- 1Anhui University of Chinese Medicine, Hefei, China
- 2The First Affiliated Hospital of Anhui University of Chinese Medicine, Hefei, China
- 3Anhui Province Key Laboratory of the Application and Transformation of Traditional Chinese Medicine in the Prevention and Treatment of Major Pulmonary Diseases, Hefei, China
Background: Jin Shui Liu Jun Decoction (JLD) is a classical prescription in Traditional Chinese Medicine (TCM). In recent years, JLD has shown beneficial effects on patients with chronic obstructive pulmonary disease (COPD). However, the existing clinical research results are contradictory, and high-quality, evidence-based medical evidence is lacking. Therefore, the exact therapeutic efficacy of JLD has not been fully evaluated.
Objectives: This study aimed to ascertain the precise therapeutic efficacy of JLD in treating COPD.
Data sources and Method: A search of 10 electronic databases was conducted up to 30 November 2024. The standardized mean difference (SMD) was used to assess continuous variables, while the relative risk (RR) was calculated to evaluate dichotomous variables. The Luis Furuya-Kanamori (LFK) asymmetry index, along with the Doi plot, Egger’s test, and Begg’s test, were used to assess publication bias. Sensitivity analysis was performed to evaluate the stability of the conclusions. Furthermore, trial sequential analysis (TSA) was used to assess the risk of false-positive results and to estimate the required sample size for the meta-analysis. Finally, single-factor and multi-factor meta-regression were conducted to analyze the sources of heterogeneity.
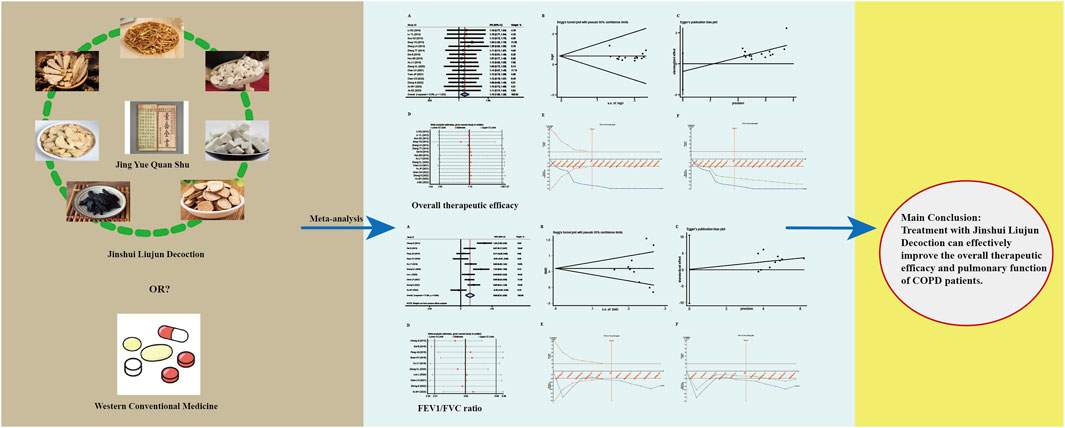
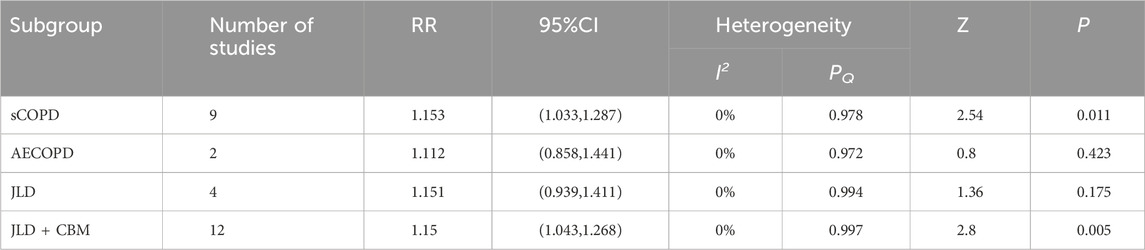
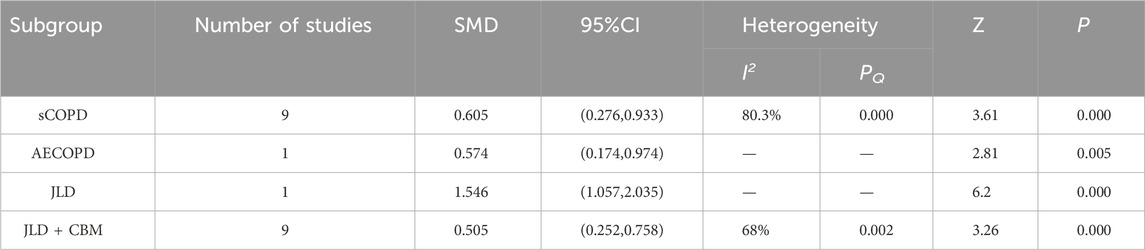
Results: A total of 22 trials meeting the inclusion criteria were included, including 1,817 COPD participants. The meta-analysis indicated that JLD could improve overall treatment efficacy (RR = 1.15, 95% CI: 1.053–1.256, PZ = 0.002) and pulmonary function (FEV1: SMD = 0.661, 95% CI: 0.276–1.046, PZ = 0.001; FEV1% Predicted: SMD = 0.368, 95% CI: 0.067–0.669, PZ = 0.017; FVC: SMD = 0.814, 95% CI: 0.392–1.235, PZ < 0.001; FEV1/FVC Ratio: SMD = 0.602, 95% CI: 0.311–0.893, PZ < 0.001) in COPD, potentially offering benefits in traditional Chinese medicine syndrome scores (SMD = 0.936, 95% CI: 0.301–1.571, PZ = 0.004) and 6-min walk distance (6MWD: SMD = 0.744, 95% CI: 0.182–1.306, PZ = 0.009). The subgroup analysis revealed a higher overall efficacy and improvement in FEV1/FVC Ratio of JLD treatment for stable chronic obstructive pulmonary disease (sCOPD) compared to the acute exacerbation of chronic obstructive pulmonary disease (AECOPD) group (SMD:0.605 v.s. 0.574, PZ < 0.05). Additionally, compared to the simple JLD treatment plan, the conventional biomedicine (CBM)+JLD scheme showed superior overall efficacy in treating COPD patients (RR = 1.15, 95% CI: 1.043–1.268, PZ = 0.005).
Conclusion: Treatment with JLD can effectively improve the overall efficacy and pulmonary function (FEV1%pred requires more research for confirmation) of COPD patients. However, the methodological quality of the included trials may limit the generalizability of the study’s findings. Sources of heterogeneity were partially identified through meta-regression, but further rigorous randomized controlled trials are still required.
Systematic Review Registration: https://osf.io/msw7b.
1 Introduction
Chronic obstructive pulmonary disease (COPD) is a globally prevalent disease characterized primarily by persistent and progressive airflow obstruction (Agustí et al., 2023). According to a survey on the burden of disease, COPD ranks among the top ten causes of mortality globally, with an age-standardized mortality rate of 45.2 per 100,000 individuals. In China, the annual death toll attributed to COPD accounts for approximately 31.1% of the global total (Ong et al., 2024; Qi et al., 2022),. Considering the chronic and incurable nature of COPD, its frequent exacerbations impose a substantial societal and economic burden. In clinical practice, the primary therapeutic approach for COPD focuses on delaying the degradation of the condition. Common treatments include the use of corticosteroids, anticholinergic medications, and beta-2 agonists (Bollmeier and Hartmann, 2020). These medications are effective in managing COPD but also increase the risk of opportunistic infections and cardiovascular events (Walters et al., 2018; Michele et al., 2010; Vanfleteren et al., 2018; Linhui et al., 2021). Hence, the long-term efficacy and safety of these treatments remain controversial.
Jin Shui Liu Jun Decoction (JLD), a commonly used prescription for the treatment of COPD, was first documented in the “Jing Yue Quan Shu Xin Fang Ba Zhen”. The prescription comprises seven traditional Chinese medicinal ingredients. Randomized controlled trials revealed that treatment of COPD with modifications of JLD yielded a higher rate of therapeutic efficacy and significantly improved patients’ lung function. However, due to the limited sample size of these studies, the conclusions still lack credibility. Owing to the absence of high-quality evidence, JLD has not yet been recommended in guidelines in China (MedicineWorld Federation of Chinese Medicine Societies Specialty Committee of Internal, 2023). In addition, Traditional Chinese Medicine (TCM) treatment encompasses personalized therapy and flexible modification of prescriptions. Nevertheless, these characteristics increase clinical research variability, complicating meta-analysis and heterogeneity analysis (Fayang et al., 2023). Finally, traditional meta-analyses may be prone to Type I errors, which are false positives, and may lead to inefficient medical resources allocations due to the inability to provide the desired responsiveness index score (RIS) (Hong et al., 2016).
Therefore, this study aimed to address the aforementioned issues by conducting a meta-analysis of randomized controlled trials (RCTs) on the treatment of COPD with JLD. To provide a more comprehensive evidence base, the study employed a combination of many analytical methods, including sensitivity analysis, meta-regression, and trial sequential analysis (TSA). These approaches were integrated to generate a detailed and robust chain of evidence regarding the efficacy of JLD in treating COPD. As the first systematic evaluation of JLD’s therapeutic effects on COPD, this study not only addresses existing research gaps but also offers clinicians objective and actionable guidance for its clinical application. By objectively estimating the RIS through TSA, the study further strengthens the reliability of its conclusions, thereby contributing to evidence-based decision-making in COPD management.
2 Methods
Our protocol was registered in the Open Science Framework (https://osf.io/msw7b). This systematic review and meta-analysis were conducted in accordance with the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement” (Page et al., 2021).
2.1 Taxonomic validation of the key botanical drug
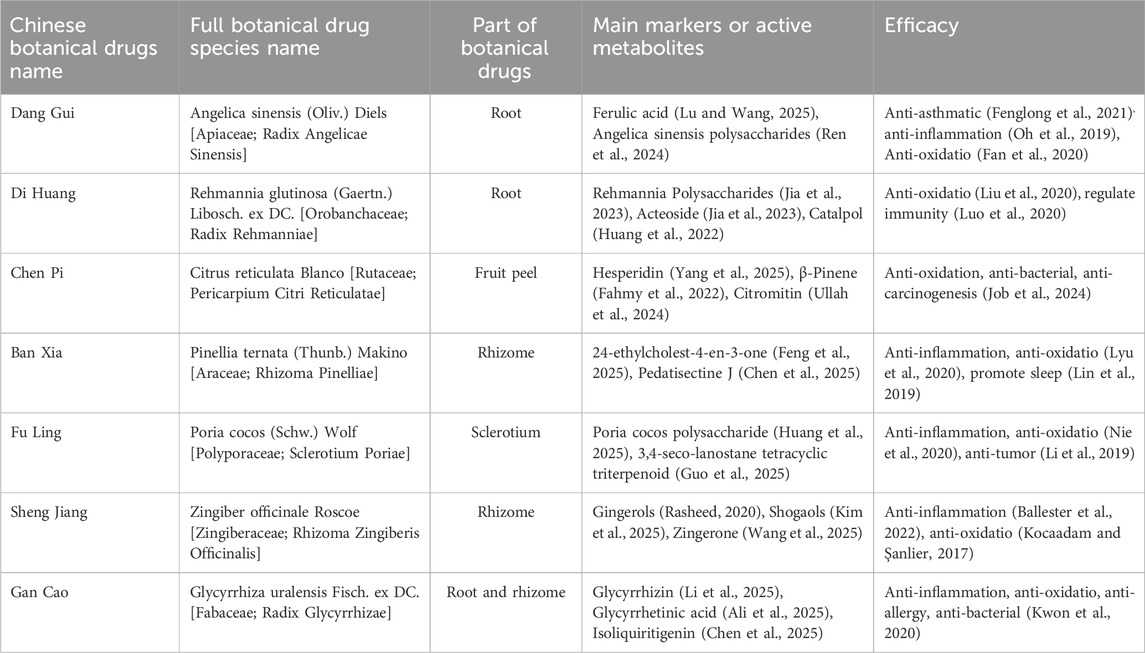
The taxonomic validation of the key botanical drugs in JLD has been verified in the Medicinal Plant Names Services (MPNS) (https://mpns.science.kew.org/mpns-portal/) and the MYCOBANK Database (https://www.mycobank.org/), as detailed in Table 1.
