Abstract
Benzoylaconine (BAC), a key active metabolite in traditional Chinese medicine, is derived from the subsoil roots of Fuzi (Aconitum carmichaelii Debx [Ranunculaceae, Aconitum carmichaelii Debx roots]). BAC has garnered considerable research attention because of its therapeutic effects against cardiovascular disease, inflammation, and arthritis, and this has led to continual updates in the literature. This systematic review summarizes evidence on the pharmacological effects, molecular mechanisms, and pharmacokinetics of BAC. PubMed and Web of Science were searched for relevant articles published between January 2000 and November 2024. Genes, proteins, and pathways related to the activity and therapeutic effects of BAC were identified. BAC usually targets proteins such as ACE2, IL-6, MAPK, PI3K, Akt, STAT3, TNF-α, and VEGF. The identified genes and proteins were subjected to protein–protein interaction analysis, molecular docking between BAC and protein hubs, and bioinformatic analyses (gene ontology, Kyoto Encyclopedia of Genes and Genomes, and disease ontology analyses). Protein–protein interaction analysis and molecular docking indicated IL-6, Akt1, and STAT3 as key targets of BAC. These findings offer theoretical insights into the potential therapeutic mechanisms of BAC and may inform its future development as a pharmacological agent.
1 Introduction
Benzoylaconine (BAC) is a white crystalline compound that is soluble in organic solvents such as methanol, ethanol, isopropanol, and chloroform and slightly soluble in water. The oral bioavailability (OB) of BAC is 12.8, indicating limited absorption through the oral route. Nonetheless, BAC has a relatively high drug-likeness value of 0.25. BAC, a common monoester diterpenoid alkaloid (MDA), is a major active metabolite in the traditional Chinese medicine (TCM) Fuzi. Fuzi is widely used in TCM formulations such as Shenfu-Tang, Fuzi-Tang, Zhenwu-Tang, and Sini-Tang for cardiac support, diuresis, vasodilation, circulatory enhancement, and central analgesia. BAC is the hydrolysis product of aconitine (AC) (He et al., 2022) (Figure 1), which is derived during the processing of Aconitum carmichaelii. (He et al., 2022). The toxicity of BAC is approximately 100 times lower than that of AC. (Zhang et al., 2016; Ye et al., 2012). BAC has proven effective in mitigating arthritis, inflammation, cardiac injury, and psoriasis. (Li et al., 2021; Li et al., 2023; Yu et al., 2020; Zhang et al., 2024).
FIGURE 1
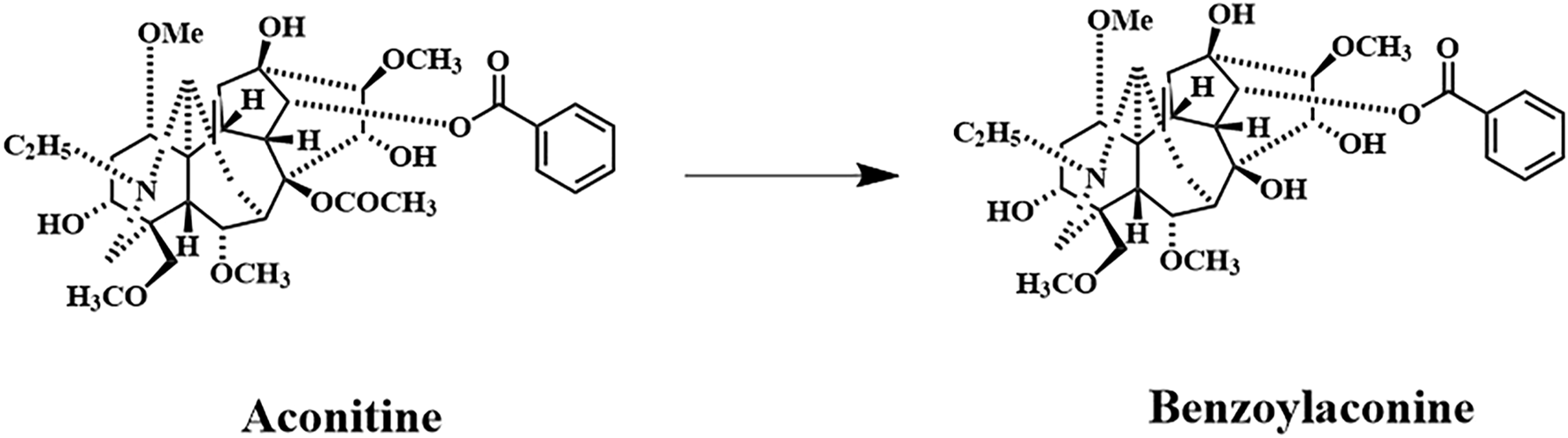
Primary metabolic pathway involving AC and BAC. Benzoylaconine; Chemical Abstracts Service registry number: 466–24-0; molecular formula: C32H45NO10; molecular weight: 603.7 g/mol.
The present systematic review summarizes evidence on the pharmacological effects, molecular mechanisms, and pharmacokinetics of BAC. This study was conducted adhering to standard methods: inclusion and exclusion criteria were predefined to ensure reproducibility, comprehensive search strategies were used across multiple databases (PubMed and Web of Science), and structured data extraction and quality assessment were performed in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines. The use of these standard methods differentiates the present study from narrative reviews, which typically offer a broader thematic overview without a systematic search or quality appraisal. Our ultimate goal was to provide valuable insights for future translational research.
2 Methods
PubMed and Web of Science were systematically searched for BAC-related studies published between January 2000 and November 2024. The article language was restricted to English. The search strategy was as follows: “(Benzoylaconine) AND (“2000/01/01” [Date - Publication]: “2024/11/30” [Date - Publication])” and “(ALL=(Benzoylaconine)) and DOP=(20000101/20241130).” A total of 111 records were retrieved from PubMed and 119 from Web of Science. Duplicate articles (n = 56) were removed using Zotero Literature Manager (https://www.zotero.org/). Articles unrelated to BAC were manually excluded by reviewing titles and abstracts. Moreover, studies focusing on the synthesis and development of BAC formulations were excluded. The final analysis included 53 articles. A flowchart depicting the article selection process (Figure 2) was created by following the PRISMA guidelines. (Page et al., 2021). The study protocol was registered with the International Prospective Register of Systematic Reviews database (identifier: CRD420250639795).
FIGURE 2
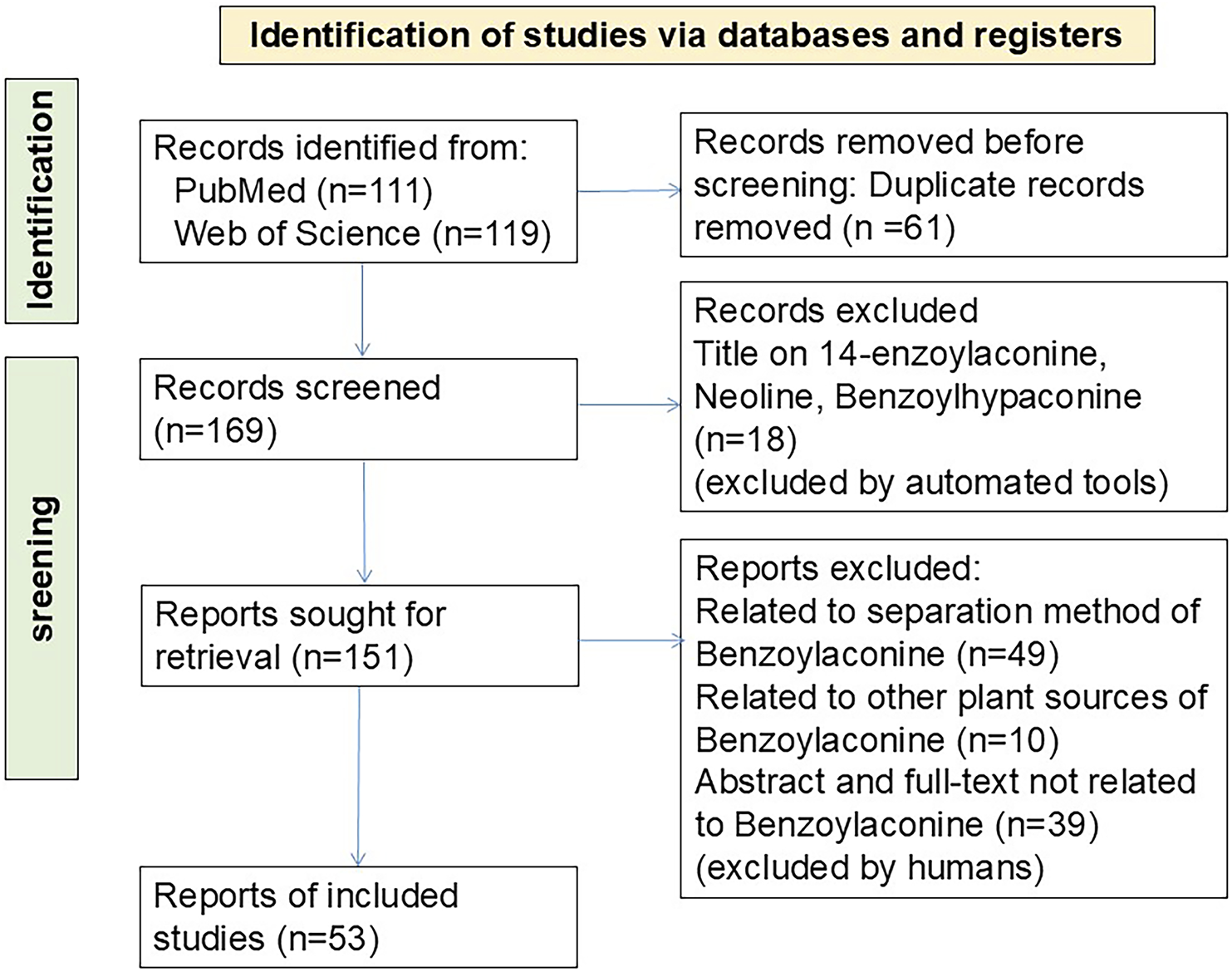
PRISMA flowchart depicting article selection.
In this comprehensive review, we explored the pharmacokinetics, toxicity, and pharmacological effects of BAC. In addition, through protein–protein interaction (PPI) analysis, we investigated the genes and proteins that mediate BAC’s therapeutic effects against various diseases. PPI revealed multiple high-node-degree or hub proteins for molecular docking with BAC. We further performed bioinformatic analyses, which involved gene ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), and disease ontology (DO) analyses. Our findings may inform future research on BAC and have implications for the safety and clinical application of BAC-containing TCMs.
3 Pharmacokinetics
3.1 Pharmacokinetic profiles
Pharmacokinetic studies provide valuable insights into in vivo drug metabolism and efficacy, toxicity reduction, and clinical application. High-performance liquid chromatography–mass spectrometry can be used to simultaneously determine the in vivo concentration–time profiles of several Aconitum alkaloids, such as BAC, AC, mesaconitine (MA), and hypaconitine (HA). (Fan et al., 2021). Fan et al. reported that BAC had a peak residence time of 60.75–69.59 min and an average residence time of 284.57–292.56 min in rats, indicating slow clearance. (Fan et al., 2021). High dosages consistently increased the apparent volume of distribution/OB ratio of Aconitum alkaloids, thereby reducing the OB of each alkaloid. (Fan et al., 2021).
Ye et al. intravenously injected rats with a mixture of eight alkaloids (AC, MA, HA, and BAC) and their parent compounds and eventually found these alkaloids in the rats’ blood. (Ye et al., 2012). Therefore, the parent compounds, which were more toxic than were the corresponding secondary metabolites, were rapidly eliminated from the body. Zhang et al. demonstrated that the maximum plasma concentrations of BAC, AC, and aconine (ACN) were reached within 1 h of the injection, indicating the rapid absorption of these alkaloids into circulation. (Zhang et al., 2016). Notably, the clearance rate and absorption efficiency were higher for AC than for BAC and ACN. AC primarily accumulates in muscles, whereas BAC and ACN primarily accumulate in the heart and kidneys. The bloodstream does not easily absorb BAC, and BAC is consequently excreted mainly through feces.
