- 1First Clinical Medical College, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
- 2Shandong University of Traditional Chinese Medicine Affiliated Hospital, Jinan, Shandong, China
Objective: This study aims to analyze potential adverse events (AEs) associated with ripretinib and sunitinb in gastrointestinal stromal tumor (GIST) treatment using data from the FDA Adverse Event Reporting System (FAERS). The findings provide insights for future research to improve the safety and clinical management of ripretinib and sunitinib.
Methods: Adverse Drug Event (ADE) reports related to ripretinib and sunitinib were extracted from the FAERS database, covering the period from Q2 2020 to Q4 2024 and Q1 2006 to Q4 2024, respectively. ADEs were classified and described according to Preferred Terms (PTs) and System Organ Classes (SOCs) in the Medical Dictionary for Regulatory Activities (MedDRA). Disproportionality analysis, including Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Multi-Item Gamma Poisson Shrinker (MGPS), was employed to identify significant signals.
Results: A total of 3,636 and 34,768 ADE reports related to ripretinib and sunitinib were identified using four disproportionality analysis methods. The top five ADR signals for ripretinib include hepatic embolization, tumor compression, hyperkeratosis, tumor excision and tumor pain. For sunitinib, the five strongest ADR signals are metastatic renal cell carcinoma, diffuse uveal melanocytic proliferation, renal cancer metastasis, connective tissue neoplasm and salivary gland fistula. Both drugs share significant ADRs including palmar-plantar erythrodysesthesia syndrome, disease progression and hyperkeratosis. Furthermore, subgroup analysis was conducted to explore sex difference in ripretinib and sunitinib.
Conclusion: This study validated known AEs and identified new potential safety signals associated with ripretinib and sunitinib in GIST treatment. These findings contribute to the understanding of ripretinib and sunitinib, providing valuable evidence for improving its clinical use.
1 Introduction
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor of the gastrointestinal tract, primarily affecting the stomach and small intestine, with an estimated global incidence of 10–15 cases per million individuals (Søreide et al., 2016) (Kelly et al., 2021). Targeted cancer therapies have emerged as a significant advancement in prolonging the survival of patients with advanced GIST (3). Approximately 80% of GIST cases harbor activating mutations in the stem cell factor receptor (KIT) receptor tyrosine kinase gene, while 5%–10% involve mutations in the platelet-derived growth factor receptor alpha (PDGFRA) gene (Dhillon, 2020). Identifying the molecular subtypes of GIST is of critical importance in clinical treatment. Although imatinib is highly effective as first-line therapy for GISTs, secondary resistance mutations frequently arise, necessitating subsequent second-line treatment with sunitinib (Cohen et al., 2021). Sunitinib, an oral multi-targeted tyrosine kinase inhibitor (TKI), mediates antitumor activity by simultaneously inhibiting angiogenesis and tumor cell proliferation through targeting vascular endothelial growth factor receptors (VEGFRs), platelet-derived growth factor receptors (PDGFRs), and KIT (6).
In GIST patients exhibiting progression after sequential treatment with imatinib and sunitinib, tumor cells develop complex kinase conformational alterations via acquired mutations, significantly reducing the efficacy of conventional TKIs. This clinical challenge prompted the development of novel targeted therapies, notably ripretinib, a breakthrough fourth-line agent. Ripretinib, the first FDA-approved broad-spectrum kinase inhibitor for fourth-line GIST treatment (Ripretinib for gastrointestinal stromal tumours, 2021), uniquely targets both the kinase activation loop and switch pocket, effectively suppressing diverse resistance mutations including KIT and PDGFRA variants (Goggin et al., 2022).
In May 2020, ripretinib received FDA approval based on results from the Phase III INVICTUS trial, indicated for advanced GIST patients with disease progression following previous treatments with imatinib, sunitinib, and regorafenib. Its favorable safety profile and enhanced tolerability facilitate prolonged disease control in advanced stages, representing a significant advancement in addressing TKI resistance. Although Ripretinib demonstrates favorable safety and tolerability profiles in clinical trials, adverse events (AEs) remain inevitable in real-world applications. In the phase III INVICTUS trial, the most common AEs (incidence >20%) included alopecia, myalgia, nausea, fatigue, palmar–plantar erythrodysesthesia, and diarrhoea (Blay et al., 2020). Identifying these AEs is crucial for ensuring patient safety and optimizing clinical outcomes, necessitating the application of data mining algorithms to detect potential safety signals of Ripretinib in real-world settings.
The U.S. Food and Drug Administration Adverse Event Reporting System (FAERS) is one of the largest post-marketing safety monitoring databases, and the reliability and validity of its data have been widely recognized in the industry (Chen et al., 2021; Yu et al., 2021). Using the FAERS database, pharmacovigilance research related to ripretinib and sunitinib was conducted to identify adverse events (AEs) not described on the drug label. These findings not only enhance research on ripretinib by comparing classical drug-sunitinib, providing valuable references for clinical drug use.
2 Materials and methods
2.1 Data source
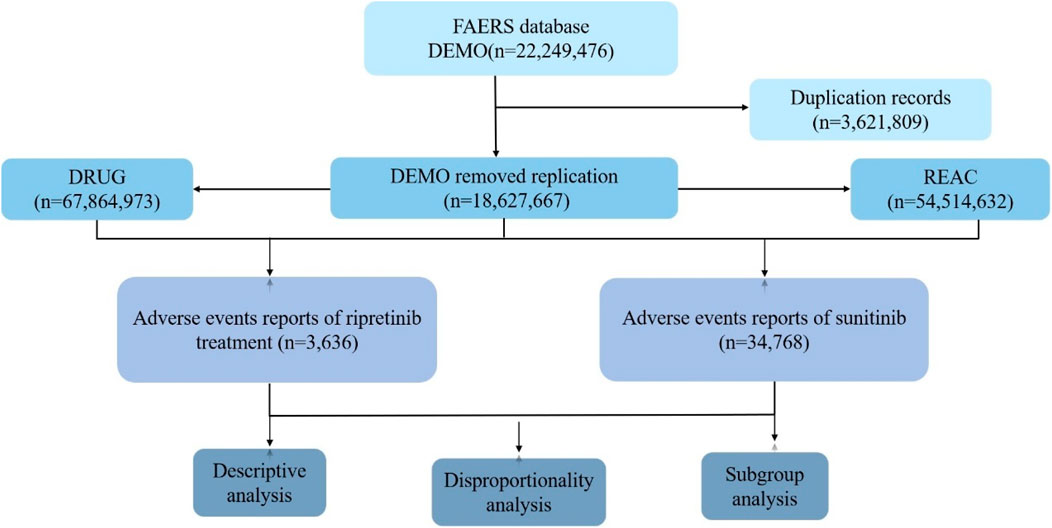
The FAERS database is a publicly accessible, voluntary, and spontaneous reporting system designed for post-marketing surveillance of FDA-approved drugs. It collects adverse drug event (ADE) reports from healthcare professionals, patients, and pharmaceutical manufacturers worldwide, reflecting real-world ADE occurrences (Guo et al., 2022; Pan et al., 2024). This study aims to systematically evaluate the post-marketing safety of ripretinib and sunitinib (Figure 1). Only reports of ripretinib and sunitinib as primary suspect drugs were included. For cases with duplicate CASEIDs, the record with the most recent FDA_DT or the highest PRIMARYID was retained. Subsequently, AEs were standardized, classified, and described based on the Preferred Terms (PT) and System Organ Classes (SOC) defined in the Medical Dictionary for Regulatory Activities (MedDRA 26.1). Some of the analysis results were computed using R software 4.4.3, with packages including “dplyr”, “ggplot2″, “forestplot” and “data.table” for data handling and visualization.
