- 1Department of Pharmacy, Shenzhen People’s Hospital (The Second Clinical Medical College, Jinan University, The First Affiliated Hospital, Southern University of Science and Technology), Shenzhen, China
- 2Department of Pharmacy, General Hospital of Southern Theatre Command, PLA, Guangzhou, China
- 3Department of Pharmacy, The Third People’s Hospital of Longgang District Shenzhen, Shenzhen, China
- 4Department of Pharmacy, Shaoxing People’s Hospital, Shaoxing, China
- 5Health Management Center, Shenzhen University General Hospital, Shenzhen, China
Aim: This study was to investigate the association between oral anticoagulant use and 28-day mortality and in-hospital mortality in patients with acute respiratory distress syndrome (ARDS).
Methods: A total of 1754 ARDS patients were identified in database from 2008 to 2022 in this cohort study. Univariable and multivariable cox regression models were applied to assess the associations of oral anticoagulant use with the risk of 28-day mortality and in-hospital mortality. Propensity score matching (PSM) was performed in ARDS patients according to whether they were taking oral anticoagulants or not to control potential bias. Subgroup analysis was performed according to severity of ARDS (mild, moderate, and severe), and comorbidities (atrial fibrillation, sepsis, and AKI). Hazards ratio (HR) and respective confidence interval (CI) were presented.
Results: In total, 7758 patients not receiving oral anticoagulant and 905 patients receiving oral anticoagulant. The reduced risk of 28-day mortality in ARDS patients was identified in those undergoing oral anticoagulant use (HR = 0.32, 95%CI: 0.24–0.44). Oral anticoagulant use was associated with reduced risk of in-hospital mortality (HR = 0.27, 95%CI: 0.20–0.37). After adjusting for the respective confounding factors, the associations of Warfarin with decreased risk of 28-day and in-hospital mortality were not significant (P > 0.05).
Conclusion: Oral anticoagulant was related to decreased risk of 28-day/in-hospital mortality in patients with ARDS. Warfarin and novel oral anticoagulants showed no significant difference on 28-day/in-hospital mortality in patients with ARDS.
Introduction
Acute respiratory distress syndrome (ARDS) is a clinical syndrome characterized by acute respiratory failure resulting from non-cardiogenic pulmonary edema (Bos and Ware, 2022). ARDS is among the most prevalent conditions in intensive care units and constitutes a significant cause of short-term mortality, imposing an enormous disease burden globally (Meyer et al., 2021). ARDS is defined by inflammatory lung injury and is associated with a global mortality rate approaching 40% (Bellani et al., 2016). To identify factors associated with short-term mortality was essential for improving the prognosis of these patients.
Pulmonary coagulopathy is a vital participant in the pathophysiology of ARDS, which is characterized by activated coagulation and decreased fibrinolysis (Camprubí-Rimblas et al., 2018; Nieuwenhuizen et al., 2009). Previous evidence indicated that coagulation function is an important factor affecting organ failure and in-hospital mortality in ARDS patients, which may be related to the increased level of inflammation associated with hypercoagulability (Gando et al., 2020; Li and Pan, 2024). Thus, the function of anticoagulants has been explored. Studies have found that early use of heparin may be associated with a reduced risk of short-term death in patients with ARDS (Xiao et al., 2024), but the conclusions are not consistent (Hofstra et al., 2012). Previous evidence also indicated that the impact of chronic oral anticoagulation therapy on the clinical outcomes of hospitalized patients with COVID-19 remains a subject of ongoing debate (Flam et al., 2021; Denas et al., 2021). Russo et al. revealed that oral anticoagulation therapy did not influence the risk of ARDS or death in patients hospitalized with COVID-19 (Russo et al., 2022). Wenzler et al. identified that apixaban for therapeutic anticoagulation was safe and efficacious in ICU patients with severe COVID-19 respiratory disease (Wenzler et al., 2020). Thus, the role of oral anticoagulation use on the prognosis of ARDS patients still require investigation. In addition, due to the need for injection of heparin and its possible adverse reactions such as heparin-induced thrombocytopenia (HIT), oral anticoagulants are gradually being used in critically ill patients (Wahab et al., 2021). In critically ill patients with both atrial fibrillation and sepsis or atrial fibrillation and acute kidney injury, the use of oral anticoagulants was found to be significantly associated with reduced 30-day mortality risk, whereas no significant difference was found between warfarin and novel oral anticoagulants (Ge et al., 2024; Bo et al., 2023). To date, no studies have investigated the relationship between the use of oral anticoagulants and the short-term prognosis of patients with ARDS.
The objectives of this study were to investigate the association between oral anticoagulant use and 28-day mortality and in-hospital mortality in patients with ARDS. The effects of warfarin and novel oral anticoagulants on short-term mortality in patients with ARDS were also compared.
Materials and methods
Study design and population
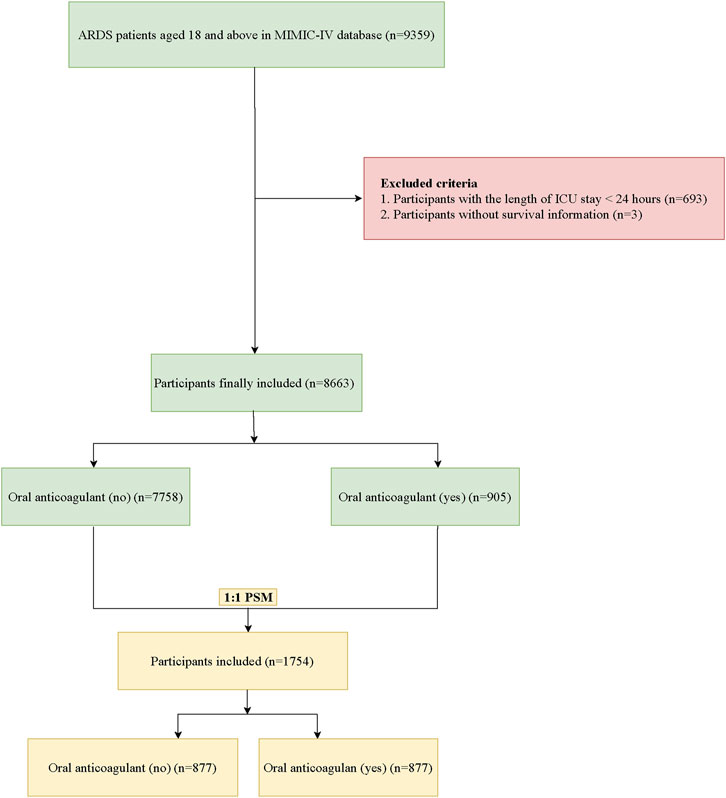
A total of 9359 participants ≥18 years diagnosed as ARDS were identified in database from 2008 to 2022 in this cohort study. The MIMIC-IV database consists of comprehensive and high-quality medical records from patients admitted to the intensive care units of Beth Israel Deaconess Medical Center (Johnson et al., 2023). In our study, the included criteria were that 1) age ≥ 18 years, 2) diagnosis of ARDS at admission (Berlin criteria). The Berlin criteria for ARDS included acute onset, partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≤300 mmHg, positive end-expiratory pressure (PEEP) ≥ 5 cm H2O, bilateral infiltrates on chest radiograph, and absence of heart failure. Mild, moderate, and severe ARDS were classified based on the PaO2/FiO2 ratios of the patients: mild (200 mmHg < PaO2/FiO2 ≤ 300 mmHg), moderate (100 mmHg < PaO2/FiO2 ≤ 200 mmHg), and severe (PaO2/FiO2 ≤ 100 mmHg) (Ranieri et al., 2012). The severity of ARDS was defined as mild 200 mmHg < PaO2/FIO2 ≤ 300 mmHg with PEEP or CPAP≥5 cm H2O, moderate 100 mmHg < PaO2/FIO2 ≤ 200 mmHg with PEEP ≥5 cm H2O, and severe PaO2/FIO2 ≤ 100 mmHg with PEEP ≥5 cm H2O (Ranieri et al., 2012). Patients were excluded if they stayed in ICU < 24 h, and missing the survival information.
