- 1Department of Biomedical Sciences for Health, University of Milan, Milan, Italy
- 2UO di Medicina, Centro Angioedema, I.R.C.C.S. Policlinico San Donato, Milano, Italy
- 3Unit of Clinical Pathology, Luigi Sacco University Hospital, Milan, Italy
- 4Department of Biomedical and Clinical Sciences, Luigi Sacco Hospital, University of Milan, Milan, Italy
- 5Department of Systems Medicine, University Hospital of Padua, Padua, Italy
- 6UOC di Patologia Clinica e Immunologia, Azienda Ospedaliera Ospedali Riuniti Villa Sofia-Cervello, Palermo, Italy
- 7Allergology Unit, IRCCS San Martino Polyclinic Hospital, Genoa, Italy
- 8SSD Dermatologia e Allergologia, Ospedale Beauregard, Aosta, Italy
- 9Allergy and Clinical Immunology Unit, Azienda Sanitaria Locale Di Pescara, Pescara, Italy
- 10Department of Allergology, University Hospital “Maggiore della Carità” of Novara, Novara, Italy
- 11Department of Medical Sciences and Public Health, University of Cagliari, Cagliari, Italy
- 12Department of Internal Medicine, Istituti Clinici Scientifici Maugeri IRCCS, Milan, Italy
- 13Division of Allergy and Clinical Immunology, University of Salerno, Salerno, Italy
- 14Department of Translational Medical Sciences, University of Naples Federico II, Naples, Italy
- 15Internal Medicine Department, Fatebenefratelli and Sacco Hospitals, Milan, Italy
- 16Allergy Unit, Hospital of Civitanova Marche, Civitanova Marche, Italy
- 17Department of Clinical and Experimental Medicine, School and Operative Unit of Allergy and Clinical Immunology, University of Messina, Messina, Italy
- 18Allergy and Clinical Immunology Unit, Department of Medical Sciences, University of Torino and Mauriziano Hospital, Torino, Italy
- 19Immunoallergology Unit, University Hospital of Careggi, Florence, Italy
- 20Rheumatology, Allergology and Clinical Immunology, Department of Systems Medicine, University of Roma Tor Vergata, Rome, Italy
- 21Department of Clinical Immunology, Azienda Ospedaliera Universitaria Ospedali Riuniti di Ancona, Ancona, Italy
- 22Internal Medicine Unit, Azienda Ospedaliero-Universitaria Policlinico “G.Rodolico-San Marco”, Catania, Italy
- 23U.O.C. di Nefrologia e Dialisi, Ospedale Generale Regionale “F. Miulli”, Acquaviva delle Fonti (BA), Italy
Background: Danazol is regularly used as a prophylactic treatment in patients with Hereditary angioedema due to C1-inhibitor deficiency (HAE-C1INH). However, this drug is characterized by a risk of drug-drug interactions (DDIs). Berotralstat, the first oral kallikrein inhibitor, has been recently approved for the prevention of HAE attacks. Here, we sought to compare the risk of potential DDIs in real-life HAE patients hypothetically given Danazol or Berotralstat.
Methods: Our clinic’s database was retrospectively reviewed to identify patients diagnosed with HAE who were treated with at least one concomitant medication. The DDIs were assessed using three freely available drug interaction checkers and scored based on their severity. The agreement between the three drug checkers was evaluated using weighted Cohen’s kappa coefficient.
Results: 75 HAE patients (64% female, mean age 56 ± 21 years) were considered. They were mainly treated with antihypertensives (37%), hypoglycemic (19%), and hypolipemic agents (17%). Significant discrepancies among the three-drug interaction checkers were found. The first checker identified 18 potential DDIs, all involving Danazol and a statin (simvastatin). The second checker identified, respectively, 66 and 14 DDIs for Danazol (20% severe, regarding Simvastatin and Rivaroxaban) and Berotralstat (0% severe). The third checker identified 49 and 43 DDIs for Danazol (22% severe, regarding Simvastatin) and Berotralstat (0%).
Conclusion: Berotralstat was consistently associated with a reduced risk of DDIs compared with Danazol. A rational assessment of DDIs would help select the best prophylactic treatment for HAE.
Introduction
Hereditary angioedema (HAE) due to C1-inhibitor (C1INH) deficiency (HAE-C1INH) is an autosomal dominant disease caused by a deficient or dysfunctional C1-inhibitor. This naturally occurring molecule inhibits kallikrein, the protease which liberates bradykinin (BK) from plasma kininogen (Miyata and Horiuchi, 2023). The disease is characterized by painful, recurrent, unpredictable, and debilitating episodes of submucosal and/or subcutaneous tissue swelling, which may be life-threatening if the larynx is involved. HAE treatment options include on-demand therapy for acute attacks, short-term prophylaxis, and long-term prophylaxis (LTP), which should be considered on every visit to achieve complete control of the disease (Maurer et al., 2022).
Danazol and Stanazolol are synthetic attenuated androgens (AA) that effectively prevent HAE attacks. These medications have been used for decades before specific treatments became available (Maurer et al., 2022) and are still used in some countries (Guryanova et al., 2021). However, their side-effect profile can be problematic because it can predispose to cardiovascular and metabolic complaints (Zanichelli et al., 2024; Johnston et al., 2021). For this reason, the latest international WAO/EAACI guidelines suggest their use only as a second choice and propose the switch to a new LTP (Maurer et al., 2022).
