- 1Department of Pharmacology, Dr M.G.R. Educational and Research Institute, Chennai, Tamil Nadu, India
- 2Institute of Pharmaceutical Research, GLA University, Mathura, Uttar Pradesh, India
- 3Department of Pharmaceutical Chemistry, Dr M.G.R. Educational and Research Institute, Chennai, Tamil Nadu, India
- 4Department of pharmaceutical analysis, Dr M.G.R. Educational and Research Institute, Chennai, Tamil Nadu, India
Ischemic stroke remains a leading cause of mortality and long-term disability worldwide, despite advancements in acute intervention and rehabilitation strategies. Traditional, Complementary, and Integrative Medicine (TCIM) systems; including herbal medicine, acupuncture, and mind-body interventions are increasingly being explored as adjunct therapies in stroke management. This narrative review evaluates the current evidence supporting TCIM approaches for ischemic stroke, highlighting their potential neuroprotective, anti-inflammatory, antioxidant, and cerebrovascular effects. Particular emphasis is placed on well-studied botanical interventions such as Salvia miltiorrhiza Bunge [Lamiaceae; Salviae miltiorrhizae radix et rhizoma], Ginkgo biloba L. [Ginkgoaceae; Ginkgo folium], and Panax ginseng C.A. Mey. [Araliaceae; Ginseng radix]. The review discusses the mechanisms of action, clinical trial outcomes, and integration challenges, while underscoring the need for standardization, quality control, and rigorous scientific validation. This work aims to support informed decisions regarding the integration of evidence-based TCIM practices into conventional stroke care protocols.
1 Introduction
Ischemic stroke is a sudden neurological impairment triggered by an interruption in the blood supply to a section of the brain. At the molecular level, cerebral blood perfusion causes an acute loss of oxygen and glucose, which lowers the generation of adenosine triphosphate (ATP), causes lactic acidosis, and disrupts cellular homeostasis (Haupt et al., 2023). Ischemic stroke includes an imbalance of ions, abnormal activation of immune cells, and neuroinflammation, which can lead to neuron death (Zhu et al., 2022). It accounts for the second leading cause of death worldwide, accounting for 5.9 million deaths and 102 million disability-adjusted life years lost (Qin et al., 2022a). Several risk factors have been associated with the development of stroke, including diabetes mellitus, smoking, hyperlipidemia, and hypertension (McFarlane et al., 2005). The underlying causes of ischemic stroke primarily involve in situ small vessel disease, artery-to-artery embolism, and cardioembolic events (Chen et al., 2019). Clinically, ischemic stroke often presents with sudden-onset symptoms such as non-orthostatic vertigo, slurred speech, diplopia, sensory disturbances (numbness), and unilateral motor weakness or paralysis (Qin et al., 2022b). Interrupting the blood supply causes several pathophysiological alterations that lead to irreversible neural disruption, such as the production of oxygen free radicals and cerebral edema, reactive oxygen species, neuroinflammation, blood-brain barrier (BBB) destruction, and local inflammatory cell infiltration (Wang et al., 2021).
Herbal medicines are being explored as potential neuroprotective agents for ischemic stroke, based on their long history of use in traditional medicine. Plants naturally produce chemical compounds to protect against diseases, predators, and environmental stress. Many of these phytochemicals have anti-inflammatory, antioxidant, and anti-apoptotic properties, which may help in understanding and managing the underlying causes of ischemic stroke (Ranjan, 2023). Acute ischemic stroke can currently be effectively treated with thrombectomy and thrombolysis. However, only a small percentage of patients can benefit from these techniques due to time frame restrictions (Tong et al., 2021; Hurd et al., 2021). Neuroprotective drugs have not worked well in clinical trials because most treatment targets are discovered in animal studies, not in humans. Therefore, there is an urgent need for effective treatments that can be widely used for ischemic stroke (Zhu et al., 2021). Traditional Chinese, Malay, and Indian (Ayurvedic/Siddha/Unani) medicines, as well as homoeopathic remedies, are part of alternative medicine. Acupuncture, homoeopathy, Chinese medicine, Ayurveda, herbal therapies, mind-body practices, and physiotherapy are all included in Complementary alternative medicine (CAM) for stroke (Rajahthurai et al., 2022).
Integrative medicine was established in the 1980s. Using the complementary advantages of macro and micro, global and local, structure and function, traditional and modern, disease differentiation, and syndrome differentiation in Western medicine and Traditional Chisnese Medicine, integrative medicine pioneers have been developing new theories of medicine and pharmacology based on the tenet of “system learning, comprehensively improving mastering, and sorting” (Wang and Xiong, 2012). As integrative medicine continues to evolve, a growing number of stroke centres in China are incorporating TCM to address the increasing demands of the patient population. More standardised and holistic stroke treatment protocols are being developed nationwide. This comprehensive strategy, which combines conventional and traditional therapies, is referred to as integrative medicine rehabilitation (Saceleanu et al., 2023).
An imperative feature of TCM is acupuncture, which involves putting tiny needles into the skin or deep tissues of particular body areas (acupoints). In addition to being safe, effective, cost-effective, and easy to use, acupuncture can be administered by hand, electric stimulation, or warmth. Acupuncture has been shown in numerous clinical and experimental studies to ameliorate neurological impairments caused by ischemic stroke, particularly those related to stroke outcomes. To produce stronger proof, researchers should keep refining the design of clinical trials, expanding the sample size, standardising and quantifying acupuncture procedures, and utilising interdisciplinary approaches (Qin et al., 2022a). The aim of this review is to critically evaluate the current scientific evidence supporting the use of TCIM approaches in ischemic stroke, identify key pharmacological mechanisms and therapeutic targets, highlight limitations in existing studies, and propose directions for future research. This review also assesses the feasibility of integrating evidence-based TCIM practices into conventional stroke management frameworks.
2 Literature search strategy
A comprehensive literature search was conducted using four major databases: PubMed/MEDLINE, Scopus, Web of Science, and Google Scholar (for supplementary and grey literature). The search was limited to articles published between January 2000 and January 2025 to capture recent and relevant advances in the field of traditional, complementary, and integrative medicine (TCIM) for ischemic stroke. We used a combination of Medical Subject Headings (MeSH) and free-text terms with Boolean operators such as (“Ischemic Stroke” OR “Cerebral Infarction” OR “Brain Ischemia”) AND (“Traditional Medicine” OR “Complementary Medicine” OR “Integrative Medicine” OR “Ayurveda” OR “Herbal Medicine” OR “Chinese Medicine” OR “Acupuncture” OR “Yoga”) AND (“Treatment” OR “Therapy” OR “Intervention” OR “Management” OR “Neuroprotection”). Only peer-reviewed English-language articles were included, covering in vitro, in vivo, or human studies that evaluated TCIM interventions specifically for ischemic stroke and discussed mechanisms of action, pharmacological effects, or clinical outcomes. We excluded non-English publications, case reports, editorials, conference abstracts, non-peer-reviewed sources, studies unrelated to ischemic stroke, and those focused on hemorrhagic stroke unless ischemic models were explicitly addressed. The screening process involved an initial review of titles and abstracts by two independent reviewers, followed by full-text screening for eligible studies. Any disagreements during selection were resolved through discussion or consultation with a third reviewer.
3 Pathophysiology of ischemia stroke
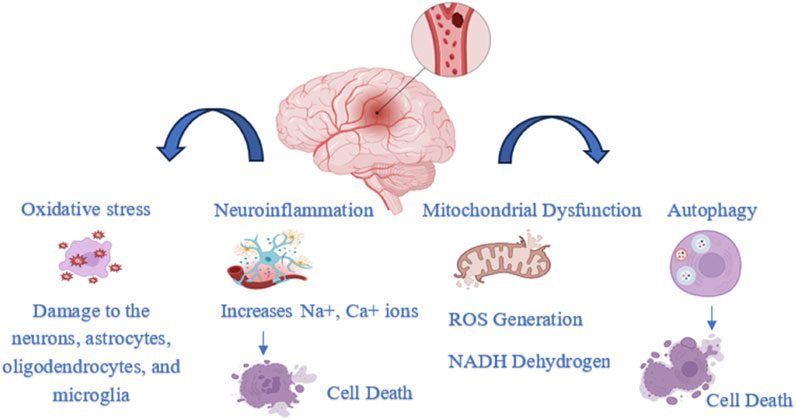
Ischemic occlusions account for approximately 85% of stroke-related deaths, while the remaining cases are due to intracerebral hemorrhage. Ischemic strokes are primarily caused by embolism or brain thrombosis. Beyond the initial vascular occlusion, several secondary pathophysiological mechanisms contribute to the progression of stroke injury. These include inflammation, energy failure, loss of cellular homeostasis, acidosis, elevated intracellular calcium levels, excitotoxicity, cytokine- and free radical-mediated cytotoxicity, complement system activation, disruption of the blood-brain barrier, activation of glial cells, oxidative stress, and leukocyte infiltration (Figure 1) (Kuriakose and Xiao, 2020).
3.1 Oxidative stress
Oxidative stress occurs when the production of reactive oxygen species (ROS) exceeds the body’s ability to neutralize and eliminate them (Juan et al., 2021). The brain contains low levels of antioxidant enzymes and cytochrome c oxidase, making it prone to superoxide generation during ATP production. Its high membrane surface-to-volume ratio and lipid-rich plasmalemma increase vulnerability to oxidative damage. Neurotransmitter metabolism leads to calcium overload and ROS formation, while iron released from damaged tissue further promotes free radical production (Jurcau and Ardelean, 2022). ROS are primarily generated as byproducts of mitochondrial electron transport and metal-catalyzed oxidation. Both internal and external factors contribute to their production. Elevated ROS levels are strongly associated with risk factors for ischemic stroke, especially in older adults. (Koutsaliaris et al., 2021). When blood flow is restored to the brain, the return of oxygen can trigger the “oxygen paradox,” leading to a surge in reactive oxygen species that further damages neurons, oligodendrocytes, microglia, and astrocytes (Granger and Kvietys, 2015). Ischemia-reperfusion injury (IRI) causes oxidative stress that leads to endothelial damage, inflammation, and disruption of the blood-brain barrier. This triggers microglial activation, lipid peroxidation, and cell death through processes like ferroptosis, pyroptosis, necroptosis, autophagy, and apoptosis. Long-term damage results from glial scar formation, poor axonal regrowth, ongoing inflammation, and reduced new blood vessel formation and remyelination (Briones-Valdivieso et al., 2024).
Reactive nitrogen species (RNS) are highly reactive molecules produced during nitrogen metabolism. They play key roles in both normal and disease processes, like regulating blood vessel function. Nitrite (NO2−), a compound made of nitrogen and oxygen, can form salts such as sodium nitrite (NaNO2) or potassium nitrite (KNO2). These nitrite salts, along with SNO-Hb, serve as reservoirs for nitric oxide (NO), which can be released to promote vasodilation and increase blood flow to distant tissues (Salvagno et al., 2024). During ischemia-reperfusion damage in the brain, an increase in both nitric oxide (NO) and superoxide (O2) leads to the rapid formation of peroxynitrite (ONOO) through their direct reaction. Peroxynitrite is more toxic than its precursors. It can nitrify protein tyrosine, forming 3-nitrotyrosine (3-NT), which is believed to carry the signature of ONOO damage (Chen et al., 2018). The generation of reactive compounds like NO2+, NO2, and OH leads to a series of redox reactions, contributing to secondary nitroxidative stress. Compared to eNOS and nNOS, iNOS produces much higher levels of NO•. This makes iNOS, often called the ‘pathological’ type of NO• synthase, capable of generating ONOO− and highly reactive hydroxyl radicals. NO• plays a critical role in the brain, affecting the neurovascular unit. NO signaling, mediated by constitutive NOS isoforms (nNOS and eNOS), is essential for regulating blood vessel dilation, neuronal excitability, and glial cell function (Wierońska et al., 2021).
