- 1Department of Pharmacy, The Wenzhou Third Clinical Institute Affiliated to Wenzhou Medical University, Wenzhou, Zhejiang, China
- 2Department of Pharmacy, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, China
- 3Ningbo Municipal Hospital of Traditional Chinese Medicine (TCM), Affiliated Hospital of Zhejiang Chinese Medical University, Ningbo, Zhejiang, China
The aim of this study was to investigate the impact of calcium channel blockers (CCBs) and antihypertensive traditional Chinese medicine (TCM) on the metabolism of quetiapine. In vitro, two incubation systems of rat liver microsomes (RLM) and human liver microsomes (HLM) were established and optimized to explore potential interactions between five kinds CCBs (nicardipine, dilthiazem, lercanidipine, nimodipine, nitrendipine), five kinds antihypertensive TCM (quercetin, fangchinoline, apigenin, tetrandrine, and berberine) and quetiapine, and to evaluate their underlying inhibition mechanisms. In vivo, Sprague-Dawley rats were used to assess the interaction between quetiapine and nicardipine. The results showed that nicardipine had the highest inhibition rate (79.22%) against quetiapine metabolism among those drugs screened. The half-maximal inhibitory concentration (IC50) values for the inhibition of quetiapine metabolism by nicardipine in RLM and HLM were similar, at 10.29 ± 0.06 μM and 13.23 ± 0.37 μM, respectively. In RLM, nicardipine exhibited a mixed mechanism of competitive and non-competitive inhibition, while in HLM, it displayed a non-competitive and un-competitive inhibition mechanism. In vivo results indicated that nicardipine could significantly increase the main pharmacokinetic parameters AUC(0-t),
1 Introduction
At present, mental disorders including bipolar disorder, major depressive disorder, and schizophrenia, remain a leading cause of the global disease burden (Collaborators, 2022). Second-generation antipsychotic drugs (SGAs) constitute a novel class of antipsychotic medications utilized in the treatment of mental illnesses such as bipolar disorder and schizophrenia (Krejci et al., 2024).
Quetiapine, a dibenzothiazepine derivative, is classified as a SGA (Maneeton et al., 2016). It was approved by the U.S. Food and Drug Administration in 1997 for the treatment of schizophrenia, bipolar disorder, and depressive episodes (Han et al., 2024). Quetiapine has been a widely used antipsychotic for more than 30 years, and it has good efficacy in treating depressive episodes associated with bipolar disorder and major depressive disorder (Bakken et al., 2011; Sarris et al., 2022). Additionally, quetiapine demonstrates greater clinical efficacy as a first-line augmentation option for alleviating symptoms of treatment-resistant depression in the long-term management of this condition (Cleare et al., 2025). In a recent clinical study, when quetiapine was used in the treatment of refractory depression, the incidence of adverse reactions was 78.0% and the incidence of serious adverse reactions was 5.7% (Reif et al., 2023). Response and remission rates were highest for quetiapine in 6- or 8-week monotherapy, but the likelihood of discontinuation due to adverse events was higher (Li S. et al., 2024). In outpatient prescriptions, the combination of escitalopram and quetiapine is a common potential drug interaction (Chen and Ding, 2023). A clinical study demonstrated that the plasma concentration of quetiapine is dose-dependently affected by lamotrigine, suggesting the need for therapeutic drug monitoring of quetiapine (Hole et al., 2024). Quetiapine undergoes complete oral absorption and is primarily metabolized by cytochrome P450 (CYP) 3A4 into various metabolites (Grimm et al., 2006; Zubiaur et al., 2021), among which N-desalkylquetiapine is the principal active metabolite of quetiapine (Jensen et al., 2008). Recent studies indicate a potential for drug-drug interactions (DDI) between phenacetin and the CYP3A4 substrate quetiapine (Watermeyer et al., 2024). When quetiapine is used in combination with methadone, the plasma concentration levels of quetiapine metabolites increase (Heisel et al., 2024). Therefore, it is necessary to conduct essential drug-drug interactions studies, particularly for drugs that have not yet been reported and possess the potential for drug interactions.
Hypertension is prevalent in patients with bipolar disorder and anxiety (Johannessen et al., 2006), primarily due to patients with hypertension are more likely to experience negative emotions such as anxiety, stress, and depression (Kretchy et al., 2014). Consequently, there exists a significant possibility of antihypertensive drugs co-administrated with quetiapine. Harrison PJ, et al. have shown that calcium channel blockers (CCBs) targeting the L-type voltage-gated calcium channels play an essential role in the fundamental neuronal processes related to mental illnesses, and CCBs can also reduce psychiatric hospitalization and self-harm in patients with bipolar disorder (Hayes et al., 2019; Lintunen et al., 2022). Furthermore, attention to drug interactions mediated by natural products is increasing. However, there are few reports on the interactions between quetiapine and CCBs or traditional Chinese medicine (TCM).
First, we developed a rapid, sensitive, and accurate UPLC-MS/MS method for detecting the concentration of quetiapine and its metabolite, N-desalkylquetiapine, based on previous studies (Fisher et al., 2012; Pan et al., 2012; Xiong et al., 2013; Li et al., 2017; Luo et al., 2023). In this study, we selected five kinds L-type voltage-gated calcium channel CCBs and five kinds TCM with antihypertension to explore their interactions with quetiapine. Initially, in vitro, we utilized rat liver microsomes (RLM) and human liver microsomes (HLM) to investigate their effects on the metabolism of quetiapine and their underlying mechanisms. Subsequently, in vivo, based on the results of the in vitro experiments, we employed Sprague-Dawley rats to examine the impact of nicardipine on the pharmacokinetic parameters of quetiapine. The schematic of the experiment method is shown in Figure 1. We expected to provide some data support for the individualized treatment of quetiapine in clinical practice.
2 Materials and methods
2.1 Chemicals and reagents
Quetiapine, N-desalkylquetiapine and lurasidone (internal standard, IS) were obtained from Shanghai Chuangsai Technology Co., Ltd. (Shanghai, China). Ten kinds drugs (nicardipine, dilthiazem, lercanidipine, nimodipine, nitrendipine, quercetin, fangchinoline, apigenin, tetrandrine, and berberine) were also got from Shanghai Chuangsai Technology Co., Ltd. (Shanghai, China). The RLM and HLM were purchased from iPhase Pharmaceutical Services Co., Ltd. (Jiangsu, China). Reduced nicotinamide adenine dinucleotide phosphate (NADPH) was procured from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Methanol and acetonitrile were provided by Merck Company (Darmstadt, Germany).
