Abstract
Ceftazidime/avibactam is effective for treating infections caused by multidrug-resistant gram-negative bacteria and is widely used. The pharmacokinetic data of ceftazidime and avibactam in patients receiving continuous renal replacement therapy (CRRT) are limited. It is challenging to dose ceftazidime/avibactam, as excessive exposure is associated with central nervous system (CNS) adverse events, especially in older patients. This case reported the pharmacokinetic parameters of ceftazidime and avibactam (1.25 g every 8 h) in an elderly patient during and after CRRT (continuous veno venous hemofiltration mode), which were estimated based on a first-order elimination equation and a two-point sampling strategy. CRRT accounted for 84.9% of the total clearance rate of ceftazidime and 77.1% of the total clearance rate of avibactam. Excessive drug exposure (plasma concentrations of ceftazidime and avibactam were 109 and 20.6 mg/L, respectively) 5 days after discontinuation of CRRT resulted in adverse CNS reactions, which manifested as involuntary convulsions and abnormal brain discharge. This case study provides pharmacokinetic data of ceftazidime and avibactam in patient during and after CRRT and information about the possible relationship between concentrations and CNS adverse reactions.
1 Introduction
Ceftazidime/avibactam is a novel β-lactam/β-lactamase inhibitor antibiotic used to address the increasing prevalence of multidrug-resistant (MDR) gram-negative bacteria (GNB), such as carbapenem-resistant Enterobacteriaceae and Pseudomonas aeruginosa (Thomsen et al., 2023; Govindaraj and Vanitha, 2018). It has demonstrated high effectiveness in the treatment of complex abdominal infections, hospital-acquired pneumonia and ventilator-associated pneumonia caused by these pathogens (Tacconelli et al., 2018; Yu et al., 2023).
With the increasing use of ceftazidime/avibactam, its safety profile receives attention. Central nervous system (CNS)-related adverse events are among the most reported severe events in clinical trials and post-marketing reports (Torres et al., 2018; Carmeli et al., 2016). It has been reported that ceftazidime/avibactam is potentially associated with nervous system disorders in patients older than 65 years and with long treatment courses (Guo et al., 2022). Importantly, the occurrence of adverse events in the CNS is often concentration dependent (Martinez-Rodriguez et al., 2001; Chuang et al., 2004). As ceftazidime and avibactam are mainly eliminated by kidney, patients with chronic kidney disease (CKD) are much more susceptible to high-concentration exposure because of decreased renal clearance. Moreover, these patients frequently receive continuous renal replacement therapy (CRRT) when admitted to the ICU (Aloy et al., 2020). There are limited data regarding the pharmacokinetic parameters for CKD patients during CRRT and after its cessation, and it is difficult to dose ceftazidime/avibactam in these patients (Yu et al., 2023; Collignon et al., 2024). Moreover, although CNS adverse events associated with ceftazidime/avibactam have been reported, the threshold concentration is unknown.
Therefore, this case reports the pharmacokinetic parameters of ceftazidime and avibactam in an elderly CKD patient during and after CRRT. Moreover, CNS adverse events caused by ceftazidime/avibactam were observed in this patient, which allowed us to estimate the possible associations between CNS adverse effects and drug exposure.
2 Case presentation
A 91-year-old male patient (body weight 60kg, height 168 cm) was admitted to the hospital due to severe COVID-19 infection more than 1 year prior. Owing to disturbances in consciousness and low oxygen saturation, the patient was transferred to the intensive care unit (ICU) for monitoring after admission. The patient had a long stay in the ICU because his respiratory function could not recover, and mechanical ventilation could not be removed. He had a history of hypertension, gout, chronic kidney disease (G3b--G4, classified by Kidney Disease Improving Global Outcomes), nephrolithiasis and kidney cysts for more than 30 years. During hospitalization in the ICU, he experienced left cerebral hemorrhage (6 months after admission), extensive left cerebral infarction (6 months after admission), subfalcine herniation, arrhythmia (paroxysmal atrial fibrillation) and gastrointestinal bleeding. He received CRRT for approximately 5 times every month due to renal failure. In our hospital, the duration of CRRT is usually 72 h as filters need to be replaced after such a period, and the physician would decide whether a next round of CRRT is needed.
