- 1School of Basic Medical Sciences, Chengdu Medical College, Chengdu, China
- 2Department of Dermatology, Chengdu Second People‘s Hospital, Chengdu, Sichuan Province, China
- 3School of Clinical Medicine, Chengdu Medical College, Chengdu, China
- 4Department of Medical Aesthetics, Nanbu People‘s Hospital, Nanchong, China
Photoaging, the premature aging of skin due to chronic ultraviolet (UV) exposure, is a growing concern in dermatology and cosmetic science. While UV radiation is known to induce DNA damage, oxidative stress, and inflammation in skin cells, recent research unveils a promising countermeasure: autophagy. This review explores the intricate relationship between autophagy and photoaging, highlighting how this cellular recycling process can mitigate UV-induced damage. We begin by examining the differential impacts of UVA and UVB radiation on skin cells and the role of oxidative stress in accelerating photoaging. Next, we delve into the molecular mechanisms of autophagy, including its various forms and regulatory pathways. Central to this review is the discussion of autophagy’s protective functions, such as the clearance of damaged organelles and proteins, and its role in maintaining genomic integrity. Furthermore, we address the current challenges in harnessing autophagy for therapeutic purposes, including the need for selective autophagy inducers and a deeper understanding of its context-dependent effects. By synthesizing recent advancements and proposing future research directions, this review underscores the potential of autophagy modulation as a novel strategy to prevent and treat photoaging. This comprehensive analysis aims to inspire further investigation into autophagy-based interventions, offering new hope for preserving skin health in the face of environmental stressors.
1 Introduction
Photoaging, the premature aging of the skin due to prolonged exposure to ultraviolet (UV) radiation, represents a major concern in dermatology and cosmetic science (Havas et al., 2022; Jin et al., 2022). It is estimated that approximately 80% of visible skin aging signs are caused by UV exposure, with photoaging being more prevalent in regions with high UV radiation exposure, such as tropical and subtropical areas (Yaar and Gilchrest, 2007; Krutmann et al., 2017). This condition arises from morphological and functional changes in the skin, influenced by a complex interplay of factors including genetic susceptibility, environmental stressors, and lifestyle choices (Ryu et al., 2022; Xu et al., 2022b; Yoon et al., 2022). Beyond its cosmetic implications, photoaging impacts health, psychological wellbeing, and socio-economic status, as visible aging signs can diminish self-esteem and affect social interactions (Shivakumar and Jafferany, 2020). Understanding its mechanisms is thus critical for both scientific inquiry and practical applications. A key cellular process implicated in this context is autophagy, a tightly regulated mechanism that degrades and recycles damaged organelles and misfolded proteins to maintain cellular homeostasis (Tang et al., 2021; Song et al., 2024). Autophagy plays an essential role in responding to stressors like oxidative damage, which is a hallmark of UV-induced skin aging (Machala et al., 2019; Ocansey et al., 2024). The relationship between photoaging and autophagy is particularly significant. For instance, UV exposure triggers oxidative stress that disrupts autophagic processes, leading to exacerbated cellular damage and perpetuating a cycle that amplifies photoaging effects (Popp et al., 2018; Kikuchi et al., 2020). Conversely, enhancing autophagic activity has emerged as a promising therapeutic strategy to mitigate these effects, underscoring its protective role in preserving skin integrity (Arensman and Eng, 2018).
Despite the individual attention given to photoaging and autophagy, their intersection remains underexplored, leaving a notable gap in the literature (Arensman and Eng, 2018; Arzalluz-Luque and Conesa, 2018). Current treatments for photoaging—such as topical antioxidants, retinoids, sunscreens, and procedures like chemical peels and laser therapies—focus primarily on reducing UV damage (Chan et al., 2024). However, emerging research suggests that targeting autophagy could offer novel avenues for intervention. For instance, studies using autophagy-enhancing compounds like rapamycin and metformin have shown encouraging results, improving skin health and reducing aging signs (Bharath et al., 2020; Ma J. et al., 2022; Ikutama et al., 2023). This highlights the potential of autophagy as a cornerstone for innovative anti-aging strategies.
This review aims to provide a comprehensive overview of the interplay between autophagy and photoaging. By examining the underlying mechanisms, synthesizing current research, and identifying knowledge gaps, we seek to lay the groundwork for future studies that could lead to effective therapies. Ultimately, our goal is to explore how autophagy’s protective potential can be harnessed to counteract the detrimental effects of photoaging, offering new possibilities for maintaining skin health in the face of environmental challenges.
2 Molecular mechanisms of photoaging
Photoaging differs from intrinsic aging, as it is mainly caused by UV-induced molecular damage that impacts cellular components such as proteins, lipids, and DNA (Widmer et al., 2006; Seo et al., 2009). The mechanisms of photoaging are characterized by intricate molecular pathways, which involve the production of reactive oxygen species (ROS), which, along with the activation of specific signaling cascades (Fan et al., 2024; Lu et al., 2024), and the breakdown of the extracellular matrix (Tang Z. et al., 2024), play a central role in the process. Here, we focus on the detailed molecular mechanisms by which UVA and UVB radiation, particularly through ROS generation, contribute to skin aging.
2.1 The impact of UV radiation on skin
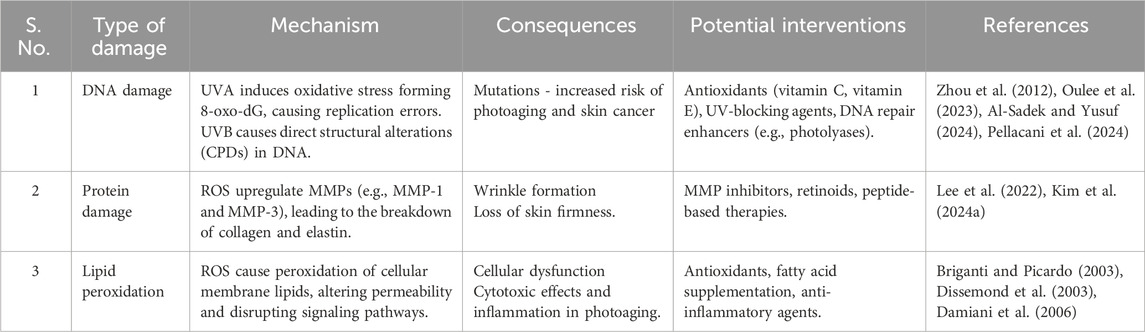
Under normal conditions, UV radiation is classified into three types based on wavelength by the WHO: UVC (100–280 nm), UVB (280–315 nm), and UVA (315–400 nm) (Al-Sadek and Yusuf, 2024). However, UVC radiation is largely absorbed by the Earth’s ozone layer and does not reach the surface under natural conditions. Therefore, this discussion focuses on UVA and UVB, which are the primary contributors to photoaging through distinct mechanisms determined by their wavelength-related energy properties and penetration depths into the skin. UVA and UVB differ in their energy levels and penetration depths, leading to distinct mechanisms of skin damage. Shorter wavelengths, such as those of UVB, carry higher energy, while longer wavelengths, like UVA, penetrate deeper into the skin but with lower energy (Gęgotek and Skrzydlewska, 2022; Salminen et al., 2022; Tang X. et al., 2024) (Table 1). UVA constitutes about 95% of the UV radiation that reaches the Earth’s surface (Hargreaves et al., 2007; Narayanapillai et al., 2012), it penetrates deeper into the dermis where collagen, elastin, and fibroblasts are located (Xia et al., 2024; Yuan et al., 2024). Although it has lower energy than UVB, UVA induces significant damage over time by generating ROS, which cause oxidative damage to cellular components such as proteins, lipids, and DNA (Bernerd et al., 2022; Negre-Salvayre and Salvayre, 2022). This oxidative damage compromises the structural integrity and function of dermal cells, leading to visible signs of aging such as loss of skin elasticity, wrinkle formation, and collagen degradation over time. In contrast to UVA, UVB accounts for only about 5% of UV radiation but possesses higher energy, primarily affecting the epidermis (Calzari et al., 2023). The higher energy of UVB directly damages DNA by inducing cyclobutane pyrimidine dimers (CPDs) and pyrimidine (6-4) photoproducts, leading to mutations that can trigger carcinogenesis and inflammatory responses (Wang Z. et al., 2022; Oulee et al., 2023). Additionally, UVB exposure stimulates keratinocyte apoptosis and activates inflammatory cytokines (Yano et al., 2003; Glady et al., 2018; Min et al., 2022), further accelerating skin damage. UVB radiation is primarily responsible for sunburn. Chronic exposure to UVB can also lead to thickening of the epidermis, pigment changes, and premature aging (Ichihashi and Ando, 2014). Understanding the distinct mechanisms of UVA and UVB radiation—including their energy levels, penetration depths, and types of molecular damage—is crucial for comprehending how UV radiation leads to molecular damage and ultimately results in photoaging.

Table 1. Comparative summary of UVA and UVB: physicochemical parameters, molecular targets, biochemical pathways, and pathological outcomes.
Thus, UVA predominantly contributes to deep dermal damage and oxidative stress by generating ROS that degrade structural proteins like collagen and elastin. In contrast, UVB primarily causes direct DNA damage in the epidermis through the formation of CPDs and induces epidermal inflammation. Both types of radiation act synergistically to accelerate skin aging, a process encompassing both intrinsic aging—caused by genetic and physiological factors—and extrinsic aging, driven by environmental influences such as chronic UV exposure.
2.2 ROS generation and damage
One of the key contributors to photoaging is the UV-induced generation of ROS, which play a pivotal role in initiating and propagating molecular damage in skin cells (Son et al., 2024; Yu Z.-W. et al., 2024). Both UVA and UVB radiation can lead to the formation of ROS, but the mechanisms and subsequent cellular effects differ between the two types of UV radiation (Svobodova et al., 2006; Manosalva et al., 2024). While UVB directly damages DNA by forming cyclobutane pyrimidine dimers (CPDs), UVA does not cause such direct DNA damage. Instead, UVA penetrates deeply into the skin and interacts with endogenous chromophores (e.g., flavins, porphyrins) to induce oxidative stress (Brem et al., 2017; Yagura et al., 2017; Bernerd et al., 2022). This interaction leads to the generation of ROS, including superoxide anions (O2−), hydroxyl radicals (⋅OH), and hydrogen peroxide (H2O2) (Zou et al., 2017; Brown et al., 2021). These ROS contribute to widespread oxidative damage by initiating lipid peroxidation, protein oxidation, and indirect DNA damage. For example, ROS can oxidize guanine bases in DNA, forming 8-oxo-deoxyguanosine (8-oxo-dG), a marker of oxidative DNA damage associated with mutagenesis (Bacqueville et al., 2021; Wang et al., 2024b).
VB radiation primarily damages DNA directly by forming CPDs when UVB photons are absorbed by DNA bases, leading to covalent bonds between adjacent pyrimidine bases. Beyond this direct damage, UVB also induces the generation of ROS by activating enzymatic pathways such as NADPH oxidases in keratinocytes. ROS are highly reactive molecules that disrupt cellular homeostasis, leading to oxidative stress—a state where the cellular antioxidant defenses are overwhelmed, resulting in damage to DNA, proteins, and lipids (Oulee et al., 2023; Al-Sadek and Yusuf, 2024; Pellacani et al., 2024). Specifically, UVB-induced ROS cause additional DNA damage beyond the initial CPDs, such as strand breaks and base oxidations, through a process known as secondary oxidative stress (Tuteja et al., 2009). This secondary oxidative stress arises when ROS generated by UVB exposure exacerbate initial cellular damage, contrasting with primary oxidative stress, which refers to the immediate oxidative damage caused directly by ROS upon their generation. Table 2 summarizes the mechanisms and consequences of ROS damage on DNA, proteins, and lipids.
In conclusion, UVA and UVB accelerate photoaging through complementary mechanisms: UVA penetrates the dermis to generate ROS, causing oxidative stress and structural protein degradation, while UVB affects the epidermis by directly damaging DNA, triggering inflammation and hyperproliferation, together contributing to skin aging. Future research should further elucidate the molecular pathways linking UVA and UVB exposure to specific photoaging outcomes, enabling more targeted prevention and intervention strategies (Kim et al., 2022).
2.3 Signaling pathways associated with photoaging
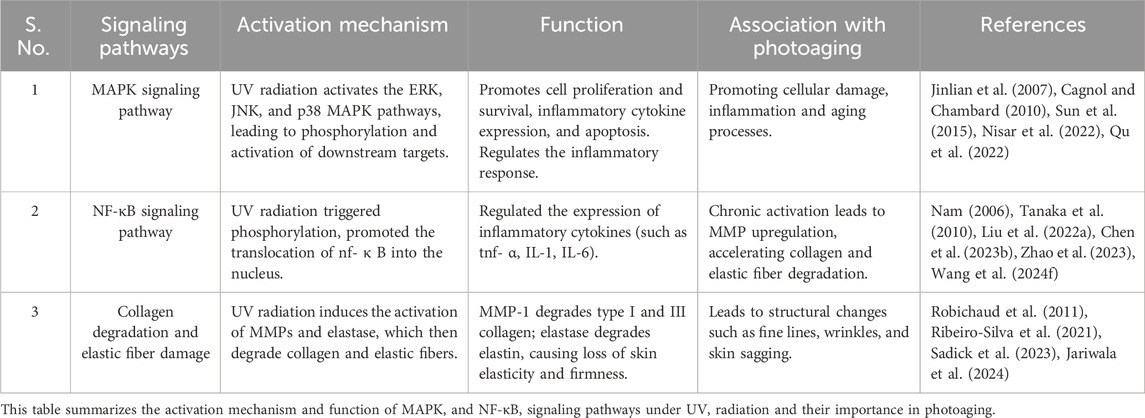
Photoaging results from signaling pathways activated by UV radiation, which cause cellular damage, inflammation, and aging (Li et al., 2024c; Wang K. et al., 2024). Two of the most well-characterized pathways are the Mitogen-Activated Protein Kinase (MAPK) and Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathways (Ge et al., 2024; Zhang et al., 2024). These pathways regulate the expression of matrix metalloproteinases (MMPs), enzymes that degrade collagen and elastic fibers, thereby contributing to the structural damage characteristic of photoaged skin (Han et al., 2022). The breakdown of these extracellular matrix components weakens the skin’s structural integrity and promotes wrinkle formation, which are hallmark features of photoaging (Hajialiasgary Najafabadi et al., 2024).
2.3.1 MAPK signaling pathway
The MAPK signaling pathway plays a pivotal role in mediating cellular responses to various stressors, including UV radiation (Bosch et al., 2015). UV exposure activates several MAPK family members—ERK (Extracellular Signal-Regulated Kinase), JNK (c-Jun N-terminal Kinase), and p38 MAPK—through a series of upstream signaling events (Madson and Hansen, 2007; López-Camarillo et al., 2012). UV radiation induces DNA damage, oxidative stress, and the activation of cell surface receptors such as epidermal growth factor receptor (EGFR). These receptors trigger a cascade of phosphorylation events mediated by small GTPases like Ras and Rac, leading to the sequential activation of MAPK kinase kinases (MAP3Ks), MAPK kinases (MAP2Ks), and ultimately MAPKs. Each of these kinases has distinct roles in the context of photoaging. ERK is primarily involved in cell proliferation and survival (Cagnol and Chambard, 2010; Sun et al., 2015). UV-induced activation of ERK promotes cell cycle progression and proliferation (Djavaheri-Mergny and Dubertret, 2001). However, prolonged or chronic activation, often resulting from repeated UV exposure, can lead to cellular senescence—a hallmark of aging characterized by irreversible cell cycle arrest and the secretion of pro-inflammatory factors, known as the senescence-associated secretory phenotype (SASP) (Victorelli et al., 2023; Ouvrier et al., 2024). JNK is activated by stress signals, including UV radiation. Once activated, JNK promotes the expression of pro-inflammatory cytokines and mediates apoptosis in severely damaged cells (Chen et al., 2018b; Zhang C. et al., 2022). This pathway contributes to the inflammatory response associated with photoaging and is linked to the induction of senescence in keratinocytes (Piao et al., 2015; Wu et al., 2016). p38 MAPK is particularly responsive to UV-induced stress and plays a critical role in regulating inflammatory responses (Jinlian et al., 2007). It activates transcription factors that induce the expression of MMPs (matrix metalloproteinases) and other inflammatory mediators (Mavrogonatou et al., 2018), facilitating collagen degradation and the subsequent loss of skin elasticity. Chronic activation of p38 is associated with sustained inflammatory states, exacerbating skin aging (Lee et al., 2009; Li H. et al., 2024). The proposed mechanism involves MAPK activation leading to a cascade of events that impact cellular responses in photoaging. Specifically, MAPK pathways contribute to oxidative stress, inflammation, and matrix degradation (Xu et al., 2022a). ERK promotes cell survival and proliferation, while prolonged activation may lead to senescence (Byun et al., 2023). JNK contributes to inflammation and apoptosis, and p38 MAPK regulates inflammation and collagen degradation (Ma et al., 2011). This coordinated activation of pathways under UV stress ultimately accelerates skin aging.
Thus, the MAPK signaling pathway serves as a crucial mediator of the cellular response to UV radiation, orchestrating a balance between proliferation, survival, inflammation, and cellular senescence (Yan et al., 2023; Yang T. et al., 2024). Dysregulation of this pathway can lead to enhanced photoaging and skin damage.
2.3.2 NF-κB signaling pathway
The interaction between NF-κB and MAPK pathways highlights the complex signaling involved in UV exposure, linking inflammation, cellular damage, and aging. The NF-κB signaling pathway plays a critical role in the inflammatory response to UV radiation (Bang et al., 2021; Han et al., 2022). Upon UV exposure, NF-κB is activated through phosphorylation events involving IκB kinase (IKK), leading to the degradation of IκB proteins and translocation of NF-κB into the nucleus (Tsuchiya et al., 2010; Bang et al., 2021). Once there, NF-κB promotes the transcription of inflammatory cytokines such as TNF-α, IL-1, and IL-6, which amplify inflammation and attract immune cells like neutrophils and macrophages to the damaged site (Mussbacher et al., 2023; Nam, 2006; Wang Z. et al., 2024). This recruitment of immune cells and the subsequent release of pro-inflammatory cytokines and ROS play a central role in perpetuating inflammation and oxidative stress. This inflammatory environment exacerbates the oxidative stress and cellular damage initiated by UV radiation by promoting the release of additional ROS and nitrogen species from recruited immune cells (Kim et al., 2022). The elevated ROS levels overwhelm the cellular antioxidant defenses, creating a feedback loop where oxidative stress amplifies inflammation, perpetuating cellular damage and structural degradation. Chronic activation of NF-κB contributes significantly to extracellular matrix breakdown. Chronic activation of NF-κB, through sustained inflammatory responses, contributes to the breakdown of the extracellular matrix, as it enhances the transcription of MMP (Yuan et al., 2022). While sustained expression of inflammatory cytokines leads to increased transcription of MMPs, MMPs are synthesized as inactive zymogens that require post-translational activation (Tanaka et al., 2010; Fu et al., 2024). This activation process involves proteolytic cleavage by enzymes such as plasmin or other active MMPs, as well as oxidative modifications induced by ROS, which enhance the proteolytic activity of MMPs (Tanaka et al., 2010; Liu S. et al., 2022). Once activated, MMPs degrade collagen, elastin, and other extracellular matrix components, weakening the skin’s structural integrity and contributing to wrinkle formation and the development of solar elastosis (Heng, 2013). Thus, NF-κB activation plays a critical role in both initiating and amplifying the inflammatory cascade that accelerates photoaging by promoting matrix degradation and enhancing ROS production. This highlights that both increased MMP transcription and their subsequent activation are critical steps in the progression of photoaging.
2.3.3 UV radiation-driven signaling pathways contributing to photoaging
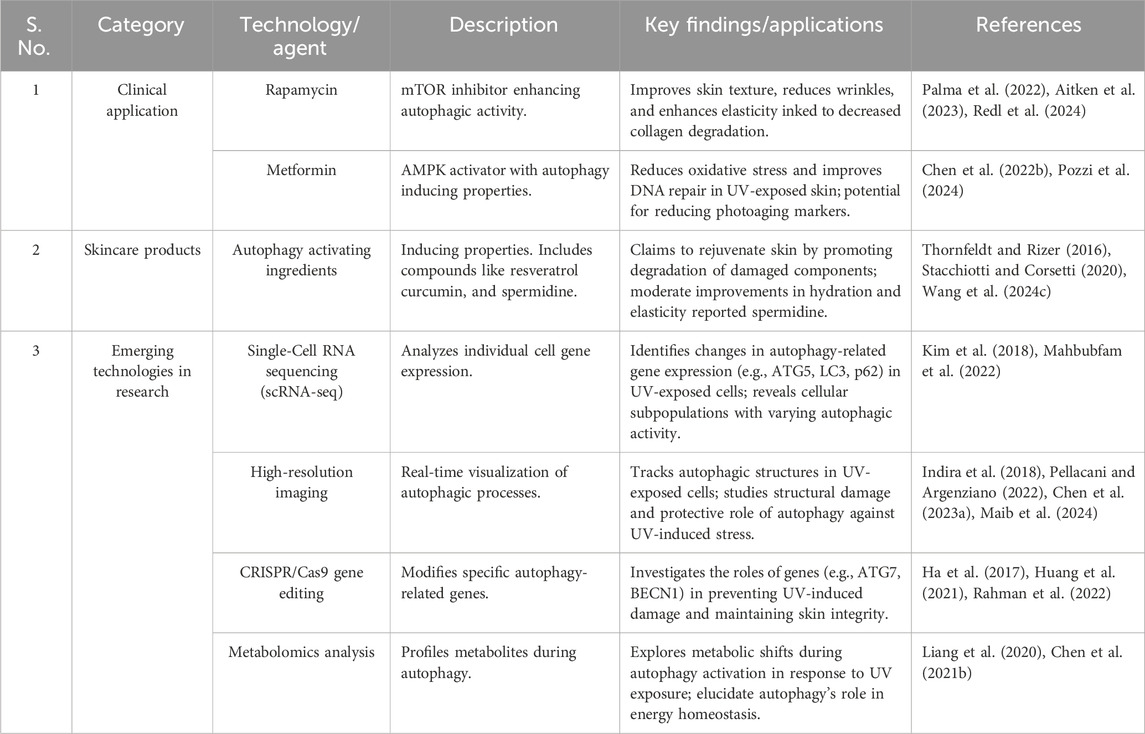
Photoaging is a complex process influenced by various signaling pathways activated in response to UVR. These pathways can either protect against or contribute to cellular damage, inflammation, and the eventual aging of skin tissue (De Magis et al., 2023) (Figure 1). UVR generates ROS, which activate several critical cellular pathways that modulate the skin’s response to damage. One of the key protective pathways is the Nrf2 pathway (Kahremany et al., 2022). In response to oxidative stress induced by ROS, Nrf2 dissociates from the Keap1 complex, translocases into the nucleus, and triggers the transcription of various antioxidant enzymes such as GCL, GST, HO-1, and NQO-1 (Kahremany et al., 2022; Robinson et al., 2022; Cai et al., 2023). These enzymes help mitigate ROS-induced cellular damage and play an essential role in maintaining cellular homeostasis.
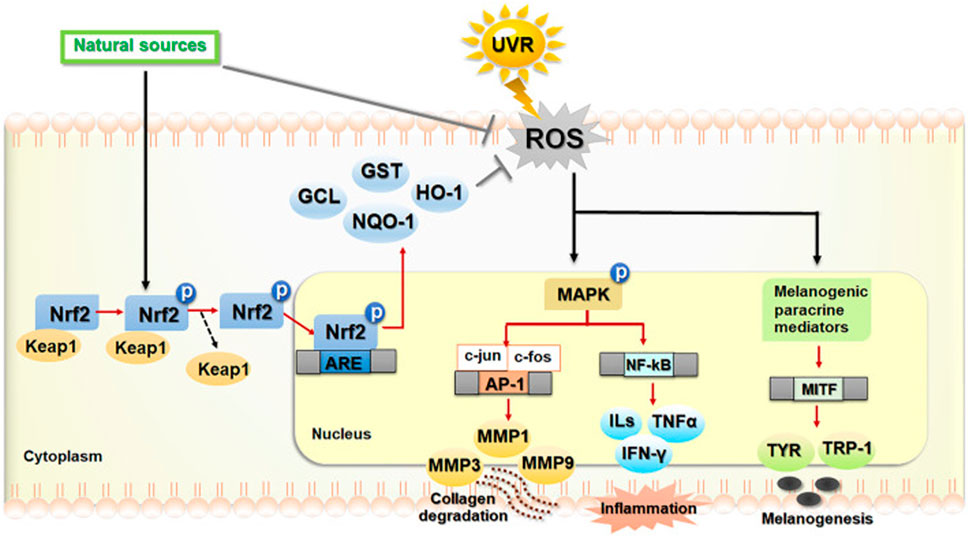
Figure 1. UV radiation-driven signaling pathways contributing to photoaging. The extended content further elaborates on the roles of specific signaling pathways in photoaging. It maintains a strong academic tone by detailing how each pathway (Nrf2, MAPK, NF-κB) contributes to different aspects of skin damage and aging. This expanded explanation provides a clear and coherent description of the complex molecular events involved, ensuring that readers can follow the intricate relationships between ROS, cellular signaling, and the eventual skin aging process caused by UV exposure. Abbreviations: UVR: Ultraviolet Radiation; ROS: Reactive Oxygen Species; Nrf2: Nuclear factor erythroid 2-related factor 2; Keap1: Kelch-like ECH-associated protein 1; GCL: Glutamate-cysteine ligase; GST: Glutathione S-transferase; HO-1: Heme oxygenase 1; NQO-1: NAD(P)H quinone dehydrogenase 1; MAPK: Mitogen-activated protein kinase; ERK: Extracellular signal-regulated kinase; JNK: c-Jun N-terminal kinase; p38: p38 mitogen-activated protein kinase; AP-1: Activator protein 1; NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells; MMPs: Matrix metalloproteinases; MMP1: Matrix metalloproteinase 1; MMP3: Matrix metalloproteinase 3; MMP9: Matrix metalloproteinase 9; ILs: Interleukins; TNF-α: Tumor necrosis factor alpha; IFN-γ: Interferon gamma; MITF: Microphthalmia-associated transcription factor; TYR: Tyrosinase; TRP-1: Tyrosinase-related protein 1. Reproduced from Chaiprasongsuk and Panich (2022). Role of Phytochemicals in Skin Photoprotection via Regulation of Nrf2. Frontiers in pharmacology, 13, 823881. Copyright © 2022 by (Chaiprasongsuk and Panich, 2022).
