- 1Department of Geriatrics, Zhejiang Medical & Health Group Quzhou Hospital (Zhejiang Quhua Hospital), Quzhou, Zhejiang, China
- 2Department of Geriatrics, Wenzhou Geriatric Hospital, Wenzhou, Zhejiang, China
Sarcopenia is characterized by the progressive loss of muscle mass, strength, and function, predominantly affecting the elderly population, and has emerged as a significant public health concern in the context of global aging. Its pathogenesis is multifactorial, involving aging-related processes, imbalances in skeletal muscle homeostasis, mitochondrial dysfunction, immune-mediated inflammation, and gut microbiota dysbiosis. Recent studies suggest that traditional therapies, including traditional Chinese medicine (TCM)—such as herbal medicine, acupuncture, and qigong—when integrated with modern medical treatments, may offer a more personalized therapeutic approach for older adults with sarcopenia. This integrative strategy has demonstrated considerable potential to improve muscle mass, enhance strength, decelerate the aging process, and ultimately improve patients’ quality of life. This review aims to summarize the clinical research and applications of TCM in sarcopenia management, explore the potential mechanisms underlying TCM’s therapeutic effects, and discuss future research directions and their clinical relevance.
1 Introduction
Sarcopenia, commonly referred to as muscle wasting, has seen continuous evolution in its definition and diagnostic criteria since the term was first introduced in the 1980s. Working Group on Sarcopenia in Older People (EWGSOP) proposed the first widely accepted diagnostic criteria, identifying low muscle mass, reduced muscle strength, and poor physical performance as the principal diagnostic indicators (Cruz-Jentoft et al., 2010). Recognizing the need for clarity and clinical relevance, EWGSOP revised its criteria in 2018, officially defining sarcopenia as a progressive and systemic skeletal muscle disorder characterized by accelerated loss of muscle mass and function. Importantly, the updated guidelines emphasized low muscle strength as the primary characteristic, with diagnosis confirmed by assessing muscle quantity and quality. Poor physical performance was further specified as an indicator of severe sarcopenia (Cruz-Jentoft and Sayer, 2019; Cruz-Jentoft et al., 2019).
In the Chinese population, where aging is rapidly accelerating, sarcopenia has attracted increasing concern. Epidemiological studies report that sarcopenia affects 5.7%–23.9% of individuals aged 60 and older, with higher prevalence observed in men and in eastern regions compared to western areas. The incidence also rises substantially with advancing age (Cui et al., 2023). Extensive research has confirmed that sarcopenia is strongly linked to frailty, increased risk of falls and fractures, functional impairment, cognitive decline, and elevated morbidity and mortality rates. Furthermore, with rising global life expectancy, sarcopenia has emerged as a critical public health issue, posing significant challenges to healthcare systems and profoundly affecting the quality of life of older adults (Beaudart et al., 2023; Zuo et al., 2023).
Despite advances in exercise therapy, nutritional supplementation, and pharmacological treatments, managing sarcopenia remains challenging, especially in elderly patients with multimorbidity and limited mobility. Traditional Chinese Medicine (TCM) has gained attention for its holistic approach, multi-target mechanisms, and favorable safety profile. TCM therapies—including herbal medicine, acupuncture, and traditional exercises like Tai Chi and Baduanjin—not only improve muscle mass and strength but also modulate inflammation, oxidative stress, mitochondrial function, and neuromuscular connectivity. This review summarizes recent advances in TCM research for sarcopenia and explores its mechanisms and therapeutic potential (The literature search strategy is detailed in the Supplementary Material).
2 Mechanisms of sarcopenia
Muscle mass and strength progressively decline with age, particularly in individuals over 75 years old. Studies indicate that the annual loss of muscle mass averages 0.64%–0.7% in women and 0.8%–0.98% in men, with losses typically more pronounced in the lower limbs than in the upper limbs. Notably, muscle function deteriorates even more rapidly: by age 75, muscle strength declines by approximately 3%–4% per year in men and 2.5%–3% in women (Amaral et al., 2014; Wilkinson et al., 2018a).
This reduction in strength is not solely attributable to the loss of lean muscle mass but also reflects a range of physiological and neural alterations, including diminished autonomic drive, impaired neuromuscular control (e.g., reduced motor neuron firing rates and slower nerve conduction velocity), increased non-contractile adipose infiltration, and reduced efficiency of excitation-contraction coupling (Gonzalez-Freire et al., 2014; Distefano and Goodpaster, 2018). Collectively, these factors accelerate the decline in muscle strength and function, adversely affecting mobility and quality of life in older adults.
The pathogenesis of sarcopenia is a multifactorial and multi-pathway process, integrating diverse cellular and molecular mechanisms. Beyond the classical imbalance between protein synthesis and degradation, recent research highlights additional contributors such as disruption of skeletal muscle homeostasis, compromised mitochondrial quality control, dysregulated immune responses, and alterations in gut microbiota composition, all of which play critical roles in the onset and progression of sarcopenia (Cruz-Jentoft and Sayer, 2019; Picca and Calvani, 2021) (Figure 1).
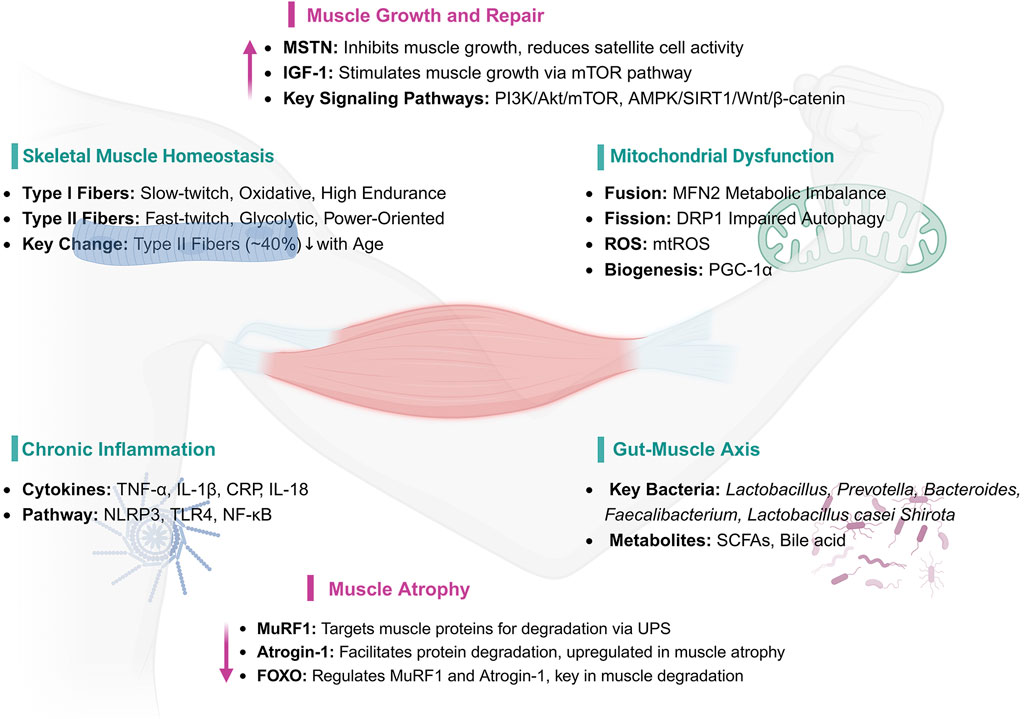
Figure 1. Key Mechanisms and Pathways in Muscle Health and Atrophy. IGF-1: Insulin-like Growth Factor 1, MSTN: Myostatin (Growth Differentiation Factor 8, GDF-8), AKT1: Protein Kinase B, PI3K: Phosphoinositide 3-Kinase, mTOR: Mechanistic Target of Rapamycin, AMPK: AMP-activated Protein Kinase, SIRT1: Sirtuin 1, PGC-1α: Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-alpha, MuRF1: Muscle Ring Finger 1, Atrogin-1: Atrophy-related ubiquitin ligase 1, FOXO: Forkhead Box O, MFN2: Mitofusin 2, DRP1: Dynamin-related Protein 1, ROS: Reactive Oxygen Species, mtROS: Mitochondrial Reactive Oxygen Species, TNF-α: Tumor Necrosis Factor-alpha, IL-1β: Interleukin-1 beta, CRP: C-reactive Protein, IL-18: Interleukin-18, NLRP3: NOD-like Receptor Pyrin Domain Containing 3, TLR4: Toll-like Receptor 4, NF-κB: Nuclear Factor-kappa B, UPS: Ubiquitin-Proteasome System, SCFAs: Short-chain Fatty Acids.
2.1 Skeletal muscle homeostasis
The dynamic balance of skeletal muscle proteins is governed by the interplay between protein synthesis and degradation, with the net balance reflecting the difference between these two processes. This equilibrium is essential for preserving muscle mass and function (Schiaffino et al., 2013). Under normal physiological conditions, skeletal muscle homeostasis is maintained through intricate regulatory mechanisms that balance muscle hypertrophy and regeneration. However, aging progressively disrupts this balance, resulting in declines in both muscle mass and function. Muscle atrophy—a morphological adaptation—manifests as narrower muscle fibers, reductions in total muscle mass and protein content, diminished force production, and shifts in muscle fiber type composition (Tieland et al., 2018).
Skeletal muscle, the largest tissue in the human body, possesses robust regenerative capacity. It comprises bundles of type I (slow oxidative) and type II (fast oxidative/glycolytic) fibers and hosts a variety of mononuclear cells within its specialized microenvironment. Type I fibers primarily depend on oxidative metabolism, whereas type II fibers generate energy predominantly through glycolysis. With aging, the number of type II fibers declines by approximately 40%, contributing to substantial reductions in lean muscle mass. The preferential loss of type II fibers is a hallmark of sarcopenia and plays a central role in its pathogenesis (Deschenes, 2004; Phu et al., 2015).
2.2 Mitochondrial dysfunction
Mitochondrial dysfunction has been recognized as a central mechanism in skeletal muscle aging and sarcopenia.Mitochondria, double-membraned organelles present in most cells, serve as the primary site for cellular aerobic respiration and function as central hubs for metabolism and signal transduction. They play a critical role in maintaining mitochondrial dynamics—including fission, fusion, and autophagy—and are involved in processes such as ATP synthesis, fatty acid oxidation, calcium homeostasis, phospholipid synthesis, and the generation of reactive oxygen species (ROS) (Hood et al., 2019; Lin et al., 2022). When mitochondrial quality control mechanisms fail, structural and functional damage ensues, leading to muscle degeneration. In aging muscle cells specifically, significant changes in mitochondrial morphology and function are observed, accompanied by increased ROS levels, further exacerbating the aging process. Age-related impairments in mitochondrial fission and fusion diminish the cell’s capacity to clear damaged mitochondria, resulting in ROS-induced DNA damage and accelerated aging (Li J. et al., 2022).
During aging, reduced levels of the mitochondrial fusion protein mitofusin 2 (Mfn2) are closely associated with metabolic dysregulation and sarcopenia. Loss of Mfn2 triggers aging-related features, such as decreased mitophagy and impaired mitochondrial function, leading to skeletal muscle metabolic imbalance and muscle wasting. Research has shown that Mfn2 deficiency weakens autophagy processes, reduces mitochondrial quality, and further worsens age-related mitochondrial dysfunction and muscle atrophy (Sebastián et al., 2016). Additionally, muscle-specific deletion of the fission protein dynamin-related protein 1 (DRP1) results in significant muscle atrophy and weakness. Persistent DRP1 deficiency not only suppresses normal muscle growth but can also cause lethality, while inducible deficiency leads to muscle atrophy and degeneration. DRP1-deficient mice exhibit abnormal mitochondrial morphology, including increased size and functional impairment. Dysfunctional mitochondria further activate nuclear signaling pathways, inducing the ubiquitin-proteasome system and the unfolded protein response (Favaro et al., 2019; Dulac et al., 2021).
Mitochondrial biogenesis, referring to the formation of new mitochondria within cells, involves mitochondrial DNA replication, transcription, and the synthesis of associated proteins. This process is regulated by key transcription factors such as peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and is essential for maintaining cellular energy metabolism, antioxidant capacity, and stress-adaptive responses. During mitochondrial biogenesis, Migliavacca et al. conducted transcriptomic analyses on muscle biopsy samples from 119 elderly men and found that patients with sarcopenia commonly exhibited significant mitochondrial dysfunction. This dysfunction was characterized by downregulation of the PGC-1α/estrogen-related receptor alpha (ERRα) signaling pathway and reduced expression of genes involved in oxidative phosphorylation (Migliavacca et al., 2019). Further studies demonstrated that overexpression of PGC-1α activated mitochondrial metabolism and angiogenesis, significantly improving endurance, suggesting its potential role in preventing age-related sarcopenia (Yang et al., 2020). Subsequent experiments revealed that muscle-specific PGC-1α knockout mice displayed more severe sarcopenia compared to wild-type mice, with significantly reduced levels of Sestrin2 and phosphorylated ribosomal protein S6 kinase one (p-S6K1) in the white gastrocnemius muscle. Treatment with recombinant Sestrin2 improved muscle function and restored p-S6K1 levels in these mice (Fu et al., 2024).
Muscle stem cells, or satellite cells, play a crucial role in muscle regeneration following injury. Mitochondrial dysfunction not only damages mature muscle fibers but also impairs satellite cells, which are essential for muscle repair and regeneration. Age-related mitochondrial dysfunction leads to a decrease in satellite cell numbers and regenerative capacity, further accelerating the progression of sarcopenia. Disruption of mitochondrial homeostasis within satellite cells impairs their self-renewal and differentiation abilities, limiting muscle repair and the capacity to adapt to physical stress (Brooks et al., 2018; Greising et al., 2019; Marzetti et al., 2024).
Moreover, mitochondrial dysfunction interacts with chronic inflammation, creating a vicious cycle that accelerates muscle loss. Damaged mitochondria produce excessive ROS, which activate inflammatory pathways such as the nuclear factor kappa B (NF-κB), leading to upregulation of pro-inflammatory cytokines including interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). These cytokines, in turn, further impair mitochondrial function, establishing a persistent feed-forward loop that promotes inflammation and muscle degeneration (Marzetti et al., 2013; Reid and Alexander, 2021).