After 11 months of hospitalization in the ICU, the patient developed hospital-acquired pneumonia, and the pathogen was carbapenem-resistant Klebsiella pneumoniae, which was identified via sputum culture. The patient experienced an increase in body temperature and infection indicators. The initial antibiotic regimen was 50 mg of tigecycline every 12 h combined with 1.00 g/0.25 g of ceftazidime/avibactam every 8 h over 1-h infusion (The first day of ceftazidime/avibactam administration is defined as Day 1). The patient was undergoing CRRT, and the dosing of ceftazidime/avibactam was selected on the basis of the most used recommendation (Nekidy et al., 2023). The patient was subjected to CRRT in continuous veno venous hemofiltration (CVVH) mode with a dose of 38.6 mL/kg/h (a post-dilution replacement fluid rate of 2,000 mL/h, and an ultrafiltration rate of 50 mL/h). Other related medications, body temperature and laboratory tests of the patient are detailed in Table 1. There was a significant decrease in body temperature and inflammatory marker levels over the next several days.
TABLE 1
| DAY | Day-3 | Day-2 | Day-1 | Day1 | Day2 | Day3 | Day4 | Day5 | Day6 | Day7 | Day8 | Day9 | Day10 | Day11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event | TDM | Occasional tremor can be seen | Severe AE and TDM | |||||||||||
| CRRT | ||||||||||||||
| CAZ/AVB | ||||||||||||||
| Tigecycline | ||||||||||||||
| Voriconazole | ||||||||||||||
| Methylprednisolone | ||||||||||||||
| Amiodarone | ||||||||||||||
| 24-hour urine output (mL) | 850 | 800 | 1050 | 1650 | 1150 | 800 | 1300 | 1350 | 2150 | 1700 | 1000 | 1400 | ||
| 24-hour fluid input/output (mL) | 1807/1710 | 2055/1430 | 2804/1800 | 1924/2721 | 1340/2794 | 1775/1410 | 2627/1760 | 2534/1910 | 2430/2660 | 2379/2350 | ||||
| hsCRP (mg/L) | 23.1 | 23.3 | 73.2 | 182.8 | 198.2 | 205.2 | 136.4 | 79.5 | 39.2 | 28.4 | 19 | 12.2 | 10.4 | 9.6 |
| PCT (ng/ml) | 0.26 | 0.21 | 0.37 | 0.63 | 0.74 | 0.82 | 0.64 | 0.62 | 0.51 | 0.48 | 0.41 | 0.31 | 0.28 | 0.21 |
| IL-6 (pg/ml) | - | - | - | 1111 | 820.8 | 173.5 | 116.3 | 46.5 | 34.6 | 29.1 | 30.2 | 21.8 | 22.2 | 53.2 |
| WBC (109/L) | 14.4 | 12.9 | 13.1 | 13.2 | 11.3 | 13.5 | 10.3 | 11.3 | 11.4 | 12.4 | 10.3 | 7.1 | 6.9 | 5.6 |
| N% | 90.1 | 92 | 88.3 | 90.2 | 91.6 | 94.7 | 92.8 | 91.4 | 91.4 | 93.1 | 93.4 | 90.1 | 90.1 | 87.6 |
| T | 37.8 | 37.8 | 38.3 | 38 | 39 | 36.8 | 37.2 | 37.3 | 37.2 | 37.1 | 37.1 | 36.9 | 36.8 | 37.9 |
| SCR (umol/L) | 95 | 60 | 79 | 118 | 150 | 161 | 64 | 88 | 112 | 123 | 129 | 128 | 119 | 110 |
Treatment course and laboratory test results.
