- 1Guangxi University of Chinese Medicine, Nanning, China
- 2Guangxi Key Laboratory of Efficacy Study on Chinese Materia Medicine, Nanning, China
Ficus hirta Vahl, a member of the Moraceae family and genus Ficus, is a traditional medicinal plant with diverse metabolites. Traditionally, Ficus hirta Vahl has been used in Asian countries to treat various ailments including indigestion, loss of appetite, cold, pneumonia, tuberculosis, cough, asthma, excessive sweating, backache, bruises, arthritis, liver diseases, skin conditions, gynecological disorders, and pediatric illnesses. As a botanical drug with both medicinal and nutritional applications, Ficus hirta Vahl is an integral part of China’s multi-ethnic medical system, serving as a tonic for many years. Approximately 130 chemical metabolites have been identified from Ficus hirta Vahl to date, including flavonoids, phenylpropanoids, phenolics, phenol glycosides, terpenoids, sterols, quinones, and esters. The bioactive properties of Ficus hirta Vahl extracts include immune modulation, enhancement of digestive system function, antitussive and antiasthmatic effects, as well as antibacterial, anti-inflammatory, antioxidant, anti-aging, hepatoprotective, anti-radiation, and antitumor activities. Toxicological assessments confirmed the safety and nontoxicity of Ficus hirta Vahl A thorough review of the literature underscores the significant potential of Ficus hirta Vahl across multiple domains such as medicine, food, and agriculture. However, research on Ficus hirta Vahl remains limited, necessitating further investigation into its pharmacokinetics and mechanisms of action.
1 Introduction
Ficus hirta (Moraceae) Vahl, termed “Wuzhi Maotao” in Chinese, is a traditional botanical drug from the genus Ficus and occupies a prominent position in the Moraceae family (Lu et al., 2022; Zhong and Xu, 2000). Ficus hirta Vahl is extensively recorded in literature, demonstrating the significance of its use as a medicinal plant. Owing to its long history of use and extensive distribution, Ficus hirta Vahl has taken on numerous aliases. After literature analysis, it was determined that the name originated from the coarse leaves of Ficus of the Moraceae family (Singh and Sharma, 2023). Roots, used either fresh or dried, are the most commonly used plant parts used in herbal medicine. After a thorough examination of classical Chinese herbal medicine books and ethnopharmacology data, Ficus hirta Vahl was found to be used either alone or in combination with other botanical drugs to treat a variety of diseases, including loss of appetite, indigestion, hypogalactia, tuberculosis, cough, asthma, and edema (Lin et al., 2013; Bhambhani et al., 2021). Furthermore, the fruit of Ficus hirta Vahl is nutrient-rich, nourishes body fluids, acts as a laxative, stimulates lactation, and enhances immunity. It is also used to treat constipation and hypolactation (Wang and Chen, 2016). Phytochemical studies revealed that Ficus hirta Vahl is an essential source of flavonoids, phenylpropanoids, phenolics, phenolic glycosides, and other metabolites. The main metabolites of Ficus hirta Vahl are listed in Table 1. It has various pharmacological effects, including antibacterial (Chen et al., 2020b), anti-inflammatory (Ye et al., 2020), antioxidant (Ye et al., 2016; Yi et al., 2013), hepatoprotective (Du, 2021), and immunoregulatory (Yang et al., 2015) activities.
Table 1. The main metabolites in Ficus hirta Vahl are listed in this table https://data.rbge.org.uk/herb/E00364493.
In addition to its use as a traditional Chinese medicine (TCM), the fruit of Ficus hirta Vahl is gaining traction in the food industry. The roots of Ficus hirta Vahl can be used to make tea bags, medicinal wine, functional drinks, ingredients, or drugs (Lin et al., 2013; Bhambhani et al., 2021). In recent years, to better utilize Ficus hirta Vahl as a resource, effectively promote the study of its pharmacological effects, and explore its clinical applications, there has been a significant investment in research on Ficus hirta Vahl However, despite progress in this field, there has been no comprehensive and systematic overview of the current research findings. Therefore, this study aimed to comprehensively summarize the latest overview of the plant characteristics, ethnopharmacology, phytochemistry, pharmacology, different potential uses, and toxicity of Ficus hirta Vahl to lay the foundation for further studies on this botanical drug, as well as to accelerate the application of Ficus hirta Vahl in the sustainable development of future agriculture, food, and pharmaceutical industries.
2 Methods
The available information on Ficus hirta Vahl was collected from scientific databases published from 1993 up to 2025. Information on Ficus hirta Vahl was obtained from published sources, including monographs on medicinal plants, ancient and modern recorded classics, the Chinese Pharmacopoeia and electronic databases, such as Web of Science, PubMed, CNKI, Wanfang DATA, Google Scholar, Baidu Scholar and Flora of China (FOR). The search terms used for this review included “Ficus hirta Vahl,” “wuzhimaotao,” “Ficus hirta Vahl,” “tuhaungqi,” “radix fici simplicissimae,” “Fici Hirtae Radix,” “Hairy Fig,” and “wuzhualong” all of which are accepted names and synonyms, “botanical characterization,” “compounds,” “traditional uses,” “pharmacology,” “toxicology,” “quality standard,” “extraction and purification” and “applications”. No language restrictions were applied during the search.
3 Botanical characterization and distribution of Ficus hirta Vahl
Interestingly, the name “Wuzhi Maotao” is derived from the plant’s characteristics, as it typically has five-lobed leaves shaped like five fingers, which is described as “Wuzhi.” Ficus hirta Vahl is also known as Niunai Mu or Wuzhua Long. The fruit of Ficus hirta Vahl resembles a peach and is covered with fluff. The leaves are alternate, oblong, lanceolate, or narrowly ovate, with microwave-like serrations. Most leaves have five lobes, yet some may have one, three, or seven (Yu et al., 2018). Ficus hirta Vahl often matures in autumn and flowers in the summer. The crisp and tasty fruit develops from the bottom to the top, growing in pairs with branches (Tian et al., 2023). Plants also produce milky juice. It is a common plant that grows in valleys, woodlands, and at sea level on slopes above 500–1000 m, and it is an annual botanical reaching a height of 1–2 m (Zhou, 2013). Ficus hirta Vahl is widespread in Southeast Asian countries, such as Nepal, Bhutan, northeastern India, Vietnam, Myanmar, Thailand, Malaysia, Cambodia, and Indonesia. In China, Ficus hirta Vahl is mainly distributed in Yunnan, Guizhou, Guangxi, Canton, Hainan, Hunan, Fujian, Jiangxi, and other regions. It is ranked among the ten most famous botanical drugs in Southeast China (Zhang and Du, 2021a). Studies have shown that Ficus hirta Vahl has various pharmacological effects, abundant metabolites, rich nutritional value, and a strong application potential (Gu et al., 2022; Huang et al., 2020). Plant specimen (A https://www.gbif.org/occurrence/4850269523), leafs (B), stem (C), fruits (D), roots(E) and the world distribution map (F) of Ficus hirta Vahl in Figures 1A–F.
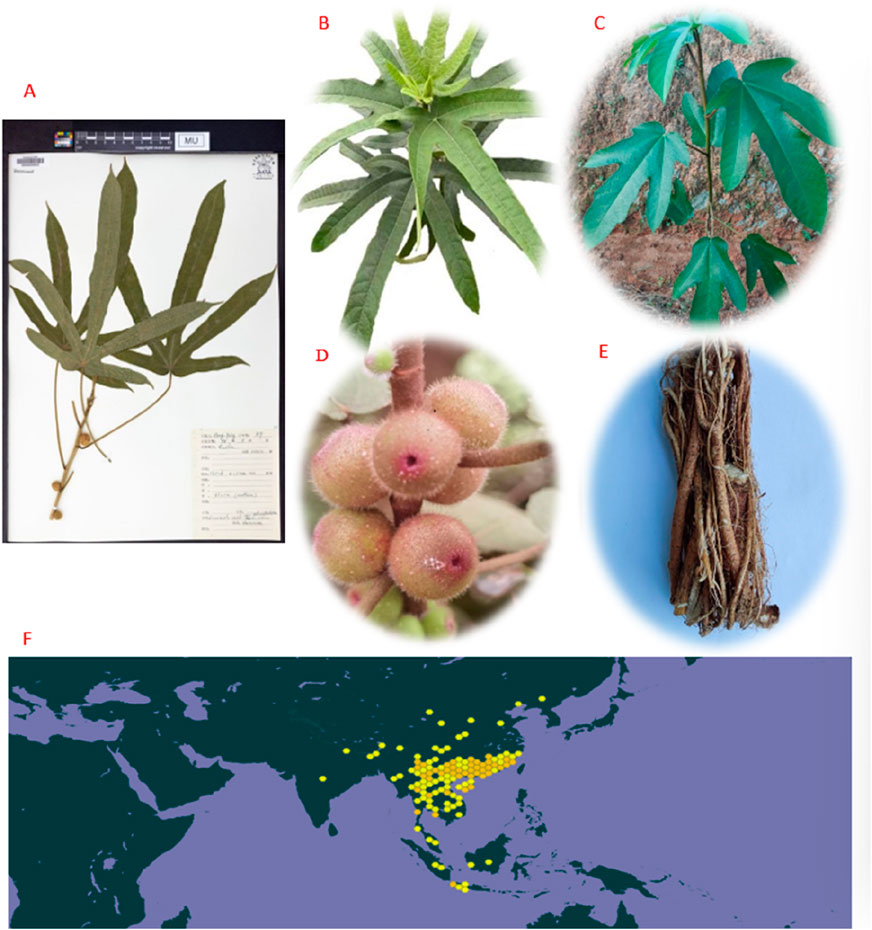
Figure 1. Plant specimen (A) (https://www.gbif.org/occurrence/4850269523), leafs (B), stem (C), fruits (D), roots (E), and the world distribution map (F) (https://www.gbif.org/species/8213018; https://image.baidu.com) of Ficus hirta Vahl.
4 Traditional use
Medicinal records of Ficus hirta Vahl first appeared during the Qing Dynasty and have been continuously enriched in subsequent generations. The medicinal properties and clinical applications of Ficus hirta Vahl recorded in ancient times are consistent with those reported in literature (Priya et al., 2015). According to TCM theory, the taste of Ficus hirta Vahl is sweet and when taken as a medicine, it is distributed to the lung, spleen, and liver meridians (Luo, 2009). Additionally, Ficus hirta Vahl is a multi-ethnic Chinese herbal medicine, which was recorded in Yao, Zhuang, Dai, Dong, Li, Hani, and other ethnic medicine books. These books record the medicinal value, original plants, and geographical distribution of Ficus hirta Vahl (Shi et al., 2018).
During the Qing Dynasty, Ficus hirta Vahl was first documented in the Shengcao Yaoxing Beiyao. It has been used to treat scabies, moss, and hemorrhoids. It is also used to wash hemorrhoids and relieve pain and swelling in the skin. Zhiwu Mingshi Tukao found that Ficus hirta Vahl is effective in treating arthritis and swelling. The records of Lingnan Caiyaolu in 1932 on its medicinal efficacy were similar to that reported previously. According to Zhongyao Zhi (1959), this plant is used in traditional medicine to stimulate the spleen, tonify the lungs, remove moisture, and soothe tendons (Zhou, 2013). It adds the effect of energizing the spleen, tonifying the lung, and it is often applied in gynecological diseases. The descriptions of Ficus hirta Vahl in the four ancient medicinal books from Guangxi folk commonly used herbal manual, New Medical Newsletter, Anthology of single prescription of Hunan Chinese herbal medicine, and National C. herbal medicine compilation are relatively brief (Zhang and Qu, 2021). The clinical applications reported in Practical C. herbal medicine include treating stomach pain, bronchitis, and mastitis. In 1977, Pharmacopoeia of the People’s Republic of China (Volume I) documented its use in the treatment of dropsy, rheumatic arthralgia, and lower back pain. This indicates that Ficus hirta Vahl has a statutory standard, but its records are relatively brief. In 1984, Zhongguo Minzu Yaozhi reported that Ficus hirta Vahl can be used to treat indigestion, abdominal distension, abdominal pain, cough, neurasthenia, asthma, chronic hepatitis, and maternal hypolactation (Luo, 2009). In 1999, Zhonghua Bencao expanded on the clinical applications and efficacy of Ficus hirta Vahl No new clinical applications have been suggested in the Canton Provincial Standards of Chinese Medicinal Materials. In 2013, Ficus hirta Vahl was included in the Guangxi Zhuang Autonomous Region Yao medicine quality standard (Volume I) as a Yao medicine. In 2015, the Chinese Pharmacopoeia (Volume I) recorded Ficus hirta Vahl as a medicinal material for the prescription of Chinese patent medicines (Zhong and Xu, 2000; Wu and Zhou, 2023). Not only is the root used medicinally but recent literature on Ficus hirta Vahl also lists the fruit as a medicinal component (Chen et al., 2024). Nevertheless, there are few clinical studies on the fruit and other plant parts such as leaves and stems, and the use of these parts are rarely supported by the literature, warranting further discussion and study. To optimize the benefits of Ficus hirta Vahl, the mixed use of medicinal and non-medicinal parts should be rigorously controlled because of variations in the effective components of the different parts (Vu et al., 2023).
Overall, traditional application records indicate that Ficus hirta Vahl can be widely used to treat indigestion, loss of appetite, cold, pneumonia, tuberculosis, cough, asthma, night sweats, low back pain, bruises, arthritis, liver disease, skin diseases, gynecological diseases, and pediatric diseases, with almost no adverse reactions. Ficus hirta Vahl is particularly beneficial for treating gastrointestinal issues. According to TCM, the therapeutic benefits of Ficus hirta Vahl in strengthening the lungs and spleen, promoting qi, and removing dampness are important focal points. A summary of the traditional uses of Ficus hirta Vahl is presented in Table 2.
5 Phytochemistry
5.1 Flavonoids
Flavonoids have attracted attention in the field of drug research and development owing to their multiple biological activities and complex mechanisms of action (Wang et al., 2019; Panchal et al., 2021). Flavonoids are the principal active metabolites in Ficus hirta Vahl They play an essential role in the growth and development of plants, enhance plant resistance to unfavorable environmental conditions, and promote human wellbeing. Additionally, certain flavonoids can alter the coloration of flowers and fruits, while enhancing the flavor of food (Li et al., 2014; Sun et al., 2013). They have a general 2-phenylchromone structure (Liu and Xue, 2016). Flavonoids derived from Ficus hirta Vahl exhibit a wide range of biological activities, including, among others, anti-inflammatory, antibacterial, antioxidant, anti-aging, antiviral, and anticancer effects (Wen et al., 2021). Two novel flavonoids, naringenin-7-O-β-D-glucoside and pinocembrin-7-O-β-D-glucoside were isolated from Ficus hirta Vahl and their structures were elucidated using spectroscopic methods, including nuclear magnetic resonance (NMR) spectroscopy and electrospray ionization mass spectrometry (ESI-MS). These flavonoids have remarkable anti-cancer and free radical-scavenging properties (Wan et al., 2017). Four common flavonoids (tricin, quercetin, vitexin, and cyclomorusin) were identified using a general method, and quercetin was found to possess significant anti-inflammatory properties (Zheng et al., 2013a). Flavonoids in Ficus hirta Vahl seedlings mainly accumulate in the leaves and stems (Chen et al., 2021a). In another study, the roots and leaves of Ficus hirta Vahl had higher flavonoid pigment extract contents than other plant parts (Liu et al., 2023). These two experiments showed that the flavonoid content in Ficus hirta Vahl was closely related to the planting environment and planting time; therefore, the flavonoid content may vary with different planting areas and increase with extended planting time. Each 100 g of Ficus hirta Vahl contains approximately 351.68–352.18 mg of flavonoids, which supports the subsequent development and utilization of the plant (Tang et al., 2021).
