- 1School of Pharmaceutical Sciences, Changchun University of Chinese Medicine, Changchun, China
- 2Jilin Ginseng Academy, Changchun University of Chinese Medicine, Changchun, China
- 3Jilin Provincial Science and Technology Innovation Center of Health Food of Chinese Medicine, Changchun University of Chinese Medicine, Changchun, China
Background: Pueraria lobata (Willd.) Ohwi, a perennial vine native to China, has significant medicinal and edible value. Its roots are used as medicine and are known as kudzu (Chinese: Gegen) and were first recorded in Shen Nong Ben Cao Jing. In addition, its roots and powder can be made into food.
Aim of the study: To integrate information on the source of Pueraria lobata (PL), summarize the evolution of its medicinal and edible value, and generalize its chemical composition, biosynthetic pathways, metabolism, and biological activity.
Materials and Methods: A comprehensive literature search (1975–2025) was conducted across ScienceDirect, Google Scholar, PubMed, Web of Science, CNKI, WFO (www.worldfloraonline.org), MPNS (https://mpsn.kew.org), Changchun University of Chinese Medicine Library collections, and SciFinder. Ancient applications were validated through analysis of classic Chinese medical literature.
Results: PL is predominantly found in Asia, Europe, and America, with significant populations in China’s Zhejiang, Jiangxi, Jiangsu, Guangdong, and Guangxi provinces. The plant comprises flavonoids, triterpenoids, and coumarins, including isoflavonoids like daidzein and puerarin, which are synthesized via diverse pathways. Metabolites produced from liver or intestinal reactions are crucial to PL’s effectiveness. Key components include puerarin, daidzein, genistein, biochanin A, and formononetin. In China, PL is incredibly versatile, being used in a wide range of foods, teas, preservatives, dairy products, etc.
Conclusion: Its extensive biological activities benefit the human body, with particular emphasis on liver protection, anti-osteoporosis, and anti-diabetic effects. These attributes highlight the potential for developing health foods, revealing PL’s promising prospects in the pharmaceuticals and nutritional healthcare industries.
1 Introduction
Pueraria lobata (Willd.) Ohwi is a perennial vine native to China, mostly found in Asia, and later introduced to the Americas and Europe, that contains micromolecules such as flavonoids, triterpenoids, and coumarins, as well as macromolecules such as polysaccharides and proteins (Gao et al., 2024). PL has a wide range of biological activities, such as lowering blood pressure, lowering sugar, detoxification, anti-virus, anti-osteoporosis, anti-inflammatory, antioxidant, and anti-aging (Zhou et al., 2014), which makes it an unparalleled delicacy. It has been used for thousands of years, as early as the Shen Nong Ben Cao Jing; it is categorized as a middle grade. Ben Cao Jing Ji Zhu recorded its edible nature, and it has been used until now.
Although PL has been used in China for thousands of years, the records of PL in successive Materia Medica may be somewhat confusing due to climatic changes and dynastic changes. In order to clarify the origin and improve the quality of PL, it is important to verify the name, place of origin, plant morphology, and records of medicinal use and consumption of PL in ancient times. With the current demand for a healthier state of affairs, herbs such as PL, which can be used both medicinally and as a food, have gradually gained popularity, and the number of related studies has been increasing in recent years. In addition, at this stage, the research on the synthesis pathway and metabolic pathway of isoflavones of kudzu lacks generalization and summarization, and the medicinal values and food functions of PL are not comprehensively expressed, which seriously affects its development. The present review is intended to provide a comprehensive representation of PL in terms of its basal origin, phytochemical profile, synthesis, metabolism, bioactivities, and comprehensive utilization for food consumption and medicinal use.
2 Materials and methods
2.1 Search strategy
An online literature search was carried out at ScienceDirect, Google Scholar, PubMed, Web of Science, CNKI, WFO (https://www.worldfloraonline.org), MPNS (https://mpsn.kew.org), Changchun University of Chinese Medicine Library collections, and SciFinder, covering 1975 to 2025. The following keywords were used: “Pueraria lobata” and “phytochemistry” or “pharmacology” or “edibility.” The references of all retrieved articles were also reviewed to include relevant literature. Consistent selection criteria were applied across all databases, with duplicate studies eliminated through a two-step process: automated detection using Zotero software, followed by manual cross-checking. Ancient applications were validated through analysis of classic Chinese medical literature. Comprehensive searches encompassed medicinal and dietary records containing the following nomenclature: “Ge Gen,” “Ge,” “Lu Huo,” “Huang Jin,” “Ye Ge,” and “Gan Ge.” Relevant prescriptions were compiled following duplicate removal via cross-database verification, with remaining materials organized chronologically based on textual origins.
2.2 Selection criteria
The inclusion criteria are as follows: PL, PL extracts, and its compounds are the subjects of the study; the diseases and physiological processes targeted by PL; the study design is clear, and the study results involve the exploration of relevant mechanisms; the latest research on PL’s clinical applications and formulations is included; the literature was published within the past 50 years, unless it holds significant historical value; the research must explicitly describe molecular mechanisms or signaling pathways and their effects on bioavailability or efficacy.
Exclusion criteria comprised: 1) literature reviews lacking clear research parameters (subjects/methods/mechanisms), 2) methodologically deficient studies (small sample sizes, unreliable results, and low quality), 3) duplicate publications, 4) non-data-driven articles (conference abstracts, editorials, and opinion pieces), and 5) non-peer-reviewed/unpublished research. Standardized extraction protocols ensured data uniformity, with dual independent review by researchers enhancing analytical rigor.
3 Herbal textual research
3.1 Name
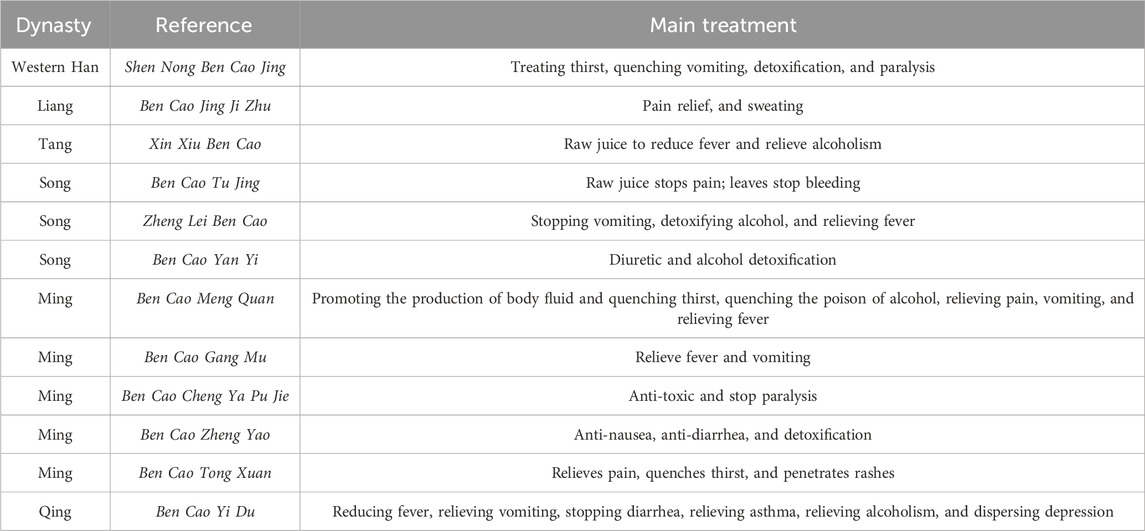
The name of Ge Gen was first mentioned in Shen Nong Ben Cao Jing and recorded as the proper name of this product. Other herbal works, such as Xin Xiu Ben Cao, Zheng Lei Ben Cao, Ben Cao Gang Mu, and Ben Cao Cong Xin, in the record of this product, use Ge Gen as the proper name, which has been used until now. The synonyms are Ji Qi Gen (Shen Nong Ben Cao Jing and Ben Cao Jing Ji Zhu), Lu Huo (Ben Cao Jing Ji Zhu and Ming Yi Bie Lu), Huang Jin (Ben Cao Jing Ji Zhu and Ming Yi Bie Lu), Ge Dou (Ben Cao Pin Hui Jing Yao), and Gan Ge (Pao Zhi Quan Shu).
3.2 Place
There are different opinions about the origin of PL. Shen Nong Ben Cao Jing records that it “grows in mountain valleys,” and Ben Cao Tu Jing states that it is especially abundant in Jiangsu and Zhejiang provinces. “The essence of the herb” pointed to Jiangsu, Zhejiang, and Jiangxi provinces as the road to the production area. The name of the plant in the real map test recorded PL as a cultivated plant; there are also wild versions. Nowadays, there is much PL in Guangdong, Guangxi, Hainan, Zhejiang, and Jiangsu provinces, and wild PL is found in Hebei, Henan, Jiangxi, Hubei, and Hunan provinces (Table 1). Therefore, PL-producing areas are Zhejiang, Jiangxi, Jiangsu, Guangdong, Guangxi, Hainan, Hebei, Henan, Hubei, and Hunan provinces. With the change of climate, PL is now mainly grown commercially in Hunan, Zhejiang, Jiangxi, and Hubei provinces.
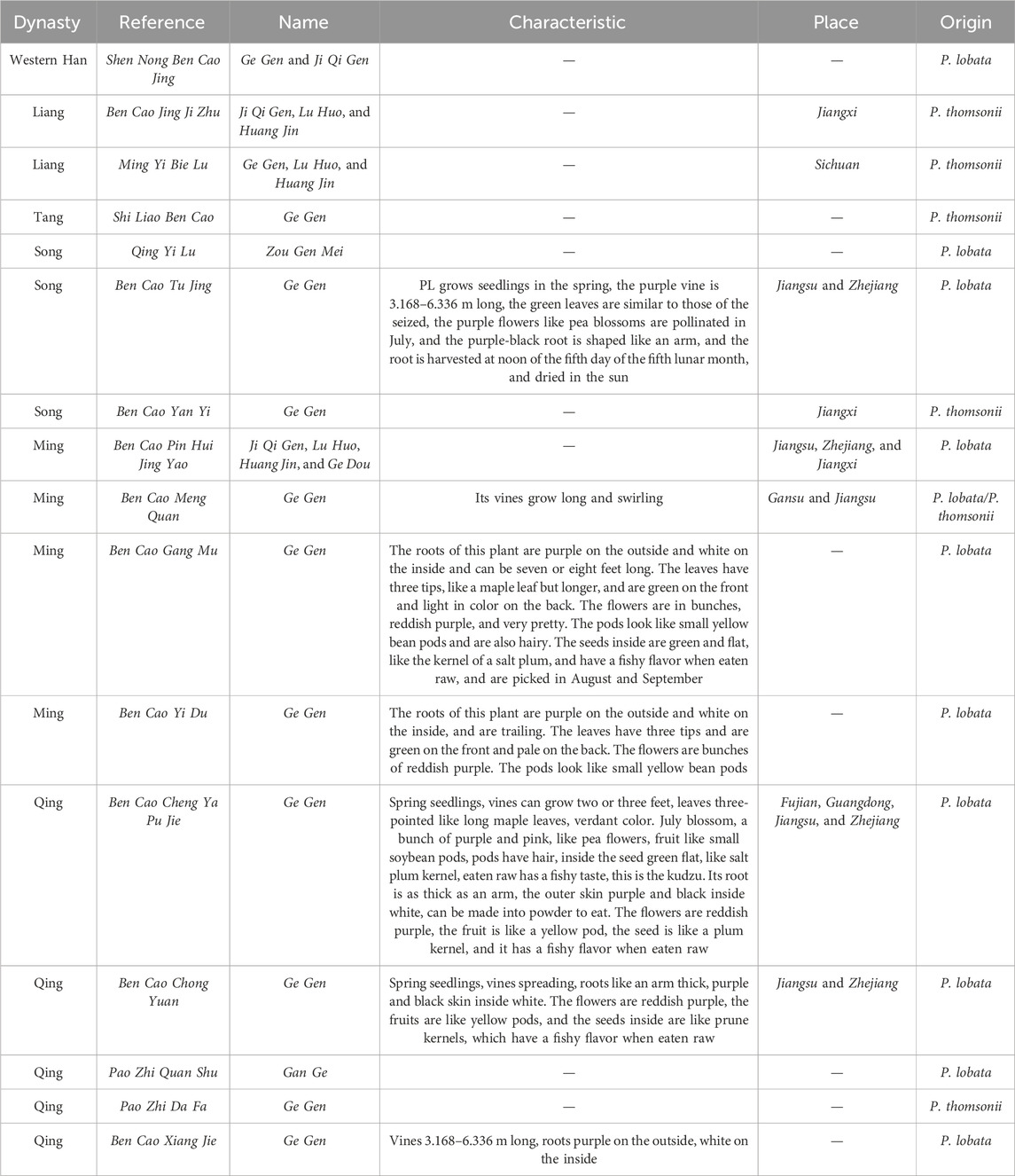
Table 1. Names, characteristics, and places of Pueraria lobata (Willd.) Ohwi in Materia Medica throughout the ages.
3.3 Origin
After integrating the herbs of the past dynasties, Figure 1 was obtained. Before the Song Dynasty, the basal origin was not clearly defined, and there was often a mixed use; after the Song Dynasty, both P. lobata and Pueraria thomsonii were used as the source of kudzu. From the Ming and Qing dynasties to modern times, P. lobata was mainly used as a medicine and was categorized as the authentic source of P. lobata in the Chinese Pharmacopoeia.
3.3.1 Song and pre-Song dynasties
Before the Song Dynasty, there was no clear description of the plant of PL. Nan Fang Cao Mu Zhuang clearly records that “Ye Ge is a kind of Duan Chang Cao, which is called Huo Ba Hua by people in southern Yunnan province.” Combined with the historical background and the depiction in the picture, based on the available literature and speculation, what is depicted in this book may be Gelsemium elegans, which is also known as Gou Wen. It is highly poisonous, and G. elegans is not a source of medicinal plants. It suggests that there may have been cases of PL with the same name before the Han Dynasty. The form of PL can be traced back to the Northern Song Dynasty. The Ben Cao Tu Jing contains: “PL grows seedlings in the spring, the purple vine is 3.168–6.336 m long, the green leaves are similar to those of the seized, the purple flowers like pea blossoms are pollinated in July, and the purple-black root is shaped like an arm, and the root is harvested at noon of the fifth day of the fifth lunar month and dried in the sun.” This is the first description of the morphology of the PL, with illustrations of Haizhou (Jiangsu province) kudzu and Chengzhou (Gansu province) kudzu, the latter of which has a single leaf and is clearly not a Pueraria plant, whereas the Haizhou kudzu has three leaflets, pods, and may be P. lobata. From the above, it can be seen that during the Song Dynasty, different varieties of plants were already used as kudzu mixes. The Northern Song Dynasty’s Ben Cao Yan Yi recorded that: “During the winter, fresh PL is kneaded in water to produce starch, which settles and is made into starch cubes. Boil water first, then break the starch block into small pieces and put it in to boil, until the color is like glue and it becomes very tough. Fish it out and mix it in honey water to eat; add some grated ginger for better flavor. Some people also add these starch pieces to the tea to cook to entertain guests, but just sweet, nothing special benefits.” The use of P. thomsonii is clearly reflected here, and it can be assumed that after the Song Dynasty, the species of kudzu gradually diversified, with both P. lobata and P. thomsonii being used.
3.3.2 Ming and Qing dynasties
By the Ming Dynasty, the phytomorphological drawings of PL contained in Jiu Huang Ben Cao, and other herbaceous texts were all likely to be P. lobata, but P. thomsonii had been recognized as having a less medicinal, but rather powdery and edible character, but its phytomorphology had not been described. It is stated that “the sweet-tasting kudzu is sweet kudzu, and the bitter-tasting kudzu is bitter kudzu,” and the sweet kudzu it describes is probably the edible kudzu, Pueraria edulis, which is clearly distinguished from the other kudzu plants by the distinctly terete tuberous roots and the banded, rectangular-shaped pods in Dian Nan Ben Cao. Alternatively, bitter kudzu may be Pueraria peduncularis, also known as Yunnan Geteng, which is not used medicinally. Ben Cao Pin Hui Jing Yao redrew the Haizhou kudzu and Chengzhou kudzu from the Ben Cao Tu Jing, adding color to the previous black-and-white illustration, and it is clear that both are kudzu, with Haizhou kudzu morphologically characterized by a trilobed terminal leaflet, occasionally entire, presumed to be PL. The morphology of Chengzhou kudzu is characterized by trilobed terminal leaflets, occasionally entire, and a large degree of tuberous expansion, presumably P. thomsonii.
In more recent times, the morphology of P. thomsonii has been more carefully drawn in the Long Men Min Jian Cao Yao, and the expanded state of its tuberous roots has generally become an identifying feature. Therefore, the Chinese Pharmacopoeia specifies the authentic source of PL (China, 2020), which reinforces the fact that PL has been used as a medicine for thousands of years.
3.4 Usage
3.4.1 Medicinal use
The use of PL was first described in the Shen Nong Ben Cao Jing. It is mainly used for treating thirst, fever, vomiting, and various kinds of paralysis. Since then, the medicinal effects of PL have been documented throughout the ages, including relieving pain, sweating, penetrating rashes, and relieving depression. Xin Xiu Ben Cao recorded that “its flowers and small bean flowers dried powder, take a little; when poor drinking, that is, the flower of PL for a good antidote to alcohol.” The efficacy of raw and cooked products is distinguished in the Ben Cao Meng Quan: raw can abort fetuses, steamed and cooked to remove the poison of wine (Table 2). In summary, PL is sweet, pungent, cool, and non-toxic, with medicinal values of relieving muscle fever, generating fluids to quench thirst, penetrating rashes, raising the sun to stop diarrhea, invigorating the meridians, and detoxifying alcohol, which is in line with the Chinese Pharmacopoeia (China, 2020).