Note: The colored sections in the table represent the use of items on the specified days. CRRT, continuous renal replacement therapy; CAZ/AVB, ceftazidime/avibactam (1.25 g q8h via 1-h infusion, the dose was not changed from Day1 to Day9); TDM, therapeutic drug monitoring; AE, adverse event; hsCRP, hypersensitive C-reactive protein; PCT, procalcitonin; IL-6, interleukin-6; WBC, white blood cell; N%, neutrophil granulocyte; T, body temperature; SCR, serum creatinine.
The patient underwent TDM with ceftazidime/avibactam using CRRT (CVVH model) on day 3 after the sixth dose. CRRT was ceased on day 4 but the dose of ceftazidime/avibactam was not changed, which may be excessive for CKD patients without CRRT. Occasional tremors and increased heart rates can be observed in this patient over the next several days. On Day 10, the patient experienced severe tremor of the whole body and an increased heart rate above 100 beats per minute. In particular, the patient’s left upper limb presented with involuntary convulsions. The patient’s Glasgow Coma Scale (GCS) score at that time was 4 + T + 5, which was the same as before ceftazidime/avibactam use. The patient’s creatine clearance (CrCL) during the period when patient experienced severe tremor and elevated heart rate was difficult to estimate due to instable renal function, but the high serum creatine level (1.35 mg/dL) indicated low drug clearance. The moderate to highly abnormal EEG on Day 10 revealed that the α waves could not be traced and that moderate low-amplitude fast waves were visible, with low to moderate 4–5 Hz medium-amplitude θ waves and more 2–3 Hz medium to high-amplitude unilateral δ waves and irregular recombination δ diffuse distributions, with basic symmetry on both sides (Figure 1, top half). During the process, the patient’s left upper limb was involuntarily flapped, and EEG revealed synchronized motion artifacts with similar rhythms (Figure 1, bottom half, blue line in the figure). We hypothesized that this was a CNS adverse reaction associated with ceftazidime/avibactam. The Naranjo score was used to evaluate the