- 1Internal Medicine and Stroke Care Ward, University Hospital “P. Giaccone”, Palermo, Italy
- 2Department of Health Promotion, Mother and Child Care, Internal Medicine and Medical Specialties (ProMISE), University of Palermo, Palermo, Italy
- 3Clinical Molecular Medicine and Clinical Laboratory Medicine, Department of Biomedicine, Neurosciences and Advanced Diagnostics, Institute of Clinical Biochemistry, University of Palermo, Palermo, Italy
- 4Immunopathology and Cancer Biomarkers Unit, Department of Cancer Research and Advanced Diagnostics, CRO Aviano National Cancer Institute IRCCS, Aviano, Italy
- 5Department of Laboratory Medicine, University Hospital “P. Giaccone”, Palermo, Italy
- 6PhD programme of Molecular and Clinical Medicine, University of Palermo, Palermo, Italy
Background: Renal Salt Wasting Syndrome (RSW) is a clinical syndrome with laboratory characteristics completely overlapping with the syndrome of inappropriate antidiuretic hormone secretion (SIADH). No studies have yet investigated the potential role of urodilatin as a diagnostic marker or its involvement in the pathogenesis of RSW.
Methods: We performed a retrospective observational case-control study, the patients were divided into 3 groups: a group of hyponatremic patients with RSW (cases) and two control groups (subjects without hyponatremia and subjects with hyponatremia from other causes). Main outcomes were assessing the differences in urinary urodilatin values in patients with RSW compared to both control groups and to evaluate the diagnostic power of urodilatin with the analysis of ROC curves.
Results: Patients with RSW display significantly higher mean urodilatin levels than both patients with (median 5.46 vs. 0.57 ng/mL, p = 0.006) or without hyponatremia (median 5.46 vs. 0.27 ng/mL, p < 0.001). Diagnostics performances of mean urodilatin levels for RSW diagnosis were evaluated by ROC curve, AUC was 0.94 (95%CI 0.86–1.00).
Conclusion: This case-control study has shown interesting results regarding the dosage of urinary urodilatin in patients with RSW, with potentially clarifying implications both regarding the pathogenesis of this syndrome and regarding the diagnosis and therefore the clinical management of patients.
Introduction
Renal Salt Wasting Syndrome (RSW) is a clinical syndrome with laboratory characteristics completely overlapping with the syndrome of inappropriate antidiuretic hormone secretion (SIADH) such as hyponatremia, normal adrenal, thyroid and renal function, concentrated urine with urinary sodium (Una) usually>30 mEq/L and hypouricemia (Maesaka et al., 2020; Assadi and Mazaheri, 2020; Bitew et al., 2009; Schwartz et al., 1957). The fundamental difference between the two syndromes lies in the extracellular volume (ECV) and therefore in the water balance. In fact, in the RSW we will have a reduction of the ECV due to a renal loss of water and sodium with negative water balance, while in the SIADH we will have a normal or slightly increased ECV with a balance substantially in equilibrium (Maesaka et al., 2020; Assadi and Mazaheri, 2020; Bitew et al., 2009; Schwartz et al., 1957). Consequently we will have the diametrically opposite therapeutic goals of water-restricting patients with SIADH or administering salt and water to patients with RSW (Maesaka et al., 2020; Assadi and Mazaheri, 2020; Bitew et al., 2009; Schwartz et al., 1957). Differentiating extracellular volume (ECV) status in clinical practice is challenging, complicating the differential diagnosis of the two syndromes. Furthermore, in SIADH a precise pathogenetic mechanism has been identified together with its protagonist (antidiuretic hormone, ADH) (Schwartz et al., 1957). This has not yet happened for RSW, contributing not only to making diagnosis difficult but also to feed a certain skepticism towards the very existence of the syndrome by some authors (Maesaka et al., 1999; Oh and Carroll, 1999; Singh et al., 2002; Peters et al., 1950).
Clarifying the pathogenesis of RSW is crucial for establishing it as a recognized cause of hyponatremia, dispelling skepticism, and facilitating diagnosis.
As regards the pathogenetic mechanisms so far called to explain RSW, we can recall an alteration of the renal sympathetic afferents and the involvement of natriuretic peptides (NPs) such as the Atrial natriuretic peptide (ANP) (Cerdà-Esteve et al., 2008; Fukuoka et al., 2017; Bitew et al., 2009; Maesaka et al., 2007; Maesaka et al., 2009; Oh and Shin, 2015; Youmans et al., 2013; Della Corte et al., 2018; DiBona, 2000).
Urodilatin
Urodilatin (URO) is a 32-amino acid peptide belonging to the family of natriuretic peptides first isolated from human urine in 1988 by Forssmann and colleagues. It comprises the amino acid sequence 95–126 of cardiac proANP and shares an identical structure with the circulating 28-amino acid human atrial natriuretic peptide (ANP), except for the addition of four amino acids (Thr-Ala-Pro-Arg) at the NH2 terminus (Potter et al., 2009; Lisy et al., 1999; Hirsch et al., 2006; Forte, 2004; Costa et al., 2009; Zhang et al., 2010; Askar and Tarif, 2007; Leonard et al., 2015; Berendes et al., 1997; Kurokawa et al., 1996; Tsubokawa et al., 2003; McGirt et al., 2004; Tomida et al., 1998; Gao et al., 2006; Goetz et al., 1990; Drummer et al., 1996; Della Corte et al., 2023).
Urodilatin (URO) is detectable in human urine but not in plasma, suggesting that it is synthesized and secreted exclusively by the kidneys. It is likely produced in the distal cortical nephron and secreted into the lumen of the kidney tubules, where it exerts paracrine effects. Like atrial natriuretic peptide (ANP), URO binds to natriuretic peptide type A receptors in the inner medullary collecting duct, triggering an increase in intracellular cyclic guanosine monophosphate (cGMP) levels and promoting renal sodium and water excretion (Potter et al., 2009; Lisy et al., 1999; Hirsch et al., 2006; Forte, 2004; Costa et al., 2009; Zhang et al., 2010; Askar and Tarif, 2007; Leonard et al., 2015; Berendes et al., 1997; Kurokawa et al., 1996; Tsubokawa et al., 2003; McGirt et al., 2004; Tomida et al., 1998; Gao et al., 2006; Goetz et al., 1990; Drummer et al., 1996; Della Corte et al., 2023).
