- 1Department of Infectious Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, China
The Annexin (ANXA) protein family, which is ubiquitously expressed across various tissues, encodes versatile calcium (Ca2+)- and phospholipid-binding proteins that play crucial roles in modulating inflammation and cell signaling pathways. This family significantly influences several essential cellular processes, including cell adhesion, proliferation, migration, differentiation, and apoptosis. ANXAs are integral to physiological regulation and the pathological states associated with liver diseases. Dysregulated expression of ANXAs has been linked to a spectrum of liver conditions, including metabolic dysfunction, hepatocyte damage, fibrosis, and tumor formation. In this review, we outline recent advancements in understanding the roles of ANXAs in liver diseases. Further investigation into the roles of ANXAs in the liver could enhance our understanding of the mechanisms underlying liver diseases and may identify biomarkers and therapeutic targets for liver diseases in the future.
1 Introduction
Liver diseases encompass pathological alterations in the liver induced by a diverse array of internal and external pathogenic factors, thereby disrupting normal physiological functions. Liver diseases can be triggered by various causes, including viral infections, metabolic disorders, drug-induced effects, excessive alcohol consumption, and autoimmune aberrations (Asrani et al., 2019). Depending on their etiology and pathogenesis, liver diseases are predominantly classified into viral hepatitis, metabolic dysfunction-associated fatty liver disease (MAFLD), drug-induced liver injury (DILI), alcoholic liver disease (ALD), autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), cirrhosis, and hepatocellular carcinoma (HCC) (Xiao et al., 2019). In recent decades, liver diseases have become one of the leading causes of death and illness worldwide (Xiao et al., 2019), resulting in over two million fatalities annually and constituting 4% of global deaths (Devarbhavi et al., 2023). Understanding the mechanisms underlying liver diseases and advancing targeted treatment strategies are crucial for improving clinical outcomes.
Annexins (ANXAs) constitute a family of calcium-regulated, membrane-associated proteins (Gerke et al., 2024). The first ANXA proteins were identified through biochemical methods that capitalized on their unique membrane-binding properties. Over the past few decades, extensive research has revealed that ANXAs are involved in a wide array of physiological and pathological processes, profoundly influencing diverse cellular functions (Li Y. Z. et al., 2022; Xi et al., 2020). A complex relationship between ANXA expression and various liver diseases has also been established. In recent years, there has been increasing evidence of a strong association between ANXAs and various liver diseases. Notably, ANXAs play an important role in the pathogenesis of liver diseases by regulating liver metabolism, inflammation, fibrosis, immune cell function, regeneration, and tumor development (Wu et al., 2024). In addition, certain types of ANXAs can be detected in the extracellular environment, such as in the blood, where they correlate with disease processes, suggesting their potential as biomarkers. In this review, we evaluate numerous studies on the role of ANXAs in liver disease, explore potential therapeutic strategies targeting ANXAs, and provide an outlook for future research.
2 Functions of annexins
ANXAs were originally discovered in the late 1970s to early 1980s. The term “Annexin” is derived from the Latin word anectere, meaning “to bind or connect,” reflecting their capacity to bind phospholipids in a calcium-dependent manner (Vedeler et al., 2025). The 12 vertebrate annexins, designated ANXA1 through ANXA11 and ANXA13, belong to the Annexin A subfamily (White et al., 2024). All ANXA proteins contain conserved core domains of approximately 70 amino acids and are characterized by variable N-terminal regions and calcium-binding sites that facilitate membrane interaction (Hakami Zanjani et al., 2024). These proteins also contain binding sites for cytoplasmic protein ligands and can be targeted to cellular membranes through their core-mediated phospholipid-binding activity (Hakami Zanjani et al., 2024).
ANXAs are widely expressed across various tissues (Supplementary Table S1) and are implicated in numerous cellular processes, including membrane scaffolding, ion channel regulation, vesicular trafficking, membrane repair, cell signaling, proliferation, differentiation, apoptosis, and migration (Gerke et al., 2024; Gerke et al., 2005; Gerke and Moss, 2002). Although traditionally regarded as intracellular proteins, some ANXAs have also been detected in the extracellular space. However, the mechanisms governing their extracellular secretion and their functions outside the cell remain poorly understood.
3 Annexin A protein family in liver diseases
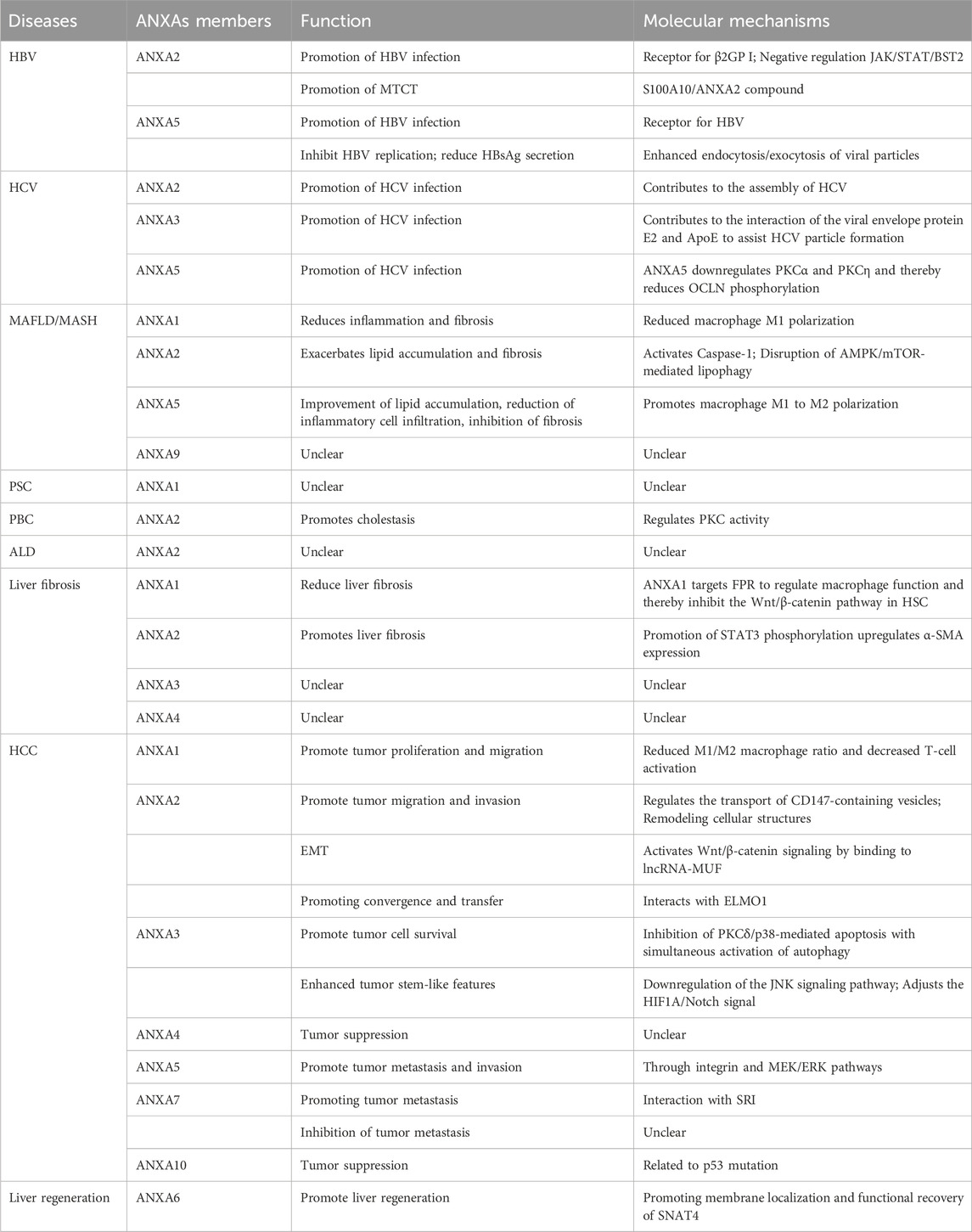
The ANXAs play an important role in liver diseases such as HCC, MAFLD, liver fibrosis, and viral hepatitis (Table 1). Importantly, ANXAs are expected to serve as biomarkers and potential therapeutic targets for liver diseases, thus advancing the diagnosis and treatment of liver diseases in the future.
3.1 Viral hepatitis B
Hepatitis B virus (HBV) infection remains to be a significant global health issue, with chronic infection potentially progressing to cirrhosis or even HCC (Feng M. et al., 2022). β2-glycoprotein I (β2GP I), a plasma glycoprotein, has been shown to bind to recombinant hepatitis B surface antigen (rHBsAg), suggesting a role in facilitating HBV entry into hepatocytes. A recent study found that ANXA2, located on the membrane of the SMMC-7721 HCC cell line, served as a receptor for β2GP I, suggesting that ANXA2 may play a bridging role in HBV infection of hepatocytes (Figure 1A) (Gao et al., 2007). Furthermore, upregulation of ANXA2 has been observed in HBV-replicating Hep RG cells (Narayan et al., 2009) and in Hep G2 cells transfected with HBV X protein (HBx) (Feng et al., 2010). Mechanistically, HBV infection increases the expression of ETS variant 4 (ETV4), which in turn enhances ANXA2 expression at the transcriptional level through binding to the ANXA2 promoter (Sun and Zhang, 2021). Moreover, CD40 mediates anti-HBV effects by upregulating the Janus kinase (JAK)/Signal Transducer and Activator of Transcription (STAT)/bone marrow stromal cell antigen 2 (BST2) axis, which is negatively regulated by ANXA2 (Chen et al., 2023).