relationship between the drug and adverse events, and the score was 8 (details in Supplementary Table S1), which indicated a “Probable” relationship. Ceftazidime/avibactam was suspended, and a midazolam injection was administered to alleviate symptoms. We collected the blood samples at 17 h and 24 h after the last dose. The patient still presented mild CNS adverse reactions 24 h after the last dose, which may provide information about possible relationship between concentrations and CNS adverse reactions.
FIGURE 1
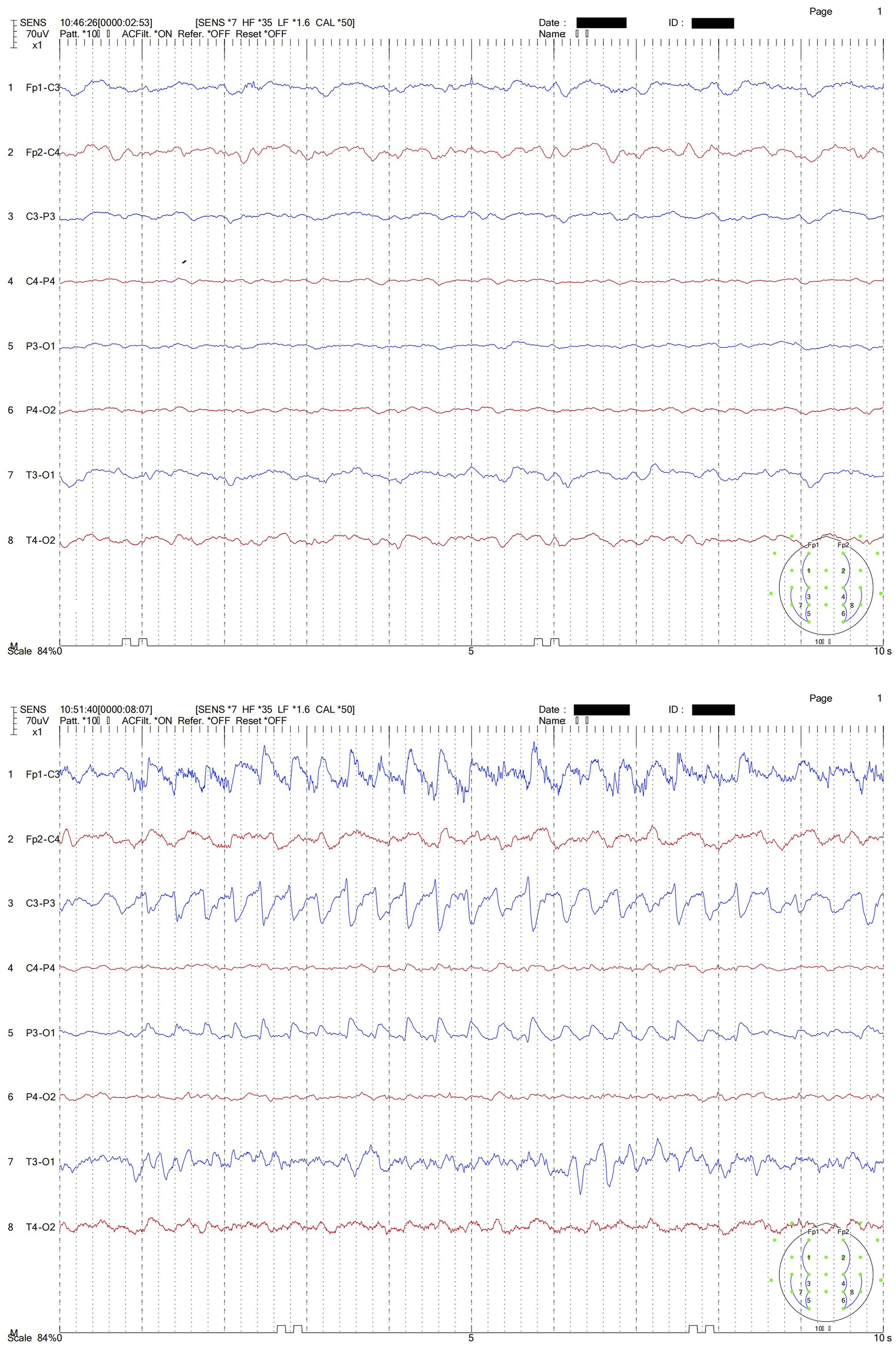
EEG of the patient. Note: The top half shows the abnormal slow waves in EEG; the bottom half shows the synchronized motion artifacts in EEG during the patient’s left upper limb rhythmic involuntary flapping.
3 PK parameter calculation
Blood samples were collected at predetermined time points, and the plasma was separated via centrifugation at 3,000 g for 10 min and then stored at −80°C before analysis. The concentrations were determined via a validated liquid chromatography–tandem mass spectrometry (Ab Sciex Triple Quad™ 4500 LC‒MS/MS) method (Cheng et al., 2022). The pharmacokinetics of both ceftazidime and avibactam could be considered one-compartment models with first-order elimination (Li et al., 2024). Therefore, PK parameters for ceftazidime and avibactam during and after CRRT were estimated via the Equations 1–5 below.where k is the elimination rate constant, t1/2 is the half-life, Cpeak is the peak blood drug concentration after infusion, tn represents the corresponding time and Cn represents the corresponding blood drug concentration. We assumed that Non-CRRT drug clearance remained constant during these days.
The sampling times after the 6th (undergoing CRRT) and 27th (after the cessation of CRRT for 6 days) administrations and the corresponding concentrations at the respective time points are listed in Table 2. The PK parameters of ceftazidime and avibactam are also listed in Table 2. CRRT accounted for 84.9% of the total elimination of ceftazidime and 77.1% of the total elimination of avibactam during CRRT treatment days.