Since the FAERS database is publicly accessible and patient records are anonymized and de-identified, this study does not involve informed consent or ethical approval.
2.2 Methods of data analysis
Disproportionality analysis is a tool used to generate hypotheses about specific drugs and AEs and to clinically evaluate potential case reports (Zh et al., 2025). Four methods were employed for ADE signal mining, including Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Multi-item Gamma Poisson Shrinker (MGPS) (Luo et al., 2025). The ROR and PRR algorithms are non-Bayesian methods, with the advantage of ROR being its ability to correct bias when event reports are limited, while the advantage of PRR is lower sensitivity by the omission of certain AEs (Li et al., 2025). The MHRA method extends PRR by combining PRR values with absolute report numbers and chi-square values, ensuring a minimum number of case combinations (Zhou et al., 2023). The BCPNN method can perform early signal detection even with limited or missing data, and as the number of reports increases, the detection results become more stable (Godfrey et al., 2025). Compared to non-Bayesian algorithms, Bayesian algorithms have higher specificity, signal stability, and lower misclassification probabilities. The data analysis process in this study is illustrated in Figure 1. The formulas and signal detection criteria of the four methods refer to the methods given in previous literature (Xiong et al., 2023; Kuai et al., 2024). In this study, AE signals were considered significant only if they met the criteria for all four algorithms simultaneously.
3 Results
3.1 General analysis of AEs in ripretinib and sunitinib
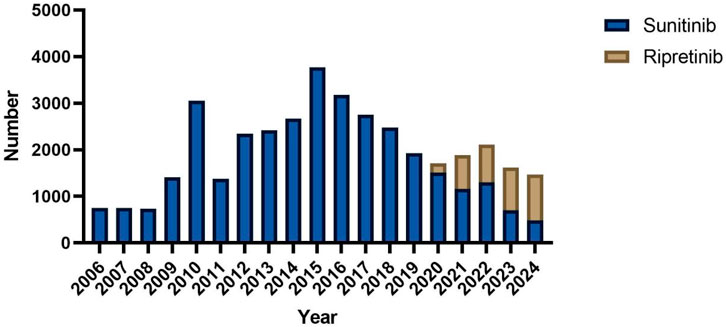
A total of 3,636 and 34,768 individual AE reports for ripretinib and sunitinib, respectively, were extracted from the FAERS database (Table 1). In terms of gender distribution, 54.2% of ripretinib-related reports were male, compared to 59.3% in the sunitinib group. Female patients accounted for 43.7% in the ripretinib group and 31.8% in the sunitinib group. Age information was missing in 52.0% of ripretinib cases and 24.8% of sunitinib cases. Among the available data, patients aged ≥65 years comprised the largest proportion in both groups (29.5% for ripretinib and 38.1% for sunitinib), followed by those aged 18–64 years (18.4% vs 36.9%). Reports involving patients under 18 years were rare in both groups. Weight data were largely incomplete, with 91.1% missing in the ripretinib group and 73.4% in the sunitinib group. Among the reports with available weight, most patients weighed between 50 and 100 kg in both groups. Regarding reporter type, most ripretinib cases were submitted by consumers (61.4%), whereas sunitinib cases were more frequently reported by physicians (29.9%) and consumers (38.7%). Reports from other healthcare professionals, such as pharmacists and non-physician health professionals, were more prevalent in the sunitinib group. The largest proportion of outcomes reported for ripretinib was categorized as “hospitalization,” accounting for 18.2% of the total cases expect missing cases. “other outcomes” and “death” were also important outcomes, comprising 11.4% and 10.6% of total reports. For sunitinib, death was the most frequent outcome (29.8% of cases), followed by “other outcomes” (22.1%) and “hospitalization” (21.3%). Geographically, the majority of ripretinib reports originated from the United States (93.1%), whereas sunitinib reports were more geographically diverse, with 49.1% from the United States, followed by notable contributions from Japan (5.6%), Argentina (5.3%), China (5.2%), and India (4.0%). The annual distribution of reports revealed distinct patterns between sunitinib and ripretinib. Sunitinib-related reports have been submitted consistently since 2006, with a marked increase beginning in 2010 and peaking around 2016. After 2017, the number of reports gradually declined. In contrast, ripretinib-related reports only appeared from 2020 onwards, aligning with its later market approval. Since then, the number of ripretinib reports has shown a steady increase, surpassing sunitinib in total annual reports by 2022 (Figure 2).
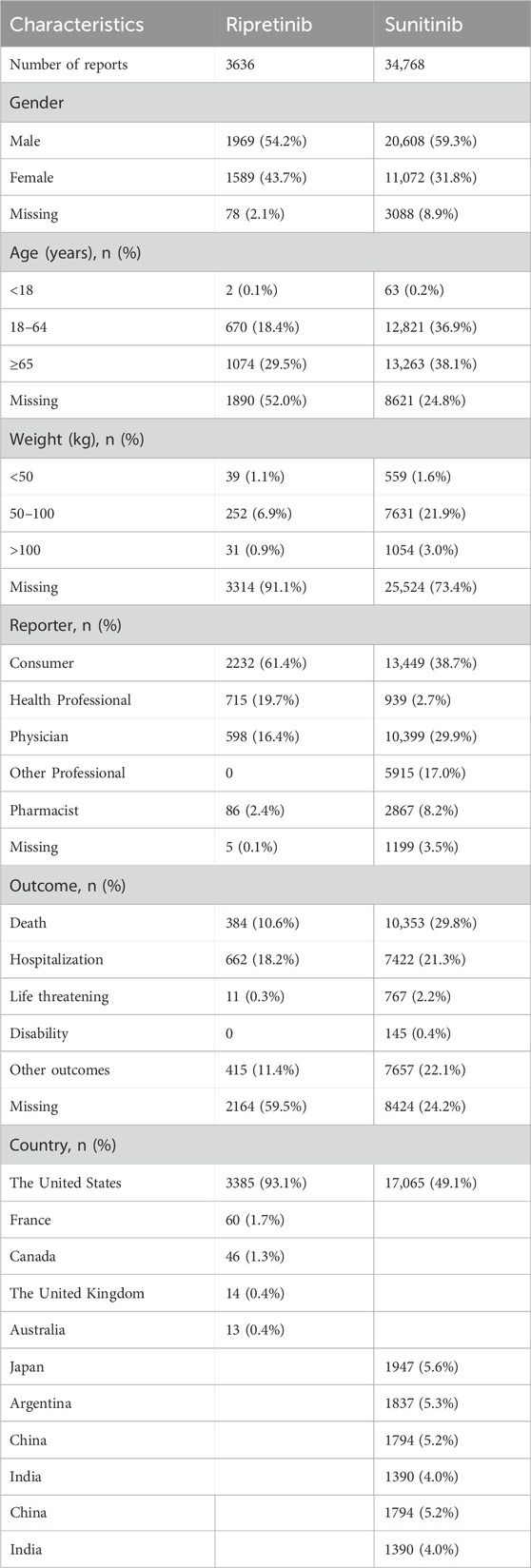
Table 1. Clinical characteristics of patients with GIST in sunitinib and ripretinib from the FAERS database.