The plasma kallikrein inhibitor Berotralstat, which prevents tissue edema elicited by BK during acute episodes of HAE-C1INH, has been approved for prophylaxis to prevent attacks of HAE-C1INH in adults and adolescent patients aged 12 years or older. This drug, easily administered orally like AA, might theoretically represent the most practical alternative to androgens for LTP. Phase III trials and real-world data showed that Berotralstat reduced the number of acute attacks and improved the quality of life in HAE patients (Zuraw et al., 2021; Kiani-Alikhan et al., 2024).
Thanks to recent advances in HAE-C1INH diagnosis and therapy, the life expectancy of those patients is similar to that of the general population (Perego et al., 2020). For this reason, the incidence of comorbidities and the risk of drug-drug interactions (DDIs) due to polypharmacy, which increase with age, can become significant issues even in patients with HAE-C1INH. (Perrella et al., 2024; Goetschi et al., 2024; Chuang et al., 2023; McDonald et al., 2024; Zidan and Awaisu, 2024; Randles et al., 2022).
In this context, both Danazol and Berotralstat may predispose to DDIs. The risk of drug-related adverse events and DDIs with co-medications - including statins, antiepileptics, immunosuppressive agents, and anticoagulants - is well known for Danazol (Stankovic et al., 2010; Andreou and Ledger, 2003; Small et al., 1982; Goulbourne and Macleod, 1981; Krämer et al., 1986; Ross et al., 1986; Zielinski et al., 1987; Watson et al., 1993; Shapiro et al., 1993; Blatt et al., 1996). Even if Berotralstat seems to have a lower propensity to cause DDIs, a recent paper suggested potential interactions with immunosuppressive drugs (Tacrolimus and Prednisone) (Adatia and Magerl, 2024; Gidaro et al., 2024). However, no studies have formally investigated the risk of potential DDIs in HAE-C1INH patients on Berotralstat so far.
To address this issue, we analyzed the potential DDIs between Danazol and associated therapies in a cohort of patients from the Italian network for Hereditary and Acquired angioedema (ITACA). After that, we simulated the potential interactions of Berotralstat in the same patients.
Materials and methods
Patient selection and study design
This retrospective cohort study involved adult patients diagnosed with HAE-C1INH referred to ITACA’s angioedema centers from 1979 to 2019, 1 year before Berotralstat became available.
All data used in the study were anonymized following the requirements of the Italian Data Protection Code (leg. decree 196/2003) and the general authorizations issued by the Data Protection Authority.
Enrollment in the ITACA Registry was approved by the ethics committee of the coordinating center (Comitato etico Milano area 1, Italy) on 5 May 2017. According to the Ethics Committee, all patients signed written informed consent.
In the analyses, we considered only co-medications given chronically, excluding drugs given on demand. We also collected the patients’ main demographic characteristics. Subsequently, we assessed the potential DDIs, simulating a hypothetical scenario in which all enrolled patients were treated with Danazol (the most frequent drug used for the prophylaxis of HAE-C1INH before the developing of new therapy) or Berotralstat (a novel prophylactic treatment for HAE).
The risk of DDIs between Danazol or Berotralstat and the co-medications was assessed using INTERcheck WEB (https://intercheckweb.marionegri.it, last access 24 September 2024), Medscape Drug Interaction checker (https://reference.medscape.com/drug-interactionchecker, last access 24 September 2024) and UpToDate (https://www.uptodate.com/drug-interactions, last access 24 September 2024). These checkers were selected because they are freely available.
Based on their severity and clinical relevance, the potential DDIs were classified as red flag (drug combinations that should be avoided), orange flag (drug combinations that may require close monitoring and/or drug dose adjustments due to potentially severe clinical consequences), or yellow flag (drug combinations with minor clinical relevance).
Statistical analyses
The Kolmogorov-Smirnov test was done to evaluate the data distribution’s normality. Continuous variables were expressed as mean, standard deviation, median, and range. Categorical variables were expressed as absolute numbers or percentages.
The agreement between the three drug checkers was evaluated using weighted Cohen’s kappa coefficient.
Results
Patient characteristics
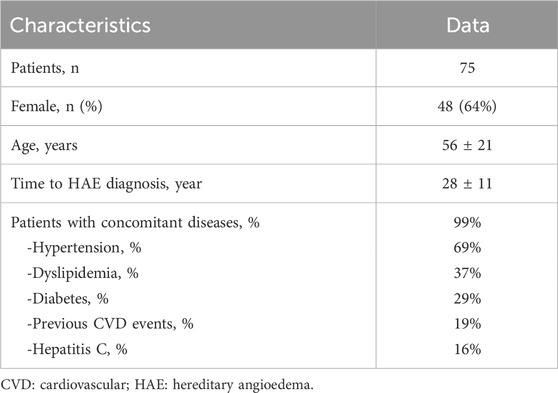
A total of 446 patients from the ITACA dataset with a HAE-C1INH diagnosis were considered. Among the 221 patients who had used AA long-term prophylaxis from 1979 to 2019, 75 had concomitant medications, fulfilled inclusion criteria, and were included in the present study. The main demographic and clinical features are shown in Table 1. The majority were female (64%), with a mean age of 56 ± 21 years, and 36% were over 65. The mean time from onset of symptoms to HAE-C1INH diagnosis was 28 ± 11 years.
60/75 HAE-C1INH patients had been treated only with Danazol as long-term prophylaxis, and this number reflects the fact that, for most of the period under consideration, specific prophylactic medications such as i.v. or s.c. Plasma-derived C1INH, Lanadelumab, and Berotralstat were not yet available. Stanozolol or Tranexamic acid had been previously prescribed to 11 and 4 patients, respectively. Patients receiving tranexamic acid were children under 12 years old who were not taking any other concomitant medications. The most frequent comorbidities were hypertension (diagnosed in 69% of HAE-C1INH patients), dyslipidemia (37%), and diabetes (29%).