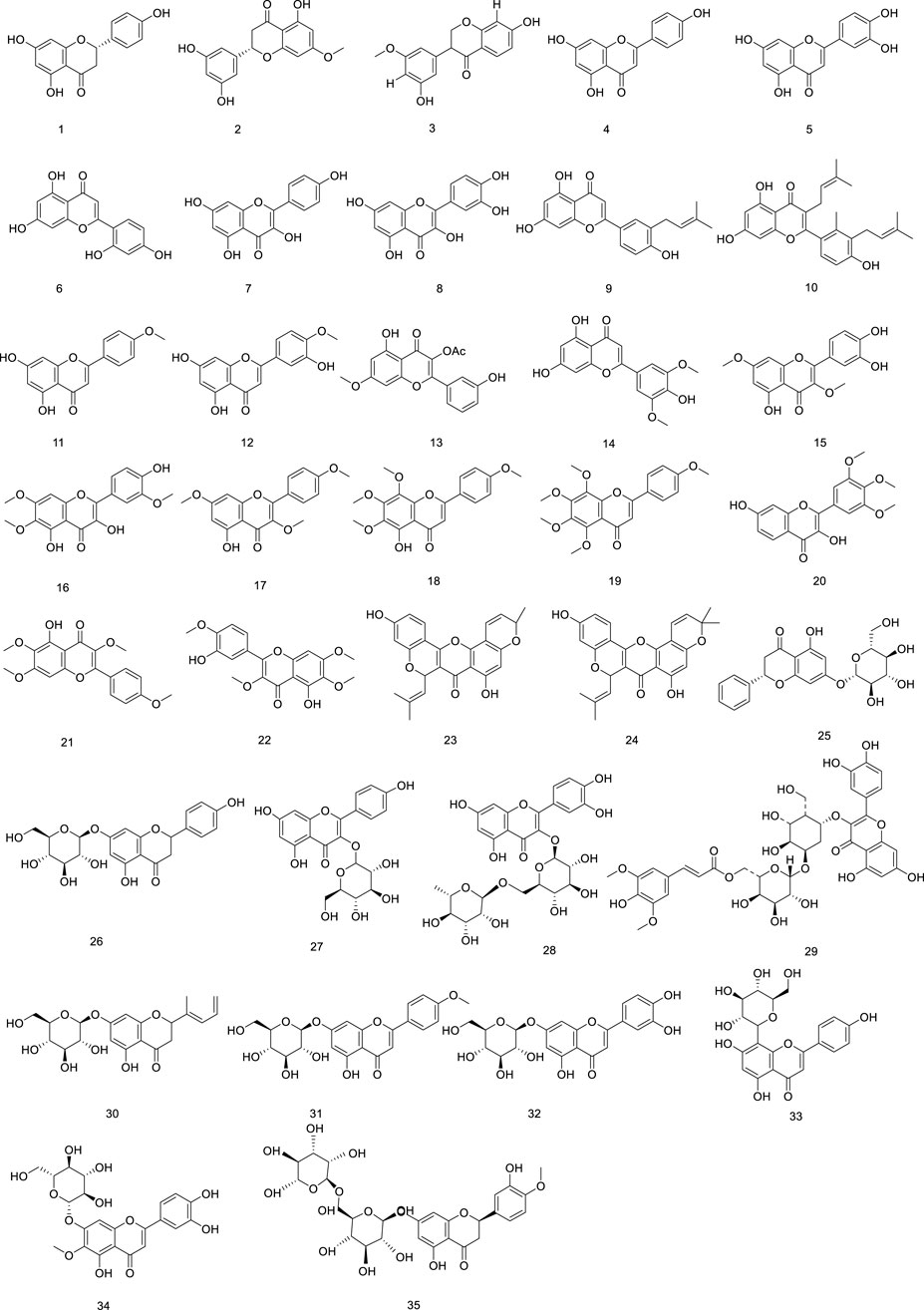
To date, a total of 34 flavonoids have been isolated from Ficus hirta Vahl Apigenin, luteolin, kaempferol, quercetin, and rutin were identified and quantified. Although many flavonoids have been discovered and isolated from Ficus hirta Vahl, detailed pharmacological studies of each flavonoid have not yet been conducted (Cheng et al., 2017). Additionally, some flavonoids from Ficus hirta Vahl lack distinctive biological actions, and we anticipate the isolation of new flavonoids from Ficus hirta Vahl The flavonoids (compounds 1–35) isolated from Ficus hirta Vahl are presented in Table 1; Figure 2.
5.2 Phenylpropanoids
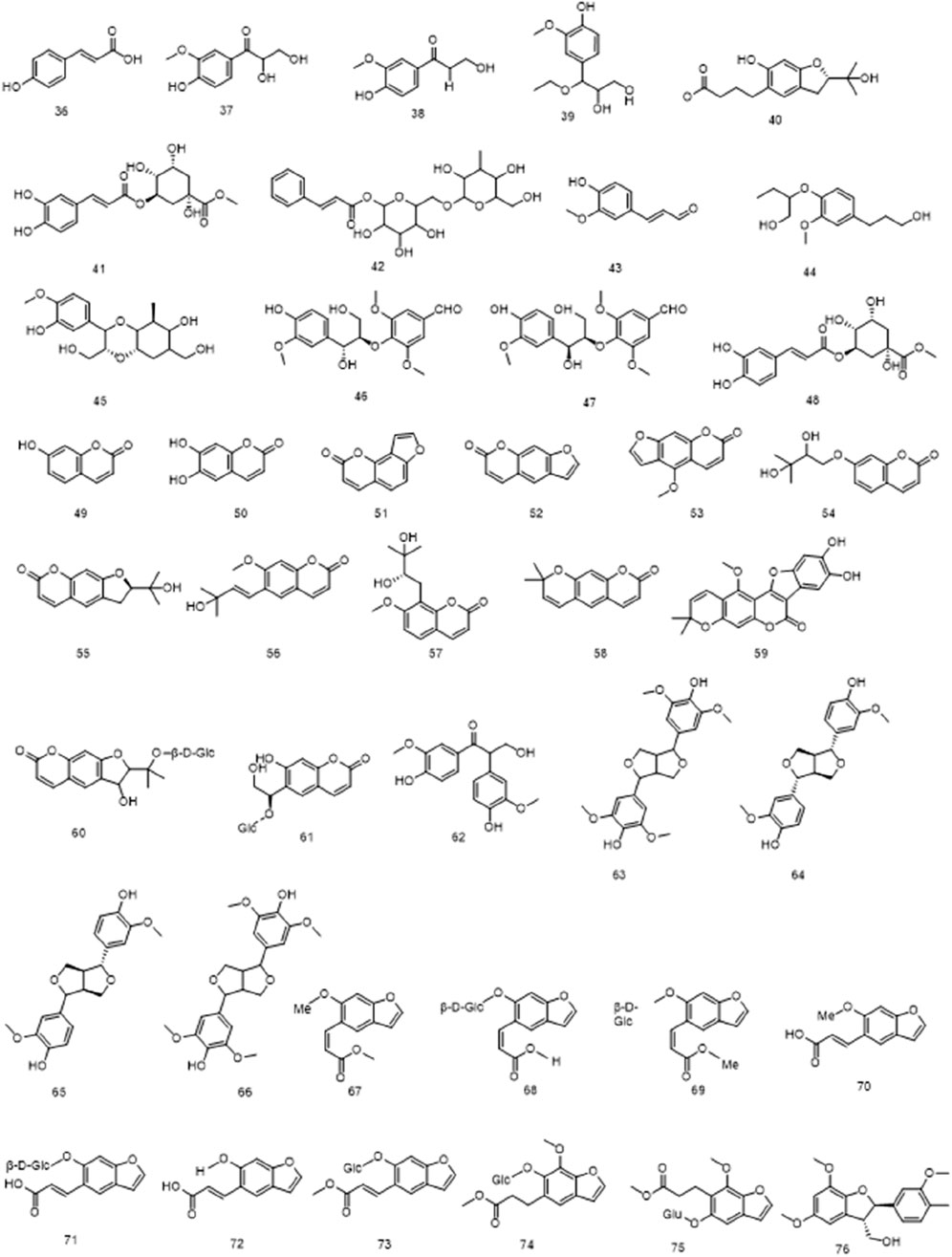
Phenylpropanoid metabolites in Ficus hirta Vahl can be divided into several categories, including simple phenylpropanoids (36–48), coumarins (49–61), simple lignans (62), bisepoxylignans (63–66), and benzofuran lignans (67–76). Among these, coumarin metabolites represent an important type of naturally occurring and synthetic oxygen-containing heterocyclic compounds with a typical benzopyrone framework. Chemical modifications of coumarins have been shown to improve their antibacterial activity (Annunziata et al., 2020; Tu et al., 2021). Coumarin metabolites exhibit various biological properties, including anti-inflammatory, anti-cancer, and hepatoprotective effects. They can also be used to treat metabolic diseases (Bhattarai et al., 2021). The structure of (E)-suberenol was established based on extensive spectroscopic analyses, including NMR spectroscopy and high-resolution (HR)ESI-MS. Additionally, meranzin hydrate has been discovered in Ficus hirta Vahl using MS and infrared (IR), ultraviolet (UV), and NMR spectroscopy (Zheng et al., 2013b). Both coumarins exhibit remarkable antioxidant and anti-inflammation properties (Ya et al., 2010; Kang et al., 2020). A previous study revealed that psoralen is the main metabolite of the total coumarins in Ficus hirta Vahl, and pharmacological studies have shown that psoralen has strong antibacterial, anticancer, anti-osteoporosis, and neuroprotective activities (Tao et al., 2011; Zhong et al., 1993). In recent years, coumarins have been suggested to have high activity and toxicity, some of which include hepatotoxicity and nephrotoxicity (Garg et al., 2020; Srikrishna et al., 2018). Overall, although many coumarins have been discovered and isolated from Ficus hirta Vahl, detailed pharmacological studies of each coumarin have not yet been conducted. The stems, leaves, flowers, fruits, roots, and other components of Ficus hirta Vahl contain abundant simple lignans with a range of structural variants and biological roles. Plant lignans can only be converted into animal lignans in the microecological environment of the gastrointestinal tract (Nawfetrias et al., 2024). Lignans play an important role in regulating estrogen levels, and neurodegenerative diseases. They also exhibit antitumor effects (Li et al., 2023a; Wang et al., 2022a). Lignans are powerful weapons used by plants to resist stress and have diverse bioactive functions that protect human health (Li et al., 2023b). Lignans are plant-active metabolites with estrogen- or anti-estrogen-like activities that can bind to estrogen receptors (ER) and play an estrogen-like role; therefore, they are classified as phytoestrogens. Clinical applications of Ficus hirta Vahl therefore include stimulating mammary gland milk secretion, improving postpartum mammary gland milk secretion, and improving the quality and quantity of breast milk (Peñalvo et al., 2008). However, these metabolites are present in other plant species and are not unique to Ficus hirta Vahl The phenylpropanoids (compounds 36–76) isolated from Ficus hirta Vahl are presented in Table 1; Figure 3.
5.3 Phenolics and phenolic glycosides
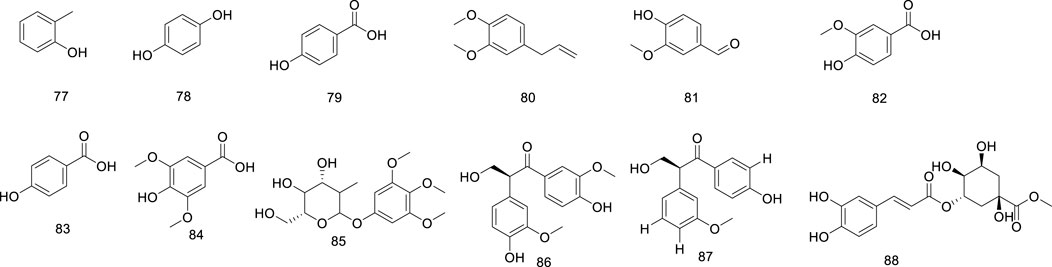
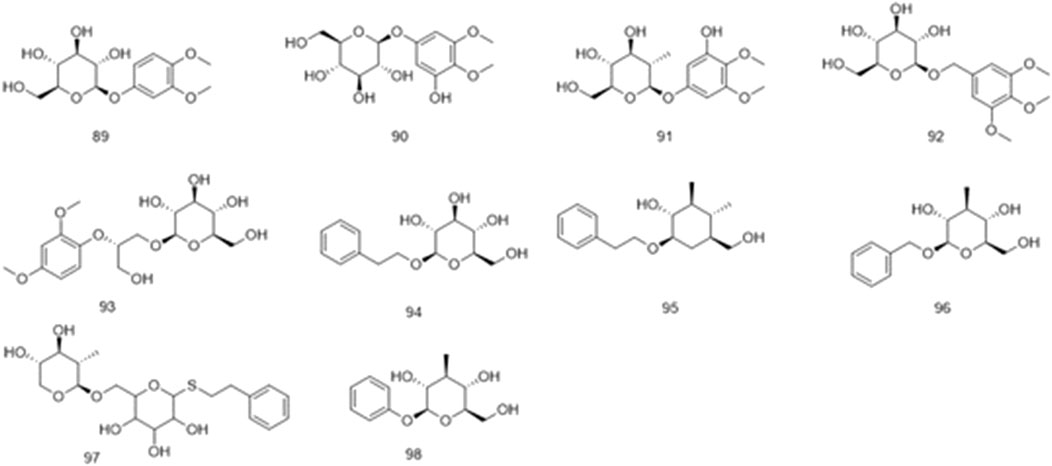
Phenolics are ubiquitous in the plant kingdom and are distributed in the roots, stems, leaves, and fruits of plants (Amadi et al., 2022; Ou et al., 2019). Phenolic substances are organic metabolites with a benzene ring structure, most of which have an aroma (Alara et al., 2021). The phenolic metabolites of Ficus hirta Vahl exert anticancer effects (Yi et al., 2019). HRESI-MS, HPLC techniques, and NMR, IR, and UV spectroscopy were used to detect vanillin, vanillic acid, p-hydroxybenzoic acid, syringic acid, ficuglucoside, and evofolin B. (8R)-4, 5′ dihydroxy-8 hydroxymethyl-3′-methoxy deoxybenzoin, methyl chlorogenic acid, hydroquinone and cresol were further obtained by ultrasonic-assisted extraction of the active metabolites in Ficus hirta Vahl and determination using high-performance liquid chromatography-mass spectrometry (HPLC-MS/MS). In the experiment, evofolin B showed a strong inhibitory effect on the lipopolysaccharide (LPS)-induced release of nitric oxide (NO) from Raw 264.7 (IC50 10.10–59.86 μmol/L) and exhibited potent anti-inflammatory activity (Chen, 2017). Spectroscopic techniques such as IR, UV, NMR, and ESI-MS have been used to isolate methyleugenol from Ficus hirta Vahl and identify its structure (Zheng et al., 2013a). Phenolic glycosides are formed by condensation of the phenol hydroxyl group in the aglycone molecule and the end-group carbon atom of the sugar (Guo et al., 2014). Phenolic glycosides, constituents of Ficus hirta Vahl, are natural metabolites ubiquitously found in various food sources, such as fruits, vegetables, and Chinese medicinal botanical drugs (Murugesu et al., 2021). Some well-known Chinese botanical drugs, such as Leonurus japonicus Houtt and Curculigo orchioides Gaertn, are rich in phenolic glycosides and exhibit anti-inflammatory activities (Liu et al., 2022). Phenolic glycosides decrease neuroinflammation in BV2 microglia cells, which might be regulated by inhibiting protein activity in the NF-κB and MAPK (JNK and ERK1/2) signaling pathways. Thus, there is a good prospect that it can be utilized to treat neurodegenerative diseases (Ye et al., 2020). It possesses antioxidant, anti-cancer, and immunomodulatory properties (Conforti and Menichini, 2011). Phenolic glycosides in Ficus hirta Vahl are not only used in the drug development but also in food production; they can enhance the taste of food and have anti-corrosive and preservative effects (Sun and Shahrajabian, 2023; Lu et al., 2006). The phenolics (compounds 77–88) phenol glycosides (compounds 89–98) isolated from Ficus hirta Vahl are presented in Table 1; Figures 4, 5.
