- 1Department of Gastroenterology, Jian Yang Hospital of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 2The General Hospital of Western Theater Command, Chengdu, Sichuan, China
- 3School of Sports Medicine and Health, Chengdu Sport University, Chengdu, Sichuan, China
- 4Department of Oncology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
Gastric cancer (GC) is the fifth most common malignant tumor that imposes heavily public health burdens worldwide. Systemic therapies for gastric cancer (GC), such as chemotherapy, targeted therapy, and immunotherapy, have undergone significant advancements. Nevertheless, the extensive application of anti-cancer agents has resulted in an increasing array of challenges related to drug resistance, presenting a substantial barrier in GC treatment. Tumor-associated macrophages (TAMs) as essential immunomodulators within the tumor immune microenvironment (TIME) of GC, providing novel therapeutic targets due to their capacity for plasticity in reaction to environmental signals. They create a complex network of communication with various immune and stromal cell types, thereby contributing to the immunosuppressive nature of the TME in GC. In this review, we establish the map of the origin and polarization of macrophages in GC. During the process of carcinogenesis, macrophages undergo dynamic phenotypic transitions. Additionally, the interactions between TAMs and tumor cells significantly influence the progression of GC, affecting tumor growth, metastasis, angiogenesis, and drug resistance. Furthermore, this intricate immunomodulatory axis notably enhances resistance to immunotherapy, suggesting that targeting TAMs presents substantial therapeutic opportunities for patients with GC. Approaches such as TAM elimination, TAM repolarization, and CAR-M therapy have been validated in numerous studies. We also elaborate on the challenges faced by the development of targeting TAMs, which may provide innovative perspectives on the GC treatment.
1 Introduction
Gastric cancer (GC) ranks as the fifth most prevalent malignant tumor and is the fifth leading cause of cancer-related mortality globally based on the GLOBOCAN 2022 report (Bray et al., 2024). It was estimated that approximately 968,000 new cases and 65,900 deaths of GC patients worldwide in 2022 (Bray et al., 2024). The combination of chemotherapy and molecular targeted therapy has clearly extended overall survival time and enhanced the quality of life for GC patients with advanced stages (Guan et al., 2023). However, the widespread use of anti-cancer agents encounters an increasing number of challenges related to drug resistance, which presents a significant obstacle in clinical oncology (Liu J. et al., 2024).
The pathogenesis of GC is closely linked to immune cells and various types of mesenchymal stromal cells within the tumor microenvironment (TME) (Yasuda and Wang, 2024). While immunotherapy has emerged as a potent clinical approach for the treatment of cancer, its clinical efficacy is limited in the context of advanced GC (Yu Y. et al., 2023). Moreover, there are significant differences in immune responses among individuals. These unfavorable therapeutic responses significantly impede the prognosis of GC patients, which await further investigations to prompt the understanding of the spatial arrangement and functional network within the tumor immune microenvironment (TIME) of GC (Xu et al., 2023).
The TIME comprises a variety of non-tumor stromal cells that play a crucial role in GC progression and immune evasion (Yu Y. et al., 2023; Huang et al., 2024a). It contains various immune cells, such as natural killer (NK) cells, cancer-associated fibroblasts (CAFs), Myeloid-Derived Suppressor Cells (MDSCs), dendritic cells (DCs), tumor-associated macrophages (TAMs), cytotoxic T lymphocytes (CTLs), and regulatory T (Treg) cells, along with the extracellular matrix (ECM) and various immunosuppressive cytokines (Yasuda and Wang, 2024; Mou et al., 2023), providing novel therapeutic targets for cancer therapy. Within the TIME, macrophages possess the ability to mediate vascular damage and induce tumor necrosis, as well as stimulate tumor resistance mechanisms mediated by innate or adaptive lymphocytes (Mantovani et al., 2022). Conversely, in the context of progressed tumor masses, macrophages transform into TAMs, which facilitate malignant progression and immune evasion by modulating TIME (Nasir et al., 2023). Additionally, they represent a significant target for contemporary checkpoint blockade immunotherapy due to their expression of inhibitory counter-receptors, such as programmed cell death ligand 1 (PD-L1), B7-1 and B7-2, which trigger immunosuppressive activity by binging to PD-1 and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) (Zhang H. et al., 2023; Sharma et al., 2024). The dual potential of macrophages in cancer progression has underscored their therapeutic promising for immunotherapy. In this review, we elaborate on the properties and dynamic transitions of macrophages in TIME of GC. Moreover, we highlight the crosstalk between TAMs and GC cells, emphasizing the essential role of TAMs in cancer progression and the critical modulations of TIME, as well as elucidating current therapeutic regimes by targeting TAMs in the field of GC, which may pave the way for the translation of TAMs-based immunotherapy into clinical course.
2 Characteristics and functions of macrophages in the TME of GC
2.1 The origin and dynamic transitions of macrophages in GC
Macrophages exhibit a diverse array of profiles that reflect their inherent plasticity, a characteristic crucial for responding to various danger signals and preserving tissue homeostasis across human organs (Nobs and Kopf, 2021). They are long-lived and recognized as the most versatile cells within significant functional diversity (Wynn et al., 2013). Resident macrophages play crucial roles in multiple aspects of biological progresses, encompassing organ development, tissue homeostasis, and host inflammatory responses against pathogens. During these adaptive processes, macrophages primarily originate from monocyte reservoirs, which are mainly derived from hematopoietic stem cells located in the bone marrow (Guilliams et al., 2018). These macrophages exhibit various tissue-specificities including Osteoclasts, Kupffer cells, Microglia (Locati et al., 2020). However, under some pathological conditions, these homeostatic functions mediated by macrophages can be compromised by ongoing insults, resulting in various disease states, such as obesity, neurodegenerative disorders and cancer (Razi et al., 2023), which suggest that macrophages constitute a highly diverse group of cells that constantly modify their functional states in response to alterations in tissue physiology or environmental cues (Razi et al., 2023) (Figure 1).
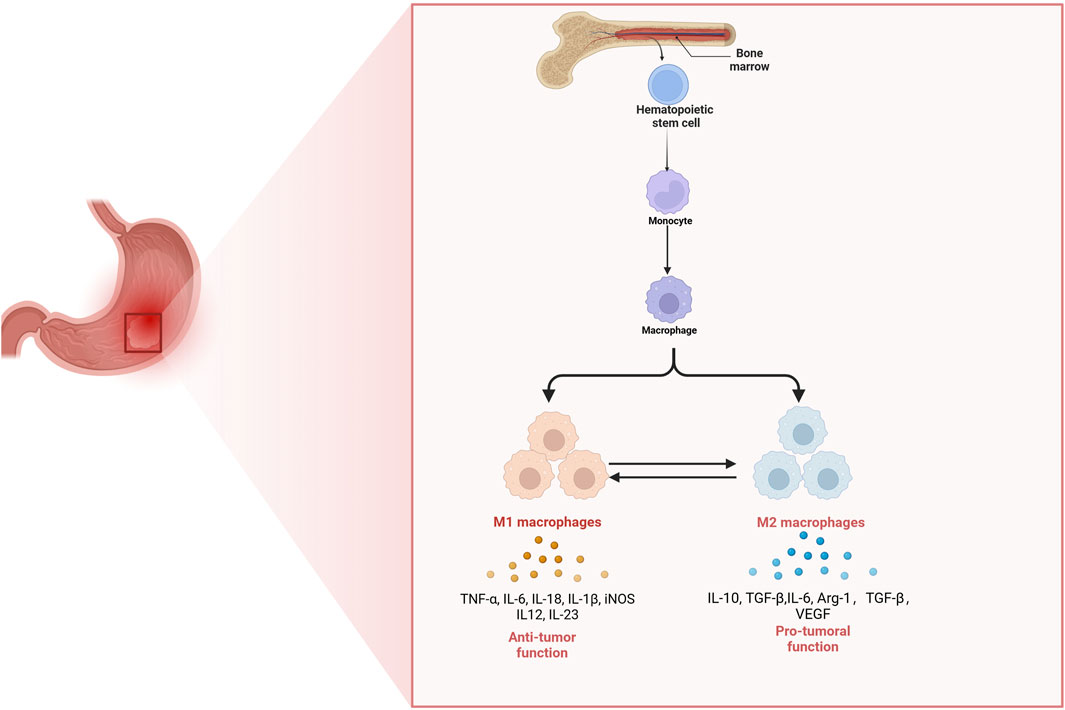
Figure 1. The origin of tumor-associated macrophages and their functions in gastric cancer. Hematopoietic stem cells in the bone marrow differentiate into monocytes, which subsequently migrate to tissues and develop into macrophages. In the gastric tumor microenvironment, macrophages can polarize into either M1 or M2 phenotypes. M1 macrophages secrete pro-inflammatory cytokines (TNF-α, IL-6, IL-18, IL-1β, iNOS, IL-12, and IL-23) and exhibit anti-tumor functions. In contrast, M2 macrophages produce anti-inflammatory and tumor-promoting factors (IL-10, TGF-β, IL-6, Arg-1, and VEGF) and support tumor growth and immune suppression. The balance between M1 and M2 polarization shapes the immune landscape and progression of gastric cancer.
In the context of GC, macrophages can be broadly categorized into two opposing groups: classically activated macrophages (M1-like) and alternatively activated macrophages (M2-like) (Li M. et al., 2023). M1 macrophages are typically induced by granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-gamma (IFN-γ) and lipopolysaccharide, which significantly secret pro-inflammatory factors such as tumor necrosis factor-alpha (TNF-α), interleukin-12 (IL-12), IL-18, IL-1β, and inducible nitric oxide synthase (iNOS) along with large amounts of intracellularly nitric oxide (NO) production (Yunna et al., 2020). M1 macrophages play a crucial role in exerting anti-tumor effects by mediating phagocytosis and antibody-dependent cell-mediated cytotoxicity to eliminate tumor cells (Mantovani et al., 2004; Mantovani et al., 2017). Additionally, M2 macrophages are generally activated by factors such as colony-stimulating factor 1 (CSF1) transforming growth factor-beta (TGF-β), IL-4, IL-10, IL-13, prostaglandin E2 (PGE2) and parasites or fungi. M2 macrophages have the ability to secrete a diverse array of complex cytokines including IL-1β, IL-6, IL-10 as well as Arg-1 (Mantovani et al., 2004), which regulates Th2-type immune responses and promotes tumor growth and metastasis. Additionally, they can induce the expression of vascular endothelial growth factor (VEGF), thus prompting tumor angiogenesis (Mantovani et al., 2017). In response to different stimuli, four subtypes of M2 macrophages are known: M2a, M2b, M2c, and M2d. M2a macrophages are activated by IL-4 and IL-13, while M2b macrophages are activated by Toll-like receptor ligands, and IL-1β. Additionally, M2c macrophages are induced by IL-10, TGF-β and glucocorticoids, while M2d macrophages are induced by IL-6 and adenosine (Rőszer, 2015). While macrophage activation and polarization allow them to adopt specific phenotypes, their inherent plasticity enables these immune cells to shift between different phenotypes (Smith et al., 2017). Metabolic changes in fatty acid and glucose utilization drive macrophage polarization towards the M1 or M2 subtype, thus affecting their plasticity (El Kasmi and Stenmark, 2015). Macrophages are highly adaptable, with polarization occurring along a continuum that encompasses various intermediate phenotypes (Zhang et al., 2022). This plasticity is supported by a responsive epigenome influenced by significant changes in DNA methylation (Li Z. et al., 2024). Such characteristics provide macrophages with heterogeneity and the ability to modify their functional phenotype in response to microenvironmental stimuli (Smith et al., 2017). In diseases like GC, the dysregulation of macrophage phenotypes is believed to contribute to pathogenesis. This functional adaptability holds considerable therapeutic promise, as it can potentially be leveraged to restore equilibrium among different macrophage subsets (Zhang et al., 2022). Consequently, it is essential to investigate the diversity of macrophage phenotypes in the context of GC development and how environmental factors affect the behavior of these immune cells (Zhang et al., 2022). Though M1 and M2 macrophages exhibit distinctive functions in biological activities, they can transform into each other, which presenting a promising therapeutic target for GC.
Recent studies utilizing single-cell RNA sequencing have offered a static transcriptional overview of the diversity of TAMs (Xu et al., 2024). Consequently, new molecular definitions for macrophage states are being applied in immuno-oncology and are being evaluated for their prognostic and predictive significance (Pittet et al., 2022). Based on these findings, the M1-M2 classification of macrophages has faced criticism due to the dynamic nature of macrophages, whose gene expression profiles can shift in response to changes in the microenvironment (Gao et al., 2025). In recent years, TAMs can be categorized into several subtypes, distinguished by their signature genes, signaling pathways, and functional characteristics: interferon-primed TAMs (IFN-TAMs), immune-regulatory TAMs, inflammatory cytokine-enriched TAMs, and proliferative TAMs (Prolif-TAMs) (Ma et al., 2022). Immune regulatory TAMs display characteristics similar to alternatively activated macrophages, which have been identified in a variety of tumors (Wu et al., 2025; Yu et al., 2025). IFN-TAMs are defined by the high expression of interferon-regulated genes, including CXCL10 and ISG15 (Cheng et al., 2021). However, contrary to the common assumption that M1 macrophages possess antitumor properties, IFN-TAMs demonstrate immunosuppressive functions. They have been shown to suppress immune responses through mechanisms such as tryptophan degradation and the recruitment of Tregs (Sadik et al., 2020). Prolif-TAMs exhibit high levels of Ki-67 and cell division cycle 45, which indicates a potential proinflammatory phenotype (Peng C. et al., 2024). However, it remains uncertain whether these cells represent a temporary state that quickly differentiates into other TAM subsets or if they persist as cycling progenitors, which await further investigations.
