- 1Ophthalmology Department, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, China
- 2Guizhou Eye Hospital, Zunyi, Guizhou, China
- 3Guizhou Branch Center of National Clinical Research Center for Ophthalmology, Zunyi, Guizhou, China
- 4Key Laboratory of Eye Diseases, High Education Institution in Guizhou Province, Zunyi, Guizhou, China
Retinal neovascularization (RNV) is a critical pathological feature of vision-threatening ocular diseases, such as diabetic retinopathy, retinopathy of prematurity, and wet age-related macular degeneration, presenting a persistent therapeutic conundrum. Current clinical treatments primarily rely on anti-vascular endothelial growth factor (VEGF) drugs and laser therapies, which face limitations including drug resistance, high costs, and potential damage to normal tissues. This underscores the need to develop novel therapeutic targets and cost-effective pharmacological interventions with improved safety profiles. Recent investigations highlight Dendrobium polysaccharides (DP), the primary bioactive components of the traditional medicinal herb Dendrobium, as promising multi-target therapeutic candidates. Studies have shown that Dendrobium polysaccharides significantly inhibits pathological angiogenesis by regulating the VEGF signaling pathway, inhibiting inflammatory response and oxidative stress, protecting the extracellular matrix, and reversing intestinal microecological disorders. This review systematically summarizes the structural and functional properties of DP, explores their mechanism of action and experimental evidence in retinal neovascularization, and analyzes their potential as a new therapeutic strategy for retinal diseases. This review also highlights the main limitations of current research: the uncertain relationship between the structure and activity of DP, the differences between pre-clinical models and human diseases, and the potential for structural optimization and the development of delivery systems.
1 Introduction
Retinal neovascularization (RNV) is an important factor in overall visual impairment, particularly among working-age adults and premature infants. This condition not only means advanced retinopathy, but is often associated with severe complications, including vascular leakage, hemorrhage, fibrotic marks and tractional retinal detachment, all of which profoundly compromise visual function (Arrigo and Bandello, 2021). RNV formation is an important pathological mechanism in chronic eye diseases such as age-related macular degeneration (AMD), retinopathy of prematurity (ROP), proliferative diabetic retinopathy (PDR), oxygen-induced retinopathy (OIR) and retinal vein occlusion (Grossniklaus et al., 2010). Dysregulation of the VEGF signaling pathway has been identified as a critical driver of neovascular pathogenesis (Penn et al., 2008). Current clinical interventions, including intravitreal anti-VEGF therapy and laser photocoagulation, effectively reduce vascular proliferation and promote neovascular regression. Despite these advances, significant challenges remain, such as the need for repeated intraocular injections, adverse systemic effects and suboptimal patient compliance. Moreover, resistance to anti-VEGF therapy observed in a subset of patients further exacerbates poor visual outcomes (Arrigo and Bandello, 2021). Therefore, identifying new therapies with higher efficacy and lower toxicity remains a priority in ophthalmic research.
Traditional Chinese medicine recognizes Dendrobium species as “eye strengthening and Yin strengthening” agents, their therapeutic applications being documented in the Materia Medica Compendium (Xie et al., 2023; Zhang et al., 2018). Several experimental studies have shown that natural compounds derived from Dendrobium have significant anti-angiogenic properties. Specifically, gigantol performs its therapeutic effects by inhibiting the AKT and ERK1/2 signaling pathways, effectively inhibiting cell migration and reproduction, as well as apoptosis and inflammatory responses of endothelial cells, ultimately inhibiting pathological angiogenesis in corneal and retinal tissues (Jiang et al., 2024; Liu B. et al., 2021). Benzyl compounds extracted from Dendrobium showed inhibitory effects on the ERK1/2-NF-κB signaling cascade in both in vitro and in vivo models. This substance ultimately protects the integrity of BRB in the DR by reducing inflammatory factors caused by microglial activation while upregulating the expression of tight junction proteins (claudine-1, occludin) and downregulating VEGF expression (Zhang et al., 2019). The aqueous extract of Dendrobium demonstrates attenuation of vascular endothelial inflammation through modulation of the TLR4/NF-κB/ICAM-1 axis (Chen T. E. et al., 2024). Eryanin, a bioactive component isolated from Dendrobium, showed significant anti-inflammatory and antiangiogenic effects in vitro and in vivo models by modulating the expression of components of the HIF-1α/VEGF signaling pathway (Yu et al., 2016). Dendrobium ethanolic extract also attenuated inflammation in the retina by inhibiting the NF-κB signaling pathway and restoring tight junction protein expression to physiological levels (Yu et al., 2015). Mechanistically, the extract exerts dual therapeutic effects by targeting VEGF/VEGFR2 signaling and inhibiting other pro-angiogenic factors (MMP-2/-9, PDGF-A/B, bFGF, IGF-1), thereby attenuating pathological angiogenesis in DR (Gong et al., 2014). Recent pharmacological advancements highlight the therapeutic potential of plant polysaccharides, particularly their multi-target efficacy and favorable safety profile, such as Lycium barbarum polysaccharides, tea polysaccharides, and astragalus polysaccharides (Chen L. et al., 2022). Among these, Dendrobium polysaccharides (DP), the main active ingredient of Dendrobium species, have been pharmacologically evaluated to exert antioxidant, anti-inflammatory and immunomodulatory bioactivities (Xie et al., 2023; Zhang et al., 2018). Emerging evidences further support the anti-angiogenic properties of DP. As a study found that different concentrations of DP improved oxidative stress and apoptosis in vascular endothelial cells induced by hyperglycemia, thereby preserving endothelial cell function (Chen et al., 2020). Further studies revealed that DP could inhibit the protein expression of angiogenic markers (CD31, CD34, and VWF) and downregulate pro-angiogenic proteins (VEGF, VEGFR2, and angiopoietin-1) (Yang, 2023). DP can inhibit pathological angiogenesis through multiple mechanisms, including modulating the VEGF signaling pathway, relieving inflammatory-oxidative cascades, protecting extracellular matrix integrity, and restoring gut microbial homeostasis. However, studies on the mechanism of action of DP in RNV are still relatively limited. This review summarizes the multi-targeted mechanism of action of DP in RNV formation, explores its potential as a novel therapeutic strategy for retinal diseases, and suggests strategic directions for future mechanistic studies through a systematic analysis of the existing literature.
2 Pathological mechanisms of retinal neovascularization
RNV formation is a complex pathological process involving the interaction of multiple cell types, cytokines and signaling pathways, such as endothelial cell apoptosis, pericyte loss, immune cell dysfunction, activation of multiple growth factors and dysregulation of signaling pathways (Figure 1).
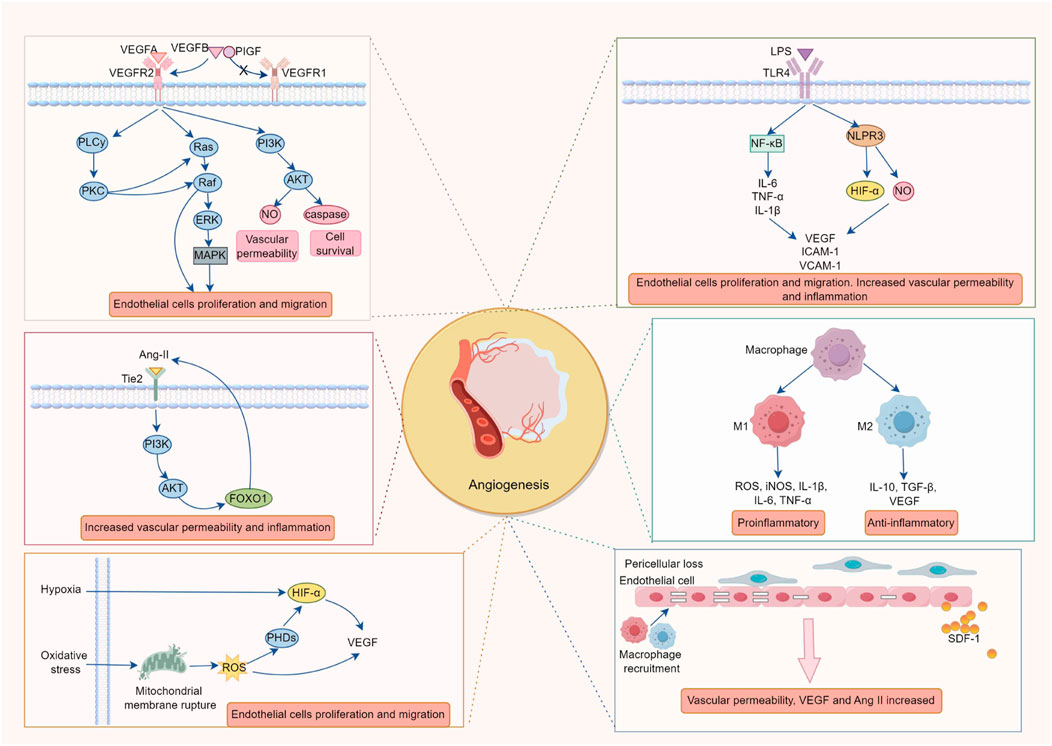
Figure 1. The Pathological Mechanisms of Retinal Neovascularization. The formation of retinal neovascularization (RNV) is driven by a multi-factorial cascade reaction, triggered by a vicious cycle of hypoxia, inflammation, and oxidative stress. The key mechanisms include: 1. The ischemic and hypoxic microenvironment activates the VEGF/VEGFR2 signaling axis via HIF-1α, in conjunction with the imbalance of AngII/Tie2 and disruption of the PDGF-B/PDGFRβ pathway, promoting endothelial cell proliferation, migration, and lumen formation while compromising the integrity of the blood-retinal barrier (BRB). 2. Chronic inflammation (mediated by NF-κB and the release of IL-6, TNF-α) and ROS bursts enhance HIF-1α stability and upregulate VEGF expression, further exacerbating endothelial cell activation and vascular leakage, thus intensifying the vicious cycle of worsening hypoxia → amplified inflammation → oxidative damage. 3. Polarization of M2 macrophages (secreting VEGF and IL-10) and abnormal recruitment of microglial cells release pro-angiogenic factors, which, in conjunction with the loss of pericytes, contribute to vascular instability and promote pathological angiogenesis. 4. Pericyte apoptosis, through the FOXO1-AngII positive feedback loop, increases endothelial cell sensitivity to VEGF, accelerating the formation of non-perfused areas and vascular leakage, ultimately leading to fibrosis and tractional retinal detachment.
2.1 Regulatory network of angiogenesis
Angiogenesis is a complex biological process. Under normal physiological conditions, it remains relatively quiescent, but external stimuli can induce pathological angiogenesis to repair damaged tissues. Among these factors, VEGF plays a crucial role as the most potent pro-angiogenic factor. VEGF directly stimulates endothelial cell proliferation, migration, and lumen formation, significantly increasing vascular permeability (Fu et al., 2023). Its biological effects are primarily mediated through binding to membrane-bound receptors VEGFR1 and VEGFR2, with VEGFR2 being more abundantly expressed in endothelial cells. However, studies suggest that under physiological conditions, VEGF binding to soluble or membrane-bound VEGFR1 may inhibit VEGF-VEGFR2 signaling through higher affinity binding, exerting anti-angiogenic effects to maintain balanced regulation in the physiological state (Uemura et al., 2021; Wu et al., 2010). Under pathological conditions such as hypoxia and inflammation, the expression of the VEGF family is significantly upregulated, leading to VEGFR1 competitively binding with placental growth factor (PlGF) and VEGF-B, which in turn promotes the activation of VEGFR2 (Robciuc et al., 2016; Yang et al., 2013). The binding of VEGF to VEGFR2 continues to activate several downstream signaling pathways, including the PLCγ/PKC, Ras/Raf/ERK/MAPK, and PI3K/AKT signaling pathways. These pathways collectively regulate vascular permeability, promote endothelial cell proliferation and anti-apoptotic activity, and influence endothelial cell migration, actin reorganization, and cytoskeletal remodeling, ultimately leading to the formation of pathological neovascularization (Claesson-Welsh, 2012; Melincovici et al., 2018). The VEGF-VEGFR2 signaling cascade has been demonstrated to critically impair endothelial adhesion and intercellular connections, leading to increased vascular permeability and fluid extravasation, and subsequent breakdown of the blood-retinal barrier (BRB) (Simons et al., 2016). Although the VEGF-VEGFR2 signaling pathway is recognized as an important angiogenic pathway, the regulatory role of VEGFR1 is still controversial: most current evidence on the mechanisms of VEGFR1 inhibition is based on in vitro or animal models, and its regulatory role in human diseases remains unclear.
The fibroblast growth factor (FGF) serves as a pivotal regulator of vascular development and tissue regeneration. Mechanistically, FGF induces endothelial cell proliferation and migration while simultaneously facilitating pericyte recruitment, processes that collectively enhance vascular integrity through regulation of endothelial permeability and microvascular network formation (Seghezzi et al., 1998; Ucuzian et al., 2010). FGF1 and FGF2 isoforms have been shown to have significant proangiogenic effects on endothelial cells, they can not only promote angiogenesis independently, but also synergistically interact with VEGF through VEGFR2-FGFR1 complex formation to promote neovascularization (Hori et al., 2017; Melincovici et al., 2018). In addition, endothelial-derived platelet-derived growth factor B (PDGF-B) exerts angiogenic functions by binding to its receptor PDGFR, activating downstream signaling pathways similar to those induced by VEGF (Vimalraj, 2022), and participating in pericyte recruitment and new blood vessel formation (Tang et al., 2023). During tumor angiogenesis, abnormal PDGF signaling recruits pericytes to blood vessels, releasing pro-angiogenic factors that further stimulate endothelial cell migration, proliferation, and lumen formation, thereby accelerating lymphangiogenesis and invasion (Vimalraj, 2022). Angiopoietins (Ang-I and Ang-II) are also key factors in regulating angiogenesis and vascular remodeling. In endothelial cells, Ang-II binds to the Tie2 receptor, inhibits the PI3K/Akt pathway, and activates the forkhead box transcription factor FOXO1. FOXO1 activation further upregulates Ang-II, establishing a positive feedback loop that promotes angiogenesis, vascular permeability, and inflammation (Augustin et al., 2009; Kusuhara et al., 2018). In pathological conditions such as DR, AMD, and retinal vein occlusion, the expression of Ang-II is significantly upregulated, increasing endothelial cell sensitivity to pro-angiogenic, pro-permeability, and pro-inflammatory stimuli (Augustin et al., 2009; Regula et al., 2016; Watanabe et al., 2005). Under conditions of ischemia, hypoxia, and inflammation, not only are VEGF and HIF-1α highly expressed, but Ang-II also acts as an antagonist to Ang-I, disrupting Ang-I mediated vascular homeostasis, impairing endothelial and supporting cell interactions, and inducing endothelial cell apoptosis, vascular instability, and neovascular formation (Huang and Bao, 2004).
