- 1Department of Pharmacy, Shengli Clinical Medical College of Fujian Medical University, Fuzhou University Affiliated Provincial Hospital, Fuzhou, China
- 2College of Pharmacy, Fujian Medical University, Fuzhou, China
Background: Inclisiran is an siRNA-based cholesterol-lowering drug with N-acetylgalactosamine carbohydrate (GalNAc) and is used for the treatment of hypercholesterolemia or dyslipidemia. It reduces LDL-C by 50%, with a convenient dosing schedule and fewer adverse events. Unlike statins, inclisiran has not been associated with an increased risk of muscle or liver adverse events in clinical studies. This favorable safety profile makes inclisiran a valuable alternative for patients who are intolerant of statins due to muscle or hepatic side effects. However, its impact on glycemic control and diabetes risk is unclear and understudied.
Methods and results: The US Food and Drug Administration Adverse Event Reporting System (FAERS) study analyzed hyperglycemia and diabetes risks for inclisiran, atorvastatin, and evolocumab. Data from 2021 to 2024 were assessed for Medical Dictionary (MedDRA) terms, and SAS 9.4 with a reporting advantage ratio (ROR) and Bayesian credible interval progressive neural network (BCPNN) was used for analysis. Systematic review and meta-analysis were conducted using PubMed, Embase, and specific search terms. Two research workers extracted data independently, and the study quality was assessed with the Cochrane and Newcastle–Ottawa scales. RevMan 5.4 and Stata 18.0 were used for analyses. Ethical approval was waived due to the use of public, anonymous data. From 2015 Q1 to 2024 Q1, 12,821,285 adverse events were reported in FEARS, with 3,375 inclisiran, 126,620 evolocumab, and 42,228 atorvastatin cases. Atorvastatin had a higher ROR for type 2 diabetes (195.03) than inclisiran (0.95) and evolocumab, but it was not statistically significant. Glucose intolerance and blood glucose issues showed weak signals for inclisiran and atorvastatin. A literature search yielded 16 relevant articles, including six cohort studies and 10 RCTs, totaling 297,863 patients. The incidence of new-onset diabetes was higher with atorvastatin than with inclisiran, placebo, and evolocumab. The SUCRA rankings were atorvastatin > inclisiran > placebo > evolocumab for new diabetes incidence.
Conclusion: The FAERS study and meta-analysis indicate that inclisiran may carry a lower risk of new-onset diabetes than atorvastatin, warranting further investigation into inclisiran’s impact on glycemic control.
Introduction
Inclisiran is a novel cholesterol-lowering small interfering RNA (siRNA) combined with three-stranded N-acetylgalactosamine carbohydrate (GalNAc) and is used as an adjunctive dietary therapy for primary hypercholesterolemia or mixed dyslipidemia (Lamb, 2021). Clinical trials have shown that subcutaneous inclisiran effectively reduces LDL-C levels by approximately 50%, with the advantages of a favorable administration regimen (1–90–180 days) and a lower incidence of adverse events (AEs), which provide better compliance (Sinning and Landmesser, 2020; Merćep et al., 2022).
Studies have shown that 3-hydroxymethylglutaryl-coenzyme A reductase (HMG-CoA) inhibitors (e.g., atorvastatin) adversely affect glycemic control and increase the risk of new-onset diabetes mellitus (NOD) by 9%–46% (Laakso and Fernandes Silva, 2023; Cederberg et al., 2015). Recently, a real-world pharmacovigilance study also demonstrated that PCSK9 inhibitors (e.g., evolocumab) have adverse effects on glycemic control (Goldman et al., 2022). These findings link the use of certain lipid-lowering drugs with the risk of impaired glycemic control and NOD (Kosmas et al., 2018), whereas diabetes is a well-established cardiovascular risk factor that accelerates atherosclerosis and microvascular and macrovascular lesions (Kosmas et al., 2020). The risk of hyperglycemia and new onset of diabetic AEs in patients receiving inclisiran remains unknown and has rarely been studied in pre-approved clinical trials. Unlike statins, inclisiran has not been associated with an increased risk of muscle or liver AEs in clinical studies. This favorable safety profile makes inclisiran a valuable alternative for patients who are intolerant of statins due to muscle or hepatic side effects. However, its impact on glycemic control and diabetes risk is unclear and understudied.
Here, we retrospectively analyzed the data on glycemic control of inclisiran, evolocumab, and atorvastatin in the US Food and Drug Administration Adverse Event Reporting System (FAERS) and combined a meta-analysis of randomized controlled trials (RCTs) to comprehensively evaluate the occurrence of AEs of diabetes to provide a useful reference for clinical practice.
Materials methods
Pharmacovigilance study
A pharmacovigilance study was conducted using the FAERS database to assess the risk of target drugs for hyperglycemia and NOD in a large population. All AE reports including inclisiran from 2021 Q1 to 2024 Q1 (first marketed in the EU in December 2020) and atorvastatin and evolocumab from 2015 Q1 to 2024 Q1 (evolocumab was first marketed in Japan in March 2015) were screened. Each report in the FAERS database was coded with preferred terms (PTs) including “type 2 diabetes,” “elevated blood glucose,” “impaired glucose tolerance,” and “elevated glycated hemoglobin” from the Medical Dictionary (MedDRA) version 27.0. We used SAS 9.4 for statistical analysis (SAS software is one of the statistical analysis software applications recommended by FDA FAERS database mining), using the reporting advantage ratio (ROR) and Bayesian credible interval progressive neural network (BCPNN) methods as the target ADE signal mining approaches. These are common techniques for detecting disproportionality in passive monitoring databases. A statistical association between a drug and an AE was considered significant only when both statistical measures, ROR and IC025, exceeded their respective significance thresholds (95% confidence interval greater than one and positive IC025 value). Adverse drug event associations were not considered statistically significant only when the significance threshold was exceeded (Goldman et al., 2022).
Systematic evaluation and meta-analysis
Literature search and study selection
We searched the electronic literature from when it was built to August 18, 2024; the search terms were “Inclisiran,” “Evolocumab,” “Atorvastatin,” “New onset diabetes,” “Randomized Controlled Trials as Topic,” and “Cohort study” in PubMed, Cochrane Library, Embase, Web of Science, and the US clinical trial registration platform, and literature search was conducted by combining the subject words and free words. According to the inclusion criteria, the study subjects were patients with confirmed hyperlipidemia; the intervention drugs were inclisiran, evolocumab, and atorvastatin; and the control group was the RCT and cohort study taking placebo. Two investigators (Ye and Li) independently extracted and processed information data on the literature of the article, such as the author, title, published year, journal, cases in the trial and control groups, and the number of new diabetes cases in the test and control groups. Studies with case reports, experience summaries, conference abstracts, and incomplete study data or articles were excluded, and literature included in the meta-analysis was determined after reaching consensus through discussion.
Evaluation of literature quality
Two investigators independently evaluated the literature quality, and the RCT was evaluated in strict accordance with the Cochrane risk of bias tool. The evaluation indicators included seven items in a total of six aspects: selection bias (random sequence generation and allocation concealment), reporting bias (selective reporting findings), implementation bias (blinded to investigators and subjects), detection bias (blind evaluation of study outcome), loss-to-visit bias (incomplete outcome data), and other bias (other sources of bias). Each entry was evaluated as “low risk,” “high risk,” or “unclear,” and then the risk of bias was drawn using RevMan 5.4 software. The cohort study was evaluated in strict accordance with the Newcastle–Ottawa Scale (NOS) for selection, comparability, and outcome, with a total score of 9; the literature with a score of 6 was generally considered high-quality literature. If there was any disagreement, the third investigator will intervene in the discussion and make the final decision.
