- 1Department of Medicinal Chemistry, Faculty of Pharmacy, King Salaman International University (KSIU), South Sinai, Egypt
- 2Department of Pharmaceutical Medicinal Chemistry and Drug Design, Faculty of Pharmacy (Girls), Al-Azhar University, Cairo, Egypt
- 3Pharmacology and Toxicology Department, Faculty of Pharmacy, Badr University in Cairo (BUC), Cairo, Egypt
- 4Department of Cytology and Histology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
- 5Faculty of Veterinary Medicine, King Salman International University, South Sinai, Egypt
- 6Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, October 6 University, Giza, Egypt
- 7Department of Medical Physiology, College of Medicine, King Khalid University, Asir, Saudi Arabia
- 8Department of Pediatrics, Faculty of Medicine, Tanta University, Tanta, Egypt
- 9Department of Pharmacology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt
- 10Department of Physiology, and Pharmacology, Faculty of Veterinary Medicine, King Salman International University, South Sinai, Egypt
- 11Department of Pharmacology and Toxicology, Faculty of Pharmacy, Menoufia University, Menoufia, Egypt
- 12Department of Pharmacology and Toxicology, Faculty of Pharmacy, Menoufia National University, Menoufia, Egypt
- 13Department of Industrial Pharmacy, College of Pharmaceutical Sciences and Drug Manufacturing, Misr University for Science and Technology, Giza, Egypt
- 14Department of Pharmaceutical Sciences, College of Pharmacy and Thumbay Research Institute for Precision Medicine, Gulf Medical University, Ajman, United Arab Emirates
- 15Department of Pharmaceutical Organic Chemistry, Faculty of Pharmacy, Cairo University, Cairo, Egypt
- 16Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Kafrelsheikh University, Kafrelsheikh, Egypt
- 17Department of Pharmaceutics, College of Pharmaceutical Sciences and Drug Manufacturing, Misr University for Science and Technology, Giza, Egypt
Introduction: Topical delivery of anti-inflammatory drugs is an important strategy for enhancing therapeutic efficacy while minimizing systemic side effects. This study focuses on improving the anti-inflammatory activity of Dexketoprofen by developing zein nanoparticles (ZNs) as a novel topical carrier system, aiming to optimize drug delivery and patient outcomes.
Methods: Dexketoprofen-loaded ZNs were prepared using an ethanol injection technique and optimized via a 23 full factorial design. The effects of three variables—phosphatidylcholine (PC) amount (X1), type of surface-active agent (SAA, X2), and SAA amount (X3)—were evaluated on entrapment efficiency (EE%), particle size (PS), polydispersity index (PDI), and zeta potential (ZP). Design-Expert® software was employed to identify the optimal formulation. Additionally, molecular docking studies were performed to explore interactions between Dexketoprofen and formulation components. The selected formulation (F7) was further characterized for morphology using scanning electron microscopy. In vivo efficacy was assessed using a formalin-induced paw edema model in rats, and histopathological analysis was conducted to evaluate skin irritation potential.
Results: The optimal formulation (F7), prepared with 200 mg PC and 20 mg Pluronic F127, demonstrated an entrapment efficiency of 92.44 ± 7.21%, particle size of 91.88 ± 3.01 nm, PDI of 0.42 ± 0.02, and zeta potential of −24.10 ± 0.29 mV. F7 exhibited a smooth, spherical morphology. In vivo studies revealed significantly enhanced anti-inflammatory activity compared to free Dexketoprofen. Histopathological examination confirmed the non-irritant nature of the formulated ZNs on rat skin.
Discussion: These findings highlight the effectiveness of zein nanoparticles as a promising topical delivery system for Dexketoprofen. The optimized ZNs not only improved drug entrapment and stability but also provided superior anti-inflammatory efficacy and excellent skin tolerability, suggesting their potential for the treatment of inflammatory skin conditions.
Introduction
Nanoparticles are colloidal drug carriers made of natural or synthetic polymers (Albash et al., 2025). They have demonstrated considerable interest in drug delivery (Mitchell et al., 2021). Different nanoparticles have been prepared to improve the therapeutic and pharmacological properties of free drugs, including instability (Yetisgin et al., 2020), serious side effects, low bioavailability, and reduced aqueous solubility, and some of them can improve the absence of targeted delivery (Chenthamara et al., 2019). They have high drug-loading capacity and can encapsulate different drugs with different solubilities. Protein can be used to prepare nanoparticles as it can provide many advantages, such as environmental tolerance, wide availability, biodegradability, and high drug-binding capacity (Hong et al., 2020). Proteins may be animal-derived, such as elastin, gelatin, albumin, casein, lysozyme, and lactoferrin (Kianfar, 2021), or plant-derived, such as zein, soy protein isolate, glutenin, gliadin, and walnut protein isolate (Liu et al., 2023). Plant-derived proteins are cheaper, decrease the risk of spreading diseases, and are less immunogenic (Reddy and Rapisarda, 2021).
Zein is the main corn storage protein (Zhang et al., 2021), which contains 44%–79% of the endosperm protein. It is widely available in nature and can be extracted from corn easily. Zein is soluble in aqueous ethanol and aqueous acetone and insoluble in water and absolute ethanol (Wu et al., 2023). It is also a prolamin and has four classes (α, β, γ, and δ) with different molecular sizes, solubility, and peptide chains. The richest constituent is α-zein, containing approximately 80% of the whole zein, followed by γ-zein, which contains approximately 15%. The available zein is commercially presented in yellow and white forms. Yellow zein is 90% pure and contains xanthophyll pigments in a high concentration, while white zein has higher purity with a minor amount of xanthophylls (De Marco, 2022). Zein has the advantage of being non-toxic, economical, biodegradable, and biocompatible (Radwan et al., 2020). Furthermore, previous studies included zein nanoparticles for the delivery of anti-inflammatory drugs, such as quercetin and resveratrol (Penalva et al., 2017; Liu et al., 2021). Zein has attracted growing attention as a feasible material for drug delivery systems based on nanoparticles because of its amphiphilic nature and biocompatibility, which make it particularly suitable for carrier applications.
Dexketoprofen is a non-steroidal anti-inflammatory propionic acid derivative with the ability to treat acute and chronic pain and inflammation (Moore and Barden, 2008). It has antipyretic, anti-inflammatory, and analgesic effects. Its action is caused by the inhibition of cyclooxygenase-1 (Cox-1), which is involved in the production of prostaglandins with physiological functions, and cyclooxygenase-2 (Cox-2), which facilitates the synthesis of pro-inflammatory prostaglandins at the inflammation site (Kuczyńska et al., 2022). Dexketoprofen inhibits prostaglandin synthesis strongly. It is a ketoprofen (S+) enantiomer but with a stronger effect and fewer serious adverse effects. It is effective at low doses and well tolerated, making it a readily used preparation (Gaskell et al., 2017). Dexketoprofen has a melting point of 104.94°C and Log P = 2.65, which indicates ambient lipophilicity, making the drug perfect for topical administration (Carpenter et al., 2024).
Inflammation is a protective approach in response to tissue injury, microbial infection, or other detrimental conditions (Soliman et al., 2023; Abdelbari et al., 2023). The typical symptoms of inflammation include pain, redness, swelling, and heat (Ahmed, 2011). Edema is a condition characterized by fluid accumulation resulting from an abnormal increase in interstitial fluid volume, typically due to inflammation (Trayes et al., 2013).
The aim of this study was to prepare zein nanoparticles (ZNs) loaded with dexketoprofen for the effective management of inflammation. ZNs are biocompatible and have the capacity to encapsulate both hydrophilic and hydrophobic drugs with good stability (Elmahboub et al., 2024). Dexketoprofen-loaded ZNs were prepared using Pluronic F127 (PF127) or Pluronic F86 (PF86) as a surface-active agent (SAA) to improve the drug’s penetration. For the optimization of the prepared ZNs, a 23 full-factorial design was applied to assess the influence of different variables on the characterization of the prepared ZN formulations and determine the best formulation. Phosphatidylcholine (PC) amount (mg) (X1), type of SAA (X2), and SAA amount (mg) (X3) were chosen as independent variables, while the responses were entrapment efficiency (EE%) (Y1), particle size (PS) (Y2), polydispersity index (PDI) (Y3), and zeta potential (ZP) (Y4). The optimal ZN morphology was investigated. Furthermore, this is the first report on the in vivo anti-inflammatory potential of the optimal dexketoprofen-loaded ZN formulation against formalin-induced paw edema. Safety assessment was also evaluated using a histopathological study.
Materials
Dexketoprofen was sourced from Utopia Pharmaceuticals, Cairo, Egypt; formalin, zein (90% purity), PF127, and PF86 were purchased from Sigma-Aldrich, MO, United States. All chemicals used were of the highest available analytical quality.
Methods
Fabrication of dexketoprofen-loaded ZNs
ZNs were synthesized using an ethanol injection method with slight modifications (Abdelbari et al., 2021). Initially, zein (10 mg) and PC with surfactants at varying concentrations were measured and dissolved in 2 mL of ethanol (95%) (1:5, v/v%). Dexketoprofen (50 mg) was dissolved in 10 mL of distilled water, and the resulting solution was injected into the lipophilic mixture and stirred using a magnetic stirrer (Model MSH-20D, GmbH, Berlin, Germany) at 1,500 rpm for 30 min at room temperature to ensure complete solvent evaporation. A probe sonicator (JY-92-II, Xinzhi, China) was used to enhance the homogeneity of the particles in the dispersion medium of ZNs, with sonication conducted at 40% amplitude for 5 min, consisting of 3 s on and 3 s off. Finally, the formulations were stored in a refrigerator for maturity.