2.2 The malignant cycle of hypoxia-inflammation-oxidative stress
Under ischemic and hypoxic conditions, cells respond to environmental changes by upregulating HIF-1α, which activates a series of hypoxic response genes and induces HIF-1α-dependent VEGF transcription (Rattner et al., 2019). This results in increased capillary permeability, neovascular formation, and disruption of the BRB integrity. Clinical studies have demonstrated that intravitreal anti-VEGF therapy reduces circulating VEGF levels in patients and effectively decreases the release of inflammatory cytokines (Mastropasqua et al., 2018; Raczyńska et al., 2018), thus mechanistically linking chronic inflammatory cascades to retinal RNV pathogenesis. When exposed to external stimuli, various pattern recognition receptors (such as TLRs and NLRs) recognize exogenous or damaged molecules (e.g., LPS, CpG DNA, lipoproteins, flagellin) (Wang R. et al., 2023), activating intracellular inflammasomes and the NF-κB signaling pathway. This process triggers the release of numerous pro-inflammatory cytokines (e.g., IL-1β, IL-6, TNF-α) and other inflammatory mediators (e.g., HIF-1α, NO). While this response helps repair damaged tissue somewhat, it can also lead to sustained tissue damage, further activating endothelial cells. These cells secrete leukocyte adhesion molecules (e.g., ICAM-1, VCAM-1), cytokines, and chemokines, increasing VEGF expression (Fu et al., 2020; Heloterä and Kaarniranta, 2022), promoting endothelial cell proliferation and migration, and resulting in angiogenesis and neovascular formation in avascular regions.
Oxidative stress plays an important role in the development of RNV. When the antioxidant defense system is impaired or reactive oxygen species (ROS) are overproduced, an imbalance between ROS generation and clearance significantly increases intracellular oxidative stress levels. This is especially true when excessive activation of the mitochondrial electron transport chain results in ROS accumulation, directly damaging mitochondria and DNA and inducing endothelial cell apoptosis. ROS not only increases the transcriptional activity of HIF-1α, promoting VEGF expression, but also activates a series of pro-inflammatory responses, accelerating capillary permeability and neovascular formation (Cheung et al., 2010; Fu et al., 2020; Yang et al., 2022). Chronic oxidative stress also induces glial cell secretion of inflammatory factors, maintaining a persistent chronic inflammatory state in retinal cells, and ultimately promoting retinal neurodegeneration and microvascular lesions (Batliwala et al., 2017). Although the HIF-1α-VEGF axis is recognized as an important signaling pathway, most studies are based on acute hypoxia models and have not adequately modeled the dynamic process of hypoxia and repetitive reperfusion that occurs in chronic retinal disease. This may lead to an underestimation of the complexity of endothelial cell adaptation to hypoxia. The dual role of ROS in RNV, promoting angiogenesis and triggering cell apoptosis, is well established. Still, in current experimental systems, it is often not possible to separate the specific contribution of different forms of ROS (such as O2−, H2O2, H2O2, and OH−) at different pathological stages. This uncertainty at the molecular level limits the accuracy of targeted antioxidant therapy.
2.3 Mononuclear phagocyte system
Monocytes/macrophages, as key sources of angiogenic factors, play a significant role in RNV, particularly in PDR and OIR models (Tu et al., 2024). The chronic inflammatory microenvironment in retinal diseases is often accompanied by the infiltration of mononuclear phagocytes, including circulating monocytes/macrophages and resident microglia in tissues (Nathan and Ding, 2010; Rashid et al., 2019; Uemura et al., 2021). Studies have indicated that activated macrophages exhibit significant pro-angiogenic effects in vivo and in vitro (Hagbi-Levi et al., 2017). Upon stimulation, monocytes “polarize” into two macrophage subtypes: M1 and M2. M1 macrophages produce large amounts of ROS, inducible nitric oxide synthase (iNOS), IL-1β, IL-6, TNF-α, and other inflammatory mediators, exhibiting antimicrobial and pro-inflammatory actions while inhibiting angiogenesis. M2 macrophages, on the other hand, secrete anti-inflammatory mediators, growth factors, and pro-angiogenic factors (Nicholas and Mysore, 2021), such as IL-10, TGF-β, and VEGF, playing roles in anti-inflammation, tissue repair, and angiogenesis (Tu et al., 2024). In PDR and OIR models, increased expression of M2 macrophages is closely associated with the progression of pathological RNV, while the number of M1 macrophages is significantly reduced (Wang et al., 2024). Under hypoxic conditions, HIF-1α upregulates inflammatory cytokines (such as VEGF, IL-6, IL-8) and chemokines (such as MCP-1), promoting the recruitment and migration of peripheral microglia to the retina (Chen Z. Y. et al., 2024). This triggers the continued secretion of pro-inflammatory chemokines and cytokines, establishing a vicious cycle that promotes angiogenesis (Grossniklaus et al., 2002). It has been found that genetic ablation of the VEGFR1 TK structural domain in mice permitted normal vascular development but significantly inhibited VEGF-induced macrophage migration (Uemura et al., 2021), suggesting that activation of VEGFR1 also generates pro-inflammatory and pro-angiogenic mediators in macrophages and microglia (Carmeliet et al., 2001; Crespo-Garcia et al., 2017).
2.4 Loss of pericytes
Pericytes are mesenchymal cells that surround the endothelial cells of capillaries and work together with endothelial cells to maintain vascular integrity and stability. A decrease in the ratio of pericytes to endothelial cells leads to increased vascular permeability and cell-free capillary formation, which exacerbates microangiomas, vascular occlusion, and local retinal hypoxia, further promoting RNV formation (Mrugacz et al., 2021). Pericyte loss is usually induced by advanced glycation end products (AGEs), oxidative stress, inflammation and other factors, which destroy vascular structure through an apoptosis mechanism and play a key role in the development of blinding eye diseases (Han et al., 2023). In patients with DR, pericyte loss compromises endothelial cell integrity, and internal BRB function is consequently disrupted, inducing retinal microangiopathy, including microaneurysms, oedema, haemorrhage, decellularised capillaries and areas of capillary non-perfusion (Arboleda-Velasquez et al., 2015; Kusuhara et al., 2018), and this idea has also been supported in animal models of diabetes mellitus (Valdez et al., 2014). Relevant studies have shown that pericyte loss not only disrupts the tight junctions between endothelial cells, but also increases the sensitivity of endothelial cells to VEGF, thus leading to a positive feedback loop between FOXO1 and Ang II (Park et al., 2017), which further promotes abnormal vascular proliferation, vascular leakage, and inflammatory responses. Vascular leakage and occlusion in turn exacerbate retinal ischaemia, upregulate HIF-1α and further promote the expression of VEGF and Ang-2 (Rattner et al., 2019). Pericyte loss also releases chemokines such as stromal cell-derived factor-1 (SDF-1), which induces inflammatory responses in endothelial cells, macrophages and microglia through perivascular infiltration. VEGF is secreted to activate VEGFR2 in endothelial cells and a series of downstream signaling cascades, further damaging vascular integrity (Ogura et al., 2017). In addition, weak vascular walls and abnormal blood flow shear forces in the bloodstream caused by pericyte loss further aggravate local vasodilation (Tian et al., 2022). Due to the lack of pericyte coverage, neovascularization can be prone to hemorrhage and fibrosis, eventually leading to a tractional retinal detachment (Zhang J. et al., 2025).
3 Characteristics and biological activities of Dendrobium polysaccharides
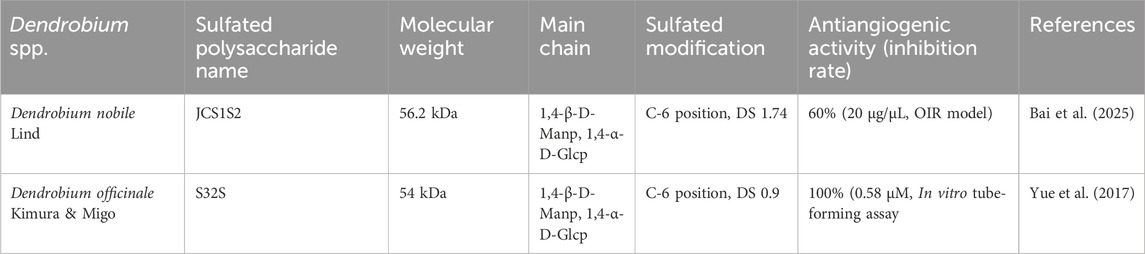
Dendrobium spp., which has a long medical history in traditional Chinese medicine, includes various varieties such as Dendrobium nobile Lindl, Dendrobium officinale Kimura & Migo, and Dendrobium huoshanense Z.Z.Tang & S.J.Cheng as documented in the Chinese Pharmacopoeia (2020 edition) (Zhang et al., 2018). These botanicals are rich in bioactive components, mainly polysaccharides, flavonoids, alkaloids, phenols and amino acids, whose pharmacological properties and health-beneficial functions have been extensively studied. It is important to note that the main polysaccharides are composed of carbohydrates called glycans, which are complex macromolecules composed of more than 10 monosaccharide units linked together. These glycans have various biological activities, including lowering blood sugar levels, immunomodulatory properties, antioxidants and gastrointestinal protection (Fan et al., 2023; Gong et al., 2014; Wang H. Y. et al., 2019). DP is mainly obtained by extraction with water, alcohol, ultrasound, etc. Their structure is complex and variable, and they are usually found in pyranose form, consisting mainly of glucose (Glcp), mannose (Manp), galactose (Galp), arabinose (Arap), xylose (Xylp), rhamnose, and other monosaccharides. The content of polysaccharides varies from genus to genus depending on factors such as climate, temperature, humidity and soil. The structure of polysaccharides varies from genus to genus, and the differences lie mainly in the type and proportion of monosaccharides, the molecular weight, and the type of glycosidic bonds and their linkages, which can lead to different pharmacological activities (Bu et al., 2019). Different extraction methods can also affect the content and pharmacological effects of different polysaccharides by changing the proportion of simple sugars rather than their type. For example, ultrasonic extraction leads to a decrease in the molecular weight of polysaccharides and the degradation of their main chains, a decrease in viscosity, and an increase in water solubility and biological activity (He et al., 2018). Most current studies of polysaccharides from Dendrobium officinale Kimura & Migo (DOP) and Dendrobium huoshanense Z.Z.Tang & S.J.Cheng (DHP) were identified as glucomannan bases containing 1,4-β-D-Manp and 1,4-β-D-Glcp units. The molecular mass of DOP ranged from 0.46 KDa to 2.27 × 106 kDa and that of DHP from 2.55 KDa to 770 KDa, with different degrees of acetylation or branching of the glucan residues (Hsieh et al., 2008; Deng et al., 2016; Chen W. et al., 2021; Li et al., 2022; Wu et al., 2023; Zhang M. et al., 2024). Most of the D. nobile Lindl (DNP) polysaccharides have been identified as glucomannan with glycosidic bonds such as α-(1→4), α-(1→6), β-(1→4), β-(1→6) and others, including the acetylation of mannose residues at various sites, with or without branched chains (Luo et al., 2009; Chen H. et al., 2022; Li et al., 2024). The keyword “Dendrobium polysaccharides” was searched in PubMed, Web of Science, Google Scholar, CNKI and Wanfang databases to retrieve experimental articles on the assessment of the effectiveness of DP in the past 10 years, and summarized and analyzed in Table 1.
4 Mechanism of Dendrobium polysaccharides in inhibiting retinal neovascularization
4.1 Inhibition of the VEGF signaling pathway
Under retinal ischemia and hypoxia, the abnormal activation of the HIF-1α/VEGF signaling axis is one of the core mechanisms that promote the formation of pathological RNV. In an ischemic hypoxia model, DP was found to downregulate HIF-1α expression in a dose-dependent manner (Li and Hong, 2020), which might involve the classical PHD2/HIF-1α pathway, whereby the activation of prolyl hydroxylase 2 (PHD2) accelerates oxygen-dependent HIF-1α proteasomal degradation (Majmundar et al., 2010). Under hypoxic conditions, as the stability and accumulation of HIF-1α decrease, the binding of HIF-1α to the hypoxia-responsive element (HRE) in the promoter region of VEGF is also attenuated, which reduces transcriptional activation of VEGF and inhibits angiogenesis. Most current studies on DP have indirectly exerted anti-angiogenic effects based on anti-inflammatory and antioxidant properties. Researchers isolated the JCS1 polysaccharide from the stem of D. nobile Lindl and found that its backbone consists of (1→4)-β-D-Manp and (1→4)-α-D-Glcp (Wang Z. et al., 2019). They then modified the monosaccharide residues to C-6 in the main chain by sulfation to obtain a new derivative, JCS1S2. JCS1S2 showed significant inhibitory effects in wound healing and angiogenesis assays in vitro by antagonizing the low affinity of VEGFR2 and binding directly to VEGF, thus blocking VEGF/VEGFR2 signaling. At the same time, this polysaccharide reduces the phosphorylation of VEGFR2 and ERK and decreases the expression of VEGF and its transcription factor AP-1, thereby inhibiting neovascularization. Notably, JCS1S2 inhibited the migration of endothelial cells without affecting their proliferation (Bai et al., 2025). injected JCS1S2 into the vitreous of rats with OIR and found that the expression of the VEGF family, especially VEGF-A, VEGF-B, VEGF-D, VEGFR1, and VEGFR2, was significantly reduced, and neovascularization in the retina and areas not subject to perfusion was also significantly reduced after treatment. Other researchers have studied sulfated polysaccharides and angiogenesis (Yue et al., 2017). They extracted the S32 polysaccharides from the stem of Dendrobium officinale Kimura & Migo and analyzed their structures, which consist mainly of 1,4-β-D-xylp and 1,2,4-β-D-xylp, and modified C-2 or C-3 of the 1,2,4-β-D-xylp residues by sulfation to obtain the sulfated S32S polysaccharides. In this study, S32S showed a dose-dependent inhibitory effect on tube formation by HMEC-1 cells and significantly inhibited endothelial cell migration at a low concentration of 0.29 μM. Although S32S has potent antiangiogenic effects, it does not have significant cytotoxicity. This class of polysaccharides has a great influence in fighting against angiogenesis, is potentially valuable, and deserves more attention.
4.2 Anti-inflammatory and immune microenvironment remodelling
DP has significant anti-inflammatory efficacy, which reduces the infiltration of inflammatory cells such as neutrophils and macrophages by inhibiting the activation of inflammatory signaling pathways such as NF-κB and decreases the release of pro-inflammatory factors such as TNF-α and IL-6 (Li, 2016; Li et al., 2016) as well as promotes the expression of anti-inflammatory factors such as IL-10 (Xie et al., 2022), which inhibits the activation of the transcripts of pro-angiogenesis-related genes that are associated with inflammation. DP also reduces retinal inflammatory microenvironment by inhibiting NLRP3 inflammasome activation, reducing the inclusion and activation of caspase-1/GSDMD, and reducing the release of pro-inflammatory cytokines (e.g., IL-1β) to reduce macrophage damage caused by the pathogen (Zhang et al., 2022). This process not only alleviates cell damage due to the inflammatory response but also enhances the activity of immune cells and downregulates VEGF expression, thereby reducing its stimulating effect on angiogenesis. In addition to inhibiting inflammatory responses, DP has been shown to play an important role in regulating immune cell function. Studies have shown that DP affects microglia polarization, promotes conversion from pro-inflammatory to anti-inflammatory phenotypes, reduces the secretion of pro-inflammatory cytokines, improves immune regulation, and increases vascular permeability and apoptosis (Feng et al., 2019). DP may also have direct immunomodulatory effects by binding to polysaccharide receptors on the surface of immune system cells (e.g., macrophages or lymphocytes), such as the mannitol receptor (Tao et al., 2019; Wu et al., 2019), enhancing lymphocyte proliferation and promoting macrophage activation and phagocytosis (Bai et al., 2025). DP can also promote the secretion of interferon-γ (IFN-γ), IL-12, IL-10 and other factors by immune cells to inhibit angiogenesis (Meng et al., 2013; Xie et al., 2022). Within adaptive immunity frameworks, DP can selectively regulate the secretion of Th1-type cytokines, elevate CD4+/CD8+ T lymphocytes ratios, and stimulate non-specific immunoglobulin synthesis (IgA, IgM, IgG), collectively improving systemic immune responsiveness (Li Z. et al., 2020; Wang K. et al., 2018). In particular, DP increases the immune activity of monocytes by activating the ERK and NF-κB signaling pathway, which manifests itself by stimulating immune cell proliferation, increasing cytokine secretion, NO and phagocytosis synthesis, and ultimately immune competence at the tissue level (Wei et al., 2016).
