- 1Chongqing Key Laboratory of High Active Traditional Chinese Drug Delivery system, Chongqing Medical and Pharmaceutical College, Chongqing, China
- 2Chongqing Key Laboratory of New Drug Screening from Traditional Chinese Medicine, Integrative Science Center of Germplasm Creation in Western China (Chongqing) Science City and Southwest University, Chongqing, China
- 3SWU-TAAHC Medicinal Plant Joint R&D Centre, College of Pharmaceutical Sciences, Southwest University, Chongqing, China
The plant genus, Rhododendron constitutes an important part of the treasure trove of traditional Chinese medicine and have made outstanding contributions to human health for centuries. There are approximately 25 species of Rhododendron plants in China that have been used in folk medicine. Among these, Dali, which is known as little Rhododendron, is one of the most commonly utilized species. Modern chemical and pharmacological studies have shown that the genus contains diverse chemical constituents, including terpenes, diterpenes, triterpenes, sesquiterpenes, monoterpenes of the resveratrol type, heteroterpenes, meroterpenoids, flavonoids, lignin, phenolic acids. Meroterpenoids are derived from terpenoid biogenic pathways, with a biosynthesis involving shikimic acid terpenoid adducts. Heteroterpenes, a class of terpenoids with diverse properties, are mainly derived from plants of the Rhododendron genus. This review manuscript collates 113 different terpenoid monomers identified in Rhododendron plants. Extracts of Rhododendron genus plants and purified terpenoid monomers exhibit numerous pharmacological effects, with anti-inflammatory, anticancer, analgesic, antibacterial, antioxidant, expectorant, anti-asthmatic, cough suppressant, and smooth muscle relaxation properties. The meroterpenoids and heteroterpenes have been shown to exhibit significant therapeutic effects in conditions such as ischemia-reperfusion injury and ischemic heart disease. The purpose of this article is to provide an overview of the chemical and pharmacological research on Rhododendron plants over the past 20 years, which may be of value in the development of new drugs or food supplements.
1 Introduction
Rhododendron is the largest genus in the Ericaceae family, and China boasts approximately 571 species of this genus, 409 of which are endemic. They are distributed throughout the country, except for the provinces of Xinjiang and Ningxia, but are predominantly concentrated in southwestern China in the regions of Tibet, Yunnan, and Sichuan (Liu et al., 2024; Hu, 2005; Yang et al., 2020; Fan et al., 2022). Among the most highly valued and prevalent landscape plants, most species in the Rhododendron genus exhibit exceptional ornamental qualities. Furthermore, approximately 25 species of Rhododendron in China have found application in traditional Chinese medicine and are extensively utilized in the treatment of acute and chronic tracheobronchitis, cough, rheumatism, rheumatoid arthritis, osteomyelitis, nephritis, venereal sores, abdominal pain, blood stagnation, menstrual irregularities, and various other ailments (Liu et al., 2024; Wang et al., 2010; Zreik, 2024; Heyadri et al., 2015; Fang et al., 2007).
Dari, also known as Paru or the Chinese name for little azalea, is primarily derived from the dried leaves and flowers of R. cerasinum Tagg (Rhododendron primuliflorum) and R. anthopogonoides Maxim, both belonging to the Ericaceae family. It is one of the most commonly used Tibetan medicines but, due to the different distribution areas of the two plants, there are regional differences in their application. Specifically, in Sichuan, Yunnan, and Tibetan traditional medicine, flowers of the cherry grass azalea R. anthopogonoides are used primarily to formulate medicines for internal use, while the leaves are generally utilized in medicinal baths to treat skin diseases. Conversely, Qinghai, Sichuan, and Tibetan doctors believe that both the leaves and flowers of R. cerasinum can be used internally (Beckwith, 1979; Reuter et al., 2013).
Dari is renowned for its properties of clearing heat, reducing swelling, and tonifying the kidneys. The Compendium of Materia Medica has systematically standardized the properties and functions of Dari: “Its leaves are bitter, astringent, and develop a bitter taste after digestion. Its efficacy includes treating cold stomach, improper diet, skin diseases, and stiffness of the limbs, with properties that are hot and sharp. Its flowers have a sweet, bitter, and astringent taste, which turns sweet after digestion. Their effects are light, hot, and dry, and they are primarily used to treat edema, water and soil disorders, lung diseases, bronchitis, weakness, and muteness.” (Popescu and Kopp, 2013; Qiang et al., 2011).
Among the medicinal plants of the Rhododendron genus, R. molle (Blume) G. Don has the longest history of use. The flowers of R. molle serve as the primary ingredient in hemp boiling powder, a renowned pain reliever and anesthetic (Zheng et al., 2024a). The earliest surviving classic medical monograph, the Shennong Ben Cao Jing (Classic of the Materia Medica of the Divine Husbandman), initially documented the flowers of R. molle as the “haunted goat’s flower,” highlighting their use in treating pain while cautioning that they were toxic (Jin et al., 2013). The 2020 edition of the Chinese Pharmacopoeia states that R. molle possesses a warm nature and a pungent flavor and is commonly utilized for the treatment of rheumatic arthralgia and pain (Xu et al., 2021). Clinically, the roots, flowers, and fruits of R. molle are employed in the treatment of rheumatoid arthritis, traumatic pain, scabies, severe hypertension, and supraventricular tachycardia (Emmerick, 1975; Yang et al., 2022; Luo et al., 2023; Zhou et al., 2017; Mei et al., 2023). It also presents insecticidal action, achieved through contact, fumigation, repellency, and growth inhibition (Zheng et al., 2024a).
Another commonly used folk medicine applies the branches and leaves of R. micranthum Turcz in the treatment of pain-related conditions like rheumatoid arthralgia, lumbago, post-partum arthralgia, dysentery, and bone fractures (Jin et al., 2021; Zhang et al., 2013; Sun et al., 2018; Zhou et al., 2020). Studies have revealed that the primary active constituents of R. molle and R. micranthum are wood veratridane-type diterpenes. These diterpenes are currently only known to exist in the Rhododendron genus (Zheng et al., 2024b; Cai et al., 2018; Li et al., 2013). With their complex and novel skeletal structures, as well as their extensive and remarkable biological activities, wood veratridine-type diterpenes have emerged as a focal point in phytochemical research on the Rhododendron genus.
The dried leaves of R. dauricum L., possess a slightly pungent aroma and a bitter taste (Leong et al., 2020). They are known for their efficacy in alleviating cough and promoting expectoration of phlegm, and have traditionally been employed as a folk remedy for the treatment of acute and chronic bronchitis, asthma, hypertension, and coughing, among other conditions. R. dauricum is clinically utilized for the treatment of asthma and thick phlegm resulting from bronchitis, demonstrating remarkable efficacy. Nowadays, it is extensively incorporated into Chinese medicines, including Man Shan Hong Syrup and Man Shan Hong Capsule (Feng et al., 2023; Liang et al., 2023). Dari (Emmerick, 1975; Li et al., 2013; Leong et al., 2020), which is also utilized as an anti-inflammatory agent in Tibetan medicine for the treatment of rheumatoid arthritis and chronic bronchitis (Reuter et al., 2013; Yang et al., 2011; Shi et al., 2020; Liu et al., 2021). In addition, the flowers of R. arboreum Smith, commonly known in Tibet as Ta Ma, are efficacious in alleviating cough and asthma (Li, 2007). Furthermore, previous studies have reported that extracts from its petals possess the ability to inhibit the replication of severe acute respiratory syndrome coronavirus (SARS-CoV)-2 in vitro (Lingwan et al., 2023). R. auriculatum Hemsl. is also a widely recognized folk medicine whose bark and roots are utilized to treat coughs (Sun et al., 2019). Additionally, R. decorum Franch. is used externally for the treatment of bruises, rheumatism, and various pains (Zhu et al., 2018).
Modern pharmacological studies have demonstrated that Rhododendron species are abundant in diverse chemical constituents, including wood veratrylane-type diterpenes, heteroterpenes, triterpenes, flavonoids, lignans, phenolic acids, sesquiterpenes, and monoterpenes respectively (Beckwith, 1979; Popescu and Kopp, 2013; Qiang et al., 2011). They exhibit a broad spectrum of significant pharmacological effects, with particularly notable anti-inflammatory, analgesic, antibacterial, and proteintyrosine phosphatase-1B (PTP1B) inhibitory activities (Liu et al., 2024; Popescu and Kopp, 2013). Furthermore, previous research has indicated that their protective effect against ischemia-reperfusion injury offers a promising pathway for the treatment of ischemic heart disease and ischemic stroke (Jiang et al., 2023; Zhou et al., 2022), highlighting their significant potential both for research and in medicinal applications.
Various heteroterpene and meroterpenoid constituents have been identified in flowering Rhododendrons, including R. dauricum and R. anthopogonoides. These compounds have demonstrated a wide array of biological activities, including anti-inflammatory, PTP1B-inhibitory, anti-histamine-releasing, anti-human immunodeficiency virus (HIV), anti-herpes simplex virus (HSV)-1, α-glycosidase inhibition, and other properties (Liao et al., 2015; Zhang et al., 2022; Shi et al., 2020). In addition to their anti-acute kidney injury (AKI) effects in mice (Ashe and Zahs, 2010; Wu et al., 2021; Mizuta et al., 2009), other in vivo studies have demonstrated improvements in learning and memory ability, and anticoagulation in Alzheimer’s disease (AD) mice (Burchill et al., 2021; Jeon et al., 2022; Day et al., 2017; Wu et al., 2017). This comprehensive review aims to offer a summary of the chemical and pharmacological studies conducted on Rhododendron plants over the last 2 decades, potentially contributing to the advancement of novel drugs or food supplements.
