Abstract
Background:
Cabozantinib combined with atezolizumab has been shown to prolong progression-free survival in first-line treatment of advanced hepatocellular carcinoma (HCC). However, the cost-effectiveness of this regimen remains unknown. This study aimed to evaluate the cost-effectiveness of cabozantinib plus atezolizumab compared with sorafenib for first-line treatment of advanced HCC from the perspectives of the Chinese health system and the US payers.
Methods:
A partitioned survival model was constructed based on a phase III randomized clinical trial (COSMIC-312) to compare the health benefits and economic outcomes of cabozantinib plus atezolizumab versus sorafenib for the treatment of hepatocellular carcinoma. Costs and utilities were obtained from published literature. Data recorded included quality-adjusted life years (QALYs), life years (LYs), and incremental cost-effectiveness ratio (ICER). One-way and probabilistic sensitivity analyses were conducted to test the robustness of the results. Subgroup analyses were also performed.
Results:
From the perspectives of the US payers and the Chinese health system, the base-case ICER values for cabozantinib plus atezolizumab compared to sorafenib were $-2,731,994.74/QALY and $-2,225,520.14/QALY, respectively. Sorafenib achieved an absolute dominance in terms of cost-effectiveness, offering greater benefits at a lower cost. The models were most sensitive to the utility values for progression-free survival and overall survival. Subgroup analyses also demonstrated that cabozantinib plus atezolizumab was unlikely to be cost-effective as a first-line treatment for advanced HCC.
Conclusion:
Cabozantinib plus atezolizumab was not a cost-effective treatment option for HCC when compared to sorafenib from both the Chinese healthcare system and the US payer perspectives.
1 Introduction
Hepatocellular carcinoma (HCC) ranks as the sixth most frequently diagnosed cancer and stands as the third leading cause of cancer-related mortality on a global scale (Bray et al., 2024). HCC and intrahepatic cholangiocarcinoma (iCCA) constitute the two major histological subtypes of primary liver cancer (Rumgay et al., 2022). HCC accounts for approximately 80% of all liver cancer cases worldwide, with an age-standardized incidence rate (ASR) of 7.3 per 100,000 person-years, making it the predominant pathological type of primary liver cancer (Rumgay et al., 2022). The prognosis of HCC depends on the tumor stage, the degree of underlying liver dysfunction, and the patient’s performance status (Forner et al., 2018). While early-stage HCC may be addressed with local therapeutic interventions such as surgical resection, radiotherapy, and transarterial chemoembolization (TACE), the majority of patients are diagnosed at an advanced stage, rendering them ineligible for surgical treatment. Consequently, systemic therapy emerges as a vital treatment approach for these individuals (Finn et al., 2020).
It is estimated that approximately 50%–60% of HCC patients will receive systemic therapy. Currently developed systemic treatments include tyrosine kinase inhibitors (TKIs), immune checkpoint inhibitors (ICIs), and anti-vascular endothelial growth factor (VEGF) antibodies (Llovet et al., 2021). Despite the notable progress in systemic therapies over recent years, the prognosis for the majority of patients with HCC remains grim, highlighting the urgent need for additional effective treatment options to serve a broader patient population (Yang et al., 2024). Sorafenib has been shown to significantly improve overall survival (OS) and has been the gold standard for the systemic treatment of advanced HCC since its approval in 2007 (Llovet et al., 2008). However, sorafenib resistance develops in most patients, limiting its clinical benefits (Gauthier and Ho, 2013).
Studies on treatment regimens incorporating ICIs are currently a focus in HCC nowadays, especially in combination with TKI drugs (Zheng et al., 2025). Cabozantinib is a multi-targeted TKI that exhibits significant activity in inhibiting HCC tumor cells. In the CELESTIAL trial, cabozantinib demonstrated a significant improvement in median overall survival and progression-free survival (PFS) compared to placebo in patients who had failed sorafenib therapy (Abou-Alfa et al., 2018). The activity of cabozantinib may be attributed to its dual inhibition of VEGFR2 and MET signaling pathways. Given that one of the mechanisms of sorafenib resistance is the overexpression of MET in HCC cells (Wu et al., 2024; Xiang et al., 2014), the additional inhibitory effects of cabozantinib on MET make it a preferred second-line treatment option for patients who have developed resistance to sorafenib.
The potential of combination therapies has been underscored by the positive outcomes of the IMbrave150 and HIMALAYA trials, which demonstrated promising activity and safety profiles (Finn et al., 2020; Sangro et al., 2024). The COSMIC-312 trial further highlighted the clinical benefits of combining cabozantinib, a TKI, with the immune checkpoint inhibitor atezolizumab in the treatment of solid tumors, including advanced HCC. This trial evaluated the efficacy of cabozantinib plus atezolizumab compared with sorafenib as first-line systemic therapy for advanced hepatocellular carcinoma. The study reported that the combination therapy significantly extended PFS compared to sorafenib (6.9 months vs. 4.3 months; hazard ratio [HR], 0.74; 99% confidence interval [CI], 0.56–0.97), but there was no significant difference in OS between the two treatment groups (16.5 months vs. 15.5 months; HR, 0.98; 96% CI, 0.78–1.24) (Yau et al., 2024). Although no significant differences in overall survival rates were observed, the benefits of the combination therapy regimen on progression-free survival and disease control still noteworthy, may become a new treatment option. In terms of cost, the combination therapy is expensive, and its economic assessment in regions with different economic conditions remains unclear. As a developed country, the United States spends 20% of its gross domestic product on healthcare (Alspaugh et al., 2021). The high medical costs will bring enormous financial pressure to the healthcare system. After the implementation of the Affordable Care Act, the Budget Control Act, and other policies, the ability of some patients to afford medical services has been somewhat improved, but for many others, it is still far from sufficient. (Jones and Kantarjian, 2019; Zheng and Sandhu, 2023; Mullangi and Eagle, 2023). As the largest developing country, China has achieved near-universal health insurance coverage, which benefits a large number of people, but this also means increased financial pressure (Chen et al., 2022; Yip et al., 2019). Decisions on healthcare policy and resource allocation will significantly affect the overall efficiency and effectiveness of the healthcare system. Therefore, this study aims to construct a partitioned survival model based on the data from the COSMIC-312 trial to compare the cost-effectiveness of cabozantinib plus atezolizumab versus sorafenib as first-line treatment for advanced, unresectable hepatocellular carcinoma from the perspectives of the Chinese healthcare system and the US payers, providing a reference for clinical drug selection decisions.
