- 1Department of Thoracic Surgery, Ganzhou People’s Hospital, Ganzhou, China
- 2Department of Oncology, Ganzhou People’s Hospital, Ganzhou, China
Background: Lung cancer can result in malignant pericardial effusion (MPE), impacting patient prognosis. Intrapericardial infusion of bevacizumab was an alternative treatment for MPE.
Case presentation: We present the case of a 48-year-old female with stage IV lung adenocarcinoma and MPE. MPE was managed by intrapericardial infusion of bevacizumab. The first intrapericardial infusion of bevacizumab effectively controlled the MPE for 8 months. Cardiotoxicity quickly emerged after the second intrapericardial infusion of bevacizumab. After intensive treatment, the symptoms of cardiotoxicity resolved within 10 days.
Conclusion: The present case indicates that intrapericardial infusion of bevacizumab could lead to cardiotoxicity in MPE patient. Cardiac examinations should be conducted before and after anti-vascular endothelial growth factor treatment.
Introduction
Malignant pericardial effusion (MPE) commonly develops in cancer patients, leading to refractory cardiac dysfunction and pericardial tamponade (Chahine et al., 2021). MPE is linked to high recurrence rates and a poor prognosis (Gornik et al., 2005; Dequanter et al., 2008). Commom clinical treatments for MPE include pericardiocentesis, percutaneous catheter drainage, pericardial window, systemic antineoplastic treatment and intrapericardial infusion of therapeutic agents (Zhang et al., 2020). Intrapericardial infusion of bevacizumab was an alternative treatment for MPE due to its effectiveness and safety (Chen et al., 2015; Chen et al., 2016; Ueda et al., 2016; Del Rosario et al., 2016). Here, we reported a MPE patient who experienced cardiotoxicity after intrapericardial infusion of bevacizumab.
Case report
A 48-year-old female was admitted to a local hospital because of tussiculation and chest tightness on 23 March 2013. Chest computed tomography revealed a right hilar mass (Figure 1A), pleural effusion (Figure 1B), and pericardial effusion (Figure 1B). Right thoracic puncture and drainage were performed, and pleural effusion examination found adenocarcinoma cells. Needle aspiration cytology of the right supraclavicular lymph node indicated adenocarcinoma. The patient came to our center for further treatment on 31 March 2021. Cervical lymph node biopsy indicated metastatic adenocarcinoma which came from lung. Pericardiocentesis and drainage were performed, and pericardial effusion examination found adenocarcinoma cells. The patient was diagnosed with right lung adenocarcinoma with pleural and pericardial metastases (cT1N3M1a). Next-generation sequencing showed no driver gene mutations. On 1 April 2021, the patient received the first intrapericardial infusion of bevacizumab (300 mg). From April 8 to 17 August 2021, she completed six cycles of first-line therapy with pemetrexed and cisplatin combined with camrelizumab. This was followed by three cycles of camrelizumab maintenance therapy from September 9 to 22 October 2021. Due to disease progression, treatment was switched to sintilimab plus pemetrexed on 14 November 2021. Throughout this period, electrocardiograms and myocardial enzyme levels remained within normal limits, and the pericardial effusion was well-controlled, with only a minimal amount remaining. On 10 December 2021, the patients received the second intrapericardial infusion of 200 mg of bevacizumab due to worsening pericardial effusion. The following day (December 11), the patient developed acute chest pain. Myocardial enzymes (Figure 2) were significantly elevated and electrocardiography (Supplementary Data Sheet S1) showed ST-T changes and QTc prolongation. The N-terminal pro-brain natriuretic peptide level was 11,507 pg/mL and echocardiogram showed a normal ejection fraction. Bevacizumab-associated cardiotoxicity (myocardial ischemia) was initially suspected. Methylprednisolone, aspirin, and heparin were administered for treatment. The myocardial enzyme levels trended downward within 2 days, and the chest pain resolved after 10 days. Unfortunately, the patient died of central respiratory failure on 10 January 2022. Figure 3 presented the patient’s treatment timeline.
