- 1Changchun University of Chinese Medicine, Changchun, China
- 2Department of Orthopedics, The Third Affiliated Clinical Hospital of Changchun University of Chinese Medicine, Changchun, China
- 3Department of Endocrinology, Changchun Hospital of Chinese Medicine, Changchun, China
- 4Department of Orthopedics, The Affiliated Hospital of Changchun University of Chinese Medicine. Changchun, China
- 5Department of Geriatrics, The Affiliated Hospital of Changchun University of Chinese Medicine. Changchun, China
Metabolic bone disease (MBD), as one of the most severe metabolic disorders, remains a focal point and challenge in medical research. Numerous studies have demonstrated the efficacy of Traditional Chinese Medicine (TCM) in preventing and treating MBD. However, the inherent complexity of TCM metabolites poses significant limitations in elucidating their mechanisms of action. The advancement of omics technologies, including metabolomics, proteomics, and transcriptomics, has greatly facilitated research on MBD. These approaches enable the identification of potential biomarkers and the exploration of metabolic pathways and mechanisms underlying TCM interventions for MBD. Evidence indicates that TCM monomers, single botanical drugs, and herbal formulations are effective, safe, and well-tolerated in MBD prevention and treatment. This review summarizes recent applications and key findings of transcriptomics, proteomics, and metabolomics in studying the mechanisms of TCM interventions for MBD. It highlights the role of omics technologies in uncovering relevant metabolites and pathways under TCM treatment, providing valuable insights and clinical references for TCM-based strategies in managing MBD.
1 Introduction
Metabolic Bone Disease (MBD) encompasses a group of disorders characterized by abnormalities in bone metabolism, structure, or mineralization, and is clinically significant due to its impact on skeletal health. Clinically, MBD is primarily manifested by abnormal bone turnover rates, accompanied by symptoms such as bone pain, deformities, and fractures. It belongs to a distinct category within metabolic diseases (Seemann et al., 2024). Bone tissue undergoes continuous remodeling, a dynamic process involving daily bone formation and resorption. An adult’s skeleton is entirely renewed approximately every 10 years (Hart et al., 2020). The stability of bone metabolism and bone mass depends on the dynamic balance between osteoblast-mediated bone formation and osteoclast-mediated bone resorption, which is maintained by a normal bone marrow microenvironment. Disruption of this balance leads to MBD, increasing the risk of skeletal abnormalities and fractures. MBD include Osteoporosis (OP), Osteomalacia, Mucopolysaccharidosis, Marfan Syndrome, Paget’s Disease, and Renal Osteodystrophy, and so on (Feng and McDonald, 2011). Among these, OP is the most prevalent form of MBD (Anonymous, 1993). Globally, approximately 18.3% of the population suffers from OP, with a significantly higher incidence in women (23.1%) than in men (11.7%). Current treatment strategies focus on supplementing vitamin D, calcium, estrogen replacement, parathyroid hormone analogs, or bisphosphonates. These interventions enhance bone response, inhibit osteoclast activity, and slow down bone metabolism (Johnston and Dagar, 2020).
In traditional Chinese medicine (TCM), MBD is named as “Guwei,” “Guku,” and “Gubi” based on their clinical manifestations. Its pathogenesis is closely linked to the kidney, bone, and marrow. Classical theories posit that “marrow is generated from kidney essence; sufficient essence leads to abundant marrow, and abundant marrow results in strong bones.” This highlights the intrinsic connection between sufficient kidney essence, abundant marrow, and robust bones. Therefore, the occurrence of bone diseases is closely associated with the functional state of the kidney. The pathogenesis of MBD may involve multiple factors, including insufficient innate endowment, age-related decline in kidney essence, liver stagnation, spleen deficiency, impaired generation of qi and blood, inadequate nourishment of kidney essence and bone marrow, and poor blood circulation. Clinically, TCM employs botanical drugs that nourish the liver and kidney, strengthen the spleen and kidney, and invigorate the tendons and bones. These botanical drugs also promote blood circulation and remove blood stasis, strengthen the kidneys and bones, thereby alleviating clinical symptoms such as weakness, fatigue, and soreness in the lower back and knees.
In recent years, active metabolites of TCM, such as icariin and achyranthes bidentata polysaccharides, have been scientifically validated for their ability to enhance bone mineral density (BMD) and improve bone microstructure (Xue et al., 2016; Zhang et al., 2018). Its mechanisms of action primarily involve regulating metabolite levels, protein expression ratios, restoring metabolite diversity, and modulating gene expression levels, effectively delaying the onset and progression of MBD. Studies have shown that both single botanical drugs and Chinese herbal formula can improve bone turnover processes, optimize bone tissue structure, and reverse declining bone density by regulating serum biochemical indicators. These findings demonstrate the significant role of TCM in regulating bone metabolism, offering new therapeutic strategies for MBD prevention and treatment.
In recent years, as an emerging interdisciplinary field, omics technologies, including proteomics, transcriptomics, metabolomics, and others, have attracted significant scientific interest. Omics technologies enable the systematic profiling of genetic information, transcriptional data, proteins, and metabolites from an entire organism, tissue, organ, or single cell, providing in-depth analysis of the composition and interrelationships of these metabolites. This is highly consistent with the holistic concept of TCM. Metabolomics focuses on the quantitative profiling of all small-molecule metabolites within an organism using techniques such as gas chromatography-mass spectrometry, liquid chromatography-mass spectrometry (LC-MS), and nuclear magnetic resonance. It establishes various mathematical models to identify differential metabolites through statistical methods and conducts bioinformatics analysis to reveal the pathophysiological implications of metabolic changes in the overall system caused by diseases or interventions (Huang R. et al., 2023). Proteomics, based on protein separation techniques, mass spectrometry identification, and bioinformatics, investigates protein functions, structures, and interactions. Commonly used methods include two-dimensional gel electrophoresis, mass spectrometry, and isobaric tags for relative and absolute quantification (Deng et al., 2022). Transcriptomics employs high-throughput RNA sequencing, single-cell sequencing, and microarray technologies to comprehensively characterize all RNA within cells during specific periods, thereby uncovering gene expression patterns and regulatory mechanisms (Chambers et al., 2019). The samples involved in these studies include blood, urine, bone, liver, and kidney tissues, among others (Tao et al., 2017; Li X. et al., 2022).
The rapid development and widespread application of omics technologies have provided new perspectives for exploring the etiology and pathogenesis of MBD, both epistemologically and methodologically. They promote the standardization of syndrome differentiation treatment and provide scientific evidence for analyzing the material basis of TCM and Chinese herbal formula. Omics technologies also provide technical support for revealing the molecular mechanisms of complex drug systems, enabling intervention in diseases at multiple levels and dimensions. With the rapid development of modern systems biology, continuous innovation in proteomics, metabolomics, and transcriptomics, and the refinement of bioinformatics methods, research on TCM for preventing and treating MBD is expected to achieve significant breakthroughs.
Through a systematic review and comprehensive analysis of the literature, this study aims to evaluate the current research status of MBD treatment based on metabolomics, proteomics, and transcriptomics. Furthermore, it seeks to elucidate the underlying mechanisms of action, assess their clinical efficacy, and provide evidence-based recommendations for future research directions.
2 Methods
2.1 Search strategy
This review searched literature from the establishment of the databases up to December 2024. The databases searched include CNKI, Wanfang, VIP, and PubMed. Key terms used in the search were “metabolomics,” “proteomics,” “transcriptomics,” “metabolic bone disease,” “osteoporosis,” “diabetic bone disease,” “renal osteodystrophy,” “Paget’s disease,” and “TCM.” Both Chinese and English literature were included.
2.2 Selection criteria
First, we conducted a title screening of the literature to assess its relevance to the research topic. Subsequently, based on the results of the title screening, we performed a secondary screening by reviewing the abstracts of the papers. Finally, we conducted a full-text read of the selected literature to ensure that its content was consistent with the objectives of the review.
During the screening process, we rigorously applied pre-defined inclusion and exclusion criteria. The inclusion criteria were as follows: studies must focus on omics (e.g., metabolomics, proteomics, transcriptomics), MBDs, TCM, or natural products. Additionally, studies must address the pathophysiological mechanisms of MBDs or the prevention and treatment of these diseases using TCM. Furthermore, studies must explicitly investigate the role of omics technologies in elucidating the mechanisms of TCM for preventing or treating MBDs. The exclusion criteria included: studies with unclear themes, methods, or mechanisms; publications of unreliable or substandard quality; duplicate publications or studies; studies with incomplete or insufficient data, dissertations, or studies for which the full text was inaccessible. To ensure objectivity and reliability in the screening process, two researchers (WC and LJ) independently conducted the literature screening and analysis. Discrepancies were resolved through discussion to reach a consensus, and unresolved issues were adjudicated by a third researcher (CZ). This approach significantly reduced subjective bias and guaranteed the scientific rigor and reliability of the final included literature.
3 Pathogenesis of MBD
Omics technologies hold significant value in the field of disease research, as they not only enable differential analysis of metabolites and proteins but also correlate data with biological significance. In the context of MBD, omics technologies have provided powerful tools for elucidating their pathogenesis. Current research indicates that the pathogenesis of MBD primarily involves four key aspects: abnormal bone resorption, abnormal bone growth, abnormal mineral deposition, and metabolic factors, as illustrated in Figure 1.

Figure 1. Mechanistic map of metabolic bone disease: The pathogenesis of MBD primarily involves four key pathological dimensions: abnormal bone resorption, abnormal bone formation, abnormal mineral deposition, and metabolic factors. ① the core regulatory pathways for abnormal bone resorption are the RANKL/RANK/OPG system, NF-κB and Ca2+-NFATc1 signaling pathway. PTH and vitamin D hormones also significantly influence bone resorption. ② Abnormal bone formation mainly involves osteoblast dysfunction. The Wnt/β-catenin and BMP/Smad signaling pathway play key roles in osteoblast differentiation and function. Other signaling pathways such as Hedgehog, Notch, and FGF also participate in the regulation of osteoblasts. ③ Abnormal mineral deposition is primarily manifested as disorders in calcium and phosphorus metabolism. Vitamin D deficiency and parathyroid dysfunction can lead to imbalances in calcium and phosphorus metabolism. Additionally, FGF23 plays a crucial role in the regulation of mineral metabolism. ④ The impact of metabolic factors mainly involves the effects of abnormalities in sugar, fat, and protein metabolism on bone metabolism.
3.1 Abnormal bone resorption
Abnormal bone resorption can be classified into physiological and pathological types, primarily associated with factors such as age, genetics, hormones, inflammatory responses, and lifestyle. For example, hormones such as parathyroid hormone and vitamin D influence osteoclast activity, thereby altering the bone resorption process. During the bone resorption phase, osteoclasts attach to the bone surface and secrete acids and proteases to degrade the bone matrix, leading to reduced bone mass and decreased bone strength. Subsequently, during the bone formation phase, osteoblasts migrate to the resorption site, secrete bone matrix, and promote its mineralization. These changes not only exacerbate bone metabolic disorders but may also affect the function of other organ systems, creating a vicious cycle. The RANKL/RANK/OPG is considered the core regulatory pathway controlling this process (Udagawa et al., 2002). Additionally, signaling pathways such as NF-κB and Ca2+-NFATc1 play crucial roles in regulating bone resorption (Russo et al., 2022).
