- 1Department of Pharmacy, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 2Clinical Pharmacy Center, Nanfang Hospital, Southern Medical University, Guangzhou, China
Background: Alirocumab and evolocumab are proprotein convertase subtilisin/kexin type 9 inhibitors that significantly reduce the relative risk of cardiovascular events. However, the relative efficacy and safety of alirocumab and evolocumab in different patient groups still warrant further indirect comparison. This systematic review and network meta-analysis indirectly compared the efficacy and safety of alirocumab and evolocumab on major cardiovascular events.
Methods: PUBMED, EMBASE, Web of Science, and Cochrane Central Register of Controlled Trials (CENTRAL) databases were comprehensively searched to extract randomized controlled trials (RCTs) regarding alirocumab and evolocumab published from inception to 17 August 2024. The meta-analysis was performed using Software Review Manager 5.4 and R 4.1.0 software.
Results: This network meta-analysis included 26 RCTs with 64,921 patients. Among these, 13 RCTs included patients receiving alirocumab or placebo (n = 13,365) and 13 RCTs included patients receiving evolocumab or placebo (n = 22,048). Compared with the placebo, treatment with alirocumab and evolocumab significantly reduced the relative risk of major adverse cardiovascular and cerebrovascular events (MACCE), myocardial infarction, stroke, and coronary revascularization. Furthermore, alirocumab and evolocumab groups did not show significant differences in MACCE [relative risk (RR): 0.99, 95% confidence interval (CI): 0.88–1.11], cardiovascular death (RR: 0.83, 95% CI: 0.65–1.06), myocardial infarction (RR: 0.87, 95% CI: 0.74–1.03), stroke (RR: 0.96, 95% CI: 0.71–1.29), coronary revascularization (RR: 0.88, 95% CI: 0.77–1.01), and any adverse event (RR: 0.91, 95% CI: 0.76–1.09). Moreover, the all-cause mortality rates were lower for patients treated with alirocumab compared to those treated with evolocumab (RR: 0.84, 95% CI: 0.70–1.00), but the difference was not statistically significant.
Conclusion: Alirocumab and evolocumab demonstrated comparable efficacy in reducing the relative risk of major cardiovascular events. The all-cause mortality rates were lower in patients treated with alirocumab compared to those treated with evolocumab but the differences were not statistically significant, probably due to heterogeneity in the sample size and follow-up duration between different studies. Both drugs exhibited comparable safety profiles.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/myprospero, identifier CRD42024505327.
1 Introduction
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death globally and low-density lipoprotein cholesterol (LDL-C) is a key, modifiable risk factor of ASCVD events (Diao et al., 2024). Reducing LDL-C levels is the primary target of lipid-lowering treatment to prevent and manage ASCVD (Mhaimeed et al., 2024). Statins are considered as the first-line therapeutics for reducing elevated LDL-C levels according to the current clinical practice guidelines. Proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i) are considered as second line therapy for patients that do not achieve optimal goals of statin therapy (Grundy et al., 2019; Bhatia et al., 2024). Statins are effective in reducing LDL-C levels in only approximately 50% of patients with dyslipidemia (Ray et al., 2021; van de Borne et al., 2024). More than 50% of patients with familial hypercholesterolemia (FH) fail to achieve the LDL-C treatment goals even after receiving the highest tolerated statin dose (Schreuder et al., 2023). Furthermore, there is a strong consensus that statin intolerance leads to poor adherence and persistence with statins, and contributes to worsening cardiovascular outcomes (Banach et al., 2023). Therefore, patients with statin tolerance require alternate LDL-C targeting therapies.
PCSK9i are effective in lowering LDL-C levels and reducing the risk of ASCVD events by competitively inhibiting the binding of PCSK9 to the LDL receptors (LDLRs), thereby maintaining higher hepatic LDLR density and enhancing LDL-C clearance (Hummelgaard et al., 2023). Several humanized monoclonal antibodies that selectively target PCSK9 receptors have been developed for clinical practice. Alirocumab and evolocumab are the two most extensively studied PCSK9i that have shown high safety and efficacy in managing patients with hypercholesterolemia and ASCVD. These two antibodies bind to PCSK9 at sites overlapping with the binding site of LDLR, thereby effectively outcompeting the interaction between PCSK9 and LDLR. PCSK9i such as inclisiran and toralizumab are only approved for treating primary hypercholesterolemia and mixed dyslipidemia, but not for reducing cardiovascular risk. According to the 2021 European Society of Cardiology guidelines, early combination therapy with a PCSK9i is recommended for patients who fail to achieve their lipid goals with a maximum tolerated dose of a statin and ezetimibe (Visseren et al., 2022). However, further evidence is required regarding the long-term safety and efficacy of evolocumab and alirocumab in reducing ASCVD events because of the high yearly cost for these two drugs at £45,279 and £46,375, respectively (Michaeli et al., 2022).
Large randomized controlled trials (RCTs) have confirmed that PCSK9i are highly effective in lowering LDL-C levels and are associated with significant beneficial outcomes, including a reduction in all-cause mortality, cardiovascular events, and cardiovascular mortality (Sabatine et al., 2017; Schwartz et al., 2018). However, two large RCTs evaluating the use of alirocumab (ODYSSEY OUTCOMES trial) and evolocumab (FOURIER trial) enrolled patients with distinct clinical profiles, resulting in contradictory conclusions. According to currently available evidence, alirocumab demonstrates better outcomes in subjects with a higher risk of ASCVD, whereas evolocumab shows higher efficacy in patients with heterozygous familial hypercholesterolemia. However, direct comparison between these two agents has not been performed in clinical trials. An indirect comparison of the safety and efficacy of alirocumab and evolocumab based on a systematic review and network meta-analyses of RCTs was performed in 2021 and demonstrated comparable safety and efficacy profiles despite heterogeneity in the study populations (Guedeney et al., 2021). In recent years, several new large RCTs of alirocumab or evolocumab have been reported but have not been evaluated through meta-analyses. While recent studies have demonstrated the robust efficacy of alirocumab and evolocumab in reducing major cardiovascular events across various patient populations, the relative efficacy and safety of these two agents still warrant further indirect comparison. Therefore, in this network meta-analysis, we compared the efficacy and safety of alirocumab and evolocumab on major cardiovascular events by indirectly evaluating the results of RCTs, including those from newer RCTs. We included RCTs with data on outcomes such as major adverse cardiovascular and cerebrovascular events (MACCE), all-cause mortality, cardiovascular deaths, myocardial infarction, stroke, and coronary revascularization.
2 Methods
2.1 Protocol registration
The protocol for this systematic review was registered in the PROSPERO database (No. CRD42024505327). This study was approved by the Ethics Committee of Nanfang Hospital (Approval No. NFEC-2023-208).
We searched the PUBMED, EMBASE, Web of Science, and Cochrane Central Register of Controlled Trials databases from inception to 17 August 2024, using the following search terms: ‘alirocumab’ OR ‘evolocumab’ AND ‘randomized controlled trial’. The searches were not limited by any publication or language restrictions.
2.2 Selection criteria and outcomes
We included only RCTs that compared alirocumab or evolocumab with placebo in patients with dyslipidemias or cardiovascular disease and reported cardiovascular events and other adverse events. To reduce small-study effects and increase the reliability of our findings, we specifically included studies with a minimum of 100 participants and a follow-up period of at least 8 weeks.
This study focused only on alirocumab and evolocumab. RCTs comparing alirocumab or evolocumab with other lipid-lowering medications but lacking a placebo group were also excluded from the analysis.
MACCE was the primary composite efficacy outcome and defined as the occurrence of all-cause mortality, cardiovascular death, myocardial infarction, stroke, or coronary revascularization. Individual components of MACCE, namely, all-cause mortality, cardiovascular death, myocardial infarction, stroke, and coronary revascularization were included as secondary efficacy outcomes. Safety endpoints encompassed any reported adverse events.
2.3 Data extraction and analysis
Two researchers (CG and LX) independently extracted data from eligible studies using a pre-specified data collection form. The extracted data included study characteristics (authors, year of publication, and number of patients), study design (double-blind or open-label), characteristics of the patients enrolled, treatment protocols (name, dosage, and follow-up time), as well as the efficacy and safety outcomes.
