1 Introduction
Prodrug–carboxypeptidase G2 (CPG2) is a promising anticancer strategy: clinical trials confirm that it can realize a targeted therapy, with a specific advantage of lacking human CPG2 analogues; further, preclinical trials demonstrate that it is effective against chemoresistant ovarian and lung cancers (Sharma and Bagshawe, 2017; Liu et al., 2020; Liu et al., 2022; Yu and Li, 2023; Qoura et al., 2024). CPG2 cleaves the amidic bond to release the active drug from the prodrug. Therefore, mutating or modifying CPG2 to improve the safety and efficacy is a hot topic, e.g., CPG2 is linked to an antibody or a ligand to realize the antibody/ligand directed prodrug therapy, and mutating CPG2 to alter the affinity or specific activity to a specific prodrug.
Native CPG2 (Pseudomonas sp. RS-16) has 23–415 and 26–415 variants due to removal of the signal peptide. These two forms have equal specific activities (400–600 U/mg) and Km (8 μM), when using methotrexate (MTX) as the substrate. The catalytic domain contains 23–213 and 326–415 residues (Rowsell et al., 1997). Recombinant CPG2(26–415) has been clinically approved to rescue MTX intoxication; this form is therefore the preferred reference to determine the data reliability when developing next-generation CPG2.
2 Clinical info of licensed CPG2(26–415) (voraxaze)
Clinical data of voraxaze provide references for understanding the safety profile of CPG2. Voraxaze hydrolyzed MTX to decrease the toxic plasma MTX level in patients receiving high-dose MTX (>1 g/m2) such as treatments of osteosarcoma, leukemia and lymphoma, thereby rescuing MTX toxication (Bielack et al., 2024). The safety was verified in clinical trials and post-marketing experiences: no drug-related serious adverse events, and no common or very common adverse events were reported (US Center for Drug Evaluation and Research, 2012; EU Committee for Medicinal Products for Human Use, 2021).
Antidrug antibodies (ADA) were detected in 21% patients after injecting voraxaze. ADA did not impact on the safety profile, and was not a major concern because of the immunosuppressed status of cancer patients and a short treatment time (only one dose on most cases) (US Center for Drug Evaluation and Research, 2012). The findings are favorable to prodrug–CPG2 therapy, considering that immunogenicity of CPG2 is commonly considered as a defect (Sharma and Bagshawe, 2017; Yu and Li, 2023).
3 Concerns on mutated/modified CPG2 for prodrug–CPG2 therapy
Here analyses were based on mutated or modified CPG2 reported in 2000–2024. Those CPG2 molecules were developed for prodrug–CPG2 therapy, and therefore certain pharmacological and clinical concerns were discussed.
3.1 Affinity and catalytic efficacy
Specific activities of almost all novel CPG2 were not quantified, but which was a characteristic parameter of an enzyme (Supplementary Table S1). The CPG2 activity based on MTX utilizes the kinetic method, i.e., the reaction rate is linear in a short time that reflects the activity. However, the absorbance decrease within 10–60 min was used to compare activities between CPG2 in certain trials (Rashidi et al., 2018; AlQahtani et al., 2019; Al-Qahtani et al., 2019; Al-mansoori et al., 2020; Al-mansoori et al., 2021). The specific activity determines the CPG2 dose required for hydrolyzing an amount of prodrug, and can mirror the purity of CPG2 protein that impacts on the safety profile. Therefore, no specific activity is a major flaw.
CPG2 was mutated or modified to modulate the affinity. CPG2(23–415).A1extM-1;I99T, CPG2(23–415).A1extM-1;G122S or CPG2(23–415).A1extM-1;T328A had a higher affinity with Km of 63–82 μM MTX, where Km of CPG2(23–415).A1extM-1 was 172 μM (Al-Qahtani et al., 2019). Km of pegylated Xen-CPG2 or human serum albumin–linker–Xen-CPG2 (70/66 μM MTX) was higher than that of Xen-CPG2 (51 μM; Xenophilus azovorans SN213), while Km of CPG2(26–415).Q1extM-1 was 172 μM (Rashidi et al., 2018; AlQahtani et al., 2019). Km demonstrated a lower affinity of CNGRC–Xen-CPG2–CNGRC, pegylated Xen-CPG2, pegylated CNGRC–Xen-CPG2 or pegylated CNGRC–Xen-CPG2–CNGRC (287–676 μM MTX) in comparison with Xen-CPG2 (236 μM) (Supplementary Table S1) (Al-mansoori et al., 2020; Al-mansoori et al., 2021). Km of native CPG2 was >>8 μM MTX in those trials. Therefore, those data cannot be directly compared, and require particular concerns. Actual Km of those CPG2 remain unclear. A drastic difference of Km of Xen-CPG2 (4.7-fold) between trials indicates that novel CPG2 should be characterized under a standard operating procedure where the range of each character of the reference CPG2(26–415) should be preset.
