- 1Department of Respiratory and Critical Care Medicine, No.2 People’s Hospital of Fuyang City, Fuyang, Anhui, China
- 2Fuyang Infectious Disease Clinical Collage of Anhui Medical University, Fuyang, Anhui, China
- 3Department of Respiratory and Critical Care Medicine, Funan County People’s Hospital, Fuyang, Anhui, China
- 4Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China
- 5Institute of Respiratory Diseases, Second Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China
Background: Ferritin is one of the major intracellular iron storage proteins and is implicated in the pathophysiological processes of many inflammatory diseases, but its function in community-acquired pneumonia (CAP) was unclear. The purpose of this study was to evaluate the expression of ferritin and analyze the relationship of serum ferritin with CAP.
Methods: Severe CAP patients and age- and gender-matched healthy participants were recruited for the study. Serum ferritin was detected through ELISA. Physiological characteristics were recorded.
Results: The serum ferritin level was significantly increased in severe CAP patients upon initial hospitalization compared to healthy participants, and it decreased after therapy. Correlative analyses hinted that there were obvious relationships of serum ferritin with the indicators of blood routine, liver function, and inflammation. Moreover, linear and logistic regression analyses confirmed that the serum ferritin level was positively related to the scores of CURB-65, CRB-65, and PSI. The poor prognosis including mechanical ventilation, vasoactive agent, ICU admission, death, and longer hospital stays were assessed in severe CAP cases during hospitalization. Multivariate logistic regression showed that higher serum ferritin levels were closely linked to the poor prognostic outcomes.
Conclusion: There is significantly positive association between the serum ferritin level upon initial hospitalization with the severity and poor prognosis. Thus, serum ferritin could be an indicator for the determination of severity and prognosis among CAP cases.
Introduction
Community-acquired pneumonia (CAP) is a frequent cause of acute inflammatory illness in different aged populations and one of the most common reasons of hospital admission. Mounting pieces of evidence have revealed that CAP is strongly associated with mortality and hospitalization across the whole world (Brown and Lerner, 1998; Marrie, 2000). The mortality rate is quite high in hospitalized patients of all ages, ranging from 5% to 15% (Chang et al., 2016). Rapid and reasonable treatment can effectively downregulate the mortality, and earlier discrimination of the state of illness is significant for ameliorating the severity in CAP patients (Jiang et al., 2021; Wang et al., 2021; Feng et al., 2021a). Though there have been great advancements in treatment tools and detection methods, the clinical, radiological, and biological characteristics are minimally sensitive or specific (Christ-Crain and Opal, 2010). Moreover, many microbiological detection methods often are slow and invalid. Hence, new indicators might assist in evaluating the illness among CAP cases.
Iron is essential for survival and reproduction in nearly all life forms. Iron application can affect the growth and pathogenic potential for microbes (Ganz and Nemeth, 2015). Various defense mechanisms have gradually been formed in the bodies’ immune systems, which then control iron metabolism and contribute to an equilibrium competition pattern between the microbes and the host (Sangkhae and Nemeth, 2017). An increasing number of studies have revealed that iron deposition is implicated in many physiological and pathological processes of several diseases (Fei et al., 2024; Xu et al., 2023). Ferritin is the major intracellular iron storage protein, which exerts a significant function in regulating iron homeostasis (Orino and Watanabe, 2008; Wang et al., 2010). It is known that ferritin is highly expressed in the cytoplasm, nucleus, and mitochondrion (Beazley et al., 2009; Surguladze et al., 2005). Previous research studies have hinted that ferritin elevation is implicated in various cancers, acute infectious diseases, and inflammatory and autoimmune diseases (Kirkali et al., 1999; Ganz and Nemeth, 2009; Zandman-Goddard and Shoenfeld, 2007; Chen et al., 2020). Recent studies have revealed that the level of ferritin is closely related to the severity in coronavirus disease 2019 (COVID-19) (Zhou et al., 2020; Feld et al., 2020). A report from our laboratory has found that pulmonary ferritin is dramatically elevated in arsenic-incurred acute lung injury models (Li et al., 2022). Moreover, plasma ferritin can indicate macrophage activation-like syndrome and microbial etiology of CAP patients (Brands et al., 2021; Oppen et al., 2021). However, the concrete molecular mechanism and biological function of ferritin remained unclear in CAP patients.
So far, the exact evidence about the role of ferritin was absent in CAP. Moreover, the link of ferritin with CAP was also obscure. As a result, the goal was to estimate the relationship of serum ferritin with CAP. A prospective cohort study was designed and carried out. Eligible cases with severe CAP were recruited, and biological samples were obtained. Our results first provided evidence that ferritin might take part in the process of CAP.
Materials and methods
Subjects
According to the diagnostic criteria of CAP (Cao et al., 2018), CAP patients were selected from the Department of Respiratory and Critical Care Medicine in No. 2 People’s Hospital of Fuyang City and the Second Affiliated Hospital of Anhui Medical University. All CAP patients included must have had at least one of the following clinical symptoms: cough; expectoration; the number of white blood cells (WBC) was higher than 10 × 109/L or less than 4 × 109/L; radiographic detection found the characteristics of patchy, lobulated, and alveolar high-density infiltrating lesions; the temperature was up to 38.0°C; there were positive pathogen detection and moist rales; and partial cases may be accompanied with dyspnea. In addition, other examinations were conducted, and heart failure, organizing pneumonia, pulmonary embolism, tuberculosis, and other diseases were all eliminated in the current research. Finally, 273 patients confirmed with CAP were selected, and fasting blood samples were collected from CAP patients on admission before any intervention and treatment. Simultaneously, clinical characteristics and demographic information were obtained from all the participants. The inclusion criteria were as follows: conformed to the diagnostic criteria, older than 18 years, occurred in the community, participated in this research voluntarily, and there was no oral or intravenous iron treatment one month before hospitalization. The exclusion criteria were as follows: pregnant women; complicated with other pulmonary diseases; cancer, with hematological malignancies in particular; and complicated with immunodeficiency diseases, severe liver dysfunction, renal dysfunction, anemia, and malnutrition (Feng et al., 2021b; Zheng et al., 2021a; Xu et al., 2022). The severe and very severe patients were in the intensive care unit, and mild and moderate cases were in the general ward. We used CAP severity scores to evaluate pneumonia severity. Moreover, primal prognostic outcomes were tracked up and observed in the general ward or the intensive care unit during hospitalization (Cao et al., 2022; Liu et al., 2021). Finally, CAP cases with incomplete information, unavailable serum specimen, and without prognosis were all ruled out; 273 CAP subjects were recruited in this study (Supplementary Figure S1). In order to compare the level of serum ferritin, healthy volunteers who did not have a history of pulmonary disease were selected from the physical examination center. At last, sex- and age-matched healthy volunteers were included in this project. In addition, serum specimens were collected from partial patients before being discharged.
