- Department of Pharmacy, Xiamen Cardiovascular Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, China
Objective: This study aimed to systematically and scientifically investigate the potential associations between the use of fluoroquinolone antibiotics (ciprofloxacin, levofloxacin, moxifloxacin, ofloxacin, norfloxacin, and delafloxacin) and suicidal thoughts and behaviors using data from the Food and Drug Administration Adverse Event Reporting System (FAERS) database.
Methods: The FAERS database was queried from the first quarter of 2004 to the fourth quarter of 2023. Disproportionality analysis was conducted using the reporting odds ratio (ROR) and empirical Bayes geometric mean (EBGM).
Results: A total of 737 cases of suicidal thoughts and behaviors associated with fluoroquinolones (FQs) were reported in the FAERS database during the study period. Overall, FQs did not demonstrate a disproportionate increase in overall cases of suicidal thoughts and behaviors (ROR: 0.74, 95% CI: 0.69–0.79, P < 0.001; EBGM05: 0.69). Stratified analyses revealed no safety signals for suicidal thoughts and behaviors associated with FQs in either females or males. However, subgroup analyses by age groups demonstrated slightly elevated RORs for suicidal thoughts and behaviors in the <18 years age group (ROR: 1.51, 95% CI: 1.05–2.19, P = 0.03) and the 18–24 years age group (ROR: 2.31, 95% CI: 1.75–3.06, P < 0.001), although the EBGM05s values remained below two in both populations. No significant safety signals were observed in the other age groups.
Conclusion: The analysis of reported cases of suicidal thoughts and behaviors in the FAERS database does not indicate an overall safety signal associated with fluoroquinolones (FQs) at present. Subgroup analysis revealed a slight increase in the RORs for suicidal thoughts and behaviors in the <18 years and the 18–24 years age group; however, no significant safety signal was detected based on the EBGM05s in these populations. Further comprehensive and prospective studies are necessary to confirm and validate these findings.
1 Introduction
Fluoroquinolones (FQs) are a group of antibiotics commonly used to treat a variety of bacterial infections, including urinary tract infections, gastrointestinal infections, respiratory tract infections, sexually transmitted diseases, bacterial bronchitis, pneumonia, sinusitis, septicemia, intra-abdominal infections, joint and bone infections and skin infections (Brar et al., 2020; Choi et al., 2023; Silva et al., 2024; Collins et al., 2022). As the first fully synthetic antibiotics, FQs exert their antimicrobial effects by inhibiting DNA gyrase and topoisomerase IV, thereby disrupting bacterial DNA synthesis and ultimately leading to rapid bacterial cell death (Blondeau, 2004). Owing to their favorable pharmacokinetic characteristics, high oral bioavailability, and wide range of antimicrobial effectiveness, fluoroquinolones were the third most frequently prescribed class of antibiotics in the United States in 2011 (Hicks et al., 2015). Despite their clinical efficacy, FQs are associated with a range of well-documented adverse effects on the gastrointestinal, dermatological, cardiovascular, and nervous systems (Rubinstein, 2001; Huruba et al., 2021; Appelbaum and Hunter, 2000). However, the potential impact of FQs on mental health remains insufficiently understood. Among the reported psychiatric side effects of FQs, suicidal thoughts and behaviors have raised significant concerns. Cases of suicidal ideation, suicide attempts, and completed suicides have been reported following the initiation of FQ therapy (Zhang et al., 2021), (Ahmed et al., 2011; Labay-Kamara et al., 2012; Dyer, 2023).
In August and September 2023, the UK Medicines and Healthcare Products Regulatory Agency (MHRA) issued an alert highlighting the psychiatric risks associated with fluoroquinolones (FQs), including ciprofloxacin, delafloxacin, levofloxacin, moxifloxacin, and ofloxacin, which may lead to suicidal thoughts and behaviors (MHRA, 2024; MHRA, 2024). This alert was prompted by a coroner’s report investigating the death of a respected consultant cardiologist who retired in May 2022 at the age of 63 and tragically died by suicide 11 days after initiating ciprofloxacin treatment for prostatitis symptoms. The coroner emphasized the rare but severe side effects of ciprofloxacin, particularly in patients without a prior history of mental health issues (Dyer, 2023; MHRA, 2024). However, the MHRA alert did not specify whether males or females were more susceptible to the psychiatric risks associated with fluoroquinolones, nor did it provide details on the age range of individuals affected by these risks (MHRA, 2024; MHRA, 2024).Consequently, based on the available information, it is not possible to determine which demographic groups are more vulnerable, underscoring the need for further research to clarify these critical aspects and inform targeted interventions. In light of the widespread global use of fluoroquinolones, it is essential to investigate potential relationships between fluoroquinolones and suicidal thoughts and behaviors. This inquiry is highly important for ensuring public safety and optimizing the clinical use of these widely prescribed antibiotics.
2 Methods
2.1 Data source
The FDA Adverse Event Reporting System (FAERS) is a publicly available database created to support the FDA’s efforts in monitoring the safety of drugs and therapeutic biologic products after they have been marketed (Ding et al., 2022). Reports were evaluated quantitatively through signal detection, with a signal indicating an adverse event (AE) related to a drug. Researchers have started analyzing various drugs and diseases using the FAERS database to identify potential AEs that warrant special attention, with the goal of offering guidance for clinical medication and treatment (Chen et al., 2023a; Chen et al., 2023b). AE symptoms are classified using the internationally recognized and clinically validated Medical Dictionary for Regulatory Activities (MedDRA) terminology (Kumar, 2019). FAERS allows for the analysis of unexpected adverse event trends that may be overlooked in clinical trials owing to limitations in participant diversity (Yu et al., 2021; Xuan et al., 2023).
