Abstract
Medicinal plants have garnered significant attention for their potential in treating various human diseases. Many of these plants exhibit antioxidant and anti-inflammatory properties, which are crucial for mitigating the impact of oxidative stress and inflammation implicated in numerous clinical conditions. This review investigated the antioxidant and anti-inflammatory properties of medicinal plants, particularly Bidens pilosa, and their potential applications in disease management. A structured systematic approach was employed for this analysis. Scopus, PubMed, and Web of Science were searched using the following search algorithm: (“Bidens pilosa”) AND (“antioxidant”) AND (“anti-inflammatory” OR “anti-inflammatory”) on the second of April 2025 without any filters. At the end of the title, abstract and full text screening, only 50 articles met the inclusion criteria and hence included in the study. Most (35/50, 70%) were published within the years 2014–2024. Of the 50 studies, 23 (46%) were done in Africa, 14 (28%) in Asia, and 11 (22%) in South America. Most studies were done in a laboratory dish (29/50, 58%), with a smaller number done in animals (12/50, 24%). Fourteen percent (14%) of the studies used both in vivo and in vitro methods, and 4% were carried out on people. Out of the 50 studies, leaves were looked at most often (23 or 46%), followed by the whole plant (19 or 38%). Bidens pilosa lowered the tissue levels of tumor necrosis factor (TNF) and interleukin (IL)-6, IL-1β, and IL-8. It also improved the tissue levels of antioxidants glutathione while reducing lipid peroxidation via malondialdehyde (MDA). In conclusion, all the studies examined in the present study reported that Bidens pilosa possess antioxidant and anti-inflammatory potential, hence holding great promise in the management of oxidative stress and inflammation-related conditions.
1 Introduction
Oxidative stress is implicated in the pathogenesis of various diseases, including Alzheimer’s disease, chronic obstructive pulmonary disease, cancer and atherosclerosis (Forman and Zhang, 2021). It is usually a consequence of an imbalance in the pro-oxidant and antioxidant ratio (Panfoli et al., 2018). Pro-oxidants are pro-biotics or xenobiotics that result in oxidative stress and its related damage; and could include free radicals or reactive oxygen species. Free radicals are associated with radical chain reaction, hence are referred to as oxidants (Tchekalarova and Tzoneva, 2023). Free radicals can be generated exogenously or endogenously; exogenous free radical generation can be through exposure to heavy metal, radiation exposure, alcohol consumption, and cigarette smoking (Ozsurekci and Aykac, 2016). On the other hand, the exogenous free radical generation can be triggered by stress, cancer, excessive exercise, immune cell activation, aging, ischemia, infection, and inflammation (Ozsurekci and Aykac, 2016).
Based on the fact that oxidative stress has been implicated negatively in various diseases, both natural and synthetic antioxidants have been increasingly recognized as promising therapeutic options (Daglia et al., 2014; Selamoglu-Talaz et al., 2013; Selamoglu et al., 2008; Sureda et al., 2023). Gallic acid and curcumin are examples of polyphenols that lessen the risk of multi-faceted mechanistic clinical conditions by reducing neuroinflammation and oxidative stress, consequently protecting against neurotoxicity (Daglia et al., 2014; Sureda et al., 2023). Phytomedicine has highlighted the antioxidant properties of plants such as propolis and synthetic organoselenium compounds (Selamoglu-Talaz et al., 2013; Selamoglu et al., 2008). Furthermore, through antioxidant and cholinesterase-inhibiting qualities, Octaviania asterosperma, Scrophularia amplexicaulis, and Neurada procumbens promote nerve regeneration and cognition (Hamedi et al., 2020; Rasul et al., 2019; Sevindik et al., 2021).
Inflammation is a complex biological response crucial for tissue repair and defense against pathogens. It involves distinct phases: an initial pro-inflammatory response mediated by the innate immune system, followed by a resolution phase characterized by a macrophage phenotypic switch and a return to tissue homeostasis through inflammatory cell apoptosis (Kulkarni et al., 2016). However, when dysregulated, inflammation can contribute to the pathogenesis of various pathologies (Kulkarni et al., 2016; Rauf et al., 2022; Teleanu et al., 2022). Natural and synthetic anti-inflammatory agents have been effective in preventing neuroinflammation, which is heightened in these pathologic conditions (Hamsalakshmi et al., 2022; Kalra et al., 2022; Kaur and Singh, 2022; Yousaf et al., 2022).
Most of these effective antioxidant and anti-inflammatory agents are derived from easily accessible and cultivable plants. This makes medicinal plants to gain significant global attention as therapeutic alternatives (Ayuba et al., 2022; Onohuean et al., 2021; Onohuean et al., 2024; Salihu et al., 2022; Usman et al., 2016; Usman et al., 2022; Yusuf et al., 2023).
Bidens pilosa, a plant native to South America but widely distributed worldwide, including Africa, China, Japan and the Americas, has emerged as a promising candidate due to its rich phytochemical profile (Bartolome et al., 2013). Bidens pilosa is rich in phytocompounds such as saponins, alkaloids, polyacetylenes, flavonoids, and diterpenes (Abdel-ghany et al., 2016). Studies have reported that Bidens pilosa possess anti-hyperglycemic, antiulcerogenic, antihypertensive, hepatoprotective, antipyretic, anti-malarial, anti-leukemic, anti-bacterial, anticancer, antioxidant, immunosuppressive and anti-inflammatory (Silva et al., 2011). In some parts of Uganda, the plant is used for wound healing; however, there is limited information or studies on specific phytochemicals to enable the development of useful products for the treatment and management of neurodegenerative diseases. Hence, the present review examined the antioxidant and anti-inflammatory potential of Biden Pilosa, highlighting this plant’s promising therapeutic potential for variable yet related disease conditions heightened by oxidative stress and inflammation.
2 Methodology
2.1 Search strategy
The antioxidant and anti-inflammatory potential of Bidens pilosa, was examined using a structured and systematic approach. Scopus, PubMed, and Web of Science were searched using the following search algorithm: (“Bidens pilosa”) AND (“antioxidant”) AND (“anti-inflammatory” OR “anti-inflammatory”) on the second of March 2024 without any filters.
2.2 Study selection criteria
The database (Scopus, PubMed, and Web of Science) search yielded 65 bibliographies and extracted as comma-separated value (CSV) files, saved, and merged. Duplicates were sorted and removed, the remaining documents were retrieved for title and abstract screening, followed by full-text screening and data extraction. Only peer-reviewed articles on the antioxidant and anti-inflammatory properties of Bidens pilosa published in English were included in the present review. At the end of the screening exercise, only 12 articles met the selection criteria, hence used for data extraction (Figure).
2.3 Quality assessment
The risk of bias assessment was done using the Systematic Review Center for Laboratory Animal Experimentation (SYRCLE) Checklist for the invivo studies (Hooijmans et al., 2014); on the other hand, Science in Risk Assessment and Policy (SCIRAP tool) as modified and used by Almeida et al. (2021) was adapted to assess the reporting quality for invitro studies since we could not find another tool (Roth et al., 2021). The findings from the risk of bias assessment were presented using the ROBVIS tool.
2.4 Data extraction
The data was extracted and classified as the following information: Author, Country, Study Design, Animals Number, participants or animal gender and strain, invivo/invitro experiments, disease model or cells used, part of the plant, extract/molecule, phytochemical analysis and key compounds identified, intervention on antioxidant and anti-inflammmatory potentials of B. Pilosa and study outcomes (Tables 1–3) and (Supplementary Tables S1–S3).
TABLE 1
| S/N | Study design | Plant part used | Animal or cells/Evaluation system | Gender/Age (wks) | Disease model | Country | References |
|---|---|---|---|---|---|---|---|
| 1 | In vivo | Not mentioned | Swiss mice | Male; 8–10 | Intestinal mucositis | Brazil | Bastos et al. (2016) |
| 2 | In vivo | Leaves | Mus musculus mice and Wistar rats | Both sexes (mice 11–12 and rats 7–8) | Analgesic models (acetic acid-induced writhing, hot-plate, capsaicin-induced neurogenic pain, and formalin-induced), and anti-inflammatory models (carrageenan, dextran, histamine, and serotonin-induced paw edema) | Cameroon | Fotso et al. (2014) |
| 3 | In vivo | Whole plant | Wistar rats | Male Age not mentioned | Carrageenan-triggered paw swelling and arthritis induced by Complete Freund’s adjuvant | Taiwan | Chih et al. (1995) |
| 4 | In vitro | Whole plant | Mouse Mononuclear leukemia cells (RAW 264.7) | NA | Leukemia | China | Yan et al. (2022) |
| 5 | In vitro | Aerial part | Lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages and human leukemia monocytic cells (THP-1) | NA | Leukemia | Korea | Xin et al. (2021) |
| 6 | In vivo and In vitro | Leaves | Peripheral blood mononuclear cells (PMBC) and Murine Lymphocytes (in vitro); B10.ArSg SnJ mice (in vivo) | Not mentioned | Human lymphocyte proliferation; Murine lymphocyte proliferation; and Zymosan-induced arthritis in Mice | Brazil | Pereira et al. (1999) |
| 7 | In vivo and Invitro | Whole plant | Transgenic G93A Mice (with SOD 1 mutation)-in vivo and LPS-BV2 microglial cells | Not mentioned | Amyotrophic Lateral Sclerosis Mouse and Lipopolysaccharide (LPS)-Stimulated BV-2 Microglia | Japan | Tsuruta et al. (2023) |
| 8 | In vivo | Whole plant | Wistar/ST rats, ddY mice and BALB/c mice | Male, age not specified | Allergic response (IgE production), passive cutaneous anaphylaxis (PCA), inflammatory skin reactions, and histamine release from mast cells | Japan | Horiuchi and Seyama (2008) |
| 9 | In vivo | Aerial part | Wistar rats and Swiss mice | Male, age not specified | 2,4,6-Trinitrobenzenesulfonic acid (TNBS)-induced colitis | Brazil | Quaglio et al. (2020) |
| 10 | In vitro | Whole plant | Oral squamous carcinoma cell line (SCC-4) | NA | Oral carcinoma | Brazil | Arantes et al. (2021a) |
| 11 | In vivo | Whole plant | BALB/c mice | Female; 6–8 | Ovalbumin-induced Th2-mediated airway inflammation | Taiwan | Chang et al. (2005) |
| 12 | In vivo and In vitro | Whole plant | Rat cardiac microvascular endothelial cells (CMECs) from hearts of Sprague Dawley rats | Gender not specified for In vitro (1–3 days) | Rat model of Chronic Heart Failure; and Cultured Microvascular Endothelial Cells (CMEC) inflammation models | China | Y. Yang et al. (2018) |
| 13 | In vivo | Whole plant excluding the roots | Wistar rats | Adult males | Carbon tetrachloride (CCl4)-induced cardiac and hepato-toxicity | Brazil | Pegoraro et al. (2018) |
| 14 | In vivo | Not mentioned | Swiss mice | Male; 7–8 | 5-Fluorouracil (5-FU)-induced intestinal mucositis in mice | Brazil | de Ávila et al. (2015) |
| 15 | In vivo | Whole plant to formulate FITOPROT | Human | Male and Female, 18–65 years of age | Oral mucositis but the study included healthy participants with adequate bone marrow, liver, renal, glycemic parameters, and heart function, alongside health assessments such as oral intake and gingival indices, with participants abstaining from specific vegetal extracts during the study | Brazil | Santos Filho et al. (2018) |
| 16 | In vivo and In vitro | Whole plant | Wistar albino rats (in vivo), DPPH and ABTS radical scavenging assay (in vitro) | Male (in vivo) | Oxalate induced liver and kidney inflammation and toxicity | Egypt | Mohamed et al. (2024) |
| 17 | In vitro | Leaves | DPPH scavenging assay | NA | NA | South Africa | Akula and Odhav (2008) |