3.2 Neuroinflammation
Inflammation is another critical factor in ischemic stroke. It worsens nerve tissue damage and cell death through an inflammatory cascade triggered by cerebral ischemia and subsequent reperfusion. This cascade involves oxidative stress, excitotoxicity, inflammatory cell infiltration, and the production of harmful inflammatory mediators (Mo et al., 2020). Consequently, the buildup of Na+ and Ca2+ ions leads to cell death, membrane damage, and organelle dysfunction. Impaired ATP production also reduces glutamate uptake, causing its excessive accumulation outside cells, which contributes to neuronal death in the ischemic penumbra. The overactivation of glutamate receptors further amplifies excitotoxicity and calcium overload, ultimately leading to mitochondrial failure and cell death (Pawluk et al., 2020). During ischemia, glial cells (microglia, astrocytes), blood cells (like leukocytes), and endothelial cells release various inflammatory mediators, including pro-inflammatory enzymes, cytokines, and chemokines. Alongside these cellular responses, genetic factors that regulate inflammation also significantly contribute to the progression of stroke-related inflammatory processes (Pawluk et al., 2020). During a stroke, astrocytes are rapidly activated by damage-associated molecular pattern molecules (DAMPs) released from injured neurons and glial cells. This activation contributes to blood–brain barrier (BBB) disruption and the recruitment of peripheral leukocytes, driven by proinflammatory cytokines, chemokines, and matrix metalloproteinases such as MMP-9 secreted by reactive astrocytes. These events collectively lead to secondary brain tissue damage (Xu et al., 2020). Following a haemorrhagic stroke, two types of damage occur: primary and secondary. The immune response is believed to play a major role in the secondary phase. Several immunological and inflammatory mechanisms contribute to this stage. For instance, thrombin and blood clots can activate the protease and complement systems, leading to receptor activation and cell lysis. Inflammation during secondary injury can also disrupt the BBB through matrix metalloproteinases (MMPs) and cytokines, increasing capillary permeability and worsening brain edema (Zhao et al., 2022).
3.3 Mitochondrial dysfunction
Intracellular organelles like mitochondria have a double membrane and play a crucial role in energy production, cell cycle regulation, and apoptosis. Under low oxygen conditions, the mitochondrial respiratory chain is disrupted, halting ATP synthesis. Electrons accumulate at complexes I and III because they enter complex I more rapidly than they move through complex IV. This imbalance slows the electron transport chain and proton pumping across the inner mitochondrial membrane, ultimately reducing the mitochondrial membrane potential (Jurcau and Ardelean, 2021). Mitochondrial complex I (NADH dehydrogenase), the first component of the mitochondrial respiratory chain (MRC), is strongly implicated in many neurodegenerative diseases due to its role in excessive ROS production and membrane polarization regulation. While complex II (succinate dehydrogenase) also contributes to ROS generation and apoptotic cell death, its involvement in ischemia-induced MRC dysfunction is comparatively limited. Following ischemic injury, both intrinsic and extrinsic apoptotic pathways are activated, involving mitochondrial membrane potential loss, MRC alterations, cytochrome c release, disrupted redox balance, and impaired antioxidant defenses (Reverse Electron Transport at Mitochondrial, 2024).
3.4 Autophagy
Controlled cell death is vital for maintaining host defense and tissue homeostasis. PANoptosis, a newly identified form of regulated cell death, integrates features of pyroptosis, apoptosis, and necroptosis. A key link between these pathways is the Caspase family, which plays roles in both pyroptosis and apoptosis, indicating a shared evolutionary origin. Caspases are broadly classified into inflammatory caspases, such as caspase-1, -4, -5, and -11, and apoptotic caspases, such as caspase-3, -6, -7, -8, -9, and -10, reflecting their distinct but interconnected functions (Tian et al., 2025). Pyroptosis is a form of programmed inflammatory cell death driven by caspase-1 activation, characterized by cell swelling and membrane rupture. This process leads to the release of pro-inflammatory cytokines like interleukin-1β and interleukin-18. A key mediator of pyroptosis is the Gasdermin D (GSDMD) protein, which forms pores in the cell membrane, triggering inflammation and cell death. These mechanisms are closely associated with the progression of inflammation-related conditions, including stroke, neurodegenerative diseases, and brain injury (Sun and Zhu, 2023). Apoptosis, triggered through both intrinsic and extrinsic pathways, contributes significantly to neuronal death following cerebral ischemia/reperfusion (I/R). In parallel, necroptosis, a regulated form of necrotic cell death, is primarily controlled by RIPK1, RIPK3, and MLKL. Emerging evidence suggests that necroptosis plays a crucial role in the development of ischemic stroke and related conditions. In experimental models, inhibiting necroptosis has been shown to reduce brain infarct size and improve motor and cognitive outcomes, indicating its potential neuroprotective value. Another form of programmed cell death, pyroptosis, is characterized by rapid plasma membrane rupture and the release of proinflammatory contents, further contributing to post-ischemic inflammation (Shu et al., 2022).
Autophagy is a cellular process in which cytoplasmic proteins or organelles are engulfed into vesicles that fuse with lysosomes to form autolysosomes, where the contents are subsequently degraded (Mo et al., 2020). Autophagy acts as a double-edged sword, capable of either repairing or degrading damaged neurons following an ischemic insult. However, excessive or prolonged activation of autophagy can lead to neuron damage and trigger cell death (Ajoolabady et al., 2021).
3.5 Ferroptosis
Iron is involved in several mechanisms of neuronal damage following ischemic stroke. It plays a key role in major molecular processes like free radical production, excitotoxicity, and neuroinflammation. Additionally, its accumulation can lead to ferroptosis (Guo et al., 2023). Existing research shows that ferroptosis regulation is primarily governed by the control of iron, lipid peroxidation, and several antioxidant systems, including the GSH/GPX4 axis, CoQ10/FSP1 axis, and others (Xu et al., 2023). Glutathione (Glu-Cyc-Gly), a tripeptide, helps protect cells from oxidative damage by interacting with free radicals. When glutathione (GSH) is converted to oxidized glutathione (GSSG), the antioxidant enzyme Gpx4 reduces harmful lipid peroxides to alcohols. During ferroptosis, redox-active iron accumulates through the Fenton reaction, depleting GSH reserves, inhibiting Gpx4, and triggering an excessive antioxidant response (Liu et al., 2020). Ferroptosis, a unique form of programmed cell death (PCD), is characterized by extreme iron overload and lipid peroxidation driven by ROS production. This pathway plays a crucial role in neuron death. Unlike other forms of cell death, ferroptosis is marked by the reduction or loss of mitochondrial cristae and a condensed mitochondrial membrane structure (Wang et al., 2020). Ferroptosis is characterized by distinct morphological changes in mitochondria, such as fragmentation, reduced size, rupture of the outer mitochondrial membrane (OMM), and the disappearance of mitochondrial cristae (She et al., 2023). Ferritin-rich brain tissue and iron-rich blood are the result of direct compression and stimulation of the hematoma, which weakens the blood-brain barrier and causes primary brain injury (Mo et al., 2020). The release of iron from the hematoma after intracerebral hemorrhage (ICH) can trigger perihematomal edema, oxidative stress, and increased ROS levels, ultimately leading to ferroptosis (Dong et al., 2023).
4 Traditional medicines in stroke management
4.1 Traditional Chinese medicine
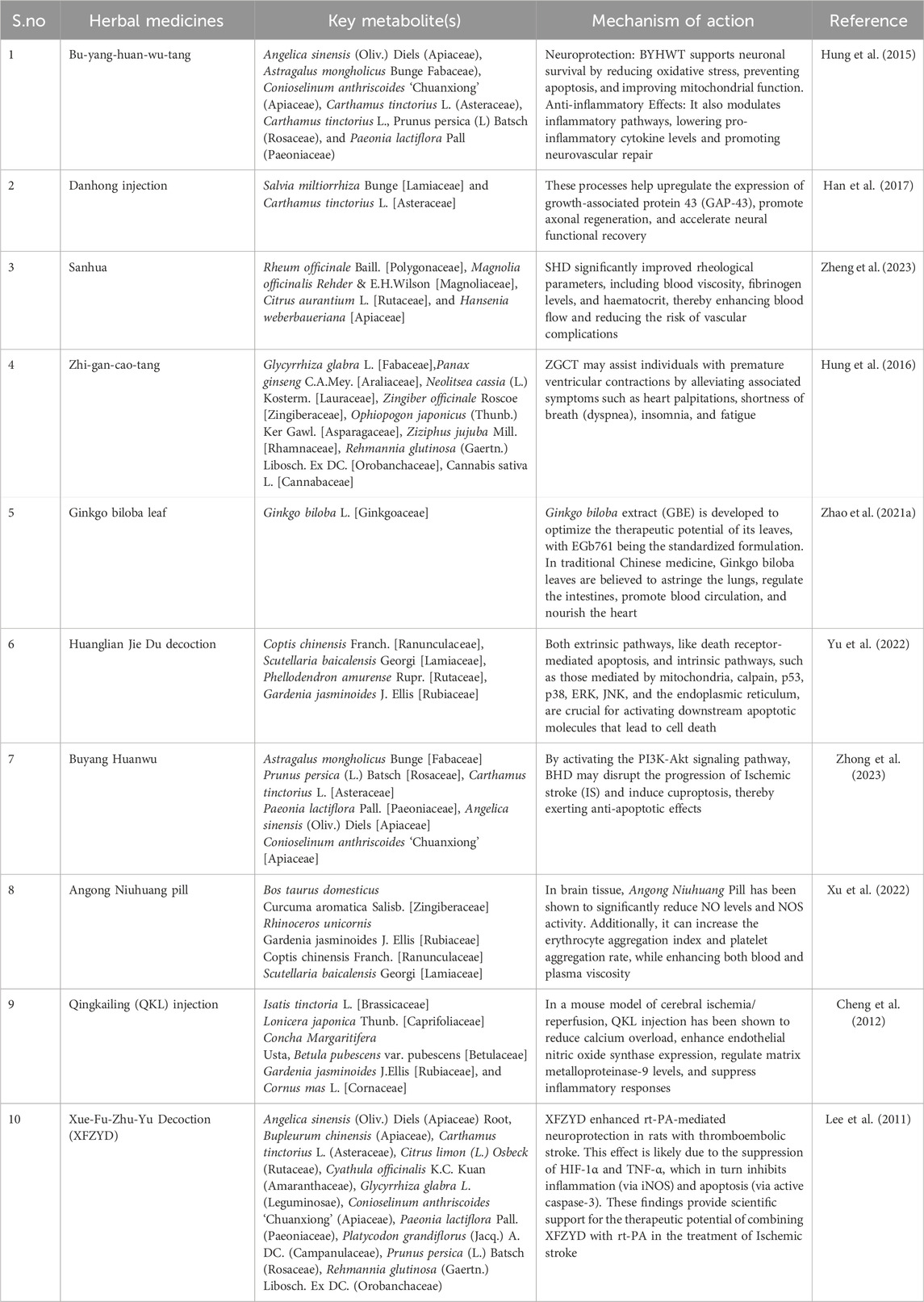
Traditional Chinese medicine has been utilised to prevent various illnesses in Asian nations, particularly China. Chinese Materia medica, TCM preparation, and their active metabolite(s) may help treat brain damage brought on by ischemia/reperfusion (Table 1). Numerous studies on TCM’s ability to protect the BBB have been published in recent years (Li et al., 2019).The botanical drug(s) resistance to platelets, such as Toxicodendron vernicifluum (Anacardiaceae), Salvia miltiorrhiza Bunge (Lamiaceae), Biancaea sappan. (Fabaceae), Curcuma aromatica Salisb.(Zingiberaceae),CinnamomumcassiaPresl (Lauraceae),Paeonialactiflora (Paeoniaceae),PanaxginsengC.A.Mey.(Araliaceae),Carthamus tinctoriusa (Asteraceae) (Kim and Park, 2019). Traditional Chinese medicine (TCM) offers several notable advantages in treating multisite, multitarget disorders and general regulation. Certain TCMS, including Gastrodia elata (Orchidaceae), Rehmannia glutinosa (Orobanchaceae), Ginkgo biloba (Ginkgoaceae), and Panax notoginseng (Araliaceae), show higher therapeutic effects on neurological illnesses than on other conditions. NBP, or dl-3-n-butylphthalide, has been shown to possess neuroprotective properties (Zhu et al., 2021). Phenolic glycosides are characteristic of Salidroside, which is obtained from the traditional Tibetan medicinal plant Rhodiola rosea (Crassulaceae). Traditional Chinese medicine is commonly used to increase the body’s resistance to weariness. Numerous studies have demonstrated that it has various biological effects, including antioxidant properties (Zuo et al., 2018).