2.2 Data queries
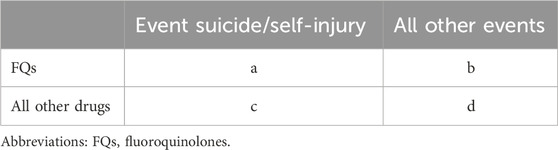
The FAERS database was queried from the first quarter (Q1) of 2004 to the fourth quarter (Q4) of 2023 to analyze disproportionality and investigate the associations between FQs and AEs involving suicidal thoughts and behaviors. Each report was categorized according to the contingency table (Table 1), where “a” denotes the count of suicide reports associated with FQs, “b” indicates the count of reports for FQs that do not involve suicide, “c” represents the number of suicide reports related to all other drugs, and “d” signifies the reports for all other drugs that do not involve suicide. Reports related to FQs were identified when any of the six FQs (ciprofloxacin, levofloxacin, moxifloxacin, ofloxacin, norfloxacin, and delafloxacin) were categorized as either “primary suspected” or “secondary suspected” drugs in the reported cases. Instances of suicide and self-injuries were examined using the MedDRA 27.0 preferred terms (PTs) associated with the standard MedDRA query (SMQ) for “suicide/self-injury.” The specific terminology employed included “assisted suicide,” “abnormal Columbia Suicide Severity Rating Scale”, “completed suicide”, “depression with suicidal tendencies”, “intentional overdose”, “deliberate self-injury”, “intentional poisoning”, “self-injurious thoughts”, “suicidal actions”, “suicidal thoughts”, “suicide attempt”, “suicide threat”, “suspected suicide”, and “suspected suicide attempt”. Using universally recognized and clinically validated Medical Subject Headings (MeSH) terms, we were able to address reports relevant to the objectives of this study effectively. The comprehensive data processing procedure is illustrated in Figure 1.
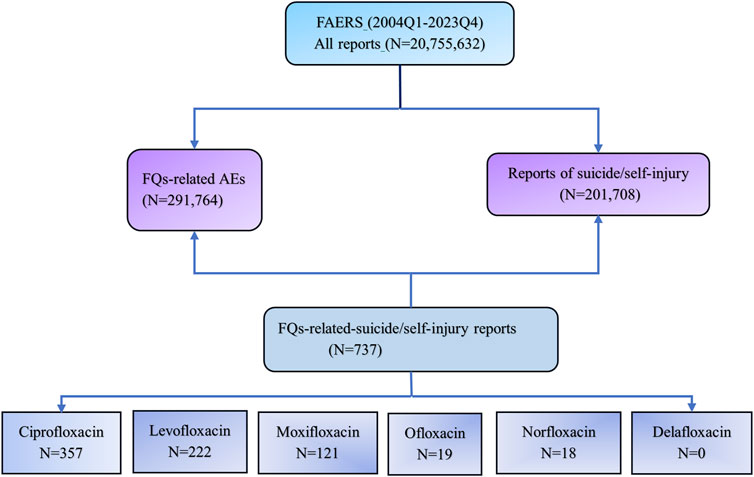
Figure 1. Flow chart of data queries within the FAERS database. FAERS, the U.S. Food and Drug Administration (FDA) Adverse Event Reporting System; Q1, the first quarter; Q4, the fourth quarter; FQs, fluoroquinolones.
2.3 Statistical analysis
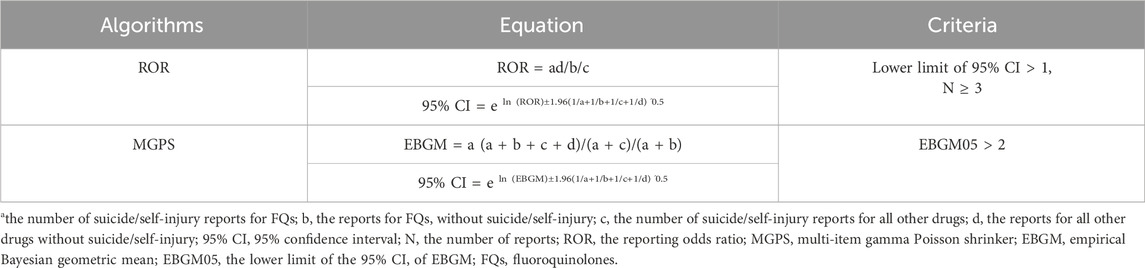
A population-based pharmacovigilance study using case/noncase methodology was conducted to explore the possible link between FQs and reports of suicidal behavior and/or self-injury. This approach is commonly used in pharmacovigilance research to identify safety signals (Dong and Sun, 2022; Ji et al., 2023; Al-Yafeai et al., 2024). From a mathematical standpoint, the case/noncase methodology involves comparing the incidence rate of a specific AE in patients who have taken a certain medication with the incidence rate of the same AE in patients who have not taken that medication (Rothman et al., 2004; Sakaeda et al., 2013). In this study, we assessed disproportionality using the empirical Bayes geometric mean (EBGM) derived from the multi-item gamma Poisson shrinker (MGPS) and the reporting odds ratio (ROR). A signal was identified when the EBGM05 metric, which represents the lower one-sided 95% confidence limit of the EBGM, was greater than or equal to 2.0 (26) or when the lower limits of the 95% confidence intervals (95% CIs) for the ROR exceeded one in at least three reports (Chen L. et al., 2024). The formulas for calculating the EBGM and ROR are provided in Table 2. All data processing and statistical analyses were performed using SPSS software (version 29.0).