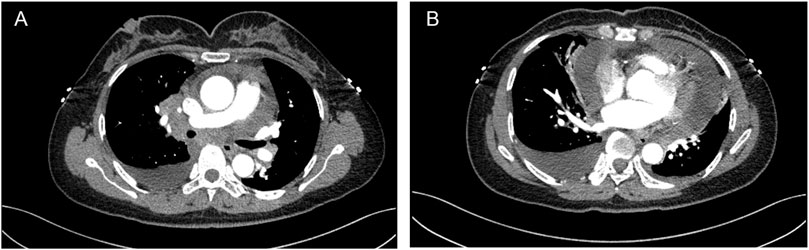
Figure 1. Chest computed tomography revealed a right hilar mass (A), pleural and pericardial effusion (B).
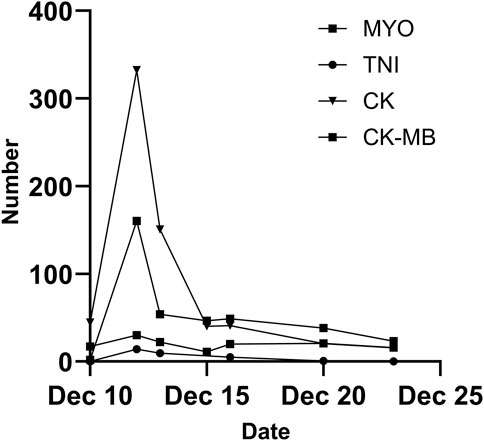
Figure 2. Alterations in myocardial enzyme levels during treatment. MYO, myoglobin; TNI, troponin I; CK, creatine kinase; CK-MB, creatine kinase isoenzyme MB.
Discussion
Malignant tumors are a common cause of pericardial effusion, with lung cancer being the most prevalent. 9 Some studies have shown that intrapericardial infusion of bevacizumab is a safe and effective treatment for managing MPE (Chen et al., 2015; Chen et al., 2016; Chen et al., 2017; He et al., 2022). Bevacizumab seldom leads to cardiotoxicity, a potentially life-threatening side effect (Abdel-Qadir et al., 2017). To the best of our knowledge, this is the first report on a MPE patient who underwent myocardial ischemia possibly due to intrapericardial infusion of bevacizumab.
There is currently no standard effective treatment for MPE. The treatment of MPE focuses on alleviating symptoms, ensuring hemodynamic stability, and preventing fluid recurrence. Systemic chemotherapy is effective for MPE and intracardiac drug injection benefits patients without recurrence (Zhang et al., 2020). Bevacizumab is a recombinant humanized monoclonal antibody that inhibits the binding of human vascular endothelial growth factor (VEGF) to its receptors (Presta et al., 1997). It has been shown to inhibit the growth of 13 types of malignant cells and reduce the density, diameter, and permeability of blood vessels (Takahashi et al., 1997). Bevacizumab is typically given intravenously, but its intracavitary administration is a safe and effective method for managing MPE (Chen et al., 2015; Chen et al., 2016; Chen et al., 2017; He et al., 2022). A randomized clinical study demonstrated that intrapleural infusion of bevacizumab is more effective and safer than intravenous infusion for managing malignant pleural effusion due to lung cancer (Nie et al., 2020). Compared to chemotherapy drugs like platinum alone, intracardial injection of bevacizumab decreased side effects and had favorable outcomes in patients with MPE (Chen et al., 2016; Chen et al., 2017; He et al., 2022). The most common adverse events associated with bevacizumab were hypertension, proteinuria, asthenia, and diarrhea (Geiger-Gritsch et al., 2010). A meta-analysis revealed that VEGF signaling pathway inhibitors significantly raise the odds of hypertension, cardiac ischemia, arterial thromboembolism, and cardiac dysfunction by 5.3, 2.8, 1.5, and 1.4 times, respectively. The risk of a fatal cardiovascular event with VEGF inhibitor therapy is only 0.25% (Abdel-Qadir et al., 2017). Diagnosis and treatment of chronic and acute coronary syndromes following VEGF inhibitor therapy adhere to ESC Guidelines on cardio-oncology (Lyon et al., 2022). The patient developed myocardial ischemia symptoms 1 day after a pericardial infusion of bevacizumab, having undergone multiple previous antineoplastic treatments without cardiac toxicity. The symptoms of myocardial ischemia subsided after treatment, but the ECG revealed a prolonged QTc interval. QTc interval prolongation is linked to the activation of cardiomyocyte potassium channel proteins and can be induced by VEGF inhibitors (Mihalcea et al., 2023). Management and