Abstract
Objective:
The main aim of this study was to identify adverse events (AEs) in neonates admitted to a Neonatal Intensive Care Unit (NICU) using a trigger-based approach.
Methods:
A retrospective observational study was conducted at Hospital Estadual Sumaré -Dr. Leandro Franceschini, Sumaré, SP, Brazil, over 6 months in 2021. Data from 120 electronic medical records of neonates hospitalized for ≥48 h and prescribed at least one medication were analyzed. Seventeen triggers, such as healthcare-associated infections (HAIs), antimicrobial use, accidental extubation, electrolyte disorders, and others, were employed to identify AEs, including those specific to adverse drug reaction (ADRs). AE severity was assessed using the Neonatal Adverse Event Severity Scale (NAESS) and the World Health Organization (WHO) classification, while ADR causality was evaluated using the WHO criteria and the algorithm proposed by Du et at. Risk factors such as gestational age, birth weight, and length of hospital stay were also analyzed.
Results:
A total of 249 triggers identified 168 confirmed AEs, resulting in a Positive Predictive Value (PPV) of 67.5%. At least one AE was observed in 50.0% of neonates and 40.8% experienced ADRs. The most frequent triggers that identified AEs included HAIs and antimicrobial use (30.8/100 records, each), followed by hyperglycemia (22.5/100 records), increased frequency of bowel movements (16.7/100 records), and hyponatremia (10.8/100 records). Severe complications such as necrotizing enterocolitis (2.5/100 records) and accidental extubation (5.0/100 records) were also recorded. Triggers with a PPV of 100% included necrotizing enterocolitis, accidental extubation, hypocalcemia, HAIs, and antimicrobial use. According to the NAESS, most AEs were classified as grade 2 - moderate, (44.0%) or grade 3 – severe (51.2%). Critical events, such as life-threatening conditions (grade 4) and death (grade 5), were less common, totaling 4.8%. Regarding ADRs, the majority were classified as possible or unlikely by both methods. The distribution of AEs varied by neonatal subgroups, with extremely preterm showing higher rates of AEs, including hyponatremia (53.8%) and accidental extubation (66.7%). Among all events, elevated serum creatinine (75.0%), necrotizing enterocolitis (66.7%), and hypercalcemia (100.0%) predominantly occurred in neonates with extremely low birth weight (ELBW). In contrast, neonates with appropriate birth weight experienced fewer AEs and lower AE severity. This association was not assessed for gestational age.
Conclusion:
The findings suggest that prematurity, low birth weight, and prolonged hospitalization are relevant risk factors for AEs in NICUs. Nonetheless, trigger tools proved effective in identifying severe events and enhancing patient safety in this high-risk setting. Prevention strategies based on these findings can help mitigate risks and optimize neonatal care.
1 Introduction
Patient safety is an essential priority in healthcare, particularly for vulnerable populations such as neonates admitted to Neonatal Intensive Care Unit (NICU). Neonatology in compasses distinct subgroups with unique physiological characteristics influenced by ontogeny, which varies significantly across gestational ages and postnatal ages (Alcorn and McNamara, 2003). These developmental differences crucially impact pharmacokinetics, including absorption, distribution, metabolism, and excretion, thereby increasing the susceptibility of neonates to severe adverse drug events (ADEs) and drug-drug interactions (DDIs) (Tayman et al., 2011; Lim and Pettit, 2019). Additionally, the off-label and unlicensed use of medications, prevalent in NICUs (Samiee-Zafarghandy et al., 2023) due to limited availability of pediatric-specific drugs, further heightens these risks (Domingues et al., 2023). Studies have consistently reported high rates of adverse drug reactions (ADRs) in neonates, ranging from 27.4% (De Las Salas and Díaz-Agudelo, 2017) to 42.6%, with associated mortality rates reaching up to 41.6% (Kaguelidou et al., 2016). Beyond ADEs, other adverse events (AEs) such as skin injuries (Broom et al., 2019) unplanned extubation, healthcare-associated infections (HAIs), and extravasations (Mccullen and Pieper, 2006) frequently occur in NICUs, often resulting in outcomes distinct from those observed in adults (Dillner et al., 2023). These AEs can prolong hospitalization, increase healthcare costs, and negatively impact neonatal outcomes. The variability in AE incidence is largely attributed to differences in detection methods, inconsistent terminology, and the exclusion of mild or self-limiting events in certain studies (Naessens et al., 2009; Meyer-Massetti et al., 2011). Prematurity and low birth weight (Canto-Rodríguez et al., 2023) are recognized as significant risk factors for AEs, particularly HAIs (Moura et al., 2020; Magluta, 2021), which are among the leading contributors to neonatal morbidity and mortality (Dias et al., 2022). These conditions, coupled with prolonged mechanical ventilation, parenteral nutrition, and invasive procedures, further compound the risks of AEs (Moura et al., 2020; Dias et al., 2022).
NICUs play a pivotal role in addressing these challenges, providing high-complexity care delivered by multidisciplinary teams with specialized training (Batista et al., 2021). However, the incidence, severity, and causes of AEs in this setting remain underexplored, particularly regarding medication safety. Several tools for the identification of AEs have been proposed, each presenting a variety of methods with distinct advantages and limitations. To enhance the efficiency of analyses and ensure more accurate identification, trigger-based approaches have been developed as indicators or warning signals that, when detected, require additional evaluation (Classen et al., 1991; Griffin and Classen, 2008). This approach enables targeted investigation, contributing to the detection of events that might otherwise go unnoticed in traditional analyses (Resar et al., 2006; Sharek et al., 2006) and employed predefined indicators, including abnormal laboratory findings, medication modifications, and clinical signs indicative of potential harm. Medical records flagged by these triggers underwent systematic and detailed analysis to confirm the occurrence of true AEs. However, despite these advancements, the identification of AEs in NICUs remains a complex and underexplored area, particularly concerning medication safety. Addressing this knowledge gap requires rigorous research to monitor drug use in neonatology, identify the causes and consequences of AEs and implement targeted interventions to enhance patient safety.
This study aimed to provide information on the following: 1) Estimate the incidence of neonates in NICUs experiencing AEs using specific trigger tools; 2) Analyze the relationship between the occurrence of AEs and neonatal clinical variables; 3) Estimate the frequency of ADRs and characterize them in terms of causality; 4) Identify drug classes associated with ADRs in NICUs; 5) Evaluate the performance of selected triggers for identifying AEs in neonates in NICUs.
2 Methods
2.1 Study design, setting, and population
We conducted a retrospective observational study between January 1 and April 30, and between September 1 and 31 October 2021, utilizing data from neonates admitted to the NICU at the Hospital Estadual Sumaré - Dr. Leandro Franceschini, Sumaré, SP, Brazil. This public university hospital serves as a referral center for high-risk pregnancies and deliveries across six regional cities, performing an average of 2,200 deliveries annually. The study population included all neonates admitted to the NICU at Hospital Estadual Sumaré - Dr. Leandro Franceschini during the study periods, regardless of birth weight, gestational age, sex, or diagnostic hypothesis, provided they met the predefined inclusion criteria.
2.2 Inclusion and exclusion criteria
Inclusion criteria required that neonates were admitted to the NICU during the period study, had been prescribed at least one medication and had a hospitalization period exceeding 48 h. The neonatal period was defined as the first 28 days of life (World Health Organization, 2014). Exclusion criteria encompassed medical records with incomplete data.
2.3 Ethics committee approval
The study was approved by the Ethics Committee of the Universidade Estadual de Campinas, Campinas, SP, Brazil under protocol number 39936920.0.0000.5404. It was conducted in accordance with the ethical principles outlined in the 2013 Declaration of Helsinki for medical research involving human subjects and Resolution No. 510 of 7 April 2016, issued by the Ministry of Health, concerning ethics in human subject research.
2.4 Sampling
Following the methodology established by the Institute for Healthcare Improvement (IHI) (Institute for Healthcare Improvement IHI, 2009), we randomly selected 20 medical records per month, resulting in a total of 120 neonates. This sampling strategy is widely recognized in scientific literature as a robust and practical approach for detecting AEs without compromising analytical rigor or overburdening the review process. This sample size has been validated in several studies as sufficient to identify patterns and trends in the occurrence of AEs, allowing for consistent inferences regarding the quality and safety of care without requiring an exhaustive review of all available records. Furthermore, this strategy enables monitoring of temporal variations in AE incidence, which is essential for evaluating the impact of interventions and continuous improvement policies (Institute for Healthcare Improvement IHI, 2009; Child Health Corporation of America, 2007).