3.2 Abnormal bone growth
Abnormal bone growth primarily involves osteoblast dysfunction. Under normal conditions, osteoblasts form a matrix containing collagen and proteoglycans, which gradually mineralizes into osteoid tissue. However, certain genetic disorders or metabolic abnormalities may impair osteoblast function, thereby affecting bone formation. The differentiation and function of osteoblasts are regulated by multiple signaling pathways, with the Wnt/β-catenin pathway playing a pivotal role. Wnt proteins bind to Frizzled receptors and LRP5/6 co-receptors, stabilizing β-catenin and promoting its nuclear translocation, which activates the expression of osteoblast-specific genes such as Runx2 and Osterix (Guo et al., 2021). Simultaneously, the BMP/Smad pathway regulates osteoblast differentiation through the phosphorylation and nuclear translocation of Smad1/5/8 (Fernandes and Gomes, 2016). Furthermore, signaling pathways such as Hedgehog, Notch, and FGF are also involved in osteoblast regulation. These pathways interact in complex ways to coordinate bone formation (Vlashi et al., 2023).
3.3 Abnormal mineral deposition
Abnormal mineral deposition is primarily characterized by disturbances in calcium and phosphorus metabolism. Calcium and phosphorus are the main metabolites of bone mineralization, and their metabolism is tightly regulated. In MBDs, various factors, such as vitamin D deficiency and parathyroid dysfunction, can disrupt calcium and phosphorus homeostasis. These disturbances directly affect bone mineralization, leading to reduced or abnormal mineral deposition. Recent studies have identified fibroblast growth factor 23 (FGF23) as a key regulator of mineral metabolism (Smith et al., 2014). FGF23, primarily produced by osteocytes, regulates phosphorus metabolism through a dual mechanism: on one hand, it inhibits phosphate reabsorption in the renal proximal tubules, and on the other hand, it suppresses the synthesis of 1,25-dihydroxyvitamin D (Sirikul et al., 2022). In patients with chronic kidney disease (CKD) and other MBDs, elevated FGF23 levels lead to hypophosphatemia and abnormal vitamin D metabolism, ultimately impairing normal mineral deposition and exacerbating skeletal pathology (Llorente-Pelayo et al., 2024).
3.4 Influence of metabolic factors
Metabolic disorders play a critical role in the development and progression of MBDs, with abnormalities in glucose, lipid, and protein metabolism having particularly significant impacts on bone metabolism. In terms of glucose metabolism, hyperglycemia affects bone metabolism balance through multiple mechanisms: on one hand, high glucose levels directly inhibit osteoblast differentiation and function while promoting osteoclast formation and activity (Sheu et al., 2024); on the other hand, the accumulation of advanced glycation end products, insulin resistance, and abnormalities in the insulin-like growth factor-1signaling pathway also contribute to glucose metabolism-related bone disorders (Liu et al., 2023; Mohan and Baylink, 1996). Lipid metabolism disorders not only induce osteoblast apoptosis and promote osteoclast differentiation, thereby inhibiting bone formation, but also alter the bone microenvironment by increasing bone marrow adipose tissue, ultimately disrupting bone remodeling balance (Zhang et al., 2024). Additionally, oxidized low-density lipoprotein promotes osteoclastogenesis by activating the NF-κB signaling pathway, further exacerbating bone resorption (Wang et al., 2022). Protein metabolism dysregulation affects bone metabolism primarily through bone matrix synthesis and osteocyte function. Inadequate protein intake reduces bone matrix synthesis, impairing bone mineralization, while abnormal amino acid metabolism alters osteocyte energy metabolism and signal transduction, thereby affecting bone remodeling (Srivastava et al., 2022). Notably, metabolic abnormalities of specific amino acids, such as arginine and glutamine, may directly influence the dynamic balance between bone formation and resorption (Devignes et al., 2022).
4 Application of omics technologies in the diagnosis of MBD
Omics technologies, as a pivotal technical system in systems biology research, integrate multidimensional data from transcriptomics, proteomics, and metabolomics to systematically elucidate dynamic changes in molecular networks within organisms. In the field of MBDs, these technologies not only comprehensively reveal the molecular mechanisms underlying bone metabolism abnormalities but also enable precise disease classification, dynamic monitoring of disease progression, and prognostic evaluation through biomarker screening. This provides novel perspectives and methodologies for clinical diagnosis and treatment.
Chen et al. conducted a systematic analysis of exosomal proteins in patients with OP at different disease stages using quantitative proteomics. They identified and validated four exosomal proteins—PSMB9, PCBP2, VSIR, and AARS—with diagnostic potential, offering new molecular targets for the clinical diagnosis of OP (Chen et al., 2020). Huo et al. employed a quantitative proteomics technologies to compare serum microvesicle protein expression profiles between OP patients and healthy controls, revealing significant differential expression of Vinculin, Filamin A, and Profilin 1. Notably, Profilin 1 expression was markedly elevated in the osteoporosis group, suggesting its potential as a valuable diagnostic indicator for OP. This finding provides critical experimental evidence for establishing a dynamic monitoring system for bone metabolism based on body fluid analysis (Huo et al., 2019). Wu Xiuhua et al. identified 165 differentially expressed genes, including Acot1, Mpig6b, Gp9, Ppbp, and Slc2a9, in a ketogenic diet-induced osteoporosis model compared to a normal diet group using transcriptomic sequencing. These genes are involved in signaling pathways such as Apelin, PI3K-Akt, and ECM-receptor interaction (Wu X. et al., 2023). Achim Buck et al. utilized spatial metabolomics to reveal complex metabolic alterations in Hyp mice, including significant changes in the pentose phosphate pathway, galactose metabolism, purine metabolism, ascorbate and aldarate metabolism, cysteine and methionine metabolism, pyrimidine metabolism, and arginine metabolism. Additionally, decreased levels of glycerophospholipids, phosphosphingolipids, fatty acids, amino acids, and peptides were observed, while carbohydrates, nucleic acids, vitamins, cofactors, and organic sulfates were elevated (Buck et al., 2022).
Notably, the application of omics technologies can not only facilitate the early diagnosis of MBDs but also provide a basis for MBDs classification by identifying distinct characteristics in metabolites, proteins, genes, and other molecular features.
The molecular characteristics uncovered by omics technologies allow for the classification of MBD into specific types, such as OP, osteomalacia, primary hyperparathyroidism, and Marfan syndrome. These classifications not only reflect the diverse etiologies of the diseases but also underscore the associated alterations in metabolites, proteins, and other molecular metabolites in vivo.
4.1 The most common MBD (OP)
OP is the most common MBD. OP is primarily classified into primary and secondary types based on etiology. Primary OP includes postmenopausal osteoporosis (PMOP), senile osteoporosis (SOP), and idiopathic osteoporosis. Secondary OP encompasses glucocorticoid-induced osteoporosis (GIOP), CKD-related osteoporosis, diabetic osteoporosis (DOP) and others.
4.1.1 Senile osteoporosis
Yahui Wu et al. analyzed plasma from 379 elderly OP patients using untargeted liquid chromatography/gas chromatography-mass spectrometry (LC/GC-MS) and identified associations with retrograde endocannabinoid signaling, glycerophospholipid metabolism, steroid hormone biosynthesis, and xenobiotic metabolism by cytochrome P450. Among these, 2-aminomuconic acid semialdehyde showed diagnostic value in male OP, while tetradecanedioic acid exhibited diagnostic potential in female OP patients (Wu Y. et al., 2023). Xu Zhou et al. based on label-free quantitative proteomics (Easy-nLC1000 and Q-exactive) and Western blotting to discover that Abl Interactor 1 was significantly downregulated in elderly Chinese men with extremely low BMD. A similar trend was observed in elderly women. Functional studies demonstrated that Abl Interactor 1, at an optimal concentration of 2.0 ng/mL, significantly promoted osteoblast growth, expression of osteogenesis-related genes (OPN, ALP, COL1A1), and osteoblast differentiation. These findings suggest Abl Interactor 1 as a potential biomarker for diagnosing OP and osteoporotic fractures (Zhou et al., 2019).
4.1.2 Postmenopausal osteoporosis
PMOP is primarily associated with declining estrogen levels (Li et al., 2020). When distinguishing OP between men and postmenopausal women, combining relevant metabolites with traditional bone turnover markers enhances diagnostic sensitivity, offering a foundation for early diagnosis and mechanistic studies of PMOP (Wang et al., 2019). Xin Li et al. analyzed metabolic interactions among the kidneys, bone marrow, and bones from a metabolomics perspective, identifying co-metabolites and pathways. Analysis of metabolites such as creatine, glutamine, β-hydroxybutyrate, and α-tocopherol further clarified the close relationship between energy metabolism, fatty acid metabolism, and ammonia metabolism (Li X. et al., 2022). Hongxia Zhao et al. conducted bone metabolomics-lipidomics using ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry, revealing significant changes in fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, and sterols. Disruptions in amino acid metabolism, nucleotide metabolism, and lipid metabolism were closely linked to the imbalance between bone resorption and formation, potentially underpinning PMOP (Zhao et al., 2018). Numerous metabolomics studies have shown that OP pathogenesis is closely related to amino acid metabolism, lipid metabolism, energy metabolism, and gut microbiota dysbiosis (Fan et al., 2021). Compared to healthy women, PMOP may involve disruptions in glucose, lipid, and amino acid metabolism, particularly associated with 18 metabolites, including isothreonic acid and ornithine, which are considered potential biomarkers for PMOP (Kou et al., 2022). A cross-sectional study of 517 perimenopausal and early postmenopausal women revealed associations between metabolites and BMD changes. Four fatty acids, three glycerophospholipids, three sterol lipids, two peptides, and eight other organic metabolites were significantly correlated with BMD changes. Notably, a functional metabolite module composed of fatty acids was closely related to BMD changes (Gong et al., 2021). Metabolomics has also played a significant role in TCM syndrome differentiation for OP. Compared to non-OP women, S-lactoylglutathione levels were significantly elevated in OP women with kidney-yang deficiency, suggesting its potential as a key indicator for TCM-based OP diagnosis (Yin et al., 2022).