3.2 Signal detection at the SOC level
Ripretinib and snuitinib related AEs were associated with 27 distinct system organ classes (SOCs), of which 7 and 10 met the criteria of all four disproportionality analysis methods (Figure 3). Among all SOCs, the most commonly reported in ripretinib were general disorders and administration site conditions (n = 2579), skin and subcutaneous tissue disorders (n = 1637) and gastrointestinal disorders (n = 1632). General disorders and administration site conditions (n = 27,124), gastrointestinal disorders (n = 23,592) and investigations (n = 11,178) were most frequent SOCs in snuitinb, which mostly consistent with ripretinib. Moreover, as for ripretinib, attention should also be paid to less common SOCs such as musculoskeletal and connective tissue disorders and neoplasms benign, malignant and unspecified (incl cysts and polyps) in clinical practice.
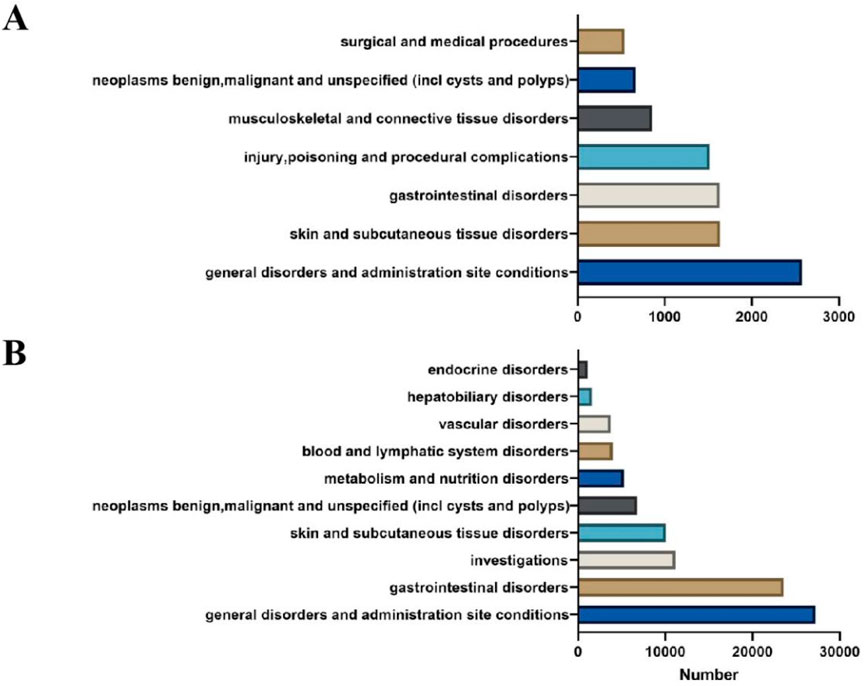
Figure 3. Comparation between ripretinib and sunitinib at the SOC level. (A) AE signals at the SOC level in ripretinib. (B) AE signals at the SOC level in sunitinib.
3.3 Signal detection at the PT level
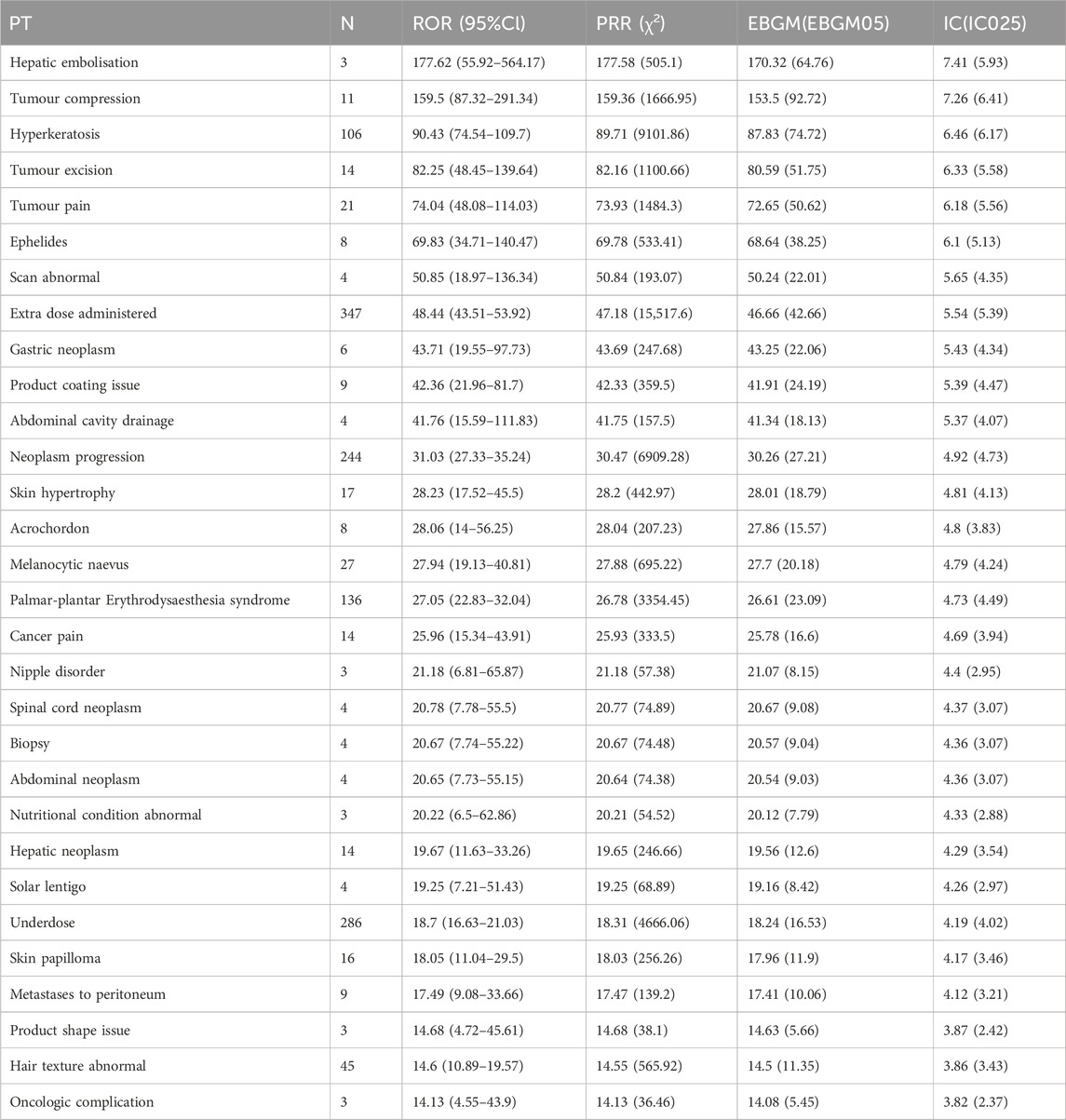
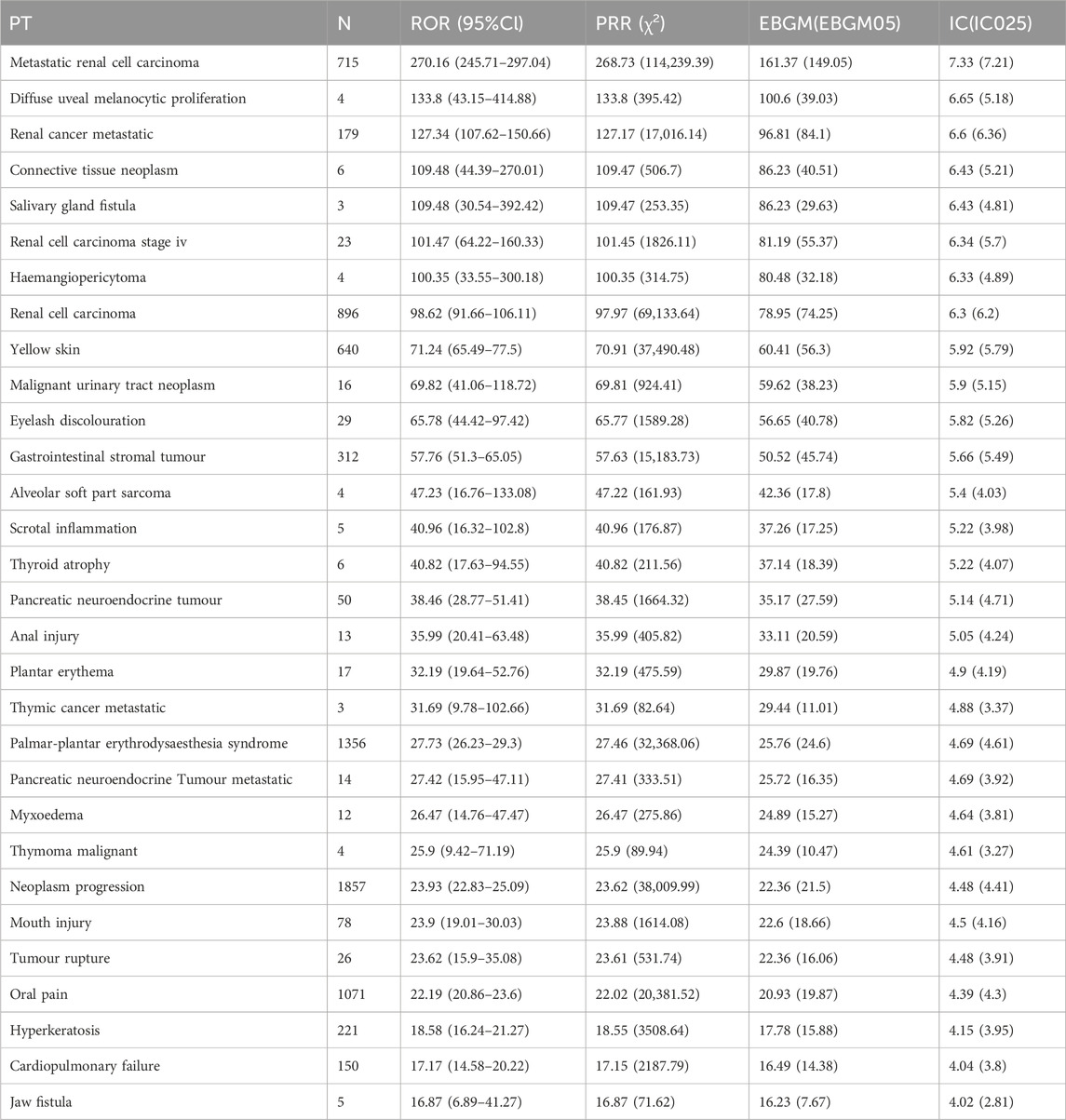
Based on disproportionality analysis, both ripretinib and sunitinib exhibit significant ADR signals in Table 2, 3. The top five ADR signals for ripretinib include hepatic embolization (ROR = 177.62, 95%CI: 55.92–564.17), tumor compression (ROR = 159.5, 95%CI: 87.32–291.34), hyperkeratosis (ROR = 90.43, 95%CI: 74.54–109.7), tumor excision (ROR = 82.25, 95%CI: 48.45–139.64), and tumor pain (ROR = 74.04, 95%CI: 48.08–114.03). For sunitinib, the five strongest ADR signals are metastatic renal cell carcinoma (ROR = 270.16, 95%CI: 245.71–297.04), diffuse uveal melanocytic proliferation (ROR = 133.8, 95%CI: 43.15–414.88), renal cancer metastasis (ROR = 127.34, 95%CI: 107.62–150.66), connective tissue neoplasm (ROR = 109.48, 95%CI: 44.39–270.01), and salivary gland fistula (ROR = 109.48, 95%CI: 30.54–392.42).
Both drugs share significant ADRs including palmar-plantar erythrodysesthesia syndrome (Ripretinib: ROR = 27.05; Sunitinib: ROR = 27.73), disease progression (Ripretinib: ROR = 31.03; Sunitinib: ROR = 23.93), and hyperkeratosis (Ripretinib: ROR = 90.43; Sunitinib: ROR = 18.58), indicating possible similarities in pharmacological effects or biological mechanisms.
However, significant differences exist in the ADR profiles between the two drugs. Ripretinib tends to cause tumor-associated direct complications such as tumor compression and surgical-related reactions, while Sunitinib predominantly involves metastasis of specific tumor types and rare pathological conditions like renal cancer metastasis and uveal melanocytic proliferation. Consequently, individualized risk management strategies should be implemented based on their distinct ADR characteristics in clinical practice.