TABLE 2
| Ceftazidime | Avibactam | |||
|---|---|---|---|---|
| Day3 (on CRRT) | Day10 (off CRRT) | Day3 (on CRRT) | Day10 (off CRRT) | |
| Time of sampling (after dose) and concentrations (mg/L) |
4 h: 63.6 8 h: 36.5 |
17 h: 127 24 h: 109 |
4 h: 16.0 8 h: 10.7 |
17 h: 24.1 24 h: 20.6 |
| Extrapolated Cpeak (mg/L) | 114.7 | 181.9 | 24.5 | 35.8 |
| kTotal (h-1) | 0.139 | - | 0.100 | - |
| kNon-CRRT (h-1) | - | 0.0210 | - | 0.0229 |
| kCRRT (h-1) | 0.118 | 0.0771 | ||
| T1/2 (h) | 4.99 | 33.0 | 6.89 | 30.3 |
Pharmacokinetic parameters of ceftazidime/avibactam during and after CRRT.
Note: k, elimination rate constant; T1/2, half-life. On Day 3, the results were under the CRRT; on Days 10, the results were obtained after the cessation of CRRT, for 6 days and 24 h after the cessation of administration.
4 Discussion
This case reported the pharmacokinetics of ceftazidime and avibactam during and after CRRT in an elderly patient with CKD and, for the first time, related the plasma concentration of ceftazidime/avibactam to its adverse effects on the CNS. The PK parameters revealed that after the withdrawal of CRRT, the total drug clearance decreased, and the drug accumulated, leading to a severe increase in drug exposure in patients. Clinical and EEG manifestations of CNS adverse events caused by ceftazidime/avibactam accumulation were presented, which occurred when the total plasma concentrations of ceftazidime and avibactam were above 109 and 20.6 mg/L, respectively.
There is limited clinical evidence regarding the use of ceftazidime/avibactam in patients undergoing CRRT. Comprehensive data on its pharmacokinetics under CRRT (CVVH) conditions remain insufficient. An intro study found that for avibactam, CL of CRRT ranged from 15.07 to 18.82 mL/min for CVVH and CVVHD (Alarcia-Lacalle et al., 2023). Wenzler’s study evaluated the pharmacokinetics in a critically ill patient undergoing CVVH (1.25 g q8h for ceftazidime/avibactam; the blood and ultrafiltration flow rates were fixed at 200 mL/min and 2 L/h, respectively, and replacement fluid was added prefiltration for CVVH). Drug concentrations and postfiltration and ultrafiltrate concentrations were measured. The t1/2 for ceftazidime was 6.07 h, whereas that for avibactam was 6.78 h. The k value of ceftazidime was inferred to be 0.114 h−1, whereas the k value of avibactam was inferred to be 0.102 h−1. These results, especially those of avibactam, are similar to ours. This study also revealed that CVVH accounted for 57.1% of the total clearance of ceftazidime and 54.3% of the total clearance of avibactam, as calculated on the basis of postfiltration and ultrafiltrate concentrations (Wenzler et al., 2017). Soukup et al. reported a case of clearance under CVVHDF mode (2.5 g, q8 h), with half-lives of 5.17 h and 5.92 h, respectively. However, the CRRT clearance rate was not calculated separately (Soukup et al., 2019). Zhang et al. reported a clearance case under CVVHD mode (2.5 g q12 h), with half-lives of 4.99 h and 9.93 h, respectively, but CRRT clearance rates were also not mentioned in the report (Zhang et al., 2022). A recent study including 4 CVVHDF patients found that the median total clearance and volume of distribution were 4.54 L/h and 73.2 L for ceftazidime and 10.5 L/h and 102 L for avibactam, respectively (O'Jeanson et al., 2025). Although the variance of parameters from different studies are not large, the CRRT modality has a significant effect on drug clearance, and those cases are less informative for CVVH patients. Moreover, there are many potential factors that would influence drug clearance, such as CRRT setting and patients’ residual renal function. This case has added pharmacokinetic data of ceftazidime and avibactam in CRRT patients, which would benefit future dosing in these patients. Additionally, we used first-order elimination equation and a two-point sampling strategy to estimate the PK parameters during CRRT. The steady status is not required for this method and it would be more practical in clinical scenarios. In this case, the half-lives of ceftazidime and avibactam under CRRT were 4.99 and 6.89 h, respectively. These results were similar to previous report in patients with various model of CRRT. When CRRT was ceased, the half-lives of ceftazidime and avibactam were prolonged significantly. Although the parameters of this case have large uncertainty, it is important to reduce the dose after the withdrawal of CRRT.
