- 1MSN, RN, Fellow,Nursing department, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 2MSN, RN, Chief Head Nurse, Nursing department, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 3MSN, RN, Teaching Supervision,Nursing department, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
Objective: To compare the efficacy of different anesthetic adjuvants combined with sevoflurane across specific surgical sites using a Bayesian network meta-analysis.
Methods: A systematic review was conducted, following PRISMA guidelines, including 100 randomized controlled trials (RCTs) involving 8,800 pediatric patients undergoing various surgeries. The network meta-analysis evaluated 22 drug interventions, with log risk ratios (logRR) and Surface Under the Cumulative Ranking (SUCRA) probabilities calculated for each drug or combination.
Results: Among all interventions, dexmedetomidine combined with alfentanil was the most effective in reducing ED risk for tonsillectomy/adenoidectomy, achieving a SUCRA ranking of 94.63% (logRR = −2.82). For ophthalmic surgery, propofol and midazolam showed the highest efficacy (logRR = −1.83, SUCRA: 86.03%). Dexmedetomidine combined with midazolam was the optimal combination for inguinal hernia/hypospadias (logRR = −2.16, SUCRA: 81.73%) and dental/oral repairs (logRR = −1.83, SUCRA: 94.85%). For cleft lip/palate repair, dexmedetomidine alone showed significant efficacy (logRR = −1.65, SUCRA: 89.15%). In myringotomy/cochlear implantation, fentanyl was the most effective adjuvant (logRR = −1.17, SUCRA: 80.02%).
Conclusion: Targeted use of dexmedetomidine-based combinations was found to be particularly effective across various surgeries, while fentanyl and propofol-midazolam combinations excelled in specific contexts. This study underscores the importance of tailoring anesthetic adjuvant strategies to specific surgical sites to reduce the risk of ED in pediatric patients, and provides a valuable reference for optimizing anesthetic care in this vulnerable population.
1 Introduction
Sevoflurane is the most commonly used drug for induction and maintenance of anesthesia in pediatric patients (Sakai et al., 2005). It provides rapid induction and recovery, with easy adjustment of anesthetic depth. Additionally, sevoflurane has minimal impact on heart rate and little airway irritation, allowing for muscle relaxation. However, its use is associated with a high incidence of postoperative emergence delirium (ED) in the pediatric population (Park et al., 2014; Dahmani et al., 2010; Kulka et al., 2001), potentially leading to self-injury, delayed discharge, and increased medical costs (Park et al., 2014; Dahmani et al., 2010; Kulka et al., 2001).
Anesthetic adjuvants such as dexmedetomidine, ketamine, propofol, fentanyl, midazolam, sufentanil, remifentanil, and clonidine are effective in preventing ED (Fang et al., 2016). A network meta-analysis (NMA) demonstrated that dexmedetomidine combined with sevoflurane appeared to be the most effective strategy for reducing the risk of ED in pediatric anesthesia, compared to other single adjuvant agents (Wang et al., 2017). Previous randomized controlled trials (RCTs) and meta-analyses have also shown that combination therapies may have a synergistic effect in preventing ED. Specifically, the combination of dexmedetomidine, midazolam, and an antiemetic was identified as the most effective strategy to prevent ED (Wang et al., 2021). However, these meta-analyses did not differentiate between various surgical sites and types, treating all pediatric surgical populations as a single homogeneous group, which may introduce bias. Previous meta-analyses have indicated that head and neck surgeries are associated with a higher incidence of pediatric ED (Chen et al., 2024). Furthermore, ophthalmic and ear, nose, and throat (ENT) surgeries are considered risk factors for ED (Dahmani et al., 2010). Children undergoing ophthalmic and ENT surgeries often experience anxiety related to visual impairment, a sense of choking from restricted speech, or discomfort with swallowing, which may increase the risk of ED (Joo et al., 2014; Eckenhoff et al., 1961). In cleft palate repairs, anatomical reconstruction of the soft and hard palates may result in significant postoperative oropharyngeal pain and bleeding (Liu et al., 2021), and pain is a major risk factor for ED. Inadequate pain management may lead to delirium. For pediatric inguinal hernia repair, the incidence of ED significantly decreases when sevoflurane anesthesia is combined with caudal block (Aouad et al., 2005).
Currently, meta-analyses comparing the efficacy of different anesthetic adjuvants for preventing ED in specific surgical sites are limited to three studies: one focusing on ophthalmic surgery (Tan et al., 2019), another on tonsillectomy/adenoidectomy (Jiao et al., 2020), and the third on cleft palate surgery (Liu et al., 2021). However, the first two studies did not comprehensively compare the combined effects of different anesthetic adjuvants, and the last one only analyzed the effect of dexmedetomidine. To compare the efficacy of combination or single therapies in preventing ED across different surgical sites, we used a Bayesian network to identify which anesthetic adjuvants combined with sevoflurane influence the incidence of ED in pediatric surgical patients and to determine the best strategy for guiding anesthetic practice.
2 Methods
2.1 Study strategy and selection criteria
This NMA follows the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 guidelines and is registered in PROSPERO. A systematic review was conducted of publications retrieved from PubMed, Embase, Cochrane Library, and ClinicalTrials.gov databases, covering records up until 29 September 2024. The search terms used included: “ (anesthesia) and (sevoflurane) and [(delirium) or (agitation)] and [(ancillary drug) or (ketamine) or (propofol) or (dexmedetomidine) or (clonidine) or (midazolam) or (fentanyl) or (remifentanil) or (sufentanil) or (melatonin)],” along with their synonyms. No restrictions were placed on language or publication date. For non-English articles, we used Google Translate for translation. Additionally, we manually searched reference lists of review articles and pairwise meta-analyses for potentially eligible studies. As this study is a systematic review and meta-analysis, no new human or animal data were collected. Ethical approval was not required. All included RCTs had previously obtained ethical approval from their respective institutions.
2.2 Inclusion and exclusion criteria
The PICO criteria applied for this NMA are as follows: (1) Patients or Population: Pediatric patients (under 18 years old) undergoing general anesthesia with sevoflurane, with specific surgical sites reported; (2) Intervention: Drug interventions administered during sevoflurane general anesthesia; (3) Control: Placebo control or active control groups; (4) Outcome: Incidence of ED following sevoflurane anesthesia.
Exclusion criteria included: (1) Studies that were not randomized controlled trials (RCTs), (2) Studies not reporting ED incidence, (3) Studies unrelated to drug interventions targeting the risk of ED, or (4) Studies involving patients not receiving sevoflurane anesthesia. In cases of duplicate data, only the report with more information and a larger sample size was included.
2.3 Data extraction
Titles and abstracts identified through the database search were exported to end note X9, with duplicates removed. Two researchers independently screened the titles and abstracts for eligibility, followed by full-text reviews of potentially relevant studies to determine final inclusion. Discrepancies during the selection process were resolved by a third reviewer. From each included study, we extracted the following information: first author, year of publication, type of surgery, type of anesthetic adjuvants, patient demographics, sample size, and the number of ED cases.
2.4 Quality assessment
The quality of the included studies was assessed using the Cochrane Risk of Bias tool, which evaluates the following domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other potential sources of bias.
2.5 Statistical analysis
Data were analyzed using R version 4.3.3, and network evidence along with comparison-adjusted funnel plots were generated using Stata 17. For categorical outcomes, log risk ratios (logRRs) and 95% credible intervals (CrIs) were calculated. The NMA was conducted within a Bayesian framework using Markov Chain Monte Carlo (MCMC) simulation. Leverage plots were used to evaluate model convergence (Supplementary Figure S1). The Bayesian approach was chosen over frequentist methods due to its flexibility in incorporating prior information and fully probabilistic interpretation of treatment effects and rankings. Unlike traditional meta-analytic techniques, which provide point estimates and confidence intervals, the Bayesian framework allows direct probability statements about treatment rankings and uncertainty. This is particularly valuable in NMA, where indirect and mixed comparisons are synthesized simultaneously (Béliveau et al., 2019).
To aid the interpretation of treatment rankings, Surface Under the Cumulative Ranking (SUCRA) values were calculated. SUCRA represents the percentage of efficacy/safety that an intervention achieves relative to a hypothetical ideal treatment that is always the best. A SUCRA of 1 (or 100%) indicates the treatment is most likely to be the best, whereas 0 indicates the least effective, which can support clinical decision-making by offering a comparative measure of benefit across multiple treatments (Mbuagbaw et al., 2017).
For the sensitivity analysis, meta-regression analyses were also conducted within a Bayesian framework to investigate the influence of confounding factors, including participants’ mean age (continuous variable), proportion of males (continuous variable), time of prescription (before or at the start of anesthesia and surgery = 1, during anesthesia in surgery = 2, near the end or at the end of surgery = 3), and risk of bias in studies (low = 1, some concerns = 2, high = 3). These models employed the MCMC method with 10,000 burn-in iterations and an additional 500,00 simulations, utilizing four chains with different initial values to derive medians and 95% credible intervals (CrIs) (Neupane et al., 2014). Comparison-adjusted funnel plots were employed to assess the presence of small-study effects.