Co-medications
The 75 patients were chronically treated with 161 co-medications in addition to LTP for HAE-C1INH, with a mean of 2,1±1,4 drugs per patient. The most frequent drug classes were anti-hypertensives (37%, mainly amlodipine and doxazosin), hypoglycemic agents (19%, primarily metformin), hypolipemic agents (17%, primarily simvastatin and atorvastatin), diuretics (15%, mainly hydrochlorothiazide) and antithrombotics (6%, primarily clopidogrel).
Assessment of the potential drug-drug interactions
The weighted kappa value of 0.367 (95% CI: 0.254–0.481) indicates fair agreement (p < 0.001) between INTERCheck web and Medscape, and the same level of agreement is observed at 0.256 (95% CI: 0.16–0.352) between INTERCheck web and UpToDate (p < 0.001). Lastly, a moderate agreement of 0.511 (95% CI: 0.389–0.632) was found between Medscape and UpToDate (p < 0.001).
INTERCheck WEB
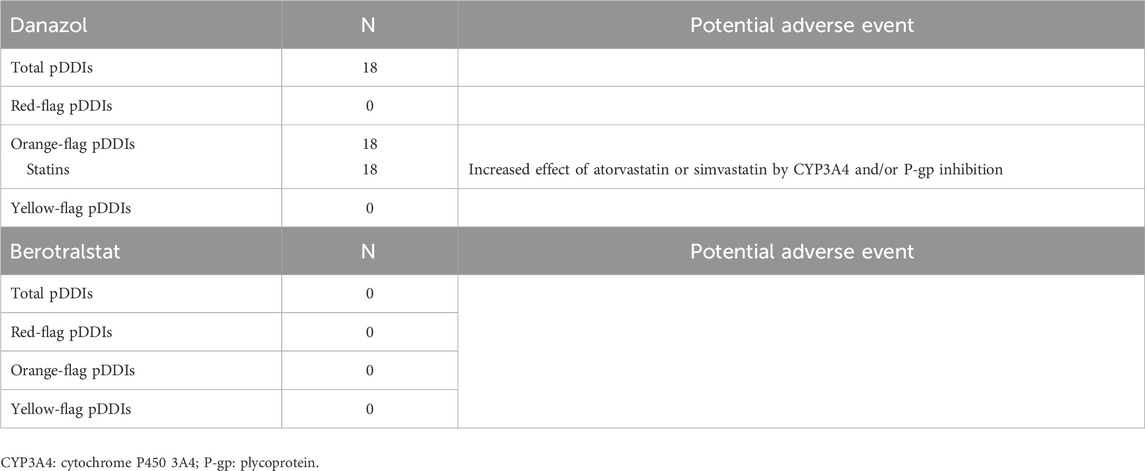
As shown in Table 2, 18 potential DDIs were identified for Danazol, all categorized as orange-flag DDIs. Among statins, Simvastatin or Atorvastatin could be involved in DDIs, while Rosuvastatin and Fluvastatin did not.
No DDIs were identified for Berotralstat.
Medscape drug interaction checker
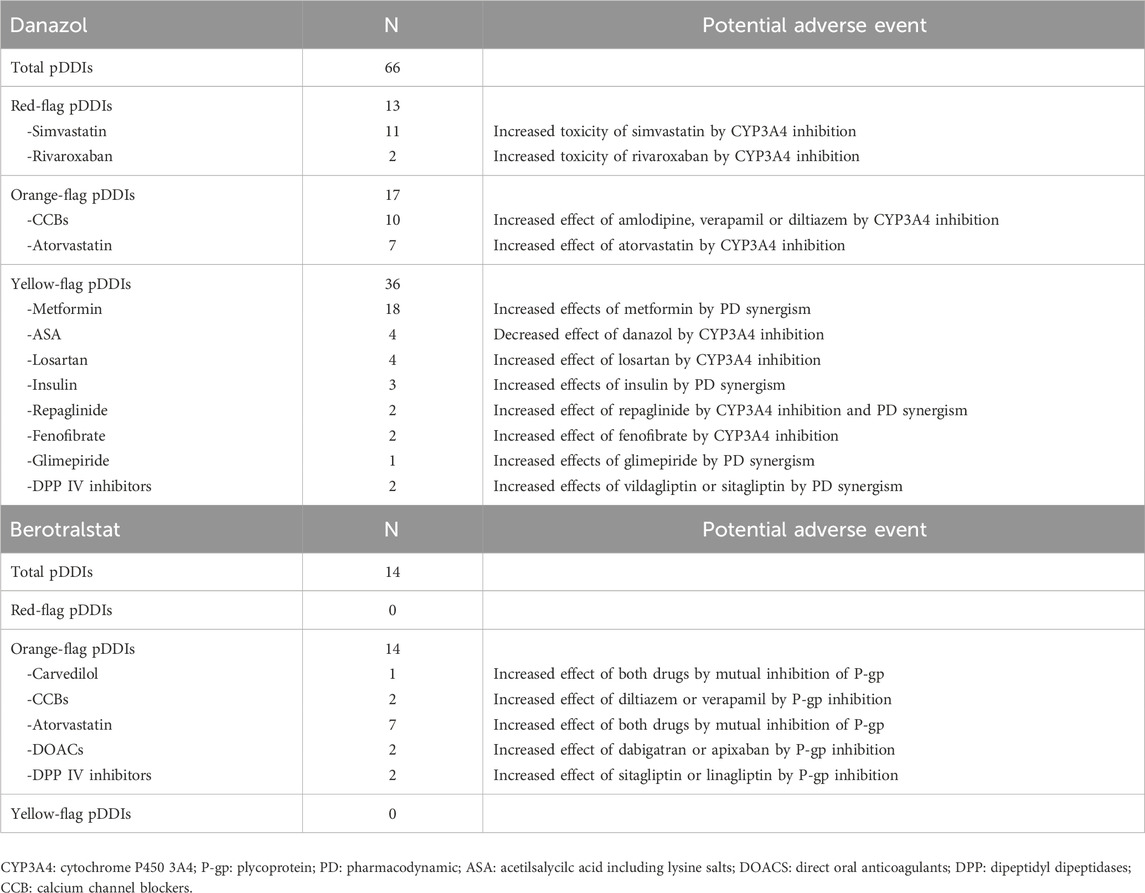
In total, 66 potential DDIs involving Danazol were identified (Table 3) and categorized, respectively, as red-flag (19.7%), orange-flag (25.8%), and yellow-flag DDIs (54.5%). The red-flag DDIs involved Simvastatin (n = 11) or Rivaroxaban (n = 2) due to Danazol’s inhibitory effect on their metabolism, potentially resulting in an increased risk of drug-related toxicity.
