- 1School of Traditional Chinese Medicine, Hunan University of Chinese Medicine, Changsha, China
- 2Hunan Key Laboratory of Traditional Chinese Medicine Prescription and Syndromes Translational Medicine, Hunan University of Chinese Medicine, Changsha, China
Context: Autophagy plays a pivotal role in the pathogenesis of DKD, and the mechanistic target of rapamycin (mTOR) pathway, which regulates nutrient sensing and oxidative stress responses, is a key regulator of autophagy. Traditional Chinese Medicine (TCM) has garnered attention for its potential to treat DKD by modulating the mTOR signaling pathway, reducing oxidative stress, and restoring autophagic function.
Objective: The objective of this study is to examine how mTOR-mediated regulation of nutrient sensing and oxidative stress impacts autophagy in DKD, and to explore how TCM modulates these pathways to improve the condition.
Methods: A systematic review was conducted using PubMed, Web of Science, Wanfang Data, and China National Knowledge Infrastructure (CNKI), with the search extended to December 2024. The search subject terms included ‘diabetic kidney disease,’ ‘Traditional Chinese Medicine,’ ‘mTOR,’ ‘nutrient sensing,’ and ‘oxidative stress.’ Studies were rigorously screened by two investigators.
Results: This review systematically examines the pathogenesis of mTOR-mediated nutrient sensing dysfunction and oxidative stress in DKD, highlighting their impact on autophagy. It further clarifies how these mechanisms are targeted by Chinese medicine in the treatment of DKD. The review summarizes the potential mechanisms by which TCM, including monomers (e.g., Astragaloside IV), individual botanical drugs (e.g., Dendrobium nobile Lindl.), and compound formulations (e.g., Tongluo Digui Decoction), regulate autophagy in DKD through pathways such as AMP-activated protein kinase (AMPK), mTOR, sirtuins (Sirt), and the phosphatidylinositol three kinase (PI3K)/Akt/mTOR signaling pathway. TCM compound formulas share a common foundational framework, with the majority being formulated based on therapeutic principles such as ‘Yiqi’, ‘Yangyin’, ‘Tongluo’, and ‘Huashi’.
Conclusion: TCM shows promise in treating DKD, with unique advantages in modulating key signaling pathways. However, the underlying mechanisms remain complex and warrant further investigation.
1 Introduction
Diabetic kidney disease (DKD) is one of the most prevalent microvascular complications in diabetic patients and a leading cause of end-stage renal disease (ESRD) (Młynarska et al., 2024). The hallmark pathological features of DKD include thickening of the glomerular basement membrane, renal tubulointerstitial fibrosis, and proteinuria. The treatment options for advanced DKD remain limited, underscoring the urgent need for novel therapeutic strategies (Zoja et al., 2020). Recent studies have shown that autophagy is impaired in the renal cells of patients with DKD (Han et al., 2023), with dysfunction exacerbated under pathological conditions such as hyperglycemia and oxidative stress (Parmar et al., 2022; Zhang et al., 2024).
Autophagy is a cellular process that degrades cytoplasmic proteins and damaged organelles through lysosomal activity, under the regulation of autophagy-related genes (Atg). There are two main types of autophagy: nonselective autophagy, which indiscriminately isolates and degrades parts of the cytoplasm (including organelles and macromolecular complexes), and selective autophagy, which targets specific, often harmful, substrates for degradation (Vargas et al., 2022). Selective autophagy is initiated by the ubiquitination of cargo, which binds to specific receptor proteins such as p62, TAX1BP1, NDP52, NBR1, optineurin, etc., (Johansen and Lamark, 2020). These receptors facilitate the core autophagy process by interacting with ubiquitin-like proteins (UBLs) from the Atg8/LC3/GABARAP family and by directly interacting with Atg11 (in yeast) or FIP200 (in mammals) (Kirkin and Rogov, 2019). Functionally, various autophagy-related proteins regulate different stages of the autophagic pathway. In mammalian cells, autophagy is primarily controlled by approximately 20 core ATG proteins and is tightly regulated by the mechanistic target of rapamycin (mTOR) signaling pathway (Zhang Y. et al., 2022).
Autophagy is closely associated with the onset and progression of DKD. In DKD, an imbalance between protein synthesis and degradation leads to protein accumulation, which exacerbates glomerular hypertrophy and further renal damage, thereby promoting the development of DKD (Dronavalli et al., 2008). Autophagy plays a crucial role in protein degradation, and defects in autophagy caused by diabetes may significantly contribute to the initiation and progression of DKD. Various renal cells involved in glomerular filtration—such as podocytes, mesangial cells, endothelial cells, and renal tubular epithelial cells—are all closely linked to autophagy. Autophagy in these cells plays a protective role in the kidney and is considered a key target in DKD (Zhang Z. et al., 2022). Among these cells, glomerular podocytes exhibit higher basal autophagy levels (Liu et al., 2017), making them a major focus of research (Audzeyenka et al., 2022). Studies have shown that knockout of podocyte-specific autophagy-related genes (ATG5) in mice rapidly induces proteinuria, suggesting that basal autophagy is essential for maintaining podocyte function (Hartleben et al., 2010; Tang et al., 2020). Additionally, Remah et al. (Yassin et al., 2021) also found that the expression of autophagy markers, including ATG5 and LC3B, was downregulated in peripheral blood monocytes from DKD patients and in kidney tissues of DKD mice. However, some studies suggest that excessive autophagy may have detrimental effects on kidney function. Continuous activation of autophagy may not always provide protective benefits and could worsen podocyte injury (Lenoir et al., 2015; Yu et al., 2018). Therefore, maintaining a balance in autophagy is essential for the proper physiological function of the glomeruli.
Malnutrition and metabolic disorders are significant contributors to DKD progression. mTOR is a protein kinase complex that exists in two main forms: mTORC1 and mTORC2. Of these, mTORC1 is the most critical, regulating cell growth, metabolism, proliferation, and autophagy through downstream effector molecules. mTORC1 plays a central role in nutrient sensing. The development of DKD is closely linked to disturbances in glucose metabolism, which can alter mTORC1 activity and, consequently, affect autophagic processes. mTORC2 primarily regulates cytoskeletal reorganization, cell survival, and metabolic homeostasis. Although Akt signaling plays a critical role in insulin resistance, the role of mTORC2 in the pathological processes of diabetic nephropathy is more indirect, and its activation level is likely weaker compared to mTORC1.
Oxidative stress is another critical factor in the progression of DKD. Hyperglycemia-induced oxidative stress damages renal cells, promoting inflammation and fibrosis. mTOR’s role in oxidative stress regulation is multifaceted. First, oxidative stress can activate mTOR. Reactive oxygen species (ROS) signaling can stimulate mTORC1 activation. In the context of DKD, chronic oxidative stress may lead to the over-activation of mTORC1, which further inhibits autophagy, exacerbating cellular damage. At the same time, mTOR itself can suppress the autophagic response to oxidative stress. mTORC1 inhibits autophagy through downstream targets such as ULK1, reducing the cell’s ability to clear damaged components. As a result, cells become more vulnerable to oxidative damage, and the inability to repair damaged cells via autophagy accelerates the deterioration of DKD.
In recent years, increasing attention has been focused on enhancing autophagy in DKD through modulation of the mTOR-mediated nutrient sensing and oxidative stress pathways (Tang et al., 2021). The inherent complexity of DKD, poses significant limitations to conventional therapeutic strategies reliant on singular molecular targeting. In this context, TCM has emerged as a promising alternative, leveraging its multi-target regulatory networks (e.g., mTOR pathway modulation, oxidative stress mitigation, and autophagic flux restoration) to address systemic imbalances in DKD progression. For example, active metabolites such as berberine and Astragaloside IV have been shown to alleviate glomerular and tubular damage by restoring the balance of the mTOR pathway and enhancing autophagic activity. Given these findings, exploring the mechanisms by which Traditional Chinese Medicine (TCM) regulates the mTOR signaling pathway offers promising potential for the development of new therapeutic strategies for DKD (Xue et al., 2024). This review systematically summarizes research on how TCM can improve autophagy through mTOR-mediated nutrient sensing and oxidative stress pathways. It aims to analyze the therapeutic potential of TCM in DKD and propose new avenues for future treatment.
2 The mechanism of autophagy regulated by mTOR-mediated nutrient sensing and oxidative stress in DKD
2.1 mTOR-mediated autophagy and DKD
In the context of DKD, mTOR expression has garnered significant attention, largely due to its role in dysregulated autophagy. Specifically, the overactivation of the mTOR signaling pathway, a known negative regulator of autophagy, is observed in podocytes in DKD (Leventhal et al., 2017). Numerous studies have demonstrated elevated mTOR activity in db/db mice, streptozotocin (STZ)-induced diabetic rats, and DKD patients (Sakai et al., 2019; Song et al., 2019). Given that autophagy is essential for maintaining podocyte homeostasis, it has been found that inhibiting the mTOR pathway using rapamycin enhances autophagy and reduces podocyte injury (Xiao et al., 2014). Multi-cohort (CRIC/SMART2D/American Indian) studies established UAdCR as a mechanistic ESKD biomarker; proximal tubule mTOR signaling underlies normoalbuminuric pathology (Sharma et al., 2023).Dysregulated autophagy drives diabetic kidney disease (DKD) progression. Clinical studies show reduced p-mTOR/mTOR in DKD (Xiong et al., 2019), while podocyte mTORC1 hyperactivation in obesity CKD exacerbates renal dysfunction (Iwata et al., 2020).
TCM has been shown to inhibit the mTOR pathway, promote autophagy, protect renal function, and reduce renal fibrosis (Xuan et al., 2021). For instance, Akt and AMPK signaling pathways activate mTOR, inhibiting autophagy. Conversely, activation of mTOR by AMPK and p53 signaling can promote autophagy. The pathogenesis of autophagy dysfunction in DKD is linked to the inhibition of AMPK and SIRT1 and the subsequent activation of mTOR (Ren et al., 2020), as well as the regulation of the PI3K/Akt/mTOR pathways (Tang et al., 2022).
It is important to note that autophagy is not a simple binary process, but rather a dynamic and finely regulated mechanism. The regulation of autophagy in the kidney must strike a balance: insufficient autophagy can lead to the accumulation of harmful substances, while excessive autophagy may impose a detrimental burden on cells. Furthermore, the regulatory effects of TCM on mTOR-related pathways in the context of DKD warrant further exploration and investigation.
