- 1Chalmers P. Wylie Ambulatory Care Center, Columbus, OH, United States
- 2Kyowa Kirin, Inc., Princeton, NJ, United States
- 3KMK Consulting Inc., Morristown, NJ, United States
- 4Texas Movement Disorder Specialists, PLLC., Georgetown, TX, United States
Background: Medication adherence and persistence are associated with better outcomes for patients with Parkinson’s disease.
Objectives: To evaluate 12-month adherence and persistence with istradefylline and identify factors associated with persistence among patients.
Methods: A retrospective cohort study was conducted using IQVIA longitudinal prescription (LRx) and medical (Dx) claims data. Adult patients initiating istradefylline between 2019 and 2022 with ≥1 LRx and ≥1 Dx claim every 6 months over 12-month baseline and follow-up periods were included. Adherence was measured by the proportion of days covered (PDC) and medication possession ratio (MPR). Persistence was measured by the duration of prescription fills, allowing for a ≤60-day gap. Multivariate logistic regression was used to evaluate factors associated with 12-month persistence.
Results: Among 2,045 patients, 76.0% were covered by Medicare Advantage and 23.9% were commercially insured. Over 12 months of follow-up, median adherence (both PDC and MPR) was 74.0% and median persistence was 276 days. In a subgroup analysis, median adherence and persistence were significantly greater in patients with Medicare Advantage versus commercial insurance (PDC and MPR: 82.2% vs. 49.3%; persistence: 365 days vs. 180 days; all P < 0.001). Treatment initiated at 40 mg versus 20 mg (odds ratio [OR]: 1.33 [95% confidence interval [CI]: 1.10, 1.62]) and Medicare Advantage versus commercial insurance (OR: 1.79 [95% CI: 1.42, 2.26]) were independently associated with longer persistence.
Conclusion: Patients initiating istradefylline at 40 mg versus 20 mg and those with Medicare Advantage versus commercial insurance were more likely to have higher adherence and longer persistence with istradefylline.
1 Introduction
Parkinson’s disease (PD), a progressive, degenerative disorder characterized by motor and non-motor symptoms, is estimated to affect between 6 and 8.5 million people worldwide (Armstrong and Okun, 2020; American Academy of Neurology Institute, 2021; World Health Organization, 2023; Hindle, 2010; National Institute of Neurological Disorders and Stroke, 2023). In the United States (US), cases of PD are projected to rise to more than 1.2 million by 2030 as the population ages (American Academy of Neurology, 2021; Marras et al., 2018; United States Census Bureau, 2020).
The “gold standard” treatment option for the motor symptoms of PD is levodopa, typically formulated as carbidopa–levodopa (American Academy of Neurology, 2021; Schade et al., 2020; Cummins and Cates, 2022; Schapira, 2007; Grimes et al., 2019; National Institute for Health and Care Excellence, 2017). Although levodopa can provide robust symptom control it cannot stop disease progression and eventually becomes less effective in most patients, leading to motor symptom fluctuations and dyskinesia (Armstrong and Okun, 2020; National Institute of Neurological Disorders and Stroke, 2023; Schapira, 2007; Berger et al., 2020; Isaacson et al., 2022a; Isaacson et al., 2022b). Patients consequently experience “OFF” episodes between medication doses, when worsening of symptoms and functional impairment occur (Armstrong and Okun, 2020; Chou et al., 2018). After 5 years of levodopa therapy, up to 50% of patients will experience “OFF” episodes, increasing to almost 100% after 10 years (Isaacson et al., 2022a; Perepezko et al., 2020; Kalia and Lang, 2015). As PD progresses, patients often require increased dosages of levodopa and the use of additional medications to manage symptoms. Adjunctive therapies to levodopa that target the dopaminergic system, including dopamine agonists, COMT (catechol-O-methyl transferase) inhibitors, and MAO-B (monoamine oxidase-B) inhibitors, may be added to the treatment regimen of patients to help control “OFF” episodes (American Academy of Neurology, 2021; Schapira, 2007; National Institute for Health and Care Excellence, 2017; Berger et al., 2020; Isaacson et al., 2022a).
Istradefylline is the first non-dopaminergic adjunctive therapy to carbidopa–levodopa that specifically targets the adenosine A2A pathway. While levodopa and other dopaminergic therapies restore dopaminergic stimulation of the indirect GABAergic striatal output pathway to reverse the enhanced GABAergic tone to the external globus pallidus (GPe) and thereby improve motor function, this effect is opposed by the excitatory effects of adenosine, which stimulates the A2A receptor to increase GABAergic transmission. By comparison, as an adenosine A2A antagonist istradefylline is believed to block adenosine A2A receptors in the striatum and GPe, thereby reducing the excessive output of the indirect striatal output pathway of the basal ganglia and improving motor control by removing a “brake” from the indirect pathway (Jenner et al., 2021; Hauser et al., 2021). The treatment, which is indicated as adjunctive therapy to carbidopa–levodopa for adult patients with PD who experience “OFF” episodes between medication doses, was approved in the US in 2019 (Armstrong and Okun, 2020; Chou et al., 2018; NOURIANZ, 2023). Across multiple clinical trials and real-world studies, istradefylline was well tolerated and demonstrated a sustained reduction in “OFF” time (National Institute for Health and Care Excellence, 2017; Hauser et al., 2021; LeWitt et al., 2008; Mizuno et al., 2010; Kondo et al., 2015; Di Luca et al., 2022; Takahashi et al., 2022; Hatano et al., 2024; Hattori et al., 2023).
Medication non-compliance has been reported to be between 10% and 67% in patients with PD (Malek and Grosset, 2015; Davis et al., 2010; Richy et al., 2013; Tarrants et al., 2010). As PD is a chronic, progressive disease that requires ongoing pharmacological treatment, the burden of increasingly complex medication regimens tends to affect treatment compliance, as well as quality of life (Malek and Grosset, 2015; Castro et al., 2021; Soileau et al., 2023; Zipprich et al., 2021; Wei et al., 2013). Adherence and persistence with PD medications have been shown to be associated with better patient outcomes and a lower healthcare burden (Malek and Grosset, 2015; Davis et al., 2010; Richy et al., 2013; Castro et al., 2021; Shoval et al., 2017). Patients with PD who are compliant with therapy are more likely to achieve the treatment goals of improved motor and non-motor functions, have slower disease progression, and experience improved quality of life (Straka et al., 2019; Grosset et al., 2009). Thus, it is important to understand whether suboptimal outcomes in patients with PD reflect the effectiveness of a given treatment regimen or might be attributable to poor treatment compliance (Tarrants et al., 2010). Insights into treatment compliance with newer medications such as istradefylline that have distinct mechanisms of action would therefore be valuable for clinical decision-making.
To date, no study has examined treatment adherence and persistence with istradefylline in real-world clinical practice. This study was performed to understand the real-world utilization patterns of istradefylline among patients with PD in the US and to assess adherence and persistence with treatment, including an examination of potential factors associated with persistence.