Statistical treatment
The results of the meta-analysis were statistically analyzed on the data using the RevMan 5.4 software. Outcome measures were dichotomous variables, using the odds ratio (OR) as the efficacy analysis statistic with 95% confidence intervals (95% CIs). Heterogeneity among studies and outcomes was included using the chi-square values test. If I2 > 50% or p 0.1, there was significant heterogeneity among the results of the index, which was analyzed using the random effects model, sensitivity analysis was performed to find the sources of heterogeneity, and subgroup analysis of factors was performed, leading to heterogeneity if necessary; if I2 at 50% and p > 0.1, a fixed-effects model was used to produce a forest plot. Reticular meta-analysis was performed using Stata 18.0 software to plot the mesh evidence, and for studies with closed rings, inconsistency testing and model species were selected. The index of this study was dichotomous variable, so the OR combined statistics were used, and 95% CI was calculated with p < 0.05; the cumulative ranking curve of each intervention (the surface under the cumulative ranking curve, SUCRA) and the ranking plot were drawn.
Ethical statement
As the FAERS database and RCTs are open to the public and patient records are anonymous and de-identified, ethical permission and informed consent were not required for this study (Zhou et al., 2023).
Results
Descriptive results of the pharmacovigilance analysis
From 2015 Q1 to 2024 Q1, a total of 12,821,285 patient AEs were reported in the FEARS database, including 3,375 for inclisiran, 126,620 for evolocumab, and 42,228 for atorvastatin. The median age of patients in the three groups was similar (IQR: 66∼70 years), and the proportion of women (48.39%∼53.95%) was higher than that of men. The number of reported cases of evolocumab and atorvastatin (53.56% and 53.38%) was higher than that of consumers (47.76% and 45.45%), and the number of reported cases (57.04%) was higher than that of health professionals (42.79%). A total of 1,228 (36.39%) AEs reported the time of AEs, 10,749 (8.49%) and 9,752 (23.09%), and the highest proportion between days 0–30 after administration (26.19%, 4.5%, and 11.67%, respectively). The results are shown in Table 1.
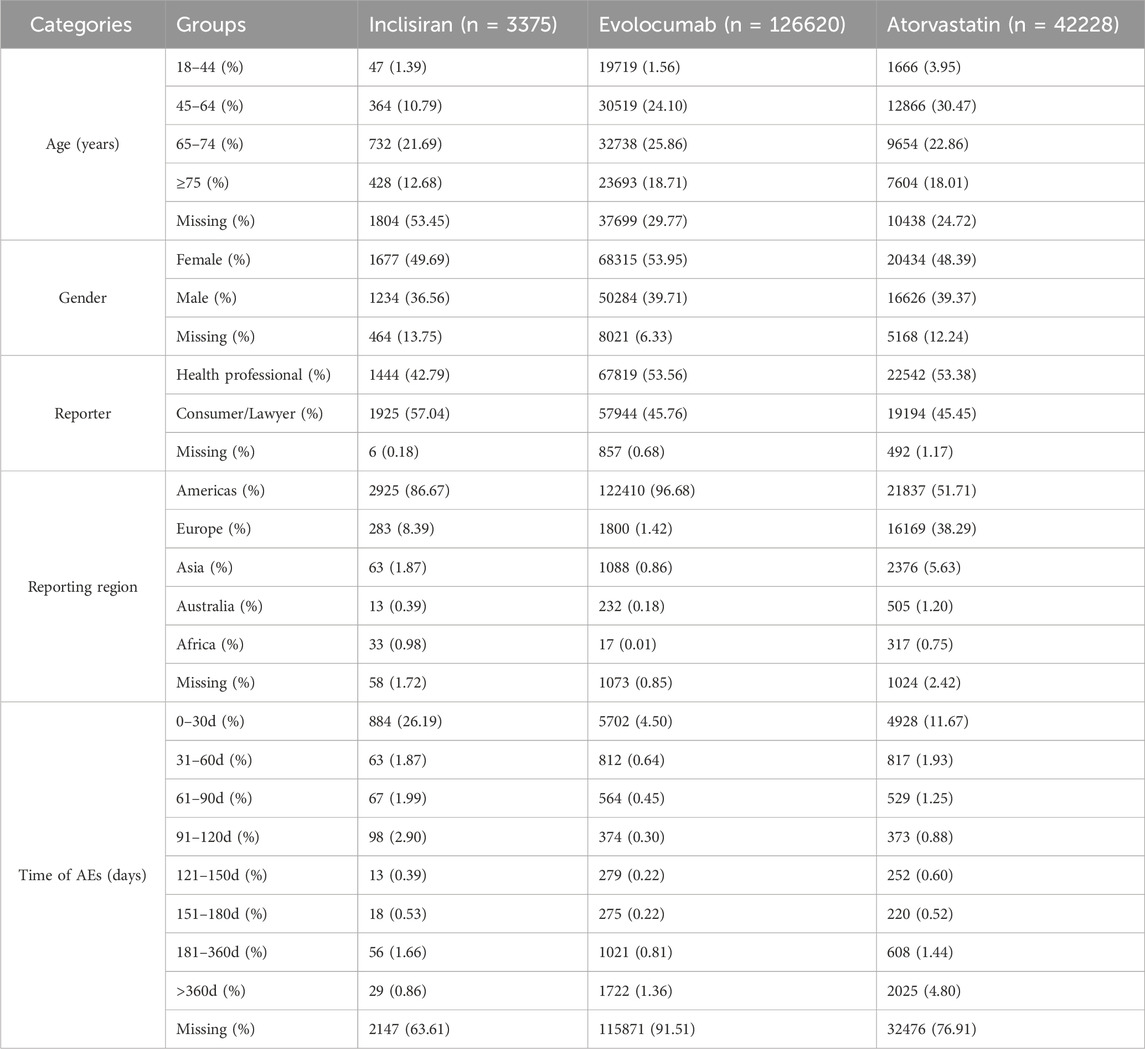
Table 1. Demographic characteristics of patients treated with inclisiran, evolocumab, and atorvastatin in the FAERS database.
Disproportionality analysis of diabetes-related adverse events
As shown in Table 2, the analysis of AE associations revealed distinct profiles between treatment groups. For type 2 diabetes AEs, inclisiran showed no statistically significant association [n = 3/3,375, ROR = 0.95 (0.31–2.96), IC025 = −1.49]. This lack of association for inclisiran stands in contrast to atorvastatin, which demonstrated a very strong positive association [n = 6,168/42,228, ROR = 195.03 (188.86–201.41), IC025 = 6.78]. For impaired glucose tolerance (IGT) AEs, the association strength observed with inclisiran [n = 5/3,375, ROR = 6.25 (2.60–15.05), IC025 = 0.55] was weaker than that observed with atorvastatin [n = 90/42,228, ROR = 9.11 (7.38–11.23), IC025 = 2.72]. No statistically significant difference in IGT AE association was found between inclisiran and evolocumab. Analyses identified weak signals for inclisiran treatment-related blood glucose increase [n = 42/3,375, 1.24%, ROR = 2.04 (1.51–2.77), IC025 = 0.55] and elevated HbA1c [n = 10/3,375, 0.30%, ROR = 2.72 (1.46–5.06), IC025 = 0.36]. Atorvastatin did not show significant signals for these specific glucose-related AEs.