Only 14 potential DDIs were identified for Berotralstat, and all scored as orange-flag DDIs. The most frequent DDI concerned Atorvastatin, which acted both as victim and perpetrator because of the mutual inhibitory effect of both drugs on p-glycoprotein.
UpToDate drug interaction checker
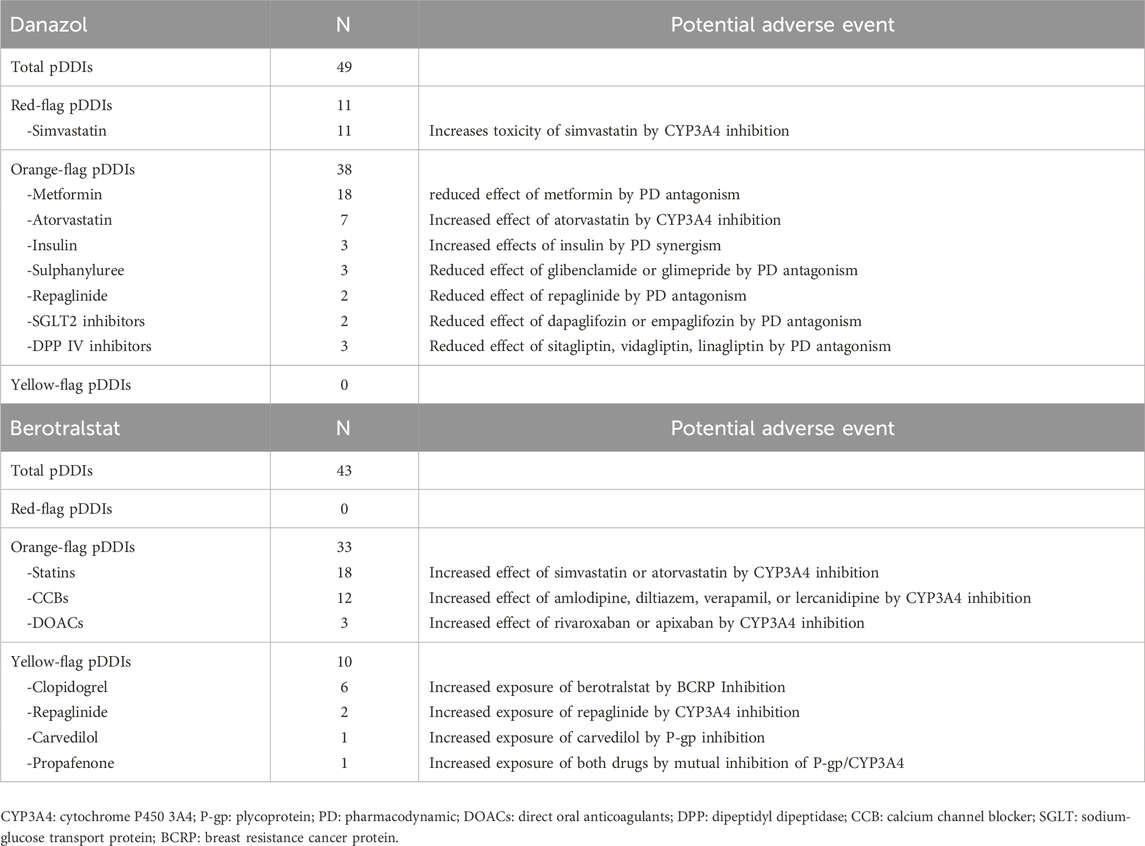
As shown in Table 4, 49 potential DDIs were identified for Danazol and categorized as red-flag (22,4%) or orange-flag DDIs (77,6%). All the red-flag DDIs involved Simvastatin as a victim of the inhibitory effect of Danazol, potentially resulting in an increased risk of drug-related toxicity. The large majority of orange-flag DDIs (81,7%) involved hypoglycemic agents, whose therapeutic effect could be diminished by concomitant Danazol administration.
Overall, 43 potential DDIs were identified for Berotralstat, categorized as orange-flag (76,7%) or yellow-flag DDIs (23,3%). Most of the orange-flag DDIs were related to the inhibitory effect of Berotralstat on CYP3A4, potentially increasing the exposure and/or effects of statins, calcium channel blockers, or direct oral anticoagulants.