2.2 UPLC-MS/MS condition
The chromatographic separation of quetiapine, N-desalkylquetiapine, and IS was achieved utilizing an ultra performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) equipped with a Waters Acquity BEH C18 chromatographic column (2.1 mm × 50 mm, 1.7 μm; Milford, MA, United States). At a column temperature of 40 °C, the mobile phase, composed of 0.1% formic acid (mobile phase A) and acetonitrile (mobile phase B), was eluted at a gradient flow rate of 0.4 mL/min for a duration of 2.0 min. Mass spectrometry information was obtained using a Waters Xevo TQS triple quadrupole tandem mass spectrometer equipped with an electrospray ionization (ESI) source (Milford, MA, United States), operating in positive mode with multiple reaction monitoring (MRM). The ion transitions for quetiapine, N-desalkylquetiapine, and IS were m/z 384.04 → 252.95, m/z 295.99 → 209.93, and m/z 493.05 → 165.99, respectively.
2.3 Incubation condition
200 μL enzymatic kinetic incubation system was adjusted according to the measurement purpose with the corresponding incubation conditions. The determination of enzymatic kinetic parameters of quetiapine in RLM and HLM were performed under the following conditions: 1–200 μM quetiapine, 0.3 mg/mL RLM or HLM, 0.1 M PBS buffer, 1 mmol/L NADPH, with triplicate samples for each concentration. The mixture without NADPH was pre-incubated at 37 °C in a water bath for 5 min, then 10 μL NADPH (20 mM) was added to initiate the reaction. Following a further 30 min incubation, the reaction was terminated by freezing at −80 °C. Prior to sample thawing, 400 μL acetonitrile was added to precipitate proteins, and 20 μL lurasidone (200 ng/mL) was added as IS. After complete dissolution of the samples, they were vortexed for 2 min and centrifuged for 10 min (13,000 rpm), then the supernatants were taken for UPLC-MS/MS detection and analysis.
The potential interactions between quetiapine and five kinds L-type voltage-gated CCBs (nicardipine, dilthiazem, lercanidipine, nimodipine, nitrendipine) and five kinds of TCM (quercetin, fangchinoline, apigenin, tetrandrine, and berberine) with antihypertension were investigated. The 200 μL incubation system was comprised of PBS buffer (0.1 M), RLM or HLM (0.3 mg/mL), NADPH (1 mmol/L), quetiapine and 10 kinds drugs (100 μM). There is a high potential for drug-drug interactions between these ten drugs that could have effects on the metabolic process of quetiapine. The concentration of quetiapine is based on the Michaelis-Menten constant (Km).
Subsequently, the half-maximal inhibitory concentration (IC50) of nicardipine on quetiapine in RLM or HLM was determined, with nicardipine concentrations set at: 0, 0.01, 0.1, 1, 10, 25, 50, and 100 μM. The concentration of quetiapine based on Km was 48.79 μM in RLM and 50.80 μM in HLM, respectively. Finally, the potential mechanism type of inhibition of quetiapine metabolism by nicardipine was further examined. The concentration of quetiapine was set according to the corresponding Km values, at 12.20, 24.40, 48.79, 97.58 μM in RLM, and 12.70, 25.40, 50.80, 101.60 μM in HLM, respectively. Moreover, the concentration of nicardipine was set according to the corresponding IC50 values, at 0, 2.57, 5.15, 10.29 μM in RLM, and 0, 6.62, 13.23, 26.46 μM in HLM, respectively. Sample processing was as described above.
2.4 Pharmacokinetics research
Due to the similarity between their hepatic enzyme system and humans, Sprague-Dawley male rats are widely used in pharmacokinetics (Martignoni et al., 2006; Riccardi et al., 2018). This pharmacokinetic study was conducted on eight healthy male Sprague-Dawley rats provided by the Animal Experiment Center of the First Affiliated Hospital of Wenzhou Medical University (Zhejiang, China). Animals were housed in an environment with a temperature of 20 °C–26 °C, relative humidity of 55% ± 15%, and a light-dark cycle of 12 h/day, in strict following the guidelines for the care and use of laboratory animals by the National Research Council. The research protocol was adhered to the ARRIVE guidelines and was approved by the Experimental Animal Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University (WYYY-IACUC-AEC-2023–064). Before the formal experiment, in order to mitigate the influence of food on drug absorption, Sprague-Dawley rats were fasted for 12 h and then randomly divided into two groups. Nicardipine and quetiapine are dissolved using corn oil. In the co-administration group (n = 4), each rat was gavaged with 4 mg/kg nicardipine, whereas in the control group (n = 4), each rat was given an equal amount of corn oil orally to reduce solvent interference. 30 min later, both groups of Sprague-Dawley rats were given 15 mg/kg quetiapine by gavage. Subsequently, blood samples were collected from the rat’s tail vein into heparinized centrifuge tubes at 15 min, 30 min, 45 min, and 1, 1.5, 2, 3, 4, 6, 8, 12, 24 h post-quetiapine administration. Following centrifugation at 8,000 rpm for 10 min, 100 μL of plasma supernatant was precisely taken into a new clean and dry 1.5 mL EP tube, where 300 μL acetonitrile and 10 μL IS working solution (200 ng/mL) were added, and after vortexing and centrifugation, the supernatants were taken for injection and analysis.
2.5 Statistical analysis
The Michaelis-Menten curves, IC50 curves, Lineweaver–Burk plots, and the mean plasma concentration–time curves were generated using GraphPad Prism software (version 9.5; GraphPad Software Inc., San Diego, CA). The pharmacokinetic parameters were derived using the non-compartmental model analysis with Drug and Statistics software (version 3.0 software, Mathematical Pharmacology Professional Committee of China, Shanghai, China). Statistical differences between the co-administered group and the control group were analyzed using the t-test of SPSS (version 24.0; SPSS Inc., Chicago, IL, USA), with P < 0.05 considered statistically significant.
