- Department of Neurology, People’s Hospital of Dong Xihu, Wuhan, China
Parkinson’s disease (PD) is currently a highly prevalent neurodegenerative disease. Its pathogenesis is complex, and there is no effective method to prevent the disease progression. Traditional Chinese medicine (TCM) has a unique advantage in treating PD through the approach of syndrome differentiation. TCM prescriptions for PD can reduce Unified Parkinson’s Disease Rating Scale (UPDRS) scores, improve non-motor symptoms, and decrease adverse drug reactions. Bioactive polysaccharides extracted from prescribed Chinese herbs exhibit diverse biological activities due to their wide range of botanical sources. This review summarizes the pharmacological mechanisms of polysaccharides derived from TCM in managing PD, including inhibition of apoptosis, activation of autophagy, regulation of inflammation, anti-oxidative stress, improvement of mitochondrial function, and neuroprotective effects, aiming to provide a theoretical basis for future research and treatment.
1 Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder (Tolosa et al., 2021; Steinmetz et al., 2024). First described by British physician James Parkinson in 1817 (Morris, 1989), PD was once considered rare. However, its global prevalence increased by 74% between 1990 and 2016 (Ben-Shlomo et al., 2024). Since the beginning of the 21st century, PD has become more prevalent, driven by aging populations, longer life spans, declining smoking rates, and the by-products of industrialization. The global burden of PD is projected to exceed 12.9 million by 2040, with China expected to have five million cases by 2030, representing nearly half of the global patients (Dorsey and Bloem, 2018; Chinese Society of Parkinson’s Disease and Movement Disorders, 2020). The prevalence of PD is 1.7% in individuals over 65 years old and exceeds 4% in those over 80 years old.
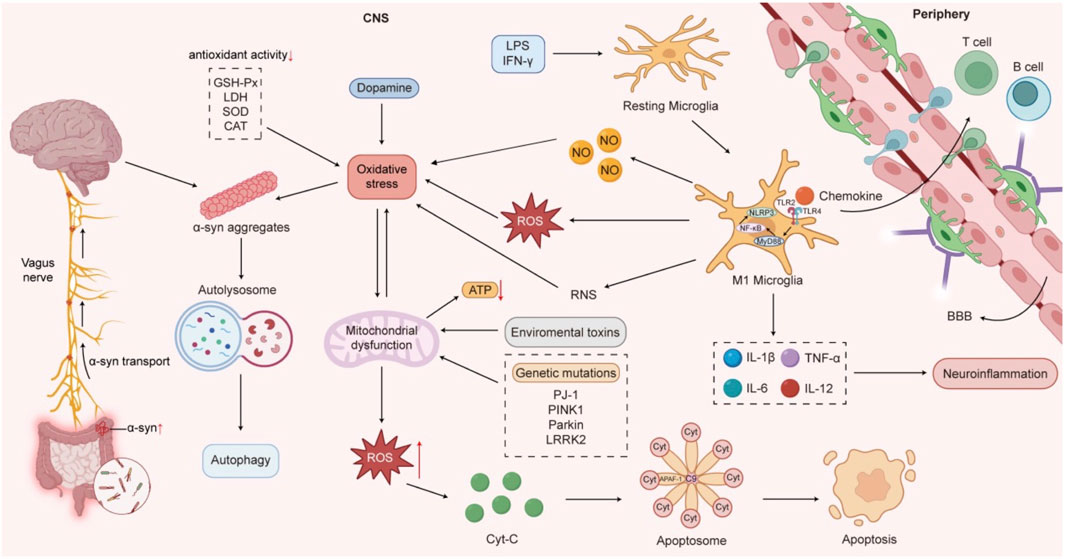
The pathogenesis of PD involves multiple factors, including age, environmental triggers, hereditary and genetic mutations, neuroinflammation, oxidative stress, mitochondrial dysfunction, abnormal aggregation of α-synuclein (α-syn), lysosomal dysfunction, gut microbiota dysbiosis, and dysregulated α-syn transmission along the gut-brain axis (Figure 1). Current pharmacological treatments face challenges, such as diminishing efficacy and emerging complications as the disease progresses. In contrast, TCM exhibits multi-target mechanisms and enhanced safety profiles, improving both motor and non-motor symptoms while potentially slowing disease progression (Yang et al., 2024).
Polysaccharides derived from TCM demonstrate significant therapeutic potential in PD through diverse biological activities. This paper aims to review the sources and mechanisms of polysaccharides from TCM in PD prevention and treatment to facilitate their further development in this field.
2 Sources and components of polysaccharides from traditional Chinese medicine
2.1 Sources of polysaccharides
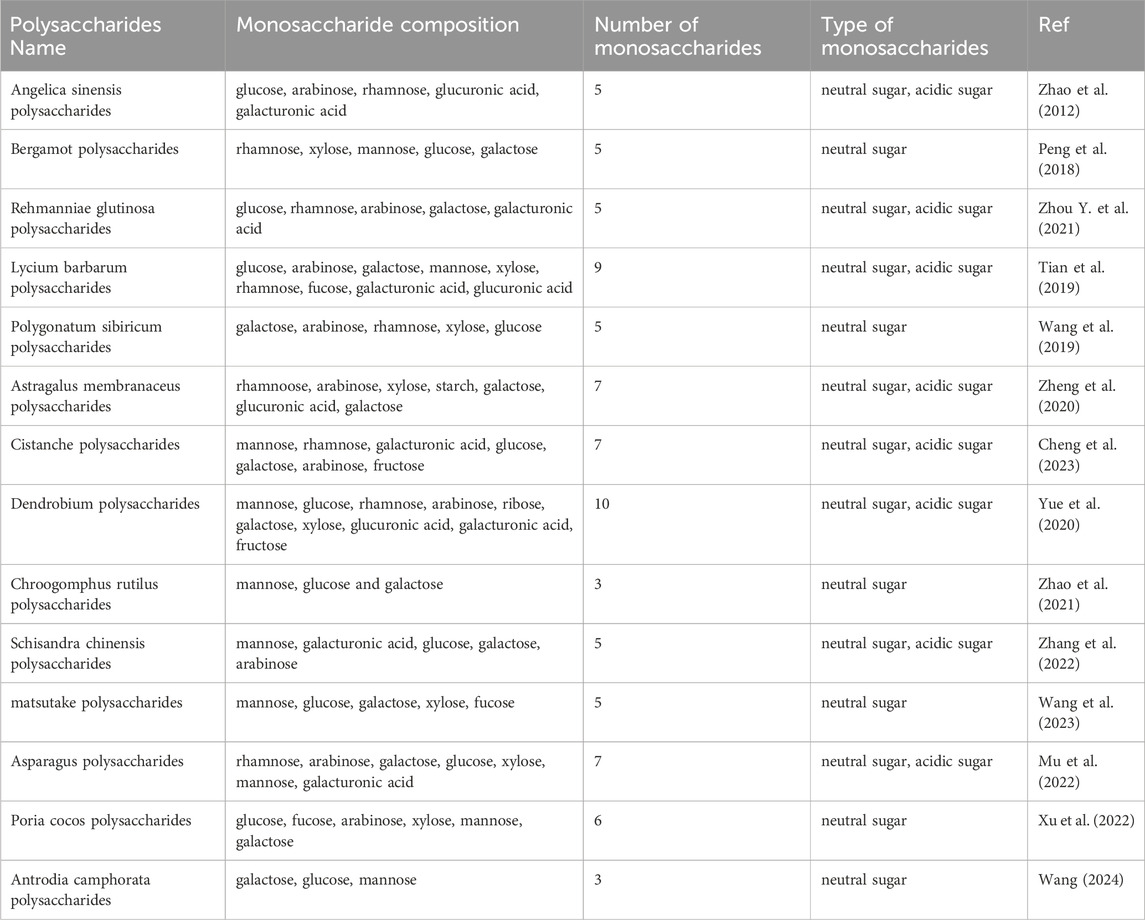
Polysaccharides from Traditional Chinese medicine refer to bioactive substances containing a large number of polysaccharides extracted from traditional Chinese medicine, which have diverse sources from fungi, plants, animals, microorganisms and marine organisms. Polysaccharides from fungi, such as Antrodia camphorata polysaccharides, Chroogomphus rutilus polysaccharides, morchella polysaccharides and bergamot polysaccharides, are typically obtained through fermentation or cultivation in specific media. Plant-derived polysaccharides are the most extensively studied, including Lycium barbarum polysaccharides (LBP), ginseng polysaccharides, Angelica sinensis polysaccharides (ASP), Astragalus polysaccharides (APS), Poria cocos polysaccharides, and Dendrobium polysaccharides (DP) etc. They are usually extracted from Chinese medicinal herbs through traditional methods like water extraction and alcohol precipitation. Animal polysaccharides, such as heparin, chitin and chondroitin sulfate, are usually obtained by extracting from animal organs and tissues. Microbial polysaccharides, such as hyaluronic acid and gel polysaccharides, are primarily produced through microbial fermentation. Marine biogenic polysaccharides, such as spirulina and wakame, are derived from marine organisms through extraction.
2.2 Components of polysaccharides from traditional Chinese medicine
The structure of polysaccharides from TCM is complex, and the extraction and purification process have not been fully standardized. Polysaccharides from TCM are mainly composed of 3–10 monosaccharides, including glucose, arabinose, rhamnose, xylose, fucose, galacturonic acid and glucuronic acid, with neutral polysaccharides being the main type. The specific components of polysaccharides from TCM are shown in Table 1.
3 Biological activities of polysaccharides from common traditional Chinese medicine
The composition of polysaccharides is relatively complex. For the same traditional Chinese medicine, due to different varieties, origins and growth time, the extracted polysaccharide components have slight differences in molecular weights, monosaccharide composition, glycosidic bonds and biological functions (Zeng et al., 2019). The structural complexity of polysaccharides leads to the diversity of their biological activities. Because of their unique sugar chain structures and functional groups, different polysaccharides can interact with biological macromolecules, such as receptors and enzymes, to exert their therapeutic effects. Polysaccharides have various biological activities, such as antiviral (Hao et al., 2024), anti-inflammatory, anti-oxidative stress (Huang et al., 2017), anti-tumor (Wang et al., 2024) and immunomodulatory effects (Li W. et al., 2020). A recent study has found that polysaccharides from traditional Chinese medicine also exhibit significant anti-aging effects in animal models (Song et al., 2024).
Statistical analysis on the frequency of use of individual herbs shows that cooked Rehmannia glutinosa (Gaertn.) Libosch. ex DC. [Orobanchaceae](processed), Astragalus mongholicus Bunge [Fabaceae], Gastrodia elata Blume [Orchidaceae], A. sinensis (Oliv.) Diels [Apiaceae], L. barbarum L.[Solanaceae], P. cocos (Schw.) Wolf. [Polyporaceae], Cistanche deserticola Ma [Orobanchaceae], and Salvia miltiorrhiza Bunge [Lamiaceae], etc. are used more frequently. Polysaccharide components of many Chinese medicines are important active agents for the treatment of PD models, such as those possessing the activity of mitigating oxidative stress and modulating inflammatory pathways.
3.1 Astragalus membranaceus polysaccharides
Astragalus mongholicus Bunge [Fabaceae] is the root of the leguminous plant Astragalus membranaceus (Fisch.) BGe.var.mongholicus (Bge.) Hsiao or Astragalus membranaceus (Fisch.) Bge. APS can be used as an immune promoter or regulator, and has pharmacological effects, such as anti-inflammatory, immune modulatory, and neurodegeneration-delaying (Du et al., 2022). The chemical characterization of APS (e.g., branching degree, molecular weight) is closely related to its immunomodulatory functions, while its bioavailability and bioactivity can be enhanced through structural modifications (e.g., sulfation) or processing methods (e.g., honey-processing). Specifically, APS with highly branched chains, low-to-medium molecular weights, large surface areas, or selenated modifications significantly promotes immune cells’ activities. Additionally, honey-processed APS with low-to-medium molecular weights exerts anti-inflammatory activities by regulating immune cells, immune organs and immune factors (Zhao et al., 2024).
3.2 Angelica sinensis polysaccharides
Angelica sinensis is derived from the dried root of the umbelliferous plant Angelica sinensis (Oliv.) Diels. It has a wide range of pharmacological effects, including hematopoietic promotion, immune enhancement, antitumor activity, and antioxidant properties (Jin et al., 2012). Studies have linked these biological activities to the structural features of its polysaccharides, showing that a high content of galacturonic acid has stronger protection and anti-oxidation effect on macrophages (Jin et al., 2022).
3.3 Lycium barbarum polysaccharides
Goji berries are tonic medicines used for deficiency syndromes. As the dried mature fruits of the Solanaceae family, LBP are water-soluble compounds with multiple biological activities, including immune enhancement, anti-aging, anti-tumor effects, free radical scavenging, anti-fatigue, anti-radiation, and hepatoprotective and reproductive system protection. Structural analysis reveals that LBP1 (Mw = 1.207 × 106) exhibits significantly stronger immunomodulatory activity than LBP2 (Mw = 1.25 × 105) (Wang Y. et al., 2021). Crude LBPs containing sulfated groups, carboxyl moieties, and higher protein content demonstrate superior antioxidant and immunoregulatory properties compared to purified LBPs (Luo et al., 2004; Wang et al., 2010).
3.4 Rehmanniae glutinosa polysaccharides
This herb is the dried root or freshly processed product of Rehmannia glutinosa (Scrophulariaceae family). Polysaccharides from the cooked Rehmannia are one of the major active components, exhibiting pharmacological effects, such as antioxidant, anti - aging, anticancer and immunomodulatory activities (Bian et al., 2023). Polysaccharides of the cooked rehmannia have complex compositions, and their content after processing and steaming is higher than that in the raw Rehmanna glutinosa (Geng, 2023). Rehmannia Radix polysaccharides (RRPs) with high content of total lactose and glucose show strong biological activities. For example, the extract RGP70 with high galactose content exhibits the highest antioxidant and anti-aging activity (Huang et al., 2024). RRPs containing high levels of glucuronic acid also demonstrate antioxidant activities (Ren et al., 2022).
3.5 Poria cocos polysaccharides
This herb is the dried sclerotium of the Tuckahoe fungus. Poria cocos Pachyman (PCP) is the main active component, accounting for about 70%–90% of the sclerotia’s dry weight. It has biological functions, such as anti-oxidation, anti-aging, anti-inflammation, immune regulation, antibacterial activity, hepatoprotection and anti-cancer effects (Li et al., 2022). The phagocytotic capacity of RAW264.7 cells is enhanced to the greatest extent when PCP is combined with lentinan (Dong et al., 2021).