2.2 Search strategy
Two researchers independently searched databases including PubMed, Embase, Web of Science, Cochrane Library, SCOPUS, Sinomed, CNKI, Proquest, Wanfang Data, and the VIP Chinese Journal Service Platform. The search period spanned from the inception of each database to 30 November 2024. To avoid omissions, the references of the included studies were manually searched when necessary. The search terms included “Jin Shui Liu Jun Jian”, “Jinshui Liujun Decoction”, and “Chronic Obstructive Pulmonary Diseases”. The detailed search strategies are provided in Supplementary Table S1.
2.3 Inclusion criteria
2.3.1 Research type
Only randomized controlled trials evaluating the efficacy and safety of JLD in treating COPD were included, without restrictions on language, blinding, publication type, etc.
2.3.2 Participants
Participants were diagnosed with COPD according to the Global Initiative for Chronic Obstructive Lung Disease or the Chinese expert consensus on the diagnosis and treatment of chronic obstructive pulmonary disease (COPD), without restrictions based on age, gender, region, or demographic characteristics.
2.3.3 Type of intervention
Participants in the control group received CBM treatment. CBM encompasseds essential treatments: (1) General supportive therapy, such as (A) oxygen supplementation, (B) necessary cardiac examinations and treatments, (C) monitoring of temperature, (D) monitoring of blood pressure, (E) monitoring of blood glucose levels, (F) nutritional support; (2) Common medications for COPD treatment, such as (A) β2 receptor agonists, (B) anticholinergic drugs, (C) theophylline, (D) ICS (inhaled corticosteroids), (E) mucolytics; (3) Anti-infective therapy, primarily involving antibiotics. All treatment regimens and dosages were included in the meta-analysis.
The treatment group included patients treated with JLD alone or with modified JLD. JLD’s formulation comprises a decoction (Tang) consisting of Angelica sinensis, Rehmannia glutinosa, Citrus reticulata peel, Pinellia ternata, Poria cocos, Glycyrrhiza uralensis (baked), and fresh ginger. Modified JLD refers to JLD supplemented with additional traditional Chinese botanical drugs based on syndrome differentiation, the modified JLD, comprises similar primary ingredients and exerts similar therapeutic effects to JLD. Interventions combining JLD with CBM therapy were also included. No restrictions were imposed on the dosage and formulation of JLD; standard dose ranges were deemed acceptable, with a minimum treatment duration of 10 days.
2.3.4 Outcome measures
The outcome measures included overall therapeutic efficacy, lung function, 6-min walking distance (6MWD), traditional Chinese medicine syndrome score, interleukin-6 levels, etc.
2.4 Exclusion criteria
This meta-analysis excluded the following studies: 1. Quasi-randomized controlled trials that allocated participants based on birth dates, medical record numbers, admission dates, or ID numbers; 2. Studies involving mechanically ventilated patients; 3. Studies involving comorbidities such as bronchial asthma, lung cancer, bronchiectasis, pulmonary hypertension, or other mental disorders/chronic diseases like depression, anxiety, or malnutrition; 4. Studies without a control group; 5. Non-randomized controlled trials (RCTs) including abstracts, reviews, case reports, expert opinions, animal experiments, etc.; 6. Studies with duplicated data; 7. Studies lacking extractable data from text/tables or obtainable data from authors; 8. Studies with significant errors in data processing and statistical methods; 9. Studies where the intervention group used external therapies or exercise therapies in addition to JLD, such as respiratory exercises, Baduanjin, Tai Chi, rehabilitation training, moxibustion, acupoint application, etc.; 10. Studies that have modified the Jin Shui Liu Jun Decoction (JLD) to include more than six botanical drugs.
2.5 Data extraction and quality assessment
Based on the inclusion and exclusion criteria, data extraction was independently conducted by two researchers and were subsequently cross-verified. Discrepancies were resolved through discussion or by involving a third researcher. The fundamental study details (authors, publication date, title, etc.), participant characteristics (sample size, gender, age, etc.), intervention specifics (therapy types and durations for both groups), and outcome measures (primary and secondary endpoints, adverse events, etc.) were extracted from the studies. For studies reporting multiple measurements, data from the final observation period were utilized for analysis.
The methodological quality of the retrieved research literature was evaluated using the modified Jadad scoring scale. The evaluation criteria included random sequence generation, allocation concealment, blinding, and withdrawals and dropouts. Studies were classified as low quality (1–3 points) or high quality (4–7 points), where 7 points indicating the highest quality.
2.6 Data analysis methods
A meta-analysis of the included studies was performed using Stata (Version 14.0) and trial sequential analysis (TSA v0.9.5.10 Beta) software. Standardized mean differences (SMD) were utilized to access continuous variables, while relative risk (RR) with 95% confidence intervals (CI) were computed for dichotomous variables. Heterogeneity among study results was evaluated using the Q test, employing a fixed-effects model when I2 ≤ 50% and PQ ≥ 0.10. In contrast, a random-effects model was applied when I2 ≥ 50% and PQ < 0.10. When the number of studies included in the analysis was less than 10, publication bias was evaluated using Doi plots and the Luis Furuya-Kanamori (LFK) asymmetry index, where LFK index values within ±1, ±1 to ±2, and ±2 or greater indicated no asymmetry, slight asymmetry, and significant asymmetry, respectively (Furuya-Kanamori et al., 2018; Li et al., 2023). When more than ten studies were included in the analysis, Egger’s test and Begg’s test were employed to assess publication bias, with P ≥ 0.05 indicating no significant publication bias. Sensitivity analysis was conducted using the leave-one-out method. Furthermore, subgroup analyses were performed to explore the efficacy of different treatment regimens for COPD patients and the efficacy of JLD across different stages of COPD, with each subgroup containing at least three studies. Meta-regression was employed to analyze variables that may influence the outcomes, with P < 0.05 indicating statistical significance. Subgroup analyses and meta-regression included at least 10 studies for each outcome measure.
3 Results
3.1 Selection and description of studies
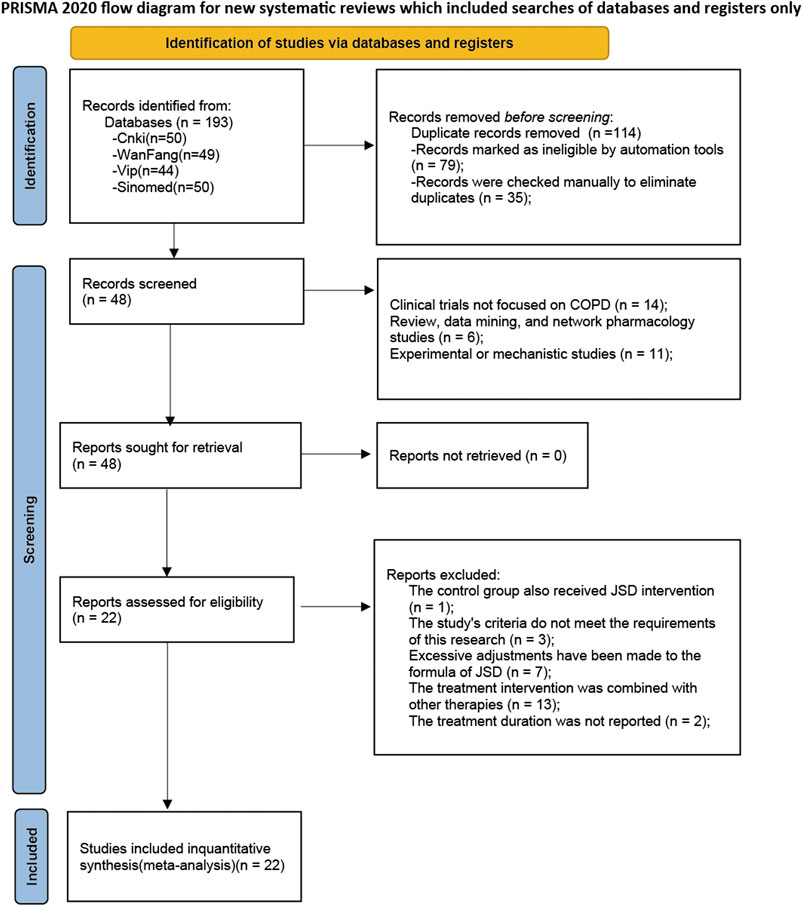
Through database searches, a total of 193 records were initially retrieved. Subsequently, 79 records identified as duplicates by the software due to multi-database searches were removed, along with 35 additional duplicates detected through manual review. The titles and abstracts were examined, and 11 records were identified as experimental or mechanistic studies, 6 records as reviews, data mining, or network pharmacology studies, and 14 records as non-COPD studies. Upon reading the full texts, one record was found to have a control group that also used JLD intervention, 3 records had study indicators that did not meet our criteria, 7 records had excessive modifications to the ingredients of JLD, 13 records combined other therapies, and 2 records did not report the treatment course. Therefore, all these records were excluded from the analysis. Finally, 22 studies were included (Xiaoqi, 2010; Rongzhao et al., 2011; Qi, 2012; Yunlong et al., 2012; Guizhen, 2013; Lihua et al., 2013; Yuqin et al., 2013; Tiantao et al., 2014; Baosong et al., 2015; Bei et al., 2015; Jingqin et al., 2015; Kaiyue et al., 2018; Liyan et al., 2019; Chuanlin, 2020; Jingqin et al., 2020; Li and Liu, 2020; Jiping, 2021; Linhui et al., 2021; Cangsong et al., 2022; Shan, 2022; Baozheng et al., 2023; Mengyao, 2023), involving 1817 participants in this meta-analysis, with 908 cases in the experimental group and 909 cases in the control group. The inclusion and exclusion process is displayed in Figure 1.