Enzyme-linked immunosorbent assay (ELISA)
Analysis of ferritin was carried out in serum samples by ELISA. Ferritin (CSB-E05187h) commercial ELISA kits were bought from Cusabio (https://www.cusabio.com/). After hospitalization, peripheral blood samples were collected. Restricting the diet and water intake was necessary in CAP patients when we collected peripheral blood samples. Then, serum samples were obtained and stored in our biological sample bank (Fu et al., 2021; Fei et al., 2019). The concentration of ferritin was measured according to the manufacturer’s instructions with minor adjustments (Zheng et al., 2021b). Each standard or sample was detected in duplicate.
Statistical analysis
All statistical analyses were conducted using SPSS 19.0 software. All continuous variables were represented as mean or median. The categorical variables were expressed as frequency. According to the tertiles of ferritin levels, CAP cases were divided into three groups. Demographics information, clinical characteristics, and laboratory data were compared via Student’s t-test, one-way ANOVA test, or the chi-square test among the three groups. The relationship of serum ferritin with clinical indicators was evaluated by performing the Spearman or Pearson correlative analysis. Linear and logistic regression models were used to establish the correlations of serum ferritin with CAP severity scores. Potential confounding factors were controlled. The relevancy of serum ferritin content with the prognosis was explored by the logistic regression model. Nonparametric receiver operating characteristic (ROC) curves of serum ferritin and other clinical parameters were constructed to discriminate the predictive powers. Statistical significance was defined as p-value < 0.05.
Results
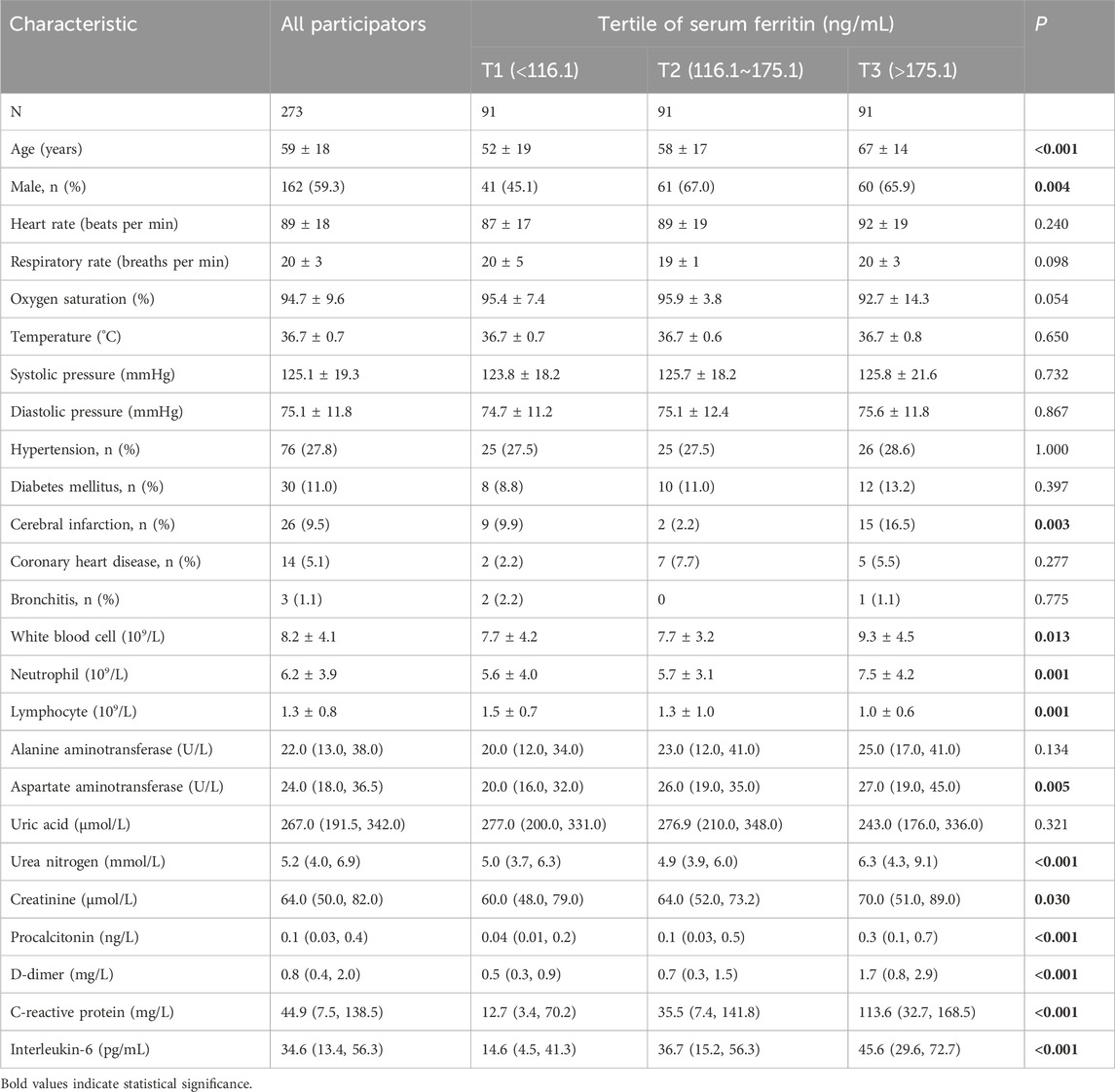
Demographic information and laboratory data
Demographic information of all CAP patients was estimated. Demographic information and laboratory data are represented in Table 1. Among 540 CAP patients, the average age was 59 ± 18 years, and there were 162 (59.3%) male subjects. Moreover, the comorbidities were recorded in the participants. The number of cerebral infarctions was gradually elevated along with serum ferritin. In addition, routine blood indexes, liver function, renal function, and inflammatory cytokines were assessed. As represented in Table 1, the levels of white blood cell (WBC), neutrophil, aspartate aminotransferase (AST), urea nitrogen, creatinine, procalcitonin (PCT), D-dimer, C-reactive protein (CRP), and interleukin-6 (IL-6) were elevated with increasing serum ferritin. On the contrary, the count of lymphocyte gradually reduced in parallel with increasing serum ferritin (Table 1).