4.1.3 Glucocorticoid-induced osteoporosis(GIOP)
Glucocorticoids, commonly used anti-inflammatory drugs, directly or indirectly affect bone remodeling and are a leading cause of secondary OP (Hu and Adachi, 2019). They impair the osteogenic capacity of mesenchymal stem cells (MSCs) and promote their differentiation into adipocytes (Lane, 2019). Additionally, glucocorticoids inhibit osteoblast maturation, shorten their lifespan, reduce their function, and induce osteocyte apoptosis, ultimately leading to bone loss and OP (Weinstein, 2012). Using LC-MS metabolomics in a glucocorticoid-induced OP model, ovariectomy (OVX) alone or combined with glucocorticoids altered metabolite and lipid profiles in sheep, suggesting that phenylalanine, tyrosine, and tryptophan biosynthesis, as well as cysteine, methionine, and branched-chain amino acid metabolism, may be key pathways regulating bone loss in OVX sheep (Weinstein, 2012). Ying et al. employed transcriptomics to analyze GIOP characteristics, identifying 158 glucocorticoid-related candidate genes significantly enriched in OP-related pathways, providing new insights into the diagnosis and treatment of GIOP (Ying et al., 2020).
4.1.4 Diabetic osteoporosis
DOP, a form of secondary OP, is characterized by reduced bone mineral content (BMC), decreased BMD, and bone structure deterioration due to hyperglycemia and metabolic alterations. Wang Yan et al. integrated dynamic contrast-enhanced magnetic resonance imaging Ktrans mapping texture analysis with metabolomics to evaluate early bone marrow microvascular lesions in diabetes, revealing associations with lipid metabolism abnormalities, particularly in linoleic acid metabolism (Wang Y. et al., 2024). W.-D. Liang et al. studied 18 DOP patients and found decreased levels of O-acetyl glycoprotein, proline, 1-methylhistidine, and tricarboxylic acid (TCA) cycle products, alongside elevated levels of branched-chain amino acids, choline, creatine, myo-inositol, glutamine, glutamate, aspartate, alanine, glycine, and citrulline. These changes may serve as early diagnostic markers for DOP (Liang et al., 2020). Kefeng Wu et al. observed significant increases in fatty acyls, glycerophospholipids, and phosphatidylethanolamines in T2DOP mice, with downregulated amino acid pathway metabolites. Dysregulation of lipid and glutathione pathways was identified as a major contributor to T2DOP progression in C57BKS mice (Wu et al., 2024).
4.1.5 Renal osteodystrophy
Renal osteodystrophy, a MBD secondary to CKD, arises from calcium, phosphorus, and vitamin D metabolism disorders, secondary hyperparathyroidism, and acid-base imbalances. Clinical manifestations include OP, bone and joint pain, pathological fractures, and soft tissue and vascular calcification. The Wnt/β-catenin signaling pathway plays a crucial regulatory role in renal osteodystrophy, affecting the dynamic balance between bone formation and resorption (Wang et al., 2016). Aline L. Baptista et al. used nuclear magnetic resonance metabolomics to identify elevated levels of dimethyl sulfone, glycine, citrate, and N-acetylornithine in high bone turnover patients, while low ethanol levels were associated with abnormal bone mineralization, and low carnitine levels were linked to low bone volume. These findings aid in assessing bone phenotypes in chronic kidney disease-mineral and bone disorder (CKD-MBD) patients (Baptista et al., 2020). Other studies have shown that CKD-MBD pathogenesis is related to changes in protein synthesis and metabolism, amino acid metabolism, energy metabolism, and steroid hormone metabolism pathways, involving metabolites such as N-(1-Deoxy-1-fructosyl)tryptophan, glycylprolylhydroxyproline, and aminohippuric acid (Wu et al., 2015). Murat Kasap et al. identified seven protein spots in MSCs isolated from uremic bone disease patients with chronic renal failure using proteomics and molecular methods, discussing their potential relationships (Kasap et al., 2015).
4.2 Other MBDs
Although less studied, other MBDs have also been explored using omics technologies. Yuan Xiaozhou et al. employed magnetic bead-based weak cation exchange chromatography combined with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry to identify four upregulated and three downregulated mass spectrometry peaks in mucopolysaccharidosis type I patients compared to healthy controls, offering a non-invasive diagnostic method for mucopolysaccharidosis type I (Yuan et al., 2017). Clarisse L. Torres et al. used untargeted liquid chromatography-high-resolution mass spectrometry metabolomics to reveal elevated levels of dipeptides, amino acids, and their derivatives, as well as N-acetylgalactosamine 4- or 6-sulfate (key metabolites of glycosaminoglycans) in mucopolysaccharidosis patients, providing new insights into its pathological mechanisms (Torres et al., 2023). Other MBDs, such as Marfan syndrome, have been less studied using omics technologies.
The application of omics technologies in the pathological classification and TCM syndrome differentiation of MBDs is still in its infancy. Current evidence-based medical data remain insufficient, highlighting the need for systematic and in-depth research. Such studies will provide molecular-level scientific evidence for TCM interventions in MBDs, facilitating precision medicine approaches.
5 Application of omics technologies in identifying potential targets of TCM monomers for treating MBD
TCM monomers, as the key active metabolites, are responsible for the pharmacological effects of TCM. Different botanical drugs may contain identical monomeric metabolites, which exhibit diverse medicinal properties and serve as crucial targets for TCM research and development. Table 1 summarizes the progress in metabolomics, proteomics, and transcriptomics studies of commonly used TCM monomers in treating MBDs. Figure 2 displays the chemical structures of common monomers.
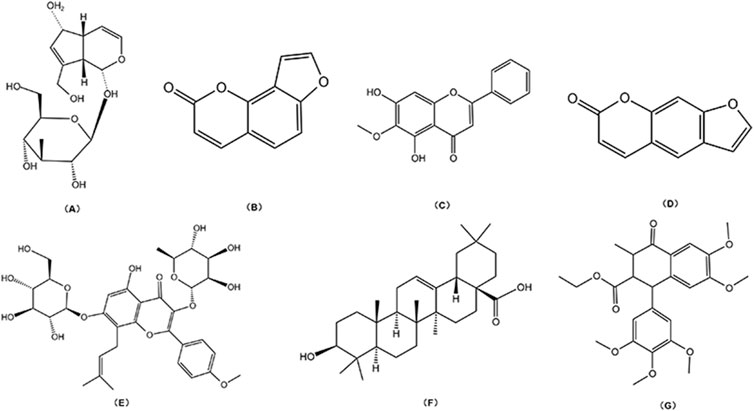
Figure 2. Chemical structural: (A) Aucubin; (B) Isopsoralen; (C) Oroxylin A; (D) Psoralen; (E) Icariin; (F) Oleanolic Acid; (G) Lignan.
5.1 Metabolomics
Metabolomics studies have demonstrated that TCM monomers can ameliorate bone metabolism imbalance by multidimensionally regulating metabolic networks. Icariin, one of the most widely used TCM monomers for MBDs, promotes osteoblast-mediated bone formation and inhibits osteoclast differentiation. It achieves this by regulating lipid metabolism, cytoskeleton remodeling, and energy metabolism homeostasis. These effects result in increased BMD and BMC, along with reduced levels of the bone resorption marker CTX-I. Icariin systematically improves the bone metabolic microenvironment by modulating energy metabolism, lipid metabolism, amino acid metabolism, phospholipid metabolism, and the gut microbiota axis. And this leads to increased bone mass (Pan et al., 2016). Metabolomics analysis based on UPLC/Q-TOF-MS revealed that puerarin improves osteoporosis and lipid metabolism in OVX rats. It specifically regulates phospholipid metabolism homeostasis and polyunsaturated fatty acid biosynthesis pathways, reducing adipogenic differentiation and promoting osteogenesis (Li B. et al., 2022). Aucubin, a major metabolite of Eucommia ulmoides Oliv. and Plantago asiatica L., promotes osteoblast differentiation and inhibits osteoclast formation in GIOP mice. It achieves this by regulating the arachidonic acid metabolism pathway (Wang H. et al., 2024). Isopsoralen significantly improves bone microstructure mechanics and strength parameters in GIOP mice. It activates the cGMP/PKG signaling axis to regulate purine metabolism homeostasis. Additionally, it increases serum calcium, phosphorus, and alkaline phosphatase levels, thereby promoting bone homeostasis reconstruction (Liu et al., 2024).
Mengliu Zhang et al. discovered that Achyranthes bidentata polysaccharides significantly increase biomarkers such as glutarylcarnitine, lysoPC (18:1), and 9-cis-retinoic acid through UPLC/Q-TOF-MS-based metabolomics. These changes improve BMD and trabecular bone structure by regulating lipid metabolism (Zhang et al., 2018). Oroxylin A, an active metabolites of Scutellaria baicalensis Georgi root, significantly improves bone density and trabecular structure in VOX mice. It specifically regulates the phenylalanine, tyrosine, and tryptophan biosynthesis pathways, affecting key metabolites such as L-phenylalanine, L-tryptophan, lysophosphatidylcholine (18:2), PC (22:5 (4Z, 7Z, 10Z, 13Z, 16Z)/16:1 (9Z)), and taurocholic acid (Xian et al., 2023).
5.2 Transcriptomics
Gao Feng et al. found that icariin significantly alters gene expression in ovariectomized rats, with 532 genes showing notable changes compared to the model group. Notably, Sp3, Pctp, MMP8, and MT3 were the most significantly altered genes. These genes potentially regulate lipid metabolism, signal transduction, transcription factor activity, collagen degradation, and immune responses, thereby improving osteoporosis (Gao et al., 2013). Bian Qin et al. used gene chip technology to compare the regulatory networks of icariin, psoralen, and oleanolic acid on bone marrow mesenchymal stem cells (BMSCs) in a corticosterone-induced rat model. They identified five common genes regulated by all three metabolites, including osteogenic differentiation-related molecules, cell cycle regulators, cytokines, and the Notch signaling pathway molecule Adam17. This suggests that kidney-tonifying TCM may promote BMSC osteogenic differentiation by regulating the cell cycle and metabolism, ultimately treating osteoporosis (Bian et al., 2011).
These studies reveal that TCM monomers exhibit multidimensional regulatory properties. Single metabolites can simultaneously act on gene expression, signal transduction, and the metabolic microenvironment related to bone metabolism. Homologous monomers exhibit distinct regulatory networks depending on the etiology, such as hormone deficiency or glucocorticoid excess. Furthermore, integrated multi-omics analysis (metabolomics-transcriptomics-proteomics) can systematically elucidate the cascade regulatory relationships of “metabolite -target-pathway-phenotype.” This is achieved through differential metabolite screening, key gene co-expression network construction, and pathway enrichment analysis. These findings provide a theoretical foundation and research paradigm for developing novel anti-MBD drugs based on the multi-target properties of TCM.
6 Application of omics technologies in identifying potential targets of single botanical drugs for treating MBD
TCM botanical drugs contain multiple active metabolites, enabling multi-target effects even without combination with other botanical drugs, effectively functioning as a small formula. In TCM practice, kidney-tonifying and bone-strengthening single botanical drugs are commonly used to treat MBDs. These botanical drugs not only ameliorate bone loss caused by metabolic disorders but also promote bone formation. Based on this, we summarize high-frequency single botanical drugs used for treating MBDs and elucidate their mechanisms from metabolomics, proteomics, and transcriptomics perspectives, as detailed in Table 2. In Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (http://tcmspw.com/tcmsp.php), set the screening criteria to DL ≥ 0.18, OB ≥ 30%, Caco-2 ≥ −0.4 and HL ≥ 4 or HERB (http://herb.ac.cn/Search/), set the screening criteria to ingredient weight≤500 and inquire the drug metabolites of which is shown in Supplementary Table S1.