One of the authors, LX, independently assessed the risk of bias according to the criteria outlines in the Cochrane Handbook for systematic reviews of interventions for RCTs. The methodological quality of all studies included in the analysis was assessed using the Review Manager (RevMan, Version 5.4; The Cochrane Collaboration). This assessment included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. The risk of bias was judged as low, unclear, and high.
Publication bias was evaluated with a combination of methods using R, including visual analysis with funnel plots and statistical tests like Begg’s test, Egger’s test, and Thompson-Sharp’s test. The threshold for statistical significance was set at p < 0.05.
A league table was generated for all pairwise comparisons in the meta-analysis using the odds ratios (OR) and 95% confidence intervals (95% CI) to directly compare the direction and magnitude of treatment effects. The analysis was performed using Software Review Manager 5.4 (RRID:SCR_003581) and R 4.1.0 software (RRID:SCR_001905).
3 Results
3.1 Literature search results
Based on searches in the four electronic databases, we initially included 1,727 studies for this updated systematic review. After removing duplicate publications, 1,309 articles were selected for further evaluation. Among these, we eliminated 1,069 articles that were reviews, comments, short reports, and case studies. Furthermore, 92 articles were excluded because of the following reasons: (i) efficacy and safety of the study drug was evaluated but major cardiovascular events were not analyzed (n = 68); (ii) patients received the study drug but did not undergo randomization (n = 2); (iii) sub-analyses (n = 10); (iv) pooled analyses (n = 9); (v) absence of comparison with a placebo arm and differences in the background lipid-lowering therapy (n = 4). Finally, this study enrolled 26 studies that met the inclusion criteria (Figure 1), including 13 RCTs comparing alirocumab with placebo and 13 RCTs comparing evolocumab with placebo. Overall, the data included 64,921 patients, with 24,851 patients allocated to alirocumab and 40,070 patients allocated to evolocumab. The corresponding network diagram is shown in Figure 2.
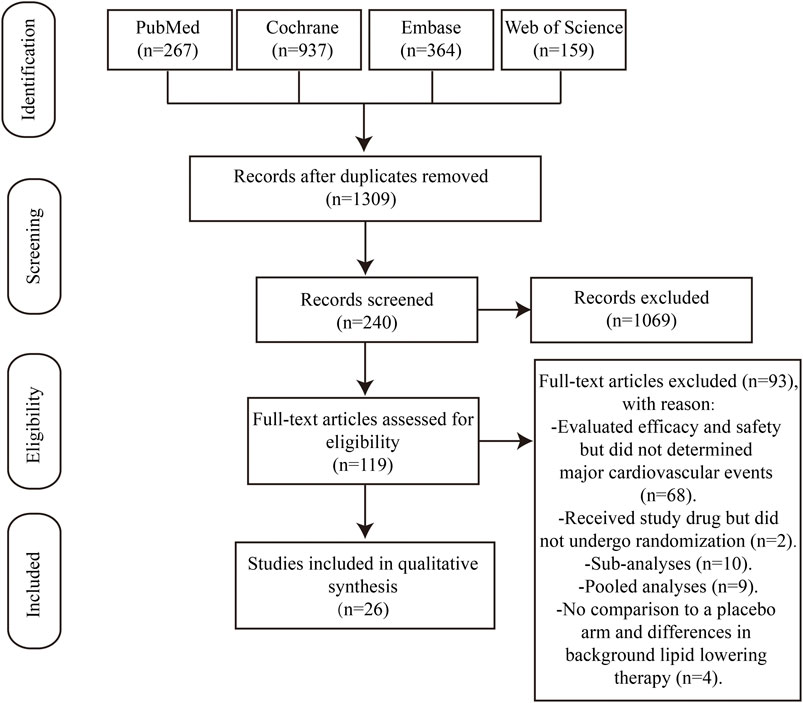
Figure 1. PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analysis. A total of 1727 articles were retrieved, and 26 studies were selected for network meta-analysis.
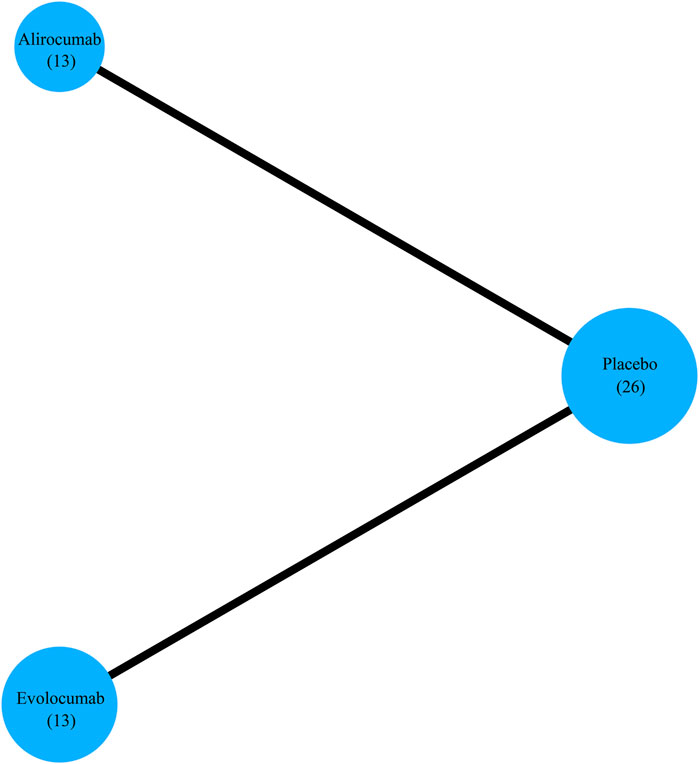
Figure 2. Network diagram shows the total number of RCTs analyzed for each treatment arm to evaluate efficacy endpoints.
3.2 Overview of study and patient characteristics
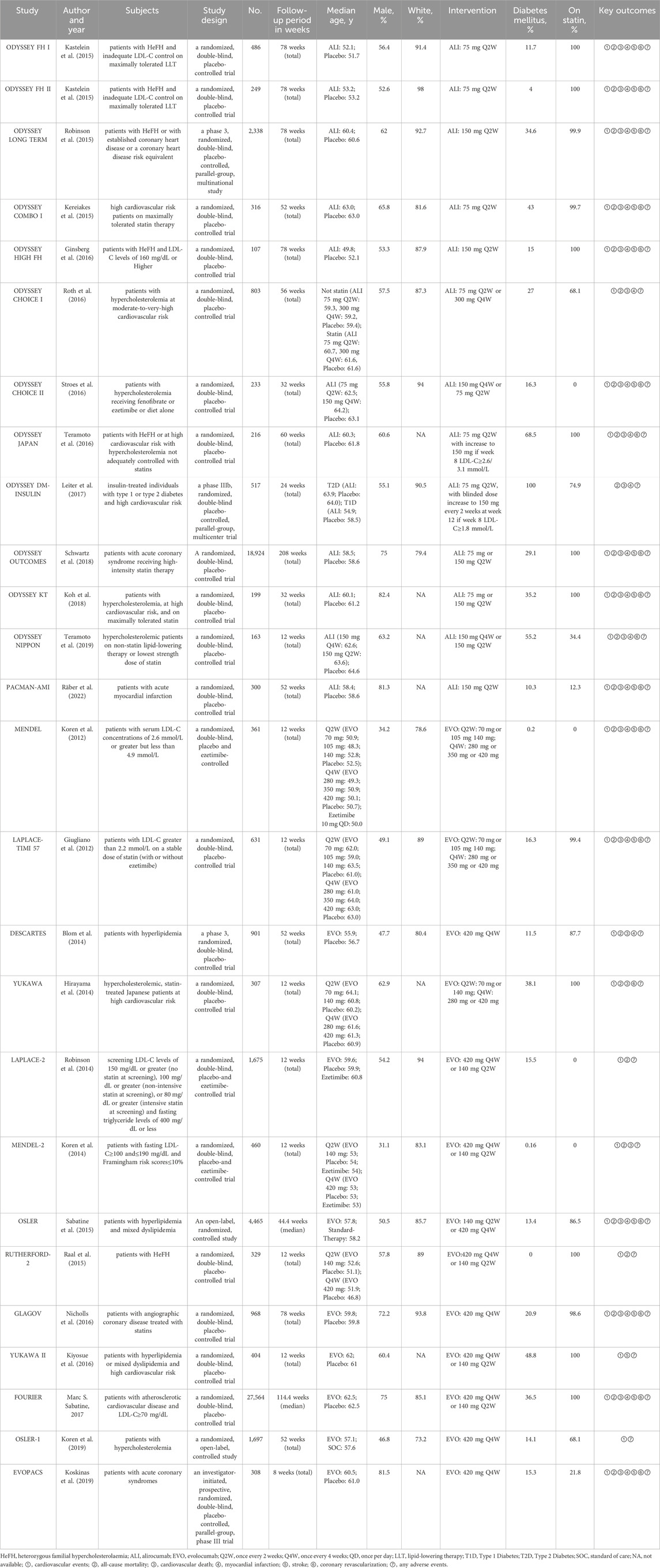
This study enrolled data from 26 studies with 64,921 participants (107–27,564 patients per study) (Kastelein et al., 2015; Robinson et al., 2015; Kereiakes et al., 2015; Ginsberg et al., 2016; Roth et al., 2016; Stroes et al., 2016; Teramoto et al., 2016; Leiter et al., 2017; Schwartz et al., 2018; Koh et al., 2018; Teramoto et al., 2019; Räber et al., 2022; Koren et al., 2012; Giugliano et al., 2012; Blom et al., 2014; Hirayama et al., 2014; Robinson et al., 2014; Koren et al., 2014; Sabatine et al., 2015; Raal et al., 2015; Nicholls et al., 2016; Kiyosue et al., 2016; Sabatine et al., 2017; Koren et al., 2019; Koskinas et al., 2019). The median age of the participants was similar across all studies (Table 1). The doses of alirocumab and evolocumab varied. The dosage for alirocumab were 75 mg Q2W, 150 mg Q2W, 150 mg Q4W, or 300 mg Q4W, and the dosage for evolocumab were 70 mg Q2W, 105 mg Q2W, 140 mg Q2W, 280 mg Q4W, 350 mg Q4W, or 420 mg Q4W (Table 1). Figure 2 shows a network of eligible treatment comparisons for major cardiovascular events.