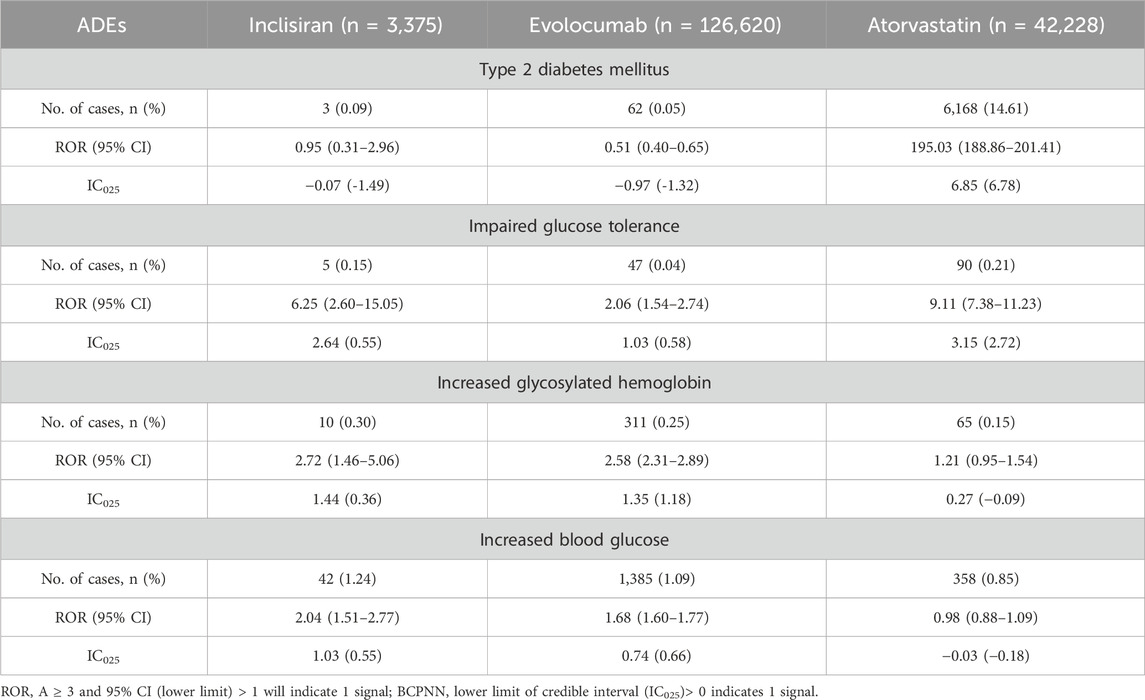
Table 2. Disproportionality analysis of NOD and hyperglycemic AEs associated with inclisiran, evolocumab, and atorvastatin as compared to the full FAERS database.
Results of literature screening by meta-analysis
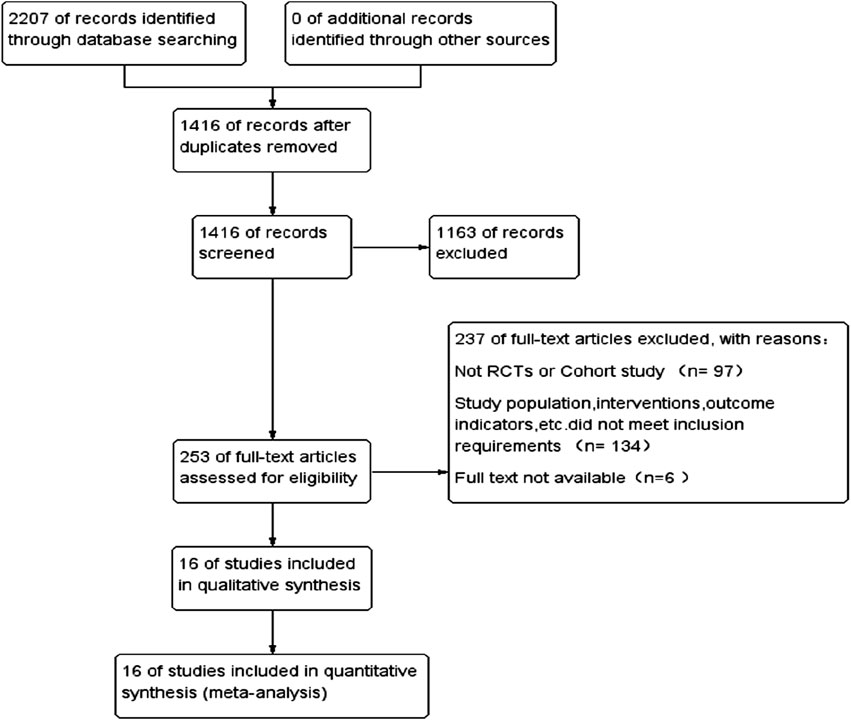
Through the search strategy, 2,207 articles were searched in electronic databases, and 1,416 articles were obtained after excluding duplicates. After reading the articles and abstracts, 1,163 articles were selected, including case reports, basic research, systematic review, and meta-analysis; 253 articles were screened for full screening. The full text was read through and screened one by one according to the inclusion and exclusion criteria. Finally, 16 articles met the criteria and were included in this study, involving six cohort studies and 10 randomized controlled trials for a total of 297,863 patients. The literature search and screening process is shown in Figure 1.