6.1 Metabolomics
Classic kidney-tonifying botanical drugs (e.g., Rehmannia glutinosa (Gaertn.) Libosch. ex DC., Epimedium brevicornu Maxim., Drynaria roosii Nakaike, and Achyranthes bidentata Blume) maintain bone metabolic balance by regulating steroid hormone biosynthesis, phenylalanine/tyrosine metabolism, glycerophospholipid metabolism, and arachidonic acid metabolism. These botanical drugs upregulate metabolites such as p-coumaric acid, tyrosine, glucose, tagatose, and 2-hydroxycinnamic acid, while downregulating gluconic acid, adenylosuccinic acid, paxilline, ophthalmic acid, glycocyamine, lysine, and biotin (Ping et al., 2022; Huang et al., 2014; Li J. et al., 2024). Eucommia ulmoides Oliv. significantly reverses abnormal expression of glycine, lysine, docosahexaenoic acid, and glucose in the serum of ovariectomized osteoporotic rats by regulating amino acid metabolism and oxidative stress pathways, while restoring tryptophan levels (Fang-Jie et al., 2020). Qiu Yuezhi et al. found that Panax quinquefolius L. effectively improves abnormal expression of osteoblast- and osteoclast-related genes in a GIOP zebrafish model by participating in purine metabolism, the TCA cycle, and the pentose phosphate pathway, modulating 10 key biomarkers (Oiu et al., 2023). Achyranthes bidentata Blume protects against osteoporosis in rats by regulating galactose metabolism, the urea cycle, arginine and proline metabolism, alanine metabolism, lactose degradation, ammonia recycling, and glycine and serine metabolism, promoting the restoration of biomarkers to normal levels (Tao et al., 2019).
6.2 Proteomics
Zeng Jianchun’s team systematically analyzed the mechanism of Eucommia ulmoides Oliv. in inducing osteogenic differentiation of MSCs using two-dimensional electrophoresis. They identified 641 differentially expressed protein spots, demonstrating that Eucommia ulmoides Oliv. Primarily promotes cell differentiation, participates in bone mineralization, and regulates cell proliferation and differentiation through six functional proteins, significantly improving osteoporosis (Zeng et al., 2010). Hui Su et al. used TMT-labeled quantitative proteomics to reveal that Drynaria roosii Nakaike reverses osteoclast differentiation and enhances osteogenic activity by regulating mitochondrial function, RNA and protein receptor signaling, while inhibiting oxidative stress, improving lipid metabolism, and reducing inflammation, forming a dual regulatory mechanism for bone metabolism (Su et al., 2024). Additionally, Liu Yang et al. found that Achyranthes bidentata Blume extract significantly improves bone density and trabecular fractures in GIOP rats, enhances osteoblast differentiation, and inhibits osteoclast-mediated collagen dissolution. TMT-based proteomics identified 93 differentially expressed proteins (61 upregulated and 32 downregulated), primarily related to oxidative phosphorylation pathways, transmembrane transport, exocytosis, cell development, and ATP metabolism. MT-CYB and NDUFA9 were identified as potential biomarkers (Yang et al., 2022).
6.3 Transcriptomics
Shuo Tian et al. discovered that Cistanche deserticola Ma improves bone metabolic disorders in OVX rats by regulating skeletal muscle function, calcium homeostasis, adipogenic-osteogenic capacity, and osteoblast differentiation, potentially related to the myosin superfamily (MYH6, MYH7, MYL1, MYL3) and skeletal muscle (ACTN2, LDB3, CSRP3, MYOZ2, TNNT3, CAV3) (Tian et al., 2023). Salt-processed Eucommia ulmoides Oliv. may improve renal injury and bone microstructure in CKD-MBD mice by affecting the AMPK, cAMP, insulin, and PI3K/Akt signaling pathways, promoting trabecular bone growth and improving femoral tissue morphology (Shen et al., 2023).
These studies demonstrate that various single botanical drugs, despite their differences, can improve bone metabolism by regulating the expression or transcriptional regulation patterns of the same genes, highlighting the metabolomic characteristics of single botanical drugs in treating MBDs.
7 Application of omics technologies in identifying targets of Chinese herbal formula for treating MBD
In TCM theory, formulations are carefully designed based on syndrome differentiation principles. Botanical drugs in a formulation have distinct roles as “monarch,” “minister,” “assistant,” and “guide,” working synergistically or antagonistically to address different aspects of disease progression and exert comprehensive therapeutic effects through multiple pathways and targets.
In the treatment of MBDs, many classic formulations are widely used, such as Du-Huo Ji Sheng Tang, Zuogui Wan, and Er-Xian Tang. These formulations effectively maintain the dynamic balance of bone metabolism by promoting bone mineralization, alleviating clinical symptoms, and significantly improving therapeutic outcomes. Current research on the omics mechanisms of Chinese herbal formula in treating MBDs is summarized in Table 3, providing critical insights into their therapeutic principles and clinical optimization. The drug metabolites of Chinese herbal formula is shown in Supplementary Table S2.
7.1 Metabolomics
Chinese herbal formula primarily regulate bone metabolism by modulating amino acid metabolism and unsaturated fatty acid pathways to enhance osteoblast activity. Gushukang Capsule, a classic anti-OP formula listed in the Chinese Pharmacopoeia, consists of Epimedium brevicornu Maxim., Rehmannia glutinosa (Gaertn.) Libosch. ex DC., Drynaria roosii Nakaike., Astragalus mongholicus Bunge, Salvia miltiorrhiza Bunge., and Auricularia auricula (L.ex Hook.) Underw. This formulation achieves bone metabolic homeostasis through multi-target synergistic regulation: at the protein level, it corrects abnormal expression of key functional proteins such as Cant1 (calcium signaling regulator), Gstz1 (glutathione transferase), Aldh3b1 (aldehyde dehydrogenase), Bid (apoptosis regulator), and Slc1a3 (glutamate transporter); at the metabolite level, it systematically regulates the bioavailability of 12 bone metabolism-related substances, including tyramine, thymidine, deoxycytidine, cytosine, and L-aspartic acid; and by intervening in 11 core metabolic pathways, including purine metabolism, pyrimidine metabolism, histidine metabolism, and β-alanine metabolism, it significantly improves bone density and trabecular structure parameters (thickness, number, and connectivity density) in OVX model rats, validating the scientific basis of the TCM theory “kidney governs bone” and “tonifying the kidney to strengthen bone” (Lin et al., 2022).
DOP, with its multi-system involvement, is traditionally attributed to kidney essence deficiency, spleen dysfunction, and blood stasis, involving energy metabolism imbalance, impaired bone matrix synthesis, and microcirculation abnormalities (Liang and Xiong, 2013). Modern studies show that different Chinese herbal formula can achieve precise intervention through specific metabolic pathways. Huilin Li et al. found that Zishen Jiangtang Pill improves glucose metabolism, bone metabolism, and metabolic disorders in DOP mice by reducing levels of metabolic markers such as acetate, urea, acetone, and citrulline in the blood (Li et al., 2018). Shuang Huang Yi Gu Fang improves bone trabecular structure in DOP patients by upregulating citrulline, valine, betaine, choline, proline, glutamine, O-acetyl glycoprotein, N-acetyl glycoprotein, pyruvate, 1-methylhistidine, and TCA cycle products (α-ketoglutarate, citrate), while downregulating branched-chain amino acids (leucine, isoleucine), glucose, tyrosine, and alanine, thereby regulating bone proteins and treating the disease (Xiong et al., 2014). Wen Shen Tong Luo Zhi Tong Decoction effectively reverses bone loss in steroid-induced osteoporosis model mice by targeting central carbon metabolism, steroidogenesis, cholesterol metabolism, and the TCA cycle, improving bone microstructure and homeostasis, increasing BMD, bone volume/total volume, trabecular number, and trabecular thickness, and reducing trabecular separation (Li et al., 2025).
Chinese herbal formula can also treat MBDs based on syndrome differentiation. Huang Chen et al. used Shen Yang Fang to treat kidney-yang deficiency OP mice, showing that the formulation corrects amino acid and lipid metabolism disorders by significantly regulating 98 metabolites (89 upregulated and 9 downregulated) (Huang C. et al., 2023). Er Zhi Wan improves bone strength and microstructure in kidney-yin deficiency osteoporotic rats by regulating arginine and proline metabolism, glycine, serine, and threonine metabolism, and alanine, aspartate, and glutamate metabolism, while alleviating kidney-yang deficiency symptoms (Shang et al., 2024). Additionally, Gushudan prevents and treats kidney-yang deficiency OP in rats through multiple targets, with 11 differential metabolites, including N,N-dimethylglycine, guanidinoacetic acid, and glycolic acid, identified for the first time (Feng et al., 2022). Yi Guan Jian decoction, a classic formula for liver-kidney yin deficiency, increases BMD, improves bone microstructure, and reverses bone loss in GIOP mice by regulating taurine and hypotaurine metabolism and improving alkaline phosphatase and osteocalcin levels (Yin et al., 2023).
These studies collectively demonstrate that Chinese herbal formula dynamically regulate bone metabolism through synergistic multi-pathway effects, providing new theoretical foundations for precision medicine in MBDs.
7.2 Proteomics
Wang Haibin et al. identified ubiquitin-conjugating enzyme as an estrogen-related protein involved in disease progression and reported the biological functions of thioredoxin peroxidase 1 and myosin light polypeptide 2 as novel osteoporosis-related proteins. Notably, Shengmai Cheng gu Capsule significantly regulates bone metabolism by modulating the expression levels of these proteins (Wang et al., 2006). Gushukang granule improves BMD and regulates bone metabolism in OP model rats by regulating differential proteins and pathways such as tyrosine metabolism, ethanol metabolism, and JAK-STAT signaling (Lin et al., 2021). Liu Bo et al. systematically analyzed the anti-osteoporosis targets of the classic formula Er Xian Tang using iTRAQ isotope labeling combined with NanoLC-LTQ-Orbitrap high-resolution mass spectrometry, validating the dynamic regulation of key differential proteins such as cytochrome b-245 light chain, MAP kinase-1, and RAC1 during TCM intervention (Liu et al., 2019).