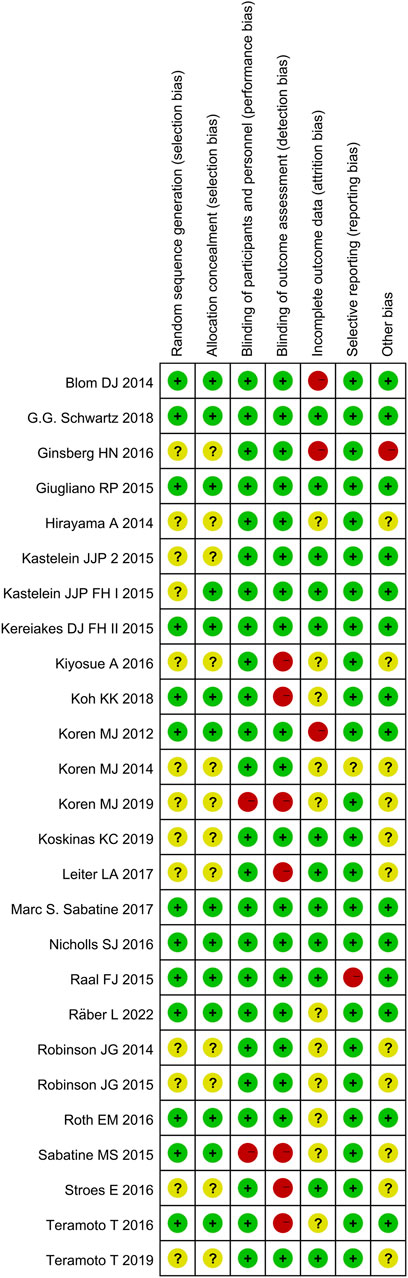
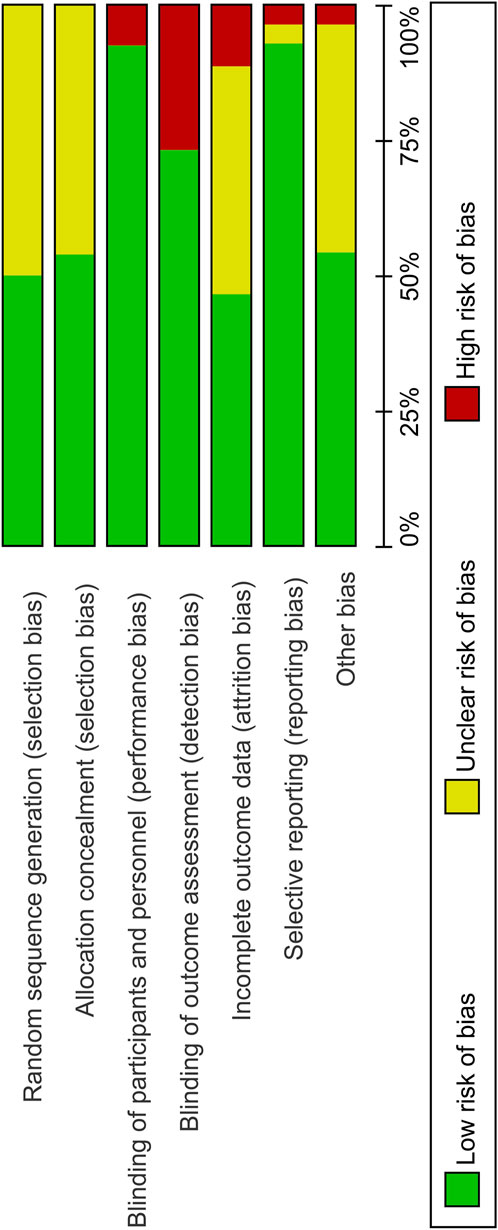
3.3 Risk of bias for the included studies
The quality of included studies was assessed using the Cochrane Risk of Bias Tool. A low risk of bias was identified across several domains because all the included studies were randomized and majority of the studies were double-blinded for both participants and personnel. There were fewer instances of selective reporting. However, approximately half of the studies did not adequately address random sequence generation and allocation concealment, which are indicators of selection bias.
The risk of bias summary and graph for the included studies are shown in Figures 3, 4, respectively.
3.4 Efficacy endpoints
3.4.1 Major adverse cardiac and cerebrovascular events
This meta-analysis included 25 trials that reported MACCE. As shown in Figure 5A, the risk of MACCE was significantly reduced in patients treated with alirocumab (RR: 0.84, 95% CI: 0.77–0.92) or evolocumab (RR: 0.83, 95% CI: 0.77–0.89). Furthermore, there were no significant differences in the risk of MACCE between patients receiving the two drugs (RR: 0.99, 95%CI: 0.88–1.11).
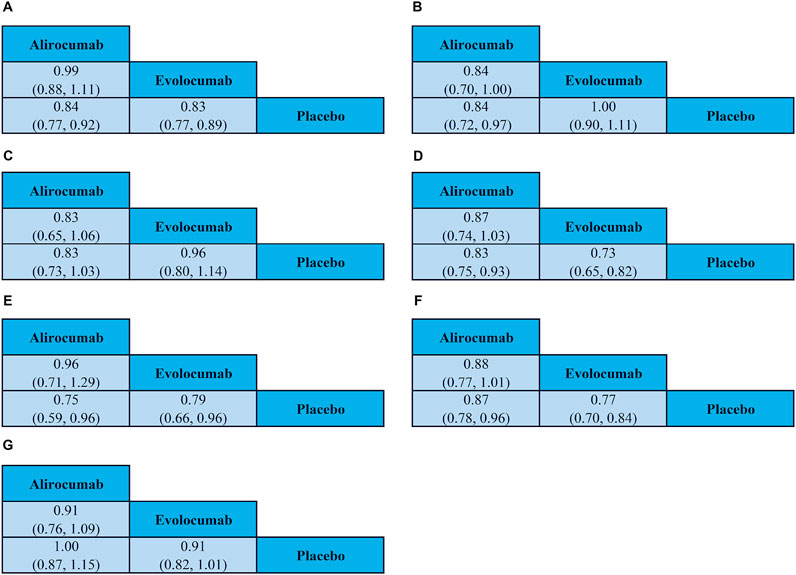
Figure 5. League table highlights the main findings of the outcome analysis. (A) Major adverse cardiac and cerebrovascular events; (B) All-cause mortality; (C) Cardiovascular death; (D) Myocardial infarction; (E) Stroke; (F) Coronary revascularization; (G) Any adverse events. For each comparison, odds ratios and 95% confidence intervals are provided.