In the context of GC, the traditional M1-M2 classification has also been challenged. For example, Du et al. revealed the five types of TAM subset marker genes in GC tissues. However, the co-expression of M1 and M2 gene signatures in all five macrophage subtypes indicated that macrophages exhibit a greater complexity, challenging the traditional binary classification of M1 and M2 TAMs (Du et al., 2024). Among them, SPP1 + TAMs possessed higher M2 signatures that played an essential immunosuppressive role in the TME of GC (Du et al., 2024). Additionally, the CTSB+ macrophages also played a role in sustaining the immunosuppressive environment of GC (Wang Q. et al., 2025). In line with this, Li et al. reported that all TAM clusters did not fit within the M1/M2 dichotomy in GC tissues with peritoneal metastasis. Thus, they divided TAMs into different groups based on diverse expression profiles of CTS and C1Q-associated genes. This suggested that the expression variation of CTS and C1Q genes in ascitic TAMs represented a continuum rather than a binary polarization (Li Y. et al., 2024). Additionally, pathway analyses of single-cell RNA sequencing data have revealed the presence of diverse metabolic processes within TAM clusters (Qian et al., 2024). Furthermore, growing evidence indicates a significant correlation between the phenotypic characteristics and metabolic profiles of TAMs (Qian et al., 2024). These investigations have demonstrated that metabolism can drive the phenotypic and functional changes of TAMs in response to variations in the tumor microenvironment. Moreover, epigenetic changes, such as DNA methylation and histone modification, play a crucial role in defining the characteristics and functions ofTAMs. Investigating this area can aid researchers in understanding how the tumor microenvironment influences the state of TAMs through epigenetic mechanisms, while also exploring potential intervention strategies (Ji et al., 2025). The longitudinal and local heterogeneity of TAM states is now achievable through single-cell and spatial transcriptomics. This comprehensive understanding of macrophage classification is laying the groundwork for a new era of GC treatment, ultimately leading to improved effectiveness of therapeutic strategies.
2.2 The crosstalk between TAMs and other immune cells within the TME of GC
TIME is an intricate microenvironment, which possesses various immune cells and non-malignant mesenchymal cells (Franzese, 2024). In the TIME, TAMs hold a significant position by interacting with other types of immune cells, thus modulating immune response in the context of GC. These communications can influence the predominant type of TAMs, M2 TAMs, through the secretion of various communicative mediators, thereby creating an immunosuppressive microenvironment that is optimal for cancer progression (Li D. et al., 2023; Zhang G. et al., 2023; Su et al., 2022).
2.2.1 TAMs interact with T cells via PD-1 and STING pathway
SIGLEC10 was found to inhibit functional activation and proliferation of tumor-infiltrating CD8+ T cells by Akt/P38/Erk cascade in GC. Guo et al. identified the infiltration of SIGLEC10+ macrophages in GC tissues via analyzing single-cell RNA sequencing data. Moreover, SIGLEC10+ macrophages exhibited M2-like phenotype, which displayed high expression of exhaustion markers such as PD-1, T-cell Immunoglobulin and Mucin-Domain Containing-3 (TIM-3) and CD39, which was also associated with exhaustion of CD8+ T cells, proving novel target to improve T cell function for GC therapy (Guo et al., 2023). Moreover, Lin et al. claimed that CSF-2 promoted the M2 macrophage-mediated C-X-C Motif Chemokine Ligand 8 (CXCL8) secretion, leading to upregulated expression of PD-L1 and activation of signal transducer and activator of transcription 3 (STAT3) pathway, which further downregulated tumor-infiltrating CD8+ T cells as well as promoted functional exhaustion of T cells, exhibiting unfavorable prognosis in GC patients (Lin et al., 2019). IL-4 mediated M2 macrophage polarization also promoted phosphoinositide 3-kinase (PI3K)/AKT/mechanistic target of rapamycin (mTOR) pathway, resulting in prompt glycolysis and upregulated the expression of Fcγ receptor IIB (FcγRIIB), which ultimately functional dysregulation of CD8+ T cells in GC (Zhang J. et al., 2024). Miao et al. reported that the repression of STING pathway and its activation by 2'3'-c-GAMP promoted the polarization of TAMs towards a pro-inflammatory subtype through the IL6R-JAK-IL24 pathway. Additionally, these changes significantly decreased the number of tumor-infiltrating CD4+ T cells and increased the CD8+/CD4+ ratio in GC tumors (Miao et al., 2020).
Existing evidence has demonstrated that human TAMs expressed PD-1. PD-1 expression of TAM was inversely related to the phagocytic ability against tumor cells. Recent studies have revealed that obesity could induce expression of PD-1 in TAMs, which significantly provided negative feedback to TAMs including the suppression of their glycolysis, phagocytosis, and their T cell stimulatory potential (Bader et al., 2024). Moreover, PD-1 deficiency slowed tumor growth, enhanced TAMs glycolysis and antigen-presentation capability, and increased CD8+ T cell activity while reducing markers of exhaustion (Bader et al., 2024). Additionally, TAM secreted Itaconate that enhanced the expression of PD-1 by promoting the upregulation of H3K4me3 at the Eomes promoter, thus promoting the tumor evasion by causing CD8+ T-cell exhaustion. (Bader et al., 2024). In vivo blockade of the PD-1-PD-L1 interaction enhanced macrophage phagocytosis, reduced tumor growth, and prolonged survival in mouse cancer models (Gordon et al., 2017). The prevalence of PD-1+ macrophages was notably elevated in GC tissues. In terms of phagocytic activity, PD-1+ macrophages exhibited significant impairment compared to their PD-1− counterparts. Furthermore, the frequency of PD-1+ macrophages were identified as an independent prognostic factor for patients survival (Kono et al., 2020). Wang et al. identified a specific subset of TAMs that highly expressed PD-1 in GC patients with advanced stages. These PD-1+ TAMs displayed M2-like characteristic, could significantly impede CD8+ T-cell function by triggering PD-1 signaling pathway. Further mechanistic studies revealed that tumor cell-derived exosomes upregulated the transformation from monocytes into PD-1+ TAMs, leading to an immunosuppressive TIME (Wang et al., 2018).
Additionally, TAMs are capable of expressing TIM-3, which could trigger functional inactivation of T cell (Wang et al., 2017). M2 TAMs also promoted the acclamations of lipids in the TIME, thus impairing T cell function (Luo et al., 2020). Furthermore, M1 TAMs enhanced the immune clearance of GC cells by improving the functionality of CD8+ T cells through the increased secretion of TNF-α in the TME (Li et al., 2019). M1 TAMs also secreted exosomes contained miR-16-5p that reduced expression of PD-L1 on GC cells, resulting in activated T cells (Li et al., 2020). These findings indicated that targeting these molecules that mediated the functional regulation of CD8+ T cells presented a promising immunotherapeutic approach.
2.2.2 TAMs interact with CAFs via targeting functional proteins
CAFs play a pivotal role in shaping the TME of GC, particularly by modulating TAMs polarization. In the context of GC, CAFs were found to highly express insulin-like growth factor binding protein 7 (IGFBP7), which induced the polarization of M2 TAMs via the fibroblast growth factor 2 (FGF2)/FGFR1/PI3K/AKT signaling pathway (Li D. et al., 2023). In line with this, CAFs secreted POSTN to promote M2 TAMs polarization in GC (You et al., 2023). Recently, extracellular vesicles (EVs) secreted by CAFs were found to induce the polarization of M2 macrophages by delivering miR-4253 that directly targeted IL6R (Duan et al., 2024). CAFs also expressed FERMT2 that promoted M2-like TAMs polarization (Yin et al., 2024), suggesting a fibroblast/FERMT2/M2 TAM axis for GC treatment. SPP1+ macrophages was a novel type of TAMs that have been identified by single-cell RNA sequencing. Notably, SPP1+ macrophages interacted with FAP-positive CAFs, contributing to the establishment of a hypoxic tumor microenvironment, which is known to promote tumor progression and resistance to therapy (Su et al., 2024).
2.2.3 TAMs interact with MSCs via NF-kB and STAT3 pathway
GC cells secreted exosomes activated mesenchymal stem cells (MSCs) by prompting NF-kB signaling pathway, leading to enhanced TAM functions and supported tumor growth (Shen et al., 2019). Moreover, MSCs derived from GC cells activated the Janus kinase 2 (JAK2)/STAT3 signaling pathway, thereby enhancing the M2 TAMs polarization through increased secretion of IL-6 and IL-8 (Li et al., 2022).
2.2.4 TAMs interact with NK cells and B-lymphocytes via TGF-β pathway
NK cells are a crucial component of the antitumor immune response in GC. Isolated TAMs have the ability to suppress the expression of IFN-γ, TNF-α, and Ki-67 in NK cells. Blocking TGF-β1 alleviated the inhibitory effects of TAMs on the functions of NK cells (Peng et al., 2017). Moreover, Peng et al. depicted dynamics communications within TIME in advanced GC patients that TAMs recruited CXCL2+CAFs and CMTM2+ neutrophils through complement 3/ complement C3a Receptor 1 (C3/ C3AR1) and annexin axis (Peng H. et al., 2024). TAMs also interacted with B-lymphocytes by the TGF-β signaling pathway, while they also secreted CCL3 to enhanced the recruitment of neutrophils (Peng H. et al., 2024). Furthermore, mast cells were activated by IL-11/IL-33 axis, which released macrophage-related factors including GM-CSF, CCL3, and IL-6 that promoted TAMs polarization. These findings indicate that TAMs interact with various cell types within the TIME, collectively influencing the progression of GC (Figure 2). As additional sources of TAMs are identified, their diversity and complexity within the TME will be more extensively elucidated, which may pave the way for GC treatment.
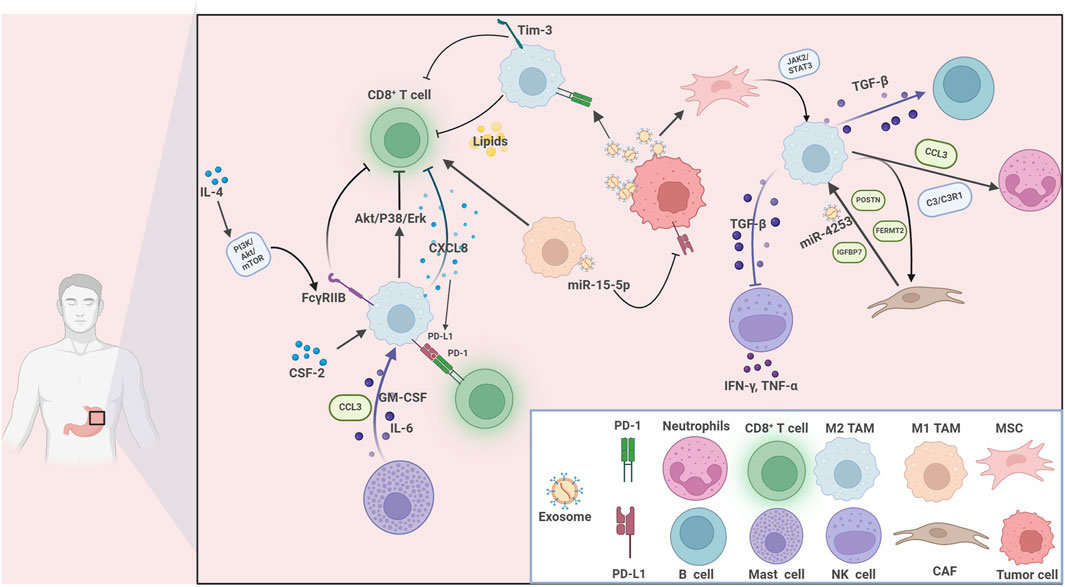
Figure 2. The communications between tumor-associated macrophages and various immune cells within the tumor immune microenvironment in gastric cancer. Immune cells interplay with tumor cells in the gastric cancer TME. Key players include CD8+ T cells, M1 and M2 tumor-associated macrophages (TAMs), neutrophils, B cells, mast cells, NK cells, mesenchymal stem cells (MSCs), cancer-associated fibroblasts (CAFs), and tumor cells. Multiple cytokines, chemokines, and signaling pathways mediate these interactions. For example, GM-CSF, IL-6, CXCL8, and CCL3 facilitate cellular communication and recruitment. The PI3K/Akt/mTOR, Akt/P38/Erk, TGF-β/STAT3, and JAK2/STAT3 pathways are involved in immune modulation, cell polarization, and tumor progression. Immune checkpoints (PD-1, PD-L1, Tim-3), exosomes (carrying miR-15-5p, miR-4253), and lipids further contribute to immunosuppression and tumor immune escape. Key molecules such as POSTN, FERMT2, and C3/C3R1 regulate the behavior of stromal and immune cells.
3 Functions and mechanisms of TAMs in GC progression
3.1 TAMs in tumor proliferation, invasion and metastasis in GC
Emerging evidence has demonstrated that TAMs and cancer cells engage in interactions mediated by various functional proteins and cytokines, which affect cell proliferation, invasion, migration in the setting of GC (Zhang G. et al., 2023; Deng et al., 2024; Piao et al., 2022). Within the hypoxia microenvironment, TAMs were stimulated to secret CXCL8, which significantly prompted cell proliferation and invasion in the cell lines of GC. Further mechanistic investigations have revealed that hypoxia activated C-X-C Motif Chemokine Receptor 1/2 (CXCR1/2), leading to the activation of JAK/STAT1 pathway, which subsequently triggered the secretion of IL-10 to facilitate macrophages polarization towards M2 phenotype, further established a reciprocal loop between TAMs and tumor cells (Piao et al., 2022). Moreover, GC cell-derived exosomes packaged circATP8A1, which sponged miR-1-3p and activated STAT6 cascade, resulting in promoted cell proliferation and metastasis by inducing M2 polarization of TAMs to enhance the secretion of IL-10, TGF-β, and CXCL1 (Deng et al., 2024). Additionally, M2 TAMs secreted EVs packaged apolipoprotein to activate PI3K/Akt signaling pathway, leading to enhanced cell proliferation, invasion and tumor growth in GC (Z et al., 2018). In line with this, TAMs enhanced TNF-α secretion by triggering the TNFR1/ERK/VGLL1 axis, facilitating the cell viability in the setting of GC (Hwang et al., 2022). Moreover, the MIR181A2HG/miR-5680 axis acted as a novel ceRNA pathway promoting versican (VCAN) expression. VCAN, via paracrine signaling, bound CD44 on TAMs to induce M2 polarization and increase VEGF-C secretion, facilitating lymphangiogenesis. Meanwhile, autocrine VCAN binding to CD44 on GC cells activated the Hippo pathway and SP1, further upregulating MIR181A2HG and forming a positive feedback loop that promotes lymphatic metastasis (Zang et al., 2025). Additionally, CEBPB-driven transcription elevated SERPINE1 levels, which, through autocrine signaling, activated PI3K/AKT and EMT pathways to enhance anoikis resistance and metastatic capacity in GC cells. Paracrine SERPINE1 also promoted M2 macrophage polarization via LRP1, leading to reduced CD8+ T-cell infiltration and suppressed antitumor immunity. Thus, SERPINE1 is a key mediator of GC progression and immune evasion, representing both a potential therapeutic target (Wang B. et al., 2025). These findings revealed that TAMs play an essential role in cell proliferation and invasion in the context of GC.