Associations between serum ferritin and CAP severity scores
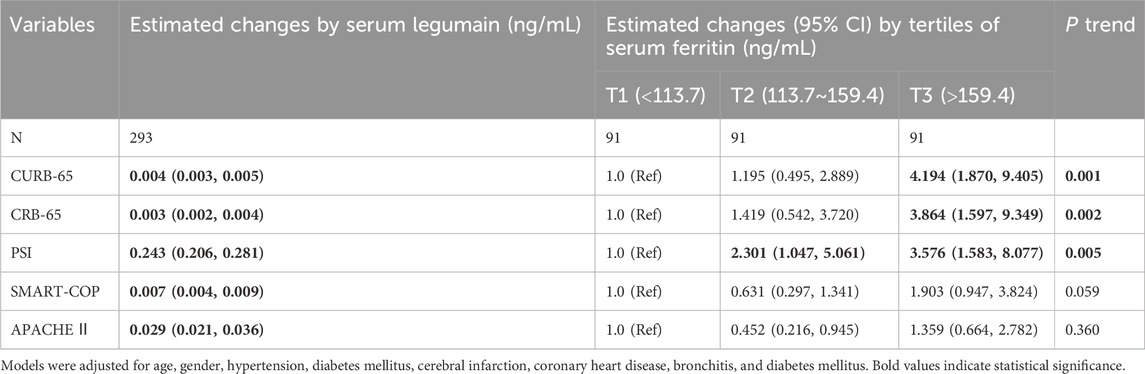
As represented in Figure 1A, the level of serum ferritin was markedly higher in CAP patients (156.1 ± 87.5 ng/mL) than in healthy volunteers (85.6 ± 6.6 ng/mL) (p < 0.01). Moreover, serum ferritin was decreased when the patients were discharged from hospital after therapy (122.1 ± 53.3 ng/mL versus 86.9 ± 40.8 ng/mL) (p < 0.01) (Figure 1B). Then, the associations of serum ferritin with the severity scores were analyzed. The potential confounding factors, such as age, gender, hypertension, diabetes mellitus, cerebral infarction, coronary heart disease, bronchitis, and diabetes mellitus, were controlled in the mixed logistic regression models. As shown in Table 2, linear aggression analysis found that each 1 ng/mL elevation of serum ferritin was related to score upregulations of 0.004 [(95% confidence interval (CI): 0.002∼0.005)], 0.003 (95% CI: 0.002∼0.004), 0.243 (95% CI: 0.206∼0.281), 0.007 (95% CI: 0.004∼0.009), and 0.029 (95% CI: 0.021∼0.036) in CURB-65, CRB-65, PSI, SMART-COP, and APACHE Ⅱ, respectively. In addition, logistic regression analysis indicated that serum ferritin concentration was positively correlated with CURB-65 (p-trend = 0.001), CRB-65 (p-trend = 0.002), and PSI (p-trend = 0.005) scores among CAP patients.
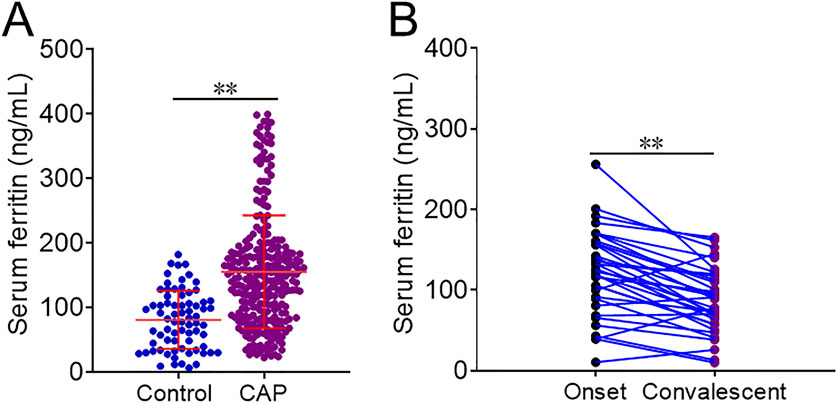
Figure 1. Levels of serum ferritin in different populations. (A,B) Levels of serum ferritin were detected and compared in different groups. (A) CAP patients versus control subjects. (B) CAP patients in the onset phase versus convalescent stage. **p < 0.01.
Associations between serum ferritin and clinical parameters
As represented in Figure 2, there were dramatic and positive correlations of serum ferritin with WBC (r = 0.191; p = 0.002) and neutrophil (r = 0.252; p < 0.001), and it was inversely associated with lymphocyte (r = −0.288; p < 0.001) in CAP patients. In addition, serum ferritin was positively and weakly related to urea nitrogen (r = 0.286; p < 0.001), creatinine (r = 0.223; p < 0.001), alanine aminotransferase (ALT) (r = 0.131; p = 0.030), AST (r = 0.215; p < 0.001), PCT (r = 0.343; p < 0.001), and D-dimer (r = 0.401; p < 0.001). In addition, the positive correlations of serum ferritin with inflammatory cytokines, including IL-6 (r = 0.350; p < 0.001) and CRP (r = 0.347; p < 0.001), were observed.