Discussion
Using a real-world dataset of HAE-C1INH patients from the ITACA network, we documented in this simulation that potential DDIs with additional chronic treatments in patients on LTP are lower with Berotralstat than with Danazol. Remarkably, no red-flag DDIs have been identified for Berotralstat, whereas Danazol was associated with nearly 20% of red-flag DDIs by two out of the three drug interaction checkers used.
This is not an unexpected finding, as Danazol is a moderate/strong inhibitor of intestinal and hepatic CYP450 3A4, 3A5 (CYP3A4/5), and 2D6 (CYP2D6), the isoenzymes responsible for the metabolic clearance of the large majority of drugs available on the market (Adatia and Magerl, 2024; Lee et al., 2012). This accounts for why clinically relevant DDIs have been extensively reported involving Danazol as the perpetrator and several drugs as victims, such as statins, antiepileptics, immunosuppressive drugs, anticoagulants, etc. (Stankovic et al., 2010; Andreou and Ledger, 2003; Small et al., 1982; Goulbourne and Macleod, 1981; Krämer et al., 1986; Ross et al., 1986; Zielinski et al., 1987; Watson et al., 1993; Shapiro et al., 1993; Blatt et al., 1996). Indeed, the Danazol-related inhibition of drug metabolism increases the exposure and the activity of most of the co-medications and the risk of drug-related toxicity (or the risk of reduced drug efficacy if the co-medication is a prodrug that requires CYP3A4 to be converted in the active metabolite). Conversely, according to the available literature, Berotralstat has a lower propensity to be involved in DDIs, being only a moderate inhibitor of CYP3A4 and CYP2D6 (Adatia and Magerl, 2024; EMA, 2024). The drug is also a substrate and a weak inhibitor of P-glycoprotein and BCRP, two drug transporters regulating drug distribution in the body compartments (Adatia and Magerl, 2024). As a result, the three-drug interaction checkers consistently reported not only fewer DDIs between Berotralstat and the co-medications but, most importantly, a reduced number of DDIs scored as potentially clinically relevant (red-flag or orange-flag) compared with Danazol. In particular, the red-flag DDIs of Danazol involved the potential risk of hepatic and muscular toxicity or the risk of bleeding if co-administered, respectively, with Simvastatin or with Rivaroxaban; for both drugs, the orange-flag DDIs involved a potential risk to increase the exposure and the effects of anti-hypertensives (calcium channel blockers, beta-blockers) and other statins (Atorvastatin).
As an additional finding of the present study, we observed significant heterogeneity and inconsistencies in the number and the severity of potential DDIs involving Danazol or Berotralstat reported by the three-drug interaction checkers used in our simulation. The hypoglycemic agents give a critical example: according to INTERCheck, no DDIs are expected between Danazol and these drugs; MEDSCAPE reports that Danazol may increase the effect of Metformin, dipeptidyl peptidase IV inhibitors, or glinides, whereas for UpToDate, Danazol is a hyperglycemia-associated agent who could diminish the therapeutic effects of antidiabetic agents, glifozins included. Such inconsistencies between the drug interaction checkers, which have been extensively described in the literature (Iversen et al., 2022; Carollo et al., 2024a; Carollo et al., 2024b; Günay et al., 2022; Roca and Roca, 2022; Monteith and Glenn, 2019), may be related to the lack of standardized methods and criteria used to classify DDIs or, in the case of new drugs like Berotralstat, by the lack of data on their use in real-life settings. Taken together, these results highlight the challenges that healthcare professionals need to face in their daily clinical practice when assessing the risk of DDIs and the safety of medications. The availability of multidisciplinary teams involving clinical pharmacologists/clinical pharmacists might help to address this issue by removing inappropriate drugs and/or guiding in the interpretation of the clinical relevance of potential DDIs when data from interaction checkers are conflicting, as we previously reported in people living with HIV, and in patients with mycobacterial or fungal infections (Cattaneo et al., 2023a; Cattaneo et al., 2020; Cattaneo et al., 2024; Cattaneo et al., 2023b).
Some important information can be retrieved from our study despite the limited overlap between the three-drug interaction checkers. For instance, all checkers consistently reported DDIs between danazol simvastatin (considered a red flag DDIs by 2 out of the three checkers) and, to a lesser extent, with atorvastatin. These DDIs, which are likely to become even more clinically relevant when these statins are used at high doses (i.e., simvastatin at 80 mg and atorvastatin at 40 mg), may require a close monitoring of transaminases and creatinine phosphokinases. Other important DDIs involving danazol may be related to the opposite and poorly predictable effect of this drug on hypoglycemic agents and insulin. Indeed, danazol may reduce the effect of metformin, sulphonylurea, SGLT2, and DPP IV inhibitors by pharmacodynamic antagonism, increasing at the same time the effect of insulin by PD synergism. The take-home message is that diabetic patients undergoing LTP with danazol require strict, intensive metabolic control. The potential DDIs between danazol or berotralstat and CCBs, although scored as orange-flag DDIs, might be less clinically relevant considering that our patients are used to monitoring BP regularly. Conversely, the orange-flag DDIs involving berotralstat and DOACs may be more challenging, possibly requiring proper drug dose adjustments to avoid the risk of bleeding.