3 Results
3.1 General characteristics
During the study period, a total of 20,755,632 unique records were extracted from the FAERS database. Of these, 201,708 AEs were associated with FQs, and 291,764 reports involved suicide or self-injury. Among these, FQs were identified as the suspected drug linked to suicide or self-injury in 737 reports.
The clinical characteristics of the cases involving suicide or self-injury associated with FQs are summarized in Table 3. Among these cases, 53.46% of the individuals were female, whereas 40.57% were male. The majority of reported incidents occurred in adults aged 25–64 years, accounting for 60.52% of the total cases. Reports were predominantly submitted by consumers (46.40%), followed by physicians (20.22%), pharmacists (8.41%), and other healthcare providers (12.21%). Notably, AEs related to suicide or self-injury associated with FQs were most frequently recorded in 2023. A detailed review of all reported cases revealed that 57.53% exhibited symptoms consistent with suicidal behavior, 13.70% resulted in completed suicide, and 11.40% involved suicide attempts.
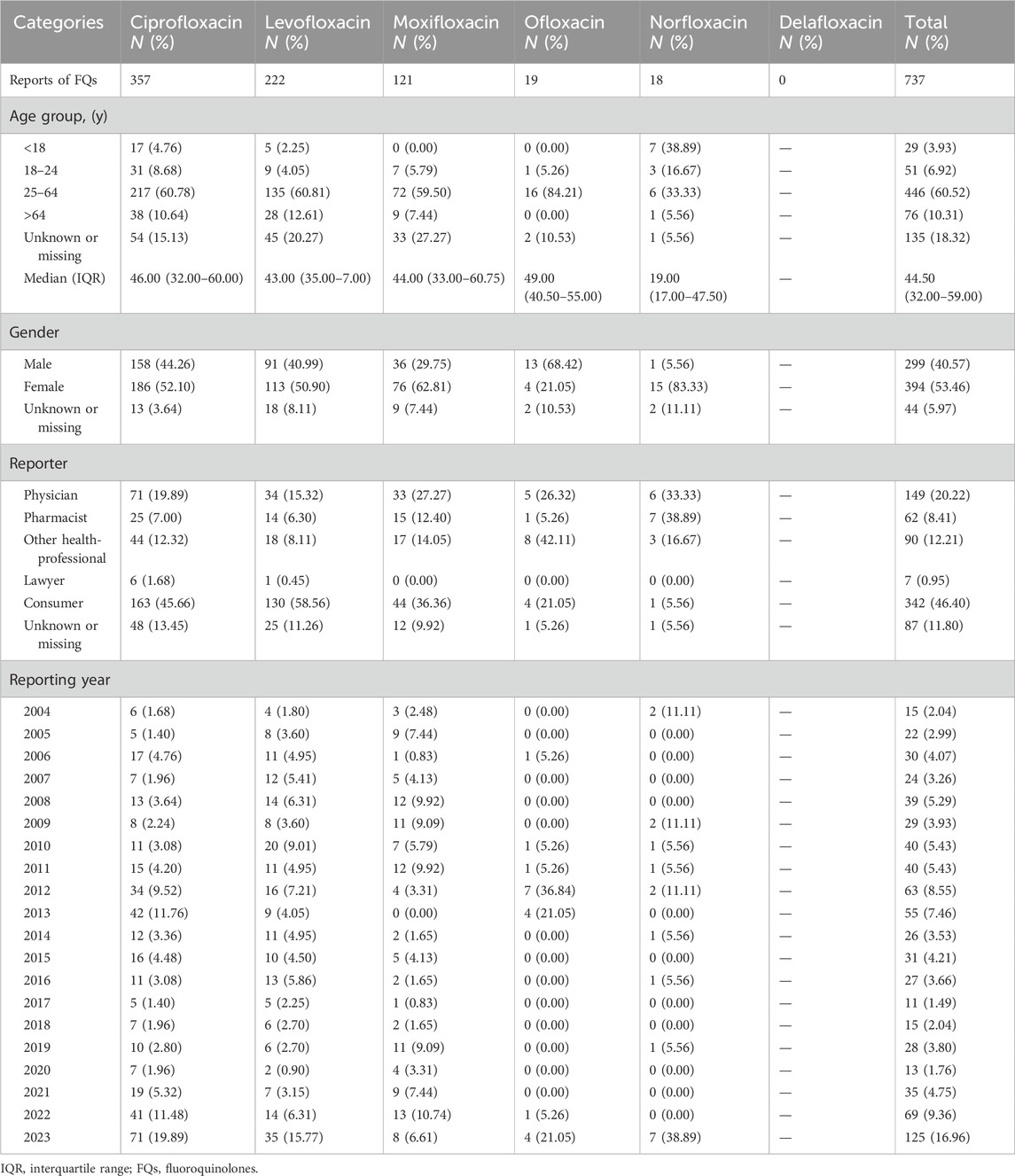
Table 3. Clinical features of suicide/self-injury cases associated with FQs reported in the FAERS during the study period.