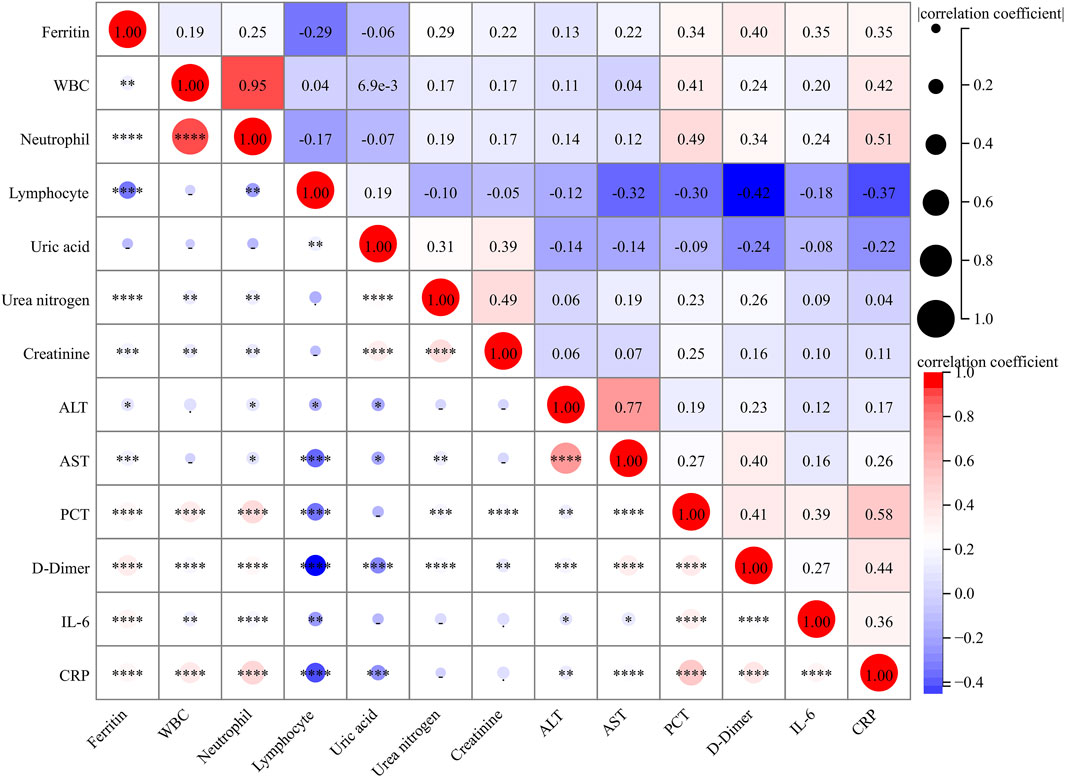
Figure 2. Correlations between serum ferritin and clinical parameters. The correlations of serum ferritin with clinical parameters were analyzed using Spearman correlation coefficient or Pearson rank correlation.
Associations between serum ferritin and prognostic outcomes
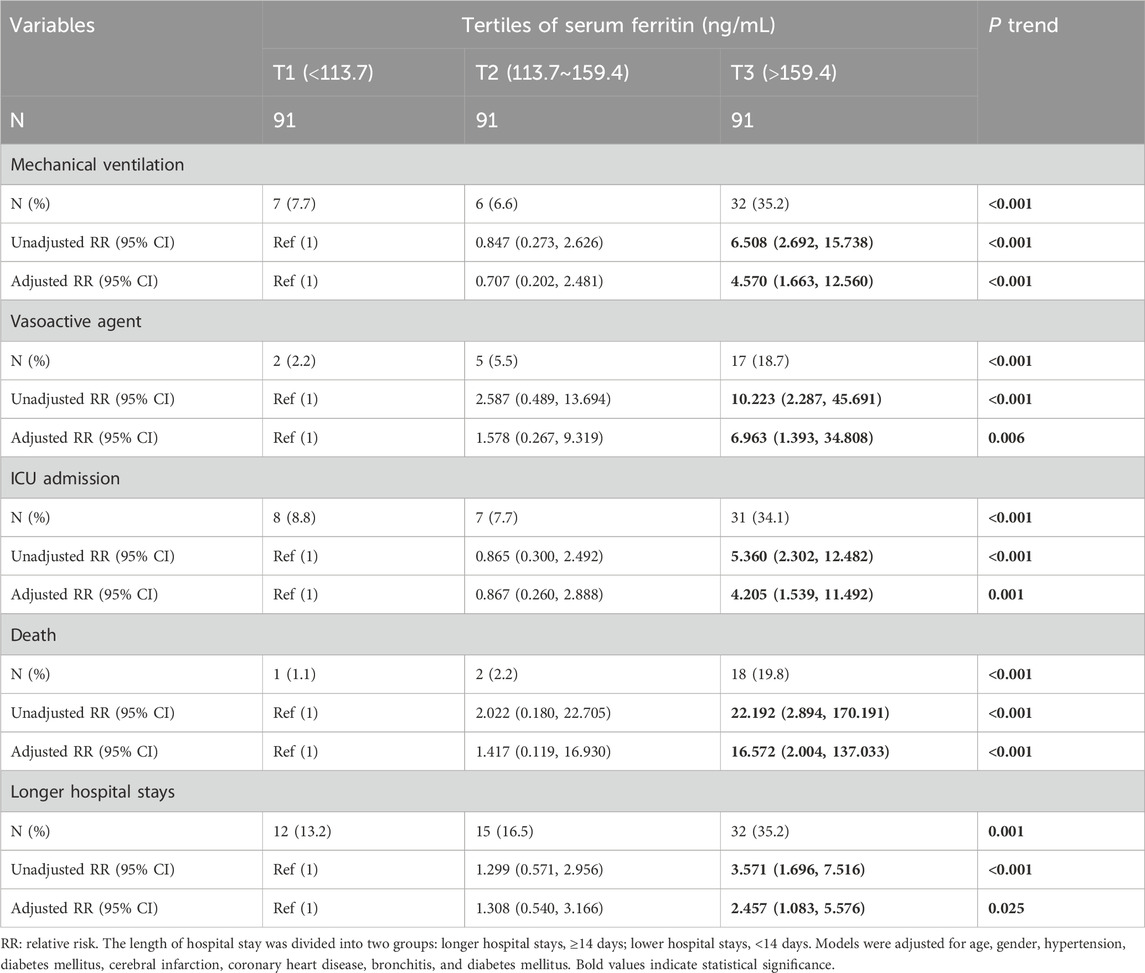
During hospitalization, prognostic outcomes were tracked up and analyzed. As represented in Supplementary Figures S2A–C, the content of serum ferritin at admission was prominently elevated in cases with mechanical ventilation, vasoactive agent, and ICU admission compared with cases without the above prognostic outcomes. Furthermore, the content of serum ferritin was higher in dead patients than in surviving subjects (Supplementary Figures S2D). Additionally, serum ferritin was significantly increased in subjects with more than 14 hospital stays than in subjects with other hospital stays (Supplementary Figures S2E). Furthermore, compared with CAP patients in tertile 1, the numbers of CAP patients with mechanical ventilation, vasoactive agent usage, ICU admission, death, and longer hospital stays were evidently higher in tertile 3 (Table 3). Logistic regression analysis suggested that the relative risk (RR) of the poor prognosis was remarkably upregulated with elevated serum ferritin in CAP patients (Table 3).