The statistical power of this study was calculated using G*Power software (version 3.1.9.4), considering an effect size of 0.30—classified as a medium effect according to Cohen’s conventions (small = 0.10; large = 0.40) and a significance level of 0.05. With a sample of 120 participants and comparative analyses involving a maximum of three groups (sex, gestational age, birth weight, and length of hospital stay), the estimated statistical power was 0.8358, which is considered sufficient for robust statistical inference.
2.5 Data collection
Demographic and clinical variables were collected from records. Neonate sex was the demographic variable included. General clinical data included gestational age (≤195 days [extremely preterm]; 196–237 days [moderately preterm]; 238–258 days [late preterm]; 259–293 days [full-term]), birth weight (≤999 g [extremely low birth weight–ELBW]; 1,000–1,499 g [very low birth weight–VLBW]; 1,500–2,499 g [low birth weight–LBW]; 2,500–2,999 g [insufficient weight–IW]; ≥3,000 g [adequate weight–AW]), and length of hospitalization (days).
2.6 Record review and method of assessment
A trigger-based methodology was employed, using specific indicators in medical records to signal potential AEs. We applied 17 neonatology-specific triggers derived from previous studies (Sharek et al., 2006; Ventura et al., 2012; Fabretti et al., 2018; Feng et al., 2022), as well as general pediatric trigger frameworks (Takata et al., 2008; Matlow et al., 2011; Unbeck et al., 2014). The final trigger list was developed and refined in consultation with specialists and clinical pharmacists (Sharek et al., 2006; Ventura et al., 2012; Fabretti et al., 2018; Feng et al., 2022; Brasil. Ministério da Saúde. Atenção à Saúde do Recém-Nascido, 2014; Brasil. Agência Nacional de Vigilância Sanitária, 2017; Marba and MezzaCappa, 2008; Martin et al., 2017; Krebs et al., 2020). These indicators include signs, symptoms, abnormal laboratory findings, medication prescriptions and procedure-related complications (Supplementary Table S1).
Two senior reviewers (clinical pharmacists) independently analyzed the medical records using the previously cited trigger-based methodology. In cases of disagreement, a third reviewer was consulted. The data collection followed a randomized sequence, and analysis adhered to the chronological order of events from admission to discharge, transfer, death, or the 28th day of life.
Medical records were accessed through the institutional electronic system. All documents related to each day of hospitalization were reviewed, including admission records, nursing and medical progress notes, prescriptions, and laboratory results. Discharge summaries provided additional information on diagnostic hypotheses, discharge date, death, transfers, surgeries, and procedures.
Medical progress notes and prescriptions were analyzed alongside laboratory results, with particular attention to clinical changes and medication dosage adjustments. Clinical progression, diagnostic hypotheses, and altered laboratory results were analyzed in the context of patient-specific characteristics such as prematurity, physiological adaptation, natural disease progression, and treatment complications. Prescribed medications were evaluated for dosage per kilogram per day and per dose, considering the daily weight of the neonates. Additionally, medication dilutions were verified in accordance with recommendations from the scientific literature.
After trigger identification, medical records were thoroughly reviewed to assess whether the triggers were associated with suspected AEs, ensuring a systematic and evidence-based evaluation. Repeated triggers in the same patient on different dates were counted only once, except for HAIs and antimicrobial use.
2.7 Definition and classification of AEs
AEs were defined as incidents arising from healthcare processes that resulted in patient harm (World Health Organization - WHO, 2009). Once an AE was confirmed, additional information was recorded, including the date of first occurrence and severity classification. Two validated severity grading systems were employed: the World Health Organization (WHO) classification (Uppsala Monitoring Centre and Organização Mundial da Saúde, 2005), which categorizes AEs into four levels—mild, moderate, severe, and death—and the Neonatal Adverse Event Severity Scale (NAESS), a tool specifically adapted to neonatal populations (Salaets et al., 2019). The NAESS ranks severity on a five-point scale: grade 1 (mild), grade 2 (moderate), grade 3 (severe), grade 4 (life-threatening), and grade 5 (death) (Salaets et al., 2019). In this study, AEs were further subdivided into non-medication-related and medication-related AEs; however, within the latter category, only ADRs were considered. Other medication-related AEs (e.g., DDIs, medication errors) were not included. ADRs are unintentional and harmful events that occur during the use of standard therapeutic doses of medications for prophylaxis, diagnosis, treatment, or modification of physiological functions (World Health Organization - WHO, 2009).
ADRs were classified according to causality, using the neonatology-specific algorithm (Du et al., 2013), which considers the clinical context and documented evidence in neonates. The likelihood of an association between the medication and the ADR was categorized into four levels based on the final score: definite (≥14), probable (Kaguelidou et al., 2016; Broom et al., 2019; Mccullen and Pieper, 2006; Dillner et al., 2023; Naessens et al., 2009; Meyer-Massetti et al., 2011; Canto-Rodríguez et al., 2023), possible (Lim and Pettit, 2019; Samiee-Zafarghandy et al., 2023; Domingues et al., 2023; De Las Salas and Díaz-Agudelo, 2017), and unlikely (≤2). Additionally, causality was assessed using the WHO criteria, which includes the following categories: certain, probable, possible, unlikely, conditional, and unclassifiable (Uppsala Monitoring Centre and Organização Mundial da Saúde, 2005). Medications associated with ADRs were classified using the Anatomical Therapeutic Chemical (ATC) system (World Health Organization Collaborating Centre for Drug Statistics Methodology, 2024).
2.8 Outcome measures
The incidence of patients with AEs was calculated as the proportion of individuals who experienced at least one AE, divided by the total number of patients included in the study, and expressed as a percentage. The rate of AEs was calculated as the number of AEs identified during the study period divided by the total number of patient-days, multiplied by 1,000. Results are expressed as AEs per 1,000 patient-days. Patient-days were calculated by summing the total length of hospital stay (in days) for all patients included in the study.
Trigger frequency was calculated as the number of times a trigger was identified divided by the total number of medical records reviewed, multiplied by 100. The AE occurrence rate was calculated by dividing the number of AEs identified through triggers by the total number of medical records reviewed, also multiplied by 100. The positive predictive value (PPV) of each trigger, expressed as a percentage, represents its ability to detect true AEs. The PPV was determined using the method proposed by Handler et al., which accounts for both the frequency of triggers and the incidence rate of AEs (Handler et al., 2007). It was calculated by dividing the AE occurrence rate by the trigger frequency and multiplying the result by 100, reflecting each trigger’s performance in identifying AEs.
2.9 Data analysis
The independent variables were described using absolute and relative frequencies, mean, standard deviation. Triggers and AEs were also reported as absolute and relative frequencies. The occurrence of AEs was expressed for each birth weight in absolute frequencies. To assess the association between birth weight categories and the occurrence of specific AEs, the Chi-square test or Fisher’s exact test was applied, depending on the expected frequencies in each cell counts.
A significant level of 5% was adopted for all analysis. Statistical analyses were performed using R software, version 4.4.2.
3 Results
3.1 Study population
During the study period, 202 neonates were admitted to the NICU. After applying inclusion criteria, cases were excluded, leaving 178 eligible for randomization. Each month, 20 neonates were randomly selected for detailed analysis, resulting in a sample of 120 neonates, representing 67.4% of the eligible population (Figure 1).