4.3 Antioxidant effects and mitochondrial protection
As a powerful natural antioxidant, DP demonstrates potent antioxidant properties through mechanisms related to scavenging free radicals and maintaining calcium homeostasis. They can effectively mitigate damage from intracellular oxidation by suppressing lipid peroxidation processes, evidenced by reduced malondialdehyde (MDA) accumulation. Concurrently, they increase cellular antioxidant protection through activating the superoxide dismutase (SOD) and glutathione (GSH) systems (Fan et al., 2020; Guo et al., 2024; Li B. et al., 2021). This mechanism preserves endothelial integrity by inhibiting apoptotic pathways and relieving inflammatory cascades caused by oxidative stress. Subsequent studies indicated that the antioxidative effect of DP may be closely related to the activation of the Nuclear factor E2-related factor 2 (Nrf2) pathway and downstream antioxidant enzymes, which can reduce the damage caused by ROS (Xu et al., 2022). Nrf2 is a key transcription factor involved in the regulation of the cellular antioxidant response. DP activates the Nrf2-Keap1 signaling pathway (by increasing Nrf2 expression in the nucleus and decreasing Keap1 expression in the cytoplasm) and triggers dissociation of the Nrf2-Keap1 complex, thereby enabling Nrf2 to avoid Keap1-mediated ubiquitination degradation (Lin et al., 2018). After the activation of Nrf2 nuclear translocation, it binds to the antioxidant response elements (ARE) and encodes transcriptional expression of antioxidant enzyme genes, thereby regulating intracellular antioxidant synthesis and directly inactivating ROS to reduce oxidative damage (Xu et al., 2022; Zhao et al., 2019). Under hyperglycemic conditions, inducible nitric oxide synthase (iNOS) triggers excessive NO production, which rapidly reacts with superoxide anion (O2−) to generate peroxynitrite (ONOO−), a cytotoxic oxidant that has a more potent oxidative effect than conventional reactive oxygen species. Experimental evidence suggests that DP attenuates oxidative cascades by down-regulating iNOS expression and inhibiting AGE formation (Fan et al., 2020).
Mitochondria as the core regulators of energy metabolism in cells, and their dysfunction is often associated with oxidative stress and cellular damage. DP has been found to significantly increase the expression of mitochondrial autophagy-related proteins such as PINK1, Parkin, and LC3B, reducing the accumulation of the P62 autophagy receptor (Li H. et al., 2023). This not only scavenges ROS to resist oxidative damage, but also maintains intracellular metabolic homeostasis by promoting mitochondrial autophagy to eliminate functionally impaired mitochondria, which improves insulin resistance and repairs DNA damage. Notably, the protective effects of DP on mitochondrial function were also involved in increasing the activity of the mitochondrial respiratory chain complex, improving the AMPK signaling pathway and improving the efficiency of the tricarboxylic acid cycle, which ultimately inhibited neuronal apoptosis effectively (Chen L. et al., 2023). However, the mechanism of action of DP is significantly heterogeneous in some specific pathological situations. In the study of colon cancer CT26 cells, DP were found to be able to interfere with mitochondrial function by stimulating ROS bursts, lowering mitochondrial membrane potential and inhibiting ATP synthesis, while activating the AMPK-mTORC1-ULK1 pathway to cause excessive autophagy in cells, inhibit tumor cell proliferation and promote their death (Zhang et al., 2021) (Figure 2).
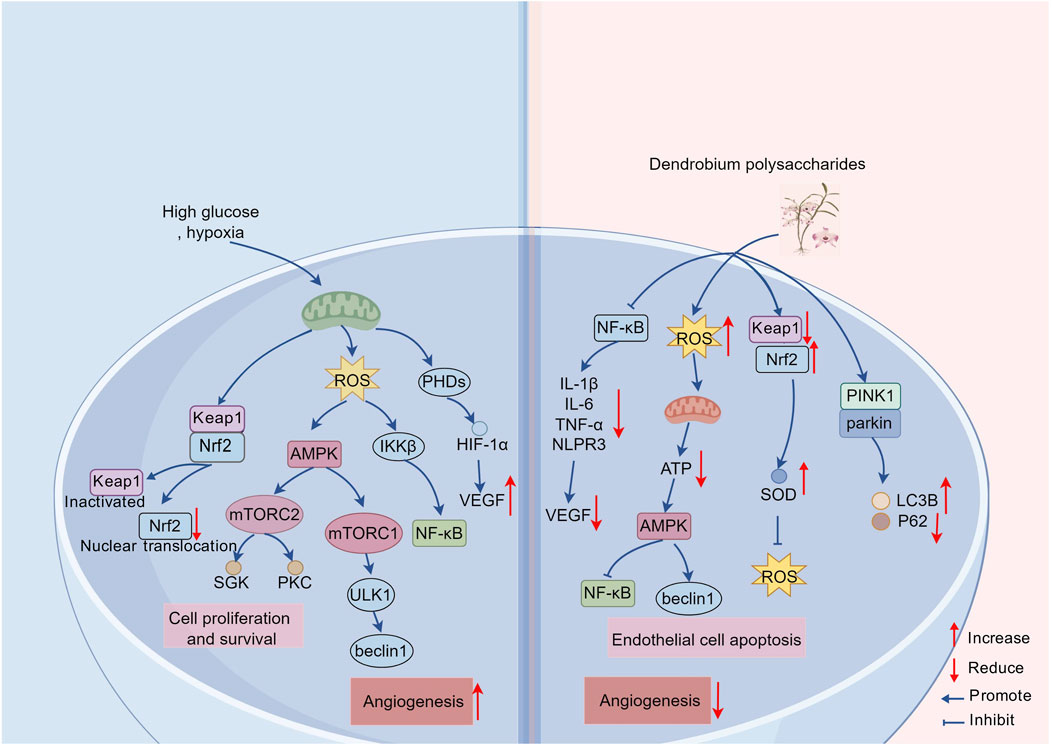
Figure 2. Schematic representation of anti-inflammatory, antioxidant and immune-enhancing properties of DP. DP demonstrates multimodal therapeutic effects through coordinated molecular interventions. They attenuate inflammatory cascades and oxidative stress while suppressing VEGF expression, thereby inhibiting vascular endothelial cell proliferation and migration. Enhanced phagocytic capacity of immunocytes is concurrently observed, coupled with angiogenesis suppression via mechanisms of macrophage protection and inflammatory pathway inhibition. It stimulates the expression of Nrf2 and reduces the activity of Keap1, activating the Nrf2-Keap1 pathway and prompting Nrf2 to escape from the ubiquitination degradation mediated by Keap1, thus binding to the downstream elements of the antioxidant response after nuclear translocation and stimulating the production of antioxidant enzymes sufficient for the elimination of ROS. The polysaccharides promote cellular homeostasis through PINK1/Parkin-mediated mitophagy activation, effectively counteracting ROS-induced damage. Paradoxically, controlled ROS elevation induces metabolic reprogramming via AMPK pathway activation, which suppresses ATP biosynthesis while potentiating autophagic flux. This dual regulatory mechanism culminates in endothelial cell apoptosis through excessive autophagy induction.
4.4 Regulation of extracellular matrix remodeling
The remodeling of extracellular matrix (ECM) and the destruction of dynamic balance play a central role in the formation of RNV. The composition of ECM includes components such as fibronectin, collagen, and laminin, and these molecules not only provide structural support for tissues but also regulate the migration, proliferation, and invasion of endothelial cells. However, pathological conditions, especially metabolic abnormalities such as hyperglycemia, can induce abnormal deposition of ECM components, leading to capillary basement membrane thickening (Yang and Liu, 2022). The thickened basement membrane enhances the sensing of mechanical tension of endothelial cells and promotes their proliferation and migration by enhancing the activity of integrin-focal adhesion kinase (FAK) signaling pathway (Katoh, 2024), thus providing favorable conditions for pathological RNV. In a therapeutic study targeting renal fibrosis (Shi et al., 2023), Dendrobium polysaccharide showed a significant reduction in ECM components, such as collagen I, collagen IV, and fibronectin, thereby alleviating the over-deposition of abnormal ECM and improving basement membrane thickening in a dose-dependent manner. The study also found that DP inhibited the expression of transforming growth factor beta 1 (TGF-β1) and connective tissue growth factor (CTGF), thereby reducing collagen synthesis. This suggests that DP can help to maintain the dynamic balance between ECM synthesis and degradation.
The excessive deposition of ECM and basement membrane thickening promotes increased expression and activity of matrix metalloproteinases (MMPs) during angiogenic processes. MMPs mediate the proteolytic almost all components of the ECM, thereby destabilizing the basement membrane’s structural organization and establishing permissive microenvironments for endothelial migration and subsequent angiogenic progression (Cabral-Pacheco et al., 2020). In endothelial cells, oxidative stress can promote the expression and activation of MMPs, promoting the degradation of ECM components. In inflammatory vascular lesions, the loss of tissue MMP inhibitors (TIMPs) is considered an important factor in vascular lesions. The imbalance between MMPs and TIMPs affects the degradation and innovation of ECM components and is considered a potential biomarker for diabetes and its complexity (Cabral-Pacheco et al., 2020; Fiorentino et al., 2013). Mediation of DP has been shown to significantly reduce the expression levels of MMP-1 and MMP-3, reduce collagen degradation and reduce the pro-degradation effect of MMPs by eliminating ROS, thus maintaining ECM stability and significantly inhibiting excessive endothelial cell migration and angiogenesis (Guo et al., 2024). In an ethanol-induced gastric ulcer study, SOD activity increased and MDA content decreased in gastric mucosal tissues treated with DP, while the levels of MMP-2, MMP-9, p-JNK, and p-ERK proteins were also significantly descreased. This also verified the effect of DP on the regulation of MMPS expression (Zhang et al., 2018). In another experiment with human skin fibroblasts in a hyperglycemic environment, DP increased collagen and TIMP2 levels and decreased MMP2 expression in a concentration-dependent manner (Liu F. Y. et al., 2021). This suggests the role of DP in the regulation of MMP2 and TIMP expression. In addition, membrane metalloproteinase type 1 (MT1-MMP) has also received special attention in angiogenesis (Sounni et al., 2002). This study demonstrates that MT1-MMP promotes endothelial cell migration through proteolytic degradation of the ECM and stimulates angiogenesis through VEGF expression modulation, providing a new perspective on molecular targets of DP for RNV inhibition. While there are still few studies on the regulation of TIMPs by DP, available research shows that the expression of TIMPs is regulated by oxidative stress and inflammatory responses (Amălinei et al., 2010). Unfortunately, the regulation of MMP and TIMP by DP has rarely been discussed in the field of ophthalmology, but the above studies indicate that DP may enhance the dynamic balance between MMPs and TIMPs and promote the regulation of ECM homeostasis, which may shed light on the mechanism of angiogenesis inhibition.
4.5 Intervention of retinal vascular diseases through the “gut-eye” axis
Systematic links between the gut microbiota and eye diseases are gradually being discovered (Thakur et al., 2022; Tîrziu et al., 2024). The intestinal flora and its metabolites can break through the intestinal mucosal barrier and enter the circulation through the cascade of the “intestinal barrier-circulation-target organ”, causing endotoxicity and systemic metabolic imbalance, and ultimately leading to pathological damage of the distal organs (e.g., the retina). In pathological states, when the balance of probiotic/pathogenic bacteria in the intestine is disturbed, not only does the increase in intestinal permeability cause localized inflammation, but the microorganisms can also cause extravagant inflammation throughout the body and reside in the intraocular lumen, which can lead to a variety of oxidative damage, Inflammation, immunodeficiency, metabolic disorders and other ocular events (Bai et al., 2023; Deng et al., 2021). In numerous human and animal studies, GM-derived metabolites, such as lipopolysaccharide (LPS), short-chain fatty acids (SCFAs), bile acids (BAs), and branched-chain amino acids (BCAAs), are involved in the regulation of retinal immune responses, energy metabolism homeostasis, and cellular autophagy through multiple targets, thereby affecting retinal angiogenesis (Xue et al., 2021). LPS is a characteristic metabolite of Gram-negative bacteria and is produced in increased amounts in a variety of ocular diseases, mainly due to increased abundance of Bacteroides, Proteobacteria, Enterobacteria and Escherichia (Qin and Zou, 2022). LPS exhibits dual pathological effects during retinal angiogenesis: on the one hand, as a pattern recognition receptor agonist, it specifically activates TLR4 on the surface of the retinal microvascular system and glial cells, triggering a cascade amplification of the NF-κB signaling pathway, which promotes downstream inflammatory factor expression (Xu et al., 2016). On the other hand, it significantly upregulates VEGF transcriptional expression through an inflammatory mechanism, induces pathologic angiogenesis, and disrupts BRB integrity, accelerating retinal microcirculatory dysfunction (Wang et al., 2020). BCAAs, including leucine, isoleucine, and valine, have high circulating levels due to increased GM synthesis in disease states and decreased metabolism. BCAAs can gradually dissolve retinal neurovascular device structures by activating the mTORC1 signaling pathway, exacerbating insulin resistance and oxidative stress, and inhibiting mitochondrial autophagy and promoting apoptosis in neurons and endothelial cells (Gong et al., 2022). GM produces SCFAs through the digestion of dietary vitamins, mainly acetate, propionate and butyrate, which have a significant role in defense against pathogenic bacteria and the development of the host immune system (Xue et al., 2021). It promotes intestinal GLP-1 secretion through activation of G protein-coupled receptors (GPR41/43) and inhibits histone deacetylase (HDAC) activity, promotes the differentiation of regulatory T cells (Treg), which modulates the body’s inflammatory response and glucose metabolism, and inhibits Th17 cell-mediated autoimmune retinal damage (R Muralitharan et al., 2025; Zhang Y. et al., 2025). Intravitreal injection of sodium butyrate significantly reduced the size of choroidal neovascularization (CNV) lesions in a mouse model of laser-induced CNV, and sodium butyrate nanoparticles also showed antiangiogenic activity in a chicken chorioallantoic membrane (CAM) experiment (Ciurariu et al., 2025). Concurrently, in a high glucose experiment with human retinal endothelial microvascular cells (HRMEC), sodium butyrate was also found to inhibit the ROS/NF-κB/NLRP3 signaling pathway and angiogenesis (Cao et al., 2025). Bile acids (BAs), whose conversion from primary (PBAs) to secondary (SBAs) is accomplished by the GM in the gut, have an important role in the absorption of dietary lipids and fat-soluble vitamins, and be neuroprotective and antiangiogenic in a variety of retinal diseases, such as DR and ROP (Win et al., 2021). SBAs reduce mitochondrial membrane potential and inflammatory responses by activating the G-protein-coupled receptor (TGR5) and the nuclear receptor (FXR), reducing Ca2+ overload and ROS bursts, inhibiting proinflammatory gene expression by antagonizing NF-κB and AP-1, and negatively regulating NLRP3 production, thereby reducing retinal Müller cell apoptosis, retinal vascular leakage, and inflammatory responses (Bertolini et al., 2022; Li J. et al., 2023). In vitro studies, lithocholic acid (LCA), glycocholic acid (GCA), and glycoursodeoxycholic acid (GUDCA) have been shown to significantly inhibit tube formation and endothelial cell proliferation and migration (Kundu et al., 2017; Warden and Brantley, 2021). In vivo experiments, ursodeoxycholic acid (UDCA) suppressed pathological retinal vasodilation by reducing the expression of inflammatory factors and normalizing VEGF-STAT3 signaling (Thounaojam et al., 2020).