3.4.2 All-cause mortality
Twenty-four of the included evaluated all-cause mortality outcomes. As shown in Figure 5B, patients treated with alirocumab demonstrated reduced risk of all-cause mortality (RR: 0.84, 95% CI: 0.72–0.97), but those treated with evolocumab did not show statistically significant decrease in the all-cause mortality events (RR: 1.00, 95% CI: 0.90–1.11). Furthermore, comparative analysis demonstrated that the risk of all-cause mortality was lower in those treated with alirocumab compared to those treated with evolocumab (RR: 0.84, 95% CI: 0.70–1.00). However, since the 95% confidence interval included the null value (1.00), the observed differences did not demonstrate statistical significance and cannot be considered as conclusive evidence of superiority.
3.4.3 Cardiovascular death
Cardiovascular death outcomes were reported in 24 studies. As shown in Figure 5C, we did not observe significant reduction in the cardiovascular death events among patients receiving alirocumab (RR: 0.83, 95% CI: 0.73–1.03) or evolocumab (RR: 0.96, 95% CI: 0.80–1.14). Furthermore, there were no significant differences in the risk of cardiovascular death between patients receiving alirocumab or evolocumab (RR: 0.83, 95% CI: 0.65–1.06).
3.4.4 Myocardial infarction
Myocardial infarction outcomes were reported in 19 studies. As shown in Figure 5D, the risk of myocardial infarction was reduced in patients treated with alirocumab (RR: 0.83, 95% CI: 0.75–0.93) or evolocumab (RR: 0.73, 95% CI: 0.65–0.82). However, we did not observe any statistically significant differences in the risk of myocardial infarction risk between patients receiving the two drugs (RR: 0.87, 95% CI: 0.74–1.03).
3.4.5 Stroke
Stroke outcomes were reported in 15 studies. As shown in Figure 5E, the risk of stroke was lower in those treated with alirocumab (RR: 0.75, 95% CI: 0.59–0.96) or evolocumab (RR: 0.79, 95% CI: 0.66–0.96). However, we did not observe any statistically significant difference in the risk of stroke between patients receiving the two drugs (RR: 0.96, 95% CI: 0.71–1.29).
3.4.6 Coronary revascularization
Coronary revascularization outcomes were evaluated by 18 studies. As shown in Figure 5F, patients treated with alirocumab (RR: 0.87, 95%: CI 0.78–0.96) or evolocumab (RR: 0.77, 95% CI: 0.70–0.84) were associated with a lower risk of coronary revascularization. However, the risk of coronary revascularization was comparable between those treated with alirocumab or evolocumab (RR 0.88, 95% CI 0.77–1.01).
3.5 Safety endpoints
Safety outcomes were reported in all the included studies. As shown in Figure 5G, there was no significant difference in the incidence rates of adverse events between those treated with evolocumab or alirocumab. Furthermore, there were no significant differences in the risk of adverse events leading to treatment discontinuation between patients treated with evolocumab or alirocumab (RR: 0.91, 95% CI: 0.76–1.09).
3.6 Analysis of publication bias
The funnel plots for all the studies are shown in Figures 6A–G. These plots were visually symmetrical. The results of Begg’s test, Egger’s test, and Thompson-Sharp’s test did not demonstrate any evidence of publication bias (P ≥ 0.05).
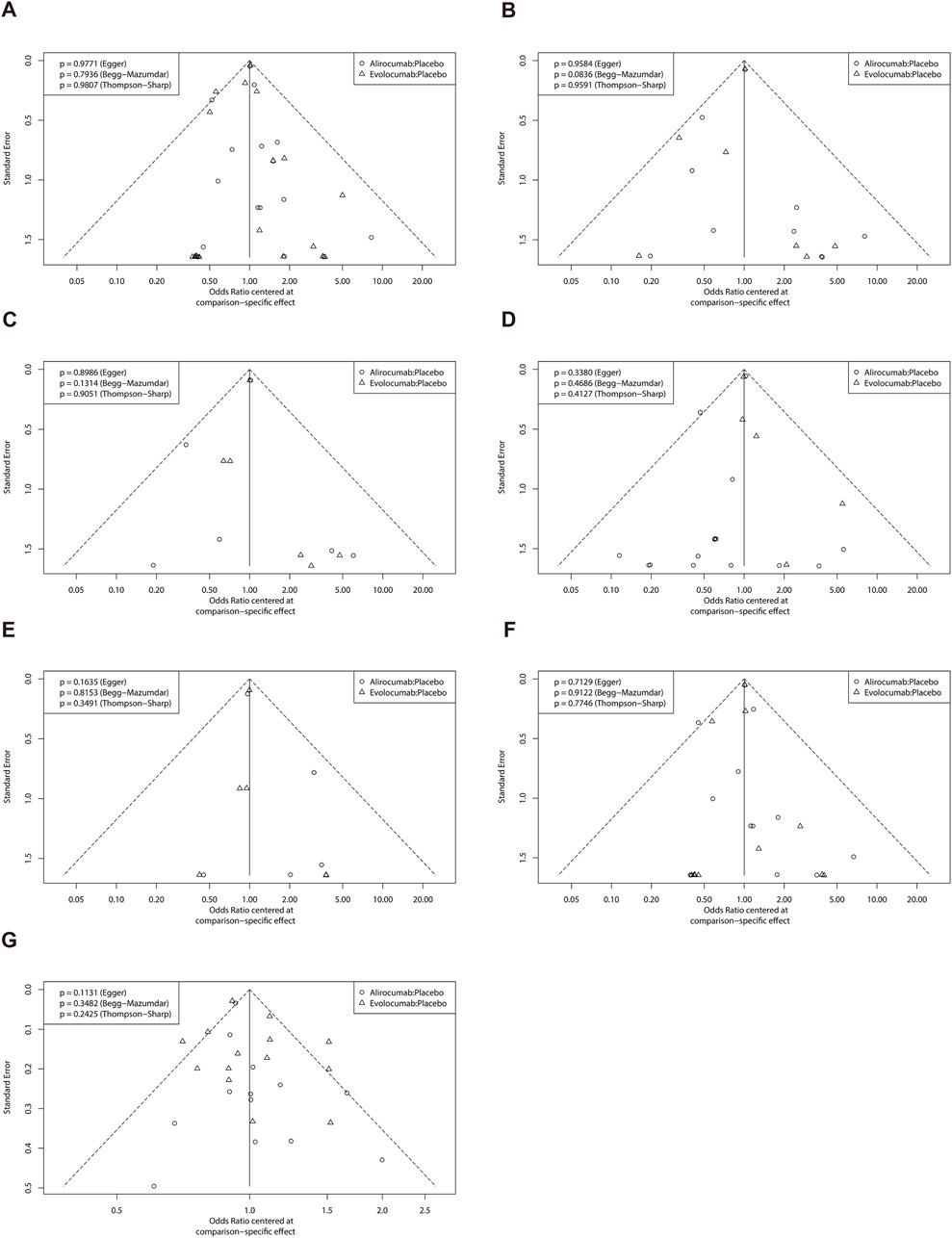
Figure 6. Funnel plot of efficacy and safety endpoints. (A) Major adverse cardiac and cerebrovascular events; (B) All-cause mortality; (C) Cardiovascular death; (D) Myocardial infarction; (E) Stroke; (F) Coronary revascularization; (G) Any adverse events.
4 Discussion
In this systematic review and meta-analysis, we analyzed data from 26 separate RCTs to compare the efficacy and safety of alirocumab and evolocumab in reducing major cardiovascular events among ASCVD patients. Both alirocumab and evolocumab showed comparable efficacy in reducing MACCE, cardiovascular death, myocardial infarction, stroke, and coronary revascularization. The all-cause death rates were lower in patients treated with alirocumab compared to those treated with evolucumab, but the differences were not statistically significant. Furthermore, there were not significant differences between the two drugs in terms of safety endpoints. To the best of our knowledge, this is the first network meta-analysis that indirectly evaluated the effectiveness and safety of alirocumab and evolocumab on major cardiovascular events.
