- 1Department of Respiratory Medicine, First Clinical Medical College, Ningxia Medical University, Yinchuan, China
- 2Department of Respiratory and Critical Care Medicine, General Hospital of Ningxia Medical University, Yinchuan, China
- 3Department of Internal Medicine, Ningxia Yiyang Hospital, Yinchuan, China
- 4Department of Respiratory Medicine, People’s Hospital of Ningxia Hui Autonomous Region, Ningxia Medical University, Yinchuan, China
Objectives: To evaluate the therapeutic efficacy of different doses of tocilizumab (TCZ) in patients with severe or critical COVID–19.
Methods: In this single–center retrospective cohort study conducted from January 2023 to January 2024, 56 hospitalized patients with severe or critical COVID–19 who received TCZ were included. Patients were categorized into three groups based on the number of TCZ doses administered: one dose (n = 16), two doses (n = 32), and three doses (n = 8). The primary outcomes were in–hospital mortality and 30–day mortality following the first dose. Secondary outcomes included changes in inflammatory marker levels, length of hospital stay, duration of mechanical ventilation, and incidence of complications during hospitalization.
Results: After adjusting for potential confounders, there were no statistically significant differences in 30–day mortality (one dose vs. two doses HR 0.39; 95% CI, 0.15–1.04; P = 0.060 and one dose vs. three doses HR 0.27; 95% CI, 0.06–1.07; P = 0.067) or in–hospital mortality (one dose vs. two doses HR 0.65; 95% CI, 0.35–1.25; P = 0.090 and one dose vs. three doses HR 0.70; 95% CI, 0.40–1.50; P = 0.300) among the three groups. However, all groups showed a favorable response in inflammatory markers. Interleukin–6 (IL–6) levels initially increased after TCZ administration but subsequently declined in a fluctuating pattern. C–reactive protein (CRP) levels decreased consistently across all groups, while procalcitonin showed a modest decline. The number of TCZ doses had no significant impact on length of hospital stay, duration of mechanical ventilation, or the incidence of complications such as respiratory failure requiring mechanical ventilation, heart failure, secondary infections, thrombotic/embolic events, transaminase elevation, neutropenia, GI perforation/Haemorrhage, or acute kidney injury.
Conclusion: Administering additional doses of TCZ beyond the initial dose was not associated with further reductions in mortality or improvements in other major clinical outcomes in patients with severe or critical COVID–19.
1 Introduction
Since its first outbreak in late 2019, Coronavirus Disease 2019 (COVID–19) has rapidly developed into a major global public health crisis. Caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS–CoV–2), the infection can lead to pneumonia and, in severe cases, progress to Acute Respiratory Distress Syndrome (ARDS) and Multiple Organ Dysfunction Syndrome (MODS) (Hu et al., 2021). As patients deteriorate into stages involving ARDS, sepsis, and MODS, this phase is often characterized as cytokine storm or cytokine release syndrome (CRS). Although the virus itself may no longer be the primary driver at this stage, immune dysregulation, triggered by multiple underlying factors, leads to systemic inflammation and life–threatening multiorgan failure due to hyperactivation of the immune system. Cytokine storms have been directly associated with disease severity, morbidity, and mortality (Oran and Topol, 2021). As a result, therapeutic agents targeting interleukin–6 (IL–6) have been evaluated in several studies for their potential benefit in critically ill patients (Hermine et al., 2021; Salama et al., 2021).
Tocilizumab (TCZ) is a recombinant humanized IgG1 monoclonal antibody targeting the interleukin–6 receptor (IL–6R), originally developed for the treatment of certain rheumatologic conditions and later approved for use in patients with COVID–19. By blocking IL–6 signaling, TCZ is also used to manage cytokine storm induced by chimeric antigen receptor T–cell (CAR–T) therapy (Mahallawi et al., 2018). It acts as a competitive antagonist of both soluble and membrane–bound IL–6 receptors, thereby inhibiting both the cis–and trans–signaling pathways (Jones et al., 2005). As a consequence of this inhibition, serum IL–6 levels often increase following TCZ administration. This elevation is thought to reflect ongoing endogenous IL–6 production and underlying inflammatory activity, rather than diminished efficacy of the drug (Nishimoto et al., 2008). C–reactive protein (CRP) is commonly used as a surrogate marker to assess IL–6 bioactivity, as it correlates more reliably with the anti–inflammatory effects of IL–6 inhibitors, particularly in patients with severe disease. Moreover, clinical studies have shown that IL–6R inhibitors demonstrate similar safety and efficacy profiles compared to direct IL–6 inhibitors (Kaly and Rosner, 2012). Reducing IL–6 activity may lead to improved outcomes in patients with SARS–CoV–2 infection, as IL–6 plays a central role in both the pathophysiology of the disease and the severity of cytokine storm.
In previous studies, patients typically received an initial single dose of TCZ, with a second dose administered 8–72 h later if clinical improvement was not observed (Gordon et al., 2021; Horby et al., 2021; Veiga et al., 2021). These studies primarily focused on comparing TCZ treatment with standard care or placebo. However, in some cases, patients who received two full doses of TCZ showed limited clinical improvement, with persistent symptoms such as fever, dyspnea, poor oxygenation indices, and inflammatory infiltrates on lung imaging. At the same time, a severe cytokine storm driven by immune overactivation often remained active, continuing to inflict systemic damage. In such challenging scenarios, clinicians may cautiously consider administering a third dose of TCZ based on the patient’s overall condition, underlying comorbidities, current levels of inflammatory markers, and organ function status. Despite these considerations, there has been no comprehensive or systematic investigation into the potential benefits of different dosing regimens of TCZ on clinical outcomes, mortality, or laboratory indicators.
2 Materials and methods
2.1 Study design and patient recruitment
This single–center retrospective cohort study was conducted at the People’s Hospital of Ningxia Hui Autonomous Region and aimed to analyze the clinical characteristics of patients with severe or critical COVID–19 who were treated with TCZ between January 1, 2023, and January 1, 2024. A total of 56 patients were included based on the following criteria: age ≥18 years; confirmed SARS–CoV–2 infection by reverse transcription polymerase chain reaction (RT–PCR) from a nasopharyngeal or oropharyngeal swab; diagnosis of severe or critical illness according to national clinical guidelines (PRC and Medicine, 2023); and receipt of at least one dose of TCZ during hospitalization. Exclusion criteria included death within 48 h of hospital admission, prior treatment with TCZ before admission, or known allergy to any component of TCZ. All patients were followed until either hospital discharge or in–hospital death, whichever occurred first.
2.2 Treatment regimens
Written informed consent was obtained from patients or their accompanying relatives prior to the administration of TCZ. All patients initially received corticosteroid therapy, which failed to improve their clinical condition or prevent further deterioration due to cytokine storm. According to the 10th edition of the Diagnostic and Treatment Protocol for Novel Coronavirus Infections issued by the National Health Commission of the People’s Republic of China (PRC and Medicine, 2023), TCZ may be considered for patients with severe or critical COVID–19 if laboratory results show significantly elevated IL–6 levels. The recommended initial dose ranges from 4 to 8 mg/kg, typically administered as 400 mg diluted in 100 mL of saline and infused over the course of 1 h. If the initial dose is ineffective, the same dosage may be repeated after 12 h, not exceeding a maximum single dose of 800 mg. For a small number of patients whose symptoms do not significantly improve after two doses, and who continue to exhibit elevated IL–6 levels, a third dose may be considered based on individual clinical judgment.
In this study, the 56 patients were divided into three groups according to the number of TCZ doses received: a one–dose group, a two–dose group, and a three–dose group. All patients were administered TCZ at the standard dose of 400 mg per infusion. For patients suspected of having bacterial co–infections, based on indicators such as elevated procalcitonin or persistent fever, antibiotic therapy was initiated at the discretion of the treating physician. Prophylactic anticoagulation therapy was provided based on individual body weight and renal function. Additionally, all patients with a nucleic acid cycle threshold (CT) value below 30 received antiviral treatment.
