulating neurotransmitter levels, reducing neuroinflammation, and providing neuroprotection. Additionally, TCM formulations like Chaihu Shugan Powder (CSP) and Shugan Jieyu Capsules (SG) further enhance these effects by promoting gut microbiota homeostasis and restoring metabolic balance. This review delves into the biological mechanisms underlying PSD, with a particular focus on neuroinflammation, neurotransmitter dysregulation, and gut-brain axis dysfunction. It also explores the potential of integrating TCM with advanced multi-omics technologies—such as metabolomics, metagenomics, and transcriptomics—to develop personalized treatment strategies for PSD. By combining the holistic principles of TCM with modern Western medicine and cutting-edge omics technologies, this integrative approach offers a comprehensive framework for managing PSD, with the potential to significantly improve recovery outcomes and enhance the quality of life for stroke survivors.
1 Introduction
Stroke, a major cause of disability and mortality worldwide, occurs when cerebral blood flow is interrupted, leading to neuronal damage and functional impairment (Hankey, 2014; Campbell et al., 2019). Among survivors, over one-third develop post-stroke depression (PSD), characterized by persistent low mood, reduced interest in activities, and cognitive decline (Villa et al., 2018; Cai et al., 2019; Medeiros et al., 2020; Guo et al., 2022). PSD prevalence can reach 31% within 5 years post-stroke, significantly hindering recovery and posing a public health burden (Carnes-Vendrell et al., 2019; Lanctot et al., 2020; Frank et al., 2022)
PSD is frequently associated with gastrointestinal dysfunction, reflecting the intricate relationship between the nervous and gastrointestinal systems (Frank et al., 2022). The gut microbiota, a key regulator of immune function, metabolism, and brain activity, plays a crucial role in stroke recovery. Stroke-induced dysbiosis not only alters the production of metabolites like Trimethylamine-N-oxide (TMAO) and Short-chain fatty acids (SCFAs) but also triggers chronic inflammation and neurotransmitter imbalances (e.g., serotonin and dopamine), exacerbating depressive symptoms. Additionally, inflammatory pathways activated by dysbiosis impair neuroprotection and brain recovery, establishing a key link in the development of PSD (Zhu et al., 2016; Lee et al., 2020). Dysregulated microbiota further influences mood by modulating neurotransmitter pathways (Liang S. et al., 2018; Waclawikova and El Aidy, 2018; Ge et al., 2021; Bai et al., 2022). These findings highlight the gut-brain axis as a promising therapeutic target in PSD (Jiang et al., 2021; Zhong et al., 2022).
Despite the widespread use of selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for PSD treatment, these drugs are often limited by side effects, such as insomnia and gastrointestinal disturbances, which complicate recovery (Mikami et al., 2011; Mortensen et al., 2013). Furthermore, many patients exhibit resistance to these therapies, with trials showing no significant differences between antidepressants and placebos in symptom relief (Robinson et al., 2000; Savadi Oskouie et al., 2017).These challenges highlight the need for alternative, multi-targeted approaches to PSD management.
Traditional Chinese Medicine (TCM) has demonstrated therapeutic efficacy in other neuropsychiatric conditions, including anxiety, depression, and cognitive impairment, by modulating neurotransmitter systems and immune responses (Guo et al., 2021). These findings suggest that TCM may offer unique advantages in managing PSD through its multi-targeted approach. By integrating multiple herbal components tailored to individual symptoms, TCM formulations modulate neurotransmitter levels, immune responses, and gut microbiota composition, addressing the diverse pathways involved in PSD pathogenesis (Li et al., 2020; Li et al., 2022). Compared to single-target pharmacotherapies, TCM’s holistic approach offers a broader framework for managing PSD, targeting both emotional disturbances and physical dysfunctions.
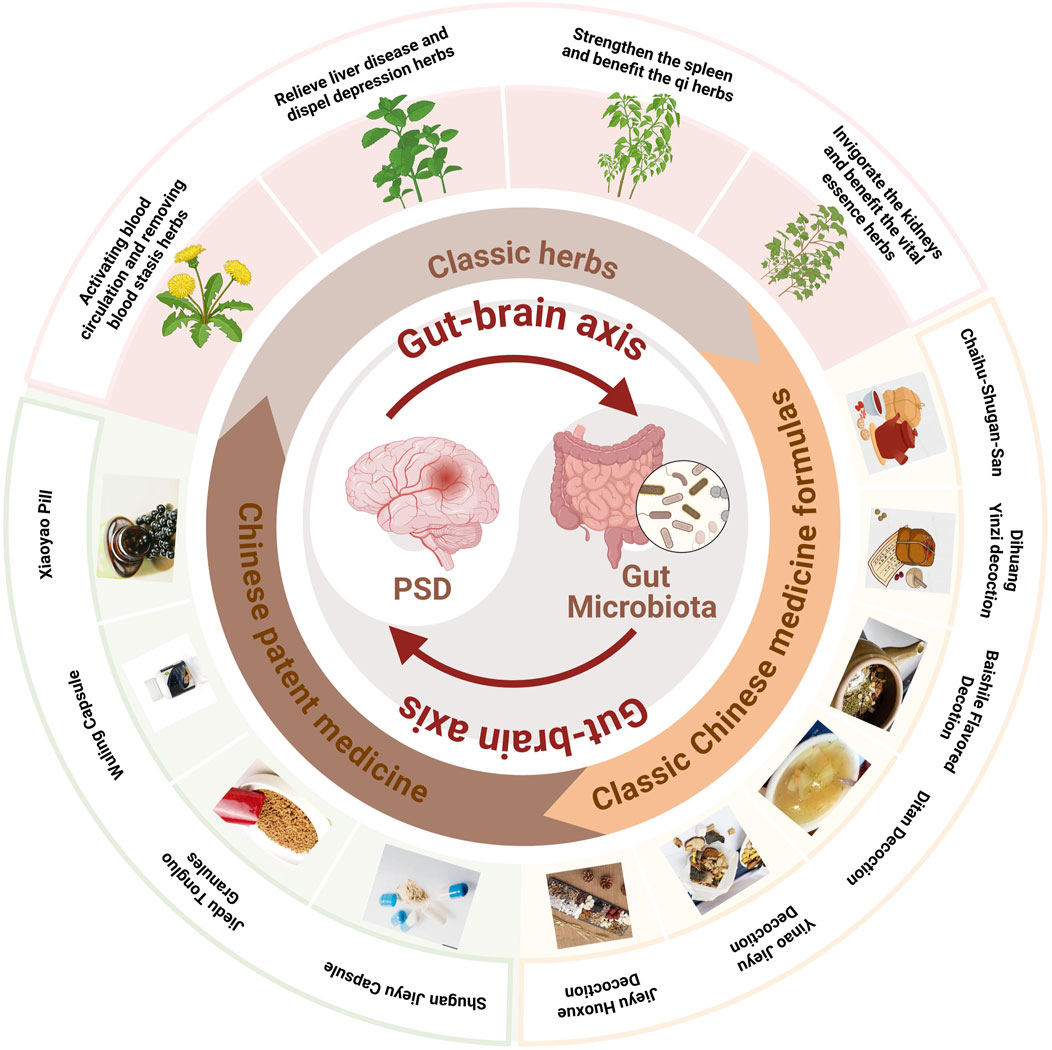
However, the use of TCM in PSD management remain under explored. Integrating TCM with Western medicine could bridge the gaps in existing treatments, offering a complementary strategy to address the complex pathophysiology of PSD. This review aims to analyze the biological mechanisms underlying PSD and explore the therapeutic potential of TCM interventions targeting the gut-brain axis and neuroinflammation. By integrating insights from both Western medicine and TCM, this work aims to inform the development of innovative therapeutic strategies for improving the quality of life in PSD patients (Figure 1).
2 TCM symptoms related to PSD
In TCM, PSD is conceptualized as a dual phenomenon encompassing both “Stroke” and “Depression.” Stroke leads to qi and blood stagnation, blocking cerebral circulation and causing symptoms like paralysis, speech impairment, and numbness. Depression arises from qi stagnation, heart and spleen deficiencies, and phlegm-blood accumulation. Emotional disturbances block the flow of qi, leading to liver qi stagnation, which manifests as low mood, chest tightness, and pain. Prolonged stagnation can cause phlegm obstruction, resulting in palpitations, excessive phlegm, and chest constriction. Unresolved emotional stress worsens heart and spleen deficiencies, causing fatigue, appetite loss, and insomnia.
The core pathological mechanism of PSD involves blocked qi flow and impaired circulation (Huang et al., 2018). Stroke-induced stagnation disrupts these pathways, and depression intensifies the imbalance, creating a vicious cycle that worsens physical and emotional symptoms. Key organs include the liver, kidney, heart, and spleen. The liver, crucial for regulating blood, qi, and emotions, plays a central role, as impaired liver function can aggravate depressive symptoms. Liver qi stagnation is especially critical, forming a cycle where emotional distress worsens stagnation, which deepens depression.
Understanding Qi deficiency and Yin-Yang (nourishing-activating) imbalances in PSD is crucial for effective treatment. Qi deficiency, linked to decreased energy metabolism, leads to neuroinflammation and immune dysfunction, exacerbating depressive symptoms. In PSD, this results in the increased release of pro-inflammatory cytokines (e.g., IL-6, TNF-α), damaging neurons and impairing synaptic function (Yan, 2018; Fu et al., 2021; Feng et al., 2022).Yin-Yang imbalances further disrupt neurotransmitter regulation and the HPA axis. Yin and Yang play opposite roles in regulating the body. Excessive Yang can overactivate the HPA axis, while Yin deficiency impairs neurotransmitter production and mood regulation, worsening depressive symptoms in PSD patients (Ding et al., 2024).
The interaction between Qi deficiency and Yin-Yang imbalances may also affect the gut-brain axis. Qi deficiency is associated with gut dysbiosis, where pathogenic bacteria increase and beneficial bacteria decrease, leading to systemic inflammation and neuroinflammation. Yin deficiency may exacerbate these issues, worsening neurotransmitter imbalances and inflammation (Jiang et al., 2023). Herbal formulas (e.g., Chaihu Shugan Powder (CSP), Salvia miltiorrhiza Bunge (Lamiaceae; Dan Shen), Astragalus membranaceus Fisch. ex Bunge (Fabaceae; Huang Qi) targeting these TCM syndromes can regulate these molecular pathways and improve clinical outcomes for PSD patients (Kwon et al., 2019).
3 The underlying molecular mechanism in PSD
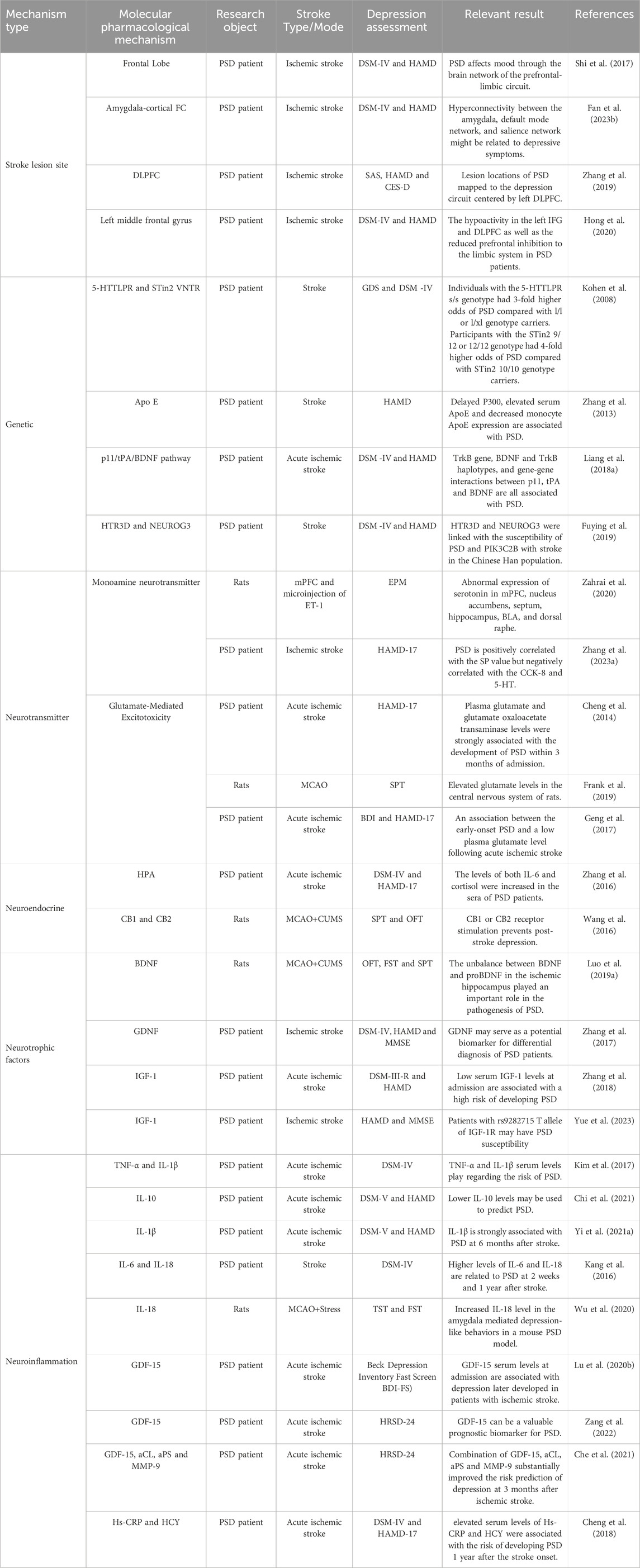
The pathogenesis of PSD remains complex and not fully understood, with research indicating contributions from stroke lesion locations, genetic predispositions, neurotransmitter imbalances, neuroendocrine alterations, neurotrophic factors, and neuroinflammatory processes (Figure 2). This section focuses on the molecular pathways most relevant to TCM interventions, including BDNF regulation, HPA axis modulation, neurotransmitter balance, and neuroinflammatory suppression. In addition, for the sake of completeness, all other relevant molecular mechanism pathway diagrams are presented in Table 1.
3.1 Overview of brain lesions, genetic factors, and glutamate excitotoxicity
Stroke-related lesions in areas such as the thalamus, basal ganglia, and prefrontal cortex impair neurotransmitter systems, contributing to depressive symptoms (Shi et al., 2017). For instance, left frontal lobe lesions correlate with serotonin and norepinephrine depletion, exacerbating emotional dysregulation (Klingbeil et al., 2021). Functional imaging studies reveal disrupted amygdala-prefrontal cortex connectivity, further linking brain damage to depressive symptoms (Zhang et al., 2019; Fan Y. et al., 2023).