3.4 Gender-based differences in AEs
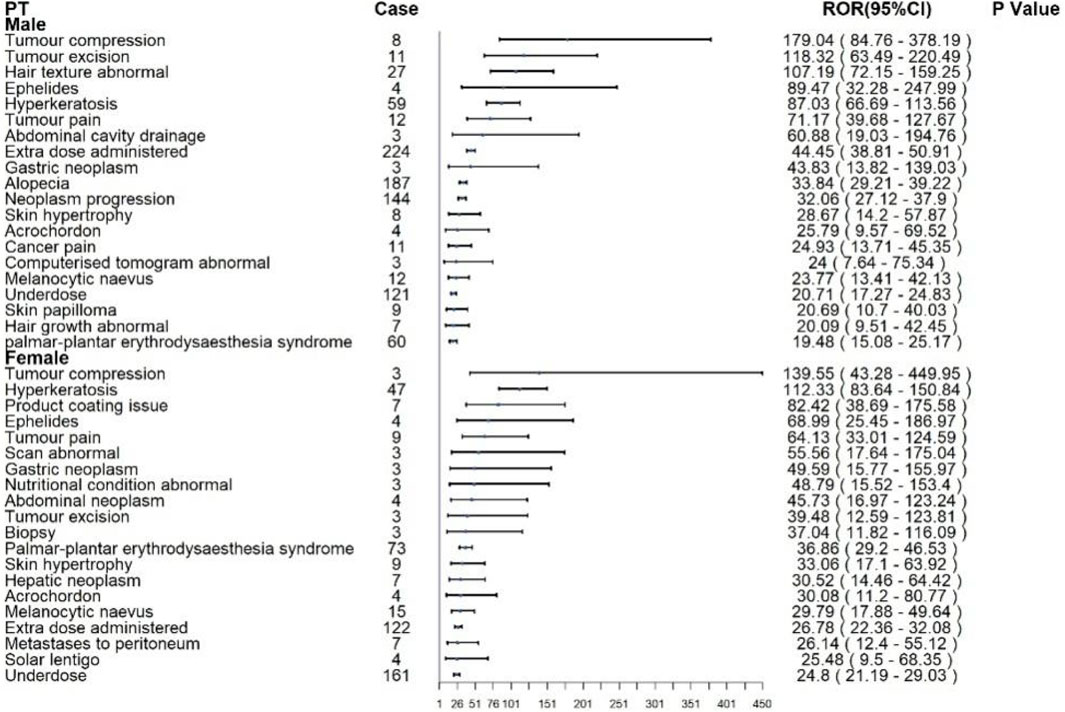
Subgroup analyses were conducted to identify sex-specific patterns of AEs associated with ripretinib and sunitinib. For ripretinib in Figure 4, male patients most frequently reported alopecia (n = 187), extra dose administered (n = 224), and neoplasm progression (n = 144), with significant disproportionality signals (RORs: 33.84, 44.45, and 32.06, respectively). Notably, tumor compression (ROR: 179.04; 95% CI: 84.76–378.19), tumor excision (ROR: 118.32), and hair texture abnormal (ROR: 107.19) showed the strongest associations. Other relevant AEs included hyperkeratosis (n = 59, ROR: 87.03) and palmar-plantar erythrodysaesthesia syndrome (n = 60, ROR: 19.48).Among females, the top reported AEs were underdose (n = 161), extra dose administered (n = 122), and palmar-plantar erythrodysaesthesia syndrome (n = 73). Tumor compression (ROR: 139.55; 95% CI: 43.28–449.95), hyperkeratosis (ROR: 112.33), and product coating issues (ROR: 82.42) demonstrated strong disproportionality.
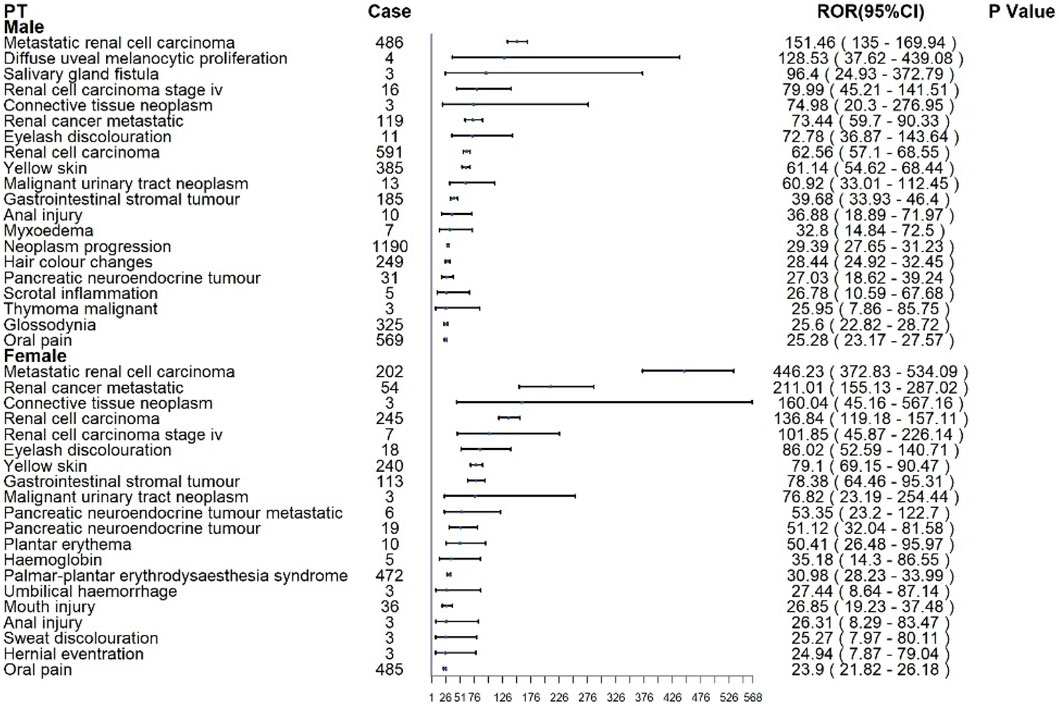
For sunitinib in Figure 5, males most commonly reported renal cell carcinoma (n = 591), metastatic RCC (n = 486), neoplasm progression (n = 1,190), and oral pain (n = 569). The strongest disproportionality was observed for metastatic RCC (ROR: 151.46; 95% CI: 135.0–169.94) and diffuse uveal melanocytic proliferation (BDUMP) (ROR: 128.53; 95% CI: 37.62–439.08), the latter being a rare but fatal ocular event. In female patients, renal cell carcinoma (n = 245), metastatic RCC (n = 202), and oral pain (n = 485) were the most frequent. Disproportionality was greatest for metastatic RCC (ROR: 446.23; 95% CI: 372.83–534.09), renal cancer metastatic (ROR: 211.01), and connective tissue neoplasm (ROR: 160.04).
4 Discussion
The ADR analysis derived from the FAERS database reveal critical similarities and differences between ripretinib and sunitinib, two widely used multi-target TKIs. Expanding upon previous studies that examined ripretinib individually, this analysis includes a comparative approach incorporating subgroup analysis to provide a more comprehensive evaluation of their ADR characteristics.