Encephalopathy, seizure and myoclonus are the most common CNS adverse events associated with ceftazidime/avibactam, and their occurrence may be related to drug concentrations (Guo et al., 2022). However, the threshold concentration is unknown. Fortunately, some cases of ceftazidime-induced encephalopathy are related to drug exposure. Suda reported a case of nonconvulsive status epilepticus as an adverse reaction to ceftazidime. The ceftazidime dosing regimen was 1000 mg q12 h, and the peak plasma concentration collected after the adverse reaction occurred was 105.2 μg/mL (Vannaprasaht et al., 2006). Chuang et al. reported a case of ceftazidime-induced Creutzfeldt‒Jakob-like EEG, with a peak drug concentration reaching 480 μg/mL before hemodialysis (Chuang et al., 2004). However, Guo et al. noted that the combination of ceftazidime and avibactam is associated with a greater likelihood of encephalopathy and myoclonus than ceftazidime alone, and the threshold would also be different (Guo et al., 2022). The clinical and EEG manifestations of CNS adverse events caused by ceftazidime/avibactam accumulation were presented in our patient. When the ADR occurred, the plasma concentrations of ceftazidime and avibactam were above 109 and 20.6 mg/L, respectively. Subsequent midazolam was administered to control for adverse events, and further ADR-exposure relationships could not be observed. Our study provides potential information for the possible relationship between concentrations of ceftazidime/avibactam and CNS adverse reactions. Continuous infusion or prolonged infusion is a promising dosing strategy for ceftazidime/avibactam, as it may achieve PK/PD target at a lower dose than intermittent infusion (Han et al., 2024). It should be noted that some studies have reported similar drug levels in patients without CNS events (Fresan et al., 2023; Yasmin et al., 2023), and the link to CNS toxicity in this case is speculative.
This case study has several limitations. Although the Naranjo score indicated a possible relationship between ceftazidime/avibactam use and ADR, the polypharmacy and underlying diseases would be confounding factors. The PK parameters were calculated based on limited samples, which may cause random error in parameter estimation. Considering the additional uncertainty in clinical setting, the estimated PK parameters should be interpreted with caution. The eGFR of patients, especially post-CRRT, was difficult to be estimated, which would undermine the strength of clearance distribution. With respect to the exposure‒ADR relationship, the drug concentrations in cerebrospinal fluid may be relatively important. However, we did not perform lumbar puncture to obtain and measure drug concentrations in the cerebrospinal fluid due to safety and benefit-risk evaluations, and this should be investigated in future studies.
5 Conclusion
This case reported the pharmacokinetic parameters of ceftazidime/avibactam in an elderly patient during and after CRRT. CRRT constituted approximately 80% of the total clearance of ceftazidime and avibactam. Owing to the removal of CRRT and elevated plasma drug concentrations, excessive drug exposure has caused severe CNS adverse events, as proven by clinical manifestations and EEG. The pharmacokinetic data of this case could benefit future dosing in these patients and finding a safety threshold. More data about the pharmacokinetics in CRRT patients are needed, as well as the upper limit of exposure to ceftazidime/avibactam.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the patient’s legal representative for the publication of any potentially identifiable images or data included in this article.