After the isolation of the urodilatin natriuretic peptide from human urine, more and more data have been generated supporting the view that in fact not ANP but urodilatin appears to be the natriuretic peptide responsible for renal manipulation of sodium (Goetz et al., 1990; Drummer et al., 1996; Della Corte et al., 2023; Drummer et al., 1993; Herten et al., 1998; Zaid et al., 2007; Anker et al., 2015; Emmeluth et al., 1992; Meyer et al., 1996; Lenz et al., 1999; Forssmann et al., 1998; Maesaka et al., 2021). On the contrary, due to the rapid secretion of ANP as a response to some cardiovascular stimuli and due to numerous effects on the cardiovascular system, it seems reasonable to postulate that the primary target of ANP is the cardiovascular system and not the kidney (Hirsch et al., 2006; Goetz et al., 1990; Della Corte et al., 2023).
Unlike ANP, which is rapidly degraded and inactivated in the kidney by a metalloendoprotease derived from the renal cortex membrane, URO remains active, suggesting that its primary physiological target is the intrarenal ANP-receptor/guanylate cyclase system rather than systemic ANP. However, the mechanisms regulating URO production and excretion are not yet fully understood (Tomida et al., 1998; Gao et al., 2006; Goetz et al., 1990; Drummer et al., 1996; Della Corte et al., 2023).
Studies indicate that renal perfusion pressure, left atrial distension, and cephalic sodium concentration influence URO excretion. Heringlake and colleagues demonstrated that changes in arterial and renal perfusion pressure affect URO release, with experiments in isolated perfused rat kidneys confirming that renal blood flow and pressure are key determinants. Additionally, left atrial elongation has been shown to stimulate URO excretion (Tomida et al., 1998; Gao et al., 2006; Goetz et al., 1990; Drummer et al., 1996; Della Corte et al., 2023).
In conscious dogs, Goetz and colleagues observed that sodium excretion correlated more closely with urinary URO than with plasma ANP, and this effect was abolished in heart-denervated dogs, suggesting a neuronal connection between the heart and kidneys. Further studies using the split infusion technique showed that hypertonic saline infusion into the carotid artery increased URO and sodium excretion, indicating a possible link between cephalic sodium chemoreceptors and renal URO secretion. However, the effect persisted even in denervated kidneys, implying the involvement of an additional humoral factor that transmits brain sodium levels to the kidneys (Tomida et al., 1998; Gao et al., 2006; Goetz et al., 1990; Drummer et al., 1996; Della Corte et al., 2023).
Moreover, URO secretion has been associated with immersion in water in humans, possibly mediated by the renal sympathetic nervous system or dopaminergic nerves. Taken together, these findings suggest that multiple factors regulate URO release, including left atrial distension, sodium concentration, renal perfusion pressure, and neural or hormonal pathways. While the exact initial stimulus remains unclear, extracellular sodium levels appear to play a central role in this regulatory process (Tomida et al., 1998; Gao et al., 2006; Goetz et al., 1990; Drummer et al., 1996; Della Corte et al., 2023).
The kidney seems to have a central role in RSW and no studies have yet been done on the only natriuretic peptide produced in the kidney and with action on the same organ, the urodilatin. Based on the above, we conducted this case-control study to determine the differences in urinary urodilatin levels in patients with RSW (cases) compared to controls and the possible diagnostic power of urodilatin.
Materials and methods
Study population
We performed a retrospective observational case-control study at the University hospital “P. Giaccone” of Palermo. We enrolled patients admitted to the Internal Medicine and Stroke Care ward of this hospital.
The patients recruited were divided into 3 groups including a case group and two control groups based on blood sodium values (hyponatraemia was defined as blood sodium values < 135 mEq/L):
• a group of patients without hyponatremia (controls)
• a group of hyponatremic patients with RSW (cases)
• a group of patients with hyponatremia from other causes (controls)
We divided the population this way to verify that differences in urinary urodilatin levels depend on the cause of hyponatremia rather than on reduced plasma sodium levels. The Institutional Ethics Committee of “P. Giaccone” University Hospital approved the study protocol. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
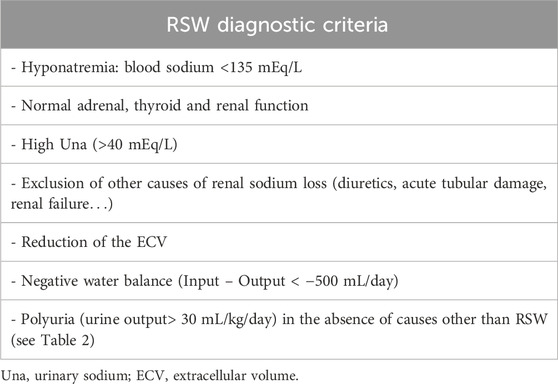
Diagnostic criteria of RSW and patient recruitment
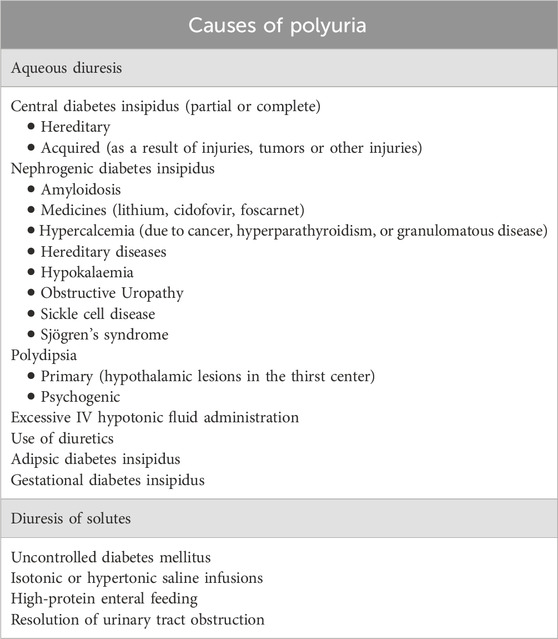
Diagnosis of RSW was made when all the criteria in Table 1 were met. In particular, only patients with polyuria (defined as urine output> 30 mL/kg/day) (Borgmann et al., 2014) not explainable with causes other than RSW (see Table 2) in association with a clearly negative water balance (output - input> 500 mL/day) and a reduction of ECV were included in the RSW group. Selecting this patient population ensured a more accurate diagnosis of RSW. Indeed, since the main diagnostic problem is that of the differential diagnosis with SIADH, these criteria have allowed us to exclude patients with SIADH with reasonable accuracy, who cannot by definition have (given the intrinsic function of ADH which is precisely an antidiuretic hormone) polyuria in the absence of the common causes listed in Table 2. (Schwartz et al., 1957). The assessment of the water balance and the ECV were performed according to the common methods of clinical practice.