3.6 Cistanche polysaccharides
This herb belongs to a tall herbaceous plant genus of Cistanche Hoffing. et Link and the family of Orobanchaceae. It has multiple pharmacological effects including improving the learning and memory ability, regulating gut microbiome, anti-inflammation, anti-aging, anti-oxidation (Zhang et al., 2016), anti-depression, regulating immune functions, moistening the bowel, managing osteoporosis, and protecting the body from chronic alcoholic liver disease (Guo et al., 2016), ischemic stroke and oxidative stress injury (Xue et al., 2024). Polysaccharides of different molecular weights promote cell proliferation at low concentrations and inhibit cell proliferation at high concentrations (Gao, 2022).
4 Clinical application and mechanisms of Chinese medicine prescriptions
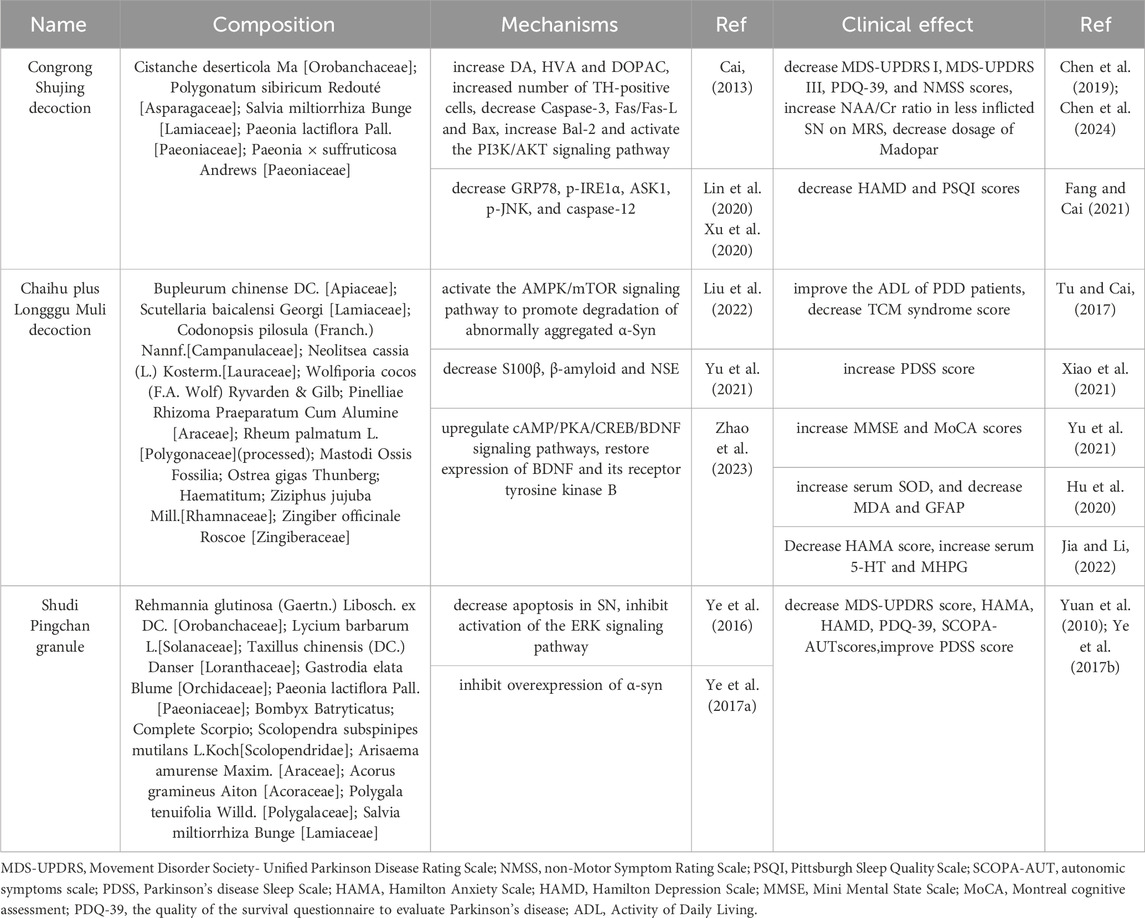
At present, many formulations containing polysaccharides from Chinese herbs have been clinically proven to improve the motor and non-motor symptoms of PD patients. Mechanistic studies further confirmed that these formulations play a role through anti-oxidation, anti-apoptosis, anti-inflammatory, inhibition of α-syn aggregation and other pathways. The mechanisms of these formulations directly correlate with the pharmacological properties of polysaccharides. Three clinically validated prescriptions, such as Cong Rong Shu Jing Granules and Chaihu Jia Longgu Muli Decoction, demonstrate these synergistic effects.
4.1 Cong Rong Shu Jing compound (CRSJ)
Chen et al. (2019), Chen et al. (2024) evaluated the efficacy and safety of Cong Rong Shu Jing granules in substantia nigra metabolites in PD patients. Compared with the control group, the treatment group demonstrated significant reductions in MDS-UPDRS I, MDS-UPDRS III, PDQ-39, and NMSS scores, indicating that CRSJ can improve both motor and non-motor symptoms as well as PD patients’ quality of life. Magnetic resonance spectroscopy (MRS) analysis showed that the N-acetylaspartic acid/creatine (NAA/Cr) ratio in the mild side of substantia nigra in the treatment group was increased. Since NAA reflects neuronal activity in the brain and Cr serves as an internal benchmark to evaluate metabolic rates, these findings suggest that CRSJ granules may improve the symptoms of PD patients by increasing the activity of substantia nigra neurons. Safety assessments, including blood tests, urinalysis, liver/kidney function tests, electrocardiograms and recorded adverse events, showed that all patients had good safety indicators. The dosage of Madopar decreased in the CRSJ group. A study (Fang and Cai, 2021) has compared the effect of CRSJ granules on improving sleep with Western medicine. The results showed significantly lower scores on both the Hamilton Depression Scale (HAMD) and Pittsburgh Sleep Quality Index (PSQI) in the experimental group compared to the control group.
A PD mouse model was established with MPTP (1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine). Their results showed as follows (Cai, 2013): the contents of DA and its metabolites (HVA and DOPAC) in the CRSJ group were significantly higher than those in the model group, the number of tyrosine hydroxylase (TH) positive cells in the CRSJ group was significantly increased. Under electron microscopy, most neurons still maintained their normal morphology. Compared with the model group, Caspase-3, Fas/FAS-L and Bax levels in the CRSJ group were significantly reduced, while Bcl-2 was increased. Bcl-2 is a protective factor against apoptosis, indicating that nerve cells in the CRSJ group initiated self-protection. This formula could significantly reduce the apoptosis of nigrostriatal neurons in PD animal models, alleviate structural damage to nigrostriatal neurons, reverse reduced dopamine levels and increase the level of the TH in dopamine anabolism.
CRSJ granule can increase the level of cerebral dopamine neurotrophic factor (CDNF) and the mesencephalic astrocyte-derived neurotrophic factor (MANF). It also activates the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway-related proteins to inhibit cell apoptosis. CRSJ granule can also decrease the expression of α-syn and the level of endoplasmic reticulum stress (ERS) related proteins, such as glucose regulatory protein 78 (GRP78), inositol-requiring enzyme one (p-IRE1α), apoptosis signal-regulating kinase 1 (ASK1), c-Jun N-terminal kinase (p-JNK) and caspase-12. This leads to reduced ERS in PD rats, inhibited apoptosis of dopaminergic (DA) neurons, increased numbers of TH positive cells, and improved the motor functions of mice (Lin et al., 2020; Xu et al., 2020).
4.2 Chaihu Jia Longgu Muli Decoction
Tu and Cai, (2017) conducted a randomized controlled study and found that Chaihu Jia Longgu Muli Decoction improved the depressive symptoms in PD patients with liver-qi depression (PDD). The decoction showed early-stage clinical efficacy by improving daily living ability and reducing TCM syndrome scores in PDD patients. The mechanism may be related to the increased serum levels of 5-HT and NA. Xiao et al. (2021) applied Chaihu Jia Longgu Muli Decoction to treat sleep disorders in PD patients. Their study revealed a significant increase in Parkinson’s Sleep Scale score (PDSS) among 30 patients after treatment compared to baseline.
Another study (Yu et al., 2021) found that Chaihu Jia Longgu Muli Decoction reduced Ashworth scores (indicating improved muscle tone) and elevated scores of life quality, MoCA and MMSE in PD patients with dementia, compared to a western medicine-only group. After treatment, the decoction group exhibited lower levels of S100β, Aβ, and neuron-specific enolase (NSE) biomarkers. These results suggest that Chaihu Jia Longgu Muli Decoction may reverse cognitive decline and improve clinical outcomes in dementia-associated PD.
A study (Hu et al., 2020) reported that Chaihu Jia Longgu Muli Decoction combined with acupuncture treatment increased serum superoxide dismutase (SOD) levels, decreased malondialdehyde (MDA) and glial fibrillary acidic protein (GFAP) levels, and ameliorated patients’ cognitive and motor functions as shown by UPDRS. These effects may result from suppressing oxidative stress-induced damage to DA neurons.
Another study (Liu et al., 2022) demonstrated that Chaihu Jia Longgu Muli Decoction could improve the depression state of PD rats, evidenced by increased total horizontal movement distance, prolonged central area exploration time in the open field test, and enhanced pole-climbing performance. The number of DAergic neurons in the substantia nigra, enlarged neuronal Soma size, and increased striatal DA and serotonin (5-HT) levels. The treatment significantly downregulated α-Syn expression, upregulated LC3-II/LC3-I and p-AMPK/AMPK ratios, while inhibiting p-mTOR/mTOR signaling. The therapeutic mechanism likely involves activating the AMPK/mTOR pathway to enhance autophagy-mediated clearance of pathological α-Syn aggregates.
4.3 Shudi Pingchan decoction
A Multi-clinical study by Yuan et al. (2010) showed that Shudi Pingchan decoction could significantly alleviate motor symptoms of PD patients manifested by decreased UPDRS-II and UPDRS-III scores, prolonged on-period and shortened off-period with no apparent side effects observed. This indicates that the decoction potentiates therapeutic efficacy while attenuating drug-induced toxicity in PD management.
Another study on this herbal decoction and repetitive transcranial magnetic stimulation (rTMS) (Ye et al., 2017b) demonstrated a good therapeutic effect on PD patients. The high-frequency rTMS group showed superior UPDRS-III score reductions versus controls at 8-week and 12-week. Non-motor improvements included lower HAMA, Hamilton Depression Rating Scale (HAMD), SCOPA-AUT and Parkinson’s Disease Questionnaire-39 (PDQ-39) scores, alongside higher PDSS (all p < 0.05). Both trials confirmed the decoction’s safety profile, with adverse event incidence comparable to controls and only mild transient reactions reported.
In an animal study, Ye et al. (2016) observed that Shudi Pingchan granule significantly reduced rotational behavior and the duration of spins in PD rats, and reduced substantia nigra apoptosis. Mechanistically, the decoction inhibited ERK1/2 phosphorylation at Thr202/Tyr204, attenuated nuclear translocation of ERK1/2, and subsequently decreased the Bax/Bcl-2 ratio while upregulating TH expression. These changes are related to neuronal survival and apoptosis. Shudi Pingchan granules can also inhibit α-syn overexpression (Ye et al., 2017a), attenuate the injury to dopaminergic neuron, regulate the imbalance between the direct and indirect pathways mediated by D1 and D2 receptors and effectively alleviate PD symptoms. A summary of mechanistic and clinical studies on TCM formulae can be found in Table 2.
5 Anti-PD mechanisms of traditional Chinese medicine polysaccharides
5.1 Inhibition of apoptosis
Dopaminergic neurons are a type of highly differentiated and non-renewable cell type. Due to their high energy demands, they are particularly vulnerable to cell death signals and more susceptible to degeneration. Currently, there are over ten known cell death pathways, among which the apoptosis of dopaminergic neurons is considered an important mechanism underlying PD. A notable feature of apoptosis is the formation of apoptotic bodies. Apoptosis primarily occurs via three pathways (Yang X. et al., 2018): the death receptor pathway, the mitochondrial pathway, and the endoplasmic reticulum pathway. The mitochondrial pathway, also known as the intrinsic apoptotic pathway, operates as follows: under the pro-apoptotic stimulation, the permeability of the outer mitochondrial membrane changes, and the mitochondrial permeability transition pore opens, leading to the release of cytochrome C (Cyt C) from the mitochondrial intermembrane space into the cytosol. The released Cyt C combines with apoptotic protease activating factor-1 (Apaf-1) and procaspase-9 to form an apoptotic complex, thereby activating caspase-9 and caspase-3, ultimately triggering apoptosis (Wu and Bratton, 2013). The BCL-2 protein family plays a crucial role in regulating mitochondrial membrane permeability and is a core factor in modulating the mitochondrial apoptotic pathway. Key members include pro-apoptotic proteins, such as BAX and anti-apoptotic proteins, such as BCL-2. When apoptotic signals are activated, BAX undergoes mitochondrial translocation, inserts into the mitochondrial membrane, and polymerizes to form transmembrane channels. This process increases mitochondrial membrane permeability, facilitating the release of Cyt C. BCL-2 inhibits the homodimerization of BAX and its impact on mitochondrial membrane permeability by forming heterodimers with BAX.
The apoptotic pathway is precisely regulated by multiple signaling pathways (He et al., 2022), including the p38 MAPK, JNK/SAPK, Notch, PI3K/AKT/mTOR, and Nrf2/ARE pathways. These pathways form an intricate regulatory network that collectively controls the initiation and progression of apoptosis.