Yougui-Wan, a TCM preparation used for treating osteoporosis due to kidney yang deficiency, contains Fuzi. (Wang et al., 2023). The rates of BAC absorption and clearance in rats with osteoporosis due to kidney yang deficiency were higher and lower, respectively, than in those with osteoporosis due to kidney yin deficiency. Yougui-Yin, another TCM preparation for treating osteoporosis, contains six types of Aconitum alkaloids, among which BAC has the lowest clearance rate and longest residence time. (Zhang J. et al., 2019). Pharmacokinetic studies involving Shenfu-Tang revealed that although the ginsenoside Rg1 markedly enhanced BAC absorption and accelerated AC metabolism, it exerted nonsignificant effects on ACN. (Xu et al., 2020). BAC is the primary active metabolite in Sini-Tang, which is used to treat myocardial infarction (MI) and heart failure. (Zhou et al., 2019). A study highlighted a lower level of systemic BAC exposure and a lower rate of drug clearance in MI rats than in control rats. A Phase I clinical trial analyzed the pharmacokinetics of BAC after intravenous injection of Shenfu-Tang powder in 18 healthy volunteers. (Zhang et al., 2008). The pharmacokinetic half-life of BAC was relatively short (<1 h). Furthermore, the plasma concentration of BAC peaked at 30 min for moderate doses and 45 min for high and low doses.
Combining Fuzi with other TCM preparations can reduce its toxicity and improve therapeutic efficacy. (Zhang M. et al., 2019). A pharmacokinetic study underscored the therapeutic potential of BAC in rats orally treated with Fuzi plus Dahuang Fuzi decoction. (Liu X. et al., 2014). Notably, Ganjiang (Zingiber officinale Roscoe [Zingiberaceae; Zingiberis Rhizoma]) may promote AC clearance and enhance BAC absorption when coadministered with Fuzi. (Peng et al., 2013). Due to the reduced toxicity of Chuanwu (Aconitum carmichaelii Debx. [Ranunculaceae; Aconiti Radix]) when used in combination with honey, Chuanwu has been used in combination with honey for a long time. Wu et al. reported that a combination of Chuanwu and honey resulted in a higher peak plasma concentration, a higher area under the plasma concentration–time curve, a shorter time to reach the peak plasma concentration, and a longer half-life of clearance for BAC than did Chuanwu alone. (Wu et al., 2022). The detoxifying and synergistic effects of honey may be attributable to its ability to reduce the toxicity of diester diterpenoid alkaloids (DDAs) while increasing the in vivo bioactivity of MDAs. In their pharmacokinetic study on BAC in rats, Zhi et al. elucidated the mechanisms underlying the detoxifying effect of Chaihu on Caowu, supporting the rationale for using steaming and boiling methods in Caowu-based treatments. (Zhi et al., 2020).
The pharmacokinetic properties of drugs are influenced by protein binding. Zhou et al. performed multispectroscopic analysis, molecular docking, and dynamic simulation to investigate the interaction between human serum albumin and BAC. (Zhou et al., 2023). Molecular docking indicated that the electronic domains of BAC’s nitrogen and benzene ring skeletons were essential for complex formation. In silico simulation revealed stability changes and key residues involved in the binding of AC analogs with human serum albumin—for example, TRP-214, LEU-219, and LEU-238.
The key features of pharmacokinetic studies on BAC are summarized in Table 1. Further animal and human studies are required to analyze the pharmacokinetic properties of BAC when administered alone.
TABLE 1
| Species | Research objects | Dose (mg/kg) | Pharmacokinetic parameters | Reference | |||
|---|---|---|---|---|---|---|---|
| AUC(0-t) (ng/mL·h) | Tmax (h) | Cmax (ng/mL) | T1/2 (h) | ||||
| Administration route:Gavage | |||||||
| SD rats | BAC | 0.5 | 121.4 ± 45.77 | 1.16 ± 0.59 | 34.51 ± 21.62 | 5.479 ± 2.276 | Fan et al. (2021) |
| 1 | 280.6 ± 107.6 | 0.841 ± 0.327 | 122.8 ± 62.49 | 4.508 ± 1.424 | |||
| 2 | 987.7 ± 382.9 | 1.013 ± 0.392 | 359.9 ± 89.75 | 4.985 ± 1.471 | |||
| SD rats | CW | 92.34 ± 0.49 | 698.3 ± 52.41 | 0.75 ± 0 | 1.54 ± 0.09 | 6.057 ± 0.163 | Wu et al. (2022) |
| CW–honey | 90.65 ± 5.96 | 1239.8 ± 41.13 | 0.25 ± 0 | 12.26 ± 0.73 | 8.309 ± 0.148 | ||
| SD rats | BAC | 20 | 215 ± 38.1 | 0.333 ± 0.105 | 35.2 ± 11.5 | 11.0 ± 2.85 | Xu et al. (2020) |
| BAC + Rg1 | 20 + 20 | 318 ± 72.5 | 0.333 ± 0.00 | 63.1 ± 11.1 | 10.3 ± 4.21 | ||
| SD rats | CW | 0.001 | 6.74 ± 0.68 | 0.5 ± 0.00 | 4.44 ± 0.72 | 1.12 ± 0.18 | Zhi et al. (2020) |
| Hezi-CW | 2.97 ± 0.60 | 0.83 ± 0.29 | 1.79 ± 0.25 | 0.58 ± 0.17 | |||
| SD rats | SND | 1.895 mg/mL | 40.44 ± 13.61 | 0.71 ± 0.13 | 12.82 ± 5.80 | 12.38 ± 4.02 | Zhou et al. (2019) |
| MI SD rats | 10.79 ± 7.53 | 1.44 ± 0.17 | 3.56 ± 2.10 | 15.49 ± 2.99 | |||
| Wistar rats | Fuzi | 10 mL/kg | 8.2 ± 2.7 | 0.63 ± 0.14 | 3.4 ± 0.6 | 3.69 ± 0.25 | Song et al. (2015) |
| Fuzi-Mahuang | 12.2 ± 2.3 | 0.67 ± 0.20 | 3.1 ± 0.3 | 4.36 ± 1.13 | |||
| SD rats | Fuzi | 0.45 g/mL | 5.928 ± 0.324 | 0.586 ± 0.098 | 1.16 ± 0.05 | 3.11 ± 0.235 | Peng et al., 2013) |
| Fuzi–Ganjiang | 6.958 ± 0.392 | 0.365 ± 0.149 | 2.08 ± 0.16 | 3.154 ± 0.155 | |||
| Administration route: Oral | |||||||
| SD rats | CW | 0.1963 | 32.45 ± 9.17 | 0.19 ± 0.04 | 7.887 ± 4.192 | 13.82 ± 3.10 | Liu J. et al. (2014) |
| 0.2067 | 40.43 ± 13.04 | 4.17 ± 0.75 | 4.813 ± 3.923 | 9.9 ± 1.8 | |||
| SD rats | Fuzi | 0.2813 | 486.9 ± 255.5 | 0.6 ± 0.3 | 151.6 ± 129.3 | 9.4 ± 2.3 | Liu X. et al. (2014) |
| DFD | 0.2778 | 144.6 ± 36.8 | 0.2 ± 0.2 | 17.2 ± 10.7 | 28.7 ± 12.5 | ||
| SD rats | Shen-Fu prescription | 0.93 | 23.34 ± 13.01 | 0.29 ± 0.17 | 6.56 ± 3.32 | 1.10 ± 0.68 | Ouyang et al. (2018) |
| Kidney yang deficiency SD rats | YGW | 1764 | 8.87 ± 3.94 | 0.17 ± 0.00 | 13.25 ± 1.38 | 5.08 ± 1.29 | Wang et al. (2023) |
| Kidney yin deficiency SD rats | 2.65 ± 0.35 | 0.17 ± 0.00 | 6.64 ± 0.87 | 0.40 ± 0.12 | |||
| SD rats | BAC | 1 | 13.54 ± 2.29 | 0.31 ± 0.17 | 3.99 ± 1.20 | 9.49 ± 0.49 | Zhang et al. (2016) |
| SD rats | YGY | 15 mL/kg | 271.3 ± 39.4 | 8.4 ± 5.6 | 18.3 ± 3.8 | 10.7 ± 3.6 | Zhang J. et al. (2019) |
| Administration route:Intravenous injection | |||||||
| healthy volunteers | Shen-Fu powder | 1.1035 | 14.12 ± 3.31 | 0.75 ± 0 | 9.120 ± 2.02 | 0.739 ± 0.031 | Zhang et al. (2008) |
| 1.4677 | 21.87 ± 1.31 | 0.5 ± 0 | 11.80 + 0.290 | 1.036 ± 0.047 | |||
| 1.8428 | 25.47 ± 0.54 | 0.75 ± 0 | 17.58 + 1.76 | 0.8 ± 0.23 | |||
Pharmacokinetic studies on BAC.
Abbreviations: SD, sprague dawley; AUC0–t, area under the plasma concentration–time curve from 0 to the last quantifiable time point; Cmax, peak plasma concentration; Tmax, time to reach Cmax; T1/2, half-life of clearance; CW (Caowu), Aconiti Kusnezoffii Radix; Fuzi, Aconiti Lateralis Radix Praeparata; DFD, Dahuang Fuzi decoction (composed of Radix et Rhizoma Rhei, Fuzi, and Radix et Rhizoma Asari); YGW, You-Gui-Wan; YGY, You-Gui-Yin; Hezi-CW, caowu processed with chebulae fructus; SND, sini decoction; MI, myocardial infarction; Mahuang, Ephedrae Herba; Ganjiang, Rhizoma Zingiberis.
3.2 Effects of BAC on efflux transporters
Efflux transporters—such as P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and multidrug resistance–associated protein 2 (MRP2)—are key regulators of drug pharmacokinetics and herb–herb or herb–drug interactions. (Ye et al., 2013). P-gp and BCRP primarily mediate the transport of AC, whereas MRP2 mediates that of BAC. (Dai et al., 2015). A study demonstrated that Aconitum alkaloids upregulated P-gp expression in LS174T and Caco-2 cells; the order of the effect magnitude was as follows: AC > BAC > ACN. (Wu et al., 2016). Moreover, AC and BAC increased the transport activity of P-gp. Intracellular BAC can increase adenosine triphosphate (ATP) levels and mitochondrial mass. Furthermore, BAC considerably upregulates the expression of MRP2 and BCRP and increases the efflux activity of MRP2 by activating the Nrf2-mediated pathway. (Wu et al., 2018). Therefore, BAC may serve as a quality indicator for Aconitum-derived botanical drugs.
4 Toxicity
Aconitum alkaloids have acute and high toxicity, which induces severe arrhythmia that can result in death. AC, MA, and HA are the main and highly toxic alkaloids in the genus Aconitum. (Ji et al., 2019; Ye et al., 2019). Accidental ingestion of Aconitum can be fatal. (Dickens et al., 1994). Although Aconitum roots are highly toxic, (Ito et al., 1996), they have for centuries been used in traditional medicine across East Asia. According to the 2020 Chinese Pharmacopoeia, only processed Aconitum roots are permitted for clinical use in Fuzi preparations. Aconitum alkaloids are metabolized primarily by esterases. AC is hydrolyzed at the C-8 position to form BAC and at both the C-8 and C-14 positions to form ACN (Figure 1). (Mizugaki et al., 1998; Wada et al., 2005).