2 Materials and methods
2.1 Study design
This was a US retrospective cohort study based on de-identified, patient-level administrative claims data from the longitudinal IQVIA Anonymized Patient Level Database (APLD). Medical data in the APLD are drawn from approximately 1 billion outpatient medical claims per year and pharmacy data consist of claims covering approximately 90% of all dispensed prescriptions from US retail pharmacies.
Adults (≥18 years) with PD who initiated istradefylline treatment between September 1, 2019 and March 31, 2022 were included in the study. Patients were required to have ≥1 medical claim and ≥1 pharmacy claim every 6 months over 12-month baseline and follow-up periods. The diagnosis of PD was verified by having ≥1 medical claim with an International Classification of Diseases 10th Revision, Clinical Modification (ICD-10-CM) diagnosis code of G20.x, G21.x during the 12-month baseline period. Patients with evidence of clinical trial participation (ICD-10-CM of Z00.6) during the study period were excluded.
Patients were evaluated overall. In addition, select comparisons were made between subgroups of patients with Medicare Advantage versus commercial insurance.
2.2 Outcomes and measures
Patient characteristics were evaluated in the baseline period and included age, gender, geographic region, and insurance type, as well as Charlson Comorbidity Index (CCI; Quan adaption) (Quan et al., 2005; Quan et al., 2011) scores, dose of levodopa-containing medications, levodopa equivalent daily dose (LEDD), and the presence of common PD-related conditions. The LEDD is an artificial calculation of the total levodopa dose a patient is receiving daily across multiple PD treatments. LEDD is frequently used in research and clinical practice settings as a standardized method for comparing different PD medication regimens (Schade et al., 2020; Tomlinson et al., 2010; Verber et al., 2020; Nyholm and Jost, 2021; Julien et al., 2021).
Istradefylline utilization and treatment adherence and persistence were evaluated over the 12-month follow-up period. Adherence was measured using two methods: the proportion of days covered (PDC), calculated as the percentage of days covered by filled istradefylline prescriptions over 12 months; and medication possession ratio (MPR), calculated as the percentage of days of supply of istradefylline prescriptions over 12 months, which credits overlapped prescriptions. Persistence was measured as the duration of time a patient continued to fill istradefylline prescriptions over 12 months, allowing for a 60-day grace period (i.e., gap of ≤60 days).
Additionally, a risk factor analysis was conducted to explore potential variables associated with 12-month persistence with istradefylline treatment.
2.3 Statistical analyses
Continuous variables were summarized with mean, standard deviation (SD), median, interquartile range (IQR), and minimum and maximum values. Categorical variables were described with counts and percentages. Subgroup comparisons were performed using the Wilcoxon signed-rank test. Kaplan–Meier (KM) curves were used to assess 12-month persistence, as determined by the time to treatment discontinuation.
Multivariate logistic regression was used to evaluate factors associated with 12-month persistence, including patient demographics, CCI, istradefylline initial dose, use of PD adjunctive medications by class, number of PD medications, levodopa (carbidopa–levodopa, carbidopa–levodopa–entacapone, or levodopa inhalation powder) dose, LEDD, and presence of PD-related conditions.
3 Results
3.1 Sample selection and patient characteristics
Overall, 2,045 patients who initiated istradefylline from September 1, 2019 to March 31, 2022 were identified and included in the study (Figure 1), including 1,554 (76.0%) patients categorized as having Medicare Advantage healthcare coverage and 488 (23.9%) categorized as commercially insured (Table 1). As only two patients (0.1%) in the study had other insurance coverage (Medicaid), they were included within the Medicare Advantage group (three patients classified as self-pay were not included in the subgroup analyses).
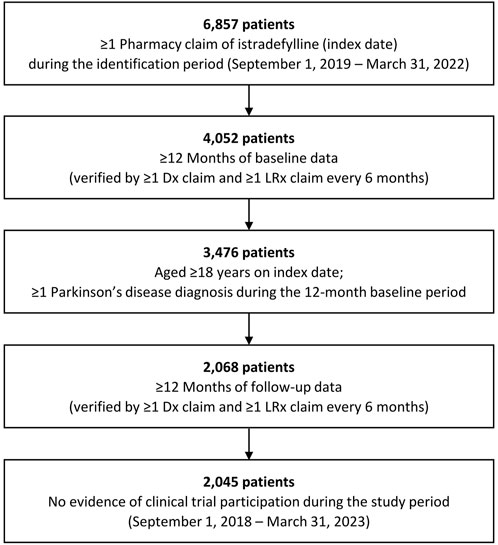
Figure 1. Selection for study cohort of patients with Parkinson’s disease with istradefylline prescription fills. Dx: medical; LRx: prescription.
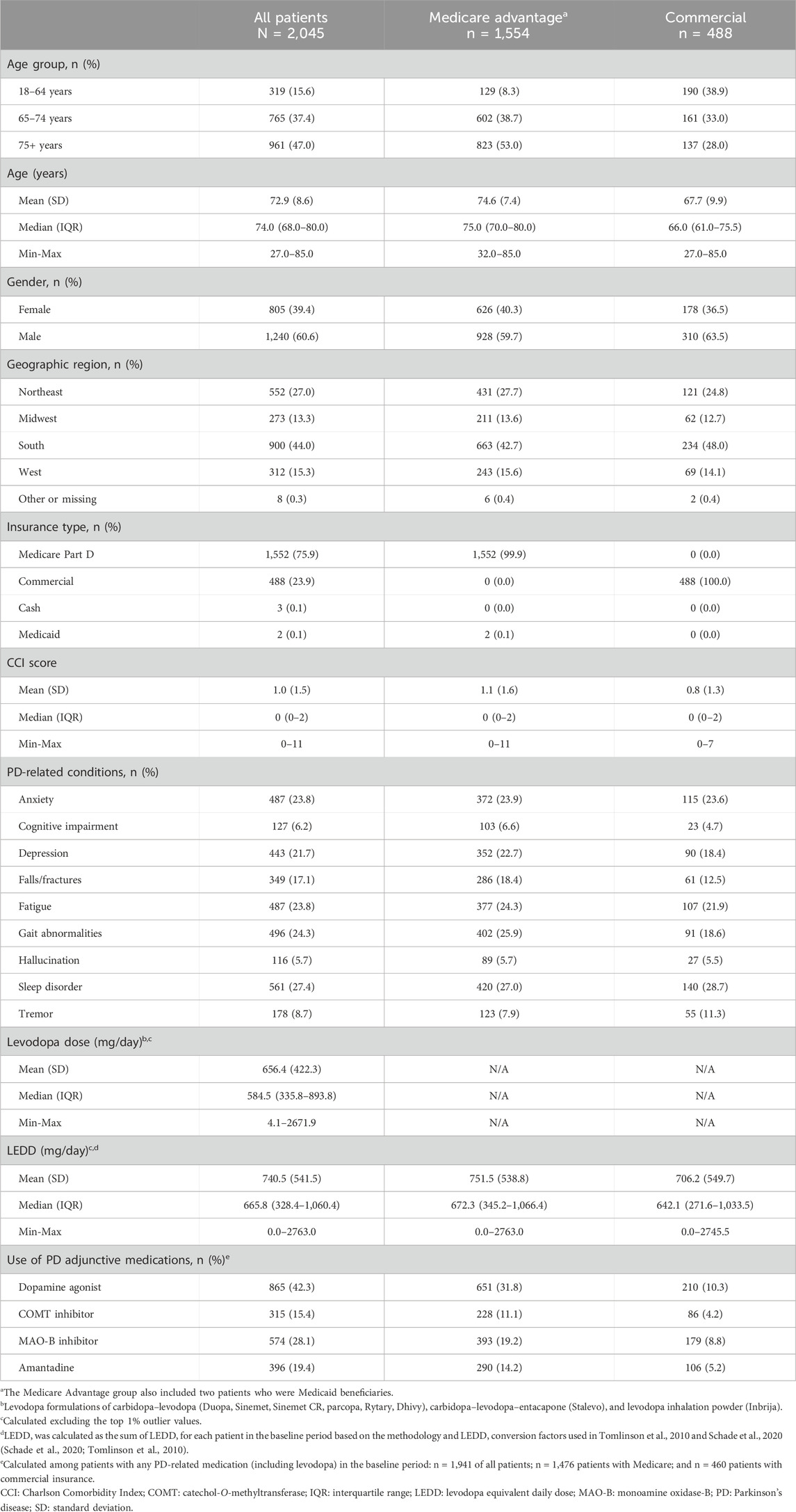
Table 1. Baseline characteristics of patients with Parkinson’s disease with istradefylline prescription fills, overall and by insurance status.