2 An overview of the research on meroterpenoids and heteroterpenes in Rhododendron species
Meroterpenoids partially originate from the terpenoidogenic pathway and are biosynthesized through the combination of mangiferic acid and terpenoids (Pedraza-Chaverri et al., 2008; Jiang et al., 2002). This class of compounds, mainly found in algae, fungi, bacteria and some higher plants, shows a wide range of biological activities and chemical structures with unique backbones (Cornforth, 1968; Li et al., 2018; Zhao et al., 2017; Zhao et al., 2021). Rhododendron heteroterpenes are derivatives of chromane and chromene synthesized through a polyketide-terpene pathway.
Compounds 66 and 67 constitute the first pair of 6/6/6-ring heterobespene enantiomers to be isolated from Rhododendron, featuring a hexahydroxyanthracene moiety (Shi et al., 2020). Compounds 99 and 100 represent the first pair of heteromonoterpene enantiomers to feature a unique benzo [b]-2-oxa-[5.1.0]undecane 6/6/6/4-ring skeleton (Liao et al., 2015). Similarly, compounds 106 and 107 constitute the first pair of heteromonoterpene enantiomers with a benzo [d]-2,6-dioxa-tricyclo [5.2.2.0]undecane 6/6/6/5-ring system (Liao et al., 2017). Furthermore, compounds 112 and 113 are the second known pair of heteromonoterpene enantiomers to possess a benzo [c]-2,5,7-trioxa-tricyclo [7.2.1.0]dodecane 6/7/5/5-ring framework (Huang et al., 2018).
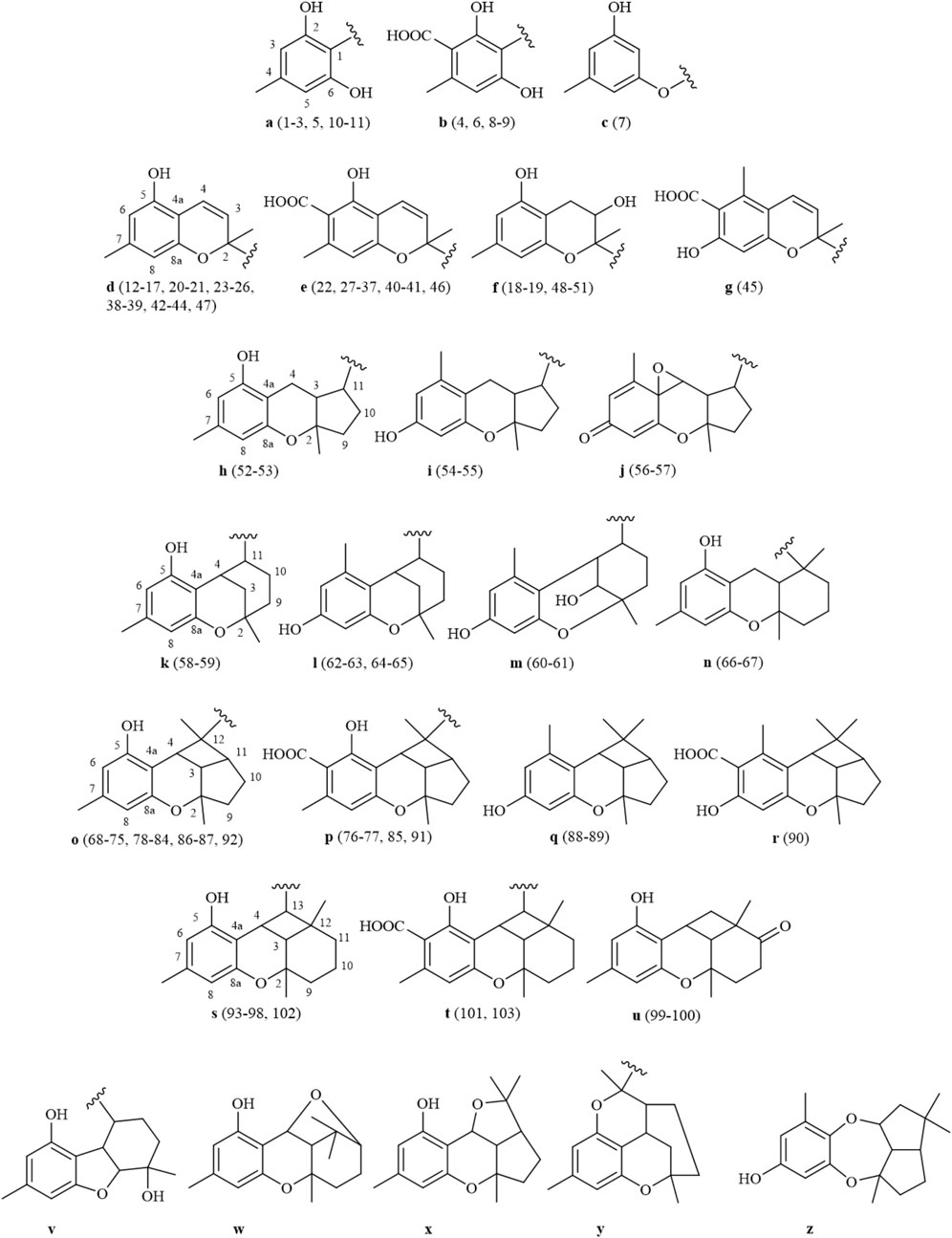
Grifolin derivatives are considered as the significant precursors in the biosynthetic pathway of this class of compounds. Through cyclization and oxidation processes, these grifolin derivatives give rise to polycyclic heteroterpenes that exhibit complex and diverse skeletal structures (Shi et al., 2020; De Loose, 1969; Kashiwada et al., 2001). Furthermore, meroterpenoids of the Rhododendron genus primarily exist in the form of enantiomers, and heteroterpenoids from this genus encompass a variety of structural types, including bicyclic (12–51), 6/5/6-ring (104–105), 6/6/5-ring (52–57), 6/6/6-ring (58–67), 6/6/5/4-ring (68–92), and 6/6/6/4-ring (93–103) structures, among others (Figure 1). The bicyclic and 6/6/5/4-ring structures are the most prevalent among these.
Nuclear magnetic resonance (NMR) spectroscopy is a crucial and indispensable tool for elucidating the structure of compounds. The spectroscopic features of a particular class of chemical constituents can offer valuable guidance for their targeted separation and structural identification (Pedraza-Chaverri et al., 2008; Jiang et al., 2002). Therefore, a concise summary of the structures of meroterpenoids isolated from Rhododendron and their respective NMR spectroscopic characteristics is provided, aiming to establish a foundation for future research endeavors.
2.1 Structures of grifolin derivatives and their NMR spectroscopic characterization
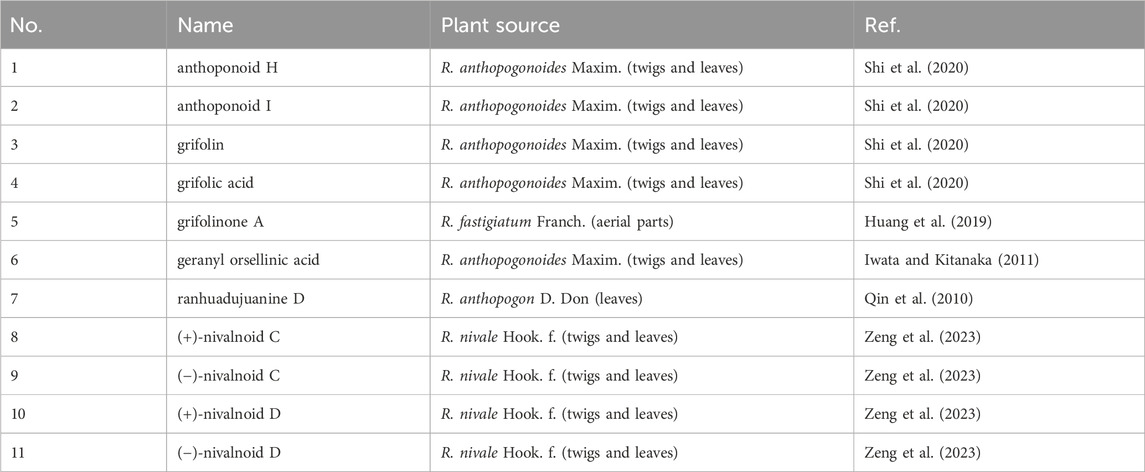
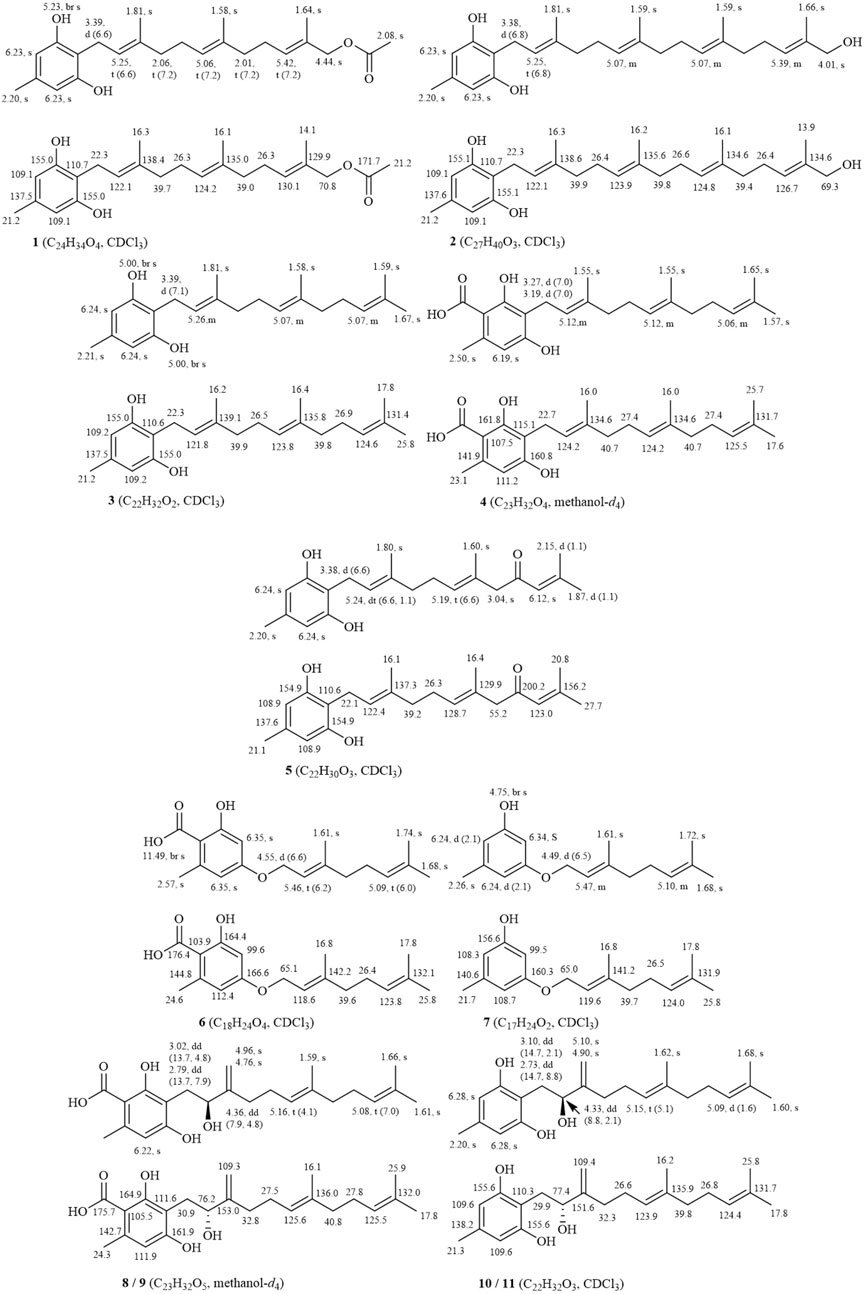