3.4.2 Edible use
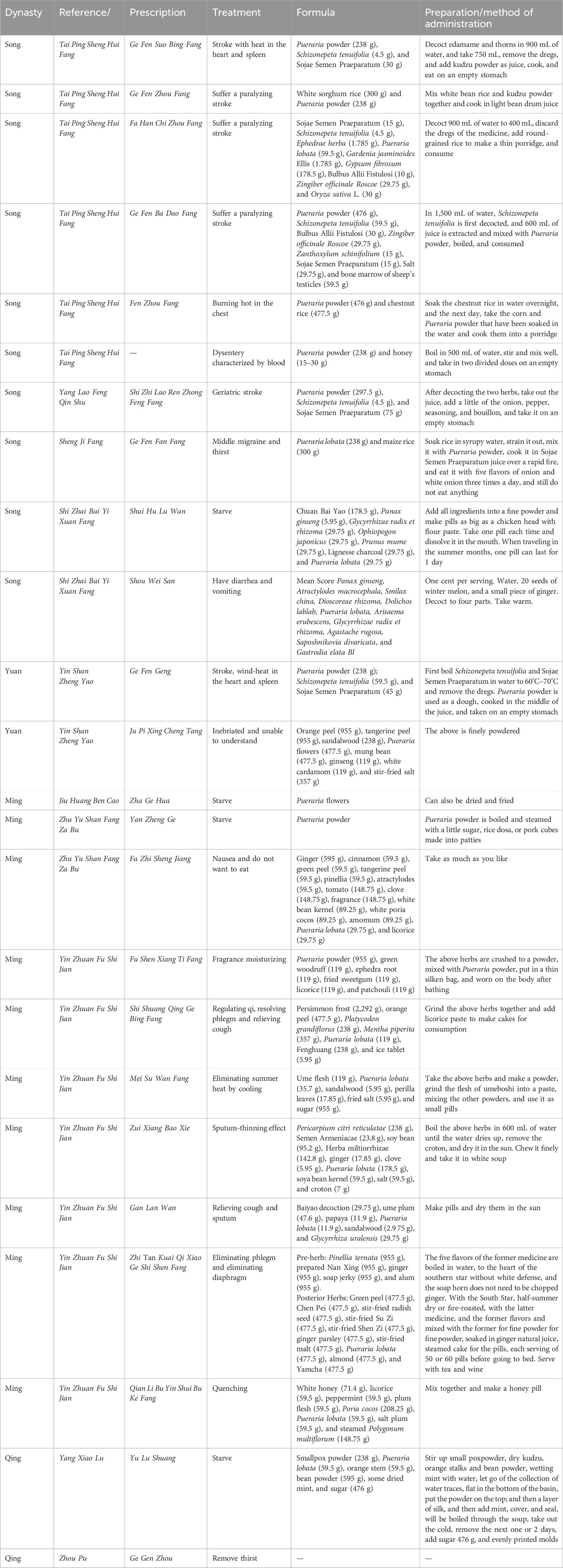
Ancient texts on the use of PL as a food application can be traced as far back as the Northern and Southern Dynasties (220–589), and Ben Cao Jing Ji Zhu clearly states that kudzus are steamed and eaten by people. The earliest application of kudzu powder can be traced back to the Tang Dynasty (713–741), Shi Liao Ben Cao recorded that: raw kudzu is harvested in winter, kneaded out of the powder with water, made into pallets, fried in boiling soup, removed, and put into the soup liquid, waiting for its color like glue, with white and toughness. Mixed with honey and eaten, crushed ginger is also good to treat fever, drunkenness, and thirst; it can facilitate urination and is also refreshing; when cut and eaten as a tea, it is very sweet, and raw kudzu directly simmered has a nourishing effect. In 978, Tai Ping Sheng Hui Fang was published, in which are recorded the Ge Fen Suo Bing Fang, Ge Fen Zhou Fang, Fa Han Chi Zhou Fang, Ge Fen Ba Dao Fang, and Fen Zhou Fang. Thereafter, during the Song and Yuan dynasties, PL was consumed mainly for the treatment of thirst and stroke in old age. In the Ming Dynasty, the therapeutic applications of PL were more extensive, including relieving cough, resolving phlegm, quenching thirst, filling hunger, and quenching summer heat, etc., and there were even external prescriptions for moisturizing and perfuming the skin and body, such as the Fu Shen Xiang Ti Fang (Table 3).
Modern research has shown that PL has a rich nutritional value because it contains a large amount of starch, polyphenols, amino acids, etc. (Xu et al., 2022; Wang J. et al., 2023). Yang et al. (2022) showed that the apparent content of resistant starch of PL was 23.14%, the molecular weight was 1.93 × 107 Da, and the solubility and solubility of starch of PL were 38.51% and 28.10 g/g, respectively. This suggests that PL could be a potential source of dietary fiber and that the starch of PL has a better application in food processing. Pang et al. (2023) found that the foaming capacity (200%) and foaming stability (97.5%) of PL’s protein increased with hydrolysis. This shows that hydrolysis contributes to the alteration of the functional properties of PL, offering the possibility of its food applicability. In addition, the leaf extract of PL contains amino acids such as arginine, valine, and proline, including 0.23% Na, 3.5% K, and 0.24% Ca (Kemertelidze et al., 2008). This reinforces the richness of PL’s prospects for food development.
Nutritional analysis revealed PL exhibits a distinct nutritional profile characterized by: 449.85 mg/g starch content, 3.81 g/100 g soluble dietary fiber, 10.23 g/100 g insoluble dietary fiber, and 106.24 g/kg crude protein content (Fu M., 2023). Compared to other products that provide dietary fiber (sweet potato 2.21%, yam 7.3%), PL is high in dietary fiber and low in calories, which provides a unique basis for its food value (Zhang Z. et al., 2023; Li Y. et al., 2023). Among them, Puerariae lobatae Radix-resistant starch is a potential novel prebiotic that is able to repair the intestinal barrier and reverse the pathology of the organism, with obvious health effects (Lian et al., 2023). Additionally, its homogeneous polysaccharide supports intestinal homeostasis by increasing short-chain fatty acid levels and regulating gut microbiota composition (Cao et al., 2025). Additionally, kudzu starch had a lower estimated glycemic index value than other starches (Ge et al., 2014).
3.5 Worldwide distribution
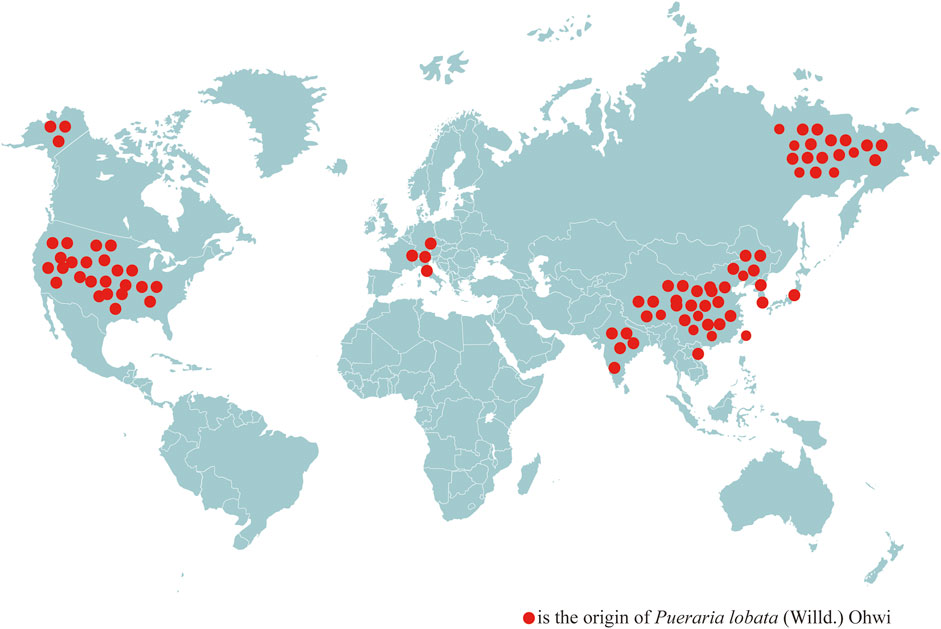
Countries other than China have recorded the use of PL in their herbal works. For example, Da He Ben Cao, written in Japan at the beginning of the Edo period (approximately 1603 A.D.), clearly records that the vine of PL can be used as a rope, the skin of PL can be used as a cloth, mourning clothes, etc., the leaves of PL can be used as fodder for horses, the powder of PL can quench thirst, stop diarrhea, and treat thirst, and the flowers of PL can quench alcohol. This indicates that the edible and medicinal functions of PL have been used for hundreds of years in many Asian countries. Combined with the Materia Medica examination, it can be seen that the medicinal and edible properties of PL have been uninterrupted since ancient times, and throughout the world, PL is mainly distributed in Asia and some parts of Europe and America (Figure 2). PL is found in 13 countries, including China, North Korea (Mun and Mun, 2015), Japan, South Korea, India (Jin et al., 2012), the Russian Far East (Kozhevnikov et al., 2017), Thailand, Vietnam (Nguyen et al., 2009), the United States (Pappert et al., 2000), Switzerland, Austria, Slovenia, and Italy (Follak, 2011). It is widely used in the food and pharmaceutical industries in Asia, whereas PL from Europe and the United States is often treated as an invasive species due to climatic and geographic differences, which has led to its lack of widespread use.
4 Phytochemistry
4.1 Chemical composition
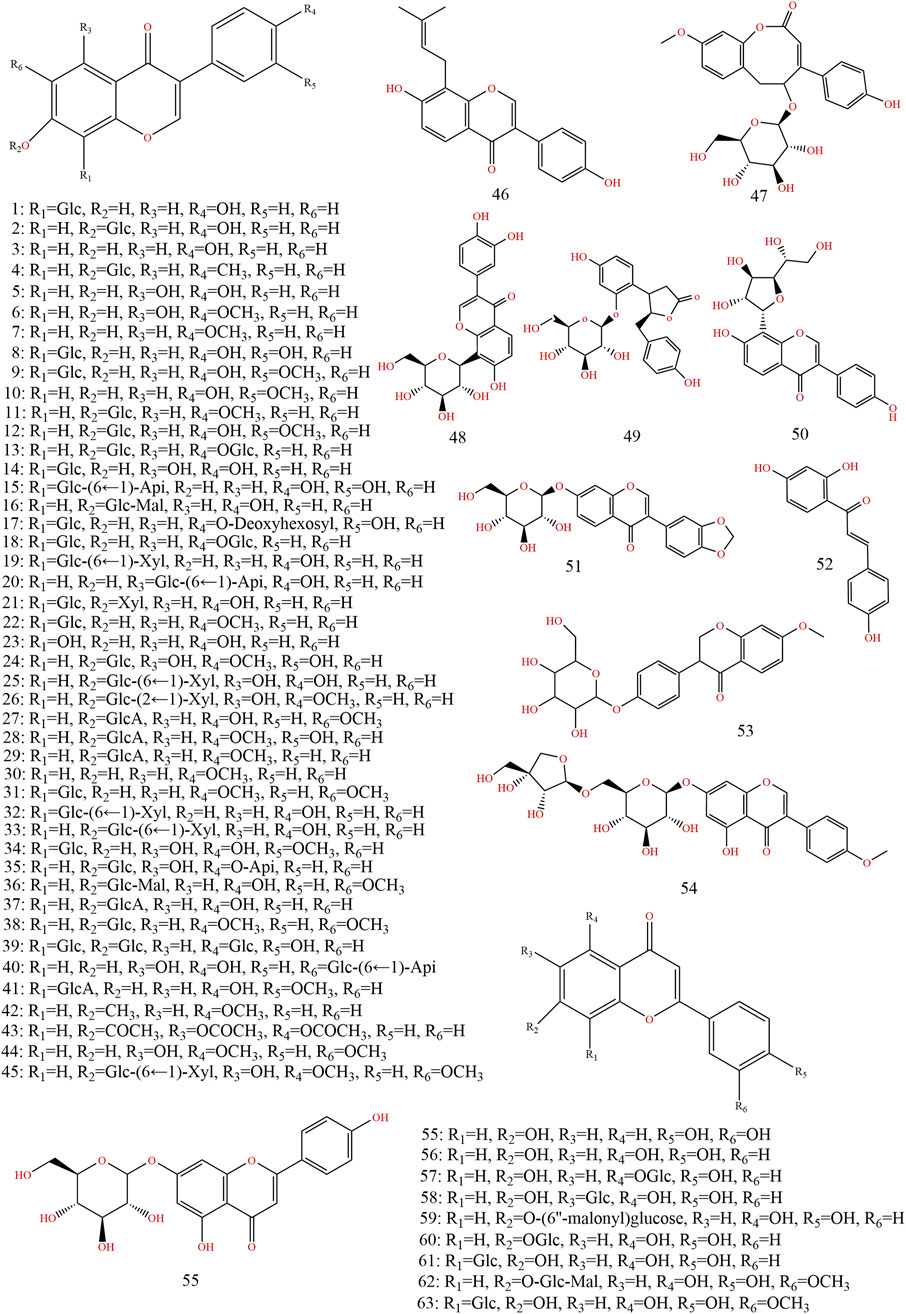
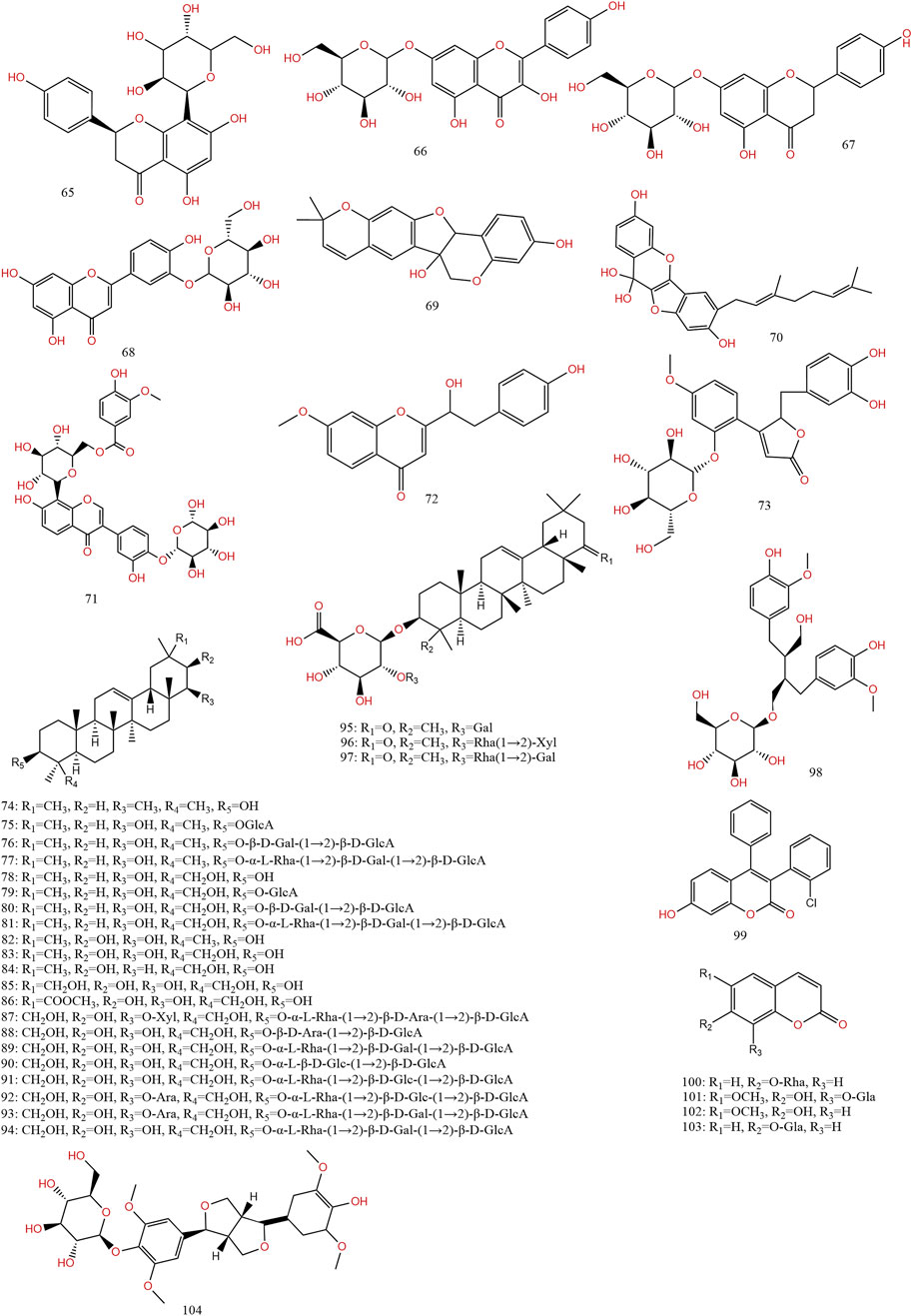
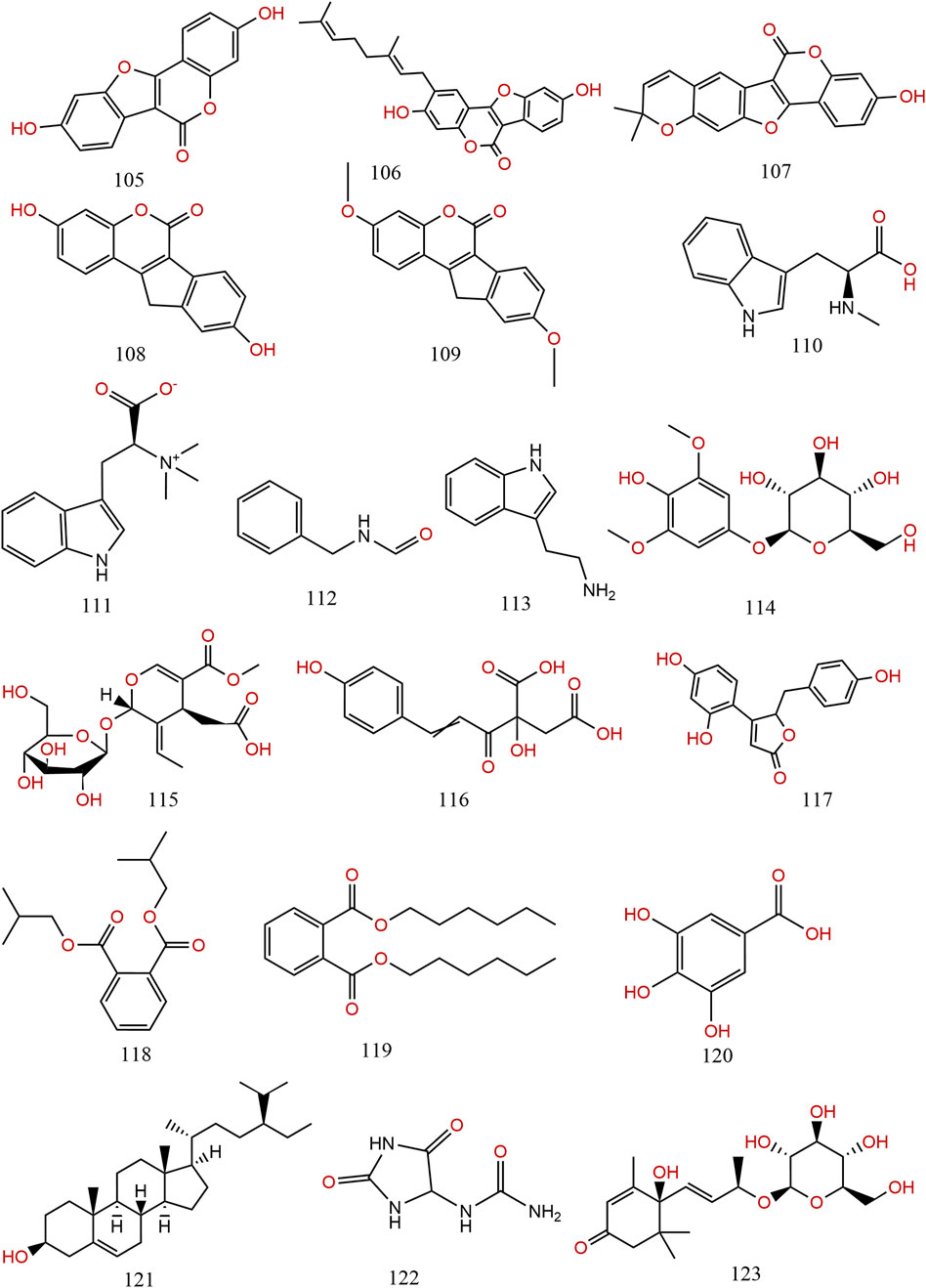
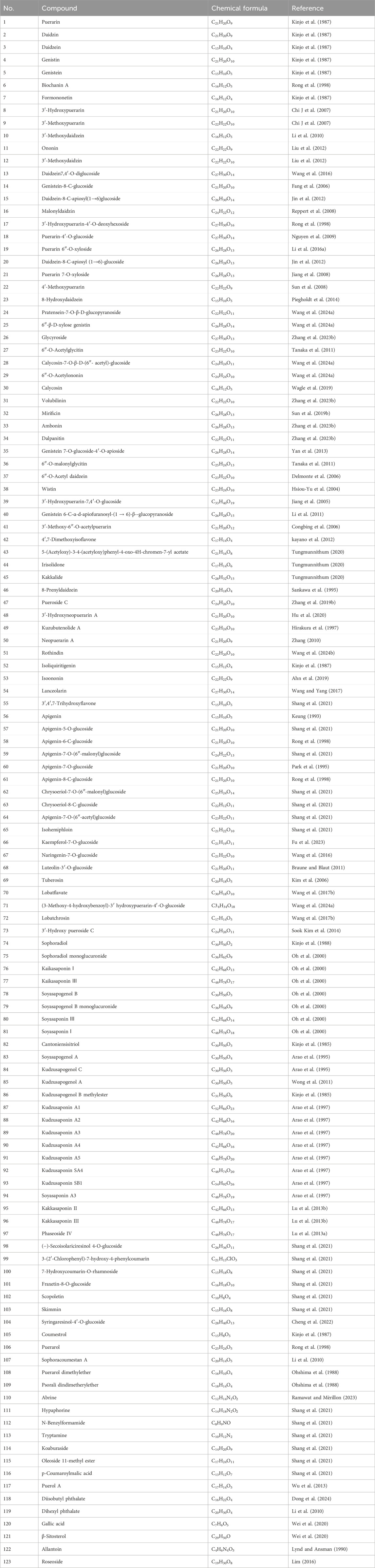
Previous information on the chemical composition of PL is shown in Figures 3–5, which summarize a total of 123 compounds in PL, of which 1–73 are flavonoids, 74–97 are triterpenoids, 98–109 are lignans and coumarins, 110–113 are alkaloids, and 114–123 are others (Table 4).