The mean age of the study population was 72.9 years and approximately 40% of patients were female. The mean CCI score was 1. PD-related conditions observed in >20% of patients included anxiety, depression, fatigue, gait abnormalities, and sleep disorders. Among all patients, the mean baseline dose of levodopa-containing medications was 656.4 mg/day, while the mean LEDD was 740.5 mg/day. Among patients with any recorded PD-related medication in the baseline period (n = 1,941), the most used PD adjunctive medications were dopamine agonists (42.3% of patients) and MAO-B inhibitors (28.1% of patients).
3.2 Istradefylline utilization
Over the 12-month follow-up period, patients had a median of 5 (IQR: 2–11) istradefylline prescription fills, with a higher number of prescriptions observed among those with Medicare Advantage coverage than commercial insurance (5 [IQR: 2–11] vs. 4 [IQR: 2–10]) (Table 2). Approximately two-thirds of patients (67.3%) initiated istradefylline treatment at a dose of 20 mg. Among patients who initiated istradefylline at 20 mg, 80.1% had prescription fills for 20 mg at 12-month follow-up, while 19.8% had increased their dose to 40 mg (Figure 2). Among patients who initiated istradefylline at 40 mg (32.1% of all patients), 95.7% had prescription fills for 40 mg at 12-month follow-up, while 4.3% had changed to 20 mg.
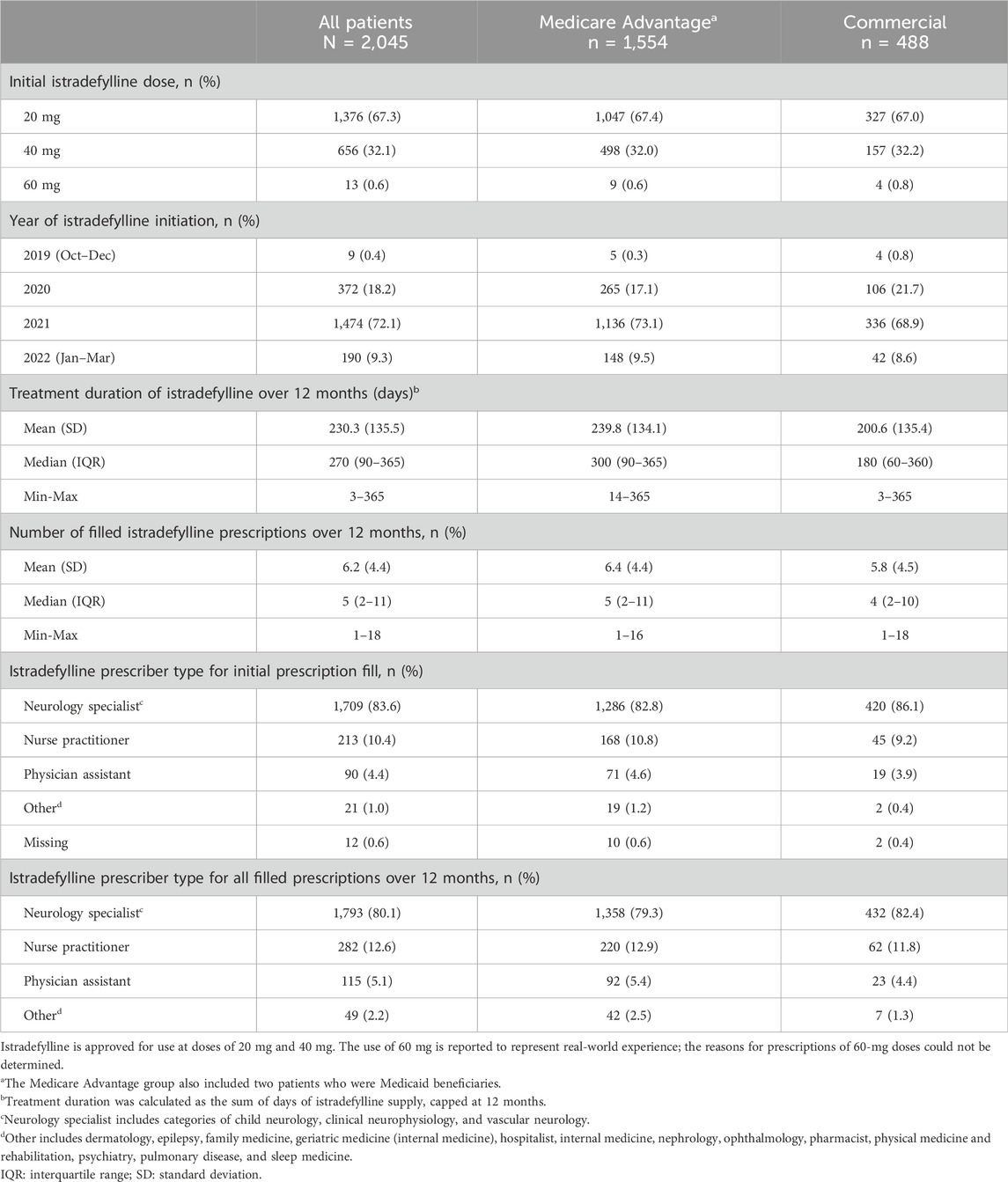
Table 2. Istradefylline prescription patterns in the 12 months after patients initiated this treatment, overall and by insurance status.
