Introduction
Autoimmune diseases (AIDs) are a series of diseases caused by the reduced or destroyed immune tolerance of the immune system to its own components due to some reasons, resulting in auto-antibodies or (and) sensitized lymphocytes damaging its own organs and tissues (Balogh et al., 2024). Clinically, AIDs are categorized into organ-specific and systemic types. Organ-specific AIDs include inflammatory bowel disease (IBD), lupus nephritis (LN), multiple sclerosis (MS), ankylosing spondylitis (AS), IgA nephropathy (IgAN), type 1 diabetes (T1D), autoimmune liver disease (AILD), and autoimmune thyroiditis (AIT). Although termed “organ-specific”, these disorders often involve damage to multiple tissues and organs. Systemic AIDs, on the other hand, include rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), scleroderma, Sjögren’s syndrome (SS), and so on. At present, there are many kinds of treatment methods for AIDs with obvious advantages and disadvantages. Thus, it is still urgent to explore effective methods to control AIDs.
Natural compounds, known for their structural diversity and broad biological activities, serve as a critical resource for drug discovery as well as lead compound development, playing a pivotal role in innovative drug research. Historically, scientists have made significant strides in pharmacology, drug formulation, and novel drug development. Notably, Chinese scientist Tu Youyou was awarded the 2015 Nobel Prize in Physiology or Medicine for her groundbreaking work on artemisinin (Qinghaosu), highlighting the immense potential of natural compounds in global drug development (Gao et al., 2024). Magnolol and honokiol (Figure 1), two isomeric neolignans, represent the key bioactive constituents of Magnolia officinalis, and have garnered increasing attention from researchers due to their wide-ranging biological properties, including anti-inflammatory, anti-cancer, anti-bacterial, anti-fungal, hepatoprotective, cardiovascular protective, neuroprotective, anti-diabetic, anti-viral, and anti-oxidant effects (Dai et al., 2024). Currently, there is no documented evidence of human toxicity associated with the herbal formulations of magnolol and honokiol (Dai et al., 2024). Moreover, these compounds are also commonly incorporated into consumer products such as mints, toothpaste, and chewing gum (Dai et al., 2024). Consequently, structural modifications of magnolol and honokiol may represent a viable strategy for developing novel therapeutics for AIDs.
FIGURE 1
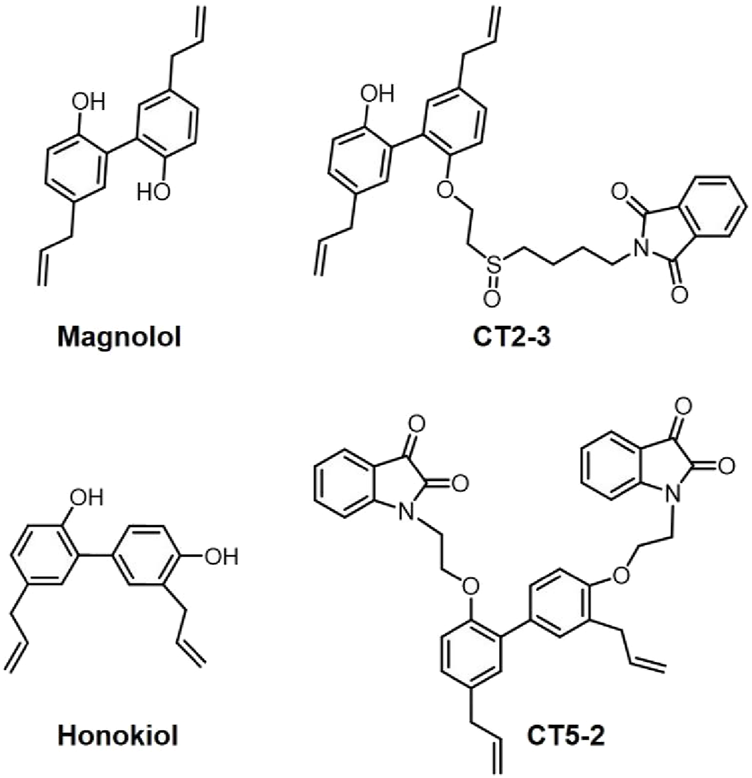
Structures of Magnolol, honokiol, CT2-3, and CT5-2.
Magnolol, honokiol and their derivatives for the treatment of common AIDs
IBD
IBD is an inflammatory bowel disease. Recent study by Zhao et al. have demonstrated that magnolol alleviates inflammation in dextran sulfate sodium (DSS)-induced colitis by restoring altered tryptophan metabolism (Zhao et al., 2017). Similarly, honokiol has been found to mitigate IBD symptoms by enhancing endothelial barrier function via its interaction with the TRPV4 channel (Niu et al., 2024). These findings suggest that magnolol and honokiol have therapeutic potential for IBD.