| 18 | In vitro | Whole plant | FRAP and DPPH assays | NA | NA | South Africa | Tesfay et al. (2016) |
| 19 | In vitro | Leaves | DPPH scavenging assay | NA | NA | Indonesia | Yuniastri et al. (2022) |
| 20 | In vivo | Whole plant | Wistar rats | Female; Age not specified | 2,4,6 trinitrobenzene sulfonic acid (TNBS) induced colitis | Nigeria | Abiodun et al. (2020) |
| 21 | In vitro | Leaves | DPPH, FRAB and ABTS assays | NA | NA | South Africa | Nxumalo et al. (2023) |
| 22 | In vitro | Leaves | Bacteria: S. typhimurium, S. boydii, V. parahaemolyticus, and E. coli/DPPH assay | NA | Common diarrhea | South Africa | Shandukani et al. (2018) |
| 23 | In vitro | Leaves | DPPH, reducing power, and β-carotene-linoleate assays | NA | NA | Cameroon | Goudoum et al. (2016) |
| 24 | In vivo and In vitro | Whole plant | In vivo: BALB/c and C57BL/6 mice. In vitro: Hela cells or peritoneal cells from C57BL/6 mice. PMI-1640 medium supplemented with 25 mM HEPES, 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin | Male; 6–10 | Toxoplasma gondii infected mice | Brazil | Mota et al. (2019) |
| 25 | In vitro | Leaves | DPPH, FRAP and hydrogen peroxide scavenging assays | NA | NA | Zambia | Phiri et al. (2024) |
| 26 | In vitro | Flower and leaves | ABTS and DPPH assays | NA | NA | Vietnam | Nguyen et al. (2023) |
| 27 | In vitro | Leaves | HepG2 cell line, derived from human hepatocellular carcinoma | NA | NA | Egypt | Said et al. (2024) |
| 28 | In vitro | Leaves, root, stem and whole plant | DPPH, ABTS and FRAP | NA | NA | Italy | Angelini et al. (2021) |
| 29 | In vitro | Leaves | DPPH, ABTS and Nitric oxide radical scavenging systems. Low Density Lipoprotein Oxidation system | NA | NA | Thailand | U-Yatung et al. (2020) |
| 30 | In vitro | Leaves | DPPH and FRAP and total antioxidant activity evaluating systems | NA | NA | Nigeria | Oduntan et al. (2018) |
| 31 | In vitro | Leaves | DPPH, ABTS and TBARS systems | NA | NA | South Africa | Falowo et al. (2017) |
| 32 | In vitro | Leaves | Human epidermoid carcinoma (KB-3-1) cells; DPPH and ABTS systems | NA | Cancer | India | Singh et al. (2017) |
| 33 | In vitro | Aerial part | HepG2 (cancerous), and Vero (non-can-cerous) cell lines; ABTS and FRAP systems | NA | Hepatocellular carcinoma | South Africa | Idris et al. (2023) |
| 34 | In vivo | Whole plant | Chickens | 1 day | Eimeria infection | China | Memon et al. (2020) |
| 35 | In vivo and In vitro | Whole plant | Fruit fly (Drosphilia melanogaster); DPPH, ABTS, FRAP and Phosphomolybdenum total antioxidant activity systems | Males, 1–2 days | Paraquat induced oxidative stress | Vietnam | Son et al. (2022) |
| 36 | In vitro | Whole plant | mycobacterium tuberculosis; DPPH and ABTS | NA | Tuberculosis | South Africa | Mashinini et al. (2023) |
| 37 | Invivo | Whole plant to formulate FITOPROT | Human | Both genders; dentulous and edentulous | Oral mucositis caused by radio-chemotherapy was observed in patients with head and neck cancer (HNC) who had been referred for adjuvant or neoadjuvant RT/RCT treatment | Brazil | Arantes et al. (2021b) |
| 38 | In vitro | Leaves and flowers | DPPH and β-carotene bleaching methods | NA | Bacterial and fungal models | Japan | Deba et al. (2007) |
| 39 | In vitro | Whole plant | RAW 264.7 cells, Murine macrophage cell line; DPPH, Superoxide scavenging and Nitric oxide inhibition systems | NA | Inflammatory disease | Taiwan | Chiang et al. (2004) |
| 40 | In vitro | Whole plant | DPPH and ABTS | NA | Colonic cancer | China | Yi et al. (2016) |
| 41 | In vitro | Not mentioned | Radical scavenging systems on DPPH, Hydroxyl group, superoxide and ferric reducing systems | NA | Oxidative stress and cancer | Taiwan | Liu et al. (2013) |
| 42 | In vitro | Whole plant | DPPH, Reducing power, β-carotene-linoleate system and phospholipid peroxidation assays | NA | Oxidative stress and Lipid peroxidation model | Zimbabwe | Muchuweti et al. (2007) |
| 43 | In vivo/In vitro | Aerial part | Balb-c mice; In vivo: MDA using TBARS, Glutathione (GSH) and Catalase (CAT); In vitro: DPPH, Hydroxyl radicals, and FRAB | Male | Carbon tetrachloride (CCl4) induced lipid peroxidation and oxidative stress | Brazil | Kviecinski et al. (2011) |
| 44 | In vivo | Whole plant | ICR mice; TBARS system | Male | Lipid peroxidation and acute gastric mucosal lesion | Japan | Horiuchi et al. (2010) |
| 45 | In vitro | Leaves and shoots | DPPH radical scavenging, reducing power and β-carotene systems | NA | Oxidative stress | Zimbabwe | Chipurura et al. (2013) |
| 46 | In vitro | Whole plant | DPPH, BHT, FRAP and ABTS scavenging assays | NA | Oxidative stress | South Africa | Adeolu Adedapo and AuthorAnonymous (2011) |
| 47 | In vivo | Leaves | Mice and Sprague–Dawley rats; MDA, SOD and GSH-Peroxidase assays | Male | CCl4-induced liver damage and fibrosis | China | Yuan et al. (2008) |
| 48 | In vitro | Aerial part | DPPH, and ABTS radical scavenging assays | NA | Oxidative stress | Brazil | Cortés-Rojas et al. (2011) |
| 49 | In vitro | Whole plant | Antioxidant evaluation by DPPH, ABTS and FRAP assays; Cytotoxicity by MTT method | NA | Human tumor cells, namely MCF-7, HepG2, MGC 803 and RKO; Colorectal cancer | China | Wu et al. (2012) |
| 50 | In vitro | Whole plant | Human erythrocytes; MDA LPO evaluation using TBARS, Antioxidant evaluation using GSH and SOD | NA | Oxidative hemolysis and lipid/protein peroxidation of erythrocytes induced by the aqueous peroxyl radical [2,20-azobis(2-amidinopropane) dihydrochloride (AAPH)] | Taiwan | H. L. Yang et al. (2006) |
Study characteristics and summarized data from articles reporting antioxidant and anti-inflammatory potentials of Bidens pilosa.
NA- not applicable.
TABLE 2
| S/N | Plant part | Phytocompound/extract or fraction | Dosage | Route of administration | Duration of administration | References |
|---|---|---|---|---|---|---|
| 1 | Not mentioned | Mucoadhesive or non-mucoadhesive formulations | 75 mg/kg BP; 100 mg/kg BP; 125 mg/kg BP | Orally (gavage) | 6 days | Bastos et al. (2016) |
| 2 | Leaves | Ethyl acetate fraction | 50, 100 and 200 mg/kg | Topically | 1–6 h | Fotso et al. (2014) |
| 3 | Whole plant | Aqueous extract | 150, 300 and 500 mg/kg | Stomach tube | 28 days | Chih et al. (1995) |
| 4 | Whole plant | Phytocompounds (1)4-O-b-D-glucopyranosyloxy-1-hydroxy- 6-(E)-tetradecene-8,10,12-triyne, (2) 3-O-b-D-glucopyrano-syloxy-1-hydroxy-6-(E)-tetradec- ene-8,10,12-triyne, (3) 2-O-b-D-glu-copyranosyloxy-1-hydroxy-5-(E)-tetradecene-7,9,11-triy-ene and (4) icthyothereol acetate | 50 μg/mL | Cultured with cells | 24 h | Yan et al. (2022) |
| 5 | Aerial part | Isookanin | 1, 5, and 10 μg/mL | Cultured with cells | 24 h | Xin et al. (2021) |
| 6 | Leaves | Methanolic extract and the polyacetylene [2-O-b-D-glucosyltrideca-11E-en-3,5,7,9-tetrayn-1,2-diol (PA-1)] | 1, 5, or 10 μg/mL | Cultured with cells or intraperitoneal (i.p.) administration | cells- 72 h, Mice - 2–6 days | Pereira et al. (1999) |
| 7 | Not mentioned | Not mentioned | cells: 2–1000 μg/mL Mice: 2 g/kg/day | Cultured with cells or orally | cells- 72 h mice: 1 week | Tsuruta et al. (2023) |
| 8 | Aerial parts | Enzymatic digested Bidens pilosa | 100, 250, and 500 mg/kg | Oral | 10 days | Horiuchi and Seyama (2008) |
| 9 | Aerial parts | Nonpolar fatty acid enriched extract | 25, 50, or 100 mg/kg | Orally | 7 days | Quaglio et al. (2020) |
| 10 | Leaves | Not mentioned | 0.01%–2% | Cultured with cells | 24 h | Arantes et al. (2021a) |
| 11 | Not mentioned | Butanol fraction | 10 mg/kg | Intra-peritoneal (i.p.) injections | 35 days | Chang et al. (2005) |
| 12 | Whole plant | 4-O-(2″-O-acetyl-6″-O- p-coumaroyl-β-D-glucopyranosyl) -p-coumaric acid | 15, 30, and 60 mg/kg/d | Intragastric administration | 8 weeks | Y. Yang et al. (2018) |
| 13 | Aerial parts | Bidens pilosa tea | orally; 0.5 mL of BP/100 g daily; topicaly, 500 mL | Oral and topical | 10 weeks | Pegoraro et al. (2018) |
| 14 | Leaves | B. pilosa glycolic extract (Mucoadhesive formulation) | 100 mg/kg | Orally (gavage) | 6 days | de Ávila et al. (2015) |
| 15 | Not mentioned | B. pilosa glycolic extract (Mucoadhesive formulation) | 15 mL | Gargle | 10 days | Santos Filho et al. (2018) |
| 16 | Not mentioned | Aqueous extracts | 100 mg/kg | Orally | 21 days | Mohamed et al. (2024) |
| 17 | Leaves | Aqueous and methanolic extracts | NA | NA | NA | Akula and Odhav (2008) |
| 18 | Leaves | Perchloric acid extract | NA | NA | NA | Tesfay et al. (2016) |
| 19 | Leaves | Ethanol and aqua dest extracts | NA | NA | NA | Yuniastri et al. (2022) |
| 20 | Whole plant | Methanol extract | 50,100, 200, and 400 mg/kg | Orally | 9 days | Abiodun et al. (2020) |
| 21 | Leaves | 50% methanol/1% formic acid | NA | NA | NA | Nxumalo et al. (2023) |
| 22 | Leaves | Hexane, dichloromethane, ethyl acetate, acetone and methanol | 2.5–0.02 mg/mL | Culture | 24 h | Shandukani et al. (2018) |
| 23 | Leaves | Distillated oil | NA | NA | NA | Goudoum et al. (2016) |
| 24 | Whole plant | Total extract and acetonic fraction | 100 μg/mL or 300 μg/mL | Not mentioned | 3 days | Mota et al. (2019) |
| 25 | Leaves | Crude methanolic and ethanolic extract | NA | NA | NA | Phiri et al. (2024) |
| 26 | Flowers and leaves | Water, methanol, acetone, and ethyl acetate | NA | NA | NA | Nguyen et al. (2023) |
| 27 | Leaves | DMSO extraction | 500 μg/mL | Cell culture | Overnight | Said et al. (2024) |
| 28 | Leaves, roots, stems and whole plants | Methanol extract | NA | NA | NA | Angelini et al. (2021) |
| 29 | Leaves | Aqueous extracts | 500 μg/mL | NA | NA | U-Yatung et al. (2020) |
| 30 | Leaves | Aqueous extracts | NA | NA | NA | Oduntan et al. (2018) |
| 31 | Leaves | Ethanol-water extract | NA | NA | NA | Falowo et al. (2017) |
| 32 | Leaves | Methanolic extract | 1–10 mg/mL | Culture | 36 h | Singh et al. (2017) |
| 33 | Aerial parts | Aqueous and ethanolic extracts | 15.6, 31.25, 62.5, 125, 250, 500, and 1000 μg/mL | Cell culture | 24 h | Idris et al. (2023) |
| 34 | Whole plants | NA | 0.5 g/kg of feed | Orally | 29 days | Memon et al. (2020) |
| 35 | Whole plants | Ethanol extracts | 0.5 mg/mL | Medium | 20 days | Son et al. (2022) |
| 36 | Whole plant | Water, acetone, methanol, hexane, and ethanol extracts | 100 μg/mL | Culture | 24 h | Mashinini et al. (2023) |
| 37 | Not mentioned | Mucoadhesive | 10 mL | Gargle | Not mentioned | Arantes et al. (2021b) |
| 38 | Leaves and flowers | Essential oils from Bidens pilosa | NA | NA | NA | Deba et al. (2007) |
| 39 | Whole plant | Crude extract ethyl acetate and butanolic fractions | 100, 200, 300, 400, 500 μg/mL | Cell culture | 24 h | Chiang et al. (2004) |
| 40 | Whole herb | Petroleum ether, ethyl acetate (EE-BP), n-BuOH and water fractions | 50, 100, 200 and 400 μmol/L | Cell culture | 24 h | Yi et al. (2016) |
| 41 | NA | NA | NA | NA | NA | Liu et al. (2013) |
| 42 | Not mentioned | Aqueous methanol | NA | NA | NA | Muchuweti et al. (2007) |
| 43 | Aerial parts | Hydroethanol crude extract and chloroform, ethyl acetate, and methanol fraction | 1.5, 15, 150, or 300 mg/kg | Not mentioned | 10 days | Kviecinski et al. (2011) |
| 44 | Aerial parts | Suspension in 0.25% sodium carboxymethyl cellulose | 0.1, 0.25, 0.5 mg/kg | Oral | 3 weeks | Horiuchi et al. (2010) |
| 45 | Plant leaves and shoots | Methanolic extracts | NA | NA | NA | Chipurura et al. (2013) |
| 46 | Leaves | Acetone, methanol and aqueous extracts | 0.1, 0.5, 1.0, 2.5 and 5.0 mg/mL | Cell culture | 24–48 h | Adeolu Adedapo and AuthorAnonymous (2011) |
| 47 | Leaves | Total flavonoid extract | 20, 50 and 100 mg/kg | Not mentioned | 10 days | Yuan et al. (2008) |
| 48 | Aerial parts | Hydroalcoholic extract | NA | NA | NA | Cortés-Rojas et al. (2011) |
| 49 | Whole plant | Petroleum ether, ethyl acetate (EE-BP), and n-BuOH fractions | 100, 200, 300, 400, 500 μg/mL | Cell culture | 24 h | Wu et al. (2012) |
| 50 | Whole plant | Ethanol and ethyl acetate/ethanol extracts | 50–150 and 25–75 ug/mL | Incubation | 30 min | Yang et al. (2006) |
Summarized data on intervention and dosages from included Studies.
NA- not applicable.