PCSK9i reduce the risk of developing ASCVD through LDLR-dependent and LDLR-independent mechanisms, including inflammation, plaque formation, and thrombosis (Luquero et al., 2021; Hummelgaard et al., 2023). In 2015, two antibody-based PCSK9i─alirocumab and evolocumab─were approved by the FDA and the European Medicines Agency (EMA) (González-Lleó et al., 2024) to reduce cholesterol levels. Several large RCTs have investigated the efficacy and safety of alirocumab (Schwartz et al., 2018; Bittner et al., 2020) and evolocumab (Sabatine et al., 2015; Sabatine et al., 2017) in reducing cardiovascular events. Furthermore, one study performed indirect comparative analysis of the efficacy and safety of alirocumab versus evolocumab (Guedeney et al., 2021), but their effects on major cardiovascular events are not clear. Therefore, we extracted data from large trials in which cardiovascular events and other adverse events were reported, and aimed to improve the evaluation and reporting of the efficacy and safety profiles of alirocumab and evolocumab on major cardiovascular events.
Systematic meta-analysis of data from 26 RCTs demonstrated that alirocumab or evolocumab were associated with a significant reduction of MACCE, myocardial infarction, stroke, or coronary revascularization compared to the placebo control. The FOURIER trial showed that evolocumab significantly improved the composite cardiovascular outcomes among participants with a baseline LDL-C level of ≥70 mg/dL and those with an average baseline LDL-C level of 90 mg/dL (Blom DJ, et al., 2014). The ODYSSEY OUTCOMES trial showed that alirocumab significantly reduced the risk of cardiovascular outcomes in participants with a baseline LDL cholesterol level of ≥100 mg/dL (Schwartz et al., 2018). The current trial showed that both alirocumab and evolocumab were highly effective in reducing major cardiovascular events in the high-risk ASCVD patients. Previous studies reported continued cardiovascular benefit even when LDL cholesterol levels were reduced to levels below the current target range of 20–25 mg/dL (Writing Committee et al., 2016; Landmesser et al., 2017; Sabatine, 2017).
Lowering blood cholesterol levels, especially LDL-C, can significantly reduce the risk of ASCVD, including coronary artery disease, one of the leading causes of death worldwide (Hummelgaard et al., 2023). PCSK9 binds to the epidermal growth factor-like repeat A domain of the LDLR. This interaction is increased by about150-fold under acidic pH conditions in the endosomes. This increased affinity directs the LDLR-PCSK9 complex to lysosomal degradation and prevents its recycling to the cell surface. Heparan sulfate proteoglycans act as co-receptors of PCSK9 on the surface of the hepatocytes and promote depletion of LDLR, thereby elevating plasma LDL-C levels (Park et al., 2024). PCSK9 also contributes to the development of cardiovascular disease in a LDLR-dependent or LDLR-independent manner, and is involved in the promotion of inflammation, plaque development, and thrombosis (Luquero et al., 2021; D’Onofrio et al., 2023). In macrophages, PCSK9 upregulates scavenger receptors (SRA, CD36, and LOX-1), and promotes uptake of oxidized LDL and secretion of pro-inflammatory cytokines. PCSK9i mitigate thrombosis risk by reducing platelet activation and neutrophil extracellular trap formation (Hummelgaard et al., 2023). Large cohort RCTs have demonstrated the effectiveness of these two drugs in lowering the risk of composite cardiovascular outcomes, including cardiovascular death, myocardial infarction, stroke, and unstable angina (Sabatine et al., 2017; Schwartz et al., 2018). The efficacy and safety of alirocumab and evolocumab has been further validated in real-world settings. A multicenter observational study involving 798 patients confirmed that both drugs were safe and effective in clinical practice and demonstrated high treatment adherence and persistence, with most patients achieving the guideline-recommended LDL-C target levels (Gargiulo et al., 2023). Furthermore, intensive and early lipid-lowering therapy with PCSK9i was safe and effective in acute coronary syndrome (ACS) patients (strike early-strike strong strategy) and associated with reduced residual cardiovascular risk (Gargiulo et al., 2024). Previous meta-analyses have primarily assessed the efficacy of PCSK9i through direct comparisons with the placebo (Dicembrini et al., 2019; Imbalzano et al., 2023). Few meta-analyses have indirectly compared alirocumab with evolocumab (Guedeney et al., 2021; Wang et al., 2022). This meta-analysis aimed to update the comparison of the efficacy and safety profiles of alirocumab and evolocumab by incorporating data from several new RCTs that have been published regarding the efficacy and safety of these two PCSK9i.
Regarding all-cause mortality, our findings were consistent with those reported in an earlier meta-analysis, which indirectly compared the efficacy and safety of these two agents (Guedeney et al., 2021). Alirocumab was associated with a lower relative risk of all-cause mortality compared to evolocumab but the differences were not statistically significant. This may be attributed to differences in the sample size and follow-up duration between different studies. According to the cochrane handbook for systematic reviews of interventions, we enrolled a minimum of 100 participants in order to decrease the standard error. However, the sample size with a minimum of 100 participants can influence study outcomes by introducing biases. In addition, the duration of the study is one of the important study design description of each included study. We collected the varying treatment durations (ranging from 8 to 208 weeks) to facilitate assessment of the risk of bias in each included study. Furthermore, this probably due to heterogeneity in the design of the ODYSSEY OUTCOMES trial. In the ODYSSEY OUTCOMES trial, while key secondary endpoints were examined via a hierarchical statistical approach to control type I error. The analysis of all-cause mortality was outside of this formal hierarchical testing approach. Therefore, the results were considered exploratory. Our data showed that all-cause mortality rates between the two drugs were not statistically significant. This may be a result of including clinical trials that assessed both cardiovascular events and adverse events. Furthermore, we evaluated a higher number of patients receiving evolocumab (n = 22,048) than in the previous study (n = 17,931). The observed difference in mortality trend requires cautious interpretation because we cannot fully exclude residual confounding from heterogeneity in the trial population. Another meta-analysis failed to identify an overall mortality advantage associated with the use of PCSK9i. However, alirocumab was associated with a lower risk of all-cause mortality when compared with the placebo control, but this effect was not observed with evolocumab (Guedeney et al., 2019). Furthermore, a previous study reported that evolocumab was associated with higher all-cause mortality compared to alirocumab, but the reasons for this phenomenon have not been thoroughly discussed (Wang et al., 2022).