7.3 Transcriptomics
Recent advances in transcriptomics have significantly contributed to understanding the mechanisms of Chinese herbal formula in treating osteoporosis. Multiple studies show that traditional Chinese herbal formula regulate bone metabolic balance through multi-pathway synergistic effects. Zhuanggu Zhitong Formula significantly affects the expression of 149 genes in ovariectomized rats, with Nlrp12, Oscar, LOC100359515, and Csf3r playing key roles in osteoblast and osteoclast differentiation, suggesting that the formulation improves osteoporosis by simultaneously regulating osteoblasts and osteoclasts through TNF and IL-17 signaling pathways (Chen et al., 2021). Bajitianwan Formula improves bone microstructure in iron overload-induced osteoporosis by activating the RAGE/PI3K/AKT pathway and delaying oxidative stress (Xu et al., 2024). Notably, kidney-tonifying formulations exhibit multidimensional regulatory advantages: Heng-Gu-Gu-Shang-Yu-He-Ji significantly enhances bone biomechanical properties in OVX rats by activating the PI3K/AKT/mTORC1 pathway through 94 key genes, including Sirt7 and Nog (Xie et al., 2024). Bushen Huoxue decoction increases BMC and density, promotes bone formation, and regulates bone metabolism by downregulating TLR4, MyD88, and NF-κB expression and inhibiting inflammatory cytokines such as IL-6, IL-1β, and TNF-α(96). Bu Shen Jian Pi Huo Xue Formula reshapes bone remodeling balance by regulating 18 differentially expressed genes and over 20 signaling pathways, involving mechanisms such as upregulation of osteoblast proliferation genes and inhibition of osteoclast-related genes (Chen et al., 2008). In terms of immune regulation, Zhang Yu et al. found that Bushen Huatan Recipe regulates bone immunity and inflammation through Toll-like/T cell receptor signaling, TNF/HIF-1 signaling, and osteoclast differentiation pathways, achieving prevention and treatment of PMOP by participating in osteoblast/osteoclast differentiation, proliferation, and apoptosis, providing a reference for treating PMOP from the perspective of phlegm (Zhang et al., 2022). Yuanyuan Zhong et al. discovered that Bu-Shen-Yi-Qi Decoction improves chronic obstructive pulmonary disease-related osteoporosis by upregulating genes related to antioxidant stress and aerobic respiration and activating the MAPK and PI3K/AKT pathways (Zhong et al., 2023). Yiwei decoction, an effective formula for yangming deficiency closely related to OP, reduces gonadotropin-releasing hormone and follicle-stimulating hormone levels, decreases bone loss, promotes osteoblast formation, inhibits osteoclast formation, balances bone metabolism, and increases BMD in rats with ovarian insufficiency-related OP by regulating calcium signaling, growth hormone synthesis, secretion, and action, and the renin-angiotensin system, as well as key targets such as ALB, C3, SNCA, and CLTC (Fan et al., 2023).
These studies collectively reveal the multi-level characteristics of Chinese herbal formula in treating MBDs: (1) bidirectional regulation of bone metabolism through key signaling pathways (e.g., PI3K/AKT, MAPK); (2) reshaping the bone microenvironment by regulating specific gene expression (e.g., Sirt7, Nog); (3) improving the bone immune microenvironment by inhibiting inflammatory cytokine release; and (4) restoring bone metabolic homeostasis by regulating endocrine axis function. These findings provide molecular biological evidence for the modernization of TCM, but further exploration is needed to understand the synergistic mechanisms between different formulations and the spatiotemporal-specific regulation of key targets.
8 Discussion and conclusion
In recent years, with the rapid advancement of omics technologies, metabolomics, proteomics, and transcriptomics have been extensively applied in the research of MBDs. These technologies have comprehensively elucidated the pathogenesis of MBDs at the levels of metabolites, proteins, and genes, and have deeply explored the mechanisms of intervention by TCM monomers, single botanical drugs, and Chinese herbal formula, providing new insights and scientific evidence for the prevention and treatment of MBDs.
8.1 Advantages and disadvantages of TCM in preventing and treating MBD
Omics technologies can comprehensively reflect the multi-metabolite, multi-target, and multi-pathway characteristics of TCM, which aligns with the holistic principles of TCM. Moreover, omics technologies enable researchers to reveal the mechanisms underlying the active metabolites of TCM and their regulatory effects on bone metabolism-related genes, proteins, and metabolites. By leveraging the technical advantages of omics and the therapeutic potential of TCM, researchers can significantly advance the systematic and in-depth analysis of TCM efficacy mechanisms, integrating “molecular networks-pharmacological activities-disease-syndrome effects” at multiple levels. However, TCM emphasizes syndrome differentiation and individualized treatment, while omics research often struggles to fully capture the complexity of personalized therapies. Additionally, it is challenging to correlate omics data analysis results with clinical symptoms and therapeutic outcomes. Therefore, strengthening the integration of omics technologies with TCM is crucial to enhance the clinical relevance and practical utility of data analysis.
8.2 Literature quality assessment
We included high-quality studies published in both Chinese and English, which provide comprehensive details on the materials and methods used in the research process, particularly those pertaining to TCM and its metabolites. Specific aspects, including experimental models, administration methods, dosing schedules, and drug application sites, are thoroughly documented, thereby ensuring the reproducibility and reliability of the research findings. However, most of these studies are confined to animal experiments or in vitro cell models and lack relevant clinical trials. This limitation hinders the further validation of their precise efficacy and potential adverse effects.
8.3 Limitations and prospects
However, current research still faces several limitations: Firstly, limited scope of disease research: existing studies primarily focus on common MBDs such as OP, with less attention paid to relatively rare MBDs like osteomalacia, mucopolysaccharidosis, and Marfan Syndrome. Future research should expand the application of omics technologies to strengthen the study of other types of MBDs. Secondly, insufficient multi-omics integration analysis: current research predominantly employs single omics technologies, making it difficult to fully reveal the complex molecular regulatory networks of MBDs. Future efforts should enhance the combined use of metabolomics, proteomics, and genomics to construct a more comprehensive molecular mechanism map of MBDs. Thirdly, inadequate capacity for big data analysis: The vast amount of data generated by omics studies presents substantial challenges in collection, organization, and analysis, developing efficient and precise data analysis methods, in conjunction with artificial intelligence and machine learning technologies, is crucial for addressing this issue in the future. Lastly, insufficient integration of omics technologies with TCM theory: Future research should focus on how to deeply integrate omics strategies with “TCM theory” and “TCM metabolite compatibility” studies, using modern scientific data to interpret the theories and methods of TCM in preventing and treating MBDs. This will help promote the modernization and internationalization of TCM, providing more comprehensive solutions for the prevention and treatment of MBDs.
In summary, omics technologies have provided powerful tools for the research and prevention of MBDs, but existing challenges must be overcome. In the future, by expanding the scope of research, strengthening multi-omics integration, enhancing data analysis capabilities, and deepening the integration of omics technologies with TCM theory, we will further advance the in-depth development of MBD research and lay a solid foundation for developing more effective prevention and treatment strategies.
Author contributions
WC: Writing – original draft, Writing – review and editing. LJ: Writing – original draft, Writing – review and editing. CZ: Writing – original draft, Writing – review and editing. XZ: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by a grant from the Natural Science Foundation of Jilin Province (YDZJ202401134ZYTS); Science and Technology Research Project of Jilin Provincial Department of Education (JJKH20241061KJ).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1576286/full#supplementary-material
Abbreviations
BMC, bone mineral content; BMD, bone mineral density; DOP, diabetic osteoporosis; FGF23, Fibroblast growth factor 23; GIOP, Glucocorticoid-induced osteoporosis; LC-MS, liquid chromatography-mass spectrometry; MBD, Metabolic bone disease; MSCs, mesenchymal stem cells; OP, Osteoporosis; OVX, Ovariectomized; PMOP, Postmenopausal osteoporosis; TCA, tricarboxylic acid; TCM, traditional Chinese medicine; UPLC/Q-TOF-MS, ultraperformance liquid chromatography/quadrupole time-of-flight.
References
Anonymous, (1993). Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 94 (6), 646–650. doi:10.1016/0002-9343(93)90218-e
Baptista, A. L., Padilha, K., Malagrino, P. A., Venturini, G., Zeri, A. C., Dos Reis, L. M., et al. (2020). Potential biomarkers of the turnover, mineralization, and volume classification: results using NMR metabolomics in hemodialysis patients. JBMR plus 4 (7), e10372. doi:10.1002/jbm4.10372
Bian, Q., Huang, J. H., Yang, Z., Ning, Y., Zhao, Y. J., Wang, Y. J., et al. (2011). Effects of active ingredients in three kidneytonifying Chinese herbal drugs on gene expression profile of bone marrow stromal cells from a rat model of corticosteroneinduced osteoporosis. J. Chin. Integr. Med. 9 (02), 179–185. doi:10.3736/jcim20110211