Biochemical analysis
Routine clinical chemistry parameters were measured for each patient at the hospital admission immediately after sample collection. Urodilatin was evaluated on urine samples collected at 6 a.m. and 2 p.m. in the first 24 h of hospital admission and stored at −80°C until analysis. pro-ANP was assessed on plasma obtained by centrifugation of whole blood sample collected in K3-EDTA tubes upon admission and stored at −80°C until analysis. Serum and urinary biochemical parameters, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, serum creatinine (sCR), uric acid, glucose, TSH, albumin, cortisol, sodium and potassium, were measured on Cobas® 8000's (Roche, Basel, Switzerland), according to the manufacturer’s procedures. The glomerular filtration rate (GFR) was estimated by was calculated using the Chronic Kidney Disease EPIdemiology collaboration (CKD-EPI) equation expressed for the specified race, gender, and sCR in mg/dL (Levey and Stevens, 2010). Urodilatin and pro-ANP levels were measured by commercially available enzyme linked immunosorbent assay (ELISA) kits, according to the manufacturer’s instructions (BMA Biomedicals, Switzerland and Biomedica Medizinprodukte GmbH, respectively). Hematological tests, including hematocrit, were performed on Sysmex XN-9000 hematology analyzer (Sysmex Corporation, Kobe, Japan). All routine biochemical analyses were performed at the Department of Laboratory Medicine-University Hospital “P. Giaccone” of Palermo, and the measurement of urodilatin and pro-ANP was performed at the Institute of Clinical Biochemistry, Clinical Molecular Medicine and Clinical Laboratory Medicine- University Hospital “P. Giaccone” of Palermo.
Statistical analysis
Statistical analyses were performed by SPSS statistical software v.17.0 (SPSS Inc., Chicago, IL, United States) and R Language v.4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). Normality distribution was assessed preliminarily by q-q plot and Shapiro–Wilk test. Quantitative variables were expressed by the median and interquartile range (IQR), while qualitative variables by absolute or relative frequency. Differences between groups for continuous variables were estimated by Kruskal–Wallis (>2groups) or Mann-Whitney test (2 groups) with Bonferroni’s correction when needed. Differences between paired groups were studied by nonparametric Wilcoxon Signed Ranks Test. The Correlation was evaluated by the nonparametric Spearman test. Diagnostic accuracy for RSW diagnosis was evaluated by Receiver Operating Characteristic (ROC) curve analysis and reported as Area Under the Curve (AUC) and 95% confidence interval calculated by the DeLong method. The Best cut-off was evaluated by the Youden’s index.
Results
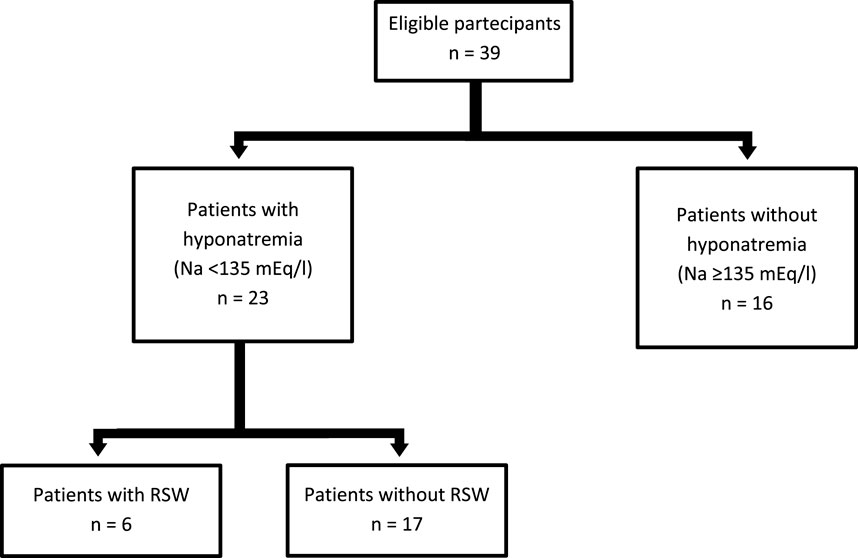
Thirty-nine subjects were studied. They were sub-grouped, according to previous clinical diagnosis or sodium levels, into patients with Renal Salt Wasting (RSW) syndrome (n = 6 patients with diagnosis made when all the criteria in Table 1 were met), patients with hyponatremia (n = 17 patients with blood sodium values < 135 mEq/L) and patients without hyponatremia (n = 16 patients with blood sodium values ≥ 135 mEq/L). Demographic, clinical and biochemical characteristics of the study population are shown in Table 3. In our study, 23 patients with hyponatremia were recruited. Of these, 6 patients met the diagnostic criteria of RSW, 2 had SIADH, 5 had hyponatraemia related to renal insufficiency (GFR <45 mL/min), 1 case of hypothyroidism, 7 cases of drug-related hyponatremia (use of diuretics predominantly). About the 6 patients with RSW, 3 had intracranial disease (2 with ischemic stroke and 1 with lung cancer brain metastases). The other 3, on the other hand, did not have intracranial pathologies and were hospitalized for the following reasons: 1 case of pneumonia, 1 case of respiratory failure in a patient with lung cancer and 1 case of alterations in consciousness due to hyponatremia. As urodilatin levels measured at 6 a.m. and 2 p.m. were not significantly different (p = 0.05), their mean value was used for subsequent analyses. Interestingly, mean levels of urodilatin were significantly different among the 3 subgroups (overall KW test p = 0.003) (Figure 1). Moreover, taking into account the Bonferroni’s correction, patients with RSW display significantly higher mean urodilatin levels than both patients with (median 5.46 vs. 0.57 ng/mL, p = 0.006) or without hyponatremia (median 5.46 vs. 0.27 ng/mL, p < 0.001) (Figure 1). Statistically significant higher mean levels of urodilatin were also observed when patients with RSW were compared with the other two groups of patients considered together (5.46 vs. 0.32 ng/mL, MW test p < 0.001). Conversely, proANP levels were not statistically different among the 3 subgroups (overall KS test p = 0.266) or between patients with RSW and patients with/without hyponatremia (4.9 vs. 9.7 nM, MW test p = 0.122). In the whole sample investigated, no association was evident between proANP and mean urodilatin levels (Speaman’s rho = 0.059, p = 0.739). However, few correlations were found to be statistically significant when the analysis was performed within subgroups. In particular, in RSW patients proANP was highly inversely correlated with 24 h urine output (rho = −0.975, p = 0.005). Diagnostics performances of mean urodilatin levels for RSW diagnosis were evaluated by ROC curve (Figure 2). Area under the curve (AUC) was 0.94 (95%CI 0.86–1.00). Best cut-off for mean urodilatin levels, according to Youden’s index, was 2.87 ng/mL. At this cut-off sensitivity, specificity, positive predictive value and negative predictive value were, respectively, 1.00, 0.88, 0.60 and 1.00.
