- 1Department of Pharmacology, Dr. M.G.R. Educational and Research Institute, Chennai, Tamil Nadu, India
- 2Department of Pharmaceutical Analysis, Dr. M.G.R. Educational and Research Institute, Chennai, Tamil Nadu, India
- 3Institute of Pharmaceutical Research, GLA University, Mathura, Uttar Pradesh, India
This review explores the potential of Traditional, Complementary, and Integrative Medicine (TCIM) as an adjunct to conventional therapies for Alzheimer’s Disease (AD). Unlike pharmaceutical treatments that primarily offer symptomatic relief, TCIM encompasses holistic approaches that target multiple pathophysiological pathways involved in AD, including tau pathology, oxidative stress, mitochondrial dysfunction, and neuroinflammation. Herbal therapies such as Withania somnifera, Ginkgo biloba, and Curcuma longa have shown promising neuroprotective effects in preclinical and limited clinical studies. Mind-body practices like Kirtan Kriya meditation have also demonstrated stress-reduction benefits, addressing modifiable risk factors for AD. While current evidence highlights the potential of TCIM interventions to complement standard care, rigorous validation through high-quality randomized controlled trials remains essential. This review underscores the need for integrative, personalized approaches that synergize traditional and modern medical systems to enhance therapeutic outcomes in AD.
1 Introduction
Globally, Alzheimer’s disease (AD) is the primary cause of dementia and cognitive loss in those over 65. Its frequency in this age group varies from 1.9 to 5.8 instances per 100 individuals, and rates rise significantly with age, especially in females (Atri, 2019) (Rocca et al., 1986). Alzheimer’s disease is caused by tau tangles, Aβ plaques, and neuroinflammation, all contributing to the disease (Kabra, 2022; Webers et al., 2020). Aβ aggregation influences tau hyperphosphorylation and microglial inflammation, linking these clinical hallmarks (Kabra, 2022). Pathophysiological changes that characterize this progressive neurodegenerative disease include amyloid-beta plaque buildup, hyperphosphorylated tau protein neurofibrillary tangles, and neurodegeneration due to inflammation and microglial activation (Jack et al., 2013; Li et al., 2022).
In its clinical form, AD first appears as cognitive decline and memory loss, which progresses to neuropsychiatric symptoms including mood swings, disorientation, and severe hallucinations (Harada et al., 2013). Traditional, Complementary, and Integrative Medicine (TCIM) may offer therapeutic alternatives for AD. Mind-body therapy and lifestyle treatments are examples of TCIM techniques that report the multidimensional aspect of AD and complement the limited pharmacological choices available (Nguyen et al., 2024). However, despite the increased interest in TCIM for AD, the number of existing studies and the quality of research are still quite limited. Most research is discredited due to inadequate procedures, such as the lack of strong statistical analysis or RCTs. For this reason, very intensive assessment and cross-disciplinary research must be conducted for TCIM methodologies to be validated and integrated with conventional treatments (Kong et al., 2023). Glutamate antagonists and cholinesterase inhibitors are two examples of current treatments for Alzheimer’s disease that target symptoms rather than the fundamental causes of the illness. This restriction emphasizes how urgently therapies that focus on the intricate pathophysiological pathways of AD are needed (Miculas et al., 2022). TCIM is emphasized in treating Alzheimer’s disease because it considers the human person as a totality-the environmental, behavioral, emotional, and physical factors all contribute to total wellness. More compelling arguments include the less-than-desirable effects of present-day pharmaceutical therapy, the harmful effects they often incur, and further factors. With the complicated nature of AD, this all-embracing approach makes TCIM a useful additional care and preventive approach (Nguyen et al., 2024). The incidence of AD is predicted to triple by 2050, impacting one in every 85 individuals, making it a rapidly expanding worldwide health concern (Brookmeyer et al., 2007). With direct medical expenses varying by country and disease severity, Alzheimer’s disease has a significant financial impact (Takizawa et al., 2014).
Although studies frequently have methodological issues, such as small sample sizes, a lack of controls, and inconsistent results, TCIM shows promise for treating AD. Another issue is publication bias, which favors favorable outcomes while keeping negative discoveries unreported. Extensive, multi-center RCTs are necessary to validate the safety and effectiveness of TCIM. While numerous reviews have explored the potential of Traditional, Complementary, and Integrative Medicine (TCIM) in Alzheimer’s disease (AD), the present manuscript addresses a critical gap by focusing on the multi-targeted and synergistic therapeutic mechanisms of TCIM interventions. Unlike monotherapeutic strategies that often target a single pathological hallmark of AD, TCIM modalities offer a unique advantage through their ability to modulate multiple pathways simultaneously, including oxidative stress, neuroinflammation, mitochondrial dysfunction, and cholinergic deficits. This review consolidates current evidence on the pleiotropic effects of TCIM interventions, offering a systems-level perspective that has not been comprehensively synthesized before. In doing so, we aim to provide a nuanced understanding of how TCIM-based multi-target strategies could complement conventional treatments and improve therapeutic outcomes.
2 Methodology
2.1 Search strategy
A comprehensive literature search was conducted across PubMed, Scopus, and Web of Science databases from inception to December 2024. The search strategy included combinations of keywords and MeSH terms such as “Alzheimer’s Disease,” “Traditional Medicine,” “Complementary and Integrative Medicine (TCIM),” “Herbal Therapies,” “Neuroinflammation,” “Cognitive Decline,” and “Botanical Drugs.” The Boolean operators “AND” and “OR” were used to refine and expand the search results.
2.2 Inclusion and exclusion criteria
To preserve the review’s quality and applicability, stringent inclusion and exclusion criteria were used. Clinical or preclinical results about neuroprotection, anti-inflammatory qualities, and cognitive function improvement were specifically highlighted in studies that examined the therapeutic potential of TCIM in Alzheimer’s disease. Additionally, articles that addressed how TCIM addresses fundamental pathogenic pathways, including amyloid-beta pathology and oxidative stress, were given priority.
On the other hand, research that was unrelated to Alzheimer’s disease or published in a language other than English was disqualified. Furthermore, papers that were only abstracts, editorials, or comments, or those that were not available in full text, were not included. This guaranteed that only comprehensive, peer-reviewed research publications were included in the study.
3 Pathogenic pathways in Alzheimer's disease
Alzheimer’s disease involves a complex interplay of multiple pathological mechanisms including amyloid-beta (Aβ) accumulation, tau protein hyperphosphorylation, oxidative stress, mitochondrial dysfunction, neuroinflammation, and cholinergic deficits. These interconnected pathways contribute to progressive synaptic failure and cognitive decline. A schematic overview of these multifactorial pathogenic mechanisms is presented in Figure 1.
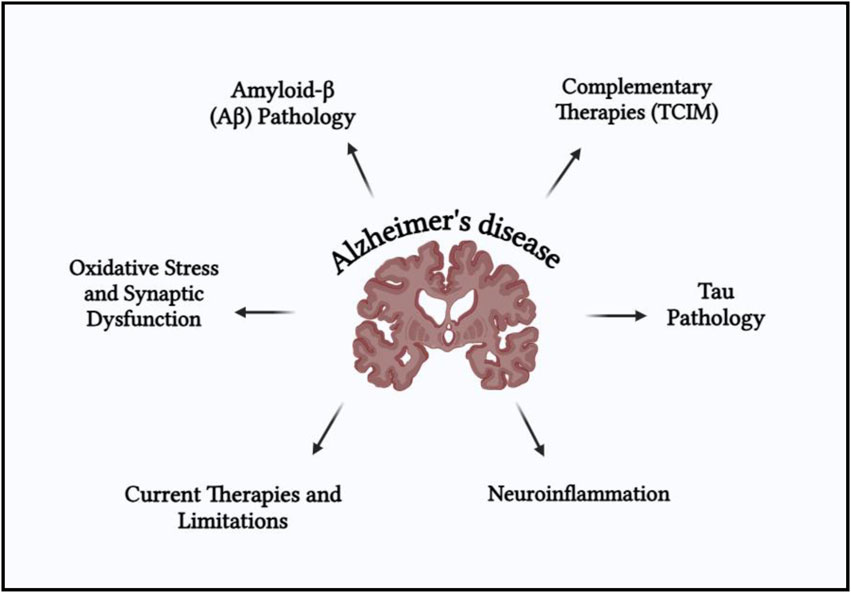
Figure 1. Schematic representation of the multifactorial pathogenesis of Alzheimer’s disease (AD), highlighting key neurobiological mechanisms.
4 TCIM approaches for AD
4.1 Foundations and therapeutic principles of TCIM
TCIM, which refers to traditional, complementary, and integrative medicine, is are health practice not very commonly applied by mainstream medicine, but rather serves to supplement the conventional ways of doing things. WHO describes the collective knowledge, skills, and practices acquired from culture and used for the discovery, treatment, and prevention of illness and health-related problems as traditional medicine (Ng et al., 2024). According to the WHO, complementary medicine refers to medical procedures that are utilized in addition to traditional medicine but are not entirely included in the prevailing healthcare system (Ventola, 2010). The term “integrative health,” which refers to the systematic blending of complementary and conventional treatments for patient-centered care, is further expanded by the National Center for Complementary and Integrative Health (NCCIH) (Adib-Hajbaghery et al., 2021). By combining conventional medical procedures with complementary therapies including acupuncture, botanical drugs (e.g., phytochemicals with known antioxidant or anti-inflammatory effects), and to a lesser extent, practices like yoga or meditation that may influence neuroendocrine or oxidative stress pathways. Activities like Kirtan Kriya, a meditation technique that incorporates hand gestures, chanting, and concentrated attention, have demonstrated the potential to lower stress, which is an acknowledged risk factor for AD (Ashford et al., 2015). New studies further reveal that TCIM can be used for the treatment of biological factors involved in AD, such as inflammation and insulin resistance. TCIM thus provides a hopeful scope for management and prevention, yet its validity, safety, and assimilation into conventional practice have to be proved by deeper exploration (Phutrakool and Pongpirul, 2022).
4.2 Botanical drugs and phytochemicals
4.2.1 Botanical drugs overview
The three oldest and most vigorous active medical practices are traditional Chinese medicine, traditional Indian medicine, and Ayurveda. The two traditions have sound empirical, experimental, and philosophical evidence. Alternative and complementary medicine have regained popularity for many reasons, such as growing side effects, microbial resistance, emergent diseases, new medications’ high costs, and no efficient treatments for chronic diseases for which no cure is known (Schaffner, 2001). Over 1,500 botanical drugs sold as supplements were among the alternative medicines that two-thirds of Americans were predicted to utilize by 2010 (Legal Status of Traditional Medicine, 2001). With projects in China, Singapore, and Europe, pharmaceutical companies worldwide concentrate on natural product medication development, effectively globalizing Traditional Chinese Medicine (TCM). India’s Ayurveda is also becoming more popular worldwide thanks to government initiatives. According to the Western scientific community, despite their increasing popularity, traditional medicines need scientific confirmation and high-quality research to gain worldwide recognition (Patwardhan and Vaidya, 2014; Patwardhan et al., 2005). In vitro models, which lack the intricacy of genuine AD pathophysiology, are used in many TCIM investigations. Although botanical drugs may exhibit anti-inflammatory properties in computational models or cell cultures, their practical effectiveness is still unknown in the absence of in vivo validation or clinical studies. Human trials with appropriate controls and well-planned animal experiments should be given top priority in future research.
4.2.2 Specific botanical drugs
Although they are becoming ever more popular, most herbal treatments still do not have concrete scientific evidence. Traditional use oftentimes relies upon empirical use or centuries of experience based on anecdotes, while Western biologic systems require evidence from systematic clinical experience and RCTs. As an example:
Although it is used extensively to enhance memory, in AD research, ginkgo biloba had contradictory effects (Xie et al., 2022). There has been little or uncontrolled investigation of the potential cognitive benefit of Bacopa monnieri, a leading Ayurvedic herb [Bacopa_monnieri_UPDATE_(supplements)]. While curcumin, the active ingredient of turmeric, possesses anti-inflammatory and antioxidant activity, its clinical bioavailability remains challenging (Lopresti, 2018). The disconnect between ancient claims and modern pharmaceutical standards is illustrated by these scenarios, where safety, dose control, repeatability, and bioavailability must be taken into careful consideration (Barnes, 2003).
4.2.3 Mechanisms of action
Several herbal compounds demonstrate interesting biological activity in early-stage investigations, yet much of the data is from in vitro studies that fail to convey the sophistication of AD pathology. Many of the studies of TCIM are based on cell models that greatly simplify processes such as amyloid deposition, oxidative stress, and neuroinflammation (shortt et al., 2017).