In vitro characterization of dexketoprofen-loaded ZNs
EE %
The percentage of dexketoprofen encapsulated in ZNs was assessed using a spectrophotometer. Cooling ultracentrifuge (Sigma 3 K 30, Germany) was used for 1 hour at 4°C and 20,000 rpm (Adel et al., 2023). The precipitate was then lysed using 10 mL methanol at room temperature, and 1 mL of the solution was measured using an ultraviolet spectrophotometer at λmax 242 nm (Carpenter et al., 2024) (Shimadzu UV1650 Spectrophotometer, Kyoto, Japan). The drug’s EE% was calculated thrice using Equation 1:
The average PS and PDI of the prepared ZNs were measured using a Zetasizer (Malvern Instrument Ltd., United Kingdom), applying the light-scattering procedure to the ZN suspension in distilled water (Abdelbari et al., 2024). Using the same Zetasizer, the electrophoretic mobility of the prepared ZNs was measured to determine the ZP. All measurements were performed in triplets (Mosallam et al., 2021a).
Experimental design and optimization
The influence of the variables on the characterization of the prepared ZNs was determined using a 23 full-factorial design with Design-Expert® software version 11 (Stat-Ease, Inc., Minneapolis, MN, United States). The investigated variables were PC amount (mg) (X1), type of SAA (X2), and SAA amount (mg) (X3), each variable with two levels, as displayed in Table 1. An analysis of variance (ANOVA) test was used to evaluate parameters and determine the optimal formulation. P ≤ 0.05 reflected a significant difference.
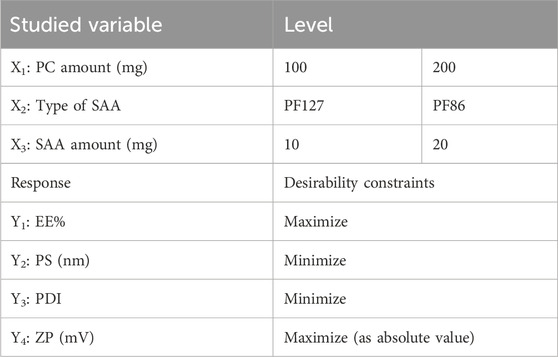
Table 1. Variables and levels used in the preparation of dexketoprofen-loaded ZNs using a (23) full-factorial design and the statistical analysis summary of the full-factorial design (23) used for the optimization of the prepared ZNs.
Docking studies
The docking studies were assessed using molecular operating environment (MOE) software version 2019.0102 (Hassab et al., 2021). The compounds were drawn using ChemDraw 20.1.1 and transformed using MOE to three-dimensional (3D) structures. The energy of the four compounds (dexketoprofen, lecithin, zein, and PF127) was minimized using AMBER10:EHT before initiating the docking. Each component (lecithin, zein, and PF127) was solvated in distilled water and then used as a potential receptor to bind with dexketoprofen. The docking studies were directed at maximum accuracy using induced fit protocol and triangle matcher as the scoring function. The results were examined based on docking scores and molecular interactions.
Characterization of the optimal dexketoprofen-loaded ZNs
Determination of the amount of drug release
USP dissolution apparatus II (Pharma Test, Hainburg, Germany) was used to assess in vitro drug release for 12 h at 37°C. An amount of 2 mL of the optimal ZN formulation containing 5 mg of dexketoprofen was put into tubes with a permeation area (3.14 cm2), with one end firmly sealed with a cellulose membrane and the other end linked to the shaft of the dissolution equipment rather than the baskets. The receptor medium was 50 mL of phosphate buffer saline solution (pH 7.4). Aliquots were withdrawn at different time intervals up to 12 h. The samples were examined using a UV spectrophotometer set to λmax = 242 nm. The measurements were carried out in triplicate.
Stability study
The optimal ZN formulation was stored at 4°C for 3 months. The initial measurements were compared with results obtained after storage to evaluate physical stability. The EE%, PS, PDI, ZP, and amount of drug released after 6 h (Q6h; %) from the nanoparticles were measured. Statistical significance was analyzed using Student’s t-test in SPSS® software 22.0. A difference at p ≤ 0.05 was considered significant.
In vivo studies
Experimental animals
Male Wistar albino rats, aged 7 weeks and weighing from 200 to 250 g, were obtained from the animal house at the Faculty of Pharmacy, Badr University, Cairo, Egypt. The rats were provided with unrestricted access to standard rat food and water in a controlled environment (23°C ± 1°C and 40%–60% humidity). After 1 week of acclimatization, the experiment was conducted in accordance with NIH guidelines (NIH Publication No. 85–23, revised 2011) for the care and use of animals, and it was accepted by the research ethics committee of the Faculty of Pharmacy, Badr University, Cairo (approval number, IACUC/PHA/162/A/2024).
Animal groups
Rats were randomly assigned to four groups, each consisting of six animals, and were labeled as follows:
• Group I: control group (CTRL).
• Group II: paw edema group (PE).
• Group III: standard group (DKP).
• Group IV: dexketoprofen-loaded ZN formulation group (NanoDKP).
During the experiment, rats were pre-treated with a single oral dose of dexketoprofen-loaded ZN formulation (0.1 mg/kg, p.o.) for Group IV (Soyocak et al., 2019). The control group received the vehicle orally at a matched dose volume of 1 mL/kg, and the standard group received dexketoprofen (0.1 mg/kg, p.o.) (Soyocak et al., 2019). One hour after the final administration of the test drug or vehicle, all rats in groups II, III, and IV were given a sub-plantar injection of 0.1 mL of 5% formalin to induce edema in the left paw (Abdel Mageed et al., 2022; Bancroft and Gamble, 2008). To ensure dose equivalence, the dose of dexketoprofen-loaded Zn nanoparticles administered to Group IV was calculated to deliver an amount of dexketoprofen equivalent to 0.1 mg/kg body weight (p.o.), consistent with the standard group.
Formalin-induced paw edema in rats
Formalin-induced edema was induced in the left hind paw of rats in all groups via a sub-plantar injection of 0.1 mL of 5% formalin, as previously described (Abdel Mageed et al., 2022; Bancroft and Gamble, 2008), except for the control group, which received normal saline. All groups received their respective treatments 1 h prior to the formalin injection. Paw thickness was measured immediately after the formalin injection (time 0) and at 1, 2, 4, and 24 h using a Vernier caliper to assess the degree of inflammation. At the end of the experiment, rats were euthanized under anesthesia using thiopental (50 mg/kg, i.p) (Mostafa et al., 2023); their paws were removed, and blood samples were collected.
Evaluation of oxidative stress and inflammatory markers
ELISA technique
In compliance with the manufacturer’s instructions, the ELISA technique was conducted using the Thermo Scientific Multiskan FC Microplate Reader (Thermo Fisher Scientific, United States) to estimate myeloperoxidase (MPO) (Cat#: E4581-100; Bio Vision Incorporated, Milpitas, CA, United States), Cox-2 (Cat#: A74115, antibodies, United Kingdom), and interleukin 6 (IL-6) (Cat#: MBS269892, MyBioSource, United States) in tissue homogenate, whereas tumor necrosis factor-α (TNF-α) (Cat#: MBS2507393; MyBioSource, United States) and prostaglandin E2 (PGE2) (Cat#: ab287802, Abcam, United Kingdom) were estimated in the serum sample.
Colorimetric analysis
Malondialdehyde (MDA) (Abcam, Cat# ab118970) and reduced glutathione (GSH) (Novus Biologicals, Cat# NBP3-25794) were assessed in tissue homogenate using colorimetric reagents, in compliance with the manufacturer’s instructions, using a UV–Visible spectrophotometer (Shimadzu UV-1800, Japan).
In vivo histopathological examination
Skin tissue from all groups was dissected, fixed in 10% neutral buffered formalin (10% NBF) for 48 h, washed, and embedded for dehydration in ascending ethanol grades. The dehydrated sections were cleared in xylene, embedded in paraffin wax, sectioned at 3–4 µm thickness, deparaffinized, and stained with hematoxylin and eosin (H&E) stain (Bancroft and Gamble, 2008). The stained slides were inspected using a light microscope (Leica DM500), and the images were captured using a camera (Leica ICC50 HD) attached to the microscope and examined using image analysis software [Leica Microsystems, LAS version 3.8.0 (Build: 878); Leica image analyzer computer system] at the Light Microscopy Unit, Cairo University Research Park (CURP), Faculty of Agriculture, Cairo University, Giza, Egypt.
Statistical analysis
The data were presented as the mean ± standard deviation (SD) and analyzed using one-way ANOVA to investigate group differences, followed by Tukey’s post hoc test for comparison. Results reflected a significant difference if p ≤ 0.05. All statistical analyses were executed using GraphPad Prism version 8 (San Diego, California, United States).
Results and discussion
Factorial design outcomes
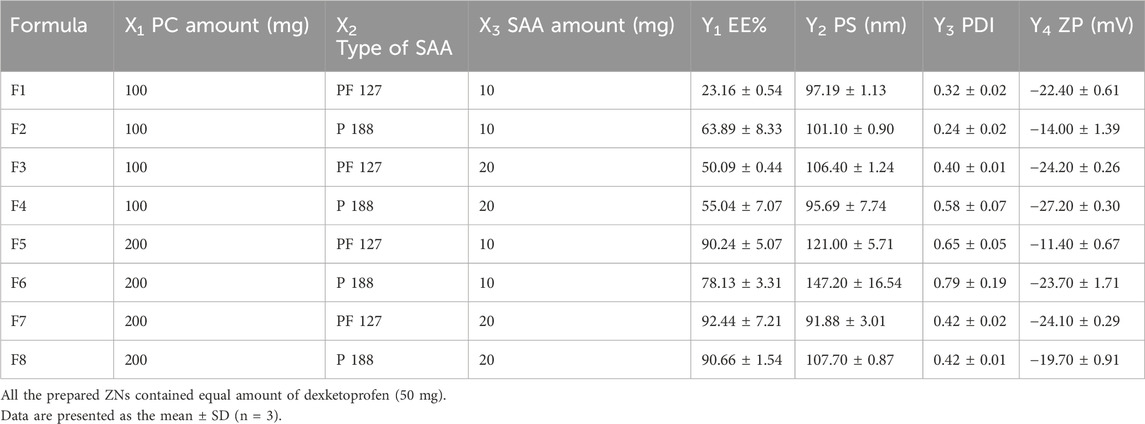
A 23 full-factorial design involved eight formulations (F1–F8). All the measured responses are displayed in Table 1. Adequate precision is the signal-to-noise ratio (Mostafa et al., 2023) that reflects the model’s ability to navigate the design space. It is preferable for this value to exceed 4 (Abdelbary et al., 2016), and this was achieved in all responses, as displayed in Table 1. The predicted R2 also affects the models’ quality (Abdelbari et al., 2021). It is necessary that the adjusted and predicted R2 show an acceptable agreement (Al-mahallawi et al., 2015), which is displayed in all the response outcomes (Table 1).