Pharmacological experiments in Sprague Dawley rats indicated that 0.01 mg/kg AC and 10 mg/kg ACN improved cardiac function, whereas 2 mg/kg BAC impaired it. (Liu et al., 2017). DDAs are 100–700 times more toxic than are MDAs. During aconite processing, Aconitum alkaloids are converted from DDAs into MDAs and then into alkylamine diterpenoid alkaloids, thereby reducing toxicity. (Bisset, 1981). However, caution should be exercised to avoid overprocessing and overhydrolysis of BAC into ACN. (Li et al., 2016). A study measuring BAC and similar alkaloids in rapidly dried and fresh-cut Aconitum revealed that although traditional processing reduces toxicity, it leads to >85.2% alkaloid loss. (Zhang D. K. et al., 2017).
Ephedrae Herba (Mahuang) (Ephedra sinica Stapf [Ephedraceae; Ephedrae Herba)–Fuzi is a traditional formula used to treat the common cold, asthma, and rheumatoid arthritis (RA). A study reported that the combination of Aconitum and Ephedra poses a risk of Aconitum alkaloid poisoning, as evidenced by the widespread distribution of nine alkaloids, including BAC, in the heart, liver, spleen, lungs, kidneys, and brain of treated individuals. (Ren et al., 2017). Prolonged use of this formula may lead to drug accumulation. Therefore, patients using formulations that contain ephedrine and Aconitum alkaloids should be closely monitored to prevent adverse effects on the cardiovascular and central nervous systems. (Song et al., 2015). Oral administration of AC induced bradycardia and hypotension in rats, consistent with AC poisoning in humans. (Zhang P. et al., 2017). These findings indicate that the metabolites of AC and BAC have antihypertensive properties.
5 Pharmacological effects
5.1 Cardioprotective effects
Fuzi is widely used for treating heart failure and related cardiac diseases. BAC, a major active metabolite in Fuzi, holds promise for the prevention and treatment of cardiovascular diseases, inflammation, arthritis, and other conditions (Table 2).
TABLE 2
| Cell/Animal | Model | BAC dose | Effect | Molecular mechanisms | References |
|---|---|---|---|---|---|
| Cardiovascular system protection | |||||
| Wistar Rat | MI | Gavaged, 0.0068 mg/kg | Promoting cardiac function, alleviating myocardial hypoxia, inhibiting inflammatory response fibrosis in heart tissue | ↓VEGF, ↓PKM2, ↓GLUT-1, ↓LDHA, ↓TNF-α, ↓IL-1β, ↓IL-6, ↓COX2 | Xing et al. (2022) |
| C57BL/6 mice, ACE2−/− mice | HF | Oral, 3, 10, 30 mg/kg | Enhancing cardiac function in heart failure | ACE2, ↓p38, ↓ERK, Mitochondrial, ↓ROS, ↓NF-κB | Zhang et al. (2024) |
| SD rats | IR | 20 mg/kg | Alleviated myocardial injury | ↑AMPK, ↑PGC-1 | Chen Z. et al. (2022) |
| H9c2 cells | 50, 75 µM | improves mitochondrial function | ↑ROS, ↑LDH | ||
| Anti-inflammatory activity and Antirheumatic activities | |||||
| SD rats | OA | Gavaged, FZD, 0.2114 ± 0.028 mg/kg | Anti-OA, restored cartilage degeneration, ameliorating pain behavior, benefitting cartilage anabolism, increased cell viability and wound healing capacity, recovering histopathological alterations | ↑Col2, ↓MMP13, ↓Col10, ↓PI3K, ↓Akt | Chen L. et al. (2022) |
| SD rats | CIA | Gavaged, 1.5 mg/kg | Alleviate the degree of swelling, arthritis index and pathological lesions of the sacroiliac gland | ↓PGE2, ↓IL-1β, ↓TNF-α, ↓VEGF, ↓IgG, ↓STAT1, ↓STAT3 | Li et al. (2022) |
| KM mice | inflammatory ear, paw edema | intravenous injection, 10 mg/kg | Anti-inflammatory for RA therapy | ↓TNF-α, ↓IL-1β, ↓NF-κB, ↓p65 | Gai et al. (2020) |
| RAW264.7 cells | 5, 18, 40 μg/mL | reduce the viability of activated macrophages |
|||
| Wistar rats | AIA | Gavage, 0.126, 0.252, 0.504 mg/kg | Anti-inflammatory, inhibiting immune organs (spleen and thymus), attenuating paw swelling, infiltration of inflammatory cells and synovial hyperplasia | ↓IL-1β, ↓IL-17A, ↓COX-2 | Li et al. (2021) |
| SW982 cell | 0, 5.10 µM | Anti-inflammatory | ↓IL-6, ↓MAPK, ↓Akt, ↓IκB-α, ↓p65, ↓IL-8 | Yu et al. (2020) | |
| SD rats and KM mice | OA | Orally, 536.6 ± 6.16, 813.1 ± 3.5 mg/kg | Against OA, attenuated joint pain, prevented articular degeneration, suppress chondrocyte hypertrophy and extracellular matrix degradation | ↓Col10, ↓Mmp2, ↓Sox5, ↓Adamts4/5/9, ↑Col2 | Zhang et al. (2020) |
| HFLS-RA fibroblast-like synoviocytes | RA | 1,000 μg/mL | Anti-inflammatory and anti-rheumatic activities | ↓PGE2, ↓IL-6, ↓IL-1β, ↓TNF-α, ↓TLR4, ↓HIF-1α, ↓VEGFA | Zhang et al. (2021) |
| SD rats | SH | 0.6, 2, 6; 3, 10, 30 mg/kg | Antihypertensive effects, enhancing vasodilation and alleviating vascular inflammation | ↑Akt/eNOS, ↑NO, ↑Ang (1–7), ACE2; ↓TNF-α, ↓IL6, ↓COX-2, ↓ACE, ↓AngII, ↓IKB-α | Zhang et al. (2022) |
| HUVECs cell | 25, 50, 100 μM | ||||
| RAW264.7 macrophage cells | 0.1, 1, 10, 100, 500 μM | Anti-inflammatory | ↓IL-6, ↓TNF-α, ↓IL-1β, ↓ROS, ↓NO, ↓PGE2, ↓iNOS, ↓COX-2, ↓NF-κB, ↓IκBα, ↓p65, ↓TLR4, ↓TAK1, ↑JNK, ↑p38, ↑ERK | Zhou et al. (2021) | |
| Others | |||||
| HepG2 cells | 25, 50, 75 µM | Induces mitochondrial biogenesis, increased mitochondrial mass | ↑mtDNA copy number, ↑cellular ATP, ↑OXPHOS, ↑AMPK | Deng et al. (2019) | |
| Balb/c mice | 10 mg/kg | ||||
| SD rats | Mitochondrial abnormalities | ↑mitochondrial energy metabolism | Zhang D. K. et al. (2017) | ||
| HaCaT keratinocytes | Psoriasis | 10, 20, 40 µM | Anti-psoriasis, inhibiting cell proliferation, the release of inflammatory factors, and the accumulation of Th17 cells | ↓STAT3 | Li et al. (2023) |
| C57BL/6 mice | 1, 3 mg/kg | ||||
| C57BL/6J mice | cholestatic mouse | Oral, YCZFD, 2.383 ± 0.103, 4.765 ± 0.205 μg/kg | Against CLF, decreased liver injury, and fibrosis biochemical indicators | ↓PDGFRβ, ↓PI3K-Akt | Meng et al. (2024) |
| Wistar rats and KM mice | 350 μg/cm2 | analgesic and anti-inflammatory effects | ↑surface tension, ↑skin permeation, ↑interaction strength | Liu et al. (2019) | |
| SD rats | IR | intraperitoneal injections, 5,10,20 mg/kg | against skeletal muscle I/R injury |
↑IF1, ↑AMPK, ↑Nrf2, ↑HO-1 | Cui et al. (2024) |
| C2C12 cells | 60 μM | ||||
Molecular mechanisms underlying the pharmacological effects of BAC.
Abbreviations: SD, sprague dawley; MI, myocardial infarction; HF, heart failure; I/R, ischemia/reperfusion; AIA, adjuvant-induced arthritis; LDH, lactate dehydrogenase; OA, osteoarthritis; FZD, fuzi decoction; CIA, collagen-induced arthritis; KM, kunming; RA, rheumatoid arthritis; SH, spontaneous hypertension; YCZFD, yinchenzhufu decoction.
BAC considerably reduces the serum levels of superoxide dismutase, MDA, creatine kinase–myocardial band, cardiac troponin T, and cardiac troponin I in MI rats and downregulates the expression of hypoxia- and inflammation-related genes such as VEGF, PKM2, GLUT-1, LDHA, TNF-α, IL-1β, IL-6, and COX2. (Xing et al., 2022). Furthermore, BAC markedly improves cardiac function, reduces infarct size, inhibits inflammatory cell infiltration, and mitigates myocardial fibrosis.
BAC was demonstrated to inhibit angiotensin II-induced cellular hypertrophy and fibrosis in primary cardiomyocytes and fibroblasts from rats and mitigate cardiac dysfunction and remodeling in mice with thoracic aortic constriction. (Zhang et al., 2024). Regarding heart failure treatment, BAC directly targets angiotensin-converting enzyme 2 (ACE2), thereby inhibiting the activation of the p38/extracellular signal-regulated kinase signaling–mediated mitochondrial reactive oxygen species (ROS) and nuclear factor (NF)-κB pathways. BAC appears to be a promising ACE2 agonist and therapeutic agent for heart failure because it can regulate mitochondrial ROS release and inflammatory activation, thereby improving cardiac function. (Zhang et al., 2024). An in vitro study indicated that BAC enhanced the survival rate of H9c2 cells subjected to oxygen–glucose deprivation and reperfusion. (Chen L. et al., 2022). Furthermore, BAC upregulates the expression of phosphorylated adenosine monophosphate–activated protein kinase (AMPK) and peroxisome proliferator-activated receptor gamma coactivator 1α. It also improves mitochondrial function, reduces oxidative stress and apoptosis, and mitigates myocardial injury in vivo.
The mechanisms through which BAC treats cardiovascular disease, as outlined in the literature, are depicted in Figure 3 and Table 2. Nonetheless, further studies are required to comprehensively identify the targets of BAC and the pathways mediating its cardioprotective effects.