5.4 Terpenoids
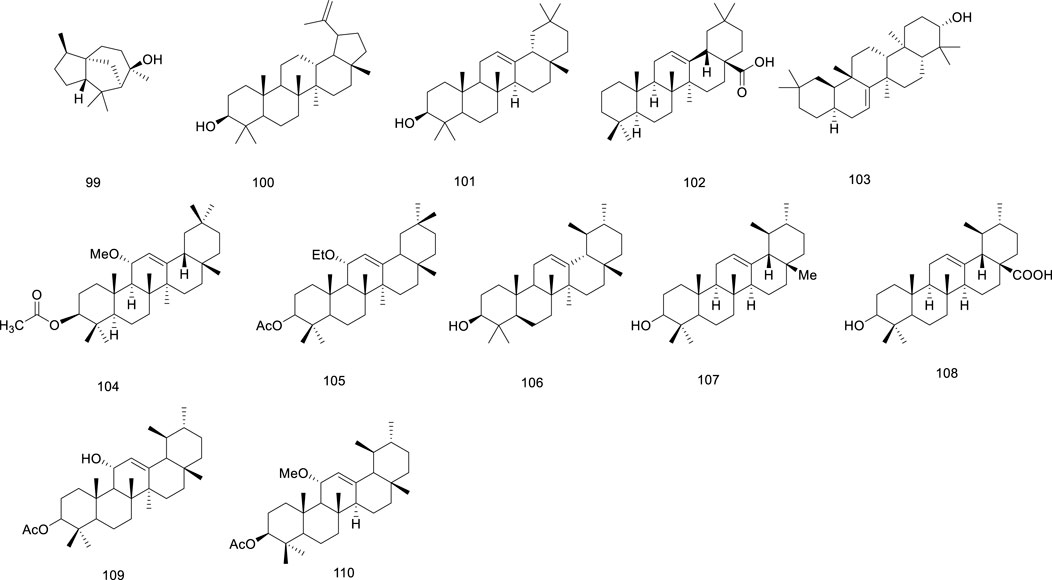
Terpenoids are important secondary metabolites in medicinal plants and triterpenoids are the most commonly extracted components from Ficus hirta Vahl (Bergman et al., 2019; Tholl, 2015). Terpenoids are vital organic molecules present in plants and are considered to have a variety of biological activities, including anti-inflammatory, antibacterial, antiviral, antitumor, antioxidant, and cardioprotective effects (Li et al., 2023c). The terpenoids (compounds 99–110) isolated from Ficus hirta Vahl are presented in Table 1; Figure 6.
5.5 Sterols
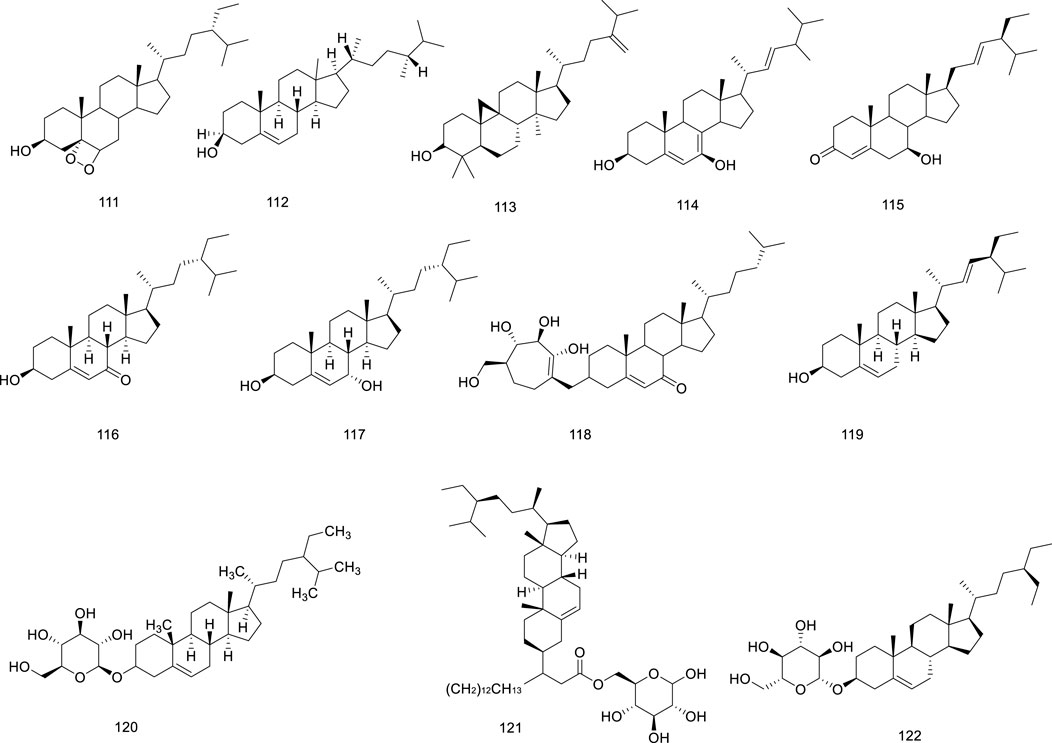
Twelve sterols have been identified in Ficus hirta Vahl Sterols are structural components of Ficus hirta Vahl plant membranes that regulate membrane fluidity and permeability to molecules such as proteins (Ferrer et al., 2017). Sterols play an important role in the growth and development of organisms and are precursors of various steroid hormones in eukaryotes; therefore, they are also called sterols (Evtyugin et al., 2023). Sterols perform a wide range of biological functions, including anti-oxidant, anti-cancer, and blood lipid level reducing activities (Couder-García et al., 2019; Cusack et al., 2013). The structure of β-daucosterol isolated from Ficus hirta Vahl was elucidated using UV and NMR spectroscopy and ESI-MS (Lao et al., 2018). The sterols (compounds 111–122) isolated from Ficus hirta Vahl are presented in Table 1; Figure 7.
5.6 Quinones
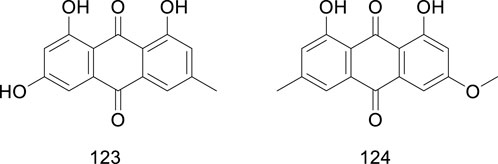
Quinones are a family of natural and synthetic metabolites characterized by a fully conjugated cyclic unsaturated dione structure. Emodin (1,3,8-trihydroxy-6-methyl-anthraquinone) is a natural anthraquinone derivative present in numerous globally renowned herbal medicines. Studies have shown that quinones display diverse biological activities, including neuroprotective, antitumor, and renoprotective effects (Li, 2019; Zhao et al., 2023). Emodin was isolated in Ficus hirta Vahl (Li et al., 2009); interestingly, emodin was found to have diuretic and purgative effects, echoing the traditional use of Ficus hirta Vahl in dehumidifying and reducing swelling. In addition to emodin, physcion ether has been isolated from Ficus hirta Vahl (Zhao et al., 2008). Physcion ether plant fungicides can not only directly inhibit the growth of plant pathogens, but also inhibit the activity of citrus postharvest pathogens and activate the active immune system of plants. The quinones (compounds 123–124) isolated from Ficus hirta Vahl are presented in Table 1; Figure 8.
5.7 Esters
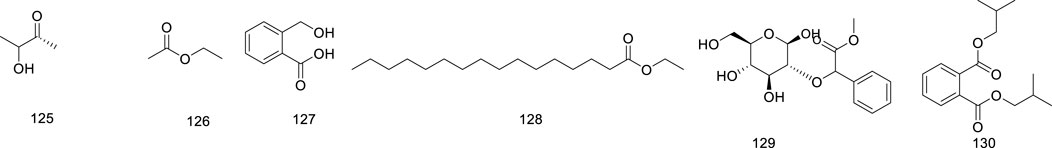
Esters, which make up vegetable oils and contribute to the fragrant flavor of plants, are essential plant components (Derbassi et al., 2022). Esters are a class of metabolites formed when acids and alcohols are dehydrated (Saini et al., 2024). Esters have been known to be applicable in medicine, food, and cosmetics (Chen, 2020; Wang et al., 2022b). The volatile composition of Ficus hirta Vahl was found to contain 7%–15% ethyl acetate, which was obtained by steam distillation (Lin et al., 2000). The Esters (compounds 125–130) isolated from Ficus hirta Vahl are presented in Table 1; Figure 9.
5.8 Other metabolites
Eight aldehydes have been isolated from Ficus hirta Vahl, namely, hexanal, heptanal, octanal, nonanal, (2E)-2-octenal, 1-decanal, (2E)-2-nonenal, and 2-butyloct-2-enal (Li et al., 2010). These chemicals were identified using GC-MS. It was found that the aldehydes changed significantly with an increase in the drying temperature. The higher the hot-air drying temperature, the higher was the relative content of aldehydes, with aldehyde contents of 6.86% at 40°C and 11.02% at 70°C (Gui et al., 2021). Both plants and humans require mineral elements for their healthy growth and development. Atomic absorption spectroscopy was used to determine the mineral contents which were present in the following order: Ca > Mg > Mn > Fe > Cu > Pb (Wen et al., 2010). The aboveground parts of Ficus hirta Vahl were rich in mineral elements. The mineral content in leaves and fruits was higher than that in stems. Intercropping under rubber plantations increased the contents of N, K, Mg, Mn, Cu, and Zn and reduced the contents of Ca and Fe (Yuan et al., 2023).
6 Pharmacological effects and therapeutic potential of Ficus hirta Vahl
Pharmacological studies have shown that Ficus hirta Vahl has immunoregulatory, digestive system function promoting, antitussive, antiasthmatic, antibacterial, anti-inflammatory, antioxidant, anti-aging, hepatoprotective, anti-radiation, and antitumor effects. The pharmacological effects target of Ficus hirta Vahl is summarized in Figure 10. Pharmacological studies of Ficus hirta Vahl in Table 3.

Figure 10. Pharmacological effects of Ficus hirta Vahl (created by Figdraw: https://www.figdraw.com).
6.1 Immunoregulatory effect
The immune system is the most important defense mechanism against pathogen invasion. Because many plants have immunomodulatory properties, they have been used to treat many illnesses. An increasing number of studies have demonstrated that Ficus hirta Vahl can strengthen non-specific and specific immunity, improve immune system control, and be used to treat a variety of illnesses, including bone marrow suppression anemia, hemorrhagic anemia, myasthenia gravis, and other diseases (Yang et al., 2015; Huang et al., 2020). Ficus hirta Vahl significantly increased the charcoal clearance coefficient in cyclophosphamide-induced immunosuppressed mice, increased the weight index of the thymus and spleen, and increased serum hemolysin levels. These results indicate that Ficus hirta Vahl has a regulatory effect on immune function (Liu et al., 2004a). Fifty mice were randomly divided into the normal saline control, cyclophosphamide model, and Ficus hirta Vahl aqueous extract (6.6, 4.4, and 2.2 g/kg) groups. An immunocompromised mouse model was established using intraperitoneal injection of cyclophosphamide. After continuous intragastric administration for 14 days, Ficus hirta Vahl aqueous extract treatment (6.6 and 4.4 g/kg) significantly improved the spleen index of cyclophosphamide immunosuppressed mice. The phagocytic function of macrophages and the proliferation ability and killing activity of T lymphocytes were also significantly enhanced. The number of T cell subpopulations and the of interleukin (IL)-1 and interferon-γ levels increased. This indicates that Ficus hirta Vahl aqueous extract improved immune function in immunosuppressed mice, and its mechanism is related to the regulation of cellular immunity (Yang et al., 2015). Ficus hirta Vahl aqueous and alcohol extracts also have anti-stress effects, regulate immune function regulation, and improve anemia (Zhou et al., 2009a). These results provide scientific justification for Ficus hirta Vahl’s traditional usage as an immunoregulatory botanical drug, and offer a foundation for the development of innovative immunoregulatory medicines.
6.2 Digestion-promoting effect
As a well-known traditional botanical drug in various Asian nations, Ficus hirta Vahl has demonstrated remarkable efficacy in treating digestive system diseases by promoting food digestion and regulating the spleen and gastrointestinal tract (Hou et al., 2016; Theobald, 2015; Zheng et al., 2013b). Ficus hirta Vahl is frequently used to treat digestive disorders. In one study, normal rats were used as a control group, while spleen-deficient model mice were orally administered 100% rhubarb water infusion decoction. The gastric emptying and intestinal propulsion speed of mice were determined. Administration of Ficus hirta Vahl aqueous extract delayed the gastric emptying and small intestinal propulsion rates in a mouse model of spleen deficiency, thus enhancing digestion and absorption (Luo et al., 2012a). Consistent with the findings of previous studies, another study demonstrated that Ficus hirta Vahl has a two-way effect of strengthening and inhibiting small intestinal propulsion, which could improve gastrointestinal motility and promote digestion and absorption (Li et al., 2008).
In a further study, normal rats were used as control group, the researchers established a rat model of pyloric ligation-induced gastric mucosal damage and discovered that Ficus hirta Vahl aqueous extract (2 g/mL) reduced gastric acid secretion and pepsin activity in rats, and had protective effects against gastric mucosal injury (Wang et al., 2011a). In another study, indomethacin was subcutaneously injected into rats to establish a gastric mucosal injury model. Forty-two SD male rats were randomly divided into normal, model, and high-dose and low-dose groups (14 and 7 g/kg) Ficus hirta Vahl water extract groups. Gastric mucosal damage was observed in the model group, which was ameliorated in mice following intragastric administration of Ficus hirta Vahl The mechanism of action was found to be related to the effect of Ficus hirta Vahl on the expression of connexin and the transcription factor SOX2 (Zeng et al., 2020). In one study, 60 rats were randomly divided into six groups. The Astragalus water extract-treated rats acted as a control group and the Ficus hirta Vahl water extract groups were divided into high, medium, and low dose groups, with 10 rats in each group. Rats in the all groups except the control were subcutaneously injected with reserpine (1 mg/kg) daily to establish a spleen deficiency model. The spleen deficiency model group was intragastrically administered purified water at 10 mL/kg. The Astragalus water extract group and the high-, medium-, and low-dose Ficus hirta Vahl water extract groups were administered the corresponding drugs intragastrically at the same dose. The study showed that Ficus hirta Vahl aqueous extract increased the β-endorphin (β-EP), motilin (MTL) and gastrin (GAS) levels in the plasma of rats with spleen deficiency, thereby regulating the movement of the gastrointestinal tract (Yang and Xia, 2012). Therefore, the role of Ficus hirta Vahl in promoting the digestive system deserves attention.
6.3 Antitussive and antiasthmatic effects
Several studies have shown that Ficus hirta Vahl can be used to treat respiratory conditions, such as cough and asthma. The effects of the drugs on digestive tract motor function were measured using the intestinal propulsion method. The antitussive effect of the drug on mice was measured using a spray-induced cough model. The expectorant effect of the drug in mice was determined using tracheal segment secretion spectrophotometry. Ficus hirta Vahl improved gastrointestinal motor function, promoted digestion and absorption, had obvious cough-suppressant effects, had certain expectorant effects, and improved respiratory system function (Li et al., 2008). In one study, seventy-two mice, half male and half female, were randomly divided into six groups: control, positive control, Astragalus aqueous extract (10 g/kg), and Ficus hirta Vahl aqueous extract high (20 g/kg), medium (10 g/kg), and low (5 g/kg) dosage groups. The positive control group (10 mg/kg) was administered Astragalus aqueous extract intragastrically and the control group was administered an equal volume of distilled water for five consecutive days. Ficus hirta Vahl aqueous extract reduced the number of cough reactions induced by aqueous ammonia in mice, indicating that it had an antitussive effect. For the antiasthmatic experiment, guinea pigs were divided into six groups: normal control group, positive control group, Astragalus water extract group (8 g/kg), and Ficus hirta Vahl aqueous extract high (16 g/kg), medium (8 g/kg), and low (4 g/kg) dose groups. The treatments were administered continuously for 7 days. Different doses of Ficus hirta Vahl aqueous extract prolonged periods of asthma relief, indicating that Ficus hirta Vahl has an antiasthmatic effect (Zhou et al., 2009b). In this experiment, the vagus nerve in the afferent pathway of the cough reflex was stimulated with electricity to measure its threshold value for inducing cough. The results showed that the threshold value for inducing the cough voltage increased significantly with Ficus hirta Vahl treatment. The research showed that the ethanolic extract of Ficus hirta Vahl exhibited an antitussive effect. Research has demonstrated that Ficus hirta Vahl ethanol extract has potent anti-asthmatic effects and considerably extends the asthma incubation period (Zeng et al., 2002). According to recent clinical studies, Ficus hirta Vahl preparations can help reduce asthma symptoms (Dogra et al., 2015; N’guessan-Irié et al., 2020). This study demonstrated that Ficus hirta Vahl effectively reduced bronchiolitis in smoked + LPS chronic obstructive pulmonary disease model rats, as well as reducing cough, expectoration, and asthma in patients and preventing the decline of lung function (Zhou et al., 2008). Network pharmacology studies have revealed that Ficus hirta Vahl plays antitussive and antiasthmatic roles by acting on targets, such as estrogen receptor 1, epidermal growth factor receptor, cyclooxygenase, phosphatidylinositol, glycogen synthase kinase, and other signaling pathways (Huang and Huang, 2023). These investigations offer a foundation for the development of novel antitussive and antiasthmatic medications by elucidating the mechanism of action of Ficus hirta Vahl in relieving cough, decreasing phlegm, and relieving asthma.