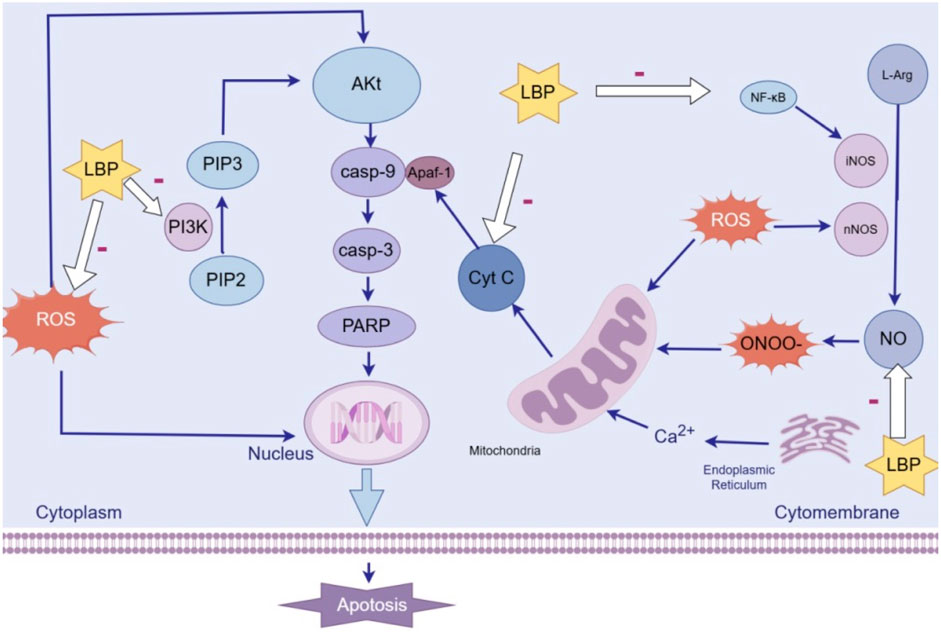
Polysaccharides, such as LBP and ASP, enhance anti-apoptotic mechanisms through reducing reactive oxygen species (ROS) accumulation, inhibiting Cyt-C release, thereby suppressing activation of the intrinsic apoptotic pathway. The neurotoxin MPP+ inhibits mitochondrial complex I, elevating ROS accumulation and triggering Cyt-C release. This cascade triggers Cyt-C release from mitochondria, which sequentially activates Caspase-9 and Caspase-3, while dysregulating Bcl-2 family proteins: pro-apoptotic Bax increases, whereas anti-apoptotic Bcl-2 decreases. LBP scavenges ROS and stabilizes mitochondrial integrity, thereby blocking Cyt-C translocation to the cytoplasm and subsequent caspase activation (Wang and Han, 2021). After pretreating SH-SY5Y cells with LBP for 1 h, it was observed that LBP downregulated MPP+-triggered overexpression of pro-apoptotic proteins, ultimately, significantly reversed MPP+-induced apoptosis. This study utilized SH-SY5Y cells but lacked validation using primary neurons or co-culture with glial cells. LBP pretreatment lasted only 1 h and whether longer pretreatment durations would enhance the protective effect was not verified. Similarly, ASP treatment (Zhang et al., 2024) attenuated astrocytic damage and apoptosis in MPTP-injected mice by modulating Bax/Bcl-2/caspase-3, thereby mitigating MPTP-induced PD-like behavioral and molecular alterations. Consistent with molecular changes, behavioral tests demonstrated that ASP significantly improved limb grip strength and latency time in MPTP-treated mice, alleviating motor deficits in PD models. This experiment evaluated motor function only using a grip strength meter and rotarod apparatus, core behavioral phenotypes for PD, such as gait analysis or open field test, were not included.
The PI3K/Akt pathway is an important pathway of anti-apoptotic signal transduction mechanism, which can protect cells from being stimulated by many apoptotic signals. When PI3K is activated, it phosphorylates PIP2 to PIP3 and promotes the phosphorylation of Akt. Phosphorylated Akt can inhibit the expression and activation of various pro-apoptotic proteins, thereby exerting anti-apoptotic effects. It was shown that LBP (Li P. et al., 2020) could reduce the apoptosis rate of PC12 cells induced by MPP+. After pretreatment with a PI3K inhibitor LY 294002, the protective effect of LBP was blocked. These findings indicate that LBP activates the PI3K/Akt pathway to reduce apoptosis and protect neurons. Mechanisms underlying the apoptosis effect of LBP is presented in Figure 2.
Another study showed (Li et al., 2016) that ASP alleviated the degeneration of dopaminergic neurons and defects in food sensing behavior in nematodes treated with 6-OHDA, and also prolonged the lifespan of nematodes exposed to 6-OHDA. Real time quantitative PCR analysis showed that among programmed cell death related proteins like CED-4, CED-3, CED-9, and EGL-1, ASP significantly reduced the mRNA level of EGL-1 in Caenorhabditis elegans, suggesting the role of ASP in regulating the expression of programmed cell death genes. Research has shown that (Wang and Han, 2021) hypoxia induces an increase in the expression of urothelial cancer associated antigen 1 (UCA1) in neurons. UCA1 can promote the progression of PD by regulating the SNCA gene, and its small interfering RNA (si-UCA1) is used for gene silencing. LBP + si-UCA1 is more effective in improving cell survival and reducing apoptosis rate than LBP or si-UCA1 alone, indicating a synergistic anti apoptotic effect of LBP and si-UCA1.
5.2 Anti-oxidative stress
During normal life activities, free radicals are constantly produced in the body. Cellular antioxidant defense systems efficiently neutralize these radicals, thereby protecting cells from oxidative damage. However, when the dynamic balance between the oxidation and antioxidation systems is disrupted-particularly when excessive reactive oxygen species (ROS) production overwhelms the antioxidant clearance capacity, oxidative stress occurs. Excessive ROS induces macromolecular structural damage, ultimately leading to cell dysfunction. Notably, dopaminergic neurons in the substantia nigra are highly susceptible to ROS-mediated oxidative injury, a key pathological mechanism in PD.
Studies have shown that the oxidative stress factors leading to PD mainly include the following aspects (Zhu et al., 2019): Firstly, mitochondrial dysfunction, impaired mitochondrial complex I activity substantially increases ROS generation; Secondly, neuroimmune inflammatory responses induce the massive release of pro-inflammatory cytokines (e.g., TNF-α, IL-1β, IL-2), enhances iNOS activities, promoting ROS and ONOO- formation (Przedborski and Dawson, 2001). In addition, abnormal dopamine metabolism, iron accumulation, calcium ion overload, and compromised antioxidant defense system - manifested as reduced activity of antioxidant key enzymes, such as glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), catalase (CAT), lactate dehydrogenase (LDH), and vitamin C (VC).
From a genetic perspective, mutations in mitochondrial quality control genes (e.g., DJ-1, PINK1, and Parkin) disrupt dopaminergic neuron mitochondrial homeostasis (Hu et al., 2021), initiating oxidative cascades. Clinical studies also show that levels of inflammatory factors (IL-6) and oxidative stress markers (SOD, MDA, NOS) in patients with PD are significantly higher than healthy controls (Nagatsu et al., 2000). These results further confirm the role of oxidative stress in the pathogenesis of PD.
Treatment of PC12 cells (a cell line derived from rat pheochromocytoma) with 6-OHDA significantly reduced cell survival and induced typical apoptotic features, such as pyknosis, karyorrhexis, and formation of apoptotic bodies. Excessive ROS accumulation occurs intracellularly. ROS, as the initial trigger of apoptosis, activates p38 mitogen-activated protein kinase (MAPK), induce scaspase-9 and caspase-3 activation, inhibits Akt phosphorylation, and ultimately induces apoptosis. LBP effectively suppressed intracellular ROS generation, blocked p38 activation, reduced 6-OHDA-induced cleavage of caspase-3, and thereby attenuated apoptotic cell death (Gao et al., 2014).
Polysaccharides from TCM significantly inhibit nitric oxide (NO) accumulation and NF-κB activation. The peroxynitrite (ONOO−) generated from NO metabolism can Inhibit mitochondrial complexes, induce Ca2+ release from the endoplasmic reticulum, trigger Cyt C release, activate apoptosis. Neuronal nitric oxide synthase (nNOS) and inducible nitric oxide synthase (iNOS) are the catalytic enzymes for NO production. 6-OHDA causes overexpression of nNOS and iNOS in PC12 cells, increases intracellular NO and its metabolites, raises Ca2+ levels, activates NF-κB in apoptosis-associated signaling pathways, and upregulates p53. After pretreating PC12 cells with different concentrations of LBP, intracellular Ca2+ levels decreased, and the expression of nNOS and iNOS as well as the activation of NF-κB were suppressed in a dose-dependent manner (Gao et al., 2014). This indicates that LBP prevents 6-OHDA-induced cell apoptosis by blocking nNOS and iNOS expression, and downregulating the intracellular Ca2+and NF-κB pathways. This study used ELISA to measure protein-bound compound to assess the level of nitrosative stress mediated by nitrogen oxides, which is susceptible to non-specific interference. If validated with immunohistochemistry or Western blotting, the conclusion would have greater specificity.
Chen et al. (2018) used LBP to study the impact on antioxidant capacity of brain tissue in Parkinson’s model mice. Their findings revealed a paradoxical dissociation between behavioral outcomes and neuroprotective efficacy: LBP-treated (50 mg/kg, 100 mg/kg) mice exhibited a significant improvement in pole-climbing scores compared to the MPTP group, the pole - climbing scores of LBP - treated mice remained lower than those of the L - DOPA group. This suggests that while LBP partially alleviates motor deficits, its symptomatic efficacy is statistically inferior to conventional L-DOPA therapy. However, the number of tyrosine hydroxylase-positive (TH+) neurons in the substantia nigra pars compacta (SNpc) of the LBP group was larger than that of the MPTP model and the L-DOPA group, suggesting direct neuroprotection. The activities of antioxidant enzymes SOD, GSH Px, and CAT in the LBP group were significantly increased compared to the MPTP group or the L-DOPA group, while the content of oxidative product MDA, a lipid peroxidation marker, was significantly decreased compared to the above two groups. These results highlight LBP’s unique disease-modifying potential through antioxidant and neuroprotective synergy, contrasting with L-DOPA’s transient symptomatic relief. However, the incomplete behavioral recovery implies that dose optimization or combinatorial therapies may be required for clinical translation. The article proposes that LBP protects neurons through antioxidant effects, but it does not specify the pathways and lacks evidence at the molecular level.
When APS was used in combination with L-dopa, the content of glutathione (GSH), activities of the GSH-Px and SOD in L-dopa-induced astrocytes were similar to those of the blank control group, and the content of MDA was not significantly higher than that in the blank control group, and both were better than those in the L-dopa alone group. These reactions were evident 4 h after administering these compounds (Li et al., 2006), indicating that the protective effect of APS was time-dependent. Mechanism studies have shown that L-DOPA produces a large number of free radicals during metabolism, leading to oxidative stress and toxic effects on various neurons. ASP can effectively eliminate free radicals and protect astrocytes treated with L-DOPA from injury by increasing the activity of antioxidant enzymes. Similarly, this study has not explored the specific signaling pathway through which APS modulates oxidative stress levels, which would require further investigation. It is recommended that subsequent research designs quantitatively analyze the key molecular targets of APS through Western blotting and ROS fluorescent probes.
When Gao et al. (2015b) used sulfated Poria polysaccharides (SP) to treat MPP + pretreated cells, it was observed that polysaccharides significantly increased the activity of intracellular antioxidant enzymes (such as GSH Px, SOD, and CAT), while reducing the content of oxidative products (such as hydrogen peroxide-H2O2 and MDA), thereby reducing the degree of cellular oxidative injury. Further in vivo experiments showed that injecting MPTP with SP (Gao et al., 2015a) or Ganoderma lucidum polysaccharides (Yang et al., 2017) significantly increased the activity of antioxidant enzymes and the content of superoxide anions, such as SOD, GSH Px, CAT, LDH in the striatum of Parkinson’s mice, while reducing the content of H2O2, MDA, etc., indicating that SP also has strong antioxidant activity in vivo. Histologically, a significant increase in the number of neurons was observed in hippocampal slices of the mouse brain tissue, with larger cell bodies, denser and more organized arrangement, reduced intercellular gaps, and significant restoration of adhesion ability, indicating good recovery of cell morphology and function. Although SP was confirmed to reduce oxidative stress markers, like SOD, GSH-Px, CAT, MDA, etc. in both in vitro and in vivo experiments, the study failed to detect core pathological markers of PD, such as the loss of dopaminergic neurons in the substantia nigra, thereby rendering the neuroprotective conclusions indirect. In summary, polysaccharides can enhance the antioxidant enzyme activity in the striatum of mice and protect dopamine neurons in the brain.
In a study on crude polysaccharides from Chroogomphis rutilus (Zhang et al., 2011) showed that they can inhibit lipid peroxidation in the liver of MPTP injected PD model mice. The results showed that the minimum antioxidant activity of crude polysaccharides from Chroogomphis rutilus was between 2 and 4 mg/mL. With increasing concentration, the antioxidant activity of polysaccharides from Chroogomphis rutilus was significantly enhanced, even reaching 70% of the antioxidant activity of vitamin C. The number of TH positive cells in the substantia nigra of mice in the Chroogomphis rutilus group increased with the increase of polysaccharide concentration, significantly higher than that in the PD model group. Behavioral observations showed significant improvement in the climbing, swimming, and hanging tests compared to the PD group. These results indicate that polysaccharides from Chroogomphis rutilus have good antioxidant activity, ameliorate injury to dopaminergic neurons, and improve the behavioral status of experimental animals. However, the article did not further explore the specific antioxidant mechanism of polysaccharides from Chroogomphis rutilus. This study conducted detailed behavioral observations and comparisons in mice, but the small sample size may compromise the statistical power. Additionally, the assessment of antioxidant capacity relied solely on hepatic lipid peroxidation assays, without evaluating critical antioxidant enzyme activities or intracerebral ROS levels.
5.3 Improve mitochondrial functions
Mitochondria play a central role in cellular energy metabolism (Morris, et al., 2024), providing energy to cells through oxidative phosphorylation. Their regulation of cellular bioenergetic states critically determines whether cells survive or undergo apoptosis. Notably, mitochondrial dysfunction constitutes one of the early pathogenic drivers in PD progression. Experimental models demonstrate that MPTP undergoes bioactivation to MPP+, which is selectively internalized via dopamine transporters into dopaminergic neurons. This metabolite potently inhibits mitochondrial complex I activity, precipitating bioenergetic failure and ultimately provoking dopaminergic neurodegeneration with PD-like symptoms. Under physiological conditions, mitochondrial-derived ROS maintain cellular homeostasis. However, pathological ROS accumulation during cellular stress induces oxidative damage to biomolecules, such as proteins, DNA, and RNA, thereby exacerbating mitochondrial impairment. Crucially, mitochondrial morphology and functionality exhibit tight coupling. Both exogenous stressors and intracellular oxidative stress disrupt mitochondrial morphology and bioenergetic function. The reticular network within mitochondria represents the integrity and damage of organelle health manifests as: (1) abnormal grid morphology, (2) elevated membrane potential (Δψm), (3) aberrant opening of mitochondrial permeability transition pores (mPTP), (4) altered inner membrane permeability, and (5) depressed enzymatic activity, collectively culminating in functional deficits. The mitochondrial membrane potential is also affected by the stimulation of cell apoptosis. After receiving the apoptosis signal, a decrease in mitochondrial membrane potential can be observed in the early stage, and membrane permeability increases, thereby establishing a pro-apoptotic cascade. The excessive accumulation of ROS leads to structural damage marked by mitochondrial membrane potential collapse, which in turn triggers the release of Cyt C from mitochondria, ultimately inducing apoptosis.