4.2 Biosynthetic and metabolic pathways
4.2.1 Biosynthetic pathways
The biosynthetic pathways of isoflavonoid metabolites in PL mainly include the mangiferolic acid, phenylpropionic acid, and puerarin pathways. After the production of p-coumaroyl-CoA, isoliquiritigenin was catalyzed by CHS and CHR, liquiritigenin was produced under the catalysis of CHI, 2,7,4′-trihydroxyisoflavanone was produced under the catalysis of 2-HIS, and daidzein wasp roduced under the catalysis of HID. Daidzein can produce puerarin and puerarin xyloside via PIUGT43. 3′-Hydroxydaidzein produces 3′-hydroxypuerarin via PIUGT43, and daidzein produces formononetin via PIOMT9. 3′-Hydroxypuerarin can be converted to 3-methoxy puerarin when catalyzed by PIOMT4. In addition, p-coumaroyl-CoA catalyzed by CHS directly produced naringenin chalcone, naringenin chalcone catalyzed by CHI produced naringenin, naringenin catalyzed by 2-HIS produced 2,5,7,4′-tetra- hydroxyisoflavanone, 2,5,7,4′-tetra-hydroxyisoflavanone catalyzed by HID produced genistein, and post-genistein can undergo the action of PIUGT43 or UGT71T5 to produce genistein-8-C-glucoside (Figure 6).
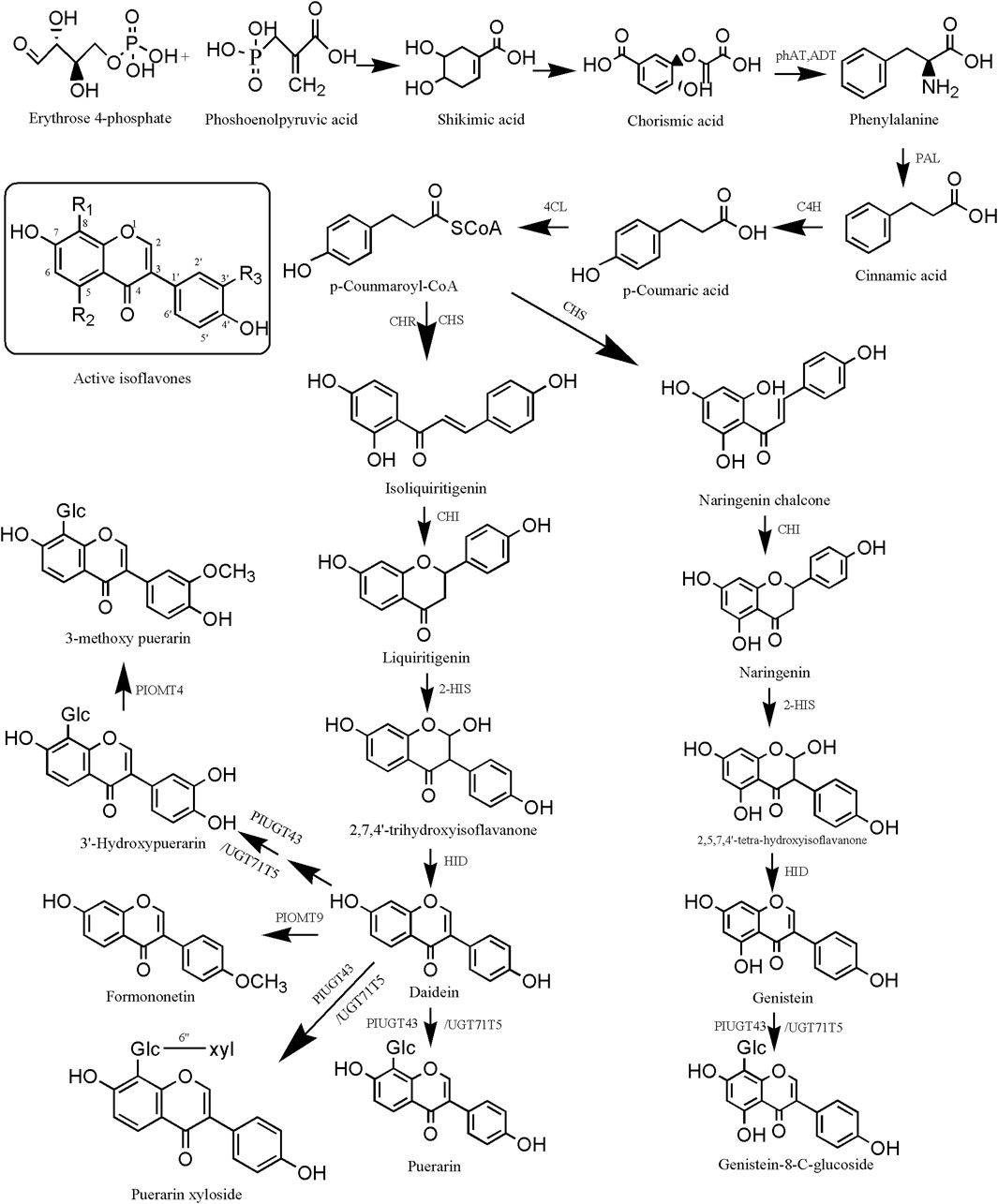
Figure 6. Biosynthesis pathway of isoflavonoid metabolites in Pueraria lobata (Willd.) Ohwi. phAT, plant phosphotransacetylase; ADT, plant aronic acid dehydratase; PAL, phenylalanine ammonia-lyase; C4H, cinnamate 4-hydroxylase; 4CL, 4-coumarate-CoA ligase; CHR, chalcone reductase; CHS, chalcone synthase; CHI, chalcone isomerase; HID, 2-hydroxyisoflavanone dehydratase.
From the above, it can be seen that the active isoflavone metabolites are the common skeleton with the presence of a wide range of isoflavonoid metabolites in PL, daidzein, puerarin, genistein, and so on. They are the mass signature metabolites. 6-C, 8-C, 3′-C, and 4′-C are the active sites of methylation and glycosylation that are susceptible to them (Li and Zhang, 2023). Of these, there are two theories for glycosylation at the 8-C position. One theory is that it is regulated through the PIUGT43 gene (Liga and Paul, 2024). The second theory is regulation through the UGT71T5 gene (Adolfo et al., 2022). Li et al. (2021) investigated the response of PL to selenium (Se) stimulation and found that five miRNAs targeting nine regulatory protein genes, which may be involved in the biosynthesis of isoflavonoids. Li et al. (2016b) found that PlOMT9 plays an important role in the biosynthesis of formononetin. PlOMT4 is a key enzyme in the 3′O-methylation of active isoflavones to enhance the lipophilicity of similar metabolites (Li et al., 2016c).
4.2.2 Metabolic pathways
Isoflavones are susceptible to oxidation in the presence of intestinal endophytes to produce ethylphenol derivatives. The isoflavones in PL undergo two main types of reactions in the gut. In the case of puerarin, for example, one is the generation of daidzein by strain CG19-1/PUE or DgpA + DgpBC bacteria (Choi et al., 2023). Subsequently, daidzein was converted to O-desmethylangolensin by Eubacterium ramulus (ER), and O-desmethylangolensin was catalyzed by CG19-1 to produce resorcinol, 2-(4-hydroxyphenyl)-propionic acid (Braune and Blaut, 2011). The second is the generation of dihydrodaidzein from daidzein catalyzed by key bacteria such as PUE and DZE, which ultimately generates (3S)-equol (Jin et al., 2008) (Figure 7). Puerarin effectively attenuated M1-like macrophage activation mediated through Akkermansia muciniphila-derived protein Amuc_2172, consequently ameliorating pathological alterations in the dextran sulfate sodium (DSS) model (Tao et al., 2024b). The metabolites demonstrate preventive effects against high-fat-diet-induced obesity by inhibiting A. muciniphila activity (Wang et al., 2019b; Yang C. W. et al., 2024).
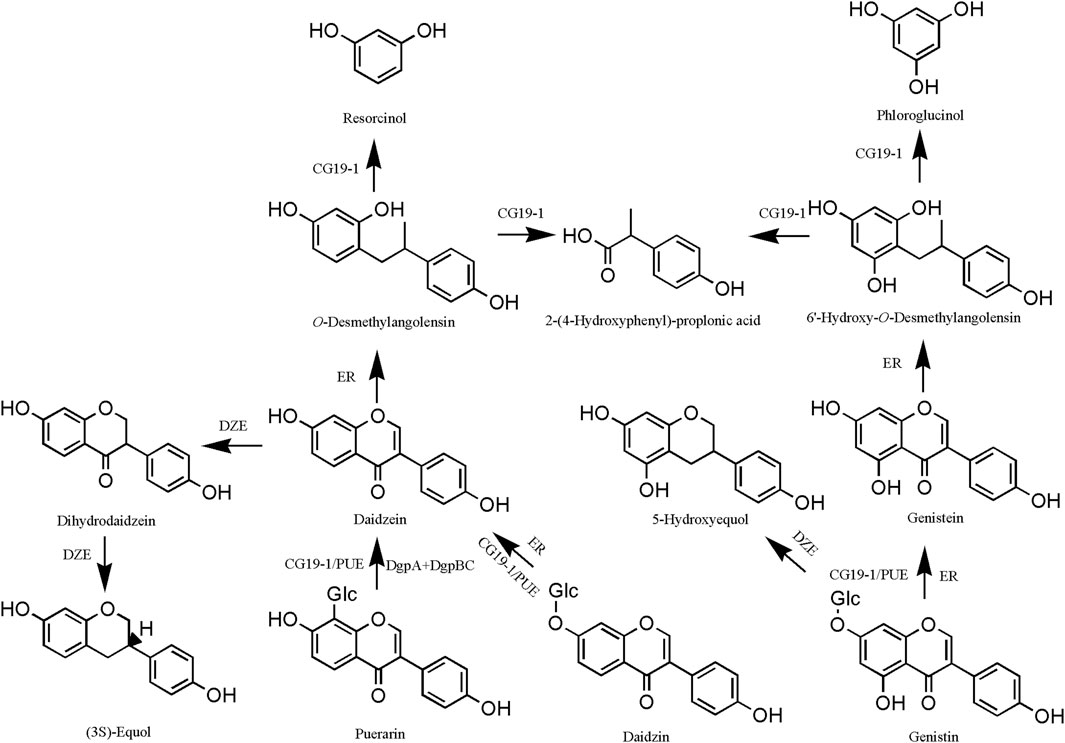
Figure 7. Intestinal metabolism of Pueraria lobata (Willd.) Ohwi isoflavones. DgpA, GenBank, BBG22493.1; DgpBC, PDBID, EM Map EMD-30808; PUE, 1.96 0.09 optical density (O.D.); DZE, 0.30 0.02 O.D.
In the liver, the isoflavones of PL often undergo glucuronidation, methylation, and reduction reactions. The hepatic metabolism of isoflavones was elucidated using daidzein and puerarin as examples (Figure 8). First, puerarin undergoes glucuronidation catalyzed by UGTA1 (UDP-glucuronosyltransferase 1A1: Gene ID 54658) to produce puerarin-7-O-glucuronide, or it undergoes hydrolysis to produce daidzein (Luo et al., 2012). In addition, daidzein is converted to formononetin catalyzed by CYP1A2 (cytochrome P450 family 1 subfamily A polypeptide 2: Gene ID 1543) enzyme and may also produce dihydrodaidzein, equol, and others (Atherton et al., 2006). It has been confirmed that the products after glucuronidation and reduction reactions are one of the important material bases for the action of puerarin, so the research on the hepatic metabolism of the isoflavones of PL is of great significance. Furthermore, puerarin demonstrates targeted therapeutic effects on hepatocytes. As evidenced by Fang et al. (2024), this metabolite facilitates macrophage phenotype switching from M1 to M2 type through ULK-1 upregulation and PAI-1 downregulation. This mechanism specifically restores hepatocyte autophagy homeostasis and ameliorates pathological alterations.
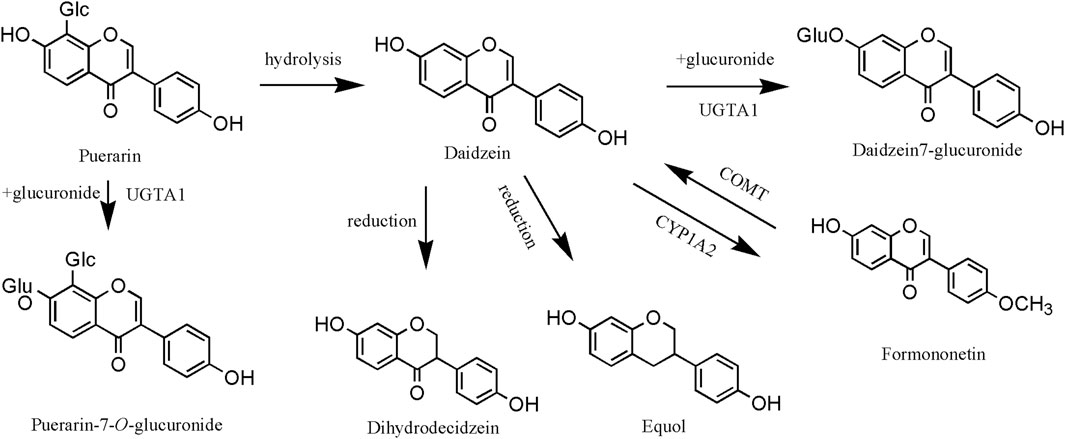
Figure 8. Liver metabolism of Pueraria lobata (Willd.) Ohwi isoflavones. UGTA1, UDP-glucuronosyltransferase 1A1; COMT, catechol-O-methyltransferase, Gene ID 1312; CYP1A2, cytochrome P450 family 1 subfamily A polypeptide 2.
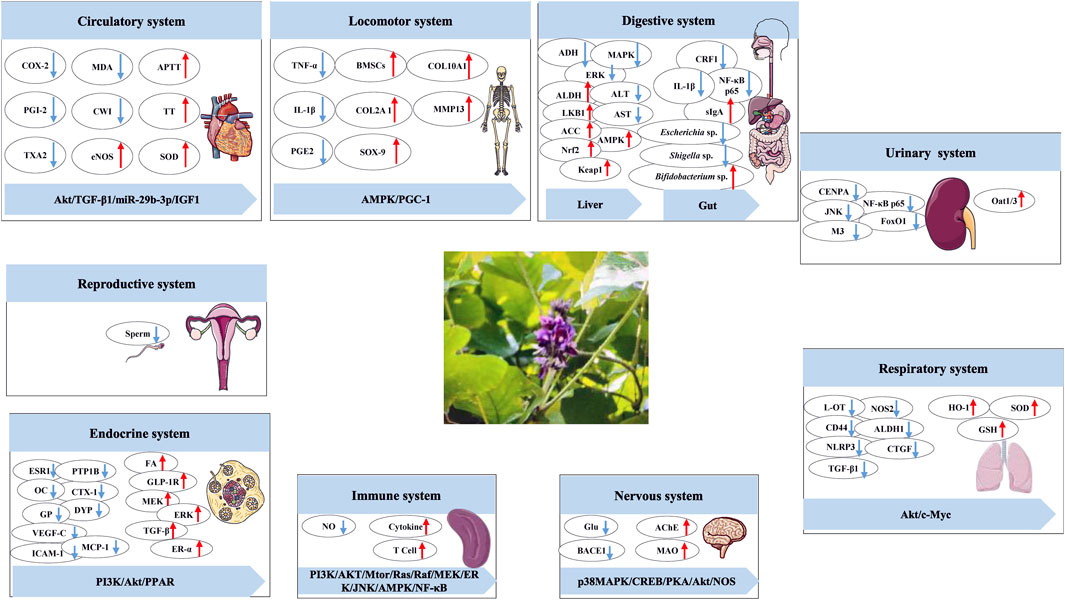
5 Pharmacology
PL has a wide range of biological activities, which are summarized according to the classification of the nine major systems of the human body in biology. The results are shown in Figure 9 and Table 5–8.