6.4 Antibacterial effect
It is well known that Ficus hirta Vahl has antimicrobial properties. The minimum inhibitory concentration (MIC) of the Ficus hirta Vahl aqueous extract was 1 g/mL, which effectively inhibited the growth of pathogenic bacteria such as E. coli, Bacillus subtilis, and S. aureus (Wang et al., 2010a). Additionally, researchers discovered that Ficus hirta Vahl aqueous extract exhibited antibacterial activity against Staphylococcus aureus and Escherichia coli, with MICs of 11.21 and 14.07 mg/mL, respectively (Chen and Ye, 2012). These findings indicate that Ficus hirta Vahl possesses antibacterial activity, although its inhibitory effect varies among the different strains.
Ficus hirta Vahl has significant anti-P. italiana and anti-Penicillium digitata activity. Studies have shown that Ficus hirta Vahl causes cell death by destroying the membranes and cell walls of mycelia. The antifungal activity of the flavonone pinocembroside metabolite obtained from the Ficus hirta Vahl fruit was evaluated against P. italicum. Pinocembroside showed antifungal activity against the in vitro mycelial growth of P. italicum, with an MIC and minimum fungicidal concentration of 200 and 800 mg/L, respectively (Chen et al., 2020a; Chen et al., 2020b). A study showed that pinocembrin-7-O-β-D-glucoside was isolated from Ficus hirta Vahl, and it was discovered to be an effective concentration-dependent antibacterial metabolite (Xia et al., 2016; Chen et al., 2017). An in vitro experiment was conducted to evaluate the effects of Ficus hirta Vahl in LPS-induced apoptosis. In this study, Fas, an apoptosis receptor, was cloned, which included a 5′-UTR of 39 bp, an ORF of 951 bp, a protein of 316 amino acids, and a 3′-UTR of 845 bp. EcFas was the most strongly expressed in the spleen tissue of orange-spotted groupers. In addition, LPS-induced apoptosis in the fish spleen cells was concentration-dependent. These findings provide evidence that Ficus hirta Vahl treatment alleviates LPS-induced apoptosis by activating miR-411 to inhibit Fas expression and, therefore, provided possible strategies for bacterial infections in fish (Feng et al., 2024). These results indicate that Ficus hirta Vahl can destroy Penicillium italiana and P. digitatum. As a result, the above studies have verified the antibacterial activity of Ficus hirta Vahl, which is worthy of further development and utilization.
6.5 Anti-inflammatory effect
A mouse model of xylene-induced ear edema was used to study the anti-inflammatory effects of Ficus hirta Vahl Sixty male and female mice were randomly divided into the normal group, positive control group, and Ficus hirta Vahl aqueous extract high (20 g/kg), medium (10 g/kg), and low dose (5 g/kg) groups. Mice in the positive control group were administered 25 mg/kg indomethacin intragastrically, and the normal group was intragastrically administered the same volume of distilled water once daily for six consecutive days. Treatment with Ficus hirta Vahl aqueous extract considerably reduced mouse ear enlargement and the increased number of abdominal capillaries, thus showing anti-inflammatory effects (Zhou et al., 2008). The anti-inflammatory activity of bergapten in Ficus hirta Vahl was studied using color mass spectrometry, LCMS-IT-TOF, and GC-MS. In a zebrafish model of inflammation induced by cutting, bergapten significantly inhibited the recruitment of neutrophils and macrophages to the injured site, as well as the production and re-release of reactive oxygen species and NO. Bergamot may be helpful in the treatment of chronic inflammatory illnesses, according to a different study (Yang et al., 2018). Ficus hirta Vahl is frequently used to treat inflammatory diseases and nerve injuries because of its anti-inflammatory properties.
6.6 Anti-oxidant and anti-aging effects
Antioxidant effects are essential for human health because they can inhibit or reduce the damage caused by free radicals in cells. In one study, the polysaccharides, flavonoids, polyphenols, and amino acids in Ficus hirta Vahl demonstrated strong free radical scavenging ability, their IC50 values for 1, 1-diphenyl-2-picrylhdrazyl (DPPH) free radicals were 15.13, 103.72, 3.63, and 13.64 μg/mL, respectively (Kuang et al., 2015). The antioxidant capacities of luteolin, apigenin, and psoralen in Ficus hirta Vahl were determined using the DPPH method, and it was found that the antioxidant capacity of luteolin was the strongest (Yi et al., 2013). In a previous study, in vitro antioxidant activity test results showed that total coumarins from Ficus hirta Vahl have significant antioxidant activity. The median IC50 values of total coumarins from the root of Ficus hirta Vahl were 2.23 mg/mL and 0.33 mg/mL for DPPH free radicals and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonicacid) (ABTS) free radicals, respectively (Wang et al., 2023). According to one study, the antioxidant activity and total phenolic content of Ficus hirta Vahl in different leaf types varied, and the antioxidant activity and total phenolic content of Ficus hirta Vahl in the seven-finger type were significantly higher than those in the three- and five-finger types. Therefore, the total phenol content of plants is a significant indicator of the antioxidant capacity of Ficus hirta Vahl (Chen et al., 2021b; Qin et al., 2021).
Malondialdehyde (MDA) and superoxide dismutase (SOD) are recognized indicators of antioxidant drug-induced damage. A total of 100 mice were divided into five groups: normal control; model control; and Ficus hirta Vahl high-, medium-, and low-dose groups. The high-, medium-, and low-dose groups were administered Ficus hirta Vahl at doses of 2, 1, and 0.5 g/kg, respectively, for 9 consecutive days. Research found that Ficus hirta Vahl aqueous extract could prevent and treat fatigue in mice by improving SOD activity and the total antioxidant capacity of skeletal muscle, reducing MDA production, enhancing antioxidant capacity, and reducing oxidative stress injury (Luo et al., 2012b). To study the effects of different doses (36, 18, 9 g/kg/d) on memory function of Alzheimer’s disease (AD) mice, the study showed, using water maze experiments, that Ficus hirta Vahl could improve memory function and peroxide damage in AD mice by reducing MDA content and increasing SOD activity in brain tissue (Feng and Li, 2015).
Free radicals are recognized as primary culprits in the aging process. Owing to its rich antioxidant components, Ficus hirta Vahl extract can effectively scavenge free radicals. This antioxidant activity plays a crucial role in safeguarding cells from the detrimental effects of free radical damage, thereby preventing cellular senescence and ultimately delaying the overall aging process. Seventy experimental mice were randomly divided into 7 groups: normal group, model group, vitamin E (100 mg/g) group, Ficus hirta Vahl aqueous extract high (20 g/kg) and low (10 g/kg) dose groups, and Ficus hirta Vahl alcohol extract (20 g/kg) and low (10 g/kg) dose groups of. The index of the thymus and spleen of mice was calculated, and the MDA content and SOD activity in the serum, glutathione peroxidase (GSH-Px) activity in the liver, and catalase (CAT) activity in the brain were measured. Both the aqueous and alcohol extracts of Ficus hirta Vahl could increase thymus and spleen indices in the D-galactose-induced aging mouse model, increase SOD activity, GSH-Px activity, and CAT activity in aging mice to varying degrees, and reduce MDA content, which may be related to its antioxidant effect (Wang et al., 2018; Ye et al., 2017).
6.7 Hepatoprotective effect
Ficus hirta Vahl has hepatoprotective effects against acute and chronic liver injury, alcoholic liver injury, non-alcoholic fatty liver disease, liver fibrosis, and liver cancer (Yang et al., 2024). Ficus hirta Vahl protected the liver of mice with acute liver injury caused by cocaine, xylene, and dimethylformamide. Following the injection of Ficus hirta Vahl aqueous extract, mouse liver tissue showed reduced pathological damage and lower levels of serum transaminases (alanine transaminase [ALT] and aspartate transaminase [AST]), CAT, and other enzymes, suggesting that Ficus hirta Vahl can reduce these enzymes and withstand lipid peroxidation (Cai et al., 2007; Zhou et al., 2008). In another study, Ficus hirta Vahl aqueous extract exhibited a protective effect against restraint stress-induced liver injury in mice. The aqueous extract of Ficus hirta Vahl has the potential to significantly reduce plasma ALT activity, liver tissue MDA, and NO content; significantly increase liver tissue oxidative free radical absorption capacity and glutathione (GSH) content; and improve glutathione peroxidase (GSH-Px) and glutathione S-transferase activity. This experiment verified that Ficus hirta Vahl protected the liver by reducing oxidative stress (Wang et al., 2015). Carbon tetrachloride (CCL4)-induced liver injury is a commonly used model of acute liver injury. For the first time, LC-MS-based metabolomics was used to elucidate the hepatoprotective effects of Ficus hirta Vahl on CCl4-induced acute liver injury in mice. The mechanism participates in the regulation of oxidative stress and energy homeostasis, and reverses the metabolomic characteristics. In particular, the metabolism of amino and fatty acids can be improved by Ficus hirta Vahl pretreatment (Zhou et al., 2022). This provides insights into potential metabolic mechanisms for future research.
Oxidative stress is considered the main cause of alcoholic liver disease (Li et al., 2015). As alcohol metabolism produces a large amount of ROS and active nitrogen free radicals, it causes oxidative stress damage to the liver when it exceeds the body’s ability to remove them (Neuman et al., 2015). Researchers have studied the effect of the alcohol extract of Ficus hirta Vahl on alcoholic liver injury in mice, and the results have shown that Ficus hirta Vahl alcohol extract significantly increases the activity of antioxidant enzymes in the liver. The mechanism may be to activate Nrf2, induce the regulation of downstream antioxidant factors or antioxidant systems, inhibit the abnormal activation of CYP2E1, reduce the oxidative stress response, and thereby reduce alcohol damage in the livers of mice (Zhang et al., 2021). Besides, a study revealed that the protective mechanism of Ficus hirta Vahl against alcoholic liver injury involves decreasing the levels of inflammatory factors such as NF-κB, tumor necrosis factor (TNF)-α, IL-1β, and IL-6 while increasing the levels of GSH and SOD (Zhang and Du, 2021b). In another study, Ficus hirta Vahl extract reduced alcohol-induced liver damage by lowering the levels of inflammatory markers such as IL-6, IL-1β, TNF-α, and NF-κB in liver tissue, which may be connected to coumarin and flavonoid contents (Zhang and Du, 2021a). In addition, the study found that aqueous and alcohol extracts of Ficus hirta Vahl could reduce the activity of ALT and AST to different degrees, indicating that Ficus hirta Vahl can reduce liver damage caused by alcohol and protect the liver. Further studies have found that Ficus hirta Vahl could reduce the levels of MDA, the product of lipid peroxidation, and increase SOD and GSH levels, GSH-Px activity, and the expression levels of Nrf2 and its downstream related antioxidant enzymes HO-1 and SOD-1 proteins to play an antioxidant role (Du, 2021). In another study, Ficus hirta Vahl to was administered to alcohol-induced liver injury model mice. As a result, the pathological changes in liver lipids and inflammatory cell infiltration were reduced, the concentrations of AST, ALT, alkaline phosphatase, and lactate dehydrogenase were significantly reduced, and the liver sinuses were normal. It was concluded that Ficus hirta Vahl extract had obvious preventive and treatment effects on alcohol-induced liver injury in mice (Feng et al., 2018). Ficus hirta Vahl also has preventive and therapeutic effects on non-alcoholic liver disease, mainly through the regulation of lipid metabolism and inflammation. In the process of lipid metabolism regulation, CD36, SREBP-1, SCD1, PPARγ, ACOX1, and CPT1α are the key factors in Ficus hirta Vahl treatment of nonalcoholic fatty liver disease. It was found that SREBP-1, ACACA, and SCD1 levels were significantly reduced, while those of CPT1α, ACOX1, and PPARα were significantly increased. During the inflammatory process, the downregulation of IL-6, IL-1β, and TNF-α is involved in the remission of nonalcoholic fatty liver disease (Quan et al., 2022).
Furthermore, many TCM prescriptions for the clinical treatment of liver illnesses include Ficus hirta Vahl, suggesting that its importance in liver protection cannot be overlooked according to the currently available literature (Qiu et al., 2012). Moreover, Ficus hirta Vahl decoction has been found to improve clinical signs, liver function, and liver fibrosis in patients with post-hepatitis B cirrhosis (You et al., 2011). Tea bags made with Ficus hirta Vahl are suitable for people with hepatitis B. Ficus hirta Vahl can improve immunity, promote antibody production, strengthen liver protection, and prevent the reduction of liver glycogen levels (Li, 2005). Thus, these studies suggest that Ficus hirta Vahl has the potential to develop into a hepatinica. Part of the hepatoprotective mechanism of Ficus hirta Vahl is shown in Figure 11.
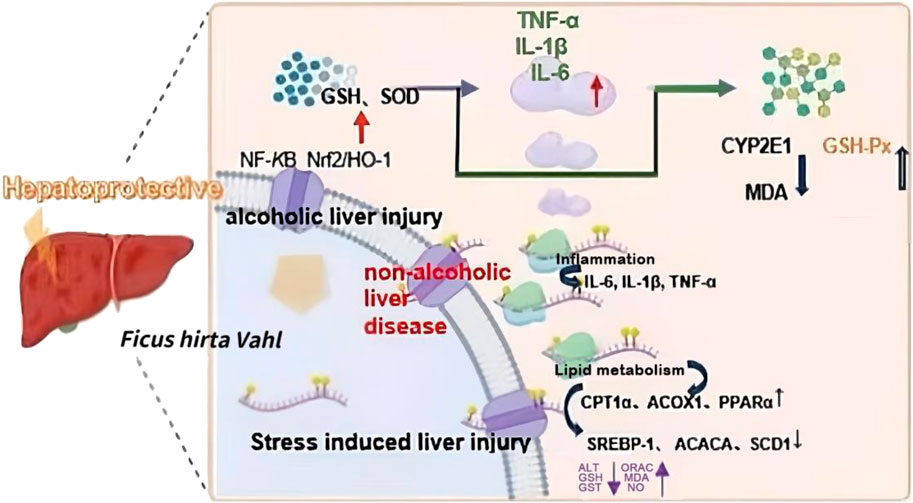
Figure 11. Part of the hepatoprotective mechanism of Ficus hirta Vahl is shown in this figure. Abbreviations: nuclear factor kappa-B (NF-κB); Nuclear factor erythropoietin-2-related factor 2/heme oxygenase 1 (Nrf2/HO-1); glutathione (GSH); Superoxide dismutase (SOD); tumor necrosis factor α (TNF-α); interleukin-1β (IL-1β); interleukin-6 (IL-6); Cytochrome P450 Family 2 Subfamily E Member 1 (CYP2E1); Malondialdehyde (MDA); Glutathione peroxidase (GSH-Px); Carnitine palmitoyltransferase 1α (CPT1α); Acyl-CoA Oxidase 1 (ACOX1); Peroxisome proliferator-activated receptor-α (PPARα); Sterol regulatory element-binding proteins (SREBP-1); Acetyl-CoA Carboxylase Alpha (ACACA); Stearoyl-CoA desaturase-1 (SCD1); alanine aminotransferase (ALT); nitric oxide (NO); oxygen radical absorbance capacity (ORAC); Glutathione-s-transferase (GST) (created by Figdraw: https://www.figdraw.com).
6.8 Radiation-protective effect
The radiation-protective potential of Ficus hirta Vahl extracts was investigated. Psoralen in Ficus hirta Vahl can improve the survival rate of mice with bone marrow injury and plays an effective anti-radiation role in radiation damage to hematopoietic tissue (Zhang et al., 2024). Several studies revealed that Ficus hirta Vahl had protective and repairable anti-radiation effects on the DNA of bone marrow and lung tissues in mice damaged by 60Coγ rays (Wang et al., 2010b; Wang et al., 2011b; Wang et al., 2010c).