Mitochondrial function is finely regulated by various upstream regulatory factors (He et al., 2023), including: AMPK, p38 MAPK, SIRT1. Peroxisome proliferators activated receptors gamma coactivator one alpha (PGC-1α) is key gene regulating mitochondrial biogenesis. It can activate various transcription factors, such as nuclear respiratory factor-1/2 (NRF-1/2), estrogen-related receptors (ERRs), and PPARγ, promoting mitochondrial function and energy metabolism.
Polysaccharides from TCM protect mitochondrial morphology and structure. A study on tricholoma matsutake polysaccharides (TMP) (Lv et al., 2024) demonstrated that the TMP-H (high concentration of TMP) group and TMP-M (medium concentration of TMP) group showed an increase in the average length and number of mitochondrial network branches, as well as an increase in mitochondrial membrane potential (ΔΨm), which stabilized the morphology of the mitochondrial network and significantly increased the cell survival rate and the number of cell body protrusions. This indicates that TMP can repair MPP + - induced injury to the mitochondrial network morphology in PC12 cells, stabilize mitochondrial membrane potential, and protect mitochondrial morphology and structure.
6-OHDA causes an increase in ROS, which leads to damage and a decrease in mitochondrial membrane potential (Δ ψ m), while sea cucumber polysaccharides (Cui et al., 2016) can effectively reduce ROS levels, stabilize mitochondrial membrane potential, and protect mitochondrial structure from damage. It can also activate the PI3K/Akt signaling pathway, which plays a crucial role in maintaining mitochondrial function and cell survival. Activation of this pathway helps to inhibit cell apoptosis and protect normal mitochondrial function. In addition, sea cucumber polysaccharides (Cui et al., 2016) can inhibit the activation of NF- κB, thereby reducing the expression of iNOS and the production of NO, which helps protect mitochondria from oxidative stress injury.
Fucoidan protected mitochondrial function through the PGC-1 α/NRF2 signaling pathway, which enhances antioxidant enzyme activity to improve mitochondrial antioxidant capacity and maintain normal mitochondrial function. Mitochondrial respiratory capacity is a critical determinant of cell survival. Rats treated solely with rotenone exhibited significantly reduced mitochondrial respiratory function, including basal respiration, ATP production, and maximal respiration. Fucoidan at 140 mg/kg completely restored the levels of all three parameters (Zhang et al., 2018), indicating a protective effect on the mitochondrial respiratory chain. In a study on LBP (Dai et al., 2022), it was reported that LBP demonstrated a protective effect on mitochondrial respiratory function. It alleviated the increase of mitochondrial oxidative respiratory enzyme I in the striatum cells of PD rats induced by rotenone, causing only reversible and mild mitochondrial edema, and alleviating mitochondrial damage in the substantia nigra and striatum of PD rats.
Research on the mechanisms of polysaccharides from the sea cucumber is relatively comprehensive. However, the high cost of obtaining it may limit their clinical application. While these studies emphasize mitochondrial protection, deeper mechanistic links (e.g., direct binding targets of polysaccharides) and in vivo pharmacokinetics remain underexplored. Future studies could further investigate whether polysaccharides exert their effects by inhibiting mitophagy (via the PINK1/Parkin pathway, etc.) or modulating endoplasmic reticulum stress (through the PERK/eIF2α pathway, etc.). Comparative analyses of polysaccharide efficacy across models could strengthen translational relevance.
5.4 Induction of autophagy and inhibition of aggregation of abnormal proteins
Autophagy refers to the process in which cells engulf, degrade, and recycle damaged and aging organelles, clearing abnormal protein aggregates to maintain cellular homeostasis. Autophagy is crucial for maintaining neuronal metabolism. Dysfunction of autophagy can lead to the accumulation of pathological proteins that can not be cleared and cause neurotoxicity, which is considered one of the important mechanisms for inducing PD. The AMPK/mTOR signaling pathway is currently widely recognized as an autophagy regulatory signaling pathway. AMPK is an important energy receptor in cells. Under conditions, such as hunger and hypoxia, the AMP/ATP ratio increases, promoting AMPK phosphorylation and activation (Yang L. C. et al., 2018). Conversely, mTOR is a member of the PI3K protein kinase family and a negative regulator of autophagy. It can inhibit the activity of ULK1-Atg13-FIP200 initiation complex, a key target of cellular autophagy. Activated AMPK converts mTOR to an inactive p-mTOR state. Consequently, its inhibitory effect on autophagy is removed and autophagy is induced (Liang et al., 2024). The PI3K/AKT/mTOR signaling pathway is involved in the regulation of autophagy, with PTEN serving as a negative regulator of PI3K. After PI3K is activated, it promotes the conversion of PIP2 to PIP3 and phosphorylates Akt. The activated Akt directly activates the mammalian target of rapamycin complex 1 (mTORC1), which can inhibit the initiation stage of autophagy. The activated mTOR can inhibit autophagy by affecting the formation of the mTORC1 complex. LC3І/П and Beclin are autophagy markers and are often used to evaluate autophagy levels.
The experimental results of rotenone-induced PD rat model showed that the p-AMPK/AMPK ratio in the striatum of rats in the PD group was significantly decreased, and LBP significantly reversed this decrease in the p-AMPK/AMPK ratio in the striatum, increased the phosphorylation level of AMPK and the autophagy level (Dai et al., 2022). These indicate that LBP can regulate autophagy through the AMPK/mTOR signaling pathway, eliminate abnormal proteins, alleviate injury to neurons caused by the accumulation of abnormal proteins, and slow down the PD progression.
To determine whether LBP regulates autophagy through the AKT/mTOR pathway, Western blot analysis was conducted to assess the protein levels of p-AKT and p-mTOR in different groups. It was found that levels of phosphorylated AKT and mTOR in the substantia nigra of mice decreased after MPTP treatment. However, LBP attenuated this decrease of p-Akt and p-mTOR (Wang et al., 2018). Further studies found that the protein expression of PTEN also decreased after LBP treatment. This indicates that LBP treatment exerts its neuroprotective effect and alleviates neuronal damage caused by abnormally aggregated α-syn by inhibiting PTEN and regulating excessive autophagy of dopamine neurons through the AKT/mTOR pathway.
In a study on APS, it was reported that APS could promote the transformation from LC3-I to LC3-II, simultaneously downregulate the expressions of pAKT and pmTOR, upregulate the expression of PTEN, and increase cell viability and autophagy. When the PI3K protein in PC12 cells is knocked down (KD), the effect of APS is reversed in PI3K KD cells (Tan et al., 2020). It indicates that APS activates autophagy through the PI3K/AKT/mTOR pathway, thereby combating PD. Super-resolution microscopy (SIM) and transmission electron microscopy (TEM) observed that the formation of autophagosomes increased in PC12 cells treated with APS, while the formation of autophagosomes decreased in PI3K KD cells. This further confirms that APS regulates autophagy levels through the PI3K/AKT/mTOR pathway, improves cell proliferation and exerts anti-PD effects. These two conclusions seem to be contradictory. LBP suppresses autophagy, while APS activates it, yet both have been reported to possess anti-PD effects. This paradoxical phenomenon likely arises from the dual-phase role of autophagy in PD pathogenesis: moderate autophagy clears α-synuclein aggregates, whereas excessive autophagy triggers neuronal apoptosis. The regulatory direction (suppression vs activation) is contingent upon the cellular damage stage and microenvironmental context. However, the underlying mechanisms require further investigation to elucidate the precise molecular basis. LBP and APS regulate autophagy through the PI3K/AKT/mTOR pathway (Figure 3).
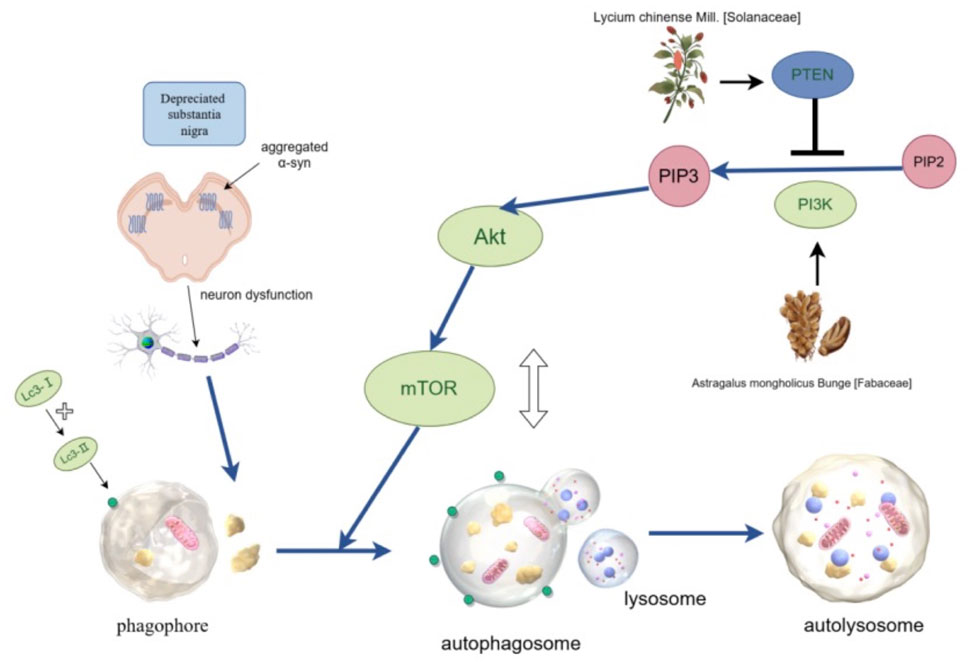
Figure 3. LBP and APS regulate autophagy through the PI3K/AKT/mTOR pathway. The activity of signaling pathway is regulated by phosphorylation of AKT and mTOR, and PTEN is a negative regulator. This pathway has a bidirectional regulatory effect on autophagy: it can maintain the basic level of autophagy while avoiding excessive autophagy.
Oligomers formed by abnormal aggregation of α-syn manifest as the primary cytotoxic species targeting dopaminergic neurons (Sun et al., 2023). These insoluble aggregates preferentially accumulate within midbrain substantia nigra neurons, forming characteristic Lewy bodies - a cardinal histopathological hallmark of PD. Such pathological inclusions directly trigger caspase-mediated apoptotic cascades and neuronal demise. A study showed that the level of aggregated α-syn in the substantia nigra of mice injected with MPTP was significantly increased. LBP significantly reversed this increase (Wang et al., 2018), confirming that LBP can effectively inhibit the aggregation of α-syn.
The ThT fluorescence kinetics experiment is a classic experiment used for analyzing amyloid protein aggregation. The intensity of fluorescence is proportional to the number of protein β-folded structures. Li (2020) first incubated in the α-syn solution to explore the effect of lentinan (LNT) on the aggregation behavior of α-syn. The results showed that the ThT fluorescence signal of the α-syn group increased over time, indicating that the content of β-folding structures in the α-syn group increased with the extension of time. After adding LNT to the α-syn solution, the fluorescence signals of the samples increased only slightly over time. When the LNT concentration rose to 0.8 mg/mL, the fluorescence intensity was only 5% of that in the α-syn group. This result indicates that LNT can effectively inhibit the generation of β-folded structure substances by α-syn. Further research found that LNT can also trigger collective fragmentation and dispersion of the already aggregated α-syn fibers in the sample, and the protein fiber structure was gradually destroyed and finally disappeared, indicating that LNT can depolymerize α-syn fibers. When cells containing α-syn were incubated with a high concentration of LNT (0.8 mg/mL), the cell survival rate was increased by 30%, indicating that the high concentration of LNT reduced the cytotoxicity of the α-syn complex. In conclusion, LNT can counteract the toxicity of α-syn from three aspects: inhibiting the aggregation of α-syn, deaggregating α-syn, and reducing the toxicity of α-syn. This study pioneers the use of LNT to modulate α-synuclein (α-syn) aggregation and explores novel properties of LNT. However, whether LNT eliminates α-syn aggregates through autophagy induction requires further experimental validation.
5.5 Alleviation of neuroinflammation
Neuroinflammation plays an important role in the pathogenesis of PD. It mainly involves the activation of glial cells, the release of inflammatory factors, and the involvement of the peripheral immune system. Among them, inflammation of the central nervous system is mainly due to activation of microglia, as well as astrocytes. The peripheral immune response includes infiltration of T cells, dysfunction of regulatory T cells, and abnormal activation of neutrophils. Microglia exhibit two phenotypes after activation: pro-inflammatory M1 type and anti-inflammatory M2 type. It is worth noting that in PD patients, there is a significant increase in M1 type microglia, and activated M1 type cells can release various pro-inflammatory factors, including chemokines (Joers et al., 2016). These inflammatory mediators (such as TNF α, IL-1 β, IL-6, IL-2, IL-4) and enzymes (such as iNOS and COX-2) orchestrate a self-perpetuating cascade through NF-κB and JAK-STAT signaling pathways, simultaneously compromising blood-brain barrier (BBB) integrity via matrix metalloproteinase-9 (MMP-9) mediated tight junction degradation. Activated microglia release chemokines, facilitating more immune cells to participate in the inflammatory response. Peripheral immune cells (particularly T cells and B cells) migrate to the central nervous system. This process establishes bidirectional crosstalk between brain and peripheral inflammation, thereby synergistically promoting the progression of PD. These pro-inflammatory cytokines induce neuronal damage through the following pathways (Shao et al., 2020): pro-inflammatory cytokines (such as TNF - α and IL-1 β) can directly destroy the structure and function of neurons. Induced oxidative stress, excessive production of ROS and reactive nitrogen species (RNS) leads to oxidative damage to neurons. Inflammatory factors activate the MAPK and NF-κB signaling pathways, promote the misfolding and aggregation of α-syn, and cause a decrease in the number of dopaminergic neurons. Combined effects reduce TH + neurons in substantia nigra pars compacta, accelerating Lewy pathology progression.
In an APS study on PD rats, the therapeutic effect and mechanism of APS on PD in a rat model induced by 6-OHDA was investigated. The study adopted a random grouping design, dividing the experimental rats into the following four groups: PD model group, PD model + L-DOPA group (positive control group), PD model + APS group and a normal control group. It was found that the number of rotations in the PD model + APS group and the PD model + L-DOPA group decreased by 20%, 11%, and 16%, respectively, on the first, seventh, and 14th day. Furthermore, the rotational speed shows non-uniformity, and the maximum rotational speed was reduced to 5–7 r/min (Chen and Zhou, 2015). This result indicates that APS exhibits good therapeutic effects in terms of behavior and are comparable to the efficacy of L-DOPA. Further exploration of the mechanism revealed that the expression levels of inflammatory cytokines IL-6, TNF and IL-2 in the brain tissue of rats in the PD model group + APS group were significantly lower than those in the PD model group on the 14th day. These suggest that APS may exert therapeutic effects on PD by reducing the levels of pro-inflammatory cytokines in the brain and improving the neuroimmune inflammatory response of the body.