2.3 Chronic inflammation
Aging is characterized by chronic, low-grade inflammation. As individuals age, the accumulation of inflammatory factors promotes muscle catabolism, contributing to the loss of muscle mass and strength and exacerbating age-related conditions such as sarcopenia (Jimenez-Gutierrez et al., 2022). A meta-analysis covering 17 studies and involving 11,249 participants (3,072 with sarcopenia and 8,177 without) showed that C-reactive protein (CRP) levels were significantly higher in sarcopenia patients compared to the control group, suggesting an association between sarcopenia and elevated CRP levels (Bano et al., 2017). 2019 clinical study conducted in Beijing, it was found that serum inflammatory markers, such as TNF-α, TNF-like weak inducer of apoptosis (TWEAK), IL-18, were significantly elevated in sarcopenia patients, while insulin-like growth factor 1 (IGF-1) and adiponectin, which are associated with metabolism, were significantly lower. Furthermore, enhanced lifestyle interventions significantly improved muscle mass and reduced serum levels of related inflammatory factors in sarcopenia patients (Li C. et al., 2019).
At the molecular level, inflammatory factors contribute to pyroptosis—a form of programmed cell death—in muscle cells, accelerating catabolism and further reducing muscle mass and strength. The NLRP3 (NOD-like receptor family pyrin domain containing 3) inflammasome plays a pivotal role in this process by promoting the maturation and secretion of pro-inflammatory cytokines such as IL-1β and IL-18. This activation enhances both local and systemic pyroptotic and inflammatory responses, damages mitochondrial function, and reduces glycolytic capacity in muscle cells, all of which contribute to muscle wasting and impaired fiber growth (Busch et al., 2021; Ma, 2023).
Inflammatory stimuli also activate NLRP3 to induce acute mitochondrial reactive oxygen species (mtROS) production, triggering oxidative stress that damages muscle cells, inhibits growth, and accelerates atrophy (Antuña et al., 2024; Yungi et al., 2024). Additionally, the NF-κB signaling pathway plays a critical role in sarcopenia pathogenesis. NF-κB overactivation inhibits the proliferation and differentiation of muscle satellite cells, reducing muscle regenerative capacity, while also promoting oxidative stress through increased mtROS production, further impairing muscle cell function and growth (Sun et al., 2018; Lee et al., 2024).Furthermore, Alexander et al. found that Toll-like receptor 4 (TLR4) promotes muscle protein degradation by coordinating the activation of the ubiquitin-proteasome pathway and the autophagy-lysosome pathway, leading to muscle atrophy. This finding provides a potential intervention target for inflammation-related muscle atrophy treatment (Doyle et al., 2011).
2.4 Gut-muscle axis
The human gastrointestinal tract harbors a vast microbiome, containing approximately 100 trillion microorganisms, including bacteria, archaea, and fungi (Eckburg et al., 2005). These microorganisms are crucial for human health and many functional characteristics of the gut microbiome. They assist in the digestion of dietary polysaccharides, metabolize xenobiotic drugs, promote immune responses, and prevent pathogen invasion (Zmora et al., 2019; Erttmann et al., 2022). Dysbiosis of the gut microbiome is closely associated with the decline in muscle mass and physical function, particularly in the elderly population. As individuals age, the composition of the gut microbiome undergoes changes, which may be closely linked to sarcopenia and the decline in physical function during aging. Increasing research has begun to explore the interaction between the gut microbiome and muscle health, revealing the potential mechanisms behind the so-called “gut-muscle axis” (Liu et al., 2021; Zhang T. et al., 2022).
Active adults typically have higher muscle mass and better physical function than sedentary adults, and their gut microbiomes also exhibit different dominant bacterial populations (Bressa et al., 2017). One study showed that in the stool samples of elderly individuals with higher frailty scores, the number of Lactobacillus was significantly reduced, with a decrease of up to 26-fold. Additionally, populations of Bacteroides/Prevotella and Firmicutes were also significantly reduced, by 3-fold and 4-fold, respectively (van Tongeren et al., 2005). Moreover, some studies have also explored the effects of probiotic supplements on lean body mass and physical performance, finding that supplementation with probiotics improved muscle strength, athletic performance, and recovery in athletes (Marttinen et al., 2020; Przewłócka et al., 2020).
The gut microbiome and its metabolites play a critical role in maintaining muscle health and function. Specifically, bile acids and short-chain fatty acids (SCFAs) are considered important mediators through which gut microbiota influence muscle health. Dysbiosis of the gut microbiota can lead to a range of negative effects, one of which is excessive muscle atrophy (Mancin et al., 2023). Germ-free mice, lacking a healthy gut microbiota, exhibited significant skeletal muscle atrophy, closely associated with the upregulation of muscle atrophy-related genes such as Forkhead box O3 (FoxO3), Atrogin-1, and Muscle Ring Finger-1 (MuRF-1) in their skeletal muscle. At the same time, genes related to muscle generation and functional recovery, particularly myosin heavy chain genes, were significantly downregulated in these mice (Lee et al., 2023; Kang et al., 2024). Lahiri et al. further revealed that the muscle atrophy in germ-free mice was closely related to a reduction in IGF-1 levels and a decline in the transcription of genes associated with skeletal muscle growth and mitochondrial function. Encouragingly, when the gut microbiota from pathogen-free mice was transplanted into germ-free mice, the skeletal muscle mass of the germ-free mice was significantly restored, muscle atrophy markers decreased, and the expression of genes related to neuromuscular junction formation, such as Rapsyn and Lrp4, was significantly increased. Additionally, SCFA treatment also effectively improved the skeletal muscle health of germ-free mice, partially reversing muscle damage induced by gut microbiota dysbiosis (Lahiri et al., 2019). Recent research further confirms the impact of the gut microbiome on age-related muscle degeneration. Researchers found that treatment with Lactobacillus casei Shirota for 12 weeks in SAMP8 mice (Senescence-Accelerated Mouse Prone 8) significantly reduced age-related muscle mass and strength decline. This study also suggested that L. casei Shirota treatment might slow muscle degeneration during aging by restoring SCFA levels, modulating inflammation, improving mitochondrial function, and optimizing the diversity of the gut microbiome (Chen et al., 2022).
2.5 Cancer-associated sarcopenia
Cancer-associated sarcopenia refers to the progressive decline in skeletal muscle mass, strength, and function observed in patients with malignancies. Unlike primary sarcopenia associated with aging, cancer-associated sarcopenia tends to develop earlier, progress more rapidly, and is frequently accompanied by cancer cachexia. Epidemiological studies indicate that approximately 50%–80% of patients with advanced cancer experience varying degrees of muscle wasting (Zhang et al., 2023). There exists a complex and bidirectional relationship between sarcopenia and cancer. On one hand, cancer induces sarcopenia through mechanisms such as systemic inflammation, metabolic dysregulation, elevated catabolic cytokines (e.g., IL-6, TNF-α), and the direct myotoxic effects of chemotherapy and radiotherapy. Cancer cachexia represents the extreme manifestation of this process, characterized by severe weight loss, muscle atrophy, and elevated systemic inflammation. On the other hand, sarcopenia itself acts as an independent adverse prognostic factor. Patients with cancer-related sarcopenia exhibit reduced chemotherapy tolerance, increased incidence of treatment-related toxicities, diminished physical performance, prolonged hospitalization, and significantly higher mortality rates (Williams et al., 2021; Fang et al., 2023; Kiss et al., 2023).Additionally, sarcopenia limits patients’ eligibility for surgical or targeted therapies, further compromising treatment outcomes (Levolger et al., 2015). Breaking this vicious cycle between cancer and sarcopenia is therefore critical for improving clinical prognosis.
At the mechanistic level, cancer-associated sarcopenia is driven by a series of interconnected pathological processes (Moreira-Pais et al., 2021). Systemic inflammation leads to sustained elevation of pro-inflammatory cytokines, which activate key catabolic signaling pathways such as NF-κB and Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3) axes. These pathways upregulate the expression of muscle-specific E3 ubiquitin ligases, including muscle RING-finger protein-1 (MuRF1) and Atrogin-1, accelerating skeletal muscle proteolysis through the ubiquitin-proteasome system (Martin et al., 2023). Metabolic disorders, including insulin resistance and elevated basal energy expenditure, combined with mitochondrial dysfunction, further impair muscle energy homeostasis (Gicquel et al., 2024).
Recently, accumulating evidence has indicated that TCM may provide a promising complementary approach for alleviating cancer-associated sarcopenia (Li S. et al., 2025). Astragalus membranaceus extracts, particularly astragaloside IV, have demonstrated the ability to suppress oxidative stress and inflammation, modulate the activity of the ubiquitin-proteasome system and autophagy-lysosomal pathways, and consequently reduce skeletal muscle proteolysis (Liu et al., 2025). In addition, Polygonatum sibiricum polysaccharides have been reported to activate the Phosphatidylinositol 3-kinase/Protein kinase B/Mammalian target of rapamycin (PI3K/Akt/mTOR) signaling pathway, thereby improving mitochondrial dysfunction, enhancing muscle energy metabolism, and promoting muscle cell homeostasis (Li Y. et al., 2025).
3 Clinical research on traditional Chinese medicine and sarcopenia
In TCM, sarcopenia is not explicitly discussed as a standalone condition, but its clinical manifestations are often integrated into the broader categories of “Wei syndrome,” “deficiency syndrome,” “bi syndrome of flesh,” and “bi syndrome of bone.” The clinical symptoms of Wei syndrome were first described in the Huangdi Neijing (Yellow Emperor’s Inner Classic), where it is noted: “Wei means weakness, and weakness is caused by movement.” Wei syndrome primarily affects the muscles and bones of the limbs, with the lesions of the five viscera (zangfu organs) potentially leading to Wei syndrome. Wei Lun (in the “Plain Questions” of the Huangdi Neijing) emphasized that “in the Yangming, the sea of the five zangfu and six fu is primarily responsible for running the zongjin, and the zongjin is responsible for binding the bones, but also for strengthening the organs.” It also presents the therapeutic principle of “treating erectile dysfunction by regulating the Yangming,” underscoring the importance of the spleen and stomach in addressing dysfunctions of other zangfu organs.
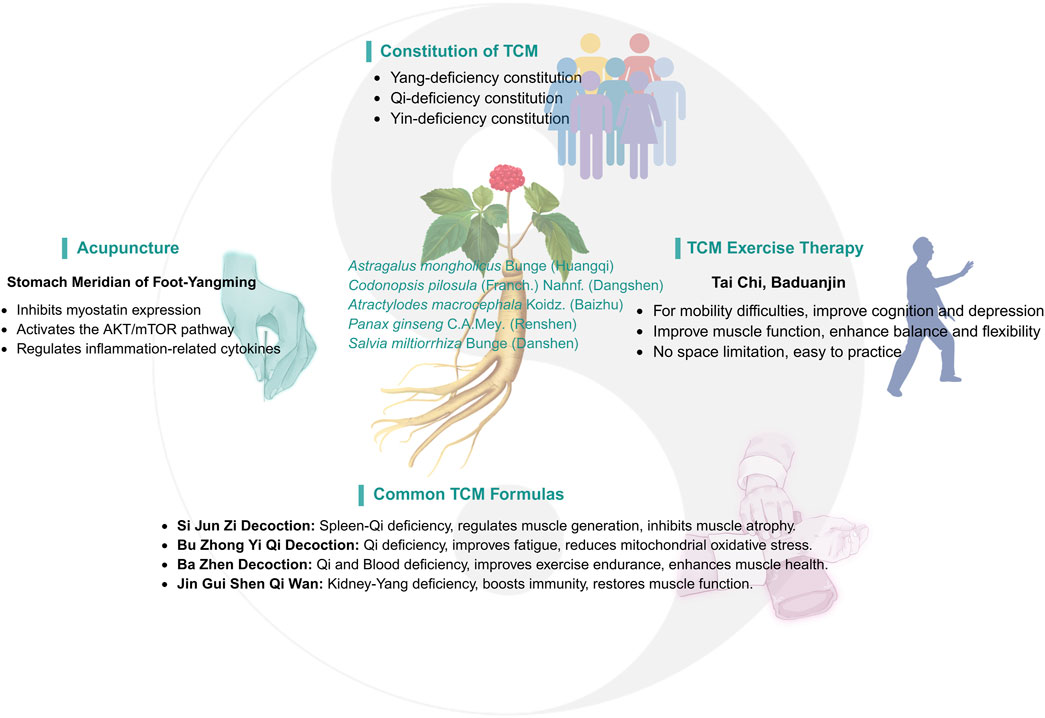
As part of a comprehensive approach, TCM has demonstrated significant efficacy and unique advantages in treating sarcopenia. TCM emphasizes a holistic approach and syndrome differentiation, aiming to restore balance in the body by regulating Yin and Yang, qi and blood, and the zangfu organs. This balance helps enhance the body’s self-healing ability. TCM treatments for sarcopenia are varied and flexible, including Chinese herbal medicine (CHM), acupuncture, massage, qigong, and moxibustion. These therapies not only improve muscle mass and function but also promote overall health, particularly among the elderly (Figure 2).
3.1 Traditional Chinese medicine constitution
In TCM theory, the classification of constitution types provides a personalized treatment framework for clinical treatment. Professor Wang Qi, a great physician of Chinese medicine, proposed nine constitutions classification (Li L. et al., 2019),including one balanced constitution (neutral constitution) and eight biased constitutions (Qi deficiency, Yang deficiency, Yin deficiency, blood stasis, Qi stagnation, dampness and heat, phlegm dampness and special constitution), which provides a new perspective for the diagnosis and treatment of senile diseases such as sarcopenia.
With aging, elderly individuals experience a gradual decline in nutritional intake and immune function, along with reduced ability to eat, digest, and absorb nutrients. This can lead to muscle strength loss, decreased energy, and reduced internal reserves, contributing to the development of various biased constitutions. Research has shown that constitutional types such as Qi deficiency, Yang deficiency, Yin deficiency, and blood stasis are closely associated with sarcopenia, particularly in elderly males. Qi deficiency and Yang deficiency are considered potential risk factors for sarcopenia, providing targets for prevention and intervention in clinical treatment (Shi et al., 2018; Nie et al., 2024). Another study indicated that Yin deficiency was independently correlated with sarcopenia, and may serve as a potential risk factor for the condition (Wang C. et al., 2024).