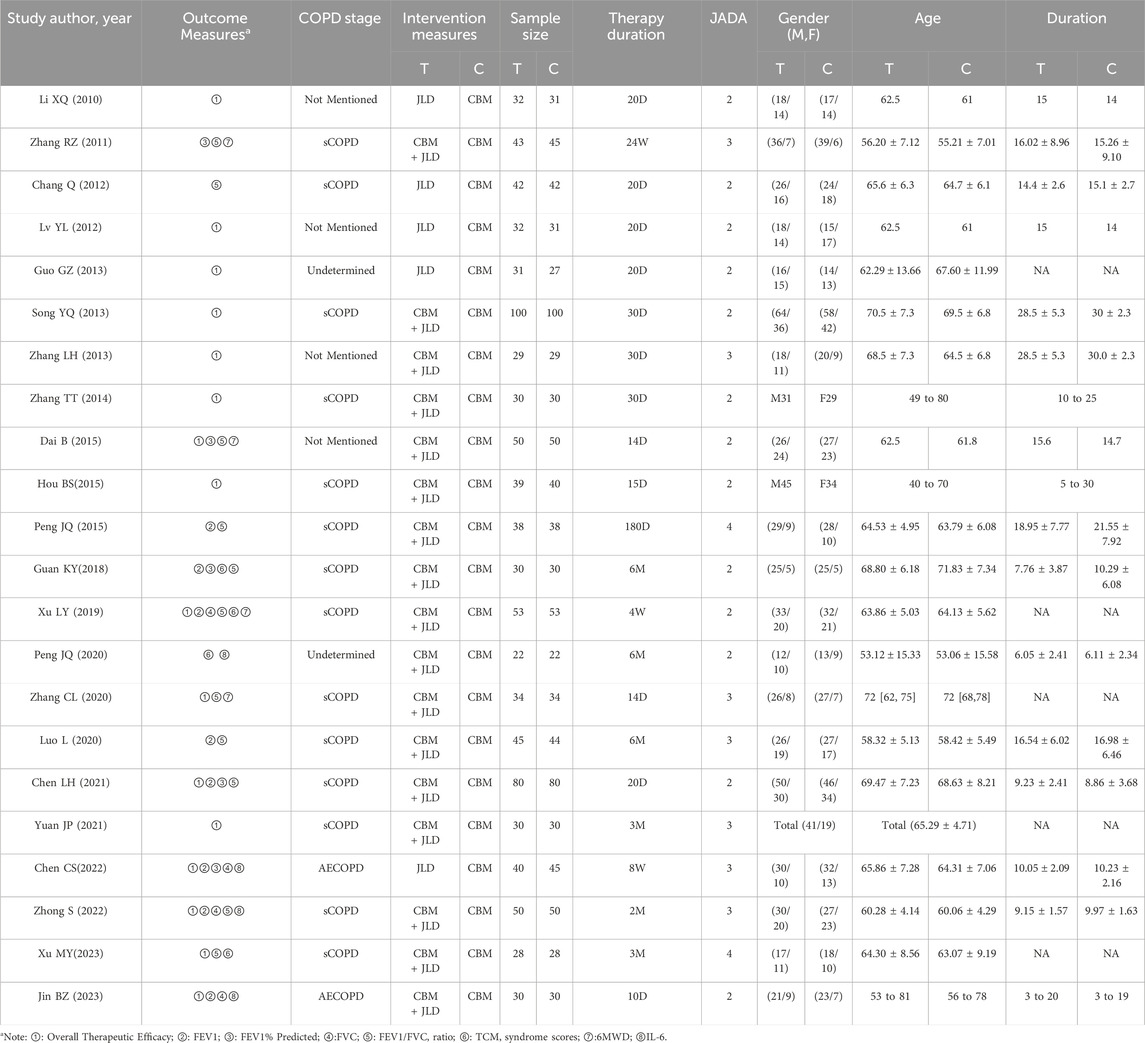
All the studies were published between 2010 and 2023, with treatment durations ranging from 10 days to 6 months. Fourteen studies focused on the stable phase of COPD, 2 studies on AECOPD, and 6 studies did not specify and the disease condition could not be determined based on their content. Only one study did not mention the dosage form, while the remaining studies all used decoction for administration. All the experimental groups received JLD or JLD combined with CBM treatment, while the control groups received CBM treatment. Sixteen studies reported the overall efficacy rate; seven studies reported forced expiratory volume in one second (FEV1); eleven studies reported the ratio of FEV1 to forced vital capacity (FVC), which is expressed as FEV1/FVC(%); five studies reported FEV1%predicted; and four studies each reported FVC, TCM syndrome scores, 6MWT, and IL-6. Three studies reported dropouts and losses to follow-up, 3 studies reported whether adverse events occurred, and 1 study reported follow-up conditions. All details of the included studies can be found in Table 2.
3.2 Effects of JLD on overall therapeutic efficacy
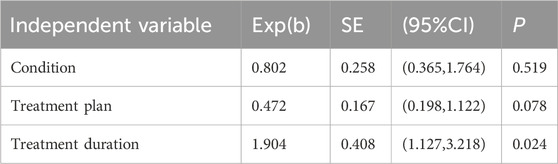
A total of 16 studies reported the overall therapeutic efficacy of JLD in the treatment of COPD, including a total of 1,376 patients (688 in both the experimental group and the control group, Figure 2A).No significant heterogeneity was observed among the studies (I2 = 0.000, PQ = 1), and a fixed-effects model was used for the meta-analysis, which showed a significantly higher overall therapeutic efficacy in the experimental group compared to the control group (RR = 1.15, 95% CI: 1.053–1.256, PZ = 0.002). The Egger test and Begg test indicated that the sample distribution was essentially symmetrical, with P values greater than 0.05 (PEgger = 0.381, PBegg = 0.28, Figures 2B,C), suggesting no publication bias. Sensitivity analysis revealed that the conclusion was stable (Figure 2D). Subgroup analysis indicated that the therapeutic effect of JLD in treating stable COPD was more significant compared to AECOPD (RR = 1.153, P = 0.011, Table 3). Moreover, compared with the JLD group, the CBM + JLD group achieved superior therapeutic effects (RR = 1.15, P = 0.005, Table 3).
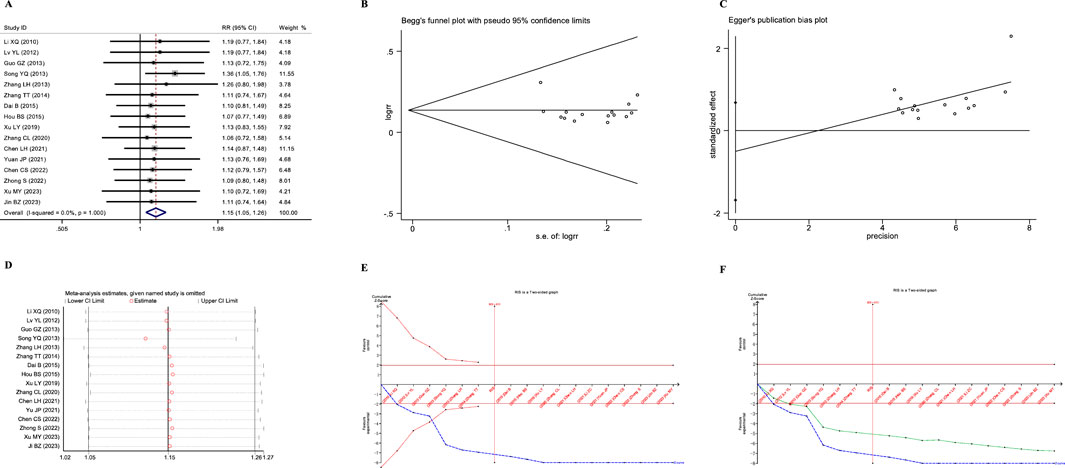
Figure 2. Meta-analysis diagrams of overall therapeutic efficacy. (A) Forest plot. (B) Begg's funnel plot. (C) Egger's publication bias plot. (D) Sensitivity analysis. (E) TSA analysis. (F) Penalized statistical analysis.
The TSA results showed that the cumulative Z-curve crossed the conventional boundary after the inclusion of the first study, crossed the TSA boundary after the inclusion of the fourth study, and the sample size exceeded the RIS (RIS: 455) after the inclusion of the seventh study (Figure 2E). Further penalized statistical analysis indicated that the penalized Z-curve crossed the conventional boundary after the inclusion of the second study (Figure 2F). Both the results of the meta-analysis and TSA confirmed that JLD effectively improved the overall therapeutic efficacy of the treatment for COPD.
3.3 Meta-analysis of pulmonary function
3.3.1 Effects of JLD on FEV1 of COPD patients
A total of seven studies reported the impact of JLD treatment on the FEV1 of patients with COPD. One study was excluded due to significant data errors (Li and Liu, 2020). The remaining seven studies involved 647 patients (321 in the experimental group and 326 in the control group). Heterogeneity was found among the studies (I2 = 82%, PQ < 0.001), and a random-effects model was employed for the meta-analysis. The results showed that the therapeutic effect of the experimental group on improving pulmonary function, specifically FEV1, was significantly superior to that of the control group (SMD = 0.661, 95% CI: 0.276–1.046, PZ = 0.001), as shown in Figure 3A.
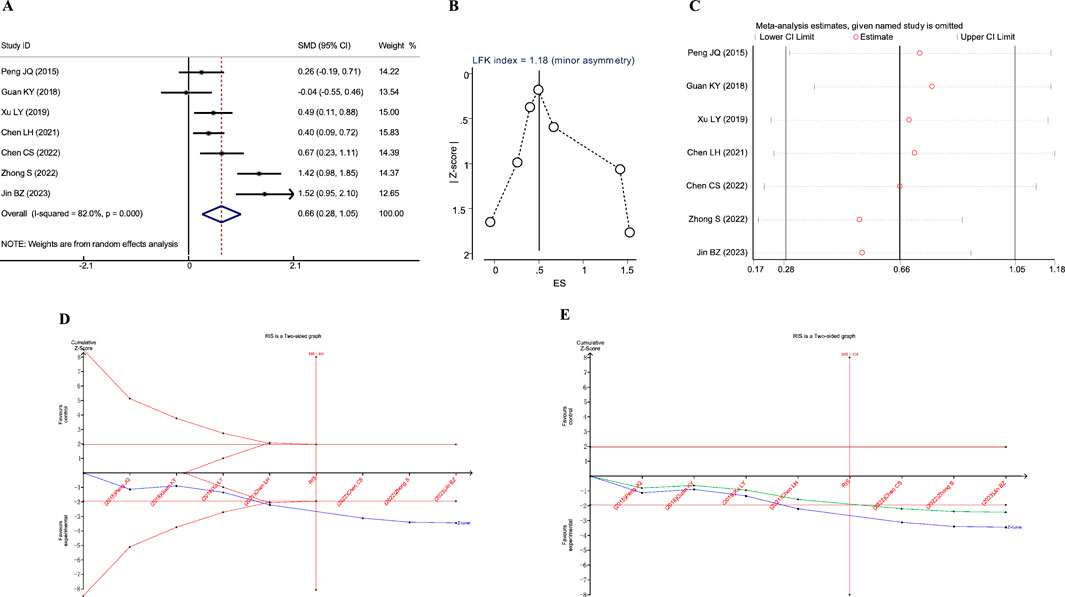
Figure 3. Meta-analysis diagrams of FEV1. (A) Forest plot. (B) Doi plot. (C) Sensitivity analysis. (D) TSA analysis. (E) Penalized statistical analysis.