In this context, DP, as a microecological regulator of the gut, exhibits multidimensional mediation potential (Liu et al., 2023; Wu et al., 2023; Zhang and Mo, 2023). DP can optimize the composition of GM by increasing the relative abundance of beneficial bacteria (e.g., Bifidobacterium, Lactobacillus) and reducing the number of harmful bacteria (e.g., Escherichia coli, Staphylococcus) (Chen X. et al., 2023; Wu et al., 2023), which maintains the structural balance of GM and prevents overproduction of harmful metabolites. DP has been shown to improve the protective function of the intestine (Xie et al., 2019). By regulating the expression of the connection proteins of the intestinal mucosa (e.g., Occludin and ZO-1) and increasing the secretion of sIgA, β-defensin and mucin-2, it has been able to improve the morphology and structure of the intestinal mucosa and increase the activity of immune cells in the intestinal tract and blood circulation, thus increasing intestinal barrier function and systemic immune response. Meanwhile, this study also found that it reduced the abundance of the Proteobacteria derived from LPS and also reduced the abundance of the Firmicutes and Bacteroidetes phyla. Therefore, DP can inhibit LPS production and LPS transmission of TLR4-NF-κB signaling pathways (Wang et al., 2020), reduce the release of pro-inflammatory and pro-angiogenic factors, and reduce intestinal hyperpermeability, thereby reducing retinal damage caused by pathogenic bacteria and the release of harmful toxins. It is interesting to note that the increase in Firmicutes episodes is associated with high intestinal permeability, chronic low-grade inflammation and has been shown to increase neovascularization (Zinkernagel et al., 2017). DP has been shown to alter BCAA metabolism by suppressing genomic expression associated with BCAA biosynthesis and increasing regulation of the catabolic BCAAs enzymes (BCATs, BCKDH) (Chen H. et al., 2021). This reduces the systemic accumulation of BCAAs, thereby mitigating the expression of mTOR-mediated inflammatory factors and VEGF activation. At the same time, polysaccharides attenuate ROS generation while maintaining mitochondrial integrity while maintaining cellular stability. In addition, DP increases microbial populations of SCFAs-producing taxa (e.g., Lactobacillus), stimulates the biosynthesis of SCFAs and improves receptor signaling pathways (FFAR2/FFAR3). These microbial metabolites act as metabolic substrates for intestinal epithelial cells, combat mitochondrial damage caused by ROS, and exhibit a variety of bioactivities, including antioxidant, anti-inflammatory and immunoregulatory properties (Liu et al., 2023; Zeng et al., 2024). Another study has demonstrated that DP enhances vascular endothelial functionality by activating the gut-vascular SCFA-GPCR43/41 signaling axis (Li Y. et al., 2021). DP can restore metabolic homeostasis of BAs in the systemic circulation, thereby improving fat absorption and potentiating insulin secretion under pathological conditions (Yang J. et al., 2020). From a mechanistic point of view, these polysaccharides suppress retinopathic progression via dual pathways: (1) facilitating interactions of BAs-TGR5 on macrophage membranes to induce anti-inflammatory polarization of M2 and reduce phagocytic activity and pro-inflammatory cytokine secretion; (2) upregulating interleukin-10 (IL-10) expression to antagonize angiogenesis-promoting microenvironments (Feng et al., 2019; Liu H. et al., 2021). Interestingly, BAs can transport fatty acids to the apical membrane of intestinal epithelial cells and absorb them into the bloodstream, after which they are absorbed by VEGF-B controlled vascular endothelium mediated by vascular fatty acid transport proteins, thereby participating in endothelial energy metabolism (Hagberg et al., 2010). This process may be related to VEGF-B/VEGFR1 signaling to regulate the expression of fatty acid transporter proteins in an endothelium-mediated manner, which may suggest that the future therapeutic potential of DP for anti-angiogenesis could be explored through the fatty acid/VEGF-B/VEGFR1 pathway (Figure 3).
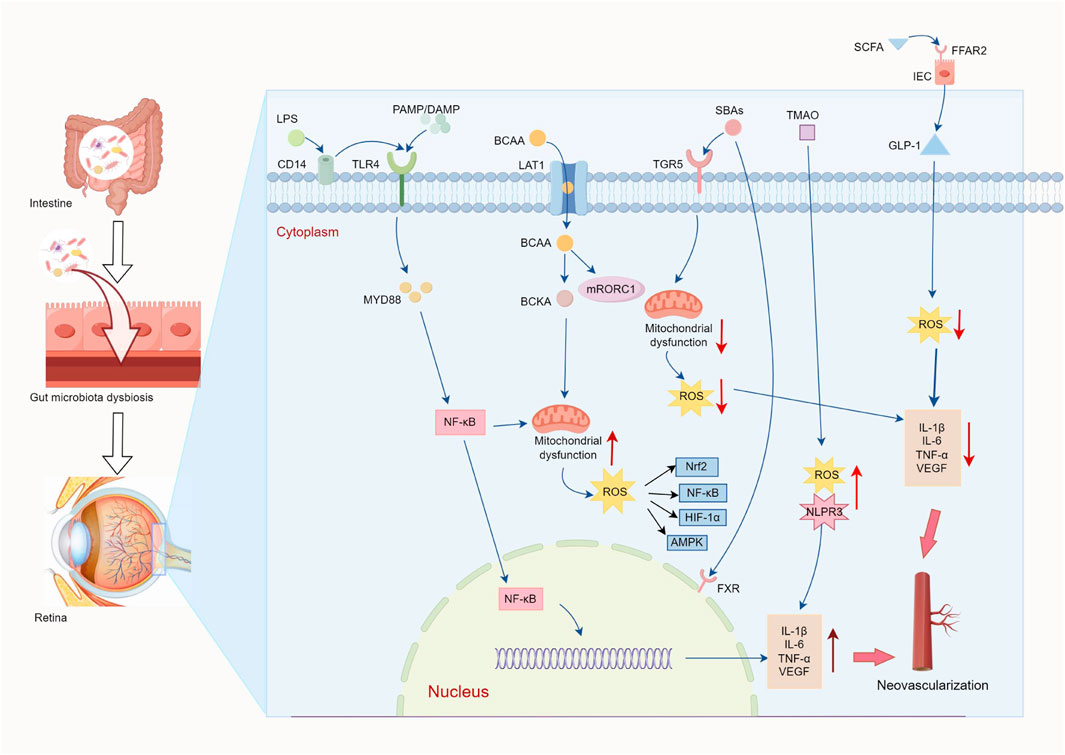
Figure 3. Schematic diagram of the “gut-blood-retina” regulatory mechanism. Poor microecological intestinal regulation of disease status (pathogenic bacteria↑/probiotic bacteria↓) leads to a weakening of intestinal barrier function, and intestinal hyperpermeability induces the entry of harmful metabolites, such as LPS and BCAA, into the bloodstream through the portal vein, and reduces beneficial metabolites, such as SCFAs and BAs. LPS regulates the expression of VEGF and pro-inflammatory factors (TNF-α, IL-6) by signaling TLR4/NF-κB. BCAAs enter retinal cells via the amino acid transporter (LAT1) in retinal cells, activating the mTORC1 pathway to stimulate insulin resistance, inflammation, ROS production and mitochondrial dysfunction, resulting in reduced cell activity. SCFAs increase GLP-1 secretion through FFARs and inhibit ROS-induced pro-inflammatory factors and VEGF expression. BAs improve mitochondrial function through TGR5/FXR receptor function, as well as inhibition of inflammatory responses and anti-angiogenesis. While the above gut microbial and metabolite disorders ultimately lead to BRB disruption, vascular leakage and neovascularization, DP address this process through a triple mechanism: (1) modification of GM composition and reduction of LPS and BCAAs biosynthesis; (2) improve intestinal barrier (tight junction proteins↑, sIgA↑) and prevent enterotoxin penetration into the bloodstream; (3) metabolic remodeling: promoting the production of SCFAs (FFAR2/3↑), activation of the SCFA-GPCR41/43 pathway to improve endothelial function; Positive regulation of the catabolic BCAAs enzymes (BCATs, BCKDH), inhibiting the over-activation of mTORC1; Regulate BAs metabolism (TGR5↑), stimulate polarization of M2 macrophages and reduce phagocytic activity and inflammatory response. In addition, DP can regulate endothelial energy metabolism via the fatty acid/VEGF-B/VEGFR1 axis, thus providing a new target for anti-angiogenic treatment (↑increase; ↓decrease).
4.6 The structure-activity relationship between Dendrobium polysaccharides and anti-angiogenesis
Polysaccharides are complex macromolecules that are widespread and commonly found in animals and plants. The biological activity of these polymers, which are composed of several glycosidically linked monosaccharide units, is closely related to their structural properties, including monosaccharide composition, molecular weight, types of glycosidic bonds and structural modifications (He et al., 2018; Bu et al., 2019). DP is no exception, and their antiangiogenic activity is closely related to the complex features of their molecular structure, particularly their monosaccharide composition, sulfate modifications and molecular weight. As shown in Table 1, the main monosaccharides of DP are Glcp and Manp, which are also frequently found in polysaccharides from various plant sources. These monosaccharides are alternatively linked by (1→4)-α/β-D-Manp or (1→4)-α/β-D-Glcp, forming the basis of glucan or heteropolysaccharides (Li Y. et al., 2023). Although the composition of polysaccharide monosaccharides varies among Dendrobium species, some common structural features are associated with their antiangiogenic properties. In particular, the addition of modifying groups, such as sulfate and acetyl groups at the C-2, C-3, or C-6 positions of monosaccharide residues, is known to significantly increase the antiangiogenic properties of DP.
Several natural polysaccharides containing sulfate groups have been shown to have a wide range of biological effects, including anticoagulant, antiviral, and anticancer properties (Chen et al., 2010). In recent years, the addition of sulfate groups to specific regions of DP has been used to alter their molecular conformation and increase their binding affinity to targets. This modification has attracted much attention due to its unique structural patterns and potent anti-angiogenic activity. The data are summarized in Table 2. For example, JCS1S2 from Dendrobium nobile Lind (sulfate group at C-6 position, sulfation degree 1.74, Mw 56.2 kDa), whose modification by sulfation significantly increased its affinity for VEGF (KD = 4.82 × 10−9) and had potent antiangiogenic effects in vitro and ex vivo by competitively inhibiting VEGF-VEGFR2 signaling (p < 0.001) (Wang H. Y. et al., 2019). In the OIR model, it reduced the area of neovascularization via the TLR4/p-NF-κB/VEGF pathway by about 60%, and significantly reduced the retinal vessel non-perfusion area (p < 0.05) and Müller cell activation (Bai et al., 2025). This is crucial for the treatment of retinal neovascularization (RNV) and indicates that JCS1S2 has therapeutic potential to inhibit RNV formation and is associated with downregulation of VEGF production and inflammatory events. High levels of sulfation are known to significantly increase the binding affinity of polysaccharides to their targets and directly influence their anti-angiogenic effects (Chen et al., 2012). Similarly, sulfated Dendrobium officinale Kimura & Migo polysaccharide S32S (degree of sulfation 0.9, Mw 54 kDa) inhibits endothelial cell tube formation at a low concentration of 0.29 μM and stops tube formation completely at 0.58 μM (Yue et al., 2017). S32S shows dose-dependent antiangiogenic effects without significant cytotoxicity, which is superior to the unmodified natural polysaccharide S32 (up to 13.51 μM). Although polysaccharides from different Dendrobium sources (e.g., D. nobile Lindl JCS1S2 and Dendrobium officinale Kimura & Migo S32S) differ in monosaccharide composition (Table 1), the main chain in both consists of (1→4)-β-D-Manp and (1→4)-α-D-Glcp with sulfation sites concentrated at the C-6 position. This structural homology may be the common basis for their anti-RNV activity. The polysaccharides DHPD1 (sulfation 1.473, Mw 3.2 kDa) (Qian et al., 2014) and DHPD2 (sulfation 0.94, Mw 8,090 kDa) (Li et al., 2014) are different sources of the above polysaccharides, but both show higher antiglycation activity after sulfation at the C-2 or C-6 position. They are expected to alleviate the progression of diabetic vascular complications by inhibiting the protein glycation process. These studies suggest that the synergistic effects of sulfation modification and specific glycosidic linkages, rather than the plant origin itself, increase the aqueous solubility of polysaccharides and alter their spatial conformation, which may be critical for activity against RNV.
Further structure-activity studies showed a close correlation between the molecular weight of DP and its biological activity. Studies have shown that low molecular weight DP possess potent antioxidant activity (Wang et al., 2022), such as DOP 80 and DOP 70 from Dendrobium officinale Kimura & Migo (Fan et al., 2018; Liu Y. et al., 2021), whereas higher molecular weight polysaccharides exhibit stronger antitumor and immunomodulatory activity (Feng et al., 2020). This phenomenon may be closely related to the structural complexity of polysaccharide molecular chains, glycosyl patterns and types of glycosidic linkages. For example, lower molecular weight polysaccharides of Dendrobium often contain higher amounts of arabinose and rhamnose, which are closely related to their higher antioxidant properties (Fan et al., 2018). Conversely, higher molecular weight polysaccharides, due to their longer molecular chains and more complex conformation, may bind more efficiently to angiogenesis-related targets such as VEGF, thereby inhibiting the formation of new blood vessels.
Moreover, acetylation modification of DP significantly affects their bioactivity, and the higher the degree of substitution, the higher the antioxidant activity (Wang et al., 2022). Studies have shown that DP with O-acetyl groups have higher immunomodulatory and anticancer activity. The linear chain structure formed by (1→6)-Glcp and (1→4)-Manp is probably the structural basis for this activity, and their antiangiogenic activity is closely related to their molecular weight (He et al., 2024). For example, polysaccharides isolated from Dendrobium huoshanense Z.Z.Tang & S.J.Cheng containing O-acetyl groups showed significantly greater inhibition of angiogenesis than their non-acetylated counterparts. Moreover, the antiangiogenic activity of these O-acetylated polysaccharides was proportional to their molecular weight, further supporting that the degree of acetylation and molecular weight play a role in modulating the antiangiogenic potential of polysaccharides (Liu H. et al., 2021).