Based on the varying physical manifestations, TCM offers tailored treatment programs. For example, for patients with yang deficiency, commonly used herbs to warm and tonify kidney yang include Zingiber officinale Roscoe (Ganjiang), Cinnamomum cassia (L.) D. Don (Rougui), Aconitum carmichaelii Debeaux (Fuzi), and others to warm yang and dispel cold. For those with qi deficiency, herbs such as Astragalus mongholicus Bunge (Huangqi), Atractylodes macrocephala Koidz. (Baizhu), Panax ginseng C.A.Mey. (Renshen) and Codonopsis pilosula (Franch.) Nannf. (Dangshen) are used to tonify qi and replenish vitality. For individuals with a blood stasis constitution, blood-activating herbs such as Salvia miltiorrhiza Bunge (Danshen), Kalanchoe pinnata (Lam.) Pers. (Sanqi), and Carthamus tinctorius L. (Honghua) are commonly used to promote blood circulation and alleviate blood stasis symptoms (Table 1).
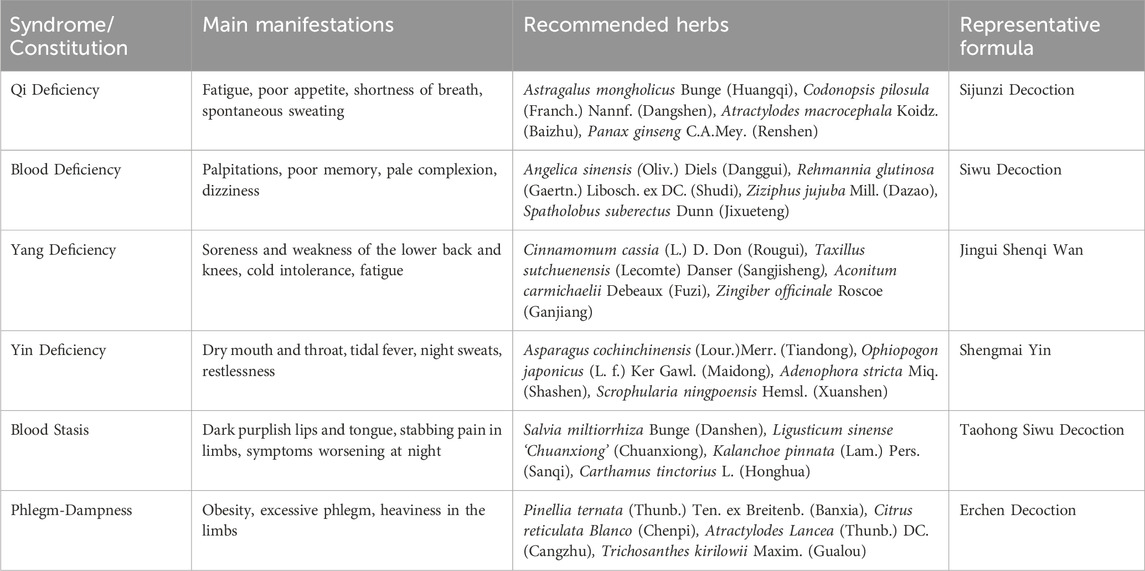
Table 1. Body constitution/patterns associated with sarcopenia, recommended herbs, and Representative Prescriptions.
3.2 Chinese herbal medicine
Chinese herbal medicine (CHM), as a core component of TCM, has attracted increasing attention and recognition in recent years. Since the discovery of artemisinin by Tu Youyou, the efficacy of CHM in treating various diseases has been supported by numerous high-quality studies (Tu, 2016; Chen W.-J. et al., 2023; Yang Y. et al., 2023).
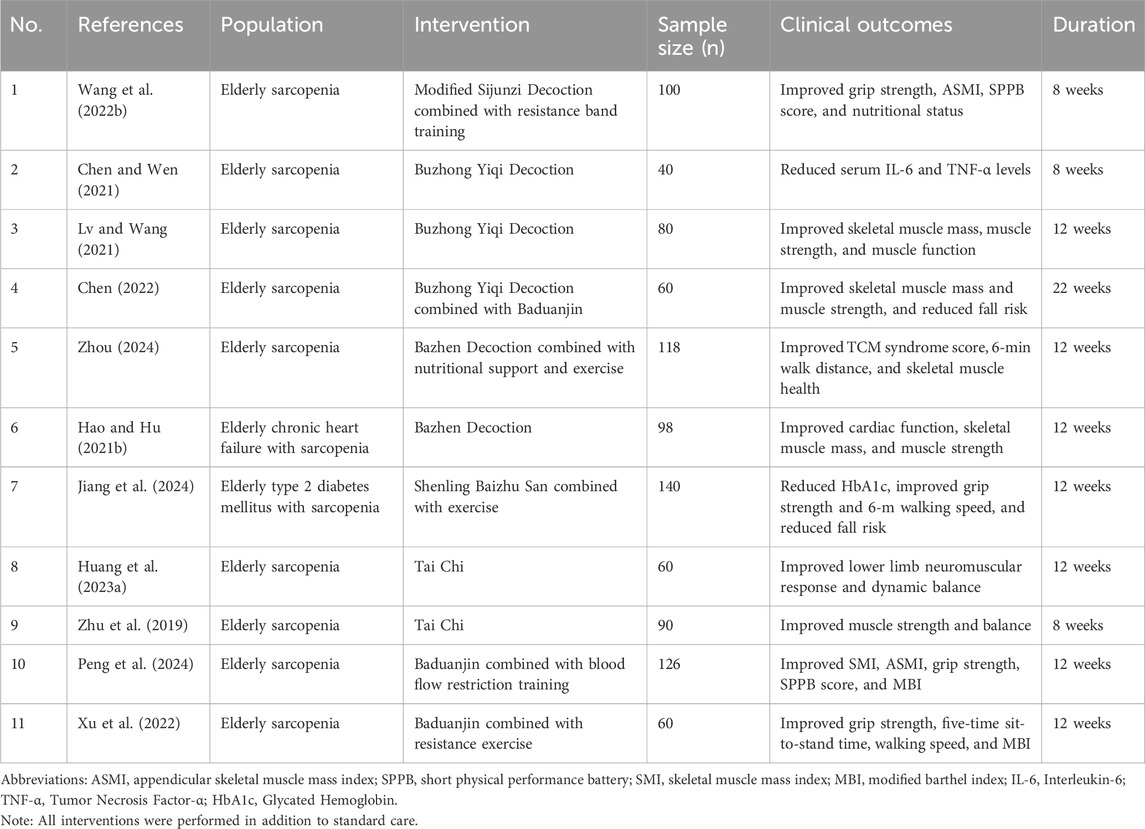
CHM not only treats diseases that Western medicine has not yet effectively addressed but also demonstrates unique therapeutic advantages in many fields. In recent years, more studies have shown that CHM has a significant effect on sarcopenia as a complementary treatment. A systematic review and meta-analysis of 17 randomized controlled trials involving 1,440 participants demonstrated that CHM significantly improved multiple clinical outcomes in sarcopenic patients, including total effective rate (RR = 1.29, 95% CI: 1.21–1.36), muscle mass (SMD = 1.02, 95% CI: 0.55–1.50), grip strength (SMD = 0.66, 95% CI: 0.36–0.96), 6-m walking speed (SMD = 1.34, 95% CI: 0.60–2.08), and scores on the Short Physical Performance Battery (SPPB) (mean increase: 1.50 points, 95% CI: 1.05–1.95) (Zhang et al., 2024a). These findings suggest that CHM, either alone or combined with conventional treatments, holds promise in improving muscle strength, mobility, and physical performance among elderly patients with sarcopenia.
From TCM perspective, sarcopenia is often associated with spleen-qi deficiency and kidney-yang deficiency. Commonly prescribed formulas include Sijunzi Decoction, Buzhong Yiqi Decoction, Bazhen Decoction, and Jingui Shenqi Wan. Clinical studies have shown that combining these herbal therapies with conventional treatments can significantly alleviate sarcopenia symptoms, particularly in elderly populations (Zhang et al., 2024b) (Table 2).
Sijunzi Decoction is composed of P. ginseng C.A.Mey. (Renshen), A. macrocephala Koidz. (Baizhu), Poria (Fulin) and Glycyrrhiza uralensis Fisch. (Gancao), which is mainly used for the treatment of spleen-stomach qi deficiency syndrome. The principle of treatment is mainly to benefit qi and strengthen the spleen, which can improve fatigue, regulate muscle production, inhibit muscle atrophy, and improve mitochondrial function (Chen J.-M. et al., 2019). Growing evidence suggests that integrating herbal therapy with physical rehabilitation may provide superior outcomes in managing sarcopenia. A randomized controlled trial involving 100 elderly patients demonstrated that an 8-week intervention with modified Sijunzi Decoction combined with resistance band training significantly improved clinical parameters compared to conventional therapy alone, including increased grip strength (23.75 ± 3.86 kg vs 20.05 ± 3.69 kg), appendicular skeletal muscle mass index (ASMI) (6.53 ± 0.41 kg/m2 vs 6.26 ± 0.32 kg/m2), and SPPB scores (9.54 ± 1.85 vs 8.14 ± 1.96), with all differences reaching statistical significance (Wang Q. et al., 2022).
Buzhong Yiqi Decoction is traditionally used to treat symptoms of qi deficiency, such as fatigue, tiredness, and loss of appetite. Its therapeutic effects are attributed to the regulation of the antioxidant system, thereby reducing mitochondrial oxidative stress damage, alleviating muscle weakness, and enhancing both physical health and cognitive function (Ding et al., 2024; Dong et al., 2024). A randomized controlled study demonstrated that modified Buzhong Yiqi Decoction combined with conventional intervention significantly reduced serum levels of IL-6 and TNF-α in elderly patients with sarcopenia after 2 months of treatment compared to conventional intervention alone (P < 0.05). Importantly, no significant changes were observed in white blood cell count, alanine aminotransferase, or serum creatinine, indicating that the combination therapy maintained a good safety profile (Chen and Wen, 2021).
Bazhen Decoction is composed of eight Chinese herbs, such as P. ginseng C.A.Mey. (Renshen), A. macrocephala Koidz. (Baizhu), Rehmannia glutinosa (Gaertn.) Libosch. ex DC. (Shudi), Radix Angelicae sinensist (Dangui), Paeonia lactiflora Pall. (Baishao). It has the functions of tonifying qi, nourishing blood, invigorating spleen and nourishing qi. This recipe is widely used to treat a variety of symptoms caused by qi and blood deficiency, especially in the elderly group, often used to intervene in sub-health status and prevent age-related degenerative diseases (Li C. et al., 2024). Recent clinical evidence supports its efficacy in sarcopenia management. A randomized controlled trial involving 118 elderly patients demonstrated that a 12-week intervention with Bazhen Decoction combined with nutrition and exercise significantly improved clinical outcomes compared to conventional therapy alone. Specifically, the combined treatment reduced clinical symptom scores (from 13.70 ± 2.68 to 3.89 ± 1.01 vs 13.56 ± 2.77 to 6.69 ± 1.30), increased 6-min walking distance (570.04 ± 35.01 m vs 533.08 ± 33.67 m), enhanced muscle function (1.01 ± 0.13 m/s vs 0.91 ± 0.11 m/s), improved muscle strength (23.38 ± 2.40 kg vs 22.49 ± 2.14 kg), and elevated muscle mass (5.87 ± 0.85 kg/m2 vs 5.40 ± 0.83 kg/m2), indicating superior clinical effectiveness (Zhou, 2024).
CHM has also shown good efficacy in the treatment of sarcopenia combined with other diseases. For example, patients with heart failure and sarcopenia treated with trimetazidine combined with Bazhen decoction can not only improve cardiac function, but also improve muscle mass and strength (Hao and Hu, 2021a). Similarly, recent clinical evidence from a randomized controlled trial involving 70 elderly patients with type 2 diabetes mellitus and sarcopenia showed that a 12-week intervention with Shenling Baizhu San combined with exercise achieved superior outcomes compared to conventional therapy alone. The combined treatment significantly lowered HbA1c levels (7.66% ± 0.58% vs 7.90% ± 0.70%), enhanced grip strength (24.00 ± 4.68 kg vs 22.24 ± 4.76 kg), and increased 6-m walking speed (1.06 ± 0.15 m/s vs 0.99 ± 0.16 m/s), although no significant difference was observed in skeletal muscle mass index (SMI) (Jiang et al., 2024). These findings suggest that CHM, when integrated with conventional therapies, may offer additional benefits in improving metabolic control, muscle strength, and physical performance in sarcopenic patients with comorbidities.
3.3 Traditional Chinese exercise
3.3.1 Tai Chi
Exercise therapy, particularly resistance and aerobic training, is a primary approach for treating sarcopenia in the elderly (Bårdstu et al., 2020; Kakehi et al., 2022). Traditional Chinese exercises, originating from TCM with a history of over 3,000 years, integrate therapeutic, aerobic, and mind-body elements. Representative practices such as Tai Chi, Baduanjin, Wuqinxi, and Yijinjing are characterized by gentle movements and emphasis on relaxation. Studies have shown that these exercises promote muscle hypertrophy, enhance strength and endurance, prevent muscle atrophy, and improve physiological functions related to frailty, including blood pressure regulation and vascular function (Kasim et al., 2020; Niu et al., 2022; Wang L. et al., 2022).
Among these traditional sports, Tai Chi, as a combination of Tuina and boxing, integrates aerobic training, resistance training and flexibility training, and is considered to significantly promote the overall health of the elderly (Siu et al., 2021). A systematic review of 11 randomized controlled trials involving 1,676 older adults with sarcopenia or frailty found that Tai Chi significantly improved lower-limb function, including the 30-s chair stand test (WMD = 2.36, 95% CI: 1.50–3.21) and the timed up-and-go test (WMD = −0.72, 95% CI: −1.10 to −0.34), and reduced fall incidence (WMD = −0.41, 95% CI: −0.64 to −0.17) (Huang et al., 2022).
The elderly are often accompanied by mental health problems such as anxiety and depression. Tai Chi not only helps to enhance physical strength and flexibility, but also plays an important role in improving mental health, especially in the antidepressant effect. Studies have shown that Tai chi helps relieve depressive symptoms by regulating the autonomic nervous system, improving mood-related neural networks, and its anti-inflammatory effects. In addition, Tai chi can also reduce the susceptibility to dopaminergic neurodegeneration, thereby helping the elderly maintain cognitive function and emotional stability, and reduce the occurrence and development of depressive symptoms (Li G. et al., 2022).
3.3.2 Baduanjin
It may be difficult for most older adults to adhere to the high-load resistance training programs recommended by the American College of Sports Medicine and the American Heart Association. Moreover, conventional exercise rehabilitation is often time-consuming, costly, and complex. Therefore, it is particularly important to identify safe and feasible exercise programs for elderly patients with sarcopenia.
