- 1Department of Education and Support for Regional Medicine (General and Kampo Medicine), Tohoku University Hospital, Sendai, Japan
- 2Department of Kampo and Integrative Medicine, Graduate School of Medicine, Tohoku University, Sendai, Japan
- 3Department of Pharmaceutical Sciences, Tohoku University Hospital, Sendai, Japan
- 4Division of Molecular Epidemiology, Department of Preventive Medicine and Epidemiology, Tohoku Medical Megabank Organization, Tohoku University, Sendai, Japan
- 5Laboratory of Biomolecule and Pathophysiological Chemistry, Graduate School of Pharmaceutical Sciences, Tohoku University, Sendai, Japan
- 6Division of Clinical Pharmacology and Therapeutics, Tohoku University Graduate School of Pharmaceutical Sciences, Sendai, Japan
- 7Drug Informatics, Faculty of Pharmacy, Meijo University, Nagoya, Japan
- 8Department of Obstetrics and Gynecology, Tohoku University Hospital, Sendai, Japan
Introduction: Traditional Japanese (Kampo) medicine containing Prunus persica kernel (KPK) is prescribed for treating menstrual- and pregnancy-related symptoms. However, no safety information is available regarding its use in pregnant women. In this study, we examined the associations of KPK prescriptions during the first trimester of pregnancy with preterm births and major congenital malformations (MCMs) in newborns.
Methods: From a large-scale Japanese health insurance claims database, we included pregnant women enrolled with the same healthcare insurer from 3 months before pregnancy to the date of delivery, who gave birth between 2010 and 2019, and whose data were linked to their infants. We then selected pregnant women who were prescribed KPK during the first trimester as the exposure group, and those who were prescribed tokishakuyakusan (TSS), commonly used for pregnancy-related symptoms, during the same period as controls. The association between KPK prescriptions and preterm birth or MCM among infants was examined using a multivariate logistic regression analysis.
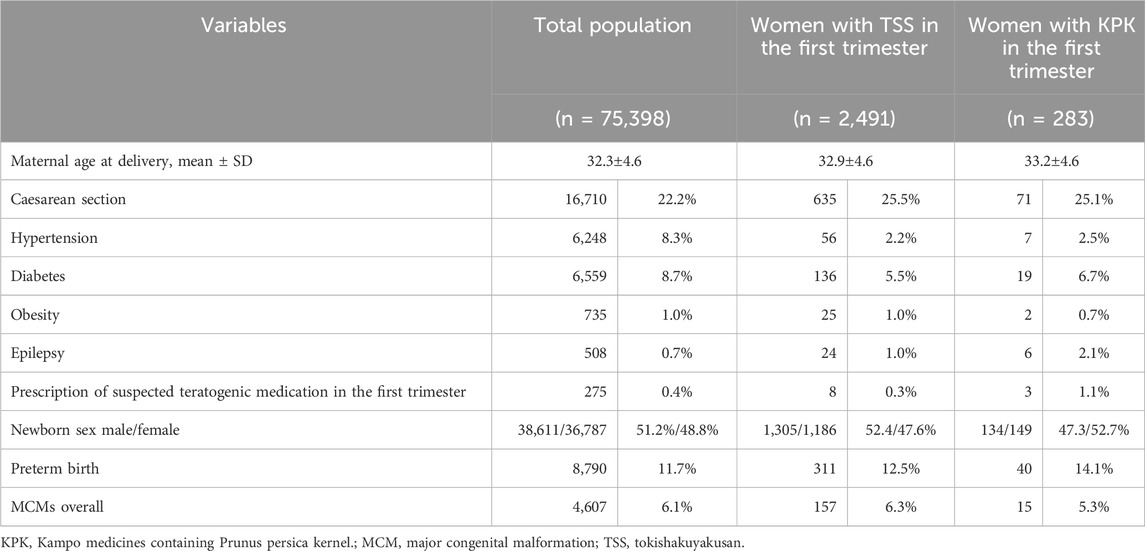
Results: Of the 75,398 infants, TSS and KPK was prescribed to 2,548 (3.38%) and 283 (0.38%) women during the first trimester, respectively. In the TSS group, 311 of 2,491 infants (12.5%) experienced preterm births, whereas 40 of 283 infants (14.1%) in the KPK group experienced preterm births. The risk of preterm birth in the KPK group was not significantly different from that in the TSS group (adjusted risk ratio, 1.122; 95% confidence interval, 0.827–1.521). In the TSS group, 157 of 2,491 infants (6.3%) had MCMs, whereas 15 of 283 infants (5.3%) in the KPK group had MCMs. There was no significant difference in the incidence of MCM in the first year after birth between infants in the KPK and TSS groups (adjusted odds ratio, 0.820; 95% confidence interval, 0.475–1.415).
Conclusion: There was no significant difference in the risk of preterm birth or MCMs between pregnant women prescribed KPK and those prescribed TSS during the first trimester.
1 Introduction
Pregnant women experience a variety of pregnancy-related symptoms. In the first trimester, nausea, vomiting, pelvic cavity pain, and back pain are the most common symptoms, with 88% of women reporting multiple symptoms (Lutterodt et al., 2019). However, the use of medications during pregnancy requires careful consideration because of the potential for adverse maternal and fetal outcomes. Given the limitations in using conventional medications during pregnancy due to potential risks, alternative treatments such as Kampo medicine are often considered.
Japanese traditional (Kampo) medicine is derived from Chinese herbal medicines and was uniquely developed in Japan (Motoo et al., 2011). Kampo formulas are composed of multiple natural herbs and are used to treat pregnancy-related symptoms by the national health insurance system in Japan (Yoshino et al., 2023). Tokishakuyakusan (TSS) is the most commonly used Kampo formula for treating pregnancy-related symptoms, such as edema, headache, and dizziness, and for supporting fetal development, so its continuous use during pregnancy has empirically been a common practice. Our previous study investigated 8% of pregnant women prescribed TSS in a Japanese large-scale claims database. (Suzuki et al., 2021).