3 Results
3.1 Study characteristics
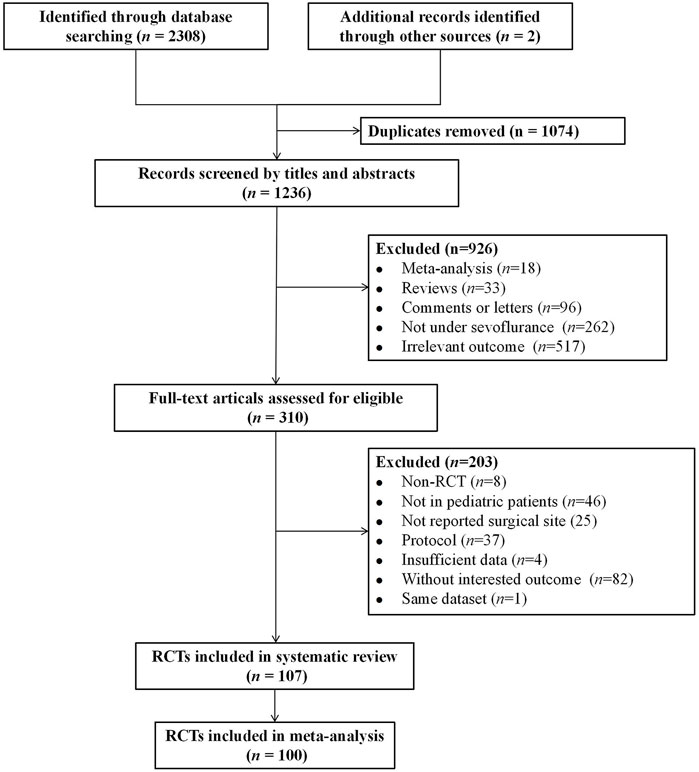
A total of 2,310 records were identified through the literature search. Of these, 1,074 were duplicate articles. After reviewing the titles and abstracts, 926 unrelated articles were excluded, and an additional 203 articles were excluded for not meeting the inclusion criteria. 107 eligible articles were included in the systematic review. Among them, seven studies investigating surgical types that involved non-invasive and painless MRI procedures were excluded (Abu-Shahwan, 2008; Bong et al., 2015; Costi et al., 2015; Cravero et al., 2003; Dalens et al., 2006; Isik et al., 2006; Moawad and El-Diasty, 2015). Ultimately, 100 studies were included in the final NMA (Abbas et al., 2019; Abdelaziz et al., 2016; Abdelhalim and Alarfaj, 2013; Abdelmawgoud and Mohy, 2012; Abu-Shahwan and Chowdary, 2007; Akin et al., 2012; Alansary et al., 2023; Ali and Abdellatif, 2013; Ali et al., 2020; Almenrader et al., 2007; Amer et al., 2022; Amer et al., 2022; Aouad et al., 2007; Asaad et al., 2011; Bae et al., 2010; Baek et al., 2022; Bakhame et al., 2009; Bedirli et al., 2017; Bergendahl et al., 2004; Bilgen et al., 2014; Bromfalk et al., 2023; Cai et al., 2021; Cai et al., 2021; Cai et al., 2024; Chen et al., 2018; Chen et al., 2010; Chen et al., 2013; Chen et al., 2023; Cho et al., 2014; Choi et al., 2016; Demirbilek et al., 2004; Di et al., 2017; Di et al., 2014; Di et al., 2014; Dong et al., 2010; Erdil et al., 2009; Finkel et al., 2001; Galinkin et al., 2000; Ghai et al., 2010; Ghosh et al., 2011; Golmohammadi et al., 2024; Guler et al., 2005; Hadi et al., 2015; Hauber et al., 2015; Hauber et al., 2015; He et al., 2023; Heinmiller et al., 2013; Huang et al., 2022; Ibacache et al., 2004; Ibrahim et al., 2023; Jangra et al., 2022; Jayaraj et al., 2023; Jeong et al., 2012; Ju et al., 2013; Jun et al., 2018; Jun et al., 2018; Jung et al., 2010; Kawai et al., 2019; Khalifa and Hassanin, 2013; Kim et al., 2009; Kim et al., 2016; Kim et al., 2013; Kim et al., 2014; Kim et al., 2011; Komazaki et al., 2020; Lankinen et al., 2006; Lankinen et al., 2006; Lee C. J. et al., 2010; Lee Y. S. et al., 2010; Li et al., 2011; Li et al., 2013; Li et al., 2024; Liang et al., 2014; Lili et al., 2012; Lin et al., 2017; Lin et al., 2016; Liu et al., 2022; Liu et al., 2022; Lundblad et al., 2015; Luo et al., 2017; Meng et al., 2012; Mizrak et al., 2011; Mizrak et al., 2010; Mohamed Maaly et al., 2024; Na et al., 2013; Patel et al., 2010; Peng and Zhang, 2015; Pestieau et al., 2011; Pestieau et al., 2011; Rashad and Soud, 2014; Saadawy et al., 2009; Sahmeddini et al., 2024; Shen et al., 2012; Sheta et al., 2014; Shi et al., 2019; Soliman and Alshehri, 2015; Song et al., 2016; Sousa-Júnior et al., 2021; Thomas et al., 2015; Thomas et al., 2015; Vettuvanthodi et al., 2024; Viitanen et al., 1999; Xi et al., 2012; Xiao et al., 2012; Xing et al., 2024; Yang et al., 2022; Yao et al., 2015; Yun et al., 2016; Zhang et al., 2022). The selection process is illustrated in Figure 1. The basic characteristics of the included studies are presented in Supplementary Table S1. In total, 8,800 children with varying health conditions were covered in the studies. Twenty-three studies were three-arm trials, while the rest were two-arm studies.
3.2 Risk of bias assessment
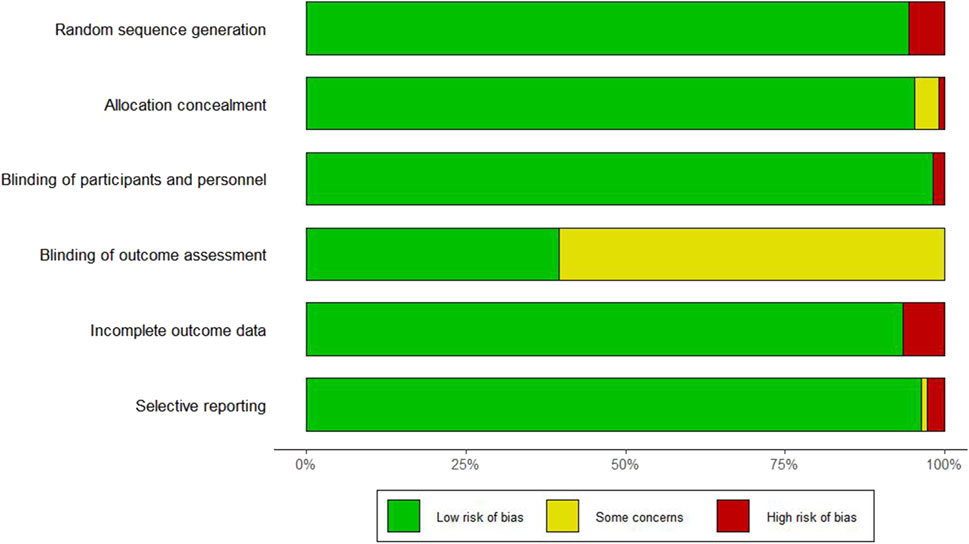
The risk of bias assessment for the seven domains across the 106 studies is provided in Supplementary Table S2 and Figure 2. Six studies did not report the use of randomization methods, one study did not specify allocation concealment, and two studies did not apply blinding for outcome assessment.