TABLE 3
| S/N | Plant part, extract/phytocompound | Mechanism of action | Treatment outcome | References |
|---|---|---|---|---|
| 1 | NA | increased levels of IL-10 in intestinal tissue homogenate | Diminished inflammatory response | Bastos et al. (2016) |
| 2 | Leaf/methanol | Reduced the edema formation of the rat paw | Diminished inflammatory response | Fotso et al. (2014) |
| 3 | Whole plant | Decreased paw edema formation in rat paw | Inhibited paw edema | Chih et al. (1995) |
| 4 | Whole plant/ethanol | Reduced release of IL-6 and NO in RAW 264.7 cells | have anti-inflammatory activity | Yan et al. (2022) |
| 5 | flavonoids-type phytochemical, isookanin | Inhibited proinflammatory cytokines, interleukin-6, interleukin-8 and interleukin-1β. Inhibiting the expression of inducible nitric oxide synthase and cyclooxygenase-2 | reduces the production of proinflammatory mediators (nitric oxide, prostaglandin E2) in mouse macrophages | Xin et al. (2021) |
| 6 | leaf/methanol | Inhibits the proliferative response of mouse lymphocytes | Possess anti-inflammatory and immunosuppressive activity | Pereira et al. (1999) |
| 7 | NA | Suppressed the induction of pro-inflammatory cytokine | Possess anti -inflammatory activity | Tsuruta et al. (2023) |
| 8 | Aerial parts/carboxy-methyl-cellulose sodium (CMC-Na) solution | Inhibits histamine release from mast cell pellet in peritoneal fluid supernatant | Possess antiinflammatory and antiallergic effects via the inhibition of histamine release from mast cells in peritoneal fluid supernatant of mice | Horiuchi and Seyama (2008) |
| 9 | Aerial parts/nonpolar fatty acid enriched extract | Inhibit IL-6, IL-1β and TNF-α and increment of anti-inflammatory IL-10 cytokine | Has anti-inflammatory activity and modulates the immune response | Quaglio et al. (2020) |
| 10 | mucoadhesive formulation | Reduced IL-8 production in an oral squamous cell carcinoma cell line (SCC-4), but had no observable effect on the production of the cytokines TNF-α, IL-6, IL-1β, IL-12p70, and IL-10 by the SCC-4 cell | exhibits anti-inflammatory properties by reducing IL-8 production in oral squamous cell carcinoma cell line (SCC-4) | Arantes et al. (2021a) |
| 11 | whole plants/butanol fraction | increased Th2 cytokines (IL-4 and/or IL-5), but decreased Th1 cytokine (IFN-c), increased Th2 cytokine-regulated IgE production in mouse serum | The butanol fraction of B. pilosa enhances Th2-mediated pulmonary inflammation by stimulating the production of Th2 cytokines and increasing IgE levels | Chang et al. (2005) |
| 12 | whole plants/ethanol | The inflammatory markers including tumor necrosis factor-α, (TNF-α), Interleukin-6 (IL-6), and Interleukin-1β (IL-1β) decreased | Suppress the expression of inflammatory mediators and effectively inhibit the inflammatory cytokines | Y. Yang et al. (2018) |
| 13 | Aerial parts/aqueous | 10 high-power fields (HPF), Inflammatory cell present (polymorphonuclear and/or morphonuclear via presence in intestinal tissue. Presented mild inflammation | Absence of hepatic inflammation and inflammation in the intestinal mucosa | Pegoraro et al. (2018) |
| 14 | Glycolic extract | decreased MPO activities in 5-FU-induced intestinal mucositis in mice | Regulated inflammatory infiltration in the intestinal mucosa in mice via the inhibition of MPO enzyme activities | de Ávila et al. (2015) |
| 15 | Glycolic extract | Alteration in the concentrations of pro-inflammatory cytokines | No significant difference was found in the levels of pro-inflammatory cytokines between participants who used FITOPROT A versus FITOPROT B | Santos Filho et al. (2018) |
| 16 | Leaf/aqueous extracts | The kidney inflammation parameters TNF-α, IL 6, and TGF 1-β were analyze. All inflammation markers were notably declined in kidney homogenate of rats treated with BP extract compared to the untreated group | BP has an ability to reduce inflammation parameters in renal homogenates | Mohamed et al. (2024) |
| 17 | Leaf/Aqueous and methanolic extracts | B. Pilosa inhibit 5-lipoxygenase (5-Lox) activity | Bidens pilosa showed maximum antiinflammatory activity by inhibiting 5-lipoxygenase activity | Akula and Odhav (2008) |
| 18 | NA | NA | NA | Tesfay et al. (2016) |
| 19 | NA | NA | NA | Yuniastri et al. (2022) |
| 20 | whole plant/methanol | Myeloperoxidase activity (MPO), TNF-α were estimated in colon homogenate. BP decrease TNF-α and inhibited leukocytes infiltration (MPO activity) | BP significantly reduced leukocytes infiltration, and TNF-α level in comparison to untreated colitic rats | Abiodun et al. (2020) |
| 21 | Leaf/methanol extract | NA | NA | Nxumalo et al. (2023) |
| 22 | Hexane, dichloromethane, ethyl acetate, acetone and methanol | NA | NA | Shandukani et al. (2018) |
| 23 | leaf/methanol | NA | NA | Goudoum et al. (2016) |
| 24 | Whole plant/aqueous, Acetonic fraction | Bidens pilosa inhibits T. gondii via the lectin maturase K, disrupting protein synthesis in the parasite’s apicoplast, while maintaining low cytotoxicity to host cells | B. pilosa induce significant decrease of the parasite burden in brain tissue of T. gondii-infected mice. This suggests its potential as a therapeutic alternative for toxoplasmosis | Mota et al. (2019) |
| 25 | Leaf/aqueous | NA | NA | Phiri et al. (2024) |
| 26 | Flowers and leaves/water, methanol, acetone, and ethyl acetate | NA | NA | Nguyen et al. (2023) |
| 27 | Leaf/DMSO | The ELISA assay indicated that treatment with B. pilosa led to a significant increase in the pro-inflammatory cytokines IL-1α and IL-1β in response to HepG2 cells | B. pilosa extract exhibited potential inhibitory effects on Raf-1 and MEK-1 gene expression without causing detectable cytotoxicity, along with a significant reduction in autophagic activity following treatment | Said et al. (2024) |
| 28 | Leaf, roots, stems and whole plants/methanol | NA | NA | Angelini et al. (2021) |
| 29 | Leaf/aqueous | NA | NA | U-Yatung et al. (2020) |
| 30 | Leaf/aqueous | NA | NA | Oduntan et al. (2018) |
| 31 | Leaf/ethanol-water solution | NA | NA | Falowo et al. (2017) |
| 32 | Leaf/methanol | NA | NA | Singh et al. (2017) |
| 33 | Aerial parts/aqueous | NA | NA | Idris et al. (2023) |
| 34 | Whole plants | Pro-inflammatory cytokines (IL-6 and IL-8) aecal samples of chicks were assessed. BP upregulated IL-6 and IL-8 | B. pilosa and probiotic + B. pilosa diets enhanced the activities of pro-inflammatory cytokines by increasing IL-6 and IL-8 | Memon et al. (2020) |
| 35 | whole part/ethanol | NA | NA | Son et al. (2022) |
| 36 | whole plant/aqueous, acetone, methanol, hexane, and ethanol | NA | NA | Mashinini et al. (2023) |
| 37 | Not mentioned | Salivary concentrations of the pro-inflammatory cytokines were evaluated | Exhibits effective anti-inflammatory properties | Arantes et al. (2021b) |
| 38 | Leaves and flowers oil/diethyl ether | NA | NA | Deba et al. (2007) |
| 39 | whole plant/ethanol | Inhibited nitric oxide production in RAW 264.7 murine macrophage cell line | Showed strong antiinflammatory activity in cells | Chiang et al. (2004) |
| 40 | whole plan/ethanol | NA | NA | Yi et al. (2016) |
| 41 | Honeys from the nectar of BP | Cytokine IL-8 of human colon carcinoma cell line (WiDr) was evaluated. B. pilosa exhibited low inhibition for IL-8 secretion by the WiDr | Immunomodulatory effects of the honey from BP seem to be attributed to components other than phenols as honey from B.pilosa had the lowest inhibition of IL-8 production by WiDr cells | Liu et al. (2013) |
| 42 | Methanol | NA | NA | Muchuweti et al. (2007) |
| 43 | Aerial parts/ethanol-water | NA | NA | Kviecinski et al. (2011) |
| 44 | Aerial parts/sodium carboxymethyl cellu-lose (CMC-Na) | MMBP reduced severity of gastric lesions induced by NSAID | Possess suppressing effects against the NSAID-induced gastric lesion model via reducing severity of gastric lesions | Horiuchi et al. (2010) |
| 45 | Leaf/total phenolic content | NA | NA | Chipurura et al. (2013) |
| 46 | Leaf/acetone and methanol | NA | NA | Adeolu Adedapo and AuthorAnonymous (2011) |
| 47 | Leaf/ethanol | nuclear factor-B (NF-B) expression of the liver were assessed. Marked reduction in the NF-B protein expression in rat hepatocytes | Exhibited NF-B activation inhibition property by B. Pilosa inhibited NF-B activation in liver fibrosis of rats | Yuan et al. (2008) |
| 48 | Aerial parts | NA | NA | Cortés-Rojas et al. (2011) |
| 49 | whole plant/ethanol | NA | NA | Wu et al. (2012) |
| 50 | whole plant/ethanol | NA | NA | H. L. Yang et al. (2006) |
Antiinflammatory potentials of Bidens pilosa as reported in the included studies.
NF-κB: Nuclear Factor kappa-light-chain-enhancer of activated B cells; IL-8: Interleukin 8; IL-6: Interleukin 6; IL-1β: Interleukin 1 beta; IL-1α: Interleukin 1 alpha; TNF-α: tumor necrosis factor alpha; COX-2: Cyclooxygenase-2.
3 Result
3.1 Study selection
Our search of three database searches returned 414 bibliographies from which 175 duplicates were removed. The remaining 239 articles were used for title and abstract screening, at the end of which 168 articles were excluded. The report sorted for retrieval was 71, out of which we were unable to retrieved 4 articles despite all our efforts. The reports assessed for eligibility were 67, out of which 16 articles were excluded for been unrelated and 1 article for been written in Chinese. At the end of the entire screening process, 50 articles were included in the study for data extraction and synthesis (Figure 1).
FIGURE 1

Prisma flow chart.
3.2 Risk of bias assessment
The risk of bias assessment using SYRCLE Checklist for the in vivo studies based on the 10 domains revealed a general low risk of bias (Figure 2); however, 11 articles had no information about “D5 - Were the caregivers and/or investigators blinded from knowledge which intervention each animal received during the experiment?” and “D6 - Were animals selected at random for outcome assessment?”. Ten articles had no information on “D7 - Was the outcome assessor blinded?” (Figure 3).
FIGURE 2

Risk of bias for the 10 domains for the in vivo studies.
FIGURE 3
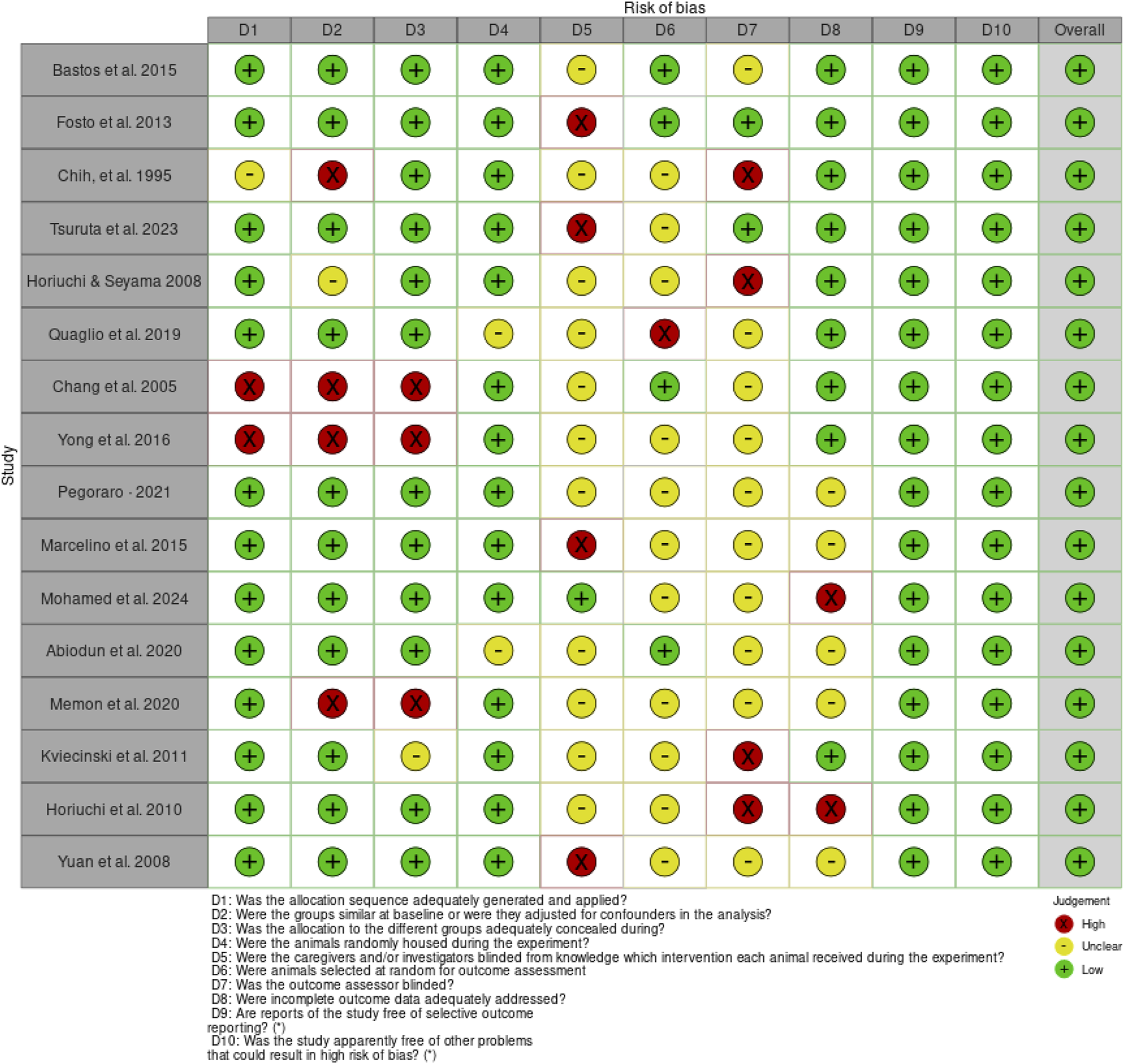
Risk of bias for the individual articles on the in vivo studies.
The SciRAP tool evaluation covered five key components: test compound and controls, test system, administration of the test compound, data collection, and analysis, encompassing a total of 23 topics. In assessing the test compound and controls, 36 studies were found to be partially fulfilled. For the test system and administration of the test compound, 20 studies met the criteria. Data collection and analysis were fulfilled in 37 studies. Regarding funding and competing interests, 13 articles met the criteria, while 19 did not (Figure 4).