Baduanjin, an ancient Chinese qigong practice originating in the Song Dynasty, involves gentle, rhythmic movements and has been shown to effectively improve the overall physical condition of the elderly. Regular practice of Baduanjin enhances balance, gait, and flexibility while also alleviating common psychological issues such as anxiety and depression. For elderly patients with sarcopenia, Baduanjin not only improves muscle function but also enhances overall quality of life, making it a highly suitable exercise modality for this population (Luo et al., 2023; Yang W. et al., 2023).
Compared with Tai Chi, Baduanjin is relatively static, involves a lower exercise load, and is easier to learn. It is not restricted by venue and can be practiced in any suitable space, which is particularly beneficial for older adults with mobility impairments. Studies have demonstrated that incorporating Baduanjin training into conventional rehabilitation programs significantly improves muscle mass, enhances muscle function, and promotes better daily activity performance and quality of life among the elderly (Xu et al., 2022; Zhou, 2024).
In a randomized trial with 126 older sarcopenic patients, Baduanjin combined with blood flow restriction training over 12 weeks led to notable improvements: handgrip strength rose by approximately 7 kg, Barthel Index improved by nearly 15 points, and SPPB scores increased by about two points. ASMI also showed significant gains. All these changes were markedly greater compared to the control group (P < 0.05), highlighting the effectiveness of the combined intervention in enhancing muscle strength, physical performance, and functional independence (Peng et al., 2024).
3.4 Acupuncture
Acupuncture, a core component of TCM with a history of over 3,000 years, has become one of the most widely recognized forms of alternative medicine worldwide. Techniques such as manual acupuncture, electroacupuncture, and transcutaneous electrical acupoint stimulation have been shown to exert therapeutic effects by stimulating specific acupoints, particularly in diseases that are challenging for modern medicine to treat (Liu et al., 2017; Wang Y. et al., 2023).
In clinical studies, the effects of acupuncture on sarcopenia have shown some variability. In a randomized controlled trial involving 60 patients with sarcopenia, twice-weekly electroacupuncture at yangming meridian acupoints—including bilateral Binao (LI14), Quchi (LI11), Zusanli (ST36), and Yanglingquan (GB34)—over 12 weeks significantly improved ASMI, grip strength, and body moisture percentage, while reducing 6-m walking time and body fat percentage compared with nutritional therapy alone (Ma et al., 2023). Similarly, another randomized controlled trial involving 42 elderly patients with sarcopenia (21 per group) reported that electroacupuncture combined with rehabilitation exercise training for 4 weeks significantly increased SPPB scores, 6-min walking distance, grip strength, and relative ASMI, and decreased SARC-F scores compared to exercise training alone (Ling and Huang, 2022).
However, contrasting results were observed in a study conducted in Brazil. In this trial, sarcopenic older adults (n = 11) received acupuncture treatment (24 sessions over 8 weeks), but no significant improvements were detected in lean mass (38.9 ± 6.7 kg, P = 0.348), body fat percentage (33.4% ± 6.1%, P = 0.358), handgrip strength (left hand at Post 3: 25.40 ± 8.54 kg), or functional performance as assessed by the Timed Up and Go (TUG) test (P = 0.86). Although IL-6 levels showed a slight reduction at Post 2 (2.31 ± 2.26 pg/mL, P = 0.05), TNF-α and IL-10 levels did not change significantly. Despite the lack of objective improvements, most participants reported subjective benefits, including pain relief and enhanced mobility (Soares Mendes Damasceno et al., 2019).
The mechanisms by which acupuncture treats sarcopenia involve multiple biological pathways, with electroacupuncture playing a key role in regulating skeletal muscle repair and regeneration. First, electroacupuncture inhibits the expression of myostatin, a negative regulator of muscle growth, and promotes satellite cell proliferation, thereby accelerating skeletal muscle repair and regeneration (Takaoka et al., 2007). Additionally, it suppresses the overactivation of the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING)-NF-κB signaling pathway, effectively reducing exercise-induced muscle inflammation and protecting skeletal muscle from damage (Li H. et al., 2024). Further studies have demonstrated that electroacupuncture can inhibit D-galactose-induced skeletal muscle atrophy, delay mitochondrial dysfunction associated with aging, and reduce muscle nuclear apoptosis (Jin et al., 2023).
Moreover, electroacupuncture significantly improves physical performance, muscle mass, and the cross-sectional area of the gastrocnemius muscle in SAMP8 mice. It promotes angiogenesis by activating AKT/mTOR/p70S6 kinase signaling pathway, upregulating hypoxia-inducible factor 1-alpha (HIF-1α) and vascular endothelial growth factor A (VEGF-A), and inhibiting the expression of muscle atrophy-related genes such as muscle ring finger protein-1 (MuRF1) and muscle atrophy F-box (MAFbx) (Zhu et al., 2020). In addition, in a rat model of spinal cord injury, electroacupuncture has demonstrated the potential to prevent neuromuscular junction degeneration, reduce muscle atrophy, improve neural function, and promote axonal regeneration (Zhang X. et al., 2022).
4 Basic research on sarcopenia of traditional Chinese medicine
4.1 Muscle synthesis and degradation pathways
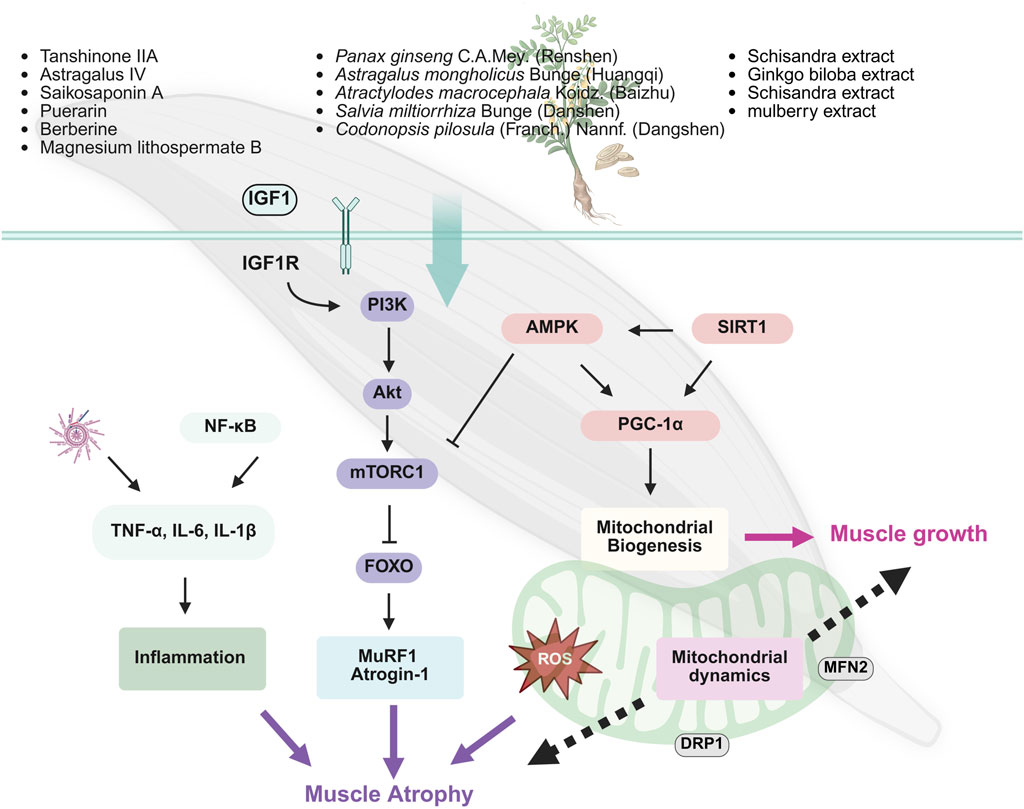
The occurrence of sarcopenia is closely related to the dysfunction of multiple signaling pathways regulating protein synthesis, particularly the imbalance between protein synthesis and degradation (Wilkinson et al., 2018b). Key mechanisms include the inhibition of PI3K/Akt/mTOR pathway and the overactivation of the adenosine 5′-monophosphate-activated protein kinase (AMPK) pathway. The inhibition of the PI3K/Akt/mTOR pathway reduces protein synthesis, while the activation of AMPK further suppresses mTOR activity, limiting muscle growth and repair and promoting muscle atrophy (Gellhaus et al., 2023; Kerr et al., 2024) (Figure 3).
TCM exhibits unique advantages in mitigating sarcopenia, especially by modulating these pathways and restoring energy balance (Ali et al., 2022; Zhou et al., 2023; Wang D. et al., 2024; Zhao et al., 2022; Qi et al., 2024). For instance, A. mongholicus Bunge [Fabaceae] (Huangqi), a widely used herbal remedy for boosting Qi and blood, is effective in elderly health management (Yao et al., 2024). Studies have demonstrated that Huangqi enhances the phosphorylation of the PI3K/Akt/mTOR signaling pathway, thereby activating muscle protein synthesis and significantly increasing the diameter and thickness of C2C12 myotubes. This provides potential therapeutic benefits for preventing and alleviating sarcopenia in older adults (Yeh et al., 2022). Codonopsis pilosula (Franch.) Nannf. (Dangshen), known for its traditional role in tonifying Qi and nourishing Yin, promotes muscle protein synthesis through the activation of the PI3K/Akt/mTORC1 pathway and inhibits the expression of muscle-specific E3 ubiquitin ligases such as MuRF1 and atrogin-1. Moreover, it enhances mitochondrial biogenesis via Sirtuin 1 (SIRT1)/PGC-1α pathway, further improving muscle function (Han and Choung, 2022; Kim et al., 2022). Puerarin, a natural bioactive compound extracted from Radix Puerariae (Gegen), activates the Akt/mTOR signaling pathway and inhibits autophagy-related pathways (e.g., LC3/p62), effectively reducing the expression of muscle atrophy markers MuRF1 and atrogin-1 in diabetic rat models. Consequently, it alleviates skeletal muscle atrophy and restores muscle strength (Yin et al., 2021). Similarly, herbal agents such as Rhizoma Dioscoreae (Shanyao), quercetin, and black ginseng extract demonstrate muscle-promoting effects by activating the Akt/mTOR axis, facilitating muscle synthesis and inhibiting muscle degradation (Lee et al., 2018; Kim et al., 2023; She et al., 2023).
4.2 Mitochondrial metabolism and oxidative stress
In skeletal muscle, mitochondria are the primary source of ROS. Excessive accumulation of ROS triggers oxidative stress, leading to cellular damage, particularly mitochondrial dysfunction, which impairs muscle health and function. Thus, maintaining ROS homeostasis is crucial for preserving mitochondrial integrity (Chen X. et al., 2023). Recent studies have shown that natural monomers, such as naringin (Zhang et al., 2020), Saikosaponin A (Huang M. et al., 2023) can mitigate age-related loss of muscle mass and strength by protecting mitochondria from oxidative damage (Zhang et al., 2020).Further mechanistic insights reveal that myricanol, a natural compound isolated from Myrica species, exerts protective effects on skeletal muscle by activating peroxiredoxin-5 (PRDX5), which reduces ROS accumulation and mitochondrial DNA damage in C2C12 myotubes (Shen et al., 2024).
Mitochondrial energy metabolism dysfunction is a key mechanism underlying sarcopenia, and SIRT1 plays a crucial regulatory role in skeletal muscle metabolism. SIRT1 directly deacetylates PGC-1α and FoxO transcription factors, modulating their nuclear translocation and activity. This regulation promotes mitochondrial biogenesis, maintains muscle mass, and prevents muscle function loss (Alamdari et al., 2012; Gellhaus et al., 2023). Numerous TCM compounds have been identified as SIRT1 activators with anti-aging and anti-sarcopenic effects. For instance, myricanol, a natural compound derived from Myrica species, enhances SIRT1 activity (Shen et al., 2019). Additionally, Fructus Lycii (gouqi) has been shown to activate SIRT1, supporting its role in combating sarcopenia and promoting muscle health (Zhou et al., 2024).
In addition to enhancing mitochondrial biogenesis through SIRT1 pathway, natural plant-derived compounds improve skeletal muscle function via complementary mechanisms. Flavonoids extracted from mulberry (Morus alba L.) leaves increase the phosphorylation of AMPK, PGC-1α. This activation promotes mitochondrial biogenesis and enhances mitochondrial function. Concurrently, these flavonoids upregulate glucose transporter type 4 (GLUT4) expression on the skeletal muscle cell membrane (m-GLUT4) and in total cellular content (T-GLUT4), improving glucose uptake and insulin sensitivity (Meng et al., 2020). Similarly, berberine and Korean cultivated wild ginseng pharmacopuncture extracts (KCWGP) regulate the expression of mitochondrial-related factors, including PGC-1α, nuclear respiratory factor 1 (NRF1), mitochondrial transcription factor A (TFAM), and SIRT1, through the AMPK signaling pathway. These mechanisms contribute to mitigating pathological changes associated with muscle degenerative diseases (Yu et al., 2018; Hwang et al., 2022).
4.3 Inflammation
Chronic low-grade inflammation is another prominent feature of sarcopenia, particularly common among older adults. The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway plays a crucial role in immune and inflammatory responses. Its hyperactivation upregulates pro-inflammatory cytokines such as IL-6 and TNF-α, promoting muscle protein degradation. Similarly, NF-κB pathway contributes significantly to inflammation. Excessive activation of this pathway stimulates the expression of muscle atrophy-related genes, such as MuRF-1 and muscle-specific ubiquitin ligase Atrogin-1, further accelerating protein breakdown (Liang et al., 2022).
Magnesium lithospermate B (MLB), a major hydrophilic component of S. miltiorrhiza Bunge (Danshen), has shown significant anti-inflammatory effects in animal studies. MLB markedly reduced the levels of TNF-α, IL-6, and their receptor TNFRI in mice fed a high-fat diet and inhibited the activation of the NF-κB pathway, thereby mitigating inflammation-induced muscle degradation (Cheng et al., 2021). Additionally, tanshinone IIA, another active compound in S. miltiorrhiza Bunge (Danshen), effectively suppressed lipopolysaccharide (LPS)-induced excessive mitophagy and reduced mtROS accumulation. This helped alleviate inflammation-induced muscle protein degradation and muscle fiber atrophy (Han et al., 2024). Furthermore, the Huanglian Wendan decoction was found to attenuate skeletal muscle damage caused by inflammation through modulating NLRP3 inflammasome pathway (Dong et al., 2021).