For instance, it is known to be observed that Icariin inhibits tau phosphorylation and Aβ deposition by modulating the PI3K/Akt/GSK-3β pathway (Wu et al., 2024; Yan et al., 2023).
Ashwagandha and Centella asiatica are said to possess anti-inflammatory activity through modulation of cytokines like TNF-α and IL-6, according to studies (Weerasinghe, 2024).
Acetylcholinesterase-inhibiting and able to cross the blood-brain barrier, huperzine A, which is extracted from Chinese club moss, has, in some experiments, been found to bring about symptomatic relief equivalent to that of existing medication, though with fewer side effects (Zhang, 2012).
It is important to validate these pathways using human trials and properly designed in vitro animal models. The transition from bench to clinical use has to be bridged in future research to allow for the safe and effective incorporation of candidate herbal therapies into AD therapy.
4.3 The role of traditional medicine in Alzheimer’s disease
Although AD is becoming increasingly prevalent across the world and much research is conducted to find a cure, a long-term remedy has yet to be seen. Concerning the devastating loss of cognitive function that patients afflicted with AD experience, powerful therapeutic and preventive treatments are desperately needed to neutralize it. This is very timely as traditional medicine has brought out several promising remedies and drugs for neurodegenerative disorders (Cooper and Ma, 2017). TCM has been practiced for over 2,000 years. TCM or modern pharmacological theories have recently served as the foundation for Chinese botanical drugs used to treat AD; this method has discovered an impact on the causes and mechanisms of AD, TCM treatment, and natural extracts that effectively treat AD. Additionally, there is proof that Chinese botanical drugs may offer some additional cognitive advantages for single-target antagonists (Liu et al., 2014). Tetradium ruticarpum (A.Juss.) T.G. Hartley—Family: Rutaceae Bentham is one neuroprotective plant that is frequently used in Traditional Chinese Medicine (TCM) as a herbal remedy. Evodiamine, an extract of E. rutaecarpa Bentham, has a wide range of advantageous qualities, including anti-inflammatory and anti-AD roles, as well as antiobesity, antinociceptive, anticancer, antinomic, and antimetastatic effects, all of which are extremely beneficial for treating neurodegenerative diseases (Liu et al., 2013). Additionally, TCM has provided us with substances that need to be improved or substituted for the current AD. Targetin, an agonist of the retinoid X receptor (RXR), effectively treats AD in mouse models. Strong contenders for RXR agonists include the TCM substances sulfonic acid and β-lipoic acid. These TCM chemicals show promise for becoming anti-dementia medications by forming strong interactions via the RXR protein receptors (Chen et al., 2014). The citrus flavonoid nobiletin, which is used in Kampo, or Traditional Japanese Medicine, may be a natural anti-dementia treatment, according to research. Despite these results, the majority of the data supporting Kampo and TCM’s cognitive advantages comes from preclinical or small-scale research. These claims are limited in their robustness by methodological errors, a dearth of placebo-controlled studies, and variations in herbal composition. It has been demonstrated to improve memory and learning, reduce oxidative stress, combat neurodegeneration, encourage synaptic plasticity, and stop plaque development. In a similar vein, ninjin’yoeito, another Kampo drug, has been investigated for enhancing mood and cognitive abilities in Alzheimer’s patients. Depression was decreased, and cognitive function was enhanced when both therapies were combined (Cooper and Ma, 2017). Centella asiatica (L.) Urb. — Family: Apiaceae, a plant used as a brain tonic, has been shown to have neuroprotective benefits such as decreasing oxidative stress, inhibiting enzymes, and stopping amyloid plaque formation in AD. Ayurveda, a traditional Indian medicine, has been discovered to include valuable substances for treating problems with the nervous system, such as memory-related conditions like dementia (Nishteswar et al., 2014). Key pathogenic processes in Alzheimer’s disease, such as Aβ aggregation and enzymes involved in the generation of Aβ peptide, can be inhibited by bioactive metabolites found in Traditional Chinese Medicine (TCM). This realization emphasizes the necessity of further research into TCM-derived plant metabolites to identify new mechanisms and synergistic pathways that conventional treatments may influence. Certain TCM elements can reduce inflammation and neuronal cytotoxicity brought on by Aβ. The review raises the idea of using TCM to treat AD and the opportunity for further TCM ingredient screening for therapeutic uses (Cheung et al., 2015). AD may benefit from the antioxidant and anti-apoptotic properties of several herbal medications and phytochemicals. Herbal medications and substances have been shown in preclinical investigations in animal models of AD to have antioxidant properties, enhance cognition, and offer neuroprotection. However, drug-herb interactions, dosage consistency, and long-term effectiveness must all be carefully considered when combining conventional therapy with contemporary pharmaceutical treatments. Guidelines based on evidence would aid in bridging the gap between conventional medicine. According to traditional wisdom, herbal treatments may be able to address the pathophysiology of AD at several molecular and cellular levels (Wadhawan et al., 2024). Medicinal plants, such as Tinospora cordifolia (Willd.) Miers—Family: Menispermaceae, Hericium erinaceus (Bull.) Pers. — Family: Hericiaceae, Commiphora wightii (Arn.) Bhandari—Family: Burseraceae, Hypericum perforatum L. — Family: Hypericaceae, Convolvulus pluricaulis Choisy—Family: Convolvulaceae, Rhodiola rosea L. — Family: Crassulaceae, Moringa oleifera Lam. — Family: Moringaceae, and Camellia sinensis (L) Kuntze—Family: Theaceae are a few examples of medicinal plants that have both preventative and therapeutic effects on AD. Information from observational research is scarce (Perry et al., 1999; Sutalangka et al., 2013; Zieneldien et al., 2022). Although Randomized controlled trials and well-designed longitudinal investigations are required to determine the effectiveness, safety, and therapeutic range of traditional herbal treatments, to expand the body of evidence.
• Ginkgo biloba L. — Family: Ginkgoaceae, a well-studied herbal extract in the context of neurodegenerative diseases, has shown promising effects in various experimental models. In vitro studies reveal that it exerts antioxidant and neuroprotective effects by protecting neurons from oxidative stress and β-amyloid-induced cytotoxicity. In vivo findings in animal models of Alzheimer’s disease demonstrate improved cognitive performance, enhanced cerebral blood flow, and restored mitochondrial function. However, while some clinical trials have reported improved memory and cognitive scores, particularly with long-term administration, the overall quality of evidence remains mixed. Meta-analyses indicate heterogeneity in outcomes, underscoring the need for more robust, large-scale randomized controlled trials (RCTs) (Yang et al., 2015).
• Huperzine A, an alkaloid derived from Huperzia serrata (Thunb.) Trevis. — Family: Lycopodiaceae, has been identified as a potent reversible inhibitor of acetylcholinesterase (AChE). In vitro, it shows neuroprotective activity against glutamate-induced excitotoxicity and oxidative injury. In vivo studies report enhancements in memory retention, learning behavior, and synaptic density in rodent models of Alzheimer’s disease. In clinical settings, small-scale RCTs have demonstrated cognitive improvements in individuals with Alzheimer’s and vascular dementia, with favorable tolerability. Despite encouraging findings, caution is warranted when interpreting these results due to limitations in trial design and sample size (Rafii et al., 2011).
• Centella asiatica (L.) Urb. — Family: Apiaceae has long been used in traditional medicine for cognitive support. Its in vitro profile includes promotion of neurite outgrowth, enhanced collagen synthesis, and modulation of antioxidant enzymes. In in vivo models, it has been shown to improve spatial learning and memory, increase hippocampal dendritic arborization, and support synaptic plasticity. Preliminary clinical evidence supports its potential, particularly in elderly populations where improvements in mood and memory have been observed. Nonetheless, these results require validation through well-powered clinical studies (Wright et al., 2022).
• Curcuma longa L. — Family: Zingiberaceae, the principal curcuminoid of C. longa, exhibits a diverse range of bioactivities. In vitro, it demonstrates antioxidant, anti-inflammatory, and anti-amyloidogenic effects by modulating signaling pathways like NF-κB, Nrf2, and β-secretase activity. In vivo animal models show that C. longa L. — Family: Zingiberaceae can reduce amyloid plaque burden, promote neurogenesis, and enhance memory performance. However, clinical trials have yielded inconsistent outcomes, largely due to curcumin’s poor oral bioavailability. While some trials suggest modest cognitive benefits, further studies employing bioavailability-enhanced formulations are necessary (Kuszewski et al., 2018).
• Withania somnifera (L.) Dunal—Family: Solanaceae has demonstrated promising neuroprotective effects across multiple experimental levels. In vitro, it stimulates neurite outgrowth, suppresses oxidative stress, and downregulates inflammatory mediators. In vivo studies reveal its ability to reverse cognitive deficits in stress-induced and transgenic mouse models of Alzheimer’s disease, with noted improvements in learning and memory. Clinical investigations have also shown favorable results in improving attention, executive function, and information processing speed in both cognitively impaired and healthy adults. Despite its traditional use and growing scientific support, large-scale, blinded RCTs are still needed to substantiate its clinical efficacy in dementia care (Xing et al., 2022)
4.4 Complementary and integrative medicine
The National Center for Complementary and Integrative Health (NCCIH) classifies TCIM approaches into three categories (a) mind-body practices (e.g., yoga, meditation, acupuncture): that may exert effects through modulation of stress hormones like cortisol or neurotrophic factors such as BDNF; (b) traditional medical systems such as Ayurveda and Traditional Chinese Medicine (TCM), which offer pharmacologically active herbal agents; and (c) natural products, including botanical medicines, vitamins, and probiotics with potential neuroprotective or anti-inflammatory properties (Nguyen et al., 2022).
It has been shown that using these adjunct methods along with drug treatments results in synergistic outcomes. For example, in AD patients, donepezil and yoga or mindfulness-based stress reduction have been associated with improved mood, reduced anxiety, and enhanced cognitive functioning. Cholinesterase inhibitors also seem to facilitate neuroprotection and delay cognitive decline when used along with nutritional therapies like the Mediterranean diet. These combined strategies illustrate how an integrated, multimodal approach could enhance overall outcomes of Alzheimer’s disease treatment (García-Casares et al., 2021).
Although preclinical and clinical research have shown encouraging benefits, there is still conflicting evidence in favor of complementary and integrative therapies for AD. Thorough clinical studies with specified objectives are required to confirm their effectiveness and clarify the exact processes behind their possible advantages.
4.5 Modulation of the microbiota-gut-brain axis in Alzheimer’s disease through TCIM approaches
The microbiota-gut-brain axis includes bidirectional communication via neuroimmune, neuroendocrine, and direct neural routes, such as the vagus nerve (Peterson, 2020). The condition contributes to AD pathology by impairing host immunological responses and increasing inflammation, potentially acting as a trigger for the start and development of AD. TCM is a viable resource for treating Alzheimer’s disease because of its chemical diversity and multi-target properties. It modulates the gut microbiota, which is a key target for its ability to cure Alzheimer’s disease by modifying the microbiota-gut-brain axis (Ma et al., 2023).
Traditional Chinese medicine can prevent and treat Alzheimer’s disease by regulating the gut microbiota (Long et al., 2024).
Mechanism: Changes in the gut plants may contribute to the buildup of amyloid beta. The relationship between the gut and the brain, and metabolites produced by the gastrointestinal microbiome, is involved in Alzheimer’s disease pathogenesis.
Clinical trial evidence: Recent preclinical and clinical research suggests that gut microbiota changes may play a role in Alzheimer’s disease. Antibiotics, prebiotics, probiotics, fecal microbiota transplantation, and dietary changes are all possible treatments.
Emphasizes the efficacy of antibiotics, prebiotics, probiotics, feces microbe transplantation, and dietary changes as complementary therapy for Alzheimer’s disease, demonstrating their incorporation in mainstream pharmacological practice (Guo et al., 2020).