Predictive capacities for prognostic outcomes
Predictive capacities of different clinical parameters for prognostic outcomes were evaluated through ROC area under the curve (AUC). We found that the distinguishing capacities for mechanical ventilation, vasoactive agent usage, and ICU admission were similar between serum ferritin and CAP severity scores, and they were higher than PCT, IL-6, CRP, and D-dimer (Figure 3). In addition, the predictive power for death was elevated in serum ferritin compared with other CAP severity scores and inflammatory cytokines (Figure 3). The cutoff level of ferritin was 203.8 ng/mL, and it had 85.7% sensitivity and 71.8% specificity for death prediction. On the contrary, the predictive efficiency of serum ferritin for longer hospital stay was lower than those of CAP severity scores, and it was similar with blood routine indexes, such as PCT, D-dimer, IL-6, and CRP (Figure 3).
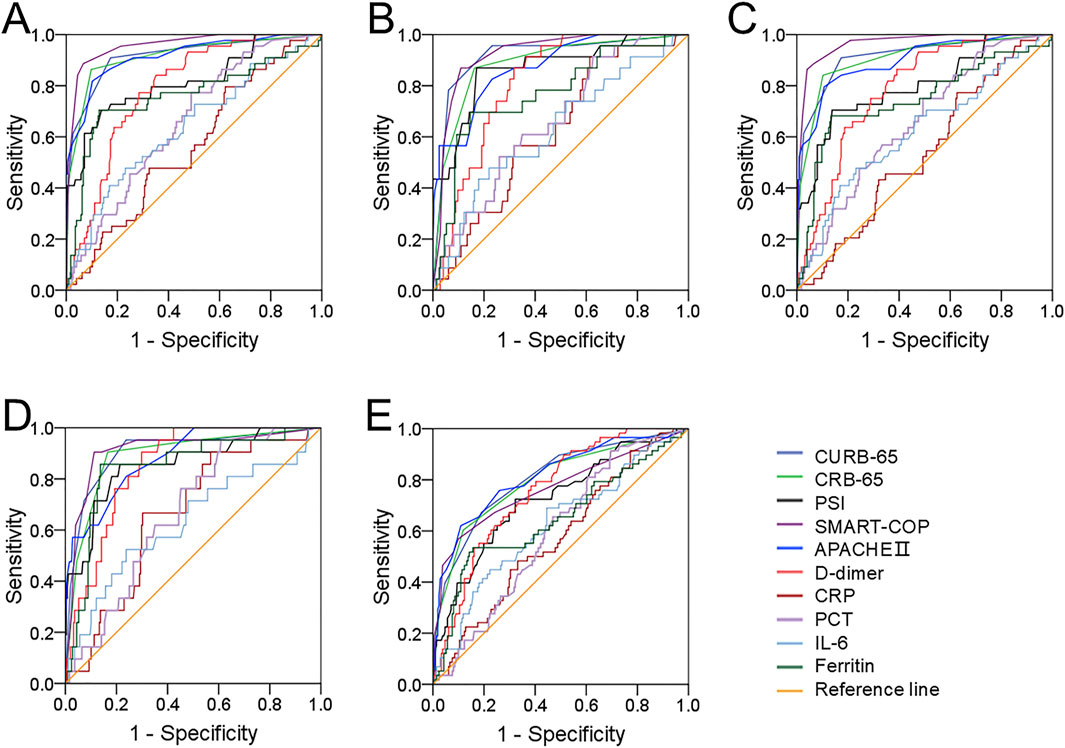
Figure 3. Predictive capacities for prognostic outcomes. (A–E) Predictive powers of different indicators for prognostic outcomes were evaluated in CAP patients through ROC curve. (A) Mechanical ventilation. (B) Vasoactive agent. (C) ICU admission. (D) Death. (E) Longer hospital stays prediction.
Discussion
In the current investigation, CAP cases were enrolled in the study, and the level of serum ferritin was analyzed. We then explored the diagnostic and predictive powers of serum ferritin for CAP patients via a prospective cohort. We found that the concentration of serum ferritin was prominently upregulated in CAP patients compared with healthy volunteers. In addition, there were obvious associations between serum ferritin at admission and CAP severity scores. Moreover, serum ferritin was strongly related to several parameters. In addition, serum ferritin at admission was positively associated with poor prognosis. Not only that but also there was a higher predictive power for death in serum ferritin than those in other severity scores and blood routine indices.
Ferritin is a ubiquitously expressed spherical protein and is the major intracellular iron storage protein, which regulates iron homeostasis (Wang et al., 2010; Torti and Torti, 2002). Previous research studies have shown that ferritin is involved in the pathogenesis of various inflammatory diseases. Increasing ferritin contents are associated with acute or chronic inflammatory conditions whether evoked by infections or not (Sharif et al., 2018; Ueda and Takasawa, 2018). A retrospective longitudinal analysis found higher ferritin level in COVID-19 patients (Qeadan, et al., 2021). Additionally, the initial serum ferritin is upregulated in acute respiratory distress syndrome (ARDS) (Sharkey, et al., 1999). In addition, the expression of ferritin is increased and inversely associated with the pulmonary function level in chronic obstructive pulmonary disease (COPD) (Zhang et al., 2020). Nevertheless, the evidence about the role of ferritin in CAP remained lacking. Therefore, we measured the serum ferritin level in the participants. Our results found that the initial serum ferritin level was increased in CAP patients compared with healthy volunteers, and it gradually upregulated with increased CAP severity scores. Correlative analysis unveiled that the initial serum ferritin level was strongly associated with many clinical indicators. Moreover, regression analysis confirmed a positive relationship of the serum ferritin level with CAP severity scores. So, it clearly stated that ferritin participated in the pathogenesis of CAP.
Cumulative proof suggested that ferritin expression is closely related to many prognostic outcomes in lots of diseases. An earlier report indicated that the initial serum ferritin content is notably increased in COVID-19 patients with severe acute liver injury, ICU transfer, and mechanical ventilation (Cheng et al., 2020). Moreover, higher serum ferritin content elevates the risk of metabolic syndrome throughout childhood (Suárez-Ortegón et al., 2019). Higher expression of ferritin mRNA in kidney is positively correlated with metastasis and bad prognosis in renal cell carcinoma (Huang et al., 2019). Interestingly, serum ferritin expression at admission was increased in CAP patients, which occurred with various poor prognoses. Then, the logistic regression model showed that the serum ferritin level at admission was positively linked to poor prognosis. Moreover, ROC curve analysis indicated that the discriminability for death was considerably higher in serum ferritin than in CAP severity scores and blood routine indices. In short, these data provided evidence that higher serum ferritin at admission increases the incidence of bad prognosis in CAP patients during hospitalization.