2.3 Data collection
Research data were collected from the patient’s electronic medical records. For all three groups receiving different dosages of TCZ, demographic information, comorbidities, treatment regimens, in–hospital complications, and clinical outcomes were collected. In addition, laboratory test results obtained during hospitalization were recorded, including complete blood count, coagulation parameters (activated partial thromboplastin time and D–dimer), and inflammatory markers such as C–reactive protein, procalcitonin, ferritin, hyaluronic acid, and IL–6.
2.4 Study outcomes
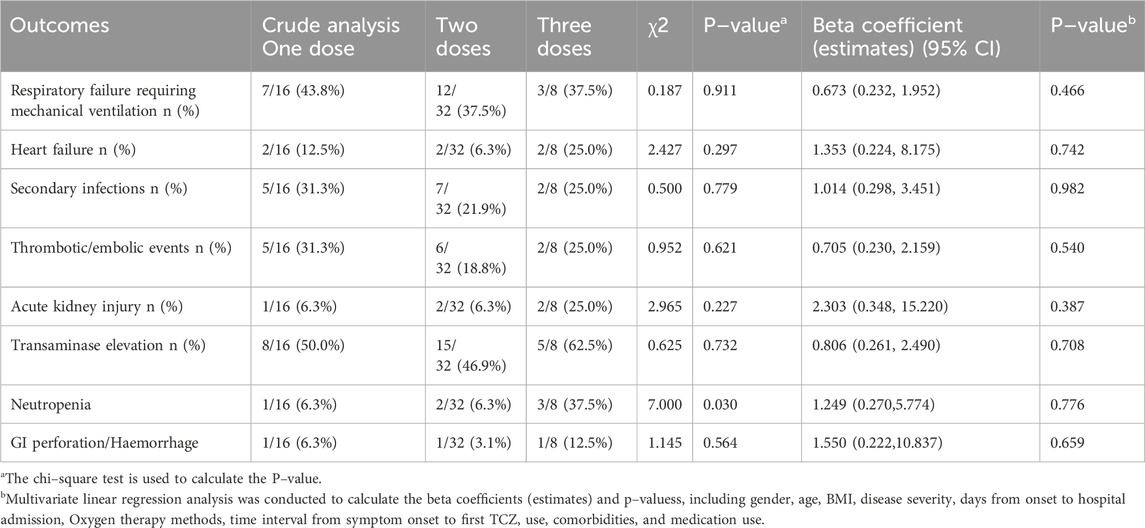
Clinical parameters and inflammatory marker data were collected prior to TCZ administration and within 1 week following each dose. The primary outcome of the study was to compare in–hospital mortality and 30–day mortality following the first dose among patients with severe or critical COVID–19 receiving different dosages of TCZ. Secondary outcomes included changes in inflammatory marker levels, length of hospital stay, duration of mechanical ventilation, and the incidence of in–hospital complications, such as respiratory failure requiring mechanical ventilation, heart failure, secondary infections, thrombotic/embolic events, transaminase elevation, neutropenia, GI perforation/Haemorrhage, or acute kidney injury.
2.5 Statistical analysis
Statistical analyses and data visualization were performed using SPSS version 27.0 and GraphPad Prism version 9. For continuous variables, the Shapiro–Wilk test was used to assess normality. Normally distributed data were presented as mean ± standard deviation (±SD), and comparisons among groups were conducted using one–way analysis of variance (ANOVA). Non–normally distributed data were expressed as median with interquartile range (P25, P75), and group comparisons were performed using the Kruskal–Wallis H test. Categorical variables were reported as frequencies and percentages (n%), and comparisons between groups were made using the chi–square (χ2) test or Fisher’s exact test, as appropriate. Standardized mean difference (SMD) calculated using Cohen’s h formula. Kaplan–Meier survival analysis was used to evaluate survival outcomes, and the log–rank test was applied to compare unadjusted survival rates across treatment groups. Time–dependent COX regression was used to analyze 30–day mortality and in–hospital mortality, as well as the interaction of cortisol hormone combined with TCZ. Additionally, generalized multivariate regression analysis was used to further assess other clinical outcome variables. All statistical tests were two–sided, with a significance level set at p < 0.05.
3 Results
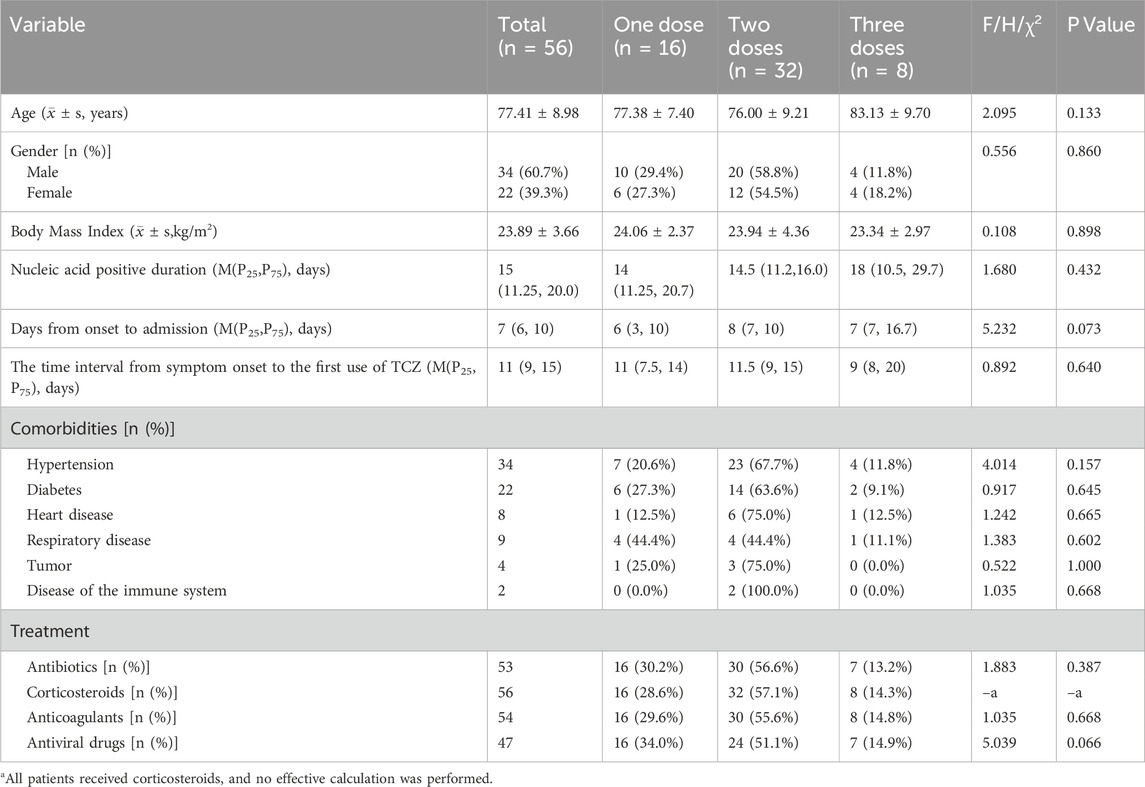
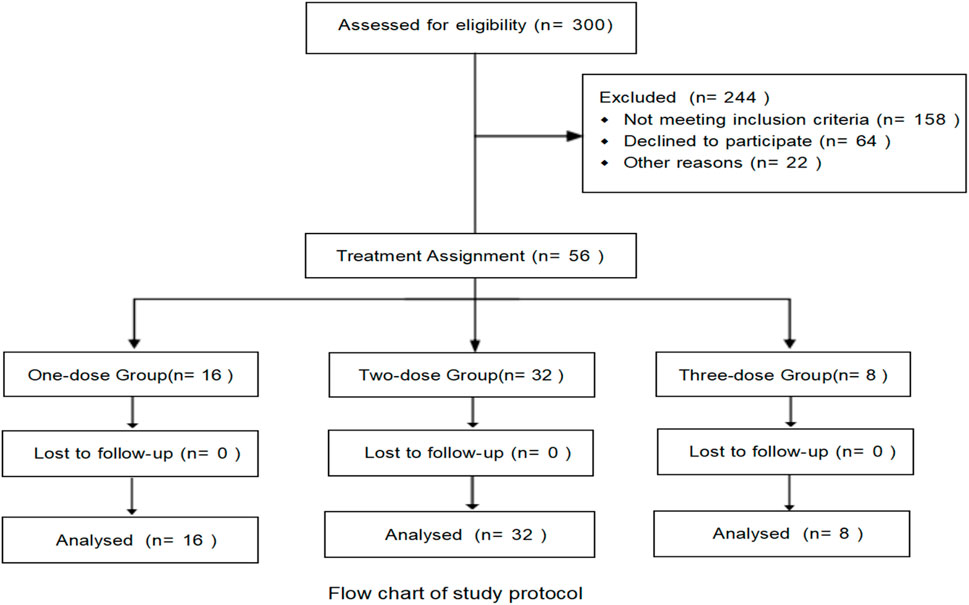