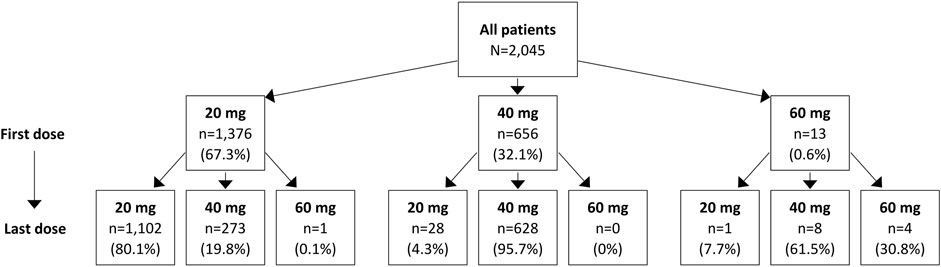
Figure 2. Istradefylline dose change for overall study population of patients with Parkinson’s disease with istradefylline prescription fills. Istradefylline is approved for use at doses of 20 mg and 40 mg. The use of 60 mg is reported to represent real-world experience; the reasons for prescriptions of 60-mg doses could not be determined.
The mean (SD) duration of istradefylline treatment was 230.3 (135.5) days, which was similar regardless of insurance status (Table 2). Istradefylline was initially prescribed by neurology specialists (including child neurology, clinical neurophysiology, and vascular neurology sub-specialties) in >80% of cases across the study population, and this proportion remained similar across all istradefylline prescriptions during the study period.
3.3 Istradefylline adherence and persistence
Over 12 months, the median treatment adherence with istradefylline was 74.0% as measured by both PDC and MPR and the median duration of persistence was 276 days (Table 3). Median adherence was higher and persistence was longer in patients with Medicare Advantage coverage than among those with commercial insurance (PDC: 82.2% vs. 49.3%; MPR: 82.2% vs. 49.3%; persistence: 365 days vs. 180 days; P < 0.001 for all comparisons). An adherence rate of at least 80% (PDC or MPR ≥0.8) over 12 months was achieved by almost half of all patients as measured by both PDC (47.1%) and by MPR (46.8%). Approximately 40% of patients had a 12-month adherence rate of less than 50% (PDC <0.5: 41.4%; MPR <0.5: 41.5%). Slightly over half of all patients (52.1%) discontinued treatment over the 12-month follow-up period (Figure 3). Treatment discontinuation rates were lower among patients with Medicare Advantage coverage (48.6%) than those with commercial insurance (62.9%).
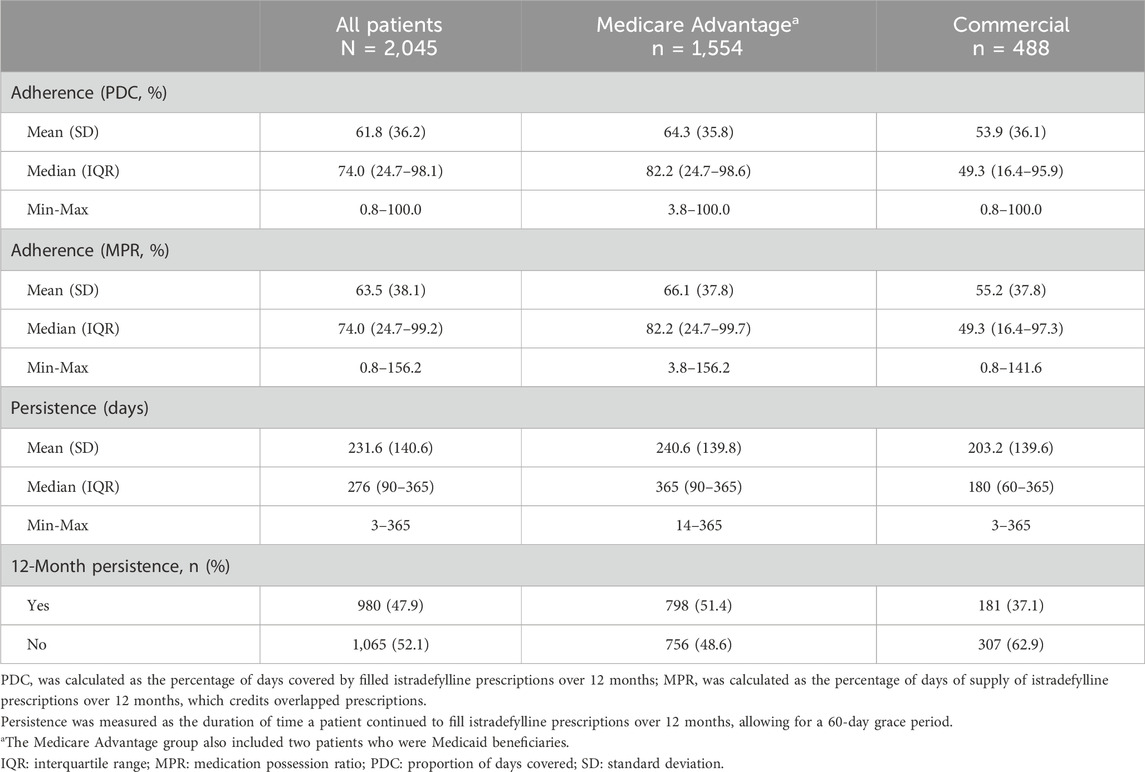
Table 3. Adherence and persistence to istradefylline over 12 months, overall and by insurance status.
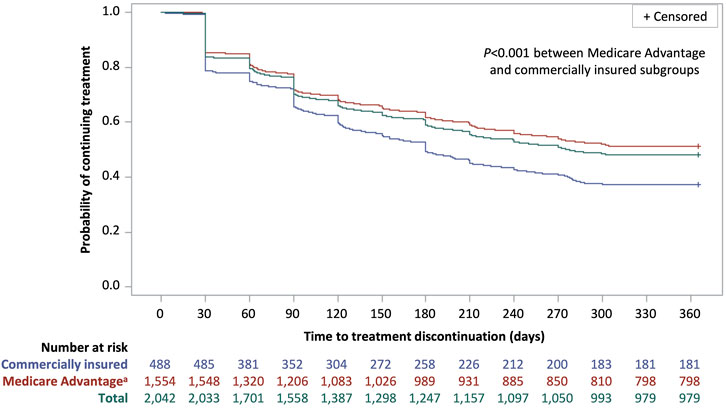
Figure 3. Kaplan–Meier curve of time to treatment discontinuation for patients with Parkinson’s disease with istradefylline prescription fills, overall and by insurance status. Discontinuation was defined as a treatment gap of >60 days between the exhaustion of a prescription supply and the next prescription refill. Patients who did not discontinue treatment were considered to be persistent to treatment. aThe Medicare Advantage group also included two patients who were Medicaid beneficiaries.
In the multivariate regression analysis, istradefylline initiated at a dose of 40 mg (OR: 1.33 [95% CI: 1.10, 1.62]) and Medicare Advantage coverage (OR: 1.79 [95% CI: 1.42, 2.26]) were both independently associated with longer persistence with treatment in this study (Table 4).