Conversely, UVR also activates the MAPK signaling pathway, which involves three major kinases: ERK, JNK, and p38 (Xu et al., 2022c). The activation of these kinases leads to the downstream activation of key transcription factors, including AP-1 and NF-Κb (Kim K. et al., 2024). These factors regulate inflammatory responses and contribute to the degradation of the extracellular matrix, a crucial event in the aging process of skin cells. AP-1 activation increases the expression of MMPs, such as MMP1, MMP3, and MMP9, which degrade collagen and elastin, leading to skin’s loss of structural integrity and the formation of wrinkles (Liu Y. et al., 2022; Zhang et al., 2024). Additionally, NF-κB activation further exacerbates the inflammatory environment by upregulating pro-inflammatory cytokines like interleukins (ILs), TNF-α, and IFN-γ (Qu et al., 2022; Yuksel Egrilmez et al., 2022). This cascade amplifies cellular and tissue damage, contributing to the breakdown of skin architecture and accelerating photoaging. Furthermore, ROS can stimulate the melanogenic pathway, leading to increased activity or expression of mediators such as MITF, tyrosinase, TYR, and TRP-1, which regulate pigmentation changes in the skin (Charachit et al., 2022). This pathway, in combination with the other signaling events, plays a pivotal role in the appearance of age spots and uneven pigmentation, which are hallmarks of photoaging (Li et al., 2024d).
In summary, UV radiation-induced ROS activates the protective Nrf2 pathway, reducing damage; MAPK pathway promotes inflammation; The NF-κB pathway leads to inflammation and matrix degradation; and the melanin production pathway, which triggers changes in pigmentation. The balance of these pathways determines the degree of photoaging, including wrinkle formation, decreased skin elasticity, and pigment changes, and provides potential targets for prevention or treatment strategies.
2.3.4 Collagen degradation and elastic fiber damage in photoaging
Photoaging is characterized by the breakdown of collagen and damage to elastic fibers, primarily due to the activity of enzymes such as MMPs (Kong et al., 2023; Jariwala et al., 2024). UV radiation triggers a series of events that lead to the upregulation and activation of these enzymes. Specifically, UV exposure increases the expression of MMPs, including MMP-1 (collagenase), MMP-3 (stromelysin), and MMP-9 (gelatinase), which degrade collagen and other extracellular matrix components (Lee et al., 2023; Oh S. et al., 2023; Feng C. et al., 2024). These enzymes degrade collagen and other extracellular matrix components, resulting in structural changes in the skin (Jariwala et al., 2024). For example, MMP-1 specifically targets type I and III collagen, which are the major components of the dermal matrix, leading to the typical loss of skin firmness and elasticity seen in photoaged skin (Robichaud et al., 2011). In addition to collagen, MMPs also play a role in the breakdown of elastic fibers (Xiong et al., 2008; Ribeiro-Silva et al., 2021). UV radiation promotes the expression of elastases, enzymes that specifically degrade elastin. The destruction of elastic fibers leads to a reduction in skin turgor and resilience, contributing to the formation of fine lines and sagging skin (Sadick et al., 2023; Jariwala et al., 2024).
The activity of MMPs is influenced by signaling pathways activated by UV exposure. For example, the NF-κB pathway can induce the expression of inflammatory cytokines, which subsequently activate MMPs, while the p38 MAPK pathway plays a role in the activation of MMPs through post-translational modifications. These signaling pathways play an integral role in amplifying the inflammatory response and contributing to the chronic activation of MMPs. The activation of MMPs is closely linked to the signaling pathways activated by UV exposure (Yuksel Egrilmez et al., 2022; Oh J. H. et al., 2023). These pathways are involved in the inflammatory response, which can indirectly influence the activation of MMPs and contribute to the degradation of collagen and elastic fibers in photoaged skin. For example, the NF-κB pathway stimulates the expression of MMPs in response to inflammatory cytokines, while the p38 MAPK pathway enhances MMP transcription as part of the cellular stress response (Li et al., 2022; Zhang Y. et al., 2022; Jeon et al., 2024). This coordinated response ultimately leads to a significant alteration of the extracellular matrix, resulting in photoaging.
In conclusion, the signaling pathways triggered by UV radiation, especially the MAPK and NF-κB pathways, are crucial in mediating the cellular response to UV-induced damage (Ge et al., 2024). These pathways orchestrate a feedback loop between inflammation, oxidative stress, and MMP activation, driving the degradation of collagen and elastic fibers (Kim et al., 2014; Liu et al., 2019). This cascade results in the characteristic features of photoaged skin (Li L. et al., 2019; Choi et al., 2020) (Table 3). Gaining a deeper understanding of these mechanisms offers valuable insights into potential therapeutic approaches to counteract the effects of photoaging and maintain skin health.
2.4 Epigenetics and photoaging
Epigenetics refers to mechanisms that alter gene expression through environmental factors or lifestyle choices without modifying the DNA sequence (He et al., 2022; Zand et al., 2024). Recently, interest in epigenetics has surged due to its role in various diseases and its influence on cellular responses to environmental stimuli, such as UV radiation, particularly in skin aging (Yuksel, 2020; Barnes et al., 2024). Research indicates that epigenetic modifications significantly affect how cells respond to UV radiation and contribute to photoaging progression (Lee Y. et al., 2021; Kim et al., 2023; Lin et al., 2024a). Two key mechanisms of epigenetic regulation, DNA methylation and histone modification, are particularly relevant in this context (Lee Y. et al., 2021; Zand et al., 2024). UV radiation can induce alterations in DNA methylation patterns, leading to changes in gene expression. For example, UV exposure has been shown to promote the hypermethylation of specific genes involved in the skin’s protective mechanisms and DNA repair processes (Crochemore et al., 2023; Johann To Berens et al., 2024). This hypermethylation silences essential genes, resulting in reduced expression of proteins critical for cellular repair and maintenance, thereby contributing to the aging process (de Oliveira et al., 2020). Such epigenetic modifications can increase the skin’s vulnerability to further UV damage, establishing a cycle of degradation (Qin H. et al., 2018). Similarly, UV radiation influences histone modifications, such as acetylation and methylation, which affect chromatin structure and gene accessibility (Xie L. et al., 2022; Wen et al., 2024). For instance, increased histone acetylation can enhance the expression of pro-inflammatory genes, while decreased acetylation may silence genes responsible for cell proliferation and repair (Lee Y. et al., 2021; Negre-Salvayre and Salvayre, 2022). Similarly, alterations in histone methylation can either activate or suppress gene expression, influencing processes critical to skin homeostasis (Negre-Salvayre and Salvayre, 2022).
Furthermore, epigenetic modifications interact with autophagy regulation, influencing photoaging (Durocher et al., 2019; Patra et al., 2019; Zha et al., 2019). For example, DNA methylation and histone modifications can affect the expression of autophagy-related genes (such as ATG genes) (Peixoto et al., 2019; Szustka et al., 2024), thereby regulating autophagic flux. Disruptions in autophagy due to these epigenetic changes can result in the accumulation of damaged proteins and organelles (Alves-Fernandes and Jasiulionis, 2019), which in turn exacerbates oxidative stress and cellular senescence. This interaction highlights the complexity of the mechanisms underlying photoaging and emphasizes the necessity for additional research to explore how these epigenetic alterations could be targeted to alleviate the effects of UV-induced skin aging (Jeremian et al., 2024; Lin et al., 2024a).
3 Molecular mechanisms of autophagy pathways
3.1 Classification of autophagy
Autophagy is a highly regulated cellular degradation process that maintains cellular homeostasis by removing damaged organelles, misfolded proteins, and other cellular debris (Chen et al., 2024; Kim J. et al., 2024). It can be broadly classified into three types: macroautophagy, microautophagy, and selective autophagy (Yan et al., 2023; Yamamoto and Matsui, 2024). Each type possesses unique characteristics and mechanisms, which are essential for the specific cellular contexts in which they function. Next, we will introduce these different autophagy types (Figure 2).
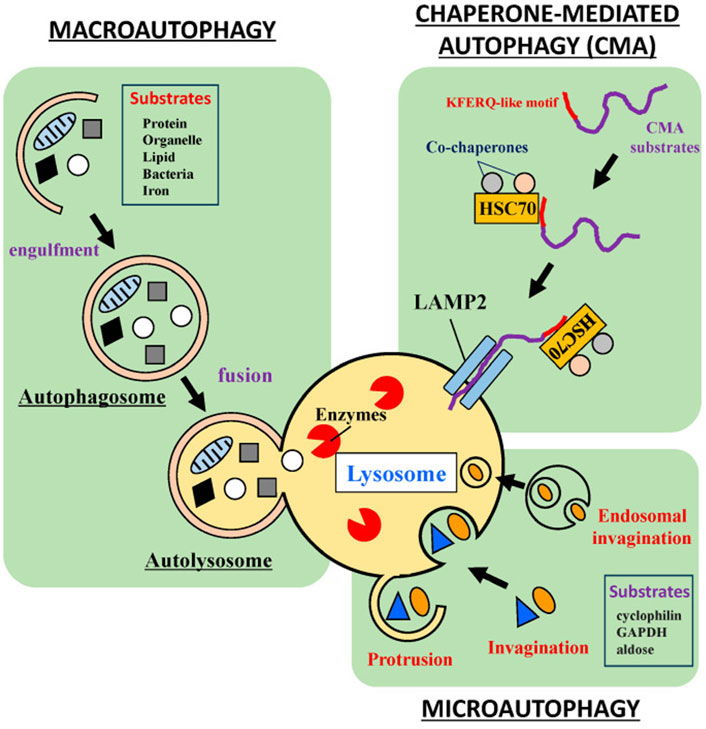
Figure 2. Classification of autophagy. Three main autophagy subtypes involved in cellular metabolism and balance, including macroautophagy, microautophagy, and a selective autophagy process: Chaperone-Mediated Autophagy (CMA). Reproduced from Watanabe et al. (2023). Roles of Stress Response in Autophagy Processes and Aging-Related Diseases. International journal of molecular sciences vol. 24, 18 13804. 7, Copyright © 2023 by (Yan et al., 2023).
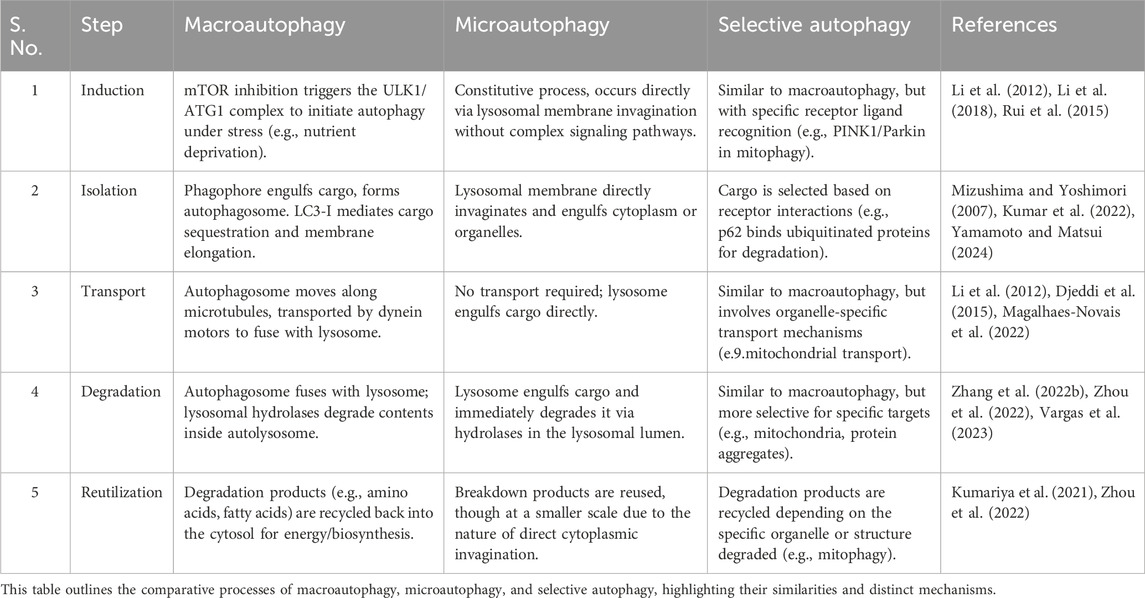
At present, macroautophagy, the most extensively studied form of autophagy, involves the formation of double-membrane structures called autophagosomes (Klionsky and Codogno, 2013; Feng Y. et al., 2024). Macroautophagy begins with the induction of autophagy, often triggered by cellular stressors such as nutrient deprivation or oxidative stress (Lyamzaev et al., 2018; Beccari et al., 2023). Oftentimes, the initiation of macroautophagy is regulated by various signaling pathways (Popelka and Klionsky, 2015), primarily the mechanistic target of rapamycin (mTOR) pathway (Pattingre et al., 2008; Ott et al., 2016). Once the process begins, the isolation membrane, referred to as the phagophore, elongates to enclose cytoplasmic components, ultimately completing the formation of an autophagosome (Egan et al., 2015; Wang et al., 2018). The autophagosome then fuses with a lysosome to form an autolysosome, where lysosomal hydrolases degrade the contents, and the resulting breakdown products are recycled back into the cytosol (Cao et al., 2021; Barnaba et al., 2023).
Microautophagy, unlike macroautophagy, involves the direct invagination of the lysosomal membrane to engulf cytoplasmic components (Pereira et al., 2012; Lemasters, 2014; Oku et al., 2017). This process occurs through the protrusion of lysosomal membrane invaginations, which directly internalize the cargo (Wang L. et al., 2023; Yamamoto and Matsui, 2024). While microautophagy is not as extensively studied as macroautophagy, it is thought to contribute to the breakdown of smaller proteins and organelles (Kuchitsu and Taguchi, 2024; Yamamoto and Matsui, 2024). Importantly, this type of autophagy is considered a constitutive process and may operate continuously to support the maintenance of cellular homeostasis.
Selective autophagy encompasses a variety of mechanisms that target specific cellular components for degradation, and one of the most common forms is chaperone-mediated autophagy (CMA) (Krause et al., 2023; Yan et al., 2023). This also includes the removal of damaged mitochondria (mitophagy), peroxisomes (pexophagy), and protein aggregates (aggrephagy) (Guan et al., 2022; Rubio-Tomás et al., 2023; Bajdzienko and Bremm, 2024). Pexophagy specifically targets damaged peroxisomes, which are organelles responsible for detoxifying ROS and metabolizing fatty acids (Dolese et al., 2022; Manandhar et al., 2024). In the context of photoaging, pexophagy helps prevent the accumulation of dysfunctional peroxisomes that can exacerbate oxidative stress and contribute to skin aging (Gallagher and Holzbaur, 2023). Aggrephagy, on the other hand, is responsible for the degradation of aggregated proteins, which may accumulate due to cellular stressors such as UV exposure (Li et al., 2023). The removal of these protein aggregates is critical for maintaining cellular integrity and preventing the detrimental effects of protein aggregation on skin cells (Rai et al., 2019). Different from macroautophagy and microautophagy, selective autophagy relies on specific receptors and cargo recognition mechanisms, ensuring that only designated targets are engulfed by the autophagic machinery (Mochida and Nakatogawa, 2022). For instance, mitophagy is facilitated by receptors such as PINK1 and Parkin, which are instrumental in marking dysfunctional mitochondria for degradation (Koentjoro et al., 2017; Narendra and Youle, 2024). Mitochondrial processes are particularly relevant in selective autophagy because mitochondria are not only key energy producers in the cell but also play a central role in maintaining cellular homeostasis (Yang et al., 2019; Feng Y. et al., 2024). In skin cells, mitochondria damaged by UV radiation may accumulate excessive ROS, exacerbating oxidative stress and leading to cellular damage, inflammation, and accelerated aging (Dunaway et al., 2018; Umar et al., 2019; Prasert et al., 2023). Mitophagy helps remove these damaged mitochondria, thereby maintaining cellular energy balance and preventing further functional decline during the photoaging process (Ding and Yin, 2012). In photoaging, impaired mitophagy can worsen oxidative damage in skin cells, promote collagen degradation, and damage elastic fibers, thus accelerating the appearance of aging signs (Panwar et al., 2018; Chen et al., 2020). Furthermore, an imbalance in mitophagy can contribute to various diseases, including neurodegenerative disorders, cancer, and aging, which are closely associated with the onset and progression of photoaging (Gaetano et al., 2021; Ghosh and Kumar, 2024). Therefore, mitophagy plays a crucial role in preserving mitochondrial integrity and maintaining overall cellular function, especially in skin cells exposed to UV-induced damage.
Macroautophagy is responsible for the large-scale degradation of cellular components through the formation of autophagosomes, whereas microautophagy operates via the direct inward folding of the lysosomal membrane. Selective autophagy specifically targets and eliminates particular cellular structures. A clear understanding of these distinctions is vital for unraveling the roles of autophagy in various physiological and pathological conditions. Recognizing these differences is essential for providing deeper insights into the involvement of autophagy in diverse biological processes.
3.2 Basic pathways of autophagy
Autophagy involves five primary steps—induction, isolation, transport, degradation, and reutilization—that are essential to its three main types: macroautophagy, microautophagy, and selective autophagy (Table 4) (Wurzer et al., 2015; Bradley et al., 2022; Lim S. H. Y. et al., 2024; Majumder et al., 2024) These steps, illustrated in Figure 3, enable macroautophagy to degrade bulk cytoplasmic materials, microautophagy to engulf materials directly via the lysosomal membrane, and selective autophagy to target specific cellular components.
Figure 3. Macroautophagy, Microautophagy, and Selective Autophagy in the Autophagic Process. (A) Macroautophagy: during macroautophagy, cytosolic substrates, including proteins and organelles, are sequestered by the autophagosome. The fusion of the autophagosome with the lysosome to form the autolysosome is a crucial step for degradation, ensuring that the sequestered contents are efficiently processed. (B) Chaperone-Mediated Autophagy (a kind of Selective Autophagy): in CMA, proteins containing the pentapeptide KFERQ-like sequence are recognized by the Hsc70 chaperone. This chaperone binds to the target proteins and associates with the lysosomal membrane protein LAMP-2A, initiating its oligomerization. This event facilitates the translocation of the target protein into the lysosomal lumen, a process that is Hsc70-dependent. (C) Microautophagy: in microautophagy, lysosomes directly engulf cytosolic components through invagination or protrusion of the lysosomal membrane, without the prior formation of an autophagosome. This process allows for the direct sequestration of cellular material for degradation. Reproduced from Assaye M. A., Gizaw S. T. Chaperone-Mediated Autophagy and Its Implications for Neurodegeneration and Cancer. Int J Gen Med., Copyright © 2024 by (Assaye and Gizaw, 2022).
The process begins with the induction phase, where stressors like nutrient deprivation, oxidative stress, or hypoxia trigger autophagy These signals are recognized by receptors like mTOR (mechanistic target of rapamycin), which, when inhibited by stress signals, activates the autophagy machinery (Iannucci et al., 2021; Jarisarapurin et al., 2021). The isolation phase involves the formation of a membrane structure called the phagophore. This structure expands to surround cellular cargo, such as damaged organelles or protein aggregates, and sequesters them within the growing vesicle (Saetre et al., 2015; Schmitt et al., 2022; Shatz and Elazar, 2024). Following this, the transport phase occurs, where the autophagosome, now encapsulating the cargo, moves along microtubules toward the lysosome. This process is facilitated by motor proteins such as dynein and kinesin, which guide the autophagosome to the lysosome for fusion (Bozic et al., 2020). Once the autophagosome fuses with the lysosome, its contents are degraded within the autolysosome by hydrolytic enzymes. Finally, the reutilization phase involves the recycling of macromolecules such as amino acids, lipids, and sugars, which are released back into the cytosol for reuse in cellular metabolism (Feng et al., 2014; Cerda-Troncoso et al., 2020).
Recent studies have illuminated the intricate regulation of autophagy, showing how processes like macroautophagy, microautophagy, and selective autophagy are governed by signaling pathways such as mTOR, PI3K, and AMPK. These pathways coordinate the initiation, elongation, and maturation of autophagic vesicles in response to cellular stressors like nutrient deprivation, oxidative stress, and hypoxia (Beyaz et al., 2023; Chien et al., 2023; Kaur et al., 2023). For example, research by Inmaculada Navarro-Lérida et al. has illustrated how different autophagic pathways can be selectively activated by specific stimuli such as damage to organelles or the presence of protein aggregates, revealing the dynamic nature of autophagy in cellular adaptation and survival (Navarro-Lérida et al., 2022).
This deeper understanding of autophagic regulation enhances our grasp of cellular homeostasis and opens avenues for therapeutic interventions targeting autophagic dysfunction in diseases such as skin aging, photoaging, and neurodegeneration (Wang et al., 2019; Lim et al., 2020; Chu et al., 2023). By carefully examining these pathways and their roles in autophagy, researchers can gain deeper insights into the cellular processes underlying aging and disease, paving the way for future treatments.
3.3 Key regulatory molecules and signaling pathways
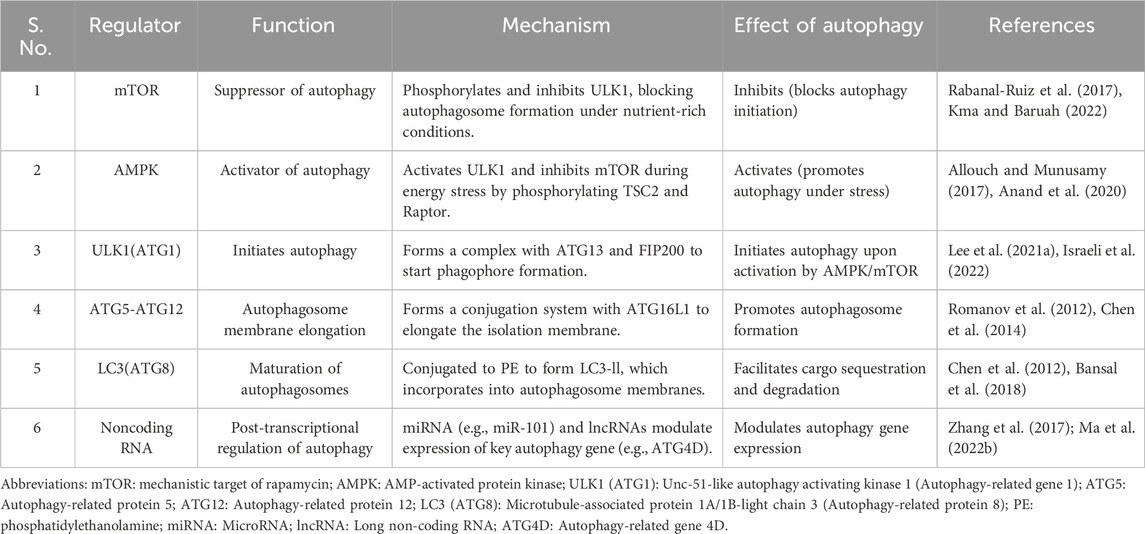
This section delves deeper into the molecular players and signaling pathways that regulate autophagy, providing a comprehensive understanding of their roles and interrelationships. Autophagy is a highly regulated process, controlled by various key molecules and signaling pathways that govern its initiation, progression, and completion (Selarka and Shravage, 2024; Wu et al., 2024; Yao et al., 2024). Critical regulators include the ATG gene family, the mTOR and AMPK pathways (Luo, 2014; Foerster et al., 2022), as well as recently discovered modulators like non-coding RNAs, which collectively ensure that autophagy occurs under the correct cellular conditions, such as nutrient deprivation or stress (Liu M. et al., 2024; Mirabdali et al., 2024). These regulators orchestrate the balance between cellular adaptation and homeostasis, playing an essential role in maintaining cellular function and survival (Rodríguez-Vargas et al., 2019; Yun et al., 2021) (Table 5).