2 Methods
2.1 Population and interventions
The target patient population for this analysis was assumed to be similar to that of the COSMIC-312 trial, consisting of patients aged 18 years or older with histologically confirmed, previously untreated advanced HCC, Barcelona Clinic Liver Cancer (BCLC) stage B or C, Child-Pugh class A, and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Two treatment regimens for advanced HCC were compared: cabozantinib plus atezolizumab combination (cabozantinib 40 mg orally once daily plus atezolizumab 1200 mg intravenously every 3 weeks) versus sorafenib monotherapy (sorafenib 400 mg orally twice daily) as first-line therapy. Treatment was discontinued upon disease progression or intolerable adverse events, and patients then received best supportive care (BSC) and subsequent treatments until death. All deceased patients were considered to receive end-of-life care.
2.2 Model overview
This study reports on the basis of the Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) checklist (Husereau, et al., 2022), which is included as Supplementary Table S1 within the Supplementary Materials. A partitioned survival was constructed using Excel for cost-effectiveness analysis (as depicted in Figure 1). Patients were assumed to be in the PFS state at baseline, and could progress to death from any state. Time horizon was set to 5 years, with a cycle length of 3 weeks. By the end of the final cycle, the cumulative mortality rate was projected to exceed 95%. The study was conducted from the dual perspectives of the US payers and the Chinese healthcare system. The primary outcomes measured in the analysis were costs, life-years (LYs), quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratio (ICER). Willingness-to-pay (WTP) thresholds were set at $150,000/QALY and $37,863/QALY for the US and China, respectively (Neumann et al., 2014; Liu et al. 2020). A treatment strategy was deemed not cost-effective if its ICER surpassed these respective WTP thresholds. Annual discount rates for both costs and utilities were set at the commonly used rate of 5% (Attema et al., 2018).
FIGURE 1
Model Structure. (A) Partitioned survival model overview. (B) Interventions for advanced hepatocellular carcinoma in first-line treatment.
2.3 Survival estimate
Given the limited follow-up time in the COSMIC-312 trial, extrapolation of the reported survival curves is necessary to obtain the required survival data. Using algorithms developed by Guyot and Wei (Guyot et al., 2012; Wei and Royston, 2017), pseudo-patient individual data was generated using Stata 17 (StataCorp LLC. College Station, Texas), and the survival curve was reconstructed and extended. The individual patient data were obtained from published clinical trials by digitizing the Kaplan-Meier survival curves using GetData Graph Digitizer version 2.25. Parametric survival models were fitted to these data using various distributions, including Weibull, Exponential, Gompertz, Log-logistic, and Log-normal. To reflect the most realistic clinical outcomes and physiological course, the distributions with the lowest Akaike information criterion (AIC) and Bayesian information criterion (BIC) values were not exclusively used in this study. Instead, both the Weibull and Log-normal distributions were fitted to the OS and PFS curves for both treatment arms. The AIC and BIC values of different Kaplan–Meier curve fitting distributions, as well as the goodness of fit, are presented in the Supplementary Table S2; Supplementary Figure S1. The distribution parameters of the survival curves are shown in Table 1.
TABLE 1
| Parameter | Cabozantinib plus atezolizumab | Sorafenib | Distribution | References |
|---|---|---|---|---|
| Weibull survival model for OS | scale = 0.016686 shape = 1.319320 |
scale = 0.032411 shape = 1.079312 |
Yau et al. (2024) | |
| Log-normal survival model for PFS | μ = 1.911015 σ = 1.086205 |
μ = 1.615714 σ = 1.113573 |
Yau et al. (2024) | |
| Incidence of grade 3/4 AEs | ||||
| PPES | 0.08 | 0.08 | Beta (88.277, 1015.183) | Kelley et al. (2022) |
| AST increased | 0.09 | 0.035 | Beta (87.306, 882.765) (92.644, 2554.316) | Kelley et al. (2022) |
| ALT increased | 0.09 | 0.025 | Beta (87.306, 882.765) (93.614, 3650.946) | Kelley et al. (2022) |
| Hypertension | 0.09 | 0.08 | Beta (87.306, 882.765) (88.277, 1015.183) | Kelley et al. (2022) |
| Fatigue | 0.03 | 0.04 | Beta (93.129, 3011.165) (92.158, 2211.802) | Kelley et al. (2022) |
| Utilities | ||||
| PFS | 0.76 (0.61–0.91) | Beta (22.908, 7.234) | Su et al. (2021) | |
| PD | 0.68 (0.54–0.82) | Beta (28.322, 13.328) | Su et al. (2021) | |
| Disutility due to AEs | ||||
| PPES | −0.116 | Beta (84.783, 646.108) | Parikh et al. (2017) | |
| AST increased | 0 | Beta | Xu et al. (2019) | |
| ALT increased | 0 | Beta | Xu et al. (2019) | |
| Hypertension | −0.012 | Beta (94.876, 7811.418) | Meng et al. (2022) | |
| Fatigue | −0.11 | Beta (85.366, 690.685) | Chiang et al. (2021) | |
| Proportion receiving subsequent treatment | 0.2 | 0.37 | Beta (76.632, 306.528) (60.135, 102.392) | Kelley et al. (2022) |
Key clinical parameters and health utility inputs.
AEs, adverse events; ALT, alanine aminotransferase; AST, aspartate aminotransferase; OS, overall survival; PD, progression disease; PFS, progression-free survival; PPES, Palmar-plantar erythrodysaesthesia syndrome.
2.4 Cost and utility
For this analysis, only direct medical costs were considered. These costs primarily included: drug costs, administration fees, follow-up care, imaging costs, best supportive care costs, treatment costs for serious adverse events, and costs of subsequent and end-of-life care. To facilitate comparison, all costs for both countries were reported in the US dollars. The exchange rate between the United States dollar (USD) and the Chinese Yuan (CNY) in 2024 was as follows: 1 USD = 7.23 CNY (State Administration of Foreign Exchange, 2024).