4.4 Other pathways
The Wnt/β-catenin signaling pathway plays a critical role in muscle generation and repair. Wnt2 promotes the accumulation ofβ-catenin, thereby activating the proliferation and differentiation of muscle progenitor cells such as satellite cells, which supports the repair and regeneration of muscle tissue. Activation of the Wnt/β-catenin pathway also delays cellular senescence and maintains muscle regenerative capacity (Lin et al., 2024).Although studies on TCM targeting the Wnt/β-catenin signaling pathway for treating sarcopenia are relatively limited, existing research highlights promising directions. For instance, polydatin has been shown to promote the proliferation and osteogenic differentiation of human bone marrow mesenchymal stem cells (hBMSCs) via the bone morphogenetic protein 2 (BMP2)-Wnt/β-catenin pathway, indicating its potential in both skeletal and muscular repair (Chen X.-J. et al., 2019). Moreover, Sijunzi decoction may improve bone quality in diabetic mice by modulating the Wnt/β-catenin pathway and maintaining redox homeostasis (Dai et al., 2024).
In East Asia, P. ginseng C.A.Mey. (Renshen) has a long history of use, traditionally believed to greatly replenish the body’s vital energy, making it suitable for long-term consumption, with purported benefits such as anti-aging and life extension (Mancuso and Santangelo, 2017). Mountain ginseng (P. ginseng C.A. Meyer) is a medicinal plant known for its immunomodulatory properties, anti-muscle damage effects, and promotion of energy metabolism. Studies have shown that mountain ginseng effectively mitigates muscle weight loss and collagen deposition induced by dexamethasone (DEXA) in rats, potentially by inhibiting FOXO3a signaling pathway and downregulating MuRF1 and muscle atrophy atrogin-1 expression (Seok et al., 2021). Another study revealed that long-term intake of Korean Red Ginseng (RGNS) significantly improved aging-related losses in muscle mass and strength. Additionally, RGNS treatment markedly delayed the shift in soleus (SOL) muscle fiber type, slowing the conversion from fast-twitch muscle fibers to slow-twitch fibers (Cho et al., 2022).
Astragaloside IV (ASIV) is the main active component of A. mongholicus Bunge [Fabaceae](Huangqi). Research indicates that ASIV can reduce muscle mass loss, muscle fiber cross-sectional area shrinkage, and atrophy in C2C12 myotubes induced by sepsis in mice. The mechanism of action may involve inhibiting the transforming growth factor-beta 1 (TGF-β1)/Smad signaling pathway, thereby reducing the expression of E3 ubiquitin ligases such as MuRF1 and atrogin-1, effectively slowing down muscle atrophy progression (Dai et al., 2023).
Osteocalcin, secreted by osteoblasts, regulates skeletal muscle function through various mechanisms. Its deficiency leads to reduced muscle mass and motor ability in mice. Ginkgo biloba extract can promote muscle regeneration by upregulating osteocalcin expression, providing potential protective effects (Wang B. Y.-H. et al., 2023). Naringin enhances skeletal muscle function by activating the Sp1-estrogen-related receptor gamma (ERRγ) transcriptional axis, promoting an increase in oxidative muscle fibers and improving aerobic metabolism (Lv et al., 2023).
Schisandra chinensis extract also reduces muscle cell apoptosis by inhibiting the expression of caspase-3 and poly (ADP-ribose) polymerase (PARP), enhancing muscle adaptability and slowing the progression of sarcopenia (Kim et al., 2018). These herbal components, through regulating multiple signaling pathways, particularly in slowing muscle degeneration and promoting muscle repair, show immense potential and provide new approaches and therapeutic directions for improving sarcopenia in the elderly.
5 Potential side effects and contraindications of traditional Chinese medicine treatments
TCM, including herbal medicine, acupuncture, and qigong, has been widely used for managing sarcopenia and related disorders. Although generally considered safe when administered by qualified practitioners, potential side effects and contraindications should not be overlooked. Herbal medicines can occasionally cause gastrointestinal disturbances, allergic reactions, hepatotoxicity or, nephrotoxicity particularly when used inappropriately or combined with other medications without professional supervision (Yang et al., 2018; Li X. et al., 2022). For instance, certain herbs containing aristolochic acid are nephrotoxic and strictly contraindicated (Ji et al., 2021). Acupuncture, while minimally invasive, may lead to minor adverse events such as bruising, dizziness, or, rarely, infection at needle sites (Huang et al., 2024). Caution is advised in patients with bleeding disorders or those receiving anticoagulant therapy. Qigong and other exercise-based interventions are generally safe but may not be suitable for individuals with severe cardiovascular or musculoskeletal conditions without medical clearance. Furthermore, patient-specific factors, such as age, comorbidities, and concurrent use of Western medications, should be considered to avoid herb-drug interactions and ensure optimal treatment outcomes. Future clinical research should include systematic safety assessments to better inform clinical guidelines and enhance patient safety.
6 Integration of traditional Chinese medicine with conventional therapies
Sarcopenia involves reductions in muscle mass, strength, and physical performance, with complex etiologies that include aging-related mitochondrial dysfunction, chronic inflammation, neuromuscular dysregulation, as well as contributions from malnutrition, physical inactivity, and various chronic diseases. Modern medical treatments primarily rely on exercise interventions (such as resistance and balance training), nutritional support (including protein and vitamin supplementation), and, to a lesser extent, pharmacological therapies (such as selective androgen receptor modulators and muscle growth factors). Although exercise and nutritional interventions are currently the cornerstone of sarcopenia management, many elderly patients suffer from comorbid chronic conditions, limited mobility, malabsorption, or poor tolerance to drug therapies. These factors result in limited efficacy of monotherapy approaches, significant individual variability, poor compliance, and suboptimal long-term outcomes.
TCM emphasizes a holistic approach and syndrome differentiation, aiming to reinforce vital energy, activate blood circulation, unblock meridians, and strengthen the spleen and kidneys. TCM treatments not only alleviate symptoms of sarcopenia but also regulate the internal environment and address the fundamental pathological states of the disease. Modern research has confirmed that herbal formulations, acupuncture, Tai Chi, and Baduanjin modulate multiple molecular pathways, including mTOR, PI3K/Akt, and AMPK signaling, promote protein synthesis, reduce muscle inflammation, improve mitochondrial function, regulate gut microbiota, and enhance neuromuscular connectivity. These interventions offer multi-targeted and multi-level therapeutic advantages.
Integrated approaches that combine standardized modern medical management with the individualized treatment principles of TCM allow for comprehensive and flexible intervention strategies tailored to different stages of sarcopenia and patient heterogeneity. While modern medicine provides evidence-based foundational treatments, TCM complements these by improving systemic regulation, delaying the aging process, and enhancing patient compliance and tolerability. Clinical studies have shown that integrative interventions significantly improve muscle mass, strength, and physical function, enhance mobility and activities of daily living, reduce the risk of falls and fractures, and may mitigate the long-term adverse outcomes of sarcopenia. Moreover, TCM therapies are generally well-tolerated with few side effects, making them particularly suitable for long-term management in elderly patients with complex health profiles.
As the aging population increases, single-modality medical approaches are insufficient to meet the diverse needs of sarcopenia management. Integrative medicine, combining TCM and Western therapies, represents a multidisciplinary and comprehensive strategy that not only holds significant practical value but also offers promising directions for future precision medicine and individualized healthcare management.
7 Discussion
This review summarized recent advances in the use of TCM for the treatment of sarcopenia, encompassing interventions such as herbal formulas, acupuncture, and traditional exercises like Tai Chi and Baduanjin. These therapies have demonstrated potential to improve muscle mass, strength, and physical performance through a multi-target and multi-pathway approach.
Mechanistically, TCM has been shown to regulate key molecular pathways—including PI3K/Akt/mTOR, AMPK, and NLRP3—involved in inflammation, oxidative stress, mitochondrial dysfunction, and energy metabolism. Additionally, TCM may modulate gut microbiota and enhance neuromuscular connectivity, offering systemic physiological support essential for managing sarcopenia. Acupuncture and traditional physical therapies also contribute by improving balance and neuromuscular coordination, which are vital for fall prevention. Despite promising evidence, most mechanistic studies focus on isolated compounds rather than holistic formulas, and the lack of integrated omics analyses limits our understanding of synergistic effects. Future research should incorporate transcriptomics, metabolomics, and proteomics to elucidate the dynamic network mechanisms of multi-component interventions.
Clinically, several studies have demonstrated that classical formulas such as Buzhong Yiqi Decoction and Sijunzi Decoction, when combined with physical training, significantly improve grip strength, skeletal muscle mass, and gait speed in elderly sarcopenia patients. Some trials also suggest reduced risk of falls and fractures. Compared with monotherapies, integrative approaches combining TCM and Western medicine offer greater adaptability, systemic regulation, and patient tolerance, making them particularly suitable for older adults with complex comorbidities or intolerance to conventional therapies.
However, several challenges hinder the broader application of TCM in clinical sarcopenia management. First, the diagnostic criteria for TCM syndromes remain inconsistent, and classification of common patterns (e.g., Qi deficiency, spleen-kidney Yang deficiency) varies across studies, undermining reproducibility and generalizability. Second, the complex composition of herbal medicines poses uncertainties regarding efficacy and safety. Variation in raw material sourcing, manufacturing techniques, and storage conditions may result in batch-to-batch inconsistency, affecting therapeutic outcomes. Furthermore, many herbal formulas lack standardized dosage guidelines and robust toxicological assessments. In elderly patients with reduced hepatic or renal function, dosage control is especially critical to avoid accumulation-related toxicity.
Moreover, polypharmacy is common in geriatric patients, and co-administration of TCM and Western drugs without rigorous evaluation may lead to herb-drug interactions (e.g., CYP450 enzyme modulation), increasing the risk of adverse effects or therapeutic antagonism. Therefore, future efforts should focus on establishing traceable quality control systems, standardizing dosages, enhancing safety monitoring, and studying pharmacokinetic interactions to ensure clinical efficacy and safety.
The nursing aspect of TCM in sarcopenia care also warrants attention. Incorporating TCM modalities—such as internal herbal administration, topical applications, acupressure, moxibustion, and traditional exercise—into nursing protocols may promote more individualized rehabilitation. Nurses play a pivotal role in patient education, treatment implementation, adherence monitoring, and functional assessments using tools such as the SARC-F or SPPB. Development of standardized nursing pathways that integrate TCM techniques could also be valuable for community-based screening and early intervention.
In summary, TCM provides a multi-dimensional, individualized, and system-oriented approach to sarcopenia management, which effectively complements conventional Western therapies. Future research should prioritize syndrome classification standardization, network pharmacology and omics-based mechanism exploration, multicenter randomized controlled trials, long-term follow-up studies, and integration into clinical nursing practice. With the continuous accumulation of high-quality evidence, TCM is well positioned to be incorporated into international sarcopenia management guidelines, thereby expanding therapeutic options and supporting personalized geriatric care.
8 Conclusion
TCM offers a promising complementary approach for managing sarcopenia through multi-target interventions that improve muscle mass, strength, and physical function. Integrating TCM with conventional therapies can address the complex and multifactorial nature of sarcopenia, particularly in elderly patients with comorbidities. Despite encouraging evidence, challenges such as the standardization of TCM diagnostic criteria, limited large-scale clinical trials, and incomplete understanding of multi-target mechanisms remain. Future research should focus on rigorous clinical studies, molecular mechanism exploration, and the development of personalized integrative treatment strategies to enhance clinical outcomes and promote healthy aging.
Author contributions
JY: Conceptualization, Writing – original draft, Writing – review and editing. SX: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We want to express our gratitude for the drawing materials provided by BioRender.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1541373/full#supplementary-material
References
Alamdari, N., Aversa, Z., Castillero, E., Gurav, A., Petkova, V., Tizio, S., et al. (2012). Resveratrol prevents dexamethasone-induced expression of the muscle atrophy-related ubiquitin ligases atrogin-1 and MuRF1 in cultured myotubes through a SIRT1-dependent mechanism. Biochem. Biophys. Res. Commun. 417, 528–533. doi:10.1016/j.bbrc.2011.11.154
Ali, S., Ahmad, K., Shaikh, S., Lim, J. H., Chun, H. J., Ahmad, S. S., et al. (2022). Identification and evaluation of traditional Chinese medicine natural compounds as potential myostatin inhibitors: an in silico approach. Molecules 27, 4303. doi:10.3390/molecules27134303
Amaral, J. F., Alvim, F. C., Castro, E. A., Doimo, L. A., Silva, M. V., and Novo Júnior, J. M. (2014). Influence of aging on isometric muscle strength, fat-free mass and electromyographic signal power of the upper and lower limbs in women. Braz. J. Phys. Ther. 18, 183–190. doi:10.1590/s1413-35552012005000145
Antuña, E., Potes, Y., Baena-Huerta, F. J., Cachán-Vega, C., Menéndez-Coto, N., Álvarez Darriba, E., et al. (2024). NLRP3 contributes to sarcopenia associated to dependency recapitulating inflammatory-associated muscle degeneration. Int. J. Mol. Sci. 25, 1439. doi:10.3390/ijms25031439
Bano, G., Trevisan, C., Carraro, S., Solmi, M., Luchini, C., Stubbs, B., et al. (2017). Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas 96, 10–15. doi:10.1016/j.maturitas.2016.11.006
Bårdstu, H. B., Andersen, V., Fimland, M. S., Aasdahl, L., Raastad, T., Cumming, K. T., et al. (2020). Effectiveness of a resistance training program on physical function, muscle strength, and body composition in community-dwelling older adults receiving home care: a cluster-randomized controlled trial. Eur. Rev. Aging Phys. Act. Off. J. Eur. Group Res. Elder. Phys. Act. 17, 11. doi:10.1186/s11556-020-00243-9
Beaudart, C., Demonceau, C., Reginster, J.-Y., Locquet, M., Cesari, M., Cruz Jentoft, A. J., et al. (2023). Sarcopenia and health-related quality of life: a systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 14, 1228–1243. doi:10.1002/jcsm.13243
Bressa, C., Bailén-Andrino, M., Pérez-Santiago, J., González-Soltero, R., Pérez, M., Montalvo-Lominchar, M. G., et al. (2017). Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 12, e0171352. doi:10.1371/journal.pone.0171352
Brooks, M. J., Hajira, A., Mohamed, J. S., and Alway, S. E. (2018). Voluntary wheel running increases satellite cell abundance and improves recovery from disuse in gastrocnemius muscles from mice. J. Appl. Physiol. Bethesda Md 1985 124, 1616–1628. doi:10.1152/japplphysiol.00451.2017
Busch, K., Kny, M., Huang, N., Klassert, T. E., Stock, M., Hahn, A., et al. (2021). Inhibition of the NLRP3/IL-1β axis protects against sepsis-induced cardiomyopathy. J. Cachexia Sarcopenia Muscle 12, 1653–1668. doi:10.1002/jcsm.12763
Chen, C., and Wen, C. (2021). Effect of modified Buzhongyiqi decoction on inflammatory factors in elderly patients with sarcopenia. Med. Sci. China 11, 13–16.