3.3 Outcomes
3.3.1 Overall surgical sites
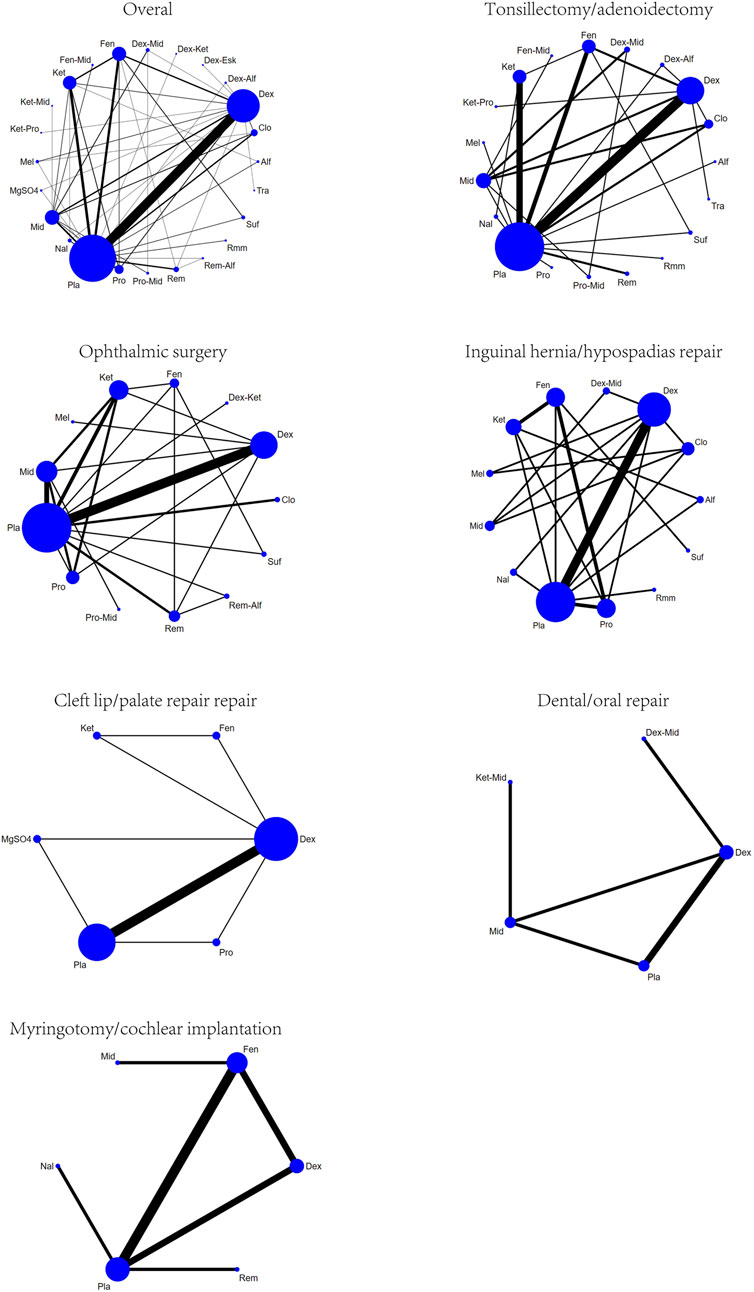
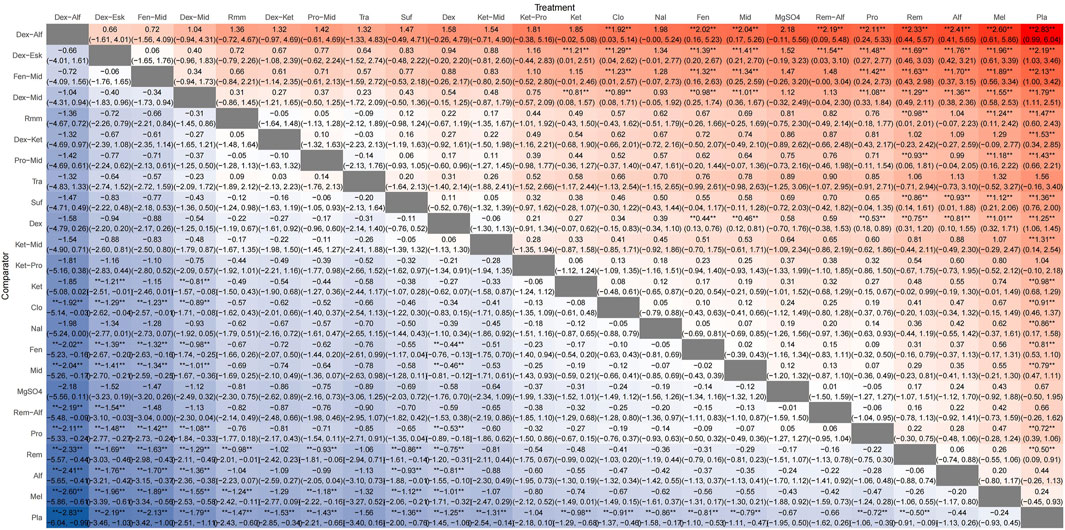
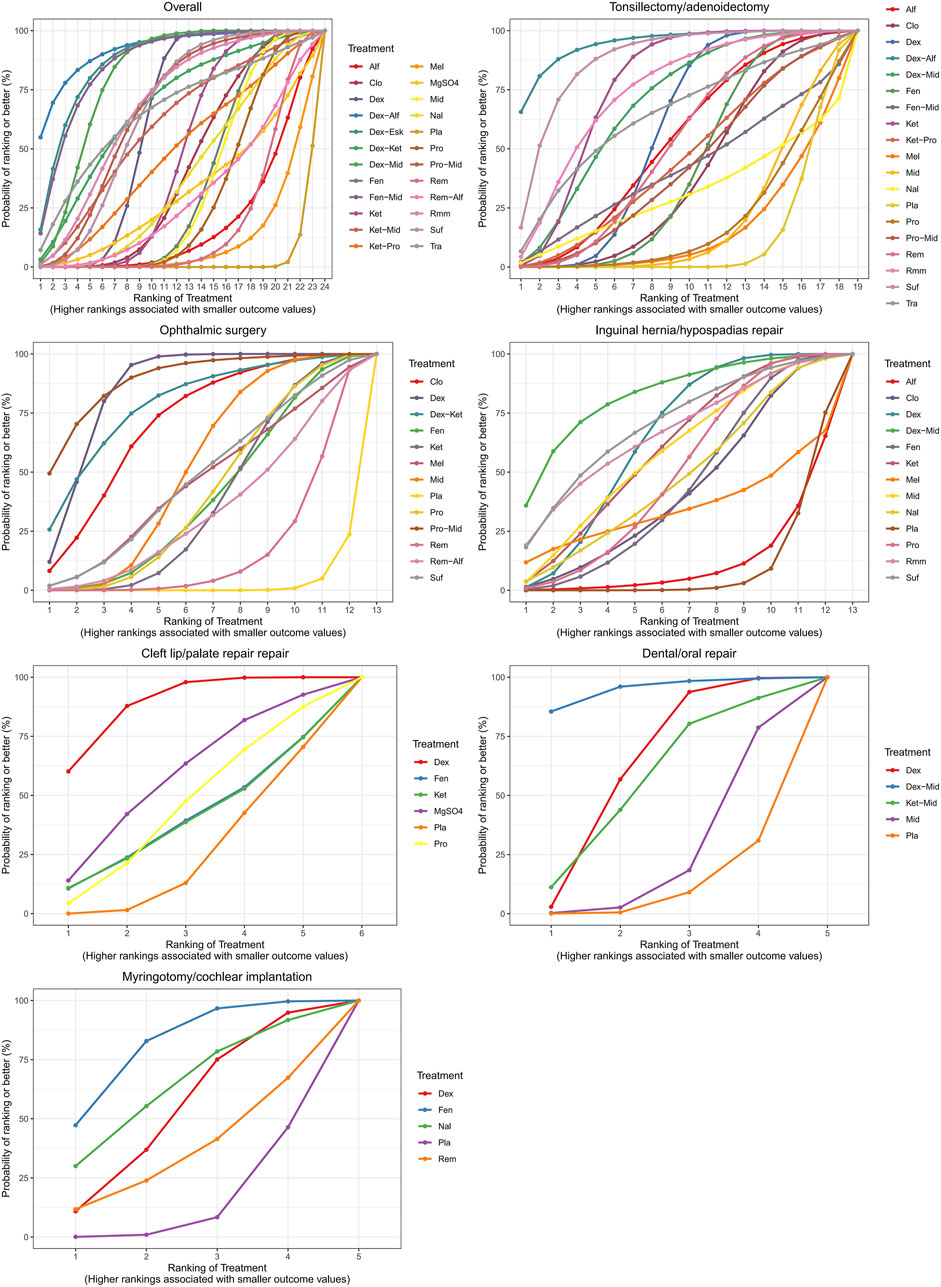
A total of 100 studies were included, covering 24 different drugs or drug combinations. The network evidence diagram is presented in Figure 3. Compared to placebo, most drug interventions were significantly associated with a reduced incidence of ED (Figure 4). The top three most effective single drugs or combinations were dexmedetomidine + alfentanil (logRR = −2.83, 95% CrI: −6.04, −0.99; SUCRA: 91.7%), dexmedetomidine + esketamine (logRR = −2.19, 95% CrI: −3.46, −1.03; SUCRA: 85.9%), and fentanyl + midazolam (logRR = −2.13, 95% CrI: −3.42, −1.00; SUCRA: 82.8%) (Figures 5, 6).
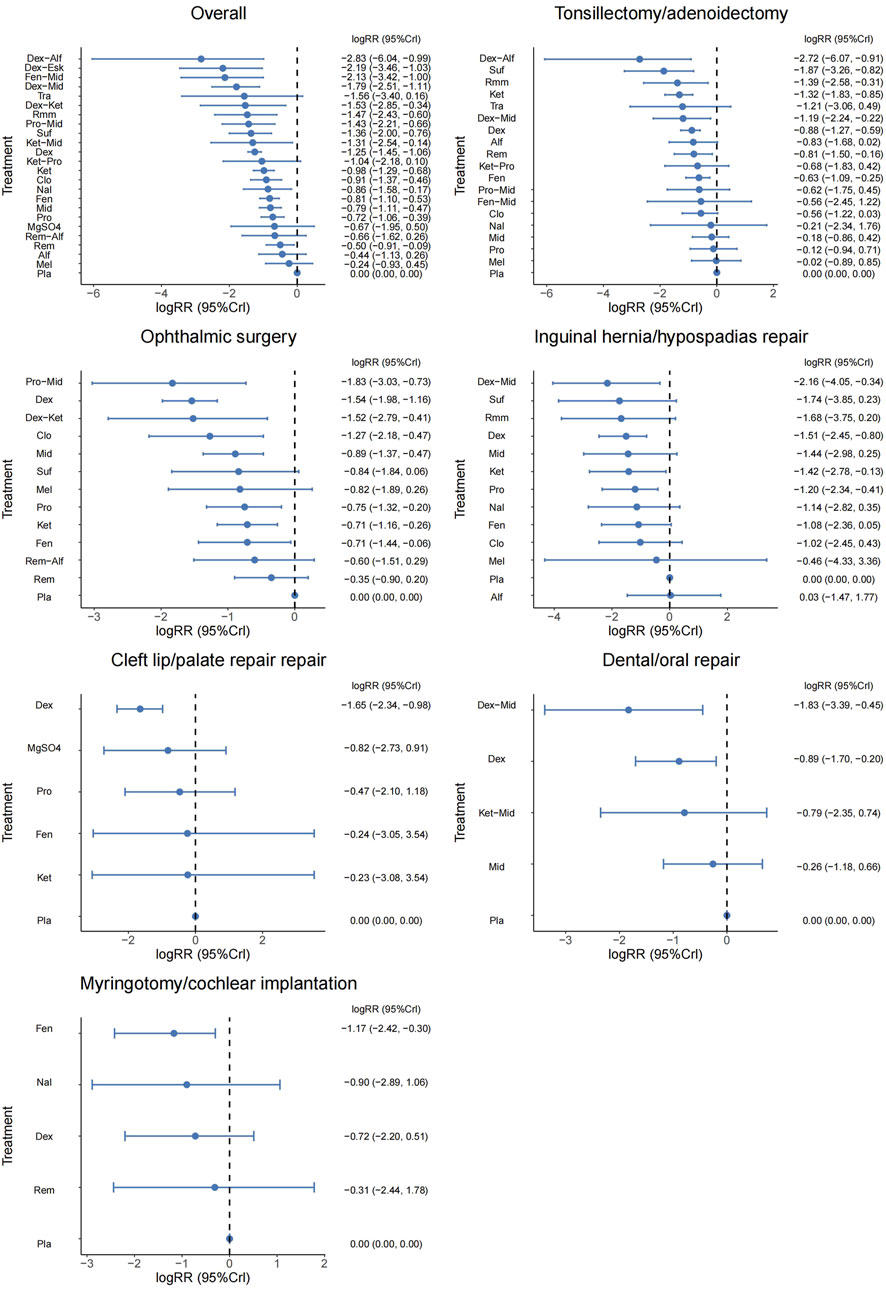
Figure 4. Forest plot presenting the results for all interventions directly compared with the placebo group.
3.3.2 Tonsillectomy/adenoidectomy
A total of 35 studies involving 19 different drugs or drug combinations and 3,238 children were included. The network evidence diagram is shown in Figure 3. Compared to placebo/control, the top three most effective drugs or combinations were dexmedetomidine + alfentanil (logRR = −2.72, 95% CrI: −6.07, −0.91; SUCRA: 94.63%), sufentanil (logRR = −1.87, 95% CrI: −3.26, −0.82; SUCRA: 87.84%), and remimazolam (logRR = −1.39, 95% CrI: −2.58, −0.31; SUCRA: 76.62%) (Figures 4, 6 and Supplementary Figure S2).
3.3.3 Ophthalmic surgery
A total of 26 studies involving 13 different drugs or drug combinations and 2,019 children were included. The network evidence diagram is shown in Figure 3. Compared to placebo/control, the top three most effective drugs or combinations were propofol + midazolam (logRR = −1.83, 95% CrI: −3.03, −0.73; SUCRA: 89.6%), dexmedetomidine (logRR = −1.54, 95% CrI: −1.98, −1.16; SUCRA: 86.0%), and dexmedetomidine + ketamine (logRR = −1.52, 95% CrI: −2.79, −0.41; SUCRA: 79.5%) (Figures 4, 6 and Supplementary Figure S3).
3.3.4 Inguinal hernia/hypospadias repair
A total of 17 studies involving 13 different drugs or drug combinations and 1,576 children were included. The network evidence diagram is shown in Figure 3. The most effective treatments compared to control were dexmedetomidine + midazolam (logRR = −2.16, 95% CrI: −4.05, −0.34; SUCRA: 81.7%), dexmedetomidine (logRR = −1.51, 95% CrI: −2.45, −0.80; SUCRA: 62.4%) and ketamine (logRR = −1.42, 95% CrI: −2.78, −0.13; SUCRA: 57.4%) (Figures 4, 6 and Supplementary Figure S4).
3.3.5 Cleft lip/palate repair
A total of 10 studies involving 6 different drugs or drug combinations and 541 children were included. The network evidence diagram is shown in Figure 3. Compared to the control group, only dexmedetomidine (logRR = −1.65, 95% CrI: −2.34, −0.98; SUCRA: 89.2%) showed a statistically significant reduction in the incidence of ED (Figures 4, 6 and Supplementary Figure S5).
3.3.6 Dental/oral repair
A total of 6 studies involving 5 different drugs or drug combinations and 450 children were included. The network evidence diagram is shown in Figure 3. Dexmedetomidine + midazolam (logRR = −1.83, 95% CrI: −3.39, −0.45; SUCRA: 94.9%) and dexmedetomidine (logRR = −0.89, 95% CrI: −1.70, −0.20; SUCRA: 63.3%) were associated with a significant reduction in the incidence of ED (Figures 4, 6 and Supplementary Figure S6).
3.3.7 Myringotomy/cochlear implantation
A total of 6 studies involving 6 different drugs or drug combinations and 576 children were included. The network evidence diagram is shown in Figure 3. Fentanyl (logRR = −1.17, 95% CrI: −2.42, −0.30; SUCRA: 80.02%) showed significantly better effects compared to placebo (Figures 4, 6 and Supplementary Figure S7).