Drug prices in this study were sourced from online databases (Menet, 2024; Drugshk, 2024; Medicare Part B, 2024; Medicare Part D, 2024). Other costs were obtained from published literature (Su et al., 2021; Parikh et al., 2017; Xu et al., 2019; Meng et al., 2022; Wu et al., 2018; Lu et al., 2017; Sun et al., 2022; Chen et al., 2023; Shi et al., 2018; Qin et al., 2018; Kobayashi et al., 2019; Soto-Perez-De-Celis et al., 2019; Li et al., 2022; Zhan et al., 2021; Sieg et al., 2020). As cabozantinib is not yet available in mainland China, we referenced prices from Hong Kong. Serious adverse events (AEs) were defined as grade 3 or 4 adverse reactions. The incidence of AEs was derived from the COSMIC-312 study (Kelley et al., 2022), including hypertension, increased aspartate aminotransferase/alanine aminotransferase, fatigue, and hand-foot syndrome. Costs associated with AEs were calculated by multiplying the probability of experiencing an AE by the cost per event. And we assume that all AEs occur in the first cycle and only happen once. After disease progression, 20% of patients in the combination therapy group and 37% of patients in the sorafenib group received subsequent systemic therapy. All costs are shown in Table 2.
TABLE 2
| Parameter | China mean (range) | US mean (range) | Distribution |
|---|---|---|---|
| Cost ($) | |||
| Cabozantinib per 40 mg tablet | 299.67 (239.74–359.60) (Drugshk, 2024) | 898.34 (718.67–1078.01) (Medicare Part D, 2024) | Gamma (96.053, 3.120) (96.038, 9.354) |
| Atezolizumab per 1200 mg | 4574.38 (3659.50–5489.26) (Menet, 2024) | 11,177.52 (8942.02–13413.02) (Medicare Part B, 2024) | Gamma (95.997, 47.641) (96.083, 116.332) |
| Sorafenib per 200 mg | 12.35 (9.88–14.82) (Menet, 2024) | 179.23 (143.38–215.08) (Medicare Part D, 2024) | Gamma (96.040, 0.129) (96.019, 1.867) |
| Drug administration | 41.00 (32.80–49.2) (Wu, et al., 2018) | 147.44 (117.95–176.93) (Zhang, et al., 2021) | Gamma (96.040, 0.427) (96.027, 1.535) |
| Follow-up | 39.66 (31.73–47.59) (Chen et al., 2023) | 212.00 (169.60–254.40) (Li et al., 2022) | Gamma (96.088, 0.413) (96.040, 2.207) |
| CT imaging (per 6 weeks) | 85.01 (68.01–102.01) (Sun et al., 2022) | 783.00 (626.40–939.60) (Zhang et al., 2021) | Gamma (96.063, 0.885) (96.040, 8.153) |
| Best support care | 337.50 (270.00–405.00) (Lu et al., 2017) | 2871.00 (2296.80–3445.20) (Su et al., 2021) | Gamma (96.040, 3.514) (96.040, 29.894) |
| End-of-life care | 278.21 (222.57–333.85) (Sun et al., 2022) | 7739.00 (6191.20–9286.80) (Soto-Perez-De-Celis et al., 2019) | Gamma (96.047, 2.897) (96.040, 80.581) |
| Subsequent treatment | |||
| Cabozantinib plus Atezolizumab | 394.73 (315.78–473.67) (Menet, 2024; Kelley et al., 2022) | 5712.40 (4569.92–6854.88) (Medicare Part B, 2024; Medicare Part D, 2024; Kelley et al., 2022) | Gamma (96.042, 4.110) (96.040, 59.479) |
| Sorafenib | 1355.79 (1084.63–1626.95) (Menet, 2024; Kelley et al., 2022) | 12,715.35 (10,172.28–15258.42) (Medicare Part B, 2024; Medicare Part D, 2024; Kelley et al., 2022) | Gamma (96.039, 14.117) (96.040, 132.396) |
| Cost of managing AEs (grade 3/4) per event | |||
| PPES | 12.00 (9.60–14.40) (Shi et al., 2018) | 385.00 (308.00–462.00) (Sieg et al., 2020) | Gamma (96.040, 0.125) (96.040, 4.009) |
| AST/ALT increased | 59.00 (47.2–70.8) (Qin et al., 2018) | 59.00 (47.20–70.80) (Qin et al., 2018) | Gamma (96.040, 0.614) |
| Hypertension | 37.00 (29.6–44.4) (Qin, et al., 2018) | 78.00 (62.40–93.60) (Sieg et al., 2020) | Gamma (96.040, 0.385) (96.040, 0.812) |
| Fatigue | 3.00 (2.4–3.6) (Zhou et al., 2022) | 93.00 (74.40–111.60) (Chiang et al., 2021) | Gamma (96.040, 0.031) (96.040, 0.968) |
| Discount rate | 0.05 (0–0.08) (Attema et al., 2018) | Beta (5.652, 107.395) | |
Cost inputs.
AEs, adverse events; ALT, alanine aminotransferase; AST, aspartate aminotransferase; OS, overall survival; PD, progression disease; PFS, progression-free survival; PPES, Palmar-plantar erythrodysaesthesia syndrome.
The baseline utility value for patients in the PFS state was 0.76 for both groups, and 0.68 (Su et al., 2021) for those in the PD state. Negative utility values associated with grade 3/4 AEs were also incorporated into this analysis.
2.5 Sensitivity analysis
To assess the robustness of the model, both one-way sensitivity analysis and probabilistic sensitivity analysis were conducted. The one-way sensitivity analysis was used to examine the impact of varying each parameter on the outcomes, with the results graphically depicted in a tornado diagram. In instances where the value range for a parameter was not available, a ±20% variation was assumed for the analysis. The probabilistic sensitivity analysis was performed using Excel, with 1000 Monte Carlo simulations. The findings from this analysis were visualized through a cost-effectiveness acceptability curve, which provides a comprehensive view of the probability that each treatment strategy is cost-effective at various WTP thresholds.
2.6 Scenario analysis and subgroup analysis
A scenario analysis with a 10-year time horizon was conducted to evaluate long-term cost-effectiveness. Subgroup analyses were conducted on predefined subgroups from the COSMIC-312 trial. These analyses adjusted the HRs for PFS and OS to explore the influence of these subgroups on the overall results. The calculation method for subgroup analysis is derived from Hoyle et al. (2010), Collett (2014). According to the methods mentioned in these studies, when the OS and PFS of the subgroup and the overall population meet the proportional hazards assumption, the relationship between the survival rates of the two groups and the HR can be derived: the survival rate of the subgroup is equal to the survival rate of the overall population raised to the power of the hazard ratio.
3 Results
3.1 Base-case results
The results of the baseline analysis were presented in Table 3. The cumulative cost for the sorafenib group in the US was $363,441.38, while for the cabozantinib plus atezolizumab group, it was $539,208.05. In China, the cumulative costs were $30,492.33 for the sorafenib group and $173,674.26 for the cabozantinib plus atezolizumab group. Sorafenib demonstrated a higher number of QALYs with 1.21 compared to 1.15 for the combination group, and a higher number of LYs with 1.70 versus 1.58for the combination group. The combination group incurring a higher cost, and it yielded a lower QALY and a lower LY, resulting in a negative ICER. Consequently, the regimen of cabozantinib in combination with atezolizumab was deemed not cost-effective in both the US and China.