FIGURE 1
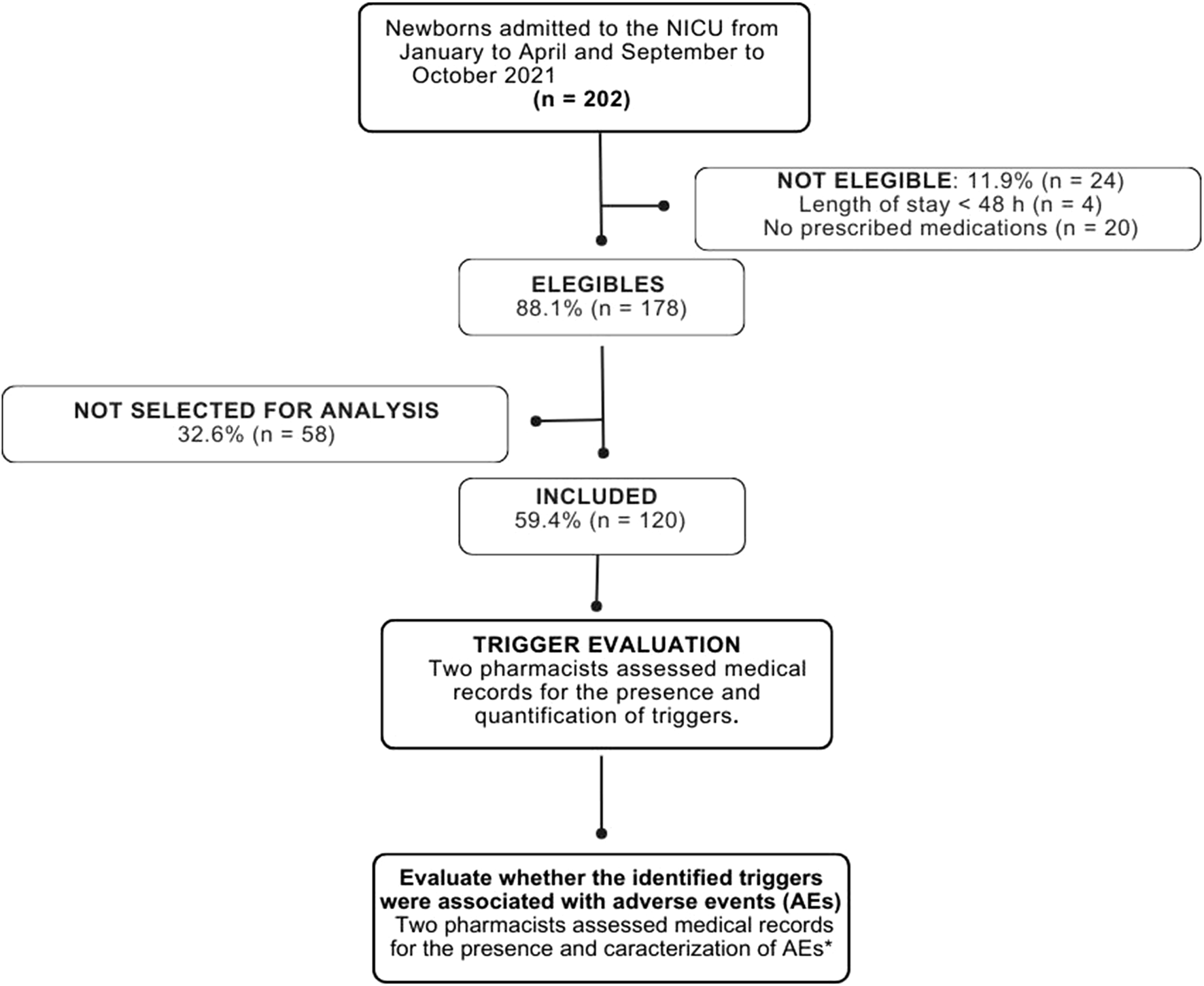
Flow diagram of neonate selection and randomization. NICU: Neonatal Intensive Care Unit. *AEs were defined as incidents arising from healthcare processes that resulted in patient harm (World Health Organization - WHO, 2009). In this study, AEs were further subdivided into non-medication-related and medication-related AEs; however, within the latter category, only ADRs were considered. Other medication-related AEs (e.g., drug interactions, medication errors) were not included. ADRs are unintentional and harmful events that occur during the use of standard therapeutic doses of medications for prophylaxis, diagnosis, treatment, or modification of physiological functions (World Health Organization - WHO, 2009).
There was a predominance of male neonates (56.7%). The mean gestational age was 33.6 ± 3.7 weeks, with 76.7% of neonates classified as preterm, including 10.0% with extreme prematurity. Based on birth weight, 77.5% of the participants weighed less than 2,500 g. In terms of length of stay, 50.0% of hospitalizations exceeded 21 days (Table 1). The total number of patient-days was 3,423.
TABLE 1
| Variables | % (n) | Mean ± SD |
|---|---|---|
| Gestational age (days) | ||
| ≤195 days (Extremely Preterm) | 10.0 (12) | 183.7 ± 9.3 |
| 196–237 days (Moderately Preterm | 35.0 (42) | 222.2 ± 11.0 |
| 238–258 days (Late Preterm) | 31.7 (38) | 245.3 ± 5.7 |
| 259–293 days (Full-Term) | 23.3 (28) | 269.2 ± 7.4 |
| TOTAL | 100.0 (120) | 235.2 ± 26.1 |
| BIRTH WEIGHT 9 g) | ||
| ≤999 g (ELBW) | 9.1 (11) | 779.5 ± 146.3 |
| 1,000–1,499 g (VLBW) | 16.7 (20) | 1301.2 ± 136.5 |
| 1,500–2,499 g (LBW) | 51.7 (62) | 2010.7 ± 265.9 |
| 2,500–2,999 g (IW) | 10.0 (12) | 2708.2 ± 138.3 |
| ≥3,000 g (AW) | 12.5 (15) | 3373.0 ± 330.5 |
| TOTAL | 100.0 (120) | 2019.6 ± 750.5 |
| LENGTH OF STAY (days) | ||
| 2–5 | 5.8 (7) | 3.6 ± 1.0 |
| 6–10 | 12.5 (15) | 7.8 ± 1.4 |
| 11–15 | 17.5 (21) | 13.0 ± 1.2 |
| 16–20 | 14.2 (17) | 17.7 ± 1.4 |
| 21–27 | 17.5 (21) | 23.2 ± 1.7 |
| ≥28 | 32.5 (39) | 57.0 ± 26.0 |
| TOTAL | 100.0 (120) | 28.5 ± 25.3 |
General clinical characteristics of the study population (n = 120).
g, grams; AW, adequate weight; ELBW, extremely low birth weight; IW, insufficient weight; LBW, low birth weight; VLBW, very low birth weight.
3.2 Triggers performance
The study identified a total of 249 triggers in 70.8% (n = 85) of the medical records, with a mean of 2.1 ± 2.2 triggers per patient. The number of triggers identified per neonate varied from 0 to 9. The five most frequently identified triggers were hyperglycemia, increased frequency of bowel movements, HAIs, antimicrobial use, and hypotension (Table 2). Flumazenil, hypernatremia, and naloxone were not identified in the reviewed medical records, suggesting either their absence or potential underreporting in clinical documentation (Table 2).
TABLE 2
| Triggers | Trigger/100 records | AE/100 records | PPV (%) |
|---|---|---|---|
| Necrotizing Enterocolitis | 2.5 | 2.5 | 100.0 |
| Accidental Extubation | 5.0 | 5.0 | 100.0 |
| Hypocalcemia | 4.2 | 4.2 | 100.0 |
| HAIs | 30.8 | 30.8 | 100.0 |
| Antimicrobials Use | 30.8 | 30.8 | 100.0 |
| Hyponatremia | 13.3 | 10.8 | 81.3 |
| Increased Serum Creatinine | 5.0 | 3.3 | 66.7 |
| Hypokalemia | 7.5 | 5.0 | 66.7 |
| Hyperglycemia | 38.3 | 22.5 | 58.7 |
| Increased Frequency of Bowel Movements | 34.2 | 16.7 | 48.8 |
| Hyperkalemia | 5.8 | 2.5 | 42.9 |
| Hypotension | 14.2 | 5.0 | 35.3 |
| Hypercalcemia | 10.0 | 0.8 | 8.3 |
| Phenobarbital | 5.8 | 0.0 | 0.0 |
| Flumazenil | 0.0 | 0.0 | 0.0 |
| Hypernatremia | 0.0 | 0.0 | 0.0 |
| Naloxone | 0.0 | 0.0 | 0.0 |
| TOTAL | 207.5 | 140.0 | 67.5 |
Performance of neonatal adverse event triggers based on Positive Predictive Value.
HAIs, Healthcare-associated infections; AE, adverse event; PPV, preditive positive value.