5 Mechanisms of action in TCIM
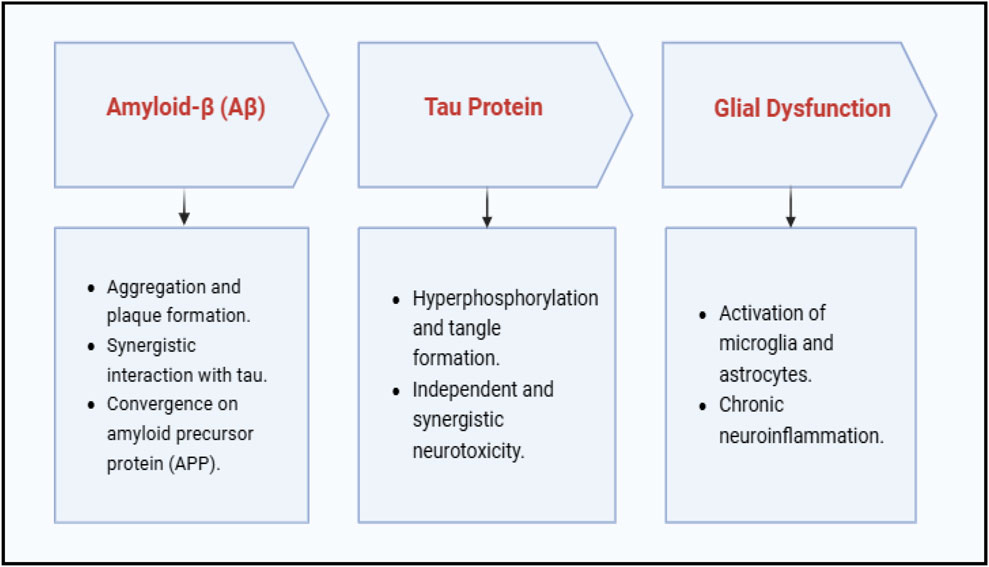
Amyloid β, tau, and glial dysfunction are the main contributors to the growth of AD (Han et al., 2020). Key Contributors and Therapeutic Pathways in AD are illustrated in Figure 2.
Curcuma longa L. — Family: Zingiberaceae has been explored as a treatment that influences pathways such as PI3K/Akt signaling and neurotrophin signaling (Bashir et al., 2022). TMS has been shown to promote synaptic plasticity, change gene expression, and boost cognitive function in AD patients (Zhao et al., 2019). In AD mouse models, trans-cinnamaldehyde (TCA) has also been demonstrated to have neuroprotective effects via improving NMDA receptor activity and decreasing neuroinflammation. TCA lowers pro-inflammatory mediator levels and microglial activation by blocking the NF-κB pathway (Prasad et al., 2002). AD is a neurodegenerative condition complicated by oxidative stress, neuroinflammation, and loss of cognitive functions (Papagiouvannis et al., 2021) (Murphy and Park, 2017). Studies indicate that treatment of AD is possibly more effective using the multi-target approach, such as including antioxidants, anti-inflammatory drugs, and neurotrophic factors, rather than single-modal therapy (Papagiouvannis et al., 2021) (Onaolapo et al., 2021).
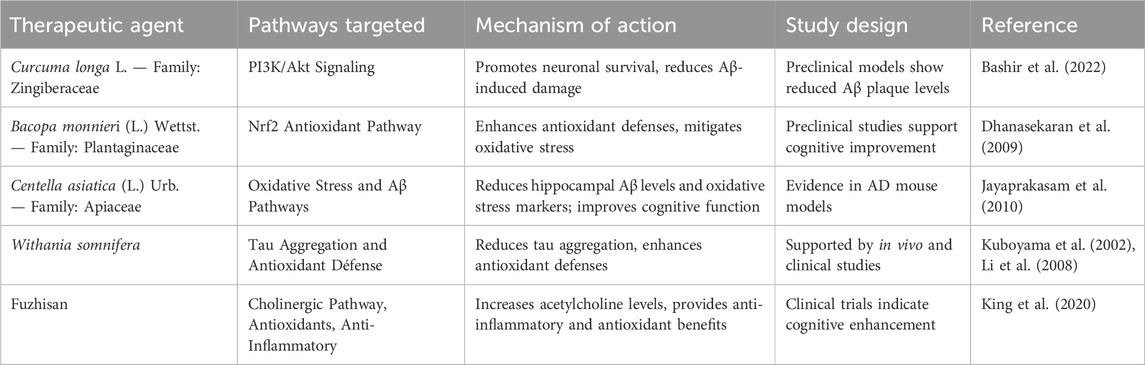
Activating the Nrf2-mediated antioxidant defense system and stimulating the neurotrophic signaling pathway can potentially change AD pathology (Onaolapo et al., 2021). Additionally, nutritional treatment and lifestyle modifications may be crucial in lowering the oxidative stress and neuroinflammation associated with Alzheimer’s. Though preclinical studies have shown that anti-inflammatory medicines are beneficial, this finding has yet to be confirmed in clinical investigations, necessitating additional studies into more viable treatment strategies (Ghosh et al., 2013). Tau immunotherapy can regulate both tau and upstream amyloid pathology, while persistent interleukin-1β overexpression worsens tau pathology despite reduced amyloid burden (Castillo-Carranza et al., 2015) (Haut et al., 2023). The “Amyloid Cascade Hypothesis,” which holds that tau and Aβ separately produce neurotoxicity, is called into question by these findings (Busche and Hyman, 2020) Research shows that tau and Aβ work in concert, convergently acting on the amyloid precursor protein (APP) as a common downstream effector (Busche and Hyman, 2020) (Wang C. et al., 2014). This emphasizes the necessity of concurrently focusing efforts on both (Wang C. et al., 2014). It has been shown in traditional medicine that C. longa, or C. longa L. — Family: Zingiberaceae, activates the PI3K/Akt pathway, increasing neuronal survival and lowering damage brought on by Aβ (Limpeanchob et al., 2008). Bacopa monnieri (L.) Wettst. — Family: Plantaginaceae also improves Nrf2 pathway activity, which reduces oxidative stress and increases cellular resilience (Dhanasekaran et al., 2009). According to recent research, Centella asiatica (L.) Urb. — Family: Apiaceae extracts considerably lower oxidative stress indicators and hippocampus Aβ levels in AD mouse models, confirming its function in protective effects on neurons and cognitive improvement (Jayaprakasam et al., 2010). Clinical investigations verifying these cognitive advantages are still few, despite preclinical research suggesting C. asiatica (L.) Urb. — Family: Apiaceae may have a neuroprotective function. Furthermore, in vivo and clinical studies have demonstrated that Withania somnifera reduces tau aggregation and enhances antioxidant defense (Kuboyama et al., 2002; Li et al., 2008). TCIM drugs like Fuzhisan that increase acetylcholine content with additional anti-inflammatory and antioxidant activities share the same aim of achieving and maintaining adequate acetylcholine levels that conventional therapy, including cholinesterase inhibitors, aims to achieve. Because TCIM mechanisms can target various pathways implicated in AD pathogenesis, they can complement traditional therapies, which highlights the promise of integrative therapy methods (King et al., 2020). However, the absence of established procedures, dosage fluctuations, and a dearth of solid clinical data pose serious obstacles to the broader use of TCIM. Evidence-based studies that align TCIM techniques with traditional medical procedures may offer the necessary scientific support for their broad application.
TCIM-based therapies have tremendous promise for the treatment of Alzheimer’s disease, as they can target most of the pathogenic pathways, such as oxidative stress, inflammation, tau pathology, and cholinergic deficits. Overcoming critical obstacles such as bioavailability, pharmacokinetics, and herbal metabolite standardization will, however, need to be overcome for preclinical success to translate to clinical application. Integrative techniques that combine TCIM with conventional medicines have the potential to provide synergistic advantages in AD management.
5.1 Pharmacological and clinical validation of herb-derived agents in Alzheimer’s disease
5.1.1 Pharmacological mechanisms of action
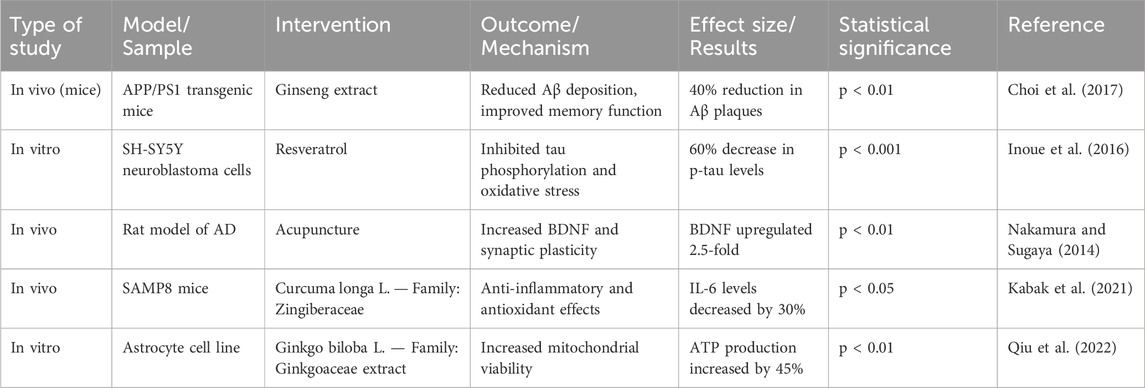
Herb-derived phytochemicals target multiple pathogenic pathways in AD (Table 1). The pharmacological mechanisms of key herbal agents in AD are summarized in Table 1.
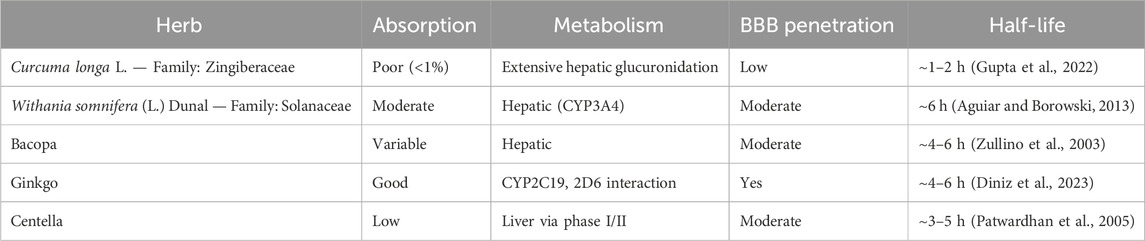
Table 1. Pharmacological mechanisms of action of selected herb-derived agents in Alzheimer’s disease.
Curcuma longa L. — Family: Zingiberaceae: Inhibits Aβ aggregation, activates PI3K/Akt pathway, and downregulates NF-κB-mediated neuroinflammation. [IC50 for AChE inhibition: ∼67.69 µM] (Sehgal et al., 2012).
Withania somnifera (L.) Dunal—Family: Solanaceae: Inhibits acetylcholinesterase, reduces TNF-α and IL-6 levels, and promotes neurogenesis via LRP1 receptor upregulation (Huang et al., 2008).
Bacopa monnieri (L.) Wettst. — Family: Plantaginaceae: Enhances BDNF expression and reduces oxidative stress through upregulation of antioxidant enzymes (SOD, catalase) (Yang et al., 2015).
Ginkgo biloba L. — Family: Ginkgoaceae: Reduces tau hyperphosphorylation, enhances cerebral blood flow, and exerts antioxidant effects (Yin et al., 2009).
Centella asiatica (L.) Urb. — Family: Apiaceae: Protects neurons via antioxidant activity and modulates MAPK/ERK and PI3K/Akt signaling pathways (Anand et al., 2007).
5.1.2 Pharmacokinetics and pharmacodynamics (PK/PD)
Despite promising effects, botanical drugs face PK/PD challenges: Table 2 outlines the absorption, metabolism, BBB penetration, and half-life of commonly used herbs in AD.
5.1.3 Quality control aspects
Standardization and quality assurance of botanical drugs remain significant concerns in TCIM. Phytochemical content variation can arise due to environmental factors like climate, harvesting season, and post-harvest storage conditions (Saper et al., 2008). This inconsistency affects therapeutic efficacy and reproducibility. Additionally, herbal products may be contaminated with pesticides, heavy metals, or microbial agents, posing potential health risks (WHO, 2024). Regulatory agencies, including the World Health Organization (WHO), advocate for the implementation of Good Agricultural and Collection Practices (GACP) and standardized manufacturing methods such as High-Performance Liquid Chromatography (HPLC) to ensure quality and safety (Kunle, 2012). Identification and quantification of specific marker metabolites, such as withanolides in Withania somnifera (L.) Dunal—Family: Solanaceae, bacosides in Bacopa monnieri (L.) Wettst. — Family: Plantaginaceae, and the standardized extract EGb761 in Ginkgo biloba L. — Family: Ginkgoaceae, are essential for product consistency and efficacy validation (Flory et al., 2021).