The mechanism of ferritin upregulation was obscure in CAP. Under normal physiological conditions, ferritin synthesis is regulated by and relies on the iron content. Several animal experiments showed that ferritin secretion is evoked through elevating serum iron levels (Zhang et al., 2021). Not only that, many studies stated that ferritin synthesis is also regulated by inflammation, oxidative stress, growth factors, hypoxia–ischemia, and hyperoxia (Koorts and Viljoen, 2007). Moreover, ferritin is one of the downstream target genes of several transcription factors, including NF-κB and Nrf-2. Research found that NF-κB can bind to DNA elements of ferritin promoter and regulate ferritin expression (Torti and Torti, 2002). In addition, Nrf-2 mediates transcriptional activation of ferritin and upregulates ferritin expression by combining with the binding site of electrophile/antioxidant responsive element (EpRE/ARE) (Pietsch et al., 2003). Due to the inflammatory and infectious disease (CAP), Nrf-2 and NF-κB are activated in the animal models of pneumonia (LaCanna et al., 2019; Sun et al., 2020). Several inflammatory cytokines can elicit the release of ferritin into the blood stream (Fahmy and Young, 1993). Therefore, we thought that different pathogenic microbes’ infection may evoke an inflammatory reaction and activate nuclear transcription factors, such as NF-κB and Nrf-2, in pulmonary epithelial cells. At last, the transcriptional expression of ferritin is upregulated in CAP.
This investigation found that serum ferritin concentration was positively associated with the severity scores and poor prognosis via a prospective cohort study. These results hinted that serum ferritin can predict the risk of poor prognosis among CAP patients in advance. In addition, the clinician can take intervention measures to avoid the adverse progress ahead, such as ICU admission, mechanical ventilation, noninvasive positive pressure ventilation, and vasoactive agent usage, to improve the prognosis. However, this study was only an observational study, and the potential mechanism of ferritin elevation was ambiguous. The effect of ferritin inhibition on the progression of CAP was unclear. We did not know whether ferritin expression can be used as a therapeutic target for CAP. In the future, more animal and cellular experiments can solve these doubts. Therefore, for the abovementioned reasons, we thought that serum ferritin can be used a biomarker to estimate the severity and adverse prognosis in CAP patients. Although there were some advantages of this study, we must also recognize the inevitable limitations. First, this investigation was a single-center study with a small sample size; a multicenter study with a large sample size is needed to further confirm the current conclusion. Second, this study only detected the level of ferritin in serum, and the local expression of ferritin cannot be evaluated.
Conclusion
This prospective cohort study assessed the relationships of the serum ferritin level with severity and prognosis. Our data suggested that serum ferritin is upregulated in CAP cases compared with control subjects. Additionally, the serum ferritin level at admission is strongly and prominently related to the disease severity and prognosis. Furthermore, serum ferritin has a higher predictive power for death than CAP severity scores and blood routine indices. These results provide evidence that ferritin may participate in the process of CAP, but the target cells and specific mechanisms remain to be illuminated. Consequently, more experiments should be conducted to understand the function of ferritin elevation and ascertain the therapeutic role of anti-ferritin treatment in CAP patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Second Affiliated Hospital of Anhui Medical University (YX2021-147). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JX: data curation, investigation, methodology, software, and writing – original draft. M-FH: conceptualization, data curation, methodology, software, and writing – original draft. K-SM: conceptualization, data curation, investigation, methodology, and writing – original draft. TZ: conceptualization, data curation, investigation, methodology, and writing – original draft. R-RW: conceptualization, data curation, investigation, methodology, and writing – original draft. J-JZ: conceptualization, data curation, investigation, Methodology, and writing – original draft. HZ: conceptualization, funding acquisition, investigation, methodology, supervision, and writing – original draft. LF: conceptualization, funding acquisition, investigation, supervision, writing – original draft, and writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Research Funds of Center for Big Data and Population Health of IHM (JKS2022007 and JKS2023010), the Key Developmental Program of Anhui Province (2022e07020084), and the University Natural Science Research Project of Anhui Province (2023AH030117).
Acknowledgments
The authors thank all patients and their families involved in this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1556185/full#supplementary-material
References
Beazley, K. E., Canner, J. P., and Linsenmayer, T. F. (2009). Developmental regulation of the nuclear ferritoid-ferritin complex of avian corneal epithelial cells: roles of systemic factors and thyroxine. Exp. Eye Res. 89 (6), 854–862. doi:10.1016/j.exer.2009.07.007
Brands, X., de Vries, F. M. C., Uhel, F., Haak, B. W., Peters-Sengers, H., Schuurman, A. R., et al. (2021). Plasma ferritin as marker of macrophage activation-like syndrome in critically ill patients with community-acquired pneumonia. Crit. Care Med. 49 (11), 1901–1911. doi:10.1097/CCM.0000000000005072
