- 1Department of Pharmacy, Renmin Hospital of Wuhan University, Wuhan, China
- 2Department of Pharmacy, Qingdao Third People’s Hospital Affiliated to Qingdao University, Qingdao, China
- 3Department of Pharmacy, First Affiliated Hospital of Army Medical University, Chongqing, China
Background: The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has led to global health crisis. Although several antiviral drugs have been used to mitigate the severity and mortality of COVID-19, the safety profile remained a critical concern. Azvudine, a new nucleoside analog, has been approved for emergency use in China for COVID-19. However, the incidence and risk factors associated with Azvudine-induced hepatotoxicity in hospitalized patients remained unclear.
Objects: To assess the prevalence, risk factors, clinical patterns, and outcomes of Azvudine-induced hepatotoxicity by real-world data.
Methods: We conducted a single-center retrospective case-control study at Renmin Hospital of Wuhan University, including patients administered Azvudine for COVID-19 treatment between December 2022 and May 2023. Univariate and multivariate logistic regression analyses were preformed to assess risk factors for Azvudine-associated or -induced hepatotoxicity. Receiver operating characteristic (ROC) curve analysis was performed to calculate the area under the ROC curve (AUC).
Results: In total, 669 patients were included in the Azvudine-associated hepatotoxicity research. 47.1% patients exhibited hepatotoxicity, abnormal liver function on admission [OR: 5.55 (3.94–7.90), P < 0.001] and antithrombotic drugs [OR: 1.79 (1.27–2.54), P = 0.001] were independent predictors of Azvudine-associated hepatotoxicity, with the area under the ROC curve (AUC) was 0.756 [95% CI: 0.719–0.792, P < 0.001]. Further studies of Azvudine-induced hepatotoxicity revealed 294 cases, of which 27.2% showed hepatotoxicity. The concomitant use of antivirals [OR: 3.80 (1.47–10.1), P = 0.006] and anticoagulant drugs [OR: 3.12 (1.77–5.61), P < 0.001], particularly Ganciclovir [OR: 4.11 (1.45–12.2), P = 0.008], Low-Molecular-Weight Heparin Calcium [OR: 3.00 (1.69–5.33), P < 0.001], and Enoxaparin [OR: 2.68 (0.99–7.10), P = 0.047], were significantly associated with an increased risk of hepatotoxicity. Most hepatotoxicity cases were mild, and recovered or improved after drug withdrawal and treatment, whereas severe cases contributed to the progression of the primary disease and increased mortality risk.
Conclusion: Our study provided evidence of the significant association between Azvudine and hepatotoxicity in hospitalized COVID-19 patients. These findings underscored the importance of monitoring liver function during Azvudine treatment and caution against concomitant use of certain medications. Further research was warranted to elucidate the mechanisms underlying Azvudine-induced hepatotoxicity and optimize clinical management strategies.
1 Introduction
The COVID-19 pandemic, triggered by the SARS-CoV-2 virus, has led to millions of fatalities worldwide, with enduring repercussions on global health systems, economic stability and social structures (Kevadiya et al., 2021; Kwok, 2022; Baden et al., 2021; Rubin, 2021). As of 2024, the COVID-19 pandemic remains a persistents public health challenge, characterized by the emergence of novel variants and sustained international efforts in disease mitigation (Chow et al., 2023; Nanaw J, 2024). To solve the problem, several antiviral drugs, such as Remdesivir, Molnupiravir, and Nirmatrelvir-Ritonavir, have been utilized in the treatment of COVID-19 following to WTO guidelines (Han et al., 2024a; Grein et al., 2020). Additionally, Azvudine (2′-deoxy-2′-β-fluoro-4′-azidocytidine), a novel nucleoside analog, has gained emergency approval for the Omicron variant surge in China (General Office of the National Health Commission, 2022; Deng et al., 2023).
Azvudine is a pro-drug that undergoes intracellular phosphorylation by deoxycytidine kinase phosphorylated in the cytoplasm to form its active metabolite. The active compound exerts antiviral effects primarily through inhibition of the viral RNA-dependent RNA polymerase (RdRp) of viruses (Zhang et al., 2021; Yu and Chang, 2020). Initially developed for HIV treatment, Azvudine has demonstrated favorable efficacy and tolerability profiles in Chinese patients during 48-week therapeutic regiments. Recent studies have revealed its potent activity against SARS-CoV-2 virus with dual mechanisms: effective suppression of viral RNA replication and immunodulatory effects through thymus-mediated lymphocyte profile enhancement (Ren et al., 2020; Yang et al., 2024a). The pharmacological properities make Azudine a promising therapeutic candidate for COVID-19. Clinical trials have demonstrated Azvudine significant antiviral efficacy against COVID-19. Prior studies have revealed comparable clinical outcomes between Azvudine and Nirmatrelvir-Ritonavir in hospitalized patients, with no statistically significant differences observed in 28 days all-cause mortality, composite disease progression, and clinical improvement (Han et al., 2024b; Shang et al., 2024). The findings provide robust evidence supporting Azvudine effectiveness as an alternative treatment for COVID-19, with safety and efficacy profile. Compared to Nirmatrelvir-Ritonavir, Azvudine demonstrated comparable clinical efficacy while showing a significantly lower risks of composite disease progression in non-severe COVID-19 patients, indicated Azvudine might offer superior clinical benefits over Nirmatrelvir-Ritonavir in certain patient populations (Yu and Chang, 2022; Wang S. et al., 2024). Further supporting evidence from a retrospective case-control study by Su et al. demonstrated comparable therapeutic efficacy between Azvudine and Nirmatrelvir-Ritonavir, with no statistically significant differences observed in key clinical outcomes including recovery time, mortality rates, and hospitalization duration (Su et al., 2024). Meta-analysis further indicated that Azvudine may be preferable to Nirmatrelvir-Ritonavir for elderly patients (aged over 75 years old), primarily due to its significantly lower risk of drugs interaction while maintaining comparable efficacy (Wang Y. et al., 2024; Shang et al., 2024). Furthermore, pharmacoeconomic analyses demonstrate that Azvudine exhibited a siginicantly lower incremental cost-effectiveness ratio (ICER) compare to Nirmatrelvir-Ritonavir (Yang et al., 2024b). The enhanced cost-efficiency profile improved treatment accessibitity, particularly in resource-limited and lower-income populations. In summary, current clinical and pharmacoeconomic evidence positions Azvudine as a clinically effective and cost-efficient therapeutic option for COVID-19 management, particularly valuable for resource-constrained healthcare systems.
Previous research has demonstrated that SARS-CoV-2 infection not only affects the respiratory system but also involves multiple organ dysfunction (MODS), thus inevitably leading to the increase in all-cause mortality (Zhong et al., 2020; Alqahtani and Schattenberg, 2020). Notably, hepatic dysfunction occurs in up to half of all reported cases (Alqahtani and Schattenberg, 2020; Allison et al., 2023). Medications have been recognized as one of the contributing factor to hepatocellular injure in COVID-19 infections (Zhong et al., 2020; Wen-Shu Hu, 2022; Rodriguez-Espada et al., 2024). Notably, antiviral drugs such as Remdesivir, and Nirmatrelvir-Ritonavir have been associated with an elevated risk of drug-induced hepatotoxicity (Allison et al., 2023; Xie et al., 2020; Naseralallah et al., 2022). The safety and tolerability of Azvudine as a novel nucleoside analog has remain critical considerations, particularly in patients with pre-exsiting liver impairment. According to the manufacturer’s label, Azvudine may cause mild-to-moderate liver dysfunction in isolated instances, with approximately 35% of patients exhibiting modest elevation in liver enzyme. Clinical studies have reported hepatotoxicity ranges from 13.5% to 38.4%, udine among COVID-19 patients receiving Azvudine (Lan et al., 2024; Zhi et al., 2023; He et al., 2023; Liu et al., 2024; Yang et al., 2023; Liu S. et al., 2023). However, the generalizability of these findings remains uncertain due to limited sample size. Consequently, further large-scale studies are warranted to clarify the safety profile of Azvudine in COVID-19 treatment.
This study utilized real world data to evaluate the prevalence, risk factors, clinical manifestations, and outcomes of Azvudine-associated hepatotoxicity among in hospitalized COVID-19 patients. The findings aim to establish a comprehensive safety profile of Azvudine and provide evidence-based guidance for clinical decision-making in COVID-19 management.
2 Methods
2.1 Study design and ethics approval
This single-center retrospective case-control study was conducted at the Renmin Hospital of Wuhan University, a tertiary medical center with approximately 7300 beds in China. This study protocol was approved by the Ethics Committee of the Renmin Hospital of Wuhan University (Approval No.: WDRY2024-K003), with waiver of informed consent granted due to the retrospective study.
2.2 Patient inclusion
The diagnosis of COVID-19 has made by infectious disease and internal medicine clinicians based on clinical signs, a chest computed tomography scan, and a positive COVID-19 nucleic acid test result. Patients with respiratory rates≥30 breaths/minute, SpO2≤93% on room air, or PaO2/FiO2 ≤ 300 mmHg were categorized as severe cases, while other cases were classified as non-severe (General Office of the National Health Commission, 2022). Patients who were Azvudine for COVID-19 treatment between December 2022 to May 2023 were included. Exclusion criteria for Azvudine associated hepatotoxicity based on the following criteria: 1) Negative COVID-19 nucleic acid test results, 2) Incomplete laboratory data (lack of baseline liver function data within 7 days prior to treatment initiation or absence of follow up, and 3) Azvudine treatment less than 3 days (Shi et al., 2021; Zhao et al., 2024; Lu et al., 2024). Additional exclusion criteria for Azvudine-induced hepatotoxicity included: 1) pre-existing liver disease and 2) abnormal liver function tests at admission.