The most frequently observed AEs were identified through the triggers HAIs, antimicrobial use, hyperglycemia, increased bowel movement frequency, and hyponatremia (Table 2).
The triggers with the highest performance, based on their PPV, were necrotizing enterocolitis, accidental extubation, hypocalcemia, HAIs, and antimicrobials use, all of which demonstrated a PPV of 100% (Table 2). The hyponatremia trigger also showed good performance, with a PPV of 81.3%. Additionally, triggers such as increased serum creatinine, hypokalemia, and hyperglycemia demonstrated PPVs between 50.0% and 70.0%, indicating a moderate likelihood of detecting true AEs. In contrast, increased frequency of bowel movements, hyperkalemia, hypotension, hypercalcemia, and phenobarbital had PPVs below 50.0%, reflecting a substantial proportion of false-positive results (Table 2). Overall, 168 of the 249 identified triggers were associated with the occurrence of an AE, resulting in a PPV of 67.5% (Table 2).
3.3 AEs incidence and characterization
As previously mentioned, a total of 168 AEs were identified through trigger evaluation, with a mean of 1.4 per patient. Half of the neonates (n = 60; 50.0%) experienced at least one AE, and 40.8% (n = 49) had at least one ADR. The incidence was 49.1 per 1,000 patient-days.
Regarding severity, using the WHO classification, 44.6% were considered moderate, 54.8% severe, and 0.6% fatal. No events were classified as mild, suggesting that AEs in the NICU tend to be significant. Based on the NAESS, AEs were classified as follows: 44.0% as grade 2 (moderate), 51.2% as grade 3 (severe), 4.2% as grade 4 (life-threatening), and 0.6% as grade 5 (death). According to the NAESS, increased bowel movement frequency and hyperglycemia were considered moderate (grade 2), along with some HAIs of cutaneous origin. Severe AEs (grade 3) included increased serum creatinine, hypotension, and electrolyte imbalances such as hypercalcemia, hyperkalemia, hypocalcemia, hypokalemia, and hyponatremia. Life-threatening AEs (grade 4) included necrotizing enterocolitis, accidental extubation, antimicrobial use, and 67.4% of identified HAIs. The only fatal case was attributed to necrotizing enterocolitis. Of the total AEs identified (n = 168), 47.6% were categorized as non-medication-related AEs (n = 80), whereas 52.4% were classified as ADRs (n = 88; 40.8% of the included neonates), with a mean of 0.7 ± 1.1 ADRs per patient.
Among the 60 neonates who experienced AEs, 81.7% (n = 49) presented with an ADRs. Of these, 59.2% were male neonates (p = 0.643). The analysis identified 21 drugs responsible for a total of 88 ADRs. Cardiac therapy drugs, used in the management of cardiovascular disorders, accounted for 34.1% of ADRs. Caffeine, utilized for apnea treatment, contributed 27.3%, whereas systemic antimicrobials accounted for 23.9% of ADRs (Table 3).
TABLE 3
| ATC groups | ATC CODE | % (N) |
|---|---|---|
| Multivitamins | A11 | 2.2 (2) |
| Multivitamins | A11JB | 1.1 (1) |
| Vitamin A, D, E (combination) | A11JA | 1.1 (1) |
| Blood Substitutes and perfusion solutions | B05 | 7.9 (7) |
| 50% Glucose | B05CX | 6.8 (6) |
| Potassium chloride | B05XA01 | 1.1 (1) |
| Cardiac therapy | C01; C03; C07; C09 | 34.1 (30) |
| Alprostadil | C01EA01 | 4.5 (4) |
| Ibuprofen | C01EB16 | 3.4 (3) |
| Dobutamine | C01CA07 | 2.3 (2) |
| Dopamine | C01CA04 | 4.5 (4) |
| Furosemide | C03CA01 | 12.5 (11) |
| Propranolol | C07AA05 | 2.3 (2) |
| Captopril | C09AA01 | 4.5 (4) |
| Systemic antimicrobials | J01 | 23.9 (21) |
| Amikacin | J01GB06 | 10.2 (9) |
| Ampicilin | J01CA01 | 4.5 (4) |
| Ampicilin + Sulbactam | J01CR01 | 1.1 (1) |
| Cefepime | J01DE01 | 1.1 (1) |
| Cefalexin | J01DB01 | 1,1 (1) |
| Oxacilin | J01CF04 | 2.3 (2) |
| Potassium Penicillin G | J01CE01 | 1.1 (1) |
| Vancomycin | J01XA01 | 2.3 (2) |
| Psychoanaleptics | N06 | 27.3 (24) |
| Caffeine | N06BC01 | 27.3 (24) |
| Drugs used in obstructive respiratory diseases | R03 | 4.5 (4) |
| Aminophylline | R03DA06 | 4.5 (4) |
Medications related to ADRs (n = 88) according to the Anatomical Therapeutic Chemical Classification.
ATC, anatomical therapeutic chemical.
Bold values indicate therapeutic groups according to the second level of the ATC classification and their corresponding absolute and relative frequencies.
The results for the causality assessment using the Du et al. algorithm indicated that 25.1% were probable, 26.4% possible, and 48.5% unlikely. According to the WHO criteria, 7.2% were classified as probable, 44.3% as possible, and 48.5% as unlikely. No ADRs were classified as definitive.
3.4 Clinical factors associated with AEs
Of the 60 patients who experienced AE, 55% (n = 33) were male (p = 0.712). The distribution of the number of AEs according to birth weight revealed that 28.0% (n = 47) occurred in neonates with ELBW, 25.0% (n = 42) in those with VLBW, 24.4% (n = 41) in those with LBW, 15.5% (n = 26) in infants with IW, and 7.1% (n = 12) in those with AW (p < 0.0001). Table 4 details the distribution of positive triggers stratified by birth weight category.
TABLE 4
| Positive triggers | <999 g ELBW | 1,000–1,499 g VLBW | 1,500–2,499 g LBW | 2,500–2,999 g IW | ≥3,000 g AW | p-valuea |
|---|---|---|---|---|---|---|
| Increased Serum Creatinine | 75.0 | 0.0 | 0.0 | 25.0 | 0.0 | 0.07 |
| Increased Frequency of Bowel Movements | 10.0 | 30.0 | 35.0 | 15.0 | 10.0 | 0.24 |
| Necrotizing Enterocolitis | 66.7 | 0.0 | 0.0 | 33.3 | 0.0 | 0.25 |
| Accidental Extubation | 50.0 | 16.7 | 16.7 | 16.7 | 0.0 | 0.41 |
| Hypercalcemia | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Hyperkalemia | 0.0 | 33.3 | 33.3 | 33.3 | 0.0 | 0.40 |
| Hyperglycemia | 22.2 | 33.3 | 33.3 | 7.4 | 3.8 | 0.03 |
| Hypocalcemia | 0.0 | 40.0 | 60.0 | 0.0 | 0.0 | 0.09 |
| Hypokalemia | 33.3 | 33.3 | 33.3 | 0.0 | 0.0 | 0.41 |
| Hyponatremia | 46.2 | 38.5 | 0.0 | 7.7 | 7.7 | 0.02 |
| Hypotension | 66.7 | 0.0 | 33.3 | 0.0 | 0.0 | 0.03 |
| HAIs | 24.3 | 21.6 | 21.6 | 21.6 | 10.8 | 0.73 |
| Antimicrobial use | 24.3 | 21.6 | 21.6 | 21.6 | 10.8 | 0.73 |
| TOTAL | 28.0 | 25.0 | 24.4 | 15.5 | 7.1 |
Distribution (%) of adverse events identified by triggers according birth weight.
Chi-square test.
AW, adequate weight; ELWB, extremely low birth weight; g, grams; HAIs, Healthcare-associated infections; IW, insufficient weight; LBW, low birth weight; VLBW, very low birth weight.
According to the NAESS, grade 3 (severe) AEs were most frequent among ELBW (70.2%) and VLBW (54.8%) neonates. Conversely, neonates with IW and AW exhibited higher proportions of grade 2 (moderate) AEs (50.0% and 58.3%, respectively). Grade 4 (life-threatening) AEs were predominantly observed in ELBW (8.5%) and IW neonates (3.8%). One fatal case (grade 5) was reported in the IW group (3.8%) (p = 0.015).