Influence of variables on EE%
The EE% of dexketoprofen in the prepared ZNs ranged from 23.16% ± 0.54% to 92.44% ± 7.21% (Table 2). The influences of PC amount (mg) (X1), type of SAA (X2), and SAA amount (mg) (X3) on EE% are demonstrated as linear plots in Figures 1A–C. Statistical analysis of the data revealed that the three variables significantly influence the EE% (p < 0.05).
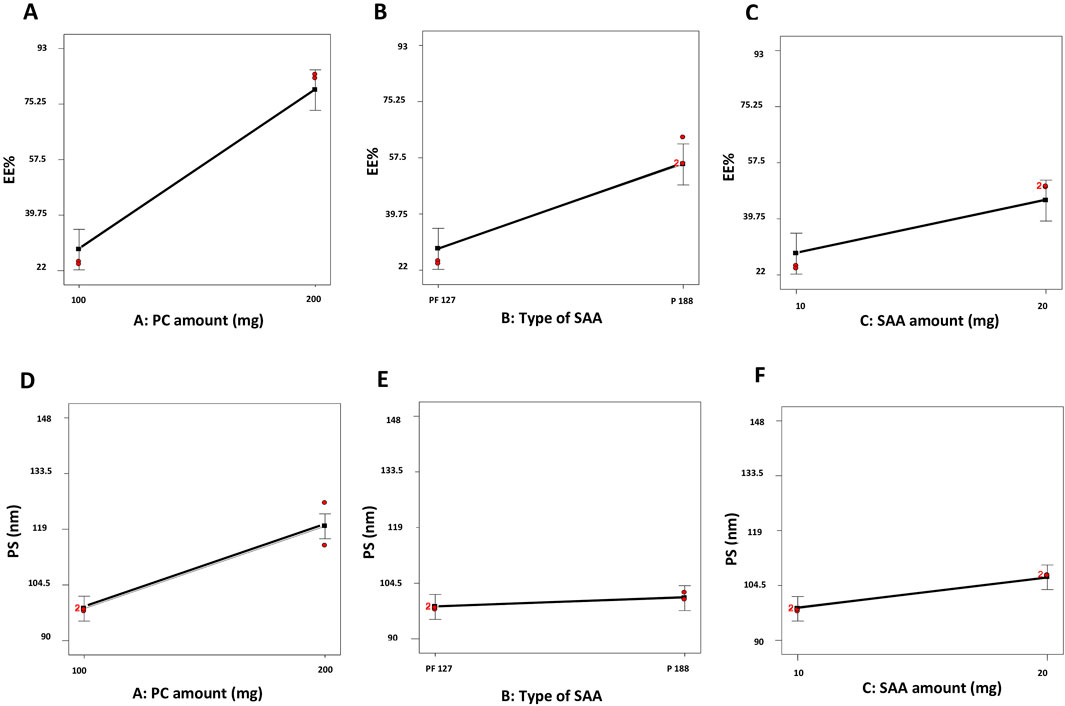
Figure 1. Linear plots demonstrating the effect of the PC amount (mg) (X1), type of SAA (X2), and SAA amount (mg) (X3) on EE% (A–C) and PS (D–F) of dexketoprofen-loaded ZNs. PC, phosphatidylcholine; EE%, encapsulation efficiency percent; PS, particle size; ZNs, zein nanoparticles.
First, increasing the PC amount (mg) (X1) significantly improved the EE% of the prepared ZNs (Tefas et al., 2017); this might have resulted from the hydrophobic interactions with the PC bilayer structure (Were et al., 2003), which decreases the membrane’s fluidity, therefore increasing the drug’s EE% in the prepared ZNs (Albash et al., 2024). Additionally, a higher PC amount increases viscosity, which might reduce the external diffusion of dexketoprofen (Albash et al., 2021).
Next, the type of SAA (X2) had a significant influence on the prepared ZNs’ EE% as those prepared using PF86 had a higher EE% than those prepared using PF127. This result is attributed to the surfactant PF86, which helps stabilize and solubilize the drug molecule, allowing it to be encapsulated within the lipid matrix and at the surface of ZNs (Ali et al., 2023). This result can also be explained through the HLB values of both SAAs, which are 22 and 29 for PF127 and PF86, respectively (Mostafa et al., 2023; Abdelbary et al., 2016); as the drug is hydrophilic, it had a higher EE% when combined with PF86, which has a higher HLB value and, consequently, lower lipophilicity (Albash et al., 2021).
Finally, the SAA amount (mg) (X3) also had a significant influence on EE% as increasing the SAA amount (mg) increased the EE% of the prepared ZNs, which can be attributed to the effect of the high SAA amount in forming a layer that enhances the vesicle interface stability, offering more room inside ZNs to encapsulate more drug (Abdelbari et al., 2021).
Influence of variables on PS
All the prepared ZNs had low PS, which ranged from 91.88 ± 3.01 to 147.20 ± 16.54 nm (Table 2). Low PS ensures the production of a stable system and enhances skin penetration (Roberts et al., 2017). The influences of PC amount (mg) (X1), type of SAA (X2), and SAA amount (mg) (X3) on PS are demonstrated as linear plots in Figures 1D–F. Statistical analysis of the data revealed that the three variables significantly influence the PS (p < 0.05).
Increasing the PC amount (mg) (X1) significantly increased PS. This result agrees with the EE% results, where increasing the PC amount (mg) resulted in a higher EE% (Albash et al., 2021). Moreover, the type of SAA significantly affected the PS of the prepared ZNs as those prepared using PF86 had a larger PS than those prepared using PF127. The higher amount of dexketoprofen encapsulated within the prepared ZNs might be the reason behind increasing their PS (Mosallam et al., 2021b). Finally, the SAA amount (mg) (X3) also had a significant influence on the PS (Rania et al., 2024).
Influence of variables on PDI
The PDI reflects the system’s heterogeneity, and it occurs between 0 and 1 (Soliman S et al., 2022). The PDI of the prepared ZNs ranged between 0.24 ± 0.02 and 0.79 ± 0.19 (Table 2), reflecting that some of the prepared formulations are polydisperse. Factorial analysis showed that PC amount (mg) (X1) significantly influenced the PDI (p < 0.05). Increasing the PC amount significantly increased the PDI values, probably due to the high PC amount that reached maximum coverage of the surface of the prepared ZNs, which formed a superficial adsorption layer producing steric resistance (Wang et al., 2019). However, both types of SAA (X2) and SAA amount (mg) (X3) had a non-significant influence on the PDI of the prepared ZNs. Results are demonstrated as linear plots in Figures 2A–C.
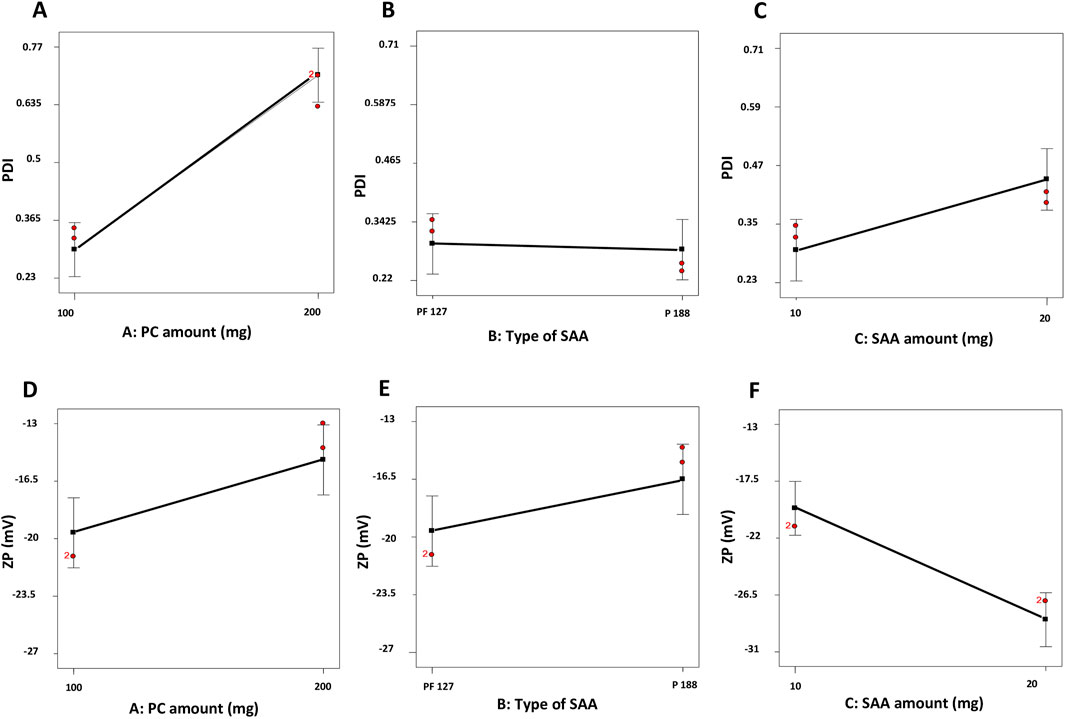
Figure 2. Linear plots demonstrating the effect of the PC amount (mg) (X1), type of SAA (X2), and SAA amount (mg) (X3) on PDI (A–C) and ZP (D–F) of dexketoprofen-loaded ZNs. PC, phosphatidylcholine; PDI, polydispersity index; ZP, zeta potential; ZNs, zein nanoparticles.