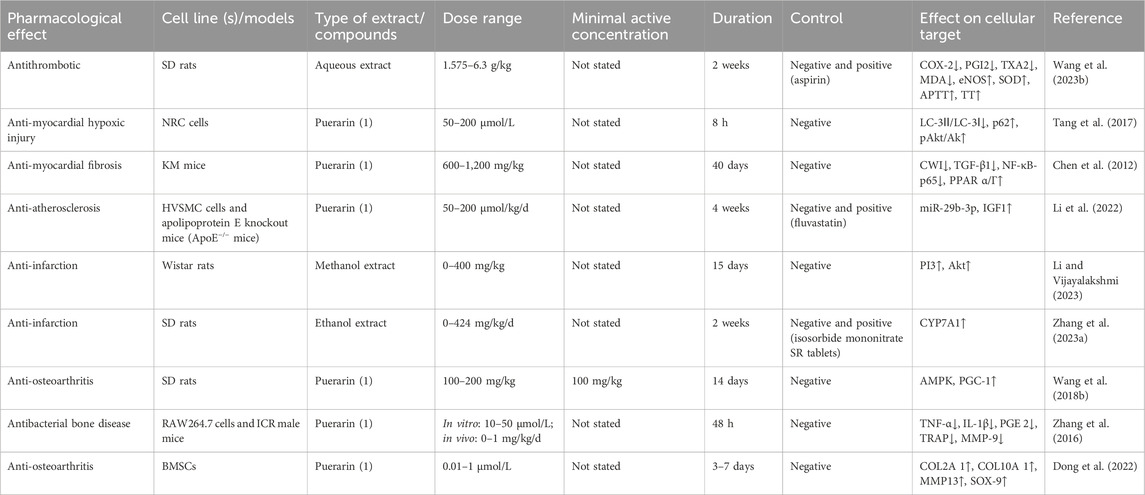
Table 5. Bioactivities of extracts or compounds from Pueraria lobata (Willd.) Ohwi in the circulatory and locomotor systems.
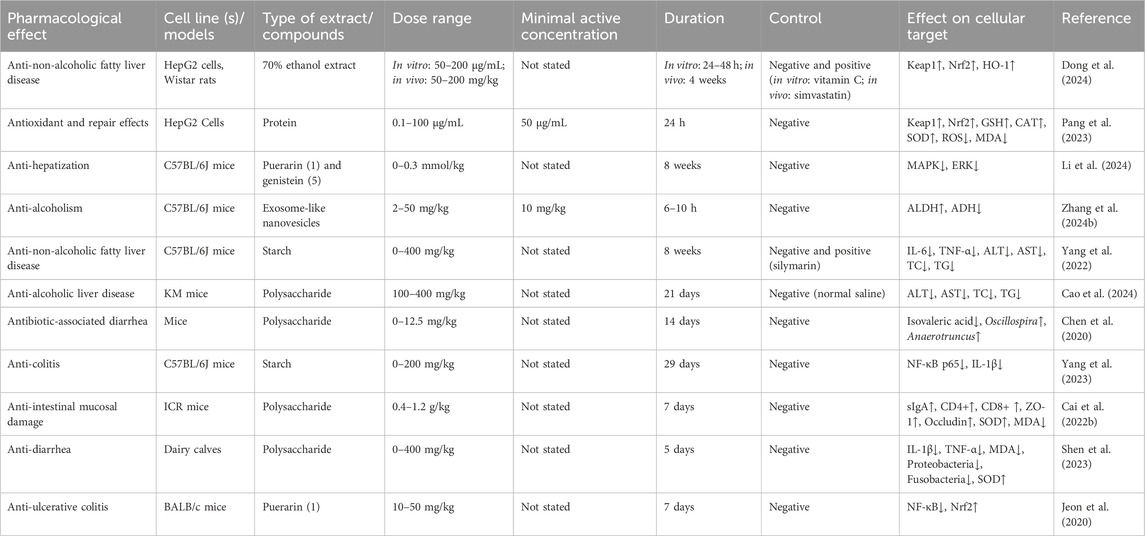
Table 6. Bioactivities of extracts or compounds from Pueraria lobata (Willd.) Ohwi in the digestive system.
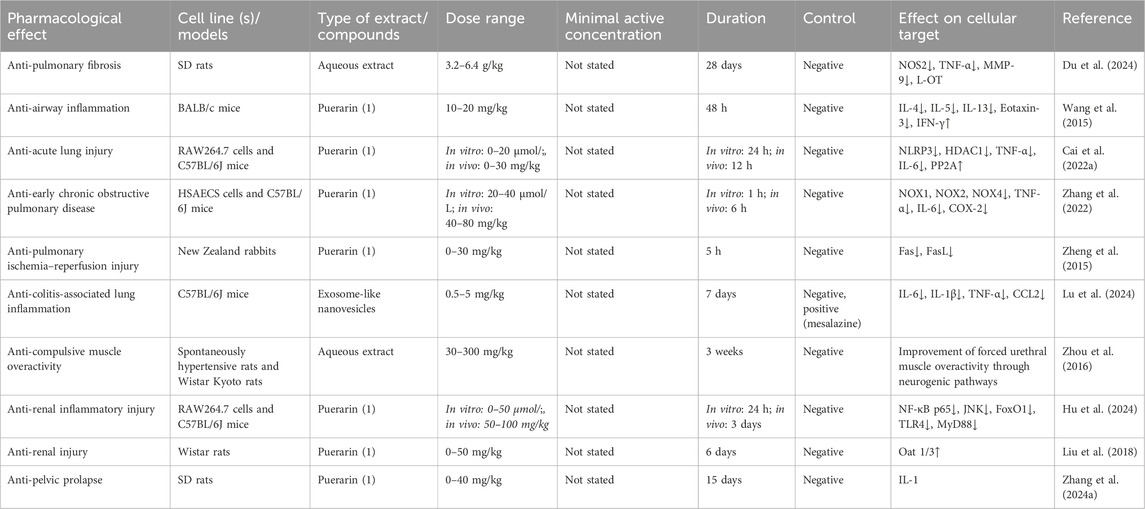
Table 7. Bioactivities of extracts or compounds from Pueraria lobata (Willd.) Ohwi in the respiratory and urinary systems.
Table 8. Bioactivities of extracts or compounds from Pueraria lobata (Willd.) Ohwi in the endocrine, nervous, and immune systems.
5.1 Circulatory system
PL has obvious antithrombotic effects, and its aqueous extract was able to reduce cyclooxygenase-2 (COX-2), prostaglandin-I-2 (PGI2), thromboxane A2 (TXA2), and malondialdehyde (MDA). The extract can elevate endothelial nitric oxide synthase (eNOS), superoxide dismutase (SOD), activated partial thromboplastin time (APTT), and thrombin time (TT) in an arteriovenous bypass thrombosis model in rats (Wang S. et al., 2023). Puerarin has a significant inhibitory effect on a hypoxia-induced myocardial ischemia model in mammary rat cardiomyocytes, as evidenced by the reduction of LC-3II/LC-3Ⅰ and the elevation of p62 and protein kinase B (pAkt)/Akt expression (Tang et al., 2017). In addition, puerarin was able to prevent subcutaneous isoprenaline-induced myocardial fibrosis by decreasing cell-wall integrity (CWI), transforming growth factor-beta (TGF-β) 1, nuclear factor kappa-B (NF-κB) -p65, and elevating peroxisome proliferator-activated receptors (PPAR) α/Γ (Chen et al., 2012). Puerarin also inhibits vascular smooth muscle proliferation in atherosclerosis via the miR-29b-3p/IGF1 (insulin-like growth factor 1) pathway (Li et al., 2022). This suggests that PL has an irreplaceable effect on the circulatory system, both from a compositional and ancient formulas. A study has confirmed that an extract of PL leaves can improve experimental myocardial infarction in Wistar rats through the (phosphatidylinositol 3-kinase) PI3K/Akt signaling pathway (Li and Vijayalakshmi, 2023). Zhang P. et al. (2023) combined the joint analysis of intestinal flora and bile acid metabolism and found that the ethanol extract of PL was able to effectively improve the bile acid level in rats with acute myocardial infarction by increasing the expression of cholesterol 7 alpha-hydroxylase (CYP7A1) and restoring the diversity of intestinal flora.
5.2 Locomotor system
Due to the presence of isoflavonoids such as puerarin, which has been described as a “natural phytoestrogen” (Wang X. et al., 2024), and the fact that estrogens have been shown to promote bone growth and development (Rizzoli and Bonjour, 1997), the motor systemic activity of PL should not be overlooked. Wang L. et al. (2018) found that puerarin could alleviate mitochondrial dysfunction and reduce osteoarthritis in osteoarthritic rats by upregulating the adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK)/(peroxisome proliferator-activated receptor-γ coactivator) PGC-1 signaling pathway. Zhang et al. (2016) found that puerarin downregulated the mRNA expression of osteoclastogenesis-related genes, anti-tartrate acid phosphatase (TRAP), histone K, and (matrix metalloprotein-9), MMP-9 by inhibiting the production of osteoclast-activating tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and prostaglandin E2 (PGE 2). This informs the prevention and treatment of bacterial bone-destructive diseases. Xin-Ran Dong’s group found that puerarin promotes the proliferation and induces the differentiation of bone marrow mesenchymal stem cells (BMSCs) to chondrocytes by increasing the protein expression of collagen type II alpha 1 chain (COL2A 1), collagen type X alpha 1 chain (COL10A 1), MMP13, and SRY-related high-mobility-group box (SOX)-9, which has potential therapeutic effects in osteoarthritis (Dong et al., 2022).
5.3 Digestive system
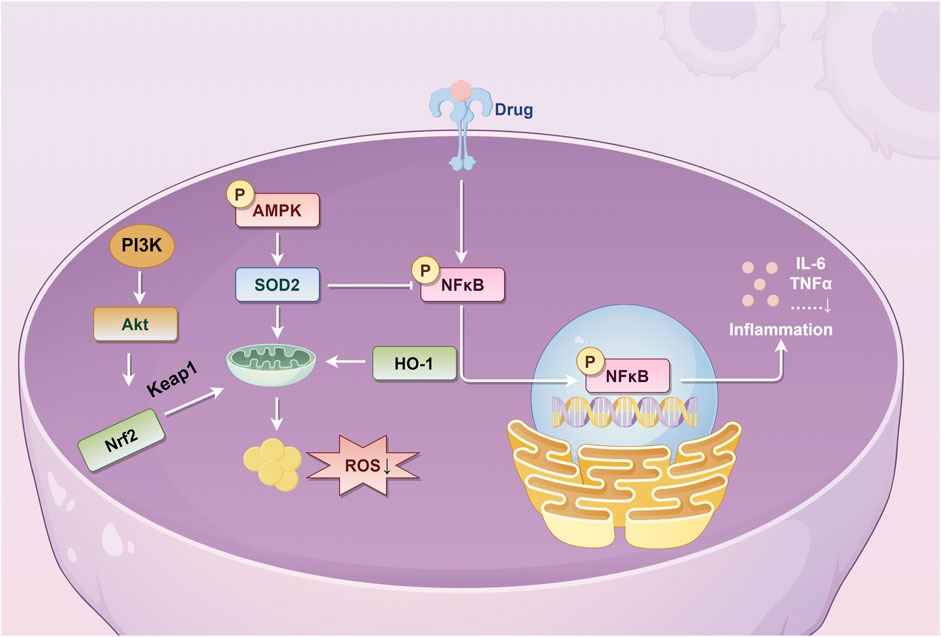
The main organs of the digestive system involved in the action of PL are the liver and intestines. First, isoflavones and triterpenoid saponins are the important material basis for liver protection of PL (He et al., 2021). Our previous study found that a 70% ethanol extract of PL could improve non-alcoholic fatty liver by changing liver fat accumulation and activating the Kelch-like ECH-associated protein 1 (Keap1)/nuclear respiratory factor 2 (Nrf2)/heme oxygenase (HO)-1 signaling pathway (Dong et al., 2024). In addition, our group also confirmed the functional characteristics of the protein of PL and reduced the accumulation of reactive oxygen species and malondialdehyde after oxidative damage of HepG2 cells by activating the Nrf2/Keap1 signaling pathway, increased superoxide dismutase activity, peroxidase activity, and increased GSH content (Pang et al., 2023). Li et al. (2024) used network pharmacology and experimental validation methods to confirm that puerarin and genistein in PL could increase ferredoxin levels by blocking the MAPK/ERK (extracellular signal-regulated kinase) signaling pathway, thereby attenuating alcohol-induced iron overload, hepatic injury, oxidative stress, and hepatocellular apoptosis. It was found that PL nanovesicles possessed exosomal properties, enhanced acetaldehyde dehydrogenase (ALDH) activity, inhibited alcohol dehydrogenase (ADH) activity, and improved the anti-intoxication and alcohol metabolizing ability of acute alcohol-poisoned mice (Zhang W. et al., 2024). Moreover, it was found that the component promoting alcohol metabolism might not be puerarin, and the key substances and mechanisms need to be explored in depth. The starch of PL can improve non-alcoholic fatty liver disease by adjusting intestinal microecology, providing a basis for its food function (Yang et al., 2022). Water-soluble polysaccharides of PL regulate lipid metabolism by inhibiting oxidative stress and increasing the production of short-chain fatty acids, thereby ameliorating liver injury in mice with acute alcoholic liver disease (Cao et al., 2024).
Chen et al. (2020) found that PL polysaccharides could treat antibiotic-associated diarrhea by attenuating the colonic lesions and dysbiosis of intestinal flora. Furthermore, the starch of PL and its branched chain starches attenuated DSS-induced colitis by decreasing the expression of NF-κB p65 and IL-1β protein levels and correcting gut microbiota disturbances (Yang et al., 2023). Cai G. et al. (2022) found that kudzu polysaccharides significantly improved intestinal mucosal immune function in cyclophosphamide-treated mice and increased secretory immunoglobulin A (sIgA) secretion and also repaired cyclophosphamide-induced mechanical barrier damage in the small intestine of mice, which could provide a basis for the development of anticancer drugs and chemotherapeutic agents. The kudzu polysaccharide ameliorates diarrhea in newborn calves by increasing the relative abundance of beneficial bacteria such as Bifidobacterium spp. and decreasing the relative abundance of pathogenic or diarrhea-associated bacteria such as Escherichia spp. and Shigella spp. (Shen et al., 2023). In addition, puerarin may improve the symptoms of abdominal pain and diarrhea in irritable bowel syndrome (IBS-D) rats by inhibiting the expression of corticotropin-releasing hormone receptor (CRF-1), which suppresses the activity of the hypothalamic–pituitary–adrenal (HPA) axis, promotes the proliferation of colonic epithelial cells by upregulating the expression of p-ERK/ERK, and repairs the colonic mucus by upregulating the expression of occludin, a protein that repairs the colonic mucus barrier (Wang Q.-S. et al., 2021). Puerarin also alleviates ulcerative colitis through the NF-κB/Nrf2 pathway (Jeon et al., 2020).
5.4 Respiratory system
PL modulates arginine metabolic pathways and attenuates bleomycin-induced pulmonary fibrosis symptoms in rats by decreasing nitric oxide synthase 2 (NOS 2)-related signaling molecules (Du et al., 2024). With the deepening of research, more and more results confirm that the components with respiratory system-promoting effects in PL are mainly isoflavonoid components. For example, puerarin can attenuate airway inflammation by inhibiting eotaxin-3 expression (Wang et al., 2015), ameliorate acute lung injury by downregulating NLRP3 (NOD-like receptor thermal protein domain-associated protein 3) inflammatory vesicle-induced pyroptosis (Cai D. et al., 2022), and have therapeutic effects on early chronic obstructive pulmonary disease (Zhang et al., 2022). It also ameliorates rabbit lung ischemia-reperfusion injury by inhibiting Fas/Factor-related apoptosis ligand (FasL) mRNA expression (Zheng et al., 2015). With the advancement of technology, kudzu-derived exosome-like nanovesicles were able to effectively attenuate inflammatory changes in the lungs by modulating the gut microbiota (Lu et al., 2024).
5.5 Urinary system
An aqueous extract of PL can ameliorate the overactivity of the forced urethral muscle in spontaneously hypertensive rats via a neurogenic pathway (Zhou et al., 2016). Combined with the existing studies, puerarin is one of the important active substances in PL with urinary effects. First, puerarin may bind to the Toll/interleukin 1 receptor (TIR) structural domain of the myeloid differentiation primary response protein 88 (MyD88) protein, impede its binding to Toll-like receptor 4 (TLR4), and downregulate NF-κB p65 and the c-Jun N-terminal kinase (JNK)/Forkhead box transcription factor O1 (FoxO1) pathway activity to attenuate lipopolysaccharide (LPS)- and unilateral ureteral obstruction (UUO)-induced renal inflammatory injury in C57BL/6J mice (Hu et al., 2024). Second, puerarin may upregulate renal Oat 1/3 by way of B-cell lymphoma 6 (BCL-6) upregulation of renal Oat 1/3 in methotrexate-induced kidney injury in Wistar rats (Liu et al., 2018). It is worth noting that some studies have confirmed that puerarin is the precursor component. In addition, a novel patch combining puerarin and polylactic acid using electrostatic spinning technology has good repair and pre-collagen synthesis properties for pelvic organ prolapse (Zhang D. et al., 2024), and it is expected to be an alternative to the traditional topical patch.
5.6 Reproductive system
As isoflavones with estrogen-like effects inhibit male spermatogenesis and female fertility to varying degrees (Sleiman et al., 2021), care should be taken with the dosage of the isoflavones of PL during physiologic states. It has been shown that the component of PL that inhibits sperm activity is not puerarin but other isoflavones (Gray et al. 2015).
5.7 Endocrine system
Extracts of PL have good anti-diabetic activity. For example, PL upregulates fatty acyl groups (FA), downregulates the levels of metabolites such as glycerophospholipids (GP) (Yang H.-B. et al., 2024), and interferes with the PI3K signaling pathway (Zhang et al., 2021) to ameliorate type II diabetes. In addition, kudzu extract may improve the development of diabetes by downregulating the PTP1B pathway (Sun R. et al., 2019), and kudzu polysaccharides may treat diabetes by modulating the PPAR signaling pathway and the structure of intestinal flora (Wang et al., 2022). It is worth mentioning that the effects of puerarin for the treatment of diabetes mellitus are manifested in various aspects such as lowering blood glucose levels, improving insulin resistance, and protecting pancreatic islets (Chen et al., 2019). As well, puerarin was able to promote pancreatic β-cell neogenesis by upregulating glucagon-like peptide 1 receptor (GLP-1R) expression, thereby ameliorating high-fat-diet-induced glycemic abnormalities in mice (Wang et al., 2020), and it also inhibited type II diabetic nephropathy by regulating iron homeostasis and attenuating iron death (Hou et al., 2024).