Kcat is measured under full substrate saturation, reflecting the initial reaction rate (Davidia et al., 2016; Lu et al., 2017). Thus, Kcat cannot be used to predict the activation of prodrugs at therapeutic doses in vitro and in vivo. Kcat for MTX or ZD2767P was 10 or 30 s-1, respectively (Niculescu-Duvaz and Springer, 1996; Lee et al., 2023). In in vitro therapy, the catalytic efficacy of CPG2(26–415) for MTX or ZD2767P was 1 or 1/40 μmol/(min·U), respectively (Liu et al., 2020). Inconsistencies indicate that the catalytic efficacy to a specific prodrug should be specifically calibrated under therapeutic doses and conditions, with MTX as the reference. The time required for activating a definite amount of prodrug, and the yield of active drug after a definite time can be calculated:
The amount of CPG2 required to activate a definite amount of prodrug in a definite time can be assessed:
Therefore, the catalytic efficacy of CPG2 to a prodrug is the critical parameter in formulating a therapy plan. Aforementioned verdicts can be used to predict the feature of intratumoral accumulation of active drugs vs. time (i.e., intratumoral pharmacokinetics (PK)), thereby assessing the pharmacodynamic effect, particularly when all independent variables in equations are characterized in cancer tissues (Zhang et al., 2017; Chang et al., 2023).
3.2 PK property
The clinical PK property of CPG2 drastically differed from that of a prodrug (Figure 1). The half-life was 16 or 10 min for CMDA (4-[(2-chloroethyl) (2-mesyloxylethyl)amino]benzoyl-L-glutamic acid) or ZD2767P (4-[N,N-bis(2-iodoethyl)amino]phenoxycarbonyl-L-glutamic acid), and was 5 or 3 h for CPG2(26–415) or CPG2(23–415) linked to anti-carcinoembryonic antigen F(ab)2 antibody, respectively (Napier et al., 2000; Francis et al., 2002; Mayer et al., 2006; Yu and Li, 2023). Unmatched PK is a challenge: prodrugs should be administrated when the plasma CPG2 level decreases to a very low level to reduce off-target activation; a rapid elimination of prodrugs (although a short residence time favors the safety) indicates that enough amounts of prodrugs and CPG2 should be transferred into the tumor in a short time, i.e., a narrow therapeutic time window. The residence time of active drugs in cancer cells is longer that in plasma (e.g., ZD2767D (4-[N,N-bis(2-iodoethyl)amino]phenol), the active form of ZD2767P, had a half-life of ≈2 min in plasma, but the mean residence time was 14–28 min in cancer cells) (Yu et al., 2024). Therefore, more prodrugs should be activated and those active drugs should be released into cancer cells in a short time to combat the rapid elimination of prodrugs from blood and the tumor. These depend on the intratumoral total activity of CPG2 (amount × specific activity).