This simulation used data collected mainly from patients diagnosed with HAE-C1INH referred to the Sacco Hospital in Milan. Therefore, selection bias and/or underestimating potential DDIs cannot be ruled out. For instance, nearly 20% of HAE-C1INH patients from our cohort were treated with proton pump inhibitors (PPIs), possibly reflecting the local approach to prevent potential gastric side effects, with no detailed information in the database on the timing of administration of these drugs (i.e., chronic versus on demand) or the type of PPI. This prevents a proper assessment of the pDDIs because, even if INTERCheck WEB and UpToDate Drug Interaction Checker do not report any DDIs between PPIs and Danazol or Berotralstat, for MEDSCAPE drug interaction checker Omeprazole, Rabeprazole, or Esomeprazole co-administration might result in yellow-flag DDIs with Danazol (increased effect of the PPIs by CYP3A4 inhibition). In contrast, Pantoprazole co-administration may result in an orange-flag DDI with Berotralstat (increased effect of Berotralstat by BCRP inhibition).
In conclusion, despite the significant discrepancies among the three-drug interaction checkers, Berotralstat was consistently associated with a reduced risk of potential DDIs compared with Danazol, which has been extensively used in the past for LTP in Italy and is still extensively used in countries with limited access to innovative, pathway-specific prophylactic treatments. A rational assessment of DDIs would contribute to better selecting the best prophylactic treatment for HAE-C1INH patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Enrollment in the ITACA Registry was approved by the ethics committee of the coordinating center (Comitato etico Milano area 1, Italy) on 5 May 2017. According to the Ethics Committee, all patients signed written informed consent.
Author contributions
AZ: Conceptualization, Data curation, Writing – review and editing. DC: Conceptualization, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review and editing. AG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review and editing. RS: Data curation, Writing – review and editing. FA: Data curation, Writing – review and editing. PA: Data curation, Writing – review and editing. DB: Data curation, Writing – review and editing. PB: Data curation, Writing – review and editing. CaC: Data curation, Writing – review and editing. TD: Data curation, Writing – review and editing. DF: Data curation, Writing – original draft. FP: Data curation, Writing – review and editing. MT: Data curation, Writing – review and editing. GS: Data curation, Writing – review and editing. ChC: Data curation, Writing – review and editing. EB: Data curation, Writing – review and editing. VP: Data curation, Writing – review and editing. MG: Data curation, Writing – review and editing. PQ: Data curation, Writing – review and editing. LB: Data curation, Writing – review and editing. OR: Data curation, Writing – review and editing. PT: Data curation, Writing – review and editing. SA: Data curation, Writing – review and editing. FG: Data curation, Writing – review and editing. VM: Data curation, Writing – review and editing. MC: Data curation, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adatia, A., and Magerl, M. (2024). Berotralstat for hereditary angioedema with C1 inhibitor deficiency: a practical guide for clinicians. Front. Immunol. 15, 1442671. doi:10.3389/fimmu.2024.1442671
Andreou, E. R., and Ledger, S. (2003). Potential drug interaction between simvastatin and danazol causing rhabdomyolysis. Can. J. Clin. Pharmacol. 10 (4), 172–174.
Blatt, J., Howrie, D., Orlando, S., and Burckart, G. (1996). Interaction between cyclosporine and danazol in a pediatric patient. J. Pediatr. Hematol. Oncol. 18 (1), 95. doi:10.1097/00043426-199602000-00019
Carollo, M., Crisafulli, S., Ciccimarra, F., Andò, G., Diemberger, I., and Trifirò, G. (2024b). Exploring the level of agreement among different drug-drug interaction checkers: a comparative study on direct oral anticoagulants. Expert Opin. Drug Metab. Toxicol. 20 (3), 157–164. doi:10.1080/17425255.2024.2322134
Carollo, M., Crisafulli, S., Selleri, M., Piccoli, L., L'Abbate, L., and Trifirò, G. (2024a). Agreement of different drug-drug interaction checkers for proton pump inhibitors. JAMA Netw. Open 7 (7), e2419851. doi:10.1001/jamanetworkopen.2024.19851
Cattaneo, D., Formenti, T., Gidaro, A., Merlo, A., and Gervasoni, C. (2020). Use of direct oral anticoagulants in people living with HIV: a single-center experience. Semin. Thromb. Hemost. 46 (8), 999–1001. doi:10.1055/s-0040-1718398
Cattaneo, D., Oreni, L., Meraviglia, P., Minisci, D., Astuti, N., Antinori, S., et al. (2023a). Polypharmacy and aging in people living with HIV: 6 Years of experience in a multidisciplinary outpatient clinic. Drugs Ag-ing 40 (7), 665–674. doi:10.1007/s40266-023-01037-1
Cattaneo, D., Torre, A., Schiuma, M., Civati, A., Casalini, G., Gori, A., et al. (2024). Management of polypharmacy and potential drug-drug interactions in patients with pulmonary aspergillosis: a 2-year study of a multidisciplinary outpatient clinic. J. Fungi (Basel) 10 (2), 107. doi:10.3390/jof10020107
Cattaneo, D., Torre, A., Schiuma, M., Civati, A., Lazzarin, S., Rizzardini, G., et al. (2023b). Management of polypharmacy and potential drug-drug interactions in patients with mycobacterial infection: a 1-year experience of a multidisciplinary outpatient clinic. Antibiot. (Basel) 12 (7), 1171. doi:10.3390/antibiotics12071171
Chuang, Y. N., Chen, C. C., Wang, C. J., Chang, Y. S., and Liu, Y. H. (2023). Frailty and polypharmacy in the community-dwelling elderly with multiple chronic diseases. Psychogeriatrics 23 (2), 337–344. doi:10.1111/psyg.12936
EMA (2024). Berotralstat summary of product characteristics. Available online at: https://www.ema.europa.eu/en/documents/product-information/orladeyo-epar-product-information_en.pdf (Accessed December 15, 2024).