PL improves multiple female sexual dysfunctions in postmenopausal women by attenuating total cholesterol, triglycerides, alanine aminotransferase (ALT/GPT), and aspartate aminotransferase (AST/GOT) in ovariectomized rats (Oh et al., 2019). As for perimenopausal syndrome, osteoporosis is one of the most common disorders. An extract of PL can ameliorate postmenopausal osteoporosis by decreasing the levels of bone markers C-telopeptide of crosslinked collagen type I (CTX-1), osteocalcin (OC), and dye-decolorizing peroxidase (DYP) and upregulating ER-α protein expression in ovariectomized rats (Lee et al., 2021). Isoflavones from high-dose PL improved lipid and bone metabolism in ovariectomized mice by affecting elevated plasma estrogen levels, decreasing abdominal adipose tissue weight, and elevating femur BMD (Cho et al., 2012). Some studies have confirmed that puerarin has the effect of increasing localized new bone formation and can be used for bone grafting or osteoinduction (Wong and Rabie, 2007). In addition, puerarin promotes cell proliferation by upregulating Bcl-xL expression, promoting osteoblast proliferation and differentiated material through activation of MEK/ERK and PI3K/Akt pathways (Wang et al., 2013), and also inhibits osteoclastogenesis by reducing the pathway in which MCP-1 prevents migration of osteoclast precursor cells (OCPs) (Lin et al., 2020) and is expected to be a drug to prevent or delay osteoporosis. Wang et al. (2014) demonstrated that puerarin may exert anti-osteoporotic effects by inhibiting receptor activator of nuclear factor-κB ligand (RANKL) and interleukin-6 through increasing the expression of ERα, ERβ and steroid hormone receptor coactivator (SRC)-1. Subsequent studies have confirmed that puerarin inhibits osteoclastogenesis via autophagy response regardless of the presence of RANKL (Zhang G. et al., 2019), but the mechanism of autophagy in the anti-osteoporotic effect of puerarin has yet to be further explored. The combination of puerarin and zinc can be anti-osteoporotic by elevating serum osteocalcin, bone marrow stromal cell (BMSC) proliferation, and alkaline phosphatase (ALP) levels and decreasing serum lipocalin levels (Li H. et al., 2016). In addition to puerarin, other isoflavones in PL also have promising anti-osteoporotic effects, such as genistein and zinc that increase bone mass in de-ovulated rats (Qi, 2018) and puerarin 6″-O-Xyloside that is capable of anti-osteoporosis by elevating blood calcium, blood phosphorus, ALP, and OPG levels in a de-ovulated mouse model of anti-osteoporosis (Li H. et al., 2016).
Studies have shown that puerarin is able to alleviate Hashimoto’s thyroiditis by regulating macrophages (Tao et al., 2024a), which further confirms the favorable hormone-like effects of PL. Because PL contains estrogen-like isoflavones, it can also treat breast diseases.
5.8 Nervous system
High doses of PL isoflavonoids can promote the metabolism of dopamine (DA) and 5-hydroxytryptamine (5-HT), reduce the level of extracellular Glu, and have a good therapeutic effect on neurodegenerative diseases (Xiao et al., 2017), so the isoflavonoids of PL are one of the pharmacological material bases for the neurological activity of PL. First, PL has the most prominent therapeutic effect on Alzheimer’s disease. Puerarin forms a stable complex with calcium/calmodulin kinase IIα (CaMK IIα), a key protein for synaptic plasticity, which can alleviate the synaptic plasticity damage caused by Alzheimer’s disease through p38MAPK/cAMP-response element binding protein (CREB) signaling pathway (Liu S. et al., 2021) and inhibit the activity of acetylcholinesterase (AChE) in brain tissue. It also downregulated the expression of cyclooxygenase-2 (COX-2) and cysteine-3 (C3) to exert anti-Alzheimer’s disease effects (Liu et al., 2019) and decreased cognitive deficits in lead acetate-induced neurotoxicity mice by elevating the activities of acetylcholinesterase (AChE) and monoamine oxidase (MAO) and modulating the PKA/Akt/NOS signaling pathway (Liu et al., 2013). For Aβ1-42-induced cognitive deficits and neurodegenerative lesions, puerarin also has a favorable protective effect, mainly by elevating the levels of brain-derived neurotrophic factor, decreasing AChE, etc., in the hippocampus and cerebral cortex (Wu et al., 2017). Furthermore, the lupane triterpenoids isolated from PL can attenuate Alzheimer’s disease by inhibiting beta-site APP cleaving enzyme 1 (BACE1) (Koirala et al., 2017). Not to be overlooked, PL and its active components also inhibit hypoxic brain edema (Wang C. et al., 2018). It also attenuates ischemic stroke injury by modulating intestinal flora (Lian et al., 2023) and is anti-stroke by inhibiting lactate dehydrogenase (Li et al., 2017).
5.9 Immune system
PL has anticancer and immunity-enhancing effects on the immune system. First, isoflavones (biochanin A and genistein) of PL with 5,7-dihydroxy groups on the A-ring in their structure can exert anticancer effects by inhibiting NO production (Jun et al., 2005). Puerarin’s anticancer effects may be carried out through PI3K/AKT/mammalian target of rapamycin (mTOR), Ras-Raf-MEK-ERK, JNK, AMPK, and NF-κB pathways (Ahmad et al., 2020). Second, studies on the immunity-enhancing effects of PL mainly include the ability of polysaccharides of PL to inhibit cyclophosphamide-induced immunocompromise by increasing immune organ index, cytokine content, and T-lymphocyte subsets (Cai et al., 2023).
5.10 Other activities
With the help of the STRING database (https://string-db.org/) and Cytoscape 3.8.2 software (https://cytoscape.org/download.html), the targets described above were visualized (Figure 10). In addition to the role of the reproductive system, the results of the PPI network diagram visualization of the other eight systems showed that AKT, IL-6, TGF-β, AMPK, SOD, TNF, and MMP were the core targets for PL to play the above roles. In addition, PL also has the effects of skin barrier damage protection (Lee et al., 2024), anti-fatigue (Xiaoming et al., 2012), suggesting it could be added to cosmetics as a whitening agent or to food as an anti-fatigue agent, etc.. It has been shown to have a good safety profile in normal doses (Chen et al., 2021), with no obvious toxic side effects, and it has great potential for food development.
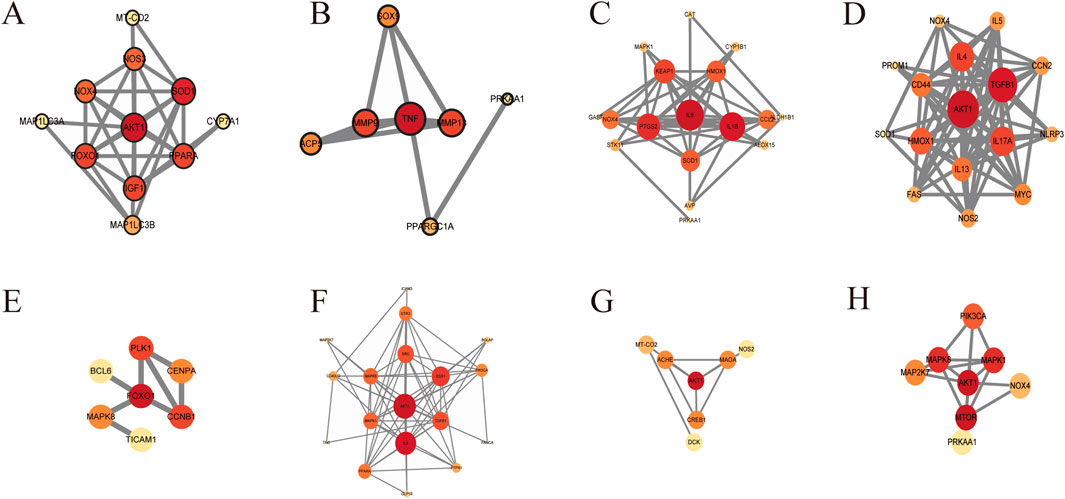
Figure 10. PPI network diagrams of targets in the (A) circulatory, (B) locomotor, (C) digestive, (D) respiratory, (E) urinary, (F) endocrine, (G) nervous, and (H) immune systems.
In summary, the ancient usage of PL has certain modern scientific connotations, such as the ability to detoxify muscles and reduce fever, corresponding to anti-inflammation, and its mechanism mainly includes inhibiting COX-2 enzyme activity, decreasing PGE2 synthesis, and decreasing the expression of pro-inflammatory factors, such as TNF-alpha and IL-6. In addition, ancient books report that PL can treat thirst and relieve alcoholism, which corresponds to the modern studies showing that it can regulate insulin resistance and treat alcoholic liver injury, respectively. However, the mechanisms remain to be further elucidated, and subsequent studies on PL should focus on the above points.
6 Food utilization
6.1 Preservative
The key enzyme in this process is polyphenol oxidase, which is responsible for the reduction of the nutritional value of PL due to enzymatic browning during storage and processing. Liu J. et al. (2021) showed that the polyphenol oxidase of PL has a high thermal stability, and that the food components pectin, bovine serum proteins, starch, and sucrose showed a decreasing trend in their protective effect against thermal processing. This further suggests that the addition of sucrose and starch during processing or storage is effective in reducing enzymatic browning and enhancing the quality of PL. Researchers added a probiotic solution to the herbal combination consisting of chuanxiong, licorice, forsythia, safflower, kudzu, and galangal, and fermented it at 30°C and pH 6.5 for 8 days. The effect on citrus fruit decay was later tested, and it was found that the herbal fermentation solution was able to inhibit decay significantly. It is a novel, highly effective, and natural citrus freshness preservative (Wang et al., 2019a).
6.2 Tea
Researchers used PL as the main ingredient and added herbs such as maitake, Ganoderma lucidum, and American ginseng, which are functional foods and medicines, to make Ge Gen Mai Dong Cha, a health food that has the potential to lower glucose and combat obesity (Li X. et al., 2023). The extracts of PL tea have good preservative and bacteriostatic effects and can effectively inhibit the growth of Gram-negative and positive pathogenic bacteria in various food products (Kim and Fung, 2004). Koreans use kudzu as a non-alcoholic beverage by soaking it in water or adding sweeteners such as honey and sugar, called yakjaecha (Lee, 2022).
6.3 Fermented products
Wang Y. et al. (2023) found that the mixed fermentation of A. erythropolis with rice, PL, and yam resulted in the conversion of soybean glycosides and diosgenin to daidzein and diosgenin, respectively, which were more efficiently digested and absorbed in vivo.
6.4 Kudzu starch with food activity
Kudzu starch is a powdered substance extracted from dried tubers of PL after water milling that has been shown to have circulation-enhancing and antispasmodic properties (Mocan et al., 2018). Robert D. Tanner et al. devised a lysine-enriched baker’s yeast process and found that kudzu starch was similar to commercially available fermented starches (Tanner and Hussain, 1979). Due to the rich nutrient content of kudzu starch, it is used as a drinkable solid beverage in Asia (Liang et al., 2017). Researchers have studied the properties of three kudzu starches (Duan et al., 2023) that can be used as an ingredient for cookies based on the properties. He et al. (2024) improved the kudzu starch extraction method and obtained kudzu starch that could be used as a binder and thickener after treatment with an alcohol base and a urea base. Because kudzu starch is rich in nutrients, it is often developed as a functional food with health benefits in East Asian countries such as China and Japan (Van Hung and Morita, 2007; Jóźwiak et al., 2018).
6.5 Others
In India, PL is commonly used as an additive in dairy products and may help regulate blood pressure by stimulating the body’s physiological systems (Maji et al., 2023). Yu et al. (2017) constructed a large-scale knowledge graph to demonstrate the nutritional value of PL, using Ge Gen Fen Zhou as an example.
iu-Zhi Fan et al. found that the use of substrates of mulberry branches and PL residues for the cultivation of monkey head mushrooms, on the one hand, could improve the yield and biological efficiency; on the other hand, the mushrooms harvested from the alternative substrates were rich in flavonoids, polysaccharides, and other active constituents, which significantly improved their antioxidant and hypoglycemic activities and enhanced the value of monkey head mushrooms (Fan et al., 2021).
Corley et al. (1997), using the above-ground parts (leaves and stems) and roots of PL, found that the chemical composition and digestive properties of these parts were very close to those of other commonly used feedstuffs, showing its potential as a feed for ruminants. In addition, the addition of PL leaves to feeds significantly increased broiler growth performance, immunity, and antioxidant capacity (Gong et al., 2022).
7 Toxicity
It can be concluded from the above ancient texts that kudzu is not recorded as poisonous in most of the Materia Medica texts, but rather that most of the texts mention its edible properties. Song et al. (2020) found that the LD50 (lowest dose causing death) of oral PL extract was greater than 5,000 mg/kg, and no acute toxicity was observed. Although no significant toxicity has been observed with oral administration of extracts of PL, injections of high concentrations of Pueraria mirifica have been reported in the literature to cause hemolysis (0.2–1 mM without significant side effects) (Hou et al., 2011). In addition, high concentrations (>1 mg/kg) of puerarin had deleterious effects in vitro and in vivo on oocyte maturation, fertilization, and subsequent embryonic development in mice (Huang and Chan, 2016). Therefore, the dosage of the medicinal components of Pueraria mirifica should be controlled in the actual application process to ensure that no toxicity or side effects will occur.
8 Discussion
8.1 Industrialization challenges in the medicinal–edible process
The thermal stability of Pueraria isoflavones (notably puerarin and daidzein) constitutes a critical challenge in industrial processing, as these bioactive compounds exhibit susceptibility to thermal degradation under conventional thermal treatments. Such thermal degradation directly compromises the bioactivity of Pueraria extracts, necessitating the development of thermal-protective strategies during processing to preserve critical components like heat-sensitive isoflavones (e.g., genistein). Recent work by Ekumah et al. (2025) demonstrated that a kudzu starch hydrogel functionalized with rutin and ferulic acid (RF-KSH) reduces component loss by 18%–22% while increasing antioxidant activity by 1.3-fold compared to conventional hydrogels. The system was further optimized through octenyl succinic anhydride modification, specifically designed to enhance thermal stability during processing.
Conventional ethanol-based extraction methods are constrained by a fundamental compromise between polysaccharide retention and industrial costs. Higher ethanol concentrations not only induce polysaccharide precipitation but also lead to significantly increased solvent recovery costs, creating dual challenges for process optimization. To address these limitations, researchers are developing innovative extraction strategies. Emerging green techniques such as ultrasound-assisted and microwave-assisted extraction demonstrate dual benefits: enhanced recovery of polysaccharides and isoflavones coupled with reduced environmental impact, while simultaneously improving cost efficiency through optimized energy consumption (Xu et al., 2025). The industrial utilization of PL requires systematic optimization to fully realize its strategic potential in functional foods and pharmaceuticals; this demands coordinated efforts in both fundamental research and applied technological development to effectively harness its medicinal-food duality.
8.2 Future research directions
Studies have confirmed the anti-inflammatory and immunomodulatory properties of Pueraria isoflavones (e.g., puerarin and daidzein). However, critical knowledge gaps remain regarding signaling pathway interactions between their microbial metabolites (e.g., equol) and specific immune cell populations (macrophages and T lymphocytes). Key unresolved issues encompass 1) gut microbiota-mediated modulation of isoflavone metabolic pathways during immunoregulation and 2) epigenetic regulation through DNA methylation/histone modifications induced by isoflavones. Both require systematic investigation via integrated application of single-cell sequencing and metabolomics. While the hypoglycemic properties of Pueraria polysaccharide have been extensively studied, the relationship between its structural parameters (particularly branching degree and glycosidic linkage types) and immunomodulatory potential remains unelucidated. To elucidate the stereocomplementary interaction mechanisms between polysaccharides and immune receptors, structural biology approaches, including molecular dynamics simulations and ligand–receptor binding assays, should be systematically implemented.
Pueraria isoflavones face significant application constraints due to poor aqueous solubility and low intestinal absorption rates. Current strategies employing liposome encapsulation technology and pH-responsive release systems demonstrate bioavailability enhancement potential. However, nanodrug delivery systems with therapeutic payloads remain at experimental stages. Critical challenges persist regarding nanomaterial accumulation-related chronic toxicity in hepatic/renal tissues and the development of scalable manufacturing processes.
9 Conclusion
In this article, the research progress of PL is systematically summarized from the aspects of basal origin, chemical composition, synthesis, metabolism, biological activity, and comprehensive utilization of food. This is of great significance for the further development and application of PL. PL is mainly distributed in Asia, America, and Europe and has been used as a medicinal herb and food for thousands of years in Asia (especially in China), while the utilization value of Pueraria lobata in Europe and the United States has yet to be further developed. PL mainly contains isoflavonoids, and the methylation and glycosylation of 6-C, 8-C, 3′-C, 4′-C are its main biogenic pathways. Hepatic and intestinal metabolism, glucuronidation, methylation, reduction, and oxidation reactions are the main types of reactions. Resorcinol, (3S)-equol, and equol are its metabolic end products, and puerarin, daidzein, genistein, biochanin A, and formononetin are the key components affecting its quality and activity.
As a natural herb with both medicinal and functional properties, PL exhibits three pivotal advantages for modern applications. First, its multi-target regulatory capacity extends beyond modulating key signaling molecules (e.g., AKT, IL-6, and TGF-β) to bidirectional coordination of the Nrf2/AMPK pathways, a critical mechanism for achieving therapeutic-nutritional synergy (Figure 11). Second, PL demonstrates exceptional food matrix compatibility through heat-resistant starch that stabilizes bioactive isoflavonoids during thermal processing, enabling functional food development without compromising bioactivity. Third, its ecological sustainability is evidenced by significantly higher biomass utilization efficiency than conventional medicinal plants, which facilitates green manufacturing through full-plant utilization and byproduct valorization. These attributes collectively position PL as a strategic candidate for developing next-generation nutraceuticals and sustainable bioproducts.
PL can be used not only as food, but also as a tea, a preservative, and as an ingredient for making porridge, dairy products, food additives, cookies, jellies, puddings, and so on. Kudzu has a wide range of biological activities on the nine systems of the human body and can be used as nutritional enhancers and supplements. The main role is focused on liver function protection, anti-osteoporosis, and anti-diabetes, AKT, IL-6, TGF-β, AMPK, SOD, TNF, MMP, etc., for the PL to play the role of the above core targets. Building on these strengths, two industrial implementation strategies emerge: 1) Development of gut microbiota-personalized nutraceuticals leveraging PL’s ability to modulate equol-producing bacterial communities and 2) advanced 3D food printing technologies for creating bone-targeted functional matrices that combine puerarin’s bioactivity with structural biomimicry. These approaches collectively position PL as a next-generation platform for precision nutrition and sustainable bioproduct development.