Enhanced angiogenesis is the critical process in the formation of the pre-metastatic niche (Liu and Cao, 2016). For example, epiregulin, cyclooxygenase-2, MMP-1, and MMP-2, expressed by cancer cells, collaboratively orchestrate a multifunctional vascular remodeling program that enhanced vascular permeability and drives the progression of lung metastasis (Gupta et al., 2007). Moreover, TAMs are capable of antigen presentation and initiating immune responses, leading to production of various tumor-derived secreted factors (TDSFs), EVs, and other molecular components (Nasrollahzadeh et al., 2020). These substances are released into the bloodstream, and accumulated at the distant site, the microenvironment gradually transforms into a premetastatic niche that is conducive to the colonization and growth of tumor cells (Wang Y. et al., 2024). In GC patients with liver metastasis, serum exosomal miR-519a-3p (exo-miR-519a-3p) was elevated and associated with poor prognosis. Furthermore, exo-miR-519a-3p activated the mitogen-activated protein kinase (MAPK)/ERK signaling pathway via dual specific phosphatase 2 (DUSP2), leading to M2-type polarization of TAMs. These changes promoted angiogenesis, leading to formation of intrahepatic premetastatic niches and further facilitated the liver metastasis (Qiu et al., 2022). Li and colleagues further expanded the molecular mechanisms on liver metastasis of GC. Their study indicated that TAMs activated epithelial-mesenchymal transition (EMT) to suppress MAPK4 expression, resulting in enhanced secretion of macrophage migration inhibitory factor (MIF), which further polarized TAMs towards M2 phenotype. The M2 TAMs-mediated MAPK4 downregulation facilitated liver metastasis, which might provide novel strategy for GC treatment (Li S. et al., 2023). TAMs transcriptionally increased the expression of glial cell-derived neurotrophic factor (GDNF) in GC, which formed GDNF/ GDNF family receptor alpha 1 (GFRA1) axis to modulate lysosomal functions, leading to inhibited cell apoptosis and autophagy flux, leading to enhanced liver metastasis under the metabolic stress (Ni et al., 2023). Additionally, emerging evidence has further exploited molecular mechanism underlying the tumor-promoting role of TAMs in GC cell proliferation, growth, invasion and metastasis (Eum et al., 2020; Gao et al., 2024; Ito et al., 2021; Shen et al., 2013; Tang et al., 2021; Wang et al., 2020; Xiao et al., 2024; Yang et al., 2021; Yao et al., 2023; Zhang S. et al., 2023), which highlight that targeting TAMs may be promising strategy for GC treatment (Figure 3). In conclusion, TAMs play an essential role in GC cell proliferation and metastasis, which may provide potential therapeutic targets.
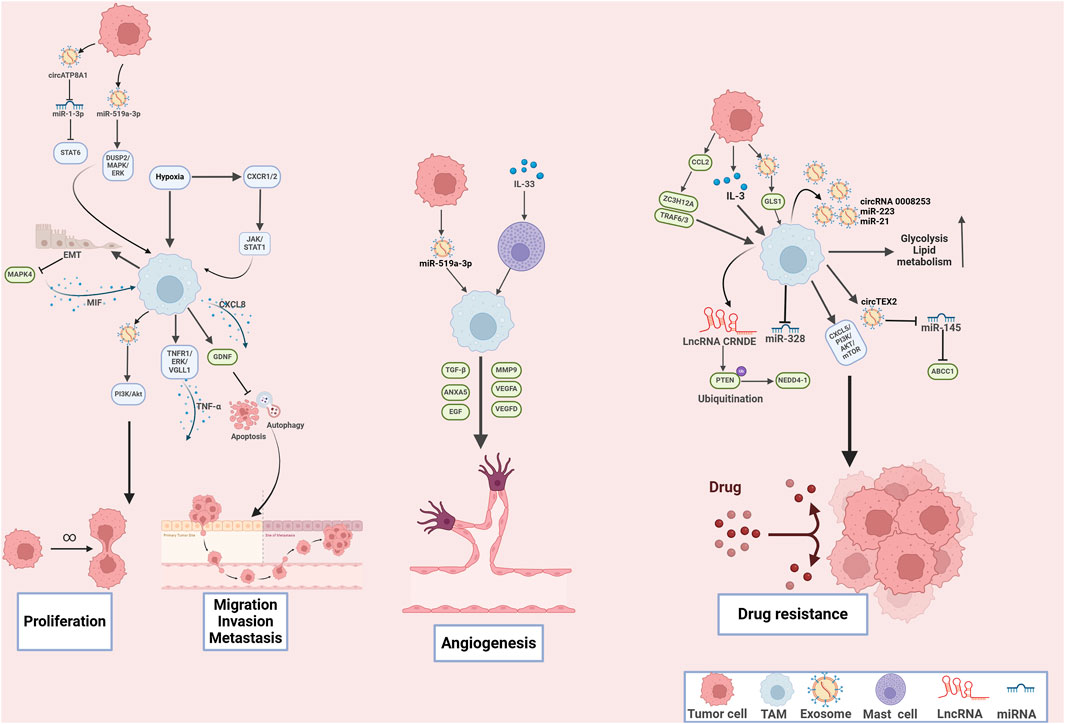
Figure 3. The mechanisms of tumor-associated macrophages in tumor cell proliferation, metastasis, angiogenesis and drug resistance in gastric cancer. Tumor-associated macrophages play multifaceted roles in driving gastric cancer cell proliferation, metastasis, angiogenesis, and drug resistance through complex molecular interactions and signaling pathways.
3.2 TAMs in the angiogenesis in GC
Angiogenesis is a multifaceted process that is crucial for the growth and development of organs, the regeneration of tissues, and the progression of various pathological conditions including cancer (Giubelan et al., 2023). Research has demonstrated that TAMs contribute to tumor angiogenesis by secreting various pro-angiogenic factors, including VEGF-A, epidermal growth factor (EGF), TNF-α, and CXCL12 (Martin and Gurevich, 2021). For example, exosomal miR-519a-3p-devried from GC cells that promoted M2-like macrophage polarization, leading to upregulated levels of VEGFA, VEGFD, TGF-β,thus promoting angiogenesis (Qiu et al., 2022). ANXA5 was identified as the TAM-related gene that promoted angiogenesis in GC (Hong et al., 2024). IL-33/mast cells mediated TAM polarization significantly promoted tumor angiogenesis in GC (Eissmann et al., 2019). Moreover, the expression of cyclooxygenase-2 (COX-2) in TAMs played a crucial role in regulating angiogenesis. Recent studies indicated that when co-cultured with GC cells, M2-polarized TAMs secreted matrix metalloproteinase 9 (MMP9) as a result of COX-2 upregulation, thereby facilitating tumor angiogenesis (Oshima et al., 2011). Several studies also demonstrated the stimulatory effects of M2 TAMs on angiogenesis in the setting of GC (Tang et al., 2021; Guo et al., 2022; Mu et al., 2021), suggesting the essential role of TAMs in angiogenesis. Moreover, SPP1+ macrophages interacted with FAP+ CAFs, and endothelial cells through regulating VEGF pathway, ECM remodeling, and hypoxia pathway, collectively facilitating angiogenesis in GC (Su et al., 2024). The aforementioned research demonstrated that TAMs play an essential role in tumor angiogenesis, collectively influencing the progression of GC and severing as promising anti-angiogenesis targets.
3.3 TAMs in drug resistance in GC
During the treatment with chemotherapeutic drugs, TAMs were recruited and polarized to the M2-like phenotype (Ishimoto et al., 2014). M2 TAMs increased CD44 expression by suppressing miR-328, thereby promoting cancer progression through enhanced ROS defense and resistance to chenotherapy (Ishimoto et al., 2014). Oxaliplatin, the third-generation platinum-based chemotherapeutic agent, sever as first-line anti-cancer drug GC (Lordick et al., 2014). However, resistance to oxaliplatin significantly worsened clinical prognosis (Lordick et al., 2014). M2-polarized TAMs secreted exosomes (M2-Exos), which could transfer circRNA 0008253, miR-223 and miR-21 from TAMs to tumor cells, resulting in reduced cell apoptosis and enhanced resistance to oxaliplatin, doxorubicin, and cisplatin (Yu D. et al., 2023; Gao et al., 2020; Zheng et al., 2017). The transfer of exosomal circTEX2 from M2 TAMs to tumor cells significantly promoted cisplatin resistance via sponging miR-145 and indirectly targeting ATP Binding Cassette Subfamily C Member 1 (ABCC1) (Qu et al., 2024). TAMs also secreted EVs packaged long noncoding RNA (LncRNA) CRNDE, which facilitated the ubiquitination-mediated degradation of phosphatase and tensin homolog (PTEN) and activating the neural precursor cell expressed developmentally downregulated protein 4-1 (NEDD4-1) axis, thus increased resistance to cisplatin in the cell lines of GC (Xin et al., 2021). Moreover, 5-fluorouracil (5-FU) continues to be the first-line chemotherapeutic agent for gastric cancer; however, the development of chemoresistance often leads to unsatisfactory clinical outcomes (Liu J. et al., 2024). During the treatment with chemotherapeutic drugs, TAMs were recruited within the TME. TAMs conferred 5-FU chemoresistance to GC cells by activating CXCL5/PI3K/AKT/mTOR signaling pathway (Su et al., 2022). Furthermore, GC cells secreted IL-3 to induce a shift in macrophage polarization towards a M2-like phenotype, which further promoted GLUT3-mediated glycolysis reprogramming to secret CCL8 for activating JAK/STAT pathway that promoted 5-FU resistance (He et al., 2021). Moreover, GC patients with peritoneal metastasis (GCPM) frequently exhibit a rapidly declining clinical course marked by chemotherapeutic resistance and dismal prognosis (Li Z. et al., 2023). Single-cell RNA sequencing analysis revealed that C1Q+ macrophages infiltrated in the TME of GCPM therapeutic failure cases, which exhibited high M2_scores. C1Q+ macrophages might contribute chemotherapeutic resistance through enhancing lipid metabolism activity, glycolytic activity and activating the complement and annexin pathways to interplay with CAFs and neutrophils within the TME, providing an intracrine molecular network that could be targeted for GCPM treatment (Peng H. et al., 2024).
Trastuzumab serves as a primary targeted treatment for GC patients with positive human epidermal growth factor receptor-2 (HER2) expression, which has significantly impeded clinical efficacy due to drug resistance (Malla et al., 2024). Tumor cells produced microvesicles packaged glutaminase 1 (GLS1) via cell division cycle 42 (CDC42) that promoted M2 TAMs polarization and contributed to trastuzumab resistance (Hu et al., 2023). Moreover, tumor cells enhanced the expression of CCL2 that impaired M1 macrophages polarization via activating the transcription factor ZC3H12A and regulating TRAF6/3, thus promoting trastuzumab resistance (Sun et al., 2022). These findings revealed that targeting TAMs and disrupting the interactions between TAMs and tumor cells could be promising therapeutic approaches for overcoming drug resistance in GC patients.
3.4 TAMs in the immunotherapy resistance of GC
Recent advancements in immune checkpoint inhibitors (ICIs) have shown significant potential in the treatment of GC. Nevertheless, only a small proportion of GC patients exhibit durable responses to treatment with PD-1 or PD-L1 inhibitors. This limited efficacy may be attributed to the heterogeneity present within the TIME (Yasuda and Wang, 2024), as well as resistance to immunotherapy (Liu et al., 2023). In the TME, high levels of macrophage infiltration are often strongly associated with resistance to PD-1/PD-L1 immune checkpoint inhibitors (Kim et al., 2020). TAMs could secrete TGF-β to alter PD-L1 expression. For instance, TGF-β induced PD-L1 expression in tumor cells and enhanced angiogenesis, potentially through succinate accumulation in tumor cells (Gómez et al., 2020). Additionally, TAMs increased PD-L1 expression in tumor-infiltrating myeloid cells via the COX-2/mPGES1/PGE2 pathway, leading to the exclusion of CD8+ T cells (Prima et al., 2017). Additionally, researchers have observed IL-4 induced glycolysis in TAMs by activating the PI3K/AKT/mTOR signaling pathway. Ultimately, these changes led to functional impairment of CD8+ T cells in GC and contribute to resistance to PD-1 antibody therapy (Zhang J. et al., 2024).