3.3.1 ATG gene family (autophagy-related genes)
The ATG (Autophagy-related) gene family plays a crucial role in regulating the autophagic process, with each member contributing to distinct stages, from initiation to degradation (Li and Zhang, 2019). Here, we list some key members of the ATG gene family and their specific roles (Figure 4). ULK1, the human counterpart of yeast ATG1, is a component of the ULK1 complex, which also includes ATG13 and FIP200 (Mizushima, 2010; Tabata et al., 2024). This complex is essential for the induction of autophagy and is directly activated or inhibited by upstream signals like mTOR and AMPK (Chen et al., 2018a). Upon activation, ULK1 phosphorylates other downstream ATG proteins to trigger the formation of the phagophore (Zachari and Ganley, 2017). The ATG5-ATG12/ATG16L1 complex functions as a conjugation system, playing an essential role in the elongation of the autophagosome membrane (Hwang et al., 2012; Tan et al., 2020). Specifically, ATG12 covalently binds to ATG5, and together with ATG16L1, they form a complex that facilitates autophagosome formation (Hwang et al., 2012; Ji et al., 2019). This complex recruits LC3 (ATG8) to the membrane, enabling its expansion and closure around the cargo (Park et al., 2022; Varga et al., 2022). While LC3 (ATG8) is a critical protein involved in the maturation of the autophagosome (Jacquet et al., 2021; Gluschko et al., 2022). It exists in two forms: LC3-I (cytosolic) and LC3-II (membrane-bound) (Hu et al., 2014; Zou et al., 2015). During autophagy, LC3 is conjugated to phosphatidylethanolamine (PE) (Nakatogawa, 2013), which then converts LC3-I to LC3-II (Hu et al., 2014). LC3-II is then incorporated into the autophagosomal membrane. This modification marks the cargo and promotes autophagosome formation, as well as its fusion with lysosomes. ATG9, the only known transmembrane ATG protein, is involved in supplying membranes to the growing phagophore (Tamura et al., 2010). It cycles between different membrane compartments, supplying lipids for autophagosome formation (Choi et al., 2024).
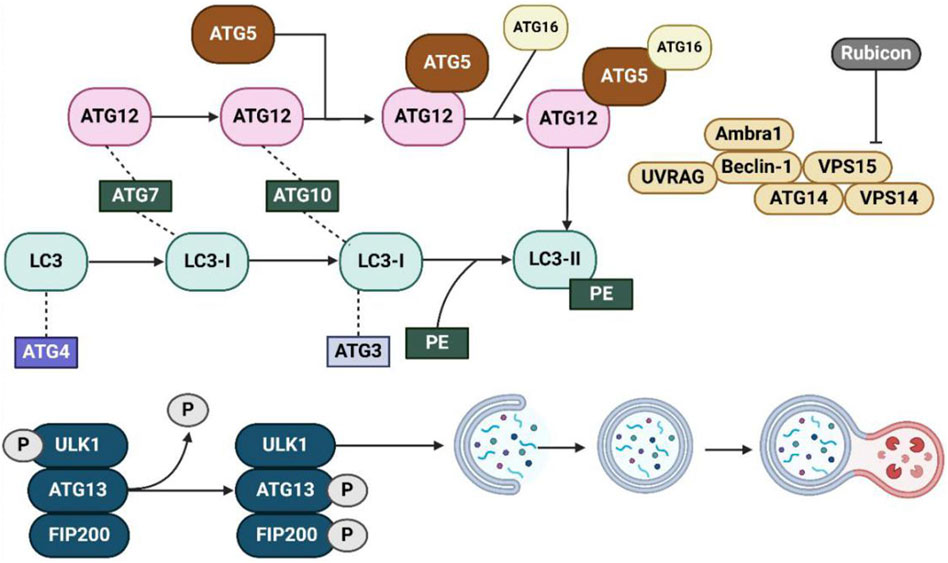
Figure 4. The autophagy pathway and key regulatory proteins involved in autophagosome formation and maturation. This diagram provides an overview of the molecular machinery involved in the formation and maturation of autophagosomes. The process begins with the activation of the ULK1 complex (composed of ULK1, ATG13, and FIP200), which is regulated by phosphorylation (indicated by P). This complex initiates the formation of the phagophore, which is extended and closed to form the autophagosome. Several key autophagy-related proteins (ATGs), such as ATG12, ATG5, ATG7, and ATG16, are involved in this process, facilitating the elongation of the autophagosomal membrane and the incorporation of LC3 (converted from LC3-I to LC3-II via conjugation with PE). Beclin-1, UVRAG, and other associated proteins like Ambra1, VPS15, ATG14, and VPS14 regulate the nucleation and expansion of the autophagosome. The pathway also includes the regulatory role of Rubicon, a key protein that inhibits the activation of the PI3K complex, thereby influencing the autophagic process. This network of interactions ensures the proper execution of autophagy, including the removal of damaged cellular components and the maintenance of cellular homeostasis. Abbreviations: ULK1: Unc-51-like autophagy activating kinase 1; ATG: Autophagy-related gene; LC3: Microtubule-associated protein 1A/1B-light chain 3; PE: Phosphatidylethanolamine; Rubicon: RUN domain and cysteine-rich domain-containing protein; Beclin-1: Autophagy-related protein 6; UVRAG: UV radiation resistance-associated gene; Ambra1: Autophagy and Beclin-1 regulator 1; VPS15: Vacuolar protein sorting 15; VPS14: Vacuolar protein sorting 14; FIP200: Focal adhesion kinase family interacting protein of 200 kDa. Reproduced from Liu, Beibei et al. Targeting cell death mechanisms: the potential of autophagy and ferroptosis in hepatocellular carcinoma therapy. Frontiers in immunology vol. 15 1450487, Copyright © 2024 by (Liu B. et al., 2024).
3.3.2 mTOR and AMPK signaling pathways
Autophagy is primarily regulated by the mTOR and AMPK signaling pathways, which respond to cellular energy levels and nutrient availability (Li Y.-Y. et al., 2024; Mundo Rivera et al., 2024). Typically, mTOR functions as a key inhibitor of autophagy by suppressing the ULK1 complex. Under nutrient-rich conditions, mTOR is active and phosphorylates ULK1, thereby preventing the initiation of autophagy (Nazio and Cecconi, 2017; Xu and Wan, 2023). This mechanism ensures that, when energy levels are high, the cell prioritizes growth and biosynthesis over degradation. Conversely, when mTOR activity is diminished, such as during starvation or oxidative stress, ULK1 undergoes dephosphorylation and activation, initiating autophagy to degrade cellular components and provide energy (D’Amico et al., 2022). While AMPK is activated in response to low cellular energy (high AMP/ATP ratio). When it is activated, AMPK promotes autophagy by inhibiting mTOR through direct phosphorylation of TSC2 and Raptor (negative regulators of mTOR) (Lee et al., 2010). Additionally, AMPK can directly activate ULK1, bypassing mTOR inhibition and stimulating autophagy (Egan et al., 2011; Wang S. et al., 2022). We believe this dual regulation ensures that autophagy is rapidly triggered in response to cellular energy stress, and providing an adaptive mechanism to restore metabolic balance. The interplay between mTOR and AMPK is a finely tuned system that ensures autophagy is only activated under conditions of stress, where catabolic processes like autophagy are necessary for survival. For example, in photoaging, excessive UV-induced oxidative stress can inhibit mTOR activity, thereby promoting autophagy to clear damaged proteins and organelles and protect the skin from further damage (Wang X. et al., 2023).
3.3.3 Recent advances in autophagy regulation
Recent research has uncovered additional regulators of autophagy, expanding our understanding of this complex process (Chowdhury and Karmakar, 2023; Almujri and Almalki, 2024; Lin et al., 2024b). Several novel ATG proteins have been identified, enhancing the diversity of autophagy regulation. For example, ATG101, a relatively recent discovery, has been shown to stabilize the ULK1 complex and facilitate the early stages of autophagy (Gallagher and Chan, 2013). This protein plays a critical role in the proper formation of autophagic initiation complexes, particularly under stress conditions (Mizushima, 2010; Corona Velazquez and Jackson, 2018). Furthermore, emerging evidence indicates that non-coding RNAs (ncRNAs), including microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), are significantly involved in autophagy regulation (Almujri and Almalki, 2024; Liu M. et al., 2024; Yu S. et al., 2024). Specifically, these ncRNAs regulate the expression of autophagy-related genes at the post-transcriptional level. For example, miR-101 has been shown to inhibit autophagy by directly targeting ATG4D, a key protein in the LC3 processing pathway (Fu et al., 2017). Likewise, certain lncRNAs, such as HOTAIR, have been implicated in either promoting or suppressing autophagy depending on the context (Li et al., 2021; Luo et al., 2024). These findings underscore the intricate regulatory network of autophagy, encompassing both traditional protein pathways and novel RNA-based mechanisms.
So far, these advances have indicated that autophagy is regulated by a complex interplay of protein-based pathways and non-coding RNA molecules (Kolapalli et al., 2023; Kumar et al., 2023), which opens new avenues for therapeutic interventions. For example, modulating autophagy through small molecules, gene therapy, or RNA-based therapies could offer potential treatments for conditions related to autophagy dysfunction, such as neurodegenerative diseases, cancer, and photoaging (Li T. et al., 2019). In the context of skin photoaging, strategies aimed at enhancing autophagic activity could help clear damaged cellular components, reduce oxidative stress, and improve skin regeneration (Suresh et al., 2020; Whitmarsh-Everiss and Laraia, 2021). Understanding these mechanisms is essential for identifying innovative strategies to modulate autophagy in disease contexts, including skin photoaging, where autophagy dysregulation may worsen aging-related cellular damage (Yu T. et al., 2015; Wang et al., 2019).
The regulation of autophagy involves an intricate network of signaling pathways, gene products, and emerging molecular regulators, such as non-coding RNAs. These pathways and molecules collaborate to preserve cellular homeostasis, especially in response to stress factors like those implicated in skin photoaging. Gaining a deeper understanding of these regulatory systems provides valuable insights into potential therapeutic targets for diseases associated with autophagy dysfunction.
4 Interaction between autophagy and photoaging
4.1 Protective role of autophagy in counteracting photoaging
Autophagy can facilitate the removal of damaged cellular components and maintains cellular homeostasis, and at the same time, it also plays a vital protective role in alleviating the effects of photoaging in this way (Hao et al., 2019; Ma J. et al., 2022). One of the primary functions of autophagy is the elimination of dysfunctional organelles and aggregated proteins, thereby reducing the production of ROS (Peng et al., 2024; Vikram et al., 2024). Specifically, autophagy targets damaged mitochondria through a selective process, which is known as mitophagy (Tran and Reddy, 2020; Shang et al., 2022). By degrading these impaired mitochondria, autophagy prevents the excessive ROS production that arises from mitochondrial dysfunction (Xu et al., 2024). This reduction in oxidative stress is particularly critical for protecting skin cells from the harmful effects of UV exposure, thereby contributing to skin health and enhancing resilience against aging (Bai et al., 2021).
Moreover, autophagy plays an integral role in maintaining DNA stability and promoting DNA repair mechanisms (Zhao et al., 2012). During photoaging, UV radiation induces various forms of DNA damage, which can lead to mutations that accelerate cellular aging (Al-Sadek and Yusuf, 2024; Markovitsi, 2024). However, autophagy aids in the removal of damaged proteins and organelles that may carry genetic defects, thereby minimizing the accumulation of harmful mutations (Vlada et al., 2015; Luo et al., 2023). Additionally, autophagy is associated with the expression of proteins involved in DNA repair pathways (Ye et al., 2017), ensuring efficient repair of UV-induced DNA damage. Therefore, by preserving genomic integrity and enhancing repair processes, autophagy significantly reduces the risk of genetic mutations associated with photoaging, reinforcing its critical role in skin protection and longevity (Vikram et al., 2024; Zhong et al., 2024).
4.2 Effects of UV radiation on autophagy
UV radiation has a dual effect on autophagy, acting both as an inducer and a disruptor of this essential cellular process. Specifically, UV exposure can trigger autophagy by activating pathways that inhibit mTOR or by stimulating AMPK.
4.2.1 UV-induced autophagy activation
We find that UV radiation can inhibit mTOR, which is a key negative regulator of autophagy (Yang et al., 2013; Huangfu et al., 2023). To be brief, when mTOR activity is reduced, the autophagic machinery is activated, enabling cells to initiate the degradation of damaged organelles and proteins (Al-Bari and Xu, 2020). The inhibition of mTOR allows for the upregulation of ATGs, which are crucial for the formation of autophagosomes. This initiates the sequestration of damaged components such as proteins, lipids, and damaged mitochondria (Boutouja et al., 2019). Furthermore, UV radiation can activate AMPK, an energy sensor that plays a crucial role in responding to cellular energy depletion (Lim C. et al., 2024; Xia et al., 2024). AMPK activation further supports autophagy by increasing the catabolic processes within the cell, including the enhancement of lysosomal function and the promotion of autophagosome-lysosome fusion (Paquette et al., 2021; Xu and Wan, 2023). As cellular energy levels drop due to UV-induced stress, AMPK activation promotes autophagy as a protective mechanism (Zhao et al., 2016). By activating this dual pathway, cells efficiently clear damaged components, alleviate oxidative stress, and ultimately adapt to the challenges imposed by UV exposure (Cao and Wan, 2009; Lee et al., 2014).
4.2.2 Autophagy dysfunction from excessive UV radiation
However, excessive UV radiation can overwhelm the cellular repair mechanisms, then leading to autophagy dysfunction finally (Wang B.-J. et al., 2024). Because too prolonged or high doses of UV exposure can cause a state of autophagic flux disruption (Wang B.-J. et al., 2024), which is characterized by impaired fusion with lysosomes, resulting in the accumulation of autophagosomes. This accumulation prevents the clearance of damaged cellular materials and instead leads to their buildup (Farizatto et al., 2017; Zhang et al., 2021). This disruption of autophagic flux is linked to the overproduction of ROS and the depletion of cellular ATP, which further inhibit the proper functioning of autophagic machinery (Yamamoto et al., 2021).
In extreme cases, excessive UV radiation can trigger autophagic cell death, where the autophagic process, initially serving a protective role, becomes a mechanism for cell demise (Hansda and Ghosh, 2022; Guerrero-Navarro et al., 2024). In this context, excessive autophagy leads to the degradation of essential cellular components, contributing to cell death and tissue damage, which worsens the overall effect of UV-induced photoaging (Xia et al., 2024). The dysregulation of autophagy in this scenario is associated with the overproduction of ROS, which not only overwhelms the cell’s antioxidant defenses but also interferes with the signaling pathways necessary for maintaining proper autophagic function (Wang D.-K. et al., 2022). This intricate interplay between autophagy activation and dysfunction emphasizes the delicate balance cells must maintain when responding to UV stress, highlighting the dual nature of autophagy as both a protective and potentially harmful process in the context of photoaging (Lamore and Wondrak, 2013).
4.3 Regulation of photoaging-related processes by autophagy
Autophagy plays a multifaceted role in regulating several processes associated with photoaging, including inflammation, cell cycle regulation, and apoptosis (Chen et al., 2017; Yang Y. et al., 2024). Through these mechanisms, autophagy mitigates cellular damage and slows down skin aging caused by UV radiation (Figure 5). However, as autophagic function declines with age, the ability to combat these effects diminishes, which leads to accelerated photoaging (Martic et al., 2020).
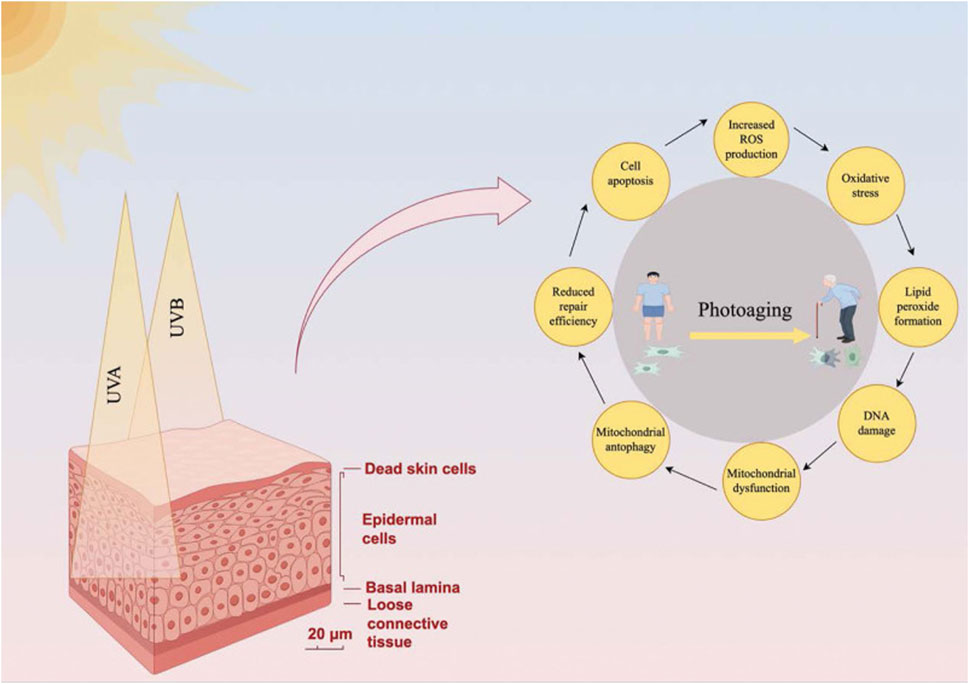
Figure 5. Regulation of photoaging-related processes by autophagy. This diagram illustrates how autophagy mitigates photoaging induced by UV radiation (UVA and UVB). The left side presents a cross-sectional view of skin (scale bar: 20 μm), showing epidermal cells, basal lamina, and loose connective tissue, with UV penetration causing dead skin cell accumulation. The right side features a circular flowchart centered on photoaging—depicted as a young individual aging into an elderly person—outlining key mechanisms: 1) mitochondrial dysfunction, 2) reduced repair efficiency, 3) cell apoptosis, 4) increased reactive oxygen species (ROS) production, 5) lipid peroxide formation, and 6) DNA damage. These processes are interconnected, with arrows indicating their cyclical nature. Autophagy regulates inflammation (e.g., via NF-κB suppression and NLRP3 inflammasome inhibition), cell cycle progression (e.g., halting the cycle for DNA repair), and apoptosis (balancing cell survival and death). Mitophagy, a subset of autophagy, counters mitochondrial dysfunction by removing damaged mitochondria, reducing ROS and oxidative stress. However, as autophagic function declines with age—due to reduced expression of genes like ATG7 and LC3—the skin’s ability to combat UV-induced damage diminishes, accelerating photoaging. Reproduced from Zhong et al. Role of autophagy in skin photoaging: A narrative review. Medicine, Copyright © 2022 by (Zhong et al., 2024).
4.3.1 Regulation of inflammatory responses
Chronic low-grade inflammation, often termed inflammaging, is a hallmark of both chronological aging and photoaging (Salminen, 2022; Salminen et al., 2022). Autophagy plays a crucial role in regulating inflammation by modulating key inflammatory signaling pathways (Figure 5). One of the primary mechanisms involves the suppression of NF-κB signaling, which is a central pathway in the inflammatory response (Chaudhary et al., 2024; Xue et al., 2024). For instance, when UV radiation induces oxidative stress and DNA damage, NF-κB is activated (Chen B. et al., 2022), then leading to the production of pro-inflammatory cytokines such as IL-6 and TNF-α (Tomasello et al., 2022). Moreover, autophagy limits this inflammatory response by selectively degrading inflammatory mediators and damaged organelles, including dysfunctional mitochondria, which are significant sources of ROS (Liu H. et al., 2024).
Additionally, autophagy interacts with the NLRP3 inflammasome, a critical component responsible for producing IL-1β, a potent pro-inflammatory cytokine (Gupta et al., 2024), a potent pro-inflammatory cytokine (Gupta et al., 2024; Wang T. et al., 2024). By degrading damaged mitochondria and preventing the release of mitochondrial DNA and ROS, autophagy inhibits inflammasome activation (Cui et al., 2023), thus reducing chronic inflammation. This anti-inflammatory effect of autophagy helps protect skin cells from the persistent inflammation that accelerates photoaging (Liu et al., 2013).
4.3.2 Cell cycle regulation and apoptosis
Autophagy exerts significant influence over the regulation of the cell cycle and apoptosis, playing a dual role in determining cell fate in response to UV-induced stress (Sample and He, 2017). On the one hand, autophagy helps to maintain proper cell cycle progression by degrading cyclins and other cell cycle regulators (Rambold and Lippincott-Schwartz, 2011; Mestre Citrinovitz et al., 2019). Specifically, in response to UV-induced DNA damage, autophagy temporarily halts the cell cycle to allow DNA repair (Lo et al., 2005; Wu et al., 2007), preventing the propagation of mutations that contribute to aging and carcinogenesis. This process not only preserves genomic stability but also serves as a protective mechanism to slow the photoaging process (Wang et al., 2021; Xie H. et al., 2022).
Autophagy and apoptosis are interconnected processes, with autophagy often acting as a survival mechanism to delay or prevent apoptosis under moderate stress conditions (Yeo et al., 2016; Saoudaoui et al., 2021). However, when stress becomes excessive, such as with prolonged UV exposure, autophagy may promote apoptotic cell death (Vikram et al., 2024). This dual role allows autophagy to balance cell survival and death, ensuring that severely damaged cells are removed while preserving those with repairable damage. Furthermore, chronic inflammation, known as inflammaging, can be triggered by excessive UV exposure and prolonged cellular stress (Lee Y. I. et al., 2021). Inflammaging refers to the persistent low-grade inflammation that occurs with aging and has been linked to various age-related diseases, including skin aging (Lee Y. I. et al., 2021; Pilkington et al., 2021). The interplay between autophagy, inflammation, and apoptosis is crucial in modulating skin aging, as sustained inflammation exacerbates cellular damage and accelerates the aging process. By regulating apoptosis, autophagy prevents the accumulation of dysfunctional cells, thereby mitigating skin aging (Song et al., 2017; Ho and Dreesen, 2021).
4.3.3 Autophagy decline and accelerated photoaging
As age progresses, autophagic function gradually declines, reducing the cellular capacity to manage UV-induced damage (Wang et al., 2019). This decline in autophagy is primarily due to age-related alterations in the autophagic machinery, including decreased expression of autophagy-related genes and impaired autophagic flux (Chen et al., 2018a). These changes result in an inability to efficiently remove damaged proteins, organelles, and other cellular debris, which accumulate over time (Lim S. H. Y. et al., 2024). Studies have shown that this age-associated decline in autophagic activity contributes to the impaired response of skin cells to environmental stressors like UV radiation (Huang et al., 2019). Consequently, this decline in autophagy exacerbates photoaging by failing to prevent the buildup of damaged proteins, organelles, and ROS (Figure 5). For instance, mitophagy—the selective removal of damaged mitochondria—becomes less efficient, leading to increased mitochondrial dysfunction and oxidative stress (Di Rienzo et al., 2024; Kong et al., 2024). As a result, the skin becomes more vulnerable to UV-induced DNA damage, protein aggregation, and chronic inflammation, all of which accelerate the aging process (Chaudhary et al., 2023).
A notable example of age-related decline in autophagy is the reduced expression of autophagy-related genes, such as ATG7 and LC3, in older individuals (Yu P. et al., 2015). Lower levels of these crucial proteins impair the autophagic process, resulting in the accumulation of cellular debris and increased susceptibility to UV-induced damage (Baechler et al., 2019; Caution et al., 2019). This reduction in autophagic activity associated with aging not only accelerates photoaging but also increases the risk of skin disorders, including carcinogenesis. Understanding the role of autophagy in these processes emphasizes its potential as a therapeutic target to alleviate the effects of UV-induced skin aging.
In conclusion, autophagy plays a crucial role in regulating inflammatory responses, cell cycle progression, and apoptosis in the context of photoaging (Zhao et al., 2022; Xia et al., 2024). However, as autophagic function declines with age, the skin becomes more susceptible to UV-induced damage, inflammation, and premature aging.
5 Recent research progress
5.1 Findings from in vitro and in vivo studies
In vitro studies have significantly advanced our understanding of autophagy’s role in responding to UV exposure and its protective effects against photoaging (Prasanth et al., 2019; Martic et al., 2020). Research has shown that UV radiation stimulates autophagic activity in skin cells, primarily through mechanisms involving the inhibition of mTOR and activation of AMPK (Lee et al., 2019; Chu et al., 2023). This autophagic activity aids in the clearance of damaged mitochondria and proteins, thus contributing to cellular protection (Gao et al., 2023; Zamanian et al., 2024). Building on these findings, in vivo studies using animal models, such as mice and zebrafish, reinforce autophagy’s critical role in mitigating UV-induced skin damage (Li M. et al., 2024; Tang Q.-Q. et al., 2024). Mice with impaired autophagic function exhibit increased sensitivity to UV radiation, leading to more pronounced signs of photoaging (Wang et al., 2019; Wang et al., 2021). While models with enhanced autophagic activity show reduced skin damage and better preservation of skin integrity (Konger et al., 2016; de Medeiros et al., 2018; Khater et al., 2024).