In this study, a retrospective analysis was conducted on 300 patients with severe or critical COVID–19 between January 1, 2023, and January 1, 2024, of whom 56 met the inclusion criteria and were enrolled. Among these, 16 patients (28.5%) received a single dose of TCZ, 32 patients (57.2%) received two doses, and eight patients (14.3%) received three doses. There was no significant difference in gender distribution across the three groups, and the mean age was 77.41 years (SD ± 8.98). The most common comorbidity was hypertension (60.7%), followed by cardiovascular disease (39.3%) and type 2 diabetes (14.3%). The median time from symptom onset to COVID–19 diagnosis was 7 days (interquartile range [IQR]: 6–10 days). During the disease course, 54 patients (96.4%) received antimicrobial therapy, 53 (94.6%) received anticoagulation, all 56 patients (100.0%) received corticosteroids, and 47 (83.9%) received antiviral treatment (Table 1).
Notably, the most commonly used corticosteroid was dexamethasone sodium phosphate (n = 38), followed by methylprednisolone sodium succinate (n = 18) (Supplementary Table S1). The mean daily dose for dexamethasone was 7.32 mg (range: 5–10 mg), administered for an average duration of 6.45 days (range: 2–10 days). In contrast, methylprednisolone was given at a higher mean daily dose of 50.0 mg (range: 40–80 mg), also for approximately 6.83 days on average (range: 5–9 days).
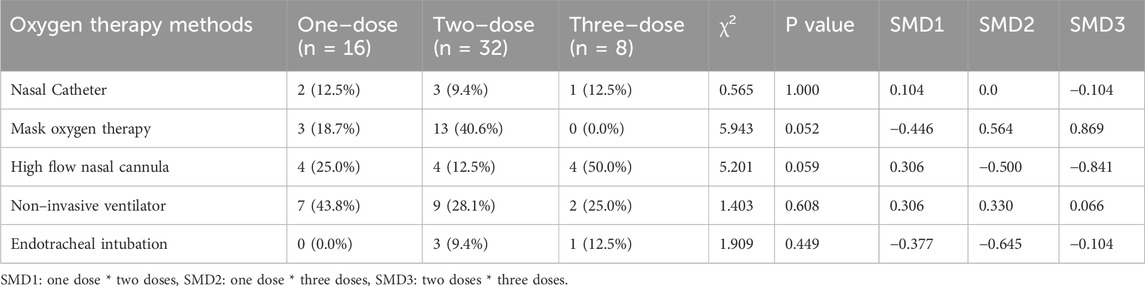
5 patients did not require oxygen therapy at admission, but all had progressed to severe or critical illness by the time of TCZ administration (Table 2). At that point, 18 patients required non–invasive ventilation (NIV), and 4 required endotracheal intubation. High–flow nasal catheter (HFNC) use was higher in the three groups than in the one/two dose group (one dose 25.0% vs. two doses 12.5% vs. three doses 50.0%), and Mask oxygen therapy was not used in the three doses (one dose 18.7% vs two doses 40.6%). Although the p–value of the chi–square test did not reach statistical significance (HFNC: P = 0.059, Mask oxygen therapy: P = 0.052), the standardized mean difference (SMD) further supported the existence of a difference between the groups: in Mask oxygen therapy, SMD1: – 0.446, SMD2: 0.564, SMD3:0.869, in HFNC, SMD1:0.306, SMD2: −0.500, SMD3: −0.841.
In addition, the clinical indications for repeated second and third doses of TCZwere analyzed. For increased oxygen demand (≥20% increase in FiO2 or change to advanced respiratory support): the incidence of one, two, and three doses was 31.1% (5/16), 50.0% (16/32), and 87.5% (7/8), in that order, with the Cochran–Armitage trend test showing an increasing trend (P = 0.013). For SaO2 decline (<90% or ≥5% from baseline): one–, two–, and three–dose incidence was 37.5% (6/16), 65.5% (21/32), and 87.5% (7/8), in that order, with a trend test of P = 0.018. Exacerbation of lung shadowing (≥50% increase in shadowing area or the development of ARDS): three doses incidence (62.5%) was though higher than one dose (50.0%), but the difference between groups was not statistically significant (trend test P = 0.678) (Table 3). In addition, baseline laboratory parameters were analyzed for patients receiving different doses of TCZ (Table 4).
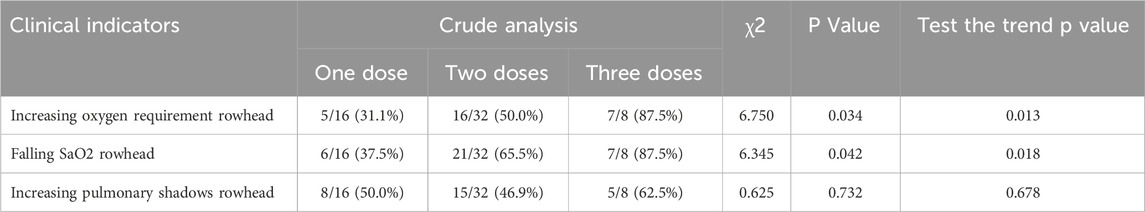
Table 3. Analysis of clinical indicators and cochran–armitage trend test for different doses of TCZ.
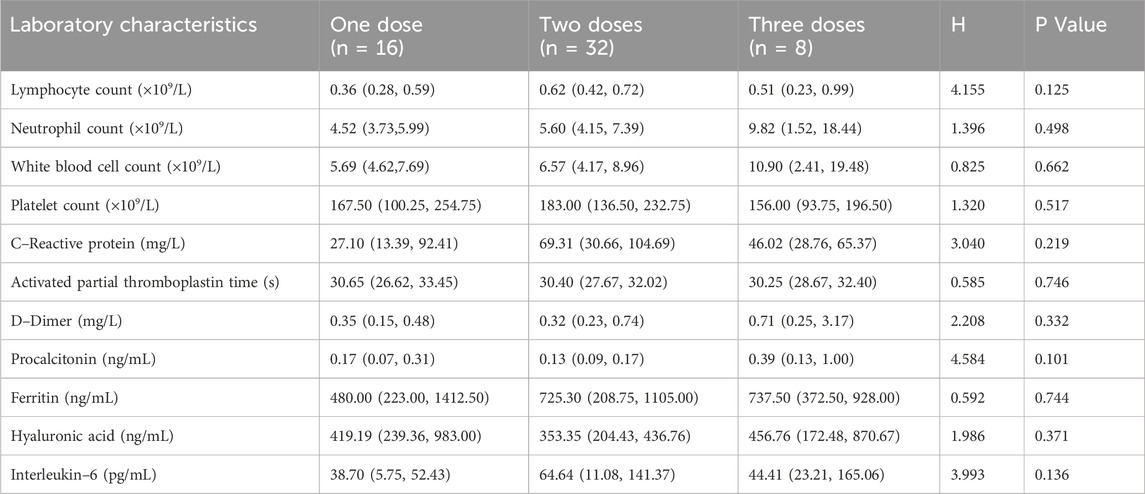
Table 4. Baseline laboratory characteristics of patients treated with different doses of tocilizumab.