FIGURE 3
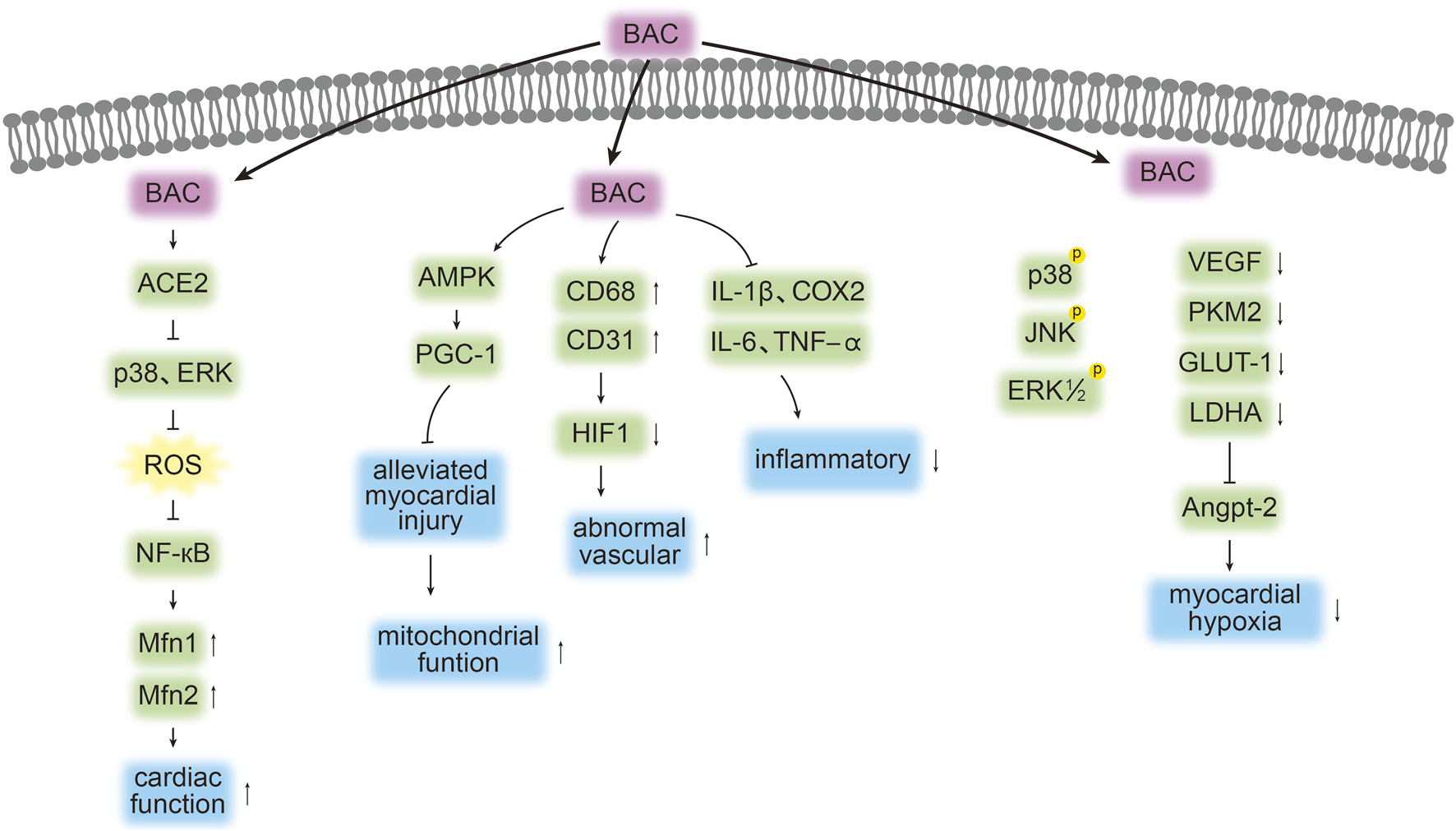
Targets and pathways associated with the cardioprotective effects of BAC. The symbols “↓”, “↑”, “┤,” and p denote protein downregulation, upregulation, inhibition, and phosphorylation, respectively. Arrows (“→”) indicate signal transduction or promotion.
5.2 Anti-inflammatory and antiarthritic effects
BAC exhibits strong anti-inflammatory activity in lipopolysaccharide (LPS)-stimulated macrophages. It was demonstrated to reduce primary and secondary paw swelling in rats with adjuvant-induced arthritis and to mitigate collagen-induced arthritis. (Gu et al., 2018). In addition, BAC markedly alleviated joint tissue inflammation, prevented bone destruction, and reduced serum interleukin (IL)-1β and IL-17A levels. It also downregulated the expression of cyclooxygenase (COX)-1 and COX-2 in synovial tissues. (Li et al., 2021). BAC further inhibited IL-1β-induced gene and protein expression of IL-6 and IL-8 in human synovial SW982 cells. Moreover, BAC suppressed the activation of mitogen-activated protein kinase (MAPK) and protein kinase B (Akt), inhibited the degradation of inhibitor of κB-α, and prevented the phosphorylation and nuclear translocation of p65. (Yu et al., 2020). One study reported that BAC strongly inhibited the proliferation of human-fibroblast-like synoviocytes derived from adult RA synovial tissues, (Zhang et al., 2021), highlighting the in vitro antirheumatic activity of this alkaloid. This activity may be mediated through downregulation of inflammatory cytokines, such as prostaglandin (PGE)2, IL-6, IL-1β, and tumor necrosis factor (TNF)-α; hypoxia-inducible factor (HIF)-1α; vascular endothelial growth factor (VEGF); and Toll-like receptor (TLR)4 expression.
The aforementioned findings are consistent with those reported by another study, in which BAC considerably inhibited the release of proinflammatory cytokines and mediators, such as IL-6, IL-1β, TNF-α, ROS, nitric oxide, and PGE2. (Zhou et al., 2021). BAC also dose-dependently blocked LPS-induced increases in the protein levels of inducible nitric oxide synthase and COX-2. Moreover, it suppressed the phosphorylation and degradation of inhibitor of κB-α and the nuclear translocation of p65, thereby inhibiting LPS-induced NF-κB activation. In addition, BAC blocked LPS-induced increases in the levels of phosphorylated c-Jun N-terminal kinase, p38, and extracellular signal-regulated kinase. It further inhibited LPS-induced phosphorylation of transforming-growth-factor-β-activated kinase one in activated RAW264.7 macrophages. (Zhou et al., 2021). Considering BAC’s anti-inflammatory properties, Gai et al. developed a drug delivery system by encapsulating BAC in nanoparticles to regulate inflammatory responses. (Gai et al., 2020). Activated macrophages treated using this system exhibited 70% and 66% lower TNF-α and IL-1β levels, respectively, compared with corresponding levels in the control without BAC nanoparticles. Liu et al. reported that the use of penetration enhancers in BAC-loaded transdermal patches intensified the analgesic and anti-inflammatory effects of BAC, supporting its potential for treating inflammatory pain. (Liu et al., 2019).
RA is a chronic, systemic autoimmune disease of the joints. BAC has been demonstrated to substantially reduce swelling and arthritis index scores in rats with collagen-induced arthritis and downregulate IL-1β, VEGF, PGE2, TNF-α, and immunoglobulin G by inhibiting the Janus kinase/STAT pathway. In a rat model of osteoarthritis, BAC downregulated aberrant expression of Col10, Mmp2, Sox5, and Adamts4/5/9 and upregulated that of Col2 in cartilage. (Zhang et al., 2020). In vitro experiments using rat cells revealed that treatment with BAC-containing serum considerably promoted chondrocyte proliferation and regulated Col2, Mmp1, Adamts9, and Aggrecan expression. These findings highlight the molecular mechanisms underlying BAC-mediated inhibition of chondrocyte hypertrophy and extracellular matrix degradation. Thus, BAC serves as an analgesic and a regulatory agent. In a study on osteoarthritis, Fuzi-Tang was found to involve similar molecular mechanisms. (Chen Z. et al., 2022). BAC, the main active metabolite in Fuzi-Tang, improved pain-related parameters, mitigated histopathological changes, promoted cartilage anabolism (by upregulating Col2 expression), and inhibited cartilage catabolism (by downregulating matrix metalloproteinase 13 and Col10 expression), thereby reversing cartilage degeneration in rats with osteoarthritis. Experiments and network pharmacology analyses have indicated that the phosphoinositide 3-kinase/Akt pathway mediates the antiarthritic effect of BAC. Notably, BAC may be the active metabolite responsible for the anti-inflammatory and immunosuppressive effects of Mahuang–Fuzi–Xixin-Tang. (Tang et al., 2015).
The primary targets of BAC and the pathways that mediate its activity against inflammation and RA are depicted in Figure 4 and Table 2. Further studies are required to comprehensively explore BAC targets in inflammation and RA as well as associated therapeutic pathways.
FIGURE 4
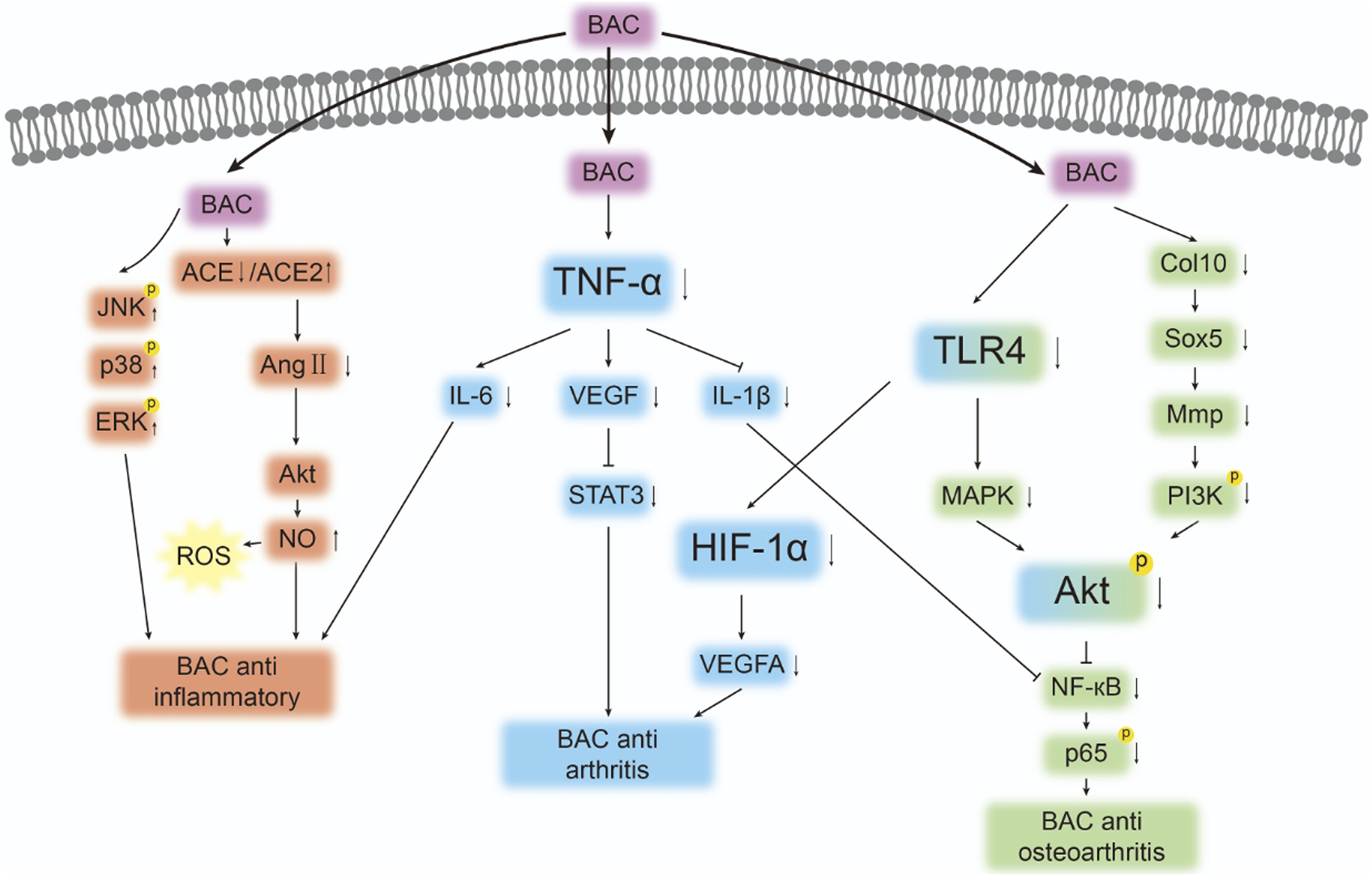
Targets and pathways associated with the anti-inflammatory and antirheumatic effects of BAC. The symbols “↓”, “↑”, “┤,” and p denote protein downregulation, upregulation, inhibition, and phosphorylation, respectively. Arrows (“→”) indicate signal transduction or promotion.
5.3 Others
BAC modulates mitochondrial energy metabolism and exerts antipsoriatic and anticholestatic effects against liver fibrosis.
In HepG2 cells, BAC dose-dependently increased the mass of the mitochondria, copy number of mitochondrial DNA, cytosolic level of ATP, and expression of proteins involved in oxidative phosphorylation. (Deng et al., 2019). Moreover, this alkaloid dose-dependently upregulated the expression of proteins involved in the AMPK pathway both in vivo and in vitro. In HepG2 cells, BAC increased cell viability without influencing cell proliferation. In vitro data suggest that BAC increases the rate of oxygen consumption in mice and activates AMPK signaling in the heart, liver, and muscles. Notably, the ester bond at the C-8 position, hydroxyl group at the C-3 position, and ethyl group on the nitrogen atom in BAC substantially contribute to its effects on mitochondrial energy metabolism. (Zhang D. K. et al., 2017). These findings highlight the therapeutic potential of BAC against diseases involving mitochondrial dysfunction.