3.4 Network meta-regression and publication bias
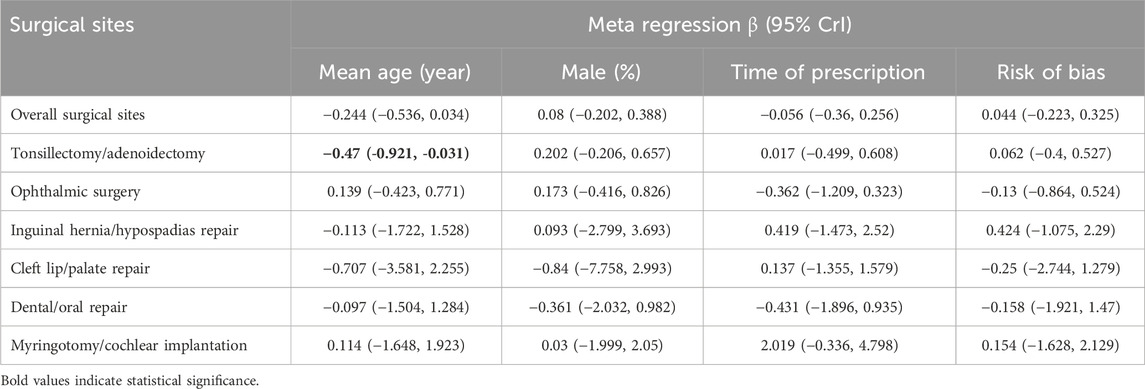
Results of network meta-regression found that, among the various surgical types, the Tonsillectomy/adenoidectomy group showed mean age potentially influences the association between anesthetic adjuvants and sevoflurane-related ED (β = −0.470, 95% CrI: −0.921, −0.031), with a 95% credible interval that does not include zero (Table 1). However, in all other surgical subgroups, none of the analyzed variables demonstrated statistical significance, further supporting the robustness of our main findings, which remain unaffected by these confounding factors.
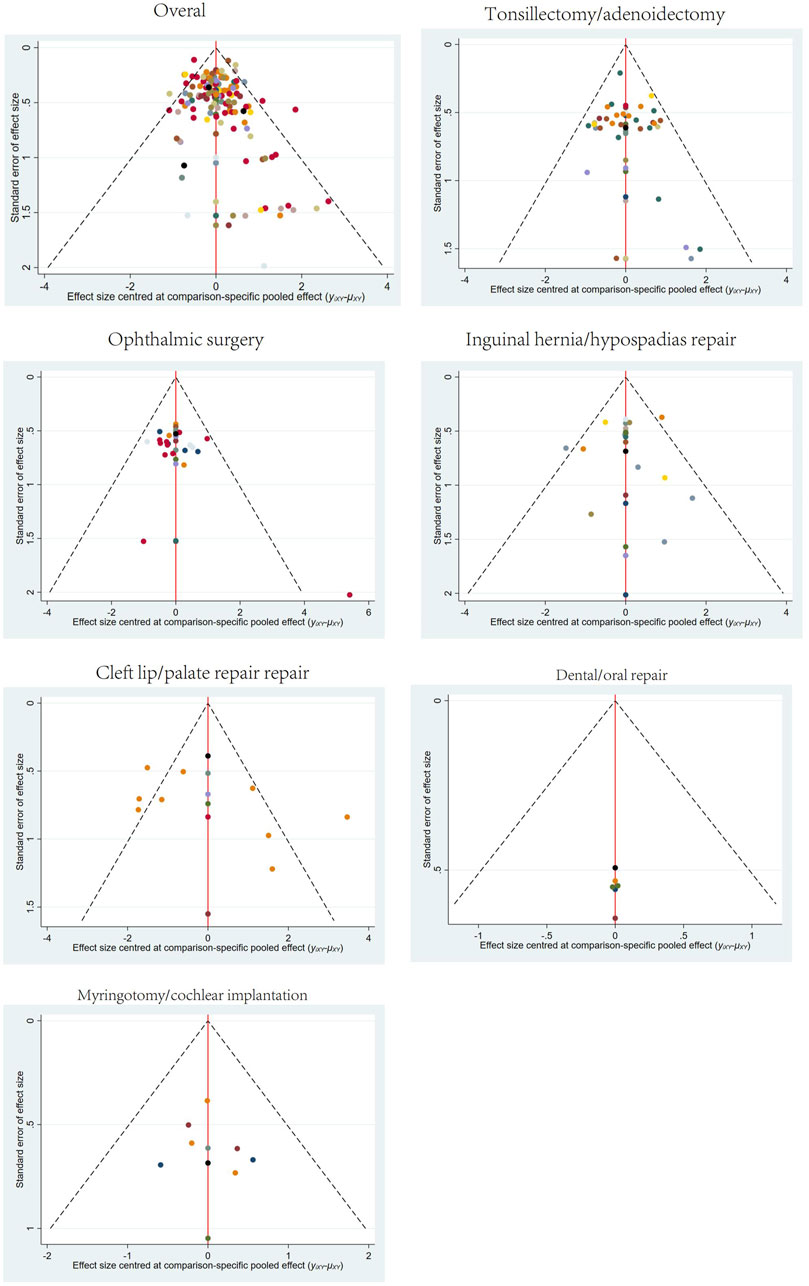
Publication bias tests were conducted for different surgical sites using comparison-adjusted funnel plots. The results showed some asymmetry in the scatter points for overall surgical sites, tonsillectomy/adenoidectomy, and cleft lip/palate repair, indicating the potential presence of publication bias and small sample size effects. The funnel plots for all outcomes are presented in Figure 7.
4 Discussions
Sevoflurane is widely used in pediatric anesthesia due to its low blood-gas solubility, offering a smooth and predictable induction and maintenance process. However, many pediatric patients experience ED during the early recovery from sevoflurane anesthesia, a troublesome complication. Several studies have attempted to explain the mechanisms by which sevoflurane induces ED in pediatric patients. Sevoflurane mediates inhibitory postsynaptic currents by binding to gamma-aminobutyric acid receptors and exerts a biphasic effect on the central nervous system. At high concentrations, sevoflurane enhances inhibitory postsynaptic currents, producing anesthesia, but at lower concentrations, it reverses inhibitory currents, leading to agitation or delirium (Zhao et al., 2020). Additionally, a previous study demonstrated that sevoflurane directly stimulates locus coeruleus neurons, increasing the release of norepinephrine (Yasui et al., 2007). Locus coeruleus neurons are primarily involved in controlling alertness and wakefulness. Moreover, higher brain concentrations of glucose and lactate associated with sevoflurane use have been positively correlated with the incidence of ED (Jacob et al., 2012).
The incidence of ED varies between 30% and 80%, with a maximum rate reported as high as 90.5% (Wang et al., 2023). This significant variation across studies may largely be attributed to differences in the use of hypnotic agents and the surgical sites involved (Lee and Sung, 2020; Kanaya, 2016). Previous studies have shown that head and neck surgeries, including ophthalmic and ENT surgeries, are associated with a higher incidence of pediatric ED (Dahmani et al., 2010; Chen et al., 2024). Skilled anesthesiologists strive to provide a safe and comfortable anesthetic experience for patients; therefore, developing targeted anesthetic strategies tailored to specific surgical characteristics is essential. However, relevant research in this area remains limited.
Since each anesthetic adjuvant has unique benefits and potential adverse side effects (Wang et al., 2016), choosing the best single treatment can be challenging. In a previous NMA, the combination of dexmedetomidine and midazolam had the highest cumulative ranking probability and seemed to perform better than dexmedetomidine alone. Our findings are similar. We found that dexmedetomidine consistently performed well overall and across various specific surgery types, especially when combined with other drugs such as alfentanil, midazolam, and esketamine, effectively reducing the incidence of ED. Compared to previous meta-analyses that focused solely on single-drug treatments (Wang et al., 2017; Tan et al., 2019; Jiao et al., 2020), our results suggest that the efficacy of dexmedetomidine alone might have been overestimated in these studies.
Dexmedetomidine is a highly selective α-2 agonist that acts on the brain, peripheral nervous system, and spinal cord (Dahmani et al., 2010) As a highly selective α-2 agonist (Dahmani et al., 2010), dexmedetomidine possesses anxiolytic, sedative, and analgesic properties, making it the first-choice adjuvant for preventing delirium during sevoflurane anesthesia (Wang et al., 2017). The European Society of Anesthesiology guidelines recommend the use of α-2 receptor agonists, such as dexmedetomidine or clonidine, to prevent ED (Aldecoa et al., 2017). Our study shows that when dexmedetomidine is combined with alfentanil, there is a trend toward enhanced preventive effects, particularly in tonsillectomy/adenoidectomy procedures. The mechanism of alfentanil in preventing ED after sevoflurane anesthesia may be related to its analgesic and mildly sedative effects (Choi et al., 2016). One study included in this review showed that intravenous alfentanil (10 μg/kg and 20 μg/kg) administered during the induction phase of anesthesia in children undergoing adenotonsillectomy could reduce the incidence of ED (Kim et al., 2009). Another study, also in children undergoing adenotonsillectomy, used the same doses of alfentanil with an additional dose of dexmedetomidine 10 min after induction, which significantly reduced ED compared to dexmedetomidine alone (Zhang et al., 2022). These studies suggest that alfentanil may have a preventive role in ED. However, as alfentanil has a rapid onset but a short duration of action, its effect may have already faded by the time of emergence from anesthesia if administered solely during induction. A possible explanation for the observed effects in these studies could be the higher doses of alfentanil used, combined with relatively short surgery durations. In our study, however, the use of alfentanil alone did not show statistically significant results (logRR = −0.83, 95% CrI: −1.68, 0.02). Therefore, the combination effect of dexmedetomidine and alfentanil should be interpreted with caution, as the observed benefits are likely more attributable to the effects of dexmedetomidine.
This study also found that among the limited comparisons of individual or combined drugs, the combination of propofol and midazolam showed significant advantages in ophthalmic surgeries. For inguinal hernia/hypospadias repair and dental/oral repair, the available evidence suggested that the combination of dexmedetomidine and midazolam was the most effective. Fentanyl and sufentanil demonstrated high efficacy and were widely used in specific surgeries, such as ENT procedures. Drug selection should thus vary depending on the surgery type, patient condition, and needs for postoperative pain control and recovery.
Given the potential impact of different demographic characteristics, medication strategies and timing, and study quality on the results (Wang et al., 2021), we performed a network meta-regression for sensitivity analysis. The results showed that mean age (years), male percentage (%), time of prescription, and risk of bias had no significant effect on the association between anesthetic adjuvants and sevoflurane-related ED. This further supports the robustness of our main findings, which remain unaffected by these confounding factors.
Potential safety concerns or adverse effects of specific drug combinations, particularly for sedative combinations, should be addressed. In previous meta-analyses, most individual and combination treatments, including dexmedetomidine, showed no significant differences compared to the placebo group in terms of extubation time, emergence time, or duration of post anesthesia care unit stay (Wang et al., 2021; Wang et al., 2016). Additionally, in the studies included in this review regarding the combination of dexmedetomidine and alfentanil, no significant increase in respiratory depression or other adverse events was observed (Bilgen et al., 2014; Kim et al., 2009; Zhang et al., 2022).
This study has several limitations. First, literature and sample sizes for certain surgical sites, such as cleft lip/palate repair, dental/oral repair, and myringotomy/cochlear implantation, are relatively scarce, which affects the quality of the evidence. Second, although our study found that the combination of anesthetic adjuvants might be more effective in reducing ED compared to single-drug use, research on combination therapies is still relatively limited and requires further supporting evidence. Finally, clinical heterogeneity is introduced by differences in doses, administration methods, and patient age across the studies included in the literature.