It was reported by another study that Polygonatum sibiricum polysaccharide (PSP), when intragastrically administered for six consecutive weeks to 6-OHDA induced PD model rats, increased the expression of PPAR-γ in the brain tissue of PD model rats at the eighth week (Chen et al., 2010). PPAR-γ, a ligand-dependent nuclear hormone receptor, belongs to the nuclear hormone receptor superfamily and plays an important role in regulating gene expression. PPAR-γ has the potential to delay the course of neurodegenerative diseases, such as PD and Alzheimer’s disease, by regulating the production of inflammatory factors. This study indicates that the therapeutic effect of PSP may be related to up-regulating PPAR-γ expression and inhibiting the generation of inflammatory factors.
Comparative analysis reveals both APS and PSP target neuroinflammation through distinct mechanisms: (1) APS directly suppresses cytokine production (IL-6/TNF-α/IL-2), whereas (2) PSP activates PPAR-γ-mediated transcriptional regulation. These findings position plant-derived polysaccharides as multi-target neuroprotective agents against PD-associated neuroinflammation.
The NLRP3 inflammasome plays an important role in neuroinflammation (Paik et al., 2021). NLRP3 is activated by sensing pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). After activation, it recruits ASC and forms the NLRP3 inflammasome with caspase-1. The activated caspase-1 cleaves the precursors of IL-1β and IL-18, releasing mature pro-inflammatory factors, and induces pyroptosis. In PD model mice, Dendrobium huoshanense polysaccharide (DHP) (Shang et al., 2023) significantly improved the motor ability, including the performance in the balance beam experiment, suspension experiment and rod rotation experiment. The mechanisms include: alleviating the injury of dopaminergic neurons in the substantia nigra of the midbrain and increasing the expression of tyrosine hydroxylase (TH); inhibiting the inflammatory response by reducing the levels of pro-inflammatory factors IL-1β, TNF-α and IL-18 in microglia; reducing the expressions of NLRP3, ASC, Caspase-1 and IL-1β, thereby inhibiting the activation of the NLRP3 inflammasome and reducing the release of pro-inflammatory factors. Antrodia polysaccharides (Yang Y. et al., 2018) also had a similar effect through reducing the expression of pro-inflammatory factors IL-1β, TNF-α and IL-6, inhibiting the excessive activation of microglia, down-regulating the expression of NLRP3-Caspase1 inflammasome and its downstream inflammatory factors, and alleviating the injury to dopaminergic neurons.
These polysaccharides achieve their therapeutic benefits primarily by inhibiting the activation of the NLRP3 inflammasome pathway. Both studies employed ELISA to quantify inflammatory cytokines (IL-1β↑, IL-4↓, IL-6↑) in murine brain tissues, coupled with Western blot analysis of pro-caspase-1, activated caspase-1, and NLRP3 expression. These approaches provided reliable quantification of CNS neuroinflammation and inflammasome activation status. However, the mechanistic interpretations remain correlative, failing to elucidate whether polysaccharides directly bind to NLRP3 or modulate upstream signaling pathways (e.g., NF-κB priming or K+ efflux).
Polysaccharides in TCM regulate signaling pathways and mitigate neuroinflammation. TNF-α, IL-1, and the NF-κB pathway can trigger the activation of the NLRP3 inflammasome, and the latter promotes the synthesis and secretion of pro-inflammatory factors. A high-dose of Pholidota chinensis lindl polysaccharides significantly reduced the expression levels of TNF-α, IL-1β and NF-κB in rats, and increased the number of TH-positive neurons (Zhou N. et al., 2021). These indicate that Pholidota chinensis lindl polysaccharides inhibit the activation of the NLRP3 inflammasome by regulating the NF-κB pathway and exerts anti-inflammatory and neuroprotective effects. Momordica charantia polysaccharides (MCPs) can reverse the increase of TLR4, MyD88, and p-p65 proteins induced by MPP+. MCPs inhibit the generation of NLRP3 inflammasome through the TLR4/MyD88/NF-κB signaling pathway and exert an anti-neuroinflammatory effect (Guo et al., 2021).
5.6 DA neuroprotection
In recent years, significant progress has been made in research on dopaminergic neuroprotection, particularly in revealing the relationship between cellular aging and neurodegenerative diseases. Research has shown that aging and death of dopaminergic neurons in the substantia nigra are the core pathological mechanisms of PD. Senescence markers, such as β-galactosidase (β-gal), telomerase, p16INK4a, matrix metalloproteinase-3 (MMP-3), and nuclear membrane protein Lamin B1, are closely related to the cellular aging process in PD (Zhang Y. et al., 2023). As a classic marker of cellular aging, β-gal elevation mirrors lysosomal stress in senescent neurons. Telomerase preserves telomeric TTAGGG repeats, delaying replicative senescence. p16INK4a enforces G1 cell cycle arrest via CDK4/6 inhibition, while MMP-3 degrades extracellular matrix components, disrupting neuron-glia crosstalk. Lamin B1 plays a crucial role in maintaining nuclear structure and cell cycle regulation, and its reduction may be associated with neuronal aging and degeneration.
In terms of signaling pathways, the Wnt1 and β-catenin pathways in the substantia nigra are involved in processes, such as cell proliferation, differentiation, migration, and maintaining tissue homeostasis. In MPTP induced PD animal models, activation of the Wnt/β-catenin signaling pathway can improve nigrostriatal injury and reduce the death of dopaminergic neurons. In vitro experiments have also shown that activated Wnt signaling pathway can improve MPTP induced dopaminergic neuron cytotoxicity (Wang et al., 2007). GSK-3 β is a key regulatory factor in the Wnt/β - catenin signaling pathway, and its phosphorylation level directly affects the stability and pathway activity of β - catenin.
DHP may protect neurons by delaying the aging of astrocytes. Compared with the normal control group, mice treated with DHP exhibited the following characteristics: the proportion of cells in the G0/G1 phase decreased, the proportion of cells in the S phase increased, the positive rate of β-gal decreased, the expression of telomerase increased, the expressions of p16INK4a and MMP-3 decreased, and the fluorescence expression of Lamin B1 in mice of the DHP 100 mg/kg group and the DHP 350 mg/kg group increased (Zhang P. et al., 2023). All these were related to cellular senescence. These results indicate that DHP can inhibit astrocyte senescence through different mechanisms and pathways, thereby alleviating neuronal damage.
In another study, 6-OHDA induced PD rats were treated with Cistanches Herba polysaccharides (CDPS) (Yin et al., 2020). It was found that after CDPS intervention, the autonomous activity of SD rats was improved, shortened swimming static time and the decreased number of autonomous standing activities were restored, and the protein expression of Wnt1 and β-catenin in the substantia nigra was increased compared with the PD model group. The expression of GSK-3β mRNA was inhibited. It is speculated that CDPS may protect dopaminergic neurons by inhibiting the activity of GSK-3β, up-regulating the expression of β-catenin protein, activating the Wnt/β-catenin signaling pathway, promoting the proliferation and differentiation of dopaminergic cells. However, the mechanistic study did not conclusively prove the direct association between β-catenin and neuroprotection. It is necessary to verify the key nodes of the signaling pathway (such as detecting the nuclear translocation of β-catenin) to prove that the neuroprotective effect stems from the activation of the pathway. Moreover, the experimental design lacked reverse verification using a Wnt pathway inhibitor; for instance, employing a GSK-3β activator to counteract CDPS effects would strengthen the mechanistic argument.
Basic fibroblast growth factor (bFGF), an important neuroprotective factor, has been proven to inhibit the degeneration of dopaminergic neurons and promote their survival and functional recovery by activating the PI3K/Akt and ERK1/2 signaling pathways. A study found that on the 14th day, the TH content in the substantia nigra of rats in the PD model + APS group was significantly higher than that in the PD group (Chen and Zhou, 2015). In addition, the expression level of bFGF protein in substantia nigra was significantly lower than that in the PD model group. The authors interpreted the APS-mediated reduction of the 6-OHDA-induced bFGF increase as suggestive of a direct protective effect on dopaminergic neurons. Nevertheless, the observed decrease in bFGF expression presents a conceptual challenge. As bFGF is a well-established neuroprotective factor, its downregulation would typically be associated with exacerbated damage rather than protection. Therefore, the authors’ speculation that the decrease in bFGF is linked to the neuroprotective effect of APS requires rigorous experimental verification to establish a plausible causal relationship.
In a cell culture study, SH-SY 5Y cells pretreated with crude polysaccharides from Chroogomphus rutilus, followed by a thorough wash and adding MPP + to verify whether they have direct protective effect on neural cells. The results showed that the survival rate of SH-SY 5Y cells pretreated with 800 μg/mL of crude polysaccharides from Chroogomphus rutilus was significantly higher than that of the control group (Zhang et al., 2013). This indicates that the protective effect of crude polysaccharides from Chroogomphus rutilus on MPP + damaged cells stems from the direct protective effect of polysaccharides on cells, rather than the direct combination of polysaccharides with MPP+. This study features a well-designed approach to eliminate interference. By thoroughly washing the cells after polysaccharide pretreatment and before adding the neurotoxin MPP+, the study effectively rules out false positives arising from direct binding between the polysaccharides and MPP+. However, the research utilizes crude polysaccharides. Without identifying the specific active monomers responsible for the effect (such as determining molecular weight or monosaccharide composition), standardization of the active component remains challenging. Future directions should include: isolating and purifying individual polysaccharide fractions, elucidating their structures using techniques like mass spectrometry (MS) and nuclear magnetic resonance (NMR), and screening these purified components to identify the core active ingredient.
LBP has a significant protective effect on PC12 cells induced by MPP+. A study showed that after 12 h and 24 h of LBP pretreatment, the cell survival rate increased significantly. The increase in cell survival rate was the most prominent after 24 h of pretreatment with 100 μmol/L LBP (Li P. et al., 2020). This indicates that LBP can counteract the neurotoxicity of MPP+ in a concentration-dependent manner and protect dopaminergic neurons.
In a PD silkworm model (Song et al., 2022), it was found that LBP coated on mulberry leaves significantly improved the motor symptoms of silkworms. UPLC detection showed that the dopamine content in the brains of PD silkworms significantly increased after LBP administration. ELISA results showed that LBP significantly enhanced the TH protein level of PD silkworms. Immunofluorescence quantification revealed an increase in the TH protein level, and the mRNA expression levels of downstream DDC and PRKN genes, indicating the protective effect of LBP on the gene expression and synthesis process of dopamine in dopaminergic neurons. This study demonstrates systematic model construction through comprehensive detection methodologies, including motor function assessment via behavioral trajectory analysis, dopamine metabolism quantification using UPLC, oxidative stress evaluation (T-AOC/MDA biomarkers), critical gene expression profiling (TH/PRKN/DDC). However, notable methodological limitations exist: insufficient LBP dosing, with only two doses (50/100 mg/kg) tested, which fails to establish dose-response relationships and an unvalidated positive control, as the LD-CD (levodopa-carbidopa, 4 mM) group used a single concentration without a justification for the dose.
5.7 Intestinal flora imbalance and microbial-gut-brain axis regulation mechanism
Recent studies have shown that gut microbial dysbiosis may play an important role in the pathogenesis of PD. Metagenomic profiling reveals PD patients exhibit significant ecological shifts in gut microbiota composition, with a decrease in beneficial bacteria and an increase in harmful bacteria, as well as a decrease in the abundance of gut microbiota characterized by depletion of butyrate-producing taxa (e.g., Faecalibacterium prausnitzii) and concomitant enrichment of pro-inflammatory species (e.g., Enterobacteriaceae), alongside reduced alpha diversity indices. This disruption of gut microbiota may lead to increased permeability of the intestinal epithelial barrier, triggering bacterial and their products (such as lipopolysaccharides) translocation, activating TLR4/MyD88-dependent innate immunity and instigating systemic low-grade inflammation.
In addition, abnormal deposition of α-syn within enteric plexuses precede motor manifestations by 5–10 years, correlating with prodromal gastrointestinal dysfunction. Dysbiosis of gut microbiota can also elevate levels of inflammatory cytokines, such as TNF-α and IL-6, which in turn activate gut glial cells and promote misfolding of α-synuclein. Retrograde propagation studies validate prion-like α-synuclein trafficking along vagal afferents, culminating in nigrostriatal dopaminergic denervation.
Active polysaccharides can be used by intestinal flora as the main energy source, and the degraded monosaccharides promote the colonization of probiotics and maintain the homeostasis of intestinal flora. In vitro fermentation studies showed that Dendrobium officinale polysaccharides increased the abundance of some beneficial bacteria in the gut, including Lactobacillus, Faecalibacterium and Rummeliibacillus, while reducing the abundance of some harmful bacteria, such as Shewanella, Geodermatophilus, Peptostreptococcaceae, and Mycobacterium (Peng et al., 2023). Polygonum polysaccharide can regulate the composition of intestinal microorganisms in mice and enhance the integrity of intestinal barrier (Jiang et al., 2024). This regulatory effect helps to improve the balance of the gut microbiome, thereby promoting gut health.
In the process of polysaccharide degradation, intestinal flora produced a large amount of short-chain fatty acids (SCFA), which reduced the release of intestinal pro-inflammatory cytokines like TNF-α and IL-6, and inhibited intestinal inflammation. Dendrobium officinale polysaccharides increased the content of short-chain fatty acids, such as acetic acid, propionic acid and butyric acid in the gut, which improved intestinal health (Fu et al., 2019).
Mediated by enteric nervous system inflammation, microglia are abnormally activated, promoting the release of IL-1β, TNF-α, IL-6, NO and proteases in the brain, inducing neuroinflammation and eliciting injury to dopaminergic neurons. Dendrobium officinale polysaccharidesdown-regulated the expression of pro-inflammatory cytokines, such as TNF-α, IFN-γ, IL-4 and IL-6, and increased the expression of anti-inflammatory cytokine IL-10, improved gut microbiome mediated immune response and restored gut flora (Liang et al., 2022).
5.8 Multifunctional mechanisms of polysaccharides in PD treatment
5.8.1 Regulation of neurotransmitter systems
PD patients not only have degeneration of DA neurons, which affects motor regulation (such as gait and balance control), but also demonstrate strong associations with dementia-related cognitive decline. The cholinergic neurons also show reduced expression of N-acetylcholine receptor (nAChR) α4β2 subtypes along with upregulated M-acetylcholine receptor (mAChR) M1/M4 subtype activity (Golan et al., 2022; Ni, 2023).