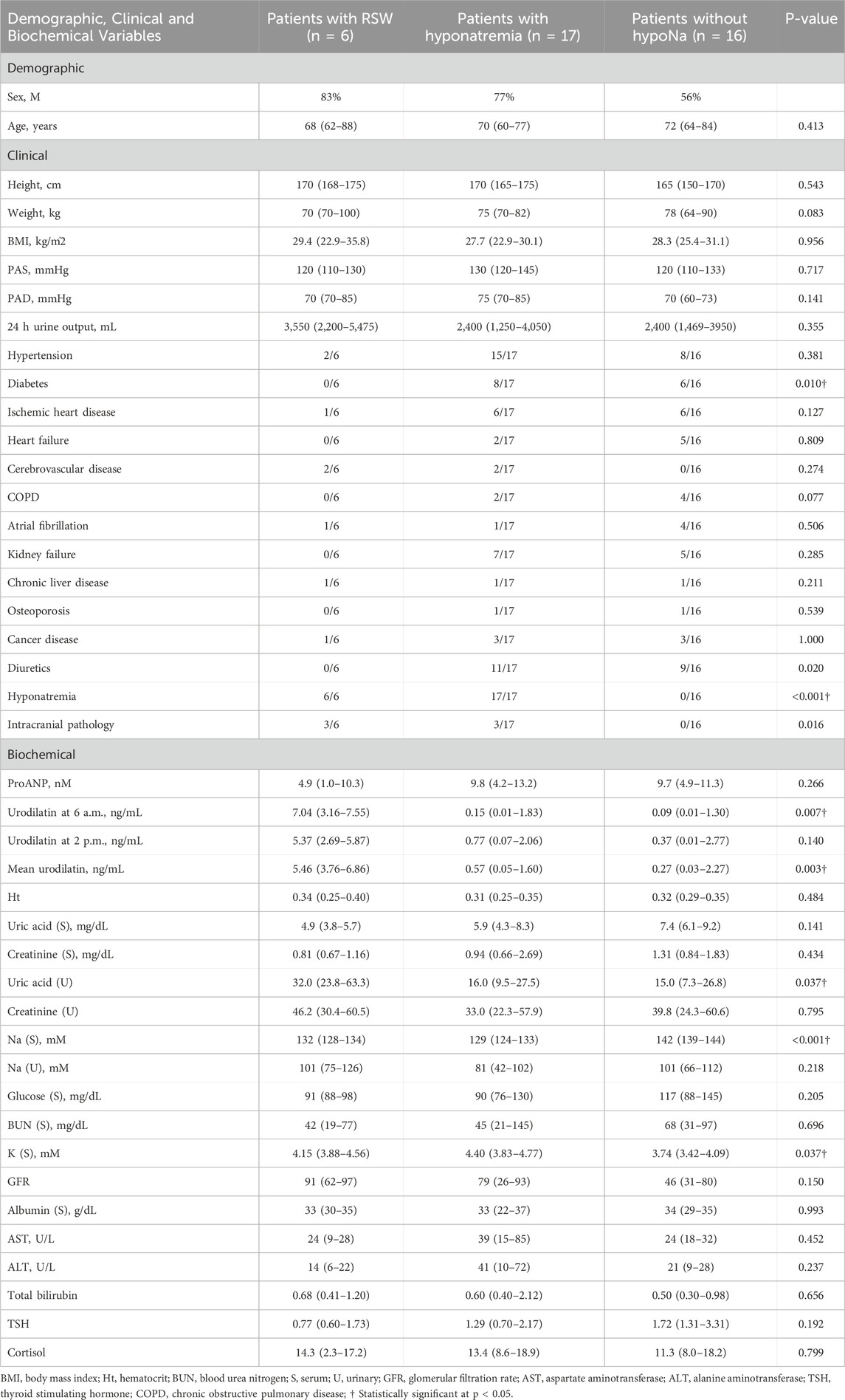
Table 3. Demographic and clinical characteristics of patients in the study, stratified by RSW status and presence of hyponatremia. Data are presented as median (IQR) or frequency (%). Comparisons between groups were performed using the Kruskal–Wallis test (continuous variables) or Chi-square test (categorical variables).
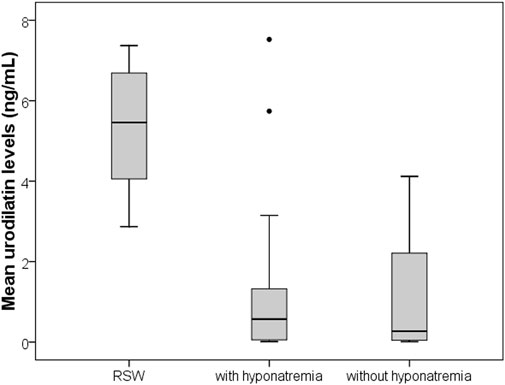
Figure 1. Boxplots comparing mean urinary urodilatin levels among patients with RSW, hyponatremia from other causes, and controls without hyponatremia. RSW patients show significantly higher urodilatin levels (p < 0.001).
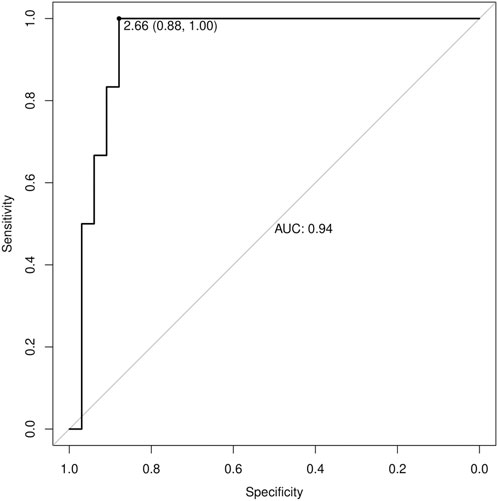
Figure 2. Receiver Operating Characteristic (ROC) curve for urinary urodilatin levels in diagnosing RSW. The AUC is 0.94, indicating excellent diagnostic performance. The optimal cutoff value of 2.87 ng/mL provides 100% sensitivity and 88% specificity.