In general, the antiangiogenic activity of DP is not only closely related to monosaccharide composition and skeletal structure, but also depends on factors such as the degree of chemical modification, including sulfation and acetylation, molecular weight and glycosylation pattern. By modifying their structural properties, notably through sulfation and acetylation, the antiangiogenic activity of these polysaccharides can be significantly enhanced, reinforcing their potential as anti-RNV agents. Although the exact relationship between the structural properties of DP and their antiangiogenic activity is still under investigation, the available data indicate that factors such as structural homology, the addition of modifying groups and molecular mass play an important role in their anti-RNV activity. Future studies should therefore continue to investigate the relationship between the structure and activity of DP, and optimize their biological activity through chemical modifications that could ultimately enhance their use in ophthalmology.
5 Discussion
RNV is a challenging problem in many clinical fundus diseases, such as AMD and DR. Developing strategies to inhibit RNV has become a major focus of ophthalmic pharmacology, but the number of effective, safe and long-term treatment options is still limited. RNV formation is a complex process involving multiple mechanisms, with the VEGF signaling pathway, which controls endothelial cell proliferation, migration and lumen formation, playing a central role. In addition, factors such as disruption of the BRB, inflammation, oxidative stress and endothelial cell damage contribute to RNV formation. This process is accompanied by the loss of pericytes, the formation of non-perfusion areas and vascular leakage, ultimately leading to a loss of vascular stability and the formation of pathological blood vessels. In response to these mechanisms, DP, especially sulfated polysaccharides, have been regarded as potent candidates for anti-RNV therapy due to their low cytotoxicity and significant antiangiogenic activity, as well as their unique advantages in inhibiting the formation of RNV at multiple targets. For example,: (1) Direct binding to VEGF: SPR analysis shows that JCS1S2’s affinity for VEGF (KD = 4.82 × 10−9) is much higher than that of VEGFR2 (KD = 1.50 × 10−7) and higher than that of some clinical anti-VEGF drugs (e.g., bevacizumab with KD = 58 pM) (Papadopoulos et al., 2012); (2) Remarkable in vivo efficacy: intravitreal injection of JCS1S2 reduces VEGF-A expression by about 60% and reduces the area of neovascularization by about 50% in the OIR model (Bai et al., 2025); (3) High safety: no toxicity for HUVECs at concentrations of 50–800 μg/mL (Li B. et al., 2021). Despite the growing evidence of the anti-RNV properties of DP, several challenges remain in this area of research.
DP derived from various plant species exhibits differences in extraction methods, molecular weight, glycosidic linkage types, and monosaccharide composition. These variations constitute a primary reason for the inconsistency observed in their bioactivities, posing significant challenges to elucidating their structure-activity relationships. The key limitations include (1) Debate on critical functional groups: Sulfation is widely recognized as a key modification enhancing anti-angiogenic activity. However, quantitative consensus regarding the impact of sulfate group position (C-2 or C-6) and degree of substitution (DS, ranging from 0.9 to 1.74) on bioactivity remains elusive across different studies. However, the influence of the sulphate position (C-2 or C-6) and the degree of substitution (0.9–1.74) on activity is inconsistent across studies, making standardized comparisons of biological activity difficult (Li et al., 2014; Qian et al., 2014; Yue et al., 2017). (2) Impact of structural heterogeneity: While DP from different botanical sources may possess variations in minor monosaccharide constituents or specific glycosidic bond structures, the extent to which these structural differences significantly influence their anti-angiogenic potency is poorly understood. This uncertainty makes it almost impossible to make meaningful comparisons or draw general conclusions based on the “structure-activity relationships” described in the various studies. (3) Molecular weight-activity correlation: High-MW polysaccharides, characterized by extended chain length and complex conformation, potentially exhibit higher VEGF binding efficiency. Conversely, low-MW polysaccharides demonstrate superior capacity to penetrate the BRB, thereby facilitating antioxidant effects and barrier protection (Yue et al., 2017; Wang et al., 2022). (4) Lack of standardized extraction: Different extraction techniques (e.g., ultrasound-assisted, enzymatic, or acid extraction) can induce variations in MW, glycosidic bond conformation, and the degree of functional group modifications. These alterations consequently affect the resulting biological activity profiles. Future studies should therefore systematically evaluate the specific influence of sulfate degree, localization and molecular weight on the antiangiogenic activity of DP using standardized extraction protocols, structure characterization and activity assessment to ensure the reliability of polysaccharide assays.
Although DP demonstrates significant anti-RNV activity in animal models (e.g., OIR rats) and in vitro assays (e.g., HUVEC cells), its reliability may decrease because of small sample sizes and the use of a single model and the short treatment duration. Furthermore, the effectiveness on real-life clinical outcomes, like functional outcomes (e.g., visual improvement, vascular leakage reduction), is currently unknown. Otherwise, although DP exhibits nontoxicity in vitro and experimental animals, not many studies refer to the long-term toxicity of DP and its clinical use. Most toxicity studies are based on HUVEC cell models, do not investigate effects on other cell types (e.g., retinal pigment epithelial cells, Müller cells, neurons), and do not consider long-term effects. Therefore, future research should focus on comparative studies between different animal species, especially by selecting animal and cell models that mimic human retinal pathology to increase the clinical value of the studies.
DP has shown favorable bioactivity in recent studies, but the problem of their low bioavailability after oral administration due to their high molecular weight and viscosity remains unresolved (Li et al., 2019; Wu et al., 2022). According to the existing hypothesis of the intestinal-gut axis, DP in appropriate concentrations modulates the integrity of the intestinal barrier, alters the composition of the gut microbiota, and restores the metabolic homeostasis of the intestinal environment and systemic circulation, thus exerting bioactivity indirectly. Studies have shown that in the presence of polysaccharide utilization loci (PULs), DP may be degraded by the phylum Bacteroidetes and the genus Prevotella into small molecules such as manno-oligosaccharides, mannose, and SCFAs, thereby indirectly affecting the retinal microenvironment (Li et al., 2019; Lei et al., 2022; Zhao et al., 2023). However, these studies lack data on the retinal degradation of DP, and it remains unclear whether it crosses the BRB. Consequently, future investigations should integrate metagenomics and metabolomics to determine how gut microbiota-derived metabolites of DP (e.g., SCFAs, oligosaccharides) regulate the retinal microenvironment through circulatory or neural signaling. Furthermore, highly sensitive tracking techniques using isotope labeling and imaging would be required to look at the possibility of oral DP and/or its key metabolites reaching the retina and their concentrations in the upcoming research. In terms of clinical applications, based on standardized DP, the relationship between dose and effect, the impact of individual microbiota variations on efficacy, and the consistency of efficacy across different species, should be validated through more in vivo and clinical trials.
Furthermore, the optimal clinical delivery route for DP needs to be further investigated. While oral administration offers enhanced patient compliance and the potential to indirectly modulate the retinal microenvironment via the gut-retina axis, this route is significantly limited by low bioavailability and a strong dependence on gut microbiota composition and activity. Intravitreal injection or ocular implants can bypass the BRB, delivering drugs directly to the site of pathology with controlled dosages and rapid onset of action. This approach addresses the issue of poor bioavailability. However, these methods are invasive and may induce immune responses or cause vitreous opacities due to the large molecular size of polysaccharides. Additionally, no data on the ocular tolerance of DP are available at present, necessitating further evaluation of dose-dependent toxicity and long-term safety. Designing nanoparticles capable of crossing the BRB, such as liposomes, exosomes, or chitosan nanoparticles, encapsulating large molecular polysaccharides, can prevent degradation in the gastrointestinal tract, enhance blood concentration, and facilitate delivery to the retina via systemic or local administration. This approach may enable low-toxicity and long-lasting therapeutic effects of polysaccharides. However, the long-term toxicity of nanoparticle carriers has yet to be evaluated, and the production process is complex. Additionally, the in vivo metabolic pathways remain unclear, necessitating careful assessment of the safety of the carriers. Structural modifications, such as reducing molecular weight, increasing water solubility, or altering glycosidic bond structures, can enhance the transmembrane absorption of DP. However, these modifications may alter the natural conformation of the polysaccharides. And, there is currently a lack of systematic studies to assess the retention of activity and the potential new toxicity of the modified products. Therefore, a balance between structural modification and safety must be maintained. Furthermore, co-administration of probiotics and prebiotics as a pretreatment may help improve the bioavailability of DP. Tailoring dietary plans based on individual gut microbiomes could further optimize the biological activity of DP.
In summary, DP has been regarded as a promising therapeutic candidate for the treatment of retinal diseases due to its unique polypharmacological properties: inhibition of angiogenesis, immunomodulatory and ROS-reducing ability, combined with low cytotoxicity in preclinical studies. The follow-up study should cover the existing gaps in the study area. For instance, the regulatory mechanisms of DP in VEGF-independent metabolic pathways should be further investigated. The human retinal organoid model should be created, and the combination of tracking techniques and multiomic studies should be used to investigate the accumulation and metabolism of DP in the retina. Computer-aided design should be used to predict the binding conformations of DP-VEGF, and standardized methods of extraction, purification, and specific structural characterization should be used for accurate structural optimization. Phase I clinical trials should be directed at finding the advantages and disadvantages of the different routes of administration prescribed (oral, injection, nanoparticle carriers, etc.) and their long-term toxicity. The development and optimization of combined oral and ocular strategies should be investigated to improve clinical applicability and ultimately ensure sustainable treatment of retinal diseases.
Author contributions
DL: Conceptualization, Investigation, Writing – original draft. XC: Writing – review and editing. SC: Supervision, Visualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China Project 82460211, and Guizhou Science and Technology Cooperation support [2023] general 265.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amălinei, C., Căruntu, I. D., Giuşcă, S. E., and Bălan, R. A. (2010). Matrix metalloproteinases involvement in pathologic conditions. Romanian J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 51, 215–228. Available online at: https://pubmed.ncbi.nlm.nih.gov/20495735/.
Arboleda-Velasquez, J. F., Valdez, C. N., Marko, C. K., and D’Amore, P. A. (2015). From pathobiology to the targeting of pericytes for the treatment of diabetic retinopathy. Curr. Diab. Rep. 15, 573. doi:10.1007/s11892-014-0573-2
Arrigo, A., and Bandello, F. (2021). Molecular features of classic retinal drugs, retinal therapeutic targets and emerging treatments. Pharmaceutics 13, 1102. doi:10.3390/pharmaceutics13071102
Augustin, H. G., Koh, G. Y., Thurston, G., and Alitalo, K. (2009). Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 10, 165–177. doi:10.1038/nrm2639
Bai, X., Xu, Q., Zhang, W., and Wang, C. (2023). The gut-eye axis: correlation between the gut microbiota and autoimmune dry eye in individuals with Sjögren syndrome. Eye Contact Lens 49, 1–7. doi:10.1097/ICL.0000000000000953