5 Conclusion
This study comprehensively and systematically reviewed various anesthetic adjuvants and combinations to prevent sevoflurane-related ED across different surgical sites. The results indicated that overall, as well as for tonsillectomy/adenoidectomy, the combination of dexmedetomidine and alfentanil was the best option; for ophthalmic surgery, the combination of propofol and midazolam was optimal; and dexmedetomidine and midazolam showed the best effectiveness in inguinal hernia/hypospadias repair and dental/oral repair. Dexmedetomidine and fentanyl performed well in cleft lip/palate repair and myringotomy/cochlear implantation, respectively. These findings highlight the importance of selecting targeted anesthetic adjuvants based on the specific surgical site.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
C-JZ: Conceptualization, Data curation, Methodology, Resources, Writing – original draft. HC: Formal Analysis, Supervision, Writing – review and editing. KZ: Data curation, Writing – review and editing. XQ: Data curation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (2024D28).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1573640/full#supplementary-material
References
Abbas, M. S., El-Hakeem, E. E. A., and Kamel, H. E. (2019). Three minutes propofol after sevoflurane anesthesia to prevent emergence agitation following inguinal hernia repair in children: a randomized controlled trial. Korean J. Anesthesiol. 72 (3), 253–259. doi:10.4097/kja.d.18.00345
Abdelaziz, H. M. M., Bakr, R. H., and Kasem, A. A. (2016). Effect of intranasal dexmedetomidine or intranasal midazolam on prevention of emergence agitation in pediatric strabismus surgery: a randomized controlled study. Egypt. J. Anaesth. 32 (2), 285–291. doi:10.1016/j.egja.2015.11.009
Abdelhalim, A. A., and Alarfaj, A. M. (2013). The effect of ketamine versus fentanyl on the incidence of emergence agitation after sevoflurane anesthesia in pediatric patients undergoing tonsillectomy with or without adenoidectomy. Saudi J. Anaesth. 7 (4), 392–398. doi:10.4103/1658-354X.121047
Abdelmawgoud, A., and Mohy, A. (2012). Effect of oral dextromethorphan versus oral ketamine on sevoflurane related emergence agitation in children undergoing adenotonsillectomy. Egypt. J. Anaesth. 28 (4), 243–248. doi:10.1016/j.egja.2012.05.005
Abu-Shahwan, I. (2008). Effect of propofol on emergence behavior in children after sevoflurane general anesthesia. Paediatr. Anaesth. 18 (1), 55–59. doi:10.1111/j.1460-9592.2007.02376.x
Abu-Shahwan, I., and Chowdary, K. (2007). Ketamine is effective in decreasing the incidence of emergence agitation in children undergoing dental repair under sevoflurane general anesthesia. Paediatr. Anaesth. 17 (9), 846–850. doi:10.1111/j.1460-9592.2007.02298.x
Akin, A., Bayram, A., Esmaoglu, A., Tosun, Z., Aksu, R., Altuntas, R., et al. (2012). Dexmedetomidine vs midazolam for premedication of pediatric patients undergoing anesthesia. Paediatr. Anaesth. 22 (9), 871–876. doi:10.1111/j.1460-9592.2012.03802.x
Alansary, A. M., Ali, M. M., and Elshafie, M. A. (2023). A randomized controlled trial of dexmedetomidine vs magnesium sulfate as adjuvants to bupivacaine in infraorbital nerve block for perioperative analgesia in pediatric patients undergoing cleft lip surgery. Anaesth. Pain Intensive Care 27 (4), 514–522. doi:10.35975/apic.v27i4.2261
Aldecoa, C., Bettelli, G., Bilotta, F., Sanders, R. D., Audisio, R., Borozdina, A., et al. (2017). European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur. J. Anaesthesiol. 34 (4), 192–214. doi:10.1097/EJA.0000000000000594
Ali, M. A., and Abdellatif, A. A. (2013). Prevention of sevoflurane related emergence agitation in children undergoing adenotonsillectomy: a comparison of dexmedetomidine and propofol. Saudi J. Anaesth. 7 (3), 296–300. doi:10.4103/1658-354X.115363
Ali, S. T., Asthana, V., Gupta, D., and Singh, S. K. (2020). A comparative evaluation of oral clonidine, Dexmedetomidine, and Melatonin as premedicants in Pediatric patients undergoing Subumbilical surgeries. Romanian J. Anaesth. Intensive Care 27 (1), 35–42. doi:10.2478/rjaic-2020-0006
Almenrader, N., Passariello, M., Coccetti, B., Haiberger, R., and Pietropaoli, P. (2007). Premedication in children: a comparison of oral midazolam and oral clonidine. Paediatr. Anaesth. 17 (12), 1143–1149. doi:10.1111/j.1460-9592.2007.02332.x
Amer, G. F., and Abdallah, M. Y. (2022). Dexmedetomidine versus propofol for prevention of emergence delirium in pediatric cataract surgery: double blinded randomized study. Egypt. J. Anaesth. 38 (1), 300–304. doi:10.1080/11101849.2022.2077049
Aouad, M. T., Kanazi, G. E., Siddik-Sayyid, S. M., Gerges, F. J., Rizk, L. B., and Baraka, A. S. (2005). Preoperative caudal block prevents emergence agitation in children following sevoflurane anesthesia. Acta Anaesthesiol. Scand. 49 (3), 300–304. doi:10.1111/j.1399-6576.2005.00642.x
Aouad, M. T., Yazbeck-Karam, V. G., Nasr, V. G., El-Khatib, M. F., Kanazi, G. E., and Bleik, J. H. (2007). A single dose of propofol at the end of surgery for the prevention of emergence agitation in children undergoing strabismus surgery during sevoflurane anesthesia. Anesthesiology 107 (5), 733–738. doi:10.1097/01.anes.0000287009.46896.a7
Asaad, O. M., Hafez, M., Mohamed, M. Y., and El-mahgoup, S. S. (2011). Comparative study between prophylactic single dose of fentanyl and dexmedetomidine in the management of agitation after sevoflurane anesthesia in children. Egypt. J. Anaesth. 27 (1), 31–37. doi:10.1016/j.egja.2010.12.005
Bae, J. H., Koo, B. W., Kim, S. J., Lee, D. H., Lee, E. T., and Kang, C. J. (2010). The effects of midazolam administered postoperatively on emergence agitation in pediatric strabismus surgery. Korean J. Anesthesiol. 58 (1), 45–49. doi:10.4097/kjae.2010.58.1.45
Baek, J., Park, S. J., Kim, J. O., Kim, M., Kim, D. Y., and Choi, E. K. (2022). The effects of remifentanil and fentanyl on emergence agitation in pediatric strabismus surgery. Children 9 (5), 606. doi:10.3390/children9050606
Bakhamees, H. S., Mercan, A., and El-Halafawy, Y. M. (2009). Combination effect of low dose fentanyl and propofol on emergence agitation in children following sevoflurane anesthesia. Saudi Med. J. 30 (4), 500–503.
Bedirli, N., Akcabay, M., and Emik, U. (2017). Tramadol vs dexmedetomidine for emergence agitation control in pediatric patients undergoing adenotonsillectomy with sevoflurane anesthesia: prospective randomized controlled clinical study. BMC Anesthesiol. 17, 41. doi:10.1186/s12871-017-0332-4
Béliveau, A., Boyne, D. J., Slater, J., Brenner, D., and Arora, P. (2019). BUGSnet: an R package to facilitate the conduct and reporting of Bayesian network Meta-analyses. BMC Med. Res. Methodol. 19 (1), 196. doi:10.1186/s12874-019-0829-2
Bergendahl, H. T., Lönnqvist, P. A., Eksborg, S., Ruthström, E., Nordenberg, L., Zetterqvist, H., et al. (2004). Clonidine vs. midazolam as premedication in children undergoing adeno-tonsillectomy: a prospective, randomized, controlled clinical trial. Acta Anaesthesiol. Scand. 48 (10), 1292–1300. doi:10.1111/j.1399-6576.2004.00525.x
Bilgen, S., Köner, Ö., Karacay, S., Sancar, N. K., Kaspar, E. C., and Sözübir, S. (2014). Effect of ketamine versus alfentanil following midazolam in preventing emergence agitation in children after sevoflurane anaesthesia: a prospective randomized clinical trial. J. Int. Med. Res. 42 (6), 1262–1271. doi:10.1177/0300060514543039
Bong, C. L., Lim, E., Allen, J. C., Choo, W. L. H., Siow, Y. N., Teo, P. B. Y., et al. (2015). A comparison of single-dose dexmedetomidine or propofol on the incidence of emergence delirium in children undergoing general anaesthesia for magnetic resonance imaging. Anaesthesia 70, 393–399. doi:10.1111/anae.12867
Bromfalk, Å., Hultin, M., Myrberg, T., Engström, Å., and Walldén, J. (2023). Postoperative recovery in preschool-aged children: a secondary analysis of a randomized controlled trial comparing premedication with midazolam, clonidine, and dexmedetomidine. Paediatr. Anaesth. 33 (11), 962–972. doi:10.1111/pan.14740
Cai, Y. H., Wang, C. Y., Li, Y., Chen, J., Li, J., Wu, J., et al. (2021). Comparison of the effects of oral midazolam and intranasal dexmedetomidine on preoperative sedation and anesthesia induction in children undergoing surgeries. Front. Pharmacol. 12, 648699. doi:10.3389/fphar.2021.648699
Cai, Y. H., Zhong, J. W., Ma, H. Y., Szmuk, P., Wang, C. Y., Wang, Z., et al. (2024). Effect of remimazolam on emergence delirium in children undergoing laparoscopic surgery: a double-blinded randomized trial. Anesthesiology 141 (3), 500–510. doi:10.1097/ALN.0000000000005077
Chen, F., Wang, C., Lu, Y., Huang, M., and Fu, Z. (2018). Efficacy of different doses of dexmedetomidine as a rapid bolus for children: a double-blind, prospective, randomized study. BMC Anesthesiol. 18, 103. doi:10.1186/s12871-018-0562-0
Chen, J., Li, W., Hu, X., and Wang, D. (2010). Emergence agitation after cataract surgery in children: a comparison of midazolam, propofol and ketamine. Paediatr. Anaesth. 20, 873–879. doi:10.1111/j.1460-9592.2010.03375.x
Chen, J. Y., Jia, J. E., Liu, T. J., Qin, M. J., and Li, W. X. (2013). Comparison of the effects of dexmedetomidine, ketamine, and placebo on emergence agitation after strabismus surgery in children. Can. J. Anesth. 60 (4), 385–392. doi:10.1007/s12630-013-9886-x