Gidaro, A., La Cava, L., Donadoni, M., Popescu Janu, V., Cogliati, C., Brucato, A. L., et al. (2024). Lanadelumab in a kidney transplant patient with hereditary angioedema due to C1-inhibitor deficiency and high cardiovascular risk - a case report. Front. Immunol. 15, 1472390. doi:10.3389/fimmu.2024.1472390
Goetschi, A. N., Verloo, H., Wernli, B., Wertli, M. M., and Meyer-Massetti, C. (2024). Prescribing pattern insights from a longitudinal study of older adult inpatients with polypharmacy and chronic non-cancer pain. Eur. J. Pain 28, 1645–1655. doi:10.1002/ejp.2298
Goulbourne, I. A., and Macleod, D. A. (1981). An interaction between danazol and warfarin. Case report. Br. J. Obstet. Gynaecol. 88 (9), 950–951. doi:10.1111/j.1471-0528.1981.tb02235.x
Günay, A., Demirpolat, E., Ünal, A., and Aycan, M. B. (2022). A comparison of four drug-drug interaction databases for patients undergoing haematopoietic stem cell transplantation. J. Clin. Pharm. Ther. 47 (10), 1711–1719. doi:10.1111/jcpt.13728
Guryanova, I., Suffritti, C., Parolin, D., Zanichelli, A., Ishchanka, N., Polyakova, E., et al. (2021). Hereditary angioedema due to C1 inhibitor deficiency in Belarus: epidemiology, access to diagnosis and seven novel mutations in SERPING1 gene. Clin. Mol. Allergy 19 (1), 3. doi:10.1186/s12948-021-00141-0
Iversen, D. B., Andersen, N. E., Dalgård Dunvald, A. C., Pottegård, A., and Stage, T. B. (2022). Drug metabolism and drug transport of the 100 most prescribed oral drugs. Basic Clin. Pharmacol. Toxicol. 131 (5), 311–324. doi:10.1111/bcpt.13780
Johnston, D. T., Henry, L. H., Craig, T. J., Bernstein, J. A., Anderson, J., Joseph, K., et al. (2021). Androgen use in hereditary angioedema: a critical appraisal and approaches to transitioning from androgens to other therapies. Allergy Asthma Proc. 42 (1), 22–29. doi:10.2500/aap.2021.42.200106
Kiani-Alikhan, S., Gower, R., Craig, T., Wedner, H. J., Kinaciyan, T., Aygören-Pürsün, E., et al. (2024). Once-daily oral berotralstat for long-term prophylaxis of hereditary angioedema: the open-label extension of the APeX-2 randomized trial. J. Allergy Clin. Immunol. Pract. 12 (3), 733–743.e10. doi:10.1016/j.jaip.2023.12.019
Krämer, G., Theisohn, M., von Unruh, G. E., and Eichelbaum, M. (1986). Carbamazepine-danazol drug interaction: its mechanism examined by a stable isotope technique. Ther. Drug Monit. 8 (4), 387–392. doi:10.1097/00007691-198612000-00001
Lee, C. A., Jones, J. P., Katayama, J., Kaspera, R., Jiang, Y., Freiwald, S., et al. (2012). Identifying a selective substrate and inhibitor pair for the evaluation of CYP2J2 activity. Drug Metab. Dispos. 40 (5), 943–951. doi:10.1124/dmd.111.043505
Maurer, M., Magerl, M., Betschel, S., Aberer, W., Ansotegui, I. J., Aygören-Pürsün, E., et al. (2022). The international WAO/EAACI guideline for the management of hereditary angioedema-The 2021 revision and update. Allergy 77 (7), 1961–1990. doi:10.1111/all.15214
McDonald, E. G., Lundby, C., Thompson, W., Boyd, C., Farrell, B., Gagnon, C., et al. (2024). Reducing potentially inappropriate polypharmacy at a national and international level: the impact of deprescribing networks. Expert Rev. Clin. Pharmacol. 17 (5-6), 433–440. doi:10.1080/17512433.2024.2355270
Miyata, T., and Horiuchi, T. (2023). Biochemistry, molecular genetics, and clinical aspects of hereditary angioedema with and without C1 inhibitor deficiency. Allergol. Int. 72 (3), 375–384. doi:10.1016/j.alit.2023.04.004
Monteith, S., and Glenn, T. (2019). A comparison of potential psychiatric drug interactions from six drug interaction database programs. Psychiatry Res. 275, 366–372. doi:10.1016/j.psychres.2019.03.041
Perego, F., Gidaro, A., Zanichelli, A., Cancian, M., Arcoleo, F., Senter, R., et al. (2020). Life expectancy in Italian patients with hereditary angioedema due to C1-inhibitor deficiency. J. Allergy Clin. Immunol. Pract. 8 (5), 1772–1774. doi:10.1016/j.jaip.2020.01.007
Perrella, L., Mucherino, S., Casula, M., Illario, M., Orlando, V., and Menditto, E. (2024). Polypharmacy management in chronic conditions: a systematic literature review of Italian interventions. J. Clin. Med. 13 (12), 3529. doi:10.3390/jcm13123529
Randles, M. A., O'Mahony, D., and Gallagher, P. F. (2022). Frailty and potentially inappropriate prescribing in older people with polypharmacy: a Bi-directional relationship? Drugs Aging 39 (8), 597–606. doi:10.1007/s40266-022-00952-z
Roca, B., and Roca, M. (2022). Assessment of drug interactions with online electronic checkers in multi-pathological patients. Pharmacology 107 (1-2), 111–115. doi:10.1159/000518439
Ross, W. B., Roberts, D., Griffin, P. J., and Salaman, J. R. (1986). Cyclosporin interaction with danazol and norethisterone. Lancet 1 (8476), 330. doi:10.1016/s0140-6736(86)90867-6
Shapiro, R., Venkataramanan, R., Warty, V. S., Scantlebury, V. P., Rybka, W., McCauley, J., et al. (1993). FK 506 interaction with danazol. Lancet 341 (8856), 1344–1345. doi:10.1016/0140-6736(93)90852-8
Small, M., Peterkin, M., Lowe, G. D., McCune, G., and Thomson, J. A. (1982). Danazol and oral anticoagulants. Scott Med. J. 27 (4), 331–332. doi:10.1177/003693308202700414
Stankovic, I., Vlahovic-Stipac, A., Putnikovic, B., Cvetkovic, Z., and Neskovic, A. N. (2010). Concomitant administration of simvastatin and danazol associated with fatal rhabdomyolysis. Clin. Ther. 32 (5), 909–914. doi:10.1016/j.clinthera.2010.04.017
Watson, S. A., Crosbee, D. M., Dilks, K. L., Robertson, J. F., and Hardcastle, J. D. (1993). Interactions between oestradiol and danazol on the growth of gastrointestinal tumour cells. Anticancer Res. 13 (1), 97–102.