Brown, P. D., and Lerner, S. A. (1998). Community-acquired pneumonia. Lancet 352 (9136), 1295–1302. doi:10.1016/S0140-6736(98)02239-9
Cao, B., Huang, Y., She, D. Y., Cheng, Q. J., Fan, H., Tian, X. L., et al. (2018). Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese Thoracic Society, Chinese Medical Association. Clin. Respir. J. 12 (4), 1320–1360. doi:10.1111/crj.12674
Cao, L. F., Cheng, J. Y., Xu, Z., Feng, C. M., Zhao, H., Wang, X. M., et al. (2022). Serum 8-hydroxydeoxyguanosine is a potential indicator for the severity and prognosis in patients with community-acquired pneumonia: a prospective cohort study. J. Immunol. 208 (2), 321–327. doi:10.4049/jimmunol.2100711
Chang, P. Y., Tsao, S. M., Chang, J. H., Chien, M. H., Hung, W. Y., Huang, Y. W., et al. (2016). Plasma levels of soluble intercellular adhesion molecule-1 as a biomarker for disease severity of patients with community-acquired pneumonia. Clin. Chim. Acta. 463, 174–180. doi:10.1016/j.cca.2016.10.030
Chen, Y., Hu, Y., Chen, H., Li, X., and Qian, P. (2020). A ferritin nanoparticle vaccine for foot-and-mouth disease virus elicited partial protection in mice. Vaccine 38 (35), 5647–5652. doi:10.1016/j.vaccine.2020.06.063
Cheng, L., Li, H., Li, L., Liu, C., Yan, S., Chen, H., et al. (2020). Ferritin in the coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J. Clin. Lab. Anal. 34 (10), e23618. doi:10.1002/jcla.23618
Christ-Crain, M., and Opal, S. M. (2010). Clinical review: the role of biomarkers in the diagnosis and management of community-acquired pneumonia. Crit. Care 14 (1), 203. doi:10.1186/cc8155
Fahmy, M., and Young, S. P. (1993). Modulation of iron metabolism in monocyte cell line U937 by inflammatory cytokines: changes in transferrin uptake, iron handling and ferritin mRNA. Biochem. J. 296 (Pt 1), 175–181. doi:10.1042/bj2960175
Fei, J., Fu, L., Cao, W., Hu, B., Zhao, H., and Li, J. B. (2019). Low vitamin D status is associated with epithelial-mesenchymal transition in patients with chronic obstructive pulmonary disease. J. Immunol. 203 (6), 1428–1435. doi:10.4049/jimmunol.1900229
Fei, J., Liu, L., Li, J. F., Zhou, Q., Wei, Y., Zhou, T. D., et al. (2024). Associations of vitamin D with GPX4 and iron parameters in chronic obstructive pulmonary disease patients: a case-control study. Can. Respir. J. 2024, 4505905. doi:10.1155/2024/4505905
Feld, J., Tremblay, D., Thibaud, S., Kessler, A., and Naymagon, L. (2020). Ferritin levels in patients with COVID-19: a poor predictor of mortality and hemophagocytic lymphohistiocytosis. Int. J. Lab. Hematol. 42 (6), 773–779. doi:10.1111/ijlh.13309
Feng, C. M., Cheng, J. Y., Xu, Z., Liu, H. Y., Xu, D. X., Fu, L., et al. (2021a). Associations of serum resistin with the severity and prognosis in patients with community-acquired pneumonia. Front. Immunol. 12, 703515. doi:10.3389/fimmu.2021.703515
Feng, C. M., Wang, X. M., Li, M. D., Xu, Z., Hua, D. X., Cheng, J. Y., et al. (2021b). Serum interleukin-17 predicts severity and prognosis in patients with community acquired pneumonia: a prospective cohort study. BMC Pulm. Med. 21 (1), 393. doi:10.1186/s12890-021-01770-6
Fu, L., Fei, J., Tan, Z. X., Chen, Y. H., Hu, B., Xiang, H. X., et al. (2021). Low vitamin D status is associated with inflammation in patients with chronic obstructive pulmonary disease. J. Immunol. 206 (3), 515–523. doi:10.4049/jimmunol.2000964
Ganz, T., and Nemeth, E. (2009). Iron sequestration and anemia of inflammation. Semin. Hematol. 46 (4), 387–393. doi:10.1053/j.seminhematol.2009.06.001
Ganz, T., and Nemeth, E. (2015). Iron homeostasis in host defence and inflammation. Nat. Rev. Immunol. 15 (8), 500–510. doi:10.1038/nri3863
Huang, H., Qiu, Y., Huang, G., Zhou, X., Zhou, X., and Luo, W. (2019). Value of ferritin heavy chain (FTH1) expression in diagnosis and prognosis of renal cell carcinoma. Med. Sci. Monit. 25, 3700–3715. doi:10.12659/MSM.914162
Jiang, X., Huang, C. M., Feng, C. M., Xu, Z., Fu, L., and Wang, X. M. (2021). Associations of serum S100A12 with severity and prognosis in patients with community-acquired pneumonia: a prospective cohort study. Front. Immunol. 12, 714026. doi:10.3389/fimmu.2021.714026
Kirkali, Z., Güzelsoy, M., Mungan, M. U., Kirkali, G., and Yörükoglu, K. (1999). Serum ferritin as a clinical marker for renal cell carcinoma: influence of tumor size and volume. Urol. Int. 62 (1), 21–25. doi:10.1159/000030349
Koorts, A. M., and Viljoen, M. (2007). Ferritin and ferritin isoforms I: structure-function relationships, synthesis, degradation and secretion. Arch. Physiol. Biochem. 113 (1), 30–54. doi:10.1080/13813450701318583
LaCanna, R., Liccardo, D., Zhang, P., Tragesser, L., Wang, Y., Cao, T., et al. (2019). Yap/Taz regulate alveolar regeneration and resolution of lung inflammation. J. Clin. Invest. 129 (5), 2107–2122. doi:10.1172/JCI125014
Li, M. D., Fu, L., Lv, B. B., Xiang, Y., Xiang, H. X., Xu, D. X., et al. (2022). Arsenic induces ferroptosis and acute lung injury through mtROS-mediated mitochondria-associated endoplasmic reticulum membrane dysfunction. Ecotoxicol. Environ. Saf. 238, 113595. doi:10.1016/j.ecoenv.2022.113595
Liu, H. Y., Xiang, H. X., Xiang, Y., Xu, Z., Feng, C. M., Fei, J., et al. (2021). The associations of serum S100A9 with the severity and prognosis in patients with community-acquired pneumonia: a prospective cohort study. BMC. Infect. Dis. 21 (1), 327. doi:10.1186/s12879-021-06020-y
Marrie, T. J. (2000). Community-acquired pneumonia in the elderly. Clin. Infect. Dis. 31 (4), 1066–1078. doi:10.1086/318124