FIGURE 4
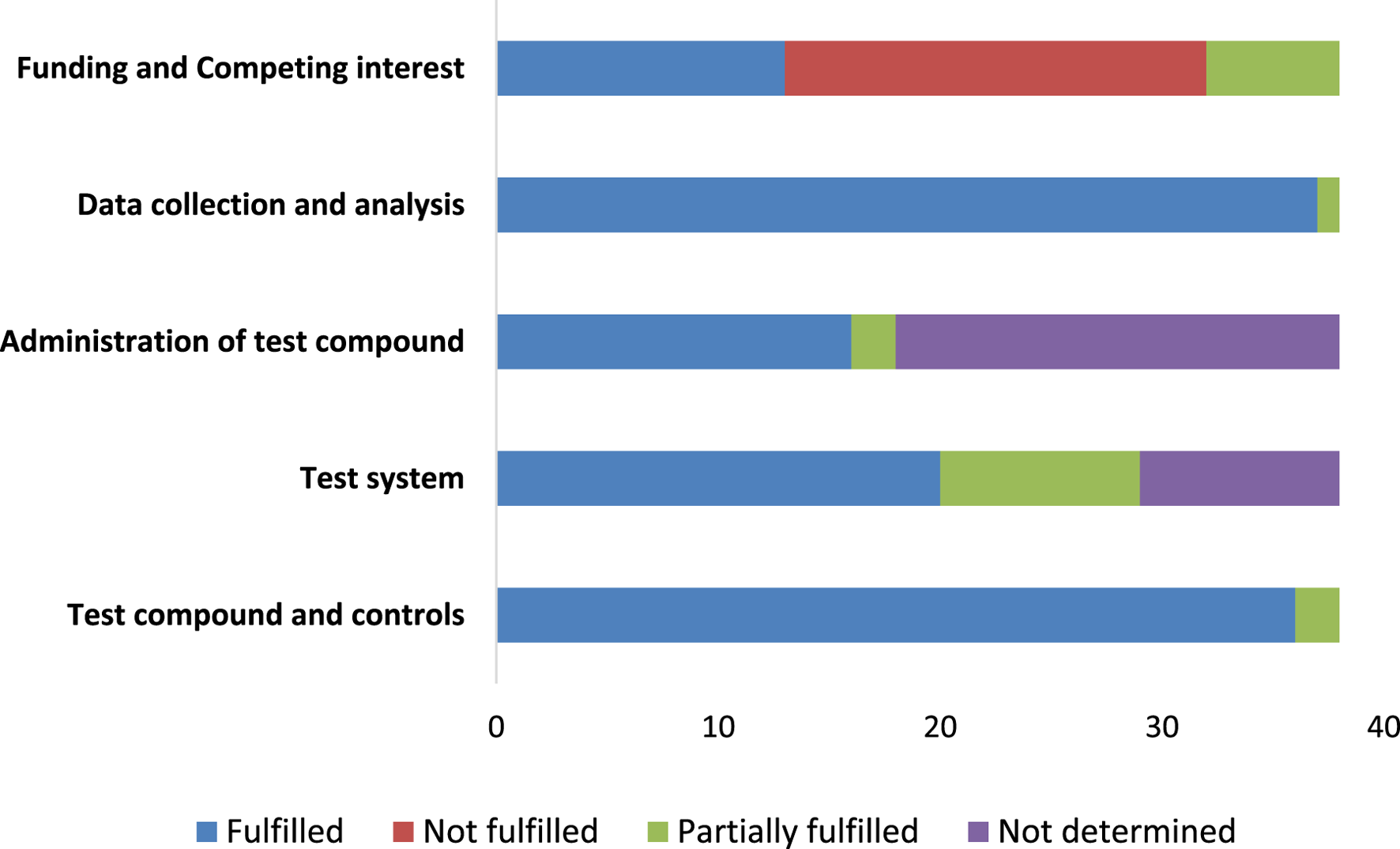
The reporting quality for the in vitro studies.
3.3 Study characteristics
We reviewed 50 studies, and most (35/50, 70%) were published within the years 2014–2024. Of the 50 studies, 23 (46%) were done in Africa, 14 (28%) in Asia, and 11 (22%) in South America. Most studies were done in a laboratory dish (29/50, 58%), with a smaller number done in animals (12/50, 24%). Fourteen percent (14%) of the studies used both in vivo and in vitro methods, and 4% were carried out on people. Out of the 50 studies, leaves were looked at most often (23 or 46%), followed by the whole plant (19 or 38%). Most of the studies (44/50, 88%) concentrated on the roots, stems, or leaves, but the aerial parts were studied in just 4/50 (8%) of the studies (Table 1). To study antioxidant, anti-inflammatory, analgesic, anticancer and immunomodulatory effects, the researchers used both living and non-living models. For mucositis and oxidative stress, male Swiss mice (7–10 weeks) were used in vivo; for TNBS-induced colitis, carrageenan-induced edema and CCl4-induced toxicity, Wistar rats and BALB/c mice were used. Ovalbumin-induced airway inflammation was studied in female BALB/c mice, while transgenic G93A mice represented ALS. Chickens were used in Eimeria infection models and Drosophila were used to study the effects of paraquat on oxidative stress. RAW 264.7, THP-1, BV-2, SCC-4, HepG2, Vero, HeLa and KB-3-1 cell lines were used in in vitro systems to investigate leukemia, inflammation and several cancers. Both healthy volunteers and patients who had oral mucositis while receiving radio-chemotherapy were studied in this research. The antioxidant property and toxicity of the extracts were measured by DPPH, ABTS, FRAP, TBARS, SOD, GSH, catalase, β-carotene bleaching, phosphomolybdenum and MTT assays. They gave a complete understanding of oxidative stress, the immune system, inflammation and cancer development.
3.4 Summary of intervention and dosages of Bidens pilosa from the included studies
The included studies used different preparations of B. pilosa such as aqueous, methanolic, ethanolic and glycolic extracts, as well as isolated phytocompounds and essential oils. Most of the plants used were leaves, aerial parts and whole plants (Table 2). The amount and frequency of the extracts were different for each type of extract and model in the studies. In animals, researchers most often gave the compound via gavage or feed and doses varied from 15 mg/kg to 2 g/kg each day for up to 10 weeks (Bastos et al., 2016; Abiodun et al., 2020; Yang et al., 2018). In a few studies, intraperitoneal injection was used, with a 10 mg/kg butanol fraction given for 35 days (Chang et al., 2005). The plant was given to patients in human clinical trials either as a gargle (15 mL for 10 days) or in the form of a mucoadhesive (at 100 mg/kg by mouth) (Santos Filho et al., 2018; de Ávila et al., 2015) (Table 2). In vitro studies most often used Bidens pilosa phytocompounds at concentrations between 1 μg/mL and 1000 μg/mL and left the cells in contact with them for 24–72 h (Xin et al., 2021; Pereira et al., 1999; Chang et al., 2005). Interestingly, some research used mixtures of phytochemicals, for example, glucopyranosylated polyacetylenes (Yan et al., 2022) and essential oils (Deba et al., 2007), but did not always mention how much or for how long the compounds were given. Plant extracts were also used in foods or as ingredients in teas, much like they have been used in the past (Pegoraro et al., 2018). Less specific detail on how the botanical drugs were given was observed in some studies (Akula and Odhav, 2008; Yuniastri et al., 2022).
3.5 Phytochemical analysis of Bidens pilosa extracts and key phytocompounds identified
A variety of methods have been used to study the phytochemical content of Bidens Pilosa (Supplementary Table S1). Ultraviolet-Visible spectrophotometry (UV-Vis Spec.) was widely used to measure the total phenolic and flavonoid content, as shown by Mohamed et al. (2024), Akula and Odhav (2008) and Phiri et al. (2024). Specific phenolic acids and flavonoids were identified and quantified using HPLC, as was done by Nguyen et al. (2023), Yang et al. (2018) and Singh et al. (2017) (Supplementary Table S1). Using LC-MS/MS and LC-HR-MS, researchers were able to identify a wider range of secondary metabolites such as coumarins, ellagitannins and hydroxybenzoic acids (Angelini et al., 2021; Idris et al., 2023; Nxumalo et al., 2023). GC-MS was found to be the best method for detecting fatty acids and terpenoids among volatile and lipophilic compounds (Goudoum et al., 2016; Abiodun et al., 2020; Falowo et al., 2017) (Supplementary Table S1).
The included studies found that B. pilosa contains many bioactive compounds, suggesting it may be useful in medicine. The phytocompounds are shown in Supplementary Table S2. Quercetin, isookanin, rutin, and glucuronylated quercetin were found in every extraction method studied by Fotso et al. (2014), Xin et al. (2021), and Santos Filho et al. (2018). Ethyl acetate and n-butanol extracts from the plant were commonly found to contain 4-O-β-D-glucopyranosyloxy-1-hydroxy-6-(E)-tetradecene-8,10,12-triyne (Yan et al., 2022; Chang et al., 2005) (Supplementary Table S2). Caffeic acid, gallic acid, and chlorogenic acid were other phenolic acids that were commonly found, according to Horiuchi and Seyama (2008) and Nguyen et al. (2023). GC-MS tests also found that the main fatty acids and volatile oils in these oils are palmitic acid, oleic acid, α-pinene, and phytol (Quaglio et al., 2020; Goudoum et al., 2016) (Supplementary Table S2). They highlight the plant’s rich chemical makeup and its ability to help with diseases linked to oxidative stress, lipid peroxidation, and inflammation.
3.6 Bidens pilosa antioxidant mechanisms and outcome as reported in the included studies
It was shown that Bidens pilosa acts as a powerful antioxidant by removing free radicals and helping the body produce its own antioxidants (Supplementary Table S3). Methanolic, aqueous, ethanolic and ethyl acetate extracts from the plant showed strong DPPH, ABTS and FRAP scavenging abilities, similar to those of ascorbic acid and BHT (Chipurura et al., 2013; Adeolu Adedapo and AuthorAnonymous, 2011; Cortés-Rojas et al., 2011; Wu et al., 2012). There was a strong relationship between these activities and the total phenolic and flavonoid contents. In addition to neutralizing radicals, B. pilosa changed the activity of SOD, GSH and GSH-Px which resulted in a decrease in lipid peroxidation and MDA levels in both hepatic and erythrocyte models (Yuan et al., 2008; Yang et al., 2006). The gastric models showed that its formulations greatly reduced thiobarbituric acid reactive substances (TBARS) which indicated that it helped protect the stomach by reducing oxidative stress (Horiuchi et al., 2010) (Supplementary Table S3). In therapeutic terms, these antioxidant properties resulted in protecting cells from damage, including the liver, stomach and colon cancer cells (Wu et al., 2012). In general, the powerful ability to scavenge radicals, alter enzymes and provide protection make B. pilosa a promising natural antioxidant for disease prevention and management. All the studies included in this review have shown that Bidens Pilosa has beneficial antioxidant properties (Supplementary Table S3). A formulation of B. pilosa (MMPB) minimized oxidative markers such as MDA and TBARS, improved antioxidant enzymes (SOD, GSH, GSH-Px) and reduced tissue damage in models of liver, stomach and erythrocyte injury (Horiuchi et al., 2010; Yuan et al., 2008; Yang et al., 2006). Radical scavenging tests revealed that methanolic, ethanolic and ethyl acetate extracts have potential for treating conditions related to oxidative stress (Chipurura et al., 2013; Adeolu Adedapo and AuthorAnonymous, 2011; Wu et al., 2012). The ethyl acetate fraction caused cancer cells to die which suggests it may be useful in treating colorectal cancer. These reports reveal that B. pilosa can be used in various ways as an antioxidant and has many therapeutic benefits.
3.7 Bidens pilosa anti-inflammatory mechanisms and outcome as reported in included studies
Anti-inflammatory activity in Bidens pilosa has been shown in many studies using different plant materials and types of extracts (Table 3). Bastos et al. (2016), Quaglio et al. (2020), Pegoraro et al. (2018), and de Ávila et al. (2015) found that B. pilosa affects cytokines, boosting IL-10 and lessening IL-1β, IL-6, and TNF-α in tissues affected by inflammation. The anti-inflammatory and immunosuppressive effects of leaves and whole plants from this plant were confirmed by reducing paw edema, lymphocyte proliferation, and histamine release in animal experiments (Fotso et al., 2014; Chih et al., 1995; Pereira et al., 1999; Horiuchi and Seyama, 2008). Experiments done in a laboratory have found that it is effective. Yan et al. (2022) and Yang et al. (2018) found that ethanolic extracts of B. pilosa reduced IL-6, NO, and TNF-α in macrophages, while isookanin flavonoids from the plant blocked COX-2 and iNOS enzymes (Xin et al., 2021) (Table 3). According to Tsuruta et al. (2023), they discovered that pro-inflammatory cytokines in the spinal cord were selectively blocked in G93A mice. Meanwhile, Santos Filho et al. (2018) reported no changes in human inflammation, which suggests that clinical application varies. The reports show that B. Pilosa, helps reduce inflammation by controlling cytokines, limiting inflammatory mediators, and regulating the immune system.
Experiments across multiple models showed that B. pilosa has anti-inflammatory effects. The outcome revealed that extracts help reduce edema in acute inflammation, decrease inflammatory cytokine levels in both the intestine and kidney, and decrease the number of leukocytes and MPO activity in colitis models (Fotso et al., 2014; Chih et al., 1995; Yang et al., 2018; Mohammed et al., 2024; Abiodun et al., 2020) (Table 3). The treatment decreased IL-8 release in oral carcinoma (Arantes et al., 2021a). Specifically, the plant’s ability to fight colitis was linked to higher IL-10, lower IL-6, and lower TNF-α levels (Quaglio et al., 2020). Mucoadhesive gels showed advantages for certain tissues while still keeping the overall cytokine profile unchanged (as shown by Santos Filho et al., 2018) (Table 3).
4 Discussion
4.1 Study characteristics and scientific methodological overview
This review looks at fifty studies on Bidens Pilosa (B. pilosa), mainly focusing on its antioxidant, anti-inflammatory, and immunomodulatory effects. Most of the studies, 70%, were published during 2014–2024, which points to a growing interest worldwide in using this plant as a botanical drug because of its important metabolites and many medical uses. Significantly, studies from Africa, Asia, and South America confirm that people from different regions recognize how bioactive this plant and its metabolites can be.