Chen, J.-M., Yang, T.-T., Cheng, T.-S., Hsiao, T.-F., Chang, P. M.-H., Leu, J.-Y., et al. (2019a). Modified Sijunzi decoction can alleviate cisplatin-induced toxicity and prolong the survival time of cachectic mice by recovering muscle atrophy. J. Ethnopharmacol. 233, 47–55. doi:10.1016/j.jep.2018.12.035
Chen, L.-H., Chang, S.-S., Chang, H.-Y., Wu, C.-H., Pan, C.-H., Chang, C.-C., et al. (2022). Probiotic supplementation attenuates age-related sarcopenia via the gut-muscle axis in SAMP8 mice. J. Cachexia Sarcopenia Muscle 13, 515–531. doi:10.1002/jcsm.12849
Chen, W.-J., Livneh, H., Li, H.-H., Wang, Y.-H., Lu, M.-C., Tsai, T.-Y., et al. (2023a). Use of Chinese herbal medicine was related to lower risk of osteoporotic fracture in sarcopenia patients: evidence from population-based health claims. Int. J. Gen. Med. 16, 3345–3354. doi:10.2147/IJGM.S416705
Chen, X. (2022). Observation on the effect of Buzhong Yiqi Decoction combined with Baduanjin exercise and intensive nutritional support in the treatment of elderly sarcopenia. J. Pract. Tradit. Chin. Med. 38, 179–180.
Chen, X., Ji, Y., Liu, R., Zhu, X., Wang, K., Yang, X., et al. (2023b). Mitochondrial dysfunction: roles in skeletal muscle atrophy. J. Transl. Med. 21, 503. doi:10.1186/s12967-023-04369-z
Chen, X.-J., Shen, Y.-S., He, M.-C., Yang, F., Yang, P., Pang, F.-X., et al. (2019b). Polydatin promotes the osteogenic differentiation of human bone mesenchymal stem cells by activating the BMP2-Wnt/β-catenin signaling pathway. Biomed. Pharmacother. Biomedecine Pharmacother. 112, 108746. doi:10.1016/j.biopha.2019.108746
Cheng, T.-L., Lin, Z.-Y., Liao, K.-Y., Huang, W.-C., Jhuo, C.-F., Pan, P.-H., et al. (2021). Magnesium lithospermate B attenuates high-fat diet-induced muscle atrophy in C57bl/6J mice. Nutrients 14, 104. doi:10.3390/nu14010104
Cho, D.-E., Choi, G.-M., Lee, Y.-S., Hong, J.-P., Yeom, M., Lee, B., et al. (2022). Long-term administration of red ginseng non-saponin fraction rescues the loss of skeletal muscle mass and strength associated with aging in mice. J. Ginseng Res. 46, 657–665. doi:10.1016/j.jgr.2021.12.001
Cruz-Jentoft, A. J., Baeyens, J. P., Bauer, J. M., Boirie, Y., Cederholm, T., Landi, F., et al. (2010). Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older People. Age Ageing 39, 412–423. doi:10.1093/ageing/afq034
Cruz-Jentoft, A. J., Bahat, G., Bauer, J., Boirie, Y., Bruyère, O., Cederholm, T., et al. (2019). Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48, 16–31. doi:10.1093/ageing/afy169
Cruz-Jentoft, A. J., and Sayer, A. A. (2019). Sarcopenia. Lancet lond. Engl. 393, 2636–2646. doi:10.1016/S0140-6736(19)31138-9
Cui, H., Wang, C., Wu, J., and Liu, Y. (2023). Chinese expert consensus on prevention and control of sarcopenia in the elderly. Chin. J. Geriatr. 42, 144–153. doi:10.3760/cma.j.issn.0254-9026.2023.02.002
Dai, H., Zheng, Y., Chen, R., Wang, Y., Zhong, Y., Zhou, C., et al. (2023). Astragaloside IV alleviates sepsis-induced muscle atrophy by inhibiting the TGF-β1/Smad signaling pathway. Int. Immunopharmacol. 115, 109640. doi:10.1016/j.intimp.2022.109640
Dai, X., Liu, Y., Liu, T., Zhang, Y., Wang, S., Xu, T., et al. (2024). SiJunZi decoction ameliorates bone quality and redox homeostasis and regulates advanced glycation end products/receptor for advanced glycation end products and WNT/β-catenin signaling pathways in diabetic mice. J. Ethnopharmacol. 319, 117167. doi:10.1016/j.jep.2023.117167
Deschenes, M. R. (2004). Effects of aging on muscle fibre type and size. Sports Med. Auckl. N. Z. 34, 809–824. doi:10.2165/00007256-200434120-00002
Ding, P., Zhou, Y., Zhi, L., Yang, M., Long, K., and Zhang, S. (2024). Case report: antisynthetase syndrome with positive anti-PL7/SSA/RO52 antibodies. Heliyon 10, e36880. doi:10.1016/j.heliyon.2024.e36880
Distefano, G., and Goodpaster, B. H. (2018). Effects of exercise and aging on skeletal muscle. Cold Spring Harb. Perspect. Med. 8, a029785. doi:10.1101/cshperspect.a029785
Dong, W.-R., Li, H., Li, Y.-F., Wang, N., Ma, B.-Y., Lu, G.-L., et al. (2021). Mechanism of Huanglian Wendan Decoction in improving impaired glucose tolerance based on skeletal muscle NLRP3/caspase-1/IL-1β, IL-18 pathway. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Medica 46, 4480–4487. doi:10.19540/j.cnki.cjcmm.20210621.401
Dong, Y.-X., Li, T.-H., Wang, S.-S., Hu, Y.-H., Liu, Y., Zhang, F., et al. (2024). Bu zhong Yiqi Decoction ameliorates mild cognitive impairment by improving mitochondrial oxidative stress damage via the SIRT3/MnSOD/OGG1 pathway. J. Ethnopharmacol. 331, 118237. doi:10.1016/j.jep.2024.118237
Doyle, A., Zhang, G., Fattah, E. A. A., Eissa, N. T., and Li, Y.-P. (2011). Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin-proteasome and autophagy-lysosome pathways. FASEB J. 25, 99–110. doi:10.1096/fj.10-164152
Dulac, M., Leduc-Gaudet, J.-P., Cefis, M., Ayoub, M.-B., Reynaud, O., Shams, A., et al. (2021). Regulation of muscle and mitochondrial health by the mitochondrial fission protein Drp1 in aged mice. J. Physiol. 599, 4045–4063. doi:10.1113/JP281752
Eckburg, P. B., Bik, E. M., Bernstein, C. N., Purdom, E., Dethlefsen, L., Sargent, M., et al. (2005). Diversity of the human intestinal microbial flora. Science 308, 1635–1638. doi:10.1126/science.1110591
Erttmann, S. F., Swacha, P., Aung, K. M., Brindefalk, B., Jiang, H., Härtlova, A., et al. (2022). The gut microbiota prime systemic antiviral immunity via the cGAS-STING-IFN-I axis. Immunity 55, 847–861.e10. doi:10.1016/j.immuni.2022.04.006
Fang, P., Zhou, J., Xiao, X., Yang, Y., Luan, S., Liang, Z., et al. (2023). The prognostic value of sarcopenia in oesophageal cancer: a systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 14, 3–16. doi:10.1002/jcsm.13126
Favaro, G., Romanello, V., Varanita, T., Desbats, M. A., Morbidoni, V., Tezze, C., et al. (2019). DRP1-mediated mitochondrial shape controls calcium homeostasis and muscle mass. Nat. Commun. 10, 2576. doi:10.1038/s41467-019-10226-9
Fu, Y., Tao, L., Wang, X., Wang, B., Qin, W., and Song, L. (2024). PGC-1α participates in regulating mitochondrial function in aged sarcopenia through effects on the Sestrin2-mediated mTORC1 pathway. Exp. Gerontol. 190, 112428. doi:10.1016/j.exger.2024.112428
Gellhaus, B., Böker, K. O., Schilling, A. F., and Saul, D. (2023). Therapeutic consequences of targeting the IGF-1/PI3K/AKT/FOXO3 Axis in sarcopenia: a narrative review. Cells 12, 2787. doi:10.3390/cells12242787
Gicquel, T., Marchiano, F., Reyes-Castellanos, G., Audebert, S., Camoin, L., Habermann, B. H., et al. (2024). Integrative study of skeletal muscle mitochondrial dysfunction in a murine pancreatic cancer-induced cachexia model. eLife 13, RP93312. doi:10.7554/eLife.93312
Gonzalez-Freire, M., Cabo, R. de, Studenski, S. A., and Ferrucci, L. (2014). The neuromuscular junction: aging at the crossroad between nerves and muscle. Front. Aging Neurosci. 6, 208. doi:10.3389/fnagi.2014.00208
Greising, S. M., Corona, B. T., McGann, C., Frankum, J. K., and Warren, G. L. (2019). Therapeutic approaches for volumetric muscle loss injury: a systematic review and meta-analysis. Tissue Eng. Part B Rev. 25, 510–525. doi:10.1089/ten.TEB.2019.0207
Han, D., Chen, Y.-B., Zhao, K., Li, H.-Z., Chen, X.-Y., Zhu, G.-Z., et al. (2024). Tanshinone IIA alleviates inflammation-induced skeletal muscle atrophy by regulating mitochondrial dysfunction. Arch. Biochem. Biophys. 762, 110215. doi:10.1016/j.abb.2024.110215
Han, M. J., and Choung, S.-Y. (2022). Codonopsis lanceolata ameliorates sarcopenic obesity via recovering PI3K/Akt pathway and lipid metabolism in skeletal muscle. Phytomedicine Int. J. Phytother. Phytopharm. 96, 153877. doi:10.1016/j.phymed.2021.153877
Hao, M. J., and Hu, J. (2021a). Clinical effect of trimetazidine combined with Bazhen decoction in the treatment of elderly patients with chronic heart failure and sarcopenia. Inn. Mong. J. Chin. Med. 40, 57–58. doi:10.16040/j.cnki.cn15-1101.201.03.036
Hao, M. J., and Hu, J. (2021b). Clinical effect of trimetazidine combined with Bazhen Decoction in the treatment of elderly patients with chronic heart failure complicated by sarcopenia. Inn. Mong. J. Tradit. Chin. Med. 3, 57–58. doi:10.16040/j.cnki.cn15-1101.2021.03.036
Hood, D. A., Memme, J. M., Oliveira, A. N., and Triolo, M. (2019). Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annu. Rev. Physiol. 81, 19–41. doi:10.1146/annurev-physiol-020518-114310
Huang, C.-C., Kotha, P., Tu, C.-H., Huang, M.-C., Chen, Y.-H., and Lin, J.-G. (2024). Acupuncture: a review of the safety and adverse events and the strategy of potential risk prevention. Am. J. Chin. Med. 52, 1555–1587. doi:10.1142/S0192415X24500617
Huang, C.-Y., Mayer, P. K., Wu, M.-Y., Liu, D.-H., Wu, P.-C., and Yen, H.-R. (2022). The effect of Tai Chi in elderly individuals with sarcopenia and frailty: a systematic review and meta-analysis of randomized controlled trials. Ageing Res. Rev. 82, 101747. doi:10.1016/j.arr.2022.101747
Huang, D., Ke, X., Jiang, C., Song, W., Feng, J., Zhou, H., et al. (2023a). Effects of 12 weeks of Tai Chi on neuromuscular responses and postural control in elderly patients with sarcopenia: a randomized controlled trial. Front. Neurol. 14, 1167957. doi:10.3389/fneur.2023.1167957
Huang, M., Yan, Y., Deng, Z., Zhou, L., She, M., Yang, Y., et al. (2023b). Saikosaponin A and D attenuate skeletal muscle atrophy in chronic kidney disease by reducing oxidative stress through activation of PI3K/AKT/Nrf2 pathway. Phytomedicine 114, 154766. doi:10.1016/j.phymed.2023.154766
Hwang, J. H., Kang, S. Y., and Jung, H. W. (2022). Effects of American wild ginseng and Korean cultivated wild ginseng pharmacopuncture extracts on the regulation of C2C12 myoblasts differentiation through AMPK and PI3K/Akt/mTOR signaling pathway. Mol. Med. Rep. 25, 192. doi:10.3892/mmr.2022.12708
Ji, H., Hu, J., Zhang, G., Song, J., Zhou, X., and Guo, D. (2021). Aristolochic acid nephropathy: a scientometric analysis of literature published from 1971 to 2019. Med. Baltim. 100, e26510. doi:10.1097/MD.0000000000026510
Jiang, H., Fen, J., Shen, H., and Chen, Y. (2024). To evaluate the effect of Shenlingbaizhu powder combined with exercise intervention on patients with diabetes mellitus complicated with sarcopenia. Hebei Med. 46, 1647–1651.