3.4.1 TAM-mediated immunotherapy resistance via PD-L1/CXCL8
Additionally, emerging evidence has revealed that TAMs play an essential role in immunotherapy resistance (Shi B. et al., 2024). Comprehensive biomarker profiling based on REGOMUNE phase II study has demonstrated that M2-like macrophages were significant abundance in the TIME of GC (Cousin et al., 2024). Further mechanistic studies have revealed that M2 macrophage-derived exosomes (M2-Exos) upregulated PD-L1, leading to inhibited T cell activation and proliferation, thus contributing to resistance to ICIs (Wang et al., 2021). TAMs secreted CXCL8 to upregulate PD-L1 on TAMs, thus determining immune evasion and resistance to ICIs (Lin et al., 2019). Moreover, TAMs also promoted ICI resistance via CXCL16-CXCR6. For example, TAMs also recruited CD8+ T cells that solely expressed NKG2A via the CXCL16-CXCR6 pathway, leading to anti-PD-1 resistance in GC patients (Li G. et al., 2024), supporting the potential of NKG2A as a novel immunotherapeutic target and present fresh perspectives on combination strategies involving ICIs in GC (Li G. et al., 2024). These evidence has revealed that TAMs could promoted immunotherapy resistance by regulating PD-L1/ CXCL8 and CXCL16-CXCR6 pathway.
In GC, Quiescent cancer cells (QCCs) were found to promote the glycolytic reprogramming of M2 macrophages, which promoted hypoxia inducible factor 1 alpha (HIF1A)-mediated T-cell exhaustion and conferred cell resistance to ICIs (Qi et al., 2024). Moreover, melatonin significantly enhanced the production of cancer-derived exosomes and releasing exosomal miRNAs, which significantly enhanced anti-tumor immunity. Mechanistically, exosomal miR-20b-5p, miR-17-5p, and miR-93-5p transcriptionally and translationally reduced PD-L1 levels in TAMs, which facilitated the infiltration of CD8+ T cells into the tumor site and boosted anti-tumor immune responses (Wang et al., 2023). Furthermore, GC cells secreted legumain upon binding to the integrin αvβ3 on the cell surface, subsequently activating the αvβ3/PI3K/AKT/mTORC2 signaling pathway. This activation promoted metabolic reprogramming and facilitated the polarization of macrophages from the M1 phenotype to the M2 phenotype, leading to anti-PD-1 immunotherapy resistance (Pei et al., 2024). MUC1+ GC cells secreted growth differentiation factor 15 (GDF15) to facilitate the immunosuppressive functions of M2 TAMs, which in turn influenced the functions of T lymphocytes via the SPP1-CD44 interaction, thus promoting immunotherapy resistance (Peng H. et al., 2024). IL-4 skew TAM polarization to M2-like phenotype, which further enhanced FcγRIIB expression and promoted PI3K/AKT/mTOR axis, resulting in immune resistance to anti-PD-1 treatment (Zhang J. et al., 2024). Dickkopf-1 (DKK1) mediated the induction of M2 TAMs impaired the clinical effacicy of PD-1 antibody treatment by interplaying with cytoskeleton-associated protein 4 (CKAP4) and facilitating PI3K-AKT pathway (Shi et al., 2022). Through the exploration of these complex interactions, these findings deliver important insights into precision medicine and aims to improve therapeutic outcomes in the treatment of GC.
4 Therapeutic interventions of TAMs in GC
An increasing body of evidence is enhancing our perspectives on TAMs in the tumorigenesis of GC. TAMs play an essential role in cell proliferation, invasion, migration and drug resistance of GC, which hold immense potential in cancer therapy. There are various strategies specifically designed to target macrophages in the preclinical and clinical settings, which including targeting TAM infiltering and macrophage polarization. Additionally, engineered macrophages such as chimeric antigen receptor macrophages (CAR-M) have progressed into clinical evaluation, which demonstrated promising results in GC treatment (Table 1).
4.1 Inhibiting the infiltration of M2 TAMs in TME
Given the essential role of TAMs in the progression of GC, eliminating TAMs within the TIME could benefit GC patients. Gastrointestinal peptide gastrin has been reported to activate the function of TAMs by increasing the expression of IL-1β, IL-3Rβ, and SDF-1α, which fostered the formation of TME (Han et al., 2013). Moreover, gastrin has been demonstrated to facilitate tumor growth via paracrine and autocrine mechanisms mediated by the cholecystokinin-B receptor (CCK-BR), which has been found to be expressed in approximately 50% of GC patients (Duan et al., 2022). Polyclonal antibody stimulator (PAS), a specific immunogen vaccine that could stimulate antibodies targeting gastrin peptide, thus impeding GC progression. Research performed by Simth et al. reported that either PAS monotherapy or its combination with PD-1 antibody led to a decrease in the number of M2-type TAMs (Smith et al., 2021). High expression of CCL2 was found to be associated with increased the proportion of M2-like phenotype macrophages, which could be attributed to activated ZC3H12A-TRAF6/3 axis, which could be reversed by CD40-HER2 bispecific antibody that recovered the ubiquitination process of TRAF6/3, leading to decreased M2-like TAMs in the TIME of HER2-positive GC (Sun et al., 2022). Mannose-conjugated chlorin (M-chlorin), a specific type of photosensitizer that could effectively bind to mannose receptors, which are prominently expressed on TAMs. Photodynamic therapy with M-chlorin were demonstrated to induce effectively cell apoptosis of TAMs and tumor cells within the TME, thus blocking GC progression (Hayas et al., 2015). Modified Jianpi Yangzheng (mJPYZ), an empirical decoction derived from Traditional Chinese medicine has been found to significantly increase the survival duration of patients suffering from advanced-stage GC. Mechanistically, mJPYZ reduced the transfer of exosomal pyruvate kinase M2 (PKM2) from GC cells to macrophages, mitigating the infiltering of M2-type TAMs within the TIME, thereby ultimately impairing tumorigenesis in GC experimental models (Wu et al., 2022). These findings reveal that mJPYZ, PAS and M-chlorin function as essential therapeutic agents for GC treatment by inhibiting the infiltration of M2-like TAMs. Although significant progress has been made in preventing tumor progression by inhibiting the recruitment and activity of TAMs. However, it is crucial to accurately assess both the extent and duration of TAM depletion. An excessive reduction in macrophage levels may inadvertently promote tumor progression (Bercovici et al., 2019). Increasing the drug dosage to deplete TAMs can also lead to a higher incidence of adverse events. Consequently, this technique requires further clinical exploration to mature and establish its safety and efficacy. Future research should focus on addressing these issues, thus paving the way for GC treatment.
4.2 Repolarization of macrophages
Mounting experimental data indicate that targeting the polarization of TAMs may be advantageous in cancer immunotherapy (Anderson et al., 2021). Researchers are increasingly utilizing the plasticity of macrophages to enhance polarization towards M1 phenotype or inhibit polarization towards M2 phenotype (Zhang and Sioud, 2023). Ubiquitin-specific protease 14 (USP14) served as an immunosuppressive factor in GC and associated with the polarization of M2 TAMs. The activation of USP14 stabilized SIRT1, which played an essential role in the fatty acid oxidation of TAMs. Blocking USP14- SIRT1 axis by IU1, the USP14 inhibitor, significantly impeded macrophage polarization towards M2 phenotype, thus inhibited tumor metastasis (He et al., 2023). The PI3K-γ inhibitor (IPI-549) was found to restore phagocytosis and polarization of M2 TAMs that were suppressed by lipid accumulation, thereby enhancing anti-tumor responses (Luo et al., 2020). In line with this, neutrophil extracellular trap (NET) degrader DNase-1 mitigated the effects of triggering receptor expressed on myeloid cell 1 (TREM1)-induced M2 macrophage polarization (Yu et al., 2024). Dickkopf-1 (Dkk1) functions as an inhibitor of Wnt signaling pathway through a negative regulatory mechanism, which is associated with poor prognosis in patients with various types of cancers (Wall et al., 2020). DKN-01, the specific antibody that targets DKK1, has been reported to inhibit GC progression by promoting M1 polarization and suppressing M2 polarization of TAMs via promoting the activation of cGAS/STING signaling pathway (Yang X. et al., 2024). Methionine restriction enhanced the M1-type polarization via suppressing MIF derived from tumor cells, highlighting a novel avenue for developing therapeutic drugs for GC (Xin et al., 2025). Additionally, Opioid-free anesthesia promoted the convention of M2 TAMs into M1 TAMs, thus reducing perioperative immunosuppression, which provides immense potential for GC treatment (Liu W. et al., 2024). Aurora kinase inhibitors Barasertib-HQPA and Danusertib could promoted cellular senescence, thus upregulating MCP-1/CCL2, ultimately inducing macrophage to M2-type polarization (Yang R. et al., 2024), indicating that clinicians should consider a sequential therapy involving senescent cell clearance following aurora kinase inhibitors treatment. Currently, the CD47-SIRPα axis is being extensively studied within the “do not eat me” signaling pathways (Jia et al., 2021). CD47 levels are consistently elevated across various tumor types and play a crucial role in inhibiting cellular function by binding to the transmembrane protein SIRPα on phagocytic cells (Jia et al., 2021). Furthermore, IHC analysis of GC samples demonstrated a positive correlation between CD47 expression and the infiltration of TAMs. The CD47 antibody increased the phagocytic activity of TAMs and enhanced secretion of IFN-β in Epstein–Barr virus-associated GC (Duan et al., 2023). These findings are paving the way for the clinical translation of TAMs as therapeutic targets.
Natural products and their extracts have a rich history of extensive use in the treatment of cancers (Wang Z. et al., 2024). Yi et al. found that epigallocatechin gallate (EGCG) extracted from Hedyotis diffusa Willd injection (HDI), significantly interacted with STAT3, leading to its reduced nuclear localization. These changes promoted the transcriptional suppression of PLXNC1, resulting in repolarization of M2 TAMs induced by exosomal miR-92b-5p derived from tumor cells, ultimately leading to diminished tumor progression in GC (Yi et al., 2024). In addition, Zhang and colleagues revealed that Qingrexiaoji Recipe, the traditional Chinese medicine, exhibited tumor-suppressive functions on GC cells by converting the M2-type TAMs into M1-type TAMs via modulating miR-29a-3p/HDAC4 axis (Zhang Y. et al., 2024). Additionally, rehmannia polysaccharide, dendrobium officinale polysaccharides, and diosmetin have been demonstrated to regulate M2-type TAM polarization (Liu X. et al., 2024; Zhao et al., 2023; Zhang and Luo, 2023).
Toll-like receptors (TLRs) are crucial for the activation of macrophages and the regulation of immune responses (Gallego et al., 2011). TLR4, the most prominent pattern recognition receptors involved in innate immunity, affecting malignant progression of human tumors (Shetab Boushehri and Lamprecht, 2018). Sophoridine has been shown to reduce CD8+ T cell exhaustion as well as converting M2-type into M1-type TAMs through toll-like receptor 4 (TLR4)–interferon regulatory factor 3 (IRF3) axis (Zhuang et al., 2020). The dual combination application of Palmitic acid and γ-interferon(γ-IFN), the TLR4 agonists, significantly increased M1-type macrophages and impeded M2-type macrophages polarization, thus suppressing GC progression (Zhang YY. et al., 2023). Furthermore, emerging studies have revealed that Calcitriol (Jie et al., 2024), Betulinic Acid (Chen et al., 2023), Flavokawain B (Zhu et al., 2022), Dextran Sulfate (Guo et al., 2022) have been involved in the regulation of M1/M2 macrophages polarization, thus providing novel therapeutic strategies for GC treatment. However, this therapeutic strategy encounters several challenges. Strategies aimed at inhibiting M2 TAMs may also affect other immune cell types, leading to alterations in the overall immune response. This could weaken the body’s ability to combat infections and other diseases. Even if M2 TAMs are successfully inhibited, tumor cells may develop resistance through alternative mechanisms, such as acquiring a more aggressive phenotype or modifying their microenvironment, allowing them to continue growing and metastasizing (Tan et al., 2025). Based on these preclinical evidence, more clinical trials are needed to pave the way for the development of TAMs as therapeutic targets in clinical translation.
4.3 Combination therapeutic strategies involved TAMs for GC
Significant clinical benefits have been observed with antiangiogenic therapy in multiple types of human cancers. However, the effectiveness of these therapies is hindered by the immunosuppressive tumor microenvironment. Pan et al. developed mAb04-MICA, a fusion antibody that targeting VEGFR2 that is linked to the α1-α2 ectodomain of MHC class I chain-related molecules A (MICA), which exhibited great synergistic effects with anti-PD-1 therapy in GC by repolarization of TAMs (Pan et al., 2023). Furthermore, OBP-702, the p53-expressing oncolytic adenovirus has exhibited significant impacts on the repolarization of TAMs by the combination of anti-PD-1 antibody, leading to suppressed peritoneal metastasis of GC (Tabuchi et al., 2024). Additionally, gastrin vaccine, both as a standalone treatment and in combination with the PD-1 antibody, inhibited GC progression through the reduction of M2 TAMs within the TIME (Smith et al., 2021). The aforementioned facts suggest that targeting TAMs, either alone or in conjunction with ICIs, could represent a promising therapeutic strategy for GC, which await further investigations.
5 CAR-M in GC therapy: advancements and challenges
The application of CARs to macrophages represents a promising strategy for enhancing cellular immunotherapy against solid tumors (Liu Y. et al., 2024). CAR-M represents an innovative form of immunotherapy that involves genetically modifying macrophages to express a specific CAR, which enhances the phagocytic activity and antigen presentation of macrophages, leading to greater specificity in targeting tumors and increased anti-tumor effectiveness (Liu Y. et al., 2024; Shi Y. et al., 2024). CAR-M demonstrates enhanced migratory and infiltrative capabilities by producing matrix metalloproteinases, which can degrade the ECM, allowing it to overcome the challenges posed by dense extracellular matrices (Zhang et al., 2019; Liu et al., 2022). By modulating the composition of the extracellular matrix and reducing immunosuppressive factors, CAR-M can enhance the activity and efficacy of other immune cells, such as T cells and NK cells (Maalej et al., 2023). Additionally, it possesses the ability to modulate the phenotype of TAMs (Noy et al., 2014; Lee et al., 2013). Small molecule inhibitors typically target specific signaling pathways to suppress tumor cells; however, they often lack selectivity, potentially affecting healthy cells, and require continuous administration to maintain efficacy, leading to drug resistance (Dong et al., 2021). Additionally, these inhibitors typically do not adapt to changes in the tumor microenvironment, resulting in diminished effectiveness in certain scenarios (Dong et al., 2021). In contrast, CAR-M demonstrates greater adaptability by responding to the biological characteristics and microenvironment of tumors, offering enhanced flexibility. Antibody-based therapies, such as monoclonal antibodies, possess high specificity and can directly target tumor cells or influence signaling pathways (Zinn et al., 2023). Nevertheless, their anti-tumor effects are frequently compromised by immunosuppression within the tumor microenvironment, limiting their efficacy (Zinn et al., 2023). Moreover, unlike CAR-M, which can modulate the local immune environment and alter the tumor microenvironment, antibody therapies generally focus on single antigens, resulting in lower adaptability to various tumor types.