3.1 Primary outcomes
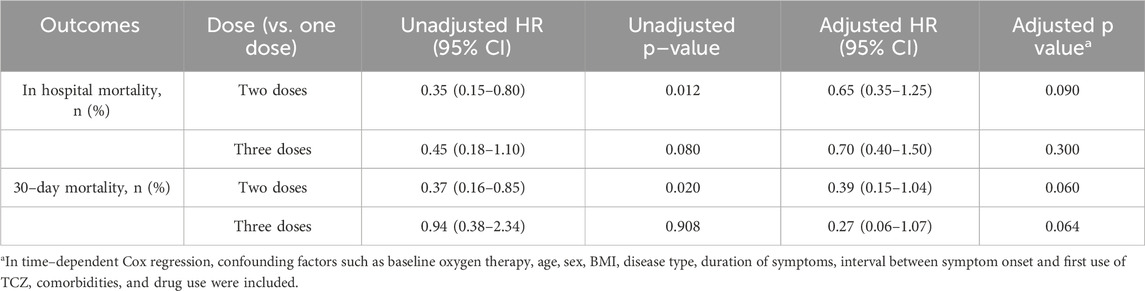
In–hospital mortality was 25.0% (4 patients) in the one–dose group, 9.3% (3 patients) in the two–dose group, and 37.5% (3 patients) in the three–dose group. The timing of TCZ administration was not standardized, and the dosing measure was modeled as a time–dependent covariate in order to eliminate the monumental time bias, and the timing of TCZ administration is shown in Supplementary Table S2. For in–hospital mortality, the unadjusted risk ratios (HRs) compared with the one–dose group showed an HR of 0.35 (95% CI: 0.15–0.80, P = 0.012) for the two–dose group and the HR for the three–dose group was 0.45 (95% CI: 0.18–1.10, P = 0.080). However, after adjusting for confounders such as baseline oxygen therapy, age, gender, BMI, disease type, duration of symptoms, interval between symptom onset and first TCZ use, comorbidities, and medication use, the adjusted HRs for the two–dose group and three–dose group were 0.65 (95% CI: 0.35–1.25, P = 0.090) and 0.70 (95% CI: 0.40–1.50, P = 0.300). Overall, the difference in in–hospital mortality between the three groups was not statistically significant. A 30–days follow–up from the first dose of TCZ showed that one patient in the two–dose group died of respiratory failure the day after discharge. Compared with the one–dose group, unadjusted HRs showed 0.37 (95% CI: 0.16–0.85, P = 0.020) for the two–dose group and 0.94 (95% CI: 0.38–2.34, P = 0.908) for the three–dose group. Adjusted HRs were 0.39 (95% CI: 0.15–1.04, P = 0.060) and 0.27 (95% CI: 0.06–1.07, P = 0.064) for the two–dose and three–dose groups, respectively. There was also no statistically significant difference in 30–day mortality between the three groups (Table 5). Kaplan–Meier survival curves for both in–hospital and 30–day follow–up periods also showed no significant differences between the groups (Figure 1).
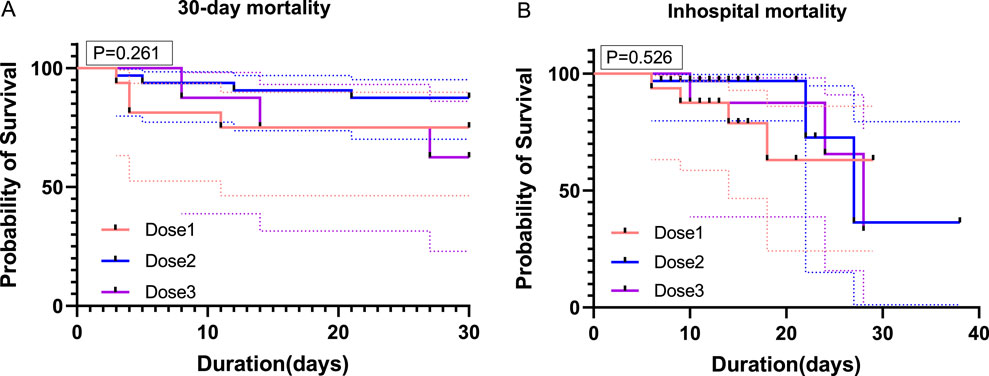
Figure 1. Survival curves for patients receiving different doses of TCZ. (A) shows the survival curves of the three groups of patients over 30 days of follow–up from the start of the first TCZ treatment. (B) shows the survival curves during hospitalization for the three groups of patients. On the horizontal axis, it shows time (in days), while on the vertical axis, it shows the probability of survival (in percentage). Dose1 (one–dose); Dose 2 (two–dose); Dose 3 (three–dose).
All patients received corticosteroids therapy, and the interaction between corticosteroids regimen and TCZ was analyzed by time–dependent Cox regression. In the univariate analysis, none of the associations of corticosteroid type, dose, and treatment duration with hospitalization outcome events were statistically significant (P > 0.05). After adjusting for confounders in the multifactorial analysis, the first two remained non–significantly associated, but corticosteroid dose approached the level of significance (HR 1.521; 95% CI 0.996–2.322, P = 0.050). In terms of interaction, the interaction terms of TCZ with corticosteroid dose (HR 1.029; 95% CI 1.002–1.056, P = 0.032), and treatment duration (HR 0.880; 95% CI 0.788–0.984, P = 0.025) were statistically significant and suggesting the presence of an interaction affecting outcome, with no significant association for the interaction term of TCZ with corticosteroid type (HR 0.580; 95% CI 0.230–1.460, P = 0.247) (Table 6).
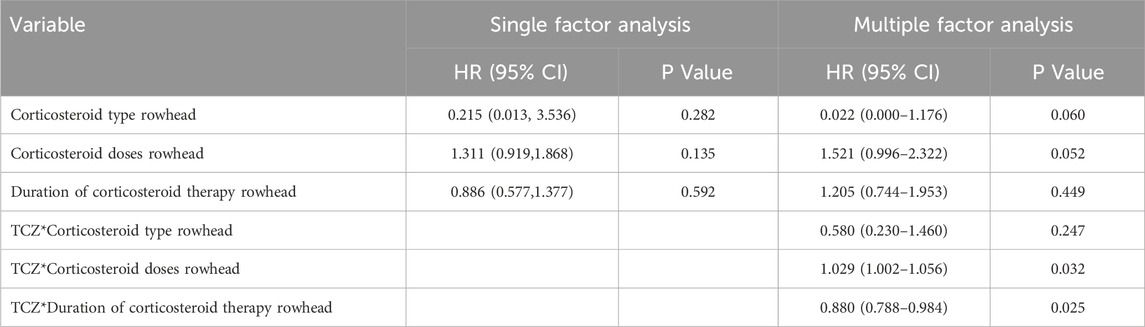
Table 6. Time-dependent Cox regression analysis of the interaction between corticosteroids regimen and tolizumab.