3.2 Signal detection
Figure 2A illustrates the number of reports associated with each specific FQ drug. Among the reports of suicidal and self-injurious behavior related to FQs, ciprofloxacin accounted for 357 cases, levofloxacin accounted for 222 cases, moxifloxacin accounted for 121 cases, ofloxacin accounted for 19 cases, and norfloxacin accounted for 18 cases. No reports were linked to delafloxacin. Further stratified analyses revealed low ROR and EBGM05 values for suicide/self-injury across all FQs, suggesting that none of the six FQ drugs investigated in this study were associated with an increased risk of suicide/self-injury overall (Figures 2B, C).
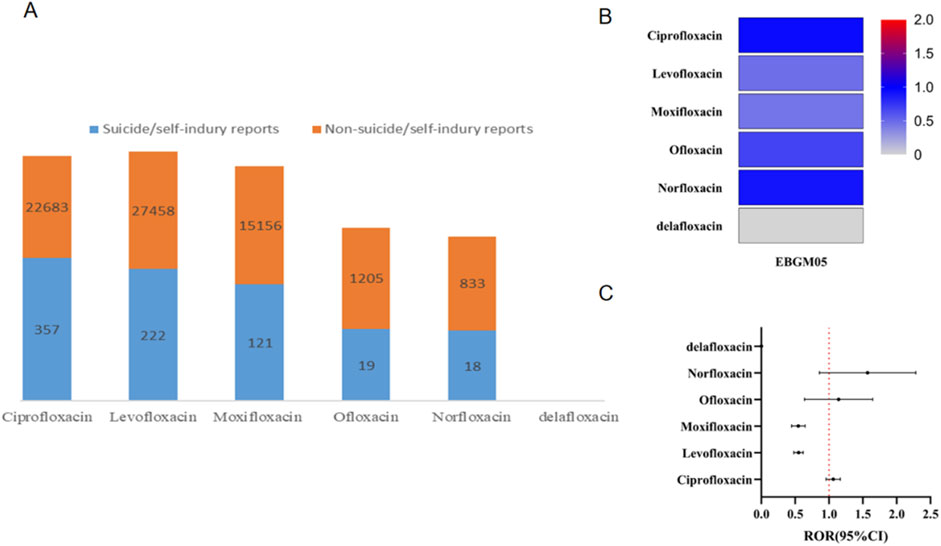
Figure 2. Results of disproportionality analysis for suicidal and self-injurious reports associated with FQs at the drug level. (A) Number of suicide/self-injury and nonsuicide/self-injury reports for each FQ drug. (B) EBGM05 of FQ-associated suicide/self-injury for each distinct FQ drug. (C) RORs (95% CI) of FQ-associated suicide/self-injury for each distinct FQ drug. ROR, reporting odds ratio; FQ, fluoroquinolone; EBGM05, lower one-sided 95% confidence limit (95% CI) of the empirical Bayes geometric mean.
To explore individual characteristics more thoroughly, separate subanalyses were performed on the basis of sex and age (Figure 3). In both females (ROR 0.64, 95% CI 0.59–0.71, P < 0.001; EBGM05 0.58) and males (ROR 0.77, 95% CI 0.69–0.86, P < 0.001; EBGM05 0.69), no safety signal indicating suicide/self-injury associated with FQs was identified. Stratified analysis based on age revealed that the RORs for suicide/self-injury associated with FQs was slightly elevated in the <18 years age group (ROR: 1.51, 95% CI: 1.05–2.19, P = 0.028) and the 18–24 years age group (ROR: 2.31, 95% CI: 1.75–3.06, P < 0.001). However, the EBGM05s values remained below two in these populations. No significant signals were observed in the other age groups.
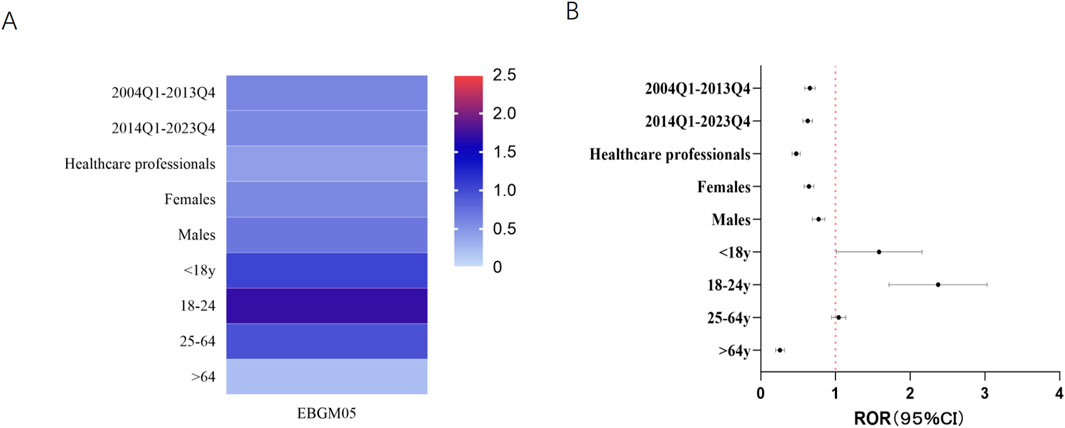
Figure 3. Results of subgroup disproportionality analysis of FQs associated with suicide/self-injury on the basis of age, sex and reporting time. (A) EBGM05s of FQ-associated suicide/self-injury in different sex, age, reporter-type and reporting time groups. (B) RORs (95% CI) of FQ-associated suicide/self-injury in different sex, age, reporter-type and reporting time groups. ROR, reporting odds ratio; 95% CI, 95% confidence limit; FQs, fluoroquinolones; EBGM05, lower one-sided 95% CI of the empirical Bayes geometric mean.