Composition of the traditional Chinese medicine of some remedies used for ischemic stroke. The botanical herbs used for the preparation of Xue-Fu-Zhu-Yu decoction and the Amount used are Angelica sinensis (Oliv) Diels (Apiaceae) Root 4.5 g, Bupleurum chinensis (Apiaceae) Root 1.5 g, Carthamus tinctorius L. (Asteraceae) Flower 4.5 g, Citrus × limon (L.) Osbeck (Rutaceae) Fruit 3.0 g, Cyathula officinalis K.C.Kuan (Amaranthaceae) Root 4.5 g, Glycyrrhiza glabra L. (Leguminosae) Root 1.5 g, Conioselinum anthriscoides ‘Chuanxiong’ (Apiaceae) Root 2.3 g, Paeonia lactiflora Pall. (Paeoniaceae) Root 3.0 g, Platycodon grandiflorus (Jacq.) A. DC. (Campanulaceae) Root 2.3 g, Prunus persica (L.). Batsch (Rosaceae) Seed 6.0 g, R. glutinosa (Gaertn.) Libosch. ex DC. (Orobanchaceae) Root 4 g (Lee et al., 2011). Buyang Huanwu decoction consist of seven botanical herbs such as A. sinensis (Oliv.) Diels (Apiaceae), Astragalus mongholicus Bunge (Fabaceae) (120 g) dried roots, C. anthriscoides “Chuanxiong:” (Apiaceae) 3 g dried rhizomes, C. tinctorius L. (Asteraceae) 3 g dried flowers of C. tinctorius L., Prunus persica (L) Batsch (Rosaceae) 3 g dried seeds, and P. lactiflora Pall (Paeoniaceae) 4.5 g dried roots (Zhang et al., 2018).
Notably, Table 2 summarizes several in vivo studies showcasing the molecular and functional outcomes of these TCMs, supporting their potential as adjunct therapies in ischemic stroke treatment.
4.2 Herbal medicines
“In the last 30 years, polyphenols and micronutrients in foods have gained attention for their antioxidant effects and potential to prevent diseases like cancer, heart problems, and brain disorders linked to oxidative stress. Polyphenols, made by plants, help protect against UV rays and infections. People consume about 1 g of polyphenols daily, much more than vitamin E or C, making them a major source of dietary antioxidants (Pacifici et al., 2021). Polyphenols are plant compounds classified into five groups: lignans, curcumins, stilbenes, phenolic acids, and flavonoids. In stroke models, they show neuroprotective effects by reducing brain damage and aiding recovery. Their antioxidant properties come from their ability to bind metals and neutralize reactive oxygen species. Key polyphenols like gallic acid, resveratrol, and quercetin help protect against ischemic stroke by targeting multiple pathways involved in damage and repair (Abdelsalam et al., 2023). Polyphenols have gained attention for their health benefits, especially their antioxidant and neuroprotective effects after ischemic stroke, as shown in recent studies (Zhou et al., 2021).
4.2.1 Resveratrol
Resveratrol (3,5,4′-trihydroxy-trans-diphenyl-ethylene) is a natural antioxidant that helps combat inflammation and neutralize free radicals in the body (Fodor et al., 2018). Resveratrol promotes neuroprotection in ischemic stroke by activating SIRT1 and NRF2 pathways. This enhances mitochondrial function, antioxidant defense, and stress response, helping cells survive and reducing apoptosis (Owjfard et al., 2024).
4.2.2 Gallic acid
Gallic acid is a well-absorbed polyphenol that helps reduce cerebral edema, a common issue after ischemic stroke. It also promotes microglial activation and shifts their phenotype, aiding in stroke recovery (Qu et al., 2022). Mechanistically, GA has been demonstrated to control M1-type macrophage polarization processes and, in part, suppress inflammatory responses by preventing M1-type macrophage polarization. Furthermore, it has been demonstrated that GA–chitosan complexes alter inflammatory responses in lipopolysaccharide-stimulated RAW264.7 cells (macrophage line) via affecting the cellular NF-κB, AP-1, and MAPK pathways (Qu et al., 2022).
4.2.3 Curcumin
One of the primary active metabolite(s) in Chinese botanical drug(s), curcumin, regulates the NLRP3 signalling pathway to promote neuronal repair and neuroprotection (Du et al., 2023).According to a study, pretreatment with curcumin reduces neuronal mortality in the CA1 region of the hippocampus of rats with LPS-induced depression, which is accompanied by an improvement in synaptic function (Fan et al., 2021).Additionally, curcumin therapy improved cell survival, reduced cell apoptosis, and increased Bcl-2 protein levels while decreasing caspase-3 expressions and Bax in mouse N2a cells after OGD/R injury. Curcumin treatment also stopped Bax activation and maintained the integrity of the mitochondrial membrane (Xie et al., 2018).
4.2.4 Flavonoid
Flavonoids, which are abundant in fruits and vegetables and other foods we eat every day, have been proven to have positive therapeutic effects on cerebral ischemia injury by reducing neuroinflammation. Apigenin, baicalein, Naringenin, EGCG, quercetin, rutin, and other flavonoids, for instance, have been found to inhibit and limit neuroinflammation following ischemic stroke by affecting the activation of astrocytes and microglia (Lu et al., 2024).Flavonoids are classified into six groups based on the various structures that link the two benzene rings: flavanols, flavanones, flavonols, isoflavones, flavones, and anthocyanidins (Liu S. et al., 2022).The degree of unsaturation, the location of the benzoid substituent, and the orientation of hydroxylation or methylation are some characteristics that distinguish different flavonoids from one another. Their antiproliferative and antioxidant properties are limited by their structural features (Cebova and Pechanova, 2020).The neuroprotective properties of flavonoids are found in Chinese herbal medicine. By reducing excitotoxicity, oxidative stress, inflammation, thrombin toxicity, and cell deaths, as well as by preserving the blood-brain barrier, Ca2+ overloading and neurogenesis, flavonoids, a type of botanical medication, have a neuroprotective effect (Zhou et al., 2024).
4.2.5 Quercetin
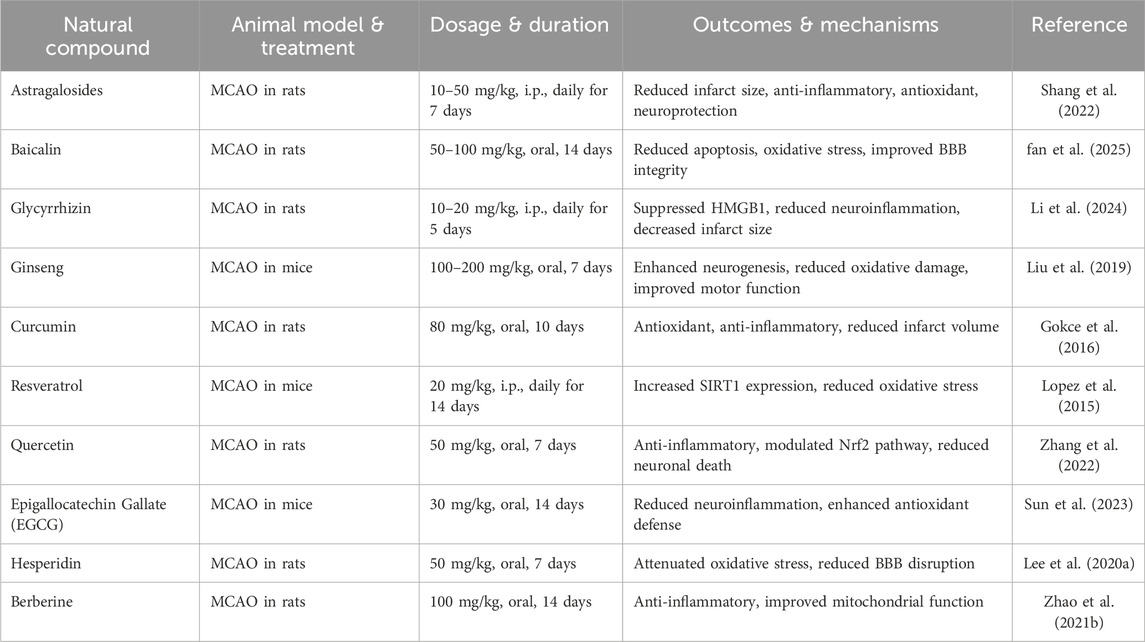
Quercetin reduces blood-brain interference and condenses the quantity of MMP-9 in tests for cerebral ischemia, according to antioxidant analyses that are similar to those of green tea polyphenols. By reducing lipid peroxidation and ion channel acid sensing, two factors that contribute to ion channel dysregulation, quercetin helps prevent ischemic injury (Maqbool and Zehravi, 2021). Quercetin prevents platelet aggregation by inhibiting agonists like thrombin, collagen, and ADP. It also blocks calcium signaling and binds to the GPIIb/IIIa receptor to reduce platelet activity. In C57BL/6 rats, this action helps reduce thrombosis and improves blood flow after FeCl3-induced carotid artery injury (Zhang et al., 2022). Table 3 illustrates In vivo studies performed in the ischemic stroke using herbal medicines.