3.2 Secondary outcomes
3.2.1 Between–group differences in laboratory parameters following different doses of TCZ treatment
Following administration of different doses of TCZ, D–dimer levels were higher in the one–dose group compared to the two–dose group (0.89 vs 0.44 mg/L, P = 0.030). However, no significant differences were observed between the one–dose and three–dose groups (0.89 vs 0.83 mg/L, P = 1.000), or between the two–dose and three–dose groups (0.44 vs 0.83 mg/L, P = 0.152) (Figure 2A). IL–6 levels were significantly elevated in the three–dose group compared to the two–dose group (775.43 vs. 138.15 pg/mL, P = 0.006). However, the differences between the one–dose and two–dose groups (278.98 vs 138.15 pg/mL, P = 0.134) and between the one–dose group and the three–dose group (278.98 vs. 775.43 pg/mL, P = 0.478) were not statistically significant (Figure 2B). In addition, no significant differences were found among the three groups in post–treatment levels of serum ferritin, hyaluronic acid, CRP, PCT, or other inflammatory markers (Supplementary Table S3).
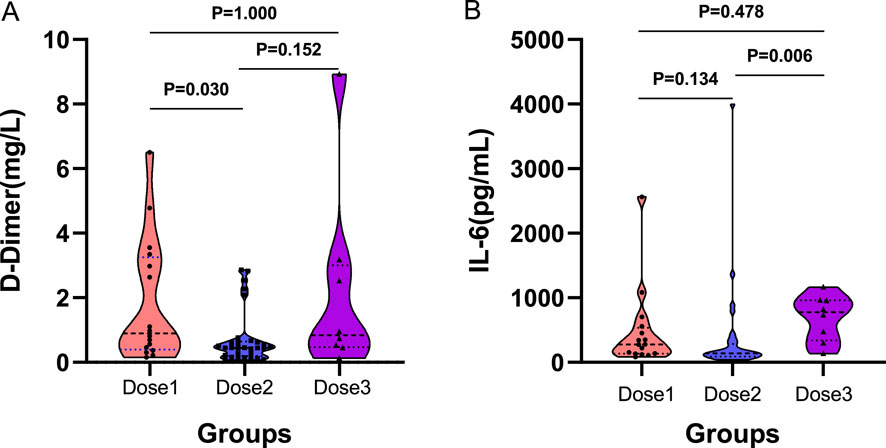
Figure 2. Comparison between groups after different doses of TCZ treatment. (A) shows the between–group differences after D–dimer treatment. (B) shows the between–group differences after interleukin–6 treatment. (A) D–Dimer; (B) IL–6. IL–6, Interleukin–6. Dose1 (one–dose); Dose 2 (two–dose); Dose 3 (three–dose).
3.2.2 Changes in laboratory parameters within groups following different TCZ dosing regimens
Immediately after the completion of TCZ treatment, hematological parameters were assessed for each group using the blood test results closest to the final dose. The findings revealed that median IL–6 levels increased significantly in all three groups compared to baseline (One–dose: 38.70 vs. 278.98 pg/mL, P < 0.001; Two–dose: 64.64 vs. 138.15 pg/mL, P < 0.001; Three–dose: 44.41 vs. 775.43 pg/mL, P = 0.012) (Figure 3A). In contrast, median CRP levels decreased significantly after treatment (One–dose: 27.10 vs 10.00 mg/L, P = 0.011; Two–dose: 69.31 vs. 18.85 mg/L, P < 0.001; Three–dose: 46.02 vs 19.30 mg/L, P = 0.017) (Figure 3B). PCT levels also declined significantly in the two–dose (P = 0.033) and three–dose groups (P = 0.036), whereas the decrease in the one–dose group was not statistically significant (P = 0.070) (Figure 3C). Detailed changes in laboratory parameters before and after treatment for each group are provided in Supplementary Table S4.
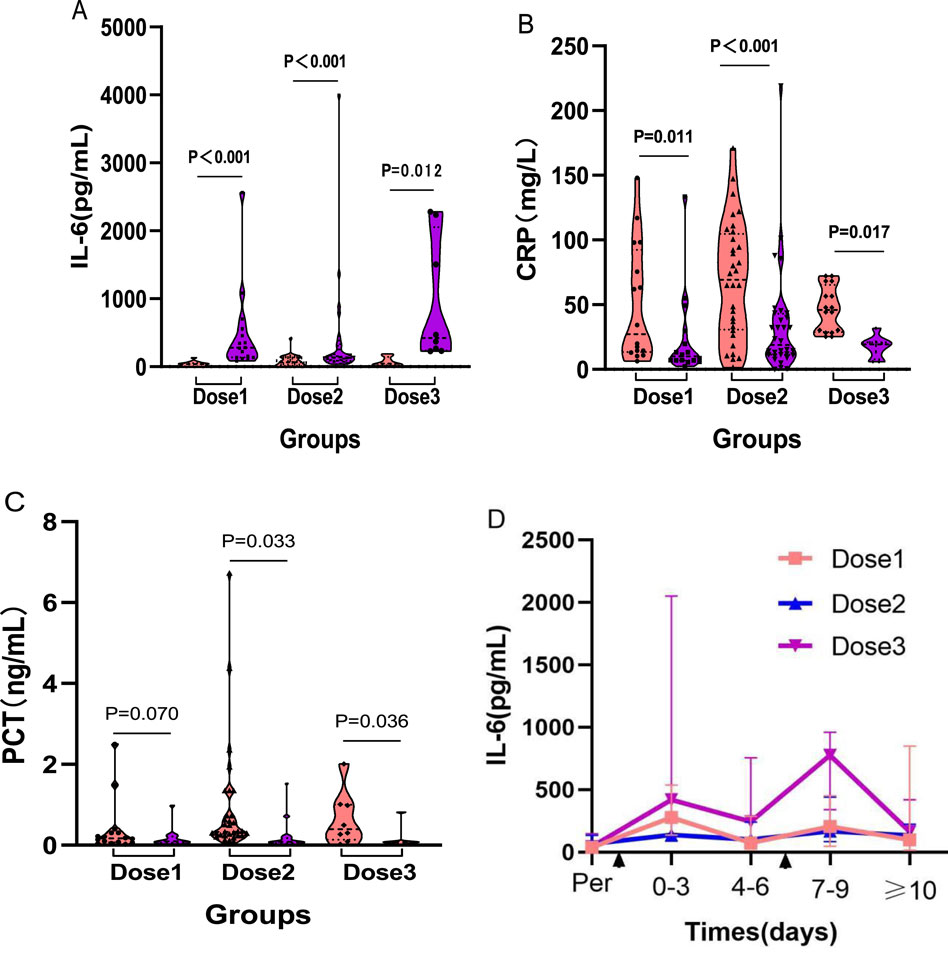
Figure 3. Comparison between groups before and after different doses of TCZ treatment. (A) shows the changes in IL–6 levels before and after TCZ treatment in the three groups. (B) shows the changes in CRP levels before and after TCZ treatment in the three groups. (C) shows the changes in PCT levels before and after TCZ treatment in the three groups. (D) shows the dynamic trend of IL–6 levels after TCZ administration on the horizontal axis indicates the time of TCZ administration; the first appearance represents the time of one and two doses of TCZ in the three groups of patients, and the second appearance represents the time of the third dose of TCZ in the three–dose group. Dose1 (one–dose); Dose2 (two–dose); Dose3 (three–dose).