Acetylcholine (ACh) plays a crucial role in regulating behaviors, such as movement, foraging, mating, and egg laying in C. elegans. The experiment on the regulatory effect of APS on acetylcholinesterase (AChE) activity in BZ555 nematodes showed that BZ555 nematodes exposed to 6-OHDA exhibited significant downregulation of AChE activity. However, when exposed to a solution containing 2.0 mg/mL APS, its AChE activity and behavioral activity was significantly normalized (Li et al., 2016). It is speculated that the protective effect of APS may be related to protecting AChE from damage and restoring acetylcholine system function. APS effectively inhibits the neurotoxicity caused by 6-OHDA, thus playing a neuroprotective role at the system level. This study showed concentration-dependent neuroprotection within the range of 0.5–4.0 mg/mL APS with 4.0 mg/mL being the optimal concentration. However, nematodes lack the blood-brain barrier, immune system, and complex neural circuits of mammals, making it impossible to directly translate these findings to human physiology.
5.8.2 Behavioral improvement in PD models
Accumulating preclinical evidence demonstrates that botanical polysaccharides exhibit significant motor improvement effects in a mouse model of PD. Among them, LBP ameliorates PD-like symptoms including hypophagia, gait instability, and static tremors, as well as motor coordination, such as pole climbing and grasping. LBP can significantly improve cognitive deficits in Morris Water Maze Test and significantly shorten their escape latency (Wang et al., 2007). ASP can significantly improve the limb grip and suspension dwell time of PD mice, and alleviate motor dysfunction (Zhang et al., 2024). PSP showed good performance in gait regulation and paw extension tests, while CDPS significantly increased the spontaneous activity and standing frequency of PD mice. The polysaccharides from Chroogomphis rutilus also showed significant improvement effects in suspension experiments, pole climbing, and swimming tests, further demonstrating the potential value of polysaccharides from TCM in PD treatment.
5.8.3 Suppression of astrocyte activation
The activation of astrocytes is a hallmark of neurodegeneration, mainly manifested by an increase in GFAP levels. MPTP injection into mice resulted in a significant increase in GFAP positive cells, enlarged cell spheres, and more cell protrusions in the ventral striatum. The LBP treatment group showed a decrease in GFAP, which alleviated the activation of astrocytes in PD mice and exerted its protective effect on dopaminergic neurons.
5.8.4 Multi-target mechanistic actions
Polysaccharides, composed of monosaccharides, usually have unique biological activities. These components often regulate multiple targets in the body and work synergistically. For example, the effect of Stichopus japonicus polysaccharide (SJP) on the SH-SY5Y PD model of human neuroepithelial tumor cells induced by 6-OHDA was investigated. It was demonstrated that SJP inhibited intracellular ROS generation and reduced oxidative stress damage. It inhibited the expression of cyclin D3, leading to the arrest of the cell cycle in the G1/S phase. In the meantime, it reversed the change in the Bax/Bcl-2 ratio and activated both p38/c-Jun N-terminal kinase (JNK)-MAPK and PI3K/Akt pathways, which play a crucial role in cell apoptosis. Furthermore, the activation of NF-κB, preventing the upregulation of iNOS and NO release in cells, and alleviating mitochondrial dysfunction were observed (Wang J. et al., 2021). SJP effectively protects dopaminergic neurons through the above five mechanisms.
Similarly, LBP can exert strong antioxidant effects, effectively suppress α-syn aggregation, and inhibit cell apoptosis through the PI3K/Akt pathway. As mentioned earlier, the mechanism of LBP in anti-PD involves multiple pathways, including: 1) eliminating ROS, reducing oxidative stress, reducing Cyt C release, and anti-apoptosis; 2) inhibiting the increase of mitochondrial oxidative respiratory enzyme Ⅰ in cells and alleviating mitochondrial oxidative damage; 3) enhancing the activity of antioxidant enzymes to combat oxidative stress; 4) inhibiting the aggregation of α-syn and alleviating the damage to DA neurons caused by its toxic effects; 5) activating the PI3K/Akt pathway to resist apoptosis; 6) regulating autophagy in cells through the AMPK/mTOR signaling pathway.
Ganoderma lucidum polysaccharides not only reduces the generation of ROS, upregulates the activity of SOD, alleviates oxidative stress damage, but also reduces the expression of pro-inflammatory factors IL-1 β, TNF α, and IL-6, inhibits excessive activation of microglia, and exerts anti neuroinflammatory effects. They can also alleviate inflammation by downregulating the expression of NLRP3-Caspase1 inflammasome and its downstream inflammatory factors (Yang Y. et al., 2018).
Tricholoma matsutake polysaccharides can stabilize mitochondrial network morphology and membrane potential, protect mitochondrial function, and reduce cell apoptosis by reversing MPTP induced decrease in the Bax/Bcl-2 ratio.
Momordica charantia polysaccharides (MCPs) can reduce the expression of inflammatory factors like TNF - α and IL-1 β, increase the expression of anti-apoptotic protein Bcl-2, and regulate anti-apoptosis. MCPs can also exert anti-inflammatory and anti-apoptotic effects by regulating the TLR4/MyD88/NF-κB signaling pathway. A summary of mechanisms underlying the therapeutic effect of TCM on PD can be found in Table 3.
6 Application of polysaccharides from traditional Chinese medicine
As previously described, Chinese Medicine Prescriptions containing Astragalus, Lycium barbarum, Cistanche deserticola, etc., have been proven to improve symptoms of PD in clinical trials. The mechanisms include regulating the PI3K/Akt/mTOR pathway to inhibit neuronal apoptosis, suppressing caspase cascade reactions, and inhibiting α-syn accumulation. These mechanisms overlap with the action mechanisms of polysaccharides derived from TCM, suggesting the universality of polysaccharide components in the treatment of PD. The strong preclinical evidence for polysaccharides from TCM indicates that they can alleviate motor and cognitive defects in PD mouse models. These findings collectively suggest that polysaccharides from TCM may exert their effects through synergistic mechanisms. Their ability to simultaneously relieve motor and non-motor symptoms in PD models makes them an important candidate in developing comprehensive treatment strategies for neurodegenerative diseases.
At present, hundreds of polysaccharides have been discovered, but due to their high molecular weight, complex structure, and difficulty in purifying bioactive compounds, only seven polysaccharides have been approved to enter the market. Among them, the most widely used are anticoagulant heparin and chondroitin sulfate for the treatment of osteoarthritis. ASP is used for diabetic nephropathy due to its anti-inflammatory effect (Zhou G. et al., 2021), which can ameliorate inflammatory reaction and reduce urinary protein with a good safety profile. Although there is currently no clinical research on polysaccharides in the treatment of PD, it has been confirmed in in vitro cell experiments and animal experiments that polysaccharides from TCM have multiple biological functions, such as immune regulation, antioxidant, lowering blood glucose, and protecting the nervous system. The safety and efficacy of traditional Chinese medicine polysaccharides have been verified.
7 Conclusion and future perspectives
In conclusion, polysaccharides derived from TCM demonstrate potential in treating PD through multi-target mechanisms. Although over a dozen of polysaccharides have been shown to enhance cell survival, improve motor function in animal models, and histologically delay PD pathological progression, there remains a notable absence of clinical trials validating their efficacy. Current research faces limitations, such as the vast majority of studies have a relatively small sample size; cellular investigations predominantly focus on signaling pathways, with insufficient depth of exploration. Most literature only detect the expression of key proteins (e.g., Bax/Bcl-2 and caspase-3 expression), lacking exploration of upstream regulators (miRNAs, epigenetic modifications) and pathway crosstalk (e.g., PI3K/Akt-NF-κB interactions); animal experiments primarily employ single neurotoxin models that only partially replicate human PD pathology, failing to capture complex etiological factors like α-syn aggregation and neuroinflammatory cascades.
In the future, network pharmacology can be combined with metabolomics, transcriptomics, and neuroimaging to decipher precise neuroprotective mechanisms of polysaccharides and their interactions. Large-scale randomized controlled trials with extended observation periods are required. Standardized extraction/purification protocols are needed to address compositional variation. Advanced pharmaceutical engineering strategies, such as chemical modifications (sulfation/selenization) to enhance bioavailability, nano-delivery systems for improved blood-brain barrier penetration, can be improved to promote the translation of herbal polysaccharides from basic research to clinical practice and accumulate more high - quality clinical data.
While polysaccharides from TCM exhibit promising multi-target anti-PD properties, their clinical translation requires optimized formulation strategies and high-quality evidence generation to advance from basic research to therapeutic applications.
Author contributions
XZ: Writing – original draft, Data curation, Formal Analysis, Software, Visualization. QP: Conceptualization, Writing – review and editing, Methodology, Validation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank Hao Zhang in the Traditional Chinese Medicine Department for his help with interpreting TCM theories of various herbal recipes.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ben-shlomo, Y., Darweesh, S., Llibre-guerra, J., Marras, C., Luciano, M. S., Tanner, C., et al. (2024). The epidemiology of parkinson's disease. Lancet 403 (10423), 283–292. doi:10.1016/s0140-6736(23)01419-8
Bian, Z., Zhang, R., Zhang, X., Zhang, J., Xu, L., Zhu, L., et al. (2023). Extraction, structure and bioactivities of polysaccharides from rehmannia glutinosa: a review. J. Ethnopharmacol. Apr 6, 116132. doi:10.1016/j.jep.2022.116132
Cai, J. (2013). Chinese herbal compound preparation for improving neurotransmitter metabolism in parkinson's disease. China, 2013–3.
Chen, H., Zhang, H., Shi, L., Li, Y., Ren, Y., Jing, W., et al. (2018). Antioxidative and neuroprotective effects of Lycium barbarum polysaccharide on parkinson's disease mice. Chin. J. Nerv. Ment. Dis. 44 (10), 613–618. doi:10.3969/j.issn.1002-0152.2018.10.008
Chen, J., Li, Y., Tian, W., and Cheng, W. (2010). Effect of polygona-polysaccharose (PP) on expression of PPAR-γ in brain tissue of rats with parkinson disease. Prog. Mod. Biomed. 10 (05), 814–817+792. doi:10.13241/j.cnki.pmb.2010.05.016
Chen, L., and Zhou, Q. (2015). Astragalus polysaccharide alleviates symptoms of rat with parkinson's disease. World Sci-technol RD 37 (02), 168–171. doi:10.16507/j.issn.1006-6055.2015.02.013
Chen, S., Li, Q., Zhang, C., Chen, W., Chen, X., Yin, L., et al. (2024). Effectiveness of congrong shujing granules on expression of substantia nigra metabolites in parkinson's disease patients: a randomized controlled trial. Chin. J. Integr. Tradit. West. Med. 44 (03), 296–302. doi:10.7661/j.cjim.20240131.010
Chen, S., Xiao, S., Lin, Y., Yang, S., Xu, Q., Cai, J., et al. (2019). Observation on the effects of congrongjing granules and Western medicine in the treatment of parkinson’s disease. Chin. J. Integr. Med. Cardio-Cerebrovasc. Dis. 17 (19), 2914–2917. doi:10.12102/j.issn.1672-1349.2019.19.007
Cheng, N., Wang, H., Hao, H., Rahman, F., and Zhang, Y. (2023). Research progress on polysaccharide components of Cistanche deserticola as potential pharmaceutical agents. Eur. J. Med. Chem. 245 (Pt1), 114892. doi:10.1016/j.ejmech.2022.114892
Chinese Society of Parkinson’s Disease and Movement Disorders (2020). Chinese guidelines for the treatment of Parkinson’s disease (4th edition). Chin. J. Neurol. 53 (12), 973–986. doi:10.3760/cma.j.cn113694-20200331-00233
Cui, C., Cui, N., Wang, P., Song, S., Liang, H., and Ji, A. (2016). Neuroprotective effect of sulfated polysaccharide isolated from sea cucumber Stichopus japonicus on 6-OHDA-induced death in SH-SY5Y through inhibition of MAPK and NF-κB and activation of PI3K/Akt signaling pathways. Biochem. Biophys. Res. Commun. 470 (2), 375–383. doi:10.1016/j.bbrc.2016.01.035
Dai, Z., Yao, X., Xu, X., Xiao, X., Guan, J., Wang, Y., et al. (2022). Study on the neuro-protective effect of Lycium barbarum polysaccharide on neurons of rats with rotenone-induced Parkinson’s disease via AMPK-mTOR signaling pathway. Mod. J. Integr. Tradit. Chin.Western Med. 31 (15), 2080–2083+2158. doi:10.3969/j.issn.1008-8849.2022.15.006
Dong, N., Li, M., Xu, D., Chen, X., Zhan, S., Pan, S., et al. (2021). Immunomodulatory effects of polysaccharides derived from five kinds of edible fungi and their compound polysaccharides on RAW264.7 cells. Food Res. Dev. 42 (9). 1–10. doi:10.12161/j.issn.1005-6521.2021.09.001
Dorsey, E. R., and Bloem, B. R. (2018). The parkinson Pandemic-a call to action. JAMA Neurol. 75 (1), 9–10. doi:10.1001/jamaneurol.2017.3299
Du, Y., Wan, H., Huang, P., Yang, J., and He, Y. (2022). A critical review of astragalus polysaccharides: from therapeutic mechanisms to pharmaceutics. Biomed. Pharmacother. Mar. 147, 112654. doi:10.1016/j.biopha.2022.112654
Fang, L., and Cai, J. (2021). Observation on the curative effect and sleep quality of patients with Parkinson’s disease depression treated with congrong shujing granules. World J. Sleep. Med. 8 (08), 1346–1348. doi:10.3969/j.issn.2095-7130.2021.08.019
Fu, Y., Zhang, J., Chen, K., Xiao, C., Fan, L., Zhang, B., et al. (2019). An in vitro fermentation study on the effects of Dendrobium officinale polysaccharides on human intestinal microbiota from fecal microbiota transplantation donors. J. Funct. Food. 53, 44–53. doi:10.1016/j.jff.2018.12.005
Gao, G., Wang, J., Jiang, F., Li, G., Liu, X., Xue, H., et al. (2015a). The neuroprotective effect of sulfated pachymaran on the MPTP-Induced parkinson mice. Chin. Pharmacol. Bull. 31 (12), 1699–1704. doi:10.3969/j.issn.1001-1978.2015.12.014
Gao, G., Wu, C., Chen, Z., and Liu, X. (2015b). The protective effect of sulfation pachymaran on the damage of PC12 cells induced by MPP+. J. Huaibei Norm. Univ. Nat. Scie. Ed. 36 (04), 52–58. doi:10.3969/j.issn.2095-0691.2015.04.011
Gao, K., Liu, M., Cao, J., Yao, M., Lu, Y., Li, J., et al. (2014). Protective effects of Lycium barbarum polysaccharide on 6-OHDA-induced apoptosis in PC12 cells through the ROS-NO pathway. Molecules 20 (1), 293–308. doi:10.3390/molecules20010293
Gao, Z. (2022). The structure characterization and immunological activity of polysaccharides derived from Cistanche deserticola. Tianjin, China: Master’s Theses Full-text Database.