The incidence of neonates experiencing at least one AE varied according to gestational age, with rates of 75.0% for extremely preterm, 54.8% for very preterm, 44.7% for late preterm, and 39.3% for term neonates (p = 0.036). Overall, 82.7% (n = 139) of AEs were identified in neonates with some degree of prematurity, with 46 occurring in extremely preterm neonates, 61 in very preterm, 32 in late preterm, and 29 in term neonates (p = 0.012). Among extremely preterm neonates, the most common complications included increased serum creatinine (75.0%), accidental extubation (66.7%), hypercalcemia (100.0%), hyponatremia (53.8%), and hypotension (50.0%).
Regarding the length of hospital stay, the incidence rates for the 2–5 days and 11–15 days intervals were identical, at 14.3%. Similar rates were observed for stays of 6–10 days and 16–20 days (46.7% and 47.1%, respectively). The highest incidence was found in hospitalizations longer than 28 days (76.9%), followed by the 21–27 days interval, during which 52.4% of patients experienced at least one AE (p < 0.001).
4 Discussion
In this study, a predominance of male neonates was observed among those admitted to the NICU, with a mean gestational age of 33.6 weeks. The majority of neonates were preterm (76.7%), with 10% classified as extremely preterm. This clinical profile aligns with findings in Brazil that also report a higher frequency of prematurity and a predominance of male sex among NICU admissions international research has emphasized the increased susceptibility of preterm neonates to critical conditions requiring intensive care (Ventura et al., 2012; Ligi et al., 2008).
The observed average length of hospital stay among the neonates in this study was 28.5 days, a duration that is consistent with findings reported in international literature, although variability exists depending on gestational age, birth weight, and the complexity of clinical conditions. Similarly, research by Oza et al. (2013) encompassing data from low- and middle-income countries, highlighted prolonged hospital stays—often exceeding four weeks—among neonates with VLBW or severe complications, emphasizing the burden on healthcare systems in resource-limited settings. Moreover, longer hospital stays have been critically associated with increased risk of adverse outcomes, including hospital-acquired infections (Stoll et al., 2010) reinforcing the importance of implementing targeted strategies to reduce length of stay without compromising safety (Ravi et al., 2019).
A high variability was observed in the number of triggers identified, with a mean of 2.2 ± 2.4, a finding consistent with previous studies (Sharek et al., 2006; Ventura et al., 2012; Feng et al., 2022). In contrast, Fabretti et al. (2018) reported a significantly higher average of 7.4 triggers per patient, using an expanded set of 48 triggers. This discrepancy in identification frequency may be attributed to differences in the trigger sets applied (Pierdevara et al., 2017), which range from general AEs (Dillner et al., 2023; Sharek et al., 2006; Ventura et al., 2012), ADEs (Fabretti et al., 2018), non-specific categories (Dillner et al., 2023), medication errors (Maziero et al., 2021), and specific clinical conditions such as nasal injuries and thermoregulation disorders (Ventura et al., 2012).
The performing triggers in detecting AEs were evaluated using both overall and individual PPVs. Triggers with low PPVs or that identify events less frequently may decrease the overall performance of the method (Classen et al., 1991; Giordani et al., 2012). The overall PPV was 67.5%, consistent with the findings of Feng et al., (2022) and higher than those reported in other studies, which ranged from 22.5% to 38.0% (Sharek et al., 2006; Fabretti et al., 2018; Unbeck et al., 2014).
These variations may be explained by differences in population characteristics, such as gestational age and birth weight, as well as the specificity and composition of the trigger sets used. High-performing triggers—such as necrotizing enterocolitis, accidental extubation, hypocalcemia, HAIs, and antimicrobial use—achieved a PPV of 100.0%. This may be due to the fact that these represent direct AEs and are commonly used as quality-of-care indicators (Sharek et al., 2006; Snijders et al., 2009). In specific cases of hypocalcemia, high PPV may be associated with the off-label use of furosemide in preterm neonates for the treatment of symptomatic patent ductus arteriosus (Backes et al., 2022; Vuralli, 2019). Necrotizing enterocolitis showed a higher PPV than those reported in other investigations possibly due to differences in diagnostic classification criteria and the clinical characteristics of the neonatal subgroups studied (Sharek et al., 2006; Fabretti et al., 2018).
Hyponatremia also stood out, with a PPV of 81.3%, higher than that reported by Fabretti et al. (2018). Previous studies analyzed electrolyte abnormalities as grouped conditions rather than as specific triggers, which may reduce accuracy in detecting individual events (Sharek et al., 2006; Barrionuevo and Esandi, 2010).
Among intermediate-performing triggers, elevated serum creatinine and hypokalemia both had a PPV of 66.7%. Variability in PPVs for creatinine across studies (11.0%–100.0%) likely reflects differences in diagnostic criteria, monitoring frequency, and nephrotoxic drug exposure (Sharek et al., 2006; Fabretti et al., 2018). Hyperglycemia, an established risk factor for neonatal mortality (Mesotten et al., 2018), showed a PPV of 58.7%, similar to Fabretti et al. (2018) but lower than Sharek et al. (2006). Its detection may have been limited by demand-based rather than continuous monitoring and the absence of a standardized definition in neonates.
Variations in definitions and thresholds for electrolyte disturbances also affected trigger performance. For example, hyperkalemia was inconsistently defined, with serum potassium cutoffs ranging from >5.5 to >8.0 mEq/L across studies (Sharek et al., 2006; Li et al., 2014; Ni et al., 2018), hindering comparability.
Triggers with PPVs below 50%—such as increased frequency of bowel movements, hyperkalemia, hypercalcemia, hypotension, and phenobarbital—often reflect clinical variability rather than true AEs. Diarrhea had limited utility due to inconsistent bowel patterns and reliance on documentation. Hypotension showed low predictive value (35.2%), likely influenced by infrequent recordings and lack of gestational age-adjusted criteria. The absence or low yield of triggers such as flumazenil, naloxone, and hypernatremia may result from appropriate medication use, incomplete records, or the limited sample size.
The trigger set identified 168 AEs, averaging 1.4 events per patient, aligning with prior findings (1.7 events) (Feng et al., 2022), but exceeding rates from studies with narrower trigger sets or different populations (Sharek et al., 2006; Fabretti et al., 2018). The AE rate of 49.1 per 1,000 patient-days was also higher than previously reported (Sharek et al., 2006; Yalçın et al., 2022), which may reflect differences in definitions, sample characteristics, inclusion of specific triggers (e.g., electrolyte disturbances), and documentation practices.
Approximately 50.0% of neonates experienced at least one AE, a rate comparable to other studies (Fabretti et al., 2018; Feng et al., 2022). The scope of the trigger set directly influences the incidence observed, as demonstrated by Sharek et al. (2006), who reported a 74.0% incidence when including death and cardiopulmonary arrest, and by Cossul et al. (2021), who found a 70.0% incidence by considering technical complaints, medication errors, and injuries related to central and peripheral venous access. Ventura et al. (2012), in a prospective analysis reported a high rate of 84.0%.
The frequency of AEs like HAIs observed in this study was comparable to findings from other investigations using similar definitions (World Health Organization, 2014; Institute for Healthcare Improvement IHI, 2009; Child Health Corporation of America, 2007), although variations were noted due to differences in diagnostic criteria and identification methods. Hyperglycemia had a prevalence of 16.1%, with substantial variability across studies (Ventura et al., 2012). The incidence of increased frequency of bowel movements was lower than that reported in other studies (Fabretti et al., 2018; Feng et al., 2022), possibly reflecting differences in standardization criteria and assessment methods. Accidental extubation may have been underestimated, potentially due to the retrospective nature of the study. Hypotension and elevated serum creatinine levels showed frequencies consistent with the literature but were influenced by varying definitions and monitoring approaches. Necrotizing enterocolitis was infrequent, likely due to dependence on clinical diagnosis and proper documentation. No adverse events were identified related to the use of phenobarbital, naloxone, or flumazenil, despite their inclusion as triggers in previous studies.