To further investigate the temporal relationship between TCZ administration and IL–6 levels, a dynamic trend chart was generated (Figure 3D). In the one–dose and two–dose groups, IL–6 levels showed a transient rise within the first 0–3 days post–treatment, followed by a gradual decline. In the three–dose group, IL–6 levels followed a similar pattern after the first and second doses. However, after an initial drop, levels remained elevated. Following administration of the third dose, IL–6 levels spiked again, and although they eventually began to decline, the decrease was slower and less pronounced.
3.2.3 Multivariable regression analysis of other secondary outcomes
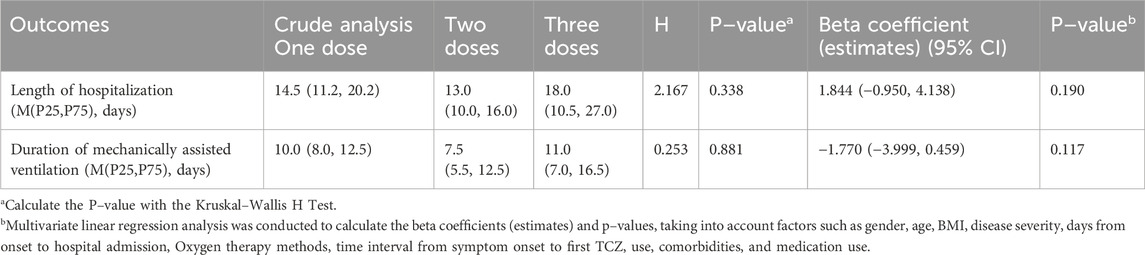
Multivariable regression analysis was conducted to evaluate additional secondary outcomes. The average length of hospital stay for the one–dose, two–dose, and three–dose groups was 14.5, 13.0, and 18.0 days, respectively (P = 0.338), while the duration of mechanical ventilation was 10.0, 7.5, and 11.0 days, respectively (P = 0.881). Neither outcome showed statistically significant differences, and these results remained unchanged after adjusting for potential confounding variables (Table 7). Furthermore, analysis of in–hospital complications revealed no significant differences among the groups in the incidence of respiratory failure requiring mechanical ventilation, heart failure, secondary infections, thrombotic/embolic events, transaminase elevation, neutropenia, GI perforation/Haemorrhage, or acute kidney injury (Table 8).
4 Discussion
This retrospective cohort study evaluated the effects of different TCZ dosing regimens (one, two, or three 400–mg doses) on clinical outcomes in patients with severe or critical COVID–19. The results of the study showed that there were differences in in–hospital mortality and 30–days mortality after the first dose of TCZ in patients with severe or critical neocoronary disease treated with one or two TCZs, but no such significant differences were observed between patients treated with one and three TCZs. Similarly, the incidence of in–hospital complications, length of stay, and duration of mechanical ventilation were comparable across the three groups. Although baseline characteristics were well balanced, adjustments were made for potential confounding factors, including the small sample size, variable dosing intervals, and the circulation of different viral variants. However, even after adjustment, patients did not show significant improvement in clinical outcomes. Notably, all groups showed a favorable response in terms of reduction in inflammatory markers.
TCZ has been shown to significantly reduce mortality in critically ill COVID–19 patients (De Roquetaillade et al., 2022; De Rossi et al., 2020; Rezaei et al., 2021), and its safety and efficacy have been validated in large–scale studies (Shankar–Hari et al., 2021). Although the therapeutic benefits of TCZ are widely recognized, the optimal dosing regimen remains a subject of debate. Guaraldi and co–workers (Guaraldi et al., 2020) suggested that critically ill patients may require a second dose to maintain adequate plasma drug concentrations, an approach supported by pharmacokinetic studies in cytokine release syndrome caused by CAR–T cell therapy (Le et al., 2018). Similarly, the tenth edition of China’s COVID–19 Diagnosis and Treatment Plan (PRC and Medicine, 2023) recommends the use of TCZ in severe and critical cases with markedly elevated IL–6 levels, allowing up to two cumulative doses. However, some studies have questioned the benefit of multiple doses. For instance, Mughal and colleagues (Mughal et al., 2020) found that administering two or more doses did not reduce all–cause mortality or improve secondary outcomes such as ICU admission, acute kidney injury (AKI), acute respiratory distress syndrome (ARDS), acute cardiac injury (ACI), thrombotic events, septic shock, or overall hospital stay. Al Sulaiman and co–workers (Al Sulaiman et al., 2021) also reported that multiple doses of TCZ did not confer additional survival benefits or reduce ICU length of stay or mechanical ventilation requirements in critically ill patients. Although the theoretical justification for repeat dosing, supported by pharmacokinetic models derived from cytokine release syndrome and national treatment guidelines such as China’s COVID–19 protocol, remains biologically plausible, our findings do not provide empirical support for its clinical efficacy in patients with COVID–19.
In contrast to our findings, large randomized controlled trials such as RECOVERY and REMAP–CAP reported significant mortality reductions with TCZ in hospitalized patients with COVID–19 (RECOVERY Collaborative Group. (2021); Gordon et al., 2021). Several factors may account for these differences. First, our cohort included a higher proportion of critically ill patients, many of whom received TCZ later in the disease course, potentially beyond the optimal therapeutic window. In RECOVERY and REMAP–CAP, earlier intervention, particularly within the hyperinflammatory phase, may have contributed to improved outcomes. Second, corticosteroid co–treatment was uniformly applied in our cohort, whereas in RECOVERY, corticosteroid use was not universal at the time of enrollment. This could have attenuated the incremental benefit of additional TCZ dosing in our study. Third, unlike these trials, our retrospective design lacked a control group that received no TCZ, limiting our ability to assess the absolute efficacy of a single dose. These differences emphasize the importance of patient selection, timing of administration, and treatment synergy in evaluating TCZ’s clinical impact.
In the present study, we found that the interaction between dose and treatment duration in the combination regimen of TCZ and corticosteroids has an impact on clinical outcomes. In terms of dose–dependent synergistic effects, corticosteroids may enhance TCZ efficacy through dual inhibition of the IL–6 pathway with systemic inflammatory responses. However, the marginal significance of the corticosteroid dose in this study (HR = 1.521, P = 0.052) also suggests the need to be alert to the potential risk of excessive immunosuppression. Notably, the negative interaction of TCZ with corticosteroid regimen suggests that early initiation of TCZ and a shorter corticosteroid regimen may optimize efficacy, consistent with the findings of the 2022 multicenter study (Jing et al. (2022)). However, the small sample size resulted in insufficient statistical power, and wide confidence intervals (e.g., the lower limit of the CI for the HR for corticosteroid dosing was close to 1.0) suggest the need for validation in larger studies. In the future, prospective designs are needed to clarify the optimal corticosteroid dose threshold and TCZ dosing timing, and to explore biomarker–guided individualized combination strategies to balance efficacy and infection risk.
In the present study, we maintained strict control over the dosage and frequency of TCZ administration. Our analysis showed that the two–dose group did not exhibit any improvement in in–hospital or 30–day mortality compared to the one–dose group. Additionally, no significant differences were found among the groups in terms of complication rates, length of hospital stay, or duration of mechanical ventilation. We also specifically evaluated the effect of a third dose to determine whether increased dosing might lead to improved clinical outcomes. Although the three–dose group did not show better outcomes compared to the one–or two–dose groups, it also did not present an increased risk of secondary infections. This contrasts with findings from Al Sulaiman et al. (2021), who reported a higher incidence of hospital–or ventilator–associated pneumonia with multiple doses. This difference may stem from multiple factors. In terms of patients’ baseline characteristics, those who received the third dose in this study had a higher oxygen demand at baseline than the other groups, and there was a difference in the choice of oxygen therapy modalities, which may have relied more on modalities such as high–flow oxygen therapy or noninvasive ventilation. Meanwhile, IL–6 levels in the three groups, although not different from the other two groups at baseline, were more significantly elevated in the third dose during follow–up, reflecting a more intense inflammatory response and greater disease severity. These factors may have acted together to cause the difference in results from other studies.