Potential covariables and definitions
Age (years), gender (female or male), ethnicity (White, Black or other), weight (kg), ARDS severity (mild, moderate, or severe), atrial fibrillation or not, diabetes or not, cerebral infarction or not, pneumonia or not, sepsis or not, acute kidney injury (AKI) or not, heart rate (bpm), mean arterial pressure (mmHg), respiratory rate (insp/min), temperature (Deg.C), sepsis related organ failure assessment (SOFA), Charlson comorbidity index (CCI), white blood cell count (K/uL), red blood cell volume distribution width (RDW) (%), hematocrit (%), creatinine (mg/dL), blood urea nitrogen.
(BUN) (mg/dL), glucose (mg/dL), anion gap (mEq/L), calcium (mg/dL), oxygen saturation (SpO2) (%), partial pressure of carbon dioxide in arterial blood (PaCO2) (mmHg), partial thromboplastin time (PTT) (sec), mechanical ventilation duration (<48 h or ≥48 h), using vasopressors or not, using antibiotics or not, and using renal replacement therapy (RRT) or not were potential confounding factors. All the data were analyzed used the first measurement during 24 h ICU admission.
Mechanical ventilation were calculated based on parameters including peak inspiratory pressure (Ppeak), positive end-expiratory pressure, measured respiratory rate, and tidal volume. Atrial fibrillation was diagnosed based on International Classification of Diseases (ICD)-9 codes (42,731, and 42,732), and ICD-10 with first three digits of I48. Diabetes was diagnosed based on ICD-9 with first three digits of 250, and ICD-10 with first three digits of E10, E11, E12, E13, and E14. Cerebral infarction was diagnosed based on ICD-9 (433.01-433.91, and 434.01-91), and ICD-10 (I63). Pneumonia was diagnosed based on ICD-9 with first three digits of 480 to 486, and ICD10 with first three digits of J12 to J18.
Main variable
Data on anticoagulant drugs were extracted based on the Prescription list in MIMIC-IV database. Patients with the use oral anticoagulant drugs including Warfarin, Apixaban, Rivaroxaban, and Dabigatran during the time from ICU admission to ICU discharge were regarded to have oral anticoagulant use. Patients used Apixaban, Rivaroxaban, or Dabigatran were regraded to use novel oral anticoagulant drugs.
Outcome variables
The 28-day mortality and in-hospital mortality were outcomes. The follow-up of 28-day mortality was ended if the patients died within 28 days or follow-up to 28-day if the patients survived. The median follow-up time of 28-day mortality was 28 days. In-hospital mortality was followed-up to the time of in-hospital death or time of discharge from the hospital. The median follow-up time of in-hospital mortality was 7.34 days.
Statistical analysis
The measurement data with normal distribution were described by Mean ± standard deviation (Mean ± SD), and the t-test was used to compare the differences between groups. The median and quartiles were used to describe the distribution of measurement data that did not obey the normal distribution, and the Wilcoxon rank sum test was used to compare the differences between group. The enumeration data were described as number and percentage, and the chi-square test was used to compare the differences between groups. Missing values were presented in Supplementary Table S1. The missing variables were filled by multiple interpolation method, and data were compared before and after missing value imputation (Supplementary Table S2). Univariable cox regression model was constructed to evaluate the covariables associated with 28-day mortality and in-hospital mortality, respectively. Univariable and multivariable cox regression models were applied to assess the associations of oral anticoagulant use with the risk of 28-day mortality and in-hospital mortality. Propensity score matching (PSM) was performed in ARDS patients according to whether they were taking oral anticoagulants or not to control potential bias. Subgroup analysis was performed according to severity of ARDS (mild, moderate, and severe), and comorbidities (atrial fibrillation, sepsis, and AKI). Hazards ratio (HR) and respective confidence interval (CI) were presented. Kaplan-Meier curves were plotted to compare the survival probability of patients receiving oral anticoagulant or not. Data analysis was conducted using R [R version 4.4.0 (2024-04–24 ucrt)]. P < 0.05 was regarded to be statistically significant.
Results
Comparisons of characteristics of participants receiving oral anticoagulant or not receiving oral anticoagulant
In total, 9359 ARDS patients aged ≥18 years were identified in the MIMIC-IV database. Among them, patients who stayed in ICU < 24 h were excluded. Also, those without survival information were not included. Finally, 8,663 participants were included with 7758 patients not receiving oral anticoagulant and 905 patients receiving oral anticoagulant. After PSM, 1754 participants were analyzed. The screening process of participants was shown Figure 1.