Genetic predispositions also influence PSD vulnerability. Polymorphisms in 5-HTTLPR, MTHFR, and ApoE have been associated with a higher risk of PSD (Kohen et al., 2008; Zhang et al., 2013). Additionally, variations in the p11/tPA/BDNF pathway affect depressive outcomes following stroke (Liang J. et al., 2018).
Glutamate excitotoxicity triggered by ischemia and hypoxia can cause neuronal damage and synaptic dysfunction. Elevated glutamate levels in cerebrospinal fluid have been associated with PSD symptoms, though plasma concentrations may vary (Cheng et al., 2014; Geng et al., 2017). TCM therapies, such as S. miltiorrhiza Bunge (Lamiaceae; Dan Shen), indirectly mitigate excitotoxicity by promoting synaptic plasticity and neurotransmitter balance.
3.2 Brain-derived neurotrophic factor (BDNF) regulation
BDNF plays a crucial role in synaptic plasticity, neuronal survival, and emotional regulation. Stroke impairs BDNF signaling, disrupting neurogenesis and axonal regeneration, which increases the risk of developing PSD. BDNF exerts its effects through p75 neurotrophin receptor (p75NTR) and tropomyosin receptor kinase B (TrkB). However, the imbalance between BDNF and proBDNF promotes neuronal apoptosis, as proBDNF activates the RhoA-JNK signaling pathway, inhibiting synaptic recovery. TCM interventions, such as Bupleurum chinense DC. (Apiaceae; Chai Hu) and S. miltiorrhiza Bunge (Lamiaceae; Dan Shen), enhance BDNF levels through the ERK-CREB-BDNF pathway, promoting emotional recovery (Yang et al., 2021). Maintaining the BDNF/proBDNF balance is essential for neuroprotection and functional recovery. Both aerobic exercise and TCM therapies have been found to enhance this balance, promoting axonal regeneration and improving mood regulation in PSD patients (Luo L. et al., 2019).
Additional neurotrophic factors, such as insulin-like growth factor-1 (IGF-1) and glial cell line-derived neurotrophic factor (GDNF), also support neuronal recovery. GDNF promotes axon regeneration and enhances brain tissue plasticity (Beker et al., 2020; Zhang et al., 2018). Clinical studies have further shown that GDNF levels are negatively correlated with Hamilton Depression Rating Scale (HAMD) scores, suggesting that GDNF may serve as a diagnostic marker for PSD (Zhang et al., 2017). Variants in the IGF-1R gene, particularly the T allele at the s9282715 locus, have also been linked to an increased risk of PSD (Yue et al., 2023).
3.3 HPA axis modulation
The HPA axis plays a key role in regulating the stress response, emotional stability, and immune function. Stroke acts as both a direct and indirect stressor, disrupting the HPA axis and leading to excessive glucocorticoid (GC) production, primarily cortisol. Elevated cortisol levels have been strongly linked to depressive symptoms in PSD patients (Zhanina et al., 2022; Zhang et al., 2016). Dysregulation of the HPA axis contributes to persistent stress responses, immune dysfunction, and inflammation, further exacerbating depressive behavior (Wang et al., 2016).
Following a stroke, the hippocampus and adjacent brain regions send signals to the hypothalamus, stimulating the release of corticotropin-releasing hormone (CRH). This triggers the pituitary gland to release adrenocorticotropic hormone (ACTH), which, in turn, stimulates the adrenal cortex to produce glucocorticoids. While glucocorticoids regulate metabolism and immune response, chronic overproduction disrupts emotional regulation and impairs neuronal function by affecting neurogenesis and neurotransmitter levels (Zhou et al., 2022).
TCM interventions have shown potential in modulating the HPA axis. Shugan Jieyu Capsule (SG) and Glycyrrhiza uralensis Fisch. ex DC. (Fabaceae; Gan Cao) help restore cortisol homeostasis by suppressing excessive GC production and reducing neuroinflammation. This modulation of the HPA axis has been associated with improved emotional regulation and mood stability in PSD patients. Activation of CB1 and CB2 receptors has also been shown to mitigate depressive-like behavior by regulating HPA axis activity in rodent models (Wang et al., 2016; Zhang S. et al., 2023).
3.4 Neurotransmitter imbalances
Neurotransmitter imbalances, particularly in 5-HT, DA, and NE, play a critical role in the development of PSD. Stroke lesions in regions such as the basal ganglia, prefrontal cortex, and thalamus impair neurotransmitter synthesis, release, and reuptake, disrupting emotional regulation and cognition. Left frontal lobe damage is especially associated with significant 5-HT and NE reduction, increasing depression risk.
The monoaminergic system is vital for regulating mood, sleep, and cognition. Stroke disrupts this system, limiting neurotransmitter release and axonal regeneration. For example, reduced 5-HT levels in the frontal lobe and hippocampus correlate with depressive behaviors (Zahrai et al., 2020), while disruptions in the GR/ERβ/TPH2 pathway impact serotonin synthesis and depressive symptoms (Zhang X. et al., 2023).
TCM interventions restore neurotransmitter balance. Salvia miltiorrhiza Bunge (Lamiaceae; Dan Shen) boosts serotonin and dopamine levels, enhancing mood and cognition, while Bupleurum chinense DC. (Apiaceae; Chai Hu) modulates neurotransmitter activity through the ERK-CREB-BDNF pathway, promoting emotional stability (Yang et al., 2021; Zhang S. et al., 2023). Though glutamate excitotoxicity contributes to stroke-related neuronal damage, TCM focuses on monoamine regulation to improve synaptic plasticity. Early interventions targeting neurotransmitter imbalances, such as restoring 5-HT levels, show promise for improving PSD outcomes (Cheng et al., 2014; Geng et al., 2017).
3.5 Neuroinflammatory processes
Neuroinflammation plays a crucial role in the pathogenesis of PSD, contributing to neuronal damage, synaptic dysfunction, and emotional disturbances. Stroke induces the release of pro-inflammatory cytokines, such as interleukin-6 (IL-6), interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and interleukin-18 (IL-18), while reducing anti-inflammatory cytokines like IL-10 and IL-13 (Kang et al., 2016; Yi Ye et al., 2021; Yi X. et al., 2021). Dysregulated cytokine levels impair synaptic plasticity and worsen depressive symptoms (Kim et al., 2017). In addition, reduced oxygen and ATP concentrations in brain tissues further impair neuronal function, increasing the vulnerability to depression (Che et al., 2021).
In animal models of PSD, Wu et al. demonstrated that stroke combined with chronic stress elevated IL-18 levels, promoting depressive-like behaviors through the IL-18 receptor/NKCC1 signaling pathway (Wu et al., 2020). Other studies have identified elevated levels of growth differentiation factor-15 (GDF-15) as a biomarker for PSD. Lu et al. found that GDF-15 levels were over twice as high in PSD patients compared to non-depressed stroke patients, and Zang et al. reported that GDF-15 was independently associated with PSD (Lu X. et al., 2020; Zang et al., 2022). Additional biomarkers, including homocysteine (Hcy) and high-sensitivity C-reactive protein (Hs-CRP), have also been linked to increased PSD risk, suggesting that chronic inflammation is closely tied to its pathogenesis (Tang et al., 2016; Cheng et al., 2018). TCM interventions modulate neuroinflammatory responses. Poria cocos inhibits the NLRP3 inflammasome, reducing pro-inflammatory cytokine production and restoring immune balance. Salvia miltiorrhiza Bunge (Lamiaceae; Dan Shen) suppresses IL-6 production, alleviating depressive symptoms and promoting emotional stability (Bian et al., 2023).
4 Gut-brain axis and PSD: a complex network of interactions
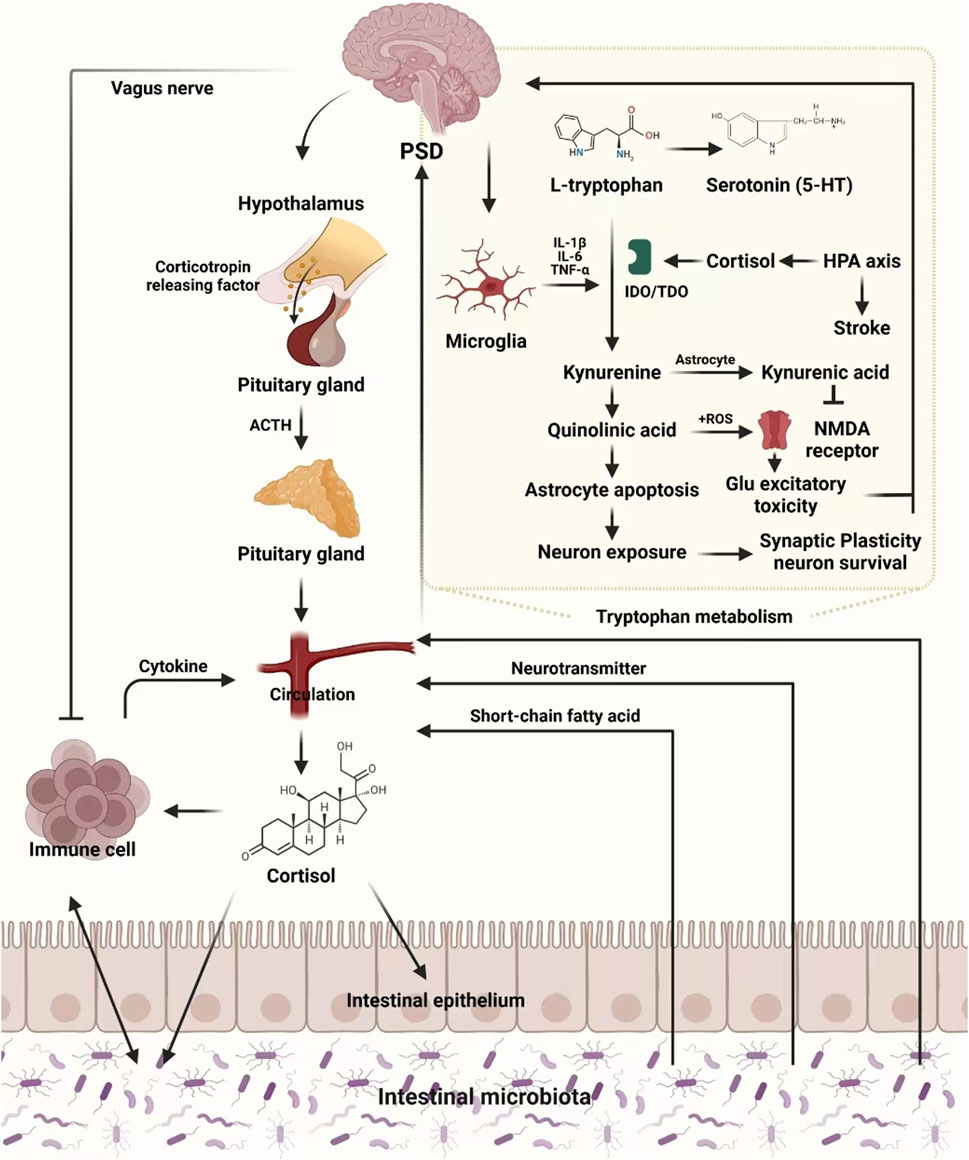
The enteric nervous system (ENS), forming part of the gut-brain axis, is a vast network of neurons within the gastrointestinal tract. It enables bidirectional communication between gut microbiota and the brain through neuroendocrine, immune, and metabolic pathways, thus influencing emotional regulation, cognition, and systemic health (Begum et al., 2022). Gut microorganisms, including bacteria and fungi, play a pivotal role in fermenting undigested food to produce essential energy sources and metabolites that support immune and digestive functions. The gut microbiota communicates with the brain via the ENS and vagus nerve, impacting central nervous system (CNS) processes, including mood and behavior (Han et al., 2022).
4.1 Gut dysbiosis and neurotransmitter imbalance in PSD
Dysbiosis, or the imbalance of gut microbial populations, has been strongly associated with PSD. Stroke survivors with PSD often exhibit reduced microbial diversity, marked by an increase in pathogenic bacteria and a decrease in anti-inflammatory species (Liang et al., 2015). This imbalance interferes with neurotransmitter metabolism, particularly serotonin (5-HT) and norepinephrine (NE), contributing to depressive symptoms (Jiang et al., 2021). Experimental studies have shown that transplanting gut microbiota from PSD patients into healthy rodents induces depressive behaviors, such as weight loss, decreased activity, and anhedonia (Frank et al., 2019). Additionally, gut dysbiosis may disrupt the synthesis of essential cofactors, such as vitamin B12 and folic acid, critical for homocysteine metabolism. Elevated homocysteine levels, commonly observed in PSD patients, impair monoamine neurotransmitter synthesis, contributing to depressive symptoms (Geng et al., 2017; Hu S. et al., 2019)
4.2 HPA axis dysregulation and the gut-brain axis in PSD
The hypothalamic-pituitary-adrenal (HPA) axis is closely linked to the gut-brain axis. Dysbiosis affects the HPA axis by altering microbial metabolites, which influence the release of corticotropin-releasing hormone (CRH). In stressful situations, activation of the HPA axis leads to increased cortisol levels, which impair gut barrier function, disrupt microbial balance, and exacerbate mood disturbances (Zhanina et al., 2022). PSD patients often exhibit elevated cortisol levels, underscoring the contribution of HPA axis dysregulation to the development of depressive symptoms (Yang et al., 2021). The overlapping mechanisms—reduced neurotransmitter synthesis and HPA axis dysregulation—highlight the importance of maintaining a balanced gut-brain axis for effective PSD management.
4.3 Neuroinflammation and the role of gut dysbiosis in PSD
Chronic neuroinflammation is a hallmark of PSD, often driven by microbial by-products such as lipopolysaccharides (LPS) entering circulation through a compromised intestinal barrier (Maes et al., 2012). Elevated levels of pro-inflammatory cytokines—such as interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α)—have been reported in PSD patients, along with a reduction in brain-derived neurotrophic factor (BDNF) (Rao et al., 2021). Gut dysbiosis also contributes to blood-brain barrier (BBB) dysfunction, allowing neurotoxins to reach the brain, further aggravating depressive symptoms.
4.4 TCM interventions targeting the gut-brain axis in PSD
Given the intricate relationship between the gut-brain axis and PSD, TCM offers promising therapeutic strategies. TCM formulations such as Chaihu Shugan Powder (CSP) promote the growth of beneficial gut bacteria and reduce pro-inflammatory species, alleviating depressive symptoms (Liu et al., 2021). Salvia miltiorrhiza Bunge (Lamiaceae; Dan Shen) has been shown to modulate the PI3K-AKT pathway and enhance vagus nerve signaling, improving gut-brain axis communication and emotional regulation (Bian et al., 2023).