5.2 Clinical studies and applications
The potential of autophagy modulators, such as rapamycin and metformin, has attracted considerable clinical interest for addressing photoaging (Zhang et al., 2016; Qin D. et al., 2018). Rapamycin, a well-known mTOR inhibitor, has shown promise in clinical trials by enhancing autophagic activity (Palma et al., 2022; Aitken et al., 2023; Redl et al., 2024). It facilitates the clearance of damaged cellular components, resulting in improvements in skin texture, wrinkle depth, and elasticity in older individuals (Rangwala et al., 2014). Similarly, metformin, an AMPK activator commonly used in the treatment of diabetes (Pozzi et al., 2024), has demonstrated the ability to reduce oxidative stress and promote DNA repair in UV-exposed skin (Chen Q. et al., 2022). However, the use of pharmaceuticals like rapamycin and metformin can also present potential side effects, including immune suppression, metabolic disturbances, and gastrointestinal issues, which need to be carefully considered in long-term applications (Foretz et al., 2023). Beyond pharmaceuticals, the skincare industry has started incorporating autophagy-activating ingredients like resveratrol, curcumin, and spermidine into topical anti-aging products (Mundo Rivera et al., 2024). These compounds are known to stimulate autophagic processes within the skin, potentially aiding in the degradation of damaged proteins and organelles (Mostafa et al., 2021). Despite their potential, there are concerns about the long-term use of these compounds, as they may cause skin irritation or allergic reactions in some individuals. Moreover, the efficacy of these ingredients in stimulating autophagy through topical application is still debated, as their absorption and bioavailability may be limited by the skin barrier (Huang, 2020). While pharmaceutical agents like rapamycin and metformin exert systemic effects through oral ingestion, topical skincare ingredients are intended to work locally, directly affecting the skin where UV-induced damage occurs (Bai et al., 2021; Chen Q. et al., 2022). Theoretically, these ingredients might promote skin rejuvenation by enhancing the skin’s autophagic capacity, thereby addressing photoaging at a cellular level (Mostafa et al., 2021). However, the delivery of these compounds through the skin and their effectiveness in activating autophagy remain areas that need further research, as the bioavailability and penetration of topical agents differ significantly from oral pharmaceuticals (Stacchiotti and Corsetti, 2020; Chen M. et al., 2021). While initial studies report improvements in skin hydration, elasticity, and fine lines, further research is needed to validate the long-term effects of these agents on skin health and photoaging prevention (Thornfeldt and Rizer, 2016; Wang et al., 2024c; Xia et al., 2024).
5.3 Application of emerging technologies in research
Emerging technologies, such as single-cell RNA sequencing (scRNA-seq) and high-resolution imaging, have significantly advanced our understanding of autophagy and its role in photoaging (Pan et al., 2021; Lin et al., 2022). scRNA-seq enables researchers to analyze gene expression at the individual cell level (Popp et al., 2002; Bell et al., 2018), revealing changes in autophagy-related genes such as ATG5, LC3, and p62 in UV-exposed skin cells (Kim et al., 2018; Mahbubfam et al., 2022). This approach has identified distinct subpopulations of cells with varying levels of autophagic activity, helping to pinpoint which cells are more susceptible to UV-induced damage (Bernard et al., 2020). Complementing this, high-resolution imaging techniques, such as live-cell confocal microscopy, provide real-time visualization of autophagic structures and processes in UV-exposed cells (Chen R. et al., 2023; Maib et al., 2024), including mitochondrial dysfunction and protein aggregation (Beyer et al., 2024). Other innovative methods, like CRISPR/Cas9 gene editing, have been employed to investigate specific autophagy-related genes (Rahman et al., 2022), enhancing our understanding of their roles in protecting against UV damage (Huang et al., 2021). Furthermore, metabolomics analysis complements these technologies by profiling metabolic changes during autophagy activation in response to UV exposure, shedding light on the metabolic support autophagy provides for cellular repair in photoaging (Liang et al., 2020).
Next, Table 6 summarizes the clinical applications and emerging technologies related to autophagy modulation and their potential for mitigating photoaging, highlighting key agents and methodologies used in recent research.
6 Conclusion
Autophagy is essential for protecting against photoaging by eliminating damaged organelles, breaking down aggregated proteins, and sustaining cellular homeostasis under UV-induced stress. The molecular processes underlying autophagy’s protective action include the removal of ROS-damaged mitochondria, regulation of inflammatory pathways, and facilitation of DNA repair. The modulation of autophagy via pathways such as mTOR and AMPK, alongside emerging regulators like non-coding RNAs, underscores its critical function in mitigating skin damage caused by UV radiation. Despite its promising therapeutic potential for counteracting photoaging, several challenges remain. These include the difficulty of monitoring autophagy dynamically in real-time, particularly in vivo, and the complex, context-dependent role of autophagy in photoaging. Additionally, the long-term safety and efficacy of autophagy modulators, as well as the consistency of benefits across diverse populations, require further evaluation.
In summary, while significant progress has been made in understanding autophagy’s role in photoaging, addressing these challenges through continued research will be essential to realizing its full therapeutic potential.
7 Future direction
To overcome the challenges in autophagy research related to photoaging, a comprehensive research strategy is necessary. Mechanistic studies should further explore the molecular processes by which autophagy regulates UV-induced DNA repair, inflammation, and mitochondrial function under oxidative stress. Investigating how autophagy interacts with other pathways involved in photoaging, such as apoptosis and cellular senescence, could enhance our understanding of its broader role in skin aging (Mostafa et al., 2022; Zhao et al., 2022; Han et al., 2024; Xia et al., 2024). The identification of novel autophagy regulators presents an exciting opportunity for advancement. Beyond well-established regulators like mTOR and AMPK, less-explored pathways involving non-coding RNAs and post-translational modifications could offer new approaches to fine-tune autophagic responses to UV damage (Türei et al., 2015; Li et al., 2020). Discovering molecules that modulate autophagy with minimal side effects could pave the way for targeted anti-photoaging therapies (Bu and Singh, 2021; Qu and Lin, 2021). Collaborative, multidisciplinary efforts will be critical to advancing this field. Integrating cellular biology, molecular genomics, and clinical medicine could translate mechanistic insights into practical therapeutic strategies. The use of advanced tools such as CRISPR/Cas9, combined with cutting-edge imaging and metabolomics, could provide deeper insights into autophagy’s role in skin aging. Furthermore, personalized clinical trials based on individual autophagy profiles could optimize therapeutic approaches for preventing and treating photoaging (Kaeberlein, 2017; Schmidt et al., 2021; Guan et al., 2024). Both basic and clinical research will play pivotal roles in harnessing autophagy regulation to prevent photoaging and promote healthier skin aging (Voegeli et al., 2017; Vikram et al., 2024).
Author contributions
ZZ: Conceptualization, Data curation, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing. RT: Conceptualization, Data curation, Supervision, Writing – review and editing. ZX: Software, Validation, Visualization, Writing – original draft. YF: Supervision, Conceptualization, Writing – review and editing. LC: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Clinical Science Research Foundation of Chengdu Medical College & Nanbu People’s Hospital (2024LHFBM1-04), University-Level Natural Science Foundation General Project of Chengdu Medical College (2024CDYXY-01), Clinical Science Research Foundation of Chengdu Medical College & the First Affiliated Hospital of Chengdu Medical College (24LHLNYX1-08), Clinical Science Research Foundation of Chengdu Medical College & Chengdu Pidu People’s Hospital (2024LHFYSZ1-41) and Key Research Project of Science and Technology Department of Sichuan Province (2022YFQ0055).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aitken, P., Stanescu, I., Boddington, L., Mahon, C., Fogarasi, A., Liao, Y.-H., et al. (2023). A novel rapamycin cream formulation improves facial angiofibromas associated with tuberous sclerosis complex: a double-blind randomized placebo-controlled trial. Br. J. Dermatol. 189, 520–530. doi:10.1093/bjd/ljad243
Al-Bari, M. A. A., and Xu, P. (2020). Molecular regulation of autophagy machinery by mTOR-dependent and -independent pathways. Ann. N. Y. Acad. Sci. 1467, 3–20. doi:10.1111/nyas.14305
Allouch, S., and Munusamy, S. (2017). Metformin attenuates albumin-induced alterations in renal tubular cells in vitro. J. Cell. Physiol. 232, 3652–3663. doi:10.1002/jcp.25838
Almujri, S. S., and Almalki, W. H. (2024). The paradox of autophagy in cancer: NEAT1’s role in tumorigenesis and therapeutic resistance. Pathol. Res. Pract. 262, 155523. doi:10.1016/j.prp.2024.155523
Al-Sadek, T., and Yusuf, N. (2024). Ultraviolet radiation biological and medical implications. Curr. Issues Mol. Biol. 46, 1924–1942. doi:10.3390/cimb46030126
Alves-Fernandes, D. K., and Jasiulionis, M. G. (2019). The role of SIRT1 on DNA damage response and epigenetic alterations in cancer. Int. J. Mol. Sci. 20, 3153. doi:10.3390/ijms20133153
Anand, S. K., Sharma, A., Singh, N., and Kakkar, P. (2020). Activation of autophagic flux via LKB1/AMPK/mTOR axis against xenoestrogen Bisphenol-A exposure in primary rat hepatocytes. Food Chem. Toxicol. 141, 111314. doi:10.1016/j.fct.2020.111314
Arensman, M. D., and Eng, C. H. (2018). Self-digestion for lifespan extension: enhanced autophagy delays aging. Mol. Cell. 71, 485–486. doi:10.1016/j.molcel.2018.08.002
Arzalluz-Luque, Á., and Conesa, A. (2018). Single-cell RNAseq for the study of isoforms-how is that possible? Genome Biol. 19, 110. doi:10.1186/s13059-018-1496-z
Assaye, M. A., and Gizaw, S. T. (2022). Chaperone-mediated autophagy and its implications for neurodegeneration and cancer. Int. J. Gen. Med. 15, 5635–5649. doi:10.2147/IJGM.S368364
Bacqueville, D., Jacques-Jamin, C., Dromigny, H., Boyer, F., Brunel, Y., Ferret, P. J., et al. (2021). Phenylene Bis-Diphenyltriazine (TriAsorB), a new sunfilter protecting the skin against both UVB + UVA and blue light radiations. Photochem. Photobiol. Sci. 20, 1475–1486. doi:10.1007/s43630-021-00114-x
Baechler, B. L., Bloemberg, D., and Quadrilatero, J. (2019). Mitophagy regulates mitochondrial network signaling, oxidative stress, and apoptosis during myoblast differentiation. Autophagy 15, 1606–1619. doi:10.1080/15548627.2019.1591672
Bai, G.-L., Wang, P., Huang, X., Wang, Z.-Y., Cao, D., Liu, C., et al. (2021). Rapamycin protects skin fibroblasts from UVA-induced photoaging by inhibition of p53 and phosphorylated HSP27. Front. Cell. Dev. Biol. 9, 633331. doi:10.3389/fcell.2021.633331
Bajdzienko, J., and Bremm, A. (2024). Mammalian pexophagy at a glance. J. Cell. Sci. 137, jcs259775. doi:10.1242/jcs.259775
Bang, E., Kim, D. H., and Chung, H. Y. (2021). Protease-activated receptor 2 induces ROS-mediated inflammation through Akt-mediated NF-κB and FoxO6 modulation during skin photoaging. Redox Biol. 44, 102022. doi:10.1016/j.redox.2021.102022
Bansal, M., Moharir, S. C., Sailasree, S. P., Sirohi, K., Sudhakar, C., Sarathi, D. P., et al. (2018). Optineurin promotes autophagosome formation by recruiting the autophagy-related Atg12-5-16L1 complex to phagophores containing the Wipi2 protein. J. Biol. Chem. 293, 132–147. doi:10.1074/jbc.M117.801944
Barnaba, C., Broadbent, D. G., Perez, G. I., and Schmidt, J. C. (2023). AMPK regulates phagophore-to-autophagosome maturation. BioRxiv Prepr. Serv. Biol. 2023, 559981. doi:10.1101/2023.09.28.559981
Barnes, B. M., Shyne, A., Gunn, D. A., Griffiths, C. E. M., and Watson, R. E. B. (2024). Epigenetics and ultraviolet radiation: implications for skin ageing and carcinogenesis. Skin. Health Dis. 4, e410. doi:10.1002/ski2.410
Beccari, S., Sierra-Torre, V., Valero, J., Pereira-Iglesias, M., García-Zaballa, M., Soria, F. N., et al. (2023). Microglial phagocytosis dysfunction in stroke is driven by energy depletion and induction of autophagy. Autophagy 19, 1952–1981. doi:10.1080/15548627.2023.2165313
Bell, A., Bell, D., Chakravarti, N., Ma, J., Henton, N., and Prieto, V. G. (2018). Detection of a MicroRNA molecular signature of ultraviolet radiation in the superficial regions of melanocytic nevi on sun-exposed skin. Mod. Pathol. 31, 1744–1755. doi:10.1038/s41379-018-0088-5
Bernard, M., Yang, B., Migneault, F., Turgeon, J., Dieudé, M., Olivier, M.-A., et al. (2020). Autophagy drives fibroblast senescence through MTORC2 regulation. Autophagy 16, 2004–2016. doi:10.1080/15548627.2020.1713640
Bernerd, F., Passeron, T., Castiel, I., and Marionnet, C. (2022). The damaging effects of long UVA (UVA1) rays: a major challenge to preserve skin health and integrity. Int. J. Mol. Sci. 23, 8243. doi:10.3390/ijms23158243
Beyaz, S., Aslan, A., Gok, O., Ozercan, I. H., and Agca, C. A. (2023). Fullerene C60 reduces acute lung injury by suppressing oxidative stress-mediated DMBA-induced apoptosis and autophagy by regulation of cytochrome-C/caspase-3/beclin-1/IL-1α/HO-1/p53 signaling pathways in rats. Free Radic. Res. 57, 373–383. doi:10.1080/10715762.2023.2247555
Beyer, M., Hladun, C., and Bou-Abdallah, F. (2024). Detection of proteins with ascorbic acid-capped gold nanoparticles: a simple and highly sensitive colorimetric assay. Anal. Methods Adv. Methods Appl. 16, 5391–5398. doi:10.1039/d4ay01146e
Bharath, L. P., Agrawal, M., McCambridge, G., Nicholas, D. A., Hasturk, H., Liu, J., et al. (2020). Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell. Metab. 32, 44–55.e6. doi:10.1016/j.cmet.2020.04.015
Bosch, R., Philips, N., Suárez-Pérez, J. A., Juarranz, A., Devmurari, A., Chalensouk-Khaosaat, J., et al. (2015). Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with Phytochemicals. Antioxid. Basel Switz. 4, 248–268. doi:10.3390/antiox4020248
Boutouja, F., Stiehm, C. M., and Platta, H. W. (2019). mTOR: a cellular regulator interface in health and disease. Cells 8, 18. doi:10.3390/cells8010018
Bozic, M., van den Bekerom, L., Milne, B. A., Goodman, N., Roberston, L., Prescott, A. R., et al. (2020). A conserved ATG2-GABARAP family interaction is critical for phagophore formation. EMBO Rep. 21, e48412. doi:10.15252/embr.201948412
Bradley, S. T., Lee, Y.-S., Gurel, Z., and Kimple, R. J. (2022). Autophagy awakens-the myriad roles of autophagy in head and neck cancer development and therapeutic response. Mol. Carcinog. 61, 243–253. doi:10.1002/mc.23372
Brem, R., Macpherson, P., Guven, M., and Karran, P. (2017). Oxidative stress induced by UVA photoactivation of the tryptophan UVB photoproduct 6-formylindolo[3,2-b]carbazole (FICZ) inhibits nucleotide excision repair in human cells. Sci. Rep. 7, 4310. doi:10.1038/s41598-017-04614-8
Briganti, S., and Picardo, M. (2003). Antioxidant activity, lipid peroxidation and skin diseases. What’s new. J. Eur. Acad. Dermatol. Venereol. JEADV 17, 663–669. doi:10.1046/j.1468-3083.2003.00751.x
Brown, O. I., Bridge, K. I., and Kearney, M. T. (2021). Nicotinamide adenine dinucleotide phosphate oxidases in glucose homeostasis and diabetes-related endothelial cell dysfunction. Cells 10, 2315. doi:10.3390/cells10092315
Bu, S., and Singh, K. K. (2021). Epigenetic regulation of autophagy in cardiovascular pathobiology. Int. J. Mol. Sci. 22, 6544. doi:10.3390/ijms22126544
Byun, K.-A., Kim, H. M., Oh, S., Son, K. H., and Byun, K. (2023). Radiofrequency irradiation attenuated UVB-induced skin pigmentation by modulating ATP release and CD39 expression. Int. J. Mol. Sci. 24, 5506. doi:10.3390/ijms24065506
Cagnol, S., and Chambard, J.-C. (2010). ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J. 277, 2–21. doi:10.1111/j.1742-4658.2009.07366.x
Cai, C., Ma, H., Peng, J., Shen, X., Zhen, X., Yu, C., et al. (2023). USP25 regulates KEAP1-NRF2 anti-oxidation axis and its inactivation protects acetaminophen-induced liver injury in male mice. Nat. Commun. 14, 3648. doi:10.1038/s41467-023-39412-6
Calzari, P., Vaienti, S., and Nazzaro, G. (2023). Uses of polypodium leucotomos extract in oncodermatology. J. Clin. Med. 12, 673. doi:10.3390/jcm12020673
Camillo, L., Gironi, L. C., Zavattaro, E., Esposto, E., and Savoia, P. (2022). Nicotinamide attenuates UV-induced stress damage in human primary keratinocytes from cancerization fields. J. Invest. Dermatol. 142, 1466–1477.e1. doi:10.1016/j.jid.2021.10.012
Cao, C., and Wan, Y. (2009). Parameters of protection against ultraviolet radiation-induced skin cell damage. J. Cell. Physiol. 220, 277–284. doi:10.1002/jcp.21780
Cao, W., Li, J., Yang, K., and Cao, D. (2021). An overview of autophagy: mechanism, regulation and research progress. Bull. Cancer (Paris) 108, 304–322. doi:10.1016/j.bulcan.2020.11.004
Caution, K., Pan, A., Krause, K., Badr, A., Hamilton, K., Vaidya, A., et al. (2019). Methylomic correlates of autophagy activity in cystic fibrosis. J. Cyst. Fibros. 18, 491–500. doi:10.1016/j.jcf.2019.01.011
Cerda-Troncoso, C., Varas-Godoy, M., and Burgos, P. V. (2020). Pro-tumoral functions of autophagy receptors in the modulation of cancer progression. Front. Oncol. 10, 619727. doi:10.3389/fonc.2020.619727
Chaiprasongsuk, A., and Panich, U. (2022). Role of Phytochemicals in skin photoprotection via regulation of Nrf2. Front. Pharmacol. 13, 823881. doi:10.3389/fphar.2022.823881
Chan, L. K. W., Lee, K. W. A., Lee, C. H., Lam, K. W. P., Lee, K. F. V., Wu, R., et al. (2024). Cosmeceuticals in photoaging: a review. Skin. Res. Technol. 30, e13730. doi:10.1111/srt.13730
Charachit, N., Sukhamwang, A., Dejkriengkraikul, P., and Yodkeeree, S. (2022). Hyperoside and quercitrin in Houttuynia cordata extract attenuate UVB-induced human keratinocyte cell damage and oxidative stress via modulation of MAPKs and Akt signaling pathway. Antioxid. Basel Switz. 11, 221. doi:10.3390/antiox11020221
Chaudhary, M. R., Chaudhary, S., Sharma, Y., Singh, T. A., Mishra, A. K., Sharma, S., et al. (2023). Aging, oxidative stress and degenerative diseases: mechanisms, complications and emerging therapeutic strategies. Biogerontology 24, 609–662. doi:10.1007/s10522-023-10050-1
Chaudhary, R., Azam, M. A., Dowand, B., Singh, A., Rehman, M., Agarwal, V., et al. (2024). Chronic stress-mediated dysregulations in inflammatory, immune and oxidative circuitry impairs the therapeutic response of methotrexate in experimental autoimmune disease models. Naunyn. Schmiedeb. Arch. Pharmacol. 398, 4305–4334. doi:10.1007/s00210-024-03529-2
Chen, B., Chen, H., Qu, H., Qiao, K., Xu, M., Wu, J., et al. (2022a). Photoprotective effects of Sargassum thunbergii on ultraviolet B-induced mouse L929 fibroblasts and zebrafish. BMC Complement. Med. Ther. 22, 144. doi:10.1186/s12906-022-03609-x
Chen, D., Fan, W., Lu, Y., Ding, X., Chen, S., and Zhong, Q. (2012). A mammalian autophagosome maturation mechanism mediated by TECPR1 and the Atg12-Atg5 conjugate. Mol. Cell. 45, 629–641. doi:10.1016/j.molcel.2011.12.036
Chen, G., Kroemer, G., and Kepp, O. (2020). Mitophagy: an emerging role in aging and age-associated diseases. Front. Cell. Dev. Biol. 8, 200. doi:10.3389/fcell.2020.00200
Chen, M., Yang, D., Sun, Y., Liu, T., Wang, W., Fu, J., et al. (2021a). In situ self-assembly nanomicelle microneedles for enhanced photoimmunotherapy via autophagy regulation strategy. ACS Nano 15, 3387–3401. doi:10.1021/acsnano.0c10396
Chen, Q., Zhang, H., Yang, Y., Zhang, S., Wang, J., Zhang, D., et al. (2022b). Metformin attenuates UVA-induced skin photoaging by suppressing mitophagy and the PI3K/AKT/mTOR pathway. Int. J. Mol. Sci. 23, 6960. doi:10.3390/ijms23136960
Chen, R., Wang, L., Ding, G., Han, G., Qiu, K., Sun, Y., et al. (2023a). Constant conversion rate of endolysosomes revealed by a pH-sensitive fluorescent probe. ACS Sens. 8, 2068–2078. doi:10.1021/acssensors.3c00340
Chen, R.-J., Lee, Y.-H., Yeh, Y.-L., Wu, W.-S., Ho, C.-T., Li, C.-Y., et al. (2017). Autophagy-inducing effect of pterostilbene: a prospective therapeutic/preventive option for skin diseases. J. Food Drug Anal. 25, 125–133. doi:10.1016/j.jfda.2016.10.022
Chen, W., Rao, J., Liu, Z., You, X., Yuan, F., Le, F., et al. (2021b). Integrated tissue proteome and metabolome reveal key elements and regulatory pathways in cutaneous squamous cell carcinoma. J. Proteomics 247, 104320. doi:10.1016/j.jprot.2021.104320
Chen, X., Li, L., Xu, S., Bu, W., Chen, K., Li, M., et al. (2018a). Ultraviolet B radiation down-regulates ULK1 and ATG7 expression and impairs the autophagy response in human keratinocytes. J. Photochem. Photobiol. B 178, 152–164. doi:10.1016/j.jphotobiol.2017.08.043
Chen, X., Li, X., Zhang, W., He, J., Xu, B., Lei, B., et al. (2018b). Activation of AMPK inhibits inflammatory response during hypoxia and reoxygenation through modulating JNK-mediated NF-κB pathway. Metabolism 83, 256–270. doi:10.1016/j.metabol.2018.03.004
Chen, Y., Guo, W., Lu, W., Guo, X., Gao, W., and Yin, Z. (2023b). SNIP1 reduces extracellular matrix degradation and inflammation via inhibiting the NF-κB signaling pathway in osteoarthritis. Arch. Biochem. Biophys. 747, 109764. doi:10.1016/j.abb.2023.109764
Chen, Y., Wu, Y., Tian, X., Shao, G., Lin, Q., and Sun, A. (2024). Golgiphagy: a novel selective autophagy to the fore. Cell. Biosci. 14, 130. doi:10.1186/s13578-024-01311-8