4 Discussion
The neuropsychiatric safety of fluoroquinolones remains a pressing area of investigation, given their extensive global use and potential to adversely affect mental health. This concern has been underscored by the MHRA alert, which highlights reports of suicidal thoughts and behaviors associated with FQ use. In response, we conducted a comprehensive analysis to evaluate the potential risk of suicide linked to various FQs.
The central nervous system (CNS) toxicity of quinolone antibiotics is well documented and manifests as a range of symptoms, such as hallucinations, dizziness, and headaches. Several potential neurobiological mechanisms have been proposed to explain the associations between fluoroquinolones and CNS events (Wierzbiński et al., 2023; Scavone et al., 2020; Wang et al., 2022). Owing to the structural resemblance of fluoroquinolones to γ-aminobutyric acid (GABA) agonists, fluoroquinolones may interact with GABA receptors in the brain, potentially resulting in neurotoxicity (Wierzbiński et al., 2023; Scavone et al., 2020; Wang et al., 2022). It has been proposed that FQs might activate excitatory N-methyl-d-aspartate (NMDA) and adenosine receptors, and this could lead to noticeable CNS symptoms if FQs penetrate the CNS sufficiently (Wierzbiński et al., 2023; Wang et al., 2022). Some researchers suggest that fluoroquinolones may contribute to neuro-AEs by inducing a reduction in serotonin levels, increasing oxidative stress, lowering antioxidant levels, and altering various microRNAs (Wang et al., 2022). However, whether these mechanisms contribute to suicidal thoughts or self-injurious behaviors remains a matter of debate, and further research is needed.
The existing evidence regarding the association between FQs and the risk of suicidal or self-injurious behavior remains limited. Wang and colleagues [30] conducted a nationwide cohort study involving over one million U.S. patients treated with fluoroquinolones for pneumonia or urinary tract infections (UTIs). Their analysis revealed no increased risk of suicidality associated with short-term use of fluoroquinolones compared with azithromycin or trimethoprim-sulfamethoxazole, with adjusted hazard ratios of 1.01 (95% confidence interval: 0.76–1.36) for the pneumonia cohort and 1.03 (95% confidence interval: 0.91–1.17) for the UTI cohort. Similarly, a nested case‒control study by Jick et al. involving 348 individuals with suicidal ideation, attempts, or completed suicides and 808 controls reported no significant association between quinolone antibiotics and an increased risk of suicidal behaviors. Our study aligns with these findings, as subgroup analyses revealed no safety signals related to suicide or self-injury for any of the six fluoroquinolones investigated. This finding is consistent with previous research and further supports the conclusion that fluoroquinolones, as a class, are not associated with an increased risk of suicidal thoughts or behaviors. Additionally, the subgroup analysis enhances our understanding of the safety profiles of individual FQ drugs in relation to these neuropsychiatric outcomes, reinforcing their clinical applicability under appropriate use.
We identified 737 cases of suicidal and self-injurious behavior associated with FQs in the FAERS database from 2004Q1 to 2023Q4. Among these patients, 53.46% were female, and 40.57% were male. Epidemiological data on suicide suggest that the 12-month prevalence of suicidal thoughts is generally higher in females than in males, whereas suicide attempt rates are comparable between the sexes (Borges et al., 2010). In our study, 424 cases (57.53%) involved reports of suicidal ideation, potentially explaining the greater proportion of females in this group. Subgroup disproportionality analysis by sex revealed no significant safety signals for either females or males, indicating no notable differences in the incidence of these adverse events, which requires further investigation.
For adolescents and young adults, suicide continues to be a leading cause of mortality worldwide and is a significant public health issue (McLoughlin et al., 2015; Bertuccio et al., 2024; Hutchinson et al., 2025). Suicide is the second leading cause of mortality among individuals aged 10–24 in the United States (US) (Graham et al., 2025). According to the Centers for Disease Control and Prevention, suicidality and suicidal behavior among youth continue to increase significantly each year (Scudder et al., 2022). The suicide rates among youth have risen precipitously over the past 3 decades with a 52.2% increase between 2000 and 2021 (35). We performed a subgroup analysis by age to evaluate the proportional imbalance of adverse events related to suicide and self-injury associated with FQs across various age groups. In particular, among children and young adults, the RORs showed a slight increase in the reporting of suicide and self-injury. However, it is important to interpret these results cautiously, as the EBGM05 values for fluoroquinolone-associated suicide and self-injury in children and young adults were less than 2, indicating a lack of strong signal strength. Although the stratified analyses were based on a limited number of exposed patients, the elevated RORs for suicidal and self-injurious behavior in the <18 years age group and 18–24 years age group suggest that young patients may be at increased risk during fluoroquinolone use.