treatment of cardiac ischemia induced by VEGF inhibitors should be tailored to the patient’s cancer severity, life expectancy, comorbidities, and available highly effective antineoplastic alternatives (Lyon et al., 2022). Bevacizumab carries a theoretical risk of inducing myocardial ischemia through mechanisms such as inhibition of angiogenesis, impairment of coronary microcirculation, or induction of localized vasospasm (Mihalcea et al., 2023). Although the onset of myocardial ischemic symptoms coincided temporally with the second intracardiac administration of bevacizumab, the potential cumulative cardiotoxic effects of prior treatments—including cisplatin, camrelizumab, and sintilimab—cannot be ruled out as contributing factors (Senkus and Jassem, 2011). The existing literature primarily highlights its efficacy and short-term tolerability, but robust data from large, prospective trials are lacking. Therefore, the occurrence of a serious adverse event like myocardial ischemia in our patient underscores the potential risks and the necessity for heightened vigilance.
In conclusion, the present case indicates that intrapericardial infusion of bevacizumab could lead to cardiotoxicity in MPE patient. Intrapericardial infusion of bevacizumab is a safe and effective treatment for managing MPE, but monitoring for adverse effects is essential. The efficacy of bevacizumab in treating cancer must be weighed against its potential cardiotoxicity.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing. YL: Data curation, Investigation, Writing – original draft. CX: Validation, Writing – original draft. HL: Data curation, Methodology, Supervision, Validation, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1573297/full#supplementary-material
Abbreviations
MPE, Malignant pericardial effusion; VEGF, human vascular endothelial growth factor.
References
Abdel-Qadir, H., Ethier, J. L., Lee, D. S., Thavendiranathan, P., and Amir, E. (2017). Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: a systematic review and meta-analysis. Cancer Treat. Rev. 53, 120–127. doi:10.1016/j.ctrv.2016.12.002
Chahine, J., Shekhar, S., Mahalwar, G., Imazio, M., Collier, P., and Klein, A. (2021). Pericardial involvement in cancer. Am. J. Cardiol. 145, 151–159. doi:10.1016/j.amjcard.2020.12.092
Chen, D., Zhang, Y., Shi, F., Li, M., Zhu, H., Kong, L., et al. (2015). Sustained response of malignant pericardial effusion to intrapericardial bevacizumab in an advanced lung cancer patient: a case report and literature review. OncoTargets Ther. 8, 2767–2770. doi:10.2147/OTT.S90145
Chen, D., Zhang, Y., Shi, F., Zhu, H., Li, M., Luo, J., et al. (2016). Intrapericardial bevacizumab safely and effectively treats malignant pericardial effusion in advanced cancer patients. Oncotarget 7 (32), 52436–52441. doi:10.18632/oncotarget.9420
Chen, D., Song, X., Shi, F., Zhu, H., Wang, H., Zhang, N., et al. (2017). Greater efficacy of intracavitary infusion of bevacizumab compared to traditional local treatments for patients with malignant cavity serous effusion. Oncotarget 8 (21), 35262–35271. doi:10.18632/oncotarget.13064
Del Rosario, M., Tsai, H., and Dasanu, C. A. (2016). Prolonged survival in Colon cancer with malignant pericardial effusion and pulmonary lymphangitic carcinomatosis: a case for monoclonal antibodies? Conn. Med. 80 (8), 483–485.