Author contributions
BK: data curation, formal analysis, investigation, methodology, visualization, writing – original draft. LM: conceptualization, writing – review and editing. MZ: visualization, writing – review and editing. HW: data curation, writing – review and editing. CL: supervision, writing – review and editing. MY: conceptualization, funding acquisition, methodology, supervision, writing – review and editing. GL: conceptualization, funding acquisition, resources, supervision, writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Natural Science Foundation of China (No. 22301020), the Science and Technology Development Plan Project of Jilin Province (No. YDZJ202301ZYTS114) and the Budgetary Fund for Basic Construction (Innovative Capacity Building) Project of Jilin Province (2024C012-2).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adolfo, L. M., Burks, D., Rao, X., Alvarez-Hernandez, A., and Dixon, R. A. (2022). Evaluation of pathways to the C-glycosyl isoflavone puerarin in roots of kudzu (Pueraria Montana lobata). Plant Direct 6 (9), e442. doi:10.1002/pld3.442
Ahmad, B., Khan, S., Liu, Y., Xue, M., Nabi, G., Kumar, S., et al. (2020). Molecular mechanisms of anticancer activities of puerarin. Cancer Manag. Res. 12, 79–90. doi:10.2147/cmar.S233567
Ahn, S. Y., Jo, M. S., Lee, D., Baek, S. E., Baek, J., Yu, J. S., et al. (2019). Dual effects of isoflavonoids from Pueraria lobata roots on estrogenic activity and anti-proliferation of MCF-7 human breast carcinoma cells. Bioorg Chem. 83, 135–144. doi:10.1016/j.bioorg.2018.10.017
Arao, T., Kinjo, J., Nohara, T., and Isobe, R. (1995). Oleanene-type triterpene glycosides from Puerariae Radix. II. Isolation of saponins and the application of tandem mass spectrometry to their structure determination. Chem. Pharm. Bull. (Tokyo) 43 (7), 1176–1179. doi:10.1248/cpb.43.1176
Arao, T., Kinjo, J., Nohara, T., and Isobe, R. (1997). Oleanene-type triterpene glycosides from Puerariae radix. IV. six new saponins from Pueraria lobata. Chem. Pharm. Bull. (Tokyo) 45 (2), 362–366. doi:10.1248/cpb.45.362
Atherton, K. M., Mutch, E., and Ford, D. (2006). Metabolism of the soyabean isoflavone daidzein by CYP1A2 and the extra-hepatic CYPs 1A1 and 1B1 affects biological activity. Biochem. Pharmacol. 72 (5), 624–631. doi:10.1016/j.bcp.2006.05.015
Braune, A., and Blaut, M. (2011). Deglycosylation of puerarin and other aromatic C-glucosides by a newly isolated human intestinal bacterium. Environ. Microbiol. 13 (2), 482–494. doi:10.1111/j.1462-2920.2010.02352.x
Cai, D., Zhao, Y., and Yu, F. (2022a). Puerarin ameliorates acute lung injury by modulating NLRP3 inflammasome-induced pyroptosis. Cell Death Discov. 8 (1), 368. doi:10.1038/s41420-022-01137-8
Cai, G., Wu, C., Mao, N., Song, Z., Yu, L., Zhu, T., et al. (2022b). Isolation, purification and characterization of Pueraria lobata polysaccharide and its effects on intestinal function in cyclophosphamide-treated mice. Int. J. Biol. Macromol. 218, 356–367. doi:10.1016/j.ijbiomac.2022.07.153
Cai, G., Wu, C., Zhu, T., Peng, S., Xu, S., Hu, Y., et al. (2023). Structure of a Pueraria root polysaccharide and its immunoregulatory activity on T and B lymphocytes, macrophages, and immunosuppressive mice. Int. J. Biol. Macromol. 230, 123386. doi:10.1016/j.ijbiomac.2023.123386
Cao, W., Lv, Q., Yu, J., He, S., Hou, X., Zhou, L., et al. (2025). Structural analysis and anti-hepatic fibrosis effects of a homogeneous polysaccharide from Radix Puerariae lobatae (Willd.) Ohwi roots. Int. J. Biol. Macromol. 298, 140028. doi:10.1016/j.ijbiomac.2025.140028
Cao, W., Wu, J., Zhao, X., Li, Z., Yu, J., Shao, T., et al. (2024). Structural elucidation of an active polysaccharide from Radix Puerariae lobatae and its protection against acute alcoholic liver disease. Carbohydr. Polym. 325, 121565. doi:10.1016/j.carbpol.2023.121565
Chen, L., Jiang, E., Guan, Y., Xu, P., Shen, Q., Liu, Z., et al. (2021). Safety of high-dose Puerariae Lobatae Radix in adolescent rats based on metabolomics. Food Sci. Nutr. 9 (2), 794–810. doi:10.1002/fsn3.2044
Chen, R., Liu, B., Wang, X., Chen, K., Zhang, K., Zhang, L., et al. (2020). Effects of polysaccharide from Pueraria lobata on gut microbiota in mice. Int. J. Biol. Macromol. 158, 740–749. doi:10.1016/j.ijbiomac.2020.04.201
Chen, R., Xue, J., and Xie, M. (2012). Puerarin prevents isoprenaline-induced myocardial fibrosis in mice by reduction of myocardial TGF-β1 expression. J. Nutr. Biochem. 23 (9), 1080–1085. doi:10.1016/j.jnutbio.2011.05.015
Chen, X., Yu, J., and Shi, J. (2019). Management of diabetes mellitus with puerarin, a natural isoflavone from Pueraria lobata. Am. J. Chin. Med. 46 (08), 1771–1789. doi:10.1142/s0192415x18500891
Cheng, H., Huang, X., Wu, S., Wang, S., Rao, S., Li, L., et al. (2022). Chromosome-level genome assembly and multi-omics dataset provide insights into isoflavone and puerarin biosynthesis in Pueraria lobata (Wild.) Ohwi. Biomolecules 12 (12), 1731. doi:10.3390/biom12121731
Chi, J., Zhang, G., and P, L. (2007). Chemical constituents of radix puerariae from anhui province. Chin. J. Med. Chem. 17 (1), 47.
China (2020). Pharmacopoeia of the people’s Republic of China. Beijing: National Pharmacopoeia Commission.
Cho, H. J., Jun, H. J., Lee, J. H., Jia, Y., Hoang, M. H., Shim, J. H., et al. (2012). Acute effect of high-dose isoflavones from Pueraria lobata (Willd.) Ohwi on lipid and bone metabolism in ovariectomized mice. Phytother. Res. 26 (12), 1864–1871. doi:10.1002/ptr.4669
Choi, J., Kim, Y., Eser, B. E., and Han, J. (2023). Theoretical study on the glycosidic C–C bond cleavage of 3″-oxo-puerarin. Sci. Rep. 13 (1), 16282. doi:10.1038/s41598-023-43379-1
Congbing, F., Xiaochun, W., Huarong, T., and Jiang, C. (2006). Identification of isoflavonoids in several kudzu samples by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J. Chromatogr. Sci. 44 (2), 57–63. doi:10.1093/chromsci/44.2.57
Corley, R. N., Woldeghebriel, A., and Murphy, M. R. (1997). Evaluation of the nutritive value of kudzu (Pueraria lobata) as a feed for ruminants. Animal Feed Sci. Technol. 68 (1-2), 183–188. doi:10.1016/s0377-8401(97)00038-2
Delmonte, P., Perry, J., and Rader, J. I. (2006). Determination of isoflavones in dietary supplements containing soy, Red Clover and kudzu: extraction followed by basic or acid hydrolysis. J. Chromatogr. A 1107 (1-2), 59–69. doi:10.1016/j.chroma.2005.11.060
Dong, H., Zhao, Y., Teng, H., Jiang, T., Yue, Y., Zhang, S., et al. (2024). Pueraria lobata antioxidant extract ameliorates non-alcoholic fatty liver by altering hepatic fat accumulation and oxidative stress. J. Ethnopharmacol. 333, 118468. doi:10.1016/j.jep.2024.118468
Dong, X.-R., Hu, M.-J., Pan, H.-X., Li, K.-F., and Cui, Y.-L. (2022). Chondrogenic differentiation of rat bone marrow mesenchymal stem cells induced by puerarin and tetrandrine. Acupunct. Herb. Med. 2 (2), 130–138. doi:10.1097/hm9.0000000000000031
Du, H., Shao, M., Xu, S., Yang, Q., Xu, J., Ke, H., et al. (2024). Integrating metabolomics and network pharmacology analysis to explore mechanism of Pueraria lobata against pulmonary fibrosis: involvement of arginine metabolism pathway. J. Ethnopharmacol. 332, 118346. doi:10.1016/j.jep.2024.118346
Duan, X. T., Guan, Y. M., Dong, H. H., Yang, M., Chen, L. H., Zhang, H., et al. (2023). Study on structural characteristics and physicochemical properties of starches extracted from three varieties of kudzu root (Pueraria lobata starch). J. Food Sci. 88 (3), 1048–1059. doi:10.1111/1750-3841.16472
Ekumah, J. N., Zhong, M., Sun, Y., Liang, Q., Rashid, A., Virk, M. S., et al. (2025). Enhancement of kudzu starch hydrogels with rutin and ferulic acid: an approach to improve cold storage stability, mechanical properties, in vitro digestibility, functional properties and consumer acceptance. Int. J. Biol. Macromol. 311 (Pt 1), 143709. doi:10.1016/j.ijbiomac.2025.143709
Fan, X.-Z., Yao, F., Yin, C.-M., Shi, D.-F., and Gao, H. (2021). Mulberry twig and Pueraria root residue improve the biological efficiency and potential functional value of Hericium erinaceus. Sci. Hortic. 286, 110214. doi:10.1016/j.scienta.2021.110214
Fang, C., Wan, X., Tan, H., and Jiang, C. (2006). Identification of isoflavonoids in several kudzu samples by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J. Chromatogr. Sci. 44 (2), 57–63.
Fang, X., Lan, X., Zhu, M., He, M., Sun, M., Cao, Y., et al. (2024). Puerarin induces macrophage M2 polarization to exert antinonalcoholic steatohepatitis pharmacological activity via the activation of autophagy. J. Agric. Food Chem. 72 (13), 7187–7202. doi:10.1021/acs.jafc.3c09601
Follak, S. (2011). Potential distribution and environmental threat of Pueraria lobata. Central Eur. J. Biol. 6 (3), 457–469. doi:10.2478/s11535-010-0120-3
Fu, M., Jahan, M. S., Tang, K., Jiang, S., Guo, J., Luo, S., et al. (2023). Comparative analysis of the medicinal and nutritional components of different varieties of Pueraria thomsonii and Pueraria lobata. Front. Plant Sci. 14, 1115782. doi:10.3389/fpls.2023.1115782
Gao, E., Wang, W., Huang, Y., Luo, Z., Chen, B., Xiao, S., et al. (2024). Puerariae lobatae radix: progress in extraction, separation methods and pharmacological activities research. Separations 11 (7), 195. doi:10.3390/separations11070195
Ge, P. Z., Fan, D. C., Ding, M., Wang, D., and Zhou, C. Q. (2014). Characterization and nutritional quality evaluation of several starch noodles. Starch-Starke 66 (9-10), 880–886. doi:10.1002/star.201300288
Gong, J., Wu, D., Li, M., Qiu, X., Jiang, Y., Zhang, J., et al. (2022). Effects of Pueraria lobata leaf powder as a feed additive on the immune system and growth performance of broiler chickens. Turkish J. Veterinary Animal Sci. 46 (4), 666–673. doi:10.55730/1300-0128.4238
Gray, S. L., Lackey, B. R., and Boone, W. R. (2015). Impact of kudzu and puerarin on sperm function. Reprod. Toxicol. 53, 54–62. doi:10.1016/j.reprotox.2015.03.010
He, H., Peng, S., Song, X., Jia, R., Zou, Y., Li, L., et al. (2021). Protective effect of isoflavones and triterpenoid saponins from Pueraria lobata on liver diseases: a review. Food Sci. Nutr. 10 (1), 272–285. doi:10.1002/fsn3.2668
He, R., Li, M., Huang, B., Zou, X., Li, S., Sang, X., et al. (2024). Comparative analysis of multi-angle structural alterations and cold-water solubility of kudzu starch modifications using different methods. Int. J. Biol. Macromol. 264 (Pt 1), 130522. doi:10.1016/j.ijbiomac.2024.130522
Hirakura, K., Morita, M., Nakajima, K., Sugama, K., Takagi, K., Niitsu, K., et al. (1997). Phenolic glucosides from the root of Pueraria lobata. Phytochemistry 46 (5), 921–928. doi:10.1016/s0031-9422(97)00371-3
Hou, B., Ma, P., Yang, X., Zhao, X., Zhang, L., Zhao, Y., et al. (2024). In silico prediction and experimental validation to reveal the protective mechanism of Puerarin against excessive extracellular matrix accumulation through inhibiting ferroptosis in diabetic nephropathy. J. Ethnopharmacol. 319, 117281. doi:10.1016/j.jep.2023.117281
Hou, S. Z., Su, Z. R., Chen, S. X., Ye, M. R., Huang, S., Liu, L., et al. (2011). Role of the interaction between puerarin and the erythrocyte membrane in puerarin-induced hemolysis. Chem. Biol. Interact. 192 (3), 184–192. doi:10.1016/j.cbi.2011.03.007
Hsiou-Yu, D., Yi-Ying, C., Wen-Liang, C., and Lin, H. (2004). Flavonoids from the flowers of Pueraria lobata. J. Chin. Chem. Soc. 51 (6), 1425–1428. doi:10.1002/jccs.200400210
Hu, Q., Xiang, H., Shan, J., Jiao, Q., Lv, S., Li, L., et al. (2020). Two pairs of diastereoisomeric isoflavone glucosides from the roots of Pueraria lobata. Fitoterapia 144, 104594. doi:10.1016/j.fitote.2020.104594
Hu, Z., Chen, D., Yan, P., Zheng, F., Zhu, H., Yuan, Z., et al. (2024). Puerarin suppresses macrophage M1 polarization to alleviate renal inflammatory injury through antagonizing TLR4/MyD88-mediated NF-κB p65 and JNK/FoxO1 activation. Phytomedicine 132, 155813. doi:10.1016/j.phymed.2024.155813
Huang, F. J., and Chan, W. H. (2016). Apoptotic effects on maturation of mouse oocytes, fertilization and fetal development by puerarin. Drug Chem. Toxicol. 39 (4), 380–387. doi:10.3109/01480545.2015.1126842
Jeon, Y.-D., Lee, J.-H., Lee, Y.-M., and Kim, D.-K. (2020). Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed. Pharmacother. 124, 109847. doi:10.1016/j.biopha.2020.109847
Jiang, J.-r., Yuan, S., Ding, J.-f., Zhu, S.-c., Xu, H.-d., Chen, T., et al. (2008). Conversion of puerarin into its 7-O-glycoside derivatives by Microbacterium oxydans (CGMCC 1788) to improve its water solubility and pharmacokinetic properties. Appl. Microbiol. Biot. 81 (4), 647–657. doi:10.1007/s00253-008-1683-z
Jiang, R. W., Lau, K. M., Lam, H. M., Yam, W. S., Leung, L. K., Choi, K. L., et al. (2005). A comparative study on aqueous root extracts of Pueraria thomsonii and Pueraria lobata by antioxidant assay and HPLC fingerprint analysis. J. Ethnopharmacol. 96 (1-2), 133–138. doi:10.1016/j.jep.2004.08.029
Jin, J. S., Nishihata, T., Kakiuchi, N., and Hattori, M. (2008). Biotransformation of C-glucosylisoflavone puerarin to estrogenic (3S)-equol in co-culture of two human intestinal bacteria. Biol. Pharm. Bull. 31 (8), 1621–1625. doi:10.1248/bpb.31.1621
Jin, S. E., Son, Y. K., Min, B. S., Jung, H. A., and Choi, J. S. (2012). Anti-inflammatory and antioxidant activities of constituents isolated from Pueraria lobata roots. Arch. Pharm. Res. 35 (5), 823–837. doi:10.1007/s12272-012-0508-x
Jóźwiak, B., Orczykowska, M., and Dziubiński, M. (2018). Rheological properties of kuzu starch pastes with galactomannans. J. food Sci. Technol. 55 (4), 1575–1581. doi:10.1007/s13197-018-3047-8
Jun, M., Hong, J., Jeong, W. S., and Ho, C. T. (2005). Suppression of arachidonic acid metabolism and nitric oxide formation by kudzu isoflavones in murine macrophages. Mol. Nutr. Food Res. 49 (12), 1154–1159. doi:10.1002/mnfr.200500103
Kayano, S. I, Matsumura, Y., Kitagawa, Y., Kobayashi, M., Nagayama, A., Kawabata, N., et al. (2012). Isoflavone C -glycosides isolated from the root of kudzu (Pueraria lobata) and their estrogenic activities. Food Chem. 134 (1), 282–287. doi:10.1016/j.foodchem.2012.02.137
Kemertelidze, É. P., Syrov, V. N., Alaniya, M. D., Kavtaradze, N. S., and Khushbaktova, Z. A. (2008). Chemical composition and pharmacological activity of the leaves of Pueraria hirsuta L. grown in Georgia. Pharm. Chem. J. 42 (6), 340–343. doi:10.1007/s11094-008-0131-9
Keung, W. M. (1993). Biochemical studies of a new class of alcohol dehydrogenase inhibitors from Radix puerariae. Alcohol Clin. Exp. Res. 17 (6), 1254–1260. doi:10.1111/j.1530-0277.1993.tb05238.x
Kim, J. M., Lee, Y. M., Lee, G. Y., Jang, D. S., Bae, K. H., and Kim, J. S. (2006). Constituents of the roots of Pueraria lobata inhibit formation of advanced glycation end products (AGEs). Arch. Pharm. Res. 29 (10), 821–825. doi:10.1007/bf02973900
Kim, S., and Fung, D. (2004). Antibacterial effect of water-soluble arrowroot (Puerariae radix) tea extracts on foodborne pathogens in ground beef and mushroom soup. J. Food Prot. 67 (9), 1953–1956. doi:10.4315/0362-028x-67.9.1953
Kinjo, J., Furusawa, J.-i., Baba, J., Takeshita, T., Yamasaki, M., and Nohara, T. (1987). Studies on the constituents of Pueraria lobata. III. Isoflavonoids and related compounds in the roots and the voluble stems. Chem. Pharm. Bull. 35, 4846–4850. doi:10.1248/cpb.35.4846
Kinjo, J., Miyamoto, I., Murakami, K., Kida, K., Tomimatsu, T., Yamasaki, M., et al. (1985). Oleanene-sapogenols from puerariae radix. Chem. Pharm. Bull. 33 (3), 1293–1296. doi:10.1248/cpb.33.1293
Kinjo, J. E., Takeshita, T., Abe, Y., Terada, N., Yamashita, H., Yamasaki, M., et al. (1988). Studies on the constituents of Pueraria lobata. IV. chemical constituents in the flowers and the leaves. Chem. Pharm. Bull. 36 (3), 1174–1179. doi:10.1248/cpb.36.1174
Koirala, P., Seong, S. H., Jung, H. A., and Choi, J. S. (2017). Comparative molecular docking studies of lupeol and lupenone isolated from Pueraria lobata that inhibits BACE1: probable remedies for Alzheimer’s disease. Asian Pac. J. Trop. Med. 10 (12), 1117–1122. doi:10.1016/j.apjtm.2017.10.018
Kozhevnikov, A. E., Kozhevnikova, Z. V., Lee, B. Y., and Kwak, M. (2017). Comparative floristic diversity of Southwest primorye and neighboring areas of the Russian Far East. J. species Res. 6 (1), 59–67. doi:10.12651/JSR.2017.6.1.059
Lee, C.-H. (2022). “The development and distinctive features of Korean non-alcoholic beverages,” in Korean food and foodways., 183–205.
Lee, M.-R., Kim, B., Lee, Y., Park, S.-Y., Shim, J.-H., Chung, B.-H., et al. (2021). Ameliorative effects of Pueraria lobata extract on postmenopausal symptoms through promoting estrogenic activity and bone markers in ovariectomized rats. Evidence-Based Complementary Altern. Med. 2021, 7924400–7924408. doi:10.1155/2021/7924400
Lee, S., Oh, S., Zheng, Q., Zheng, S., Kim, M., Park, S., et al. (2024). Photoprotective effects of lithospermum erythrorhizon and Pueraria lobata extracts on UVB-induced photoaging: a study on skin barrier protection. Photodermatol. Photoimmunol. Photomed. 40 (1), e12950. doi:10.1111/phpp.12950
Li, C., and Zhang, Y. (2023). Glycosylation and methylation in the biosynthesis of isoflavonoids in Pueraria lobata. Front. Plant Sci. 14, 1330586. doi:10.3389/fpls.2023.1330586
Li, G., Zhang, Q., and Wang, Y. (2010). Chemical constituents from roots of Pueraria lobata. Zhongguo Zhong Yao Za Zhi 35 (23), 3156–3160.