Our study had several limitations. First, the majority of reports lacked evidence to establish a definitive causal relationship between the reported AEs and fluoroquinolone exposure. The inability to conclusively determine causality is a limitation that is prevalent in all pharmacovigilance studies (Chen J. et al., 2024; Zhao et al., 2024; Cho et al., 2024). Second, cases in the FAERS database often contain incomplete information, such as details on dosage, comorbidities, time to onset, and other relevant factors. This lack of comprehensive data limits the ability to assess a drug’s safety profile completely. As a result, our study cannot provide a complete evaluation of the relationship between FQ dosages or indications and the risk of suicide or self-injury. Additionally, this study does not have the capacity to assess other potential risk factors or comorbid conditions thoroughly. To address these challenges, we conducted a detailed stratified analysis to explore potential factors influencing the neuropsychiatric safety of fluoroquinolones. The risk of neuropsychiatric adverse events may be associated with multiple variables, including gender, age, reporting year, and reporter type. This study is a pharmacovigilance analysis based on an adverse event reporting database, aiming to detect post-marketing safety signals. The fundamental principle of disproportionality analysis relies on the imbalance in reporting proportions, which can be extracted from real-world data. In our study, beyond assessing the overall risk, we performed stratified analyses based on gender, age, and reporting year to determine whether the risk is elevated under specific conditions and to assess the influence of these factors. Furthermore, to improve the reliability of our findings and minimize confounding biases, we conducted a secondary analysis (Model 2), which included only adverse event reports submitted by healthcare professionals. Reports from healthcare professionals generally undergo a more rigorous causality assessment, enhancing reliability and potentially reducing confounding influences. By incorporating these analytical strategies, we aimed to refine the interpretation of fluoroquinolone-associated neuropsychiatric risks while acknowledging the inherent limitations of spontaneous reporting data. Consequently, any conclusions derived from the pharmacovigilance analysis should be understood in light of these limitations, and additional research with a wider focus may be needed to provide a more comprehensive understanding of the relationships involved. Despite these limitations, disproportionality analysis continues to be an essential tool for identifying potential safety signals related to medications and directing further investigations (Yu et al., 2021; Nair et al., 2023). Importantly, our study provides an initial overview on the basis of the data and methodologies currently available. This finding requires validation through additional clinical studies.
5 Conclusion
Concerns regarding the potential risk of suicide associated with FQs have primarily stemmed from anecdotal case reports. This study contributes postmarket evidence to the understanding of the neuropsychiatric safety profile of FQs. Our analysis of suicide and self-injury cases reported in the FAERS database does not indicate any significant safety signals directly linked to FQs. However, it is essential to recognize that this study provides a preliminary assessment on the basis of the limitations of the available data and methodologies. To confirm these findings, larger-scale and more comprehensive prospective studies are urgently needed.
Data availability statement
The datasets presented in this study can be accessed online. The names of the repository/repositories and accession number(s) can be found below: https://www.fda.gov/drugs/questions-and-answers-fdasadverse-event-reporting-system-faers/fda-adverse-event-reportingsystem-faers-public-dashboard.
Author contributions
LY: Data curation, Writing – original draft, Writing – review and editing. CC: Data curation, Writing – original draft, Writing – review and editing. LD: Data curation, Writing – original draft, Writing – review and editing. TL: Data curation, Writing – original draft, Writing – review and editing. XL: Data curation, Writing – original draft, Writing – review and editing. JX: Conceptualization, Methodology, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Genertive AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed, A. I., van der Heijden, F. M., van den Berkmortel, H., and Kramers, K. (2011). A man who wanted to commit suicide by hanging himself: an adverse effect of ciprofloxacin. Gen. Hosp. Psychiatry 33 (1), 82.e5–e7. doi:10.1016/j.genhosppsych.2010.07.002
Al-Yafeai, Z., Sondhi, M., Vadlamudi, K., Vyas, R., Nadeem, D., Alawadi, M., et al. (2024). Novel anti-psoriasis agent-associated cardiotoxicity, analysis of the FDA adverse event reporting system (FAERS). Int. J. Cardiol. 402, 131819. doi:10.1016/j.ijcard.2024.131819
Appelbaum, P. C., and Hunter, P. A. (2000). The fluoroquinolone antibacterials: past, present and future perspectives. Int. J. Antimicrob. Agents 16 (1), 5–15. doi:10.1016/s0924-8579(00)00192-8
Bertuccio, P., Amerio, A., Grande, E., La Vecchia, C., Costanza, A., Aguglia, A., et al. (2024). Global trends in youth suicide from 1990 to 2020: an analysis of data from the WHO mortality database. EClinicalMedicine 70, 102506. doi:10.1016/j.eclinm.2024.102506
Blondeau, J. M. (2004). Fluoroquinolones: mechanism of action, classification, and development of resistance. Surv. Ophthalmol. 49 (Suppl. 2), S73–S78. doi:10.1016/j.survophthal.2004.01.005
Borges, G., Nock, M. K., Haro Abad, J. M., Hwang, I., Sampson, N. A., Alonso, J., et al. (2010). Twelve-month prevalence of and risk factors for suicide attempts in the world health organization world mental health surveys. J. Clin. Psychiatry 71 (12), 1617–1628. doi:10.4088/JCP.08m04967blu
Brar, R. K., Jyoti, U., Patil, R. K., and Patil, H. C. (2020). Fluoroquinolone antibiotics: An overview. Adesh. Univ. J. Med. Sci. Res. 2 (1), 26–30.