The patients’ comprehensive data including: demographic characteristics, COVID-19 severity, Azvudine dosage, duration of treatment liver disease history, serial liver function tests, comorbidities, and concomitant medications. A schematic flowchart delineating the methodology for identifying Azvudine-associated and Azvudine-induced hepatotoxicity cases was shown in Figure 1.
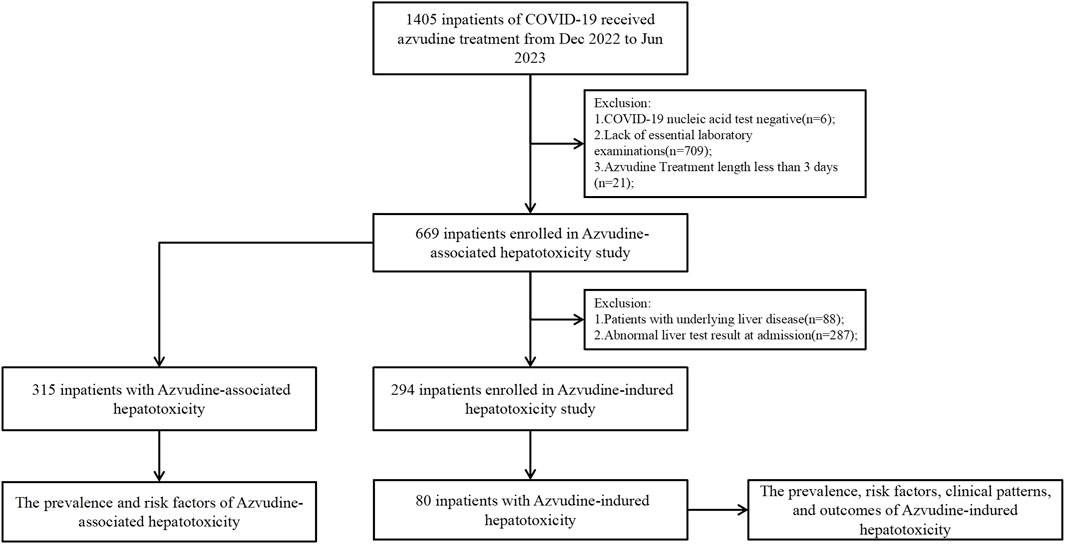
Figure 1. Identification of Azvudine-associated or -induced hepatotoxicity among COVID-19 hospitalized patients during the study period.
2.3 Evaluation of hepatotoxicity
Hepatotoxicity was defined as liver function biochemical abnormalities s when meet at least one of the following biochemical criteria: 1) alanine aminotransferase (ALT) > 40U/L; 2) aspartate transaminase (AST) > 35U/L; 3) alkaline phosphatase (ALP) > 135U/L; 4) gamma–glutamyltransferase (GGT) > 45U/L; 5) total bilirubin (TB) > 23 μmol/L (Yu et al., 2017). All values represent the upper limit of normal (ULN) for our laboratory reference ranges. In this study, patients who developed hepatotoxicity after Azvudine treatment were categorized into the Abnormal group, while those without hepatotoxicity were classified as the Normal group.
The causality of Azvudine-induced hepatotoxicity was evaluated using the updated Roussel Uclaf Causality Assessment Method (RUCAM) (Danan and Teschke, 2016; Zhao et al., 2021), with cases scoring below 3 on the RUCAM scale being excluded from analysis due to insufficient evidence for establishing causal relationship between liver injury and Azvudine administration. To classify the clinical type of Azvudine-induced hepatotoxicity, the R value was calculated using the following formula: R = [ ALT/ (ALT ULN)]/ [ALP/ (ALP ULN)]. Based on established criteria (Andrade et al., 2019a; Andrade et al., 2019b), drug-induced liver injury was categorized into three clinical types: 1) hepatocellular injury (R ≥ 5); 2) cholestatic injury (R ≤ 2); 3) mixed injury (2 < R < 5).
The severity of Azvudine-induced hepatotoxicity was classfied according to the biological criteria Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0, based on the ULN thresholds of ALT, ALP, and GGT as follows: 1) Grade 1 (Mild): elevation of ALT or ALT ≥1 × ULN and <3 × ULN, elevation of ALP or GGT ≥1 × ULN and <2.5 × ULN; 2) Grade 2 (Moderate): elevation of ALT or ALT ≥3 × ULN and <5 × ULN and elevation of ALP or GGT ≥2.5 × ULN and <5 × ULN; 3) Grades 3 and 4 (severe induced liver injury, DILI): elevation of ALT or ALT ≥5 × ULN and elevation of ALP or GGT ≥5 × ULN.
2.4 Prognosis of azvudine-induced hepatotoxicity
The prognosis of Azvudine-induced hepatotoxicity was categorized based on post-treatment liver function records was as follows: 1) Complete Recovery: liver enzyme levels returned to within normal limits (below ULN) or baseline values; 2) Partial Improvement: ALT/AST decreased to below 3 × ULN or ALP/GGT decreased to below 2.5 × ULN; 3) Aggravation: liver test values exceeded previous peak levels; 4) Unknown: Insufficient follow-up data for proper assessment.
2.5 Statistical analysis
Statistical analysis was conducted using SPSS Statistics version 26.0 (IBM Corp., Armonk, NY, USA). Continuous variables with normal variables were reported as mean ± standard deviation (SD), whereas non-normally distributed variables were presented as medians (interquartile range, IQR). Independent sample t-tests and Mann-Whitney U tests were for comparisons. Categorical variables were analyszed using either chi-square test or fisher’s exact test, as appropriate. For multivariable analysis, variables demonstrating a potential association (P < 0.20) in univariate analysis were entered into a logistic regression modelIndependent risk factors for Azvudine-associated and Azudine-induced hepatoxicity were identified through stepwise logistic regression. A two-tailed P value < 0.05 was considered statistically significant for all analysis.
We performed sensitivity analyses to evaluate the robustness and generalizability of primary findings, following established methodological approaches (Hykin et al., 2019; Liu ZQ. et al., 2023). In the current analysis, we mainly conducted sensitivity analyses on two specific subgroups. Age-stratified analysis: We introduced an age-treatment interaction term in the regression model to specifically examine potential effect modification in patients aged more than 35 years old. Allergy adjusted analysis: we systematically exclude patients with documented drug allergy histories and re-analyzed the primary outcomes to assess the potential confounding effect of medication hypersensitivity. These rigorous sensitivity assessments were designed to:
1) Verify the consistency of treatment effects across clinically relevant subgroup
2) Examine the potential influence of key confounding variables
3) Enhance the external validity of the conclusion for diverse patient population.
3 Results
3.1 Patients enrolled
We initially identified 1,405 hospitalized COVID-19 patients through electronic medical record review, we implement the following exclusion criteria: Six patients (0.4%) with negative COVID-19 nucleic acid tests, 709 (50.5%) lacking essential laboratory examinations, and 21 (1.5%) with follow-up durations of less than 3 days were excluded; 669 patients (47.6%) were included in the Azvudine-associated hepatotoxicity research (Figure 1). The enrolled population was subsequently stratified into two groups according to hepatotoxicity status, with comprehensive clinical characteristics presented in Table 1.
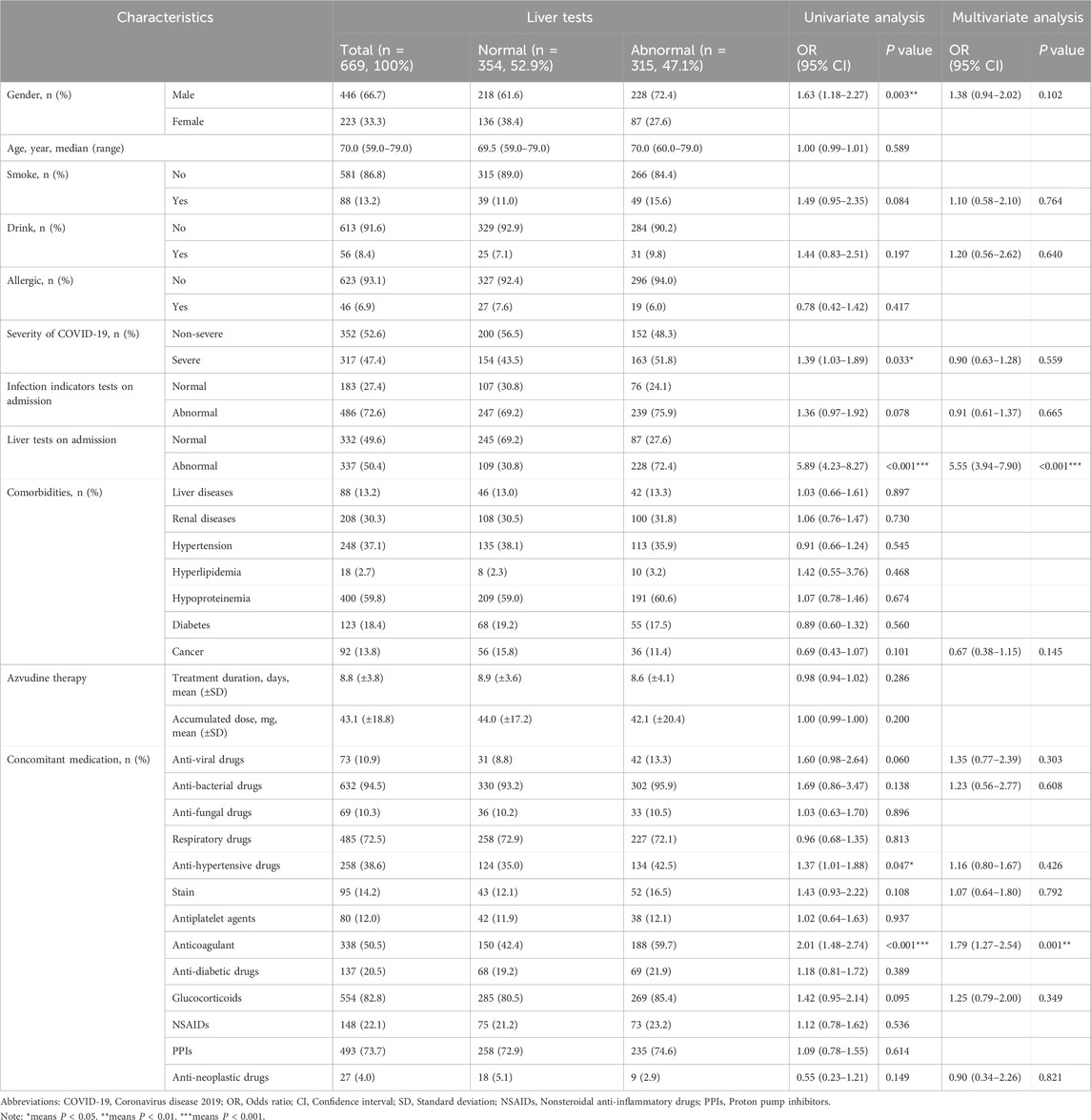
Table 1. Demographic characteristics of hospitalized COVID-19 patients in Azvudine-associated hepatotoxicity study.