Buck, A., Prade, V. M., Kunzke, T., Erben, R. G., and Walch, A. (2022). Spatial metabolomics reveals upregulation of several pyrophosphate-producing pathways in cortical bone of Hyp mice. JCI insight 7 (20), e162138. doi:10.1172/jci.insight.162138
Chambers, D. C., Carew, A. M., Lukowski, S. W., and Powell, J. E. (2019). Transcriptomics and single-cell RNA-sequencing. Respirology 24 (1), 29–36. doi:10.1111/resp.13412
Chang, Y. S., Zhu, L. L., Kuang, H. M., Yuan, Y. W., Ye, Z. F., Zhong, X. Y., et al. (2023). Serum metabonomics study of anti-osteoporosis effect of jingangwan in ovariectomized rats. Chin. J. Osteoporos. 29 (02), 179–185+94. doi:10.3969/j.issn.1006-7108.2023.02.005
Chen, H. Q., Yang, S. S., Chen, Y., Yu, J., and Lei, X. M. (2021). Effect of zhuanggu zhitong formula on differential genes expression of OvariectomizedRats with osteoporosis based on transcriptome sequencing. Inf. Traditional Chin. Med. 38 (06), 25–30. doi:10.19656/j.cnki.1002-2406.210605
Chen, M., Li, Y., Lv, H., Yin, P., Zhang, L., and Tang, P. (2020). Quantitative proteomics and reverse engineer analysis identified plasma exosome derived protein markers related to osteoporosis. J. proteomics 228, 103940. doi:10.1016/j.jprot.2020.103940
Chen, X., Liang, Z. J., Shao, M., Wei, G. Q., Han, Q. M., and Zhuang, H. (2008). Regulatory effect of bushen jianpi huoxue formula on signal transduction gene expression in osteoporosis. J. NEW Chin. Med. (03), 60–62. doi:10.13457/j.cnki.jncm.2008.03.043
Chen, Z. B., Zhao, J. L., Hong, S. H., and Tang, H. Y. (2023). Role of the signal axis of NLRP3/caspase-1/GSDMD for preventing and treatingosteoporosis by liuwei dihuang pill decoction in rats. Chinese journal of osteoporosis and. Bone Mineral Res. 16 (06), 559–567. doi:10.3969/j.issn.1674-2591.2023.06.005
Deng, H. B., Wang, Z. Q., Cai, Y. J., Hu, Z. J., Zhang, M., Gong, Y. B., et al. (2022). Progress of proteomics technology application in traditional Chinese medicine syndrome Differentiation,Diagnosis and treatment of malignant tumors. J. Oncol. Chin. Med. 4 (01), 36–42. doi:10.19811/j.cnki.ISSN2096-6628.2022.01.007
Devignes, C. S., Carmeliet, G., and Stegen, S. (2022). Amino acid metabolism in skeletal cells. Bone Rep. 17, 101620. doi:10.1016/j.bonr.2022.101620
Dong, X., Wang, J. Q., Zhang, X. Y., Zhang, Z. Y., Lu, J. K., Gao, J. P., et al. (2023). The improvement effect and metabolic mechanism of Echinops sphaerocephalus extract on osteoporosis model rat. China Pharm. 34 (04), 413–418. doi:10.6039/j.issn.1001-0408.2023.04.06
Fan, J., Jahed, V., and Klavins, K. (2021). Metabolomics in bone research. Metabolites 11 (7), 434. doi:10.3390/metabo11070434
Fan, W., Meng, Y., Zhang, J., Li, M., Zhang, Y., Qu, X., et al. (2023). To investigate the mechanism of Yiwei Decoction in the treatment of premature ovarian insufficiency-related osteoporosis using transcriptomics, network pharmacology and molecular docking techniques. Sci. Rep. 13 (1), 19016. doi:10.1038/s41598-023-45699-8
Fang, Y. Q., Shen, Y., Zhang, Q., Wang, N., Liu, Y. L., Zhu, L. L., et al. (2022). Exploring the mechanism of Jintiange capsule in preventing and treating osteoporosis based on UPLC-Q-TOF-MS metabolomics. Chin. J. Osteoporos. 28 (06), 848–856. doi:10.3969/j.issn.1006-7108.2022.06.013
Feng, Q., Tong, L., Lu, Q., Liu, S., Zhao, L., and Xiong, Z. (2022). (1)H NMR serum metabolomics and its endogenous network pharmacological analysis of Gushudan on kidney-yang-deficiency-syndrome rats. Anal. Biochem. 643, 114580. doi:10.1016/j.ab.2022.114580
Feng, X., and McDonald, J. M. (2011). Disorders of bone remodeling. Annu. Rev. Pathol. 6, 121–145. doi:10.1146/annurev-pathol-011110-130203
Fernandes, M. H., and Gomes, P. S. (2016). Bone cells dynamics during peri-implantitis: a theoretical analysis. J. Oral Maxillofac. Res. 7 (3), e6. doi:10.5037/jomr.2016.7306
Gao, F., Mo, X. M., and Li, J. P. (2013). Analysis of genes expression profiles of lcariin in treating osteoporosis of ovariectomized rats. Chin. J. Inf. TCM 20 (02), 43–45. doi:10.3969/j.issn.1005-5304.2013.02.014
Gong, R., Xiao, H. M., Zhang, Y. H., Zhao, Q., Su, K. J., Lin, X., et al. (2021). Identification and functional characterization of metabolites for bone mass in peri- and postmenopausal Chinese women. J. Clin. Endocrinol. metabolism 106 (8), e3159–e3177. doi:10.1210/clinem/dgab146
Guo, Y. W. L., Liu, Y., Ding, Y. J., Li, Y. X., Zhu, H. M., and Feng, D. H. (2021). Advances in research of canonical Wnt/β ⁃ catenin signaling pathway in bone metabolic diseases. J. Nanjing Med. Univ. Sci. 41 (03), 460–464. doi:10.7655/NYDXBNS20210326
Hart, N. H., Newton, R. U., Tan, J., Rantalainen, T., Chivers, P., Siafarikas, A., et al. (2020). Biological basis of bone strength: anatomy, physiology and measurement. J. Musculoskelet. Neuronal Interact. 20 (3), 347–371.
Hu, K., and Adachi, J. D. (2019). Glucocorticoid induced osteoporosis. Expert Rev. Endocrinol. Metab. 14 (4), 259–266. doi:10.1080/17446651.2019.1617131
Huang, C., Shi, Q., Wang, Y. J., and Tang, D. Z. (2023b). A study of mechanism of Shenyang Fangin treatment of osteoporosis with syndrome of kidney-yang deficiency based on metabonomics technology. J. Trad. Chin. Orthop. Trauma 35 (09), 1–7+29.
Huang, R., Ji, X., and Xiong, D. (2023a). Advances and applications of metabolomics technology in the diagnosis and treatment of malignant tumors. Chin. J. Clin. Lab. Sci. 41 (11), 854–857. doi:10.13602/j.cnki.jcls.2023.11.11
Huang, Y., Liu, X., Zhao, L., Li, F., and Xiong, Z. (2014). Kidney tissue targeted metabolic profiling of glucocorticoid-induced osteoporosis and the proposed therapeutic effects of Rhizoma Drynariae studied using UHPLC/MS/MS. Biomed. Chromatogr. BMC 28 (6), 878–884. doi:10.1002/bmc.3194
Huo, C., Li, Y., Qiao, Z., Shang, Z., Cao, C., Hong, Y., et al. (2019). Comparative proteomics analysis of microvesicles in human serum for the evaluation of osteoporosis. Electrophoresis 40 (14), 1839–1847. doi:10.1002/elps.201900130
Jiang, T., Li, C., Li, Y., Hu, W., Guo, J., Du, X., et al. (2025). Multi-omics and bioinformatics for the investigation of therapeutic mechanism of roucongrong pill against postmenopausal osteoporosis. J. Ethnopharmacol. 337 (Pt 2), 118873. doi:10.1016/j.jep.2024.118873
Jiang, Y. C., Li, Y. F., Zhou, L., and Zhang, D. P. (2020). UPLC-MS metabolomics method provides valuable insights into the effect and underlying mechanisms of Rhizoma Drynariae protecting osteoporosis. J. Chromatogr. B, Anal. Technol. Biomed. life Sci. 1152, 122262. doi:10.1016/j.jchromb.2020.122262
Johnston, C. B., and Dagar, M. (2020). Osteoporosis in older adults. Med. Clin. North Am. 104 (5), 873–884. doi:10.1016/j.mcna.2020.06.004
Kasap, M., Yeğenağa, I., Akpinar, G., Tuncay, M., Aksoy, A., and Karaoz, E. (2015). Comparative proteome analysis of hAT-MSCs isolated from chronic renal failure patients with differences in their bone turnover status. PloS one 10 (11), e0142934. doi:10.1371/journal.pone.0142934
Kou, J., He, C., Cui, L., Zhang, Z., Wang, W., Tan, L., et al. (2022). Discovery of potential biomarkers for postmenopausal osteoporosis based on untargeted GC/LC-MS. Front. Endocrinol. 13, 849076. doi:10.3389/fendo.2022.849076
Lane, N. E. (2019). Glucocorticoid-induced osteoporosis: new insights into the pathophysiology and treatments. Curr. Osteoporos. Rep. 17 (1), 1–7. doi:10.1007/s11914-019-00498-x
Li, B., Wang, Y., Gong, S., Yao, W., Gao, H., Liu, M., et al. (2022b). Puerarin improves OVX-induced osteoporosis by regulating phospholipid metabolism and biosynthesis of unsaturated fatty acids based on serum metabolomics. Phytomedicine Int. J. phytotherapy Phytopharm. 102, 154198. doi:10.1016/j.phymed.2022.154198
Li, C., Lin, X., Lin, Q., Lin, Y., and Lin, H. (2024b). Jiangu granules ameliorate postmenopausal osteoporosis via rectifying bone homeostasis imbalance: a network pharmacology analysis based on multi-omics validation. Phytomedicine Int. J. phytotherapy Phytopharm. 122, 155137. doi:10.1016/j.phymed.2023.155137
Li, H., Chu, S., Zhao, H., Liu, D., Liu, X., Qu, X., et al. (2018). Effect of zishen Jiangtang pill, a Chinese herbal product, on rats with diabetic osteoporosis. Evidence-based complementary Altern. Med. eCAM. 2018, 7201914. doi:10.1155/2018/7201914
Li, J., Chen, X., Lu, L., and Yu, X. (2020). The relationship between bone marrow adipose tissue and bone metabolism in postmenopausal osteoporosis. Cytokine Growth Factor Rev. 52, 88–98. doi:10.1016/j.cytogfr.2020.02.003
Li, J., Zou, Z., Su, X., Xu, P., Du, H., Li, Y., et al. (2024a). Cistanche deserticola improves ovariectomized-induced osteoporosis mainly by regulating lipid metabolism: insights from serum metabolomics using UPLC/Q-TOF-MS. J. Ethnopharmacol. 322, 117570. doi:10.1016/j.jep.2023.117570
Li, M., Niu, Y., Zhang, T., Yang, H., Tian, L., Zhou, S., et al. (2025). Wen-Shen-Tong-Luo-Zhi-Tong-Decoction inhibits bone loss in senile osteoporosis model mice by promoting testosterone production. J. Ethnopharmacol. 338 (Pt 2), 119033. doi:10.1016/j.jep.2024.119033
Li, X., Wang, Y., Gao, M., Bao, B., Cao, Y., Cheng, F., et al. (2022a). Metabolomics-driven of relationships among kidney, bone marrow and bone of rats with postmenopausal osteoporosis. Bone 156, 116306. doi:10.1016/j.bone.2021.116306
Liang, W. D., Huang, P. J., Xiong, L. H., Zhou, S., Ye, R. Y., Liu, J. R., et al. (2020). Metabolomics and its application in the mechanism analysis on diabetic bone metabolic abnormality. Eur. Rev. Med. Pharmacol. Sci. 24 (18), 9591–9600. doi:10.26355/eurrev_202009_23047
Liang, W. D., and Xiong, L. H. (2013). Research on traditional Chinese medicine for diabetic osteoporosis from a metabolomics perspective. Chin. J. Inf. TCM 20 (07), 5–7. doi:10.3969/j.issn.1005-5304.2013.07.003
Lin, R., Xie, B., Xie, L., Ge, J., and Li, S. (2022). Integrated proteomics and metabolomics analysis of lumbar in a rat model of osteoporosis treated with Gushukang capsules. BMC complementary Med. Ther. 22 (1), 333. doi:10.1186/s12906-022-03807-7
Lin, R. H., Chen, S. N., Ye, Y. J., Chen, J., Xie, L. H., Huang, J. W., et al. (2021). Protective mechanism of Gushukang granule in a rat osteoporosis model based on TMT proteomic analysis. Chin. J. Tissue Eng. Res. 25 (32), 5141–5147.