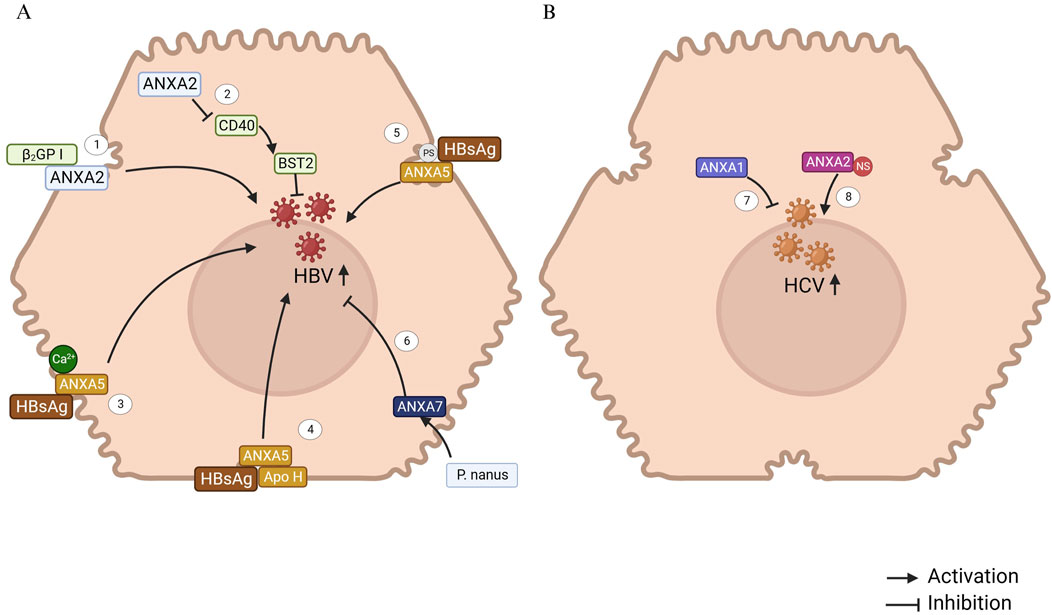
Figure 1. ANXAs involved in Viral hepatitis B and C. (A) 1. ANXA2, located on the hepatocyte membrane, acts as the receptor for β(2)GP I, potentially facilitating the interaction between HBV and infected hepatocytes (Gao et al., 2007). 2. ANXA2, a regulatory protein, downregulates CD40 expression, which subsequently reduces the expression of the IFN-stimulated gene BST2, thereby promoting HBV replication and transcription (Chen et al., 2023). 3. Hepatic plasma membrane protein ANXA5 binds specifically to HBsAg in a Ca2+-dependent manner, indicating its crucial role in the progression of HBV infection (Hertogs et al., 1993). 4. ANXA5, along with apolipoprotein H (Apo H), binds to the hepatitis B surface antigen (HBsAg). It is hypothesized that these proteins specifically interact with the HBsAg S protein, playing a significant role in the initiation of HBV infection (Neurath and Strick, 1994). 5. Phosphatidylserine, a phospholipid component of the HBV envelope, plays a crucial role in the binding of ANXA5 to the HBV envelope and is essential for HBV infection in vitro (De Meyer et al., 1999). 6. An ethanolic extract of Phyllanthus nanus (P. nanus) demonstrated strong antiviral activity against HBV. ANXA7 may significantly contribute to the therapeutic effects of the active components in P. nanus (Lam et al., 2006). (B) 7. ANXA1 inhibits HCV RNA replication but does not affect the viral entry process into hepatocytes (Hiramoto et al., 2015). 8. HCV RNA replication in hepatocytes occurs through the formation of a replication complex, composed of both viral nonstructural (NS) proteins and host cell proteins. ANXA2 is involved in this complex, where it interacts with the NS proteins (Saxena et al., 2012).
Interestingly, ANXA2 expression is downregulated in primary duck liver cells (PDHs) infected with duck hepatitis B virus (DHBV) (Zhao et al., 2010). The HepG2.2.15 cell line, a human HCC line stably transfected with the HBV genome, exhibits sustained HBV expression. In contrast, the parental HepG2 cell line lacks integrated HBV DNA and does not support viral replication. Notably, ANXA2 expression is significantly downregulated in HepG2.2.15 cells compared with HBV-negative HepG2 cells, suggesting a potential functional link between ANXA2 and HBV infection or replication (Niu et al., 2009). ANXA2 expression exhibits divergent patterns during HBV infection, possibly due to variations in species or cell models. Future investigations should standardized experimental conditions to definitively characterize the mechanistic role of ANXA2 in HBV pathogenesis.
Mother-to-child transmission (MTCT) is a primary route for chronic HBV infection. In intrauterine HBV infection and MTCT, a portion of the virus utilizes the autophagic protein secretion pathway, translocating across the trophoblastic layer via exocytosis facilitated by the S100 Calcium Binding Protein A10 (S100A10)/ANXA2 complex and polyvesicular bodies (Bai et al., 2022). This study identifies a potential therapeutic target for disrupting the mechanisms underlying HBV intrauterine transmission and vertical mother-to-child infection.
ANXA5, a protein present in fetal tissue, may also serve as an HBV receptor across various tissues. Elevated ANXA5 levels in the liver might contribute to the organ’s increased susceptibility to HBV infection (Ye et al., 2006). The binding of viral envelope proteins to specific receptors on hepatocytes is a crucial step in HBV infection. A previous study has demonstrated that ANXA5, a human liver plasma membrane protein, specifically binds to small hepatitis B surface antigen (HBsAg) in a calcium-dependent manner, highlighting its potential role in HBV infection (Hertogs et al., 1993). ANXA5 plays a key role in the early stages of HBV infection, and further research suggests that species-specific susceptibility to HBV infection and replication in hepatocytes is linked to ANXA5 expression (Gong et al., 1999). In rat hepatocyte primary cultures, ANXA5 promotes HBV entry, facilitating successful infection, while in human hepatocyte primary cultures, ANXA5 does not prevent HBV infection (De Meyer et al., 2000). Additionally, ANXA5 and apolipoprotein H have been shown to bind to HBsAg, specifically interacting with the HBsAg S protein, which is essential for initiating HBV infection (Neurath and Strick, 1994). The binding of phosphatidylserine and non-phospholipid components of the HBV envelope to ANXA5 also contributes to the infection process (De Meyer et al., 1999). Collectively, the evidence indicates that ANXA5 plays a crucial role in HBV infection through facilitating viral entry into hepatocytes, highlighting its potential as a therapeutic target for anti-HBV drug development.
ANXA7 plays a critical role in modulating HBsAg release during HBV infection. It has been demonstrated that the ethanolic extract of Phyllanthus nanus upregulates ANXA7 expression in HBV-infected hepatoma cells, which correlates with suppressed HBV replication and reduced HBsAg secretion. ANXA7 localizes near secretory vesicles and may inhibit HBV by enhancing endocytosis/exocytosis of viral particles or interfering with HBsAg release. Functional studies have confirmed that ANXA7 overexpression in HBV-integrated Alexander cells markedly reduces extracellular HBsAg levels (Lam et al., 2006). These findings position ANXA7 as a promising molecular target for novel HBV therapeutics aimed at blocking viral propagation by hijacking vesicular trafficking pathways.
3.2 Viral hepatitis C
Chronic infection with hepatitis C virus (HCV) often progresses to chronic hepatitis, which has a high likelihood of advancing to cirrhosis and HCC (Hajarizadeh et al., 2023). ANXA1 plays a role in inhibiting HCV RNA replication, although it does not affect the initial viral entry into human hepatocytes (Hiramoto et al., 2015).
HCV RNA replication complex (RC) is formed by viral nonstructural (NS) proteins and host cell proteins, enabling the replication of the viral RNA genome associated with the cell membrane (Lai et al., 2008). The enzymatic activity of these proteins plays a crucial role in the HCV replication process. Previous studies have shown that ANXA2 interacts with NS3/NS4A (Lai et al., 2008) and helps recruit HCV NS proteins, concentrating them to form replication complexes (Figure 1B) (Saxena et al., 2012). Although silencing ANXA2 expression does not affect viral RNA replication, it results in a significant reduction in both extracellular and intracellular viral titers. This suggests that ANXA2 likely contributes to HCV assembly rather than genome replication or the release of viral particles. Colocalization studies of separately expressed HCV NS proteins have indicated that NS5A may specifically recruit ANXA2 through indirect mechanisms (Backes et al., 2010).
Knocking down ANXA3 does not affect HCV RNA replication, but it does significantly disrupt the production of viral particles. Mechanistically, ANXA3 plays a critical role in the interaction between the viral envelope protein E2 and apolipoprotein E (ApoE), as well as in the transport (but not the lipidogenesis) of ApoE in HCV-infected cells (Rösch et al., 2016). Therefore, ANXA3 may serve as a co-factor for HCV particle production.
The disruption of occludin (OCLN) distribution facilitates HCV infection. Normal distribution of OCLN is regulated by phosphorylation. Knockout of ANXA5 results in decreased phosphorylation of OCLN, thereby leading to its disrupted distribution and promoting HCV infection. Protein kinase C (PKC) subtypes, such as PKCα and PKCη, play a crucial role in regulating ANXA5-mediated OCLN phosphorylation and distribution, which in turn helps limit HCV infection. HCV infection downregulates the expression of PKCα and PKCη, thereby reducing OCLN phosphorylation (Abe et al., 2023). Collectively, the data indicate that ANXA5 mimics could function as effective HCV entry inhibitors.
3.3 Metabolic dysfunction-associated fatty liver disease
MAFLD has emerged as the most prevalent chronic liver disorder worldwide, affecting an estimated 38% of the global population (Wong et al., 2023). This disease encompasses a broad spectrum of severity, ranging from simple steatosis to metabolic dysfunction-associated steatohepatitis (MASH), cirrhosis, and HCC (Munk Lauridsen et al., 2025). MAFLD is intrinsically linked to metabolic comorbidities, particularly obesity, insulin resistance, type 2 diabetes mellitus, and atherogenic dyslipidemia (Mejía-Guzmán et al., 2025). Compared with the general population, MAFLD patients exhibit increased risks of liver-related, kidney-related, cardiovascular, and all-cause mortality (Cao et al., 2021; Mann et al., 2020). Emerging mechanistic evidence highlights ANXAs as critical regulators in MAFLD pathogenesis, modulating key processes such as lipid metabolism, inflammatory signaling, and fibrosis progression.
Exogenous treatment with ANXA1 has demonstrated efficacy in counteracting the progression of MASH, primarily through its anti-inflammatory and anti-fibrotic properties, although its impact on hepatic steatosis appears to be limited. In preclinical models, MASH was induced in mice via feeding of a methionine-choline deficient (MCD) diet or a Western diet (WD). Once MASH was established, the animals received daily intraperitoneal (IP) injections of human recombinant ANXA1 (hrANXA1; 1 μg) or saline for 4–6 weeks. Across both experimental paradigms, hrANXA1 treatment significantly alleviated liver injury and reduced inflammatory cell infiltration, without influencing the degree of steatosis (Gadipudi et al., 2022). Mechanistically, macrophage-derived ANXA1 ameliorates hepatic inflammation and fibrosis by reducing macrophage M1 polarization during MASH progression (Locatelli et al., 2014). However, the molecular mechanisms governing ANXA1-mediated regulation of macrophage polarization and function remain elusive. This knowledge gap represents a crucial area that merits comprehensive investigation in future research endeavors.
ANXA2 is significantly upregulated in both MAFLD patients and high-fat diet (HFD)-fed mouse models, where it exacerbates MAFLD-associated lipid accumulation and fibrosis (Sobolewski et al., 2020), and ANXA2 expression exhibits a positive correlation with the progression of MAFLD-associated hepatocyte pyroptosis and fibrosis (Feng Y. et al., 2022). Bioinformatics analysis showed that ANXA2 could act as a core gene driving MASH progression (Arendt et al., 2019; Li X. et al., 2022; Qin et al., 2023; Chen et al., 2024; Fan et al., 2024). Mechanistic studies have shown that ANXA2 activates Caspase-1-mediated MASH hepatocyte pyroptosis and fibrosis (Feng Y. et al., 2022). Another study showed that ANXA2 promotes lipid accumulation and liver injury by disrupting AMP-activated protein kinase (AMPK)/mechanistic target of rapamycin (mTOR)-mediated lipophagy (Wu et al., 2024). In addition, increased ANXA2 expression in hepatocytes promotes MASH-associated hepatic fibrosis by increasing the expression of osteopontin (Wang et al., 2022). Thus, future studies may focus on investigating the potential of ANXA2 as a pathological predictor for MAFLD and a promising therapeutic target.