This data shows studies ranging from in vitro designs to in vivo experiments, while some studies combined the two approaches. Although currently in an early translational phase, the few human clinical trials (Arantes et al., 2021a; Santos Filho et al., 2018) form a solid base for future studies. These results agree with previous studies (Abiodun et al., 2020; Rodríguez Mesa et al., 2023), highlighting the importance of bringing preclinical results into clinical use.
Although preclinical studies prove that B. pilosa has antioxidant and anti-inflammatory properties, very few clinical trials have been done. The lack of translation may be caused by problems in the way this plant is extracted, the variation in doses, and the different active compound concentrations found in studies, making it hard to standardize how cannabis should be used in humans. In addition, the information from preclinical studies is usually not robust enough in pharmacokinetics and toxicity to get ethical approval for clinical trials. Limits on regulations, money, and the belief that herbal remedies are not scientifically proven hold back progress to human studies. Future work should focus on standardizing B. pilosa extracts to guarantee that results from different studies are the same. Immense pharmacological and toxicological evaluations can strengthen the drug design and safety, thereby helping it gain approval from regulators.
In this current review, tests using Swiss mice for intestinal mucositis, oxidative stress, and inflammatory bowel models in BALB/c and Wistar rats, as well as transgenic mouse models of ALS, show the wide range of conditions studied with B. pilosa. Furthermore, the choice of RAW 264.7 (macrophages), THP-1 (monocytes), BV-2 (microglia) and SCC-4 (oral cancer) cell lines demonstrates how the plant relates to immunological, oxidative, and oncogenic pathways.
The results also highlight that B. pilosa is a multi-purpose botanical drug. In particular, leaves and whole plant extracts were most studied, pointing to their high content of bioactive substances. This matches earlier research that found the leaves’ extracts in water and methanol could scavenge radicals (Akula and Odhav, 2008; Fotso et al., 2014) and whole plant extracts helped treat inflammation and oxidative damage in colitis and hepatic toxicity (Abiodun et al., 2020; Mashinini et al., 2023). Despite this, phytochemical analysis of the aerial parts (8%) and roots/flowers has yet to be done, and these plant parts should be studied further.
In all these experiments, DPPH, ABTS, TBARS, and FRAP were chosen to evaluate radical scavenging activity. MTT, catalase, and GSH assays were used to measure how the compounds affected cells and enzymes. Together, these assays measure the plant’s capacity to clear free radicals, control redox balance, and reduce the release of inflammatory cytokines, all of which are important for many diseases (Rodríguez Mesa et al., 2023; Santos Filho et al., 2018).
These findings have important consequences. Since B. pilosa can affect many biological systems and diseases, it is a good candidate for making multi-targeted phytotherapeutics. Because it works well in both immune and oxidative models, it is well suited to be a dual-action agent, which is important when diseases negatively impact multiple biological pathways. As a result, we require botanical drugs that treat the main condition and its linked problems, such as oxidative stress, which is found in many chronic inflammatory and neurodegenerative diseases.
4.2 Intervention strategies and dosage patterns in Bidens pilosa administration
The results from this review show a lot of variation in how B. pilosa was studied and given to participants. Such variety demonstrates the plant’s many uses and the absence of a set way to use it in preclinical and clinical research.
Different extracts of B. pilosa were used in the studies. For example, aqueous, methanolic, ethanolic, glycolic, and butanolic fractions, isolated phytocompounds, and essential oils. Most studies used leaves, aerial parts, or the whole plant to make interventions, reflecting the multiple therapeutic components in B. pilosa (Bastos et al., 2016; Abiodun et al., 2020; Chang et al., 2005; Deba et al., 2007). The results add to earlier studies showing that B. pilosa contains many metabolites, especially polyacetylenes, flavonoids, and phenolic compounds, which have been found to have various biological activities (Yan et al., 2022; Xin et al., 2021).
In animal experiments, the majority of researchers gave B. pilosa by gavage or mixed it into the feed, using amounts from 15 mg/kg to 2 g/kg each day for periods of 6 days–10 weeks (Yang et al., 2018; Abiodun et al., 2020). Since the best dose is not feasible, the broad range of doses used in research makes it challenging to see how effective the treatment is in various models. Significant outcomes were obtained by Chang et al. (2005) when they treated mice with a butanol fraction at 10 mg/kg daily for 35 days by injection into the abdomen. Since drugs are delivered and stay in the body differently, pharmacokinetic research is done to determine the best doses and improve outcomes.
The majority of in vitro studies used extracts at concentrations from 1 to 1000 μg/mL for 24–72 h (Pereira et al., 1999; Chang et al., 2005; Xin et al., 2021). Even so, the findings indicate that B. pilosa metabolites affect cells in a way that depends on both the dose and the time, even though a lack of standard endpoints prevents direct comparison between studies. Furthermore, research with complex mixtures, like glucopyranosylated polyacetylenes (Yan et al., 2022) and essential oils (Deba et al., 2007), often failed to clearly state the amounts and lengths of exposure, which weakens their reproducibility and usefulness for translation.
Fortunately, research on humans, while limited, has shown that clinical use is possible. Santos Filho et al. (2018) showed that a B. pilosa gargle (15 mL/day for 10 days) could be therapeutic, and de Ávila et al. (2015) gave 100 mg/kg orally in the form of a mucoadhesive preparation. They are especially important because they look at how these approaches are delivered and whether people will accept them in practice. Even so, the limited number of patients, short follow-up, and lack of pharmacokinetic data weaken these results and suggest that further studies are needed.
Our results are consistent with earlier reviews and analyses, which confirm that B. pilosa has broad bioactivity and may be developed as phytopharmaceutical agents (Pegoraro et al., 2018; Pereira et al., 1999). Still, the differences found and the challenges in methods suggest that we need strong standardization and better ways to move from research to clinical use.
4.3 Bidens pilosa phytochemical analysis, composition, and pharmacological importance
The phytochemical analysis of Bidens pilosa presented in this review confirms the plant’s status as a chemically diverse medicinal herb with high therapeutic potential. Through various analytical platforms and extraction methodologies, researchers have consistently identified a spectrum of secondary metabolites, including flavonoids, phenolic acids, polyacetylenes, fatty acids, and terpenoids. These findings provide a robust chemical foundation to support B. pilosa’s ethnomedicinal applications in managing oxidative stress, lipid peroxidation, inflammation, and metabolic disorders.
The repeated use of UV-visible spectrophotometry across studies, such as those by Mohamed et al. (2024), Akula and Odhav (2008), and Phiri et al. (2024), has facilitated rapid quantification of total phenolic and flavonoid content, which are important classes of antioxidants. These bulk metrics serve as initial indicators of pharmacological relevance, given the well-established role of flavonoids and phenolics in neutralizing free radicals and mitigating oxidative damage.
More targeted profiling was achieved by High-Performance Liquid Chromatography (HPLC), which allowed the specific identification and quantification of key compounds such as caffeic acid, chlorogenic acid, and quercetin derivatives (Nguyen et al., 2023; Yang et al., 2018; Singh et al., 2017). These phenolic acids and flavonoids are recognized for their antioxidant, anti-inflammatory, and vasoprotective activities, suggesting that B. pilosa could exert multifaceted biological effects relevant to chronic inflammatory diseases.
Advanced metabolomic platforms like Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS) and Liquid Chromatography–High-Resolution Mass Spectrometry (LC-HR-MS) revealed an even broader spectrum of bioactive constituents, including coumarins, ellagitannins, and hydroxybenzoic acids (Angelini et al., 2021; Idris et al., 2023; Nxumalo et al., 2023). The identification of such structurally diverse molecules underscores the plant’s chemotypic complexity and supports its potential utility in drug discovery pipelines. Notably, polyacetylenic glucosides, especially 4-O-β-D-glucopyranosyloxy-1-hydroxy-6-(E)-tetradecene-8,10,12-triyne, were consistently isolated in ethyl acetate and n-butanol extracts (Yan et al., 2022; Chang et al., 2005). These compounds are known for their immunomodulatory and cytotoxic properties, and their recurrent detection suggests they may serve as chemotaxonomic markers or lead molecules for pharmaceutical development.
In parallel, Gas Chromatography–Mass Spectrometry (GC-MS) analyses were pivotal in identifying lipophilic and volatile compounds, including palmitic acid, oleic acid, α-pinene, phytol, and other terpenoids (Goudoum et al., 2016; Abiodun et al., 2020; Falowo et al., 2017; Quaglio et al., 2020). These molecules, often associated with anti-inflammatory and antimicrobial effects, are key to understanding the broader pharmacological effects of B. pilosa, especially in topical and nutraceutical applications.
The consistency in identification of certain compounds across studies, such as quercetin, rutin, isookanin, and glucuronylated quercetin (Fotso et al., 2014; Xin et al., 2021; Santos Filho et al., 2018), adds to the credibility of these findings. These flavonoids are among the most pharmacologically relevant, with a wide range of reported activities including enzyme inhibition, reactive oxygen species scavenging, and modulation of intracellular signaling pathways. Their ubiquity in B. pilosa across various extraction methods and geographic sources strengthens the case for their use as quality control markers in future standardization protocols.
Specifically, we identified 35 key metabolites with proven pharmacological relevance in B. Pilosa. These compounds were relevant particularly in modulating oxidative stress, lipid peroxidation, and inflammation, which are drivers of various diseases, including neurodegenerative and non-communicable diseases. Key phenolic acids like caffeic acid, gallic acid, ferulic acid, and chlorogenic acid (for example, 1-caffeoylquinic acid and 5Z-caffeoylquinic acid) exhibit antioxidant and anti-inflammatory properties by suppressing ROS generation, NF-κB activation, and pro-inflammatory cytokines such as TNF-α and IL-6. Flavonoids, including quercetin, kaempferol, rutin, isoquercetin, catechin, and luteolin derivatives, enhance cellular defenses through Nrf2 pathway activation, PI3K/Akt signaling, and membrane stabilization, mitigating neuroinflammation and mitochondrial dysfunction. Fatty acids like oleic acid, palmitic acid, and linoleic acid, with their esters (such as ethyl linoleate), support membrane integrity and exhibit anti-inflammatory and hypolipidemic effects. Terpenoids such as phytol, beta-caryophyllene, limonene, alpha-pinene, cadinene, and delta-cadinene contribute to antimicrobial, analgesic, and anti-inflammatory actions. Phytosterols like stigmasterol and beta-sitosterol have cholesterol-lowering and anti-diabetic properties, while tannic acid and vanillin exert broad-spectrum antioxidant and neuroprotective roles. Notably, paclitaxel, though rare in plants outside the Taxus genus, suggests a possible antitumor synergy within B. pilosa. Collectively, the phytocompounds in B. pilosa demonstrate a strong potential for integrative therapeutic strategies targeting oxidative damage, inflammation, and chronic degenerative diseases.
The presence of these diverse and bioactive metabolites aligns with findings from previous phytochemical reviews of B. pilosa and related Asteraceae species (Pegoraro et al., 2018; Pereira et al., 1999). The current synthesis, however, benefits from recent technological advancements in mass spectrometry and chromatography, allowing for a more comprehensive phytochemical fingerprint. Furthermore, the correlation between specific compounds (e.g., chlorogenic acid, rutin, phytol) and pharmacological outcomes such as antioxidant and anti-inflammatory effects reinforces the mechanistic plausibility of B. pilosa’s traditional uses in managing inflammatory and oxidative stress-related conditions.
4.4 Combined antioxidant and lipid peroxidation reducing potential of Bidens pilosa
According to the studies included, Bidens pilosa possesses a strong and varied antioxidant ability, working by both trapping radicals and influencing the body’s methods of protection. A number of extracts from B. pilosa, for example methanolic, aqueous and tea, have been shown to have strong free radical-scavenging effects in tests such as DPPH, ABTS and FRAP, proving that they can block reactive oxygen species and protect against oxidative damage (Akula and Odhav, 2008; Mohamed et al., 2024; Nxumalo et al., 2023; Phiri et al., 2024). Significant decreases in malondialdehyde (MDA) levels which indicate lipid peroxidation, are also observed in both laboratory and animal experiments (Abiodun et al., 2020; de Ávila et al., 2015; Bastos et al., 2016; Santos Filho et al., 2018). Lipid peroxidation plays a significant role in systemic inflammatory diseases, cardiovascular conditions, and metabolic disorders (Moretti et al., 2024; Wazir et al., 2023). Damage to cell membranes caused by oxidative stress can trigger immune responses, contribute to chronic inflammation, and exacerbate conditions such as atherosclerosis, diabetes, and autoimmune diseases (Bhol et al., 2024; Sadiq, 2021). Studies suggest that Bidens pilosa, a plant rich in flavonoids and phenolic acids, may offer protective benefits by reducing oxidative stress and inflammation (Varadhan et al., 2022). Through its antioxidant and anti-inflammatory properties, Bidens pilosa may help maintain cellular integrity, protect against membrane destabilization, and support overall physiological function (Aborode et al., 2022; Alqahtani et al., 2023; Tchekalarova and Tzoneva, 2023; Teleanu et al., 2022). The ability of B. pilosa to reduce MDA is important because lipid peroxidation is a main cause of cell membrane damage in diseases such as colitis and mucositis.
As well as direct radical removal, B. pilosa also activates the body’s internal antioxidant systems. Several studies show that in rodents and plants, extracts can boost the activity of SOD, CAT, POD and GSH (Tesfay et al., 2016; Mohamed et al., 2024; Abiodun et al., 2020). Because these enzymes are the first line of defense against oxidative stress, their increased levels by B. pilosa suggest both quick and lasting protection against redox imbalances. Removing radicals and supporting the body’s defense system makes the therapeutic potential of this plant more promising.
Importantly, these antioxidant activities are strongly associated with the phytochemical richness of B. pilosa, particularly its high total phenolic and flavonoid contents. Studies have consistently reported that flavonoids such as quercetin and phenolic acids like chlorogenic and caffeic acids contribute to its antioxidant effects by donating hydrogen atoms, chelating pro-oxidant metal ions, and modulating redox-sensitive signaling pathways (Yuniastri et al., 2022; Goudoum et al., 2016; Shandukani et al., 2018; Phiri et al., 2024). Through metal ion chelation, flavonoids including quercetin and kaempferol neutralize free radicals and prevent oxidative damage (Gulcin and Alwasel, 2022). By contributing hydrogen atoms to combat reactive oxygen species (ROS), phenolic acids, such as caffeic acid and chlorogenic acid, demonstrate strong radical-scavenging action (Mashinini et al., 2023). Additionally, by modulating oxidative stress signaling pathways, terpenoids like β-sitosterol strengthen cellular antioxidant defenses (Dwivedi et al., 2023; Liu et al., 2013)
These mechanisms collectively contribute to its broad-spectrum cytoprotective benefits, especially in inflammatory and oxidative-stress-related conditions.