TABLE 3
| Strategy | Cost ($) | QALYs | ICER ($/QALY) | LYs |
|---|---|---|---|---|
| Base-case result | ||||
| US | ||||
| Sorafenib | 363,441.38 | 1.21 | 1.70 | |
| Cabozantinib plus Atezolizumab | 539,208.05 | 1.15 | −2,731,994.74 | 1.58 |
| China | ||||
| Sorafenib | 30,492.33 | 1.21 | 1.70 | |
| Cabozantinib plus Atezolizumab | 173,674.26 | 1.15 | −2,731,994.74 | 1.58 |
| Scenario analysis result | ||||
| US | ||||
| Sorafenib | 386,702.46 | 1.27 | 1.79 | |
| Cabozantinib plus Atezolizumab | 558,137.28 | 1.16 | −1,621,800.98 | 1.60 |
| China | ||||
| Sorafenib | 32,825.14 | 1.27 | 1.79 | |
| Cabozantinib plus Atezolizumab | 180,330.28 | 1.16 | −1,395,422.36 | 1.60 |
Summary of base-case result and scenario analysis.
ICER, incremental cost-effectiveness ratio; LY, life year; QALY, quality-adjusted life year.
3.2 Sensitivity analyses
In this model, the tornado diagram from the univariate sensitivity analysis (Figure 2) showed that in the US, the utility values for patients in the PD and PFS states had the greatest impact on the results. These were trailed by the unit cost of cabozantinib, sorafenib and atezolizumab. The costs of subsequent treatment in both the cabozantinib and sorafenib groups exerted a significant impact. In China, utility values for patients with PD and PFS status exert the most significant influence, while discount rates and the unit costs of cabozantinib and atezolizumab also carry substantial weight. Even with ±20% variations in baseline parameter values, conclusions remain consistent with the baseline case analysis, demonstrating the model’s robustness.
FIGURE 2
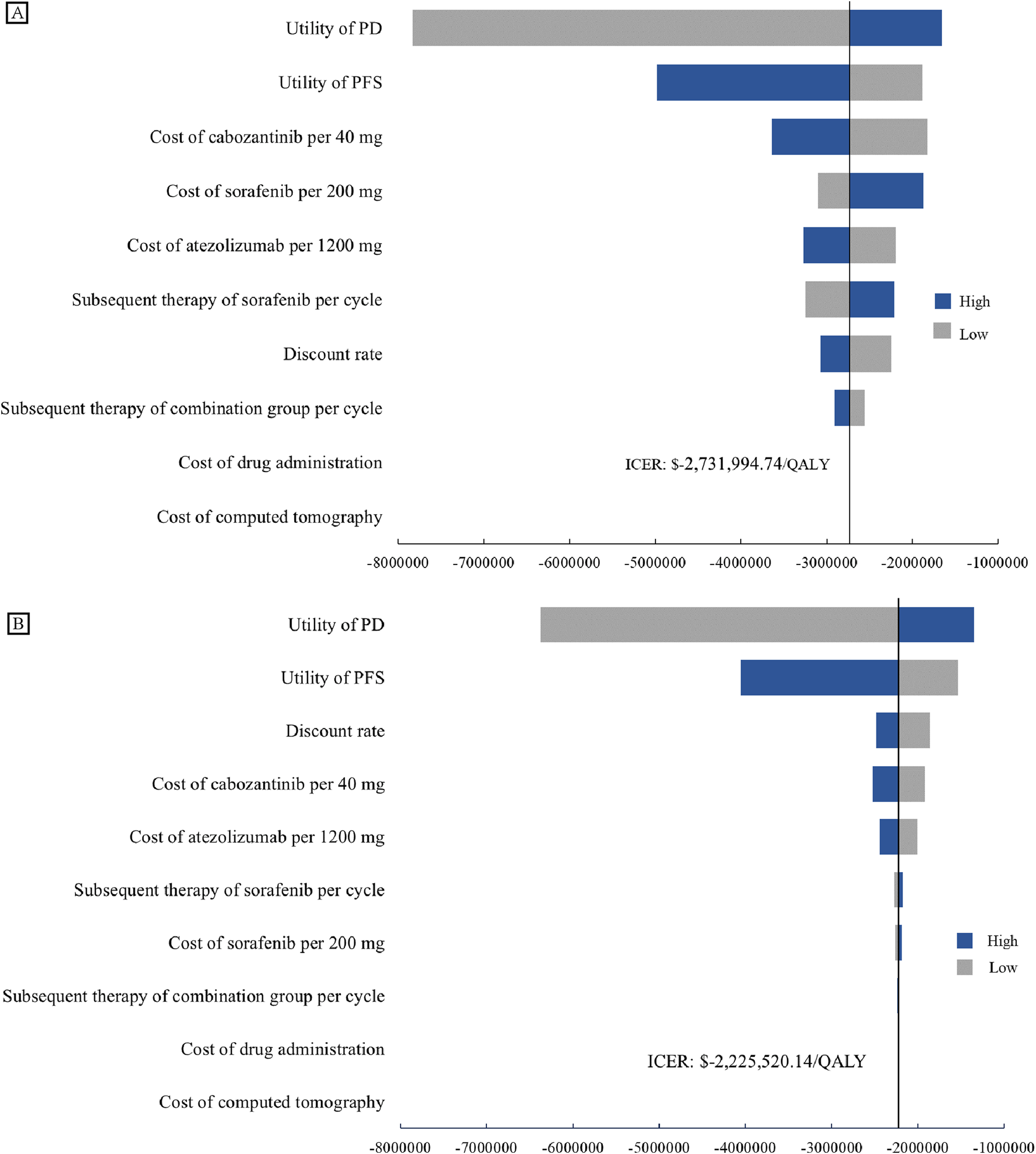
One-way Sensitivity Analyses. Tornado diagrams show the impact on the model from the perspectives of the US (A) and China (B). Both tornado diagrams showing one-way sensitivity analyses for each variable in the ±20% range. BSC, best support care; ICER, incremental cost-effectiveness ratio; PD, progression disease; PFS, progression-free survival.
The cost-effectiveness acceptance curve (as shown in Figure 3) indicates that sorafenib demonstrates superior cost-effectiveness compared to the cabozantinib plus atezolizumab regimen for treating advanced hepatocellular carcinoma, regardless of the United States or China.