FIGURE 1
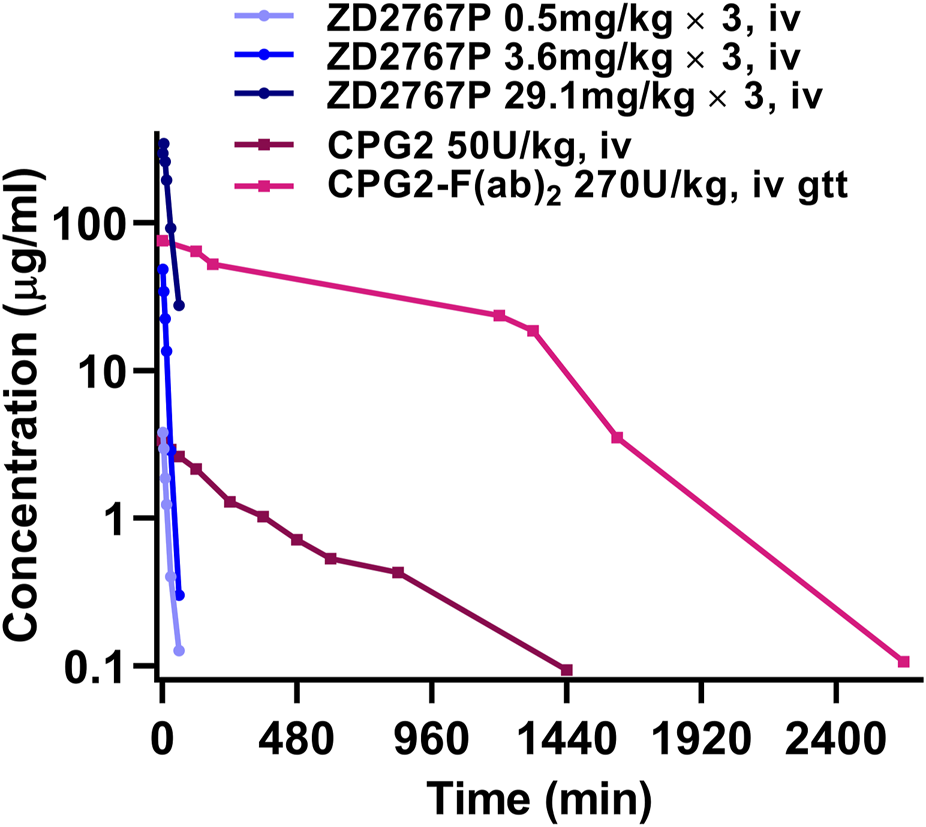
Blood drug level vs. time curves of ZD2767P and CPG2: PK property of CPG2 did not match with that of ZD2767P (data were from Napier et al., 2000; Francis et al., 2002; Mayer et al., 2006; Yu and Li, 2023).
A narrow time window indicates that a higher yield rate of active drugs is favorable, depending on a higher specific activity of CPG2 (Liu et al., 2020). Unfortunately, specific activities of certain mutated/modified CPG2 were not quantitatively calibrated using CPG2(26–415) as the reference, limiting analyses of clinical potentials (Supplementary Table S1). Linking an antibody or a peptide to CPG2 decreases the specific activity, e.g., specific activities of CPG2, and CPG2 linked to anti-carcinoembryonic antigen single chain Fv antibody or to F(ab)2 antibody are 460, 128 and 80 U/mg, respectively (Yu and Li, 2023). Therefore, a higher dose of CPG2 conjugate is required to realize an equal total activity of CPG2.
Another PK-related issue is the ratio of CPG2 in tumor to blood, which determines the therapeutic precision. Intravenously infusing CPG2 linked to anti-carcinoembryonic antigen F(ab)2 antibody led to a median value of 0.4 (0–10.4) on prodrug day, i.e., low selectivity (Francis et al., 2002). The high variance is due to heterogeneity of in vivo distribution, and will complicate the outcome due to PK incoordination between CPG2 and prodrugs.
3.3 ADA against CPG2
ADA may impact on the behavior and activity of CPG2, and therefore reducing immunogenicity is in development (e.g., pegylation or modifying immunogenic epitopes) (Sharma and Bagshawe, 2017; Yu and Li, 2023; Qoura et al., 2024). A recent clinical trial demonstrated positive serum ADA at baseline in 3/20 cases, where the PK feature of CPG2(26–415) did not differ from that in cases with negative ADA. Further, CPG2(26–415) efficiently hydrolyzed MTX to reduce the plasma MTX concentration to a safe level in 1/4 ADA-positive patient. These data suggest that ADA against CPG2 may not be a critical concern for prodrug–CPG2 therapy. The catalytic domain of carboxypeptidase is highly conserved between species, and therefore human has partial tolerance to CPG2 (US Center for Drug Evaluation and Research, 2012).
3.4 His-tag and Met in the N-terminus
Most mutated/modified CPG2 was with poly(His) and/or Met in the N-terminus (Supplementary Table S1). The extension does not affect the specific activity, but may give rise to extra safety risks. Those CPG2 hardly have chances of being approved according to the drug regulations for therapeutic biologics (i.e., clinical futureless). Therefore, preclinical data using those CPG2 have poor clinical relevancies, and preclinical and translational trials should base on tag/Met-free CPG2.