4.3 Ayurveda
The oldest and most significant tradition in India, Ayurveda, is founded on experimentation and philosophy. With an emphasis on strengthening the host’s immune system, Ayurveda has a database of numerous medicinal botanical drug(s) and is accessible in several regional languages. According to several classical books, Ayurveda mentions 1200 illnesses. The 700 therapeutic botanical drug(s) in the Atharveda (c. 1200 BC), Charak Samhita, and Sushrat Samhita (c. 1000–500 BC) (Ara et al., 2022).Adoxa moschatellina, Bacopa monnieri, Centella asiatica, Mucuna urens, Phyllanthus emblica, Terminalia arjuna, and Withania somnifera are the plants that are the basis for the Mentat, also known as BR-16A. Because of its free-radical-scavenging qualities and antioxidant, it functions as a neuroprotective agent and may be utilized to help patients recover from ischemic stroke (Ibáñez et al., 2023). Ashwagandha (W. somnifera) is a traditional, well-known Indian medicinal plant that is frequently used as a component in Ayurvedic formulations sold to treat neurological conditions. It is helpful. Inhibition of gelatinases (MMP-9) Therapeutically active in ischemic stroke and hemorrhagic stroke (Zahiruddin et al., 2020).Ayurveda views hemiplegia as a clinical entity, “Pakshaghata,” grouped under “Vatavyadhi.” The central role of Ayurveda lies during the time of stroke rehabilitation. It employs internal medicines along with procedures, including Panchakarma therapies. Evidence-based medicine (EBM) requires proof of effectiveness, efficiency, and safety for decision-making in patient care. Integrating individual clinical expertise with the best external clinical evidence is the core of EBM practice. Thus, empirical Ayurvedic knowledge accrued over the years can be tapped for integrated stroke rehabilitation after proper evaluation (M. A et al., 2025).
5 Mechanisms of action involved in TCIM approaches
Studies show that acupuncture combined with the Tianma Gouteng Yin formula is effective in treating acute cerebral infarction. This combination significantly improves vascular endothelial function and cerebral vascular function, including measures like pulsatility index, cerebrovascular reserve, neurological function, platelet activation, and inflammation (IL-6, hs-CRP). It also positively impacts NIHSS and BI scores (Zhang et al., 2023). TCM can treat stroke through multiple targets and pathways with lower side effects. It affects pathways like AKT/PI3K and SLC7A11/GPX4, which promote angiogenesis, neuroprotection, and reduce ferroptosis. This multi-target approach may lead to more effective stroke treatment (Fu et al., 2022). A study found that Astragaloside IV could boost the levels of SLC7A11 by activating the Nrf2/HO-1 signalling pathway. and GPX4 and ROS. In an ICH model with intravascular perforation, this activation resulted in increased antioxidant capacity and lipid peroxidation inhibition (Liu Z. et al., 2022). Through several processes, TCM has been utilized to treat stroke either alone or in conjunction with other treatments. such include promoting neurogenesis and angiogenesis, regulating the blood-brain barrier, inhibiting platelet activation, preventing inflammation, oxidative stress, and apoptosis (Chen and Jin, 2023).
6 Complementary medicines
Complementary alternative medicine encompasses a variety of goods, techniques, and structures that are not typically associated with traditional care. Geographical and cultural disparities may cause different CAMs to be utilized in Asian and African nations to treat stroke. Over the past 10 years, stroke patients’ interest in complementary and alternative medicine has rapidly increased (Chang et al., 2016). Integrative medicine addresses patients’ complicated demands by combining alternative therapies with traditional medical treatments, especially in cases of ischemic stroke. Nowadays, a lot of people use Chinese medicine in conjunction with conventional therapy to prevent and cure stroke (Ni et al., 2020).
6.1 Yoga
Yoga therapy is based on the Panchakosha concept from the Taittiriya Upanishad, which views a person as having five layers: physical (Annamaya), energy or life force (Pranamaya), mind (Manomaya), intellect (Vijnanamaya), and bliss (Anandamaya) (Jasti et al., 2022). Yoga combines breathing, stretching, and balance to improve both mental and physical wellbeing. It helps calm the mind and build focus. Breathing is coordinated with various poses, whether standing, sitting, lying, or prone (Pal et al., 2023). Yoga may help control key risk factors for stroke, especially high blood pressure—the most modifiable risk factor. Evidence suggests it can safely and effectively reduce hypertension in stroke patients (Thayabaranathan et al., 2018). Yoga boosts hemoglobin and red blood cell levels, improving oxygen delivery to cells. It also helps lower blood pressure, reducing the risk of stroke and heart failure caused by blood clots (Dubey, 2024). In a pilot study by Arlene et al., 47 chronic stroke patients were randomized to either therapeutic yoga (n = 37) or a control group (n = 10). After 8 weeks, the yoga group showed significant improvements in physical function. This is important, as chronic stroke often leads to disabilities that affect daily activities and social participation (Schmid et al., 2014).
6.2 Acupuncture
Acupuncture, a key part of Traditional Chinese Medicine, involves inserting fine needles into specific body areas (Mu et al., 2023). Acupuncture has been used to treat stroke since ancient times, including body, scalp, eye, and electro-acupuncture methods. Choosing the correct points is crucial for effective treatment (Li et al., 2022). Acupuncture supports neurogenesis after ischemic injury by boosting neural stem cell activity and reducing local inhibitory factors, creating a healing-friendly environment (Mu et al., 2023). Current studies suggest that acupuncture may aid in treating ischemic stroke by reducing oxidative stress, protecting the blood-brain barrier, and regulating exosomes involved in neuroprotection, neuroplasticity, cell growth, apoptosis, immunity, and inflammation (Zhu et al., 2024).
6.2.1 Mechanism of action
Acupuncture may help reduce inflammation in ischemic stroke by lowering proinflammatory cytokines like IL-1β, IL-6, and TNF-α in the brain and blood. This effect is likely linked to inhibition of NF-κB activation, which reduces cytokine production (Kuang et al., 2024). The following sections discuss methods to prevent oxidative stress, including repairing damaged proteins, lipids, or DNA, limiting ROS-induced cell death or autophagy, reducing ROS production, and neutralizing ROS through antioxidant enzymes or other signaling pathways (Poljsak, 2011).
Acupuncture and low-intensity laser (LA) therapy are used to treat various conditions. In rats with right middle cerebral artery blockage, LA at GV20 reduced MDA levels and increased the activity of GPx, CAT in the cerebral cortex, and SOD in mitochondria (Jittiwat, 2017). A randomized controlled trial assessed acupuncture’s effectiveness in ischemic stroke rehabilitation. Patients were divided into two acupuncture groups and one control group with only rehabilitative instructions. After 2 weeks, the acupuncture groups showed significant improvements in NIHSS scores, and the second acupuncture group had higher BI scores than the control. These results suggest that acupuncture may aid in functional rehabilitation post-stroke (Li et al., 2022).
6.3 Physical exercise
Exercise promotes neuroplasticity, neurotrophins, and cognitive function, while improving overall health and reducing the risk of hypokinetic diseases associated with a sedentary lifestyle (Sakakima, 2019). After a stroke, physical therapy aids in regaining function and movement. While task-specific training was once the main focus, recent attention has shifted to exercise-based rehabilitation (Pogrebnoy and Dennett, 2020). Exercise after a stroke may enhance the expression of Ang-1 and Tie-2, aiding brain recovery. Ischemic stroke can impair memory by increasing apoptosis and affecting BDNF levels in the hippocampus. Treadmill training helps recover memory by boosting BDNF expression, promoting cell growth, and reducing apoptosis (Xing et al., 2018). Cerebral ischemia triggers a cascade of events that increase cerebrovascular permeability and disrupt the blood-brain barrier (BBB), leading to brain edema. MMP-9 contributes to BBB failure by breaking down the extracellular matrix (Zhang et al., 2019). Exercise preconditioning strengthens the basal lamina and reduces blood-brain barrier (BBB) dysfunction in ischemic stroke by increasing MMP-9. Chronic cerebral hypoperfusion can be countered by treadmill exercise, which boosts MMP-9 levels, lowers occludin, and degrades zonula occludens-1 (ZO-1) (Lee SS. et al., 2020).
6.4 Dietary and nutritional supplementation
Nutrition plays a key role in stroke risk, which can be reduced by up to 80% with a healthy lifestyle, also lowering stroke risk factors (Spence, 2019). The former treatment regimen involves consuming specific nutrients (e.g., metals, fibers, fatty acids, vitamins), while the latter refers to time-restricted feeding, fasting, or nutrient restriction (e.g., carbs, amino acids). Both approaches, used in preclinical or clinical settings, help maintain nutritional balance and may slow the progression of neurological diseases (Mao et al., 2021). The former treatment regimen involves consuming specific nutrients (e.g., metals, fibers, fatty acids, vitamins), while the latter refers to time-restricted feeding, fasting, or nutrient restriction (e.g., carbs, amino acids). Both approaches, used in preclinical or clinical settings, help maintain nutritional balance and may slow the progression of neurological diseases (Ciancarelli et al., 2024). Research shows that omega-3 fatty acids, found in fish, nut oils, and leafy vegetables, improve neurotransmission and prevent membrane fluidity loss caused by cholesterol. These effects help maintain cell signaling and synaptic function. Regular intake of polyphenol-rich foods can enhance cerebral blood flow, cognition, and promote neurogenesis and synaptogenesis. Flavonoids, present in many edible plants, may support neuroplasticity, neurogenesis, and cerebral blood flow, thanks to their anti-inflammatory and antioxidant properties (Siotto et al., 2022).
6.4.1 Vitamins
Vitamins and minerals can support neuroprotection and recovery by boosting antioxidant capacity and aiding functional recovery in stroke patients. Antioxidants like vitamin E and C help reduce oxidative damage (Lieber et al., 2018). Vitamin C administration improves microcirculation, neurological outcomes, and survival, while reducing oxidative stress, heart damage, and arrhythmias (Spoelstra-de Man et al., 2018). The study showed that supplementing with tetrahydrobiopterin, arginine, and vitamin C improved blood flow during ischemia by reducing oxidative stress and increasing endothelial NO synthase activity (Tang et al., 2022). Vitamin D may have antithrombotic effects by reducing cytokine-induced changes in tissue factor and thrombomodulin, as suggested by several experimental studies (Cui et al., 2024). EPA metabolites from Omega-3 fatty acids reduce inflammation, lower monocyte adhesion and platelet aggregation, and improve endothelial function by inhibiting COX-1/2 enzymes, leading to less production of inflammatory prostaglandins (Ueno et al., 2019).
6.4.2 Minerals
Copper (Cu) and zinc (Zn) are the most common metals in the human body and are found in high concentrations in the brain. While their exact roles in inflammation are still being studied, they are essential for controlling oxidative stress and regulating the production of free radicals (Wessels et al., 2017). Selenium (Se) is an essential mineral with roles in immune function, cancer prevention, and cell regulation. As part of the antioxidant system, selenium and its proteins may also offer neuroprotective benefits (Mirończuk et al., 2021).