Macrophages can be precisely modified through modern techniques. Zheng et al. performed bioinformatics analyses, which revealed that c-MET was elevated in pancreatic cancer tissues. Furthermore, CAR-M-c-MET cells were developed and exhibited specific binding to cancer cells, which significantly enhanced the phagocytic and cytotoxic capabilities of macrophages and resulted in remarkable tumor suppression in the murine models (Zheng et al., 2024). Although great advancements of CAR-M have been made in solid tumors, there are few studies that have investigated CAR-M based treatment in the setting of GC. Dong and colleagues created a novel CAR-M by genetically modifying macrophages to express a HER2-FcεR1γ-CAR (HF-CAR), which specifically targeted HER2-positive GC and exhibited great synergistic effects in combination with oxaliplatin, leading to significant anti-tumor functions in mouse models (Dong et al., 2023).
Although CAR-M represents a promising approach in cancer immunotherapy, it encounters several challenges, particularly regarding the limited availability of cells and their low proliferation capacity in vivo, which can adversely affect treatment efficacy (Li J. et al., 2024). Additionally, macrophages exhibit a lower propensity to circulate in the bloodstream, making the collection and development of these cells a significant challenge in CAR-M production (Shi Y. et al., 2024). Additionally, the heterogeneity of human macrophages in contrast to mouse macrophages, along with the limited understanding of human macrophage biology, presents further difficulties in the production of CAR-M (Wang et al., 2022). On the other hand, due to the multifunctional role of macrophages in immune regulation, patient safety and treatment outcomes may have different implications (Locati et al., 2020; Klichinsky et al., 2020). Research has shown that macrophages play a crucial role in the onset of cytokine release syndrome (CRS) associated with CAR-T therapy, primarily through the production of IL-6, IL-1, and NO (Giavridis et al., 2018; Huang et al., 2024b). Given that CAR-M has the potential to profoundly alter immune system dynamics, the long-term effects of CAR-M are particularly contentious. With advancements in research and the implementation of various innovative solutions, these concerns can be alleviated and CAR-M would be regarded as a promising therapeutic approach in GC.
6 Conclusions and perspectives
TAMs function as the essential immunomodulators within the TME of GC. TAMs establish a complex communication network with other types of immune cells or stromal cells, thus contributing to the immunosuppressive TME in GC. Moreover, the crosstalk between TAMs and tumor cells significantly impacts GC progression including tumor growth, metastasis, angiogenesis and drug resistance. As one of the most abundant cell types in solid tumors, TAMs contribute to increased PD-L1 expression in tumor cells and other immunosuppressive cells through the secretion of cytokines. Furthermore, the regulation of the epigenetic state of TAMs, influencing gene expression and thereby enhancing their immunosuppressive functions, which is crucial for the ICI resistance (Zhang and Jagannath, 2025). This understanding not only sheds light on the biological functions of TAMs but also presents potential targets and strategies for future therapeutic interventions.
Besides, the intricate immunomodulatory axis remarkably promotes immunotherapy resistance, thus indicating that targeting TAMs hold immense therapeutic potential for GC patients. Strategies such as eliminating TAMs, repolarization of TAMs, and CAR-M has been verified in multiple studies. Nevertheless, introduction of suitable therapeutic strategies targeting TAMs presents several challenges due to the heterogeneity and complexity of macrophage. Firstly, current studies on TAMs are conducted in the experimental mouse models, which typically exhibit notable differences from the pathogenesis and therapeutic responses observed in humans (Sica and Mantovani, 2012). Studies focusing on the development of feasible inhibitors targeting human TAMs within the TIME of GC are critical for TAM-based treatment. Furthermore, the diversity and plasticity of TAMs in human tumors are significantly evident by the distinct characteristics of TAMs at different stages of carcinogenesis, suggesting that relying on a single specific blocking antibody might not effectively eliminate tumors (Nasir et al., 2023). Therefore, investigating the diversity of TAMs and their developmental relationships with tumor progression at the single-cell level could yield valuable insights into approaches for selectively targeting specific subpopulations, which could be applicable in the clinical settings for GC treatment (Ma et al., 2022). In addition, emerging strategies targeting TAMs such as CAR-M and developing specific inhibitors could remove TAMs within TME, which might obstruct the intrinsic communication among TAMs and other types of cells, leading to dysregulated immune system dynamics. These changes might result in unforeseen toxicities, which await further investigation in the clinical settings. And the elimination of TAMs could promote the compensatory emergence of other types of immunosuppressive cells. More basic studies should in-depth explore the molecular mechanism underlying the role of TAMs in GC, which might pave the way for developing feasible strategies targeting TAMs, ultimately improving the prognosis of GC patients.
Author contributions
CTW: Conceptualization, Data curation, Software, Writing – original draft, Writing – review and editing, Project administration, Supervision. JD: Investigation, Methodology, Conceptualization, Validation, Writing – review and editing. YZ: Writing – original draft, Writing – review and editing, Methodology, Validation, Data curation. LW: Writing – review and editing, Methodology. LYX: Writing – review and editing, Data curation. QC: Writing – review and editing, Investigation. CZ: Data curation, Investigation, Writing – review and editing. JH: Conceptualization, Data curation, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anderson, N. R., Minutolo, N. G., Gill, S., and Klichinsky, M. (2021). Macrophage-based approaches for cancer immunotherapy. Cancer Res. 81 (5), 1201–1208. doi:10.1158/0008-5472.Can-20-2990
Bader, J. E., Wolf, M. M., Lupica-Tondo, G. L., Madden, M. Z., Reinfeld, B. I., Arner, E. N., et al. (2024). Obesity induces PD-1 on macrophages to suppress anti-tumour immunity. Nature 630 (8018), 968–975. doi:10.1038/s41586-024-07529-3
Bercovici, N., Guérin, M. V., Trautmann, A., and Donnadieu, E. (2019). The remarkable plasticity of macrophages: a chance to fight cancer. Front. Immunol. 10, 1563. doi:10.3389/fimmu.2019.01563
Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., et al. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74 (3), 229–263. doi:10.3322/caac.21834
Chen, J. L., Tai, Y. S., Tsai, H. Y., Hsieh, C. Y., Chen, C. L., Liu, C. J., et al. (2023). Betulinic acid inhibits the stemness of gastric cancer cells by regulating the GRP78-TGF-β1 signaling pathway and macrophage polarization. Molecules 28 (4), 1725. doi:10.3390/molecules28041725
Cheng, S., Li, Z., Gao, R., Xing, B., Gao, Y., Yang, Y., et al. (2021). A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell 184 (3), 792–809.e23. doi:10.1016/j.cell.2021.01.010
Cousin, S., Guégan, J. P., Shitara, K., Palmieri, L. J., Metges, J. P., Pernot, S., et al. (2024). Identification of microenvironment features associated with primary resistance to anti-PD-1/PD-L1 + antiangiogenesis in gastric cancer through spatial transcriptomics and plasma proteomics. Mol. Cancer 23 (1), 197. doi:10.1186/s12943-024-02092-x
Deng, C., Huo, M., Chu, H., Zhuang, X., Deng, G., Li, W., et al. (2024). Exosome circATP8A1 induces macrophage M2 polarization by regulating the miR-1-3p/STAT6 axis to promote gastric cancer progression. Mol. Cancer 23 (1), 49. doi:10.1186/s12943-024-01966-4
Dong, J., Cheng, X. D., Zhang, W. D., and Qin, J. J. (2021). Recent update on development of small-molecule STAT3 inhibitors for cancer therapy: from phosphorylation inhibition to protein degradation. J. Med. Chem. 64 (13), 8884–8915. doi:10.1021/acs.jmedchem.1c00629
Dong, X., Fan, J., Xie, W., Wu, X., Wei, J., He, Z., et al. (2023). Efficacy evaluation of chimeric antigen receptor-modified human peritoneal macrophages in the treatment of gastric cancer. Br. J. Cancer 129 (3), 551–562. doi:10.1038/s41416-023-02319-6
Du, Y., Lin, Y., Gan, L., Wang, S., Chen, S., Li, C., et al. (2024). Potential crosstalk between SPP1 + TAMs and CD8 + exhausted T cells promotes an immunosuppressive environment in gastric metastatic cancer. J. Transl. Med. 22 (1), 158. doi:10.1186/s12967-023-04688-1
Duan, S., Rico, K., and Merchant, J. L. (2022). Gastrin: from physiology to gastrointestinal malignancies. Funct. (Oxf) 3 (1), zqab062. doi:10.1093/function/zqab062
Duan, X., Yu, X., and Gan, J. (2024). Extracellular vesicle-packaged miR-4253 secreted by cancer-associated fibroblasts facilitates cell proliferation in gastric cancer by inducing macrophage M2 polarization. Cancer Biol. Ther. 25 (1), 2424490. doi:10.1080/15384047.2024.2424490
Duan, Y., Li, S., Huang, B., Dou, Y., Kong, P., Kang, W., et al. (2023). CD47-targeted immunotherapy unleashes antitumour immunity in Epstein-Barr virus-associated gastric cancer. Clin. Immunol. 247, 109238. doi:10.1016/j.clim.2023.109238
Eissmann, M. F., Dijkstra, C., Jarnicki, A., Phesse, T., Brunnberg, J., Poh, A. R., et al. (2019). IL-33-mediated mast cell activation promotes gastric cancer through macrophage mobilization. Nat. Commun. 10 (1), 2735. doi:10.1038/s41467-019-10676-1
El Kasmi, K. C., and Stenmark, K. R. (2015). Contribution of metabolic reprogramming to macrophage plasticity and function. Semin. Immunol. 27 (4), 267–275. doi:10.1016/j.smim.2015.09.001
Eum, H. H., Kwon, M., Ryu, D., Jo, A., Chung, W., Kim, N., et al. (2020). Tumor-promoting macrophages prevail in malignant ascites of advanced gastric cancer. Exp. Mol. Med. 52 (12), 1976–1988. doi:10.1038/s12276-020-00538-y
Franzese, O. (2024). Tumor microenvironment drives the cross-talk between Co-stimulatory and inhibitory molecules in tumor-infiltrating lymphocytes: implications for optimizing immunotherapy outcomes. Int. J. Mol. Sci. 25 (23), 12848. doi:10.3390/ijms252312848
Gallego, C., Golenbock, D., Gomez, M. A., and Saravia, N. G. (2011). Toll-like receptors participate in macrophage activation and intracellular control of Leishmania (Viannia) panamensis. Infect. Immun. 79 (7), 2871–2879. doi:10.1128/iai.01388-10
Gao, H., Ma, J., Cheng, Y., and Zheng, P. (2020). Exosomal transfer of macrophage-derived miR-223 confers doxorubicin resistance in gastric cancer. Onco Targets Ther. 13, 12169–12179. doi:10.2147/ott.S283542
Gao, J., Zhao, Z., Pan, H., and Huang, Y. (2024). Significance of dysregulated M2 macrophage and ESR2 in the ovarian metastasis of gastric cancer. Transl. Cancer Res. 13 (6), 2674–2690. doi:10.21037/tcr-24-124
Gao, Y., Zhang, M., Wang, G., Lai, W., Liao, S., Chen, Y., et al. (2025). Metabolic cross-talk between glioblastoma and glioblastoma-associated microglia/macrophages: from basic insights to therapeutic strategies. Crit. Rev. Oncol. Hematol. 208, 104649. doi:10.1016/j.critrevonc.2025.104649
Giavridis, T., van der Stegen, S. J. C., Eyquem, J., Hamieh, M., Piersigilli, A., and Sadelain, M. (2018). CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 24 (6), 731–738. doi:10.1038/s41591-018-0041-7
Giubelan, A., Stancu, M. I., Honţaru, S. O., Mălăescu, G. D., Badea-Voiculescu, O., Firoiu, C., et al. (2023). Tumor angiogenesis in gastric cancer. Rom. J. Morphol. Embryol. 64 (3), 311–318. doi:10.47162/rjme.64.3.03
Gómez, V., Eykyn, T. R., Mustapha, R., Flores-Borja, F., Male, V., Barber, P. R., et al. (2020). Breast cancer-associated macrophages promote tumorigenesis by suppressing succinate dehydrogenase in tumor cells. Sci. Signal 13 (652), eaax4585. doi:10.1126/scisignal.aax4585
Gordon, S. R., Maute, R. L., Dulken, B. W., Hutter, G., George, B. M., McCracken, M. N., et al. (2017). PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545 (7655), 495–499. doi:10.1038/nature22396
Guan, W. L., He, Y., and Xu, R. H. (2023). Gastric cancer treatment: recent progress and future perspectives. J. Hematol. Oncol. 16 (1), 57. doi:10.1186/s13045-023-01451-3
Guilliams, M., Mildner, A., and Yona, S. (2018). Developmental and functional heterogeneity of monocytes. Immunity 49 (4), 595–613. doi:10.1016/j.immuni.2018.10.005
Guo, J., Li, Z., Ma, Q., Li, M., Zhao, Y., Li, B., et al. (2022). Dextran sulfate inhibits angiogenesis and invasion of gastric cancer by interfering with M2-type macrophages polarization. Curr. Cancer Drug Targets 22 (11), 904–918. doi:10.2174/1568009622666220705095403
Guo, Y., Ke, S., Xie, F., Chen, J., Liu, X., Wang, Z., et al. (2023). SIGLEC10(+) macrophages drive gastric cancer progression by suppressing CD8(+) T cell function. Cancer Immunol. Immunother. 72 (10), 3229–3242. doi:10.1007/s00262-023-03488-2
Gupta, G. P., Nguyen, D. X., Chiang, A. C., Bos, P. D., Kim, J. Y., Nadal, C., et al. (2007). Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature 446 (7137), 765–770. doi:10.1038/nature05760
Han, Y. M., Park, J. M., Park, S. H., Hahm, K. B., Hong, S. P., and Kim, E. H. (2013). Gastrin promotes intestinal polyposis through cholecystokinin-B receptor-mediated proliferative signaling and fostering tumor microenvironment. J. Physiol. Pharmacol. 64 (4), 429–437.