Influence of variables on ZP
The stability of a system can be evaluated through the ZP; it is a value that reflects the total charges gained by the particles. A ZP value between ±30 mV reflects a stable system as it indicates a sufficient electric repulsive force between particles (Salama et al., 2022). ZP values of the prepared ZNs ranged from −11.40 ± 0.67 to −27.20 ± 0.30 mV (Table 2), which indicates the high stability of the formulated ZNs. Factorial analysis exposed that the SAA amount (mg) (X3) had a significant influence on ZP (p < 0.05). As shown in Figures 2D–F, increasing the SAA amount increased the ZP as an absolute value. Neither the PC amount (mg) (X1) nor the type of SAA (X2) had a significant influence on the ZP of the prepared ZNs.
Determination of the optimal dexketoprofen-loaded ZNs
Design-Expert® software was used to select the optimal ZN formulation by evaluating the results statistically. The optimal formulation had the highest EE% and most stable ZP and the lowest PS and PDI. F7 was selected as the optimal formulation, with a desirability of 0.787. F7 was prepared using 10 mg zein, 200 mg PC, and 20 mg PF127 and had an EE% of 92.44% ± 7.21%, a PS of 91.88 ± 3.01 nm, a PDI of 0.42 ± 0.02, and a ZP of −24.10 ± 0.29 mV.
Docking studies
The study was conducted to explore the potential interactions between dexketoprofen and the components of the formula. Each component was assessed for its ability to bind with dexketoprofen. Notably, dexketoprofen showed favorable binding interactions with all three components of the formulation. The recorded binding affinities were −5.8, −6.6, and −5.3 kcal/mol for PC, zein, and Pluronic F127, respectively. As illustrated in Figure 3, dexketoprofen is involved in multiple interactions with these components. The docking analysis particularly highlighted the strong binding affinity of zein for dexketoprofen, suggesting that incorporating zein into the formulation may be essential.
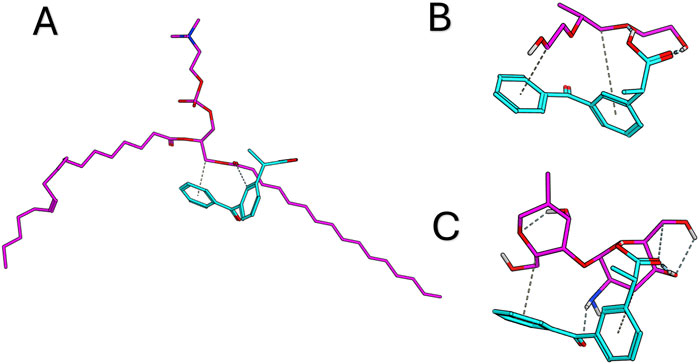
Figure 3. Docking results for dexketoprofen with the formulation components and the 3D interaction diagram between dexketoprofen, (A) lecithin, (B) Pluronic F127, and (C) zein.
Characterization of the optimal dexketoprofen-loaded ZNs
In vitro release profile
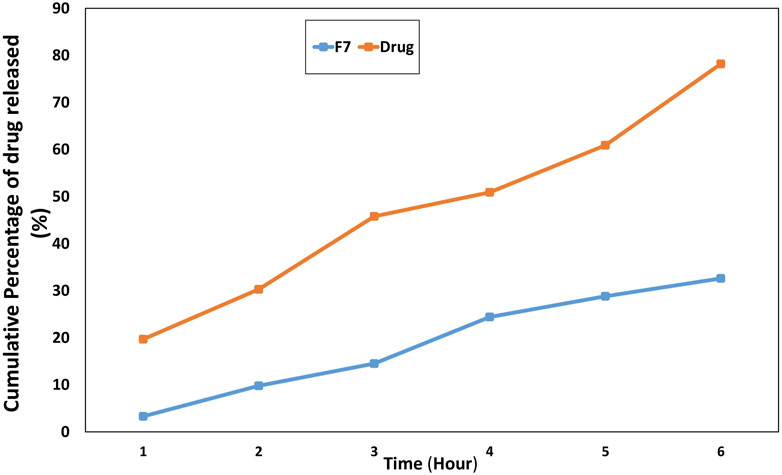
The adverse effects can be reduced in the modified release manner as the drug exists at the target site for an extended time (Sankhyan and Pawar, 2013). F7 exhibited sustained in vitro release behavior of the drug, with approximately 78% of the drug released within 12 h, compared to approximately 100% from the drug solution, as shown in Figure 4. The presence of the hydrophobic carrier zein played an essential role in obtaining a modified release manner (Franco and De Marco, 2020).
Stability study
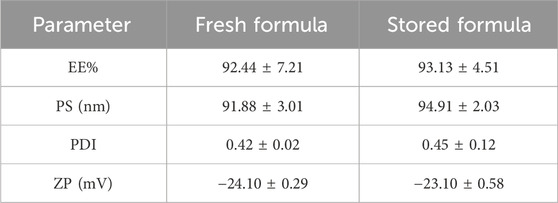
The results revealed that F7 exhibited respectable storage stability. The stored F7 showed non-significant deviations in appearance, EE%, PS, PDI, and ZP (p > 0.05) compared to the fresh F7 (Table 3).
In vivo studies (effect of F7)
The effects of F7 on formalin-induced paw edema inflammation in rats were investigated. As depicted in Figure 5, no significant difference was observed in p-values for paw edema at the time of formalin injection compared to other groups. Paw edema was significantly induced at 1-, 2-, 4-, and 24-h post-injection, with notable significance at 4 h (p < 0.0001), indicating the establishment of formalin-induced inflammation. These results are consistent with previous studies (Soyocak et al., 2019; Lee and Jeong, 2002), which reported that formalin administration resulted in swelling of the paws and an increase in vascular permeability. Group III (DKP group), which received the drug only, showed a mild ameliorative effect on the inflammation (p < 0.05) compared to group II (PE group), while group IV (DKP Nano group), which received the optimal ZN formulation (F7), demonstrated a markedly greater reduction in inflammation (p < 0.001), with no significant difference from the control group.
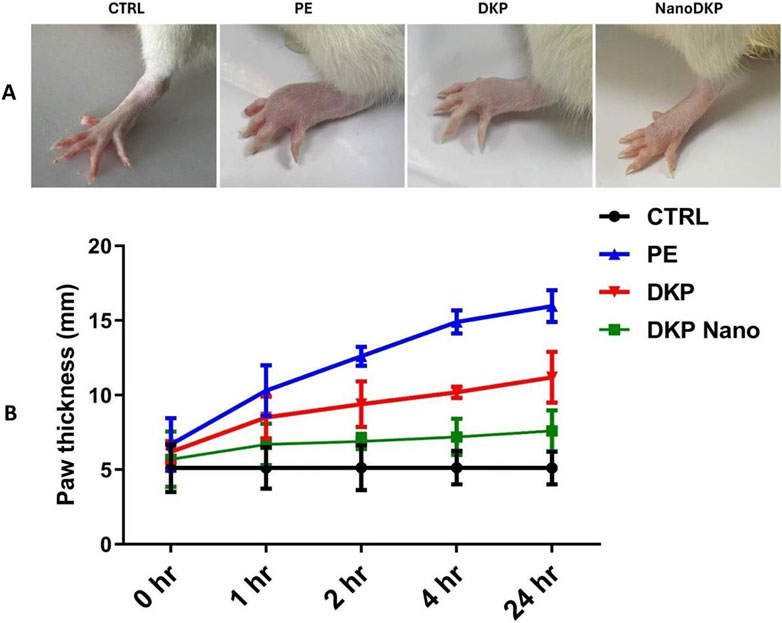
Figure 5. Effect of the optimal ZN formulation (F7) on formalin-induced paw edema in rats. (A) Representative images show foot swelling in each group 24 h after edema induction. (B) Change in paw thickness (mm) in the experimental rats. Edema was induced by injecting 0.1 mL of a 5% formalin solution into the sub-plantar surface of the left-hind paw. The mean values ±SD for six rats per group (n = 6) are shown. Groups are CTRL (control group), PE (paw edema group), DKP (dexketoprofen group), and NanoDKP: (the optimal dexketoprofen ZN formulation group) (significant at p < 0.05).