3 Results
3.1 Chromatographic characterization and mass spectrometric information of analytes
The UPLC-MS/MS analytical method was developed according to the Guideline on bioanalytical method validation published by EMA (EMA, 2015). It could be seen from Figure 2 that the analytes were well separated within an elution time of 2.0 min, and there was no interference from endogenous substances. The retention times of quetiapine and its main metabolite (N-desalkylquetiapine), and IS were 1.22 min, 1.22 min, and 1.29 min, respectively. Quetiapine and N-desalkylquetiapine exhibited good linearity within the range of 0.1–100 ng/mL and 0.1–500 ng/mL, respectively. The linear regression equation for quetiapine was y = 0.101313x + 0.011457, r2 = 0.9946, and for N-desalkylquetiapine was y = 0.000869966x + 0.0000589335, r2 = 0.9987, respectively. Additionally, the lower limit of quantification (LLOQ) of quetiapine and N-desalkylquetiapine both were 0.1 ng/mL.
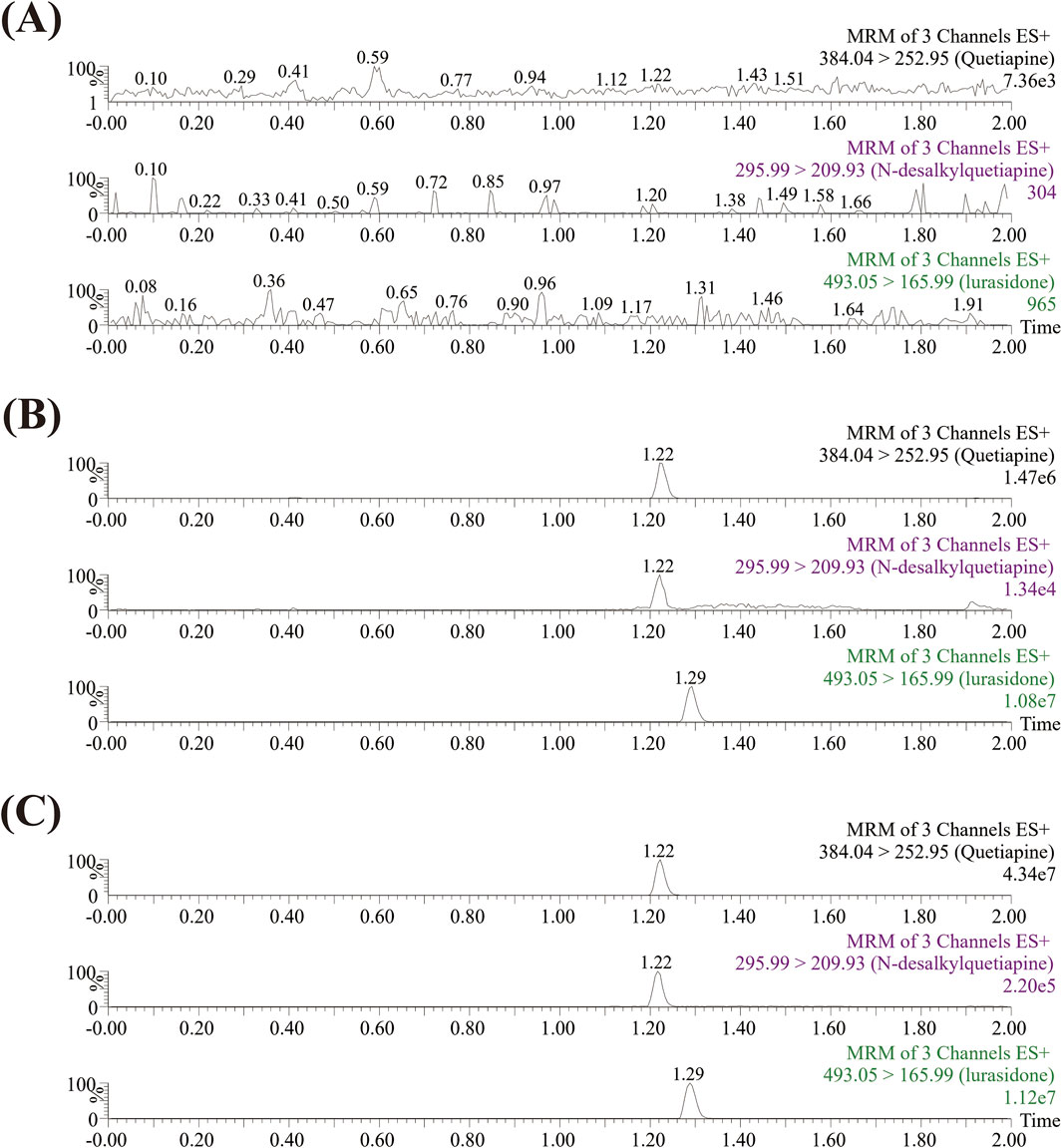
Figure 2. Typical UPLC-MS/MS chromatograms of quetiapine, N-desalkylquetiapine, and IS. Blank rat plasma (A); Blank rat plasma with 1 ng/mL standards (B); Rat plasma sample from the formal animal experiment (C).
3.2 Nicardipine had the strongest suppression strength on quetiapine metabolism among the 10 kinds drugs
Figure 3 shows that nicardipine was the drug that most inhibited the metabolism of quetiapine among the five kinds L-type voltage-gated CCBs, and the strongest inhibitory ability among the five kinds antihypertensive TCM was quercetin. However, the inhibition rate of nicardipine was 79.22%, which was still higher than that of quercetin (61.47%). The results indicated that nicardipine had the strongest inhibitory effect on quetiapine metabolism among the 10 screened drugs.
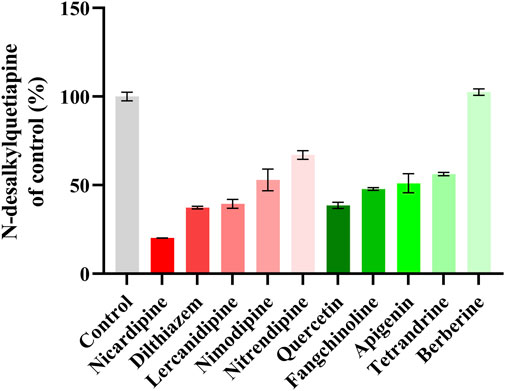
Figure 3. A comparison of the inhibitory effects on the formation of N-desalkylquetiapine by five kinds L-type voltage-gated CCBs and five kinds antihypertensive TCM in RLM. Data are expressed as the mean ± standard deviation (SD). N = 2.