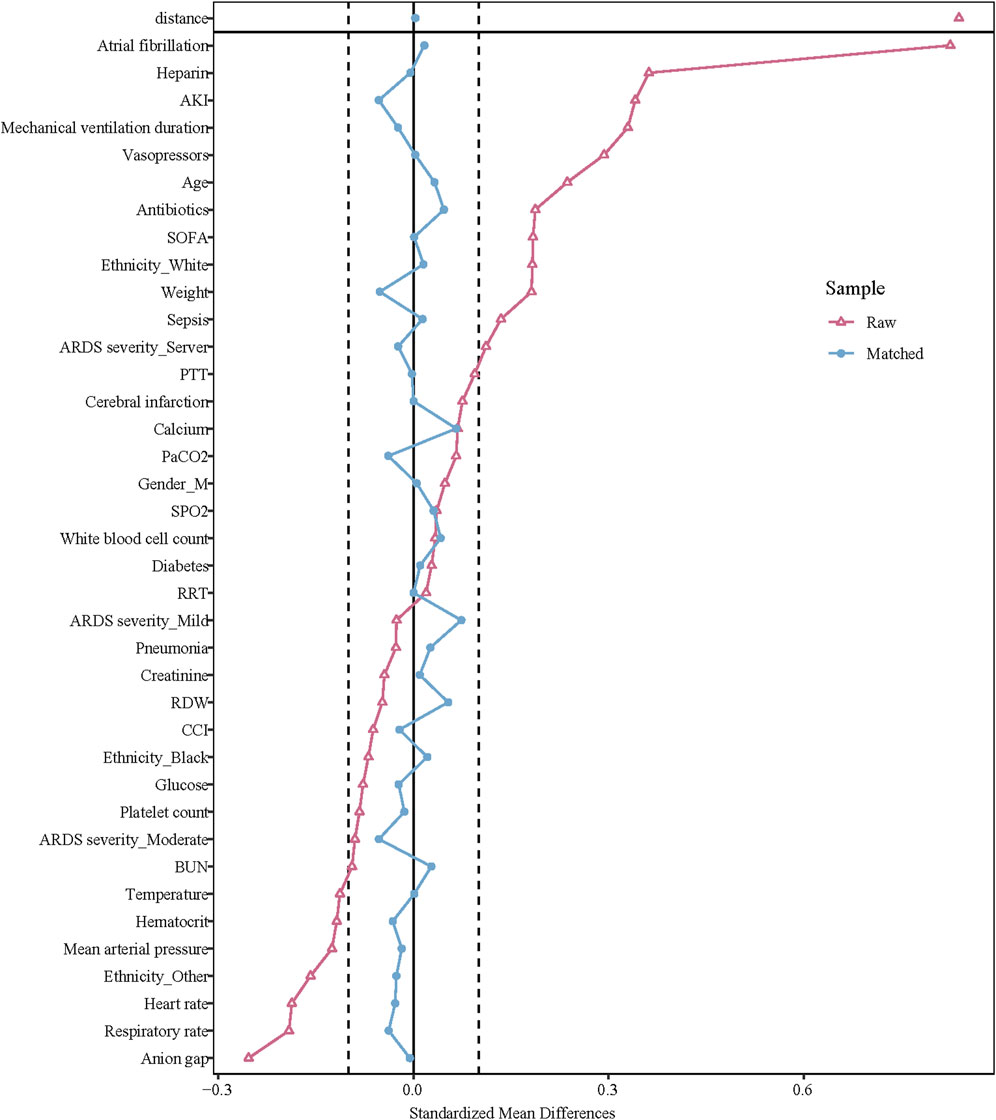
Before PSM, the mean age of the oral anticoagulant group was older than the non-oral anticoagulant group (64.59 years vs. 61.29 years). The percentage of atrial fibrillation patients was higher in the oral anticoagulant group relative to the non-oral anticoagulant group (62.98% vs. 23.14%). The percentage of cerebral infarction patients was higher in the oral anticoagulant group compared to the non-oral anticoagulant group (0.88% vs. 0.18%). The median SOFA score in the oral anticoagulant group was higher than the non-oral anticoagulant group (3.00 vs. 2.00). After PSM, the data in the oral anticoagulant group and the non-oral anticoagulant group were equilibrium. More information of the characteristics was exhibited in Table 1. The standardized mean difference (SMD) of data before PSM and after PSM was presented in Figure 2.
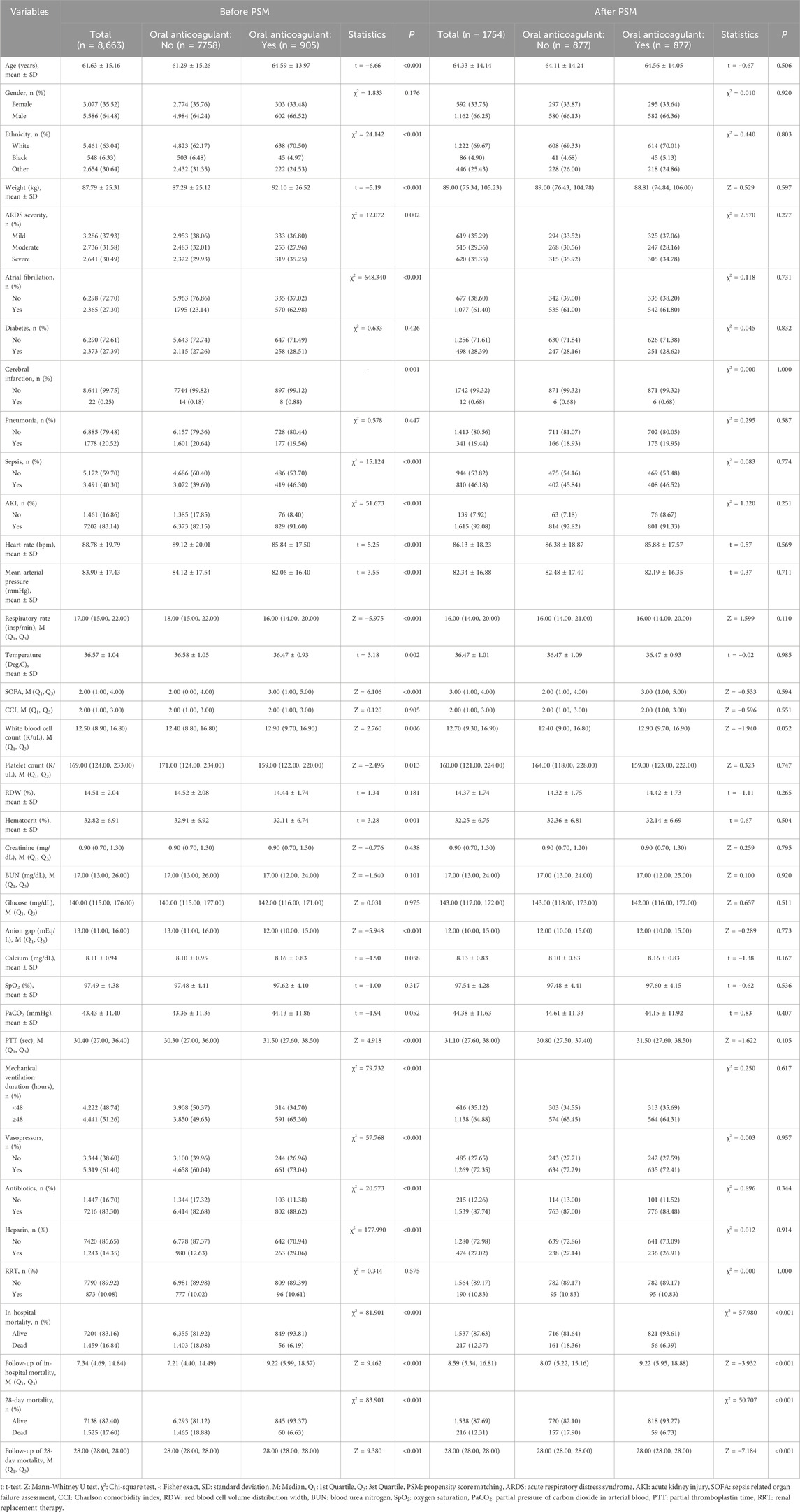
Table 1. Characteristics of participant with or without oral anticoagulant use before and after PSM.
Association between oral anticoagulant use with the risk of 28-day and in-hospital mortality
As delineated in gender, ethnicity, ARDS severity, cerebral infarction, pneumonia, sepsis, AKI, heart rate, respiratory rate, SOFA, CCI, WBC, creatinine, BUN, glucose, anion gap, SpO2, PTT, mechanical ventilation duration, antibiotics, and RRT were confounding factors related to 28-day mortality (Table 2). Compared to ARDS patients without oral anticoagulant use, the risk of 28-day mortality might be reduced in those receiving oral anticoagulant use (HR = 0.35, 95%CI: 0.26–0.47). After adjusting for the confounding factors, the reduced risk of 28-day mortality in ARDS patients was identified in those undergoing oral anticoagulant use (HR = 0.32, 95%CI: 0.24–0.44).