Chen, Y., Ru, F., Ye, Q., Wu, X., Hu, X., Zhang, Y., et al. (2023). Effect of S-ketamine administered at the end of anesthesia on emergence delirium in preschool children undergoing tonsillectomy and/or adenoidectomy. Front. Pharmacol. 14. doi:10.3389/fphar.2023.1044558
Chen, Y. C., Foster, J., Wang, M. L., Rohmah, I., Tseng, Y. H., and Chiu, H. Y. (2024). Global prevalence and risk factors of emergence delirium in pediatric patients undergoing general anesthesia: a systemic review and meta-analysis. J. Pediatr. Nurs. 77, 74–80. doi:10.1016/j.pedn.2024.03.010
Cho, E. J., Yoon, S. Z., Cho, J. E., and Lee, H. W. (2014). Comparison of the effects of 0.03 and 0.05 mg/kg midazolam with placebo on prevention of emergence agitation in children having strabismus surgery. Anesthesiology 120 (6), 1354–1361. doi:10.1097/ALN.0000000000000181
Choi, Y. H., Kim, K. M., Lee, S. K., Kim, Y. S., Kim, S. J., Hwang, W. S., et al. (2016). Effects of remifentanil and remifentanil-alfentanil administration on emergence agitation after brief ophthalmic surgery in children. BMC Anesthesiol. 16 (1), 50. doi:10.1186/s12871-016-0213-2
Costi, D., Ellwood, J., Wallace, A., Ahmed, S., Waring, L., and Cyna, A. (2015). Transition to propofol after sevoflurane anesthesia to prevent emergence agitation: a randomized controlled trial. Paediatr. Anaesth. 25 (5), 517–523. doi:10.1111/pan.12617
Cravero, J. P., Beach, M., Thyr, B., and Whalen, K. (2003). The effect of small dose fentanyl on the emergence characteristics of pediatric patients after sevoflurane anesthesia without surgery. Anesth. Analgesia 97 (2), 364–367. doi:10.1213/01.ANE.0000070227.78670.43
Dahmani, S., Stany, I., Brasher, C., Lejeune, C., Bruneau, B., Wood, C., et al. (2010). Pharmacological prevention of sevoflurane- and desflurane-related emergence agitation in children: a meta-analysis of published studies. Br. J. Anaesth. 104 (2), 216–223. doi:10.1093/bja/aep376
Dalens, B. J., Pinard, A. M., Létourneau, D. R., Albert, N. T., and Truchon, R. J. Y. (2006). Prevention of emergence agitation after sevoflurane anesthesia for pediatric cerebral magnetic resonance imaging by small doses of ketamine or nalbuphine administered just before discontinuing anesthesia. Anesth. Analgesia 102 (4), 1056–1061. doi:10.1213/01.ane.0000200282.38041.1f
Demirbilek, S., Togal, T., Cicek, M., Aslan, U., Sizanli, E., and Ersoy, M. O. (2004). Effects of fentanyl on the incidence of emergence agitation in children receiving desflurane or sevoflurane anaesthesia. Eur. J. Anaesthesiol. 21 (7), 538–542. doi:10.1017/s0265021504007069
Di, M., Han, Y., Yang, Z., Liu, H., Ye, X., Lai, H., et al. (2017). Tracheal extubation in deeply anesthetized pediatric patients after tonsillectomy: a comparison of high-concentration sevoflurane alone and low-concentration sevoflurane in combination with dexmedetomidine pre-medication. BMC Anesthesiol. 17, 28. doi:10.1186/s12871-017-0317-3
Di, M., Huang, C., Chen, F., Zeng, R., Yu, C., Shangguan, W., et al. (2014). Effect of single-dose dexmedetomidine on recovery profiles after sevoflurane anesthesia with spontaneous respiration in pediatric patients undergoing cleft lip and palate repair. Zhonghua Yi Xue Za Zhi 94, 1466–1469. doi:10.3760/cma.j.issn.0376-2491.2014.19.009
Dong, Y. X., Meng, L. X., Wang, Y., Zhang, J. J., Zhao, G. Y., and Ma, C. H. (2010). The effect of remifentanil on the incidence of agitation on emergence from sevoflurane anaesthesia in children undergoing adenotonsillectomy. Anaesth. Intensive Care 38 (4), 718–722. doi:10.1177/0310057X1003800416
Eckenhoff, J. E., Kneale, D. H., and Dripps, R. D. (1961). The incidence and etiology of postanesthetic excitment. A clinical survey. Anesthesiology 22, 667–673. doi:10.1097/00000542-196109000-00002
Erdil, F., Demirbilek, S., Begec, Z., Ozturk, E., Ulger, M. H., and Ersoy, M. O. (2009). The effects of dexmedetomidine and fentanyl on emergence characteristics after adenoidectomy in children. Anaesth. Intensive Care 37 (4), 571–576. doi:10.1177/0310057X0903700405
Fang, X. Z., Gao, J., Ge, Y. L., Zhou, L. J., and Zhang, Y. (2016). Network meta-analysis on the efficacy of dexmedetomidine, midazolam, ketamine, propofol, and fentanyl for the prevention of sevoflurane-related emergence agitation in children. Am. J. Ther. 23 (4), e1032–e1042. doi:10.1097/MJT.0000000000000321
Finkel, J. C., Cohen, I. T., Hannallah, R. S., Patel, K. M., Kim, M. S., Hummer, K. A., et al. (2001). The effect of intranasal fentanyl on the emergence characteristics after sevoflurane anesthesia in children undergoing surgery for bilateral myringotomy tube placement. Anesth. Analgesia 92 (5), 1164–1168. doi:10.1097/00000539-200105000-00016
Galinkin, J. L., Fazi, L. M., Cuy, R. M., Chiavacci, R. M., Kurth, C. D., Shah, U. K., et al. (2000). Use of intranasal fentanyl in children undergoing myringotomy and tube placement during halothane and sevoflurane anesthesia. Anesthesiology 93 (6), 1378–1383. doi:10.1097/00000542-200012000-00006
Ghai, B., Ram, J., Chauhan, S., and Wig, J. (2010). Effects of clonidine on recovery after sevoflurane anaesthesia in children undergoing cataract surgery. Anaesth. Intensive Care 38 (3), 530–537. doi:10.1177/0310057X1003800319
Ghosh, S. M., Agarwala, R. B., Pandey, M., and Vajifdar, H. (2011). Efficacy of low-dose caudal clonidine in reduction of sevoflurane-induced agitation in children undergoing urogenital and lower limb surgery: a prospective randomised double-blind study. Eur. J. Anaesthesiol. 28 (5), 329–333. doi:10.1097/EJA.0b013e3283416754
Golmohammadi, M., Sane, S., Ghavipanjeh Rezaei, S., Hosseini, R., Alwaily, E. R., Hussien, B. M., et al. (2024). Investigating the effect of dexmedetomidine in controlling postoperative emergence agitation in children under sevoflurane anesthesia. Anesthesiol. Res. Pract. 2024, 2024. doi:10.1155/2024/6418429
Guler, G., Akin, A., Tosun, Z., Ors, S., Esmaoglu, A., and Boyaci, A. (2005). Single-dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomy. Paediatr. Anaesth. 15 (9), 762–766. doi:10.1111/j.1460-9592.2004.01541.x
Hadi, S. M., Saleh, A. J., Tang, Y. Z., Daoud, A., Mei, X., and Ouyang, W. (2015). The effect of KETODEX on the incidence and severity of emergence agitation in children undergoing adenotonsillectomy using sevoflurane based-anesthesia. Int. J. Pediatr. Otorhinolaryngology 79 (5), 671–676. doi:10.1016/j.ijporl.2015.02.012
Hauber, J. A., Davis, P. J., Bendel, L. P., Martyn, S. V., McCarthy, D. L., Evans, M. C., et al. (2015). Dexmedetomidine as a rapid bolus for treatment and prophylactic prevention of emergence agitation in anesthetized children. Anesth. Analgesia 121, 1308–1315. doi:10.1213/ANE.0000000000000931
He, H., Cui, Q., Chen, H., Huang, X., Wang, S., Yu, T., et al. (2023). The effect of intranasal dexmedetomidine on emergence delirium prevention in pediatric ambulatory dental rehabilitation under general anesthesia: a randomized clinical trial. Drug Des. Devel Ther. 17, 3563–3570. doi:10.2147/DDDT.S427291
Heinmiller, L. J., Nelson, L. B., Goldberg, M. B., and Thode, A. R. (2013). Clonidine premedication versus placebo: effects on postoperative agitation and recovery time in children undergoing strabismus surgery. J. Pediatr. Ophthalmol. Strabismus 50 (3), 150–154. doi:10.3928/01913913-20130205-02
Huang, L., Wang, L., Peng, W., and Qin, C. (2022). A comparison of dexmedetomidine and propofol on emergence delirium in children undergoing cleft palate surgery with sevoflurane-based anesthesia. J. Craniofac Surg. 33 (2), 650–653. doi:10.1097/SCS.0000000000008343
Ibacache, M. E., Muñoz, H. R., Brandes, V., and Morales, A. L. (2004). Single-dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children. Anesth. Analgesia 98 (1), 60–63. doi:10.1213/01.ANE.0000094947.20838.8E
Ibrahim, D. M. H. A., Mostafa, Z. A., Ismail, Y. A. A., and Ashoor, T. M. A. (2023). The effect of low dose nalbuphine or ketamine in the prevention of emergence agitation after sevoflurane anesthesia in children undergoing tonsillectomy with or without adenoidectomy. Egypt. J. Anaesth. 39 (1), 894–899. doi:10.1080/11101849.2023.2287794
Isik, B., Arslan, M., Tunga, A. D., and Kurtipek, O. (2006). Dexmedetomidine decreases emergence agitation in pediatric patients after sevoflurane anesthesia without surgery. Paediatr. Anaesth. 16 (7), 748–753. doi:10.1111/j.1460-9592.2006.01845.x
Jacob, Z., Li, H., Makaryus, R., Zhang, S., Reinsel, R., Lee, H., et al. (2012). Metabolomic profiling of children's brains undergoing general anesthesia with sevoflurane and propofol. Anesthesiology 117 (5), 1062–1071. doi:10.1097/ALN.0b013e31826be417
Jangra, S., Ashok, V., Sethi, S., and Ram, J. (2022). Atomised intranasal dexmedetomidine versus oral melatonin in prevention of emergence delirium in children undergoing ophthalmic surgery with sevoflurane: a randomised double-blind study. Eur. J. Anaesthesiol. 39 (11), 868–874. doi:10.1097/EJA.0000000000001727
Jayaraj, P. S., Raj, R. L., Rosebell, J., and Joenit, G. (2023). Effect of dexmedetomidine versus ketofol on the incidence of emergence agitation associated with sevoflurane based anaesthesia in children undergoing adenotonsillectomy. Int. J. Acad. Med. Pharm. 5 (1), 711–718. doi:10.47009/jamp.2023.5.1.148
Jeong, W. J., Kim, W. Y., Moon, M. G., Min, D. J., Lee, Y. S., Kim, J. H., et al. (2012). The effect of ketamine on the separation anxiety and emergence agitation in children undergoing brief ophthalmic surgery under desflurane general anesthesia. Korean J. Anesthesiol. 63 (3), 203–208. doi:10.4097/kjae.2012.63.3.203