Both ripretinib and sunitinib exhibited overlapping ADRs, predominantly palmar-plantar erythrodysesthesia syndrome (PPES), hyperkeratosis, and paradoxical disease progression. Mechanistic analysis suggests that PPES may originate from potent VEGFR signaling inhibition by both agents, leading to microvascular dysfunction and subsequent inflammatory tissue damage (Zhang et al., 2023). Notably, a multicenter phase III trial revealed significantly higher PPES incidence in the treatment group compared with placebo (12.5% vs. 0.8%), establishing it as the second most frequent ADR following hypertension (Lin et al., 2023), which corroborates our findings. The comparable hyperkeratosis rates observed with both TKIs imply a class effect potentially mediated through EGFR or PDGFR pathway inhibition, which may disrupt keratinocyte proliferation and differentiation homeostasis (Malovitski et al., 2023). However, current pharmacovigilance data present an apparent discrepancy: while case report document ripretinib-associated hyperkeratosis (Muskat et al., 2022), no published clinical studies explicitly link sunitinib to this dermatological manifestation. This comparative analysis provides clinical evidence that hyperkeratosis represents a TKI-class adverse effect rather than a ripretinib-specific phenomenon. Of particular clinical significance was the identification of disease progression as an ADR, which likely reflects treatment failure mechanisms involving secondary kinase mutations that confer therapeutic resistance or insufficient target inhibition. This paradoxical phenomenon underscores the need for molecular monitoring during TKI therapy escalation.
Subgroup analysis by gender offers critical insights into drug-specific ADRs. For ripretinib, both male and female patients exhibited elevated risks of tumor compression, hyperkeratosis, and tumor-related surgical interventions. Notably, female patients demonstrated higher incidences of tumor-associated pain and abnormal imaging findings, while males were more frequently affected by direct tumor-related complications, such as tumor excision and abdominal drainage. These disparities may reflect sex-specific pharmacokinetic and pharmacodynamic profiles, including hormonal influences and differences in metabolic pathways. In contrast, gender differences in sunitinib-related ADRs were more pronounced. Male patients showed higher frequencies of metastatic renal cell carcinoma, salivary gland fistula, and eyelash discoloration, whereas females exhibited increased risks of renal cancer metastasis and yellow skin pigmentation. The ocular and cutaneous toxicities are likely attributable to sunitinib’s potent inhibition of VEGFR, which disrupts melanocyte function and melanin synthesis (Jin et al., 2023; Shah et al., 2021).
Despite these observations, research on sex-specific genetic mechanisms underlying ripretinib and sunitinib responses in GIST remains limited. However, genomic analyses in clear cell renal cell carcinoma (CCRCC) have revealed that female patients with elevated DKC1 expression also exhibit increased TERC levels, a pattern associated with reduced therapeutic response and shorter progression-free survival (Yuan et al., 2023). These findings suggest sex-dependent interactions between telomerase-related genes and tyrosine kinase inhibitor efficacy. Given the documented gender disparities in clinical ADRs among GIST patients—particularly cutaneous and ocular toxicities—comprehensive investigations into sex-specific molecular mechanisms are warranted. Key areas include kinase resistance pathways, telomerase regulatory networks, and polymorphisms affecting drug metabolism. Such research is essential to elucidate the biological underpinnings of therapeutic heterogeneity and to inform precision oncology through gender-stratified treatment strategies. A comparative mechanistic analysis further distinguishes ripretinib and sunitinib. Ripretinib is a novel type II switch control tyrosine kinase inhibitor designed to broadly inhibit both primary and secondary KIT and PDGFRA mutations associated with the progression of GIST (28, 29). Ripretinib exhibits high potency against the inactive form of receptor tyrosine kinases (RTKs) by binding to the switch pocket and activation loop, stabilizing the protein in an inactive conformation and thereby inhibiting its active state. This mechanism makes ripretinib a “switch control inhibitor,” with broad inhibitory activity against various secondary mutations, including KIT exons 13 (V654A), 14 (T670I), 17 (D816), and 18 (A829P) (Yu et al., 2021; Janku et al., 2020). The drug was approved by the U.S. FDA in May 2020 for patients with advanced GIST who have failed at least three prior kinase inhibitors. Relevant studies support that ripretinib provides clinical benefits at a daily dose of 150mg, with better safety and patient tolerability compared to other treatments (Zalcberg).
Through a comprehensive analysis of ripretinib-related AEs in the FAERS database, we observed that the adverse signals associated with ripretinib primarily involved categories such as gastrointestinal disorders, general disorders and administration site reactions, and skin and subcutaneous tissue diseases. These results are consistent with the common adverse events reported in ripretinib’s drug labeling and clinical trials, such as nausea, vomiting, and fatigue (Dhillon, 2020). Ripretinib predominantly induces ADRs reflecting significant tumor volume changes and vascular alterations, such as hepatic embolization, which might be attributed to the profound vascular disruption caused by VEGFR pathway inhibition (Roskoski, 2021). Tumor compression, excision, and associated pain likely reflect the drug’s effective antitumor activity leading to rapid tumor cell necrosis, edema formation, and structural deformation, necessitating medical interventions (Schwartz, 2022; Lostes-Bardaji et al., 2021). Furthermore, this study identified some high-signal adverse events not mentioned in the drug’s prescribing information, such as skin papillomas, melanocytic nevi, and blood iron reduction. These new findings indicate that ripretinib may have potential effects on the skin, blood, and metabolism, which clinicians should monitor closely (Mühlenberg et al., 2024). In addition, the study also identified signals for adverse events associated with severe outcomes. Among the reported serious adverse outcomes of ripretinib, 18.2% required hospitalization, and 10.6% involved deaths, some of which may be related to disease progression caused by the tumor. However, the possibility of severe reactions triggered by the drug itself cannot be ruled out. Notably, rare but high-signal adverse events, such as hepatic neoplasm, were reported, which are not explicitly described in the prescribing information. Their biological mechanisms and clinical significance require further research and validation.
Conversely, AEs of sunitinib prominently features distinct pathologic conditions such as diffuse uveal melanocytic proliferation, connective tissue neoplasm, and salivary gland fistula. Ocular adverse events associated with sunitinib therapy necessitate increased clinical vigilance. A case report described a male patient with renal cell carcinoma who developed bilateral diffuse uveal melanocytic proliferation (BDUMP) during sunitinib treatment, resulting in a fatal outcome (Parakh et al., 2022). BDUMP is a rare paraneoplastic ocular syndrome, typically occurring in patients with advanced, often occult, systemic malignancies (Tong et al., 2021). It serves as both a poor prognostic indicator and a marker of disease progression. The pathogenesis is hypothesized to involve ectopic production of growth factors or hormones that exert paracrine effects on distant ocular tissues. Experimental studies have demonstrated that IgG-enriched plasma fractions contain cultured melanocyte elongation and proliferation-stimulating factors (CMEP), which induce abnormal proliferation of choroidal melanocytes and retinal pigment epithelial cells (Hu et al., 2023). These pathological changes disrupt retinal pigment epithelium function, ultimately compromising the outer blood-retinal barrier (Przeździecka-Dołyk et al., 2020; Sarkar et al., 2022). Additionally, unique ocular AEs like and eyelash discoloration could result from disrupted melanocyte function and pigmentary alterations secondary to VEGFR inhibition.