ADR frequency reached 40.8%, with a mean of 0.7 per patient, consistent with larger neonatal cohorts (Kaguelidou et al., 2016). Cardiovascular drugs—especially furosemide, dopamine, and captopril—were the main culprits, as previously reported (Leopoldino et al., 2023; Sugioka et al., 2020; Workineh and Workie, 2022). Caffeine, widely used for apnea of prematurity, accounted for nearly a third of ADRs, while antibiotics were linked to most cases of diarrhea and electrolyte imbalances, while ibuprofen was linked to a case of necrotizing enterocolitis. Causality analysis using the algorithm by Du et al. showed lower ADR rates in all categories compared to earlier studies, except for the “unlikely” category. No ADRs were classified as definite, which may be related to the absence of documented practices such as drug detection in blood or fluids and the use of doses above recommended levels (Du et al., 2013; Sugioka et al., 2020).
Lower gestational age and reduced birth weight are well-established risk factors for the occurrence of AEs in the neonatal population, as the physiological immaturity characteristic of these subgroups predisposes them to greater susceptibility to clinical complications and drug-induced toxicity (Canto-Rodríguez et al., 2023; Giesinger and McNamara, 2016).
In the present study, a higher prevalence of AEs was observed among extremely preterm and very preterm neonates, as well as those classified as having ELBW and VLBW, corroborating existing literature that links these factors to increased clinical vulnerability (Srulovici et al., 2012). Among the AEs evaluated, hyperglycemia, hyponatremia, and hypotension demonstrated statistically significant associations when stratified by birth weight, reinforcing the relationship between low birth weight and a heightened predisposition to metabolic and hemodynamic disturbances (Inage et al., 2022). Hyperglycemia was notably more prevalent in neonates weighing less than 2,500 g. This finding aligns with previous studies that associate prematurity and intrauterine growth restriction with altered glucose homeostasis, potentially driven by immature insulin secretion, exaggerated stress responses, and the frequent use of parenteral nutrition in this population (Inage et al., 2022; Beardsall, 2021).
Hyponatremia was similarly more frequent among ELBW and VLBW neonates, likely reflecting the interplay of renal immaturity, altered fluid homeostasis, and pharmacological interventions such as diuretic administration (Hao, 2019). These data highlight the importance of vigilant electrolyte monitoring in high-risk neonates to mitigate further clinical deterioration. Hypotension, significantly more prevalent in ELBW infants, underscores the inherent hemodynamic instability of this subgroup, necessitating individualized cardiovascular monitoring and support strategies. Moreover, a prolonged duration of hospitalization was associated with an increased prevalence of AEs, particularly in neonates with NICU stays exceeding 28 days. Extended hospitalization inherently increases exposure to invasive procedures, broad-spectrum antimicrobials, and nosocomial pathogens, thereby amplifying the cumulative risk of AEs. These findings emphasize the critical need for preventive measures and tailored surveillance protocols for neonates with prolonged NICU admissions (Bartelink et al., 2006).
This study is subject to several methodological limitations that warrant consideration. The retrospective design, coupled with exclusive reliance on data extracted from electronic medical records, inherently constrains the detection of AEs that were either undocumented or insufficiently recorded. As such, the findings are intrinsically dependent on the accuracy, completeness, and consistency of clinical documentation by healthcare professionals, introducing potential information bias. Moreover, the study sample was derived from a single center and may not be representative of other neonatal care settings or broader pediatric populations, thereby limiting the external validity and generalizability of the results. The temporal scope of data collection also constitutes a relevant constraint, as the selected study period may not capture seasonal trends or temporal shifts in clinical practices and institutional protocols. Additionally, the review of medical records was not conducted across consecutive months due to adjustments in the research protocol necessitated by restrictions related to the COVID-19 pandemic. This discontinuity may have introduced variability in case selection and hindered a more homogeneous temporal analysis of AE occurrence.
The findings of this study highlight the complexity and fragility of neonatal care in intensive care settings, particularly among highly vulnerable populations such as preterm and ELBW neonates. The high frequency of AEs and ADRs, combined with variability in the performance of the trigger tools used, underscores not only the sensitivity of the methodology but also the inherent challenges in accurately identifying such events in real-world clinical contexts.
The high PPV observed for certain triggers—such as accidental extubation, necrotizing enterocolitis, and HAIs—demonstrates their potential as robust indicators of neonatal care quality. However, the heterogeneity in the performance of intermediate and low-yield triggers reveals the need for ongoing refinement of trigger sets to enhance their specificity and clinical utility, particularly in neonatal subpopulations at higher risk.
Furthermore, the observed association between physiological immaturity, prolonged hospitalization, and increased prevalence of AEs reinforces the importance of individualized clinical strategies guided by evidence-based practices. Early interventions, rigorous monitoring of hemodynamic and metabolic parameters, and judicious pharmacological management are essential to mitigate risks and promote patient safety.
5 Conclusion
In summary, this study offers important contributions to the understanding of neonatal patient safety in intensive care environments, demonstrating the feasibility of using trigger-based tools for the systematic detection of AEs and ADRs. The results suggest that the adoption of standardized approaches, adapted to the specific context of neonatal units, can strengthen surveillance systems and foster sustained improvements in care quality. Nevertheless, further research, particularly through prospective and multicenter designs, is essential to validate these findings and broaden them generalizability. Ultimately, the integration of clinical best practices, structured monitoring tools, and an institutional culture focused on neonatal patient safety represents a promising path toward reducing preventable harm and ensuring safer, more effective, and humanized care.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the University of Campinas under protocol number 39936920.0.0000.5404. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
FA: Conceptualization, Data curation, Formal Analysis, Methodology, Validation, Writing – original draft, Writing – review and editing. DV: Conceptualization, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review and editing. MP: Conceptualization, Investigation, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review and editing. RS: Investigation, Writing – review and editing. MM: Formal analysis, Writing – review and editing. MV: Conceptualization, Methodology, Validation, Writing – original draft. PM: Conceptualization, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) – Funding Code 001. The study was also financed by CAPES (AUXPE) –Process N° 88881.859210/2023-01F.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1539687/full#supplementary-material
Nomenclature
- AE
Adverse Event
- ADE
Adverse Drug Event
- ADR
Adverse Drug Reaction
- AW
Adequate weight
- DDI
Drug Interactions
- ELBW
Extremely low birth weight
- HAIs
Healthcare-associated infections
- IHI
Institute for Healthcare Improvement
- IW
Insufficient weight
- LBW
Low birth weight
- NAESS
Neonatal Adverse Event Severity Scale
- NICU
Neonatal Intensive Care Unit
- PPV
Positive Predictive Value
- VLBW
Very low birth weight
References
1
Alcorn J. McNamara P. (2003). Pharmacokinetics in the newborn. Adv. Drug Deliv. Rev.55, 667–686. 10.1016/S0169-409X(03)00030-9
2
Backes C. Hill K. Shelton E. Slaughter J. Lewis T. Weisz D. et al (2022). Patent ductus arteriosus: a contemporary perspective for the pediatric and adult cardiac care provider. J. Am. Heart Assoc.11, 25784. 10.1161/JAHA.122.025784
3
Barrionuevo L. Esandi M. (2010). Epidemiología de eventos adversos en el servicio de neonatología de un hospital público regional en la Argentina. Arch. Argent. Pediatr.108, 303–310. 10.1590/S0325-00752010000400003
4
Bartelink I. Rademaker C. Schobben A. Van Den Anker J. (2006). Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin. Pharmacokinet.45 (11), 1077–1097. 10.2165/00003088-200645110-00003
5
Batista G. J. Pereira C. T. J. Felipe F. R. Luz K. M. G. Cruz M. M. Silva D. J. et al (2021). Unidade de terapia intensiva neonatal (utin): A importância na sobrevida dos recém nascidos. Res. Soc. Dev., 10. 10.33448/rsd-v10i6.15884
6
Beardsall K. (2021). Hyperglycaemia in the newborn infant. Physiology verses pathology. Front. Pediatr.9, 641306. 10.3389/fped.2021.641306
7
Brasil. Agência Nacional de Vigilância Sanitária. Critérios Diagnósticos de Infecção Associada à Assistência à Saúde Neonatologia. Série Segurança do Paciente e Qualidade em Serviços de Saúde. Vol 3. Brasília, DF: Ministério da Saúde. (2017).