4 Conclusion
CPG2 is the pivotal determinant in prodrug–CPG2 therapy. Intravenously injecting CPG2 or a CPG2 conjugate leads to a low amount of CPG2 in cancer tissues, although the expected goal of using a CPG2 conjugate is to realize an intratumoral enrichment of CPG2. Intratumoral application may be a solution considering the complexity of in vivo distribution. The catalytic efficacy to a specific prodrug should be specifically calibrated under therapeutic doses and conditions, and translational trials should utilize tag/Met-free CPG2.
Statements
Author contributions
TY: Formal Analysis, Validation, Writing – original draft, Writing – review and editing. TY: Formal Analysis, Writing – original draft. XL: Formal Analysis, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1560834/full#supplementary-material
References
1
Al-mansoori L. Bashraheel S. S. Al Qahtani A. D. O'Connor C. D. Elsinga P. Goda S. K. (2020). In vitro studies on CNGRC-CPG2 fusion proteins for ligand directed enzyme prodrug therapy for targeted cancer therapy. Oncotarget11, 619–633. 10.18632/oncotarget.27478
2
Al-mansoori L. Al Qahtani A. D. Elsinga P. Goda S. K. (2021). Production of long-acting CNGRC-CPG2 fusion proteins: new derivatives to overcome drug immunogenicity of ligand-directed enzyme prodrug therapy for targeted cancer treatment. Technol. Cancer Res. Treat.20, 15330338211057371. 10.1177/15330338211057371
3
AlQahtani A. D. Al-mansoori L. Bashraheel S. S. Rashidi F. B. Al-Yafei A. Elsinga P. et al (2019). Production of “biobetter” glucarpidase variants to improve drug detoxification and antibody directed enzyme prodrug therapy for cancer treatment. Eur. J. Pharm. Sci.127, 79–91. 10.1016/j.ejps.2018.10.014
4
Al-Qahtani A. D. Bashraheel S. S. Rashidi F. B. O'Connor C. D. Romero A. R. Domling A. et al (2019). Production of “biobetter” variants of glucarpidase with enhanced enzyme activity. Biomed. Pharmacother.112, 108725. 10.1016/j.biopha.2019.108725
5
Bielack S. S. Soussain C. Fox C. P. Houillier C. Murciano T. Osborne W. et al (2024). A European consensus recommendation on the management of delayed methotrexate elimination: supportive measures, leucovorin rescue and glucarpidase treatment. J. Cancer Res. Clin. Oncol.150, 441. 10.1007/s00432-024-05945-6
6
Chang H. P. Le H. K. Shah D. K. (2023). Pharmacokinetics and pharmacodynamics of antibody-drug conjugates administered via subcutaneous and intratumoral routes. Pharmaceutics15, 1132. 10.3390/pharmaceutics15041132
7
Davidia D. Noorb E. Liebermeisterc W. Bar-Even A. Flamholz A. Tummler K. et al (2016). Global characterization of in vivo enzyme catalytic rates and their correspondence to in vitro Kcat measurements. Proc. Natl. Acad. Sci. U. S. A.113, 3401–3406. 10.1073/pnas.1514240113
8
EU Committee for Medicinal Products for Human Use (2021). Voraxaze: assessment report.