The defining characteristic of the chemical structure of Grifolin derivatives (1–11) is a 1,2,3,5-tetrasubstituted benzene ring bearing a long single chain, frequently attached at the C-1 position. The side chain typically comprises two to four isopentenyl units. Compound 2, which is the firstly heteroditerpene isolated from Rhododendron (Shi et al., 2020), possesses a side chain comprising four interconnected isopentenyl units, with a hydroxyl substituent on the methyl group of the final unit. Furthermore, some compounds feature a side chain with a 5-position hydroxyl group (6 and 7). When compounds exhibit a carboxyl group at the C-3 position (4, 6, 8, and 9), the aryl ring engages in π-π conjugation with the -COOH group. This interaction leads to a shift in the electron cloud towards the more electronegative O atoms, causing the chemical shifts of the C-2, C-4, and C-6 positions to appear at lower field compared to those of the C-1, C-3, and C-5 positions that are relatively shielded. Consequently, the chemical shifts of their carbon spectra are typically 2.0–6.0 ppm lower than those observed in compounds lacking a C-3 carboxyl group (comparing 3 with 4, 6 with 7, and 8/9 with 10/11). Without C-3 carboxyl substitution, a pair of magnetically equivalent aromatic proton signals typically appears in the low-field region of the 1H-NMR spectra for this class of compounds (1–3, 5, 7, 10, and 11). This characteristic, along with the presence of multiple isopentenyl signals, is commonly observed by 1H and 13C-NMR spectroscopy, serving as the primary method for distinguishing grifolin derivatives from other types of heteroterpene monomeric compounds. The names of the compounds, along with their plant sources and chemical structures, are provided in Table 1 and Figure 2.
2.2 Structures of bicyclic heteroterpenes or meroterpenoids and their NMR spectroscopic characterization
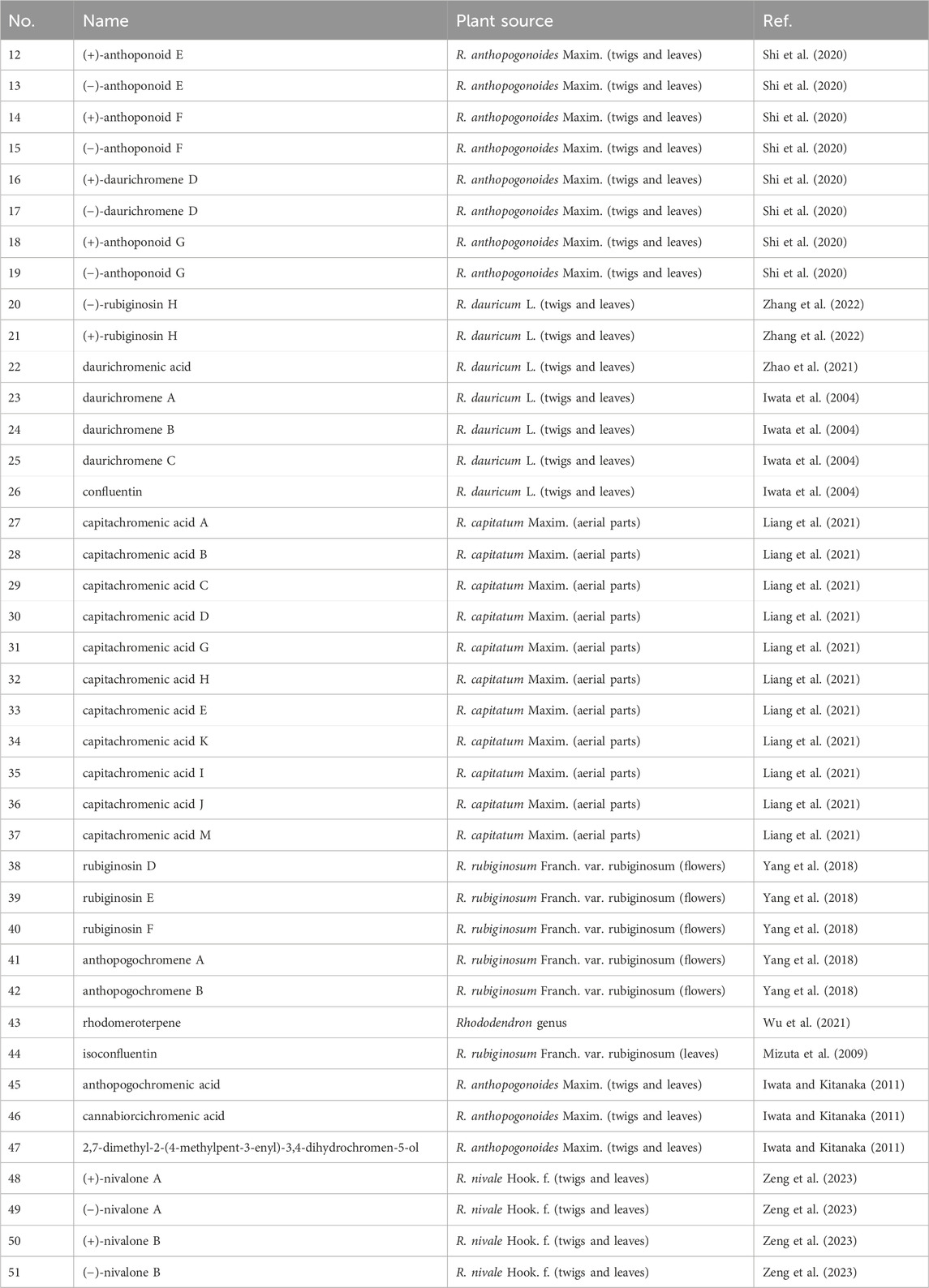
When there is no substituent at the C-6 position, bicyclic heteroterpenes or meroterpenoids (12–51) typically exhibit a sequential pattern of double-single-single-double peak signals in their 1H-NMR spectra in the range 5.40–6.80 ppm. This pattern consists of a set of cis-coupled olefinic proton signals and two aromatic proton signals (δH: 6.10–6.30 ppm). This feature is primarily utilized to distinguish bicyclic heteroterpenes with a double bond at the 3,4-position from other types of heteroterpenes. Typically, the chemical shift values for H-4 are around 6.60–6.80 ppm, and for H-3, they are approximately 5.40–5.50 ppm. Additionally, the coupling constant value J is generally around 10.0 Hz. Furthermore, a small number of bicyclic heteroterpenes undergo addition reactions at the 3,4-positions, particularly when there is hydroxyl substitution at the C-3 position (18–19 and 48–51). In such cases, H-3 typically appears as a triplet (t-peak) with a chemical shift value of approximately 3.88 ppm, while H2-4 exhibits a doublet of doublets (dd-peak) with chemical shift values of around 2.89 ppm and 2.63 ppm. Certain bicyclic meroterpenoids frequently undergo substitution at the C-6 position by a carboxyl group, and their carbon spectral chemical shift values are similar to those observed in grifolin derivatives (21/40, 41/42, and 46/47). The C-9 position of bicyclic meroterpenoids is commonly linked to a side chain comprising either one or two isopentenyl units. The absolute configuration of the 2-position in bicyclic meroterpenoids can be deduced using the chromane/chromene helix rule (Liang et al., 2021; Presley et al., 2018; Górecki et al., 2014; Makoto et al., 2008; Lin et al., 2015). The names, source plants, and chemical structures of bicyclic meroterpenoids are presented in Table 2 and Figures 1–3, 3-2, and 3-3.