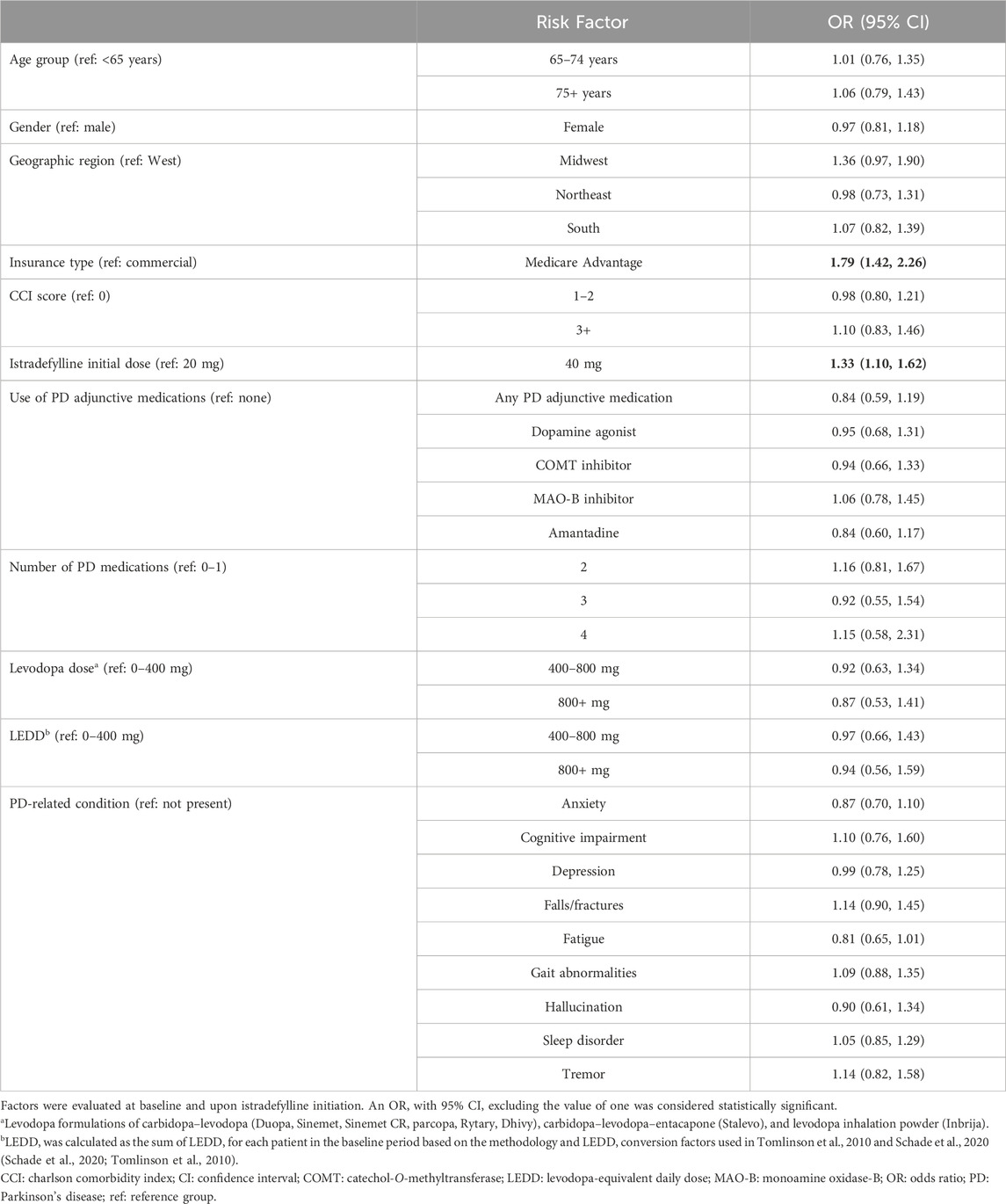
Table 4. Factors associated with 12-month persistence with istradefylline among patients with Parkinson’s disease.
4 Discussion
This retrospective cohort study provides insights into the real-world use of istradefylline in patients with PD who initiated treatment since the drug’s US approval in 2019. While treatment adherence or persistence with dopaminergic PD medications has been evaluated in previous studies, to our knowledge this study provides the first analysis of adherence and persistence with istradefylline, a non-dopaminergic adjunctive therapy to carbidopa–levodopa.
Among patients who initiated istradefylline at a 20-mg dose, approximately 80% had prescription fills for 20 mg at 12 months, while nearly 20% had increased their dose to 40 mg. By comparison, nearly all patients (96%) who initiated istradefylline at 40 mg had prescription fills for 40 mg at 12 months, with only a small proportion (<5%) lowering their dose to 20 mg. Over 12 months of follow-up from initiation of istradefylline, a median treatment adherence of 74% (using both measures of PDC and MPR) and a median persistence duration of 276 days were observed.
Given that previous studies in PD assessed treatment patterns with dopaminergic agents, comparisons with the current analysis of istradefylline are limited. Nevertheless, in previous studies evaluating treatment adherence with dopaminergic PD medications using commercial and Medicare claims data, rates of adherence by patients were reported to vary between 37% and 73% (Davis et al., 2010; Richy et al., 2013; Tarrants et al., 2010; Wei et al., 2013; Johnsrud et al., 2021; Kulkarni et al., 2008). A more recent study in the Medicaid population reported higher adherence and persistence in patients initiating levodopa (considered the gold standard for treatment of PD) than those initiating a dopamine agonist, but overall <50% of patients in either group were considered adherent (PDC threshold for adherence: ≥0.8) and only approximately 50% of patients initiating levodopa and 40% initiating a dopamine agonist remained persistent with treatment over 12 months (Johnsrud et al., 2021). In another study using commercial and Medicare claims data and conducted prior to the availability of istradefylline, mean adherence (as measured by MPR) following initiation of approved medications for PD was 58% over 12 months, and an adherence rate of at least 80% (MPR ≥0.8) was achieved by <40% of patients (Davis et al., 2010). In this study of istradefylline, median adherence was 74.0% and an adherence rate of at least 80% (PDC or MPR ≥0.8) over 12 months was achieved by almost half of patients, suggesting that adherence with istradefylline is comparable or favorable to adherence with dopaminergic treatments.
Several previous analyses have identified multiple factors associated with treatment adherence in PD. These factors have included age, gender, motor and non-motor symptoms, depressive symptoms, cognition, use of dopamine agonists, number of PD medications, and LEDD exposure (Richy et al., 2013; Tarrants et al., 2010; Castro et al., 2021; Zipprich et al., 2021; Wei et al., 2013; Straka et al., 2019; Johnsrud et al., 2021; Kulkarni et al., 2008; Aggarwal et al., 2021; Daley et al., 2012; Dahodwala et al., 2021; Frazer et al., 2022; Radojević et al., 2022; Yi et al., 2024). For example, in a retrospective US claims data analysis of commercially insured patients, the odds of non-adherence (MPR) was lower in patients <80 years than those ≥80 years and was higher in those with a diagnosis of depression (Richy et al., 2013). In a claims data analysis of patients with Medicare coverage, an LEDD of >1,000 mg/day was associated with lower adherence (PDC) than >800 mg/day (Dahodwala et al., 2021). In addition, in a claims analysis of patients receiving Medicaid, use of levodopa was associated with higher adherence than the use of dopamine agonists (PDC: 62.1% vs. 54.6%) (Johnsrud et al., 2021). Comparisons with such previous studies are limited, however, given that these analyses were conducted in different populations using varying measures of treatment compliance, and also generally evaluated potential factors affecting treatment adherence rather than persistence. As noted, most previous studies also assessed compliance with dopaminergic agents and did not include istradefylline.