Zanichelli, A., Senter, R., Merlo, A., Gidaro, A., Popescu Janu, V., Cogliati, C. B., et al. (2024). Comorbidities in angioedema due to C1-inhibitor deficiency: an Italian survey. J. Allergy Clin. Immunol. Pract. 12 (4), 1029–1036. doi:10.1016/j.jaip.2023.12.046
Zidan, A., and Awaisu, A. (2024). Inappropriate polypharmacy management versus deprescribing: a review on their relationship. Basic Clin. Pharmacol. Toxicol. 134 (1), 6–14. doi:10.1111/bcpt.13920
Zielinski, J. J., Lichten, E. M., and Haidukewych, D. (1987). Clinically significant danazol-carbamazepine interaction. Ther. Drug Monit. 9 (1), 24–27. doi:10.1097/00007691-198703000-00005
Zuraw, B., Lumry, W. R., Johnston, D. T., Aygören-Pürsün, E., Banerji, A., Bernstein, J. A., et al. (2021). Oral once-daily berotralstat for the prevention of hereditary angioedema attacks: a randomized, double-blind, placebo-controlled phase 3 trial. J. Allergy Clin. Immunol. 148 (1), 164–172.e9. doi:10.1016/j.jaci.2020.10.015
Keywords: hereditary angioedema, C1-inhibitor deficiency, drug-drug interactions, long-term prophylaxis, bradykinin, danazol, berotralstat, ITACA
Citation: Zanichelli A, Cattaneo D, Gidaro A, Senter R, Arcoleo F, Accardo P, Bignardi D, Borrelli P, Colangelo C, De Pasquale T, Firinu D, Perego F, Triggiani M, Spadaro G, Cogliati C, Bizzi E, Popescu Janu V, Guarino MD, Quattrocchi P, Brussino L, Rossi O, Triggianese P, Agolini S, Giardino F, Montinaro V and Cancian M (2025) Assessment of potential drug-drug interactions in patients with hereditary angioedema from the ITACA cohort: simulations from a real-life dataset considering danazol versus berotralstat. Front. Pharmacol. 16:1550133. doi: 10.3389/fphar.2025.1550133
Received: 02 January 2025; Accepted: 10 April 2025;
Published: 25 April 2025.
Edited by:
Maurizio Margaglione, University of Foggia, ItalyReviewed by:
Milos Jesenak, Comenius University, SlovakiaIsabelle Boccon-gibod, Centre Hospitalier Universitaire de Grenoble, France
Copyright © 2025 Zanichelli, Cattaneo, Gidaro, Senter, Arcoleo, Accardo, Bignardi, Borrelli, Colangelo, De Pasquale, Firinu, Perego, Triggiani, Spadaro, Cogliati, Bizzi, Popescu Janu, Guarino, Quattrocchi, Brussino, Rossi, Triggianese, Agolini, Giardino, Montinaro and Cancian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio Gidaro, Z2lkYXJvLmFudG9uaW9AYXNzdC1mYmYtc2FjY28uaXQ=
†These authors have contributed equally to this work
 Andrea Zanichelli
Andrea Zanichelli Dario Cattaneo
Dario Cattaneo Antonio Gidaro
Antonio Gidaro Riccardo Senter
Riccardo Senter Francesco Arcoleo6
Francesco Arcoleo6 Pietro Accardo
Pietro Accardo Donatella Bignardi
Donatella Bignardi Davide Firinu
Davide Firinu Francesca Perego
Francesca Perego Massimo Triggiani
Massimo Triggiani Giuseppe Spadaro
Giuseppe Spadaro Chiara Cogliati
Chiara Cogliati Emanuele Bizzi
Emanuele Bizzi Maria Domenica Guarino
Maria Domenica Guarino Luisa Brussino
Luisa Brussino Stefano Agolini
Stefano Agolini Francesco Giardino
Francesco Giardino Vincenzo Montinaro
Vincenzo Montinaro Mauro Cancian
Mauro Cancian