ANXA5 attenuated MASH-associated hepatic lipid accumulation, reduced inflammatory cell infiltration, and suppressed fibrosis. Mechanistically, in hepatic macrophages, ANXA5 directly binds to pyruvate kinase M2 (PKM2) at the ASP101, LEU104, and ARG106 residues. This interaction facilitates the assembly of active PKM2 tetramers while inhibiting PKM2 Y105 phosphorylation. By enhancing PKM2’s pyruvate kinase activity, ANXA5 drives metabolic reprogramming in M1 macrophages, shifting their energy metabolism from glycolysis to oxidative phosphorylation (OXPHOS). Consequently, ANXA5 promotes a phenotypic switch of hepatic macrophages from pro-inflammatory M1 to anti-inflammatory M2 polarization, thereby mitigating MASH progression (Figure 2) (Xu et al., 2020). Notably, intravenous administration of ANXA5 in HFD-induced MASH mice alleviates hepatic lipotoxicity, inflammation, and fibrosis, underscoring its therapeutic potential for clinical translation.
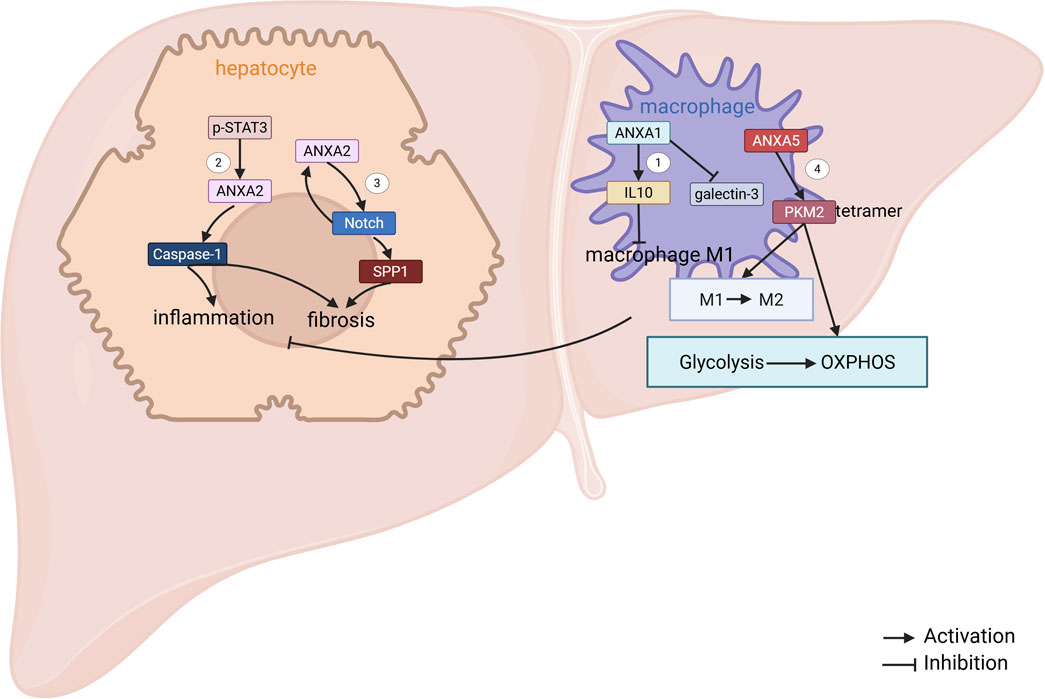
Figure 2. ANXAs involved in MAFLD. 1. In NASH mice, macrophage-derived ANXA1 reduces M1 polarization by promoting interleukin-10 (IL-10) production. Additionally, ANXA1 downregulates the expression of galactoglucan-3 (Locatelli et al., 2014). 2. Phosphorylated STAT3 (p-STAT3) enhances the transcriptional expression of ANXA2, which in turn triggers Caspase-1-mediated pyroptosis in NASH hepatocytes, contributing to liver fibrosis. 3. In hepatocytes, the ANXA2-Notch positive regulatory loop activates hepatic stellate cells (HSCs) by upregulating SPP1 expression, thus promoting liver fibrosis in NASH (Wang et al., 2022). 4. ANXA5 directly interacts with pyruvate kinase M2 (PKM2), which leads to a shift in metabolism from glycolysis to oxidative phosphorylation (OXPHOS), induces phenotypic changes in hepatic macrophages, and alleviates NASH (Xu et al., 2020).
Furthermore, bioinformatics analysis identified ANXA9 as a key driver gene in MAFLD pathogenesis, indicating its potential utility as a diagnostic biomarker and therapeutic target (Song et al., 2023a).
3.4 Autoimmune liver disease
PSC is a chronic cholestatic liver disease characterized by bile duct stenosis due to inflammation and fibrosis of the intrahepatic and extrahepatic bile ducts, which can ultimately progress to cirrhosis and liver failure (Manns et al., 2025). The pathogenesis of PSC has not been fully elucidated yet, and it may be due to a combination of factors such as genetics, environment, immunity, bile acid metabolism disorders, and dysfunction of intestinal flora (Tan et al., 2023).
ANXA1 expression was significantly upregulated in liver tissues and bile of PSC patients. T cell infiltration, which is thought to play a crucial role in PSC. Bioinformatics analysis of one study showed that ANXA1 is a key gene associated with high risk and infiltration of immune cells, especially T cells, in PSC (Zhang J. et al., 2023). Staining of hepatic tissues for ANXA1 showed that it was significantly upregulated around the hepatic tissues of the portal vein in patients with PSC. Although ANXA1 was not expressed predominantly on T cells, the areas of high ANXA1 expression were also accompanied by a greater number of CD3+ T cells infiltrating (Zhang J. et al., 2023). In another study, bile proteomic analysis showed that ANXA1 was significantly upregulated in the bile of patients with PSC, and immunostaining of hepatic tissue for ANXA1 showed that, in addition to its expression in cholangiocytes and vascular endothelial cells, it also highly expressed in inflammatory cells infiltrating the peripheral bile ducts (Kan et al., 2023). Both studies only demonstrated the expression of ANXA1 in patients with PSC and did not investigate the biological role of ANXA1 in PSC. Previous studies have shown that ANXA1 belongs to the group of anti-inflammatory proteins (Perretti and D'Acquisto, 2009; Perretti and Dalli, 2023; Gavins and Hickey, 2012), and its anti-inflammatory effects are exerted by inhibiting the release of inflammatory mediators (e.g., prostaglandin E2 and leukotrienes), promoting tissue repair, and enhancing leukocyte migration (Purvis et al., 2019). However, the exact role of ANXA1 in PSC, the specific mechanism of its action, and the mechanism for the increased expression of ANXA1 in bile remain to be elucidated.
PBC is an autoimmune liver disease characterized by progressive destruction of intrahepatic bile ducts leading to cholestasis, cirrhosis and liver failure (Tanaka, 2024). Proteomic analysis of PBC patients showed significant upregulation of ANXA2 expression in cholangiocytes. Preliminary functional analyses suggest that the upregulation of ANXA2 expression in cholangiocytes may promote cholestasis by regulating protein kinase C (PKC) activity to compensate for the impaired anion exchanger (AE) activity in cholangiocytes in PBC, i.e., bicarbonate-rich ductal secretion and bile formation. However, the specific regulatory mechanisms, such as molecule-to-molecule interactions, by which ANXA2 functions in PBC remain to be further explored (Kido et al., 2009).
3.5 Alcohol-related liver disease
ALD encompasses a range of hepatic pathologies, including steatosis, hepatitis and cirrhosis, that develop secondary to prolonged alcohol abuse. While the progression of ALD is primarily influenced by the amount and duration of alcohol intake, and it is also shaped by genetic, epigenetic, and environmental factors (Liu S. Y. et al., 2021). It has been demonstrated that ANXA2 expression is significantly elevated in alcohol-induced cell lines, in mouse and baboon models of ALD, and in liver tissues of ALD patients (Seth et al., 2008; Seth et al., 2003; Zhang et al., 2011). Although the relationship between ANXAs and ALD has not been extensively studied, emerging evidence suggests that ANXAs may play a role in the progression of liver disease. Further research is needed to clarify their mechanisms in ALD.
3.6 Liver fibrosis
Liver fibrosis is associated with chronic liver injury including viral hepatitis, autoimmune liver disease and MAFLD. As the number of patients affected by virus-related liver disease decreases with the availability of antiviral drugs, the increase in fibrosis in patients with MAFLD has now become one of the most critical issues in the field of hepatology. The progression of liver fibrosis in response to injury involves complex interactions between multiple cell types in the liver, and there is a close link between hepatocellular injury, activation of innate immune cells, and the production of extracellular matrixc (ECM) (Rieder et al., 2025). MAFLD-associated liver fibrosis has been demonstrated in the “Metabolic dysfunction-associated fatty liver disease” chapter.
ANXA1 is upregulated in fibrosis. Functional studies showed that ANXA1 attenuated CCl4-induced hepatic fibrosis in mice, and the mechanism may be that ANXA1 targets the N-formylpeptide receptor (FPR) to regulate macrophage function and thus inhibits Wnt/β-catenin pathway activation in hepatic stellate cell (HSC) (Fan et al., 2023). Thus, the fibrosis inhibitory effect of ANXA1 makes it a potential for future development of drugs for the treatment of liver fibrosis.
ANXA2 was significantly upregulated in HBV and alcohol-induced liver fibrosis (Seth et al., 2003; Zhang et al., 2010). ANXA2 levels were significantly elevated in patients with S4 stage of fibrosis compared to those with S0-1 stage of fibrosis (Zhang et al., 2010). Recent research has indicated that isoliquiritigenin suppresses ANXA2 expression. Subsequently, this inhibition reduces the phosphorylation of signal transducer and activator of transcription 3 (STAT3) in downstream signaling pathways (Figure 3). The reduced STAT3 activity downregulates α–smooth muscle actin (α-SMA) expression, ultimately reversing HSC activation and alleviating liver fibrosis (Liu N. et al., 2023). Additionally, the mouse ANXA6/miR-9-5p/ANXA2 axis, along with the PI3K/Akt signaling pathway, may play a role in promoting liver fibrosis mediated by lncRNA ANXA2P2 (Liao et al., 2022). However, whether ANXA2 can be used as a noninvasive biomarker in HBV and alcohol-induced liver fibrosis deserves further investigation.