Furthermore, combining probiotics with TCM formulations has yielded promising results by enhancing anti-inflammatory cytokine production and reducing serum cortisol levels, leading to improved mood and reduced neuroinflammation (Rao et al., 2021). This integrative approach demonstrates the potential of personalized medicine strategies that target e gut-brain axis to treat PSD.
5 Herbal interventions for enhancing recovery in PSD
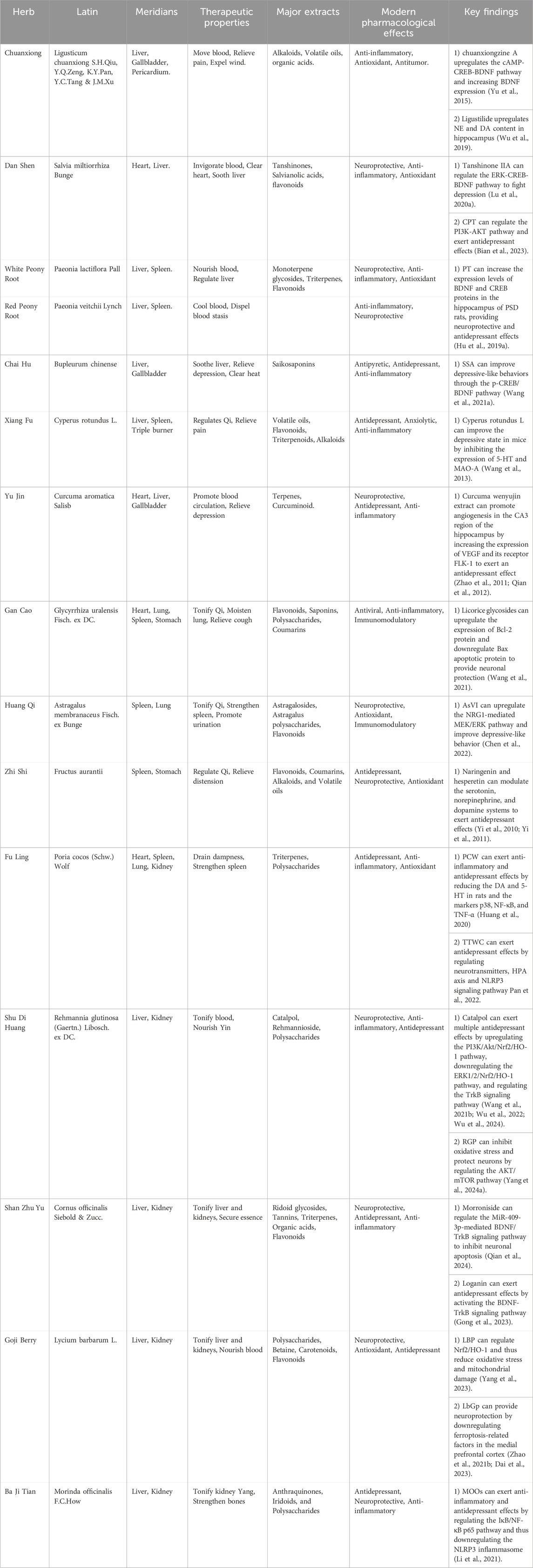
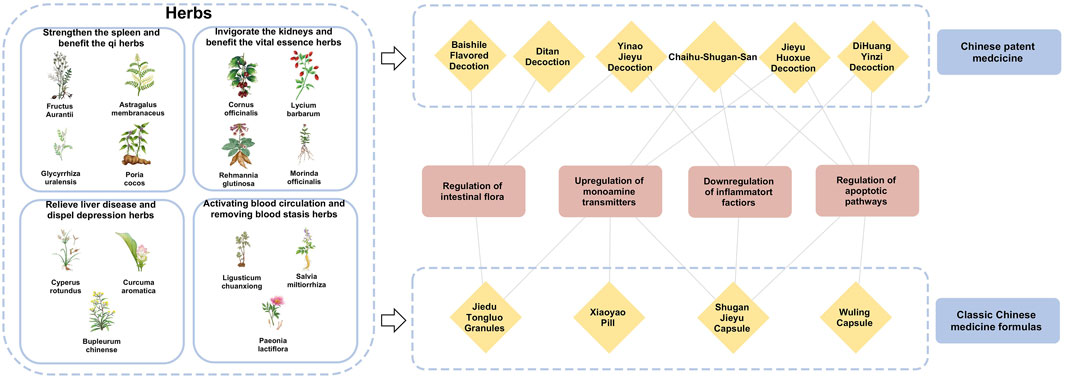
The theories of Chinese medicine emphasize individualized diagnosis and treatment according to each patient and their environment. The main methods of treating PSD include invigorating blood circulation and removing blood stasis, detoxifying the liver and relieving depression, invigorating the spleen and strengthening qi, and tonifying the kidneys and essence. Invigorating blood circulation and removing blood stasis aim to improve qi and blood circulation by dredging meridians and collaterals, using medicines such as Ligusticum chuanxiong S.H.Qiu, Y.Q.Zeng, K.Y.Pan, Y.C.Tang & J.M.Xu (Apiaceae; Chuan Xiong), S. miltiorrhiza Bunge (Lamiaceae; Dan Shen), and Paeonia lactiflora Pall. (Paeoniaceae; Shao Yao). Detoxifying the liver and relieving depression focus on regulating the liver, qi, and calming the mind, with herbs like Bupleurum chinense DC. (Apiaceae; Bei Chai Hu), Cyperus rotundus L. (Cyperaceae; Xiang Fu), and Curcuma aromatica Salisb. (Zingiberaceae; Yu Jin). Strengthening the spleen and vital energy is particularly for patients with deficiency of the heart and spleen, employing medicines G. uralensis Fisch. ex DC. (Fabaceae; Gan Cao), A. membranaceus Fisch. ex Bunge (Fabaceae; Huang Qi), Fructus aurantii (Rutaceae; Zhi Shi), Poria cocos (Schw.) Wolf (Polyporaceae; Fu Ling). The kidney tonic drugs are for patients with deficiency of spleen and kidney, such as the use of Rehmannia glutinosa (Gaertn.) Libosch. ex DC. (Orobanchaceae; Di Huang), Cornus officinalis Siebold & Zucc. (Cornaceae; Shan Zhu Yu), Lycium barbarum L. (Solanaceae; Goji Berry) and Morinda officinalis F.C.How (Rubiaceae; Ba Ji Tian). By regulating the internal organs with these herbal treatments, the functions of the liver, heart, spleen, and kidneys are restored on an individual basis, achieving balance and coordination among the internal organs (Table 2).
5.1 Activating blood circulation and removing blood stasis herbs
Ligusticum chuanxiong S.H.Qiu, Y.Q.Zeng, K.Y.Pan, Y.C.Tang & J.M.Xu (Apiaceae; Chuan Xiong): Contains alkaloids and volatile oils that activate the cAMP-CREB-BDNF pathway, increasing NE and DA, improving synaptic plasticity and mood stability (Wu et al., 2019; Yu et al., 2022).
Salvia miltiorrhiza Bunge (Lamiaceae; Dan Shen): Offers neuroprotection through tanshinones and salvianolic acids. Tanshinone IIA activates the ERK-CREB-BDNF pathway to a lleviate depression (Lu J. et al., 2020). Sodium tanshinone IIA sulfonate enhances function in ischemic stroke models (Wang Z. et al., 2022). and cryptotanshinone regulates the PI3K-AKT pathway and gut microbiota (Bian et al., 2023).
Paeonia lactiflora Pall. (Paeoniaceae; Shao Yao) and Paeonia veitchii Lynch (Paeoniaceae; Chuan Chi Shao): Known for anti-inflammatory effects, these herbs modulate neurotransmitter levels, reducing oxidative stress. Paeoniflorin boosts BDNF, enhancing synaptic plasticity and cognitive function (Hu M. Z. et al., 2019; Wang X. L. et al., 2021).
5.2 Relieving liver disease and dispel depression herbs
Bupleurum chinense DC. (Apiaceae; Chai Hu): Saikosaponins increase serotonin and dopamine via the p-CREB/BDNF pathway (Wang A. R. et al., 2021). Ping et al. reported improved pharmacokinetics and enhanced antidepressant effects when saikosaponin A (SSA) was combined with paeoniflorin, suggesting a synergistic action. Additionally, other components such as saikosaponin D (SSD), quercetin, bupleurum polysaccharides, kaempferol, and baicalin have demonstrated antidepressant properties (Yin et al., 2023).
Cyperus rotundus L (Cyperaceae; Xiang Fu): balances qi and soothes the liver, essential in TCM for regulating emotions. Its extracts have been shown to improve depressive symptoms by increasing 5-HT levels and inhibiting monoamine oxidase A (MAO-A) activity (Lu et al., 2022; Wang F. et al., 2022).
Curcuma aromatica Salisb (Zingiberaceae; Yu Jin): Curcumin, its primary bioactive compound, has exhibited significant antidepressant effects through behavioral models, including the tail suspension test. Furthermore, curcumin promotes hippocampal angiogenesis by upregulating vascular endothelial growth factor (VEGF) and FLK-1 expression, thereby improving cognitive function and mood stability (Zhao et al., 2011; Qian et al., 2012).
5.3 Strengthen the spleen and benefit the qi herbs
Glycyrrhiza uralensis Fisch. ex DC (Fabaceae; Gan Cao): contains glycyrrhizic acid, which exhibits anti-inflammatory properties. It promotes neuronal survival by enhancing Bcl-2 expression, reducing neuroinflammation, and alleviating depressive symptoms (Wang et al., 2023; Wang et al., 2021).
Astragalus membranaceus Fisch. ex Bunge (Fabaceae; Huang Qi): activates the EGFR/MAPK pathway, promoting neuronal recovery and emotional stability. modulates the gut-brain axis, and supports mood and cognitive function in PSD patients (Chen et al., 2022).
Fructus aurantii (Rutaceae; Zhi Ke): exerts antidepressant effects through its flavonoid content, such as naringenin and hesperetin, which regulate dopamine receptor activity. These active compounds contribute to emotional wellbeing by restoring neurotransmitter balance, enhancing mood, and supporting cognitive function (Yi et al., 2010; Yi et al., 2011).
Poria cocos (Schw.) Wolf (Polyporaceae; Fu Ling): inhibits the NLRP3 inflammasome, reducing depressive behaviors and inflammatory markers (Huang et al., 2020). Additionally, the total triterpenes of Poria cocos have been shown to exhibit antidepressant effects through modulation of neurotransmitter pathways, further validating its role in PSD management (Pan et al., 2022).
5.4 Invigorating the kidneys and benefit the vital essence herbs
Rehmannia glutinosa (Gaertn.) Libosch. ex DC. (Orobanchaceae; Shu Di Huang): mitigates oxidative stress via the PI3K/Akt/Nrf2 pathway, with catalpol enhancing synaptic plasticity and neurogenesis through the TrkB pathway (Bhattamisra et al., 2019; Song et al., 2021; Sun et al., 2021; Wang Y. L. et al., 2021). Rehmannia glutinosa polysaccharides (RGP) further mitigate oxidative stress and promote autophagy, providing neuroprotection in PSD models (Yang Y. et al., 2024; Wang J. et al., 2021; Wu et al., 2022; Wu et al., 2024).
Cornus officinalis Siebold & Zucc. (Cornaceae; Shan Zhu Yu): alleviates depressive-like symptoms by activating the BDNF/TrkB signaling pathway. Morroniside has been shown to reduce PSD-related symptoms by improving synaptic function and enhancing hippocampal plasticity through miRNA modulation (Qian et al., 2024). Additionally, logani exhibits neuroprotective and anti-inflammatory properties, further contributing to mood stabilization (Gong et al., 2023).
Lycium barbarum L. (Solanaceae; Goji Berry), linked to liver and kidney meridians, is traditionally used to address fatigue and yin deficiency. Its polysaccharides (LBP), constituting a major portion of its active compounds, have demonstrated antidepressant effects by reducing oxidative stress through the Nrf2/HO-1 pathway and mitigating anxiety-like behaviors (Zhou et al., 2021; Dai et al., 2023; Yang et al., 2023; Zhao F. et al., 2021).
Morinda officinalis F.C.How (Rubiaceae; Ba Ji Tian): is known for treating kidney yang deficiency and rheumatic pain. Morinda officinalis oligosaccharides (MOOs), inhibit NLRP3 inflammasome activation, reducing neuroinflammation and alleviating depressive behaviors in PSD models (Li et al., 2021). This dual anti-inflammatory and neuroprotective effect underscores its therapeutic relevance in managing PSD.
6 Classical formulations and Chinese patent medicines
TCM formulas, composed of multiple herbs, offer a more comprehensive approach to managing PSD by addressing both emotional and physiological imbalance (Table 3). In addition, the close connections and related potential mechanisms among herbal medicines, classic Chinese medicine formulas and Chinese patent medicines for the treatment of stroke depression have been presented in Figure 3.