Prunus persica kernel (PK) is also a natural herb used to treat gynecological diseases. The current Japanese Pharmacopoeia classifies PK as seeds of P. persica Batsch or P. persica Batsch var. davidiana Maximowicz (Rosaceae), which contains no less than 1.2% amygdalin (The Ministry of HealthLabour and Welfare, 2021). PK exerts multiple pharmacological effects, including anti-inflammatory, antioxidant, and immune regulatory effects, and is clinically used not only for gynecological diseases but also for cardiovascular, anal, digestive, and orthopedic conditions (Nowicka and Wojdyło, 2019; Liu et al., 2024).
There is limited discussion on the lack of modern scientific evidence regarding PK’s contraindication during pregnancy. Package inserts of Kampo formulas containing PK (KPK) indicate that their use is not recommended for pregnant women or women who may possibly be pregnant and that PK may cause preterm birth or abortion (Tsumura and Co, 2007), though it is not prohibited. This recommendation is based on classical books on traditional Chinese medicine. For example, The Compendium of Materia Medica (Honzokomoku in Japanese, Bencao Gangmu in Chinese), published in the 16th century, describes PK as a natural herb prohibited during pregnancy. A literature search revealed one article in Chinese that reported the teratogenic effects of PK; however, detailed information on the study design and results is lacking (Xi et al., 2013). Despite traditional contraindications, there is a notable absence of modern clinical studies evaluating the safety of PK during pregnancy. Given the ongoing use of KPKs among pregnant women and the lack of empirical safety data, it is imperative to investigate the potential risks associated with their use.
Our previous research investigating large-scale claims data showed that KPKs (e.g., keishibukuryogan, tokakujokito, and junchoto) were used by pregnant women (Suzuki et al., 2021). The prescription of KPK might occur when physicians have concluded that the benefits are greater than the potential risks. However, the safety of KPK in pregnant women and infants has not been investigated. The aim of this study was to investigate the associations of first-trimester exposure to KPK with preterm birth and major congenital malformations (MCMs) in infants using a large claims database.
2 Methods
2.1 Database
Data were obtained from JMDC Inc. (Tokyo, Japan) (Kimura et al., 2010). This database is anonymous and privacy-protected, and includes details such as sex, birth year, and month. We extracted insurance claims information, diagnoses defined by the International Classification of Diseases, 10th Revision (ICD-10) codes, drug prescriptions covered by health insurance at hospitals, including outpatient and inpatient care, dispensing at pharmacies, and procedures including operations. The Institutional Review Board of Tohoku University School of Medicine approved this study on 22 January 2024 (registration number: 2023-1-808). Informed consent was not required because the obtained data were de-identified.
2.2 Study population
We used the dataset available on 8 May 2020, for this study, which included 7,447,761 men and women covered by health insurance between January 2005 and November 2019, similar to our previous study (Suzuki et al., 2023). The insured individuals had anonymized family and personal identification numbers. Therefore, we identified mother-child relationships among individuals if data regarding their newborns were entered by an identical health insurer with matching family identification numbers. In addition, this dataset allowed us to identify the birth year and month (information regarding the date of birth was not included). The study population included mothers who met the following eligibility criteria: mothers who were linked to their infants whose birth month was in accordance with the month of enrollment in the health insurance, mothers who continued to be covered under the same health insurance from 3 months prior to pregnancy until delivery, and mothers whose dates of pregnancy onset and delivery were estimable. Figure 1 shows the flowchart of the patient selection process. The database contained a limited number of women who gave birth before 2009; thus, we included only those women who became pregnant between 2010 and 2019. Only one pregnancy per woman, which was the first pregnancy estimated in the database, was included. Furthermore, to investigate the risk of preterm birth or MCM due to exposure to KPK in first-trimester pregnancy, mothers whose newborns were covered by the same health insurance during their birth month for more than a year after birth were included. Conversely, mothers with multiple gestations were excluded because the risk of congenital disabilities increases with multiple births (Zhang et al., 2011). Mothers whose infants had chromosomal abnormalities (ICD-10 codes Q90–Q99) (Huybrechts et al., 2019) were also excluded because they were not associated with exposure, and their inclusion would reduce the detection of exposure-related risk.
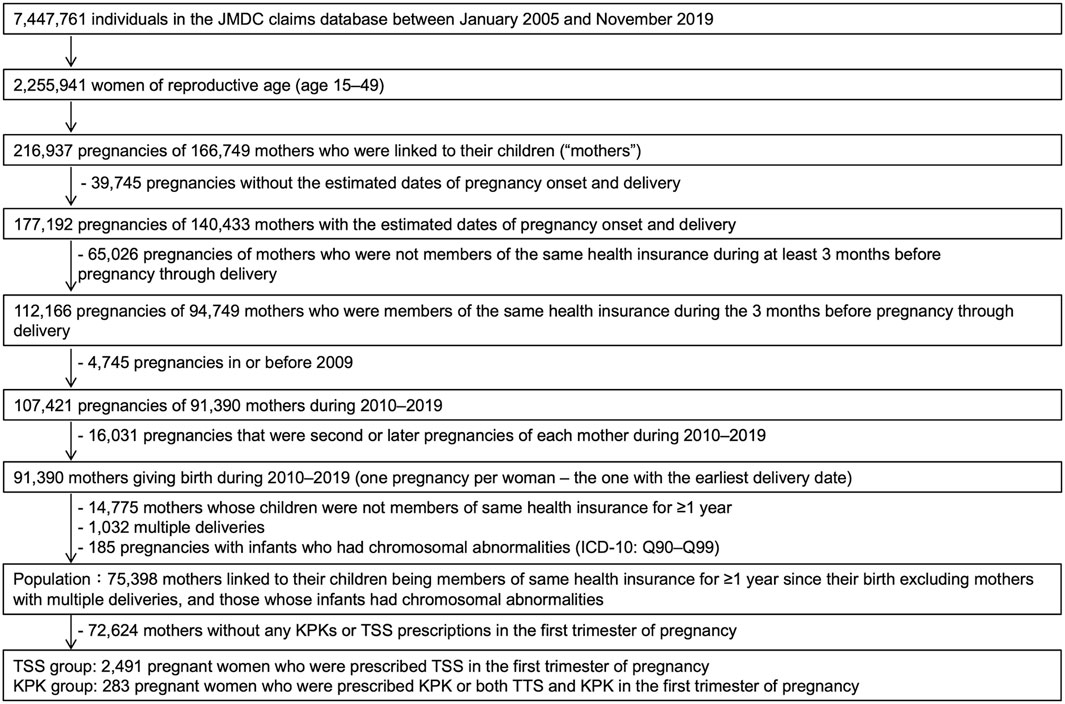
Figure 1. Flow chart illustrating the selection of the study population for data analysis. ICD, international classification of disease; KPK, Kampo medicines containing Prunus persica kernel; TSS, tokishakuyakusan.