Chen, Z.-H., Cao, J.-F., Zhou, J.-S., Liu, H., Che, L.-Q., Mizumura, K., et al. (2014). Interaction of caveolin-1 with ATG12-ATG5 system suppresses autophagy in lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 306, L1016–L1025. doi:10.1152/ajplung.00268.2013
Chien, L.-H., Deng, J.-S., Jiang, W.-P., Chou, Y.-N., Lin, J.-G., and Huang, G.-J. (2023). Evaluation of lung protection of Sanghuangporus sanghuang through TLR4/NF-κB/MAPK, keap1/Nrf2/HO-1, CaMKK/AMPK/Sirt1, and TGF-β/SMAD3 signaling pathways mediating apoptosis and autophagy. Biomed. Pharmacother. Biomedecine Pharmacother. 165, 115080. doi:10.1016/j.biopha.2023.115080
Choi, H.-J., Alam, M. B., Baek, M.-E., Kwon, Y.-G., Lim, J.-Y., and Lee, S.-H. (2020). Protection against UVB-induced photoaging by Nypa fruticans via inhibition of MAPK/AP-1/MMP-1 signaling. Oxid. Med. Cell. Longev. 2020, 2905362. doi:10.1155/2020/2905362
Choi, J., Jang, H., Xuan, Z., and Park, D. (2024). Emerging roles of ATG9/ATG9A in autophagy: implications for cell and neurobiology. Autophagy 20, 2373–2387. doi:10.1080/15548627.2024.2384349
Chowdhury, S. G., and Karmakar, P. (2023). Revealing the role of epigenetic and post-translational modulations of autophagy proteins in the regulation of autophagy and cancer: a therapeutic approach. Mol. Biol. Rep. 51, 3. doi:10.1007/s11033-023-08961-w
Chu, J., Xiang, Y., Lin, X., He, M., Wang, Y., Ma, Q., et al. (2023). Handelin protects human skin keratinocytes against ultraviolet B-induced photodamage via autophagy activation by regulating the AMPK-mTOR signaling pathway. Arch. Biochem. Biophys. 743, 109646. doi:10.1016/j.abb.2023.109646
Corona Velazquez, A. F., and Jackson, W. T. (2018). So many roads: the multifaceted regulation of autophagy induction. Mol. Cell. Biol. 38, e00303–e00318. doi:10.1128/MCB.00303-18
Crochemore, C., Chica, C., Garagnani, P., Lattanzi, G., Horvath, S., Sarasin, A., et al. (2023). Epigenomic signature of accelerated ageing in progeroid Cockayne syndrome. Aging Cell. 22, e13959. doi:10.1111/acel.13959
Cui, H., Banerjee, S., Xie, N., Dey, T., Liu, R.-M., Sanders, Y. Y., et al. (2023). MafB regulates NLRP3 inflammasome activation by sustaining p62 expression in macrophages. Commun. Biol. 6, 1047. doi:10.1038/s42003-023-05426-5
Damiani, E., Rosati, L., Castagna, R., Carloni, P., and Greci, L. (2006). Changes in ultraviolet absorbance and hence in protective efficacy against lipid peroxidation of organic sunscreens after UVA irradiation. J. Photochem. Photobiol. B 82, 204–213. doi:10.1016/j.jphotobiol.2005.03.011
D’Amico, R., Impellizzeri, D., Cordaro, M., Siracusa, R., Interdonato, L., Marino, Y., et al. (2022). Complex interplay between autophagy and oxidative stress in the development of endometriosis. Antioxid. Basel Switz. 11, 2484. doi:10.3390/antiox11122484
De Magis, A., Limmer, M., Mudiyam, V., Monchaud, D., Juranek, S., and Paeschke, K. (2023). UV-induced G4 DNA structures recruit ZRF1 which prevents UV-induced senescence. Nat. Commun. 14, 6705. doi:10.1038/s41467-023-42494-x
de Medeiros, W. A., da Silva, L. A., Dall’Igna, D. M., Michels, M., Manfredini, A., Dos Santos Cardoso, J., et al. (2018). N-acetylcysteine effects on a murine model of chronic critical limb ischemia. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 454–463. doi:10.1016/j.bbadis.2017.10.027
de Oliveira, N. F. P., de Souza, B. F., and de Castro Coêlho, M. (2020). UV radiation and its relation to DNA methylation in epidermal cells: a review. Epigenomes 4, 23. doi:10.3390/epigenomes4040023
Diffey, B. (2015). Solar spectral irradiance and summary outputs using excel. Photochem. Photobiol. 91, 553–557. doi:10.1111/php.12422
Ding, W.-X., and Yin, X.-M. (2012). Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol. Chem. 393, 547–564. doi:10.1515/hsz-2012-0119
Di Rienzo, M., Romagnoli, A., Refolo, G., Vescovo, T., Ciccosanti, F., Zuchegna, C., et al. (2024). Role of AMBRA1 in mitophagy regulation: emerging evidence in aging-related diseases. Autophagy 20, 2602–2615. doi:10.1080/15548627.2024.2389474
Dissemond, J., Schneider, L. A., Brenneisen, P., Briviba, K., Wenk, J., Wlaschek, M., et al. (2003). Protective and determining factors for the overall lipid peroxidation in ultraviolet A1-irradiated fibroblasts: in vitro and in vivo investigations. Br. J. Dermatol. 149, 341–349. doi:10.1046/j.1365-2133.2003.05457.x
Djavaheri-Mergny, M., and Dubertret, L. (2001). UV-A-induced AP-1 activation requires the Raf/ERK pathway in human NCTC 2544 keratinocytes. Exp. Dermatol. 10, 204–210. doi:10.1034/j.1600-0625.2001.010003204.x
Djeddi, A., Al Rawi, S., Deuve, J. L., Perrois, C., Liu, Y.-Y., Russeau, M., et al. (2015). Sperm-inherited organelle clearance in C. elegans relies on LC3-dependent autophagosome targeting to the pericentrosomal area. Dev. Camb. Engl. 142, 1705–1716. doi:10.1242/dev.117879
Dolese, D. A., Junot, M. P., Ghosh, B., Butsch, T. J., Johnson, A. E., and Bohnert, K. A. (2022). Degradative tubular lysosomes link pexophagy to starvation and early aging in C. elegans. Autophagy 18, 1522–1533. doi:10.1080/15548627.2021.1990647
Dunaway, S., Odin, R., Zhou, L., Ji, L., Zhang, Y., and Kadekaro, A. L. (2018). Natural antioxidants: multiple mechanisms to protect skin from solar radiation. Front. Pharmacol. 9, 392. doi:10.3389/fphar.2018.00392
Durocher, M., Ander, B. P., Jickling, G., Hamade, F., Hull, H., Knepp, B., et al. (2019). Inflammatory, regulatory, and autophagy co-expression modules and hub genes underlie the peripheral immune response to human intracerebral hemorrhage. J. Neuroinflammation 16, 56. doi:10.1186/s12974-019-1433-4
Egan, D., Kim, J., Shaw, R. J., and Guan, K.-L. (2011). The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 7, 643–644. doi:10.4161/auto.7.6.15123
Egan, D. F., Chun, M. G., Vamos, M., Zou, H., Rong, J., Miller, C. J., et al. (2015). Small molecule inhibition of the autophagy kinase ULK1 and identification of ULK1 substrates. Mol. Cell. 59, 285–297. doi:10.1016/j.molcel.2015.05.031
Fan, L., Luan, X., Jia, Y., Ma, L., Wang, Z., Yang, Y., et al. (2024). Protective effect and mechanism of lycium barbarum polysaccharide against UVB-induced skin photoaging. Photochem. Photobiol. Sci. 23, 1931–1943. doi:10.1007/s43630-024-00642-2
Farizatto, K. L. G., Ikonne, U. S., Almeida, M. F., Ferrari, M. F. R., and Bahr, B. A. (2017). Aβ42-mediated proteasome inhibition and associated tau pathology in hippocampus are governed by a lysosomal response involving cathepsin B: evidence for protective crosstalk between protein clearance pathways. PLoS One 12, e0182895. doi:10.1371/journal.pone.0182895
Feng, C., Chen, X., Yin, X., Jiang, Y., and Zhao, C. (2024a). Matrix metalloproteinases on skin photoaging. J. Cosmet. Dermatol. 23, 3847–3862. doi:10.1111/jocd.16558
Feng, Y., Chen, Y., Wu, X., Chen, J., Zhou, Q., Liu, B., et al. (2024b). Interplay of energy metabolism and autophagy. Autophagy 20, 4–14. doi:10.1080/15548627.2023.2247300
Feng, Y., He, D., Yao, Z., and Klionsky, D. J. (2014). The machinery of macroautophagy. Cell. Res. 24, 24–41. doi:10.1038/cr.2013.168
Fernández-Carro, E., and Ciriza, J. (2025). Generation and characterization of a new aging skin human dermal extracellular matrix scaffold. Methods Mol. Biol. Clifton NJ. doi:10.1007/7651_2024_579
Foerster, E. G., Mukherjee, T., Cabral-Fernandes, L., Rocha, J. D. B., Girardin, S. E., and Philpott, D. J. (2022). How autophagy controls the intestinal epithelial barrier. Autophagy 18, 86–103. doi:10.1080/15548627.2021.1909406
Foretz, M., Guigas, B., and Viollet, B. (2023). Metformin: update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. 19, 460–476. doi:10.1038/s41574-023-00833-4
Fu, Y., Xie, J.-L., Zhang, W.-T., Zhang, X.-L., Zhang, X.-M., Xu, M.-M., et al. (2024). Synergistic delivery of hADSC-Exos and antioxidants has inhibitory effects on UVB-induced skin photoaging. Heliyon 10, e34321. doi:10.1016/j.heliyon.2024.e34321
Fu, Z., Luo, W., Wang, J., Peng, T., Sun, G., Shi, J., et al. (2017). Malat1 activates autophagy and promotes cell proliferation by sponging miR-101 and upregulating STMN1, RAB5A and ATG4D expression in glioma. Biochem. Biophys. Res. Commun. 492, 480–486. doi:10.1016/j.bbrc.2017.08.070
Gaetano, A. D., Gibellini, L., Zanini, G., Nasi, M., Cossarizza, A., and Pinti, M. (2021). Mitophagy and oxidative stress: the role of aging. Antioxidants 10, 794. doi:10.3390/antiox10050794
Gallagher, E. R., and Holzbaur, E. L. F. (2023). SQSTM1/P62 promotes lysophagy via formation of liquid-like condensates maintained by HSP27. Autophagy 19, 3029–3030. doi:10.1080/15548627.2023.2210943
Gallagher, L. E., and Chan, E. Y. W. (2013). Early signalling events of autophagy. Essays Biochem. 55, 1–15. doi:10.1042/bse0550001
Gao, Y., Liu, J., Li, K., Li, T., Li, R., Zhang, W., et al. (2023). Metformin alleviates sepsis-associated myocardial injury by enhancing AMP-activated protein kinase/mammalian target of rapamycin signaling pathway-mediated autophagy. J. Cardiovasc. Pharmacol. 82, 308–317. doi:10.1097/FJC.0000000000001463
Ge, Y., Li, M., Bai, S., Chen, C., Zhang, S., Cheng, J., et al. (2024). Doxercalciferol alleviates UVB-induced HaCaT cell senescence and skin photoaging. Int. Immunopharmacol. 127, 111357. doi:10.1016/j.intimp.2023.111357
Gęgotek, A., and Skrzydlewska, E. (2022). The role of ABC transporters in skin cells exposed to UV radiation. Int. J. Mol. Sci. 24, 115. doi:10.3390/ijms24010115
Ghosh, D., and Kumar, A. (2024). Harnessing mitophagy for therapeutic advances in aging and chronic neurodegenerative diseases. Neuroglia 5, 391–409. doi:10.3390/neuroglia5040026
Glady, A., Tanaka, M., Moniaga, C. S., Yasui, M., and Hara-Chikuma, M. (2018). Involvement of NADPH oxidase 1 in UVB-induced cell signaling and cytotoxicity in human keratinocytes. Biochem. Biophys. Rep. 14, 7–15. doi:10.1016/j.bbrep.2018.03.004
Gluschko, A., Farid, A., Herb, M., Grumme, D., Krönke, M., and Schramm, M. (2022). Macrophages target Listeria monocytogenes by two discrete non-canonical autophagy pathways. Autophagy 18, 1090–1107. doi:10.1080/15548627.2021.1969765
Guan, X., Iyaswamy, A., Sreenivasmurthy, S. G., Su, C., Zhu, Z., Liu, J., et al. (2022). Mechanistic insights into selective autophagy subtypes in Alzheimer’s disease. Int. J. Mol. Sci. 23, 3609. doi:10.3390/ijms23073609
Guan, Y., Spaulding, H., Yu, Q., Zhang, M., Willoughby, O., Drake, J. C., et al. (2024). Ulk1 phosphorylation at S555 is not required for endurance training-induced improvements in exercise and metabolic capacity in mice. J. Appl. Physiol. (1985) 137, 223–232. doi:10.1152/japplphysiol.00742.2023
Guerrero-Navarro, L., Jansen-Dürr, P., and Cavinato, M. (2024). Synergistic interplay of UV radiation and urban particulate matter induces impairment of autophagy and alters cellular fate in senescence-prone human dermal fibroblasts. Aging Cell. 23, e14086. doi:10.1111/acel.14086
Gupta, S., Cassel, S. L., Sutterwala, F. S., and Dagvadorj, J. (2024). Regulation of the NLRP3 inflammasome by autophagy and mitophagy. Immunol. Rev. 329, e13410. doi:10.1111/imr.13410
Ha, S., Jeong, S.-H., Yi, K., Chung, K. M., Hong, C. J., Kim, S. W., et al. (2017). Phosphorylation of p62 by AMP-activated protein kinase mediates autophagic cell death in adult hippocampal neural stem cells. J. Biol. Chem. 292, 13795–13808. doi:10.1074/jbc.M117.780874
Hajialiasgary Najafabadi, A., Soheilifar, M. H., and Masoudi-Khoram, N. (2024). Exosomes in skin photoaging: biological functions and therapeutic opportunity. Cell. Commun. Signal. 22, 32. doi:10.1186/s12964-023-01451-3
Han, S., Liu, P., Yan, Q., Cen, Y., Wu, G., Chen, Z., et al. (2024). Seawater pearl hydrolysate inhibits photoaging via decreasing oxidative stress, autophagy and apoptosis of Ultraviolet B-induced human skin keratinocytes. J. Cosmet. Dermatol. 23, 256–270. doi:10.1111/jocd.15916
Han, S. H., Ballinger, E., Choung, S.-Y., and Kwon, J. Y. (2022). Anti-photoaging effect of hydrolysates from pacific whiting skin via MAPK/AP-1, NF-κB, TGF-β/Smad, and Nrf-2/HO-1 signaling pathway in UVB-induced human dermal fibroblasts. Mar. Drugs 20, 308. doi:10.3390/md20050308
Hansda, S., and Ghosh, R. (2022). Bystander effect of ultraviolet A radiation protects A375 melanoma cells by induction of antioxidant defense. J. Environ. Sci. Health Part C Toxicol. Carcinog. 40, 46–67. doi:10.1080/26896583.2021.1994820
Hao, D., Wen, X., Liu, L., Wang, L., Zhou, X., Li, Y., et al. (2019). Sanshool improves UVB-induced skin photodamage by targeting JAK2/STAT3-dependent autophagy. Cell. Death Dis. 10, 19. doi:10.1038/s41419-018-1261-y
Hargreaves, A., Taiwo, F. A., Duggan, O., Kirk, S. H., and Ahmad, S. I. (2007). Near-ultraviolet photolysis of beta-phenylpyruvic acid generates free radicals and results in DNA damage. J. Photochem. Photobiol. B 89, 110–116. doi:10.1016/j.jphotobiol.2007.09.007
Harwansh, R. K., and Deshmukh, R. (2023). Recent insight into UV-induced oxidative stress and role of herbal bioactives in the management of skin aging. Curr. Pharm. Biotechnol. 25, 16–41. doi:10.2174/1389201024666230427110815
Havas, F., Krispin, S., Cohen, M., Loing, E., Farge, M., Suere, T., et al. (2022). A Dunaliella salina extract counteracts skin aging under intense solar irradiation thanks to its antiglycation and anti-inflammatory properties. Mar. Drugs 20, 104. doi:10.3390/md20020104
He, J., He, H., Qi, Y., Yang, J., Zhi, L., and Jia, Y. (2022). Application of epigenetics in dermatological research and skin management. J. Cosmet. Dermatol. 21, 1920–1930. doi:10.1111/jocd.14355
Heng, M. C. Y. (2013). Signaling pathways targeted by curcumin in acute and chronic injury: burns and photo-damaged skin. Int. J. Dermatol. 52, 531–543. doi:10.1111/j.1365-4632.2012.05703.x
Ho, C. Y., and Dreesen, O. (2021). Faces of cellular senescence in skin aging. Mech. Ageing Dev. 198, 111525. doi:10.1016/j.mad.2021.111525
Hu, M., Huang, H., Zhao, R., Li, P., Li, M., Miao, H., et al. (2014). AZD8055 induces cell death associated with autophagy and activation of AMPK in hepatocellular carcinoma. Oncol. Rep. 31, 649–656. doi:10.3892/or.2013.2890
Huang, D.-R., Dai, C.-M., Li, S.-Y., and Li, X.-F. (2021). Obacunone protects retinal pigment epithelium cells from ultra-violet radiation-induced oxidative injury. Aging 13, 11010–11025. doi:10.18632/aging.202437
Huang, Y., Li, Y., Qu, Y., Zheng, Y., Ouyang, M., Zhang, Y., et al. (2019). UVA-induced photoaging inhibits autophagic degradation by impairing lysosomal function in dermal fibroblasts. Biochem. Biophys. Res. Commun. 518, 611–618. doi:10.1016/j.bbrc.2019.08.103
Huangfu, L., Wang, X., Tian, S., Chen, J., Wang, X., Fan, B., et al. (2023). Piceatannol enhances Beclin-1 activity to suppress tumor progression and its combination therapy strategy with everolimus in gastric cancer. Sci. China Life Sci. 66, 298–312. doi:10.1007/s11427-022-2185-9
Hwang, S., Maloney, N. S., Bruinsma, M. W., Goel, G., Duan, E., Zhang, L., et al. (2012). Nondegradative role of Atg5-Atg12/Atg16L1 autophagy protein complex in antiviral activity of interferon gamma. Cell. Host Microbe. 11, 397–409. doi:10.1016/j.chom.2012.03.002
Iannucci, L. F., Di Benedetto, G., and Lefkimmiatis, K. (2021). PRKA/PKA signals and autophagy: space matters. Autophagy 17, 1563–1564. doi:10.1080/15548627.2021.1924501
Ichihashi, M., and Ando, H. (2014). The maximal cumulative solar UVB dose allowed to maintain healthy and young skin and prevent premature photoaging. Exp. Dermatol. 23 (1), 43–46. doi:10.1111/exd.12393
Ikutama, R., Peng, G., Tsukamoto, S., Umehara, Y., Trujillo-Paez, J. V., Yue, H., et al. (2023). Cathelicidin LL-37 activates human keratinocyte autophagy through the P2X7, mechanistic target of rapamycin, and MAPK pathways. J. Invest. Dermatol. 143, 751–761.e7. doi:10.1016/j.jid.2022.10.020
Indira, D., Varadarajan, S. N., Subhasingh Lupitha, S., Lekshmi, A., Mathew, K. A., Chandrasekharan, A., et al. (2018). Strategies for imaging mitophagy in high-resolution and high-throughput. Eur. J. Cell. Biol. 97, 1–14. doi:10.1016/j.ejcb.2017.10.003
Israeli, T., Riahi, Y., Garzon, P., Louzada, R. A., Werneck-de-Castro, J. P., Blandino-Rosano, M., et al. (2022). Nutrient sensor mTORC1 regulates insulin secretion by modulating β-cell autophagy. Diabetes 71, 453–469. doi:10.2337/db21-0281
Jacquet, M., Guittaut, M., Fraichard, A., and Despouy, G. (2021). The functions of Atg8-family proteins in autophagy and cancer: linked or unrelated? Autophagy 17, 599–611. doi:10.1080/15548627.2020.1749367
Jarisarapurin, W., Kunchana, K., Chularojmontri, L., and Wattanapitayakul, S. K. (2021). Unripe carica papaya protects methylglyoxal-invoked endothelial cell inflammation and apoptosis via the suppression of oxidative stress and Akt/MAPK/NF-κB signals. Antioxid. Basel Switz. 10, 1158. doi:10.3390/antiox10081158
Jariwala, N., Ozols, M., Eckersley, A., Mambwe, B., Watson, R. E. B., Zeef, L., et al. (2024). Prediction, screening and characterization of novel bioactive tetrapeptide matrikines for skin rejuvenation. Br. J. Dermatol. 191, 92–106. doi:10.1093/bjd/ljae061
Jeon, Y., Kim, J., Kwon, H., Yeon, Y. J., Kim, T., Ham, J., et al. (2024). Cannabiorcol as a novel inhibitor of the p38/MSK-1/NF-κB signaling pathway, reducing matrix metalloproteinases in osteoarthritis. Phytomedicine Int. J. Phytother. Phytopharm. 135, 156141. doi:10.1016/j.phymed.2024.156141
Jeremian, R., Malinowski, A., Lytvyn, Y., Georgakopoulos, J. R., Muntyanu, A., Mufti, A., et al. (2024). Skin photoageing following sun exposure is associated with decreased epigenetic and biologic age, and correlates with basal cell carcinoma phenotype. Br. J. Dermatol. 190, 590–592. doi:10.1093/bjd/ljad527
Ji, E., Kim, C., Kang, H., Ahn, S., Jung, M., Hong, Y., et al. (2019). RNA binding protein HuR promotes autophagosome formation by regulating expression of autophagy-related proteins 5, 12, and 16 in human hepatocellular carcinoma cells. Mol. Cell. Biol. 39, e00508–e00518. doi:10.1128/MCB.00508-18
Jin, Y., Liu, D., Lu, Z., Yang, L., Chen, J., Zhou, X., et al. (2022). Preparation and evaluation of liposomes and niosomes containing total ginsenosides for anti-photoaging therapy. Front. Bioeng. Biotechnol. 10, 874827. doi:10.3389/fbioe.2022.874827
Jinlian, L., Yingbin, Z., and Chunbo, W. (2007). p38 MAPK in regulating cellular responses to ultraviolet radiation. J. Biomed. Sci. 14, 303–312. doi:10.1007/s11373-007-9148-4
Johann To Berens, P., Peter, J., Koechler, S., Bruggeman, M., Staerck, S., and Molinier, J. (2024). The histone demethylase JMJ27 acts during the UV-induced modulation of H3K9me2 landscape and facilitates photodamage repair. Nat. Plants 10, 1698–1709. doi:10.1038/s41477-024-01814-9
Kaeberlein, M. (2017). Translational geroscience: a new paradigm for 21st century medicine. Transl. Med. Aging 1, 1–4. doi:10.1016/j.tma.2017.09.004
Kahremany, S., Hofmann, L., Gruzman, A., Dinkova-Kostova, A. T., and Cohen, G. (2022). NRF2 in dermatological disorders: pharmacological activation for protection against cutaneous photodamage and photodermatosis. Free Radic. Biol. Med. 188, 262–276. doi:10.1016/j.freeradbiomed.2022.06.238
Kaur, K., Narang, R. K., and Singh, S. (2023). Role of Nrf2 in oxidative stress, neuroinflammation and autophagy in Alzheimer’s disease: regulation of Nrf2 by different signaling pathways. Curr. Mol. Med. 25, 372–387. doi:10.2174/1566524023666230726145447
Khater, S. I., El-Emam, M. M. A., Abdellatif, H., Mostafa, M., Khamis, T., Soliman, R. H. M., et al. (2024). Lipid nanoparticles of quercetin (QU-Lip) alleviated pancreatic microenvironment in diabetic male rats: the interplay between oxidative stress - unfolded protein response (UPR) - autophagy, and their regulatory miRNA. Life Sci. 344, 122546. doi:10.1016/j.lfs.2024.122546
Kikuchi, Y., Nakamura, S., Woodson, J. D., Ishida, H., Ling, Q., Hidema, J., et al. (2020). Chloroplast autophagy and ubiquitination combine to manage oxidative damage and starvation responses. Plant Physiol. 183, 1531–1544. doi:10.1104/pp.20.00237
Kim, C.-W., Alam, M. B., Song, B.-R., Lee, C. H., Kim, S. L., and Lee, S.-H. (2024a). γ-Mangosteen, an autophagy enhancer, prevents skin-aging via activating KEAP1/NRF2 signaling and downregulating MAPKs/AP-1/NF-κB-mediated MMPs. Phytomed. Int. J. Phytother. Phytopharm. 132, 155815. doi:10.1016/j.phymed.2024.155815
Kim, H. S., Park, S.-Y., Moon, S. H., Lee, J. D., and Kim, S. (2018). Autophagy in human skin fibroblasts: impact of age. Int. J. Mol. Sci. 19, 2254. doi:10.3390/ijms19082254
Kim, J., Yu, Y. S., Choi, Y., Lee, D. H., Han, S., Kwon, J., et al. (2024b). USF2 and TFEB compete in regulating lysosomal and autophagy genes. Nat. Commun. 15, 8334. doi:10.1038/s41467-024-52600-2
Kim, K., Kim, C.-E., Baek, D.-J., Park, E.-Y., and Oh, Y. S. (2024c). Prevention of UVB-induced photoaging by an ethyl acetate fraction from allomyrina dichotoma larvae and its potential mechanisms in human dermal fibroblasts. Int. J. Mol. Sci. 25, 7850. doi:10.3390/ijms25147850
Kim, K. S., Choi, Y. J., Jang, D. S., and Lee, S. (2022). 2-O-β-d-Glucopyranosyl-4,6-dihydroxybenzaldehyde isolated from morus alba (Mulberry) fruits suppresses damage by regulating oxidative and inflammatory responses in TNF-α-induced human dermal fibroblasts. Int. J. Mol. Sci. 23, 14802. doi:10.3390/ijms232314802
Kim, M.-J., Woo, S. W., Kim, M.-S., Park, J.-E., and Hwang, J.-K. (2014). Anti-photoaging effect of aaptamine in UVB-irradiated human dermal fibroblasts and epidermal keratinocytes. J. Asian Nat. Prod. Res. 16, 1139–1147. doi:10.1080/10286020.2014.983092
Kim, M.-K., Shin, H. S., Shin, M. H., Kim, H., Lee, D. H., and Chung, J. H. (2023). Dual role of enhancer of zeste homolog 2 in the regulation of ultraviolet radiation-induced matrix metalloproteinase-1 and type I procollagen expression in human dermal fibroblasts. Matrix Biol. J. Int. Soc. Matrix Biol. 119, 112–124. doi:10.1016/j.matbio.2023.04.001
Klionsky, D. J., and Codogno, P. (2013). The mechanism and physiological function of macroautophagy. J. Innate Immun. 5 (5), 427–433. doi:10.1159/000351979
Kma, L., and Baruah, T. J. (2022). The interplay of ROS and the PI3K/Akt pathway in autophagy regulation. Biotechnol. Appl. Biochem. 69, 248–264. doi:10.1002/bab.2104