3.3 Nicardipine inhibited the metabolism of quetiapine by a mixed mechanism in both RLM and HLM
Figure 4 reveals that the Km of quetiapine in RLM and HLM were close, at 48.79 μM and 50.80 μM, respectively, suggesting that the activity of RLM in catalyzing the metabolism of quetiapine may be similar to that of HLM. Km is the concentration of the substrate when the enzymatic reaction reaches half of the maximum speed. It is a characteristic physical quantity of the enzyme and its magnitude is related to the nature of the enzyme. Using Km as the substrate concentration, the effect of other factors on the reaction rate was clearly observed. According to Figure 5, the IC50 of nicardipine inhibiting quetiapine metabolism in RLM and HLM was also not significantly different, with IC50 values of 10.29 ± 0.06 μM and 13.23 ± 0.37 μM, respectively. Interestingly, Figure 6 indicates that nicardipine inhibited quetiapine metabolism in both RLM and HLM through a mixed mechanism type. In RLM, nicardipine exhibited a mixed mechanism combining competition and non-competition, with Ki = 0.08 μM and αKi = 10.05 μM, while in HLM, it showed a combination of non-competition and un-competition, with Ki = 32.63 μM and αKi = 17.91 μM.
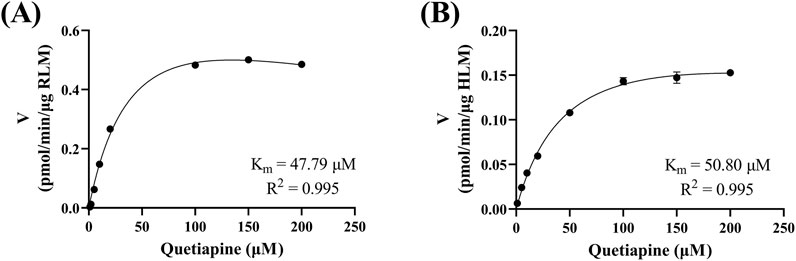
Figure 4. Michaelis-Menten curves of quetiapine in RLM (A) and HLM (B). Data are expressed as the mean ± SD, n = 3.
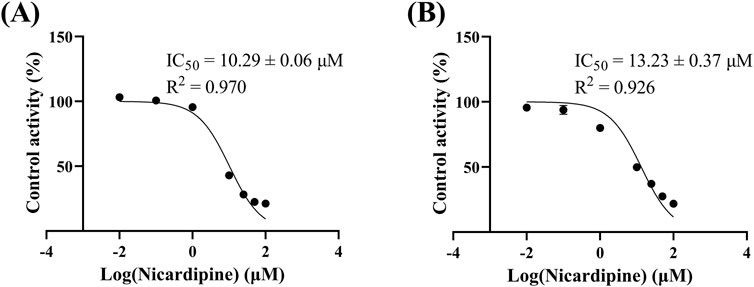
Figure 5. IC50 curves of nicardipine at different concentrations (0.01, 0.1, 1, 10, 25, 50, and 100 μM) for the inhibition of quetiapine in RLM (A) and HLM (B). Data are expressed as the mean ± SD, n = 3.
![Graphs show Lineweaver-Burk plots and secondary plots for quetiapine and nicardipine interactions. (A) Rat liver microsomes: plots display 1/V versus 1/[quetiapine] with slopes and intercepts for various concentrations. (B) Human liver microsomes: similar setup with different K_i and R^2 values, indicating interaction effects at differing concentrations.](https://www.frontiersin.org/files/Articles/1567044/fphar-16-1567044-HTML/image_m/fphar-16-1567044-g006.jpg)
Figure 6. Lineweaver-Burk plots, Ki secondary plots, and αKi secondary plots of nicardipine at different concentrations inhibiting quetiapine metabolism in RLM (A) and HLM (B). Data are expressed as the mean ± SD, n = 3.
3.4 Nicardipine enhanced the drug exposure of quetiapine in Sprague-Dawley rats
The mean plasma concentration-time curves of quetiapine and its main metabolite (N-desalkylquetiapine) are shown in Figure 7. The results indicated that the co-administration of nicardipine not only increased the drug exposure to quetiapine in Sprague-Dawley rats, but also reduced the production of N-desalkylquetiapine. According to the pharmacokinetic parameters in Table 1, nicardipine increased the AUC(0-t),
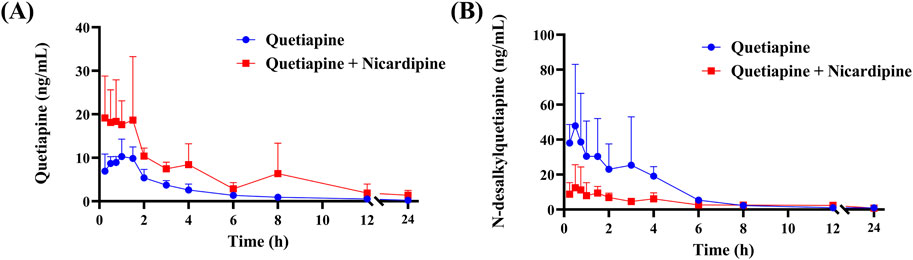
Figure 7. Mean plasma concentration-time curve of quetiapine (A) and N-desalkylquetiapine (B) in two groups of rats. Data are presented as the means ± SD, n = 4.
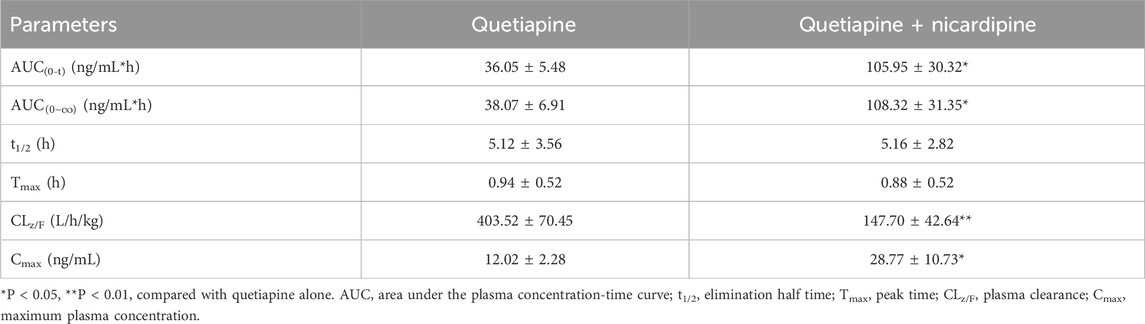
Table 1. Main pharmacokinetic parameters of quetiapine in the two groups of Sprague-Dawley rats (n = 4, mean ± SD).