Basic characteristics of the literature in meta-analysis
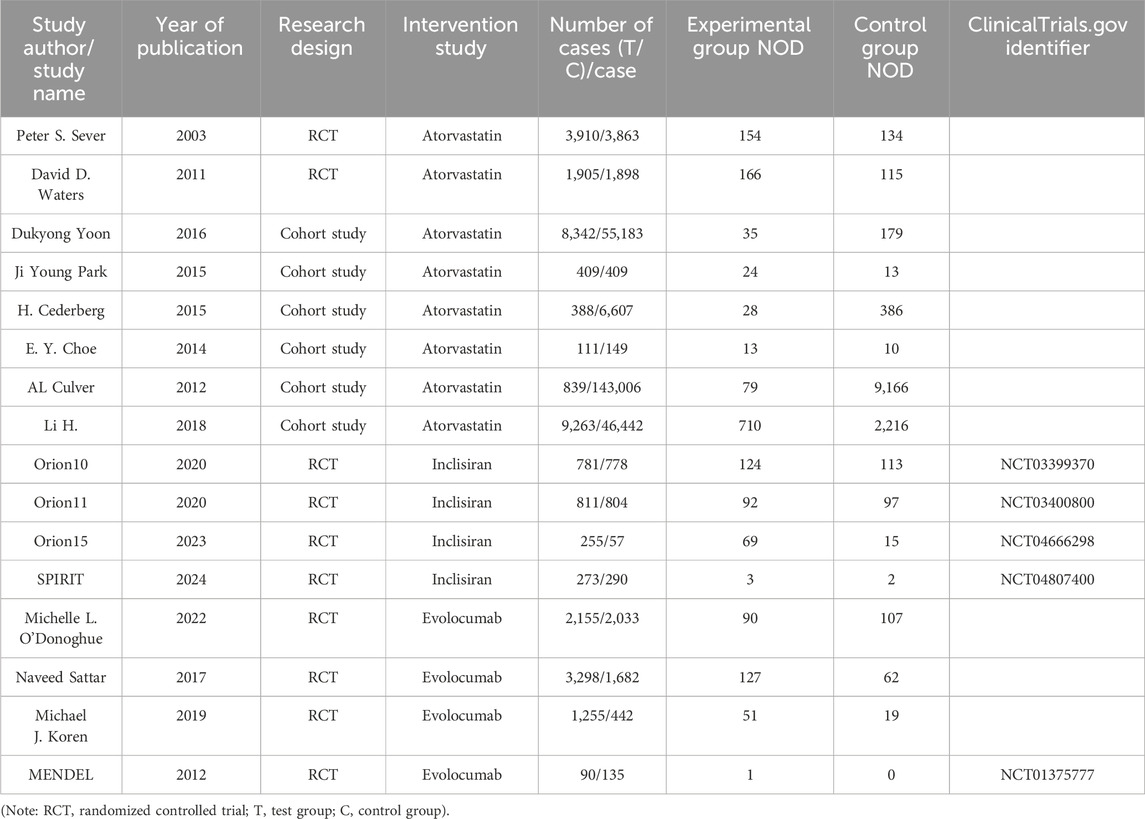
Basic information of the 16 articles in this study includes the following: study author/study name, year of publication, type of study, intervention measures, number of cases in the experimental group/control group, number of NOD in the experimental group, number of NOD in the control group, ClinicalTrials.gov identifier, including 10 RCT (Mayer et al., 2006; Waters et al., 2011; O’Donoghue et al., 2022; Sattar et al., 2017; Koren et al., 2019), and six retrospective cohort studies (Cederberg et al., 2015; Li et al., 2018; Park et al., 2015; Choe et al., 2014; Culver et al., 2012; Yoon et al., 2016) published from 2003 to 2024, including 297,863 patients, which were studied in both arms. Four studies addressed the comparison of effects on NOD between inclisiran and placebo, four on the effects of evolocumab and placebo, and eight on the effects of diabetes between atorvastatin and placebo. The basic characteristics of the included literature are presented in Table 3.
Results of literature quality evaluation in meta-analysis
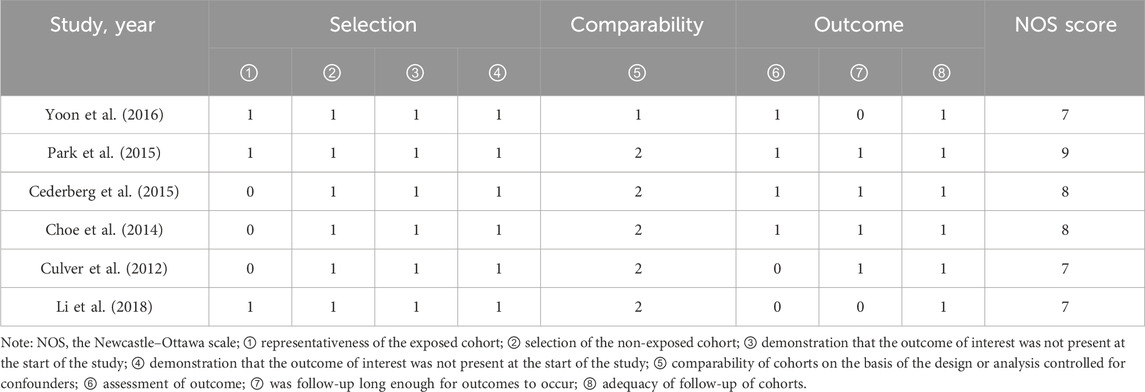
All 10 included RCT articles were randomized allocation trials, with only one literature (O’Donoghue et al., 2022) specifically describing the random method, four papers were double-blind, and four papers were not blinded. All RCT study outcomes were complete, the study results were not selectively reported, they had no other sources of bias, and the results of the RCT risk assessment are shown in Figures 2, 3. The NOS score of the six retrospective cohort studies was 19 points, 38 points, and 27 points; three did not truly represent the community population, one did not describe the evaluation method of outcome events, and two had a short follow-up period. The results are shown in Table 4.
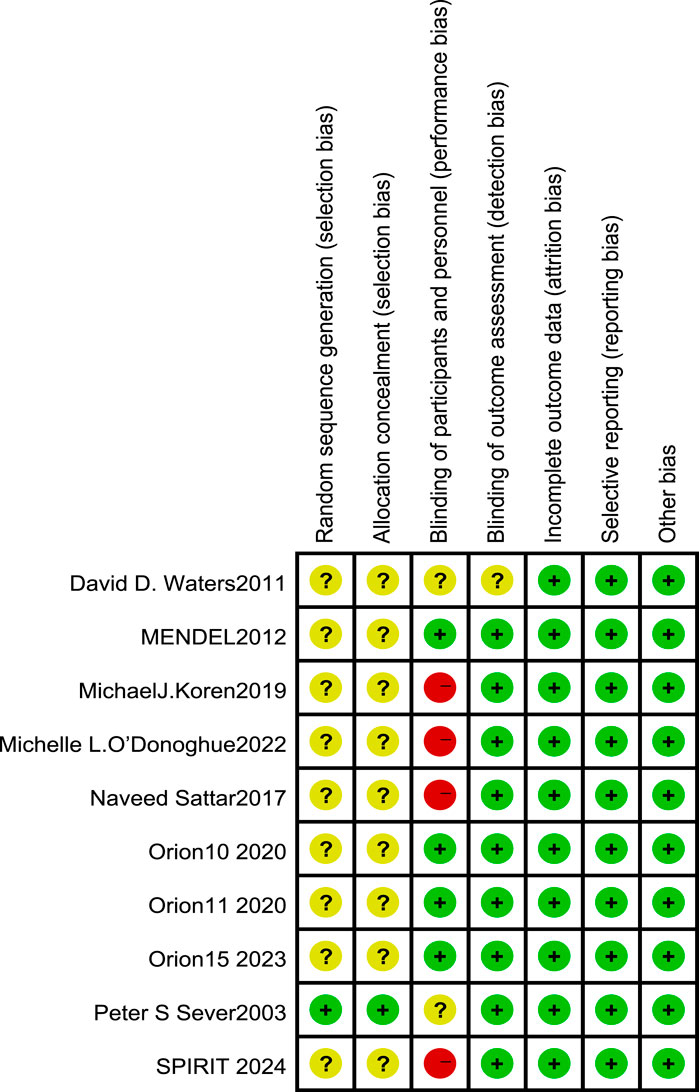
Figure 2. Risk of bias in randomized controlled trials [note: (+), low risk; (−), high risk; (?), NK].