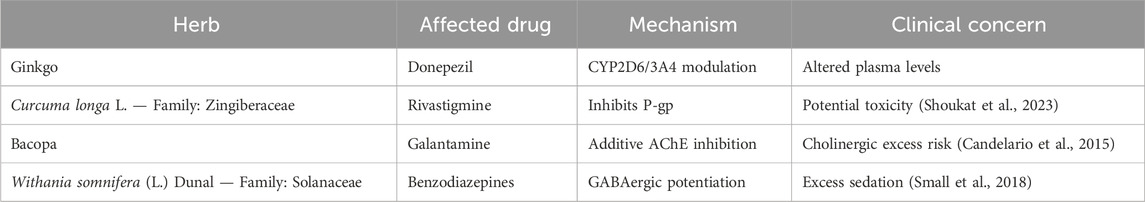
5.1.4 Herb-drug interactions
Several herb-drug interactions can affect conventional AD treatments. Common herb-drug interactions that may influence pharmacotherapy in AD are detailed in Table 3.
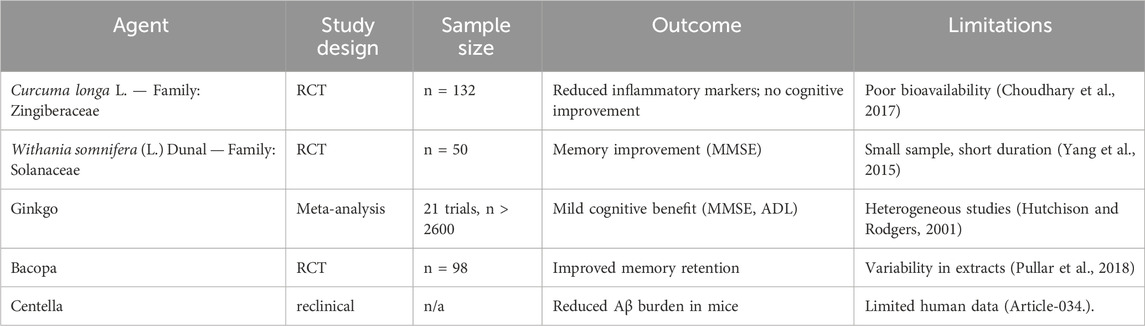
5.1.5 Clinical evidence and efficacy evaluation
Several human studies have explored herbal efficacy, but limitations remain.
6 Enhanced therapeutic potential of herbal combinations
Multi-metabolite botanical drugs are widely recognized for potential use in treating complex diseases such as AD. These alternatives have several key advantages over more conventional treatments, among which are improved safety profiles, relative cost-effectiveness, and potential multi-target efficacy. Traditional Oriental herbal formulations have been shown to address multiple aspects of AD by diverse mechanisms (Jeon et al., 2019). Illustrations of Synergistic Properties of Multi-metabolite Herbal Therapies in Figure 3.
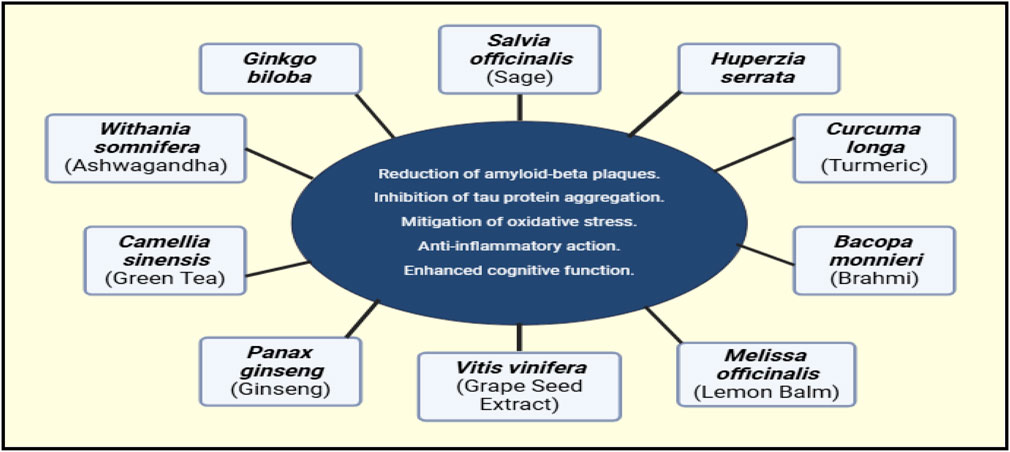
Figure 3. Proposed synergistic effects of various TCIM interventions in AD. This figure is based on theoretical predictions derived from existing literature and mechanistic insights, rather than direct experimental evidence.
The multi-metabolite nature of herbal preparations and the existence of unknown plant metabolites pose challenges in metabolism studies. Nevertheless, recent improvements in bioanalytical technologies have improved our capacity to assess the pharmacokinetics and metabolic interactions of plant metabolites (Xin et al., 2011).
These treatments have also been able to counteract some of the chemotherapy-induced toxicities, including neurotoxicity and cardiotoxicity (Fu et al., 2018). The neuropathological factors involved, such as tau, α-synuclein, and β-amyloid (Aβ), are synergistic and interact with each other in a complex manner, different from the classical linear progression model (Clinton et al., 2010). Thus, a single protein cannot be targeted, since the anti-Aβ clinical studies were not successful enough (Wang J. et al., 2014). Promising approaches are multi-target therapies, which include polyphenolic metabolites. Comparison between grape seed extract, resveratrol, and purple grape juice extract showed better outcomes than single metabolite treatment on cognition scores and lowering of amyloid loads in AD models of animal systems (Calfio et al., 2020). It forms a significant problem in producing effective treatments against AD. Recent studies focused on using the possibilities of multi-target treatments of natural substances and phytochemicals, which could serve to mitigate Alzheimer’s disease (Tuzimski and Petruczynik, 2022) (Calfio et al., 2020). Multi-target therapies, including polyphenolic compounds, have emerged as promising strategies. Research comparing grape seed extract, resveratrol, and purple grape juice extract with single-metabolite treatments found improved cognitive outcomes and reduced amyloid load in animal models of AD (Tuzimski and Petruczynik, 2022) (Iqubal et al., 2021). Natural compounds, however, tend to attack the complexities of Alzheimer’s disease from various angles, whereas synthetic drugs tend to target a particular target (Wang J. et al., 2014). Unlike synthetic drugs, which often target a single pathway, natural compounds exhibit a broader therapeutic scope. Plant extracts and isolated phytochemicals are gaining attention as potential AD therapeutics, with many showing promise in preclinical studies (Bhat et al., 2022; Oxford et al., 2020).
7 Evaluation of clinical studies and therapeutic efficacy
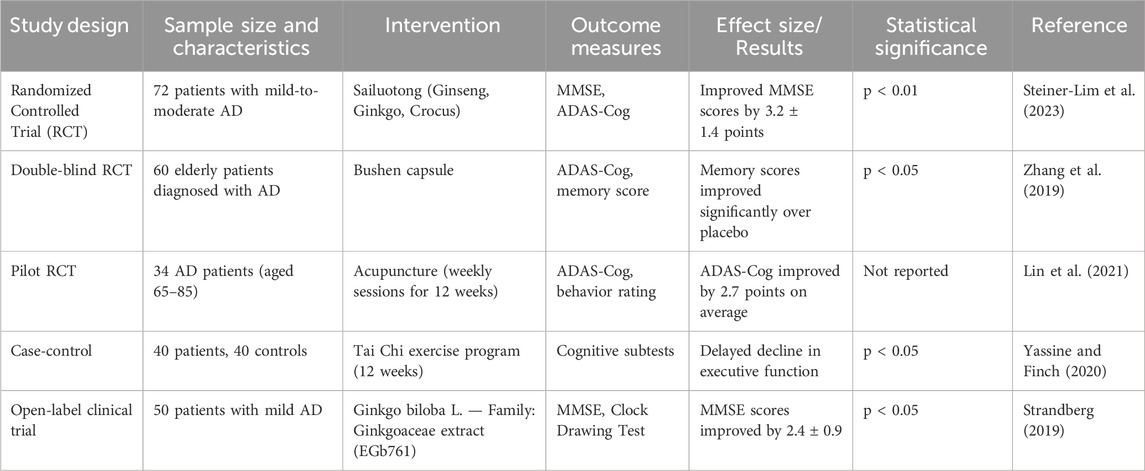
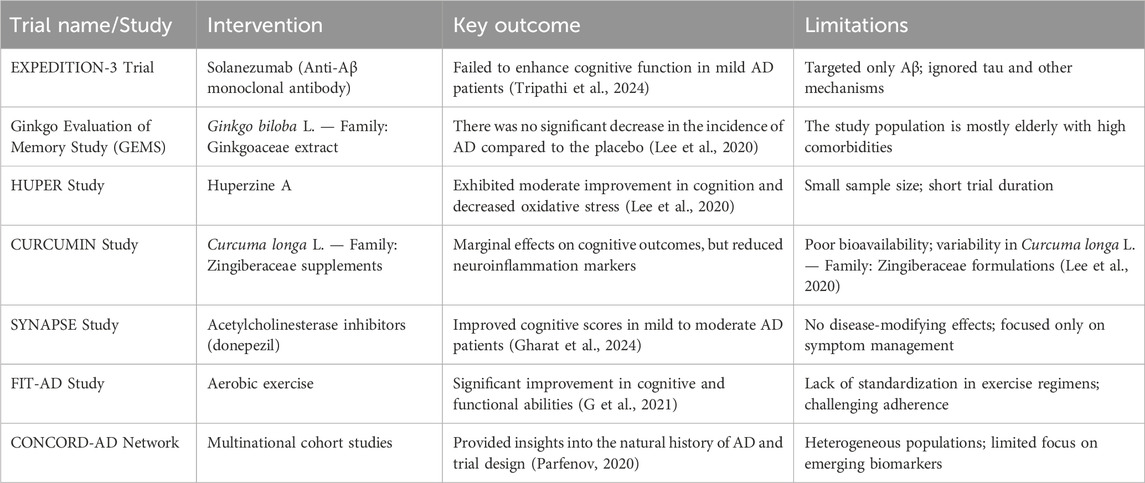
Recent AD pharmacological trials have focused on beta-amyloid, which has usually failed to enhance cognitive function (Tripathi et al., 2024). The other targets that scientists consider include tau protein, neuroinflammation, and oxidative stress. A summary of clinical investigations evaluating TCIM interventions in Alzheimer’s disease is provided in Table 4. Similarly, Table 5 provides an overview of experimental studies elucidating the mechanisms of action of TCIM approaches in Alzheimer’s disease. Selected landmark clinical trials investigating both conventional and herbal interventions in Alzheimer’s disease are summarized in Table 6. Plant-based traditional remedies like Ginkgo biloba L. — Family: Ginkgoaceae, Huperzia serrata (Thunb.) Trevis. — Family: Lycopodiaceae, and Curcuma longa have been shown to influence these targets through plant metabolites (Lee et al., 2020). Randomized controlled trials have been conducted to look at botanical drug therapies for AD, and Mini-Mental State Checkup scores have been used as a key outcome measure (Gharat et al., 2024). Cholinesterase inhibitors are already in use for the treatment of Alzheimer’s disease symptoms, and there is an urgent need for disease-modifying medication (Cocchiara et al., 2020). Management of AD requires initial diagnosis and proper treatment, encompassing both drug and nondrug therapies (Pimperkhede et al., 2023).
Acetylcholinesterase inhibitors and memantine are the two most commonly prescribed pharmacotherapies; however, herbal treatments are also being researched (Pavlik et al., 2022). The CONCORD-AD network consists of seven multinational cohorts and aims to provide greater insight into the natural history of AD, thereby informing the design of clinical trials (Parfenov, 2020). However, management mistakes prevail; these include underdiagnosis, misunderstanding contemporary medicines, and the use of anti-dementia drugs. Non-pharmacological interventions like stimulation, exercise, and antioxidant foods are often overlooked (Andrade et al., 2022). The education and support of the caregivers are essential to provide excellent patient care (Pimperkhede et al., 2023). More knowledge among healthcare providers, improved diagnosis techniques, and access to effective medications would be vital in enhancing the management of AD (Andrade et al., 2022) (Pimperkhede et al., 2023). Meta-analyses and systematic reviews in recent times have shown significant insights into the prevention and treatment of AD. Exercise, specifically aerobic and multimodal exercise, has been proven to decrease AD symptoms, most importantly, cognitive (G et al., 2021).
Some gene variations, including CD33, BIN1, and MTHFR, have been identified as contributing to the risk of AD in several studies (Yu et al., 2020). A comprehensive assessment of modifiable factors disclosed 21 evidence-based notions for preventing AD, with great evidence for education, cognitive activity, and health conditions (Ou et al., 2020). Lastly, infectious agents have also been linked to the risk for Alzheimer’s disease, which has been found to increase with many infections, including Chlamydia pneumoniae, Human herpesvirus-6, and Herpes simplex virus-1, with significant connections (Kumar et al., 2016). New-generation improvements in diagnostic tools and biomarker-based assessments will even increase trial reliability. Currently, the landscape of AD clinical trials is characterized by narrow target approaches, potential biases, small sample sizes, and variability in interventions. These must be overcome with multi-target therapeutic approaches, long follow-ups, and standardized protocols in drug and non-drug therapy.