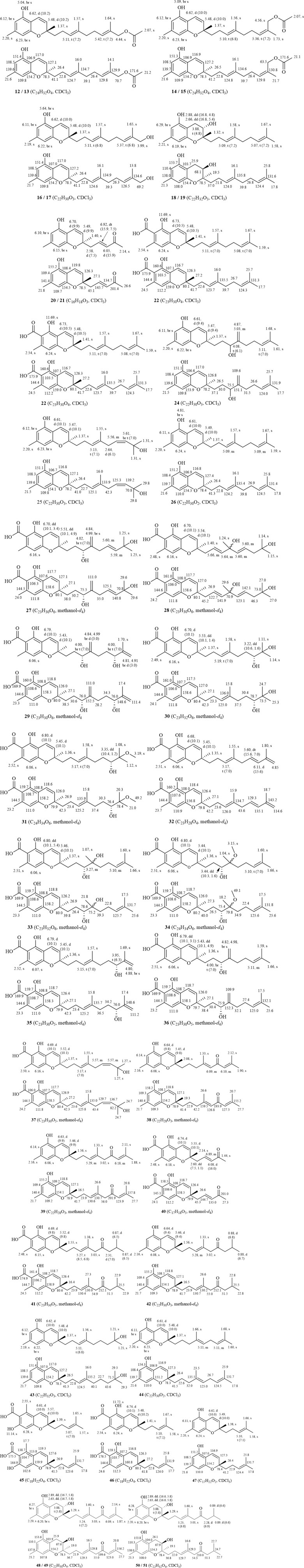
Figure 3. (1–3). Structures and 1H-NMR/13C-NMR data of bicyclic heteroterpenes or meroterpenoids from genus Rhododendron.
2.3 Structures of polycyclic heteroterpenes or meroterpenoids and their NMR spectroscopic characterization
Polycyclic heteroterpenes or meroterpenoids have been reported predominantly to possess a 6/6/5/4-ring skeleton (68–92). The chemical shift values for H-3 in this type of compound range from 2.50 to 2.70 ppm and are displayed as dd peaks in the 1H-NMR spectra. The J-values associated with these peaks are in the ranges 9.0–10.0 Hz and 7.0–8.0 Hz. The chemical shift values for the H-4 position range between 3.00 and 3.30 ppm, exhibiting d-peaks with J-values in the range 9.0–10.0 Hz. Conversely, the chemical shift values for H-11 lie between 2.40 and 2.50 ppm, displaying td-peaks. The proton signals of the 6/6/5/4-ring meroterpenoids, specifically H-3, H-4, and H-11, serve as distinguishing features that differentiate them from other heteroterpenes. Additionally, the values of the coupling constants between H-3 and H-4 (J3-4) and between H-3 and H-11 (J3-11) can be utilized in conjunction with the rotating frame overhauser effect spectroscopy (ROESY) spectra of the compounds to determine their relative configuration (Shi et al., 2020; Zhao et al., 2021; Huang et al., 2018; Huang et al., 2019). In the 6/6/5/4-ring heteroterpenes derived from Rhododendron, H-3, H-4, and H-11 typically reside on the same side of the plane. Furthermore, compounds of this type generally possess a side chain comprising an isopentenyl unit attached at the C-13 position.
A total of eleven 6/6/6/4-ring heteroterpene monomers (93–103) have been reported in Rhododendron. In comparison to the 6/6/5/4-ring heteroterpenes, this type of compound exhibits a d-peak for H-3, with a chemical shift in the range 1.80–1.90 ppm, and a t-peak for H-4, typically showing a chemical shift at 3.80–4.30 ppm. H-13 displays a d-peak with chemical shift values in the range 2.90–4.00 ppm and J-values between 9.0 and 10.0 Hz. Notably, the hydrogen signals of H-3 and H-4 are the primary features that differentiate these compounds from 6/6/5/4-ring cycloheteroterpenoids. The values of the coupling constants between H-3 and H-4 (J3-4) can be utilized in conjunction with ROESY spectra to ascertain the relative configuration of the compounds. Additionally, in the compounds reported to date (Zhang et al., 2022), H-3 and H-4 are positioned on the same side of the plane. For meroterpenoid 6/6/6-ring compounds (58–65), H-4 chemical shifts range from 3.20–3.50 ppm and typically appear as t-peaks or broad singlets (br s). Coupling constants J4-11 (between H-4 and H-11), rather than J3-4, can determine relative configuration (Huang et al., 2018). Compounds of this type may feature a C-3 hydroxyl group (60–61) and, when determining their relative configuration, it is often necessary to consider the influence of the γ-gauche effect arising from this 3-OH. In such cases, the values of the coupling constants between H-3 and H-4 (J3-4) can serve as a reference for establishing the relative configuration (Huang et al., 2018; Wang et al., 2017). Furthermore, the 6/6/5- and 6/6/6-ring heteroterpenes frequently undergo a positional shift of the aromatic methyl group and the 5-OH (52/53, 54/55, 58/59, and 64/65). Similarly, the 6/6/5/4-ring heteroterpenes exhibit the same phenomenon when lacking side-chain substitution at the C-13 position (88/89 and 92, as well as 90 and 91). The names, source plants, and chemical structures of the polycyclic heteroterpenes are presented in Table 3 and Figures 1–4, 4–2, 4-3, and 4-4.
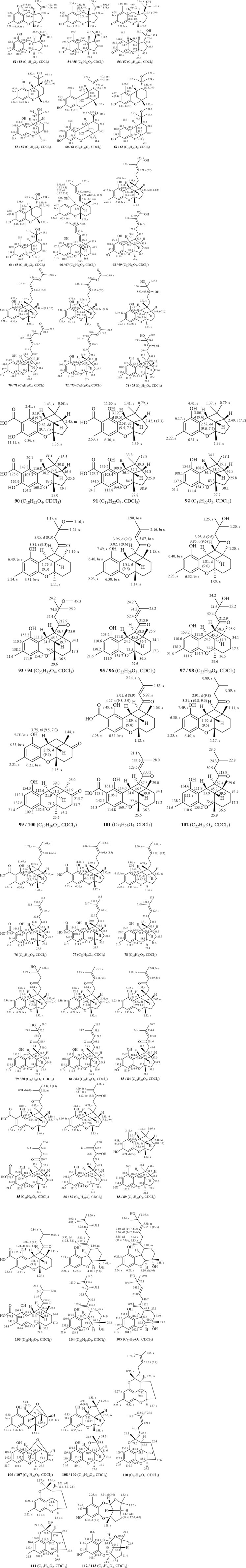
Figure 4. (1–4). Structures and 1H-NMR/13C-NMR data of polycyclic heteroterpenes or meroterpenoids from genus Rhododendron.
3 Overview of the biological activities of heteroterpenes or meroterpenoids in the Rhododendron genus
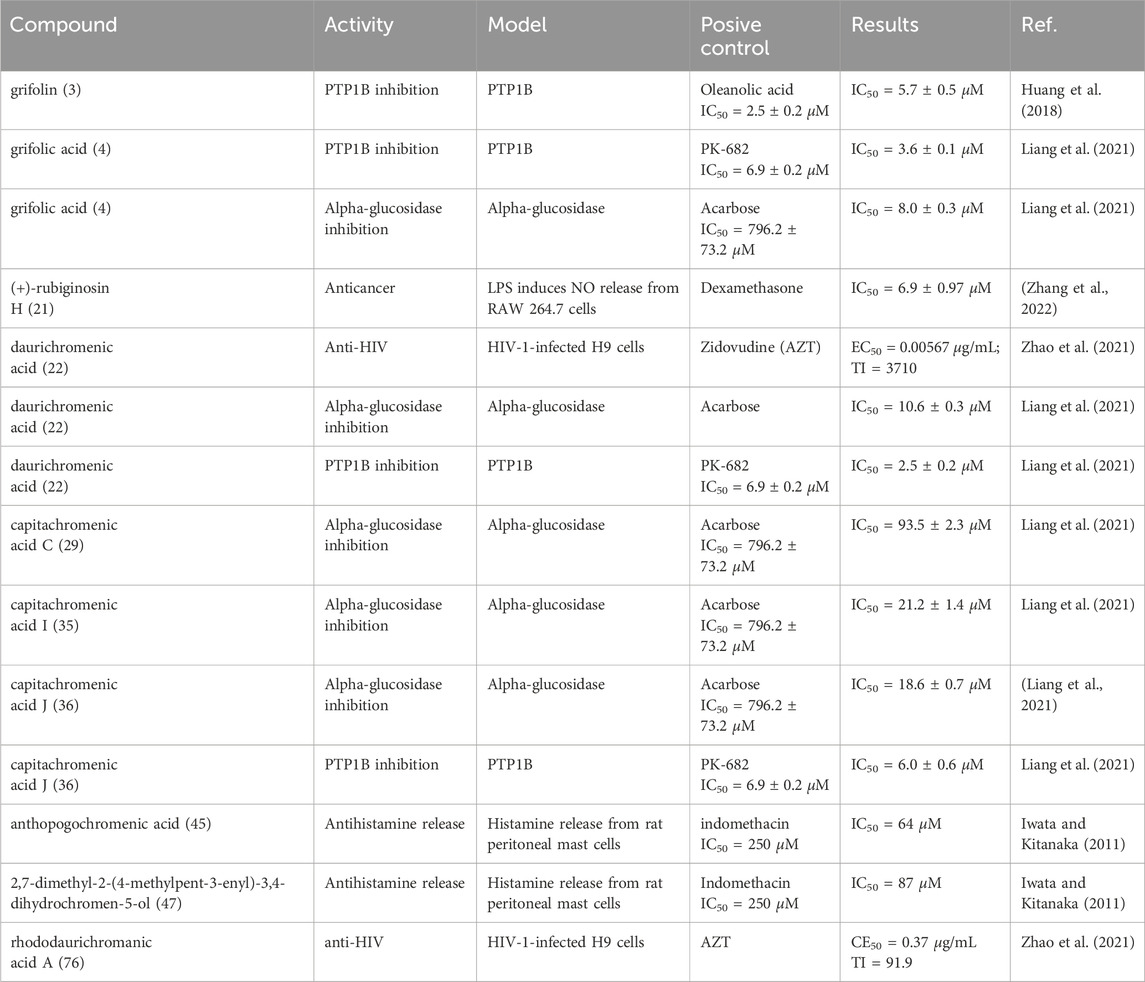
Most of the heteroterpenes or meroterpenoids isolated from Rhododendron have been reported to exhibit a wide range of bioactivities, including anti-inflammatory, PTP1B inhibitory, antihistamine-releasing, anti-HIV, anti-herpes simplex virus (HSV-1), anti-tumor, and α-glucosidase inhibitory activities. The anti-inflammatory and PTP1B-inhibitory activities are particularly significant among these (Liang et al., 2021). Hou et al. (Wu et al., 2021) discovered that the novel compound rhodomeroterpene (43) exhibited ameliorative effects in various models of renal injury. Pretreatment with this compound (30 mg/kg/d, ip, 3d) significantly suppressed the acute inflammatory response in LPS-induced septic mice. The mechanism of action may involve the regulation of inflammatory signaling pathways, such as IKK/NF-кB and PI3K/PDK1/Akt, especially in macrophages. This study indicates that rhodomeroterpene holds potential as a lead compound for the treatment of acute kidney injury (AKI).