Geng, X. (2023). Prepared radix rehmanniae research progress in chemical composition changes and pharmacological effects. Brand Stand. 3, 106–108. doi:10.3969/j.issn.1674-4977.2023.03.035
Golan, H., Volkov, O., and Shalom, E. (2022). Nuclear imaging in Parkinson’s disease: the past, the present, and the future. J. Neurol. Sci. 436, 120220. doi:10.1016/j.jns.2022.120220
Guo, D., Zhou, J., Zhang, M., Taximaimaiti, R. Y. S., Wang, X., and Wang, H. (2021). Momordica charantia polysaccharides attenuates MPP+ -Induced injury in parkinson’s disease mice and cell models by regulating TLR4/MyD88/NF-κB pathway. Int. J. Poly. Sci. 2021, 1–15. doi:10.1155/2021/5575636
Guo, Y., Cao, L., Zhao, Q., Zhang, L., Chen, J., Liu, B., et al. (2016). Preliminary characterizations, antioxidant and hepatoprotective activity of polysaccharide from Cistanche deserticola. Int. J. Biol. Macromol. 93 (Pt A), 678–685. doi:10.1016/j.ijbiomac.2016.09.039
Hao, C. ., Xu, Z., Xu, C., and Yao, R. (2024). Anti-herpes simplex virus activities and mechanisms of marine derived compounds. Front. Cell. Infect. Microbiol. 13, 1302096. doi:10.3389/fcimb.2023.1302096
He, H., Chen, B., Shi, Q., Zheng, L., Li, X., Li, D., et al. (2022). Advances in molecular mechanisms related to parkinson's disease. J. Reg. Anat. Oper. Surg. 31 (5), 456–460. doi:10.11659/jjssx.11E021034
He, L., Fan, H., Li, Y., Jia, L., Qin, S., Wang, L., et al. (2023). Mitochondrial dysfunction and Parkinson’s disease. Chin. J. Pract. Nerv. Dis. 26 (10), 1305–1310. doi:10.12083/SYSJ.230261
Hu, A., Yin, C., Guo, Y., Wang, B., and Liu, X. (2021). Role of mitochondrial dysfunction and oxidative stress in parkinson's disease. Med. Recapitul. (15), 2929–2934. doi:10.3969/j.issn.1006-2084.2021.15.004
Hu, K., Ren, Y., and Jia, D. (2020). Clinical study on chaihu jia longgu muli tang combined with three needles for tremor in treating parkinson disease. J. New. Chin. Med. 52 (14), 24–27. doi:10.13457/j.cnki.jncm.2020.14.006
Huang, G., Mei, X., and Hu, J. (2017). The antioxidant activities of natural polysaccharides. Curr. Drug. Targets 18 (11), 1296–1300. doi:10.2174/1389450118666170123145357
Huang, L., Zhang, H., Xia, W., Yao, N., Xu, R., He, Y., et al. (2024). Structural characteristics, biological activities and market applications of rehmannia radix polysaccharides: a review. Int. J. Biol. Macromol. 282 (Pt 1), 136554. doi:10.1016/j.ijbiomac.2024.136554
Jia, L., and Li, X. (2022). Analysis of the efficacy and safety of the treatment of depression with the addition of chaihu longgu muli decoction. Chin. Pract. Med. 17 (10), 30–33. doi:10.14163/j.cnki.11-5547/r.2022.10.009
Jiang, Y., Zeng, X., Dai, H., Luo, S., and Zhang, X. (2024). Polygonatum sibiricum polysaccharide regulation of gut microbiota: a viable approach to alleviate cognitive impairment. Int. J. Bio. Macromol. 277, 134494. doi:10.1016/j.ijbiomac.2024.134494
Jin, M., Zhao, K., Huang, Q., Xu, C., and Peng, S. (2012). Isolation, structure and bioactivities of the polysaccharides from Angelica sinensis (oliv.) diels: a review. Carbohydr. Polym. 89 (1), 713–722. doi:10.1016/j.carbpol.2012.04.049
Jin, Y., Ge, J., Liu, S., Chen, J., and Li, Y. (2022). Advances in chemical structure,pharmacological action and structure-activity relationship of angelica polysaccharide. Inf. Tradit. Chin. Med. 39 (02), 69–77. doi:10.19656/j.cnki.1002-2406.20220213
Joers, V., Tansey, M. G., Mulas, G., and Carta, A. R. (2016). Microglial phenotypes in Parkinson’s disease and animal models of the disease. Prog. Neurobiol. 207, 57–75. doi:10.1016/j.pneurobio.2016.04.006
Li, H., Shi, R., Ding, F., Wang, H., Han, W., Ma, F., et al. (2016). Astragalus polysaccharide suppresses 6-Hydroxydopamine-Induced neurotoxicity in Caenorhabditis elegans. Oxid. Med. Cell. Longev. 2016, 4856761. doi:10.1155/2016/4856761
Li, L., Zuo, Z. T., and Wang, Y. (2022). The traditional usages, chemical components and pharmacological activities of wolfiporia cocos: a review. Am. J. Chin. Med. 50 (2), 389–440. doi:10.1142/s0192415x22500161
Li, P., Du, Y., Wen, J., Shi, Y., Yan, W., Li, X., et al. (2020). Lycium barbarum polysaccharide regulate MPP+-Induced parkinson's disease model cell apoptosis by activating the PI3K/Akt pathway. Chin. Remedies. Clin. 20 (01), 28–30. doi:10.11655/zgywylc2020.01.007
Li, W., Hu, X., Wang, S., Jiao, Z., Sun, T., Liu, T., et al. (2020). Characterization and anti-tumor bioactivity of astragalus polysaccharides by immunomodulation. Int. J. Biol. Macromol. 145 (15), 985–997. doi:10.1016/j.ijbiomac.2019.09.189
Li, W., Sun, S., Yuan, H., Wang, H., Zhang, Y., and Sun, B. (2006). Time dependency of astragalus polysaccharides against systemic injury of free radical in astrocyte culture medium. Chin. J. Clin. Rehabil. 10 (11), 59–61. doi:10.3321/j.issn:1673-8225.2006.11.023
Li, Y. (2020). A study on the regulation of α-synuclein aggregation by lentinan. Chin. [Master's thesis]. Master's Theses Full-text Database. doi:10.27157/d.cnki.ghzku.2020.004324
Liang, Y., Huang, Y., Liu, J., Ou, Y., Li, Y., and Zhang, R. (2024). Autophagy and neurological diseases. Chin. J. Comp. Med. 34 (3), 111–119. doi:10.3969/j.issn.1671-7856.2024.03.014
Liang, Y., Liu, G., Xie, L., Su, K., Chang, X., Xu, Y., et al. (2022). Dendrobium candidum polysaccharide reduce atopic dermatitis symptoms and modulate gut microbiota in DNFB-Induced AD-like mice. Front. Physiol. 13, 976421. doi:10.3389/fphys.2022.976421
Li, Y. (2020). A study on the regulation of α-synuclein aggregation by lentinan. Chin. [Master’s thesis]. Master’s Theses Full-text Database. doi:10.27157/d.cnki.ghzku.2020.004324
Lin, Y., Tang, L., Huang, P., Liu, T., Zhong, J., Xu, Q., et al. (2020). Mechanisms of cong rong shu jing compound effects on endoplasmic reticulum stress in a rat model of parkinson's disease. Altern. Med. 2020 1818307 2020, 1818307. doi:10.1155/2020/1818307
Liu, W., Cao, J., Jing, Z., Cui, T., Yin, M., Liu, X., et al. (2022). Neurorotective effect of chaihu jia longgu mulitang on parkinson's disease with depression model rats and its mechanism based on AMPK/m TOR signaling pathway. Chin. J. Exp. Tradit. Med. Form. 28 (08), 21–29. doi:10.13422/j.cnki.syfjx.20220838
Luo, Q., Cai, Y., Yan, J., Sun, M., and Corke, H. (2004). Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 76 (2), 137–149. doi:10.1016/j.lfs.2004.04.056
Lv, H., Shen, X., Zhao, F., and Zhu, M. (2024). Effects of Tricholoma matsutake polysaccharides on 1-methy-4-pehnyl-pyridine ion-induced PC12 cell damage. Acta Anat. Sin. 55 (01), 49–54. doi:10.16098/j.issn.0529-1356.2024.01.007
Morris, H. R., Spillantini, M. G., Sue, C. M., and Williams-Gray, C. H. (2024). The pathogenesis of parkinson's disease. Lancet 403 (10423), 293–304. doi:10.1016/S0140-6736(23)01478-2
Mu, D., ShaRen, G. W., Bao, L., Chen, Y., and Sha, R. (2022). Structure, immunomodulatory and antioxidant activities in vitro of ploysaccharides from Radix asparagi. J. Chin. Inst. Food Sci. Technol. 22 (08), 51–60. doi:10.16429/j.1009-7848.2022.08.006
Nagatsu, T., Mogi, M., Ichinose, H., and Togari, A. (2000). Changes in cytokines and neurotrophins in parkinson's disease. J. Neural. Transm. Suppl. (60), 277–290. doi:10.1007/978-3-7091-6301-6_19
Ni, R. (2023). PET imaging in animal models of Parkinson’s disease. Behav. Brain Res. 438, 114174. doi:10.1016/j.bbr.2022.114174
Paik, S., Kim, J. K., Silwal, P., Sasakawa, C., and Jo, E. K. (2021). An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell. Mol. Immunol. 18 (5), 1141–1160. doi:10.1038/s41423-021-00670-3
Peng, B., Wen, Y., Yu, R., and Zhu, J. (2018). Advances in extraction,structure characterization and bioactivities of citrus medica polysaccharide. Food Drug 20 (03), 236–241. doi:10.3969/j.issn.1672-979X.2018.03.017
Peng, D., Tian, W., An, M., Chen, Y., Zeng, W., Zhu, S., et al. (2023). Characterization of antidiabetic effects of Dendrobium officinale derivatives in a mouse model of type 2 diabetes mellitus. Food Chem. 399, 133974. doi:10.1016/j.foodchem.2022.133974
Przedborski, S., and Dawson, T. M. (2001). The role of nitric oxide in parkinson's disease. Mol. Med. 62, 113–136. doi:10.1385/1-59259-142-6:113
Ren, H., Li, Z., Gao, R., Zhao, T., Luo, D., Yu, Z., et al. (2022). Structural characteristics of Rehmannia glutinosa polysaccharides treated using different decolorization processes and their antioxidant effects in intestinal epithelial cells. Foods 11 (21), 3449. doi:10.3390/foods11213449
Shang, X., Xu, C., Dong, J., Ci, C., Zhang, P., Jin, H., et al. (2023). Mechanism of Dendrobium huoshanense polysaccharide against neuroinflammation in parkinson's disease model: based on NLRP3 inflammasome. Chin. J. Exp. Tradit. Med. Form. 29 (11), 97–105. doi:10.13422/j.cnki.syfjx.20230437
Shao, F., Liang, S., and Qin, H. (2020). The role of microglia in Parkinson’s disease and its research progress. Chin. J. Neuroanat. 36 (5), 565–569. doi:10.16557/j.cnki.1000-7547.2020.05.015
Song, J., Liu, L., Li, Z., Mao, T., Zhang, J., Zhou, L., et al. (2022). Lycium barbarum polysaccharide improves dopamine metabolism and symptoms in an MPTP-Induced model of parkinson's disease. BMC Med. 20 (1), 412. doi:10.1186/s12916-022-02621-9
Song, Z., Huang, Y., Wang, Y., Deng, J., Zhen, D., Tan, N., et al. (2024). Antioxidant and anti-aging effects and mechanism of poria cocos polysaccharides in Caenorhabditis elegans. Chin. Tradit. Herb. Drugs. 55 (04), 1133–1144. doi:10.7501/j.issn.0253-2670.2024.04.008
Steinmetz, J. D., Seeher, K. M., Schiess, N., Nichols, E., Cao, B., Servili, C., et al. (2024). Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: a systematic analysis for the global burden of disease study 2021. Lancet Neurol. 23 (4), 344–381. doi:10.1016/s1474-4422(24)00038-3
Sun, S., Xiao, Q., Fan, L., He, L., Li, Y., Fan, H., et al. (2023). Research status of α-synuclein and parkinson's disease. Chin. J. Clin. Pharmacol. 39 (8), 1230–1234. doi:10.13699/j.cnki.1001-6821.2023.08
Tan, Y., Yin, L., Sun, Z., Shao, S., Chen, W., Man, X., et al. (2020). Astragalus polysaccharide exerts anti-Parkinson via activating the PI3K/AKT/mTOR pathway to increase cellular autophagy level in vitro. Int. J. Biol. Macromol. Jun 15, 349–356. doi:10.1016/j.ijbiomac.2020.02.282
Tian, X., Liang, T., Liu, Y., Ding, G., Zhang, F., Ma, Z., et al. (2019). Extraction, structural characterization, and biological functions of Lycium barbarum polysaccharides: a review. Biomolecules 9 (9), 389. doi:10.3390/biom9090389
Tolosa, E., Garrido, A., Scholz, S. W., and Poewe, W. (2021). Challenges in the diagnosis of parkinson's disease. Lancet Neurol. 20 (5), 385–397. doi:10.1016/s1474-4422(21)00030-2
Tu, Y., and Cai, J. G. J. (2017). Chaihu jialonggu muli decoction for parkinson's disease with depression in 34 cases. Guangming J. Chin. Med. 32 (04), 520–523. doi:10.3969/j.issn.10038914.2017.04.02
Wang, J., Hu, Y., Wang, D., Liu, J., Zhang, J., Abula, S., et al. (2010). Sulfated modification can enhance the immune-enhancing activity of Lycium barbarum polysaccharides. Cell. Immunol. 263 (2), 219–223. doi:10.1016/j.cellimm.2010.04.001
Wang, J., Zhong, Q., Dong, C., Li, H., Wang, H., Xia, F., et al. (2021). Recent progress in the biological activity and mechanism of action of sea cucumber polysaccharides. Food Sci. 42 (23), 370–380. doi:10.7506/spkx1002-6630-20200705-055
Wang, L., and Han, J. (2021). Mechanism of Lycium barbarum polysaccharide and UCA1 siRNA single or combined treatment in the MPP+-Induced Parkinson’s disease cell model. Chin. J. Comp. Med. 31 (11), 55–61. doi:10.3969/j.issn.1671-7856.2021.11.009
Wang, R., Santos, J. M., Dufour, J. M., Stephens, E. R., Miranda, J. M., Washburn, R. L., et al. (2022). Ginger root extract improves GI health in diabetic rats by improving intestinal integrity and mitochondrial function. Nutrients 14 (20), 4384. doi:10.3390/nu14204384