To establish a baseline of normal liver function prior to treatment, we implement additional exclusion criteria: 88 (13.2%) cases were excluded due to liver disease, and 287 (42.9%) cases were excluded due to abnormal liver function, the remaining 294 (43.9%) cases enrolled in the study of Azvudine-induced hepatotoxicity (Figure 1). The final cohort was dichotomized based on the development of treatment-emergent liver injury, with comprehensive demographic and clinical characteristics detailed in Table 2.
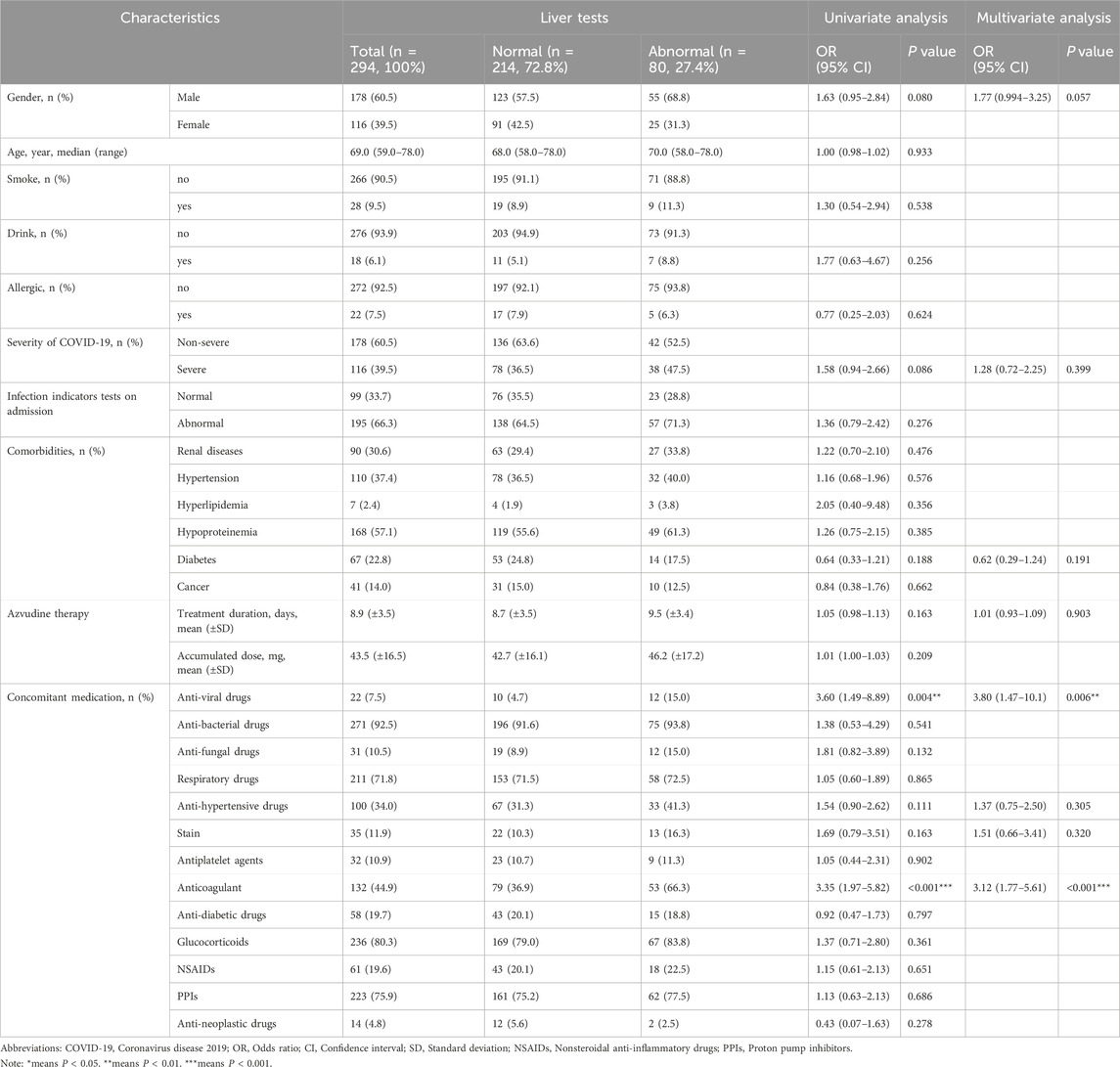
Table 2. Demographic characteristics of hospitalized COVID-19 patients in Azvudine-induced hepatotoxicity study.
3.2 Characteristics of COVID-19 patients with Azvudine-associated hepatotoxicity
Our analysis of 669 hospitalized COVID-19 patients revealed that 315 cases (47.1%) exhibited abnormal liver function test, whereas 354 patients (52.9%) maintained normal hepatic parameters, as shown in Table 1. Multivariate logistic regression identified two significant risk factors for hepatoxicity. (1) male gender (OR 1.63, 95% CI 1.18–2.27, P = 0.003), and (2) severe COVID-19 infection (OR 1.39, 95% CI 1.03–1.89, P = 0.033) The conclusion suggest that male patients and those with severe COVID-19 manifestations demonstrate increased susceptibility to Azvudine-associated injury.
The analysis identified several significant predictors of Azvudine associated hepatotoxicity Notably, abnormal liver function tests at admission demonstrated the strongest association (OR 5.89, 95% CI 4.28–8.27, P < 0.001). Interestingly, the presence of comorbidities did not significantly affect liver function disorders in hospitalized patients. However, the concurrent use of antihypertensive (OR1.37, 95% CI 1.01–1.88, P = 0.047) and anti-thrombotic drugs (OR 2.01, 95% CI 1.48–2.74, P < 0.001) was significantly correlated with abnormal liver function. Comparative analysis of baseline laboratory parameters (Figure 2) revealed significant differences (P < 0.05) in multiple hepatic markers (ALT, AST, ALP, GGT, TB, and DB), and hematological indices (WBC, RBC, HGB, and PLT) between groups. Notably, coagulation such as the international normalized ratio (INR) and renal function parameters such as estimated glomerular filtration rate (eGFR) showed no significant difference intergroup variation.
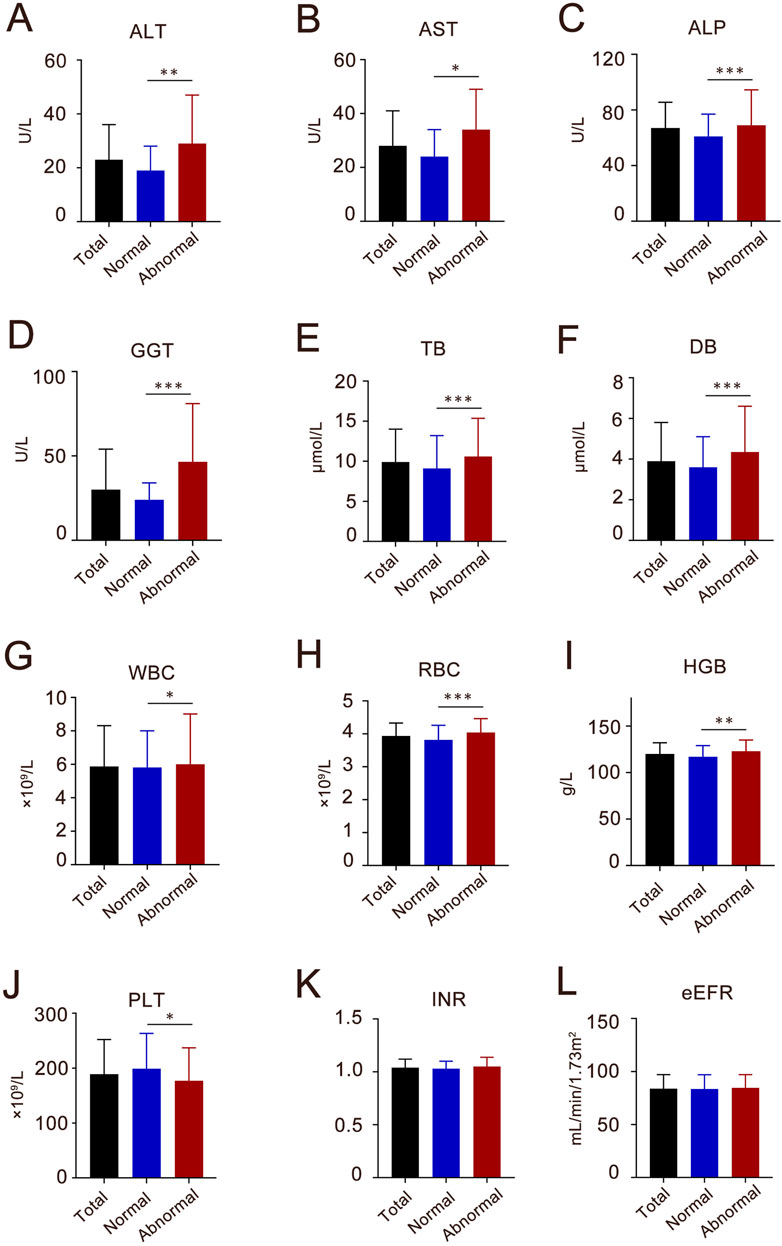
Figure 2. The baseline characteristics of liver enzymes, blood components, coagulate and kidney function index of the participants in Azvudine-associated hepatotoxicity study. (A) ALT, alanine aminotransferase; (B) AST, aspartate transaminase; (C) ALP, alkaline phosphatase; (D) GGT, gamma–glutamyltransferase; (E) TB, total bilirubin; (F) DB, direct bilirubin; (G) WBC, white blood cell; (H) RBC, red blood cell; (I) HGB, hemoglobin; (J) PLT, platelet count; (K) INR, international normalized ratio; (L) eGFR, estimated glomerular filtration rate. Each column shows the median (interquartile range, IQR). *P < 0.05, **P < 0.01, ***P < 0.001.