2.3 Pregnancy onset and delivery date estimations
As no data on pregnancy onset or delivery date were obtained from this database, we used previously reported estimation methods (Ishikawa et al., 2018; Ishikawa et al., 2019; Ishikawa et al., 2020; Suzuki et al., 2021; Ishikawa et al., 2022; Ishikawa et al., 2023; Suzuki et al., 2023). The pregnancy onset date was calculated by subtracting the gestational age associated with the diagnosis from the date of the disease diagnosis (Ishikawa et al., 2018; Ishikawa et al., 2019; Ishikawa et al., 2020; Suzuki et al., 2021; Ishikawa et al., 2022; Ishikawa et al., 2023). In cases where a mother had several visits with diagnoses, the most recent gestational age was considered because a more accurate diagnosis could be made in the later stages of pregnancy (Committee on Obstetric Practicethe American Institute of Ultrasound in Medicinethe Society for Maternal-Fetal Medicine, 2017). A study based on university hospital records demonstrated that approximately 90% of the pregnancy onset dates estimated using this method fell within ±1 week of the true pregnancy onset, indicating a high accuracy (Ishikawa et al., 2018).
The delivery date was estimated from delivery-related entries and infant birth months using a previously reported algorithm (Ishikawa et al., 2019; Suzuki et al., 2021). A study based on university hospital records demonstrated that approximately 95% of the estimated delivery dates were within 1 week of the actual delivery date (Ishikawa et al., 2018). In Japan, health insurance does not always cover deliveries that do not require procedures or medication. Therefore, in cases where delivery data were unavailable, the 15th day of the month of the birth month was considered as the delivery date. Induction of labor is recommended in women with a pregnancy duration of over 294 days because of increased perinatal mortality (Minakami et al., 2014). When the difference between the estimated dates of delivery and pregnancy onset was >294 days, the gestational period was set at 294 days and pregnancy onset was subtracted from the estimated delivery date by 294 days. The first trimester was defined as the duration from pregnancy onset up to week 13 and day 6 of gestation, the second trimester as from week 14 days 0 to week 27 day 6 of gestation, and the third trimester as week 28 day 0 until delivery (Minakami et al., 2014).
2.4 Exposures
We set tokishakuyakusan (TSS) as a competitor drug. TSS is a Kampo medicine that does not contain PK and has been used to treat pregnancy-related symptoms, such as edema, headache, and dizziness (Morohashi et al., 2020; Nakayama et al., 2021; Suzuki et al., 2021; Toda et al., 2021). The package insert of TSS states its indications for symptoms during pregnancy (Tsumura and Co, 2014). The TSS was composed of peony root, Atractylodes lancea rhizome, Alisma rhizome, Poria sclerotium, Cnidium rhizome, and Japanese Angelica root (Supplementary Table S1). TSS has been reported to activate blood (Shimizu et al., 2022), have blood-replenishing effects (Akase et al., 2007), prevent miscarriage (Nagamatsu et al., 2018), and promote fetal growth (Li et al., 2013); therefore, TSS is used to manage various conditions in pregnant women. Our previous studies have shown that TSS is one of the frequently prescribed Kampo medicines during pregnancy (Suzuki et al., 2021; Noda et al., 2024).
The exposure drugs are KPK, which include the following eight Kampo formulations; choyoto, daiobotampito, junchoto, keishibukuryogan, keishibukuryogankayokuinin, sokeikakketsuto, tokakujokito. The composition crude drugs are listed in Supplementary Table S1. The maximum daily amount of PK are 2–5 g. The exposure group comprised pregnant women who were prescribed KPK and crude P. persica kernel, including pregnant women who received both TSS and KPK. We used the methods reported in a previous study to estimate the timing of exposure to these medications (Suzuki et al., 2023).
The timing of drug exposure was based on the day of dispensing. In Japan, dispensing dates are recorded on receipts primarily for outpatient prescriptions filled at external pharmacies; they are missing for inpatient prescriptions administered during hospital stays. If the dispensing date was not available in the database, the date of admission was considered for calculation. If neither the dispensing date nor the admission date could be obtained, the 15th of the month was used as the dispensing date because the month and year were listed on each claim. The exposure period was calculated as the number of days exposed to each prescription. We extracted drug exposures during the first trimester of pregnancy, which is the most critical period for organogenesis, to analyze their association with maternal and fetal outcomes.
2.5 Outcomes
Preterm birth was defined as an estimated pregnancy period of <37 weeks (Goldenberg et al., 2008). Supplementary Table S2 shows the MCMs definition with ICD-10 codes (Q00–Q89). Minor congenital malformations were excluded, and diagnoses of MCMs administered to Infants during the first year of life were extracted (Ishikawa et al., 2021). Additionally, MCMs were grouped separately based on individual organ systems that were affected. A study based on university hospital records demonstrated that MCMs in claims were validated against medical records, and the overall positive predictive value of MCMs was approximately 90% (Ishikawa et al., 2021).