The Doi plots displayed slight asymmetry (LFK index = 1.18, Figure 3B), suggesting the possibility of publication bias. Sensitivity analysis confirmed the stability of the conclusions (Figure 3C). The TSA results revealed that the cumulative Z-curve crossed both the conventional boundary and the TSA-adjusted boundary after the inclusion of the fourth study, and the sample size had reached the required information size (RIS: 416, Figure 3D). Further penalized statistical analysis indicated that the penalized Z-curve surpassed the conventional boundary after the inclusion of the fifth study (Figure 3E). Both the meta-analysis and TSA results confirmed that JLD treatment in COPD patients can improve FEV1.
3.3.2 Effects of JLD on FEV1% predicted of COPD patients
A total of five studies reported the impact of JLD treatment on the FEV1% predicted in COPD patients, including 493 patients (243 in the experimental group and 250 in the control group). Heterogeneity was observed among the studies (I2 = 62.9%, PQ = 0.029), and a random-effects model was utilized for the meta-analysis. The meta-analysis results indicated a significantly higher efficacy in improving pulmonary function, specifically FEV1% predicted, in the experimental group to the control group (SMD = 0.368, 95% CI: 0.067–0.669, PZ = 0.017), as depicted in Figure 4A.
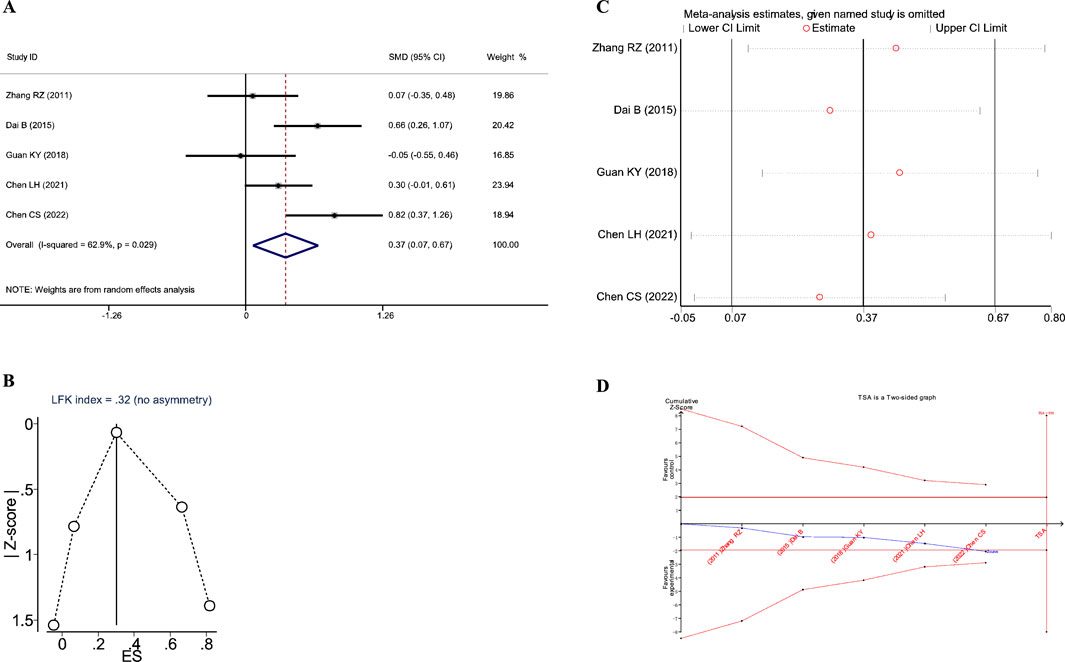
Figure 4. Meta-analysis diagrams of FEV1% Predicted. (A) Forest plot. (B) Doi plot. (C) Sensitivity analysis. (D) TSA analysis.
The Doi plots demonstrated no asymmetry (LFK index = 0.32, Figure 2B), suggesting no publication bias. Furthermore, sensitivity analysis confirmed the stability of the conclusions (Figure 2C). The TSA revealed that the cumulative Z-curve crossed the conventional boundary after the first study. However, the cumulative Z-curve did not cross the TSA-adjusted boundary, and the required information size (RIS: 930) was not achieved. These findings suggest that further research is needed to confirm the efficacy of JLD treatment on the FEV1% Predicted of COPD patients.
3.3.3 Effects of JLD on FVC of COPD patients
A total of four studies reported the impact of JLD treatment on the FVC in COPD patients, including 351 patients (173 in the experimental group and 178 in the control group). Heterogeneity was observed among the studies (I2 = 72.3%, PQ = 0.013), and a random-effects model was applied for the meta-analysis. The meta-analysis results revealed that the therapeutic effect of the experimental group on improving lung function, specifically FVC, was significantly superior to that of the control group (SMD = 0.814, 95% CI: 0.392–1.235, PZ<0.001), as shown in Figure 5A.
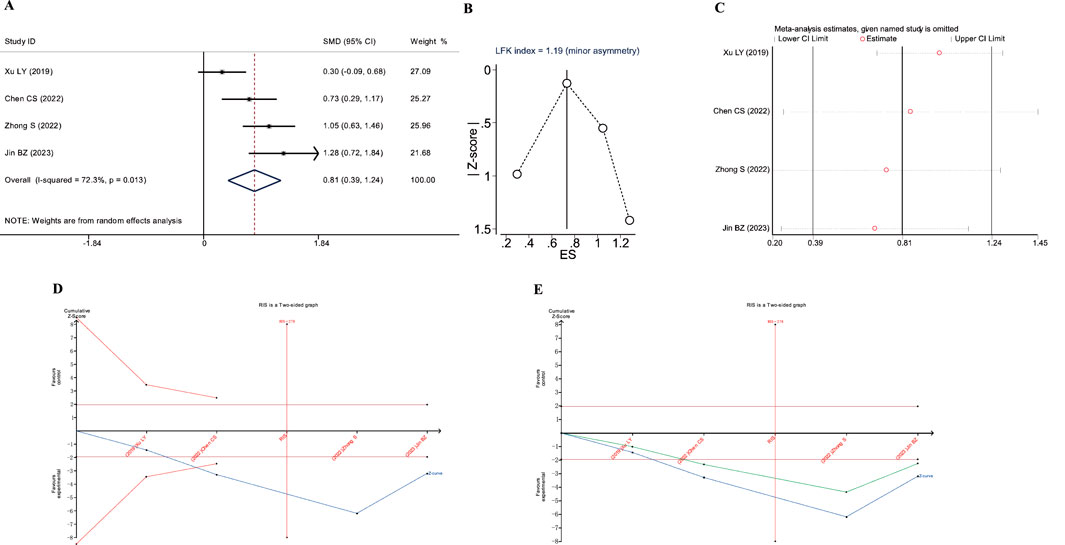
Figure 5. Meta-analysis diagrams of FVC. (A) Forest plot. (B) Doi plot. (C) Sensitivity analysis. (D) TSA analysis. (E) Penalized statistical analysis.
The Doi plots displayed slight asymmetry (LFK index = 1.19, Figure 5B), suggesting the possibility of publication bias. Sensitivity analysis confirmed the stability of the conclusions (Figure 5C). The TSA results showed that the cumulative Z-curve crossed both the conventional boundary and the TSA-adjusted boundary after the inclusion of the second study, and the sample size had reached the required information size (RIS: 278, Figure 5D). In addition, the penalized statistical analysis indicated that the penalized curve surpassed the conventional boundary after the inclusion of the second study, further confirming the conclusion (Figure 5E). Both the meta-analysis and TSA results substantiated that JLD treatment for COPD can improve FVC.
3.3.4 Effects of JLD on FEV1/FVC ratio of COPD patients
A total of 10 studies reported the impact of JLD treatment on the FEV1/FVC ratio in COPD patients, involving 899 patients (450 in the experimental group and 449 in the control group). Heterogeneity was present among the studies (I2 = 77.8%, PQ < 0.001), so a random-effects model was employed for the meta-analysis. The meta-analysis results indicated a significantly higher improvement in the lung function ratio, specifically FEV1/FVC, in the experimental group compared to the control group (SMD = 0.602, 95% CI: 0.311–0.893, PZ < 0.001), as shown in Figure 6A. Further subgroup analysis confirmed the therapeutic effect of JLD on the FEV1% of patients with AECOPD (SMD = 0.574, P = 0.005) and sCOPD (SMD = 0.605, P < 0.001) (Table 4). Compared with the control group, both the CBM + JLD group (SMD = 0.521, P = 0.001) and the JLD group (SMD = 1.546, P < 0.001) significantly improved the patients’ FEV1/FVC ratio (Table 4).
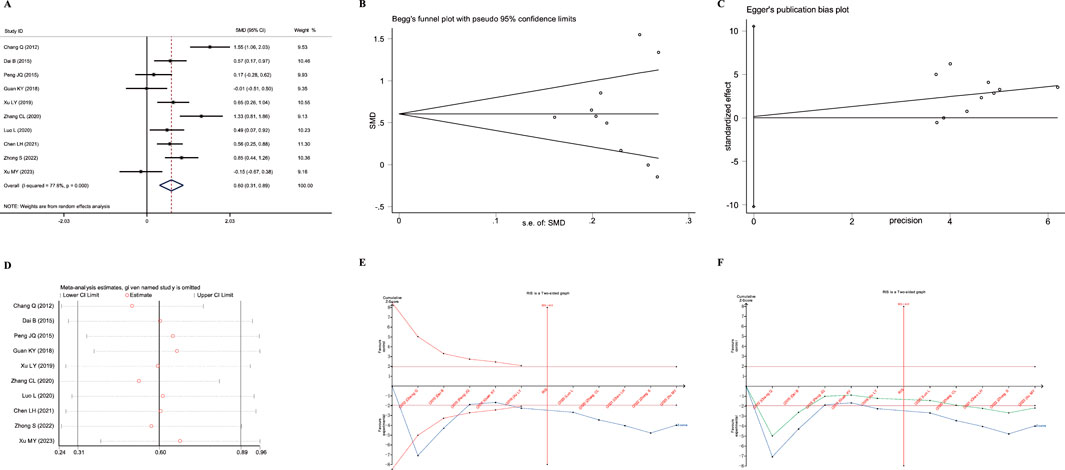
Figure 6. Meta-analysis diagrams of FEV1/FVC Ratio. (A) Forest plot. (B) Begg's funnel plot. (C) Egger's publication bias plot. (D) Sensitivity analysis. (E) TSA analysis. (F) Penalized statistical analysis.
The Egger test and Begg test showed that the sample distribution was essentially symmetrical, with P values greater than 0.05 (PEgger = 0.975, PBegg = 0.592); these results suggested no publication bias, as shown in Figures 5B,C. Sensitivity analysis confirmed the stability of the conclusions (Figure 5D). Moreover, TSA results showed that the cumulative Z-curve crossed both the conventional boundary and the TSA-adjusted boundary after the inclusion of the fifth study, with the sample size reaching the required information size (RIS: 445). Further penalized statistical analysis indicated that the penalized curve surpassed the conventional boundary after the inclusion of the eighth study, confirming the conclusion. Both the meta-analysis and TSA results provided evidence that JLD treatment for COPD can improve the FEV1/FVC ratio in patients.
3.3.5 Effects of JLD on TCM syndrome scores of COPD patients
A total of four studies reported the impact of JLD on the total TCM syndrome scores in COPD patients, involving 266 patients (133 in both the experimental and control groups). Heterogeneity was observed among the studies (I2 = 82.6%, PQ = 0.001), and a random-effects model was adopted for the meta-analysis. The meta-analysis results showed that the experimental group yielded a significantly higher improvement in TCM syndrome scores compared to the control group (SMD = 0.936, 95% CI: 0.301–1.571, PZ = 0.004), as depicted in Figure 7A.