Yang et al. reported that isoconfluentin (44), a novel bicyclic heteroterpenoid compound isolated from R. nivale Hook.f., exhibited significant anticoagulant effects in a dose-dependent manner (Mizuta et al., 2009). The compounds (+)-nivalnoid C (8) and (+)-nivalone B (50) isolated from R. nivale demonstrated protective effects against oxidative damage in nerve cells (Zeng et al., 2023). Xu et al. screened for the activity of heteroterpenoid cannabichromeorcinic acid (CA) from R. primuliflorum, finding anti-acetylcholinesterase activity (Iwata and Kitanaka, 2011). This compound significantly improved the memory and learning ability of mice and had a notable inhibitory effect on acetylcholinesterase in the brain tissue and serum of mice with Alzheimer’s disease (AD). Therefore, CA can be considered a promising lead compound for the development of therapeutic drugs for AD. Tables 1–4 present the significant active meroterpenoids derived from the genus Rhododendron. Based on this literature review, it appears that grifolin derivatives and bicyclic meroterpenoids exhibit superior anti-inflammatory, PTP1B, and α-glycosidase inhibitory activities. However, there are relatively few studies on the bioactivity of this class of constituents, and the available experimental data is insufficient to draw definitive conclusions.
4 Discussion
Rhododendron are quintessential alpine plants. As dominant or constructive species, they give rise to characteristic scrub communities in subtropical regions. These plants are predominantly distributed across the alpine and subalpine tree-line areas, as well as regions above the tree line, in western and southwestern China (Ștefănescu et al., 2019). They exert a substantial influence on various aspects, including the climate-change response of their distribution areas, the biogeochemical cycle, and the sustainable livelihoods of mountain-dwelling communities (Luo, 2020). Rhododendron possess a branching structure and are firmly rooted. The taxa that thrive in the alpine zone hold significant ecological importance, not only for water conservation and the maintenance of inter-regional hydrothermal balance, but also for defining the distinctive functions and services of mountain ecosystems, as well as contributing to biodiversity (Tomoki et al., 2019). The principal chemical constituents of Rhododendron encompass flavonoids, diterpenoids, triterpenoids, phenols, tannins, and volatile oils, among others. These compounds exhibit a range of pharmacological properties, including expectorant, cough-suppressant, anti-rheumatoid arthritis, anti-chronic bronchitis, cardiovascular-disease-treating, neuro-regulatory, anti-inflammatory, analgesic, stomachic, decongestant, and immune-modulating effects (Quang et al., 2006). Given their high medicinal value, the application of Rhododendron in the field of healthcare holds significant untapped potential. Volatile oils are extracted from aromatic plant materials. For instance, the leaves and flowers of the strongly-scented azalea, along with the leaves and shoots of the beauty azalea, are particularly rich in volatile oils. Additionally, these plant parts also contain ellagitannic substances, from which valuable extracts can be derived (Nukata et al., 2002). Furthermore, Rhododendron serve as a common source of animal fodder. In certain ethnic-populated regions, the local inhabitants still maintain the practice of consuming rhododendron crowns (Mao et al., 2017). Additionally, some species of Rhododendron boast dense, finely-grained wood, which makes them highly suitable for craft-making purposes. The aforementioned points collectively illustrate that the diversity of ecosystem functions and services provided by Rhododendron remains a central focus of both national and international research endeavors (Srivastava, 2012).
Currently, the taxonomic study of the genus Rhododendron has embraced modern technological advancements, enabling researchers to largely identify its related species. However, the majority of existing classification efforts for this genus are confined to specific major classes or subgenera within a given class (Dampc and Luczkiewicz, 2013). Furthermore, there is an absence of scientifically sound and standardized criteria for species classification, as well as uniform nomenclature guidelines. Additionally, the existing classification systems lack a comprehensive corresponding collection of specimens (Cross, 1975). Now, there exists a dearth of more systematic investigations into the relationship between the chemical constituents and pharmacological activities of Rhododendron species (Chamberlain et al., 1996). Consequently, research on their pharmacological effects requires further enhancement. The pharmacological mechanisms of action underlying the active ingredients in these species remain unclear and warrant in-depth exploration (Liu et al., 2024; Vengrytė and Raudonė, 2024; Sun et al., 2024; Zhang et al., 2024). Moreover, Rhododendron species have received relatively limited attention with regard to the establishment of quality standards (Yu et al., 2024; Kukhtenko et al., 2024; Mangral et al., 2025).
5 Conclusion
The genus Rhododendron is abundant in phytochemical resources, with numerous medicinal plants that exhibit significant anti-inflammatory and analgesic properties with great potential therapeutic value (Islam et al., 2023a). Furthermore, genus-specific heteroterpene/meroterpenoid constituents show significant activities in anti-inflammation, anti-histamine release, PTP1B inhibition, and α-glucosidase inhibition. The genus has also been applied in the emerging field of cyberpharmacology (Islam et al., 2023b). Meroterpenoids and heteroterpenes are secondary metabolites with structures partially derived from terpenoid pathways, and are research hotspots in the study of the chemical composition of Rhododendron species (Gu et al., 2025). To date, 113 monomers with diverse ring system skeletons and a wide variety of structural types have been isolated in Rhododendron. Among them, bicyclic and 6/6/5/4-ring heteroterpenes are predominant (Pan et al., 2023). These compounds may be differentiated based on the characteristics of their 1H-NMR spectra. However, most of the reported compounds are enantiomers obtained through chiral column separation of the racemate (Lin, 2024). Research on this class of constituents is insufficient, highlighting the need for further studies on the chemical composition of Rhododendron with the aim of discovering heteroterpene or meroterpenoid constituents with novel skeletal structures and significant pharmacological activities (Shi et al., 2020). This will provide potential active lead compounds for the research and development of new drugs, and also offer a reference for the development and application of Rhododendron-related medicinal plants (Li et al., 2008; Jing et al., 2015). In addition, by combining computer methods in the future, innovative momentum can be injected into the research of natural medicine chemistry and pharmacology from multiple dimensions (Sang et al., 2024; Iwata and Kitanaka, 2010; Nakatsuka et al., 2005; Wei et al., 2023). Through data integration, model construction, and intelligent analysis, the dual improvement of research efficiency and accuracy can be achieved.
Author contributions
JM: Writing – original draft, Funding acquisition. MY: Writing – original draft. TL: Writing – original draft. YS: Writing – review and editing. ZD: Writing – review and editing. HZ: Writing – review and editing. MC: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Opening Foundation of Chongqing Key Laboratory of High Active Traditional Chinese Drug Delivery System (kfkt202301), Key Scientific and Technological Research Project of Chongqing Municipal Education Commission (KJZD-K202302801), Chongqing Municipal Education Commission Youth Project (KJQN202402816), and Scientific Research Project of Chongqing Medical Pharmaceutical College (ygzrc2024104, ygz2022104, and ygz2024117) respectively.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ashe, K. H., and Zahs, K. R. (2010). Probing the biology of alzheimer's disease in mice. Neuron 66 (5), 631–645. doi:10.1016/j.neuron.2010.04.031
Beckwith, C. I. (1979). The introduction of Greek medicine into Tibet in the seventh and eighth centuries. J. Am. Orient. Soc. 99, 297–313. doi:10.2307/602665
Burchill, L., Day, A. J., Yahiaoui, O., and George, J. H. (2021). Biomimetic total synthesis of the rubiginosin meroterpenoids. Org. Lett. 23 (2), 578–582. doi:10.1021/acs.orglett.0c04117
Cai, Y. Q., Hu, J. H., Qin, J., Sun, T., and Li, X. L. (2018). Rhododendron molle (ericaceae): phytochemistry, pharmacology, and toxicology. Chin. J. Nat. Med. 16 (6), 401–410. doi:10.1016/S1875-5364(18)30073-6
Chamberlain, D., Hyam, R., Argent, G., Fairweather, G., and Walter, K. S. (1996). The genus rhododendron: its classification and synonymy.