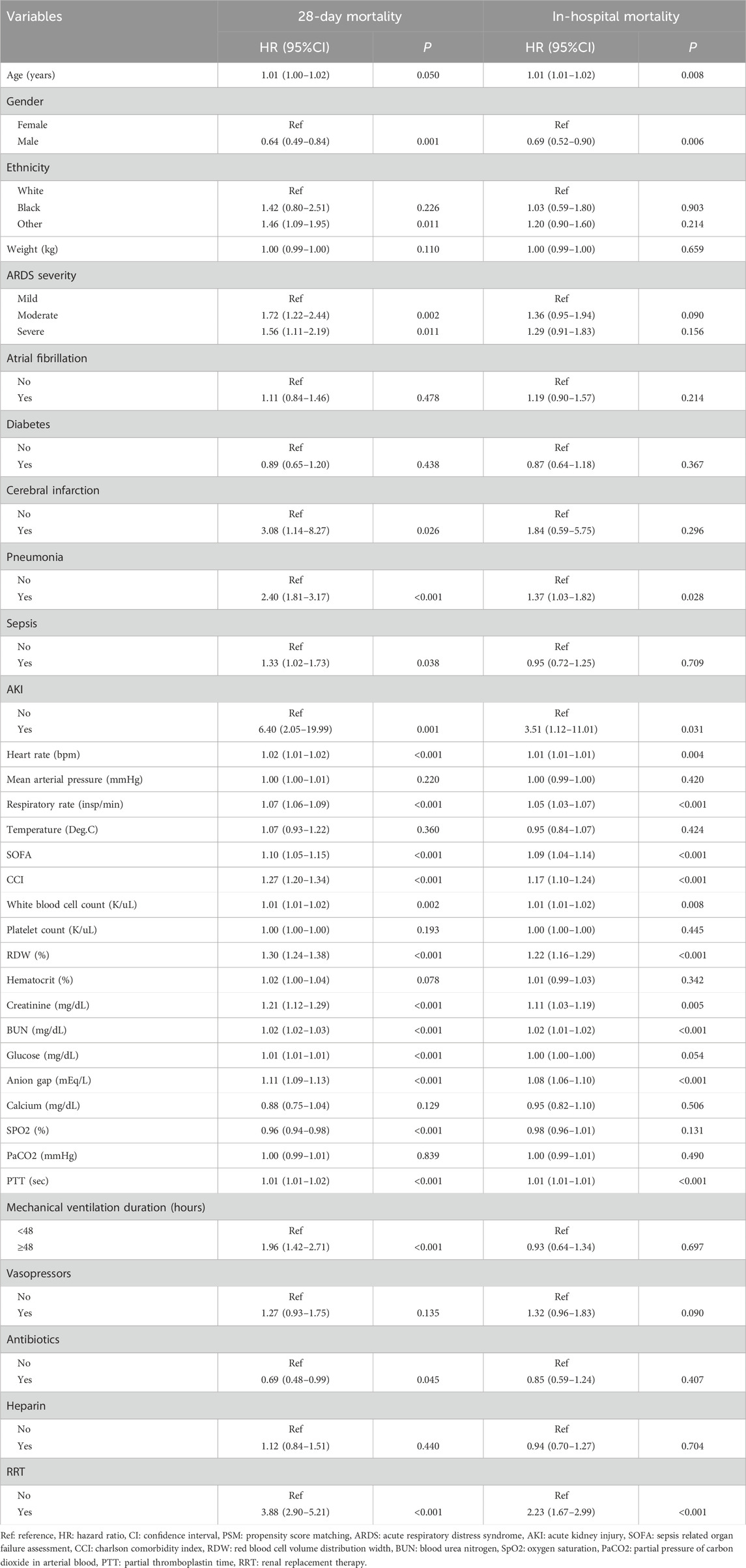
Table 2. Potential confounding factors associated with 28-day mortality and in-hospital mortality (after PSM, n = 1754).
Age, gender, pneumonia, AKI, heart rate, respiratory rate, SOFA, CCI, WBC, RDW, creatinine, BUN, anion gap, PTT, and RRT were confounding factors associated with in-hospital mortality (Table 2). The risk of in-hospital mortality might be decreased in patients who had oral anticoagulant in comparison with those did not have (HR = 0.29, 95%CI: 0.22–0.40). In the adjusted model, the association of oral anticoagulant use with reduced risk of in-hospital mortality was significant (HR = 0.27, 95%CI: 0.20–0.37) (Table 3). The 28-day survival probability of patients receiving oral anticoagulant was higher than those without oral anticoagulant use (Figure 3). ARDS patients had oral anticoagulant showed higher in-hospital survival probability than patients who did not use oral anticoagulant (Figure 4).
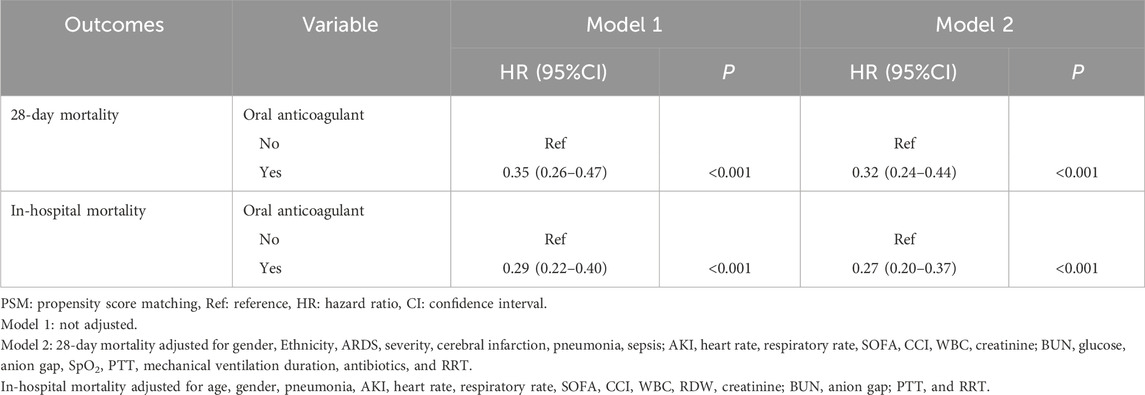
Table 3. Association between oral anticoagulant use with the risk of 28-day and in-hospital mortality (after PSM).