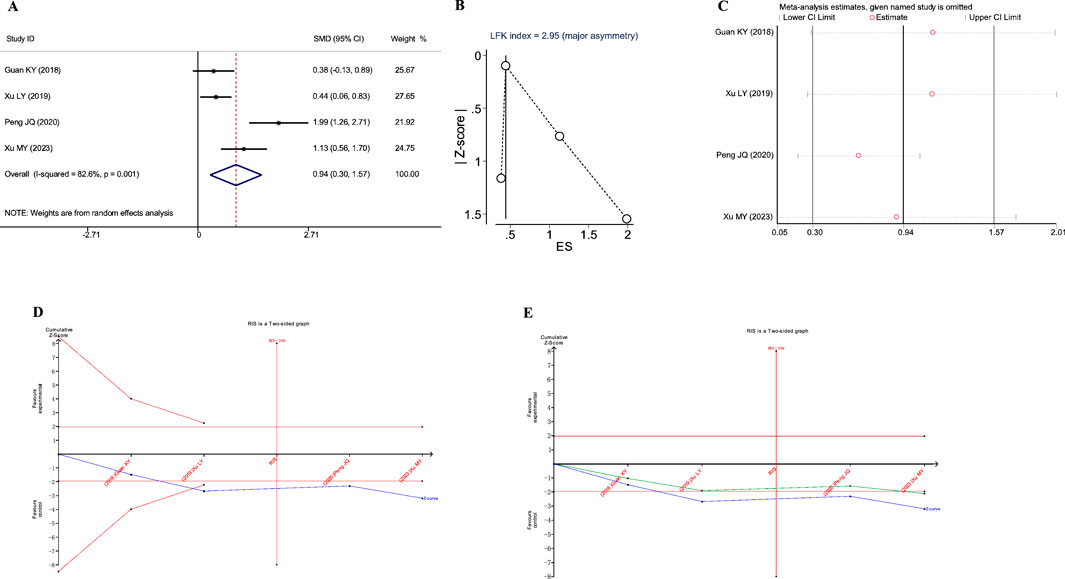
Figure 7. Meta-analysis diagrams of TCM Syndrome Scores. (A) Forest plot. (B) Doi plot. (C) Sensitivity analysis. (D) TSA analysis. (E) Penalized statistical analysis.
The Doi plots exhibited significant asymmetry (LFK index = 2.95, Figure 7B), suggesting the potential presence of publication bias. Sensitivity analysis confirmed the stability of the conclusions (Figure 7C). The TSA results indicated that the cumulative Z-curve crossed the conventional boundary after the inclusion of the second study and had already reached the required information size (RIS: 206, Figure 7D). Further penalized statistical analysis indicated that the penalized curve surpassed the conventional boundary after the inclusion of the fourth study, further confirming the conclusion (Figure 7E). Both the meta-analysis and TSA results substantiated that JLD treatment for COPD can improve TCM syndrome scores in patients.
3.3.6 Effects of JLD on 6MWD of COPD patients
A total of four studies reported the impact of JLD on the 6MWD in COPD patients, including 362 subjects (treatment group 180, control group 182). Significant heterogeneity was observed (I2 = 85.1%, PQ<0.001), prompting the use of a random-effects model for meta-analysis. The results indicated a statistically significant improvement in 6MWD in the treatment group (SMD = 0.744, 95% CI: 0.182–1.306, PZ = 0.009), as illustrated in Figure 8A. This suggests that JLD can effectively enhance the 6MWD in COPD patients.
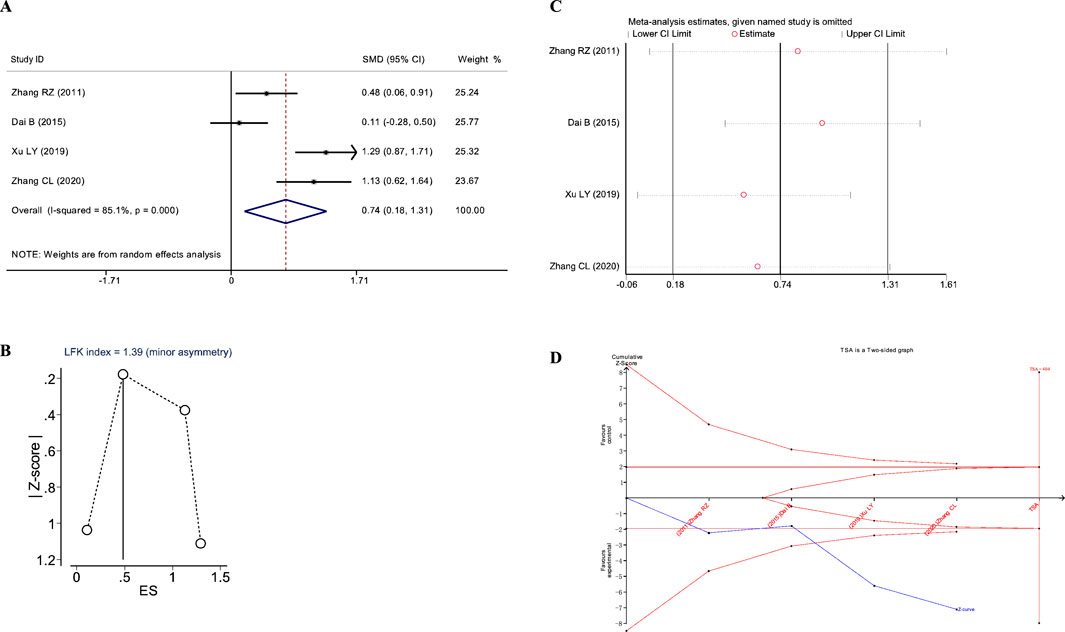
Figure 8. Meta-analysis diagrams of 6MWD. (A) Forest plot. (B) Doi plot. (C) Sensitivity analysis. (D) TSA analysis.
The Doi plots asymmetry test indicated slight asymmetry (LFK index = 1.39, Figure 8B), suggesting potential publication bias. Furthermore, sensitivity analysis confirmed the stability of the conclusions (Figure 8C). TSA indicated that the cumulative Z-curve crossed the conventional boundary after the first study and the TSA-adjusted boundary after the third study; however, the RIS was not reached (RIS: 404, Figure 8D). These findings suggest that further research is needed to confirm the efficacy of JLD treatment on the FVC of COPD patients.
3.3.7 Effects of JLD on IL-6 of COPD patients
Four studies reported the impact of JLD treatment on IL-6 levels in COPD patients, including 289 subjects (treatment group 142, control group 147). Significant heterogeneity was observed among the studies (I2 = 78%, PQ = 0.003), leading to the use of a random-effects model was adopted for the meta-analysis. The meta-analysis results indicated no statistically significant difference in improving IL-6 between the treatment and control groups (SMD = 0.372, 95% CI: −0.143 to 0.887, PZ = 0.156), as shown in Figure 9A.
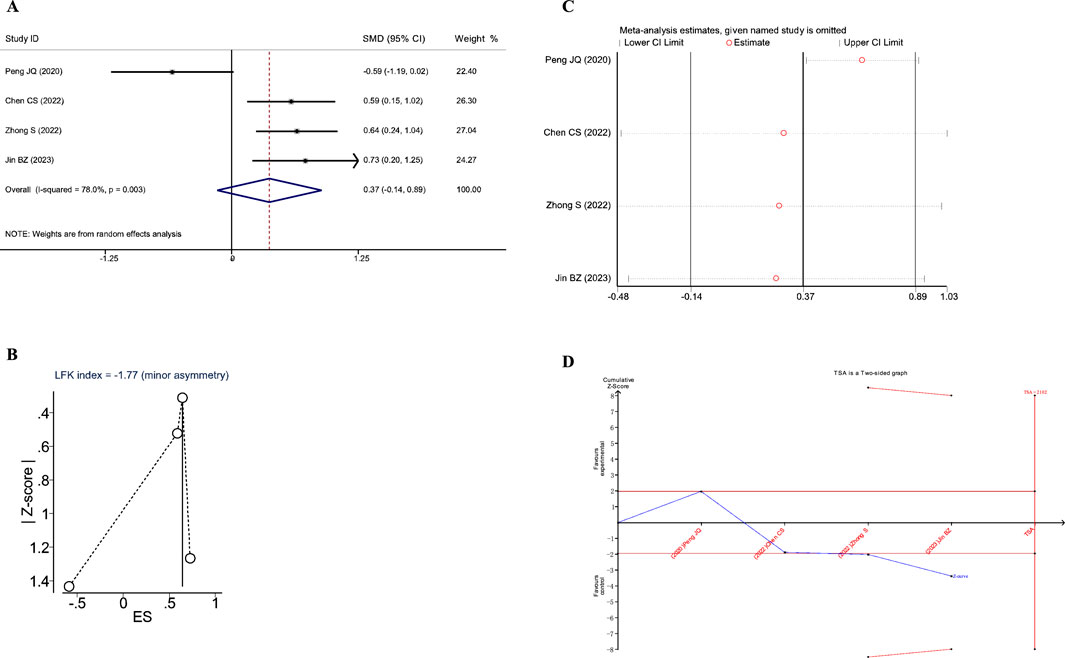
Figure 9. Meta-analysis diagrams of IL-6. (A) Forest plot. (B) Doi plot. (C) Sensitivity analysis. (D) TSA analysis.
The Doi plots showed significant asymmetry (LFK index = −1.77, Figure 9B), suggesting the possibility of publication bias. Sensitivity analysis demonstrated that the conclusions were stable (Figure 9C). Moreover, the TSA results indicated that the cumulative Z-value crossed the conventional boundary after the third study but did not cross the TSA-adjusted boundary and did not reach the RIS (RIS: 2102, Figure 9D). These findings suggest that further research is needed to confirm the impact of JLD on IL-6 in COPD patients.
3.4 Meta-regression
3.4.1 Univariate meta-regression
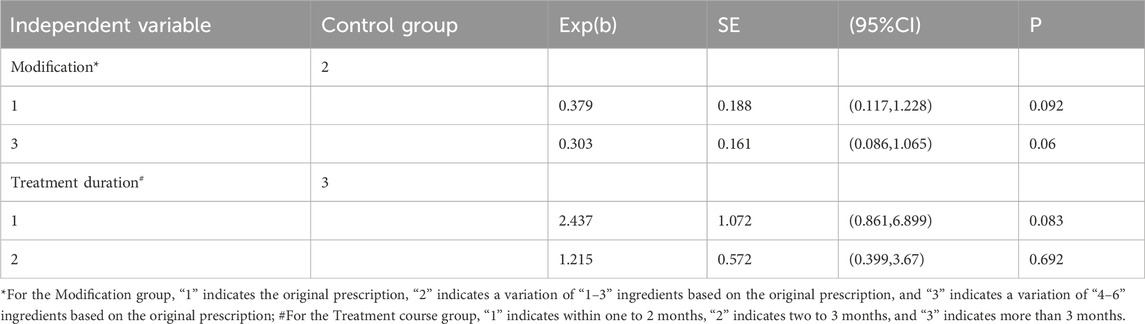
The modification of botanical drugs in TCM prescriptions (in the following table, “modification” is used as a substitute) and the duration of treatment in each study were taken as independent variables, and the FEV1/FVC (expressed as a percentage) as the dependent variable. Univariate meta-regression was employed to further explore the sources of heterogeneity. The results of the univariate meta-regression indicated that the “modification” and “treatment duration” were not significantly influenced the effect of JLD on FEV1/FVC (P < 0.05) (Table 5).