Author contributions
HH: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Writing – review and editing. YH: Data curation, Formal Analysis, Visualization, Writing – original draft. YW: Investigation, Project administration, Resources, Supervision, Writing – original draft. FG: Conceptualization, Project administration, Resources, Supervision, Writing – review and editing. ZY: Conceptualization, Supervision, Validation, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1569715/full#supplementary-material
References
1
Alarcia-Lacalle A. Barrasa H. Maynar J. Canut-Blasco A. Solinis M. A. Isla A. et al (2023). Ceftaroline and avibactam removal by continuous renal replacement therapies: an in vitro study. Blood Purif.52 (5), 464–473. 10.1159/000529264
2
Aloy B. Launay-Vacher V. Bleibtreu A. Bortolotti P. Faure E. Filali A. et al (2020). Antibiotics and chronic kidney disease: dose adjustment update for infectious disease clinical practice. Med. Mal. Infect.50 (4), 323–331. 10.1016/j.medmal.2019.06.010
3
Carmeli Y. Armstrong J. Laud P. J. Newell P. Stone G. Wardman A. et al (2016). Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Lancet Infect. Dis.16 (6), 661–673. 10.1016/S1473-3099(16)30004-4
4
Cheng Y. Chen M. Zhang B. Lin H. Li X. Cai Y. et al (2022). Rapid, simple, and economical LC-MS/MS method for simultaneous determination of ceftazidime and avibactam in human plasma and its application in therapeutic drug monitoring. J. Clin. Pharm. Ther.47 (9), 1426–1437. 10.1111/jcpt.13693
5
Chuang C. L. Chen K. P. Kwan S. Y. Yang W. C. (2004). Creutzfeldt-Jakob-like EEG in a patient with end-stage renal failure. Nephrol. Dial. Transpl.19 (1), 252–254. 10.1093/ndt/gfg476
6
Collignon C. Benaboud S. Gana I. Bendavid M. Fournier B. Oualha M. et al (2024). Pharmacokinetic of ceftazidime-avibactam in a critically ill patient under high-volume continuous venovenous haemodiafiltration: a first paediatric case report. Br. J. Clin. Pharmacol.90 (3), 890–894. 10.1111/bcp.15993
7
Fresan D. Luque S. Benítez-Cano A. Sorlí L. Milagro Montero M. De-Antonio M. et al (2023). Pharmacokinetics/pharmacodynamics and therapeutic drug monitoring of ceftazidime/avibactam administered by continuous infusion in patients with MDR Gram-negative bacterial infections. J. Antimicrob. Chemother.78, 678–683. 10.1093/jac/dkac439
8
Govindaraj V. A. Vanitha A. (2018). WHO global priority pathogens list on antibiotic resistance: an urgent need for action to integrate One Health data. Perspect. Public Health138 (2), 87–88. 10.1177/1757913917743881
9
Guo X. Guo M. Li J. Cui X. (2022). Central nervous system adverse events of ceftazidime/avibactam: a retrospective study using Food and Drug Administration Adverse Event Reporting System. J. Clin. Pharm. Ther.47 (12), 2369–2372. 10.1111/jcpt.13796
10
Han Y. Zhu J. Liu J. Zheng Y. Liang G. Yang Y. et al (2024). Adequacy of the dosing and infusion time of ceftazidime/avibactam for the treatment of gram-negative bacterial infections: a PK/PD simulation study. Infect. Drug Resist17, 2823–2832. 10.2147/IDR.S469313
11
Li M. Gao L. Wang Z. Zeng L. Chen C. Wang J. et al (2024). Population pharmacokinetics and dose optimization of ceftazidime in critically ill children. Front. Pharmacol.15, 1470350. 10.3389/fphar.2024.1470350
12
Martinez-Rodriguez J. E. Barriga F. J. Santamaria J. Iranzo A. Pareja J. A. Revilla M. et al (2001). Nonconvulsive status epilepticus associated with cephalosporins in patients with renal failure. Am. J. Med.111 (2), 115–119. 10.1016/s0002-9343(01)00767-7
13
Nekidy W. Ali M. Abidi E. Ghazi I. Attallah N. Lababidi R. et al (2023). Microbiologic outcomes of ceftazidime-avibactam dosing in patients with sepsis utilizing renal replacement therapies. Hemodial. Int.27 (3), 289–295. 10.1111/hdi.13090