6.5 Aromatherapy
Essential oils are used therapeutically in aromatherapy as a supplemental medicine to cure a variety of mental and physical illnesses and to advance patient wellbeing (Reis and Jones, 2017). Essential oil components can enter the bloodstream and cross the blood-brain barrier through various routes such as topical, subcutaneous, oral, intraperitoneal, or inhalation applications. Aromatic oils like lavender, bergamot, and curcuma have shown numerous beneficial effects (Contrada et al., 2021). It has been demonstrated that essential oils contain anti-inflammatory and antioxidant qualities in addition to psychological benefits that might lessen emotional and physical ailments like exhilaration, exhaustion, and delirium (Lim and Kim, 2024). Neurotransmitter systems involved in neuronal survival and plasticity are influenced by specific essential oils. For example, it has been demonstrated that chemicals in sandalwood oil interact with gamma-aminobutyric acid (GABA) receptors, modifying excitatory and inhibitory neurotransmission to promote neuroprotective effects (Younis and Mohamed, 2020). Essential oils like bergamot and lavender, known for their antioxidant properties, help reduce oxidative stress during ischemia. They boost antioxidant enzymes like glutathione peroxidase and superoxide dismutase, which lower ROS production and cellular damage. Additionally, these oils have anti-inflammatory effects by suppressing pro-inflammatory cytokines, reducing inflammation linked to ischemic stroke (Contrada et al., 2021).
6.6 Music therapy
Music therapy uses musical elements to improve mood and neurological function. It has shown positive results in stroke rehabilitation across various studies (Xu et al., 2022). A home-based music therapy program was developed for self-rehabilitation in chronic stroke patients. Music therapy has a significant impact on improving neurological function and can change the brain’s structure and function in stroke patients (Huang et al., 2021). Thirty stroke patients received music therapy for 4 weeks. Those in the music group showed significantly better performance on the Wolf Motor Function Test, with improved timing and motion quality, compared to the mute group. Most patients in the music group could complete the task independently (Tong et al., 2015). Music can impact the brain stem, stimulate the cerebral cortex, regulate peripheral nerves, strengthen muscles, and boost physical vitality in stroke patients (Barclay et al., 2020).
7 Clinical trials of ischemia stroke
A randomized clinical trial showed that patients with acute ischemic stroke who received Tongxinluo along with their usual medication within 72 h of symptom onset had a better functional outcome compared to those who received a placebo (Dong et al., 2024). Acupuncture, as a complementary treatment, has shown benefits for ischemic stroke patients in randomized controlled trials (Zhang et al., 2015). A study using Wen Dan Decoction in ischemic stroke patients showed it helps improve and stabilize atherosclerotic plaques (Xu et al., 2015). Table 4 illustrates Clinical studies of ischemic stroke using traditional medicines and complementary medicines.

Table 4. Clinical studies of ischemic stroke using traditional medicines and complementary medicines.
8 Limitations and future directions
Traditional and herbal formulations often vary in preparation methods, dosages, and phytochemical profiles, leading to inconsistent therapeutic outcomes. Most evidence supporting TCIM approaches is based on preclinical (in vitro/in vivo) models, while high-quality, large-scale randomized controlled trials (RCTs) in humans remain scarce. Many studies evaluate outcomes over brief periods, failing to assess long-term safety, efficacy, or the risk of relapse. Additionally, most clinical trials involve homogenous populations, limiting the applicability of results across diverse demographics and co-morbid conditions. Potential interactions between herbal remedies and standard stroke medications are seldom explored, raising concerns about safety. Methodological flaws, such as inadequate randomization, lack of placebo controls, and absence of blinding, further compromise the reliability of findings. Studies also vary widely in their outcome measures and definitions of key clinical endpoints like “functional recovery” and “neuroprotection,” which affects comparability and reproducibility. Furthermore, several trials do not adequately justify the choice of dose, frequency, or duration of treatment. Although some studies demonstrate beneficial effects, they often lack detailed investigations into the molecular mechanisms involved, which are essential for scientific validation and regulatory approval.
To overcome these limitations, future research should prioritize the development of standardized formulations with defined phytochemical profiles and optimized dosing protocols. Multicenter, double-blind, placebo-controlled RCTs involving larger and more diverse patient populations are needed to establish clinical efficacy. Mechanistic studies employing systems biology and omics technologies (e.g., genomics, proteomics, metabolomics) can help elucidate how TCIM therapies exert their effects. Comprehensive safety evaluations, including herb-drug interaction studies and long-term toxicity assessments, are crucial. Integrative models that combine conventional and traditional medicine could support a more personalized approach to stroke management. Finally, the use of advanced imaging techniques and validated biomarkers would enable more objective and reliable assessment of therapeutic outcomes.
9 Conclusion
This review underscores the potential of Traditional, Complementary, and Integrative Medicine (TCIM) in addressing the multifactorial pathophysiology of ischemic stroke. The neuroprotective, anti-inflammatory, and antioxidant properties of various herbal and alternative therapies may support improved neurological recovery and reduce the risk of stroke recurrence. Integrating TCIM into conventional stroke care requires a multidisciplinary framework that fosters collaboration among clinicians, researchers, and traditional medicine practitioners. However, to enable safe and effective implementation, further rigorous studies are needed to standardise formulations, establish therapeutic efficacy, and evaluate safety profiles. Such evidence-based integration could ultimately transform the global paradigm of ischemic stroke management.
Author contributions
SS: Writing – original draft, Data curation. RB: Formal Analysis, Resources, Supervision, Validation, Visualization, Writing – review and editing. LD: Data curation, Investigation, Project administration, Supervision, Writing – review and editing. PR: Writing – original draft. NH: Investigation, Project administration, Resources, Supervision, Visualization, Writing – review and editing. ASS: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelsalam, S. A., Renu, K., Zahra, H. A., Abdallah, B. M., Ali, E. M., Veeraraghavan, V. P., et al. (2023). Polyphenols mediate neuroprotection in cerebral ischemic stroke—an update. Nutrients 15, 1107. doi:10.3390/nu15051107
Ajoolabady, A., Wang, S., Kroemer, G., Penninger, J. M., Uversky, V. N., Pratico, D., et al. (2021). Targeting autophagy in ischemic stroke: from molecular mechanisms to clinical therapeutics. Pharmacol. Ther. 225, 107848. doi:10.1016/j.pharmthera.2021.107848
Akashlal, M., Nair, P.P., Nair, D.R., Ahmad, A., Chandrasekhararao, B., and Sudhakar, D. (2025). A systematic review on safety and efficacy of ayurvedic interventions in hemiplegia (pakshaghata). J. Evid. Based Integr. Med. 30, 2515690X241304523. doi:10.1177/2515690X241304523
Ara, I., Maqbool, M., Gani, I., Kashmir, O., and Kashmir, I. (2022). Neuroprotective activity of herbal medicinal products: a review. Int. J. Cyrrent Reseach Physiology Pharmacol. 6. Available online at: https://ijcrpp.com/index.php/ijcrpp/article/view/53.
Barclay, R. E., Stevenson, T. J., Poluha, W., Semenko, B., and Schubert, J. (2020). Mental practice for treating upper extremity deficits in individuals with hemiparesis after stroke. Cochrane Database Syst. Rev. 2020. doi:10.1002/14651858.cd005950.pub5
Briones-Valdivieso, C., Briones, F., Orellana-Urzúa, S., Chichiarelli, S., Saso, L., and Rodrigo, R. (2024). Novel multi-antioxidant approach for ischemic stroke therapy targeting the role of oxidative stress. Biomedicines 12, 501. doi:10.3390/biomedicines12030501
Cebova, M., and Pechanova, O. (2020). Protective effects of polyphenols against ischemia/reperfusion injury. Molecules 25, 3469. doi:10.3390/molecules25153469
Chang, C. C., Lee, Y. C., Lin, C. C., Chang, C. H., Chiu, C. D., Chou, L. W., et al. (2016). Characteristics of traditional Chinese medicine usage in patients with stroke in Taiwan: a nationwide population-based study. J. Ethnopharmacol. 186, 311–321. doi:10.1016/j.jep.2016.04.018
Chen, B., and Jin, W. (2023). A comprehensive review of stroke-related signaling pathways and treatment in western medicine and traditional Chinese medicine. Front. Neurosci. 17, 1200061. doi:10.3389/fnins.2023.1200061
Chen, H., Chen, X., Luo, Y., and Shen, J. (2018). Potential molecular targets of peroxynitrite in mediating blood–brain barrier damage and haemorrhagic transformation in acute ischaemic stroke with delayed tissue plasminogen activator treatment. Free Radic. Res. 52 (11–12), 1220–1239. doi:10.1080/10715762.2018.1521519
Chen, Z., Mo, J., Xu, J., Qin, H., Zheng, H., Pan, Y., et al. (2019). Risk profile of ischemic stroke caused by small-artery occlusion vs. Deep intracerebral hemorrhage. Front. Neurol. 10, 10. doi:10.3389/fneur.2019.01213
Cheng, F., Wang, X., Lu, Y., Zhong, X., Zhao, Y., and Wang, Q. (2012). Chinese medicine injection qingkailing for treatment of acute ischemia stroke: a systematic review of randomized controlled trials. Evidence-Based Complementary Altern. Med. 2012, 213172–213177. doi:10.1155/2012/213172
Ciancarelli, I., Morone, G., Iosa, M., Cerasa, A., Calabrò, R. S., and Tozzi Ciancarelli, M. G. (2024). Neuronutrition and its impact on post-stroke neurorehabilitation: modulating plasticity through diet. Nutrients 16 (21), 3705. doi:10.3390/nu16213705
Contrada, M., Cerasa, A., Tonin, P., Bagetta, G., and Scuteri, D. (2021). Aromatherapy in stroke patients: is it time to begin? Front. Behav. Neurosci. 15, 749353. doi:10.3389/fnbeh.2021.749353
Cui, P., Hou, H., Song, B., Xia, Z., and Xu, Y. (2024). Vitamin D and ischemic stroke - association, mechanisms, and therapeutics. Ageing Res. Rev. 96, 102244. doi:10.1016/j.arr.2024.102244
Deijle, I. A., Jonkers, I. M., Hooghiemstra, A. M., Engels, G., Twisk, J. W. R., Weinstein, H. C., et al. (2024). Effects of a 1 year aerobic and strength training on cognitive functioning after transient ischemic attack or minor stroke: a randomized controlled trial. J. Stroke Cerebrovasc. Dis. 33 (1), 107441. doi:10.1016/j.jstrokecerebrovasdis.2023.107441
Dong, W., Gong, F., Zhao, Y., Bai, H., and Yang, R. (2023). Ferroptosis and mitochondrial dysfunction in acute central nervous system injury. Front. Cell. Neurosci. 17, 1228968. doi:10.3389/fncel.2023.1228968
Dong, Y., Jiang, K., Li, Z., Zhou, Y., Ju, B., Min, L., et al. (2024). Tongxinluo and functional outcomes among patients with acute ischemic stroke: a randomized clinical trial. JAMA Netw. Open 7 (9), e2433463. doi:10.1001/jamanetworkopen.2024.33463
Du, X., Amin, N., Xu, L., Botchway, B. O. A., Zhang, B., and Fang, M. (2023). Pharmacological intervention of curcumin via the NLRP3 inflammasome in ischemic stroke, 14. Frontiers Media SA. Frontiers in Pharmacology. doi:10.3389/fphar.2023.1249644
Dubey, S. (2024). A review on yoga practice and its effects. Available online at: https://www.researchgate.net/publication/359157970.