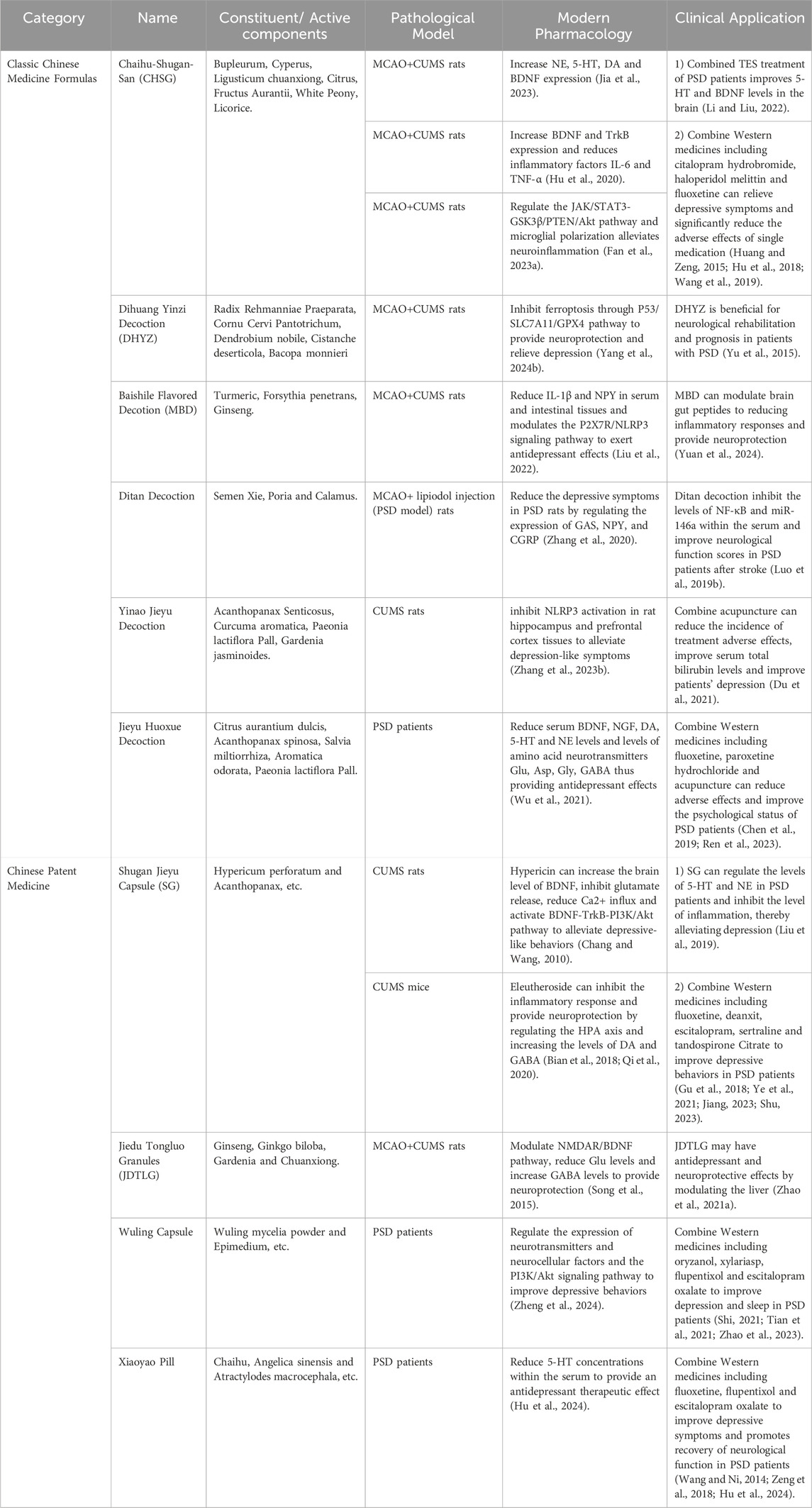
Table 3. Modern pharmacologic mechanisms and clinical studies of classical formulas and proprietary Chinese medicines for the treatment of PSD.
6.1 Chaihu Shugan Powder (CSP)
This classical TCM formula, documented in the Ming Dynasty’s Jingyue Quanshu, has been used for nearly four centuries to treat emotional disorders, particularly those associated with liver qi stagnation and depression. CSP consists of seven core herbs: Bupleurum chinense DC. (Apiaceae; Chai Hu), C. rotundus L. (Cyperaceae; Xiang Fu), L. chuanxiong S.H.Qiu, Y.Q.Zeng, K.Y.Pan, Y.C.Tang & J.M.Xu (Apiaceae; Chuan Xiong), Citrus reticulata Blanco (Rutaceae; Chen Pi), Citrus aurantium L (Rutaceae; Zhi Ke), P. lactiflora Pall. (Paeoniaceae; Bai Shao), and G. uralensis Fisch. ex DC. (Fabaceae; Gan Cao). These herbs work synergistically to relieve lumbar pain, regulate qi, and alleviate emotional distress. Recent pharmacological studies show that CSP significantly enhances monoamine neurotransmitter levels in PSD patients, promoting emotional stabilization (Liu et al., 2020; Gao et al., 2022). Furthermore, CSP has been shown to reduce neuroinflammation by lowering serum TNF-α levels and hippocampal NF-κB expression, with higher dosages correlating with stronger anti-inflammatory effects (Fan Q. et al., 2023). Gao et al. further demonstrated reductions in IL-6 and TNF-α, confirming the anti-inflammatory potential of this formula (Gao et al., 2021; Hu et al., 2020; Jia et al., 2023).
In clinical practice, CSP has proven effective when combined with western antidepressants such as citalopram and haloperidol, enhancing therapeutic outcomes while reducing adverse side effects (Kwon et al., 2019; Hu et al., 2018; Huang and Zeng, 2015; Li et al., 2022; Wang et al., 2019) This integration of TCM with conventional medicine highlights CSP’s potential as an adjunct treatment for PSD, offering both mood stabilization and neuroprotection.
6.2 Dihuang Yinzi decoction (DHYZ)
First documented in the Xuan Ming Lun Fang, DHYZ consists of twelve herbs, including R. glutinosa (Gaertn.) Libosch. ex DC. (prepared root) (Orobanchaceae; Di Huang), Gynochthodes officinalis (F.C.How) Razafim. & B.Bremer (Rubiaceae; Ba Ji Tian), C. officinalis Siebold & Zucc. (Cornaceae; Shan Zhu Yu), Dendrobium nobile Lindl. (Orchidaceae; Shi Hu), Cistanche deserticola Ma (Orobanchaceae; Rou Cong Rong), Aconitum carmichaelii Debx. (Ranunculaceae; Fu Zi), Schisandra chinensis (Turcz.) Baill. (Schisandraceae; Wu Wei Zi), Cinnamomum cassia Nees (Lauraceae; Guan Gui), Wolfiporia extensa (Peck) E. Horak. (Polyporaceae; Fu Ling), Ophiopogon japonicus (Thunb.) Ker Gawl. (Asparagaceae; Mai Dong), Acorus gramineus Aiton (Acoraceae; Shi Chang Pu), and Polygala tenuifolia Willd. (Polygalaceae; Yuan Zhi). Traditionally used to treat neurological disorders, DHYZ has shown effectiveness in rodent models by reducing apoptosis and enhancing memory (Yu et al., 2015; An et al., 2017). Researchers found that DHYZ alleviates PSD symptoms by inhibiting ferroptosis through the P53/SLC7A11/GPX4 pathway, providing neuroprotection (Yang Z. et al., 2024).
Other classical formulas, such as Baishile Flavored Decotion (MBD) (Liu et al., 2022), Ditan Decoction (Zhang et al., 2020), Yinao Jieyu Decoction (Zhang X. et al., 2023) and Jieyu Huoxue Decoction (Wu et al., 2021), have also demonstrated effectiveness in managing PSD, either as standalone treatments or combined with Western therapies.
6.3 Shugan Jieyu Capsule (SG)
Approved by the China National Medical Products Administration in 2008, SG is the first herbal product specifically indicated for depression. Its key components are Hypericum perforatum L. (Hypericaceae; Guan Ye Jin Si Tao) and Acanthopanax (Decne. & Planch.) Witte (Araliaceae; Ci Wu Jia), which exhibit calming, cognitive-enhancing, and anti-inflammatory properties. Hypericin modulates the HPA axis, inhibits glutamate release, and boosts BDNF expression (Chang and Wang, 2010). Quercetin activates the BDNF-TrkB-PI3K/Akt pathway, further alleviating depressive symptoms (Qi et al., 2020). Acanthopanax’s active compounds, eleutherosides B and E, reduce depressive behaviors, while syringin increases dopamine and GABA levels (Bian et al., 2018). Additional components, such as emodin and syringaresinol, also exhibit antidepressant properties (Bonaterra et al., 2020; Zhang et al., 2021). Clinical studies show that SG increases norepinephrine and serotonin levels, with enhanced outcomes when combined with fluoxetine (Yao et al., 2020; Jiang et al., 2023; Liu et al., 2019; Shu et al., 2018).
6.4 Jiedu Tongluo Granules (JDTLG)
A proprietary TCM formulation, contains Panax ginseng C.A.Mey. (Araliaceae; Ren Shen), Scutellaria baicalensis Georgi (Lamiaceae; Huang Qin), Ginkgo biloba L. (Ginkgoaceae; Yin Xing Ye), H. perforatum L. (Hypericaceae; Guan Ye Lian Qiao), Gardenia J.Ellis (Rubiaceae; Zhi Zi Hua), Gastrodia elata Blume (Orchidaceae; Tian Ma), and L. chuanxiong S.H.Qiu, Y.Q.Zeng, K.Y.Pan, Y.C.Tang & J.M.Xu (Apiaceae; Chuan Xiong). It enhances physical recovery and alleviates depressive symptoms in PSD patients (Song et al., 2015). Zhao et al. demonstrated that JDTLG exerts neuroprotective effects by modulating the NMDAR/BDNF pathway, lowering glutamate levels, and increasing GABA concentrations, stabilizing mood (Zhao A. et al., 2021).
Additionally, other compound Chinese medicines, such as Wuling Capsule (Zheng et al., 2024) and Xiaoyao Pills (Hu et al., 2024) have been shown to improve depressive behaviors, either alone or in combination with Western pharmacotherapies.
6.5 Baishile flavored decoction
Baishile Flavored Decoction, containing Curcuma longa L. (Zingiberaceae; Jiang Huang), Forsythia suspensa (Thunb.) Vahl (Oleaceae; Lian Qiao), and Panax ginseng C.A.Mey. (Araliaceae; Ren Shen), exerts antidepressant effects primarily through modulation of the P2X7R/NLRP3 signaling pathway. Studies in MCAO + CUMS rat models have shown that Baishile significantly reduces IL-1β and neuropeptide Y (NPY) levels in serum and intestinal tissues, leading to reduced neuroinflammation and improved neurological function (Liu et al., 2022). Moreover, Clinical studies have demonstrated the ability of MBD to exert neuroprotective effects and reduce inflammatory responses by modulating brain-gut peptides (Yuan et al., 2024).
6.6 Ditan decoction
Ditan Decoction, composed of Pinellia ternata (Thunb.) Bremer (Araceae; Ban Xia), Poria cocos (Schw.) Wolf (Polyporaceae; Fu Ling), Arisaema cum bile L. (Araceae; Tan Nan Xing), Acorus calamus L. (Acoraceae; Shi Chang Pu), Citri Grandis Exocarpium (Rutaceae; Ju Hong), Poncirus trifoliata (L.) Raf. (Rutaceae; Zhi Shi), Bambusae Caulis In Taenias (Poaceae; Zhu Ru), Panax ginseng C.A.Mey. (Araliaceae; Ren Shen) and G. uralensis Fisch. ex DC. (Fabaceae; Gan Cao) has been found to regulate key neurotransmitters, including GAS, NPY, and CGRP, thereby alleviating depression in PSD rat models (Zhang et al., 2020). Clinical studies further indicate that Ditan Decoction inhibits NF-κB and miR-146a expression in serum, which correlates with reduced neuroinflammatory responses and improved neurological function scores in PSD patient post-stroke (Luo W. et al., 2019).
6.7 Yinao jieyu decoction
Yinao Jieyu Decoction, containing Acanthopanax senticosus (Rupr. et Maxim.) Harms (Araliaceae; Ci Wu Jia), C. aromatica Salisb. (Zingiberaceae; Yu Jin), S. chinensis (Turcz.) Baill. (Schisandraceae; Wu Wei Zi) and Gardenia jasminoides J.Ellis (Rubiaceae; Zhi Zi Hua), has been reported to alleviate depressive-like symptoms in CUMS rat models via NLRP3 inflammasome inhibition in hippocampal and prefrontal cortex tissues (Zhang S. et al., 2023). Additionally, when combined with acupuncture, Yinao Jieyu Decoction has been observed to reduce the incidence of adverse effects, improve serum bilirubin levels, and enhance PSD recovery (Du et al., 2021).
6.8 Jieyu huoxue decoction
Jieyu Huoxue Decoction, formulated with P. trifoliata (L.) Raf (Rutaceae; Zhi Shi), Acanthopanax spinosa (L.). Siebold & Zuccarin (Araliaceae; Ci Wu Jia), S. miltiorrhiza Bunge (Lamiaceae; Dan Shen), C. rotundus L. (Cyperaceae; Xiang Fu), Paeoniae Radix Alba (Paeoniaceae; Bai Shao), Bupleurum chinense DC. (Apiaceae; Chai Hu) and Angelica sinensis (Oliv.) Diels (Apiaceae; Dang Gui), exerts antidepressant effects by regulating monoamine neurotransmitters (BDNF, NGF, DA, 5-HT, and NE) and amino acid neurotransmitters (Glu, Asp, Gly, and GABA) (Wu et al., 2021).Clinical data suggest that combining Jieyu Huoxue Decoction with Western antidepressants, such as fluoxetine and paroxetine hydrochloride, as well as acupuncture, enhances psychological recovery and reduces medication side effects in PSD patients (Chen et al., 2019; Ren et al., 2023).
6.9 Wuling capsule
Wuling Capsule, derived from Wuling Mycelia Powder, acts via the PI3K/Akt signaling pathway, which is crucial for neuroprotection and synaptic plasticity. Studies in PSD patients have confirmed that Wuling Capsule modulates neurotransmitter expression and enhances neurocellular factor activity, leading to improved depressive symptoms and sleep quality (Zheng et al., 2024; Shi, 2021; Tian et al., 2021; Zhao et al., 2023).
6.10 Xiaoyao pills
Xiaoyao Pills containing Bupleurum chinense DC. (Apiaceae; Chai Hu), A. sinensis (Oliv.) Diels (Apiaceae; Dang Gui), Paeoniae Radix Alba (Paeoniaceae; Bai Shao), Atractylodes macrocephala Koidz. (Asteraceae; Bai Zhu), W. extensa (Peck) E. Horak. (Polyporaceae; Fu Ling), Mentha canadensis L. (Lamiaceae; Bo he), Zingiber officinale Roscoe (Zingiberaceae; Sheng Jiang) and Glycyrrhizae radix et rhizoma praeparata (Fabaceae; Mi Zhi Gan Cao) is widely used in PSD patients due to its ability to modulate 5-HT levels in serum, directly impacting mood regulation (Hu et al., 2024; Wang et al., 2013; Zeng et al., 2018).