3.5 Multivariate meta-regression
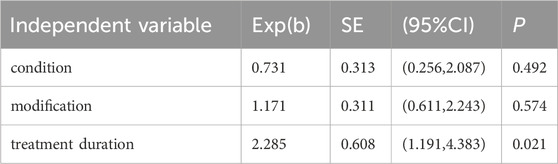
A multivariate meta-regression was conducted with treatment duration, patient condition severity at inclusion (in the following table, “condition” is used as a substitute), the modification of botanical drugs in TCM prescriptions (in the following table, “modification” is used as a substitute) and treatment protocols in the treatment group as independent variables; the FEV1/FVC (expressed as a percentage) was set as the dependent variable. Considering the collinearity between the independent variables “modification” and “treatment plan” in the treatment group, two separate analyses were performed. The results indicated that treatment duration significantly influenced the FEV1/FVC (P < 0.05) (Tables 6, 7).
4 Discussion
To date, multiple studies have demonstrated the therapeutic effects of JLD on COPD; however, high-quality evidence-based medical evidence remains scarce. The only meta-analysis conducted to date included only eight studies, and was limited by a small sample size and the absence of subgroup analysis, which have constrained the generalizability of the conclusions. This study represents the first large-scale meta-analysis of JLD treatment for COPD, aiming to evaluate the therapeutic efficacy of JLD and provide a reliable reference for clinical application.
In terms of overall efficacy, this study yielded surprising results. JLD was found to enhance the overall efficacy of COPD treatment, with no evidence of publication bias. The possibility of a false positive was ruled out by TSA, reaching RIS, thereby further confirming the therapeutic effect of JLD. Subgroup analyses revealed that JLD is particularly effective in treating sCOPD and shows better efficacy when combined with CBM. The conclusions regarding AECOPD and sole JLD treatment were not statistically significant, which may be attributed to a lack of relevant studies.
Irreversible airflow limitation is a key characteristic of COPD (Yanqin et al., 2024), and pulmonary function plays a crucial role in assessing the severity of COPD (Yahong, 2022) and guiding personalized medication for COPD patients (Global Initiative for Chronic Obstructive Lung Disease, 2024). This study conducted a comprehensive analysis of the relevant published literature to evaluate the impact of JLD on pulmonary function indicators in COPD patients, including FEV1, FEV1%pred, FVC, and FEV1/FVC(%). The results showed that JLD significantly improved these indicators. However, subgroup analyses indicated that JLD had a positive effect on FEV1% in both AECOPD and sCOPD patients, and the CBM + JLD combined therapy as well as JLD monotherapy could enhance FEV1%. Still, given the limited number of studies, further research is required to confirm the efficacy in AECOPD patients and the JLD monotherapy approach.
TCM syndrome scores reflect COPD patients’ conditions, including cough, phlegm, dyspnea, and quality of life. The meta-analysis results suggest that JLD significantly improves TCM syndrome scores, and TSA indicates that RIS was achieved. However, significant publication bias was observed, and the Deeks’ funnel plot showed significant asymmetry. Considering that TCM syndrome scores are based on questionnaires, the results are influenced by patients’ and researchers’ subjectivity, which may contribute to the publication bias.
Previous studies reported that COPD can lead to osteoporosis (Fiorentino et al., 2020), muscle dysfunction (Peñailillo et al., 2022) and even atrophy in patients, potentially impairing exercise function. The 6MWD reflects the exercise capacity of COPD patients and is a strong prognostic factor (Perez et al., 2019). Despite the encouraging preliminary results, TSA analysis indicated that additional studies are required to confirm the stability and reproducibility of these findings. Furthermore, the potential presence of publication bias in existing studies may affect a comprehensive assessment of JLD efficacy.
Inflammatory responses are integral to the progression of COPD. Chronic inflammation leads to airway remodeling and exacerbates hypoxia (Barnes, 2016). Prolonged hypoxic conditions, in turn, promote inflammation, further contributing to airway remodeling (Berggren-Nylund et al., 2023), thereby creating a vicious cycle of “inflammation-airway remodeling-hypoxia-inflammation”. Aslani MR et al. found significantly higher serum IL-6 levels in the COPD population compared to healthy individuals (Aslani et al., 2022). Nicolai Obling et al. reported that inflammatory cytokines such as IL-6 were closely related to the progression of COPD (Obling et al., 2022). In addition, the present study revealed that JLD can improve IL-6 levels in COPD patients. However, TSA analysis suggests that more research is needed for confirmation, and the current evidence may be subject to publication bias. Notably, inflammatory cytokines may be the focus of clinical observation in future JLD treatment for COPD.
With the evolution of the medical treatment system, individualized disease management has become increasingly important (Brandsma et al., 2020). The greatest advantage of TCM is its flexible and adaptable prescription formulation, which allows for individualized treatment (Aijun et al., 2024). Clinical use of JLD encompasses various scenarios. First, slight variations in the TCM prescriptions were observed due to different TCM syndrome types. Second, enrolled patients may be in different disease stages, such as the acute exacerbation phase and the stable phase. Third, the duration of JLD treatment varied due as no standardized protocol has been established. Lastly, interventions in the two groups differed across studies. For instance, Chen Cangsong et al. used JLD alone for treatment, while Jin Baozheng et al. adopted an integrated TCM and biomedicine approach. Based on these discrepancies, some indicators in this study exhibited high heterogeneity. Considering that multiple subgroup analyses may increase the probability of Type I errors and the use of Bonferroni correction may lead to insufficient statistical power (Kai, 2023), univariate and multivariate meta-regressions were combined. The “treatment duration,” “the modification of botanical drugs in TCM prescriptions,” “disease condition,” and “treatment plan” were incorporated into the regression equation. The analysis revealed that “treatment duration” was the main cause of heterogeneity (P > 0.05). Additionally, considering the potential collinearity between “the modification of botanical drugs in TCM prescriptions” and “treatment plan”, two multivariate meta-regressions were conducted, and revealing that the conclusions remained unchanged. This suggests that future research design should focus on “treatment duration” to facilitate the production of high-quality clinical evidence.
Although the Egger test is considered more sensitive to small samples compared to the Begg test (Zhiqiang, 2013; Jie et al., 2022), some studies have indicated a low sensitivity is lower, especially with fewer than five studies (only 18.5%) (Furuya-Kanamori et al., 2018). To avoid incorrect estimation of publication bias that could affect the interpretation of the results, the LKF index and the doi plots were used to assess publication bias for outcomes with fewer than ten included studies (Li et al., 2023). The doi plots illustrated the relationship between the effect size and the normality quantile (Z-score), which helps in more intuitively identifying and assessing the asymmetry of study effects. Combined with the LKF index, which quantifies asymmetry by comparing the area differences between the two sides of the Deeks’ funnel plot, publication bias can be evaluated more accurately in small-sample outcomes. In this study, FEV1, FEV1%pred, FVC, TCM syndrome scores, 6MWD, and IL-6 were assessed using this method, and publication bias was found in all indicators except for FEV1%pred.
In terms of safety, three of the included studies specifically reported whether adverse events occurred, including cardiovascular incidents and withdrawals due to intolerance to the strong or distinctive odor of the botanical drug decoction (Rongzhao et al., 2011; Yunlong et al., 2012; Mengyao, 2023). Both were unrelated to JLD. No significant reports of adverse reactions or drug interactions related to JLD were identified in the remaining studies. Based on current clinical experience, the botanical drugs in JLD are mild in nature and demonstrate a favorable safety profile (Baozheng et al., 2023; Mengyao, 2023).
While the majority of conclusions of this study support the positive effects of JLD on the treatment of COPD, several limitations are noted: ① All included literature originated from the Chinese region, which may bias the conclusions towards beneficial effects; ② Most of the literature included in this study only mentioned “randomization” but provided no detail concerning the method of random sequence generation, potentially contributing to high heterogeneity in conclusions; ③ Few included studies reported patients’ GOLD staging. Although this study conducted meta-regression with patient conditions as a factor, the conclusions remained unchanged. However, we still believe that the therapeutic effects of JLD may vary among patients with different GOLD stages.
In summary, this study demonstrates that treatment with JLD can effectively improve the overall response rate and pulmonary function in COPD patients. Further clinical research is needed to confirm these findings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
WH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing. HW: Writing – review and editing, Data curation, Investigation. DW: Conceptualization, Writing – review and editing, Investigation. LZ: Conceptualization, Investigation, Writing – review and editing. JT: Conceptualization, Data curation, Writing – review and editing. MY: Conceptualization, Data curation, Writing – review and editing. ZL: Funding acquisition, Project administration, Supervision, Writing – review and editing. QY: Conceptualization, Supervision, Visualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The National Natural Science Foundation of China (82374399); Key Project of the Scientific Research Program for Higher Education Institutions in Anhui Province (2024AH051047); The Study on the Intervention of Qi Bai Pingfei Capsule on the Pyroptosis of COPD Pulmonary Arterial Adventitial Fibroblasts by Modulating the NLRP3/Casepase-1/GSDMD Pathway (82205074).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fphar.2025.1643998.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1567452/full#supplementary-material
Abbreviations
COPD, Chronic obstructive pulmonary disease; TCM, Traditional Chinese Medicine; CBM, Conventional Biomedicine; JLD, Jinshui Liujun Decoction; AECOPD, Acute exacerbation of chronic obstructive pulmonary disease; sCOPD, Stable chronic obstructive pulmonary disease; TSA, Trial sequential analysis; RIS, Responsiveness index score; FEV1, Forced expiratory volume in one second; FVC, Forced vital capacity; FEV1/FVC, the ratio of FEV1 to FVC; 6MWD, 6-min walk distance; IL-6, Interleukin-6; RR, Relative risk; SMD, Standardized mean difference.