8
Brasil. Ministério da Saúde. Atenção à Saúde do Recém-Nascido (2014). Guia para Profissionais de Saúde. Problemas Respiratórios, Cardiocirculatórios, Metabólicos, Neurológicos, Ortopédicos e Dermatológicos. 2nd ed. Vol. 3, DF. Brasília: Ministério da Saúde.
9
Broom M. Dunk A. M. Mohamed A. L. (2019). Predicting neonatal skin injury: the first step to reducing skin injuries in neonates. Heal Serv. Insights12, 1–10. 10.1177/1178632919845630
10
Canto-Rodríguez L. Y. Lara-Aké N. J. Torres-Romero J. C. Paulino González-Mateos A. (2023). Adverse drug reactions in neonatal intensive care unit: characteristics and risk factors. Int. J. Biol. Pharm. Sci. Arch.5, 15–23. 10.53771/ijbpsa.2023.5.2.0033
11
Child Health Corporation of America (2007). Pediatric trigger toolkit: measuring adverse drug events in the children’s hospital. Shawnee Mission, KS. Available online at: https://toolkit-child-health-corporation-of-america (Accessed April 15, 2025).
12
Classen D. Pestotnik S. Evans R. Burke J. (1991). Computerized surveillance of adverse drug events in hospital patients. JAMA266, 2847–2851. 10.1001/jama.1991.03470200059035
13
Cossul M. Neiva L. Silveira A. (2021). Notificação de eventos adversos em uma unidade de terapia intensiva neonatal. Rev. Enferm. UFPE online.15 (1), e246969. 10.5205/1981-8963.2021.246969
14
De Las Salas R. Díaz-Agudelo D. (2017). Reacciones adversas a medicamentos en neonatos hospitalizados en unidades de cuidado intensivo neonatal en Barranquilla, Colombia. Biomedica37 (1), 33–42. 10.7705/biomedica.v37i1.3192
15
Dias B. Leal M. Martinelli K. Nakamura-Pereira M. Esteves-Pereira A. Neto E. (2022). Recurrent preterm birth: data from the study “Birth in Brazil”. Rev. Saude Publica56, 7. 10.11606/s1518-8787.2022056003527
16
Dillner P. Unbeck M. Norman M. Nydert P. Härenstam K. Lindemalm S. et al (2023). Identifying neonatal adverse events in preterm and term infants using a paediatric trigger tool. Acta Paediatr.112, 1670–1682. 10.1111/apa.16814
17
Domingues C. Jarak I. Veiga F. Dourado M. Figueiras A. (2023). Pediatric drug development: reviewing challenges and opportunities by tracking innovative therapies. Pharmaceutics15 (2431), 2431. 10.3390/pharmaceutics15102431
18
Du W. Lehr V. T. Lieh‐Lai M. Koo W. Ward R. M. Rieder M. J. et al (2013). An algorithm to detect adverse drug reactions in the neonatal intensive care unit. J. Clin. Pharmacol.53 (1), 87–95. 10.1177/0091270011433327
19
Fabretti S. C. Brassica S. C. Cianciarullo M. A. Romano-Lieber N. S. (2018). Rastreadores para a busca ativa de eventos adversos a medicamentos em recém-nascidos. Cad. Saude Publica, 34. 10.1590/0102-311X00069817
20
Feng K. Zhang L. He H. You X. Zhang Q. Wei H. et al (2022). Neonatal adverse events’ trigger tool setup with random forest. J. Patient Saf.18, e585–e590. 10.1097/PTS.0000000000000871
21
Giesinger R. E. McNamara P. J. (2016). Hemodynamic instability in the critically ill neonate: an approach to cardiovascular support based on disease pathophysiology. Semin. Perinatol.40, 174–188. 10.1053/j.semperi.2015.12.005
22
Giordani F. Rozenfeld S. Oliveira D. F. M. de Versa G. L. G. da S. Terencio J. S. Caldeira L. de F. et al (2012). Vigilância de eventos adversos a medicamentos em hospitais: aplicação e desempenho de rastreadores. Rev. Bras. Epidemiol.15 (3), 455–467. 10.1590/s1415-790x2012000300002
23
Griffin F. Classen D. (2008). Detection of adverse events in surgical patients using the Trigger Tool approach. Qual. Saf. Heal Care17, 253–258. 10.1136/qshc.2007.025080
24
Handler S. M. Altman R. L. Perera S. Hanlon J. T. Studenski S. A. Bost J. E. et al (2007). A systematic review of the performance characteristics of clinical event monitor signals used to detect adverse drug events in the hospital setting. J. Am. Med. Inf. Assoc.14 (4), 451–458. 10.1197/jamia.M2369
25
Hao T. (2019). Prevalence and risk factors for hyponatremia in preterm infants. Open Access Maced. J. Med. Sci.7 (19), 3201–3204. 10.3889/oamjms.2019.558
26
Inage Y. Hirano D. Nakagawa A. Yamada S. Kotake Y. Ikoma N. et al (2022). Risk factors for hyperglycemia in extremely low birth weight infants during the first 14 days. Pediatr. Neonatol.63 (1), 13–18. 10.1016/j.pedneo.2021.07.001
27
Institute for Healthcare Improvement (IHI) (2009). IHI global trigger tool for measuring adverse events. 2nd end. Cambridge, MA: Institute for Healthcare Improvement. Available online at: https://www.ihi.org/resources/white-papers/ihi-global-trigger-tool-measuring-adverse-events (Accessed April 15, 2025).
28
Kaguelidou F. Beau-Salinas F. Jonville-Bera A. Jacqz-Aigrain E. (2016). Neonatal adverse drug reactions: an analysis of reports to the French pharmacovigilance database. Br. J. Clin. Pharmacol. 82:1058–1068. 10.1111/bcp.13034
29
Krebs V. L. J. Durante P. P. (2020). “Água, eletrólitos e glicose,” in PEDIATRIA neonatologia. Editors SchvartsmanB. G.MalufJr P. T.Carneiro-SampaioM.2nd ed. (Barueri: Manole), 342–363.
30
Leopoldino R. Marques D. Rocha L. Fernandes F. Oliveira A. Martins R. (2023). Temporal profile of adverse drug reactions and associated clinical factors: a prospective observational study in a neonatal intensive care unit. BMJ Open13, e073304. 10.1136/bmjopen-2023-073304
31
Li Q. Melton K. Lingren T. Kirkendall E. Hall E. Zhai H. et al (2014). Phenotyping for patient safety: algorithm development for electronic health record based automated adverse event and medical error detection in neonatal intensive care. Am. Med. Inf. Assoc.21 (5), 776–784. 10.1136/amiajnl-2013-001914
32
Ligi I. Arnaud F. Jouve E. Tardieu S. Sambuc R. Simeoni U. (2008). Iatrogenic events in admitted neonates: a prospective cohort study. Lancet371 (Feb), 404–410. 10.1016/S0140-6736(08)60204-4
33
Lim S. Pettit R. (2019). Pharmacokinetic considerations in pediatric pharmacotherapy. Am. J. Heal Syst. Pharm.76, 1472–1480. 10.1093/ajhp/zxz161
34
Magluta C. (2021). Internação de Recém-Nascidos de Risco em Unidades de Terapia Intensiva Neonatal no Brasil: uma análise especial. Rio de Janeiro: Fundação Oswaldo Cruz, 32.
35
Marba S. T. M. MezzaCappa F. (2008). Manual de Neonatologia UNICAMP. CAISM- Centro de Atenção Integral à Saúde da Mulher. 2nd ed.Rio de Janeiro: Revinter, 504.
36
Martin R. Fanaroff A. Walsh M. (2017). Fanaroff and Martin’s Medicina Neonatal e Perinatal: Doenças do Feto e do Neonato. 10th ed.Rio de Janeiro: Elsevier Ltd., 1684.