8 Novel therapeutic targets: advances and emerging strategies
AD is a neurodegenerative illness caused by tau tangles, β-amyloid plaques, and neuroinflammation. Research efforts are directed toward novel targets and multifunctional compounds to address the multifaceted aspect of Alzheimer’s pathogenesis. Computational biology, particularly molecular docking, has emerged as an effective methodology for discovering new therapeutic candidates. Alzheimer’s disease is linked to multiple signaling pathways that have been involved in its etiology. Some key processes are abnormal calcium homeostasis, amyloid-beta plaque formation, and tau protein hyperphosphorylation. The PI3K/AKT pathway regulates cell survival and death, which is important for neuroprotection (Long et al., 2021). Autophagy, which interacts with several AD-related processes, is critical for maintaining cellular homeostasis and removing harmful proteins (Ramachandra et al., 2021).
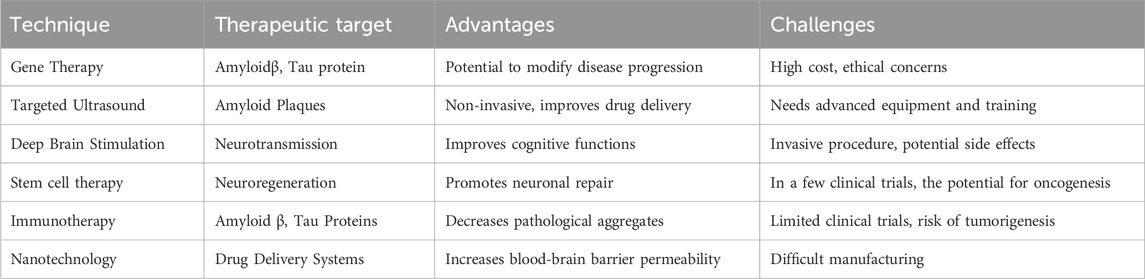
APOE, GSK-3β, Notch, and Wnt signaling have also recently been associated with AD (Long et al., 2021). Some studies are currently on multi-convergence, with studies suggesting that autophagy serves as a potential therapeutic target, with its critical role and multiple interactions (Gadhave et al., 2021). In addition, natural compounds are identified as activators of PI3K/AKT; hence, they may come into use as neuroprotective drugs in the pharmacotherapy of AD. Current treatments focus on early intervention and a holistic approach to the various disease processes. The targets include amyloid beta, tau proteins, neurotransmission, inflammation, metabolism, and oxidative stress (Hroudová and Fišar, 2024). There are only a few medications that are accepted for the treatment of AD, such as cholinesterase inhibitors, NMDA antagonists, and anti-Aβ monoclonal antibodies (Abdallah, 2024) (Athar et al., 2021). The researchers are working on innovative therapeutic methods such as immunotherapy and neuroinflammation (Abdallah, 2024). An overview of promising therapeutic innovations, including gene therapy, nanotechnology, and neuroregenerative strategies, is summarized in Table 7. Other promising alternative techniques for AD treatment include targeted deep brain stimulation, ultrasound, stem cell therapy, and gene therapy, as represented in Table 4 for Innovative Approaches in AD Management.
Yet, there is still the issue of presymptomatic neuronal injury, undesirable effects of medication, and poor clinical trial design (Tatulian, 2022). Future research may be focused on AD pathophysiology, biomarkers, and new diagnostic tools, thus allowing for the development of more effective therapy strategies in the future (Hroudová and Fišar, 2024) (Tatulian, 2022). The limited absorption and quick metabolism of botanical drugs such as Curcuma longa L. — Family: Zingiberaceae and Bacopa monnieri (L.) Wettst. — Family: Plantaginaceae makes it difficult to translate preclinical findings of TCIM treatments for AD into human trials. Variability in extract quality and irregular dosage further restricts reproducibility. The multi-target mechanisms by which TCIM interventions modulate Alzheimer’s pathology are illustrated in Table 8. Animal models often cannot mimic the intricate pathology of Alzheimer’s disease observed in humans, which adds to discrepancies between preclinical and clinical outcomes. In addition, short-term follow-up studies with smaller sample sizes limit the capacity of the trials to reveal important disease-modifying benefits. In addition, there are no standard procedures followed, and only a few biomarkers are used, hence, it is impossible to replicate reproducible results. Future studies would be more optimized in drug delivery systems, and further standardization of formulations can be done using advanced biomarkers and lengthier studies to decide the therapeutic efficacy of TCIM.
9 Innovation and future directions
There are numerous limits to current Alzheimer’s disease research. While artificial intelligence and language processing show potential for detecting cognitive decline, low standardization and restricted result comparability impede the clinical application (de la Fuente Garcia et al., 2020). Existing pharmacological treatments only provide symptomatic alleviation, while disease-modifying medicines remain unavailable. Central nervous system drug delivery is still challenging. However, nanoliposomes and exosomes have the potential to improve brain function (Passeri et al., 2022). The preclinical animal models are poor at simulating human AD pathology, leading to a poor transformation of hopeful applicants into clinical trials (Akhtar et al., 2022). Neuroimaging techniques have emerged as promising tools for the early diagnosis of Alzheimer’s disease and the identification of biomarkers. However, their clinical utility varies in terms of precision and effectiveness (Kim et al., 2022).
Future drugs will focus on the fundamental mechanisms of diseases like Aβ plaques and neurofibrillary tangles, besides clinical relief (Yiannopoulou and Papageorgiou, 2020). Biomarkers of Aβ, tau pathology, neurodegeneration, synaptic dysfunction, and inflammation are important for selecting the right people to be studied and assessing the degree of their improvement (Zetterberg and Bendlin, 2021). The growing interest in psychedelic therapy for Alzheimer’s disease raises ethical concerns, for example, about the effect on autonomy, consent, and caregiving, not to mention the potential exploitation of desperation in patients (Peterson et al., 2023). As science moves forward toward personalized medicine, various biomarkers combined with cognitive and neuroimaging data might guide treatment regimens (Yiannopoulou and Papageorgiou, 2020). In preparing healthcare systems for disease-modifying medications and addressing the ethical concerns surrounding psychedelic research, much advancement is set for Alzheimer’s treatment and research. Among the new treatment options, there are targeting butyrylcholinesterase, tau proteins, and endocannabinoid system involvement. The role of microRNAs in AD research. Advanced diagnostic techniques, including qRTPCR and iPSCs, alter early detection (Sharma et al., 2024). The focus has shifted to studies on prodromal phases and biomarkers for early detection of the disease at preclinical levels (Rayathala et al., 2022). The goal of future drugs is the modulation of pathology via alterations to amyloid beta plaques and neurofibrillary tangles, whereas nowadays therapies are mainly trying to level neurotransmitter abnormalities (Ramachandra et al., 2021). To better, the complex causative basis of Alzheimer’s, multiple metabolites like inflammation, the microbiota, hormones, as well as changes in the neurovascular unit, etc., are now being searched for (Giannoni et al., 2020). The discipline is shifting toward personalized medicine, which incorporates different therapies based on individual biomarkers and clinical manifestations (Yiannopoulou and Papageorgiou, 2020). Future Alzheimer’s disease research should prioritize the thorough validation of novel medicines via well-designed clinical trials to ensure reproducibility and effectiveness. Regulatory approvals must include rigorous safety and efficacy studies, particularly for new medicines like psychedelics, stem cells, and nanotechnology-based interventions. Integrating evidence-based conventional treatments is critical for developing holistic approaches that address disease pathophysiology and improve cognitive function. There are still several gaps in understanding the multiple causal metabolites of Alzheimer’s disease, including neuroinflammation, gut-brain interactions, hormone imbalances, and alterations in neurovascular variables. Further research in new diagnostic tools, such as qRT-PCR and iPSCs, for earlier detection of AD, together with the development of biomarkers to allow for personalized treatment regimens, shall be promising in the future. Standardization of protocols, drug delivery systems, and combination therapies will be the keys to further improvements in AD treatment.
9.1 Limitations and future research priorities
Despite the promising evidence presented for the use of TCIM in managing Alzheimer’s disease (AD), several limitations hinder its clinical translation. A major concern is the lack of large-scale, multicenter randomized controlled trials (RCTs) that would provide robust evidence on safety and efficacy. Many existing studies suffer from small sample sizes, inadequate control groups, short follow-up durations, and variability in herbal formulations. The bioavailability and pharmacokinetic profiles of key herbal agents, such as curcumin and bacosides, remain suboptimal, restricting their clinical effectiveness.
Standardization of herbal products is also a critical issue. Variability in active compound concentrations due to differences in cultivation, harvesting, and processing conditions can lead to inconsistent therapeutic outcomes. Moreover, herb-drug interactions are not well-characterized, posing potential safety risks when TCIM is combined with conventional therapies.
Future research should prioritize:
• Rigorous, multicenter RCTs with standardized interventions and outcome measures.
• Development of novel drug delivery systems (e.g., nanocarriers) to improve bioavailability.
• Comprehensive studies on pharmacokinetics and herb-drug interactions.
• Identification and validation of biomarkers to assess treatment response.
• Integration of personalized medicine approaches, leveraging genomics and neuroimaging data.
10 Conclusion
In conclusion, Alzheimer’s disease is a very complex and fast-growing global health challenge; hence, an innovative and holistic solution is required. TCIM is a way that might be adopted as a strategy for the nature of the disease; current pharmaceutical treatments offer little symptomatic relief for the condition. Key pharmacologically active agents derived from TCIM, such as C. longa L. — Family: Zingiberaceae, B. monnieri (L.) Wettst. — Family: Plantaginaceae, Centella asiatica (L.) Urb. — Family: Apiaceae, and Withania somnifera, exhibit multi-targeted mechanisms, ranging from PI3K/Akt and Nrf2 pathway activation to tau aggregation inhibition and cholinergic enhancement. These compounds demonstrate potential for disease modification in Alzheimer’s through antioxidant, anti-inflammatory, and neuroprotective actions. Future integration of such agents into conventional frameworks may open new avenues for holistic yet mechanism-based Alzheimer’s therapeutics.
Collaboration between conventional scientists and TCIM practitioners is pivotal to optimizing TCIM’s strengths in Alzheimer’s treatment. Efficacy, safety, and long-term outcomes will have to be validated by significant, well-planned clinical trials on established protocols. Such an integration will hasten the move of TCIM from adjunct therapies to an established part of mainstream Alzheimer’s care.
Emerging therapeutics include multi-targeted approaches, gene therapy, and nanotechnology. New techniques must be established and tested scientifically and in a multidisciplinary collaboration with other traditional healthcare practitioners before they are included in conventional healthcare. Early diagnosis, specific care, and new treatments might reduce the effects of Alzheimer’s disease in the future and may even help improve people’s lives.