The multivariate logistic regression analysis incorporating demographic characteristics (gender, smoking, drinking), clinical factors (severity of COVID-19), admission laboratory tests (infection indicator and liver function), and medication history (antiviral drugs, antibacterial drugs, antihypertensive drugs, statins, anticoagulants, glucocorticoids, and antineoplastic drugs) on adimission were subsequently incorporated to further identified their associations. (1) Abnormal liver function tests on admission (OR 5.55, 95% CI 3.94–7.90, P < 0.001). (2)Antithrombotic medication management (OR 1.79, 95% CI 1.27–2.54, P = 0.001). The model demonstrated good discriminatory ability with an area under the ROC curve of 0.756, 95% CI 0.719–0.792, P < 0.001), indicating moderate predictive accuracy for liver injury risk in COVID-19 patients receiving Azudine.
3.3 Characteristics of COVID-19 patients with Azvudine-induced hepatotoxicity
Overall, 80 (27.2%) patients developed hepatoxicity after Azvudine treatment, while 214 (72.8%) maintained normal. Multivariate analysis identified two independent predictors: concomitant antiviral therapy (adjusted OR 3.60, 95%CI 1.49–8.89, P = 0.004); Antithrombotic medication management (adjusted OR 3.35, 95% CI 1.77–5.61, P < 0.010). Additionally, the regression model demonstrated acceptable discrimination (AUC 0.712, 95% CI 0.648–0.775, P < 0.001). For laboratory examination, significant post-treatment elevations in hepatic enzymes (baseline levels of ALT, AST, GGT, WBC, HGB, and PLT) were significantly associated with liver injury (all P < 0.05, Figure 3). Gender, COVID-19 severity, diabetes, Azvudine therapy, antiviral drugs, and antithrombotic drugs were then incorporated into a multivariate LR model. It was determined that anti-viral (OR 3.80, 95% CI 1.47–10.1, P = 0.006) and Anti-thrombotic drugs (OR 3.12, 95%CI 1.77–5.61, P < 0.001) were both indendent predictors of Azudine hepatotoxicity. The sensitivity analysis results were consistent with the primary analysis, thus lending support of the accuracy of the conclusion (Supplementary Table S1). Additionally, a significant elevation in liver function (ALT, AST, and GGT) was revealed following Azvudine administration (Table 3), indicating the occurrence of liver injury (P < 0.05).
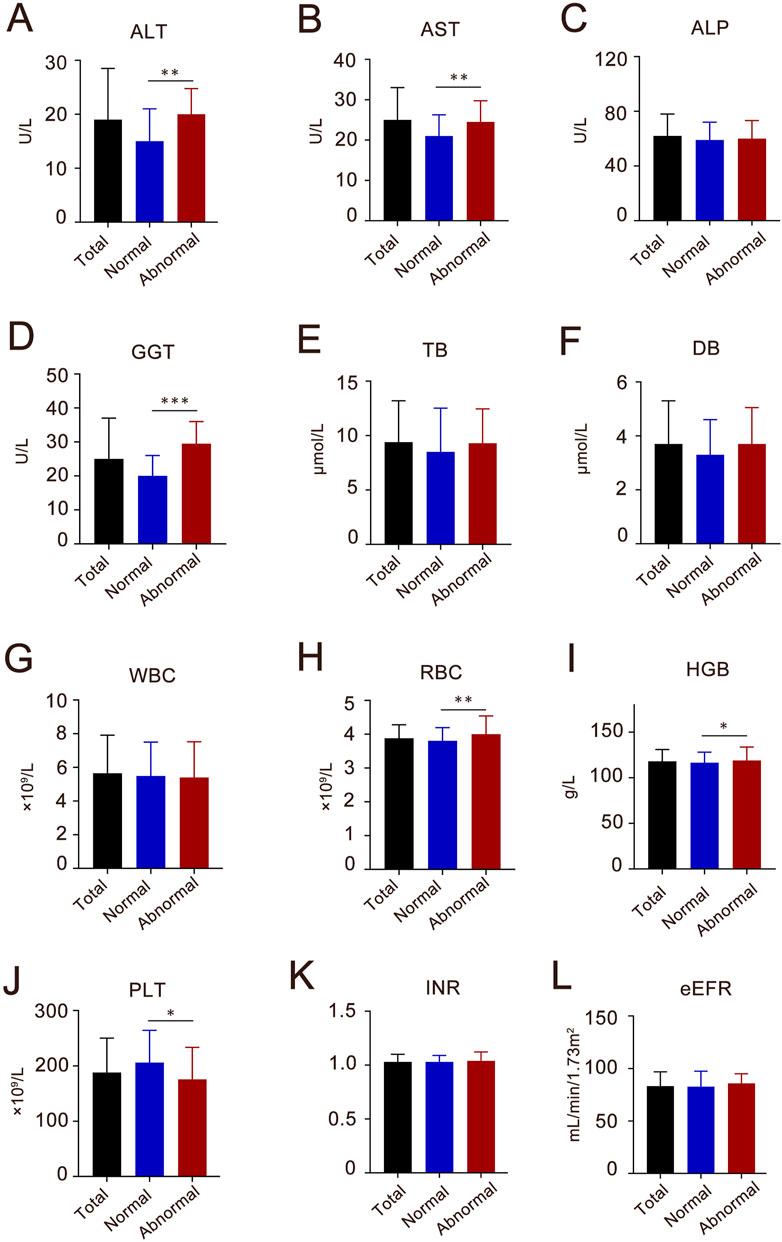
Figure 3. The baseline characteristics of liver enzymes, blood components, coagulative and kidney function index of the participants in Azvudine-induced hepatotoxicity study. (A) ALT, alanine aminotransferase; (B) AST, aspartate transaminase; (C) ALP, alkaline phosphatase; (D) GGT, gamma–glutamyltransferase; (E) TB, total bilirubin; (F) DB, direct bilirubin; (G) WBC, white blood cell; (H) RBC, red blood cell; (I) HGB, hemoglobin; (J) PLT, platelet count; (K) INR, international normalized ratio; (L) eGFR, estimated glomerular filtration rate. Each column shows the median (interquartile range, IQR). *P < 0.05, **P < 0.01, ***P < 0.001.
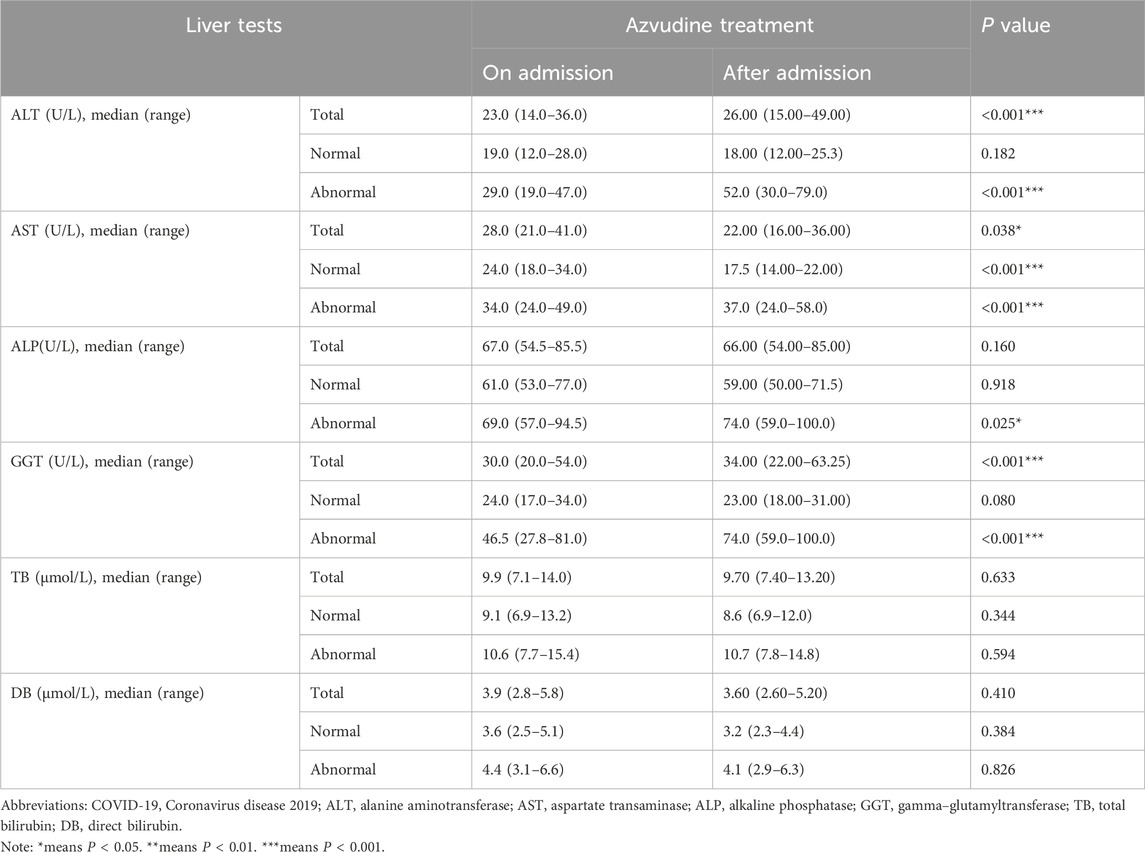
Table 3. The liver tests result of Azvudine on and after admission among hospitalized COVID-19 patients with Azvudine-induced hepatotoxicity.