Koentjoro, B., Park, J.-S., and Sue, C. M. (2017). Nix restores mitophagy and mitochondrial function to protect against PINK1/Parkin-related Parkinson’s disease. Sci. Rep. 7, 44373. doi:10.1038/srep44373
Kolapalli, S. P., Nielsen, T. M., and Frankel, L. B. (2023). Post-transcriptional dynamics and RNA homeostasis in autophagy and cancer. Cell. Death Differ. 32, 27–36. doi:10.1038/s41418-023-01201-5
Kong, L., Li, S., Fu, Y., Cai, Q., Du, X., Liang, J., et al. (2024). Mitophagy in relation to chronic inflammation/ROS in aging. Mol. Cell. Biochem. 480, 721–731. doi:10.1007/s11010-024-05042-9
Kong, S., Lv, L., Guo, J., Yang, X., Liao, M., Zhao, T., et al. (2023). Preparation of Cod skin collagen peptides/chitosan-based temperature-sensitive gel and its anti-photoaging effect in skin. Drug Des. devel. Ther. 17, 419–437. doi:10.2147/DDDT.S391812
Konger, R. L., Derr-Yellin, E., Hojati, D., Lutz, C., and Sundberg, J. P. (2016). Comparison of the acute ultraviolet photoresponse in congenic albino hairless C57BL/6J mice relative to outbred SKH1 hairless mice. Exp. Dermatol. 25, 688–693. doi:10.1111/exd.13034
Krause, G. J., Kirchner, P., Stiller, B., Morozova, K., Diaz, A., Chen, K.-H., et al. (2023). Molecular determinants of the crosstalk between endosomal microautophagy and chaperone-mediated autophagy. Cell. Rep. 42, 113529. doi:10.1016/j.celrep.2023.113529
Krutmann, J., Bouloc, A., Sore, G., Bernard, B. A., and Passeron, T. (2017). The skin aging exposome. J. Dermatol. Sci. 85, 152–161. doi:10.1016/j.jdermsci.2016.09.015
Kuchitsu, Y., and Taguchi, T. (2024). Lysosomal microautophagy: an emerging dimension in mammalian autophagy. Trends Cell. Biol. 34, 606–616. doi:10.1016/j.tcb.2023.11.005
Kumar, A., Girisa, S., Alqahtani, M. S., Abbas, M., Hegde, M., Sethi, G., et al. (2023). Targeting autophagy using long non-coding RNAs (LncRNAs): new landscapes in the arena of cancer therapeutics. Cells 12, 810. doi:10.3390/cells12050810
Kumar, A. V., Mills, J., and Lapierre, L. R. (2022). Selective autophagy receptor p62/SQSTM1, a pivotal player in stress and aging. Front. Cell. Dev. Biol. 10, 793328. doi:10.3389/fcell.2022.793328
Kumariya, S., Ubba, V., Jha, R. K., and Gayen, J. R. (2021). Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective. Autophagy 17, 2706–2733. doi:10.1080/15548627.2021.1938914
Kunisada, M., Hosaka, C., Takemori, C., Nakano, E., and Nishigori, C. (2017). CXCL1 inhibition regulates UVB-induced skin inflammation and tumorigenesis in Xpa-deficient mice. J. Invest. Dermatol. 137, 1975–1983. doi:10.1016/j.jid.2017.04.034
Lamore, S. D., and Wondrak, G. T. (2013). UVA causes dual inactivation of cathepsin B and L underlying lysosomal dysfunction in human dermal fibroblasts. J. Photochem. Photobiol. B 123, 1–12. doi:10.1016/j.jphotobiol.2013.03.007
Lee, E. K., Lee, Y. S., Lee, H., Choi, C. Y., and Park, S. H. (2009). 14-3-3epsilon protein increases matrix metalloproteinase-2 gene expression via p38 MAPK signaling in NIH3T3 fibroblast cells. Exp. Mol. Med. 41, 453–561. doi:10.3858/emm.2009.41.7.050
Lee, J., Kim, J., Shin, J., Kang, Y., Choi, J., and Cheong, H. (2021a). ATG101 degradation by HUWE1-mediated ubiquitination impairs autophagy and reduces survival in cancer cells. Int. J. Mol. Sci. 22, 9182. doi:10.3390/ijms22179182
Lee, J. H., Park, J., and Shin, D. W. (2022). The molecular mechanism of polyphenols with anti-aging activity in aged human dermal fibroblasts. Mol. Basel Switz. 27, 4351. doi:10.3390/molecules27144351
Lee, J. W., Park, S., Takahashi, Y., and Wang, H.-G. (2010). The association of AMPK with ULK1 regulates autophagy. PLoS One 5, e15394. doi:10.1371/journal.pone.0015394
Lee, K.-S., Lee, M.-G., Woo, Y.-J., and Nam, K.-S. (2019). The preventive effect of deep sea water on the development of cancerous skin cells through the induction of autophagic cell death in UVB-damaged HaCaT keratinocyte. Biomed. Pharmacother. 111, 282–291. doi:10.1016/j.biopha.2018.12.083
Lee, T.-H., Chen, J.-L., Tsai, M.-M., Wu, Y.-H., Tseng, H.-C., Cheng, L.-C., et al. (2023). Protective effects of sophoraflavanone G by inhibiting TNF-α-induced MMP-9-mediated events in brain microvascular endothelial cells. Int. J. Mol. Sci. 25, 283. doi:10.3390/ijms25010283
Lee, Y., Shin, M. H., Kim, M.-K., Kim, Y. K., Shin, H. S., Lee, D. H., et al. (2021c). Increased histone acetylation and decreased expression of specific histone deacetylases in ultraviolet-irradiated and intrinsically aged human skin in vivo. Int. J. Mol. Sci. 22, 2032. doi:10.3390/ijms22042032
Lee, Y.-H., Wang, E., Kumar, N., and Glickman, R. D. (2014). Ursolic acid differentially modulates apoptosis in skin melanoma and retinal pigment epithelial cells exposed to UV-VIS broadband radiation. Apoptosis Int. J. Program. Cell. Death 19, 816–828. doi:10.1007/s10495-013-0962-z
Lee, Y. I., Choi, S., Roh, W. S., Lee, J. H., and Kim, T.-G. (2021b). Cellular senescence and inflammaging in the skin microenvironment. Int. J. Mol. Sci. 22, 3849. doi:10.3390/ijms22083849
Lemasters, J. J. (2014). Variants of mitochondrial autophagy: types 1 and 2 mitophagy and micromitophagy (Type 3). Redox Biol. 2, 749–754. doi:10.1016/j.redox.2014.06.004
Li, H., Zhu, L., Weng, Z., Fu, H., Liu, J., Mao, Q., et al. (2024a). Sesamin attenuates UVA-induced keratinocyte injury via inhibiting ASK-1-JNK/p38 MAPK pathways. J. Cosmet. Dermatol. 23, 316–325. doi:10.1111/jocd.15951
Li, L., Hongying, C., and Heng, G. (2023). Autophagy-related LncRNA PRDM10-DT responds to UVB radiation in keratinocytes. Biochem. Biophys. Res. Commun. 677, 105–112. doi:10.1016/j.bbrc.2023.08.013
Li, L., Ngo, H. T. T., Hwang, E., Wei, X., Liu, Y., Liu, J., et al. (2019a). Conditioned medium from human adipose-derived mesenchymal stem cell culture prevents UVB-induced skin aging in human keratinocytes and dermal fibroblasts. Int. J. Mol. Sci. 21, 49. doi:10.3390/ijms21010049
Li, M., Mo, Y., Yu, Q., Anayyat, U., Yang, H., Zhang, F., et al. (2024b). Rotating magnetic field improves cognitive and memory impairments in APP/PS1 mice by activating autophagy and inhibiting the PI3K/AKT/mTOR signaling pathway. Exp. Neurol. 383, 115029. doi:10.1016/j.expneurol.2024.115029
Li, S., Khoi, P. N., Yin, H., Sah, D. K., Kim, N.-H., Lian, S., et al. (2022). Sulforaphane suppresses the nicotine-induced expression of the matrix metalloproteinase-9 via inhibiting ROS-mediated AP-1 and NF-κB signaling in human gastric cancer cells. Int. J. Mol. Sci. 23, 5172. doi:10.3390/ijms23095172
Li, T., Yue, J., Huang, L., and Yang, M. (2019b). Autophagy inhibitor Vacuolin-1 interferes with lipid-based small interference RNA delivery. Biochem. Biophys. Res. Commun. 510, 427–434. doi:10.1016/j.bbrc.2019.01.121
Li, W., Li, J., and Bao, J. (2012). Microautophagy: lesser-known self-eating. Cell. Mol. Life Sci. 69, 1125–1136. doi:10.1007/s00018-011-0865-5
Li, W., Yang, Q., and Mao, Z. (2018). Signaling and induction of chaperone-mediated autophagy by the endoplasmic reticulum under stress conditions. Autophagy 14, 1094–1096. doi:10.1080/15548627.2018.1444314
Li, W., and Zhang, L. (2019). Regulation of ATG and autophagy initiation. Adv. Exp. Med. Biol. 1206, 41–65. doi:10.1007/978-981-15-0602-4_2
Li, X., Zhou, Y., Yang, L., Ma, Y., Peng, X., Yang, S., et al. (2020). LncRNA NEAT1 promotes autophagy via regulating miR-204/ATG3 and enhanced cell resistance to sorafenib in hepatocellular carcinoma. J. Cell. Physiol. 235, 3402–3413. doi:10.1002/jcp.29230
Li, Y., Gao, J., Liu, S., Chen, S., Wei, X., Guan, Y., et al. (2024c). Ergothioneine protects against UV-induced oxidative stress through the PI3K/AKT/Nrf2 signaling pathway. Clin. Cosmet. Investig. Dermatol. 17, 1309–1319. doi:10.2147/CCID.S449987
Li, Y., Liang, Z., He, H., Huang, X., Mo, Z., Tan, J., et al. (2021). The lncRNA HOTAIR regulates autophagy and affects lipopolysaccharide-induced acute lung injury through the miR-17-5p/ATG2/ATG7/ATG16 axis. J. Cell. Mol. Med. 25, 8062–8073. doi:10.1111/jcmm.16737
Li, Y., Ma, Y., Yao, Y., Ru, G., Lan, C., Li, L., et al. (2024d). Protective effect of isoquercitrin on UVB-induced injury in HaCaT cells and mice skin through anti-inflammatory, antioxidant, and regulation of MAPK and JAK2-STAT3 pathways. Photochem. Photobiol. 100, 1507–1518. doi:10.1111/php.13919
Li, Y.-Y., Qin, Z.-H., and Sheng, R. (2024e). The multiple roles of autophagy in neural function and diseases. Neurosci. Bull. 40, 363–382. doi:10.1007/s12264-023-01120-y
Liang, W., Yamahara, K., Hernando-Erhard, C., Lagies, S., Wanner, N., Liang, H., et al. (2020). A reciprocal regulation of spermidine and autophagy in podocytes maintains the filtration barrier. Kidney Int. 98, 1434–1448. doi:10.1016/j.kint.2020.06.016
Lim, C., Piao, M. J., Kang, K. A., Fernando, P. D. S. M., Herath, H. M. U. L., Kim, D. W., et al. (2024a). Inhibitory action of 1,3,5-trihydroxybenzene on UVB-induced NADPH oxidase 4 through AMPK and JNK signaling pathways. Biomol. Ther. 32, 499–507. doi:10.4062/biomolther.2024.054
Lim, G.-E., Park, J. E., Cho, Y. H., Lim, D. S., Kim, A.-J., Moh, S. H., et al. (2020). Alpha-neoendorphin can reduce UVB-induced skin photoaging by activating cellular autophagy. Arch. Biochem. Biophys. 689, 108437. doi:10.1016/j.abb.2020.108437
Lim, S. H. Y., Hansen, M., and Kumsta, C. (2024b). Molecular mechanisms of autophagy decline during aging. Cells 13, 1364. doi:10.3390/cells13161364
Lin, Y., Cao, Z., Lyu, T., Kong, T., Zhang, Q., Wu, K., et al. (2022). Single-cell RNA-seq of UVB-radiated skin reveals landscape of photoaging-related inflammation and protection by vitamin D. Gene 831, 146563. doi:10.1016/j.gene.2022.146563
Lin, Y., Sun, Y., Hou, W., Chen, X., Zhou, F., Xu, Q., et al. (2024a). FTO-mediated regulation of m6A methylation is closely related to apoptosis induced by repeated UV irradiation. J. Dermatol. Sci. 114, 124–132. doi:10.1016/j.jdermsci.2024.01.001
Lin, Y., Wu, X., Yang, Y., Wu, Y., Xiang, L., and Zhang, C. (2024b). The multifaceted role of autophagy in skin autoimmune disorders: a guardian or culprit? Front. Immunol. 15, 1343987. doi:10.3389/fimmu.2024.1343987
Liu, B., Liu, L., and Liu, Y. (2024a). Targeting cell death mechanisms: the potential of autophagy and ferroptosis in hepatocellular carcinoma therapy. Front. Immunol. 15, 1450487. doi:10.3389/fimmu.2024.1450487
Liu, H., Zhen, C., Xie, J., Luo, Z., Zeng, L., Zhao, G., et al. (2024b). TFAM is an autophagy receptor that limits inflammation by binding to cytoplasmic mitochondrial DNA. Nat. Cell. Biol. 26, 878–891. doi:10.1038/s41556-024-01419-6
Liu, M., Jiang, H., and Momeni, M. R. (2024c). Epigenetic regulation of autophagy by non-coding RNAs and exosomal non-coding RNAs in colorectal cancer: a narrative review. Int. J. Biol. Macromol. 273, 132732. doi:10.1016/j.ijbiomac.2024.132732
Liu, S., Mohri, S., Manabe, Y., Ejima, A., Sato, K., and Sugawara, T. (2022a). Gly-Pro protects normal human dermal fibroblasts from UVA-induced damages via MAPK-NF-κB signaling pathway. J. Photochem. Photobiol. B 237, 112601. doi:10.1016/j.jphotobiol.2022.112601
Liu, W., Otkur, W., Zhang, Y., Li, Q., Ye, Y., Zang, L., et al. (2013). Silibinin protects murine fibroblast L929 cells from UVB-induced apoptosis through the simultaneous inhibition of ATM-p53 pathway and autophagy. FEBS J. 280, 4572–4584. doi:10.1111/febs.12426
Liu, X.-Y., Hwang, E., Park, B., Xiao, Y.-K., and Yi, T.-H. (2019). Photoprotective and anti-inflammatory properties of vina-ginsenoside R7 ameliorate ultraviolet B-induced photodamage in normal human dermal fibroblasts. Appl. Biochem. Biotechnol. 189, 729–744. doi:10.1007/s12010-019-03027-9
Liu, Y., Qu, L., Wan, S., Li, Y., and Fan, D. (2022b). Ginsenoside Rk1 prevents UVB irradiation-mediated oxidative stress, inflammatory response, and collagen degradation via the PI3K/AKT/NF-κB pathway in vitro and in vivo. J. Agric. Food Chem. 70, 15804–15817. doi:10.1021/acs.jafc.2c06377
Lo, H.-L., Nakajima, S., Ma, L., Walter, B., Yasui, A., Ethell, D. W., et al. (2005). Differential biologic effects of CPD and 6-4PP UV-induced DNA damage on the induction of apoptosis and cell-cycle arrest. BMC Cancer 5, 135. doi:10.1186/1471-2407-5-135
López-Camarillo, C., Ocampo, E. A., Casamichana, M. L., Pérez-Plasencia, C., Alvarez-Sánchez, E., and Marchat, L. A. (2012). Protein kinases and transcription factors activation in response to UV-radiation of skin: implications for carcinogenesis. Int. J. Mol. Sci. 13, 142–172. doi:10.3390/ijms13010142
Lu, F., Zhou, Q., Liang, M., Liang, H., Yu, Y., Li, Y., et al. (2024). α-Arbutin ameliorates UVA-induced photoaging through regulation of the SIRT3/PGC-1α pathway. Front. Pharmacol. 15, 1413530. doi:10.3389/fphar.2024.1413530
Luo, C., Wei, L., Qian, F., Bo, L., Gao, S., Yang, G., et al. (2024). LncRNA HOTAIR regulates autophagy and proliferation mechanisms in premature ovarian insufficiency through the miR-148b-3p/ATG14 axis. Cell. Death Discov. 10, 44. doi:10.1038/s41420-024-01811-z
Luo, J. (2014). Autophagy and ethanol neurotoxicity. Autophagy 10, 2099–2108. doi:10.4161/15548627.2014.981916
Luo, N., Shang, D., Tang, Z., Mai, J., Huang, X., Tao, L.-Z., et al. (2023). Engineered ATG8-binding motif-based selective autophagy to degrade proteins and organelles in planta. New Phytol. 237, 684–697. doi:10.1111/nph.18557
Lyamzaev, K. G., Tokarchuk, A. V., Panteleeva, A. A., Mulkidjanian, A. Y., Skulachev, V. P., and Chernyak, B. V. (2018). Induction of autophagy by depolarization of mitochondria. Autophagy 14, 921–924. doi:10.1080/15548627.2018.1436937
Ma, F. Y., Tesch, G. H., Ozols, E., Xie, M., Schneider, M. D., and Nikolic-Paterson, D. J. (2011). TGF-β1-activated kinase-1 regulates inflammation and fibrosis in the obstructed kidney. Am. J. Physiol. Ren. Physiol. 300, F1410–F1421. doi:10.1152/ajprenal.00018.2011
Ma, J., Teng, Y., Huang, Y., Tao, X., and Fan, Y. (2022a). Autophagy plays an essential role in ultraviolet radiation-driven skin photoaging. Front. Pharmacol. 13, 864331. doi:10.3389/fphar.2022.864331
Ma, Q., Long, S., Gan, Z., Tettamanti, G., Li, K., and Tian, L. (2022b). Transcriptional and post-transcriptional regulation of autophagy. Cells 11, 441. doi:10.3390/cells11030441
Machala, M., Procházková, J., Hofmanová, J., Králiková, L., Slavík, J., Tylichová, Z., et al. (2019). Colon cancer and perturbations of the sphingolipid metabolism. Int. J. Mol. Sci. 20, 6051. doi:10.3390/ijms20236051
Madson, J. G., and Hansen, L. A. (2007). Multiple mechanisms of Erbb2 action after ultraviolet irradiation of the skin. Mol. Carcinog. 46, 624–628. doi:10.1002/mc.20335
Magalhaes-Novais, S., Blecha, J., Naraine, R., Mikesova, J., Abaffy, P., Pecinova, A., et al. (2022). Mitochondrial respiration supports autophagy to provide stress resistance during quiescence. Autophagy 18, 2409–2426. doi:10.1080/15548627.2022.2038898
Mahbubfam, S., Rezaie, J., and Nejati, V. (2022). Crosstalk between exosomes signaling pathway and autophagy flux in senescent human endothelial cells. Tissue Cell. 76, 101803. doi:10.1016/j.tice.2022.101803
Maib, H., Adarska, P., Hunton, R., Vines, J. H., Strutt, D., Bottanelli, F., et al. (2024). Recombinant biosensors for multiplex and super-resolution imaging of phosphoinositides. J. Cell. Biol. 223, e202310095. doi:10.1083/jcb.202310095
Majumder, P., Shukla, C., Arya, A., Sharma, S., and Datta, B. (2024). G-quadruplexes in MTOR and induction of autophagy. Sci. Rep. 14, 2525. doi:10.1038/s41598-024-52561-y
Manandhar, L., Dutta, R. K., Devkota, P., Chhetri, A., Wei, X., Park, C., et al. (2024). TFEB activation triggers pexophagy for functional adaptation during oxidative stress under calcium deficient-conditions. Cell. Commun. Signal. 22, 142. doi:10.1186/s12964-024-01524-x
Manosalva, C., Bahamonde, C., Soto, F., Leal, V., Ojeda, C., Cortés, C., et al. (2024). Linoleic acid induces metabolic reprogramming and inhibits oxidative and inflammatory effects in keratinocytes exposed to UVB radiation. Int. J. Mol. Sci. 25, 10385. doi:10.3390/ijms251910385
Markovitsi, D. (2024). On the use of the intrinsic DNA fluorescence for monitoring its damage: a contribution from fundamental studies. ACS Omega 9, 26826–26837. doi:10.1021/acsomega.4c02256
Martic, I., Wedel, S., Jansen-Dürr, P., and Cavinato, M. (2020). A new model to investigate UVB-induced cellular senescence and pigmentation in melanocytes. Mech. Ageing Dev. 190, 111322. doi:10.1016/j.mad.2020.111322
Mavrogonatou, E., Konstantinou, A., and Kletsas, D. (2018). Long-term exposure to TNF-α leads human skin fibroblasts to a p38 MAPK- and ROS-mediated premature senescence. Biogerontology 19, 237–249. doi:10.1007/s10522-018-9753-9
Mestre Citrinovitz, A. C., Strowitzki, T., and Germeyer, A. (2019). Decreased autophagy impairs decidualization of human endometrial stromal cells: a role for ATG proteins in endometrial physiology. Int. J. Mol. Sci. 20, 3066. doi:10.3390/ijms20123066
Min, X., Zheng, M., Yu, Y., Wu, J., Kuang, Q., Hu, Z., et al. (2022). Ultraviolet light induces HERV expression to activate RIG-I signalling pathway in keratinocytes. Exp. Dermatol. 31, 1165–1176. doi:10.1111/exd.14568
Mirabdali, S., Ghafouri, K., Farahmand, Y., Gholizadeh, N., Yazdani, O., Esbati, R., et al. (2024). The role and function of autophagy through signaling and pathogenetic pathways and lncRNAs in ovarian cancer. Pathol. Res. Pract. 253, 154899. doi:10.1016/j.prp.2023.154899
Mizushima, N. (2010). The role of the Atg1/ULK1 complex in autophagy regulation. Curr. Opin. Cell. Biol. 22, 132–139. doi:10.1016/j.ceb.2009.12.004
Mizushima, N., and Yoshimori, T. (2007). How to interpret LC3 immunoblotting. Autophagy 3, 542–545. doi:10.4161/auto.4600
Mochida, K., and Nakatogawa, H. (2022). ER-phagy: selective autophagy of the endoplasmic reticulum. EMBO Rep. 23, e55192. doi:10.15252/embr.202255192
Mostafa, D. K., Nayel, O. A., Abdulmalek, S., Abdelbary, A. A., and Ismail, C. A. (2022). Modulation of autophagy, apoptosis and oxidative stress: a clue for repurposing metformin in photoaging. Inflammopharmacology 30, 2521–2535. doi:10.1007/s10787-022-01041-8
Mostafa, D. K., Omar, S. I., Abdellatif, A. A., Sorour, O. A., Nayel, O. A., and Abod Al Obaidi, M. R. (2021). Differential modulation of autophagy contributes to the protective effects of resveratrol and Co-enzyme Q10 in photoaged mice. Curr. Mol. Pharmacol. 14, 458–468. doi:10.2174/1874467213666200730114547
Mundo Rivera, V. M., Tlacuahuac Juárez, J. R., Murillo Melo, N. M., Leyva Garcia, N., Magaña, J. J., Cordero Martínez, J., et al. (2024). Natural autophagy activators to fight age-related diseases. Cells 13, 1611. doi:10.3390/cells13191611
Mussbacher, M., Derler, M., Basílio, J., and Schmid, J. A. (2023). NF-κB in monocytes and macrophages - an inflammatory master regulator in multitalented immune cells. Front. Immunol. 14, 1134661. doi:10.3389/fimmu.2023.1134661
Nakatogawa, H. (2013). Two ubiquitin-like conjugation systems that mediate membrane formation during autophagy. Essays Biochem. 55, 39–50. doi:10.1042/bse0550039
Nam, N.-H. (2006). Naturally occurring NF-kappaB inhibitors. Mini Rev. Med. Chem. 6, 945–951. doi:10.2174/138955706777934937
Narayanapillai, S., Agarwal, C., Tilley, C., and Agarwal, R. (2012). Silibinin is a potent sensitizer of UVA radiation-induced oxidative stress and apoptosis in human keratinocyte HaCaT cells. Photochem. Photobiol. 88, 1135–1140. doi:10.1111/j.1751-1097.2011.01050.x
Narendra, D. P., and Youle, R. J. (2024). The role of PINK1-Parkin in mitochondrial quality control. Nat. Cell. Biol. 26, 1639–1651. doi:10.1038/s41556-024-01513-9
Navarro-Lérida, I., Aragay, A. M., Asensio, A., and Ribas, C. (2022). Gq signaling in autophagy control: between chemical and mechanical cues. Antioxid. Basel Switz. 11, 1599. doi:10.3390/antiox11081599
Nazio, F., and Cecconi, F. (2017). Autophagy up and down by outsmarting the incredible ULK. Autophagy 13, 967–968. doi:10.1080/15548627.2017.1285473
Neale, P. J., Williamson, C. E., Banaszak, A. T., Häder, D.-P., Hylander, S., Ossola, R., et al. (2023). The response of aquatic ecosystems to the interactive effects of stratospheric ozone depletion, UV radiation, and climate change. Photochem. Photobiol. Sci. 22, 1093–1127. doi:10.1007/s43630-023-00370-z
Negre-Salvayre, A., and Salvayre, R. (2022). Post-translational modifications evoked by reactive carbonyl species in ultraviolet-A-exposed skin: implication in fibroblast senescence and skin photoaging. Antioxid. Basel Switz. 11, 2281. doi:10.3390/antiox11112281
Nisar, M. F., Liu, T., Wang, M., Chen, S., Chang, L., Karisma, V. W., et al. (2022). Eriodictyol protects skin cells from UVA irradiation-induced photodamage by inhibition of the MAPK signaling pathway. J. Photochem. Photobiol. B 226, 112350. doi:10.1016/j.jphotobiol.2021.112350
Ocansey, D. K. W., Qian, F., Cai, P., Ocansey, S., Amoah, S., Qian, Y., et al. (2024). Current evidence and therapeutic implication of PANoptosis in cancer. Theranostics 14, 640–661. doi:10.7150/thno.91814
Oh, J. H., Karadeniz, F., Lee, J. I., Seo, Y., and Kong, C.-S. (2023a). Oleracone C from Portulaca oleracea attenuates UVB-induced changes in matrix metalloproteinase and type I procollagen production via MAPK and TGF-β/Smad pathways in human keratinocytes. Int. J. Cosmet. Sci. 45, 166–176. doi:10.1111/ics.12828
Oh, S., Zheng, S., Fang, M., Kim, M., Bellere, A. D., Jeong, J., et al. (2023b). Anti-photoaging effect of phaseolus angularis L. Extract on UVB-exposed HaCaT keratinocytes and possibilities as cosmetic materials. Mol. Basel Switz. 28, 1407. doi:10.3390/molecules28031407
Oku, M., Maeda, Y., Kagohashi, Y., Kondo, T., Yamada, M., Fujimoto, T., et al. (2017). Evidence for ESCRT- and clathrin-dependent microautophagy. J. Cell. Biol. 216, 3263–3274. doi:10.1083/jcb.201611029
Ott, C., König, J., Höhn, A., Jung, T., and Grune, T. (2016). Macroautophagy is impaired in old murine brain tissue as well as in senescent human fibroblasts. Redox Biol. 10, 266–273. doi:10.1016/j.redox.2016.10.015
Oulee, A., Ahn, G. S., Javadi, S. S., and Wu, J. J. (2023). Phototherapy and DNA damage: a systematic review. J. Clin. Aesthetic Dermatol. 16, 55–58.