Author contributions
RS: Data curation, Writing – original draft. TP: Data curation, Resources, Writing – original draft. TV: Data curation, Formal Analysis, Resources, Visualization, Writing – review and editing. VS: Data curation, Investigation, Resources, Supervision, Visualization, Writing – review and editing. NH: Formal Analysis, Investigation, Project administration, Resources, Supervision, Writing – review and editing. RB: Investigation, Methodology, Resources, Supervision, Validation, Writing – review and editing. SA: Conceptualization, Investigation, Methodology, Resources, Supervision, Validation, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdallah, A. E. (2024). Review on anti-alzheimer drug development: approaches, challenges and perspectives. RSC Adv. 14 (16), 11057–11088. doi:10.1039/d3ra08333k
Adib-Hajbaghery, M., Fattahi Ardakani, M., Sotoudeh, A., and Asadian, A. (2021). Prevalence of complementary and alternative medicine (CAM) among diabetic patients in Eastern Mediterranean country members of the World Health Organization (WHO): a review. J. Herb. Med. 29, 100476. doi:10.1016/j.hermed.2021.100476
Aguiar, S., and Borowski, T. (2013). Neuropharmacological review of the nootropic herb Bacopa monnieri. Rejuvenation Res. 16 (4), 313–326. doi:10.1089/rej.2013.1431
Akhtar, A., Gupta, S. M., Dwivedi, S., Kumar, D., Shaikh, M. F., and Negi, A. (2022). Preclinical models for Alzheimer’s disease: past, present, and future approaches. ACS Omega 7 (51), 47504–47517. doi:10.1021/acsomega.2c05609
Anand, P., Kunnumakkara, A. B., Newman, R. A., and Aggarwal, B. B. (2007). Bioavailability of curcumin: problems and promises. Mol. Pharm. 4 (6), 807–818. doi:10.1021/mp700113r
Andrade, A., Siqueira, T. C., D’Oliveira, A., and Dominski, F. H. (2022). Effects of exercise in the treatment of Alzheimer’s disease: an umbrella review of systematic reviews and meta-analyses. J. Aging Phys. Act. 30 (3), 535–551. doi:10.1123/japa.2021-0033
Ashford, J. W., Mahoney, L., and Burkett, T. (2015). A role for complementary and integrative medicine in Alzheimer’s disease prevention. J. Alzheimer’s Dis. 48 (1), 13–14. doi:10.3233/JAD-150505
Athar, T., Al Balushi, K., and Khan, S. A. (2021). Recent advances on drug development and emerging therapeutic agents for Alzheimer’s disease. Mol. Biol. Rep. 48 (7), 5629–5645. doi:10.1007/s11033-021-06512-9
Atri, A. (2019). The Alzheimer's disease clinical spectrum: diagnosis and management. Med. Clin. N. Am. 103 (2), 263–293. doi:10.1016/j.mcna.2018.10.009
Barnes, J. (2003). Quality, efficacy and safety of complementary medicines: fashions, facts and the future. Part I. Regulation and quality. Br. J. Clin. Pharmacol. 55 (3), 226–233. doi:10.1046/j.1365-2125.2003.01810.x
Bashir, S., Uzair, M., Abualait, T., Arshad, M., Khallaf, R. A., Niaz, A., et al. (2022). Effects of transcranial magnetic stimulation on neurobiological changes in Alzheimer's disease (Review). Mol. Med. Rep. 25 (4), 109. doi:10.3892/mmr.2022.12625
Bhat, B. A., Almilaibary, A., Mir, R. A., Aljarallah, B. M., Mir, W. R., Ahmad, F., et al. (2022). Natural therapeutics in aid of treating Alzheimer’s disease: a green gateway toward ending quest for treating neurological disorders. Front. Neurosci. 16, 884345. doi:10.3389/fnins.2022.884345
Brookmeyer, R., Johnson, E., Ziegler-Graham, K., and Arrighi, H. M. (2007). Forecasting the global burden of Alzheimer’s disease. Alzheimer’s and Dementia 3 (3), 186–191. doi:10.1016/j.jalz.2007.04.381
Busche, M. A., and Hyman, B. T. (2020). Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 23 (10), 1183–1193. doi:10.1038/s41593-020-0687-6
Calfio, C., Gonzalez, A., Singh, S. K., Rojo, L. E., and Maccioni, R. B. (2020). The emerging role of nutraceuticals and phytochemicals in the prevention and treatment of Alzheimer’s disease. J. Alzheimer’s Dis. 77 (1), 33–51. doi:10.3233/JAD-200443
Candelario, M., Cuellar, E., Reyes-Ruiz, J. M., Darabedian, N., Feimeng, Z., Miledi, R., et al. (2015). Direct evidence for GABAergic activity of Withania somnifera on mammalian ionotropic GABAA and GABAρ receptors. J. Ethnopharmacol. 171, 264–272. doi:10.1016/j.jep.2015.05.058
Castillo-Carranza, D. L., Guerrero-Muñoz, M. J., Sengupta, U., Hernandez, C., Barrett, A. D. T., Dineley, K., et al. (2015). Tau immunotherapy modulates both pathological tau and upstream amyloid pathology in an Alzheimer’s disease mouse model. J. Neurosci. 35 (12), 4857–4868. doi:10.1523/jneurosci.4989-14.2015
Chen, K. C., Liu, Y. C., Lee, C. C., and Chen, C. Y. C. (2014). Potential retinoid X receptor agonists for treating Alzheimer’s disease from traditional Chinese medicine. Evidence-Based Complementary Altern. Med. 2014 (1), 278493. doi:10.1155/2014/278493
Cheung, T. S., Song, T. H., Ng, T. B., Wu, F. H., Lao, L. X., Tang, S. C. W., et al. (2015). Therapeutic effects of herbal chemicals in traditional Chinese medicine on Alzheimer’s disease. Curr. Med. Chem. 22 (19), 2392–2403. doi:10.2174/0929867322666150520095509
Choi, M. R., Kwak, S. M., Bang, S. H., Jeong, J. E., and Kim, D. J. (2017). Chronic saponin treatment attenuates damage to the pancreas in chronic alcohol-treated diabetic rats. J. Ginseng Res. 41 (4), 503–512. doi:10.1016/j.jgr.2016.09.002
Choudhary, D., Bhattacharyya, S., and Bose, S. (2017). Efficacy and safety of ashwagandha (Withania somnifera (L.) dunal) root extract in improving memory and cognitive functions. J. Diet. Suppl. 14 (6), 599–612. doi:10.1080/19390211.2017.1284970
Clinton, L. K., Blurton-Jones, M., Myczek, K., Trojanowski, J. Q., and LaFerla, F. M. (2010). Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J. Neurosci. 30 (21), 7281–7289. doi:10.1523/JNEUROSCI.0490-10.2010
Cocchiara, R. A., de Lucia, F., Koci, L., Lisanti, E., Petruccini, G., and la Torre, G. (2020). Management of the early stage of Alzheimer’s disease: a systematic review of literature over the past 10 years. Clin. Ter. Soc. Ed. Universo 171, E357–E368. doi:10.7417/CT.2020.2239
Cooper, E. L., and Ma, M. J. (2017). Alzheimer Disease: clues from traditional and complementary medicine. J. Tradit. Complement. Med. 7 (4), 380–385. doi:10.1016/j.jtcme.2016.12.003
de la Fuente Garcia, S., Ritchie, C. W., and Luz, S. (2020). Artificial intelligence, speech, and language processing approaches to monitoring Alzheimer’s disease: a systematic review. J. Alzheimer’s Dis. 78 (4), 1547–1574. doi:10.3233/JAD-200888
Dhanasekaran, M., Holcomb, L. A., Hitt, A. R., Tharakan, B., Porter, J. W., Young, K. A., et al. (2009). Centella asiatica extract selectively decreases amyloid β levels in hippocampus of Alzheimer’s disease animal model. Phytotherapy Res. 23 (1), 14–19. doi:10.1002/ptr.2405
Diniz, L. R. L., Calado, L. L., Duarte, A. B. S., and de Sousa, D. P. (2023). Centella asiatica and its metabolite asiatic acid: wound healing effects and therapeutic potential. Metabolites 13 (2), 276. doi:10.3390/metabo13020276
Flory, S., Männle, R., and Frank, J. (2021). The inhibitory activity of curcumin on P-glycoprotein and its uptake by and efflux from LS180 cells is not affected by its galenic formulation. Antioxidants (Basel) 10 (11), 1826. doi:10.3390/antiox10111826
Fu, B., Wang, N., Tan, H. Y., Li, S., Cheung, F., and Feng, Y. (2018). Multi-component herbal products in the prevention and treatment of chemotherapy-associated toxicity and side effects: a review on experimental and clinical evidences. Front. Pharmacol. 9, 1394. doi:10.3389/fphar.2018.01394
G, N. S. H. S., Marise, V. L. P., Satish, K. S., Yergolkar, A. V., Krishnamurthy, M., Ganesan, R. S., et al. (2021). Untangling huge literature to disinter genetic underpinnings of Alzheimer’s Disease: a systematic review and meta-analysis. Ageing Res. Rev. 71, 101421. doi:10.1016/j.arr.2021.101421
Gadhave, K., Kumar, D., Uversky, V. N., and Giri, R. (2021). A multitude of signaling pathways associated with Alzheimer’s disease and their roles in AD pathogenesis and therapy. Med. Res. Rev. 41 (5), 2689–2745. doi:10.1002/med.21719
García-Casares, N., Gallego Fuentes, P., Barbancho, M. Á., López-Gigosos, R., García-Rodríguez, A., and Gutiérrez-Bedmar, M. (2021). Alzheimer’s disease, mild cognitive impairment and mediterranean diet. A systematic review and dose-response meta-analysis. J. Clin. Med. 10 (20), 4642. doi:10.3390/jcm10204642
Gharat, R., Dixit, G., Khambete, M., and Prabhu, A. (2024). Targets, trials and tribulations in Alzheimer therapeutics. Eur. J. Pharmacol. 962, 176230. doi:10.1016/j.ejphar.2023.176230
Ghosh, S., Wu, M. D., Shaftel, S. S., Kyrkanides, S., LaFerla, F. M., Olschowka, J. A., et al. (2013). Sustained interleukin-1β overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer’s mouse model. J. Neurosci. 33 (11), 5053–5064. doi:10.1523/JNEUROSCI.4361-12.2013
Giannoni, P., Fossati, S., Marcello, E., and Claeysen, S. (2020). Editorial: identification of multiple targets in the fight against Alzheimer’s disease. Front. Aging Neurosci. 12, 169. doi:10.3389/fnagi.2020.00169
Guo, T., Zhang, D., Zeng, Y., Huang, T. Y., Xu, H., and Zhao, Y. (2020). Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 15 (1), 40. doi:10.1186/s13024-020-00391-7
Gupta, S. K., Jadhav, S., Gohil, D., Panigrahi, G. C., Kaushal, R. K., Gandhi, K., et al. (2022). Safety, toxicity and pharmacokinetic assessment of oral Withaferin-A in mice. Toxicol. Rep. 9, 1204–1212. doi:10.1016/j.toxrep.2022.05.012
Hannan, M. A., Dash, R., Haque, M. N., Sohag, A. A. M., and Moon, I. S. (2020). Mechanistic insights into the curcumin-mediated neuroprotection in alzheimer’s disease: an integrated system. Pharmacol. Mol. Simul. Study. doi:10.3233/JAD-200581
Harada, C. N., Natelson Love, M. C., and Triebel, K. L. (2013). Normal cognitive aging. Clin. Geriatr. Med. 29 (4), 737–752. doi:10.1016/j.cger.2013.07.002
Haut, F., Argyrousi, E. K., and Arancio, O. (2023). Re-arranging the puzzle between the amyloid-beta and tau pathology: an APP-centric approach. Int. J. Mol. Sci. 25 (1), 259. doi:10.3390/ijms25010259
Hroudová, J., and Fišar, Z. (2024). Alzheimer’s disease approaches - focusing on pathology, biomarkers and clinical trial candidates. Prog. Neuropsychopharmacol. Biol. Psychiatry 134, 111069. doi:10.1016/j.pnpbp.2024.111069
Huang, L., Yagura, T., and Chen, S. (2008). Sedative activity of hexane extract of Keampferia galanga L. and its active compounds. J. Ethnopharmacol. 120 (1), 123–125. doi:10.1016/j.jep.2008.07.045
Hutchison, C. W., and Rodgers, T. (2001). The chronic effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy human subjects. Psychopharmacol. Berl. 156 (4), 481–484. doi:10.1007/s002130100815
Inoue, Y., Hayashi, Y., Kangawa, K., Suzuki, Y., Murakami, N., and Nakahara, K. (2016). Des-acyl ghrelin prevents heatstroke-like symptoms in rats exposed to high temperature and high humidity. Neurosci. Lett. 615, 28–32. doi:10.1016/j.neulet.2016.01.003
Iqubal, A., Rahman, S. O., Ahmed, M., Bansal, P., Haider, M. R., Iqubal, M. K., et al. (2021). Current quest in natural bioactive compounds for Alzheimer’s disease: multi-targeted-designed-ligand based approach with preclinical and clinical based evidence. Curr. Drug Targets 22 (6), 685–720. doi:10.2174/1389450121999201209201004
Jack, C. R., Knopman, D. S., Jagust, W. J., Petersen, R. C., Weiner, M. W., Aisen, P. S., et al. (2013). Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12 (2), 207–216. doi:10.1016/S1474-4422(12)70291-0
Jayaprakasam, B., Padmanabhan, K., and Nair, M. G. (2010). Withanamides in Withania somnifera fruit protect PC-12 cells from β-amyloid responsible for Alzheimer’s disease. Phytotherapy Res. 24 (6), 859–863. doi:10.1002/ptr.3033
Jeon, S. G., Song, E. J., Lee, D., Park, J., Nam, Y., Kim, J., et al. (2019). Traditional oriental medicines and Alzheimer’s disease. Aging Dis. 10 (2), 307–328. doi:10.14336/AD.2018.0328
Kabak, M., Çil, B., and Hocanlı, I. (2021). Relationship between leukocyte, neutrophil, lymphocyte, platelet counts, and neutrophil to lymphocyte ratio and polymerase chain reaction positivity. Int. Immunopharmacol. 93, 107390. doi:10.1016/j.intimp.2021.107390
Kabra, A. (2022). A systematic review of amyloid-β and tau’s contribution to neuroinflammation in Alzheimer’s disease progression. Int. J. High Sch. Res. 4 (6), 55–61. doi:10.36838/v4i6.10
Kim, J., Jeong, M., Stiles, W. R., and Choi, H. S. (2022). Neuroimaging modalities in Alzheimer’s disease: diagnosis and clinical features. Int. J. Mol. Sci. 23 (11), 6079. doi:10.3390/ijms23116079
King, M., Tavoulari, S., Mavridou, V., King, A., Mifsud, J., and Kunji, E. (2020). A single cysteine residue in the translocation pathway of the mitosomal ADP/ATP carrier from cryptosporidium parvum confers a broad nucleotide specificity. Int. J. Mol. Sci. 21 (23), 8971. doi:10.3390/ijms21238971
Kong, X., Ma, Z., Tang, R., Wang, X., Wei, K., Yang, G., et al. (2023). Efficacy of acupuncture in patients with mild Alzheimer’s disease and its impact on gut microbiota: study protocol for a randomized sham-controlled trial. Front. Med. (Lausanne) 10, 1014113. doi:10.3389/fmed.2023.1014113
Kuboyama, T., Tohda, C., Zhao, J., Nakamura, N., Hattori, M., and Komatsu, K. (2002). Axon- or dendrite-predominant outgrowth induced by constituents from Ashwagandha. Neuroreport 13 (14), 1715–1720. doi:10.1097/00001756-200210070-00005
Kumar, A., Nisha, C. M., Silakari, C., Sharma, I., Anusha, K., Gupta, N., et al. (2016). Current and novel therapeutic molecules and targets in Alzheimer’s disease. J. Formos. Med. Assoc. 115 (1), 3–10. doi:10.1016/j.jfma.2015.04.001
Kunle, O. F. (2012). Standardization of herbal medicines - a review. Int. J. Biodivers. Conserv. 4 (3). doi:10.5897/ijbc11.163
Kuszewski, J. C., Wong, R. H. X., and Howe, P. R. C. (2018). Can curcumin counteract cognitive decline? Clinical trial evidence and rationale for combining ω-3 fatty acids with curcumin. Adv. Nutr. 9 (2), 105–113. doi:10.1093/advances/nmx013
Lee, J., Jin, C., Cho, S. Y., Park, S. U., Jung, W. S., Moon, S. K., et al. (2020). Herbal medicine treatment for Alzheimer disease: a protocol for a systematic review and meta-analysis. Medicine 99 (33), e21745. doi:10.1097/MD.0000000000021745
Legal Status of traditional medicine (2001). Legal Status of traditional medicine and complementary/alternative medicine: a worldwide review.