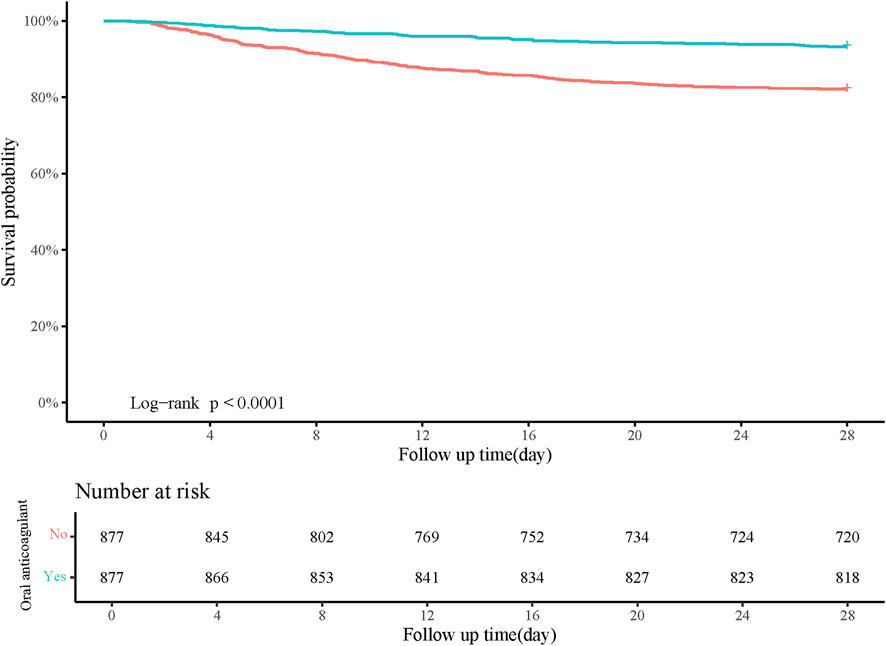
Figure 3. Kaplan-Meier curve of 28-day survival probability of patients receiving oral anticoagulant or not.
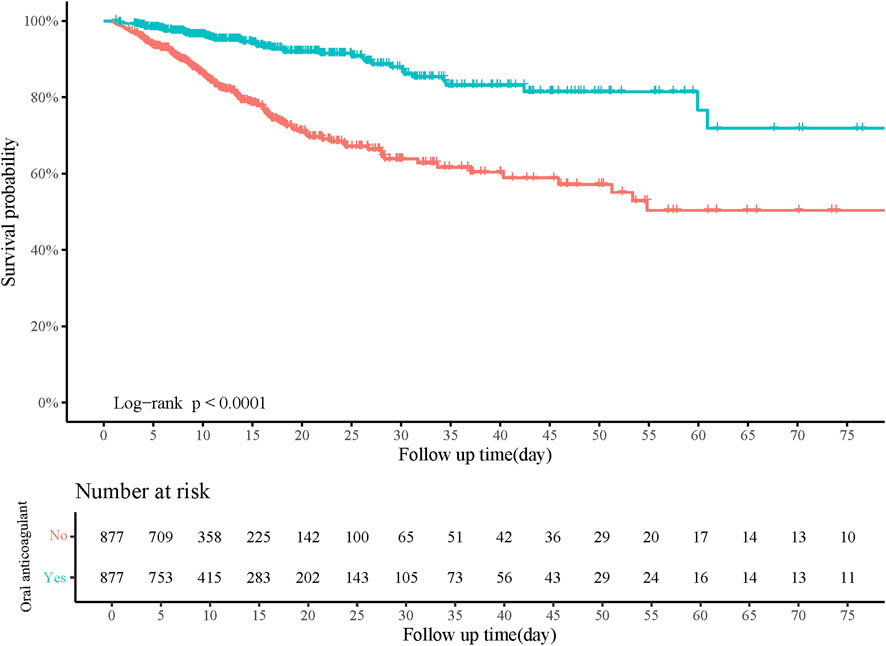
Figure 4. Kaplan-Meier curve of in-hospital survival probability of patients receiving oral anticoagulant or not.
Subgroup analysis on the association between oral anticoagulant use with the risk of 28-day and in-hospital mortality
The risk of 28-day mortality was identified to be decreased in both the ARDS mild (HR = 0.31, 95%CI: 0.16–0.58) or moderate/severe (HR = 0.33, 95%CI: 0.23–0.47) patients who received oral anticoagulant. In both the ARDS mild group (HR = 0.34, 95%CI: 0.18–0.65) and the moderate/severe group (HR = 0.25, 95%CI: 0.18–0.36), the risk of in-hospital mortality was reduced in patients with oral anticoagulant use. In ARDS patients complicated with sepsis or not, the risk of 28-day mortality and in-hospital mortality was decreased. Also, regardless ARDS patients complicated with atrial fibrillation or not, the risk of 28-day mortality and in-hospital mortality was decreased in those with oral anticoagulant use (Table 4).
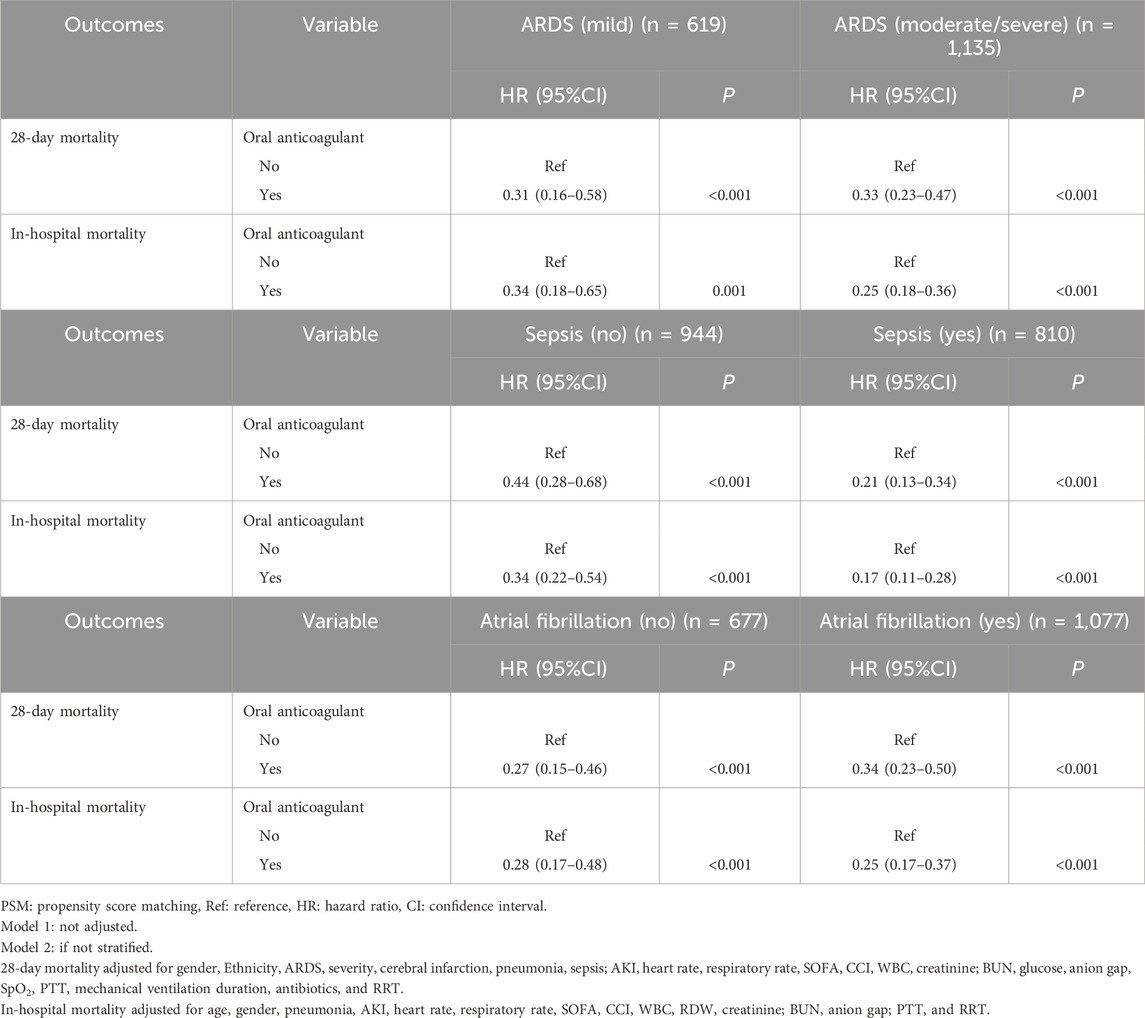
Table 4. Subgroup analysis of the association between oral anticoagulant use with the risk of 28-day and in-hospital mortality (after PSM).