2.2 Studies on the mechanism of autophagy regulated by the mTOR-mediated nutrient sensing pathway in DKD
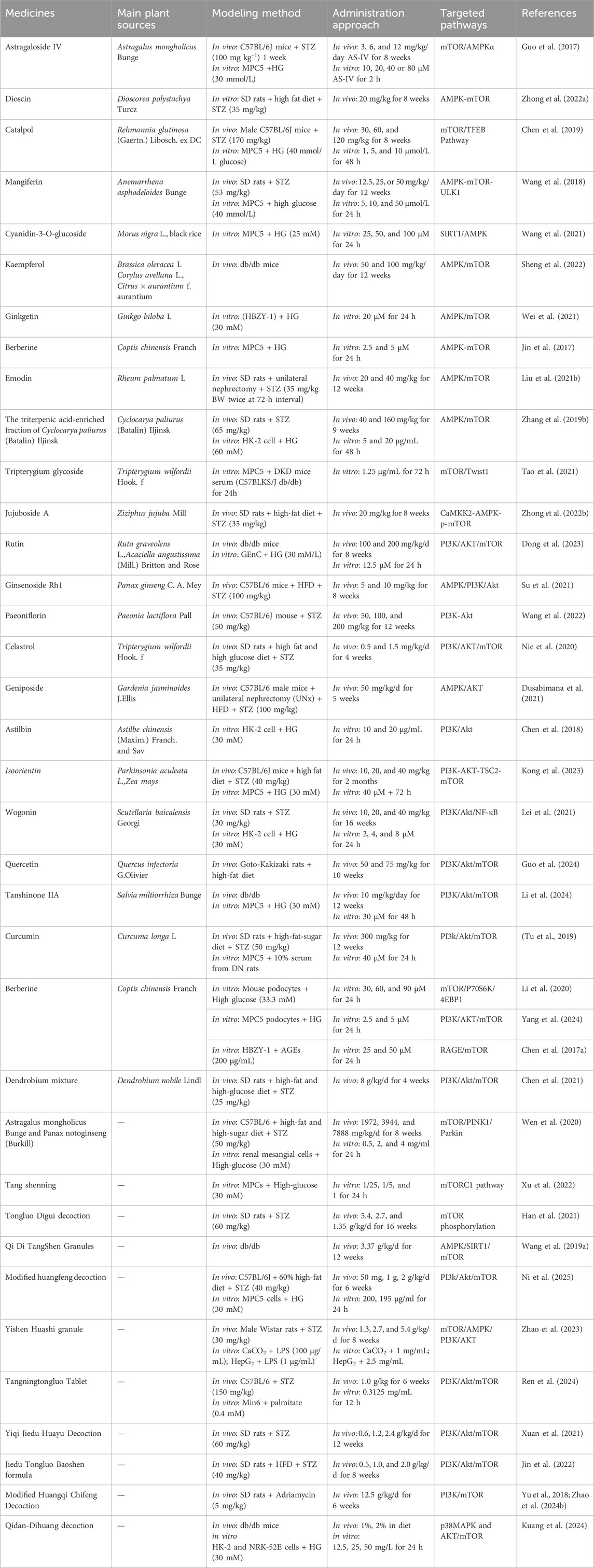
Nutrient sensing pathways, including those involving AMPK, mTOR, and Sirt1, have been widely recognized for their roles in regulating autophagy in diabetic complications (Parmar et al., 2022). In general, proper regulation of AMPK, SIRT1, and mTOR activities—especially enhancing AMPK and SIRT1 function while inhibiting mTOR signaling—could help restore or enhance autophagic activity, potentially mitigating the pathological changes associated with DKD. The crosstalk of the trophic pathways is shown in Figure 1.
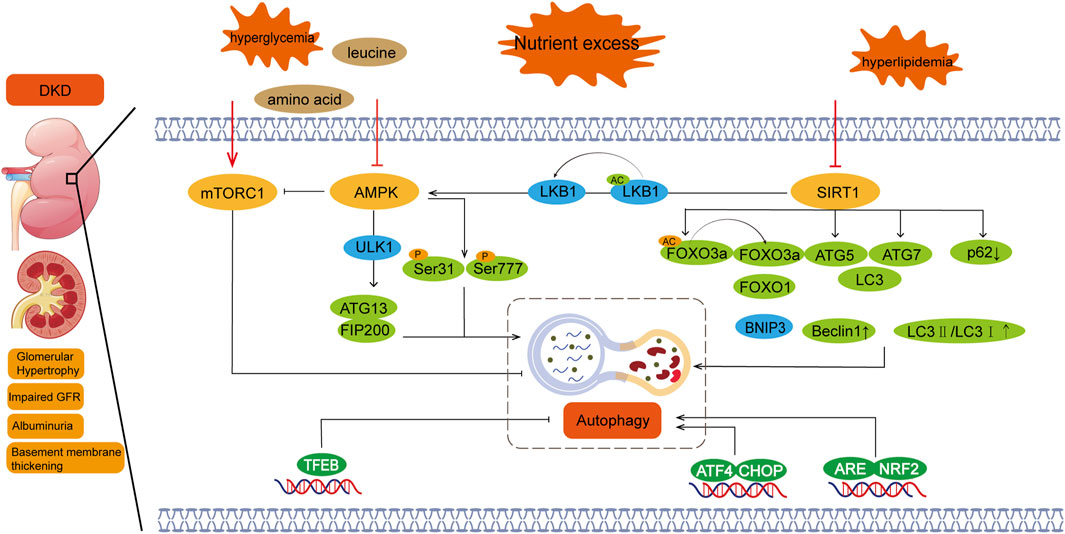
Figure 1. Nutrient sensing pathway and autophagy in diabetic nephropathy. Regulation of autophagy involves three major nutrition-sensing pathways: mTOR, AMPK, and SIRT1 signaling pathways. Excessive nutritional conditions, such as hyperglycemia, activate mTOR and inhibit AMPK and SIRT1. There is also crosstalk between these pathways, with SIRT1 activating AMPK and AMPK inhibiting mTOR. mTORC1:Mechanistic target of rapamycin complex 1; AMPK:Adenosine 5‘-monophosphate (AMP)-activated protein kinase; SIRT1:Silent information regulator 1; DKD:Diabetic kidney disease.
mTORC1 regulates autophagy through nutrient-sensing signals, such as amino acids, glucose, and energy status. The development of DKD is closely linked to disturbances in glucose metabolism, which can lead to abnormal activation of mTORC1 and inhibition of autophagy, thereby exacerbating kidney damage (Hinden et al., 2022). In the nutrient sensing pathway, amino acids, particularly leucine, are potent activators of mTORC1 (Zhao et al., 2024a). In diabetic patients, metabolic abnormalities can lead to alterations in amino acid signaling pathways, which, in turn, affect mTORC1 activity and disrupt the autophagy process (Corsetti et al., 2024; Singh et al., 2024). Additionally, HG levels activate mTORC1 through various mechanisms, including the PI3K/Akt signaling pathway. Overactivation of mTORC1 inhibits autophagy, impairing the kidney’s ability to clear accumulated damaged proteins and thus promoting the progression of DKD (Wang H. et al., 2024). From the perspective of energy sensing, AMPK acts as a sensor of cellular energy status. Under low energy conditions, AMPK inhibits mTORC1 activity and activates autophagy to help cells restore energy balance (Deng et al., 2024; Huynh et al., 2023). In DKD, however, AMPK activity may be inhibited, disrupting the regulation of mTORC1 and impairing autophagic function (Oza et al., 2021).
AMPK is an energy-sensing enzyme that plays a pivotal role in initiating autophagy by directly activating ULK1/2 (autophagy-initiating kinases 1/2). Upon activation, AMPK can directly phosphorylate ULK1 or engage in crosstalk with mTOR to induce autophagy (Haq et al., 2019). When mTOR is inhibited by rapamycin, the interaction between ULK1 and AMPK is enhanced, and ULK1 phosphorylation by AMPK increases. This complex crosstalk and feedback between these interconnected proteins fine-tune the autophagic response under metabolic stress conditions (Hou et al., 2023).
In hyperglycemic states, AMPK activity is typically inhibited, which reduces its ability to promote autophagy (Wang et al., 2020). AMPK acts as a central mediator in cellular responses to energy stress and mitochondrial damage, regulating various aspects of autophagy and mitochondrial function (Herzig and Shaw, 2018). As a positive regulator of autophagy, AMPK levels are found to be decreased in DKD. Inhibition of AMPK prevents the ULK1-ATg13-FIP200 complex from inhibiting autophagy, since the ULK1-FIP200 complex is the initiator of autophagy through ULK1 phosphorylation.
In DKD, TCM exerts a renoprotective effect by inhibiting apoptosis and enhancing podocyte autophagy through the AMPK/mTOR signaling pathway (Liu H. et al., 2021). By activating the AMPK/mTOR-mediated autophagy pathway, TCM can reduce mesangial cell proliferation, oxidative stress, inflammation, and ECM accumulation. This regulation of glomerular mesangial cell dysfunction helps alleviate the progression of DKD. Targeting AMPK in TCM represents a promising therapeutic strategy for DKD (Wei et al., 2021). In clinical studies, AMPK signaling has been shown to play a crucial role in improving DKD. To exert its protective effects, AMPK signaling interacts with other molecular pathways, including PGC-1α, PI3K/Akt, NOX4, and NF-κB (Entezari et al., 2022).
Sirt1 is another key regulator in the autophagy process, particularly in metabolic diseases such as DKD (Cantó et al., 2009; Kitada et al., 2017; Wang W. et al., 2019). Sirt1 is a potent positive regulator of autophagy, but its expression is often suppressed in the kidneys of various animal models of diabetes. Sirt1 regulates autophagy-related gene expression by deacetylating the transcription factor FOXO, and increase the expression of BNIP3,thereby promoting autophagy and reducing cell damage in DKD (Han et al., 2023). SIRT1 also increased the levels of Beclin1, ATG5, ATG7 and LC3, increased the ratio of LC3II/LC3I and decreased p62.Additionally, Sirt1 interacts with AMPK and mTOR. The AMPK/Sirt1 axis plays a protective role in DKD by not only restoring autophagic function but also maintaining mitochondrial health (Siddhi et al., 2022). In type 2 diabetic patients, inhibition of Sirt1 and AMPK significantly reduces autophagy levels in glomerular podocytes and renal tubules, which can increase susceptibility to kidney injury. Conversely, under conditions of nutrient deprivation and hypoxia, the AMPK/Sirt1 axis, along with hypoxia-inducible factors (HIF-1α and HIF-2α), drives autophagy and protects the kidney by restoring autophagy, regulating sodium transport, and modulating inflammatory pathways (Jin et al., 2023; Liu H. et al., 2021; Packer, 2020). Therefore, Sirt1 agonists and bromodomain inhibitors may emerge as therapeutic strategies for diabetic nephropathy and could potentially offer new treatment directions for other organ damage caused by diabetes (Zhong et al., 2018).
AMPK/SIRT1/mTOR signaling exhibits stage-specific regulation in DKD. Early-stage compensatory autophagy is maintained through AMPK-mediated ULK1 phosphorylation at Ser317 and SIRT1’s dual deacetylation of LKB1/TSC2, forming a protective loop that suppresses mTORC1 (Yuan et al., 2023). In advanced stages, NAD + depletion inactivates SIRT1, impairing AMPK activation and unleashing mTORC1-driven ULK1 hyperphosphorylation at Ser757, culminating in autophagic failure (Yau et al., 2019). This regulatory shift underlies the pathological transition from adaptive autophagy to proteotoxic stress (Suda et al., 2019).
2.3 Study on the mechanism of autophagy regulated by mTOR-mediated oxidative stress pathway in DKD
Oxidative stress refers to cellular damage caused by the accumulation of ROS, while autophagy is a cellular mechanism that helps cells respond to various stressors by degrading and recycling damaged components to maintain survival. The relationship between oxidative stress and autophagy is complex (Sherkhane et al., 2023). Under normal conditions, moderate levels of ROS can promote autophagy to maintain cellular homeostasis. However, in DKD, when cellular damage, such as oxidative stress and protein accumulation, begins to occur, ROS can act as signaling molecules that induce autophagy through oxidative modifications of proteins (Tang et al., 2020). In this context, autophagy serves a protective role by repairing damaged organelles and proteins, thus helping to maintain kidney function and structure (Jaikumkao et al., 2024).
Oxidative stress and DKD are closely linked in the pathogenesis of the disease (Ma F. et al., 2023). In certain stages of DKD or under pathological conditions such as hyperglycemia and hyperlipidemia, excessive ROS production can overwhelm the antioxidant defense capacity of cells, leading to impaired autophagy and extensive damage to cellular structures and functions. However, under conditions of persistent oxidative stress, autophagy can become dysregulated and over-activated, leading to autophagic cell death. Therefore, it is essential to restore autophagic homeostasis by inhibiting both oxidative stress and excessive autophagy under these conditions (Dusabimana et al., 2021; Liu et al., 2025).