Liu, B., Liu, Z. W., Wu, Q., Xie, H. H., Yang, S., and Wang, Y. (2019). Analysis of the differentially expressed proteins of ovariectomized osteoporosis rats with Er-xian decoction intervention by iTRAQ coupled with NanoLC-LTQ-orbitrap. Traditional Chinese drug research and clinical. Pharmacology 30 (02), 194–199. doi:10.19378/j.issn.1003-9783.2019.02.010
Liu, C. J., Yang, X., Wang, S. H., Wu, X. T., Mao, Y., Shi, J. W., et al. (2023). Preventing disused bone loss through inhibition of advanced glycation end products. Int. J. Mol. Sci. 24 (5), 4953. doi:10.3390/ijms24054953
Liu, D., Ma, L., Zheng, J., Zhang, Z., Zhang, N., Han, Z., et al. (2024). Isopsoralen improves glucocorticoid-induced osteoporosis by regulating purine metabolism and promoting cGMP/PKG pathway-mediated osteoblast differentiation. Curr. drug Metab. 25 (4), 288–297. doi:10.2174/0113892002308141240628071541
Liu, S., Yuan, X., Ma, C., Zhao, J., and Xiong, Z. (2020). (1)H-NMR-based urinary metabolomic analysis for the preventive effects of gushudan on glucocorticoid-induced osteoporosis rats. Anal. Biochem. 610, 113992. doi:10.1016/j.ab.2020.113992
Liu, W. J., Yang, J., Huang, M. Y., Zhang, C. T., Sun, P., and Huang, Y. M. (2021). Serum metabonomics study of anti-osteoporosis effeet of Jiangu granule in ovariectomized rats. Chin. J. Osteoporos. 27 (12), 1820–1826. doi:10.3969/j.issn.1006-7108.2021.12.018
Llorente-Pelayo, S., Docio, P., Arriola, S., Lavin-Gomez, B. A., Garcia-Unzueta, M. T., Ballesteros, M. A., et al. (2024). Role of fibroblast growth factor-23 as an early marker of metabolic bone disease of prematurity. BMC Pediatr. 24 (1), 418. doi:10.1186/s12887-024-04897-7
Mai, W. X., Xie, Y. X., Zhang, Y. L., Zhu, Z. S., and Tang, H. Y. (2023). The mechanism of reinforcing kidney and activating blood decoction in the prevention and treatment of osteoporosis. Chin. J. Osteoporos. 29 (05), 660–664+764.
Mohan, S., and Baylink, D. J. (1996). Insulin-like growth factor system components and the coupling of bone formation to resorption. Horm. Res. 45 (Suppl. 1), 59–62. doi:10.1159/000184833
Oiu, Y. Z., Wang, C. S., Xu, F. H., Zhang, X. M., Wang, L. Z., Li, P. H., et al. (2023). Anti-osteoporosis mechanism of Panax quiquefolium L. based onzebrafish model and metabonomic. Acta Pharm. Sin. 58 (07), 1894–1903. doi:10.16438/j.0513-4870.2022-1293
Pan, S., Chen, A., Han, Z., Wang, Y., Lu, X., and Yang, Y. (2016). (1)H NMR-based metabonomic study on the effects of Epimedium on glucocorticoid-induced osteoporosis. J. Chromatogr. B, Anal. Technol. Biomed. life Sci. 1038, 118–126. doi:10.1016/j.jchromb.2016.10.015
Ping, F., Zhu, L., Shen, X., Han, Q. F., Liu, Y., Yan, Y. G., et al. (2022). Study on the molecular mechanism of Cinnamomi Cortex-Rehmanniae Radix in preventing and treating osteoporosis based on multi-directional regulation of inflammatory factors, estrogen and bone homeostasis. Acta Pharm. Sin. 57 (12), 3644–3652. doi:10.16438/i.0513-4870.2022-0879
Russo, S., Scotto di Carlo, F., and Gianfrancesco, F. (2022). The osteoclast traces the route to bone tumors and metastases. Front. Cell Dev. Biol. 10, 886305. doi:10.3389/fcell.2022.886305
Seemann, L. L., Hanos, C. T., and Pujalte, G. G. A. (2024). Metabolic bone disease. Prim. Care 51 (3), 445–454. doi:10.1016/j.pop.2024.04.005
Shang, G., Zhou, X., Yin, J., Niu, X., Zhao, Y., Li, X., et al. (2024). Multi-omics analysis of kidney, bone and bone marrow explored potential mechanisms of Erzhi Wan against osteoporosis with kidney-Yin deficiency. J. Pharm. Biomed. analysis 246, 116211. doi:10.1016/j.jpba.2024.116211
Shen, J., Liu, Y., Wang, Q., Chen, H., Hu, Y., Guo, X., et al. (2023). Integrated network pharmacology, transcriptomics, and metabolomics analysis to reveal the mechanism of salt Eucommiae cortex in the treatment of chronic kidney disease mineral bone disorders via the PPARG/AMPK signaling pathway. J. Ethnopharmacol. 314, 116590. doi:10.1016/j.jep.2023.116590
Sheng, L. L., Li, X. N., Huo, J. H., Tang, Q. F., Yan, Y., and W, W. M. (2020). Study on mechanism of anti-osteoporosis of soybean meal based on urine metabolomics. Chin. Pharmacol. Bull. 36 (02), 182–190.
Sheu, A., White, C. P., and Center, J. R. (2024). Bone metabolism in diabetes: a clinician's guide to understanding the bone-glucose interplay. Diabetologia 67 (8), 1493–1506. doi:10.1007/s00125-024-06172-x
Sirikul, W., Siri-Angkul, N., Chattipakorn, N., and Chattipakorn, S. C. (2022). Fibroblast growth factor 23 and osteoporosis: evidence from bench to bedside. Int. J. Mol. Sci. 23 (5), 2500. doi:10.3390/ijms23052500
Smith, E. R., McMahon, L. P., and Holt, S. G. (2014). Fibroblast growth factor 23. Ann. Clin. Biochem. 51 (Pt 2), 203–227. doi:10.1177/0004563213510708
Srivastava, R. K., Sapra, L., and Mishra, P. K. (2022). Osteometabolism: metabolic alterations in bone pathologies. Cells 11 (23), 3943. doi:10.3390/cells11233943
Su, H., Yan, B., Wang, R., Li, Z., Xu, Z., Xue, H., et al. (2024). Proteomic analysis based on TMT regarding the therapeutic action of rhizoma drynariae on rats in an osteoporosis model. Comb. Chem. and high throughput Screen. 27 (15), 2223–2238. doi:10.2174/0113862073261905231110061401
Tang, Z. C., Zhao, Y. C., Zhang, R. K., Yan, K., Li, G. J., and Yao, X. M. (2023). The effect of yigu decoction on bone tissue protein expression in osteoporotic rats. Zhejiang Clin. Med. J. 25 (12), 1754–1757.
Tao, Y., Chen, X., Li, W., Cai, B., Di, L., Shi, L., et al. (2017). Global and untargeted metabolomics evidence of the protective effect of different extracts of Dipsacus asper Wall. ex C.B. Clarke on estrogen deficiency after ovariectomia in rats. J. Ethnopharmacol. 199, 20–29. doi:10.1016/j.jep.2017.01.050
Tao, Y., Huang, S., Yan, J., Li, W., and Cai, B. (2019). Integrated metallomic and metabolomic profiling of plasma and tissues provides deep insights into the protective effect of raw and salt-processed Achyranthes bidentata Blume extract in ovariectomia rats. J. Ethnopharmacol. 234, 85–95. doi:10.1016/j.jep.2019.01.033
Tian, S., Guo, L., Song, Y., Miao, J., Peng, M., Fang, X., et al. (2023). Transcriptomic analysis the mechanisms of anti-osteoporosis of desert-living Cistanche herb in ovariectomized rats of postmenopausal osteoporosis. Funct. and Integr. genomics 23 (3), 237. doi:10.1007/s10142-023-01154-5
Torres, C. L., Scalco, F. B., de Oliveira, M. L. C., Peake, R. W. A., and Garrett, R. (2023). Untargeted LC-HRMS metabolomics reveals candidate biomarkers for mucopolysaccharidoses. Clin. Chim. Acta 541, 117250. doi:10.1016/j.cca.2023.117250
Udagawa, N., Kotake, S., Kamatani, N., Takahashi, N., and Suda, T. (2002). The molecular mechanism of osteoclastogenesis in rheumatoid arthritis. Arthritis Res. 4 (5), 281–289. doi:10.1186/ar431
Vlashi, R., Zhang, X., Wu, M., and Chen, G. (2023). Wnt signaling: essential roles in osteoblast differentiation, bone metabolism and therapeutic implications for bone and skeletal disorders. Genes Dis. 10 (4), 1291–1317. doi:10.1016/j.gendis.2022.07.011
Wang, B., Wang, H., Li, Y., and Song, L. (2022). Lipid metabolism within the bone micro-environment is closely associated with bone metabolism in physiological and pathophysiological stages. Lipids Health Dis. 21 (1), 5. doi:10.1186/s12944-021-01615-5
Wang, F. J., Wang, T., Luo, F. M., Zhang, C. X., and Liu, S. (2020). Study on anti-osteoporosis effect of Eucommiae Cortex based on GC-MS metabonomics. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J. Chin. Materia Medica 45 (22), 5555–5560. doi:10.19540/j.cnki.cjcmm.20200624.202
Wang, H., Zhao, Y., Liu, H., Zhang, X., Lv, S., Zhou, T., et al. (2024b). Untargeted metabolomics revealed the mechanism of aucubin on glucocorticoid-induced osteoporosis in mice through modulating arachidonic acid metabolism. J. Pharm. Biomed. analysis 248, 116273. doi:10.1016/j.jpba.2024.116273
Wang, H. B., Liu, J. R., Fan, Y. G., Xiong, L. H., He, W., Peng, X. X., et al. (2006). Bone proteomic analysis about Chinese medicine action on rat ovariectomy model of osteoporosis. J. Sichuan Traditional Chin. Med. (08), 9–13.