3.4 Concomitant medication of Azvudine-induced hepatotoxicity in COVID-19 patients
To identify concomitant medications significantly associated with Azvudine-induced hepatotoxicity, we conducted focused analysis of pharmacological agents that demonstrated statistical significance (P < 0.05) in our multivariate regression model, as shown in Table 4. Significant Hepatotoxicity Associations: Ganciclovir (adjusted OR 4.22, 95% CI 1.56–12.0, P = 0.005). LMWH showed significant risk (OR 3.01, 95% CI 1.73–5.23, P < 0.001). Multivariate Analysis Results: The final predictive model identified three independent pharmacological risk factors: Ganciclovir (adjusted OR 4.11, 95% CI 1.45–12.2, P = 0.008). LMWH calcium (adjusted OR 3.00, 95% CI 1.69–5.33, P < 0.001). Enoxaparin (adjusted OR 2.68, 95% CI 0.99–7.10, P = 0.047). Model Performance: The model achieved acceptable discrimination (AUC 0.674, 95% CI 0.602–0.746, P < 0.001). Validation: Robustness confirmed through consistent sensitivity analyses (Supplementary Table S2). All significant associated maintained statistical significance in validation testing.
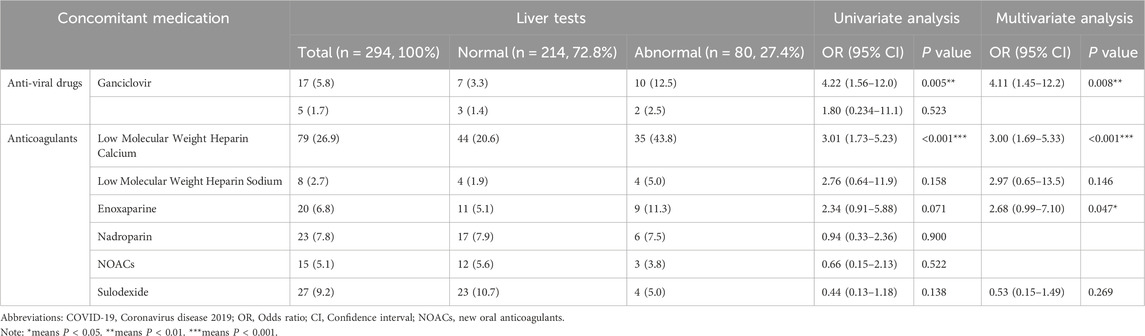
Table 4. Concomitant medication of hospitalized COVID-19 patients in Azvudine-induced hepatotoxicity study.
3.5 Clinical outcome of Azvudine-induced hepatotoxicity, severity, and treatment
The characteristics and outcomes of the Azvudine-induced hepatotoxicity was shown in Table 5. Severity Distribution: Grade 1 (Mild): 64 cases (80.0%); Grade 2 (Moderate): 14 cases (17.5%); Grade 3 (Grade 3+): 2 cases (2.5%). The onset of liver injury occurred at approximately 7 days (range 5–10 days) and showed no significant differences across severity grades.
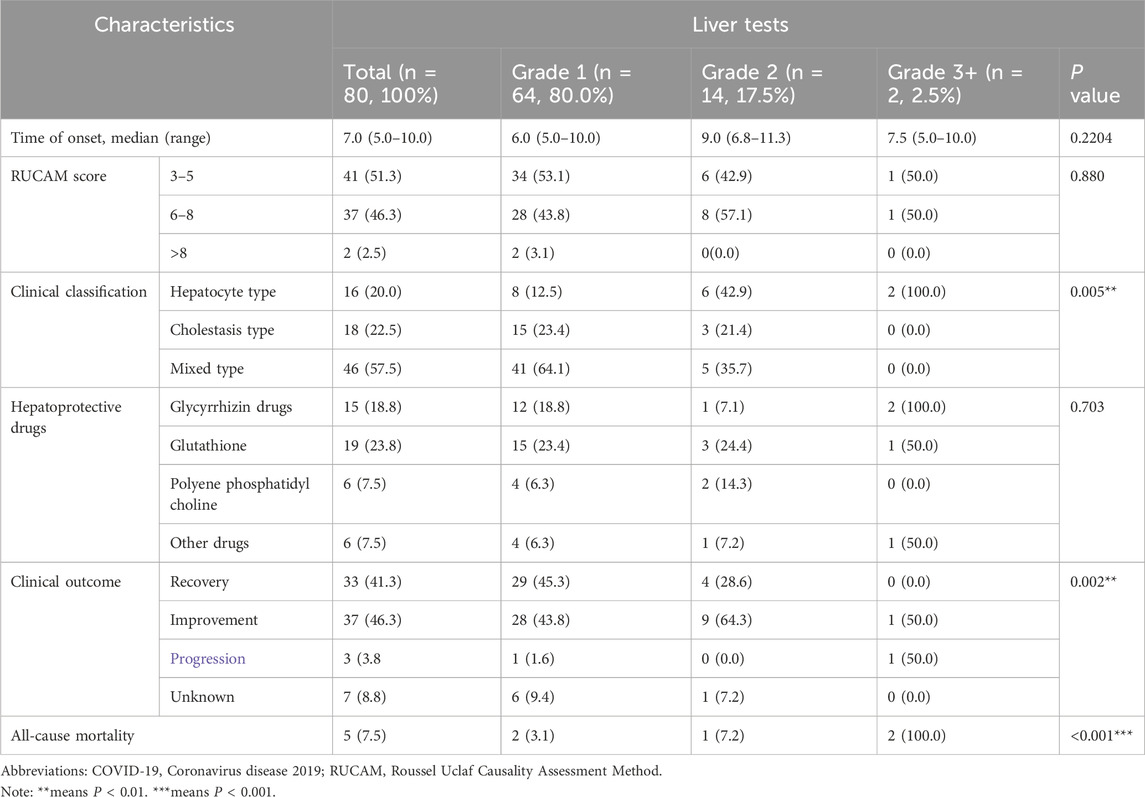
Table 5. Clinical characteristics and outcome of hospitalized COVID-19 patients in Azvudine-induced hepatotoxicity study.
Causality and Phenotypic Characterization of Azvudline-Induced Hepatotoxicity. RUCAM-based Causality Assessment: Possible association (score 3–5): 41 cases (51.3%); Probable association (score 6–8): 37 cases (46.2%). Highly probable association (score>8): 2 cases (2.5%). Injury Patterns at Presentation: Hepatocellular (R ≥ 5): 16 cases (20.0%); Cholestatic (R ≤ 2): 18 cases (22.5%). Mixed (2 < R < 5):46 cases (57.5%).
There was a significant difference between the level of liver injury: mild cases predominantly showed mixed pattern, while severe cases demonstrated hepatocellular predominance. Notably, two cases in the hepatocellular pattern reached grade 3 and one case achieved grade 4, with an increasing AST value of 585 U/L (>10X the upper limit of normal, ULN).
As for treatment patterns: 32/80 (40.0%) patients received hepatoprotective therapy. Medication utilization:Glutathione (19, 23.75%) was the most frequently administered treatment for DILI, followed by Glycyrrhizin (15, 18.75%) and Polyene phosphatidylcholine (6, 7.5%). Treatment Outcome: The majority of patients achieved recovery or improvement after therapy, although two patients showed disease progression despite hepatoprotection. Case presentations of progressive liver injury: One patient initially presented with mild liver injury (Grade 1) with cholestatic pattern that progressed to acute liver failure within 1 week, exhibiting terminal hepatocellular pattern changes with AST levels of 684 U/L, ALT >6000 U/L, ALP 161 U/L, and GGT 79 U/L, whereas TB and DB levels remained normal at 8.3 μmol/L and 5.3 μmol/L, respectively. Another patient persistent AST level of >200 U/L with severe liver injury maintained and fatal outcome due to severe pneumonia complications 4 days post discontinuation of Azvudine. Clinical Observation: Although progress cases received hepatoprotective therapy, rapid biochemical deterioration occurred despite standard interventions. Non-hepatic comorbidties contributed to mortality in severe cases.
Mortality analysis in the study cohort. Overall Mortality in entire cohort, 14 (4.76%, 14/294) died after Azvudine therapy. In the normal liver function group, the all-cause mortality rate was 4.21% (9/214), with five patients dying from severe pneumonia, one from MODS, and one from acute heart failure (AHF). The all-cause mortality by hepatotoxicity severity in the liver injury group was 6.25% (5/80), with 2 cases (3.13%, 2/64) Grade 1, 1 case (7.14%, 1/14) Grade 2, and 2 cases (100%, 2/2) Grade 3 or higher. Among which, four patients died from severe pneumonia and one died from MODS. Although there was no significant difference between the normal and abnormal liver function groups, severe liver injury significantly contributed to the progression of the main disease. Liver injury severity correlated with worse clinical outcomes, particularly in patients with pre-existing severe COVID-19. Hepatotoxicity may potentiate disease progression in critical ill patients.