This study demonstrates that patients showed favorable changes in inflammatory markers following treatment with varying doses of TCZ. IL–6, a pivotal cytokine in the inflammatory cascade triggered by SARS–CoV–2 infection, plays a dual role, exhibiting both pro–and anti–inflammatory effects, and is critically involved in disease progression (Fajgenbaum and June, 2020). IL–6 signals through two pathways: classical signaling via membrane–bound IL–6 receptors (mIL–6R) and trans–signaling via soluble IL–6 receptors (sIL–6R). While low serum IL–6 levels are associated with anti–inflammatory responses, elevated IL–6 levels promote a pro–inflammatory state through trans–signaling, thereby activating a broader range of cells (Wang et al., 2022). This dysregulation is a hallmark of the hyperinflammatory response observed in severe COVID–19.
In this study, all three treatment groups exhibited increases in IL–6 levels following TCZ administration, typically showing a transient spike followed by a gradual decline with some fluctuation, a trend consistent with findings from a recent study by Liang and colleagues (Liang et al., 2024). This pattern aligns with TCZ’s mechanism of action: by inhibiting IL–6 receptors, it prevents IL–6 from binding, leading to an accumulation of unbound IL–6 in the serum (Killer et al., 2024). Notably, Mughal and colleagues (Mughal et al., 2020) reported no significant difference in post–treatment peak IL–6 levels between patients receiving a single dose versus multiple doses. Similarly, our study found comparable IL–6 levels after treatment in the one–dose and two–dose groups. However, patients in the three–dose group exhibited a more pronounced increase in IL–6 levels after treatment. This could be attributed to a cumulative pharmacological effect, individual variability in immune responses, or a more intense cytokine storm in these patients.
Simultaneously, this study found no statistically significant differences in CRP levels among the three TCZ treatment groups, which are consistent with findings from previous studies (Mughal et al., 2020). This suggests that the extent of CRP reduction may not be dependent on the number of TCZ doses administered. CRP levels decreased in all three groups after treatment compared to baseline, indicating that TCZ contributed to effective inflammation control (Calderon–Ochoa et al., 2024). In prior follow–up studies, patients receiving multiple doses of TCZ exhibited higher post–treatment PCT levels, which were associated with an increased risk of hospital–or ventilator–acquired infections (Al Sulaiman et al., 2021). However, in the present study, the risk of secondary infections did not appear to rise with additional doses of TCZ, and no significant differences in PCT levels were observed among the three groups after treatment. The variations in post–treatment PCT levels relative to baseline may reflect the influence of other endogenous anti–inflammatory factors, synergistic immune regulatory mechanisms, and individual patient variability.
Although different dosing regimens of TCZ demonstrated some positive effects on inflammatory markers, these improvements did not translate into reduced mortality or better clinical outcomes in critically ill COVID–19 patients. However, there were limitations in this study, with small and unbalanced sample sizes in the three groups (n = 16/32/8), resulting in the study lacking sufficient statistical power to detect moderate effect sizes, and upon post hoc efficacy analyses, the statistical power of the moderate differences in 30–day deaths and hospitalized deaths was found to be approximately 39.8%–41.2% and 38.7%–42.1%, which is well below the conventional threshold of 80%. This further confirms the lack of statistical validity. Thus, no firm conclusions can be drawn regarding the dose–response relationship based on the statistical power limitations of the current study. Also, the clinical benefit of the second dose is questionable due to the limitations of the current study, although the limited sample size may be one of the main influencing factors. In addition, there were deficiencies in safety reporting. Although no increase in secondary infections was observed between dose groups, this finding needs to be interpreted with caution given the incomplete surveillance of infections and the lack of detailed follow–up of adverse events.
This study has several limitations that warrant consideration. First, its retrospective, single–center design and small, imbalanced sample size (n = 16/32/8 across groups) limit generalizability and reduce statistical power to detect moderate effect sizes, as reflected in the wide confidence intervals observed for key outcomes. Accordingly, the findings should be interpreted as exploratory and hypothesis–generating rather than definitive. Second, the timing and frequency of TCZ administration were not standardized and were left to physician discretion, introducing potential for confounding by indication and immortal–time bias. Although time–dependent COX regression analyses were performed, fewer outcome events occurred before the typical window period for tolizumab administration, which influenced the impact of immortalization time on our specific cohort. Third, baseline severity may have varied between groups, especially in terms of oxygen treatment modality, oxygen demand, and SaO2, as echoed in our data, which may have led to bias in the comparison of treatment response. Fourth, subgroup analyses of type, dose, and duration of corticosteroids were not performed, and specific cutoff values for the effect on outcomes were not obtained, which affected the analysis of temporal synergism between corticosteroids and TCZ. Finally, although adverse events have been refined, the timing of their occurrence is difficult to obtain, the temporal association between multiple TCZ and complications is difficult to effectively address, and future prospective studies need to evaluate safety outcomes more systematically.
5 Conclusion
In this study of patients with severe and critical COVID–19, no additional benefit was observed with further dosing; larger studies are needed to confirm this finding. Due to the lack of a control group that did not receive TCZ, the efficacy of a single dose cannot be determined. These findings highlight the need for careful consideration of individual patient characteristics when determining dosing strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the Ningxia Medical University General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JeG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Writing – original draft. WZ: Formal Analysis, Investigation, Methodology, Writing – original draft. YD: Investigation, Software, Supervision, Writing – original draft. XP: Data curation, Investigation, Methodology, Software, Writing – original draft. JW: Investigation, Methodology, Project administration, Writing – original draft. YL: Formal Analysis, Methodology, Software, Supervision, Writing – original draft. JnG: Conceptualization, Methodology, Writing – original draft. JC: Project administration, Software, Validation, Writing – original draft. WZ: Conceptualization, Funding acquisition, Resources, Validation, Visualization, Writing – review and editing. SZ: Conceptualization, Funding acquisition, Resources, Supervision, Visualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Ningxia Hui Autonomous Region’s project “Study on Factors and Infectivity Related to the Recovery of Nucleic Acid in COVID-19 Patients in Ningxia” (Grant Number: 2022BEG03111), as well as the Autonomous Region Major Scientific and Technological Achievements Transformation Project “Clinical Translation of cfRNA Sequencing Technology for Auxiliary Diagnosis of Major Respiratory Diseases” (Grant Number: 2022CJE09009).