LN
LN, an immune-complex-mediated kidney disease associated with systemic lupus erythematosus (SLE), significantly contributes to patient mortality and morbidity. Research has indicated that magnolol exhibits protective properties against LN progression by modulating the NLRP3 inflammasome as well as suppressing the NF-κB pathway (Huang et al., 2017). Similarly, honokiol has demonstrated therapeutic potential in LN. Studies by Ma et al. revealed that honokiol alleviates renal damage in MRL/lpr mice and disrupts pathological interactions between renal macrophages and tubular epithelial cells in LN by inhibiting the NLRP3/IL-33/ST2 signaling axis (Ma et al., 2023).
MS
MS is a chronic inflammatory disorder marked by immune-mediated demyelination in the central nervous system. In a 2023 study, Chen et al. revealed that magnolol reduced body weight loss and disease severity in experimental autoimmune encephalomyelitis (EAE) mice, a model for MS. Their findings indicated that magnolol specifically suppresses T helper 17 (Th17) cell differentiation and cytokine production by selectively inhibiting STAT3 signaling, leading to an altered Th17/Treg cell balance (Chen J. Y. et al., 2023). This highlights magnolol’s potential as a novel STAT3 inhibitor for MS treatment.
T1D
T1D stems from pancreatic beta cell destruction by islet reactive immune cells. In 2022, Yang demonstrated that magnolol significantly improved neurological impairments in type 1 diabetic mice (Yang et al., 2022). Similarly, honokiol has been shown to alleviate myocardial ischemia/reperfusion injury in type 1 diabetic rats by mitigating oxidative stress and apoptosis via activation of the SIRT1-Nrf2 pathway (Zhang et al., 2018). These findings highlight the therapeutic potential of magnolol and honokiol in managing T1D-related complications.
RA
RA is a persistent autoimmune disorder that can result in disability, marked by progressive joint deterioration and symptoms beyond the joints. Studies have indicated that honokiol showed anti-inflammatory properties in RA and might act as a promising suppressor of TNF-α-triggered inflammatory factor expression in RA synovial fibroblasts (Sun et al., 2022; Li et al., 2011). Interestingly, our group successfully synthesized a magnolol derivative, namely, CT2-3 (Figure 1) that had the ability to induce cell cycle arrest and apoptosis in RA-fibroblast-like synoviocytes (FLSs) via modulating the PI3K/AKT pathway (Chen J. et al., 2023). Moreover, we also designed and synthesized a honokiol derivative (isatin-honokiol hybrid) namely, CT5-2 (Figure 1) that inhibited proliferation and triggered cell cycle arrest and apoptosis of RA-FLSs through modulation of the c-Myc/CDCA7/p65 pathway (Chen et al., 2022). These studies indicated that CT2-3 and CT5-2 may serve as promising lead compounds for the research and development of new anti-RA drugs.
Discussion
Currently, over 100 AIDs have been identified that collectively contribute to a significant worldwide disease burden (Lenti et al., 2022). While the exact causes and mechanisms of AIDs remain unclear, they are often spontaneous or idiopathic, and most lack definitive diagnostic markers. Extensive research indicates that the onset and progression of AIDs are strongly associated with factors such as genetic predisposition, infections, hormonal imbalances, smoking, educational background, and the use of certain medications. Recent studies further classify AIDs as a significant subset of chronic inflammatory conditions. Additionally, AIDs are closely linked to the development of various cancers, making them a critical risk factor for tumorigenesis. Over the past decades, the development of new drugs to effectively control AIDs is still one of the hot spots of scientists. However, the journey of discovering new drugs is fraught with numerous obstacles and challenges. To guarantee the safety and effectiveness of medications for patients, the process involves an extensive screening phase aimed at identifying promising compounds while discarding those with significant adverse effects. Consequently, lead compounds play a pivotal role in the discovery of innovative drugs. Amidst these challenges and opportunities, natural compounds offer significant benefits owing to their diverse structures and broad biological activities. Enhanced research in this area is expected to drive the development of novel drugs with proprietary intellectual property. As previously outlined, magnolol and honokiol demonstrate diverse biological activities in treating AIDs, including IBD, MS, LN, T1D, RA, et al., positioning them as promising candidates for structural optimization. Notably, their derivatives, CT2-3 and CT5-2, have shown significant efficacy in managing RA, laying the groundwork for the development of innovative drugs for AIDs based on magnolol and honokiol frameworks.