7 Future directions: multi-omics approaches to optimize TCM interventions through gut-host interaction
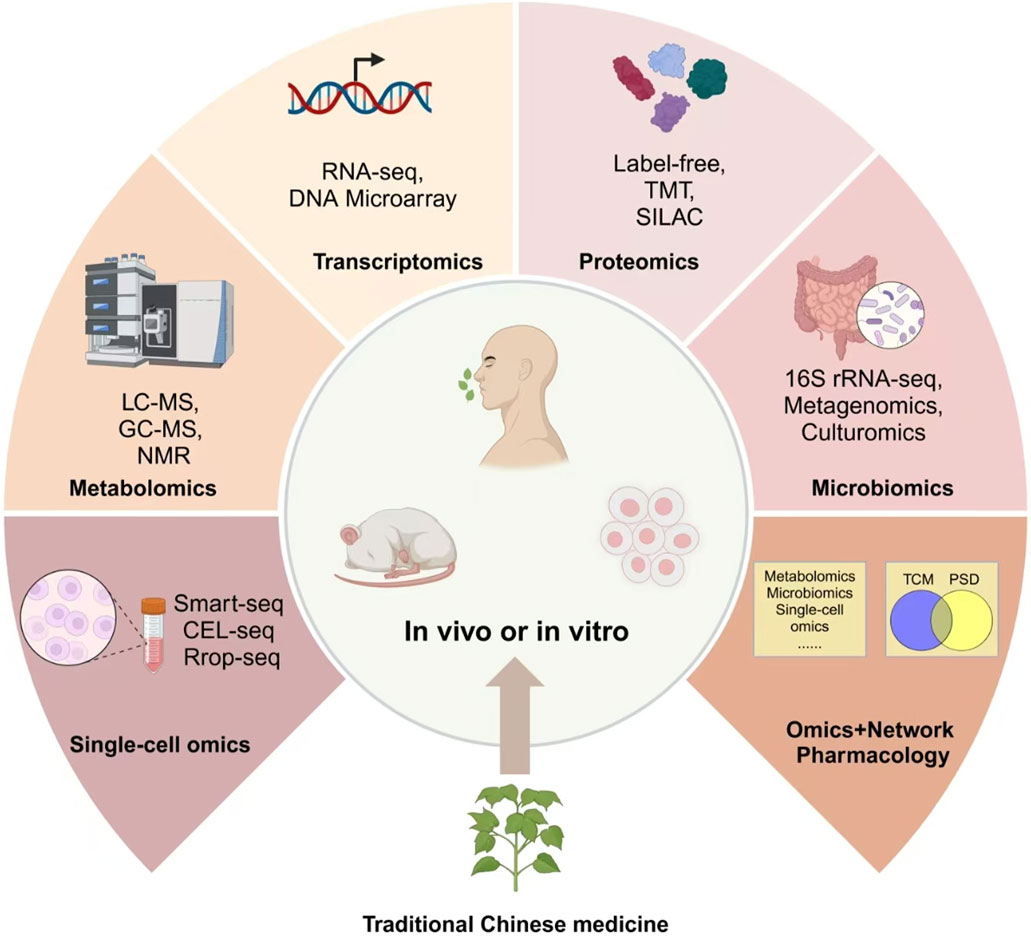
As scientific understanding deepens, integrating TCM with multi-omics technologies opens new frontiers in enhancing therapeutic precision. TCM has shown remarkable potential in modulating gut microbiota and influencing host metabolism, especially in treating metabolic and neurological disorders. However, the interactions between gut microbiota, host metabolism, and TCM interventions are complex and dynamic. Traditional research approaches struggle to capture these intricate mechanisms, making multi-omics technologies indispensable for precise and individualized interventions. To bridge this gap, recent studies have started applying multi-omics technologies, including metabolomics and metagenomics, to better understand the therapeutic mechanisms of TCM in diseases like PSD (Feng et al., 2022; Wang et al., 2022; Meng et al., 2025).
Omics platforms such as metabolomics, metagenomics, proteomics, and single-cell omics offer new dimensions for understanding how active compounds in TCM reshape the gut microbiota and modulate biochemical pathways at various levels. These multi-layered insights allow researchers to unravel the intricate relationship between the gut and brain, identify key biomarkers, and optimize treatment strategies in diseases such as PSD (Figure 4). TCM’s active components—such as polysaccharides, flavonoids, and alkaloids—function by enhancing microbial diversity, supporting beneficial bacteria, and suppressing pathogens (Xia et al., 2022; Wang et al., 2024). Astragalus polysaccharides promote the growth of Lactobacillus and Bifidobacterium, while alkaloids in Coptis chinensis Franch. (Ranunculaceae; Huang Lian) inhibit pathogenic bacteria, thereby maintaining gut homeostasis (Bot et al., 2020; Du et al., 2021; Amin et al., 2023). These effects not only amplify the therapeutic impact of TCM but also encourage the production of key metabolites. Baicalin, for example, is transformed into baicalein by gut bacteria, enhancing anti-inflammatory and neuroprotective effects (Du et al., 2021). Similarly, ginsenosides from ginseng are metabolized into rare bioactive forms that improve glucose metabolism and reduce inflammation (Bot et al., 2020).
In patients with PSD, disturbances in the gut microbiota and alterations in metabolic pathways can exacerbate depressive symptoms. One significant mechanism is the shift in tryptophan metabolism towards the kynurenine pathway, which reduces serotonin levels, potentially intensifying mood disorders. Research has demonstrated that S. miltiorrhiza Bunge (Lamiaceae; Dan Shen) effectively restores metabolic balance by enhancing butyrate production, which offers both anti-inflammatory and neuroprotective effects, thereby alleviating symptoms of PSD (Badini et al., 2022). Further, the integration of metabolomics and metagenomics has deepened our understanding of how gut microbiota affects neurotransmitter production, energy metabolism, and immune function. This multi-omics approach provides a framework for personalized therapeutic strategies by adapting TCM interventions to individual microbial and metabolic profiles. Specifically, formulations containing S. miltiorrhiza Bunge (Lamiaceae; Dan Shen) and Astragalus membranaceus have been proven to regulate neurotransmitter balance, thus improving gut-brain communication.
Metabolomics offers insights into how TCM compounds modulate metabolic pathways associated with neurotransmitter synthesis, energy metabolism, and inflammation. For instance, Astragalus polysaccharides promote the growth of Lactobacillus and Bifidobacterium, while alkaloids in C. chinensis Franch. (Ranunculaceae; Huang Lian) inhibit pathogenic bacteria, thereby maintaining gut homeostasis (Bot et al., 2020; Du et al., 2021; Amin et al., 2023). In PSD, disruptions in gut microbiota and altered metabolic pathways exacerbate depressive symptoms. A key mechanism involves a shift in tryptophan metabolism toward the kynurenine pathway, reducing serotonin levels and intensifying mood disorders. Metagenomics deciphers the structural and functional composition of gut microbiota in response to TCM interventions. Research has shown that ginsenosides from Panax ginseng C.A.Mey. (Araliaceae; Ren Shen) are transformed by gut microbiota into rare bioactive metabolites, which enhance glucose metabolism and suppress neuroinflammation (Bot et al., 2020).Transcriptomics and proteomics allow for the exploration of gene expression changes and protein-level modifications triggered by TCM therapies. Baicalin, a flavonoid from S. baicalensis Georgi (Lamiaceae; Huang Qin), is metabolized by gut bacteria into baicalein, which enhances anti-inflammatory pathways and promotes neuronal survival (Du et al., 2021). These effects not only amplify the therapeutic impact of TCM but also encourage the production of key metabolites. Proteomic studies have also identified that Danshenextracts regulate neurotransmitter-related proteins, particularly those involved in serotonin and dopamine signaling, which are disrupted in PSD. Single-cell omics provides unprecedented resolution in identifying cellular heterogeneity within the gut-brain axis, revealing how specific immune cells, neurons, and glial cells respond to TCM-derived compounds. By mapping cellular interactions at the single-cell level, researchers can decipher the precise molecular targets of TCM therapies, refining treatment strategies for PSD and other neurological disorders. (Badini et al., 2022).
Multi-omics techniques elucidate the complex interactions between the gut and brain, laying a foundation for precision medicine in PSD. These methods facilitate personalized treatments by integrating TCM with contemporary diagnostic tools, enhancing TCM’s capacity to regulate inflammation, neurotransmitter functions, and metabolic processes, thus supporting early diagnosis and tailored treatments for PSD patients.
8 Conclusion and perspectives
PSD affects over one-third of stroke survivors, driven by complex factors like genetic predisposition, neurotransmitter imbalances, neuroinflammation, and gut-brain axis disruptions. While conventional treatments are effective for some, side effects and drug resistance highlight the need for alternative approaches. TCM offers a holistic strategy, targeting neurotransmitter regulation, neuroprotection, neuroinflammation, and gut microbiota modulation.
However, its clinical application faces challenges, including lack of standardized dosing, quality control variability, potential herb-drug interactions, and limited large-scale randomized controlled trials (RCTs). Additionally, integrating TCM with multi-omics technologies remains complex, requiring standardized methodologies to bridge traditional knowledge with modern precision medicine.
Future research should focus on standardized clinical trials and molecular mechanisms, including neurotrophic factors and microbial interactions. By addressing these challenges and leveraging multi-omics technologies, TCM can complement conventional therapies, optimizing recovery and improving quality of life for stroke survivors.
Author contributions
LZ: Writing – original draft, Writing – review and editing. RH: Writing – review and editing, Writing – original draft. LH: Writing – review and editing, Writing – original draft. BP: Writing – review and editing. WZ: Writing – review and editing. YL: Funding acquisition, Writing – review and editing. XL: Funding acquisition, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was financially supported by the Cross-disciplinary Research Fund of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (JYJC202131).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amin, N., Liu, J., Bonnechere, B., MahmoudianDehkordi, S., Arnold, M., Batra, R., et al. (2023). Interplay of metabolome and gut microbiome in individuals with major depressive disorder vs control individuals. JAMA Psychiatry 80 (6), 597–609. doi:10.1001/jamapsychiatry.2023.0685
An, H. M., Lin, C., Gu, C., Chen, J. J., Sun, W. X., Jin, M., et al. (2017). Di-Huang-Yi-Zhi herbal formula attenuates amyloid-beta-induced neurotoxicity in PC12 cells. Exp. Ther. Med. 13 (6), 3003–3008. doi:10.3892/etm.2017.4368
Badini, I., Coleman, J. R. I., Hagenaars, S. P., Hotopf, M., Breen, G., Lewis, C. M., et al. (2022). Depression with atypical neurovegetative symptoms shares genetic predisposition with immuno-metabolic traits and alcohol consumption. Psychol. Med. 52 (4), 726–736. doi:10.1017/S0033291720002342
Bai, S., Bai, H., Li, D., Zhong, Q., Xie, J., and Chen, J. J. (2022). Gut microbiota-related inflammation factors as a potential biomarker for diagnosing major depressive disorder. Front. Cell. Infect. Microbiol. 12, 831186. doi:10.3389/fcimb.2022.831186
Begum, N., Mandhare, A., Tryphena, K. P., Srivastava, S., Shaikh, M. F., Singh, S. B., et al. (2022). Epigenetics in depression and gut-brain axis: a molecular crosstalk. Front. Aging Neurosci. 14, 1048333. doi:10.3389/fnagi.2022.1048333
Beker, M., Caglayan, A. B., Beker, M. C., Altunay, S., Karacay, R., Dalay, A., et al. (2020). Lentivirally administered glial cell line-derived neurotrophic factor promotes post-ischemic neurological recovery, brain remodeling and contralesional pyramidal tract plasticity by regulating axonal growth inhibitors and guidance proteins. Exp. Neurol. 331, 113364. doi:10.1016/j.expneurol.2020.113364
Bhattamisra, S. K., Yap, K. H., Rao, V., and Choudhury, H. (2019). Multiple biological effects of an iridoid glucoside, catalpol and its underlying molecular mechanisms. Biomolecules 10 (1), 32. doi:10.3390/biom10010032
Bian, X., Liu, X., Liu, J., Zhao, Y., Li, H., Cai, E., et al. (2018). Study on antidepressant activity of chiisanoside in mice. Int. Immunopharmacol. 57, 33–42. doi:10.1016/j.intimp.2018.02.007
Bian, L. H., Wang, S. Q., Li, W. J., Li, J., Yin, Y., Ye, F. F., et al. (2023). Cryptotanshinone regulates gut microbiota and PI3K-AKT pathway in rats to alleviate CUMS induced depressive symptoms. Biomed. Pharmacother. 169, 115921. doi:10.1016/j.biopha.2023.115921
Bin, Y., Ming, R., Li, X., and Hongyi, L. (2022). Chuanxiong injection improves neurological function in rats with post-stroke depression via cAMP-CREB-BDNF pathway. Chin. Pharmacol. Bull. 38 (08), 1246–1251. doi:10.2360/CPB202202035
Bonaterra, G. A., Mierau, O., Hofmann, J., Schwarzbach, H., Aziz-Kalbhenn, H., Kolb, C., et al. (2020). In vitro effects of st. John's wort extract against inflammatory and oxidative stress and in the phagocytic and migratory activity of mouse SIM-A9 microglia. Front. Pharmacol. 11, 603575. doi:10.3389/fphar.2020.603575
Bot, M., Milaneschi, Y., Al-Shehri, T., Amin, N., Garmaeva, S., Onderwater, G. L. J., et al. (2020). Metabolomics profile in depression: a pooled analysis of 230 metabolic markers in 5283 cases with depression and 10,145 controls. Biol. Psychiatry 87 (5), 409–418. doi:10.1016/j.biopsych.2019.08.016
Cai, W., Mueller, C., Li, Y. J., Shen, W. D., and Stewart, R. (2019). Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta-analysis. Ageing Res. Rev. 50, 102–109. doi:10.1016/j.arr.2019.01.013
Campbell, B. C. V., De Silva, D. A., Macleod, M. R., Coutts, S. B., Schwamm, L. H., Davis, S. M., et al. (2019). Ischaemic stroke. Nat. Rev. Dis. Prim. 5 (1), 70. doi:10.1038/s41572-019-0118-8
Carnes-Vendrell, A., Deus, J., Molina-Seguin, J., Pifarre, J., and Purroy, F. (2019). Depression and apathy after transient ischemic attack or minor stroke: prevalence, evolution and predictors. Sci. Rep. 9 (1), 16248. doi:10.1038/s41598-019-52721-5
Chang, Y., and Wang, S. J. (2010). Hypericin, the active component of St. John's wort, inhibits glutamate release in the rat cerebrocortical synaptosomes via a mitogen-activated protein kinase-dependent pathway. Eur. J. Pharmacol. 634 (1-3), 53–61. doi:10.1016/j.ejphar.2010.02.035
Che, B., Zhu, Z., Bu, X., Yin, J., Han, L., Xu, T., et al. (2021). Multiple biomarkers covering several pathways for the prediction of depression after ischemic stroke. J. Affect Disord. 280 (Pt A), 442–449. doi:10.1016/j.jad.2020.10.075
Chen, F., Tan, W., and Zhu, Y. (2019). Clinical efficacy evaluation of Jieyu Huoxue Decoction combined with fluoxetine capsules in the treatment of post-stroke depression. Electron. J. Mod. Med. Health Res. 3 (07), 25–26+28.