Clinically, these differential AEs underscore the necessity of tailored patient risk assessment and individualized monitoring strategies. For ripretinib, clinicians should closely monitor hepatic function, tumor-related symptoms, and dermatologic health. For sunitinib, rigorous ophthalmologic evaluations, renal function monitoring, and attention to pigmentation changes are recommended, particularly in long-term therapy.
This study has several limitations. First, the FAERS database is a spontaneous reporting system that relies on voluntarily submitted adverse event reports. This reliance on spontaneous reporting may lead to underreporting of mild or common events or unusual events may be overreported. Second, the underlying conditions treated with certain drugs may predispose patients in GIST, acting as a confounding factor. Furthermore, establishing causality in pharmacovigilance and observational cohort studies is inherently challenging due to the lack of complete information in FAERS cases, such as dosage, frequency, duration of exposure, patient comorbidities, onset times, and other critical clinical details. This missing information limits the ability to fully analyze potential associations. Consequently, although we achieved a comprehensive analysis of ripretinib’s adverse reactions by removing indication restrictions, it should be noted that this generalized analytical approach may introduce data from non-target indications, potentially creating biases in identifying adverse reaction characteristics specific to GIST patients.
5 Conclusion
This study provides a comprehensive evaluation between ripretinib and sunitinib based on FAERS data. Known AEs were validated, and new potential safety signals were identified. The common AEs and differences between ripretinib and sunitinib should be taken into account to adjust clinical medication strategies. These findings contribute to the optimization of ripretinib and sunitinib’s clinical use and emphasize the importance of continued pharmacovigilance.
Author contributions
XC: Writing – original draft, Conceptualization, Data curation, Formal Analysis. YZ: Funding acquisition, Supervision, Writing – review and editing, Resources, Validation, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research work was supported by the Traditional Chinese Medicine Science and Technology Project (Z-2023025) of the Shandong Provincial Health Commission.
Acknowledgments
We extend our gratitude to the US FDA for providing free access to the data utilized in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fphar.2025.1689499.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Blay, J.-Y., Serrano, C., Heinrich, M. C., Zalcberg, J., Bauer, S., Gelderblom, H., et al. (2020). Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet, Oncol. 21, 923–934. doi:10.1016/S1470-2045(20)30168-6
Chen, C., Wu, B., Zhang, C., and Xu, T. (2021). Immune-related adverse events associated with immune checkpoint inhibitors: an updated comprehensive disproportionality analysis of the FDA adverse event reporting system. Int. Immunopharmacol. 95, 107498. doi:10.1016/j.intimp.2021.107498
Cohen, P., Cross, D., and Jänne, P. A. (2021). Kinase drug discovery 20 years after imatinib: progress and future directions. Nat. Rev. Drug Discov. 20, 551–569. doi:10.1038/s41573-021-00195-4
Godfrey, H., Leibovit-Reiben, Z., Jedlowski, P., and Thiede, R. (2025). Alopecia associated with the use of semaglutide and tirzepatide: a disproportionality analysis using the FDA adverse event reporting system (FAERS) from 2022 to 2023. J. Eur. Acad. Dermatol Venereol. JEADV 39, e153–e154. doi:10.1111/jdv.20197
Goggin, C., Stansfeld, A., Mahalingam, P., Thway, K., Smith, M. J., Huang, P., et al. (2022). Ripretinib in advanced gastrointestinal stromal tumors: an overview of current evidence and drug approval. Future Oncol. lond Engl. 18, 2967–2978. doi:10.2217/fon-2022-0226
Guo, M., Shu, Y., Chen, G., Li, J., and Li, F. (2022). A real-world pharmacovigilance study of FDA adverse event reporting system (FAERS) events for niraparib. Sci. Rep. 12, 20601. doi:10.1038/s41598-022-23726-4
Hu, B., Flemetakis, E., Liu, Z., Hänsch, R., and Rennenberg, H. (2023). Significance of nitrogen-fixing actinorhizal symbioses for restoration of depleted, degraded, and contaminated soil. Trends Plant Sci. 28, 752–764. doi:10.1016/j.tplants.2023.03.005
Janku, F., Abdul Razak, A. R., Chi, P., Heinrich, M. C., von Mehren, M., Jones, R. L., et al. (2020). Switch control inhibition of KIT and PDGFRA in patients with advanced gastrointestinal stromal tumor: a phase I study of ripretinib. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 38, 3294–3303. doi:10.1200/JCO.20.00522
Jin, J., Xie, Y., Zhang, J.-S., Wang, J.-Q., Dai, S.-J., He, W.-F., et al. (2023). Sunitinib resistance in renal cell carcinoma: from molecular mechanisms to predictive biomarkers. Drug Resist Updat. Rev. Comment Antimicrob. Anticancer Chemother. 67, 100929. doi:10.1016/j.drup.2023.100929
Kelly, C. M., Gutierrez Sainz, L., and Chi, P. (2021). The management of metastatic GIST: current standard and investigational therapeutics. J. Hematol. Oncol. 14, 2. doi:10.1186/s13045-020-01026-6
Klug, L. R., Khosroyani, H. M., Kent, J. D., and Heinrich, M. C. (2022). New treatment strategies for advanced-stage gastrointestinal stromal tumours. Nat. Rev. Clin. Oncol. 19, 328–341. doi:10.1038/s41571-022-00606-4
Kuai, Z., Ye, Y., Zhang, X., Gao, L., Tang, G., and Yuan, J. (2024). Exploring SGLT-2 inhibitors and sarcopenia in FAERS: a post-marketing surveillance study. Expert Opin. Drug Saf., 1–8. doi:10.1080/14740338.2024.2412234
Kumar, V., Doros, L., Thompson, M., Mushti, S. L., Charlab, R., Spehalski, E. I., et al. (2023). FDA approval summary: ripretinib for advanced gastrointestinal stromal tumor. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 29, 2020–2024. doi:10.1158/1078-0432.CCR-22-2400
Li, X., Sun, Y.-Q., Huang, Q.-L., Zhang, Z.-J., Shi, L.-Q., Tang, J.-F., et al. (2025). Drug-related macular edema: a real-world FDA adverse event reporting system database study. BMC Pharmacol. Toxicol. 26, 23. doi:10.1186/s40360-025-00856-9