Discussion
This is the first study in the literature, to the best of our knowledge, in which urinary urodilatin levels were measured in patients with RSW. On a preliminary basis, we can note how this study confirms the data that has already emerged from the literature which maintains that RSW is a cause of hyponatremia even more common than SIADH (we recruited 23 patients with hyponatremia, of which 6 patients met the diagnostic criteria of RSW and 2 had a SIADH). (Maesaka et al., 2018; Musch and Decaux, 2019). We also observed how patients with RSW display significantly higher mean urodilatin levels than both patients with (median 5.46 vs. 0.57 ng/mL, p = 0.006) or without hyponatremia (median 5.46 vs. 0.27 ng/mL, p < 0.001) (Figure 1). Statistically significant higher mean levels of urodilatin were also observed when patients with RSW were compared with the other two groups of patients considered together (5.46 vs. 0.32 ng/mL, MW test p < 0.001). This suggests a possible role of urodilatin in the pathogenesis of RSW. Indeed the statistically significant increase in the levels of this molecule in patients with RSW was found not only against the subjects without hyponatremia, but also against the subjects with hyponatremia from other causes. This data leads us to believe that the increase in urinary urodilatin levels is quite peculiar to subjects with RSW, and that this could be the cause of hyponatremia in these subjects, while several other mechanisms are involved in the genesis of hyponatremia from causes other than RSW. The potential role of urodilatin in RSW is supported by the lack of significant differences in proANP levels across the three groups (overall KS test p = 0.266) or between RSW patients and controls with or without hyponatremia (4.9 vs. 9.7 nM, MW test p = 0.122). Therefore, as other studies in the literature have shown, circulating natriuretic peptides such as ANP do not seem to play a role in this syndrome (Cerdà-Esteve et al., 2008). Another data supporting this issue highlighted in this study is that, in the group of patients with RSW, the levels of proANP are inversely related to the urinary output of 24 h (rho = −0.975, p = 0.005). This suggests that the main cause of massive diuresis in these patients is urodilatin, while the circulating ANP levels are instead reduced due to a probable negative feedback mechanism put in place to try to contain the renal loss of water and sodium in these patients. This consideration is also supported by the work of Youmans et al. who found in vitro natriuretic activity in urine of neurosurgical patients with RSW (Youmans et al., 2013). The main diagnostic challenge of RSW lies in the distinction between RSW and SIADH. In this regard, the gold standard for the determination of the extracellular volume is represented by the dilution methods with radioisotopes (e.g., techniques using red blood cells labeled with radiochromate or albumin labeled with radioiodine) as they are reliable and highly reproducible (Recommended methods for measurement of, 1980; Nelson et al., 1981; Wijdicks et al., 1985; Sivakumar et al., 1994). However, these methods are of little use in clinical practice as they do not give immediate results, are rather complex to perform and have a high cost. An alternative method for the distinction between RSW and SIADH has recently been proposed, represented by the dosage of the excreted fraction of urate (Fractional Excretion urate, FEurate) (Assadi and Mazaheri, 2020; Musch and Decaux, 2019; Maesaka et al., 2014). Maesaka et al. observed that both in SIADH and RSW we will find a high FEurate (>11%). With the correction of blood sodium, the FEurate will tend to normalize in the SIADH (4%–11%) while it will remain >11% in the RSW.51 However, this method may not be easy to apply in clinical practice. In fact, the need to calculate the FEurate after correction of natremia values can collide with the difficulties of clinical management of acutely ill patients. It can be difficult to bring natremia back to the normal range in good time, and it may be necessary for various reasons to introduce drugs into therapy that could interfere with urate excretion and therefore with the calculation of FEurate. Furthermore, this method has not been validated by other authors. For these reasons it was not used in the present study. The above mentioned important difference in urinary urodilatin levels could be very useful in the diagnostic setting and can help overcome the diagnostic approaches used up to now. The analysis of the ROC curves (Figure 2) showed an area under the curve (AUC) of 0.94 (95% CI 0.86–1.00), with the best cut-off as a urodilatin value of 2.87 ng/mL. This value corresponds to a sensitivity of 1.00, specificity of 0.88, positive predictive value of 0.60 and negative of 1.00. These data suggest a strong diagnostic power of urodilatin and, if confirmed by further studies, it could be a breakthrough in clinical management and research concerning RSW. It would be the first finding of a diagnostic marker that would greatly facilitate not only the differential diagnosis with SIADH, but the recognition of RSW in general. In fact, as we have seen, the statistically significant increase of urinary urodilatin levels in patients with RSW was found not only against patients without hyponatremia, but also against those with hyponatremia from other causes. This confers a particular sensitivity of the urodilatin towards RSW diagnosis. A diagnostic marker for RSW would allow to overcome the problems of the diagnostic approaches adopted up to now, namely,: the need to have an accurate and reliable evaluation of the ECV; the need to calculate the FEurate twice, the second time after correction of the blood sodium.
Limitations
The possible limitations of this study must be considered, first of all the small number of recruited patients with RSW (Figure 3). This was due both to the fact that RSW is a relatively infrequent syndrome, and to the use of very restrictive criteria for the diagnosis of RSW. On the other hand, the use of these diagnostic criteria has allowed us to make an accurate differential diagnosis between SIADH and RSW. It is important to emphasize that all other studies in the literature on natriuretic peptide measurement in RSW have included populations of fewer than 10 individuals. Therefore, the size of our population is consistent with previous studies and may be sufficient to provide preliminary results on urinary urodilatin measurement in this context, encouraging further research. It must be pointed out that the kit used for the urodilatin dosage does not distinguish urodilatin from ANP, and this could cast doubts on the specificity of the urinary urodilatin dosage which could be altered by the presence of ANP in the urine. However, it should be considered that ANP is rapidly degraded in the renal tubule as opposed to urodilatin which is instead resistant to this degradation, and that the use of specific kits in the past has shown that almost all of the natriuretic peptides that can be measured in urine is represented precisely by urodilatin (Zaid et al., 2007; Drummer et al., 1991; Feller et al., 1989). Furthermore, the statistical analysis did not show any correlation between the circulating levels of proANP and urinary urodilatin levels in the entire population studied (Speaman’s rho = 0.059, p = 0.739). It must also be considered that, having included in the group of cases with RSW only patients with polyuria that cannot be explained by the causes listed in Table 2, it is possible that we have selected only a subpopulation of patients with RSW in the “hyperacute” phase. In fact, we know that RSW can be an insidious syndrome, with an oscillating trend and with effects on natremia and diuresis that are not always so pronounced (Maesaka et al., 2020; Della Corte et al., 2018; Musch and Decaux, 2019). Therefore it must be clarified whether urodilatin secretion remains significantly elevated even in patients with a “moderate” RSW, or whether it is instead a characteristic of patients with RSW in the “acute” phase. Further studies will certainly help us resolve this question as well.