FIGURE 3
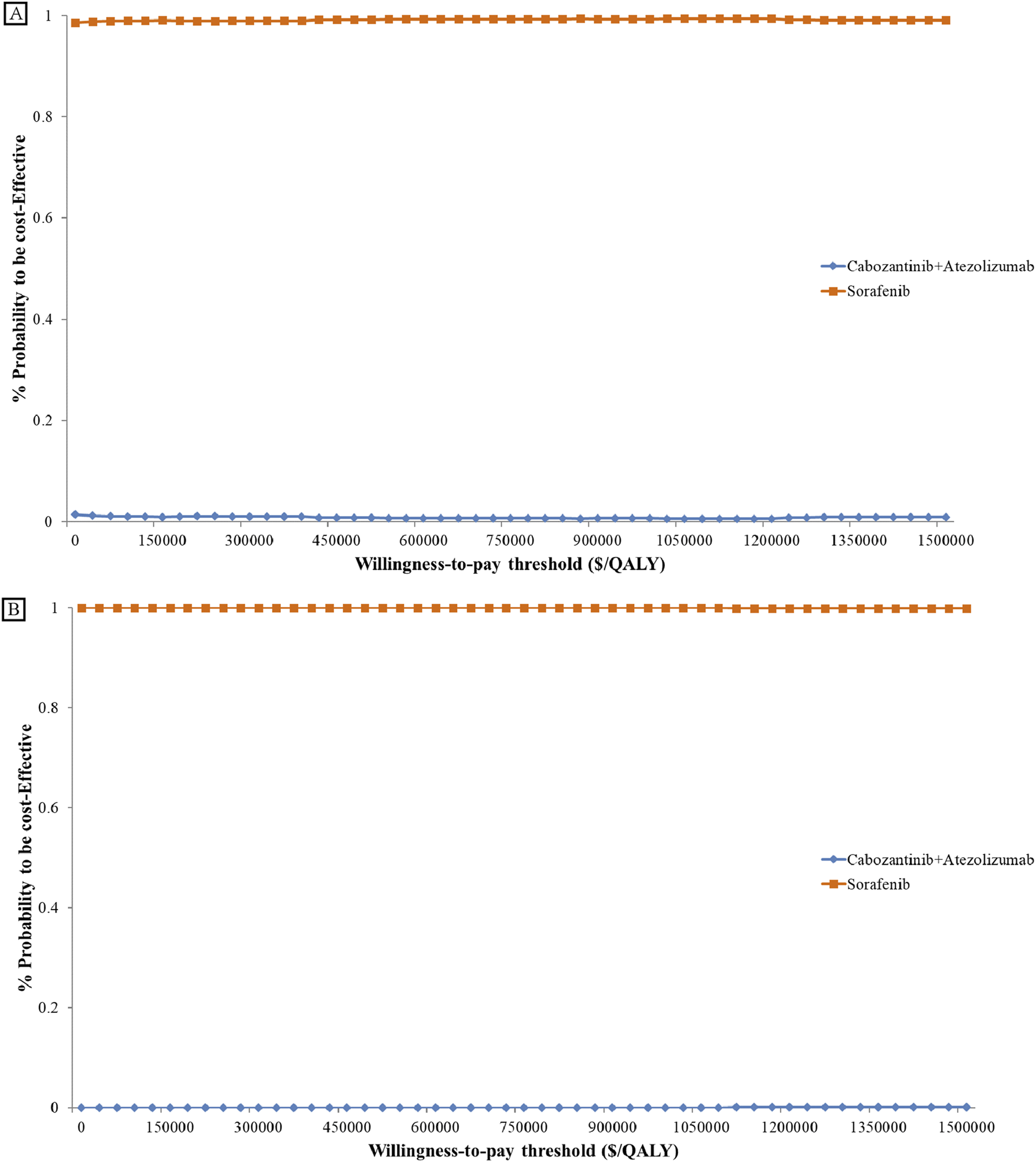
Cost-effectiveness Acceptability Curve. Cost-effectiveness acceptability curves present the probability to be cost-effective of cabozantinib plus atezolizumab compare sorafenib at different WTP thresholds from the US (A) and China (B). The y-axis in the graph represents the percentage acceptable and the x-axis represents the willingness-to-pay thresholds QALY, quality-adjusted life-year.
3.3 Scenario analysis and subgroup analyses
Scenario analysis results indicate that, over a 10-year period, the combination group gained 1.19 QALYs, while the sorafenib group gained 1.30 QALYs. Total costs for the combination group were $639,483.16 in the United States and $194,828.76 in China. The total costs for the sorafenib group were $526,145.83 and $47,101.83, respectively. The ICER for cabozantinib plus atezolizumab was -$971,191.65/QALY in the United States and -$1,265,877.51/QALY in China (Table 3).
Subgroup analyses (provided in the Supplementary Table S3) revealed that patients from the Asian region, or with BCLC stage C, hepatitis B virus related liver cancer, or extrahepatic disease or macrovascular invasion, experienced greater survival benefits with cabozantinib plus atezolizumab compared to sorafenib. However, the ICERs in these subgroups still significantly exceeded the WTP threshold. In other subgroups, sorafenib remained dominant, offering more QALYs at a lower cost.
4 Discussion
This study constructed a partitioned survival model based on data from the COSMIC-312 trial and relevant literature to evaluate the cost-effectiveness of cabozantinib plus atezolizumab versus sorafenib as first-line treatments for advanced hepatocellular carcinoma from the perspectives of the US payers and the Chinese healthcare system. The results showed that the combination therapy yielded fewer QALYs and LYs over a five-year time horizon compared to sorafenib. Moreover, the combination therapy incurred significantly higher costs than sorafenib in both the US and China. Consequently, the combination of cabozantinib and atezolizumab is not likely to be considered cost-effective as a first-line treatment option in either country. Although subgroup analyses suggested that certain subgroups derived more significant survival benefits from the combination therapy, the substantial costs of the drugs made the combination therapy an unlikely cost-effective choice.
Due to the high cost of healthcare in the United States, the total cost of the two treatment strategies and their difference are proportionally smaller. In China, although the total cost of the combination therapy group is nearly four times that of the sorafenib group, the relative cost difference is not significantly different from the values observed in the United States. Therefore, the ICER is similar in both countries. Regarding the WTP threshold, the threshold we assumed for the United States is approximately four times that of China, which is related to the development levels and national consumption capacity of the two countries. Although the threshold setting is rough, it can serve as a reasonable assumption when analyzing whether an investment is justified (Neumann et al., 2014).