A previous retrospective cohort study evaluating treatment adherence with high-dose PD medications in Medicare beneficiaries found that few patients with advanced disease sustained a high-dose medication regimen (LEDD >1,000 mg) over 12 months, but most could sustain a substantially lower-dose regimen (Dahodwala et al., 2021). Real-world analyses have demonstrated a long-term slowing of LEDD escalation in patients who initiated treatment with istradefylline, particularly for those who were receiving higher doses of levodopa (Hattori et al., 2023; Hattori et al., 2022). Patients who initiated istradefylline early and were not receiving concomitant COMT inhibitors or MAO-B inhibitors also showed a reduced escalation in LEDD (Hattori et al., 2023; Hattori et al., 2022). Thus, by contributing to a slowing of LEDD escalation, the use of istradefylline early in the treatment of PD might enable patients to have a lower likelihood of experiencing levodopa-induced complications and thus be more compliant with their PD medication regimens.
Given that a dose change to 40 mg at 12 months of follow-up was observed for almost 20% of patients and the higher likelihood of persistence with a 40-mg initial dose, initiating istradefylline treatment at a 40-mg dose could be preferable to a 20-mg dose to reduce the risk of treatment discontinuation. Istradefylline has been shown to effectively reduce “OFF” time at both 20- and 40-mg doses (Hauser et al., 2021; Takahashi et al., 2022). In a recent analysis, an increase in istradefylline dosing from 20 mg to 40 mg did not substantially increase the incidence of adverse drug reactions, supporting the safety of the 40-mg dose (Takahashi et al., 2022). Unlike dopaminergic agents, the adenosine A2A antagonism of istradefylline acts through a mechanism beyond the dopaminergic system, thereby offering a distinct approach for treating “OFF” time in patents with PD (Jenner et al., 2021). Further research is necessary to evaluate considerations for initial treatment dosing with istradefylline in clinical practice. Additionally, the higher odds of treatment persistence and lower rates of discontinuation observed in our study with Medicare Advantage coverage compared with commercial insurance may be related to reimbursement issues (Fusco et al., 2023; IQVIA Institute for Human Data Science, 2020) and warrant further investigation.
4.1 Study limitations
This study had several limitations inherent to its retrospective, real-world study design and use of administrative claims data. The data provide only indirect measures of treatment adherence and persistence and associated risk factors. Pharmacy prescription fills cannot account for whether the medication was taken as prescribed. Additionally, given the nature of claims data, only those disease entities and variables with specific billing codes could be captured. The types of measurable data available were thus limited, including potential risk factors for treatment compliance, such as markers of disease severity. Reasons for treatment discontinuation with istradefylline could also not be determined. As with all claims data, there was also the potential for underestimation of rates of health events and missed events, as well as misclassification due to coding errors. In addition, given that adherence and persistence were evaluated in this study over a 12-month period, further research would be needed to determine longer-term treatment compliance with istradefylline.
Finally, the IQVIA APLD claims database used in this study provides a large, representative sample of patients with Medicare Advantage or commercial insurance coverage in the US. However, the study outcomes may not be generalizable to all patients with PD, such as those with other insurance types.
4.2 Conclusions
This real-world US study among 2,045 patients with PD demonstrated a median adherence of 74% and a median persistence duration of 276 days over 12 months. Patients who initiated istradefylline at 40 mg or who had Medicare Advantage healthcare coverage were more likely to have higher adherence and longer persistence over 12 months than those who started dosing at 20 mg or who were commercially insured. Further research into factors associated with adherence and persistence with istradefylline would be beneficial to support treatment decision-making in clinical practice.
Data availability statement
The datasets presented in this article are not readily available because the data were used under license from IQVIA. Data availability is subject to third-party restrictions. Requests to access the datasets should be directed to Joyce Qian, am95Y2UucWlhbi44eUBreW93YWtpcmluLmNvbQ==.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
RJ: Conceptualization, Supervision, Writing – review and editing. JQ: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review and editing. HC: Conceptualization, Investigation, Validation, Writing – review and editing. Y-HC: Investigation, Methodology, Project administration, Software, Validation, Writing – review and editing. ST: Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Writing – review and editing. MS: Conceptualization, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by Kyowa Kirin, Inc., Princeton, NJ, USA. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
Medical writing support, under the direction of the authors, was provided by Liza Sovran, BSc (Hons) at Asclepius Analytics, New York, NY, USA.
Conflict of interest
Authors JQ and HC were employed by Kyowa Kirin, Inc.
Authors Y-HC and ST were employed by KMK Consulting Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aggarwal, S., Paul, G., Paul, B. S., Mahendru, D., and Goyal, S. (2021). Factors affecting adherence to pharmacotherapy in Parkinson's disease. Ann. Indian Acad. Neurol. 24 (6), 879–884. doi:10.4103/aian.AIAN_143_21
American Academy of Neurology (2021). Dopaminergic therapy for motor symptoms in early Parkinson disease practice guideline. Available online at: https://www.aan.com/Guidelines/Home/GuidelineDetail/1043 (Accessed November 20, 2024).
American Academy of Neurology Institute (2021). Parkinson’s disease quality measurement set: 2020 update. Available online at: https://www.aan.com/siteassets/home-page/policy-and-guidelines/quality/quality-measures/movement-disorders/2020-parkinson-disease-measurement-set-final.pdf [Accessed November 20, 2024].