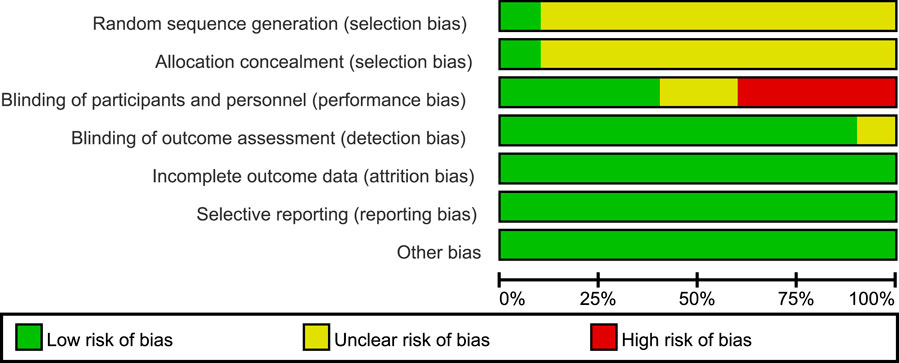
Figure 3. Overall risk of bias assessment diagram of four RCT studies. Note: the bias indicator shows the risk distribution in a percentage manner. The red part indicates the proportion of studies with high risk of bias in all studies, the yellow part indicates the proportion of studies with unclear risk of bias in all studies, and the green part indicates the proportion of studies with low risk of bias in all studies.
Results of the meta-analysis
Meta-analysis was performed using RevMan 5.4 software. Heterogeneity tests indicated low between-study heterogeneity in the three intervention comparisons (atorvastatin, inclisiran, and evolocumab versus placebo) (p > 0.05, I2 < 50%). When p > 0.05 (no significant heterogeneity) and I2 < 50% (low heterogeneity), the observed differences among the included studies are primarily attributable to random error rather than variability in true effect sizes. Under this condition, the fixed-effect model, which assumes a common true effect size across all studies, provides a more precise estimate of the pooled effect by accounting solely for within-study variation. Therefore, the fixed-effect model was uniformly applied for direct pairwise comparisons. The incidence of NOD in the atorvastatin group (4.80%) was higher than that in the placebo group (4.74%), and the result was statistically significant [OR = 1.54, 95% CI (1.44, 1.66), p < 0.05] (Figure 4C). There were no significant statistical differences in the incidence of NOD between the inclisiran group and the placebo group [OR = 1.03, 95% CI (0.85, 1.25), p > 0.05] and between the evolocumab group and the placebo group [OR = 0.91, 95% CI (0.75, 1.10), p > 0.05] (Figures 4A,B).
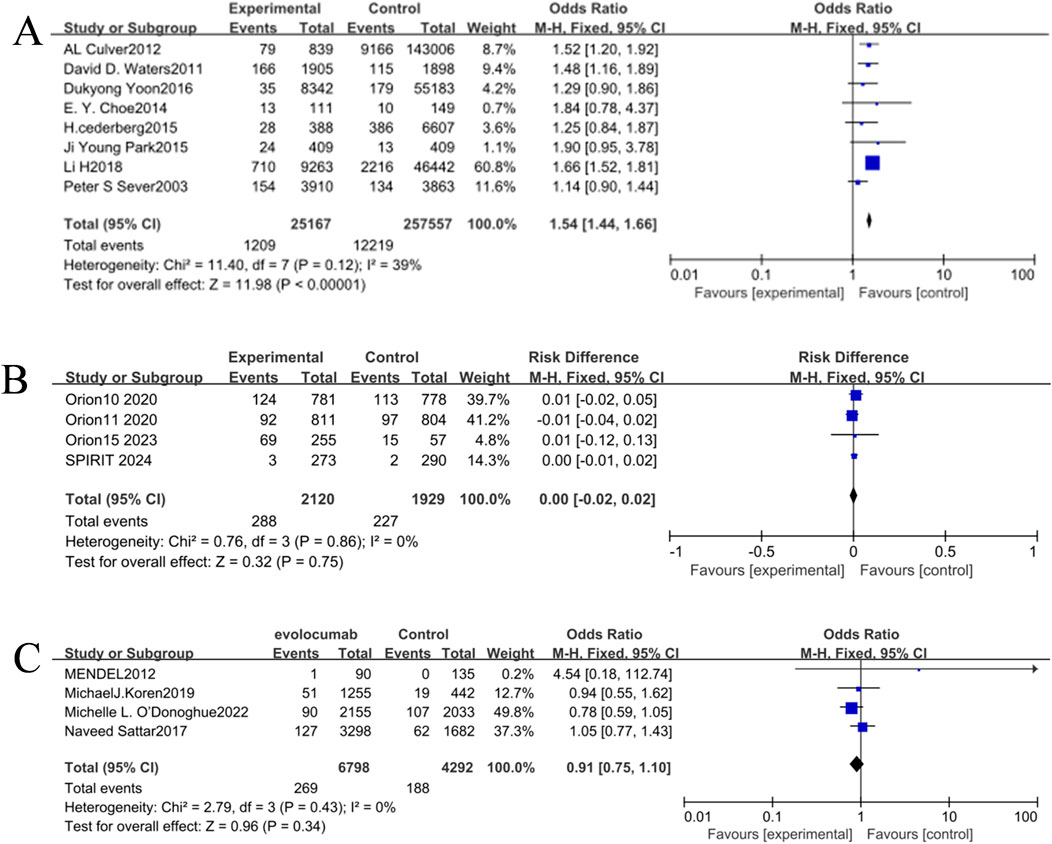
Figure 4. (A) Forest plot of the incidence of NOD in the inclisiran and control groups. (B) Forest plot of the incidence of NOD in the evolocumab and control groups. (C) Forest plot of the incidence of NOD in the atorvastatin and control groups.
Reticular meta-analysis results
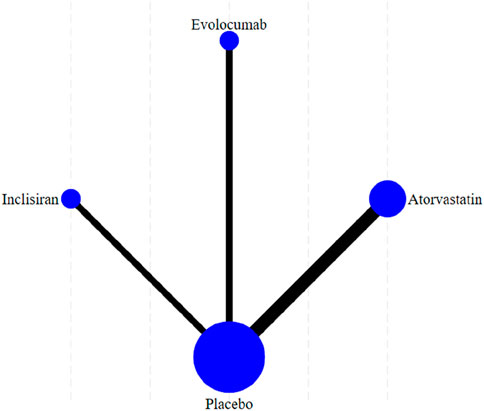
Figure 5 shows the reticular diagram for each intervention. Each node (blue dot) in the figure represents an intervention; the size of each node represents the number of participants included in the intervention, and the larger nodes indicate a larger number of participants. The connection between the two points was a direct comparison between the two interventions, and the thicker the line segment, the more studies were compared between the two interventions. There were no connecting lines between different intervention methods, and indirect comparisons can be made using reticular meta-analysis.