Chen, C., Ding, L., Fu, F., and Xiao, J. (2023b). Updated insights on dementia-related risk of sacubitril/valsartan: a real-world pharmacovigilance analysis. CNS Neurosci. Ther. 29 (9), 2548–2554. doi:10.1111/cns.14195
Chen, C., Zhou, R., Fu, F., and Xiao, J. (2023a). Postmarket safety profile of suicide/self-injury for GLP-1 receptor agonist: a real-world pharmacovigilance analysis. Eur. Psychiatry 66 (1), e99. doi:10.1192/j.eurpsy.2023.2474
Chen, J., Xu, S., Yu, W., Sun, C., and Zhang, W. (2024b). Evaluating cardiac disorders associated with triazole antifungal agents based on the US Food and Drug Administration Adverse Event reporting system database. Front. Pharmacol. 15, 1255918. doi:10.3389/fphar.2024.1255918
Chen, L., Bao, R., and Tian, X. (2024a). Safety profile of levonorgestrel intrauterine system: analysis of spontaneous reports submitted to FAERS. Heliyon 10 (17), e37112. doi:10.1016/j.heliyon.2024.e37112
Cho, H., Yoo, K. Y., Shin, J. Y., Lee, E. K., and Choi, B. (2024). Comparison of thrombotic adverse events in patients treated with factor VIII products and emicizumab using the 2018-2022 US Food and Drug Administration Adverse Event Reporting System data. J. Thromb. Haemost. 22 (6), 1640–1648. doi:10.1016/j.jtha.2024.02.009
Choi, S. H., Cesar, A., Snow, T. A. C., Saleem, N., Arulkumaran, N., and Singer, M. (2023). Respiratory fluoroquinolone monotherapy vs. β-lactam plus macrolide combination therapy for hospitalized adults with community-acquired pneumonia: a systematic review and meta-analysis of randomized controlled trials. Int. J. Antimicrob. Agents 62 (3), 106905. doi:10.1016/j.ijantimicag.2023.106905
Collins, J. P., King, L. M., Collier, S. A., Person, J., Gerdes, M. E., Crim, S. M., et al. (2022). Antibiotic prescribing for acute gastroenteritis during ambulatory care visits-United States, 2006-2015. Infect. Control Hosp. Epidemiol. 43 (12), 1880–1889. doi:10.1017/ice.2021.522
Ding, L., Chen, C., Yang, Y., Fang, J., Cao, L., and Liu, Y. (2022). Musculoskeletal adverse events associated with PCSK9 inhibitors: disproportionality analysis of the FDA adverse event reporting system. Cardiovasc Ther. 2022, 9866486. doi:10.1155/2022/9866486
Dong, S., and Sun, C. (2022). Can glucagon-like peptide-1 receptor agonists cause acute kidney injury? An analytical study based on post-marketing approval pharmacovigilance data. Front. Endocrinol. (Lausanne) 13, 1032199. doi:10.3389/fendo.2022.1032199
Dyer, C. (2023). Coroner asks drug regulator to review advice about ciprofloxacin after doctor's suicide. Bmj 381, 1418. doi:10.1136/bmj.p1418
Graham, L. M., Kafka, J. M., and AbiNader, M. A. (2025). Co-Occurrence of intimate partner violence and suicide mortality among adolescents and young adults in the United States. J. Adolesc. Health 76 (2), 283–290. doi:10.1016/j.jadohealth.2024.09.019
Hicks, L. A., Bartoces, M. G., Roberts, R. M., Suda, K. J., Hunkler, R. J., Taylor, T. H., et al. (2015). US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin. Infect. Dis. 60 (9), 1308–1316. doi:10.1093/cid/civ076
Huruba, M., Farcas, A., Leucuta, D. C., Bucsa, C., Sipos, M., and Mogosan, C. (2021). A VigiBase descriptive study of fluoroquinolone induced disabling and potentially permanent musculoskeletal and connective tissue disorders. Sci. Rep. 11 (1), 14375. doi:10.1038/s41598-021-93763-y
Hutchinson, E., Scott, L., Choukas-Bradley, S., and Silk, J. (2025). Interpersonal risk factors for suicide in daily life among young people: a review of intensive longitudinal studies. Dev. Psychopathol., 1–21. doi:10.1017/S0954579424001810
Ji, L. H., Zhao, C. L., Wang, Y. Q., and Fu, Z. H. (2023). Bisphosphonates-related tendinopathies and ligament disorders: cases analysis from the U.S. Food and Drug Administration adverse event reporting system. Bone 177, 116919. doi:10.1016/j.bone.2023.116919
Kumar, A. (2019). The newly available FAERS public dashboard: implications for health care professionals. Hosp. Pharm. 54 (2), 75–77. doi:10.1177/0018578718795271
Labay-Kamara, U., Manning, S., and McMahon, T. (2012). Fluoroquinolone-induced suicidal ideation and suicidality. Psychosomatics 53 (1), 97–98. doi:10.1016/j.psym.2011.05.003