TNF-α, IL-6, PGE2, and COX-2 are pivotal mediators in inflammatory paw edema, each contributing to distinct mechanistic pathways. TNF-α drives edema formation by enhancing vascular permeability and neutrophil migration via p55 receptor activation, as demonstrated in carrageenan-induced edema models where TNF-α neutralization or receptor knockout significantly reduced edema and mechanical hypersensitivity (Rocha et al., 2006; Mansouri et al., 2015). IL-6 exhibits systemic pro-inflammatory effects, with serum levels directly correlating with paw swelling kinetics in adjuvant arthritis, while pharmacological inhibition of IL-6 pathways ameliorates both edema and cytokine activity (Leisten et al., 1990; Karim et al., 2019). PGE2, synthesized through COX-2 upregulation, mediates vasodilation and nociception via EP3 receptor-dependent signaling cascades involving phospholipase C and protein kinase C (Claudino et al., 2006; Damas and Liégeois, 1999). The critical role of COX-2 in sustaining PGE2 production is highlighted by its selective inhibition, which rapidly normalizes prostaglandin levels and resolves edema (Karim et al., 2019; Anderson et al., 1996). These mediators collectively represent interconnected facets of inflammation—TNF-α and IL-6 modulate immune cell recruitment and cytokine amplification, while the COX-2/PGE2 axis regulates vascular dynamics and pain sensitization. Targeting these pathways through dexketoprofen-loaded zein nanoparticles likely achieves synergistic anti-inflammatory effects by suppressing COX-2-mediated prostaglandin synthesis (Jori et al., 2024) and attenuating cytokine-driven neutrophil infiltration (Mansouri et al., 2015), thereby disrupting the inflammatory cascade at multiple nodes. As illustrated in Figure 6, the levels of inflammatory cytokines IL-6, TNF-α, PGE2, and COX2 were assessed using the ELISA technique to verify the anti-inflammatory effect of dexketoprofen. Twenty-four hours after formalin injection, there was a significant increase in IL-6 [F (3, 20) = 631.9, p < 0.0001], TNF-α [F (3, 20) = 84.61, p < 0.0001], PGE2 [F (3, 20) = 312.8, p < 0.0001], and COX2 [F (3, 20) = 216.1, p < 0.0001] by 8.7-, 5.4-, 13-, and 4.3-fold, respectively, compared to the control group. The prior administration of dexketoprofen significantly mitigated the formalin-induced elevation in these inflammatory markers by 31%, 34%, 27.5%, and 32%, respectively, compared to the formalin group. Notably, F7 exhibited a marked anti-inflammatory effect, restoring the inflammatory markers to near-normal levels by decreasing IL-6, TNF-α, PGE2, and COX2 levels by 76%, 55%, 79%, and 53%, respectively, compared to the DKP group.
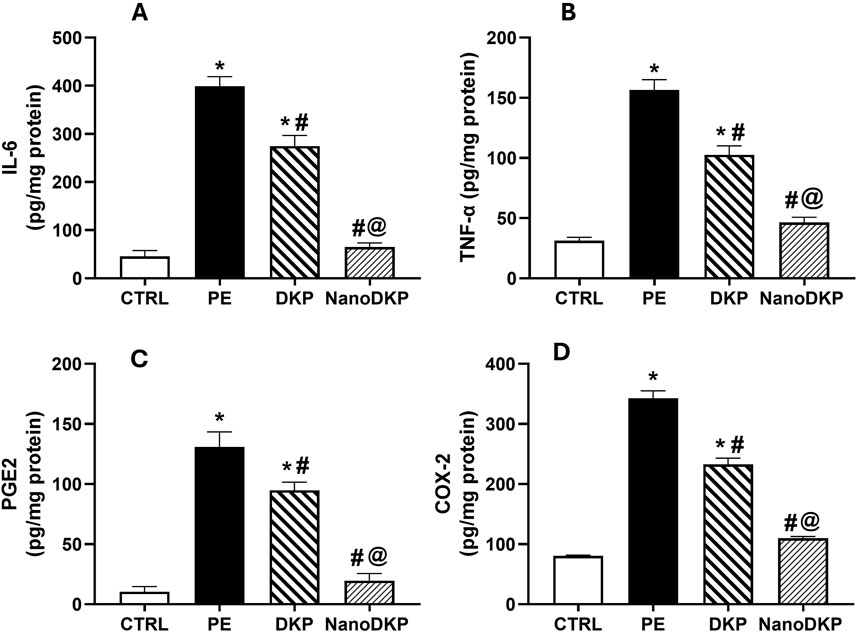
Figure 6. Effect of F7 on the level of inflammatory cytokines in rats with foot swelling. (A) IL-6, (B) TNF-α, (C) PGE2, and (D) COX2. Data are represented as the mean ± SD; *, #, and @ p < 0.05 compared to CRTL, PE, and DKP groups, respectively, using one-way ANOVA, followed by Tukey’s multiple comparisons test. CTRL, control group; PE, paw edema group; DKP, dexketoprofen group; and NanoDKP, the optimal dexketoprofen ZN formulation group.
MDA and GSH are critical biomarkers of oxidative stress-mediated inflammation in paw edema, offering complementary insights into redox balance and tissue damage. MDA, a terminal product of lipid peroxidation, directly reflects ROS-induced membrane damage during inflammation. Its accumulation triggers pro-inflammatory signaling by forming cytotoxic adducts with macromolecules, activating NF-κB-driven pathways that amplify cytokine production (e.g., TNF-α and IL-6) and neutrophil infiltration, exacerbating edema (Zhang et al., 2020). For instance, in carrageenan-induced edema, elevated MDA correlates with increased vascular leakage and hyperalgesia, while antioxidant therapies that reduce MDA concurrently suppress inflammation (Zhang et al., 2020). Conversely, GSH, the primary intracellular antioxidant, counteracts oxidative stress by scavenging ROS and maintaining cellular redox homeostasis. Its depletion disrupts PTEN/PI3K/AKT signaling, leading to unchecked ROS production and heightened pro-inflammatory cytokine release (e.g., TNF-α and IL-1β), which perpetuates edema and tissue injury (Albarakati, 2022). Studies on adjuvant-induced arthritis show that GSH replenishment attenuates edema by restoring redox balance and suppressing NF-κB activation. The findings of our study align with these previous reports, showing that the administration of formalin significantly elevated oxidative stress damage, as indicated by a 3.2-fold increase in MDA levels [F (3, 20) = 69.61, p < 0.0001] and a 64% reduction in GSH levels [F (3, 20) = 38.59, p < 0.0001] compared to the control group (Figure 7). However, pre-treatment with dexketoprofen effectively mitigated these detrimental effects, resulting in a 32% reduction in MDA levels (p < 0.0107) and a 1.7-fold increase in GSH levels (p < 0.0001) relative to the formalin group. Notably, treatment with the F7 (NanoDKP) formulation further enhanced the antioxidant response, producing a 36% reduction in MDA levels (p = 0.0006) and a 49% increase in GSH levels (p = 0.0028) compared to the DKP group. These values were statistically indistinguishable from those of the control group (p > 0.05), indicating that NanoDKP normalized oxidative stress markers to near-baseline levels.
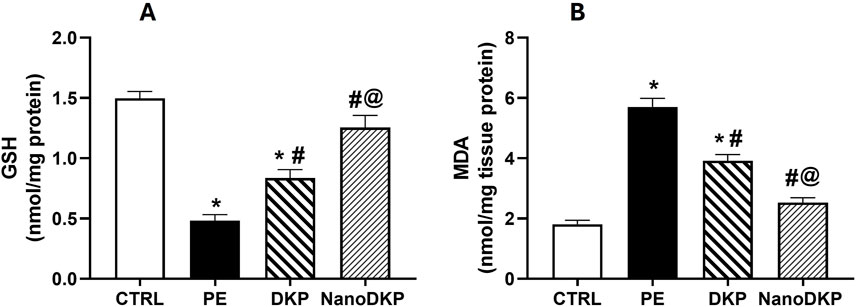
Figure 7. Effect of F7 on the level of oxidative stress biomarkers in rats with foot swelling. (A) GSH and (B) MDA. Data are represented as the mean ± SD; *, #, and @ p < 0.05 compared to control, PE, and DKP groups, respectively, using one-way ANOVA, followed by Tukey’s multiple comparisons test. CTRL, control group; PE, paw edema group; DKP, dexketoprofen group; and NanoDKP, the optimal dexketoprofen ZN formulation group.
MPO is a critical mediator of neutrophil-driven inflammation in paw edema, serving as both a biomarker of neutrophil infiltration and a direct contributor to oxidative tissue damage. Released by activated neutrophils, MPO catalyzes the production of hypochlorous acid (HOCl) and other reactive oxygen species (ROS), exacerbating oxidative stress and lipid peroxidation in inflamed tissues (Soyocak et al., 2019). Elevated MPO activity correlates strongly with the severity of inflammation, as demonstrated in carrageenan-induced paw edema models where MPO levels surge alongside neutrophil recruitment and edema formation (Soyocak et al., 2019). Pharmacological interventions, such as tannic acid and memantine, attenuate paw swelling by suppressing MPO activity, thereby reducing ROS generation and subsequent lipid peroxidation (e.g., MDA levels) (Soyocak et al., 2019; Azarbaijani et al., 2021). MPO’s role extends beyond oxidative damage; it amplifies pro-inflammatory signaling by promoting cytokine release and endothelial activation, creating a feedback loop that sustains neutrophil influx (Puttaswamy et al., 2018; Kothari et al., 2011). In formalin-induced edema, MPO inhibition coincides with diminished vascular permeability and pain sensitization, underscoring its dual role in inflammation and tissue injury (Soyocak et al., 2019). In accordance, the MPO activity in paw tissue was elevated by 3.7-fold 24 h after formalin injection compared to the control group [F (3, 20) = 91.58, p < 0.0001]. As illustrated in Figure 8, pre-treatment with dexketoprofen significantly reduced the increase in MPO activity in formalin-treated paw tissue by 32% (p < 0.0001). Notably, treatment with dexketoprofen ZNs resulted in a significant 41% reduction in MPO activity (p < 0.0001) compared to the DKP group, nearly restoring levels to those observed in the control group.
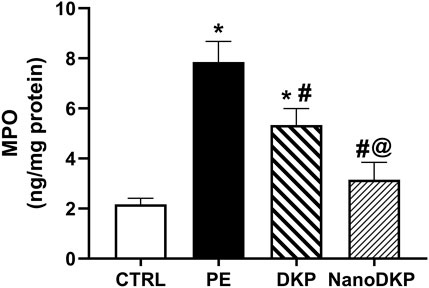
Figure 8. Effect of F7 on the activity of MPO biomarkers in rats with foot swelling. Data are represented as the mean ± SD; *, #, and @ p < 0.05 compared to control, PE, and DKP groups, respectively, using one-way ANOVA, followed by Tukey’s multiple comparisons test. CTRL, control group; PE, paw edema group; DKP, dexketoprofen group; and NanoDKP, the optimal dexketoprofen ZN formulation group.