Conclusion
This case-control study provides novel insights into urinary urodilatin levels in RSW patients, with potential implications for understanding the syndrome’s pathogenesis, refining diagnostic criteria, and improving clinical management. Further studies will be needed to confirm our findings by expanding the study population, including cases of chronic RSW, and utilizing an external validation cohort. We hope that further scientific research will provide greater insight into this fascinating topic.
Clinical significance
Renal Salt Wasting Syndrome (RSW) is a clinical syndrome with laboratory characteristics completely overlapping with the syndrome of inappropriate antidiuretic hormone secretion (SIADH). The differential diagnosis is still difficult, and the pathogenesis of this syndrome has not been clarified. In this case-control study, urinary urodilatin levels in patients with RSW were found up to 10 times higher than in controls, the analysis of ROC curves showed good diagnostic power of urinary urodilatin toward RSW diagnosis. Urinary urodilatin assay could be potentially used as a new diagnostic marker of Renal Salt Wasting Syndrome. Furthermore, these data may provide important clues to the underlying pathogenesis of RSW.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee Azienda Ospedaliera Universitaria Policlinico Paolo Giaccone di Palermo, Italy. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
VD: Writing – original draft, Writing – review and editing, Conceptualization, Methodology. LA: Data curation, Formal Analysis, Methodology, Resources, Validation, Visualization, Writing – original draft. RN: Data curation, Investigation, Methodology, Visualization, Writing – review and editing. Mca: Data curation, Investigation, Methodology, Visualization, Writing – review and editing. FD: Data curation, Methodology, Formal Analysis, Software, Writing – review and editing. RP: Data curation, Investigation, Visualization, Writing – review and editing. CM: Data curation, Investigation, Visualization, Writing – review and editing. CG: Data curation, Methodology, Validation, Visualization, Writing – review and editing. MD: Data curation, Funding acquisition, Visualization, Writing – review and editing. RG: Data curation, Formal Analysis, Investigation, Validation, Writing – review and editing. MCi: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – original draft. AT: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anker, S. D., Ponikowski, P., Mitrovic, V., Peacock, W. F., and Filippatos, G. (2015). Ularitide for the treatment of acute decompensated heart failure: from preclinical to clinical studies. Eur. Heart J. 36 (12), 715–723. Epub 2015 Feb 10. PMID: 25670819; PMCID: PMC4368857. doi:10.1093/eurheartj/ehu484
Askar, A., and Tarif, N. (2007). Cerebral salt wasting in a patient with head trauma: management with saline hydration and fludrocortisone. Saudi J. Kidney Dis. Transpl. 18 (1), 95–99. PMID: 17237900.
Assadi, F., and Mazaheri, M. (2020). Differentiating syndrome of inappropriate ADH, reset osmostat, cerebral/renal salt wasting using fractional urate excretion. J. Pediatr. Endocrinol. Metab. 34 (1), 137–140. PMID: 33180045. doi:10.1515/jpem-2020-0379
Berendes, E., Walter, M., Cullen, P., Prien, T., Van Aken, H., Horsthemke, J., et al. (1997). Secretion of brain natriuretic peptide in patients with aneurysmal subarachnoid haemorrhage. Lancet 349 (9047), 245–249. PMID: 9014912. doi:10.1016/s0140-6736(96)08093-2
Bitew, S., Imbriano, L., Miyawaki, N., Fishbane, S., and Maesaka, J. K. (2009). More on renal salt wasting without cerebral disease: response to saline infusion. Clin. J. Am. Soc. Nephrol. 4 (2), 309–315. PMID: 19201917; PMCID: PMC2637602. doi:10.2215/CJN.02740608
Borgmann, H. (2014). “Polyuria,” in Urology at a glance. Editors A. Merseburger, M. Kuczyk, and J. Moul (Berlin, Heidelberg: Springer). doi:10.1007/978-3-642-54859-8_5
Cerdà-Esteve, M., Cuadrado-Godia, E., Chillaron, J. J., Pont-Sunyer, C., Cucurella, G., Fernández, M., et al. (2008). Cerebral salt wasting syndrome: review. Eur. J. Intern Med. 19 (4), 249–254. Epub 2008 Mar 7. PMID: 18471672. doi:10.1016/j.ejim.2007.06.019
Costa, K. N., Nakamura, H. M., Cruz, L. R., Miranda, L. S. V. F. d., Santos-Neto, R. C. d., Cosme, S. d. L., et al. (2009). Hyponatremia and brain injury: absence of alterations of serum brain natriuretic peptide and vasopressin. Arq. Neuropsiquiatr. 67 (4), 1037–1044. PMID: 20069215. doi:10.1590/s0004-282x2009000600014
Della Corte, V., Pacinella, G., Todaro, F., Pecoraro, R., and Tuttolomondo, A. (2023). The natriuretic peptide system: a single entity, pleiotropic effects. Int. J. Mol. Sci. 24 (11), 9642. PMID: 37298592; PMCID: PMC10253422. doi:10.3390/ijms24119642
Della Corte, V., Tuttolomondo, A., Pecoraro, R., and Pinto, A. (2018). Chronic hyponatremia in a patient with renal salt wasting and without cerebral disease: relationship between RSW, risk of fractures and cognitive impairment. Intern Emerg. Med. 13 (8), 1167–1171. Epub 2018 Aug 13. PMID: 30105494. doi:10.1007/s11739-018-1926-7
DiBona, G. F. (2000). Neural control of the kidney: functionally specific renal sympathetic nerve fibers. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279 (5), R1517–R1524. PMID: 11049831. doi:10.1152/ajpregu.2000.279.5.R1517
Drummer, C., Fiedler, F., Bub, A., Kleefeld, D., Dimitriades, E., Gerzer, R., et al. (1993). Development and application of a urodilatin (CDD/ANP-95-126)-specific radioimmunoassay. Pflugers Arch. 423 (5-6), 372–377. PMID: 8351194. doi:10.1007/BF00374930
Drummer, C., Fiedler, F., König, A., and Gerzer, R. (1991). Urodilatin, a kidney-derived natriuretic factor, is excreted with a circadian rhythm and is stimulated by saline infusion in man. J. Am. Soc. Nephrol. 1 (9), 1109–1113. doi:10.1681/ASN.V191109
Drummer, C., Franck, W., Heer, M., Forssmann, W. G., Gerzer, R., and Goetz, K. (1996). Postprandial natriuresis in humans: further evidence that urodilatin, not ANP, modulates sodium excretion. Am. J. Physiol. 270 (2 Pt 2), F301–F310. PMID: 8779891. doi:10.1152/ajprenal.1996.270.2.F301
Emmeluth, C., Drummer, C., Gerzer, R., and Bie, P. (1992). Roles of cephalic Na+ concentration and urodilatin in control of renal Na+ excretion. Am. J. Physiol. 262 (3 Pt 2), F513–F516. PMID: 1313647. doi:10.1152/ajprenal.1992.262.3.F513
Feller, S. M., Gagelmann, M., and Forssmann, W. G. (1989). Urodilatin: a newly described member of the ANP family. Trends Pharmacol. Sci. 10 (3), 93–94. PMID: 2531950. doi:10.1016/0165-6147(89)90199-5
Forssmann, W. G., Richter, R., and Meyer, M. (1998). The endocrine heart and natriuretic peptides: histochemistry, cell biology, and functional aspects of the renal urodilatin system. Histochem Cell Biol. 110 (4), 335–357. PMID: 9792413. doi:10.1007/s004180050295
Forte, L. R. (2004). Uroguanylin and guanylin peptides: pharmacology and experimental therapeutics. Pharmacol. Ther. 104 (2), 137–162. PMID: 15518884. doi:10.1016/j.pharmthera.2004.08.007
Fukuoka, T., Tsurumi, Y., and Tsurumi, A. (2017). Cerebral salt-wasting syndrome caused by minor head injury. Case Rep. Emerg. Med. 2017, 8692017. Epub 2017 Jan 17. PMID: 28194285; PMCID: PMC5282430. doi:10.1155/2017/8692017
Gao, Y. L., Xin, H. N., Feng, Y., and Fan, J. W. (2006). Human plasma DNP level after severe brain injury. Chin. J. Traumatol. 9 (4), 223–227. PMID: 16848994.