Psoriasis is a common polygenic skin condition characterized by inflammatory infiltrates, keratinocyte hyperproliferation, and immune cell accumulation. BAC may ameliorate psoriasis symptoms by inhibiting keratinocyte proliferation, inflammatory factor release, and Th17 cell accumulation. (Li et al., 2023). In TNF-α/LPS-stimulated HaCaT keratinocytes, BAC markedly reduced the protein and mRNA levels of inflammatory cytokines by inhibiting STAT3 phosphorylation. Therefore, BAC may slow psoriasis progression and thus serve as a promising therapeutic agent for psoriasis.
Yinchenzhufu decoction (YCZFD) is a TCM preparation with hepatoprotective effects. YCZFD contains seven primary metabolites, including BAC, which can considerably reduce serum metabolite levels, liver injury, and fibrosis index scores in mice with cholestatic liver fibrosis (CLF). (Meng et al., 2024). This study revealed that the expression of platelet-derived growth factor receptor-β (PDGFRβ) was upregulated in the liver of mice with CLF. (Meng et al., 2024). However, YCZFD treatment downregulated the expression of PDGFRβ. The protective effect of BAC against CLF is mediated primarily through regulation of the PDGFRβ/phosphoinositide 3-kinase/Akt pathway.
BAC has been demonstrated to protect skeletal muscle tissue from ischemia/reperfusion injury; increase cell viability; elevate the superoxide dismutase level; and reduce creatine kinase, lactate dehydrogenase, ROS, MDA, and proapoptotic factor levels both in vivo and in vitro. (Cui et al., 2024). Mechanistically, BAC upregulates ATPase inhibitory factor 1 expression, promotes AMPK phosphorylation, facilitates Nrf2 nuclear translocation, and induces heme oxygenase-1 expression.
6 Network pharmacology analysis
To investigate the potential relationships of BAC with its target proteins and genes, we performed PPI network analysis by using a previously reported method (Xie et al., 2020). The resulting network contained 31 nodes and 146 edges, excluding hidden standalone nodes. IL-6, Akt1, and STAT3 emerged as the primary proteins with high connectivity in the PPI graph (Figure 5).
FIGURE 5
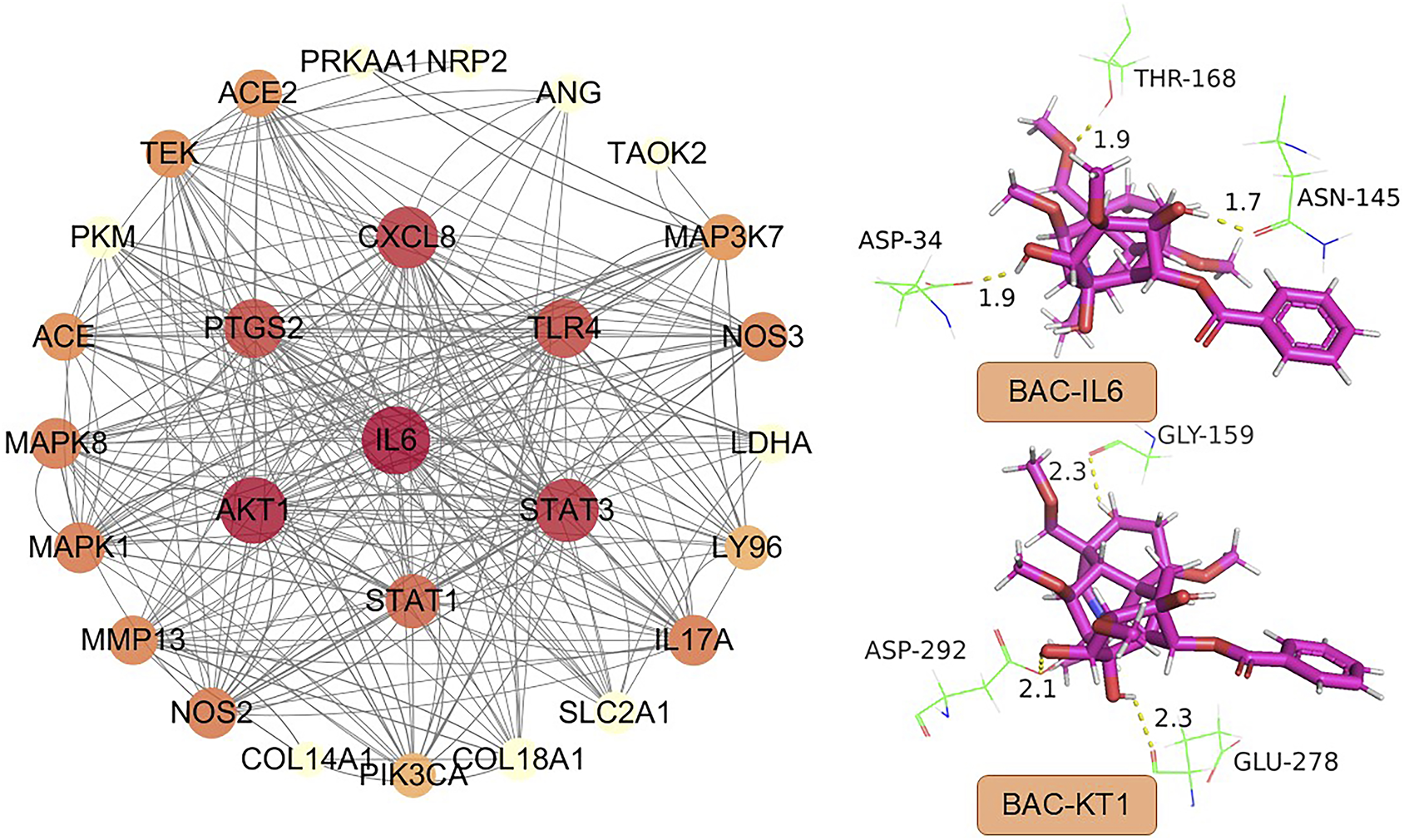
PPI network constructed using BAC targets and high-node-degree proteins.
Molecular docking performed using SYBYL-X (version 1.3; Tripos Inc., St. Louis, MO, United States) revealed three key amino acid residues involved in the binding of IL6 with BAC: Asp, Thr, and Asn. These residues formed three intramolecular hydrogen bonds, each of length <2 Å: 1.9 Å for Asp34, 1.9 Å for Thr168, and 1.7 Å for Asn145. The amino acid residues involved in the binding of Akt1 with BAC were Asp, Gly, and Glu. The corresponding hydrogen bonds had length smaller than 2.5 Å; the lengths were 2.1 Å for Asp292, 2.3 Å for Gly159; and 2.3 Å for Glu278, indicating strong binding affinity (Figure 5). The score for molecular docking between IL-6 and BAC was 6.04, whereas that for molecular docking between Akt1 and BAC was 7.53. To simplify the simulation, explicit water molecules were excluded from the docking system. Our findings indicate that BAC strongly interacts with IL-6 and Akt1, highlighting the need for further in-depth mechanistic studies. IL-6 and Akt1 appear to play key roles in the PPI network of BAC, as indicated by their high degrees and docking affinities. A study demonstrated that, under hypoxic conditions, IL-6 promoted apoptosis and inhibited autophagy in cardiac stem cells by suppressing the phosphorylation of STAT3, suggesting the existence of a regulatory axis. (Li et al., 2025). Regarding systemic inflammation, cannabidiol mitigates cardiovascular injury by downregulating IL-6, STAT3, and HIF-1α and upregulating endothelial nitric oxide synthase; these findings implicate the IL-6/STAT3 pathway in the regulation of oxidative stress and inflammation. (Tepebasi et al., 2024). In pulmonary hypertension, the IL-6/gp130 pathway in CD4+ T cells drives pathogenesis through STAT3 phosphorylation and Th17 cell activation. Notably, IL-6 knockdown or gp130 deficiency can ameliorate pulmonary hypertension. (Ishibashi et al., 2024). In RA, the IL-6/STAT3 pathway serves as an “autoimmune adaptive axis,” enabling immune cells and synovial fibroblasts to perpetuate inflammation and resist treatment through epigenetic and noncoding RNA–mediated mechanisms. (Kumar and Mangla, 2025). Together, the findings indicate that the IL-6/STAT3 pathway mediates apoptosis, inflammation, and immune regulation in cardiovascular disease and arthritis. (Qin et al., 2025; Abdelaziz et al., 2025). Thus, this pathway holds promise as a therapeutic target for BAC.
After constructing the PPI network, we performed gene enrichment analyses—GO, KEGG, and DO analyses—with the identified proteins and genes. These analyses were performed using relevant bioinformatic tools (http://www.bioinformatics.com.cn/). (Sherman et al., 2022)
GO is used to determine the properties of genes and gene products. A biological analysis revealed that the key processes influenced by BAC include inflammation, angiogenesis, LPS-mediated signaling, positive regulation of smooth muscle cell proliferation, positive regulation of IL-8 production, positive regulation of IL-1β production, and phosphorylation of the stress-activated MAPK cascade (Figure 6A), supporting our conclusions. A cellular component analysis indicated localization in the extracellular region, endoplasmic reticulum lumen, cytoplasm, caveolae, cytosol, LPS receptor complex, plasma membrane, and extracellular space (Figure 6B). A molecular function analysis suggested that the function of BAC primarily includes protein serine/threonine kinase activity, identical protein binding, protein homodimerization, MAP kinase activity, and ATP binding (Figure 6C).
FIGURE 6
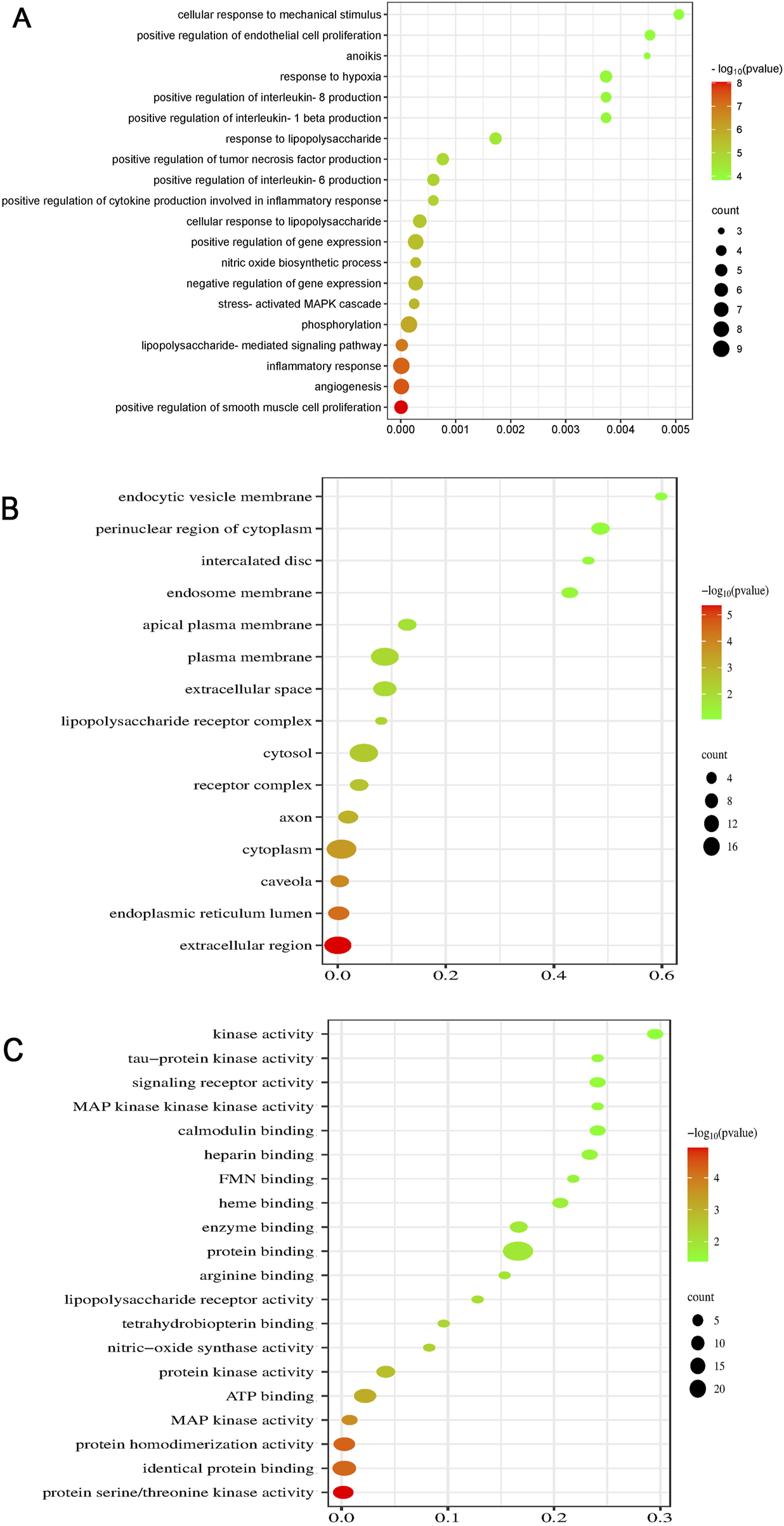
Dot plots for GO analysis: (A) BP, (B) CC, and (C) MF analyses.