2.6 Covariates
We considered the following variables as covariates: maternal age at birth; birth year; medical history of epilepsy, diabetes, and obesity; and prescription of teratogenic drugs in the first trimester of pregnancy. These covariates were defined on the basis of previous studies (Eriksson et al., 2000; Oliveira and Fett-Conte, 2013; Minakami et al., 2014; Alvarado-Terrones et al., 2018; Mbizvo et al., 2020) (Supplementary Table S3). These covariates were considered a possible determinant of exposure, outcome, or both (VanderWeele, 2019).
2.7 Statistical analyses
To investigate the preterm birth risk and the MCM risk exposure among the KPK group prescribed in the first trimester of pregnancy, two groups were selected from the study population: pregnant women who were prescribed TSS in the first trimester of pregnancy (control group), and those who were prescribed KPK in the first trimester of pregnancy (KPK group). Women prescribed both TSS and KPK were placed in KPK group.
Between the two groups, the risk ratios (RR) and 95% confidence intervals (CI) for preterm birth were evaluated using modified Poisson regression analysis and the odd ratios (OR) and 95% CI for MCM were evaluated using logistic regression analysis. The results were assessed after adjusting for multiple covariates. Sensitivity analysis was also performed. To analyze the risk of preterm birth, the analysis was conducted after excluding pregnant women with combined TSS and KPK prescriptions. To analyze MCM risk, the analyses were repeated after 1) including only women who did not receive a prescription for a suspected teratogenic drug in the first trimester of pregnancy, 2) including only pregnant women who had been prescribed TSS and KPK for more than 30 days during the first trimester, and 3) excluding pregnant women with combined prescriptions of TSS and KPK.
Differences were considered significant when the 95% CIs did not cross 1.0. Statistical significance was set at P < 0.05. difference. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, United States).
3 Results
3.1 Population characteristics
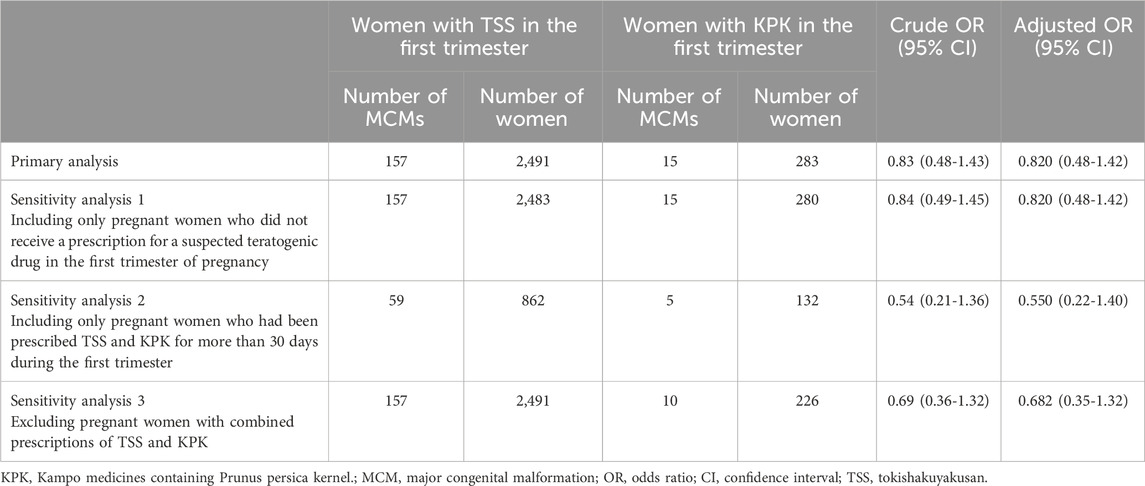
We identified 75,398 women who were eligible for screening in this study (Figure 1). The distribution of the methods of estimating delivery dates was as follows: 37,213 (49.4%) based on the dates of specific diagnoses and procedures for the aforementioned algorithms; 7,117 (9.4%) based on the dates of other delivery-related entries; 31,608 (41.2%) based on the 15th day of the neonatal birth month; and 1,134 (1.5%) evenly assigned 294 days as the duration of gestation.
During pregnancy, TSS and KPK was prescribed to 6,576 (8.72%) and 411 (0.55%) women, respectively (Table 1). Among the prescriptions of KPK, keishibukuryogan (203, 0.27%) was the most frequently prescribed, followed by tokakujokito (74, 0.10%), keishibukuryogankayokuinin (58, 0.08%), and junchoto (51, 0.07%). TSS and KPK was prescribed to 2,548 (3.38%) and 283 (0.38%) women during the first trimester, respectively.
3.2 Preterm birth risks of first-trimester exposure to KPK
Of the 75,398 infants, 8,790 (11.7%) experienced preterm births. In the TSS group, 311 of 2,491 infants (12.5%) experienced preterm births, whereas 40 of 283 infants (14.1%) in the KPK group experienced preterm births (Table 2). The risk of preterm birth in the KPK group was not significantly different from the risk in the TSS group (crude RR, 1.132; 95%CI, 0.834-1.536; adjusted RR, 1,122; 95%CI, 0.827-1.521) (Table 3). Sensitivity analysis produced a result similar to those of the primary analysis.
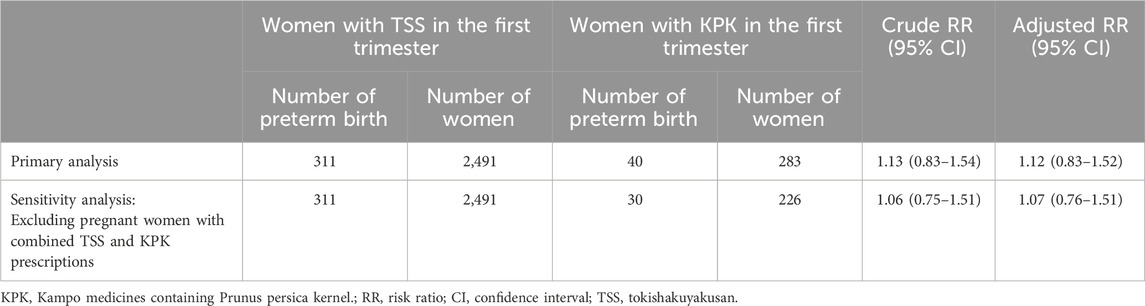
Table 3. Associations between KPK prescribed during the first trimester of pregnancy and preterm birth.