Jimenez-Gutierrez, G. E., Martínez-Gómez, L. E., Martínez-Armenta, C., Pineda, C., Martínez-Nava, G. A., and Lopez-Reyes, A. (2022). Molecular mechanisms of inflammation in sarcopenia: diagnosis and therapeutic update. Cells 11, 2359. doi:10.3390/cells11152359
Jin, J., Yang, Z., Liu, H., Guo, M., Chen, B., Zhu, H., et al. (2023). Effects of acupuncture on the miR-146a-mediated IRAK1/TRAF6/NF-κB signaling pathway in rats with sarcopenia induced by D-galactose. Ann. Transl. Med. 11, 47. doi:10.21037/atm-22-6082
Kakehi, S., Wakabayashi, H., Inuma, H., Inose, T., Shioya, M., Aoyama, Y., et al. (2022). Rehabilitation nutrition and exercise therapy for sarcopenia. World J. Mens. Health 40, 1–10. doi:10.5534/wjmh.200190
Kang, M., Kang, M., Yoo, J., Lee, J., Lee, S., Yun, B., et al. (2024). Dietary supplementation with Lacticaseibacillus rhamnosus IDCC3201 alleviates sarcopenia by modulating the gut microbiota and metabolites in dexamethasone-induced models. Food Funct. 15, 4936–4953. doi:10.1039/d3fo05420a
Kasim, N. F., Veldhuijzen van Zanten, J., and Aldred, S. (2020). Tai Chi is an effective form of exercise to reduce markers of frailty in older age. Exp. Gerontol. 135, 110925. doi:10.1016/j.exger.2020.110925
Kerr, H. L., Krumm, K., Anderson, B., Christiani, A., Strait, L., Li, T., et al. (2024). Mouse sarcopenia model reveals sex- and age-specific differences in phenotypic and molecular characteristics. J. Clin. Invest. 134, e172890. doi:10.1172/JCI172890
Kim, J.-W., Shin, S.-K., and Kwon, E.-Y. (2023). Luteolin protects against obese sarcopenia in mice with high-fat diet-induced obesity by ameliorating inflammation and protein degradation in muscles. Mol. Nutr. Food Res. 67, e2200729. doi:10.1002/mnfr.202200729
Kim, K.-Y., Ku, S.-K., Lee, K.-W., Song, C.-H., and An, W. G. (2018). Muscle-protective effects of Schisandrae Fructus extracts in old mice after chronic forced exercise. J. Ethnopharmacol. 212, 175–187. doi:10.1016/j.jep.2017.10.022
Kim, T.-Y., Park, K.-T., and Choung, S.-Y. (2022). Codonopsis lanceolata and its active component Tangshenoside I ameliorate skeletal muscle atrophy via regulating the PI3K/Akt and SIRT1/PGC-1α pathways. Phytomedicine Int. J. Phytother. Phytopharm. 100, 154058. doi:10.1016/j.phymed.2022.154058
Kiss, N., Prado, C. M., Daly, R. M., Denehy, L., Edbrooke, L., Baguley, B. J., et al. (2023). Low muscle mass, malnutrition, sarcopenia, and associations with survival in adults with cancer in the UK Biobank cohort. J. Cachexia Sarcopenia Muscle 14, 1775–1788. doi:10.1002/jcsm.13256
Lahiri, S., Kim, H., Garcia-Perez, I., Reza, M. M., Martin, K. A., Kundu, P., et al. (2019). The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 11, eaan5662. doi:10.1126/scitranslmed.aan5662
Lee, E.-J., Charles, J. F., Sinha, I., and Neppl, R. L. (2024). Loss of HNRNPU in skeletal muscle increases intramuscular infiltration of Ly6C positive cells, leading to muscle atrophy through activation of NF-κB signaling. Adv. Biol. 8, e2400152. doi:10.1002/adbi.202400152
Lee, J.-Y., Shin, S.-K., Bae, H. R., Ji, Y., Park, H.-J., and Kwon, E.-Y. (2023). The animal protein hydrolysate attenuates sarcopenia via the muscle-gut axis in aged mice. Biomed. Pharmacother. Biomedecine Pharmacother. 167, 115604. doi:10.1016/j.biopha.2023.115604
Lee, S.-Y., Go, G.-Y., Vuong, T. A., Kim, J. W., Lee, S., Jo, A., et al. (2018). Black ginseng activates Akt signaling, thereby enhancing myoblast differentiation and myotube growth. J. Ginseng Res. 42, 116–121. doi:10.1016/j.jgr.2017.08.009
Levolger, S., van Vugt, J. L. A., de Bruin, R. W. F., and Ijzermans, J. N. M. (2015). Systematic review of sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. Br. J. Surg. 102, 1448–1458. doi:10.1002/bjs.9893
Li, C., Yang, J., Chu, L., Tian, J., Xiao, J., Huang, Y., et al. (2024a). The function of Bazhen decoction in rescuing progeroid cell senescence via facilitating G-quadruplex resolving and telomere elongation. J. Ethnopharmacol. 323, 117694. doi:10.1016/j.jep.2023.117694
Li, C., Yu, K., Shyh-Chang, N., Li, G., Jiang, L., Yu, S., et al. (2019a). Circulating factors associated with sarcopenia during ageing and after intensive lifestyle intervention. J. Cachexia Sarcopenia Muscle 10, 586–600. doi:10.1002/jcsm.12417
Li, G., Huang, P., Cui, S.-S., Tan, Y.-Y., He, Y.-C., Shen, X., et al. (2022a). Mechanisms of motor symptom improvement by long-term Tai Chi training in Parkinson’s disease patients. Transl. Neurodegener. 11, 6. doi:10.1186/s40035-022-00280-7
Li, H., Zhou, R., Guo, Y., Gao, M., Xu, G., Wen, J., et al. (2024b). Acupuncture pretreatment reduces skeletal muscle inflammatory response in EIMD rats by activating cGAS-STING-NF-κB signaling pathway. Altern. Ther. Health Med. 30, 214–221.
Li, J., Wang, Z., Li, C., Song, Y., Wang, Y., Bo, H., et al. (2022b). Impact of exercise and aging on mitochondrial homeostasis in skeletal muscle: roles of ROS and epigenetics. Cells 11, 2086. doi:10.3390/cells11132086
Li, L., Yao, H., Wang, J., Li, Y., and Wang, Q. (2019b). The role of Chinese medicine in health maintenance and disease prevention: application of constitution theory. Am. J. Chin. Med. 47, 495–506. doi:10.1142/S0192415X19500253
Li, S., Chen, X., Shi, H., Yi, M., Xiong, B., and Li, T. (2025a). Tailoring traditional Chinese medicine in cancer therapy. Mol. Cancer 24, 27. doi:10.1186/s12943-024-02213-6
Li, X., Tang, J., and Mao, Y. (2022c). Incidence and risk factors of drug-induced liver injury. Liver Int. Off. J. Int. Assoc. Study Liver 42, 1999–2014. doi:10.1111/liv.15262
Li, Y., Liu, Z., Yan, H., Zhou, T., Zheng, L., Wen, F., et al. (2025b). Polygonatum sibiricum polysaccharide ameliorates skeletal muscle aging and mitochondrial dysfunction via PI3K/Akt/mTOR signaling pathway. Phytomedicine Int. J. Phytother. Phytopharm. 136, 156316. doi:10.1016/j.phymed.2024.156316
Liang, Z., Zhang, T., Liu, H., Li, Z., Peng, L., Wang, C., et al. (2022). Inflammaging: the ground for sarcopenia? Exp. Gerontol. 168, 111931. doi:10.1016/j.exger.2022.111931
Lin, J., Duan, J., Wang, Q., Xu, S., Zhou, S., and Yao, K. (2022). Mitochondrial dynamics and mitophagy in cardiometabolic disease. Front. Cardiovasc. Med. 9, 917135. doi:10.3389/fcvm.2022.917135
Lin, W., Chow, S. K. H., Cui, C., Liu, C., Wang, Q., Chai, S., et al. (2024). Wnt/β-catenin signaling pathway as an important mediator in muscle and bone crosstalk: a systematic review. J. Orthop. Transl. 47, 63–73. doi:10.1016/j.jot.2024.06.003
Ling, F., and Huang, F. (2022). Clinical observation of electroacupuncture combined with rehabilitation exercise training for elderly patients with sarcopenia. Chin. Folk. Ther. 30, 52–55. doi:10.19621/j.cnki.11-3555/r.2022.0619
Liu, C., Cheung, W.-H., Li, J., Chow, S. K.-H., Yu, J., Wong, S. H., et al. (2021). Understanding the gut microbiota and sarcopenia: a systematic review. J. Cachexia Sarcopenia Muscle 12, 1393–1407. doi:10.1002/jcsm.12784
Liu, H., Wang, K., Shang, T., Cai, Z., Lu, C., Shen, M., et al. (2025). Astragaloside IV improves muscle atrophy by modulating the activity of UPS and ALP via suppressing oxidative stress and inflammation in denervated mice. Mol. Neurobiol. 62, 4689–4704. doi:10.1007/s12035-024-04590-x
Liu, Z., Liu, Y., Xu, H., He, L., Chen, Y., Fu, L., et al. (2017). Effect of electroacupuncture on urinary leakage among women with stress urinary incontinence: a randomized clinical trial. JAMA 317, 2493–2501. doi:10.1001/jama.2017.7220
Luo, X., Zhao, M., Zhang, Y., and Zhang, Y. (2023). Effects of baduanjin exercise on blood glucose, depression and anxiety among patients with type II diabetes and emotional disorders: a meta-analysis. Complement. Ther. Clin. Pract. 50, 101702. doi:10.1016/j.ctcp.2022.101702
Lv, C., and Wang, (2021). Clinical observation of modified Buzhong Yiqi Decoction as an adjuvant treatment for elderly patients with sarcopenia. North. Pharm. 11, 29–31.
Lv, Z., Meng, J., Yao, S., Xiao, F., Li, S., Shi, H., et al. (2023). Naringenin improves muscle endurance via activation of the Sp1-ERRγ transcriptional axis. Cell Rep. 42, 113288. doi:10.1016/j.celrep.2023.113288
Ma, Q. (2023). Pharmacological inhibition of the NLRP3 inflammasome: structure, molecular activation, and inhibitor-NLRP3 interaction. Pharmacol. Rev. 75, 487–520. doi:10.1124/pharmrev.122.000629
Ma, S.-F., Lv, W.-Y., Zhu, Q.-Y., Li, H.-J., Li, J.-J., Shi, Q., et al. (2023). Electroacupuncture at acupoints of yangming meridians for sarcopenia: a randomized controlled trial. Zhongguo Zhen Jiu Chin. Acupunct. Moxibustion 43, 1114–1117. doi:10.13703/j.0255-2930.20221202-k0001
Mancin, L., Wu, G. D., and Paoli, A. (2023). Gut microbiota-bile acid-skeletal muscle axis. Trends Microbiol. 31, 254–269. doi:10.1016/j.tim.2022.10.003
Mancuso, C., and Santangelo, R. (2017). Panax ginseng and Panax quinquefolius: from pharmacology to toxicology. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 107, 362–372. doi:10.1016/j.fct.2017.07.019
Martin, A., Gallot, Y. S., and Freyssenet, D. (2023). Molecular mechanisms of cancer cachexia-related loss of skeletal muscle mass: data analysis from preclinical and clinical studies. J. Cachexia Sarcopenia Muscle 14, 1150–1167. doi:10.1002/jcsm.13073
Marttinen, M., Ala-Jaakkola, R., Laitila, A., and Lehtinen, M. J. (2020). Gut microbiota, probiotics and physical performance in athletes and physically active individuals. Nutrients 12, 2936. doi:10.3390/nu12102936
Marzetti, E., Calvani, R., Cesari, M., Buford, T. W., Lorenzi, M., Behnke, B. J., et al. (2013). Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int. J. Biochem. Cell Biol. 45, 2288–2301. doi:10.1016/j.biocel.2013.06.024
Marzetti, E., Lozanoska-Ochser, B., Calvani, R., Landi, F., Coelho-Júnior, H. J., and Picca, A. (2024). Restoring mitochondrial function and muscle satellite cell signaling: remedies against age-related sarcopenia. Biomolecules 14, 415. doi:10.3390/biom14040415
Meng, Q., Qi, X., Fu, Y., Chen, Q., Cheng, P., Yu, X., et al. (2020). Flavonoids extracted from mulberry (Morus alba L.) leaf improve skeletal muscle mitochondrial function by activating AMPK in type 2 diabetes. J. Ethnopharmacol. 248, 112326. doi:10.1016/j.jep.2019.112326
Migliavacca, E., Tay, S. K. H., Patel, H. P., Sonntag, T., Civiletto, G., McFarlane, C., et al. (2019). Mitochondrial oxidative capacity and NAD+ biosynthesis are reduced in human sarcopenia across ethnicities. Nat. Commun. 10, 5808. doi:10.1038/s41467-019-13694-1
Moreira-Pais, A., Ferreira, R., Oliveira, P. A., and Duarte, J. A. (2021). Sarcopenia versus cancer cachexia: the muscle wasting continuum in healthy and diseased aging. Biogerontology 22, 459–477. doi:10.1007/s10522-021-09932-z
Nie, X., Wang, C., Zhang, H., Liu, Q., Hou, L., Deng, Y., et al. (2024). The original scores of traditional Chinese medicine constitutions are risk and diagnostic factors in middle-aged and older adults with sarcopenia. Aging Med. 7, 334–340. doi:10.1002/agm2.12328
Niu, K., Liu, Y.-L., Yang, F., Wang, Y., Zhou, X.-Z., and Qu, Q. (2022). Efficacy of traditional Chinese exercise for sarcopenia: a systematic review and meta-analysis of randomized controlled trials. Front. Neurosci. 16, 1094054. doi:10.3389/fnins.2022.1094054
Peng, N., Miao, D., Wang, H., and Yuan, L. (2024). Application effect of Baduanjin combined with blood flow restriction training on senile sarcopenia. Chin. J. Health Care Med. 26, 332–335.