Previous trials in advanced HCC have reported clinical trial results for ICIs or TKIs as monotherapy, or combination strategies involving multiple ICIs (Finn et al., 2020; Llovet et al., 2021; Abou-Alfa et al., 2018; Yau et al., 2022). The combination of cabozantinib and atezolizumab represented the first Phase III randomized controlled trial to assess the synergistic effect of a TKI and an ICI. While this combination demonstrated some potential in delaying disease progression, clinical studies have shown that this benefit in PFS did not translate into OS benefit. In fact, the overall survival of the combination therapy group showed a declining trend compared to the standard first-line treatment, sorafenib. In our study, leveraging the fitted survival data and the extrapolation of survival curves, sorafenib even demonstrated superior survival benefits after disease progression compared to the combination therapy (0.67 QALYs vs. 0.46 QALYs). The reasons for this finding are currently unknown, but it is speculated that the higher toxicity associated with the combination therapy in clinical trials may have led to dose reductions or treatment interruptions, ultimately affecting overall survival. Additionally, the rate of accepted subsequent treatment in the sorafenib group was higher than that in the combination therapy group, and this rate was influenced by multiple factors. Overall, the results for overall survival may be underestimated.
Systemic therapies for unresectable advanced HCC have seen significant advancements in recent years. The success of atezolizumab plus bevacizumab and durvalumab plus tremelimumab has marked a breakthrough in systemic treatment, transcending the traditional standard of care that relied solely on TKIs. Immunotherapy is poised to become a more popular option. As clinical trial results for these new therapies have been published, accompanying pharmacoeconomic analyses have been undertaken. However, almost all studies have indicated that, in comparison to sorafenib, combination immunotherapy is unlikely to be cost-effective Wen et al. (2021), Su et al. (2021). Evaluated the cost-effectiveness of atezolizumab plus bevacizumab versus sorafenib as first-line treatments for advanced HCC patients from the perspectives of the Chinese healthcare system and the US payers, respectively. Both studies found that even though the combination of atezolizumab and bevacizumab significantly improved PFS and OS, its high cost made the regimen unaffordable, and the ICER of the combination therapy exceeded the WTP threshold. In our study, univariate sensitivity analyses demonstrated that drug pricing was a substantial determinant of cost-effectiveness, suggesting that reductions in drug prices could render the combination therapy more cost-effective. When the total prices of cabozantinib and atezolizumab were reduced to 59.8% and 13.1% of their baseline values in the US and China, respectively, the combination therapy became more favorable. This means that the cabozantinib plus atezolizumab strategy achieves a slightly lower QALY at a lower cost, making it a reasonable choice compared to sorafenib.
Our study had several limitations. Firstly, the majority of the cost data in this study were obtained from published literature rather than from real-world data, which may introduce some bias. However, univariate sensitivity analysis showed that these cost data had a relatively small impact on the results. Secondly, utility values, as key parameters in pharmacoeconomic evaluations, are influenced by regional, ethnic, and cultural differences. The utility values utilized in this study were extracted from a clinical trial that spanned 178 centers across 32 countries, encompassing regions such as Asia and Europe. Additionally, univariate sensitivity analysis showed that utility values for patients in the PD and PFS states had the greatest impact on the results, potentially leading to biased outcomes. Thirdly, to streamline the model, only severe adverse events (grade 3/4) were included in this study, which might introduce some bias. However, sensitivity analysis showed that these parameters had a limited impact on the results. Furthermore, the follow-up time in the COSMIC-312 trial was relatively short, and the extrapolation of long-term efficacy through survival curve fitting may not accurately reflect the actual long-term effects in real-world settings.
5 Conclusion
In conclusion, from the perspectives of the US payers and the Chinese healthcare system, cabozantinib plus atezolizumab as first-line therapy for HCC is unlikely to be cost-effective compared to sorafenib, despite the potential progression-free survival benefits.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’; legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
YZ: Conceptualization, Methodology, Writing – original draft. CH: Data curation, Methodology, Writing – original draft. S-CC: Formal Analysis, Writing – review and editing. H-JL: Investigation, Writing – review and editing. R-XC: Conceptualization, Writing – review and editing. Z-JB: Formal Analysis, Writing – review and editing. T-XX: Methodology, Visualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Science and Technology Program of Guizhou Province [grant number QKHJC-zk [2025]mianshang409]; Health Commission of Guizhou Province [grant number gzwkj 2025-537], [grant number gzwkj 2022-220].
Acknowledgments
We sincerely thank all the professors who have provided us with guidance and technical assistance.
Conflict of interest
Author R-XC was employed by Sinopharm Group Tongjitang (Guizhou) Pharmaceutical Co., Ltd. Author Z-JB was employed by Guangxi Shenli Pharmaceutical Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fphar.2025.1735585.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1556304/full#supplementary-material
References
1
Abou-Alfa G. K. Meyer T. Cheng A.-L. El-Khoueiry A. B. Rimassa L. Ryoo B.-Y. et al (2018). Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med.379 (1), 54–63. 10.1056/NEJMoa1717002
2
Alspaugh A. Lanshaw N. Kriebs J. Van Hoover C. (2021). Universal health care for the united states: a primer for health care providers. J. Midwifery Womens Health66 (4), 441–451.
3
Attema A. E. Brouwer W. B. F. Claxton K. (2018). Discounting in economic evaluations. PharmacoEconomics36 (7), 745–758. 10.1007/s40273-018-0672-z
4
Bray F. Laversanne M. Sung H. Ferlay J. Siegel R. L. Soerjomataram I. et al (2024). Global cancer statistics 2022: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.74 (3), 229–263. 10.3322/caac.21834
5
Chen X. Giles J. Yao Y. Yip W. Meng Q. Berkman L. et al (2022). The path to healthy ageing in China: a Peking University-Lancet Commission. Lancet400 (10367), 1967–2006.
6
Chen X. Zhao M. Tian L. (2023). Economic evaluation of five first-line Pd-(L)1 inhibitors for treating non-squamous non-small cell lung cancer in China: a cost-effectiveness analysis based on network meta-analysis. Front. Pharmacol.14, 1119906. 10.3389/fphar.2023.1119906
7
Chiang C. L. Chan S. K. Lee S. F. Wong I. O. L. Choi H. C. W. (2021). Cost-Effectiveness of pembrolizumab as a second-line therapy for hepatocellular carcinoma. Jama Netw. Open4 (1), e2033761. 10.1001/jamanetworkopen.2020.33761
8
Collett D. (2014). Modelling survival data in medical research. 3rd ed.Chapman and Hall/CRC.