Goetz, K., Drummer, C., Zhu, J. L., Leadley, R., Fiedler, F., and Gerzer, R. (1990). Evidence that urodilatin, rather than ANP, regulates renal sodium excretion. J. Am. Soc. Nephrol. 1 (6), 867–874. PMID: 1966524. doi:10.1681/ASN.V16867
Herten, M., Lenz, W., Gerzer, R., and Drummer, C. (1998). The renal natriuretic peptide urodilatin is present in human kidney. Nephrol. Dial. Transpl. 13 (10), 2529–2535. PMID: 9794555. doi:10.1093/ndt/13.10.2529
Hirsch, J. R., Meyer, M., and Forssmann, W. G. (2006). ANP and urodilatin: who is who in the kidney. Eur. J. Med. Res. 11 (10), 447–454. PMID: 17107879.
Kurokawa, Y., Uede, T., Ishiguro, M., Honda, O., Honmou, O., Kato, T., et al. (1996). Pathogenesis of hyponatremia following subarachnoid hemorrhage due to ruptured cerebral aneurysm. Surg. Neurol. 46 (5), 500–508. PMID: 8874554. doi:10.1016/s0090-3019(96)00034-1
Lenz, W., Herten, M., Gerzer, R., and Drummer, C. (1999). Regulation of natriuretic peptide (urodilatin) release in a human kidney cell line. Kidney Int. 55 (1), 91–99. PMID: 9893117. doi:10.1046/j.1523-1755.1999.00242.x
Leonard, J., Garrett, R. E., Salottolo, K., Slone, D. S., Mains, C. W., Carrick, M. M., et al. (2015). Cerebral salt wasting after traumatic brain injury: a review of the literature. Scand. J. Trauma Resusc. Emerg. Med. 23, 98. PMID: 26561391; PMCID: PMC4642664. doi:10.1186/s13049-015-0180-5
Levey, A. S., and Stevens, L. A. (2010). Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am. J. Kidney Dis. 55 (4), 622–627. PMID: 20338463; PMCID: PMC2846308. doi:10.1053/j.ajkd.2010.02.337
Lisy, O., Jougasaki, M., Heublein, D. M., Schirger, J. A., Chen, H. H., Wennberg, P. W., et al. (1999). Renal actions of synthetic dendroaspis natriuretic peptide. Kidney Int. 56 (2), 502–508. PMID: 10432389. doi:10.1046/j.1523-1755.1999.00573.x
Maesaka, J. K., Gupta, S., and Fishbane, S. (1999). Cerebral salt-wasting syndrome: does it exist? Nephron 82 (2), 100–109. PMID: 10364700. doi:10.1159/000045384
Maesaka, J. K., Imbriano, L., Mattana, J., Gallagher, D., Bade, N., and Sharif, S. (2014). Differentiating SIADH from cerebral/renal salt wasting: failure of the volume approach and need for a new approach to hyponatremia. J. Clin. Med. 3 (4), 1373–1385. PMID: 26237607; PMCID: PMC4470189. doi:10.3390/jcm3041373
Maesaka, J. K., Imbriano, L. J., Ali, N. M., and Ilamathi, E. (2009). Is it cerebral or renal salt wasting? Kidney Int. 76 (9), 934–938. Epub 2009 Jul 29. PMID: 19641485. doi:10.1038/ki.2009.263
Maesaka, J. K., Imbriano, L. J., and Miyawaki, N. (2018). High prevalence of renal salt wasting without cerebral disease as cause of hyponatremia in general medical wards. Am. J. Med. Sci. 356 (1), 15–22. Epub 2018 Apr 7. PMID: 30049325. doi:10.1016/j.amjms.2018.03.020
Maesaka, J. K., Imbriano, L. J., and Miyawaki, N. (2020). Evolution and evolving resolution of controversy over existence and prevalence of cerebral/renal salt wasting. Curr. Opin. Nephrol. Hypertens. 29 (2), 213–220. PMID: 31904619. doi:10.1097/MNH.0000000000000592
Maesaka, J. K., Imbriano, L. J., Pinkhasov, A., Muralidharan, R., Song, X., Russo, L. M., et al. (2021). Identification of a novel natriuretic protein in patients with cerebral-renal salt wasting-implications for enhanced diagnosis. Am. J. Med. Sci. 361 (2), 261–268. Epub 2020 Jul 13. PMID: 33526214. doi:10.1016/j.amjms.2020.07.015
Maesaka, J. K., Miyawaki, N., Palaia, T., Fishbane, S., and Durham, J. H. (2007). Renal salt wasting without cerebral disease: diagnostic value of urate determinations in hyponatremia. Kidney Int. 71 (8), 822–826. Epub 2007 Feb 21. PMID: 17311074. doi:10.1038/sj.ki.5002093
McGirt, M. J., Blessing, R., Nimjee, S. M., Friedman, A. H., Alexander, M. J., Laskowitz, D. T., et al. (2004). Correlation of serum brain natriuretic peptide with hyponatremia and delayed ischemic neurological deficits after subarachnoid hemorrhage. Neurosurgery 54 (6), 1369–1374. PMID: 15157293. doi:10.1227/01.neu.0000125016.37332.50
Meyer, M., Richter, R., Brunkhorst, R., Wrenger, E., Schulz-Knappe, P., Kist, A., et al. (1996). Urodilatin is involved in sodium homeostasis and exerts sodium-state-dependent natriuretic and diuretic effects. Am. J. Physiol. 271 (3 Pt 2), F489–F497. PMID: 8853410. doi:10.1152/ajprenal.1996.271.3.F489
Musch, W., and Decaux, G. (2019). Hyponatremia secondary to transient renal salt wasting (TRSW): a not so uncommon observation in the elderly. Clin. Nephrol. 91 (6), 344–352. PMID: 30935460. doi:10.5414/CN109472