Comparisons of the effect of warfarin with novel oral anticoagulants on the risk of 28-day and in-hospital mortality
In total, 777 patients receiving warfarin, and 116 patients receiving novel oral anticoagulants. The percentage of participants receiving mechanical ventilation duration ≥48 h was lower in warfarin group relative to novel oral anticoagulants group (62.93% vs. 78.45%). The percentage of patients receiving antibiotics was higher in warfarin group than novel oral anticoagulants group (91.63% vs. 68.97%). Detailed information on the characteristics of patients receiving warfarin and novel oral anticoagulants was presented in Supplementary Table S3.
According to the data in Supplementary Table S4, age, weight, ARDS severity, cerebral infarction, pneumonia, heart rate, respiratory rate, CCI, RDW, creatinine, BUN, anion gap, SpO2, PaCO2, and RRT were covariates related to 28-day mortality. Age, gender, weight, pneumonia, sepsis, heart rate, respiratory rate, CCI, RDW, BUN, and anion gap were covariates related to in-hospital mortality. In the crude model, Warfarin might be related to decreased risk of 28-day mortality (HR = 0.35, 95%CI: 0.20–0.61) and in-hospital mortality (HR = 0.53, 95%CI: 0.30–0.95). After adjusting for the respective confounding factors, the associations of Warfarin with decreased risk of 28-day and in-hospital mortality were not significant (P > 0.05) (Table 5).
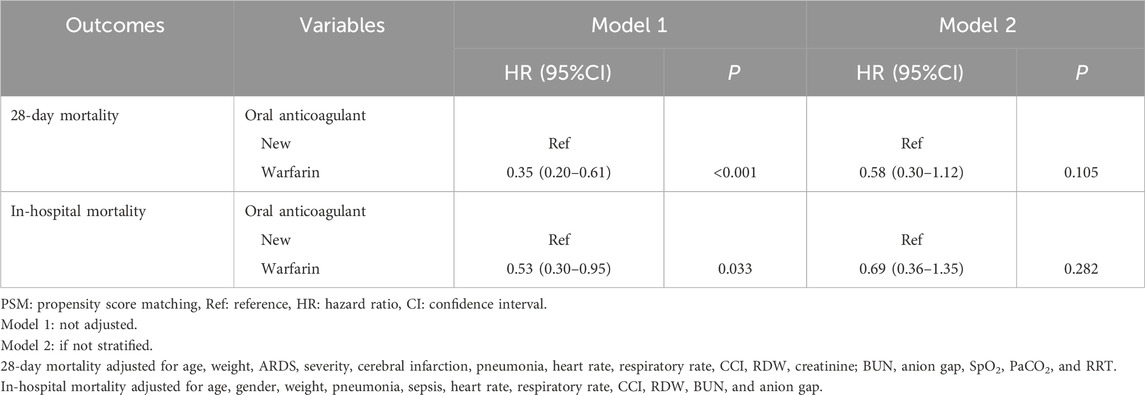
Table 5. Comparisons of the effect of warfarin with novel oral anticoagulants on the risk of 28-day and in-hospital mortality.
Discussion
The present study evaluated the association between oral anticoagulant use and 28-day and in-hospital mortality in patients with ARDS. We also compared the effects of warfarin and novel oral anticoagulants on 28-day and in-hospital mortality in patients with ARDS. The results indicated that oral anticoagulant use was related to reduced 28-day and in-hospital mortality risk in patients with ARDS. No significant difference on the 28-day and in-hospital mortality risk was observed between patients receiving warfarin and novel oral anticoagulants. The findings might provide a reference for making future medication strategies to improve the prognosis of ARDS patients.
Previously, increasing evidence has demonstrated the effect of anticoagulation administration on several lung-related diseases. Tang et al. revealed that anticoagulation therapy mainly with low molecular weight heparin was correlated with a reduced risk of mortality in patients with severe forms of coronavirus disease 2019 (COVID-19) (Tang et al., 2020). In hospitalized COVID-19 patients in the absence of contraindications, pharmacological antithrombotic prophylaxis was recommended (Thachil et al., 2020; American Society of Hematology, 2025). Nebulised unfractionated heparin was found to have strong scientific and biological rationale of its therapeutic potential, for patients with COVID-19 requiring invasive mechanical ventilation (van Haren et al., 2022). Also, in patients with or at risk of ARDS, nebulised heparin was well tolerated and exploratory outcomes suggest less progression of lung injury and earlier return home (Dixon et al., 2021). The lungs of ARDS patients are characterized by inflammation and elevated levels of procoagulant factors, the absence of hydrostatic pulmonary edema, and disruption of the alveolar-capillary barrier, leading to increased permeability of proteins (Ware and Matthay, 2000; Gonzales et al., 2015). This leads to the activation of pulmonary macrophages toward a proinflammatory phenotype, an increase in both intravascular and extravascular neutrophils, platelets, and fibrin, as well as endothelial and epithelial damage (Camprubí-Rimblas et al., 2018). The severe inflammatory response and disseminated intravascular coagulation, in conjunction with virus-induced local inflammatory reactions, impairing endothelial cell function, results in vessel wall damage and subsequent microvascular thrombosis (Scudiero et al., 2021). Functional implications encompass a progressively deteriorating ventilation/perfusion mismatch and a diminished hypoxic pulmonary vasoconstriction reflex, accompanied by a significant component of microvascular pulmonary thrombosis (Ciceri et al., 2020). This mechanism, primarily characterized by endothelial injury and microvascular thrombosis, indicates that microvascular obstructive thrombo-inflammatory syndrome in the lungs may represent an atypical form of ARDS in patients with COVID-19 (Ciceri et al., 2020). Pulmonary coagulopathy in the pathophysiology of ARDS is characterized by an activation of the coagulation system and a concurrent reduction in fibrinolytic activity (Schultz et al., 2004; Tuinman et al., 2012). Various pathways of the coagulation cascade, such as the tissue factor (TF) pathway, the protein C pathway, and the regulation of fibrinolysis through the plasminogen activator (PA) and inhibitor pathways, play crucial roles in the pathophysiology of ARDS (Camprubí-Rimblas et al., 2018). The pathophysiological roles of the coagulation and fibrinolysis systems in ARDS have been extensively investigated through both experimental and clinical studies (Sonobe et al., 2023). Patients with coagulopathy exhibited adverse outcomes and persistent hypoxemia, indicating a significant association between coagulopathy and the development of ARDS. Given the established association between coagulopathy and inflammation as reported in prior studies, ARDS accompanied by coagulopathy may manifest as a hyperinflammatory phenotype. This condition is linked to multiple organ dysfunction syndrome and adverse outcomes (Shankar-Hari et al., 2019; Gando et al., 2019). Pulmonary and extrapulmonary microvascular thrombosis may significantly contribute to the development of acute lung injury and multiple organ dysfunction (Russo et al., 2020). In the present study, oral anticoagulant was identified to be related to more favorable short-term prognosis.