Chen, X., Shen, J., Zhou, Q., Jin, X., Liu, H., and Gao, R. (2022). Astragaloside VI ameliorates post-stroke depression via upregulating the NRG-1-mediated MEK/ERK pathway. Pharm. (Basel) 15 (12), 1551. doi:10.3390/ph15121551
Cheng, S. Y., Zhao, Y. D., Li, J., Chen, X. Y., Wang, R. D., and Zeng, J. W. (2014). Plasma levels of glutamate during stroke is associated with development of post-stroke depression. Psychoneuroendocrinology 47, 126–135. doi:10.1016/j.psyneuen.2014.05.006
Cheng, L. S., Tu, W. J., Shen, Y., Zhang, L. J., and Ji, K. (2018). Combination of high-sensitivity C-reactive protein and homocysteine predicts the post-stroke depression in patients with ischemic stroke. Mol. Neurobiol. 55 (4), 2952–2958. doi:10.1007/s12035-017-0549-8
Chi, C. H., Huang, Y. Y., Ye, S. Z., Shao, M. M., Jiang, M. X., Yang, M. Y., et al. (2021). Interleukin-10 level is associated with post-stroke depression in acute ischaemic stroke patients. J. Affect Disord. 293, 254–260. doi:10.1016/j.jad.2021.06.037
Dai, Y., Guo, J., Zhang, B., Chen, J., Ou, H., He, R. R., et al. (2023). Lycium barbarum (Wolfberry) glycopeptide prevents stress-induced anxiety disorders by regulating oxidative stress and ferroptosis in the medial prefrontal cortex. Phytomedicine 116, 154864. doi:10.1016/j.phymed.2023.154864
Ding, W., Wang, L., Li, L., Li, H., Wu, J., Zhang, J., et al. (2024). Pathogenesis of depression and the potential for traditional Chinese medicine treatment. Front. Pharmacol. 15, 1407869. doi:10.3389/fphar.2024.1407869
Du, F., Liang, F., Zhang, Z., Huang, Y., Zheng, F., and Shan, X. (2021). Clinical observation of Yinao Jieyu Decoction combined with acupuncture in the treatment of post-stroke depression. Chin. Med. Innov. 18 (09), 94–98.
Fan, Q., Liu, Y., Sheng, L., Lv, S., Yang, L., Zhang, Z., et al. (2023a). Chaihu-Shugan-San inhibits neuroinflammation in the treatment of post-stroke depression through the JAK/STAT3-GSK3β/PTEN/Akt pathway. Biomed. Pharmacother. 160, 114385. doi:10.1016/j.biopha.2023.114385
Fan, Y., Wang, L., Jiang, H., Fu, Y., Ma, Z., Wu, X., et al. (2023b). Depression circuit adaptation in post-stroke depression. J. Affect Disord. 336, 52–63. doi:10.1016/j.jad.2023.05.016
Feng, L., Xing, H., and Zhang, K. (2022). The therapeutic potential of traditional Chinese medicine in depression: targeting adult hippocampal neurogenesis. Phytomedicine 98, 153980. doi:10.1016/j.phymed.2022.153980
Frank, D., Kuts, R., Tsenter, P., Gruenbaum, B. F., Grinshpun, Y., Zvenigorodsky, V., et al. (2019). The effect of pyruvate on the development and progression of post-stroke depression: a new therapeutic approach. Neuropharmacology 155, 173–184. doi:10.1016/j.neuropharm.2019.05.035
Frank, D., Gruenbaum, B. F., Zlotnik, A., Semyonov, M., Frenkel, A., and Boyko, M. (2022). Pathophysiology and current drug treatments for post-stroke depression: a review. Int. J. Mol. Sci. 23 (23), 15114. doi:10.3390/ijms232315114
Fu, R., Li, J., Yu, H., Zhang, Y., Xu, Z., and Martin, C. (2021). The yin and yang of traditional Chinese and western medicine. Med. Res. Rev. 41, 3182–3200. doi:10.1002/med.21793
Fuying, Z., Yingying, Y., Shining, Z., Kezhong, Z., Yanyan, S., Xuemei., Z., et al. (2019). Novel susceptibility genes were found in a targeted sequencing of stroke patients with or without depression in the Chinese han population. J. Affect. Disord. 255, 1–9. doi:10.1016/j.jad.2019.05.023
Gao, Q. S., Wang, L. C., Ma, A. J., Cheng, L. L., Wu, W. F., and Wei, Z. X. (2021). Efficacy of modified Chaihu Shugan Powder in the treatment of post-ischemic stroke depression with hyperlipidemia and its effects on serum TNF-α, CRP, and IL-6 levels. J. Mod. Integr. Traditional Chin. West. Med. 30 (28), 3138–3142. doi:10.3969/j.issn.1008-8849.2021.28.013
Gao, Z., Wang, Y., and Yu, H. (2022). A Chinese classical prescription Chaihu shugan powder in treatment of post-stroke depression: an overview. Med. Kaunas. 59 (1), 55. doi:10.3390/medicina59010055
Ge, T., Yao, X., Zhao, H., Yang, W., Zou, X., Peng, F., et al. (2021). Gut microbiota and neuropsychiatric disorders: implications for neuroendocrine-immune regulation. Pharmacol. Res. 173, 105909. doi:10.1016/j.phrs.2021.105909
Geng, L. Y., Qian, F. Y., Qian, J. F., and Zhang, Z. J. (2017). The combination of plasma glutamate and physical impairment after acute stroke as a potential indicator for the early-onset post-stroke depression. J. Psychosom. Res. 96, 35–41. doi:10.1016/j.jpsychores.2017.01.006
Gong, M., Wang, J., Song, L., Wu, X., Wang, Y., Li, B., et al. (2023). Role of BDNF-TrkB signaling in the antidepressant-like actions of loganin, the main active compound of Corni Fructus. CNS Neurosci. Ther. 29 (12), 3842–3853. doi:10.1111/cns.14305
Gu, D. J., Zheng, H. S., Li,, J. Z., Liu, F., Shuai, G. Y., Wang, Z. Y., et al. (2018). Clinical efficacy of Shugan Jieyu Capsule combined with Delixin in the treatment of post-stroke depression. Systems Medicine 3 (11), 18–19+22. doi:10.19368/j.cnki.2096-1782.2018.11.018
Guo, Y., Wang, T., Chen, W., Kaptchuk, T. J., Li, X., Gao, X., et al. (2021). Acceptability of traditional Chinese medicine in Chinese People based on 10-year's real world study with multiple big data mining. Front. Public Health 9, 811730. doi:10.3389/fpubh.2021.811730
Guo, J., Wang, J., Sun, W., and Liu, X. (2022). The advances of post-stroke depression: 2021 update. J. Neurol. 269 (3), 1236–1249. doi:10.1007/s00415-021-10597-4
Han, W., Wang, N., Han, M., Ban, M., Sun, T., and Xu, J. (2022). Reviewing the role of gut microbiota in the pathogenesis of depression and exploring new therapeutic options. Front. Neurosci. 16, 1029495. doi:10.3389/fnins.2022.1029495
Hankey, G. J. (2014). Secondary stroke prevention. Lancet Neurol. 13 (2), 178–194. doi:10.1016/S1474-4422(13)70255-2
Hong, W., Zhao, Z., Wang, D., Li, M., Tang, C., Li, Z., et al. (2020). Altered gray matter volumes in post-stroke depressive patients after subcortical stroke. Neuroimage Clin. 26, 102224. doi:10.1016/j.nicl.2020.102224
Hu, D., Ling, Z. X., and Sheng, L. (2018). Clinical study on the effect of modified Chaihu Shugan Powder combined with western medicine on sleep in patients with post-stroke depression - attached with clinical data of 32 cases. Jiangsu J. Traditional Chin. Med. 50 (04), 43–45.
Hu, M. Z., Wang, A. R., Zhao, Z. Y., Chen, X. Y., Li, Y. B., and Liu, B. (2019a). Antidepressant-like effects of paeoniflorin on post-stroke depression in a rat model. Neurol. Res. 41 (5), 446–455. doi:10.1080/01616412.2019.1576361
Hu, S., Li, A., Huang, T., Lai, J., Li, J., Sublette, M. E., et al. (2019b). Gut microbiota changes in patients with bipolar depression. Adv. Sci. (Weinh) 6 (14), 1900752. doi:10.1002/advs.201900752
Hu, D., Liu, Y. Y., and Sheng, L. (2020). Effects of Chaihu Shugan San on BDNF/TrkB signaling pathway and inflammatory indicators in rats with post-stroke depression model. Jiangsu J. Traditional Chin. Med. 52 (08), 78–81. doi:10.19844/j.cnki.1672-397X.2020.08.028
Hu, X. Q., Tan, L., and Ji, J. (2024). Clinical observation of Jiawei Xiaoyao Pills combined with escitalopram oxalate tablets in the treatment of post-stroke depression. J. Pract. Chin. Med. 40 (05), 956–958.
Huang, W. B., and Zeng, Y. Q. (2015). Observation on the efficacy of Chaihu Shugan San combined with fluoxetine in the treatment of post-stroke depression. J. Traditional Chin. Med. 21 (13), 79–81. doi:10.13862/j.cnki.cn43-1446/r.2015.13.030
Huang, W., Liao, X., Tian, J., Wu, J., Shan, Y., and Zhou, W. (2018). Traditional Chinese medicine for post-stroke depression: a systematic review and network meta-analysis (Protocol). Medicine 97, e13840. doi:10.1097/MD.0000000000013840
Huang, Y. J., Hsu, N. Y., Lu, K. H., Lin, Y. E., Lin, S. H., Lu, Y. S., et al. (2020). Poria cocos water extract ameliorates the behavioral deficits induced by unpredictable chronic mild stress in rats by down-regulating inflammation. J. Ethnopharmacol. 258, 112566. doi:10.1016/j.jep.2020.112566
Jia, K., Feng, L., Huang, C. J., and Li, J. Y. (2023). Effects of Chaihu Shugan San on behavior and expression of 5-HT2A and BDNF in brain regions of rats with post-stroke depression. Chin. Pat. Med. 45 (12), 3943–3949. doi:10.3969/j.issn.1001-1528.2023.12.014
Jiang, W., Gong, L., Liu, F., Ren, Y., and Mu, J. (2021). Alteration of gut microbiome and correlated lipid metabolism in post-stroke depression. Front. Cell. Infect. Microbiol. 11, 663967. doi:10.3389/fcimb.2021.663967
Jiang, H., Deng, S., Zhang, J., Chen, J., Li, B., Zhu, W., et al. (2023). Acupuncture treatment for post-stroke depression: intestinal microbiota and its role. Front. Neurosci. 17, 1146946. doi:10.3389/fnins.2023.1146946
Jiang, R. S. (2023). Efficacy and safety of shugan Jieyu capsule combined with tandospirone citrate in the treatment of post-stroke depression. Clin. Ration. Drug Use 16 (06), 47–50. doi:10.15887/j.cnki.13-1389/r.2023.06.014
Kang, H. J., Bae, K. Y., Kim, S. W., Kim, J. T., Park, M. S., Cho, K. H., et al. (2016). Effects of interleukin-6, interleukin-18, and statin use, evaluated at acute stroke, on post-stroke depression during 1-year follow-up. Psychoneuroendocrinology 72, 156–160. doi:10.1016/j.psyneuen.2016.07.001
Kim, J. M., Kang, H. J., Kim, J. W., Bae, K. Y., Kim, S. W., Kim, J. T., et al. (2017). Associations of tumor necrosis factor-α and interleukin-1β levels and polymorphisms with post-stroke depression. Am. J. Geriatr. Psychiatry 25 (12), 1300–1308. doi:10.1016/j.jagp.2017.07.012
Klingbeil, J., Brandt, M. L., Wawrzyniak, M., Stockert, A., Schneider, H. R., Baum, P., et al. (2021). Association of lesion location and depressive symptoms poststroke. Stroke 52 (3), 830–837. doi:10.1161/STROKEAHA.120.031889
Kohen, R., Cain, K. C., Mitchell, P. H., Becker, K., Buzaitis, A., Millard, S. P., et al. (2008). Association of serotonin transporter gene polymorphisms with poststroke depression. Arch. Gen. Psychiatry 65 (11), 1296–1302. doi:10.1001/archpsyc.65.11.1296
Kwon, C. Y., Lee, B., Chung, S. Y., Kim, J. W., Shin, A., Choi, Y. Y., et al. (2019). Efficacy and safety of Sihogayonggolmoryeo-tang (Saikokaryukotsuboreito, Chai-Hu-Jia-Long-Gu-Mu-Li-Tang) for post-stroke depression: a systematic review and meta-analysis. Sci. Rep. 9 (1), 14536. doi:10.1038/s41598-019-51055-6
Lanctot, K. L., Lindsay, M. P., Smith, E. E., Sahlas, D. J., Foley, N., Gubitz, G., et al. (2020). Canadian stroke best practice recommendations: mood, cognition and fatigue following stroke, 6th edition update 2019. Int. J. Stroke 15 (6), 668–688. doi:10.1177/1747493019847334
Lee, J., d'Aigle, J., Atadja, L., Quaicoe, V., Honarpisheh, P., Ganesh, B. P., et al. (2020). Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circ. Res. 127 (4), 453–465. doi:10.1161/CIRCRESAHA.119.316448
Li, Q., and Liu, X. H. (2022). Effects of modified Chaihu Shugan powder combined with transcranial electrical stimulation on 5-HT and BDNF levels in patients with post-stroke depression. J. Integr. Traditional Chin. West. Med. Cardiovasc. Dis. 20 (10), 1755–1759. doi:10.12102/j.issn.1672-1349.2022.10.007
Li, Y. C., Zheng, X. X., Xia, S. Z., Li, Y., Deng, H. H., Wang, X., et al. (2020). Paeoniflorin ameliorates depressive-like behavior in prenatally stressed offspring by restoring the HPA axis- and glucocorticoid receptor-associated dysfunction. J. Affect Disord. 274, 471–481. doi:10.1016/j.jad.2020.05.078
Li, Z., Xu, H., Xu, Y., Lu, G., Peng, Q., Chen, J., et al. (2021). Morinda officinalis oligosaccharides alleviate depressive-like behaviors in post-stroke rats via suppressing NLRP3 inflammasome to inhibit hippocampal inflammation. CNS Neurosci. Ther. 27 (12), 1570–1586. doi:10.1111/cns.13732
Li, L., Huo, B., Wang, Y., Wang, Y., Gong, Y., Zhang, Y., et al. (2022). Efficacy of Chinese herbal medicine on poststroke depression in animal models: a systematic review and meta-analysis. Front. Neurol. 13, 1095444. doi:10.3389/fneur.2022.1095444
Liang, S., Wang, T., Hu, X., Luo, J., Li, W., Wu, X., et al. (2015). Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 310, 561–577. doi:10.1016/j.neuroscience.2015.09.033