Nelson, P. B., Seif, S. M., Maroon, J. C., and Robinson, A. G. (1981). Hyponatremia in intracranial disease: perhaps not the syndrome of inappropriate secretion of antidiuretic hormone (SIADH). J. Neurosurg. 55 (6), 938–941. PMID: 7299468. doi:10.3171/jns.1981.55.6.0938
Oh, J. Y., and Shin, J. I. (2015). Syndrome of inappropriate antidiuretic hormone secretion and cerebral/renal salt wasting syndrome: similarities and differences. Front. Pediatr. 2, 146. PMID: 25657991; PMCID: PMC4302789. doi:10.3389/fped.2014.00146
Oh, M. S., and Carroll, H. J. (1999). Cerebral salt-wasting syndrome. We need better proof of its existence. Nephron 82 (2), 110–114. PMID: 10364701. doi:10.1159/000045385
Peters, J. P., Welt, L. G., Sims, E. A., Orloff, J., and Needham, J. (1950). A salt-wasting syndrome associated with cerebral disease. Trans. Assoc. Am. Physicians 63, 57–64. PMID: 14855556.
Potter, L. R., Yoder, A. R., Flora, D. R., Antos, L. K., and Dickey, D. M. (2009). Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb. Exp. Pharmacol. (191), 341–366. PMID: 19089336; PMCID: PMC4855512. doi:10.1007/978-3-540-68964-5_15
Recommended methods for measurement of red-cell and plasma volume: international Committee for Standardization in Haematology (1980). J. Nucl. Med.;21(8):793–800. PMID: 7400838.
Schwartz, W. B., Bennett, W., Curelop, S., and Bartter, F. C. (1957). A syndrome of renal sodium loss and hyponatremia probably resulting from inappropriate secretion of antidiuretic hormone. Am. J. Med. 23 (4), 529–542. PMID: 13469824. doi:10.1016/0002-9343(57)90224-3
Singh, S., Bohn, D., Carlotti, A. P., Cusimano, M., Rutka, J. T., and Halperin, M. L. (2002). Cerebral salt wasting: truths, fallacies, theories, and challenges. Crit. Care Med. 30 (11), 2575–2579. PMID: 12441772. doi:10.1097/00003246-200211000-00028
Sivakumar, V., Rajshekhar, V., and Chandy, M. J. (1994). Management of neurosurgical patients with hyponatremia and natriuresis. Neurosurgery 34 (2), 269–274. PMID: 8177388. doi:10.1227/00006123-199402000-00010
Tomida, M., Muraki, M., Uemura, K., and Yamasaki, K. (1998). Plasma concentrations of brain natriuretic peptide in patients with subarachnoid hemorrhage. Stroke 29 (8), 1584–1587. PMID: 9707197. doi:10.1161/01.str.29.8.1584
Tsubokawa, T., Kurita, H., Kaneko, N., Iino, N., and Shiokawa, Y. (2003). Clinical significance of natriuretic peptides in patients with aneurysmal subarachnoid hemorrhage. No To Shinkei 55 (11), 953–960. Japanese. PMID: 14727535.
Wijdicks, E. F., Vermeulen, M., ten Haaf, J. A., Hijdra, A., Bakker, W. H., and van Gijn, J. (1985). Volume depletion and natriuresis in patients with a ruptured intracranial aneurysm. Ann. Neurol. 18 (2), 211–216. doi:10.1002/ana.410180208
Youmans, S. J., Fein, M. R., Wirkowski, E., and Maesaka, J. K. (2013). Demonstration of natriuretic activity in urine of neurosurgical patients with renal salt wasting. F1000Res 2, 126. PMID: 24358843; PMCID: PMC3752684. doi:10.12688/f1000research.2-126.v2
Zaid, A., Eliahu, G., Klein, H., and Keiser Harry, (2007). Urodilatin: a natriuretic peptide of renal origin. Cardiovasc. Drug Rev. 10, 199–210. doi:10.1111/j.1527-3466.1992.tb00246.x
Keywords: renal salt wasting syndrome, urodilatin, ANP, natriuretic peptide, hyponatremia
Citation: Della Corte V, Agnello L, Norrito R, Cataldi M, Del Ben F, Pecoraro R, Maida C, Gambino CM, Daidone M, Giglio RV, Ciaccio M and Tuttolomondo A (2025) Urinary urodilatin levels in patients with renal salt wasting syndrome as a possible new diagnostic marker. A pilot study. Front. Pharmacol. 16:1579699. doi: 10.3389/fphar.2025.1579699
Received: 19 February 2025; Accepted: 09 May 2025;
Published: 11 June 2025.
Edited by:
Daniele Bani, University of Florence, ItalyReviewed by:
Marcos A Lessa, The University of Iowa, United StatesAbeje Abebayehu Silte, Virginia Commonwealth University, United States
Copyright © 2025 Della Corte, Agnello, Norrito, Cataldi, Del Ben, Pecoraro, Maida, Gambino, Daidone, Giglio, Ciaccio and Tuttolomondo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vittoriano Della Corte, dml0dG9yaWFuby5kY0BnbWFpbC5jb20=
†These authors share senior authorship
 Vittoriano Della Corte
Vittoriano Della Corte Luisa Agnello
Luisa Agnello Rosario Norrito
Rosario Norrito Marco Cataldi1,2
Marco Cataldi1,2 Rosaria Vincenza Giglio
Rosaria Vincenza Giglio Marcello Ciaccio
Marcello Ciaccio Antonino Tuttolomondo
Antonino Tuttolomondo