Oppen, K., Ueland, T., Siljan, W. W., Skadberg, Ø., Brede, C., Lauritzen, T., et al. (2021). Hepcidin and ferritin predict microbial etiology in community-acquired pneumonia. Open Forum Infect. Dis. 8 (4), ofab082. doi:10.1093/ofid/ofab082
Orino, K., and Watanabe, K. (2008). Molecular, physiological and clinical aspects of the iron storage protein ferritin. Vet. J. 178 (2), 191–201. doi:10.1016/j.tvjl.2007.07.006
Pietsch, E. C., Chan, J. Y., Torti, F. M., and Torti, S. V. (2003). Nrf2 mediates the induction of ferritin H in response to xenobiotics and cancer chemopreventive dithiolethiones. J. Biol. Chem. 278 (4), 2361–2369. doi:10.1074/jbc.M210664200
Qeadan, F., Tingey, B., Gu, L. Y., Packard, A. H., Erdei, E., and Saeed, A. I. (2021). Prognostic values of serum ferritin and D-dimer trajectory in patients with COVID-19. Viruses 13 (3), 419. doi:10.3390/v13030419
Sangkhae, V., and Nemeth, E. (2017). Regulation of the iron homeostatic hormone hepcidin. Adv. Nutr. 8 (1), 126–136. doi:10.3945/an.116.013961
Sharif, K., Vieira, B. V., Zandman-Goddard, G., and Shoenfeld, Y. (2018). Eppur Si Muove: ferritin is essential in modulating inflammation. Clin. Exp. Immunol. 191 (2), 149–150. doi:10.1111/cei.13069
Sharkey, R. A., Donnelly, S. C., Connelly, K. G., Robertson, C. E., Haslett, C., and Repine, J. E. (1999). Initial serum ferritin levels in patients with multiple trauma and the subsequent development of acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 159 (5 Pt 1), 1506–1509. doi:10.1164/ajrccm.159.5.9809027
Suárez-Ortegón, M. F., Blanco, E., McLachlan, S., Fernandez-Real, J. M., Burrows, R., Wild, S. H., et al. (2019). Ferritin levels throughout childhood and metabolic syndrome in adolescent stage. Nutr. Metab. Cardiovasc. Dis. 29 (3), 268–278. doi:10.1016/j.numecd.2018.11.008
Sun, W., Cheng, Z., Chen, H., Lin, G., and Chen, H. (2020). Tetrahydropyrimidines, ZL-5015 alleviated lipopolysaccharide (LPS)-Induced acute pneumonia in rats by activating the NRF-2/HO-1 pathway. Med. Sci. Monit. 26, e924482. doi:10.12659/MSM.924482
Surguladze, N., Patton, S., Cozzi, A., Fried, M. G., and Connor, J. R. (2005). Characterization of nuclear ferritin and mechanism of translocation. Biochem. J. 388 (Pt 3), 731–740. doi:10.1042/BJ20041853
Torti, F. M., and Torti, S. V. (2002). Regulation of ferritin genes and protein. Blood 99 (10), 3505–3516. doi:10.1182/blood.v99.10.3505
Ueda, N., and Takasawa, K. (2018). Impact of inflammation on ferritin, hepcidin and the management of iron deficiency anemia in chronic kidney disease. Nutrients 10 (9), 1173. doi:10.3390/nu10091173
Wang, J. L., Chen, X., Xu, Y., Chen, Y. X., Wang, J., Liu, Y. L., et al. (2021). The associations of serum IL-37 with the severity and prognosis in patients with community-acquired pneumonia: a retrospective cohort study. Front. Immunol. 12, 636896. doi:10.3389/fimmu.2021.636896
Wang, W., Knovich, M. A., Coffman, L. G., Torti, F. M., and Torti, S. V. (2010). Serum ferritin: past, present and future. Biochim. Biophys. Acta. 1800 (8), 760–769. doi:10.1016/j.bbagen.2010.03.011
Xu, X., Zhou, M., Wu, X., Zhao, F., Luo, X., Li, K., et al. (2023). Increased iron deposition in nucleus accumbens associated with disease progression and chronicity in migraine. BMC Med. 21 (1), 136. doi:10.1186/s12916-023-02855-1
Xu, Z., Wang, X. M., Cao, P., Zhang, C., Feng, C. M., Zheng, L., et al. (2022). Serum IL-27 predicts the severity and prognosis in patients with community-acquired pneumonia: a prospective cohort study. Int. J. Med. Sci. 19 (1), 74–81. doi:10.7150/ijms.67028
Zandman-Goddard, G., and Shoenfeld, Y. (2007). Ferritin in autoimmune diseases. Autoimmun. Rev. 6 (7), 457–463. doi:10.1016/j.autrev.2007.01.016
Zhang, N., Yu, X., Xie, J., and Xu, H. (2021). New insights into the role of ferritin in iron homeostasis and neurodegenerative diseases. Mol. Neurobiol. 58 (6), 2812–2823. doi:10.1007/s12035-020-02277-7
Zhang, W. Z., Oromendia, C., Kikkers, S. A., Butler, J. J., O'Beirne, S., Kim, K., et al. (2020). Increased airway iron parameters and risk for exacerbation in COPD: an analysis from SPIROMICS. Sci. Rep. 10 (1), 10562. doi:10.1038/s41598-020-67047-w
Zheng, L., Fei, J., Feng, C. M., Xu, Z., Fu, L., and Zhao, H. (2021a). Serum 8-iso-PGF2α predicts the severity and prognosis in patients with community-acquired pneumonia: a retrospective cohort study. Front. Med. (Lausanne) 8, 633442. doi:10.3389/fmed.2021.633442
Zheng, L., Jiang, Y. L., Fei, J., Cao, P., Zhang, C., Xie, G. F., et al. (2021b). Circulatory cadmium positively correlates with epithelial-mesenchymal transition in patients with chronic obstructive pulmonary disease. Ecotoxicol. Environ. Saf. 215, 112164. doi:10.1016/j.ecoenv.2021.112164
Keywords: ferritin, community-acquired pneumonia, severity, prognosis, longitudinal relationship
Citation: Xu J, Han M-F, Ma K-S, Zhang T, Wang R-R, Zhang J-J, Zhao H and Fu L (2025) Longitudinal relationships between serum ferritin and prognosis among severe community-acquired pneumonia patients. Front. Pharmacol. 16:1556185. doi: 10.3389/fphar.2025.1556185
Received: 06 January 2025; Accepted: 17 April 2025;
Published: 20 May 2025.
Edited by:
Chun Xu, The University of Sydney, AustraliaReviewed by:
Eylem Acartürk Tuncay, Martyr Dr. İlhan Varank Sancaktepe Training and Research Hospital, TürkiyeHabib Ghedira, Independent Researcher, Tunis, Tunisia
Copyright © 2025 Xu, Han, Ma, Zhang, Wang, Zhang, Zhao and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Fu, ZnVsaW5AYWhtdS5lZHUuY24=; Hui Zhao, emhhb2h1aWNoZW54aUAxMjYuY29t
†These authors have contributed equally to this work
 Jing Xu1,2†
Jing Xu1,2† Hui Zhao
Hui Zhao Lin Fu
Lin Fu