Li, Q., Wu, Y., Chen, J., Xuan, A., and Wang, X. (2022). Microglia and immunotherapy in Alzheimer’s disease. Acta Neurol. Scand. 145 (3), 273–278. doi:10.1111/ane.13551
Li, X. L., Wang, D. S., Zhao, B. Q., Li, Q., Qu, H. Y., Zhang, T., et al. (2008). Effects of Chinese herbal medicine fuzhisan on aged rats. Exp. Gerontol. 43 (9), 853–858. doi:10.1016/j.exger.2008.05.018
Limpeanchob, N., Jaipan, S., Rattanakaruna, S., Phrompittayarat, W., and Ingkaninan, K. (2008). Neuroprotective effect of Bacopa monnieri on beta-amyloid-induced cell death in primary cortical culture. J. Ethnopharmacol. 120 (1), 112–117. doi:10.1016/j.jep.2008.07.039
Lin, J. C. W., Srivastava, G., and Tseng, V. S. (2021). International journal of interactive multimedia and artificial intelligence. London, United Kingdom: Universidad Internacional de la Rioja. Vol. 6, 4–5.
Liu, A. J., Wang, S. H., Hou, S. Y., Lin, C. J., Chiu, W. T., Hsiao, S. H., et al. (2013). Evodiamine induces transient receptor potential vanilloid-1-mediated protective autophagy in U87-MG astrocytes. Evidence-Based Complementary Altern. Med. 2013, 354840–354849. doi:10.1155/2013/354840
Liu, P., Kong, M., Yuan, S., Liu, J., and Wang, P. (2014). History and experience: a survey of traditional Chinese medicine treatment for Alzheimer’s disease. Evidence-Based Complementary Altern. Med. 2014 (1), 642128. doi:10.1155/2014/642128
Long, H. Z., Cheng, Y., Zhou, Z. W., Luo, H. Y., Wen, D. D., and Gao, L. C. (2021). PI3K/AKT signal pathway: a target of natural products in the prevention and treatment of Alzheimer’s disease and Parkinson’s disease. Front. Pharmacol. 12, 12. doi:10.3389/fphar.2021.648636
Long, J., Zhang, J., Zeng, X., Wang, M., and Wang, N. (2024). Prevention and treatment of Alzheimer’s disease via the regulation of the gut microbiota with traditional Chinese medicine. CNS Neurosci. Ther. 30 (11), e70101. doi:10.1111/cns.70101
Lopresti, A. L. (2018). The problem of curcumin and its bioavailability: could its gastrointestinal influence contribute to its overall health-enhancing effects? Adv. Nutr. 9 (1), 41–50. doi:10.1093/advances/nmx011
Ma, L., Jiang, X., Huang, Q., Chen, W., Zhang, H., Pei, H., et al. (2023). Traditional Chinese medicine for the treatment of Alzheimer’s disease: a focus on the microbiota–gut–brain axis. Biomed. and Pharmacother. 165, 115244. doi:10.1016/j.biopha.2023.115244
Miculas, D. C., Negru, P. A., Bungau, S. G., Behl, T., Hassan, S. S., and Tit, D. M. (2022). Pharmacotherapy evolution in Alzheimer’s disease: current framework and relevant directions. Cells 12 (1), 131. doi:10.3390/cells12010131
Murphy, K., and Park, J. (2017). Can Co-activation of Nrf2 and neurotrophic signaling pathway slow Alzheimer’s disease? Int. J. Mol. Sci. 18 (6), 1168. doi:10.3390/ijms18061168
Nakamura, K., and Sugaya, K. (2014). Neuromelanin-sensitive magnetic resonance imaging: a promising technique for depicting tissue characteristics containing neuromelanin. Neural Regen. Res. 9 (7), 759–760. doi:10.4103/1673-5374.131583
Ng, J. Y., Cramer, H., Lee, M. S., and Moher, D. (2024). Traditional, complementary, and integrative medicine and artificial intelligence: novel opportunities in healthcare. Integr. Med. Res. 13 (1), 101024. doi:10.1016/j.imr.2024.101024
Nguyen, S. A., Ajam Oughli, H., and Lavretsky, H. (2024). Use of complementary and integrative medicine for Alzheimer's disease and cognitive decline. J. Alzheimers Dis. 97, p. 523–540. doi:10.3233/JAD-230710
Nguyen, S. A., Oughli, H. A., and Lavretsky, H. (2022). Complementary and integrative medicine for neurocognitive disorders and caregiver health. Curr. Psychiatry Rep. 24 (9), 469–480. doi:10.1007/s11920-022-01355-y
Nishteswar, K., Joshi, H., and Karra, R. (2014). Role of indigenous herbs in the management of Alzheimer′s disease. Anc. Sci. Life 34 (1), 3–7. doi:10.4103/0257-7941.150763
Onaolapo, O. J., Olofinnade, A. T., Ojo, F. O., and Onaolapo, A. Y. (2021). Neuroinflammation and oxidative stress in Alzheimer’s disease; can nutraceuticals and functional foods come to the rescue? Antiinflamm. Antiallergy Agents Med. Chem. 21 (2), 75–89. doi:10.2174/1871523021666220815151559
Ou, Y. N., Zhu, J. X., Hou, X. H., Shen, X. N., Xu, W., Dong, Q., et al. (2020). Associations of infectious agents with Alzheimer’s disease: a systematic review and meta-analysis. J. Alzheimer’s Dis. 75 (1), 299–309. doi:10.3233/JAD-191337
Oxford, A. E., Stewart, E. S., and Rohn, T. T. (2020). Clinical trials in Alzheimer’s disease: a hurdle in the path of remedy. Int. J. Alzheimers Dis. 2020, 5380346. doi:10.1155/2020/5380346
Papagiouvannis, G., Theodosis-Nobelos, P., Kourounakis, P. N., and Rekka, E. A. (2021). Multi-target directed compounds with antioxidant and/or anti- inflammatory properties as potent agents for Alzheimer’s disease. Med. Chem. (Los Angeles) 17 (10), 1086–1103. doi:10.2174/1573406416666201013161303
Parfenov, V. A. (2020). Alzheimer’s disease: clinical management errors. Meditsinskiy Sov. = Med. Counc. (19), 23–28. doi:10.21518/2079-701x-2020-19-23-28
Passeri, E., Elkhoury, K., Morsink, M., Broersen, K., Linder, M., Tamayol, A., et al. (2022). Alzheimer’s disease: treatment strategies and their limitations. Int. J. Mol. Sci. 23 (22), 13954. doi:10.3390/ijms232213954
Patwardhan, B., Warude, D., Pushpangadan, P., and Bhatt, N. (2005). Ayurveda and traditional Chinese medicine: a comparative Overview. Evidence-Based Complementary Altern. Med. 2 (4), 465–473. doi:10.1093/ecam/neh140
Patwardhan, B. K., and Vaidya, A. D. B. (2014). Herbal remedies and the bias against Ayurveda arvind chopra centre for rheumatic diseases pune SEE PROFILE. Available online at: https://www.researchgate.net/publication/230607149.
Pavlik, V. N., Burnham, S. C., Kass, J. S., Helmer, C., Palmqvist, S., Vassilaki, M., et al. (2022). Connecting cohorts to diminish Alzheimer’s disease (CONCORD-AD): a report of an international research collaboration network. J. Alzheimer’s Dis. 85 (1), 31–45. doi:10.3233/JAD-210525
Perry, E. K., Pickering, A. T., Wang, W. W., Houghton, P. J., and Perry, N. S. L. (1999). Medicinal plants and Alzheimer’s disease: from ethnobotany to phytotherapy. J. Pharm. Pharmacol. 51 (5), 527–534. doi:10.1211/0022357991772808
Peterson, A., Largent, E. A., Lynch, H. F., Karlawish, J., and Sisti, D. (2023). Journeying to ixtlan: ethics of psychedelic medicine and research for Alzheimer’s disease and related dementias. AJOB Neurosci. 14 (2), 107–123. doi:10.1080/21507740.2022.2148771
Peterson, C. T. (2020). Dysfunction of the microbiota-gut-brain Axis in neurodegenerative disease: the promise of therapeutic modulation with prebiotics, medicinal herbs, probiotics, and synbiotics. J. Evid. Based Integr. Med. 25, 2515690X20957225. doi:10.1177/2515690X20957225
Phutrakool, P., and Pongpirul, K. (2022). Acceptance and use of complementary and alternative medicine among medical specialists: a 15-year systematic review and data synthesis. Syst. Rev. 11 (1), 10. doi:10.1186/s13643-021-01882-4
Pimperkhede, M., Vaibhav, M., and Kute, G. (2023). A systemic review on management of Alzheimer’s disease. Int. J. Res. Publ. Rev. 4 (4), 4637–4645. doi:10.55248/gengpi.4.423.38127
Prasad, K. N., Cole, W. C., and Prasad, K. C. (2002). Risk factors for Alzheimer’s disease: role of multiple antioxidants, non-steroidal anti-inflammatory and cholinergic agents alone or in combination in prevention and treatment. J. Am. Coll. Nutr. 21 (6), 506–522. doi:10.1080/07315724.2002.10719249
Pullar, J., Carr, A., Bozonet, S., and Vissers, M. (2018). High vitamin C Status is associated with elevated mood in male tertiary students. Antioxidants 7 (7), 91. doi:10.3390/antiox7070091
Qiu, Z. D., Wei, X. Y., Chen, Z. Y., Guo, J., Huang, L. Q., and Lai, C. J. S. (2022). Discovery of the directionally detoxification effect and chemical mechanism of Ginseng-Fuzi co-decoction based on real-time online filtration electrospray ionization mass spectrometry. Phytomedicine 100, 154059. doi:10.1016/j.phymed.2022.154059
Rafii, M. S., Walsh, S., Little, J. T., Behan, K., Reynolds, B., Ward, C., et al. (2011). A phase II trial of huperzine A in mild to moderate Alzheimer disease. Neurology 76 (16), 1389–1394. doi:10.1212/WNL.0b013e318216eb7b
Ramachandran, A. K., Das, S., Joseph, A., Shenoy, G. G., Alex, A. T., and Mudgal, J. (2021). Neurodegenerative pathways in Alzheimer’s disease: a review. Curr. Neuropharmacol. 19 (5), 679–692. doi:10.2174/1570159X18666200807130637
Rayathala, J., C, K. K., and P, V. (2022). Review on Alzheimer’s disease: past, present and future. J. Innovations Appl. Pharm. Sci. (JIAPS), 28–31. doi:10.37022/jiaps.v7i1.274
Rocca, W. A., Amaducci, L. A., and Schoenberg, B. S. (1986). Epidemiology of clinically diagnosed Alzheimer’s disease. Ann. Neurol. 19 (5), 415–424. doi:10.1002/ana.410190502
Saper, R. B., Phillips, R. S., Sehgal, A., Khouri, N., Davis, R. B., Paquin, J., et al. (2008). Lead, mercury, and arsenic in US- and Indian-manufactured ayurvedic medicines sold via the internet. JAMA 300 (8), 915–923. doi:10.1001/jama.300.8.915
Schaffner, T. E. (2001). The role of complementary and alternative medicine: accommodating pluralism. Available online at: https://books.google.co.in/books?id=ryEnQlgMCT0C.