The KEGG database contains information on known intermolecular interactions, such as biochemical and metabolic reactions. Figure 7A presents the major pathways potentially influenced by BAC—for example, HIF-1 signaling, cancer development, TLR signaling, advanced glycation end product–receptor for advanced glycation end product signaling (in diabetes-related complications), lipid metabolism and atherosclerosis development, and hepatitis B development. The predicted pathways, particularly those involving cancer and HIF-1α signaling, should be validated through Western blotting and reverse transcription quantitative polymerase chain reaction to elucidate the precise mechanisms underlying the therapeutic effects of BAC.
FIGURE 7
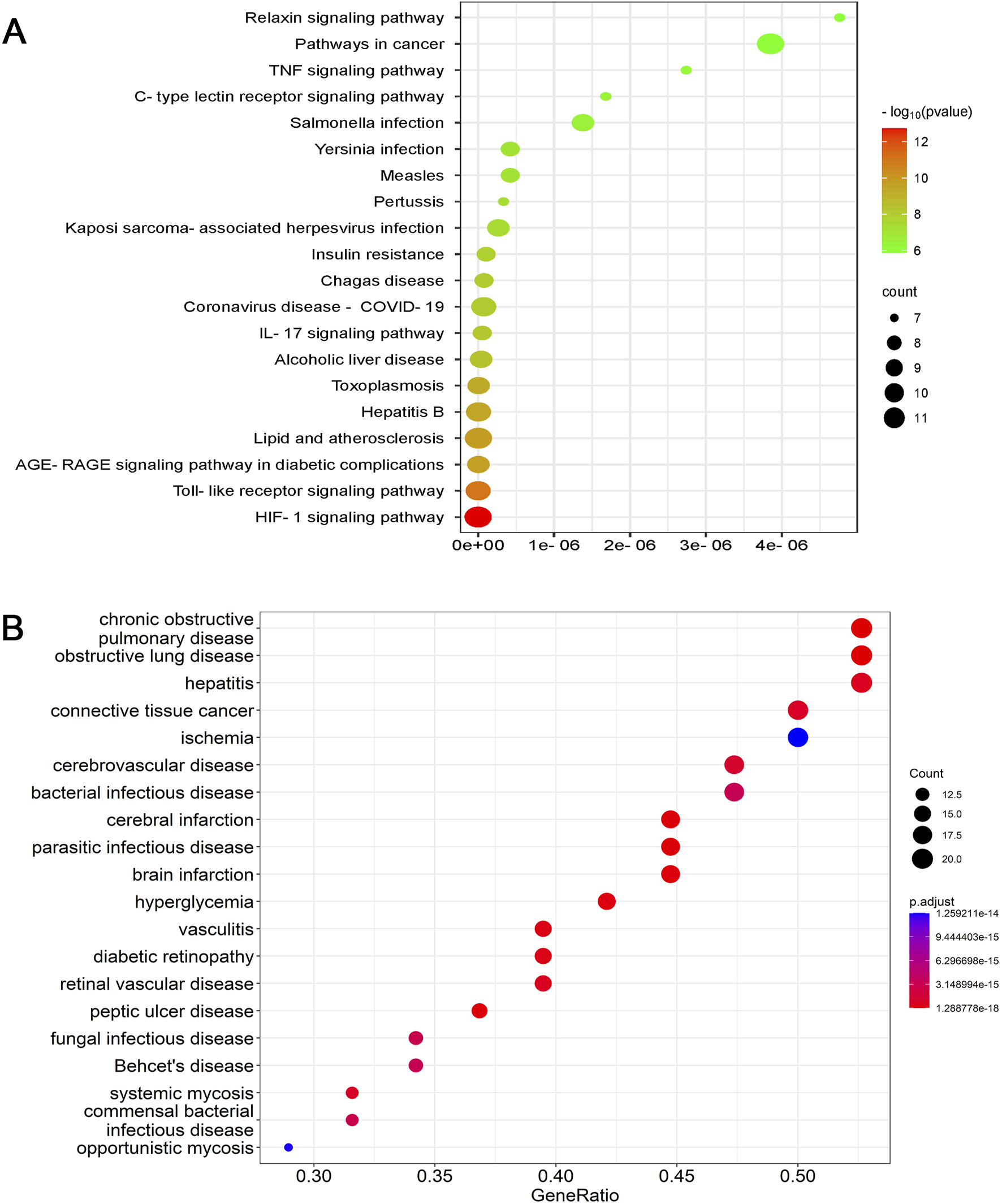
Dot plots for (A) KEGG and (B) DO analyses.
DO is a key analytical tool used in studies concerning the genetic basis of pathogenesis. In the present study, DO analysis indicated that genes and proteins associated with BAC were also associated with chronic obstructive pulmonary disease, obstructive pulmonary disease, hepatitis, connective tissue cancer, cerebrovascular disease, bacterial infectious diseases, cerebral infarction, parasitic infections, brain infarction, and ischemia (Figure 7B). Although BAC is known to alleviate inflammation and reduce cardiocerebrovascular risk, very few studies have investigated its effects against chronic obstructive pulmonary disease, connective tissue cancer, bacterial infections, and myocardial ischemia, indicating a need for further investigation.
On the basis of the literature, we hypothesize that BAC exhibits immunomodulatory activity in infectious diseases. Some studies have underscored the vital role of TLR4–NF-κB signaling in parasitic infections. For instance, in poultry birds with Heterakis gallinarum infection, elevated TLR4 level was associated with elevated proinflammatory cytokine (e.g., IL-1β and interferon-γ) levels and tissue damage. (El-Saied et al., 2024). Similarly, Plasmodium falciparum elicited a TLR-mediated proinflammatory response, upregulating TNF-α and downregulating IL-10. (McCall et al., 2007). This mechanism may be analogous to the immunomodulatory effect of BAC. In viral hepatitis (hepatitis B or C), dysregulated microbiota and cytokine profiles (e.g., IL-6 and TNF-α) exacerbate liver pathology, (Padilha et al., 2024), highlighting the need to regulate the crosstalk between TLR and NF-κB, as demonstrated in Echinococcus granulosus infection. (Taha et al., 2025). Parasitic nematodes such as Trichinella spiralis reduce the levels of proinflammatory cytokines (e.g., IL-6 and TNF-α) to ameliorate metabolic disorders. (Kang and Yu, 2024). BAC may mimic this immunoregulatory mechanism.
7 Conclusion and implications
We systematically reviewed BAC-related studies published between January 2000 and November 2024. This review focused on the therapeutic effects of BAC against cardiovascular and cerebrovascular diseases, inflammation, arthritis, and related conditions. Our results indicate that BAC regulates multiple signaling cascades, exerting diverse biological effects on various targets.
BAC enhances cardiac function, alleviates myocardial hypoxia, and inhibits inflammatory response–induced fibrosis in cardiac tissue. It also exerts antiarthritic effects by restoring degenerated cartilage, alleviating joint pain, reversing histopathological changes, promoting cartilage anabolism, inhibiting cartilage catabolism, and improving chondrocyte viability and wound healing capacity. Moreover, BAC reduces sacroiliac gland swelling, foot and plantar swelling, arthritis index scores, and the extent of pathological lesions (e.g., inflammatory cell infiltration and synovial hyperplasia). It further suppresses immune organs (e.g., the spleen and thymus) and inhibits splenocyte proliferation, exerting strong anti-inflammatory effects.
Although promising findings have been obtained, several limitations persist in BAC-related research. (1) Animal Models versus human diseases: Although animal models are indispensable in preclinical research, interspecies differences in genetics, metabolism, and disease pathophysiology limit their translational relevance. For example, murine models often fail to fully replicate human immune responses and complex comorbidities. (2) Effect of low OB: Low OB (attributable to factors such as first-pass metabolism or poor intestinal absorption) can reduce systemic exposure and thus drug efficacy. In such cases, high dosing (which carries a high risk of toxicity) or alternative delivery methods (e.g., nanocarriers and prodrugs) should be considered to achieve therapeutic concentrations. (3) Delivery route trade-offs: Oral administration offers convenience at the cost of variable absorption; it is thus suitable for chronic conditions. Intravenous delivery, suitable for acute conditions, ensures full bioavailability but is invasive. Subcutaneous or intramuscular routes strike a balance between bioavailability and patient compliance, particularly for biologics. Thus, the delivery route should be tailored to drug characteristics and clinical indications to optimize treatment efficacy and adherence. However, few studies have compared the various routes of BAC monotherapy delivery. Further research in this area is warranted.
Owing to its multitarget mechanism and low toxicity, BAC offers therapeutic advantages over conventional anti-inflammatory and cardiovascular drugs. Unlike single-target biologics (e.g., anti-IL-6 receptor monoclonal antibodies) and small-molecule kinase inhibitors (e.g., Janus kinase/STAT blockers), BAC simultaneously modulates IL-6/STAT3 signaling and activates ACE2. This dual mechanism makes BAC suitable for treating a wide range of comorbidities such as RA and cardiovascular diseases.
In summary, although the cardioprotective and anti-inflammatory effects of BAC are well known, systematic investigations into its multitarget mechanisms remain limited. Future studies should explore the relationships between BAC’s physiological activities and investigate its multipathway–multitarget synergistic mechanisms. Furthermore, researchers should incorporate network pharmacology analyses and perform systematic analyses from a holistic perspective. To enhance translational relevance, additional in vitro and in vivo experiments should be conducted to validate BAC’s interactions with IL-6, STAT3, and Akt1.
8 Future perspectives and unresolved challenges
Although BAC has considerable therapeutic potential, several challenges hinder its clinical application. First, sustained-release formulations must be developed to address the metabolite’s short half-life and improve treatment adherence in patients with chronic diseases such as arthritis. Second, leveraging the ACE2 agonist activity of BAC in precision medicine represents a promising area of research. For instance, stratifying patients with heart failure by biomarkers may help predict each patient’s response to BAC’s dual effects: cardioprotective (through STAT3 inhibition) and vasodilatory (through the ACE2/angiotensin one to seven pathway) effects. Finally, the synergistic effects of BAC with other drugs should be systematically evaluated, particularly in comparison of BAC with mainstream drugs such as IL-6 inhibitors for arthritis or SGLT2 inhibitors for heart failure, to determine whether BAC can improve treatment efficacy or reduce adverse reactions. Addressing these research gaps through interdisciplinary collaboration will expedite the clinical translation of BAC.