Li, G. H., Zhang, Q. W., Wang, L., Zhang, X. Q., Ye, W. C., and Wang, Y. T. (2011). New isoflavone C-glycosides from Pueraria lobata. Helvetica Chim. Acta 94 (3), 423–428. doi:10.1002/hlca.201000205
Li, H., Chen, B., Pang, G., Chen, J., Xie, J., and Huang, H. (2016a). Anti-osteoporotic activity of puerarin 6″-O-xyloside on ovariectomized mice and its potential mechanism. Pharm. Biol. 54 (1), 111–117. doi:10.3109/13880209.2015.1017885
Li, J., Li, C., Gou, J., Wang, X., Fan, R., and Zhang, Y. (2016b). An alternative pathway for formononetin biosynthesis in Pueraria lobata. Front. Plant Sci. 7, 861. doi:10.3389/fpls.2016.00861
Li, J., Li, C., Gou, J., and Zhang, Y. (2016c). Molecular cloning and functional characterization of a novel isoflavone 3′-O-methyltransferase from Pueraria lobata. Front. Plant Sci. 7, 793. doi:10.3389/fpls.2016.00793
Li, J., Li, Y., Yuan, X., Yao, D., Gao, Z., Niu, Z., et al. (2022). The effective constituent puerarin, from Pueraria lobata, inhibits the proliferation and inflammation of vascular smooth muscle in atherosclerosis through the miR-29b-3p/IGF1 pathway. Pharm. Biol. 61 (1), 1–11. doi:10.1080/13880209.2022.2099430
Li, L., and Vijayalakshmi, A. (2023). Protective effect of Pueraria lobata leaves on doxorubicin-induced myocardial infarction in experimental Wistar rats. Biotechnol. Appl. Biochem. 70 (5), 1641–1651. doi:10.1002/bab.2462
Li, S., Li, S., Liu, C., Liu, C., and Zhang, Y. (2017). Extraction and isolation of potential anti-stroke compounds from flowers of Pueraria lobata guided by in vitro PC12 cell model. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1048, 111–120. doi:10.1016/j.jchromb.2017.02.009
Li, X., Liu, L., Wan, M. X., Gong, L. M., Su, J., and Xu, L. (2024). Active components of Pueraria lobata through the MAPK/ERK signaling pathway alleviate iron overload in alcoholic liver disease. Chem. Biodivers. 21 (5), e202400005. doi:10.1002/cbdv.202400005
Li, X., Wang, S., Chen, M., Xi, Y., Shan, Y., and Lü, X. (2023a). Evaluation of storage stability and safety of hypoglycemic Pueraria-Ophiopogon tea. J. Stored Prod. Res. 102, 102124. doi:10.1016/j.jspr.2023.102124
Li, Y., He, M., Li, J., Yao, Y., Zhu, L., and Wu, B. (2021). Regulatory protein genes and microRNAs in response to selenium stimuli in Pueraria lobata (Willd.) Ohwi. Metallomics 13 (1), mfaa004. doi:10.1093/mtomcs/mfaa004
Li, Y., Ji, S., Xu, T., Zhong, Y., Xu, M., Liu, Y., et al. (2023b). Chinese yam (Dioscorea): nutritional value, beneficial effects, and food and pharmaceutical applications. Trends Food Sci. Technol. 134, 29–40. doi:10.1016/j.tifs.2023.01.021
Lian, Z., Xu, Y., Wang, C., Chen, Y., Yuan, L., Liu, Z., et al. (2023). Gut microbiota-derived melatonin from Puerariae Lobatae Radix-resistant starch supplementation attenuates ischemic stroke injury via a positive microbial co-occurrence pattern. Pharmacol. Res. 190, 106714. doi:10.1016/j.phrs.2023.106714
Liang, J., Maeda, T., Tao, X. L., Wu, Y. H., and Tang, H. J. (2017). Physicochemical properties of Pueraria root starches and their effect on the improvement of buckwheat noodle quality. Cereal Chem. 94 (3), 554–559. doi:10.1094/cchem-08-16-0219-r
Liga, S., and Paul, C. (2024). Puerarin-A promising flavonoid: biosynthesis, extraction methods, analytical techniques, and biological effects. Int. J. Mol. Sci. 25 (10), 5222. doi:10.3390/ijms25105222
Lim, T. K. (2016). “Pueraria Montana var. lobata,” in Edible medicinal and non-medicinal plants: volume 10, modified stems, roots, bulbs, (Dordrecht: Springer Netherlands), 482–540.
Lin, S., Ke, D., Lin, Y., Fu, X., and Yu, Y. (2020). Puerarin inhibits the migration of osteoclast precursors and osteoclastogenesis by inhibiting MCP-1 production. Biosci. Biotechnol. Biochem. 84 (7), 1455–1459. doi:10.1080/09168451.2020.1738912
Liu, C.-M., Zheng, G.-H., Ming, Q.-L., Sun, J.-M., and Cheng, C. (2013). Protective effect of puerarin on lead-induced mouse cognitive impairment via altering activities of acetyl cholinesterase, monoamine oxidase and nitric oxide synthase. Environ. Toxicol. Pharmacol. 35 (3), 502–510. doi:10.1016/j.etap.2013.02.009
Liu, J., Zhang, J., Liao, T., Zhou, L., Zou, L., Liu, Y., et al. (2021a). Thermal inactivation kinetics of kudzu (Pueraria lobata) polyphenol oxidase and the influence of food constituents. Foods 10 (6), 1320. doi:10.3390/foods10061320
Liu, L., Ma, Y., Chen, X., Xiong, X., and Shi, S. (2012). Screening and identification of BSA bound ligands from Puerariae lobata flower by BSA functionalized Fe3O4 magnetic nanoparticles coupled with HPLC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 887-888, 55–60. doi:10.1016/j.jchromb.2012.01.008
Liu, Q., Liu, Z., Huo, X., Wang, C., Meng, Q., Sun, H., et al. (2018). Puerarin improves methotrexate-induced renal damage by up-regulating renal expression of Oat1 and Oat3 in vivo and in vitro. Biomed. Pharmacother. 103, 915–922. doi:10.1016/j.biopha.2018.04.122
Liu, S., Cao, X.-L., Liu, G.-Q., Zhou, T., Yang, X.-L., and Ma, B.-X. (2019). Thein silicoandin vivoevaluation of puerarin against Alzheimer’s disease. Food Funct. 10 (2), 799–813. doi:10.1039/c8fo01696h
Liu, S., Zhou, T., Chen, D., Liu, R., Qin, H.-H., Min, Z.-L., et al. (2021b). In silico-determined compound from the root of Pueraria lobate alleviates synaptic plasticity injury induced by Alzheimer’s disease via the p38MAPK-CREB signaling pathway. Food Funct. 12 (3), 1039–1050. doi:10.1039/d0fo02388d
Lu, J., Sun, J. H., Tan, Y., Kano, Y., and Yuan, D. (2013a). New triterpenoid saponins from the flowers of Pueraria thomsonii. J. Asian Nat. Prod. Res. 15 (10), 1065–1072. doi:10.1080/10286020.2013.802690
Lu, J., Xie, Y., Tan, Y., Qu, J., Matsuda, H., Yoshikawa, M., et al. (2013b). Simultaneous determination of isoflavones, saponins and flavones in Flos Puerariae by ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Chem. Pharm. Bull. (Tokyo) 61 (9), 941–951. doi:10.1248/cpb.c13-00271
Lu, Y., Xu, J., Tang, R., Zeng, P., Li, Z., You, J., et al. (2024). Edible Pueraria lobata-derived exosome-like nanovesicles ameliorate dextran sulfate sodium-induced colitis associated lung inflammation through modulating macrophage polarization. Biomed. Pharmacother. 170, 116098. doi:10.1016/j.biopha.2023.116098
Luo, C. F., Cai, B., Hou, N., Yuan, M., Liu, S. M., Ji, H., et al. (2012). UDP-glucuronosyltransferase 1A1 is the principal enzyme responsible for puerarin metabolism in human liver microsomes. Arch. Toxicol. 86 (11), 1681–1690. doi:10.1007/s00204-012-0874-7
Lynd, J. Q., and Ansman, T. R. (1990). Exceptional forage regrowth, nodulation and nitrogenase activity of kudzu (Pueraria lobata (Willd.) Ohivi) grown on eroded Dougherty loam subsoil. J. Plant Nutr. 13 (7), 861–885. doi:10.1080/01904169009364122
Maji, S., Bumbadiya, M., and Sao, K. (2023). Value addition of traditional Indian dairy products using herbs and spices- an overview. Int. J. Bio-resource Stress Manag. 14, 400–406. doi:10.23910/1.2023.3363a
Mocan, A., Carradori, S., Locatelli, M., Secci, D., Cesa, S., Mollica, A., et al. (2018). Bioactive isoflavones from Pueraria lobata root and starch: different extraction techniques and carbonic anhydrase inhibition. Food Chem. Toxicol. 112, 441–447. doi:10.1016/j.fct.2017.08.009
Mun, S.-C., and Mun, G.-S. (2015). Dynamics of phytoestrogen, isoflavonoids, and its isolation from stems of Pueraria lobata (Willd.) Ohwi growing in Democratic People’s Republic of Korea. J. food drug analysis 23 (3), 538–544. doi:10.1016/j.jfda.2015.04.003
Nguyen, V. D., Min, B. C., Kyung, M. O., Park, J. T., Lee, B. H., Choi, C. H., et al. (2009). Identification of a naturally-occurring 8-[alpha-D-glucopyranosyl-(1--6)-beta-D-glucopyranosyl]daidzein from cultivated kudzu root. Phytochem. Anal. 20 (6), 450–455. doi:10.1002/pca.1146
Oh, J. H., Baek, S.-E., Lee, W.-Y., Baek, J. Y., Trinh, T. A., Park, D. H., et al. (2019). Investigating the systems-level effect of Pueraria lobata for menopause-related metabolic diseases using an ovariectomized rat model and network pharmacological analysis. Biomolecules 9 (11), 747. doi:10.3390/biom9110747
Oh, S. R., Kinjo, J., Shii, Y., Ikeda, T., Nohara, T., Ahn, K. S., et al. (2000). Effects of triterpenoids from Pueraria lobata on immunohemolysis: β-D-glucuronic acid plays an active role in anticomplementary activity in vitro. Planta Medica 66 (6), 506–510. doi:10.1055/s-2000-8614
Ohshima, Y., Okuyama, T., Takahashi, K., Takizawa, T., and Shibata, S. (1988). Isolation and high performance liquid chromatography (HPLC) of isoflavonoids from the Pueraria root. Planta Med. 54 (3), 250–254. doi:10.1055/s-2006-962420
Pang, H., Yue, Y., Dong, H., Jiang, T., Zhang, H., Zhao, Y., et al. (2023). Structural properties of Kudzu protein enzymatic hydrolysate and its repair effect on HepG2 cells damaged by H2O2 oxidation. Food Funct. 14 (21), 9872–9891. doi:10.1039/d3fo02988c
Pappert, R. A., Hamrick, J., and Donovan, L. A. (2000). Genetic variation in Pueraria lobata (Fabaceae), an introduced, clonal, invasive plant of the southeastern United States. Am. J. Bot. 87 (9), 1240–1245. doi:10.2307/2656716
Park, H.-H., Hakamatsuka, T., Sankawa, U., and Ebizuka, Y. (1995). Rapid metabolism of isoflavonoids in elicitor-treated cell suspension cultures of Pueraria lobata. Phytochemistry 38 (2), 373–380. doi:10.1016/0031-9422(94)00640-F
Piegholdt, S., Pallauf, K., Esatbeyoglu, T., Speck, N., Reiss, K., Ruddigkeit, L., et al. (2014). Biochanin A and prunetin improve epithelial barrier function in intestinal CaCo-2 cells via downregulation of ERK, NF-κB, and tyrosine phosphorylation. Free Radic. Biol. Med. 70, 255–264. doi:10.1016/j.freeradbiomed.2014.02.025
Qi, S. (2018). Synergistic effects of genistein and zinc on bone metabolism and the femoral metaphyseal histomorphology in the ovariectomized rats. Biol. Trace Elem. Res. 183 (2), 288–295. doi:10.1007/s12011-017-1134-8
Ramawat, K. G., and Mèrillon, J. M. (2023). “An introduction to specialized metabolites from plants, their biological and ecological roles, and biotechnological interventions to improve the production,” in Plant specialized metabolites: phytochemistry, ecology and biotechnology, (Cham: Springer Nature Switzerland), 1–21.
Reppert, A., Yousef, G. G., Rogers, R. B., and Lila, M. A. (2008). Isolation of radiolabeled isoflavones from kudzu (Pueraria lobata) root cultures. J. Agric. Food Chem. 56 (17), 7860–7865. doi:10.1021/jf801413z
Rizzoli, R., and Bonjour, J.-P. (1997). Hormones and bones. Lancet 349, S20–S23. doi:10.1016/s0140-6736(97)90007-6
Rong, H., Stevens, J. F., Deinzer, M. L., Cooman, L. D., and Keukeleire, D. D. (1998). Identification of isoflavones in the roots of Pueraria lobata. Planta Med. 64 (7), 620–627. doi:10.1055/s-2006-957534
Sankawa, U., Hakamatsuka, T., Shinkai, K., Yoshida, M., and Ebizuka, Y. (1995). Changes of secondary metabolism by elicitor treatment in Pueraria lobata cell cultures. Netherlands: Springer, 595–604.