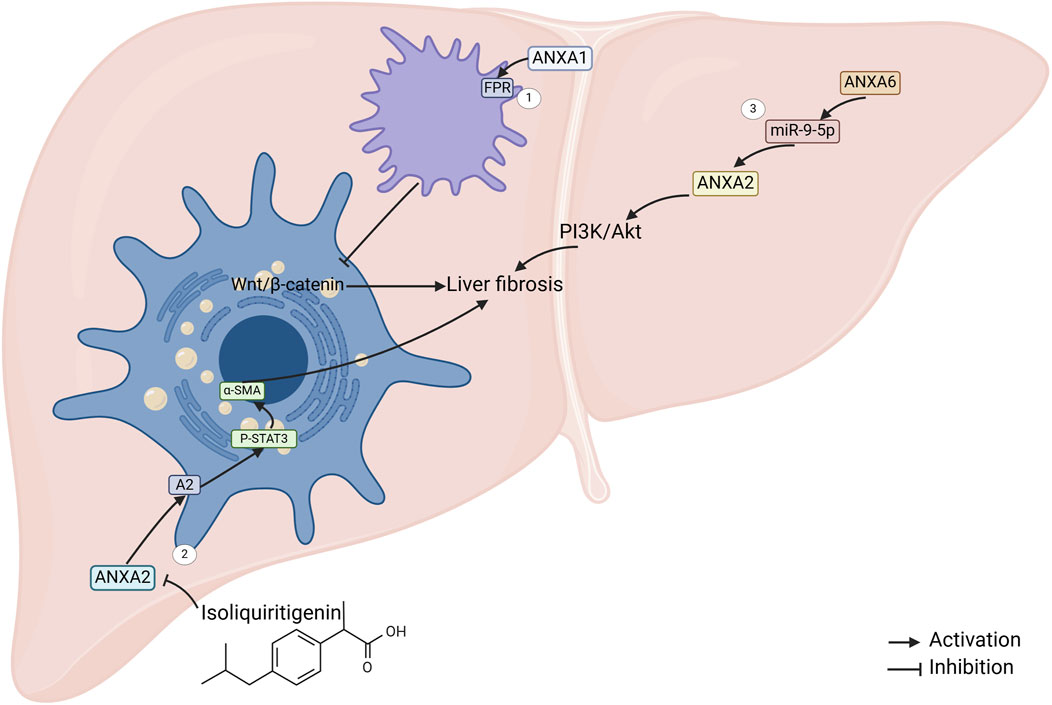
Figure 3. ANXAs involved in liver fibrosis. 1. ANXA1 regulates macrophage activity by targeting formylpeptide receptors, which in turn suppresses the activation of the Wnt/β-catenin pathway in hepatic stellate cells (HSCs), ultimately inhibiting liver fibrosis (Fan et al., 2023). 2. Isoflavones inhibit ANXA2 expression, which leads to a reduction in the phosphorylation of transcription activator 3 (STAT3) and subsequently downregulates the expression of α-SMA (Liu N. et al., 2023). 3. The mouse ANXA6/miR-9-5p/ANXA2 axis, along with the PI3K/Akt pathway, may contribute to liver fibrosis promotion through the long non-coding RNA ANXA2P2 (mouse ANXA6) (Liao et al., 2022).
Furthermore, ANXA3 expression was reduced in liver tissues of mouse models of fibrosis induced by ethanol, olive oil and pyrazole (Jia et al., 2012). In patients with advanced fibrosis associated with HBV infection, ANXA4 expression was significantly increased in liver tissue (Katrinli et al., 2016). These findings suggest that different members of ANXAs may play different roles in the onset and progression of liver fibrosis, and the exact functions of ANXA3 and ANXA4 in liver fibrosis remain unclear warranting further study in the future.
3.7 Hepatocellular carcinoma
Liver cancer ranks as the eighth most prevalent cancer and the third leading cause of cancer-related mortality worldwide (Sung et al., 2021). HCC accounts for approximately 80% of all liver cancer cases (Chrysavgis et al., 2022). Although surgery, liver transplantation, chemotherapy, and targeted therapy are the most effective treatment options currently available, the overall survival rate for patients with HCC remains unsatisfactory (Chen et al., 2020). The prognosis is particularly grim for individuals with recurrent disease or distant metastases (Goyal et al., 2013). This highlights the urgent need for further research to develop more effective therapeutic strategies for HCC.
In earlier studies, elevated expression of ANXA1 was identified as a predictor of poor prognosis in HCC and was shown to enhance malignant cell behaviors (Lin et al., 2014). Recent studies have revealed that ANXA1 is highly expressed in mesenchymal cells, particularly macrophages, in liver cancer tissues in humans. Furthermore, ANXA1 expression in mesenchymal cells is associated with programmed cell death-ligand 1 (PD-L1) levels. Suppression of ANXA1 expression inhibits HCC cell proliferation and migration by increasing the M1/M2 macrophage ratio and stimulating T-cell activation (Figure 4) (Song et al., 2023b).
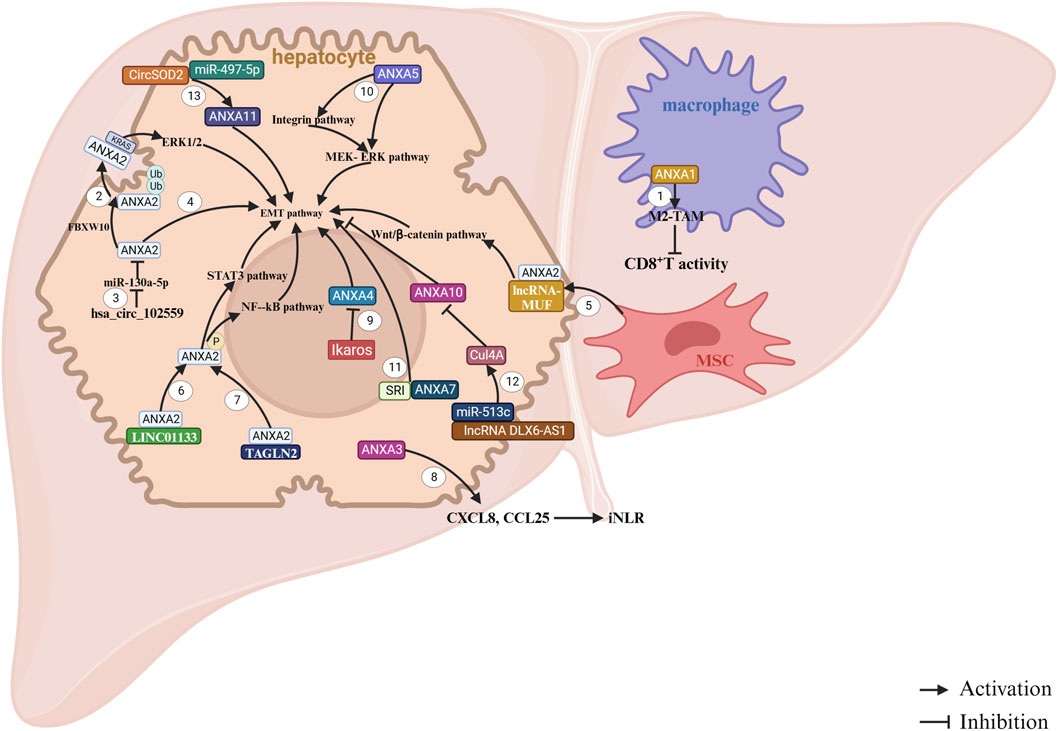
Figure 4. ANXAs involved in HCC. 1. Overexpression of ANXA1 in macrophages promotes malignant growth and metastasis by enhancing the infiltration and M2 polarization of tumor-associated macrophages (TAMs). This process creates an immunosuppressive tumor microenvironment (TME) and suppresses the antitumor response of CD8+ T cells (Song et al., 2023b). 2. FBXW10 promotes the polyubiquitination and activation of ANXA2. Once activated, ANXA2 translocates from the cytoplasm to the cell membrane, where it binds KRAS and activates the MEK/ERK pathway, leading to the proliferation of hepatocellular carcinoma (HCC) cells (Liu Z. Y. et al., 2023). 3. The upregulation of hsa_circ_102559 in HCC inhibits the expression of miR-130a-5p, which in turn promotes ANXA2 expression, triggering epithelial–mesenchymal transition (EMT), as well as cell proliferation and migration in HCC (Li et al., 2020). 4. ANXA2 contributes to increased cell proliferation and motility (Shi et al., 2016). 5. LncRNA-MUF binds to ANXA2 and activates Wnt/β-catenin signaling, thereby promoting EMT (Yan et al., 2017). 6. LINC01133 interacts with ANXA2 to activate the ANXA2/STAT3 signaling pathway (Yin et al., 2021). 7. The NF-kB signaling pathway participates in HCC progression through TAGLN2, which interacts with ANXA2 (Shi et al., 2020). 8. ANXA3 enhances the immune response in HCC by inducing the release of chemokines CXCL8 and CCL25, remodeling the immune microenvironment of HCC (Zhu et al., 2020). 9. Overexpression of ANXA4 promotes HCC cell proliferation, but Ikaros can inhibit ANXA4 expression by repressing its promoter activity (Liu et al., 2017). 10. Downregulation of ANXA5 suppresses the expression of molecules in the integrin pathway, such as CRKI/II, DOCK180, and RAC1, as well as key elements in the MEK-ERK pathway (e.g., p-MEK, p-ERK, c-Myc, and MMP-9), along with VIMINTIN in Hca-P cells (Sun et al., 2018). 11. ANXA7 promotes EMT by interacting with SRI, which contributes to the aggressiveness of HCC (Ling et al., 2021). 12. LncRNA DLX6-AS1 increases Cul4A expression by competitively binding to miR-513c. Cul4A then promotes the degradation of ANXA10 through the ubiquitin-proteasome pathway, facilitating the development of HCC (Liu X. et al., 2021). 13. CircSOD2 induces the upregulation of ANXA11 expression by interacting with miR-497-5p (Ye et al., 2023).
Extensive research has highlighted the role of ANXA2 in facilitating the onset and progression of liver cancer. ANXA2 has emerged as a promising prognostic biomarker and modulator of tumor immune microenvironment in various malignancies, including HCC (Ning et al., 2023). Initial findings suggest that ANXA2 is upregulated in human liver cancer tissues and cell lines (Yoon et al., 2006). Subsequent studies have identified ANXA2 as a potential novel marker for tumor angiogenesis in HCC (Yu et al., 2007; Mohammad et al., 2008; Ji et al., 2009; Longerich et al., 2011; Zhang et al., 2012; Sun et al., 2013; Tang et al., 2019; Huang et al., 2021; Herrera-López et al., 2023). Mechanistic investigations have provided further insights. One study has demonstrated that ANXA2 promotes HCC cell migration and invasion in vitro by regulating the trafficking of CD147-harboring microvesicles (Zhang W. et al., 2013). In addition, ANXA2 enhances the malignant properties of HCC cells, primarily by remodeling cellular structures (Shi et al., 2016). Additionally, lncRNA-MUF binds to ANXA2, activating the Wnt/β-catenin signaling and epithelial–mesenchymal transition (EMT) (Yan et al., 2017). Furthermore, ANXA2 interacts with engulfment and cell motility protein 1 (ELMO1) to regulate HCC chemotaxis and metastasis (Li et al., 2019). In terms of therapeutic potential, it has been shown that silencing ANXA2 using shRNA effectively reduces hepatoma cell invasion, migration, and tumorigenicity (Zhang H. J. et al., 2013; Dong et al., 2014). Furthermore, ANXA2 is integral in HCC, and its downregulation enhances the efficacy of chemotherapeutic agents such as 5-fluorouracil (Wang et al., 2015). However, serum or tissue ANXA2 levels are not reliable diagnostic markers for HCC in patients with HBV-related cirrhosis and are not associated with patient prognosis.