3.3 MCM risks of first-trimester exposure to KPK
Of 75,398 infants, 4,607 (6.1%) were diagnosed with MCMs within the first year after birth. In the TSS group, 157 of 2,491 infants (6.3%) had MCMs, whereas 15 of 283 infants (5.3%) in the KPK group had MCMs (Table 2). There was no significant difference in terms of the assignment of MCM in the first year after birth between the births among pregnant women in the TSS and KPK groups (crude OR, 0.832; 95% CI, 0.483–1.434; adjusted OR, 0.820; 95% CI, 0.475–1.1415). The sensitivity analyses supported the main analysis (Table 4).
4 Discussion
In this study, using a large-scale clinical database, no significant association was found between the frequency of MCMs in the infants of pregnant women prescribed KPK and TSS during the first trimester of pregnancy. Furthermore, there was no association between the frequency of preterm delivery in pregnant women prescribed KPK and those prescribed TSS during the first trimester of pregnancy. These novel findings suggest that KPK can be safely administered during the first trimester of pregnancy.
While previous article has reported teratogenic and abortifacient effects of PK according to literature search (Xi et al., 2013), our findings did not observe significant association with MCMs, suggesting the opposite indication. While literature reviews often base their findings on animal studies or classical texts, which may involve different administration dosage or periods than our research, our findings suggest that the risk of using KPK during the first trimester warrants re-evaluation. Furthermore, the association with other pregnancy-related outcomes beyond MCM and miscarriage needs to be clarified through future research.
KPK is known to promote uterine contractions and has been used to expel the retained placenta after delivery. Two cases have been reported in the literature where retained placenta was successfully expelled without surgery using traditional medicines containing PK (Nishida et al., 2011; Huang et al., 2021). A recent meta-analysis from China revealed that supplementation with Shenghua decoction, which contains PK, was associated with a higher complete abortion rate in women with early medical abortion and was associated with no adverse events (Li et al., 2022). The use of PK during pregnancy has not been recommended in classical, traditional Chinese medicine books or modern Kampo formula package inserts because of this potential risk of preterm birth. However, our findings challenge the conventional wisdom that PK should be avoided during pregnancy due to its potential risks.
Previous studies using traditional medicine database do not describe PK or KPK. A database study from Taiwan reported that 20%–33% of pregnant women used traditional medicine, with greater tendency to use it among patients with threatened abortion (Yeh et al., 2009; Chuang et al., 2009; Wen et al., 2020). Furthermore, while Coptis rhizome and An-Tai-Yin use during the first trimester was associated with an increased risk of some congenital malformations (Chuang, et al., 2006.), no papers mentioned the risk associated with PK. We hope that the results of this study will contribute to the choice of KPK for use during pregnancy.
According to the traditional framework of Kampo medicine, PK is used to address the blood stasis pattern (TM1). This condition is clinically characterized by features such as darkened complexion, localized bluish or purplish lumps, localized pain, bleeding with dark-colored blood and clots, a purple or spotted tongue, purple lips, and a wiry, firm, or choppy pulse. These manifestations are commonly associated with various menstrual disorders (WHO, 2024). Kampo prescriptions, particularly KPKs, are often administered to women with infertility attributed to blood-related abnormalities (Yoshida-Komiya et al., 2021). They are typically recommended for women of reproductive age who present with dysmenorrhea in conjunction with evidence of blood disorders. Consequently, it is critical to establish robust safety parameters for the use of KPK during pregnancy to alleviate concerns among pregnant women and their families who are prescribed these treatments.
We conducted several sensitivity analyses. Almost of all analyses revealed similar results to those in primary analyses. In the sensitivity analysis two for MCM risk, the point estimate of OR in KPK group was 0.536. The reason for this may be that the sample size was reduced by limiting women prescribed laxatives for more than 30 days during the first trimester of pregnancy, resulting in a lower prevalence of MCM, especially in the KPK group. Therefore, it should be noted that the prevalence is not necessarily higher in the TSS group.
This study is subject to several limitations that warrant discussion. First, the prescription of KPK in the first trimester was smaller than that of TSS (0.38% and 3.38%, respectively). Despite the possibility that the infrequent prescription of KPK to pregnant women indicates adherence to non-recommendations, the limited exposure numbers might have hindered a robust calculation of the actual risk of adverse events. Second, there is less evidence of the safety of TSS, the competitor drug in this study. TSS has been utilized for pregnancy-related symptoms for hundreds of years and was prescribed to 8% of pregnant women (Suzuki et al., 2021). Its tolerability has historically not been a significant concern. A recent basic study showed the safety of TSS on the hatching tare from embryo and morphology of the juvenile of Danio rerio (Luo et al., 2024). Further basic and clinical studies to elucidate the safety of TSS during pregnancy are warranted. Third, Kampo medicine prescriptions may not accurately reflect the actual consumption of the prescribed substances. If patients did not adhere to the prescribed regimen, the findings might be influenced by exposure misclassification bias. However, the utilization of prescription data minimizes recall bias that could arise from reliance on self-reported information. the safety of TSS is historically assured, though scientific evidence is limited. Forth, TSS and KPK are available at pharmacies as over-the-counter drugs; however, data on those purchased outside health insurance could not be obtained. Fifth, we could not obtain data on smoking habit or folic acid supplementation which are associated with MCMs (Harris et al., 2017), because the database used in this study was based on insurance claim data, and it did not include this information. Sixth, our previous report has shown that in the case of preterm births, the percentage of births within 7 days of the gold standard birth date estimated from office data is about 85% (Ishikawa et al., 2021), which may cause slight misclassification. There are no reports on positive predictive value (PPV) with preterm birth as an outcome, and future studies on the accuracy of preterm birth estimation based on Japanese claims data and other data are desirable. Seventh, while this study evaluated MCMs and preterm births, it excluded data on abortions and stillbirths due to the study design prioritizing linkage between mother and child. The lack of research on the effects of KPK on stillbirths and miscarriages presents a gap that may contribute to underestimation of its harm. Additionally, the effect of KPK exposure after the second trimester of pregnancy on preterm births should also be evaluated. However, we first analyzed KPK during the first trimester of pregnancy in the same population as the MCM assessment, with priority given to obtaining bias-free results. Future studies should adopt alternative designs to address this limitation and assess the impact of KPK on these outcomes. Lastly, the maximum daily dosage of PK in Japanese Kampo extract formulations is relatively low, capped at 5 g (Supplementary Table S1). More amount of PK could be consumed when multiple PKs are prescribed, or when decoction formula containing PK is used for severe disease conditions. Further research is essential to investigate whether higher dosages of PK during pregnancy might influence the incidence of MCM or preterm births in infants, because higher dosage are generally associated with an increased risk of adverse effects. Despite the research limitations described, the present study is the first study based on a quantitative analysis rather than a literature search, using a large-scale clinical database, of the association between KPK during the first trimester of pregnancy and risk of MCMs and preterm births.