There are concerns regarding the long-term safety outcomes of alirocumab and evolocumab. The current study did not identify overall safety issues with either drug, but two studies with the highest follow-up duration were only 2.2 years and 2.8 years (Sabatine et al., 2017; Schwartz et al., 2018). In the open-label, long-term FOURIER-OLE trial, all participants (n = 6,635) were treated with evolocumab for a median follow-up of 5.0 years. The maximum exposure to evolocumab in this trial was 8.4 years. The sustained reduction in the LDL-C levels with evolocumab was associated with reduced adverse event rates for a duration of over 8 years, and did not exceed the adverse event rates observed in the original placebo group during the parent study (O’Donoghue et al., 2022). Another clinical trial with a median follow-up period of 3.3 years enrolled patients who participated in the ODYSSEY OUTCOMES study with follow-up ranging from 3 to 5 years (n = 8,242). In this trial, the incidence rates of new-onset diabetes, worsening or complications of diabetes, and neurocognitive events were comparable between the alirocumab and placebo groups. The tolerability profile of alirocumab was comparable with the placebo, except for an a slight increase in reactions at the local injection site. During a follow-up period of 4 years, the overall occurrence of the first local injection site reaction was less than 5%, and most reactions occurred within the first 6 months (Goodman et al., 2023). The EBBINGHAUS trial specifically assessed neurocognitive safety using validation tools and demonstrated no significant differences in executive function, working memory, or psychomotor speed between evolocumab and placebo groups over a period of 19 months (Da Dalt et al., 2025). Extended follow-up in the EBBINGHAUS-OLE trial for 5.1 years further confirmed the absence of neurocognitive impairment in the ASCVD patients even when the LDL-C levels were maintained below 20 mg/dL (Zimerman et al., 2025). Several clinical trials have also demonstrated that treatment with alirocumab did not induce neurocognitive dysfunction (Kastelein et al., 2015; Leiter et al., 2017; Janik et al., 2021). Treatment with alirocumab and evolocumab did not increase the risk of hospitalization for congestive heart failure compared to placebo (1.9% vs. 1.9% with alirocumab in ODYSSEY OUTCOMES, and 2.9% vs. 3.0% with evolocumab in FOURIER) (Sabatine et al., 2017; Schwartz et al., 2018). In the extended follow-up of patients from the ODYSSEY LONG TERM study, congestive heart failure requiring hospitalization occurred in 0.6% of patients in the alirocumab group and 0.4% of patients in the placebo group, and the incidences of non-ischemic cardiac diseases were comparable between the two groups (Goodman et al., 2023). This suggested that PCSK9i were safe, effective, and well-tolerated lipid-lowering therapeutics. Long-term maintenance of very low levels of LDL-C below 20 mg per deciliter (0.5 mmol per liter) are associated with reduced risk of adverse cardiovascular events in patients with ASCVD, and these very low LDL-C levels do not show any significant safety concerns (Gaba et al., 2023). However, in primary prevention, the relative therapeutic efficacy of lowering LDL-C may decrease with age (Burger et al., 2024). It is a matter of debate whether lower LDL-C levels are associated with significant adverse clinical outcomes such as hemorrhagic stroke or new-onset diabetes. A recent review evaluated familial genetic conditions associated with lifelong, very low LDL-C levels (<30 mg/dL) and observed severe neurocognitive impairment and hepatic steatosis in abetalipoproteinemia and familial hypobetalipoproteinemia, respectively (Karagiannis et al., 2021). However, these complications were caused by mechanisms that were not related with extremely low LDL-C levels. Conversely, individuals with loss of function PCSK9 mutations or familial combined hypolipidemia maintain lifelong low LDL-C levels for decades. Individuals with loss-of-function PCSK9 mutations are healthy and do not show evidence of neurocognitive impairment, increased incidence of diabetes, cataracts, or stroke. This highlights that different genetic causes of low LDL-C levels can lead to distinct health outcomes (Karagiannis et al., 2021).
This study has a few limitations. First, this study indirectly compared the efficacy and safety of alirocumab and evolocumab because head-to-head RCT of these two drugs has not been conducted yet. Therefore, we included trials in which alirocumab or evolocumab were compared with a control group. Furthermore, clinical trials are necessary to evaluate the efficacy and safety of different types of PCSK9i in the future. Second, the experimental design varied between the studies included in this meta-analysis, especially regarding the inclusion and exclusion criteria. Third, the duration of follow-up varied between studies. For example, the follow-up duration in the EVOPACS trial was shortest among the included studies at 8 weeks, and whereas the mean follow-up duration in the FOURIER trial was 114.4 weeks. Differences in follow-up duration may introduce heterogeneity and potentially affect the effect size. Fourth, MACCE was not reported in one study. Therefore, we calculated MACCE based on the sub-outcomes reported in studies. This reconstruction approach may introduce potential biases, including variations in event definitions across different studies and the possibility of double-counting the same event. Although we made rigorous efforts to prevent duplicate counting, the results still need to be interpreted with caution. Fifth, only two types of PCSK9i were analyzed in this study. In the future, we plan to compare these with other PCSK9i or other novel lipid-lowering therapies to provide more comprehensive analysis for the clinical application of lipid-lowering regimens. Finally, most of the included trials have been previously included in earlier systematic reviews and meta-analyses of RCTs that compare alirocumab or evolocumab with the control groups. This limits the novelty of this study.
5 Conclusion
This updated network meta-analysis demonstrated that alirocumab and evolocumab shared a similar efficacy profile in reducing LDL-C levels. Although all-cause mortality rates were lower in ASCVD patients treated with alirocumab compared to those treated with evolocumab (RR 0.84, 95% CI 0.70–1.00), but the difference was not statistically significant. There were no significant differences in other safety endpoints between the two drugs. Compared to the placebo, both these drugs were associated with lower relative risk for MACCE, myocardial infarction, stroke, and coronary revascularization. In the future, RCTs are required to directly assess the efficacy of these two drugs on major cardiovascular events to confirm these findings and provide evidence-based guidance for clinical management.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
LX: Writing – original draft, Writing – review and editing, Data curation, Formal Analysis. ML: Formal Analysis, Writing – original draft, Writing – review and editing. LL: Project administration, Writing – review and editing. YL: Conceptualization, Writing – review and editing. CG: Methodology, Writing – review and editing, Formal Analysis, Writing – original draft. PZ: Methodology, Writing – review and editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from the Foundation of GuangDong Pharmaceutical Association (No. 2023JZ04) to PZ, the Foundation of GuangDong Pharmaceutical Association (No. 2022-1115-25) to LL, the President Foundation of Nanfang Hospital, Southern Medical University (No. 2022A006), and GuangDong Basic and Applied Basic Research Foundation (No. 2023A1515110921) to CG.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Banach, M., Cannon, C. P., Paneni, F., and Penson, P. E.endorsed by the International Lipid Expert Panel (ILEP) (2023). Individualized therapy in statin intolerance: the key to success. Eur. Heart J. 44, 544–546. doi:10.1093/eurheartj/ehac556