Bai, Y., Wang, R., Jin, C., Wang, L., Tang, Y., Wang, H., et al. (2025). Impacts of sulfate polysaccharide JCS1S2 on retinal neovascularization in oxygen-induced retinopathy rats. Front. Pharmacol. 16, 1499420. doi:10.3389/fphar.2025.1499420
Batliwala, S., Xavier, C., Liu, Y., Wu, H., and Pang, I. H. (2017). Involvement of Nrf2 in ocular diseases. Oxid. Med. Cell. Longev. 2017, 1703810. doi:10.1155/2017/1703810
Bertolini, A., Fiorotto, R., and Strazzabosco, M. (2022). Bile acids and their receptors: modulators and therapeutic targets in liver inflammation. Semin. Immunopathol. 44, 547–564. doi:10.1007/s00281-022-00935-7
Bu, W. C., Yu, Y. N., and Cheng, J. M. (2019). Overview of the pharmacological activity of Dendrobium polysaccharides. Chin. J. Ethnomedicine Ethnopharmacy 28, 61–64. doi:10.3969/j.issn.1007-8517.2019.2.zgmzmjyyzz201902017
Cabral-Pacheco, G. A., Garza-Veloz, I., Castruita-De la Rosa, C., Ramirez-Acuña, J. M., Perez-Romero, B. A., Guerrero-Rodriguez, J. F., et al. (2020). The roles of matrix metalloproteinases and their inhibitors in human diseases. Int. J. Mol. Sci. 21, 9739. doi:10.3390/ijms21249739
Cao, X., Di, Y., Tian, Y. J., Huang, X. B., Zhou, Y., Zhang, D. M., et al. (2025). Sodium butyrate inhibits activation of ROS/NF-κB/NLRP3 signaling pathway and angiogenesis in human retinal microvascular endothelial cells. Int. Ophthalmol. 45, 108. doi:10.1007/s10792-025-03458-w
Carmeliet, P., Moons, L., Luttun, A., Vincenti, V., Compernolle, V., De Mol, M., et al. (2001). Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 7, 575–583. doi:10.1038/87904
Chen, H., Nie, Q., Hu, J., Huang, X., Yin, J., and Nie, S. (2021). Multiomics approach to explore the amelioration mechanisms of glucomannans on the metabolic disorder of type 2 diabetic rats. J. Agric. Food Chem. 69, 2632–2645. doi:10.1021/acs.jafc.0c07871
Chen, H., Shi, X., Zhang, L., Yao, L., Cen, L., Li, L., et al. (2022). Ultrasonic extraction process of polysaccharides from Dendrobium nobile Lindl.: optimization, physicochemical properties and anti-inflammatory activity. Foods Basel Switz. 11, 2957. doi:10.3390/foods11192957
Chen, L., He, C., Zhou, M., Long, J., and Li, L. (2022). Research progress on the mechanisms of polysaccharides against gastric cancer. Mol. Basel Switz. 27, 5828. doi:10.3390/molecules27185828
Chen, L., He, X., Wang, H., Fang, J., Zhang, Z., Zhu, X., et al. (2023). Dendrobium officinale polysaccharide prevents neuronal apoptosis via TET2-dependent DNA demethylation in high-fat diet-induced diabetic mice. Int. J. Biol. Macromol. 233, 123288. doi:10.1016/j.ijbiomac.2023.123288
Chen, L., Zhang, S., Liu, X., Dai, J., Yan, Z., and Wu, C. (2020). Effects of Dendrobium polysaccharides on human brain microvascular endothelial cell injury induced by ox-LDL via regulating the miR-378 expression. Cell. Mol. Biol. Noisy--Gd. Fr. 66, 66–71. doi:10.14715/cmb/2020.66.7.11
Chen, S., Wang, J., Xue, C., Li, H., Sun, B., Xue, Y., et al. (2010). Sulfation of a squid ink polysaccharide and its inhibitory effect on tumor cell metastasis. Carbohydr. Polym. 81, 560–566. doi:10.1016/j.carbpol.2010.03.009
Chen, T. E., Lo, J., Huang, S. P., Chang, K. C., Liu, P. L., Wu, H. E., et al. (2024). Glaucine inhibits hypoxia-induced angiogenesis and attenuates LPS-induced inflammation in human retinal pigment epithelial ARPE-19 cells. Eur. J. Pharmacol. 981, 176883. doi:10.1016/j.ejphar.2024.176883
Chen, W., Wu, J., Li, X., Lu, J., Wu, W., Sun, Y., et al. (2021). Isolation, structural properties, bioactivities of polysaccharides from Dendrobium officinale Kimura et. Migo: a review. Int. J. Biol. Macromol. 184, 1000–1013. doi:10.1016/j.ijbiomac.2021.06.156
Chen, X., Chen, C., and Fu, X. (2023). Dendrobium officinale polysaccharide alleviates type 2 diabetes mellitus by restoring gut microbiota and repairing intestinal barrier via the LPS/TLR4/TRIF/NF-kB Axis. J. Agric. Food Chem. 71, 11929–11940. doi:10.1021/acs.jafc.3c02429
Chen, X., Xiao, F., Wang, Y., Fang, J. P., and Ding, K. (2012). Structure-activity relationship study of WSS25 derivatives with anti-angiogenesis effects. Glycoconj. J. 29, 389–398. doi:10.1007/s10719-012-9424-z
Chen, Z. Y., Yan, M. T., Dong, Y. J., Su, J., Yu, J. J., Lin, Q. W., et al. (2024). Effect of Dendrobii officinalis Caulis water extract on hyperviscosity rats based on TLR4/NF-κB/ICAM-1 signaling pathway. China J. Chin. Mater. Medica 49, 5261–5272. doi:10.19540/j.cnki.cjcmm.20240604.401
Cheung, N., Mitchell, P., and Wong, T. Y. (2010). Diabetic retinopathy. Lancet Lond. Engl. 376, 124–136. doi:10.1016/S0140-6736(09)62124-3
Ciurariu, E., Tirziu, A. T., Varga, N. I., Hirtie, B., Alexandru, A., Ivan, C. S., et al. (2025). Short-chain fatty acids and the gut-retina connection: a systematic review. Int. J. Mol. Sci. 26, 2470. doi:10.3390/ijms26062470
Claesson-Welsh, L. (2012). Blood vessels as targets in tumor therapy. Ups. J. Med. Sci. 117, 178–186. doi:10.3109/03009734.2012.660550
Crespo-Garcia, S., Corkhill, C., Roubeix, C., Davids, A. M., Kociok, N., Strauss, O., et al. (2017). Inhibition of placenta growth factor reduces subretinal mononuclear phagocyte accumulation in choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 58, 4997–5006. doi:10.1167/iovs.16-21283
Deng, Y., Chen, L., Han, B., Wu, D., Cheong, K., Chen, N., et al. (2016). Qualitative and quantitative analysis of specific polysaccharides in Dendrobium huoshanense by using saccharide mapping and chromatographic methods. J. Pharm. Biomed. Anal. 129, 163–171. doi:10.1016/j.jpba.2016.06.051
Deng, Y., Ge, X., Li, Y., Zou, B., Wen, X., Chen, W., et al. (2021). Identification of an intraocular microbiota. Cell Discov. 7, 13. doi:10.1038/s41421-021-00245-6
Deng, Y., Li, M., Chen, L. X., Chen, X. Q., Lu, J. H., Zhao, J., et al. (2018). Chemical characterization and immunomodulatory activity of acetylated polysaccharides from Dendrobium devonianum. Carbohydr. Polym. 180, 238–245. doi:10.1016/j.carbpol.2017.10.026
Fan, C., Sun, X., Wang, X., and Yu, H. (2023). Therapeutic potential of the chemical composition of Dendrobium nobile Lindl. Front. Pharmacol. 14, 1163830. doi:10.3389/fphar.2023.1163830
Fan, H., Meng, Q., Xiao, T., and Zhang, L. (2018). Partial characterization and antioxidant activities of polysaccharides sequentially extracted from Dendrobium officinale. J. Food Meas. Charact. 12, 1054–1064. doi:10.1007/s11694-018-9721-8
Fan, X., Han, J., Zhu, L., Chen, Z., Li, J., Gu, Y., et al. Protective Activities of Dendrobium huoshanense C. Z (2020). Protective Activities of Dendrobium huoshanense C. Z. Tang et S. J. Cheng Polysaccharide against High-Cholesterol Diet-Induced Atherosclerosis in Zebrafish. Oxid. Med. Cell. Longev. 2020, 8365056. doi:10.1155/2020/8365056
Feng, C., Cao, L., Luo, D., Ju, L., Yang, J., Xu, X., et al. (2019). Dendrobium polysaccharides attenuate cognitive impairment in senescence-accelerated mouse prone 8 mice via modulation of microglial activation. Brain Res. 1704, 1–10. doi:10.1016/j.brainres.2018.09.030
Feng, Y. Q., Zhang, J. X., Wen, C. T., Dzah, C. S., Juliet, I. C., Duan, Y. Q., et al. (2020). Recent advances in Agaricus bisporus polysaccharides: extraction, purification, physicochemical characterization and bioactivities. Process Biochem. 94, 39–50. doi:10.1016/j.procbio.2020.04.010
Fiorentino, L., Cavalera, M., Menini, S., Marchetti, V., Mavilio, M., Fabrizi, M., et al. (2013). Loss of TIMP3 underlies diabetic nephropathy via FoxO1/STAT1 interplay. EMBO Mol. Med. 5, 441–455. doi:10.1002/emmm.201201475
Fu, X., Chen, S., Xian, S., Wu, Q., Shi, J., and Zhou, S. (2023). Dendrobium and its active ingredients: emerging role in liver protection. Biomed. Pharmacother. Biomedecine Pharmacother. 157, 114043. doi:10.1016/j.biopha.2022.114043
Fu, Z., Sun, Y., Cakir, B., Tomita, Y., Huang, S., Wang, Z., et al. (2020). Targeting neurovascular interaction in retinal disorders. Int. J. Mol. Sci. 21, 1503. doi:10.3390/ijms21041503
Gong, C., Yu, Z., Lu, B., Yang, L., Sheng, Y., Fan, Y., et al. (2014). Ethanol extract of Dendrobium chrysotoxum Lindl ameliorates diabetic retinopathy and its mechanism. Vasc. Pharmacol. 62, 134–142. doi:10.1016/j.vph.2014.04.007
Gong, Q., Zhang, R., Wei, F., Fang, J., Zhang, J., Sun, J., et al. (2022). SGLT2 inhibitor-empagliflozin treatment ameliorates diabetic retinopathy manifestations and exerts protective effects associated with augmenting branched chain amino acids catabolism and transportation in db/db mice. Biomed. Pharmacother. Biomedecine Pharmacother. 152, 113222. doi:10.1016/j.biopha.2022.113222
Grossniklaus, H. E., Kang, S. J., and Berglin, L. (2010). Animal models of choroidal and retinal neovascularization. Prog. Retin. Eye Res. 29, 500–519. doi:10.1016/j.preteyeres.2010.05.003
Grossniklaus, H. E., Ling, J. X., Wallace, T. M., Dithmar, S., Lawson, D. H., Cohen, C., et al. (2002). Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol. Vis. 8, 119–126. Available online at: http://www.molvis.org/molvis/v8/a16/.
Guo, L., Yang, Y., Pu, Y., Mao, S., Nie, Y., Liu, Y., et al. (2024). Dendrobium officinale Kimura and Migo polysaccharide and its multilayer emulsion protect skin photoaging. J. Ethnopharmacol. 318, 116974. doi:10.1016/j.jep.2023.116974
Hagberg, C. E., Falkevall, A., Wang, X., Larsson, E., Huusko, J., Nilsson, I., et al. (2010). Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature 464, 917–921. doi:10.1038/nature08945
Hagbi-Levi, S., Grunin, M., Jaouni, T., Tiosano, L., Rinsky, B., Elbaz-Hayoun, S., et al. (2017). Proangiogenic characteristics of activated macrophages from patients with age-related macular degeneration. Neurobiol. Aging 51, 71–82. doi:10.1016/j.neurobiolaging.2016.11.018
Han, C., Zhai, L., Shen, H., Wang, J., and Guan, Q. (2023). Advanced glycation end-products (AGEs) promote endothelial cell pyroptosis under cerebral ischemia and hypoxia via HIF-1α-RAGE-NLRP3. Mol. Neurobiol. 60, 2355–2366. doi:10.1007/s12035-023-03228-8
He, L., Yan, X., Liang, J., Li, S., He, H., Xiong, Q., et al. (2018). Comparison of different extraction methods for polysaccharides from Dendrobium officinale stem. Carbohydr. Polym. 198, 101–108. doi:10.1016/j.carbpol.2018.06.073
He, Y., Jiang, P., Bian, M., Xu, G., Huang, S., and Sun, C. (2024). Structural characteristics and anti-tumor effect of low molecular weight Dendrobium officinale polysaccharides by reconstructing tumor microenvironment. J. Funct. Foods 119, 106314. doi:10.1016/j.jff.2024.106314
Heloterä, H., and Kaarniranta, K. (2022). A linkage between angiogenesis and inflammation in neovascular age-related macular degeneration. Cells 11, 3453. doi:10.3390/cells11213453
Hori, Y., Ito, K., Hamamichi, S., Ozawa, Y., Matsui, J., Umeda, I. O., et al. (2017). Functional characterization of VEGF- and FGF-induced tumor blood vessel models in human cancer xenografts. Anticancer Res. 37, 6629–6638. doi:10.21873/anticanres.12120
Hsieh, Y. S. Y., Chien, C., Liao, S. K. S., Liao, S., Hung, W. T., Yang, W., et al. (2008). Structure and bioactivity of the polysaccharides in medicinal plant Dendrobium huoshanense. Bioorg. Med. Chem. 16, 6054–6068. doi:10.1016/j.bmc.2008.04.042
Hua, Y. F., Zhang, M., Fu, C. X., Chen, Z. H., and Chan, G. Y. S. (2004). Structural characterization of a 2-O-acetylglucomannan from Dendrobium officinale stem. Carbohydr. Res. 339, 2219–2224. doi:10.1016/j.carres.2004.05.034
Huang, Z., and Bao, S. D. (2004). Roles of main pro- and anti-angiogenic factors in tumor angiogenesis. World J. Gastroenterol. 10, 463–470. doi:10.3748/wjg.v10.i4.463
Jiang, B., Tian, S., Li, T., Li, C. X., and Zhou, X. D. (2024). Experimental study of the inhibition of gigantol on corneal neovascularization in rats by alkali burn. Acta Lab. Anim. Sci. Sin. 32, 846–855. doi:10.3969/j.issn.1005-4847.2024.07.004
Jin, C., Du, Z., Lin, L., Zhou, L., Li, S., Liu, Q., et al. (2017). Structural characterization of mannoglucan from Dendrobium nobile Lindl and the neuritogenesis-induced effect of its acetylated derivative on PC-12 cells. Polymers 9, 399. doi:10.3390/polym9090399
Katoh, K. (2024). Signal transduction mechanisms of focal adhesions: src and FAK-mediated cell response. Front. Biosci. Landmark Ed. 29, 392. doi:10.31083/j.fbl2911392
Kundu, S., Bansal, S., Muthukumarasamy, K. M., Sachidanandan, C., Motiani, R. K., and Bajaj, A. (2017). Deciphering the role of hydrophobic and hydrophilic bile acids in angiogenesis using in vitro and in vivo model systems. Medchemcomm 8, 2248–2257. doi:10.1039/c7md00475c
Kusuhara, S., Fukushima, Y., Ogura, S., Inoue, N., and Uemura, A. (2018). Pathophysiology of diabetic retinopathy: the old and the new. Diabetes Metab. J. 42, 364–376. doi:10.4093/dmj.2018.0182
Lei, X., Zeng, X., Liu, Y., Wu, Z., Zheng, X., and Zhang, X. (2023). Inhibitory effect of Dendrobium officinale polysaccharide on oxidative damage of glial cells in aging mice by regulating gut microbiota. Int. J. Biol. Macromol. 247, 125787 doi:10.1016/j.ijbiomac.2023.125787
Lei, X. C., Huo, P., Xie, Y. J., Wang, Y. H., Liu, G. H., Tu, H. Y., et al. (2022). Dendrobium nobile Lindl polysaccharides improve testicular spermatogenic function in streptozotocin-induced diabetic rats. Mol. Reprod. Dev. 89, 202–213. doi:10.1002/mrd.23556
Li, J. (2016). Effects of polysaccharides of Dendrobium Candidum on protects of retinal in diabetic rats. Master’s thesis. Bengbu: Bengbu Medical University.