In addition, we found that novel oral anticoagulants had similar effect with warfarin on the risk of 28-day and in-hospital mortality. Previous evidence has suggested that the incidence of major and minor bleeding complications among patients in the emergency department who are on novel oral anticoagulants is comparable to that of patients receiving warfarin therapy as no significant differences between the warfarin and novel oral anticoagulants groups in the frequency of minor bleeding complications, major bleeding complications, and intracranial bleeding were identified (Dogan et al., 2022). Anderson et al. demonstrated that both novel oral anticoagulants and warfarin are safe and effective options for anticoagulation in patients experiencing postoperative atrial fibrillation (POAF) following coronary artery bypass grafting (CABG), and neither group had a major bleeding event during the initial hospitalization (Anderson et al., 2015).
This study explored the relationship between the use of oral anticoagulants and the risk of short-term mortality in patients with ARDS, which provided a reference for the formulation of medication strategies to improve the prognosis of ARDS patients. The study population was based on a large sample of critically ill patients with MIMICIV, and a variety of possible confounding factors including comorbidities, laboratory indicators, treatment and so on were controlled by PSM. There were several limitations. Firstly, this was a retrospective cohort study, the potential recall bias cannot be avoided. Secondly, the study population was from a single center in the United States, and the generalization of the results to other population should be conducted with caution. Thirdly, the total dose of patients receiving oral anticoagulant could not be calculated based on the data from the MIMIC-IV database, and the results from our study still requires verification in more studies. Fourthly, the number of patients with COVID ARDS was limited (n = 2), and whether COVID-9 caused ARDS could not be future analyzed. Fifthly, the cause of ARDS was not specific, which might affect the results. The association between oral anticoagulants and short-term prognosis in patients with ARDS still needs further validation in randomized controlled trials.
Conclusions
The association between oral anticoagulant use and 28-day/in-hospital mortality in patients with ARDS was explored in the current study. The results delineated that oral anticoagulant was related to decreased risk of 28-day/in-hospital mortality in patients with ARDS. Warfarin and novel oral anticoagulants showed no significant difference on 28-day/in-hospital mortality in patients with ARDS. The findings might provide a reference for making future medical strategies for ARDS to improve the prognosis of these patients.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: MIMIC-IV database, https://mimic.physionet.org/iv/.
Ethics statement
The requirement of ethical approval was waived by Shenzhen University General Hospital as the data were obtained from a public database MIMIC-IV. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JH: Conceptualization, Methodology, Project administration, Writing – original draft, Writing – review and editing. HA: Data curation, Formal Analysis, Investigation, Methodology, Writing – review and editing. LC: Data curation, Formal Analysis, Investigation, Methodology, Writing – review and editing. WL: Data curation, Formal Analysis, Investigation, Methodology, Writing – review and editing. KZ: Data curation, Formal Analysis, Investigation, Methodology, Writing – review and editing. DS: Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1565312/full#supplementary-material
References
American Society of Hematology (2025). COVID-19-Hematology.org sgouoaipw. Available online at:https://www.hematology.org:443/education/clinicians/guidelines-and-quality-care/clinical-practice-guidelines/venous-thromboembolism-guidelines/ash-guidelines-on-use-of-anticoagulation-in-patients-with-covid-19.
Anderson, E., Johnke, K., Leedahl, D., Glogoza, M., Newman, R., and Dyke, C. (2015). Novel oral anticoagulants vs warfarin for the management of postoperative atrial fibrillation: clinical outcomes and cost analysis. Am. J. Surg. 210 (6), 1095–1103. doi:10.1016/j.amjsurg.2015.07.005
Bellani, G., Laffey, J. G., Pham, T., Fan, E., Brochard, L., Esteban, A., et al. (2016). Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. Jama 315 (8), 788–800. doi:10.1001/jama.2016.0291
Bo, D., Wang, X., and Wang, Y. (2023). Survival benefits of oral anticoagulation therapy in acute kidney injury patients with atrial fibrillation: a retrospective study from the mimic-iv database. BMJ open 13 (1), e069333. doi:10.1136/bmjopen-2022-069333
Bos, L. D. J., and Ware, L. B. (2022). Acute respiratory distress syndrome: causes, pathophysiology, and phenotypes. Lancet 400 (10358), 1145–1156. doi:10.1016/s0140-6736(22)01485-4
Camprubí-Rimblas, M., Tantinyà, N., Bringué, J., Guillamat-Prats, R., and Artigas, A. (2018). Anticoagulant therapy in acute respiratory distress syndrome. Ann. Transl. Med. 6 (2), 36. doi:10.21037/atm.2018.01.08
Ciceri, F., Beretta, L., Scandroglio, A. M., Colombo, S., Landoni, G., Ruggeri, A., et al. (2020). Microvascular covid-19 lung vessels obstructive thromboinflammatory syndrome (microclots): an atypical acute respiratory distress syndrome working hypothesis. Crit. care Resusc. J. Australas. Acad. Crit. Care Med. 22 (2), 95–97. doi:10.51893/2020.2.pov2
Denas, G., Gennaro, N., Ferroni, E., Fedeli, U., Lorenzoni, G., Gregori, D., et al. (2021). Reduction in all-cause mortality in COVID-19 patients on chronic oral anticoagulation: a population-based propensity score matched study. Int. J. Cardiol. 329, 266–269. doi:10.1016/j.ijcard.2020.12.024
Dixon, B., Smith, R. J., Campbell, D. J., Moran, J. L., Doig, G. S., Rechnitzer, T., et al. (2021). Nebulised heparin for patients with or at risk of acute respiratory distress syndrome: a multicentre, randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir. Med. 9 (4), 360–372. doi:10.1016/s2213-2600(20)30470-7
Dogan, Y., Az, A., Sogut, O., Akdemir, T., and Kaplan, O. (2022). Bleeding events in the emergency department with warfarin versus novel oral anticoagulants: a five-year analysis. Niger. J. Clin. Pract. 25 (8), 1308–1317. doi:10.4103/njcp.njcp_125_22
Flam, B., Wintzell, V., Ludvigsson, J. F., Mårtensson, J., and Pasternak, B. (2021). Direct oral anticoagulant use and risk of severe COVID-19. J. Intern. Med. 289 (3), 411–419. doi:10.1111/joim.13205
Gando, S., Fujishima, S., Saitoh, D., Shiraishi, A., Yamakawa, K., Kushimoto, S., et al. (2020). The significance of disseminated intravascular coagulation on multiple organ dysfunction during the early stage of acute respiratory distress syndrome. Thrombosis Res. 191, 15–21. doi:10.1016/j.thromres.2020.03.023
Gando, S., Shiraishi, A., Yamakawa, K., Ogura, H., Saitoh, D., Fujishima, S., et al. (2019). Role of disseminated intravascular coagulation in severe sepsis. Thrombosis Res. 178, 182–188. doi:10.1016/j.thromres.2019.04.025
Ge, G., Bo, D., Jiang, R., Zhao, W., and Lu, Y. (2024). Oral anticoagulants increased 30-day survival in sepsis patients complicated with atrial fibrillation: a retrospective analysis from mimic-iv database. Front. Cardiovasc. Med. 11, 1322045. doi:10.3389/fcvm.2024.1322045
Gonzales, J. N., Lucas, R., and Verin, A. D. (2015). The acute respiratory distress syndrome: mechanisms and perspective therapeutic approaches. Austin J. Vasc. Med. 2 (1), 1009.