Bhatia, H. S., Wandel, S., Willeit, P., Lesogor, A., Bailey, K., Ridker, P. M., et al. (2024). Independence of lipoprotein(a) and low-density lipoprotein cholesterol-mediated cardiovascular risk: a participant-level meta-analysis. Circulation 151, 312–321. doi:10.1161/CIRCULATIONAHA.124.069556
Bittner, V. A., Szarek, M., Aylward, P. E., Bhatt, D. L., Diaz, R., Edelberg, J. M., et al. (2020). Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J. Am. Coll. Cardiol. 75, 133–144. doi:10.1016/j.jacc.2019.10.057
Blom, D. J., Hala, T., Bolognese, M., Lillestol, M. J., Toth, P. D., Burgess, L., et al. (2014). A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N. Engl. J. Med. 370, 1809–1819. doi:10.1056/NEJMoa1316222
Burger, P. M., Dorresteijn, J. A. N., Koudstaal, S., Holtrop, J., Kastelein, J. J. P., Jukema, J. W., et al. (2024). Course of the effects of LDL-cholesterol reduction on cardiovascular risk over time: a meta-analysis of 60 randomized controlled trials. Atherosclerosis 396, 118540. doi:10.1016/j.atherosclerosis.2024.118540
Da Dalt, L., Baragetti, A., and Norata, G. D. (2025). Targeting PCSK9 beyond the liver: evidence from experimental and clinical studies. Expert Opin. Ther. Targets 29 (3), 137–157. doi:10.1080/14728222.2025.2482545
Diao, J. A., Shi, I., Murthy, V. L., Buckley, T. A., Patel, C. J., Pierson, E., et al. (2024). Projected changes in statin and antihypertensive therapy eligibility with the AHA PREVENT cardiovascular risk equations. JAMA 332, 989–1000. doi:10.1001/jama.2024.12537
Dicembrini, I., Giannini, S., Ragghianti, B., Mannucci, E., and Monami, M. (2019). Effects of PCSK9 inhibitors on LDL cholesterol, cardiovascular morbidity and all-cause mortality: a systematic review and meta-analysis of randomized controlled trials. J. Endocrinol. Invest. 42, 1029–1039. doi:10.1007/s40618-019-01019-4
D’Onofrio, N., Prattichizzo, F., Marfella, R., Sardu, C., Martino, E., Scisciola, L., et al. (2023). SIRT3 mediates the effects of PCSK9 inhibitors on inflammation, autophagy, and oxidative stress in endothelial cells. Theranostics 13, 531–542. doi:10.7150/thno.80289
Gaba, P., O’Donoghue, M. L., Park, J.-G., Wiviott, S. D., Atar, D., Kuder, J. F., et al. (2023). Association between achieved low-density lipoprotein cholesterol levels and long-term cardiovascular and safety outcomes: an analysis of FOURIER-OLE. Circulation 147, 1192–1203. doi:10.1161/CIRCULATIONAHA.122.063399
Gargiulo, P., Basile, C., Cesaro, A., Marzano, F., Buonocore, D., Asile, G., et al. (2023). Efficacy, safety, adherence and persistence of PCSK9 inhibitors in clinical practice: a single country, multicenter, observational study (AT-TARGET-IT). Atherosclerosis 366, 32–39. doi:10.1016/j.atherosclerosis.2023.01.001
Gargiulo, P., Basile, C., Galasso, G., Bellino, M., D’Elia, D., Patti, G., et al. (2024). Strike early-strike strong lipid-lowering strategy with proprotein convertase subtilisin/kexin type 9 inhibitors in acute coronary syndrome patients: real-world evidence from the AT-TARGET-IT registry. Eur. J. Prev. Cardiol. 31, 1806–1816. doi:10.1093/eurjpc/zwae170
Ginsberg, H. N., Rader, D. J., Raal, F. J., Guyton, J. R., Baccara-Dinet, M. T., Lorenzato, C., et al. (2016). Efficacy and safety of alirocumab in patients with heterozygous familial hypercholesterolemia and LDL-C of 160 mg/dl or higher. Cardiovasc Drugs Ther. 30, 473–483. doi:10.1007/s10557-016-6685-y
Giugliano, R. P., Desai, N. R., Kohli, P., Rogers, W. J., Somaratne, R., Huang, F., et al. (2012). Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet 380 (9858), 2007–2017. doi:10.1016/S0140-6736(12)61770-X
González-Lleó, A. M., Sánchez-Hernández, R. M., Plana, N., Ibarretxe, D., Rehues, P., Ribalta, J., et al. (2024). Impact of PCSK9 inhibitors in glycaemic control and new-onset diabetes. Cardiovasc Diabetol. 23, 4. doi:10.1186/s12933-023-02077-y
Goodman, S. G., Steg, P. G., Poulouin, Y., Bhatt, D. L., Bittner, V. A., Diaz, R., et al. (2023). Long-term efficacy, safety, and tolerability of alirocumab in 8242 patients eligible for 3 to 5 years of placebo-controlled observation in the ODYSSEY OUTCOMES trial. J. Am. Heart Assoc. 12, e029216. doi:10.1161/JAHA.122.029216
Grundy, S. M., Stone, N. J., Bailey, A. L., Beam, C., Birtcher, K. K., Blumenthal, R. S., et al. (2019). 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 73, e285–e350. doi:10.1016/j.jacc.2018.11.003
Guedeney, P., Giustino, G., Sorrentino, S., Claessen, B. E., Camaj, A., Kalkman, D. N., et al. (2019). Efficacy and safety of alirocumab and evolocumab: a systematic review and meta-analysis of randomized controlled trials. Eur. Heart J. 43, e17–e25. doi:10.1093/eurheartj/ehz430
Guedeney, P., Sorrentino, S., Giustino, G., Chapelle, C., Laporte, S., Claessen, B. E., et al. (2021). Indirect comparison of the efficacy and safety of alirocumab and evolocumab: a systematic review and network meta-analysis. Eur. Heart J. Cardiovasc Pharmacother. 7, 225–235. doi:10.1093/ehjcvp/pvaa024
Hirayama, A., Honarpour, N., Yoshida, M., Yamashita, S., Huang, F., Wasserman, S. M., et al. (2014). Effects of evolocumab (AMG 145), a monoclonal antibody to PCSK9, in hypercholesterolemic, statin-treated Japanese patients at high cardiovascular risk: – primary results from the Phase 2 YUKAWA study. Circ. J. 78, 1073–1082. doi:10.1253/circj.CJ-14-0130
Hummelgaard, S., Vilstrup, J. P., Gustafsen, C., Glerup, S., and Weyer, K. (2023). Targeting PCSK9 to tackle cardiovascular disease. Pharmacol. Ther. 249, 108480. doi:10.1016/j.pharmthera.2023.108480
Imbalzano, E., Ilardi, F., Orlando, L., Pintaudi, B., Savarese, G., and Rosano, G. (2023). The efficacy of PCSK9 inhibitors on major cardiovascular events and lipid profile in patients with diabetes: a systematic review and meta-analysis of randomized controlled trials. Eur. Heart J. - Cardiovasc. Pharmacother. 9, 318–327. doi:10.1093/ehjcvp/pvad019
Janik, M. J., Urbach, D. V., van Nieuwenhuizen, E., Zhao, J., Yellin, O., Baccara-Dinet, M. T., et al. (2021). Alirocumab treatment and neurocognitive function according to the CANTAB scale in patients at increased cardiovascular risk: a prospective, randomized, placebo-controlled study. Atherosclerosis 331, 20–27. doi:10.1016/j.atherosclerosis.2021.06.913
Kastelein, J. J. P., Ginsberg, H. N., Langslet, G., Hovingh, G. K., Ceska, R., Dufour, R., et al. (2015). ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur. Heart J. 36, 2996–3003. doi:10.1093/eurheartj/ehv370
Kereiakes, D. J., Robinson, J. G., Cannon, C. P., Lorenzato, C., Pordy, R., Chaudhari, U., et al. (2015). Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am. Heart J. 169, 906–915. doi:10.1016/j.ahj.2015.03.004
Kiyosue, A., Honarpour, N., Kurtz, C., Xue, A., Wasserman, S. M., and Hirayama, A. (2016). A phase 3 study of evolocumab (AMG 145) in statin-treated Japanese patients at high cardiovascular risk. Am. J. Cardiol. 117, 40–47. doi:10.1016/j.amjcard.2015.10.021
Koh, K. K., Nam, C. W., Chao, T.-H., Liu, M.-E., Wu, C.-J., Kim, D.-S., et al. (2018). A randomized trial evaluating the efficacy and safety of alirocumab in South Korea and Taiwan (ODYSSEY KT). J. Clin. Lipidol. 12, 162–172. doi:10.1016/j.jacl.2017.09.007
Koren, M. J., Lundqvist, P., Bolognese, M., Neutel, J. M., Monsalvo, M. L., Yang, J., et al. (2014). Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J. Am. Coll. Cardiol. 63, 2531–2540. doi:10.1016/j.jacc.2014.03.018
Koren, M. J., Sabatine, M. S., Giugliano, R. P., Langslet, G., Wiviott, S. D., Ruzza, A., et al. (2019). Long-term efficacy and safety of evolocumab in patients with hypercholesterolemia. J. Am. Coll. Cardiol. 74, 2132–2146. doi:10.1016/j.jacc.2019.08.1024
Koren, M. J., Scott, R., Kim, J. B., Knusel, B., Liu, T., Lei, L., et al. (2012). Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet 380 (9858), 1995–2006. doi:10.1016/S0140-6736(12)61771-1
Koskinas, K. C., Windecker, S., Pedrazzini, G., Mueller, C., Cook, S., Matter, C. M., et al. (2019). Evolocumab for early reduction of LDL cholesterol levels in patients with acute coronary syndromes (EVOPACS). J. Am. Coll. Cardiol. 74, 2452–2462. doi:10.1016/j.jacc.2019.08.010