Dequanter, D., Lothaire, P., Berghmans, T., and Sculier, J. P. (2008). Severe pericardial effusion in patients with concurrent malignancy: a retrospective analysis of prognostic factors influencing survival. Ann. Surg. Oncol. 15 (11), 3268–3271. doi:10.1245/s10434-008-0059-z
Geiger-Gritsch, S., Stollenwerk, B., Miksad, R., Guba, B., Wild, C., and Siebert, U. (2010). Safety of bevacizumab in patients with advanced cancer: a meta-analysis of randomized controlled trials. Oncol. 15 (11), 1179–1191. doi:10.1634/theoncologist.2009-0155
Gornik, H. L., Gerhard-Herman, M., and Beckman, J. A. (2005). Abnormal cytology predicts poor prognosis in cancer patients with pericardial effusion. J. Clin. Oncol. 23 (22), 5211–5216. doi:10.1200/JCO.2005.00.745
He, D., Guo, Z., Xie, Z., Zhang, Y., Deng, Q., and Yang, H. (2022). Efficacy of intrapleural or intrapericardial injection of single bevacizumab in the treatment of lung cancer-mediated malignant effusion. Can. Respir. J. 2022, 6763625. doi:10.1155/2022/6763625
Lyon, A. R., López-Fernández, T., Couch, L. S., Asteggiano, R., Aznar, M. C., Bergler-Klein, J., et al. (2022). ESC guidelines on cardio-oncology developed in collaboration with the european hematology association (EHA), the european society for therapeutic radiology and oncology (ESTRO) and the international cardio-oncology society (IC-OS). Eur. Heart J. 43 (41), 4229–4361.
Mihalcea, D., Memis, H., Mihaila, S., and Vinereanu, D. (2023). Cardiovascular toxicity induced by vascular endothelial growth factor inhibitors. Life (Basel, Switzerland) 13 (2), 366. doi:10.3390/life13020366
Nie, K., Zhang, Z., You, Y., Zhuang, X., Zhang, C., and Ji, Y. (2020). A randomized clinical study to compare intrapleural infusion with intravenous infusion of bevacizumab in the management of malignant pleural effusion in patients with non-small-cell lung cancer. Thorac. Cancer 11 (1), 8–14. doi:10.1111/1759-7714.13238
Presta, L. G., Chen, H., O'Connor, S. J., Chisholm, V., Meng, Y. G., Krummen, L., et al. (1997). Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 57 (20), 4593–4599.
Senkus, E., and Jassem, J. (2011). Cardiovascular effects of systemic cancer treatment. Cancer Treat. Rev. 37 (4), 300–311. doi:10.1016/j.ctrv.2010.11.001
Takahashi, Y., Tucker, S. L., Kitadai, Y., Koura, A. N., Bucana, C. D., Cleary, K. R., et al. (1997). Vessel counts and expression of vascular endothelial growth factor as prognostic factors in node-negative colon cancer. Archives Surg. 132 (5), 541–546. doi:10.1001/archsurg.1997.01430290087018
Ueda, T., Tsubamoto, H., Eguchi, A., Terada, T., and Shibahara, H. (2016). Bevacizumab helped resolve pericardial and pleural effusion that was associated with malignant ovarian clear cell carcinoma. Gynecol. Oncol. Rep. 16, 11–13. doi:10.1016/j.gore.2016.01.006
Keywords: bevacizumab, cardiotoxicity, myocardial ischemia, lung cancer, malignant pericardial effusion
Citation: Wu S, Lu Y, Xu C and Liu H (2025) Cardiotoxicity related to intrapericardial infusion of bevacizumab in the treatment of lung cancer-mediated malignant pericardial effusion: a case report. Front. Pharmacol. 16:1573297. doi: 10.3389/fphar.2025.1573297
Received: 08 February 2025; Accepted: 06 October 2025;
Published: 17 October 2025.
Edited by:
Yuan Tang, University of Toledo, United StatesReviewed by:
Xian Xiao, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, ChinaWeir Chiang You, Taichung Veterans General Hospital, Taiwan
Copyright © 2025 Wu, Lu, Xu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huafeng Liu, aHVhZmVuZ19sMTk4MUAxNjMuY29t
 Shilong Wu
Shilong Wu Yanjun Lu2
Yanjun Lu2