37
Matlow A. G. Cronin C. M. G. Flintoft V. Nijssen-Jordan C. Fleming M. Brady-Fryer B. et al (2011). Description of the development and validation of the Canadian paediatric trigger tool. BMJ Qual. Saf.20 (5), 416–423. 10.1136/bmjqs.2010.041152
38
Maziero E. C. S. Cruz E. D. A. Batista J. Alpendre F. T. Brandão M. B. Krainski E. T. (2021). Association between professional qualification and adverse events in neonatal and pediatric intensive treatment units. Rev. Gaúcha Enferm.42, e20210025. 10.1590/1983-1447.2021.20210025
39
Mccullen K. L. Pieper B. (2006). A retrospective chart review of risk factors for extravasation among neonates receiving peripheral intravascular fluids. J. Wound Ostomy Cont. Nurs.33, 133–139. 10.1097/00152192-200603000-00006
40
Mesotten D. Joosten K. Van Kempen A. Verbruggen S. ESPGHAN/ESPEN/ESPR/CSPEN working group on pediatric parenteral nutrition (2018). ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: carbohydrates. Clin. Nutr.37 (6), 2337–2343. 10.1016/j.clnu.2018.06.947
41
Meyer-Massetti C. Cheng C. Schwappach D. Paulsen L. Ide B. Meier C. et al (2011). Systematic review of medication safety assessment methods. Am. J. Heal Syst. Pharm.68, 227–240. 10.2146/ajhp100019
42
Moura B. Alencar G. Silva Z. Almeida M. (2020). Fatores associados à internação e à mortalidade neonatal em uma coorte de recém-nascidos do Sistema Único de Saúde, no município de São Paulo. Rev. Bras. Epidemiol., 23. 10.1590/1980-549720200088
43
Naessens J. M. Campbell C. R. Huddleston J. M. Berg B. P. Lefante J. J. Williams A. R. et al (2009). A comparison of hospital adverse events identified by three widely used detection methods. Int. J. Qual. Heal Care21, 301–307. 10.1093/intqhc/mzp027
44
Ni Y. Lingren T. Hall E. Leonard M. Melton K. Kirkendall E. (2018). Designing and evaluating an automated system for real-time medication administration error detection in a neonatal intensive care unit. J. Am. Med. Inf. Assoc.25 (5), 555–563. 10.1093/jamia/ocx156
45
Oza S. Lawn J. E. Hogan D. R. Mathers C. Cousens S. N. (2013). Neonatal cause-of-death estimates for the early and late neonatal periods for 194 countries: (2000–2013). Bull. World Health Organ93 (1), 19–28. 10.2471/BLT.14.139790
46
Pierdevara L. Ventura I. Eiras M. Gracias A. (2017). Trigger Tool na Segurança do Doente: Uma Revisão Sistemática de Literatura. Port. J. Public Heal35, 69–76. 10.1159/000479606
47
Ravi D. Sigurdson K. Profit J. (2019). Improving quality of care can mitigate persistent disparities. Pediatrics144 (3), e20192002. 10.1542/peds.2019-2002
48
Resar R. Rozich J. Simmonds T. Haraden C. (2006). A trigger tool to identify adverse events in the intensive care unit. J. Comm. J. Qual. Patient Saf.32 (10), 585–590. 10.1016/s1553-7250(06)32076-4
49
Salaets T. Turner M. A. Short M. Ward R. M. Hokuto I. Ariagno R. L. et al (2019). Development of a neonatal adverse event severity scale through a Delphi consensus approach. Arch. Dis. Child.104, 1167–1173. 10.1136/archdischild-2019-317399
50
Samiee-Zafarghandy S. Van den Anker J. Allegaert K. (2023). Roadmap to optimal pharmacovigilance practice in neonatal intensive care units. Br. J. Clin. Pharmacol.89, 523–525. 10.1111/bcp.15465
51
Sharek P. Horbar J. Mason W. Bisarya H. Thurm C. Suresh G. et al (2006). Adverse events in the neonatal intensive care unit: development, testing, and findings of an NICU-focused trigger tool to identify harm in north American NICUs. Pediatrics118, 1332–1340. 10.1542/peds.2006-0565
52
Snijders C. van Lingen R. A. Klip H. Fetter W. P. F. van der Schaaf T. W. Molendijk H. A. et al (2009). Specialty-based, voluntary incident reporting in neonatal intensive care: description of 4846 incident reports. Arch. Dis. Child. - Fetal Neonatal Ed.94 (3), F210–F215. 10.1136/adc.2007.135020
53
Srulovici E. Ore L. Shinwell E. Blazer S. Zangen S. Riskin A. et al (2012). Factors associated with iatrogenesis in neonatal intensive care units: an observational multicenter study. Eur. J. Pediatr.171, 1753–1759. 10.1007/s00431-012-1799-0
54
Stoll B. J. Hansen N. I. Bell E. F. Shankaran S. Laptook A. R. Walsh M. C. et al (2010). Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics126 (3), 443–456. 10.1542/peds.2009-2959
55
Sugioka M. Tachi T. Mizui T. Koyama A. Murayama A. Katsuno H. et al (2020). Effects of the number of drugs used on the prevalence of adverse drug reactions in children. Sci. Rep.10 (21341), 21341. 10.1038/s41598-020-78358-3
56
Takata G. S. Mason W. Taketomo C. Logsdon T. Sharek P. J. (2008). Development, testing, and findings of a pediatric-focused trigger tool to identify medication-related harm in US children's hospitals Children’s Hospitals. Pediatrics. (121, (4):e927–e935. 10.1542/peds.2007-1779
57
Tayman C. Rayyan M. Allegaert K. (2011). Neonatal pharmacology: extensive interindividual variability despite limited size. J. Pediatr. Pharmacol. Ther.16 (3), 170–184. 10.5863/1551-6776-16.3.170
58
Unbeck M. Lindemalm S. Nydert P. Ygge B.-M. Nylén U. Berglund C. et al (2014). Validation of triggers and development of a pediatric trigger tool to identify adverse events. BMC Health Serv. Res.14 (1), 655. 10.1186/s12913-014-0655-5
59
Uppsala Monitoring Centre, Organização Mundial da Saúde (2005). Monitorização da segurança de medicamentos: diretrizes para criação e funcionamento de um centro de farmacovigilância. Brasília, DF, 28.
60
Ventura C. Alves J. Meneses J. (2012). Adverse events in a neonatal intensive care unit. Rev. Bras. Enferm.65, 49–55. 10.1590/s0034-71672012000100007
61
Vuralli D. (2019). Clinical approach to hypocalcemia in newborn period and infancy: who should Be treated?Int. J. Pediatr.2019, 4318075. 10.1155/2019/4318075
62
Workineh Y. Workie H. (2022). Adverse neonatal outcomes and associated risk factors: a case-control study. Glob. Pediatr. Heal9, 2333794X221084070. 10.1177/2333794X221084070
63
World Health Organization (2014). Every newborn: an action plan to end preventable deaths. Geneva: WHO Press.
64
World Health Organization Collaborating Centre for Drug Statistics Methodology (2024). ATC/DDD index 2024 Norwegian Institute of public health. Available online at: https://atcddd.fhi.no/atc_ddd_index/(Accessed April 15, 2025).
65
World Health Organization - WHO (2009). Conceptual framework for the international classification for patient safety. Version 1.1. Final Technical Report Geneva. Available online at: https://apps.who.int/iris/handle/10665/70882.
66
Yalçın N. Kaşıkcı M. Çelik H. Allegaert K. Demirkan K. Yiğit Ş. et al (2022). An artificial intelligence approach to support detection of neonatal adverse drug reactions based on severity and probability scores: a new risk score as web-tool. Children9 (12), 1826. 10.3390/children9121826
Summary
Keywords
adverse events, adverse drug events, trigger tool, neonatal intensive care unit, low birth weight
Citation
Albanese FB, Ventura DdS, Perroud MW Jr, Nogueira de Souza R, Morau MV, Visacri MB and Moriel P (2025) Adverse events in the neonatal intensive care unit identified by triggers. Front. Pharmacol. 16:1539687. doi: 10.3389/fphar.2025.1539687
Received
04 December 2024
Accepted
05 May 2025
Published
30 May 2025
Volume
16 - 2025
Edited by
Wei Luan, Shuguang Hospital Affiliated to Shanghai University of TCM, China
Reviewed by
Karel Allegaert, KU Leuven, Belgium
Christopher A. W. David, University of Liverpool, United Kingdom
Updates
Copyright
© 2025 Albanese, Ventura, Perroud, Nogueira de Souza, Morau, Visacri and Moriel.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patricia Moriel, patricia.moriel@fcf.unicamp.br
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.