Since mTORC1 plays a central role in cellular metabolism, ROS can influence the overall metabolic state of cells by regulating mTORC1 activity (Yasuda-Yamahara et al., 2021). Therapeutically, the inhibition of mTORC1 activity serves as a primary approach. Studies have demonstrated that mTOR inhibitors, such as rapamycin, can reduce mTORC1 activity, thereby promoting autophagy, reducing oxidative stress and inflammation, and alleviating the progression of DKD (Lin et al., 2021). Second, mTORC1 activity can be modulated indirectly by regulating AMPK and Akt signaling pathways, which further influence autophagy. For example, mTORC1 activity is affected by inhibiting the PI3K/Akt signaling pathway (Kma and Baruah, 2022). Akt, under normal conditions, promotes cell survival and proliferation by activating mTORC1. However, its inhibition leads to an increase in autophagic activity (Liu et al., 2020). Thus, the PI3K/Akt/mTOR axis represents a key pathway for regulating oxidative stress and autophagy. Third, the activation of mTORC1 driven by oxidative stress can be mitigated by antioxidant treatments aimed at reducing ROS generation or enhancing the antioxidant capacity of cells (Zhou et al., 2024). For example, the use of antioxidants such as N-acetyl-L-cysteine and vitamin C has been shown to reduce oxidative stress in DKD (Ma X. et al., 2023). A demonstration of these signaling pathways is shown in Figure 2.
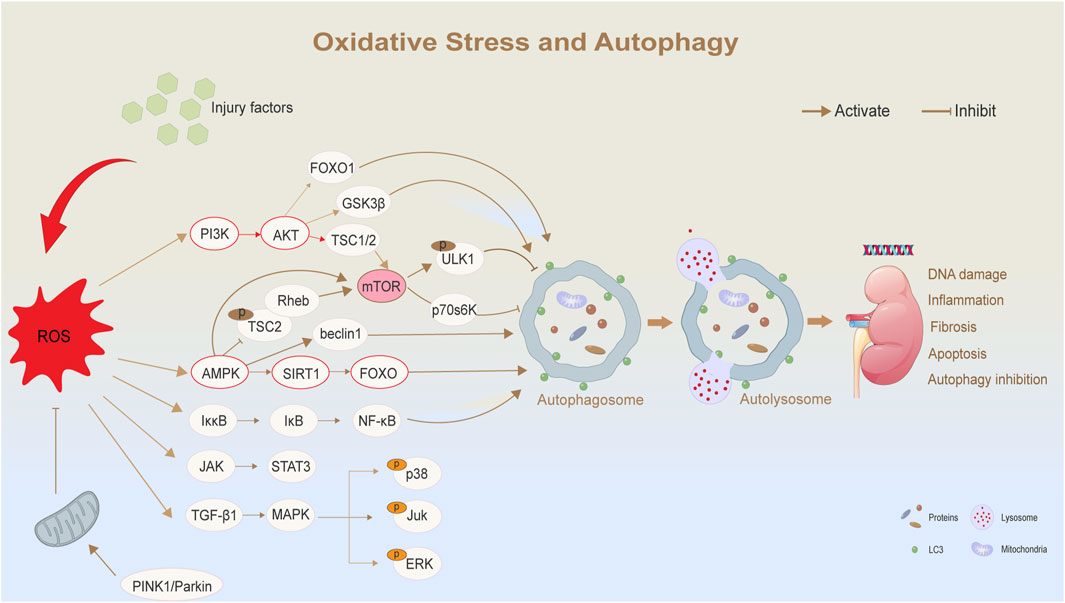
Figure 2. Oxidative stress and autophagy in diabetic nephropathy. ROS regulates autophagy and its related biological effects through a variety of signaling pathways. Elevated levels of ROS are triggered by external injury factors or mitochondrial dysfunction. As a central regulator, ROS affects multiple signaling pathways, such as AMPK/SIRT1/mTOR and PI3K/AKT/mTOR pathways, thereby regulating autophagy. ROS:Reactive Oxygen Species; PI3K:Phosphatidylinositol 3-kinase; LC3:microtubule-associated-proteinlight-chain-3; FOXO:Forkhead box O.
At the same time, hyperglycemia-induced oxidative stress is also recognized as a significant factor contributing to excessive autophagy in DKD. While autophagy initially acts as a self-protective mechanism, severe oxidative stress can result in the overactivation of autophagy, leading to excessive degradation of organelles and eventual cell death. This dysregulated autophagy further exacerbates kidney injury.
2.4 Relationship between nutrient sensing and oxidative stress in DKD
In DKD, a complex interplay exists between the nutrient sensing pathway and oxidative stress (Wang M. et al., 2024). The overactivation of the mTOR pathway exacerbates oxidative stress, which in turn disrupts normal nutrient sensing responses by aberrantly activating signaling pathways. This feedback loop further promotes the progression of DKD (Wang and Zhang, 2024). The relationship between metabolic dysregulation and oxidative stress in DKD is intricate, with both processes interacting to drive renal cell damage, inflammation, and fibrosis.
In the context of metabolic dysregulation, the PI3K/Akt/mTOR pathway is often abnormally activated in DKD (Dong et al., 2023; Lai et al., 2023). This excessive activation contributes to renal cell proliferation, fibrosis (e.g., the overproliferation of renal tubulointerstitial cells), and apoptosis. Additionally, it impairs autophagy, resulting in defective cellular clearance and accumulation of damaged proteins and organelles (Kaushal et al., 2020). The activation of the mTOR pathway increases the metabolic burden on cells, further amplifying the inflammatory response and oxidative stress.
In addition, in the context of hyperglycemia and hyperlipidemia, DKD is characterized by a state of nutrient metabolism disorder, which directly contributes to the excessive production of free radicals and ROS. ROS not only causes direct damage to kidney tissues but also activates various signaling pathways, such as the NF-κB and JNK pathways, thereby exacerbating the progression of DKD (Wang Y. et al., 2024). Oxidative stress further damages renal cells, triggers inflammatory responses, activates the mTOR pathway, and inhibits autophagy, leading to aggravated intracellular damage. Interestingly, ROS can also activate signaling molecules such as AMPK, which in turn inhibits mTOR activity. This may represent an adaptive response by cells to mitigate oxidative stress (Yang et al., 2020). However, prolonged high levels of oxidative stress can disrupt the regulation of the PI3K/Akt/mTOR pathway, resulting in metabolic dysfunction and progressive renal impairment.
3 TCM regulates autophagy through mTOR-mediated nutrient sensing and oxidative stress pathways to intervene in DKD
In the pathological state of DKD, TCM has shown potential in regulating the mTOR pathway to mitigate oxidative stress and reduce cellular damage caused by hyperglycemia. Additionally, TCM can improve nutrient sensing, helping cells adapt to diabetes-related metabolic imbalances. By promoting the activation of autophagy, TCM facilitates the removal of damaged organelles and excess proteins, thereby reducing inflammatory and fibrotic responses in kidney tissue. This review focuses on the role of TCM and its active ingredients in treating DKD by targeting the mTOR pathway to improve oxidative stress and nutrient sensing, ultimately modulating autophagy. These mechanisms are shown schematically in Figure 3.
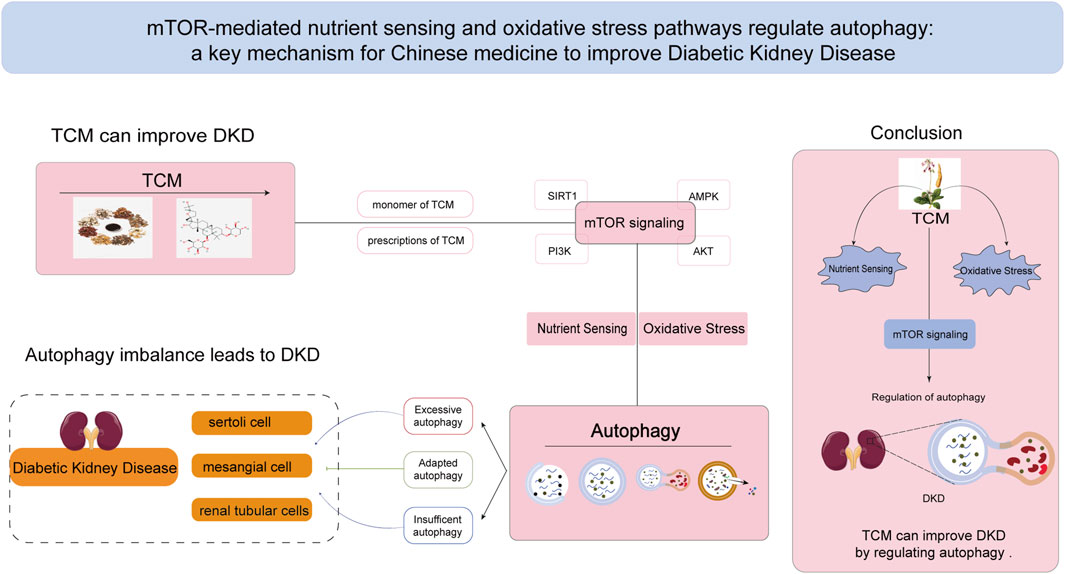
Figure 3. TCM ameliorates DKD by regulating mTOR-mediated nutrient sensing pathway and oxidative stress pathway to affect autophagy. mTOR signaling pathway plays an important role in the regulation of autophagy, and it is also a crucial mechanism by which Chinese medicine affects the balance of autophagy in the treatment of DKD. TCM:Traditional Chinese Medicine.
3.1 Single botanical drugs and monomers in DKD that regulate the AMPK/SIRT1/mTOR pathway to mediate autophagy
Numerous TCMs and their active monomers, such as Rehmannia glutinosa (Gaertn.) Libosch. ex DC., Astragalus mongholicus Bunge, Anemarrhena asphodeloides Bunge, Tripterygium wilfordii Hook.f., Coptis chinensis Franch., and their derivatives, have been shown to modulate the AMPK/SIRT1/mTOR autophagy pathway in DKD. These TCMs are widely applied in clinical practice and have demonstrated efficacy in improving DKD by regulating autophagy.We elaborate on each category based on their distinct classifications.
The terpenoid compounds are as follows:Astragaloside IV, derived from A. mongholicus Bunge, is another widely used compound with a significant effect on DKD. Animal studies have demonstrated that it reduces podocyte injury in STZ-induced DKD rats by inducing autophagy via AMPK activation. Notably, this effect is associated with SERCA2-dependent mitigation of endoplasmic reticulum stress (ERS) (Guo et al., 2017). Dioscin, another bioactive compound, ameliorates DKD by inhibiting oxidative stress caused by mitochondria and ERS. It also enhances autophagy and improves mitochondrial quality and quantity control through the AMPK-mTOR pathway (Zhong et al., 2022a). Catalpol belongs to iridoid glycosides. Catalpol, an iridoid glycoside extracted from R. glutinosa (Gaertn.) Libosch. ex DC—a commonly used TCM for treating DKD—has been shown to enhance autophagy and lysosomal function by inhibiting mTOR activity and promoting TFEB nuclear translocation. This mechanism stabilizes the podocyte cytoskeleton and restores impaired podocyte autophagy, thereby improving DKD outcomes (Chen et al., 2019).
The flavonoid compounds are as follows:Mangiferin, extracted from A. asphodeloides Bunge, has been shown to increase autophagosome formation in podocytes affected by DKD. Its protective effect on podocytes is achieved through upregulation of p-AMPK, downregulation of p-mTOR, and upregulation of p-ULK1 (Wang et al., 2018).Cyanidin-3-O-glucoside (C3G), derived from Morus alba L., black rice, and other sources, protects against HG-induced podocyte dysfunction by improving autophagy and reducing cell apoptosis and EMT. These effects are mediated via activation of the SIRT1/AMPK pathway (Wang et al., 2021). Kaempferol (KPF), found in various green plants such as Brassica oleracea L., Corylus avellana L., propolis, Citrus × aurantium f. aurantium, and tea, exerts a protective effect on DKD by reducing apoptosis and enhancing podocyte autophagy. This protective mechanism is associated with the regulation of the AMPK/mTOR pathway (Sheng et al., 2022). Experimental studies have also highlighted the protective effects of Ginkgetin in DKD (Wei et al., 2021). Its mechanism involves reducing HG-induced oxidative stress, inflammation, and extracellular matrix (ECM) deposition via the AMPK/mTOR-mediated autophagy axis. Puerarin, the active compound of Pueraria montana var. lobata (Willd.) Maesen and S.M.Almeida ex Sanjappa and Predeep, inhibits HG-induced apoptosis. Research has shown (Li et al., 2020) that puerarin promotes AMPK-dependent autophagy by reducing liver kinase B1 (LKB1) acetylation in DKD. Additionally, puerarin can upregulate HMOX1 and Sirt1-mediated autophagy, thereby mitigating diabetes-induced podocyte injury.