6.9 Antitumour effect
Ficus hirta Vahl has shown promising results as an antitumor botanical drug. An Ficus hirta Vahl extract inhibited the proliferation of HepG2 cells, decreased the mRNA and protein expression of Bcl-2, and increased the mRNA and protein expression of Bax, thereby accelerating the apoptosis of cancer cells (Yang et al., 2019; Cen et al., 2010). In one study, extracts of Ficus hirta Vahl had a strong cytotoxic effect on HepG2 cells, inducing apoptosis in HepG2 cells through the JNK and p38MAPK signaling pathways. In another study, the MTT assay was used to detect the cytotoxic activity of Ficus hirta Vahl against HeLa, MCF-7, and H460 cell lines, in which the isoprene flavonoids inhibited the proliferation of HeLa cells with an IC50 value of 28.88 μM. HeLa cell apoptosis is induced through the MAPK and AKT signaling pathways (Ye et al., 2022). Water, ethyl acetate, and n-butanol Ficus hirta Vahl extracts inhibited HeLa cell proliferation and induced HeLa cell apoptosis. The extracts decreased the viability of HeLa cells in a dose-dependent manner and induced a sub-G1 peak in the flow cytometry histogram of treated cells compared to the control (Zeng et al., 2012). A previous study revealed that psoralen inhibited the proliferation of gastric cancer BGC-803 cells and induced tumor cell apoptosis (Yan et al., 2015). It also inhibits the proliferation of the human breast cancer MCF-7 cell line (Jiang et al., 2016). Part of the antitumous mechanism of Ficus hirta Vahl is shown in Figure 12.
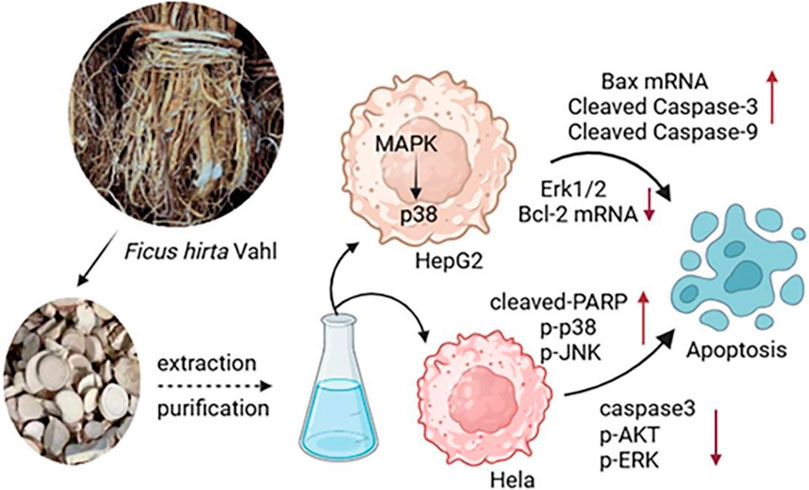
Figure 12. Part of the antitumous mechanism of Ficus hirta Vahl is shown in this figure. Abbreviations: mitogen-activated protein kinase (MAPK); human hepatocellular carcinomas (HepG2); extracellular signal-regulated kinase 1/2 (Erk1/2); B-cell lymphoma-2 (Bcl-2); cleaved-Poly ADP-ribose polymerase (cleaved-PARP); Phospho-p38 (p-p38); Phospho-c-Jun N-terminal kinase (p-JNK); cysteinyl aspartate specific proteinase3 (caspase3); Phospho-protein kinase B (p-AKT); Phospho-ERK (p-ERK) (created by BioRender: https://biorender.com/).
Additionally, Endomelanconiopsis, an endophytic fungal strain isolated from Ficus hirta Vahl, has anti-tumor activity (Liang, 2014). However, in another study on Ficus hirta Vahl endophytic fungi, no metabolites exhibited anticytotoxic activity against SF-268, MCF-7, NCI-H460, or HepG2 cells (Sun et al., 2016). Therefore, it remains to be determined whether Ficus hirta Vahl endophytic fungi exert anti-tumor effects.
7 Nutritional composition and other potential uses of Ficus hirta Vahl
7.1 Nutritional value and the food industry
Ficus hirta Vahl is a TCM with value as both medicine and food. It has high nutritional value and is rich in minerals, vitamins, amino acids, and plant proteins. It is also a plant that has been investigated by nutritionists. Ficus hirta Vahl, often known as “Canton ginseng,” is a popular soup ingredient in the Canton and Guangxi areas of China due to its therapeutic effects. Ficus hirta Vahl soup smells strongly like botanical drugs and also smells somewhat similar to coconut. It can be used as a seasoning for cooking a variety of dishes. People also use Ficus hirta Vahl to make wine, which is golden in color, fragrant, sweet, mellow in flavor, and has a well-balanced medicinal taste. Ficus hirta Vahl not only has good health-promoting and curative effects, but also has high food and drug value. Some studies have reported the brewing process steps of Ficus hirta Vahl sweet wine and medicinal liquor (Kuang, 1999), which provide a basis for the development and utilization of Ficus hirta Vahl medicinal liquor.
A list of products, including candies, drinks, and tea substitutes, that use Ficus hirta Vahl as a raw ingredient has been published (Zhang et al., 2009; Yue, 2021). Studies have indicated that essential nutrients, such as amino acids, organic acids, carbohydrates, and volatile fragrance metabolites, are present in Ficus hirta Vahl Amino acid content and composition can be used as indicators to evaluate the nutritional and health value of Ficus hirta Vahl (Liu et al., 2014). Both essential and nonessential amino acids are present in Ficus hirta Vahl According to previous research, the non-essential amino acids in Ficus hirta Vahl are L-arginine (4.549 mg/g), L-alanine (3.989 mg/g), and L-proline (3.818 mg/g) (Du et al., 2023). Ficus hirta Vahl is rich in minerals, such as Ca, K, Mg, Fe, Cu, Zn, Mg, Mn, and Pb. It also contains large amounts of vitamins, of which vitamin C is the most abundant (Su et al., 2007). A study showed that Ficus hirta Vahl polysaccharide is a key metabolite that regulates the function of intestinal flora, revealing the potential of Ficus hirta Vahl polysaccharide as a natural and effective prebiotic (Xiao, 2022). Therefore, Ficus hirta Vahl polysaccharide can be used as a natural new functional food metabolite or biological material, and the application of Ficus hirta Vahl in the development of functional beverages, dietary supplements, and pharmaceutical preparations is deepened.
Moreover, the flesh of Ficus hirta Vahl fruit is a low-fat, low-calorie food that can be consumed by individuals who want to maintain a good blood lipid profile and weight. Other studies have shown that the four different split-leaf Ficus hirta Vahl fruits, including whole leaves (pinnate leaf and intact leaf), three split leaves (palmate leaves deeply split into three pieces), five split leaves (palmate leaf deeply split into five pieces) and 7-lobed leaf (palmate leaf deeply split into seven pieces), are rich in amino acids. The fruit of Ficus hirta Vahl may be developed as a medicinal plant with a high protein content, making it more suitable for further processing and utilization (Chen et al., 2024).
Because of its potential nutritional and therapeutic properties, Ficus hirta Vahl has become increasingly popular in modern society. Researchers have used Ficus hirta Vahl juice (30%), sugar (16%), jelly powder, and water as raw materials to develop a fragrant and resilient nutritional health jelly, which has opened up a new way for the development of functional health foods (Huang et al., 2009). In a study on the relationship between the composition and metabolic function of the human intestinal microflora and the Ficus hirta Vahl diet, it was found that the Ficus hirta Vahl diet changed the fermentation of carbohydrates by intestinal microorganisms, resulting in more short-chain fatty acids, which were more abundant in amino acid synthesis and the citric acid cycle (Xiao et al., 2022). These results indicate that Ficus hirta Vahl diet can optimize the structure and metabolism of the intestinal microbial community and form a healthy internal environment.
Ficus hirta Vahl and its pharmaceutical preparations, such as Ficus hirta Vahl granules, Xichuan pills, Ficus hirta Vahl Yangxue Fuzheng Decoction, Ficus hirta Vahl Huoxue Yangyan Decoction, can be used in the treatment of digestive, liver, and respiratory diseases in clinical practice,. The production of medicinal powders and decoctions is worthy of market promotion, which not only maintains the nature and taste of the TCM decoction, but also melts quickly, makes full use of its medicinal activity, and further optimizes its application value. It is also promising to formulate Ficus hirta Vahl into a tablet with healthcare functions.
7.2 Agriculture
The bacteriostatic effects of Ficus hirta Vahl have also been exploited for the production of plant pesticides (Xing et al., 2022). Citrus rot is commonly caused by P. italiana and Penicillium digitum. Ficus hirta Vahl has a clear inhibitory effect on Penicillium growth and infection (Chen, 2021). A study found that (2R) methyl 2-o-β-D-glucopyrano2-phenylacetate was isolated from Ficus hirta Vahl fruit, which showed antibacterial activity against P. italiana, indicating that it may be a potential natural fungicide (Wan et al., 2016; Le et al., 2021; Ye et al., 2020). Therefore, it is anticipated that Ficus hirta Vahl can be used to develop natural preservatives for citrus. Another study showed that Ficus hirta Vahl aqueous extract was added to chitosan and coated on the surface of citrus. This reduced the decay, weight loss, and respiration rates, and the MDA content of the fruit. Thus, the content of ascorbic acid and the activity of peroxidase in fruit were reduced, reducing oxidative stress and delaying the effect of lipid peroxidation to improve fruit preservation (Chen et al., 2019).
The success or failure of shrimp farming largely depends on the quality of the water used for aquaculture. Ammonia nitrogen and pH are important indicators of aquaculture water quality. Ammonia nitrogen is produced by the decomposition of nitrogen-containing organic matter such as shrimp residue and excrement (Drzeżdżon et al., 2018). The breakdown of organic waste containing nitrogen, such as shrimp leftovers and excrement, produces ammonia nitrogen. Shrimp immunity, susceptibility to pathogens, and the ability to withstand disease are all compromised by high levels of ammonia and nitrogen stress, endangering their survival and ability to thrive. Nitrogen and oxygen stresses are common environmental stress factors in shrimp cultures. To address this problem, it has been found that supplementing shrimp feed with Ficus hirta Vahl can improve the antioxidant capacity and ammonia nitrogen tolerance of shrimp. These effects are mediated by reducing excessive ROS by inhibiting the expression of miR-2765 in shrimp and activating antioxidant enzyme activity (Liang et al., 2021). In conclusion, Ficus hirta Vahl offers a novel approach to enhance the aquatic habitat of shrimp raised for aquaculture and merits further investigation and advancement.
8 Breeding and cultivation technology
Market demand for Ficus hirta Vahl increases as more individuals become aware of the medical value and health benefits of the plant. Therefore, Ficus hirta Vahl cultivation technology is urgently required for sustainable planting. Cultivation technology is the 'cornerstone’ of the Ficus hirta Vahl planting industry. Selecting seedlings, planting, managing weeds, pruning, controlling pests, harvesting, and other related tasks are essential (Li et al., 2023d). There are numerous advantages to developing and planting Ficus hirta Vahl beneath the forest, however, due to Ficus hirta Vahl seeds being small, the seeds can be combined with plant ash prior to planting. The ideal temperature for raising seedling is 25°C–30°C. (Dong et al., 2014). The effects of different ratios of N, P, and K fertilizers on the yield of Ficus hirta Vahl were studied. It was found that the optimum fertilization was 145.8 kg/h m2 for N, 83.2 kg/h m2 for phosphorus (P2O5), and 186.9 kg/h m2 for potassium (K2O). The yield-increasing effects were N, P, and K fertilizers, with yield-increasing rates of 78.2%, 69.5%, and 54.6%, respectively (Li and Wang, 2020). Innovations in cultivation techniques and new agricultural technologies, such as hydrogen water treatment, can increase the phenylpropanoid and flavonoid contents. In the future, efficacy may be enhanced directionally by regulating plant hormone signals. In summary, Ficus hirta Vahl has the potential for sustainable agricultural development.
9 Toxicology
To assess the safety of Ficus hirta Vahl, researchers observed the death, activity, nutrition, body weight, anatomical organs, and pathological examination of mice using the acute toxicity test method. The maximum dosage of this experiment was 370 times the human dosage, which showed the safety of the clinical daily dosage of the raw drug at 50 g/d. The results showed that there was no death in the mice, and there was no significant difference in activity, diet, body weight, anatomical organs, or pathological examination compared to the control group (Luo et al., 2009). It is well known that pesticide residues are an important factor affecting drug safety. Pesticide residues in Ficus hirta Vahl were determined using GC-MS. The pesticide residues in Ficus hirta Vahl samples were minimal, with the exception of γ-666, which had a residue of 0.0005 mg/kg, and the rest were not detected (Zhen et al., 2018). These findings provide a theoretical basis for safe use of Ficus hirta Vahl, as it is a demonstrably safe medicinal plant. Of course, it is necessary to pay attention to the choice of dosage and the compatibility relationship to make more rational use of this resource.
10 Identification methods
Plant misidentification is a common problem during the identification of TCM. Therefore, an energy-saving onsite identification method was developed to quickly identify Ficus hirta Vahl Researchers can use a rapid DNA extraction scheme called Recombinase Polymerase Amplification of DNA Barcoding (Tian et al., 2017). Ficus hirta Vahl may not only be confused with different species, but also with plants of the same genus. Researchers have created DNA barcodes for Ficus hirta Vahl (Vu et al., 2023). This provides a basis for the accurate and comprehensive identification of Ficus hirta Vahl.
11 Discussion
Ficus hirta Vahl, a plant with medicinal and edible properties, has a significant value in traditional medicine and modern research. Based on the main findings and their implications, Ficus hirta Vahl has diverse characteristics and potential applications. In terms of botanical characteristics, their unique morphologies and wide distributions provide a basis for resource development. In traditional medicine, Ficus hirta Vahl is used to treat a variety of diseases, covering multiple fields such as the digestive system, respiratory system, and liver diseases. This reflects its important position in folk healthcare and provides a theoretical basis for modern drug research and development.
Modern studies have isolated 130 metabolites from Ficus hirta Vahl, such as flavonoids and phenylpropanoids. These metabolites endow Ficus hirta Vahl with multiple biological activities and play roles in immunoregulation, digestion promotion, and antibacterial and anti-inflammatory effects, providing a scientific basis for its applications in the medical, food, and agricultural fields. In terms of nutritional components, Ficus hirta Vahl is rich in minerals and vitamins and can be developed as a nutritional supplement. At the same time, its role in improving the intestinal flora provides a direction for the research and development of functional foods. In agriculture, the antibacterial properties of Ficus hirta Vahl can be used for plant pesticides and fruit preservation. It can also improve the shrimp farming environment, which has positive implications for the sustainable development of agriculture.
However, there are still some gaps in current research on Ficus hirta Vahl Although numerous metabolites have been identified, relatively few studies have been conducted on their chemical properties and pharmacological effects. There is a lack of in-depth research on specific metabolites, and unique plant metabolites have not yet been reported. In terms of biological activity, most studies have focused on extracts, and the pharmacological mechanisms of specific metabolites remain unclear. In addition, research on the mechanism of action and pharmacokinetics of Ficus hirta Vahl is insufficient, and the lack of relevant information limits its further development and utilization. Although it has a wide range of traditional applications, data from large-scale clinical trials are lacking.
In the future, it will be necessary to strengthen in-depth research on Ficus hirta Vahl metabolites and clarify their pharmacological mechanisms and pharmacokinetic characteristics. Bottlenecks in clinical translation exist, although there are abundant achievements in in vitro and animal experiments, there is a lack of large - scale clinical trial data. Further high-quality clinical studies should be conducted to verify its efficacy and safety. At the same time, formulation technology should be optimized to improve the bioavailability of active ingredients and promote the sustainable development and application of Ficus hirta Vahl in the medical, food, agricultural, and other fields.
12 Conclusion and prospects
Ficus hirta Vahl is a TCM notable value for both its medicinal qualities and use as a food. Ancient records, experimental research, and clinical practice have shown that Ficus hirta Vahl is widely used for the treatment of dyspepsia, stomach disease, hepatitis, rheumatism, edema, postpartum agalactia, respiratory diseases, gynecological disorders, and pediatric diseases. Research has shown that it has a significant therapeutic value and is safe. Despite the abundance of ethnopharmacological documentation on Ficus hirta Vahl, there is insufficient scientific research. Ancient literature indicates that Ficus hirta Vahl can be used to treat hematuria, anal prolapse, eczema, and urticaria. This suggests that it has potential applications for the treatment of urinary and skin diseases. However, related experimental studies are scarce.