5 Conclusion
This study found no significant differences in the risk of preterm birth or MCMs between pregnancies exposed to KPK and those exposed to TSS. These findings suggest that the use of KPK during the first trimester of pregnancy does not pose additional risks for these outcomes compared to TSS prescriptions, providing important preliminary evidence to inform the safe use of KPK in clinical practice. Further research is needed to validate these findings and explore their implications for maternal and fetal health.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Institutional Review Board of The Tohoku University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
SS: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review and editing. RA: Methodology, Resources, Visualization, Writing – original draft, Writing – review and editing. TO: Conceptualization, Data curation, Methodology, Supervision, Writing – original draft, Writing – review and editing. TI: Conceptualization, Data curation, Methodology, Supervision, Writing – original draft, Writing – review and editing. TK: Writing – review and editing. TS: Methodology, Writing – review and editing. AN: Writing – review and editing. GS: Writing – review and editing. MI: Writing – review and editing. MOr: Methodology, Writing – review and editing. SK: Writing – review and editing. MOh: Methodology, Writing – review and editing. KH: Methodology, Writing – review and editing. NM: Writing – review and editing. AK: Writing – review and editing. ST: Methodology, Writing – review and editing. TI: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the Ministry of Health, Labour, and Welfare of Japan (H23-iyaku-ippan-006), the Japan Society for the Promotion of Science (19K09746 and 20K16070), and the Japan Agency for Medical Research and Development (JP23lk0310095). Funding sources had no role in the study design; collection, analysis, or interpretation of the data; writing of the manuscript; or decision to submit the paper for publication.
Acknowledgments
This manuscript underwent English language editing by Editage (www.editage.com).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1562724/full#supplementary-material
References
Akase, T., Hihara, E., Shimada, T., Kojima, K., Akase, T., Tashiro, S.-I., et al. (2007). Efficacy of tokishakuyakusan on the anemia in the iron-deficient pregnant rats. Biol. Pharm. Bull. 30, 1523–1528. doi:10.1248/bpb.30.1523
Alvarado-Terrones, E. G., Perea-Cabrera, M., Klünder-Klünder, M., Segura-Stanford, B., Erdmenger-Orellana, J. R., Lopez-Yañez Blanco, A., et al. (2018). Maternal obesity as a risk factor for the development of total anomalous pulmonary venous connection in their offspring. Arch. Med. Res. 49, 109–113. doi:10.1016/j.arcmed.2018.06.001
Chuang, C. H., Doyle, P., Wang, J. D., Chang, P. J., Lai, J. N., and Chen, P. C. (2006). Herbal medicines used during the first trimester and major congenital malformations: an analysis of data from a pregnancy cohort study. Drug Saf. 29, 537–548. doi:10.2165/00002018-200629060-00006
Chuang, C. H., Chang, P. J., Hsieh, W. S., Tsai, Y. J., Lin, S. J., and Chen, P. C. (2009). Chinese herbal medicine use in Taiwan during pregnancy and the postpartum period: a population-based cohort study. Int. J. Nurs. Stud. 46, 787–795. doi:10.1016/j.ijnurstu.2008.12.015
Committee on Obstetric Practice, the American Institute of Ultrasound in Medicine, the Society for Maternal-Fetal Medicine (2017). Committee Opinion No 700: methods for estimating the due date. Obstet. Gynecol. 129, e150–e154. doi:10.1097/AOG.0000000000002046
Eriksson, U. J., Borg, L. A., Cederberg, J., Nordstrand, H., Simán, C. M., Wentzel, C., et al. (2000). Pathogenesis of diabetes-induced congenital malformations. Ups. J. Med. Sci. 105, 53–84. doi:10.1517/03009734000000055
Goldenberg, R. L., Culhane, J. F., Iams, J. D., and Romero, R. (2008). Epidemiology and causes of preterm birth. Lancet 371, 75–84. doi:10.1016/s0140-6736(08)60074-4
Harris, B. S., Bishop, K. C., Kemeny, H. R., Walker, J. S., Rhee, E., and Kuller, J. A. (2017). Risk factors for birth defects. Obstet. Gynecol. Surv. 72, 123–135. doi:10.1097/OGX.0000000000000405
Huang, H., Wang, J., Li, K., and Ma, H. (2021). Successful conservative treatment of placenta accreta with traditional Chinese medicine: a case report. Med. (Baltim.). 100, e24820. doi:10.1097/MD.0000000000024820
Huybrechts, K. F., Bateman, B. T., and Hernández-Díaz, S. (2019). Use of real-world evidence from healthcare utilization data to evaluate drug safety during pregnancy. Pharmacoepidemiol. Drug Saf. 28, 906–922. doi:10.1002/pds.4789
Ishikawa, T., Obara, T., Nishigori, H., Miyakoda, K., Inoue, R., Hoshiai, T., et al. (2018). Development of algorithms to determine the onset of pregnancy and delivery date using health care administrative data in a university hospital in Japan. Drug Saf. 27, 751–762. doi:10.1002/pds.4444
Ishikawa, T., Obara, T., Jin, K., Nishigori, H., Miyakoda, K., Suzuka, M., et al. (2019). Examination of the prescription of antiepileptic drugs to prenatal and postpartum women in Japan from a health administrative database. Drug Saf. 28, 804–811. doi:10.1002/pds.4749
Ishikawa, T., Obara, T., Kikuchi, S., Kobayashi, N., Miyakoda, K., Nishigori, H., et al. (2020). Antidepressant prescriptions for prenatal and postpartum women in Japan: a health administrative database study. J. Affect. Disord. 264, 295–303. doi:10.1016/j.jad.2020.01.016
Ishikawa, T., Oyanagi, G., Obara, T., Noda, A., Morishita, K., Takagi, S., et al. (2021). Validity of congenital malformation diagnoses in healthcare claims from a university hospital in Japan. Drug Saf. 30, 975–978. doi:10.1002/pds.5244