Hofstra, J. J., Vlaar, A. P., Prins, D. J., Koh, G., Levi, M., Schultz, M. J., et al. (2012). Early intravenous unfractionated heparin and outcome in acute lung injury and acute respiratory distress syndrome: a retrospective propensity matched cohort study. BMC Pulm. Med. 12, 43. doi:10.1186/1471-2466-12-43
Johnson, A. E. W., Bulgarelli, L., Shen, L., Gayles, A., Shammout, A., Horng, S., et al. (2023). Mimic-iv, a freely accessible electronic health record dataset. Sci. Data 10 (1), 1. doi:10.1038/s41597-022-01899-x
Li, J., and Pan, G. (2024). Association of coagulation function with the risk of in-hospital mortality in patients with severe acute respiratory distress syndrome. Am. J. Med. Sci. 368 (2), 143–152. doi:10.1016/j.amjms.2024.04.012
Meyer, N. J., Gattinoni, L., and Calfee, C. S. (2021). Acute respiratory distress syndrome. Lancet 398 (10300), 622–637. doi:10.1016/s0140-6736(21)00439-6
Nieuwenhuizen, L., de Groot, P. G., Grutters, J. C., and Biesma, D. H. (2009). A review of pulmonary coagulopathy in acute lung injury, acute respiratory distress syndrome and pneumonia. Eur. J. Haematol. 82 (6), 413–425. doi:10.1111/j.1600-0609.2009.01238.x
Ranieri, V. M., Rubenfeld, G. D., Thompson, B. T., Ferguson, N. D., Caldwell, E., Fan, E., et al. (2012). Acute respiratory distress syndrome: the Berlin definition. Jama 307 (23), 2526–2533. doi:10.1001/jama.2012.5669
Russo, V., Bottino, R., D'Andrea, A., Silverio, A., Di Maio, M., Golino, P., et al. (2022). Chronic oral anticoagulation and clinical outcome in hospitalized COVID-19 patients. Cardiovasc. Drugs Ther. 36 (4), 705–712. doi:10.1007/s10557-021-07194-y
Russo, V., Di Maio, M., Attena, E., Silverio, A., Scudiero, F., Celentani, D., et al. (2020). Clinical impact of pre-admission antithrombotic therapy in hospitalized patients with covid-19: a multicenter observational study. Pharmacol. Res. 159, 10496. doi:10.1016/j.phrs.2020.104965
Schultz, M. J., Millo, J., Levi, M., Hack, C. E., Weverling, G. J., Garrard, C. S., et al. (2004). Local activation of coagulation and inhibition of fibrinolysis in the lung during ventilator associated pneumonia. Thorax 59 (2), 130–135. doi:10.1136/thorax.2003.013888
Scudiero, F., Silverio, A., Di Maio, M., Russo, V., Citro, R., Personeni, D., et al. (2021). Pulmonary embolism in covid-19 patients: prevalence, predictors and clinical outcome. Thrombosis Res. 198, 34–39. doi:10.1016/j.thromres.2020.11.017
Shankar-Hari, M., Fan, E., and Ferguson, N. D. (2019). Acute respiratory distress syndrome (ards) phenotyping. Intensive care Med. 45 (4), 516–519. doi:10.1007/s00134-018-5480-6
Sonobe, S., Kitabatake, M., Hara, A., Konda, M., Ouji-Sageshima, N., Terada-Ikeda, C., et al. (2023). The critical role of the histone modification enzyme Setdb2 in the pathogenesis of acute respiratory distress syndrome. Shock (Augusta, Ga) 60 (1), 137–145. doi:10.1097/shk.0000000000002145
Tang, N., Bai, H., Chen, X., Gong, J., Li, D., and Sun, Z. (2020). Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thrombosis Haemostasis JTH 18 (5), 1094–1099. doi:10.1111/jth.14817
Thachil, J., Tang, N., Gando, S., Falanga, A., Cattaneo, M., Levi, M., et al. (2020). Isth interim guidance on recognition and management of coagulopathy in covid-19. J. Thrombosis Haemostasis JTH 18 (5), 1023–1026. doi:10.1111/jth.14810
Tuinman, P. R., Dixon, B., Levi, M., Juffermans, N. P., and Schultz, M. J. (2012). Nebulized anticoagulants for acute lung injury - a systematic review of preclinical and clinical investigations. Crit. care 16 (2), R70. doi:10.1186/cc11325
van Haren, F. M. P., Laffey, J. G., Artigas, A., Page, C., Schultz, M. J., Cosgrave, D., et al. (2022). Can nebulised HepArin Reduce morTality and time to Extubation in patients with COVID-19 Requiring invasive ventilation Meta-Trial (CHARTER-MT): protocol and statistical analysis plan for an investigator-initiated international meta-trial of prospective randomised clinical studies. Br. J. Clin. Pharmacol. 88 (7), 3272–3287. doi:10.1111/bcp.15253
Wahab, A., Patnaik, R., and Gurjar, M. (2021). Use of direct oral anticoagulants in icu patients. Part Ii - clinical evidence. Anaesthesiol. Intensive Ther. 53 (5), 440–449. doi:10.5114/ait.2021.110608
Ware, L. B., and Matthay, M. A. (2000). The acute respiratory distress syndrome. N. Engl. J. Med. 342 (18), 1334–1349. doi:10.1056/nejm200005043421806
Wenzler, E., Engineer, M. H., Yaqoob, M., and Benken, S. T. (2020). Safety and efficacy of apixaban for therapeutic anticoagulation in critically ill ICU patients with severe COVID-19 respiratory disease. TH Open 4 (4), e376–e382. doi:10.1055/s-0040-1720962
Keywords: oral anticoagulant, ARDS, 28-day mortality, in-hospital mortality, warfain
Citation: Huang J, An H, Cheng L, Li W, Zhang K and Su D (2025) Roles of oral anticoagulant use on the risk of 28-day mortality and in-hospital mortality in patients with acute respiratory distress syndrome. Front. Pharmacol. 16:1565312. doi: 10.3389/fphar.2025.1565312
Received: 23 January 2025; Accepted: 25 April 2025;
Published: 14 May 2025.
Edited by:
Giuseppa Pistritto, Italian Medicines Agency (AIFA), ItalyReviewed by:
Antonio Artigas, Parc Taulí Foundation, SpainPradana Zaky Romadhon, Airlangga University Hospital, Indonesia
Copyright © 2025 Huang, An, Cheng, Li, Zhang and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Su, c3VkYW5fc2hlbnpoZW5AMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Jiayang Huang1†
Jiayang Huang1† Dan Su
Dan Su