Sehgal, N., Gupta, A., Valli, R. K., Joshi, S. D., Mills, J. T., Hamel, E., et al. (2012). Withania somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc. Natl. Acad. Sci. 109 (9), 3510–3515. doi:10.1073/pnas.1112209109
Sharma, M., Pal, P., and Gupta, S. K. (2024). Advances in Alzheimer’s disease: a multifaceted review of potential therapies and diagnostic techniques for early detection. Neurochem. Int. 177, 105761. doi:10.1016/j.neuint.2024.105761
shortt, J., Ott, C. J., Johnstone, R. W., and Bradner, J. E. (2017). Erratum: a chemical probe toolbox for dissecting the cancer epigenome. Nat. Rev. Cancer 17 (4), 268. doi:10.1038/nrc.2017.26
Shoukat, S., Zia, M. A., Uzair, M., Attia, K. A., Abushady, A. M., Fiaz, S., et al. (2023). Bacopa monnieri: a promising herbal approach for neurodegenerative disease treatment supported by in silico and in vitro research. Heliyon 9 (11), e21161. doi:10.1016/j.heliyon.2023.e21161
Small, G. W., Siddarth, P., Li, Z., Miller, K. J., Ercoli, L., Emerson, N. D., et al. (2018). Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: a double-blind, placebo-controlled 18-month trial. Am. J. Geriatric Psychiatry 26 (3), 266–277. doi:10.1016/j.jagp.2017.10.010
Steiner-Lim, G. Z., Bensoussan, A., Andrews-Marney, E. R., Al-Dabbas, M. A., Cave, A. E., Chiu, C. L., et al. (2023). A randomized, double-blind, placebo-controlled, parallel-group 12-week pilot phase II trial of SaiLuoTong (SLT) for cognitive function in older adults with mild cognitive impairment. Alzheimer’s and Dementia Transl. Res. and Clin. Interventions 9 (4), e12420. doi:10.1002/trc2.12420
Strandberg, T. E. (2019). Challenges of a statin trial in older people. J. Am. Geriatr. Soc. 67 (4), 856–857. doi:10.1111/jgs.15763
Sutalangka, C., Wattanathorn, J., Muchimapura, S., and Thukham-mee, W. (2013). Moringa oleifera mitigates memory impairment and neurodegeneration in animal model of age-related dementia. Oxid. Med. Cell. Longev. 2013, 695936–695939. doi:10.1155/2013/695936
Takizawa, C., Thompson, P. L., van Walsem, A., Faure, C., and Maier, W. C. (2014). Epidemiological and economic burden of Alzheimer’s disease: a systematic literature review of data across Europe and the United States of America. J. Alzheimer’s Dis. 43 (4), 1271–1284. doi:10.3233/JAD-141134
Tatulian, S. A. (2022). Challenges and hopes for Alzheimer’s disease. Drug Discov. Today 27 (4), 1027–1043. doi:10.1016/j.drudis.2022.01.016
Tripathi, P., Lodhi, A., Rai, S., Nandi, N., Dumoga, S., Yadav, P., et al. (2024). Review of pharmacotherapeutic targets in Alzheimer’s disease and its management using traditional medicinal plants. Degener. Neurol. Neuromuscul. Dis. 14, 47–74. doi:10.2147/DNND.S452009
Tuzimski, T., and Petruczynik, A. (2022). Determination of anti-alzheimer’s disease activity of selected plant ingredients. Molecules 27 (10), 3222. doi:10.3390/molecules27103222
Ventola, C. L. (2010). Current issues regarding complementary and alternative medicine (CAM) in the United States: Part 1: the widespread use of CAM and the need for better-informed health care professionals to provide patient counseling. P T 35 (8), 461–468.
Wadhawan, S., Biswkarma, V. K., Chaudhary, A., and Masand, P. (2024). A critical review based on preclinical studies of medicinal plants for the management of Alzheimer’s disease. Curr. Bioact. Compd. 20 (4). doi:10.2174/1573407219666230807150426
Wang, C., Zhang, X., Teng, Z., Zhang, T., and Li, Y. (2014a). Downregulation of PI3K/Akt/mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic mice. Eur. J. Pharmacol. 740, 312–320. doi:10.1016/j.ejphar.2014.06.051
Wang, J., Bi, W., Cheng, A., Freire, D., Vempati, P., Zhao, W., et al. (2014b). Targeting multiple pathogenic mechanisms with polyphenols for the treatment of Alzheimer’s disease-experimental approach and therapeutic implications. Front. Aging Neurosci. 6, 6. doi:10.3389/fnagi.2014.00042
Webers, A., Heneka, M. T., and Gleeson, P. A. (2020). The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol. Cell. Biol. 98 (1), 28–41. doi:10.1111/imcb.12301
Weerasinghe, P. (2024). Molecular targets of ashwagandha (Withania somnifera), Korean ginseng (panax ginseng), brahmi (Bacopa monneri) and gotu kola (Centella asiatica) in the treatment of autism spectrum disorder (asd): a global health perspective. Adv. Complementary and Altern. Med. 8 (3). doi:10.31031/acam.2024.08.000687
WHO (2024). WHO guidelines on good agricultural and collection practices (GACP) for medicinal plants.
Wright, K. M., Bollen, M., David, J., Speers, A. B., Brandes, M. S., Gray, N. E., et al. (2022). Pharmacokinetics and pharmacodynamics of key components of a standardized Centella asiatica product in cognitively impaired older adults: a phase 1, double-blind, randomized clinical trial. Antioxidants 11 (2), 215. doi:10.3390/antiox11020215
Wu, S., Zheng, L., Huang, J., Wang, S., Huang, Q., Guo, S., et al. (2024). Icariin, a natural flavonoid glucoside, inhibits neuroinflammation in mice with triple-transgenic Alzheimer’s disease by regulating the Akt/GSK-3β signaling pathway. J. Funct. Foods 118, 106263. doi:10.1016/j.jff.2024.106263
Xie, L., Zhu, Q., and Lu, J. (2022). Can we use ginkgo biloba extract to treat Alzheimer’s disease? Lessons from preclinical and clinical studies. Cells 11 (3), 479. doi:10.3390/cells11030479
Xin, G. Z., Qi, L. W., Shi, Z. Q., Li, P., Hao, H. P., Wang, G. J., et al. (2011). Strategies for integral metabolism profile of multiple compounds in herbal medicines: pharmacokinetics, metabolites characterization and metabolic interactions. Curr. Drug Metab. 12 (9), 809–817. doi:10.2174/138920011797470164
Xing, D., Yoo, C., Gonzalez, D., Jenkins, V., Nottingham, K., Dickerson, B., et al. (2022). Effects of acute ashwagandha ingestion on cognitive function. Int. J. Environ. Res. Public Health 19 (19), 11852. doi:10.3390/ijerph191911852
Yan, F., Liu, J., Chen, M. X., Zhang, Y., Wei, S. J., Jin, H., et al. (2023). Icariin ameliorates memory deficits through regulating brain insulin signaling and glucose transporters in 3×Tg-AD mice. Neural Regen. Res. 18 (1), 183–188. doi:10.4103/1673-5374.344840
Yang, G., Wang, Y., Sun, J., Zhang, K., and Liu, J. (2015). Ginkgo biloba for mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis of randomized controlled trials. Curr. Top. Med. Chem. 16 (5), 520–528. doi:10.2174/1568026615666150813143520
Yassine, H. N., and Finch, C. E. (2020). APOE alleles and diet in brain aging and Alzheimer’s disease. Front. Aging Neurosci. 12, 12. doi:10.3389/fnagi.2020.00150
Yiannopoulou, K. G., and Papageorgiou, S. G. (2020). Current and future treatments in alzheimer disease: an update. J. Cent. Nerv. Syst. Dis. 12, 117957352090739. doi:10.1177/1179573520907397
Yin, H., Zhang, J., Lin, H., Qiao, Y., Wang, R., Lu, H., et al. (2009). Effect of traditional Chinese medicine Shu-mai-tang on angiogenesis, arteriogenesis and cardiac function in rats with myocardial ischemia. Phytotherapy Res. 23 (1), 92–98. doi:10.1002/ptr.2565
Yu, J. T., Xu, W., Tan, C. C., Andrieu, S., Suckling, J., Evangelou, E., et al. (2020). Evidence-based prevention of Alzheimer’s disease: systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J. Neurol. Neurosurg. Psychiatry 91 (11), 1201–1209. doi:10.1136/jnnp-2019-321913
Zetterberg, H., and Bendlin, B. B. (2021). Biomarkers for Alzheimer’s disease—preparing for a new era of disease-modifying therapies. Mol. Psychiatry 26 (1), 296–308. doi:10.1038/s41380-020-0721-9
Zhang, H. (2012). New insights into huperzine A for the treatment of Alzheimer’s disease. Acta Pharmacol. Sin. 33 (9), 1170–1175. doi:10.1038/aps.2012.128
Zhang, J., Yang, C., Wei, D., Li, H., Leung, E. L. H., Deng, Q., et al. (2019). Long-term efficacy of Chinese medicine Bushen Capsule on cognition and brain activity in patients with amnestic mild cognitive impairment. Pharmacol. Res. 146, 104319. doi:10.1016/j.phrs.2019.104319
Zhao, Y., Deng, H., Li, K., Wang, L., Wu, Y., Dong, X., et al. (2019). Trans-cinnamaldehyde improves neuroinflammation-mediated NMDA receptor dysfunction and memory deficits through blocking NF-κB pathway in presenilin1/2 conditional double knockout mice. Brain Behav. Immun. 82, 45–62. doi:10.1016/j.bbi.2019.07.032
Zieneldien, T., Kim, J., and Cao, C. (2022). The multifaceted role of neuroprotective plants in Alzheimer’s disease treatment. Geriatrics 7 (2), 24. doi:10.3390/geriatrics7020024
Keywords: Alzheimer’s disease (AD), traditional medicine, complementary and integrative medicine (TCIM), herbal therapies, neuroinflammation, cognitive decline
Citation: Sneha Sri R, Pavithra T, Vinciya T, Santhosh Kumar V, Harikrishnan N, Begum RF and Ankul Singh S (2025) Integrative approaches in Alzheimer’s disease: evaluating the potential of traditional, complementary, and integrative medicine (TCIM). Front. Pharmacol. 16:1561702. doi: 10.3389/fphar.2025.1561702
Received: 16 January 2025; Accepted: 04 June 2025;
Published: 30 June 2025.
Edited by:
Chun Guang Li, Western Sydney University, AustraliaReviewed by:
Majid Sharifi-Rad, Zabol University, IranHee Geun Jo, Gachon University, Republic of Korea
Copyright © 2025 Sneha Sri, Pavithra, Vinciya, Santhosh Kumar, Harikrishnan, Begum and Ankul Singh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: S. Ankul Singh, YW5rdWxzaW5naDU3QGdtYWlsLmNvbQ==; Rukaiah Fatma Begum, cnVrYWlhaDc4NkBnbWFpbC5jb20=
 R. Sneha Sri1
R. Sneha Sri1 Rukaiah Fatma Begum
Rukaiah Fatma Begum S. Ankul Singh
S. Ankul Singh