Jiao, H., Wang, H., Jiang, Z., and Hu, J. (2020). Comparative efficacy of ancillary drugs in sevoflurane-related emergence agitation after paediatric adenotonsillectomy: a Bayesian network meta-analysis. J. Clin. Pharm. Ther. 45 (5), 1039–1049. doi:10.1111/jcpt.13133
Joo, J., Lee, S., and Lee, Y. (2014). Emergence delirium is related to the invasiveness of strabismus surgery in preschool-age children. J. Int. Med. Res. 42 (6), 1311–1322. doi:10.1177/0300060514549783
Ju, P., Cheng, Y., and Ya, J. (2013). Efficacy of dexmedetomidine on recovery period in infants undergoing cleft lip and palate repair. Guangdong Med. J. 34, 1439–1441. doi:10.13820/j.cnki.gdyx.2013.09.061
Jun, P., Jun, L., and Jie, L. (2018). Efficacy of dexmedetomidine on emergence agitation during recovery time in children after cleft lip and palate surgery. Zhejiang Trauma. J. 23, 1250–1251. doi:10.3969/j.issn.1009-7147.2018.06.088
Jung, H. J., Kim, J. B., Im, K. S., Oh, S. H., and Lee, J. M. (2010). Effect of ketamine versus thiopental sodium anesthetic induction and a small dose of fentanyl on emergence agitation after sevoflurane anesthesia in children undergoing brief ophthalmic surgery. Korean J. Anesthesiol. 58 (2), 148–152. doi:10.4097/kjae.2010.58.2.148
Kanaya, A. (2016). Emergence agitation in children: risk factors, prevention, and treatment. J. Anesth. 30 (2), 261–267. doi:10.1007/s00540-015-2098-5
Kawai, M., Kurata, S., Sanuki, T., Mishima, G., Kiriishi, K., Watanabe, T., et al. (2019). The effect of midazolam administration for the prevention of emergence agitation in pediatric patients with extreme fear and non-cooperation undergoing dental treatment under sevoflurane anesthesia, a double-blind, randomized study. Drug Des. Devel Ther. 13, 1729–1737. doi:10.2147/DDDT.S198123
Khalifa, O. S. M., and Hassanin, A. A. M. (2013). Melatonin, ketamine and their combination in half doses for management of sevoflurane agitation in children undergoing adenotonsillectomy. Egypt. J. Anaesth. 29 (4), 337–341. doi:10.1016/j.egja.2013.05.006
Kim, J. Y., Chang, Y. J., Lee, J. Y., Park, H. Y., and Kwak, H. J. (2009). Post-induction alfentanil reduces sevoflurane-associated emergence agitation in children undergoing an adenotonsillectomy. Acta Anaesthesiol. Scand. 53 (5), 678–681. doi:10.1111/j.1399-6576.2009.01943.x
Kim, K. M., Lee, K. H., Kim, Y. H., Ko, M. J., Jung, J. W., and Kang, E. (2016). Comparison of effects of intravenous midazolam and ketamine on emergence agitation in children: randomized controlled trial. J. Int. Med. Res. 44 (2), 258–266. doi:10.1177/0300060515621639
Kim, M. S., Moon, B. E., Kim, H., and Lee, J. R. (2013). Comparison of propofol and fentanyl administered at the end of anaesthesia for prevention of emergence agitation after sevoflurane anaesthesia in children. Br. J. Anaesth. 110, 274–280. doi:10.1093/bja/aes382
Kim, N. Y., Kim, S. Y., Yoon, H. J., and Kil, H. K. (2014). Effect of dexmedetomidine on sevoflurane requirements and emergence agitation in children undergoing ambulatory surgery. Yonsei Med. J. 55 (1), 209–215. doi:10.3349/ymj.2014.55.1.209
Kim, Y. H., Yoon, S. Z., Lim, H. J., and Yoon, S. M. (2011). Prophylactic use of midazolam or propofol at the end of surgery may reduce the incidence of emergence agitation after sevoflurane anaesthesia. Anaesth. Intensive Care 39 (5), 904–908. doi:10.1177/0310057X1103900516
Komazaki, M., Mihara, T., Nakamura, N., Ka, K., and Goto, T. (2020). Preventive effect of ramelteon on emergence agitation after general anaesthesia in paediatric patients undergoing tonsillectomy: a randomised, placebo-controlled clinical trial. Sci. Rep. 10 (1), 21996. doi:10.1038/s41598-020-79078-4
Kulka, P. J., Bressem, M., and Tryba, M. (2001). Clonidine prevents sevoflurane-induced agitation in children. Anesth. Analg. 93 (2), 335–338. doi:10.1097/00000539-200108000-00019
Lankinen, U., Avela, R., and Tarkkila, P. (2006). The prevention of emergence agitation with tropisetron or clonidine after sevoflurane anesthesia in small children undergoing adenoidectomy. Anesth. Analgesia 102 (5), 1383–1386. doi:10.1213/01.ane.0000205745.84044.31
Lee, C. J., Lee, S. E., Oh, M. K., Shin, C. M., Kim, Y. J., Choe, Y. K., et al. (2010a). The effect of propofol on emergence agitation in children receiving sevoflurane for adenotonsillectomy. Korean J. Anesthesiol. 59 (2), 75–81. doi:10.4097/kjae.2010.59.2.75
Lee, S. J., and Sung, T. Y. (2020). Emergence agitation: current knowledge and unresolved questions. Korean J. Anesthesiol. 73 (6), 471–485. doi:10.4097/kja.20097
Lee, Y. S., Kim, W. Y., Choi, J. H., Son, J. H., Kim, J. H., and Park, Y. C. (2010b). The effect of ketamine on the incidence of emergence agitation in children undergoing tonsillectomy and adenoidectomy under sevoflurane general anesthesia. Korean J. Anesthesiol. 58 (5), 440–445. doi:10.4097/kjae.2010.58.5.440
Li, J., Huang, Z. L., Zhang, X. T., Luo, K., Zhang, Z. Q., Mao, Y., et al. (2011). Sufentanil reduces emergence agitation in children receiving sevoflurane anesthesia for adenotonsillectomy compared with fentanyl. Chin. Med. J. 124 (22), 3682–3685. doi:10.3760/cma.j.issn.0366-6999.2011.22.015
Li, X., Zhang, Y., Zhou, M., Xia, Q., Li, W., and Lu, Q. (2013). The effect of small dose sufentanil on emergence agitation in preschool children following sevoflurane anesthesia for elective repair of unilateral inguinal hernia. Saudi Med. J. 34 (1), 40–45.
Li, Y., Li, Q., Zhao, G., Zhang, H., Zhong, H., and Zeng, Y. (2024). Nalbuphine in pediatric emergence agitation following cochlear implantation: a randomized trial. Drug Des. Dev. Ther. 18, 2837–2845. doi:10.2147/DDDT.S451089
Liang, P., Zhou, C., Ni, J., Luo, Z., and Liu, B. (2014). Single-dose sufentanil or fentanyl reduces agitation after sevoflurane anesthesia in children undergoing ophthalmology surgery. Pak. J. Med. Sci. 30, 1059–1063. doi:10.12669/pjms.305.4483
Lili, X., Jianjun, S., and Haiyan, Z. (2012). The application of dexmedetomidine in children undergoing vitreoretinal surgery. J. Anesth. 26 (4), 556–561. doi:10.1007/s00540-012-1354-1
Lin, L., Yueming, Z., Meisheng, L., Jiexue, W., and Yang, J. (2017). Effect of dexmedetomidine on emergence agitation after general anesthesia in children undergoing odontotherapy in day-surgery operating room. Hua Xi Kou Qiang Yi Xue Za Zhi 35, 613–617. doi:10.7518/hxkq.2017.06.010
Lin, Y., Chen, Y., Huang, J., Chen, H., Shen, W., Guo, W., et al. (2016). Efficacy of premedication with intranasal dexmedetomidine on inhalational induction and postoperative emergence agitation in pediatric undergoing cataract surgery with sevoflurane. J. Clin. Anesth. 33, 289–295. doi:10.1016/j.jclinane.2016.04.027
Liu, D., Gao, Y., Liu, J., Pan, L., Feng, L., Li, X., et al. (2021). Efficaciousness of dexmedetomidine in children undergoing cleft lip and palate repair: a systematic review and meta-analysis. BMJ Open 11 (8), e046798. doi:10.1136/bmjopen-2020-046798
Liu, W., Sun, R., Gao, X., and Wang, S. (2022). Effects of preoperative nasal spray esketamine on separation anxiety and emergence agitation in pediatric strabismus surgery: a randomized clinical trial. Med. (United States) 101 (51), e32280. doi:10.1097/MD.0000000000032280
Lundblad, M., Marhofer, D., Eksborg, S., and Lönnqvist, P. A. (2015). Dexmedetomidine as adjunct to ilioinguinal/iliohypogastric nerve blocks for pediatric inguinal hernia repair: an exploratory randomized controlled trial. Paediatr. Anaesth. 25 (9), 897–905. doi:10.1111/pan.12704
Luo, K., Xu, J. M., Cao, L., and Gao, J. (2017). Effect of dexmedetomidine combined with sufentanil on preventing emergence agitation in children receiving sevoflurane anesthesia for cleft palate repair surgery. Exp. Ther. Med. 14, 1775–1782. doi:10.3892/etm.2017.4660
Mbuagbaw, L., Rochwerg, B., Jaeschke, R., Heels-Andsell, D., Alhazzani, W., Thabane, L., et al. (2017). Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst. Rev. 6 (1), 79. doi:10.1186/s13643-017-0473-z
Meng, Q. T., Xia, Z. Y., Luo, T., Wu, Y., Tang, L. H., Zhao, B., et al. (2012). Dexmedetomidine reduces emergence agitation after tonsillectomy in children by sevoflurane anesthesia: a case-control study. Int. J. Pediatr. Otorhinolaryngology 76 (7), 1036–1041. doi:10.1016/j.ijporl.2012.03.028
Mizrak, A., Erbagci, I., Arici, T., Avci, N., Ganidagli, S., and Oner, U. (2011). Dexmedetomidine use during strabismus surgery in agitated children. Med. Princ. Pract. 20 (5), 427–432. doi:10.1159/000324554
Mizrak, A., Erbagci, I., Arici, T., Ozcan, I., Ganidagli, S., Tatar, G., et al. (2010). Ketamine versus propofol for strabismus surgery in children. Clin. Ophthalmol. 4, 673–679. doi:10.2147/opth.s11336
Moawad, H. E. S., and El-Diasty, T. (2015). Efficacy of ketamine in prevention of agitation in children undergoing magnetic resonance imaging under face mask sevoflurane: a randomized trial. Egypt. J. Anaesth. 31 (2), 121–125. doi:10.1016/j.egja.2015.01.005
Mohamed Maaly, A., Mahgoub, A., Osman, Y., Abdelhalim, A. A., and Gomaa, M. (2024). Comparison between nalbuphine versus dexmedetomidine for prevention of emergence agitation in pediatrics during sevoflurane anesthesia: prospective randomized controlled clinical trial. Alexandria J. Med. 60 (1), 200–207. doi:10.1080/20905068.2024.2378237
Na, H. S., Song, I. A., Hwang, J. W., Do, S. H., and Oh, A. Y. (2013). Emergence agitation in children undergoing adenotonsillectomy: a comparison of sevoflurane vs. sevoflurane-remifentanil administration. Acta Anaesthesiol. Scand. 57 (1), 100–105. doi:10.1111/aas.12006