14
O'Jeanson A. Ioannidis K. Nielsen E. I. Galani L. Ginosyan A. Paskalis H. et al (2025). Ceftazidime-avibactam (CAZ-AVI) pharmacokinetics in critically ill patients undergoing continuous venovenous hemodiafiltration (CVVHDF). Int. J. Antimicrob. Agents65 (1), 107394. 10.1016/j.ijantimicag.2024.107394
15
Soukup P. Faust A. C. Edpuganti V. Putnam W. C. Mckinnell J. A. (2019). Steady-state ceftazidime-avibactam serum concentrations and dosing recommendations in a critically ill patient being treated for Pseudomonas aeruginosa pneumonia and undergoing continuous venovenous hemodiafiltration. Pharmacotherapy39 (12), 1216–1222. 10.1002/phar.2338
16
Tacconelli E. Carrara E. Savoldi A. Harbarth S. Mendelson M. Monnet D. L. et al (2018). Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis.18 (3), 318–327. 10.1016/S1473-3099(17)30753-3
17
Thomsen J. Menezes G. A. Abdulrazzaq N. M. Moubareck C. A. Senok A. Everett D. B. et al (2023). Evolving trends among Pseudomonas aeruginosa: a 12-year retrospective study from the United Arab Emirates. Front. Public Health11, 1243973. 10.3389/fpubh.2023.1243973
18
Torres A. Zhong N. Pachl J. Timsit J. F. Kollef M. Chen Z. et al (2018). Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect. Dis.18 (3), 285–295. 10.1016/S1473-3099(17)30747-8
19
Vannaprasaht S. Tawalee A. Mayurasakorn N. Yodwut C. Bansong R. Reungjui S. et al (2006). Ceftazidime overdose-related nonconvulsive status epilepticus after intraperitoneal instillation. Clin. Toxicol. (Phila).44 (4), 383–386. 10.1080/15563650600671753
20
Wenzler E. Bunnell K. L. Bleasdale S. C. Benken S. Danziger L. H. Rodvold K. A. (2017). Pharmacokinetics and dialytic clearance of ceftazidime-avibactam in a critically ill patient on continuous venovenous hemofiltration. Antimicrob. Agents Chemother.61 (7). 10.1128/AAC.00464-17
21
Yasmin M. Nutman A. Wang L. Marshall S. Chen K. Wang J. et al (2023). Utilizing ceftazidime/avibactam therapeutic drug monitoring in the treatment of neurosurgical meningitis caused by difficult-to-treat resistant Pseudomonas aeruginosa and KPC-producing enterobacterales. Open Forum Infect. Dis.10, ofad507–6. 10.1093/ofid/ofad507
22
Yu J. Zuo W. Fan H. Wu J. Qiao L. Yang B. et al (2023). Ceftazidime-avibactam for carbapenem-resistant gram-negative bacteria infections: a real-world experience in the ICU. Infect. Drug Resist16, 6209–6216. 10.2147/IDR.S422545
23
Zhang X. S. Wang Y. Z. Shi D. W. Xu F. M. Yu J. H. Chen J. et al (2022). Efficacy and pharmacodynamic target attainment for ceftazidime-avibactam off-label dose regimens in patients with continuous or intermittent venovenous hemodialysis: two case reports. Infect. Dis. Ther.11 (6), 2311–2319. 10.1007/s40121-022-00621-z
Summary
Keywords
ceftazidime/avibactam, continuous renal replacement therapy, pharmacokinetic, electroencephalogram, adverse events
Citation
Huang H, Han Y, Wu Y, Guo F and Yu Z (2025) Case Report: Pharmacokinetics of ceftazidime and avibactam during and after CRRT in an elderly patient and their associations with CNS adverse effects. Front. Pharmacol. 16:1569715. doi: 10.3389/fphar.2025.1569715
Received
03 February 2025
Accepted
30 April 2025
Published
21 May 2025
Volume
16 - 2025
Edited by
Muhammad Fawad Rasool, Bahauddin Zakariya University, Pakistan
Reviewed by
Susan J Lewis, University of Findlay, United States
Dayu Chen, Nanjing Drum Tower Hospital the Affiliated Hospital of Nanjing University Medical School, China
Updates
Copyright
© 2025 Huang, Han, Wu, Guo and Yu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Guo, 3408003@zju.edu.cn; Zhenwei Yu, yzw_srrsh@zju.edu.cn
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.