Berberine belongs to alkaloids. Coptis chinensis Franch., another commonly used TCM in diabetes and DKD, contains berberine, an active compound with multiple therapeutic effects. Berberine enhances AMPK activation and autophagy while inhibiting mTOR activation and reducing podocyte apoptosis caused by HG or AMPK inhibitors (Jin et al., 2017). Notably, the effect of berberine resembles siRNA-mediated mTOR silencing, as it suppresses P70S6k and 4EBP1 phosphorylation (Li et al., 2020). In addition, berberine interferes with the PI3K/Akt/mTOR signaling pathway by targeting VEGFR2, further inhibiting abnormal mesangial cell proliferation (Yang et al., 2024). Berberine is also linked to the PI3K/Akt/AS160/GLUT1 signaling pathway (Ni et al., 2022), highlighting its broad range of effects and its potential as a therapeutic agent for DKD.
Emodin belongs to anthraquinones. Emodin, a natural modulator, has been found to reduce proteinuria and alleviate renal fibrosis. Previous evidence has demonstrated (Liu Y. et al., 2021) that emodin mediates autophagy through the AMPK/mTOR signaling pathway, thereby reducing apoptosis and podocyte injury in DKD rats. Cyclocarya paliurus (Batalin) Iljinsk., a botanical drug, contains triterpenic acids that have been shown to prevent kidney injury and apoptosis in DKD by regulating autophagy. Studies have found that these triterpenic acids increase AMPK phosphorylation, reduce the downstream phosphorylation of mTOR, and activate the AMPK-mTOR pathway to modulate autophagy (Zhang L. et al., 2019). Tripterygium glycoside, exerts its bioactivity through synergistic interactions among multiple constituents, including diterpene lactones (e.g., Triptolide), alkaloids, and triterpenes. Tripterygium glycoside, widely studied in both clinical and experimental settings, has demonstrated significant therapeutic effects in DKD. In vivo studies have shown that it prevents podocyte injury in DKD mice by upregulating autophagy and downregulating β-arrestin-1 (Zhan et al., 2019). In vitro experiments further revealed that Tripterygium glycoside regulates autophagy via the mTOR/Twist1 pathway, inhibiting epithelial-to-mesenchymal transition (EMT) and apoptosis in podocytes (Tao et al., 2021). Jujuboside A belongs to triterpenoid saponins. Jujuboside A, another TCM monomer, inhibits mitochondrial and ERS-mediated oxidative stress and apoptosis. It regulates the CaMKK2-AMPK-p-mTOR and PINK1/Parkin signaling pathways to enhance autophagy and alleviate DKD-related damage (Zhang Y. et al., 2022).
3.2 Single botanical drug and monomers in DKD that regulate the PI3K/Akt/mTOR pathway to mediate autophagy
Dendrobium nobile Lindl. has demonstrated significant renoprotective effects in DKD. Animal studies have shown that it reduces kidney damage by inhibiting PI3K, Akt, and mTOR phosphorylation while increasing LC3 and Beclin-1 protein and mRNA levels in renal tissues. These effects are attributed to the inhibition of the PI3K/Akt/mTOR signaling pathway and the activation of renal autophagy (Chen et al., 2021).
Ginsenoside Rh1 and paeoniflorin belong to terpenoids, specifically categorized as triterpenoid saponins and iridoid glycosides, respectively. Ginsenoside Rh1 has been reported to improve DKD by reducing inflammation and apoptosis via the AMPK/PI3K/Akt-mediated signaling pathway (Su et al., 2021). Similarly, paeoniflorin (PF) alleviates diabetic kidney injury by restoring autophagy and inhibiting apoptosis through the VEGFR2-mediated PI3K/Akt pathway (Wang et al., 2022). Paeoniflorin has also been shown to improve advanced glycation end products (AGE)-induced mesangial cell damage via the RAGE/mTOR/autophagy pathway (Chen H. et al., 2017). Celastrol, a potential therapeutic agent for DKD, has been shown to achieve podocyte homeostasis and reduce kidney injury through the PI3K/Akt pathway. High doses of celastrol improve renal function, reduce blood glucose, and decrease 24-h urinary albumin in DKD rats. The mechanism involves increasing LC3-II and renin expression while downregulating PI3K, p-Akt, and NF-κB/mTOR mRNA levels (Nie et al., 2020). Geniposide enhances autophagy by activating AMPK and inhibiting Akt via the ULK1-mediated pathway. This reduces proteinuria, podocyte injury, inflammation, and fibrosis, ultimately improving the development and progression of DKD (Dusabimana et al., 2021).
Astilbin, Rutin, Isoorientin, Wogonin and quercetin belong to flavonoids, specifically categorized into dihydroflavonol glycosides, flavonol glycosides, C-glycosylflavones, and methoxyflavones, respectively. Astilbin, a flavonoid compound, has shown protective effects in DKD by reversing high glucose (HG)-induced autophagy inhibition and regulating the PI3K/Akt pathway to reduce autophagy and apoptosis in HK-2 cells (Chen et al., 2018). Rutin, a polyphenolic flavonoid found in plants such as Ruta graveolens L., Acaciella angustissima (Mill.) Britton and Rose, A. mongholicus Bunge, and Fagopyrum esculentum Moench, has shown delayed protective effects on DKD. It inhibits HDAC1 via the PI3K/Akt/mTOR pathway to restore autophagy and alleviate endothelial-to-mesenchymal transition (EndMT) in DKD (Dong et al., 2023). Using a proteomic approach, Kong et al. (2023) demonstrated that isoorientin reverses hyperphosphorylation of TSC2 (S939) under HG conditions, stimulating autophagy by inhibiting the PI3K/Akt/TSC2/mTOR pathway.Wogonin targets the PI3K/Akt/NF-κB signaling pathway to regulate autophagy and inflammation. It reduces the expression of pro-inflammatory cytokines, mitigates autophagy dysfunction, and alleviates renal fibrosis and injury in DKD (Lei et al., 2021). Similarly, animal studies have demonstrated (Guo et al., 2024) that quercetin significantly improves liver, spleen, and kidney injuries in Goto-Kakizaki (GK) rats by promoting autophagy through the PI3K/Akt/mTOR pathway.
Tanshinone IIA belongs to diterpenoid quinones. Tanshinone IIA has been found to alleviate DKD by inhibiting the PI3K/Akt/mTOR pathway in podocytes, regulating autophagy, and reducing inflammation (Li et al., 2024).
Curcumin belongs to polyphenols, specifically categorized as a diarylheptanoid within the curcuminoid subclass. Curcumin plays a protective role in DKD by upregulating E-cadherin and LC3 protein expression while downregulating TWIST1, p62, p-mTOR, p-Akt, and PI3K levels. By inhibiting the PI3K/Akt/mTOR pathway, curcumin induces autophagy and reduces podocyte EMT (Tu et al., 2019).
3.3 TCM prescriptions regulating mTOR-related pathways to mediate autophagy in DKD
TCM offers significant advantages in the prevention and treatment of DKD, particularly due to its multi-target and multi-pathway therapeutic properties. Among the mechanisms of TCM formulations in treating DKD, mTOR serves as a key target for regulating autophagy. Of the mTOR-related autophagy pathways, the AMPK/SIRT1/mTOR and PI3K/Akt/mTOR pathways are the most extensively studied. Due to the multi-pathway nature of TCM formulas, many formulations have been shown to regulate and improve DKD through crosstalk and interactions between multiple signaling pathways.Details of these formulas are provided in Table 1.
TCM compound formulas share a common foundational framework, with the majority being formulated based on therapeutic principles such as ‘Yiqi’, ‘Yangyin’, ‘Tongluo’, and ‘Huashi’. Through in vivo and in vitro experiments, the combination of A. mongholicus Bunge and Panax notoginseng (Burkill) F.H.Chen has been demonstrated to enhance autophagy by suppressing mTOR and activating the PINK1/Parkin signaling pathway, thereby mitigating inflammatory kidney damage in DKD. (Wen et al., 2020). In vitro studies have shown that Tangshenning reduces HG-induced podocyte injury by inhibiting the mTORC1 pathway (including p-mTOR, mTOR, p-p70S6K, p70S6K, ULK1, and 4EBP1) and its downstream targets, while restoring podocyte autophagy (Xu et al., 2022). Tongluo Digui Decoction increases autophagy by inhibiting mTOR phosphorylation, thereby protecting podocytes and slowing the progression of DKD (Han et al., 2021). Wang X. et al. (2019) demonstrated that QiDi TangShen Granules effectively improve DKD by regulating nutrient-sensing molecules involved in autophagy (AMPK, SIRT1, and mTOR). In vitro experiments with db/db mice showed that QDTS treatment increased SIRT1 expression and the p-AMPK/AMPK ratio while decreasing the p-mTOR/mTOR ratio. These findings align with previous reports on the active metabolites of QDTS and their effects on nutrient-sensing pathways. Ni et al. (2025) demonstrated through animal experiments that Modified Huangfeng Decoction (MHD) activates podocyte autophagy via the PI3K/AKT/mTOR pathway and regulates gut microbiota and their metabolites, thereby potentially ameliorating diabetic nephropathy. Yishen Huashi Granule improves mitophagy and inhibits the mTOR/AMPK/PI3K/Akt signaling pathway, further protecting against DKD-related kidney damage (Zhao et al., 2023). Using molecular docking, network pharmacology analysis, and animal experiments, Tangningtongluo Tablets have been shown to reduce pancreatic injury in diabetic mice by inhibiting the PI3K/Akt/mTOR signaling pathway and regulating autophagy (Ren et al., 2024).
Animal studies (Xuan et al., 2021) have demonstrated that Yiqi Jiedu Huayu Decoction improves DKD by further inhibiting the mTOR pathway through the regulation of PI3K/Akt and AMPK pathways. This decoction upregulates the expression of autophagy-related proteins LC3-II and Beclin-1 while downregulating p62 expression, thereby promoting autophagy to alleviate podocyte injury, protect renal function, and reduce renal fibrosis. Additionally, it inhibits the PI3K/Akt/mTOR signaling pathway, enhances podocyte autophagy, and reduces proteinuria in DKD. The Jiedu Tongluo Baoshen Formula similarly inhibits the PI3K/Akt/mTOR signaling pathway, enhancing podocyte autophagy and reducing podocyte injury, thus offering an effective treatment for DKD (Jin et al., 2022). Meanwhile, Qidan Dihuang Decoction protects against renal fibrosis in DKD by inhibiting EMT and inflammatory responses via the p38MAPK and AKT/mTOR signaling pathways (Kuang et al., 2024). Interestingly, reducing excessive autophagy is also critical for mitigating kidney injury. Huangqi Chifeng Decoction has been found to activate the PI3K/Akt/mTOR pathway while inhibiting the AMPK/mTOR pathway, exploring the interplay between these two pathways to suppress excessive autophagy and protect the kidneys (Yu et al., 2018; Zhao et al., 2024a). TCMs have shown significant efficacy in the clinical treatment of DKD, and numerous experimental results have provided robust evidence for the role and mechanism of TCM in improving DKD (Zhang L. et al., 2019). These studies offer both a theoretical and scientific foundation for TCM-based therapies for DKD. However, given the complex composition of TCM formulations and the broad range of pathways involved, further research is needed to fully explore their mechanisms and therapeutic potential (The schematic diagram is illustrated in Figure 4).