The therapeutic utility of B. pilosa is further validated by in vivo models. Notably, formulations such as FITOPROT and Ecobidens® significantly attenuated oxidative stress and inflammatory markers in models of 5-fluorouracil-induced oral and intestinal mucositis, respectively (Bastos et al., 2016; de Ávila et al., 2015; Santos Filho et al., 2018). These findings highlight the practical relevance of B. pilosa-based formulations in mitigating chemotherapy-induced toxicity. Moreover, studies on B. pilosa tea demonstrated its effectiveness in reversing oxidative damage in rats fed a high-oxalate diet, including restoration of GSH levels and reduction in MDA concentrations (Mohamed et al., 2024).
Another noteworthy observation is that methanolic and acetonic extracts of B. pilosa have shown antioxidant activities comparable to, or even surpassing, those of synthetic antioxidants such as butylated hydroxytoluene (BHT) (Goudoum et al., 2016). This not only positions B. pilosa as a promising candidate for inclusion in nutraceutical and pharmaceutical formulations but also aligns with current trends favoring natural over synthetic antioxidant sources due to safety concerns.
Collectively, these findings affirm that B. pilosa possesses strong antioxidant potential mediated by both its phytochemical constituents and its ability to modulate oxidative stress pathways. Its versatile applications across disease models, dietary supplementation, and food preservation further underscore its therapeutic and preventive promise.
4.5 Anti-inflammatory potential of Bidens pilosa
Our review confirms that Bidens pilosa possesses potent anti-inflammatory properties, mediated by a broad range of bioactive compounds that influence multiple immunological and molecular pathways. The findings reported across diverse studies highlight the plant’s capacity to downregulate pro-inflammatory mediators while promoting anti-inflammatory responses, indicating its promising therapeutic potential in managing inflammatory conditions.
Notably, B. pilosa inhibits major pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-8, while concurrently upregulating IL-10, a critical anti-inflammatory cytokine (Bastos et al., 2016; Quaglio et al., 2020). These effects suggest that the plant may restore immune homeostasis, particularly in pathological states marked by cytokine imbalances. The suppression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) by isookanin, leading to decreased nitric oxide and prostaglandin E2 levels, further affirms the mechanistic depth of B. pilosa’s anti-inflammatory action (Xin et al., 2021). These outcomes are consistent with those of Caporali et al. (2022) and Chiang et al. (2004), who previously demonstrated that flavonoids in medicinal plants inhibit these same enzymes, key drivers of inflammation.
Additionally, B. pilosa modulates non-cytokine pathways involved in inflammation. It reduces histamine release from mast cells (Horiuchi and Seyama, 2008), inhibits the 5-lipoxygenase pathway (Akula and Odhav, 2008), and suppresses lymphocyte proliferation (Pereira et al., 1999), suggesting both early-phase and chronic inflammatory processes are targeted. These findings resonate with those by Tahya et al. (2023), who identified flavonoid glycosides in B. pilosa capable of regulating early immunologic responses.
Evidence from cellular models further strengthens these conclusions. Extracts from B. pilosa significantly inhibited IL-6 and nitric oxide production, downregulated NF-κB activation, and reduced myeloperoxidase (MPO) activity in macrophages and colon cell lines (Yan et al., 2022; Abiodun et al., 2020; Mohammed et al., 2024). These molecular effects suggest interference with canonical inflammatory signaling pathways such as NF-κB and MAPK, which are known to orchestrate a cascade of pro-inflammatory gene transcription (Wang et al., 2023; Detka et al., 2024). Importantly, the reduction in MPO activity and leukocyte infiltration in colitis models aligns with the anti-colitic effects previously reported by Abiodun et al. (2020) and Quaglio et al. (2020).
Animal studies provided compelling in vivo validation of these cellular findings. For instance, ethanol and aqueous extracts of B. pilosa significantly reduced paw edema and tissue inflammation in acute and chronic models (Fotso et al., 2014; Chih et al., 1995), while also mitigating cytokine expression in intestinal and renal tissues (Yang et al., 2018; Mohammed et al., 2024). These observations parallel those of Rodríguez Mesa et al. (2023), who reported that stigmasterol and friedelan-3-one in B. pilosa effectively attenuate inflammation through both COX-2 inhibition and antioxidant mechanisms.
Interestingly, the anti-inflammatory outcomes appear to be tissue-specific in certain delivery systems. For instance, FITOPROT-based mucoadhesive gels provided localized anti-inflammatory benefits without altering systemic cytokine profiles (Santos Filho et al., 2018). This property may be advantageous in the targeted treatment of oral and mucosal inflammations, as further demonstrated by Arantes et al. (2021b) in oral carcinoma and salivary epithelial models.
Despite these encouraging findings, the immunological effects of B. pilosa are not universally anti-inflammatory. Chang et al. (2005) reported that in certain conditions, the plant promotes a Th2-skewed immune response with elevated IgE levels, implying potential allergenic or pro-inflammatory consequences under specific immunological contexts. This duality underscores the importance of dosage, route of administration, and the physiological state of the host in mediating B. pilosa’s outcomes.
Collectively, these findings present B. pilosa as a multi-targeted anti-inflammatory agent with broad therapeutic applications. Its ability to modulate cytokine profiles, inflammatory enzymes, immune cell activity, and signaling pathways positions it as a strong candidate for managing inflammatory disorders such as colitis, arthritis, and mucosal inflammations. Given the increasing burden of chronic inflammatory diseases worldwide, especially in resource-limited settings, B. pilosa could be a cost-effective, plant-based therapeutic option.
Furthermore, its actions on oxidative and inflammatory pathways suggest potential for use in neuroinflammatory and metabolic conditions where similar mechanisms are implicated (Iqbal et al., 2025; Schreiner and Popescu, 2022). These findings also justify a deeper investigation into its role in modulating inflammasome activation, especially in diseases like Alzheimer’s and diabetes, where NLRP3 and oxidative stress play central roles (Qin et al., 2024; Alqahtani et al., 2023).
4.6 Limitations and future perspective
Although Bidens pilosa has many pharmacological uses, several obstacles prevent it from being used in clinical practice. Since there is a wide variety in what parts of plants are used, the solvents chosen, and the methods applied, it is difficult to compare results between studies. Due to the inconsistency in phytochemical analysis and no standard ways to dose herbal products, it is difficult to connect a dose with a specific effect and to pinpoint the active compounds. Also, because information on how these drugs are processed in the body and tested in humans is limited, it is difficult to apply findings to real clinical practice. The way phytochemicals work together to produce health benefits is not well understood. Despite these problems, the positive results from our systematic review highlight that more research is needed on Bidens pilosa to include it in health-promoting programs dealing with tissue toxicity, oxidative stress, lipid peroxidation, and inflammation.
To enhance a better use of B. pilosa, future studies should standardize how it is extracted and should test and measure all important active compounds in the plant. Bioassay-guided fractionation and using omics methods (metabolomics, transcriptomics and network pharmacology) can help find the targets and explain how they work. Evaluating the pharmacokinetics and toxicology of polyacetylenes and quercetin derivatives in major metabolites is essential. In particular, clinical trials that compare a treatment to a placebo and use standardized formulations should be given priority for conditions linked to oxidative stress and inflammation. Advanced computer models and systems biology methods should be used to study how various treatments work together and how to improve their results.
5 Conclusion
The review shows that Bidens pilosa is a medicinal plant with strong antioxidant and anti-inflammatory properties, confirmed by several in vitro and in vivo studies. The multiple metabolites in it, such as flavonoids, phenolic acids, polyacetylenes, and fatty acids, affect various biological functions and seem safe in animal studies. To make the most of its therapeutic benefits, people from different fields should collaborate to create standard ways, explore how it works, and confirm its safety and effectiveness in important clinical studies. With all these discoveries, B. pilosa has a strong chance to help develop useful plant-based treatments for diseases caused by oxidative stress, lipid peroxidation, and inflammation.
According to the findings of this systematic review, the following recommendations are presented to help direct future studies and the development of
Bidens pilosaas a standardized phytotherapeutic agent:
i) Standardization of Extracts and Fractions of B. pilosa plant parts: Creating standard, detailed procedures for extracting metabolites and active components, their proportions, the temperature, the time involved, and the quantity of phytochemicals yielded will enable easy comparison of outcomes and ensure reproducibility.
ii) Separating the most active metabolites: Working on isolating and structurally defining the main bioactive compounds such as flavonoids, phenolic acids, and polyacetylenes, by using HPLC, LC-MS/MS, and NMR will allow better targeting of pharmacological experiments and improvement of compounds.
iii) Performing in silico pharmacokinetic (ADME) and toxicological predictions: Predicting whether key metabolites are bioavailable, safe, and can be properly formulated using Lipinski’s Rule of Five and SwissADME will facilitate the drug discovery and design process.
iv) Employing Molecular Docking and Molecular Dynamic Simulation Approaches: Molecular docking can be used to predict how B. pilosa compounds interact with proteins related to oxidative stress, inflammation, and metabolic disorders (such as COX-2, TNF-α, Nrf2, MAPKs). In addition, molecular dynamics simulations should be carried out to understand how strongly the ligand binds, how its structure is affected, and how the interaction with the protein changes over time.
v) Performing long-term and disease-specific research: By carrying out long-term in vivo experiments with tested animal models, the long-term effects of B. pilosa’s metabolites can be revealed. These studies should target inflammation using LPS-induced systemic inflammation and not only carrageenan-induced paw edema models. Models of metabolic disorders, diabetes, neurodegeneration, and cancer should also be used, along with testing antiproliferative and cytotoxic effects on both human tumor xenografts and cancer cell cultures, in vitro.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
EE: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review and editing. IU: Conceptualization, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing. AO: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Writing – review and editing. VO: Conceptualization, Investigation, Methodology, Project administration, Visualization, Writing – review and editing. IJ: Formal Analysis, Investigation, Methodology, Project administration, Visualization, Writing – review and editing. WM: Conceptualization, Data curation, Methodology, Project administration, Visualization, Writing – review and editing. MD: Data curation, Formal Analysis, Investigation, Methodology, Resources, Writing – review and editing. EO: Investigation, Methodology, Project administration, Visualization, Writing – review and editing. AP: Data curation, Investigation, Methodology, Project administration, Visualization, Writing – review and editing. JI: Conceptualization, Data curation, Methodology, Project administration, Visualization, Writing – review and editing. IF: Conceptualization, Investigation, Methodology, Project administration, Visualization, Writing – review and editing. VA: Conceptualization, Investigation, Methodology, Project administration, Resources, Writing – review and editing. EA: Conceptualization, Methodology, Project administration, Supervision, Visualization, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1569527/full#supplementary-material
Abbreviations
Bidens pilosa, Bidens pilosa; SYRCLE, Systematic Review Centre for Laboratory Animal Experimentation; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RoB, – Risk of Bias; SciRAP, Science in Risk Assessment and Policy; TPC, Total Phenolic Content; TFC, Total Flavonoid Content; TTC, Total Tannin Content; TSC, Total Saponin Content; UV Spec, Ultraviolet Spectrophotometry; GC-MS, Gas Chromatography-Mass Spectrometry; HPLC, High-Performance Liquid Chromatography; LC-MS/MS, Liquid Chromatography-Tandem Mass Spectrometry; LC-HR-MS, Liquid Chromatography-High-Resolution Mass Spectrometry; NA, Not Applicable; CID, Compound Identifier.
References
1
Abdel-ghany R. H. Barakat W. M. Shahat A. Abd-allah W. E. Ali E. A. (2016). In vitro and in vivo hepatoprotective activity of extracts of aerial parts of Bidens pilosa L (asteraceae). Trop. J. Pharm. Res.15 (November), 2371–2381. 10.4314/tjpr.v15i11.11
2
Abiodun O. O. Sosanya A. S. Nwadike N. Oshinloye A. O. (2020). Beneficial effect of Bidens pilosa L. (asteraceae) in a rat model of colitis. J. Basic Clin. Physiology Pharmacol.31 (6), 1–9. 10.1515/jbcpp-2019-0166
3
Aborode A. T. Pustake M. Awuah W. A. Alwerdani M. Shah P. Yarlagadda R. et al (2022). Targeting oxidative stress mechanisms to treat alzheimer’s and parkinson’s disease: a critical review. Oxidative Med. Cell. Longev.2022 (1), 7934442. 10.1155/2022/7934442
4
Adeolu Adedapo F. J. A A. (2011). Comparison of the nutritive value and biological activities of the acetone, methanol and water extracts of the leaves of Bidens pilosa and Chenopodium album - PubMed. Acta Poloniae Pharm. ñ Drug Res.68 (1). 83ñ92, 2011.
5
Akula U. S. Odhav B. (2008). vitro 5-Lipoxygenase inhibition polyphenolic antioxidants undomesticated plants S. Afr.2 (9), 207–212.