A total of 130 metabolites were isolated from Ficus hirta Vahl The flavonoids possess various biological activities, including antioxidant, anti-inflammatory, antibacterial, antiviral, and antitumor effects. The most effective flavonoids in Ficus hirta Vahl possess unique pharmacological properties. Among the flavonoid metabolites, apigenin and hesperidin are responsible for their antioxidant and immunomodulatory effects. Among the phenylpropanoid metabolites, psoralen and bergapten play a leading role in anti - inflammatory and antitumor effects. Although many phytochemicals have been isolated from Ficus hirta Vahl, there have been few studies on their chemical properties and pharmacological effects. Unique plant metabolites in Ficus hirta Vahl have not yet been reported, and whether there are specific unique plant metabolites in Ficus hirta Vahl requires further study. In terms of bioactivity, extracts from plant species have been shown to regulate the immune system, promote digestive system function, and exhibit antitussive, antiasthmatic, hepatoprotective, and radiation-protective properties. However, most of these compounds are concentrated in the extracts and the pharmacological effects of their specific metabolites are not clear. Therefore, it is necessary to elucidate the pharmacological effects of these metabolites. In addition, research on its mechanism of action is insufficient and lacks information on its pharmacokinetics; therefore, it is necessary to study its mechanism of action and pharmacokinetics in depth.
Author contributions
MC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. JL: Writing – original draft. SZ: Writing – review and editing. JY: Methodology, Writing – review and editing. JP: Writing – review and editing. ZL: Writing – review and editing. YW: Writing – review and editing. BZ: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Sciences Foundation of China (82360960) and the Guangxi Key Laboratory of Chinese Medicine Foundation Research (20-065-53-07).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
TCM, traditional Chinese medicine; ODS, Octadecylsilyl; HPLC, High Performance Liquid Chromatography; UV, ultraviolet spectroscopy; IR, infrared spectroscopy; NMR, Nuclear Magnetic Resonance; GC, gas chromatography; MS, mass spectrometry; ESI, electrospray ionization; 1D-NMR, one-dimensional nuclear magnetic resonance; 2D-NMR, two-dimensional nuclear magnetic resonance; HRESI-MS, high-resolution electrospray ionization mass spectrometry; UPLC, Ultra Performance Liquid Chromatography; LPS, Lipopolysaccharides; MSD, Mean Square Displacement; DRP1, dynamin-related protein 1; SCFA, CTX MAPK1 STAT3 MAPK3 AKT1 STAT1 NSAID SOX2 Short-chain fatty acids Cyclophosphamide mitogen-activated protein kinase 1 signal transducer and activator of transcription 3 mitogen-activated protein kinase 3 protein kinase B 1 signal transducer and activator of transcription Nonsteroidal Antiinflammatory Drugs SRY-Box Transcription Factor 2; IC50, Half of Inhibition Concentration; 5-LOX, 5-Lipoxygenase; LCMS-IT-TOF, Liquid chromatography-mass spectrometry–ion trap–time of flight; MDA, Malondialdehyde; SOD, Superoxide Dismutase; GSH, Glutathione; GSH-Px, Glutathione peroxidase; DNA, Deoxyribonucleic acid.
References
Alara, O. R., Abdurahman, N. H., and Ukaegbu, C. I. (2021). Extraction of phenolic compounds: a review. Curr. Res. Food Sci. 4, 200–214. doi:10.1016/j.crfs.2021.03.011
Amadi, C. N., Orish, C. N., Frazzoli, C., and Orisakwe, O. E. (2022). Dietary interventions for autism spectrum disorder: an updated systematic review of human studies. Psychiatriki 33 (3), 228–242. doi:10.22365/jpsych.2022.073
Annunziata, F., Pinna, C., Dallavalle, S., Tamborini, L., and Pinto, A. (2020). An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int. J. Mol. Sci. 21 (13), 4618. doi:10.3390/ijms21134618
Bergman, M. E., Davis, B., and Phillips, M. A. (2019). Medically useful plant terpenoids: biosynthesis, occurrence, and mechanism of action. Molecules 24 (21), 3961. doi:10.3390/molecules24213961
Bhambhani, S., Kondhare, K. R., and Giri, A. P. (2021). Diversity in chemical structures and biological properties of plant alkaloids. Molecules 26 (11), 3374. doi:10.3390/molecules26113374
Bhattarai, N., Kumbhar, A. A., Pokharel, Y. R., and Yadav, P. N. (2021). Anticancer potential of coumarin and its derivatives. Mini-reviews Med. Chem. 21 (19), 2996–3029. doi:10.2174/1389557521666210405160323
Cai, Q. Y., Chen, H. B., Cai, S. Q., Zhao, Z. Z., Ruan, M., Jia, F. L., et al. (2007). Effect of roots of Ficus hirta on cocaine-induced hepatotoxicity and active components. China J. Chin. Materia Medica 32 (12), 1190–1193.
Cen, Y. W., Wang, X. P., Huang, X., Li, J. L., and Lu, Q. F. (2010). The effects of Ficus hirta Vahl on cyclophosphamide-induced geneti injury. J. Yulin Normal Univ. 31 (05), 77–79+83. doi:10.13792/j.cnki.cn45-1300/z.2010.05.012
Chen, C., Chen, J., and Wan, C. (2020a). Pinocembrin-7-Glucoside (P7G) reduced postharvest blue mold of navel orange by suppressing penicillium italicum growth. Microorganisms 8 (4), 536. doi:10.3390/microorganisms8040536
Chen, C., Nie, Z., Wan, C., and Chen, J. (2019). Preservation of xinyu tangerines with an edible coating using Ficus hirta Vahl. fruits extract-incorporated chitosan. Biomolecules 9 (2), 46. doi:10.3390/biom9020046
Chen, C., Wan, C., Peng, X., and Chen, J. (2020b). A flavonone pinocembroside inhibits Penicillium italicum growth and blue mold development in 'Newhall' navel oranges by targeting membrane damage mechanism. Pestic. Biochem. Physiol. 165, 104505. doi:10.1016/j.pestbp.2019.11.025
Chen, C. Y. (2020). “A new phenylacetate compound and its antibacterial activity,”. Jiangxi: Jiangxi Agricultural University.
Chen, J. (2017). The research on the bioactive constituents of the roots of Hairy Fig (Ficus hirta Vahl.). Guangzhou: Canton Pharmaceutical University.
Chen, J. Y. (2021). Research on the development and application of “green pesticide” ethnic medicine-Wuzhi Maotao and Bai Wei as examples. Jiangxi: Jiangxi Agricultural University.
Chen, Q., and Ye, S. X. (2012). Antibacterial activity of radix fici hirta by chromotest microassay. Anhui Agric. Sci. 40 (15), 8452–8454+8461. doi:10.13989/j.cnki.0517-6611.2012.15.108
Chen, R. Z., Li, Z., Lin, M. Z., Wang, X. P., and Lai, Z. X. (2021a). Transcriptome analysis of Ficus hirta Vahl and expression profiling of key genes involved in flavonoid biosynthesis. J. Trop. Crop 42 (03), 675–684.
Chen, R. Z., Wang, X. P., and Li, Z. (2021b). Comparative analysis about total phenol content, polyphenol oxidase activity and antioxidant. Fujian Sci. and Technol. Trop. Crops. 46 (04), 14–17+28.
Chen, Z. R., Wang, X. P., Gao, W. C., Luo, X. F., Zhou, J. J., and Lin, M. Z. (2024). Comparative analysis of amino acids composition in fruits of four types Ficus hirta with lobed leaf and evaluation of their nutritional value. Chin. Food Addit. 35 (01), 238–246. doi:10.19804/j.issn1006-2513.2024.1.028
Cheng, J., Yi, X., Chen, H., Wang, Y., and He, X. (2017). Anti-inflammatory phenylpropanoids and phenolics from Ficus hirta Vahl. Fitoterapia 121, 229–234. doi:10.1016/j.fitote.2017.07.018
Conforti, F., and Menichini, F. (2011). Phenolic compounds from plants as nitric oxide production inhibitors. Curr. Med. Chem. 18 (8), 1137–1145. doi:10.2174/092986711795029690
Couder-García, B. D. C., Jacobo-Herrera, N. J., Zentella-Dehesa, A., Rocha-Zavaleta, L., Tavarez-Santamaría, Z., and Martínez-Vázquez, M. (2019). The phytosterol peniocerol inhibits cell proliferation and tumor growth in a colon cancer xenograft model. Front. Oncol. 9, 1341. doi:10.3389/fonc.2019.01341
Cusack, L. K., Fernandez, M. L., and Volek, J. S. (2013). The food matrix and sterol characteristics affect the plasma cholesterol lowering of phytosterol/phytostanol. Adv. Nutr. 4 (6), 633–643. doi:10.3945/an.113.004507
Deng, S. B., Chen, J. P., Yu, C. Q., Wu, S. H., Yang, Y., and Du, M. J. (2018). Analysis of Ficus hirta Vahl fragrance extract. Chin. Pat. Drug 40 (10), 2337–2339.
Derbassi, N. B., Pedrosa, M. C., Heleno, S., Carocho, M., Ferreira, I. C., and Barros, L. (2022). Plant volatiles: using scented molecules as food additives. Trends Food Sci. and Technol. 122, 97–103. doi:10.1016/j.tifs.2022.02.002
Dogra, K. S., Chauhan, S., and Jalal, J. S. (2015). Assessment of Indian medicinal plants for the treatment of asthma. J. Med. Plants Res. 9 (32), 851–862. doi:10.5897/jmpr2015.5890
Dong, Q. S., Yan, Z. G., Wei, S. G., Ke, F., and Wu, Q. H. (2014). Ficus hirta Vahl biological characteristics of seeds. Jiangsu Agric. Sci. 42 (11), 278–280. doi:10.15889/j.issn.1002-1302.2014.11.100
Drzeżdżon, J., Jacewicz, D., and Chmurzyński, L. (2018). The impact of environmental contamination on the generation of reactive oxygen and nitrogen species - consequences for plants and humans. Environ. Int. 119, 133–151. doi:10.1016/j.envint.2018.06.019
Du, J. (2021). Study on the protection and action mechanism of Ficus hirta Vahl on alcoholic liver injury. Heilongjiang: Harbin University of Commerce. doi:10.27787/d.cnki.ghrbs.2021.000674
Du, L. M., Lin, X. J., Yang, L. W., Huang, G. N., li, X. B., and Guo, X. (2023). Analysis of nutrient components in roots of different phenotypes of Ficus hirta vahl. Mol. Plant Breed., 1–13.
Evtyugin, D. D., Evtuguin, D. V., Casal, S., and Domingues, M. R. (2023). Advances and challenges in plant sterol research: fundamentals, analysis, applications and production. Molecules 28 (18), 6526. doi:10.3390/molecules28186526
Feng, J., and Li, X. (2015). Effects of Radix Fici Hirtae on the memory function and oxidative damage in alzheimer’s mice. J. Hunan Univ. 35, 31–32.
Feng, X., Li, K., Tan, F., Zhu, M., Zhou, J., Lai, Y., et al. (2018). Assessment of the hepatoprotective potential of Radix Fici Hirtae on alcohol-induced liver injury in Kunming mice. Biochem. Biophys. Rep. 16, 69–73. doi:10.1016/j.bbrep.2018.10.003
Feng, Y., Liu, Z., Han, C., Chen, J., Lin, X., Du, W., et al. (2024). Ficus hirta Vahl. alleviate LPS induced apoptosis via down-regulating of miR-411 in orange-spotted grouper (Epinephelus coioides) spleen cell. Dev. And Comp. Immunol. 157, 105191. doi:10.1016/j.dci.2024.105191
Ferrer, A., Altabella, T., Arró, M., and Boronat, A. (2017). Emerging roles for conjugated sterols in plants. Prog. Lipid Res. 67, 27–37. doi:10.1016/j.plipres.2017.06.002
Garg, S. S., Gupta, J., Sharma, S., and Sahu, D. (2020). An insight into the therapeutic applications of coumarin compounds and their mechanisms of action. Eur. J. Pharm. Sci. 152, 105424. doi:10.1016/j.ejps.2020.105424
Gu, X., Hao, D., and Xiao, P. (2022). Research progress of Chinese herbal medicine compounds and their bioactivities: fruitful 2020. Chin. Herb. Med. 14 (2), 171–186. doi:10.1016/j.chmed.2022.03.004
Gui, Q., Zhou, L. J., Zheng, D. H., Huang, J., Pang, J., Chen, J. M., et al. (2021). Effects of hot-air drying temperature on the quality of Ficus hirta vahl. Food Ind. Technol. 42 (21), 249–261. doi:10.13386/j.issn1002-0306.2021010005
Guo, N., Zhu, M. X., Han, X. J., and Yang, Q. (2014). Advances in studies on intestinal absorption kinetics of phenolic glycosides. Chin. J. Biochem. Drugs 34 (03), 180–184.
Hou, W., Zhong, D., Zhang, P., Li, Y., Lin, M., Liu, G., et al. (2016). A strategy for the targeted metabolomics analysis of 11 gut microbiota-host co-metabolites in rat serum, urine, and feces by ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. 1429, 207–217. doi:10.1016/j.chroma.2015.12.031
Huang, B. W., Lu, J. J., Lin, H., Pang, Y., Tang, M. L., and Yu, M. P. (2020). Advances in the study on the chemical composition, pharmacological effect, and clinical application of radix fici simplicissimae, and clinical application of radix fici simplicissimae. J. Liaoning Univ. Traditional Chin. Med. 22 (12), 93–96. doi:10.13194/j.issn.1673-842x.2020.12.022
Huang, M. W., and Huang, S. H. (2023). Based on network pharmacology, the mechanism of the immunosuppressive, cough, phlegm-relieving, and anti-inflammatory effects of the Ficus hirta Vahl was studied. 8(08), 70–73.
Huang, Y. N., Zhon, M. H., and Zhang, M. H. (2009). Study on the processing technology of Radix Fici Simplicissimae juice jelly. Food Sci. Technol. 34 (01), 66–68.
Jiang, J., Wang, X., Cheng, K., Zhao, W., Hua, Y., Xu, C., et al. (2016). Psoralen reverses the p-glycoprotein-mediated multidrug resistance in human breast cancer MCF-7/ADR cells. Mol. Med. Rep. 13 (6), 4745–4750. doi:10.3892/mmr.2016.5098
Kang, M., Tao, N. P., Yu, J., Wang, X. C., Le, C. H., Yin, M. Y., et al. (2020). Comparison of texture quality and volatile components of dried figs by different drying methods. Food Industry Sci. Technol. 44 (06), 58–65. doi:10.13386/j.issn1002-0306.2022050189
Kuang, W., Liu, Z. W., Zhang, C., and Li, B. L. (2015). Study on the antioxidant activity of the Ficus hirta Vahl. Canton Chem. Ind. 42 (19), 42–43.
Lao, J. L., Yu, X. D., and Luo, J. J. (2018). Ficus hirta Vahl progress in chemical composition and pharmacological effects. Trop. Agric. Sci. 38 (05), 82–87.
Le, Y., Yang, L. L., Xiong, S. Q., Li, H. Y., and Huang, Y. J. (2021). Professor Xuan Guowei's experience in the treatment of skin diseases with Ficus hirta Vahl. Chin. Med. Guide 27 (10), 171–173. doi:10.13862/j.cnki.cn43-1446/r.2021.10.034
Li, C., Bu, P. B., and Sun, Y. F. (2006). Chemical constituents from roots of Ficus hirta. Chin. J. Traditional Chin. Med. (02), 131–133.
Li, C., Zha, W., Li, W., Wang, J., and You, A. (2023a). Advances in the biosynthesis of terpenoids and their ecological functions in plant resistance. Int. J. Mol. Sci. 24 (14), 11561. doi:10.3390/ijms241411561
Li, C. P., Lu, K., Liao, Y. E., and Su, Y. Y. (2023b). Ficus hirta Vahl cultivation and precision fertilization technology. Mod. Agric. Sci. Technol. (12), 84–86.
Li, H. Y., Li, Z. Y., Wang, C. X., Hang, X. W., Liang, Z. X., Li, L. E., et al. (2008). Effects of Ridix Fici Hirtae on the respiratory tract and digestive tract. China: Chinese Journal of Modern Drug Application, 50–51.
Li, J., Ma, X., Luo, L., Tang, D., and Zhang, L. (2023c). The what and who of dietary lignans in human health: special attention to estrogen effects and safety evaluation. J. Agric. Food Chem. 71 (44), 16419–16434. doi:10.1021/acs.jafc.3c02680
Li, J., and Wang, X. Q. (2020). Study on the fertilizing amount applied to Ficus hirta Vahl intercropping in the mature rubber plantations. China Trop. Agric. (06), 73–76.