Ouvrier, B., Ismael, S., and Bix, G. J. (2024). Senescence and SASP are potential therapeutic targets for ischemic stroke. Pharm. Basel Switz. 17, 312. doi:10.3390/ph17030312
Palma, J.-A., Martinez, J., Millar Vernetti, P., Ma, T., Perez, M. A., Zhong, J., et al. (2022). mTOR inhibition with sirolimus in multiple system atrophy: a randomized, double-blind, placebo-controlled futility trial and 1-year biomarker longitudinal analysis. Mov. Disord. 37, 778–789. doi:10.1002/mds.28923
Pan, M.-H., Zhu, C.-C., Ju, J.-Q., Xu, Y., Luo, S.-M., Sun, S.-C., et al. (2021). Single-cell transcriptome analysis reveals that maternal obesity affects DNA repair, histone methylation, and autophagy level in mouse embryos. J. Cell. Physiol. 236, 4944–4953. doi:10.1002/jcp.30201
Panwar, P., Butler, G. S., Jamroz, A., Azizi, P., Overall, C. M., and Brömme, D. (2018). Aging-associated modifications of collagen affect its degradation by matrix metalloproteinases. Matrix Biol. 65, 30–44. doi:10.1016/j.matbio.2017.06.004
Paquette, M., El-Houjeiri, L., C Zirden, L., Puustinen, P., Blanchette, P., Jeong, H., et al. (2021). AMPK-dependent phosphorylation is required for transcriptional activation of TFEB and TFE3. Autophagy 17, 3957–3975. doi:10.1080/15548627.2021.1898748
Park, N. Y., Jo, D. S., and Cho, D.-H. (2022). Post-translational modifications of ATG4B in the regulation of autophagy. Cells 11, 1330. doi:10.3390/cells11081330
Patra, S., Panigrahi, D. P., Praharaj, P. P., Bhol, C. S., Mahapatra, K. K., Mishra, S. R., et al. (2019). Dysregulation of histone deacetylases in carcinogenesis and tumor progression: a possible link to apoptosis and autophagy. Cell. Mol. Life Sci. 76, 3263–3282. doi:10.1007/s00018-019-03098-1
Pattingre, S., Espert, L., Biard-Piechaczyk, M., and Codogno, P. (2008). Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie 90, 313–323. doi:10.1016/j.biochi.2007.08.014
Peixoto, P., Grandvallet, C., Feugeas, J.-P., Guittaut, M., and Hervouet, E. (2019). Epigenetic control of autophagy in cancer cells: a key process for cancer-related phenotypes. Cells 8, 1656. doi:10.3390/cells8121656
Pellacani, G., and Argenziano, G. (2022). New insights from non-invasive imaging: from prospection of skin photodamages to training with mobile application. J. Eur. Acad. Dermatol. Venereol. 36 (6), 38–50. doi:10.1111/jdv.18197
Pellacani, G., Lim, H. W., Stockfleth, E., Sibaud, V., Brugués, A. O., and Saint Aroman, M. (2024). Photoprotection: current developments and controversies. J. Eur. Acad. Dermatol. Venereol. 38 (5), 12–20. doi:10.1111/jdv.19677
Peng, Y., Tao, Y., Liu, L., Zhang, J., and Wei, B. (2024). Crosstalk among reactive oxygen species, autophagy and metabolism in myocardial ischemia and reperfusion stages. Aging Dis. 15, 1075–1107. doi:10.14336/AD.2023.0823-4
Pereira, L., Girardi, J. P., and Bakovic, M. (2012). Forms, crosstalks, and the role of phospholipid biosynthesis in autophagy. Int. J. Cell. Biol. 2012, 931956. doi:10.1155/2012/931956
Piao, M. J., Susara Ruwan Kumara, M. H., Kim, K. C., Kang, K. A., Kang, H. K., Lee, N. H., et al. (2015). Diphlorethohydroxycarmalol suppresses ultraviolet B-induced matrix metalloproteinases via inhibition of JNK and ERK signaling in human keratinocytes. Biomol. Ther. 23, 557–563. doi:10.4062/biomolther.2015.054
Pilkington, S. M., Bulfone-Paus, S., Griffiths, C. E. M., and Watson, R. E. B. (2021). Inflammaging and the skin. J. Invest. Dermatol. 141, 1087–1095. doi:10.1016/j.jid.2020.11.006
Popelka, H., and Klionsky, D. J. (2015). One step closer to understanding mammalian macroautophagy initiation: interplay of 2 HORMA architectures in the ULK1 complex. Autophagy 11, 1953–1955. doi:10.1080/15548627.2015.1087635
Popp, L., Tran, V., Patel, R., and Segatori, L. (2018). Autophagic response to cellular exposure to titanium dioxide nanoparticles. Acta Biomater. 79, 354–363. doi:10.1016/j.actbio.2018.08.021
Popp, S., Waltering, S., Herbst, C., Moll, I., and Boukamp, P. (2002). UV-B-type mutations and chromosomal imbalances indicate common pathways for the development of Merkel and skin squamous cell carcinomas. Int. J. Cancer 99, 352–360. doi:10.1002/ijc.10321
Pozzi, M., Vantaggiato, C., Brivio, F., Orso, G., and Bassi, M. T. (2024). Olanzapine, risperidone and ziprasidone differently affect lysosomal function and autophagy, reflecting their different metabolic risk in patients. Transl. Psychiatry 14, 13. doi:10.1038/s41398-023-02686-x
Prasanth, M. I., Sivamaruthi, B. S., Chaiyasut, C., and Tencomnao, T. (2019). A review of the role of green tea (Camellia sinensis) in antiphotoaging, stress resistance, neuroprotection, and autophagy. Nutrients 11, 474. doi:10.3390/nu11020474
Prasert, S., Gavin, S., and Sawaek, W. (2023). Oxidative stress and inflammation: the root causes of aging. Explor. Med., 127–156. doi:10.37349/emed.2023.00129
Qin, D., Ren, R., Jia, C., Lu, Y., Yang, Q., Chen, L., et al. (2018a). Rapamycin protects skin fibroblasts from ultraviolet B-induced photoaging by suppressing the production of reactive oxygen species. Cell. Physiol. Biochem. 46, 1849–1860. doi:10.1159/000489369
Qin, H., Zhang, G., and Zhang, L. (2018b). GSK126 (EZH2 inhibitor) interferes with ultraviolet A radiation-induced photoaging of human skin fibroblast cells. Exp. Ther. Med. 15, 3439–3448. doi:10.3892/etm.2018.5863
Qu, C., Li, N., Liu, T., He, Y., and Miao, J. (2022). Preparation of CPD photolyase nanoliposomes derived from antarctic microalgae and their effect on UVB-induced skin damage in mice. Int. J. Mol. Sci. 23, 15148. doi:10.3390/ijms232315148
Qu, J., and Lin, Z. (2021). Autophagy regulation by crosstalk between miRNAs and ubiquitination system. Int. J. Mol. Sci. 22, 11912. doi:10.3390/ijms222111912
Rabanal-Ruiz, Y., Otten, E. G., and Korolchuk, V. I. (2017). mTORC1 as the main gateway to autophagy. Essays Biochem. 61, 565–584. doi:10.1042/EBC20170027
Rahman, M. A., Engelsen, A. S. T., Sarowar, S., Bindesbøll, C., Birkeland, E., Goplen, D., et al. (2022). Bortezomib abrogates temozolomide-induced autophagic flux through an ATG5 dependent pathway. Front. Cell. Dev. Biol. 10, 1022191. doi:10.3389/fcell.2022.1022191
Rai, S., Arasteh, M., Jefferson, M., Pearson, T., Wang, Y., Zhang, W., et al. (2019). The ATG5-binding and coiled coil domains of ATG16L1 maintain autophagy and tissue homeostasis in mice independently of the WD domain required for LC3-associated phagocytosis. Autophagy 15, 599–612. doi:10.1080/15548627.2018.1534507
Rambold, A. S., and Lippincott-Schwartz, J. (2011). Mechanisms of mitochondria and autophagy crosstalk. Cell. Cycle Georget. Tex 10, 4032–4038. doi:10.4161/cc.10.23.18384
Rangwala, R., Chang, Y. C., Hu, J., Algazy, K. M., Evans, T. L., Fecher, L. A., et al. (2014). Combined MTOR and autophagy inhibition: phase I trial of hydroxychloroquine and temsirolimus in patients with advanced solid tumors and melanoma. Autophagy 10, 1391–1402. doi:10.4161/auto.29119
Redl, A., Doberer, K., Unterluggauer, L., Kleissl, L., Krall, C., Mayerhofer, C., et al. (2024). Efficacy and safety of mTOR inhibition in cutaneous sarcoidosis: a single-centre trial. Lancet Rheumatol. 6, e81–e91. doi:10.1016/S2665-9913(23)00302-8
Ribeiro-Silva, J. C., Nolasco, P., Krieger, J. E., and Miyakawa, A. A. (2021). Dynamic crosstalk between vascular smooth muscle cells and the aged extracellular matrix. Int. J. Mol. Sci. 22, 10175. doi:10.3390/ijms221810175
Robichaud, T. K., Steffensen, B., and Fields, G. B. (2011). Exosite interactions impact matrix metalloproteinase collagen specificities. J. Biol. Chem. 286, 37535–37542. doi:10.1074/jbc.M111.273391
Robinson, K. S., Toh, G. A., Rozario, P., Chua, R., Bauernfried, S., Sun, Z., et al. (2022). ZAKα-driven ribotoxic stress response activates the human NLRP1 inflammasome. Science 377, 328–335. doi:10.1126/science.abl6324
Rodríguez-Vargas, J. M., Oliver-Pozo, F. J., and Dantzer, F. (2019). PARP1 and Poly(ADP-ribosyl)ation signaling during autophagy in response to nutrient deprivation. Oxid. Med. Cell. Longev. 2019, 2641712. doi:10.1155/2019/2641712
Romanov, J., Walczak, M., Ibiricu, I., Schüchner, S., Ogris, E., Kraft, C., et al. (2012). Mechanism and functions of membrane binding by the Atg5-Atg12/Atg16 complex during autophagosome formation. EMBO J. 31, 4304–4317. doi:10.1038/emboj.2012.278
Rubio-Tomás, T., Sotiriou, A., and Tavernarakis, N. (2023). The interplay between selective types of (macro)autophagy: mitophagy and xenophagy. Int. Rev. Cell. Mol. Biol. 374, 129–157. doi:10.1016/bs.ircmb.2022.10.003
Rui, Y.-N., Xu, Z., Chen, Z., and Zhang, S. (2015). The GST-BHMT assay reveals a distinct mechanism underlying proteasome inhibition-induced macroautophagy in mammalian cells. Autophagy 11, 812–832. doi:10.1080/15548627.2015.1034402
Ryu, T.-K., Roh, E., Shin, H.-S., and Kim, J.-E. (2022). Inhibitory effect of lotusine on solar UV-induced matrix metalloproteinase-1 expression. Plants Basel Switz. 11, 773. doi:10.3390/plants11060773
Sadick, N., Pannu, S., Abidi, Z., and Arruda, S. (2023). Topical treatments for photoaged skin. J. Drugs Dermatol. 22, 867–873. doi:10.36849/JDD.7753
Saetre, F., Hagen, L. K., Engedal, N., and Seglen, P. O. (2015). Novel steps in the autophagic-lysosomal pathway. FEBS J. 282, 2202–2214. doi:10.1111/febs.13268
Salminen, A. (2022). Clinical perspectives on the age-related increase of immunosuppressive activity. J. Mol. Med. 100, 697–712. doi:10.1007/s00109-022-02193-4
Salminen, A., Kaarniranta, K., and Kauppinen, A. (2022). Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Inflamm. Res. 71, 817–831. doi:10.1007/s00011-022-01598-8
Sample, A., and He, Y.-Y. (2017). Autophagy in UV damage response. Photochem. Photobiol. 93, 943–955. doi:10.1111/php.12691
Saoudaoui, S., Bernard, M., Cardin, G. B., Malaquin, N., Christopoulos, A., and Rodier, F. (2021). mTOR as a senescence manipulation target: a forked road. Adv. Cancer Res. 150, 335–363. doi:10.1016/bs.acr.2021.02.002
Schmidt, M. F., Gan, Z. Y., Komander, D., and Dewson, G. (2021). Ubiquitin signalling in neurodegeneration: mechanisms and therapeutic opportunities. Cell. Death Differ. 28, 570–590. doi:10.1038/s41418-020-00706-7
Schmitt, D., Bozkurt, S., Henning-Domres, P., Huesmann, H., Eimer, S., Bindila, L., et al. (2022). Lipid and protein content profiling of isolated native autophagic vesicles. EMBO Rep. 23, e53065. doi:10.15252/embr.202153065
Selarka, K., and Shravage, B. V. (2024). Illuminating intercellular autophagy: a comprehensive review of cell non-autonomous autophagy. Biochem. Biophys. Res. Commun. 716, 150024. doi:10.1016/j.bbrc.2024.150024
Seo, M.-Y., Chung, S.-Y., Choi, W.-K., Seo, Y.-K., Jung, S.-H., Park, J.-M., et al. (2009). Anti-aging effect of rice wine in cultured human fibroblasts and keratinocytes. J. Biosci. Bioeng. 107, 266–271. doi:10.1016/j.jbiosc.2008.11.016
Shang, H., VanDusseldorp, T. A., Ma, R., Zhao, Y., Cholewa, J., Zanchi, N. E., et al. (2022). Role of MST1 in the regulation of autophagy and mitophagy: implications for aging-related diseases. J. Physiol. Biochem. 78, 709–719. doi:10.1007/s13105-022-00904-6
Shatz, O., and Elazar, Z. (2024). The physiological relevance of autophagosome morphogenesis. Trends Biochem. Sci. 49, 569–572. doi:10.1016/j.tibs.2024.05.002
Shivakumar, S., and Jafferany, M. (2020). “The unfair drive to be fair”: psychosocial aspects and implications of the use of skin lightening agents. Dermatol. Ther. 33, e14091. doi:10.1111/dth.14091
Son, J., Bailey, J. T., Worrell, S., and Glick, A. B. (2024). IRE1α regulates ROS and immune responses after UVB irradiation. Redox Exp. Med. 2024, e230030. doi:10.1530/rem-23-0030
Song, H., Zhu, Y., Hu, C., Liu, Q., Jin, Y., Tang, P., et al. (2024). Selective autophagy receptor NBR1 retards nucleus pulposus cell senescence by directing the clearance of SRBD1. Int. J. Biol. Sci. 20, 701–717. doi:10.7150/ijbs.90186
Song, X., Narzt, M. S., Nagelreiter, I. M., Hohensinner, P., Terlecki-Zaniewicz, L., Tschachler, E., et al. (2017). Autophagy deficient keratinocytes display increased DNA damage, senescence and aberrant lipid composition after oxidative stress in vitro and in vivo. Redox Biol. 11, 219–230. doi:10.1016/j.redox.2016.12.015
Stacchiotti, A., and Corsetti, G. (2020). Natural compounds and autophagy: allies against neurodegeneration. Front. Cell. Dev. Biol. 8, 555409. doi:10.3389/fcell.2020.555409
Sun, Y., Liu, W.-Z., Liu, T., Feng, X., Yang, N., and Zhou, H.-F. (2015). Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 35, 600–604. doi:10.3109/10799893.2015.1030412
Suresh, S. N., Chakravorty, A., Giridharan, M., Garimella, L., and Manjithaya, R. (2020). Pharmacological tools to modulate autophagy in neurodegenerative diseases. J. Mol. Biol. 432, 2822–2842. doi:10.1016/j.jmb.2020.02.023
Svobodova, A., Walterova, D., and Vostalova, J. (2006). Ultraviolet light induced alteration to the skin. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czechoslov. 150, 25–38. doi:10.5507/bp.2006.003
Szustka, A., Kozal, K., and Krześlak, A. (2024). RING1 inhibition has a cell-specific antitumoral role by promoting autophagy in endometrial cancer cells. Cell. Physiol. Biochem. 58, 1–13. doi:10.33594/000000679
Tabata, K., Imai, K., Fukuda, K., Yamamoto, K., Kunugi, H., Fujita, T., et al. (2024). Palmitoylation of ULK1 by ZDHHC13 plays a crucial role in autophagy. Nat. Commun. 15, 7194. doi:10.1038/s41467-024-51402-w
Tamura, H., Shibata, M., Koike, M., Sasaki, M., and Uchiyama, Y. (2010). Atg9A protein, an autophagy-related membrane protein, is localized in the neurons of mouse brains. J. Histochem. Cytochem. 58, 443–453. doi:10.1369/jhc.2010.955690
Tan, M., Zhang, Q.-B., Liu, T.-H., Yang, Y.-Y., Zheng, J.-X., Zhou, W.-J., et al. (2020). Autophagy dysfunction may be involved in the pathogenesis of ankylosing spondylitis. Exp. Ther. Med. 20, 3578–3586. doi:10.3892/etm.2020.9116
Tanaka, K., Asamitsu, K., Uranishi, H., Iddamalgoda, A., Ito, K., Kojima, H., et al. (2010). Protecting skin photoaging by NF-kappaB inhibitor. Curr. Drug Metab. 11, 431–435. doi:10.2174/138920010791526051
Tang, Q.-Q., Wang, Z.-D., An, X.-H., Zhou, X.-Y., Zhang, R.-Z., Zhan, X., et al. (2024a). Apigenin ameliorates H2O2-induced oxidative damage in melanocytes through nuclear factor-E2-related factor 2 (Nrf2) and phosphatidylinositol 3-kinase (PI3K)/Protein kinase B (Akt)/Mammalian target of rapamycin (mTOR) pathways and reducing the generation of reactive oxygen species (ROS) in zebrafish. Pharm. Basel Switz. 17, 1302. doi:10.3390/ph17101302
Tang, X., Yang, T., Yu, D., Xiong, H., and Zhang, S. (2024b). Current insights and future perspectives of ultraviolet radiation (UV) exposure: friends and foes to the skin and beyond the skin. Environ. Int. 185, 108535. doi:10.1016/j.envint.2024.108535
Tang, Y., Liu, J., Wang, Y., Yang, L., Han, B., Zhang, Y., et al. (2021). PARP14 inhibits microglial activation via LPAR5 to promote post-stroke functional recovery. Autophagy 17, 2905–2922. doi:10.1080/15548627.2020.1847799
Tang, Z., Liu, Z., Zhang, Y., Luo, S., Xu, Y., and Ren, L. (2024c). Functional hyaluronic acid microneedles for skin photoaging based on collagen induction and oxidative stress regulation strategies. Int. J. Biol. Macromol. 277, 134080. doi:10.1016/j.ijbiomac.2024.134080
Thornfeldt, C. R., and Rizer, R. L. (2016). Superior efficacy of an herbal-based cosmeceutical compared with common prescription and cosmetic antiaging therapies. J. Drugs Dermatol. 15, 218–223.