Neupane, B., Richer, D., Bonner, A. J., Kibret, T., and Beyene, J. (2014). Network meta-analysis using R: a review of currently available automated packages. PloS One 9 (12), e115065. doi:10.1371/journal.pone.0115065
Park, J. H., Lim, B. G., Kim, H. Z., Kong, M. H., Lim, S. H., Kim, N. S., et al. (2014). Comparison of emergence agitation between sevoflurane/nitrous oxide administration and sevoflurane administration alone in children undergoing adenotonsillectomy with preemptive ketorolac. Korean J. Anesthesiol. 66 (1), 34–38. doi:10.4097/kjae.2014.66.1.34
Patel, A., Davidson, M., Tran, M. C. J., Quraishi, H., Schoenberg, C., Sant, M., et al. (2010). Dexmedetomidine infusion for analgesia and prevention of emergence agitation in children with obstructive sleep apnea syndrome undergoing tonsillectomy and adenoidectomy. Anesth. Analg. 111, 1004–1010. doi:10.1213/ANE.0b013e3181ee82fa
Peng, W., and Zhang, T. (2015). Dexmedetomidine decreases the emergence agitation in infant patients undergoing cleft palate repair surgery after general anesthesia. BMC Anesthesiol. 15, 145. doi:10.1186/s12871-015-0124-7
Pestieau, S. R., Quezado, Z. M. N., Johnson, Y. J., Anderson, J. L., Cheng, Y. I., McCarter, R. J., et al. (2011). The effect of dexmedetomidine during myringotomy and pressure-equalizing tube placement in children. Paediatr. Anaesth. 21 (11), 1128–1135. doi:10.1111/j.1460-9592.2011.03615.x
Rashad, M. M., and Soud, D. E. M. (2014). The effect of different drugs on sevoflurane emergence agitation in pediatric patients undergoing hypospadias repair surgery. Egypt. J. Anaesth. 30 (2), 123–127. doi:10.1016/j.egja.2013.12.005
Saadawy, I., Boker, A., Elshahawy, M. A., Almazrooa, A., Melibary, S., Abdellatif, A. A., et al. (2009). Effect of dexmedetomidine on the characteristics of bupivacaine in a caudal block in pediatrics. Acta Anaesthesiol. Scand. 53 (2), 251–256. doi:10.1111/j.1399-6576.2008.01818.x
Sahmeddini, M. A., Jamshidi, M., Panah, A., Salari, M., Banifatemi, M., and Kanaani Nejad, F. (2024). The effect of post-anesthetic administration of dexmedetomidine versus remifentanil on postoperative agitation of strabismus surgery in children: a randomized double-blind clinical trial. Strabismus 32, 243–251. doi:10.1080/09273972.2024.2368703
Sakai, E. M., Connolly, L. A., and Klauck, J. A. (2005). Inhalation anesthesiology and volatile liquid anesthetics: focus on isoflurane, desflurane, and sevoflurane. Pharmacotherapy 25 (12), 1773–1788. doi:10.1592/phco.2005.25.12.1773
Shen, X., Hu, C., and Li, W. (2012). Tracheal extubation of deeply anesthetized pediatric patients: a comparison of sevoflurane and sevoflurane in combination with low-dose remifentanil. Paediatr. Anaesth. 22 (12), 1179–1184. doi:10.1111/j.1460-9592.2012.03906.x
Sheta, S. A., Al-Sarheed, M. A., and Abdelhalim, A. A. (2014). Intranasal dexmedetomidine vs midazolam for premedication in children undergoing complete dental rehabilitation: a double-blinded randomized controlled trial. Paediatr. Anaesth. 24 (2), 181–189. doi:10.1111/pan.12287
Shi, M., Miao, S., Gu, T., Wang, D., Zhang, H., and Liu, J. (2019). Dexmedetomidine for the prevention of emergence delirium and postoperative behavioral changes in pediatric patients with sevoflurane anesthesia: a double-blind, randomized trial. Drug Des. Devel Ther. 13, 897–905. doi:10.2147/DDDT.S196075
Soliman, R., and Alshehri, A. (2015). Effect of dexmedetomidine on emergence agitation in children undergoing adenotonsillectomy under sevoflurane anesthesia: a randomized controlled study. Egypt. J. Anaesth. 31 (4), 283–289. doi:10.1016/j.egja.2015.04.006
Song, I. A., Seo, K. S., Oh, A. Y., Baik, J. S., Kim, J. H., Hwang, J. W., et al. (2016). Dexmedetomidine injection during strabismus surgery reduces emergence agitation without increasing the oculocardiac reflex in children: a randomized controlled trial. PLoS One 11 (9), e0162785. doi:10.1371/journal.pone.0162785
Sousa-Júnior, F. A., Souza, A. S. R., Lima, L. C., Santos, Í. G. M., Menezes, L. A. P., Ratis, P. A. P. L., et al. (2021). Intraoperative clonidine to prevent postoperative emergence delirium following sevoflurane anesthesia in pediatric patients: a randomized clinical trial. Braz J. Anesthesiol. 71 (1), 5–10. doi:10.1016/j.bjane.2020.12.003
Tan, D., Xia, H., Sun, S., and Wang, F. (2019). Effect of ancillary drugs on sevoflurane related emergence agitation in children undergoing ophthalmic surgery: a Bayesian network meta-analysis. BMC Anesthesiol. 19 (1), 138. doi:10.1186/s12871-019-0810-y
Thomas, D., Lagoo, J., and Kilpadi, K. (2015). Emergence agitation in children after sevoflurane anaesthesia: a comparative evaluation of ketamine and varying doses of fentanyl. Sri Lankan J. Anaesthesiol. 23 (1), 10–16. doi:10.4038/slja.v23i1.7514
Vettuvanthodi, T., Abdul, B. P. M., Subramonian, M., and Krishnankutty, R. (2024). Effect of dexmedetomidine versus propofol on sevoflurane related emergence agitation in paediatric patients: a randomised clinical study. J. Clin. Diagnostic Res. 18 (5), UC11–UC15. doi:10.7860/jcdr/2024/68717.19374
Viitanen, H., Annila, P., Viitanen, M., and Tarkkila, P. (1999). Premedication with midazolam delays recovery after ambulatory sevoflurane anesthesia in children. Anesth. Analgesia 89 (1), 75–79. doi:10.1097/00000539-199907000-00014
Wang, H. Y., Chen, T. Y., Lin, P. Y., Su, K. P., Chiang, M. H., Carvalho, A. F., et al. (2021). Association of pharmacological prophylaxis with the risk of pediatric emergence delirium after sevoflurane anesthesia: an updated network meta-analysis. J. Clin. Anesth. 75, 110488. doi:10.1016/j.jclinane.2021.110488
Wang, W., Huang, P., Gao, W., Cao, F., Yi, M., Chen, L., et al. (2016). Efficacy and acceptability of different auxiliary drugs in pediatric sevoflurane anesthesia: a network meta-analysis of mixed treatment comparisons. Sci. Rep. 6, 36553. doi:10.1038/srep36553
Wang, X., Deng, Q., Liu, B., and Yu, X. (2017). Preventing emergence agitation using ancillary drugs with sevoflurane for pediatric anesthesia: a network meta-analysis. Mol. Neurobiol. 54 (9), 7312–7326. doi:10.1007/s12035-016-0229-0
Wang, C. M., Zhang, Y., Chen, W. C., Lin, S., and He, H. F. (2023). Effects of pharmacological intervention on recovery after sevoflurane anesthesia in children: a network meta-analysis of randomized controlled trials. Mol. Neurobiol. 60 (8), 4488–4501. doi:10.1007/s12035-023-03349-0
Xi, H., Zu, H., and Shou, S. (2012). The effect of recovery quality of dexmedetomidine for cleft lip and palate repair in children. Guangdong Med. J. 33, 2490–2492. doi:10.13820/j.cnki.gdyx.2013.09.061
Xiao, L., Long, C., and Jun, F. (2012). Comparison of dexmedetomidine, ketamine and fentanyl in children undergoing cleft lip and palate surgery. Zhejiang Pract. Med. 17, 173–175. doi:10.16794/j.cnki.cn33-1207/r.2012.03.006
Xing, F., Zhang, T. T., Yang, Z., Qu, M., Shi, X., Li, Y., et al. (2024). Comparison of dexmedetomidine and a dexmedetomidine-esketamine combination for reducing dental anxiety in preschool children undergoing dental treatment under general anesthesia: a randomized controlled trial. J. Affect. Disord. 347, 569–575. doi:10.1016/j.jad.2023.12.011
Yang, X., Lin, C., Chen, S., Huang, Y., Cheng, Q., and Yao, Y. (2022). Remimazolam for the prevention of emergence delirium in children following tonsillectomy and adenoidectomy under sevoflurane anesthesia: a randomized controlled study. Drug Des. Dev. Ther. 16, 3413–3420. doi:10.2147/DDDT.S381611
Yao, Y., Qian, B., Lin, Y., Wu, W., Ye, H., and Chen, Y. (2015). Intranasal dexmedetomidine premedication reduces minimum alveolar concentration of sevoflurane for laryngeal mask airway insertion and emergence delirium in children: a prospective, randomized, double-blind, placebo-controlled trial. Paediatr. Anaesth. 25, 492–498. doi:10.1111/pan.12574
Yasui, Y., Masaki, E., and Kato, F. (2007). Sevoflurane directly excites locus coeruleus neurons of rats. Anesthesiology 107 (6), 992–1002. doi:10.1097/01.anes.0000291453.78823.f4
Yun, L., Zhen, L., and Xu, Y. (2016). Effects of intranasal dexmedetomidine for children undergoing cleft lip and palate repair surgery. Int. J. Stomatology 43, 401–405. doi:10.7518/gjkq.2016.04.008
Zhang, Y. Z., Wei, X. L., Tang, B., Qin, Y. Y., Ou, M., Jiang, X. H., et al. (2022). The effects of different doses of alfentanil and dexmedetomidine on prevention of emergence agitation in pediatric tonsillectomy and adenoidectomy surgery. Front. Pharmacol. 13, 648802. doi:10.3389/fphar.2022.648802
Keywords: emergence delirium, pediatric, adjuvant, sevoflurane-related, network meta-analysis
Citation: Zhang C-J, Chen H, Zou K and Qu X (2025) Optimizing adjuvant strategies for sevoflurane-related emergence delirium: a Bayesian network meta-analysis in pediatric surgery. Front. Pharmacol. 16:1573640. doi: 10.3389/fphar.2025.1573640
Received: 09 February 2025; Accepted: 02 June 2025;
Published: 04 July 2025.
Edited by:
Wei Zhao, Shandong University, ChinaReviewed by:
Catherine M. T. Sherwin, University of Western Australia, AustraliaNadia Najafi, University Hospital Brussels, Belgium
Copyright © 2025 Zhang, Chen, Zou and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Chen, aG9wbjEyMTNAdGpoLnRqbXUuZWR1LmNu
†ORCID: Hong Chen, orcid.org/0000-0002-8304-0352
 Chun-Jin Zhang
Chun-Jin Zhang Hong Chen2*†
Hong Chen2*†