Shang, X., Huang, D., Wang, Y., Xiao, L., Ming, R., Zeng, W., et al. (2021). Identification of nutritional ingredients and medicinal components of Pueraria lobata and its varieties using UPLC-MS/MS-Based metabolomics. Molecules 26 (21), 6587. doi:10.3390/molecules26216587
Shen, L., Shen, Y., You, L., Zhang, Y., Su, Z., Peng, G., et al. (2023). Pueraria lobata polysaccharides alleviate neonatal calf diarrhea by modulating gut microbiota and metabolites. Front. Veterinary Sci. 9, 1024392. doi:10.3389/fvets.2022.1024392
Sleiman, H. K., de Oliveira, J. M., and Langoni de Freitas, G. B. (2021). Isoflavones alter male and female fertility in different development windows. Biomed. Pharmacother. 140, 111448. doi:10.1016/j.biopha.2021.111448
Song, J., Kim, Y. S., Lee, D., and Kim, H. (2020). Safety evaluation of root extract of Pueraria lobata and Scutellaria baicalensis in rats. BMC Complement. Med. Ther. 20 (1), 226. doi:10.1186/s12906-020-02998-1
Sook Kim, Y., Soo Lee, I., and Sook Kim, J. (2014). Protective effects of Puerariae radix extract and its single compounds on methylglyoxal-induced apoptosis in human retinal pigment epithelial cells. J. Ethnopharmacol. 152 (3), 594–598. doi:10.1016/j.jep.2014.01.008
Sun, R., Deng, X., Zhang, D., Xie, F., Wang, D., Wang, J., et al. (2019a). Anti-diabetic potential of Pueraria lobata root extract through promoting insulin signaling by PTP1B inhibition. Bioorg. Chem. 87, 12–15. doi:10.1016/j.bioorg.2019.02.046
Sun, Y., Zhang, H., Cheng, M., Cao, S., Qiao, M., Zhang, B., et al. (2019b). New hepatoprotective isoflavone glucosides from Pueraria lobata (Willd.) Ohwi. Nat. Prod. Res. 33 (24), 3485–3492. doi:10.1080/14786419.2018.1484461
Sun, Y. G., Wang, S. S., Feng, J. T., Xue, X. Y., and Liang, X. M. (2008). Two new isoflavone glycosides from Pueraria lobata. J. Asian Nat. Prod. Res. 10 (7-8), 729–733. doi:10.1080/10286020802016198
Tanaka, T., Tang, H., Yu, F., Michihara, S., Uzawa, Y., Zaima, N., et al. (2011). Kudzu (Pueraria lobata) vine ethanol extracts improve ovariectomy-induced bone loss in female mice. J. Agric. food Chem. 59 (24), 13230–13237. doi:10.1021/jf2031617
Tang, H., Song, X., Ling, Y., Wang, X., Yang, P., Luo, T., et al. (2017). Puerarin attenuates myocardial hypoxia/reoxygenation injury by inhibiting autophagy via the Akt signaling pathway. Mol. Med. Rep. 15 (6), 3747–3754. doi:10.3892/mmr.2017.6424
Tanner, R. D., and Hussain, S. S. (1979). Kudzu (Pueraria lobata) root starch as a substrate for the lysine-enriched baker’s yeast and ethanol fermentation process. J. Agric. Food Chem. 27 (1), 22–27. doi:10.1021/jf60221a029
Tao, Q., Chen, Y., Liang, Q., Shi, J., Wang, Z., Min, H., et al. (2024a). Puerarin alleviates experimental autoimmune thyroiditis by regulating macrophages. J. Immunol. 212 (11), 1670–1679. doi:10.4049/jimmunol.2300779
Tao, Q., Liang, Q., Fu, Y., Qian, J., Xu, J., Zhu, Y., et al. (2024b). Puerarin ameliorates colitis by direct suppression of macrophage M1 polarization in DSS mice. Phytomedicine 135, 156048. doi:10.1016/j.phymed.2024.156048
Tungmunnithum, D., Intharuksa, A., and Sasaki, Y. (2020). A Promising View of Kudzu Plant, Pueraria montana var. lobata (Willd.) Sanjappa & Pradeep: flavonoid phytochemical compounds, taxonomic data, traditional uses and potential biological activities for future cosmetic application. Cosmetics. 7. doi:10.3390/cosmetics7010012
Van Hung, P., and Morita, N. (2007). Chemical compositions, fine structure and physicochemical properties of kudzu (Pueraria lobata) starches from different regions. Food Chem. 105 (2), 749–755. doi:10.1016/j.foodchem.2007.01.023
Wagle, A., Seong, S. H., Jung, H. A., and Choi, J. S. (2019). Identifying an isoflavone from the root of Pueraria lobata as a potent tyrosinase inhibitor. Food Chem. 276, 383–389. doi:10.1016/j.foodchem.2018.10.008
Wang, C., Yan, M., Jiang, H., Wang, Q., He, S., Chen, J., et al. (2018a). Mechanism of aquaporin 4 (AQP 4) up-regulation in rat cerebral edema under hypobaric hypoxia and the preventative effect of puerarin. Life Sci. 193, 270–281. doi:10.1016/j.lfs.2017.10.021
Wang, C., Yao, J., Ju, L., Wen, X., and Shu, L. (2020). Puerarin ameliorates hyperglycemia in HFD diabetic mice by promoting β-cell neogenesis via GLP-1R signaling activation. Phytomedicine 70, 153222. doi:10.1016/j.phymed.2020.153222
Wang, F. R., and Yang, X. W. (2017). Absorption and transport of isoflavonoid compounds from Tongmai formula across human intestinal epithelial (Caco-2) cells in vitro. Zhongguo Zhong Yao Za Zhi 42 (16), 3206–3212. doi:10.19540/j.cnki.cjcmm.20170705.003
Wang, J., Dai, G., Shang, M., Wang, Y., Xia, C., Duan, B., et al. (2023a). Extraction, structural-activity relationships, bioactivities, and application prospects of Pueraria lobata polysaccharides as ingredients for functional products: a review. Int. J. Biol. Macromol. 243, 125210. doi:10.1016/j.ijbiomac.2023.125210
Wang, J., Zhang, T., Ma, C., and Wang, S. (2015). Puerarin attenuates airway inflammation by regulation of eotaxin-3. Immunol. Lett. 163 (2), 173–178. doi:10.1016/j.imlet.2014.12.002
Wang, L., Ning, T., and Chen, X. (2019a). Postharvest storage quality of citrus fruit treated with a liquid ferment of Chinese herbs and probiotics. Sci. Hortic. 255, 169–174. doi:10.1016/j.scienta.2019.03.030
Wang, L., Shan, H., Wang, B., Wang, N., Zhou, Z., Pan, C., et al. (2018b). Puerarin attenuates osteoarthritis via upregulating AMP-activated protein kinase/proliferator-activated receptor-γ coactivator-1 signaling pathway in osteoarthritis rats. Pharmacology 102 (3-4), 117–125. doi:10.1159/000490418
Wang, L., Wu, Y., Zhuang, L., Chen, X., Min, H., Song, S., et al. (2019b). Puerarin prevents high-fat diet-induced obesity by enriching Akkermansia muciniphila in the gut microbiota of mice. PLoS One 14 (6), e0218490. doi:10.1371/journal.pone.0218490
Wang, Q.-S., Wang, Y.-L., Zhang, W.-Y., Li, K.-D., Luo, X.-F., and Cui, Y.-L. (2021). Puerarin from Pueraria lobata alleviates the symptoms of irritable bowel syndrome-diarrhea. Food Funct. 12 (5), 2211–2224. doi:10.1039/d0fo02848g
Wang, S., Yao, W., Zhu, X., Wang, J., Lu, L., Zhu, N., et al. (2023b). Exploring the mechanism of the antithrombotic effects of Pueraria lobata and Pueraria lobata var. thomsonii based on network pharmacology. J. Ethnopharmacol. 300, 115701. doi:10.1016/j.jep.2022.115701
Wang, W., Liu, Y., Liu, D., Zhou, H., Li, Y., Yuan, W., et al. (2024a). Profiling of antidiabetic bioactive flavonoid compounds from an edible plant kudzu (Pueraria lobata). J. Agric. Food Chem. 72 (28), 15704–15714. doi:10.1021/acs.jafc.4c02564
Wang, X., Chen, B., Fang, X., Zhong, Q., Liao, Z., Wang, J., et al. (2024b). Soy isoflavone-specific biotransformation product S-equol in the colon: physiological functions, transformation mechanisms, and metabolic regulatory pathways. Crit. Rev. Food Sci. Nutr. 64 (16), 5462–5490. doi:10.1080/10408398.2022.2154744
Wang, X., Fan, R., Li, J., Li, C., and Zhang, Y. (2016). Molecular cloning and functional characterization of a novel (Iso)flavone 4',7-O-diglucoside glucosyltransferase from Pueraria lobata. Front. Plant Sci. 7, 387. doi:10.3389/fpls.2016.00387
Wang, X. L., Jiao, F. R., Yu, M., Lin, L. B., Xiao, J., Zhang, Q., et al. (2017b). Constituents with potent α-glucosidase inhibitory activity from Pueraria lobata (Willd.) Ohwi. Bioorg Med. Chem. Lett. 27 (9), 1993–1998. doi:10.1016/j.bmcl.2017.03.013
Wang, Y., Gao, C., Long, P., Hu, Z., Zhu, L., Wang, L., et al. (2023c). Dynamic changes of active substances of rice, Pueraria and yam fermentation by Monascus ruber. Lwt 183, 114925. doi:10.1016/j.lwt.2023.114925
Wang, Y., Wang, W. L., Xie, W. L., Li, L. Z., Sun, J., Sun, W. J., et al. (2013). Puerarin stimulates proliferation and differentiation and protects against cell death in human osteoblastic MG-63 cells via ER-dependent MEK/ERK and PI3K/Akt activation. Phytomedicine 20 (10), 787–796. doi:10.1016/j.phymed.2013.03.005
Wang, Y., Yang, C., Xie, W. L., Zhao, Y. W., Li, Z. M., Sun, W. J., et al. (2014). Puerarin concurrently stimulates osteoprotegerin and inhibits receptor activator of NF-κB ligand (RANKL) and interleukin-6 production in human osteoblastic MG-63 cells. Phytomedicine 21 (8-9), 1032–1036. doi:10.1016/j.phymed.2014.04.012
Wang, Z., Du, H., Peng, W., Yang, S., Feng, Y., Ouyang, H., et al. (2022). Efficacy and mechanism of Pueraria lobata and Pueraria thomsonii polysaccharides in the treatment of type 2 diabetes. Nutrients 14 (19), 3926. doi:10.3390/nu14193926
Wei, L., Zhu, P., Chen, X., Wang, Y., and Xu, Y. (2020). An ultra high performance liquid chromatography with tandem mass spectrometry method for simultaneous determination of thirteen components extracted from Radix Puerariae in rat plasma and tissues: application to pharmacokinetic and tissue distribution study. J. Sep. Sci. 43 (2), 418–437. doi:10.1002/jssc.201900824
Wong, K. H., Li, G. Q., Li, K. M., Razmovski-Naumovski, V., and Chan, K. (2011). Kudzu root: traditional uses and potential medicinal benefits in diabetes and cardiovascular diseases. J. Ethnopharmacol. 134 (3), 584–607. doi:10.1016/j.jep.2011.02.001
Wong, R., and Rabie, B. (2007). Effect of puerarin on bone formation. Osteoarthr. Cartil. 15 (8), 894–899. doi:10.1016/j.joca.2007.02.009
Wu, L., Tong, T., Wan, S., Yan, T., Ren, F., Bi, K., et al. (2017). Protective effects of puerarin against Aβ1–42-induced learning and memory impairments in mice. Planta Medica 83 (3-04), 224–231. doi:10.1055/s-0042-111521
Wu, S., Xu, W., and Yang, X. W. (2013). Studies on biotransformation of chemical constituents of tongmai formula by human intestinal flora. Zhongguo Zhong Yao Za Zhi 38 (20), 3510–3519.
Xiao, B., Sun, Z., Cao, F., Wang, L., Liao, Y., Liu, X., et al. (2017). Brain pharmacokinetics and the pharmacological effects on striatal neurotransmitter levels of Pueraria lobata isoflavonoids in rat. Front. Pharmacol. 8, 599. doi:10.3389/fphar.2017.00599
Xiaoming, W., Ling, L., and Jinghang, Z. (2012). Antioxidant and anti-fatigue activities of flavonoids from Puerariae radix. Afr. J. Tradit. Complement. Altern. Med. 9 (2), 221–227. doi:10.4314/ajtcam.v9i2.6
Xu, X., Guo, Y., Chen, S., Ma, W., Xu, X., Hu, S., et al. (2022). The positive influence of polyphenols extracted from Pueraria lobata root on the gut microbiota and its antioxidant capability. Front. Nutr. 9, 868188. doi:10.3389/fnut.2022.868188
Xu, Y., Cao, H., and He, J. (2025). Research advances in okra polysaccharides: green extraction technology, structural features, bioactivity, processing properties and application in foods. Food Res. Int. 202, 115686. doi:10.1016/j.foodres.2025.115686
Yan, Y., Chai, C.-Z., Wang, D.-W., Yue, X.-Y., Zhu, D.-N., and Yu, B.-Y. (2013). HPLC-DAD-Q-TOF-MS/MS analysis and HPLC quantitation of chemical constituents in traditional Chinese medicinal formula Ge-Gen Decoction. J. Pharm. Biomed. 80, 192–202. doi:10.1016/j.jpba.2013.03.008
Yang, C. W., Liu, H. M., Chang, Z. Y., Liu, G. H., Chang, H. H., Huang, P. Y., et al. (2024a). Puerarin modulates hepatic farnesoid X receptor and gut microbiota in high-fat diet-induced obese mice. Int. J. Mol. Sci. 25 (10), 5274. doi:10.3390/ijms25105274
Yang, H.-B., Song, J.-Y., Xu, C., Li, J., Zhang, C., Xie, S., et al. (2024b). Interventional effects of Pueraria oral liquid on T2DM rats and metabolomics analysis. Biomed. Pharmacother. 175, 116780. doi:10.1016/j.biopha.2024.116780
Yang, Y., Li, M., Liu, Q., Zhao, Q., Zeng, J., Wang, Q., et al. (2023). Starch from Pueraria lobata and the amylose fraction alleviates dextran sodium sulfate induced colitis in mice. Carbohydr. Polym. 302, 120329. doi:10.1016/j.carbpol.2022.120329
Yang, Y., Li, M., Wang, Q., Huang, H., Zhao, Y., Du, F., et al. (2022). Pueraria lobata starch regulates gut microbiota and alleviates high-fat high-cholesterol diet induced non-alcoholic fatty liver disease in mice. Food Res. Int. 157, 111401. doi:10.1016/j.foodres.2022.111401
Yu, T., Li, J., Yu, Q., Tian, Y., Shun, X., Xu, L., et al. (2017). Knowledge graph for TCM health preservation: design, construction, and applications. Artif. Intell. Med. 77, 48–52. doi:10.1016/j.artmed.2017.04.001
Zhang, D., Xu, D., Huang, X., Wei, Y., Tang, F., Qin, X., et al. (2024a). Puerarin-loaded electrospun patches with anti-inflammatory and pro-collagen synthesis properties for pelvic floor reconstruction. Adv. Sci. 11 (21), e2308590. doi:10.1002/advs.202308590
Zhang, G., Wang, Y., Tang, G., and Ma, Y. (2019a). Puerarin inhibits the osteoclastogenesis by inhibiting RANKL-dependent and -independent autophagic responses. BMC Complementary Altern. Med. 19 (1), 269. doi:10.1186/s12906-019-2691-5
Zhang, H.-J., Yang, X.-P., and Wang, K.-W. (2010). Isolation of two new C-glucofuranosyl isoflavones from Pueraria lobata (Wild.) Ohwi with HPLC-MS guiding analysis. J. Asian Nat. Prod. Res. (3/4), 12. doi:10.1080/10286021003745445
Zhang, P., Fang, Z., Zhao, M., Yi, B., Huang, Y., Yang, H., et al. (2023a). Ethanol extract of Pueraria lobata improve acute myocardial infarction in rats via regulating gut microbiota and bile acid metabolism. Phytother. Res. 37 (12), 5932–5946. doi:10.1002/ptr.8005
Zhang, P., Zhang, Y., Wang, L., Wang, X., Xu, S., Zhai, Z., et al. (2022). Reversal of NADPH oxidase-dependent early oxidative and inflammatory responses in chronic obstructive pulmonary disease by puerarin. Oxidative Med. Cell. Longev. 2022, 5595781–5595824. doi:10.1155/2022/5595781
Zhang, S., Ge, Q., Chen, L., and Chen, K. (2021). Studies of the anti-diabetic mechanism of Pueraria lobata based on metabolomics and network pharmacology. Processes 9 (7), 1245. doi:10.3390/pr9071245
Zhang, W., Song, Q., Bi, X., Cui, W., Fang, C., Gao, J., et al. (2024b). Preparation of Pueraria lobata root-derived exosome-like nanovesicles and evaluation of their effects on mitigating alcoholic intoxication and promoting alcohol metabolism in mice. Int. J. Nanomedicine 19, 4907–4921. doi:10.2147/ijn.S462602
Zhang, Y., Xu, D., Xing, X., Yang, H., Gao, W., and Li, P. (2023b). The chemistry and activity-orientedcharacterization of isoflavones difference between roots of Pueraria lobata and P. thomsonii guided by feature-based molecular networking. Food Chem. 422, 136198. doi:10.1016/j.foodchem.2023.136198
Zhang, Y., Yan, M., Yu, Q.-f., Yang, P.-f., Zhang, H.-d., Sun, Y.-h., et al. (2016). Puerarin prevents LPS-induced osteoclast formation and bone loss via inhibition of Akt activation. Biol. Pharm. Bull. 39 (12), 2028–2035. doi:10.1248/bpb.b16-00522
Zhang, Y. N., Zhu, S. J., Li, N., Jing, Y. N., and Yue, X. F. (2019b). Screening and identification of the active components from Puerariae Radix by HUVEC/CMC-LC-MS(2). J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1132, 121825. doi:10.1016/j.jchromb.2019.121825
Zhang, Z., Liu, B., Liu, X., Hu, W., Zhang, C., Guo, Y., et al. (2023c). Effects of steaming on sweet potato soluble dietary fiber: content, structure, and lactobacillus proliferation in vitro. Foods 12 (8), 1620. doi:10.3390/foods12081620
Zheng, M., Song, D., Luo, Z., Lu, Z., Wu, Y., and Wang, W. (2015). Effect of puerarin on expression of Fas/FasL mRNA in pulmonary injury induced by ischemia-reperfusion in rabbits. Nat. Product. Commun. 10 (2), 253–256. doi:10.1177/1934578x1501000209
Zhou, X., Lam, W. P., Tang, H. C., Koon, C.-M., Cheng, L., Lau, C.B.-S., et al. (2016). Effects of Gegen (Puerariae lobatae Radix) water extract on improving detrusor overactivity in spontaneously hypertensive rats. Phytomedicine 23 (6), 672–678. doi:10.1016/j.phymed.2016.03.002
Zhou, Y. X., Zhang, H., and Peng, C. (2014). Puerarin: a review of pharmacological effects. Phytother. Res. 28 (7), 961–975. doi:10.1002/ptr.5083
Glossary
4CL 4-Coumarate-CoA ligase
5-HT 5-Hydroxytryptamine
ACC Acetyl-CoA carboxylase
AChE Acetylcholinesterase
ADH Alcohol dehydrogenase
ADT Plant aronic acid dehydratase
Akt Protein kinase B
ALD Alcoholic liver disease
ALDH Acetaldehyde dehydrogenase
ALOX15 Arachidonic acid 15-lipoxygenase
ALP Alkaline phosphatase
ALT Alanine aminotransferase
ALT/GPT Alanine aminotransferase
AMPK Adenosine 5′-monophosphate (AMP)-activated protein kinase
APTT Activated partial thromboplastin time
AST Aspartate transaminase
AST/GOT Aspartate aminotransferase
BACE1 Beta-site APP cleaving enzyme 1
BCL 6 B-cell lymphoma 6
BMD Bone mineral density
BMSCs Bone marrow mesenchymal stem cells
C3 Cysteine-3
C4H Cinnamate 4-hydroxylase
CaMK IIα Calcium/calmodulin kinase IIα
CAT Catalase
CCNB 1 Cell cycle protein B1
CHI Chalcone isomerase
CHR Chalcone reductase
CHS Chalcone synthase
COL10A 1 Collagen type X alpha 1 chain
COL2A 1 Collagen type II alpha 1 chain
COX-2 Cyclooxygenase-2
CREB cAMP-response element binding
CRF Corticotropin-releasing hormone receptor
CTGF Connective tissue growth factor
CTX-1 C-telopeptide of crosslinked collagen type I
CWI Cell-wall integrity
CYP1B1 Cytochrome P450 family 1 subfamily B member 1
CYP7A1 Cholesterol 7 alpha-hydroxylase
DA Dopamine
DgpA GenBank: BBG22493.1
DgpBC PDBID: EM Map EMD-30808
DYP Dye-decolorizing peroxidase
DZE 0.30 0.02 O. D
eNOS Endothelial nitric oxide synthase
ERK Extracellular signal-regulated kinase
FA Fatty acyl groups
FasL Factor-related apoptosis ligand
FoxO1 Forkhead box transcription factor O1
GLP-1R Glucagon-like peptide 1 receptor
GP Glycerophospholipids
GR Glutathione reductase
GSH Glutathione
HID 2-Hydroxyisoflavanone dehydratase
HO-1 Heme oxygenase-1
HPA Hypothalamic–pituitary–adrenal
IBS-D Irritable bowel syndrome
IGE Immunoglobulin E
IGF Insulin-like growth factor
IgM Immunoglobulin
IL Interleukin
JNK c-Jun N-terminal kinase
Keap1 Kelch-like ECH-associated protein 1
LKB1 Liver kinase B1
LPS Lipopolysaccharide
LTC4 Leukotriene C4
M3 Muscarinic receptor-3
MAO Monoamine oxidase
MCP Monocyte chemotactic protein
MDA Malondialdehyde
MMP Matrix metalloproteinase
MMP-9 Matrix metalloprotein-9
mTOR Mammalian target of rapamycin.
MyD88 Myeloid differentiation primary response protein 88
NF-κB Nuclear factor kappa-B
NLRP3 NOD-like receptor thermal protein domain-associated protein 3
NOS 2 Nitric oxide synthase 2
Nrf2 Nuclear respiratory factor 2
OC Osteocalcin
OCPs Osteoclast precursor cells
PAL Phenylalanine ammonia-lyase
PGC-1 Peroxisome proliferator-activated receptor-γ coactivator
PGE 2 Prostaglandin E2
PGI2 Prostaglandin-I-2
phAT Plant phosphotransacetylase
PI3K Phosphatidylinositol 3-kinase
PL Pueraria lobata
PLK 1 Polo-like kinase 1
PPAR Peroxisome proliferator-activated receptors
PTGS2 Prostaglandin-endoperoxide synthase 2
PUE 1.96 0.09 optical density (O.D.)
RANKL Receptor activator of nuclear factor-κB ligand
sIgA Secretory immunoglobulin A
SOD Superoxide dismutase
SOX SRY-related high-mobility-group box
SRC Steroid hormone receptor coactivator
TC Total cholesterol
TG Triglycerides
TGF-β Transforming growth factor-beta
Th 17 T helper cell 17
Th 2 T helper 2 cell
TIR Toll/interleukin 1 receptor
TLR4 Toll-like receptor 4
TNF Tumor necrosis factor
TRAP Tartrate acid phosphatase
TT Thrombin time
TXA2 Thromboxane A2
UUO Unilateral ureteral obstruction
Keywords: Pueraria lobata (Willd.) Ohwi, herbal textual research, phytochemistry, pharmacology, edibility, puerarin
Citation: Kou B, Meng L, Zhao M, Wang H, Lu C, Yan M and Li G (2025) Unveiling the power of Pueraria lobata: a comprehensive exploration of its medicinal and edible potentials. Front. Pharmacol. 16:1578472. doi: 10.3389/fphar.2025.1578472
Received: 17 February 2025; Accepted: 19 May 2025;
Published: 10 July 2025.
Edited by:
Michael Heinrich, University College London, United KingdomReviewed by:
Stefania Schiavone, University of Foggia, ItalyJunbo Gou, Hubei University of Chinese Medicine, China
Jie Guo, Jishou University, China
Copyright © 2025 Kou, Meng, Zhao, Wang, Lu, Yan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingming Yan, Mzg2NzU5MTAyQHFxLmNvbQ==; Guangzhe Li, bGlnekBuZW51LmVkdS5jbg==
 Baixin Kou
Baixin Kou Lingkun Meng1
Lingkun Meng1