Statements
Author contributions
HZ: Investigation, Methodology, Writing – original draft. HY: Conceptualization, Investigation, Methodology, Project administration, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1571153/full#supplementary-material
Glossary
- BAC
benzoylaconine
- IL-6
interleukin-6
- MAPK
mitogen-activated protein kinase
- PI3K
Phosphatidylinositol 3-kinase
- Akt
protein kinase B
- STAT3
signal transducer and activator of transcription 3
- MDA
monoester diterpenoid alkaloid
- TCM
traditional Chinese medicine
- AC
aconitine
- PPI
protein–protein interaction
- GO
gene ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- DO
disease ontology
- MA
mesaconitine
- HA
hypaconitine
- ACN
aconine
- MI
myocardial infarction
- DDA
diester diterpenoid alkaloid
- P-gp
P-glycoprotein
- ROS
reactive oxygen species
- BCRP
breast cancer resistance protein
- MRP2
multidrug resistance–associated protein 2
- ATP
adenosine triphosphate
- ACE2
angiotensin-converting enzyme 2
- RA
rheumatoid arthritis
- LPS
lipopolysaccharide
- YCZFD
Yinchenzhufu decoction
- IL
interleukin
- PGE2
prostaglandin 2
- TNF-α
tumor necrosis factor α
- HIF-α
hypoxia-inducible factor α
- VEGF
vascular endothelial growth factor
- TLR
toll-like receptor
- CLF
cholestatic liver fibrosis
- PDGFRβ
platelet-derived growth factor receptor-β.
References
1
Abdelaziz H. M. Abdelmageed M. E. Suddek G. M. (2025). Molsidomine ameliorates DEXA-Induced insulin resistance: involvement of HMGB1/JAK1/STAT3 signaling pathway. Eur. J. Pharmacol.1002, 177832. 10.1016/j.ejphar.2025.177832
2
Bisset N. G. (1981). Arrow poisons in China. Part II. Aconitum–botany, chemistry, and pharmacology. J. Ethnopharmacol.4 (3), 247–336. 10.1016/0378-8741(81)90001-5
3
Chen L. Yan L. Zhang W. (2022). Benzoylaconine improves mitochondrial function in oxygen-glucose deprivation and reperfusion-induced cardiomyocyte injury by activation of the AMPK/PGC-1 axis. Korean J. Of Physiology and Pharmacol.26, 325–333. 10.4196/kjpp.2022.26.5.325
4
Chen Z. Zhou L. Ge Y. Chen J. Du W. Xiao L. et al (2022). Fuzi decoction ameliorates pain and cartilage degeneration of osteoarthritic rats through PI3K-Akt signaling pathway and its clinical retrospective evidence. Phytomedicine100, 154071. 10.1016/j.phymed.2022.154071
5
Cui Y. Liu Q. Zhang Q. Di X. Zhang H. (2024). Benzoylaconine protects skeletal muscle against ischemia-reperfusion injury through activation of IF1-Dependent AMPK/Nrf2 axis. Drug Des. Dev. Ther.18, 2125–2142. 10.2147/DDDT.S456699
6
Dai P. M. Zhu L. J. Yang X. S. Zhao M. Shi J. Wang Y. et al (2015). Multidrug resistance-associated protein 2 is involved in the efflux of aconitum alkaloids determined by MRP2-MDCKII cells. Life Sci.127, 66–72. 10.1016/j.lfs.2015.02.011
7
Deng X. H. Liu J. J. Sun X. J. Dong J. C. Huang J. H. (2019). Benzoylaconine induces mitochondrial biogenesis in mice via activating AMPK signaling Cascade. Acta Pharmacol. Sin.40, 658–665. 10.1038/s41401-018-0174-8
8
Dickens P. Tai Y. T. But P. P. H. Tomlinson B. Ng H. K. Yan K. W. (1994). Fatal accidental Aconitine poisoning following ingestion of Chinese herbal medicine: a report of two cases. Forensic Sci. Int.67, 55–58. 10.1016/0379-0738(94)90412-X
9
El-Saied M. A. Attia M. M. Ibrahim M. A. Elaish M. Mousa M. R. (2024). Morphomolecular identification of heavy parasitic typhlitis in layer flocks: tissue response and cell-mediated reaction. ACTA VETERINARIA Scand.66, 27. 10.1186/s13028-024-00748-8
10
Fan B. Xu S. Bi J. Huang S. Zu Z. Qian C. (2021). Simultaneous determination of six alkaloids in rat plasma by SPE-HPLC-MS/MS and their pharmacokinetics after oral administration of Radix aconiti preparata extract. ACS Pharmacol. and Transl. Sci.4, 118–127. 10.1021/acsptsci.0c00133
11
Gai W. Hao X. Zhao J. Wang L. Liu J. Jiang H. et al (2020). Delivery of benzoylaconitine using biodegradable nanoparticles to suppress inflammation via regulating NF-κB signaling. Colloids Surfaces B Biointerfaces191, 110980. 10.1016/j.colsurfb.2020.110980
12
Gu P. Li J. Tong R. (2018). Anti-inflammation and analgesia effects of combination therapy with benzoylaconine and paeoniflorin. Chin. J. Mod. Appl. Pharm.35, 1212–1216. 10.13748/j.cnki.issn1007-7693.2018.08.021
13
He Y. N. Chen L. M. Liu Y. Ma H. Y. Hu Q. Cao Z. X. et al (2022). New understanding of Aconitine hydrolysis pathway: isolation, identification and toxicity evaluation based on intermediate products. Arabian J. Of Chem.15 (11), 1–10. 10.1016/j.arabjc.2022.104255
14
Ishibashi T. Inagaki T. Okazawa M. Yamagishi A. Ohta-Ogo K. Asano R. et al (2024). IL-6/gp130 signaling in CD4+T cells drives the pathogenesis of pulmonary hypertension. PNAS121 (16), e2315123121. 10.1073/pnas.2315123121
15
Ito K. Ohyama Y. Hishinuma T. Mizugaki M. (1996). Determination of aconitum alkaloids in the tubers of Aconitum japonicum using gas chromatography/selected ion monitoring. Planta Medica62, 57–59. 10.1055/s-2006-957798
16
Ji X. Yang M. Or K. H. Yim W. S. Zuo Z. (2019). Tissue accumulations of toxic aconitum alkaloids after short-term and long-term oral administrations of clinically used Radix aconiti lateralis preparations in rats. Toxins (Basel).11, 353. 10.3390/toxins11060353
17
Kang S. A. Yu H. S. (2024). Anti-obesity effects by parasitic nematode (Trichinella spiralis) total lysates. Front. Cell. Infect. Microbiol.13, 1285584. 10.3389/fcimb.2023.1285584
18
Kumar P. Mangla B. (2025). Reframing IL-6/STAT3 signalling pathways as an autoimmune adaptability network in rheumatoid arthritis. Med. HYPOTHESES200, 111681. 10.1016/j.mehy.2025.111681
19
Li J. Gu P. Tong R. Wang J. Zhang S. He D. et al (2022). Therapeutic effects of benzoylaconitine and paeoniflorin in rats with collagen-induced arthritis. Braz. J. Of Pharm. Sci.58. 10.1590/s2175-97902022e191132
20
Li S. Li R. Xu Y. X. Baak J. P. A. Gao J. H. Shu J. Q. et al (2021). Traditional Chinese medicine aconiti radix cocta improves rheumatoid arthritis via suppressing COX-1 and COX-2. Evidence-based Complementary Altern. Med.2021, 1–11. 10.1155/2021/5523870
21
Li T. F. Gong N. Wang Y. X. (2016). Ester hydrolysis differentially reduces aconitine-induced anti-hypersensitivity and acute neurotoxicity: involvement of spinal microglial dynorphin expression and implications for aconitum processing. Front. Pharmacol.7, 367. 10.3389/fphar.2016.00367
22
Li X. Y. Zhang Y. Zhang G. W. (2025). Effect of miR-98/IL-6/STAT3 on autophagy and apoptosis of cardiac stem cells under hypoxic conditions in vitro. Curr. STEM CELL Res. and Ther.20 (5), 592–604. 10.2174/011574888X294637240517050849
23
Li Y. Guo D. Wang Q. Li A. Yin S. Li S. et al (2023). Benzoylaconitine alleviates progression of psoriasis via suppressing STAT3 phosphorylation in keratinocytes. Molecules28, 4473. 10.3390/molecules28114473
24
Liu C. Farah N. Weng W. Jiao B. Shen M. Fang L. (2019). Investigation of the permeation enhancer strategy on benzoylaconitine transdermal patch: the relationship between transdermal enhancement strength and physicochemical properties of permeation enhancer. Eur. J. Of Pharm. Sci.138, 105009. 10.1016/j.ejps.2019.105009
25
Liu J. Li Q. Yin Y. Liu R. Xu H. Bi K. (2014). Ultra-fast LC-ESI-MS/MS method for the simultaneous determination of six highly toxic aconitum alkaloids from aconiti kusnezoffii radix in rat plasma and its application to a pharmacokinetic study. J. Sep. Sci.37, 171–178. 10.1002/jssc.201300775
26
Liu M. Cao Y. Lv D. Zhang W. Zhu Z. Zhang H. et al (2017). Effect of processing on the alkaloids in aconitum tubers by HPLC-TOF/MS. J. Pharm. Analysis7, 170–175. 10.1016/j.jpha.2017.01.001
27
Liu X. X. Li H. Song X. Qin K. Guo H. Wu L. et al (2014). Comparative pharmacokinetics studies of benzoylhypaconine, benzoylmesaconine, benzoylaconine and hypaconitine in rats by LC-MS method after administration of Radix aconiti lateralis praeparata extract and dahuang fuzi decoction. Biomed. Chromatogr.28, 966–973. 10.1002/bmc.3102
28
McCall M. B. B. Netea M. G. Hermsen C. C. Jansen T. Jacobs L. Golenbock D. et al (2007). Plasmodium falciparum infection causes proinflammatory priming of human TLR responses. J. Immunol.19 (1), 162–171. 10.4049/jimmunol.179.1.162
29
Meng Q. Zhu H. Li Y. Peng X. Wang T. Huang H. et al (2024). Quantitative proteomics reveals the protective effects of yinchenzhufu decoction against cholestatic liver fibrosis in mice by inhibiting the PDGFRβ/PI3K/AKT pathway. Front. Pharmacol.15, 1341020. 10.3389/fphar.2024.1341020
30
Mizugaki M. Ito K. Ohyama Y. Konishi Y. Tanaka S. Kurasawa K. (1998). Quantitative analysis of aconitum alkaloids in the urine and serum of a Male attempting suicide by oral intake of aconite extract. J. Anal. Toxicol.22, 336–340. 10.1093/jat/22.4.336
31
Ouyang H. Liu F. Tang Z. Chen X. Bo F. Liu H. et al (2018). A validated LC-MS/MS method for simultaneous determination of six aconitum alkaloids and seven ginsenosides in rat plasma and application to pharmacokinetics of Shen-Fu prescription. Evidence-based Complementary Altern. Med.2018, 5107083. 10.1155/2018/5107083
32
Padilha M. D. M. Melo F. T. D. Laurentino R. V. da Silva ANMR Feitosa R. N. M. (2024). Dysregulation in the microbiota by HBV and HCV infection induces an altered cytokine profile in the pathobiome of infection. Braz. J. Infect. Dis.29 (1), 104468. 10.1016/j.bjid.2024.104468
33
Page M. J. McKenzie J. E. Bossuyt P. M. Boutron I. Hoffmann T. C. Mulrow C. D. et al (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Brittish Med. J.372, n71. 10.1136/bmj.n71
34
Peng W. W. Li W. Li J. S. Cui X. B. Zhang Y. x. Yang G. M. et al (2013). The effects of rhizoma zingiberis on pharmacokinetics of six aconitum alkaloids in herb couple of Radix aconiti lateralis-rhizoma zingiberis. J. Of Ethnopharmacol.148, 579–586. 10.1016/j.jep.2013.04.056
35
Qin Y. Y. Qin Z. Y. Liang T. Qin D. e. Hua S. y. Tan M. h. et al (2025). Phellodendrine chloride alleviates gouty arthritis through IL-6/STAT3 signaling pathway. Immunobiology230, 152918. 10.1016/j.imbio.2025.152918
36
Ren M. Song S. Liang D. Hou W. Tan X. Luo J. (2017). Comparative tissue distribution and excretion study of alkaloids from herba ephedrae-radix aconiti lateralis extracts in rats. J. Pharm. Biomed. Analysis134, 137–142. 10.1016/j.jpba.2016.11.027
37
Sherman B. T. Hao M. Qiu J. Jiao X. Baseler M. W. Lane H. C. et al (2022). DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res.50 (W1), W216–W221. 10.1093/nar/gkac194
38
Song S. Tang Q. Huo H. Li H. Xing X. Luo J. (2015). Simultaneous quantification and pharmacokinetics of alkaloids in herba ephedrae-radix aconiti lateralis extracts. J. Anal. Toxicol.39, 58–68. 10.1093/jat/bku113
39
Taha N. M. Salem M. A. El-Saied M. A. Mohammed F. F. Kamel M. El-Bahy M. M. et al (2025). Multifaceted analysis of equine cystic echinococcosis: genotyping, immunopathology, and screening of repurposed drugs against E. equinus protoscolices. E. equinus protoscolices21 (1), 178. 10.1186/s12917-025-04616-z
40
Tang F. Liang S. Chen F. Tang Q. Tan X. (2015). Study on material basis of mahuang fuzi xixin decoction for anti-inflammation and immune suppression based on combined method of serum pharmacochemistry and serum pharmacology. China J. Chin. Materia Medica40, 1971–1976.