Ishikawa, T., Obara, T., Akazawa, M., Noda, A., Oyanagi, G., Morishita, K., et al. (2022). Risk of major congenital malformations associated with first-trimester exposure to propulsives: a health administrative database study in Japan. Drug Saf. 31, 196–205. doi:10.1002/pds.5370
Ishikawa, T., Nishigori, H., Akazawa, M., Miyakoda, K., Noda, A., Ishikuro, M., et al. (2023). Risk of major congenital malformations associated with first-trimester antihypertensives, including amlodipine and methyldopa: a large claims database study 2010-2019. Pregnancy Hypertens. 31, 73–83. doi:10.1016/j.preghy.2023.01.001
Kimura, S., Sato, T., Ikeda, S., Noda, M., and Nakayama, T. (2010). Development of a database of health insurance claims: standardization of disease classifications and anonymous record linkage. J. Epidemiol. 20, 413–419. doi:10.2188/jea.je20090066
Li, H., Wada, E., and Wada, K. (2013). Maternal administration of the herbal medicine toki-shakuyaku-san promotes fetal growth and placental gene expression in normal mice. Am. J. Chin. Med. 41, 515–529. doi:10.1142/S0192415X13500377
Li, H.-F., Chen, W.-M., Shen, H.-L., Feng, Z.-F., Yang, Y., and Shen, Q.-H. (2022). The efficacy of Shenghua Decoction supplementation after early medical abortion: a meta-analysis of randomized controlled trials. Complement. Ther. Med. 69, 102848. doi:10.1016/j.ctim.2022.102848
Liu, Y.-Q., Wu, H.-L., Zhang, Z.-Q., Wang, W. L., Han, G.-Q., Zhang, C.-H., et al. (2024). Traditional use, phytochemistry, pharmacology, toxicology and clinical applications of Persicae Semen: a review. Chin. J. Integr. Med. 30, 1137–1147. doi:10.1007/s11655-024-3815-4
Luo, R., He, C., He, J., Li, Z., Wang, Y., Hou, M., et al. (2024). Acute toxicology on Danio rerio embryo and adult from Chinese traditional medicine preparation Danggui Shaoyao san. J. Ethnopharmacol. 321, 117528. doi:10.1016/j.jep.2023.117528
Lutterodt, M. C., Kähler, P., Kragstrup, J., Nicolaisdottir, D. R., Siersma, V., and Ertmann, R. K. (2019). Examining to what extent pregnancy-related physical symptoms worry women in the first trimester of pregnancy: a cross-sectional study in general practice. BJGP Open 3, bjgpopen19X101674. doi:10.3399/bjgpopen19X101674
Mbizvo, G. K., Bennett, K. H., Schnier, C., Simpson, C. R., Duncan, S. E., and Chin, R. F. M. (2020). The accuracy of using administrative healthcare data to identify epilepsy cases: a systematic review of validation studies. Epilepsia 61, 1319–1335. doi:10.1111/epi.16547
Minakami, H., Maeda, T., Fujii, T., Hamada, H., Iitsuka, Y., Itakura, A., et al. (2014). “Guidelines for obstetrical practice,” in J obstet gynaecol res. Guidelines for obstetrical practice in Japan 40. 2014 ed. Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists JAOG, 1469–1499.
Minakami, H., Maeda, T., Fujii, T., Hamada, H., Iitsuka, Y., Itakura, A., et al. (2014). Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2014 edition. J. Obstet. Gynaecol. Res. 40, 1469–1499. doi:10.1111/jog.12419
Motoo, Y., Seki, T., and Tsutani, K. (2011). Traditional Japanese medicine, Kampo: its history and current status. Chin. J. Integr. Med. 17, 85–87. doi:10.1007/s11655-011-0653-y
Nagamatsu, T., Fujii, T., Schust, D. J., Tsuchiya, N., Tokita, Y., Hoya, M., et al. (2018). Tokishakuyakusan, a traditional Japanese medicine (Kampo) mitigates iNKT cell-mediated pregnancy loss in mice. Am. J. Reprod. Immunol. 80, e13021. doi:10.1111/aji.13021
Nakayama, T., Tawara, F., Murabayashi, N., So, S., Yamaguchi, W., Miyano, N., et al. (2021). Significance of combined use of Kamishoyosan and tokishakuyakusan for general infertility treatment. Kampo Med. 72, 361–367. doi:10.3937/kampomed.72.361
Nishida, Y., Narahara, H., and Oribe, K. (2011). A case of placenta accreta successfully treated with tokakujokito. Kampo Med. 62, 34–37. doi:10.3937/kampomed.62.34
Noda, A., Arita, R., Obara, T., Suzuki, S., Ohsawa, M., Obara, R., et al. (2024). The use of Japanese traditional (Kampo) medicines before and during pregnancy in Japan: the Tohoku medical megabank project birth and three-generation cohort study. Pharmacoepidemiol. Drug Saf. 33, e70033. doi:10.1002/pds.70033
Nowicka, P., and Wojdyło, A. (2019). Content of bioactive compounds in the peach kernels and their antioxidant, anti-hyperglycemic, anti-aging properties. Eur. Food Res. Technol. 245, 1123–1136. doi:10.1007/s00217-018-3214-1
Oliveira, C. I. F., and Fett-Conte, A. C. (2013). Birth defects: risk factors and consequences. J. Pediatr. Genet. 2, 85–90. doi:10.3233/PGE-13052
Shimizu, T., Terawaki, K., Sekiguchi, K., Sanechika, S., Ohbuchi, K., Matsumoto, C., et al. (2022). Tokishakuyakusan ameliorates lowered body temperature after immersion in cold water through the early recovery of blood flow in rats. J. Ethnopharmacol. 285, 114896. doi:10.1016/j.jep.2021.114896
Suzuki, S., Obara, T., Ishikawa, T., Noda, A., Matsuzaki, F., Arita, R., et al. (2021). Prescription of Kampo formulations for pre-natal and post-partum women in Japan: data from an administrative health database. Front. Nutr. 8, 762895. doi:10.3389/fnut.2021.762895
Suzuki, S., Obara, T., Ishikawa, T., Noda, A., Matsuzaki, F., Arita, R., et al. (2023). No association between major congenital malformations and exposure to Kampo medicines containing rhubarb rhizome: a Japanese database study. Front. Pharmacol. 14, 1107494. doi:10.3389/fphar.2023.1107494
The Ministry of Health, Labour and Welfare (2021). The Japanese Pharmacopoeia. 18th ed. Available online at: https://www.mhlw.go.jp/content/11120000/000945683.pdf (Accessed October 23, 2024).