ANXA3 has been identified as a key factor in conferring resistance to sorafenib in HCC cells, it is enriched in sorafenib-resistant HCC cells and patient-derived xenografts. Mechanistically, ANXA3 overexpression in these cells inhibits the PKCδ/p38-mediated apoptotic pathways while activating autophagy to support cell survival (Tong et al., 2018). Additionally, ANXA3 contributes to chemotherapy resistance in HCC (Pan et al., 2015a). Emerging evidence underscores the pivotal role of ANXA3 in liver cancer stem cell (CSC) maintenance and tumor progression through multiple molecular mechanisms. Studies have demonstrated that both intracellular and secreted ANXA3 significantly enhance the malignant and stem-like properties of CD133+ liver CSCs by dysregulating c-Jun N-terminal kinase (JNK) signaling (Tong et al., 2015). Complementary research indicates that ANXA3 sustains HCC CSC activity, potentially through modulation of the hypoxia inducible factor-1A (HIF1A)/Notch signaling axis (Pan et al., 2015b). Additionally, ANXA3 influences chemokine signaling to reshape the infiltrated neutrophil-to-lymphocyte ratio, thereby promoting tumorigenicity in HCC (Zhu et al., 2020). ANXA3 has been identified as an HCC-associated gene, representing a potential therapeutic target for HCC treatment.
The serum level of ANXA4 has been suggested as a potential biomarker for the early detection of HCC (Herrera-López et al., 2023; Saad et al., 2020). Moreover, it has been indicated that reducing ANXA4 expression suppressed HCC cell proliferation and tumorigenesis both in vitro and in vivo (Liu et al., 2017). Mechanistically, ANXA5 can promote HCC progression and metastasis through the integrin- and mitogen-activated extracellular signal-regulated kinase (MEK)/extracellular regulated protein kinase (ERK) pathway (Sun et al., 2018).
Dysregulation of ANXA7 has been implicated in tumorigenesis, invasion, metastasis, and progression across multiple cancer types, though its functional role appears context-dependent. In vitro studies in the human HCC cell line Hep G2 demonstrated that ANXA7 knockdown suppressed cell migration, suggesting its pro-metastatic role in this context (Ibrahim et al., 2013). Similarly, in Hca-F cells—a mouse HCC model with high lymphatic metastatic potential—miR-124-3p exerts tumor-suppressive effects by targeting ANXA7, thereby inhibiting tumor growth, invasion, and lymphatic metastasis (Wang et al., 2020). Mechanistically, ANXA7 interacts with Sorcin (SRI), and their cooperation facilitates EMT, further driving HCC proliferation, invasion, and migration (Ling et al., 2021). However, contrasting findings have been reported. For instance, one study revealed that ANXA7 upregulation suppresses HCC lymph node metastasis, whereas its knockdown exacerbates metastatic spread (Jin et al., 2013).
Unlike other ANXAs, ANXA10 is a tumor suppressor gene (Zhang X. et al., 2023). Elevated ANXA10 expression has been shown to inhibit HCC cell viability, invasion and migration (Liu X. et al., 2021). Conversely, reduced ANXA10 levels in HCC are linked to vascular invasion, early recurrence and poor prognosis, particularly in synergy with p53 mutations (Liu et al., 2002).
3.8 Liver regeneration
ANXA6 plays a crucial role in acute liver regeneration. Loss of ANXA6 markedly impairs liver regeneration capacity and reduces survival in mice following partial hepatectomy (PHx) (Enrich et al., 2017). Mechanistic studies revealed that ANXA6 modulates alanine-dependent gluconeogenesis by facilitating the membrane localization and functional recovery of sodium-coupled neutral amino acid transporter 4 (SNAT4). Since alanine is a critical substrate for hepatic gluconeogenesis, ANXA6 deficiency disrupts SNAT4-mediated alanine uptake in hepatocytes, thereby impairing glucose production from alanine. This metabolic disturbance results in paradoxical hepatic alanine underutilization despite elevated plasma alanine levels, ultimately leading to a blockade of the gluconeogenesis pathway and compromised regenerative capacity. Notably, either liver-specific ANXA6 reconstitution or exogenous glucose administration effectively restores normoglycemia and improves survival in PHx mice (Alvarez-Guaita et al., 2020). These findings identify ANXA6 as a key metabolic regulator that orchestrates energy homeostasis during liver regeneration, offering novel insights into the metabolic reprogramming essential for hepatic repair.
4 Conclusions and future perspectives
ANXAs represent a family of multifunctional proteins that play significant roles in the pathogenesis of various liver diseases, particularly MAFLD and HCC. This article reviews the progress in research on the functions and mechanisms of ANXAs in liver diseases and offers insights for future research and therapeutic development in this field.
The involvement of ANXA7 in HCC development has been extensively studied; however, its precise function remains controversial. Discrepancies in experimental outcomes may be attributed to several factors: differences in genetic backgrounds, epigenetic modifications, or mutational profiles of HCC cell lines (e.g., Hep G2 vs. Hca-F) across studies could result in divergent ANXA7-associated functions. For example, Hca-F cells exhibit high lymphatic metastatic potential, whereas Hep G2 cells may depend on alternative metastatic pathways. Additionally, due to the complexity of the underlying molecular mechanisms, ANXA7 may exert its effects via different downstream molecules or interacting proteins, the expression or activity of which may vary across cell types. Moreover, variations in gene manipulation techniques (e.g., knockdown vs. knockout), assay conditions, or analytical approaches could also contribute to inconsistent findings. Given the potential dual role of ANXA7 in HCC progression, future research should prioritize the use of standardized model systems and consistent experimental conditions to clarify its function in HCC.
The treatment of liver disease is an ongoing area of research, and patient survival rates remains low once progression to end-stage liver disease occurs. Despite a surge of research and findings regarding ANXAs in recent years, gaps still exist in the study of ANXAs and their relationship to liver disease (Figure 5).
Although preliminary studies suggest that ANXAs could serve as promising diagnostic biomarkers for liver diseases, their clinical utility remains uncertain due to the absence of large-scale, multicenter validation studies. Further research is needed to rigorously assess their diagnostic sensitivity and specificity, ideally through comprehensive comparisons with established liver disease biomarkers.
MAFLD has now become the leading cause of chronic liver disease. Although the epidemiology of MAFLD has been extensively studied, the underlying mechanisms and effective therapeutic targets remain unclear. Members of ANXAs, such as ANXA1 and ANXA5, have demonstrated significant improvement of inflammation and antifibrotic effects in MAFLD. However, there are some ANXAs with unknown roles in MAFLD. Considering the similarities among members of the same family, we hypothesize that other ANXAs also play important roles in MAFLD, but the specific roles and mechanisms require further investigation in the future.
Notably, while the secretion of certain ANXAs into the extracellular environment has been reported, the precise mechanisms governing their secretion and their functional consequences in the extracellular milieu remain poorly characterized. Moreover, although several ANXAs have established biological roles, the molecular mechanisms mediating these functions are incompletely understood.
An increasing body of evidence highlights the therapeutic potential of targeting ANXAs for liver diseases treatment. For instance, ANXA6 administration significantly enhances survival in PHx mouse models, suggesting its promise as a regeneration-promoting drug for the treatment of acute liver injury and even liver failure. Additionally, ANXA5 has demonstrated strong ability to improve lipid metabolism, inflammation and fibrosis in MAFLD mice. The development of mimics or neutralizing antibodies against ANXAs with great therapeutic potential for clinical use would bring new hope to patients with liver diseases.
In summary, ANXAs hold great promise as biomarkers and therapeutic targets for liver diseases. Understanding their nuanced roles in disease-specific contexts will be crucial for translating these insights into effective and targeted therapies.
Author contributions
MF: Writing – original draft. YH: Writing – review and editing. HW: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This paper was funded by the Key Discipline Project (GWVI-11.1-12) of Shanghai Public Health System Construction Three-year Action Plan (2023–2025).
Acknowledgments
We thank LetPub (www.letpub.com.cn) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1569927/full#supplementary-material
References
Abe, T., Marutani, Y., Deng, L., Matsui, C., Fukasawa, M., Suzuki, R., et al. (2023). Hepatitis C virus disrupts annexin 5-Mediated occludin integrity through downregulation of protein kinase Cα (PKCα) and PKCη expression, thereby promoting viral propagation. J. Virol. 97 (6), e0065523. doi:10.1128/jvi.00655-23
Alvarez-Guaita, A., Blanco-Muñoz, P., Meneses-Salas, E., Wahba, M., Pollock, A. H., Jose, J., et al. (2020). Annexin A6 is critical to maintain glucose homeostasis and survival during liver regeneration in mice. Hepatology 72 (6), 2149–2164. doi:10.1002/hep.31232
Arendt, B. M., Teterina, A., Pettinelli, P., Comelli, E. M., Ma, D. W. L., Fung, S. K., et al. (2019). Cancer-related gene expression is associated with disease severity and modifiable lifestyle factors in non-alcoholic fatty liver disease. Nutrition 62, 100–107. doi:10.1016/j.nut.2018.12.001
Asrani, S. K., Devarbhavi, H., Eaton, J., and Kamath, P. S. (2019). Burden of liver diseases in the world. J. Hepatol. 70 (1), 151–171. doi:10.1016/j.jhep.2018.09.014
Backes, P., Quinkert, D., Reiss, S., Binder, M., Zayas, M., Rescher, U., et al. (2010). Role of annexin A2 in the production of infectious hepatitis C virus particles. J. Virol. 84 (11), 5775–5789. doi:10.1128/JVI.02343-09
Bai, X., Ran, J., Zhao, X., Liang, Y., Yang, X., and Xi, Y. (2022). The S100A10-AnxA2 complex is associated with the exocytosis of hepatitis B virus in intrauterine infection. Lab. Invest 102 (1), 57–68. doi:10.1038/s41374-021-00681-8
Cao, Y., Deng, Y., Wang, J., Zhao, H., Zhang, J., and Xie, W. (2021). The association between NAFLD and risk of chronic kidney disease: a cross-sectional study. Ther. Adv. Chronic Dis. 12, 20406223211048649. doi:10.1177/20406223211048649
Chen, C., Yang, K., Zhang, Y., Lu, M., Zhao, X., and Wan, Z. (2024). Pathogenic gene connections in type 2 diabetes and non-alcoholic fatty liver disease: a bioinformatics analysis and mouse model investigations experiments. Nutr. Diabetes 14 (1), 60. doi:10.1038/s41387-024-00323-0
Chen, J., Chen, H., Mai, H., Lou, S., Luo, M., Xie, H., et al. (2023). A functional variant of CD40 modulates clearance of hepatitis B virus in hepatocytes via regulation of the ANXA2/CD40/BST2 axis. Hum. Mol. Genet. 32 (8), 1334–1347. doi:10.1093/hmg/ddac284
Chen, Z., Xie, H., Hu, M., Huang, T., Hu, Y., Sang, N., et al. (2020). Recent progress in treatment of hepatocellular carcinoma. Am. J. Cancer Res. 10 (9), 2993–3036.