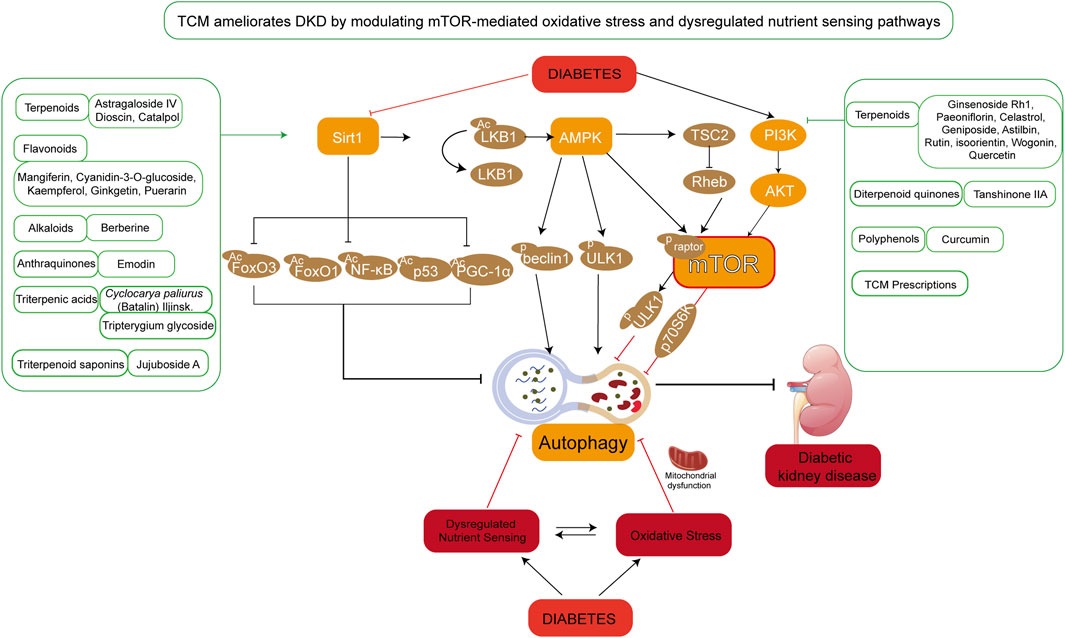
Figure 4. TCM ameliorates DKD by modulating mTOR-mediated oxidative stress and nutrient sensing pathways. Under hyperglycemic conditions, aberrant activation of mTOR-associated signaling pathways leads to dysregulated nutrient sensing and oxidative stress, resulting in mitochondrial dysfunction, suppressed autophagy, and aggravated renal injury. The figure highlights TCM compounds primarily targeting the SIRT1/AMPK/mTOR and PI3K/AKT/mTOR signaling axes. These phytochemicals are categorized into terpenoids, flavonoids, alkaloids, anthraquinones, and other classes.
4 Discussion
4.1 The unique advantages of TCM
This review highlights the critical role of autophagy regulated by mTOR-mediated nutrient sensing and oxidative stress pathways in the pathogenesis of DKD. It also underscores the therapeutic potential of TCM in treating DKD through these mechanisms. TCM offers distinct advantages in DKD by multi-target regulation, early intervention, and safety. Unlike single-target drugs (e.g., ACEI/ARBs or SGLT-2 inhibitors), TCM formulations holistically improve autophagy, oxidative stress, and metabolic inflammation, achieving albuminuria reversal of early-stage patients and delaying ESRD (Liu et al., 2022; Shen et al., 2024; Wu et al., 2022). TCM also mitigates subjective symptoms (fatigue, edema) unaddressed by conventional therapies and poses lower risks of hypotension/electrolyte disorders, even in advanced CKD (Chen J. et al., 2017). While standardization and large-scale RCTs remain challenges, integrating TCM with emerging strategies (e.g., FDA-aligned quality control) could optimize DKD management through synergistic, patient-centered approaches.
4.2 Nutrient-sensing mechanisms: Key players in DKD pathogenesis
While malnutrition is indeed a secondary manifestation of metabolic and renal dysfunction, nutrient sensing dysregulation (e.g., via AMPK/SIRT1/mTORC1 pathways) constitutes a pivotal mechanism in DKD progression, deeply intertwined with oxidative stress and autophagy impairment. These pathways form a triad of pathogenesis: hyperglycemia-induced mitochondrial ROS overproduction suppresses AMPK activity, exacerbating mTORC1-driven anabolic resistance and autophagic flux blockage, which collectively accelerate tubular atrophy and protein-energy wasting.These pathways act as metabolic “hubs,” linking hyperglycemia-induced mitochondrial dysfunction, redox imbalance, and cellular autophagy.
TCM addresses this not by direct nutritional supplementation but by rectifying upstream pathologies: (1) improving tubular energy metabolism via AMPK/SIRT1 to reduce protein catabolism; (2) restoring gut-kidney axis homeostasis to enhance nutrient absorption; (3) mitigating inflammation-driven muscle wasting. This multi-dimensional regulation distinguishes TCM from conventional nutritional therapies, which often neglect underlying disease mechanisms.
4.3 Categorization of TCM ingredients by autophagy modulation stages
TCM compounds that regulate autophagy in DKD can be classified into multiple categories: flavonoids, terpenoids, alkaloids, anthraquinones, etc., each demonstrating unique pharmacological characteristics in autophagy modulation. Flavonoids (e.g., astragaloside IV, baicalin) enhance autophagosome initiation and lysosomal fusion by activating AMPK/mTORC1 and upregulating Rab7/LAMP2. Alkaloids like berberine and rhein regulate lysosomal maturation and substrate degradation—berberine elevates lysosomal pH to prolong autophagosome retention, while rhein activates Nrf2-driven mitophagy, reducing renal oxidative damage. Terpenoids (e.g., tanshinone IIA) coordinate autophagy-inflammation crosstalk by inhibiting NF-κB/NLRP3, decreasing IL-1β-induced p62 accumulation in podocytes, while triptolide adaptively regulates AMPK/ULK1 to prevent apoptosis in advanced DKD.
These metabolites classes collectively address DKD’s multifaceted pathogenesis: flavonoids and polysaccharides dominate early-stage interventions by improving autophagic efficiency and metabolic reprogramming, whereas alkaloids and terpenoids target late-stage complications through lysosomal refinement and inflammation resolution.
4.4 Existing shortcomings and future research prospects
While TCM shows great promise for DKD management, further research is needed to elucidate its precise mechanisms of action and optimize its clinical application. Future research should delve deeper into the intricate relationship between mTOR and autophagy in DKD and investigate how TCM modulates autophagy and inhibits oxidative stress by targeting upstream signaling molecules of mTOR. Advanced technologies such as high-throughput screening, proteomics, and genomics are essential for comprehensively analyzing the molecular mechanisms of TCM in the treatment of DKD.
Robust clinical trials, particularly randomized controlled trials (RCTs), are needed to evaluate the efficacy and safety of TCM, either as standalone treatments or in combination with modern medicines. Such trials should focus on key efficacy indicators, including renal function, urinary albumin levels, and blood glucose control, while also assessing safety profiles. Existing clinical studies are frequently limited by methodological shortcomings, including small sample sizes, non-standardized TCM formulations, and inadequate blinding protocols. These limitations may exaggerate placebo effects or obscure true treatment outcomes.This direction warrants prioritized attention from the research community.
Furthermore, studies should investigate the therapeutic effects of TCM across different stages of DKD. Given the multi-component nature of TCM, studies on dose optimization and drug interactions are vital. Future research should conduct dose-escalation studies to identify the optimal dose ranges for TCM ingredients, minimizing adverse reactions and interactions with conventional antidiabetic drugs. This approach will enhance both the safety and efficacy of TCM in clinical settings. Patients with DKD exhibit considerable individual variability, necessitating personalized treatment strategies. Combining cutting-edge technologies, such as genomics and metabolomics, with TCM could enable the development of tailored therapeutic approaches. These strategies could address variations in genotypes or clinical subtypes, providing more precise and effective treatments for DKD patients. In conclusion, integrating TCM with modern medical treatments may offer innovative approaches for the comprehensive management of DKD.
Emerging evidence highlights mTOR as a pivotal yet underexplored target in DKD. Conventional mTOR inhibitors (e.g., everolimus) show promise in preclinical models but require rigorous clinical validation for renal safety and efficacy. Meanwhile, TCM-derived metabolites like Astragaloside IV and rhein offer multi-pathway modulation, indirectly targeting mTOR while mitigating oxidative stress and inflammation. Future studies should prioritize TCM-based mTOR inhibitors with refined pharmacokinetic profiles to bridge traditional knowledge and modern pharmacology.
5 Conclusion
In the pathological process of DKD, the mTOR signaling pathway plays a critical role in regulating autophagy and maintaining the balance of intracellular metabolism and oxidative stress, thereby mitigating kidney injury. Particularly under conditions of excessive oxidative stress or metabolic dysregulation, the activation or inhibition of the mTOR pathway becomes crucial for the regulation of autophagy, significantly influencing the prevention and treatment of DKD. This review highlights the importance of mTOR-mediated nutrient sensing and oxidative stress pathways in autophagy regulation by analyzing extensive literature. It also emphasizes the potential mechanisms by which TCM improves DKD through these pathways. TCM enhances autophagy in DKD and slows disease progression by modulating mTOR-mediated signaling pathways, particularly the AMPK/SIRT1/mTOR and PI3K/Akt/mTOR pathways. The regulation of autophagy via mTOR-mediated nutrient sensing and oxidative stress pathways represents a key mechanism of TCM intervention in DKD treatment. This underscores the unique advantages of TCM in both the prevention and treatment of DKD, offering promising therapeutic strategies for the effective management of this disease.