4 Discussion
During the COVID-19 pandemic, the urgent need for the effective antiviral medication has highlighted the importance of rigorous drug safety evaluation. Azvudine has emerged as a promising newly marketed medication, its safety profile, particularly regarding hepatotoxicity remains incompletely characterized, especially for off-label use in hospitalized patients with prolonged symptomatic combined with complex commodities. In the initial studies, the medication did not demonstrate any significant adverse effects, indicating a seemingly safe profile for its application. Nevertheless, this is the first large-scale evaluation of Azvudine hepatotoxicity in real-world hospitalized patients. The research focus on high-risk population with symptoms prolonged more than 5 days with multiple comorbidities/concomitant medications. Sample size (n = 1405) substantially exceeds previous clinical trial cohorts.
Previous studies have documented that hepatocellular injury in 14%–53% of hospitalized COVID-19 patients, typically characterized by an elevation in aminotransferase levels below 5-fold ULN in initial stage of pandemic (Gupta et al., 2020). In our prior single center data demonstrated progress from 51.2% admission prevalence to 70.0% during hospitalization (Chen et al., 2023). Recently, multicenter study enrolling 1246 hospitalized adult patients identified that approximately 58.7% patients had presented with abnormal liver biochemistry and 47.7% had persistent abnormalities up to 6 months post-infection (Rajaram et al., 2024). In our study, the overall hepatoxicity prevalence: 47.1% consistent with history ranges. Characteristic pattern: isolated with elevated levels of ALT, AST, and GGT, indicating that Azvudine did not increase the risk of hepatotoxicity compared with other anti-viral therapies in COVID-19 treatment.
In our cohort, the incidence of Azvudine-induced hepatotoxicity was 27.2%. Most of cases were characterized by mild-to-moderate hepatic impairment, corresponding to Grades 1 and 2. In previous studies, varying incidences of hepatic impairment have been documented among COVID-19 patients of diverse types when administered Azvudine therapy, with reported rates of liver injury ranging from 10.5% (Lan et al., 2024) to 17.8% (Yang et al., 2023), 18.6% (Liu S. et al., 2023), 23.0% (Zhi et al., 2023) and 38.4% (He et al., 2023). The retrospective study conducted by Liu et al., among 490 patients with mild COVID-19 who underwent pharmacological treatment, 91 individuals presented with aberrant hepatic function, predominantly manifested as elevated liver enzyme levels (Liu ZQ. et al., 2023). The meticulous study conducted by Li et al., with cohort of 190 patients who received Azvudine demonstrated an incidence of elevated ALT of 38.4% (73/190) (He et al., 2023). The majority of these cases, specifically 93.2% (68/73) patients exhibited an increase within one-fold the ULN and zero case up to 5-fold, which is indicative of a benign pattern typically observed in clinical trials. Age specific hepatotoxicity profile of Azvudine therapy. Elderly patients aged over 75 years old with the rate of abnormal liver function of 23.0% (17/74), among which 6 occurrences of elevated ALT, 4 of elevated AST, and 7 of elevated GGT (Zhi et al., 2023). Notably, the incidence of Azvudine-induced DILI was observed to be relatively as low as 0.68% (2/294) in our study. Consistent findings were reported in another cohort by Zhou et al., with Azvudine-induced DILI of 0.64% (2/311) in the treatment for moderate to severe COVID-19 patients (Zhou et al., 2023). Liu et al. conducted the study involving 294 patients, of which 17 cases of DILI were identified (Liu et al., 2024). The higher incidence may be attributed to the inclusion of patients with pre-existing elevated liver function at the enrollment stage, which could not only increase the risk of occurrence but also confounds non-pharmacological factors. In summary, the aggregate data from these studies suggest that Azvudine was associated with a favorable safety profile in the context of COVID-19 therapy.
Medications have been identified as one of the causes of hepatocellular damage in COVID-19 infections (Zhong et al., 2020; Wen-Shu Hu, 2022; Rodriguez-Espada et al., 2024). In previous researches, antiviral drugs, Remdesivir, and Nirmatrelvir-Ritonavir have been reported to have greater risk of developing drug-induce hepatotoxicity (Allison et al., 2023; Xie et al., 2020; Naseralallah et al., 2022). During the Azvudine therapy in our cohort, the value of liver enzymes increased significantly, revealing the potential hepatotoxicity. However, the mechanism underlying Azvudine-induced hepatic injury in COVID-19 patients remained unclear. Naveen et al. have demonstrated that Azvudine could cause mitochondrial ROS induction by two functional groups on sugar, Azido at 4′-poisoning and fluorine at 2′-poisoning, induce ROS generation with a time- and dose-dependent manner, prompt mitochondrial-mediated apoptosis (Kumar et al., 2024b; Kumar et al., 2024a). However, due to its minimal plasma protein binding affinity, Azvudine exhibited a propensity for accumulation in the thymus and was excreted in its parent form via renal clearance, which seems to be a safety pattern in the liver system (He et al., 2023; Liu S. et al., 2023; Sheng et al., 2024). Based on current pharmacokinetic and toxicity data, we assumed that the administration of Azvudine might lead to a dose-dependent intrinsic hepatotoxicity due to the accumulation exceeding the threshold level as a consequence of impaired excretion.
Interestingly, in the present study only concomitant antiviral and anticoagulant drugs were independent risk factors for Azvudine-induced hepatotoxicity, whereas other factors, including gender, age, severity of COVID-19, and comorbidities, were not significantly associated with the risk of liver injury. Further examination revealed that the co-administration of Ganciclovir, Low-Molecular-Weight Heparin Calcium, and Enoxaparin increased the risk. Literatures have indicated an increased likelihood of adverse drug reactions with the concurrent use of multiple antiviral agents (Liu et al., 2024). This might be attributed to competitive targeting by these drugs, resulting in cellular damage (Abdallah et al., 2014). Therefore, the concomitant use of multiple antiviral agents in clinical settings was not advocated. Additionally, it is suggested the concurrent use of anticoagulant drugs contributed not only the Azvudine but also Azvudine-induced hepatotoxicity. These conclusions aligned with previous studies, which identified anticoagulant as an independent risk factor for liver injury and increased 28-day mortality in COVID-19 patients, underscoring the potential correlation that warrants further investigation (Chen et al., 2024; Lu et al., 2024). Retrospective cohort study conducted by Yang et al., anticoagulants (LMWH and Fondaparinux) were associated with liver dysfunction with occurrence rate of 17.1% (79/463) in pulmonary embolism patients, recommencing transit to oral anticoagulants if possible (Yang et al., 2020). These findings were also recommended in our research as the new oral anticoagulants (NOACs) showed no significant difference in hepatotoxicity. Elder age and hypoproteinemia have been identified as risk factors for the progress of Azvudine-induced hepatotoxicity in previous studies (Lan et al., 2024; Zhi et al., 2023; Lu et al., 2024). However, the factors were not obvious in our study, possibly due to the inclusion of patients with a median age of >65 years, and the prevalence of critically ill patients contributed to lower baseline albumin level, which could have obscured the observed differences in the analysis.
In this study, the relatively low RUCAM scores, with only two cases indicating a highly probable association, suggested a tenuous causal relationship between Azvudine and hepatotoxicity (Danan and Teschke, 2018; Caines and Moonka, 2024; Zhao et al., 2024). This observation likely stems from multiple factors. Notably, polypharmacy was prevalent, the concurrent use of hepatotoxic agents with established hepatotoxic potential, such as statins and NSAIDs, complicated the assessment of Azvudine’s specific contribution to hepatotoxicity (Teschke et al., 2022; Russo et al., 2014; Zoubek et al., 2020). Additionally, the absence of rechallenge data posed a significant challenge. Most patients did not reintroduce the suspected medication post-discontinuation, creating an evidentiary gap in a critical RUCAM evaluation component. This lack of rechallenge documentation made definitively establishing a drug-hepatotoxicity link particularly difficult. Furthermore, the study’s relatively small positive sample size may have contributed to the lower RUCAM scores. Future research will address these limitations through large-scale studies and more comprehensive hepatotoxicity assessments, with the goal of clarifying the causal relationship between Azvudine and hepatotoxicity.
Although our study has several limitations, the conclusion provide great significance insights into hepatotoxicity associated with antiviral therapy in COVID-19 patients. First, as restrospective analysis, potential biases may existed due to incomplete or inconsistent data recording in electronic medical records (e.g., Incomplete body mass index). Second, the available data on combining medications were insufficient for robust statistical analysis, and the result was still suspicious and required further validation in future studies. Additionlly, the lack of standardized follow up protocols limited our ability to monitor liver injury progression and management during Azvudine therapy in real time. To address these limitations, multicenter prospective study with larger sample sizes to comprehensively assess the real-world hepatoxicity profile of Azudine. The future research will incorporate structed follow-up schedules, standardized laboratory monitoring, and detailed documentation of concomitant medications to enhance data reliability and clinical applicability.
5 Conclusion
Our investigation demonstrated a significant association between Azvudine and hepatotoxicity, with a notably elevated incidence of 27.2%. Most of these hepatotoxic events were characterized by mild severity and were reversible upon drug withdrawal. Thus, it was essential for clinicians to monitor the hepatic function vigilantly throughout the therapeutic course. Moreover, the incidence of Azvudine-induced hepatotoxicity appeared to be exacerbated by the concomitant administration of antiviral and anticoagulant therapies, specifically Ganciclovir, Low-molecular-weight heparin calcium, and Enoxaparin. Therefore, we do not recommend the concurrent use of Ganciclovir with Azvudine, and instead we suggest considering NOACs as a preferable alternative to LMWH for anticoagulation therapy in patients receiving Azvudine therapy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Renmin Hospital of Wuhan University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because This study is a single-center retrospective cohort study.