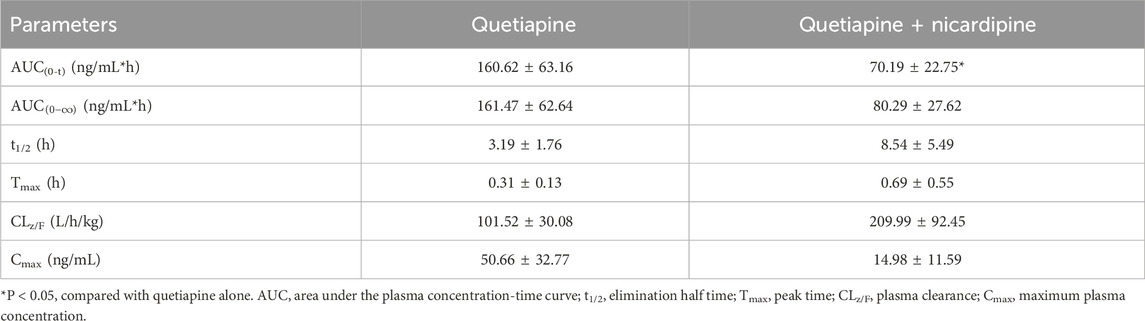
Table 2. Main pharmacokinetic parameters of N-desalkylquetiapine in the two groups of Sprague-Dawley rats (n = 4, mean ± SD).
4 Discussion
Atypical antipsychotics, also known as SAGs, include clozapine, olanzapine, risperidone, quetiapine, aripiprazole, and ziprasidone, which have the advantages of a broad spectrum of action, good efficacy, and a good safety profile compared with traditional antipsychotics, and can improve the quality of life of patients (Mauri et al., 2018). They are now considered as the first-line treatments for schizophrenia (Hamsa et al., 2023). Quetiapine primarily exerts its pharmacological activity by binding to dopamine D2 and serotonin (5-HT) receptors(Mauri et al., 2018). Studies have shown that quetiapine has similar efficacy to first-generation antipsychotics, but it does not increase the level of hyperprolactinemia and related somatic severe adverse reactions (Krøigaard et al., 2022). Thus, it serves as an excellent alternative for patients sensitive to other antipsychotic drugs like clozapine or olanzapine. However, a study indicates that potent and moderate CYP3A4 inducers can increase the apparent clearance of quetiapine by approximately fourfold, while potent CYP3A4 inhibitors reduce it by 93%. DDI can affect the elimination of quetiapine in the body, and conducting relevant research can reduce the risk of potential DDI occurring.
It has been observed that patients with bipolar disorder or schizophrenia have higher cardiovascular morbidity and mortality, partly due to an increased risk of hypertension or poor treatment outcomes, with an overall incidence ratio of hypertension of 1.27 (Ayerbe et al., 2018). One study reported a 39% prevalence of hypertension in schizophrenia and related disorders(Sudarshan and Cheung, 2023). Consequently, there is a high probability that patients with mental disorders would co-administration of quetiapine with antihypertensive medications. It is also known that CCBs targeting the L-type voltage-gated calcium channels play a key role in the fundamental neuronal processes associated with mental illnesses (Harrison et al., 2022). Moreover, there is a growing preference for using natural products as dietary supplements, food additives, and medications to enhance health. Thus, this study systematically screened five kinds CCBs targeting the L-type voltage-gated calcium channels and five kinds antihypertensive TCM to explore their impact on quetiapine metabolism.
Based on previous literature, we have developed a UPLC-MS/MS analytical method to rapidly and sensitively determine the concentration of quetiapine and its metabolite N-desalkylquetiapine. Quetiapine and its metabolite N-desalkylquetiapine have good linearity. Based on this analytical method, we quantified quetiapine and N-desalkylquetiapine in vitro and in vivo to study the effect of nicardipine on quetiapine metabolism.
In vitro results indicated that nicardipine inhibited 79.22% of quetiapine metabolism, making it to be the most potent inhibitor among the 10 drugs. Previous studies have found that nicardipine can significantly affect drugs metabolized via CYP3A4, resulting in slower metabolism and increased drug exposure (Xia et al., 2012; Liu et al., 2023; Chen et al., 2024). Its IC50 values for quetiapine metabolism in RLM and HLM were 10.29 ± 0.06 μM and 13.23 ± 0.37 μM, respectively. It has been reported that nicardipine is a relatively potent inhibitor of human CYP3A4 (Nakamura et al., 2005). Additionally, research by Ya-nan Liu, et al. has shown that nicardipine can significantly inhibit the metabolism of alectinib, a CYP3A4 substrate, both in vitro and in vivo (Liu et al., 2023). Quetiapine is extensively metabolized in the liver by the CYP450 system, primarily via CYP3A4. Therefore, nicardipine may reduce the metabolic rate of quetiapine in vitro by inhibiting CYP3A4 activity. In terms of the inhibition mechanism, in RLM, nicardipine inhibited quetiapine metabolism through a mixed mechanism of competition and non-competition, with Ki = 0.08 μM and αKi = 10.05 μM, and exhibited a non-competitive and uncompetitive inhibition mechanism in HLM, with Ki = 32.63 μM and αKi = 17.91 μM. Competitive inhibition occurs when the inhibitor competes with the substrate for the enzyme’s active site. Non-competitive inhibition occurs when the inhibitor binds to an allosteric site, altering the enzyme’s activity regardless of substrate binding. Uncompetitive inhibition occurs when the inhibitor binds only to the enzyme-substrate complex, stabilizing it and preventing the reaction. Mixed inhibition is a combination, where the inhibitor can bind both to the free enzyme and the enzyme-substrate complex, affecting enzyme activity in multiple ways (Zhou et al., 2007; Kamel and Harriman, 2013; Ye et al., 2024). Ki reflects the inhibitory strength of the inhibitor against the target, thus suggesting that nicardipine may have a stronger inhibitory effect on quetiapine metabolism in RLM than in HLM. The human liver primarily expresses CYP3A4/5, while the rat liver expresses CYP3A1/2. These isoforms share ∼70–85% sequence identity but differ in substrate/inhibitor binding patterns (Fujino et al., 2021). The rat CYP3A isoforms often show lower abundance (<1 fmol/μg), compared to 30–90 fmol/μg for human CYP3A4 (Hammer et al., 2021). Human CYP3A4 exhibits a highly flexible and large (∼520 Å3) active site, enabling it to accommodate diverse ligands (Wright et al., 2019). By contrast, rat CYP3A1 enzyme have more restricted active sites with different key residues, which direct distinct ligand orientations (Handa et al., 2013). These differences may have led to different inhibitory mechanisms. Although RLM provide valuable mechanistic insights, the discrepancies emphasize the need for caution when interpreting animal data and underscore the importance of confirming inhibitory mechanism in HLM or clinical studies.