6
Almeida N. C. O. S. Silva F. R. P. Carneiro A. L. B. Lima E. S. Barcellos J. F. M. Furtado S. C. (2021). Libidibia ferrea (Jucá) anti-inflammatory action: a systematic review of in vivo and in vitro studies. PLOS ONE16 (11), e0259545. 10.1371/journal.pone.0259545
7
Alqahtani T. Deore S. L. Kide A. A. Shende B. A. Sharma R. Dadarao Chakole R. et al (2023). Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease, and Parkinson’s disease, Huntington’s disease and amyotrophic lateral sclerosis -An updated review. Mitochondrion71, 83–92. 10.1016/J.MITO.2023.05.007
8
Angelini P. Matei F. Flores G. A. Pellegrino R. M. Vuguziga L. Venanzoni R. et al (2021). Metabolomic profiling, antioxidant and antimicrobial activity of Bidens pilosa. Processes9 (6), 903. 10.3390/PR9060903
9
Arantes D. A. C. da Silva A. C. G. Freitas N. M. A. Lima E. M. de Oliveira A. C. Marreto R. N. et al (2021a). Safety and efficacy of a mucoadhesive phytomedication containing curcuminoids and Bidens pilosa L. extract in the prevention and treatment of radiochemotherapy-induced oral mucositis: triple-Blind, randomized, placebo-controlled, clinical trial. Head Neck43 (12), 3922–3934. 10.1002/hed.26892
10
Arantes D. A. C. da Silva A. C. G. Lima E. M. Alonso E. C. P. Marreto R. N. Mendonça E. F. et al (2021b). Biological effects of formulation containing curcuminoids and Bidens pilosa L. in oral carcinoma cell line. Braz. Oral Res.35, e063. 10.1590/1807-3107BOR-2021.VOL35.0063
11
Ayuba J. T. Okesina A. A. Usman I. M. Ajenikoko M. K. Pius T. Kusiima N. et al (2022). Galinsoga parviflora restored associated motor coordination through increased linear distribution of purkinje cells in Mercury chloride-induced toxicity of mices cerebellum. Afr. J. Cell. Pathology14 (1), 1–8. 10.5897/AJCPATH2022.0035
12
Bartolome A. P. Villaseñor I. M. Yang W. (2013). Bidens pilosa L. (Asteraceae): botanical properties, traditional uses. Pharmacogn. Phytochem. 10.1155/2013/340215
13
Bastos C. C. C. Ávila P. H. M. de Filho E. X. dos S. Ávila R. I. de Batista A. C. et al (2016). Use of Bidens pilosa L. (asteraceae) and Curcuma longa L. (zingiberaceae) to treat intestinal mucositis in mice: toxico-Pharmacological evaluations. Toxicol. Rep.3, 279–287. 10.1016/j.toxrep.2015.10.013
14
Bhol N. K. Bhanjadeo M. M. Singh A. K. Dash U. C. Ojha R. R. Majhi S. et al (2024). The interplay between cytokines, inflammation, and antioxidants: mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. Biomed. and Pharmacother.178, 117177. 10.1016/J.BIOPHA.2024.117177
15
Caporali S. De Stefano A. Calabrese C. Giovannelli A. Pieri M. Savini I. et al (2022). Anti-inflammatory and active biological properties of the plant-derived bioactive compounds Luteolin and Luteolin 7-Glucoside. Nutrients. 14 (6), 1155. 10.3390/NU14061155
16
Chang C. L. T. Kuo H. K. Chang S. L. Chiang Y. M. Lee T. H. Wu W. M. et al (2005). The distinct effects of a butanol fraction of Bidens pilosa plant extract on the development of Th1-mediated diabetes and Th2-mediated airway inflammation in mice. J. Biomed. Sci.12 (1), 79–89. 10.1007/s11373-004-8172-x
17
Chiang Y. M. Chuang D. Y. Wang S. Y. Kuo Y. H. Tsai P. W. Shyur L. F. (2004). Metabolite profiling and chemopreventive bioactivity of plant extracts from Bidens pilosa. J. Ethnopharmacol.95 (2–3), 409–419. 10.1016/J.JEP.2004.08.010
18
Chih H. W. Lin C. C. Tang K. S. (1995). Anti-inflammatory activity of Taiwan folk medicine “ham-hong-chho” in rats. Am. J. Chin. Med.23 (3–4), 273–278. 10.1142/S0192415X95000328
19
Chipurura B. Muchuweti M. Bhebhe M. (2013). An assessment of the phenolic content, composition and antioxidant capacity of selected Indigenous vegetables of Zimbabwe. Acta Hortic.979, 611–620. 10.17660/ACTAHORTIC.2013.979.66
20
Cortés-Rojas D. F. Souza C. R. F. Oliveira W. P. (2011). Optimisation of the extraction of phenolic compounds and antioxidant activity from aerial parts of Bidens pilosa L. using response surface methodology. Int. J. Food Sci. Technol.46 (11), 2420–2427. 10.1111/J.1365-2621.2011.02765.X
21
Daglia M. Di Lorenzo A. F. Nabavi S. S. Talas Z. Nabavi M. (2014). Polyphenols: well beyond the antioxidant capacity: gallic acid and related compounds as neuroprotective agents: you are what you eat. Curr. Pharm. Biotechnol.15 (4), 362–372. 10.2174/138920101504140825120737
22
de Ávila P. H. M. de Ávila R. I. dos Santos Filho E. X. Cunha Bastos C. C. Batista A. C. Mendonça E. F. et al (2015). Mucoadhesive formulation of Bidens pilosa L. (asteraceae) reduces intestinal injury from 5-fluorouracil-induced mucositis in mice. Toxicol. Rep.2, 563–573. 10.1016/J.TOXREP.2015.03.003
23
Detka J. Płachtij N. Strzelec M. Manik A. Sałat K. (2024). p38α mitogen-activated protein kinase—an emerging drug target for the treatment of alzheimer’s disease. Molecules. 29 (18), 4354. 10.3390/MOLECULES29184354
24
Deba F. Xuan T. D. Yasuda M. Tawata S. (2007). Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa linn. Var. radiata. Food control.19 (4), 346–352. 10.1016/J.FOODCONT.2007.04.011
25
Dwivedi J. Sachan P. Wal P. (2023). A mechanistic approach on structural, analytical and pharmacological potential of beta-sitosterol: a promising nutraceutical. Curr. Nutr. and Food Sci.20 (8), 932–951. 10.2174/0115734013245468230927042947
26
Falowo A. B. Muchenje V. Hugo A. Aiyegoro O. A. Fayemi P. O. (2017). Antioxidant activities of Moringa oleifera L. and Bidens pilosa L. leaf extracts and their effects on oxidative stability of ground raw beef during refrigeration storage. CyTA - J. Food15 (2), 249–256. 10.1080/19476337.2016.1243587
27
Forman H. J. Zhang H. (2021). Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov.20 (9), 689–709. 10.1038/s41573-021-00233-1
28
Fotso A. F. Longo F. Djomeni P. D. D. Kouam S. F. Spiteller M. Dongmo A. B. et al (2014). Analgesic and antiinflammatory activities of the ethyl acetate fraction of Bidens pilosa (asteraceae). Inflammopharmacology22 (2), 105–114. 10.1007/s10787-013-0196-2
29
Goudoum A. Abdou A. B. Ngamo L. S. T. Ngassoum M. B. Mbofung C. M. F. (2016). Antioxidant activities of essential oil of Bidens pilosa (linn. Var. Radita) used for the preservation of food qualities in north Cameroon. Food Sci. Nutr.4 (5), 671–678. 10.1002/FSN3.330
30
Gulcin İ. Alwasel S. H. (2022). Metal ions, metal chelators and metal chelating assay as antioxidant method. Processes10 (1), 132. 10.3390/PR10010132
31
Hamedi A. Zengin G. Aktumsek A. Selamoglu Z. Pasdaran A. (2020). In vitro and in silico approach to determine neuroprotective properties of iridoid glycosides from aerial parts of Scrophularia amplexicaulis by investigating their cholinesterase inhibition and anti-oxidant activities. Biointerface Res. Appl. Chem.10 (3), 5429–5454. 10.33263/BRIAC103.429454
32
Hamsalakshmi Alex A. M. Arehally Marappa M. Joghee S. Chidambaram S. B. (2022). Therapeutic benefits of flavonoids against neuroinflammation: a systematic review. Inflammopharmacology30 (1), 111–136. 10.1007/S10787-021-00895-8
33
Hooijmans C. R. Rovers M. M. de Vries R. B. M. Leenaars M. Ritskes-Hoitinga M. Langendam M. W. (2014). SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol.14, 43. 10.1186/1471-2288-14-43
34
Horiuchi M. Seyama Y. (2008). Improvement of the antiinflammatory and antiallergic activity of Bidens pilosa L. Var. radiata SCHERFF treated with enzyme (cellulosine). J. Health Sci.54 (3), 294–301. 10.1248/jhs.54.294
35
Horiuchi M. Wachi H. Seyama Y. (2010). Effects of Bidens pilosa L. Var. radiata scherff on experimental gastric lesion. J. Nat. Med.64 (4), 430–435. 10.1007/s11418-010-0426-5
36
Idris O. A. Kerebba N. Horn S. Maboeta M. S. Pieters R. (2023). Phytochemical-based evidence of the health benefits of Bidens pilosa extracts and cytotoxicity. Chem. Afr.6 (4), 1767–1788. 10.1007/s42250-023-00626-2
37
Iqbal U. Malik A. Ibrahim L. Sial N. T. Mehmood M. H. (2025). Natural and synthetic potential drug leads for rheumatoid arthritis probing innovative target: mitochondrial dysfunction and NLRP3 inflammasome activation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1–18. 10.1007/S00210-025-03897-3/METRICS
38
Kalra S. Malik R. Singh G. Bhatia S. Al-Harrasi A. Mohan S. et al (2022). Pathogenesis and management of traumatic brain injury (TBI): role of neuroinflammation and anti-inflammatory drugs. Inflammopharmacology30 (4), 1153–1166. 10.1007/S10787-022-01017-8
39
Kaur B. Singh P. (2022). Inflammation: biochemistry, cellular targets, anti-inflammatory agents and challenges with special emphasis on cyclooxygenase-2. Bioorg. Chem.121, 105663. 10.1016/J.BIOORG.2022.105663
40
Kulkarni O. P. Lichtnekert J. Anders H. J. Mulay S. R. (2016). The immune system in tissue environments regaining homeostasis after injury: is “inflammation” always inflammation?Mediat. Inflamm.2016 (Figure 1), 2856213. 10.1155/2016/2856213
41
Kviecinski M. R. Felipe K. B. Correia J. F. G. Ferreira E. A. Rossi M. H. Gatti F. de M. et al (2011). Brazilian Bidens pilosa linné yields fraction containing quercetin-derived flavonoid with free radical scavenger activity and hepatoprotective effects. Libyan J. Med.6 (1), 1–8. 10.3402/LJM.V6I0.5651
42
Liu J. R. Ye Y. L. Lin T. Y. Wang Y. W. Peng C. C. (2013). Effect of floral sources on the antioxidant, antimicrobial, and anti-inflammatory activities of honeys in Taiwan. Food Chem.139 (1–4), 938–943. 10.1016/j.foodchem.2013.02.015
43
Mashinini P. P. Chihomvu P. Pillay M. Takaidza S. (2023). Phytochemical analysis and anti-mycobacterium activity of Bidens pilosa crude extracts. J. Biotech Res.15 (September), 116–137.
44
Memon F. Q. Yang Y. Lv F. Soliman A. M. Chen Y. Sun J. et al (2020). Eff. probiotics Bidens pilosa Perform. gut health Chick. Dur. Induc. E. tenella Infect.10.21203/RS.3.RS-18318/V1
45
Mohamed R. S. Ramadan M. M. Fouda K. Ghanem K. Z. Omara E. A. Abdel-Aziz S. A. (2024). Preventive impacts of black tea, green tea and bidens pilosa on renal stone formation in rats: antioxidant and anti-inflammatory pathways. Egypt. J. Chem.67 (12), 423–432. 10.21608/EJCHEM.2024.317701.10337
46
Moretti E. P. de L. Torres R. C. Laurindo K. Sloan L. F. Sloan K. P. Castro L. A. et al (2024). Glycolipid metabolic disorders, metainflammation, oxidative stress, and cardiovascular diseases: unraveling pathways. Biology13 (7), 519. 10.3390/BIOLOGY13070519
47
Mota C. M. Santiago F. M. Cardoso M. de R. D. Rostkowska C. de Oliveira T. C. Silva D. A. de O. et al (2019). Acetonic fraction of Bidens pilosa enriched for maturase K is able to control cerebral parasite burden in mice experimentally infected with Toxoplasma gondii. Front. Veterinary Sci.6 (MAR), 55–13. 10.3389/fvets.2019.00055
48
Muchuweti M. Mupure C. Ndhlala A. Murenje T. Benhura M. A. N. (2007). Screening of antioxidant and radical scavenging activity of vigna ungiculata, Bidens pilosa and Cleome gynandra. Am. J. Food Technol.2 (3), 161–168. 10.3923/AJFT.2007.161.168
49
Nguyen T. H. D. Vu D. C. Hanh P. Q. P. Vo X. T. Nguyen V. C. Nguyen T. N. et al (2023). Comparative analysis of phenolic content and in vitro bioactivities of Bidens pilosa L. flowers and leaves as affected by extraction solvents. J. Agric. Food Res.14, 100879. 10.1016/J.JAFR.2023.100879
50
Nxumalo K. A. Aremu A. O. Fawole O. A. (2023). Metabolite profiling, antioxidant and antibacterial properties of four medicinal plants from Eswatini and their relevance in food preservation. South Afr. J. Bot.162, 719–729. 10.1016/J.SAJB.2023.10.008
51
Oduntan A. O. Fasoyiro S. B. Akinfasoye J. A. Adeboyejo F. O. Akintoye H. A. (2018). Antioxidant and proximate properties of underutilized vegetables in Western Nigeria. Acta Hortic.1225, 255–260. 10.17660/ACTAHORTIC.2018.1225.35
52
Onohuean H. Alagbonsi A. I. Usman I. M. Iceland Kasozi K. Alexiou A. Badr R. H. et al (2021). Annona muricata linn and Khaya grandifoliola C.DC. Reduce oxidative stress in vitro and ameliorate plasmodium berghei-Induced parasitemia and cytokines in BALB/c mice. J. Evidence-Based Integr. Med.26, 2515690X211036669. 10.1177/2515690X211036669
53
Onohuean H. Onohuean E. F. Igbinoba S. Odoma S. Usman I. Ifie J. E. et al (2024). In silico pharmacokinetic and therapeutic evaluation of Musa acuminata peels against aluminium chloride-induced hepatotoxicity in adult BALB/c mice. Silico Pharmacol.12 (1), 46–15. 10.1007/s40203-024-00216-1
54
Ozsurekci Y. Aykac K. (2016). Oxidative stress related diseases in newborns. Oxidative Med. Cell. Longev. 20162016, 2768365. 10.1155/2016/2768365
55
Panfoli I. Candiano G. Malova M. De Angelis L. Cardiello V. Buonocore G. et al (2018). Oxidative stress as a primary risk factor for brain damage in preterm newborns. Front. Pediatr.6 (November), 369–6. 10.3389/fped.2018.00369
56
Pegoraro C. M. R. Nai G. A. Garcia L. A. Serra F. de M. Alves J. A. Chagas P. H. N. et al (2018). Protective effects of Bidens pilosa on hepatoxicity and nephrotoxicity induced by carbon tetrachloride in rats. Drug and Chemical Toxicology44 (1), 64–74. 10.1080/01480545.2018.1526182
57
Pereira R. L. C. Ibrahim T. Lucchetti L. Da Silva A. J. R. De Moraes V. L. G. (1999). Immunosuppressive and anti-inflammatory effects of methanolic extract and the polyacetylene isolated from Bidens pilosa L. Immunopharmacology43 (1), 31–37. 10.1016/S0162-3109(99)00039-9
58
Phiri H. Lumai A. Zombe K. Nyirenda J. (2024). Evaluation of antioxidant activity of selected wild fruits and vegetables from Zambia. Food and Humanity3, 100390. 10.1016/J.FOOHUM.2024.100390
59
Qin P. Sun Y. Li L. (2024). Mitochondrial dysfunction in chronic neuroinflammatory diseases (Review). Int. J. Mol. Med. 53 (5). 10.3892/IJMM.2024.5371/DOWNLOAD
60
Quaglio A. E. V. Cruz V. M. Almeida-Junior L. D. Costa C. A. R. A. Di Stasi L. C. (2020). Bidens pilosa (black jack) standardized extract ameliorates acute TNBS-Induced intestinal inflammation in rats. Planta Medica86 (05), 319–330. 10.1055/A-1089-8342
61
Rasul A. Al-Shawi A. A. Malik S. A. Anwar H. (2019). Neurada procumbens promotes functions regain in a mouse model of mechanically induced sciatic nerve injury.