Fan, C., Li, Y., Lan, T., Wang, W., Mao, X., and Yu, S. Y. (2021). Prophylactic treatment of curcumin in a rat model of depression by attenuating hippocampal synaptic loss. Food Funct. 12 (22), 11202–11213. doi:10.1039/d1fo02676c
fan, Li Y., fan, Z. Y., Huang, C., and Jiang, J. (2025). Baicalin improves neurological outcomes in mice with ischemic stroke by inhibiting astrocyte activation and neuroinflammation. Int. Immunopharmacol. 149, 114186. doi:10.1016/j.intimp.2025.114186
Fodor, K., Tit, D. M., Pasca, B., Bustea, C., Uivarosan, D., Endres, L., et al. (2018). Long-Term resveratrol supplementation as a secondary prophylaxis for stroke. Oxid. Med. Cell. Longev. 2018, 4147320. doi:10.1155/2018/4147320
Fu, C., Wu, Y., Liu, S., Luo, C., Lu, Y., Liu, M., et al. (2022). Rehmannioside A improves cognitive impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2 and SLC7A11/GPX4 signaling pathway after ischemia. J. Ethnopharmacol. 289, 115021. doi:10.1016/j.jep.2022.115021
Gokce, E. C., Kahveci, R., Gokce, A., Sargon, M. F., Kisa, U., Aksoy, N., et al. (2016). Curcumin attenuates inflammation, oxidative stress, and ultrastructural damage induced by spinal cord ischemia–reperfusion injury in rats. J. Stroke Cerebrovasc. Dis. 25 (5), 1196–1207. doi:10.1016/j.jstrokecerebrovasdis.2016.01.008
Granger, D. N., and Kvietys, P. R. (2015). Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 6, 524–551. doi:10.1016/j.redox.2015.08.020
Gudarzi, S., Jafari, M., Pirzad Jahromi, G., Eshrati, R., Asadollahi, M., and Nikdokht, P. (2022). Evaluation of modulatory effects of saffron (Crocus sativus L.) aqueous extract on oxidative stress in ischemic stroke patients: a randomized clinical trial. Nutr. Neurosci. 25 (6), 1137–1146. doi:10.1080/1028415X.2020.1840118
Guo, J., Tuo, Q., and Lei, P. (2023). Iron, ferroptosis, and ischemic stroke. J. Neurochem. 165 (4), 487–520. doi:10.1111/jnc.15807
Han, S. Y., Hong, Z. Y., Xie, Y. H., Zhao, Y., and Xu, X. (2017). Therapeutic effect of Chinese herbal medicines for post stroke recovery: a traditional and network meta-analysis. Med. Madr. 96, e8830. doi:10.1097/MD.0000000000008830
Haupt, M., Gerner, S. T., Bähr, M., and Doeppner, T. R. (2023). Neuroprotective strategies for ischemic stroke—future perspectives. Int. J. Mol. Sci. 24, 4334. doi:10.3390/ijms24054334
Huang, W. H., Dou, Z. L., Jin, H. M., Cui, Y., Li, X., and Zeng, Q. (2021). The effectiveness of music therapy on hand function in patients with stroke: a systematic review of randomized controlled trials. Front. Neurol. 12, 641023. doi:10.3389/fneur.2021.641023
Hung, I. L., Hung, Y. C., Wang, L. Y., Hsu, S. F., Chen, H. J., Tseng, Y. J., et al. (2015). Chinese herbal products for ischemic stroke. Am. J. Chin. Med. 43 (7), 1365–1379. doi:10.1142/S0192415X15500779
Hung, Y. C., Cheng, Y. C., Muo, C. H., Chiu, H. E., Liu, C. T., and Hu, W. L. (2016). Adjuvant Chinese herbal products for preventing ischemic stroke in patients with atrial fibrillation. PLoS One. 11 (7), e0159333. doi:10.1371/journal.pone.0159333
Hurd, M. D., Goel, I., Sakai, Y., and Teramura, Y. (2021). Current status of ischemic stroke treatment: from thrombolysis to potential regenerative medicine. Regen. Ther. 18, 408–417. doi:10.1016/j.reth.2021.09.009
Ibáñez, B., Melero, A., Montoro, A., Merino-Torres, J. F., Soriano, J. M., and San Onofre, N. (2023). A narrative review of the herbal preparation of ayurvedic, traditional Chinese, and kampō medicines applied as radioprotectors. Antioxidants 12 (7), 1437. doi:10.3390/antiox12071437
Jasti, N., Reddy, A. V., Ramakrishna, K. K., Bhargav, H., and Kulkarni, G. B. (2022). “Role of yoga in stroke management: current evidence and future directions,” in The principles and practice of yoga in cardiovascular medicine (Springer Nature), 253–265.
Jittiwat, J. (2017). Laser acupuncture at GV20 improves brain damage and oxidative stress in animal model of focal ischemic stroke. J. Acupunct. Meridian Stud. 10 (5), 324–330. doi:10.1016/j.jams.2017.08.003
Juan, C. A., Pérez de la Lastra, J. M., Plou, F. J., and Pérez-Lebeña, E. (2021). The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 22 (9), 4642. doi:10.3390/ijms22094642
Jurcau, A., and Ardelean, A. I. (2022). Therapeutic strategies in huntington's disease: from genetic defect to gene therapy. Oxidative Stress Ischemia/Reperfusion Inj. Follow. Acute Ischemic Stroke 10, 1895. doi:10.3390/biomedicines10081895
Jurcau, A., and Ardelean, I. A. (2021). Molecular pathophysiological mechanisms of ischemia/reperfusion injuries after recanalization therapy for acute ischemic stroke. NeuroSignals 20, 727–744. doi:10.31083/j.jin2003078
Kim, K., and Park, K. (2019). A review of antiplatelet activity of traditional medicinal herbs on integrative medicine studies. Evid. Based. Complement. Altern. Med. 2019, 7125162. Il. doi:10.1155/2019/7125162
Koutsaliaris, I. K., Moschonas, I. C., Pechlivani, L. M., Tsouka, A. N., and Tselepis, A. D. (2021). Inflammation, oxidative stress, vascular aging and atherosclerotic ischemic stroke. Curr. Med. Chem. 29 (34), 5496–5509. doi:10.2174/0929867328666210921161711
Kuang, H., Zhu, X., Chen, H., Tang, H., and Zhao, H. (2024). Corrigendum: the immunomodulatory mechanism of acupuncture treatment for ischemic stroke: research progress, prospects, and future direction. Front. Immunol. 15, 1468179. doi:10.3389/fimmu.2024.1468179
Kuriakose, D., and Xiao, Z. (2020). Pathophysiology and treatment of stroke: present status and future perspectives. Int. J. Mol. Sci. 21, 7609–7624. doi:10.3390/ijms21207609
Lai, Y. T., Huang, H. L., Hsieh, C. C., Lin, C. H., Yang, J. C., Tsou, H. H., et al. (2023). The effects of yoga exercise on blood pressure and hand grip strength in chronic stroke patients: a pilot controlled study. Int. J. Environ. Res. Public Health 20 (2), 1108. doi:10.3390/ijerph20021108
Lee, B. K., Hyun, S. W., and Jung, Y. S. (2020a). Yuzu and hesperidin ameliorate blood-brain barrier disruption during hypoxia via antioxidant activity. Antioxidants 9 (9), 843. doi:10.3390/antiox9090843
Lee, J. J., Hsu, W. H., Yen, T. L., Chang, N. C., Luo, Y. J., Hsiao, G., et al. (2011). Traditional Chinese medicine, Xue-Fu-Zhu-Yu decoction, potentiates tissue plasminogen activator against thromboembolic stroke in rats. J. Ethnopharmacol. 134 (3), 824–830. doi:10.1016/j.jep.2011.01.033
Lee, S. S., Kim, C. J., Shin, M. S., and Lim, B. V. (2020b). Treadmill exercise ameliorates memory impairment through ERK-Akt-CREB-BDNF signaling pathway in cerebral ischemia gerbils. J. Exerc Rehabil. 16 (1), 49–57. doi:10.12965/jer.2040014.007
Lenoir dit, C. R., Verdun, S., Triquenot-Bagan, A., Tourny, C., and Coquart, J. (2024). Yoga in the rehabilitation of post-stroke sequelae: a non-inferiority randomized controlled trial. J. Integr. Complementary Med. 30 (6), 543–553. doi:10.1089/jicm.2023.0315
Li, L., Zhu, W., Lin, G., Chen, C., Tang, D., Lin, S., et al. (2022). Effects of acupuncture in ischemic stroke rehabilitation: a randomized controlled trial. Front. Neurol. 13, 13. doi:10.3389/fneur.2022.897078
Li, M. Z., Zhang, Y., Zou, H. Y., Ouyang, J. Y., Zhan, Y., Yang, L., et al. (2018). Investigation of Ginkgo biloba extract (EGb 761) promotes neurovascular restoration and axonal remodeling after embolic stroke in rat using magnetic resonance imaging and histopathological analysis. Biomed. & Pharmacother. 103, 989–1001. doi:10.1016/j.biopha.2018.04.125
Li, Y., Wu, J., Du, F., Tang, T., Lim, J. C. W., Karuppiah, T., et al. (2024). Neuroprotective potential of glycyrrhizic acid in ischemic stroke: mechanisms and therapeutic prospects. Pharmaceuticals 17 (11), 1493. doi:10.3390/ph17111493
Li, Y., Zhong, W., Jiang, Z., and Tang, X. (2019). New progress in the approaches for blood–brain barrier protection in acute ischemic stroke. Brain Res. Bull. 144, 46–57. doi:10.1016/j.brainresbull.2018.11.006
Lieber, A. C., Hong, E., Putrino, D., Nistal, D. A., Pan, J. S., and Kellner, C. P. (2018). Nutrition, energy expenditure, dysphagia, and self-efficacy in stroke rehabilitation: a review of the literature. Brain Sci. 8 (12), 218. doi:10.3390/brainsci8120218
Lim, A. R., and Kim, H. K. (2024). The effects of aromatherapy on stroke symptoms in stroke patients: a systematic review and meta-analysis. Korean J. Adult Nurs. 36 (2), 85. doi:10.7475/kjan.2024.36.2.85
Liu, J., Guo, Z. N., Yan, X. L., Huang, S., Ren, J. X., Luo, Y., et al. (2020). Crosstalk between autophagy and ferroptosis and its putative role in ischemic stroke. Front. Cell. Neurosci. 14, 577403. doi:10.3389/fncel.2020.577403
Liu, L., Anderson, G. A., Fernandez, T. G., and Doré, S. (2019). Efficacy and mechanism of Panax ginseng in experimental stroke. Front. Neurosci. 13, 13. doi:10.3389/fnins.2019.00294
Liu, S., Lin, F., Wang, J., Pan, X., Sun, L., and Wu, W. (2022a). Polyphenols for the treatment of ischemic stroke: new applications and insights. Molecules 27, 4181. doi:10.3390/molecules27134181
Liu, W., Zhou, X., Zeng, K., Nie, C., Huang, J., Zhu, L., et al. (2023). Study on the action mechanism of Buyang Huanwu Decoction against ischemic stroke based on S1P/S1PR1/PI3K/Akt signaling pathway. J. Ethnopharmacol. 312, 116471. doi:10.1016/j.jep.2023.116471