Armstrong, M. J., and Okun, M. S. (2020). Diagnosis and treatment of Parkinson disease: a review. JAMA 323 (6), 548–560. doi:10.1001/jama.2019.22360
Berger, A. A., Winnick, A., Welschmeyer, A., Kaneb, A., Berardino, K., Cornett, E. M., et al. (2020). Istradefylline to treat patients with Parkinson’s disease experiencing “off” episodes: a comprehensive review. Neurol. Int. 12 (3), 109–129. doi:10.3390/neurolint12030017
Castro, G. S., Aguilar-Alvarado, C. M., Zúñiga-Ramírez, C., Sáenz-Farret, M., Otero-Cerdeira, E., Serrano-Dueñas, M., et al. (2021). Adherence to treatment in Parkinson's disease: a multicenter exploratory study with patients from six Latin American countries. Park. Relat. Disord. 93, 1–7. doi:10.1016/j.parkreldis.2021.10.028
Chou, K. L., Stacy, M., Simuni, T., Miyasaki, J., Oertel, W. H., Sethi, K., et al. (2018). The spectrum of “OFF” in Parkinson’s disease: what have we learned over 40 years? Park. Relat. Disord. 51, 9–16. doi:10.1016/j.parkreldis.2018.02.001
Cummins, L., and Cates, M. E. (2022). Istradefylline: a novel agent in the treatment of “OFF” episodes associated with levodopa/carbidopa use in Parkinson disease. Ment. Health Clin. 12 (1), 32–36. doi:10.9740/mhc.2022.01.032
Dahodwala, N., Jahnke, J., Pettit, A. R., Li, P., Ladage, V. P., Kandukuri, P. L., et al. (2021). Low sustainment of high-dose oral medication regimens for advanced Parkinson's disease in Medicare beneficiaries. J. Park. Dis. 11 (2), 675–684. doi:10.3233/JPD-202147
Daley, D. J., Myint, P. K., Gray, R. J., and Deane, K. H. (2012). Systematic review on factors associated with medication nonadherence in Parkinson's disease. Park. Relat. Disord. 18 (10), 1053–1061. doi:10.1016/j.parkreldis.2012.09.004
Davis, K. L., Edin, H. M., and Allen, J. K. (2010). Prevalence and cost of medication nonadherence in Parkinson's disease: evidence from administrative claims data. Mov. Disord. 25 (4), 474–480. doi:10.1002/mds.22999
Di Luca, D. G., Reyes, N. G. D., and Fox, S. H. (2022). Newly approved and investigational drugs for motor symptom control in Parkinson’s disease. Drugs 82, 1027–1053. doi:10.1007/s40265-022-01747-7
Frazer, M., Arcona, S., Le, L., and Sasane, R. (2022). Dopamine agonist monotherapy utilization in patients with Parkinson’s disease. Clin. Park Relat. Disord. 8, 100173. doi:10.1016/j.prdoa.2022.100173
Fusco, N., Sils, B., Graff, J. S., Kistler, K., and Ruiz, K. (2023). Cost-sharing and adherence, clinical outcomes, health care utilization, and costs: a systematic literature review. J. Manag. Care Spec. Pharm., 29, 4–16. doi:10.18553/jmcp.2022.21270
Grimes, D., Fitzpatrick, M., Gordon, J., Miyasaki, J., Fon, E. A., Schlossmacher, M., et al. (2019). Canadian guideline for Parkinson disease. CMAJ 191 (36), E989–E1004. doi:10.1503/cmaj.181504
Grosset, D., Antonini, A., Canesi, M., Pezzoli, G., Lees, A., Shaw, K., et al. (2009). Adherence to antiparkinson medication in a multicenter European study. Mov. Disord. 24 (6), 826–832. doi:10.1002/mds.22112
Hatano, T., Sengoku, R., Nagayama, H., Yanagisawa, N., Yoritaka, A., Suzuki, K., et al. (2024). Impact of istradefylline on levodopa dose escalation in Parkinson’s disease: ISTRA ADJUST PD study, a multicenter, open-label, randomized, parallel-group controlled study. Neurol. Ther. 13, 323–338. doi:10.1007/s40120-023-00574-6
Hattori, N., Kabata, D., Asada, S., Kanda, T., Nomura, T., Shintani, A., et al. (2022). POSA415 association of an adenosine A2A antagonist istradefylline on the treatment paradigm of levodopa for patients with Parkinson’s disease: real-world evidence from a nationwide Japanese database over 10 years (2008-2019). Value Health 25 (1), S254. doi:10.1016/j.jval.2021.11.1240
Hattori, N., Kabata, D., Asada, S., Kanda, T., Nomura, T., Shintani, A., et al. (2023). Real-world evidence on levodopa dose escalation in patients with Parkinson’s disease treated with istradefylline. PLoS One 18 (12), e0269969. doi:10.1371/journal.pone.0269969
Hauser, R. A., Hattori, N., Fernandez, H., Isaacson, S. H., Mochizuki, H., Rascol, O., et al. (2021). Efficacy of istradefylline, an adenosine A2A receptor antagonist, as adjunctive therapy to levodopa in Parkinson's disease: a pooled analysis of 8 phase 2b/3 trials. J. Park. Dis. 11 (4), 1663–1675. doi:10.3233/JPD-212672
Hindle, J. V. (2010). Ageing, neurodegeneration and Parkinson’s disease. Age Ageing 39 (2), 156–161. doi:10.1093/ageing/afp223
IQVIA Institute for Human Data Science (2020). Medicine spending and affordability in the United States. Available online at: https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/medicine-spending-and-affordability-in-the-us (Accessed November 20, 2024).
Isaacson, S. H., Betté, S., and Pahwa, R. (2022a). Istradefylline for OFF episodes in Parkinson’s disease: a US perspective of common clinical scenarios. Degener. Neurol. Neuromuscul. Dis. 12, 97–109. doi:10.2147/DNND.S245197
Isaacson, S. H., Pagan, F. L., Lew, M. F., and Pahwa, R. (2022b). Should “on-demand” treatments for Parkinson’s disease OFF episodes be used earlier? Clin. Park Relat. Disord. 7, 100161. doi:10.1016/j.prdoa.2022.100161
Jenner, P., Mori, A., Aradi, S. D., and Hauser, R. A. (2021). Istradefylline – a first generation adenosine A2A antagonist for the treatment of Parkinson's disease. Expert Rev. Neurother. 21 (3), 317–333. doi:10.1080/14737175.2021.1880896
Johnsrud, M., Richards, K., Arcona, S., Sasané, R., and Leoni, M. (2021). An assessment of Parkinson’s disease medication treatment patterns in the Medicaid population. Clin. Park Relat. Disord. 5, 100109. doi:10.1016/j.prdoa.2021.100109
Julien, C., Hache, G., Dulac, M., Dubrou, C., Castelnovo, G., Giordana, C., et al. (2021). The clinical meaning of levodopa equivalent daily dose in Parkinson's disease. Fundam. Clin. Pharmacol. 35 (3), 620–630. doi:10.1111/fcp.12646
Kalia, L. V., and Lang, A. E. (2015). Parkinson’s disease. Lancet 386 (9996), 896–912. doi:10.1016/S0140-6736(14)61393-3
Kondo, T., and Mizuno, Y.Japanese Istradefylline Study Group (2015). A long-term study of istradefylline safety and efficacy in patients with Parkinson disease. Clin. Neuropharmacol. 38 (2), 41–46. doi:10.1097/WNF.0000000000000073
Kulkarni, A. S., Balkrishnan, R., Anderson, R. T., Edin, H. M., Kirsch, J., and Stacy, M. A. (2008). Medication adherence and associated outcomes in Medicare health maintenance organization-enrolled older adults with Parkinson's disease. Mov. Disord. 23 (3), 359–365. doi:10.1002/mds.21831
LeWitt, P. A., Guttman, M., Tetrud, J. W., Tuite, P. J., Mori, A., Chaikin, P., et al. (2008). Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces off time in Parkinson’s disease: a double-blind, randomized, multicenter clinical trial (6002-US-005). Ann. Neurol. 63, 295–302. doi:10.1002/ana.21315
Malek, N., and Grosset, D. A. (2015). Medication adherence in patients with Parkinson’s disease. CNS Drugs 29 (1), 47–53. doi:10.1007/s40263-014-0220-0
Marras, C., Beck, J. C., Bower, J. H., Roberts, E., Ritz, B., Ross, G. W., et al. (2018). Prevalence of Parkinson’s disease across north America. NPJ Park. Dis. 4, 21. doi:10.1038/s41531-018-0058-0
Mizuno, Y., Hasegawa, K., Kondo, T., Kuno, S., and Yamamoto, M.Japanese Istradefylline Study Group (2010). Clinical efficacy of istradefylline (KW-6002) in Parkinson’s disease: a randomized, controlled study. Mov. Disord. 25, 1437–1443. doi:10.1002/mds.23107
National Institute for Health and Care Excellence (2017). Parkinson’s disease in adults: diagnosis and management. NICE Guidel. NG71. Available online at: https://www.nice.org.uk/guidance/ng71/resources/parkinsons-disease-in-adults-pdf-1837629189061 (Accessed November, 202024).