Acknowledgments
We express our profound appreciation to the individuals who contributed their time and effort to participate in this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1571372/full#supplementary-material
References
Al Sulaiman, K., Aljuhani, O., Bin Salah, K., Korayem, G. B., Eljaaly, K., Al Essa, M., et al. (2021). Single versus multiple doses of tocilizumab in critically ill patients with coronavirus disease 2019 (COVID-19): a two-center, retrospective cohort study. J. Crit. Care 66, 44–51. doi:10.1016/j.jcrc.2021.08.007
Calderon–Ochoa, C., Plamenov–Donchev, N., Hernandez–Quinones, F., Mendoza–Lopez, O., Hinojos–Gallardo, L. C., Longino–Gomez, A. J., et al. (2024). Tocilizumab demonstrates superiority in decreasing C-reactive protein levels in hospitalized COVID-19 patients, compared to standard care treatment alone. Microbiol. Spectr. 12, e249823. doi:10.1128/spectrum.02498–23
De Roquetaillade, C., Guillemin, J., Picod, A., Mebazaa, A., Kimmoun, A., Chousterman, B. G., et al. (2022). Single-dose tocilizumab blunts systemic inflammation in severe COVID-19 patients. J. Crit. Care 68, 169–171. doi:10.1016/j.jcrc.2021.11.001
De Rossi, N., Scarpazza, C., Filippini, C., Cordioli, C., Rasia, S., Mancinelli, C. R., et al. (2020). Early use of low dose tocilizumab in patients with COVID-19: a retrospective cohort study with a complete follow-up. Eclinicalmedicine 25, 100459. doi:10.1016/j.eclinm.2020.100459
Fajgenbaum, D. C., and June, C. H. (2020). Cytokine storm. N. Engl. J. Med. 383, 2255–2273. doi:10.1056/NEJMra2026131
Gordon, A. C., Mouncey, P. R., Al–Beidh, F., Rowan, K. M., Nichol, A. D., Arabi, Y. M., et al. (2021). Interleukin–6 receptor antagonists in critically ill patients with covid–19. N. Engl. J. Med. 384, 1491–1502. doi:10.1056/NEJMoa2100433
Guaraldi, G., Meschiari, M., Cozzi–Lepri, A., Milic, J., Tonelli, R., Menozzi, M., et al. (2020). Tocilizumab in patients with severe covid–19: a retrospective cohort study. Lancet Rheumatol. 2, e474–e484. doi:10.1016/S2665–9913(20)30173–9
Hermine, O., Mariette, X., Tharaux, P., Resche-Rigon, M., Porcher, R., Ravaud, P., et al. (2021). Effect of tocilizumab vs usual care in adults hospitalized with covid–19 and moderate or severe pneumonia: a randomized clinical trial. Jama Intern Med. 181, 32–40. doi:10.1001/jamainternmed.2020.6820
Horby, P. W., Campbell, M., Staplin, N., Abbas, M., Abbasi, S., Abbass, H., et al. (2021). Tocilizumab in patients admitted to hospital with covid–19 (recovery): a randomised, controlled, open–label, platform trial. Lancet 397, 1637–1645. doi:10.1016/S0140–6736(21)00676–0
Hu, B., Huang, S., and Yin, L. (2021). The cytokine storm and covid–19. J. Med. Virol. 93, 250–256. doi:10.1002/jmv.26232
Jing, S. R. T., Ting, K. N., and Ee, C. X. (2022). High-dose versus low-dose corticosteroids in COVID-19 patients: a systematic review and meta-analysis. Journal of cardiothoracic and vascular anesthesia 36, 3576–3586. doi:10.1053/J.JVCA.2022.05.011
Jones, S. A., Richards, P. J., Scheller, J., and Rose-John, S. (2005). Il–6 transsignaling: the in vivo consequences. J. Interferon Cytokine Res. 25, 241–253. doi:10.1089/jir.2005.25.241
Kaly, L., and Rosner, I. (2012). Tocilizumab – a novel therapy for non–organ–specific autoimmune diseases. Best. Pract. Res. Clin. Rheumatol. 26, 157–165. doi:10.1016/j.berh.2012.01.001
Killer, A., Gliga, S., Massion, P., Ackermann, C., De Angelis, C., Flasshove, C., et al. (2024). Trajectories and predictive significance of inflammatory parameters for clinical outcome in covid–19 patients treated with tocilizumab. Infection 53, 339–348. doi:10.1007/s15010–024–02375–x
Le, R. Q., Li, L., Yuan, W., Shord, S. S., Nie, L., Habtemariam, B. A., et al. (2018). Fda approval summary: tocilizumab for treatment of chimeric antigen receptor t cell–induced severe or life–threatening cytokine release syndrome. Oncologist 23, 943–947. doi:10.1634/theoncologist.2018–0028
Liang, P., Li, Y., Meng, L., Li, Y., Mai, H., Li, T., et al. (2024). Prognostic significance of serum interleukin–6 in severe/critical covid–19 patients treated with tocilizumab: a detailed observational study analysis. Sci. Rep. 14, 29634. doi:10.1038/s41598–024–81028–3
Mahallawi, W. H., Khabour, O. F., Zhang, Q., Makhdoum, H. M., and Suliman, B. A. (2018). Mers–cov infection in humans is associated with a pro–inflammatory th1 and th17 cytokine profile. Cytokine 104, 8–13. doi:10.1016/j.cyto.2018.01.025
Mughal, M. S., Kaur, I., Kakadia, M., Wang, C., Alhashemi, R., Salloum, R., et al. (2020). Is there any additional benefit of multiple doses of tocilizumab in covid–19 patients? Cureus 12, e12214. doi:10.7759/cureus.12214
Nishimoto, N., Terao, K., Mima, T., Nakahara, H., Takagi, N., and Kakehi, T. (2008). Mechanisms and pathologic significances in increase in serum interleukin–6 (Il–6) and soluble il–6 receptor after administration of an anti–il–6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and castleman disease. Blood 112, 3959–3964. doi:10.1182/blood–2008–05–155846
Oran, D. P., and Topol, E. J. (2021). The proportion of SARS–cov–2 infections that are asymptomatic: a systematic review. Ann. Intern Med. 174, 655–662. doi:10.7326/M20–6976
Prc, N. H. C. O., and Medicine, N. A. O. T. (2023). Diagnosis and treatment plan for covid.19(trial version 10). Inter. J. Epidemiol. Infect. Dis. 36, 18–25.
RECOVERY Collaborative Group (2021). TCZ in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. The Lancet 397, 1637–1645. doi:10.1016/S0140-6736(21)00676-0
Rezaei, S., Fatemi, B., Karimi Majd, Z., Minaei, H., Peikanpour, M., Anjidani, N., et al. (2021). Efficacy and safety of tocilizumab in severe and critical COVID-19: a systematic review and meta-analysis. Expert Rev. Clin. Immunol. 17, 499–511. doi:10.1080/1744666X.2021.1908128
Salama, C., Han, J., Yau, L., Reiss, W. G., Kramer, B., Neidhart, J. D., et al. (2021). Tocilizumab in patients hospitalized with covid–19 pneumonia. N. Engl. J. Med. 384, 20–30. doi:10.1056/NEJMoa2030340
Shankar–Hari, M., Vale, C. L., Godolphin, P. J., Fisher, D., Higgins, J. P. T., Spiga, F., et al. (2021). Association between administration of il–6 antagonists and mortality among patients hospitalized for covid–19: a meta–analysis. Jama 326, 499–518. doi:10.1001/jama.2021.11330
Veiga, V. C., Prats, J. A. G. G., Farias, D. L. C., Rosa, R. G., Dourado, L. K., Zampieri, F. G., et al. (2021). Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. Bmj 372, n84. doi:10.1136/bmj.n84
Keywords: COVID-19, SARS-CoV-2, tocilizumab, IL-6, crp
Citation: Gao J, Zhang W, Ding Y, Peng X, Wang J, Li Y, Gao J, Cheng J, Zhou W and Zhang S (2025) Evaluating the efficacy of different doses of tocilizumab in treating critically ill COVID–19 patients: a single–center retrospective cohort study. Front. Pharmacol. 16:1571372. doi: 10.3389/fphar.2025.1571372
Received: 05 February 2025; Accepted: 09 July 2025;
Published: 24 July 2025.
Edited by:
Avijit Dutta, Amity University Uttar Pradesh, IndiaReviewed by:
Khin Phyu Pyar, Ministry of Health Myanmar, MyanmarJune-Youong Koh, University of California, San Diego, United States
Hong-Han Ge, Shandong First Medical University, China
Copyright © 2025 Gao, Zhang, Ding, Peng, Wang, Li, Gao, Cheng, Zhou and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhou, emh3MDMwNkAxMjYuY29t; Shuxiang Zhang, enN4OTVAMTI2LmNvbQ==
 Jie Gao
Jie Gao Wenjing Zhang1,2
Wenjing Zhang1,2 Wei Zhou
Wei Zhou Shuxiang Zhang
Shuxiang Zhang