Statements
Author contributions
XL: Funding acquisition, Writing – original draft. JC: Funding acquisition, Project administration, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. We thank the National Natural Science Foundation of China (Grant No. 82471831), the Shenzhen Science and Technology Program (Grant No. JCYJ20230807095804010), and the Scientific Research Foundation of Peking University Shenzhen Hospital KYQD202100X (Grant No. KYQD2024403) for supporting this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Balogh L. Olah K. Santa S. Majerhoffer N. Nemeth T. (2024). Novel and potential future therapeutic options in systemic autoimmune diseases. Front. Immunol.15, 1249500. 10.3389/fimmu.2024.1249500
2
Chen J. Lin X. He J. Liu J. He J. Tao C. et al (2022). Novel isatin-based hybrids as potential anti-rheumatoid arthritis drug candidates: synthesis and biological evaluation. Bioorg Chem.128, 106063. 10.1016/j.bioorg.2022.106063
3
Chen J. Lin X. Liu K. He J. Li X. Zhang C. et al (2023b). CT2-3 induces cell cycle arrest and apoptosis in rheumatoid arthritis fibroblast-like synoviocytes through regulating PI3K/AKT pathway. Eur. J. Pharmacol.956, 175871. 10.1016/j.ejphar.2023.175871
4
Chen J. Y. Tian X. Y. Wei S. S. Xu W. Pan R. R. Chen L. L. et al (2023a). Magnolol as STAT3 inhibitor for treating multiple sclerosis by restricting Th17 cells. Phytomedicine117, 154917. 10.1016/j.phymed.2023.154917
5
Dai S. Y. Qin W. X. Yu S. Li C. Yang Y. H. Pei Y. H. (2024). Honokiol and magnolol: a review of structure-activity relationships of their derivatives. Phytochemistry223, 114132. 10.1016/j.phytochem.2024.114132
6
Gao X. Lin X. Wang Q. Chen J. (2024). Artemisinins: promising drug candidates for the treatment of autoimmune diseases. Med. Res. Rev.44 (2), 867–891. 10.1002/med.22001
7
Huang F. Zhang R. Y. Song L. (2017). Beneficial effect of magnolol on lupus nephritis in MRL/lpr mice by attenuating the NLRP3 inflammasome and NF‑κB signaling pathway: a mechanistic analysis. Mol. Med. Rep.16 (4), 4817–4822. 10.3892/mmr.2017.7154
8
Lenti M. V. Rossi C. M. Melazzini F. Gastaldi M. Bugatti S. Rotondi M. et al (2022). Seronegative autoimmune diseases: a challenging diagnosis. Autoimmun. Rev.21 (9), 103143. 10.1016/j.autrev.2022.103143
9
Li J. Shao X. Wu L. Feng T. Jin C. Fang M. et al (2011). Honokiol: an effective inhibitor of tumor necrosis factor-alpha-induced up-regulation of inflammatory cytokine and chemokine production in human synovial fibroblasts. Acta Biochim. Biophys. Sin. (Shanghai)43 (5), 380–386. 10.1093/abbs/gmr027
10
Ma Q. Xu M. Jing X. Qiu J. Huang S. Yan H. et al (2023). Honokiol suppresses the aberrant interactions between renal resident macrophages and tubular epithelial cells in lupus nephritis through the NLRP3/IL-33/ST2 axis. Cell Death Dis.14 (3), 174. 10.1038/s41419-023-05680-9
11
Niu L. Wang S. Xu Y. Zu X. You X. Zhang Q. et al (2024). Honokiol targeting ankyrin repeat domain of TRPV4 ameliorates endothelial permeability in mice inflammatory bowel disease induced by DSS. J. Ethnopharmacol.325, 117825. 10.1016/j.jep.2024.117825
12
Sun J. Liu B. Yuan Y. Zhang L. Wang J. (2022). Disease markers and therapeutic targets for rheumatoid arthritis identified by integrating bioinformatics analysis with virtual screening of traditional chinese medicine. Front. Biosci. (Landmark Ed). 28;27 (9):–267. 10.31083/j.fbl2709267
13
Yang J. Wei Y. Zhao T. Li X. Zhao X. Ouyang X. et al (2022). Magnolol effectively ameliorates diabetic peripheral neuropathy in mice. Phytomedicine107, 154434. 10.1016/j.phymed.2022.154434
14
Zhang B. Zhai M. Li B. Liu Z. Li K. Jiang L. et al (2018). Honokiol ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by reducing oxidative stress and apoptosis through activating the SIRT1-nrf2 signaling pathway. Oxid. Med. Cell Longev.2018, 3159801. 10.1155/2018/3159801
15
Zhao L. Xiao H. T. Mu H. X. Huang T. Lin Z. S. Zhong L. L. D. et al (2017). Magnolol, a natural polyphenol, attenuates dextran sulfate sodium-induced colitis in mice. Molecules22 (7), 1218. 10.3390/molecules22071218
Summary
Keywords
magnolol, honokiol, autoimmune diseases, rheumatoid arthritis, lead compound
Citation
Lin X and Chen J (2025) Magnolol and honokiol: potential lead compounds for the new drug discovery in treating autoimmune diseases. Front. Pharmacol. 16:1578971. doi: 10.3389/fphar.2025.1578971
Received
19 February 2025
Accepted
08 April 2025
Published
23 April 2025
Volume
16 - 2025
Edited by
Cheorl-Ho Kim, Sungkyunkwan University, Republic of Korea
Reviewed by
Vittoria Colotta, University of Florence, Italy
Simge Unay, Uşak University, Türkiye
Updates
Copyright
© 2025 Lin and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Chen, chenjian@pkuszh.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.