Wang, W., Yang, Y., Ying, C., Li, W., Ruan, H., Zhu, X., et al. (2007). Inhibition of glycogen synthase kinase-3beta protects dopaminergic neurons from MPTP toxicity. Neuropharmacology 52 (8), 1678–1684. doi:10.1016/j.neuropharm.2007.03.017
Wang, W., Zhao, B., Zhang, Z., Kikuchi, T., Wei, L., Jantrawut, P., et al. (2024). Natural polysaccharides and their derivatives targeting the tumor microenvironment: a review. Int. J. Biol. Macromol. 268, 131789. doi:10.1016/j.ijbiomac.2024.131789
Wang, X., Pang, L., Zhang, Y., Xu, J., Ding, D., Yang, T., et al. (2018). Lycium barbarum polysaccharide promotes nigrostriatal dopamine function by modulating PTEN/AKT/mTOR pathway in a Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) murine model of parkinson's disease. Neurochem. Res. Apr 43 (4), 938–947. doi:10.1007/s11064-018-2499-6
Wang, Y. (2024). Isolation and purification of Taiwanofungus camphoratus polysaccharides and its effect on macrophage function. Nanchang University [Master's thesis]. doi:10.27232/d.cnki.gnchu.2023.000298
Wang, Y., Dong, P., Jin, C., Kang, J., Hu, Y., and Wang, Z. (2019). Analysis on composition of Polygonatum sibiricum polysaccharide and its anti-oxidant activity. Genomics Appl. Biol. 38 (05), 2191–2199. doi:10.13417/j.gab.038.002191
Wang, Y., Jin, H., Li, Y., Dong, X., Za, K., and Ma, S. (2021). Immunomodulatory effect of Lycium barbarum polysaccharide of different molecular weights on macrophage RAW264.7. Chin. J. New Drugs. 30 (12), 1079–1086. doi:10.3969/j.issn.1003-3734.2021.12.005
Wang, Y., Mo, X., Zhang, W., Li, P., Zhu, X., and Jiang, N. (2023). Research progresses on structure and pharmacological action of Tricholoma matsutake polysaccharides. Chin. Mod. Med. 30 (10), 34–38. doi:10.3969/j.issn.1674-4721.2023.10.010
Wirdefeldt, K., Adami, H. O., Cole, P., Trichopoulos, D., and Mandel, J. (2011). Epidemiology and etiology of parkinson's disease: a review of the evidence. Eur. J. Epidemiol. 26 (Suppl. 1), S1–S58. doi:10.1007/s10654-011-9581-6
Wu, C. C., and Bratton, S. B. (2013). Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid. Redox. Signal. 19 (6), 546–558. doi:10.1089/ars.2012.4905
Xiao, H., Hong, A., and Cheng, X. (2021). Clinical application of Radix bupleuri keen-oyster soup in the treatment of sleep disorders in patients with parkinson's disease. World J. Sleep. Med. 8 (07), 1161–1162. doi:10.3969/j.issn.2095-7130.2021.07.019
Xu, Q., Yang, S., Wu, F., Lin, Y., Zhong, J., Tang, L., et al. (2020). Congrong shujing granule-induced Grp78 expression reduced endoplasmic reticulum stress and neuronal apoptosis in the midbrain in a parkinson’s disease rat model. Evid. Based Complement. Altern. Med. 30, 4796236. doi:10.1155/2020/4796236
Xu, T., Zhang, H., Wang, S., Xiang, Z., Kong, H., Xue, Q., et al. (2022). A review on the advances in the extraction methods and structure elucidation of poria cocos polysaccharide and its pharmacological activities and drug carrier applications. Int. J. Biol. Macromol. Sep. 30, 536–551. doi:10.1016/j.ijbiomac.2022.07.070
Xue, T., Zheng, D., Wen, L., Hou, Q., He, S., Zhang, H., et al. (2024). Advance in Cistanche deserticola Y. C. Ma. Polysaccharides: isolation, structural characterization, bioactivities and application: a review. Int. J. Biol. Macromol. Oct. 278 (Pt 2), 134786. doi:10.1016/j.ijbiomac.2024.134786
Yang, L. C., Zhang, Y., Yu, X., Sun, J. W., Li, J. W., Zhang, X. Y., et al. (2018). Research progress of the relationship between autophagy and ULK1 protein. Med. Recapitul. 24 (23), 4635–4639. doi:10.3969/j.issn.1006-2084.2018.23.012
Yang, X., Yang, B., Zhang, Y., Liang, Y., Li, K., Xu, C., et al. (2018). The mechanism of programmed cell death of dopaminergic neurons in parkinson's disease. Neural. Inj. Funct. Reconst. 13 (6), 296–299. doi:10.16780/j.cnki.sjssgncj.2018.06.008
Yang, Y., Guan, Q., Guo, L., and Han, C. (2018). Antrodia camphorata polysaccharide improved neurobehavioral function in parkinson mice via reducing the expression of NLRP3-Caspase1 inflammatory body. Chin. J. Clin. Pharmacol. Ther. 23 (04), 406–412. doi:10.12092/j.issn.1009-2501.2018.04.008
Yang, Y., Zhao, X., Wang, Y., Guan, Q., and Han, C. (2017). Antioxidation and anti-inflammatory effects of Antrodia camphorata polysaccharide on parkinsonian mice induced by 6-OHDA. Chin. J. Mod. Appl. Pharm. 34 (07), 969–972. doi:10.13748/j.cnki.issn1007-7693.2017.07.008
Yang, Y. N., Sun, L., and Cui, X. (2024). Molecular mechanisms related to parkinson's disease and the intervention effect of traditional Chinese medicine. Pract. J. Card. Cereb. Pneum. Vasc. Dis. 32 (09), 25–31. doi:10.12114/j.issn.1008-5971.2024.00.208
Ye, Q., Wang, K., Yuan, X., He, J., Zhou, J., Yuan, C., et al. (2017a). Effects of shudi pingchan granule on behavior and alpha-synuclein expression in rats with parkinson's disease. Chin. J. Integr. Med. Cardio-Cerebrovasc. Dis. 15 (04), 419–424. doi:10.3969/j.issn.1672-1349.2017.04.009
Ye, Q., Yuan, X. L., He, J., Zhou, J., Yuan, C. X., Yang, X. M., et al. (2016). Anti-apoptotic effect of shudipingchan granule in the substantia nigra of rat models of parkinson's disease. Neural Regen. Res. 11 (10), 1625–1632. doi:10.4103/1673-5374.193242
Ye, Q., Yuan, X. L., Zhang, Y., and Yuan, C. X. (2017b). Clinical study on treatment of parkinson’s disease with shudi pingchan granule combined with repetitive transcranial magnetic stimulation. Tradit. Chin. Med. 6 (2), 60–67. doi:10.12677/tcm.2017.62011
Yin, S., Wang, H., and Yang, S. (2020). Neuroprotective effects of Cistanche deserticola polysaccharide on Parkinson’s rats induced by 6-HODA caused by activating the Wnt/β-catenin signaling pathway. Chin. J. Integr. Med. Cardio-Cerebrovascu. Dis. 18 (08), 1227–1230. doi:10.12102/j.issn.1672-1349.2020.08.011
Yu, C., Fang, X., Yu, Y. J., and Yu, Z. (2021). Effect of chaihu jialonggu oyster decoction on Parkinson's dementia and its influence on MMSE score and MoCA score. Clin. Educ. Gen. Pract. 19 (11), 985–988. doi:10.13558/j.cnki.issn1672-3686.2021.011.008
Yuan, C., Zhi, H., Chen, S., Chen, Y., Li, J., Shen, W., et al. (2010). Clinical multicenter randomized controlled study on treatment of parkinson's disease with “Shudi Pingchan Decoction” and wWstern medicine. Shanghai J. Tradit. Chin. Med. 44 (06), 3–6. doi:10.16305/j.1007-1334.2010.06.041
Yue, H., Zeng, H., and Ding, K. (2020). A review of isolation methods, structure features and bioactivities of polysaccharides from dendrobium species. Chin. J. Nat. Med. Jan. 18 (1), 1–27. doi:10.1016/s1875-5364(20)30001-7
Zeng, P., Li, J., Chen, Y., and Zhang, L. (2019). The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Mol. Biol. Transl. Sci. 163, 423–444. doi:10.1016/bs.pmbts.2019.03.003
Zhang, C., Liu, B., Yuan, Z., Ma, X., and Zeng, F. (2024). Angelica polysaccharide alleviates motor impairment in mice with Parkinson’s disease via inhibition of astrocyte apoptosis. Chin. J. Gerontolo. 44 (13), 3188–3192. doi:10.3969/j.issn.1005-9202.2024.13.030
Zhang, H., Li, F., Zhao, C., Wang, C., Yan, X., Yu, H., et al. (2022). Isolation, purification and characterization of polysaccharide from Schisandra chinensis. Tianjin J. Tradit. Chin. Med. 39 (04), 509–515. doi:10.11656/j.issn.1672-1519.2022.04.21
Zhang, L., Hao, J., Zheng, Y., Su, R., Liao, Y., Gong, X., et al. (2018). Fucoidan protects dopaminergic neurons by enhancing the mitochondrial function in a rotenone-induced rat model of parkinson’s disease. Aging Dis. 9 (4), 590–604. doi:10.14336/AD.2017.0831
Zhang, P., Dong, J., Xu, C., Wang, S., and Cheng, N. (2023). Effects of DHP on dyskinesia, neuronal damage and senescence of astrocytes in mice with parkinson's disease. Shandong Med. J. 63 (35), 24–29. doi:10.3969/j.issn.1002-266X.2023.35.006
Zhang, W., Huang, J., Wang, W., Li, Q., Chen, Y., Feng, W., et al. (2016). Extraction, purification, characterization and antioxidant activities of polysaccharides from Cistanche tubulosa. Int. J. Biol. Macromol. 93 (PtA), 448–458. doi:10.1016/j.ijbiomac.2016.08.079
Zhang, X., Sun, H., Wang, L., Wang, Y., Liu, C., Bai, Y., et al. (2011). Protective effects of crude polysaccharides from Chroogomphus rutilus on dopaminergic neurons impaired by MPTP in mice. Mycosystema 30 (01), 77–84. doi:10.13346/j.mycosystema.2011.01.020
Zhang, X., Wang, Y., Wang, L., and Guan, Z. (2013). Protective effects of crude polysaccharides from Chroogomphus rutilus on SH-SY5Y cells impaired by MPP+. Acta Physiol. Sin. 65 (02), 210–216. doi:10.13294/j.aps.2013.02.004
Zhang, Y., Fu, J., Yuan, Z., and Gu, H. (2023). The role of cellular senescence in the development and progression of neurological diseases. J. China Med. Univ. 52 (9), 847–851. doi:10.12007/j.issn.0258-4646.2023.09.013
Zhao, B., Zhao, J., Lv, M., Li, X., Wang, J., Yue, Z., et al. (2021). Comparative study of structural properties and biological activities of polysaccharides extracted from Chroogomphus rutilus by four different approaches. Int. J. Biol. Macromol. 188, 215–225. doi:10.1016/j.ijbiomac.2021.08.025
Zhao, D., Niu, J., Du, Z., Zhou, C., Ding, S., Du, M., et al. (2023). Exploration of antidepressant mechanism of chaihu and longgu mulitang based on cAMP/PKA/CREB/BDNF signaling pathway. Chin. J. Exp. Tradit. Med. Formul. 29 (03), 17–25. doi:10.13422/j.cnki.syfjx.20221709
Zhao, L., Wang, Y., Shen, H., Shen, X., Nie, Y., Wang, Y., et al. (2012). Structural characterization and radioprotection of bone marrow hematopoiesis of two novel polysaccharides from the root of Angelica sinensis (oliv.) diels. Fitoterapia 83 (8), 1712–1720. doi:10.1016/j.fitote.2012.09.029
Zhao, X., Zhao, W., Duan, C., Zheng, J., Sun, N., Yang, J., et al. (2024). Research progress on relationship between chemical characterization and immune regulation of astragalus polysaccharides. Chin. Arch. Tradit. Chin. Med. 42 (03), 108–112. doi:10.13193/j.issn.1673-7717.2024.03.020
Zheng, Y., Ren, W., Zhang, L., Zhang, Y., Liu, D., and Liu, Y. (2020). A review of the pharmacological action of astragalus polysaccharide. Front. Pharmacol. 11, 349. doi:10.3389/fphar.2020.00349
Zhou, G., Li, Y., Peng, Y., Yang, Y., Cui, J., and Lv, Q. (2021). Effects of astragalus polysaccharide injection on the levels of urine CXCL10, E-cadherin and MMP-9 in patients with diabetic nephropathy. Med. Pharm. J. Chin. PLA. 33 (1), 94–97. doi:10.3969/j.issn.2095-140X.2021.01.021
Zhou, N., Zhao, S., and Liang, X. (2021). Study on the anti-oxidative damage and neuroprotective effects of pholidota chinensis lindl. Polysaccharides on a rat model of parkinson's disease. China med. Pharm 11 (21), 32–36. doi:10.3969/j.issn.2095-0616.2021.21.009
Zhou, Y., Wang, S., Feng, W., Zhang, Z., and Li, H. (2021). Structural characterization and immunomodulatory activities of two polysaccharides from rehmanniae radix praeparata. Int. J. Biol. Macromol. Sep. 1 (186), 385–395. doi:10.1016/j.ijbiomac.2021.06.100
Keywords: polysaccharides, traditional Chinese medicine, Parkinson’s disease, molecular mechanisms, neuroprotection, research progress
Citation: Zhou X and Pan Q (2025) Research progress on the mechanisms of polysaccharides derived from traditional Chinese medicine in the treatment of Parkinson’s disease. Front. Pharmacol. 16:1566479. doi: 10.3389/fphar.2025.1566479
Received: 24 January 2025; Accepted: 14 July 2025;
Published: 08 August 2025.
Edited by:
Huazheng Liang, Monash University - Southeast University Joint Research Institute (Suzhou), ChinaReviewed by:
Hatice Kızıltaş, Yüzüncü Yıl University, TürkiyeNian Xiong, Huazhong University of Science and Technology, China
Copyright © 2025 Zhou and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Pan, bm93YXkxODE4QDE2My5jb20=
 Xiangling Zhou
Xiangling Zhou Qi Pan*
Qi Pan*