Dampc, A., and Luczkiewicz, M. (2013). Rhododendron tomentosum (ledum palustre). A review of traditional use based on current research. Fitoterapia 85, 130–143. doi:10.1016/j.fitote.2013.01.013
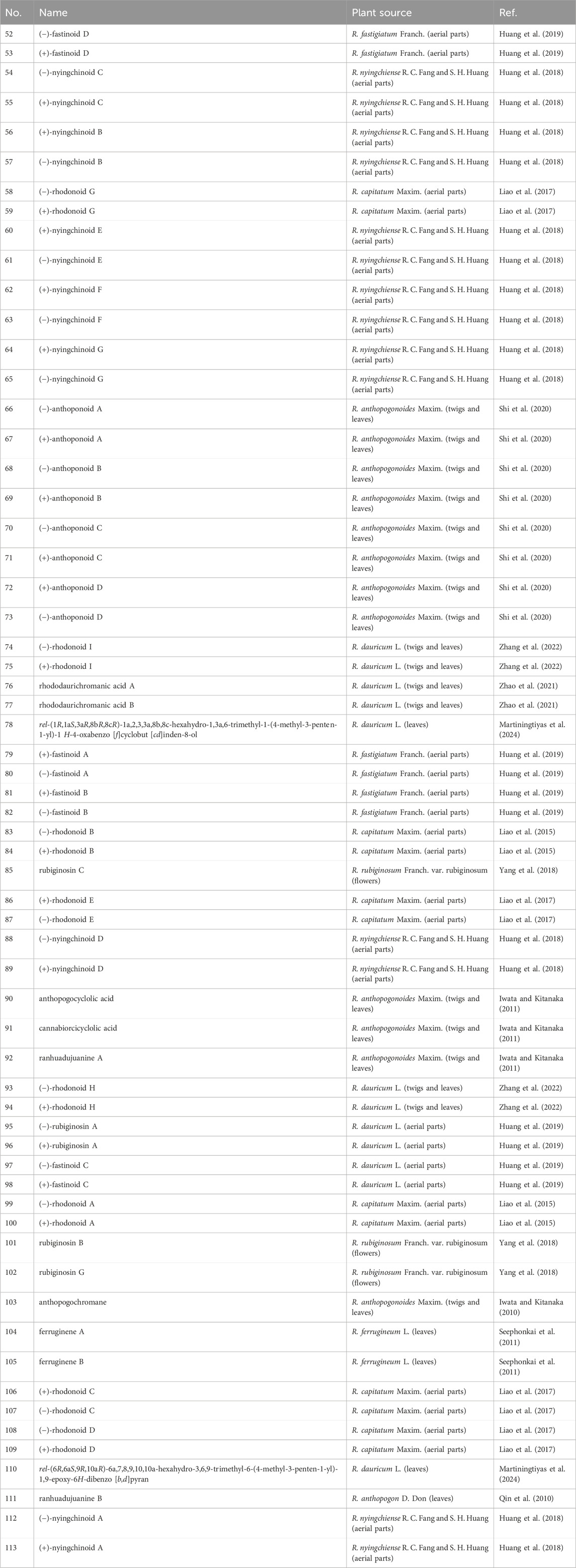
Day, A. J., Lam, H. C., Sumby, C. J., and George, J. H. (2017). Biomimetic total synthesis of rhodonoids C and D, and murrayakonine D. Org. Lett. 19 (10), 2463–2465. doi:10.1021/acs.orglett.7b00779
De Loose, R. (1969). The flower pigments of the Belgian hybrids of Rhododendron simsii and other species and varieties from rhododendron subseries obtusum. Phytochemistry 8 (1), 253–259. doi:10.1016/s0031-9422(00)85822-7
Fan, X. Z., Zhu, Y. L., Yuan, R. W., Deng, L., Hou, C., Li, W., et al. (2022). Terpenoids with α-glucosidase inhibitory activity from Rhododendron minutiflorum Hu. Phytochemistry 196, 113083. doi:10.1016/j.phytochem.2021.113083
Fang, J. Y., Guo, Z. D., Piao, S. L., and Chen, A. P. (2007). Estimation of carbon sinks in terrestrial vegetation in China from 1981 to 2000. Sci. Sin. (Terrae) 37 (6), 804–812. doi:10.3969/j.issn.1674-7240.2007.06.012
Feng, Y., Zhang, H., Gao, B., Zheng, G., Zha, S., and Yao, G. (2023). Highly oxygenated grayanane diterpenoids with structural diversity from the flowers of Rhododendron dauricum and their analgesic activities. Bioorg Chem. 132, 106374. doi:10.1016/j.bioorg.2023.106374
Górecki, M., Suszczyńska, A., Woźnica, M., Baj, A., Wolniak, M., Cyrański, M. K., et al. (2014). Chromane helicity rule--scope and challenges based on an ECD study of various trolox derivatives. Org. Biomol. Chem. 12 (14), 2235–2254. doi:10.1039/c3ob42376j
Gu, J., Zhang, J., Zeng, S., Zhang, W., Xia, R., Wang, X., et al. (2025). Artificial intelligence in tumor drug resistance: mechanisms and treatment prospects. Intell. Oncol. 1, 73–88. doi:10.1016/j.intonc.2025.02.001
Heyadri, M., Hashempur, M. H., Ayati, M. H., Quintern, D., and Nimrouzi, M. (2015). The use of Chinese herbal drugs in Islamic medicine. J. Integr. Med. 13 (6), 363–367. doi:10.1016/S2095-4964(15)60205-9
Hu, S. (2005). Food plants of China. China: Chinese University Press. Available online at: https://sc.panda985.com/#v=onepage&q=Hu%2C%20S.%20(2005).%20Food%20plants%20of%20China.%20Chinese%20University%20Press.&f=false.
Huang, G. H., Hu, Z., Lei, C., Wang, P. P., Yang, J., Li, J. Y., et al. (2018). Enantiomeric pairs of meroterpenoids with diverse heterocyclic systems from Rhododendron nyingchiense. J. Nat. Prod. 81 (8), 1810–1818. doi:10.1021/acs.jnatprod.8b00273
Huang, G. H., Lei, C., Zhu, K. X., Li, J. Y., Li, J., and Hou, A. J. (2019). Enantiomeric pairs of meroterpenoids from Rhododendron fastigiatum. Chin. J. Nat. Med. 17 (12), 963–969. doi:10.1016/S1875-5364(19)30119-0
Islam, S. U., Mangral, Z. A., Hussain, K., Tariq, L., Bhat, B. A., Khuroo, A. A., et al. (2023a). Unravelling diversity, drivers, and indicators of soil microbiome of Trillium govanianum, an endangered plant species of the himalaya. Environ. Res. 227, 115819. doi:10.1016/j.envres.2023.115819
Islam, S. U., Mangral, Z. A., Tariq, L., Ahmad Bhat, B., Waseem Tantray, W., Ahmad, R., et al. (2023b). Conservation genetics of endangered Trillium govanianum wall. Ex D. Don–A pharmaceutically prized medicinal plant from the himalaya and implications for species recovery. Gene 888, 147748. doi:10.1016/j.gene.2023.147748
Iwata, N., and Kitanaka, S. (2010). Tetracyclic chromane derivatives from Rhododendron anthopogonoides. J. Nat. Prod. 73 (7), 1203–1206. doi:10.1021/np900543r
Iwata, N., and Kitanaka, S. (2011). New cannabinoid-like chromane and chromene derivatives from Rhododendron anthopogonoides. Chem. Pharm. Bull. (Tokyo) 59 (11), 1409–1412. doi:10.1248/cpb.59.1409
Iwata, N., Wang, N., Yao, X., and Kitanaka, S. (2004). Structures and histamine release inhibitory effects of prenylated orcinol derivatives from Rhododendron dauricum. J. Nat. Prod. 67 (7), 1106–1109. doi:10.1021/np0303916
Jeon, H., Kang, G., Kim, M. J., Shin, J. S., Han, S., and Lee, H. Y. (2022). On the erosion of enantiopurity of rhodonoids via their asymmetric total synthesis. Org. Lett. 24 (11), 2181–2185. doi:10.1021/acs.orglett.2c00482
Jiang, B., Yang, H., Li, M. L., Hou, A. J., Han, Q. B., Wang, S. J., et al. (2002). Diterpenoids from isodon a denantha. J. Nat. Prod. 65 (8), 1111–1116. doi:10.1021/np020084k
Jiang, C. C., Shi, L., Zhao, X. Y., Zhang, H., Li, Z. X., Lu, J. J., et al. (2023). Mechanism of total flavonoids of rhododendra Simsii in alleviating ischemic brain injury. Zhongguo Zhong Yao Za Zhi 48 (2), 455–464. doi:10.19540/j.cnki.cjcmm.20221010.402
Jin, P., Zheng, G., Yuan, X., Ma, X., Feng, Y., and Yao, G. (2021). Structurally diverse diterpenoids with eight carbon skeletons from Rhododendron micranthum and their antinociceptive effects. Bioorg Chem. 111, 104870. doi:10.1016/j.bioorg.2021.104870
Jin, R., Lin, Z., Xue, C., and Zhang, B. (2013). An improved association-mining research for exploring Chinese herbal property theory: based on data of the Shennong's classic of materia medica. J. Integr. Med. 11 (5), 352–365. doi:10.3736/jintegrmed2013051
Jing, L., Ma, H., Fan, P., Gao, R., and Jia, Z. (2015). Antioxidant potential, total phenolic and total flavonoid contents of Rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in PC12 cells. BMC complementary Altern. Med. 15, 287–12. doi:10.1186/s12906-015-0820-3
Kashiwada, Y., Yamazaki, K., Ikeshiro, Y., Yamagishi, T., Fujioka, T., Mihashi, K., et al. (2001). Isolation of rhododaurichromanic acid B and the anti-HIV principles rhododaurichromanic acid A and rhododaurichromenic acid from Rhododendron dauricum. Tetrahedron 57 (8), 1559–1563. doi:10.1016/s0040-4020(00)01144-3
Kukhtenko, H., Bevz, N., Konechnyi, Y., Kukhtenko, O., and Jasicka-Misiak, I. (2024). Spectrophotometric and chromatographic assessment of total polyphenol and flavonoid content in Rhododendron tomentosum extracts and their antioxidant and antimicrobial activity. Molecules 29 (5), 1095. doi:10.3390/molecules29051095
Leong, F., Hua, X., Wang, M., Chen, T., Song, Y., Tu, P., et al. (2020). Quality standard of traditional Chinese medicines: comparison between european pharmacopoeia and Chinese pharmacopoeia and recent advances. Chin. Med. 15, 76–20. doi:10.1186/s13020-020-00357-3
Li, H. L., Xu, R., Li, X. M., Yang, S. Q., Meng, L. H., and Wang, B. G. (2018). Simpterpenoid A, a meroterpenoid with a highly functionalized cyclohexadiene moiety featuring gem-Propane-1,2-dione and methylformate groups, from the mangrove-derived Penicillium simplicissimum MA-332. Org. Lett. 20 (5), 1465–1468. doi:10.1021/acs.orglett.8b00327
Li, M., Jia, Z., Hu, Z., Zhang, R., and Shen, T. (2008). Experimental study on the hemostatic activity of the Tibetan medicinal herb lamiophlomis rotata. Phytotherapy Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 22 (6), 759–765. doi:10.1002/ptr.2359
Li, Y., Liu, Y. B., Zhang, J. J., Jiang, J. D., Yu, S. S., Ma, S. G., et al. (2013). Mollolide A, a diterpenoid with a new 1,10:2,3-disecograyanane skeleton from the roots of Rhododendron molle. Org. Lett. 15 (12), 3074–3077. doi:10.1021/ol401254e