9
Drugshk (2024). Search cancer drugs. Available online at: https://drugs-hk.squarespace.com/ (Accessed April 9, 2024).
10
Finn R. S. Qin S. Ikeda M. Galle P. R. Ducreux M. Kim T.-Y. et al (2020). Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med.382 (20), 1894–1905. 10.1056/NEJMoa1915745
11
Forner A. Reig M. Bruix J. (2018). Hepatocellular carcinoma. Lancet London, Engl.391 (10127), 1301–1314. 10.1016/S0140-6736(18)30010-2
12
Gauthier A. Ho M. (2013). Role of sorafenib in the treatment of advanced hepatocellular carcinoma: an update. Hepatology Res. Official J. Jpn. Soc. Hepatology43 (2), 147–154. 10.1111/j.1872-034X.2012.01113.x
13
Guyot P. Ades A. E. Ouwens M. J. N. M. Welton N. J. (2012). Enhanced secondary analysis of survival data: reconstructing the data from published kaplan-meier survival curves. BMC Med. Res. Methodol.12, 9. 10.1186/1471-2288-12-9
14
Hoyle M. Green C. Thompson-Coon J. Liu Z. Welch K. Moxham T. et al (2010). Cost-effectiveness of temsirolimus for first line treatment of advanced renal cell carcinoma. Value Health13 (1), 61–68. 10.1111/j.1524-4733.2009.00617.x
15
Husereau D. Drummond M. Augustovski F. de Bekker-Grob E. Briggs A. H. Carswell C. et al (2022). Consolidated health economic evaluation reporting standards 2022 (cheers 2022) statement: updated reporting guidance for health economic evaluations. Value Health J. Int. Soc. For Pharmacoeconomics Outcomes Res.25 (1), 3–9. 10.1016/j.jval.2021.11.1351
16
Jones G. Kantarjian H. (2019). The many roads to universal health care in the USA. Lancet Oncol.20 (10), e601–e605.
17
Kelley R. K. Rimassa L. Cheng A.-L. Ahmed K. Qin S. Zhu A. X. et al (2022). Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (Cosmic-312): a multicentre, open-label, randomised, phase 3 trial. Lancet. Oncol.23 (8), 995–1008. 10.1016/S1470-2045(22)00326-6
18
Kobayashi M. Kudo M. Izumi N. Kaneko S. Azuma M. Copher R. et al (2019). Cost-effectiveness analysis of lenvatinib treatment for patients with unresectable hepatocellular carcinoma (uhcc) compared with sorafenib in Japan. J. Gastroenterology54 (6), 558–570. 10.1007/s00535-019-01554-0
19
Li Y. Liang X. Li H. Chen X. (2022). Atezolizumab plus bevacizumab versus nivolumab as first-line treatment for advanced or unresectable hepatocellular carcinoma: a cost-effectiveness analysis. Cancer128 (22), 3995–4003. 10.1002/cncr.34457
20
Liu G. G. Hu S. Wu J. Wu J. Dong Z. Hongchao Li (2020). China guidelines for pharmacoeconomic evaluations 2020 edition. Beijing: China Market Press.
21
Llovet J. M. Ricci S. Mazzaferro V. Hilgard P. Gane E. Blanc J. F. et al (2008). Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med.359 (4), 378–390. 10.1056/NEJMoa0708857
22
Llovet J. M. Kelley R. K. Villanueva A. Singal A. G. Pikarsky E. Roayaie S. et al (2021). Hepatocellular carcinoma. Nat. Rev. Dis. Prim.7 (1), 6. 10.1038/s41572-020-00240-3
23
Lu S. Ye M. Ding L. Tan F. Fu J. Wu B. (2017). Cost-effectiveness of gefitinib, icotinib, and Pemetrexed-Based chemotherapy as first-line treatments for advanced non-small cell lung cancer in China. Oncotarget8 (6), 9996–10006. 10.18632/oncotarget.14310
24
Medicare Part B (2024). Available online at: https://www.cms.gov/medicare/payment/part-b-drugs/asp-pricing-files (Accessed April 9, 2024).
25
Medicare Part D (2024). Available online at: https://data.cms.gov/summary-statistics-on-use-and-payments/medicare-medicaid-spending-by-drug/medicare-part-d-spending-by-drug (Accessed April 9, 2024).
26
Menet (2024). Available online at: https://www.menet.com.cn/ (Accessed April 9, 2024).
27
Meng R. Cao Y. Zhou T. Hu H. Qiu Y. (2022). The cost effectiveness of donafenib compared with sorafenib for the first-line treatment of unresectable or metastatic hepatocellular carcinoma in China. Front. Public Health10, 794131. 10.3389/fpubh.2022.794131
28
Mullangi S. Eagle D. A. (2023). The inflation reduction act and community oncology practices. JAMA Oncol.9 (12), 1612–1613.
29
Neumann P. J. Cohen J. T. Weinstein M. C. (2014). Updating Cost-Effectiveness--the curious resilience of the $50,000-Per-Qaly threshold. N. Engl. J. Med.371 (9), 796–797. 10.1056/NEJMp1405158
30
Parikh N. D. Singal A. G. Hutton D. W. (2017). Cost effectiveness of regorafenib as second-line therapy for patients with advanced hepatocellular carcinoma. Cancer123 (19), 3725–3731. 10.1002/cncr.30863
31
Qin S. Kruger E. Tan S. C. Cheng S. Wang N. Liang J. (2018). Cost-effectiveness analysis of Folfox4 and sorafenib for the treatment of advanced hepatocellular carcinoma in China. Cost Eff. Resour. Allocation C/E 1616, 29. 10.1186/s12962-018-0112-0
32
Rumgay H. Ferlay J. de Martel C. Georges D. Ibrahim A. S. Zheng R. et al (2022). Global, regional and national burden of primary liver cancer by subtype. Eur. J. Cancer161, 108–118. 10.1016/j.ejca.2021.11.023
33
Sangro B. Chan S. L. Kelley R. K. Lau G. Kudo M. Sukeepaisarnjaroen W. et al (2024). Four-year overall survival update from the phase iii himalaya study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann. Oncol. Official J. Eur. Soc. For Med. Oncol.35 (5), 448–457. 10.1016/j.annonc.2024.02.005
34
Shi G. Park S. H. Ren H. Mei X. Lu X. Dong P. et al (2018). Cost analysis for different sequential treatment regimens for metastatic renal cell carcinoma in China. J. Med. Econ.21 (12), 1150–1158. 10.1080/13696998.2018.1515769
35
Sieg M. Hartmann M. Settmacher U. Arefian H. (2020). Comparative cost-effectiveness of cabozantinib as second-line therapy for patients with advanced hepatocellular carcinoma in Germany and the United States. BMC Gastroenterol.20 (1), 120. 10.1186/s12876-020-01241-y
36
Soto-Perez-de-Celis E. Aguiar P. N. Cordón M. L. Chavarri-Guerra Y. Gilberto de Lima Lopes (2019). Cost-effectiveness of cabozantinib in the second-line treatment of advanced hepatocellular carcinoma. J. Natl. Compr. Cancer Netw. JNCCN17 (6), 669–675. 10.6004/jnccn.2018.7275
37
State Administration of Foreign Exchange (2024). Available online at: https://www.safe.gov.cn/en/(Accessed May 13, 2024).