Li, J. X., Hui, J., Yang, Y. Y., and Huang, Z. Q. (2010). Component analysis of volatile oil from Ficus hirta Vahl by GC-MS. J. Anhui Agric. Sci. 38 (14), 7281–7282. doi:10.13989/j.cnki.0517-6611.2010.14.157
Li, S., Tan, H.-Y., Wang, N., Zhang, Z.-J., Lao, L., Wong, C.-W., et al. (2015). The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 16 (11), 26087–26124. doi:10.3390/ijms161125942
Li, S., Wang, Z., Ding, F., Sun, D., Ma, Z., Cheng, Y., et al. (2014). Content changes of bitter compounds in ‘Guoqing No. 1’Satsuma Mandarin (Citrus unshiu Marc.) during fruit development of consecutive 3 seasons. Food Chem. 145, 963–969. doi:10.1016/j.foodchem.2013.09.040
Li, W. B. (2005). Study on the production and identification of hepatitis B tea bag. Canton Pharm. (04), 44–45.
Li, X. (2019). Screening of antitumor active components of Chinese herbal medicine in Gansu province. Gansu: Lanzhou University of Technology.
Li, Y., Duan, J., Guo, T., Xie, W., Yan, S., Li, B., et al. (2009). In vivo pharmacokinetics comparisons of icariin, emodin, and psoralen from gan-kang granules and extracts of herba epimedii, Nepal dock root, Ficus hirta yahl. J. Ethnopharmacol. 124 (3), 522–529. doi:10.1016/j.jep.2009.05.008
Li, Z., Zheng, Y., Liu, K., Liang, Y., Lu, J., Li, Q., et al. (2023d). Lignans as multi-targeted natural products in neurodegenerative diseases and depression: recent perspectives. Phytotherapy Res. 37 (12), 5599–5621. doi:10.1002/ptr.8003
Liang, F. L. (2014). Studies on secondary metabolites and antitumor activities of two plant endophytic fungus. Guangdong: Guangdong Pharmaceutical University.
Liang, Q., Dong, W., Wang, F., Wang, W., Zhang, J., and Liu, X. (2021). Ficus hirta Vahl. promotes antioxidant enzyme activity under ammonia stress by inhibiting miR-2765 expression in Penaeus vannamei. Ecotoxicol. Environ. Saf. 228, 112989. doi:10.1016/j.ecoenv.2021.112989
Lin, H., Mei, Q. X., and Zeng, C. Y. (2013). Clinical application of radix fici simplicissimae and its preparation. Chin. Pharm. 24 (15), 1434–1435.
Lin, L., Zhong, X. Q., and Wei, G. (2000). GC-MS analysis of volatile components in root of Ficus hitra. J. Chin. Med. Mater. 23 (04), 206–207. doi:10.13863/j.issn1001-4454.2000.04.011
Liu, C. L., Wei, G., He, J. X., Xu, X. F., and Xu, H. H. (2004a). Analysis of volatile oils and ethanol-extracts from different parts of Radix Fici Hirtae. J. Guangzhou Univ. Chin. Med. (03), 204–205+210.
Liu, C. L. X., Wu, H. H., and Chen, Q. H. (2004b). Experimental study on the effect of Ficus hirta Vahl on immune function in mice. J. Chin. Med. Mater. (05), 367–368. doi:10.13863/j.issn1001-4454.2004.05.024
Liu, W. Q., Yan, H., Li, W., Wei, F., and Ma, S. C. (2014). Pharmacognostic study on Ficus hirta. Chin. Herb. Med. 45 (07), 1011–1015.
Liu, X. X., Zhang, X. Q., Guo, X. H., Wu, W. H., Li, J., and Zhang, M. (2022). Mechanism of phenolic glycosides in xianmao against collagen-induced arthritis in rats based on RNA-seg. Pharmacol. Clin. Chin. Materia Medica 38 (02), 84–90. doi:10.13412/j.cnki.zyyl.2022.02.011
Liu, Y. J., and Xue, Y. C. (2016). The research progress of flavonoids in plants. China Biotechnol. 36 (9), 81–86.
Liu, Y. T., Lin, Y. T., Yang, F., and Yang, L. (2023). Process and color stability analysis of flavonoid plant pigments from Ficus hirt. Mol. Plant Breed. 21 (21), 7186–7192. doi:10.13271/j.mpb.021.007186
Lu, Y., Chen, J., Chen, B., Liu, Q., Zhang, H., Yang, L., et al. (2022). High genetic diversity and low population differentiation of a medical plant Ficus hirta Vahl., uncovered by microsatellite loci: implications for conservation and breeding. Bmc Plant Biol. 22 (1), 334. doi:10.1186/s12870-022-03734-2
Lu, Z. L., Feng, Y., Xu, D. S., Lin, X., and Yang, Y. (2006). Application of caco-2 cell model in performing absorption of Chinese materia medica and its mechanism. China: Chinese Medicinal Herb., 616–619.
Luo, Q. (2009). The study of the main pharmacodynamics and quality control atlas of Radix Fici from the south China area where the raw material is the best. Guangdong: Guangzhou University of Traditional Chinese Medicine.
Luo, Q., Xi, P., Liao, H. Z., Cai, Q. Q., and Zhao, R. J. (2009). Experimental study on pharmacological safety of Ficus hirta Vahl decoction. Pharm. Today 19 (02), 12–13.
Luo, Q., Xi, P., Liao, X. Z., and Chen, M. Y. (2012a). Experimental study on gastrointestinal motility function of rhubarb type spleen deficiency mice with Radix Fici Hirtae decoction. Pharm. Today 22 (07), 398–399+407.
Luo, Z. M., Chen, S. H., Liu, Y. Y., Huang, Y., Ou, Y. M. Z., Xiao, Y., et al. (2012b). Study on mechanism of Ficus hirta Vahl in preventing and treating fatigue-type sub-healthy mice. J. Guiyang Coll. Traditional Chin. Med. 34 (06), 25–28.
Murugesu, S., Selamat, J., and Perumal, V. (2021). Phytochemistry, pharmacological properties, and recent applications of ficus benghalensis and ficus religiosa. Plants (Basel). 10(12), 2749. doi:10.3390/plants10122749
Nawfetrias, W., Devy, L., Esyanti, R. R., and Faizal, A. (2024). Phyllanthus lignans: a review of biological activity and elicitation. Horticulturae 10 (2), 195. doi:10.3390/horticulturae10020195
Neuman, M. G., Malnick, S., Maor, Y., Nanau, R. M., Melzer, E., Ferenci, P., et al. (2015). Alcoholic liver disease: clinical and translational research. Exp. Mol. Pathology 99 (3), 596–610. doi:10.1016/j.yexmp.2015.09.001
N’guessan-Irié, G. A., Kouakou, L. S., Djadji, T. A., Kouassi, J. A., Effo, E. K., and Siransy-Kouakou, G. N. (2020). Review of experimental pharmacological studies of plants used for asthma management in africa. Int. J. Pharm. and Pharmacol. 4 (2), 1–11. doi:10.31531/2581-3080.1000146
Ou, J., Wang, M., Zheng, J., and Ou, S. (2019). Positive and negative effects of polyphenol incorporation in baked foods. Food Chem. 284, 90–99. doi:10.1016/j.foodchem.2019.01.096
Panchal, P., Miller, A. J., and Giri, J. (2021). Organic acids: versatile stress-response roles in plants. J. Exp. Bot. 72 (11), 4038–4052. doi:10.1093/jxb/erab019
Peñalvo, J. L., Adlercreutz, H., Uehara, M., Ristimaki, A., and Watanabe, S. (2008). Lignan content of selected foods from Japan. J. Agric. And Food Chem. 56 (2), 401–409. doi:10.1021/jf072695u
Priya, D., Purnima, D., and Borthakur, S. (2015). A taxonomic account of Ficus hirta Vahl: an ethnobotanically important plant. J. Econ. Taxonomic Bot. 39 (1), 121–123.
Qin, H. Z., Deng, L. L., Zou, R., Tang, J. M., Wei, X., Qin, F., et al. (2021). Comparative study on photosynthetic characteristics of two leaf types of Ficus hirta Vahl. J. Guangxi Acad. Sci. 37 (01), 1–7. doi:10.13657/j.cnki.gxkxyxb.20210429.008
Qin, R. H., Chen, R. X., Pang, Y., Deng, Y. P., and Yang, L. X. (2020). Simultaneous determination of six flavonoids in Ficus hirta Vahl. HPLC. Chin. J. Pharm. Analysis 40 (12), 2244–2249. doi:10.16155/j.0254-1793.2020.12.19
Qin, X. L. (2006). Determination of psoralen in Ficus hirta Vahl from different regions by HPLC. J. Chin. Physician. (02), 270–271.
Qiu, J., Wang, C., and Cao, B. (2012). Professor Liu Youzhang's experience in treating viral hepatitis. Glob. Chin. Med. 5 (11): 846–847.
Quan, T., Zhou, F., Chen, H., Jian, L., Yang, Y., Xia, F., et al. (2022). Ficus hirta Vahl. ameliorates nonalcoholic fatty liver disease through regulating lipid metabolism and gut microbiota. Oxid. Med. Cell Longev. 2022, 3474723. doi:10.1155/2022/3474723
Saini, A., Seni, K., Chawla, P. A., Chawla, V., and Ganti, S. S. (2024). An insight into recent updates on analytical techniques for bioactive alkaloids. Phytochem. Anal. 35 (3), 423–444. doi:10.1002/pca.3338
Shi, Y., Mon, A. M., Fu, Y., Zhang, Y., Wang, C., Yang, X., et al. (2018). The genus Ficus (Moraceae) used in diet: its plant diversity, distribution, traditional uses, and ethnopharmacological importance. J. Ethnopharmacol. 226, 185–196. doi:10.1016/j.jep.2018.07.027
Singh, B., and Sharma, R. A. (2023). Updated review on Indian Ficus species. Arabian J. Chem. 16 (8), 104976. doi:10.1016/j.arabjc.2023.104976
Srikrishna, D., Godugu, C., and Dubey, P. K. (2018). A review on pharmacological properties of coumarins. Mini Rev. Med. Chem. 18 (2), 113–141. doi:10.2174/1389557516666160801094919
Su, X. G., Yang, C. Y. J. D., and Jiang, Y. M. (2007). Determination of metal elements in several decoction botanical drugs by high-performance liquid chromatography. Food Sci. (07), 403–406.
Sun, W., and Shahrajabian, M. H. (2023). Therapeutic potential of phenolic compounds in medicinal plants—natural health products for human health. Molecules 28 (4), 1845. doi:10.3390/molecules28041845
Sun, Y., Qiao, L., Shen, Y., Jiang, P., Chen, J., and Ye, X. (2013). Phytochemical profile and antioxidant activity of physiological drop of citrus fruits. J. Food Sci. 78 (1), C37–C42. doi:10.1111/j.1750-3841.2012.03002.x
Sun, Z. H., Liang, F. L., Chen, Y. C., Liu, H. X., Li, H. H., and Zhang, W. M. (2016). Two new xyloketals from the endophytic fungus endomelanconiopsis endophytica derived from medicinal plant Ficus hirta. J. Asian Nat. Prod. Res. 18 (11), 1036–1041. doi:10.1080/10286020.2016.1188084
Tang, S. J. X. S., Qin, Y. M., Wu, G. Y., and Zhang, P. (2021). Study on microwave extraction of total flavonoids from Ficus hirta Vah. China feed. (15), 37–42+47. doi:10.15906/j.cnki.cn11-2975/s.20211508
Tao, J., Wen, Y., Wang, H., Wang, S., and Chen, H. (2011). Determination of psoralen and bergapten in Radix Fici Hirtae by liquid-liquid microextraction coupled with liquid chromatography-mass spectrometry. Anal. Abstr. 30 (06), 618–623.
Technology, J. K. L. f.P., Fruits, N. T. o., Chen, J., and Wan, C. (2020). Antioxidant, antifungal activities of ethnobotanical Ficus hirta vahl. And analysis of main constituents by HPLC-MS. Nanchang Biomed. Jiangxi Agric. Univ. 8 (1), 15. doi:10.3390/biomedicines8010015
Theobald, P. (2015). Principles of using organic acids in animal nutrition. Agric. Food Sci. 3, 17. Available online at: https://pdfs.semanticscholar.org/3529/208446f1fd200efad0050191b0e3effd420c.
Thien, D. D., Dai, T. D., Sa, N. H., Lieu, N., Thuy, T. T., Hoang Anh, N. T., et al. (2019). A new oleanane triterpene from the leaves of Ficus hirta. Nat. Prod. Res. 33 (21), 3065–3069. doi:10.1080/14786419.2018.1517122
Tholl, D. (2015). Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Engineering-biotechnology 148, 63–106. doi:10.1007/10_2014_295
Tian, E., Liu, Q., Ye, H., Li, F., and Chao, Z. (2017). A DNA barcode-based RPA assay (BAR-RPA) for rapid identification of the dry root of Ficus hirta (Wuzhimaotao). Molecules 22 (12), 2261. doi:10.3390/molecules22122261
Tian, M. X., Gao, Y. L., Qin, X. M., Tian, M. J., Huang, G. L., Mo, Z. H., et al. (2023). Growth traits and the optimal harvest period of Ficus hirta Vahl planted in masson pine. Green Sci. Technol. 25 (03), 119–123. doi:10.16663/j.cnki.lskj.2023.03.041
Tu, Y., Yang, Y., Li, Y., and He, C. (2021). Naturally occurring coumestans from plants, their biological activities and therapeutic effects on human diseases. Pharmacol. Res. 169, 105615. doi:10.1016/j.phrs.2021.105615
Vu, T. T. T., Vu, L. T. K., Le, L. T., Lo, T. T. M., and Chu, M. H. (2023). Analysis of the chloroplast Genome of Ficus hirta Vahl collected in vietnam and proposed barcodes for identifying ficus plants. Curr. Issues Mol. Biol. 45 (2), 1024–1036. doi:10.3390/cimb45020067
Wan, C., Chen, C., Li, M., Yang, Y., Chen, M., and Chen, J. (2017). Chemical constituents and antifungal activity of Ficus hirta Vahl. Fruits. Plants. 6 (4), 44. doi:10.3390/plants6040044
Wan, C., Han, J., Chen, C., Yao, L., Chen, J., and Yuan, T. (2016). Monosubstituted benzene derivatives from Fruits of Ficus hirta and their antifungal activity against phytopathogen penicillium italicum. J. Agric. Food Chem. 64 (28), 5621–5624. doi:10.1021/acs.jafc.6b02176
Wang, C. P., and Chen, C. Y. (2016). “Chemical constituents from the fruits of Ficus hirta,” in The 30th Annual academic conference of the Chinese chemical society (China: China Conference).
Wang, D. H., Tang, K. J., Zeng, L., Wang, Z. H., Wang, S., Zhou, Y. L., et al. (2023). Extraction process optimization of total coumarins by response surface methodology from Ficus hirta and evaluation of their antioxidant activity. J. Zhongkai Univ. Agric. 36 (04), 45–51.
Wang, L. X., Wang, H. L., Huang, J., Chu, T. Z., Peng, C., Zhang, H., et al. (2022a). Review of lignans from 2019 to 2021: newly reported compounds, diverse activities, structure-activity relationships, and clinical applications. Phytochemistry 202, 113326. doi:10.1016/j.phytochem.2022.113326
Wang, M., He, R. R., Li, Y. F., Han, F. X., Qin, Y. Y., and Su, Y. B. (2015). Ficus hirta Vahl the protective effect of aqueous extract on restrained stress liver injury. Chin. J. Hosp. Pharm. 35 (06), 522–525. doi:10.13286/j.cnki.chinhosppharmacyj.2015.06.12
Wang, S. H., Hu, Y. L., and Liu, T. X. (2019). Plant distribution and pharmacological activity of flavonoids. Traditional Med. Res. 4 (5), 269–287–287. doi:10.53388/tmr20190824131
Wang, X. P., Duan, L. J., Chen, X. B., Li, L. M., Lu, Q. F., and Li, J. Q. (2010a). Ficus hirta Vahl experimental study on the antibacterial effect of aqueous extraction. Lishizhen Med. Materia Medica Res. 21 (07), 1692–1693.