Li, J. W., Li, G. W., Qin, Y., and Li, C. X. (2016). Effects of polysaccharides of Dendrobium candidum on overexpression of inflammatory factors in diabetic rats with retinopathy. China J. Chin. Ophthalmol. 26, 7–11. doi:10.13444/j.cnki.zgzyykzz.2016.01.003
Li, L., Chen, H., Huang, G., Lv, Y., Yao, L., Guo, Z., et al. (2024). Structure of polysaccharide from Dendrobium nobile Lindl. And its mode of action on TLR4 to exert immunomodulatory effects. Foods Basel Switz. 13, 1356. doi:10.3390/foods13091356
Li, L., Yao, H., Li, X., Zhang, Q., Wu, X., Wong, T., et al. (2019). Destiny of Dendrobium officinale polysaccharide after oral administration: indigestible and nonabsorbing, ends in modulating gut microbiota. J. Agric. Food Chem. 67 (21), 5968–5977. doi:10.1021/acs.jafc.9b01489
Li, M. Z., Huang, X. J., Hu, J. L., Cui, S. W., Xie, M. Y., and Nie, S. P. (2020). The protective effects against cyclophosphamide (CTX)-induced immunosuppression of three glucomannans. Food Hydrocoll. 100, 105445. doi:10.1016/j.foodhyd.2019.105445
Li, X., and Hong, M. (2020). Aqueous extract of Dendrobium officinale confers neuroprotection against hypoxic-ischemic brain damage in neonatal rats. Kaohsiung J. Med. Sci. 36, 43–53. doi:10.1002/kjm2.12139
Li, X., Zhang, Q., Zhu, Y., Li, Y., Mei, S., Luo, H., et al. (2022). Structural characterization of a mannoglucan polysaccharide from Dendrobium huoshanense and evaluation of its osteogenesis promotion activities. Int. J. Biol. Macromol. 211, 441–449. doi:10.1016/j.ijbiomac.2022.05.036
Li, X. L., Xiao, J. J., Zha, X. Q., Pan, L. H., Asghar, M. N., and Luo, J. P. (2014). Structural identification and sulfated modification of an antiglycation Dendrobium huoshanense polysaccharide. Carbohydr. Polym. 106, 247–254. doi:10.1016/j.carbpol.2014.02.029
Li, Y., Cao, Z., Jia, L., Huang, Y., Shi, M., and Li, Q. (2021). Regulation of Dendrobium polysaccharides on proliferation and oxidative stress of human umbilical vein endothelial cells in the high glucose environment. J. Diabetes Res. 2021, 6685055. doi:10.1155/2021/6685055
Li, Y., Zhu, L., Cai, M. X., Wang, Z. L., Zhuang, M., Tan, C. Y., et al. (2023). TGR5 supresses cGAS/STING pathway by inhibiting GRP75-mediated endoplasmic reticulum-mitochondrial coupling in diabetic retinopathy. Cell Death Dis. 14, 583. doi:10.1038/s41419-023-06111-5
Liang, J., Li, H., Chen, J., He, L., Du, X., Zhou, L., et al. (2019). Dendrobium officinale polysaccharides alleviate colon tumorigenesis via restoring intestinal barrier function and enhancing anti-tumor immune response. Pharmacol. Res. 148, 104417. doi:10.1016/j.phrs.2019.104417
Li, B., He, X., Jin, H., Wang, H., Zhou, F., Zhang, N., et al. (2021). Beneficial effects of Dendrobium officinale on metabolic hypertensive rats by triggering the enteric-origin SCFA-GPCR43/41 pathway. Food Amp Funct. 12, 5524–5538. doi:10.1039/D0FO02890H
Li, H., Zheng, J., Wu, Y., Zhou, H., Zeng, S., and Li, Q. (2023). Dendrobium officinale polysaccharide decreases podocyte injury in diabetic nephropathy by regulating IRS-1/AKT signal and promoting mitophagy. Aging 15, 10291–10306. doi:10.18632/aging.205075
Li, J., Wang, Y. F., Shen, Z. C., Zou, Q., Lin, X. F., and Wang, X. Y. (2023). Recent developments on natural polysaccharides as potential anti-gastric cancer substance: structural feature and bioactivity. Int. J. Biol. Macromol. 232, 123390. doi:10.1016/j.ijbiomac.2023.123390
Lin, G., Luo, D., Liu, J., Wu, X., Chen, J., Huang, Q., et al. (2018). Hepatoprotective effect of polysaccharides isolated from Dendrobium officinale against acetaminophen-induced liver injury in mice via regulation of the Nrf2-Keap1 signaling pathway. Oxid. Med. Cell. Longev. 2018, 6962439. doi:10.1155/2018/6962439
Liu, B., Li, Q. M., Shang, Z. Z., Zha, X. Q., Pan, L. H., and Luo, J. P. (2021). Anti-gastric cancer activity of cultivated Dendrobium huoshanense stem polysaccharide in tumor-bearing mice: effects of molecular weight and O-acetyl group. Int. J. Biol. Macromol. 192, 590–599. doi:10.1016/j.ijbiomac.2021.10.016
Liu, F. Y., Guo, S. X., Lin, Y. Y., Wu, H., Lai, F. R., Liu, J. G., et al. (2021). The relationship between the structure and function of Dendrobium polysaccharides: a review. Mod. Food Sci. Technol. 37, 308–338. doi:10.13982/j.mfst.1673-9078.2021.01.0790
Liu, H., Liang, J., Zhong, Y., Xiao, G., Efferth, T., Georgiev, M. I., et al. (2021). Dendrobium officinale polysaccharide alleviates intestinal inflammation by promoting small extracellular vesicle packaging of miR-433-3p. J. Agric. Food Chem. 69, 13510–13523. doi:10.1021/acs.jafc.1c05134
Liu, H., Xing, Y., Wang, Y., Ren, X., Zhang, D., Dai, J., et al. (2023). Dendrobium officinale polysaccharide prevents diabetes via the regulation of gut microbiota in prediabetic mice. Foods Basel Switz. 12, 2310. doi:10.3390/foods12122310
Liu, Y., Jin, X. D., Jia, B. H., Ma, Y., and Xu, L. M. (2021). Mechanism of inhibition of high glucose-induced apoptosis and inflammatory response in retinal vascular endothelial cells by dendrobenol. Chin. J. Gerontol. 41, 1085–1088. doi:10.3969/j.issn.1005-9202.2021.05.053
Liu, Y., Yang, L., Zhang, Y., Liu, X., Wu, Z., Gilbert, R. G., et al. (2020). Dendrobium officinale polysaccharide ameliorates diabetic hepatic glucose metabolism via glucagon-mediated signaling pathways and modifying liver-glycogen structure. J. Ethnopharmacol. 248, 112308. doi:10.1016/j.jep.2019.112308
Li, Z., Xiang, J., Hu, D., and Song, B. (2020). Naturally potential antiviral agent polysaccharide from Dendrobium nobile Lindl. Pestic. Biochem. Physiol. 167, 104598. doi:10.1016/j.pestbp.2020.104598
Luo, A., He, X., Zhou, S., Fan, Y., He, T., and Chun, Z. (2009). In vitro antioxidant activities of a water-soluble polysaccharide derived from Dendrobium nobile Lindl. extracts. Int. J. Biol. Macromol. 45, 359–363. doi:10.1016/j.ijbiomac.2009.07.008
Ma, M., Gao, L., Chen, C., Gu, F., Wu, S., Li, F., et al. (2023). Dendrobium huoshanense polysaccharide improves high-fat diet induced liver injury by regulating the gut-liver axis. Chem. Biodivers. 20, e202300980. doi:10.1002/cbdv.202300980
Majmundar, A. J., Wong, W. J., and Simon, M. C. (2010). Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 40, 294–309. doi:10.1016/j.molcel.2010.09.022
Mastropasqua, R., D’Aloisio, R., Di Nicola, M., Di Martino, G., Lamolinara, A., Di Antonio, L., et al. (2018). Relationship between aqueous humor cytokine level changes and retinal vascular changes after intravitreal aflibercept for diabetic macular edema. Sci. Rep. 8, 16548. doi:10.1038/s41598-018-35036-9
Melincovici, C. S., Boşca, A. B., Şuşman, S., Mărginean, M., Mihu, C., Istrate, M., et al. (2018). Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Romanian J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 59, 455–467. Available online at: https://pubmed.ncbi.nlm.nih.gov/30173249/.
Meng, L. Z., Lv, G. P., Hu, D. J., Cheong, K. L., Xie, J., Zhao, J., et al. (2013). Effects of polysaccharides from different species of Dendrobium (Shihu) on macrophage function. Mol. Basel Switz. 18, 5779–5791. doi:10.3390/molecules18055779
Mrugacz, M., Bryl, A., and Zorena, K. (2021). Retinal vascular endothelial cell dysfunction and neuroretinal degeneration in diabetic patients. J. Clin. Med. 10, 458. doi:10.3390/jcm10030458
Nathan, C., and Ding, A. (2010). Nonresolving inflammation. Cell 140, 871–882. doi:10.1016/j.cell.2010.02.029
Nicholas, M. P., and Mysore, N. (2021). Corneal neovascularization. Exp. Eye Res. 202, 108363. doi:10.1016/j.exer.2020.108363
Ogura, S., Kurata, K., Hattori, Y., Takase, H., Ishiguro-Oonuma, T., Hwang, Y., et al. (2017). Sustained inflammation after pericyte depletion induces irreversible blood-retina barrier breakdown. JCI Insight 2, e90905. doi:10.1172/jci.insight.90905
Pan, L. H., Li, X. F., Wang, M. N., Zha, X. Q., Yang, X. F., Liu, Z. J., et al. (2014). Comparison of hypoglycemic and antioxidative effects of polysaccharides from four different Dendrobium species. Int. J. Biol. Macromol. 64, 420–427. doi:10.1016/j.ijbiomac.2013.12.024
Papadopoulos, N., Martin, J., Ruan, Q., Rafique, A., Rosconi, M. P., Shi, E., et al. (2012). Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 15, 171–185. doi:10.1007/s10456-011-9249-6
Park, D. Y., Lee, J., Kim, J., Kim, K., Hong, S., Han, S., et al. (2017). Plastic roles of pericytes in the blood-retinal barrier. Nat. Commun. 8, 15296. doi:10.1038/ncomms15296
Penn, J. S., Madan, A., Caldwell, R. B., Bartoli, M., Caldwell, R. W., and Hartnett, M. E. (2008). Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 27, 331–371. doi:10.1016/j.preteyeres.2008.05.001
Qian, X. P., Zha, X. Q., Xiao, J. J., Zhang, H. L., and Luo, J. P. (2014). Sulfated modification can enhance antiglycation abilities of polysaccharides from Dendrobium huoshanense. Carbohydr. Polym. 101, 982–989. doi:10.1016/j.carbpol.2013.10.035
Qin, X., and Zou, H. (2022). The role of lipopolysaccharides in diabetic retinopathy. BMC Ophthalmol. 22, 86. doi:10.1186/s12886-022-02296-z
Raczyńska, D., Lisowska, K. A., Pietruczuk, K., Borucka, J., Ślizień, M., Raczyńska, K., et al. (2018). The level of cytokines in the vitreous body of severe proliferative diabetic retinopathy patients undergoing posterior vitrectomy. Curr. Pharm. Des. 24, 3276–3281. doi:10.2174/1381612824666180926110704
Rashid, K., Akhtar-Schaefer, I., and Langmann, T. (2019). Microglia in retinal degeneration. Front. Immunol. 10, 1975. doi:10.3389/fimmu.2019.01975
Rattner, A., Williams, J., and Nathans, J. (2019). Roles of HIFs and VEGF in angiogenesis in the retina and brain. J. Clin. Invest. 129, 3807–3820. doi:10.1172/JCI126655
Regula, J. T., Lundh von Leithner, P., Foxton, R., Barathi, V. A., Cheung, C. M. G., Bo Tun, S. B., et al. (2016). Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol. Med. 8, 1265–1288. doi:10.15252/emmm.201505889
R Muralitharan, R., Zheng, T., Dinakis, E., Xie, L., Barbaro-Wahl, A., Jama, H. A., et al. (2025). Gut microbiota metabolites sensed by host GPR41/43 protect against hypertension. Circ. Res. 136, e20–e33. doi:10.1161/CIRCRESAHA.124.325770
Robciuc, M. R., Kivelä, R., Williams, I. M., de Boer, J. F., van Dijk, T. H., Elamaa, H., et al. (2016). VEGFB/VEGFR1-induced expansion of adipose vasculature counteracts obesity and related metabolic complications. Cell Metab. 23, 712–724. doi:10.1016/j.cmet.2016.03.004
Seghezzi, G., Patel, S., Ren, C. J., Gualandris, A., Pintucci, G., Robbins, E. S., et al. (1998). Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. J. Cell Biol. 141, 1659–1673. doi:10.1083/jcb.141.7.1659
Shi, Y., Zhou, L., Zheng, G., Jing, Y., Zhang, X., Yuan, J., et al. (2023). Therapeutic mechanism exploration of polysaccharides from Dendrobium officinale on unilateral ureteral obstruction operation-induced renal fibrosis based on improving oxidative stress injury mediated by AhR/NOX4 pathway. Int. J. Biol. Macromol. 253, 126920. doi:10.1016/j.ijbiomac.2023.126920
Simons, M., Gordon, E., and Claesson-Welsh, L. (2016). Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 17, 611–625. doi:10.1038/nrm.2016.87
Sounni, N. E., Devy, L., Hajitou, A., Frankenne, F., Munaut, C., Gilles, C., et al. (2002). MT1-MMP expression promotes tumor growth and angiogenesis through an up-regulation of vascular endothelial growth factor expression. FASEB J. 16, 555–564. doi:10.1096/fj.01-0790com
Tang, L., Xu, G. T., and Zhang, J. F. (2023). Inflammation in diabetic retinopathy: possible roles in pathogenesis and potential implications for therapy. Neural Regen. Res. 18, 976–982. doi:10.4103/1673-5374.355743
Tao, S., Lei, Z. X., Huang, K. W., Li, Y. R., Ren, Z. Y., Zhang, X. F., et al. (2019). Structural characterization and immunomodulatory activity of two novel polysaccharides derived from the stem of Dendrobium officinale Kimura et Migo. J. Funct. Foods 57, 121–134. doi:10.1016/j.jff.2019.04.013
Thakur, P. S., Aggarwal, D., Takkar, B., Shivaji, S., and Das, T. (2022). Evidence suggesting the role of gut dysbiosis in diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 63, 21. doi:10.1167/iovs.63.8.21
Thounaojam, M. C., Jadeja, R. N., Rajpurohit, S., Gutsaeva, D. R., Stansfield, B. K., Martin, P. M., et al. (2020). Ursodeoxycholic acid halts pathological neovascularization in a mouse model of oxygen-induced retinopathy. J. Clin. Med. 9, 1921. doi:10.3390/jcm9061921
Tian, Y., Fopiano, K. A., Buncha, V., Lang, L., Rudic, R. D., Filosa, J. A., et al. (2022). Aging-induced impaired endothelial wall shear stress mechanosensing causes arterial remodeling via JAM-A/F11R shedding by ADAM17. GeroScience 44, 349–369. doi:10.1007/s11357-021-00476-1
Tîrziu, A. T., Susan, M., Susan, R., Sonia, T., Harich, O. O., Tudora, A., et al. (2024). From gut to eye: exploring the role of microbiome imbalance in ocular diseases. J. Clin. Med. 13, 5611. doi:10.3390/jcm13185611
Tu, Y., Luo, Y., Zhao, Q., Zeng, Y., Leng, K., and Zhu, M. (2024). Role of macrophage in ocular neovascularization. Heliyon 10, e30840. doi:10.1016/j.heliyon.2024.e30840
Ucuzian, A. A., Gassman, A. A., East, A. T., and Greisler, H. P. (2010). Molecular mediators of angiogenesis. J. Burn Care Res. 31, 158–175. doi:10.1097/BCR.0b013e3181c7ed82
Uemura, A., Fruttiger, M., D’Amore, P. A., De Falco, S., Joussen, A. M., Sennlaub, F., et al. (2021). VEGFR1 signaling in retinal angiogenesis and microinflammation. Prog. Retin. Eye Res. 84, 100954. doi:10.1016/j.preteyeres.2021.100954