Liang, C., Kjaerulff, L., Hansen, P. R., Kongstad, K. T., and Staerk, D. (2021). Dual high-resolution α-Glucosidase and PTP1B inhibition profiling combined with HPLC-PDA-HRMS-SPE-NMR analysis for the identification of potentially antidiabetic chromene meroterpenoids from Rhododendron capitatum. J. Nat. Prod. 84 (9), 2454–2467. doi:10.1021/acs.jnatprod.1c00454
Liang, Q., Hu, J. X., Zhang, X. M., and Xu, W. H. (2023). Traditional uses, phytochemistry, pharmacology, toxicology, and quality control of Rhododendron dauricum L. leaves: a comprehensive review. J. Ethnopharmacol. 305, 116085. doi:10.1016/j.jep.2022.116085
Liao, H. B., Huang, G. H., Yu, M. H., Lei, C., and Hou, A. J. (2017). Five pairs of meroterpenoid enantiomers from Rhododendron capitatum. J. Org. Chem. 82 (3), 1632–1637. doi:10.1021/acs.joc.6b02800
Liao, H. B., Lei, C., Gao, L. X., Li, J. Y., Li, J., and Hou, A. J. (2015). Two enantiomeric pairs of meroterpenoids from Rhododendron capitatum. Org. Lett. 17 (20), 5040–5043. doi:10.1021/acs.orglett.5b02515
Lin, H. (2024). Artificial intelligence with great potential in medical informatics: a brief review. Medinformatics 1 (1), 2–9. doi:10.47852/bonviewmedin42022204
Lin, Y. C., Chang, J. C., Cheng, S. Y., Wang, C. M., Jhan, Y. L., Lo, I. W., et al. (2015). New bioactive chromanes from Litchi chinensis. J. Agric. Food Chem. 63 (9), 2472–2478. doi:10.1021/jf5056387
Lingwan, M., Shagun, S., Pahwa, F., Kumar, A., Verma, D. K., Pant, Y., et al. (2023). Phytochemical rich himalayan Rhododendron arboreum petals inhibit SARS-CoV-2 infection in vitro. J. Biomol. Struct. Dyn. 41 (4), 1403–1413. doi:10.1080/07391102.2021.2021287
Liu, X. J., Su, H. G., Peng, X. R., Bi, H. C., and Qiu, M. H. (2024). An updated review of the genus rhododendron since 2010: traditional uses, phytochemistry, and pharmacology. Phytochemistry 217, 113899. doi:10.1016/j.phytochem.2023.113899
Liu, Z., Li, M., Tao, Y., and Olsen, R. (2021). Multivariate statistical and comparison analysis of chemical constituents in Arenaria kansuensis maxim. From different regions in qinghai-tibet Plateau. Phytochem. Anal. 32 (5), 794–803. doi:10.1002/pca.3025
Luo, G. H. (2020). Cognitive history about cuckoo and azalea in China. J. Beijing For. Univ. Soc. Sci. 19 (1), 45–51. doi:10.13931/j.cnki.bjfuss.2019127
Luo, Y., Shen, Y., Zong, L., Xie, J., Dai, L., and Luo, X. (2023). Anti-rheumatoid arthritis potential of Rhododendron molle G. Don leaf extract in adjuvant induced arthritis rats. J. Ethnopharmacol. 307, 116175. doi:10.1016/j.jep.2023.116175
Makoto, I. M., Tako, N., Hayakawa, T., Matsunaga, T., Mori, J., and Saito, H. (2008). New chromane derivatives isolated from the brown alga. Sargassum Micracanthum. Chem. Pharm. Bull. (Tokyo) 56 (1), 124–128. doi:10.1248/cpb.56.124
Mangral, Z. A., Bhat, B. A., Sheikh, S., Islam, S. U., Tariq, L., Dar, R., et al. (2025). Exploring the therapeutic potential of Rhododendron anthopogon D. Don essential oil constituents against lung cancer: a network pharmacology-based analysis with molecular docking and experimental studies. Comput. Biol. Med. 187, 109827. doi:10.1016/j.compbiomed.2025.109827
Mao, P., Huang, D. X., Wang, Y. H., Zhou, H., Wang, H. Y., and Chen, H. (2017). Big data analysis of status and trends of global change research. J. Univ. Chin. Acad. Sci. 34 (4), 439–451. doi:10.7523/j.issn.2095-6134.2017.04.006
Martiningtiyas, C. R., Banjarnahor, E., Asih, R. R. D., Anjani, D. G., Ramadhan, S., and Togatorop, D. (2024). Pelatihan Fotografi kepada Para Pelaku UMKM Azalea GDC untuk Meningkatkan Promosi Produk. J. Pengabdi. Masy. Ekon. Manaj. Dan. Akunt. (JPMEMA) 3 (1), 1–9. Available online at: https://journal.binainternusa.org/index.php/jpmema/article/view/230.
Mei, W. Y., Liu, X. D., Wang, Z. H., Yu, J. B., He, J. W., Zhang, J. Y., et al. (2023). Research progress on Rhododendron molle in treatment of rheumatoid arthritis. Zhongguo Zhong Yao Za Zhi 48 (21), 5690–5700. doi:10.19540/j.cnki.cjcmm.20230811.601
Mizuta, D., Ban, T., Miyajima, I., Nakatsuka, A., and Kobayashi, N. (2009). Comparison of flower color with anthocyanin composition patterns in evergreen azalea. Sci. Hortic. 122 (4), 594–602. doi:10.1016/j.scienta.2009.06.027
Nakatsuka, T., Nishihara, M., Mishiba, K., and Yamamura, S. (2005). Temporal expression of flavonoid biosynthesis-related genes regulates flower pigmentation in Gentian plants. Plant Sci. 168 (5), 1309–1318. doi:10.1016/j.plantsci.2005.01.009
Nukata, M., Hashimoto, T., Yamamoto, I., Iwasaki, N., Tanaka, M., and Asakawa, Y. (2002). Neogrifolin derivatives possessing anti-oxidative activity from the mushroom Albatrellus ovinus. Phytochemistry 59 (7), 731–737. doi:10.1016/s0031-9422(02)00050-x
Pan, H., Chen, S., and Xiong, H. (2023). A high-dimensional feature selection method based on modified gray wolf optimization. Appl. Soft Comput. 135, 110031. doi:10.1016/j.asoc.2023.110031
Pedraza-Chaverri, J., Cárdenas-Rodríguez, N., Orozco-Ibarra, M., and Pérez-Rojas, J. M. (2008). Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 46 (10), 3227–3239. doi:10.1016/j.fct.2008.07.024
Popescu, R., and Kopp, B. (2013). The genus rhododendron: an ethnopharmacological and toxicological review. J. Ethnopharmacol. 147 (1), 42–62. doi:10.1016/j.jep.2013.02.022
Presley, C. C., Valenciano, A. L., Fernández-Murga, M. L., Du, Y. L., ShanaiahMaria, N., Cassera, B. S., et al. (2018). Antiplasmodial chromanes and chromenes from the monotypic plant species koeberlinia Spinose. J. Nat. Prod. 81 (3), 475–483. doi:10.1021/acs.jnatprod.7b00579
Qiang, Y., Zhou, B., and Gao, K. (2011). Chemical constituents of plants from the genus rhododendron. Chem. Biodivers. 8 (5), 792–815. doi:10.1002/cbdv.201000046
Qin, C., Mei, Y., Zhou, X., Ping, A., and Huang, S. (2010). Five monoterpenes from leaves of Rhododendron anthopogon. Zhongguo Zhong Yao Za Zhi 35 (19), 2568–2571.
Quang, D. N., Harinantenaina, L., Nishizawa, T., Hashimoto, T., Kohchi, C., Soma, G. I., et al. (2006). Inhibitory activity of nitric oxide production in RAW 264.7 cells of daldinals A–C from the fungus Daldinia childiae and other metabolites isolated from inedible mushrooms. J. Nat. Med. 60 (4), 303–307. doi:10.1007/s11418-006-0010-1
Reuter, K. P., Weißhuhn, T. E. R., and Witt, C. M. (2013). Tibetan medicine: a systematic review of the clinical research available in the west. Evidence-Based Complementary Altern. Med. 2013 (1), 213407. doi:10.1155/2013/213407
Sang, Y. L., Wang, P., Pan, Z. X., Tu, X., Dai, L., Xin, Y., et al. (2024). Repellence and insecticidal activity of Rhododendron anthopogonoides EO and head transcriptome analysis. Arthropod-Plant Interact. 18 (3), 501–517. doi:10.1007/s11829-024-10043-y
Seephonkai, P., Popescu, R., Zehl, M., Krupitza, G., Urban, E., and Kopp, B. (2011). Ferruginenes A-C from Rhododendron ferrugineum and their cytotoxic evaluation. J. Nat. Prod. 74 (4), 712–717. doi:10.1021/np100778k
Shi, Q., Li, T. T., Wu, Y. M., Sun, X. Y., Lei, C., Li, J. Y., et al. (2020). Meroterpenoids with diverse structures and anti-inflammatory activities from Rhododendron anthopogonoides. Phytochemistry 180, 112524. doi:10.1016/j.phytochem.2020.112524
Srivastava, P. (2012). Rhododendron arboreum: an overview. J. Appl. Pharm. Sci., 158–162. Available online at: https://japsonline.com/admin/php/uploads/362_pdf.pdf.