Liang, J., Yue, Y., Jiang, H., Geng, D., Wang, J., Lu, J., et al. (2018a). Genetic variations in the p11/tPA/BDNF pathway are associated with post stroke depression. J. Affect Disord. 226, 313–325. doi:10.1016/j.jad.2017.09.055
Liang, S., Wu, X., Hu, X., Wang, T., and Jin, F. (2018b). Recognizing depression from the Microbiota(-)Gut(-)Brain Axis. Int. J. Mol. Sci. 19 (6), 1592. doi:10.3390/ijms19061592
Liu, S. Q., Zhang, L. N., and Yuan, C. (2019). Clinical efficacy of Shugan Jieyu capsule in the treatment of post-stroke depression and its effect on norepinephrine and 5-hydroxytryptamine levels. World J. Traditional Chin. Med. 14 (07), 1784–1788. doi:10.3969/j.issn.1673-7202.2019.07.034
Liu, Y. Y., Hu, D., Fan, Q. Q., Zhang, X. H., Zhu, Y. C., Ni, M. Y., et al. (2020). Mechanism of Chaihu shugan powder for treating depression based on network pharmacology. Chin. J. Integr. Med. 26 (12), 921–928. doi:10.1007/s11655-019-3172-x
Liu, Y., Wang, H., Gui, S., Zeng, B., Pu, J., Zheng, P., et al. (2021). Proteomics analysis of the gut-brain axis in a gut microbiota-dysbiosis model of depression. Transl. Psychiatry 11 (1), 568. doi:10.1038/s41398-021-01689-w
Liu, L., Yuan, X. H., Yi, Y. Q., Zhou, S., Liu, Y., Shao, L., et al. (2022). Experimental study on the regulation of brain-gut axis inflammatory response in post-stroke depression by Baishile Jiawei Fang through P2X7R/NLRP3. J. Traditional Chin. Med. 33 (05), 1051–1055. doi:10.3969/j.issn.1008-0805.2022.05.08
Lu, J., Zhou, H., Meng, D., Zhang, J., Pan, K., Wan, B., et al. (2020a). Tanshinone IIA improves depression-like behavior in mice by activating the ERK-CREB-BDNF signaling pathway. Neuroscience 430, 1–11. doi:10.1016/j.neuroscience.2020.01.026
Lu, X., Duan, J., Cheng, Q., and Lu, J. (2020b). The association between serum growth differentiation factor-15 and 3-month depression after acute ischemic stroke. J. Affect Disord. 260, 695–702. doi:10.1016/j.jad.2019.09.037
Lu, L., Wan, B., Li, L., and Sun, M. (2022). Hypothyroidism has a protective causal association with hepatocellular carcinoma: a two-sample Mendelian randomization study. Front. Endocrinol. (Lausanne) 13, 987401. doi:10.3389/fendo.2022.987401
Luo, L., Li, C., Du, X., Shi, Q., Huang, Q., Xu, X., et al. (2019a). Effect of aerobic exercise on BDNF/proBDNF expression in the ischemic hippocampus and depression recovery of rats after stroke. Behav. Brain Res. 362, 323–331. doi:10.1016/j.bbr.2018.11.037
Luo, W., Li, Y., and Liu, L. (2019b). Effect of modified Ditan decoction in the treatment of post-stroke depression and its effect on the expression of NF-κB and miR-146a in peripheral blood of patients. Shaanxi J. Traditional Chin. Med. 40 (08), 1029–1031+1048. doi:10.3969/j.issn.1000-7369.2019.08.012
Maes, M., Kubera, M., Leunis, J. C., and Berk, M. (2012). Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J. Affect Disord. 141 (1), 55–62. doi:10.1016/j.jad.2012.02.023
Medeiros, G. C., Roy, D., Kontos, N., and Beach, S. R. (2020). Post-stroke depression: a 2020 updated review. Gen. Hosp. Psychiatry 66, 70–80. doi:10.1016/j.genhosppsych.2020.06.011
Meng, W., Chao, W., Kaiwei, Z., Sijia, M., Jiajia, S., and Shijie, X. (2025). Bioactive compounds from Chinese herbal plants for neurological health: mechanisms, pathways, and functional food applications. Front. Nutr. 12, 1537363. doi:10.3389/fnut.2025.1537363
Mikami, K., Jorge, R. E., Adams, H. P., Davis, P. H., Leira, E. C., Jang, M., et al. (2011). Effect of antidepressants on the course of disability following stroke. Am. J. Geriatr. Psychiatry 19 (12), 1007–1015. doi:10.1097/JGP.0b013e31821181b0
Mortensen, J. K., Larsson, H., Johnsen, S. P., and Andersen, G. (2013). Post stroke use of selective serotonin reuptake inhibitors and clinical outcome among patients with ischemic stroke: a nationwide propensity score-matched follow-up study. Stroke 44 (2), 420–426. doi:10.1161/STROKEAHA.112.674242
Pan, X., Chen, K., Han, S., Luo, X., Zhang, D., Zhang, H., et al. (2022). Total triterpenes of Wolfiporia cocos (schwein.) ryvarden & gilb exerts antidepressant-like effects in a chronic unpredictable mild stress rat model and regulates the levels of neurotransmitters, HPA Axis and NLRP3 pathway. Front. Pharmacol. 13, 793525. doi:10.3389/fphar.2022.793525
Qi, Y., Zhang, H., Liang, S., Chen, J., Yan, X., Duan, Z., et al. (2020). Evaluation of the antidepressant effect of the functional beverage containing active peptides, menthol and eleutheroside and investigation of its mechanism of action in mice. Food Technol. Biotechnol. 58 (3), 295–302. doi:10.17113/ftb.58.03.20.6568
Qian, H. B., Wang, Y., and Huang, G. J. (2012). Effects of water extract of Curcuma zedoariae on behavior and angiogenesis in rats with post-stroke depression. J. Traditional Chin. Med. 23 (07), 1709–1711. doi:10.3969/j.issn.1008-0805.2012.07.051
Qian, L., Huang, S., Liu, X., Jiang, Y., Jiang, Y., Hu, Y., et al. (2024). Morroniside improves the symptoms of post-stroke depression in mice through the BDNF signaling pathway mediated by MiR-409-3p. Phytomedicine 123, 155224. doi:10.1016/j.phymed.2023.155224
Rao, X., Liu, L., Wang, H., Yu, Y., Li, W., Chai, T., et al. (2021). Regulation of gut microbiota disrupts the glucocorticoid receptor pathway and inflammation-related pathways in the mouse Hippocampus. Exp. Neurobiol. 30 (1), 59–72. doi:10.5607/en20055
Ren, S. H., Yang, C. X., and Wei, X. (2023). Clinical observation of Jieyu Huoxue decoction combined with acupuncture in the treatment of post-stroke depression of liver depression and blood stasis type. J. Guangzhou Univ. Traditional Chin. Med. 40 (10), 2443–2450. doi:10.13359/j.cnki.gzxbtcm.2023.10.005
Robinson, R. G., Schultz, S. K., Castillo, C., Kopel, T., Kosier, J. T., Newman, R. M., et al. (2000). Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. Am. J. Psychiatry 157 (3), 351–359. doi:10.1176/appi.ajp.157.3.351
Savadi Oskouie, D., Sharifipour, E., Sadeghi Bazargani, H., Hashemilar, M., Nikanfar, M., Ghazanfari Amlashi, S., et al. (2017). Efficacy of citalopram on acute ischemic stroke outcome: a randomized clinical trial. Neurorehabil Neural Repair 31 (7), 638–647. doi:10.1177/1545968317704902
Shi, Y., Zeng, Y., Wu, L., Liu, W., Liu, Z., Zhang, S., et al. (2017). A study of the brain abnormalities of post-stroke depression in frontal lobe lesion. Sci. Rep. 7 (1), 13203. doi:10.1038/s41598-017-13681-w
Shi, H. L. (2021). Effects of Wuling capsule combined with flupentixol-melitracen tablets on mood and sleep status in patients with post-stroke anxiety and depression. Chin. J. Drugs Clin. 21 (23), 3805–3808. doi:10.11655/zgywylc2021.23.002
Shu, Q. (2023). Clinical effect of Shugan Jieyu capsule combined with escitalopram in the treatment of patients with post-stroke depression. Chin. Foreign Med. Res. 21 (13), 11–14. doi:10.14033/j.cnki.cfmr.2023.13.003
Song, W. T., Xu, L., Ren, J. X., Yao, M. J., Wang, G. R., and Liu, J. X. (2015). Study on the efficacy of Jiedutongluo capsule in invigorating qi and relieving depression in rats with post-stroke depression. World Sci. Technology-Modernization Traditional Chin. Med. 17 (07), 1380–1385.
Song, L., Wu, X., Wang, J., Guan, Y., Zhang, Y., Gong, M., et al. (2021). Antidepressant effect of catalpol on corticosterone-induced depressive-like behavior involves the inhibition of HPA axis hyperactivity, central inflammation and oxidative damage probably via dual regulation of NF-κB and Nrf2. Brain Res. Bull. 177, 81–91. doi:10.1016/j.brainresbull.2021.09.002
Sun, L., Zhang, W., Ye, R., Liu, L., Jiang, L., and Xi, C. (2021). Catalpol enhanced physical exercise-mediated brain functional improvement in post-traumatic stress disorder model via promoting adult hippocampal neurogenesis. Aging (Albany NY) 13 (14), 18689–18700. doi:10.18632/aging.203313
Tang, C. Z., Zhang, Y. L., Wang, W. S., Li, W. G., and Shi, J. P. (2016). Serum levels of high-sensitivity C-reactive protein at admission are more strongly associated with poststroke depression in acute ischemic stroke than homocysteine levels. Mol. Neurobiol. 53 (4), 2152–2160. doi:10.1007/s12035-015-9186-2
Tian, X. S., Gong, Y. T., Ji, C. S., and Zhang, C. (2021). Clinical efficacy of Delexin combined with Wuling capsules in the treatment of post-stroke depression. Chin. J. Traditional Chin. Med. 38 (01), 63–66. doi:10.19656/j.cnki.1002-2406.210115
Villa, R. F., Ferrari, F., and Moretti, A. (2018). Post-stroke depression: mechanisms and pharmacological treatment. Pharmacol. Ther. 184, 131–144. doi:10.1016/j.pharmthera.2017.11.005
Waclawikova, B., and El Aidy, S. (2018). Role of microbiota and tryptophan metabolites in the remote effect of intestinal inflammation on brain and depression. Pharm. (Basel) 11 (3), 63. doi:10.3390/ph11030063
Wang, J. Q., and Ni, X. L. (2014). Observation on the efficacy of flupentixol-melitracen combined with Xiaoyao pills in the treatment of post-stroke depression. J. Pract. Cardiovasc. Dis. 22 (05), 45–46. doi:10.3969/j.issn.1008-5971.2014.05.022
Wang, J. M., Ma, Y. X., Zhang, B., Li, Q. W., and Cui, Y. (2013). Study on the antidepressant effect of Cyperus rotundus extract. J. Traditional Chin. Med. 24 (04), 779–781. doi:10.3969/j.issn.1008-0805.2013.04.005
Wang, S., Sun, H., Liu, S., Wang, T., Guan, J., and Jia, J. (2016). Role of hypothalamic cannabinoid receptors in post-stroke depression in rats. Brain Res. Bull. 121, 91–97. doi:10.1016/j.brainresbull.2016.01.006
Wang, R., Li, G. C., and Gao, X. (2019). Clinical study of Chaihu Shugan San plus or minus in the treatment of post-stroke depression. World Latest Med. Inf. Abstr. 19 (96), 211. doi:10.19613/j.cnki.1671-3141.2019.96.120
Wang, X. Y., Li, Y., Zhu, H. X., O, R. W., R, N., Xu, F. F., et al. (2021). Effects of liquiritin on brain-derived neurotrophic factor and Bax and Bcl-2 protein expression in the prefrontal cortex of rats with post-stroke depression. Chin. J. Geriatric Cardiovasc. Dis. 23 (06), 647–650. doi:10.3969/j.issn.1009-0126.2021.06.023
Wang, A. R., Mi, L. F., Zhang, Z. L., Hu, M. Z., Zhao, Z. Y., Liu, B., et al. (2021a). Saikosaponin A improved depression-like behavior and inhibited hippocampal neuronal apoptosis after cerebral ischemia through p-CREB/BDNF pathway. Behav. Brain Res. 403, 113138. doi:10.1016/j.bbr.2021.113138
Wang, J., Chen, R., Liu, C., Wu, X., and Zhang, Y. (2021b). Antidepressant mechanism of catalpol: involvement of the PI3K/Akt/Nrf2/HO-1 signaling pathway in rat hippocampus. Eur. J. Pharmacol. 909, 174396. doi:10.1016/j.ejphar.2021.174396
Wang, X. L., Feng, S. T., Wang, Y. T., Chen, N. H., Wang, Z. Z., and Zhang, Y. (2021c). Paeoniflorin: a neuroprotective monoterpenoid glycoside with promising anti-depressive properties. Phytomedicine 90, 153669. doi:10.1016/j.phymed.2021.153669
Wang, Y. L., Wu, H. R., Zhang, S. S., Xiao, H. L., Yu, J., Ma, Y. Y., et al. (2021d). Catalpol ameliorates depressive-like behaviors in CUMS mice via oxidative stress-mediated NLRP3 inflammasome and neuroinflammation. Transl. Psychiatry 11 (1), 353. doi:10.1038/s41398-021-01468-7
Wang, D., Li, T., Ron, J., Mao, M., Yang, Y., Feng, Y., et al. (2022). Efficacy and safety of Xiaoyao recipe in the treatment of poststroke depression: a systematic review and meta-analysis. Evid. Based Complement. Altern. Med. 2022, 4385783. doi:10.1155/2022/4385783
Wang, F., Zhang, S., Zhang, J., and Yuan, F. (2022a). Systematic review of ethnomedicine, phytochemistry, and pharmacology of Cyperi Rhizoma. Front. Pharmacol. 13, 965902. doi:10.3389/fphar.2022.965902
Wang, Z., Sun, Y., Bian, L., Zhang, Y., Zhang, Y., Wang, C., et al. (2022b). The crosstalk signals of Sodium Tanshinone ⅡA Sulfonate in rats with cerebral ischemic stroke: insights from proteomics. Biomed. Pharmacother. 151, 113059. doi:10.1016/j.biopha.2022.113059
Wang, R., Chen, Y., Wang, Z., Cao, B., Du, J., Deng, T., et al. (2023). Antidepressant effect of licorice total flavonoids and liquiritin: a review. Heliyon 9 (11), e22251. doi:10.1016/j.heliyon.2023.e22251