Author contributions
LL: Data curation, Writing – original draft, Conceptualization, Writing – review and editing. JZ: Methodology, Writing – review and editing. TZ: Conceptualization, Visualization, Writing – review and editing. XL: Data curation, Writing – review and editing. DT: Conceptualization, Writing – review and editing. QX: Conceptualization, Supervision, Writing – review and editing. RY: Funding acquisition, Project administration, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research was supported by [National Natural Science Foundation of China#1](Grant Numbers U21A20411, 82074400); Hunan Provincial Natural Science Foundation Innovation Research Group Project#2 (2024JJ1007); Hunan Provincial Natural Science Foundation #3 (2024JJ5303); Hunan University of Chinese Medicine Disciplinary Construction‘Revealing the List and Appointing Leaders’Project #4 (22JBZ002); A Project Supported by Scientific Research Fund of Hunan Provincial Education Department #5 (24A0263); Graduate Student Innovation Project of Hunan University of Chinese Medicine #6 (CX20230787).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Audzeyenka, I., Bierżyńska, A., and Lay, A. C. (2022). Podocyte bioenergetics in the development of diabetic nephropathy: the role of mitochondria. Endocrinology 163 (1), bqab234. doi:10.1210/endocr/bqab234
Cantó, C., Gerhart-Hines, Z., Feige, J. N., Lagouge, M., Noriega, L., Milne, J. C., et al. (2009). AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458 (7241), 1056–1060. doi:10.1038/nature07813
Chen, F., Sun, Z., Zhu, X., and Ma, Y. (2018). Astilbin inhibits high glucose-induced autophagy and apoptosis through the PI3K/Akt pathway in human proximal tubular epithelial cells. Biomed. Pharmacother. 106, 1175–1181. doi:10.1016/j.biopha.2018.07.072
Chen, H., Guo, J., Zhao, X., He, X., He, Z., Zhao, L., et al. (2017a). Retrospective analysis of the overt proteinuria diabetic kidney disease in the treatment of modified Shenzhuo formula for 2 years. Medicine 96 (12), e6349. doi:10.1097/MD.0000000000006349
Chen, J., Zhao, D., Zhu, M., Zhang, M., Hou, X., Ding, W., et al. (2017b). Paeoniflorin ameliorates AGEs-induced mesangial cell injury through inhibiting RAGE/mTOR/autophagy pathway. Biomed. Pharmacother. 89, 1362–1369. doi:10.1016/j.biopha.2017.03.016
Chen, Y., Liu, Q., Shan, Z., Mi, W., Zhao, Y., Li, M., et al. (2019). Catalpol ameliorates podocyte injury by stabilizing cytoskeleton and enhancing autophagy in diabetic nephropathy. Front. Pharmacol. 10, 1477. doi:10.3389/fphar.2019.01477
Chen, Y., Zheng, Y. F., Lin, X. H., Zhang, J. P., Lin, F., and Shi, H. (2021). Dendrobium mixture attenuates renal damage in rats with diabetic nephropathy by inhibiting the PI3K/Akt/mTOR pathway. Mol. Med. Rep. 24 (2), 590. doi:10.3892/mmr.2021.12229
Corsetti, G., Pasini, E., Scarabelli, T. M., Romano, C., Singh, A., Scarabelli, C. C., et al. (2024). Importance of energy, dietary protein sources, and amino acid composition in the regulation of metabolism: an indissoluble dynamic combination for life. Nutrients 16 (15), 2417. doi:10.3390/nu16152417
Deng, J., Yang, Q., Zhu, W., Zhang, Y., Lin, M., She, J., et al. (2024). Neuregulin 4 attenuates podocyte injury and proteinuria in part by activating AMPK/mTOR-Mediated autophagy in mice. J. Cell Biochem. 125 (10), e30634. doi:10.1002/jcb.30634
Dong, R., Zhang, X., Liu, Y., Zhao, T., Sun, Z., Liu, P., et al. (2023). Rutin alleviates EndMT by restoring autophagy through inhibiting HDAC1 via PI3K/AKT/mTOR pathway in diabetic kidney disease. Phytomedicine 112, 154700. doi:10.1016/j.phymed.2023.154700
Dronavalli, S., Duka, I., and Bakris, G. L. (2008). The pathogenesis of diabetic nephropathy. Nat. Clin. Pract. Endocrinol. Metab. 4 (8), 444–452. doi:10.1038/ncpendmet0894
Dusabimana, T., Park, E. J., Je, J., Jeong, K., Yun, S. P., Kim, H. J., et al. (2021). Geniposide improves diabetic nephropathy by enhancing ULK1-mediated autophagy and reducing oxidative stress through AMPK activation. Int. J. Mol. Sci. 22 (4), 1651. doi:10.3390/ijms22041651
Entezari, M., Hashemi, D., Taheriazam, A., Zabolian, A., Mohammadi, S., Fakhri, F., et al. (2022). AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: a pre-clinical and clinical investigation. Biomed. Pharmacother. 146, 112563. doi:10.1016/j.biopha.2021.112563
Guo, H., Wang, Y., Zhang, X., Zang, Y., Zhang, Y., Wang, L., et al. (2017). Astragaloside IV protects against podocyte injury via SERCA2-dependent ER stress reduction and AMPKα-regulated autophagy induction in streptozotocin-induced diabetic nephropathy. Sci. Rep-Uk 7 (1), 6852. doi:10.1038/s41598-017-07061-7
Guo, Z., Zhang, J., Li, M., Xing, Z., Li, X., Qing, J., et al. (2024). Mechanism of action of quercetin in regulating cellular autophagy in multiple organs of Goto-Kakizaki rats through the PI3K/Akt/mTOR pathway. Front. Med-Lausanne 11, 1442071. doi:10.3389/fmed.2024.1442071
Han, J., Zhang, Y., Shi, X., Peng, Z., Xing, Y., and Pang, X. (2021). Tongluo Digui decoction treats renal injury in diabetic rats by promoting autophagy of podocytes. J. Tradit. Chin. Med. 41 (1), 125–132. doi:10.19852/j.cnki.jtcm.2021.01.014
Han, Y. P., Liu, L. J., Yan, J. L., Chen, M. Y., Meng, X. F., Zhou, X. R., et al. (2023). Autophagy and its therapeutic potential in diabetic nephropathy. Front. Endocrinol. 14, 1139444. doi:10.3389/fendo.2023.1139444
Haq, S., Grondin, J., Banskota, S., and Khan, W. I. (2019). Autophagy: roles in intestinal mucosal homeostasis and inflammation. J. Biomed. Sci. 26 (1), 19. doi:10.1186/s12929-019-0512-2
Hartleben, B., Gödel, M., Meyer-Schwesinger, C., Liu, S., Ulrich, T., Köbler, S., et al. (2010). Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J. Clin. Invest 120 (4), 1084–1096. doi:10.1172/JCI39492
Herzig, S., and Shaw, R. J. (2018). AMPK: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Bio 19 (2), 121–135. doi:10.1038/nrm.2017.95
Hinden, L., Kogot-Levin, A., Tam, J., and Leibowitz, G. (2022). Pathogenesis of diabesity-induced kidney disease: role of kidney nutrient sensing. Febs J. 289 (4), 901–921. doi:10.1111/febs.15790
Hou, W., Zhao, F., Fang, L., Wang, X., Wu, D., Liu, C., et al. (2023). Walnut-derived peptides promote autophagy via the activation of AMPK/mTOR/ULK1 pathway to ameliorate hyperglycemia in type 2 diabetic mice. J. Agr Food Chem. 71 (8), 3751–3765. doi:10.1021/acs.jafc.2c07112
Huynh, C., Ryu, J., Lee, J., Inoki, A., and Inoki, K. (2023). Nutrient-sensing mTORC1 and AMPK pathways in chronic kidney diseases. Nat. Rev. Nephrol. 19 (2), 102–122. doi:10.1038/s41581-022-00648-y
Iwata, W., Unoki-Kubota, H., Kato, H., Shimizu, A., Matsumoto, M., Imasawa, T., et al. (2020). Podocyte-specific deletion of tubular sclerosis complex 2 promotes focal segmental glomerulosclerosis and progressive renal failure. Plos One 15 (3), e0229397. doi:10.1371/journal.pone.0229397
Jaikumkao, K., Thongnak, L., Htun, K. T., Pengrattanachot, N., Phengpol, N., Sutthasupha, P., et al. (2024). Dapagliflozin and metformin in combination ameliorates diabetic nephropathy by suppressing oxidative stress, inflammation, and apoptosis and activating autophagy in diabetic rats. Bba-Mol Basis Dis. 1870 (1), 166912. doi:10.1016/j.bbadis.2023.166912
Jin, D., Liu, F., Yu, M., Zhao, Y., Yan, G., Xue, J., et al. (2022). Jiedu Tongluo Baoshen formula enhances podocyte autophagy and reduces proteinuria in diabetic kidney disease by inhibiting PI3K/Akt/mTOR signaling pathway. J. Ethnopharmacol. 293, 115246. doi:10.1016/j.jep.2022.115246
Jin, D., Zhao, Y., Sun, Y., Xue, J., Li, X., and Wang, X. (2023). Jiedu Tongluo Baoshen formula enhances renal tubular epithelial cell autophagy to prevent renal fibrosis by activating SIRT1/LKB1/AMPK pathway. Biomed. Pharmacother. 160, 114340. doi:10.1016/j.biopha.2023.114340
Jin, Y., Liu, S., Ma, Q., Xiao, D., and Chen, L. (2017). Berberine enhances the AMPK activation and autophagy and mitigates high glucose-induced apoptosis of mouse podocytes. Eur. J. Pharmacol. 794, 106–114. doi:10.1016/j.ejphar.2016.11.037
Johansen, T., and Lamark, T. (2020). Selective autophagy: ATG8 family proteins, LIR motifs and cargo receptors. J. Mol. Biol. 432 (1), 80–103. doi:10.1016/j.jmb.2019.07.016
Kaushal, G. P., Chandrashekar, K., Juncos, L. A., and Shah, S. V. (2020). Autophagy function and regulation in kidney disease. Biomolecules 10 (1), 100. doi:10.3390/biom10010100
Kirkin, V., and Rogov, V. V. (2019). A diversity of selective autophagy receptors determines the specificity of the autophagy pathway. Mol. Cell 76 (2), 268–285. doi:10.1016/j.molcel.2019.09.005
Kitada, M., Ogura, Y., Monno, I., and Koya, D. (2017). Regulating autophagy as a therapeutic target for diabetic nephropathy. Curr. Diabetes Rep. 17 (7), 53. doi:10.1007/s11892-017-0879-y
Kma, L., and Baruah, T. J. (2022). The interplay of ROS and the PI3K/Akt pathway in autophagy regulation. Biotechnol. Appl. Bioc 69 (1), 248–264. doi:10.1002/bab.2104
Kong, Z., Xiao, M., Wang, B., Zhang, W., Che, K., Lv, W., et al. (2023). Renoprotective effect of isoorientin in diabetic nephropathy via activating autophagy and inhibiting the PI3K-AKT-TSC2-mTOR pathway. Am. J. Chin. Med. 51 (5), 1269–1291. doi:10.1142/S0192415X23500581
Kuang, L., You, Y., Qi, J., Chen, J., Zhou, X., Ji, S., et al. (2024). Qi-dan-dihuang decoction ameliorates renal fibrosis in diabetic rats via p38MAPK/AKT/mTOR signaling pathway. Environ. Toxicol. 39 (6), 3481–3499. doi:10.1002/tox.24179
Lai, W., Luo, D., Li, Y., Li, Y., Wang, Q., Hu, Z., et al. (2023). Irisin ameliorates diabetic kidney disease by restoring autophagy in podocytes. Faseb J. 37 (10), e23175. doi:10.1096/fj.202300420R
Lei, L., Zhao, J., Liu, X. Q., Chen, J., Qi, X. M., Xia, L. L., et al. (2021). Wogonin alleviates kidney tubular epithelial injury in diabetic nephropathy by inhibiting PI3K/Akt/NF-κB signaling pathways. Drug Des. Devel Ther. 15, 3131–3150. doi:10.2147/DDDT.S310882
Lenoir, O., Jasiek, M., C, H., Guyonnet, L., Hartleben, B., Bork, T., et al. (2015). Endothelial cell and podocyte autophagy synergistically protect from diabetes-induced glomerulosclerosis. Autophagy 11 (7), 1130–1145. doi:10.1080/15548627.2015.1049799