62
Rauf A. Badoni H. Abu-Izneid T. Olatunde A. Rahman M. M. Painuli S. et al (2022). Neuroinflammatory markers: key indicators in the pathology of neurodegenerative diseases. Molecules27 (10), 3194. 10.3390/MOLECULES27103194
63
Rodríguez-Mesa X. M. Contreras Bolaños L. A. Mejía A. Pombo L. M. Modesti Costa G. Santander González S. P. (2023). Immunomodulatory properties of natural extracts and compounds derived from bidens pilosa L.: literature review. Pharmaceutics. 15 (5). 10.3390/pharmaceutics15051491
64
Roth N. Zilliacus J. Beronius A. (2021). Development of the SciRAP approach for evaluating the reliability and relevance of in vitro toxicity data. Frontiers in Toxicology3, 746430. 10.3389/ftox.2021.746430
65
Sadiq I. Z. (2021). Free radicals and oxidative stress: signaling mechanisms, redox basis for human diseases, and cell cycle regulation. Current Molecular Medicine23 (1), 13–35. 10.2174/1566524022666211222161637
66
Said W. Khattab A. A. Hamed S. A. Abo-Elmaaty S. A. Khalil H. (2024). Identification of bioactive and anticancer properties of Bidens pilosa in-vitro evidence. Asian Pacific Journal of Cancer Prevention APJCP25 (10), 3551–3558. 10.31557/APJCP.2024.25.10.3551
67
Salihu M. Batiha G. E. S. Kasozi K. I. Zouganelis G. D. Sharkawi S. M. Z. Ahmed E. I. et al (2022). Crinum jagus (J. Thomps. Dandy): Antioxidant and protective properties as a medicinal plant on toluene-induced oxidative stress damages in liver and kidney of rats. Toxicology Reports9, 699–712. 10.1016/J.TOXREP.2022.03.026
68
Santos Filho E. X. dos Arantes D. A. C. Oton Leite A. F. Batista A. C. Mendonça E. F. de Marreto R. N. et al (2018). Randomized clinical trial of a mucoadhesive formulation containing curcuminoids (zingiberaceae) and Bidens pilosa linn (asteraceae) extract (FITOPROT) for prevention and treatment of oral mucositis - phase I study. Chemico-Biological Interactions291, 228–236. 10.1016/J.CBI.2018.06.010
69
Schreiner T. G. Popescu B. O. (2022). Impact of Caffeine on alzheimer’s disease pathogenesis—protective or risk factor?Life. 12 (3), 330. 10.3390/LIFE12030330
70
Selamoglu T. Z. Ozdemir I. Yilmaz I. Gok Y. Orun I. (2008). The investigation of the antioxidative properties of the novel synthetic organoselenium compounds in some rat tissues. 233(5), 575–579. 10.3181/0707-RM-191
71
Selamoglu-Talaz Z. Ozdemir I. Ciftci O. Cakir O. (2013). Propolis attenuates oxidative injury in brain and lung of nitric oxide synthase inhibited rats. Journal of Pharmaceutical Care, 45–50. Available online at: https://jpc.tums.ac.ir/index.php/jpc/article/view/11.
72
Sevindik M. Akgul H. Selamoglu Z. Braidy N. (2021). Antioxidant, antimicrobial and neuroprotective effects of Octaviania asterosperma in vitro. Mycology12 (2), 128–138. 10.1080/21501203.2020.1816584
73
Shandukani P. D. Tshidino S. C. Masoko P. Moganedi K. M. (2018). Antibacterial activity and in situ efficacy of Bidens pilosa Linn and Dichrostachys cinerea Wight et Arn extracts against common diarrhoea-causing waterborne bacteria. BMC Complementary and Alternative Medicine18 (1), 171. 10.1186/S12906-018-2230-9
74
Silva F. L. Fischer D. C. H. Tavares J. F. Silva M. S. De Athayde-Filho P. F. Barbosa-Filho J. M. (2011). Compilation of secondary metabolites from Bidens pilosa L. Molecules16 (2), 1070–1102. 10.3390/molecules16021070
75
Singh G. Passsari A. K. Singh P. Leo V. V. Subbarayan S. Kumar B. et al (2017). Pharmacological potential of Bidens pilosa L. and determination of bioactive compounds using UHPLC-QqQLIT-MS/MS and GC/MS. BMC Complementary and Alternative Medicine17 (1), 1–16. 10.1186/S12906-017-2000-0/TABLES/9
76
Son N. H. Tuan N. T. Tran T. M. (2022). Investigation of chemical composition and evaluation of antioxidant, antibacterial and antifungal activities of ethanol extract from Bidens pilosa L. Food Science and Technology42, e22722. 10.1590/FST.22722
77
Sureda A. Tejada S. Mir Khan U. Selamoglu Z. (2023). An overview of the biological function of curcumin in the processes of oxidative stress, inflammation, nervous system, and lipid levels. Central Asian Journal of Medical and Pharmaceutical Sciences Innovation3 (1), 1–11. 10.22034/CAJMPSI.2023.01.01
78
Tahya C. Y. Karnelasatri Gaspersz N. (2023). Chemical profiling and histamine inhibitory activity assessment of merremia vitifolia and bidens pilosa extracts. Molekul. 18 (1), 117–130. 10.20884/1.jm.2023.18.1.6833
79
Tchekalarova J. Tzoneva R. (2023). Oxidative stress and aging as risk factors for alzheimer’s disease and parkinson’s disease: the role of the antioxidant melatonin. International Journal of Molecular Sciences24 (3), 3022. 10.3390/ijms24033022
80
Teleanu D. M. Niculescu A. G. Lungu I. I. Radu C. I. Vladâcenco O. Roza E. et al (2022). An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. International Journal of Molecular Sciences23 (11), 5938. 10.3390/IJMS23115938
81
Tesfay S. Z. Mathe S. Modi A. T. Mabhaudhi T. (2016). A comparative study on antioxidant potential of selected African and exotic leafy vegetables. HortScience51 (12), 1529–1536. 10.21273/HORTSCI11161-16
82
Tsuruta K. Shidara T. Miyagishi H. Nango H. Nakatani Y. Suzuki N. et al (2023). Anti-inflammatory effects of miyako Bidens pilosa in a mouse model of amyotrophic lateral sclerosis and lipopolysaccharide-stimulated BV-2 microglia. International Journal of Molecular Sciences24 (18), 13698. 10.3390/IJMS241813698
83
Usman I. Buraimoh A. Ibegbu A. (2016). Histological and biochemical studies of Tamarindus indica pulp extract on the cerebral cortex in prenatal ethanol exposure in wistar rats. Journal of Experimental and Clinical Anatomy15 (2), 96. 10.4103/1596-2393.200919
84
Usman I. M. Adebisi S. S. Musa S. A. Iliya I. A. Archibong V. B. Lemuel A. M. et al (2022). Tamarindus indica ameliorates behavioral and cytoarchitectural changes in the cerebellar cortex following prenatal aluminum chloride exposure in wistar rats. Anatomy and Cell Biology55, 320–329. 10.5115/acb.22.033
85
U-Yatung S. Suebsaiprom W. Pornprom T. Chompoo J. (2020). Performance of some Thai weed extracts on antioxidants and atherosclerosis-related enzymes. Agrivita42 (2), 243–254. 10.17503/AGRIVITA.V0I0.2322
86
Varadhan S. Venkatachalam R. Perumal S. M. Ayyamkulamkara S. S. (2022). Evaluation of oxidative stress parameters and antioxidant status in coronary artery disease patients. Archives of Razi Institute77 (2), 853–859. 10.22092/ARI.2022.357069.1965
87
Wang Z. Fang C. Yao M. Wu D. Chen M. Guo T. et al (2023). Research progress of NF-κB signaling pathway and thrombosis. Front. Immunol. 14, 1257988. 10.3389/FIMMU.2023.1257988/XML
88
Wazir M. Olanrewaju O. A. Yahya M. Kumari J. Kumar N. Singh J. et al (2023). Lipid disorders and cardiovascular risk: a comprehensive analysis of current perspectives. Cureus15, e51395. 10.7759/cureus.51395
89
Wu J. Wan Z. Yi J. Wu Y. Peng W. Wu J. (2012). Investigation of the extracts from Bidens pilosa linn. Var. radiata sch. Bip. For antioxidant activities and cytotoxicity against human tumor cells. Journal of Natural Medicines 201267 (1), 17–26. 10.1007/S11418-012-0639-X
90
Xin Y.-J. Choi S. Roh K.-B. Cho E. Ji H. Weon J. B. et al (2021). Anti-inflammatory activity and mechanism of isookanin, isolated by bioassay-guided fractionation from Bidens pilosa L. Molecules26 (2), 255. 10.3390/molecules26020255
91
Yan Z. Chen Z. Zhang L. Wang X. Zhang Y. Tian Z. (2022). Bioactive polyacetylenes from Bidens pilosa L and their anti-inflammatory activity. Natural Product Research36 (24), 6353–6358. 10.1080/14786419.2022.2029432
92
Yang H. L. Chen S. C. Chang N. W. Chang J. M. Lee M. L. Tsai P. C. et al (2006). Protection from oxidative damage using Bidens pilosa extracts in normal human erythrocytes. Food and Chemical Toxicology44 (9), 1513–1521. 10.1016/J.FCT.2006.04.006
93
Yang Y. Yu K. Zhang Y. M. (2018). The cardioprotective effects of 4-O-(2″-O-acetyl-6″-O- P-coumaroyl-β-D-glucopyranosyl)-P-coumaric acid (4-ACGC) on chronic heart failure. Iranian Journal of Pharmaceutical Research IJPR17 (2), 593–600. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC5985177/.
94
Yi J. Wu J. G. Wu Y. B. Peng W. (2016). Antioxidant and anti-proliferative activities of flavonoids from Bidens pilosa L Var radiata sch bip. Tropical Journal of Pharmaceutical Research15 (2), 341–348. 10.4314/TJPR.V15I2.17
95
Yousaf M. Chang D. Liu Y. Liu T. Zhou X. (2022). Neuroprotection of cannabidiol, its synthetic derivatives and combination preparations against microglia-mediated neuroinflammation in neurological disorders. Molecules27 (15), 4961. 10.3390/MOLECULES27154961
96
Yuan L. P. Chen F. H. Ling L. Dou P. F. Bo H. Zhong M. M. et al (2008). Protective effects of total flavonoids of Bidens pilosa L. (TFB) on animal liver injury and liver fibrosis. Journal of Ethnopharmacology116 (3), 539–546. 10.1016/J.JEP.2008.01.010
97
Yuniastri R. Huzaimah N. Estiasih T. Martati E. Tarmadi D. Fatriasari W. et al (2022). A comparative evaluation of the antioxidant activity of local plants originated from sumenep regency, east java, indonesia. 87–94. 10.31788/RJC.2022.1558120
98
Yusuf H. R. Musa S. A. Agbon A. N. Eze E. D. Okesina A. A. Onanuga I. et al (2023). Hepatoprotective potential of Tamarindus indica following prenatal aluminum exposure in wistar rat pups. Toxicology Reports10, 376–381. 10.1016/J.TOXREP.2023.03.002
Summary
Keywords
Bidens pilosa , cytoprotection, antioxidant properties, anti-inflammatory properties, systematic review
Citation
Etukudo EM, Usman IM, Oviosun A, Ojiakor VO, Jama IA, Makena W, Makeri D, Owembabazi E, Aja PM, Ifie J, Fasogbon IV, Archibong VB and Anyanwu E (2025) Exploring the phytochemical profile, antioxidant and anti-inflammatory potential of Bidens pilosa: A Systematic Review. Front. Pharmacol. 16:1569527. doi: 10.3389/fphar.2025.1569527
Received
01 February 2025
Accepted
30 June 2025
Published
01 August 2025
Volume
16 - 2025
Edited by
Michael Heinrich, University College London, United Kingdom
Reviewed by
Zeliha Selamoglu, Niğde Ömer Halisdemir University, Türkiye
Tshepo Mashela, University of Limpopo, South Africa
Cica Vissiennon, Leipzig University, Germany
Updates
Copyright
© 2025 Etukudo, Usman, Oviosun, Ojiakor, Jama, Makena, Makeri, Owembabazi, Aja, Ifie, Fasogbon, Archibong and Anyanwu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ibe Michael Usman, gopama13@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.