Wang, X., Zhou, J., Jiang, T., and Xu, J. (2024). Deciphering the therapeutic potential of SheXiangXinTongNing: interplay between gut microbiota and brain metabolomics in a CUMS mice model, with a focus on tryptophan metabolism. Phytomedicine 129, 155584. doi:10.1016/j.phymed.2024.155584
Wu, L., Tang, Y., Zheng, Q., Wu, H. X., Hu, P. Y., Guo, Y. Y., et al. (2019). Study on the antidepressant effect of Chuanxiong volatile oil based on CUMS rats. World Tradit. Chin. Med. 14 (07), 1643–1648. doi:10.3969/j.issn.1673-7202.2019.07.004
Wu, D., Zhang, G., Zhao, C., Yang, Y., Miao, Z., and Xu, X. (2020). Interleukin-18 from neurons and microglia mediates depressive behaviors in mice with post-stroke depression. Brain Behav. Immun. 88, 411–420. doi:10.1016/j.bbi.2020.04.004
Wu, Z. P., Wang, Y. S., Wang, P. L., and Zhao, Y. C. (2021). Effects of Huoxue Jieyu Decoction combined with fluoxetine on serum brain-derived neurotrophic factor in post-stroke depression. Chin. J. Traditional Chin. Med. 39 (02), 185–188. doi:10.13193/j.issn.1673-7717.2021.02.047
Wu, X., Liu, C., Wang, J., Guan, Y., Song, L., Chen, R., et al. (2022). Catalpol exerts antidepressant-like effects by enhancing anti-oxidation and neurotrophy and inhibiting neuroinflammation via activation of HO-1. Neurochem. Res. 47 (10), 2975–2991. doi:10.1007/s11064-022-03641-w
Wu, X., Liu, C., Wang, J., Zhang, Y., Li, Y., Wang, Y., et al. (2024). The role of TrkB signaling-mediated synaptic plasticity in the antidepressant properties of catalpol, the main active compound of Rehmannia glutinosa Libosch. J. Ethnopharmacol. 333, 118448. doi:10.1016/j.jep.2024.118448
Xia, W., Liu, B., Tang, S., Yasir, M., and Khan, I. (2022). The science behind TCM and Gut microbiota interaction-their combinatorial approach holds promising therapeutic applications. Front. Cell. Infect. Microbiol. 12, 875513. doi:10.3389/fcimb.2022.875513
Yan, Q. (2018). Neuroimmune imbalances and yin-yang dynamics in stress, anxiety, and depression. Methods Mol. Biol. 1781, 77–85. doi:10.1007/978-1-4939-7828-1_5
Yang, B., Wang, L., Nie, Y., Wei, W., and Xiong, W. (2021). proBDNF expression induces apoptosis and inhibits synaptic regeneration by regulating the RhoA-JNK pathway in an in vitro post-stroke depression model. Transl. Psychiatry 11 (1), 578. doi:10.1038/s41398-021-01667-2
Yang, Y., Yu, L., Zhu, T., Xu, S., He, J., Mao, N., et al. (2023). Neuroprotective effects of Lycium barbarum polysaccharide on light-induced oxidative stress and mitochondrial damage via the Nrf2/HO-1 pathway in mouse hippocampal neurons. Int. J. Biol. Macromol. 251, 126315. doi:10.1016/j.ijbiomac.2023.126315
Yang, Y., Yu, L., Zhu, T., Xu, S., He, J., Mao, N., et al. (2024a). Neuroprotective effects of Rehmannia glutinosa polysaccharide on chronic constant light (CCL)-induced oxidative stress and autophagic cell death via the AKT/mTOR pathway in mouse hippocampus and HT-22 cells. Int. J. Biol. Macromol. 261 (Pt 2), 129813. doi:10.1016/j.ijbiomac.2024.129813
Yang, Z., Jiang, Y., Xiao, Y., Qian, L., Jiang, Y., Hu, Y., et al. (2024b). Di-Huang-Yin-Zi regulates P53/SLC7A11 signaling pathway to improve the mechanism of post-stroke depression. J. Ethnopharmacol. 319 (Pt 2), 117226. doi:10.1016/j.jep.2023.117226
Yao, G., Li, J., Wang, J., Liu, S., Li, X., Cao, X., et al. (2020). Improved resting-state functional dynamics in post-stroke depressive patients after shugan Jieyu capsule treatment. Front. Neurosci. 14, 297. doi:10.3389/fnins.2020.00297
Yi, L. T., Li, C. F., Zhan, X., Cui, C. C., Xiao, F., Zhou, L. P., et al. (2010). Involvement of monoaminergic system in the antidepressant-like effect of the flavonoid naringenin in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 34 (7), 1223–1228. doi:10.1016/j.pnpbp.2010.06.024
Yi, L. T., Xu, H. L., Feng, J., Zhan, X., Zhou, L. P., and Cui, C. C. (2011). Involvement of monoaminergic systems in the antidepressant-like effect of nobiletin. Physiol. Behav. 102 (1), 1–6. doi:10.1016/j.physbeh.2010.10.008
Yi, Y., Wei, Z., and Zheng, Q. (2021a). Clinical study of Shugan Jieyu capsule combined with fluoxetine in the treatment of post-stroke depression. New Chin. Med. 53 (05), 75–78. doi:10.13457/j.cnki.jncm.2021.05.019
Yi, X., Zhu, X., Zhou, Y., Zhang, D., Li, M., Zhu, Y., et al. (2021b). The combination of insulin resistance and serum interleukin-1β correlates with post-stroke depression in patients with acute ischemic stroke. Neuropsychiatr. Dis. Treat. 17, 735–746. doi:10.2147/NDT.S291164
Yin, P., Han, X., Yu, L., Zhou, H., Yang, J., Chen, Y., et al. (2023). Pharmacokinetic analysis for simultaneous quantification of Saikosaponin A-paeoniflorin in normal and poststroke depression rats: a comparative study. J. Pharm. Biomed. Anal. 233, 115485. doi:10.1016/j.jpba.2023.115485
Yu, M., Sun, Z. J., Li, L. T., Ge, H. Y., Song, C. Q., and Wang, A. J. (2015). The beneficial effects of the herbal medicine Di-huang-yin-zi (DHYZ) on patients with ischemic stroke: a Randomized, Placebo controlled clinical study. Complement. Ther. Med. 23 (4), 591–597. doi:10.1016/j.ctim.2015.06.003
Yuan, X. H., Liu, Z. H., Liu, J., Lei, S. H., Liu, Y., Li, W., et al. (2024). Protective effect and mechanism of Baishile Jiawei Fang on oxygen-glucose deprivation combined with lipopolysaccharide-induced hippocampal neuronal cell injury. Chin. J. Traditional Chin. Med. Inf. 31 (02), 116–122. doi:10.19879/j.cnki.1005-5304.202211910
Yue, Y., You, L., Zhao, F., Zhang, K., Shi, Y., Tang, H., et al. (2023). Common susceptibility variants of KDR and IGF-1R are associated with poststroke depression in the Chinese population. Gen. Psychiatr. 36 (1), e100928. doi:10.1136/gpsych-2022-100928
Zahrai, A., Vahid-Ansari, F., Daigle, M., and Albert, P. R. (2020). Fluoxetine-induced recovery of serotonin and norepinephrine projections in a mouse model of post-stroke depression. Transl. Psychiatry 10 (1), 334. doi:10.1038/s41398-020-01008-9
Zang, Y., Zhu, Z., Xie, Y., Liu, Z., Yin, J., Yang, P., et al. (2022). Serum growth differentiation factor 15 levels are associated with depression after ischemic stroke. J. Am. Heart Assoc. 11 (1), e022607. doi:10.1161/JAHA.121.022607
Zeng, M. L., Chen, L., Li, B., Chen, K. L., Zhi, H. Y., Zhang, Y. T., et al. (2018). Clinical efficacy of Xiaoyao Pills combined with fluoxetine in the treatment of post-stroke depression and its effect on serum 5-hydroxytryptamine level. Zhejiang J. Integr. Traditional Chin. West. Med. 28 (12), 997–999.
Zhang, Z., Mu, J., Li, J., Li, W., and Song, J. (2013). Aberrant apolipoprotein E expression and cognitive dysfunction in patients with poststroke depression. Genet. Test. Mol. Biomarkers 17 (1), 47–51. doi:10.1089/gtmb.2012.0253
Zhang, X. F., Zou, W., and Yang, Y. (2016). Effects of IL-6 and cortisol fluctuations in post-stroke depression. J. Huazhong Univ. Sci. Technol. Med. Sci. 36 (5), 732–735. doi:10.1007/s11596-016-1653-0
Zhang, Y., Jiang, H., Yue, Y., Yin, Y., Zhang, Y., Liang, J., et al. (2017). The protein and mRNA expression levels of glial cell line-derived neurotrophic factor in post stroke depression and major depressive disorder. Sci. Rep. 7 (1), 8674. doi:10.1038/s41598-017-09000-y
Zhang, W., Wang, W., and Kuang, L. (2018). The relation between insulin-like growth factor 1 levels and risk of depression in ischemic stroke. Int. J. Geriatr. Psychiatry 33 (2), e228–e233. doi:10.1002/gps.4774
Zhang, X. F., He, X., Wu, L., Liu, C. J., and Wu, W. (2019). Altered functional connectivity of amygdala with the fronto-limbic-striatal circuit in temporal lobe lesion as a proposed mechanism for poststroke depression. Am. J. Phys. Med. Rehabil. 98 (4), 303–310. doi:10.1097/PHM.0000000000001081
Zhang, Z. R., Zhou, Y. R., and Wang, J. W. (2020). Ditan decoction improves depressive symptoms in rats with post-stroke depression and regulates brain gut peptides. Shaanxi J. Traditional Chin. Med. 41 (02), 147–151. doi:10.3969/j.issn.1000-7369.2020.02.003
Zhang, T., Yang, C., Chu, J., Ning, L. N., Zeng, P., Wang, X. M., et al. (2021). Emodin prevented depression in chronic unpredicted mild stress-exposed rats by targeting miR-139-5p/5-lipoxygenase. Front. Cell. Dev. Biol. 9, 696619. doi:10.3389/fcell.2021.696619
Zhang, S., Li, J. L., Zhao, R. Z., Zhang, D. S., Li, X. Q., and Tang, Q. S. (2023a). Effects of Yinao Jieyu Fang on behavior and NLRP3-related inflammatory proteins in depressed rats. Acta Sin. 38 (10), 2168–2174. doi:10.16368/j.issn.1674-8999.2023.10.350
Zhang, X., Wang, C. B., Duan, L. H., Long, J. J., Xiao, P., Wang, Y. L., et al. (2023b). Correlation research of serum substance P, CCK-8, and 5-HT values with depression levels in stroke survivors. Eur. Rev. Med. Pharmacol. Sci. 27 (4), 1248–1254. doi:10.26355/eurrev_202302_31357
Zhanina, M. Y., Druzhkova, T. A., Yakovlev, A. A., Vladimirova, E. E., Freiman, S. V., Eremina, N. N., et al. (2022). Development of post-stroke cognitive and depressive disturbances: associations with neurohumoral indices. Curr. Issues Mol. Biol. 44 (12), 6290–6305. doi:10.3390/cimb44120429
Zhao, Z. G., Zhang, P., Wu, Y. G., and Liu, H. (2011). Screening of antidepressant active sites of Wenyujin. Chin. J. Traditional Chin. Med. 26 (08), 868–869.
Zhao, A., Ma, B., Xu, L., Yao, M., Zhang, Y., Xue, B., et al. (2021a). Jiedu tongluo granules ameliorates post-stroke depression rat model via regulating NMDAR/BDNF signaling pathway. Front. Pharmacol. 12, 662003. doi:10.3389/fphar.2021.662003
Zhao, F., Guan, S., Fu, Y., Wang, K., Liu, Z., and Ng, T. B. (2021b). Lycium barbarum polysaccharide attenuates emotional injury of offspring elicited by prenatal chronic stress in rats via regulation of gut microbiota. Biomed. Pharmacother. 143, 112087. doi:10.1016/j.biopha.2021.112087
Zhao, X. C., Xing, X. R., and Qin, X. (2023). Effects of Wuling capsule combined with escitalopram oxalate on psychological state and quality of life in patients with post-stroke depression. Chin. Prescr. Drugs 21 (06), 128–131.
Zheng, S. Z., Lin, J., and Li, G. R. (2024). Effect and safety analysis of Wuling capsule combined with oryzanol tablets in the treatment of post-stroke depression. Chin. J. Mod. Med. 34 (12), 62–67. doi:10.3969/j.issn.1005-8982.2024.12.011
Zhong, J., Chen, J., Cao, M., Fang, L., Wang, Z., Liao, J., et al. (2022). Elevated plasma intestinal fatty acid binding protein and aberrant lipid metabolism predict post-stroke depression. Heliyon 8 (11), e11848. doi:10.1016/j.heliyon.2022.e11848
Zhou, J., Li, H., Wang, F., Wang, H., Chai, R., Li, J., et al. (2021). Effects of 2,4-dichlorophenoxyacetic acid on the expression of NLRP3 inflammasome and autophagy-related proteins as well as the protective effect of Lycium barbarum polysaccharide in neonatal rats. Environ. Toxicol. 36 (12), 2454–2466. doi:10.1002/tox.23358
Zhou, L., Wang, T., Yu, Y., Li, M., Sun, X., Song, W., et al. (2022). The etiology of poststroke-depression: a hypothesis involving HPA axis. Biomed. Pharmacother. 151, 113146. doi:10.1016/j.biopha.2022.113146
Keywords: post-stroke depression (PSD), traditional Chinese medicine (TCM), gut-brain axis, neuroinflammation, neurotransmitter
Citation: Zhu L, Han R, He L, Pan B, Zhong W, Li Y and Liu X (2025) Innovative strategies for post-stroke depression: integrating traditional Chinese medicine with neurobiological insights, including the gut-brain axis. Front. Pharmacol. 16:1539357. doi: 10.3389/fphar.2025.1539357
Received: 04 December 2024; Accepted: 23 April 2025;
Published: 03 June 2025.
Edited by:
Alexander George Panossian, Phytomed AB, SwedenReviewed by:
Baomei Xia, Nanjing Normal University of Special Education, ChinaYue Hu, Nanjing University of Chinese Medicine, China
Chaofang Lei, Beijing University of Chinese Medicine, China
Copyright © 2025 Zhu, Han, He, Pan, Zhong, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinru Liu, bGl1eGlucnVAaG90bWFpbC5jby51aw==; Yi Li, c25haWxsaXlpQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Lin Zhu
Lin Zhu Ruina Han1†
Ruina Han1† Linxia He
Linxia He Weijie Zhong
Weijie Zhong Yi Li
Yi Li Xinru Liu
Xinru Liu