Chrysavgis, L., Giannakodimos, I., Diamantopoulou, P., and Cholongitas, E. (2022). Non-alcoholic fatty liver disease and hepatocellular carcinoma: clinical challenges of an intriguing link. World J. Gastroenterol. 28 (3), 310–331. doi:10.3748/wjg.v28.i3.310
De Meyer, S., Gong, Z., Depla, E., Maertens, G., and Yap, S. H. (1999). Involvement of phosphatidylserine and non-phospholipid components of the hepatitis B virus envelope in human Annexin V binding and in HBV infection in vitro. J. Hepatol. 31 (5), 783–790. doi:10.1016/s0168-8278(99)80278-5
De Meyer, S., Gong, Z. J., Hertogs, K., Depla, E., van Pelt, J. F., Roskams, T., et al. (2000). Influence of the administration of human annexin V on in vitro binding of small hepatitis B surface antigen to human and to rat hepatocytes and on in vitro hepatitis B virus infection. J. Viral Hepat. 7 (2), 104–114. doi:10.1046/j.1365-2893.2000.00207.x
Devarbhavi, H., Asrani, S. K., Arab, J. P., Nartey, Y. A., Pose, E., and Kamath, P. S. (2023). Global burden of liver disease: 2023 update. J. Hepatol. 79 (2), 516–537. doi:10.1016/j.jhep.2023.03.017
Dong, Z., Yao, M., Zhang, H., Wang, L., Huang, H., Yan, M., et al. (2014). Inhibition of Annexin A2 gene transcription is a promising molecular target for hepatoma cell proliferation and metastasis. Oncol. Lett. 7 (1), 28–34. doi:10.3892/ol.2013.1663
Enrich, C., Rentero, C., and Grewal, T. (2017). Annexin A6 in the liver: from the endocytic compartment to cellular physiology. Biochim. Biophys. Acta Mol. Cell Res. 1864 (6), 933–946. doi:10.1016/j.bbamcr.2016.10.017
Fan, H., Wang, R., Wen, B., and Xiong, J. (2024). Biomarkers and potential therapeutic targets driving progression of non-alcoholic steatohepatitis to hepatocellular carcinoma predicted through transcriptomic analysis. Front. Immunol. 15, 1502263. doi:10.3389/fimmu.2024.1502263
Fan, J. H., Luo, N., Liu, G. F., Xu, X. F., Li, S. Q., and Lv, X. P. (2023). Mechanism of annexin A1/N-formylpeptide receptor regulation of macrophage function to inhibit hepatic stellate cell activation through Wnt/β-catenin pathway. World J. Gastroenterol. 29 (22), 3422–3439. doi:10.3748/wjg.v29.i22.3422
Feng, H., Li, X., Niu, D., and Chen, W. N. (2010). Protein profile in HBx transfected cells: a comparative iTRAQ-coupled 2D LC-MS/MS analysis. J. Proteomics 73 (8), 1421–1432. doi:10.1016/j.jprot.2009.12.004
Feng, M., Liu, K., Zhao, G., Lou, S., An, B., Lin, L., et al. (2022). A novel model based on qAnti-HBc and conventional biomarkers for identifying significant liver injury among CHB patients with ALT ≤ ULN. Antiviral Res. 202, 105315. doi:10.1016/j.antiviral.2022.105315
Feng, Y., Li, W., Wang, Z., Zhang, R., Li, Y., Zang, L., et al. (2022). The p-STAT3/ANXA2 axis promotes caspase-1-mediated hepatocyte pyroptosis in non-alcoholic steatohepatitis. J. Transl. Med. 20 (1), 497. doi:10.1186/s12967-022-03692-1
Gadipudi, L. L., Ramavath, N. N., Provera, A., Reutelingsperger, C., Albano, E., Perretti, M., et al. (2022). Annexin A1 treatment prevents the evolution to fibrosis of experimental nonalcoholic steatohepatitis. Clin. Sci. (Lond) 136 (9), 643–656. doi:10.1042/CS20211122
Gao, P. J., Shi, Y., Gao, Y. H., Liu, Y. W., and Tan, Y. (2007). The receptor for beta(2)GP I on membrane of hepatocellular carcinoma cell line SMMC-7721 is annexin II. World J. Gastroenterol. 13 (24), 3364–3368. doi:10.3748/wjg.v13.i24.3364
Gavins, F. N., and Hickey, M. J. (2012). Annexin A1 and the regulation of innate and adaptive immunity. Front. Immunol. 3, 354. doi:10.3389/fimmu.2012.00354
Gerke, V., Creutz, C. E., and Moss, S. E. (2005). Annexins: linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 6 (6), 449–461. doi:10.1038/nrm1661
Gerke, V., Gavins, F. N. E., Geisow, M., Grewal, T., Jaiswal, J. K., Nylandsted, J., et al. (2024). Annexins-a family of proteins with distinctive tastes for cell signaling and membrane dynamics. Nat. Commun. 15 (1), 1574. doi:10.1038/s41467-024-45954-0
Gerke, V., and Moss, S. E. (2002). Annexins: from structure to function. Physiol. Rev. 82 (2), 331–371. doi:10.1152/physrev.00030.2001
Gong, Z. J., De Meyer, S., van Pelt, J., Hertogs, K., Depla, E., Soumillion, A., et al. (1999). Transfection of a rat hepatoma cell line with a construct expressing human liver annexin V confers susceptibility to hepatitis B virus infection. Hepatology 29 (2), 576–584. doi:10.1002/hep.510290238
Goyal, L., Muzumdar, M. D., and Zhu, A. X. (2013). Targeting the HGF/c-MET pathway in hepatocellular carcinoma. Clin. Cancer Res. 19 (9), 2310–2318. doi:10.1158/1078-0432.CCR-12-2791
Hajarizadeh, B., Kairouz, A., Ottaviano, S., Ireland, J., Willing, A., Cunningham, E., et al. (2023). Global, regional, and country-level coverage of testing and treatment for HIV and hepatitis C infection among people who inject drugs: a systematic review. Lancet Glob. Health 11 (12), e1885–e1898. doi:10.1016/S2214-109X(23)00461-8
Hakami Zanjani, A. A., Ebstrup, M. L., Nylandsted, J., and Khandelia, H. (2024). Modulation of annexin-induced membrane curvature by cholesterol and the anionic lipid headgroup during plasma membrane repair. J. Phys. Chem. B 128 (36), 8701–8711. doi:10.1021/acs.jpcb.4c02318
Herrera-López, E. E., Guerrero-Escalera, D., Aguirre-Maldonado, I., López-Hernández, A., Montero, H., Gutiérrez-Nava, M. A., et al. (2023). Annexins A2 and A5 are potential early biomarkers of hepatocarcinogenesis. Sci. Rep. 13 (1), 6948. doi:10.1038/s41598-023-34117-8
Hertogs, K., Leenders, W. P., Depla, E., De Bruin, W. C., Meheus, L., Raymackers, J., et al. (1993). Endonexin II, present on human liver plasma membranes, is a specific binding protein of small hepatitis B virus (HBV) envelope protein. Virology 197 (2), 549–557. doi:10.1006/viro.1993.1628
Hiramoto, H., Dansako, H., Takeda, M., Satoh, S., Wakita, T., Ikeda, M., et al. (2015). Annexin A1 negatively regulates viral RNA replication of hepatitis C virus. Acta Med. Okayama 69 (2), 71–78. doi:10.18926/AMO/53335
Huang, S. W., Chen, Y. C., Lin, Y. H., and Yeh, C. T. (2021). Clinical limitations of tissue annexin A2 level as a predictor of postoperative overall survival in patients with hepatocellular carcinoma. J. Clin. Med. 10 (18), 4158. doi:10.3390/jcm10184158
Ibrahim, M. M., Sun, M. Z., Huang, Y., Jun, M., Jin, Y., Yue, D., et al. (2013). Down-regulation of ANXA7 decreases metastatic potential of human hepatocellular carcinoma cells in vitro. Biomed. Pharmacother. 67 (4), 285–291. doi:10.1016/j.biopha.2013.02.005
Ji, N. Y., Park, M. Y., Kang, Y. H., Lee, C. I., Kim, D. G., Yeom, Y. I., et al. (2009). Evaluation of annexin II as a potential serum marker for hepatocellular carcinoma using a developed sandwich ELISA method. Int. J. Mol. Med. 24 (6), 765–771. doi:10.3892/ijmm_00000290
Jia, X., Yin, L., Feng, Y., Peng, X., Ma, F., Yao, Y., et al. (2012). A dynamic plasma membrane proteome analysis of alcohol-induced liver cirrhosis. Proteome Sci. 10 (1), 39. doi:10.1186/1477-5956-10-39
Jin, Y., Wang, S., Chen, W., Zhang, J., Wang, B., Guan, H., et al. (2013). Annexin A7 suppresses lymph node metastasis of hepatocarcinoma cells in a mouse model. BMC Cancer 13, 522. doi:10.1186/1471-2407-13-522
Kan, M., Chiba, T., Konno, R., Kouchi, Y., Mishima, T., Kawashima, Y., et al. (2023). Bile proteome analysis by high-precision mass spectrometry to examine novel biomarkers of primary sclerosing cholangitis. J. Hepatobiliary Pancreat. Sci. 30 (7), 914–923. doi:10.1002/jhbp.1299
Katrinli, S., Ozdil, K., Sahin, A., Ozturk, O., Kir, G., Baykal, A. T., et al. (2016). Proteomic profiling of HBV infected liver biopsies with different fibrotic stages. Proteome Sci. 15, 7. doi:10.1186/s12953-017-0114-4
Kido, O., Fukushima, K., Ueno, Y., Inoue, J., Jefferson, D. M., and Shimosegawa, T. (2009). Compensatory role of inducible annexin A2 for impaired biliary epithelial anion-exchange activity of inflammatory cholangiopathy. Lab. Invest 89 (12), 1374–1386. doi:10.1038/labinvest.2009.105
Lai, C. K., Jeng, K. S., Machida, K., and Lai, M. M. C. (2008). Association of hepatitis C virus replication complexes with microtubules and actin filaments is dependent on the interaction of NS3 and NS5A. J. Virol. 82 (17), 8838–8848. doi:10.1128/JVI.00398-08
Lam, W. Y., Leung, K. T., Law, P. T. W., Lee, S. M. Y., Chan, H. L. Y., Fung, K. P., et al. (2006). Antiviral effect of Phyllanthus nanus ethanolic extract against hepatitis B virus (HBV) by expression microarray analysis. J. Cell Biochem. 97 (4), 795–812. doi:10.1002/jcb.20611
Li, H., Wang, Y., and Lu, Y. (2019). Annexin A2 interacting with ELMO1 regulates HCC chemotaxis and metastasis. Life Sci. 222, 168–174. doi:10.1016/j.lfs.2019.03.003
Li, J., Yu, Z., Zhu, Q., Tao, C., and Xu, Q. (2020). hsa_circ_102559 acts as the sponge of miR-130a-5p to promote hepatocellular carcinoma progression through regulation of ANXA2. Cell Transpl. 29, 963689720968748. doi:10.1177/0963689720968748
Li, X., Zhong, S., Sun, Y., Huang, X., Li, Y., Wang, L., et al. (2022b). Integration analysis identifies the role of metallothionein in the progression from hepatic steatosis to steatohepatitis. Front. Endocrinol. (Lausanne) 13, 951093. doi:10.3389/fendo.2022.951093
Li, Y. Z., Wang, Y. Y., Huang, L., Zhao, Y. Y., Chen, L. H., and Zhang, C. (2022a). Annexin A protein family in atherosclerosis. Clin. Chim. Acta 531, 406–417. doi:10.1016/j.cca.2022.05.009
Liao, J., Zhang, Z., Yuan, Q., Luo, L., and Hu, X. (2022). The mouse Anxa6/miR-9-5p/Anxa2 axis modulates TGF-β1-induced mouse hepatic stellate cell (mHSC) activation and CCl(4)-caused liver fibrosis. Toxicol. Lett. 362, 38–49. doi:10.1016/j.toxlet.2022.04.004
Lin, Y., Lin, G., Fang, W., Zhu, H., and Chu, K. (2014). Increased expression of annexin A1 predicts poor prognosis in human hepatocellular carcinoma and enhances cell malignant phenotype. Med. Oncol. 31 (12), 327. doi:10.1007/s12032-014-0327-7
Ling, F., Zhang, H., Sun, Y., Meng, J., Sanches, J. G. P., Huang, H., et al. (2021). AnnexinA7 promotes epithelial-mesenchymal transition by interacting with sorcin and contributes to aggressiveness in hepatocellular carcinoma. Cell Death Dis. 12 (11), 1018. doi:10.1038/s41419-021-04287-2
Liu, N., Liu, M., Jiang, M., Li, Z., Chen, W., Wang, W., et al. (2023a). Isoliquiritigenin alleviates the development of alcoholic liver fibrosis by inhibiting ANXA2. Biomed. Pharmacother. 159, 114173. doi:10.1016/j.biopha.2022.114173
Liu, S. H., Lin, C. Y., Peng, S. Y., Jeng, Y. M., Pan, H. W., Lai, P. L., et al. (2002). Down-regulation of annexin A10 in hepatocellular carcinoma is associated with vascular invasion, early recurrence, and poor prognosis in synergy with p53 mutation. Am. J. Pathol. 160 (5), 1831–1837. doi:10.1016/S0002-9440(10)61129-7
Liu, S. Y., Tsai, I. T., and Hsu, Y. C. (2021a). Alcohol-related liver disease: basic mechanisms and clinical perspectives. Int. J. Mol. Sci. 22 (10), 5170. doi:10.3390/ijms22105170
Liu, X., Peng, D., Cao, Y., Zhu, Y., Yin, J., Zhang, G., et al. (2021b). Upregulated lncRNA DLX6-AS1 underpins hepatocellular carcinoma progression via the miR-513c/Cul4A/ANXA10 axis. Cancer Gene Ther. 28 (5), 486–501. doi:10.1038/s41417-020-00233-0
Liu, Y. Y., Ge, C., Tian, H., Jiang, J. Y., Zhao, F. Y., Li, H., et al. (2017). The transcription factor Ikaros inhibits cell proliferation by downregulating ANXA4 expression in hepatocellular carcinoma. Am. J. Cancer Res. 7 (6), 1285–1297.