McLoughlin, A. B., Gould, M. S., and Malone, K. M. (2015). Global trends in teenage suicide: 2003-2014. Qjm 108 (10), 765–780. doi:10.1093/qjmed/hcv026
MHRA (2024). MHRA issues further update on fluoroquinolone safety. Drug. Ther. Bull. 62 (6), 83. doi:10.1136/dtb.2024.000021
MHRA (2024). MHRA issues two updates on fluoroquinolone safety. Drug Ther. Bull. 62 (2), 19. doi:10.1136/dtb.2023.000069
Nair, H. P., Kulkarni, A. R., Eswaran, M., and Subeesh, V. (2023). Pantoprazole associated dyspepsia hypocalcemia and hyponatremia: a disproportionality analysis in FDA adverse event reporting system (FAERS) database. Arab. J. Gastroenterol. 24 (1), 1–4. doi:10.1016/j.ajg.2022.10.012
Rothman, K. J., Lanes, S., and Sacks, S. T. (2004). The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 13 (8), 519–523. doi:10.1002/pds.1001
Rubinstein, E. (2001). History of quinolones and their side effects. Chemotherapy 47 (Suppl. 3), 3–8. doi:10.1159/000057838
Sakaeda, T., Tamon, A., Kadoyama, K., and Okuno, Y. (2013). Data mining of the public version of the FDA adverse event reporting system. Int. J. Med. Sci. 10 (7), 796–803. doi:10.7150/ijms.6048
Scavone, C., Mascolo, A., Ruggiero, R., Sportiello, L., Rafaniello, C., Berrino, L., et al. (2020). Quinolones-Induced musculoskeletal, neurological, and psychiatric ADRs: a pharmacovigilance study based on data from the Italian spontaneous reporting system. Front. Pharmacol. 11, 428. doi:10.3389/fphar.2020.00428
Scudder, A., Rosin, R., Baltich Nelson, B., Boudreaux, E. D., and Larkin, C. (2022). Suicide screening tools for pediatric emergency department patients: a systematic review. Front. Psychiatry 13, 916731. doi:10.3389/fpsyt.2022.916731
Silva, J. B. B., Riester, M. R., and Zullo, A. R. (2024). Antibiotic prescribing patterns for urinary tract infections and pneumonia by prescriber type and specialty in nursing home care, 2016-2018. J. Am. Med. Dir. Assoc. 25 (5), 769–773.e9. doi:10.1016/j.jamda.2024.01.019
Wang, J., Gagne, J. J., Kattinakere-Sreedhara, S., Fischer, M. A., and Bykov, K. (2022). Association between initiation of fluoroquinolones and hospital admission or emergency department visit for suicidality: population based cohort study. Bmj 379, e069931. doi:10.1136/bmj-2021-069931
Wierzbiński, P., Hubska, J., Henzler, M., Kucharski, B., Bieś, R., and Krzystanek, M. (2023). Depressive and other adverse CNS effects of fluoroquinolones. Pharm. (Basel) 16 (8), 1105. doi:10.3390/ph16081105
Xuan, G., Zhang, Y., Cui, J., Zhou, J., and Sui, C. (2023). Propofol-associated serious adverse events: an analysis of the FAERS database. Biotechnol. Genet. Eng. Rev. 40, 2874–2887. doi:10.1080/02648725.2023.2202541
Yu, R. J., Krantz, M. S., Phillips, E. J., and Stone, C. A. (2021). Emerging causes of drug-induced anaphylaxis: a review of anaphylaxis-associated reports in the FDA adverse event reporting system (FAERS). J. Allergy Clin. Immunol. Pract. 9 (2), 819–829.e2. doi:10.1016/j.jaip.2020.09.021
Zhang, J., Liu, K., Sun, L., Yang, L., Liu, X., Zhu, Y., et al. (2021). Exposure to antibiotics and mental disorders in children: a community-based cross-sectional study. Environ. Geochem Health 43 (8), 3237–3253. doi:10.1007/s10653-021-00840-2
Keywords: FAERS, fluoroquinolones, pharmacovigilance, suicidal thoughts, suicidal behaviors
Citation: Yang L, Chen C, Ding L, Lu T, Li X and Xiao J (2025) Suicidal thoughts and behaviors associated with fluoroquinolone antibiotics: a real-world pharmacovigilance analysis. Front. Pharmacol. 16:1556159. doi: 10.3389/fphar.2025.1556159
Received: 06 January 2025; Accepted: 08 April 2025;
Published: 25 April 2025.
Edited by:
Jose Javier Miguel-Hidalgo, University of Mississippi Medical Center, United StatesReviewed by:
Andy R. Eugene, Osawatomie State Hospital, United StatesShiva Shabani, Arak science univercity, Iran
Copyright © 2025 Yang, Chen, Ding, Lu, Li and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Xiao, eWZ4amhlYXJ0QDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Lijuan Yang
Lijuan Yang Congqin Chen
Congqin Chen Lingqing Ding
Lingqing Ding Tingting Lu
Tingting Lu Xiwen Li†
Xiwen Li†