38
Su D. Wu B. Shi L. Z. (2021). Cost-effectiveness of atezolizumab plus bevacizumab Vs sorafenib as first-line treatment of unresectable hepatocellular carcinoma. Jama Netw. Open4 (2), 11 e210037. 10.1001/jamanetworkopen.2021.0037
39
Sun K.-X. Cao S.-S. Shi F.-H. Yue G. Tang M. Zhao M.-Na et al (2022). First-line treatments for advanced hepatocellular carcinoma: a network meta-analysis and cost-effectiveness analysis in China and the United States. Ther. Adv. Gastroenterology15, 17562848221140662. 10.1177/17562848221140662
40
Wei Y. Royston P. (2017). Reconstructing time-to-event data from published kaplan-meier curves. Stata J.17 (4), 786–802. 10.1177/1536867x1701700402
41
Wen F. Zheng H. Zhang P. Liao W. Zhou K. Qiu Li (2021). Atezolizumab and bevacizumab combination compared with sorafenib as the first-line systemic treatment for patients with unresectable hepatocellular carcinoma: a cost-effectiveness analysis in China and the United States. Liver Int. Official J. Int. Assoc. For Study Liver41 (5), 1097–1104. 10.1111/liv.14795
42
Wu B. Zhang Q. Sun J. (2018). Cost-effectiveness of nivolumab plus ipilimumab as first-line therapy in advanced renal-cell carcinoma. J. For Immunother. Cancer6 (1), 124. 10.1186/s40425-018-0440-9
43
Wu T.K.-H. Hui R.W.-H. Mak L.-Yi Fung J. Seto W.-K. Yuen M.-F. (2024). Hepatocellular carcinoma: advances in systemic therapies. F1000Research13, 104. 10.12688/f1000research.145493.2
44
Xiang Q. Chen W. Ren M. Wang J. Zhang H. Deng D. Y. B. et al (2014). Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of Vegfr2 and met. Clin. Cancer Res. Official J. Am. Assoc. For Cancer Res.20 (11), 2959–2970. 10.1158/1078-0432.CCR-13-2620
45
Xu Y. Mao N. Chirikov V. Du F. Yeh Y.-C. Liu Li et al (2019). Cost-effectiveness of teriflunomide compared to interferon Beta-1b for relapsing multiple sclerosis patients in China. Clin. Drug Investig.39 (3), 331–340. 10.1007/s40261-019-00750-3
46
Yang X. Yang C. Zhang S. Geng H. Zhu A. X. Bernards R. et al (2024). Precision treatment in advanced hepatocellular carcinoma. Cancer Cell42 (2), 180–197. 10.1016/j.ccell.2024.01.007
47
Yau T. Park J.-W. Finn R. S. Cheng A.-L. Mathurin P. Edeline J. et al (2022). Nivolumab versus sorafenib in advanced hepatocellular carcinoma (checkmate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet. Oncol.23 (1), 77–90. 10.1016/S1470-2045(21)00604-5
48
Yau T. Ahmed K. Cheng A.-L. Qin S. Zhu A. X. Chan S. L. et al (2024). Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (Cosmic-312): final results of a randomised phase 3 study. Lancet. Gastroenterology and Hepatology9 (4), 310–322. 10.1016/S2468-1253(23)00454-5
49
Yip W. Fu H. Chen A. T. Zhai T. Jian W. Xu R. et al (2019). 10 years of health-care reform in China: progress and gaps in Universal Health Coverage. Lancet394 (10204), 1192–1204.
50
Zhang X. Wang J. Shi J. Jia X. Dang S. Wang W. (2021). Cost-effectiveness of atezolizumab plus bevacizumab Vs sorafenib for patients with unresectable or metastatic hepatocellular carcinoma. JAMA Netw. Open4 (4), e214846. 10.1001/jamanetworkopen.2021.4846
51
Zheng J. Sandhu A. T. (2023). The inflation reduction act expands prescription drug affordability: decades in the making. J. Am. Coll. Cardiol.81 (21), 2112–2114.
52
Zheng J. Wang S. Xia L. Sun Z. Chan K. M. Bernards R. et al (2025). Hepatocellular carcinoma: signaling pathways and therapeutic advances. Signal Transduct. Target Ther.10 (1), 35.
53
Zhou T. Cao Y. Wang X. Yang L. Wang Z. Ma A. et al (2022). Economic evaluation of sintilimab plus bevacizumab versus sorafenib as a first-line treatment for unresectable hepatocellular carcinoma. Adv. Ther.39 (5), 2165–2177. 10.1007/s12325-022-02079-4
Summary
Keywords
cost-effectiveness analysis, advanced hepatocellular carcinoma, cabozantinib, atezolizumab, sorafenib
Citation
Zhu Y, He C, Cao S-C, Li H-J, Cai R-X, Bai Z-J and Xia T-X (2025) Cost-effectiveness analysis of cabozantinib plus atezolizumab for advanced hepatocellular carcinoma. Front. Pharmacol. 16:1556304. doi: 10.3389/fphar.2025.1556304
Received
06 January 2025
Accepted
30 September 2025
Published
09 October 2025
Corrected
13 November 2025
Volume
16 - 2025
Edited by
Qiang Sun, Shandong University, China
Reviewed by
Balram Chowbay, National Cancer Centre Singapore, Singapore
Chen-Han Chueh, University of Califronia, San Diego, United States
Updates
Copyright
© 2025 Zhu, He, Cao, Li, Cai, Bai and Xia.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tong-Xia Xia, xtx0925@163.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.