Lin, Y., Qin, S., Yang, H., Shi, F., Yang, A., Han, X., et al. (2023). Multicenter randomized double-blind phase III trial of donafenib in progressive radioactive iodine-refractory differentiated thyroid cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 29, 2791–2799. doi:10.1158/1078-0432.CCR-22-3613
Lostes-Bardaji, M. J., García-Illescas, D., Valverde, C., and Serrano, C. (2021). Ripretinib in gastrointestinal stromal tumor: the long-awaited step forward. Ther. Adv. Med. Oncol. 13, 1758835920986498. doi:10.1177/1758835920986498
Luo, Z.-Y., Li, X., Chen, C.-T., Kang, H.-H., Zhang, Z.-J., Wang, D., et al. (2025). Ocular adverse events associated with GLP-1 receptor agonists: a real-world study based on the FAERS database and network pharmacology. Expert Opin. Drug Saf. 24, 287–296. doi:10.1080/14740338.2024.2419989
Malovitski, K., Sarig, O., Feller, Y., Bergson, S., Assaf, S., Mohamad, J., et al. (2023). Defective cathepsin Z affects EGFR expression and causes autosomal dominant palmoplantar keratoderma. Br. J. Dermatol 189, 302–311. doi:10.1093/bjd/ljad167
Mohammadi, M., and Gelderblom, H. (2021). Systemic therapy of advanced/metastatic gastrointestinal stromal tumors: an update on progress beyond imatinib, sunitinib, and regorafenib. Expert Opin. Investig. Drugs 30, 143–152. doi:10.1080/13543784.2021.1857363
Mühlenberg, T., Falkenhorst, J., Schulz, T., Fletcher, B. S., Teuber, A., Krzeciesa, D., et al. (2024). KIT ATP-Binding pocket/activation loop mutations in GI stromal tumor: emerging mechanisms of kinase inhibitor escape. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 42, 1439–1449. doi:10.1200/JCO.23.01197
Muskat, A., Nawrocki, S., Kost, Y., Mattis, D., Amin, B., and McLellan, B. (2022). Verruca vulgaris eruption arising in the setting of a tyrosine kinase inhibitor. Cureus 14, e26006. doi:10.7759/cureus.26006
Pan, Y., Wang, Y., Zheng, Y., Chen, J., and Li, J. (2024). A disproportionality analysis of FDA adverse event reporting system (FAERS) events for ticagrelor. Front. Pharmacol. 15, 1251961. doi:10.3389/fphar.2024.1251961
Parakh, S., Maheshwari, S., Das, S., Kumar, V., Agrawal, R., Gupta, V., et al. (2022). Presumed bilateral diffuse uveal melanocytic proliferation - a case report and review of literature. Am. J. Ophthalmol. Case Rep. 27, 101582. doi:10.1016/j.ajoc.2022.101582
Przeździecka-Dołyk, J., Brzecka, A., Ejma, M., Misiuk-Hojło, M., Torres Solis, L. F., Solís Herrera, A., et al. (2020). Ocular paraneoplastic syndromes. Biomedicines 8, 490. doi:10.3390/biomedicines8110490
Ripretinib for gastrointestinal stromal tumours. Aust. Prescr. (2021). 44:112. doi:10.18773/austprescr.2021.025
Roskoski, R. (2021). Properties of FDA-Approved small molecule protein kinase inhibitors: a 2021 update. Pharmacol. Res. 165, 105463. doi:10.1016/j.phrs.2021.105463
Sarkar, P., Mehtani, A., Gandhi, H. C., Bhalla, J. S., and Tapariya, S. (2022). Paraneoplastic ocular syndrome: a pandora’s box of underlying malignancies. Eye lond Engl. 36, 1355–1367. doi:10.1038/s41433-021-01676-x
Schwartz, G. K. (2022). Ripretinib and the failure to advance GI stromal tumor therapy in the age of precision medicine. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 40, 3903–3906. doi:10.1200/JCO.22.01406
Shah, A. A., Kamal, M. A., and Akhtar, S. (2021). Tumor angiogenesis and VEGFR-2: mechanism, pathways and current biological therapeutic interventions. Curr. Drug Metab. 22, 50–59. doi:10.2174/1389200221666201019143252
Søreide, K., Sandvik, O. M., Søreide, J. A., Giljaca, V., Jureckova, A., and Bulusu, V. R. (2016). Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol. 40, 39–46. doi:10.1016/j.canep.2015.10.031
Tong, N., Wang, L., Wang, N., and Zhou, Z. (2021). Bilateral diffuse uveal melanocytic proliferation secondary to rectal adenocarcinoma: a case report and literature review. Front. Med. 8, 691686. doi:10.3389/fmed.2021.691686
Xiong, R., Lei, J., Pan, S., Zhang, H., Tong, Y., Wu, W., et al. (2023). Post-marketing safety surveillance of dalfampridine for multiple sclerosis using FDA adverse event reporting system. Front. Pharmacol. 14, 1226086. doi:10.3389/fphar.2023.1226086
Yu, R. J., Krantz, M. S., Phillips, E. J., and Stone, C. A. (2021). Emerging causes of drug-induced anaphylaxis: a review of anaphylaxis-associated reports in the FDA adverse event reporting system (FAERS). J. Allergy Clin. Immunol. Pract. 9, 819–829.e2. doi:10.1016/j.jaip.2020.09.021
Yuan, H., Qin, X., Yang, Q., Liu, L., Fang, Z., Fan, Y., et al. (2023). Dyskerin and telomerase RNA component are sex-differentially associated with outcomes and sunitinib response in patients with clear cell renal cell carcinoma. Biol. Sex. Differ. 14, 46. doi:10.1186/s13293-023-00526-7
Zalcberg, J. R. (2021). Ripretinib for the treatment of advanced gastrointestinal stromal tumor. Ther. Adv. Gastroenterol. 14, 17562848211008177. doi:10.1177/17562848211008177
Zhang, X., Zhang, P., Qiu, H., Fang, Y., Liu, H., Zhou, Y., et al. (2023). Large-scale, multicenter, prospective registry study of ripretinib in advanced GIST: a real-world study from China. Adv. Ther. 40, 3817–3829. doi:10.1007/s12325-023-02576-0
Zhou, X., Yang, G., Zeng, X., Wang, L., Xiang, J., Zhao, J., et al. (2023). Dupilumab and the potential risk of eosinophilic pneumonia: case report, literature review, and FAERS database analysis. Front. Immunol. 14, 1277734. doi:10.3389/fimmu.2023.1277734
Keywords: ripretinib, sunitinib, adverse events, FAERS, GIST treatment
Citation: Che X and Zhu Y (2025) Gastrointestinal stromal tumors with the use of ripretinib and sunitinib: real-world adverse event analysis based on the FDA adverse event reporting system (FAERS). Front. Pharmacol. 16:1561937. doi: 10.3389/fphar.2025.1561937
Received: 16 January 2025; Accepted: 16 June 2025;
Published: 26 June 2025; Corrected: 09 September 2025.
Edited by:
Yanzhu Zhu, Jilin Agricultural Science and Technology College, ChinaReviewed by:
Rui Xiong, 958 Hospital of the People’s Liberation Army, ChinaJia Wei Zhao, Capital Medical University, China
Copyright © 2025 Che and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Zhu, MTUxNjg4ODYzOTZAMTYzLmNvbQ==
 Xinzhen Che
Xinzhen Che Yong Zhu
Yong Zhu