Liu, Z., Zhou, Z., Ai, P., Zhang, C., Chen, J., and Wang, Y. (2022b). Astragaloside IV attenuates ferroptosis after subarachnoid hemorrhage via Nrf2/HO-1 signaling pathway. Front. Pharmacol. 13, 13. doi:10.3389/fphar.2022.924826
Lopez, M. S., Dempsey, R. J., and Vemuganti, R. (2015). Resveratrol neuroprotection in stroke and traumatic CNS injury. Neurochem. Int. 89, 75–82. doi:10.1016/j.neuint.2015.08.009
Lu, W., Chen, Z., and Wen, J. (2024). Flavonoids and ischemic stroke-induced neuroinflammation: focus on the glial cells. Biomed. Pharmacother. 170. doi:10.1016/j.biopha.2023.115847
Mao, X. Y., Yin, X. X., Guan, Q. W., Xia, Q. X., Yang, N., Zhou, H. H., et al. (2021). Dietary nutrition for neurological disease therapy: current status and future directions. Pharmacol. Ther. 226, 107861. doi:10.1016/j.pharmthera.2021.107861
Maqbool, M., and Zehravi, M. (2021). Neuroprotective role of polyphenols in treatment of neurological disorders: a review. Interventional Pain Med. Neuromodulation 1 (1). doi:10.5812/ipmn.117170
McFarlane, S. I., Sica, D. A., and Sowers, J. R. (2005). Stroke in patients with diabetes and hypertension. J. Clin. Hypertens. 7 (5), 286–292. doi:10.1111/j.1524-6175.2005.04379.x
Mirończuk, A., Kapica-Topczewska, K., Socha, K., Soroczyńska, J., Jamiołkowski, J., Kułakowska, A., et al. (2021). Selenium, copper, zinc concentrations and Cu/Zn, Cu/Se molar ratios in the serum of patients with acute ischemic stroke in northeastern Poland—a new insight into stroke pathophysiology. Nutrients 13 (7), 2139. doi:10.3390/nu13072139
Mo, Y., Sun, Y. Y., and Liu, K. Y. (2020). Autophagy and inflammation in ischemic stroke. Neural Regen. Res. 15, 1388–1396. doi:10.4103/1673-5374.274331
Mu, J. D., Ma, L. X., Zhang, Z., Qian, X., Zhang, Q. Y., Ma, L. H., et al. (2023). The factors affecting neurogenesis after stroke and the role of acupuncture. Front. Neurol. 14, 1082625. doi:10.3389/fneur.2023.1082625
Ni, X., Lin, H., Li, H., Liao, W., Luo, X., Wu, D., et al. (2020). Evidence-based practice guideline on integrative medicine for stroke 2019. J. Evid. Based Med. 13 (2), 137–152. doi:10.1111/jebm.12386
Owjfard, M., Rahimian, Z., Karimi, F., Borhani-Haghighi, A., and Mallahzadeh, A. (2024). A comprehensive review on the neuroprotective potential of resveratrol in ischemic stroke. Heliyon 10, e34121. doi:10.1016/j.heliyon.2024.e34121
Pacifici, F., Rovella, V., Pastore, D., Bellia, A., Abete, P., Donadel, G., et al. (2021). Polyphenols and ischemic stroke: insight into one of the best strategies for prevention and treatment. Nutrients 13, 1967. doi:10.3390/nu13061967
Pal, R., Adhikari, D., Heyat, M. B., Ullah, I., and You, Z. (2023). Yoga meets intelligent internet of things: recent challenges and future directions. Bioengineering 10 (4), 459. doi:10.3390/bioengineering10040459
Pawluk, H., Woźniak, A., Grześk, G., Kołodziejska, R., Kozakiewicz, M., Kopkowska, E., et al. (2020). The role of selected pro-inflammatory cytokines in pathogenesis of ischemic stroke. Clin. Interventions Aging 15, 469–484. doi:10.2147/CIA.S233909
Pogrebnoy, D., and Dennett, A. (2020). Exercise programs delivered according to guidelines improve mobility in people with stroke: a systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 101 (1), 154–165. doi:10.1016/j.apmr.2019.06.015
Poljsak, B. (2011). Strategies for reducing or preventing the generation of oxidative stress. Oxid. Med. Cell. Longev. 2011, 1–15. doi:10.1155/2011/194586
Qin, C., Yang, S., Chu, Y. H., Zhang, H., Pang, X. W., Chen, L., et al. (2022a). Signaling pathways involved in ischemic stroke: molecular mechanisms and therapeutic interventions. Signal Transduct. Target Ther. 7 (1), 215. doi:10.1038/s41392-022-01064-1
Qin, S., Zhang, Z., Zhao, Y., Liu, J., Qiu, J., Gong, Y., et al. (2022b). The impact of acupuncture on neuroplasticity after ischemic stroke: a literature review and perspectives. Front. Cell. Neurosci. 16, 817732. doi:10.3389/fncel.2022.817732
Qu, Y., Wang, L., and Mao, Y. (2022). Gallic acid attenuates cerebral ischemia/re-perfusion-induced blood–brain barrier injury by modifying polarization of microglia. J. Immunotoxicol. 19 (1), 17–26. doi:10.1080/1547691X.2022.2043494
Rajahthurai, S. D., Farrukh, M. J., Makmor-Bakry, M., Tan, H. J., Fatokun, O., Mohd, S. S., et al. (2022). Use of complementary and alternative medicine and adherence to medication therapy among stroke patients: a meta-analysis and systematic review. Front. Pharmacol. 13, 870641. doi:10.3389/fphar.2022.870641
Ranjan, K. N. (2023). Herbal drugs for neuroprotection in ischemic stroke. Certif. J. │ Kar World J. Pharm. Res 12. doi:10.20959/wjpr202314-29334
Reis, D., and Jones, T. (2017). Aromatherapy: using essential oils as a supportive therapy. Clin. J. Oncol. Nurs. 21 (1), 16–19. doi:10.1188/17.CJON.16-19
Reverse Electron Transport at Mitochondrial (2024). Reverse electron transport at mitochondrial complex I in ischemic stroke. Aging Age-Related Dis.
Saceleanu, V. M., Toader, C., Ples, H., Covache-Busuioc, R. A., Costin, H. P., Bratu, B. G., et al. (2023). Integrative approaches in acute ischemic stroke: from symptom recognition to future innovations. Biomedicines 11 (10), 2617. doi:10.3390/biomedicines11102617
Sakakima, H. (2019). Endogenous neuroprotective potential due to preconditioning exercise in stroke. Phys. Ther. Res. 22 (2), 45–52. doi:10.1298/ptr.R0006
Salehi, S. S., Lorigooini, Z., Jivad, N., and Ghadimi, K. (2019). Research paper: effect of aromatherapy with lavender 10% essential oil on motor function, speech and delirium in patients with acute thrombotic cerebral ischemia. Casp. J. Neurol. Sci. 5 (2), 49–55. doi:10.29252/CJNS.5.17.49
Salvagno, M., Sterchele, E. D., Zaccarelli, M., Mrakic-Sposta, S., Welsby, I. J., Balestra, C., et al. (2024). Oxidative stress and cerebral vascular tone: the role of reactive oxygen and nitrogen species. Int. J. Mol. Sci. 25 (5), 3007. doi:10.3390/ijms25053007
Sankaran, R., Kamath, R., Nambiar, V., and Kumar, A. (2019). A prospective study on the effects of Ayurvedic massage in post-stroke patients. J. Ayurveda Integr. Med. 10 (2), 126–130. doi:10.1016/j.jaim.2018.02.137
Schmid, A. A., Miller, K. K., Van Puymbroeck, M., and DeBaun-Sprague, E. (2014). Yoga leads to multiple physical improvements after stroke, a pilot study. Complement. Ther. Med. 22 (6), 994–1000. doi:10.1016/j.ctim.2014.09.005
Shah, Z. A., Nada, S. E., and Doré, S. (2011). Heme oxygenase 1, beneficial role in permanent ischemic stroke and in Gingko biloba (EGb 761) neuroprotection. Neuroscience 180, 248–255. doi:10.1016/j.neuroscience.2011.02.031
Shang, Y., Zhang, Z., Tian, J., and Li, X. (2022). Anti-inflammatory effects of natural products on cerebral ischemia. Front. Pharmacol. 13, 13. doi:10.3389/fphar.2022.914630
She, R., Liu, D., Liao, J., Wang, G., Ge, J., and Mei, Z. (2023). Mitochondrial dysfunctions induce PANoptosis and ferroptosis in cerebral ischemia/reperfusion injury: from pathology to therapeutic potential. Front. Cell. Neurosci. 17, 1191629. doi:10.3389/fncel.2023.1191629
Shu, J., Yang, L., Wei, W., and Zhang, L. (2022). Identification of programmed cell death-related gene signature and associated regulatory axis in cerebral ischemia/reperfusion injury. Front. Genet. 13, 13. doi:10.3389/fgene.2022.934154
Siotto, M., Germanotta, M., Santoro, M., Canali, R., Pascali, S., Insalaco, S., et al. (2022). Oxidative stress status in post stroke patients: sex differences. Healthcare 10 (5), 869. doi:10.3390/healthcare10050869
Spoelstra-de Man, A. M. E., Elbers, P. W. G., and Oudemans-van Straaten, H. M. (2018). Making sense of early high-dose intravenous vitamin C in ischemia/reperfusion injury. Crit. Care 22 (1), 70. doi:10.1186/s13054-018-1996-y
Sun, Y., and Zhu, C. (2023). Potential role of PANoptosis in neuronal cell death: commentary on “PANoptosis-like cell death in ischemia/reperfusion injury of retinal neurons.”. Neural Regen. Res. 18 (2), 339–340. doi:10.4103/1673-5374.346483
Sun, Y. Y., Zhu, H. J., Zhao, R. Y., Zhou, S. Y., Wang, M. Q., Yang, Y., et al. (2023). Remote ischemic conditioning attenuates oxidative stress and inflammation via the Nrf2/HO-1 pathway in MCAO mice. Redox Biol. 66, 102852. doi:10.1016/j.redox.2023.102852
Tang, X., Liu, H., Xiao, Y., Wu, L., and Shu, P. (2022). Vitamin C intake and ischemic stroke. Front. Nutr. 9, 935991. doi:10.3389/fnut.2022.935991
Thayabaranathan, T., Immink, M. A., Stevens, P., Hillier, S., Thrift, A. G., Brodtmann, A., et al. (2018). Understanding the potential for yoga and tai chi interventions to moderate risk factors for stroke – a scoping review. Future Neurol. 13 (4), 239–252. doi:10.2217/fnl-2018-0005
Tian, H. Y., Lei, Y. X., Zhou, J. T., Liu, L. J., Yang, T., Zhou, Y., et al. (2025). Insight into interplay between PANoptosis and autophagy: novel therapeutics in ischemic stroke. Front. Mol. Neurosci. 17, 17. doi:10.3389/fnmol.2024.1482015
Tong, X., Wang, Y., Fiehler, J., Bauer, C. T., Jia, B., Zhang, X., et al. (2021). Thrombectomy versus combined thrombolysis and thrombectomy in patients with acute stroke: a matched-control study. Stroke 52 (5), 1589–1600. doi:10.1161/STROKEAHA.120.031599