9
Francis R. J. Sharma S. K. Springer C. Green A. J. Hope-Stone L. D. Sena L. et al (2002). A phase I trial of antibody directed enzyme prodrug therapy (ADEPT) in patients with advanced colorectal carcinoma or other CEA producing tumours. Br. J. Cancer87, 600–607. 10.1038/sj.bjc.6600517
10
Lee J. P. Corless B. C. Gardner T. J. Scheinberg D. A. Tan D. S. (2023). Development of a p-hydroxybenzyl-alcohol-linked glutamate prodrug for activation by Pseudomonas carboxypeptidase G2. Org. Lett.25, 6295–6299. 10.1021/acs.orglett.3c02130
11
Liu Q. Zhong X. Zhang Y. Li X. Qian G. Yu T. (2020). Ultrasound enhances ZD2767P-carboxypeptidase G2 against chemoresistant ovarian cancer cells by altering the intracellular pharmacokinetics of ZD2767D. Mol. Pharm. 17, 1922–1932. 10.1021/acs.molpharmaceut.0c00008
12
Liu Q. Li X. Luo Y. Wang H. Zhang Y. Yu T. (2022). Ultrasonically enhanced ZD2767P-carboxypeptidase G2 deactivates cisplatin-resistant human lung cancer cells. Oxid. Med. Cell Longev.2022, 9191233. 10.1155/2022/9191233
13
Lu J. Dong Y. Ng E. C. Siehl D. L. (2017). Novel form of the Michaelis-Menten equation that enables accurate estimation of (kcat/KM)*KI with just two rate measurements; utility in directed evolution. Protein Eng. Des. Sel.30, 395–399. 10.1093/protein/gzx012
14
Mayer A. Francis R. J. Sharma S. K. Tolner B. Springer C. J. Martin J. et al (2006). A phase I study of single administration of antibody-directed enzyme prodrug therapy with the recombinant anti-carcinoembryonic antigen antibody-enzyme fusion protein MFECP1 and a bis-iodo phenol mustard prodrug. Clin. Cancer Res.12, 6509–6516. 10.1158/1078-0432.CCR-06-0769
15
Napier M. P. Sharma S. K. Springer C. J. Bagshawe K. D. Green A. J. Martin J. et al (2000). Antibody-directed enzyme prodrug therapy: efficacy and mechanism of action in colorectal carcinoma. Clin. Cancer Res.6, 765–772.
16
Niculescu-Duvaz I. Springer C. J. (1996). Development of prodrugs for ADEPT (antibody-directed enzyme prodrug therapy). Expert Opin. Investg. Drugs3, 289–308.
17
Qoura L. A. Morozova E. Ramaa С. S. Pokrovsky V. S. (2024). Smart nanocarriers for enzyme-activated prodrug therapy. J. Drug Target32, 1029–1051. 10.1080/1061186X.2024.2383688
18
Rashidi F. B. AlQhatani A. D. Bashraheel S. S. Shaabani S. Groves M. R. Dömling A. et al (2018). Isolation and molecular characterization of novel glucarpidases: enzymes to improve the antibody directed enzyme pro-drug therapy for cancer treatment. PLoS One13, e0196254. 10.1371/journal.pone.0196254
19
Rowsell S. Pauptit R. A. Tucker A. D. Melton R. G. Blow D. M. Brick P. (1997). Crystal structure of carboxypeptidase G2, a bacterial enzyme with applications in cancer therapy. Structure5, 337–347. 10.1016/s0969-2126(97)00191-3
20
Sharma S. K. Bagshawe K. D. (2017). Antibody directed enzyme prodrug therapy (ADEPT): trials and tribulations. Adv. Drug Deliv. Rev.118, 2–7. 10.1016/j.addr.2017.09.009
21
US Center for Drug Evaluation and Research (2012). Voraxaze: cross discipline team leader review.
22
Yu T. Li X. (2023). Development of ZD2767P-carboxypeptidase G2-ultrasound therapy against cisplatin-resistant cancer. Front. Oncol.13, 1151613. 10.3389/fonc.2023.1151613
23
Yu T. Li X. Yu T. Chen M. Sun Y. Ran R. (2024). Intracellular pharmacokinetics of activated drugs in a prodrug-enzyme-ultrasound system: evaluations on ZD2767P+CPG2+US. ACS Med. Chem. Lett.15, 739–745. 10.1021/acsmedchemlett.4c00071
24
Zhang Y. Li J. Yu T. (2017). Pharmacokinetic profiles of cancer sonochemotherapy. Expert Opin. Drug Deliv.14, 745–753. 10.1080/17425247.2016.1232248
Summary
Keywords
prodrug-carboxypeptidase G2 therapy, pharmacokinetics, enzyme kinetics, antidrug antibody, therapeutic time window
Citation
Yu T, Yu T and Li X (2025) Prodrug–carboxypeptidase G2 therapy: certain concerns on carboxypeptidase G2. Front. Pharmacol. 16:1560834. doi: 10.3389/fphar.2025.1560834
Received
15 January 2025
Accepted
15 May 2025
Published
12 June 2025
Volume
16 - 2025
Edited by
Germain Sotoing Taiwe, University of Buea, Cameroon
Reviewed by
Marco Girhard, Heinrich Heine University of Düsseldorf, Germany
Jie Yuan, Henan Normal University, China
Updates
Copyright
© 2025 Yu, Yu and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tinghe Yu, yutinghe@hotmail.com, yutinghe@cqmu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.