Hayashi, N., Kataoka, H., Yano, S., Tanaka, M., Moriwaki, K., Akashi, H., et al. (2015). A novel photodynamic therapy targeting cancer cells and tumor-associated macrophages. Mol. Cancer Ther. 14 (2), 452–460. doi:10.1158/1535-7163.Mct-14-0348
He, F., Chen, Y., He, D., and He, S. (2023). USP14-mediated deubiquitination of SIRT1 in macrophage promotes fatty acid oxidation amplification and M2 phenotype polarization. Biochem. Biophysical Res. Commun. 646:19–29. doi:10.1016/j.bbrc.2022.12.076
He, Z., Chen, D., Wu, J., Sui, C., Deng, X., Zhang, P., et al. (2021). Yes associated protein 1 promotes resistance to 5-fluorouracil in gastric cancer by regulating GLUT3-dependent glycometabolism reprogramming of tumor-associated macrophages. Arch. Biochem. Biophys. 702, 108838. doi:10.1016/j.abb.2021.108838
Hong, Z., Wen, P., Wang, K., Wei, X., Xie, W., Rao, S., et al. (2024). The macrophage-associated prognostic gene ANXA5 promotes immunotherapy resistance in gastric cancer through angiogenesis. BMC Cancer 24 (1), 141. doi:10.1186/s12885-024-11878-7
Hu, X., Ma, Z., Xu, B., Li, S., Yao, Z., Liang, B., et al. (2023). Glutamine metabolic microenvironment drives M2 macrophage polarization to mediate trastuzumab resistance in HER2-positive gastric cancer. Cancer Commun. (Lond) 43 (8), 909–937. doi:10.1002/cac2.12459
Huang, P., Wen, F., and Li, Q. (2024b). Current concepts of the crosstalk between lncRNA and E2F1: shedding light on the cancer therapy. Front. Pharmacol. 15, 1432490. doi:10.3389/fphar.2024.1432490
Huang, P., Wen, F., Tuerhong, N., Yang, Y., and Li, Q. (2024a). Neoantigens in cancer immunotherapy: focusing on alternative splicing. Front. Immunol. 15, 1437774. doi:10.3389/fimmu.2024.1437774
Hwang, M. A., Won, M., Im, J. Y., Kang, M. J., Kweon, D. H., and Kim, B. K. (2022). TNF-Α secreted from macrophages increases the expression of prometastatic integrin αV in gastric cancer. Int. J. Mol. Sci. 24 (1), 376. doi:10.3390/ijms24010376
Ishimoto, T., Sugihara, H., Watanabe, M., Sawayama, H., Iwatsuki, M., Baba, Y., et al. (2014). Macrophage-derived reactive oxygen species suppress miR-328 targeting CD44 in cancer cells and promote redox adaptation. Carcinogenesis 35 (5), 1003–1011. doi:10.1093/carcin/bgt402
Ito, A., Kagawa, S., Sakamoto, S., Kuwada, K., Kajioka, H., Yoshimoto, M., et al. (2021). Extracellular vesicles shed from gastric cancer mediate protumor macrophage differentiation. BMC Cancer 21 (1), 102. doi:10.1186/s12885-021-07816-6
Ji, Y., Xiao, C., Fan, T., Deng, Z., Wang, D., Cai, W., et al. (2025). The epigenetic hallmarks of immune cells in cancer. Mol. Cancer 24 (1), 66. doi:10.1186/s12943-025-02255-4
Jia, X., Yan, B., Tian, X., Liu, Q., Jin, J., Shi, J., et al. (2021). CD47/SIRPα pathway mediates cancer immune escape and immunotherapy. Int. J. Biol. Sci. 17 (13), 3281–3287. doi:10.7150/ijbs.60782
Jie, L., Hengyue, W., and Ting, H. (2024). Calcitriol suppresses gastric cancer progression and cisplatin resistance by inhibiting glycolysis and M2 macrophage polarization through inhibition of mTOR activation. Environ. Toxicol. 39 (2), 830–839. doi:10.1002/tox.23975
Kim, Y. J., Won, C. H., Lee, M. W., Choi, J. H., Chang, S. E., and Lee, W. J. (2020). Correlation between tumor-associated macrophage and immune checkpoint molecule expression and its prognostic significance in cutaneous melanoma. J. Clin. Med. 9 (8), 2500. doi:10.3390/jcm9082500
Klichinsky, M., Ruella, M., Shestova, O., Lu, X. M., Best, A., Zeeman, M., et al. (2020). Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 38 (8), 947–953. doi:10.1038/s41587-020-0462-y
Kono, Y., Saito, H., Miyauchi, W., Shimizu, S., Murakami, Y., Shishido, Y., et al. (2020). Increased PD-1-positive macrophages in the tissue of gastric cancer are closely associated with poor prognosis in gastric cancer patients. BMC Cancer 20 (1), 175. doi:10.1186/s12885-020-6629-6
Lee, H. W., Choi, H. J., Ha, S. J., Lee, K. T., and Kwon, Y. G. (2013). Recruitment of monocytes/macrophages in different tumor microenvironments. Biochim. Biophys. Acta 1835 (2), 170–179. doi:10.1016/j.bbcan.2012.12.007
Li, D., Xia, L., Huang, P., Wang, Z., Guo, Q., Huang, C., et al. (2023b). Cancer-associated fibroblast-secreted IGFBP7 promotes gastric cancer by enhancing tumor associated macrophage infiltration via FGF2/FGFR1/PI3K/AKT axis. Cell Death Discov. 9 (1), 17. doi:10.1038/s41420-023-01336-x
Li, G., Liu, X., Gu, C., Ma, G., Li, S., Ma, Z., et al. (2024c). Mutual exclusivity and co-occurrence patterns of immune checkpoints indicate NKG2A relates to anti-PD-1 resistance in gastric cancer. J. Transl. Med. 22 (1), 718. doi:10.1186/s12967-024-05503-1
Li, J., Chen, P., and Ma, W. (2024d). The next frontier in immunotherapy: potential and challenges of CAR-macrophages. Exp. Hematol. Oncol. 13 (1), 76. doi:10.1186/s40164-024-00549-9
Li, M., Yang, Y., Xiong, L., Jiang, P., Wang, J., and Li, C. (2023a). Metabolism, metabolites, and macrophages in cancer. J. Hematol. Oncol. 16 (1), 80. doi:10.1186/s13045-023-01478-6
Li, S., Guo, D., Sun, Q., Zhang, L., Cui, Y., Liu, M., et al. (2023c). MAPK4 silencing in gastric cancer drives liver metastasis by positive feedback between cancer cells and macrophages. Exp. Mol. Med. 55 (2), 457–469. doi:10.1038/s12276-023-00946-w
Li, T., Li, B., Sara, A., Ay, C., Leung, W. Y., Zhang, Y., et al. (2019). Docking protein-1 promotes inflammatory macrophage signaling in gastric cancer. Oncoimmunology 8 (11), e1649961. doi:10.1080/2162402x.2019.1649961
Li, W., Zhao, S. L., Zheng, P., Shi, P. Q., Zhou, Y., Zhang, T., et al. (2022). Gastric cancer-derived mesenchymal stem cells regulate the M2 polarization of macrophages within gastric cancer microenvironment via JAK2/STAT3 signaling pathway. Zhonghua Zhong Liu Za Zhi 44 (7), 728–736. doi:10.3760/cma.j.cn112152-20200106-00008
Li, Y., Jiang, L., Chen, Y., Li, Y., Yuan, J., Lu, J., et al. (2024b). Specific lineage transition of tumor-associated macrophages elicits immune evasion of ascitic tumor cells in gastric cancer with peritoneal metastasis. Gastric Cancer 27 (3), 519–538. doi:10.1007/s10120-024-01486-6
Li, Z., Luo, J., Zhao, K., Xu, J., and Xia, L. (2024a). M2 tumor-associated macrophage promoted DNA methylation in lung cancer metastasis via intensifying EZH2. Anticancer Drugs 35 (1), 22–35. doi:10.1097/cad.0000000000001538
Li, Z., Suo, B., Long, G., Gao, Y., Song, J., Zhang, M., et al. (2020). Exosomal miRNA-16-5p derived from M1 macrophages enhances T cell-dependent immune response by regulating PD-L1 in gastric cancer. Front. Cell Dev. Biol. 8, 572689. doi:10.3389/fcell.2020.572689
Li, Z., Wang, J., Wang, Z., and Xu, Y. (2023d). Towards an optimal model for gastric cancer peritoneal metastasis: current challenges and future directions. EBioMedicine 92, 104601. doi:10.1016/j.ebiom.2023.104601
Lin, C., He, H., Liu, H., Li, R., Chen, Y., Qi, Y., et al. (2019). Tumour-associated macrophages-derived CXCL8 determines immune evasion through autonomous PD-L1 expression in gastric cancer. Gut 68 (10), 1764–1773. doi:10.1136/gutjnl-2018-316324
Liu, J., Yuan, Q., Guo, H., Guan, H., Hong, Z., and Shang, D. (2024a). Deciphering drug resistance in gastric cancer: potential mechanisms and future perspectives. Biomed. Pharmacother. 173, 116310. doi:10.1016/j.biopha.2024.116310
Liu, K., Yuan, S., Wang, C., and Zhu, H. (2023). Resistance to immune checkpoint inhibitors in gastric cancer. Front. Pharmacol. 14, 1285343. doi:10.3389/fphar.2023.1285343
Liu, M., Liu, L., Song, Y., Li, W., and Xu, L. (2022). Targeting macrophages: a novel treatment strategy in solid tumors. J. Transl. Med. 20 (1), 586. doi:10.1186/s12967-022-03813-w
Liu, W., Ou, C., Xue, R., Yang, X., Ye, Y., Wang, X., et al. (2024b). Opioid-free anesthesia attenuates perioperative immunosuppression by regulating macrophages polarization in gastric cancer patients treated with neoadjuvant PD-1 inhibitor. Front. Immunol. 15, 1438859. doi:10.3389/fimmu.2024.1438859
Liu, X., Yang, F., Ren, P., Lv, W., Chen, B., Niu, B., et al. (2024c). Study on the mechanism of macrophages activated by phosphoesterified rehmanniae polysaccharide on human gastric cancer cells. Int. J. Biol. Macromol. 277 (Pt 1), 133952. doi:10.1016/j.ijbiomac.2024.133952
Liu, Y., and Cao, X. (2016). Characteristics and significance of the pre-metastatic niche. Cancer Cell 30 (5), 668–681. doi:10.1016/j.ccell.2016.09.011
Liu, Y., Xiao, L., Yang, M., Chen, X., Liu, H., Wang, Q., et al. (2024d). CAR-armored-cell therapy in solid tumor treatment. J. Transl. Med. 22 (1), 1076. doi:10.1186/s12967-024-05903-3
Locati, M., Curtale, G., and Mantovani, A. (2020). Diversity, mechanisms, and significance of macrophage plasticity. Annu. Rev. Pathol. 15, 123–147. doi:10.1146/annurev-pathmechdis-012418-012718
Lordick, F., Lorenzen, S., Yamada, Y., and Ilson, D. (2014). Optimal chemotherapy for advanced gastric cancer: is there a global consensus? Gastric Cancer 17 (2), 213–225. doi:10.1007/s10120-013-0297-z
Luo, Q., Zheng, N., Jiang, L., Wang, T., Zhang, P., Liu, Y., et al. (2020). Lipid accumulation in macrophages confers protumorigenic polarization and immunity in gastric cancer. Cancer Sci. 111 (11), 4000–4011. doi:10.1111/cas.14616
Ma, R. Y., Black, A., and Qian, B. Z. (2022). Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. 43 (7), 546–563. doi:10.1016/j.it.2022.04.008
Maalej, K. M., Merhi, M., Inchakalody, V. P., Mestiri, S., Alam, M., Maccalli, C., et al. (2023). CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances. Mol. Cancer 22 (1), 20. doi:10.1186/s12943-023-01723-z
Malla, R. R., Nellipudi, H. R., Srilatha, M., and Nagaraju, G. P. (2024). HER-2 positive gastric cancer: current targeted treatments. Int. J. Biol. Macromol. 274 (Pt 1), 133247. doi:10.1016/j.ijbiomac.2024.133247
Mantovani, A., Allavena, P., Marchesi, F., and Garlanda, C. (2022). Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 21 (11), 799–820. doi:10.1038/s41573-022-00520-5
Mantovani, A., Marchesi, F., Malesci, A., Laghi, L., and Allavena, P. (2017). Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 14 (7), 399–416. doi:10.1038/nrclinonc.2016.217
Mantovani, A., Sica, A., Sozzani, S., Allavena, P., Vecchi, A., and Locati, M. (2004). The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25 (12), 677–686. doi:10.1016/j.it.2004.09.015
Martin, P., and Gurevich, D. B. (2021). Macrophage regulation of angiogenesis in health and disease. Semin. Cell Dev. Biol. 119, 101–110. doi:10.1016/j.semcdb.2021.06.010
Miao, L., Qi, J., Zhao, Q., Wu, Q. N., Wei, D. L., Wei, X. L., et al. (2020). Targeting the STING pathway in tumor-associated macrophages regulates innate immune sensing of gastric cancer cells. Theranostics 10 (2), 498–515. doi:10.7150/thno.37745
Mou, P., Ge, Q. H., Sheng, R., Zhu, T. F., Liu, Y., and Ding, K. (2023). Research progress on the immune microenvironment and immunotherapy in gastric cancer. Front. Immunol. 14, 1291117. doi:10.3389/fimmu.2023.1291117