Liu, Z. Y., Lin, X. T., Zhang, Y. J., Gu, Y. P., Yu, H. Q., Fang, L., et al. (2023b). FBXW10-S6K1 promotes ANXA2 polyubiquitination and KRAS activation to drive hepatocellular carcinoma development in males. Cancer Lett. 566, 216257. doi:10.1016/j.canlet.2023.216257
Locatelli, I., Sutti, S., Jindal, A., Vacchiano, M., Bozzola, C., Reutelingsperger, C., et al. (2014). Endogenous annexin A1 is a novel protective determinant in nonalcoholic steatohepatitis in mice. Hepatology 60 (2), 531–544. doi:10.1002/hep.27141
Longerich, T., Haller, M. T., Mogler, C., Aulmann, S., Lohmann, V., Schirmacher, P., et al. (2011). Annexin A2 as a differential diagnostic marker of hepatocellular tumors. Pathol. Res. Pract. 207 (1), 8–14. doi:10.1016/j.prp.2010.09.007
Mann, J. P., Carter, P., Armstrong, M. J., Abdelaziz, H. K., Uppal, H., Patel, B., et al. (2020). Hospital admission with non-alcoholic fatty liver disease is associated with increased all-cause mortality independent of cardiovascular risk factors. PLoS One 15 (10), e0241357. doi:10.1371/journal.pone.0241357
Manns, M. P., Bergquist, A., Karlsen, T. H., Levy, C., Muir, A. J., Ponsioen, C., et al. (2025). Primary sclerosing cholangitis. Nat. Rev. Dis. Prim. 11 (1), 17. doi:10.1038/s41572-025-00600-x
Mejía-Guzmán, J. E., Belmont-Hernández, R. A., Chávez-Tapia, N. C., Uribe, M., and Nuño-Lámbarri, N. (2025). Metabolic-dysfunction-associated steatotic liver disease: molecular mechanisms, clinical implications, and emerging therapeutic strategies. Int. J. Mol. Sci. 26 (7), 2959. doi:10.3390/ijms26072959
Mohammad, H. S., Kurokohchi, K., Yoneyama, H., Tokuda, M., Morishita, A., Jian, G., et al. (2008). Annexin A2 expression and phosphorylation are up-regulated in hepatocellular carcinoma. Int. J. Oncol. 33 (6), 1157–1163.
Munk Lauridsen, M., Ravnskjaer, K., Gluud, L. L., and Sanyal, A. J. (2025). Disease classification, diagnostic challenges, and evolving clinical trial design in MASLD. J. Clin. Invest 135 (10), e189953. doi:10.1172/JCI189953
Narayan, R., Gangadharan, B., Hantz, O., Antrobus, R., García, A., Dwek, R. A., et al. (2009). Proteomic analysis of HepaRG cells: a novel cell line that supports hepatitis B virus infection. J. Proteome Res. 8 (1), 118–122. doi:10.1021/pr800562j
Neurath, A. R., and Strick, N. (1994). The putative cell receptors for hepatitis B virus (HBV), annexin V, and apolipoprotein H, bind to lipid components of HBV. Virology 204 (1), 475–477. doi:10.1006/viro.1994.1558
Ning, Y., Li, Y., and Wang, H. (2023). ANXA2 is a potential biomarker for cancer prognosis and immune infiltration: a systematic pan-cancer analysis. Front. Genet. 14, 1108167. doi:10.3389/fgene.2023.1108167
Niu, D., Sui, J., Zhang, J., Feng, H., and Chen, W. N. (2009). iTRAQ-coupled 2-D LC-MS/MS analysis of protein profile associated with HBV-Modulated DNA methylation. Proteomics 9 (15), 3856–3868. doi:10.1002/pmic.200900071
Pan, Q. Z., Pan, K., Wang, Q. J., Weng, D. S., Zhao, J. J., Zheng, H. X., et al. (2015b). Annexin A3 as a potential target for immunotherapy of liver cancer stem-like cells. Stem Cells 33 (2), 354–366. doi:10.1002/stem.1850
Pan, Q. Z., Pan, K., Weng, D. S., Zhao, J. J., Zhang, X. F., Wang, D. D., et al. (2015a). Annexin A3 promotes tumorigenesis and resistance to chemotherapy in hepatocellular carcinoma. Mol. Carcinog. 54 (8), 598–607. doi:10.1002/mc.22126
Perretti, M., and D'Acquisto, F. (2009). Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 9 (1), 62–70. doi:10.1038/nri2470
Perretti, M., and Dalli, J. (2023). Resolution pharmacology: focus on pro-resolving annexin A1 and lipid mediators for therapeutic innovation in inflammation. Annu. Rev. Pharmacol. Toxicol. 63, 449–469. doi:10.1146/annurev-pharmtox-051821-042743
Purvis, G. S. D., Solito, E., and Thiemermann, C. (2019). Annexin-A1: therapeutic potential in microvascular disease. Front. Immunol. 10, 938. doi:10.3389/fimmu.2019.00938
Qin, J., Cao, P., Ding, X., Zeng, Z., Deng, L., and Luo, L. (2023). Machine learning identifies ferroptosis-related gene ANXA2 as potential diagnostic biomarkers for NAFLD. Front. Endocrinol. (Lausanne) 14, 1303426. doi:10.3389/fendo.2023.1303426
Rieder, F., Nagy, L. E., Maher, T. M., Distler, J. H. W., Kramann, R., Hinz, B., et al. (2025). Publisher correction: fibrosis: cross-organ biology and pathways to development of innovative drugs. Nat. Rev. Drug Discov. 24, 399. doi:10.1038/s41573-025-01190-9
Rösch, K., Kwiatkowski, M., Hofmann, S., Schöbel, A., Grüttner, C., Wurlitzer, M., et al. (2016). Quantitative lipid droplet proteome analysis identifies Annexin A3 as a cofactor for HCV particle production. Cell Rep. 16 (12), 3219–3231. doi:10.1016/j.celrep.2016.08.052
Saad, Z. M., Fouad, Y., Ali, L. H., and Hassanin, T. M. (2020). Clinical significance of annexin A4 as a biomarker in the early diagnosis of hepatocellular carcinoma. Asian Pac J. Cancer Prev. 21 (9), 2661–2665. doi:10.31557/APJCP.2020.21.9.2661
Saxena, V., Lai, C. K., Chao, T. C., Jeng, K. S., and Lai, M. M. C. (2012). Annexin A2 is involved in the formation of hepatitis C virus replication complex on the lipid raft. J. Virol. 86 (8), 4139–4150. doi:10.1128/JVI.06327-11
Seth, D., Hogg, P. J., Gorrell, M. D., McCaughan, G. W., and Haber, P. S. (2008). Direct effects of alcohol on hepatic fibrinolytic balance: implications for alcoholic liver disease. J. Hepatol. 48 (4), 614–627. doi:10.1016/j.jhep.2007.12.015
Seth, D., Leo, M. A., McGuinness, P. H., Lieber, C. S., Brennan, Y., Williams, R., et al. (2003). Gene expression profiling of alcoholic liver disease in the baboon (Papio hamadryas) and human liver. Am. J. Pathol. 163 (6), 2303–2317. doi:10.1016/S0002-9440(10)63587-0
Shi, H., Xiao, L., Duan, W., He, H., Ma, L., Da, M., et al. (2016). ANXA2 enhances the progression of hepatocellular carcinoma via remodeling the cell motility associated structures. Micron 85, 26–33. doi:10.1016/j.micron.2016.03.008
Shi, J., Ren, M., She, X., Zhang, Z., Zhao, Y., Han, Y., et al. (2020). Transgelin-2 contributes to proliferation and progression of hepatocellular carcinoma via regulating Annexin A2. Biochem. Biophys. Res. Commun. 523 (3), 632–638. doi:10.1016/j.bbrc.2020.01.028
Sobolewski, C., Abegg, D., Berthou, F., Dolicka, D., Calo, N., Sempoux, C., et al. (2020). S100A11/ANXA2 belongs to a tumour suppressor/oncogene network deregulated early with steatosis and involved in inflammation and hepatocellular carcinoma development. Gut 69 (10), 1841–1854. doi:10.1136/gutjnl-2019-319019
Song, Z., Wang, X., Liu, X., Luo, Y., Qiu, J., Yin, A., et al. (2023b). Targeting of Annexin A1 in Tumor-associated macrophages as a therapeutic strategy for hepatocellular carcinoma. Biochem. Pharmacol. 213, 115612. doi:10.1016/j.bcp.2023.115612
Song, Z., Wang, Y., Lin, P., Yang, K., Jiang, X., Dong, J., et al. (2023a). Identification of key modules and driving genes in nonalcoholic fatty liver disease by weighted gene co-expression network analysis. BMC Genomics 24 (1), 414. doi:10.1186/s12864-023-09458-3
Sun, T., and Zhang, J. (2021). ETV4 mediates the Wnt/β-catenin pathway through transcriptional activation of ANXA2 to promote hepatitis B virus-associated liver hepatocellular carcinoma progression. J. Biochem. 170 (5), 663–673. doi:10.1093/jb/mvab088
Sun, X., Liu, S., Wang, J., Wei, B., Guo, C., Chen, C., et al. (2018). Annexin A5 regulates hepatocarcinoma malignancy via CRKI/II-DOCK180-RAC1 integrin and MEK-ERK pathways. Cell Death Dis. 9 (6), 637. doi:10.1038/s41419-018-0685-8
Sun, Y., Gao, G., Cai, J., Wang, Y., Qu, X., He, L., et al. (2013). Annexin A2 is a discriminative serological candidate in early hepatocellular carcinoma. Carcinogenesis 34 (3), 595–604. doi:10.1093/carcin/bgs372