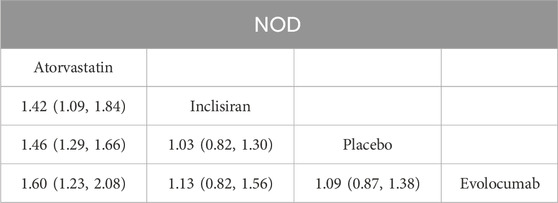
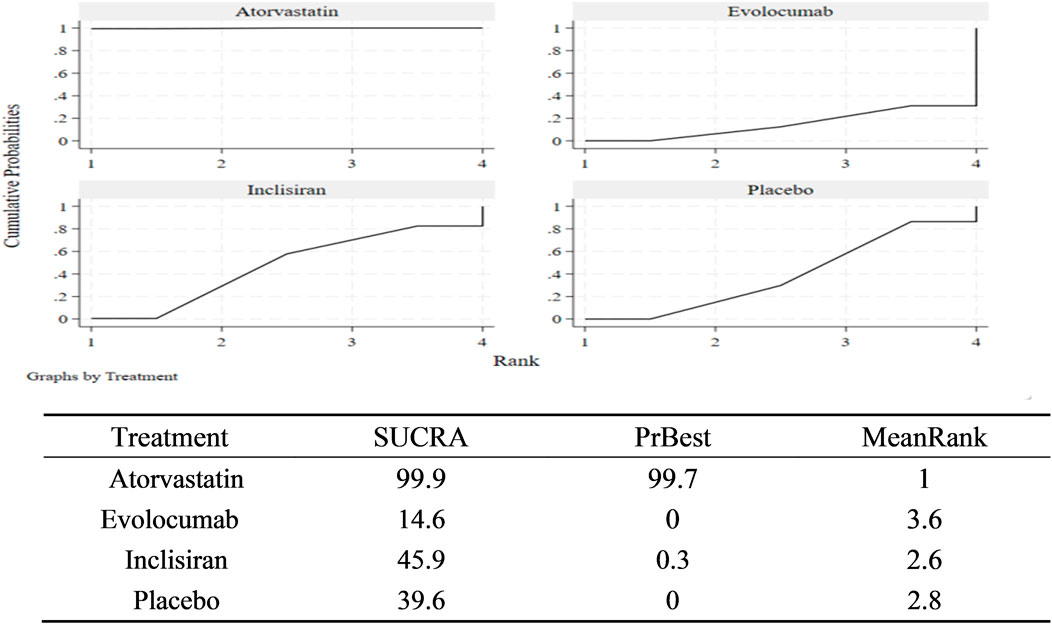
According to the match table (Table 5), the incidence of NOD was higher in the atorvastatin group than in the inclisiran group (OR = 1.42, 95% CI: 1.09–1.84), placebo group (OR = 1.46, 95% CI: 1.29–1.66), and evolocumab group (OR = 1.60, 95% CI: 1.23–2.08). However, the differences among the inclisiran, evolocumab, and placebo groups were not significant, indicating that inclisiran and evolocumab may not increase the incidence of NOD. According to the SUCRA chart (Figure 6), the incidence of NOD under the three interventions was ranked as follows: atorvastatin group (SUCRA = 99.9%)> inclisiran group (SUCRA = 45.9%)> placebo group (SUCRA = 39.6%)> evolocumab group (SUCRA = 14.6%).
Comparison of the analysis results of new diabetes incidence data-corrected funnel plot showed that the included studies were centrally distributed, basically within the funnel, and symmetrically distributed, indicating that the included studies were less likely to have publication bias (Figure 7).
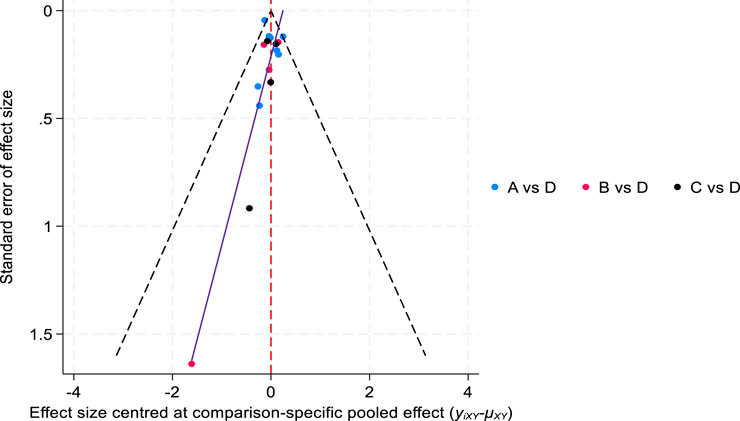
Figure 7. Comparison of the incidence of new-onset diabetes-corrected funnel plot. ((A) atorvastatin, (B) inclisiran, (C) evolocumab, and (D) placebo).
Discussion
Inclisiran has been on the market for a relatively short time, so continuous monitoring of long-term AEs should be maintained during its clinical use. Whether there is a disease correlation between NOD and dyslipidemia remains a question. As future research delves deeper into the pathophysiology of these two diseases, new discoveries may emerge. Some relatively rare clinical and epidemiological AE data are better accessible and evaluated in the real world than in registry trials (Sisi et al., 2022). Global pharmacovigilance study plays an important role in tracking differences in the safety profiles of the same class of therapeutic agents (Goldman et al., 2022), but causal inference is somewhat challenging due to the nature of the adverse reactions. Meta-analysis of RCTs provides ways to address causality information in the FAERS report that has not been validated in science or otherwise. In this study, we combined the advantages of both approaches to provide a systematic, evidence-based approach to assess the association of inclisiran with NOD and hyperglycemia.
Our pharmacovigilance analysis showed that inclisiran had no significant impact on AEs of type 2 diabetes mellitus [ROR 0.95, 95% CI (0.31–2.96)] and impaired glucose tolerance [ROR 6.25, 95% CI (2.60–15.05)]. In contrast, atorvastatin was highly associated with AEs of type 2 diabetes mellitus [ROR 195.03, 95% CI (188.86–201.41)] and IGT [ROR 9.11, 95% CI (7.38–11.23)]. However, in the FAERS database, consumers and lawyers reported more adverse Ingram (57.04%) than health professionals (42.79%), which is potentially unbalanced, and bias and differences should be interpreted with caution. Based on the results of the pharmacovigilance analysis, we introduced a meta-analysis to further explore causality, as clinical trials have addressed these safety issues reported by health professionals. We extracted 16 studies from published articles to quantify the risk of adverse drug events. Our findings are consistent with the diabetes panel of the National Lipid Association Expert Panel on evidence that statins increase the risk of NOD (Maki et al., 2014). However, there was no statistically significant difference among the inclisiran, evolocumab, and placebo groups (as shown in Table 5).
Notably, patients treated with atorvastatin had a higher risk of NOD, but there were no significant changes in glycemic control and HbA1c in some patients (Angelidi et al., 2018), which was also validated in the FAERS database. Our retrospective query of the FAERS database found that atorvastatin treatment was not significantly associated with abnormal glycemia and elevated HbA1c. Statins moderately accelerate the time to diabetes diagnosis, and this risk is mainly limited to patients who are already at high risk of developing diabetes, such as those with impaired fasting glucose, metabolic syndrome, or raised HbA1c (Ridker et al., 2012).