Landmesser, U., Chapman, M. J., Farnier, M., Gencer, B., Gielen, S., Hovingh, G. K., et al. (2017). European Society of Cardiology/European Atherosclerosis Society Task Force consensus statement on proprotein convertase subtilisin/kexin type 9 inhibitors: practical guidance for use in patients at very high cardiovascular risk. Eur. Heart J. 38, 2245–2255. doi:10.1093/eurheartj/ehw480
Leiter, L. A., Cariou, B., Müller-Wieland, D., Colhoun, H. M., Del Prato, S., Tinahones, F. J., et al. (2017). Efficacy and safety of alirocumab in insulin-treated individuals with type 1 or type 2 diabetes and high cardiovascular risk: the ODYSSEY DM-INSULIN randomized trial. Diabetes Obes. Metab. 19, 1781–1792. doi:10.1111/dom.13114
Luquero, A., Badimon, L., and Borrell-Pages, M. (2021). PCSK9 functions in atherosclerosis are not limited to plasmatic LDL-cholesterol regulation. Front. Cardiovasc Med. 8, 639727. doi:10.3389/fcvm.2021.639727
Mhaimeed, O., Burney, Z. A., Schott, S. L., Kohli, P., Marvel, F. A., and Martin, S. S. (2024). The importance of LDL-C lowering in atherosclerotic cardiovascular disease prevention: lower for longer is better. Am. J. Prev. Cardiol. 18, 100649. doi:10.1016/j.ajpc.2024.100649
Michaeli, D. T., Michaeli, J. C., Boch, T., and Michaeli, T. (2022). Cost-effectiveness of icosapent ethyl, evolocumab, alirocumab, ezetimibe, or fenofibrate in combination with statins compared to statin monotherapy. Clin. Drug Investig. 42, 643–656. doi:10.1007/s40261-022-01173-3
Nicholls, S. J., Puri, R., Anderson, T., Ballantyne, C. M., Cho, L., Kastelein, J. J. P., et al. (2016). Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA 316, 2373–2384. doi:10.1001/jama.2016.16951
O’Donoghue, M. L., Giugliano, R. P., Wiviott, S. D., Atar, D., Keech, A., Kuder, J. F., et al. (2022). Long-term evolocumab in patients with established atherosclerotic cardiovascular disease. Circulation 146, 1109–1119. doi:10.1161/CIRCULATIONAHA.122.061620
Park, C. S., Yang, H.-M., Han, K., Lee, H.-S., Kang, J., Han, J.-K., et al. (2024). J-shaped association between LDL cholesterol and cardiovascular events: a longitudinal primary prevention cohort of over 2.4 million people nationwide. J. Adv. Res. 58, 139–147. doi:10.1016/j.jare.2023.05.003
Raal, F. J., Stein, E. A., Dufour, R., Turner, T., Civeira, F., Burgess, L., et al. (2015). PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet 385 (9965), 331–340. doi:10.1016/S0140-6736(14)61399-4
Räber, L., Ueki, Y., Otsuka, T., Losdat, S., Häner, J. D., Lonborg, J., et al. (2022). Effect of alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with acute myocardial infarction: the PACMAN-AMI randomized clinical trial. JAMA 327, 1771–1781. doi:10.1001/jama.2022.5218
Ray, K. K., Molemans, B., Schoonen, W. M., Giovas, P., Bray, S., Kiru, G., et al. (2021). EU-Wide cross-sectional observational study of lipid-modifying therapy use in secondary and primary care: the DA VINCI study. Eur. J. Prev. Cardiol. 28, 1279–1289. doi:10.1093/eurjpc/zwaa047
Robinson, J. G., Farnier, M., Krempf, M., Bergeron, J., Luc, G., Averna, M., et al. (2015). Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 372, 1489–1499. doi:10.1056/NEJMoa1501031
Robinson, J. G., Nedergaard, B. S., Rogers, W. J., Fialkow, J., Neutel, J. M., Ramstad, D., et al. (2014). Effect of evolocumab or ezetimibe added to moderate-or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA 311, 1870–1882. doi:10.1001/jama.2014.4030
Roth, E. M., Moriarty, P. M., Bergeron, J., Langslet, G., Manvelian, G., Zhao, J., et al. (2016). A phase III randomized trial evaluating alirocumab 300 mg every 4 weeks as monotherapy or add-on to statin: ODYSSEY CHOICE I. Atherosclerosis 254, 254–262. doi:10.1016/j.atherosclerosis.2016.08.043
Sabatine, M. S. (2017). Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors: comparing and contrasting guidance across the Atlantic. Eur. Heart J. 38 (29), 2256–2258. doi:10.1093/eurheartj/ehw572
Sabatine, M. S., Giugliano, R. P., Keech, A. C., Honarpour, N., Wiviott, S. D., Murphy, S. A., et al. (2017). Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 376, 1713–1722. doi:10.1056/NEJMoa1615664
Sabatine, M. S., Giugliano, R. P., Wiviott, S. D., Raal, F. J., Blom, D. J., Robinson, J., et al. (2015). Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 372, 1500–1509. doi:10.1056/NEJMoa1500858
Schreuder, M. M., Hamkour, S., Siegers, K. E., Holven, K. B., Johansen, A. K., van de Ree, M. A., et al. (2023). LDL cholesterol targets rarely achieved in familial hypercholesterolemia patients: a sex and gender-specific analysis. Atherosclerosis 384, 117117. doi:10.1016/j.atherosclerosis.2023.03.022
Schwartz, G. G., Steg, P. G., Szarek, M., Bhatt, D. L., Bittner, V. A., Diaz, R., et al. (2018). Alirocumab and cardiovascular outcomes after acute coronary syndrome. N. Engl. J. Med. 379, 2097–2107. doi:10.1056/NEJMoa1801174
Stroes, E., Guyton, J. R., Lepor, N., Civeira, F., Gaudet, D., Watts, G. F., et al. (2016). Efficacy and safety of alirocumab 150 mg every 4 weeks in patients with hypercholesterolemia not on statin therapy: the ODYSSEY CHOICE II study. JAHA 5, e003421. doi:10.1161/JAHA.116.003421
Teramoto, T., Kiyosue, A., Ishigaki, Y., Harada-Shiba, M., Kawabata, Y., Ozaki, A., et al. (2019). Efficacy and safety of alirocumab 150 mg every 4 weeks in hypercholesterolemic patients on non-statin lipid-lowering therapy or lowest strength dose of statin: ODYSSEY NIPPON. J. Cardiol. 73, 218–227. doi:10.1016/j.jjcc.2018.10.004
Teramoto, T., Kobayashi, M., Tasaki, H., Yagyu, H., Higashikata, T., Takagi, Y., et al. (2016). Efficacy and safety of alirocumab in Japanese patients with heterozygous familial hypercholesterolemia or at high cardiovascular risk with hypercholesterolemia not adequately controlled with statins – ODYSSEY Japan randomized controlled trial. Circ. J. 80, 1980–1987. doi:10.1253/circj.CJ-16-0387
van de Borne, P., Peeters, A., Janssens, L., Leone, A., Lemmens, R., Verhaegen, A., et al. (2024). Lipid-lowering therapy and risk-based LDL-C goal attainment in Belgium: DA VINCI observational study. Acta Cardiol. 79, 20–29. doi:10.1080/00015385.2022.2030568
Visseren, F. L. J., Mach, F., Smulders, Y. M., Carballo, D., Koskinas, K. C., Bäck, M., et al. (2022). 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: developed by the task force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Rev. Esp. Cardiol. Engl. Ed. 75, 429. doi:10.1016/j.rec.2022.04.003
Wang, X., Wen, D., Chen, Y., Ma, L., and You, C. (2022). PCSK9 inhibitors for secondary prevention in patients with cardiovascular diseases: a bayesian network meta-analysis. Cardiovasc Diabetol. 21, 107. doi:10.1186/s12933-022-01542-4
Writing Committee Lloyd-Jones, D. M., Morris, P. B., Ballantyne, C. M., Birtcher, K. K., Daly, D. D., et al. (2016). 2016 acc expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American college of Cardiology task force on clinical expert consensus documents. J. Am. Coll. Cardiol. 68, 92–125. doi:10.1016/j.jacc.2016.03.519
Keywords: alirocumab, evolocumab, PCSK9 inhibitors, cardiovascular events, efficacy, safety, network meta-analysis
Citation: Xu L, Lei M, Li L, Li Y, Gu C and Zheng P (2025) Indirect comparison of the efficacy and safety of alirocumab and evolocumab on major cardiovascular events: a systematic review and network meta-analysis. Front. Pharmacol. 16:1555508. doi: 10.3389/fphar.2025.1555508
Received: 04 January 2025; Accepted: 10 June 2025;
Published: 24 June 2025.
Edited by:
Federica Fogacci, University of Bologna, ItalyReviewed by:
Federica Marzano, University of Naples Federico II, ItalyAndrea Baragetti, University of Milan, Italy
Shengkai Yan, Zunyi Medical University, China
Copyright © 2025 Xu, Lei, Li, Li, Gu and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Zheng, enBtMzIxQDEyNi5jb20=; Chunping Gu, Z2NwMTJAc211LmVkdS5jbg==
†These authors have contributed equally to this work
 Leyu Xu1,2†
Leyu Xu1,2† Yilei Li
Yilei Li Chunping Gu
Chunping Gu