Phu, S., Boersma, D., and Duque, G. (2015). Exercise and sarcopenia. J. Clin. Densitom. 18, 488–492. doi:10.1016/j.jocd.2015.04.011
Picca, A., and Calvani, R. (2021). Molecular mechanism and pathogenesis of sarcopenia: an overview. Int. J. Mol. Sci. 22, 3032. doi:10.3390/ijms22063032
Przewłócka, K., Folwarski, M., Kaźmierczak-Siedlecka, K., Skonieczna-Żydecka, K., and Kaczor, J. J. (2020). Gut-muscle Axis exists and may affect skeletal muscle adaptation to training. Nutrients 12, 1451. doi:10.3390/nu12051451
Qi, H., Gao, Y., Zhang, Z., Zhang, X., Tian, D., Jiang, Y., et al. (2024). HouShiHeiSan attenuates sarcopenia in middle cerebral artery occlusion (MCAO) rats. J. Ethnopharmacol. 337, 118917. doi:10.1016/j.jep.2024.118917
Reid, A. L., and Alexander, M. S. (2021). The interplay of mitophagy and inflammation in duchenne muscular dystrophy. Life Basel Switz. 11, 648. doi:10.3390/life11070648
Schiaffino, S., Dyar, K. A., Ciciliot, S., Blaauw, B., and Sandri, M. (2013). Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 280, 4294–4314. doi:10.1111/febs.12253
Sebastián, D., Sorianello, E., Segalés, J., Irazoki, A., Ruiz-Bonilla, V., Sala, D., et al. (2016). Mfn2 deficiency links age-related sarcopenia and impaired autophagy to activation of an adaptive mitophagy pathway. EMBO J. 35, 1677–1693. doi:10.15252/embj.201593084
Seok, Y. M., Yoo, J.-M., Nam, Y., Kim, J., Kim, J. S., Son, J.-H., et al. (2021). Mountain ginseng inhibits skeletal muscle atrophy by decreasing muscle RING finger protein-1 and atrogin1 through forkhead box O3 in L6 myotubes. J. Ethnopharmacol. 270, 113557. doi:10.1016/j.jep.2020.113557
She, M., Huang, M., Zhang, J., Yan, Y., Zhou, L., Zhang, M., et al. (2023). Astragulus embranaceus (Fisch.) Bge-Dioscorea opposita Thunb herb pair ameliorates sarcopenia in senile type 2 diabetes mellitus through Rab5a/mTOR-mediated mitochondrial dysfunction. J. Ethnopharmacol. 317, 116737. doi:10.1016/j.jep.2023.116737
Shen, S., Liao, Q., Liu, J., Pan, R., Lee, S. M.-Y., and Lin, L. (2019). Myricanol rescues dexamethasone-induced muscle dysfunction via a sirtuin 1-dependent mechanism. J. Cachexia Sarcopenia Muscle 10, 429–444. doi:10.1002/jcsm.12393
Shen, S., Liao, Q., Lyu, P., Wang, J., and Lin, L. (2024). Myricanol prevents aging-related sarcopenia by rescuing mitochondrial dysfunction via targeting peroxiredoxin 5. MedComm 5, e566. doi:10.1002/mco2.566
Shi, M., Shi, X., Wang, D., and Chen, Y. (2018). Relationship between traditional Chinese medicine constitution types and chronic diseases in the elderly with low body weight. China Med. Guide 15, 87–89.
Siu, P. M., Yu, A. P., Tam, B. T., Chin, E. C., Yu, D. S., Chung, K.-F., et al. (2021). Effects of Tai chi or exercise on sleep in older adults with insomnia: a randomized clinical trial. JAMA Netw. Open 4, e2037199. doi:10.1001/jamanetworkopen.2020.37199
Soares Mendes Damasceno, G., Teixeira, T. H. M. M., Souza, V. C. de, Neiva, T. S., Prudente Pereira, K., Teles Landim, M. de F., et al. (2019). Acupuncture treatment in elderly People with sarcopenia: effects on the strength and inflammatory mediators. J. Aging Res. 2019, 8483576. doi:10.1155/2019/8483576
Sun, L., Si, M., Liu, X., Choi, J. M., Wang, Y., Thomas, S. S., et al. (2018). Long-noncoding RNA Atrolnc-1 promotes muscle wasting in mice with chronic kidney disease. J. Cachexia Sarcopenia Muscle 9, 962–974. doi:10.1002/jcsm.12321
Takaoka, Y., Ohta, M., Ito, A., Takamatsu, K., Sugano, A., Funakoshi, K., et al. (2007). Electroacupuncture suppresses myostatin gene expression: cell proliferative reaction in mouse skeletal muscle. Physiol. Genomics 30, 102–110. doi:10.1152/physiolgenomics.00057.2006
Tieland, M., Trouwborst, I., and Clark, B. C. (2018). Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle 9, 3–19. doi:10.1002/jcsm.12238
Tu, Y. (2016). Artemisinin-A gift from traditional Chinese medicine to the world (nobel lecture). Angew. Chem. Int. Ed. Engl. 55, 10210–10226. doi:10.1002/anie.201601967
van Tongeren, S. P., Slaets, J. P. J., Harmsen, H. J. M., and Welling, G. W. (2005). Fecal microbiota composition and frailty. Appl. Environ. Microbiol. 71, 6438–6442. doi:10.1128/AEM.71.10.6438-6442.2005
Wang, B. Y.-H., Chen, Y.-F., Hsiao, A. W.-T., Chen, W.-J., Lee, C.-W., and Lee, O. K.-S. (2023a). Ginkgolide B facilitates muscle regeneration via rejuvenating osteocalcin-mediated bone-to-muscle modulation in aged mice. J. Cachexia Sarcopenia Muscle 14, 1349–1364. doi:10.1002/jcsm.13228
Wang, C., Zhang, H., Nie, X., Ding, F., Liu, Q., Hou, L., et al. (2024a). Traditional Chinese medicine constitution and sarcopenia: a cross-sectional study. Front. Public Health 12, 1368933. doi:10.3389/fpubh.2024.1368933
Wang, D., Qi, W., Mao, X., Zhang, Y., Miao, Z., Zhu, C., et al. (2024b). Gui Qi Zhuang Jin Decoction ameliorates mitochondrial dysfunction in sarcopenia mice via AMPK/PGC-1α/Nrf2 axis revealed by a metabolomics approach. Phytomedicine 133, 155908. doi:10.1016/j.phymed.2024.155908
Wang, L., Tian, L., Niu, Q., and Liu, W. (2022a). To explore the effects of 11 kinds of exercise on improving physical function in elderly patients with sarcopenia by network Meta-analysis. Chin. J. Nurs. 57, 2652–2660.
Wang, Q., Bai, J., Liu, Z., Liu, F., and Li, G. (2022b). To observe the effect of modified Sijunzi decoction combined with elastic band training in the treatment of senile sarcopenia with spleen-stomach qi deficiency. China Med. Guide 28, 39–44. doi:10.13862/j.cn43-1446/r.2022.04.010
Wang, Y., Yang, J.-W., Yan, S.-Y., Lu, Y., Han, J.-G., Pei, W., et al. (2023b). Electroacupuncture vs sham electroacupuncture in the treatment of postoperative ileus after laparoscopic surgery for colorectal cancer: a multicenter, randomized clinical trial. JAMA Surg. 158, 20–27. doi:10.1001/jamasurg.2022.5674
Wilkinson, D. J., Piasecki, M., and Atherton, P. J. (2018a). The age-related loss of skeletal muscle mass and function: measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 47, 123–132. doi:10.1016/j.arr.2018.0.005
Wilkinson, D. J., Piasecki, M., and Atherton, P. J. (2018b). The age-related loss of skeletal muscle mass and function: measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 47, 123–132. doi:10.1016/j.arr.2018.07.005
Williams, G. R., Dunne, R. F., Giri, S., Shachar, S. S., and Caan, B. J. (2021). Sarcopenia in the older adult with cancer. J. Clin. Oncol. 39, 2068–2078. doi:10.1200/JCO.21.00102
Xu, X., Guo, H., and Lin, J. (2022). 30 elderly patients with sarcopenia were treated with Baduanjin combined with resistance exercise. Fujian Tradit. Chin. Med. 53, 60–62. doi:10.13260/j.cnki.jfjtcm.012601
Yang, B., Xie, Y., Guo, M., Rosner, M. H., Yang, H., and Ronco, C. (2018). Nephrotoxicity and Chinese herbal medicine. Clin. J. Am. Soc. Nephrol. CJASN 13, 1605–1611. doi:10.2215/CJN.11571017
Yang, S., Loro, E., Wada, S., Kim, B., Tseng, W.-J., Li, K., et al. (2020). Functional effects of muscle PGC-1alpha in aged animals. Skelet. Muscle 10, 14. doi:10.1186/s13395-020-00231-8
Yang, W., Xu, Y., Ye, L., Rong, L., Feng, J., Huang, B., et al. (2023a). Effects of Baduanjin exercise on quality-of-life and exercise capacity in patients with heart failure: a systematic review and meta-analysis. Complement. Ther. Clin. Pract. 50, 101675. doi:10.1016/j.ctcp.2022.101675
Yang, Y., Li, X., Chen, G., Xian, Y., Zhang, H., Wu, Y., et al. (2023b). Traditional Chinese medicine compound (tongxinluo) and clinical outcomes of patients with acute myocardial infarction: the CTS-AMI randomized clinical trial. JAMA 330, 1534–1545. doi:10.1001/jama.2023.19524
Yao, J., Peng, T., Shao, C., Liu, Y., Lin, H., and Liu, Y. (2024). The antioxidant action of astragali radix: its active components and molecular basis. Mol. Basel Switz. 29, 1691. doi:10.3390/molecules29081691
Yeh, T.-S., Lei, T.-H., Liu, J.-F., and Hsu, M.-C. (2022). Astragalus membranaceus enhances myotube hypertrophy through PI3K-mediated akt/mTOR signaling phosphorylation. Nutrients 14, 1670. doi:10.3390/nu14081670
Yin, L., Chen, X., Li, N., Jia, W., Wang, N., Hou, B., et al. (2021). Puerarin ameliorates skeletal muscle wasting and fiber type transformation in STZ-induced type 1 diabetic rats. Biomed. Pharmacother. Biomedecine Pharmacother. 133, 110977. doi:10.1016/j.biopha.2020.110977
Yu, Y., Zhao, Y., Teng, F., Li, J., Guan, Y., Xu, J., et al. (2018). Berberine improves cognitive deficiency and muscular dysfunction via activation of the AMPK/SIRT1/PGC-1a pathway in skeletal muscle from naturally aging rats. J. Nutr. Health Aging 22, 710–717. doi:10.1007/s12603-018-1015-7
Yungi, Z., Xiangbin, T., Siyun, Y., Zhanglin, C., Bin, L., Zhou, Z., et al. (2024). New insights into the function of the NLRP3 inflammasome in sarcopenia: mechanism and therapeutic strategies. Metabolism 158, 155972. doi:10.1016/j.metabol.2024.155972
Zhang, F.-M., Wu, H.-F., Shi, H.-P., Yu, Z., and Zhuang, C.-L. (2023). Sarcopenia and malignancies: epidemiology, clinical classification and implications. Ageing Res. Rev. 91, 102057. doi:10.1016/j.arr.2023.102057
Zhang, T., Cheng, J.-K., and Hu, Y.-M. (2022a). Gut microbiota as a promising therapeutic target for age-related sarcopenia. Ageing Res. Rev. 81, 101739. doi:10.1016/j.arr.2022.101739
Zhang, X., Wang, P., Zhang, J., and Li, L. (2020). Naringin reduces ROS in muscle cells through Akt signaling pathway to treat disuse sarcopenia. Chongqing Med. J. 49, 2839–2843.
Zhang, X., Xu, H., Zhu, L., Huang, D., Kong, L., Wang, Z., et al. (2022b). Thoracic Jia-Ji electro-acupuncture mitigates low skeletal muscle atrophy and improves motor function recovery following thoracic spinal cord injury in rats. Am. J. Transl. Res. 14, 8103–8116.
Zhang, Y., Liu, K., Zhan, Y., Zhao, Y., Chai, Y., Ning, J., et al. (2024a). Impact of Chinese herbal medicine on sarcopenia in enhancing muscle mass, strength, and function: a systematic review and meta-analysis of randomized controlled trials. Phytother. Res. PTR 38, 2303–2322. doi:10.1002/ptr.854
Zhang, Y., Liu, K., Zhan, Y., Zhao, Y., Chai, Y., Ning, J., et al. (2024b). Impact of Chinese herbal medicine on sarcopenia in enhancing muscle mass, strength, and function: a systematic review and meta-analysis of randomized controlled trials. Phytother. Res. PTR 38, 2303–2322. doi:10.1002/ptr.8154
Zhao, X., Yuan, F., Wan, H., Qin, H., Jiang, N., and Yu, B. (2022). Mechanisms of magnoliae cortex on treating sarcopenia explored by GEO gene sequencing data combined with network pharmacology and molecular docking. BMC Genomic Data 23, 15. doi:10.1186/s12863-022-01029-x
Zhou, C., Ma, H., Liu, C., and Yang, L. (2023). Exploring traditional Chinese medicine as a potential treatment for sarcopenia: a network pharmacology and data mining analysis of drug selection and efficacy. Med. Baltim. 102, e35404. doi:10.1097/MD.0000000000035404
Zhou, J. (2024). To investigate the clinical efficacy of Bazhen decoction combined with nutritional support and physical exercise in the treatment of senile sarcopenia. Gansu Med. 43, 798–800. doi:10.15975/j.cnki.gsyy.2024.09.007
Zhou, X., Xu, S., Zhang, Z., Tang, M., Meng, Z., Peng, Z., et al. (2024). Gouqi-derived nanovesicles (GqDNVs) inhibited dexamethasone-induced muscle atrophy associating with AMPK/SIRT1/PGC1α signaling pathway. J. Nanobiotechnology 22, 276. doi:10.1186/s12951-024-02563-9
Zhu, Y.-Q., Peng, N., Zhou, M., Liu, P.-P., Qi, X.-L., Wang, N., et al. (2019). Tai Chi and whole-body vibrating therapy in sarcopenic men in advanced old age: a clinical randomized controlled trial. Eur. J. Ageing 16, 273–282. doi:10.1007/s10433-019-00498-x
Zhu, Z.-W., Tang, C.-L., Li, X.-H., Yang, Z.-X., Yang, Y.-H., Pang, F., et al. (2020). Effects of electroacupuncture on proangiogenesis process and protein turnover in a mouse model of sarcopenia. Zhen Ci Yan Jiu Acupunct. Res. 45, 973–979. doi:10.13702/j.1000-0607.200081
Zmora, N., Suez, J., and Elinav, E. (2019). You are what you eat: diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 16, 35–56. doi:10.1038/s41575-018-0061-2
Keywords: sarcopenia, traditional Chinese medicine, muscular atrophy, natural drug, complementary and alternative therapies
Citation: Yao J and Xia S (2025) Exploring the role of traditional Chinese medicine in sarcopenia: mechanisms and therapeutic advances. Front. Pharmacol. 16:1541373. doi: 10.3389/fphar.2025.1541373
Received: 07 December 2024; Accepted: 26 May 2025;
Published: 30 June 2025.
Edited by:
Rajeev K. Singla, Sichuan University, ChinaReviewed by:
Ka Yui Kum, Life Code Limited, Hong Kong SAR, ChinaTianye Li, Zhejiang University School of Medicine, China
Md Mofizur Rahman, Binghamton University, United States
Edwin Njoto, Sepuluh Nopember Institute of Technology, Indonesia
Copyright © 2025 Yao and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianjun Yao, eWFvamlhbmp1bmRvY0AxNjMuY29t
 Jianjun Yao
Jianjun Yao Sheng Xia
Sheng Xia