References
Agustí, A., Celli, B. R., Criner, G. J., Halpin, D., Anzueto, A., Barnes, P., et al. (2023). Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur. Respir. J. 61, 2300239. doi:10.1183/13993003.00239-2023
Aijun, C., Huang, S., Liu, X., Tang, Yu, Zhang, F., Kong, Y., et al. (2024). Aanlysis on quality attribute transfer law of liquid-solid preparation of shengxuebao. Chin. J. Exp. Traditional Med. Formulae, 1–7. doi:10.13422/j.cnki.syfjx.20240861
Ali, M. A. M., Matouk, A. I., Hamza, A. A., Amin, A., and Heeba, G. H. (2025). 'Gallic and glycyrrhetinic acids prevent azithromycin-induced liver damage in rats by mitigating oxidative stress and inflammation. Sci. Rep. 15, 9566. doi:10.1038/s41598-025-93120-3
Aslani, M. R., Amani, M., Moghadas, F., and Ghobadi, H. (2022). Adipolin and IL-6 serum levels in chronic obstructive pulmonary disease. Adv. Respir. Med. 90, 391–398. doi:10.3390/arm90050049
Ballester, P., Cerdá, B., Arcusa, R., Marhuenda, J., Yamedjeu, K., and Zafrilla, P. (2022). 'Effect of ginger on inflammatory diseases. Molecules 27, 7223. doi:10.3390/molecules27217223
Baosong, H., Liu, X., Song, Y., Zhang, L., and Zhang, T. (2015). Jinshui Liujun decoction for the treatment of stable phase chronic obstructive pulmonary disease: a clinical study of 79 cases. Med. Inf. doi:10.3969/j.issn.1006-1959.2015.11.344
Baozheng, J., Yan, A., and Dong, Z. (2023). Clinical curative effect observation of six gentlemen metal and water brew on 30 cases of AECOPD with turbid phlegm obstructing lung type. Smart Healthc. 9, 183–186. doi:10.19335/j.cnki.2096-1219.2023.17.043
Barnes, P. J. (2016). Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 138, 16–27. doi:10.1016/j.jaci.2016.05.011
Bei, D., Cai, Z., Dong, Y., and Chao, Ge (2015). Observational study on the therapeutic efficacy of Jin Shui Liu Jun decoction for chronic obstructive pulmonary disease. Guid. J. Traditional Chin. Med. Pharm. 21, 67–68. doi:10.13862/j.cnki.cn43-1446/r.2015.11.030
Berggren-Nylund, R., Ryde, M., Löfdahl, A., Ibáñez-Fonseca, A., Kåredal, M., Westergren-Thorsson, G., et al. (2023). Effects of hypoxia on bronchial and alveolar epithelial cells linked to pathogenesis in chronic lung disorders. Front. Physiol. 14, 1094245. doi:10.3389/fphys.2023.1094245
Bollmeier, S. G., and Hartmann, A. P. (2020). Management of chronic obstructive pulmonary disease: a review focusing on exacerbations. Am. J. Health Syst. Pharm. 77, 259–268. doi:10.1093/ajhp/zxz306
Brandsma, C. A., Van den Berge, M., Hackett, T. L., Brusselle, G., and Timens, W. (2020). Recent advances in chronic obstructive pulmonary disease pathogenesis: from disease mechanisms to precision medicine. J. Pathol. 250, 624–635. doi:10.1002/path.5364
Cangsong, C., He, J., Yiqiang, T., and Zhang, J. (2022). Clinical study on modified Jinshui Liujun decoction for chronic obstructive pulmonary disease at acute stage of Qi deficiency and phlegm obstruction type in SenilePatients. New Chin. Med. 54, 16–19. doi:10.13457/j.cnki.jncm.2022.13.004
Chen, X., Cao, Y. G., Hao, F. X., Liu, Y. L., Chi, F. G., Niu, Y., et al. (2025). Diketopiperazine and purine alkaloids from the tubers of Pinellia pedatisecta Schott and their protective activities against Aβ(25-35)-induced PC-12 cell injury. Phytochemistry 234, 114447. doi:10.1016/j.phytochem.2025.114447
Chen, Y. P., Shi, L. L., Li, Y. Y., Zhang, Y. M., Zhang, S. Z., Hou, H. D., et al. (2025). 'Isoliquiritigenin alleviates radiation-induced intestinal injury in lung cancer by inhibiting TNF-α/caspase3 signaling pathway. Naunyn Schmiedeb. Arch. Pharmacol. doi:10.1007/s00210-025-04007-z
Chuanlin, Z. (2020). Observation of the clinical efficacy of Jinshui Liujun Decoction in treatingCOPD with Yin deficiency and phlegm retention syndrome. China Mod. Dr. 58, 131–134.
Ong, K. L., Aali, A., Ababneh, H. S., Abate, Y. H., and Abbafati, C.Collaborators, GBD 2021 Causes ofDeath (2024). Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 403, 2100–2132. doi:10.1016/s0140-6736(24)00367-2
Fahmy, N. M., Elhady, S. S., Bannan, D. F., Malatani, R. T., and Gad, H. A. (2022). 'Citrus reticulata leaves essential oil as an antiaging agent: a comparative study between different cultivars and correlation with their chemical compositions. Plants (Basel) 11, 3335. doi:10.3390/plants11233335
Fan, Q., Yang, R., Yang, F., Xia, P., and Zhao, L. (2020). Spectrum-effect relationship between HPLC fingerprints and antioxidant activity of Angelica sinensis. Biomed. Chromatogr. 34, e4707. doi:10.1002/bmc.4707
Fayang, L., Xiao, Li, Jun, Z., Yi, Z., Qi, W., Wang, Z., et al. (2023). Liang fanrong's experience in treating tinnitus with the theory of wind. Mod. Traditional Chin. Med. Materia Medica-World Sci. Technol. 25, 3431–3435.
Feng, F., Hu, P., Chen, J., Peng, L., Wang, L., Tao, X., et al. (2025). 'Mechanism research on inhibition of gastric cancer in vitro by the extract of Pinellia ternata based on network pharmacology and cellular metabolomics. Open Med. (Wars) 20, 20241131. doi:10.1515/med-2024-1131
Fenglong, W., Liu, Y., Zhang, L., and Lv, J. (2021). Anti-inflammatory and analgesic activities of angelicae sinensis radix:A review. Chin. J. Exp. Traditional Med. Formulae 27, 197–209. doi:10.13422/j.cnki.syfjx.20211308
Fiorentino, G., Esquinas, A. M., and Annunziata, A. (2020). 'Exercise and chronic obstructive pulmonary disease (COPD). Adv. Exp. Med. Biol. 1228, 355–368. doi:10.1007/978-981-15-1792-1_24
Furuya-Kanamori, L., Barendregt, J. J., and Doi, S. A. R. (2018). A new improved graphical and quantitative method for detecting bias in meta-analysis. Int. J. Evid. Based Healthc. 16, 195–203. doi:10.1097/XEB.0000000000000141
Global Initiative for Chronic Obstructive Lung (2024). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2024 report. Available online at: https://goldcopd.org/2024-gold-report.
Guizhen, G. (2013). Clinical observation of modified Jin Shui Liu Jun decoction for the treatment of chronic obstructive pulmonary disease. Shanxi J. Traditional Chin. Med. 29.
Guo, Z. Y., Wu, X., Zhang, S. J., Yang, J. H., Miao, H., and Zhao, Y. Y. (2025). Poria cocos: traditional uses, triterpenoid components and their renoprotective pharmacology. Acta Pharmacol. Sin. 46, 836–851. doi:10.1038/s41401-024-01404-7
Hong, W., Sheng, Li, Zeng, X., Wu, S., Liu, Q., and Wang, X. (2016). Application of the trial sequential analysis soft ware for meta-analysis. Chin. J. Evidence-Based Med. 16, 604–611. doi:10.7507/1672-2531.20160093
Huang, Y., Yan, P., Zhu, J., Gong, Y., Liu, M., Cheng, H., et al. (2025). 'From genes to healing: the protective mechanisms of Poria cocos polysaccharide in endometrial health. Curr. Issues Mol. Biol. 47, 139. doi:10.3390/cimb47030139
Huang, Z., Gong, J., Lin, W., Feng, Z., Ma, Y., Tu, Y., et al. (2022). Catalpol as a component of Rehmannia glutinosa protects spinal cord injury by inhibiting endoplasmic reticulum stress-mediated neuronal apoptosis. Front. Pharmacol. 13, 860757. doi:10.3389/fphar.2022.860757
Jia, J., Chen, J., Wang, G., Li, M., Zheng, Q., and Li, D. (2023). Progress of research into the pharmacological effect and clinical application of the traditional Chinese medicine Rehmanniae Radix. Biomed. Pharmacother. 168, 115809. doi:10.1016/j.biopha.2023.115809
Jie, M., Huang, X., Xiao, X., and Fu, Z. (2022). Systematic evaluation and meta analysis of the impact of meta cognitive interventions on P.E. J. Wuhan Sports Univ. 56, 78–87. doi:10.15930/j.cnki.wtxb.2022.11.012
Jingqin, P., Shu, Yu, and Zhu, F. (2015). The influence of longkui Liujun decoction on lung function and quality of life in patients with stable chronic obstructive pulmonary disease. Yunnan J. Traditional Chin. Med. Materia Medica 36. doi:10.16254/j.cnki.53-1120/r.2015.05.026
Jingqin, P., Ye, W., and Fan, F. (2020). The impact of Jinshui Liujun decoction on clinical symptoms and inflammatory responses in patients with severe and very severe chronic obstructive pulmonary disease. Chin. J. Gerontology 40, 2759–2762. doi:10.3969/j.issn.1005-9202.2020.13.023
Jiping, Y. (2021). The efficacy of Jinshui Liujun decoction in patients with stable chronic obstructive pulmonary disease. Chin. Sci. Technol. J. Database Full-text Ed. Med. Health Sci.
Job, J. T., Visakh, N. U., Pathrose, B., Alfarhan, A., Rajagopal, R., Thayyullathil, J., et al. (2024). Chemical composition and biological activities of the essential oil from Citrus reticulata blanco peels collected from agrowastes. Chem. Biodivers. 21, e202301223. doi:10.1002/cbdv.202301223
Kai, W. (2023). “Comparison of intravenous anesthesia with propofol and inhalation anesthesia with sevoflurane on the quality of anesthesia for bariatric surgery”, Master. Air Force Medical University, Xi'an, China. doi:10.27002/d.cnki.gsjyu.2023.000195
Kaiyue, G., Yan, L., Ling, C., Zhengqiu, Yu, and Cai, W. (2018). Clinical observation on modified Jinshui Liujun jian combined with shengmai powder for COPD in stable phase. New Chin. Med. 50, 83–86. doi:10.13457/j.cnki.jncm.2018.12.024
Kim, J. H., Yoon, H. J., Choi, Y., Kim, J. S., Ju, I. G., Eo, H., et al. (2025). 6-Shogaol, a neuro-nutraceutical derived from ginger, alleviates motor symptoms and depression-like behaviors and modulates the release of monoamine neurotransmitters in Parkinson's disease mice. Eur. J. Nutr. 64, 116. doi:10.1007/s00394-025-03639-4
Kocaadam, B., and Şanlier, N. (2017). Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 57, 2889–2895. doi:10.1080/10408398.2015.1077195
Kwon, Y. J., Son, D. H., Chung, T. H., and Lee, Y. J. (2020). A review of the pharmacological efficacy and safety of licorice root from corroborative clinical trial findings. J. Med. Food 23, 12–20. doi:10.1089/jmf.2019.4459
Li, A., Yuan, S. Y., Li, Q. G., Li, J. X., Yin, X. Y., and Liu, N. N. (2023). Prevalence and risk factors of malnutrition in patients with pulmonary tuberculosis: a systematic review and meta-analysis. Front. Med. (Lausanne) 10, 1173619. doi:10.3389/fmed.2023.1173619
Li, L., and Liu, X. (2020). The influence of longkui Liujun decoction on lung function and quality of life in patients with stable chronic obstructive pulmonary disease. Heilongjiang J. Traditional Chin. Med. 49, 158–159.