Tomasello, B., Malfa, G. A., Acquaviva, R., La Mantia, A., and Di Giacomo, C. (2022). Phytocomplex of a standardized extract from red orange (Citrus sinensis L. Osbeck) against photoaging. Cells 11, 1447. doi:10.3390/cells11091447
Tran, M., and Reddy, P. H. (2020). Defective autophagy and mitophagy in aging and Alzheimer’s disease. Front. Neurosci. 14, 612757. doi:10.3389/fnins.2020.612757
Tsuchiya, Y., Asano, T., Nakayama, K., Kato, T., Karin, M., and Kamata, H. (2010). Nuclear IKKbeta is an adaptor protein for IkappaBalpha ubiquitination and degradation in UV-induced NF-kappaB activation. Mol. Cell. 39, 570–582. doi:10.1016/j.molcel.2010.07.030
Türei, D., Földvári-Nagy, L., Fazekas, D., Módos, D., Kubisch, J., Kadlecsik, T., et al. (2015). Autophagy Regulatory Network - a systems-level bioinformatics resource for studying the mechanism and regulation of autophagy. Autophagy 11, 155–165. doi:10.4161/15548627.2014.994346
Tuteja, N., Ahmad, P., Panda, B. B., and Tuteja, R. (2009). Genotoxic stress in plants: shedding light on DNA damage, repair and DNA repair helicases. Mutat. Res. 681, 134–149. doi:10.1016/j.mrrev.2008.06.004
Umar, S. A., Tanveer, M. A., Nazir, L. A., Divya, G., Vishwakarma, R. A., and Tasduq, S. A. (2019). Glycyrrhizic acid prevents oxidative stress mediated DNA damage response through modulation of autophagy in ultraviolet-B-irradiated human primary dermal fibroblasts. Cell. Physiol. Biochem. 53, 242–257. doi:10.33594/000000133
Varga, V. B., Keresztes, F., Sigmond, T., Vellai, T., and Kovács, T. (2022). The evolutionary and functional divergence of the Atg8 autophagy protein superfamily. Biol. Futura 73, 375–384. doi:10.1007/s42977-022-00123-6
Vargas, J. N. S., Hamasaki, M., Kawabata, T., Youle, R. J., and Yoshimori, T. (2023). The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell. Biol. 24, 167–185. doi:10.1038/s41580-022-00542-2
Victorelli, S., Salmonowicz, H., Chapman, J., Martini, H., Vizioli, M. G., Riley, J. S., et al. (2023). Apoptotic stress causes mtDNA release during senescence and drives the SASP. Nature 622, 627–636. doi:10.1038/s41586-023-06621-4
Vikram, A., Patel, S. K., Singh, A., Pathania, D., Ray, R. S., Upadhyay, A. K., et al. (2024). Natural autophagy activators: a promising strategy for combating photoaging. Phytomedicine 132, 155508. doi:10.1016/j.phymed.2024.155508
Vlada, C. A., Kim, J.-S., and Behrns, K. E. (2015). Autophagy: self-preservation through cannibalism of proteins and organelles. Surgery 157, 1–5. doi:10.1016/j.surg.2014.07.014
Voegeli, R., Monneuse, J.-M., Schoop, R., Summers, B., and Rawlings, A. V. (2017). The effect of photodamage on the female Caucasian facial stratum corneum corneome using mass spectrometry-based proteomics. Int. J. Cosmet. Sci. 39, 637–652. doi:10.1111/ics.12426
Wang, B.-J., Chen, Y.-Y., Chang, H.-H., Chen, R.-J., Wang, Y.-J., and Lee, Y.-H. (2024a). Zinc oxide nanoparticles exacerbate skin epithelial cell damage by upregulating pro-inflammatory cytokines and exosome secretion in M1 macrophages following UVB irradiation-induced skin injury. Part. Fibre Toxicol. 21, 9. doi:10.1186/s12989-024-00571-z
Wang, C., Wang, H., Zhang, D., Luo, W., Liu, R., Xu, D., et al. (2018). Phosphorylation of ULK1 affects autophagosome fusion and links chaperone-mediated autophagy to macroautophagy. Nat. Commun. 9, 3492. doi:10.1038/s41467-018-05449-1
Wang, D.-K., Zheng, H.-L., Zhou, W.-S., Duan, Z.-W., Jiang, S.-D., Li, B., et al. (2022a). Mitochondrial dysfunction in oxidative stress-mediated intervertebral disc degeneration. Orthop. Surg. 14, 1569–1582. doi:10.1111/os.13302
Wang, J., Gu, L., Shi, Z., Xu, Z., Zhai, X., Zhou, S., et al. (2024b). 5-aminolevulinic acid photodynamic therapy protects against UVB-induced skin photoaging: a DNA-repairing mechanism involving the BER signalling pathway. J. Cell. Mol. Med. 28, e18536. doi:10.1111/jcmm.18536
Wang, J., Huang, H., Tao, K., Guo, L., Hu, X., and Chang, H. (2024c). Novel Thermus thermophilus and Bacillus subtilis mixed-culture ferment extract provides potent skin benefits in vitro and protects skin from aging. J. Cosmet. Dermatol. 23, 4334–4342. doi:10.1111/jocd.16531
Wang, K., Hu, X., Xie, X.-L., Huang, M., Wang, D., and Yu, F.-L. (2024d). Phytocosmetic potential of Blumea balsamifera oil in mitigating UV-induced photoaging: evidence from cellular and mouse models. J. Ethnopharmacol. 334, 118535. doi:10.1016/j.jep.2024.118535
Wang, L., Klionsky, D. J., and Shen, H.-M. (2023a). The emerging mechanisms and functions of microautophagy. Nat. Rev. Mol. Cell. Biol. 24, 186–203. doi:10.1038/s41580-022-00529-z
Wang, M., Charareh, P., Lei, X., and Zhong, J. L. (2019). Autophagy: multiple mechanisms to protect skin from ultraviolet radiation-driven photoaging. Oxid. Med. Cell. Longev. 2019, 8135985. doi:10.1155/2019/8135985
Wang, M., Lei, M., Chang, L., Xing, Y., Guo, Y., Pourzand, C., et al. (2021). Bach2 regulates autophagy to modulate UVA-induced photoaging in skin fibroblasts. Free Radic. Biol. Med. 169, 304–316. doi:10.1016/j.freeradbiomed.2021.04.003
Wang, S., Li, H., Yuan, M., Fan, H., and Cai, Z. (2022b). Role of AMPK in autophagy. Front. Physiol. 13, 1015500. doi:10.3389/fphys.2022.1015500
Wang, T., Sun, G., and Tao, B. (2024e). Updated insights into the NLRP3 inflammasome in postoperative cognitive dysfunction: emerging mechanisms and treatments. Front. Aging Neurosci. 16, 1480502. doi:10.3389/fnagi.2024.1480502
Wang, X., Shi, W., Wang, K., Ye, Z., Wang, R., Wang, H., et al. (2023b). Rationally designed nonapeptides with great skin photoprotective effect through producing type 1 collagen and blocking the mTOR pathway. J. Med. Chem. 66, 7615–7628. doi:10.1021/acs.jmedchem.3c00503
Wang, Z., Du, J., Ma, W., Diao, X., Liu, Q., and Liu, G. (2024f). Bacteriocins attenuate Listeria monocytogenes-induced intestinal barrier dysfunction and inflammatory response. Appl. Microbiol. Biotechnol. 108, 384. doi:10.1007/s00253-024-13228-w
Wang, Z., Li, Z., Lei, Y., Liu, Y., Feng, Y., Chen, D., et al. (2022c). Recombinant photolyase-thymine alleviated UVB-induced photodamage in mice by repairing CPD photoproducts and ameliorating oxidative stress. Antioxid. Basel Switz. 11, 2312. doi:10.3390/antiox11122312
Watanabe, Y., Taguchi, K., and Tanaka, M. (2023). Roles of stress response in autophagy processes and aging-related diseases. Int. J. Mol. Sci. 24, 13804. doi:10.3390/ijms241813804
Wen, S.-Y., Ng, S.-C., Chiu, Y.-T., Tai, P.-Y., Chen, T.-J., Chen, C.-J., et al. (2024). Enhanced SIRT1 activity by galangin mitigates UVB-induced senescence in dermal fibroblasts via p53 acetylation regulation and activation. J. Agric. Food Chem. 72, 23286–23294. doi:10.1021/acs.jafc.4c05945
Whitmarsh-Everiss, T., and Laraia, L. (2021). Small molecule probes for targeting autophagy. Nat. Chem. Biol. 17, 653–664. doi:10.1038/s41589-021-00768-9
Widmer, R., Ziaja, I., and Grune, T. (2006). Protein oxidation and degradation during aging: role in skin aging and neurodegeneration. Free Radic. Res. 40, 1259–1268. doi:10.1080/10715760600911154
Wu, N.-L., Lee, T.-A., Wang, S.-F., Li, H.-J., Chen, H.-T., Chien, T.-C., et al. (2016). Green fluorescent protein chromophore derivative suppresses ultraviolet A-induced JNK-signalling and apoptosis in keratinocytes and adverse effects in zebrafish embryos. Exp. Dermatol. 25, 983–990. doi:10.1111/exd.13168
Wu, X., Shell, S. M., Liu, Y., and Zou, Y. (2007). ATR-dependent checkpoint modulates XPA nuclear import in response to UV irradiation. Oncogene 26, 757–764. doi:10.1038/sj.onc.1209828
Wu, Y., Wang, A., Feng, G., Pan, X., Shuai, W., Yang, P., et al. (2024). Autophagy modulation in cancer therapy: challenges coexist with opportunities. Eur. J. Med. Chem. 276, 116688. doi:10.1016/j.ejmech.2024.116688
Wurzer, B., Zaffagnini, G., Fracchiolla, D., Turco, E., Abert, C., Romanov, J., et al. (2015). Oligomerization of p62 allows for selection of ubiquitinated cargo and isolation membrane during selective autophagy. eLife 4, e08941. doi:10.7554/eLife.08941
Xia, Y., Zhang, H., Wu, X., Xu, Y., and Tan, Q. (2024). Resveratrol activates autophagy and protects from UVA-induced photoaging in human skin fibroblasts and the skin of male mice by regulating the AMPK pathway. Biogerontology 25, 649–664. doi:10.1007/s10522-024-10099-6
Xie, H., Zhou, L., Liu, F., Long, J., Yan, S., Xie, Y., et al. (2022a). Autophagy induction regulates aquaporin 3-mediated skin fibroblast ageing. Br. J. Dermatol. 186, 318–333. doi:10.1111/bjd.20662
Xie, L., Guo, Y., Ren, C., Cao, Y., Li, J., Lin, J., et al. (2022b). Unravelling the consecutive glycosylation and methylation of flavonols in peach in response to UV-B irradiation. Plant Cell. Environ. 45, 2158–2175. doi:10.1111/pce.14323
Xiong, W., Knispel, R. A., Dietz, H. C., Ramirez, F., and Baxter, B. T. (2008). Doxycycline delays aneurysm rupture in a mouse model of Marfan syndrome. J. Vasc. Surg. 47, 166–172. doi:10.1016/j.jvs.2007.09.016
Xu, D., Li, C., and Zhao, M. (2022a). Attenuation of UV-induced skin photoaging in rats by walnut protein hydrolysates is linked to the modulation of MAPK/AP-1 and TGF-β/Smad signaling pathways. Food Funct. 13, 609–623. doi:10.1039/d1fo02598h
Xu, D., Li, C., and Zhao, M. (2022b). Theragra chalcogramma hydrolysate, rich in Gly-Leu-pro-Ser-Tyr-Thr, alleviates photoaging via modulating deposition of collagen fibers and restoration of extracellular components matrix in SD rats. Mar. Drugs 20, 252. doi:10.3390/md20040252
Xu, D., Zhao, M., Lin, H., and Li, C. (2022c). Theragra chalcogramma hydrolysates, rich in Gly-Leu-pro-Ser-Tyr-Thr, exerts anti-photoaging potential via targeting MAPK and NF-κB pathways in SD rats. Mar. Drugs 20, 286. doi:10.3390/md20050286
Xu, Y., and Wan, W. (2023). Acetylation in the regulation of autophagy. Autophagy 19, 379–387. doi:10.1080/15548627.2022.2062112
Xu, Z., Li, J., Su, B., Gao, H., Ren, M., Lin, Y., et al. (2024). A role of ROS-dependent defects in mitochondrial dynamic and autophagy in carbon black nanoparticle-mediated myocardial cell damage. Free Radic. Biol. Med. 220, 249–261. doi:10.1016/j.freeradbiomed.2024.04.241
Xue, H., Hu, Z., Liu, S., Zhang, S., Yang, W., Li, J., et al. (2024). The mechanism of NF-κB-TERT feedback regulation of granulosa cell apoptosis in PCOS rats. PLoS One 19, e0312115. doi:10.1371/journal.pone.0312115
Yaar, M., and Gilchrest, B. A. (2007). Photoageing: mechanism, prevention and therapy. Br. J. Dermatol. 157, 874–887. doi:10.1111/j.1365-2133.2007.08108.x
Yagura, T., Schuch, A. P., Garcia, C. C. M., Rocha, C. R. R., Moreno, N. C., Angeli, J. P. F., et al. (2017). Direct participation of DNA in the formation of singlet oxygen and base damage under UVA irradiation. Free Radic. Biol. Med. 108, 86–93. doi:10.1016/j.freeradbiomed.2017.03.018
Yamamoto, H., and Matsui, T. (2024). Molecular mechanisms of macroautophagy, microautophagy, and chaperone-mediated autophagy. J. Nippon. Med. Sch. 91, 2–9. doi:10.1272/jnms.JNMS.2024_91-102
Yamamoto, T., Takabatake, Y., Minami, S., Sakai, S., Fujimura, R., Takahashi, A., et al. (2021). Eicosapentaenoic acid attenuates renal lipotoxicity by restoring autophagic flux. Autophagy 17, 1700–1713. doi:10.1080/15548627.2020.1782034
Yan, T., Huang, L., Yan, Y., Zhong, Y., Xie, H., and Wang, X. (2023). MAPK/AP-1 signaling pathway is involved in the protection mechanism of bone marrow mesenchymal stem cells-derived exosomes against ultraviolet-induced photoaging in human dermal fibroblasts. Skin. Pharmacol. Physiol. 36, 98–106. doi:10.1159/000529551
Yang, J., Zhou, R., and Ma, Z. (2019). Autophagy and energy metabolism. Adv. Exp. Med. Biol. 1206, 329–357. doi:10.1007/978-981-15-0602-4_16
Yang, T., Geng, F., Tang, X., Yu, Z., Liu, Y., Song, B., et al. (2024a). UV radiation-induced peptides in frog skin confer protection against cutaneous photodamage through suppressing MAPK signaling. MedComm 5, e625. doi:10.1002/mco2.625
Yang, W., Ju, J., Lee, K., Nam, K., Oh, S., and Shin, I. (2013). Protein kinase B/Akt1 inhibits autophagy by down-regulating UVRAG expression. Exp. Cell. Res. 319, 122–133. doi:10.1016/j.yexcr.2012.11.014
Yang, Y., Liu, L., Tian, Y., Gu, M., Wang, Y., Ashrafizadeh, M., et al. (2024b). Autophagy-driven regulation of cisplatin response in human cancers: exploring molecular and cell death dynamics. Cancer Lett. 587, 216659. doi:10.1016/j.canlet.2024.216659
Yano, S., Komine, M., Fujimoto, M., Okochi, H., and Tamaki, K. (2003). Interleukin 15 induces the signals of epidermal proliferation through ERK and PI 3-kinase in a human epidermal keratinocyte cell line, HaCaT. Biochem. Biophys. Res. Commun. 301, 841–847. doi:10.1016/s0006-291x(03)00060-3
Yao, W., Feng, Y., Zhang, Y., Yang, H., and Yi, C. (2024). The molecular mechanisms regulating the assembly of the autophagy initiation complex. BioEssays 46, e2300243. doi:10.1002/bies.202300243
Ye, Y., Lin, P., Zhang, W., Tan, S., Zhou, X., Li, R., et al. (2017). DNA repair interacts with autophagy to regulate inflammatory responses to pulmonary hyperoxia. J. Immunol. 198, 2844–2853. doi:10.4049/jimmunol.1601001
Yeo, B. K., Hong, C. J., Chung, K. M., Woo, H., Kim, K., Jung, S., et al. (2016). Valosin-containing protein is a key mediator between autophagic cell death and apoptosis in adult hippocampal neural stem cells following insulin withdrawal. Mol. Brain 9, 31. doi:10.1186/s13041-016-0212-8
Yoon, S., Kim, M., Shin, S., Woo, J., Son, D., Ryu, D., et al. (2022). Effect of Cirsium japonicum flower extract on skin aging induced by glycation. Mol. Basel Switz. 27, 2093. doi:10.3390/molecules27072093
Yu, P., Zhang, J., Yu, S., Luo, Z., Hua, F., Yuan, L., et al. (2015a). Protective effect of sevoflurane postconditioning against cardiac ischemia/reperfusion injury via ameliorating mitochondrial impairment, oxidative stress and rescuing autophagic clearance. PLoS One 10, e0134666. doi:10.1371/journal.pone.0134666
Yu, S., Li, Y., Lu, X., Han, Z., Li, C., Yuan, X., et al. (2024a). The regulatory role of miRNA and lncRNA on autophagy in diabetic nephropathy. Cell. Signal. 118, 111144. doi:10.1016/j.cellsig.2024.111144
Yu, T., Zuber, J., and Li, J. (2015b). Targeting autophagy in skin diseases. J. Mol. Med. (Berl) 93, 31–38. doi:10.1007/s00109-014-1225-3
Yu, Z.-W., Zheng, M., Fan, H.-Y., Liang, X.-H., and Tang, Y.-L. (2024b). Ultraviolet (UV) radiation: a double-edged sword in cancer development and therapy. Mol. Biomed. 5, 49. doi:10.1186/s43556-024-00209-8
Yuan, M., Fu, H., Mo, Q., Wang, S., Wang, C., Wang, D., et al. (2024). Protective mechanism of Rosa roxburghii tratt fermentation broth against ultraviolet-A-induced photoaging of human embryonic skin fibroblasts. Antioxid. Basel Switz. 13, 382. doi:10.3390/antiox13030382
Yuan, Q., Tang, B., and Zhang, C. (2022). Signaling pathways of chronic kidney diseases, implications for therapeutics. Signal Transduct. Target. Ther. 7, 182. doi:10.1038/s41392-022-01036-5
Yuksel, E. (2020). Epigenetic and environmental risk factors in skin cancer. Int. J. Sci. Technol. Res. 6, 179–183. doi:10.7176/jstr/6-03-21
Yuksel Egrilmez, M., Kocturk, S., Aktan, S., Oktay, G., Resmi, H., Simsek Keskin, H., et al. (2022). Melatonin prevents UVB-induced skin photoaging by inhibiting oxidative damage and MMP expression through JNK/AP-1 signaling pathway in human dermal fibroblasts. Life (Basel) 12, 950. doi:10.3390/life12070950
Yun, Y., Baek, A., and Kim, D.-E. (2021). Autophagy down-regulates NLRP3-dependent inflammatory response of intestinal epithelial cells under nutrient deprivation. BMB Rep. 54, 260–265. doi:10.5483/BMBRep.2021.54.5.211
Zachari, M., and Ganley, I. G. (2017). The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 61, 585–596. doi:10.1042/EBC20170021
Zamanian, M. Y., Golmohammadi, M., Yumashev, A., Hjazi, A., Toama, M. A., AbdRabou, M. A., et al. (2024). Effects of metformin on cancers in experimental and clinical studies: focusing on autophagy and AMPK/mTOR signaling pathways. Cell. Biochem. Funct. 42, e4071. doi:10.1002/cbf.4071
Zand, A., Enkhbilguun, S., Macharia, J. M., Varajti, K., Szabó, I., Gerencsér, G., et al. (2024). Betanin attenuates epigenetic mechanisms and UV-induced DNA fragmentation in HaCaT cells: implications for skin cancer chemoprevention. Nutrients 16, 860. doi:10.3390/nu16060860
Zha, S., Li, Z., Chen, S., Liu, F., and Wang, F. (2019). MeCP2 inhibits cell functionality through FoxO3a and autophagy in endothelial progenitor cells. Aging 11, 6714–6733. doi:10.18632/aging.102183
Zhang, C., Yang, Y., Gao, Y., and Sun, D. (2022a). NaF-induced neurotoxicity via activation of the IL-1β/JNK signaling pathway. Toxicology 469, 153132. doi:10.1016/j.tox.2022.153132
Zhang, J., Wang, P., Wan, L., Xu, S., and Pang, D. (2017). The emergence of noncoding RNAs as Heracles in autophagy. Autophagy 13, 1004–1024. doi:10.1080/15548627.2017.1312041
Zhang, J., Xu, Y., Ruan, X., Zhang, T., Zi, M., and Zhang, Q. (2024). Photoprotective effects of epigallocatechin gallate on ultraviolet-induced zebrafish and human skin fibroblasts cells. Mediat. Inflamm. 2024, 7887678. doi:10.1155/2024/7887678
Zhang, J.-A., Zhou, B.-R., Xu, Y., Chen, X., Liu, J., Gozali, M., et al. (2016). MiR-23a-depressed autophagy is a participant in PUVA- and UVB-induced premature senescence. Oncotarget 7, 37420–37435. doi:10.18632/oncotarget.9357
Zhang, L., Feng, G., Yang, S., Liu, B., Niu, Y., Fan, P., et al. (2021). Polyethylenimine-modified mesoporous silica nanoparticles induce a survival mechanism in vascular endothelial cells via microvesicle-mediated autophagosome release. ACS Nano 15, 10640–10658. doi:10.1021/acsnano.1c03456
Zhang, S., Peng, X., Yang, S., Li, X., Huang, M., Wei, S., et al. (2022b). The regulation, function, and role of lipophagy, a form of selective autophagy, in metabolic disorders. Cell. Death Dis. 13, 132. doi:10.1038/s41419-022-04593-3
Zhang, Y., Wang, L., Bai, L., Jiang, R., Wu, J., and Li, Y. (2022c). Ebosin attenuates the inflammatory responses induced by TNF-α through inhibiting NF-κB and MAPK pathways in rat fibroblast-like synoviocytes. J. Immunol. Res. 2022, 9166370. doi:10.1155/2022/9166370
Zhao, B., Qiang, L., Joseph, J., Kalyanaraman, B., Viollet, B., and He, Y.-Y. (2016). Mitochondrial dysfunction activates the AMPK signaling and autophagy to promote cell survival. Genes Dis. 3, 82–87. doi:10.1016/j.gendis.2015.12.002
Zhao, C., Wu, S., and Wang, H. (2025). Medicinal plant extracts targeting UV-induced skin damage: molecular mechanisms and therapeutic potential. Int. J. Mol. Sci. 26, 2278. doi:10.3390/ijms26052278
Zhao, P., Ma, G., and Ma, L. (2023). miR-181a-5p targets DDX3X to inhibit the progression of osteoarthritis via NF-ΚB signaling pathway. J. Orthop. Surg. 18, 606. doi:10.1186/s13018-023-04073-0
Zhao, Y., Lin, J., Li, J., Bwalya, C., Xu, Y., Niu, Y., et al. (2022). RhFGF21 protects epidermal cells against UVB-induced apoptosis through activating AMPK-mediated autophagy. Int. J. Mol. Sci. 23, 12466. doi:10.3390/ijms232012466
Zhao, Z., Oh, S., Li, D., Ni, D., Pirooz, S. D., Lee, J.-H., et al. (2012). A dual role for UVRAG in maintaining chromosomal stability independent of autophagy. Dev. Cell. 22, 1001–1016. doi:10.1016/j.devcel.2011.12.027
Zhong, X., Deng, Y., Yang, H., Du, X., Liu, P., and Du, Y. (2024). Role of autophagy in skin photoaging: a narrative review. Med. (Baltimore) 103, e37178. doi:10.1097/MD.0000000000037178
Zhou, B., Yin, H., Xu, Y., Wu, D., Zhang, Z., Yin, Z., et al. (2012). Baicalin protects human skin fibroblasts from ultraviolet A radiation-induced oxidative damage and apoptosis. Free Radic. Res. 46, 1458–1471. doi:10.3109/10715762.2012.726355
Zhou, J., Li, X.-Y., Liu, Y.-J., Feng, J., Wu, Y., Shen, H.-M., et al. (2022). Full-coverage regulations of autophagy by ROS: from induction to maturation. Autophagy 18, 1240–1255. doi:10.1080/15548627.2021.1984656
Zou, H., Zhuo, L., Han, T., Hu, D., Yang, X., Wang, Y., et al. (2015). Autophagy and gap junctional intercellular communication inhibition are involved in cadmium-induced apoptosis in rat liver cells. Biochem. Biophys. Res. Commun. 459, 713–719. doi:10.1016/j.bbrc.2015.03.027
Zou, Z., Chang, H., Li, H., and Wang, S. (2017). Induction of reactive oxygen species: an emerging approach for cancer therapy. Apoptosis Int. J. Program. Cell. Death 22, 1321–1335. doi:10.1007/s10495-017-1424-9
Glossary
UV Ultraviolet
UVB Ultraviolet B
UVA Ultraviolet A
ROS Reactive Oxygen Species
AMPK AMP-Activated Protein Kinase
mTOR Mechanistic Target of Rapamycin
CPD Cyclobutane Pyrimidine Dimer
MMP Matrix Metalloproteinase
NF-κB Nuclear Factor kappa-light-chain-enhancer of activated B cells
ERK Extracellular Signal-Regulated Kinase
JNK c-Jun N-terminal Kinase
ATG Autophagy-Related Gene
LC3 Microtubule-Associated Protein 1A/1B-Light Chain 3
ULK1 Unc-51-like Autophagy Activating Kinase 1
FIP200 Focal Adhesion Kinase Family Interacting Protein of 200 kDa
Beclin-1 Autophagy-Related Protein 6
UVRAG UV Radiation Resistance-Associated Gene
PINK1 PTEN-Induced Kinase 1
Parkin E3 Ubiquitin-Protein Ligase
PI3K Phosphoinositide 3-Kinase
ATG5 Autophagy-Related Protein 5
ATG7 Autophagy-Related Protein 7
ATG3 Autophagy-Related Protein 3
PE Phosphatidylethanolamine
CMA Chaperone-Mediated Autophagy
miRNA MicroRNA
lncRNA Long Non-Coding RNA
ATG8 Autophagy-Related Protein 8
mTORC2 Mechanistic Target of Rapamycin Complex 2
SRBD1 SREB Binding Domain 1
ATG4D Autophagy-Related Gene 4D.
Keywords: photoaging, autophagy, UV, ROS, skin aging
Citation: Zhang Z, Tan R, Xiong Z, Feng Y and Chen L (2025) Dysregulation of autophagy during photoaging reduce oxidative stress and inflammatory damage caused by UV. Front. Pharmacol. 16:1562845. doi: 10.3389/fphar.2025.1562845
Received: 18 January 2025; Accepted: 21 April 2025;
Published: 12 May 2025.
Edited by:
Burkhard Hinz, Rostock University Medical Center, GermanyReviewed by:
Kushneet Kaur Sodhi, University of Delhi, IndiaChandra Kant Singh, University of Delhi, India
Wahyu Widowati, Maranatha Christian University, Indonesia
Copyright © 2025 Zhang, Tan, Xiong, Feng and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanyan Feng, Znl5bWVkQDE2My5jb20=; Long Chen, Y2hlbmxvbmdAY21jLmVkdS5jbg==
 Zhongsong Zhang
Zhongsong Zhang Run Tan2,3
Run Tan2,3 Long Chen
Long Chen