Toda, T., Shiota, A., Fukushima, Y., Fujita, R., Takushima, Y., and Noumi, A. (2021). A report on three cases of infertility that resulted in pregnancy and childbirth when tokishakuyakusan was administered following symptomatic treatment resolving blood stasis or regulating qi. Kampo Med. 72, 377–382. doi:10.3937/kampomed.72.377
Tsumura and Co (2007). Tsumura keishibukuryogan extract granules for ethical use. Available online at: https://www.tsumura.co.jp/english/products/pi/JPR_T125.pdf (Accessed October 23, 2024).
Tsumura and Co (2014). Tsumura tokishakuyakusan extract granules for ethical use. Available online at: https://www.tsumura.co.jp/english/products/pi/JPR_T023.pdf (Accessed June 1, 2025).
VanderWeele, T. J. (2019). Principles of confounder selection. Eur. J. Epidemiol. 34, 211–219. doi:10.1007/s10654-019-00494-6
Wen, S. H., Chang, W. C., Shen, H. S., and Wu, H. C. (2020). Prescription patterns and factors influencing the use of Chinese herbal medicine among pregnant women in Taiwan: a population-based retrospective study. BMC Complement. Med. Ther. 20, 240. doi:10.1186/s12906-020-03032-0
WHO (2024). FIC foundation. Blood Stasis Pattern. TM1. Available online at: https://icd.who.int/dev11/f/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f1004763328.
Xi, S., Qian, L., Tong, H., Yue, L., Zhao, H., Wang, D., et al. (2013). Toxicity and clinical reasonable application of Taoren (Semen persicae) based on ancient and modern literature research. J. Tradit. Chin. Med. 33, 272–279. doi:10.1016/s0254-6272(13)60139-9
Yeh, H. Y., Chen, Y. C., Chen, F. P., Chou, L. F., Chen, T. J., and Hwang, S. J. (2009). Use of traditional Chinese medicine among pregnant women in Taiwan. Int. J. Gynaecol. Obstet. 107, 147–150. doi:10.1016/j.ijgo.2009.07.024
Yoshida-Komiya, H., Ami, M., Suganuma, R., and Mitsuma, T. (2021). Sho-based Kampo medicine combined with assisted reproductive technology is effective for refractory infertility and early recurrent miscarriage: a case report. Front. Nutr. 8, 761199. doi:10.3389/fnut.2021.761199
Yoshino, T., Kashio, A., Terasawa, Y., Hachiki, M., Yoshinaga, R., and Arita, R. (2023). The integration of traditional medicine with conventional biomedicine: a narrative review of the Japanese perspective. Med. 29, 372–379. doi:10.1089/jicm.2022.0643
Keywords: database, pregnancy, malformation, preterm birth, Prunus persica kernel, Kampo medicine, traditional medicine
Citation: Suzuki S, Arita R, Obara T, Ishikawa T, Kunitoki T, Sakai T, Noda A, Shinoda G, Ishikuro M, Orui M, Kuriyama S, Ohsawa M, Haneda K, Mano N, Kikuchi A, Takayama S and Ishii T (2025) Association of first-trimester exposure to Kampo medicines containing Prunus persica kernel with preterm birth and major congenital malformations: a Japanese database study. Front. Pharmacol. 16:1562724. doi: 10.3389/fphar.2025.1562724
Received: 18 January 2025; Accepted: 28 July 2025;
Published: 22 August 2025.
Edited by:
Yoshiaki Uyama, Pharmaceuticals and Medical Devices Agency, JapanReviewed by:
Mansoor Ahmed Mahar, Dow University of Health Sciences, PakistanChieko Ishiguro, Kokuritsu Kenko Kiki Kanri Kenkyu Kiko, Japan
Copyright © 2025 Suzuki, Arita, Obara, Ishikawa, Kunitoki, Sakai, Noda, Shinoda, Ishikuro, Orui, Kuriyama, Ohsawa, Haneda, Mano, Kikuchi, Takayama and Ishii. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shin Takayama, c2hpbi50YWtheWFtYS5jNEB0b2hva3UuYWMuanA=
 Satoko Suzuki
Satoko Suzuki Ryutaro Arita
Ryutaro Arita Taku Obara3,4
Taku Obara3,4 Tomofumi Ishikawa
Tomofumi Ishikawa Takamasa Sakai
Takamasa Sakai Masatsugu Orui
Masatsugu Orui Akiko Kikuchi
Akiko Kikuchi Shin Takayama
Shin Takayama