Wang, X. P., Huang, X., Chen, X. B., and Cen, Y. W. (2010b). Ficus hirta Vahl study on the protection and repair of ∼ (60) Co γ. J. Chin. Med. Mater. 33 (10), 1614–1618. doi:10.13863/j.issn1001-4454.2010.10.023
Wang, X. P., Huang, X., Chen, X. B., Meng, L., Li, L. M., and Lu, Q. F. (2010c). Effect of Yaoyao Ficus hirta Vahl on lung histopathomorphology of irradiated mice. J. Yulin Normal Univ. 31 (05), 80–83. doi:10.13792/j.cnki.cn45-1300/z.2010.05.013
Wang, X. P., Huang, X., Duan, L. J., Lu, Q. F., and Cen, Y. W. (2011a). Ficus hirta Vahl the protective effect of aqueous extract on DNA damage in radiation-induced lung cells in mice. China Dispens. 22 (03), 201–203.
Wang, X. Y., Gao, X. X., Wang, R. Y., and Niu, Y. L. (2022b). Advances of LSPR metal-catalyzed oxidation of alcohols to aldehydes and esters and their application in the daily chemical field. China Surfactant Deterg. and Cosmet. 52 (06), 664–671.
Wang, Y., Ye, M. R., Tang, L. H., and Zhou, T. N. (2011b). Ficus hirta Vahl experimental study on protecting gastric mucosa and improving microcirculation. Shizhen Natl. Med. 22 (05), 1181–1182.
Wang, Y. J., Zhao, R., Li, S. H., Qin, W., and Wei, N. Q. (2018). Study on the anti-aging of the different extracts of radix fici simplicissimae from Guangxi. Popular Sci. and Technol. 20 (1), 57–59.
Wen, J., Wang, X. P., Huang, W. Q., Chen, X. B., and Zhao, S. L. (2010). Ficus hirta Vahl measurement and analysis of mineral elements. Lishizhen Med. Materia Medica Res. 21 (06), 1399–1400.
Wen, K., Fang, X., Yang, J., Yao, Y., Nandakumar, K. S., Salem, M. L., et al. (2021). Recent research on flavonoids and their biomedical applications. Curr. Med. Chem. 28 (5), 1042–1066. doi:10.2174/0929867327666200713184138
Wu, W. Y., and Zhou, L. M. (2023). Research on varieties and application of radix fici Hirtae. Chin. Med. Bull. 22 (2023), 57–60. doi:10.14046/j.cnki.zyytb2002.2023.11.004
Xia, L., Chen, C. Y., Li, Q. H., Kun, J. R., Lai, W., Li, H. T., et al. (2016). Antifungal constituents from Fruits of Ficus hirta extracts against citrus postharvest pathogens. Postharvest Pathog. 39 (03), 187–191.
Xiao, R., Luo, G., Liao, W., Chen, S., Han, S., Liang, S., et al. (2022). Association of human gut microbiota composition and metabolic functions with Ficus hirta Vahl dietary supplementation. Npj Sci. Food 6 (1), 45. doi:10.1038/s41538-022-00161-3
Xiao, R. M. (2022). Effects of Ficus hirta Vahl tonic diet on gut microbial diversity and function analysis of its non-starch polysaccharides. Guangzhou: South China University of Technology.
Xing, L. G., Zou, F., Liang, J., Zhang, C. F., Li, Z. Z., Luo, D. G., et al. (2022). Control effect of Chinese herbal medicine compound pesticide on black spot disease of brassica juncea coss. var.tumida tsen et lee black spot. South China Agric. 16 (17), 90–93+97. doi:10.19415/j.cnki.1673-890x.2022.17.021
Ya, J., Zhang, X. Q., Wang, Y., Zhang, Q. W., Chen, J. X., and Ye, W. C. (2010). Two new phenolic compounds from the roots of Ficus hirta. Nat. Prod. Res. 24 (7), 621–625. doi:10.1080/14786410902847377
Yan, W. W., Zhang, Y. H., Liu, J. L., Wu, J., Zhou, X., Liu, S., et al. (2015). Effect of psoralen on proliferation and apoptosis of gastric cancer cell line BGC-803 and its mechanism. Jilin J. Chin. Med. 10, 1064–1067. doi:10.13463/j.cnki.jlzyy.2015.10.028
Yang, J., Wei, D. F., Wang, W. X., and Cheng, W. D. (2015). Effects of aqueous extract of Ficus hirta on cellular immunity in immunosuppressed mice. Pharmacol. Clin. Chin. Materia Medica. 31 (06), 111–114. doi:10.13412/j.cnki.zyyl.2015.06.033
Yang, L., Jiang, R. Q., Zhang, H. Y., Li, S. Y., Wu, M. L., and Zhang, C. J. (2023). Optimisation of the process of preparing effervescent tablets from aqueous extraction of Ficus hirta Vahl. Food Ind. Technol., 1–13. doi:10.13386/j.issn1002-0306.2023050326
Yang, M., and Xia, F. (2012). Effect of Radix Fici Hirtae on gastrointestinal function of spleen rat model. Her. Med. 31 (10), 1264–1267.
Yang, S. X., Li, W., Xie, Z. P., Lu, H., and Cheng, W. D. (2019). Study on the effect and mechanism of the extract of Ficus hirta Vahl on apoptosis of HepG2 cells. J. Chin. Med. Mater. 42 (07), 1670–1673. doi:10.13863/j.issn1001-4454.2019.07.043
Yang, Y., Chen, Y., Feng, D., Wu, H., Long, C., Zhang, J., et al. (2024). Ficus hirta Vahl. ameliorates liver fibrosis by triggering hepatic stellate cell ferroptosis through GSH/GPX4 pathway. J. Ethnopharmacol. 334, 118557. doi:10.1016/j.jep.2024.118557
Yang, Y., Zheng, K., Mei, W., Wang, Y., Yu, C., Yu, B., et al. (2018). Anti-inflammatory and pro-resolution activities of bergapten isolated from the roots of Ficus hirta in an in vivo zebrafish model. Biochem. Biophysical Res. Commun. 496 (2), 763–769. doi:10.1016/j.bbrc.2018.01.071
Yang, Y. J., Dai, J., and Chen, M. G. (2013). Studies on the chemical constituents of Ficus hirta vahl,2013 Chinese pharmaceutical congress and the 13th Chinese pharmacist week, Nanning.
Ye, B. Y., Peng, X. M., and Deng, G. H. (2016). Study on the anti-aging activity of the extraction of radix fici simplicissimae. Inn. Mong. J. Traditional Chin. 35 (12), 112–113. doi:10.16040/j.cnki.cn15-1101.2016.12.095
Ye, B. Y., Peng, X. M., and Deng, G. H. (2017). Study on the anti-aging activity of the extraction of radix fici simplicissimae. Inn. Mong. J. Traditional Chin. 36 (11), 90–91. doi:10.16040/j.cnki.cn15-1101.2017.11.091
Ye, S. X. (2021). Study on the Anti-aging activity of the extraction of Ficus hirta Vahl. Xiamen: Xiamen University.
Ye, T., Shi, R., Wu, Y., Wu, Y., Li, Y., and Yu, B. (2019). Research progress of Ficus hirta Vahl on their chemical constituents and pharmacological activities. J. Canton Pharm. Univ. 35 (4), 591–596.
Ye, X., Tian, W., Wang, G., Zhang, X., Zhou, M., Zeng, D., et al. (2020). Phenolic glycosides from the roots of Ficus hirta Vahl. and Their antineuroinflammatory activities. J. Agric. Food Chem. 68 (14), 4196–4204. doi:10.1021/acs.jafc.9b07876
Ye, X. L., Lai, J. J., and Zhang, J. B. (2021). Content determination of psoralen in five-finger figs by HPLC and quality assessment. Chin. J. Mod. Drug Appl. 15 (14), 239–242. doi:10.14164/j.cnki.cn11-5581/r.2021.14.092
Ye, X. S., Tian, W. J., Liu, X. Z., Zhou, M., Zeng, D. Q., Lin, T., et al. (2022). Lignans and phenylpropanoids from the roots of Ficus hirta and their cytotoxic activities. Nat. Prod. Res. 36 (15), 3840–3849. doi:10.1080/14786419.2021.1892099
Yi, J., Li, S., Wang, C., Cao, N., Qu, H., Cheng, C., et al. (2019). Potential applications of polyphenols on main ncRNAs regulations as novel therapeutic strategy for cancer. Biomed. Pharmacother. 113, 108703. doi:10.1016/j.biopha.2019.108703
Yi, T., Chen, Q., He, X., So, S., Lo, Y., Fan, L., et al. (2013). Chemical quantification and antioxidant assay of four active components in Ficus hirta root using UPLC-PAD-MS fingerprinting combined with cluster analysis. Chem. Central J. 7 (1), 115. doi:10.1186/1752-153x-7-115
You, H. L., Chen, Y., Xu, Q. S., Xiao, J. D., Xin, X. H., Huang, C. X., et al. (2011). Modified after finger fig treatment of hepatitis B clinical observation of 40 Cases of Liver Cirrhosis. Liaoning J. Traditional Chin. Med. 38 (04), 683–684. doi:10.13192/j.ljtcm.2011.04.112.youhl.039
Yu, H., Liang, D., Tian, E., Zheng, L., and Kjellberg, F. (2018). Plant geographic phenotypic variation drives diversification in its associated community of a phytophagous insect and its parasitoids. Bmc Ecol. Evol. 18 (1), 134. doi:10.1186/s12862-018-1239-5
Yuan, S. N., Tu, H. Q., Pang, J., Huang, J. X., and Wang, X. Q. (2023). Effects of planting patterns and planting years on biomass and mineral element content of Ficus hirta Vahl. Chin. J. Trop. Crops. 44 (10), 2043–2050.
Yue, H. C. (2021). Preparation Technology of spray drying powder of compound Ficus hirta Vahl. Harbin: Harbin University of Commerce.
Za, Q., Zang, X. Q., Wang, Y., Li, Y. L., and Ye, W. C. (2008). Studies on flavonoids and coumarins in the roots of Ficus hirta Vahl. Chem. Industry For. Prod. 28 (06), 49–52.
Zeng, X. C. C., Lai, S. H., and Chen, S. N. (2002). Ficus hirta Vahl's effect on the eliminating cough, sputum, and stridor. Chin. J. Inf. Traditional Chin. Med. (02), 30–32.
Zeng, X. P., Li, R. L., Liang, X. Y., Zhu, H. B., Zhang, D., Luo, M., et al. (2020). Experimental study on the prevention and treatment of gastric mucosal injury by Wuzhimaotao and Xianhecao. Guangzhou Univ. Traditional Chin. Med. 31 (05), 583–589. doi:10.19378/j.issn.1003-9783.2020.05.013
Zeng, Y. W., Liu, X. Z., Lv, Z. C., and Peng, Y. H. (2012). Effects of Ficus hirta Vahl. (Wuzhimaotao) extracts on growth inhibition of HeLa cells. Exp. Toxicol. Pathol. 64 (7-8), 743–749. doi:10.1016/j.etp.2011.01.009
Zhang, B., Zhang, M., Tian, J., Zhang, X., Zhang, D., Li, J., et al. (2024). Advances in the regulation of radiation-induced apoptosis by polysaccharides: a review. Int. J. Biol. Macromol. 263, 130173. doi:10.1016/j.ijbiomac.2024.130173
Zhang, M. H., Zhong, M. H., and Huang, Y. N. (2009). Study on the processing technology of Radix Fici Simplicissimae soft sweets. Sci. Technol. Food Industry (01), 217–218. doi:10.13386/j.issn1002-0306.2009.01.054
Zhang, R., and Du, J. (2021a). Progress in the study of hairy Ficus hirta Vahl. Mod. Chin. Med. 41 (05), 48–52. doi:10.13424/j.cnki.mtcm.2021.05.008
Zhang, R., and Du, J. (2021b). Protective effect of Fici Hirtae Radix on mice with alcoholic liver injury. J. Harbin Univ. Commer. Nat. Sci. Ed. 37 (02), 139–144. doi:10.19492/j.cnki.1672-0946.2021.02.002
Zhang, R., and Qu, Z. Y. D. J. (2021). Antioxidant protective mechanism of alcohol extract of Fici Hirtae Radix on mice with alcoholic liver injury through Nrf2-HO-1/CYP2E1 pathway. Traditional Chin. Drug Res. Clin. Pharmacol. 32 (12), 1769–1775. doi:10.19378/j.issn.1003-9783.2021.12.006
Zhao, L. P., Di, B., and Feng, F. (2008). Chemical constituents from the roots of Ficus hirta. Pharm. Clin. Res. (01), 5–7. doi:10.13664/j.cnki.pcr.2008.01.004
Zhao, W., Zheng, X. D., Tang, P. Y. Z., Li, H. M., Liu, X., Zhong, J. J., et al. (2023). Advances of antitumor drug discovery in traditional Chinese medicine and natural active products by using multi-active components combination. Med. Res. Rev. 43 (5), 1778–1808. doi:10.1002/med.21963
Zhen, D. D., Qiu, Q., and Zhen, H. S. (2018). Study on detection of pesticide residues in radix fici Hirtae. Guangzhou Chem. Ind. 46 (03), 100–102.
Zheng, R. R., Za, W., Wang, W. J., Yang, H. B., Zhang, Q. W., Zhang, X. Q., et al. (2013a). Chemical studies on roots of Ficus hirta. China J. Chin. Materia Medica 38 (21), 3696–3701.
Zheng, X., Qiu, Y., Zhong, W., Baxter, S., Su, M., Li, Q., et al. (2013b). A targeted metabolomic protocol for short-chain fatty acids and branched-chain amino acids. Metabolomics 9, 818–827. doi:10.1007/s11306-013-0500-6
Zhong, J. J., Liu, Z. Q., Yuan, Y. E., Jiang, B., and Xu, H. (1993). HPLC determination of psoralen and bergapten in Ficus hirta vahl. Traditional Chin. Drug Res. and Clin. Pharmacol. doi:10.19378/j.issn.1003-9783.2007.02.014
Zhong, X. Q., and Xu, H. H. (2000). Variety research and textual criticism of Ficus hirta Vahl. J. Chin. Med. Mater. (06), 361–362. doi:10.13863/j.issn1001-4454.2000.06.030
Zhou, F. Y., Quan, T., Xiang, S. J., Li, H., Chen, H. Y., Yang, Y. X., et al. (2022). Metabolomic mechanisms of Radix Fici Hirtae against carbon tetrachloride-induced acute liver damage in mice. Evidence-based Complementary Altern. Med. 2022, 9157465. doi:10.1155/2022/9157465
Zhou, J. S. (2013). Talking about nanqi. Wuzhualong. Chin. Med. Cult. 8 (06), 57–58. doi:10.16307/j.1673-6281.2013.06.023
Zhou, T. N., Tang, L. H., Huang, S. C., Liu, D. D., Wang, Y., Liu, L. F., et al. (2009a). Study on the antitussive and antiasthmatic effects of radix fici Hirtae. J. Chin. Med. Mater. 32 (04), 571–574. doi:10.13863/j.issn1001-4454.2009.04.035
Zhou, T. N., Wang, Y., Liu, D. D., Tang, L. H., Xiao, X. J., Liu, L. F., et al. (2009b). Experimental study on the beneficial effects of different extracts of Ficus hirta Vahl. J. Chin. Med. Mater. 32 (05), 753–757. doi:10.13863/j.issn1001-4454.2009.05.015
Keywords: Ficus hirta (Moraceae) Vahl, botanical characterization, traditional medicine, phytochemistry, pharmacology, toxicology
Citation: Chen M, Liu J, Zou S, Yuan J, Pan J, Lin Z, Wu Y and Zhou B (2025) A review on the ethnopharmacology, metabolites, pharmacological uses, and toxicology of Ficus hirta (Moraceae) Vahl. Front. Pharmacol. 16:1545348. doi: 10.3389/fphar.2025.1545348
Received: 14 December 2024; Accepted: 31 March 2025;
Published: 24 April 2025.
Edited by:
Adolfo Andrade-Cetto, National Autonomous University of Mexico, MexicoReviewed by:
Smith B. Babiaka, University of Tuebingen, GermanyJohra Khan, Majmaah University, Saudi Arabia
Mubarak Hussaini Ahmad, Ahmadu Bello University, Nigeria
Copyright © 2025 Chen, Liu, Zou, Yuan, Pan, Lin, Wu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanchun Wu, d3V5YW5jaHVuXzMzMzNAMTI2LmNvbQ==; Bei Zhou, em9leTYxOUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Minghui Chen
Minghui Chen Jiao Liu
Jiao Liu Sihua Zou1
Sihua Zou1 Jingquan Yuan
Jingquan Yuan