This pharmacokinetic study was conducted in a relatively small number of animals (4 rats per group). The limited number of subjects may reduce the statistical power of the analysis and restrict the generalizability of the findings. In vivo results in Sprague-Dawley rats showed that nicardipine significantly increased the AUC(0-t),
5 Conclusion
Quetiapine may be co-administered with antihypertensive drugs. Especially, nicardipine could cause fluctuations in quetiapine plasma concentration. Therefore, when quetiapine is used in combination with antihypertensive drugs in clinical practice, special attention should be paid to the potential drug-drug interactions with nicardipine. However, considering interspecies differences, further clinical studies are needed to clarify the possibility of drug interactions occurring in humans.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the Institutional Animal Care and Use Committee (IACUC) of The First Affiliated Hospital of Wenzhou Medical University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JY: Conceptualization, Data curation, Writing – original draft. HX: Investigation, Methodology, Writing – original draft. QL: Methodology, Software, Writing – original draft. RL: Visualization, Writing – original draft. LC: Formal Analysis, Project administration, Writing – original draft. CT: Resources, Supervision, Validation, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank Ren-ai Xu for his help and support in this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CCBs, calcium channel blockers; TCM, traditional Chinese medicine; RLM, rat liver microsomes; HLM, human liver microsomes; IC50, half-maximal inhibitory concentration; SGA, Second-generation antipsychotic drug; CYP, cytochrome P450; IS, internal standard; NADPH, Reduced nicotinamide adenine dinucleotide phosphate; ESI, electrospray ionization; MRM, reaction monitoring; UPLC-MS/MS, ultra performance liquid chromatography tandem mass spectrometry; Km, Michaelis-Menten constant; SD, standard deviation; DDI, drug-drug interactions.
References
Ayerbe, L., Forgnone, I., Addo, J., Siguero, A., Gelati, S., and Ayis, S. (2018). Hypertension risk and clinical care in patients with bipolar disorder or schizophrenia; a systematic review and meta-analysis. J. Affect Disord. 225, 665–670. doi:10.1016/j.jad.2017.09.002
Bakken, G. V., Rudberg, I., Molden, E., Refsum, H., and Hermann, M. (2011). Pharmacokinetic variability of quetiapine and the active metabolite N-desalkylquetiapine in psychiatric patients. Ther. Drug Monit. 33 (2), 222–226. doi:10.1097/FTD.0b013e31821160c4
Chen, Y., and Ding, L. (2023). Potential drug-drug interactions in outpatients with depression of a psychiatry department. Saudi Pharm. J. 31 (2), 207–213. doi:10.1016/j.jsps.2022.12.004
Chen, D., Chen, J., Shen, Y., Chen, X., Xia, H., Liu, Y. N., et al. (2024). Optimization of a sensitive and reliable UPLC-MS/MS method to simultaneously quantify almonertinib and HAS-719 and its application to study the interaction with nicardipine. Pharm. Biol. 62 (1), 874–881. doi:10.1080/13880209.2024.2425648
Cleare, A. J., Kerr-Gaffney, J., Goldsmith, K., Zenasni, Z., Yaziji, N., Jin, H., et al. (2025). Clinical and cost-effectiveness of lithium versus quetiapine augmentation for treatment-resistant depression: a pragmatic, open-label, parallel-group, randomised controlled superiority trial in the UK. Lancet Psychiatry 12 (4), 276–288. doi:10.1016/s2215-0366(25)00028-8
Collaborators, G. B. D. M. D. (2022). Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the global Burden of Disease Study 2019. Lancet Psychiatry 9 (2), 137–150. doi:10.1016/S2215-0366(21)00395-3
EMA (2015). EMA-guideline bioanalytical method validation Available online at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf.