Ștefănescu, B. E., Szabo, K., Mocan, A., and Crişan, G. (2019). Phenolic compounds from five ericaceae species leaves and their related bioavailability and health benefits. Molecules 24 (11), 2046. doi:10.3390/molecules24112046
Sun, N., Zheng, G., He, M., Feng, Y., Liu, J., Wang, M., et al. (2019). Grayanane diterpenoids from the leaves of Rhododendron auriculatum and their analgesic activities. J. Nat. Prod. 82 (7), 1849–1860. doi:10.1021/acs.jnatprod.9b00095
Sun, N., Zhu, Y., Zhou, H., Zhou, J., Zhang, H., Zhang, M., et al. (2018). Grayanane diterpenoid glucosides from the leaves of Rhododendron micranthum and their bioactivities evaluation. J. Nat. Prod. 81 (12), 2673–2681. doi:10.1021/acs.jnatprod.8b00490
Sun, Q., Li, X., Sun, L., Sun, M., Xu, H., and Zhou, X. (2024). Plant hormones and phenolic acids response to UV-B stress in Rhododendron chrysanthum pall. Biol. Direct 19 (1), 40. doi:10.1186/s13062-024-00483-0
Tomoki, N., Takuma, S., Nanang, R. A., Koseki, T., Aboshi, T., Murayama, T., et al. (2019). Meroterpenoids produced by pseudocosmospora sp. Bm-1-1 isolated from Acanthus ebracteatus vahl. Phytochem. Lett. 31, 85–91. doi:10.1016/j.phytol.2019.03.014
Vengrytė, M., and Raudonė, L. (2024). Phytochemical profiling and biological activities of rhododendron subsect. Ledum: discovering the medicinal potential of labrador tea species in the northern hemisphere. Plants 13 (6), 901. doi:10.3390/plants13060901
Wang, S., Lin, S., Zhu, C., Yang, Y., Zhang, J., Chen, X. G., and Shi, J. G. (2010). Highly acylated diterpenoids with a new 3,4-secograyanane skeleton from the flower buds of Rhododendron molle. Org. Lett. 12 (7), 1560–1563. doi:10.1021/ol1002797
Wang, X., Li, L., Zhu, R., Zhang, J., Zhou, J., and Lou, H. (2017). Bibenzyl-based meroterpenoid enantiomers from the Chinese liverwort Radula sumatrana. J. Nat. Prod. 80 (12), 3143–3150. doi:10.1021/acs.jnatprod.7b00394
Wei, L., Hongping, H., Chufang, L., Cuomu, M., Jintao, L., Kaiyin, C., et al. (2023). Effects of shiwei longdanhua formula on LPS induced airway mucus hypersecretion, cough hypersensitivity, oxidative stress and pulmonary inflammation. Biomed. Pharmacother. 163, 114793. doi:10.1016/j.biopha.2023.114793
Wu, H., Hsung, R. P., and Tang, Y. (2017). Total syntheses of (±)-Rhodonoids C, D, E, F, and G and ranhuadujuanine B. Org. Lett. 19 (13), 3505–3507. doi:10.1021/acs.orglett.7b01463
Wu, Y. M., Shi, Q., Zhu, P. F., Ma, H. J., Cui, S. C., Li, J., et al. (2021). Rhodomeroterpene alleviates macrophage infiltration and the inflammatory response in renal tissue to improve acute kidney injury. FASEB J. 35 (11), e21985. doi:10.1096/fj.202100981RR
Xu, X., Xu, H., Shang, Y., Zhu, R., Hong, X., Song, Z., et al. (2021). Development of the general chapters of the Chinese pharmacopoeia 2020 edition: a review. J. Pharm. analysis 11 (4), 398–404. doi:10.1016/j.jpha.2021.05.001
Yang, F. S., Nie, S., Liu, H., Shi, T. L., Tian, X. C., Zhou, S. S., et al. (2020). Chromosome-level genome assembly of a parent species of widely cultivated azaleas. Nat. Commun. 11 (1), 5269. doi:10.1038/s41467-020-18771-4
Yang, J., Zhao, J., and Zhang, J. (2022). The efficacy and toxicity of grayanoids as analgesics: a systematic review. J. Ethnopharmacol. 298, 115581. doi:10.1016/j.jep.2022.115581
Yang, K., Zhou, Y. X., Wang, C. F., Du, S. S., Deng, Z. W., Liu, Q. Z., et al. (2011). Toxicity of Rhododendron anthopogonoides essential oil and its constituent compounds towards Sitophilus zeamais. Molecules 16 (9), 7320–7330. doi:10.3390/molecules16097320
Yang, Y. X., Wang, J. X., Wang, Q., Li, H. L., Tao, M., Luo, Q., et al. (2018). New chromane and chromene meroterpenoids from flowers of Rhododendron rubiginosum franch. Var. rubiginosum. Fitoterapia 127, 396–401. doi:10.1016/j.fitote.2018.03.017
Yu, W., Gong, F., Zhou, X., Xu, H., and Lyu, J. (2024). Comparative metabolomics and transcriptome studies of two forms of Rhododendron chrysanthum pall. Under UV-B stress. Biology 13 (4), 211. doi:10.3390/biology13040211
Zeng, X., Zhao, X., Liu, W., and Yuan, T. (2023). Meroterpenoids from Rhododendron nivale. Zhongguo Zhong Yao Za Zhi 48 (5), 1273–1279. doi:10.19540/j.cnki.cjcmm.20221226.201
Zhang, M., Zhu, Y., Zhan, G., Shu, P., Sa, R., Lei, L., et al. (2013). Micranthanone A, a new diterpene with an unprecedented carbon skeleton from Rhododendron micranthum. Org. Lett. 15 (12), 3094–3097. doi:10.1021/ol401292y
Zhang, N., Xiong, L. L., Sun, D. J., Zhu, M., Zhao, Z. Y., Li, H., et al. (2022). Anti-infammatory scalemic chromanoids and chromenoids from Rhododendron dauricum. Fitoterapia 162, 105300. doi:10.1016/j.fitote.2022.105300
Zhang, Y., Wang, J., Wang, X., Wang, L., Wang, Y, Wei, J., et al. (2024). Population structures and dynamics of rhododendron communities with different stages of succession in northwest Guizhou, China. Plants 13 (7), 946. doi:10.3390/plants13070946
Zhao, H., Chen, G. D., Zou, J., He, R. R., Qin, S. Y., Hu, D., et al. (2017). Dimericbiscognienyne A: a meroterpenoid dimer from biscogniauxia sp. with new skeleton and its activity. Org. Lett. 19 (1), 38–41. doi:10.1021/acs.orglett.6b03264
Zhao, M., Tang, Y., Xie, J., Zhao, Z., and Cui, H. (2021). Meroterpenoids produced by fungi: occurrence, structural diversity, biological activities, and their molecular targets. Eur. J. Med. Chem. 209, 112860. doi:10.1016/j.ejmech.2020.112860
Zheng, G., Huang, L., Feng, Y., Zhang, H., Gao, B., Ma, X., et al. (2024a). Discovery of highly functionalized grayanane diterpenoids from the flowers of Rhododendron molle as potent analgesics. Bioorg Chem. 142, 106928. doi:10.1016/j.bioorg.2023.106928
Zheng, G., Huang, L., Feng, Y., Zhang, H., Ma, X., Gao, B., et al. (2024b). Structurally diverse analgesic diterpenoids from the flowers of Rhododendron molle. Fitoterapia 172, 105770. doi:10.1016/j.fitote.2023.105770
Zhou, J., Zhan, G., Zhang, H., Zhang, Q., Xue, Y., and Yao, G. M. (2017). Rhodomollanol A, a highly oxygenated diterpenoid with a 5/7/5/5 tetracyclic carbon skeleton from the leaves of Rhododendron molle. Org. Lett. 19 (14), 3935–3938. doi:10.1021/acs.orglett.7b01863
Zhou, L., Yang, S., and Zou, X. (2022). Farrerol alleviates myocardial ischemia/reperfusion injury by targeting macrophages and NLRP3. Front. Pharmacol. 13, 879232. doi:10.3389/fphar.2022.879232
Zhou, X., Liu, M., Lu, X., Sun, S. S., Cheng, Y. W., and Ya, H. Y. (2020). Genome survey sequencing and identification of genomic SSR markers for Rhododendron micranthum. Biosci. Rep. 40 (6), BSR20200988. doi:10.1042/BSR20200988
Zhu, Y. X., Zhang, Z. X., Yan, H. M., Lu, D., Zhang, H. P., Li, L., et al. (2018). Antinociceptive diterpenoids from the leaves and twigs of Rhododendron decorum. J. Nat. Prod. 81 (5), 1183–1192. doi:10.1021/acs.jnatprod.7b00941
Keywords: traditional Chinese medicines, Rhododendron genus, phytochemistry, biological activities, analyses
Citation: Mao J, Yang M, Li T, Sun Y, Dong Z, Zhan H and Chen M (2025) Research progress on heteroterpene and meroterpenoid compounds from the Rhododendron genus and their NMR characterization and biological activity. Front. Pharmacol. 16:1584962. doi: 10.3389/fphar.2025.1584962
Received: 28 February 2025; Accepted: 02 July 2025;
Published: 29 July 2025.
Edited by:
Hui Cui, Guangzhou University of Chinese Medicine, ChinaReviewed by:
Francis-Alfred Unuagbe Attah, University of Ilorin, NigeriaTanvir Ul Hassan Dar, Baba Ghulam Shah Badshah University, India
Copyright © 2025 Mao, Yang, Li, Sun, Dong, Zhan and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Chen, bW1pbmNoZW5Ac3d1LmVkdS5jbg==
 Jingxin Mao
Jingxin Mao Meiyan Yang2,3
Meiyan Yang2,3 Min Chen
Min Chen