Mu, G., Zhu, Y., Dong, Z., Shi, L., Deng, Y., and Li, H. (2021). Calmodulin 2 facilitates angiogenesis and metastasis of gastric cancer via STAT3/HIF-1A/VEGF-A mediated macrophage polarization. Front. Oncol. 11, 727306. doi:10.3389/fonc.2021.727306
Nasir, I., McGuinness, C., Poh, A. R., Ernst, M., Darcy, P. K., and Britt, K. L. (2023). Tumor macrophage functional heterogeneity can inform the development of novel cancer therapies. Trends Immunol. 44 (12), 971–985. doi:10.1016/j.it.2023.10.007
Nasrollahzadeh, E., Razi, S., Keshavarz-Fathi, M., Mazzone, M., and Rezaei, N. (2020). Pro-tumorigenic functions of macrophages at the primary, invasive and metastatic tumor site. Cancer Immunol. Immunother. 69 (9), 1673–1697. doi:10.1007/s00262-020-02616-6
Ni, B., He, X., Zhang, Y., Wang, Z., Dong, Z., Xia, X., et al. (2023). Tumor-associated macrophage-derived GDNF promotes gastric cancer liver metastasis via a GFRA1-modulated autophagy flux. Cell Oncol. (Dordr) 46 (2), 315–330. doi:10.1007/s13402-022-00751-z
Nobs, S. P., and Kopf, M. (2021). Tissue-resident macrophages: guardians of organ homeostasis. Trends Immunol. 42 (6), 495–507. doi:10.1016/j.it.2021.04.007
Noy, R., and Pollard, J. W. (2014). Tumor-associated macrophages: from mechanisms to therapy. Immunity 41 (1), 49–61. doi:10.1016/j.immuni.2014.06.010
Oshima, H., Popivanova, B. K., Oguma, K., Kong, D., Ishikawa, T. O., and Oshima, M. (2011). Activation of epidermal growth factor receptor signaling by the prostaglandin E(2) receptor EP4 pathway during gastric tumorigenesis. Cancer Sci. 102 (4), 713–719. doi:10.1111/j.1349-7006.2011.01847.x
Pan, M., Wang, F., Nan, L., Yang, S., Qi, J., Xie, J., et al. (2023). αVEGFR2-MICA fusion antibodies enhance immunotherapy effect and synergize with PD-1 blockade. Cancer Immunol. Immunother. 72 (4), 969–984. doi:10.1007/s00262-022-03306-1
Pei, X., Zhang, S. L., Qiu, B. Q., Zhang, P. F., Liu, T. S., and Wang, Y. (2024). Cancer cell secreted legumain promotes gastric cancer resistance to anti-PD-1 immunotherapy by enhancing macrophage M2 polarization. Pharm. (Basel) 17 (7), 951. doi:10.3390/ph17070951
Peng, C., Wang, Y., Zhang, H., and Chen, P. (2024a). The platelet-related genes associated with the prognosis of HCC by regulating cycling T cell and prolif-TAMs. Heliyon 10 (5), e26798. doi:10.1016/j.heliyon.2024.e26798
Peng, H., Jiang, L., Yuan, J., Wu, X., Chen, N., Liu, D., et al. (2024b). Single-cell characterization of differentiation trajectories and drug resistance features in gastric cancer with peritoneal metastasis. Clin. Transl. Med. 14 (10), e70054. doi:10.1002/ctm2.70054
Peng, L. S., Zhang, J. Y., Teng, Y. S., Zhao, Y. L., Wang, T. T., Mao, F. Y., et al. (2017). Tumor-associated monocytes/macrophages impair NK-cell function via TGFβ1 in human gastric cancer. Cancer Immunol. Res. 5 (3), 248–256. doi:10.1158/2326-6066.Cir-16-0152
Piao, H., Fu, L., Wang, Y., Liu, Y., Wang, Y., Meng, X., et al. (2022). A positive feedback loop between gastric cancer cells and tumor-associated macrophage induces malignancy progression. J. Exp. Clin. Cancer Res. 41 (1), 174. doi:10.1186/s13046-022-02366-6
Pittet, M. J., Michielin, O., and Migliorini, D. (2022). Clinical relevance of tumour-associated macrophages. Nat. Rev. Clin. Oncol. 19 (6), 402–421. doi:10.1038/s41571-022-00620-6
Prima, V., Kaliberova, L. N., Kaliberov, S., Curiel, D. T., and Kusmartsev, S. (2017). COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc. Natl. Acad. Sci. U. S. A. 114 (5), 1117–1122. doi:10.1073/pnas.1612920114
Qi, H., Ma, X., Ma, Y., Jia, L., Liu, K., and Wang, H. (2024). Mechanisms of HIF1A-mediated immune evasion in gastric cancer and the impact on therapy resistance. Cell Biol. Toxicol. 40 (1), 87. doi:10.1007/s10565-024-09917-x
Qian, Y., Yin, Y., Zheng, X., Liu, Z., and Wang, X. (2024). Metabolic regulation of tumor-associated macrophage heterogeneity: insights into the tumor microenvironment and immunotherapeutic opportunities. Biomark. Res. 12 (1), 1. doi:10.1186/s40364-023-00549-7
Qiu, S., Xie, L., Lu, C., Gu, C., Xia, Y., Lv, J., et al. (2022). Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. J. Exp. Clin. Cancer Res. 41 (1), 296. doi:10.1186/s13046-022-02499-8
Qu, B., Liu, J., Peng, Z., Xiao, Z., Li, S., Wu, J., et al. (2024). Macrophages enhance cisplatin resistance in gastric cancer through the transfer of circTEX2. J. Cell Mol. Med. 28 (5), e18070. doi:10.1111/jcmm.18070
Razi, S., Yaghmoorian Khojini, J., Kargarijam, F., Panahi, S., Tahershamsi, Z., Tajbakhsh, A., et al. (2023). Macrophage efferocytosis in health and disease. Cell Biochem. Funct. 41 (2), 152–165. doi:10.1002/cbf.3780
Rőszer, T. (2015). Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediat. Inflamm. 2015, 816460. doi:10.1155/2015/816460
Sadik, A., Somarribas Patterson, L. F., Öztürk, S., Mohapatra, S. R., Panitz, V., Secker, P. F., et al. (2020). IL4I1 is a metabolic immune checkpoint that activates the AHR and promotes tumor progression. Cell 182 (5), 1252–1270.e34. doi:10.1016/j.cell.2020.07.038
Sharma, N., Fan, X., Atolagbe, O. T., Ge, Z., Dao, K. N., Sharma, P., et al. (2024). ICOS costimulation in combination with CTLA-4 blockade remodels tumor-associated macrophages toward an antitumor phenotype. J. Exp. Med. 221 (4), e20231263. doi:10.1084/jem.20231263
Shen, Y., Xue, C., Li, X., Ba, L., Gu, J., Sun, Z., et al. (2019). Effects of gastric cancer cell-derived exosomes on the immune regulation of mesenchymal stem cells by the NF-kB signaling pathway. Stem Cells Dev. 28 (7), 464–476. doi:10.1089/scd.2018.0125
Shen, Z., Kauttu, T., Cao, J., Seppänen, H., Vainionpää, S., Ye, Y., et al. (2013). Macrophage coculture enhanced invasion of gastric cancer cells via TGF-β and BMP pathways. Scand. J. Gastroenterol. 48 (4), 466–472. doi:10.3109/00365521.2013.772226
Shetab Boushehri, M. A., and Lamprecht, A. (2018). TLR4-Based immunotherapeutics in cancer: a review of the achievements and shortcomings. Mol. Pharm. 15 (11), 4777–4800. doi:10.1021/acs.molpharmaceut.8b00691
Shi, B., Du, M., and Chen, Z. (2024a). Advances in tumor immunotherapy targeting macrophages. Expert Rev. Clin. Immunol. 21, 259–276. doi:10.1080/1744666x.2024.2438721
Shi, T., Zhang, Y., Wang, Y., Song, X., Wang, H., Zhou, X., et al. (2022). DKK1 promotes tumor immune evasion and impedes anti-PD-1 treatment by inducing immunosuppressive macrophages in gastric cancer. Cancer Immunol. Res. 10 (12), 1506–1524. doi:10.1158/2326-6066.Cir-22-0218
Shi, Y., Li, X., Dong, Y., Yuan, H., Wang, Y., and Yang, R. (2024b). Exploring the potential of CAR-macrophage therapy. Life Sci. 361, 123300. doi:10.1016/j.lfs.2024.123300
Sica, A., and Mantovani, A. (2012). Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest 122 (3), 787–795. doi:10.1172/jci59643
Smith, J. P., Cao, H., Chen, W., Mahmood, K., Phillips, T., Sutton, L., et al. (2021). Gastrin vaccine alone and in combination with an immune checkpoint antibody inhibits growth and metastases of gastric cancer. Front. Oncol. 11, 788875. doi:10.3389/fonc.2021.788875
Smith, T. D., Nagalla, R. R., Chen, E. Y., and Liu, W. F. (2017). Harnessing macrophage plasticity for tissue regeneration. Adv. Drug Deliv. Rev. 114, 193–205. doi:10.1016/j.addr.2017.04.012
Su, P., Jiang, L., Zhang, Y., Yu, T., Kang, W., Liu, Y., et al. (2022). Crosstalk between tumor-associated macrophages and tumor cells promotes chemoresistance via CXCL5/PI3K/AKT/mTOR pathway in gastric cancer. Cancer Cell Int. 22 (1), 290. doi:10.1186/s12935-022-02717-5
Su, Z., He, Y., You, L., Chen, J., Zhang, G., and Liu, Z. (2024). SPP1+ macrophages and FAP+ fibroblasts promote the progression of pMMR gastric cancer. Sci. Rep. 14 (1), 26221. doi:10.1038/s41598-024-76298-w
Sun, W., Wang, X., Wang, D., Lu, L., Lin, H., Zhang, Z., et al. (2022). CD40×HER2 bispecific antibody overcomes the CCL2-induced trastuzumab resistance in HER2-positive gastric cancer. J. Immunother. Cancer 10 (7), e005063. doi:10.1136/jitc-2022-005063
Tabuchi, M., Kikuchi, S., Tazawa, H., Okura, T., Ogawa, T., Mitsui, E., et al. (2024). Functional remodeling of intraperitoneal macrophages by oncolytic adenovirus restores anti-tumor immunity for peritoneal metastasis of gastric cancer. Mol. Ther. Oncol. 32 (2), 200806. doi:10.1016/j.omton.2024.200806
Tan, H., Cai, M., Wang, J., Yu, T., Xia, H., Zhao, H., et al. (2025). Harnessing macrophages in cancer therapy: from immune modulators to therapeutic targets. Int. J. Biol. Sci. 21 (5), 2235–2257. doi:10.7150/ijbs.106275
Tang, C., Lei, X., Xiong, L., Hu, Z., and Tang, B. (2021). HMGA1B/2 transcriptionally activated-POU1F1 facilitates gastric carcinoma metastasis via CXCL12/CXCR4 axis-mediated macrophage polarization. Cell Death Dis. 12 (5), 422. doi:10.1038/s41419-021-03703-x
Wall, J. A., Klempner, S. J., and Arend, R. C. (2020). The anti-DKK1 antibody DKN-01 as an immunomodulatory combination partner for the treatment of cancer. Expert Opin. Investig. Drugs 29 (7), 639–644. doi:10.1080/13543784.2020.1769065
Wang, B., Gu, B., Gao, L., Ma, C., Li, X., Wang, Y., et al. (2025b). SERPINE1 facilitates metastasis in gastric cancer through anoikis resistance and tumor microenvironment remodeling. Small 21 (19), e2500136. doi:10.1002/smll.202500136
Wang, F., Li, B., Wei, Y., Zhao, Y., Wang, L., Zhang, P., et al. (2018). Tumor-derived exosomes induce PD1(+) macrophage population in human gastric cancer that promotes disease progression. Oncogenesis 7 (5), 41. doi:10.1038/s41389-018-0049-3
Wang, K., Cai, R., Fei, S., Chen, X., Feng, S., Zhang, L., et al. (2023). Melatonin enhances anti-tumor immunity by targeting macrophages PD-L1 via exosomes derived from gastric cancer cells. Mol. Cell Endocrinol. 568-569, 111917. doi:10.1016/j.mce.2023.111917
Wang, L., Chen, X., Zhang, L., Niu, B., Li, L., Sun, Y., et al. (2022). CAR cell design strategies in solid tumors. Int. Immunopharmacol. 113 (Pt A), 109345. doi:10.1016/j.intimp.2022.109345
Wang, Q., Chen, J., Wang, Y., Li, X., Ping, X., Shen, J., et al. (2025a). The profiles of immunosuppressive microenvironment in the Lauren intestinal-type gastric adenocarcinoma. Cancer Immunol. Immunother. 74 (3), 82. doi:10.1007/s00262-024-03938-5
Wang, Y., Jia, J., Wang, F., Fang, Y., Yang, Y., Zhou, Q., et al. (2024a). Pre-metastatic niche: formation, characteristics and therapeutic implication. Signal Transduct. Target Ther. 9 (1), 236. doi:10.1038/s41392-024-01937-7
Wang, Y., Shang, K., Zhang, N., Zhao, J., and Cao, B. (2021). Tumor-associated macrophage-derived exosomes promote the progression of gastric cancer by regulating the P38MAPK signaling pathway and the immune checkpoint PD-L1. Cancer Biother Radiopharm. doi:10.1089/cbr.2021.0218
Wang, Z., Li, M., Bi, L., Hu, X., and Wang, Y. (2024b). Traditional Chinese medicine in regulating tumor microenvironment. Onco Targets Ther. 17, 313–325. doi:10.2147/ott.S444214