National Institute of Neurological Disorders and Stroke (2023). Parkinson's disease: challenges, progress, and promise. Available online at: https://www.ninds.nih.gov/current-research/focus-disorders/parkinsons-disease-research/parkinsons-disease-challenges-progress-and-promise (Accessed November 20, 2024).
NOURIANZ (2023). Prescribing information. Available online at: https://www.nourianzhcp.com/assets/pdf/nourianz-full-prescribing-information.pdf [Accessed November 20, 2024].
Nyholm, D., and Jost, W. H. (2021). An updated calculator for determining levodopa-equivalent dose. Neurol. Res. Pract. 3, 58. doi:10.1186/s42466-021-00157-6
Perepezko, K., Hinkle, J., Mills, K., and Pontone, G. (2020). “Frequency of wearing-off symptoms in Parkinson’s disease fluctuators: an on/off evaluation,” in Presented at the international Parkinson and movement disorder society MDS virtual congress 2020. September 12–16.
Quan, H., Li, B., Couris, C. M., Fushimi, K., Graham, P., Hider, P., et al. (2011). Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 173 (6), 676–682. doi:10.1093/aje/kwq433
Quan, H., Sundararajan, V., Halfon, P., Fong, A., Burnand, B., Luthi, J. C., et al. (2005). Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 43 (11), 1130–1139. doi:10.1097/01.mlr.0000182534.19832.83
Radojević, B., Dragaŝević-Miŝković, N. T., Milovanović, A., Svetel, M., Petrović, I., Pešić, M., et al. (2022). Adherence to medication among Parkinson’s disease patients using the adherence to refills and medications scale. Int. J. Clin. Pract. 2022, 6741280. doi:10.1155/2022/6741280
Richy, F. F., Pietri, G., Moran, K. A., Senior, E., and Makaroff, L. E. (2013). Compliance with pharmacotherapy and direct healthcare costs in patients with Parkinson's disease: a retrospective claims database analysis. Appl. Health Econ. Health Policy 11 (4), 395–406. doi:10.1007/s40258-013-0033-1
Schade, S., Mollenhauer, B., and Trenkwalder, C. (2020). Levodopa equivalent dose conversion factors: an updated proposal including opicapone and safinamide. Mov. Disord. Clin. Pract. 7 (3), 343–345. doi:10.1002/mdc3.12921
Schapira, A. H. V. (2007). Treatment options in the modern management of Parkinson disease. Arch. Neurol. 64 (8), 1083–1088. doi:10.1001/archneur.64.8.1083
Shoval, G., Stubbs, B., Balicer, R. D., Feldman, B., Hoshen, M., Zalsman, G., et al. (2017). Low adherence to antidepressants is associated with increased mortality in Parkinson disease patients. Park. Relat. Disord. 43, 92–96. doi:10.1016/j.parkreldis.2017.07.032
Soileau, M. J., Pagan, F. L., Fasano, A., Rodriguez-Cruz, R., Yan, C. H., Gupta, N. R., et al. (2023). Comparative effectiveness of carbidopa/levodopa enteral suspension and deep brain stimulation on pill burden reduction in Medicare fee-for-service patients with advanced Parkinson’s disease. Neurol. Ther. 12, 459–478. doi:10.1007/s40120-022-00433-w
Straka, I., Minár, M., Škorvánek, M., Grofik, M., Danterová, K., Benetin, J., et al. (2019). Adherence to pharmacotherapy in patients with Parkinson's disease taking three and more daily doses of medication. Front. Neurol. 10, 799. doi:10.3389/fneur.2019.00799
Takahashi, M., Itou, S., Tsuji, Y., and Horiguchi, S. (2022). Safety and effectiveness of istradefylline as add-on therapy to levodopa in patients with Parkinson's disease: final report of a post-marketing surveillance study in Japan. J. Neurol. Sci. 443, 120479. doi:10.1016/j.jns.2022.120479
Tarrants, M. L., Denarié, M. F., Castelli-Haley, J., Millard, J., and Zhang, D. (2010). Drug therapies for Parkinson's disease: a database analysis of patient compliance and persistence. Am. J. Geriatr. Pharmacother. 8 (4), 374–383. doi:10.1016/j.amjopharm.2010.08.001
Tomlinson, C. L., Stowe, R., Patel, S., Rick, C., Gray, R., and Clarke, C. E. (2010). Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov. Disord. 25 (15), 2649–2653. doi:10.1002/mds.23429
United States Census Bureau (2020). Demographic turning points for the United States: population projections for 2020 to 2060. Available online at: https://www.census.gov/library/publications/2020/demo/p25-1144.html (Accessed November 20, 2024).
Verber, D., Novak, D., Borovič, M., Dugonik, J., and Flisar, D. (2020). EQUIDopa: a responsive web application for the levodopa equivalent dose calculator. Comput. Methods Programs Biomed. 196, 105633. doi:10.1016/j.cmpb.2020.105633
Wei, Y.-J., Palumbo, F. B., Simoni-Wastila, L., Shulman, L. M., Stuart, B., Beardsley, R., et al. (2013). Antiparkinson drug use and adherence in Medicare Part D beneficiaries with Parkinson’s disease. Clin. Ther. 35 (10), 1513–1525.e1. doi:10.1016/j.clinthera.2013.09.001
World Health Organization (2023). Parkinson disease. Available online at: https://www.who.int/news-room/fact-sheets/detail/parkinson-disease (Accessed November 20, 2024).
Yi, Z., Mao, Y., He, C., Zhang, Y., Zhou, J., and Feng, X. L. (2024). Medication adherence and costs of medical care among patients with Parkinson’s disease: an observational study using electronic medical records. BMC Public Health 24 (1), 1202. doi:10.1186/s12889-024-18431-y
Keywords: Parkinson’s disease, adherence, persistence, istradefylline, treatment patterns
Citation: Joseph R, Qian J, Cummings H, Chen Y-H, Tewari S and Soileau M (2025) Adherence and persistence with istradefylline treatment in patients with Parkinson’s disease in the United States. Front. Pharmacol. 16:1548631. doi: 10.3389/fphar.2025.1548631
Received: 07 January 2025; Accepted: 18 April 2025;
Published: 06 May 2025.
Edited by:
Cüneyt Türkeş, Erzincan Binali Yıldırım University, TürkiyeReviewed by:
Jessa Koch, Loma Linda University School of Pharmacy, United StatesNaomi Kanzato, Okinawa Southern Medical Center, Japan
Copyright © 2025 Joseph, Qian, Cummings, Chen, Tewari and Soileau. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joyce Qian, am95Y2UucWlhbi44eUBreW93YWtpcmluLmNvbQ==
 Reversa Joseph1
Reversa Joseph1 Joyce Qian
Joyce Qian