Despite the clinical observation of a general association between LDL cholesterol and high risk of type 2 diabetes, it is far from certain that all interventions to lower LDL cholesterol increase the risk of type 2 diabetes, as the mechanisms of action of different lipid-regulating drugs may differ (Schmidt et al., 2017). Statins have been shown to increase the risk of diabetes, the underlying mechanism associated with reduced insulin secretion. The expression of LDLR in the pancreas, leading to increased LDL-C concentration in the pancreas, induces the lipotoxic effect of β cells and β-cell dysfunction, impairs insulin secretion, and promotes the development of type 2 diabetes mellitus (Laakso and Fernandes Silva, 2023; Takei et al., 2020). Regarding glucose metabolism and the risk of NOD, Langhi et al. studied the expression and function of PCSK 9 in islets and showed that PCSK 9 does not alter insulin secretion in mice (Langhi et al., 2009). However, some evidence from the Mendelian randomization study suggests that certain genetic PCSK 9 variants associated with low LDL-C levels are associated with a 29% increased risk of type 2 diabetes (Schmidt et al., 2017). Glodman et al. showed that PCSK 9 inhibitors are associated with increased reporting of hyperglycemic AEs, mainly with mild hyperglycemia (Goldman et al., 2022). The increased risk of NOD (hyperglycemia) was not observed in the longer trial of the ORION-1 study (Ray et al., 2023). Therefore, the existing studies are not sufficient to demonstrate the role of PCSK 9 and PCSK 9 monoclonal antibodies in the disruption of islet function. Moreover, the results of a post hoc pooled analysis of the phase-3 randomized trials ORION-9, ORION-10, and ORION-11 with a follow-up period of up to 540 days confirmed that inclisiran was well tolerated and did not increase the risk of NOD compared with the placebo group (Leiter et al., 2024).
In FAERS, we ranked the signal intensity of AEs in the top 30 PTs. The results indicate that evolocumab lacked administration/injection site rotation (ROR = 38.49/19.89; IC025 = 4.91/4.12) and injection fear (ROR = 14.33; IC025 = 3.70). Inclisiran reported no such obvious AEs compared with evolocumab (Supplementary Material S1). Therefore, when inclisiran is used twice a year and combined with lower rates of AEs, these give it higher treatment adherence than evolocumab (Scicchitano et al., 2021). However, given the short time of marketing of inclisiran, as with any new substance, the potential off-target effects and long-term safety of the drug should be closely monitored (Wolowiec et al., 2023). This needs to be confirmed by in-depth and large-scale long-term follow-up studies, especially with regular blood glucose monitoring in high-risk groups, and a comprehensive assessment of risk factors and possible drug interactions appears very important (Tosur et al., 2020).
Limitations
Although pharmacovigilance and RCTs are complementary in an integrated study design, our study still has some limitations. First, the FAERS database is a global spontaneous reporting system, with nonmedical professionals expanding reporting coverage, but it has unstandardized data quality, possibly with reporting bias and substantial missing data. Second, the pharmacovigilance analysis failed to verify the causal relationship between inclisiran and AEs, as the disproportionate analysis provided only an estimate of signal strength and could neither quantify the risk nor determine causality (Chen et al., 2024). Finally, most RCT studies were not designed to study the relationship between inclisiran and NOD, which may lead to heterogeneity of results. Moreover, another important point is the potential impact of the COVID-19 pandemic (e.g., infection and healthcare access disruptions) on disease control and AE reporting during our study period (2021–2024). This is a recognized limitation of our study.
Conclusion
Through a systematic evaluation of integrated pharmacovigilance and RCTs, our results showed that inclisiran may carry a lower risk of NOD than atorvastatin. However, inclisiran is a newly approved drug that has been available for a short duration in recent years, and larger long-term follow-up studies are needed to confirm these findings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
FL: Formal Analysis, Project administration, Resources, Validation, Writing – original draft. HY: Formal Analysis, Project administration, Validation, Writing – original draft. LC: Supervision, Writing – review and editing. YM: Data curation, Methodology, Supervision, Writing – review and editing. SC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Natural Science Foundation of Fujian Province, China (No. 2022J05204), the Health Technology project of Fujian Province (No. 2022GGA006), and Joint Funds for the innovation of Science and Technology of Fujian Province (No. 2023Y9274).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1554631/full#supplementary-material
References
Angelidi, A. M., Stambolliu, E., Adamopoulou, K. I., and Kousoulis, A. A. (2018). Is atorvastatin associated with new onset diabetes or deterioration of glycemic control? Systematic review using data from 1.9 million patients. Int. J. Endocrinol. 2018, 8380192. doi:10.1155/2018/8380192
Cederberg, H., Stancakova, A., Yaluri, N., Modi, S., Kuusisto, J., and Laakso, M. (2015). Increased risk of diabetes with statin treatment is associated with impaired insulin sensitivity and insulin secretion: a 6 year follow-up study of the METSIM cohort. Diabetologia 58 (5), 1109–1117. doi:10.1007/s00125-015-3528-5
Chen, K., Huang, H., Chen, Y., and He, W. (2024). Association between atorvastatin and erectile dysfunction: a comprehensive analysis incorporating real-world pharmacovigilance and Mendelian randomization. Front. Pharmacol. 15, 1382924. doi:10.3389/fphar.2024.1382924
Choe, E. Y., Wang, H. J., Kwon, O., Cho, Y., Huh, K. H., Kim, M. S., et al. (2014). HMG CoA reductase inhibitor treatment induces dysglycemia in renal allograft recipients. Transplantation 97 (4), 419–425. doi:10.1097/01.TP.0000437427.04733.ad
Culver, A. L., Ockene, I. S., Balasubramanian, R., Olendzki, B. C., Sepavich, D. M., Wactawski-Wende, J., et al. (2012). Statin use and risk of diabetes mellitus in postmenopausal women in the Women's health initiative. Arch. Intern Med. 172 (2), 144–152. doi:10.1001/archinternmed.2011.625
Goldman, A., Raschi, E., Cukierman-Yaffe, T., Dankner, R., Shouval, R., Shechter, M., et al. (2022). Hyperglycaemic disorders associated with PCSK9 inhibitors: a real-world, pharmacovigilance study. Eur. J. Prev. Cardiol. 29 (9), 1334–1342. doi:10.1093/eurjpc/zwab209
Koren, M. J., Sabatine, M. S., Giugliano, R. P., Langslet, G., Wiviott, S. D., Ruzza, A., et al. (2019). Long-term efficacy and safety of evolocumab in patients with hypercholesterolemia. J. Am. Coll. Cardiol. 74 (17), 2132–2146. doi:10.1016/j.jacc.2019.08.1024