Wang, J., Dong, X., Ma, F., Li, C., Bu, R., Lu, J., et al. (2020). Metabolomics profiling reveals Echinops latifolius Tausch improves the trabecular micro-architecture of ovariectomized rats mainly via intervening amino acids and glycerophospholipids metabolism. J. Ethnopharmacol. 260, 113018. doi:10.1016/j.jep.2020.113018
Wang, J., Yan, D., Zhao, A., Hou, X., Zheng, X., Chen, P., et al. (2019). Discovery of potential biomarkers for osteoporosis using LC-MS/MS metabolomic methods. Osteoporos. Int. 30 (7), 1491–1499. doi:10.1007/s00198-019-04892-0
Wang, Q., Shu, B., Zhao, Y. J., and Wang, Y. J. (2016). Wnt/β-catenin signal pathway in renal osteopathy. Chin. J. Osteoporos. 22 (12), 1618–1622+36. doi:10.3969/j.issn.10067108.2016.12.024
Wang, Y., Li, L., Yan, Y., Zhang, T., Hu, L., Chen, J., et al. (2024a). Integration of texture analysis based on DCE-MRI K(trans) map and metabolomics of early bone marrow microvascular changes in alloxan-induced diabetic rabbits. BMC Med. Imaging 24 (1), 247. doi:10.1186/s12880-024-01416-z
Wei, Q., Zhou, Y., Hu, Z., Shi, Y., Ning, Q., Ren, K., et al. (2024). Function-oriented mechanism discovery of coumarins from Psoralea corylifolia L. in the treatment of ovariectomy-induced osteoporosis based on multi-omics analysis. J. Ethnopharmacol. 329, 118130. doi:10.1016/j.jep.2024.118130
Weinstein, R. S. (2012). Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol. Metab. Clin. North Am. 41 (3), 595–611. doi:10.1016/j.ecl.2012.04.004
Wu, K., Wang, P., Deng, L., Li, Y., Zhang, Q., Hou, H., et al. (2024). Analysis of bone metabolic alterations linked with osteoporosis progression in type 2 diabetic db/db mice. Exp. Gerontol. 185, 112347. doi:10.1016/j.exger.2023.112347
Wu, Q., Lai, X., Zhu, Z., Hong, Z., Dong, X., Wang, T., et al. (2015). Evidence for chronic kidney disease-mineral and bone disorder associated with metabolic pathway changes. Medicine 94 (32), e1273. doi:10.1097/md.0000000000001273
Wu, X., Fan, Y., Ye, Y., Li, P., Zhu, Q., Chen, Z., et al. (2023a). A transcriptomic study of osteoporosis induced by ketogenic diet in mice. Nan fang yi ke da xue xue bao = J. South. Med. Univ. 43 (8), 1440–1446. doi:10.12122/j.issn.1673-4254.2023.08.23
Wu, Y., Yuan, C., Han, P., Guo, J., Wang, Y., Chen, C., et al. (2023b). Discovery of potential biomarkers for osteoporosis using LC/GC-MS metabolomic methods. Front. Endocrinol. 14, 1332216. doi:10.3389/fendo.2023.1332216
Xia, T., Dong, X., Jiang, Y., Lin, L., Dong, Z., Shen, Y., et al. (2019b). Metabolomics profiling reveals rehmanniae radix preparata extract protects against glucocorticoid-induced osteoporosis mainly via intervening steroid hormone biosynthesis. Mol. Basel, Switz. 24 (2), 253. doi:10.3390/molecules24020253
Xia, T., Dong, X., Lin, L., Jiang, Y., Ma, X., Xin, H., et al. (2019a). Metabolomics profiling provides valuable insights into the underlying mechanisms of Morinda officinalis on protecting glucocorticoid-induced osteoporosis. J. Pharm. Biomed. analysis 166, 336–346. doi:10.1016/j.jpba.2019.01.019
Xian, Y., Nong, Y., Gao, Y., Su, Y., Lei, Z., Lian, H., et al. (2023). UPLC/Q-TOF-MS-based metabolomics evaluate the efficacy of oroxylin A against postmenopausal osteoporosis. Biomed. Chromatogr. Bmc. 37 (5), e5609. doi:10.1002/bmc.5609
Xiao, H. H., Sham, T. T., Chan, C. O., Li, M. H., Chen, X., Wu, Q. C., et al. (2018). A metabolomics study on the bone protective effects of a lignan-rich fraction from Sambucus williamsii ramulus in aged rats. Front. Pharmacol. 9, 932. doi:10.3389/fphar.2018.00932
Xie, L., Song, X., Lei, L., Chen, C., Zhao, H., Hu, J., et al. (2024). Exploring the potential mechanism of Heng-Gu-Gu-Shang-Yu-He-Ji therapy for osteoporosis based on network pharmacology and transcriptomics. J. Ethnopharmacol. 321, 117480. doi:10.1016/j.jep.2023.117480
Xiong, L. H., Liang, W. D., Ye, R. Y., Zhou, S., Lai, R. Y., and Liu, J. R. (2014). Metabonomic study on the therapeutic mechanism of diabetic osteoporosis with shuanghuangyigu Recipe. Guid. J. Traditional Chin. Med. Pharm. 20 (10), 18–22. doi:10.13862/j.cnki.cn43-1446/r.2014.10.006
Xu, W., Jiang, T., Ding, L., Jiang, Y., Zhang, L., Xia, T., et al. (2024). Bajitianwan formula extract ameliorates bone loss induced by iron overload via activating RAGE/PI3K/AKT pathway based on network pharmacology and transcriptomic analysis. J. Nat. Med. 78 (3), 488–504. doi:10.1007/s11418-024-01779-1
Xue, L., Jiang, Y., Han, T., Zhang, N., Qin, L., Xin, H., et al. (2016). Comparative proteomic and metabolomic analysis reveal the antiosteoporotic molecular mechanism of icariin from Epimedium brevicornu maxim. J. Ethnopharmacol. 192, 370–381. doi:10.1016/j.jep.2016.07.037
Yang, J., Mo, X. M., and Li, J. P. (2012). Proteomic analysis of zhuanggu zhitong formula in treating ovariectomy-induced osteoporosis in rats. Chin. J. Basic Med. Traditional Chin. Med. 18 (02), 166–168. doi:10.19945/j.cnki.issn.1006-3250.2012.02.022
Yang, L., Hou, A., Zhang, X., Zhang, J., Wang, S., Dong, J., et al. (2022). TMT-based proteomics analysis to screen potential biomarkers of Achyranthis Bidentatae Radix for osteoporosis in rats. Biomed. Chromatogr. BMC 36 (4), e5339. doi:10.1002/bmc.5339
Yin, H., Zhu, T. C., Zhang, Y. F., and Wang, J. W. (2022). Metabolomics-based screening for potential serum biomarkers of kidney yang defi ciency syndrome in osteoporosis patients: a pilot study. Asian J. Surg. 45 (11), 2494–2495. doi:10.1016/j.asjsur.2022.05.118
Yin, M., Zhou, D., Jia, F., Su, X., Li, X., Sun, R., et al. (2023). Metabolomics analysis of the potential mechanism of Yi-Guan-Jian decoction to reverse bone loss in glucocorticoid-induced osteoporosis. J. Orthop. Surg. Res. 18 (1), 409. doi:10.1186/s13018-023-03778-6
Ying, X., Jin, X., Wang, P., He, Y., Zhang, H., Ren, X., et al. (2020). Integrative analysis for elucidating transcriptomics landscapes of glucocorticoid-induced osteoporosis. Front. Cell Dev. Biol. 8, 252. doi:10.3389/fcell.2020.00252
Yuan, X., Meng, Y., Liang, S., Jiang, W., Ge, S., Duan, J., et al. (2017). Analysis ofurinary polypeptide pattern of Mucopolysaccharidosislby magnetic beads based weakcation exchange separation technology and matrix-assisted laser desorption-inoizationtime of flight mass spectrometry technology. Life Sci. Instrum. 15 (01), 36–39+5. doi:10.11967/2017150108
Yuan, X., Wen, J., Jia, H., Tong, L., Zhao, J., Zhao, L., et al. (2020). Integrated metabolomic analysis for intervention effects of Gushudan on glucocorticoid-induced osteoporostic rat plasma based on RP/HILIC-UHPLC-Q-Orbitrap HRMS. Anal. Biochem. 591, 113559. doi:10.1016/j.ab.2019.113559
Zeng, J. C., Fan, Y. G., Liu, J. R., Yi, C. Z., and Yan, L. (2010). Study on proteame change during the process of MSCs differentiation to Osteoblas Guiding by blood serum containing Duzhong (Cortex Eucommiae). Lishizhen Med. Materia Medica Res. 21 (02), 274–277.
Zhang, J., Hu, W., Zou, Z., Li, Y., Kang, F., Li, J., et al. (2024). The role of lipid metabolism in osteoporosis: clinical implication and cellular mechanism. Genes Dis. 11 (4), 101122. doi:10.1016/j.gendis.2023.101122
Zhang, M., Wang, Y., Zhang, Q., Wang, C., Zhang, D., Wan, J. B., et al. (2018). UPLC/Q-TOF-MS-based metabolomics study of the anti-osteoporosis effects of Achyranthes bidentata polysaccharides in ovariectomized rats. Int. J. Biol. Macromol. 112, 433–441. doi:10.1016/j.ijbiomac.2018.01.204
Zhang, Y., Li, Y., Zhang, J., Liu, M. J., Zhao, H. Y., Pan, J. H., et al. (2016). Action mechanism of Zuogui Pill in ovariectomized - induced osteoporotic rats based on proteomics techniques. Hebei J. TCM. 38 (07), 1024–1028+33. doi:10.3969/j.issn.1002-2619.2016.07.018
Zhang, Y., Zhang, F. X., Zhang, H., Xue, Y. J., Zhou, G. W., Zhang, Y., et al. (2022). The mechanism of Bushen Huatan Recipe on preventing postmenopausal osteoporosis based on multi-omics techniques. J. Hubei Univ. Chin. Med. 24 (05), 48–53. doi:10.3969/j.issn.1008-987x.2022.05.11
Zhao, H., Li, X., Zhang, D., Chen, H., Chao, Y., Wu, K., et al. (2018). Integrative bone metabolomics-lipidomics strategy for pathological mechanism of postmenopausal osteoporosis mouse model. Sci. Rep. 8 (1), 16456. doi:10.1038/s41598-018-34574-6
Zhong, Y., Wang, B., Chen, W., Zhang, H., Sun, J., and Dong, J. (2023). Exploring the mechanisms of modified bu-shen-yi-qi decoction for COPD-related osteoporosis therapy via transcriptomics and network pharmacology approach. Drug Des. Dev. Ther. 17, 2727–2745. doi:10.2147/dddt.S413532
Zhou, J., Zhou, F., Yang, L., Liang, H., Zhu, Q., Guo, F., et al. (2024). Morinda officinalis saponins promote osteogenic differentiation of human umbilical cord-derived mesenchymal stem cells via the BMP-SMAD signaling pathway. Am. J. Transl. Res. 16 (10), 5441–5453. doi:10.62347/KNRS3234
Keywords: metabolic bone disease, traditional Chinese medicine (TCM), metabolomics, proteomics, transcriptomics
Citation: Cai W, Jiang L, Zhao C and Zhou X (2025) Advances in omics technologies for traditional Chinese medicine in the prevention and treatment of metabolic bone diseases. Front. Pharmacol. 16:1576286. doi: 10.3389/fphar.2025.1576286
Received: 13 February 2025; Accepted: 25 March 2025;
Published: 11 April 2025.
Edited by:
Jiankun Xu, The Chinese University of Hong Kong, ChinaReviewed by:
Feng Zhang, Nanjing University of Chinese Medicine, ChinaHongting Jin, Zhejiang Chinese Medical University, China
Puwei Yuan, Shaanxi University of Chinese Medicine, China
Mingwang Zhou, Gansu Provincial Hospital of TCM, China
Copyright © 2025 Cai, Jiang, Zhao and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changwei Zhao, emN3XzE5ODBAMTI2LmNvbQ==; Xiaoling Zhou, enhsMTk4MDAxMTBAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Wenjun Cai1,2†
Wenjun Cai1,2† Changwei Zhao
Changwei Zhao