Valdez, C. N., Arboleda-Velasquez, J. F., Amarnani, D. S., Kim, L. A., and D’Amore, P. A. (2014). Retinal microangiopathy in a mouse model of inducible mural cell loss. Am. J. Pathol. 184, 2618–2626. doi:10.1016/j.ajpath.2014.06.011
Vimalraj, S. (2022). A concise review of VEGF, PDGF, FGF, Notch, angiopoietin, and HGF signalling in tumor angiogenesis with a focus on alternative approaches and future directions. Int. J. Biol. Macromol. 221, 1428–1438. doi:10.1016/j.ijbiomac.2022.09.129
Wang, C., Xu, L., Guo, X., Cui, X., and Yang, Y. (2018). Optimization of the extraction process of polysaccharides from Dendrobium officinale and evaluation of the in vivo immunmodulatory activity. J. Food Process. Preserv. 42, e13598. doi:10.1111/jfpp.13598
Wang, F., Yuan, C., Deng, R., and Liu, Y. (2025). Multi-omics analysis reveals the pre-protective mechanism of Dendrobium flexicaule polysaccharide against alcohol-induced gastric mucosal injury. Int. J. Biol. Macromol. 291, 139191. doi:10.1016/j.ijbiomac.2024.139191
Wang, H. Y., Li, Q. M., Yu, N. J., Chen, W. D., Zha, X. Q., Wu, D. L., et al. (2019). Dendrobium huoshanense polysaccharide regulates hepatic glucose homeostasis and pancreatic β-cell function in type 2 diabetic mice. Carbohydr. Polym. 211, 39–48. doi:10.1016/j.carbpol.2019.01.101
Wang, K., Wang, H., Liu, Y., Shui, W., Wang, J., Cao, P., et al. (2018). Dendrobium officinale polysaccharide attenuates type 2 diabetes mellitus via the regulation of PI3K/Akt-mediated glycogen synthesis and glucose metabolism. J. Funct. Foods 40, 261–271. doi:10.1016/j.jff.2017.11.004
Wang, K., Yang, X., Wu, Z., Wang, H., Li, Q., Mei, H., et al. (2020). Dendrobium officinale polysaccharide protected CCl(4)-induced liver fibrosis through intestinal homeostasis and the LPS-TLR4-NF-κB signaling pathway. Front. Pharmacol. 11, 240. doi:10.3389/fphar.2020.00240
Wang, K. W., Yang, C., Yan, S. N., Wang, H., Cao, X. J., and Cheng, Y. (2022). Dendrobium hancockii polysaccharides, structure characterization, modification, antioxidant and antibacterial activity. Ind. CROPS Prod. 188, 115565. doi:10.1016/j.indcrop.2022.115565
Wang, L. X., Li, C. Y., Hu, C., Gong, P. S., and Zhao, S. H. (2021). Purification and structural characterization of Dendrobium officinale polysaccharides and its activities. Chem. Biodivers. 18, e2001023. doi:10.1002/cbdv.202001023
Wang, R., Wang, Q. Y., Bai, Y., Bi, Y. G., and Cai, S. J. (2023). Research progress of diabetic retinopathy and gut microecology. Front. Microbiol. 14, 1256878. doi:10.3389/fmicb.2023.1256878
Wang, Y., Jiang, Z., Deng, L., Zhang, G., Xu, X., Alonge, E., et al. (2023). Dendrobium offificinale polysaccharides prevents glucocorticoids-induced osteoporosis by destabilizing KEAP1-NRF2 interaction. Int. J. Biol. Macromol. 253, 126600. doi:10.1016/j.ijbiomac.2023.126600
Wang, Z., Jin, C., Li, X., and Ding, K. (2019). Sulfated polysaccharide JCS1S2 inhibits angiogenesis via targeting VEGFR2/VEGF and blocking VEGFR2/Erk/VEGF signaling. Carbohydr. Polym. 207, 502–509. doi:10.1016/j.carbpol.2018.11.091
Wang, Z., Tan, W., Li, B., Chen, J., Zhu, J., Xu, F., et al. (2024). LncRNA-MM2P regulates retinal neovascularization through M2 macrophage polarization. Exp. Eye Res. 248, 110072. doi:10.1016/j.exer.2024.110072
Warden, C., and Brantley, M. A. J. (2021). Glycine-conjugated bile acids protect RPE tight junctions against oxidative stress and inhibit choroidal endothelial cell angiogenesis in vitro. Biomolecules 11, 626. doi:10.3390/biom11050626
Watanabe, D., Suzuma, K., Suzuma, I., Ohashi, H., Ojima, T., Kurimoto, M., et al. (2005). Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am. J. Ophthalmol. 139, 476–481. doi:10.1016/j.ajo.2004.10.004
Wei, W., Feng, L., Bao, W. R., Ma, D. L., Leung, C. H., Nie, S. P., et al. (2016). Structure characterization and immunomodulating effects of polysaccharides isolated from Dendrobium officinale. J. Agric. Food Chem. 64, 881–889. doi:10.1021/acs.jafc.5b05180
Win, A., Delgado, A., Jadeja, R. N., Martin, P. M., Bartoli, M., and Thounaojam, M. C. (2021). Pharmacological and metabolic significance of bile acids in retinal diseases. Biomolecules 11, 292. doi:10.3390/biom11020292
Wu, F. T. H., Stefanini, M. O., Mac Gabhann, F., Kontos, C. D., Annex, B. H., and Popel, A. S. (2010). A systems biology perspective on sVEGFR1: its biological function, pathogenic role and therapeutic use. J. Cell. Mol. Med. 14, 528–552. doi:10.1111/j.1582-4934.2009.00941.x
Wu, W., Zhao, Z., Zhao, Z., Zhang, D., Zhang, Q., Zhang, J., et al. (2023). Structure, health benefits, mechanisms, and gut microbiota of Dendrobium officinale polysaccharides: a review. Nutrients 15, 4901. doi:10.3390/nu15234901
Wu, Y. G., Wang, K. W., Zhao, Z. R., Zhang, P., Liu, H., Zhou, G. J., et al. (2019). A novel polysaccharide from Dendrobium devonianum serves as a TLR4 agonist for activating macrophages. Int. J. Biol. Macromol. 133, 564–574. doi:10.1016/j.ijbiomac.2019.04.125
Wu, Z., Zhang, Y., Nie, G., Liu, J., Mei, H., He, Z., et al. (2022). Tracking the gastrointestinal digestive and metabolic behaviour of Dendrobium officinale polysaccharides by fluorescent labelling. Food Funct. 13, 7274–7286. doi:10.1039/d2fo01506d
Xie, H., Fang, J., Farag, M. A., Li, Z., Sun, P., and Shao, P. (2022). Dendrobium officinale leaf polysaccharides regulation of immune response and gut microbiota composition in cyclophosphamide-treated mice. Food Chem. X 13, 100235. doi:10.1016/j.fochx.2022.100235
Xie, S. Z., Liu, B., Ye, H. Y., Li, Q. M., Pan, L. H., Zha, X. Q., et al. (2019). Dendrobium huoshanense polysaccharide regionally regulates intestinal mucosal barrier function and intestinal microbiota in mice. Carbohydr. Polym. 206, 149–162. doi:10.1016/j.carbpol.2018.11.002
Xie, Z., Jiang, N., Lin, M., He, X., Li, B., Dong, Y., et al. (2023). The mechanisms of polysaccharides from tonic Chinese herbal medicine on the enhancement immune function: a review. Mol. Basel Switz. 28, 7355. doi:10.3390/molecules28217355
Xu, W. Q., Yin, J., and Wang, Y. S. (2016). Promoting effect of Toll like receptor 4 on oxygen-induced retinal neovascularization and its mechanism. Chin. J. Exp. Ophthalmol. 34, 317–323. doi:10.3760/cma.j.issn.2095-0160.2016.04.007
Xu, X., Zhang, C., Wang, N., Xu, Y., Tang, G., Xu, L., et al. (2022). Bioactivities and mechanism of actions of Dendrobium officinale: a comprehensive review. Oxid. Med. Cell. Longev. 2022, 6293355. doi:10.1155/2022/6293355
Xue, W., Li, J. J., Zou, Y., Zou, B., and Wei, L. (2021). Microbiota and ocular diseases. Front. Cell. Infect. Microbiol. 11, 759333. doi:10.3389/fcimb.2021.759333
Yang, J., Chen, H., Nie, Q., Huang, X., and Nie, S. (2020). Dendrobium officinale polysaccharide ameliorates the liver metabolism disorders of type II diabetic rats. Int. J. Biol. Macromol. 164, 1939–1948. doi:10.1016/j.ijbiomac.2020.08.007
Yang, J., and Liu, Z. (2022). Mechanistic pathogenesis of endothelial dysfunction in diabetic nephropathy and retinopathy. Front. Endocrinol. 13, 816400. doi:10.3389/fendo.2022.816400
Yang, K., Lu, T., Zhan, L., Zhou, C., Zhang, N., Lei, S., et al. (2020). Physicochemical characterization of polysaccharide from the leaf of Dendrobium officinale and effect on LPS induced damage in GES-1 cell. Int. J. Biol. Macromol. 149, 320–330. doi:10.1016/j.ijbiomac.2020.01.026
Yang, S., Qi, S., and Wang, C. (2022). The role of retinal Müller cells in diabetic retinopathy and related therapeutic advances. Front. Cell Dev. Biol. 10, 1047487. doi:10.3389/fcell.2022.1047487
Yang, X. (2023). Protective effect and mechanism of Dendrobium officinale polysaccharide on liver and intestinal mucosal barrier in rats with CCl4 induced liver fibrosis. PhD thesis. Wuhan: Huazhong University of Science and Technology. Available online at: https://d.wanfangdata.com.cn/thesis/ChhUaGVzaXNOZXdTMjAyNDA5MjAxNTE3MjUSCUQwMzYzNTk3MRoIdjZjbnExdGs%3D.
Yang, X., Zhang, Y., Yang, Y., Lim, S., Cao, Z., Rak, J., et al. (2013). Vascular endothelial growth factor-dependent spatiotemporal dual roles of placental growth factor in modulation of angiogenesis and tumor growth. Proc. Natl. Acad. Sci. U. S. A. 110, 13932–13937. doi:10.1073/pnas.1309629110
Ye, G., Li, J., Zhang, J., Liu, H., Ye, Q., and Wang, Z. (2021). Structural characterization and antitumor activity of a polysaccharide from Dendrobium wardianum. Carbohydr. Polym. 269, 118253. doi:10.1016/j.carbpol.2021.118253
Ye, H. Y., Shang, Z. Z., Gao, X., Zha, X. Q., Zhang, F. Y., Li, Q. M., et al. (2024). Dendrobium huoshanense stem polysaccharide exhibits gastroprotective effect via regulating PI3K/AKT, NF-κB and Nrf-2 signaling in high-salt diet-induced gastritis mice. Food Biosci. 62, 105309. doi:10.1016/j.fbio.2024.105309
Yu, Z., Gong, C., Lu, B., Yang, L., Sheng, Y., Ji, L., et al. (2015). Dendrobium chrysotoxum Lindl. alleviates diabetic retinopathy by preventing retinal inflammation and tight junction protein decrease. J. Diabetes Res. 2015, 518317. doi:10.1155/2015/518317
Yu, Z., Zhang, T., Gong, C., Sheng, Y., Lu, B., Zhou, L., et al. (2016). Erianin inhibits high glucose-induced retinal angiogenesis via blocking ERK1/2-regulated HIF-1α-VEGF/VEGFR2 signaling pathway. Sci. Rep. 6, 34306. doi:10.1038/srep34306
Yue, H., Liu, Y., Qu, H., and Ding, K. (2017). Structure analysis of a novel heteroxylan from the stem of Dendrobium officinale and anti-angiogenesis activities of its sulfated derivative. Int. J. Biol. Macromol. 103, 533–542. doi:10.1016/j.ijbiomac.2017.05.097
Zeng, X., Tang, S., Dong, X., Dong, M., Shao, R., Liu, R., et al. (2024). Analysis of metagenome and metabolome disclosed the mechanisms of Dendrobium officinale polysaccharide on DSS-induced ulcerative colitis-affected mice. Int. J. Biol. Macromol. 277, 134229. doi:10.1016/j.ijbiomac.2024.134229
Zhang, H., and Mo, Y. (2023). The gut-retina axis: a new perspective in the prevention and treatment of diabetic retinopathy. Front. Endocrinol. 14, 1205846. doi:10.3389/fendo.2023.1205846
Zhang, K., Zhou, X., Wang, J., Zhou, Y., Qi, W., Chen, H., et al. (2021). Dendrobium officinale polysaccharide triggers mitochondrial disorder to induce colon cancer cell death via ROS-AMPK-autophagy pathway. Carbohydr. Polym. 264, 118018. doi:10.1016/j.carbpol.2021.118018
Zhang, M., Xu, L., Chen, L., Wu, H., Jia, L., and Zhu, H. (2024). Dendrobium officinale polysaccharides as a natural functional component for acetic-acid-induced gastric ulcers in rats. Mol. Basel Switz. 29, 880. doi:10.3390/molecules29040880
Zhang, T., Ouyang, H., Mei, X., Lu, B., Yu, Z., Chen, K., et al. (2019). Erianin alleviates diabetic retinopathy by reducing retinal inflammation initiated by microglial cells via inhibiting hyperglycemia-mediated ERK1/2-NF-κB signaling pathway. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 33, 11776–11790. doi:10.1096/fj.201802614RRR
Zhang, X., Ge, R., Wu, J., Cai, X., Deng, G., Lv, J., et al. (2024). Structural characterization and improves cognitive disorder in ageing mice of a glucomannan from Dendrobium huoshanense. Int. J. Biol. Macromol. 269, 131995. doi:10.1016/j.ijbiomac.2024.131995
Zhang, X., Yan, Y., Lv, Y., Li, X., Chen, L., Huang, Z., et al. (2022). Dendrobium officinale polysaccharides attenuate uropathogenic Escherichia coli (UPEC)-induced pyroptosis in macrophage cells. Biomed. Pharmacother. Biomedecine Pharmacother. 151, 113098. doi:10.1016/j.biopha.2022.113098
Zhang, Y., Ji, W., Qin, H., Chen, Z., Zhou, Y., Zhou, Z., et al. (2025). Astragalus polysaccharides alleviate DSS-induced ulcerative colitis in mice by restoring SCFA production and regulating Th17/Treg cell homeostasis in a microbiota-dependent manner. Carbohydr. Polym. 349, 122829. doi:10.1016/j.carbpol.2024.122829
Zhang, Y., Wang, H., Mei, N., Ma, C., Lou, Z., Lv, W., et al. (2018). Protective effects of polysaccharide from Dendrobium nobile against ethanol-induced gastric damage in rats. Int. J. Biol. Macromol. 107, 230–235. doi:10.1016/j.ijbiomac.2017.08.175
Zhang, J., Sheng, X., Ding, Q., Wang, Y., Zhao, J., and Zhang, J. (2025). Subretinal fibrosis secondary to neovascular age-related macular degeneration: mechanisms and potential therapeutic targets. Neural Regen. Res. 20, 378–393. doi:10.4103/NRR.NRR-D-23-01642
Zhao, T., Yue, H., Peng, J., Nie, Y., Wu, L., Li, T., et al. (2023). Degradation of xylan by human gut Bacteroides xylanisolvens XB1A. Carbohydr. Polym. 315, 121005. doi:10.1016/j.carbpol.2023.121005
Zhao, Y., Sun, Y., Wang, G., Ge, S., and Liu, H. (2019). Dendrobium officinale polysaccharides protect against MNNG-induced PLGC in rats via activating the NRF2 and antioxidant enzymes HO-1 and NQO-1. Oxid. Med. Cell. Longev. 2019, 9310245. doi:10.1155/2019/9310245
Keywords: dendrobium polysaccharides, retinal neovascularization, vascular endothelial growth factor, antioxidant, anti-inflammatory
Citation: Liu D, Chen X and Cai S (2025) Inhibition of retinal neovascularization by Dendrobium polysaccharides: a review. Front. Pharmacol. 16:1584553. doi: 10.3389/fphar.2025.1584553
Received: 27 February 2025; Accepted: 09 June 2025;
Published: 30 June 2025.
Edited by:
Xu Zhao, Shenyang Pharmaceutical University, ChinaReviewed by:
Yuxiang Fei, China Pharmaceutical University, ChinaPan Long, General Hospital of Western Theater Command, China
Copyright © 2025 Liu, Chen and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shanjun Cai, Y2Fpc2hhbmp1bkAxNjMuY29t
 Dan Liu
Dan Liu Xingwang Chen
Xingwang Chen Shanjun Cai
Shanjun Cai