Histopathological examination
H&E-stained skin tissues from all groups were examined using light microscopy with different magnification powers. Tissues obtained from the control group (group I) revealed the normal structure of the stratified squamous keratinized epithelium of the epidermis and normal histological architecture of the dense irregular connective tissue of the dermis and dermal glands, in addition to the normal arrangement of the striated muscle fibers under the skin layers. On the other hand, group II exhibited different inflammatory changes in the skin tissue in the form of disintegration of collagen fibers of the dermis by edema, congestion of dermal blood vessels that appeared engorged with blood and edematous fluid, and vacuolation of tunica media. Moreover, keratinocytes of the epidermal layer, particularly cells of the stratum spinosum, revealed ballooning degeneration and acute cellular swelling. The striated muscle appeared deteriorated and was invaded by inflammatory cells. The previously mentioned changes were also observed in group III, which showed inflammatory changes in the dermis in the form of deteriorated collagen fibers separated by edematous fluid, along with congested blood vessels exhibiting edema and engorgement with blood. Moreover, hemorrhage and inflammatory cell infiltration of the dermal tissue were also detected. Ballooning degeneration of stratum spinosum cells that appeared swollen and pale was clearly revealed. Interestingly, the group treated with F7 (group IV) exhibited an improvement in the destructed tissue with mild inflammatory changes that exhibited mild vacuolation of the tunica media of dermal blood vessels, with normal stratified squamous keratinized epithelium of the epidermis and other structures of the dermis. Furthermore, the striated muscle of group IV showed the regular arrangement of normal striated muscle bundles surrounded by connective tissue fibers of the perimysium (Figure 9).
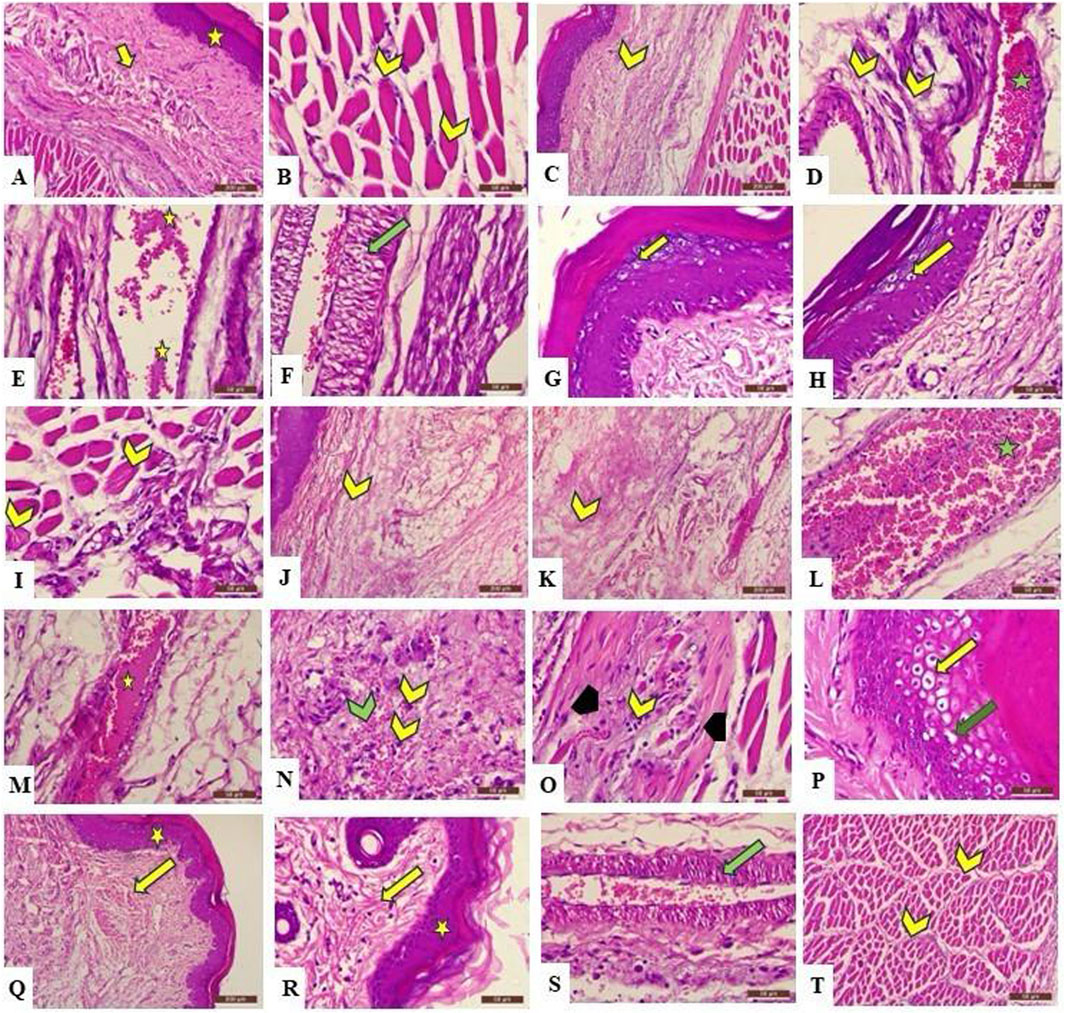
Figure 9. Photomicrograph of the H&E-stained section of rat’s skin showing A (X100) and B (X400): the control group reveals normal histological architecture of the epidermis (A) (star) and dermis (A) (arrow), which exhibits a normal arrangement of collagen fibers, in addition to normal striated muscle fibers under the skin (B) (chevron) that show peripheral nuclei in cross section. (C) (X100) and (D–I) (X400): Group 2 exhibits deterioration of the tissue in the form of dispersion of the collagen fibers in the dermis because of dermal edema (C,D) (yellow chevron). Moreover, blood vessels appear congested and engorged with blood (D) (green star), and edematous fluid is present (E) (yellow star), with vacuolation of the tunica media (F) (green arrow). In addition, ballooning degeneration and acute cellular swelling of keratinocytes are also observed (G,H) (yellow arrow). The same group also reveals accumulation of inflammatory cells between muscle fibers, which have lost their normal arrangement (I) (yellow chevron). (J,K) (X100) and (M–Q) (X400): Group 3 shows the disintegration of collagen fibers by edematous fluid (J,K) (yellow chevron), blood vessel congestion (L) (green star), edema (M) (yellow star), hemorrhage (N) (green chevron), and infiltration of inflammatory cells (N) (yellow chevron) in the dermis, in addition to the deterioration of the striated muscle fibers (O) (black arrowhead), which appear invaded by inflammatory cells (O) (yellow chevron). Moreover, ballooning degeneration (P) (yellow arrow) and acute cellular swelling (P) (green arrow) of the stratum spongiosum are observed. (Q) (X100) and (R–T) (X400): Group 4 exhibits normal microscopical structure of the epidermis (Q,R) (yellow star) and the dermis (Q,R) (yellow arrow), indicating no inflammatory changes, with less vacuolation of the tunica intima of blood vessel (S); (green arrow) and normal arrangement of striated muscle bundles surrounded by perimysium (T) (yellow chevron).
Limitation
This study used a prophylactic model, administering the NanoDKP formulation prior to formalin-induced edema, to assess its ability to modulate early inflammatory mediators (e.g., prostaglandins and histamine) and establish proof-of-concept efficacy. Although this design aligns with preclinical studies evaluating preventive strategies for anticipated inflammation (e.g., post-surgical settings), we acknowledge that therapeutic models (post-induction administration) are needed to fully extrapolate findings to clinical scenarios involving established inflammation. Future studies will explore these therapeutic contexts.