Author contributions
YX: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing. HX: Data curation, Formal Analysis, Writing – original draft. CS: Investigation, Methodology, Software, Writing – original draft. XG: Data curation, Formal Analysis, Writing – review and editing. YC: Data curation, Methodology, Resources, Writing – review and editing. CH: Data curation, Writing – review and editing. FM: Funding acquisition, Supervision, Writing – review and editing. GY: Conceptualization, Supervision, Validation, Visualization, Writing – review and editing. JY: Conceptualization, Supervision, Validation, Visualization, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We are grateful to all the participants in this study. We also extend our sincere thanks to Dr. Zhou Liu and Palpasa Sharestha for their professional language editing, which greatly improved the clarity and quality of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1558054/full#supplementary-material
References
Abdallah, E., Al-Helal, B., and Asad, R. (2014). Ganciclovir-induced acute liver injury in a patient with lupus nephritis. Saudi J. Kidney Dis. Transpl. 25 (5), 1084–1085. doi:10.4103/1319-2442.139947
Allison, R., Guraka, A., Shawa, I. T., Tripathi, G., Moritz, W., and Kermanizadeh, A. (2023). Drug induced liver injury - a 2023 update. J. Toxicol. Environ. Health, Part B 26 (8), 442–467. doi:10.1080/10937404.2023.2261848
Alqahtani, S. A., and Schattenberg, J. M. (2020). Liver injury in COVID-19: the current evidence. United Eur. Gastroenterol. J. 8 (5), 509–519. doi:10.1177/2050640620924157
Andrade, R. J., Aithal, G. P., Björnsson, E. S., Kaplowitz, N., Kullak-Ublick, G. A., Larrey, D., et al. (2019a). EASL clinical practice guidelines: drug-induced liver injury. J. Hepatol. 70 (6), 1222–1261. doi:10.1016/j.jhep.2019.02.014
Andrade, R. J., Chalasani, N., Björnsson, E. S., Suzuki, A., Kullak-Ublick, G. A., Watkins, P. B., et al. (2019b). Drug-induced liver injury. Nat. Rev. Dis. Prim. 5 (1), 58. doi:10.1038/s41572-019-0105-0
Baden, L. R., El Sahly, H. M., Essink, B., Kotloff, K., Frey, S., Novak, R., et al. (2021). Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384 (5), 403–416. doi:10.1056/NEJMoa2035389
Caines, A., and Moonka, D. (2024). Drug hepatotoxicity: causality assessment. Clin. Liver Dis. 24 (1), 25–35. doi:10.1016/j.cld.2019.09.001
Chen, Y., Lin, Y., Lu, H., Wu, X., Pan, Y., Xia, A., et al. (2024). Real-world effectiveness of molnupiravir, azvudine and paxlovid against mortality and viral clearance among hospitalized patients with COVID-19 infection during the omicron wave in China: a retrospective cohort study. Diagn. Microbiol. Infect. Dis. 109 (4), 116353. doi:10.1016/j.diagmicrobio.2024.116353
Chen, Y., Shi, C., Zhan, H., Yang, B., Liu, J., Rong, P., et al. (2023). Drug-induced liver injury in COVID-19 patients during hospitalization. Med. Baltim. 102 (11), e33294. doi:10.1097/MD.0000000000033294
Chow, E. J., Uyeki, T. M., and Chu, H. Y. (2023). The effects of the COVID-19 pandemic on community respiratory virus activity. Nat. Rev. Microbiol. 21 (3), 195–210. doi:10.1038/s41579-022-00807-9
Danan, G., and Teschke, R. (2016). RUCAM in drug and herb induced liver injury: the update. Int. J. Mol. Sci. 17 (1), 14. doi:10.3390/ijms17010014
Danan, G., and Teschke, R. (2018). Drug-induced liver injury: why is the Roussel Uclaf causality assessment method (RUCAM) still used 25 Years after its launch? Drug Saf. 41 (8), 735–743. doi:10.1007/s40264-018-0654-2
Deng, G., Li, D., Sun, Y., Jin, L., Zhou, Q., Xiao, C., et al. (2023). Real-world effectiveness of azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: a retrospective cohort study. J. Med. Virol. 95 (4), e28756. doi:10.1002/jmv.28756
General Office of the National Health Commission (2022). Notice on the issuance of diagnosis and treatment protocol for novel coronavirus infection (trial version 9). Available online at: https://www.gov.cn/zhengce/zhengceku/2022-03/15/content_5679257.htm (accessed March 15, 2022).
Grein, J., Ohmagari, N., Shin, D., Diaz, G., Asperges, E., Castagna, A., et al. (2020). Compassionate use of Remdesivir for patients with severe Covid-19. N. Engl. J. Med. 382 (24), 2327–2336. doi:10.1056/NEJMoa2007016
Gupta, A., Madhavan, M. V., Sehgal, K., Nair, N., Mahajan, S., Sehrawat, T. S., et al. (2020). Extrapulmonary manifestations of COVID-19. Nat. Med. 26 (7), 1017–1032. doi:10.1038/s41591-020-0968-3
Han, X., Gao, D., Li, C., Yuan, X., Cui, J., Zhao, W., et al. (2024a). Real-world effectiveness of nirmatrelvir-ritonavir versus azvudine in hospitalized patients with COVID-19 during the omicron wave in Beijing: a multicenter retrospective cohort study. BMC Infect. Dis. 24 (1), 57. doi:10.1186/s12879-023-08965-8
Han, X., Li, C., Yuan, X., Cui, J., Han, Z., Meng, J., et al. (2024b). Associations of nirmatrelvir-ritonavir treatment with death and clinical improvement in hospitalized patients with COVID-19 during the Omicron wave in Beijing, China: a multicentre, retrospective cohort study. Ann. Med. (Helsinki) 56 (1), 2313062. doi:10.1080/07853890.2024.2313062
He, M., Li, H., Mu, L. F., and Yang, M. (2023). Effect of azovudine on hepatic and renal function in patients with COVID-19:a case series study. Chin. General Pract. 26 (20), 2476–2481. doi:10.12114/j.issn.1007-9572.2023.0117
Hykin, P., Prevost, A. T., Vasconcelos, J. C., Murphy, C., Kelly, J., Ramu, J., et al. (2019). Clinical effectiveness of intravitreal therapy with ranibizumab vs aflibercept vs bevacizumab for macular edema secondary to central retinal vein occlusion: a randomized clinical trial. JAMA Ophthalmol. 137 (11), 1256–1264. doi:10.1001/jamaophthalmol.2019.3305
Kevadiya, B. D., Machhi, J., Herskovitz, J., Oleynikov, M. D., Blomberg, W. R., Bajwa, N., et al. (2021). Diagnostics for SARS-CoV-2 infections. Nat. Mater. 20 (5), 593–605. doi:10.1038/s41563-020-00906-z
Kumar, N., Delu, V., Ulasov, I., Kumar, S., Singh, R. K., Kumar, S., et al. (2024b). Pharmacological insights: mitochondrial ROS generation by FNC (azvudine) in dalton’s lymphoma cells revealed by super resolution imaging. Cell biochem. Biophys. 82 (2), 873–883. doi:10.1007/s12013-024-01238-4
Kumar, N., Kumar, S., and Shukla, A. (2024a). Mitochondrial-mediated apoptosis as a therapeutic target for FNC(2′-deoxy-2′-b-fuoro-4′-azidocytidine)-induced inhibition of Dalton′s lymphoma growth and proliferation. Discov. Oncol. 1 (15), 16. doi:10.1007/s12672-023-00829-6
Kwok, H. F. (2022). The significance of advanced COVID-19 diagnostic testing in pandemic control measures. Int. J. Biol. Sci. 18 (12), 4610–4617. doi:10.7150/ijbs.72837
Lan, B., Li, G. D., Li, W. S., Zhou, G. P., and Lin, C. Y. (2024). 112 cases of adverse drug reactions induced by azvudine tablets in patients with COVID-19. Cent. South Pharm. 22 (6), 1658–1662. doi:10.7539/j.issn.1672-2981.2024.06.040
Liu, S., Yang, S., Zhang, Y., and Liu, G. F. (2023a). Sensitivity analyses in longitudinal clinical trials via distributional imputation. Stat. Methods Med. Res. 32 (1), 181–194. doi:10.1177/09622802221135251
Liu, X. Z., Liu, J. J., Fang, X., Li, M., Zhao, J. J., and Xing, H. Y. (2024). A real-world study of azivudine tablets for drug-induced liver injury caused by novel coronavirus pneumonia. Chin. J. Hosp. Pharm. 44 (9), 1082–1087. doi:10.13286/j.1001-5213.2024.09.12
Liu, Z. Q., Lin, D., Liao, L. D., Gao, Y., Chen, W. T., and Hu, K. Z. (2023b). Efficacy and safety of azvudine in the treatment of COVID-19. Pract. Pharm. And Clin. Remedies 26 (10), 899–903. doi:10.14053/j.cnki.ppcr.202310007
Lu, H., Zeng, Y., Shi, Q., Liu, L., Gong, Y., Li, S., et al. (2024). Low albumin combined with low-molecular-weight heparin as risk factors for liver injury using azvudine: evidence from an analysis of COVID-19 patients in a national prospective pharmacovigilance database. Int. J. Clin. Pharmacol. Ther. 62 (05), 222–228. doi:10.5414/CP204544