Leventhal, J. S., Wyatt, C. M., and Ross, M. J. (2017). Recycling to discover something new: the role of autophagy in kidney disease. Kidney Int. 91 (1), 4–6. doi:10.1016/j.kint.2016.11.004
Li, C., Guan, X. M., Wang, R. Y., Xie, Y. S., Zhou, H., Ni, W. J., et al. (2020). Berberine mitigates high glucose-induced podocyte apoptosis by modulating autophagy via the mTOR/P70S6K/4EBP1 pathway. Life Sci. 243, 117277. doi:10.1016/j.lfs.2020.117277
Li, Y., Wu, T., Li, H., Liu, M., and Xu, H. (2024). Tanshinone IIA promoted autophagy and inhibited inflammation to alleviate podocyte injury in diabetic nephropathy. Diabet. Metab. Synd. Ob. 17, 2709–2724. doi:10.2147/DMSO.S464015
Lin, F., Liu, Y., Tang, L., Xu, X., Zhang, X., Song, Y., et al. (2021). Rapamycin protects against aristolochic acid nephropathy in mice by potentiating mammalian target of rapamycin-mediated autophagy. Mol. Med. Rep. 24 (1), 495. doi:10.3892/mmr.2021.12134
Liu, H., Wang, Q., Shi, G., Yang, W., Zhang, Y., Chen, W., et al. (2021a). Emodin ameliorates renal damage and podocyte injury in a rat model of diabetic nephropathy via regulating AMPK/mTOR-Mediated autophagy signaling pathway. Diabet. Metab. Synd. Ob. 14, 1253–1266. doi:10.2147/DMSO.S299375
Liu, J., Chang, A., Peng, H., Huang, H., Hu, P., Yao, A., et al. (2025). Isoferulic acid regulates CXCL12/CXCR4-mediated apoptosis and autophagy in podocyte and mice with STZ-induced diabetic nephropathy. Int. Immunopharmacol. 144, 113707. doi:10.1016/j.intimp.2024.113707
Liu, N., Xu, L., Shi, Y., and Zhuang, S. (2017). Podocyte autophagy: a potential therapeutic target to prevent the progression of diabetic nephropathy. J. Diabetes Res. 2017, 3560238. doi:10.1155/2017/3560238
Liu, X., Ge, M., Zhai, X., Xiao, Y., Zhang, Y., Xu, Z., et al. (2022). Traditional Chinese medicine for the treatment of diabetic kidney disease: a study-level pooled analysis of 44 randomized controlled trials. Front. Pharmacol. 13, 1009571. doi:10.3389/fphar.2022.1009571
Liu, Y., Kou, D., Chu, N., and Ding, G. (2020). Cathelicidin-BF attenuate kidney injury through inhibiting oxidative stress, inflammation and fibrosis in streptozotocin-induced diabetic rats. Life Sci. 257, 117918. doi:10.1016/j.lfs.2020.117918
Liu, Y., Liu, W., Zhang, Z., Hu, Y., Zhang, X., Sun, Y., et al. (2021b). Yishen capsule promotes podocyte autophagy through regulating SIRT1/NF-κB signaling pathway to improve diabetic nephropathy. Ren. Fail. 43 (1), 128–140. doi:10.1080/0886022x.2020.1869043
Ma, F., Li, H., Huo, H., Han, Q., Liao, J., Zhang, H., et al. (2023a). N-acetyl-L-cysteine alleviates FUNDC1-mediated mitophagy by regulating mitochondrial dynamics in type 1 diabetic nephropathy canine. Life Sci. 313, 121278. doi:10.1016/j.lfs.2022.121278
Ma, X., Ma, J., Leng, T., Yuan, Z., Hu, T., Liu, Q., et al. (2023b). Advances in oxidative stress in pathogenesis of diabetic kidney disease and efficacy of TCM intervention. Ren. Fail. 45 (1), 2146512. doi:10.1080/0886022X.2022.2146512
Młynarska, E., Buławska, D., Czarnik, W., Hajdys, J., Majchrowicz, G., Prusinowski, F., et al. (2024). Novel insights into diabetic kidney disease. Int. J. Mol. Sci. 25 (18), 10222. doi:10.3390/ijms251810222
Ni, W. J., Guan, X. M., Zeng, J., Zhou, H., Meng, X. M., and Tang, L. Q. (2022). Berberine regulates mesangial cell proliferation and cell cycle to attenuate diabetic nephropathy through the PI3K/Akt/AS160/GLUT1 signalling pathway. J. Cell Mol. Med. 26 (4), 1144–1155. doi:10.1111/jcmm.17167
Ni, Y., Yang, W., Wang, S., Pan, Y., Du, H., and Zheng, L. (2025). Modified huangfeng decoction alleviates diabetic nephropathy by activating autophagy and regulating the gut microbiot. Phytomedicine. 141, 156677. doi:10.1016/j.phymed.2025.156677
Nie, Y., Fu, C., Zhang, H., Zhang, M., Xie, H., Tong, X., et al. (2020). Celastrol slows the progression of early diabetic nephropathy in rats via the PI3K/AKT pathway. Bmc Complement. Med. 20 (1), 321. doi:10.1186/s12906-020-03050-y
Oza, M. J., Laddha, A. P., Gaikwad, A. B., Mulay, S. R., and Kulkarni, Y. A. (2021). Role of dietary modifications in the management of type 2 diabetic complications. Pharmacol. Res. 168, 105602. doi:10.1016/j.phrs.2021.105602
Packer, M. (2020). Role of impaired nutrient and oxygen deprivation signaling and deficient autophagic flux in diabetic CKD development: implications for understanding the effects of sodium-glucose cotransporter 2-inhibitors. J. Am. Soc. Nephrol. 31 (5), 907–919. doi:10.1681/ASN.2020010010
Parmar, U. M., Jalgaonkar, M. P., Kulkarni, Y. A., and Oza, M. J. (2022). Autophagy-nutrient sensing pathways in diabetic complications. Pharmacol. Res. 184, 106408. doi:10.1016/j.phrs.2022.106408
Ren, H., Shao, Y., Wu, C., Ma, X., Lv, C., and Wang, Q. (2020). Metformin alleviates oxidative stress and enhances autophagy in diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol. Cell Endocrinol. 500, 110628. doi:10.1016/j.mce.2019.110628
Ren, Y., Hu, X., Qi, M., Zhu, W., Li, J., Yang, S., et al. (2024). Tangningtongluo Tablet ameliorates pancreatic damage in diabetic mice by inducing autophagy and inhibiting the PI3K/Akt/mTOR signaling pathway. Int. Immunopharmacol. 142 (Pt A), 113032. doi:10.1016/j.intimp.2024.113032
Sakai, S., Yamamoto, T., Takabatake, Y., Takahashi, A., Namba-Hamano, T., Minami, S., et al. (2019). Proximal tubule autophagy differs in type 1 and 2 diabetes. J. Am. Soc. Nephrol. 30 (6), 929–945. doi:10.1681/ASN.2018100983
Sharma, K., Zhang, G., Hansen, J., Bjornstad, P., Lee, H. J., Menon, R., et al. (2023). Endogenous adenine mediates kidney injury in diabetic models and predicts diabetic kidney disease in patients. J. Clin. Invest 133 (20), e170341. doi:10.1172/JCI170341
Shen, S., Zhong, H., Zhou, X., Li, G., Zhang, C., Zhu, Y., et al. (2024). Advances in Traditional Chinese Medicine research in diabetic kidney disease treatment. Pharm. Biol. 62 (1), 222–232. doi:10.1080/13880209.2024.2314705
Sheng, H., Zhang, D., Zhang, J., Zhang, Y., Lu, Z., Mao, W., et al. (2022). Kaempferol attenuated diabetic nephropathy by reducing apoptosis and promoting autophagy through AMPK/mTOR pathways. Front. Med-Lausanne 9, 986825. doi:10.3389/fmed.2022.986825
Sherkhane, B., Yerra, V. G., Sharma, A., Kumar, K. A., Chayanika, G., Kumar, A. V., et al. (2023). Nephroprotective potential of syringic acid in experimental diabetic nephropathy: focus on oxidative stress and autophagy. Indian J. Pharmacol. 55 (1), 34–42. doi:10.4103/ijp.ijp_671_22
Siddhi, J., Sherkhane, B., Kalavala, A. K., Arruri, V., Velayutham, R., and Kumar, A. (2022). Melatonin prevents diabetes-induced nephropathy by modulating the AMPK/SIRT1 axis: focus on autophagy and mitochondrial dysfunction. Cell Biol. Int. 46 (12), 2142–2157. doi:10.1002/cbin.11899
Singh, A., Mahapatra, K. K., Praharaj, P. P., Patra, S., Mishra, S. R., Patil, S., et al. (2024). Prolonged glutamine starvation reactivates mTOR to inhibit autophagy and initiate autophagic lysosome reformation to maintain cell viability. Int. J. Biochem. Cell B 177, 106694. doi:10.1016/j.biocel.2024.106694
Song, S., Qiu, D., Shi, Y., Wang, S., Zhou, X., Chen, N., et al. (2019). Thioredoxin-interacting protein deficiency alleviates phenotypic alterations of podocytes via inhibition of mTOR activation in diabetic nephropathy. J. Cell Physiol. 234, 16485–16502. doi:10.1002/jcp.28317
Su, W. Y., Li, Y., Chen, X., Li, X., Wei, H., Liu, Z., et al. (2021). Ginsenoside Rh1 improves type 2 diabetic nephropathy through AMPK/PI3K/Akt-Mediated inflammation and apoptosis signaling pathway. Am. J. Chin. Med. 49 (5), 1215–1233. doi:10.1142/S0192415X21500580
Suda, K., Kaneko, A., Shimobayashi, M., Nakashima, A., Maeda, T., Hall, M. N., et al. (2019). TORC1 regulates autophagy induction in response to proteotoxic stress in yeast and human cells. Biochem. Bioph Res. Co. 511 (2), 434–439. doi:10.1016/j.bbrc.2019.02.077
Tang, C., Livingston, M. J., Liu, Z., and Dong, Z. (2020). Autophagy in kidney homeostasis and disease. Nat. Rev. Nephrol. 16 (9), 489–508. doi:10.1038/s41581-020-0309-2
Tang, G., Du, Y., Guan, H., Jia, J., Zhu, N., Shi, Y., et al. (2022). Butyrate ameliorates skeletal muscle atrophy in diabetic nephropathy by enhancing gut barrier function and FFA2-mediated PI3K/Akt/mTOR signals. Brit J. Pharmacol. 179 (1), 159–178. doi:10.1111/bph.15693
Tang, G., Li, S., Zhang, C., Chen, H., Wang, N., and Feng, Y. (2021). Clinical efficacies, underlying mechanisms and molecular targets of Chinese medicines for diabetic nephropathy treatment and management. Acta Pharm. Sin. B 11 (9), 2749–2767. doi:10.1016/j.apsb.2020.12.020
Tao, M., Zheng, D., Liang, X., Wu, D., Hu, K., Jin, J., et al. (2021). Tripterygium glycoside suppresses epithelial-to-mesenchymal transition of diabetic kidney disease podocytes by targeting autophagy through the mTOR/Twist1 pathway. Mol. Med. Rep. 24 (2), 592. doi:10.3892/mmr.2021.12231
Tu, Q., Li, Y., Jin, J., Jiang, X., Ren, Y., and He, Q. (2019). Curcumin alleviates diabetic nephropathy via inhibiting podocyte mesenchymal transdifferentiation and inducing autophagy in rats and MPC5 cells. Pharm. Biol. 57 (1), 778–786. doi:10.1080/13880209.2019.1688843
Vargas, J., Hamasaki, M., Kawabata, T., Youle, R. J., and Yoshimori, T. (2022). The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Bio 3 (24), 167–185. doi:10.1038/s41580-022-00542-2
Wang, H., Gao, L., Zhao, C., Fang, F., Liu, J., Wang, Z., et al. (2024a). The role of PI3K/Akt signaling pathway in chronic kidney disease. Int. Urol. Nephrol. 56 (8), 2623–2633. doi:10.1007/s11255-024-03989-8
Wang, M., Li, Q., Wang, S., Zuo, L., Hai, Y., Yuan, S., et al. (2024b). Astragaloside IV protects renal tubular epithelial cells against oxidative stress-induced injury by upregulating CPT1A-mediated HSD17B10 lysine succinylation in diabetic kidney disease. Phytother. Res. 38 (9), 4519–4540. doi:10.1002/ptr.8298
Wang, N., and Zhang, C. (2024). Oxidative stress: a culprit in the progression of diabetic kidney disease. Antioxidants-Basel. 13 (4), 455. doi:10.3390/antiox13040455