Li, M., Peng, S., Bu, J., Quan, S., Liu, L., Yue, Z., et al. (2025). Glycyrrhizic acid alleviates gefitinib-induced liver injury by regulating the p53/p21 pathway and releasing cell cycle arrest. Food Chem. Toxicol. 200, 115405. doi:10.1016/j.fct.2025.115405
Li, X., He, Y., Zeng, P., Liu, Y., Zhang, M., Hao, C., et al. (2019). Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J. Cell Mol. Med. 23, 4–20. doi:10.1111/jcmm.13564
Lihua, Z., Zhanfu, Yu, Song, Y., and Zhang, T. (2013). 'The integration of traditional Chinese medicine (TCM) and western medicine in the clinical observation of treating chronic obstructive pulmonary disease (COPD). Shanxi J. Traditional Chin. Med. 29, 17–18.
Lin, S., Nie, B., Yao, G., Yang, H., Ye, R., and Yuan, Z. (2019). 'Pinellia ternata (Thunb.) Makino Preparation promotes sleep by increasing REM sleep. Nat. Prod. Res. 33, 3326–3329. doi:10.1080/14786419.2018.1474466
Linhui, C., Haipeng, W., Xiang, Yu, Chen, H., and Zhang, X. (2021). Effects of Jinshui liuiun decoction in the treatment of chronic obstructive disease in stable stage (2025). World Chin. Med. 16, 1450–1453+58.
Liu, W., Yin, D. X., Zhang, T., Qiao, Q., Yang, Y. Q., and Wang, W. L. (2020). Phytochemical profiles and antioxidant activity of Rehmannia glutinosa from different production locations. Chem. Biodivers. 17, e2000341. doi:10.1002/cbdv.202000341
Liyan, Xu, Chen, W., and Yang, B. (2019). “Clinical observation on Jinshui Liujun decoction on chronic obstructive pulmonary disease with syndrome of fei(lung)and shen(Kidney)Qi deficiency”, DOCTOR, 4: 78–80.
Lu, J., and Wang, C. (2025). 'Ferulic acid from Angelica sinensis (Oliv.) Diels ameliorates lipid metabolism in alcoholic liver disease via AMPK/ACC and PI3K/AKT pathways. J. Ethnopharmacol. 338, 119118. doi:10.1016/j.jep.2024.119118
Luo, C., Qin, S., Liang, P., Ling, W., and Zhao, C. (2020). Advances in modern research on pharmacological effects of Radix Rehmanniae. AIP Conf. Proc. 2252, 020034. doi:10.1063/5.0020348
Lyu, Y., Chen, X., Xia, Q., Zhang, S., and Yao, C. (2020). Network pharmacology-based study on the mechanism of Pinellia ternata in asthma treatment. Evid. Based Complement. Altern. Med. 2020, 9732626. doi:10.1155/2020/9732626
Medicine, World Federation of Chinese Medicine Societies Specialty Committee of Internal (2023). Guideline of integrated Chinese and western medicine for diagnosis and treatment of chronic obstructive pulmonary disease. Chin. J. Evidence-Based Med. 23, 1117–1128.
Mengyao, Xu (2023). “Researching the clinical efficacy of modified JinshuiLiujun decoction,” in The treatment on stable COPD ofLung-kidney deficiency pattern based on the theory of Mutual generation about metal and water. Master. Jinan, China: Shandong University of Traditional Chinese Medicine. doi:10.27282/d.cnki.gsdzu.2023.000772
Michele, T. M., Pinheiro, S., and Iyasu, S. (2010). The safety of tiotropium--the FDA's conclusions. N. Engl. J. Med. 363, 1097–1099. doi:10.1056/NEJMp1008502
Nie, A., Chao, Y., Zhang, X., Jia, W., Zhou, Z., and Zhu, C. (2020). 'Phytochemistry and pharmacological activities of wolfiporia cocos (F.A. Wolf) ryvarden and gilb. Front. Pharmacol. 11, 505249. doi:10.3389/fphar.2020.505249
Obling, N., Backer, V., Hurst, J. R., and Bodtger, U. (2022). 'Nasal and systemic inflammation in chronic obstructive pulmonary disease (COPD). Respir. Med. 195, 106774. doi:10.1016/j.rmed.2022.106774
Oh, Y. C., Jeong, Y. H., Li, W., and Go, Y. (2019). Angelicae gigantis radix regulates LPS-induced neuroinflammation in BV2 microglia by inhibiting NF-κB and MAPK activity and inducing nrf-2 activity. Molecules 24, 3755. doi:10.3390/molecules24203755
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372, n71. doi:10.1136/bmj.n71
Peñailillo, L., Valladares-Ide, D., Jannas-Velas, S., Flores-Opazo, M., Jalón, M., Mendoza, L., et al. (2022). Effects of eccentric, concentric and eccentric/concentric training on muscle function and mass, functional performance, cardiometabolic health, quality of life and molecular adaptations of skeletal muscle in COPD patients: a multicentre randomised trial. BMC Pulm. Med. 22, 278. doi:10.1186/s12890-022-02061-4
Perez, T., Deslée, G., Burgel, P. R., Caillaud, D., Le Rouzic, O., Zysman, M., et al. (2019). Predictors in routine practice of 6-min walking distance and oxygen desaturation in patients with COPD: impact of comorbidities. Int. J. Chron. Obstruct Pulmon Dis. 14, 1399–1410. doi:10.2147/COPD.S188412
Qi, C. (2012). “Effect of “Jinshui Liujun Decoction” on respiratory function and immunologic function in patients with chronic obstructive pulmonary disease at stationary phase”, Shanghai J. Traditional Chin. Med., 46. doi:10.16305/j.1007-1334.2012.07.015
Qi, R., Zhang, H., Li, D., Gao, F., Miao, Q., Chen, S., et al. (2022). The efficacy and safety of xinjia xuanbai chengqi granules in acute exacerbation of COPD: a multicentre, randomised, double-blind, controlled trial. Evid. Based Complement. Altern. Med. 2022, 7366320. doi:10.1155/2022/7366320
Rasheed, N. (2020). Ginger and its active constituents as therapeutic agents: recent perspectives with molecular evidences. Int. J. Health Sci. (Qassim) 14, 1–3.
Ren, C., Luo, Y., Li, X., Ma, L., Wang, C., Zhi, X., et al. (2024). 'Pharmacological action of Angelica sinensis polysaccharides: a review. Front. Pharmacol. 15, 1510976. doi:10.3389/fphar.2024.1510976
Rongzhao, Z., Zheng, S., and Yan, G. (2011). Observational study on the treatment of moderate chronic obstructive pulmonary disease with yu ping feng san and Jin Shui Liu Jun decoction. China Pract. Med. 6. doi:10.14163/j.cnki.11-5547/r.2011.20.027
Shan, Z. (2022). Effects of Jinshui Liujun decoction(金水六君煎) combined with modified bufei decoction (补肺汤) on blood oxygen Indexes,Symptoms and pulmonary respiratory function in patients with stable COPD. Chin. Archives Traditional Chin. Med. 40. doi:10.13193/j.issn.1673-7717.2022.09.048
Tiantao, Z., Song, Y., Hou, B., Liu, X., and Zhang, L. (2014). Clinical observation of 60 cases of chronic obstructive pulmonary disease treated with integrated traditional Chinese and western medicine. Medicine&.people 27.
Ullah, A., Sun, Q., Li, J., Li, J., Khatun, P., Kou, G., et al. (2024). Bioactive compounds in Citrus reticulata peel are potential candidates for alleviating physical fatigue through a triad approach of network pharmacology, molecular docking, and molecular dynamics modeling. Nutrients 16, 1934. doi:10.3390/nu16121934
Vanfleteren, L., Fabbri, L. M., Papi, A., Petruzzelli, S., and Celli, B. (2018). 'Triple therapy (ICS/LABA/LAMA) in COPD: time for a reappraisal. Int. J. Chron. Obstruct Pulmon Dis. 13, 3971–3981. doi:10.2147/COPD.S185975
Walters, J. A., Tan, D. J., White, C. J., and Wood-Baker, R. (2018). 'Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 3, Cd006897. doi:10.1002/14651858.CD006897.pub3
Wang, Y., Dong, L., Han, S., You, Y., Zhang, M., Sun, B., et al. (2025). 'Zingerone alleviates inflammatory pain by reducing the intrinsic excitability of anterior cingulate cortex neurons in a mice model. Front. Pharmacol. 16, 1543594. doi:10.3389/fphar.2025.1543594
Xiaoqi, Li (2010). Clinical observation of modified Jin Shui Liu Jun decoction in the treatment of chronic obstructive pulmonary emphysema in 32 cases. Hebei J. Traditional Chin. Med. 32.
Yahong, C. (2022). Interpretation of global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2021 report. Chin. General Pract. 25, 1294–1304+308. doi:10.12114/j.issn.1007-9572.2022.01.302
Yang, F. Q., Tan, X. M., Chu, S. S., Yin, M. Z., Zhang, Z. Y., and Peng, H. S. (2025). 'UPLC-Q-TOF-MS with chemometrics approach analysis of nonvolatile compounds for medicinal Citrus reticulata with cultivar and areas variations. Phytochem. Anal. 36, 467–484. doi:10.1002/pca.3496
Yanqin, Q., Dong, H., Yang, J., Haibo, Li, Peng, Z., and Jiansheng, Li (2024). The evaluation of effective-component compatibility of bufei yishen formula Ⅲ and components compatibility in treating airway remodeling of COPD. Chin. General Pract., 1–8. doi:10.12114/j.issn.1007-9572.2023.0868
Yunlong, Lv, Hongmei, Z., and Jincun, W. (2012). The clinical effect of modified Jin Shui Liu Jun jian in the treatment ofChronic obstructive pulmonary disease(32 cases). China Health Ind. 9, 5–6. doi:10.16659/j.cnki.1672-5654.2012.36.020
Yuqin, S., Wenyuan, Z., Zhang, L., Hou, B., Zhang, T., Fan, J., et al. (2013). Clinical observation of Jin Shui Liu Jun decoction in the treatment of 100 cases with stable chronic obstructive pulmonary disease. Hebei J. Traditional Chin. Med. 35, 1650–1651.
Keywords: Jinshui Liujun decoction, traditional Chinese medicine, chronic obstructive pulmonary disease, trial sequential analysis, meta-analysis
Citation: Huang W, Wang H, Wu D, Zhang L, Tong J, Yu M, Li Z and Yang Q (2025) Efficacy of Jinshui Liujun decoction on chronic obstructive pulmonary disease patients: a systematic review and meta-analysis. Front. Pharmacol. 16:1567452. doi: 10.3389/fphar.2025.1567452
Received: 27 January 2025; Accepted: 06 May 2025;
Published: 21 May 2025; Corrected: 22 August 2025.
Edited by:
Dawei Yang, Fudan University, ChinaReviewed by:
Yuxiang Fei, China Pharmaceutical University, ChinaPan Liu, Anhui Provincial Hospital of Integrated Traditional and Western Medicine, China
Copyright © 2025 Huang, Wang, Wu, Zhang, Tong, Yu, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zegeng Li, YWh6eWZiQHNpbmEuY29t; Qinjun Yang, MTMyMjU4NTA4ODZAMTYzLmNvbQ==
 Wanqiu Huang
Wanqiu Huang Hui Wang
Hui Wang Di Wu
Di Wu Lu Zhang
Lu Zhang Jiabing Tong
Jiabing Tong Minghui Yu
Minghui Yu Zegeng Li
Zegeng Li Qinjun Yang
Qinjun Yang