41
Tepebasi M. Y. Asci H. Özmen O. Taner R. Temel E. N. Garli S. (2024). Cannabidiol ameliorates lipopolysaccharide-induced cardiovascular toxicity by its antioxidant and anti-inflammatory activity via regulating IL-6, Hif1α, STAT3, eNOS pathway. Mol. Biol. Rep.51 (825), 592–604. 10.1007/s11033-024-09772-3
42
Wada K. Nihira M. Hayakawa H. Tomita Y. Hayashida M. Ohno Y. (2005). Effects of long-term administrations of Aconitine on electrocardiogram and tissue concentrations of Aconitine and its metabolites in mice. Forensic Sci. Int.148, 21–29. 10.1016/j.forsciint.2004.04.016
43
Wang Y. Zhang J. Wang L. Yin H. (2023). Twelve-component pharmacokinetic study of rat plasma after oral administration of you-gui-wan in osteoporosis rats with kidney-yin deficiency and kidney-yang deficiency. Biomed. Chromatogr.37 (6), e5619. 10.1002/bmc.5619
44
Wu J. Duan R. Deng H. Li L. Zhao Y. Yu Z. (2022). The effect of compatibility of aconiti radix and honey on the pharmacokinetics of five aconitum alkaloids in rat plasma. Biomed. Chromatogr.36, e5453. 10.1002/bmc.5453
45
Wu J. Lin N. Li F. Zhang G. He S. Zhu Y. et al (2016). Induction of P-Glycoprotein expression and activity by aconitum alkaloids: implication for clinical drug-drug interactions. Sci. Rep.6, 25343. 10.1038/srep25343
46
Wu J. J. Zhu Y. F. Guo Z. Z. Lou Y. m. He S. g. Guan Y. et al (2018). Aconitum alkaloids, the major components of aconitum species, affect expression of multidrug resistance-associated protein 2 and breast cancer resistance protein by activating the Nrf2-Mediated signalling pathway. Phytomedicine44, 87–97. 10.1016/j.phymed.2017.12.007
47
Xie R. F. Liu Z. Z. Lin Z. Shi P. Chen B. Li S. et al (2020). Potential mechanism of action of Ixeris sonchifolia extract injection against cardiovascular diseases revealed by combination of HPLC-Q-TOF-MS, virtual screening and systems pharmacology approach. Rsc Adv.10 (63), 38497–38504. 10.1039/d0ra07038f
48
Xing Z. Yang C. He J. Feng Y. Li X. Peng C. et al (2022). Cardioprotective effects of aconite in isoproterenol-induced myocardial infarction in rats. Oxidative Med. Cell. Longev.2022, 1090893. 10.1155/2022/1090893
49
Xu Y. Yang L. Liang K. An R. Wang X. Zhang H. (2020). Pharmacokinetic effects of ginsenoside Rg1 on aconitine, benzoylaconine and aconine by UHPLC-MS/MS. Biomed. Chromatogr.34, e4793. 10.1002/bmc.4793
50
Ye L. Gao S. Feng Q. Liu W. Yang Z. Hu M. et al (2012). Development and validation of a highly sensitive UPLC-MS/MS method for simultaneous determination of Aconitine, mesaconitine, hypaconitine, and five of their metabolites in rat blood and its application to a pharmacokinetics study of Aconitine, mesaconitine, and hypaconitine. Xenobiotica42, 518–525. 10.3109/00498254.2011.641608
51
Ye L. Yang X. Yang Z. Gao S. Yin T. Liu W. et al (2013). The role of efflux transporters on the transport of highly toxic Aconitine, mesaconitine, hypaconitine, and their hydrolysates, as determined in cultured Caco-2 and transfected MDCKII cells. Toxicol. Lett.216, 86–99. 10.1016/j.toxlet.2012.11.011
52
Ye Q. Liu H. M. Fang C. X. Liu Y. S. Liu X. Liu J. et al (2019). Cardiotoxicity evaluation and comparison of diterpene alkaloids on zebrafish. Drug And Chem. Toxicol.44, 294–301. 10.1080/01480545.2019.1586916
53
Yu H. H. Li M. Li Y. B. Lei B. B. Yuan X. Xing X. K. et al (2020). Benzoylaconitine inhibits production of IL-6 and IL-8 via MAPK, akt, NF-κB signaling in IL-1β-Induced human synovial cells. Biol. and Pharm. Bull.43, 334–339. 10.1248/bpb.b19-00719
54
Zhang. D. K. Yang Z. R. Han X. Lin J. z. Li C. Y. Yang M. et al (2017). Microcalorimetric investigation of six alkaloids from radix aconite lateralis preparata (fuzi) on the metabolic activity of mitochondria isolated from rat liver. J. Therm. Analysis Calorim.130, 1707–1715. 10.1007/s10973-017-6159-x
55
Zhang F. Tang M. H. Chen L. J. Li R. Wang X. h. Duan J. g. et al (2008). Simultaneous quantitation of Aconitine, mesaconitine, hypaconitine, benzoylaconine, benzoylmesaconine and benzoylhypaconine in human plasma by liquid chromatography-tandem mass spectrometry and pharmacokinetics evaluation of “SHEN-FU” injectable powder. J. Chromatogr. B Anal. Technol. Biomed. Life Sci.873, 173–179. 10.1016/j.jchromb.2008.08.008
56
Zhang H. Sun S. Zhang W. Xie X. Zhu Z. Chai Y. et al (2016). Biological activities and pharmacokinetics of Aconitine, benzoylaconine, and aconine after oral administration in rats. Drug Test. And Analysis8 (8), 839–846. 10.1002/dta.1858
57
Zhang J. Wang L. Zhu X. Bai X. Yin H. (2019). Simultaneous quantitation of aconitum alkaloids from you-gui-yin in rat plasma by UPLC-ESI-MS and its application to a pharmacokinetic study. ACTA Chromatogr.31, 23–27. 10.1556/1326.2017.00316
58
Zhang L. Li T. Wang R. Xu J. Zhou L. Yan L. et al (2020). Evaluation of long-time decoction-detoxicated hei-shun-pian (processed Aconitum carmichaeli debeaux lateral root with peel) for its acute toxicity and therapeutic effect on mono-iodoacetate induced osteoarthritis. Front. Pharmacol.11, 1053. 10.3389/fphar.2020.01053
59
Zhang L. Siyiti M. Zhang J. Yao M. Zhao F. (2021). Anti-inflammatory and anti-rheumatic activities in vitro of alkaloids separated from Aconitum soongoricum stapf. Exp. Ther. Med.21, 493. 10.3892/etm.2021.9924
60
Zhang M. Peng Y. Wang M. Y. Gao B. B. Zhao L. J. Li X. B. (2019). The influence of compatibility of Si-Ni decoction with metabolism in intestinal bacteria on transports of toxic diterpenoid alkaloids from processed aconite root across Caco-2 monolayers. J. Of Ethnopharmacol.228, 164–178. 10.1016/j.jep.2018.09.022
61
Zhang P. Kong D. Du Q. Zhao J. Li Q. Zhang J. et al (2017). A conscious rat model involving bradycardia and hypotension after oral administration: a toxicokinetical study of Aconitine. Xenobiotica47, 515–525. 10.1080/00498254.2016.1204484
62
Zhang Q. Q. Chen F. H. Wang F. Di X. M. Li W. Zhang H. (2022). A novel modulator of the renin-angiotensin system, benzoylaconitine, attenuates hypertension by targeting ACE/ACE2 in enhancing vasodilation and alleviating vascular inflammation. Front. Pharmacol.13, 841435. 10.3389/fphar.2022.841435
63
Zhang Q. Q. Chen Q. S. Feng F. Cao X. Chen X. F. Zhang H. (2024). Benzoylaconitine: a promising ACE2-Targeted agonist for enhancing cardiac function in heart failure. Free Radic. Biol. And Med.214, 206–218. 10.1016/j.freeradbiomed.2024.02.010
64
Zhi M. Liu K. Han S. Xu J. Li W. Li F. et al (2020). Influence of different dosage forms on pharmacokinetics of 6 alkaloids in raw aconiti kusnezoffii radix (caowu) and chebulae Fructus- (Hezi-) processed caowu by UPLC-MS/MS. BioMed Res. Int.2020. 10.1155/2020/1942849
65
Zhou C. Gao J. Ji H. Li W. Xing X. Liu D. et al (2021). Benzoylaconine modulates LPS-induced responses through inhibition of toll-like receptor-mediated NF-κB and MAPK signaling in RAW264.7 cells. Inflammation44, 2018–2032. 10.1007/s10753-021-01478-z
66
Zhou J. Cheng C. Ma L. Wu Y. Zhang Y. Li L. et al (2023). Investigating the interactions of benzoylaconine and benzoylhypacoitine with human serum albumin: experimental studies and computer calculations. J. Mol. Struct.1294, 136497. 10.1016/j.molstruc.2023.136497
67
Zhou Q. Meng P. Wang H. Dong X. Tan G. (2019). Pharmacokinetics of monoester-diterpenoid alkaloids in myocardial infarction and normal rats after oral administration of sini decoction by microdialysis combined with liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr.33, e4406. 10.1002/bmc.4406
Summary
Keywords
benzoylaconine, toxicity, pharmacokinetics, pharmacological activity, molecular mechanisms
Citation
Zhuang H and Yao H (2025) Pharmacological effects, molecular mechanisms, and pharmacokinetics of benzoylaconine: a systematic review. Front. Pharmacol. 16:1571153. doi: 10.3389/fphar.2025.1571153
Received
24 March 2025
Accepted
25 July 2025
Published
05 August 2025
Volume
16 - 2025
Edited by
Qingwen Tao, China-Japan Friendship Hospital, China
Reviewed by
Tayo Alex Adekiya, Howard University, United States
Lei Dong, Southeast University, China
Updates
Copyright
© 2025 Zhuang and Yao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Yao, hongyao@mail.fjmu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.