Nanaw, J., Sherchan, J. S., Fernandez, J. R., Strassle, P. D., Powell, W., and Forde, A. T. (2024). Racial/ethnic differences in the associations between trust in the U.S. healthcare system and willingness to test for and vaccinate against COVID-19. BMC Public Health 1 (24), 1084. doi:10.1186/s12889-024-18526-6
Naseralallah, L. M., Aboujabal, B. A., Geryo, N. M., Al Boinin, A., Al Hattab, F., Akbar, R., et al. (2022). The determination of causality of drug induced liver injury in patients with COVID-19 clinical syndrome. PLoS One 17 (9), e0268705. doi:10.1371/journal.pone.0268705
Rajaram, R. B., Jayaraman, T., Khoo, X. H., Saravanaa, N., Kukreja, A., Johari, B. M., et al. (2024). Liver dysfunction in adults with COVID -19 infection: a longitudinal study with transient elastography evaluation. JGH Open 8 (8), e13118. doi:10.1002/jgh3.13118
Ren, Z., Luo, H., Yu, Z., Song, J., Liang, L., Wang, L., et al. (2020). A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and Common COVID-19, a pilot study. Adv. Sci. 7 (19), e2001435. doi:10.1002/advs.202001435
Rodriguez-Espada, A., Salgado-De La Mora, M., Rodriguez-Paniagua, B. M., Limon-De La Rosa, N., Martinez-Gutierrez, M. I., Pastrana-Brandes, S., et al. (2024). Histopathological impact of SARS-CoV-2 on the liver: cellular damage and long-term complications. World J. gastroenterology WJG 30 (22), 2866–2880. doi:10.3748/wjg.v30.i22.2866
Rubin, R. (2021). COVID-19 testing moves out of the clinic and into the home. JAMA J. Am. Med. Assoc. 326 (14), 1362–1364. doi:10.1001/jama.2021.15679
Russo, M. W., Hoofnagle, J. H., Gu, J., Fontana, R. J., Barnhart, H., Kleiner, D. E., et al. (2014). Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatology 60 (2), 679–686. doi:10.1002/hep.27157
Shang, N., Li, X., Guo, Z., Zhang, L., and Wang, S. (2024). Comparative analysis of the safety and effectiveness of nirmatrelvir-ritonavir and azvudine in older patients with COVID-19: a retrospective study from a tertiary hospital in China. Front. Pharmacol. 15, 1362345. doi:10.3389/fphar.2024.1362345
Sheng, N., Li, R., Li, Y., Wang, Z., Wang, L., Li, Y., et al. (2024). Selectively T cell phosphorylation activation of azvudine in the thymus tissue with immune protection effect. Acta Pharm. Sin. B 14 (7), 3140–3154. doi:10.1016/j.apsb.2024.03.032
Shi, X., Zuo, C., Yu, L., Lao, D., Li, X., Xu, Q., et al. (2021). Real-world data of tigecycline-associated drug-induced liver injury among patients in China: a 3-year retrospective study as assessed by the updated RUCAM. Front. Pharmacol. 12, 761167. doi:10.3389/fphar.2021.761167
Su, P., Yang, C. X., and Wang, X. G. (2024). Azvudine versus paxlovid for oral treatment of COVID-19 in Chinese patients. BMC Infect. Dis. 24 (1), 44. doi:10.1186/s12879-023-08828-2
Teschke, R., Méndez-Sánchez, N., and Eickhoff, A. (2022). Liver injury in COVID-19 patients with drugs as causatives: a systematic review of 996 DILI cases published 2020/2021 based on RUCAM as causality assessment method. Int. J. Mol. Sci. 23 (9), 4828. doi:10.3390/ijms23094828
Wang, S., Sun, J., Zhang, X., Li, M., Qin, B., Liu, M., et al. (2024a). Antiviral effectiveness and survival correlation of azvudine and nirmatrelvir/ritonavir in elderly severe patients with COVID-19: a retrospective real-world study. EClinicalMedicine 69, 102468. doi:10.1016/j.eclinm.2024.102468
Wang, Y., Xie, H., Wang, L., Fan, J., Zhang, Y., Pan, S., et al. (2024b). Effectiveness of azvudine in reducing mortality of COVID-19 patients: a systematic review and meta-analysis. Virol. J. 21 (1), 46. doi:10.1186/s12985-024-02316-y
Wen-Shu Hu, F. J. W. S., Jiang, F. Y., Shu, W., Zhao, R., Cao, J. M., and Wang, D. P. (2022). Liver injury in COVID-19: a minireview. World J. Gastroenterol. 47 (28), 6716–6731. doi:10.3748/wjg.v28.i47.6716
Xie, H., Zhao, J., Lian, N., Lin, S., Xie, Q., and Zhuo, H. (2020). Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int. 40 (6), 1321–1326. doi:10.1111/liv.14449
Yang, H., Wang, Z., Wang, C., Zhang, Y., Han, S., and An, Z. (2024a). Cost-effectiveness of azvudine for high-risk outpatients with mild-to-moderate coronavirus disease 2019 in China. Clin. Ther. 46 (9), e1–e5. doi:10.1016/j.clinthera.2024.07.009
Yang, H., Zhang, Y., Wang, Z., Xu, M., Wang, Y., Zhang, Y., et al. (2024b). Adherence and recommended optimal treatment to azvudine application for the treatment of outpatient COVID-19 patients: a real-world retrospective study. Heliyon 10 (9), e30619. doi:10.1016/j.heliyon.2024.e30619
Yang, M., Zhao, H., Xu, Z. L., Luo, X., Yang, X. X., Xiao, D. K., et al. (2023). Analysis of the efficacy and safety of azvudine in treating moderate COVID-19 in kidney transplant recipients. Chin. J. New Clin. Med. 16 (10), 1011–1015. doi:10.3969/j.issn.1674-3806.2023.10.06
Yang, X., Li, N., Guo, T., Guan, X., Tan, J., Gao, X., et al. (2020). Comparison of the effects of low-molecular-weight heparin and Fondaparinux on liver function in patients with pulmonary embolism. J. Clin. Pharmacol. 60 (12), 1671–1678. doi:10.1002/jcph.1686
Yu, B., and Chang, J. (2020). Azvudine (FNC): a promising clinical candidate for COVID-19 treatment. Signal Transduct. Target. Ther. 5 (1), 236. doi:10.1038/s41392-020-00351-z
Yu, B., and Chang, J. (2022). The first Chinese oral anti-COVID-19 drug azvudine launched. Innov. (New York, NY) 3 (6), 100321. doi:10.1016/j.xinn.2022.100321
Yu, Y. C., Mao, Y. M., Chen, C. W., Chen, J. J., Chen, J., Cong, W. M., et al. (2017). CSH guidelines for the diagnosis and treatment of drug-induced liver injury. Hepatol. Int. 11 (3), 221–241. doi:10.1007/s12072-017-9793-2
Zhang, J. L., Li, Y. H., Wang, L. L., Liu, H. Q., Lu, S. Y., Liu, Y., et al. (2021). Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal Transduct. Target. Ther. 6 (1), 414. doi:10.1038/s41392-021-00835-6
Zhao, H., Wang, Y., Zhang, T., Wang, Q., and Xie, W. (2021). Drug-induced liver injury from anti-tuberculosis treatment: a retrospective cohort study. Med. Sci. Monit. 7 (26), e920350. doi:10.12659/MSM.920350
Zhao, X., Wang, Y., Lai, R., Wang, X., Yu, Y., Li, M., et al. (2024). Validation of the revised electronic version of RUCAM for diagnosis of DILI in Chinese patients. Hepatol. Commun. 8 (4), e0235. doi:10.1097/HC9.0000000000000235
Zhi, J., Wu, L., Tan, Z., Dou, Y., and Qu, G. (2023). Adverse reaction of azvudine in elderly patients with COVID-19 over 75 years old. Drug Eval. Res. 46 (10), 2208–2213. doi:10.7501/j.issn.1674-6376.2023.10.019
Zhong, P., Xu, J., Yang, D., Shen, Y., Wang, L., Feng, Y., et al. (2020). COVID-19-associated gastrointestinal and liver injury: clinical features and potential mechanisms. Signal Transduct. Target. Ther. 5 (1), 256. doi:10.1038/s41392-020-00373-7
Zhou, Y., Liu, Y., Jiang, L., Zhang, R., Zhang, H., Shi, Q., et al. (2023). Azvudine and nirmatrelvir–ritonavir in hospitalized patients with moderate-to-severe COVID-19: emulation of a randomized target trial. J. Med. Virol. 95 (12), e29318. doi:10.1002/jmv.29318
Keywords: COVID-19, Azvudine, hepatotoxicity, risk factors, real-world data
Citation: Xiong Y, Xin H, Shi C, Guo X, Chen Y, Huang C, Ma F, Yang G and Yang J (2025) Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study. Front. Pharmacol. 16:1558054. doi: 10.3389/fphar.2025.1558054
Received: 09 January 2025; Accepted: 23 May 2025;
Published: 04 June 2025.
Edited by:
Fatma Mohamady El-Demerdash, Alexandria University, EgyptReviewed by:
Stelvio Tonello, University of Eastern Piedmont, ItalyMi Mi Tang, Central South University, China
Copyright © 2025 Xiong, Xin, Shi, Guo, Chen, Huang, Ma, Yang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fuwang Ma, MTM0Njk5OTY4MDlAcXEuY29t; Ge Yang, eWFuZ2dlQHRtbXUuZWR1LmNu; Jian Yang, cm0wMDIzOTdAb3V0bG9vay5jb20=
†These authors have contributed equally to this work and share first authorship
 Yuanguo Xiong
Yuanguo Xiong Hao Xin2†
Hao Xin2† Cai Shi
Cai Shi Xianxi Guo
Xianxi Guo Ying Chen
Ying Chen Ge Yang
Ge Yang Jian Yang
Jian Yang