Wang, S., Huang, Y., Luo, G., Yang, X., and Huang, W. (2021). Cyanidin-3-O-glucoside attenuates high glucose-induced podocyte dysfunction by inhibiting apoptosis and promoting autophagy via activation of SIRT1/AMPK pathway. Can. J. Physiol. Pharm. 99 (6), 589–598. doi:10.1139/cjpp-2020-0341
Wang, W., Sun, W., Cheng, Y., Xu, Z., and Cai, L. (2019a). Role of sirtuin-1 in diabetic nephropathy. J. Mol. Med. 97 (3), 291–309. doi:10.1007/s00109-019-01743-7
Wang, X., Gao, L., Lin, H., Song, J., Wang, J., Yin, Y., et al. (2018). Mangiferin prevents diabetic nephropathy progression and protects podocyte function via autophagy in diabetic rat glomeruli. Eur. J. Pharmacol. 824, 170–178. doi:10.1016/j.ejphar.2018.02.009
Wang, X., Jiang, L., Liu, X. Q., Huang, Y. B., Wang, A. L., Zeng, H. X., et al. (2022). Paeoniflorin binds to VEGFR2 to restore autophagy and inhibit apoptosis for podocyte protection in diabetic kidney disease through PI3K-AKT signaling pathway. Phytomedicine 106, 154400. doi:10.1016/j.phymed.2022.154400
Wang, X., Zhao, L., Ajay, A. K., Jiao, B., Zhang, X., Wang, C., et al. (2019b). QiDiTangShen Granules activate renal nutrient-sensing associated autophagy in db/db mice. Front. Physiol. 10, 1224. doi:10.3389/fphys.2019.01224
Wang, Y., Liu, Z., Shu, S., Cai, J., Tang, C., and Dong, Z. (2020). AMPK/mTOR signaling in autophagy regulation during cisplatin-induced acute kidney injury. Front. Physiol. 11, 619730. doi:10.3389/fphys.2020.619730
Wang, Y., Yang, J., Chang, X., Xue, Y., Liu, G., Zhang, T., et al. (2024c). Isoliquiritigenin alleviates diabetic kidney disease via oxidative stress and the TLR4/NF-κB/NLRP3 inflammasome pathway. Mol. Nutr. Food Res. 68 (16), e2400215. doi:10.1002/mnfr.202400215
Wei, L., Jian, P., Erjiong, H., and Qihan, Z. (2021). Ginkgetin alleviates high glucose-evoked mesangial cell oxidative stress injury, inflammation, and extracellular matrix (ECM) deposition in an AMPK/mTOR-mediated autophagy axis. Chem. Biol. Drug Des. 98 (4), 620–630. doi:10.1111/cbdd.13915
Wen, D., Tan, R. Z., Zhao, C. Y., Li, J. C., Zhong, X., Diao, H., et al. (2020). Astragalus mongholicus Bunge and Panax notoginseng (burkill) F.H. Chen formula for renal injury in diabetic nephropathy-in vivo and in vitro evidence for autophagy regulation. Front. Pharmacol. 11, 732. doi:10.3389/fphar.2020.00732
Wu, C., Fei, J., Xu, Q., Tao, Y., Zhou, Z., Wang, Y., et al. (2022). Walking exercise reduces postprandial lipemia but does not influence postprandial hemorheological properties and oxidative stress. Metabolites 12 (9), 1038. doi:10.3390/metabo12111038
Xiao, T., Guan, X., Nie, L., Wang, S., Sun, L., He, T., et al. (2014). Rapamycin promotes podocyte autophagy and ameliorates renal injury in diabetic mice. Mol. Cell Biochem. 394 (1-2), 145–154. doi:10.1007/s11010-014-2090-7
Xiong, G., Tang, W., Zhang, D., He, D., Wei, G., Atala, A., et al. (2019). Impaired regeneration potential in urinary stem cells diagnosed from the patients with diabetic nephropathy. Theranostics 9 (14), 4221–4232. doi:10.7150/thno.34050
Xu, J., Shan, X., Chen, C., Gao, Y., Zou, D., Wang, X., et al. (2022). Tangshenning attenuates high glucose-induced podocyte injury via restoring autophagy activity through inhibiting mTORC1 activation. J. Diabetes Res. 2022, 1610416. doi:10.1155/2022/1610416
Xuan, C., Xi, Y. M., Zhang, Y. D., Tao, C. H., Zhang, L. Y., and Cao, W. F. (2021). Yiqi Jiedu Huayu decoction alleviates renal injury in rats with diabetic nephropathy by promoting autophagy. Front. Pharmacol. 12, 624404. doi:10.3389/fphar.2021.624404
Xue, H. Z., Chen, Y., Wang, S. D., Yang, Y. M., Cai, L. Q., Zhao, J. X., et al. (2024). Radix astragali and its representative extracts for diabetic nephropathy: efficacy and molecular mechanism. J. Diabetes Res. 2024, 5216113. doi:10.1155/2024/5216113
Yang, L., Yuan, S., Wang, R., Guo, X., Xie, Y., Wei, W., et al. (2024). Exploring the molecular mechanism of berberine for treating diabetic nephropathy based on network pharmacology. Int. Immunopharmacol. 126, 111237. doi:10.1016/j.intimp.2023.111237
Yang, S., Lin, C., Zhuo, X., Wang, J., Rao, S., Xu, W., et al. (2020). Glucagon-like peptide-1 alleviates diabetic kidney disease through activation of autophagy by regulating AMP-activated protein kinase-mammalian target of rapamycin pathway. Am. J. Physiol-Endoc M. 319 (6), E1019-E1030–E1030. doi:10.1152/ajpendo.00195.2019
Yassin, R., Tadmor, H., Farber, E., Igbariye, A., Armaly-Nakhoul, A., Dahan, I., et al. (2021). Alteration of autophagy-related protein 5 (ATG5) levels and Atg5 gene expression in diabetes mellitus with and without complications. Diabetes Vasc. Dis. Re 18 (6), 14791641211062050. doi:10.1177/14791641211062050
Yasuda-Yamahara, M., Kume, S., and Maegawa, H. (2021). Roles of mTOR in diabetic kidney disease. Antioxidants-Basel. 10 (2), 321. doi:10.3390/antiox10020321
Yau, W. W., Singh, B. K., Lesmana, R., Zhou, J., Sinha, R. A., Wong, K. A., et al. (2019). Thyroid hormone (T(3)) stimulates brown adipose tissue activation via mitochondrial biogenesis and MTOR-mediated mitophagy. Autophagy 15 (1), 131–150. doi:10.1080/15548627.2018.1511263
Yu, Z. K., Yang, B., Zhang, Y., Li, L. S., Zhao, J. N., and Hao, W. (2018). Modified Huangqi Chifeng decoction inhibits excessive autophagy to protect against Doxorubicin-induced nephrotic syndrome in rats via the PI3K/mTOR signaling pathway. Exp. Ther. Med. 16 (3), 2490–2498. doi:10.3892/etm.2018.6492
Yuan, W., Fang, W., Zhang, R., Lyu, H., Xiao, S., Guo, D., et al. (2023). Therapeutic strategies targeting AMPK-dependent autophagy in cancer cells. Bba-Mol Cell Res. 1870 (7), 119537. doi:10.1016/j.bbamcr.2023.119537
Zhan, H., Jin, J., Liang, S., Zhao, L., Gong, J., and He, Q. (2019). Tripterygium glycoside protects diabetic kidney disease mouse serum-induced podocyte injury by upregulating autophagy and downregulating β-arrestin-1. Histol. Histopathol. 34 (8), 943–952. doi:10.14670/HH-18-097
Zhang, L., Yang, L., Shergis, J., Zhang, L., Zhang, A. L., Guo, X., et al. (2019a). Chinese herbal medicine for diabetic kidney disease: a systematic review and meta-analysis of randomised placebo-controlled trials. Bmj Open 9 (4), e025653. doi:10.1136/bmjopen-2018-025653
Zhang, X., Jackson, S., Liu, J., Li, J., Yang, Z., Sun, D., et al. (2024). Arsenic aggravates the progression of diabetic nephropathy through miRNA-mRNA-autophagy axis. Food Chem. Toxicol. 187, 114628. doi:10.1016/j.fct.2024.114628
Zhang, X. X., Jiang, C. H., Liu, Y., Lou, D. X., Huang, Y. P., Gao, M., et al. (2019b). Cyclocarya paliurus triterpenic acids fraction attenuates kidney injury via AMPK-mTOR-regulated autophagy pathway in diabetic rats. Phytomedicine 64, 153060. doi:10.1016/j.phymed.2019.153060
Zhang, Y., Guo, R., Wang, S. S., Jiang, X. Y., Cui, H. Y., Guo, Y., et al. (2022a). Autophagy-related proteins in genome stability: autophagy-dependent and independent actions. Int. J. Biol. Sci. 18 (14), 5329–5344. doi:10.7150/ijbs.76134
Zhang, Z., Sun, Y., Xue, J., Jin, D., Li, X., Zhao, D., et al. (2022b). The critical role of dysregulated autophagy in the progression of diabetic kidney disease. Front. Pharmacol. 13, 977410. doi:10.3389/fphar.2022.977410
Zhao, M., Lin, J., Wang, X., Chen, C., Li, J., Yu, J., et al. (2024a). Multi-immunometabolomics mining: NP prevents hyperimmune in ALI by inhibiting Leucine/PI3K/Akt/mTOR signaling pathway. Free Radic. Bio Med. 225, 302–315. doi:10.1016/j.freeradbiomed.2024.09.053
Zhao, M., Yin, Y., Yang, B., Chang, M., Ma, S., Shi, X., et al. (2024b). Ameliorative effects of Modified Huangqi Chifeng decoction on podocyte injury via autophagy mediated by PI3K/AKT/mTOR and AMPK/mTOR pathways. J. Ethnopharmacol. 321, 117520. doi:10.1016/j.jep.2023.117520
Zhao, T., Xiang, Q., Lie, B., Chen, D., Li, M., Zhang, X., et al. (2023). Yishen Huashi granule modulated lipid metabolism in diabetic nephropathy via PI3K/AKT/mTOR signaling pathways. Heliyon 9 (3), e14171. doi:10.1016/j.heliyon.2023.e14171
Zhong, Y., Lee, K., and He, J. C. (2018). SIRT1 is a potential drug target for treatment of diabetic kidney disease. Front. Endocrinol. 9, 624. doi:10.3389/fendo.2018.00624
Zhong, Y., Liu, J., Sun, D., Guo, T., Yao, Y., Xia, X., et al. (2022a). Dioscin relieves diabetic nephropathy via suppressing oxidative stress and apoptosis, and improving mitochondrial quality and quantity control. Food Funct. 13 (6), 3660–3673. doi:10.1039/d1fo02733f
Zhong, Y., Luo, R., Liu, Q., Zhu, J., Lei, M., Liang, X., et al. (2022b). Jujuboside A ameliorates high fat diet and streptozotocin induced diabetic nephropathy via suppressing oxidative stress, apoptosis, and enhancing autophagy. Food Chem. Toxicol. 159, 112697. doi:10.1016/j.fct.2021.112697
Zhou, M., Zhang, S., Bai, X., Cai, Y., Zhang, Z., Zhang, P., et al. (2024). Acteoside delays the fibrosis process of diabetic nephropathy by anti-oxidation and regulating the autophagy-lysosome pathway. Eur. J. Pharmacol. 978, 176715. doi:10.1016/j.ejphar.2024.176715
Keywords: autophagy, diabetic kidney disease, sense of nutrition, oxidative stress, traditional Chinese medicine, mTOR, mechanism
Citation: Li L, Zou J, Zhou T, Liu X, Tan D, Xiang Q and Yu R (2025) mTOR-mediated nutrient sensing and oxidative stress pathways regulate autophagy: a key mechanism for traditional Chinese medicine to improve diabetic kidney disease. Front. Pharmacol. 16:1578400. doi: 10.3389/fphar.2025.1578400
Received: 17 February 2025; Accepted: 08 April 2025;
Published: 23 April 2025.
Edited by:
Irina Ielciu, University of Medicine and Pharmacy Iuliu Hatieganu, RomaniaReviewed by:
Kameswara Rao Badri, Morehouse School of Medicine, United StatesJunqi Chen, Guangzhou University of Chinese Medicine, China
Siying Weng, Ningbo Municipal Hospital of TCM, China
Yan Dai, The Affiliated Hospital of Southwest Medical University, China
Ning Wang, Dalian University of Technology, China
Sara Gamal Ahmed, Minia University, Egypt
Copyright © 2025 Li, Zou, Zhou, Liu, Tan, Xiang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Yu, eXVyb25neXlkc0AxNjMuY29t
 Liu Li1
Liu Li1 Rong Yu
Rong Yu