Fisher, D. S., Handley, S. A., Taylor, D., and Flanagan, R. J. (2012). Measurement of quetiapine and four quetiapine metabolites in human plasma by LC-MS/MS. Biomed. Chromatogr. 26 (9), 1125–1132. doi:10.1002/bmc.2672
Fujino, C., Sanoh, S., and Katsura, T. (2021). Variation in expression of cytochrome P450 3A isoforms and toxicological effects: Endo- and exogenous substances as regulatory factors and substrates. Biol. Pharm. Bull. 44 (11), 1617–1634. doi:10.1248/bpb.b21-00332
Grimm, S. W., Richtand, N. M., Winter, H. R., Stams, K. R., and Reele, S. B. (2006). Effects of cytochrome P450 3A modulators ketoconazole and carbamazepine on quetiapine pharmacokinetics. Br. J. Clin. Pharmacol. 61 (1), 58–69. doi:10.1111/j.1365-2125.2005.02507.x
Hammer, H., Schmidt, F., Marx-Stoelting, P., Pötz, O., and Braeuning, A. (2021). Cross-species analysis of hepatic cytochrome P450 and transport protein expression. Arch. Toxicol. 95 (1), 117–133. doi:10.1007/s00204-020-02939-4
Hamsa, A., Karumandampalayam Shanmugaramasamy, K., Kariyarambath, P., and Kathirvel, S. (2023). Quetiapine fumarate: a review of analytical methods. J. Chromatogr. Sci. 61 (9), 892–906. doi:10.1093/chromsci/bmac100
Han, L., Gu, J. Q., Mao, J. H., Liu, X. Q., and Jiao, Z. (2024). Insights into the population pharmacokinetics and pharmacodynamics of quetiapine: a systematic review. Expert Rev. Clin. Pharmacol. 17 (1), 57–72. doi:10.1080/17512433.2023.2295428
Handa, K., Nakagome, I., Yamaotsu, N., Gouda, H., and Hirono, S. (2013). Three-dimensional quantitative structure-activity relationship analysis of inhibitors of human and rat cytochrome P4503A enzymes. Drug Metab. Pharmacokinet. 28 (4), 345–355. doi:10.2133/dmpk.dmpk-12-rg-133
Harrison, P. J., Husain, S. M., Lee, H., Los Angeles, A., Colbourne, L., Mould, A., et al. (2022). CACNA1C (Ca(V)1.2) and other L-type calcium channels in the pathophysiology and treatment of psychiatric disorders: advances from functional genomics and pharmacoepidemiology. Neuropharmacology 220, 109262. doi:10.1016/j.neuropharm.2022.109262
Hayes, J. F., Lundin, A., Wicks, S., Lewis, G., Wong, I. C. K., Osborn, D. P. J., et al. (2019). Association of Hydroxylmethyl Glutaryl coenzyme A reductase inhibitors, L-Type calcium channel antagonists, and biguanides with rates of psychiatric hospitalization and self-harm in individuals with serious mental illness. JAMA Psychiatry 76 (4), 382–390. doi:10.1001/jamapsychiatry.2018.3907
Heisel, L. S., Andersen, F. D., Joca, S., Sørensen, L. K., Simonsen, U., Hasselstrøm, J. B., et al. (2024). Combined in vivo metabolic effects of quetiapine and methadone in brain and blood of rats. Arch. Toxicol. 98 (1), 289–301. doi:10.1007/s00204-023-03620-2
Hole, K., Lorentsen, S. K., Nordby, K. L., Slettvik, M., Sørum, I. T., Molden, E., et al. (2024). Dose-dependent effect of lamotrigine on quetiapine serum concentration in patients using instant release tablets. Eur. J. Clin. Pharmacol. 80 (6), 839–845. doi:10.1007/s00228-024-03655-z
Jensen, N. H., Rodriguiz, R. M., Caron, M. G., Wetsel, W. C., Rothman, R. B., and Roth, B. L. (2008). N-desalkylquetiapine, a potent norepinephrine reuptake inhibitor and partial 5-HT1A agonist, as a putative mediator of quetiapine's antidepressant activity. Neuropsychopharmacology 33 (10), 2303–2312. doi:10.1038/sj.npp.1301646
Johannessen, L., Strudsholm, U., Foldager, L., and Munk-Jorgensen, P. (2006). Increased risk of hypertension in patients with bipolar disorder and patients with anxiety compared to background population and patients with schizophrenia. J. Affect Disord. 95 (1-3), 13–17. doi:10.1016/j.jad.2006.03.027
Kamel, A., and Harriman, S. (2013). Inhibition of cytochrome P450 enzymes and biochemical aspects of mechanism-based inactivation (MBI). Drug Discov. Today Technol. 10 (1), e177–e189. doi:10.1016/j.ddtec.2012.09.011
Ko, S., Chang, S. H., Chung, Y. W., Seo, Y. G., Kang, D. Y., Kim, K., et al. (2023). Investigation of hepatic adverse events due to quetiapine by using the common data model. Pharmacoepidemiol Drug Saf. 32 (12), 1341–1349. doi:10.1002/pds.5663
Krejci, V., Murinova, I., Slanar, O., and Sima, M. (2024). Evidence for therapeutic drug monitoring of atypical antipsychotics. Prague Med. Rep. 125 (2), 101–129. doi:10.14712/23362936.2024.10
Kretchy, I. A., Owusu-Daaku, F. T., and Danquah, S. A. (2014). Mental health in hypertension: assessing symptoms of anxiety, depression and stress on anti-hypertensive medication adherence. Int. J. Ment. Health Syst. 8, 25. doi:10.1186/1752-4458-8-25
Krøigaard, S. M., Clemmensen, L., Tarp, S., and Pagsberg, A. K. (2022). A meta-analysis of antipsychotic-induced Hypo- and Hyperprolactinemia in children and adolescents. J. Child. Adolesc. Psychopharmacol. 32 (7), 374–389. doi:10.1089/cap.2021.0140
Li, M., Zhang, S., Shi, A., Qi, W., and Liu, Y. (2017). Determination of quetiapine in human plasma by LC-MS/MS and its application in a bioequivalence study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1060, 10–14. doi:10.1016/j.jchromb.2017.05.031
Li, D., Wang, H., Qin, C., Du, D., Wang, Y., Du, Q., et al. (2024a). Drug-Induced acute pancreatitis: a real-world pharmacovigilance Study using the FDA adverse event reporting System database. Clin. Pharmacol. Ther. 115 (3), 535–544. doi:10.1002/cpt.3139
Li, S., Xu, C., Hu, S., and Lai, J. (2024b). Efficacy and tolerability of FDA-approved atypical antipsychotics for the treatment of bipolar depression: a systematic review and network meta-analysis. Eur. Psychiatry 67 (1), e29. doi:10.1192/j.eurpsy.2024.25
Lintunen, J., Lahteenvuo, M., Tanskanen, A., Tiihonen, J., and Taipale, H. (2022). Allopurinol, dipyridamole and calcium channel blockers in the treatment of bipolar disorder - a nationwide cohort study. J. Affect Disord. 313, 43–48. doi:10.1016/j.jad.2022.06.040
Liu, Y. N., Chen, J., Wang, J., Li, Q., Hu, G. X., Cai, J. P., et al. (2023). Effects of drug-drug interactions and CYP3A4 variants on alectinib metabolism. Arch. Toxicol. 97 (8), 2133–2142. doi:10.1007/s00204-023-03524-1
Luo, K., Zhao, H., Zhang, H., Huang, L., Lin, J., Cheng, Q., et al. (2023). A validated method for the determination of quetiapine fumarate tablets in human plasma by UPLC-MS/MS and its application to a pharmacokinetic study in healthy Chinese subjects. Pak J. Pharm. Sci. 36 (5), 1597–1607.