Tong, Y., Forreider, B., Sun, X., Geng, X., Zhang, W., Du, H., et al. (2015). Music-supported therapy (MST) in improving post-stroke patients’ upper-limb motor function: a randomised controlled pilot study. Neurol. Res. 37 (5), 434–440. doi:10.1179/1743132815Y.0000000034
Ueno, Y., Miyamoto, N., Yamashiro, K., Tanaka, R., and Hattori, N. (2019). Omega-3 polyunsaturated fatty acids and stroke burden. Int. J. Mol. Sci. 20 (22), 5549. doi:10.3390/ijms20225549
Wang, H., Liu, C., Zhao, Y., and Gao, G. (2020). Mitochondria regulation in ferroptosis. Eur. J. Cell. Biol. 99 (1), 151058. doi:10.1016/j.ejcb.2019.151058
Wang, J., Hu, J., Chen, X., Lei, X., Feng, H., Wan, F., et al. (2021). Traditional Chinese medicine monomers: novel strategy for endogenous neural stem cells activation after stroke. Front. Cell. Neurosci. 15, 628115. doi:10.3389/fncel.2021.628115
Wang, J., and Xiong, X. (2012). Current situation and perspectives of clinical study in integrative medicine in China. Evidence-Based Complementary Altern. Med. 2012, 268542–268611. doi:10.1155/2012/268542
Wang, T., Duan, S., Wang, H., Sun, S., Han, B., and Fu, F. (2016). Neurological function following cerebral ischemia/reperfusion is improved by the Ruyi Zhenbao pill in a rats. Biomed. Rep. 4 (2), 161–166. doi:10.3892/br.2016.568
Wessels, I., Maywald, M., and Rink, L. (2017). Zinc as a gatekeeper of immune function. Nutrients 9 (12), 1286. doi:10.3390/nu9121286
Wierońska, J. M., Cieślik, P., and Kalinowski, L. (2021). Nitric oxide-dependent pathways as critical factors in the consequences and recovery after brain ischemic hypoxia. Biomolecules 11 (8), 1097. doi:10.3390/biom11081097
Wu, L., Song, H., Zhang, C., Wang, A., Zhang, B., Xiong, C., et al. (2023). Efficacy and safety of Panax notoginseng saponins in the treatment of adults with ischemic stroke in China. JAMA Netw. Open 6 (6), e2317574. doi:10.1001/jamanetworkopen.2023.17574
Xie, C., Gu, A., Cai, J., Wu, Y., and Chen, R. (2018). Curcumin protects neural cells against ischemic injury in N2a cells and mouse brain with ischemic stroke. Brain Behav. 8 (2), e00921. doi:10.1002/brb3.921
Xing, Y., Yang, S. D., Dong, F., Wang, M. M., Feng, Y. S., and Zhang, F. (2018). The beneficial role of early exercise training following stroke and possible mechanisms. Life Sci. 198, 32–37. doi:10.1016/j.lfs.2018.02.018
Xu, C., He, Z., Shen, Z., and Huang, F. (2022). Potential Benefits of Music Therapy on Stroke Rehabilitation. Oxid Med Cell Longev. 9386095. doi:10.1155/2022/9386095
Xu, J. H., Huang, Y. M., Ling, W., Li, Y., Wang, M., Chen, X. Y., et al. (2015). Wen Dan Decoction for hemorrhagic stroke and ischemic stroke. Complement. Ther. Med. 23 (2), 298–308. doi:10.1016/j.ctim.2015.01.001
Xu, M., Wu, R. X., Li, X. L., Zeng, Y. S., Liang, J. Y., Fu, K., et al. (2022). Traditional medicine in China for ischemic stroke: bioactive components, pharmacology, and mechanisms. J. Integr. Neurosci. 21 (1), 26. doi:10.31083/j.jin2101026
Xu, S., Lu, J., Shao, A., Zhang, J. H., and Zhang, J. (2020). Glial cells: role of the immune response in ischemic stroke. Front. Immunol. 11, 294. doi:10.3389/fimmu.2020.00294
Xu, Y., Li, K., Zhao, Y., Zhou, L., Liu, Y., and Zhao, J. (2023). Role of ferroptosis in stroke. Cell. Mol. Neurobiol. 43 (1), 205–222. doi:10.1007/s10571-022-01196-6
Yan-shu, P., Yao, W., Bing, W., and Peng-tao, L. (2008). Effects of qingkailing injection on transforming growth factor-β1 expression in cerebral ischemia-reperfusion injury in rats. World Sci. Technol. 10 (5), 29–32. doi:10.1016/s1876-3553(09)60023-5
Younis, N., and Mohamed, M. (2020). Sandalwood oil neuroprotective effects on middle cerebral artery occlusion model of ischemic brain stroke. Pharmacogn. Mag. 16 (68), 117. doi:10.4103/pm.pm_398_19
Yu, C. C., Liu, L., Chen, S. Y., Wang, X. F., Wang, L., and Du, Y. J. (2022). Ancient Chinese herbal recipe huanglian jie du decoction for ischemic stroke: an overview of current evidence. Aging Dis. 13 (6), 1733–1744. doi:10.14336/AD.2022.0311
Yu, M., Sun, Z. J., Li, L. T., Ge, H. Y., Song, C. Q., and Wang, A. J. (2015). The beneficial effects of the herbal medicine Di-huang-yin-zi (DHYZ) on patients with ischemic stroke: a Randomized, Placebo controlled clinical study. Complement. Ther. Med. 23 (4), 591–597. doi:10.1016/j.ctim.2015.06.003
Zahiruddin, S., Basist, P., Parveen, A., Parveen, R., Khan, W., et al. (2020). Ashwagandha in brain disorders: a review of recent developments. J. Ethnopharmacol. 257, 112876. doi:10.1016/j.jep.2020.112876
Zhang, H., Jin, B., You, X., Yi, P., Guo, H., Niu, L., et al. (2023). Pharmacodynamic advantages and characteristics of traditional Chinese medicine in prevention and treatment of ischemic stroke. Chin. Herb. Med. 15 (4), 496–508. doi:10.1016/j.chmed.2023.09.003
Zhang, H., Lee, J. Y., Borlongan, C., and Tajiri, N. (2019). A brief physical activity protects against ischemic stroke. Brain Circ. 5 (3), 112–118. doi:10.4103/bc.bc_32_19
Zhang, L., Ma, J., Yang, F., Li, S., Ma, W., Chang, X., et al. (2022). Neuroprotective effects of quercetin on ischemic stroke: a literature review. Front. Pharmacol. 13, 854249. doi:10.3389/fphar.2022.854249
Zhang, S., Wu, B., Liu, M., Li, N., Zeng, X., Liu, H., et al. (2015). Acupuncture efficacy on ischemic stroke recovery: multicenter randomized controlled trial in China. Stroke 46 (5), 1301–1306. doi:10.1161/STROKEAHA.114.007659
Zhang, W. W., Xu, F., Wang, D., Ye, J., and Cai, S. Q. (2018). Buyang Huanwu Decoction ameliorates ischemic stroke by modulating multiple targets with multiple components: in vitro evidences. Chin. J. Nat. Med. 16 (3), 194–202. doi:10.1016/S1875-5364(18)30047-5
Zhao, H., Li, Y., Zhang, Y., He, W. Y., and Jin, W. N. (2022). Role of immune and inflammatory mechanisms in stroke: a review of current advances. Neuroimmunomodulation 29 (4), 255–268. doi:10.1159/000524951
Zhao, L., Li, H., Gao, Q., Xu, J., Zhu, Y., Zhai, M., et al. (2021b). Berberine attenuates cerebral ischemia-reperfusion injury induced neuronal apoptosis by down-regulating the CNPY2 signaling pathway. Front. Pharmacol. 12, 12. doi:10.3389/fphar.2021.609693
Zhao, S., Zheng, H., Du, Y., Zhang, R., Chen, P., Ren, R., et al. (2021a). The clinical efficacy of Ginkgo biloba leaf preparation on ischemic stroke: a systematic review and meta-analysis. Evid. Based. Complement. Altern. Med. 2021, 4265219. doi:10.1155/2021/4265219
Zheng, L., Meng, L., Liang, H., and Yang, J. (2023). Sanhua decoction: current understanding of a traditional herbal recipe for stroke. Front. Neurosci. 17, 1149833. doi:10.3389/fnins.2023.1149833
Zhong, D. Y., Cheng, H., Luo, H. S., Li, Q., Li, L., Li, H. J., et al. (2023). Study on mechanisms and molecular verification of Buyang Huanwu decoction in treating ischemic stroke from the perspective of cuproptosis. TMR Integr. Med. 7 (0), e23013. doi:10.53388/tmrim202307013
Zhou, J., Sun, F., Zhang, W., Feng, Z., Yang, Y., and Mei, Z. (2024). Novel insight into the therapeutical potential of flavonoids from traditional Chinese medicine against cerebral ischemia/reperfusion injury. Front. Pharmacol. 15, 1352760. Frontiers in Pharmacology. Frontiers Media SA. doi:10.3389/fphar.2024.1352760
Zhou, Y., Zhang, S., and Fan, X. (2021). Role of polyphenols as antioxidant supplementation in ischemic stroke. Oxid. Med. Cell. Longev. 2021, 5471347. doi:10.1155/2021/5471347
Zhu, B., Cao, H., Sun, L., Li, B., Guo, L., Duan, J., et al. (2018). Metabolomics-based mechanisms exploration of Huang-Lian Jie-Du decoction on cerebral ischemia via UPLC-Q-TOF/MS analysis on rat serum. J. Ethnopharmacol. 216, 147–156. doi:10.1016/j.jep.2018.01.015
Zhu, H., Hu, S., Li, Y., Sun, Y., Xiong, X., Hu, X., et al. (2022). Interleukins and ischemic stroke. Front. Immunol. 13. doi:10.3389/fimmu.2022.828447
Zhu, T., Wang, L., Feng, Y., Sun, G., and Sun, X. (2021). Classical active ingredients and extracts of Chinese herbal medicines: pharmacokinetics, pharmacodynamics, and molecular mechanisms for ischemic stroke. Oxid. Med. Cell. Longev. 2021, 8868941. doi:10.1155/2021/8868941
Zhu, W., Jia, Q., Ferreira, A. C., Jiang, H., Zhang, J., Li, B., et al. (2024). Acupuncture for ischemic stroke: where are we now? Acupunct. Herb. Med. 4, 36–55. doi:10.1097/hm9.0000000000000094
Keywords: ischemic stroke, traditional, complementary, and integrative medicine, neuroinflammation, oxidative stress, complementary alternative medicine
Citation: Sowmiya S, Begum RF, Dhivya LS, Rajendran P, Harikrishnan N and Singh S A (2025) Traditional, complementary, and integrative medicine in the management of ischemic stroke: a narrative review. Front. Pharmacol. 16:1561688. doi: 10.3389/fphar.2025.1561688
Received: 16 January 2025; Accepted: 12 May 2025;
Published: 30 May 2025.
Edited by:
Huazheng Liang, Monash University - Southeast University Joint Research Institute (Suzhou), ChinaCopyright © 2025 Sowmiya, Begum, Dhivya, Rajendran, Harikrishnan and Singh S. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rukaiah Fatma Begum, cnVrYWlhaDc4NkBnbWFpbC5jb20=; Ankul Singh S, YW5rdWxzaW5naDU3QGdtYWlsLmNvbQ==
 S. Sowmiya1
S. Sowmiya1 Rukaiah Fatma Begum
Rukaiah Fatma Begum Ankul Singh S
Ankul Singh S