Kosmas, C. E., Silverio, D., Sourlas, A., Garcia, F., Montan, P. D., and Guzman, E. (2018). Impact of lipid-lowering therapy on glycemic control and the risk for new-onset diabetes mellitus. Drugs Context. 7, 212562–212567. doi:10.7573/dic.212562
Kosmas, C. E., Skavdis, A., Sourlas, A., Papakonstantinou, E. J., Peña Genao, E., Echavarria Uceta, R., et al. (2020). Safety and tolerability of PCSK9 inhibitors: current insights. Clin. Pharmacol. Adv. Appl. 12, 191–202. doi:10.2147/cpaa.S288831
Laakso, M., and Fernandes Silva, L. (2023). Statins and risk of type 2 diabetes: mechanism and clinical implications. Front. Endocrinol. 14, 1239335. doi:10.3389/fendo.2023.1239335
Lamb, Y. N. (2021). Inclisiran: first approval. Drugs. 81 (3), 389–395. doi:10.1007/s40265-021-01473-6
Langhi, C., Le May, C., Gmyr, V., Vandewalle, B., Kerr-Conte, J., Krempf, M., et al. (2009). PCSK9 is expressed in pancreatic delta-cells and does not alter insulin secretion. Biochem. Biophys. Res. Commun. 390 (4), 1288–1293. doi:10.1016/j.bbrc.2009.10.138
Leiter, L. A., Raal, F. J., Schwartz, G. G., Koenig, W., Ray, K. K., Landmesser, U., et al. (2024). Inclisiran in individuals with diabetes or obesity: post hoc pooled analyses of the ORION-9, ORION-10 and ORION-11 phase 3 randomized trials. Diabetes, Obes. Metabolism 26 (8), 3223–3237. doi:10.1111/dom.15650
Li, H., Lin, H., Zhao, H., Xu, Y., Cheng, Y., Shen, P., et al. (2018). Statins use and risk of new-onset diabetes in hypertensive patients: a population-based retrospective cohort study in Yinzhou district, Ningbo city, People's Republic of China. Ther. Clin. Risk Manag. 14, 823–832. doi:10.2147/TCRM.S158850
Maki, K. C., Ridker, P. M., Brown, W. V., Grundy, S. M., Sattar, N., and The Diabetes Subpanel of the National Lipid Association Expert Panel, (2014). An assessment by the statin diabetes safety task force: 2014 update. J. Clin. Lipidol. 8 (3 Suppl. l), S17–S29. doi:10.1016/j.jacl.2014.02.012
Mayer, L. D., Harasym, T. O., Tardi, P. G., Harasym, N. L., Shew, C. R., Johnstone, S. A., et al. (2006). Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol. Cancer Ther. 5 (7), 1854–1863. doi:10.1158/1535-7163.MCT-06-0118
Merćep, I., Friščić, N., Strikić, D., Reiner, Ž., and Tomlinson, B. (2022). Advantages and disadvantages of inclisiran: a small interfering ribonucleic acid molecule targeting PCSK9-A narrative review. Cardiovasc. Ther. 2022, 8129513–8129516. doi:10.1155/2022/8129513
O’Donoghue, M. L., Giugliano, R. P., Wiviott, S. D., Atar, D., Keech, A., Kuder, J. F., et al. (2022). Long-term evolocumab in patients with established atherosclerotic cardiovascular disease. Circulation 146 (15), 1109–1119. doi:10.1161/CIRCULATIONAHA.122.061620
Park, J. Y., Rha, S. W., Choi, B., Choi, J. W., Ryu, S. K., Kim, S., et al. (2015). Impact of low dose atorvastatin on development of new-onset diabetes mellitus in Asian population: three-year clinical outcomes. Int. J. Cardiol. 184, 502–506. doi:10.1016/j.ijcard.2015.03.047
Ray, K. K., Troquay, R. P. T., Visseren, F. L. J., Leiter, L. A., Scott Wright, R., Vikarunnessa, S., et al. (2023). Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated LDL cholesterol (ORION-3): results from the 4-year open-label extension of the ORION-1 trial. Lancet Diabetes Endocrinol. 11 (2), 109–119. doi:10.1016/S2213-8587(22)00353-9
Ridker, P. M., Pradhan, A., MacFadyen, J. G., Libby, P., and Glynn, R. J. (2012). Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet 380 (9841), 565–571. doi:10.1016/S0140-6736(12)61190-8
Sattar, N., Toth, P. P., Blom, D. J., Koren, M. J., Soran, H., Uhart, M., et al. (2017). Effect of the proprotein convertase subtilisin/kexin type 9 inhibitor evolocumab on glycemia, body weight, and new-onset diabetes mellitus. Am. J. Cardiol. 120 (9), 1521–1527. doi:10.1016/j.amjcard.2017.07.047
Schmidt, A. F., Swerdlow, D. I., Holmes, M. V., Patel, R. S., Fairhurst-Hunter, Z., Lyall, D. M., et al. (2017). PCSK9 genetic variants and risk of type 2 diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol. 5 (2), 97–105. doi:10.1016/S2213-8587(16)30396-5
Scicchitano, P., Milo, M., Mallamaci, R., De Palo, M., Caldarola, P., Massari, F., et al. (2021). Inclisiran in lipid management: a literature overview and future perspectives. Biomed. Pharmacother. 143, 112227. doi:10.1016/j.biopha.2021.112227
Sinning, D., and Landmesser, U. (2020). Low-density lipoprotein-cholesterol lowering strategies for prevention of atherosclerotic cardiovascular disease: focus on siRNA treatment targeting PCSK9 (inclisiran). Curr. Cardiol. Rep. 22 (12), 176. doi:10.1007/s11886-020-01427-6
Sisi, M., Fusaroli, M., De Giglio, A., Facchinetti, F., Ardizzoni, A., Raschi, E., et al. (2022). Psychiatric adverse reactions to anaplastic lymphoma kinase inhibitors in non-small-cell lung cancer: analysis of spontaneous reports submitted to the FDA adverse event reporting system. Target Oncol. 17 (1), 43–51. doi:10.1007/s11523-021-00865-8
Takei, S., Nagashima, S., Takei, A., Yamamuro, D., Wakabayashi, T., Murakami, A., et al. (2020). β-Cell-Specific deletion of HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A) reductase causes overt diabetes due to reduction of β-Cell mass and impaired insulin secretion. Diabetes 69 (11), 2352–2363. doi:10.2337/db19-0996
Tosur, M., Viau-Colindres, J., Astudillo, M., Redondo, M. J., and Lyons, S. K. (2020). Medication-induced hyperglycemia: pediatric perspective. BMJ Open Diabetes Res. Care 8 (1), e000801. doi:10.1136/bmjdrc-2019-000801
Waters, D. D., Ho, J. E., DeMicco, D. A., Breazna, A., Arsenault, B. J., Wun, C. C., et al. (2011). Predictors of new-onset diabetes in patients treated with atorvastatin: results from 3 large randomized clinical trials. J. Am. Coll. Cardiol. 57 (14), 1535–1545. doi:10.1016/j.jacc.2010.10.047
Wolowiec, L., Osiak, J., Wolowiec, A., Wijata, A., Grześk, E., Kozakiewicz, M., et al. (2023). Inclisiran-safety and effectiveness of small interfering RNA in inhibition of PCSK-9. Pharmaceutics 15 (2), 323. doi:10.3390/pharmaceutics15020323
Yoon, D., Sheen, S. S., Lee, S., Choi, Y. J., Park, R. W., and Lim, H. S. (2016). Statins and risk for new-onset diabetes mellitus: a real-world cohort study using a clinical research database. Med. Baltim. 95 (46), e5429. doi:10.1097/MD.0000000000005429
Keywords: inclisiran, evolocumab, atorvastatin, hyperglycemia, new-onset diabetes
Citation: Li F, Ye H, Chen L, Ma Y and Chen S (2025) Systemic evaluation of inclisiran on the risk of new-onset diabetes and hyperglycemia compared to evolocumab and atorvastatin. Front. Pharmacol. 16:1554631. doi: 10.3389/fphar.2025.1554631
Received: 20 January 2025; Accepted: 25 June 2025;
Published: 15 July 2025.
Edited by:
Maria Giovanna Lupo, University of Padua, ItalyReviewed by:
Liping Jiang, University of Illinois Chicago, United StatesElena Sani, University of Verona, Italy
Copyright © 2025 Li, Ye, Chen, Ma and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sunhui Chen, YW5uYW5jaGVuQGZ6dS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Fei Li1†
Fei Li1† Sunhui Chen
Sunhui Chen