Conclusion
This research involved the preparation of ZNs as carriers for dexketoprofen for the treatment of inflammation. Dexketoprofen-loaded ZNs were produced by applying the ethanol injection process. A 23 full-factorial design was used to assess the results and conclude the optimal ZN formulation. The in silico study of the optimal formulation highlighted the strong binding affinity of zein for dexketoprofen. The optimal formulation had spherical morphology, high EE%, and small PS. The in vivo investigation of the optimal dexketoprofen-loaded ZNs has shown a more pronounced anti-inflammatory activity against formalin-induced paw edema than the drug, along with normal histological results. The optimal ZN formulation also exhibited a significant reduction in TNF-α, COX-2, IL-6, PGE2, and MDA levels while significantly increasing GSH levels compared to dexketoprofen. Concisely, the overall conclusion is that ZNs are a promising, effective, and convenient system for the topical delivery of dexketoprofen for the treatment of inflammation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by the Faculty of Pharmacy, Badr University, Cairo (approval number, IACUC/PHA/162/A/2024). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MhE: conceptualization, resources, visualization, and writing – original draft. MI: conceptualization, data curation, and writing – original draft. ShA: investigation, methodology, and writing – original draft. AM: investigation, methodology, and writing – original draft. ZO: formal analysis, methodology, and writing – original draft. SM: formal analysis, methodology, and writing – original draft. MA: investigation, visualization, and writing – review and editing. EK: funding acquisition, investigation, validation, and writing – review and editing. MaE: data curation, formal analysis, visualization, and writing – review and editing. AE: formal analysis, methodology, and writing – review and editing. SaA: formal analysis, investigation, and writing – original draft. MoE: investigation, validation, and writing – review and editing. AN: investigation, resources, visualization, and writing – original draft. WE: formal analysis, project administration, supervision, visualization, and writing – review and editing. RA: investigation, methodology, and writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through Small Research Project under grant number (RGP1/132/46).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fphar.2025.1658349.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelbari, M. A., El-Gazar, A. A., Abdelbary, A. A., Elshafeey, A. H., and Mosallam, S. (2024). Investigating the potential of novasomes in improving the trans-tympanic delivery of niflumic acid for effective treatment of acute otitis media. J. Drug Deliv. Sci. Technol. 98, 105912. doi:10.1016/j.jddst.2024.105912
Abdelbari, M. A., El-mancy, S. S., Elshafeey, A. H., and Abdelbary, A. A. (2021). Implementing spanlastics for improving the ocular delivery of clotrimazole: in vitro characterization, ex vivo permeability, microbiological assessment and in vivo safety study. IJN 16, 6249–6261. doi:10.2147/IJN.S319348
Abdelbari, M. A., Elshafeey, A. H., Abdelbary, A. A., and Mosallam, S. (2023). Implementing nanovesicles for boosting the skin permeation of non-steroidal anti-inflammatory drugs. AAPS PharmSciTech 24, 195. doi:10.1208/s12249-023-02649-x
Abdelbary, A. A., Abd-Elsalam, W. H., and Al-mahallawi, A. M. (2016). Fabrication of novel ultradeformable bilosomes for enhanced ocular delivery of terconazole: in vitro characterization, ex vivo permeation and in vivo safety assessment. Int. J. Pharm. 513, 688–696. doi:10.1016/j.ijpharm.2016.10.006
Abdel Mageed, S. S., Ammar, R. M., Nassar, N. N., Moawad, H., and Kamel, A. S. (2022). Role of PI3K/Akt axis in mitigating hippocampal ischemia-reperfusion injury via CB1 receptor stimulation by paracetamol and FAAH inhibitor in rat. Neuropharmacol. 207:108935. doi:10.1016/j.neuropharm.2021.108935
Adel, A. M., El-Gazar, A. A., Ahmed, A. A., Hassen Elshafeey, A., and Mosallam, S. (2023). Brij® integrated bilosomes for improving the transdermal delivery of niflumic acid for effective treatment of osteoarthritis: in vitro characterization, ex vivo permeability assessment, and in vivo study. Int. J. Pharm. 640, 123024. doi:10.1016/j.ijpharm.2023.123024
Ahmed, A. U. (2011). An overview of inflammation: mechanism and consequences. Front. Biol. 6, 274. doi:10.1007/s11515-011-1123-9
Albarakati, A. J. A. (2022). Protocatechuic acid counteracts oxidative stress and inflammation in carrageenan-induced paw edema in mice. Environ. Sci. Pollut. Res. Int. 29, 56393–56402. doi:10.1007/s11356-022-19688-9
Albash, R., Abdelbari, M. A., Elbesh, R. M., Khaleel, E. F., Badi, R. M., Eldehna, W. M., et al. (2024). Sonophoresis mediated diffusion of caffeine loaded Transcutol® enriched cerosomes for topical management of cellulite. Eur. J. Pharm. Sci. 201, 106875. doi:10.1016/j.ejps.2024.106875
Albash, R., Abdellatif, M. M., Hassan, M., and M Badawi, N. (2021). Tailoring terpesomes and leciplex for the effective ocular conveyance of moxifloxacin hydrochloride (comparative assessment): in-vitro, ex-vivo, and in-vivo evaluation. IJN 16, 5247–5263. doi:10.2147/ijn.s316326
Albash, R., Fahmy, A. M., Shamsel-Din, H. A., Ibrahim, A. B., Bogari, H. A., Malatani, R. T., et al. (2025). Intranasal propranolol hydrochloride-loaded PLGA-lipid hybrid nanoparticles for brain targeting: optimization and biodistribution study by radiobiological evaluation. Eur. J. Pharm. Sci. 208, 107061. doi:10.1016/j.ejps.2025.107061
Ali, A., Madni, A., Shah, H., Jamshaid, T., Jan, N., Khan, S., et al. (2023). “Solid lipid-based nanoparticulate system for sustained release and enhanced in-vitro cytotoxic effect of 5-fluorouracil on skin Melanoma and squamous cell carcinoma,”PLoS ONE. Editor K. Raza, 18. doi:10.1371/journal.pone.0281004
Al-mahallawi, A. M., Abdelbary, A. A., and Aburahma, M. H. (2015). Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int. J. Pharm. 485, 329–340. doi:10.1016/j.ijpharm.2015.03.033
Anderson, G. D., Hauser, S. D., McGarity, K. L., Bremer, M. E., Isakson, P. C., and Gregory, S. A. (1996). Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J. Clin. investigation 97, 2672–2679. doi:10.1172/jci118717
Azarbaijani, M., Kian, M., and Soraya, H. (2021). Anti-inflammatory effects of memantine in carrageenan-induced paw edema model in rats. J. Rep. Pharm. Sci. 10, 60–65. doi:10.4103/jrptps.JRPTPS_37_20
Bancroft, J. D., and Gamble, M. (2008). Theory and practice of histological techniques. Elsevier publisher: Netherlands. Available online at: https://books.google.com.eg/books?id=Dhn2KispfdQC.
Carpenter, J., Dubey, D. D., Bhargava, D. T., Dashora, D. K., Khirwadkar, P., and Mandoria, D. N. (2024). Development and evaluation of emulgel formulation of dexketoprofen trometamol.
Chenthamara, D., Subramaniam, S., Ramakrishnan, S. G., Krishnaswamy, S., Essa, M. M., Lin, F.-H., et al. (2019). Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 23, 20. doi:10.1186/s40824-019-0166-x
Claudino, R. F., Kassuya, C. A., Ferreira, J., and Calixto, J. B. (2006). Pharmacological and molecular characterization of the mechanisms involved in prostaglandin E2-induced mouse paw edema. J. Pharmacol. Exp. Ther. 318, 611–618. doi:10.1124/jpet.106.102806
Damas, J., and Liégeois, J. F. (1999). The inflammatory reaction induced by formalin in the rat paw. Naunyn-Schmiedeberg's archives Pharmacol. 359, 220–227. doi:10.1007/pl00005345
De Marco, I. (2022). Zein microparticles and nanoparticles as drug delivery systems. Polymers 14, 2172. doi:10.3390/polym14112172
Elmahboub, Y., Albash, R., Magdy, W. M., Rayan, A. H., Hamed, N. O., Ousman, M. S., et al. (2024). Metformin loaded zein polymeric nanoparticles to augment antitumor activity against ehrlich carcinoma via activation of AMPK pathway: D-optimal design optimization, in vitro characterization, and in vivo study. Molecules 29, 1614. doi:10.3390/molecules29071614
Franco, P., and De Marco, I. (2020). Supercritical antisolvent coprecipitation in the pharmaceutical field: different polymeric carriers for different drug releases. Can. J. Chem. Eng. 98, 1935–1943. doi:10.1002/cjce.23759
Gaskell, H., Derry, S., Wiffen, P. J., and Moore, R. A. (2017). Single dose oral ketoprofen or dexketoprofen for acute postoperative pain in adults. Cochrane Database Syst. Rev. 5. doi:10.1002/14651858.CD007355.pub3
Hassab, M. A. E., Fares, M., Amin, M. K. A.-H., Al-Rashood, S. T., Alharbi, A., Eskandrani, R. O., et al. (2021). Toward the identification of potential α-ketoamide covalent inhibitors for SARS-CoV-2 main protease: fragment-based drug design and MM-PBSA calculations. Processes 9, 1004. doi:10.3390/pr9061004
Hong, S., Choi, D. W., Kim, H. N., Park, C. G., Lee, W., and Park, H. H. (2020). Protein-based nanoparticles as drug delivery systems. Pharmaceutics 12, 604. doi:10.3390/pharmaceutics12070604
Jori, C., Ansari, M. M., Ahmad, A., Raza, S. S., and Khan, R. (2024). Biomaterial-based combinatorial approach of aescin-comprised zein-coated gelatin nanoparticles alleviates synovial inflammation in experimental inflammatory arthritis. Nanoscale 16, 7965–7975. doi:10.1039/D3NR06476J
Karim, N., Khan, I., Khan, W., Khan, I., Khan, A., Halim, S. A., et al. (2019). Anti-nociceptive and anti-inflammatory activities of asparacosin A involve selective cyclooxygenase 2 and inflammatory cytokines inhibition: an in-vitro, in-vivo, and in-silico approach. Front. Immunol., 10, 581–2019. doi:10.3389/fimmu.2019.00581