Maneeton, N., Maneeton, B., Woottiluk, P., Likhitsathian, S., Suttajit, S., Boonyanaruthee, V., et al. (2016). Quetiapine monotherapy in acute treatment of generalized anxiety disorder: a systematic review and meta-analysis of randomized controlled trials. Drug Des. Devel Ther. 10, 259–276. doi:10.2147/DDDT.S89485
Martignoni, M., Groothuis, G. M., and de Kanter, R. (2006). Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin. Drug Metab. Toxicol. 2 (6), 875–894. doi:10.1517/17425255.2.6.875
Mauri, M. C., Paletta, S., Di Pace, C., Reggiori, A., Cirnigliaro, G., Valli, I., et al. (2018). Clinical pharmacokinetics of atypical antipsychotics: an update. Clin. Pharmacokinet. 57 (12), 1493–1528. doi:10.1007/s40262-018-0664-3
Nakamura, K., Ariyoshi, N., Iwatsubo, T., Fukunaga, Y., Higuchi, S., Itoh, K., et al. (2005). Inhibitory effects of nicardipine to cytochrome P450 (CYP) in human liver microsomes. Biol. Pharm. Bull. 28 (5), 882–885. doi:10.1248/bpb.28.882
Pan, R. N., Kuo, B. P., and Pao, L. H. (2012). Validated LC-MS-MS method for the determination of quetiapine in human plasma: application to a pharmacokinetic study. J. Chromatogr. Sci. 50 (3), 277–282. doi:10.1093/chromsci/bms001
Reif, A., Bitter, I., Buyze, J., Cebulla, K., Frey, R., Fu, D. J., et al. (2023). Esketamine Nasal spray versus quetiapine for treatment-resistant depression. N. Engl. J. Med. 389 (14), 1298–1309. doi:10.1056/NEJMoa2304145
Riccardi, K., Ryu, S., Lin, J., Yates, P., Tess, D., Li, R., et al. (2018). Comparison of species and cell-type differences in fraction unbound of liver tissues, hepatocytes, and cell lines. Drug Metab. Dispos. 46 (4), 415–421. doi:10.1124/dmd.117.079152
Sarris, J., Ravindran, A., Yatham, L. N., Marx, W., Rucklidge, J. J., McIntyre, R. S., et al. (2022). Clinician guidelines for the treatment of psychiatric disorders with nutraceuticals and phytoceuticals: the World Federation of Societies of Biological Psychiatry (WFSBP) and Canadian Network for Mood and Anxiety Treatments (CANMAT) Taskforce. World J. Biol. Psychiatry 23 (6), 424–455. doi:10.1080/15622975.2021.2013041
Srifuengfung, M., Sukakul, T., Pummangura, C., Srifuengfung, S., and Viravan, N. (2022). Oral ulcers as an unpredictable adverse reaction to quetiapine use in a patient with schizophrenia. J. Clin. Psychopharmacol. 42 (1), 103–106. doi:10.1097/JCP.0000000000001473
Sudarshan, Y., and Cheung, B. M. Y. (2023). Hypertension and psychosis. Postgrad. Med. J. 99 (1171), 411–415. doi:10.1136/postgradmedj-2021-141386
Watermeyer, F., Gaebler, A. J., Neuner, I., Haen, E., Hiemke, C., Schoretsanitis, G., et al. (2024). Discovering interactions in polypharmacy: impact of metamizole on the metabolism of quetiapine. Br. J. Clin. Pharmacol. 90 (11), 2793–2801. doi:10.1111/bcp.16168
Wright, W. C., Chenge, J., and Chen, T. (2019). Structural perspectives of the CYP3A family and their small molecule modulators in drug metabolism. Liver Res. 3 (3-4), 132–142. doi:10.1016/j.livres.2019.08.001
Xia, Z., Wang, M., Zou, S., and Chen, R. (2012). Different effects of dihydropyridine calcium channel antagonists on CYP3A4 enzyme of human liver microsomes. Eur. J. Drug Metab. Pharmacokinet. 37 (3), 211–216. doi:10.1007/s13318-011-0076-3
Xiong, X., Yang, L., and Duan, J. (2013). Development and validation of a sensitive and robust LC-MS/MS with electrospray ionization method for simultaneous quantitation of quetiapine and its active metabolite norquetiapine in human plasma. Clin. Chim. Acta 423, 69–74. doi:10.1016/j.cca.2013.04.016
Ye, Z., Xia, H., Hu, J., Liu, Y. N., Wang, A., Cai, J. P., et al. (2024). CYP3A4 and CYP2C19 genetic polymorphisms and myricetin interaction on tofacitinib metabolism. Biomed. Pharmacother. 175, 116421. doi:10.1016/j.biopha.2024.116421
Zhou, S. F., Xue, C. C., Yu, X. Q., Li, C., and Wang, G. (2007). Clinically important drug interactions potentially involving mechanism-based inhibition of cytochrome P450 3A4 and the role of therapeutic drug monitoring. Ther. Drug Monit. 29 (6), 687–710. doi:10.1097/FTD.0b013e31815c16f5
Keywords: quetiapine, nicardipine, drug-drug interactions, UPLC-MS/MS, microsome
Citation: Yang J, Xia H, Li Q, Li R, Cao L and Tang C (2025) Evaluation the inhibitory effect of nicardipine on the metabolism of quetiapine. Front. Pharmacol. 16:1567044. doi: 10.3389/fphar.2025.1567044
Received: 26 January 2025; Accepted: 09 September 2025;
Published: 23 September 2025.
Edited by:
Yurong Lai, Gilead, United StatesReviewed by:
Basak Gokce, Süleyman Demirel University, TürkiyeSourav Poddar, Haldia Institute of Technology, India
Copyright © 2025 Yang, Xia, Li, Li, Cao and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Congrong Tang, dGNyYWIxQDE2My5jb20=
†These authors have contributed equally to this work
 Jinzhao Yang
Jinzhao Yang Hailun Xia
Hailun Xia Qingqing Li
Qingqing Li Ruibin Li2
Ruibin Li2 Congrong Tang
Congrong Tang