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tan, N., Lubel, J., Kemp, W., Roberts, S., and Majeed, A. (2023). Current therapeutics in primary sclerosing cholangitis. J. Clin. Transl. Hepatol. 11 (5), 1267–1281. doi:10.14218/JCTH.2022.00068S
Tanaka, A. (2024). New therapies on the horizon for primary biliary cholangitis. Drugs 84 (1), 1–15. doi:10.1007/s40265-023-01979-1
Tang, L., Liu, J. X., Zhang, Z. J., Xu, C. Z., Zhang, X. N., Huang, W. R., et al. (2019). High expression of Anxa2 and Stat3 promote progression of hepatocellular carcinoma and predict poor prognosis. Pathol. Res. Pract. 215 (6), 152386. doi:10.1016/j.prp.2019.03.015
Tong, M., Che, N., Zhou, L., Luk, S. T., Kau, P. W., Chai, S., et al. (2018). Efficacy of annexin A3 blockade in sensitizing hepatocellular carcinoma to sorafenib and regorafenib. J. Hepatol. 69 (4), 826–839. doi:10.1016/j.jhep.2018.05.034
Tong, M., Fung, T. M., Luk, S. T., Ng, K. Y., Lee, T. K., Lin, C. H., et al. (2015). ANXA3/JNK signaling promotes self-renewal and tumor growth, and its blockade provides a therapeutic target for hepatocellular carcinoma. Stem Cell Rep. 5 (1), 45–59. doi:10.1016/j.stemcr.2015.05.013
Vedeler, A., Tartaglia, G. G., and Pastore, A. (2025). Annexin, a protein for all seasons: from calcium dependent membrane metabolism to RNA recognition. Bioessays 47, e70019. doi:10.1002/bies.70019
Wang, C., Guo, Y., Wang, J., and Min, Z. (2015). Annexin A2 knockdown inhibits hepatoma cell growth and sensitizes hepatoma cells to 5-fluorouracil by regulating β-catenin and cyclin D1 expression. Mol. Med. Rep. 11 (3), 2147–2152. doi:10.3892/mmr.2014.2906
Wang, G., Duan, J., Pu, G., Ye, C., Li, Y., Xiu, W., et al. (2022). The Annexin A2-Notch regulatory loop in hepatocytes promotes liver fibrosis in NAFLD by increasing osteopontin expression. Biochim. Biophys. Acta Mol. Basis Dis. 1868 (8), 166413. doi:10.1016/j.bbadis.2022.166413
Wang, H., Mao, J., Huang, Y., Zhang, J., Zhong, L., Wu, Y., et al. (2020). Prognostic roles of miR-124-3p and its target ANXA7 and their effects on cell migration and invasion in hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 13 (3), 357–370.
White, Z. B., Nair, S., and Bredel, M. (2024). The role of annexins in central nervous system development and disease. J. Mol. Med. Berl. 102 (6), 751–760. doi:10.1007/s00109-024-02443-7
Wong, V. W., Ekstedt, M., Wong, G. L. H., and Hagström, H. (2023). Changing epidemiology, global trends and implications for outcomes of NAFLD. J. Hepatol. 79 (3), 842–852. doi:10.1016/j.jhep.2023.04.036
Wu, H., Zhou, M., Jin, Q., Wang, X., Xu, Y., Li, M., et al. (2024). The upregulation of Annexin A2 by TLR4 pathway facilitates lipid accumulation and liver injury via blocking AMPK/mTOR-mediated autophagy flux during the development of non-alcoholic fatty liver disease. Hepatol. Int. 18, 1144–1157. doi:10.1007/s12072-023-10622-w
Xi, Y., Ju, R., and Wang, Y. (2020). Roles of Annexin A protein family in autophagy regulation and therapy. Biomed. Pharmacother. 130, 110591. doi:10.1016/j.biopha.2020.110591
Xiao, J., Wang, F., Wong, N. K., He, J., Zhang, R., Sun, R., et al. (2019). Global liver disease burdens and research trends: analysis from a Chinese perspective. J. Hepatol. 71 (1), 212–221. doi:10.1016/j.jhep.2019.03.004
Xu, F., Guo, M., Huang, W., Feng, L., Zhu, J., Luo, K., et al. (2020). Annexin A5 regulates hepatic macrophage polarization via directly targeting PKM2 and ameliorates NASH. Redox Biol. 36, 101634. doi:10.1016/j.redox.2020.101634
Yan, X., Zhang, D., Wu, W., Wu, S., Qian, J., Hao, Y., et al. (2017). Mesenchymal stem cells promote hepatocarcinogenesis via lncRNA-MUF interaction with ANXA2 and miR-34a. Cancer Res. 77 (23), 6704–6716. doi:10.1158/0008-5472.CAN-17-1915
Ye, F., Li, S. h., Chen, T. y., Yue, Y. f., Zhang, S. l., and Bai, G. q. (2006). Expression of human annexin V in different fetal tissues. Nan Fang. Yi Ke Da Xue Xue Bao 26 (2), 193–195.
Ye, R., Lu, X., Liu, J., Duan, Q., Xiao, J., Duan, X., et al. (2023). CircSOD2 contributes to tumor progression, immune evasion and Anti-PD-1 resistance in hepatocellular carcinoma by targeting miR-497-5p/ANXA11 axis. Biochem. Genet. 61 (2), 597–614. doi:10.1007/s10528-022-10273-w
Yin, D., Hu, Z. Q., Luo, C. B., Wang, X. Y., Xin, H. Y., Sun, R. Q., et al. (2021). LINC01133 promotes hepatocellular carcinoma progression by sponging miR-199a-5p and activating annexin A2. Clin. Transl. Med. 11 (5), e409. doi:10.1002/ctm2.409
Yoon, S. Y., Kim, J. M., Oh, J. H., Jeon, Y. J., Lee, D. S., Kim, J. H., et al. (2006). Gene expression profiling of human HBV- and/or HCV-associated hepatocellular carcinoma cells using expressed sequence tags. Int. J. Oncol. 29 (2), 315–327.
Yu, G. R., Kim, S. H., Park, S. H., Cui, X. D., Xu, D. Y., Yu, H. C., et al. (2007). Identification of molecular markers for the oncogenic differentiation of hepatocellular carcinoma. Exp. Mol. Med. 39 (5), 641–652. doi:10.1038/emm.2007.70
Zhang, H. J., Yao, D. F., Yao, M., Huang, H., Wang, L., Yan, M. J., et al. (2013b). Annexin A2 silencing inhibits invasion, migration, and tumorigenic potential of hepatoma cells. World J. Gastroenterol. 19 (24), 3792–3801. doi:10.3748/wjg.v19.i24.3792
Zhang, H. J., Yao, D. F., Yao, M., Huang, H., Wu, W., Yan, M. J., et al. (2012). Expression characteristics and diagnostic value of annexin A2 in hepatocellular carcinoma. World J. Gastroenterol. 18 (41), 5897–5904. doi:10.3748/wjg.v18.i41.5897
Zhang, J., Wang, H., Liu, J., Fu, L., and Peng, S. (2023a). ANXA1 is identified as a key gene associated with high risk and T cell infiltration in primary sclerosing cholangitis. Hum. Genomics 17 (1), 86. doi:10.1186/s40246-023-00534-z
Zhang, L., Jia, X., Feng, Y., Peng, X., Zhang, Z., Zhou, W., et al. (2011). Plasma membrane proteome analysis of the early effect of alcohol on liver: implications for alcoholic liver disease. Acta Biochim. Biophys. Sin. (Shanghai) 43 (1), 19–29. doi:10.1093/abbs/gmq108
Zhang, L., Peng, X., Zhang, Z., Feng, Y., Jia, X., Shi, Y., et al. (2010). Subcellular proteome analysis unraveled annexin A2 related to immune liver fibrosis. J. Cell Biochem. 110 (1), 219–228. doi:10.1002/jcb.22529
Zhang, W., Zhao, P., Xu, X. L., Cai, L., Song, Z. S., Cao, D. Y., et al. (2013a). Annexin A2 promotes the migration and invasion of human hepatocellular carcinoma cells in vitro by regulating the shedding of CD147-harboring microvesicles from tumor cells. PLoS One 8 (8), e67268. doi:10.1371/journal.pone.0067268
Zhang, X., Zhang, C., Zhao, Q., Wang, S., Wang, L., Si, Y., et al. (2023b). Inhibition of annexin A10 contributes to ZNF281 mediated aggressiveness of hepatocellular carcinoma. J. Hepatocell. Carcinoma 10, 553–571. doi:10.2147/JHC.S400989
Zhao, Y., Ben, H., Qu, S., Zhou, X., Yan, L., Xu, B., et al. (2010). Proteomic analysis of primary duck hepatocytes infected with duck hepatitis B virus. Proteome Sci. 8, 28. doi:10.1186/1477-5956-8-28
Keywords: annexins, ANXA2, liver disease, MAFLD, HCC
Citation: Feng M, He Y and Wang H (2025) Role of the Annexin A protein family in liver diseases: insights and therapeutic opportunities. Front. Pharmacol. 16:1569927. doi: 10.3389/fphar.2025.1569927
Received: 02 February 2025; Accepted: 26 June 2025;
Published: 11 July 2025.
Edited by:
Angelo A Izzo, University of Naples Federico II, ItalyReviewed by:
Baharan Fekry, University of Texas Health Science Center at Houston, United StatesYijie Ning, Second Hospital of Shanxi Medical University, China
Copyright © 2025 Feng, He and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Wang, d2FuZ2h1aXJqQDE2My5jb20=
 Mingyang Feng1
Mingyang Feng1 Yong He
Yong He Hui Wang
Hui Wang