Wang, Z., Yang, Y., Cui, Y., Wang, C., Lai, Z., Li, Y., et al. (2020). Tumor-associated macrophages regulate gastric cancer cell invasion and metastasis through TGFβ2/NF-κB/Kindlin-2 axis. Chin. J. Cancer Res. 32 (1), 72–88. doi:10.21147/j.issn.1000-9604.2020.01.09
Wang, Z., Yin, N., Zhang, Z., Zhang, Y., Zhang, G., and Chen, W. (2017). Upregulation of T-cell Immunoglobulin and mucin-domain containing-3 (Tim-3) in monocytes/macrophages associates with gastric cancer progression. Immunol. Invest 46 (2), 134–148. doi:10.1080/08820139.2016.1229790
Wu, J., Yuan, M., Shen, J., Chen, Y., Zhang, R., Chen, X., et al. (2022). Effect of modified Jianpi Yangzheng on regulating content of PKM2 in gastric cancer cells-derived exosomes. Phytomedicine 103, 154229. doi:10.1016/j.phymed.2022.154229
Wu, Y., Park, J., Xu, E., Kim, D., Lee, J., and Oh, Y. K. (2025). MicroRNA-induced reprogramming of tumor-associated macrophages for modulation of tumor immune microenvironment. J. Control Release 381, 113593. doi:10.1016/j.jconrel.2025.113593
Wynn, T. A., Chawla, A., and Pollard, J. W. (2013). Macrophage biology in development, homeostasis and disease. Nature 496 (7446), 445–455. doi:10.1038/nature12034
Xiao, H., Fu, J., Liu, R., Yan, L., Zhou, Z., and Yuan, J. (2024). Gastric cancer cell-derived exosomal miR-541-5p induces M2 macrophage polarization through DUSP3/JAK2/STAT3 pathway. BMC Cancer 24 (1), 957. doi:10.1186/s12885-024-12672-1
Xin, L., Liu, J., Lai, J. Y., Xu, H. S., Fan, L. J., Zou, Y. H., et al. (2025). Methionine restriction promotes the polarization of macrophages towards M1 and the immunotherapy effect of PD-L1/PD-1 blockades by inhibiting the secretion of MIF by gastric carcinoma cells. Transl. Oncol. 51, 102181. doi:10.1016/j.tranon.2024.102181
Xin, L., Zhou, L. Q., Liu, C., Zeng, F., Yuan, Y. W., Zhou, Q., et al. (2021). Transfer of LncRNA CRNDE in TAM-derived exosomes is linked with cisplatin resistance in gastric cancer. EMBO Rep. 22 (12), e52124. doi:10.15252/embr.202052124
Xu, J., Yu, B., Wang, F., and Yang, J. (2024). Single-cell RNA sequencing to map tumor heterogeneity in gastric carcinogenesis paving roads to individualized therapy. Cancer Immunol. Immunother. 73 (11), 233. doi:10.1007/s00262-024-03820-4
Xu, X., Chen, J., Li, W., Feng, C., Liu, Q., Gao, W., et al. (2023). Immunology and immunotherapy in gastric cancer. Clin. Exp. Med. 23 (7), 3189–3204. doi:10.1007/s10238-023-01104-2
Yang, R., Kwan, W., Du, Y., Yan, R., Zang, L., Li, C., et al. (2024b). Drug-induced senescence by aurora kinase inhibitors attenuates innate immune response of macrophages on gastric cancer organoids. Cancer Lett. 598, 217106. doi:10.1016/j.canlet.2024.217106
Yang, X., Cai, S., Shu, Y., Deng, X., Zhang, Y., He, N., et al. (2021). Exosomal miR-487a derived from m2 macrophage promotes the progression of gastric cancer. Cell Cycle 20 (4), 434–444. doi:10.1080/15384101.2021.1878326
Yang, X., Qi, Y., and Wang, S. (2024a). DKN-01 suppresses gastric cancer progression through activating cGAS-STING pathway to block macrophage M2 polarization. Appl. Biochem. Biotechnol. 197, 1025–1038. doi:10.1007/s12010-024-05073-4
Yao, M., Mao, X., Zhang, Z., Xi, Y., Gan, H., Cui, F., et al. (2023). Tumor-derived CircRNA_102191 promotes gastric cancer and facilitates M2 macrophage polarization. Cell Cycle 22 (18), 2003–2017. doi:10.1080/15384101.2023.2271341
Yasuda, T., and Wang, Y. A. (2024). Gastric cancer immunosuppressive microenvironment heterogeneity: implications for therapy development. Trends Cancer 10 (7), 627–642. doi:10.1016/j.trecan.2024.03.008
Yi, J., Ye, Z., Xu, H., Zhang, H., Cao, H., Li, X., et al. (2024). EGCG targeting STAT3 transcriptionally represses PLXNC1 to inhibit M2 polarization mediated by gastric cancer cell-derived exosomal miR-92b-5p. Phytomedicine 135, 156137. doi:10.1016/j.phymed.2024.156137
Yin, S. Y., Liu, Y. J., Li, J. P., and Liu, J. (2024). Overexpression of FERM domain containing kindlin 2 (FERMT2) in fibroblasts correlates with EMT and immunosuppression in gastric cancer. Int. J. Genomics 2024, 4123737. doi:10.1155/2024/4123737
You, T., Tang, H., Wu, W., Gao, J., Li, X., Li, N., et al. (2023). POSTN secretion by extracellular matrix cancer-associated fibroblasts (eCAFs) correlates with poor ICB response via macrophage chemotaxis activation of Akt signaling pathway in gastric cancer. Aging Dis. 14 (6), 2177–2192. doi:10.14336/ad.2023.0503
Yu, C., Zhou, G., Shi, Z., Yu, L., and Zhou, X. (2024). TREM1 facilitates the development of gastric cancer through regulating neutrophil extracellular traps-mediated macrophage polarization. Dig. Liver Dis. 56 (7), 1237–1247. doi:10.1016/j.dld.2023.12.002
Yu, D., Chang, Z., Liu, X., Chen, P., Zhang, H., and Qin, Y. (2023b). Macrophage-derived exosomes regulate gastric cancer cell oxaliplatin resistance by wrapping circ 0008253. Cell Cycle 22 (6), 705–717. doi:10.1080/15384101.2022.2146839
Yu, X., Qian, J., Ding, L., Pan, C., Liu, X., Wu, Q., et al. (2025). Galectin-1-Induced tumor associated macrophages repress antitumor immunity in hepatocellular carcinoma through recruitment of Tregs. Adv. Sci. (Weinh) 12, e2408788. doi:10.1002/advs.202408788
Yu, Y., Wu, Y., Zhang, Y., Lu, M., and Su, X. (2023a). Oxidative stress in the tumor microenvironment in gastric cancer and its potential role in immunotherapy. FEBS Open Bio 13 (7), 1238–1252. doi:10.1002/2211-5463.13630
Yunna, C., Mengru, H., Lei, W., and Weidong, C. (2020). Macrophage M1/M2 polarization. Eur. J. Pharmacol. 877, 173090. doi:10.1016/j.ejphar.2020.173090
Zang, W., Yang, Y., Chen, J., Mao, Q., Xue, W., and Hu, Y. (2025). The MIR181A2HG/miR-5680/VCAN-CD44 Axis regulates gastric cancer lymph node metastasis by promoting M2 macrophage polarization. Cancer Med. 14 (2), e70600. doi:10.1002/cam4.70600
Zhang, F., and Luo, H. (2023). Diosmetin inhibits the growth and invasion of gastric cancer by interfering with M2 phenotype macrophage polarization. J. Biochem. Mol. Toxicol. 37 (10), e23431. doi:10.1002/jbt.23431
Zhang, G., Gao, Z., Guo, X., Ma, R., Wang, X., Zhou, P., et al. (2023b). CAP2 promotes gastric cancer metastasis by mediating the interaction between tumor cells and tumor-associated macrophages. J. Clin. Invest 133 (21), e166224. doi:10.1172/jci166224
Zhang, H., Liu, L., Liu, J., Dang, P., Hu, S., Yuan, W., et al. (2023a). Roles of tumor-associated macrophages in anti-PD-1/PD-L1 immunotherapy for solid cancers. Mol. Cancer 22 (1), 58. doi:10.1186/s12943-023-01725-x
Zhang, J., Dong, Y., Yu, S., Hu, K., Zhang, L., Xiong, M., et al. (2024a). IL-4/IL-4R axis signaling drives resistance to immunotherapy by inducing the upregulation of Fcγ receptor IIB in M2 macrophages. Cell Death Dis. 15 (7), 500. doi:10.1038/s41419-024-06875-4
Zhang, J., Zhou, X., and Hao, H. (2022). Macrophage phenotype-switching in cancer. Eur. J. Pharmacol. 931, 175229. doi:10.1016/j.ejphar.2022.175229
Zhang, K., and Jagannath, C. (2025). Crosstalk between metabolism and epigenetics during macrophage polarization. Epigenetics Chromatin 18 (1), 16. doi:10.1186/s13072-025-00575-9
Zhang, Q., and Sioud, M. (2023). Tumor-associated macrophage subsets: shaping polarization and targeting. Int. J. Mol. Sci. 24 (8), 7493. doi:10.3390/ijms24087493
Zhang, S., Ren, D., Hou, H., Yao, L., and Yuan, H. (2023c). M-CSF secreted by gastric cancer cells exacerbates the progression of gastric cancer by increasing the expression of SHP2 in tumor-associated macrophages. Aging (Albany NY) 15 (24), 15525–15534. doi:10.18632/aging.205390
Zhang, W., Liu, L., Su, H., Liu, Q., Shen, J., Dai, H., et al. (2019). Chimeric antigen receptor macrophage therapy for breast tumours mediated by targeting the tumour extracellular matrix. Br. J. Cancer 121 (10), 837–845. doi:10.1038/s41416-019-0578-3
Zhang, Y., Chen, L., Fei, Y., Chen, P., and Pan, L. (2024b). Qingrexiaoji Recipe regulates the differentiation of M2 TAM via miR-29 in GC. Comb. Chem. High. Throughput Screen 27 (18), 2764–2775. doi:10.2174/0113862073263776231009115524
Zhang, Y. Y., Li, J., Li, F., Xue, S., Xu, Q. Y., Zhang, Y. Q., et al. (2023d). Palmitic acid combined with γ-interferon inhibits gastric cancer progression by modulating tumor-associated macrophages' polarization via the TLR4 pathway. J. Cancer Res. Clin. Oncol. 149 (10), 7053–7067. doi:10.1007/s00432-023-04655-9
Zhao, Y., Lu, X., Huang, H., Yao, Y., Liu, H., and Sun, Y. (2023). Dendrobium officinale polysaccharide converts M2 into M1 subtype macrophage polarization via the STAT6/PPAR-r and JAGGED1/NOTCH1 signaling pathways to inhibit gastric cancer. Molecules 28 (20), 7062. doi:10.3390/molecules28207062
Zheng, H., Yang, X., Huang, N., Yuan, S., Li, J., Liu, X., et al. (2024). Chimeric antigen receptor macrophages targeting c-MET(CAR-M-c-MET) inhibit pancreatic cancer progression and improve cytotoxic chemotherapeutic efficacy. Mol. Cancer 23 (1), 270. doi:10.1186/s12943-024-02184-8
Zheng, P., Chen, L., Yuan, X., Luo, Q., Liu, Y., Xie, G., et al. (2017). Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J. Exp. Clin. Cancer Res. 36 (1), 53. doi:10.1186/s13046-017-0528-y
Zheng, P., Luo, Q., Wang, W., Li, J., Wang, T., Wang, P., et al. (2018). Tumor-associated macrophages-derived exosomes promote the migration of gastric cancer cells by transfer of functional Apolipoprotein E. Cell Death Dis. 9 (4), 434. doi:10.1038/s41419-018-0465-5
Zhu, Y., Fan, W., Wang, Y., Ding, H., Yang, S., and He, F. (2022). Flavokawain B weakens gastric cancer progression via the TGF-β1/SMAD4 pathway and attenuates M2 macrophage polarization. J. Immunol. Res. 2022, 4903333. doi:10.1155/2022/4903333
Zhuang, H., Dai, X., Zhang, X., Mao, Z., and Huang, H. (2020). Sophoridine suppresses macrophage-mediated immunosuppression through TLR4/IRF3 pathway and subsequently upregulates CD8(+) T cytotoxic function against gastric cancer. Biomed. Pharmacother. 121, 109636. doi:10.1016/j.biopha.2019.109636
Keywords: gastric cancer, tumor-associated macrophage, tumor microenvironment, tumor progression, immunotherapy
Citation: Wan C, Deng J, Zhu Y, Wan L, Xu L, Chen Q, Zou C and Huang J (2025) Targeting tumor-associated macrophages in gastric cancer progression and therapy: insights from molecular mechanisms to therapeutic applications. Front. Pharmacol. 16:1549694. doi: 10.3389/fphar.2025.1549694
Received: 21 December 2024; Accepted: 29 May 2025;
Published: 17 June 2025.
Edited by:
Runbin Sun, Nanjing Drum Tower Hospital, ChinaReviewed by:
Xiaosong Xiang, Nanjing University, ChinaHolya A. Lafta, Al-Karkh University of Science, Iraq
Jianfeng Yi, Gansu University of Chinese Medicine, China
Copyright © 2025 Wan, Deng, Zhu, Wan, Xu, Chen, Zou and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: ChengTao Wan, NTIyNDc2MzY3QHFxLmNvbQ==
†These authors have contributed equally to this work
 ChengTao Wan1*†
ChengTao Wan1*† Yu Zhu
Yu Zhu Ju Huang
Ju Huang