Kianfar, E. (2021). Protein nanoparticles in drug delivery: animal protein, plant proteins and protein cages, albumin nanoparticles. J. Nanobiotechnol 19, 159. doi:10.1186/s12951-021-00896-3
Kothari, N., Keshari, R. S., Bogra, J., Kohli, M., Abbas, H., Malik, A., et al. (2011). Increased myeloperoxidase enzyme activity in plasma is an indicator of inflammation and onset of sepsis. J. Crit. care 26, 435.e1–435.e4357. doi:10.1016/j.jcrc.2010.09.001
Kuczyńska, J., Pawlak, A., and Nieradko-Iwanicka, B. (2022). The comparison of dexketoprofen and other painkilling medications (review from 2018 to 2021). Biomed. and Pharmacother. 149, 112819. doi:10.1016/j.biopha.2022.112819
Lee, I. O., and Jeong, Y. S. (2002). Effects of different concentrations of formalin on paw edema and pain behaviors in rats. J. Korean Med. Sci. 17, 81–85. doi:10.3346/jkms.2002.17.1.81
Leisten, J. C., Gaarde, W. A., and Scholz, W. (1990). Interleukin-6 serum levels correlate with footpad swelling in adjuvant-induced arthritic Lewis rats treated with cyclosporin A or indomethacin. Clin. Immunol. Immunopathol. 56, 108–115. doi:10.1016/0090-1229(90)90174-o
Liu, G., An, D., Li, J., and Deng, S. (2023). Zein-based nanoparticles: preparation, characterization, and pharmaceutical application. Front. Pharmacol. 14, 1120251. doi:10.3389/fphar.2023.1120251
Liu, J., Zhang, Y., Liu, W., Gao, B., and Yu, L. (2021). A novel zein-based composite nanoparticles for improving bioaccessibility and anti-inflammatory activity of resveratrol. Foods 10, 2773. doi:10.3390/foods10112773
Mansouri, M. T., Hemmati, A. A., Naghizadeh, B., Mard, S. A., Rezaie, A., and Ghorbanzadeh, B. (2015). A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian J. Pharmacol. 47, 292–298. doi:10.4103/0253-7613.157127
Mitchell, M. J., Billingsley, M. M., Haley, R. M., Wechsler, M. E., Peppas, N. A., and Langer, R. (2021). Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124. doi:10.1038/s41573-020-0090-8
Moore, R. A., and Barden, J. (2008). Systematic review of dexketoprofen in acute and chronic pain. BMC Clin. Pharmacol. 8, 11. doi:10.1186/1472-6904-8-11
Mosallam, S., Ragaie, M. H., Moftah, N. H., Elshafeey, A. H., and Abdelbary, A. A. (2021a). Use of novasomes as a vesicular carrier for improving the topical delivery of terconazole: in vitro characterization, in vivo assessment and exploratory clinical experimentation. IJN 16, 119–132. doi:10.2147/IJN.S287383
Mosallam, S., Sheta, N. M., Elshafeey, A. H., and Abdelbary, A. A. (2021b). Fabrication of highly deformable bilosomes for enhancing the topical delivery of terconazole: in vitro characterization, microbiological evaluation, and in vivo skin deposition study. AAPS PharmSciTech 22, 74. doi:10.1208/s12249-021-01924-z
Mostafa, M. M., Amin, M. M., Zakaria, M. Y., Hussein, M. A., Shamaa, M. M., and Abd El-Halim, S. M. (2023). Chitosan surface-modified PLGA nanoparticles loaded with cranberry powder extract as a potential oral delivery platform for targeting colon cancer cells. Pharmaceutics 15, 606. doi:10.3390/pharmaceutics15020606
Penalva, R., González-Navarro, C. J., Gamazo, C., Esparza, I., and Irache, J. M. (2017). Zein nanoparticles for oral delivery of quercetin: pharmacokinetic studies and preventive anti-inflammatory effects in a mouse model of endotoxemia. Nanomedicine Nanotechnol. Biol. Med. 13, 103–110. doi:10.1016/j.nano.2016.08.033
Puttaswamy, N., Malojiao, V. H., Mohammed, Y. H. E., Sherapura, A., Prabhakar, B. T., and Khanum, S. A. (2018). Synthesis and amelioration of inflammatory paw edema by novel benzophenone appended oxadiazole derivatives by exhibiting cyclooxygenase-2 antagonist activity. Biomed. and Pharmacother. 103, 1446–1455. doi:10.1016/j.biopha.2018.04.167
Radwan, S. A. A., El-Maadawy, W. H., Yousry, C., ElMeshad, A. N., and Shoukri, R. A. (2020). Zein/phospholipid composite nanoparticles for successful delivery of gallic acid into aHSCs: influence of size, surface charge, and vitamin A coupling. IJN 15, 7995–8018. doi:10.2147/IJN.S270242
Rania, M., Hathout, A., Hathout, R. M., and Ahmed, H. E. (2024). Development and characterization of colloidal soft nano-carriers for transdermal delivery and bioavailability enhancement of an angiotensin II receptor blocker. doi:10.1016/j.ejpb.2012.07.002
Reddy, N., and Rapisarda, M. (2021). Properties and applications of nanoparticles from plant proteins. Materials 14, 3607. doi:10.3390/ma14133607
Roberts, M., Mohammed, Y., Pastore, M., Namjoshi, S., Yousef, S., Alinaghi, A., et al. (2017). Topical and cutaneous delivery using nanosystems. J. Control. Release 247, 86–105. doi:10.1016/j.jconrel.2016.12.022
Rocha, A. C., Fernandes, E. S., Quintão, N. L., Campos, M. M., and Calixto, J. B. (2006). Relevance of tumour necrosis factor-alpha for the inflammatory and nociceptive responses evoked by carrageenan in the mouse paw. Br. J. Pharmacol. 148, 688–695. doi:10.1038/sj.bjp.0706775
Salama, A., El-Hashemy, H. A., and Darwish, A. B. (2022). Formulation and optimization of lornoxicam-loaded bilosomes using 23 full factorial design for the management of osteoarthritis in rats: modulation of MAPK/Erk1 signaling pathway. J. Drug Deliv. Sci. Technol. 69, 103175. doi:10.1016/j.jddst.2022.103175
Sankhyan, A., and Pawar, P. K. (2013). Metformin loaded non-ionic surfactant vesicles: optimization of formulation, effect of process variables and characterization. DARU J. Pharm. Sci. 21, 7. doi:10.1186/2008-2231-21-7
Soliman, S. M., Teaima, M. H., Rashwan, K. O., Ali, B. M., Jasti, B. R., El-Nabarawi, M. A., et al. (2023). The deleterious effect of xylene-induced ear edema in rats: protective role of dexketoprofen trometamol transdermal invasomes via inhibiting the oxidative stress/NF-κB/COX-2 pathway. Int. J. Pharm. 631, 122525. doi:10.1016/j.ijpharm.2022.122525
Soliman S, M., Mosallam, S., Mamdouh, M. A., Hussein, M. A., and Abd El-Halim S, M. (2022). Design and optimization of cranberry extract loaded bile salt augmented liposomes for targeting of MCP-1/STAT3/VEGF signaling pathway in DMN-intoxicated liver in rats. Drug Deliv. 29, 427–439. doi:10.1080/10717544.2022.2032875
Soyocak, A., Kurt, H., Cosan, D. T., Saydam, F., Calis, I., Kolac, U., et al. (2019). Tannic acid exhibits anti-inflammatory effects on formalin-induced paw edema model of inflammation in rats. Hum. Exp. Toxicol. 38, 1296–1301. doi:10.1177/0960327119864154
Tefas, L. R., Sylvester, B., Tomuta, I., Sesarman, A., Licarete, E., Banciu, M., et al. (2017). Development of antiproliferative long-circulating liposomes co-encapsulating doxorubicin and curcumin, through the use of a quality-by-design approach. DDDT 11, 1605–1621. doi:10.2147/DDDT.S129008
Trayes, K. P., Studdiford, J. S., Pickle, S., and Tully, A. S. (2013). Edema: diagnosis and management, Am. Fam. Physician, 88, 102, 110.
Wang, C., Cui, B., Guo, L., Wang, A., Zhao, X., Wang, Y., et al. (2019). Fabrication and evaluation of lambda-cyhalothrin nanosuspension by one-step melt emulsification technique. Nanomaterials 9, 145. doi:10.3390/nano9020145
Were, L. M., Bruce, B. D., Davidson, P. M., and Weiss, J. (2003). Size, stability, and entrapment efficiency of phospholipid nanocapsules containing polypeptide antimicrobials. J. Agric. Food Chem. 51, 8073–8079. doi:10.1021/jf0348368
Wu, Y., Du, J., Zhang, J., Li, Y., and Gao, Z. (2023). pH effect on the structure, rheology, and electrospinning of maize zein. Foods 12, 1395. doi:10.3390/foods12071395
Yetisgin, A. A., Cetinel, S., Zuvin, M., Kosar, A., and Kutlu, O. (2020). Therapeutic nanoparticles and their targeted delivery applications. Molecules 25, 2193. doi:10.3390/molecules25092193
Zhang, H., Shang, C., Tian, Z., Amin, H. K., Kassab, R. B., Abdel Moneim, A. E., et al. (2020). Diallyl disulfide suppresses inflammatory and oxidative machineries following carrageenan injection-induced paw edema in mice. Mediat. Inflamm. 2020, 8508906. doi:10.1155/2020/8508906
Keywords: dexketoprofen, zein nanoparticles, inflammation, formalin-induced paw edema, topical delivery, docking
Citation: El Hassab MA, Ibrahim MH, Abdel Mageed SS, Mahmoud AMA, Othman Ahmed ZS, Mosallam S, Abdelbari MA, Khaleel EF, El Hasab MA, Elsayyad A, Allam S, Eltabeeb MA, Negmeldin AT, Eldehna WM and Albash R (2025) Formulation of zein nanoparticles for augmenting the anti-inflammatory activity of dexketoprofen. Front. Pharmacol. 16:1560585. doi: 10.3389/fphar.2025.1560585
Received: 14 January 2025; Accepted: 06 June 2025;
Published: 01 July 2025; Correctded: 07 August 2025.
Edited by:
Haohao Wu, Ocean University of China, ChinaReviewed by:
Victor Martin, University of Porto, PortugalSutapa Biswas Majee, NSHM Knowledge Campus, Kolkata, India
Copyright © 2025 El Hassab, Ibrahim, Abdel Mageed, Mahmoud, Othman Ahmed, Mosallam, Abdelbari, Khaleel, El Hasab, Elsayyad, Allam, Eltabeeb, Negmeldin, Eldehna and Albash. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mahmoud A. El Hassab, bWFobW91ZDY1NTgyQHBoYXJtLnRhbnRhLmVkdS5lZw==; Ahmed T. Negmeldin, ZHIuYWhtZWR0aGFiZXRAZ211LmFjLmFl
 Mahmoud A. El Hassab
Mahmoud A. El Hassab Mona H. Ibrahim2
Mona H. Ibrahim2 Sherif S. Abdel Mageed
Sherif S. Abdel Mageed Abdulla M. A. Mahmoud
Abdulla M. A. Mahmoud Manar Adel Abdelbari
Manar Adel Abdelbari Ahmed T. Negmeldin
Ahmed T. Negmeldin Wagdy M. Eldehna
Wagdy M. Eldehna Rofida Albash
Rofida Albash